EP3436552B1 - Verwendung von triarylbenzenderivaten als tracer zur markierung von flüssigen kraftstoffen und motorkraftstoffen, flüssige kraftstoffe und motorkraftstoffe mit solchen derivaten und entsprechende prozesse - Google Patents
Verwendung von triarylbenzenderivaten als tracer zur markierung von flüssigen kraftstoffen und motorkraftstoffen, flüssige kraftstoffe und motorkraftstoffe mit solchen derivaten und entsprechende prozesse Download PDFInfo
- Publication number
- EP3436552B1 EP3436552B1 EP17717209.5A EP17717209A EP3436552B1 EP 3436552 B1 EP3436552 B1 EP 3436552B1 EP 17717209 A EP17717209 A EP 17717209A EP 3436552 B1 EP3436552 B1 EP 3436552B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fuels
- halogen
- compound
- formula
- fact
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- PAIQXYSZUMLJGD-UHFFFAOYSA-N CCCCCCCCCCCCOc(c(-c1cccc(C(F)(F)F)c1)cc(-c1cccc(C(F)(F)F)c1)c1)c1-c1cc(C(F)(F)F)ccc1 Chemical compound CCCCCCCCCCCCOc(c(-c1cccc(C(F)(F)F)c1)cc(-c1cccc(C(F)(F)F)c1)c1)c1-c1cc(C(F)(F)F)ccc1 PAIQXYSZUMLJGD-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/003—Marking, e.g. coloration by addition of pigments
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/16—Hydrocarbons
- C10L1/1608—Well defined compounds, e.g. hexane, benzene
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/185—Ethers; Acetals; Ketals; Aldehydes; Ketones
- C10L1/1852—Ethers; Acetals; Ketals; Orthoesters
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/20—Organic compounds containing halogen
- C10L1/202—Organic compounds containing halogen aromatic bond
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/20—Organic compounds containing halogen
- C10L1/203—Organic compounds containing halogen hydroxyl compounds; ethers, acetals, ketals
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/04—Use of additives to fuels or fires for particular purposes for minimising corrosion or incrustation
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2230/00—Function and purpose of a components of a fuel or the composition as a whole
- C10L2230/16—Tracers which serve to track or identify the fuel component or fuel composition
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2270/00—Specifically adapted fuels
- C10L2270/02—Specifically adapted fuels for internal combustion engines
- C10L2270/023—Specifically adapted fuels for internal combustion engines for gasoline engines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2270/00—Specifically adapted fuels
- C10L2270/02—Specifically adapted fuels for internal combustion engines
- C10L2270/026—Specifically adapted fuels for internal combustion engines for diesel engines, e.g. automobiles, stationary, marine
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2270/00—Specifically adapted fuels
- C10L2270/04—Specifically adapted fuels for turbines, planes, power generation
Definitions
- the present invention relates to the use of triarylbenzene derivatives as labeling tracers for fuels and liquid fuels; fuels and liquid fuels containing such derivatives; a process for marking fuels and liquid fuels with such derivatives; and a method of determining whether fuels or liquid fuels initially containing these tracers have been tampered with, used illegally or stolen.
- Fraud concerning fuels or liquid fuels covers various forms of activity: falsification by laundering or cutting, illegal use, smuggling and theft.
- Falsification by laundering concerns the transformation of a fuel or liquid fuel with a low tax rate into fuel or liquid fuel with a higher tax rate, for example - in France - the transformation of domestic fuel oil into diesel.
- Falsification by cutting concerns the dilution of a fuel or liquid fuel with a high tax rate by a fuel or liquid fuel at a low tax rate.
- Illegal use refers to the illegal use of a fuel or liquid fuel with a low tax rate instead of a fuel or liquid fuel with a higher tax rate.
- Smuggling is the purchase of fuel or liquid fuel at low tax rates in one country for resale in another country where taxes are higher.
- WO2012 / 153132 A1 describes aromatic organic markers substituted by at least one bromine and / or one fluorine atom, and / or one or more fluoroalkyl groups.
- the combustion of fuels labeled with such compounds can produce hydrogen bromide and / or hydrogen fluoride in the flue gases.
- Hydrogen bromide and fluoride are known to be particularly corrosive to the engine.
- Hydrogen fluoride is well known to be highly toxic to people and the environment.
- WO2014 / 008164 describes aryl and trityl alkyl ethers as tracers for labeling hydrocarbons. Such tracers appear not to exhibit good resistance to the abovementioned elimination methods.
- triarylbenzenes are particularly effective for the labeling of fuels and liquid fuels.
- these triarylbenzenes are particularly resistant to bleaching attempts. chemical and physical.
- most of these triarylbenzenes contain only carbon and hydrogen and the combustion of these compounds used as markers produces only carbon dioxide and water.
- any atom can be replaced by one of its isotopes.
- the benzene ring A is unsubstituted or substituted by C 1 -C 18 alkoxy.
- a halogen falling within the definition of Formula I can be F, Cl, Br and I, and in particular F, Cl and Br.
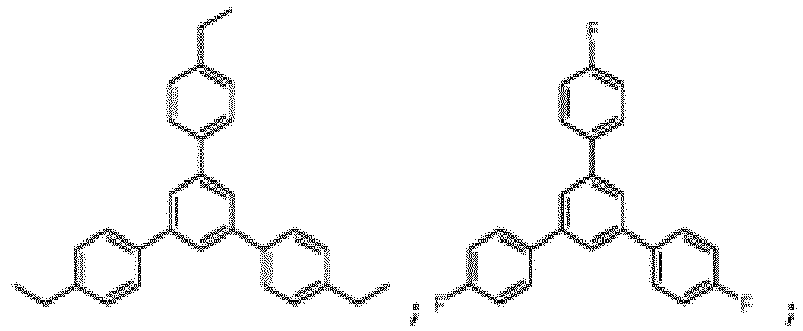
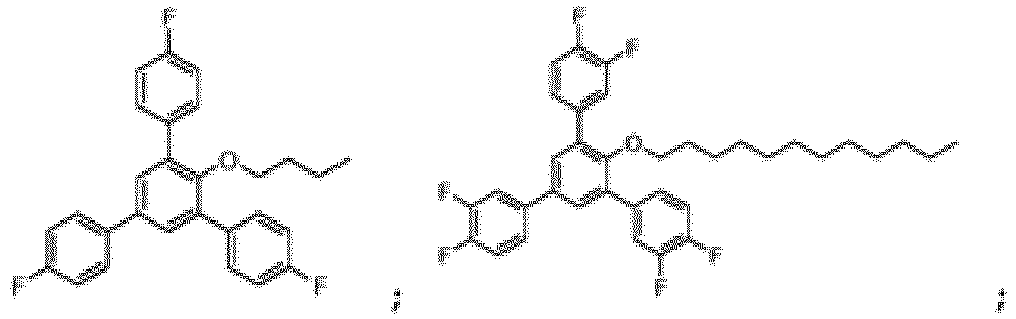
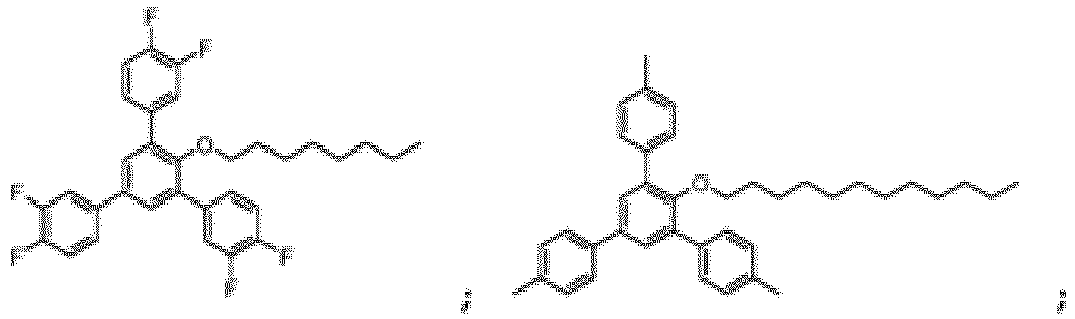
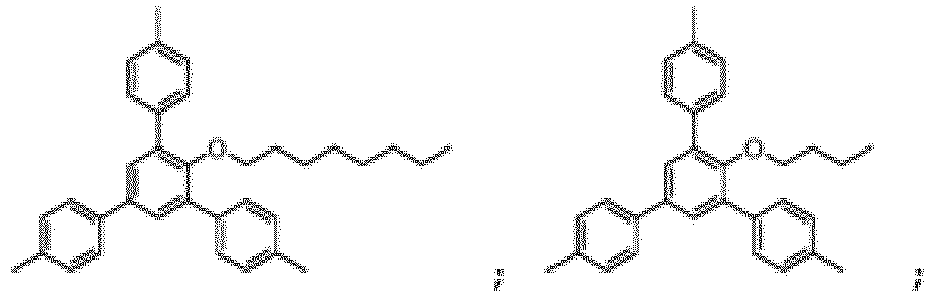
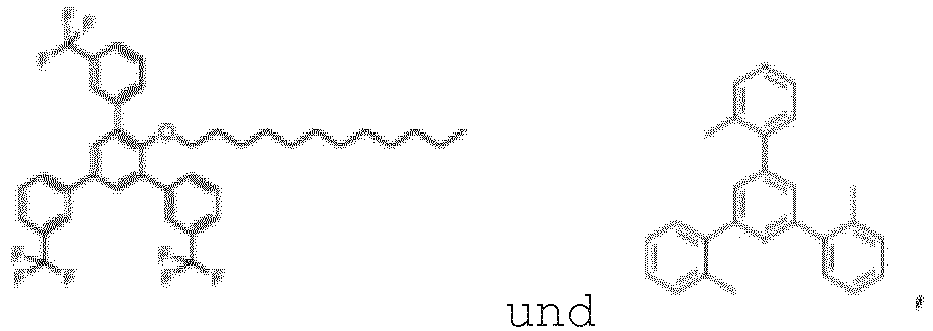
- the compound of Formula I can in particular be chosen from:
- the compound of Formula I is:
- a subject of the invention is also a fuel or liquid fuel containing as labeling tracer at least one compound of Formula I.
- Compounds of Formula I can be prepared by palladio-catalyzed Suzuki coupling between a 1,3,5-trihaloarene optionally substituted at positions 2, 4 or 6 and three equivalents of an arylboronic acid in the presence of an inorganic base:
- the compounds of Formula I not being substituted on the benzene ring A can also be prepared by cyclotrimerization of aryl methyl ketones according to:
- the reaction takes place between 80 and 150 ° C in the presence of catalysts or dehydrating agents.
- the catalysts are Bronsted or Lewis acids.
- the use of arenesulfonic acids is well described in the literature.
- the use of a catalytic amount of 4-toluenesulfonic acid monohydrate is described in Zhao, Y.; Li, J .; Li, C .; Yin, K .; Ye, D .; Jia, X. Green Chem. 2010, 12, 1370 .
- the compound of Formula I is added to the fuel or liquid fuel in an amount of 0.01 to 500 mg / L, in particular in an amount of 0.02 to 20 mg / L, and more particularly in an amount of 0.1 to 5 mg / L.
- a subject of the invention is also a process for marking fuels or liquid fuels, characterized in that a solution at 0.1 - 40% by weight of the compound of Formula I in a solvent or a mixture of solvents is prepared. and that said solution is injected into the fuel or liquid fuel, for example online in a pipeline or else at the loading arm of a tanker truck, or even in a tank to obtain the target concentration of the marker in the fuel or liquid fuel.
- a 0.2-20% by weight solution of the compound of Formula I in the solvent or mixture of solvents is prepared.
- the solvent (s) can be chosen from aromatic solvents such as toluene, ethylbenzene, xylenes, mesitylene, 1,2,4-trimethylbenzene, cumene, p-cymene, aromatic hydrocarbons (for example Shellsol ® A100, Solvarex® 10, Solvesso® 200); from aliphatic solvents such as pentane, hexanes, cyclohexane, heptanes, 2,2,4-trimethylpentane, white spirits, kerosene, isoparaffins, paraffins; from oxygenated and / or nitrogenous solvents such as ketones, ethers, esters, lactones, phenols, amides, lactams; or from halogenated solvents such as chlorobenzene, 1,2-dichlorobenzene, ⁇ , ⁇ , ⁇ -trifluorotoluene.
- aromatic solvents such as toluene, ethy
- the subject of the invention is also a method for determining whether fuels or liquid fuels have been adulterated by bleaching, characterized in that a sample of the fuel or liquid fuel which is suspected to have been adulterated is taken and that It is analyzed to determine whether the concentration of compound of Formula I in said sample is less than the initial concentration, and if so, it is inferred that the sample has been adulterated by bleaching.
- the invention also relates to a method for determining whether fuels or liquid fuels have been adulterated by cutting, or have been used illegally or have been the subject of smuggling or theft, characterized in that one take a sample of fuel or liquid fuel suspected of having been adulterated or illegally used, smuggled or stolen, and analyzed to determine if the compound of Formula I is present in said sample, and, if so, it is inferred that the sample has been tampered with or used illegally or is the subject of smuggling or theft.
- the marker (s) according to the invention can be used individually or in combination with other markers.
- Fuels or liquid fuels labeled with one or more compounds of Formula I can be analyzed qualitatively or quantitatively, for example, by the following methods: gas chromatography coupled to a flame ionization detector or to a mass spectrometer; high performance liquid chromatography coupled to a diode array detector or a mass spectrometer.
- the tube was shaken using a linear shaker at 300 strokes / min for 10 min.
- the tube was shaken using a linear shaker at 300 strokes / min for 10 min. After a few minutes of rest, the liquid constituting the phase upper was removed and then filtered through a 0.45 ⁇ m polytetrafluoroethylene (PTFE) membrane and analyzed by GC / FID.
- PTFE polytetrafluoroethylene
- a mixture of 134.2 g of 4'-methylacetophenone and 19 g of 4-toluenesulfonic acid monohydrate was heated to 140 ° C with stirring. The water was removed by distillation to reach the temperature and then during the course of the reaction. The temperature of 140 ° C was maintained for 24 h.
- Example 2 (of the invention) : 1,3,5-Tris (4-isobutylphenyl) benzene
- the title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 176.3 g of 4'-isobutylacetophenone and 19 g of 4-toluenesulfonic acid monohydrate.
- the title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 10 g of 4'-butylacetophenone and 1.1 g of 4-toluenesulfonic acid monohydrate.
- Example 5 1,3,5-Tris (4-ethylphenyl) benzene
- the title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 10 g of 4'-ethylacetophenone and 1.3 g of 4-toluenesulfonic acid monohydrate.
- Example 7 1,3,5-Tris (4-chlorophenyl) benzene
- 1,3,5-tris (4-chlorophenyl) benzene was prepared according to the procedure of Example 1, replacing 4'-methylacetophenone with 4'-chloroacetophenone.
- the crude product was recrystallized from ethanol. 36.8 g of yellowish white crystals were obtained. ⁇ u> Table 7 ⁇ /u> For 10 ml of marked diesel: Silica 0.5g Silica 2g Activated carbon 1g H 2 SO 4 at 95% 65% HNO 3 88% 58% 9% 104% 100%
- Example 8 1,3,5-Tris (4-bromophenyl) benzene
- 1,3,5-tris (4-bromophenyl) benzene was prepared according to the procedure of Example 1, replacing 4'-methylacetophenone with 4'-bromoacetophenone.
- the crude product was recrystallized from toluene. 54.3 g of yellowish white crystals were obtained.
- Table 8 ⁇ /u> For 10 ml of marked diesel: Silica 0.5g Silica 2g Activated carbon 1g H 2 SO 4 at 95% 65% HNO 3 113% 85% 0% 122% 92%
- Example 9 (of the invention) : 1-Dodecyloxy-2,4,6-tris (4-fluorophenyl) benzene
- Example 10 (of the invention): 1-Octyloxy-2,4,6-tris (4-fluorophenyl) benzene
- Example 11 (of the invention) : 1-Butoxy-2,4,6-tris (4-fluorophenyl) benzene
- Example 12 (of the invention) : 1-Dodecyloxy-2,4,6-tris (3,4-difluorophenyl) -benzene
- Example 13 (of the invention) : 1-Octyloxy-2,4,6-tris (3,4 - difluorophenyl) -benzene
- Example 14 (of the invention) : 1-Dodecyloxy-2,4,6-tris (4-methylphenyl) benzene
- Example 15 (of the invention): 1-Octyloxy-2,4,6-tris (4-methylphenyl) benzene
- Example 17 (of the invention) -: 1-Dodecyloxy-2,4,6-tris (3-trifluoromethyl-phenyl) benzene
- Example 18 (of the invention) -: 1,3,5-Tris (2-methylphenyl) benzene
- the compound of Example 2 is markedly more resistant to treatment with silica than the compound of Comparative Example 19.
- the marker of Comparative Example 19 is completely removed by nitric acid while the compound of Example 2 shows very good resistance to this chemical agent.
- the compound of Example 2 is significantly more resistant to treatment with activated carbon than TPB. In addition, the compound of Example 2 is superior to TPB for resistance to silica treatment.
- Example 21 Fuel injection nozzle clogging test according to CEC F-23-01
- the average percentage reduction in airflow through the injector at 0.1 mm needle lift is 70.8 for non-marker fuel and 69.5 for marker fuel.
- the addition of the marker therefore has no significant influence on the fouling of the injection nozzles.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Combustion & Propulsion (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Investigating Or Analyzing Non-Biological Materials By The Use Of Chemical Means (AREA)
Claims (11)
- Verwendung als Tracer zur Markierung von flüssigen Kraftstoffen und Motorkraftstoffen von mindestens einer Verbindung der Formel I:
unter der Bedingung, dass, wenn der Benzolkern A nicht substituiert ist und die Benzolringe B, C und D jeweils durch ein Methyl substituiert sind, sich letzteres in Bezug auf den Benzolkern A nicht an der para-Position befindet. - Verwendung nach Anspruch 1, dadurch gekennzeichnet, dass der Benzolkern A durch C1-C18-Alkoxy substituiert oder nicht substituiert ist.
- Verwendung nach einem der Ansprüche 1 und 2, dadurch gekennzeichnet, dass die Benzolringe B, C und D substituiert sind durch:- ein lineares, verzweigtes oder zyklisches C1-C18-, insbesondere C1-C12-, vor allem C1-C6-Alkylradikal, fakultativ substituiert durch mindestens ein Halogen; oder- mindestens ein Halogen.
- Verwendung nach einem der Ansprüche 1 bis 3, dadurch gekennzeichnet, dass ein Halogen, das in die Definition der Formel I eintritt, F, Cl, Br und I und vor allem F, Cl und Br ist.
- Flüssiger Kraftstoff und Motorkraftstoff, der mindestens eine Verbindung der Formel I nach einem der Ansprüche 1 bis 5 enthält, dadurch gekennzeichnet, dass die Verbindung der Formel I dem flüssigen Kraftstoff oder Motorkraftstoff in einem Umfang von 0,01 bis 500 mg/L, vor allem in einem Umfang von 0,02 bis 20 mg/L und insbesondere in einem Umfang von 0,1 bis 5 mg/L hinzugefügt ist.
- Verfahren zur Markierung von flüssigen Kraftstoffen oder Motorkraftstoffen, dadurch gekennzeichnet, dass eine Lösung 0,1-40 Gew.%ige Lösung der Verbindung der Formel I nach einem der Ansprüche 1 bis 5 in einem Lösungsmittel oder einem Lösungsmittelgemisch hergestellt wird und die Lösung in den flüssigen Kraftstoff oder Motorkraftstoff beispielsweise über eine Leitung in eine Pipeline oder den Ladearm eines Tankwagens oder auch in einen Kessel eingeleitet wird, um die angestrebte Konzentration des Markers in dem flüssigen Kraftstoff oder Motorkraftstoff zu erhalten.
- Verfahren nach Anspruch 7, dadurch gekennzeichnet, dass eine 0,2-20 Gew.-%ige Lösung der Verbindung der Formel I in dem Lösungsmittel oder Lösungsmittelgemisch hergestellt wird.
- Verfahren nach einem der Ansprüche 7 und 8, dadurch gekennzeichnet, dass das oder die Lösungsmittel aus den aromatischen Lösungsmitteln wie Toluen, Ethylbenzol, den Xylenen, Mesitylen, 1,2,4-Trimethylbenzol, Cumen, p-Cymen, den aromatischen Kohlenwasserstoffen (beispielsweise Shellsol® A100, Solvarex® 10, Solvesso® 200); aus den aliphatischen Lösungsmitteln wie Pentan, den Hexanen, Cyclohexan, den Heptanen, 2,2,4-Trimethylpentan, den White Spirits, Kerosin, den Isoparaffinen, den Paraffinen; aus den sauerstoff- und/oder stickstoffhaltigen Lösungsmitteln wie den Ketonen, den Ethern, den Estern, den Lactonen, den Phenolen, den Amiden, den Lactamen; oder den halogenierten Lösungsmitteln wie Chlorbenzol, 1,2-Dichlorbenzol, α,α,α-Trifluortoluen ausgewählt ist/sind.
- Verfahren zum Bestimmen, ob flüssige Kraftstoffe oder Motorkraftstoffe durch Waschen falsifiziert wurden, dadurch gekennzeichnet, dass eine Probe des flüssigen Kraftstoffs oder Motorkraftstoffs entnommen wird, bei dem der Verdacht besteht, dass er falsifiziert wurde, und dass er analysiert wird, um zu bestimmen, ob die Konzentration an Verbindung der Formel I nach einem der Ansprüche 1 bis 5 in der Probe unter der Anfangskonzentration liegt und wenn ja, daraus abgeleitet wird, dass die Probe durch Waschen falsifiziert wurde.
- Verfahren zum Bestimmen, ob flüssige Kraftstoffe und Motorkraftstoffe durch Verschneiden falsifiziert wurden oder illegal verwendet wurden oder Gegenstand von Schmuggel oder Diebstahl waren, dadurch gekennzeichnet, dass eine Probe des flüssigen Kraftstoffs oder Motorkraftstoffs entnommen wird, bei dem der Verdacht besteht, dass er falsifiziert oder illegal verwendet wurde oder Gegenstand von Schmuggel oder Diebstahl war, und dass er analysiert wird, um zu bestimmen, ob die Verbindung der Formel I nach einem der Ansprüche 1 bis 5 in der Probe enthalten ist und wenn ja, daraus abgeleitet wird, dass die Probe falsifiziert oder illegal verwendet wurde oder Gegenstand von Schmuggel oder Diebstahl war.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1652729A FR3049614B1 (fr) | 2016-03-30 | 2016-03-30 | Utilisation de derives de triarylbenzene comme traceurs de marquage de carburants et combustibles liquides, carburants et combustibles liquides contenant de tels derives et procedes correspondants |
| PCT/FR2017/050517 WO2017168066A1 (fr) | 2016-03-30 | 2017-03-09 | Utilisation de derives de triarylbenzene comme traceurs de marquage de carburants et combustibles liquides, carburants et combustibles liquides contenant de tels derives et procedes correspondants |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3436552A1 EP3436552A1 (de) | 2019-02-06 |
| EP3436552B1 true EP3436552B1 (de) | 2021-05-12 |
Family
ID=56069124
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP17717209.5A Active EP3436552B1 (de) | 2016-03-30 | 2017-03-09 | Verwendung von triarylbenzenderivaten als tracer zur markierung von flüssigen kraftstoffen und motorkraftstoffen, flüssige kraftstoffe und motorkraftstoffe mit solchen derivaten und entsprechende prozesse |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP3436552B1 (de) |
| ES (1) | ES2877166T3 (de) |
| FR (1) | FR3049614B1 (de) |
| WO (1) | WO2017168066A1 (de) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3775110B1 (de) | 2018-04-05 | 2023-04-19 | Dow Global Technologies, LLC | Substituierte dibenzofurane als brennstoffmarker |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU670427B2 (en) | 1992-01-29 | 1996-07-18 | Isotag Technology, Inc. | Method of identifying chemicals by use of non-radioactive isotopes |
| US20050019939A1 (en) * | 2003-07-25 | 2005-01-27 | Dale Spall | Combination marker for liquids and method identification thereof |
| US7858373B2 (en) | 2006-02-03 | 2010-12-28 | Rohm And Haas Company | Chemical markers |
| GB201107870D0 (en) * | 2011-05-11 | 2011-06-22 | Johnson Matthey Plc | Tracers and method of marking hydrocarbon liquids |
| MY169066A (en) * | 2011-06-24 | 2019-02-12 | Dow Global Technologies Llc | Tritylated ethers |
| TWI516468B (zh) | 2012-07-06 | 2016-01-11 | 羅門哈斯公司 | 三苯甲基化之烷基芳基醚 |
-
2016
- 2016-03-30 FR FR1652729A patent/FR3049614B1/fr active Active
-
2017
- 2017-03-09 ES ES17717209T patent/ES2877166T3/es active Active
- 2017-03-09 EP EP17717209.5A patent/EP3436552B1/de active Active
- 2017-03-09 WO PCT/FR2017/050517 patent/WO2017168066A1/fr not_active Ceased
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2877166T3 (es) | 2021-11-16 |
| EP3436552A1 (de) | 2019-02-06 |
| FR3049614A1 (fr) | 2017-10-06 |
| FR3049614B1 (fr) | 2020-02-07 |
| WO2017168066A1 (fr) | 2017-10-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Matoušek et al. | Expanding the scope of hypervalent iodine reagents for perfluoroalkylation: from trifluoromethyl to functionalized perfluoroethyl | |
| Shou et al. | Chemoselective hydro (chloro) pentafluorosulfanylation of diazo compounds with pentafluorosulfanyl chloride | |
| Roy et al. | Dissecting Alkynes: Full Cleavage of Polarized C C Moiety via Sequential Bis-Michael Addition/Retro-Mannich Cascade | |
| EP0449699A2 (de) | Pyrazol-Derivate als Angiotensin-II-Receptor-Antagonisten, Verfahren zu ihrer Herstellung und diese enthaltende pharmazeutische Zusammensetzungen | |
| Hashimoto et al. | Direct α‐Siladifluoromethylation of Lithium Enolates with Ruppert‐Prakash Reagent via C F Bond Activation | |
| Wang et al. | Copper‐Catalyzed Intramolecular Carbotrifluoromethylation of Alkynes for the Construction of Trifluoromethylated Heterocycles | |
| EP0596809A1 (de) | Beta-Diketone, Verfahren zu ihrer Herstellung und ihre Verwendung als Stabilisatoren für Polymere | |
| BE1004983A3 (fr) | Systeme catalytique d'hydrochloration et procede de fabrication de chlorure de vinyle au depart d'acetylene et de chlorure d'hydrogene en presence de ce systeme catalytique. | |
| WO2005082820A1 (fr) | Procedure de preparation d'un compose fluoroaromatique a partir d'un compose aminoaromatique. | |
| CN103910628A (zh) | α-氟代丙烯酸酯的制造方法 | |
| EP3436552B1 (de) | Verwendung von triarylbenzenderivaten als tracer zur markierung von flüssigen kraftstoffen und motorkraftstoffen, flüssige kraftstoffe und motorkraftstoffe mit solchen derivaten und entsprechende prozesse | |
| Bonge et al. | Highly efficient formation of halodiazoacetates and their use in stereoselective synthesis of halocyclopropanes | |
| Lu et al. | Iron (III) Porphyrin Catalyzed Olefination of Aldehydes with 2, 2, 2‐Trifluorodiazoethane (CF3CHN2) | |
| CA2172450A1 (fr) | Reactif et procede utiles pour greffer un groupement difluoromethyle substitue sur un compose comportant au moins une fonction electrophile | |
| BE1004984A3 (fr) | Systeme catalytique d'hydrochloration et procede de fabrication de chlorure de vinyle au depart d'acetylene et de chlorure d'hydrogene en presence de ce systeme catalytique. | |
| EP0905113B1 (de) | Verfahren zur Herstellung von 2-Chlorprop-1-en | |
| Richard | Aromatic substitution reactions of amines with ring-substituted 1-phenyl-2, 2, 2-trifluoroethyl carbocations | |
| Looker et al. | Reduction of Methyl Benzoyldiazoacetate. A New Synthesis of Allophenylserine1 | |
| EP3997055B1 (de) | Neue stabile formulierungen von 1-z-bromoalk-1-en-verbindungen und ihre verwendung zur herstellung von pheromonen | |
| JP2003519112A (ja) | エステルの製造方法 | |
| Fedorovskii et al. | C2-Alkylation in a three-component reaction of fluorocarbonyl compounds, pyrrole derivatives and malononitrile in competition with C2-oxyalkylation | |
| Kharasch et al. | Reactions of Atoms and Free Radicals in Solution. XXXIX. The Reaction of Diacetyl Peroxide with Sec-Butyl Nitrite and 3-Amyl Nitrite | |
| EP3539955B1 (de) | Verfahren für die herstellung von tetrazinen | |
| Gupta et al. | A simple and efficient method for the synthesis of gem-chloronitroso compounds | |
| CH280180A (fr) | Procédé de préparation des lactones d'acides bêta-hydroxy-carboxyliques. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: UNKNOWN |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20181026 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C10L 1/185 20060101ALI20201014BHEP Ipc: C10L 1/16 20060101ALI20201014BHEP Ipc: C10L 10/04 20060101ALI20201014BHEP Ipc: C10L 1/00 20060101AFI20201014BHEP Ipc: C10L 1/20 20060101ALI20201014BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20201117 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602017038508 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1392188 Country of ref document: AT Kind code of ref document: T Effective date: 20210615 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1392188 Country of ref document: AT Kind code of ref document: T Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210812 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2877166 Country of ref document: ES Kind code of ref document: T3 Effective date: 20211116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210912 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210813 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210913 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210812 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602017038508 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20220215 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210912 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220309 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20170309 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20250328 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20250317 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20250314 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20250314 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20250304 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250317 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20250305 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20250415 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20250326 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20250401 Year of fee payment: 9 |