EP3240580B1 - Negative pressure wound dressing with absorbent adhesive sealant layer - Google Patents
Negative pressure wound dressing with absorbent adhesive sealant layer Download PDFInfo
- Publication number
- EP3240580B1 EP3240580B1 EP15832852.6A EP15832852A EP3240580B1 EP 3240580 B1 EP3240580 B1 EP 3240580B1 EP 15832852 A EP15832852 A EP 15832852A EP 3240580 B1 EP3240580 B1 EP 3240580B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- porous layer
- wound dressing
- adhesive gel
- layer
- absorbent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000853 adhesive Substances 0.000 title claims description 184
- 230000001070 adhesive effect Effects 0.000 title claims description 184
- 230000002745 absorbent Effects 0.000 title claims description 121
- 239000002250 absorbent Substances 0.000 title claims description 121
- 239000000565 sealant Substances 0.000 title 1
- 239000012530 fluid Substances 0.000 claims description 15
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 15
- 229910000831 Steel Inorganic materials 0.000 claims description 13
- 239000010959 steel Substances 0.000 claims description 13
- 239000004599 antimicrobial Substances 0.000 claims description 11
- 230000005540 biological transmission Effects 0.000 claims description 11
- 239000013464 silicone adhesive Substances 0.000 claims description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 7
- 239000011148 porous material Substances 0.000 claims description 3
- 239000010410 layer Substances 0.000 description 170
- 239000000499 gel Substances 0.000 description 107
- 206010052428 Wound Diseases 0.000 description 95
- 208000027418 Wounds and injury Diseases 0.000 description 95
- 230000008961 swelling Effects 0.000 description 28
- 239000003795 chemical substances by application Substances 0.000 description 26
- 239000010408 film Substances 0.000 description 24
- 238000012360 testing method Methods 0.000 description 21
- 239000000017 hydrogel Substances 0.000 description 20
- 239000007788 liquid Substances 0.000 description 20
- 229920000642 polymer Polymers 0.000 description 20
- 239000000203 mixture Substances 0.000 description 19
- 239000006260 foam Substances 0.000 description 17
- 229920002554 vinyl polymer Polymers 0.000 description 16
- -1 hydroxypropyl Chemical group 0.000 description 14
- 238000000034 method Methods 0.000 description 11
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 10
- 210000000416 exudates and transudate Anatomy 0.000 description 9
- 239000000463 material Substances 0.000 description 8
- 239000004820 Pressure-sensitive adhesive Substances 0.000 description 7
- 239000000758 substrate Substances 0.000 description 7
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 6
- 238000001035 drying Methods 0.000 description 6
- 239000000416 hydrocolloid Substances 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- 239000004745 nonwoven fabric Substances 0.000 description 6
- 229920001296 polysiloxane Polymers 0.000 description 6
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 5
- 239000002202 Polyethylene glycol Substances 0.000 description 5
- 235000011187 glycerol Nutrition 0.000 description 5
- 230000035876 healing Effects 0.000 description 5
- 229920001223 polyethylene glycol Polymers 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 238000003860 storage Methods 0.000 description 5
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 4
- 244000007835 Cyamopsis tetragonoloba Species 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 4
- 238000007726 management method Methods 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- DXPPIEDUBFUSEZ-UHFFFAOYSA-N 6-methylheptyl prop-2-enoate Chemical compound CC(C)CCCCCOC(=O)C=C DXPPIEDUBFUSEZ-UHFFFAOYSA-N 0.000 description 3
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 3
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 3
- 230000003278 mimic effect Effects 0.000 description 3
- 229920000191 poly(N-vinyl pyrrolidone) Polymers 0.000 description 3
- 238000005096 rolling process Methods 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 239000002759 woven fabric Substances 0.000 description 3
- 101100065878 Caenorhabditis elegans sec-10 gene Proteins 0.000 description 2
- 229920002785 Croscarmellose sodium Polymers 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 229920002367 Polyisobutene Polymers 0.000 description 2
- 229920005830 Polyurethane Foam Polymers 0.000 description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 239000012790 adhesive layer Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 229920002678 cellulose Chemical class 0.000 description 2
- 239000001913 cellulose Chemical class 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- YZIYKJHYYHPJIB-UUPCJSQJSA-N chlorhexidine gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O.C1=CC(Cl)=CC=C1NC(=N)NC(=N)NCCCCCCNC(=N)NC(=N)NC1=CC=C(Cl)C=C1 YZIYKJHYYHPJIB-UUPCJSQJSA-N 0.000 description 2
- 229960003333 chlorhexidine gluconate Drugs 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 239000004020 conductor Substances 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 150000004676 glycans Chemical class 0.000 description 2
- 230000036571 hydration Effects 0.000 description 2
- 238000006703 hydration reaction Methods 0.000 description 2
- 229920001477 hydrophilic polymer Polymers 0.000 description 2
- 229920001600 hydrophobic polymer Polymers 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000005865 ionizing radiation Effects 0.000 description 2
- 150000003951 lactams Chemical class 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 230000035699 permeability Effects 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 239000011496 polyurethane foam Substances 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- NDVLTYZPCACLMA-UHFFFAOYSA-N silver oxide Chemical compound [O-2].[Ag+].[Ag+] NDVLTYZPCACLMA-UHFFFAOYSA-N 0.000 description 2
- 239000008279 sol Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- VAZJLPXFVQHDFB-UHFFFAOYSA-N 1-(diaminomethylidene)-2-hexylguanidine Polymers CCCCCCN=C(N)N=C(N)N VAZJLPXFVQHDFB-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- ZILVNHNSYBNLSZ-UHFFFAOYSA-N 2-(diaminomethylideneamino)guanidine Chemical compound NC(N)=NNC(N)=N ZILVNHNSYBNLSZ-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- OSDLLIBGSJNGJE-UHFFFAOYSA-N 4-chloro-3,5-dimethylphenol Chemical compound CC1=CC(O)=CC(C)=C1Cl OSDLLIBGSJNGJE-UHFFFAOYSA-N 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 108010001478 Bacitracin Proteins 0.000 description 1
- 208000034309 Bacterial disease carrier Diseases 0.000 description 1
- XNCOSPRUTUOJCJ-UHFFFAOYSA-N Biguanide Chemical compound NC(N)=NC(N)=N XNCOSPRUTUOJCJ-UHFFFAOYSA-N 0.000 description 1
- 229940123208 Biguanide Drugs 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- GHXZTYHSJHQHIJ-UHFFFAOYSA-N Chlorhexidine Chemical compound C=1C=C(Cl)C=CC=1NC(N)=NC(N)=NCCCCCCN=C(N)N=C(N)NC1=CC=C(Cl)C=C1 GHXZTYHSJHQHIJ-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 206010056340 Diabetic ulcer Diseases 0.000 description 1
- 206010063560 Excessive granulation tissue Diseases 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 229920002633 Kraton (polymer) Polymers 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 229920005987 OPPANOL® Polymers 0.000 description 1
- 206010030124 Oedema peripheral Diseases 0.000 description 1
- 229920002614 Polyether block amide Polymers 0.000 description 1
- 229920002413 Polyhexanide Polymers 0.000 description 1
- 108010093965 Polymyxin B Proteins 0.000 description 1
- 208000004210 Pressure Ulcer Diseases 0.000 description 1
- 229920002323 Silicone foam Polymers 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 206010040880 Skin irritation Diseases 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- XEFQLINVKFYRCS-UHFFFAOYSA-N Triclosan Chemical compound OC1=CC(Cl)=CC=C1OC1=CC=C(Cl)C=C1Cl XEFQLINVKFYRCS-UHFFFAOYSA-N 0.000 description 1
- NJSSICCENMLTKO-HRCBOCMUSA-N [(1r,2s,4r,5r)-3-hydroxy-4-(4-methylphenyl)sulfonyloxy-6,8-dioxabicyclo[3.2.1]octan-2-yl] 4-methylbenzenesulfonate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)O[C@H]1C(O)[C@@H](OS(=O)(=O)C=2C=CC(C)=CC=2)[C@@H]2OC[C@H]1O2 NJSSICCENMLTKO-HRCBOCMUSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 229920006322 acrylamide copolymer Polymers 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000002313 adhesive film Substances 0.000 description 1
- 239000002998 adhesive polymer Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000845 anti-microbial effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 229960003071 bacitracin Drugs 0.000 description 1
- 229930184125 bacitracin Natural products 0.000 description 1
- CLKOFPXJLQSYAH-ABRJDSQDSA-N bacitracin A Chemical compound C1SC([C@@H](N)[C@@H](C)CC)=N[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]1C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2N=CNC=2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC1 CLKOFPXJLQSYAH-ABRJDSQDSA-N 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 229960003260 chlorhexidine Drugs 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 239000013039 cover film Substances 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 210000001126 granulation tissue Anatomy 0.000 description 1
- 230000037313 granulation tissue formation Effects 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 229940035535 iodophors Drugs 0.000 description 1
- 210000000629 knee joint Anatomy 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000011278 mitosis Effects 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 238000009581 negative-pressure wound therapy Methods 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- RFJIPESEZTVQHZ-UHFFFAOYSA-N oxirane;prop-2-enoic acid Chemical compound C1CO1.OC(=O)C=C RFJIPESEZTVQHZ-UHFFFAOYSA-N 0.000 description 1
- 150000002924 oxiranes Chemical class 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229920006149 polyester-amide block copolymer Polymers 0.000 description 1
- 229920001195 polyisoprene Polymers 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 229920000024 polymyxin B Polymers 0.000 description 1
- 229960005266 polymyxin b Drugs 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920006264 polyurethane film Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- 238000010526 radical polymerization reaction Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 210000004911 serous fluid Anatomy 0.000 description 1
- 239000013514 silicone foam Substances 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 229910001923 silver oxide Inorganic materials 0.000 description 1
- 230000036556 skin irritation Effects 0.000 description 1
- 231100000475 skin irritation Toxicity 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229920001897 terpolymer Polymers 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000013271 transdermal drug delivery Methods 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 125000005208 trialkylammonium group Chemical group 0.000 description 1
- 229960003500 triclosan Drugs 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/46—Deodorants or malodour counteractants, e.g. to inhibit the formation of ammonia or bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0246—Adhesive bandages or dressings characterised by the skin-adhering layer
- A61F13/0253—Adhesive bandages or dressings characterised by the skin-adhering layer characterized by the adhesive material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/00051—Accessories for dressings
- A61F13/00063—Accessories for dressings comprising medicaments or additives, e.g. odor control, PH control, debriding, antimicrobic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0203—Adhesive bandages or dressings with fluid retention members
- A61F13/0206—Adhesive bandages or dressings with fluid retention members with absorbent fibrous layers, e.g. woven or non-woven absorbent pads or island dressings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0203—Adhesive bandages or dressings with fluid retention members
- A61F13/022—Adhesive bandages or dressings with fluid retention members having more than one layer with different fluid retention characteristics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0259—Adhesive bandages or dressings characterised by the release liner covering the skin adhering layer
- A61F13/0266—Adhesive bandages or dressings characterised by the release liner covering the skin adhering layer especially adapted for wound covering/occlusive dressings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/05—Bandages or dressings; Absorbent pads specially adapted for use with sub-pressure or over-pressure therapy, wound drainage or wound irrigation, e.g. for use with negative-pressure wound therapy [NPWT]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/24—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/26—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/425—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/44—Medicaments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/58—Adhesives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/60—Liquid-swellable gel-forming materials, e.g. super-absorbents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/04—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B25/042—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material of natural rubber or synthetic rubber
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/04—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B25/045—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material of foam
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/04—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B25/08—Layered products comprising a layer of natural or synthetic rubber comprising rubber as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/10—Layered products comprising a layer of natural or synthetic rubber next to a fibrous or filamentary layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B25/00—Layered products comprising a layer of natural or synthetic rubber
- B32B25/14—Layered products comprising a layer of natural or synthetic rubber comprising synthetic rubber copolymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B27/065—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material of foam
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B27/08—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/12—Layered products comprising a layer of synthetic resin next to a fibrous or filamentary layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/28—Layered products comprising a layer of synthetic resin comprising synthetic resins not wholly covered by any one of the sub-groups B32B27/30 - B32B27/42
- B32B27/285—Layered products comprising a layer of synthetic resin comprising synthetic resins not wholly covered by any one of the sub-groups B32B27/30 - B32B27/42 comprising polyethers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/34—Layered products comprising a layer of synthetic resin comprising polyamides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/36—Layered products comprising a layer of synthetic resin comprising polyesters
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/40—Layered products comprising a layer of synthetic resin comprising polyurethanes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B3/00—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B3/00—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form
- B32B3/26—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B3/00—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form
- B32B3/26—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer
- B32B3/266—Layered products comprising a layer with external or internal discontinuities or unevennesses, or a layer of non-planar shape; Layered products comprising a layer having particular features of form characterised by a particular shape of the outline of the cross-section of a continuous layer; characterised by a layer with cavities or internal voids ; characterised by an apertured layer characterised by an apertured layer, the apertures going through the whole thickness of the layer, e.g. expanded metal, perforated layer, slit layer regular cells B32B3/12
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/02—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by structural features of a fibrous or filamentary layer
- B32B5/022—Non-woven fabric
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/02—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by structural features of a fibrous or filamentary layer
- B32B5/024—Woven fabric
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/02—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by structural features of a fibrous or filamentary layer
- B32B5/026—Knitted fabric
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/18—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by features of a layer of foamed material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/22—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed
- B32B5/24—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed one layer being a fibrous or filamentary layer
- B32B5/245—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed one layer being a fibrous or filamentary layer another layer next to it being a foam layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/22—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed
- B32B5/24—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed one layer being a fibrous or filamentary layer
- B32B5/26—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed one layer being a fibrous or filamentary layer another layer next to it also being fibrous or filamentary
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B5/00—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts
- B32B5/22—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed
- B32B5/32—Layered products characterised by the non- homogeneity or physical structure, i.e. comprising a fibrous, filamentary, particulate or foam layer; Layered products characterised by having a layer differing constitutionally or physically in different parts characterised by the presence of two or more layers which are next to each other and are fibrous, filamentary, formed of particles or foamed at least two layers being foamed and next to each other
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
- B32B7/08—Interconnection of layers by mechanical means
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
- B32B7/12—Interconnection of layers using interposed adhesives or interposed materials with bonding properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
- B32B7/12—Interconnection of layers using interposed adhesives or interposed materials with bonding properties
- B32B7/14—Interconnection of layers using interposed adhesives or interposed materials with bonding properties applied in spaced arrangements, e.g. in stripes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/404—Biocides, antimicrobial agents, antiseptic agents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2250/00—Layers arrangement
- B32B2250/44—Number of layers variable across the laminate
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/02—Coating on the layer surface on fibrous or filamentary layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/10—Coating on the layer surface on synthetic resin layer or on natural or synthetic rubber layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/26—Polymeric coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/02—Synthetic macromolecular fibres
- B32B2262/0276—Polyester fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/02—Synthetic macromolecular fibres
- B32B2262/0292—Polyurethane fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2262/00—Composition or structural features of fibres which form a fibrous or filamentary layer or are present as additives
- B32B2262/04—Cellulosic plastic fibres, e.g. rayon
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2266/00—Composition of foam
- B32B2266/02—Organic
- B32B2266/0214—Materials belonging to B32B27/00
- B32B2266/0278—Polyurethane
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2266/00—Composition of foam
- B32B2266/06—Open cell foam
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2270/00—Resin or rubber layer containing a blend of at least two different polymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/40—Properties of the layers or laminate having particular optical properties
- B32B2307/412—Transparent
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/51—Elastic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/542—Shear strength
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/546—Flexural strength; Flexion stiffness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/558—Impact strength, toughness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/714—Inert, i.e. inert to chemical degradation, corrosion
- B32B2307/7145—Rot proof, resistant to bacteria, mildew, mould, fungi
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/718—Weight, e.g. weight per square meter
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/72—Density
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/724—Permeability to gases, adsorption
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/726—Permeability to liquids, absorption
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/726—Permeability to liquids, absorption
- B32B2307/7265—Non-permeable
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/728—Hydrophilic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/73—Hydrophobic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/732—Dimensional properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2535/00—Medical equipment, e.g. bandage, prostheses or catheter
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2556/00—Patches, e.g. medical patches, repair patches
Definitions
- NPT negative pressure therapy
- exudates i.e., body secretions
- VAC vacuum assisted closure
- NPT is useful in the treatment of a variety of wound types, including acute, subacute, chronic, traumatic, graphs, flaps, pressure ulcers, and diabetic ulcers. NPT also has been shown to facilitate healing in deep wounds or cavity wounds. In particular, it allows the dead tissue, debris, and/or exudates to be drawn from the wound area under vacuum pressure which increases the rate of healing. In addition, the use of NPT on surgically closed incision has been shown to reduce complications, increase tissue-tissue apposition, and reduce scarring.
- These methods typically include a watertight seal over the wound. Generally, the watertight seal is adhered to the portion of the outer skin which surrounds the wound area.
- One type of wound dressing inserts a porous foam into the wound. Sometimes, a drainage tube, a drainage pump, and a dressing cover are combined with the porous foam insert to form a system which siphons exudates from the wound. There are problems associated with this type of dressing.
- Another type of dressing has been developed which uses a flexible single piece dressing that has a unitary structure which combines a drainage tube as an integral part of the outer wound cover.
- WO 2012/156655 A1 describes a wound dressing comprising a cover layer, an exudate absorbing layer, a porous transmission layer and a wound contact layer with a silicone adhesive applied thereto.
- the cover layer is moisture vapour permeable and has an adhesive coated thereon.
- An orifice is provided in the cover film and in the absorbent layer to allow a negative pressure to be applied to the dressing.
- the wound contact layer comprises through holes forming an open area which comprises 20% or less of an overall area of said layer.
- the present disclosure generally relates to wound dressings.
- the present disclosure relates to wound dressings that can be connected to a source of negative pressure in order to remove undesirable and/or excess fluid at a wound site and thereby promote tissue healing.
- the wound dressing of the present disclosure advantageously provides two routes for the movement of liquid away from the wound site: 1) a manifold porous layer placed in conjunction with a liquid path leading to a vacuum source and 2) a highly moisture-transmissible laminate comprising an absorbent adhesive gel and a backing layer that provide a means for evaporative moisture loss through the wound dressing.
- the highly-conformable absorbent adhesive gel facilitates sealing of the dressing to the skin and thereby helps maintain negative pressure to the wound site even when the dressing is applied to relatively mobile anatomical sites such as a joint (e.g., a knee joint), for example.
- the gels function as absorbent reservoirs for excess wound exudate with high retention of moisture relative to other absorbent layers in the dressing, which enables the use of a smaller canister (i.e., collection reservoir) when the dressing is for negative pressure wound therapy.
- the absorbent adhesive gel provides gentle adhesion to dry periwound skin which reduces skin trauma upon removal and it also provides added securement of the wound site from undesirable shear forces.
- the present disclosure provides a wound dressing.
- the wound dressing can comprise a moisture-transmissible backing layer having a first major surface, a second major surface, a backing layer perimeter, and a first opening; an absorbent adhesive gel adhered to at least a portion of the second major surface of the backing layer, the absorbent adhesive gel comprising an adhesive gel perimeter; and a porous layer having a first side, a second side, and a porous layer perimeter.
- the second major surface has a first adhesive disposed thereon proximate the backing layer perimeter.
- the first side of the porous layer is adhered to the absorbent adhesive gel.
- the porous layer is configured to facilitate passage of fluid to the first opening.
- One-hundred percent of the adhesive gel perimeter is overlapped by the backing layer.
- the porous layer has a porous layer area defined by the porous layer perimeter, wherein the porous layer comprises at least one open area which is a through-hole that is substantially larger than the pores of the porous layer, wherein the at least one open area is greater than or equal to 5% and less than or equal to 50% of the porous layer area.
- the absorbent adhesive gel has a second opening.
- at least a portion of the first opening overlaps at least a portion of the second opening.
- At least 50% of the porous layer perimeter is overlapped by the absorbent adhesive gel. In any embodiment, more than 50% of the porous layer perimeter is overlapped by the absorbent adhesive gel.
- the absorbent adhesive gel can have a thickness of about 0.2 mm to about 4.0 mm. In any of the above embodiments, the absorbent adhesive gel can comprise less than 40% (w/w) water. In any of the above embodiments, the dressing further can comprise a perforated layer adhered to the second side of the porous layer. In any of the above embodiments; the perforated layer, when present, can have a first major surface facing the second side of the porous layer and a second major surface opposite the first major surface, wherein the second major surface includes a silicone adhesive.
- the backing layer with the absorbent adhesive gel adhered thereto has a Moisture Vapor Transmission Rate ⁇ 1 g/10 cm 2 /24 hours, as measured by EN-13726-1:2002 Section 3.3.
- the absorbent adhesive gel can have a shear modulus, G', between about 0.5 newtons/cm 2 and about 5 newtons/cm 2 at 24 °C at a shear of 1 rad/sec.
- the absorbent adhesive gel can have a loss shear modulus, G" between about 0.2 newtons/cm 2 and about 2 newtons/cm 2 at 24° C at a shear of 1 rad/sec.
- the porous absorbent layer can comprise open-cell foam (preferably, a non-water-swellable foam such as the foam used in GRANUFOAMTM dressings available from Kinetic Concepts, Inc. (San Antonio, TX) or the foam used in 3MTM TEGADERMTM High Performance Foam Adhesive Dressings available from 3M Company (St. Paul, MN)), a woven fabric, or a nonwoven fabric.
- the absorbent adhesive gel is capable of absorbing ⁇ 1.5 times its dry weight when contacted with an isotonic saline solution at 37° C for 24 hours.
- hydrogel refers to a continuous phase of a hydrophilic polymer that is capable of swelling on contact with water and other hydrophilic swelling agents. The term is used regardless of the state of hydration. Useful hydrogels will absorb at least 40% by weight based on the hydrogel's weight in an anhydrous state. Hydrogels are hydrophilic polymers characterized by their hydrophilicity (i.e., capable of absorbing large amounts of fluid such as wound exudate). The hydrogels are typically transparent or translucent, regardless of their degree of hydration. Hydrogels are generally distinguishable from hydrocolloids, which typically comprise a hydrophobic matrix that contains dispersed hydrophilic particles.
- hydrocolloid refers to a continuous phase of hydrophobic polymer containing 15-45% by weight of absorbent hydrophilic particles, polymers, or fibers dispersed therein.
- the hydrophobic polymer can be based on silicone or polyolefin.
- Useful hydrocolloids when used as the absorbent adhesive gel) will absorb at least 150% by weight based on its dry weight.
- a porous layer can be interpreted to mean “one or more” porous layers.
- connection and “coupled” are not restricted to physical or mechanical connections or couplings. It is to be understood that other embodiments may be utilized and structural or logical changes may be made without departing from the scope of the present disclosure. Furthermore, terms such as “front,” “rear,” “top,” “bottom,” and the like are only used to describe elements as they relate to one another, but are in no way meant to recite specific orientations of the device, to indicate or imply necessary or required orientations of the device, or to specify how the invention described herein will be used, mounted, displayed, or positioned in use.
- the present disclosure generally relates to negative pressure-type wound dressings. These dressings are used to manage fluid accumulation at a wound site and to create an interfacial pressure between the wound surface and the wound covering such that the formation and growth of granulation tissue is stimulated.
- the inventive dressing provides two distinct mechanisms (e.g., egress routes) for fluid management.
- the materials from which the dressing is constructed provide a surface that facilitates conformity of the wound to anatomical structure and that maintains a negative pressure environment for extended periods of time, relative to other negative pressure dressing configurations.
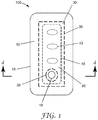
- FIG. 1 is an exploded side view and FIG. 2 is a plan view of one embodiment of a wound dressing 100 according to the present disclosure.
- the dressing 100 comprises a backing layer 10, an absorbent adhesive gel 30 adhered to at least a portion of the moisture-transmissible backing layer 10, and a porous layer 40.
- the dressing 100 optionally comprises a perforated layer 50.
- the wound dressing 100 can be formed in a variety of shapes including, for example, a circle, an oval, a trapezoid, a rectangle, and a square, which can include rounded corners for each of the various shapes.
- the backing layer 10 has a first major surface 12, a second major surface 14 opposite the first major surface, a backing layer perimeter 16 that defines the edge of the backing layer, and an opening 18 that extends from the first major surface 12 to the second major surface 14.
- the backing layer 10 is made from a flexible material (e.g., a polymeric elastic film, an elastic non-woven fabric, or combinations thereof) that is compliant enough to conform to the anatomical contours of skin surfaces.
- the backing layer perimeter 16 defines outer edges of the backing layer 10.
- Suitable backing layers 10 include, for example, nonwoven fibrous webs, woven fibrous webs, knits, films and other familiar backing materials.
- the preferred backing materials are translucent or transparent polymeric elastic films.
- the backing is a high moisture vapor permeable film backings.
- U.S. Patent No. 3,645,835 describe methods of making such films and methods for testing their moisture vapor permeability.
- the backing layer 10 has a thickness of about 15 ⁇ m to about 250 ⁇ m.
- Adhered to (e.g., coated onto) at least a portion is a first adhesive 20.
- the first adhesive 20 forms a seal (e.g., a liquid seal around the entire backing layer 16) between the backing layer 10 and the skin to which the dressing is applied.
- the backing layer/first adhesive composite should permit transmission of moisture vapor therethrough at a rate equal to or greater than human skin.
- the uncoated film i.e., the backing layer without the first adhesive coated thereon
- the uncoated backing layer transmits moisture greater than 20 g/10 cm 2 /24 hours.
- the adhesive coated portions of the backing layer transmit moisture vapor at rate of at least 1 g/10 cm 2 /24 hours, and preferably greater than 1.6 g/10 cm 2 /24 hours.
- the backing layer 10 is preferably conformable to anatomical surfaces. As such, when the backing layer is applied to an anatomical surface, it conforms to the surface even when the surface is moved.
- the preferred backing layer 10 is also conformable to animal anatomical joints. When the joint is flexed and then returned to its unflexed position, the backing layer stretches to accommodate the flexion of the joint, but is resilient enough to continue to conform to the joint when the joint is returned to its unflexed condition.
- the backing layer has an ultimate elongation of greater than 200%. More preferably, the backing layer has an ultimate elongation of greater than 400%.
- backing layers preferred for use with the wound dressing of the present disclosure can be found in issued U.S. Patent Nos. 5,088,483 and 5,160,315 .
- Particularly preferred backing layers are elastomeric polyurethane, co-polyester, or polyether block amide films. These films combine the desirable properties of resiliency, high moisture vapor permeability, and transparency found in preferred backing layers.
- any pressure sensitive adhesive can be used for the first adhesive 20
- preferred adhesives include a pressure sensitive adhesive that is reasonably skin compatible, such as soft silicone gel adhesives, Kraton® based adhesive, hydrocolloid adhesives, or the acrylate copolymers described in U.S. Patent No. RE 24,906 .
- Particularly preferred is a 97:3 iso-octyl acrylate:acrylamide copolymer.
- an 70:15:15 isooctyl acrylate: ethyleneoxide acrylate:acrylic acid terpolymer as described in U.S. Patent No. 4,737,410 (Example 31).
- Other useful adhesives for first adhesive 20 are described in U.S. Patent Nos.
- the preferred pressure sensitive adhesives for first adhesive 20 described above preferably transmit moisture vapor at rate greater to or equal to that of human skin. While such a characteristic can be achieved through the selection of an appropriate adhesive or through use of a nonwoven (e.g., melt blown) adhesive (as described in U.S. Patent No. 6,171,985 , U.S. Patent No. 6,368,687 , and PCT Publication No. WO 99/27975 ), it is also contemplated in the wound dressings of the present disclosure that other methods of achieving a high relative rate of moisture vapor transmission may be used, such as pattern coating (not shown) the first adhesive on the backing layer. In addition, this adhesive has low absorbency (i.e., less than 25% its dry weight, and preferably less than 10% its dry weight) when submerged in isotonic saline at 37 Celsius for 24 hours.
- a nonwoven (e.g., melt blown) adhesive as described in U.S. Patent No. 6,171,985 , U.S. Patent No
- the absorbent adhesive gel 30 is adhered to at least a portion of the second major surface 14 of the backing layer 10.
- the absorbent adhesive gel 30 comprises an absorbent adhesive gel perimeter 36 that defines the outer edge of the adhesive gel perimeter.
- the absorbent adhesive gel perimeter 36 is spaced apart from the backing layer perimeter 16.
- a first piece e.g. the backing layer
- the second piece e.g., the absorbent adhesive gel
- one piece can "overlap" or "overlie” another piece even though separated by a third piece.
- the absorbent adhesive gel comprises a second opening 38.
- the second opening 38 preferably is spaced apart from the absorbent adhesive gel perimeter.
- At least a portion of the first opening 18 of the backing layer 10 does not have absorbent adhesive gel 30 between it and the porous layer (e.g., the first opening is located at a portion where there is no absorbent adhesive gel between the first opening and the porous layer, as shown in the wound dressing 102 of FIG. 3A ; or the first opening overlaps at least a portion of a second opening 38 in the absorbent adhesive gel, as shown in FIG. 3B ).
- the first and second openings can be coextensive.
- the absorbent adhesive gel 30 can be relatively thin. In any embodiment, the absorbent adhesive gel has a thickness of about 0.2 mm to about 4.0 mm. In any embodiment, the absorbent adhesive gel has a thickness of about 0.4 mm to about 3 mm.
- the absorbent adhesive gel 30 is capable of absorbing and retaining an aqueous liquid having an ionic strength similar to blood. Thus, this property can be tested easily by quantifying the absorbance of physiological saline.
- the absorbent adhesive gel is capable of absorbing at least 0.4 times its dry weight; preferably, at least 1.0 times its dry weight; more preferably, greater than or equal to 1.5 times its dry weight; even more preferably, greater than or equal to 4 times its dry weight when contacted with an isotonic saline solution at 37° C for 24 hours.
- the gel also has high moisture retention in that it will retain greater than 50% of what it absorbs (i.e., residual absorbent capacity) when externally compressed with 40 mm Hg pressure.
- the absorbent adhesive gel 30 can comprise a hydrogel that includes polyglycerol-3, crosslinked polyvinylpyrrolidone, and/or hydroxypropyl guar.
- suitable absorbent adhesive hydrogels and methods of making said absorbent adhesive hydrogels are described in U.S. Patent Application No. 2009/0187130 .
- the absorbent adhesive gel may comprise an antimicrobial agent such as, for example an antimicrobial biguanide (e.g., chlorhexidine gluconate).
- Suitable hydrogel compositions include, for example, a natural hydrogel, such as pectin, gelatin, or carboxymethylcellulose (CMC) (Aqualon Corp., Wilmington, Del.), a semisynthetic hydrogel, such as cross-linked carboxymethylcellulose X4ink CMC) (e.g. Ac-Di-Sol; FMC Corp., Philadelphia, Pa.), a synthetic hydrogel, such as cross linked polyacrylic acid (PAA) (e.g., CARBOPOLTMNo. 974P; B.F. Goodrich, Brecksville, Ohio), or a combination thereof.
- PPAA cross linked polyacrylic acid
- the hydrogel adhesive comprises a swellable, crosslinked poly(N-vinyl lactam), a swelling agent and a modifying polymer present in an amount sufficient to form a cohesive, pressure-sensitive adhesive composition as described further in U.S. Patent Publication No. 2004/0247655 .
- the amount of swelling agent to be mixed with the crosslinked swellable poly(N-vinyl lactam) can range from about 50 to about 90 weight percent of the composition. Consequently, exclusive of any biocompatible and/or therapeutic and/or ionically conductive materials to be added to the composition, the weight percent of the swellable poly(N-vinyl lactam) can be from about 10 to about 50 weight percent.
- the weight percent of poly(N -vinyl pyrrolidone) can range from about 15 to about 45 percent. In particular embodiments, the poly (N -vinyl pyrrolidone) can range from about 18 percent to about 35 percent.
- the hydrogel adhesive composition of the present invention comprises a swellable, poly(N-vinyl lactam) that is radiation-crosslinked, typically while the lactam is in a solid form.
- the poly (N -vinyl) lactam is crosslinked by free-radical polymerization, either in bulk or in solution, of a precursor containing an N-vinyl lactam monomer, optionally other monomers, and a crosslinking compound as described in U.S. Patent No. 4,931,282 .
- Poly(N-vinyl lactam) useful in an absorbent adhesive of present disclosure can be provided in any form susceptible to being crosslinked such as the solid forms described in U.S.
- the poly(N-vinyl lactam) is a homopolymer of N-vinyl-2-pyrrolidone.
- poly(N-vinyl lactam) After exposure to ionizing radiation, poly(N-vinyl lactam) can have a swelling capacity in water of at least about 15, typically at least about 30, and often at least about 40 as described in U.S. Patent No 5,409,966 .
- Poly(N-vinyl lactam) in any solid form may be crosslinked for use when subjected to ionizing radiation from a high-energy source.
- the modifying polymer is present in the hydrogel adhesive composition to maintain and/or increase cohesiveness while reducing adhesiveness.
- the modifying polymer When added with the swelling agent, the modifying polymer becomes solubilized or suspended in the swelling agent.
- the modifying polymer will form a viscous solution or viscous gel when combined with the swelling agent in a ratio of modifying polymer to swelling agent of 1:9.
- modifying polymers typically will determine the appropriate modifying polymer to accomplish a reduction in adhesion while maintaining or improving cohesion of the adhesive composition.
- Modifying polymers that are poorly solubilized in one swelling agent may be highly swollen in a different swelling agent for use in the present invention.
- suitable modifying swellable polymers include, but are not limited to, polysaccharides, polysaccharide derivatives, acrylates, acrylate derivatives, cellulose, cellulose derivatives, and combinations thereof.
- modifying swellable polymers for use in the present invention are hydroxypropyl guar; guar gum; hydroxyethyl cellulose; hydroxypropyl cellulose; hydroxypropyl methylcellulose; polymeric quaternary ammonium salt of hydroxyethyl cellulose reacted with trialkyl ammonium substituted epoxide; copolymers of hydroxyethyl cellulose and diallyldimethyl ammonium chloride; and derivatives and combinations of the foregoing.
- the amount of modifying polymer can range up to about 50 weight percent of the composition. Consequently, exclusive of any biocompatible and/or therapeutic and/or ionically-conductive materials to be added to the composition, the weight percent of the modifying polymer can be from about 0.1 to about 40 weight percent. When the modifying polymer is hydroxypropyl guar, the weight percent of hydroxypropyl guar can range from about 1 to about 20 percent.
- the hydrogel adhesive composition also comprises a swelling agent which can swell both the crosslinked poly(N-vinyl lactam) polymer and the modifying polymer, and which is biocompatible with human skin.
- swelling agents useful to swell the poly(N-vinyl lactam) include monohydric alcohols (e.g., ethanol and isopropanol), polyhydric alcohols, (e.g., ethylene glycol, propylene glycol, polyethylene glycol (Molecular Weight between 200 and 600) and glycerin), ether alcohols (e.g., glycol ethers), other polyol swelling agents which do not cause skin irritation or toxic reaction, and water.
- non-volatile and/or volatile swelling agents may be used.
- One suitable swelling agent may comprise volatile swelling agent and non-volatile swelling agent, such as a mixture of glycerin or polyethylene glycol with water.
- non-volatile swelling agents may be used by themselves such as, for example, glycerin or polyethylene glycol.
- volatile swelling agents such as water may be used by themselves in the compositions of the invention.
- essentially non-volatile means that a swelling agent as used in the present invention will render the adhesive polymer, such as radiated poly(N-vinyl lactam), sufficiently cohesive and pressure sensitive adhesive, such that less than ten percent (10%) of a given volume of nonvolatile swelling agent evaporates after exposure to processing or storage conditions.
- the swelling agent can be added in an amount ranging from about 50 to about 90 weight percent of the hydrogel adhesive composition and preferably from about 60 to about 80 weight percent.
- glycerin and polyethylene glycol are chosen to be the essentially non-volatile swelling agent. Both glycerin and polyethylene glycol can comprise up to 100 weight percent of the swelling agent.
- the absorbent adhesive gel 30 is useful for containing a number of substances, optionally including antimicrobial agents, drugs for transdermal drug delivery, chemical indicators to monitor hormones or other substances in a patient, etc.
- the absorbent adhesive gel can deliver an antimicrobial agent to the skin, reducing the likeliness of an infection to a percutaneous device or to treat infections of the skin or wounds.
- the antimicrobial agent is added in levels up to 10% by weight of the total composition.
- antimicrobial agents include parachlorometaxylenol; triclosan; chlorhexidine and its salts such as chlorhexidine gluconate, polyhexamethylene biguanide and its salts such as poly hexamethylene biguanidine chloride, iodine, idodophors, fatty acid monoesters; poly-n-vinyl pyrrolidone-iodophors; silver oxide, silver and its salts, peroxides (e.g. hydrogen peroxide), antibiotics (e.g. neomycin, bacitracin, and polymyxin B).
- suitable antimicrobial agents are those listed in U.S. Patent Publication No. 2004/0247655 .
- a method of preparing a hydrogel adhesive composition of the present disclosure comprises mixing crosslinked poly(N-vinyl lactam) with a swelling agent and a modifying polymer, and other additives in a solvent which is may be somewhat volatile at or above ambient temperatures.
- the swelling agent, modifying polymer, and other additives, such as antimicrobial agents are in essentially unirradiated form.
- suitable volatile solvents include water, ethanol, methanol, and isopropanol.
- a quantity of the resulting suspension is then cast onto a surface of a substrate, such as a release liner or a backing material and then stored.
- the volatile solvent is evaporated by heating such as by the application of microwave energy, infrared energy, or by convective air flow or the like, in order to form a cohesive, pressure-sensitive adhesive composition on the substrate. Often, a drying oven heated to about 65 degree C may be employed for the evaporation step.
- a product release liner can optionally be laminated over the exposed surface of the composition to protect it from contamination.
- the absorbent adhesive gel 30 can comprise a hydrocolloid that contains polyisobutylene, a polyisoprene based polymer, a soluble absorbent, an insoluble absorbent, and a tackifier.
- Suitable hydocolloid compositions include, for example, 20-40% by weight of polyisobutylene (OPPANOL from BASF), 15-40% of a polyisprene based polymer, and a 15-45% by soluble and insoluble absorbents such as carboxymethylcellulose (CMC) (Aqualon Corp., Wilmington, Del.) and cross-linked carboxymethylcellulose X4ink CMC) (e.g. Ac-Di-Sol; FMC Corp., Philadelphia, Pa.), respectively, and less than 15% by weight of a tackifier such as WINGTACK 95 (Sartomer Co., Exton, PA).
- CMC carboxymethylcellulose
- X4ink CMC cross-linked carboxymethylcellulose X4ink CMC
- the hydrocolloid comprises a crosslinked hydrophobic silicone gel with soluble and/or insoluble absorbents dispersed therein.
- the absorbent content should be 15-45% by weight.
- the absorbent adhesive gel 30 of the present disclosure when adhered to the backing layer 10, is moisture transmissive.
- the backing layer 10 with the absorbent adhesive gel 30 adhered thereto has a Moisture Vapor Transmission Rate (MVTR) ⁇ 1 g/10 cm 2 /24 hours, as measured by EN-13726-1:2002 Section 3.3.
- this Moisture Vapor Transmission Rate is greater than 3 g/10 cm 2 /24 hours.
- the wound dressing 100 has a moisture vapor-transmissive backing layer and a moisture vapor-transmissive absorbent adhesive gel
- moisture can be transported away from the wound site and out of the dressing 100 by two means: 1) passage out of the dressing (in the liquid state) through the first opening and out of the dressing, and 2) passage through the absorbent adhesive gel and out of the intact backing layer (in the vapor state).
- a high moisture vapor transmission rate through the gel and backing layer is important because it reduces the size of the canister needed for collection of wound exudate when using vacuum therapy for wounds.
- a high MVTR gel and backing is especially useful in the embodiment when the vacuum source is a created mechanically using constant force springs such as that used in the SNaP® Wound Care System (Spiracur, Inc.; Sunnyvale, CA).
- the high MVTR gel/backing system prolongs the effective life of a mechanical vacuum system because fluid is removed via evaporation and does not fill the canister or cartridge.
- the absorbent adhesive gel before applying the wound dressing of the present disclosure to a treatment site, comprises less than 40% (w/w) water. In any embodiment, before applying the wound dressing of the present disclosure to a treatment site, the absorbent adhesive gel comprises less than 25% (w/w) water. Preferably, before applying the wound dressing of the present disclosure to a treatment site, the absorbent adhesive gel comprises less than 10% (w/w).
- the use of an absorbent adhesive gel comprising ⁇ 40% (w/w) water, ⁇ 25% (w/w) water, or ⁇ 10% (w/w) reduces the need for specialized packaging that would otherwise be needed to preserve the water in the gel during storage before use.
- the elastic properties (e.g., as indicated by the storage modulus (G')) of the absorbent adhesive gel is selected so that, within the range of thicknesses of the absorbent adhesive gel, the wound dressing is conformable, yet of high integrity so that it won't readily fall apart when stretched.
- the absorbent adhesive gel before applying the wound dressing of the present disclosure to a treatment site, has a storage shear modulus between about 5,000 pascals and about 50,000 pascals at 24 °C at a shear rate of 1 rad/sec. Preferably between 10,000 pascals and about 30, 000 pascals.
- the loss modulus (G") indicates the viscous response of the absorbent adhesive gel 30.
- the absorbent adhesive gel before applying the wound dressing of the present disclosure to a treatment site, has a loss shear modulus between about 2,000 pascals and about 20,000 pascals (inclusive) at 24 °C at a shear of 1 rad/sec. Preferably, between about 3,000 pascals and about 15, 000 pascals, inclusive.
- an absorbent adhesive gel having a loss modulus G" between about 2,000 pascals and about 20,000 pascals (inclusive) at 24 °C at a shear of 1 rad/sec is sufficiently soft to create a good vacuum seal on skin but not so soft that flows readily and easily loses its integrity.
- a wound dressing 100 comprises a porous layer 40.
- the porous layer 40 has a first side 42, a second side 44, and a porous layer perimeter 46 that defines the outer edge of the porous layer, which further defines a porous layer area.
- the first side 42 of the porous layer 40 is adhered to the absorbent adhesive gel 30.
- at least 50% of the porous layer perimeter is overlapped by the absorbent adhesive gel.
- more than 50% of the porous layer perimeter is overlapped by the absorbent adhesive gel.
- at least 60% of the porous layer perimeter is overlapped by the absorbent adhesive gel.
- at least 70% of the porous layer perimeter is overlapped by the absorbent adhesive gel.
- the porous layer perimeter is overlapped by the absorbent adhesive gel. In any embodiment, at least 80% of the porous layer perimeter is overlapped by the absorbent adhesive gel. In any embodiment, at least 85% of the porous layer perimeter is overlapped by the absorbent adhesive gel. In any embodiment, at least 90% of the porous layer perimeter is overlapped by the absorbent adhesive gel. In any embodiment, at least 95% of the porous layer perimeter is overlapped by the absorbent adhesive gel. In any embodiment, 100% of the porous layer perimeter is overlapped by the absorbent adhesive gel. Preferably, the gel extends past the porous layer perimeter (i.e., toward the backing layer perimeter) by at least 3 mm and more preferably at least 5 mm.
- the absorbent adhesive gel also acts as a reservoir for fluid such that it will take up moisture from the porous layer which facilitates keeping the porous layer free from liquid.
- the porous layer 40 is configured to facilitate passage of fluid (e.g., blood or other fluids leaking from a wound site such as an incisional wound, for example) through the porous layer to the first opening 18 of the backing layer 10.
- fluid e.g., blood or other fluids leaking from a wound site such as an incisional wound, for example
- the porous layer 40 can comprise open-cell foam, a woven fabric (e.g., gauze), or a nonwoven fabric. Multi-layer porous polymer films would also be acceptable.
- the porous layer 40 can be combination of layers of material.
- a non-woven fabric laminated (or otherwise attached) to a foam could be considered the porous layer.
- a non-woven fabric with a porous film (macroporous or microporous) laminated to one or both sides of it, that is then laminated to an open cell foam can function as a good porous layer because the multiple layers enables fluid to readily pass through the porous layer and because it is more resistant to collapse under vacuum.
- the porous layer 40 additionally comprises one or more open area 43.
- the porous layer 40 optionally comprises a plurality of open areas 43.
- the open area 43 is a through-hole that is substantially larger than the pores of the porous layer 40.
- a sum of the open areas 43 has an area that is greater than or equal to 5 percent and less than or equal to 50 percent of the porous layer area.
- the sum of the open areas 43 has an area that is greater than or equal to 5 percent and less than or equal to 20 percent of the porous layer area.
- the open areas 43 function to facilitate visualization of the wound site without removing the dressing.
- the open areas should be large enough such that the adhesive gel will conform to the sides of the open area and make contact with the skin or the perforated layer upon application of vacuum.
- the holes are ovals, circles, squares, rectangles, or diamond in shape, for example, and a hole is at least 0.4 cm 2 in area, and preferably greater than 0.75 cm 2 in area, and most preferably greater than 1.5 cm 2 in area.
- the area of an individual hole should not exceed 13 cm 2 as too large of an open area reduces the available area of porous layer for communicating negative pressure to the wound and for evacuating fluid away from the wound.
- the open areas 43 can take the form of any shape.
- the illustrated embodiment of the wound dressing 100 of FIGS. 1 and 2 comprises a plurality of oval-shaped open areas.
- Nonlimiting examples of shapes that are suitable for open areas 43 include circles, triangles, squares, rectangles, and other polygons.
- a wound dressing 100 of the present disclosure optionally comprises a perforated layer 50.
- the perforated layer 50 comprises a substrate 51 (e.g., a polymeric film substrate, a foam, a non-woven fabric, a woven fabric, or a combination of any two or more of the foregoing materials) with a first surface 52, a second surface 53, a perforated layer perimeter 56, and a plurality of perforations 54 extending therethrough.
- the perforations 54 provide a liquid pathway for exudate from the wound to pass through the substrate 51 and into the porous layer 40.
- the first surface 51 of the perforated layer 50 is adhered to the second side 44 of the porous layer 40 via a second adhesive 56, which may be coated onto the perforated layer 50.
- the second adhesive may be any suitable adhesive for adhering the perforated layer 50 to the porous layer 40.
- the perforated layer 50 may comprise a third adhesive 58 coated thereon.
- the third adhesive 58 can provide additional securement of the wound dressing 100 to the treatment site (skin).
- the third adhesive 58 can comprise a hydrophobic soft silicone adhesive.
- the second adhesive 56 and third adhesive 58 can be coated onto the perforated layer 50 prior to perforating the substrate and, thus, the second and third adhesive also comprise perforations.
- the second adhesive 56 and third adhesive 58 can be pattern coated onto the substrate 51.
- the perforations 54 in the perforated layer 50 not only provide a pathway for moisture transmission to the porous layer 40, they also render the perforated layer 50 highly conformable to the anatomical surfaces of various wound sites.
- the perforated layer does not have to be coextensive with the perimeter of porous layer or the open areas.
- the third adhesive 58 is a soft silicone adhesive and is greater than 50 microns thick; more preferably, greater than 75 microns thick.
- the third adhesive 58 provides added securement of the wound dressing to the tissue and provides improved vacuum sealing properties at the skin/adhesive interface proximate the porous layer.
- the adhesion to steel of this porous layer with a soft silicone adhesive layer is less than the adhesion to steel of the absorbent adhesive gel and/or the adhesive layer coated on the backing.
- the third adhesive has low absorbency in isotonic saline at 37 Celsius for 24 hours. (i.e., less than 25% of its dry weight, and preferably less than 10% its dry weight).
- the dressing further comprises a vacuum port (not shown in FIGS 1-3 ).
- the vacuum port is a structure that permits operatively coupling the wound dressing to a source of negative pressure, as shown in FIG. 4 .
- the vacuum port can be any suitable vacuum port known in the art that can be sealingly attached (e.g., via a pressure sensitive adhesive, a secondary transparent adhesive/film dressing, or a heat seal) to the backing layer.
- FIGS. 1 and 2 show a wound dressing 100 wherein the absorbent adhesive gel perimeter 36 completely overlaps the porous layer perimeter 46, it is contemplated that, in certain embodiments, the absorbent adhesive gel perimeter 36 does not overlap 100% of the porous layer perimeter 46, as discussed above.
- FIG. 3 shows one embodiment of a wound dressing 101 wherein the absorbent adhesive gel perimeter 36 of the absorbent adhesive gel 30 does not completely overlap the porous layer perimeter 46 of the porous layer 40.
- the backing layer perimeter 16 of the backing layer 10 completely overlaps both the absorbent adhesive gel perimeter 36 and the porous layer perimeter 46.
- FIG. 3A is the first opening 18 of the backing layer 10 and the second opening 38 of the absorbent adhesive gel 30.
- FIG. 4 shows a side view, partially in section, of one embodiment of a wound dressing 103 applied to the surface of skin 5 at a treatment site.
- the wound dressing 103 is identical to the wound dressing 100 of FIG. 1 except that, in addition to comprising a backing layer 10 with a first opening (not shown), an absorbent adhesive gel 30 comprising a second opening 38, a porous layer 40 with a plurality of open areas 43, and a perforated layer 50; the wound dressing 103 further comprises a vacuum port 70.
- the vacuum port 70 is operatively coupled to a source of negative pressure 80 (e.g., a vacuum pump (not shown)) via tubing 82.
- the first adhesive (not shown) on the second major surface 14 forms a liquid-tight seal on the skin 5.
- a wound dressing comprising a vacuum port according to the present disclosure can be operatively connected to a source of vacuum.
- the source of vacuum can be a mechanical pump, for example.
- the source of vacuum can be a mechanically-powered, portable vacuum pump.
- the mechanically-powered vacuum source comprises one or more constant force spring.
- the adhesion to steel test was conducted using 304 AISI stainless steel plates with a bright annealed finish. Dimensions of the plates were 51 mm x 127 mm x 1.6 mm.
- the test surfaces was cleaned prior to each test one time using a disposable laboratory wipe saturated with isopropanol and three times with a disposable laboratory wipe saturated with n-heptane. The surface was allowed to dry prior to placing the sample on the test surface. The length of the test sample was 1" wide. Prior to measuring the adhesive force, the adhesive side of the sample was manually pressed against the steel plate and the nonadhesive side of the sample was rolled with a roller once in each direction to press the adhesive side against the plate.
- the roller had a total diameter of 3.75" (95 mm), a face width of 1.75" (44 mm), and it was covered with approximately 0.25" (6 mm) thickness of 75 - 85 durometer hardness rubber having an effective rolling weight of 4.5 pounds or 2043 grams.
- the rolling speed was approximately 50 mm/second.
- the sample was tested immediately after rolling using a 180 degree peel at a rate of 305 mm/min. Testing was conducted in a room at 25 Celsius and 50% relative humidity.
- a test apparatus was constructed to mimic an incisional wound.
- a schematic top view of the apparatus is shown in FIG. 5 .
- the apparatus comprised a slit into which liquid was fed through three spaced-apart ports.
- the slit was intended to mimic an opening in the skin at the site of an approximately 150 mm long incision-type wound.
- the liquid fed through the spaced-apart ports was intended to mimic blood and/or serous fluid flow or leakage from an incision-type wound.
- the slit 92 was 1 mm wide, 1.6 mm deep, and 152 mm long, and two of the liquid feed port holes 94 were spaced inward 19 mm from the their respective end of the slit with the remaining feed port hole 94 in the center of the slit.
- Three vacuum measurement ports 96 were located about 2 mm from the slit. One vacuum measurement port was located proximate one end of the slit, and the other two were on opposite sides of the slit about 50 mm from their respective slit ends. All ports extended completely through the table and the ports were threaded on the bottom of the table. Hose barb fittings were connected to both the feed ports and the vacuum measurement ports at the bottom of the table for easy connection with appropriate tubing.
- fluid was fed to each of the feed ports at the bottom of the aluminum table via a three syringes disposed in a syringe pump (Model NE-1600; New Era Pump Systems, Inc.; Farmingdale, NY). Vacuum was measured using pressure transducers from Omega Engineering (Stamford, Connecticut). When testing examples with this apparatus, vacuum was applied through a port that was sealed to the top of the samples described in the examples using an ATMOS WOUND RX 041 vacuum unit (Allentown, PA). All experiments with this test apparatus were operated at ambient temperature (approximately 24 Celsius) and ambient humidity (35-65% Relative humidity). The vacuum measured was the pressure difference from atmospheric. For example, a reading of 120 mm Hg means that the pressure in the system or dressing was 120 mm Hg below atmospheric pressure.
- the adhesive side of wound dressings made according to Example 1 were pressed against the surface of the test apparatus such that the absorbent foam layer of the wound dressings overlapped the slit, the liquid feed ports, and the vacuum measurement ports.
- An adhesive-coated backing layer (such as can be obtained from 3M Transparent TEGADERMTM Film Roll 16004; 3M Company, St. Paul, MN) was cut into a rectangle approximately 10.16 cm x 27.94 cm. A 1.27 cm diameter hole was punched through the adhesive/film backing approximately 2.5" (6.35 cm) from one edge of the dressing, and approximately centered along the width of the adhesive film backing as shown in FIG. 1 . The release liner protecting the adhesive was then removed.
- a large absorbent adhesive gel was made using the method described in Example E3 of US2009/0187130 , which is incorporated herein by reference in its entirety.
- the absorbent adhesive gel was cut into a rectangle of 57 mm x 229 mm.
- a 38 mm diameter hole was punched out proximate an edge of the absorbent adhesive gel.
- the absorbent adhesive gel was then laminated to the adhesive side of the breathable backing layer such that a hole in the adhesive/film back was approximately centered with the hole in the absorbent adhesive gel.
- a 3.3 mm thick absorbent polyurethane foam (used in the 90642 3M TEGADERM Silicone Foam Border dressing; foam available from 3M Company, St. Paul, MN)was laminated to an absorbent non-woven layer used in the 3M TEGADERM+ Pad product line available from 3M Company (St. Paul, MN) using a layer of porous adhesive (approximately 25% open).
- An eight inch (20.3 cm) by two inch (5.1 cm) rectangle with rounded corners was cut out of this foam/non-woven laminate to form the porous layer. Starting approximately 12.5 mm from one end of the porous layer, five oval-shaped (16 mm x 25 mm) open areas were punched out of the rectangle.
- a perforated (ca. 15% open with 1.5 mm apertures) adhesive/polyurethane film/adhesive was then laminated to the foam side of the porous layer. The non-woven side of the porous layer was then centered over and laminated to the absorbent adhesive gel.
- a vacuum port and tubing (removed from the dressing of the PICO Single Use Negative Pressure device obtained from Smith & Nephew, Andover, MA) was then attached with an adhesive to the opening in the backing layer of the constructed sample to ensure a leak free seal at the port.
- the dressing was placed on the fluid management test apparatus (described above) with the adhesive side attached to the aluminum plate and the porous layer extended over all ports and the entire slit.
- the apparatus was connected to the vacuum system.
- a vacuum of 120 mm Hg from atmospheric was then pulled on the dressing.
- a total flow of 1 ml/hr of an aqueous solution containing 142 mmol/liter of sodium ions (from sodium chloride) and 2.5 mmol/liter of calcium ions (from calcium chloride) was then fed to the dressing.
- the data in Table 1 show the measured vacuum level in three identical dressings over time. The data demonstrate the presence of the absorbent adhesive gel helps minimize pressure drop in the dressing when the dressing is placed under vacuum and then held for periods up to about 21 1 ⁇ 2 hours..
- Table 1 show the measured vacuum level in three identical dressings over time. The data demonstrate the presence of the absorbent adhesive gel helps minimize pressure drop in the dressing when the dressing is placed under vacuum and then held for periods up to about 21 1 ⁇ 2 hours.
- Comparative Example 1 was constructed and tested in the same manner as Example 1 except that no absorbent adhesive gel was used.
- the data in Table 2 show the average measured pressure level below atmospheric pressure in the dressing over time.
- Table 2. Average pressure below atmospheric pressure in dressing that does not comprise the absorbent adhesive gel as described herein. The test was conducted under the conditions described in the Wound Dressing Vacuum Test herein. Time (hr) after applying vacuum Average Pressure Below Atmospheric Pressure in the Dressing (mm Hg) 0 120 3.3 120 6.4 120 22.4 40
- the rheological properties of the absorbent adhesive gel used in the dressings of Example 1 were measured as a function of drying temperature and time using a TA Instruments' ARES rheometer (Texas Instruments, New Castle, DE). Samples of the absorbent adhesive gel were dried at 48.9°C. The shear measurements were taken at 24°C and a frequency range from 0.1 to 500 rad/second. The adhesive gel sample was a 25 mm diameter circle with a thickness of 1.6 mm. The results for the dynamic shear viscosity are shown in Table 3. The results of the storage shear modulus (G'), and the loss shear modulus (G”) measurements are shown in Table 4. Table 3: Viscosity (poise) change with drying time at different shear rate.
- Example 3 This example is to be seen as a reference-example and is not according to the claimed invention.
- the test sample was prepared and tested the same as Example 1 except; 1) the liquid barrier contained in the vacuum port was removed, 2) the vacuum source was a SNaP® 125 mm Hg cartridge; 3) no open areas were cut out of the porous layer; and 4) the backing was a 23 micron thick urethane film with a 25 micron thick acrylate adhesive laminated to it. Two 5 cm x 5 cm cutouts in the acrylate adhesive prior to lamination to the film were removed so that the absorbent adhesive gel was in direct contact with the urethane film in these areas in order to create a high MVTR areas of the dressing.
- the urethane film was made using Estane® 58237 polymer from Lubrizol Co; Wickliffe, OH).
- the vacuum cartridge After 24 hours of testing (i.e., 24 cm 3 of solution fed to the dressing), the vacuum cartridge only changed volume by 8 cm 3 .
- the average pressure in the dressing was 120 mm Hg below atmospheric pressure after 24 hours.
- Example 4 This example is to be seen as a reference-example and is not according to the claimed invention.
- a 23 micron thick film of Estane 58237 was laminated to a discontinuous (pattern of approximately 1 mm diameter holes with 10% total open area) acrylate adhesive layer (described U.S. Patent No. RE 24,906 ; a copolymer of iso-octyl acrylate;acrylamide (97:3) with coating weight of 750 mg/200 cm 2 ).
- This film adhesive sample was equilibrated at 25 Celsius and 50% relative humidity. The adhesion to steel value of this sample was measured at 4 N/25 mm.
- Example 5 This example is to be seen as a reference-example and is not according to the claimed invention.
- a 23 micron thick film of Estane 58237 was laminated to a continuous layer of absorbent adhesive (as described in Example E3 of US2009/0187130 ) that was 1.75 mm thick.
- This film adhesive sample was equilibrated at 25 Celsius and 50% relative humidity. The adhesion to steel value of this sample was measured at 5.8 N/25 mm.
- Example 6 This example is to be seen as a reference-example and is not according to the claimed invention.
- a 30 micron thick urethane film (Texin 1209 from Bayer) was extruded onto a continuous acrylate adhesive whose coating weight was 550 mg/200 cm 2 .
- a silicone gel adhesive was then coated at 0.1 mm thick and cured (as described in and WO2010/056543 ) onto the urethane film/adhesive laminate and a protective liner put onto this silicone adhesive.
- This multilayer laminate was then mechanically perforated (1.5 mm diameter holes; 20% open).
- the acrylate side of this laminate was then laminated to a polyurethane foam cut out of a 90642 3MTM TEGADERMTM Foam Silicone Border dressing.
- the adhesion to steel of the silicone side of this foam laminate was then tested at 25 Celsius and 50% relative humidity. The adhesion to steel value was measured at 2 N/25 mm.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Materials Engineering (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Vascular Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Dermatology (AREA)
- Dispersion Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Textile Engineering (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Mechanical Engineering (AREA)
- Materials For Medical Uses (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Polymers & Plastics (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462098058P | 2014-12-30 | 2014-12-30 | |
| US201562112714P | 2015-02-06 | 2015-02-06 | |
| PCT/US2015/067661 WO2016109420A1 (en) | 2014-12-30 | 2015-12-28 | Negative pressure wound dressing with absorbent adhesive sealant layer |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3240580A1 EP3240580A1 (en) | 2017-11-08 |
| EP3240580B1 true EP3240580B1 (en) | 2020-04-15 |
Family
ID=55315699
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15832851.8A Active EP3240581B1 (en) | 2014-12-30 | 2015-12-28 | Wound dressing with multiple adhesive layers |
| EP15832852.6A Active EP3240580B1 (en) | 2014-12-30 | 2015-12-28 | Negative pressure wound dressing with absorbent adhesive sealant layer |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15832851.8A Active EP3240581B1 (en) | 2014-12-30 | 2015-12-28 | Wound dressing with multiple adhesive layers |
Country Status (5)
| Country | Link |
|---|---|
| US (3) | US10537478B2 (zh) |
| EP (2) | EP3240581B1 (zh) |
| JP (2) | JP6744315B2 (zh) |
| CN (2) | CN107106723B (zh) |
| WO (2) | WO2016109418A1 (zh) |
Families Citing this family (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0808376D0 (en) | 2008-05-08 | 2008-06-18 | Bristol Myers Squibb Co | Wound dressing |
| GB0817796D0 (en) | 2008-09-29 | 2008-11-05 | Convatec Inc | wound dressing |
| GB201020236D0 (en) | 2010-11-30 | 2011-01-12 | Convatec Technologies Inc | A composition for detecting biofilms on viable tissues |
| CN103347562B (zh) | 2010-12-08 | 2016-08-10 | 康沃特克科技公司 | 伤口分泌液系统附件 |
| EP2648793B1 (en) | 2010-12-08 | 2020-03-11 | ConvaTec Technologies Inc. | Integrated system for assessing wound exudates |
| GB201115182D0 (en) | 2011-09-02 | 2011-10-19 | Trio Healthcare Ltd | Skin contact material |
| GB2497406A (en) | 2011-11-29 | 2013-06-12 | Webtec Converting Llc | Dressing with a perforated binder layer |
| BR112015014816A2 (pt) | 2012-12-20 | 2017-07-11 | Convatec Technologies Inc | processamento de fibras celulósicas modificadas quimicamente |
| WO2016109418A1 (en) * | 2014-12-30 | 2016-07-07 | 3M Innovative Properties Company | Wound dressing with multiple adhesive layers |
| CN108601678A (zh) * | 2016-01-29 | 2018-09-28 | 王子控股株式会社 | 创可贴 |
| PL3435941T3 (pl) | 2016-03-30 | 2022-05-09 | Convatec Technologies Inc. | Wykrywanie infekcji drobnoustrojowych w ranach |
| JP2019513238A (ja) | 2016-03-30 | 2019-05-23 | クオリザイム・ダイアグノスティクス・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング・ウント・コムパニー・コマンディットゲゼルシャフトQualizyme Diagnostics Gmbh And Co. Kg | 創傷における微生物感染の検出 |
| BR112019000284A2 (pt) | 2016-07-08 | 2019-04-16 | Convatec Technologies Inc. | aparelho de coleta de fluido |
| JP7071957B2 (ja) | 2016-07-08 | 2022-05-19 | コンバテック・テクノロジーズ・インコーポレイテッド | 可撓性陰圧システム |
| MX2019000232A (es) | 2016-07-08 | 2019-11-12 | Convatec Technologies Inc | Deteccion de flujo de fluidos. |
| EP3281617B1 (en) * | 2016-08-10 | 2020-09-23 | Advanced Medical Solutions Limited | Wound dressing |
| EP3315145B1 (en) * | 2016-10-28 | 2022-06-08 | BSN medical GmbH | Multi-layer wound care product with perforated collagen layer |
| JP7231227B2 (ja) | 2017-02-22 | 2023-03-01 | コーネル ユニヴァーシティー | 小さい切開創を機械式に管理、保護及び吸引するための機械式真空ドレッシング |
| CN107574575A (zh) * | 2017-08-02 | 2018-01-12 | 六安尚荣无纺布制品有限公司 | 一种医用抗菌无纺布的制备工艺 |
| CN111032333B (zh) | 2017-08-25 | 2022-10-04 | 3M创新有限公司 | 允许无损移除的粘合剂制品 |
| TWI795430B (zh) | 2017-08-25 | 2023-03-11 | 美商3M新設資產公司 | 容許無損傷移除的黏著劑物品 |
| EP3694457A1 (en) * | 2017-10-09 | 2020-08-19 | 3M Innovative Properties Company | Securement dressing with conformal border |
| AU2018354152B2 (en) | 2017-10-23 | 2024-02-29 | Solventum Intellectual Properties Company | Low profile distribution components for wound therapy |
| US11432967B2 (en) * | 2017-10-23 | 2022-09-06 | Kci Licensing, Inc. | Fluid bridge for simultaneous application of negative pressure to multiple tissue sites |
| AU2018378653B2 (en) | 2017-12-06 | 2024-09-05 | Cornell University | Manually-operated negative pressure wound therapy (NPWT) bandage with improved pump efficiency, automatic pressure indicator and automatic pressure limiter |
| US20190351094A1 (en) * | 2018-05-21 | 2019-11-21 | Milliken & Company | Wound care device having fluid transfer and adhesive properties |
| EP3806785A1 (en) * | 2018-06-15 | 2021-04-21 | Coloplast A/S | Wound dressing with electrode multiplexing and related methods |
| US10993708B2 (en) * | 2018-07-31 | 2021-05-04 | Ethicon, Inc. | Skin closure devices with interrupted closure |
| WO2020035811A1 (en) * | 2018-08-17 | 2020-02-20 | 3M Innovative Properties Company | Wound dressing system |
| US11007083B2 (en) * | 2018-08-28 | 2021-05-18 | Aatru Medical, LLC | Dressing |
| US11666680B2 (en) | 2018-08-28 | 2023-06-06 | Aatru Medical, LLC | Dressing |
| GB2592805B (en) * | 2018-10-18 | 2023-04-12 | Smith & Nephew | Tissue treatment device |
| USD961782S1 (en) | 2018-10-23 | 2022-08-23 | Kci Licensing, Inc. | Low-profile fluid conductor for tissue therapy |
| SG11202112292QA (en) | 2019-06-03 | 2021-12-30 | Convatec Ltd | Methods and devices to disrupt and contain pathogens |
| WO2021124357A1 (en) * | 2019-12-19 | 2021-06-24 | Pankajkumar Kumanbhai Chhatrala | A mechanical barrier device for medically incorporated instruments |
| EP3838238B1 (en) * | 2019-12-19 | 2023-01-25 | Mölnlycke Health Care AB | Double coating for wound dressings |
| US11771819B2 (en) | 2019-12-27 | 2023-10-03 | Convatec Limited | Low profile filter devices suitable for use in negative pressure wound therapy systems |
| US11331221B2 (en) * | 2019-12-27 | 2022-05-17 | Convatec Limited | Negative pressure wound dressing |
| US20210244357A1 (en) * | 2020-02-07 | 2021-08-12 | Medtronic Minimed, Inc. | Systems for medical device breathability |
| EP4196062A1 (en) * | 2020-08-13 | 2023-06-21 | KCI Manufacturing Unlimited Company | Negative-pressure therapy dressing with viewing window |
| GB202013871D0 (en) * | 2020-09-03 | 2020-10-21 | Brightwake Ltd | Wound dressing |
| CN112691230A (zh) * | 2020-12-23 | 2021-04-23 | 上海弘生医疗科技有限公司 | 一种硅胶敷料胶带及其制备工艺 |
| GB202107893D0 (en) * | 2021-06-02 | 2021-07-14 | Medtrade Products Ltd | Antimicrobial wound dressing |
| CN114712088B (zh) * | 2022-04-01 | 2023-05-16 | 浙江隆腾医用新材料有限公司 | 一种羧甲基纤维素创面敷料 |
Family Cites Families (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1032400A (en) | 1911-01-21 | 1912-07-16 | Wilhelm Guenther | Process of treating ores. |
| US1013052A (en) | 1911-03-22 | 1911-12-26 | Goldman & Company Inc E | Conveyer-chain. |
| USRE24906E (en) | 1955-11-18 | 1960-12-13 | Pressure-sensitive adhesive sheet material | |
| US4112213A (en) | 1964-09-28 | 1978-09-05 | Johnson & Johnson | Pressure sensitive adhesive tapes and method of making same |
| US3389827A (en) | 1967-04-10 | 1968-06-25 | Minnesota Mining & Mfg | Easy-open container and sealing tape |
| NO134790C (no) | 1968-07-09 | 1984-03-22 | Smith & Nephew | Klebende,; trykkfoelsomt, vanndamp-permeabelt produkt for bruk paa hud hos mennesker. |
| US4310509A (en) | 1979-07-31 | 1982-01-12 | Minnesota Mining And Manufacturing Company | Pressure-sensitive adhesive having a broad spectrum antimicrobial therein |
| US4323557A (en) | 1979-07-31 | 1982-04-06 | Minnesota Mining & Manufacturing Company | Pressure-sensitive adhesive containing iodine |
| AU560088B2 (en) | 1982-04-08 | 1987-03-26 | Smith & Nephew Associated Companies Plc | Surgical adhesive dressing |
| US4737410A (en) | 1986-11-28 | 1988-04-12 | Minnesota Mining And Manufacturing Company | Polyalkyloxazoline-reinforced acrylic pressure-sensitive adhesive composition |
| US4931282A (en) | 1987-11-25 | 1990-06-05 | Minnesota Mining And Manufacturing Company | Pressure-sensitive medical sealant |
| US5225473A (en) | 1987-11-25 | 1993-07-06 | Minnesota Mining And Manufacturing Company | Pressure-sensitive adhesives |
| US5088483A (en) | 1988-11-04 | 1992-02-18 | Minnesota Mining And Manufacturing Co. | Adhesive frame bandage |
| US5261893A (en) | 1989-04-03 | 1993-11-16 | Zamierowski David S | Fastening system and method |
| US5527293A (en) | 1989-04-03 | 1996-06-18 | Kinetic Concepts, Inc. | Fastening system and method |
| US4969880A (en) | 1989-04-03 | 1990-11-13 | Zamierowski David S | Wound dressing and treatment method |
| US5160315A (en) | 1991-04-05 | 1992-11-03 | Minnesota Mining And Manufacturing Company | Combined adhesive strip and transparent dressing delivery system |
| US5276079A (en) | 1991-11-15 | 1994-01-04 | Minnesota Mining And Manufacturing Company | Pressure-sensitive poly(n-vinyl lactam) adhesive composition and method for producing and using same |
| US5244457A (en) * | 1992-03-23 | 1993-09-14 | The Kendall Company | Vented wound dressing |
| DE19727032A1 (de) | 1997-06-25 | 1999-01-07 | Hartmann Paul Ag | Pflaster |
| US6171985B1 (en) | 1997-12-01 | 2001-01-09 | 3M Innovative Properties Company | Low trauma adhesive article |
| US6071267A (en) | 1998-02-06 | 2000-06-06 | Kinetic Concepts, Inc. | Medical patient fluid management interface system and method |
| SE9801899L (sv) | 1998-05-28 | 1999-07-05 | Moelnlycke Health Care Ab | Sårförband eller fixeringstejp för hud innefattande ett laminat av en plastfilm och ett material med oregelbunden ytstruktur och som är belagt med en klibbig elastomer |
| US20020015726A1 (en) | 2000-06-30 | 2002-02-07 | Scamilla Aledo Maria Aparecida De Carvalho | Dressings and bandages comprising same |
| US6566575B1 (en) * | 2000-02-15 | 2003-05-20 | 3M Innovative Properties Company | Patterned absorbent article for wound dressing |
| CA2626268A1 (en) | 2000-03-10 | 2001-09-20 | 3M Innovative Properties Company | Medical dressings with multiple adhesives and methods of manufacturing |
| WO2003057485A1 (en) * | 2001-12-21 | 2003-07-17 | Avery Dennison Corporation | Process for the manufacture of multi-layered wound dressings |
| US7005143B2 (en) * | 2002-04-12 | 2006-02-28 | 3M Innovative Properties Company | Gel materials, medical articles, and methods |
| USD468548S1 (en) | 2002-05-25 | 2003-01-14 | H. Wayne Head, Jr. | Portable stadium seat |
| US7520872B2 (en) | 2002-09-13 | 2009-04-21 | Neogen Technologies, Inc. | Closed wound drainage system |
| US6979324B2 (en) | 2002-09-13 | 2005-12-27 | Neogen Technologies, Inc. | Closed wound drainage system |
| CN100536806C (zh) * | 2002-12-31 | 2009-09-09 | Bsn医疗有限责任公司 | 具有允许敷料膨胀的顺应元件的伤口敷料 |
| US9278155B2 (en) * | 2003-06-05 | 2016-03-08 | 3M Innovative Properties Company | Adhesive compositions, articles incorporating same and methods of manufacture |
| GB0606661D0 (en) | 2006-04-03 | 2006-05-10 | Brightwake Ltd | Improvements relating to dressings |
| EP1923077B1 (de) | 2006-11-07 | 2011-01-12 | Paul Hartmann AG | Mehrschichtige, absorbierende Wundauflage mit einer hydrophilen Wundkontaktschicht |
| US7947366B2 (en) | 2007-03-19 | 2011-05-24 | 3M Innovative Properties Company | Adhesive sheet article |
| US8211071B2 (en) | 2007-08-21 | 2012-07-03 | Kci Licensing, Inc. | Reduced-pressure system and method employing a gasket |
| BRPI0905692A2 (pt) | 2008-01-18 | 2015-07-07 | 3M Innovative Properties Co | "bandagem em forma de ilha" |
| CA2726815C (en) | 2008-05-27 | 2016-07-05 | Kalypto Medical, Inc. | Negative pressure wound therapy device |
| DE102008031183A1 (de) * | 2008-07-03 | 2010-01-07 | Paul Hartmann Ag | Wundauflage |
| US20100106120A1 (en) | 2008-10-24 | 2010-04-29 | 3M Innovative Properties Company | Wound dressing |
| WO2010056543A1 (en) | 2008-10-29 | 2010-05-20 | 3M Innovative Properties Company | Electron beam cured, nonfunctionalized silicone pressure sensitive adhesives |
| CN102232103B (zh) | 2008-10-29 | 2016-07-06 | 3M创新有限公司 | 电子束固化的有机硅材料 |
| US9421309B2 (en) | 2009-06-02 | 2016-08-23 | Kci Licensing, Inc. | Reduced-pressure treatment systems and methods employing hydrogel reservoir members |
| GB2470940A (en) | 2009-06-10 | 2010-12-15 | Systagenix Wound Man Ip Co Bv | Vacuum wound dressing with hydrogel layer |
| SE533841C2 (sv) | 2009-06-15 | 2011-02-01 | Moelnlycke Health Care Ab | Sårförband med hög vätskehanteringskapacitet |
| US8690844B2 (en) * | 2009-08-27 | 2014-04-08 | Kci Licensing, Inc. | Re-epithelialization wound dressings and systems |
| CN102686249B (zh) | 2009-11-09 | 2014-06-25 | 3M创新有限公司 | 医疗制品以及使用不能混溶的材料进行制备的方法 |
| US9358158B2 (en) * | 2010-03-16 | 2016-06-07 | Kci Licensing, Inc. | Patterned neo-epithelialization dressings, systems, and methods |
| GB201006986D0 (en) * | 2010-04-27 | 2010-06-09 | Smith & Nephew | Wound dressing |
| US9107990B2 (en) | 2011-02-14 | 2015-08-18 | Kci Licensing, Inc. | Reduced-pressure dressings, systems, and methods for use with linear wounds |
| GB201108229D0 (en) * | 2011-05-17 | 2011-06-29 | Smith & Nephew | Tissue healing |
| CN107252383A (zh) | 2011-07-14 | 2017-10-17 | 史密夫及内修公开有限公司 | 伤口敷料和治疗方法 |
| EP3296436B1 (en) | 2011-11-01 | 2019-07-24 | Brightwake Limited | Wound dressings, and fabric useful therein |
| CN108272557A (zh) | 2012-03-01 | 2018-07-13 | 爱乐康株式会社 | 伤口处理用品 |
| WO2014003957A1 (en) | 2012-06-26 | 2014-01-03 | 3M Innovative Properties Company | Medical dressing with multiple adhesives |
| JP6307504B2 (ja) | 2012-08-01 | 2018-04-04 | スミス アンド ネフュー ピーエルシーSmith & Nephew Public Limited Company | 創傷被覆材 |
| ITMI20121899A1 (it) | 2012-11-07 | 2014-05-08 | Prysmian Spa | Cavo elettrico per un impianto solare per la generazione di energia elettrica e di energia termica ed impianto che lo comprende |
| US20160120706A1 (en) * | 2013-03-15 | 2016-05-05 | Smith & Nephew Plc | Wound dressing sealant and use thereof |
| CN105188780A (zh) | 2013-03-15 | 2015-12-23 | 史密夫及内修公开有限公司 | 伤口敷料密封剂及其使用 |
| JP2017512239A (ja) | 2014-03-05 | 2017-05-18 | スリーエム イノベイティブ プロパティズ カンパニー | 皮膚に優しい(メタ)アクリレート感圧接着剤 |
| EP3666237B1 (en) | 2014-06-18 | 2023-11-01 | Smith & Nephew plc | Wound dressing |
| WO2016109418A1 (en) | 2014-12-30 | 2016-07-07 | 3M Innovative Properties Company | Wound dressing with multiple adhesive layers |
| CA3039370A1 (en) * | 2016-10-04 | 2018-04-12 | Ch2M Hill, Inc. | Passive device for vapor intrusion sampling |
-
2015
- 2015-12-28 WO PCT/US2015/067655 patent/WO2016109418A1/en active Application Filing
- 2015-12-28 US US15/539,855 patent/US10537478B2/en active Active
- 2015-12-28 EP EP15832851.8A patent/EP3240581B1/en active Active
- 2015-12-28 EP EP15832852.6A patent/EP3240580B1/en active Active
- 2015-12-28 JP JP2017534994A patent/JP6744315B2/ja active Active
- 2015-12-28 JP JP2017535053A patent/JP6838812B2/ja active Active
- 2015-12-28 CN CN201580071925.8A patent/CN107106723B/zh active Active
- 2015-12-28 CN CN201580071938.5A patent/CN107106334B/zh active Active
- 2015-12-28 WO PCT/US2015/067661 patent/WO2016109420A1/en active Application Filing
- 2015-12-28 US US15/539,866 patent/US11007086B2/en active Active
-
2019
- 2019-12-04 US US16/703,676 patent/US20200107967A1/en not_active Abandoned
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107106723B (zh) | 2020-10-23 |
| WO2016109418A1 (en) | 2016-07-07 |
| JP2018500137A (ja) | 2018-01-11 |
| JP6838812B2 (ja) | 2021-03-03 |
| CN107106334A (zh) | 2017-08-29 |
| CN107106723A (zh) | 2017-08-29 |
| JP6744315B2 (ja) | 2020-08-19 |
| CN107106334B (zh) | 2021-02-09 |
| EP3240581B1 (en) | 2020-04-15 |
| US11007086B2 (en) | 2021-05-18 |
| US20170367895A1 (en) | 2017-12-28 |
| US10537478B2 (en) | 2020-01-21 |
| JP2018508243A (ja) | 2018-03-29 |
| US20170367896A1 (en) | 2017-12-28 |
| EP3240580A1 (en) | 2017-11-08 |
| EP3240581A1 (en) | 2017-11-08 |
| US20200107967A1 (en) | 2020-04-09 |
| WO2016109420A1 (en) | 2016-07-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3240580B1 (en) | Negative pressure wound dressing with absorbent adhesive sealant layer | |
| US20210228417A1 (en) | Conformable medical dressing with self supporting substrate | |
| KR20200016934A (ko) | 음압 치료를 이용한 육아 개선 및 침연 감소용 복합 드레싱재 | |
| US20220142820A1 (en) | Antimicrobial dressing, dressing components, and methods | |
| JP2001511396A (ja) | 改良創傷包帯 | |
| RU2715718C2 (ru) | Раневая повязка | |
| HU225557B1 (en) | Absorbent wound dressing containing a hydrogel layer | |
| EP1333788A1 (en) | Hydrogel wound dressings | |
| US20140228786A1 (en) | Absorbent body for the therapeutic treatment of a wound by means of negative pressure | |
| US20140221946A1 (en) | Absorbent body for the therapeutic treatment of a wound by means of negative pressure | |
| GB2379392A (en) | Wound dressing sheet materials | |
| US9656000B2 (en) | Wound dressing | |
| KR200359390Y1 (ko) | 의료용 패치 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20170706 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20181204 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61L 15/46 20060101ALI20191007BHEP Ipc: A61L 15/60 20060101ALI20191007BHEP Ipc: A61L 15/42 20060101AFI20191007BHEP Ipc: A61L 15/58 20060101ALI20191007BHEP Ipc: A61F 13/02 20060101ALI20191007BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20191108 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602015050858 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1256492 Country of ref document: AT Kind code of ref document: T Effective date: 20200515 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20200415 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200817 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200815 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200715 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200716 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1256492 Country of ref document: AT Kind code of ref document: T Effective date: 20200415 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200715 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602015050858 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 |
|
| 26N | No opposition filed |
Effective date: 20210118 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20201228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20201231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201228 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201228 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201231 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201228 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200415 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201231 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20231121 Year of fee payment: 9 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R081 Ref document number: 602015050858 Country of ref document: DE Owner name: SOLVENTUM INTELLECTUAL PROPERTIES CO. (N.D.GES, US Free format text: FORMER OWNER: 3M INNOVATIVE PROPERTIES CO., ST. PAUL, MINN., US |