EP3768290B1 - Zusammensetzung für gewichtsmanagement - Google Patents
Zusammensetzung für gewichtsmanagement Download PDFInfo
- Publication number
- EP3768290B1 EP3768290B1 EP19771630.1A EP19771630A EP3768290B1 EP 3768290 B1 EP3768290 B1 EP 3768290B1 EP 19771630 A EP19771630 A EP 19771630A EP 3768290 B1 EP3768290 B1 EP 3768290B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- composition
- weight
- extract
- dry weight
- weight management
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/15—Vitamins
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/30—Dietetic or nutritional methods, e.g. for losing weight
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/12—Ketones
- A61K31/122—Ketones having the oxygen directly attached to a ring, e.g. quinones, vitamin K1, anthralin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
- A61K31/197—Carboxylic acids, e.g. valproic acid having an amino group the amino and the carboxyl groups being attached to the same acyclic carbon chain, e.g. gamma-aminobutyric acid [GABA], beta-alanine, epsilon-aminocaproic acid or pantothenic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
- A61K31/197—Carboxylic acids, e.g. valproic acid having an amino group the amino and the carboxyl groups being attached to the same acyclic carbon chain, e.g. gamma-aminobutyric acid [GABA], beta-alanine, epsilon-aminocaproic acid or pantothenic acid
- A61K31/198—Alpha-amino acids, e.g. alanine or edetic acid [EDTA]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
- A61K31/201—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids having one or two double bonds, e.g. oleic, linoleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
- A61K31/353—3,4-Dihydrobenzopyrans, e.g. chroman, catechin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
- A61K31/353—3,4-Dihydrobenzopyrans, e.g. chroman, catechin
- A61K31/355—Tocopherols, e.g. vitamin E
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/38—Heterocyclic compounds having sulfur as a ring hetero atom
- A61K31/385—Heterocyclic compounds having sulfur as a ring hetero atom having two or more sulfur atoms in the same ring
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/13—Coniferophyta (gymnosperms)
- A61K36/15—Pinaceae (Pine family), e.g. pine or cedar
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/21—Amaranthaceae (Amaranth family), e.g. pigweed, rockwort or globe amaranth
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/38—Clusiaceae, Hypericaceae or Guttiferae (Hypericum or Mangosteen family), e.g. common St. Johnswort
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/61—Myrtaceae (Myrtle family), e.g. teatree or eucalyptus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/74—Rubiaceae (Madder family)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/74—Rubiaceae (Madder family)
- A61K36/742—Coffea, e.g. coffee
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/82—Theaceae (Tea family), e.g. camellia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
Definitions
- the present invention generally relates to a composition and composition for use in treating obesity in a human.
- One of the most potent strategies available for reducing body weight is to participate in a structured program of physical activity, such as endurance or resistance exercise training. Studies conducted over the past several decades have repeatedly shown that exercise or physical activity increase energy expenditure to promote weight loss and improve health, however a large portion of the population in most countries is not active at recommended levels. Primary reasons for which individuals do not engage in exercise include that it is inconvenient, costly, time consuming, difficult to perform, boring or otherwise unenjoyable.
- a weight management composition has now been developed which has been determined to promote weight loss in an individual.
- a first aspect of the present invention is, a weight management composition
- a weight loss agent comprising a mixture of green tea extract, green coffee bean extract and forskolin
- a mitochondria enhancing agent comprising a mixture of beetroot extract, coenzyme Q10, alpha lipoic acid and vitamin E
- the green tea extract comprises 80% or more of catechins by dry weight, with 50% or more of the catechins being epigallocatechin gallate
- the green coffee bean extract comprises 30% or more of chlorogenic acids comprising 3-caffeoylquinic acid, 4-caffeoylquinic acid and/or 5-caffeoylquinic acid by dry weight
- the beetroot extract comprises at least 1.5% nitrates by dry weight
- the composition comprises: 5-50% by dry weight of each of the green tea extract and the green coffee bean extract, 0.05-50% by dry weight forskolin, about 1-50% by dry weight of each of beet root extract, coenzyme Q10 and
- a further aspect of the invention is, a weight management composition
- a weight management composition comprising a weight loss agent comprising a mixture of green tea extract, green coffee bean extract and forskolin, and a mitochondria enhancing agent comprising a mixture of beetroot extract, coenzyme Q10, alpha lipoic acid and vitamin E for use in the treatment of obesity in a human
- the green tea extract comprises 80% or more of catechins by dry weight, with 50% or more of the catechins being epigallocatechin gallate
- the green coffee bean extract comprises 30% or more of chlorogenic acids by dry weight
- the beetroot extract comprises at least 1.5% nitrates by dry weight
- the composition for use comprises a daily dosage of 50-1000mg of green tea extract, 50-1000mg of green coffee bean extract, 15mg-100mg of forskolin, 50-5000mg of beetroot extract, 50-900mg of coenzyme Q10, 50mg-900mg alpha lipoic acid and 50
- a weight management composition comprising a weight loss agent and a mitochondria enhancing agent is provided which is useful to treat or improve aspects of the health of a mammal such as weight management, mitochondrial capacity, fatty liver disease, dyslipidemia, oxidative stress levels, brown adipose tissue (BAT) activity and systemic inflammation levels.
- weight management is used herein to refer to achieving, progressing towards or maintaining a body weight within a normal healthy range.
- One of the most widely used metrics for measuring body weight is the "body mass index” or BMI.
- a BMI range of about 18.5 to 24.9 is considered to be the normal healthy range for the BMI index, e.g. a body weight within a range that promotes the health, fitness, well-being and physical appearance of an individual.
- Overweight and obesity are defined as the abnormal or excessive accumulation of fat that may cause health impairments.
- An individual having a BMI between about 25-29.9 is generally considered to be overweight, while an individual having a BMI equal to or greater than 30 is generally considered to be obese.
- Weight management may also refer to the maintenance of body fat levels within a healthy range, e.g. a body fat percentage within a range that promotes the health, fitness, well-being and physical appearance of an individual.
- a healthy body fat range may be an amount ranging from the essential body fat level (i.e. the amount considered essential for physical and psychological well-being) up to approximately 20% above the essential body fat level.
- the amount of fat considered to be essential for men is about 2-5% of body fat and for women is about 10-13% body fat.
- the weight management composition comprises at least one weight loss agent.
- the weight loss agent may be any suitable agent that possesses the characteristic of promoting weight loss in a mammal, e.g. by at least about 0.5%, and preferably a body weight reduction of about 1% or more, e.g. by 5%, 10%, 30%, 50%, 70% or greater of body weight in the individual prior to treatment with the present composition, or a reduction in the rate of rising body weight by at least about 1% or more, and preferably a reduction of about 5% or more, e.g. by 10%, 30%, 50%, 70%, 90% or greater to the rate of body weight increase prior to treatment.
- Suitable weight loss agents may promote weight loss by any one or more of several mechanisms including, but not limited to, the following: decreasing food intake or promoting satiety, decreasing lipid absorption, increasing energy expenditure, decreasing pre-adipocyte differentiation and proliferation, decreasing lipogenesis or increasing lipolysis or lipid oxidation.
- weight loss agents include, but are not limited to, chitosan, psyllium, guar gum, capsaicin, caffeine, Garcina cambogia , Pinus densiflora, capsaicin, yohimbe, hoodia, glucomannan, African mango, guarana, pyruvate, carnitine, beta-glucans, fucoxanthin, raspberry ketone, white kidney bean, kola nut, chromium, ginseng, psyllium, St. John's wort, dandelion, hydroxycitric acid, conjugated linoleic acid, green tea extract, black tea extract, green coffee bean extract, forskolin, bitter orange and mixtures thereof.
- the weight loss agent comprises a mixture of green tea extract, green coffee bean extract and forskolin.
- the weight loss agent comprises a mixture of green tea extract, black tea extract, green coffee bean extract, conjugated linoleic acid and forskolin.
- Green tea extract for use in the present composition may be selected from any suitable green tea leaf or green tea source such as Sencha, Fukamushi Sencha, Gyokuro, Kabusecha, Matcha, Tencha, Genmaicha, Matcha, Shincha, Hojicha, Ichibanchagreen, Nibancha and Sanbancha tea, which are derived from the Camellia sinensis leaf.
- Green tea is abundant in polyphenols such as catechins. Examples of such catechins include epigallocatechin gallate, catechin, catechin gallate, epicatechin, gallocatechin, epigallocatechin, and epicatechin gallate.
- the green tea extract for use in the composition comprises about 80% or more of catechins by dry weight, with about 50% or more of the catechins being epigallocatechin gallate.
- Green tea extract for use in the present composition may be either caffeinated or substantially decaffeinated, for example, having less than 1% caffeine by dry weight.
- the green tea extract comprises about 0.1-90% of the dry weight of the weight management composition, such as about 5-50%, or about 10-30% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 10mg-5g of green tea extract and preferably, about 50-1000mg.
- Green coffee bean extract for use in the present composition may be selected from any suitable green coffee bean source such as Coffea Arabica or Coffea canephora.
- Green coffee beans contain several types of chlorogenic acids, such as 3-caffeoylquinic acid, 4-caffeoylquinic acid and 5-caffeoylquinic acid.
- the green coffee bean extract for use in the present composition comprises about 30% or more of chlorogenic acids by dry weight.
- Green coffee bean extract for use in the present composition may be either caffeinated or substantially decaffeinated, for example, having less than 1% of caffeine by dry weight.
- the green coffee bean extract comprises at least 35% chlorogenic acids and at least 35% caffeine by dry weight.
- the green coffee bean extract comprises about 0.1-80% of the dry weight of the weight management composition, such as about 5-50%, or about 10-30% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 10mg-5g of green coffee bean extract and preferably, about 50-1000mg.
- Forskolin for use in the present composition may be obtained from any suitable source.
- Forskolin may be extracted from the Coleus forskohli plant, or synthetically produced.
- the forskolin extract is derived from the Coleus forskohli plant and is standardized to contain about 40% forskolin.
- forskolin comprises about 0.05-50% of the dry weight of the weight management composition, such as about 0.1-30%, or about 0.5-10% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 1mg-200mg of forskolin and preferably, about 15mg-100mg.
- Black tea extract for use in the present composition may be obtained from any suitable black tea leaf or black tea source including unblended black tea sources such as Congou, Assam, Darjeeling, Nilgiri or Ceylon or blended black teas such as Earl Grey, English Breakfast tea, English afternoon tea, Irish breakfast tea or Masala chai, which are derived from the Camilla sinensis leaf.
- Black tea is abundant in polyphenols such as theaflavins, thearubigins and catechins. Examples of theaflavins include theaflavin, theaflavin-3-gallate, theaflavin-3'-gallate and theaflavin-3,3'-gallate.
- the black tea extract for use in the present composition comprises 10% or more of polyphenols by dry weight.
- Black tea extract for use in the present composition may be either caffeinated or substantially decaffeinated, for example, having less than 1% of caffeine by dry weight.
- the black tea extract comprises at least about 30% polyphenols by dry weight.
- the black tea extract comprises about 0.1-80% of the dry weight of the weight management composition, such as about 5-50%, or about 10-30% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 10mg-5g of black tea extract and preferably, about 50-750mg.
- Conjugated linoleic acid for use in the present application may be obtained from any suitable source such as safflower oil, sunflower oil or grass-fed beef sources.
- the term "conjugated linoleic acid” refers to any of the at least 28 known geometric or positional isomers of linoleic acid, wherein two of the double bonds of the molecule are conjugated such as in the cis-9:trans-11 or trans-10:cis-12 form.
- the composition may include a single isomer, a mixture of isomers, natural isomers, synthetic isomers, or a pharmaceutically acceptable salt, ester, monoglyceride, diglyceride, triglyceride, or any combinations thereof.
- the conjugated linoleic acid contains about a 50:50 mixture of its cis-9:trans-11, and trans-10:cis-12 isomers.
- the conjugated linoleic acid source comprises about 1%-80% of the dry weight of the weight management composition, such as about 20-70%, or about 30-50% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 10mg-10g of conjugated linoleic acid and preferably, about 500mg-3g.
- the weight management composition comprises at least one mitochondria-enhancing agent.
- the mitochondria enhancing agent may be selected from any suitable agent which enhances mitochondrial capacity in a mammal, for example, by increasing the abundance of mitochondria, increasing the ATP generating capacity of mitochondria, protecting mitochondria from excessive oxidative stress, promoting the maintenance of mitochondrial components, ion gradients and ultrastructure, such as through increased mitochondrial autophagy (mitophagy) events or increased mitochondrial fission and fusion events.
- the mitochondria enhancing agent is selected from at least one of the following: beetroot extract, nitrates, idebenone, nicotinamide riboside, elamepratide, vitamin C, vitamin D, vitamin E, thiamine, riboflavin, magnesium, calcium, phosphate, membrane phospholipids, creatine, pyruvate, coenzyme Q10, NADH, nicotinic acid, l-carnitine, dichloroacetate, curcumin, schisandrin, resveratrol and mixtures thereof.
- the mitochondria enhancing agent comprises a mixture of beetroot extract, coenzyme Q10, alpha lipoic acid and vitamin E.
- the mitochondria enhancing agent comprises a mixture of beetroot extract, coenzyme Q10, alpha lipoic acid, creatine and vitamin E.
- the beetroot extract for use in the present composition may be selected from any suitable beetroot source including red beets such as Detroit Dark Red, Red Ace, Early Wonder Tall Top, Bull's Blood, Forono, Ruby Queen, Chioggia, Cylindra or Gladiator, yellow or gold beets such as Yellow Detroit, Golden, Touchstone Gold or Bolder or white beets such as Avalanche, Baby White, Blankoma or Sugar.
- the beetroot extract is substantially derived from the taproot portion of the beetroot.
- the beetroot extract for use in the present composition comprises at least about 1.5% nitrates by dry weight.
- the beetroot extract comprises about 0.1-90% of the dry weight of the weight management composition, such as about 1-50%, or about 5-25% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 10mg-50g of beetroot extract and preferably, about 50-5000mg.
- Coenzyme Q10 also known as ubiquinone, ubidecarenone, coenzyme Q, CoQ10, CoQ, or Q10, may assume any one of three redox states for use in the present composition, namely, fully oxidized (ubiquinone), semi-oxidized (semiquinone or ubisemiquinone), and fully reduced (ubiquinol) forms, along with oxidized mitochondrially targeted forms of this enzyme (e.g. mitoquinone mesylate (MitoQ10)).
- coenzyme Q10 may be formulated in numerous ways to improve its bioavailability or effectiveness.
- coenzyme Q10 comprises about 0.1-80% of the dry weight of the weight management composition, such as about 1-50%, or about 5-20% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 10mg-1g of coenzyme Q10 and preferably, about 50-900mg.
- Alpha lipoic acid suitable for use in the present composition may include, without limitation, alpha lipoic acid or its reduced form, dihydrolipoic acid, with R- and S-enantiomers either present individually, in racemic form or in any other mixture thereof.
- the R-enantiomer is produced naturally or synthetically, while the S-enantiomer is only produced synthetically and does not occur naturally.
- any pharmaceutically acceptable salts or derivatives thereof are suitable for use in the present composition.
- the alpha lipoic acid is present in racemic form.
- the alpha lipoic acid comprises about 0.1-90% of the dry weight of the weight management composition, such as about 5-50%, or about 10-30% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 10mg-10g of alpha lipoic acid and preferably, about 50mg-900mg.
- Creatine for use in the present composition may be in any suitable form, such as creatine monohydrate, creatine anhydrous, creatine citrate, creatine ascorbate, creatine ethyl ester, creatine nitrate, creatine magnesium chelate, creatine hydrochloride, creatine malate, creatine pyruvate, creatine phosphate, creatine citrate malate, creatine tartrate, creatine HMB ( ⁇ -hydroxy ⁇ -methylbutyrate), effervescent creatine, creatine titrate, buffered creatine, micronized creatine and any combination thereof.
- the creatine is creatine monohydrate.
- creatine comprises about 1%-90% of the dry weight of the weight management composition, such as about 20-70%, or about 30-50% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 0.1-10g of creatine and preferably, about 0.5-5g.
- Vitamin E for use in the present composition may be in the form of any one or more of the isomers thereof, including alpha-tocopherol, beta-tocopherol, gamma-tocopherol, delta-tocopherol, alpha-tocotrienol, beta-tocotrienol, gamma-tocotrienol, and delta-tocotrienol, and stereoisomers thereof.
- Vitamin E may also be used in analogue form, including, for example, vitamin E esters (such as acetate, succinate or palmitate forms) or other forms of vitamin E which have been modified for improved stability or bioavailability.
- the form of vitamin E used in the composition is alpha-tocopherol comprising biologically functional stereoisomers of alpha-tocopherol such as the naturally occurring RRR-configuration or the synthetically produced 2R-stereoisomer forms (RSR-, RRS-, and RSS-).
- the vitamin E used is D (also known as RRR)-alpha tocopheryl acetate.
- the vitamin E comprises about 0.1-80% of the dry weight of the weight management composition, such as about 1-50%, or about 3-15% of the dry weight of the composition.
- the weight management composition comprises a daily dosage of about 10mg-1g of vitamin E and preferably, about 50-900mg.
- the weight management composition comprises as the weight loss agent a mixture comprising a daily dosage of 50-1000mg of green tea extract, 50-1000mg of green coffee bean extract, 15-100mg of forskolin, and as the mitochondria enhancing agent a mixture comprising a daily dosage of 50-5000mg of beetroot extract, 50-900mg of coenzyme Q10, 50-900mg alpha lipoic acid and 50-900mg of vitamin E.
- the weight management composition comprises as the weight loss agent a mixture comprising a daily dosage of 50-500mg of green tea extract, 50-500mg of black tea extract, 50-500mg of green coffee bean extract, 500mg-3g of conjugated linoleic acid and 15mg-50mg of forskolin, and as the mitochondria enhancing agent a mixture comprising a daily dosage of 100-1000mg of beetroot extract, 50-200mg of coenzyme Q10, 50mg-500mg alpha lipoic acid, 1-5g of creatine and 50-200mg of vitamin E.
- caffeine is present in the weight management composition either as anhydrous caffeine or within a naturally occurring source such as green tea or green coffee beans.
- the anhydrous caffeine may be derived from any suitable source, such as from any one of about 60 plant species naturally containing caffeine, which include tea leaves, coffee beans, cocoa beans, yerba maté, guarana berries, guayusa, and the yaupon holly.
- the weight management composition comprises a daily dose of approximately 25-1000mg of caffeine, such as about 100-250mg.
- the weight management composition is substantially caffeine free.
- ingredients which naturally contain caffeine may be provided in decaffeinated form, such that each decaffeinated ingredient contains less than 1% of caffeine by dry weight for example. Methods for decaffeination are known in the art.
- the weight management composition may be used as a sole, primary, or supplemental source of nutrition.
- the composition will generally comprise other essential nutrients required in an adequate diet, such as vitamins, minerals, proteins, carbohydrates, fibre, and fats/lipids as would be appreciated by one of skill in the art.
- weight management composition may be formulated with at least one additional source of nutrition, including, but not limited to; proteins, carbohydrates, lipids, fibre, vitamins, minerals, antioxidants, prebiotics, probiotics, phytochemicals or phytonutrients.
- Proteins suitable for inclusion in the weight management composition include any food-grade protein which is suitable for oral administration to an individual. Proteins may be selected from any suitable protein source, including those from an animal source, a dairy source, a plant source, an insect source or any combination thereof. Non-limiting examples of insect sources of protein include: cricket protein, grasshopper protein, mealworm protein, earthworm protein and any combination thereof. Non-limiting examples of animal sources of protein include: cow protein, pig protein, goat protein, lamb protein, poultry protein (such as chicken, duck, goose, pheasant and the like), wild game protein, seafood protein (such as fish and shellfish) and any combination thereof.
- Non-limiting examples of dairy sources of protein include: whey protein, whey protein concentrate, whey protein isolate, milk protein concentrate, milk protein isolate, powdered fat and/or fat-free milk, micellar casein, acid casein, potassium caseinate, calcium caseinate, sodium caseinate and any combination thereof.
- Non-limiting examples of plant sources of protein include: pea protein, yeast protein, soy protein, corn protein, wheat protein, rice protein, canola protein, peanut protein, bean protein, lentil protein, and any combination thereof.
- the protein source may be non-hydrolyzed, partially hydrolyzed or hydrolyzed and may be in the form of an intact protein, amino acid or peptide.
- Non-limiting examples of amino acids may include essential amino acids such as: leucine, isoleucine, valine, tryptophan, methionine, threonine, phenylalanine and lysine and semi-essential amino acids such as: histidine and arginine and non-essential amino acids such as tyrosine, aspartic acid, glycine, alanine, cysteine, arginine, glutamic acid, proline, glutamine, serine, asparagine, taurine and any combination thereof.
- the protein source is a high-quality protein source, at least comprising each of the essential amino acids. More preferably, the protein source is a high-quality protein source containing an additional amount of leucine. In one embodiment of the disclosure, the present composition may include about 0.1-99% by weight protein.
- Carbohydrates suitable for inclusion in the weight management composition include any food-grade carbohydrate which is suitable for oral administration to an individual.
- Suitable carbohydrates include the following non-limiting examples: quickly-digestible carbohydrates such as monosaccharides, disaccharides or polysaccharides (e.g. glucose, fructose, sucrose, dextrose, maltodextrin and maltose), molasses, honey, maple syrup, corn syrup, high fructose corn syrup, sugar alcohols (e.g.
- the present composition may include about 0.1-99% by weight carbohydrates.
- the weight management composition may comprise any food-grade source of fibre which is suitable for oral administration to an individual.
- Suitable sources of fibre include the following non-limiting examples: water-soluble dietary fiber such as beta-glucans, pectin, xylose, plant gums, inulin and alginates, and insoluble dietary fiber such as lignin, beta-glucans, xanthan gum, resistant starches and combinations thereof.
- Fibre may be included in the composition in an amount that does not adversely affect the function of the composition.
- the weight management composition may comprise any food-grade source of lipids suitable for oral administration to an individual.
- Suitable sources of lipids include the following non-limiting examples: olive oil, safflower oil, canola oil, coconut oil, corn oil, palm oil, palm kernel oil, soybean oil, peanut oil, fish oil, almond oil, sunflower oil, butter, lard, and sources of medium chain triglycerides, long chain triglycerides, monoglycerides, diglycerides, cold water fish (e.g. cod, salmon, tuna, sardines, mackerel, krill and squid), algae, dark leafy green vegetables, plant and plant seed oils (e.g. flaxseed oil, canola oil and walnut oil), nuts (e.g. walnuts) and combinations thereof.
- Lipids may be included in the composition in an amount that does not adversely affect the function of the composition.
- the weight management composition may comprise any food-grade source of vitamins and minerals which are suitable for oral administration to an individual.
- Suitable vitamins include the following non-limiting examples: vitamin A, vitamin C, vitamin D, vitamin K, thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, folic acid, cobalamin, biotin, carotenoids (e.g.
- lutein, beta-carotene, lycopene and cryptoxanthin), choline, inositol and combinations thereof, and suitable minerals include, but are not limited to, calcium, phosphorus, selenium, chromium, zinc, molybdenum, iodine, chloride, phosphorus, manganese, fluoride, potassium, iron, copper, magnesium, sodium and combinations thereof.
- Vitamins may be included in an amount that complies with recommended daily dosages, either alone or in combination with diet. Vitamins and minerals may be included in the composition in an amount that does not adversely affect the function of the composition.
- the weight management composition may comprise any food-grade source of antioxidants which are suitable for oral administration to an individual.
- Suitable antioxidants include the following non-limiting examples: citric acid monohydrate, vitamin A, vitamin C, folic acid, and beta-carotene, iron, copper, butylated hydroxyamisole, butylated hydroxytoluene, propyl gallate, tertiary butylhydroquinone, resveratrol, and plant phytonutrients or phytochemicals (e.g. flavonoids and lignin).
- Herbs or herbal extracts e.g. oregano, Goji berry, dill, garden thyme, rosemary and peppermint
- tea leaves or tea leaf extracts e.g.
- Camellia sinensis coffee bean extracts (e.g. Coffea canephora and Coffea arabica ), brewed coffee or tea or brewed coffee or tea extracts (e.g. oolong tea and Coffea robusta ) and other plants or plant extracts (e.g. ginger root) may also be used as a source of antioxidants, as well as combinations of any of the antioxidants.
- Antioxidants may be included in the composition in an amount that does not adversely affect the function of the composition.
- the weight management composition may comprise any food-grade source of prebiotics which are suitable for oral administration to an individual.
- Suitable prebiotics include the following non-limiting examples: dietary fibers and carbohydrate polymers such as cellulose, inulin, gums, trans-galactooligosaccharide, fructans, resistant starches, xylooligosaccharides, hemicelluloses, pectin, sugar alcohols, beta-glucans and combinations thereof.
- the weight management composition may comprise any food-grade source of probiotics which are suitable for oral administration to an individual.
- Suitable probiotics include the following non-limiting examples: Lactobacillus Acidophilus, Lactobacillus Reuteri, Lactobacillus Rhamnosus, Lactobacillus Gasseri, Lactobacillus Salivarius, Lactobacillus Bulgaricus, Lactobacillus Helventicus, Lactobacillus Silivarus, Lactobacillus Plantarum, Lactobacillus Casei, Lactobacillus Paracassei, Lactobacillus Fermentum, Bifidobacterium Breve, Bifidobacterium Lactis, Bifidobacterium Longum, Bifidobacterium Bifidum, Bifidobacterium Infantis, Bifidobacterium Bifidum, Bacillus Coagulans, Saccharomyces Boulardii, Pediococcus Acidlacti and combinations thereof.
- the weight management composition may comprise any food-grade source of phytochemicals or phytonutrients which are suitable for oral administration to an individual.
- Suitable phytochemicals or phytonutrients include the following non-limiting examples: phytosterols including sterols (e.g. cempesterol) and stanol (e.g. sitostanol), soy flavonoids (e.g. genistein and glycitein), garlic and organosulfur compounds (e.g. L-cysteine sulfoxides and ⁇ -glutamyl-L-cysteine peptides), carotenoids (e.g.
- fiber e.g. lignin and cellulose
- indole 3-carbinol and condensation products e.g. 3,3'-diindolylmethane and 5,11-dihydroindolo-[3,2-b]carbazole
- Prebiotics, probiotics, phytochemicals and phytonutrients may be included in the composition in an amount that does not adversely affect the function of the composition.
- the present composition may additionally include at least one physiologically acceptable excipient.
- physiologically acceptable is used herein to refer to excipients which are food-grade and thus, acceptable for consumption or administration to a mammal.
- suitable excipients which are not to be construed as limiting, include flavouring agents, sweetening agents, anti-caking agents/ flowing agents, emulsifiers, stabilizers, masking agents, colorants, preservatives, disintegrants, binders, thickeners and pH adjusters.
- flavouring agents include natural or artificial flavours such as fruit flavours (e.g. raspberry, orange, apple, pomegranate, mixed berry, lemon, lime, watermelon, strawberry, blueberry, pineapple, coconut, grape, cherry, banana, peach, mango, kiwifruit, cranberry), sodium sources (e.g. sodium chloride and monosodium glutamate), high fructose corn syrup, vanilla, chocolate, unsweetened chocolate, honey, molasses, brown sugar, coffee, cocoa, mint, maple, almond, or extracts or combinations thereof.
- Savoury flavourings may also be used (e.g. beef, chicken or vegetable flavourings).
- Non-limiting examples of sweetening agents include natural sweeteners such as, glucose, fructose, sucrose, dextrose, maltose, brown sugar, molasses, honey, maple syrup, corn syrup, high fructose corn syrup, erythritol, xylitol, sorbitol, isomalt, monatin, monellin, curculin, brazzein, tagatose and mannitol, and artificial sweeteners such as aspartame, acesulfame K, saccharin cyclamate and sucralose.
- natural sweeteners such as, glucose, fructose, sucrose, dextrose, maltose, brown sugar, molasses, honey, maple syrup, corn syrup, high fructose corn syrup, erythritol, xylitol, sorbitol, isomalt, monatin, monellin, curculin, brazzein, tagatose and mannitol
- Non-limiting examples of further excipients include: anti-caking agents or flowing agents such as silicates (e.g. silicon dioxide) and calcium or magnesium stearates; emulsifiers such as agar, gums, egg yok, lecithin, monostearate, monosodium phosphate, monoglycerides, diglycerides and alginates; stabilizers such as glycerine, agar, gums, alginates and pectin; masking agents such as glycerine, sodium chloride, peppermint, lemon-lime, mint, cherry, black liquorice, peach, apricot, raspberry, or sweetening agents such as aspartame or sucrose; colorants such as those which are suitable for inclusion in foods, e.g.
- binders such as stearic acid, gelatin, saccharides and derivatives thereof, sugar alcohols, polyethylene glycol and cellulose
- thickeners such as polysaccharide-based thickeners such as vegetable gums, pectin and starches or protein-based thickeners such as gelatin, egg white and collagen
- pH adjusters such as citric acid, ammonium carbonate, ammonium phosphate, calcium carbonate, sodium hydroxide, malic acid and phosphoric acid.
- excipient e.g. flavouring agent, sweetener, emulsifier, preservative, etc.
- the weight management composition may be formulated for oral administration including, for example, solid, semi-solid, liquid, semi-liquid, powder, suspension, emulsion, solution, ready-to-drink beverage, gel, bar, pill, tablet or capsule form.
- oral or “orally” as used herein is intended to include any method in which the weight management composition is introduced into the digestive tract including the stomach and small intestine.
- oral administration may include administration via mouth, directly into the stomach using a feeding tube, through the nose to the stomach via a feeding tube and through the nose to the small intestine via a feeding tube.
- the weight management composition is provided as a loose powder, a bar or in capsules.
- the loose powdered composition may be reconstituted in water or any suitable liquid (such as juice, milk, saline, etc.) immediately prior to consumption.
- the weight management composition may be packaged in individual use containers, packets or sachets, or in larger bulk containers.
- any suitable capsule may be used including gelatin and hard hydroxypropyl methylcellulose (also known as Hypromellose) capsules.
- the weight management composition may also be administered parenterally, either alone or in combination with at least one pharmaceutically acceptable adjuvant, for use in methods in accordance with embodiments of the invention or disclosure.
- pharmaceutically acceptable means acceptable for use in the pharmaceutical and veterinary arts, i.e. not being unacceptably toxic or otherwise unsuitable.
- pharmaceutically acceptable adjuvants include diluents, excipients and the like. Reference may be made to " Remington's: The Science and Practice of Pharmacy", 21st Ed., Lippincott Williams & Wilkins, 2005 , for guidance on drug formulations generally. The selection of adjuvant depends on the intended mode of administration of the composition.
- the composition is formulated for administration by infusion, or by injection either subcutaneously or intravenously.
- the composition may be prepared as an aqueous solution in sterile and pyrogen-free form and optionally buffered or made isotonic.
- the composition may be administered in distilled water or, more desirably, in saline, phosphate-buffered saline or 5% dextrose solution.
- Creams, lotions and ointments may be prepared for topical application using an appropriate base such as a triglyceride base. Such creams, lotions and ointments may also contain a surface active agent. Aerosol formulations may also be prepared in which suitable propellant adjuvants are used.
- compositions may include a coating or may be encased in a protective material to prevent undesirable degradation thereof by enzymes, acids or by other conditions that may affect the therapeutic activity thereof.
- the weight management composition may be administered in a daily effective amount to a mammal in need thereof, one or more times per day, for a period ranging from one day to chronic or long-term administration.
- mammal is used herein to refer to human and non-human mammals such as domestic animals (cats, dogs, horses and other livestock).
- the term "daily effective amount” as used herein refers to an amount which achieves the effects desired in the mammal, without surpassing any amount which may cause undesirable side effects.
- a daily effective amount of the weight management composition may be administered once per day, or a daily effective amount of the weight management composition may be divided into 2, 3, 4, 5, 6 or more portions to be administered throughout the day.
- the weight management composition is administered once a day to an individual in the morning when first waking up.
- the weight management composition may be administered to an individual in need thereof for 1, 2, 3, 4, 5, 6 or 7 days in a week and for 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more weeks.
- the weight management composition is administered chronically to an individual in need thereof.
- the term "chronically” as used herein refers to the administration of the weight management composition for a period of at least 2-4 or more months, for example, administration of the weight management composition on a continual basis beyond 6 months, at a frequency of at least 2 days/week, and preferably at least 3 or more days a week.
- the daily effective amount of the weight management composition is consumed over two servings, with one serving being administered in the morning and the remaining serving being administered in the afternoon or evening for a period of 3 months. In another embodiment of the disclosure, the daily effective amount of the weight management composition is consumed over three servings, with one serving being administered in the morning, the second serving being administered around midday and the third serving being administered in the evening for a period of 3 months.
- the components of the composition may be administered in conjunction, either together, in a single composition, or individually in separate dosage forms which may be the same or different, and which may be administered at the same time or at different times.
- the weight loss agent may be administered in tablet form, while the mitochondrial enhancing agent may be administered separately in a different administrable dosage form, such as a capsule.
- the mitochondrial agent may be administered at the same time as the weight loss agent is administered, or at a different time, and at the same frequency or at a different frequency.
- the weight loss tablet may be administered twice daily, while the mitochondrial enhancing capsules are administered once or twice a day.
- each of the weight management composition components are provided in capsule form, together or separately, with the exception of creatine, which is provided in tablet form.
- the disclosed weight management composition may be used to treat or improve at least one of the following in a mammal: body weight, body fat, mitochondrial capacity, fatty liver disease, dyslipidemia, oxidative stress levels, BAT activity and systemic inflammation levels.
- body weight body fat, mitochondrial capacity, fatty liver disease, dyslipidemia, oxidative stress levels, BAT activity and systemic inflammation levels.
- the term "individual” is used herein to refer to a mammal, preferably a human.
- the method comprises administering to the individual an effective amount of the weight management composition. Any individual may be treated using the present method, including individuals of any age.
- treat means that favourably alter body weight, body fat, liver intra-cytosolic lipid deposits, blood lipid levels, oxidative stress levels, BAT activity, systemic inflammation levels and mitochondrial energy generating capacity, including those that moderate, reverse, reduce the severity of, or protect against, the progression of conditions associated with the dysregulation of body weight, body fat, liver intra-cytosolic lipid deposits, blood lipid levels, oxidative stress levels, BAT activity, systemic inflammation levels and mitochondrial energy generating capacity.
- the term "improve" is used herein with respect to the variables of body weight, body fat, liver intra-cytosolic lipid deposits, blood lipid levels, oxidative stress levels, BAT activity, systemic inflammation levels and mitochondrial energy generating capacity refer to either a healthy increase or a healthy decrease in the variable, i.e. an increase or decrease in the variable, whichever is considered to promote health.
- the treatment will achieve at least one of weight loss, an increase in mitochondrial capacity, a decrease in liver intra-cytosolic lipid deposits to treat fatty liver disease, a decrease in blood lipid levels to treat dyslipidemia, a decrease in oxidative stress levels, an increase in BAT activity and a decrease in systemic inflammation levels.
- the present method is useful for healthy individuals, as well as individuals that require treatment of one or more of body weight, body fat, fatty liver disease, dyslipidemia, oxidative stress levels, BAT activity, systemic inflammation levels and mitochondrial capacity, for example, elderly individuals, bed-ridden individuals, hospitalized individuals and individuals afflicted with a disease or condition that adversely effects one or more of body weight, body fat, fatty liver disease, dyslipidemia, oxidative stress levels, BAT activity, systemic inflammation levels and mitochondrial capacity.
- body weight refers to the total body mass of an individual or to the mass of specific regions of the body. Improved body weight is a reduction of body weight in an individual by at least about 0.5%, and preferably a reduction of about 1% or more, e.g. by 5%, 10%, 30%, 50%, 70% or greater of body weight in the individual prior to treatment with the present composition or a reduction in the rate of rising body weight by at least about 1% or more, and preferably a reduction of about 5% or more, e.g. by 10%, 30%, 50%, 70%, 90% or greater to the rate of body weight increase prior to treatment.
- the present composition surprisingly exhibits a synergistic effect, i.e.
- the effect of the combination of a weight loss agent and a mitochondrial enhancing agent results in a reduction of body weight that is greater than the additive effect of a weight loss agent alone, and a mitochondrial enhancing agent alone. This in turn will be reflected in other treatments for which the composition is useful insofar as an improvement in body weight and reduction in body fat promotes such treatments.
- body fat refers to the total fat mass of an individual or to the fat mass of specific regions of the body. During healthy weight loss, the majority of body weight loss is derived from the reduction of excess fat stores in an individual. Improved body fat is a reduction of body fat in an individual by at least about 0.5%, and preferably a reduction of about 1% or more, e.g. by 5%, 10%, 30%, 50%, 70% or greater of body fat in the individual prior to treatment with the present composition, or a reduction in the rate of rising body fat by at least about 1% or more, and preferably a reduction of about 5% or more, e.g. by 10%, 30%, 50%, 70%, 90% or greater to the rate of body fat increase prior to treatment.
- mitochondrial capacity refers to the total physiological productive capacity for mitochondria to produce cellular energy stores by carrying out cellular respiration.
- the term encompasses the mitochondrial capacity of one or more mitochondria and may be used to refer to the mitochondrial capacity of mitochondria within specific regions (such as specific muscles or organs) or within the entire body.
- Important indicators of mitochondrial capacity include the following non-limiting examples: an increase in the mRNA expression of mitochondrial capacity biomarkers such as PGC1 alpha, COX2, PPAR alpha, HSL, SCHAD, UCP3, PRDM16, CIDEA and AIPOQ, an increase in the total number of mitochondria (also known as mitochondrial volume density) in a tissue, an increase in the enzymatic capacity expressed in absolute terms or relative to the total number of mitochondria measured, the presence of biomarkers of mitochondrial integrity, such as the absence of deletions, cristae fragmentation or pleomorphism and a heightened amount (but not an excessive amount) of mitochondrial turnover via mitophagy or mitochondrial fission and fusion events.
- mitochondrial capacity biomarkers such as PGC1 alpha, COX2, PPAR alpha, HSL, SCHAD, UCP3, PRDM16, CIDEA and AIPOQ
- an increase in the total number of mitochondria also known as mitochondrial volume density
- improved mitochondrial capacity refers to an increase in the mRNA expression of mitochondrial capacity biomarkers in an individual by at least about 0.5%, and preferably an increase of about 1% or more, e.g. by 5%, 10%, 30%, 50%, 70% or greater from the mRNA expression of mitochondrial capacity biomarkers of the individual prior to treatment with the present composition, or at least a reduction in the rate of mRNA expression of mitochondrial capacity biomarker decline in an individual who is experiencing a decrease in the mRNA expression of mitochondrial capacity biomarkers by at least about 1% or more, and preferably a reduction in the rate of mitochondrial capacity loss by about 5% or more, e.g.
- Improved mitochondrial capacity may also refer to a normalization of mitochondrial capacity in one or more tissues within the levels of a healthy individual.
- fatty liver disease is intended to encompass a spectrum of metabolic fatty liver diseases, which progress in severity from fatty liver (also known as steatosis) to steatohepatitis to fibrosis/cirrhosis and potentially liver failure or hepatocellular carcinoma.
- Fatty liver disease is also intended to refer to both the nonalcoholic fatty liver disease and alcoholic liver disease (ALD).
- Indicators of the presence or development of fatty liver disease may include liver triglyceride accumulation, liver inflammation biomarkers, liver endoplasmic reticulum stress biomarkers, liver hepatocellular injury or hepatocyte death biomarkers and liver fibrosis biomarkers.
- improved fatty liver disease is a reduction of liver intra-cytosolic lipid deposits in an individual by at least about 0.5%, and preferably a reduction of about 1% or more, e.g. by 5%, 10%, 30%, 50%, 70% or greater of liver intra-cytosolic lipid deposits in the individual prior to treatment with the present composition, or a reduction in the rate of rising liver intra-cytosolic lipid deposits by at least about 1% or more, and preferably a reduction of about 5% or more, e.g. by 10%, 30%, 50%, 70%, 90% or greater to the rate of liver intra-cytosolic lipid deposit increase prior to treatment.
- the term “dyslipidemia” refers to an abnormal level of lipids in the blood, and the term commonly refers to elevated blood levels of non-HDL cholesterol (which includes chylomicrons, LDL cholesterol, IDL cholesterol and VLDL cholesterol), triglycerides or low levels of blood HDL cholesterol. Reducing the amount of total blood non-HDL cholesterol, or increasing HDL cholesterol generally leads to a reduced risk of, or slows the progression of atherogenic changes that may lead to coronary artery disease.
- One of the most accurate ways to diagnose elevated blood cholesterol is to measure the ApoB protein as this biomarker represents the non-HDL proteins in the blood, while ApoA1 is often used as a biomarker of blood HDL levels.
- improved dyslipidemia is a reduction of blood non-HDL lipids in an individual by at least about 0.5%, and preferably a reduction of about 1% or more, e.g. by 5%, 10%, 30%, 50%, 70% or greater of blood non-HDL lipids in the individual prior to treatment with the present composition, or a reduction in the rate of rising blood non-HDL lipids by at least about 1% or more, and preferably a reduction of about 5% or more, e.g. by 10%, 30%, 50%, 70%, 90% or greater to the rate of blood non-HDL lipid increase prior to treatment.
- Improved dyslipidemia may also refer to an increase in the amount of blood HDL cholesterol in an individual by at least about 0.5%, and preferably an increase of about 1% or more, e.g. by 5%, 10%, 30%, 50%, 70% or greater from the blood HDL cholesterol level of the individual prior to treatment with the present composition, or at least a reduction in the rate of blood HDL cholesterol level decline in an individual who is experiencing a decline of blood HDL cholesterol level by at least about 1% or more, and preferably a reduction in the rate of blood HDL cholesterol level decline by about 5% or more, e.g. by 10%, 30%, 50%, 70%, 90% or greater from the rate of blood HDL cholesterol level decline in the individual prior to treatment with the present composition.
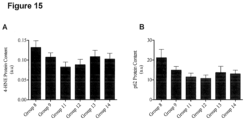
- Oxidative stress refers to the amount of free radical or free radical producing agents present in circulation and within tissues of the body. Oxidative stress is known to induce modifications which alter the functioning of proteins, lipids, DNA and other cellular components. Chronically elevated oxidative stress has been associated with and shown to exacerbate the development of obesity and obesity-associated co-morbidities such as insulin resistance, dyslipidemia, heart disease and fatty liver disease for example. Oxidative stress level is most commonly evaluated by measuring the biomarkers of oxidatively modified cellular products such as protein carbonyls and peroxidized lipids (trans-4-hydroxy-2-nonenal (4-HNE) for example).
- improved oxidative stress level is a reduction in 4-HNE protein levels in an individual by at least about 0.5%, and preferably a reduction of about 1% or more, e.g. by 5%, 10%, 30%, 50%, 70% or greater to the level of 4-HNE protein levels prior to treatment with the present composition, or at least a reduction in the rate of increase of 4-HNE protein levels by at least about 1% or more, and preferably a reduction of the rate of increase of 4-HNE protein levels by about 5% or more, e.g. by 10%, 30%, 50%, 70%, 90% or greater to the rate of increase of 4-HNE protein levels prior to treatment with the present composition.
- BAT activity refers to the total thermogenic activity of brown or beige adipocytes in an individual or to the thermogenic activity of brown or beige adipocytes in any one or more regions (such as one or more brown or beige adipocytes in an adipose tissue depot).
- Brown or beige adipose tissue is known to promote energy expenditure by increasing oxidative phosphorylation that is uncoupled from ATP generation, causing energy release in the form of heat.
- Beige adipocytes are commonly understood to be white adipocytes that have undergone transdifferentiation to exhibit an increase in "brown-like" characteristics, such as having a higher mitochondrial density and capacity for mitochondrial uncoupling.
- BAT activity Three of the common methods of improving BAT activity include the following: 1) converting one or more of the white adipocytes in an individual into beige adipocytes, 2) newly forming beige adipocytes from adipogenic progenitor cells, and 3) increasing the thermogenic activity of existing brown adipocytes.
- Activation of BAT is most commonly evaluated by measuring the mRNA expression or protein levels of BAT biomarkers such as UCP3, PRDM16, CIDEA, PPAR alpha and PGC1 alpha.
- improved BAT activity refers to an increase in the amount of mRNA expression of BAT biomarkers in an individual by at least about 0.5%, and preferably an increase of about 1% or more, e.g.
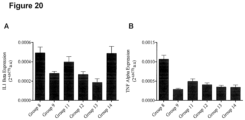
- systemic inflammation level refers to the amount of inflammation present in circulation and within tissues of the body. While acute inflammation in response to injurious events is thought to be beneficial, the presence of a chronic-low grade inflammation level is generally thought to be deleterious and likely related to the development of other chronic diseases associated with obesity. Systemic inflammation level is most commonly evaluated by measuring the mRNA expression or protein levels of pro-inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6), IL1 beta and tumor necrosis factor-alpha (TNF-alpha) in circulation.
- CRP C-reactive protein
- IL-6 interleukin-6
- TNF-alpha tumor necrosis factor-alpha
- biomarkers of systemic inflammation include pro-inflammatory cytokines such as IL-1B, IL-12 and IL-18, chemokines such as CXCL-8, CCL2, CCL3, CCL4, CCL5, CCL11 and CCXCL10 and growth factors such as GM-CSF, PDGF, TGF-Beta and VEGF.
- pro-inflammatory cytokines such as IL-1B, IL-12 and IL-18
- chemokines such as CXCL-8, CCL2, CCL3, CCL4, CCL5, CCL11 and CCXCL10
- growth factors such as GM-CSF, PDGF, TGF-Beta and VEGF.
- improved systemic inflammation level is a reduction in the mRNA expression of inflammation biomarkers in an individual by at least about 0.5%, and preferably a reduction of about 1% or more, e.g.
- the present weight management composition may be used in treating obesity and may also treat one or more obesity associated co-morbidities in an individual, such as fatty liver disease (e.g. steatosis, steatohepatitis and cirrhosis), cardiovascular disease (e.g. coronary artery disease and arrhythmias) , type 2 diabetes, hypertension, dyslipidemia (e.g. high LDL cholesterol, low HDL cholesterol, high triglycerides), gallbladder disease, osteoarthritis, sleep apnea, asthma, chronic kidney disease and depression.
- the method comprises administering to the individual a weight management composition comprising a weight loss agent and a mitochondria enhancing agent.
- the present methods of promoting weight management, reducing body weight and body fat and treating obesity in an individual may advantageously comprise administration of the weight management composition to an individual who is also performing regular exercise.
- exercise is meant to encompass endurance exercise, high-intensity interval training, resistance exercise, and the like, e.g. exercise that achieves a level of working of at least about 3-6 metabolic equivalents (METS), and combinations thereof (e.g. any combination of endurance exercise, high-intensity interval exercise, or > 50 % of the one repetition maximum (resistance exercise)).
- METS is the energy expenditure of a physical activity or exercise defined as the ratio of the metabolic rate of an exercising individual (and therefore the rate of energy consumption) during a specific physical activity to a reference basal metabolic rate. In a preferred embodiment of the disclosure, the exercise is performed on a regular basis.
- Regularly performing exercise refers to the performance of exercise for a duration of at least a month and preferably chronically such as for 2, 4 or 6 or more months, at a frequency of at least 2 days/week, and preferably at least 3 or more days a week, for a period of at least 30 consecutive minutes per day, preferably 45 minutes or greater, such as 60 minutes or greater, or 75 - 90 minutes or more.
- Exercise may include endurance activities such as brisk walking, jogging, running, dancing, swimming, bicycling, sports, interval training, resistance exercise, and the like.
- Interval training refers to repetitive bouts of exercise that may be at high or lower intensity provided it meets minimal METS requirements. High intensity interval training would include activities such as sprints (e.g.
- the term "resistance exercise” refers to weight training or other resistance exercise (plyometrics, hydraulic machines, etc.) with a resistance of at least 50% of the one repetition maximum, performed in sets of repetitions (for example, 8-15 repetitions), followed by a recovery between sets, for a period of time sufficient to achieve minimal METS requirements.
- One repetition maximum is the maximal voluntary contraction strength for a single movement where a second movement is impossible.
- the weight management composition may be administered at any time relative to the performance of exercise, i.e. before, during or following the exercise, or any combination thereof. In one embodiment of the disclosure, the weight management composition is administered to an individual immediately following exercise.
- Example 1 Weight management composition improves body weight, fat mass and mitochondrial capacity in vivo.
- a weight management composition in accordance with an embodiment of the invention, comprising a weight loss agent and a mitochondria enhancing agent could: 1) reduce body weight and body fat,, and improve mitochondrial capacity; and/or 2) enhance exercise-mediated improvements in body weight, body fat and mitochondrial capacity, high-fat diet (HFD) fed mice were administered the weight management composition or one of several control compositions and were either exercised or remained sedentary for a period of 30 days.
- HFD high-fat diet
- mice were ordered from Jackson Laboratories, which were placed on a HFD (Teklad #TD.06414) containing 60% energy from fat at 6 weeks and fed ad libitum. At approximately 12 weeks of age, mice were allocated into experimental groups which where standardized by average body weight.
- HFD control HFD control
- WL only diet HFD containing weight loss agents
- WL only diet HFD containing mitochondria enhancing agents
- ME only diet HFD containing mitochondria enhancing agents
- mice per group for Groups 1-4 were then individually housed in standard microisolator cages and fed their respective diets for 30 days.
- Group 5 consisted of another group of 10 mice which were fed the Weight management combination A diet, but were individually housed in cages containing an exercise wheel which allowed the mice to voluntarily exercise at will and were additionally exercised three times weekly on a treadmill at a speed of 15m/min for 45 minutes.
- Group 6 consisted of 10 mice which were fed the HFD control and subjected to the same housing and exercise regime as Group 5 in order to provide a reference to the effects of exercise alone on mice fed a HFD.
- Group 7 consisted of 10 C57/Bl6 mice (Jackson laboratories, Cat. # 000664), which were fed a standard mouse chow diet (referred to as Chow diet), with energy density of 3 Kcal/g; Envigo, diet # 8640) from weeks 0-12, were housed in microisolator cages and continued on Chow diet for 30 days in order to serve as a third control, which demonstrates the effects of a healthy diet on body composition.
- Chow diet standard mouse chow diet
- mice Beginning 7 days prior to the introduction of the experimental diets (i.e. day -7), baseline testing was conducted on all of the mice. Baseline testing included the following measurements: body weight, relative fat mass and lean body mass, grip strength, motor coordination and maximal running capacity.
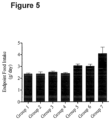
- body weight was measured daily throughout the study and food intake was measured approximately every day.
- each of the groups were then continued on their respective diets and baseline evaluations were repeated as endpoint measures over the span of a week.
- mice were anaesthetised with isoflurane, sacrificed by exsanguination and tissues were harvested.
- Relative fat mass and lean body mass were quantified using a time-domain NMR whole-body composition analyzer (minispec LF90II, Bruker; MA, USA) and normalizing values to body weight. Maximal exercise capacity was measured by exercising mice on a treadmill at starting speed of 10m/min and increasing the speed by 1m/min every 1 min until exhaustion. Motor coordination, grip strength and balance were evaluated using a rotarod apparatus (Harvard Apparatus, MA).
- mice fed the HFD control (Group 1) continued to gain weight throughout the entire study period and this HFD-induced weight gain was reduced in Groups 2 and 3.
- mice fed the Weight management combination A diet (Group 4) had a lower average body weight than that of the groups fed WL only diet (Group 2) or ME only diet (Group 3) alone, i.e. the weight management composition exhibited a synergistic or greater than additive effect with respect to weight loss.
- mice in Group 6 were protected against any HFD-induced weight gain, weight loss in Group 5 was substantially greater, with Group 5 mice achieving a healthy body weight comparable to mice fed the Chow diet (Group 7) by only the 6 th day of supplementation and maintaining this desirable body weight throughout the remainder of the study.
- body composition was also measured by MRI.
- the relative fat mass of Group 1 was increased over time as anticipated at the endpoint measurement ( Figure 2A ). While the mice in Groups 2 and 3 experienced little to no increase over the treatment period, the relative fat mass of Group 4 was reduced, despite the continued HFD feeding.
- mice in Group 5 experienced a 53% reduction in fat mass by endpoint in comparison with the 27% observed in Group 6.
- Small reductions in relative lean mass were experienced by Groups 1-3 ( Figure 2B ).
- this loss of lean mass was prevented in the Groups 4-7.
- tissue weights were recorded for an additional perspective of how the experimental diets affected body composition. Consistent with the previous findings, the relative weight of the intra-abdominal fat pads were lower in Groups 2 and 3 when compared to Group 1 and yet further reduced in Group 4 ( Figure 3A ).
- the relative intra-abdominal fat pad weight of Group 5 was 68% lower than that of the sedentary HFD control Group 1, while Group 6 was only 43% lower than the same sedentary control.
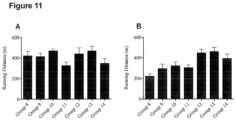
- a maximal exercise capacity test ( Figure 6A ) was performed to determine how long the mice could run at a progressively increasing speed and a rotarod performance test ( Figure 6B ) was conducted to quantify motor coordination, balance and grip strength. While mice in Group 1 and 2 experienced reductions in exercise capacity and rotarod performance over the supplementation period, mice in Groups 3 and 4 were protected against this HFD-induced impairment in performance. Consistent with the training effects from the exercise regime, mice in Groups 5 and 6 demonstrated markedly improved exercise capacity and rotarod performance by the time of the endpoint measure in comparison with baseline.
- Example 2 Alternate versions of the weight management composition improve body weight, body fat and mitochondrial capacity in vivo.
- mice were ordered from Jackson Laboratories, which were placed on a HFD (Teklad #TD.06414) containing 60% energy from fat at 6 weeks and fed ad libitum. At approximately 12 weeks of age, mice were allocated into experimental groups which where standardized by average body weight.
- HFD control diet HFD control diet
- HFD control diet HFD control diet
- Weight management combination A diet HFD control diet
- Weight management combination B diet alternate weight management composition
- Group 12 mice per group for Groups 8-11 were then individually housed in standard microisolator cages and fed their respective diets for 1 month.
- Group 13 consisted of another group of 12 mice which were fed the Weight management combination A diet, but were individually housed in cages containing an exercise wheel which allowed the mice to voluntarily exercise at will and were additionally exercised three times weekly on a treadmill at a speed of 15m/min for 45 minutes.
- Group 12 consisted of 12 mice which were fed the HFD control diet and subjected to the same housing and exercise regime as Group 13 in order to provide a reference to the effects of exercise alone on mice fed a HFD.
- Group 14 consisted of 12 C57/Bl6 mice (Jackson laboratories, Cat. # 000664), which were fed a standard mouse chow diet (referred to as Chow diet), with energy density of 3 Kcal/g; Envigo, diet # 8640) from weeks 0-12, were housed in microisolator cages and continued on Chow diet for 30 days in order to serve as a third control, which demonstrates the effects of a healthy diet on body composition.
- Chow diet standard mouse chow diet
- baseline testing was conducted on all of the mice.
- Baseline testing included the following measurements: body weight, relative fat mass and lean body mass and maximal running capacity.
- the experimental diets were introduced to all mice and the two exercise groups were placed in the running wheel cages and began the treadmill exercise regime. Body weight was measured daily throughout the study and food intake was measured weekly.
- each of the groups were then continued on their respective diets and baseline evaluations were repeated as endpoint measures over the span of a week.
- mice were anaesthetised with isoflurane, sacrificed by exsanguination and tissues were harvested. Relative fat mass, lean body mass and maximal exercise capacity was measured as described above.
- mitochondrial respiration was evaluated in permeabilized quadriceps muscle using high resolution respirometry (Oroboros Oxygraph-2k, Oroboros Instruments, Corp; Innsbruck, Australia).
- Complex I supported State 2 oxygen consumption was measured in the presence of 5mM pyruvate, 2mM malate and in the absence of ADP to provide an index of the uncoupling of mitochondrial substrate oxidation from the production of ATP.
- Complex I and II supported State 3 submaximal oxygen consumption was measured in the presence of 500uM ADP, 2mM Malate and 5mM pyruvate was added to simulate complex I-supported respiration via NADH generation.
- the viia7 system (Thermofischer Scientific) in conjunction with FAST SYBR Green (Life Technologies) was used for quantitative real-time PCR assessment of mRNA species in liver.
- the RNA (aqueous) phase was purified using the EZNA Total RNA Kit 1 (Omega Bio-Tek, Norcross, GA, USA) as per the manufacturer's instructions.
- Samples were then reverse transcribed using a high capacity cDNA reverse transcription kit (SuperScript ® VILO TM Master Mix; Invitrogen, cat. no. 11755050). Liver triglycerides were quantified using a commercially available kit (Abcam, ab65336) and protocol was carried out as per manufacturer's instructions. Lipid droplet staining was assessed in frozen liver sections using oil-red-O.
- liver sections were washed in propylene glycol and exposed to an oil-red-O solution prepared in isopropanol. Sections were subsequently washed again in propylene glycol, counter stained with hematoxylin and mounted in an aqueous medium.
- Serum alanine transaminase activity (ALT) was assessed using a commercially available kit (Cayman Chemical, 700260) and protocol was carried out as per manufacturer's instructions.
- Serum ApoB levels were quantified using a commercially available ELISA (Abeam, ab20737). Serum samples were diluted 1:5000 and protocol was carried out as per manufacturer's instructions. Serum ApoA1 levels were quantified using a commercially available ELISA (Abeam, ab238260). Serum samples were diluted 1:50,000 and protocol was carried out as per manufacturer's instructions. Serum PCSK9 levels were quantified using a commercially available ELISA (R and D Systems, MCP900). Serum samples were diluted 1:400 and protocol was carried out as per manufacturer's instructions.
- quadriceps muscle was homogenized in Lysing Matrix D tubes (MP Biomedicals, Solon, OH, USA) using the FastPrep-24 Tissue and Cell Homogenizer (MP Biomedicals, Solon, OH, USA) for 5 x 5-second cycles at a speed of 4.0 m/s with samples placed on ice for 5 minutes between cycles. Samples were homogenized in 20 volumes of buffer containing 70 mM sucrose, 220 mM mannitol, 10mM HEPES, 1mM EGTA, supplemented with protease inhibitors (Complete Mini ® , Roche Applied Science, Laval, PQ, Canada).
- oxidized cytochrome c (Sigma C7752) was reduced by sodium dithionite in 0.05 M potassium phosphate buffer (KH2PO4, pH 7.4). Twenty microliters of muscle homogenate were added to 955 ⁇ L of 0.05 M potassium phosphate buffer and 30 ⁇ L of reduced cytochrome c in a cuvette that had been warmed to 37°C. The rate of oxidation of reduced cytochrome c was measured at 550 nm for 3 min at 37°C.
- mice fed the HFD control (Group 8) continually increased over the month long period, rising 31% higher than the day 0 weight ( Figure 7 ).
- mice in group 9 fed the Weight management combination A diet had a substantially lower body weight after 1 month of supplementation when compared to Group 8.
- mice in Group 10, 11 and 12 experienced a similar protection from HFD-induced weight gain, losing 2, 3 and 4% of the starting body weight amounts respectively, despite the month of HFD feeding.
- mice fed the Weight management combination A diet in addition to being administered exercise (Group 13) had the greatest amount of weight loss of all groups (a reduction of 13%) and maintained a body weight similar to that of mice fed the Chow diet (Group 14).
- the relative intra-abdominal fat depot weights for each of the 3 groups administered one of the Weight management combination diets (Groups 9-11) and Group 12 were between 29-39% lower than the HFD control Group 8, while the exercise and Weight management combination A diet Group 13 had 63% less intra-abdominal fat than Group 8 ( Figure 9A ).
- Liver ( Figure 9B ), skeletal muscle ( Figure 9C and 10A ), heart ( Figure 10B ), pancreas ( Figure 10C ) and BAT ( Figure 10D ) weights were comparable between groups, with the exception that the relative liver, skeletal muscle and heart weights were lower in Group 8.
- an exercise capacity test was performed at baseline ( Figure 11A ) and endpoint ( Figure 11B ).
- ADP-stimulated oxygen consumption in Chow diet fed Group 14 was lower than that of the HFD control Group 8 and each of the groups administered one of the Weight management combination diets (Groups 9-11 and 13) had a respiration rate that was comparable to Chow diet fed Group 14 ( Figure 12B ).
- the ratio of enzyme activity of cytochrome c oxidase IV, the final enzyme in the electron transport chain) and citrate synthase was determined ( Figure 12C ).
- all groups had a lower cytochrome c oxidase IV to citrate synthase activity ratio, with Group 14 having the lowest ratio.
- PGC1 alpha is a transcriptional coactivator that is considered to be one of the primary promotors of mitochondrial biogenesis. PGC1 alpha expression was increased by approximately 4-fold in Groups 9, 11 and 12 in comparison to Group 8. Interestingly, PGC1 alpha expression was increased 6.0-fold and 6.4-fold in Group 13 and 14, respectively ( Figure 13A ).
- COX2 is one of the subunits of citrate synthase, a biomarker of mitochondrial density, and was lowest in the HFD control Group 8 ( Figure 13B ).
- PPAR alpha, HSL and SCHAD are three key genes that collectively, promote the breakdown of stored triglycerides into fatty acid chains, the tissue uptake of fatty acids and fatty acid oxidation in the mitochondria.
- the expression of PPAR alpha ( Figure 13C ), HSL ( Figure 13D ) and SCHAD ( Figure 13E ) were markedly lower in the HFD control Group 8 compared with the Chow diet fed Group 14.
- the amount of trans-4-hydroxy-2-nonenal (4-HNE) in skeletal muscle was measured.
- 4-HNE is generated as a product of the lipid peroxidation reaction and is a validated biomarker of oxidative stress.
- 4-HNE was 29% higher in the HFD fed control Group 8 compared with the Chow diet fed Group 14 ( Figure 15A ).
- the amount of 4-HNE was also between 18 and 37% lower in mice administered one of the Weight management combination diets or exercised (Groups 9 and 11-13). Oxidative stress is known to oxidatively damage cellular components and promote the degradation of those cellular materials by the autophagy pathway.
- p62 is an indicator of oxidative stress and the amount of cellular components which are targeted for degradation by the autophagy pathway.
- p62 abundance was between 30 and 49% lower in mice administered one of the Weight management combination diets, exercised or fed chow diet ( Figure 15B ).
- ALT levels Blood alanine aminotransferase (ALT) levels are used clinically as an indicator of hepatocellular injury or liver cell death. Serum ALT levels were elevated 2.9-fold in HFD fed Group 8 compared with Chow diet fed Group 14, but remained unchanged in Groups 11 and 12 ( Figure 17C ). To investigate a potential cause for excess lipid accumulation in the livers of HFD fed mice, and the presence of normal lipid levels in HFD mice supplemented with the weight management composition, mRNA expression levels of key liver proteins were evaluated. SREBP1, SREBP2, ADRP, and HNF1 alpha are 4 of the key genes that regulate liver lipid levels.
- the mRNA expression for these 4 genes were all substantially increased in Group 8 compared to Group 14 and for each protein, Group 11 demonstrated a reduction of mRNA expression level that was consistent with Group 12 which engaged in daily exercise ( Figure 16E and 18A ).
- the unfolded protein response occurs in response to increased endoplasmic reticulum stress, which may be caused by and further promote hepatocellular injury.
- GRP78, sXBP1, IRE1 alpha, ATF4, PERK and CHOP are 6 of the primary genes which effect the UPR and during prolonged endoplasmic reticulum stress, direct the cell towards apoptosis.
- mRNA expression for each of these UPR proteins were between 2 and 5.3-fold higher in the livers of HFD fed Group 8 mice compared to Chow diet fed Group 14 ( Figure 18B ).
- Daily exercise reduced this HFD-induced increase in UPR markers of Group 12, a protective effect that was mirrored in Group 11 fed the Weight management combination C diet.
- Casp1, Casp3, Casp7, Casp9 and PARP1 are 5 of the key genes which are promotors of apoptosis, pyroptosis, necroptosis and inflammation that initiate and effect programmed cell death.
- TGF beta and FN1 are two of the primary genes associated with the development of liver fibrosis.
- the liver expression of fibrosis markers were greatly elevated in Group 8 and lowered at or near the level of Chow diet fed mice in Groups 11 and 12 ( Figure 19B ).
- TNF alpha and IL-1 beta expression were considerably lower in the livers of Groups 11, 12 and 14 when compared to the Group 8 ( Figure 20A ). Accordingly, mRNA expression of IL1 beta in white adipose tissue was lower in each of the groups administered the Weight management combination diets or exercised (Groups 9 and 11-13) in comparison to the HFD control Group 8. The mRNA expression of TNF alpha in white adipose tissue was between 2.2 and 3.7-fold elevated when compared to Groups 9 and 11-13 ( Figure 20B ).
- Example 3 Alternate versions of the weight management composition improve fat oxidation in vivo.
- HFD fed mice were placed in metabolic cages while being administered the weight management composition.
- mice were ordered from Jackson Laboratories, which were placed on a HFD (Teklad #TD.06414) containing 60% energy from fat at 6 weeks and fed ad libitum.
- HFD Teklad #TD.06414
- mice were allocated into experimental groups which where standardized by average body weight, placed in the metabolic cages (Columbus Lab Animal Monitoring System; Columbus instruments) and allowed to acclimate to the new environment for about 12 hours. Following the acclimation period, mice remained on the 60% HFD used in Groups 1 and 8 above for a period of 1 day to collect metabolic data for each group while being fed the control HFD and prior to any treatment.
- mice were administered diets based on their allocation to the following groups: Group 15 were administered the same 60% HFD control as they were previously on, Group 16 were administered the Weight management combination A diet from Example 1 and 2 and Group 17 were administered the Weight management combination C diet from Example 2. As a standard low-fat diet control, a group of 8 mice (Group 18) which had been raised on Chow diet from Example 1 and 2 remained on Chow diet throughout the study. Following Day -1, measurements were collected for 3 days (referred to as Days 1-3) to observe any changes in metabolic activity during administration of the experimental diets. Lipid oxidation rates were collected by indirect calorimetry and were calculated based on the following equation (1.6946*VO2)-(1.7012*VCO2). Activity levels were collected by measured beam breaks across the x-axis of the metabolic cages.
- the findings from this study surprisingly reveal that the administration of the weight management composition comprising a weight loss agent and a mitochondria enhancing agent to an individual, or to an individual performing regular exercise, is an effective strategy for improving weight management, mitochondrial capacity, fatty liver disease, dyslipidemia, oxidative stress levels, brown adipose tissue activity and systemic inflammation levels in an individual.
- the present compositions and methods advantageously provide individuals with a means for achieving weight loss, with or without performing exercise or dieting.
- the present weight management composition has been scientifically validated and permits use by individuals without the expenditure of time to determine which supplements should be used and will provide a desired beneficial effect.
- the present method and composition also provides health benefits to an individual in need thereof that are synergistic with the health benefits obtained from exercise.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Mycology (AREA)
- Botany (AREA)
- Medical Informatics (AREA)
- Biotechnology (AREA)
- Alternative & Traditional Medicine (AREA)
- Microbiology (AREA)
- Nutrition Science (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Obesity (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Child & Adolescent Psychology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines Containing Plant Substances (AREA)
Claims (11)
- Zusammensetzung für Gewichtsmanagement, umfassend ein Mittel zur Gewichtsabnahme, umfassend ein Gemisch aus Grüntee-Extrakt, Extrakt aus grünen Kaffeebohnen und Forskolin, und ein Mitochondrien-verstärkendes Mittel, umfassend ein Gemisch aus Rote-Bete-Extrakt, Coenzym Q10, alpha-Liponsäure und Vitamin E, wobeii) der Grüntee-Extrakt 80 % oder mehr, bezogen auf das Trockengewicht, Catechine umfasst, wobei 50 % oder mehr der Catechine Epigallocatechingallat sind; der Extrakt aus grünen Kaffeebohnen 30 % oder mehr, bezogen auf das Trockengewicht, Chlorogensäuren umfasst, die 3-Caffeoylchinsäure, 4-Caffeoylchinsäure und/oder 5-Caffeoylchinsäure umfassen; und der Rote-Bete-Extrakt mindestens 1,5 %, bezogen auf das Trockengewicht, Nitrate umfasst; undii) die Zusammensetzung Folgendes umfasst:
5-50 %, bezogen auf das Trockengewicht, Grüntee-Extrakt und Extrakt aus grünen Kaffeebohnen, 0,05-50 %, bezogen auf das Trockengewicht, Forskolin, etwa 1-50 %, bezogen auf das Trockengewicht, Rote-Bete-Extrakt, Coenzym Q10 und Vitamin E sowie 5-50 %, bezogen auf das Trockengewicht, alpha-Liponsäure. - Zusammensetzung nach Anspruch 1, wobei das Mittel zur Gewichtsabnahme zusätzlich 5-50 %, bezogen auf das Trockengewicht, Schwarztee-Extrakt und 20-70 %, bezogen auf das Trockengewicht, konjugierte Linolsäure umfasst, wobei der Schwarztee-Extrakt 10 % oder mehr, bezogen auf das Trockengewicht, Polyphenole umfasst, ausgewählt aus Theaflavinen, Thearubiginen und/oder Catechinen, und wobei das Mitochondrien-verstärkende Mittel zusätzlich 20-70 %, bezogen auf das Trockengewicht, Kreatin umfasst.
- Zusammensetzung nach Anspruch 1, zusätzlich umfassend einen oder mehrere zusätzliche Inhaltsstoffe, ausgewählt aus Proteinen, Kohlenhydraten, Lipiden, Ballaststoffen, Vitaminen, Mineralstoffen, Antioxidantien, Präbiotika, Probiotika, Phytochemikalien und Phytonährstoffen.
- Zusammensetzung nach Anspruch 1, die entkoffeiniert ist.
- Zusammensetzung nach Anspruch 1, zur oralen Verabreichung formuliert.
- Zusammensetzung nach Anspruch 1, die in zwei verschiedenen Dosierungsformen bereitgestellt wird.
- Zusammensetzung nach Anspruch 1, wobei das Mittel zur Gewichtsabnahme in einer ersten Dosierungsform und das Mitochondrien-verstärkende Mittel in einer zweiten Dosierungsform bereitgestellt wird.
- Zusammensetzung für Gewichtsmanagement, umfassend ein Mittel zur Gewichtsabnahme, umfassend ein Gemisch aus Grüntee-Extrakt, Extrakt aus grünen Kaffeebohnen und Forskolin, und ein Mitochondrien-verstärkendes Mittel, umfassend ein Gemisch aus Rote-Bete-Extrakt, Coenzym Q10, alpha-Liponsäure und Vitamin E, zur Verwendung bei der Behandlung von Adipositas beim Menschen, wobei:i) der Grüntee-Extrakt 80 % oder mehr, bezogen auf das Trockengewicht, Catechine umfasst, wobei 50 % oder mehr der Catechine Epigallocatechingallat sind; der Extrakt aus grünen Kaffeebohnen 30 % oder mehr, bezogen auf das Trockengewicht, Chlorogensäuren umfasst; und der Rote-Bete-Extrakt mindestens 1,5 %, bezogen auf das Trockengewicht, Nitrate umfasst; undii) die Zusammensetzung zur Verwendung eine tägliche Dosis von 50-1000 mg Grüntee-Extrakt, 50-1000 mg Extrakt aus grünen Kaffeebohnen, 15 mg-100 mg Forskolin, 50-5000 mg Rote-Bete-Extrakt, 50-900 mg Coenzym Q10, 50 mg-900 mg alpha-Liponsäure und 50-900 mg Vitamin E umfasst.
- Zusammensetzung zur Verwendung nach Anspruch 8, wobei das Mittel zur Gewichtsabnahme zusätzlich eine tägliche Dosis von 50-500 mg Schwarztee-Extrakt und 500 mg-3 g konjugierter Linolsäure umfasst, wobei der Schwarztee-Extrakt 10 % oder mehr, bezogen auf das Trockengewicht, Polyphenole umfasst, ausgewählt aus Theaflavinen, Thearubiginen und/oder Catechinen, und wobei das Mitochondrien-verstärkende Mittel zusätzlich eine tägliche Dosis von 1-5 g Kreatin umfasst.
- Zusammensetzung zur Verwendung nach Anspruch 8, wobei das Mittel zur Gewichtsabnahme in einer ersten Dosierungsform verabreicht wird und das Mitochondrien-verstärkende Mittel in einer zweiten Dosierungsform verabreicht wird.
- Zusammensetzung zur Verwendung nach Anspruch 8, umfassend eine tägliche Dosis von 50-500 mg Grüntee-Extrakt, 50-500 mg Extrakt aus grünen Kaffeebohnen und 15 mg-50 mg Forskolin, 100-1000 mg Rote-Bete-Extrakt, 50-200 mg Coenzym Q10, 50 mg-500 mg alpha-Liponsäure und 50-200 mg Vitamin E, zusätzlich umfassend eine tägliche Dosis von 50-500 mg Schwarztee-Extrakt, 500 mg-3 g konjugierte Linolsäure und 1-5 g Kreatin.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201862645561P | 2018-03-20 | 2018-03-20 | |
| PCT/CA2019/050342 WO2019178689A1 (en) | 2018-03-20 | 2019-03-20 | Weight management composition |
Publications (4)
| Publication Number | Publication Date |
|---|---|
| EP3768290A1 EP3768290A1 (de) | 2021-01-27 |
| EP3768290A4 EP3768290A4 (de) | 2021-11-24 |
| EP3768290B1 true EP3768290B1 (de) | 2024-08-21 |
| EP3768290C0 EP3768290C0 (de) | 2024-08-21 |
Family
ID=67988252
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19771630.1A Active EP3768290B1 (de) | 2018-03-20 | 2019-03-20 | Zusammensetzung für gewichtsmanagement |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US11167001B2 (de) |
| EP (1) | EP3768290B1 (de) |
| CN (1) | CN112118855A (de) |
| AU (1) | AU2019239797B2 (de) |
| CA (1) | CA3050823C (de) |
| WO (1) | WO2019178689A1 (de) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11625937B2 (en) * | 2020-04-06 | 2023-04-11 | Toyota Motor Engineering & Manufacturing North America, Inc. | Methods and systems for monitoring human body weight with vehicle sensors and big data AI analytics |
| CN111449243A (zh) * | 2020-04-07 | 2020-07-28 | 中国海洋大学 | 一种辅助降脂减肥的海藻果蔬配方 |
| CN111466565A (zh) * | 2020-04-13 | 2020-07-31 | 广州市慈膳生物科技有限公司 | 一种海参肽特殊医学用途配方食品及其制备方法 |
| US12115120B2 (en) | 2020-04-17 | 2024-10-15 | The Florida State University Research Foundation, Inc. | Diagnostic and therapeutic splints and methods of use |
| CN112168837A (zh) * | 2020-06-29 | 2021-01-05 | 武汉林宝莱生物科技有限公司 | 一种线粒体素口服固体制剂配方及其制备工艺 |
| CN112546195A (zh) * | 2020-12-16 | 2021-03-26 | 刘袭文 | 红花多肽轻盈片 |
| CN115039878B (zh) * | 2021-03-09 | 2024-01-05 | 仙乐健康科技股份有限公司 | 一种调节脂肪代谢功能的组合物 |
| JP2024517556A (ja) * | 2021-03-17 | 2024-04-23 | ユ,ヒョギョン | コエンザイムq10含有液状補助飼料組成物 |
| CN113975335B (zh) * | 2021-12-29 | 2022-04-08 | 仙乐健康科技股份有限公司 | 一种控制食欲和诱导饱腹感的组合物 |
| US20240009261A1 (en) * | 2022-07-08 | 2024-01-11 | Tci Co., Ltd. | Prebiotic compositions and method for weight loss by using the same |
| FR3138770A1 (fr) * | 2022-08-10 | 2024-02-16 | Actina | Ensemble de parties destine a moduler le microbiote intestinale, reduire la prise de poids et le stockage des graisses dans le foie |
| CN115500519A (zh) * | 2022-08-31 | 2022-12-23 | 郑州瑞普生物工程有限公司 | 具有减脂减重功效的凝胶体及其制备方法 |
| AU2023371556A1 (en) * | 2022-11-03 | 2025-06-12 | Exerkine Corporation | Fertility enhancement composition |
| CN115779018A (zh) * | 2022-12-05 | 2023-03-14 | 杭州天草科技有限公司 | 一种具有降低体重作用的植物提取物组合物 |
| CN117770452B (zh) * | 2023-03-21 | 2024-08-13 | 中恩(天津)医药科技有限公司 | 一种具有减重降脂功效的膳食食品 |
| CN116849356A (zh) * | 2023-07-07 | 2023-10-10 | 北京彩晔健康管理有限公司 | 一种用于缓解肥胖的可食用组合物及其制备方法 |
| CN117179304A (zh) * | 2023-09-08 | 2023-12-08 | 福建农林大学 | 促进脂肪烧热的膳食活性成分复配口服液及其制备方法 |
| SE2550705A1 (en) * | 2024-01-09 | 2025-07-17 | Maifeng Zhuhai Tech Co Ltd | Guarana composition product absorbed through oral mucosa and preparation method thereof |
| CN117562257B (zh) * | 2024-01-15 | 2024-05-14 | 北京衡美金叶营养健康科技有限公司 | 一种高脂肪低碳水的食品组合物及其制备方法 |
| CN119547888B (zh) * | 2025-02-06 | 2025-05-02 | 中国食品发酵工业研究院有限公司 | 一种减脂减重食品组合物、压片糖果及其制备方法和应用 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2712191B1 (fr) * | 1993-11-08 | 1996-03-08 | Bioes Laboratoires | Composition pharmaceutique ou diététique associant du ginseng et du guarana. |
| US20050130933A1 (en) * | 2003-10-17 | 2005-06-16 | Ira Jacobs | Weight-loss supplement |
| US20060051435A1 (en) | 2004-08-19 | 2006-03-09 | Udell Ronald G | Nutritional supplement for body fat reduction |
| WO2006096996A1 (en) * | 2005-03-18 | 2006-09-21 | Ripped Formulations Ltd. | Compositions and methods for increasing metabolism, thermogenesis and/or muscular definition |
| US7989007B2 (en) * | 2007-07-03 | 2011-08-02 | Vincent James Enterprises, Llc | Weight loss composition |
| US20090252758A1 (en) * | 2008-04-07 | 2009-10-08 | Mazed Mohammad A | Nutritional supplement for the prevention of cardiovascular disease, alzheimer's disease, diabetes, and regulation and reduction of blood sugar and insulin resistance |
| GB201012539D0 (en) * | 2010-07-27 | 2010-09-08 | Savantium Ltd | Nutritional compositions |
| US9220741B2 (en) * | 2010-08-30 | 2015-12-29 | Northern Innovations And Formulations Corp. | Weight loss formulation |
| US8900557B2 (en) * | 2010-12-30 | 2014-12-02 | Jr Chem, Llc | Dental cleaning composition |
| CA2751218C (en) * | 2011-08-31 | 2019-09-17 | Northern Innovations And Formulations Corp. | Weight loss formulation |
| US20150250203A1 (en) * | 2012-01-26 | 2015-09-10 | Wikifoods, Inc. | Enclosing materials in natural transport systems |
| WO2014151329A1 (en) * | 2013-03-15 | 2014-09-25 | Aerodesigns, Inc. | Particles for aerosolizing apparatus |
| CA2903752A1 (en) * | 2013-03-15 | 2014-09-25 | Nusirt Sciences, Inc. | Treatment of pets with sirtuin activators |
| CN113387836A (zh) * | 2014-07-03 | 2021-09-14 | Nan全球有限责任公司 | 治疗肥胖症,防止体重增加,促进体重减轻或治疗或预防糖尿病的发展的方法和组合物 |
| DK3050561T3 (da) * | 2014-07-30 | 2020-11-16 | Shyam Prasad Kodimule | Sammensætninger af chlorogensyre og fremgangsmåder til fremstilling og anvendelse af samme i fedmehåndtering |
| JP2019512229A (ja) * | 2016-03-07 | 2019-05-16 | ネイチャーズ サンシャイン プロダクツ、インク. | 食事療法および運動での減量および心血管代謝の健康をさらに改善するための方法および組成物 |
-
2019
- 2019-03-20 CA CA3050823A patent/CA3050823C/en active Active
- 2019-03-20 WO PCT/CA2019/050342 patent/WO2019178689A1/en not_active Ceased
- 2019-03-20 EP EP19771630.1A patent/EP3768290B1/de active Active
- 2019-03-20 AU AU2019239797A patent/AU2019239797B2/en active Active
- 2019-03-20 CN CN201980032119.8A patent/CN112118855A/zh active Pending
- 2019-08-01 US US16/528,855 patent/US11167001B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US20190350997A1 (en) | 2019-11-21 |
| WO2019178689A1 (en) | 2019-09-26 |
| US11167001B2 (en) | 2021-11-09 |
| CN112118855A (zh) | 2020-12-22 |
| EP3768290A4 (de) | 2021-11-24 |
| AU2019239797A1 (en) | 2020-10-15 |
| CA3050823C (en) | 2021-02-23 |
| CA3050823A1 (en) | 2019-09-20 |
| EP3768290C0 (de) | 2024-08-21 |
| AU2019239797B2 (en) | 2024-04-04 |
| EP3768290A1 (de) | 2021-01-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3768290B1 (de) | Zusammensetzung für gewichtsmanagement | |
| US11969411B2 (en) | Administration of berberine metabolites | |
| EP2976073B1 (de) | Zusammensetzungen und verfahren zur herstellung erhöhter und verzögerter ketose | |
| JP6859336B2 (ja) | 骨格筋の健康のためポリフェノールを使用する組成物及び方法 | |
| US12419852B2 (en) | Method for treating lysosomal storage disease | |
| EP2859896B1 (de) | Pharmazeutische Zusammensetzungen zur Behandlung von Muskelkrankheiten | |
| US20130171268A1 (en) | Methods and compositions for treatment of mitochondrial toxicity | |
| US12390470B2 (en) | 1-methylxanthine-based bioactive composition and method of use thereof | |
| US20230037138A1 (en) | Paraxanthine-based caffeine substitute compositions and method of use thereof in slow caffeine metabolizers | |
| US20160303176A1 (en) | Nutritional supplement | |
| US12285456B2 (en) | Weight management composition | |
| JP2023554187A (ja) | 身体運動パフォーマンスを改善するため方法 | |
| US20220339142A1 (en) | Administration of r-beta-hydroxybutyrate salt blend and related compounds in humans | |
| US20250281438A1 (en) | Compositions and methods using at least one glycine or derivative thereof and/or at least one n-acetylcysteine or derivative thereof, and at least one thymol and/or carvacrol | |
| US20220202789A1 (en) | Administration of berberine metabolites | |
| CN117979971A (zh) | 基于1-甲基黄嘌呤的生物活性组合物及其使用方法 | |
| WO2024092369A1 (en) | Fertility enhancement composition | |
| WO2025166466A1 (en) | A method to treat body composition resistance |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20201014 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| A4 | Supplementary search report drawn up and despatched |
Effective date: 20211027 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C07D 339/04 20060101ALI20211021BHEP Ipc: C07D 311/78 20060101ALI20211021BHEP Ipc: C07C 50/28 20060101ALI20211021BHEP Ipc: A61P 3/04 20060101ALI20211021BHEP Ipc: A61K 36/74 20060101ALI20211021BHEP Ipc: A61K 36/36 20060101ALI20211021BHEP Ipc: A61K 31/385 20060101ALI20211021BHEP Ipc: A61K 31/355 20060101ALI20211021BHEP Ipc: A61K 31/353 20060101ALI20211021BHEP Ipc: A61K 31/122 20060101ALI20211021BHEP Ipc: A61K 36/82 20060101AFI20211021BHEP |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20220614 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Free format text: PREVIOUS MAIN CLASS: A61K0036820000 Ipc: A23L0033100000 Ref country code: DE Ref legal event code: R079 Ref document number: 602019057440 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: A61K0036820000 Ipc: A23L0033100000 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20240315 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61P 3/04 20060101ALI20240301BHEP Ipc: A61K 36/74 20060101ALI20240301BHEP Ipc: A61K 36/36 20060101ALI20240301BHEP Ipc: A61K 31/385 20060101ALI20240301BHEP Ipc: A61K 31/355 20060101ALI20240301BHEP Ipc: A61K 31/353 20060101ALI20240301BHEP Ipc: A61K 31/122 20060101ALI20240301BHEP Ipc: A61K 36/82 20060101ALI20240301BHEP Ipc: A61K 45/06 20060101ALI20240301BHEP Ipc: A61K 36/38 20060101ALI20240301BHEP Ipc: A61K 36/21 20060101ALI20240301BHEP Ipc: A61K 36/185 20060101ALI20240301BHEP Ipc: A61K 36/15 20060101ALI20240301BHEP Ipc: A61K 31/352 20060101ALI20240301BHEP Ipc: A61K 31/201 20060101ALI20240301BHEP Ipc: A61K 31/198 20060101ALI20240301BHEP Ipc: A23L 33/00 20160101ALI20240301BHEP Ipc: A23L 33/15 20160101ALI20240301BHEP Ipc: A23L 33/105 20160101ALI20240301BHEP Ipc: A23L 33/10 20160101AFI20240301BHEP |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602019057440 Country of ref document: DE |
|
| U01 | Request for unitary effect filed |
Effective date: 20240921 |
|
| U07 | Unitary effect registered |
Designated state(s): AT BE BG DE DK EE FI FR IT LT LU LV MT NL PT RO SE SI Effective date: 20241015 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241121 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241122 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241221 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241121 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241121 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241121 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241221 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241122 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 |
|
| U20 | Renewal fee for the european patent with unitary effect paid |
Year of fee payment: 7 Effective date: 20250205 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250130 Year of fee payment: 7 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20250522 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240821 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: H13 Free format text: ST27 STATUS EVENT CODE: U-0-0-H10-H13 (AS PROVIDED BY THE NATIONAL OFFICE) Effective date: 20251023 |