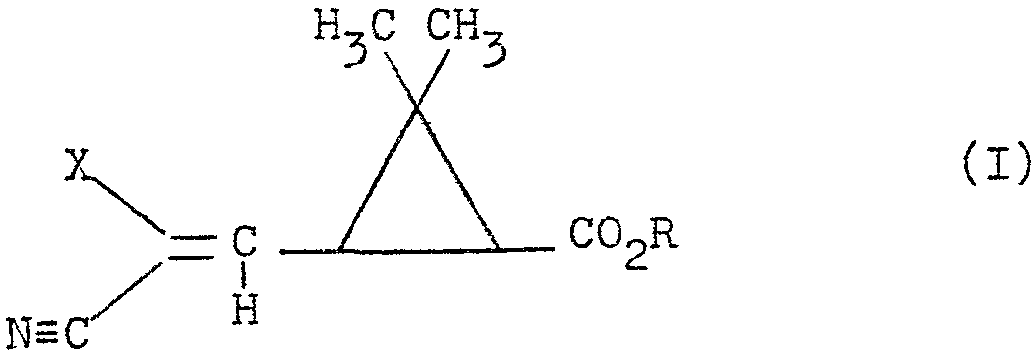FR2550191A1 - Novel cyclopropane carboxylic acid derivatives, a process for their preparation, and their use for controlling parasites. - Google Patents
Novel cyclopropane carboxylic acid derivatives, a process for their preparation, and their use for controlling parasites. Download PDFInfo
- Publication number
- FR2550191A1 FR2550191A1 FR8312858A FR8312858A FR2550191A1 FR 2550191 A1 FR2550191 A1 FR 2550191A1 FR 8312858 A FR8312858 A FR 8312858A FR 8312858 A FR8312858 A FR 8312858A FR 2550191 A1 FR2550191 A1 FR 2550191A1
- Authority
- FR
- France
- Prior art keywords
- formula
- compounds
- sep
- alcohol
- esters
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 244000045947 parasite Species 0.000 title claims abstract description 14
- 238000000034 method Methods 0.000 title claims abstract description 11
- YMGUBTXCNDTFJI-UHFFFAOYSA-N cyclopropanecarboxylic acid Chemical class OC(=O)C1CC1 YMGUBTXCNDTFJI-UHFFFAOYSA-N 0.000 title claims abstract description 10
- 238000002360 preparation method Methods 0.000 title claims abstract description 9
- 239000000203 mixture Substances 0.000 claims abstract description 44
- 150000001875 compounds Chemical class 0.000 claims abstract description 35
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims abstract description 11
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims abstract description 9
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims abstract description 7
- 229910052799 carbon Inorganic materials 0.000 claims abstract description 7
- 125000005843 halogen group Chemical group 0.000 claims abstract description 6
- 230000015572 biosynthetic process Effects 0.000 claims abstract description 5
- 238000003786 synthesis reaction Methods 0.000 claims abstract description 5
- 125000004432 carbon atom Chemical group C* 0.000 claims abstract description 4
- 229920006395 saturated elastomer Polymers 0.000 claims abstract description 4
- -1 2-fluoro-2-cyanoethenyl Chemical group 0.000 claims description 34
- 239000004480 active ingredient Substances 0.000 claims description 22
- 150000002148 esters Chemical class 0.000 claims description 17
- 241001465754 Metazoa Species 0.000 claims description 16
- 229910052731 fluorine Inorganic materials 0.000 claims description 14
- 230000000895 acaricidal effect Effects 0.000 claims description 10
- 239000000460 chlorine Chemical group 0.000 claims description 10
- 239000003795 chemical substances by application Substances 0.000 claims description 9
- 241000238876 Acari Species 0.000 claims description 8
- 241000607479 Yersinia pestis Species 0.000 claims description 8
- 239000000642 acaricide Substances 0.000 claims description 8
- 229910052801 chlorine Inorganic materials 0.000 claims description 8
- 239000011737 fluorine Substances 0.000 claims description 8
- 230000000749 insecticidal effect Effects 0.000 claims description 8
- 239000002253 acid Substances 0.000 claims description 7
- 125000001153 fluoro group Chemical group F* 0.000 claims description 7
- 229910052739 hydrogen Inorganic materials 0.000 claims description 7
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 6
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical group ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 claims description 6
- LVZWSLJZHVFIQJ-UHFFFAOYSA-N Cyclopropane Chemical compound C1CC1 LVZWSLJZHVFIQJ-UHFFFAOYSA-N 0.000 claims description 6
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 6
- 125000004429 atom Chemical group 0.000 claims description 6
- 239000001257 hydrogen Substances 0.000 claims description 6
- KGANAERDZBAECK-UHFFFAOYSA-N (3-phenoxyphenyl)methanol Chemical class OCC1=CC=CC(OC=2C=CC=CC=2)=C1 KGANAERDZBAECK-UHFFFAOYSA-N 0.000 claims description 5
- 239000005645 nematicide Substances 0.000 claims description 5
- 241000039077 Copula Species 0.000 claims description 4
- 238000007239 Wittig reaction Methods 0.000 claims description 4
- 150000001298 alcohols Chemical class 0.000 claims description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 4
- AFEOKIGLYCQHAZ-UHFFFAOYSA-N (5-benzylfuran-3-yl)methanol Chemical compound OCC1=COC(CC=2C=CC=CC=2)=C1 AFEOKIGLYCQHAZ-UHFFFAOYSA-N 0.000 claims description 3
- 150000007942 carboxylates Chemical class 0.000 claims description 3
- LLMLSUSAKZVFOA-UHFFFAOYSA-N 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid Chemical class CC1(C)C(C=C(Cl)Cl)C1C(O)=O LLMLSUSAKZVFOA-UHFFFAOYSA-N 0.000 claims description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 2
- 230000002378 acidificating effect Effects 0.000 claims description 2
- 235000019445 benzyl alcohol Nutrition 0.000 claims description 2
- 239000012374 esterification agent Substances 0.000 claims description 2
- 230000001069 nematicidal effect Effects 0.000 claims description 2
- OYDPVRNLLQZQFY-UHFFFAOYSA-N 2,2-dimethyl-3-[(2-oxothiolan-3-ylidene)methyl]cyclopropane-1-carboxylic acid Chemical class OC(=O)C1C(C)(C)C1C=C1C(=O)SCC1 OYDPVRNLLQZQFY-UHFFFAOYSA-N 0.000 claims 1
- VQXSOUPNOZTNAI-UHFFFAOYSA-N Pyrethrin I Natural products CC(=CC1CC1C(=O)OC2CC(=O)C(=C2C)CC=C/C=C)C VQXSOUPNOZTNAI-UHFFFAOYSA-N 0.000 claims 1
- 241000700605 Viruses Species 0.000 claims 1
- 239000008280 blood Substances 0.000 claims 1
- 210000004369 blood Anatomy 0.000 claims 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims 1
- HYJYGLGUBUDSLJ-UHFFFAOYSA-N pyrethrin Natural products CCC(=O)OC1CC(=C)C2CC3OC3(C)C2C2OC(=O)C(=C)C12 HYJYGLGUBUDSLJ-UHFFFAOYSA-N 0.000 claims 1
- VJFUPGQZSXIULQ-XIGJTORUSA-N pyrethrin II Chemical compound CC1(C)[C@H](/C=C(\C)C(=O)OC)[C@H]1C(=O)O[C@@H]1C(C)=C(C\C=C/C=C)C(=O)C1 VJFUPGQZSXIULQ-XIGJTORUSA-N 0.000 claims 1
- VEMKTZHHVJILDY-UXHICEINSA-N bioresmethrin Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OCC1=COC(CC=2C=CC=CC=2)=C1 VEMKTZHHVJILDY-UXHICEINSA-N 0.000 abstract description 3
- 230000008878 coupling Effects 0.000 abstract 1
- 238000010168 coupling process Methods 0.000 abstract 1
- 238000005859 coupling reaction Methods 0.000 abstract 1
- 239000000047 product Substances 0.000 description 30
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 15
- 150000003254 radicals Chemical class 0.000 description 12
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 11
- 239000000843 powder Substances 0.000 description 11
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 8
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 8
- UHOVQNZJYSORNB-UHFFFAOYSA-N monobenzene Natural products C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 8
- YMGUBTXCNDTFJI-UHFFFAOYSA-M cyclopropanecarboxylate Chemical compound [O-]C(=O)C1CC1 YMGUBTXCNDTFJI-UHFFFAOYSA-M 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 6
- 241000238631 Hexapoda Species 0.000 description 5
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 5
- 239000011541 reaction mixture Substances 0.000 description 5
- 239000000377 silicon dioxide Substances 0.000 description 5
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 4
- 239000000706 filtrate Substances 0.000 description 4
- 239000003921 oil Substances 0.000 description 4
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 4
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 4
- PUFSDEFIEAPJAJ-UHFFFAOYSA-N (bromo-fluoro-phenylmethyl)-cyanomercury Chemical compound C1(=CC=CC=C1)C(F)([Hg]C#N)Br PUFSDEFIEAPJAJ-UHFFFAOYSA-N 0.000 description 3
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 238000003776 cleavage reaction Methods 0.000 description 3
- 230000032050 esterification Effects 0.000 description 3
- 238000005886 esterification reaction Methods 0.000 description 3
- 230000003071 parasitic effect Effects 0.000 description 3
- 229960005235 piperonyl butoxide Drugs 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- 238000010992 reflux Methods 0.000 description 3
- 208000005687 scabies Diseases 0.000 description 3
- 230000007017 scission Effects 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 239000008096 xylene Substances 0.000 description 3
- QQHOVRKETYPQHY-UHFFFAOYSA-N 2-(hydroxymethyl)-4,5,6,7-tetrahydroisoindole-1,3-dione Chemical compound O=C1N(CO)C(=O)C2=C1CCCC2 QQHOVRKETYPQHY-UHFFFAOYSA-N 0.000 description 2
- VJXYSIXJDWGJNR-UHFFFAOYSA-N 2-bromo-2-fluoroacetamide Chemical compound NC(=O)C(F)Br VJXYSIXJDWGJNR-UHFFFAOYSA-N 0.000 description 2
- PABLWEPPFMSACN-UHFFFAOYSA-N 2-bromo-2-fluoroacetonitrile Chemical compound FC(Br)C#N PABLWEPPFMSACN-UHFFFAOYSA-N 0.000 description 2
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 2
- 206010063409 Acarodermatitis Diseases 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- 241000255925 Diptera Species 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- 241000257226 Muscidae Species 0.000 description 2
- 241000244206 Nematoda Species 0.000 description 2
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 2
- 241001481703 Rhipicephalus <genus> Species 0.000 description 2
- 241000447727 Scabies Species 0.000 description 2
- 240000004460 Tanacetum coccineum Species 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- ROVGZAWFACYCSP-MQBLHHJJSA-N [2-methyl-4-oxo-3-[(2z)-penta-2,4-dienyl]cyclopent-2-en-1-yl] (1r,3r)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate Chemical compound CC1(C)[C@H](C=C(C)C)[C@H]1C(=O)OC1C(C)=C(C\C=C/C=C)C(=O)C1 ROVGZAWFACYCSP-MQBLHHJJSA-N 0.000 description 2
- 235000011054 acetic acid Nutrition 0.000 description 2
- 238000005903 acid hydrolysis reaction Methods 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 239000011149 active material Substances 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- 235000013339 cereals Nutrition 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000002316 fumigant Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 235000012054 meals Nutrition 0.000 description 2
- 201000002266 mite infestation Diseases 0.000 description 2
- PSHKMPUSSFXUIA-UHFFFAOYSA-N n,n-dimethylpyridin-2-amine Chemical compound CN(C)C1=CC=CC=N1 PSHKMPUSSFXUIA-UHFFFAOYSA-N 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 230000000361 pesticidal effect Effects 0.000 description 2
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 2
- 229920000053 polysorbate 80 Polymers 0.000 description 2
- 229940015367 pyrethrum Drugs 0.000 description 2
- 238000003307 slaughter Methods 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- DLYUQMMRRRQYAE-UHFFFAOYSA-N tetraphosphorus decaoxide Chemical compound O1P(O2)(=O)OP3(=O)OP1(=O)OP2(=O)O3 DLYUQMMRRRQYAE-UHFFFAOYSA-N 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- PTQGFDXPHNRDCV-CRCLSJGQSA-N (1s,3s)-3-formyl-2,2-dimethylcyclopropane-1-carboxylic acid Chemical compound CC1(C)[C@@H](C=O)[C@@H]1C(O)=O PTQGFDXPHNRDCV-CRCLSJGQSA-N 0.000 description 1
- OOZCCPUDERLHBE-UHFFFAOYSA-M (bromo-cyano-phenylmethyl)-fluoromercury Chemical compound C1(=CC=CC=C1)C(C#N)([Hg]F)Br OOZCCPUDERLHBE-UHFFFAOYSA-M 0.000 description 1
- 125000004972 1-butynyl group Chemical group [H]C([H])([H])C([H])([H])C#C* 0.000 description 1
- 125000006023 1-pentenyl group Chemical group 0.000 description 1
- 125000006017 1-propenyl group Chemical group 0.000 description 1
- PQTDYOQEPYGGHF-UHFFFAOYSA-N 2,2-dimethylcyclopropane-1-carbaldehyde Chemical compound CC1(C)CC1C=O PQTDYOQEPYGGHF-UHFFFAOYSA-N 0.000 description 1
- VTJMSIIXXKNIDJ-UHFFFAOYSA-N 2-(4-chlorophenyl)-3-methylbutyric acid Chemical class CC(C)C(C(O)=O)C1=CC=C(Cl)C=C1 VTJMSIIXXKNIDJ-UHFFFAOYSA-N 0.000 description 1
- SGTZHNFBWALGOD-UHFFFAOYSA-N 2-[chloro(triphenyl)-$l^{5}-phosphanyl]acetonitrile Chemical compound C=1C=CC=CC=1P(CC#N)(C=1C=CC=CC=1)(Cl)C1=CC=CC=C1 SGTZHNFBWALGOD-UHFFFAOYSA-N 0.000 description 1
- ZYSXNJVFRGCTCU-UHFFFAOYSA-N 2-bromo-6-[methylidene(diphenyl)-lambda5-phosphanyl]benzonitrile Chemical compound BrC=1C(=C(C=CC=1)P(C1=CC=CC=C1)(C1=CC=CC=C1)=C)C#N ZYSXNJVFRGCTCU-UHFFFAOYSA-N 0.000 description 1
- SLRMQYXOBQWXCR-UHFFFAOYSA-N 2154-56-5 Chemical compound [CH2]C1=CC=CC=C1 SLRMQYXOBQWXCR-UHFFFAOYSA-N 0.000 description 1
- JJCKHVUTVOPLBV-UHFFFAOYSA-N 3-Methylbenzyl alcohol Chemical compound CC1=CC=CC(CO)=C1 JJCKHVUTVOPLBV-UHFFFAOYSA-N 0.000 description 1
- XNRCGJVOJYKMSA-UHFFFAOYSA-N 5-[bis[2-(2-butoxyethoxy)ethoxy]methyl]-1,3-benzodioxole Chemical compound CCCCOCCOCCOC(OCCOCCOCCCC)C1=CC=C2OCOC2=C1 XNRCGJVOJYKMSA-UHFFFAOYSA-N 0.000 description 1
- 241001124076 Aphididae Species 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- 241001674044 Blattodea Species 0.000 description 1
- FIPWRIJSWJWJAI-UHFFFAOYSA-N Butyl carbitol 6-propylpiperonyl ether Chemical compound C1=C(CCC)C(COCCOCCOCCCC)=CC2=C1OCO2 FIPWRIJSWJWJAI-UHFFFAOYSA-N 0.000 description 1
- BKISLXNRHBIYKY-UHFFFAOYSA-N C1(CC1)C(=O)O.BrC(=C)C#N Chemical compound C1(CC1)C(=O)O.BrC(=C)C#N BKISLXNRHBIYKY-UHFFFAOYSA-N 0.000 description 1
- 241000218645 Cedrus Species 0.000 description 1
- 235000013162 Cocos nucifera Nutrition 0.000 description 1
- 244000060011 Cocos nucifera Species 0.000 description 1
- 241000790917 Dioxys <bee> Species 0.000 description 1
- 235000019733 Fish meal Nutrition 0.000 description 1
- 241001337998 Machilus Species 0.000 description 1
- 241001674048 Phthiraptera Species 0.000 description 1
- 235000008331 Pinus X rigitaeda Nutrition 0.000 description 1
- 235000011613 Pinus brutia Nutrition 0.000 description 1
- 241000018646 Pinus brutia Species 0.000 description 1
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- 235000019764 Soybean Meal Nutrition 0.000 description 1
- 241000256250 Spodoptera littoralis Species 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 235000019772 Sunflower meal Nutrition 0.000 description 1
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 1
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 239000003905 agrochemical Substances 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 244000000054 animal parasite Species 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- WVDDGKGOMKODPV-UHFFFAOYSA-N benzyl alcohol Substances OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000001246 bromo group Chemical group Br* 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 210000000038 chest Anatomy 0.000 description 1
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 1
- OQNGCCWBHLEQFN-UHFFFAOYSA-N chloroform;hexane Chemical compound ClC(Cl)Cl.CCCCCC OQNGCCWBHLEQFN-UHFFFAOYSA-N 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- JEVCWSUVFOYBFI-UHFFFAOYSA-N cyanyl Chemical compound N#[C] JEVCWSUVFOYBFI-UHFFFAOYSA-N 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000004495 emulsifiable concentrate Substances 0.000 description 1
- ULNDTPIRBQGESN-UHFFFAOYSA-N ethyl 2-bromo-2-fluoroacetate Chemical compound CCOC(=O)C(F)Br ULNDTPIRBQGESN-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 239000004467 fishmeal Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 244000000013 helminth Species 0.000 description 1
- OTTZHAVKAVGASB-UHFFFAOYSA-N hept-2-ene Chemical compound CCCCC=CC OTTZHAVKAVGASB-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 239000005457 ice water Substances 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 239000002198 insoluble material Substances 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- ZGEGCLOFRBLKSE-UHFFFAOYSA-N methylene hexane Natural products CCCCCC=C ZGEGCLOFRBLKSE-UHFFFAOYSA-N 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 230000000050 nutritive effect Effects 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 238000000053 physical method Methods 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000002728 pyrethroid Substances 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 239000004449 solid propellant Substances 0.000 description 1
- 239000004550 soluble concentrate Substances 0.000 description 1
- 239000004455 soybean meal Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/62—Oxygen or sulfur atoms
- C07D213/63—One oxygen atom
- C07D213/64—One oxygen atom attached in position 2 or 6
- C07D213/647—One oxygen atom attached in position 2 or 6 and having in the molecule an acyl radical containing a saturated three-membered ring, e.g. chrysanthemumic acid esters
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N53/00—Biocides, pest repellants or attractants, or plant growth regulators containing cyclopropane carboxylic acids or derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C255/00—Carboxylic acid nitriles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D233/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings
- C07D233/54—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
- C07D233/66—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D233/72—Two oxygen atoms, e.g. hydantoin
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F3/00—Compounds containing elements of Groups 2 or 12 of the Periodic Table
- C07F3/10—Mercury compounds
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
La présente invention concerne de nouveaux dérivés de l'acide cyclopropane carboxylique, leur procédé de préparation et leur application a la lutte contre les parasites. The present invention relates to novel cyclopropane carboxylic acid derivatives, process for their preparation and their application to pest control.
L'invention a pour objet sous toutes leurs formes isomères possibles ainsi que les mélanges de ces isomères les composés de formule (I)
dans laquelle la copule acide cyclopropanique est de structure 1R cis ou 1R trans, X représente un atome d'halogene et R représente un atome d'hydrogéne, un radical alcoyle linéaire, ramifié ou cyclisé, saturé ou insaturé, renfermant jusqu'a 12 atomes de carbone ou le reste d'un alcool
R-OH utilisé dans la synthèse des pyréthrinoides, la géométrie de la double liaison portée par le carbone en 3 pouvant être de structure E ou Z.The subject of the invention is in all their possible isomeric forms as well as the mixtures of these isomers the compounds of formula (I)
in which the cyclopropanic acid copula is of 1R cis or 1R trans structure, X represents a halogen atom and R represents a hydrogen atom, a linear alkyl radical, branched or cyclized, saturated or unsaturated, containing up to 12 atoms carbon or the rest of an alcohol
R-OH used in the synthesis of pyrethroids, the geometry of the double bond carried by the carbon in 3 can be of structure E or Z.
Lorsque R représente un radical alcoyle linéaire, ramifié ou cyclisé, saturé ou insaturé, il s'agit de préférence du radical méthyle, éthyle, propyle linéaire ou ramifié, butyle linéaire ou ramifié, pentyle linéaire ou ramifié, hexyle linéaire ou ramifié, cyclopropyle, cyclobutyle, cyclopentyle, cyclohexyle, 1-propényle, 1-butynyle, 1,3-butédiényle, 1pentényle, l-cyclobutynyle, 1-cyclopentadiényle, 1-cyclohexényle. When R represents a linear, branched or cyclized, saturated or unsaturated alkyl radical, it is preferably methyl, ethyl, linear or branched propyl, linear or branched butyl, linear or branched pentyl, linear or branched hexyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, 1-propenyl, 1-butynyl, 1,3-butediyl, 1pentenyl, 1-cyclobutynyl, 1-cyclopentadienyl, 1-cyclohexenyl.
Lorsque R représente le reste d'un alcool utilisé dans la synthese des pyréthrinoîdes, il s'agit de préférence du radical choisi dans le groupe des radicaux suivants
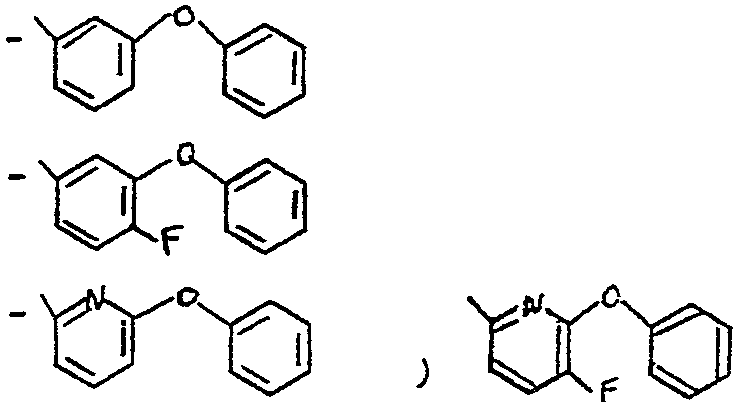
A - les radicaux -CH-Ardans lesquels Z représente un atome
z d'hydrogène, un radical éthynyle, méthyle, ou cyano et Ar représente un radical
dans lequel 5 représente un atome d'hydrogène ou de fluor et x représente
un atome de fluor, de chlore ou de broine
When R represents the residue of an alcohol used in the synthesis of pyrethroids, it is preferably the radical chosen from the group of the following radicals
A - the radicals -CH-Ardans which Z represents an atom
hydrogen, an ethynyl, methyl or cyano radical and Ar represents a radical
in which 5 represents a hydrogen or fluorine atom and x represents
a fluorine, chlorine or broine atom
<tb> <SEP> "3
<tb> B <SEP> - <SEP> le <SEP> radical
<tb>
C - le radical
<tb><SEP>"3
<tb> B <SEP> - <SEP> the radical <SEP>
<Tb>
C - the radical
D - et les radicaux
formule dans laquelle Y2 représente un atome d'hydrogène, un atome de fluor, de chlore ou de brome, un groupement -C-N ou un groupement -Cr CH, Y3, Y4, Y5 identiques ou différents, représentent un atome d'hydrogène, un atome de fluor, de chlore ou de brome, un radical alcoyle linéaire, ramifié ou cyclique, renfermant de 1 a 8 atomes de carbone, éventuellement substitué par un ou plusieurs groupements fonctionnels, identiques ou différents, un radical alcényle comportant de 2 à 8 atomes de carbone ou un radical alcynyle comportant de 2 a 8 atomes de carbone, les radicaux Y3, Y4 et Y5 peuvent former les cycles entre eux deux a deux.D - and the radicals
formula in which Y2 represents a hydrogen atom, a fluorine, chlorine or bromine atom, a group -CN or a group -Cr CH, Y3, Y4, Y5 identical or different, represent a hydrogen atom, a fluorine, chlorine or bromine atom, a linear, branched or cyclic alkyl radical containing 1 to 8 carbon atoms, optionally substituted by one or more functional groups, which may be identical or different, an alkenyl radical containing from 2 to 8 atoms of carbon or an alkynyl radical having from 2 to 8 carbon atoms, the radicals Y3, Y4 and Y5 can form the rings between them two by two.
Par atome d'halogène, on entend les atomes de fluor, de chlore ou de brome, mais l'invention a tout particulièrement pour objet les composés de formule (I) dans laquelle X représente un atome de fluor. The term "halogen atom" means fluorine, chlorine or bromine atoms, but the subject of the invention is particularly those compounds of formula (I) in which X represents a fluorine atom.
L'invention a tout spécialement pour objet les composés de formule (I) pour lesquels la géométrie de la double liaison portée par le carbone en 3 du cyclopropane est de structure Z. The subject of the invention is especially those compounds of formula (I) for which the geometry of the double bond borne by the carbon at 3 of the cyclopropane is of structure Z.
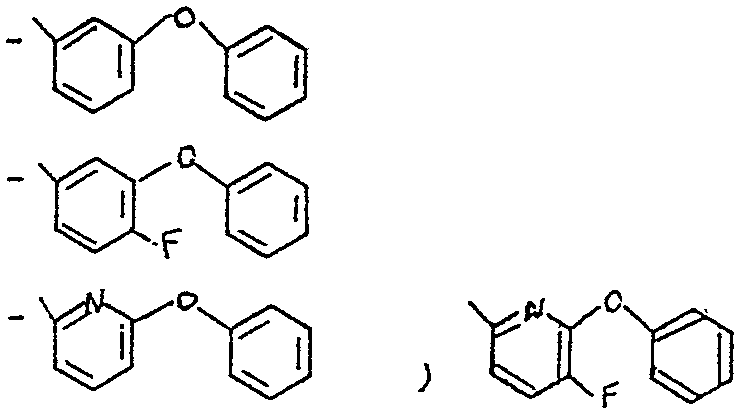
Parmi les composés de l'invention, on peut citer tout particulièrement les composés pour lesquels R représente un radical -CH-Ar, dans lequel Z Z représente un atome d'hydrogène, un radical-C -CH,CH3 ou un radical -C--N
et Ar représente un radical choisi dans le groupe des radicaux - C5H5 - C6F5
Among the compounds of the invention, mention may be made especially of the compounds for which R represents a radical -CH-Ar, in which ZZ represents a hydrogen atom, a radical-C -CH, CH3 or a radical -C- -NOT
and Ar represents a radical selected from the group of radicals - C5H5 - C6F5
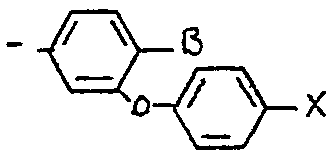
<tb> avec <SEP> B=H <SEP> ou <SEP> F
<tb> <SEP> X=F, <SEP> Cl, <SEP> Br
<tb> dans lequel B représente un atome d'hydrogène ou de fluor et X représente un atome de fluor, de chlore ou de brome et notamment les composés pour lesquels Ar représente un radical X -cyano métaphênoxy benzyle comme le 1R cis 2,2-diméthyl 3/z E 2-fluoro 2-cyano éthényl/ cyclopropane carboxylate de (S) < -cyano métaphénoxybenzyle ou l'isomereD Z correspondant. <tb> with <SEP> B = H <SEP> or <SEP> F
<tb><SEP> X = F, <SE> Cl, <SE> Br
in which B represents a hydrogen or fluorine atom and X represents a fluorine, chlorine or bromine atom and in particular the compounds for which Ar represents an X-cyano metaphenoxy benzyl radical such as 1R cis 2.2 or (S) -cyano metaphenoxybenzyl dimethyl 3 / z E 2-fluoro-2-cyano ethenyl / cyclopropane carboxylate or the corresponding Z isomer.
Les composés de formule (Ij présentent d'intéressantes propriétés qui permettent leur utilisation dans la lutte contre les parasites. Il peut s'agir par exemple de la lutte contre les parasites des végétaux, les parasites des locaux et les parasites des animaux a sang chaud. C'est ainsi que l'on peut utiliser les produits de l'invention pour lutter contre les inspectes, les nématodes et les acariens parasites des végétaux et des animaux. The compounds of formula (I) have interesting properties which allow their use in the fight against pests, which may be, for example, the control of plant pests, parasites of premises and parasites of warm-blooded animals. Thus, the products of the invention can be used to control pest, nematode and parasitic mites of plants and animals.
L'invention a donc aussi pour objet l'application des composés de formule (I) tels que définis précédemment a la lutte contre les parasites des végétaux, les parasites des locaux et les parasites des animaux à sang chaud. The invention therefore also relates to the application of the compounds of formula (I) as defined above to the control of plant pests, parasites of premises and parasites of warm-blooded animals.
Les produits de formule (I) peuvent être utilises notamment pour lutter contre les insectes dans le domaine agricole, pour lutter par exemple contre les pucerons, les larves de lépidoptères et les coléoptères. Ils sont utilisés a des doses comprises entre 10 g et 300 g de matière active a l'hectare. The products of formula (I) can be used in particular for controlling insects in the agricultural field, for example to combat aphids, lepidopteran larvae and coleopterans. They are used at doses of between 10 g and 300 g of active ingredient per hectare.
Les produits de formule (I) peuvent également être utilisés pour lutter contre les insectes dans les locaux, pour lutter notamment contre les mouches, les moustiques et les blattes
Les produits de formule (I) peuvent aussi être utilisés pour lutter contre les acariens et les nématodes parasites des végétaux.The products of formula (I) can also be used to control insects in the premises, in particular to fight against flies, mosquitoes and cockroaches.
The products of formula (I) may also be used to control plant-parasitic mites and nematodes.
Les composés de formule (I) peuvent encore être utilisés pour lutter contre les acariens parasites des animaux, pour lutter par exemple contre les tiques et notamment les tiques de l'espèce Boophilus, ceux de l'espèce Hyalomnia, ceux de l'espèce Amblyomnia et ceux de l'espèce Rhipicephalus ou pour lutter contre toutes sortes de gales et notamment la gale sarcoptique, la gale psoroptique et la gale chorioptique. Ils peuvent aussi être utilisés contre les poux et les helminthes. The compounds of formula (I) can still be used to fight against parasitic mites of animals, for example to fight against ticks and in particular ticks of the species Boophilus, those of the species Hyalomnia, those of the species Amblyomnia and those of the species Rhipicephalus or to fight against all kinds of scabies including sarcoptic mange, psoroptic mange and chorioptic mange. They can also be used against lice and helminths.
L'invention a donc également pour objet les compositions destinées a la lutte contre les parasites des végétaux, les parasites des locaux et les parasites des animaux à sang chaud, caractérisées en ce qu'elles renferment comme principe actif au moins un des produits de formule générale (I). The subject of the invention is therefore also compositions intended for combating plant pests, premises parasites and parasites of warm-blooded animals, characterized in that they contain as active principle at least one of the products of formula General (I).
L'invention a notamment pour objet les compositions insecticides renfermant comme principe actif au moins l'un des produits de formule générale (I). The invention particularly relates to the insecticidal compositions containing as active ingredient at least one of the products of general formula (I).
Parmi les compositions notamment insecticides proférées de l'invention, on peut citer tout spécialement les compositions renfermant les composés
décrits dans les exemples et notamment le (1R cis) 2,2-diméthyl 3/(S E) 2fluoro 2-cyano éthényl/ cyclopropane carboxylate de (S)d-cyano métaphénoxy
benzyle. Among the particularly insecticidal compositions of the invention, mention may be made especially of compositions containing the compounds
described in the examples and in particular (S) d-cyano metaphenoxy (1R cis) 2,2-dimethyl 3 / (SE) 2-fluoro-2-cyano ethenyl / cyclopropane carboxylate
benzyl.
Ces compositions sont préparées selon les procédés usuels de l'industrie agrochimique. Elles peuvent être additionnées éventuellement d'un ou plusieurs autres agents pesticides. Ces compositions peuvent se presenter sous forme de poudres, granulés, suspensions, émulsions, solutions, solutions pour aérosols, bandes combustibles, appâts ou autres préparations employés classiquement pour l'utilisation de ce genre de composés. These compositions are prepared according to the usual processes of the agrochemical industry. They may be optionally added with one or more other pesticidal agents. These compositions may be in the form of powders, granules, suspensions, emulsions, solutions, aerosol solutions, combustible strips, baits or other preparations conventionally used for the use of such compounds.
Outre le principe actif, ces compositions contiennent, en général un véhicule et/ou un agent tensio-actif, non ionique, assurant, en outre, une dispersion uniforme des substances constitutives du mélange. Le véhicule utilisé peut etre un liquide, tel que liteau, l'alcool, les hydrocarbures ou autres solvants organiques, une huile minérale, animale ou végétale, une poudre telle que le talc, les argiles, les silicates, le kieselguhr ou un solide combustible. In addition to the active ingredient, these compositions contain, in general, a vehicle and / or a nonionic surfactant, ensuring, in addition, a uniform dispersion of the constitutive substances of the mixture. The vehicle used can be a liquid, such as batten, alcohol, hydrocarbons or other organic solvents, a mineral oil, animal or vegetable, a powder such as talc, clays, silicates, kieselguhr or a solid fuel .
Les compositions insecticides selon l'invention contiennent de préférence 0,005 à à 10 % en poids de matière active. The insecticidal compositions according to the invention preferably contain from 0.005 to 10% by weight of active material.
Selon un mode opératoire avantageux, pour un usage dans les locaux, les compositions selon l'invention sont utilisées sous forme de compositions fumigantes. According to an advantageous procedure, for use in the premises, the compositions according to the invention are used in the form of fumigant compositions.
Les compositions selon l'invention peuvent alors être avantageusement constituees, pour la partie non active, d'un serpentin combustible,.ou ou encore d'un susbtrat fibreux incombustible. Dans ce dernier cas, le fumigant obtenu après incorporation de la matière active est place sur un appareil chauffant. The compositions according to the invention can then be advantageously constituted, for the non-active part, of a combustible coil, or a non-combustible fibrous susbtrate. In the latter case, the fumigant obtained after incorporation of the active ingredient is placed on a heating apparatus.
Dans le cas ou -l'on utilise un serpentin insecticide, le support inerte peut être. par exemple, composé de marc de pyrèthre, poudre de Tabu (ou poudre de feuilles Machilus Thumbergii, poudre de tige de pyrethre, poudre de feuille de cèdre, poudre de bois (telle que de la sciure de pin) amidon et poudre de coque de noix de coco. In the case where an insecticidal coil is used, the inert support may be. for example, composed of pyrethrum marc, Tabu powder (or Machilus Thumbergii leaf powder, pyrethrum stem powder, cedar leaf powder, wood powder (such as pine sawdust) starch and hull powder). coconut.
La dose de matière active peut alors être, par exemple,de 0,03 à 1 en poids. The dose of active ingredient can then be, for example, 0.03 to 1 by weight.
Dans le cas ou l'on utilise un support fibreux incombustible, la dose de matière active peut alors être, par exemple, de 0,03 a 25 en poids. In the case where a noncombustible fibrous support is used, the dose of active material can then be, for example, 0.03 to 25% by weight.
Les compositions selon l'invention pour un usage dans les locaux peuvent aussi être obtenues en préparant une huile pulvérisable a base de principe actif, cette huile imbibant Ta mèche d'une lampe et étant alors soumise à la combustion. The compositions according to the invention for use in the premises can also be obtained by preparing a sprayable oil based on the active ingredient, this oil soaking the wick of a lamp and then being subjected to combustion.
La concentration du principe actif incorporé à l'huile est, de préférence, de 0,03 a 25 % en poids. The concentration of the active ingredient incorporated in the oil is preferably from 0.03 to 25% by weight.
Les compositions acaricides et nématicides peuvent se présenter notamment sous forme de poudre, granulés, suspensions, émulsions, solutions. The acaricide and nematicide compositions may be in particular in the form of powder, granules, suspensions, emulsions, solutions.
Les compositions acaricides et nématicides peuvent être additionnées éventuellement d'un ou plusieurs autres agents pesticides. The acaricide and nematicide compositions may be optionally added with one or more other pesticidal agents.
Pour l'usage acaricide, on utilise de préférence des poudres mouillables, pour pulvérisation foliaire, contenant de 1 à 80 % de principe actif ou des liquides pour pulvérisation foliaire contenant de 1 a 500 g/l de principe actif. On peut également employer des poudres pour poudrage foliaires contenant de 0,05 à 3 % de matiere active. For the acaricide use, wettable powders, for foliar spraying, containing from 1 to 80% of active ingredient or foliar sprays containing from 1 to 500 g / l of active ingredient are preferably used. It is also possible to use foliar powders containing 0.05 to 3% of active ingredient.
Pour l'usage nématicide, on utilise de préférence des liquides pour traitement des sols contenant de 300 a 500 g/l de principe actif. For the nematicide use, it is preferable to use soil treatment liquids containing from 300 to 500 g / l of active ingredient.
Les composés acaricides et nématicides selon l'invention sont utilisées, de préférence, à des doses comprises entre I et 100 g de matière active a l'hectare. The acaricide and nematicide compounds according to the invention are preferably used at doses of between 1 and 100 g of active ingredient per hectare.
L'invention a donc -aussi pour objet les compositions acaricides renfermant comme principe actif au moins l'un des produits de formule générale (i) ainsi que les compositions nématicides renfermant comme principe actif au moins l'un des produits définis de formule générale (I). The invention therefore also relates to the acaricide compositions containing as active ingredient at least one of the products of general formula (i) as well as the nematicidal compositions containing as active principle at least one of the defined products of general formula ( I).
L'invention a également pour objet les compositions acaricides renfermant comme principe actif l'un au moins des composés de formule générale (I), caractérisées en ce qu'elles sont utilisées dans la lutte contre les parasites des animaux a sang chaud, notamment contre les tiques et les gales. The invention also relates to the acaricide compositions containing as active ingredient at least one of the compounds of general formula (I), characterized in that they are used in the fight against parasites of warm-blooded animals, in particular against ticks and scabies.
Lorsqu'il s'agit de lutter contre les parasites des animaux, les compositions peuvent être utilisées sous forme de spray, de bain, ou encore selon la méthode "pour-on". When it comes to combating animal parasites, the compositions may be used in the form of a spray, a bath, or according to the "for-on" method.
Lorsqu'on utilise la méthode pour-on, on utilise due préférence des solutions renfermant de 0,5 a 4 g de principe actif pour 100 cm3 de solution. When using the method for-one, it is preferable to use solutions containing 0.5 to 4 g of active ingredient per 100 cm3 of solution.
Lorsqu'il s'agit de lutter contre les acariens parasites des animaux, on peut incorporer les produits de l'invention dans des compositions alimentaires en association avec un mélange nutritif adapté a l'alimentation animale. Le mélange nutritiel peut varier selon l'espèce animale ; il peut renfermer des céréales, des sucres et des grains des tourteaux de soja, d'arachide et de tournesol, des farines d'origine animale, par exemple des farines de poissons, des acides aminés de
synthese, des sels minéraux, des vitamines et des antioxydants.When it comes to controlling animal parasitic mites, it is possible to incorporate the products of the invention into food compositions in combination with a nutritive mixture suitable for animal feed. The nutrient mixture may vary depending on the animal species; it may contain cereals, sugars and grains of soybean meal, peanut meal and sunflower meal, meal of animal origin, for example fish meal, amino acids from
synthesis, mineral salts, vitamins and antioxidants.
L'invention a donc ainsi également pour objet les compositions
destinées a l'alimentation animale renfermant comme principe actif au moins l'un des produits de formule générale (t). The invention thus also relates to the compositions
for animal feed containing as active ingredient at least one of the products of general formula (t).
L'invention a également pour objet les associations douées d'activité insecticide, acaricide ou némati ci de, caractérisés en ce qu'elles contiennent comme matière active, d'une part un au moins des composés de formule générale (I) et d'autre part, un au moins des esters pyrethrinoldes choisis dans le groupe constitué par les esters d'alléthrolones, d'alcool 3,4,5,6-tétrahydrophtalimido - méthyl ique, d'alcool 5-benzyl 3-furyl méthylique, d'alcool 3-phénoxy benzylique et d'alcools -cyano 3-phênoxy benzyliques des acides chrysanthémiques, par les esters d'alcool 5-benzyl 3-furyl méthylique des acides 2,2-diméthyl 3-(2-oxo 3-tétrahydrothiophénylidène méthyl) cyclopropane l-carboxyliques, par les esters d'alcool 3phénoxy benzylique et d'alcools -cyano 3-phénoxy benzyliques des acides 2,2-diméthyl 3-(2,2-dichlorovinyl) cyclopropane 1-carboxyliques, par les esters d'alcools o(-cyano 3-phénoxy benzyliques d'acides 2,2-diméthyl 3 (2,2-dibromovinyl) cyclopropane carboxyliques, par les esters d'alcool 3phénoxy benzylique des acides 2-parachlorophényl 2-isopropyl acétiques-, par les esters d'allêthrolones, d'alcool 3,4,5,6-tétrahydrophtalimido méthylique, d'alcool 5-benzyl 3-furyl méthylique, d'alcool 3-phénoxy benzylique et d'alcools o(-cyano 3-phénoxy benzyliques des acides 2,2diméthyl 3-(1,2-2,2-tétrahaloéthyl) cyclopropane 1-carboxyliques dans lesquels "halo" représente un atome de fluor, de chlore ou de brome, étant entendu que les copules acides et alcools des esters pyréthrinoides cidessus peuvent exister sous toutes leurs formes stéréoisomères possibles. The invention also relates to combinations endowed with insecticidal, acaricidal or nematic activity, characterized in that they contain as active ingredient, on the one hand at least one of the compounds of general formula (I) and of on the other hand, at least one of the pyrethrinol esters selected from the group consisting of esters of allethrolones, 3,4,5,6-tetrahydrophthalimido-methyl alcohol, 5-benzyl-3-furyl methyl alcohol, benzyl 3-phenoxy alcohol and 3-phenoxy benzyl alcohol-cyano chrysanthemic acids, by the alcohol esters 5-benzyl 3-furyl methyl 2,2-dimethyl-3- (2-oxo-3-tetrahydrothiophenylidene methyl) 1-carboxylic cyclopropane, with the esters of 3-phenoxybenzyl alcohol and 3-phenoxybenzyl alcohols of 2,2-dimethyl-3- (2,2-dichlorovinyl) cyclopropane-1-carboxylic acids, with the esters of alcohols o (-cyano 3-phenoxy benzyl acids 2,2-dimethyl 3 (2,2-dibromovinyl) cyclopropan carboxylic esters, by the esters of 3-phenoxy benzyl alcohol of 2-parachlorophenyl 2-isopropyl acetic acids, by esters of allethrolones, 3,4,5,6-tetrahydrophthalimido methyl alcohol, 5-benzyl alcohol 3 methyl-benzyl alcohol, 3-phenoxy benzyl alcohol and o-cyano-3-phenoxy benzyl alcohols 2,2dimethyl 3- (1,2-2,2-tetrahaloethyl) cyclopropane 1-carboxylic acids in which "halo" represents a fluorine, chlorine or bromine atom, it being understood that the acidic copula and alcohols of the pyrethrinoid esters above may exist in all their possible stereoisomeric forms.
L'inventio a pour objet les compositions insecticides définies précédemment, caractérisées en ce qu'elles renferment en outre un synergiste des pyréthrinoides
Comme synergistes classiques utilisés en pareil cas, on peut citer le 1-(2,5,8-trioxadodécyl) 9-propyl 4,5-méthylène dioxy benzène (ou butoxyde de pipéronyle) ou le N-(2-éthyl heptyl) bicyclo /2,2-1/5-heptène 2,3 di carboximi de, ou le pipéronyl-bis-2-(2n-butoxy éthoxy) éthyl acétal (ou tropital).The subject of the invention is the insecticidal compositions defined above, characterized in that they also contain a pyrethroid synergist.
As conventional synergists used in such cases, there may be mentioned 1- (2,5,8-trioxadodecyl) 9-propyl 4,5-methylene dioxy benzene (or piperonyl butoxide) or N- (2-ethyl heptyl) bicyclo / 2,2-1 / 5-heptene 2,3 di carboximi of, or piperonyl-bis-2- (2n-butoxyethoxy) ethyl acetal (or tropital).
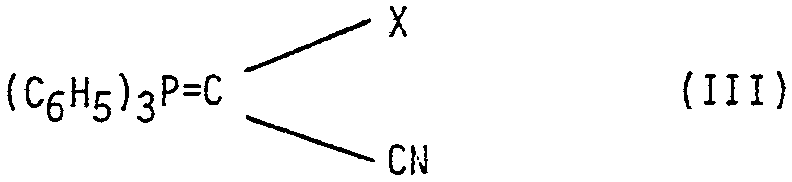
L'invention a également pour objet un procédé de préparation des composés de formule (I) tels que définis précédemment, caractérisé en ce que l'on soumet selon la réaction de Wittig un composé de formule (II)
dans laquelle R conserve la même signification que précédemment å l'action d'un composé de formule (III)
dans laquelle X conserve la même signification que précédemment pour obtenir un composé de formule (I) correspondant que l'on soumet, si désiré, à l'action d'un agent d'estérification si R représente un atome d'hydrogène, ou que l'on soumet, si désiré, à l'action d'un agent de clivage de la fonction ester, puis, si désiré, à l'action d'un agent d'estérification.The subject of the invention is also a process for the preparation of the compounds of formula (I) as defined above, characterized in that, according to the Wittig reaction, a compound of formula (II) is subjected
in which R retains the same meaning as above with the action of a compound of formula (III)
in which X has the same meaning as above to obtain a corresponding compound of formula (I) which is subjected, if desired, to the action of an esterifying agent if R represents a hydrogen atom, or if desired, the cleavage agent is subjected to the ester function and, if desired, to the action of an esterification agent.
Les composés de formule (II) et de formule (III) sont des produits connus. The compounds of formula (II) and formula (III) are known products.
Les produits de formule (III) peuvent etre préparés selon le procédé décrit dans Helv. Chem. Acta. Vol .60 (1977) p. 585. The products of formula (III) can be prepared according to the method described in Helv. Chem. Acta. Vol. 60 (1977) p. 585.
La réaction de Wittig utilisée généralement pour préparer les produits de formule (I) fournit des composés de formule (I) dont la géométrie est
E + Z.The Wittig reaction generally used to prepare the products of formula (I) provides compounds of formula (I) whose geometry is
E + Z.
Les isomères au niveau de la double liaison peuvent être séparés, si désiré, par des méthodes physiques telles que la chromatographie, soit au niveau des acides, soit au niveau des esters d'alcoyle, soit au niveau des esters finaux. The isomers at the double bond level can be separated, if desired, by physical methods such as chromatography, or at the level of the acids, or at the level of the alkyl esters, or at the level of the final esters.
L'agent de clivage du groupement CO2P est de préférence la chaleur utilisée avec un agent d'hydrolyse acide. Comme agent d'hydrolyse acide, on peut utiliser l'acide p-toluène sulfonique. The cleavage agent of the CO2P group is preferably the heat used with an acid hydrolysis agent. As acidic hydrolysis agent, p-toluenesulphonic acid may be used.
L'estérification peut être effectuée en présence d'une base tertiaire, telle que la pyridine. Cette estérification peut être effectuée avantageusement en présence d'un mélange de pyri di ne, de dicyclohexyl carbodiimide et,de de 4-diméthylaminopyridine ou de pyridine. Esterification can be carried out in the presence of a tertiary base, such as pyridine. This esterification can be carried out advantageously in the presence of a mixture of pyridine, dicyclohexyl carbodiimide and 4-dimethylaminopyridine or pyridine.
Il va de soi que la réaction de Wittig, les clivages des fonctions esters, l'estérification sont des réactions bien connues de l'homme de métier et n'ont pas à être detaillées ici. It goes without saying that the Wittig reaction, the cleavages of the ester functions, the esterification are reactions well known to those skilled in the art and need not be detailed here.
Les exemples suivants illustrent l'invention sans toutefois la limiter. The following examples illustrate the invention without limiting it.
EXEMPLE i : Acide 1R cis 2,2-diméthyl 3/(E+Z) 2-bromo 2-cyano éthényl/- cyclopropans carboxylique.EXAMPLE 1: 1 R cis 2,2-dimethyl 3 / (E + Z) 2-bromo-2-cyanoethenyl / -cyclopropanecarboxylic acid.
On mélange sous atmosphère inerte 3,5 g de bromo cyano méthylène triphényl phosphorane, 30cm3 de tétrahydrofuranne et 4cm3 de diméthyl formamide. On ajoute à la solution obtenue 1,3 9 de la lactone de l'acide 1R cis 2,2-diméthyl dihydroxy méthyl cyclopropane carboxylique et lOcm3 de tétrahydrofuranne. On maintient 16 heures à la température ambiante, amène à sec, on obtient 5,8 g d'un résidu huileux que l'on chromatographie sur silice en éluant par le mélange hexane, acétate d'éthyle,(7.3) à 1% d'a -cide acétique . On obtient le mélange des isomères #E etaZ (F=132 C). 3.5 g of bromo cyano methylene triphenyl phosphorane, 30 cm 3 of tetrahydrofuran and 4 cm 3 of dimethylformamide are mixed under an inert atmosphere. 1.39 of the 1 R cis 2,2-dimethyl dihydroxy methyl cyclopropane carboxylic acid lactone and 10 μl of tetrahydrofuran are added to the resulting solution. It is kept at room temperature for 16 hours and is evaporated to dryness. 5.8 g of an oily residue are obtained, which is chromatographed on silica, eluting with hexane, ethyl acetate (7.3) at 1% concentration. 'acetic acid . The mixture of isomers #E etaZ (F = 132 C) is obtained.
EXEMPLE 2: 1R cis 2,2-diméthyl 3-/( E) 2-bromo 2-cyano éthényll cyclopropane carboxylate de (S)α(-cyano 3-phenoxybenzyle et isomères correspondant.EXAMPLE 2: 1R cis 2,2-dimethyl-3 - [(E) 2-bromo [2-cyanoethylene] cyclopropane carboxylate of (S) -α-cyano-3-phenoxybenzyl and corresponding isomers.
On ajoute 30 mg de diméthylaminopyridine a Oe*5 C dans 10cm3 d' une solution renfermant 1,4 g d'acide 1R cis 2,2-diméthyl 3/(E+Z) 2-bromo 2cyano éthényl cyclopropane carboxylique et 1,4 g d'alcool (S)-cyano
3-phénoxybenzylique. On ajoute ensuite ,29 de dicyclohexyicarbodiimide et 5cm3 de chlorure de méthylène. On laisse le mélange réactionnel revenir à 20 C et agite pendant 3 heures. On filtre l'urée formée, rince et amène à sec le filtrat obtient 3,7 g de produit que l'on chromatographie sur silice en éluant par le benzène.On obtient d'une part 1,3 g d'isomère# E (F=600C) et d'autre part, 750 mg d'isomère Z#(F=64 C). 30 mg of dimethylaminopyridine at 0 ° C. are added in 10 cm 3 of a solution containing 1.4 g of 1R cis-2,2-dimethyl-3 / (E + Z) 2-bromo-2-cyanoethenyl cyclopropane carboxylic acid and 1.4 g g of alcohol (S) -cyano
3-phenoxybenzyl. Dicyclohexylcarbodiimide and 5 cm 3 of methylene chloride are then added. The reaction mixture is allowed to return to 20 ° C. and stirred for 3 hours. The formed urea is filtered off, rinsed and the filtrate is evaporated to dryness to obtain 3.7 g of product, which is chromatographed on silica eluting with benzene. 1.3 g of # E isomer is obtained ( F = 600C) and on the other hand, 750 mg of Z # isomer (F = 64 C).
EXEMPLE 3 : 1R cis 2,2-diméthyl 3/( Z! Z) 2-fluoro 2-cyano éthényl/ cyclopropane carboxylate de terbutyle et isomère bE correspondant.EXAMPLE 3: 1r cis 2,2-dimethyl 3 / (Z! Z) 2-fluoro-2-cyano ethenyl / cyclopropane carboxylate and terbutyl isomer bE.
On porte au reflux sous agitation pendant 1 heure un mélange renfermant 15 g de phényl bromo fluoro cyano méthyl mercure, 180cm3 de xylène,
8 g de triphénylphosphine et 5,25 g
1R, cis 2,2-diméthyl 3-formyl cyclopropane 1carboxylate de terbutyle.A mixture containing 15 g of phenyl bromo-fluoro cyano methyl mercury and 180 cm 3 of xylene is stirred under reflux for 1 hour.
8 g of triphenylphosphine and 5.25 g
1R, cis 2,2-dimethyl-3-formyl cyclopropane tetbutyl carboxylate.
On glace, filtre et amène a sec le filtrat. On obtient 20 g d'un produit que l'on chromatographie sur silice en éluant par du benzène pur. It is ice-cold, filtered and the filtrate is brought to dryness. 20 g of a product are obtained which are chromatographed on silica eluting with pure benzene.
On obtient - 3 g d'isomère #E (rf=0,4 - 1,3 g d'isomère #z (rf=0,33). 3 g of #E isomer (rf = 0.4-1.3 g of #z isomer (rf = 0.33)) are obtained.
Préparation du phényl bromo fluoro cyano methyl mercure utilisé au début de l'exemple.Preparation of phenyl bromo fluoro cyano methyl mercury used at the beginning of the example.
a) Bromo fluoro acétamide
On ajoute à +5"C 86cm3 d'ammoniaque concentré dans 79 g de bromofluoro acétate d'éthyle en agitant fortement. On maintient encore 30 minutes l'agitation et la température, évapore à sec, distille le résidu et obtient 53,6 g de produit attendu eb/0,1=82-84 C. a) Bromo fluoroacetamide
Concentrated ammonia (79 g) of ethyl bromofluoroacetate was added with concentrated stirring at +5 ° C. Stirring and temperature were continued for a further 30 minutes, evaporated to dryness and the residue distilled to give 53.6 g. expected product eb / 0,1 = 82-84 C.
b) Bromo fluoro acétonitrile
On ajoute 92 g d'anhydride phosphorique dans 183 g de bromofluoro acétamide obtenu comme précédemment chauffé suffisamment pour mélanger, puis on chauffe progressivement le mélange jusqu'à 200 C (extérieur) et distille entre 55 à 80 C 87 g de produit brut attendu.(b) Bromo fluoroacetonitrile
92 g of phosphoric anhydride are added to 183 g of bromofluoroacetamide obtained as previously heated sufficiently to mix, and then the mixture is gradually heated to 200 ° C. (outside) and 87 g of expected crude product are distilled between 55 ° and 80 ° C.
c) Phényl bromo fluoro cyano méthyl mercure
On refroidit à -50 C 15,65 g de chlorure de phényle mercurique dans 100 cm3 de tétrahydrofuranne, ajoute 10,6 g de fluoro bromo acétonitrile obtenu comme ci-dessus, puis, toujours à -50 C et sous agitation, une suspension contenant 7,85 g de terbutylate de potassium, 50 cm3 de tétrahydrofuranne et 6,6 cm3 d'alcool terbutylique.c) Phenyl bromo fluoro cyano methyl mercury
15.65 g of mercuric phenyl chloride are cooled down to -50 ° C. in 100 cm 3 of tetrahydrofuran, 10.6 g of fluoro bromoacetonitrile, obtained as above, are added, then, again at -50 ° C., with stirring, a suspension containing 7.85 g of potassium tert-butylate, 50 cm3 of tetrahydrofuran and 6.6 cc of terbutyl alcohol.
Après 30 minutes, on verse le mélange réactionnel sur de l'eau glacée renfermant 6 cm3 d'acide chlorhydrique concentré et extrait au chloroforme. After 30 minutes, the reaction mixture is poured into ice water containing 6 cm3 of concentrated hydrochloric acid and extracted with chloroform.
On sèche la phase organique et concentre à sec. On reprend le résidu par un mélange chloroforme hexane (1/1), filtre l'insoluble, glace, essore et obtient 9,3 g de produit attendu (F=130-132"C). The organic phase is dried and concentrated to dryness. The residue is taken up in a chloroform hexane (1/1) mixture, the insoluble material is filtered off, ice, filtered and 9.3 g of expected product are obtained (mp 130-132 ° C.).
EXEMPLE 4 : Acide 1R cis 2,2-diméthyl 3/( Z) 2-cyano 2-fluoro éthényl/ cyclopropane carboxylique. EXAMPLE 4 1-cis cis 2,2-dimethyl-3 ((Z) 2-cyano-2-fluoro ethenyl / cyclopropane carboxylic acid.
On porte a 120-130"C 1,3 g de 1R cis 2,2-diméthyl 3-/ < Z) 2-fluoro 2cyano éthényl/ cyclopropane carboxylate de terbutyle, 13cm3 de méthylbenzene et 130 mg d'acide paratoluènesulfonique. On maintient le mélange réactionnel 15 minutes sous agitation après le début du reflux. On ramène le mélange réactionnel a 20 C, lave à l'eau, sèche sur sulfate de
soude la solution toluénique, la filtre et l'amène a sec sous pression
réduite. On obtient 1 g de produit recherché.1.3 g of 1R cis-2,2-dimethyl-3- (2-fluoro-2-cyano-ethenyl-cyclopropanecarboxyl carboxylate, 13 cm.sup.3 of methylbenzene and 130 mg of para-toluenesulphonic acid are added at 120.degree.-130.degree. the reaction mixture is stirred for 15 minutes after the start of refluxing The reaction mixture is cooled to 20 ° C., washed with water and dried over sodium sulfate.
soda toluene solution, filter and bring it to dry under pressure
scaled down. 1 g of desired product is obtained.
EXEMPLE 5 : Acide 1R cis 2,2-diméthyl 3-/(6 E) 2-cyano 2-fluoro éthényl/ cyclopropane carboxylique. EXAMPLE 5 1R cis 2,2-dimethyl-3 - [(6E) 2-cyano-2-fluoro-ethenyl] cyclopropane carboxylic acid
En opérant comme a l'exemple 4, à partir du 1R cis 2,2-diméthyl 3/(# E) 2-fluoro 2-cyano éthényl/ cyclopropane carboxylate de terbutyle, on obtient le produit recherché. By operating as in Example 4, starting from the 1R cis 2,2-dimethyl 3 / (# E) 2-fluoro-2-cyano ethenyl / cyclopropane carboxylate terbutyl, the desired product is obtained.
EXEMPLE 6 : 1R cis 2,2-diméthyl 3/( Z) 2-fluoro 2-cyano éthényl/ cyclopropane carboxylate de (S) α-cyano 3-phénoxybentyle.EXAMPLE 6 1R cis 2,2-dimethyl 3 / (Z) 2-fluoro-2-cyano ethenyl / cyclopropane carboxylate (S) -α-cyano-3-phenoxybentyl.
On ajoute a 5 C 30 mg de diméthylaminopyridine dans une solution renfermant 1 g d'acide 1R cis 2,2-diméthyl 3/(fl Z) 2-fluoro 2-cyano éthényl/ cyclopropane carboxylique, 6cm3 de chlorure de méthylène et 1,2 g d'alcool (S) α-cyano 3-phénoxybenzylique. On ajoute ensuite 1,1 g de dicyclohexylcarbodiimide et 8cm3 de chlorure de méthylène. On laisse le mélange réactionnel revenir a 20 C et l'on maintient sous agitation pendant 2 heures. On ajoute ensuite 0,5cm3 d'acide acétique et 0,5cm3 d'éthanol. On filtre ensuite l'urée formée, la rince avec un peu de chlorure de méthylène et amène le filtrat a sec.On obtient 2,8 g de produit que l'on chromatographie sur silice en éluant par le mélange chlorure de méthylène, hexane (8-2). On obtient 1,5 g du produit recherché brut. Après recristallisation dans l'isopropanol au reflux, on obtient 1,3 g du produit recherché fondant a 90 C. 30 mg of dimethylaminopyridine are added at 5 ° C. to a solution containing 1 g of 1R cis-2,2-dimethyl-3 ((2 Z) 2-fluoro-2-cyanoethenyl / cyclopropane carboxylic acid, 6 cm 3 of methylene chloride and 1 g. 2 g of (S) -alpha-3-phenoxybenzyl alcohol. 1.1 g of dicyclohexylcarbodiimide and 8 cm3 of methylene chloride are then added. The reaction mixture is allowed to return to 20 ° C. and the mixture is stirred for 2 hours. 0.5 cm3 of acetic acid and 0.5 cm3 of ethanol are then added. The urea formed is then filtered off, rinsed with a little methylene chloride and the filtrate is brought to dryness. 2.8 g of product are obtained which is chromatographed on silica eluting with a mixture of methylene chloride and hexane ( 8-2). 1.5 g of the crude desired product are obtained. After recrystallization from refluxing isopropanol, 1.3 g of the desired product, melting at 90 ° C., are obtained.
EXEMPLE 7 : Acide 1R cis 2,2-diméthyl 3/(E+Z) 2-chloro 2-cyano éthényl/ cyclopropane carboxylique.EXAMPLE 7: 1 R cis 2,2-dimethyl 3 / (E + Z) 2-chloro-2-cyanoethenyl / cyclopropane carboxylic acid.
En opérant comme a l'exemple 1, à partir du chloro cyanométhyl triphényl phosphorane, on obtient le produit recherché (F < 50 C). By operating as in Example 1, starting from chloro cyanomethyl triphenyl phosphorane, the desired product (F <50 ° C.) is obtained.
EXEMPLE 8 : 1R cis 2,2-diméthyl 3/( j E) 2-chloro 2-cyano éthényl/ cyclopropane carboxylate de (S) -cyano 3-phénoxybenzyle et isomère #z correspondant.EXAMPLE 8: 1R cis 2,2-dimethyl 3 / (e) 2-chloro-2-cyano ethenyl / cyclopropane carboxylate (S) -cyano-3-phenoxybenzyl and isomer # z corresponding.
En opérant comme a l'exemple 2, à partir de l'acidepréparé à l'exemple 7, on a obtenu - d'une part, l'isomère #E (F=66 C) - d'autre part, l'isomère #z (F=630C). Operating as in Example 2, starting from the acid prepared in Example 7, the first isomer #E (F = 66 C) was obtained on the other hand, the isomer #z (F = 630C).
EXEMPLE - 9 : 1R trans 2,2-diméthyl 3/(X E) 2-cyano 2-fluoro éthényl/ cyclopropane carboxylate de (S)4 -cyano métaphénoxybenzyle et isomère #z correspondant.EXAMPLE 9: 1R trans 2,2-dimethyl 3 / (X E) 2-cyano-2-fluoro ethenyl / cyclopropane carboxylate (S) 4 -cyano metaphenoxybenzyl and corresponding ## isomer.
On porte au reflux sous agitation pendant 2 heures un mélange renfermant 10 g d'acide 1R trans 2,2-diméthyl 3-formyl cyclopropane carboxylique, 8 g de triphénylphosphine, 180 cm3 de xylène et 15 g-de phényl bromo cyano fluoro méthyl mercure. On glace, essore et amène à sec le filtrat. On obtient 24 g d'une huile que l'on chromatographie sur silice en éluant par le toluène. On sépare après plusieurs"HPLC",835 mg d'isomèreJZ (α D=17 + 1 5 -c=1 % dans CHCl3) et 1,8 g d'isomère #E (D=-21 + 205 c=0,5 % dans CHCi3). A mixture containing 10 g of 1R trans 2,2-dimethyl-3-formyl cyclopropane carboxylic acid, 8 g of triphenylphosphine, 180 cm 3 of xylene and 15 g of phenylbromo-cyano-fluoro-methyl-mercury is refluxed under agitation for 2 hours. . It is ice-cold, drained and the filtrate is brought to dryness. 24 g of an oil are obtained which are chromatographed on silica eluting with toluene. After several "HPLCs", 835 mg of isomer Z (alpha = 17 + 1 5 -c = 1% in CHCl 3) and 1.8 g of #E isomer (D = -21 + 205 c = 0) are separated off. , 5% in CHCl3).
EXEMPLES 10 à 22
En opérant comme précédemment, on a obtenu les produits répondant à la formule (I) suivants
EXAMPLES 10 to 22
By operating as above, the following products having the following formula (I) were obtained:
<tb> 'Exemples <SEP> X <SEP> d <SEP> Géométrie <SEP> R
<tb> <SEP> CDI)
<tb> <SEP> 10 <SEP> F <SEP> E <SEP> 1Rcis <SEP> a <SEP> "(0-7)
<tb> <SEP> 11 <SEP> Br <SEP> E <SEP> 1Rcis
<tb> <SEP> 12 <SEP> Br <SEP> E <SEP> 3 <SEP> lacis
<tb> <SEP> Z <SEP> Br
<tb> <SEP> 13 <SEP> ' <SEP> E
<tb> <SEP> ci'
<tb> <SEP> 14 <SEP> El <SEP> El <SEP> El <SEP> M
<tb> <SEP> 15 <SEP> -çD/,10
<tb> <SEP> 16 <SEP> C1 <SEP> 11 <SEP> 1 <SEP> lRtrans
<tb> <SEP> Ct.
<tb><tb>'Examples<SEP> X <SEP> d <SEP> Geometry <SEP> R
<tb><SEP> CDI)
<tb><SEP> 10 <SEP> F <SEP> E <SEP> 1Cross <SEP> a <SEP>"(0-7)
<tb><SEP> 11 <SEP> Br <SEP> E <SEP> 1Rcis
<tb><SEP> 12 <SEP> Br <SEP> E <SEP> 3 <SEP> lacis
<tb><SEP> Z <SEP> Br
<tb><SEP> 13 <SEP>'<SEP> E
<tb><SEP> ci '
<tb><SEP> 14 <SEP> El <SEP> El <SEP> El <SEP> M
<tb><SEP> 15 <SEP> -cd /, 10
<tb><SEP> 16 <SEP> C1 <SEP> 11 <SEP> 1 <SEP> ltrans
<tb><SEP> Ct.
<Tb>
<SEP> 17 <SEP> " <SEP> i <SEP> E'
<tb>
<SEP> 17 <SEP>"<SEP> i <SEP> E '
<Tb>
<SEP> 4
<tb> <SEP> Exemples <SEP> X <SEP> 4 <SEP> Géométrie <SEP> R
<tb> <SEP> o
<tb> <SEP> 18 <SEP> C1 <SEP> E <SEP> îRcis
<tb> <SEP> 'E <SEP> Z <SEP> "
<tb> <SEP> 19 <SEP> Cl <SEP> 3
<tb> <SEP> 'E <SEP> E, <SEP> -r <SEP>
<tb> <SEP> L
<tb> <SEP> 20 <SEP> ., <SEP> E+Z <SEP> "
<tb> 'a'
<tb> <SEP> 21 <SEP> lE <SEP> Il <SEP> ll <SEP> M
<tb> <SEP> 22 <SEP> F <SEP> Z <SEP> 1Rtrans
<tb>
EXEMPLE 23 : Préparation d'un concentré soluble.<SEP> 4
<tb><SEP> Examples <SEP> X <SEP> 4 <SEP> Geometry <SEP> R
<tb><SEP> o
<tb><SEP> 18 <SEP> C1 <SEP> E <SEP> Iscis
<tb><SEP>'E<SEP> Z <SEP>"
<tb><SEP> 19 <SEP> Cl <SEP> 3
<tb><SEP> E <SEP> E, <SEP> -r <SEP>
<tb><SEP> L
<tb><SEP> 20 <SEP>., <SEP> E + Z <SEP>"
<tb>'a'
<tb><SEP> 21 <SEP> I <SEP> It <SEP> ll <SEP> M
<tb><SEP> 22 <SEP> F <SEP> Z <SEP> 1Retrans
<Tb>
EXAMPLE 23 Preparation of a soluble concentrate
On a effectué un mélange homogène de - produit de l'exemple 6 ....................... 0,25 g - butoxyde de pipéronyle ....................... 1,00 g - tween 80...................................... 0,25 g - topanol A..................................... 0,1 g - eau........................................... 98,4 g
EXEMPLE 24 : Préparation d'un concentré émulsifiable.A homogeneous product mixture of Example 6 was carried out... 0.25 g - Piperonyl butoxide ... .................. 1.00 g - tween 80 ......................... ............. 0.25 g - topanol A .............................. ....... 0,1 g - water ..................................... ...... 98.4 g
EXAMPLE 24 Preparation of an Emulsifiable Concentrate
- produit de l'exemple 10............................. 0,015 g - butoxyde de pipéronyle ............................. 0,5 g - topanol A.......................................... 0,1 g - tween 80............................................ 3,5 g - xylène............................................. 95,885 g
Etude de l'effet létal sur larves de Spodoptera Littoralis.product of example 10 ............................. 0.015 g - piperonyl butoxide ........ ..................... 0.5 g - topanol A ...................... .................... 0.1 g - tween 80 ....................... ..................... 3.5 g - xylene ....................... ...................... 95.885 g
Study of the lethal effect on larvae of Spodoptera Littoralis.
Les essais sont effectués par application topique d'une solution acétonique toxique a l'aide du micro manipulateur d'Arnold sur le thorax dorsal des chenilles.Qn détermine la DL50 du produit en utilisant
15 larves par dose de produit tester Les chenilles utilisées sont des larves du quatrième stade larvaire, c' est-â-dire âgées d'environ 10 jours en élevage sur milieu artificiel (milieu de Poitout) â 24 C et 55 d'humidité relative. Apres traitement, les individus sont mis en observation sur milieu nutritif artificiel.The tests are carried out by topical application of a toxic acetone solution using the Arnold micro-manipulator on the dorsal thorax of the caterpillars.The LD50 of the product is determined using
Larvae per dose of test product The larvae used are larvae of the fourth instar larvae, that is to say, about 10 days old in culture on artificial medium (Poitout medium) at 24 ° C. and 55 relative humidity. . After treatment, the individuals are observed on artificial nutrient medium.
On effectue le contrôle des mortalités 48 heures après traitement. Mortality control is performed 48 hours after treatment.
Les résultats expérimentaux obtenus pour les produits des exemples 2, 6 et 8 sont compris entre 0,48 et 4,8 ng par insecte. The experimental results obtained for the products of Examples 2, 6 and 8 are between 0.48 and 4.8 ng per insect.
Etude de l'effet d'abattage sur mouche domestique.Study of the slaughter effect on houseflies.
Les insectes tests sont des mouches domestiques femelles âgées-de 4 a 5 jours. On opère par pulvérisation directe à la concentration de 0,25 g/l en chambre de Kearns et March en utilisant comme solvant un mélange d'acétone (5 %) et d'Isopar L (solvant pétrolier) (quantité de solvant utilisée 2 ml en une seconde). On utilise 50 insectes par traitement. On effectue les contrôles d'abattage toutes les minutes jusqu" 10 minutes, puis à 15 minutes et l'on détermine le KT 50 par les méthodes habituelles. Test insects are female house flies aged 4-5 days. It is carried out by direct spraying at a concentration of 0.25 g / l in a Kearns and March chamber using as solvent a mixture of acetone (5%) and Isopar L (petroleum solvent) (amount of solvent used 2 ml in one second). 50 insects are used per treatment. The slaughter checks are carried out every minute for up to 10 minutes, then at 15 minutes and the KT 50 is determined by the usual methods.
tes résultats expérimentaux obtenus pour les produits des exemples 2, 6, et 8 exprimés en KT 50 en minutes sont compris entre 1,8 et 3,6 minutes. the experimental results obtained for the products of Examples 2, 6 and 8 expressed in KT 50 in minutes are between 1.8 and 3.6 minutes.
Claims (14)
Priority Applications (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8312858A FR2550191B1 (en) | 1983-08-04 | 1983-08-04 | NOVEL DERIVATIVES OF CYCLOPROPANE CARBOXYLIC ACID, PROCESS FOR THEIR PREPARATION AND THEIR APPLICATION TO THE CONTROL OF PESTS |
| ZA845790A ZA845790B (en) | 1983-08-04 | 1984-07-26 | Derivatives of cyclopropane carboxylic acid,their preparation,their use in combatting parasites and the compositions containing them |
| EP84401616A EP0133406B1 (en) | 1983-08-04 | 1984-08-02 | Cyclopropanecarboxylic-acid derivatives, their preparation, their use as parasiticides and compositions containing them |
| US06/637,106 US4565822A (en) | 1983-08-04 | 1984-08-02 | 2-Fluoro-2-cyanoethenyl cyclopropane carboxylates as pesticides |
| DE8484401616T DE3463263D1 (en) | 1983-08-04 | 1984-08-02 | Cyclopropanecarboxylic-acid derivatives, their preparation, their use as parasiticides and compositions containing them |
| ES534871A ES8505336A1 (en) | 1983-08-04 | 1984-08-03 | Cyclopropanecarboxylic-acid derivatives, their preparation, their use as parasiticides and compositions containing them. |
| CA000460347A CA1222250A (en) | 1983-08-04 | 1984-08-03 | Cyclopropane carboxylic acid derivatives, their preparation and their use in pest control |
| JP16300984A JPH0723350B2 (en) | 1983-08-04 | 1984-08-03 | New derivative of cyclopropanecarboxylic acid, process for producing them, and parasite control composition containing them |
| SU843785252A SU1468410A3 (en) | 1983-08-04 | 1984-08-03 | Method of producing derivatives of cyclopropanecarboxylic acid |
| HU842964A HU196951B (en) | 1983-08-04 | 1984-08-03 | Process for production of 2,2-dimethil-3-/2-fluor-2-cyane-ethenil/-cycloprophane carbonic acid esthers |
| AU31474/84A AU576775B2 (en) | 1983-08-04 | 1984-08-03 | Derivatives of cyclopropane carboxylic acid |
| BR8403898A BR8403898A (en) | 1983-08-04 | 1984-08-03 | PROCESS FOR PREPARING UNDER ALL POSSIBLE ISOMERIC FORMS OR IN THE FORM OF MIXTURES OF THESE ISOMEROS-DERIVED FROM CYCLOPROPANIC CARBOXYLIC ACID, AS WELL AS COMPOSITES INTENDED TO COMBAT VEGETABLE PARASITES, DEASAS DE PARASITAS DE PARASISAS |
| SU884356226A SU1591807A3 (en) | 1983-08-04 | 1988-08-05 | Method of producing derivatives of cyclopropane carbolic acid |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8312858A FR2550191B1 (en) | 1983-08-04 | 1983-08-04 | NOVEL DERIVATIVES OF CYCLOPROPANE CARBOXYLIC ACID, PROCESS FOR THEIR PREPARATION AND THEIR APPLICATION TO THE CONTROL OF PESTS |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| FR2550191A1 true FR2550191A1 (en) | 1985-02-08 |
| FR2550191B1 FR2550191B1 (en) | 1986-09-26 |
Family
ID=9291393
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| FR8312858A Expired FR2550191B1 (en) | 1983-08-04 | 1983-08-04 | NOVEL DERIVATIVES OF CYCLOPROPANE CARBOXYLIC ACID, PROCESS FOR THEIR PREPARATION AND THEIR APPLICATION TO THE CONTROL OF PESTS |
Country Status (3)
| Country | Link |
|---|---|
| FR (1) | FR2550191B1 (en) |
| SU (2) | SU1468410A3 (en) |
| ZA (1) | ZA845790B (en) |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2099810A (en) * | 1981-06-09 | 1982-12-15 | Shell Int Research | Cyclopropanecarboxylate esters and their use as pesticides |
-
1983
- 1983-08-04 FR FR8312858A patent/FR2550191B1/en not_active Expired
-
1984
- 1984-07-26 ZA ZA845790A patent/ZA845790B/en unknown
- 1984-08-03 SU SU843785252A patent/SU1468410A3/en active
-
1988
- 1988-08-05 SU SU884356226A patent/SU1591807A3/en active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2099810A (en) * | 1981-06-09 | 1982-12-15 | Shell Int Research | Cyclopropanecarboxylate esters and their use as pesticides |
Also Published As
| Publication number | Publication date |
|---|---|
| FR2550191B1 (en) | 1986-09-26 |
| ZA845790B (en) | 1985-08-28 |
| SU1591807A3 (en) | 1990-09-07 |
| SU1468410A3 (en) | 1989-03-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0050534B1 (en) | Esters of cyclopropane-carboxylic acids related to pyrethric acid, their preparation and use as insecticides | |
| FR2539956A2 (en) | New cyclopropanecarboxylic acid derivatives, process for their preparation and their application in combating parasites | |
| FR2610624A1 (en) | NOVEL ESTERS OF CYCLOPROPANECARBOXYLIC ACIDS RELATED TO PYRETHRIQUE ACID, PROCESS FOR THE PREPARATION THEREOF AND THEIR USE IN THE FIGHT AGAINST PESTS | |
| EP0448427B1 (en) | Ester derivatives of 3-[2-cyano-2-haloethenyl]-2,2-dimethylcyclopropanecarboxylic acid, process for their preparation and their use as pesticides | |
| FR2642421A1 (en) | NOVEL DERIVATIVES OF 2,2-DIMETHYL 3- (2-MONOHALOETHENYL) CYCLOPROPANE CARBOXYLIC ACID, PROCESS FOR PREPARING THEM AND THEIR APPLICATION AS MEDICAMENTS | |
| EP0133406B1 (en) | Cyclopropanecarboxylic-acid derivatives, their preparation, their use as parasiticides and compositions containing them | |
| EP0267105B1 (en) | Ethylenic oxime derivatives of cyclopropanecarboxylic-acid esters, process and intermediates for their preparation and their use in combatting parasites | |
| FR2534252A1 (en) | NOVEL 3-SUBSTITUTED CYCLOPROPANE CARBOXYLIC ACID DERIVATIVES FROM A SUBSTITUTED SINGLE-VINYL CHAIN, PROCESS FOR PREPARING THEM AND THEIR APPLICATION AS PESTICIDES | |
| EP0557192B1 (en) | Pyrethrinoid esters derived from 6-trifluoromethyl benzyl alcohol, process for their preparation and their use as pesticides | |
| EP0110769B1 (en) | Esters derived from 2,2-dimethylcyclopropane-carboxylic acids and biaryl alcohols, process for their preparation, their use as parasiticides and compositions containing them | |
| EP0114012B1 (en) | Esters of cyclopropane-carboxylic acids and unsaturated aliphatic alcohols, process and intermediates of preparation and pesticidal compositions containing them | |
| EP0335801A2 (en) | Process for the preparation of trifluoromethylvinyl derivatives from the corresponding halovinyl derivatives | |
| EP0638543A1 (en) | Esters of 2,2-dimethyl 3-(3,3,3-trifluoro-1-propenyl) cyclopropanecarboxylic acid, their preparation and use as pesticides | |
| EP0228942A1 (en) | Iodine-substituted cyclopropanecarboxylic-acid derivatives, their preparation, their use as plant and animal parasiticides and compositions containing them | |
| EP0261035A1 (en) | Indole derivatives, process for their preparation, their use in combating parasites and compositions containing them | |
| FR2550191A1 (en) | Novel cyclopropane carboxylic acid derivatives, a process for their preparation, and their use for controlling parasites. | |
| FR2537973A1 (en) | Esters of cyclopropanecarboxylic acids and of unsaturated aliphatic alcohols, process for their preparation and the pesticidal compositions containing them | |
| EP0194919B1 (en) | Ester derivatives of 2,2-dimethyl-3-ethenylcyclopropane-carboxylic acid, their preparation, their use as parasiticides and compositions containing them | |
| EP0215701B1 (en) | Esters of cyclopropanecarboxylic acids and 2,3-dihydro-4-phenyl-1h-inden-2-ol, their preparation, their use as parasiticides and compositions containing them | |
| EP0364326B1 (en) | Derivatives of 2,2-dimethyl-3-(1-hydroxy-2-sulfinoethyl)-cyclopropane-carboxylic acid, method for their preparation, and their use as pesticides | |
| EP0093664B1 (en) | Ester of cyclopropanecarboxylic acid and (s)-cyano-(4-fluoro-3-phenoxyphenyl)-methyl alcohol, its preparation, its use in combating parasites and compositions containing it | |
| FR2686602A1 (en) | NOVEL DERIVATIVES OF 2,2-DIMETHYL 3 - [(2,2-DIFLUOROCYCLOPROPYLIDENE METHYL) CYCLOPROPANE CARBOXYLIC ACID, PROCESS FOR THEIR PREPARATION AND THEIR USE AS PESTICIDES | |
| FR2558466A2 (en) | New cyclopropanecarboxylic acid derivatives, process for preparing them and their use for combating parasites | |
| FR2536392A2 (en) | New cyclopropanecarboxylic acid derivatives, process for their preparation and their application to the fight against parasites. | |
| FR2601360A2 (en) | New cyclopropanecarboxylic acid derivatives containing an iodinated substituent, their preparation and their application to controlling parasites of plants and animals |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ST | Notification of lapse |