JP2005330486A - ハイオクタンガソリンの製造法 - Google Patents
ハイオクタンガソリンの製造法 Download PDFInfo
- Publication number
- JP2005330486A JP2005330486A JP2005144751A JP2005144751A JP2005330486A JP 2005330486 A JP2005330486 A JP 2005330486A JP 2005144751 A JP2005144751 A JP 2005144751A JP 2005144751 A JP2005144751 A JP 2005144751A JP 2005330486 A JP2005330486 A JP 2005330486A
- Authority
- JP
- Japan
- Prior art keywords
- paraffins
- catalyst
- dehydrogenation
- branched
- stage
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 title abstract description 19
- 238000004519 manufacturing process Methods 0.000 title abstract description 7
- 238000000034 method Methods 0.000 claims abstract description 59
- 239000003054 catalyst Substances 0.000 claims abstract description 34
- 230000008569 process Effects 0.000 claims abstract description 32
- 238000006356 dehydrogenation reaction Methods 0.000 claims abstract description 30
- 239000000203 mixture Substances 0.000 claims abstract description 26
- 238000006317 isomerization reaction Methods 0.000 claims abstract description 22
- 238000000926 separation method Methods 0.000 claims abstract description 16
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims abstract description 14
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 claims abstract description 14
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 claims abstract description 12
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 claims abstract description 12
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims abstract description 12
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims abstract description 11
- 230000002378 acidificating effect Effects 0.000 claims abstract description 10
- 239000011148 porous material Substances 0.000 claims abstract description 9
- 239000000463 material Substances 0.000 claims abstract description 8
- 229910052751 metal Inorganic materials 0.000 claims abstract description 8
- 239000002184 metal Substances 0.000 claims abstract description 8
- 229910052697 platinum Inorganic materials 0.000 claims abstract description 8
- 229910052763 palladium Inorganic materials 0.000 claims abstract description 7
- 239000000377 silicon dioxide Substances 0.000 claims abstract description 7
- 230000003197 catalytic effect Effects 0.000 claims abstract description 5
- 229910052703 rhodium Inorganic materials 0.000 claims abstract description 5
- 239000010948 rhodium Substances 0.000 claims abstract description 5
- 229910052741 iridium Inorganic materials 0.000 claims abstract description 4
- 150000001247 metal acetylides Chemical class 0.000 claims abstract description 4
- 150000004767 nitrides Chemical class 0.000 claims abstract description 4
- 239000003575 carbonaceous material Substances 0.000 claims abstract description 3
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 claims abstract description 3
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 claims abstract description 3
- 229930195733 hydrocarbon Natural products 0.000 claims description 25
- 150000002430 hydrocarbons Chemical class 0.000 claims description 25
- 239000001257 hydrogen Substances 0.000 claims description 21
- 229910052739 hydrogen Inorganic materials 0.000 claims description 21
- 239000004215 Carbon black (E152) Substances 0.000 claims description 20
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 20
- 150000001924 cycloalkanes Chemical class 0.000 claims description 12
- 125000001511 cyclopentyl group Chemical class [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 claims description 6
- 239000007788 liquid Substances 0.000 claims description 5
- 150000002739 metals Chemical class 0.000 claims description 3
- QGLKJKCYBOYXKC-UHFFFAOYSA-N nonaoxidotritungsten Chemical compound O=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1 QGLKJKCYBOYXKC-UHFFFAOYSA-N 0.000 claims description 3
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 claims description 3
- 229910052721 tungsten Inorganic materials 0.000 claims description 3
- 239000010937 tungsten Substances 0.000 claims description 3
- 229910001930 tungsten oxide Inorganic materials 0.000 claims description 3
- 241000588731 Hafnia Species 0.000 claims description 2
- 239000002253 acid Substances 0.000 claims description 2
- CJNBYAVZURUTKZ-UHFFFAOYSA-N hafnium(IV) oxide Inorganic materials O=[Hf]=O CJNBYAVZURUTKZ-UHFFFAOYSA-N 0.000 claims description 2
- 239000013335 mesoporous material Substances 0.000 claims 2
- 229910052799 carbon Inorganic materials 0.000 claims 1
- 125000000113 cyclohexyl group Chemical class [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 claims 1
- 150000002431 hydrogen Chemical class 0.000 claims 1
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 claims 1
- 229910001887 tin oxide Inorganic materials 0.000 claims 1
- 150000001491 aromatic compounds Chemical class 0.000 description 23
- 238000006243 chemical reaction Methods 0.000 description 18
- 239000012188 paraffin wax Substances 0.000 description 13
- 239000007789 gas Substances 0.000 description 9
- 239000010457 zeolite Substances 0.000 description 7
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 6
- 150000001923 cyclic compounds Chemical class 0.000 description 6
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical class CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 5
- 229910021536 Zeolite Inorganic materials 0.000 description 5
- 150000001336 alkenes Chemical class 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 5
- 150000001934 cyclohexanes Chemical class 0.000 description 4
- 238000000354 decomposition reaction Methods 0.000 description 4
- UAEPNZWRGJTJPN-UHFFFAOYSA-N methylcyclohexane Chemical compound CC1CCCCC1 UAEPNZWRGJTJPN-UHFFFAOYSA-N 0.000 description 4
- 229910021529 ammonia Inorganic materials 0.000 description 3
- 238000005899 aromatization reaction Methods 0.000 description 3
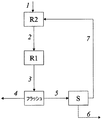
- 238000010586 diagram Methods 0.000 description 3
- 239000002808 molecular sieve Substances 0.000 description 3
- 229910000510 noble metal Inorganic materials 0.000 description 3
- 238000002407 reforming Methods 0.000 description 3
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 3
- XHCLAFWTIXFWPH-UHFFFAOYSA-N [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] XHCLAFWTIXFWPH-UHFFFAOYSA-N 0.000 description 2
- 150000001335 aliphatic alkanes Chemical class 0.000 description 2
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 239000010779 crude oil Substances 0.000 description 2
- 238000003795 desorption Methods 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- 239000000446 fuel Substances 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- GYNNXHKOJHMOHS-UHFFFAOYSA-N methyl-cycloheptane Natural products CC1CCCCCC1 GYNNXHKOJHMOHS-UHFFFAOYSA-N 0.000 description 2
- 229910001935 vanadium oxide Inorganic materials 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- VCUFZILGIRCDQQ-KRWDZBQOSA-N N-[[(5S)-2-oxo-3-(2-oxo-3H-1,3-benzoxazol-6-yl)-1,3-oxazolidin-5-yl]methyl]-2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carboxamide Chemical compound O=C1O[C@H](CN1C1=CC2=C(NC(O2)=O)C=C1)CNC(=O)C=1C=NC(=NC=1)NCC1=CC(=CC=C1)OC(F)(F)F VCUFZILGIRCDQQ-KRWDZBQOSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical class CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 229910006404 SnO 2 Inorganic materials 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 1
- VXAUWWUXCIMFIM-UHFFFAOYSA-M aluminum;oxygen(2-);hydroxide Chemical compound [OH-].[O-2].[Al+3] VXAUWWUXCIMFIM-UHFFFAOYSA-M 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 238000004517 catalytic hydrocracking Methods 0.000 description 1
- 238000001833 catalytic reforming Methods 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 150000001925 cycloalkenes Chemical class 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000011038 discontinuous diafiltration by volume reduction Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 229910021476 group 6 element Inorganic materials 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 150000002500 ions Chemical group 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000005272 metallurgy Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- UJVRJBAUJYZFIX-UHFFFAOYSA-N nitric acid;oxozirconium Chemical compound [Zr]=O.O[N+]([O-])=O.O[N+]([O-])=O UJVRJBAUJYZFIX-UHFFFAOYSA-N 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- IYDGMDWEHDFVQI-UHFFFAOYSA-N phosphoric acid;trioxotungsten Chemical compound O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.O=[W](=O)=O.OP(O)(O)=O IYDGMDWEHDFVQI-UHFFFAOYSA-N 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 230000007420 reactivation Effects 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- CGFYHILWFSGVJS-UHFFFAOYSA-N silicic acid;trioxotungsten Chemical compound O[Si](O)(O)O.O=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1.O=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1.O=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1.O=[W]1(=O)O[W](=O)(=O)O[W](=O)(=O)O1 CGFYHILWFSGVJS-UHFFFAOYSA-N 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 229910001928 zirconium oxide Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G35/00—Reforming naphtha
- C10G35/04—Catalytic reforming
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/0201—Impregnation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/40—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals of the platinum group metals
- B01J23/44—Palladium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/40—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals of the platinum group metals
- B01J23/46—Ruthenium, rhodium, osmium or iridium
- B01J23/464—Rhodium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/54—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals combined with metals, oxides or hydroxides provided for in groups B01J23/02 - B01J23/36
- B01J23/56—Platinum group metals
- B01J23/58—Platinum group metals with alkali- or alkaline earth metals
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G35/00—Reforming naphtha
- C10G35/04—Catalytic reforming
- C10G35/06—Catalytic reforming characterised by the catalyst used
- C10G35/085—Catalytic reforming characterised by the catalyst used containing platinum group metals or compounds thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
- C10G45/02—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing
- C10G45/04—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing characterised by the catalyst used
- C10G45/10—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing characterised by the catalyst used containing platinum group metals or compounds thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G59/00—Treatment of naphtha by two or more reforming processes only or by at least one reforming process and at least one process which does not substantially change the boiling range of the naphtha
- C10G59/02—Treatment of naphtha by two or more reforming processes only or by at least one reforming process and at least one process which does not substantially change the boiling range of the naphtha plural serial stages only
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Materials Engineering (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Catalysts (AREA)
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DKPA200400795 | 2004-05-18 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005330486A true JP2005330486A (ja) | 2005-12-02 |
| JP2005330486A5 JP2005330486A5 (enExample) | 2006-05-11 |
Family
ID=34934967
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2005144751A Withdrawn JP2005330486A (ja) | 2004-05-18 | 2005-05-17 | ハイオクタンガソリンの製造法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20050258076A1 (enExample) |
| EP (1) | EP1598411A1 (enExample) |
| JP (1) | JP2005330486A (enExample) |
| KR (1) | KR20060047979A (enExample) |
| CA (1) | CA2507578A1 (enExample) |
| ZA (1) | ZA200503916B (enExample) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7846880B2 (en) * | 2006-12-20 | 2010-12-07 | Chevron U.S.A. Inc. | Light base oil fraction and lubricant having low wt% noack volatility |
| US8202815B2 (en) * | 2008-12-26 | 2012-06-19 | General Electric Company | Catalyst composition for the hydro-treatment of alkanes and methods of use thereof |
| US20100331590A1 (en) * | 2009-06-25 | 2010-12-30 | Debarshi Majumder | Production of light olefins and aromatics |
| US8779224B2 (en) * | 2010-04-12 | 2014-07-15 | Shell Oil Company | Process for the production of gasoline blending components and aromatic hydrocarbons from lower alkanes |
| RU2486005C1 (ru) * | 2012-02-03 | 2013-06-27 | Федеральное Государственное Автономное Образовательное Учреждение Высшего Профессионального Образования "Сибирский Федеральный Университет" | Оксидный катализатор для изомеризации легких бензиновых фракций |
| EP2689843A1 (en) | 2012-07-26 | 2014-01-29 | Saudi Basic Industries Corporation | Alkane dehydrogenation catalyst and process for its preparation |
| US20140171702A1 (en) * | 2012-12-14 | 2014-06-19 | Uop Llc | Methods and apparatuses for increasing alkyl-cyclopentane concentrations in aromatic-rich streams |
| CN108722406B (zh) * | 2018-06-04 | 2020-10-23 | 山东麟丰化工科技有限公司 | 一种钨基催化剂、制备方法及其在异丁烷-丁烯烷基化反应中的用途 |
| BR102018072896A2 (pt) * | 2018-11-07 | 2020-05-26 | Petróleo Brasileiro S.A. - Petrobras | Processo para produzir um composto de isoparafina renovável, composto de isoparafina renovável, e, uso do composto de isoparafina renovável |
| US11066345B2 (en) * | 2019-06-27 | 2021-07-20 | Uop Llc | Processes for increasing an octane value of a gasoline component |
| US11338269B2 (en) | 2019-09-10 | 2022-05-24 | Saudi Arabian Oil Company | Catalytic hydrocarbon dehydrogenation |
| US11097257B2 (en) | 2019-09-10 | 2021-08-24 | Saudi Arabian Oil Company | Catalytic hydrocarbon dehydrogenation |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2288866A (en) * | 1939-04-17 | 1942-07-07 | Shell Dev | Treatment of hydrocarbons |
| US2767125A (en) * | 1951-06-13 | 1956-10-16 | Shell Dev | Process for improving hydrocarbon oils boiling in the gasoline range |
| US3116232A (en) * | 1961-12-01 | 1963-12-31 | Shell Oil Co | Process for upgrading cracked gasoline fractions |
| US4607129A (en) * | 1985-06-10 | 1986-08-19 | Phillips Petroleum Company | Catalytic dehydrocyclization and dehydrogenation of hydrocarbons |
| US4644089A (en) * | 1986-07-10 | 1987-02-17 | Phillips Petroleum Company | Catalytic reforming of hydrocarbons |
| FR2735488B1 (fr) * | 1995-06-16 | 1997-08-22 | Inst Francais Du Petrole | Procede de transformation catalytique d'hydrocarbures en composes aromatiques avec un catalyseur contenant des lanthanides |
| US5958217A (en) * | 1995-11-15 | 1999-09-28 | Chevron Chemical Company Llc | Two-stage reforming process that enhances para-xylene yield and minimizes ethylbenzene production |
| FR2771419B1 (fr) * | 1997-11-25 | 1999-12-31 | Inst Francais Du Petrole | Essences a haut indice d'octane et leur production par un procede associant hydro-isomerisation et separation |
| US6190534B1 (en) * | 1999-03-15 | 2001-02-20 | Uop Llc | Naphtha upgrading by combined olefin forming and aromatization |
| US6875339B2 (en) * | 2003-03-07 | 2005-04-05 | Conocophillips Company | Octane improvement of a hydrocarbon stream |
-
2005
- 2005-04-11 EP EP05007859A patent/EP1598411A1/en not_active Withdrawn
- 2005-04-26 US US11/113,993 patent/US20050258076A1/en not_active Abandoned
- 2005-05-16 ZA ZA200503916A patent/ZA200503916B/en unknown
- 2005-05-17 CA CA002507578A patent/CA2507578A1/en not_active Abandoned
- 2005-05-17 JP JP2005144751A patent/JP2005330486A/ja not_active Withdrawn
- 2005-05-18 KR KR1020050041434A patent/KR20060047979A/ko not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| US20050258076A1 (en) | 2005-11-24 |
| ZA200503916B (en) | 2006-02-22 |
| EP1598411A1 (en) | 2005-11-23 |
| CA2507578A1 (en) | 2005-11-18 |
| KR20060047979A (ko) | 2006-05-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6652737B2 (en) | Production of naphtha and light olefins | |
| JP3522797B2 (ja) | 炭化水素燃料の製造方法 | |
| EP1689834B1 (en) | Ring opening for increased olefin production | |
| JP3299538B2 (ja) | パラフィン異性化/開環を組合わせた方法 | |
| AU2001276996A1 (en) | Production of naphtha and light olefins | |
| US4935566A (en) | Dehydrocyclization and reforming process | |
| US10519387B2 (en) | Catalyst composition for converting light naphtha to aromatic compounds and a process thereof | |
| Onyestyák et al. | Cyclohexane conversion over H-zeolite supported platinum | |
| US5254787A (en) | Dehydrogenation and dehydrocyclization using a non-acidic NU-87 catalyst | |
| GB2319781A (en) | Simultaneous hydrogenation and isomerisation of benzene | |
| JP2005330486A (ja) | ハイオクタンガソリンの製造法 | |
| JP2004508161A (ja) | ナフテン環含有化合物のナフテン環を開環するための方法および触媒 | |
| US20210023537A1 (en) | Single step process for the simultaneous production of aromatics, naphthenics and isoparaffins using transition metal functionalized zeolite based catalyst | |
| EP0186479A2 (en) | Shape selective zeolite catalyst | |
| EP3237581B1 (en) | Process for producing c2 and c3 hydrocarbons | |
| US9273255B2 (en) | Production of middle distillates from an effluent originating from fischer-tropsch synthesis comprising a step of reducing the content of oxygenated compounds | |
| JP2004517711A (ja) | 分解調節剤を含む第viii族金属触媒上でのナフテン環の開環 | |
| US7037422B2 (en) | Process for producing high RON gasoline using CFI Zeolite | |
| KR0136583B1 (ko) | 디메틸부탄-비함유 탄화수소 분류물의 개질방법 | |
| WO2002007877A1 (en) | Ring opening with group viii metal catalysts supported on modified substrate | |
| EP2186785A2 (en) | Process for the separation of olefins from paraffins | |
| US20130270154A1 (en) | Optimized method for producing middle distillates from a feedstock originating from the fischer-tropsch process containing a limited quantity of oxygenated compounds | |
| EP0882002A1 (en) | Process for selectively opening naphthenic rings | |
| Traa et al. | A NOVEL PROCESS FOR CONVERTING SURPLUS AROMATICS INTO A HIGH-VALUE SYNTHETIC STEAMCRACKER FEEDSTOCK. INFLUENCE OF KEY PARAMETERS ON THE YIELDS DURING THE RING OPENING PROCESS AND ON THE PRODUCT COMPOSITION ACHIEVABLE IN THE STEAMCRACKER | |
| Al-Hassany | Effect of ZrO2, WO3 additives on catalytic performance of Pt/HY zeolite compared with Pt/γ-Al2O3 for Iraqi Naphtha transformation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20060322 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20060322 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20061121 |