JP2005524436A - 組織pHモニターのシステムおよび方法 - Google Patents
組織pHモニターのシステムおよび方法 Download PDFInfo
- Publication number
- JP2005524436A JP2005524436A JP2004500681A JP2004500681A JP2005524436A JP 2005524436 A JP2005524436 A JP 2005524436A JP 2004500681 A JP2004500681 A JP 2004500681A JP 2004500681 A JP2004500681 A JP 2004500681A JP 2005524436 A JP2005524436 A JP 2005524436A
- Authority
- JP
- Japan
- Prior art keywords
- tissue
- electrode
- acidosis
- preservation solution
- heart
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 97
- 238000012544 monitoring process Methods 0.000 title claims description 46
- 208000010444 Acidosis Diseases 0.000 claims abstract description 124
- 230000007950 acidosis Effects 0.000 claims abstract description 124
- 208000026545 acidosis disease Diseases 0.000 claims abstract description 124
- 238000001356 surgical procedure Methods 0.000 claims abstract description 79
- 208000028867 ischemia Diseases 0.000 claims abstract description 35
- 239000012530 fluid Substances 0.000 claims abstract description 32
- 238000001139 pH measurement Methods 0.000 claims abstract description 26
- 210000005240 left ventricle Anatomy 0.000 claims abstract description 12
- 210000001519 tissue Anatomy 0.000 claims description 203
- 230000002107 myocardial effect Effects 0.000 claims description 159
- 210000002216 heart Anatomy 0.000 claims description 73
- 239000000523 sample Substances 0.000 claims description 50
- 238000007675 cardiac surgery Methods 0.000 claims description 44
- 238000012937 correction Methods 0.000 claims description 44
- 239000003761 preservation solution Substances 0.000 claims description 39
- 210000004165 myocardium Anatomy 0.000 claims description 34
- 210000004351 coronary vessel Anatomy 0.000 claims description 32
- 239000000243 solution Substances 0.000 claims description 29
- 210000003748 coronary sinus Anatomy 0.000 claims description 27
- 230000000250 revascularization Effects 0.000 claims description 27
- 238000004393 prognosis Methods 0.000 claims description 26
- 238000005259 measurement Methods 0.000 claims description 24
- 238000012545 processing Methods 0.000 claims description 24
- 230000002980 postoperative effect Effects 0.000 claims description 23
- 230000004083 survival effect Effects 0.000 claims description 22
- 238000003780 insertion Methods 0.000 claims description 21
- 230000037431 insertion Effects 0.000 claims description 21
- 230000002861 ventricular Effects 0.000 claims description 20
- 230000010410 reperfusion Effects 0.000 claims description 15
- 239000003755 preservative agent Substances 0.000 claims description 10
- 230000002335 preservative effect Effects 0.000 claims description 10
- 210000005003 heart tissue Anatomy 0.000 claims description 9
- 230000001965 increasing effect Effects 0.000 claims description 9
- 210000001367 artery Anatomy 0.000 claims description 8
- 230000002411 adverse Effects 0.000 claims description 7
- 230000008520 organization Effects 0.000 claims description 6
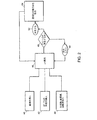
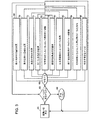
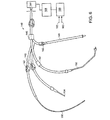
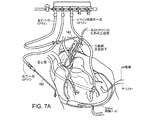
- 238000010586 diagram Methods 0.000 claims description 4
- 238000004321 preservation Methods 0.000 claims description 4
- 238000009529 body temperature measurement Methods 0.000 claims description 2
- 238000004891 communication Methods 0.000 claims description 2
- 230000004872 arterial blood pressure Effects 0.000 claims 4
- 230000037452 priming Effects 0.000 claims 1
- 230000035440 response to pH Effects 0.000 claims 1
- 230000000302 ischemic effect Effects 0.000 abstract description 27
- 230000008859 change Effects 0.000 abstract description 19
- 238000003860 storage Methods 0.000 abstract description 10
- 238000002405 diagnostic procedure Methods 0.000 abstract description 3
- 229940100084 cardioplegia solution Drugs 0.000 description 40
- 238000007726 management method Methods 0.000 description 25
- 210000004369 blood Anatomy 0.000 description 24
- 239000008280 blood Substances 0.000 description 24
- 239000000372 cardioplegic agent Substances 0.000 description 22
- 230000036961 partial effect Effects 0.000 description 19
- 239000011521 glass Substances 0.000 description 18
- 210000003734 kidney Anatomy 0.000 description 18
- 230000007774 longterm Effects 0.000 description 18
- 208000010496 Heart Arrest Diseases 0.000 description 15
- 239000000872 buffer Substances 0.000 description 15
- 230000000747 cardiac effect Effects 0.000 description 14
- 230000002612 cardiopulmonary effect Effects 0.000 description 14
- 230000007423 decrease Effects 0.000 description 14
- 230000004044 response Effects 0.000 description 14
- 239000001257 hydrogen Substances 0.000 description 13
- 229910052739 hydrogen Inorganic materials 0.000 description 13
- -1 hydrogen ions Chemical class 0.000 description 13
- 210000000056 organ Anatomy 0.000 description 13
- 230000006378 damage Effects 0.000 description 12
- 230000010412 perfusion Effects 0.000 description 12
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 10
- 230000017531 blood circulation Effects 0.000 description 10
- 239000003795 chemical substances by application Substances 0.000 description 10
- 238000009826 distribution Methods 0.000 description 10
- GPRLSGONYQIRFK-UHFFFAOYSA-N hydron Chemical compound [H+] GPRLSGONYQIRFK-UHFFFAOYSA-N 0.000 description 10
- 229910021607 Silver chloride Inorganic materials 0.000 description 9
- 230000003872 anastomosis Effects 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- HKZLPVFGJNLROG-UHFFFAOYSA-M silver monochloride Chemical compound [Cl-].[Ag+] HKZLPVFGJNLROG-UHFFFAOYSA-M 0.000 description 9
- 238000002054 transplantation Methods 0.000 description 9
- 230000001746 atrial effect Effects 0.000 description 8
- 230000006907 apoptotic process Effects 0.000 description 7
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 7
- 238000001514 detection method Methods 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 239000001301 oxygen Substances 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 210000005241 right ventricle Anatomy 0.000 description 7
- 238000012795 verification Methods 0.000 description 7
- 229910000831 Steel Inorganic materials 0.000 description 6
- 208000027418 Wounds and injury Diseases 0.000 description 6
- 238000009825 accumulation Methods 0.000 description 6
- 239000012491 analyte Substances 0.000 description 6
- 230000001186 cumulative effect Effects 0.000 description 6
- 208000014674 injury Diseases 0.000 description 6
- 230000004060 metabolic process Effects 0.000 description 6
- 208000031225 myocardial ischemia Diseases 0.000 description 6
- 230000002093 peripheral effect Effects 0.000 description 6
- 239000010959 steel Substances 0.000 description 6
- 230000009885 systemic effect Effects 0.000 description 6
- ZKHQWZAMYRWXGA-KQYNXXCUSA-J ATP(4-) Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)[C@H]1O ZKHQWZAMYRWXGA-KQYNXXCUSA-J 0.000 description 5
- ZKHQWZAMYRWXGA-UHFFFAOYSA-N Adenosine triphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O ZKHQWZAMYRWXGA-UHFFFAOYSA-N 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 210000000709 aorta Anatomy 0.000 description 5
- 210000001765 aortic valve Anatomy 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 230000003177 cardiotonic effect Effects 0.000 description 5
- 230000001976 improved effect Effects 0.000 description 5
- 230000003834 intracellular effect Effects 0.000 description 5
- 210000004185 liver Anatomy 0.000 description 5
- 206010033675 panniculitis Diseases 0.000 description 5
- 238000007639 printing Methods 0.000 description 5
- 230000000750 progressive effect Effects 0.000 description 5
- 210000004304 subcutaneous tissue Anatomy 0.000 description 5
- 230000035899 viability Effects 0.000 description 5
- 206010019280 Heart failures Diseases 0.000 description 4
- 238000005481 NMR spectroscopy Methods 0.000 description 4
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical class [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 230000036770 blood supply Effects 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 230000008602 contraction Effects 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 230000004064 dysfunction Effects 0.000 description 4
- 239000003792 electrolyte Substances 0.000 description 4
- 230000010102 embolization Effects 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 210000003752 saphenous vein Anatomy 0.000 description 4
- 239000011550 stock solution Substances 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- YZCKVEUIGOORGS-UHFFFAOYSA-N Hydrogen atom Chemical compound [H] YZCKVEUIGOORGS-UHFFFAOYSA-N 0.000 description 3
- 206010022680 Intestinal ischaemia Diseases 0.000 description 3
- 208000032382 Ischaemic stroke Diseases 0.000 description 3
- 230000006538 anaerobic glycolysis Effects 0.000 description 3
- 239000007853 buffer solution Substances 0.000 description 3
- 238000004364 calculation method Methods 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 230000000004 hemodynamic effect Effects 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 210000003405 ileum Anatomy 0.000 description 3
- 239000007943 implant Substances 0.000 description 3
- 239000005355 lead glass Substances 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 239000006174 pH buffer Substances 0.000 description 3
- 230000002265 prevention Effects 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 239000012487 rinsing solution Substances 0.000 description 3
- 239000012266 salt solution Substances 0.000 description 3
- 229910052709 silver Inorganic materials 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- 101100243025 Arabidopsis thaliana PCO2 gene Proteins 0.000 description 2
- 201000000057 Coronary Stenosis Diseases 0.000 description 2
- 206010011084 Coronary artery embolism Diseases 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 208000031481 Pathologic Constriction Diseases 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 238000002583 angiography Methods 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 230000010104 catheter embolization Effects 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 230000001010 compromised effect Effects 0.000 description 2
- 208000029078 coronary artery disease Diseases 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 230000010339 dilation Effects 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 230000004907 flux Effects 0.000 description 2
- 230000004116 glycogenolysis Effects 0.000 description 2
- 230000034659 glycolysis Effects 0.000 description 2
- 230000002631 hypothermal effect Effects 0.000 description 2
- 230000010354 integration Effects 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 239000002207 metabolite Substances 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 210000000663 muscle cell Anatomy 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 230000000399 orthopedic effect Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 230000010349 pulsation Effects 0.000 description 2
- 238000000611 regression analysis Methods 0.000 description 2
- 210000002254 renal artery Anatomy 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 210000005245 right atrium Anatomy 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 210000000813 small intestine Anatomy 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 230000036262 stenosis Effects 0.000 description 2
- 208000037804 stenosis Diseases 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 238000004659 sterilization and disinfection Methods 0.000 description 2
- 238000011477 surgical intervention Methods 0.000 description 2
- 210000000115 thoracic cavity Anatomy 0.000 description 2
- 210000002978 thoracic duct Anatomy 0.000 description 2
- 230000036962 time dependent Effects 0.000 description 2
- 230000001052 transient effect Effects 0.000 description 2
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 241001553178 Arachis glabrata Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- 208000031104 Arterial Occlusive disease Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 208000001778 Coronary Occlusion Diseases 0.000 description 1
- 206010011086 Coronary artery occlusion Diseases 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- 101710088194 Dehydrogenase Proteins 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 108010044467 Isoenzymes Proteins 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 208000009378 Low Cardiac Output Diseases 0.000 description 1
- 238000013494 PH determination Methods 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 206010063900 Steal syndrome Diseases 0.000 description 1
- 206010047139 Vasoconstriction Diseases 0.000 description 1
- 206010047281 Ventricular arrhythmia Diseases 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 210000002376 aorta thoracic Anatomy 0.000 description 1
- 238000003782 apoptosis assay Methods 0.000 description 1
- 208000021328 arterial occlusion Diseases 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 238000010009 beating Methods 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000005013 brain tissue Anatomy 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 210000005242 cardiac chamber Anatomy 0.000 description 1
- 210000004413 cardiac myocyte Anatomy 0.000 description 1
- 230000001101 cardioplegic effect Effects 0.000 description 1
- 239000008148 cardioplegic solution Substances 0.000 description 1
- 229940045200 cardioprotective agent Drugs 0.000 description 1
- 239000000496 cardiotonic agent Substances 0.000 description 1
- 210000000748 cardiovascular system Anatomy 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 230000003727 cerebral blood flow Effects 0.000 description 1
- 230000007012 clinical effect Effects 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 238000002052 colonoscopy Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 230000008828 contractile function Effects 0.000 description 1
- 239000013256 coordination polymer Substances 0.000 description 1
- 238000002586 coronary angiography Methods 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000002716 delivery method Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 210000000188 diaphragm Anatomy 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000011833 dog model Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 229920002457 flexible plastic Polymers 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 229910052816 inorganic phosphate Inorganic materials 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 210000002429 large intestine Anatomy 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 210000001349 mammary artery Anatomy 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 210000003975 mesenteric artery Anatomy 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 208000037891 myocardial injury Diseases 0.000 description 1
- 210000000107 myocyte Anatomy 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 238000011022 operating instruction Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000011224 oxide ceramic Substances 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000005522 programmed cell death Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 239000003223 protective agent Substances 0.000 description 1
- 230000000541 pulsatile effect Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000012088 reference solution Substances 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000004065 semiconductor Substances 0.000 description 1
- 208000037974 severe injury Diseases 0.000 description 1
- 230000009528 severe injury Effects 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 210000001562 sternum Anatomy 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 210000003932 urinary bladder Anatomy 0.000 description 1
- 230000025033 vasoconstriction Effects 0.000 description 1
- 210000001631 vena cava inferior Anatomy 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14539—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue for measuring pH
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/41—Detecting, measuring or recording for evaluating the immune or lymphatic systems
- A61B5/413—Monitoring transplanted tissue or organ, e.g. for possible rejection reactions after a transplant
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Physics & Mathematics (AREA)
- Heart & Thoracic Surgery (AREA)
- Molecular Biology (AREA)
- Veterinary Medicine (AREA)
- Biophysics (AREA)
- Pathology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Public Health (AREA)
- Medical Informatics (AREA)
- General Health & Medical Sciences (AREA)
- Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- Vascular Medicine (AREA)
- Transplantation (AREA)
- Immunology (AREA)
- Optics & Photonics (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Surgical Instruments (AREA)
- Measuring And Recording Apparatus For Diagnosis (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/137,709 US20030040665A1 (en) | 1999-05-28 | 2002-05-02 | Systems and methods of pH tissue monitoring |
| PCT/US2003/015328 WO2003092489A1 (en) | 1999-05-28 | 2003-05-02 | Systems and methods of ph tissue monitoring |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005524436A true JP2005524436A (ja) | 2005-08-18 |
| JP2005524436A5 JP2005524436A5 (enExample) | 2006-06-29 |
Family
ID=33550847
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004500681A Pending JP2005524436A (ja) | 2002-05-02 | 2003-05-02 | 組織pHモニターのシステムおよび方法 |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP1503661B1 (enExample) |
| JP (1) | JP2005524436A (enExample) |
| AT (1) | ATE408999T1 (enExample) |
| AU (1) | AU2003239469A1 (enExample) |
| DE (1) | DE60323746D1 (enExample) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013214317A (ja) * | 2013-06-05 | 2013-10-17 | Toshiba Tec Corp | 決済端末及びその制御プログラム |
| JP2014503235A (ja) * | 2010-11-11 | 2014-02-13 | ゾール メディカル コーポレイション | 急性期介護処置システムの計器盤 |
| JP2017512588A (ja) * | 2014-03-31 | 2017-05-25 | ガンブロ・ルンディア・エービーGambro Lundia Ab | 体外血液処理のアラームドッキング |
| US12102590B2 (en) | 2020-03-30 | 2024-10-01 | Zoll Medical Corporation | Medical device system and hardware for sensor data acquisition |
| US12214211B2 (en) | 2020-09-04 | 2025-02-04 | Zoll Medical Corporation | Medical treatment system with companion device |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2992927B1 (en) * | 2014-09-04 | 2017-04-05 | BIOTRONIK SE & Co. KG | Activity monitor and rate-adaptive implantable leadless pacemaker |
-
2003
- 2003-05-02 AU AU2003239469A patent/AU2003239469A1/en not_active Abandoned
- 2003-05-02 DE DE60323746T patent/DE60323746D1/de not_active Expired - Fee Related
- 2003-05-02 AT AT03734037T patent/ATE408999T1/de not_active IP Right Cessation
- 2003-05-02 EP EP03734037A patent/EP1503661B1/en not_active Expired - Lifetime
- 2003-05-02 JP JP2004500681A patent/JP2005524436A/ja active Pending
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014503235A (ja) * | 2010-11-11 | 2014-02-13 | ゾール メディカル コーポレイション | 急性期介護処置システムの計器盤 |
| JP2016187562A (ja) * | 2010-11-11 | 2016-11-04 | ゾール メディカル コーポレイションZOLL Medical Corporation | 急性期介護処置システムの計器盤 |
| US10485490B2 (en) | 2010-11-11 | 2019-11-26 | Zoll Medical Corporation | Acute care treatment systems dashboard |
| US10959683B2 (en) | 2010-11-11 | 2021-03-30 | Zoll Medical Corporation | Acute care treatment systems dashboard |
| US11759152B2 (en) | 2010-11-11 | 2023-09-19 | Zoll Medical Corporation | Acute care treatment systems dashboard |
| US11826181B2 (en) | 2010-11-11 | 2023-11-28 | Zoll Medical Corporation | Acute care treatment systems dashboard |
| US12207953B2 (en) | 2010-11-11 | 2025-01-28 | Zoll Medical Corporation | Acute care treatment systems dashboard |
| JP2013214317A (ja) * | 2013-06-05 | 2013-10-17 | Toshiba Tec Corp | 決済端末及びその制御プログラム |
| JP2017512588A (ja) * | 2014-03-31 | 2017-05-25 | ガンブロ・ルンディア・エービーGambro Lundia Ab | 体外血液処理のアラームドッキング |
| US12102590B2 (en) | 2020-03-30 | 2024-10-01 | Zoll Medical Corporation | Medical device system and hardware for sensor data acquisition |
| US12214211B2 (en) | 2020-09-04 | 2025-02-04 | Zoll Medical Corporation | Medical treatment system with companion device |
Also Published As
| Publication number | Publication date |
|---|---|
| DE60323746D1 (de) | 2008-11-06 |
| AU2003239469A1 (en) | 2003-11-17 |
| ATE408999T1 (de) | 2008-10-15 |
| EP1503661B1 (en) | 2008-09-24 |
| EP1503661A1 (en) | 2005-02-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6600941B1 (en) | Systems and methods of pH tissue monitoring | |
| US20030040665A1 (en) | Systems and methods of pH tissue monitoring | |
| Ready et al. | Assessment of the risk of bleeding from esophageal varices by continuous monitoring of portal pressure | |
| US6351667B1 (en) | Device for detecting pericardial effusion | |
| JP4625808B2 (ja) | 埋め込み型マルチパラメータ検出システム及びその製造方法 | |
| US8075490B2 (en) | Vascular resistance gauge | |
| CN113473904A (zh) | 使用来自多个传感器的测量数据的医疗系统 | |
| US20100057046A1 (en) | Systems for characterizing physiologic parameters and methods for use therewith | |
| US20160100797A1 (en) | Access needle with direct visualization and related methods | |
| US20100106140A1 (en) | Assay catheter with pressure monitoring | |
| Khabbaz et al. | Intraoperative metabolic monitoring of the heart: II. Online measurement of myocardial tissue pH | |
| JP2010284532A (ja) | 患者の生理的パラメータを確定するための装置、及びその方法 | |
| BRPI0513428B1 (pt) | dispositivo de detecção fisiológica para a medição de pco2 | |
| EP1503661B1 (en) | Systems of ph tissue monitoring | |
| JP4227519B2 (ja) | 後細動脈圧の測定のための方法 | |
| US20220401061A1 (en) | Device and Method for Diagnosis of Cardiac Tamponade | |
| JP2005524436A5 (enExample) | ||
| Khan et al. | Clinical application of proximal arch cannulation in the surgical treatment of acute type I aortic dissection | |
| GB2279253A (en) | Method and apparatus for haemodyamic assessment | |
| US20200164133A1 (en) | Method of sensing and sensing cannula for use during cardiac surgery | |
| Lajos et al. | A permanent experimental model for reversible myocardial anoxia | |
| SIMPSON | Monitoring During Anesthesia for Cardiac Surgery | |
| Stokes et al. | Haemodynamic monitoring with the Swan-Ganz catheter | |
| Gumbrell et al. | Development of a minimally invasive microvascular ischemia monitor: clinical detection of ischemia | |
| STEWART et al. | Instrumentation and Monitoring During Open-Heart Surgery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20060501 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20060501 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20080909 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090519 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090818 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090825 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100216 |