EP3436552B1 - Use of triarylbenzene derivatives as tracers for marking liquid fuels and motor fuels, liquid fuels and motor fuels containing such derivatives and corresponding processes - Google Patents
Use of triarylbenzene derivatives as tracers for marking liquid fuels and motor fuels, liquid fuels and motor fuels containing such derivatives and corresponding processes Download PDFInfo
- Publication number
- EP3436552B1 EP3436552B1 EP17717209.5A EP17717209A EP3436552B1 EP 3436552 B1 EP3436552 B1 EP 3436552B1 EP 17717209 A EP17717209 A EP 17717209A EP 3436552 B1 EP3436552 B1 EP 3436552B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fuels
- halogen
- compound
- formula
- fact
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- PAIQXYSZUMLJGD-UHFFFAOYSA-N CCCCCCCCCCCCOc(c(-c1cccc(C(F)(F)F)c1)cc(-c1cccc(C(F)(F)F)c1)c1)c1-c1cc(C(F)(F)F)ccc1 Chemical compound CCCCCCCCCCCCOc(c(-c1cccc(C(F)(F)F)c1)cc(-c1cccc(C(F)(F)F)c1)c1)c1-c1cc(C(F)(F)F)ccc1 PAIQXYSZUMLJGD-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/003—Marking, e.g. coloration by addition of pigments
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/16—Hydrocarbons
- C10L1/1608—Well defined compounds, e.g. hexane, benzene
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/185—Ethers; Acetals; Ketals; Aldehydes; Ketones
- C10L1/1852—Ethers; Acetals; Ketals; Orthoesters
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/20—Organic compounds containing halogen
- C10L1/202—Organic compounds containing halogen aromatic bond
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/20—Organic compounds containing halogen
- C10L1/203—Organic compounds containing halogen hydroxyl compounds; ethers, acetals, ketals
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/04—Use of additives to fuels or fires for particular purposes for minimising corrosion or incrustation
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2230/00—Function and purpose of a components of a fuel or the composition as a whole
- C10L2230/16—Tracers which serve to track or identify the fuel component or fuel composition
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2270/00—Specifically adapted fuels
- C10L2270/02—Specifically adapted fuels for internal combustion engines
- C10L2270/023—Specifically adapted fuels for internal combustion engines for gasoline engines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2270/00—Specifically adapted fuels
- C10L2270/02—Specifically adapted fuels for internal combustion engines
- C10L2270/026—Specifically adapted fuels for internal combustion engines for diesel engines, e.g. automobiles, stationary, marine
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L2270/00—Specifically adapted fuels
- C10L2270/04—Specifically adapted fuels for turbines, planes, power generation
Definitions
- the present invention relates to the use of triarylbenzene derivatives as labeling tracers for fuels and liquid fuels; fuels and liquid fuels containing such derivatives; a process for marking fuels and liquid fuels with such derivatives; and a method of determining whether fuels or liquid fuels initially containing these tracers have been tampered with, used illegally or stolen.
- Fraud concerning fuels or liquid fuels covers various forms of activity: falsification by laundering or cutting, illegal use, smuggling and theft.
- Falsification by laundering concerns the transformation of a fuel or liquid fuel with a low tax rate into fuel or liquid fuel with a higher tax rate, for example - in France - the transformation of domestic fuel oil into diesel.
- Falsification by cutting concerns the dilution of a fuel or liquid fuel with a high tax rate by a fuel or liquid fuel at a low tax rate.
- Illegal use refers to the illegal use of a fuel or liquid fuel with a low tax rate instead of a fuel or liquid fuel with a higher tax rate.
- Smuggling is the purchase of fuel or liquid fuel at low tax rates in one country for resale in another country where taxes are higher.
- WO2012 / 153132 A1 describes aromatic organic markers substituted by at least one bromine and / or one fluorine atom, and / or one or more fluoroalkyl groups.
- the combustion of fuels labeled with such compounds can produce hydrogen bromide and / or hydrogen fluoride in the flue gases.
- Hydrogen bromide and fluoride are known to be particularly corrosive to the engine.
- Hydrogen fluoride is well known to be highly toxic to people and the environment.
- WO2014 / 008164 describes aryl and trityl alkyl ethers as tracers for labeling hydrocarbons. Such tracers appear not to exhibit good resistance to the abovementioned elimination methods.
- triarylbenzenes are particularly effective for the labeling of fuels and liquid fuels.
- these triarylbenzenes are particularly resistant to bleaching attempts. chemical and physical.
- most of these triarylbenzenes contain only carbon and hydrogen and the combustion of these compounds used as markers produces only carbon dioxide and water.
- any atom can be replaced by one of its isotopes.
- the benzene ring A is unsubstituted or substituted by C 1 -C 18 alkoxy.
- a halogen falling within the definition of Formula I can be F, Cl, Br and I, and in particular F, Cl and Br.
- the compound of Formula I can in particular be chosen from:
- the compound of Formula I is:
- a subject of the invention is also a fuel or liquid fuel containing as labeling tracer at least one compound of Formula I.
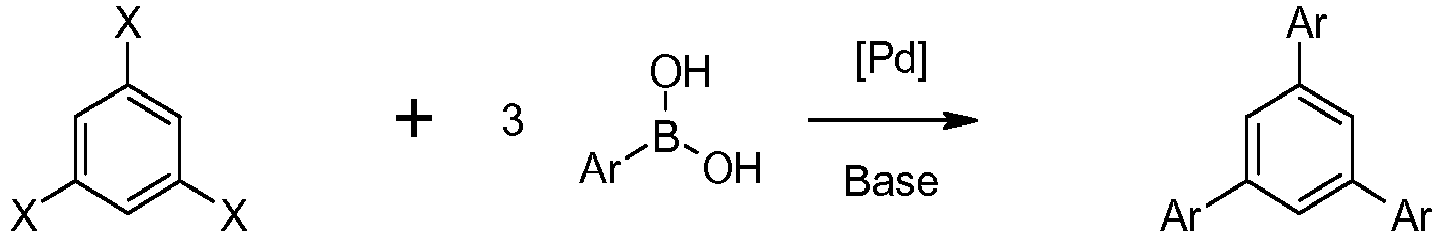
- Compounds of Formula I can be prepared by palladio-catalyzed Suzuki coupling between a 1,3,5-trihaloarene optionally substituted at positions 2, 4 or 6 and three equivalents of an arylboronic acid in the presence of an inorganic base:
- the compounds of Formula I not being substituted on the benzene ring A can also be prepared by cyclotrimerization of aryl methyl ketones according to:
- the reaction takes place between 80 and 150 ° C in the presence of catalysts or dehydrating agents.
- the catalysts are Bronsted or Lewis acids.
- the use of arenesulfonic acids is well described in the literature.
- the use of a catalytic amount of 4-toluenesulfonic acid monohydrate is described in Zhao, Y.; Li, J .; Li, C .; Yin, K .; Ye, D .; Jia, X. Green Chem. 2010, 12, 1370 .
- the compound of Formula I is added to the fuel or liquid fuel in an amount of 0.01 to 500 mg / L, in particular in an amount of 0.02 to 20 mg / L, and more particularly in an amount of 0.1 to 5 mg / L.
- a subject of the invention is also a process for marking fuels or liquid fuels, characterized in that a solution at 0.1 - 40% by weight of the compound of Formula I in a solvent or a mixture of solvents is prepared. and that said solution is injected into the fuel or liquid fuel, for example online in a pipeline or else at the loading arm of a tanker truck, or even in a tank to obtain the target concentration of the marker in the fuel or liquid fuel.
- a 0.2-20% by weight solution of the compound of Formula I in the solvent or mixture of solvents is prepared.
- the solvent (s) can be chosen from aromatic solvents such as toluene, ethylbenzene, xylenes, mesitylene, 1,2,4-trimethylbenzene, cumene, p-cymene, aromatic hydrocarbons (for example Shellsol ® A100, Solvarex® 10, Solvesso® 200); from aliphatic solvents such as pentane, hexanes, cyclohexane, heptanes, 2,2,4-trimethylpentane, white spirits, kerosene, isoparaffins, paraffins; from oxygenated and / or nitrogenous solvents such as ketones, ethers, esters, lactones, phenols, amides, lactams; or from halogenated solvents such as chlorobenzene, 1,2-dichlorobenzene, ⁇ , ⁇ , ⁇ -trifluorotoluene.
- aromatic solvents such as toluene, ethy
- the subject of the invention is also a method for determining whether fuels or liquid fuels have been adulterated by bleaching, characterized in that a sample of the fuel or liquid fuel which is suspected to have been adulterated is taken and that It is analyzed to determine whether the concentration of compound of Formula I in said sample is less than the initial concentration, and if so, it is inferred that the sample has been adulterated by bleaching.
- the invention also relates to a method for determining whether fuels or liquid fuels have been adulterated by cutting, or have been used illegally or have been the subject of smuggling or theft, characterized in that one take a sample of fuel or liquid fuel suspected of having been adulterated or illegally used, smuggled or stolen, and analyzed to determine if the compound of Formula I is present in said sample, and, if so, it is inferred that the sample has been tampered with or used illegally or is the subject of smuggling or theft.
- the marker (s) according to the invention can be used individually or in combination with other markers.
- Fuels or liquid fuels labeled with one or more compounds of Formula I can be analyzed qualitatively or quantitatively, for example, by the following methods: gas chromatography coupled to a flame ionization detector or to a mass spectrometer; high performance liquid chromatography coupled to a diode array detector or a mass spectrometer.
- the tube was shaken using a linear shaker at 300 strokes / min for 10 min.
- the tube was shaken using a linear shaker at 300 strokes / min for 10 min. After a few minutes of rest, the liquid constituting the phase upper was removed and then filtered through a 0.45 ⁇ m polytetrafluoroethylene (PTFE) membrane and analyzed by GC / FID.
- PTFE polytetrafluoroethylene
- a mixture of 134.2 g of 4'-methylacetophenone and 19 g of 4-toluenesulfonic acid monohydrate was heated to 140 ° C with stirring. The water was removed by distillation to reach the temperature and then during the course of the reaction. The temperature of 140 ° C was maintained for 24 h.
- Example 2 (of the invention) : 1,3,5-Tris (4-isobutylphenyl) benzene
- the title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 176.3 g of 4'-isobutylacetophenone and 19 g of 4-toluenesulfonic acid monohydrate.
- the title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 10 g of 4'-butylacetophenone and 1.1 g of 4-toluenesulfonic acid monohydrate.
- Example 5 1,3,5-Tris (4-ethylphenyl) benzene
- the title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 10 g of 4'-ethylacetophenone and 1.3 g of 4-toluenesulfonic acid monohydrate.
- Example 7 1,3,5-Tris (4-chlorophenyl) benzene
- 1,3,5-tris (4-chlorophenyl) benzene was prepared according to the procedure of Example 1, replacing 4'-methylacetophenone with 4'-chloroacetophenone.
- the crude product was recrystallized from ethanol. 36.8 g of yellowish white crystals were obtained. ⁇ u> Table 7 ⁇ /u> For 10 ml of marked diesel: Silica 0.5g Silica 2g Activated carbon 1g H 2 SO 4 at 95% 65% HNO 3 88% 58% 9% 104% 100%
- Example 8 1,3,5-Tris (4-bromophenyl) benzene
- 1,3,5-tris (4-bromophenyl) benzene was prepared according to the procedure of Example 1, replacing 4'-methylacetophenone with 4'-bromoacetophenone.
- the crude product was recrystallized from toluene. 54.3 g of yellowish white crystals were obtained.
- Table 8 ⁇ /u> For 10 ml of marked diesel: Silica 0.5g Silica 2g Activated carbon 1g H 2 SO 4 at 95% 65% HNO 3 113% 85% 0% 122% 92%
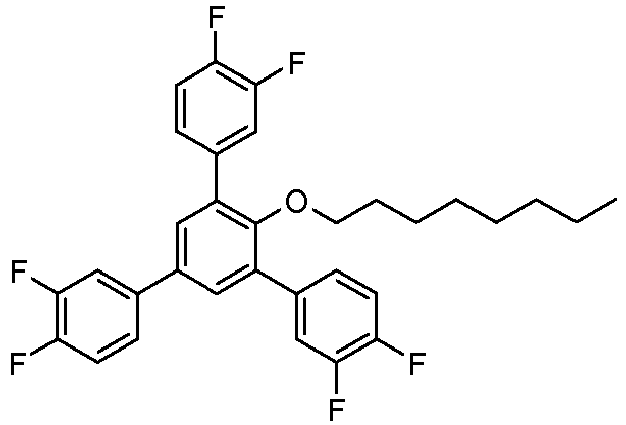
- Example 9 (of the invention) : 1-Dodecyloxy-2,4,6-tris (4-fluorophenyl) benzene
- Example 10 (of the invention): 1-Octyloxy-2,4,6-tris (4-fluorophenyl) benzene
- Example 11 (of the invention) : 1-Butoxy-2,4,6-tris (4-fluorophenyl) benzene
- Example 12 (of the invention) : 1-Dodecyloxy-2,4,6-tris (3,4-difluorophenyl) -benzene
- Example 13 (of the invention) : 1-Octyloxy-2,4,6-tris (3,4 - difluorophenyl) -benzene
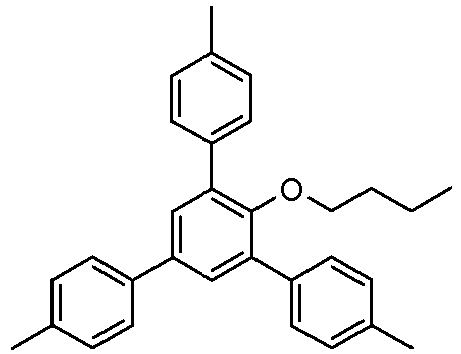
- Example 14 (of the invention) : 1-Dodecyloxy-2,4,6-tris (4-methylphenyl) benzene
- Example 15 (of the invention): 1-Octyloxy-2,4,6-tris (4-methylphenyl) benzene
- Example 17 (of the invention) -: 1-Dodecyloxy-2,4,6-tris (3-trifluoromethyl-phenyl) benzene
- Example 18 (of the invention) -: 1,3,5-Tris (2-methylphenyl) benzene
- the compound of Example 2 is markedly more resistant to treatment with silica than the compound of Comparative Example 19.
- the marker of Comparative Example 19 is completely removed by nitric acid while the compound of Example 2 shows very good resistance to this chemical agent.
- the compound of Example 2 is significantly more resistant to treatment with activated carbon than TPB. In addition, the compound of Example 2 is superior to TPB for resistance to silica treatment.
- Example 21 Fuel injection nozzle clogging test according to CEC F-23-01
- the average percentage reduction in airflow through the injector at 0.1 mm needle lift is 70.8 for non-marker fuel and 69.5 for marker fuel.
- the addition of the marker therefore has no significant influence on the fouling of the injection nozzles.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Combustion & Propulsion (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Investigating Or Analyzing Non-Biological Materials By The Use Of Chemical Means (AREA)
Description
La présente invention concerne l'utilisation de dérivés de triarylbenzène comme traceurs de marquage pour les carburants et combustibles liquides ; les carburants et combustibles liquides contenant de tels dérivés ; un procédé de marquage de carburants et combustibles liquides par de tels dérivés ; et un procédé pour déterminer si des carburants ou combustibles liquides contenant initialement ces traceurs ont été falsifiés, utilisés de manière illégale ou volés.The present invention relates to the use of triarylbenzene derivatives as labeling tracers for fuels and liquid fuels; fuels and liquid fuels containing such derivatives; a process for marking fuels and liquid fuels with such derivatives; and a method of determining whether fuels or liquid fuels initially containing these tracers have been tampered with, used illegally or stolen.
Le marquage des carburants et combustibles liquides avec différents types de marqueurs chimiques est bien connu dans l'art. Des composés variés ont été utilisés à cette fin, ainsi que de nombreuses techniques d'analyse pour la détection des marqueurs (par exemple, la chromatographie en phase gazeuse couplée à la spectrométrie de masse). Une application importante consiste à marquer les carburants combustibles selon leur niveau de taxation. L'élimination délibérée du traceur à des fins illégales est une question importante. Comme les traceurs étaient des colorants jusqu'à présent, les « méthodes d'élimination » ont été appelées « méthodes de blanchiment ». Ces méthodes sont physiques (par exemple : adsorption sur charbon ou sur gel de silice) ou chimique (par exemple : traitement acide ou basique). Par conséquent, ces marqueurs doivent être résistants à de telles méthodes d'élimination, être entièrement compatibles avec les carburants et combustibles liquides, et être identifiables par une méthode analytique appropriée.The labeling of fuels and liquid fuels with various types of chemical markers is well known in the art. A variety of compounds have been used for this purpose, as well as many analytical techniques for the detection of labels (eg, gas chromatography coupled with mass spectrometry). An important application is to mark combustible fuels according to their level of taxation. The deliberate disposal of the tracker for illegal purposes is an important issue. As tracers have been dyes heretofore, “removal methods” have been referred to as “bleaching methods”. These methods are physical (for example: adsorption on carbon or on silica gel) or chemical (for example: acid or basic treatment). Therefore, these markers must be resistant to such removal methods, be fully compatible with fuels and combustibles. liquids, and be identifiable by an appropriate analytical method.
La fraude concernant les carburants ou combustibles liquides recouvre différents agissements : la falsification par blanchiment ou par coupage, l'usage illégal, la contrebande et le vol. La falsification par blanchiment concerne la transformation d'un carburant ou combustible liquide à bas taux de taxation en carburant ou combustible liquide à taux de taxation plus élevé, par exemple - en France - la transformation du fioul domestique en gazole. La falsification par coupage concerne la dilution d'un carburant ou combustible liquide à fort taux de taxation par un carburant ou combustible liquide à bas taux de taxation. L'usage illégal concerne l'utilisation illégale d'un carburant ou combustible liquide à bas taux de taxation en lieu et place d'un carburant ou combustible liquide à taux de taxation plus élevé. La contrebande concerne l'achat d'un carburant ou combustible liquide à bas taux de taxation dans un pays pour le revendre dans un autre pays où les taxes sont plus élevées.Fraud concerning fuels or liquid fuels covers various forms of activity: falsification by laundering or cutting, illegal use, smuggling and theft. Falsification by laundering concerns the transformation of a fuel or liquid fuel with a low tax rate into fuel or liquid fuel with a higher tax rate, for example - in France - the transformation of domestic fuel oil into diesel. Falsification by cutting concerns the dilution of a fuel or liquid fuel with a high tax rate by a fuel or liquid fuel at a low tax rate. Illegal use refers to the illegal use of a fuel or liquid fuel with a low tax rate instead of a fuel or liquid fuel with a higher tax rate. Smuggling is the purchase of fuel or liquid fuel at low tax rates in one country for resale in another country where taxes are higher.
Il subsiste donc encore un besoin de marqueurs chimiques supplémentaires compatibles avec les carburants et combustibles liquides, détectables dans un tel milieu complexe et résistants aux méthodes physiques et chimiques de blanchiment, au moins à la méthode physique de blanchiment par le charbon actif et/ou par la silice.There therefore still remains a need for additional chemical markers compatible with fuels and liquid fuels, detectable in such a complex medium and resistant to physical and chemical bleaching methods, at least to the physical method of bleaching by activated carbon and / or by silica.
De manière surprenante, il a été découvert que certains triarylbenzènes sont particulièrement efficaces pour le marquage des carburants et combustibles liquides. De plus, il a été montré que ces triarylbenzènes sont particulièrement résistants aux tentatives de blanchiment chimique et physique. En outre, la plupart de ces triarylbenzènes ne contiennent que du carbone et de l'hydrogène et la combustion de ces composés utilisés comme marqueurs ne produit que du dioxyde de carbone et de l'eau.Surprisingly, it has been found that certain triarylbenzenes are particularly effective for the labeling of fuels and liquid fuels. In addition, it has been shown that these triarylbenzenes are particularly resistant to bleaching attempts. chemical and physical. In addition, most of these triarylbenzenes contain only carbon and hydrogen and the combustion of these compounds used as markers produces only carbon dioxide and water.
La présente invention a donc d'abord pour objet l'utilisation comme traceur de marquage de carburants et combustibles liquides d'au moins un composé de Formule I :
- le noyau benzénique A est facultativement substitué dans au moins une des positions 2, 4 ou 6 par :
- un radical alkyle, linéaire, ramifié ou cyclique, en C1-C18, facultativement substitué par au moins un halogène et/ou facultativement interrompu par au moins un oxygène ; ou
- un radical alcoxy, linéaire, ramifié ou cyclique, en C1-C18, facultativement substitué par au moins un halogène et/ou facultativement interrompu par au moins un oxygène ; ou
- un radical alcényle, linéaire, ramifié ou cyclique, en C2-C18, facultativement substitué par au moins un halogène et/ou facultativement interrompu par au moins un oxygène ;
- les noyaux benzéniques B, C et D sont substitués, indépendamment les uns des autres, dans au moins une position ortho, méta ou para par rapport au noyau benzénique A par :
- un radical alkyle, linéaire, ramifié ou cyclique, en C1-C18, facultativement substitué par au moins un halogène et/ou facultativement interrompu par au moins un oxygène ; ou
- un radical alcoxy, linéaire, ramifié ou cyclique, en C1-C18, facultativement substitué par au moins un halogène et/ou facultativement interrompu par au moins un oxygène ; ou
- un radical alcényle, linéaire, ramifié ou cyclique, en C2-C18, facultativement substitué par au moins un halogène et/ou facultativement interrompu par au moins un oxygène ; ou
- un halogène ;
au moins un noyau benzénique B, C et D pouvant par ailleurs comporter un cycle aliphatique à 5-7 chaînons accolé à celui-ci, par exemple un cycle à 6 chaînons,
- the benzene ring A is optionally substituted in at least one of the 2, 4 or 6 positions by:
- an alkyl radical, linear, branched or cyclic, C 1 -C 18 , optionally substituted with at least one halogen and / or optionally interrupted by at least one oxygen; or
- an alkoxy radical, linear, branched or cyclic, C 1 -C 18 , optionally substituted by at least one halogen and / or optionally interrupted by at least one oxygen; or
- an alkenyl radical, linear, branched or cyclic, C 2 -C 18 , optionally substituted with at least one halogen and / or optionally interrupted by at least one oxygen;
- the benzene rings B, C and D are substituted, independently of one another, in at least one position ortho, meta or para with respect to the benzene ring A by:
- an alkyl radical, linear, branched or cyclic, C 1 -C 18 , optionally substituted with at least one halogen and / or optionally interrupted by at least one oxygen; or
- an alkoxy radical, linear, branched or cyclic, C 1 -C 18 , optionally substituted by at least one halogen and / or optionally interrupted by at least one oxygen; or
- an alkenyl radical, linear, branched or cyclic, C 2 -C 18 , optionally substituted with at least one halogen and / or optionally interrupted by at least one oxygen; or
- a halogen;
at least one benzene ring B, C and D which may moreover contain a 5-7-membered aliphatic ring attached thereto, for example a 6-membered ring,
On notera que, dans la Formule 1, tout atome peut être remplacé par l'un de ses isotopes.Note that, in Formula 1, any atom can be replaced by one of its isotopes.
De préférence, le noyau benzénique A est non substitué ou substitué par alcoxy en C1-C18.Preferably, the benzene ring A is unsubstituted or substituted by C 1 -C 18 alkoxy.
De préférence, les noyaux benzéniques B, C et D sont substitués par :
- un radical alkyle, linéaire, ramifié ou cyclique, en C1-C18, notamment en C1-C12, en particulier en C1-C6, facultativement substitué par au moins un halogène ; ou
- au moins un halogène.
- an alkyl radical, linear, branched or cyclic, C 1 -C 18 , in particular C 1 -C 12 , in particular C 1 -C 6 , optionally substituted by at least one halogen; or
- at least one halogen.
Un halogène entrant dans la définition de la Formule I peut être F, Cl, Br et I, et en particulier F, Cl et Br.A halogen falling within the definition of Formula I can be F, Cl, Br and I, and in particular F, Cl and Br.
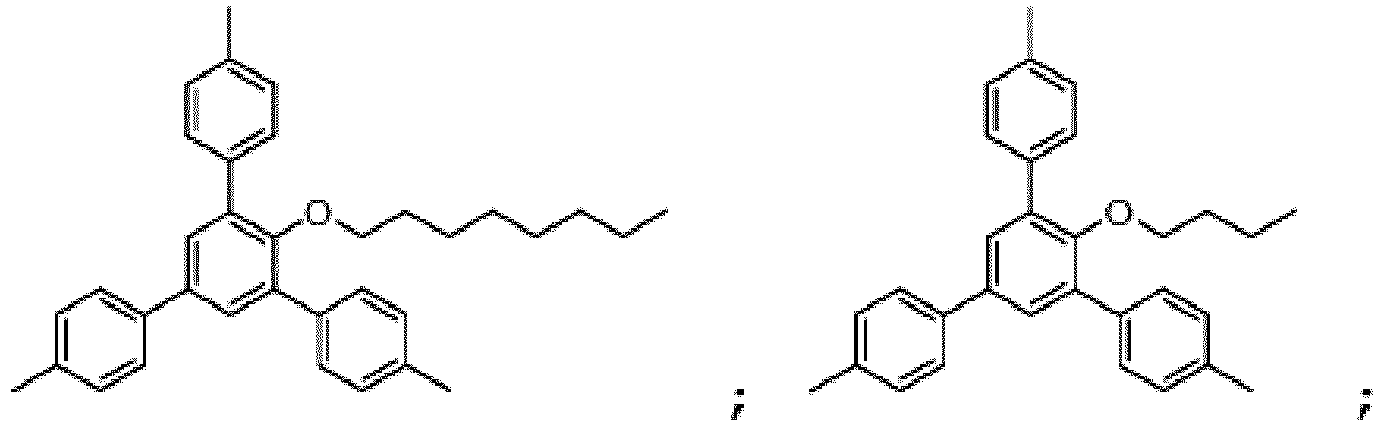
Le composé de Formule I peut notamment être choisi parmi :
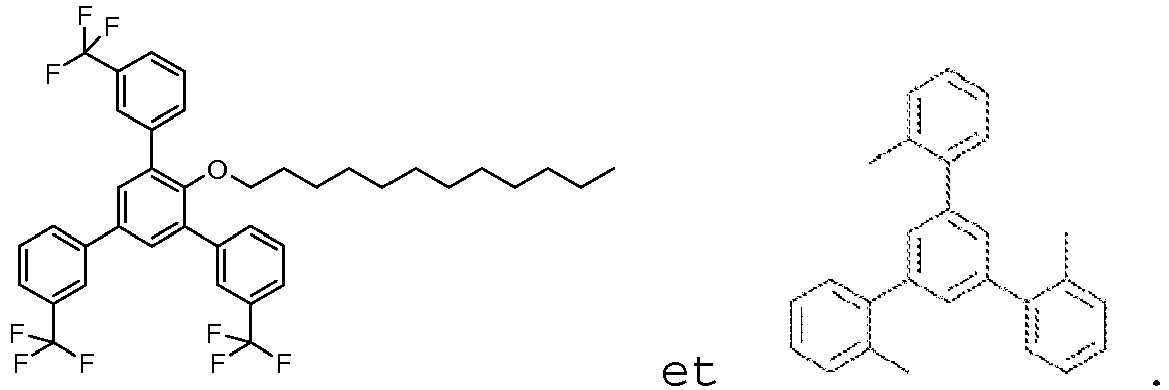
De manière particulièrement préférée, le composé de Formule I est :
L'invention a également pour objet un carburant ou combustible liquide contenant comme traceur de marquage au moins un composé de Formule I.A subject of the invention is also a fuel or liquid fuel containing as labeling tracer at least one compound of Formula I.
Comme carburant ou combustible liquide, on peut citer :
- les carburants ou combustibles liquides obtenus à partir du pétrole parmi lesquels on peut citer l'essence, le gazole, le fuel domestique, le kérosène, le pétrole lampant ;
- les carburants ou combustibles liquides obtenus à partir des végétaux tels que les biocarburants parmi lesquels on peut citer les huiles végétales, l'éthanol, le butanol, l'éthyl tert-butyl éther (ETBE), les esters méthyliques d'acide gras.
- fuels or liquid fuels obtained from petroleum, among which may be mentioned gasoline, gas oil, domestic fuel oil, kerosene, kerosene;
- fuels or liquid fuels obtained from plants such as biofuels among which there may be mentioned vegetable oils, ethanol, butanol, ethyl tert-butyl ether (ETBE), methyl esters of fatty acids.
Les composés de Formule I peuvent être préparés par couplage de Suzuki palladio-catalysé entre un 1,3,5-trihalogénoarène facultativement substitué sur les positions 2, 4 ou 6 et trois équivalents d'un acide arylboronique en présence d'une base minérale :
Les composés de Formule I n'étant pas substitués sur le noyau benzénique A peuvent aussi être préparés par cyclotrimérisation des aryl méthyl cétones selon :
La réaction a lieu entre 80 et 150°C en présence de catalyseurs ou d'agents déshydratants. Les catalyseurs sont des acides de Bronsted ou de Lewis. L'emploi des acides arènesulfoniques est bien décrit dans la littérature. En particulier l'emploi d'une quantité catalytique d'acide 4-toluènesulfonique monohydrate est décrit dans
Le composé de Formule I est ajouté au carburant ou combustible liquide à raison de 0,01 à 500 mg/L, en particulier à raison de 0,02 à 20 mg/L, et plus particulièrement à raison de 0,1 à 5 mg/L.The compound of Formula I is added to the fuel or liquid fuel in an amount of 0.01 to 500 mg / L, in particular in an amount of 0.02 to 20 mg / L, and more particularly in an amount of 0.1 to 5 mg / L.
L'invention a aussi pour objet un procédé de marquage de carburants ou combustibles liquides, caractérisé par le fait que l'on prépare une solution à 0,1 - 40 % en poids du composé de Formule I dans un solvant ou un mélange de solvants et que l'on injecte ladite solution dans le carburant ou combustible liquide par exemple en ligne dans un pipeline ou bien au bras de chargement d'un camion-citerne, ou bien encore dans une cuve pour obtenir la concentration visée du marqueur dans le carburant ou combustible liquide.A subject of the invention is also a process for marking fuels or liquid fuels, characterized in that a solution at 0.1 - 40% by weight of the compound of Formula I in a solvent or a mixture of solvents is prepared. and that said solution is injected into the fuel or liquid fuel, for example online in a pipeline or else at the loading arm of a tanker truck, or even in a tank to obtain the target concentration of the marker in the fuel or liquid fuel.
De préférence, on prépare une solution à 0,2 - 20 % en poids du composé de Formule I dans le solvant ou mélange de solvants.Preferably, a 0.2-20% by weight solution of the compound of Formula I in the solvent or mixture of solvents is prepared.
Le ou les solvants peuvent être choisis parmi des solvants aromatiques tels que le toluène, l'éthylbenzène, les xylènes, le mésitylène, le 1,2,4-triméthylbenzène, le cumène, le p-cymène, les hydrocarbures aromatiques (par exemple Shellsol® A100, Solvarex® 10, Solvesso® 200) ; parmi des solvants aliphatiques tels que le pentane, les hexanes, le cyclohexane, les heptanes, le 2,2,4-triméthylpentane, le white spirits, le kérosène, les isoparaffines, les paraffines ; parmi des solvants oxygénés et/ou azotés tels que les cétones, les éthers, les esters, les lactones, les phénols, les amides, les lactames ; ou parmi des solvants halogénés tels que le chlorobenzène, le 1,2-dichlorobenzène, l'α,α,α-trifluorotoluène.The solvent (s) can be chosen from aromatic solvents such as toluene, ethylbenzene, xylenes, mesitylene, 1,2,4-trimethylbenzene, cumene, p-cymene, aromatic hydrocarbons (for example Shellsol ® A100, Solvarex® 10, Solvesso® 200); from aliphatic solvents such as pentane, hexanes, cyclohexane, heptanes, 2,2,4-trimethylpentane, white spirits, kerosene, isoparaffins, paraffins; from oxygenated and / or nitrogenous solvents such as ketones, ethers, esters, lactones, phenols, amides, lactams; or from halogenated solvents such as chlorobenzene, 1,2-dichlorobenzene, α, α, α-trifluorotoluene.
L'invention a également pour objet un procédé pour déterminer si des carburants ou combustibles liquides ont été falsifiés par blanchiment, caractérisé par le fait que l'on prélève un échantillon du carburant ou combustible liquide dont on suspecte qu'il a été falsifié et qu'on l'analyse pour déterminer si la concentration en composé de Formule I dans ledit échantillon est inférieure à la concentration initiale, et, dans l'affirmative, on en déduit que l'échantillon a été falsifié par blanchiment.The subject of the invention is also a method for determining whether fuels or liquid fuels have been adulterated by bleaching, characterized in that a sample of the fuel or liquid fuel which is suspected to have been adulterated is taken and that It is analyzed to determine whether the concentration of compound of Formula I in said sample is less than the initial concentration, and if so, it is inferred that the sample has been adulterated by bleaching.
L'invention a aussi pour objet un procédé pour déterminer si des carburants ou combustibles liquides ont été falsifiés par coupage, ou ont été utilisés de manière illégale ou ont fait l'objet de contrebande ou de vol, caractérisé par le fait que l'on prélève un échantillon du carburant ou combustible liquide dont on suspecte qu'il a été falsifié ou utilisé de manière illégale ou fait l'objet de contrebande ou de vol, et qu'on l'analyse pour déterminer si le composé de Formule I est présent dans ledit échantillon, et, dans l'affirmative, on en déduit que l'échantillon a été falsifié ou utilisé de manière illégale ou fait l'objet de contrebande ou de vol.The invention also relates to a method for determining whether fuels or liquid fuels have been adulterated by cutting, or have been used illegally or have been the subject of smuggling or theft, characterized in that one take a sample of fuel or liquid fuel suspected of having been adulterated or illegally used, smuggled or stolen, and analyzed to determine if the compound of Formula I is present in said sample, and, if so, it is inferred that the sample has been tampered with or used illegally or is the subject of smuggling or theft.
On peut souligner ici que le ou les marqueurs selon l'invention peuvent être utilisés individuellement ou en combinaison avec d'autres marqueurs.It can be emphasized here that the marker (s) according to the invention can be used individually or in combination with other markers.
Les carburants ou combustibles liquides marqués par un ou plusieurs composés de Formule I peuvent être analysés qualitativement ou quantitativement, par exemple, par les méthodes suivantes : chromatographie en phase gazeuse couplée à un détecteur à ionisation de flamme ou à un spectromètre de masse ; chromatographie liquide à haute performance couplée à un détecteur à barrette de diodes ou à un spectromètre de masse.Fuels or liquid fuels labeled with one or more compounds of Formula I can be analyzed qualitatively or quantitatively, for example, by the following methods: gas chromatography coupled to a flame ionization detector or to a mass spectrometer; high performance liquid chromatography coupled to a diode array detector or a mass spectrometer.
Les Exemples suivants illustrent la présente invention sans toutefois en limiter la portée. Dans ces Exemples, les pourcentages sont en poids sauf indication contraire.The following Examples illustrate the present invention without, however, limiting its scope. In these Examples, the percentages are by weight unless otherwise indicated.
Les tests suivants d'élimination physique et chimique des marqueurs ont été conduits sur les composés des Exemples 1 (de référence), 2 à 18 (de l'invention) et 19 et 20 (comparatifs).The following tests for physical and chemical removal of the markers were carried out on the compounds of Examples 1 (reference), 2 to 18 (of the invention) and 19 and 20 (comparative).
Dans un tube à essai, à 10 mL de gazole de type B0 conforme à la norme EN 590 contenant 10 mg/L de marqueur chimique a été ajouté une quantité - précisée dans la suite du texte - d'agent d'élimination solide : gel de silice1, charbon actif2, alumine3.
1 0,060 - 0,200 mm, 40A, Acros 241660010
2 Comelt Carbosorb 10A
3 Sigma-Aldrich 11028In a test tube, to 10 mL of diesel fuel type B0 conforming to standard EN 590 containing 10 mg / L of chemical marker was added a quantity - specified in the rest of the text - of solid elimination agent: gel silica 1 , activated carbon 2 , alumina 3 .
1 0.060 - 0.200 mm, 40A, Acros 241660010
2 Comelt Carbosorb 10A
3 Sigma-Aldrich 11028
Le tube a été agité en utilisant un agitateur linéaire à 300 mouvements/min pendant 10 min.The tube was shaken using a linear shaker at 300 strokes / min for 10 min.
Le liquide a ensuite été filtré à travers une membrane de polytétrafluoroéthylène (PTFE) de 0,2 µm et analysé par GC/FID.
- Colonne ZB-50 de 15 m x 0,25 mm x 0,25 µm ID.
- Hélium P = 90kPa
- Ecoulement de 50 mL/min.
- Injecteur 280°C
- Détecteur 300°C
- Four : 100°C à 320°C à 20°C/min puis 320°C pendant 19 min.
- Volume injecté : 2 µL.
- ZB-50 column 15 mx 0.25 mm x 0.25 µm ID.
- Helium P = 90kPa
- Flow of 50 mL / min.
- 280 ° C injector
- 300 ° C detector
- Oven: 100 ° C to 320 ° C at 20 ° C / min then 320 ° C for 19 min.
- Volume injected: 2 µL.
Dans un tube à essai, à 10 mL de gazole de type B0 conforme à la norme EN 590 contenant 10 mg/L de marqueur chimique a été ajouté 10 mL d'agent d'élimination chimique : H2SO4, HNO3, NaOH, NaClO, HCl aux concentrations indiquées.In a test tube, to 10 mL of diesel fuel type B0 conforming to standard EN 590 containing 10 mg / L of chemical marker was added 10 mL of chemical elimination agent: H 2 SO 4 , HNO 3 , NaOH , NaClO, HCl at the concentrations indicated.
Le tube a été agité en utilisant un agitateur linéaire à 300 mouvements/min pendant 10 min. Après quelques minutes de repos, le liquide constituant la phase supérieure a été prélevé puis filtré à travers une membrane de polytétrafluoroéthylène (PTFE) de 0,45 µm et analysé par GC/FID. Les conditions d'analyse sont celles indiquées ci-dessus pour le test d'élimination physique.The tube was shaken using a linear shaker at 300 strokes / min for 10 min. After a few minutes of rest, the liquid constituting the phase upper was removed and then filtered through a 0.45 µm polytetrafluoroethylene (PTFE) membrane and analyzed by GC / FID. The analytical conditions are those indicated above for the physical elimination test.
Du gazole contenant le composé des Exemples 1 à 18 à la concentration de 10 mg/L a été soumis aux tests d'élimination physique et chimique décrits ci-dessus. La concentration de chaque composé, mesurée dans le gazole après le traitement d'élimination, est indiquée dans les Tableaux respectivement 1 à 18 en tant que pourcentage de la concentration mesurée avant le traitement d'élimination.Gas oil containing the compound of Examples 1 to 18 at a concentration of 10 mg / L was subjected to the physical and chemical elimination tests described above. The concentration of each compound, measured in the gas oil after the stripping treatment, is shown in Tables 1 to 18, respectively, as a percentage of the concentration measured before the stripping treatment.
Un mélange de 134,2 g de 4'-méthylacétophénone et de 19 g d'acide 4-toluène sulfonique monohydraté a été chauffé jusqu'à 140°C sous agitation. L'eau a été éliminée par distillation pour atteindre la température puis pendant le déroulement de la réaction. La température de 140°C a été maintenue pendant 24 h.A mixture of 134.2 g of 4'-methylacetophenone and 19 g of 4-toluenesulfonic acid monohydrate was heated to 140 ° C with stirring. The water was removed by distillation to reach the temperature and then during the course of the reaction. The temperature of 140 ° C was maintained for 24 h.
Après le refroidissement du mélange réactionnel à la température ambiante, du toluène a été ajouté pour dissoudre la masse. La solution organique a ensuite été lavée 3 fois avec de l'eau puis séchée avec du sulfate de magnésium. Le solvant a été éliminé par distillation sous vide.After the reaction mixture cooled to room temperature, toluene was added to dissolve the mass. The organic solution was then washed 3 times with water and then dried with magnesium sulfate. The solvent was removed by vacuum distillation.
92 g de 1,3,5-tris(4-méthylphényl)benzène ont été obtenus. Une purification par chromatographie sur gel de silice (cyclohexane/acétate d'éthyle : 99/1) suivie d'une recristallisation dans l'éthanol a donné une poudre cristalline beige.
Le composé de l'intitulé a été préparé selon le mode opératoire de l'Exemple 1 excepté que le mélange de départ était un mélange de 176,3 g de 4'-isobutylacétophénone et de 19 g de d'acide 4-toluènesulfonique monohydraté.The title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 176.3 g of 4'-isobutylacetophenone and 19 g of 4-toluenesulfonic acid monohydrate.
126 g d'un liquide brun très visqueux contenant 68% (m/m) de 1,3,5-tris(4-isobutylphényl)benzène ont été obtenus.
Le composé de l'intitulé a été préparé selon le mode opératoire de l'Exemple 1 excepté que le mélange de départ était un mélange de 10 g de 4'-butylacétophénone et de 1,1 g de d'acide 4-toluènesulfonique monohydraté.The title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 10 g of 4'-butylacetophenone and 1.1 g of 4-toluenesulfonic acid monohydrate.
10,1 g de 1,3,5-tris(4-butylphényl)benzène ont été obtenus sous forme d'un liquide brun très visqueux.
1,23 g de 1,3,5-triphénylbenzène ont été mis en suspension dans 10 mL de dichlorométhane et 1,5 mL de 2-chloro-2-méthylpropane. 25 mg de chlorure ferrique ont ensuite été ajoutés. Après quelques minutes, de l'eau et du dichlorométhane ont été ajoutés sous agitation. La solution organique a ensuite été lavée deux fois avec une solution aqueuse à 10 % de carbonate de sodium, séchée avec du sulfate de magnésium et évaporée sous vide. 1,88 g de 1,3,5-tris(4-tert-butylphényl)benzène a été obtenu. Un produit cristallin blanc a été obtenu après recristallisation dans le toluène.
Le composé de l'intitulé a été préparé selon le mode opératoire de l'Exemple 1 excepté que le mélange de départ était un mélange de 10 g de 4'-éthylacétophénone et de 1,3 g de d'acide 4-toluènesulfonique monohydraté.The title compound was prepared according to the procedure of Example 1 except that the starting mixture was a mixture of 10 g of 4'-ethylacetophenone and 1.3 g of 4-toluenesulfonic acid monohydrate.
7,3 g de 1,3,5-tris(4-éthylphényl)benzène ont été obtenus sous forme d'un liquide brun très visqueux.
Sous atmosphère d'argon, un mélange de 630 mg de 1,3,5-tribromobenzène, 1,007 g d'acide 4-fluorophénylboronique, 15 mg d'acétate de palladium et 52 mg de triphénylphosphine dans 4 mL de solution aqueuse de carbonate de sodium 2M et de 13 mL de 1-propanol a été chauffé au reflux sous agitation pendant 3 h.Under an argon atmosphere, a mixture of 630 mg of 1,3,5-tribromobenzene, 1.007 g of 4-fluorophenylboronic acid, 15 mg of palladium acetate and 52 mg of triphenylphosphine in 4 mL of aqueous solution of sodium carbonate. 2M sodium and 13 mL of 1-propanol was refluxed with stirring for 3 h.
20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® puis évaporée sous vide. Le produit brut a été recristallisé dans l'éthanol pour donner 0,53 g de 1,3,5-tris(4-fluorophényl)benzène sous forme d'une poudre cristalline blanc grisâtre.
Le 1,3,5-tris(4-chlorophényl)benzène a été préparé selon le mode opératoire de l'Exemple 1 en remplaçant la 4'-méthylacétophénone par la 4'-chloroacétophénone. Le produit brut a été recristallisé dans l'éthanol. 36,8 g de cristaux blanc jaunâtre ont été obtenus.
Le 1,3,5-tris(4-bromophényl)benzène a été préparé selon le mode opératoire de l'Exemple 1 en remplaçant la 4'-méthylacétophénone par la 4'-bromoacétophénone. Le produit brut a été recristallisé à partir de toluène. 54,3 g de cristaux blanc jaunâtre ont été obtenus.
Un mélange de 12,8 g de 2,4,6-tribromophénol, de 9,85 g de 1-bromododécane et de 10,35 g de carbonate de potassium dans 25 mL de N-éthylpyrrolidone a été chauffé à 115°C sous agitation pendant 2 h. Le mélange a ensuite été versé dans l'eau froide. L'acide chlorhydrique à 16% a été ajouté jusqu'à pH <2. Le produit a ensuite été extrait avec du cyclohexane. La solution organique a ensuite été lavée avec de l'eau, séchée sur carbonate de potassium puis évaporé sous vide pour donner 18,6 g de 1-dodécyloxy-2,4,6-tribromobenzène.A mixture of 12.8 g of 2,4,6-tribromophenol, 9.85 g of 1-bromododecane and 10.35 g of potassium carbonate in 25 mL of N-ethylpyrrolidone was heated to 115 ° C under stirring for 2 h. The mixture was then poured into cold water. 16% hydrochloric acid was added until pH <2. The product was then extracted with cyclohexane. The organic solution was then washed with water, dried over potassium carbonate and then evaporated in vacuo to give 18.6 g of 1-dodecyloxy-2,4,6-tribromobenzene.
Sous atmosphère d'argon, un mélange de 998 mg de 1-dodécyloxy-2,4,6-tribromobenzène, 1,007 g d'acide 4-fluorophénylboronique, 15 mg d'acétate de palladium et 52 mg de triphénylphosphine dans 4 mL de solution aqueuse de carbonate de de sodium 2M et 13 mL de 1-propanol a été chauffé à la température de reflux sous agitation pendant 3 h.Under an argon atmosphere, a mixture of 998 mg of 1-dodecyloxy-2,4,6-tribromobenzene, 1.007 g of 4-fluorophenylboronic acid, 15 mg of palladium acetate and 52 mg of triphenylphosphine in 4 mL of solution aqueous 2M sodium carbonate and 13 mL of 1-propanol was heated to reflux temperature with stirring for 3 h.
20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® et évaporée sous vide. Le produit brut a été recristallisé dans l'éthanol pour donner 0,69 g de 1-dodécyloxy-2,4,6-tris(4-fluorophényl)benzène sous forme d'une poudre cristalline blanche.
Le 1-octyloxy-2,4,6-tribromobenzène a été préparé suivant la procédure de l'Exemple 9 en remplaçant le 1-bromododécane par le 1-bromooctane.1-Octyloxy-2,4,6-tribromobenzene was prepared following the procedure of Example 9 by replacing 1-bromododecane with 1-bromooctane.
Sous atmosphère d'argon, un mélange de 1,33 g de 1-octyloxy-2,4,6-tribromobenzène, 1,51 g d'acide 4-fluorophénylboronique, 99 mg d'acétate de palladium et 297 mg de triphénylphosphine dans 6 mL de solution aqueuse de carbonate de sodium 2M et 20 mL de 1-propanol a été chauffé au reflux sous agitation pendant 2 h.Under an argon atmosphere, a mixture of 1.33 g of 1-octyloxy-2,4,6-tribromobenzene, 1.51 g of 4-fluorophenylboronic acid, 99 mg of palladium acetate and 297 mg of triphenylphosphine in 6 mL of 2M aqueous sodium carbonate solution and 20 mL of 1-propanol was refluxed with stirring for 2 h.
20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® et évaporée sous vide. Le produit brut a été recristallisé dans l'éthanol pour donner 0,4 g de 1-octyloxy-2,4,6-tris(4-fluorophényl)benzène sous forme d'une poudre cristalline blanche.
Le 1-butoxy-2,4,6-tribromobenzène a été préparé suivant la procédure de l'Exemple 9 en remplaçant le 1-bromododécane par le 1-bromobutane.1-Butoxy-2,4,6-tribromobenzene was prepared following the procedure of Example 9 by replacing 1-bromododecane with 1-bromobutane.
Sous atmosphère d'argon, un mélange de 774 mg de 1-butoxy-2,4,6-tribromobenzène, 1,007 g d'acide 4-fluorophénylboronique, 15 mg d'acétate de palladium et 52 mg de triphénylphosphine dans 4 mL d'une solution aqueuse de carbonate de sodium 2M et 13 mL de 1-propanol a été chauffé au reflux sous agitation pendant 2 h.Under an argon atmosphere, a mixture of 774 mg of 1-butoxy-2,4,6-tribromobenzene, 1.007 g of 4-fluorophenylboronic acid, 15 mg of palladium acetate and 52 mg of triphenylphosphine in 4 mL of 2M aqueous sodium carbonate solution and 13 mL of 1-propanol was heated to reflux with stirring for 2 h.
20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® puis évaporée sous vide. Le produit brut a été recristallisé dans l'éthanol pour donner 0,52 g de 1-butoxy-2,4,6-tris(4-fluorophényl)benzène sous forme d'une poudre cristalline blanche.
Sous atmosphère d'argon, un mélange de 998 mg de 1-dodécyloxy-2,4,6-tribromobenzène (voir Exemple 9), 1,14 g d'acide 3,4-difluorophénylboronique, 66 mg d'acétate de palladium et 200 mg de triphénylphosphine dans 4 mL de solution aqueuse de carbonate de sodium 2 M et 13 mL de 1-propanol a été chauffée au reflux sous agitation pendant 2 h.Under an argon atmosphere, a mixture of 998 mg of 1-dodecyloxy-2,4,6-tribromobenzene (see Example 9), 1.14 g of 3,4-difluorophenylboronic acid, 66 mg of palladium acetate and 200 mg of triphenylphosphine in 4 mL of 2 M aqueous sodium carbonate solution and 13 mL of 1-propanol was refluxed with stirring for 2 h.
20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® puis évaporée sous vide. Le produit brut a été recristallisé dans l'éthanol pour donner 0,48 g de 1-dodécyloxy-2,4,6-tris(3,4-difluorophényl)benzène sous forme d'une poudre cristalline blanche.
Sous atmosphère d'argon, un mélange de 886 mg de 1-octyloxy-2,4,6-tribromobenzène (voir Exemple 10), 1,14 g d'acide 3,4-difluorophénylboronique, 15 mg d'acétate de palladium et 52 mg de triphénylphosphine dans 4 mL de solution aqueuse de carbonate de sodium 2 M et 13 mL de 1-propanol a été chauffée au reflux sous agitation pendant 3 h.Under an argon atmosphere, a mixture of 886 mg of 1-octyloxy-2,4,6-tribromobenzene (see Example 10), 1.14 g of 3,4-difluorophenylboronic acid, 15 mg of palladium acetate and 52 mg of triphenylphosphine in 4 mL of 2 M aqueous sodium carbonate solution and 13 mL of 1-propanol was refluxed with stirring for 3 h.
20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® puis évaporée sous vide. Le produit brut a été recristallisé dans l'éthanol pour donner 0,83 g de 1-octyloxy-2,4,6-tris(3,4-difluorophényl)benzène sous forme d'une poudre cristalline grisâtre.
Sous atmosphère d'argon, un mélange de 998 mg de 1-dodécyloxy-2,4,6-tribromobenzène (voir Exemple 9), 979 mg d'acide 4-tolylboronique, 66 mg d'acétate de palladium et 199 mg de triphénylphosphine dans 4 mL de solution aqueuse de carbonate de sodium 2M et 14 mL de 1-propanol a été chauffé au reflux sous agitation pendant 4 h. 20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® puis évaporée sous vide. Le produit brut a été recristallisé dans d'éthanol pour donner 0,59 g de 1-octyloxy-2,4,6-tris(4-méthylphényl)benzène est obtenu sous forme d'une poudre cristalline blanche.
Sous atmosphère d'argon, un mélange de 886 mg de 1-octyloxy-2,4,6-tribromobenzène (voir Exemple 10), 979 mg d'acide 4-tolylboronique, 66 mg d'acétate de palladium et 199 mg de triphénylphosphine dans 4 mL de solution aqueuse de carbonate de sodium 2M et 14 mL de 1-propanol a été chauffé au reflux sous agitation pendant 2 h.Under an argon atmosphere, a mixture of 886 mg of 1-octyloxy-2,4,6-tribromobenzene (see Example 10), 979 mg of 4-tolylboronic acid, 66 mg of palladium acetate and 199 mg of triphenylphosphine in 4 mL of 2M aqueous sodium carbonate solution and 14 mL of 1-propanol was refluxed with stirring for 2 h.
20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® puis évaporée sous vide. Le produit brut a été recristallisé dans l'éthanol pour donner 0,58 g de 1-octyloxy-2,4,6-tris(4-méthylphényl)benzène sous forme d'une poudre cristalline blanche.
Sous atmosphère d'argon, un mélange de 182 mg de 1-butoxy-2,4,6-tribromobenzène (voir Exemple 11), 230 mg d'acide 4-tolylboronique, 15 mg d'acétate de palladium et 47 mg de triphénylphosphine dans 1 mL de solution aqueuse de carbonate de sodium 2M et 3,5 mL de 1-propanol a été chauffé au reflux sous agitation pendant 2 h.Under an argon atmosphere, a mixture of 182 mg of 1-butoxy-2,4,6-tribromobenzene (see Example 11), 230 mg of 4-tolylboronic acid, 15 mg of palladium acetate and 47 mg of triphenylphosphine in 1 mL of 2M aqueous sodium carbonate solution and 3.5 mL of 1-propanol was refluxed with stirring for 2 h.
20 mL d'eau ont ensuite été ajouté. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® puis évaporée sous vide. Le produit brut a été recristallisé dans l'éthanol pour donner 0,15 g de 1-octyloxy-2,4,6-tris(4-méthylphényl)benzène sous forme d'une poudre cristalline blanche.
Sous atmosphère d'argon, un mélange de 998 mg de 1-dodécyloxy-2,4,6-tribromobenzène (voir Exemple 9), 1,37 g d'acide 3-trifluorométhylphénylboronique, 66 mg d'acétate de palladium et 199 mg de triphénylphosphine dans 4 mL de solution aqueuse de carbonate de sodium 2 M et 14 mL de 1-propanol a été chauffé au reflux sous agitation pendant 3 h.Under an argon atmosphere, a mixture of 998 mg of 1-dodecyloxy-2,4,6-tribromobenzene (see Example 9), 1.37 g of 3-trifluoromethylphenylboronic acid, 66 mg of palladium acetate and 199 mg of triphenylphosphine in 4 mL of 2 M aqueous sodium carbonate solution and 14 mL of 1-propanol was refluxed with stirring for 3 h.
20 mL d'eau ont ensuite été ajoutés. Le produit a été extrait deux fois avec de l'acétate d'éthyle. La solution organique a ensuite été lavée avec une solution aqueuse de bicarbonate de sodium à 4% et avec de la saumure saturée, séchée sur du sulfate de magnésium, filtré sur Hyflo® puis évaporée sous vide. 1,62 g de 1-dodécyloxy-2,4,6-tris(3-trifluorométhylphényl)benzène sous forme d'une huile brunâtre.
Le produit est commercialisé par Sigma-Aldrich (réf. 764671.
La demande de brevet
Du gazole contenant du ((3-(sec-butyl)-4-(décyloxy)phényl)méthanetriyl)tribenzène à la concentration de 10 mg/L a été soumis aux tests d'élimination physique et chimique décrits plus haut. La concentration du composé mesurée dans le gazole après le traitement d'élimination est indiquée dans le Tableau 19 comme le pourcentage de la concentration mesurée avant le traitement d'élimination.
Le composé de l'Exemple 2 est nettement plus résistant au traitement par la silice que le composé de l'Exemple Comparatif 19. Par ailleurs, le marqueur de l'Exemple Comparatif 19 est totalement éliminé par l'acide nitrique alors que le composé de l'Exemple 2 présente une très bonne résistance à cet agent chimique.The compound of Example 2 is markedly more resistant to treatment with silica than the compound of Comparative Example 19. On the other hand, the marker of Comparative Example 19 is completely removed by nitric acid while the compound of Example 2 shows very good resistance to this chemical agent.
Le produit est commercialisé par Sigma-Aldrich (réf. T82007).The product is marketed by Sigma-Aldrich (ref. T82007).
Le composé de l'Exemple 2 est nettement plus résistant au traitement par le charbon actif que le TPB. De plus, le composé de l'Exemple 2 est supérieur au TPB pour la résistance au traitement par la silice.The compound of Example 2 is significantly more resistant to treatment with activated carbon than TPB. In addition, the compound of Example 2 is superior to TPB for resistance to silica treatment.
On a conduit ce test sur du gazole de type B0 conforme à la norme EN 590 d'une part sans marqueur et d'autre part avec le marqueur de l'Exemple 2 additionné à raison de 6,8 mg/L.This test was carried out on type B0 gas oil conforming to standard EN 590 on the one hand without a marker and on the other hand with the marker of Example 2 added at a rate of 6.8 mg / L.
Le pourcentage moyen de réduction du flux d'air dans l'injecteur à 0,1 mm de soulèvement d'aiguille est de 70,8 pour le carburant sans marqueur et de 69,5 pour le carburant avec marqueur. L'addition du marqueur n'a donc pas d'influence significative sur l'encrassement des buses d'injection.The average percentage reduction in airflow through the injector at 0.1 mm needle lift is 70.8 for non-marker fuel and 69.5 for marker fuel. The addition of the marker therefore has no significant influence on the fouling of the injection nozzles.
Claims (11)
- Use as a tracer for marking liquid fuels and motor fuels of at least one compound of Formula I:
provided that, when the benzene ring A is not substituted and the benzene rings B, C and D are each substituted by a methyl, the latter is not at para position relative to the benzene ring A. - The use according to claim 1, characterized by the fact that the benzene ring A is non-substituted or substituted by C1-C18 alkoxy.
- The use according to one of claims 1 or 2, characterized by the fact that the benzene rings B, C and D are substituted by:- a linear, branched or cyclic C1-C18 alkyl radical, in particular C1-C12, more particularly C1-C6, optionally substituted by at least one halogen; or- at least one halogen.
- The use according to one of claims 1 to 3, characterized by the fact that a halogen included in the definition of Formula I is F, Cl, Br and I, and in particular F, Cl and Br.
- Liquid fuel or motor fuel containing at least one Formula I compound such as defined in one of claims 1 to 5, characterized by the fact that the Formula I compound is added to the liquid fuel or motor fuel in a proportion of 0.01 to 500 mg/L, particularly in a proportion of 0.02 to 20 mg/L, and more particularly in a proportion of 0.1 to 5 mg/L.
- A method for marking liquid fuels or motor fuels, characterized by the fact that a solution is prepared of 0.1 - 40 weight % of the Formula I compound such as defined in one of claims 1 to 5, in a solvent or mixture of solvents, and said solution is injected into the liquid fuel or motor fuel for example in-line into a pipeline or else into a loading arm of a tanker, or else into a tank to obtain the targeted concentration of the marker in the liquid fuel or motor fuel.
- The method according to claim 7, characterized by the fact that a solution is prepared of 0.2 to 20 weight % of the Formula I compound in the solvent or mixture of solvents.
- The method according to one of claims 7 and 8, characterized by the fact that the solvent(s) are selected from among aromatic solvents such as toluene, ethylbenzene, xylenes, mesitylene, 1.2.4-trimethylbenzene, cumene, p-cymene, aromatic hydrocarbons (e.g. Shellsol® A100, Solvarex® 10, Solvesso® 200); from among aliphatic solvents such as pentane, hexanes, cyclohexane, heptanes, 2,2,4-trimethylpentane, white spirits, kerosene, isoparaffins, paraffins; from among oxygen- and/or nitrogen-containing solvents such as ketones, ethers, esters, lactones, phenols, amides, lactams; or from among halogenated solvents such as chlorobenzene, 1,2-dichlorobenzene, α,α,α-trifluorotoluene.
- A method for determining whether liquid fuels or motor fuels have been tampered with by laundering, characterized by the fact that a sample is taken of the suspected tampered liquid fuel or motor fuel and it is analysed to determine whether the concentration of Formula I compound such as defined in one of claims 1 to 5 in said sample is less than the initial concentration, and in the affirmative it is inferred that the sample has been tampered with by laundering.
- A method for determining whether liquid fuels or motor fuels have been tampered with by diluting, or have been used illegally, or have been the subject of smuggling or theft, characterized by the fact that a sample is taken of the liquid fuel or motor fuel suspected of having been tampered with or used illegally or of being the subject of smuggling or theft, and it is analysed to determine whether the Formula I compound such as defined in one of claims 1 to 5 is contained in said sample, and in the affirmative it is inferred that the sample has been tampered with or used illegally or is the subject of smuggling or theft.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1652729A FR3049614B1 (en) | 2016-03-30 | 2016-03-30 | USE OF TRIARYLBENZENE DERIVATIVES AS MARKERS FOR LIQUID FUELS AND FUELS, LIQUID FUELS AND FUELS CONTAINING SUCH DERIVATIVES AND RELATED METHODS |
| PCT/FR2017/050517 WO2017168066A1 (en) | 2016-03-30 | 2017-03-09 | Use of triarylbenzene derivatives as tracers for marking liquid fuels and motor fuels, liquid fuels and motor fuels containing such derivatives and corresponding processes |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3436552A1 EP3436552A1 (en) | 2019-02-06 |
| EP3436552B1 true EP3436552B1 (en) | 2021-05-12 |
Family
ID=56069124
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP17717209.5A Active EP3436552B1 (en) | 2016-03-30 | 2017-03-09 | Use of triarylbenzene derivatives as tracers for marking liquid fuels and motor fuels, liquid fuels and motor fuels containing such derivatives and corresponding processes |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP3436552B1 (en) |
| ES (1) | ES2877166T3 (en) |
| FR (1) | FR3049614B1 (en) |
| WO (1) | WO2017168066A1 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3775110B1 (en) | 2018-04-05 | 2023-04-19 | Dow Global Technologies, LLC | Substituted dibenzofurans as fuel markers |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU670427B2 (en) | 1992-01-29 | 1996-07-18 | Isotag Technology, Inc. | Method of identifying chemicals by use of non-radioactive isotopes |
| US20050019939A1 (en) * | 2003-07-25 | 2005-01-27 | Dale Spall | Combination marker for liquids and method identification thereof |
| US7858373B2 (en) | 2006-02-03 | 2010-12-28 | Rohm And Haas Company | Chemical markers |
| GB201107870D0 (en) * | 2011-05-11 | 2011-06-22 | Johnson Matthey Plc | Tracers and method of marking hydrocarbon liquids |
| MY169066A (en) * | 2011-06-24 | 2019-02-12 | Dow Global Technologies Llc | Tritylated ethers |
| TWI516468B (en) | 2012-07-06 | 2016-01-11 | 羅門哈斯公司 | Tritylated alkyl aryl ether |
-
2016
- 2016-03-30 FR FR1652729A patent/FR3049614B1/en active Active
-
2017
- 2017-03-09 ES ES17717209T patent/ES2877166T3/en active Active
- 2017-03-09 EP EP17717209.5A patent/EP3436552B1/en active Active
- 2017-03-09 WO PCT/FR2017/050517 patent/WO2017168066A1/en not_active Ceased
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2877166T3 (en) | 2021-11-16 |
| EP3436552A1 (en) | 2019-02-06 |
| FR3049614A1 (en) | 2017-10-06 |
| FR3049614B1 (en) | 2020-02-07 |
| WO2017168066A1 (en) | 2017-10-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Matoušek et al. | Expanding the scope of hypervalent iodine reagents for perfluoroalkylation: from trifluoromethyl to functionalized perfluoroethyl | |
| Shou et al. | Chemoselective hydro (chloro) pentafluorosulfanylation of diazo compounds with pentafluorosulfanyl chloride | |
| Roy et al. | Dissecting Alkynes: Full Cleavage of Polarized C C Moiety via Sequential Bis-Michael Addition/Retro-Mannich Cascade | |
| EP0449699A2 (en) | Pyrazole derivatives as angiotensin II-receptor antagonists, process for their preparation and pharmaceutical compositions containing them | |
| Hashimoto et al. | Direct α‐Siladifluoromethylation of Lithium Enolates with Ruppert‐Prakash Reagent via C F Bond Activation | |
| Wang et al. | Copper‐Catalyzed Intramolecular Carbotrifluoromethylation of Alkynes for the Construction of Trifluoromethylated Heterocycles | |
| EP0596809A1 (en) | Beta-diketones, process for their preparation and their use as polymer stabilizers | |
| BE1004983A3 (en) | CATALYST SYSTEM AND METHOD hydrochlorination CHLORIDE PRODUCTION START IN VINYL CHLORIDE ACETYLENE AND HYDROGEN IN THE PRESENCE OF THIS SYSTEM CATALYST. | |
| WO2005082820A1 (en) | Method for preparation of a fluoroaromatic compound from an aminoaromatic amine compound. | |
| CN103910628A (en) | Production method of α-fluoroacrylate | |
| EP3436552B1 (en) | Use of triarylbenzene derivatives as tracers for marking liquid fuels and motor fuels, liquid fuels and motor fuels containing such derivatives and corresponding processes | |
| Bonge et al. | Highly efficient formation of halodiazoacetates and their use in stereoselective synthesis of halocyclopropanes | |
| Lu et al. | Iron (III) Porphyrin Catalyzed Olefination of Aldehydes with 2, 2, 2‐Trifluorodiazoethane (CF3CHN2) | |
| CA2172450A1 (en) | Reagent and process for grafting a substituted difluoromethyl group on a compound containing a least one electrophilic function | |
| BE1004984A3 (en) | CATALYST SYSTEM AND METHOD hydrochlorination CHLORIDE PRODUCTION START IN VINYL CHLORIDE ACETYLENE AND HYDROGEN IN THE PRESENCE OF THIS SYSTEM CATALYST. | |
| EP0905113B1 (en) | Process for the preparation of 2-chloroprop-1-ene | |
| Richard | Aromatic substitution reactions of amines with ring-substituted 1-phenyl-2, 2, 2-trifluoroethyl carbocations | |
| Looker et al. | Reduction of Methyl Benzoyldiazoacetate. A New Synthesis of Allophenylserine1 | |
| EP3997055B1 (en) | New stable formulations of 1-z-bromoalk-1-ene compounds and use thereof in the manufacture of pheromones | |
| JP2003519112A (en) | Method for producing ester | |
| Fedorovskii et al. | C2-Alkylation in a three-component reaction of fluorocarbonyl compounds, pyrrole derivatives and malononitrile in competition with C2-oxyalkylation | |
| Kharasch et al. | Reactions of Atoms and Free Radicals in Solution. XXXIX. The Reaction of Diacetyl Peroxide with Sec-Butyl Nitrite and 3-Amyl Nitrite | |
| EP3539955B1 (en) | Process for the preparation of tetrazines | |
| Gupta et al. | A simple and efficient method for the synthesis of gem-chloronitroso compounds | |
| CH280180A (en) | Process for preparing beta-hydroxy-carboxylic acid lactones. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: UNKNOWN |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20181026 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C10L 1/185 20060101ALI20201014BHEP Ipc: C10L 1/16 20060101ALI20201014BHEP Ipc: C10L 10/04 20060101ALI20201014BHEP Ipc: C10L 1/00 20060101AFI20201014BHEP Ipc: C10L 1/20 20060101ALI20201014BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20201117 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602017038508 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1392188 Country of ref document: AT Kind code of ref document: T Effective date: 20210615 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1392188 Country of ref document: AT Kind code of ref document: T Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210812 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2877166 Country of ref document: ES Kind code of ref document: T3 Effective date: 20211116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210912 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210813 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210913 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210812 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602017038508 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20220215 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210912 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220309 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20170309 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210512 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20250328 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20250317 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20250314 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20250314 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20250304 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250317 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20250305 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20250415 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20250326 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20250401 Year of fee payment: 9 |