EP3554250B1 - Procédé de production d'un produit laitier concentré avec agrégation bivalente libre des cations de protéine - Google Patents
Procédé de production d'un produit laitier concentré avec agrégation bivalente libre des cations de protéine Download PDFInfo
- Publication number
- EP3554250B1 EP3554250B1 EP17835654.9A EP17835654A EP3554250B1 EP 3554250 B1 EP3554250 B1 EP 3554250B1 EP 17835654 A EP17835654 A EP 17835654A EP 3554250 B1 EP3554250 B1 EP 3554250B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- milk
- protein
- calcium
- ingredient composition
- concentrate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23C—DAIRY PRODUCTS, e.g. MILK, BUTTER OR CHEESE; MILK OR CHEESE SUBSTITUTES; MAKING OR TREATMENT THEREOF
- A23C1/00—Concentration, evaporation or drying
- A23C1/14—Concentration, evaporation or drying combined with other treatment
- A23C1/16—Concentration, evaporation or drying combined with other treatment using additives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23C—DAIRY PRODUCTS, e.g. MILK, BUTTER OR CHEESE; MILK OR CHEESE SUBSTITUTES; MAKING OR TREATMENT THEREOF
- A23C1/00—Concentration, evaporation or drying
- A23C1/04—Concentration, evaporation or drying by spraying into a gas stream
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23C—DAIRY PRODUCTS, e.g. MILK, BUTTER OR CHEESE; MILK OR CHEESE SUBSTITUTES; MAKING OR TREATMENT THEREOF
- A23C1/00—Concentration, evaporation or drying
- A23C1/14—Concentration, evaporation or drying combined with other treatment
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23C—DAIRY PRODUCTS, e.g. MILK, BUTTER OR CHEESE; MILK OR CHEESE SUBSTITUTES; MAKING OR TREATMENT THEREOF
- A23C9/00—Milk preparations; Milk powder or milk powder preparations
- A23C9/152—Milk preparations; Milk powder or milk powder preparations containing additives
- A23C9/1522—Inorganic additives, e.g. minerals, trace elements; Chlorination or fluoridation of milk; Organic salts or complexes of metals other than natrium or kalium; Calcium enrichment of milk
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23C—DAIRY PRODUCTS, e.g. MILK, BUTTER OR CHEESE; MILK OR CHEESE SUBSTITUTES; MAKING OR TREATMENT THEREOF
- A23C9/00—Milk preparations; Milk powder or milk powder preparations
- A23C9/152—Milk preparations; Milk powder or milk powder preparations containing additives
- A23C9/154—Milk preparations; Milk powder or milk powder preparations containing additives containing thickening substances, eggs or cereal preparations; Milk gels
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23G—COCOA; COCOA PRODUCTS, e.g. CHOCOLATE; SUBSTITUTES FOR COCOA OR COCOA PRODUCTS; CONFECTIONERY; CHEWING GUM; ICE-CREAM; PREPARATION THEREOF
- A23G1/00—Cocoa; Cocoa products, e.g. chocolate; Substitutes therefor
- A23G1/30—Cocoa products, e.g. chocolate; Substitutes therefor
- A23G1/56—Liquid products; Solid products in the form of powders, flakes or granules for making liquid products, e.g. for making chocolate milk, drinks and the products for their preparation, pastes for spreading or milk crumb
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23G—COCOA; COCOA PRODUCTS, e.g. CHOCOLATE; SUBSTITUTES FOR COCOA OR COCOA PRODUCTS; CONFECTIONERY; CHEWING GUM; ICE-CREAM; PREPARATION THEREOF
- A23G3/00—Sweetmeats; Confectionery; Marzipan; Coated or filled products
- A23G3/34—Sweetmeats, confectionery or marzipan; Processes for the preparation thereof
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23J—PROTEIN COMPOSITIONS FOR FOODSTUFFS; WORKING-UP PROTEINS FOR FOODSTUFFS; PHOSPHATIDE COMPOSITIONS FOR FOODSTUFFS
- A23J1/00—Obtaining protein compositions for foodstuffs; Bulk opening of eggs and separation of yolks from whites
- A23J1/20—Obtaining protein compositions for foodstuffs; Bulk opening of eggs and separation of yolks from whites from milk, e.g. casein; from whey
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23J—PROTEIN COMPOSITIONS FOR FOODSTUFFS; WORKING-UP PROTEINS FOR FOODSTUFFS; PHOSPHATIDE COMPOSITIONS FOR FOODSTUFFS
- A23J1/00—Obtaining protein compositions for foodstuffs; Bulk opening of eggs and separation of yolks from whites
- A23J1/20—Obtaining protein compositions for foodstuffs; Bulk opening of eggs and separation of yolks from whites from milk, e.g. casein; from whey
- A23J1/205—Obtaining protein compositions for foodstuffs; Bulk opening of eggs and separation of yolks from whites from milk, e.g. casein; from whey from whey, e.g. lactalbumine
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23J—PROTEIN COMPOSITIONS FOR FOODSTUFFS; WORKING-UP PROTEINS FOR FOODSTUFFS; PHOSPHATIDE COMPOSITIONS FOR FOODSTUFFS
- A23J3/00—Working-up of proteins for foodstuffs
- A23J3/04—Animal proteins
- A23J3/08—Dairy proteins
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23J—PROTEIN COMPOSITIONS FOR FOODSTUFFS; WORKING-UP PROTEINS FOR FOODSTUFFS; PHOSPHATIDE COMPOSITIONS FOR FOODSTUFFS
- A23J3/00—Working-up of proteins for foodstuffs
- A23J3/04—Animal proteins
- A23J3/08—Dairy proteins
- A23J3/10—Casein
Definitions
- the present invention relates to a method of producing a dairy concentrate as defined in claim 1.
- CN104489097A describes a process to obtain a heat convection drying protectant preparations for lactic bacteria or probiotics consisting in heat treating at 60°C a milk preparation enriched with calcium in order to induce protein aggregation and subsequently submitting the preparation to a mechanical homogenization treatment.
- This patent application does not relates to dairy concentrates.
- WO07040113A describes the production of an ingredient exhibiting high content in milk-derived complex lipids. It is obtained by precipitating the protein fractions of butter serum at pH 4.0 - 5.0 in presence of calcium and filtering the supernatant in order to concentrate the complex lipids.
- WO 06065135 A2 disclosing the production of a free divalent cations-rich liquid food product in which 20% of the lysine residues carried out by the proteins have been glycosylated in order to increase their resistance to aggregation in presence of calcium. Therefore, WO 06065135 A2 is related to preventing protein aggregation in presence of divalent cations, calcium among others.
- US20130011515 A1 is describing a process for the production of a milk protein concentrate which is enriched with whey proteins. Skimmed milk is heated in the pH range 6.5-7.0 in order to promote aggregation of whey proteins together with caseins. The heated product is subsequently submitted to filtration in order to concentrate protein aggregates and to remove lactose.
- WO2016/102503 discloses the production of dairy-based RTD.
- the milk component comprises casein and whey proteins.
- the milk proteins are partially aggregated in the presence of hydrocolloids as stabilizing system and chelated calcium salts can be added to fortify the products.
- WO2016/174651 relates to a heat-treated liquid nutritional composition
- a heat-treated liquid nutritional composition comprising 8 to 20 g of protein per 100 ml of the composition, wherein at least 40 wt.% of the protein is micellar casein and at least 10 wt.% of the protein is hydrolysed whey protein.
- WO2016/102500 relates to a milk concentrate comprising caseins and whey proteins in the ratio 90:10 to 60:40, wherein the casein/whey protein aggregates have a volume based mean diameter value Dv50 of at least 1 ⁇ m as measured by laser diffraction.
- WO2012/017043 relates to a beverage product comprising a partially denatured protein system including kappa-casein and beta-lactoglobulin, wherein said product has a pH comprised between 5.6 and 6.3.
- the pH of the product may vary and influence process and may lead to instability of the product.
- the prior art does not show how to provide food and beverage products delivering desirable taste and texture.
- the present invention provides the improvement by the use of milk protein-based aggregates by specific heat treatment in the presence of a specific concentration of added divalent cations. It was surprisingly found that there is a critical range of divalent cations addition leading to optimum protein aggregation without precipitation or gelation of the formed aggregates upon heating.
- the invention relates to a method of producing a dairy concentrate, comprising the steps of:
- a major advantage of this invention is that it allows to texturize reduced fat milk-protein based concentrates and enables a reduction or elimination of the use of additional hydrocolloids and/or emulsifiers.
- the agglomerates created with the method according to the invention and present in the product of the invention have a size of 3 - 50 microns, preferably 5 - 50 microns, more preferably 5 - 10 microns, as measured by D( 4,3) mean diameter.
- the agglomerate particle size distribution is measured (PSD) using a laser granulometer such as a Mastersizer 2000 (Malvern Instruments, UK).
- a sample may e.g. be dispersed in the Hydro SM measuring cell until an obscuration rate of 9-10% is obtained and then analysed in the Mastersizer.
- free divalent cations may be measured by means of a selective electrode.
- free (ionic) calcium concentration is determined a Mettler Toledo calcium selective electrode perfection TM DX series half cells with BNC connector P/N 51344703 connected to a 692pH/Ion meter (Metrohm Switzerland).
- % of a component means the % of weight based on the weight of the composition, i.e. weight/weight %.
- dairy concentrate may be a dairy culinary product, a soup or soup base, a dessert, a whipping cream, a tea or coffee creamer or enhancer, a dairy component in coffee mixes and dairy component for use in a beverage system such as a beverage vending system.
- stirring means moving the ingredient composition.
- the stirring may result in a shearing of the ingredient composition. If it does it is preferred that this is done without destroying the agglomerates.
- the aggregates are 5 - 30 microns, preferably 5 - 10 microns. This give a desirable mouth feel to the product without the aggregates providing grittiness.
- the divalent cations are calcium cations.
- the divalent cations are added until the free divalent cations concentration is 3.5 - 6.5 mM divalent cations. It has been found that amounts that need to be added in dairy concentrate are 3 - 25 mM.
- the divalent cations are added in form of a calcium mineral salt, selected from the group consisting of calcium chloride, calcium lactate calcium gluconate or calcium phosphate.
- the calcium salt is calcium chloride.
- the calcium is obtained from concentrated minerals from milk after separation of the protein, fat and lactose by e.g. membrane fractionation.
- the pH of the ingredient composition is preferably 6.2 - 7.1 before adding the calcium cations.
- the content of soluble protein in the ingredient composition is preferable below or equal to 30% in relation to the total protein content indicating that the majority of the proteins are in the form of aggregates.
- the ingredient composition comprises from 0 - 50 wt. % fat, preferably 1.0 - 20 wt. %, more preferably 3.0 - 15 wt. %, most preferably 5 - 10 wt. % of fat. It has been found that even with low amounts of fat the texture of the product is still perceived as creamy due to the agglomeration created within the product.
- the caseins and whey protein in the ingredient composition are preferably provided in a form selected from the group consisting of raw milk, pasteurized milk, low heat concentrated milk, low heat milk powder, milk protein concentrate, milk protein isolate in liquid or powder format or a combination thereof while the additional whey proteins are provided in a form selected from the group consisting of sweet dairy whey, whey protein concentrates, whey protein isolates in liquid, concentrate or powder format or a combination thereof.
- the ingredient composition is a concentrate comprising 6 - 55, preferably 25 - 50 wt.% milk solids.
- the concentrate is dried into powder by means of freeze drying, spray drying or roller-drying.
- low pressure drying system refers to centrifugal wheel or pneumatic atomization systems which protects the structure of the casein-whey protein aggregates. It has been observed that high pressure atomizers such as hydraulic (high) pressure-nozzle atomization results in shearing effect thus destroying the casein-whey protein aggregates and thus its unique functionality. Such high pressure atomizers are useful for making conventional milk powders; however such a high-pressure system is not suitable for producing samples of the present invention. It has however been found that spray drying using low pressure drying system preserves the functionality of the product.
- the low pressure nozzles may operate below 100 bars, more preferred below 50 bars, preferably below 20 bars.
- Milk protein-based aggregates obtained by calcium chloride addition in heated full fat milk.
- Chilled pasteurised and microfiltered full fat milk (3.5 wt.% fat) was provided by Cremo S.A. (Le Mont-sur-Lausanne, Switzerland). It had an initial pH of 6.77 as measured at 25°C.
- For calcium addition a solution of CaCl2, 2(H20) (Merck, Darmstadt, Germany) was prepared at 200mM in MilliQ water. A volume 50 mL of milk were introduced in a Pyrex glass bottle of 50 ml (Schott Duran type, Germany) for each calcium chloride solution addition to cover a free calcium addition ranging from 1 - 16 mM. Magnetic stirring was performed 300 rpm and at room temperature 20-23°C.
- the capillary viscosity was determined using Rheotest LK 2.2 (Medingen GmbH, Dresden, Germany) and the particle size distribution (PSD) using Mastersizer 2000 (Malvern Intruments, UK).
- the direct visual appearance of the tubes was done to detect the first free calcium chloride concentration where protein aggregates were formed.
- Ionic (free) calcium concentration after heating was determined a Mettler Toledo calcium selective electrode perfection TM DX series half cells with BNC connector P/N 51344703 connected to a 692pH/Ion meter (Metrohm Switzerland).
- the size of protein-based aggregates reaches a maximum at about 6 mM CaCl2 and then decreased steadily while more calcium was present in the system.
- the viscosity of the system increases with the increase of the calcium chloride content.
- micellar caseins dispersion was prepared at a protein concentration of 10 wt.%.
- Micellar caseins concentrate Promilk852B (batch 13610656) was purchased from Ingredia (Arras, France).
- the powder composition was (g/100g wet powder): protein (Nx6.38) 82.3, Ca 2.6, Mg 0.1, Na 0.07, K 0.29, Cl 0.05, P 1.56.
- the mass of powder needed to prepare the dispersion was calculated as a function of the protein content in the powder.
- micellar casein powder was hydrated in MilliQ water for 3 hours under stirring at the room temperature. After 3 hours, the protein dispersion was homogenized with an EmulsiFlex C-5 high pressure, single-stage homogenizer (Avestin ® , Canada). This treatment decreased the average particle size of micellar caseins and the amount of non-sedimentable caseins ( ⁇ , ⁇ s1; and ⁇ s2) in serum increases, it allows to stabilize the solution and avoids the sedimentation of the MCI.
- the average particle diameter was determined after the homogenization using a Nanosizer ZS (Malvern Instruments ® , UK) and it was monodisperse and around 250 nm.
- O/W emulsions were prepared by the addition of high oleic sunflower oil (Oleificio Sabo, Manno, Switzerland) to the proteins dispersions so that total sample resulted in oil content of 2.5, 5 and 10 wt.% and a constant protein content of 3 wt.%.
- the mixtures were subsequently pre-homogenized using an Ultra-Turrax T25 basic (IKA ® , Switzerland) at 11,000 rpm/min during 1 minute for a volume of 500 mL.
- the pre-homogenized emulsions were after homogenized at High Pressure with a PandaPLUS HomoGenius 2000 (GEA ® , Germany) adjusted at 50 bars for the first valve and at 250 bars for the second one, to obtain a pressure total of 300 bars.
- GAA ® PandaPLUS HomoGenius 2000
- Emulsions were homogenized twice by this method. After homogenization, pH and concentration of CaCl2 were adjusted to defined target values. Samples with different pH were heated up at 95°C during 15 min in a hot water bath just after have been prepared and 1 hour after for different concentration of CaCl2. Emulsions were after cooled in iced-water during 20 min and stored at 4°C during 1 hour.
- the samples were afterward sheared at 16,000 rpm during 2 min using a Ultra-Turrax T25 basic (IKA ® , Switzerland) in a beaker for a volume of 60 mL, thirty circles were applied in order to have the same shearing for all the volume. Emulsions were after stored at 4°C until the analyses were done.
- IKA ® Ultra-Turrax T25 basic
- dispersions and emulsions were analyzed after shearing by dynamic light scattering using a MasterSizer 3000 (Malvern Instruments Ltd ® , UK).
- the emulsion sample was dispersed in the Hydro SM measuring cell until an obscuration rate of 9-10% was obtained. Non-heated and heated samples were analyzed. Measures were performed three times and the average of the three replications was reported.
- Cryogenic cuts were done in order to analyze samples by CLSM. To this aim, sucrose and formaldehyde were added at the samples in order to conserve them (PRICE and JEROME, 2011). Percentage are for the sucrose 30 wt.% of the total volume and 3.7 wt.% for the formaldehyde. Samples were homogenized using a vortex and stored overnight at 4°C before beginning analyses.
- the cryostat sample holder was immersed in a plastic vial containing 80 mL of 2-Methylbutane (99% from Sigma Aldrich ® , US), itself immersed in Sagex box of nitrogen liquid.
- 2-Methylbutane 99% from Sigma Aldrich ® , US
- the solution of 2-Methylbutane ensures a good freezing of the sample and protects it from the drying.
- Microscope slides were previously treated with HistoGrip (50 ⁇ concentrate from ThermoFisher Scientific ® , US) for adhering tissue to glass slides and avoid to remove tissues during harsh processes.
- HistoGrip 50 ⁇ concentrate from ThermoFisher Scientific ® , US
- Nile Red is an excellent dye for the detection of intracellular lipid droplets by fluorescence microscopy, it is highly hydrophobic and fluorescent. 25 mg of Nile Red was solubilized in 100 mL of ethanol. The excitation wavelength was achieved using the 488 nm emission from the diode laser and the emitted light was collected between 488 nm and 630 nm.
- Fast Green is an organic dye, electrostatically attracted to charged groups on proteins (MERRIL and WASHART, 1998). It can bind non-covalently to the biopolymer of interest by electrostatic interactions (AUTY, 2013).
- the excitation wavelength was set using the 633 nm emission from the diode laser and the emitted light was collected between 633 nm and 740 nm.
- the Fast Green used was at 1 wt.% in water.
- Microscope slides were after analyzed using a Zeiss ® LSM 710 Confocal Scanning Microscope (Zeiss ® , Germany).
- the CLSM is equipped with lasers allowing the excitations of several fluorescent probes at the same time, this capability allows multi-imaging of a sample by selecting the correct excitation wavelength and filters to collect the emission light from a particular dye.
- emulsions were centrifuged at 16,000 g at room temperature for 20 min using an Eppendorf ® centrifuge 5418 (Vaudaux-Eppendorf AG ® , Switzerland), one day after production. Supernatant was carefully withdrawn and stored at 4°C in order to be analyzed by Reverse Phase-Ultra Performance Liquid Chromatography (RP-UPLC).
- RP-UPLC Reverse Phase-Ultra Performance Liquid Chromatography
- the UPLC system (Waters Corp Milford Ma, USA) consisted of a binary pump, a temperature controlled auto-sampler (sample manager-UPSMPM6R) and a photodiode array detector (UPPDA-E). The equipment was controlled by the Empower ® 3 software, Pro version.
- Solvent A consisted of 0.1% TFA in water and solvent B was 0.1% TFA in acetonitrile/water (90/10) (v: v). Separations were performed with a linear gradient from 15 - 35 % B in 4 min (5% B.min-1), 35 - 47% B in 24 min (0.5% B.min-1) and from 47% B - 80% B in 4 min (8.25 % B.min-1). This was followed by an isocratic elution at 80% B during 1 min. Then returned linearly to the starting condition in 2 min, followed by the rebalance of the column for 5 min.
- the flow rate was 0.6 mL.min-1 and the column temperature was kept constant at 40 ⁇ 1°C.
- Figure 2 shows that upon heat treatment and shearing, the size distribution of the emulsions at pH 7.0 exhibit a peak around 400-600 nm for the 3 sunflower oil content tested. On the contrary, larger particles are formed when the heat treatment in achieved in presence of 5 mM added fee calcium. Hence, there is a clear shift of the size distribution to around 15-25 microns, indicating that the initial oil droplets had aggregated into larger protein based particles.
- the emulsion produced at pH 7.0 exhibited a Newtonian flow behaviour with an independence of the viscosity as a function of shear rate. This is explained by the fact that viscosity is mainly driven by the oil volume fraction and that the oil droplets are not interacting. In the sample of the present invention containing 5 mM calcium, the flow behaviour is shear thinning, which is an indication that shear sensitive particles have been produced, affecting the overall flow behaviour.
- the sample viscosity is compared for the 3 sunflower oil contents tested at a shear rate of 10 s-1 which is relevant for in-mouth conditions (see Figure 6 ). It can be seen that at pH 7.0, the viscosity slightly increases with increasing the oil content.
- the viscosity was about 10 to 100 times larger than the corresponding sample at pH 7.0. This clearly indicates that the particles of the present invention enables to build viscosity at a much lower oil content, enabling fat lowering in food products, see Figure 5 .
- a set of 2 samples were produced according to the following procedure, involving: concentration of a commercial whole milk to 35% total solids (TS) content, adding a variable amount CaCl2 (5 and 10 mM) in the milk concentrate, standardized heat processing including a direct steam injection step, and spray drying to obtain a functionalized milk powder.
- the concentration process is done in recirculating batch mode, starting with milk at 4°C.
- the milk is pumped with a progressing cavity pump, from a buffer tank through a plate heat exchanger set to 40°C outlet temperature and the Centritherm ® CT1-09 evaporator, back into the buffer tank.
- the milk in the buffer tank thereby gradually increases in solid concentration and temperature.
- a critical concentration threshold is reached, the milk is brought to the desired total solids content by a final evaporator pass without remixing, and collected in a separate holding tank.
- the milk concentrate is cooled to 10°C and the required amount of CaCl2, 2H2O powder (Merck, Darmstadt, Germany) was added, under agitation, to the milk.
- the typical timeframe for calcium powder addition to a 40 kg batch is about 15 minutes.
- the cooled, calcium loaded milk concentrate was heat-processed in semi-continuous mode on a commercially available OMVE HT320-20 DSI SSHE pilot plant line (OMVE Netherlands B.V., NL). Processing steps are: preheating in the OMVE tubular heat exchanger to 60°C, direct steam injection to 95°C outlet temperature, 300 sec hot holding period at 95°C in the two scraped surface heat exchangers of the OMVE line, connected in series and running at maximum rpm, and subsequent cooling to about 23°C product outlet temperature the OMVE tubular heat exchanger cooled with ice water.
- the flow rate is set to 14 l/h to obtain a sum of approximately 300 sec residence time in the scraped surface heat exchanger units. Residence time in the OMVE cooler is about 2 minutes. The residence times are averages from volumetric flow rates and dead volume of line elements (tubular heat exchanger, scraped surface heat exchanger).
- Clogging of the DSI injector is a critical phenomenon, and the line must be carefully controlled in this respect. No flash evaporation is applied and condensing steam remains entirely in the product.
- the heat-processed milk concentrate with 5 mM calcium added was spray-dried on a Niro SD 6.3 pilot plant spray tower (GEA NIRO Process Engineering, DK), equipped with a FS1 rotary atomizer. Operating parameters are: Product feed rate 10 - 20 kg/h, product inlet temperature in the rotary atomizer 25 - 30°C, rotary atomizer speed 25000 rpm, airflow 350 - 400 kg/h (mass flow control), air inlet temperature 160°C, exhaust air temperature 80°C and exhaust air relative humidity 15%.
- the finished powder product is packed immediately in air-tight bags and has a residual humidity below 4 %.
- sample was reconstituted to 13 or 50% TS before measurements.
- Distilled water was poured into a beaker and heated up to 42 °C - 44 °C with a water bath.
- a volume of 150 mL distilled water at 42 °C - 44 °C was measured and transferred into a glass beaker using a volumetric cylinder.
- An amount of 22.5 g milk powder is added to the 150 ml distilled water at 42 °C and mixed with a spoon for 30 s.
- Table 2 Mean average diameters D 43 and D 32 and viscosity at a shear rate of 13 s -1 measured at 25°C for double concentrated milk (25% TS) before and after heat treatment in presence of CaCl2 at 95°C for 300 s.
- the slight reduction in particle size might be due to the shearing effect occurring during the spray drying of the product.
- the soluble protein content obtained after reconstitution of the powder at 13% TS was 7% of the total proteins, indicating that the majority of the milk proteins were involved in the aggregate structure.
- the microstructure of the particles can be seen on Figure 8 A and B . Aggregates were rather compacts and were composed of proteins and fat droplets with no sign of non-reacting proteins which is confirming the low amount of soluble proteins. Higher magnification of the particles on Figure 8B shows well embedded fat droplets with an average size of 1-2 microns embedded in a dense protein matrix. There is little sign of fat droplet coalescence indicating that aggregate formation arose from a flocculation mechanism.
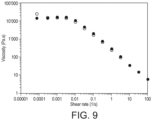
- the milk spray dried powder according to the present invention was reconstituted to 50% TS which is generally the TS at which full fat milk is spray dried. It can be seen of figure 9 that the flow behavior is strongly shear thinning, exhibiting a steep negative slope and a high low shear viscosity. This is a sign that the product upon reconstitution had built some structure and that protein aggregates were able to interact between each other. Surprisingly, the structure could be recovered upon releasing the stress on the sample as the up and down curves were almost superimposed.
- the milk concentrate is then spray dried on a Nestlé 3.5 m Egron (self-construction) by a two-phase nozzle system (1.8 mm nozzle) to maximal moisture content of 3% and packed into air tight bags.
- Conditions of spray drying were: product flow of 413 kg/h at 37°C product temperature, hot air inlet temperature of 270°C and an air flow of 4664 kg/h, outlet air temperature of 88°C.
- the milk concentrate is then spray dried on a NIRO SD6 3N spray dryer by a rotary disc nozzle system at 17,000 rpm to maximal moisture content of 3% and packed into air tight bags.
- Conditions of spray drying were: product flow of 20 L/h at 40°C product temperature, hot air inlet temperature of 160°C and an air flow of 360 m 3 /h, outlet air temperature of 80°C.
- Powdered samples were reconstituted before measurements. Distilled water was poured into a beaker and heated up to 42 °C - 44 °C with a water bath. A volume of 150 mL distilled water at 42 °C - 44 °C was measured and transferred into a glass beaker using a volumetric cylinder. An amount of 22.5 g milk powder is added to the 150 ml distilled water at 42 °C and mixed with a spoon for 30 s.
- Measurement settings used are a refractive index of 1.46 for fat droplets and 1.33 for water at an absorption of 0.01. All samples were measured at an obscuration rate of 2.0 - 2.5%.
- the two milk powders were reconstituted to 50% TS and their flow properties were compared.
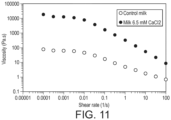
- Control full fat milk that was spray dried at 50%TS exhibited a shear thinning behavior and a low shear viscosity plateau about 100 Pa.s (see Figure 11 ).
- the milk from the present invention when reconstituted at 50% TS as well exhibited a shear thinning profile, but the low shear viscosity was 100 times larger and the shear thinning region had a much stronger slope. This is a sign of highly structured sample as well as the proof of interaction between the protein aggregates. It shows also that the present invention is clearly able to generate higher viscosity at equivalent fat content and has therefore potential for fat reduction in food products.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Biochemistry (AREA)
- Nutrition Science (AREA)
- Health & Medical Sciences (AREA)
- Zoology (AREA)
- Inorganic Chemistry (AREA)
- Dairy Products (AREA)
- Seeds, Soups, And Other Foods (AREA)
- Non-Alcoholic Beverages (AREA)
- Confectionery (AREA)
- Tea And Coffee (AREA)
Claims (10)
- Procédé de production d'un concentré laitier, comprenant les étapes consistant à :fournir une composition d'ingrédients comprenant des caséines micellaires et des protéines de lactosérum et ayant un pH de 6,1 à 7,1 et une concentration de 3 à 25 % en poids de protéines, et dans lequel la composition d'ingrédients a un rapport de la caséine à la protéine de lactosérum de 90/10 à 60/40,ajouter 3 à 25 mM de cations divalents pour fournir une concentration de 3 à 8 mM de cations divalents libres dans la composition d'ingrédients,homogénéiser la composition d'ingrédients ; et ensuitepasteuriser et agiter la composition d'ingrédients à une température de 80 ° à 105 °C pendant un laps de temps de 0,5 à 3 min pour former des protéines agglomérées comprenant des caséines et de la bêta-lactoglobuline provenant des protéines de lactosérum, les agglomérats ayant une taille de 3 à 50 micromètres telle que mesurée par le diamètre moyen D(4,3), et dans lequel la composition d'ingrédients est un concentré qui comprend 6 à 55 % en poids de solides de lait, et dans lequel les cations divalents sont Ca.
- Procédé selon la revendication 1, dans lequel les agrégats sont de 5 à 30 micromètres, de préférence de 5 à 10 micromètres.
- Procédé selon l'une quelconque des revendications précédentes, dans lequel des cations divalents sont ajoutés jusqu'à ce que la concentration libre en cations divalents soit de 3,5 à 6,5 mM de cations divalents.
- Procédé selon l'une quelconque des revendications précédentes, dans lequel les cations divalents sont ajoutés sous forme d'un sel minéral.
- Procédé selon l'une quelconque des revendications 1 à 3, dans lequel le sel de calcium est choisi dans le groupe constitué par chlorure de calcium, lactate de calcium, gluconate de calcium et phosphate de calcium.
- Procédé selon l'une quelconque des revendications précédentes, dans lequel le pH de la composition d'ingrédients va de 6,2 à 7,1 avant ajout des cations calcium.
- Procédé selon l'une quelconque des revendications précédentes, dans lequel la teneur en protéine soluble dans la composition d'ingrédients est inférieure ou égale à 30 % par rapport à la teneur totale en protéines.
- Procédé selon l'une quelconque des revendications précédentes, dans lequel la composition d'ingrédients comprend de 0 à 50 % en poids de matière grasse, de préférence 1,0 à 20 % en poids, plus préférablement 3,0 à 15 % en poids, le plus préférablement 5 à 10 % en poids de matière grasse.
- Procédé selon l'une quelconque des revendications précédentes, dans lequel les caséines et protéines de lactosérum dans la composition d'ingrédients sont fournies sous une forme choisie dans le groupe constitué par lait cru, lait pasteurisé, lait concentré à basse température, lait en poudre à basse température, concentré de protéines laitières, isolat de protéines laitières sous une forme liquide ou en poudre ou une combinaison de ceux-ci alors que les protéines de lactosérum supplémentaires sont fournies sous une forme choisie dans le groupe constitué par lactosérum laitier sucré, concentrés de protéines de lactosérum, isolats de protéines de lactosérum sous forme de liquide, de concentré ou de poudre ou une combinaison de ceux-ci.
- Procédé selon les revendications précédentes, dans lequel le concentré est séché en une poudre au moyen d'une lyophilisation, d'un séchage par atomisation ou d'un séchage au rouleau.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16205142 | 2016-12-19 | ||
| PCT/EP2017/083353 WO2018114826A1 (fr) | 2016-12-19 | 2017-12-18 | Procédé de production de concentré laitier avec agrégation protéique par cations divalents libres |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3554250A1 EP3554250A1 (fr) | 2019-10-23 |
| EP3554250B1 true EP3554250B1 (fr) | 2024-10-30 |
Family
ID=57680076
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP17835654.9A Active EP3554250B1 (fr) | 2016-12-19 | 2017-12-18 | Procédé de production d'un produit laitier concentré avec agrégation bivalente libre des cations de protéine |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US11089791B2 (fr) |
| EP (1) | EP3554250B1 (fr) |
| JP (2) | JP7004718B2 (fr) |
| CN (1) | CN109963471B (fr) |
| AU (1) | AU2017384154B2 (fr) |
| BR (1) | BR112019010190A2 (fr) |
| CA (1) | CA3044138C (fr) |
| CL (1) | CL2019001379A1 (fr) |
| ES (1) | ES3007685T3 (fr) |
| MX (1) | MX2019005950A (fr) |
| MY (1) | MY192818A (fr) |
| NZ (1) | NZ753148A (fr) |
| RU (1) | RU2761483C2 (fr) |
| WO (1) | WO2018114826A1 (fr) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3554248B1 (fr) | 2016-12-19 | 2024-11-06 | Société des Produits Nestlé S.A. | Boisson à agrégation protéique avec cations divalents libres et son procédé de production |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MY192818A (en) | 2016-12-19 | 2022-09-12 | Nestle Sa | A method of producing a dairy concentrate with free divalent cations protein aggregation |
| JP6990243B2 (ja) * | 2016-12-19 | 2022-01-12 | ソシエテ・デ・プロデュイ・ネスレ・エス・アー | 遊離二価カチオンタンパク質凝集体を有する食品又は飲料製品の製造方法 |
| MX2019014444A (es) | 2017-06-01 | 2020-01-27 | Société des Produits Nestlé SA | Un metodo para producir un producto alimenticio o de bebiba con agregacion de proteinas vegetales y de la leche de cationes divalentes libres. |
| CN120882315A (zh) * | 2023-03-31 | 2025-10-31 | 雀巢产品有限公司 | 用于改善低脂乳制奶油口感的受控热诱导蛋白质聚集 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5350590A (en) | 1992-12-15 | 1994-09-27 | Beatreme Foods Inc. | Protein fat replacer and method of manufacture thereof |
| US5855936A (en) | 1997-03-21 | 1999-01-05 | Nestec S.A. | Food fortification |
| WO2016102500A1 (fr) | 2014-12-22 | 2016-06-30 | Nestec S.A. | Concentrés de lait présentant une meilleure sensation en bouche |
| WO2016102503A1 (fr) | 2014-12-22 | 2016-06-30 | Nestec S.A. | Boissons lactées prêtes à boire présentant une texture/sensation en bouche améliorées par agrégation contrôlée de protéines, et son procédé de fabrication |
| WO2017021428A1 (fr) | 2015-08-06 | 2017-02-09 | Nestec S.A. | Boissons prêtes à boire ayant une texture améliorée par une agrégation contrôlée des protéines |
| WO2018114826A1 (fr) | 2016-12-19 | 2018-06-28 | Nestec S.A. | Procédé de production de concentré laitier avec agrégation protéique par cations divalents libres |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006065135A2 (fr) | 2004-12-15 | 2006-06-22 | Csm Nederland B.V. | Proteines modifiees avec des proprietes alterees d'agregation |
| JP4852684B2 (ja) | 2005-09-30 | 2012-01-11 | 雪印メグミルク株式会社 | 乳由来複合脂質高含有粉末 |
| ES2373400T3 (es) * | 2006-03-27 | 2012-02-03 | Nestec S.A. | Micelas de proteína láctea. |
| BRPI1008792B1 (pt) * | 2009-02-13 | 2017-11-14 | Nestec S.A. | Method for producing a frozen aerated confectionery product |
| US20130011515A1 (en) | 2009-10-19 | 2013-01-10 | Cytosport, Inc. | Milk protein concentrates |
| CN103052324B (zh) * | 2010-08-05 | 2015-02-25 | 雀巢产品技术援助有限公司 | 具有改善质构的冷冻甜食产品 |
| PH12013500217A1 (en) * | 2010-08-05 | 2017-08-23 | Nestec Sa | Milk protein containing liquid beverage products |
| US20120164277A1 (en) * | 2010-12-22 | 2012-06-28 | Starbucks Corporation D/B/A Starbucks Coffee Company | Dairy containing beverages with enhanced flavors and textures and methods of making same |
| CN104489097B (zh) | 2014-11-25 | 2018-01-23 | 南通东概念新材料有限公司 | 活性乳酸菌或益生菌的热和对流干燥保护剂及其制备方法 |
| WO2016102501A1 (fr) * | 2014-12-22 | 2016-06-30 | Nestec S.A. | Poudre de lait à sensation en bouche améliorée |
| US20190327995A1 (en) * | 2015-08-24 | 2019-10-31 | Arla Foods Amba | Stabiliser-free cottage cheese, a thickened dairy liquid suitable for its production, and related methods |
| US20190069589A1 (en) * | 2015-10-01 | 2019-03-07 | Frieslandcampina Nederland B.V. | Liquid nutritional composition comprising micellar casein and hydrolysed whey protein |
-
2017
- 2017-12-18 MY MYPI2019002598A patent/MY192818A/en unknown
- 2017-12-18 BR BR112019010190-0A patent/BR112019010190A2/pt not_active Application Discontinuation
- 2017-12-18 WO PCT/EP2017/083353 patent/WO2018114826A1/fr not_active Ceased
- 2017-12-18 JP JP2019527191A patent/JP7004718B2/ja active Active
- 2017-12-18 EP EP17835654.9A patent/EP3554250B1/fr active Active
- 2017-12-18 CN CN201780071822.0A patent/CN109963471B/zh active Active
- 2017-12-18 MX MX2019005950A patent/MX2019005950A/es unknown
- 2017-12-18 AU AU2017384154A patent/AU2017384154B2/en active Active
- 2017-12-18 US US16/462,448 patent/US11089791B2/en active Active
- 2017-12-18 CA CA3044138A patent/CA3044138C/fr active Active
- 2017-12-18 RU RU2019115969A patent/RU2761483C2/ru active
- 2017-12-18 NZ NZ753148A patent/NZ753148A/en unknown
- 2017-12-18 ES ES17835654T patent/ES3007685T3/es active Active
-
2019
- 2019-05-22 CL CL2019001379A patent/CL2019001379A1/es unknown
-
2022
- 2022-01-04 JP JP2022000050A patent/JP7349515B2/ja active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5350590A (en) | 1992-12-15 | 1994-09-27 | Beatreme Foods Inc. | Protein fat replacer and method of manufacture thereof |
| US5855936A (en) | 1997-03-21 | 1999-01-05 | Nestec S.A. | Food fortification |
| WO2016102500A1 (fr) | 2014-12-22 | 2016-06-30 | Nestec S.A. | Concentrés de lait présentant une meilleure sensation en bouche |
| WO2016102503A1 (fr) | 2014-12-22 | 2016-06-30 | Nestec S.A. | Boissons lactées prêtes à boire présentant une texture/sensation en bouche améliorées par agrégation contrôlée de protéines, et son procédé de fabrication |
| WO2017021428A1 (fr) | 2015-08-06 | 2017-02-09 | Nestec S.A. | Boissons prêtes à boire ayant une texture améliorée par une agrégation contrôlée des protéines |
| WO2018114826A1 (fr) | 2016-12-19 | 2018-06-28 | Nestec S.A. | Procédé de production de concentré laitier avec agrégation protéique par cations divalents libres |
Non-Patent Citations (17)
| Title |
|---|
| A. V. DOMINGUEZ,: "Heat induced gelation of micellar casein with and without whey proteins in the presence of polyphosphate", INTERNATIONAL DAIRY JOURNAL, vol. 104, 2020, XP086079649, DOI: 10.1016/j.idairyj.2020.104640 |
| ANONYMOUS: "Mastersizer 3000 Customer Training Course - Part 1: Basic Principles and Data Quality", MALVERN PANALYTICAL, 1 January 2017 (2017-01-01), XP093303531, Retrieved from the Internet <URL:https://www.malvernpanalytical.com/en/assets/malvern%20panalytical%20-%20mastersizer%203000%20training%20materials_tcm50-97739.pdf> |
| ANONYMOUS: "Micellar Casein Concentrate", THINK USA DAIRY, 8 June 2015 (2015-06-08), pages 1 - 2, XP055713537, Retrieved from the Internet <URL:https://www.thinkusadairy.org/resources-and-insights/resources-and-insights/product-resources/micellar-casein-concentrate> [retrieved on 20200710] |
| E. D. OMOARUKHE ET AL.: "Effects of different calcium salts on properties of milk related to heat stability", INTERNATIONAL JOURNAL OF DAIRY TECHNOLOGY, vol. 63, no. 4, November 2010 (2010-11-01), pages 504 - 511, XP072056338, DOI: 10.1111/j.1471-0307.2010.00613.x |
| GAUCHERON F: "The minerals of milk.", REPRODUCTION, NUTRITION, DEVELOPMENT., PARIS., FR, vol. 45, no. 4, 1 July 2005 (2005-07-01), FR , pages 473 - 483, XP002411682 |
| HILTON C DEETH, MICHAEL J LEWIS: "Practical consequences of calcium addition to and removal from milk and milk products", INTERNATIONAL JOURNAL OF DAIRY TECHNOLOGY, SOCIETY OF DAIRY TECHNOLOGY, HUNTINGDON,, GB, vol. 68, no. 1, 1 February 2015 (2015-02-01), GB , pages 1 - 10, XP055609304, ISSN: 1364-727X, DOI: 10.1111/1471-0307.12188 |
| J.V.V. SILVA ET AL.: "Heat-induced gelation of mixtures of micellar caseins and plant proteins in aqueous solution", FOOD RESEARCH INTERNATIONAL, vol. 116, 2019, pages 1135 - 143, XP085593753, DOI: 10.1016/j.foodres.2018.09.058 |
| JONES ALICIA NOELLE: "Density of Milk - An educational, fair use website", THE PHYSICS FACTBOOK - AN ENCYCLOPEDIA OF SCIENTIFIC ESSAYS, 1 January 2002 (2002-01-01), XP093303533, Retrieved from the Internet <URL:https://hypertextbook.com/facts/2002/AliciaNoelleJones.shtml> |
| KATJA SIEVANEN; THOM HUPPERTZ; ALAN L KELLY; PATRICK F FOX: "Influence of added calcium chloride on the heat stability of unconcentrated and concentrated bovine milk", INTERNATIONAL JOURNAL OF DAIRY TECHNOLOGY, SOCIETY OF DAIRY TECHNOLOGY, HUNTINGDON,, GB, vol. 61, no. 2, 7 April 2008 (2008-04-07), GB , pages 151 - 155, XP072056164, ISSN: 1364-727X, DOI: 10.1111/j.1471-0307.2008.00391.x |
| L. RAMASUBRAMANIAN ET AL.: "The rheological properties of calcium-induced milk gels", JOURNAL OF FOOD ENGINEERING, vol. 130, 2014, XP055395360, DOI: 10.1016/j.jfoodeng.2014.01.020 |
| MCKINNON, I.R. ; YAP, S.E. ; AUGUSTIN, M.A. ; HEMAR, Y.: "Diffusing-wave spectroscopy investigation of heated reconstituted skim milks containing calcium chloride", FOOD HYDROCOLLOIDS, ELSEVIER BV, NL, vol. 23, no. 4, 1 June 2009 (2009-06-01), NL , pages 1127 - 1133, XP025817627, ISSN: 0268-005X, DOI: 10.1016/j.foodhyd.2008.08.009 |
| MICHAEL J LEWIS: "The measurement and significance of ionic calcium in milk - A review", INTERNATIONAL JOURNAL OF DAIRY TECHNOLOGY, SOCIETY OF DAIRY TECHNOLOGY, HUNTINGDON,, GB, vol. 64, no. 1, 1 February 2011 (2011-02-01), GB , pages 1 - 13, XP055610583, ISSN: 1364-727X, DOI: 10.1111/j.1471-0307.2010.00639.x |
| MIKE BOLAND, HARJINDER SINGH, AND ABBY THOMPSON: "Milk proteins: From expression to Food", 1 January 2009, ELSEVIER , USA, ISBN: 978-0-12-374039-7, article THOMPSON ABBY, MIKE BOLAND, HARJINDER SINGH: "Interactions between denatured whey proteins and κ -CN/casein micelles", pages: 249 - 250, XP093303524 |
| NGUYEN BACH T., BALAKRISHNAN GIREESHKUMAR, JACQUETTE BORIS, NICOLAI TACO, CHASSENIEUX CHRISTOPHE, SCHMITT CHRISTOPHE, BOVETTO LION: "Inhibition and Promotion of Heat-Induced Gelation of Whey Proteins in the Presence of Calcium by Addition of Sodium Caseinate", BIOMACROMOLECULES, AMERICAN CHEMICAL SOCIETY, US, vol. 17, no. 11, 14 November 2016 (2016-11-14), US , pages 3800 - 3807, XP093303529, ISSN: 1525-7797, DOI: 10.1021/acs.biomac.6b01322 |
| ON-NOM N., GRANDISON A.S., LEWIS M.J.: "Heat stability of milk supplemented with calcium chloride", JOURNAL OF DAIRY SCIENCE, ELSEVIER, AMSTERDAM, NL, vol. 95, no. 4, 1 April 2012 (2012-04-01), AMSTERDAM, NL, pages 1623 - 1631, XP093303527, ISSN: 0022-0302, DOI: 10.3168/jds.2011-4697 |
| SIAMAND RAHELA, DEETH HILTON C., AL-SAADI JASIM M.S.: "Textural and sensory properties of a calcium-induced milk gel", JOURNAL OF FOOD ENGINEERING, ELSEVIER, AMSTERDAM, NL, vol. 139, 1 October 2014 (2014-10-01), AMSTERDAM, NL, pages 10 - 12, XP093303523, ISSN: 0260-8774, DOI: 10.1016/j.jfoodeng.2014.04.014 |
| SILANIKOVE NISSIM, SHAPIRO FIRA, SHAMAY AVI: "Use of an ion-selective electrode to determine free Ca ion concentration in the milk of various mammals", JOURNAL OF DAIRY RESEARCH, CAMBRIDGE UNIVERSITY PRESS., CAMBRIDGE, UK, vol. 70, no. 2, 1 May 2003 (2003-05-01), Cambridge, UK, pages 241 - 243, XP009562448, ISSN: 0022-0299, DOI: 10.1017/S0022029903006083 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3554248B1 (fr) | 2016-12-19 | 2024-11-06 | Société des Produits Nestlé S.A. | Boisson à agrégation protéique avec cations divalents libres et son procédé de production |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2020501528A (ja) | 2020-01-23 |
| ES3007685T3 (en) | 2025-03-20 |
| CA3044138C (fr) | 2024-01-16 |
| EP3554250A1 (fr) | 2019-10-23 |
| RU2019115969A (ru) | 2020-11-23 |
| US11089791B2 (en) | 2021-08-17 |
| RU2019115969A3 (fr) | 2021-03-15 |
| RU2761483C2 (ru) | 2021-12-08 |
| MX2019005950A (es) | 2019-10-09 |
| AU2017384154B2 (en) | 2022-06-16 |
| CN109963471B (zh) | 2022-12-30 |
| CL2019001379A1 (es) | 2019-08-30 |
| CA3044138A1 (fr) | 2018-06-28 |
| US20190373907A1 (en) | 2019-12-12 |
| MY192818A (en) | 2022-09-12 |
| NZ753148A (en) | 2023-01-27 |
| BR112019010190A2 (pt) | 2021-02-17 |
| AU2017384154A1 (en) | 2019-05-23 |
| WO2018114826A1 (fr) | 2018-06-28 |
| JP2022061990A (ja) | 2022-04-19 |
| JP7349515B2 (ja) | 2023-09-22 |
| CN109963471A (zh) | 2019-07-02 |
| JP7004718B2 (ja) | 2022-01-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2022202636B2 (en) | A method of producing a food or beverage product with free divalent cations protein aggregation | |
| EP3554250B1 (fr) | Procédé de production d'un produit laitier concentré avec agrégation bivalente libre des cations de protéine | |
| AU2018277283B2 (en) | A method of producing a food or beverage product with free divalent cations dairy and plant protein aggregation | |
| MX2008012334A (es) | Vehiculo de proteina de suero para suministro de agente activo. | |
| RU2799524C2 (ru) | Способ получения пищевого продукта или напитка с использованием агрегации молочного или растительного белка в присутствии свободных двухвалентных катионов | |
| BR112019025180B1 (pt) | Produto alimentício ou de bebida com agregação de laticínios e proteína vegetal com cátions divalentes livres, e seu método de produção |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: UNKNOWN |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20190719 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20200407 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230527 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A23J 3/08 20060101ALI20240528BHEP Ipc: A23J 1/20 20060101ALI20240528BHEP Ipc: A23G 3/34 20060101ALI20240528BHEP Ipc: A23G 1/56 20060101ALI20240528BHEP Ipc: A23C 9/156 20060101ALI20240528BHEP Ipc: A23C 9/154 20060101AFI20240528BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20240611 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602017085812 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20241030 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 3007685 Country of ref document: ES Kind code of ref document: T3 Effective date: 20250320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250228 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250228 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20250128 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1735988 Country of ref document: AT Kind code of ref document: T Effective date: 20241030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20250228 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250131 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20250225 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20250129 Year of fee payment: 8 Ref country code: GB Payment date: 20250130 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R026 Ref document number: 602017085812 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20241218 |
|
| 26 | Opposition filed |
Opponent name: FRESENIUS KABI DEUTSCHLAND GMBH Effective date: 20250728 Opponent name: COMPAGNIE GERVAIS DANONE Effective date: 20250730 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241030 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20241231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20241231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20241231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20241218 |