JP2005097082A - Method of firing ceramics at low temperature and ceramics fired at low temperature - Google Patents
Method of firing ceramics at low temperature and ceramics fired at low temperature Download PDFInfo
- Publication number
- JP2005097082A JP2005097082A JP2004149767A JP2004149767A JP2005097082A JP 2005097082 A JP2005097082 A JP 2005097082A JP 2004149767 A JP2004149767 A JP 2004149767A JP 2004149767 A JP2004149767 A JP 2004149767A JP 2005097082 A JP2005097082 A JP 2005097082A
- Authority
- JP
- Japan
- Prior art keywords
- ceramics
- firing
- low
- temperature
- ceramic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P40/00—Technologies relating to the processing of minerals
- Y02P40/60—Production of ceramic materials or ceramic elements, e.g. substitution of clay or shale by alternative raw materials, e.g. ashes
Abstract
Description
本発明は、陶磁器産業、技術に関する The present invention relates to the ceramic industry and technology.
陶器や磁器は、長石、珪石、粘土を主要構成素材とし、陶器の場合、長石プラス珪石と粘土の割合が2:3、磁器の場合、反対に3:2とされ、焼成温度はそれぞれ略800℃と略1300℃以上とされてきた。粘土鉱物は100℃〜200℃の温度で周囲に吸着している吸着水が脱水し、500〜600℃でカオリナイトの結晶を構成し、SiやAlを結合していた水酸基(OH)が結晶から追い出され、カオリナイトはメタカオリンという非晶質に代わる。 Porcelain and porcelain are mainly composed of feldspar, silica and clay. In the case of pottery, the ratio of feldspar plus silica and clay is 2: 3. In the case of porcelain, the ratio is 3: 2, and the firing temperature is approximately 800. It has been set to about 1300 ° C. or higher. The clay mineral is dehydrated by the adsorbed water adsorbed to the surroundings at a temperature of 100 ° C. to 200 ° C., forms kaolinite crystals at 500 to 600 ° C., and the hydroxyl group (OH) bonded with Si and Al is crystallized. The kaolinite is replaced by an amorphous metal called metakaolin.
1300℃になると、このメタカオリンも破壊されて、γアルミナ(γAl2O3)やムライト(3Al2O3・2SiO2)となり、これに長石の働きで二酸化珪素や珪砂(いずれもSiO2)がガラス化し、このγアルミナやムライトの結晶にこのガラスが結合し、硬くて強い陶磁器ができる。これが通常の場合の陶磁器の生成機序である。しかし落下や互いの接触による打撃には弱い。At 1300 ° C., this metakaolin is also destroyed, becoming γ-alumina (γAl 2 O 3 ) and mullite (3Al 2 O 3 · 2SiO 2 ), and silicon dioxide and silica sand (both SiO 2 ) are added to the feldspar. The glass is vitrified and the glass is bonded to the crystals of γ-alumina and mullite, making a hard and strong ceramic. This is the normal mechanism of ceramic production. However, they are vulnerable to blows caused by falling or touching each other.
ところが、日常家庭でもっとも使用される食器は、この互いの接触や落下の機会が多く、破損することが多い。ちなみに日本の家庭から廃棄される不燃ごみ(鉄類、陶器類、プラスチック類など)のうち重量の半分が、この陶磁器であるという報告もある。この不燃ごみは、ごみの最終処分場に廃棄され、今ではこの最終処分場も残余処分能力がひっ迫し、社会問題となっている。 However, the tableware most used in everyday homes has many opportunities for mutual contact and dropping, and often breaks. By the way, it is reported that half of the weight of non-combustible waste (iron, ceramics, plastics, etc.) discarded from Japanese households is this ceramic. This incombustible waste is disposed of at the final disposal site for waste, and now this final disposal site is also a social problem due to its limited residual disposal capacity.
そこで、こうした廃棄陶磁器をリサイクルして使用して行こうという試みが行われ、「陶磁器製品リサイクルの新しい可能性(LCA評価)−磁器食器のリサイクルにおける環境負荷−」(「セラミックデータブック2000・別刷」発行/工業製品技術協会<テクノプラザ>)などという形で発表されている。 Therefore, an attempt was made to recycle and use these discarded ceramics, and "New potential for recycling ceramic products (LCA evaluation)-Environmental impact in recycling porcelain tableware" ("Ceramic Data Book 2000, separate print). "Issuance / Industrial Product Technology Association <Techno Plaza>).
可塑性の良い粘土鉱物を素材とした陶磁器は、手軽にいろいろな形状に作ることが出来るが、陶磁器として完成させるためには、1300℃という高温で焼成しなければならず、そこで消費されるエネルギーコストだけでなく、CO2排出による地球環境への影響も無視できなかった。Ceramics made from clay minerals with good plasticity can be easily made into various shapes, but in order to be completed as ceramics, they must be fired at a high temperature of 1300 ° C, and the energy cost consumed there. In addition, the impact on the global environment due to CO 2 emissions could not be ignored.
また、1300℃で焼成すると釉薬として赤や緑、黄色などの原色を出そうとしても、その原色の色は飛んでしまい、いわゆる下絵として、そうした色合いを出すことは難しかった。 In addition, when firing at 1300 ° C., even if primary colors such as red, green, and yellow are produced as glazes, the primary colors fly out and it is difficult to produce such shades as a so-called sketch.
多くのエネルギーを使って作った陶磁器も破損に弱く、割れやすく現状では割れたものはそのまま廃棄されていたため、処分場問題という新たな環境問題も引き起こしてきた。 Ceramics made using a lot of energy are also vulnerable to breakage, and because they are easy to break and the cracked pieces are discarded as they are, they have also caused new environmental problems such as disposal sites.
また、先のセラミックデータブックの報告などでは、企業で生産された規格外となったペケ品や、アンテナショップで回収した生活使用後の廃棄陶磁器を、再生利用する試みが報告されている。そこではペケ品を数mmに粗粉砕した後、ボールミルで湿式粉砕する方法が行われている。ペケ品といっても一度1300℃以上に焼成しているため、次に再生利用するときは、低温焼成が可能となるはずである。ところが、その報告で見ると、再焼成の温度は1280℃となっていて、ペケ品を使用するエネルギー面でのメリットがあまり見えない。また、ペケ品等廃棄陶磁器の再利用に当たっては、ペケ品等の利用率を20%以内に抑え、その他に粘土を30%、珪石20%、長石20%を混入して再生利用品を作ったことが報告されていた。あまり効率のよい再生利用方法ではない。 In the previous report of the ceramic data book, etc., attempts have been made to recycle the non-standard pique products produced by companies and the waste ceramics collected after use at the antenna shop. In this method, a pique product is roughly pulverized to several mm and then wet pulverized by a ball mill. Even if it is a peke product, it has been fired once at 1300 ° C. or higher, so that it should be able to be fired at a low temperature the next time it is recycled. However, according to the report, the re-baking temperature is 1280 ° C., and the merit in terms of energy using the pique product is not so much visible. Also, when reusing discarded ceramics such as piques, the recycling rate was reduced to less than 20%, and recycled products were made by mixing 30% clay, 20% silica, and 20% feldspar. It was reported. It is not a very efficient recycling method.
この問題を追試するために、ペケ品を含む廃棄陶磁器を、湿式粉砕機のボールミルで粉砕し、報告に書かれている方法で再利用品を作ってみた。ボールミルを使って所定の粒度(10μm以下)にするためには、24時間近くボールミルを作動させる必要がある。その追試によって大事なことがわかった。1度焼成し、磁器化した廃棄陶磁器でもその位の時間、湿式粉砕するとソーダ分がアルカリ溶出し、その結果泥しょうは、部分的に固まり、いわゆるどぼつくことがわかった。また、ソーダ分の流出により融点が上がり、焼成温度を通常の陶磁器の焼成温度(1300℃)レベルにまで高めなければガラス化し、いわゆる磁器化しないことがわかった。 In order to test this problem, the waste ceramics containing pique products were pulverized with a ball mill of a wet pulverizer, and reusable products were made by the method described in the report. In order to obtain a predetermined particle size (10 μm or less) using a ball mill, it is necessary to operate the ball mill for nearly 24 hours. The reexamination proved important. It was found that even in waste ceramics fired once and made into porcelain, the soda content was eluted with alkali when wet pulverized for that amount of time, and as a result, the mud partially solidified and so-called stale. Further, it has been found that the melting point rises due to the outflow of soda, and the glass is vitrified and does not become so-called porcelain unless the firing temperature is raised to the level of ordinary ceramics (1300 ° C.).
つまり、既存の廃棄物処理のシステムと、既存の廃棄陶磁器の再生技術の下では、破損したり、ペケ品となった廃棄陶磁器は、次のような問題を持っていた。
▲1▼廃棄陶磁器の再利用率が略20%程度と低かった。これでは廃棄される陶磁器量の5倍ものリサイクル製品が出来ることになった。通常エコマークは、廃棄品を50%以上再利用したものとしているが、そうしなければ集まってきた廃棄品が再利用されず、不用品として山積みされることになるからである。
▲2▼廃棄陶磁器利用の特徴、低温焼成化が未確立であった。1度1300℃で焼成し、粘土のみならず長石、珪石まで結晶化(いわゆる磁器化)した陶磁器は、粉砕粉にした時も個々の粉砕粉は結晶化したものの粉である。従ってこの特徴を生かせば低温焼成が可能であった。
こうした問題点を持つ中で、循環利用する商品経済流通に乗る技術として確立されていなかった。In other words, under the existing waste disposal system and the existing recycling technology for waste ceramics, the waste ceramics that were damaged or turned into piques had the following problems.
(1) The recycling rate of discarded ceramics was as low as about 20%. This made it possible to produce a recycled
(2) The characteristics of using waste ceramics and low-temperature firing have not been established. Ceramics baked at 1300 ° C. and crystallized not only to clay but also feldspar and silica (so-called porcelain) are individual pulverized powders even when pulverized. Therefore, if this feature is utilized, low-temperature firing was possible.
In spite of these problems, it has not been established as a technology for the circulation of commodity economy for recycling.
上述した問題は、これまでの製造技術上の問題に由来していた。たとえば廃棄陶磁器は、従来の陶磁器産業で粉砕のために利用しているジョークラッシャーやロールクラッシャーを使用して粉砕した時には、数mmの大きさにしかならない。これをボールミルなどの湿式粉砕機で、数μレベルに落とすためには、10数時間から1日くらいの時間がかかる。ところがこれだけの時間、湿式粉砕機にかけると廃棄陶磁器の中からアルカリ分が溶出し、それによって粉砕粉が固まってしまったり、それで作った杯土を使用して成型すると、どぼつきが出て、手ロクロで廻した時、土がバサバサしたり、鋳込み成型の時にスムーズに鋳込みができないことが生じてしまったのである。 The problems described above have been derived from problems in manufacturing technology so far. For example, waste ceramics are only a few mm in size when pulverized using a jaw crusher or roll crusher used for pulverization in the conventional ceramic industry. In order to reduce this to a level of several μm with a wet pulverizer such as a ball mill, it takes about 10 hours to 1 day. However, when it is applied to the wet pulverizer for this amount of time, the alkaline content is eluted from the waste ceramics, causing the pulverized powder to harden. When it was turned by hand, the soil became rough and it was not possible to cast smoothly during casting.
廃棄陶磁器の再利用率を上げると、この手ロクロ時でのバサツキや、鋳込み成型時の問題はより際立つようになった。またその他に、釉薬を付けて本焼している廃棄物陶磁器の中には、鉛などの重金属を釉薬の中に混入したものが過去に使われていた。廃棄物陶磁器のリサイクルを行うとき、こうしたものが混入する恐れがある。そこで、こうした混入したものを取り除くということが課題に上った。また、こうして夾雑物が混入すると、土の地肌が濁った白地となり、使用目的が限定されてくるという問題もあった。 Increasing the reuse rate of waste ceramics made the dusting and hand casting problems more prominent. In addition, waste ceramics that have been burned with glaze have been used in the past when heavy metals such as lead are mixed in the glaze. When recycling waste ceramics, there is a risk of such contamination. Therefore, it was a challenge to remove such mixed materials. In addition, when impurities are mixed in this way, the background of the soil becomes a cloudy white background, and there is a problem that the purpose of use is limited.
そこで本発明では、廃棄陶磁器を略50%以上再生利用し、市場経済流通に適合できるシステムを考えた。そのために製造技術上の2つの問題、アルカリ溶出によって粉砕粉が固まってしまうという問題と、可塑性が欠如し、成形性が悪くなるという問題を解決することにし、そのため従来は廃棄処分されてきた破損した陶磁器や規格外のペケ品となったいわゆる廃棄陶磁器を乾式粉砕によって♯350メッシュ以下の粒径、約10μ〜20μに細かく粉砕し、再利用し再商品化することにした。また可塑性添加剤を加え成型しやすくした。可塑性添加剤としては、素材を兼ねて天然粘土を使用し、これをデカンタなどの遠心分離機にかけて、珪砂などを取り除き、可塑性部分を密度高く取り出したものを使用した。 Therefore, in the present invention, a system that can be recycled and reused by about 50% or more of discarded ceramics and adapted to market economic distribution is considered. Therefore, we decided to solve the two problems in manufacturing technology, the problem of pulverized powder becoming hardened due to alkali elution, and the problem of lack of plasticity and poor moldability. We decided to recycle and re-use commercialized ceramics and so-called waste ceramics that became non-standard peked products by dry pulverization to a particle size of # 350 mesh or less, about 10 μm to 20 μm. A plastic additive was added to facilitate molding. As the plastic additive, natural clay was used as a raw material, and this was applied to a centrifugal separator such as a decanter to remove silica sand and the like, and the plastic part was taken out with high density.
また、CO2削減、CO2税などが現実社会の課題となっている。市場経済の中で、焼成炉を使い今後も生産を続けていくためには、低温焼成で陶磁器の生産を行い、コスト上の負荷増大を避けることが大切になる。そこで一度陶磁器として作り上げたものは、焼成段階でのエネルギーを受け、組成構造を変え、いわゆる磁器化しているため、再利用するにあたっては、その点に注目し、略1150℃、いわゆる陶磁器における低温焼成で再商品化を計るように考えた。In addition, CO 2 reduction, CO 2 tax, etc. are issues in the real world. In the market economy, in order to continue production using a firing furnace, it is important to produce ceramics by low-temperature firing to avoid an increase in cost. Therefore, what was once made as ceramics receives energy at the firing stage, changes the composition structure, and so-called porcelain, so when reusing, pay attention to that point, approximately 1150 ° C, low-temperature firing in so-called ceramics I thought about re-commercialization.
また、低温焼成を可能とするために、本願では陶磁器の焼成のメカニズムに注目し、珪石を結晶化する際に、略1300℃の高温焼成が必要である点を考え、新たに加える材料として珪石は除去し、なおかつ粘土の1150℃以下における結晶化を考えてガラスやソーダ長石などからなる低融点添加剤も加えるようにした。 In order to enable low-temperature firing, the present application focuses on the firing mechanism of ceramics and considers that high-temperature firing at approximately 1300 ° C. is necessary when crystallizing the silica, and as a newly added material, silica In addition, a low melting point additive made of glass, soda feldspar or the like was added in consideration of crystallization of clay at 1150 ° C. or lower.
図7は粘土、長石、珪石とからなる陶磁器用の素地土を、200℃、400℃、600℃、800℃、1000℃、1200℃、1300℃、1400℃、1600℃のそれぞれの温度で加熱した時に、素地土がどのように変化しているのかをX線分析によって調べた図である。(「瀬戸焼1300年の伝統と技術」より) FIG. 7 is a diagram illustrating heating of a clay earthenware made of clay, feldspar, and quartzite at temperatures of 200 ° C., 400 ° C., 600 ° C., 800 ° C., 1000 ° C., 1200 ° C., 1300 ° C., 1400 ° C., and 1600 ° C. It is the figure which examined by X-ray analysis how the ground soil is changing at the time. (From "Seto ware 1300 tradition and technology")
この分析図から分かることは、以下の通りである。
▲1▼200℃、400℃で加熱しものは、加熱していない素地土と全く変わっていない。400℃の加熱ではやきものにならないこと。
▲2▼600℃で加熱したものは、粘土を示す回析角度12度と24度付近のピークが小さくなり、加熱によって粘土の一部が分解している。しかし、長石及び珪石は変化していない。600℃はやきもの−陶器になる境目の温度と考えられる。
▲3▼800℃で加熱したものは、粘土を示すピークが消失し長石、珪石は変化していない。この温度でやきもの−陶器はできる。
▲4▼1000℃で加熱したものは、長石を示す27度付近のピークが小さくなっており、長石がこの温度で溶け始めていることが示されている。
▲5▼1200℃まで加熱したものは、長石を示すピークが消え、代わりにガラス相を示すバックグランドが少し上がっている。やきもの−陶磁器はこの温度になると、粘土、長石、珪石が反応しあって、やきものの最終的な構造物であるムライト結晶とガラスが生成し始める。
▲6▼1300℃まで加熱したものは、珪石が徐々に溶け、ムライトがさらに生成されていることが分かる。What can be understood from this analysis chart is as follows.
(1) The one heated at 200 ° C. and 400 ° C. is not different from the unheated base soil. Don't be a ceramic when heated at 400 ° C.
{Circle around (2)} When heated at 600 ° C., peaks at diffraction angles of 12 degrees and around 24 degrees indicating clay are reduced, and a part of the clay is decomposed by heating. However, feldspar and quartz have not changed. 600 degrees Celsius is considered to be the temperature at the border of ceramic ware.
(3) When heated at 800 ° C., the peak indicating clay disappears and feldspar and silica do not change. Pottery-pottery can be made at this temperature.
(4) When heated at 1000 ° C., the peak around 27 degrees indicating feldspar is small, indicating that feldspar begins to melt at this temperature.
(5) In the case of heating to 1200 ° C., the peak indicating feldspar disappears, and the background indicating the glass phase is slightly raised instead. Ceramics-When ceramics reach this temperature, clay, feldspar, and silica react with each other, and mullite crystals and glass, the final structure of ceramics, begin to form.
{Circle around (6)} When heated to 1300 ° C., it can be seen that the silica is gradually dissolved and mullite is further generated.
また電子顕微鏡による焼成表面の写真や、加熱重量減分析や示差熱分析、及び熱膨張測定のデータなどを加味すると、次のように言える。
▲1▼粘土は400℃〜600℃で分解し,水を加えても粘土に戻らない物質に変化する。
▲2▼長石は800℃まではほとんど変化なく、1000℃まで加熱すると溶け始め、1200℃まで加熱すると溶けて周りと反応してガラス相を作り、やきものの反応を高める役割をする。
▲3▼珪石は1200℃くらいで長石と反応して、やや溶け始め、1400℃まででほとんど溶ける。1300℃で焼成したものは、ムライトとガラス相が発達し陶磁器となる。Moreover, it can be said as follows when the photograph of the firing surface by an electron microscope, the data on the weight loss analysis, the differential thermal analysis, and the thermal expansion measurement are taken into consideration.
(1) Clay decomposes at 400-600 ° C and changes to a substance that does not return to clay even when water is added.
(2) The feldspar hardly changes up to 800 ° C., starts to melt when heated to 1000 ° C., melts when heated to 1200 ° C., reacts with the surroundings to form a glass phase, and enhances the reaction of the ceramic.
(3) Silica reacts with feldspar at around 1200 ° C and starts to melt slightly, and almost melts up to 1400 ° C. When baked at 1300 ° C., mullite and glass phase develop and become ceramic.
陶磁器は可塑性、成形性のためには粘土が必要となり、長石には粘土や珪石を溶かすカリウムやナトリウムが含まれ、結晶化やムライト化の生成を助け、溶け残った珪石は骨材の役割をして陶磁器の強度を上げる役割をする。廃棄陶磁器の粉砕粉を利用した再生陶磁器の場合、一度1300℃で焼成され磁器化したこの粉砕粉がベースとなるため、この粉砕粉を結合させる可塑性添加剤としての粘土と、この粘土をガラス化するための低融点化添加剤としてのガラスやソーダ長石が必要となるが、粉砕粉自体が珪石に代わって骨材としての役割をもつ。この珪石が入っている時には、1200℃以上に加熱し、ムライト化を計り、陶磁器の強度確保をしなければならなかったが、珪石を抜けば、低温での焼成による陶磁器化が可能となる。 Ceramics need clay for plasticity and moldability, feldspar contains potassium and sodium that dissolve clay and silica, helping to generate crystallization and mullite, and the remaining silica plays the role of aggregate. To increase the strength of ceramics. In the case of recycled ceramics using waste ceramic ground powder, this ground powder once fired at 1300 ° C to become porcelain is the base, so clay as a plastic additive that binds this ground powder and vitrification of this clay For this purpose, glass or soda feldspar as a low melting point additive is required, but the pulverized powder itself serves as an aggregate instead of silica. When this silica stone was contained, it had to be heated to 1200 ° C. or higher to make mullite and ensure the strength of the ceramic. However, if the silica is removed, it can be made into ceramic by firing at a low temperature.
このような廃棄陶磁器の再商品化技術の確立が、本発明の第1の目的である。市町村において、廃棄陶磁器をごみとして廃棄するのではなく、資源として再利用するために分別収集し、保管する廃棄物処理のシステムができれば、本発明による再商品化技術を条件に、廃棄陶磁器の再商品化ができることになる。 The establishment of such a re-commercialization technology for discarded ceramics is the first object of the present invention. If a waste disposal system that collects and stores waste ceramics for reuse as resources, rather than disposing of waste ceramics as waste in municipalities, recycle waste ceramics on the condition of the re-commercialization technology according to the present invention. It can be commercialized.
また、廃棄陶磁器の再商品化によって作った陶磁器に重金属等を混入させないで安全なものとして、かつ地肌の白いものとして作るということが第2の目的である。本発明ではこの第2の目的のために磁気分離の技術を使い、廃棄物陶磁器の粉砕粉から鉄などの他、鉛などの夾雑物を除去するようにした。 In addition, the second purpose is to make the ceramics made by re-commercializing the discarded ceramics safe and without the heavy metal or the like, and to make the ceramics white. In the present invention, for this second purpose, magnetic separation technology is used to remove impurities such as lead as well as iron from the pulverized powder of waste ceramics.
磁気分離の技術については特公昭53−44260でも明らかにされている。あらゆる物質は、磁場の中で大なり小なり磁性を持つ。その磁性を持った微粒子をマグネット(電磁石)の磁気力によって捕獲し、除去する磁気分離の原理は30年以上も前に英国のネイチャー誌などで紹介されていたが、強磁性の鉄やニッケル、コバルトだけでなく、弱磁性や非磁性のものを分離除去するについては、強い磁力が必要であり、その電磁力を発生させるためには多くの消費電力と熱の発生がこれまで課題となっていた。また、特公昭53−44260で示された磁気分離の技術を実用化する上で現われた問題を解決する形でのいくつかの改良特許が出されている。たとえば、取りきれない非磁性物質を磁性物質と一緒に取り除くために、凝集剤を用いるようにした特公昭56−30049。磁気フィルターを金網状に作り、磁界と直行するように配置し、磁気勾配を高めた特公昭59−49044。逆洗に当たって、原水中の微粒子と逆極性のゼータ電位を有する微粒子の含有水を流し、逆洗を効率よくできるようにした特公昭60−49006。 The magnetic separation technique is also disclosed in Japanese Patent Publication No. 53-44260. Every substance is more or less magnetic in a magnetic field. The principle of magnetic separation, which captures and removes the magnetic particles by the magnetic force of a magnet (electromagnet), was introduced over 30 years ago in British Nature magazines, but ferromagnetic iron and nickel, In order to separate and remove not only cobalt but also weak magnetism and non-magnetism, a strong magnetic force is required, and in order to generate the electromagnetic force, much power consumption and heat generation have been problems until now. It was. Also, several improved patents have been issued in a form that solves the problems that have arisen in putting the magnetic separation technique shown in Japanese Patent Publication No. 53-44260 into practical use. For example, Japanese Examined Patent Publication No. 56-30049 uses a flocculant to remove non-magnetic substances that cannot be removed together with magnetic substances. Japanese Examined Patent Publication No. Sho 59-49044 with a magnetic filter made in the form of a wire mesh and arranged so as to be perpendicular to the magnetic field. Japanese Patent Publication No. 60-49006, which allows the backwashing to be performed efficiently by flowing water containing fine particles having a zeta potential opposite to that of the fine particles in the raw water.
しかし、こうした改良にかかわらず、磁気分離の技術は多くの実用上の問題を残し、超電導方式が分離精度を高める上で残された唯一の方法というのが現状である。しかし、それは研究開発と設備費に巨額のお金がかかるという問題を抱えていた。本発明では、こうした問題も解決しつつ、目的の実現を果たそうとするものである。粒子に働く磁気力の3要素として▲1▼粒子の体積、▲2▼粒子と分散媒体の磁性差、▲3▼磁気勾配が知られている。 However, despite these improvements, the magnetic separation technique has left many practical problems, and the superconducting method is the only method left to improve the separation accuracy. However, it had the problem of enormous amounts of money for research and development and equipment costs. The present invention intends to achieve the object while solving these problems. As three elements of the magnetic force acting on the particles, (1) the volume of the particles, (2) the magnetic difference between the particles and the dispersion medium, and (3) the magnetic gradient are known.
そこで本願では、▲1▼粒子の体積をできるだけ小さくするために、乾式粉砕したものをさらに湿式粉砕して、細粒にするようにした。ただし、長時間湿式粉砕するとアルカリが溶出するため、数時間程度で抑えるようにした。それでもあらかじめ乾式粉砕で細かくしているため細粒化が可能となった。 Therefore, in the present application, in order to make the volume of (1) particles as small as possible, the dry pulverized one is further wet pulverized into fine particles. However, since alkali is eluted when wet pulverized for a long time, it was suppressed in about several hours. Even so, it was made fine by dry pulverization beforehand, so it became possible to make it finer.
また、▲2▼磁気分離で除去する粒子とベースとなっている分散媒体の磁性差を保つため、複数の磁力区分を設け、電磁力を弱いものから徐々に強いものへと進むようにし、最初は鉄、ニッケル、コバルト等、電磁力が弱くとも除去できるものを除去し、最後には強い力で非磁性のものを取るように工夫した。 (2) In order to maintain the magnetic difference between the particles to be removed by magnetic separation and the base dispersion medium, a plurality of magnetic force divisions are provided so that the electromagnetic force gradually proceeds from weak to strong. Removed iron, nickel, cobalt, etc. that can be removed even if the electromagnetic force is weak, and finally devised a non-magnetic material with a strong force.
さらに、▲3▼磁気勾配をつけるために、突起型の常磁性部材を用い、その突起部においての磁気勾配を大きくつけ、比較的弱い電磁力で非磁性のものまで除去できるようにした。 (3) In order to create a magnetic gradient, a projection type paramagnetic member was used, and the magnetic gradient at the projection was increased so that even a non-magnetic material could be removed with a relatively weak electromagnetic force.
そして、これらすべてがうまく働くよう、機械系を2連、すなわち2系統にして、1連を除去機能を発揮するため着磁させている一方で、他方は消磁し、付着した対象物を実際に除去している。これらを交互に利用し、付着物の付着による分離性能の緩慢化を避けるようにした。 And in order for all of these to work well, the mechanical system is divided into two systems, that is, two systems, one of which is magnetized to exhibit the removal function, while the other is demagnetized, and the attached object is actually It has been removed. These were used alternately to avoid slowing the separation performance due to the adhesion of deposits.
以上説明してきたが、本発明は従来食器や花器などの廃棄陶磁器が、ごみとして廃棄され、それを再利用した商品として、経済社会に循環利用されてこなかった要因として、▲1▼廃棄陶磁器が資源物として分別回収され、保管する仕組みがなかった点。▲2▼廃棄陶磁器の粉砕を従来の製土産業では乾式粉砕と湿式粉砕で行っていたが、乾式粉砕では数mmまでの大きさしか粉砕せず湿式粉砕で長時間かけて10数μの大きさに粉砕してきた。しかし、湿式粉砕で長時間かけると、一度焼成したものはアルカリ溶出によってソーダ分等が流出し、粉砕後の土で製土することが難しくなり、また融点が低く焼成できるという特質をなくしていた点、そして、廃棄陶磁器中に含まれてくる古い釉薬に使われていた有害重金属等の除去が難しいという点に問題があった。 As described above, according to the present invention, waste ceramics such as tableware and flower vases have been discarded as garbage and recycled as products that have been reused as waste. There was no system for separate collection and storage as recyclable items. (2) Waste ceramics were pulverized by dry pulverization and wet pulverization in the conventional soil-making industry, but in dry pulverization, only up to several millimeters were pulverized, and wet pulverization took over 10 μm over a long time. I've been crushed. However, when wet grinding is used for a long time, once fired, the soda content will flow out due to alkali elution, making it difficult to make soil with the ground soil, and the characteristic that the melting point is low can be lost. There was also a problem in that it was difficult to remove harmful heavy metals used in the old glaze contained in the discarded ceramics.
そして▲1▼家庭や地域の小規模事業体からでる一般廃棄物を責任処理する市町村において、廃棄陶磁器を資源として分別・回収し、企業体から出るペケ品とともにこれらを収集するシステムを作り、▲2▼これまで鉱工業における工業材料素材作りとして利用されてきたローラーミル等の乾式粉砕装置で、この集めた廃棄陶磁器を微粉砕し、▲3▼可塑性添加剤と低融点化添加剤を工夫し、これを本来の陶磁器産業における、杯土作りにあたる製土(混練)、成型、焼成の事業とを結びつけ廃棄陶磁器を利用した低温焼成による食器や花器などの陶磁器の生産と、高勾配磁気分離装置によって有害重金属等を除去する技術を提案した。 ▲ 1 ▼ In municipalities responsible for general waste from households and local small-scale business entities, a system for separating and collecting waste ceramics as resources and collecting them together with pique products from the enterprise is created. 2 ▼ Using a dry mill such as a roller mill, which has been used as an industrial material material in the mining industry, finely pulverize the collected waste ceramics, and (3) devising plastic additives and low melting point additives, Combining this with the earth making (kneading), molding, and baking business for making earthen clay in the original ceramic industry, producing ceramics such as tableware and flower vase by low temperature baking using waste ceramics, and harmful by high gradient magnetic separation device A technology to remove heavy metals was proposed.
本発明による方式を使い、陶磁器生産を行なえば、廃棄陶磁器の再生利用が可能となり、▲1▼これまでごみとして処理・処分することによって処分場を確保するために自然を壊すなどの環境に与えていた負荷がなくなり、▲2▼廃棄陶磁器の特性、一度磁器化したエネルギーを効率よく利用し、これまでは1300℃以上でなければ焼成磁器化できなかったものを、1150℃以下の低温焼成で陶磁器化でき、▲3▼さらに釉薬も、1300℃では色がほとんど消えてしまった銅、亜鉛,セレンなどを原料とした釉薬も使用でき、緑や黄や色鮮やかな赤の色を出すことができるようになった。 If ceramic production is carried out using the method according to the present invention, it becomes possible to recycle discarded ceramics, and (1) it is given to the environment such as destroying nature in order to secure a disposal site by treating and disposing of it as waste. (2) Characteristics of waste ceramics, energy once made porcelain is used efficiently, and what was previously not possible to be fired porcelain at 1300 ° C or higher can be obtained by low-temperature firing at 1150 ° C or lower. It can be made into porcelain. (3) In addition, glazes can be used glazes made of copper, zinc, selenium, etc. whose color has almost disappeared at 1300 ° C, producing green, yellow and colorful red colors. I can do it now.
以下図面に基づき本発明を説明する。図1は本発明による請求項1に基づく低温焼成方法を示すブロック図である。(2)は廃棄陶磁器、(4)は追加する陶磁器原材料、(6)は乾式粉砕工程、(8)は水、(10)は可塑性添加剤、(12)は低融点化添加剤、(14)は混練工程、(16)は成型工程、(18)は低温焼成工程である。 The present invention will be described below with reference to the drawings. FIG. 1 is a block diagram showing a low-temperature firing method according to
廃棄陶磁器は現状では、一度家庭で使用され、破損したものなどはほとんどの場合、市町村による不燃ごみ収集によって集められ、最終処分場に廃棄されている。また製造過程で排出される規格外品も、生産分留の範囲で数%は出るが、ほとんどの場合、これも廃棄処分されている。一方市町村では、ビンや缶など最近ではペットボトルや白色トレー、その他の廃棄プラスチックなども、資源として分別回収し、独自の再商品化ルートに乗せて利用している。 Currently, discarded ceramics are once used at home, and most of the damaged ones are collected by non-combustible waste collection by municipalities and disposed of in final disposal sites. In addition, non-standard products discharged in the manufacturing process are several percent within the range of production fractionation, but in most cases they are also discarded. On the other hand, municipalities, such as bottles and cans, recently collect plastic bottles, white trays, and other waste plastics as separate resources and use them on their own re-commercialization routes.
そこでまず、家庭から出るいわゆる一般ごみについて、収集、処理の責任を負う市町村が、家庭から出る廃棄陶磁器について、不燃ごみとして集めるのではなく、ビンなどと同様、資源として分別回収する仕組みを作る。ビン、缶の分別資源化回収はすでに多くのところで行われている。ビンは、色や大きさによって区分けしながら分別して出し、それを回収するようにしているところもあるが、この廃棄陶磁器も白色系と色物を分けて出すようにすれば、後の処理の上でより都合がよくなる。 First of all, the municipalities responsible for collection and disposal of so-called general waste from households create a system to separate and collect waste ceramics from homes as resources, not as non-burnable waste. Recycling of bottles and cans has already been done in many places. Some bottles are separated and sorted according to color and size, and they are collected. However, if this waste ceramic is also separated from white and colored, it can be used for later processing. More convenient above.
このようにして市町村で分別収集した廃棄陶磁器と、各陶磁器メーカーなどから集めたペケ品として収集してきた、1度1300℃以上で焼成され磁器化されたものが廃棄陶磁器(2)である。低温焼成による再商品化を行う製造現場とこの廃棄陶磁器を収集する市町村とが、距離的に離れているときには、市町村が保管し、一定量ごと大量に運ぶという方法をとることになる。 In this way, waste ceramics (2) that have been baked at 1300 ° C or more and made porcelain once collected as waste ceramics collected separately in municipalities and pique goods collected from various ceramic manufacturers. When the manufacturing site that performs re-commercialization by low-temperature firing and the municipalities that collect the waste ceramics are separated from each other, the municipalities store them and transport them in large quantities.
廃棄陶磁器は一度焼成することによりガラス化を伴う磁器化を行っている。従ってそれを粉末にしても個々の粉末体は磁器化されているが、全体としては粉体になることによって形を保持するという意味での一部エネルギーを失っている。陶磁器商品とするためには、これらの粉体を結合しつつ成型し、結合体自体を改めて焼成し、再び再結晶化を含めた磁器化を行う必要がある。廃棄陶磁器は先にも説明したが、元々粘土、長石、珪石から作られ、粘土中のカオリナイトの焼成温度が上がるに従い、メタカオリンに代わり、さらにγアルミニウムやムライトとなり、これに長石の働きで珪石がガラス化し結合してできた(いわゆる磁器化)ものである。従ってこの廃棄陶磁器を再磁器化するにあたっては、微粉砕した素材があれば、あとはこれに可塑性を与え成型できるようにすれば事足りる。 Waste ceramics are made into porcelain with vitrification by firing once. Therefore, even if it is made into a powder, each powder body is made porcelain, but as a whole it loses some energy in the sense that it retains its shape by becoming a powder. In order to make a ceramic product, it is necessary to mold these powders while bonding them, fire the bonded body anew, and perform porcelain including recrystallization again. As explained earlier, waste ceramics were originally made from clay, feldspar, and silica. As the firing temperature of kaolinite in the clay increases, it becomes γ-aluminum and mullite instead of metakaolin. Is formed by vitrification and bonding (so-called porcelainization). Therefore, in order to recycle this waste ceramic, if there is a finely pulverized material, it will be sufficient if the material can be molded after being plasticized.
しかし、乾式粉砕を行なえば、アルカリ溶出はほとんどないとはいえ、それでもごく微量の溶出があるため、これを補う意味と粉体の結合体である粘土を低融点で磁化するために、原材料の長石を追加する原材料(4)として供給する。 However, if dry pulverization is performed, there is almost no alkali elution, but there is still a very small amount of elution. To compensate for this and to magnetize the clay, which is a powder combination, at a low melting point, Supply as raw material (4) to add feldspar.
乾式粉砕工程(6)ではローラーミル等を使用して廃棄陶磁器を♯350メッシュ以下の粒度すなわち平均10μmレベルに粉砕する。ローラーミル以外の乾式粉砕機でこのレベルまで粉砕してもよい。理想的には7〜10μmの大きさに粉砕することが望ましい。粉砕した廃棄陶磁器の微粉末は、βグルカン等のカードラン系の多糖類からなる可塑性添加剤(10)とガラスやソーダ長石(ナトリウム系)等の低融点化添加剤(12)と一緒に混練工程(14)で水(8)を入れて混練する。 In the dry pulverization step (6), the waste ceramic is pulverized to a particle size of # 350 mesh or less, that is, an average level of 10 μm using a roller mill or the like. You may grind | pulverize to this level with dry mills other than a roller mill. Ideally, it is desirable to grind to a size of 7 to 10 μm. The fine powder of waste ceramics after pulverization is kneaded together with plastic additives (10) consisting of curdlan polysaccharides such as β-glucan and low melting point additives (12) such as glass and soda feldspar (sodium). In step (14), water (8) is added and kneaded.
粘土の持つ可塑性については、長い間その研究が行なわれ、人工粘土の研究の中でβグルカン等のカードラン系の多糖類が関与していることが判明している。そこで本発明の1実施例として、粘土の可塑性を成立させているこの多糖類を投入することにより、直接この微粉末に可塑性を持たせるようにした。 The plasticity of clay has been studied for a long time, and it has been found that curdlan-based polysaccharides such as β-glucan are involved in the study of artificial clay. Therefore, as one embodiment of the present invention, the fine powder was directly plasticized by introducing this polysaccharide which has established the plasticity of clay.
混練した土は、成型工程(16)で食器等に成型し、低温釉を施釉した上で1150℃以下の低温焼成工程(18)で焼成した。釉薬は、1300℃以上の高温ではFe系など以外、色が飛んでしまい、銅や亜鉛、セレンなどを使った緑や青、赤などの色合いを出すことは難しかったが、低温焼成ではこうした釉薬の施釉も可能となる。混練工程(14)で投入した低融点化添加剤(12)の働きで低温焼成を可能とした。 The kneaded soil was molded into tableware or the like in the molding step (16), glazed with a low temperature glaze, and fired in a low temperature firing step (18) of 1150 ° C. or lower. At a high temperature of 1300 ° C or higher, the glaze will fly out of colors other than Fe, etc., and it was difficult to produce shades of green, blue, red, etc. using copper, zinc, selenium, etc. Glazing is also possible. Low temperature firing was made possible by the action of the low melting point additive (12) added in the kneading step (14).
図2も本発明による陶磁器の低温焼成方法の一実施例で、請求項1及び2に該当する具体的な実施例を示すブロック図である。破線部分のブロックで、図1と同じ番号は、同じ内容を示す。(20)は陶磁器の生産ないし販売企業、(22)は規格外のペケ品、(26)は収集・保管、(28)は市町村、(30)は資源物、(32)は収集・保管、(34)は長石、(36)は乾式粉砕装置、(38)は粘土、(40)はデカンター、(42)は可塑性の高い粘土成分、(44)は珪砂やキラ、(46)は混練装置、(48)はマグネサイト、(50)は成型装置、(52)は焼成炉である。 FIG. 2 is also a block diagram showing a specific embodiment corresponding to
廃棄陶磁器は一方で陶磁器生産を行なったり販売する企業(20)のペケ品(22)として、他方再商品化のための資源物(30)として、分別収集・保管した市町村から排出される資源物として、まず乾式粉砕装置(36)を持っている企業に収集運搬され保管される(26・32)。ここではローラーミル等の乾式粉砕装置(36)で、廃棄陶磁器を平均10μmくらいの大きさに粉砕する。一方、粉砕物を結合し、再生陶磁器の磁器化を助けるための長石(34)も、この乾式粉砕装置で粉砕するか一緒に行なってもよいし、生産上の流れの都合で別個に粉砕してもよい。いずれにせよこうした粉砕装置は、鉱物を粉砕し様々な素材原料を作り出している産業・企業が保有しているが、収集してきた廃棄陶磁器を保管し、音の大きい乾式粉砕装置を作動させるため、周辺環境への影響を考えた設置条件が求められる。 Waste ceramics, on the other hand, are used as a peculiar product (22) for companies that produce or sell ceramics (22), and on the other hand, they are recyclable resources (30) that are separated and collected and stored from municipalities. First, it is collected, transported and stored in a company having a dry grinding device (36) (26, 32). Here, the waste ceramics are pulverized to an average size of about 10 μm by a dry pulverization apparatus (36) such as a roller mill. On the other hand, the feldspar (34) for combining the pulverized materials and helping to make the regenerated ceramics porcelain may be pulverized by the dry pulverizer or together, or separately for convenience of production flow. May be. In any case, these crushers are owned by industries and companies that produce various raw materials by crushing minerals, but in order to store waste ceramics collected and operate dry crushers with loud sound, Installation conditions that consider the impact on the surrounding environment are required.
乾式粉砕装置(36)で平均10μmレベルに微粉砕した陶磁器は、いわゆる陶磁器のための土をつくる混練装置(46)で水(48)と低融点化添加剤(12)としてのマグネサイトの微粉末(48)と可塑性添加剤(10)と一緒に混練する。ここでは粘土(38)をデカンター(40)、いわゆる遠心分離装置で珪砂やキラ等の鉱物部分(44)を取り出し、可塑性の高い粘土分(42)を可塑性添加剤(10)として使用した。 Ceramics finely pulverized to an average level of 10 μm by a dry pulverizer (36) are made of solubilized magnesite as water (48) and low melting point additive (12) in a so-called kneading device (46) for making clay for ceramics. Knead together with powder (48) and plastic additive (10). Here, the clay (38) was decanted (40), and the mineral part (44) such as silica sand and glitter was taken out with a so-called centrifuge, and the highly plastic clay (42) was used as the plastic additive (10).
ここで長石(34)をソーダ長石などアルカリ分の多い長石を使い、低融点添加剤(12)の働きを持たせ、マグネサイト(48)を使わないようにすることもできる。 Here, the feldspar (34) can be made of feldspar with high alkalinity such as soda feldspar, can serve as a low melting point additive (12), and can avoid the use of magnesite (48).
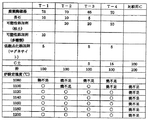
混練装置(46)で混練した「土」は、フィルタープレス等で水分をとった後、成型装置(50)で成型した。廃棄陶磁器の微粉末が「7」に対し「粘土」を「2」の割合にしたものでも成形性は確保できた。その他、図3のようにT−1からT−4まで4つの組み合わせで実験したが、成形性についてはいずれも確保できた。 The “soil” kneaded by the kneading device (46) was molded with the molding device (50) after removing moisture with a filter press or the like. The moldability was ensured even when the waste ceramic fine powder was "7" with "clay" in the ratio of "2". In addition, as shown in FIG. 3, four combinations of T-1 to T-4 were tested, but all of the moldability could be secured.
成型装置(50)で成型したものは、焼成炉(52)で焼成した。具体的には図3に示したように、様々な炉内設定温度の下で焼成し、T−1からT−3については1120℃以上、T−1とT−3では1100℃以上の設定温度で磁器化を確認した。焼成炉内では、炉内の場所によっては±50℃程度の温度の偏在があるため、1100℃以上で磁器化を確認できれば、1150℃での低温焼成による磁器化を確認できたということができる。 What was shape | molded with the shaping | molding apparatus (50) was baked with the baking furnace (52). Specifically, as shown in FIG. 3, firing is performed at various furnace set temperatures, and T-1 to T-3 are set to 1120 ° C. or higher, and T-1 and T-3 are set to 1100 ° C. or higher. The porcelain was confirmed at the temperature. In the firing furnace, there is uneven distribution of temperature of about ± 50 ° C. depending on the location in the furnace, so that if porcelainization can be confirmed at 1100 ° C. or higher, it can be said that porcelainization by low temperature firing at 1150 ° C. could be confirmed. .
図3は、本発明による低温焼成方法によって作成したテストピース(T−1からT−4)と比較用テストピースとして「比較C」を「廃棄陶磁器」「長石」、「可塑性添加剤(粘土)」、「可塑性添加剤(多糖類)」、「低融点化添加剤(マグネサイト)」、「C土−通常の陶磁器用はい土」のそれぞれの割合を変えて、成形佳と焼成温度を変えた磁器化の実験を行なった結果データである。○印は磁器化が確認できた印である。 FIG. 3 shows a test piece (T-1 to T-4) prepared by the low-temperature firing method according to the present invention and “Comparison C” as “Waste ceramic”, “feldspar”, “plastic additive (clay) as comparative test pieces. ”,“ Plastic additives (polysaccharides) ”,“ Low melting point additive (magnesite) ”,“ C soil-normal ceramic soil ”, changing the molding and firing temperature It is the result data which conducted the experiment of porcelain. The circles indicate that the porcelain has been confirmed.
この他にも、図1で言えば追加する材料(4)として、低融点化添加剤を兼ねてソーダ長石を使用したり、光ファイバーの生産過程からでるペケ品を粉砕した、ガラス粉を用い、陶磁器の再生利用品を作ることに成功した。たとえば具体的には、廃棄陶磁器、ソーダ長石もしくは光ファイバーの粉砕物、そして可塑性添加物として、デカンタなどを使って取り出した可塑性の高い粘土をそれぞれ50:15:35の割合や、60:10:30の割合で作り、実用上の物を作るのに成功した。また光ファイバーの粉砕粉を使用したときには、元々の光ファイバー自体が管系となっていて空気管を含むため、できあがった陶磁器の軽量化を計ることができた。 In addition to this, as a material to be added in FIG. 1 (4), glass powder obtained by using soda feldspar that also serves as a low melting point additive or by pulverizing a pique product from the optical fiber production process, Succeeded in making ceramic recycled products. For example, concretely, waste ceramic, soda feldspar or optical fiber pulverized product, and high plasticity clay extracted using a decanter or the like as a plastic additive are in a ratio of 50:15:35 or 60:10:30, respectively. And made a practical product. In addition, when the pulverized powder of the optical fiber was used, the original optical fiber itself was a tube system and included an air tube, so the weight of the finished ceramic could be reduced.
図4は本発明による特許請求の範囲、請求項5を含み、請求項1や請求項2による発明を説明するブロック図である。(60)は廃棄物陶磁器、(62)は乾式粉砕工程、(64)は可塑性添加剤としての粘土、(66)は低融点化添加剤としての長石、(68)はボールミル等の湿式粉砕工程、(70)は高勾配磁気分離工程、(72)は成型工程、(74)は焼成工程である。 FIG. 4 is a block diagram illustrating the invention according to
この図4のブロック図は、図1を基本としている。廃棄物陶磁器(60)を10μm前後に粉砕するためには、24時間以上の時間がかかる。湿式粉砕では水分のためそうした時間をかけるとアルカリ分が溶出し、どぼつきが出るため、実質粉砕ができなかった。そこで乾式粉砕工程(62)で10μm前後まで1度粉砕した。 The block diagram of FIG. 4 is based on FIG. It takes 24 hours or more to grind the waste ceramic (60) to around 10 μm. In wet milling, due to moisture, when such time was spent, the alkali content was eluted and a staleness was produced, so substantial milling could not be performed. Then, it grind | pulverized once to about 10 micrometers in the dry-type grinding | pulverization process (62).
廃陶磁器の再商品化用の「土」として利用するためには、10μmよりさらに細かい粒度が必要となる。また、高勾配磁気分離工程(70)に「土」を流すためには、水分を含むスラリー状にすることが必要となる。そこで、乾式粉砕工程(62)を通した上に、湿式粉砕工程(68)を通すことにした。長時間湿式粉砕工程(68)を通すと、ここでもアルカリ分の溶出の心配があり、粉砕粉の固化や成型時での、どぼつきがないように数時間に抑えるようにした。 In order to use as “soil” for re-commercialization of waste ceramics, a particle size finer than 10 μm is required. Further, in order to flow “soil” in the high gradient magnetic separation step (70), it is necessary to form a slurry containing moisture. Therefore, it was decided to pass the wet grinding process (68) after passing through the dry grinding process (62). If the wet pulverization step (68) is passed through for a long time, there is a concern that the alkali content may be dissolved out again, and the pulverized powder is suppressed to a few hours so as not to become sticky at the time of solidification or molding.
高勾配磁気分離工程(70)では、こうした分離装置としては実験に実験を重ね、いくつかの工夫の上、所定の目的にそった装置を開発し、これを使用した。その結果、鉛等の重金属の他、鉄分等の夾雑物も取り除き、成型(72)の後、焼成(74)したものは、バージン原材料で構成した「土」で作ったものと遜色ない地肌の陶磁器となった。 In the high-gradient magnetic separation step (70), as such a separation apparatus, experiments were repeated in an experiment, and an apparatus suitable for a predetermined purpose was developed and used based on some ideas. As a result, in addition to heavy metals such as lead, impurities such as iron are also removed. After molding (72) and firing (74), the surface is inferior to that made of "earth" made of virgin raw materials. It became ceramic.
ここでの湿式粉砕(68)は、短時間なので混入する粘土(64)や長石(66)などについては、あらかじめ単独で湿式や乾式粉砕を行い、条件をそろえた土で混入するようにした。 Since the wet pulverization (68) here is a short time, the clay (64) and the feldspar (66) to be mixed are preliminarily singly wet or dry pulverized so as to be mixed with soil having the same conditions.
高勾配磁気分離装置は、電磁力によって高磁場を作り、細かい線や網目状の強磁性体を入れ、磁場の強さの大きく変化する磁場勾配の高い場所を作り、その磁界中におかれたいわゆる弱磁性や非磁性の物質にまで着磁して、ベースとなっている分散媒体からその物質を分離する技術である。すでに東北大学の金属材料研究所の本河光博教授らは、超電力磁石を利用し、21万ガウスの磁力を与えることによって、水に磁力をかけ水を浮かせることに成功している。 The high gradient magnetic separator creates a high magnetic field by electromagnetic force, puts fine lines and mesh-like ferromagnets, creates a place with a high magnetic field gradient where the strength of the magnetic field changes greatly, and was placed in that magnetic field It is a technique that magnetizes even a so-called weak magnetic or non-magnetic substance and separates the substance from the base dispersion medium. Prof. Mitsuhiro Motokawa of Tohoku University's Institute for Materials Research has already succeeded in floating water by applying a magnetic force of 210,000 gauss using a super-power magnet.
この技術は、汚染水や汚染土壌他、あらゆる汚染物質を微粉体にし、スラリー状にすれば、除去したい物質を取り除くことができ、その意味で大変注目されている技術であるが、高磁力を発生させるために、2000A、400kWレベルの電力が必要であり、その消費電力と電力消費に伴って発生する熱の冷却処理が大変であった。そのため超電力磁石などの開発が不可欠とされてきた技術である。これを横型にすれば重力方向の力は考えなくともすむ場合も産み出すことが可能となった。 This technology is a technology that has attracted a great deal of attention in that sense because it can remove the polluted water, contaminated soil, and other pollutants by making them into fine powders and making them into a slurry. In order to generate it, power of 2000A, 400 kW level is necessary, and the cooling process of the heat generated with the power consumption and the power consumption is difficult. For this reason, development of super-power magnets has been indispensable. By making this a horizontal type, it is possible to produce even when force in the direction of gravity is not considered.
図5は本発明に於いて使用した高勾配磁気分離装置の1実施例であり、(80)は原料タンク、(82)はポンプ、(84)は原料弁、(86)は磁気分離機、(88)はろ過弁、(90)はろ過原料タンク、(92)及び(94)は回転軸受、(96)は油タンク、(98)はポンプ、(100)は油クーラー、(102)は着磁物タンク、(104)は冷却・洗浄タンク、(106)はポンプ、(110)は洗浄水弁、(112)は送風機、(114)は送風弁である。 FIG. 5 shows an embodiment of the high gradient magnetic separation apparatus used in the present invention. (80) is a raw material tank, (82) is a pump, (84) is a raw material valve, (86) is a magnetic separator, (88) is a filtration valve, (90) is a filtration raw material tank, (92) and (94) are rotary bearings, (96) is an oil tank, (98) is a pump, (100) is an oil cooler, and (102) is A magnetized material tank, (104) is a cooling / washing tank, (106) is a pump, (110) is a washing water valve, (112) is a blower, and (114) is a blower valve.
原料タンク(80)の中の原料(81)は、図4の湿式粉砕(68)を済ませたもので、スラリー状になっている。廃棄物陶磁器の中には、鉛等の釉薬への使用規制前に作成したものもあり、スラリー状の原料の中には、こうした重金属も含まれている。この原料を原料タンク(80)からポンプ(82)で吸引し、逆止弁にしている原料弁(84)を通して磁気分離部(86)を通す。 The raw material (81) in the raw material tank (80) has been subjected to the wet pulverization (68) of FIG. 4 and is in the form of a slurry. Some of the waste ceramics were made before the regulation of the use of lead and other glazes, and these heavy metals are also included in the slurry materials. The raw material is sucked from the raw material tank (80) by the pump (82), and is passed through the magnetic separation part (86) through the raw material valve (84) serving as a check valve.
磁気分離機(86)は回転軸受(92)(94)に軸受された筒状部材(図示せず)が中心部に設置され、この筒状部材を取り囲んで、電磁コイルが設置され、筒状部材内を吸引された原料(81)が通過し、ろ過弁(88)を通って、ろ過原料タンク(90)に送られる。 In the magnetic separator (86), a cylindrical member (not shown) supported by the rotary bearings (92) and (94) is installed at the center, and the electromagnetic coil is installed around the cylindrical member to form a cylindrical shape. The sucked raw material (81) passes through the member, passes through the filtration valve (88), and is sent to the filtered raw material tank (90).
この筒状部材内は、磁気コイルによって高磁場となるように設置しているため、その中を通過する原料中の粒子は、大小はあっても一様に磁性を持つようにしている。その上で、この筒状部材内には、小型ネジクギをたくさん詰め込み、磁性体として作動するようにしている。ネジクギの先端や、ネジ山の尖った部材に、その結果、磁場勾配の高い部分がつくられ、このネジクギは回転軸受(92)(94)に支えられた筒状部材が回転するたびに位置を変え、この磁場勾配の高い部分が様々に位置を変えることになる。 Since the inside of this cylindrical member is installed so that it may become a high magnetic field with a magnetic coil, the particles in the raw material passing through it are made to be uniformly magnetized even if large or small. In addition, the cylindrical member is packed with a lot of small screws and operates as a magnetic body. As a result, a portion with a high magnetic field gradient is created at the tip of the screw thread or a member with a sharp thread, and this screw thread is positioned each time the cylindrical member supported by the rotary bearings (92) (94) rotates. In other words, the high magnetic field gradient portion changes its position in various ways.
そのため原料(81)が、筒状部材を通過する中で、原料中の粒子部分は弱磁性や非磁性のものを含め、このネジクギに着磁し、取り除かれることになる。ろ過原料タンク(90)に貯えられるのは、こうした粒子を取り除いたろ過原料ということになる。 Therefore, while the raw material (81) passes through the cylindrical member, the particle portion in the raw material, including weak magnetic and non-magnetic ones, is magnetized on this screw and removed. What is stored in the filtration raw material tank (90) is a filtration raw material from which such particles have been removed.
この磁気分離は、図のように5つの区分に分け、それぞれ独立した電磁コイルを作動させることができるようにしている。例えば、元々強磁性の鉄やニッケルなどは、磁場を弱くしていても取り除くことができる。そのため、元々が弱磁性や非磁性の物質を取り除こうとする場合でも、そうした強磁性のものは着磁されてくる。しかし、いろいろなものが着磁すれば、ネジクギの尖った部分の尖りが失われ、高い磁場勾配ができなくなり、結果として、元々が弱磁性や非磁性の物質を取り除きにくくなる。そこで、複数区分に分けている電磁コイルによって作る磁場を最初の区分では強磁性のものが取れるくらいの弱い磁場にし、強磁性のものをとり、徐々に磁場を強め、最後には非磁性の粒子まで着磁させるようにした。 This magnetic separation is divided into five sections as shown in the figure so that independent electromagnetic coils can be operated. For example, originally ferromagnetic iron or nickel can be removed even if the magnetic field is weakened. Therefore, even when originally trying to remove a weakly magnetic or nonmagnetic material, such a ferromagnetic material is magnetized. However, if various things are magnetized, the sharpness of the pointed portion of the screw will be lost, and a high magnetic field gradient will not be possible. As a result, it will be difficult to remove materials that are originally weak or nonmagnetic. Therefore, the magnetic field created by the electromagnetic coils divided into multiple sections is made weak enough to obtain ferromagnetic materials in the first section, and ferromagnetic fields are taken, gradually increasing the magnetic field, and finally non-magnetic particles. Until it was magnetized.
このようにして、弱磁性の鉛分や混入していた非磁性のカドミ分まで着磁し、取り除くことができた。陶磁器の地肌をできるだけ白い色にするためには、鉄分を除去することが大切だが、強磁性体の鉄分に的を絞り、1回の操作でこの鉄分を取り除く際には、この複数区分をほぼ同一レベルの磁場が働くようにし、着磁・分離するという方法も考えることができる。 In this way, it was possible to magnetize and remove even weak magnetic lead and mixed non-magnetic cadmium. In order to make the ceramic background as white as possible, it is important to remove the iron, but when focusing on the iron in the ferromagnetic material and removing this iron in a single operation, the multiple sections are almost A method of making the magnetic field of the same level work and magnetizing and separating can also be considered.
こうして磁気分離機(86)を働かせることは電磁コイルに電流を流すことになり、磁気分離機(86)が発熱する。循環パイプ(図示せず)を磁気分離機(86)に取り付け、このパイプに油を流し、この熱を取るようにした。とった熱は油クーラー(100)で冷やし、再びポンプ(98)で循環させるようにした。油クーラー(100)は冷却・洗浄水タンク(104)からポンプ(106)で水を送り、油を冷却するようにしている。 When the magnetic separator (86) is operated in this way, an electric current flows through the electromagnetic coil, and the magnetic separator (86) generates heat. A circulation pipe (not shown) was attached to the magnetic separator (86), and oil was allowed to flow through the pipe to take up this heat. The taken heat was cooled by the oil cooler (100) and circulated again by the pump (98). The oil cooler (100) feeds water from the cooling / washing water tank (104) with a pump (106) to cool the oil.
図6は図5の高勾配磁気分離装置の分離稼動中及び、洗浄の過程を説明する説明図である。図6は(a)は分離稼動中で、この時には磁気分離機(86)は水平状態を保持したまま原料タンク(80)からの原料の供給を受け、着磁・分離しながらろ過液をろ過原料タンク(90)に供給している。 FIG. 6 is an explanatory view for explaining the cleaning operation and the separation operation of the high gradient magnetic separation apparatus of FIG. FIG. 6 (a) shows the separation operation. At this time, the magnetic separator (86) is supplied with the raw material from the raw material tank (80) while maintaining the horizontal state, and the filtrate is filtered while being magnetized and separated. The raw material tank (90) is supplied.
図6(b)は着磁・分離が一定の度合い進んだ後、これを取り除く過程に入り、磁気分離機(86)を斜めに傾けた上で、まず、図5の送風機(112)から送風し、磁気分離機(86)内に残っている原料ないし、ろ過原料液を原料タンク(80)に戻す。この電磁コイルは、そのまま作動させ、着磁物が落ちないように働かせている。送風によって残留物を流しだし、再処理しなければならない原料を増やすことがないように工夫した。 FIG. 6B shows a process of removing the magnetization / separation after a certain degree of progress, and after the magnetic separator (86) is tilted obliquely, the air is first blown from the blower (112) of FIG. The raw material remaining in the magnetic separator (86) or the filtered raw material liquid is returned to the raw material tank (80). The electromagnetic coil is operated as it is so that the magnetized material does not fall. Residue was poured out by blowing air, and it was devised not to increase the raw materials that have to be reprocessed.
図6(c)は残留物を出した後、磁気分離機(86)を再び水平に戻し、図5の回転軸(92)(94)を回転させながら消磁し、ネジクギに付着した粒子をネジクギから引き剥がす作業工程である。この作業の際、洗浄のため最低限の水を送る。 6 (c), after removing the residue, return the magnetic separator (86) to the horizontal again, demagnetize while rotating the rotating shafts (92) and (94) in FIG. 5, and remove the particles adhering to the screw thread. It is a work process to peel off from. During this work, send a minimum amount of water for cleaning.
図6(d)は再び磁気分離機(86)を傾け、送風し、引き剥がした着磁されていた粒子を着磁物タンク(102)に送っている。 In FIG. 6D, the magnetic separator (86) is tilted again, blown, and the magnetized particles peeled off are sent to the magnetized substance tank (102).
本発明の高勾配磁気分離装置では、実は、図5に描いたシステムを2系統備えるようにし、1系統では図6(a)で示したように、分離稼動させながら、もう1系統では図6(b)から図6(d)に示したように、着磁・分離したものを洗浄するように交互に作動させている。この作動を極短時間で切り替えることによって、着磁物が磁性体に付着し、付着物によって高勾配の磁界が働くことが鈍ることがないようにしている。つまり、少し付着し始めるとすぐに洗浄し、付着物を落とし、新たに着磁分離を開始するように工夫した。 In the high gradient magnetic separation apparatus of the present invention, actually, the system depicted in FIG. 5 is provided with two systems, while one system is separated as shown in FIG. As shown in FIG. 6 (d) to FIG. 6 (d), they are alternately operated so as to wash the magnetized / separated material. By switching this operation in a very short time, the magnetized material adheres to the magnetic material so that the high-gradient magnetic field does not dull due to the adhered material. In other words, the device was devised so that as soon as it started to attach a little, it was washed, the adhering matter was dropped, and a new magnetization separation was started.
このように本発明では、特公昭53−44260に示されて高勾配磁気分離装置をあらゆる観点から見直し工夫を加え、高電流を流すことなく非磁性体を着磁できるようにした。 As described above, in the present invention, as shown in Japanese Patent Publication No. 53-44260, the high gradient magnetic separation device has been reviewed and devised from all points of view so that a non-magnetic material can be magnetized without flowing a high current.
15kw〜100kwレベルの電力によって、弱磁性から非磁性の物質まで除去するようにした本発明の装置の特徴を以下に列挙すると
▲1▼従来、電磁力印加部(図5、図6の磁気分離機の部分)を上から下に原料を流し、例えば網目状に作製した強磁性体の着磁・分離部を通過する方法をとっていたが、この方法だと、原料を流す力と重力の方向が重なり、この二つの力を足し合わせた力に拮抗する力以上の磁力を働かせることで、粒子を着磁し、分離することが必要であった。そのため、強力な磁力が必要となったが、これを横型にすれば、重力方向の力は考えなくともすむ場合も産み出すことが可能となった。
▲2▼また、横型にすることをベースにし、電磁力印加部を複数区分に分けて設置することができるようになり、電磁力印加部を長くとることができるようになった。もし、縦型で、複数区分を設ければ、逆洗を下から上に水を流して行うとして、高さが高くなり、高圧洗浄等が必要となり、使用する水量が増え、洗浄水の処理に困ることになった。
▲3▼電磁力印加部を複数区分にすることにより、この複数区分への電磁力を変化させることができるようになった。複数区分に分けられた電磁力印加部への電磁力を、原料が流れる方向に沿って徐々に強くし、最初は強磁性の鉄やニッケル等がとれ、流れが進むに従って、弱磁性のものから非磁性のものまで着磁・分離できるようにすれば、着磁によって高勾配部が鈍り、分離力が衰えるということを防ぐことができる。The characteristics of the device of the present invention that removes from weak magnetism to non-magnetic material by power of 15 kW to 100 kW are listed below. (1) Conventionally, an electromagnetic force application unit (magnetic separation shown in FIGS. 5 and 6) For example, the material flowed from the top to the bottom of the machine and passed through the magnetized / separated part of the ferromagnetic material produced in a mesh shape. It was necessary to magnetize and separate the particles by applying a magnetic force greater than the force that antagonizes the combined force of these two forces and adding these two forces together. For this reason, a strong magnetic force was required, but if this was made horizontal, it would be possible to produce even when the force in the direction of gravity need not be considered.
(2) Further, based on the horizontal type, the electromagnetic force application part can be divided into a plurality of sections and installed, and the electromagnetic force application part can be made longer. If a vertical type and multiple sections are provided, the backwashing is performed by flowing water from the bottom to the top, the height becomes high, high pressure washing is required, the amount of water used increases, and the washing water is treated. I was in trouble.
(3) The electromagnetic force applied to the plurality of sections can be changed by dividing the electromagnetic force application section into a plurality of sections. The electromagnetic force applied to the electromagnetic force application unit divided into multiple sections is gradually increased along the direction of flow of the raw material. At first, ferromagnetic iron or nickel is removed. If it is possible to magnetize / separate even non-magnetic ones, it is possible to prevent the high gradient portion from becoming dull due to magnetization and the separation force from declining.
この装置では、磁気勾配を高めつつ同時に、着磁した粒子を洗浄して取り除くことが必要となる。これまでは、網目状に作製した強磁性体に電磁力をかけ、これに粒子が着磁し、分離除去する方法をとってきていたが、消磁後も磁力は残るため、この粒子を洗浄で洗い流すことはなかなか困難であった。そこで本発明では先の▲1▼〜▲3▼に続き、次の工夫を行った。
▲4▼電磁力印加部の中に、両端を回転軸受で支えた筒体を置き、その筒体の中に沢山の小型のネジクギをいれ、この強磁性体であるネジクギの先端やネジの山の尖った部分に磁場勾配の高い部分を作り、原料がこの筒体の中を流れる内に、流体がこのネジクギに着磁するようにした。この筒体は原料流入時だけでなく、洗浄時も作動させているため、消磁した後、ネジクギは互いにぶつかり合いながらその衝撃で速やかに着磁していた粒子部分を洗い流すことができる。
▲5▼電磁力印加部を作動工程によって水平状態と傾けた状態へと変化させ、さらに送風工程を入れて洗浄することにより、余分な水を使うことによる再処理の手間を省くことができた。
▲6▼そして、図5に示した高勾配磁気分離装置の一連の装置を2系統備え、「着磁・分離」と「洗浄」を互いに交替し、事実上「着磁・分離」を連続して行える装置とした。In this apparatus, it is necessary to clean and remove the magnetized particles while increasing the magnetic gradient. Until now, electromagnetic force was applied to a ferromagnetic material produced in a mesh shape, and particles were magnetized and separated and removed.However, since the magnetic force remains after demagnetization, the particles can be washed. It was quite difficult to wash away. Therefore, in the present invention, following the above (1) to (3), the following devices were made.
(4) Place a cylindrical body supported by rotating bearings at both ends in the electromagnetic force application section, and insert many small screw nails into the cylinder body. A part with a high magnetic field gradient was made in the pointed part of the material, and the fluid was magnetized on the screw as the raw material flowed through the cylinder. Since this cylindrical body is operated not only when the raw material flows in but also during cleaning, after degaussing, the thread can collide with each other and can quickly wash away the particles that have been magnetized by the impact.
(5) By changing the electromagnetic force application part between the horizontal state and the inclined state according to the operation process, and by washing with a blowing process, it was possible to save the trouble of reprocessing by using excess water. .
(6) Two series of high gradient magnetic separators shown in FIG. 5 are provided, and “magnetization / separation” and “cleaning” are replaced with each other. The device can be used.
Claims (7)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2004149767A JP2005097082A (en) | 2003-04-17 | 2004-04-16 | Method of firing ceramics at low temperature and ceramics fired at low temperature |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003147015 | 2003-04-17 | ||
| JP2003350102 | 2003-09-02 | ||
| JP2004149767A JP2005097082A (en) | 2003-04-17 | 2004-04-16 | Method of firing ceramics at low temperature and ceramics fired at low temperature |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005097082A true JP2005097082A (en) | 2005-04-14 |
| JP2005097082A5 JP2005097082A5 (en) | 2007-04-19 |
Family
ID=34468268
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004149767A Pending JP2005097082A (en) | 2003-04-17 | 2004-04-16 | Method of firing ceramics at low temperature and ceramics fired at low temperature |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2005097082A (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007151875A (en) * | 2005-12-06 | 2007-06-21 | Ogiso:Kk | Porcelain tableware |
| JP2009046346A (en) * | 2007-08-20 | 2009-03-05 | Takahama Industry Co Ltd | Chamotte and clay roofing tile with which chamotte is blended |
| RU2469986C1 (en) * | 2011-09-30 | 2012-12-20 | Юлия Алексеевна Щепочкина | Mass for facing tile fabrication |
| KR101667773B1 (en) * | 2015-07-24 | 2016-10-28 | 강동하 | Ceramic composition using waste ceramics, manufacturing method thereof and ceramics with high strength and transparent using thereof |
| CN110627478A (en) * | 2019-10-23 | 2019-12-31 | 翟作栋 | Carrier material prepared from chemical hazardous waste and preparation method and application thereof |
| CN114149247A (en) * | 2021-12-31 | 2022-03-08 | 新明珠集团股份有限公司 | Super-thick black coffee through-body ceramic tile and preparation method and application thereof |
| KR20230018180A (en) * | 2021-07-29 | 2023-02-07 | 이창용 | Manufacturing Method of Crack Resistance Ceramic Composition and Resistance Ceramic Composition by the same |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000256056A (en) * | 1999-03-08 | 2000-09-19 | Ozawa Kawara Kogyo:Kk | Recycled roof tile |
-
2004
- 2004-04-16 JP JP2004149767A patent/JP2005097082A/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000256056A (en) * | 1999-03-08 | 2000-09-19 | Ozawa Kawara Kogyo:Kk | Recycled roof tile |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007151875A (en) * | 2005-12-06 | 2007-06-21 | Ogiso:Kk | Porcelain tableware |
| JP2009046346A (en) * | 2007-08-20 | 2009-03-05 | Takahama Industry Co Ltd | Chamotte and clay roofing tile with which chamotte is blended |
| RU2469986C1 (en) * | 2011-09-30 | 2012-12-20 | Юлия Алексеевна Щепочкина | Mass for facing tile fabrication |
| KR101667773B1 (en) * | 2015-07-24 | 2016-10-28 | 강동하 | Ceramic composition using waste ceramics, manufacturing method thereof and ceramics with high strength and transparent using thereof |
| CN110627478A (en) * | 2019-10-23 | 2019-12-31 | 翟作栋 | Carrier material prepared from chemical hazardous waste and preparation method and application thereof |
| KR20230018180A (en) * | 2021-07-29 | 2023-02-07 | 이창용 | Manufacturing Method of Crack Resistance Ceramic Composition and Resistance Ceramic Composition by the same |
| KR102508145B1 (en) | 2021-07-29 | 2023-03-09 | 이창용 | Manufacturing Method of Crack Resistance Ceramic Composition and Resistance Ceramic Composition by the same |
| CN114149247A (en) * | 2021-12-31 | 2022-03-08 | 新明珠集团股份有限公司 | Super-thick black coffee through-body ceramic tile and preparation method and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN104624607B (en) | Domestic waste incineration residue processing method | |
| CN103752401B (en) | Potash feldspar iron removal process | |
| CN102189037A (en) | Impurity removal process for quartz sand | |
| Park et al. | The regeneration of waste foundry sand and residue stabilization using coal refuse | |
| JP2013117058A (en) | Apparatus for producing iron-based material and regenerated sand | |
| CN110328047B (en) | Method for preparing ceramic raw material from granite stone sawn mud stone powder | |
| JP2011526827A (en) | Coal ash recycling apparatus and method | |
| CN100591780C (en) | Method for producing high alumina refined powder by using alumyte | |
| CN110976077A (en) | Method for preparing high-purity quartz sand iron concentrate from magnetite associated granular quartz | |
| CN110002849A (en) | A kind of preparation method preparing high-performance abrasion-proof domestic ceramics using waste old ceramics | |
| JP2005097082A (en) | Method of firing ceramics at low temperature and ceramics fired at low temperature | |
| CN101885489A (en) | Method for preparing feldspar powder concentrate by mineral separation of aeolian sand in desert | |
| JPH0741874A (en) | Method for recovering metal slag of waste | |
| KR100442191B1 (en) | Recycling Equipment for Waste Casting Sand and Its recycling process | |
| CN108793731A (en) | A kind of preparation method of ultra-clear glasses raw material | |
| PT1414601E (en) | PRODUCTS FOR THE MANUFACTURE OF MOLDS AND MALES USED IN THE METAL FOUNDRY AND A METHOD FOR THEIR MANUFACTURE AND ITS RECYCLING FROM CRUSHED ROCK | |
| Silva et al. | Incorporation of quartzite fines in the production of red ceramics | |
| CN205893102U (en) | Ceramic recycle device of abandonment | |
| JP2000301128A (en) | Method and apparatus for recycling incineration ash of fluidized bed incinerator and incombustible material residue at bottom of gasification furnace of gasifying/ melting furnace | |
| US20190329268A1 (en) | Methods and systems for polishing and recovering aluminum from a waste material | |
| CN115446292A (en) | Cleaning and grading ferromagnetic separation method for investment shell type casting | |
| KR101525543B1 (en) | Method for recycling waste-abrasive used in the lapping and polishing of semiconductor and industry wafer | |
| JP2006111513A (en) | Production method utilizing waste across industry and product | |
| CN102806307B (en) | Recycling method of abandoned shell | |
| KR101577023B1 (en) | Effective Treatment Method of Wastes Comprising Slag, Ash and Sludge, Generated During Pyrometallurgical Copper Production |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070205 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070205 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20090225 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100406 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100817 |