EP4065062B1 - Anti-decubitus mattress or mat - Google Patents
Anti-decubitus mattress or mat Download PDFInfo
- Publication number
- EP4065062B1 EP4065062B1 EP20824642.1A EP20824642A EP4065062B1 EP 4065062 B1 EP4065062 B1 EP 4065062B1 EP 20824642 A EP20824642 A EP 20824642A EP 4065062 B1 EP4065062 B1 EP 4065062B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- mattress
- mat
- layer
- adhesive
- polymer gel
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61G—TRANSPORT, PERSONAL CONVEYANCES, OR ACCOMMODATION SPECIALLY ADAPTED FOR PATIENTS OR DISABLED PERSONS; OPERATING TABLES OR CHAIRS; CHAIRS FOR DENTISTRY; FUNERAL DEVICES
- A61G7/00—Beds specially adapted for nursing; Devices for lifting patients or disabled persons
- A61G7/05—Parts, details or accessories of beds
- A61G7/057—Arrangements for preventing bed-sores or for supporting patients with burns, e.g. mattresses specially adapted therefor
Definitions
- the present invention relates to an anti-decubitus mattress or mat and a method for its production.
- Prolonged contact with the surface of a support can in fact create lesions caused by excessive body pressure (especially in areas where the bones protrude more). If these sores are not prevented or treated, there is a risk of causing injury to the internal tissues (bone and muscle), leading to necrosis and therefore permanent damage to the same.
- Anti-decubitus mattresses have been developed specifically for ensuring defense of the skin but also for greater hygiene.
- Document EP 2095745 describes a modular supporting element for producing mattresses, pillows and the like comprising an elastically deformable body in at least one deformation direction, which comprises a first portion having a first modulus of elasticity and a second portion (305) having a second modulus of elasticity different from said first modulus of elasticity.
- Document EP 2 802 305 describes a mattress made of viscoelastic foam (polyurethane foam) which can be fixed to the medical table by means of straps so as to be able to support the patient even when the table is in a tilted position.
- viscoelastic foam polyurethane foam
- the straps however are bulky, they make the mattress expensive to construct, they require that the rigid support be of such a shape as to allow it to be hooked and are not suitable for fixing a small mattress, which only has to support a part of the patient's body, to a rigid support.
- the general objective of the present invention is to produce an anti-decubitus mattress or mat that is disposable, and therefore does not require sanitization after use, economical, hypoallergenic, thanks to the absence of latex and phthalates, suitable for supporting any part of the body and for use in any context, i.e. that can be positioned on stretchers, medical examination tables, operating tables, robotic operating tables.
- a further objective of the present invention is to provide a mattress or mat which is fast, easy and intuitive to use.
- the method, object of the present invention provides that said at least one strip, insert or layer in a compact polyurethane, adhesive polymer product or in gel form, be injected or glued directly on said at least one layer of shape-memory viscoelastic polyurethane, also called memory foam, or viscoelastic, elastic, shape-memory sponge, or on at least one non-adhesive polyurethane layer in gel form, as better described hereunder, so as to create a whole with said overlying layer.
- shape-memory viscoelastic polyurethane also called memory foam, or viscoelastic, elastic, shape-memory sponge, or on at least one non-adhesive polyurethane layer in gel form, as better described hereunder, so as to create a whole with said overlying layer.
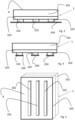
- the anti-decubitus mattress or mat, object of the present invention, indicated in the figures with 1 is composed of at least one layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 and has an upper surface A suitable for coming into contact with at least part of the patient's body (not shown) and a lower surface B suitable for being connected with a rigid support (not shown), said mattress or mat 1 comprises at least one strip or insert in adhesive polymer gel, or a compact polymer product, 102,102' which ensures the adhesion of at least part of said lower surface B to said rigid support.
- Said at least one strip or insert in gel form not only ensures the non-occurrence of the bottom-out effect, but intrinsically possesses adhesive properties and therefore attaches itself very stably (but removably) to the surface of the support without the need for straps or other mechanical fixing elements for remaining in position.
- Said at least one strip or insert in polymer gel preferably has a compact structure that is non-spongy, non-expanded, free of cells.
- Said at least one strip or insert in polymer gel is adhesive and allows the adhesion or stable but removable connection of at least part of said lower surface B to said rigid support.
- said gel, or compact adhesive polymer product is a polyurethane gel.
- It can, for example, be a soft elastomeric polyurethane gel known with the trade-name KAIROS HDR G 2015 or 207 L.
- Said one or more strips or inserts 102, 102' have a thickness ranging from 0.01 cm to 10 cm.
- Said at least one layer or sponge of viscoelastic or elastic shape-memory polyurethane foam 101 has a thickness ranging from 5 mm to 40 cm.
- the mattress 1 comprises a plurality of adhesive polymer gel strips 102 positioned parallel to each other and distributed over at least part of the lower surface B.
- said strips 102 can be positioned intersected with each other and distributed over at least part of the lower surface B.
- said inserts have a geometric shape 102' and are distributed over at least part of the lower surface B of the mattress or mat 1.
- said at least one strip or insert in adhesive polymer gel 102, 102' is distributed uniformly on at least part of the lower surface B of the mattress or mat 1.
- Said strips can obviously be understood as being inserts with an elongated shape, for example inserts with a rectangular base, whereas inserts refer to gel elements having various geometrical shapes, for example, inserts with a round, oval, square, rectangular or similar base or inserts with an irregular base.
- inserts refer to gel elements having various geometrical shapes, for example, inserts with a round, oval, square, rectangular or similar base or inserts with an irregular base. The combination of these elements guarantees the adhesion of the mattress to the support 8 (also in the absence of a single adhesive layer of gel) and at the same time avoids the bottom-out phenomenon.
- the layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 which is available in different thicknesses and sizes, suitable for operating and robotic tables, and for the practical adhesion system to surfaces, patients can be positioned in all safety in any position and inclination. All this is possible thanks to the presence, on the lower side of the mattress 1, of said inserts/strips 102, 102' having various shapes and positions, in a variable number and which are arranged both at regular intervals and randomly, produced in adhesive polyurethane gel.
- the mattress or mat 1 can be detached from the surface of the rigid support (for example an operating table) by applying only a very light force, and subsequently correctly disposed of.
- the thickness of these inserts/strips 102, 102' is preferably minimal, for example about 1-2 mm, and in any case such as not to create discomfort or pain for the patient.
- said at least one strip or insert in adhesive polyurethane gel 102, or in a compact polymer product which is also adhesive covers the whole lower surface B of the mattress or mat 1 to form a single continuous adhesive layer 102''.
- Said layer 102'' in polymeric gel which covers the whole lower surface B of the mattress or mat 1 is adhesive and allows at least the stable adhesion (but removable by applying a force on said mattress 1) of at least part of said lower surface B to said rigid support.
- Said gel layer 102'' in fact, not only ensures the non-occurrence of the bottom-out effect, but also intrinsically possesses adhesive properties and therefore adheres stably to the surface of the support without the need for straps or other mechanical fastening elements for remaining in position.
- Said single continuous layer 102'' has a thickness ranging from 5 mm to 40 cm.
- the layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 allows both the release of the contact pressures and also the correct skin aeration, whereas the gel layer 102'', i.e. the compact layer, allows the mattress 1 to stably adhere to the underlying supporting structure, stabilizes it and also avoids the bottom-out effect, as it (102'') is also deformable even if more load-bearing than the upper layer 101.
- patients can be positioned in all positions and inclinations that can be reached from any bed or operating table or generic support, keeping the patients appropriately anchored while the pressure and friction forces due to the various positions are attenuated, also ensuring the correct transpiration of the skin in contact with the upper surface A of said mattress or mat 1.
- Both the underlying layer 102'' and the inserts 102' or strips 102 in gel in fact, not only ensure the non-occurrence of the bottom-out effect, but also intrinsically possess adhesive properties and therefore adhere very stably to the surface of the table without requiring straps or other mechanical fasteners for remaining in position.
- Said at least one layer in non-adhesive polyurethane gel 103 can be inserted between at least two layers or sponges of viscoelastic or elastic shape-memory polyurethane foam 101.
- said at least one layer in non-adhesive polyurethane gel 103 is provided on the side of the mattress 1 facing the rigid support and said at least one strip or insert in adhesive polyurethane gel 102, 102', 102", suitable for at least partially adhering said lower surface B to said rigid support, is provided on said at least one layer in non-adhesive polyurethane gel 103.
- said at least one strip or insert in adhesive polyurethane gel 102, 102' which guarantees the at least partial adhesion of said lower surface B, i.e. of at least part of said lower surface B, to said rigid support, has a compactness, a thickness, an elasticity and a distribution on said lower surface sufficient for avoiding the bottom-out phenomenon, i.e. that at least part of the patient's body sinks down to the rigid support.
- the distribution and quantity of said inserts and strips 102, 102' provided on said lower surface B are in fact such as to ensure that the mattress is comfortable for the patient and that at the same time it can be securely positioned on the support.
- Said at least one non-adhesive polyurethane gel layer 103 when provided in the mattress 1, also has a compactness, a thickness, an elasticity and a distribution on said lower surface sufficient for avoiding the bottom-out phenomenon, i.e. that at least part of the patient's body sinks down to the rigid support.
- said at least one strip or insert 102, 102' or said continuous layer 102'' in adhesive polyurethane gel which guarantees at least partial adhesion of said lower surface B to said rigid support, obviously has at least one protective film 104 on the side facing the rigid support, that can be removed before use.
- Another polymer can obviously be used, alternatively or in combination with polyurethane, to form said layer 103, strips or inserts or adhesive layer 102, 102', 102'' in gel, with a compact structure.
- the present invention also relates to a method for producing an anti-decubitus mattress or mat 1 as described above wherein said at least one strip or insert or layer in adhesive polymeric gel 102, 102', 102", is injected or glued directly onto said at least one layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 or on said at least one layer of non-adhesive polyurethane gel 103 so as to create a whole with said overlying layer.
- the embodiments of the mattress or mat 1 described above are disposable and should be preferably used for a short period, i.e. for supporting the patient to be operated and transported, for example: they consequently do not require any management and sanitization, but must simply be disposed of once used.
- the mattress 1 object of the present invention can be produced in various shapes and sizes to be able to adapt to and support every part of the patient's body or the whole of the patient's body.
- Figure 1 schematically illustrates a mattress or mat 1 according to an embodiment of the present invention which comprises a layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 and a compact layer, i.e. not expanded, not with a spongy structure, in adhesive polyurethane gel 102'' suitable for adhering to or connecting with (stably but removably, i.e. with the possibility of detaching the mattress from the support 1 once its function is considered complete) the lower surface B of the mattress 1 to the rigid support.
- a layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 and a compact layer, i.e. not expanded, not with a spongy structure, in adhesive polyurethane gel 102'' suitable for adhering to or connecting with (stably but removably, i.e. with the possibility of detaching the mattress from the support 1 once its function is considered complete) the lower surface B of the mattress 1 to the rigid support.
- the adhesive side of the gel layer/insert is protected by a film 104 which is removed before use ( figure 2 ) in order to allow the mattress 1 to adhere to the support.
- This embodiment which is highly performing from an anti-decubitus point of view, is therefore composed of a single double-layer sheet: the upper layer, in contact with the patient, is made of memory foam polyurethane foam whereas the underlying layer is produced by extrusion of polyurethane gel, which is reacted and injected, or glued, directly on the memory foam surface so as to be one with the overlying layer.
- Each layer can be produced in various shapes and sizes (length and width of the two layers are variable), with a thickness of each layer ranging for example from a minimum of 5 mm to a maximum of 40 cm, always guaranteeing a result that is soft to the touch and deformable.
- the particular processing makes this product smooth and pleasant to the touch on the upper side, towards the patient, while guaranteeing a high adhesive power to the lower layer, allowing a safe and stable fixing on any surface.
- the mattress or mat 1 is detached from the surface of the rigid support by applying only a very light force, and is correctly disposed of.
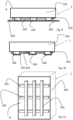
- Figures 3 to 15 show further embodiments wherein the mattress or mat 1 can further comprise at least one non-adhesive polyurethane gel layer 103 and wherein the mattress 1 does not have the lower surface B consisting of a whole layer of adhesive polyurethane gel 102" but the adhesion of the mattress 1 to the rigid support is guaranteed by one or more inserts or strips 102, 102' in adhesive polyurethane gel.
- figures 3-5 schematically illustrate a mattress 1 comprising a first layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101, a second layer in non-adhesive polyurethane gel 103 positioned below said first layer 101 and strips of adhesive polyurethane gel 102 which guarantee the adhesion of the mattress 1 to the rigid support, not shown, said layers/strips in gel preferably having a compact, non-expanded, non-foamy structure.
- the adhesive side is protected during non-use by a film 104 which covers all the strips 102 ( figure 3 ) or by various separate films 104 ( figure 4 ).
- FIGS 6-8 schematically illustrate a mattress 1 comprising a layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 and strips of adhesive polyurethane gel 102 which ensure the adhesion of the mattress 1 to the rigid support, not shown.
- the adhesive side is protected during non-use by a film 104 which covers all the strips ( figure 6 ) or by various separate films 104 ( figure 7 ).
- FIGS 9-11 schematically show a mattress 1 comprising a layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 and a lattice of strips of adhesive polyurethane gel 102 which guarantee the adhesion of the mattress 1 to the rigid support, not illustrated.
- the adhesive side of the lattice is protected during non-use by a single film 104 which covers the whole lattice ( figure 9 ) or by various separate films 104 ( figure 10 ).
- FIGS 12-14 schematically illustrate a mattress 1 comprising a layer or sponge in viscoelastic or elastic shape-memory polyurethane foam 101 and a plurality of inserts 102', of various shapes and sizes in adhesive polyurethane gel, which guarantee the adhesion of the mattress 1 to the rigid support, not illustrated.
- the adhesive side of the inserts 102' is protected during non-use by a single film 104 that covers them ( figure 12 ) or by various separate films 104 ( figure 13 ).
- Figures 15 and 16 illustrate a further embodiment wherein the mattress or mat 1 is composed, in its adhesive component, of strips of adhesive polyurethane gel 102 and, in its component for releasing the pressures exerted by at least part of a patient's body, by a sandwich structure comprising two layers or sponges in viscoelastic or elastic shape-memory polyurethane foam 101 interspersed with a layer of non-adhesive polyurethane gel 103.
- the mattress or mat 1 object of the present invention is disposable and can be simply disposed of once used, therefore without the need for sanitization.
- the mattress or mat 1 object of the present invention guarantees a first protection, i.e. emergency and not prolonged over time, for the patient against bedsores as it allows the discharge of contact pressures and allows an excellent skin ventilation; it can consequently be used in both emergency situations and in the operating theatre, thus facilitating the positioning of the patient, preferably for times not exceeding 48 hours.
- These mattresses or mats 1 are useful, quick to apply, i.e. to adhere/connect stably but removably on rigid supports (horizontal or tilted) thanks to the adhesive layers or inserts 102, 102', easy to dispose of and at low costs.
- the mattress or mat 1 object of the present invention can be stably attached to any surface without the need for using straps.
- the mattress/mat 1 can be produced in any size and shape, even with different proportions between the layers.
- the mattress/mat 1 is suitable for any handling procedure which provides, in some practices, for also keeping patients in tilted positions of many degrees, for example both in tilt, trendelenburg or antitrendelenburg.
- the mattress/mat 1 can be used for example: in first aid, on ambulances, applied on stretchers, for facilitating the air transport of injured or seriously ill patients, in operating theatres, in veterinary surgery, on robotic operating tables.
Landscapes
- Health & Medical Sciences (AREA)
- Nursing (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Mattresses And Other Support Structures For Chairs And Beds (AREA)
- Invalid Beds And Related Equipment (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| RS20250369A RS66786B1 (sr) | 2019-11-25 | 2020-11-23 | Dušek ili prostirka protiv dekubitusa |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IT102019000022050A IT201900022050A1 (it) | 2019-11-25 | 2019-11-25 | Materassino o tappetino antidecubito |
| PCT/IB2020/061036 WO2021105849A1 (en) | 2019-11-25 | 2020-11-23 | Anti-decubitus mattress or mat |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP4065062A1 EP4065062A1 (en) | 2022-10-05 |
| EP4065062B1 true EP4065062B1 (en) | 2025-02-26 |
| EP4065062C0 EP4065062C0 (en) | 2025-02-26 |
Family
ID=69904037
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP20824642.1A Active EP4065062B1 (en) | 2019-11-25 | 2020-11-23 | Anti-decubitus mattress or mat |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP4065062B1 (pl) |
| ES (1) | ES3018859T3 (pl) |
| IT (1) | IT201900022050A1 (pl) |
| PL (1) | PL4065062T3 (pl) |
| RS (1) | RS66786B1 (pl) |
| WO (1) | WO2021105849A1 (pl) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113713181B (zh) * | 2021-09-02 | 2022-11-25 | 温州医科大学附属第一医院 | 蚊香式交替自动充放气高分子材料凝胶垫及其凝胶管 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2789270B1 (en) * | 2013-04-08 | 2015-09-16 | Technogel Italia S.R.L. | Padding element for seats and method of manufacturing the same |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7682680B2 (en) * | 2006-09-30 | 2010-03-23 | Let's Gel, Inc. | Method and apparatus for fabricating an anti-fatigue mat employing multiple durometer layers |
| EP2095745A1 (en) * | 2008-02-26 | 2009-09-02 | Technogel Italia S.R.L. | Modular supporting element to make mattresses and the like |
| JP2015506747A (ja) | 2012-01-10 | 2015-03-05 | ピガッツィ, アレッショPIGAZZI, Alessio | 患者がトレンデレンブルグ体位にある場合に患者を手術台に固定する方法及びそのためのキットを含む装置 |
| US9877591B2 (en) * | 2012-09-28 | 2018-01-30 | Direct Supply, Inc. | Medical mattress with firmness adjustment |
-
2019
- 2019-11-25 IT IT102019000022050A patent/IT201900022050A1/it unknown
-
2020
- 2020-11-23 EP EP20824642.1A patent/EP4065062B1/en active Active
- 2020-11-23 ES ES20824642T patent/ES3018859T3/es active Active
- 2020-11-23 PL PL20824642.1T patent/PL4065062T3/pl unknown
- 2020-11-23 WO PCT/IB2020/061036 patent/WO2021105849A1/en not_active Ceased
- 2020-11-23 RS RS20250369A patent/RS66786B1/sr unknown
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2789270B1 (en) * | 2013-04-08 | 2015-09-16 | Technogel Italia S.R.L. | Padding element for seats and method of manufacturing the same |
Also Published As
| Publication number | Publication date |
|---|---|
| IT201900022050A1 (it) | 2021-05-25 |
| ES3018859T3 (en) | 2025-05-19 |
| WO2021105849A1 (en) | 2021-06-03 |
| PL4065062T3 (pl) | 2025-07-14 |
| EP4065062C0 (en) | 2025-02-26 |
| EP4065062A1 (en) | 2022-10-05 |
| RS66786B1 (sr) | 2025-06-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7329229B2 (en) | Orthopedic splints | |
| US7000616B2 (en) | Wound care suspension system | |
| CA2779725C (en) | Apparatus and system for turning and positioning a patient | |
| EP1522237A1 (en) | Mat | |
| JP2011518029A (ja) | 膨張式装置を用いる褥瘡の予防および治療 | |
| US7909787B2 (en) | Reconfigurable heel elevator | |
| US11026854B2 (en) | Patient stabilization, infection barrier, pressure ulcer prevention and equipment protection device and methods of use | |
| US20250205075A1 (en) | Methods for manufacturing attachment apparatuses for pressure-mitigation apparatuses and using the same | |
| US11013336B2 (en) | Kyphosis back cushion device | |
| EP4065062B1 (en) | Anti-decubitus mattress or mat | |
| US6892734B1 (en) | Wound care suspension system | |
| US20050177081A1 (en) | Wrist splint | |
| US7141713B2 (en) | Method for reducing pressure damage to skin of a person, and corresponding skin protective devices | |
| WO2011070438A2 (en) | A medical device for limb immobilization | |
| WO2004105805A2 (en) | Wound care suspension system | |
| US20060084902A1 (en) | Wound care suspension system | |
| EP1935381A2 (en) | Immobilization support device | |
| CN215875228U (zh) | 耳廓敷料贴 | |
| CN221154561U (zh) | 医用辅助翻身装置 | |
| CN215915514U (zh) | 一种骨折手术用体位垫 | |
| EP4458339A1 (en) | A moldable pad for supporting a body part | |
| EP0996405A1 (en) | Resting system | |
| US20240252371A1 (en) | Dressing kit for external fixation and extremities | |
| JPH0661353B2 (ja) | 褥瘡防止用クツシヨン体 | |
| JPH0661354B2 (ja) | 褥瘡防止体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: UNKNOWN |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20220616 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20240923 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602020046888 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| U01 | Request for unitary effect filed |
Effective date: 20250311 |
|
| U07 | Unitary effect registered |
Designated state(s): AT BE BG DE DK EE FI FR IT LT LU LV MT NL PT RO SE SI Effective date: 20250319 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 3018859 Country of ref document: ES Kind code of ref document: T3 Effective date: 20250519 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250626 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250226 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250527 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250226 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250226 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250226 |