EP3082852B1 - Mikrogeformte oder 3d-gedruckte impstoff-formulierungen mit rhythmischer freisetzung - Google Patents
Mikrogeformte oder 3d-gedruckte impstoff-formulierungen mit rhythmischer freisetzung Download PDFInfo
- Publication number
- EP3082852B1 EP3082852B1 EP14830756.4A EP14830756A EP3082852B1 EP 3082852 B1 EP3082852 B1 EP 3082852B1 EP 14830756 A EP14830756 A EP 14830756A EP 3082852 B1 EP3082852 B1 EP 3082852B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- formulation
- antigen
- release
- bsa
- plga
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203 mixture Substances 0.000 title claims description 147
- 238000009472 formulation Methods 0.000 title claims description 133
- 229960005486 vaccine Drugs 0.000 title description 50
- 230000000541 pulsatile effect Effects 0.000 title description 3
- 239000000427 antigen Substances 0.000 claims description 122
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 claims description 108
- 239000004005 microsphere Substances 0.000 claims description 101
- 102000036639 antigens Human genes 0.000 claims description 91
- 108091007433 antigens Proteins 0.000 claims description 91
- 229920000642 polymer Polymers 0.000 claims description 76
- -1 poly(lactic acid) Polymers 0.000 claims description 66
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 50
- 239000011859 microparticle Substances 0.000 claims description 36
- 229920000747 poly(lactic acid) Polymers 0.000 claims description 28
- 235000000346 sugar Nutrition 0.000 claims description 25
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 claims description 21
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 claims description 21
- 239000005720 sucrose Substances 0.000 claims description 21
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 claims description 20
- 238000010146 3D printing Methods 0.000 claims description 18
- 206010028980 Neoplasm Diseases 0.000 claims description 15
- 238000001727 in vivo Methods 0.000 claims description 14
- 208000000474 Poliomyelitis Diseases 0.000 claims description 13
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 13
- 229930006000 Sucrose Natural products 0.000 claims description 13
- 230000028993 immune response Effects 0.000 claims description 11
- 239000000347 magnesium hydroxide Substances 0.000 claims description 11
- 229910001862 magnesium hydroxide Inorganic materials 0.000 claims description 11
- 239000003381 stabilizer Substances 0.000 claims description 11
- 229920000954 Polyglycolide Polymers 0.000 claims description 10
- 150000001875 compounds Chemical class 0.000 claims description 10
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 claims description 10
- 238000001053 micromoulding Methods 0.000 claims description 10
- 239000003921 oil Substances 0.000 claims description 10
- 229920001577 copolymer Polymers 0.000 claims description 9
- 239000003094 microcapsule Substances 0.000 claims description 8
- 241000700605 Viruses Species 0.000 claims description 7
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 claims description 6
- 229910021502 aluminium hydroxide Inorganic materials 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 6
- 239000011521 glass Substances 0.000 claims description 6
- 239000012678 infectious agent Substances 0.000 claims description 6
- 239000006172 buffering agent Substances 0.000 claims description 5
- 206010022000 influenza Diseases 0.000 claims description 5
- 229920000728 polyester Polymers 0.000 claims description 5
- TWJNQYPJQDRXPH-UHFFFAOYSA-N 2-cyanobenzohydrazide Chemical compound NNC(=O)C1=CC=CC=C1C#N TWJNQYPJQDRXPH-UHFFFAOYSA-N 0.000 claims description 4
- 241000894006 Bacteria Species 0.000 claims description 4
- TUNFSRHWOTWDNC-UHFFFAOYSA-N Myristic acid Natural products CCCCCCCCCCCCCC(O)=O TUNFSRHWOTWDNC-UHFFFAOYSA-N 0.000 claims description 4
- 235000021360 Myristic acid Nutrition 0.000 claims description 4
- 150000008163 sugars Chemical class 0.000 claims description 4
- 201000005702 Pertussis Diseases 0.000 claims description 3
- 206010043376 Tetanus Diseases 0.000 claims description 3
- 150000001720 carbohydrates Chemical class 0.000 claims description 3
- 235000014633 carbohydrates Nutrition 0.000 claims description 3
- 201000005505 Measles Diseases 0.000 claims description 2
- 208000005647 Mumps Diseases 0.000 claims description 2
- 241000702670 Rotavirus Species 0.000 claims description 2
- 206010013023 diphtheria Diseases 0.000 claims description 2
- 208000006454 hepatitis Diseases 0.000 claims description 2
- 231100000283 hepatitis Toxicity 0.000 claims description 2
- 239000012729 immediate-release (IR) formulation Substances 0.000 claims description 2
- 150000002632 lipids Chemical class 0.000 claims description 2
- 208000010805 mumps infectious disease Diseases 0.000 claims description 2
- 230000002685 pulmonary effect Effects 0.000 claims description 2
- 201000005404 rubella Diseases 0.000 claims description 2
- 241000588832 Bordetella pertussis Species 0.000 claims 1
- 241000193449 Clostridium tetani Species 0.000 claims 1
- 241000186227 Corynebacterium diphtheriae Species 0.000 claims 1
- 241000233866 Fungi Species 0.000 claims 1
- 241000588650 Neisseria meningitidis Species 0.000 claims 1
- 241000193998 Streptococcus pneumoniae Species 0.000 claims 1
- 230000005867 T cell response Effects 0.000 claims 1
- 230000007062 hydrolysis Effects 0.000 claims 1
- 238000006460 hydrolysis reaction Methods 0.000 claims 1
- 229940031000 streptococcus pneumoniae Drugs 0.000 claims 1
- 238000002560 therapeutic procedure Methods 0.000 claims 1
- 229940029583 inactivated polio vaccine Drugs 0.000 description 103
- 239000002245 particle Substances 0.000 description 64
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 59
- 238000000034 method Methods 0.000 description 54
- 108090000623 proteins and genes Proteins 0.000 description 37
- 102000004169 proteins and genes Human genes 0.000 description 35
- 239000003814 drug Substances 0.000 description 30
- 238000002347 injection Methods 0.000 description 30
- 239000007924 injection Substances 0.000 description 30
- 229940079593 drug Drugs 0.000 description 29
- 239000000243 solution Substances 0.000 description 29
- 239000002904 solvent Substances 0.000 description 29
- 239000000463 material Substances 0.000 description 25
- 239000004626 polylactic acid Substances 0.000 description 24
- 238000001035 drying Methods 0.000 description 21
- 239000000839 emulsion Substances 0.000 description 20
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 18
- 239000000872 buffer Substances 0.000 description 18
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 17
- 108010010803 Gelatin Proteins 0.000 description 17
- 229940098773 bovine serum albumin Drugs 0.000 description 17
- 239000008273 gelatin Substances 0.000 description 17
- 229920000159 gelatin Polymers 0.000 description 17
- 235000019322 gelatine Nutrition 0.000 description 17
- 235000011852 gelatine desserts Nutrition 0.000 description 17
- 238000004519 manufacturing process Methods 0.000 description 17
- 239000000976 ink Substances 0.000 description 16
- 230000008569 process Effects 0.000 description 16
- 230000000717 retained effect Effects 0.000 description 16
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 16
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 14
- 238000011084 recovery Methods 0.000 description 13
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 12
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 12
- 238000000338 in vitro Methods 0.000 description 12
- 230000015572 biosynthetic process Effects 0.000 description 11
- 238000005538 encapsulation Methods 0.000 description 11
- 230000003053 immunization Effects 0.000 description 11
- 238000002649 immunization Methods 0.000 description 11
- 238000011068 loading method Methods 0.000 description 11
- 229920002451 polyvinyl alcohol Polymers 0.000 description 11
- 239000000758 substrate Substances 0.000 description 11
- 238000002255 vaccination Methods 0.000 description 11
- JVTAAEKCZFNVCJ-REOHCLBHSA-N L-lactic acid Chemical compound C[C@H](O)C(O)=O JVTAAEKCZFNVCJ-REOHCLBHSA-N 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 10
- 239000003960 organic solvent Substances 0.000 description 10
- 229920001432 poly(L-lactide) Polymers 0.000 description 10
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 9
- 238000002965 ELISA Methods 0.000 description 9
- 239000000654 additive Substances 0.000 description 9
- 230000008859 change Effects 0.000 description 9
- 238000009826 distribution Methods 0.000 description 9
- 238000004945 emulsification Methods 0.000 description 9
- 238000004108 freeze drying Methods 0.000 description 9
- 229940127241 oral polio vaccine Drugs 0.000 description 9
- 239000012071 phase Substances 0.000 description 9
- 239000000600 sorbitol Substances 0.000 description 9
- RHQDFWAXVIIEBN-UHFFFAOYSA-N Trifluoroethanol Chemical compound OCC(F)(F)F RHQDFWAXVIIEBN-UHFFFAOYSA-N 0.000 description 8
- 230000002159 abnormal effect Effects 0.000 description 8
- 238000011049 filling Methods 0.000 description 8
- 230000005847 immunogenicity Effects 0.000 description 8
- 239000011159 matrix material Substances 0.000 description 8
- 239000002480 mineral oil Substances 0.000 description 8
- 235000010446 mineral oil Nutrition 0.000 description 8
- 238000002156 mixing Methods 0.000 description 8
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 8
- 239000004372 Polyvinyl alcohol Substances 0.000 description 7
- NWGKJDSIEKMTRX-AAZCQSIUSA-N Sorbitan monooleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O NWGKJDSIEKMTRX-AAZCQSIUSA-N 0.000 description 7
- 229920002988 biodegradable polymer Polymers 0.000 description 7
- 239000004621 biodegradable polymer Substances 0.000 description 7
- 230000015556 catabolic process Effects 0.000 description 7
- 238000006731 degradation reaction Methods 0.000 description 7
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 229920001600 hydrophobic polymer Polymers 0.000 description 7
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical group CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 7
- 229910001629 magnesium chloride Inorganic materials 0.000 description 7
- 239000002953 phosphate buffered saline Substances 0.000 description 7
- 229920001223 polyethylene glycol Polymers 0.000 description 7
- 210000002966 serum Anatomy 0.000 description 7
- 238000000527 sonication Methods 0.000 description 7
- 108010058846 Ovalbumin Proteins 0.000 description 6
- 239000004373 Pullulan Substances 0.000 description 6
- 229920001218 Pullulan Polymers 0.000 description 6
- 239000002253 acid Substances 0.000 description 6
- 230000002378 acidificating effect Effects 0.000 description 6
- 239000002775 capsule Substances 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 229920001477 hydrophilic polymer Polymers 0.000 description 6
- 230000007774 longterm Effects 0.000 description 6
- 235000019423 pullulan Nutrition 0.000 description 6
- 230000002269 spontaneous effect Effects 0.000 description 6
- 230000000087 stabilizing effect Effects 0.000 description 6
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 5
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 5
- 239000002202 Polyethylene glycol Substances 0.000 description 5
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 5
- 229910001679 gibbsite Inorganic materials 0.000 description 5
- 239000012074 organic phase Substances 0.000 description 5
- 238000012667 polymer degradation Methods 0.000 description 5
- 229920000053 polysorbate 80 Polymers 0.000 description 5
- 238000007639 printing Methods 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 238000007789 sealing Methods 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 4
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 4
- 229940032046 DTaP vaccine Drugs 0.000 description 4
- 241000991587 Enterovirus C Species 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical group OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 4
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 4
- 206010033799 Paralysis Diseases 0.000 description 4
- 238000000692 Student's t-test Methods 0.000 description 4
- 239000002671 adjuvant Substances 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 239000006227 byproduct Substances 0.000 description 4
- 230000003247 decreasing effect Effects 0.000 description 4
- 239000004205 dimethyl polysiloxane Substances 0.000 description 4
- 230000003628 erosive effect Effects 0.000 description 4
- 238000007641 inkjet printing Methods 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 238000001127 nanoimprint lithography Methods 0.000 description 4
- 239000013642 negative control Substances 0.000 description 4
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 4
- 229920001281 polyalkylene Polymers 0.000 description 4
- 229920000515 polycarbonate Polymers 0.000 description 4
- 239000004417 polycarbonate Substances 0.000 description 4
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 230000001681 protective effect Effects 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000000935 solvent evaporation Methods 0.000 description 4
- TUNFSRHWOTWDNC-HKGQFRNVSA-N tetradecanoic acid Chemical compound CCCCCCCCCCCCC[14C](O)=O TUNFSRHWOTWDNC-HKGQFRNVSA-N 0.000 description 4
- 238000003260 vortexing Methods 0.000 description 4
- 238000003466 welding Methods 0.000 description 4
- 206010006187 Breast cancer Diseases 0.000 description 3
- 208000026310 Breast neoplasm Diseases 0.000 description 3
- 108010022366 Carcinoembryonic Antigen Proteins 0.000 description 3
- 102100025475 Carcinoembryonic antigen-related cell adhesion molecule 5 Human genes 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 229920002774 Maltodextrin Polymers 0.000 description 3
- 239000005913 Maltodextrin Substances 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- 239000004952 Polyamide Substances 0.000 description 3
- 229920002732 Polyanhydride Polymers 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- 102000013529 alpha-Fetoproteins Human genes 0.000 description 3
- 108010026331 alpha-Fetoproteins Proteins 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- AFYNADDZULBEJA-UHFFFAOYSA-N bicinchoninic acid Chemical compound C1=CC=CC2=NC(C=3C=C(C4=CC=CC=C4N=3)C(=O)O)=CC(C(O)=O)=C21 AFYNADDZULBEJA-UHFFFAOYSA-N 0.000 description 3
- 239000012620 biological material Substances 0.000 description 3
- 229920002678 cellulose Polymers 0.000 description 3
- 238000012512 characterization method Methods 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 239000000084 colloidal system Substances 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 238000012937 correction Methods 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 239000004310 lactic acid Substances 0.000 description 3
- 235000014655 lactic acid Nutrition 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 229940035034 maltodextrin Drugs 0.000 description 3
- 229940092253 ovalbumin Drugs 0.000 description 3
- 229920002647 polyamide Polymers 0.000 description 3
- 229920001610 polycaprolactone Polymers 0.000 description 3
- 229920002635 polyurethane Polymers 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 238000001694 spray drying Methods 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical group FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 description 3
- 210000004881 tumor cell Anatomy 0.000 description 3
- 208000030507 AIDS Diseases 0.000 description 2
- 241000709701 Human poliovirus 1 Species 0.000 description 2
- 241000709704 Human poliovirus 2 Species 0.000 description 2
- 241000709727 Human poliovirus 3 Species 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- 229920001710 Polyorthoester Polymers 0.000 description 2
- 229920000388 Polyphosphate Polymers 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 102000003425 Tyrosinase Human genes 0.000 description 2
- 108060008724 Tyrosinase Proteins 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 230000030741 antigen processing and presentation Effects 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 229920001400 block copolymer Polymers 0.000 description 2
- 230000036760 body temperature Effects 0.000 description 2
- 229920005605 branched copolymer Polymers 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 230000001186 cumulative effect Effects 0.000 description 2
- 239000007857 degradation product Substances 0.000 description 2
- 238000000151 deposition Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000011067 equilibration Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 229920005684 linear copolymer Polymers 0.000 description 2
- 238000001459 lithography Methods 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 238000005192 partition Methods 0.000 description 2
- 230000002688 persistence Effects 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- 230000004962 physiological condition Effects 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920000233 poly(alkylene oxides) Polymers 0.000 description 2
- 229920001308 poly(aminoacid) Polymers 0.000 description 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 2
- 229920002627 poly(phosphazenes) Polymers 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 229920001515 polyalkylene glycol Polymers 0.000 description 2
- 229920002643 polyglutamic acid Polymers 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 239000001205 polyphosphate Substances 0.000 description 2
- 235000011176 polyphosphates Nutrition 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 238000013268 sustained release Methods 0.000 description 2
- 239000012730 sustained-release form Substances 0.000 description 2
- 238000012353 t test Methods 0.000 description 2
- 230000003442 weekly effect Effects 0.000 description 2
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- PJRSUKFWFKUDTH-JWDJOUOUSA-N (2s)-6-amino-2-[[2-[[(2s)-2-[[(2s,3s)-2-[[(2s)-2-[[2-[[(2s)-2-[[(2s)-6-amino-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[(2-aminoacetyl)amino]-4-methylsulfanylbutanoyl]amino]propanoyl]amino]-3-hydroxypropanoyl]amino]hexanoyl]amino]propanoyl]amino]acetyl]amino]propanoyl Chemical compound CSCC[C@H](NC(=O)CN)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(N)=O PJRSUKFWFKUDTH-JWDJOUOUSA-N 0.000 description 1
- RKDVKSZUMVYZHH-UHFFFAOYSA-N 1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1 RKDVKSZUMVYZHH-UHFFFAOYSA-N 0.000 description 1
- RPZANUYHRMRTTE-UHFFFAOYSA-N 2,3,4-trimethoxy-6-(methoxymethyl)-5-[3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxyoxane;1-[[3,4,5-tris(2-hydroxybutoxy)-6-[4,5,6-tris(2-hydroxybutoxy)-2-(2-hydroxybutoxymethyl)oxan-3-yl]oxyoxan-2-yl]methoxy]butan-2-ol Chemical compound COC1C(OC)C(OC)C(COC)OC1OC1C(OC)C(OC)C(OC)OC1COC.CCC(O)COC1C(OCC(O)CC)C(OCC(O)CC)C(COCC(O)CC)OC1OC1C(OCC(O)CC)C(OCC(O)CC)C(OCC(O)CC)OC1COCC(O)CC RPZANUYHRMRTTE-UHFFFAOYSA-N 0.000 description 1
- XZKIHKMTEMTJQX-UHFFFAOYSA-N 4-Nitrophenyl Phosphate Chemical compound OP(O)(=O)OC1=CC=C([N+]([O-])=O)C=C1 XZKIHKMTEMTJQX-UHFFFAOYSA-N 0.000 description 1
- JJTUDXZGHPGLLC-IMJSIDKUSA-N 4511-42-6 Chemical compound C[C@@H]1OC(=O)[C@H](C)OC1=O JJTUDXZGHPGLLC-IMJSIDKUSA-N 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- 238000011725 BALB/c mouse Methods 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 229920000623 Cellulose acetate phthalate Polymers 0.000 description 1
- DQEFEBPAPFSJLV-UHFFFAOYSA-N Cellulose propionate Chemical compound CCC(=O)OCC1OC(OC(=O)CC)C(OC(=O)CC)C(OC(=O)CC)C1OC1C(OC(=O)CC)C(OC(=O)CC)C(OC(=O)CC)C(COC(=O)CC)O1 DQEFEBPAPFSJLV-UHFFFAOYSA-N 0.000 description 1
- 229920002284 Cellulose triacetate Polymers 0.000 description 1
- 241001112696 Clostridia Species 0.000 description 1
- 206010053567 Coagulopathies Diseases 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- CKLJMWTZIZZHCS-UWTATZPHSA-N D-aspartic acid Chemical compound OC(=O)[C@H](N)CC(O)=O CKLJMWTZIZZHCS-UWTATZPHSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UWTATZPHSA-N D-lactic acid Chemical compound C[C@@H](O)C(O)=O JVTAAEKCZFNVCJ-UWTATZPHSA-N 0.000 description 1
- 229940032024 DPT vaccine Drugs 0.000 description 1
- 241000018344 Ehrlichia sp. 'CGE agent' Species 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 241000606768 Haemophilus influenzae Species 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 1
- 241000701806 Human papillomavirus Species 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 206010061598 Immunodeficiency Diseases 0.000 description 1
- 238000012404 In vitro experiment Methods 0.000 description 1
- 108090000723 Insulin-Like Growth Factor I Proteins 0.000 description 1
- 102000004218 Insulin-Like Growth Factor I Human genes 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 241000222722 Leishmania <genus> Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102000000440 Melanoma-associated antigen Human genes 0.000 description 1
- 108050008953 Melanoma-associated antigen Proteins 0.000 description 1
- 102100034256 Mucin-1 Human genes 0.000 description 1
- 108010008707 Mucin-1 Proteins 0.000 description 1
- 208000034176 Neoplasms, Germ Cell and Embryonal Diseases 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 241000224016 Plasmodium Species 0.000 description 1
- 101100388690 Plasmodium falciparum (isolate K1 / Thailand) MEF-1 gene Proteins 0.000 description 1
- 229920006022 Poly(L-lactide-co-glycolide)-b-poly(ethylene glycol) Polymers 0.000 description 1
- 229920001389 Poly(hydroxyalkylmethacrylamide) Polymers 0.000 description 1
- 229920001305 Poly(isodecyl(meth)acrylate) Polymers 0.000 description 1
- 229920002319 Poly(methyl acrylate) Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920002873 Polyethylenimine Polymers 0.000 description 1
- 229920001273 Polyhydroxy acid Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 108700020978 Proto-Oncogene Proteins 0.000 description 1
- 102000052575 Proto-Oncogene Human genes 0.000 description 1
- 241000220317 Rosa Species 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 208000003217 Tetany Diseases 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 241000700647 Variola virus Species 0.000 description 1
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 1
- 108020005202 Viral DNA Proteins 0.000 description 1
- NNLVGZFZQQXQNW-ADJNRHBOSA-N [(2r,3r,4s,5r,6s)-4,5-diacetyloxy-3-[(2s,3r,4s,5r,6r)-3,4,5-triacetyloxy-6-(acetyloxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6s)-4,5,6-triacetyloxy-2-(acetyloxymethyl)oxan-3-yl]oxyoxan-2-yl]methyl acetate Chemical compound O([C@@H]1O[C@@H]([C@H]([C@H](OC(C)=O)[C@H]1OC(C)=O)O[C@H]1[C@@H]([C@@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](COC(C)=O)O1)OC(C)=O)COC(=O)C)[C@@H]1[C@@H](COC(C)=O)O[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O NNLVGZFZQQXQNW-ADJNRHBOSA-N 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 238000007605 air drying Methods 0.000 description 1
- 229920003232 aliphatic polyester Polymers 0.000 description 1
- 229920013820 alkyl cellulose Polymers 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 229960005261 aspartic acid Drugs 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 230000003851 biochemical process Effects 0.000 description 1
- 229920000249 biocompatible polymer Polymers 0.000 description 1
- 239000004623 biodegradable polyanhydride Substances 0.000 description 1
- 229920000229 biodegradable polyester Polymers 0.000 description 1
- 239000004622 biodegradable polyester Substances 0.000 description 1
- 239000002981 blocking agent Substances 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 229920006217 cellulose acetate butyrate Polymers 0.000 description 1
- 229940081734 cellulose acetate phthalate Drugs 0.000 description 1
- 229920003086 cellulose ether Polymers 0.000 description 1
- 229920006218 cellulose propionate Polymers 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 230000035602 clotting Effects 0.000 description 1
- 235000021310 complex sugar Nutrition 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 230000003750 conditioning effect Effects 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 238000009295 crossflow filtration Methods 0.000 description 1
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 1
- 229940022769 d- lactic acid Drugs 0.000 description 1
- DEZRYPDIMOWBDS-UHFFFAOYSA-N dcm dichloromethane Chemical compound ClCCl.ClCCl DEZRYPDIMOWBDS-UHFFFAOYSA-N 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 239000002274 desiccant Substances 0.000 description 1
- 239000000032 diagnostic agent Substances 0.000 description 1
- 229940039227 diagnostic agent Drugs 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000002296 dynamic light scattering Methods 0.000 description 1
- 238000001493 electron microscopy Methods 0.000 description 1
- 230000013020 embryo development Effects 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 235000013861 fat-free Nutrition 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 229940045808 haemophilus influenzae type b Drugs 0.000 description 1
- 208000005252 hepatitis A Diseases 0.000 description 1
- 208000002672 hepatitis B Diseases 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 229920013821 hydroxy alkyl cellulose Polymers 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 229960001438 immunostimulant agent Drugs 0.000 description 1
- 239000003022 immunostimulating agent Substances 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 229940031551 inactivated vaccine Drugs 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 239000010954 inorganic particle Substances 0.000 description 1
- 230000008944 intestinal immunity Effects 0.000 description 1
- 210000004347 intestinal mucosa Anatomy 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 238000010902 jet-milling Methods 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- JJTUDXZGHPGLLC-UHFFFAOYSA-N lactide Chemical compound CC1OC(=O)C(C)OC1=O JJTUDXZGHPGLLC-UHFFFAOYSA-N 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 125000005647 linker group Chemical group 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- UNYOJUYSNFGNDV-UHFFFAOYSA-M magnesium monohydroxide Chemical compound [Mg]O UNYOJUYSNFGNDV-UHFFFAOYSA-M 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000008099 melanin synthesis Effects 0.000 description 1
- 125000005395 methacrylic acid group Chemical group 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 239000002088 nanocapsule Substances 0.000 description 1
- 239000002086 nanomaterial Substances 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 239000002077 nanosphere Substances 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000000399 optical microscopy Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 229940126578 oral vaccine Drugs 0.000 description 1
- 150000003891 oxalate salts Chemical class 0.000 description 1
- 108700025694 p53 Genes Proteins 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 238000007427 paired t-test Methods 0.000 description 1
- 201000006995 paralytic poliomyelitis Diseases 0.000 description 1
- 230000003071 parasitic effect Effects 0.000 description 1
- 238000000059 patterning Methods 0.000 description 1
- 108010021753 peptide-Gly-Leu-amide Proteins 0.000 description 1
- 210000001986 peyer's patch Anatomy 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 229920002120 photoresistant polymer Polymers 0.000 description 1
- 238000004023 plastic welding Methods 0.000 description 1
- 229960001539 poliomyelitis vaccine Drugs 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 229920000071 poly(4-hydroxybutyrate) Polymers 0.000 description 1
- 229920000729 poly(L-lysine) polymer Polymers 0.000 description 1
- 229920001490 poly(butyl methacrylate) polymer Polymers 0.000 description 1
- 229920001483 poly(ethyl methacrylate) polymer Polymers 0.000 description 1
- 239000005014 poly(hydroxyalkanoate) Substances 0.000 description 1
- 229920001390 poly(hydroxyalkylmethacrylate) Polymers 0.000 description 1
- 229920000218 poly(hydroxyvalerate) Polymers 0.000 description 1
- 229920000212 poly(isobutyl acrylate) Polymers 0.000 description 1
- 229920000205 poly(isobutyl methacrylate) Polymers 0.000 description 1
- 229920000196 poly(lauryl methacrylate) Polymers 0.000 description 1
- 229920000184 poly(octadecyl acrylate) Polymers 0.000 description 1
- 229920001583 poly(oxyethylated polyols) Polymers 0.000 description 1
- 229920000070 poly-3-hydroxybutyrate Polymers 0.000 description 1
- 229920002721 polycyanoacrylate Polymers 0.000 description 1
- 229920006149 polyester-amide block copolymer Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 239000004633 polyglycolic acid Substances 0.000 description 1
- 229920000903 polyhydroxyalkanoate Polymers 0.000 description 1
- 229920000197 polyisopropyl acrylate Polymers 0.000 description 1
- 229920001855 polyketal Polymers 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 229920000182 polyphenyl methacrylate Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 108010000222 polyserine Proteins 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 229920001290 polyvinyl ester Polymers 0.000 description 1
- 229920001289 polyvinyl ether Polymers 0.000 description 1
- 229920001291 polyvinyl halide Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 108700042226 ras Genes Proteins 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000012770 revaccination Methods 0.000 description 1
- 238000005185 salting out Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 235000021309 simple sugar Nutrition 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 239000002195 soluble material Substances 0.000 description 1
- 239000012798 spherical particle Substances 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 238000012027 sterile manufacturing Methods 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 229940031626 subunit vaccine Drugs 0.000 description 1
- 150000003890 succinate salts Chemical class 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 231100000816 toxic dose Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 230000005740 tumor formation Effects 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 239000012646 vaccine adjuvant Substances 0.000 description 1
- 229940124931 vaccine adjuvant Drugs 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 210000003501 vero cell Anatomy 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- PAPBSGBWRJIAAV-UHFFFAOYSA-N ε-Caprolactone Chemical group O=C1CCCCCO1 PAPBSGBWRJIAAV-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55555—Liposomes; Vesicles, e.g. nanoparticles; Spheres, e.g. nanospheres; Polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/60—Medicinal preparations containing antigens or antibodies characteristics by the carrier linked to the antigen
- A61K2039/6093—Synthetic polymers, e.g. polyethyleneglycol [PEG], Polymers or copolymers of (D) glutamate and (D) lysine
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/32011—Picornaviridae
- C12N2770/32611—Poliovirus
- C12N2770/32634—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- This invention is generally in the field of injectable vaccine formulations providing multiple releases of vaccine.
- Vaccines typically involve an initial dose of antigen, followed by one or more booster doses at defined times after the initial administration, typically ten to 60 days later.
- booster doses typically limit the practicality of vaccines in much of the world, as well as increases costs and difficulties in agricultural applications.
- Polymeric microspheres have the potential to be effective vaccine delivery vehicles. They have the ability to enhance targeting of antigen presenting cells (APCs) and have the potential for controlled, sustained release of antigen-thereby potentially eliminating the need for multiple vaccination doses. Further, the polymer matrix can act as a shield from a hostile external environment and has the potential to reduce adverse reactions and abrogate problems caused by the vaccine strain in immunocompromised individuals.
- PLGA microspheres have been developed for single immunization, with and without burst release. Given the biodegradable nature and sustained release properties that PLGA offers, microspheres formulated from PLGA could be useful for the delivery of vaccines.
- PLGA based microparticles are traditionally produced by double emulsion-solvent evaporation, nano- precipitation, cross-flow filtration, salting-out techniques, emulsion-diffusion methods, jet milling, and spray drying.
- ICirby et ah Chapter 13: Formation and Characterisiation of polylactide-co-galactide PLGA microspheres (2013 ).
- PLGA microspheres can also be formulated to incorporate a range of moieties, including drugs and proteins, that can act as adjuvants.
- PGLA particles produced by these methods can be lyophilized and stored for later use and delivery.
- Sanchez et al., J. Pharmaceutical Sciences, 85(6):547-552 (1996 ) discloses microcapsule structures with oil-based cores of TT surrounded by outer PLGA polymer shells which release the antigen in pulses at two different times. The particles are prepared using an emulsion method.
- Microspheres have potential as carriers for oral vaccine delivery due to their protective effects on encapsulated antigens and their ability to be taken up by the Peyer's patches in the intestine.
- the potency of these optimal depot formulations for antigen may be enhanced by the co-delivery of vaccine adjuvants, including cytokines, that are either entrapped in the polymer matrix or, alternatively, incorporated into the backbone of the polymer itself and released concomitantly with antigen as the polymer degrades.
- vaccine adjuvants including cytokines
- a subunit vaccine e.g ., gpl20
- AIDS acquired immunodeficiency syndrome
- PLGA poly(lactic-co-glycolic acid)
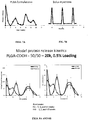
- the protein was released under physiological conditions in two discrete phases: an initial burst released over the first day and after several weeks or months, a second burst of protein was released.
- the second burst of protein was dependent upon the PLGA inherent viscosity and lactide/glycolide ratio (bulk erosion).
- Micromolded (“MM”) or three dimensional printed (“3DP”) polymeric formulations for single injection of antigen, releasing at two or more time periods, have been developed, as claimed.
- Formulations are formed of biocompatible, biodegradable polymers. Discrete regions encapsulating antigen, alone or in combination with other antigens, adjuvants, stabilizers, and release modifiers, are present in the formulations.
- Antigen is preferably present in excipient at the time of administration, or on the surface of the formulation, for immediate release, and incorporated within the formulation for release at ten to 45 days after initial release of antigen, optionally at ten to 90 day intervals for release of antigen in one or more additional time periods.
- Antigen may be stabilized through the use of stabilizing agents such as trehalose glass.
- antigen is released at the time of administration, and two, four and six months thereafter.
- leakage between bursts of release is minimal and release occurs over a narrow time frame.
- Preferred solvents include methylene chloride and chloroform
- preferred polymers are polylactic acid (“PLA”), polyglycolic acid (“PGA”), and copolymers thereof (“PLGA”).
- Formulations are designed for subcutaneous or intramuscular injection via needle or cannula, for topical injection to a mucosal region such as intranasal, or by scarification to the epidermis.
- Preferred applications are for administration of antigen eliciting an effective immune response to infectious agents such as bacteria, virus, protozoan and parasitic organisms.
- formulations may also be used for administration of other therapeutic, prophylactic or diagnostic agents, alone or in combination with antigen.
- the formulations which may be formed of microparticles, including microspheres or microcapsules and including those that are emulsion-based, or devices such as those prepared by micromolding, are formed of polymers.
- Antigen may be dispersed or encapsulated by the polymer.
- the device contains a core that only contains one or more vaccines or antigen and stabilizers and the shell or particle wall only contains one or more biodegradable polymers with or without additives. Polymer without antigen may be used to seal or separate areas of the formulation from other areas, and release at different rates.
- Polymers must be biocompatible and processible under conditions and using reagents that preserve the antigen.
- the formulation can be made with hydrophilic polymers, hydrophobic polymers, amphiphilic polymers, or mixtures thereof.
- the formulation can contain one or more hydrophilic polymers.
- Hydrophilic polymers include cellulosic polymers such as starch and polysaccharides; hydrophilic polypeptides; poly(amino acids) such as poly-L-glutamic acid (PGS), gamma-polyglutamic acid, poly-L-aspartic acid, poly-L-serine, or poly-L-lysine; polyalkylene glycols and polyalkylene oxides such as polyethylene glycol (PEG), polypropylene glycol (PPG), and poly(ethylene oxide) (PEO); poly(oxyethylated polyol); poly(oleflnic alcohol); polyvinylpyrrolidone); poly(hydroxyalkylmethacrylamide); poly(hydroxyalkylmethacrylate); poly(saccharides); poly(hydroxy acids); poly(vinyl alcohol), and copolymers thereof.
- hydrophobic polymers include polyhydroxyacids such as poly(lactic acid), poly(glycolic acid), and poly(lactic acid-co-glycolic acids); polyhydroxyalkanoates such as poly3-hydroxybutyrate or poly4-hydroxybutyrate; polycaprolactones; poly(orthoesters); polyanhydrides; poly(phosphazenes); poly(lactide-co-caprolactones); polycarbonates such as tyrosine polycarbonates; polyamides (including synthetic and natural polyamides), polypeptides, and poly(amino acids); polyesteramides; polyesters; poly(dioxanones); poly(alkylene alkylates); hydrophobic polyethers; polyurethanes; polyetheresters; polyacetals; polycyanoacrylates; polyacrylates; polymethylmethacrylates; polysiloxanes; poly(oxyethylene)/poly(oxypropylene) copolymers; polyketals; polyphosphates; polyhydroxyvalerates
- the formulation can contain one or more biodegradable polymers.
- Biodegradable polymers can include polymers that are insoluble or sparingly soluble in water that are converted chemically or enzymatically in the body into water-soluble materials.
- Biodegradable polymers can include soluble polymers crosslinked by hydolyzable cross-linking groups to render the crosslinked polymer insoluble or sparingly soluble in water.
- Biodegradable polymers include polyamides, polycarbonates, polyalkylenes, polyalkylene glycols, polyalkylene oxides, polyalkylene terepthalates, polyvinyl alcohols, polyvinyl ethers, polyvinyl esters, polyvinyl halides, polyvinylpyrrolidone, polyglycolides, polysiloxanes, polyurethanes and copolymers thereof, alkyl cellulose, hydroxyalkyl celluloses, cellulose ethers, cellulose esters, nitro celluloses, polymers of acrylic and methacrylic esters, methyl cellulose, ethyl cellulose, hydroxypropyl cellulose, hydroxy-propyl methyl cellulose, hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose acetate phthalate, carboxylethyl cellulose, cellulose triacetate, cellulose sulphate sodium salt, poly (methyl
- biodegradable polymers include polyesters, poly(ortho esters), polyethylene imines), poly(caprolactones), poly(hydroxybutyrates), poly(hydroxyvalerates), polyanhydrides, poly(acrylic acids), polyglycolides, poly(urethanes), polycarbonates, polyphosphate esters, polyphosphazenes, derivatives thereof, linear and branched copolymers and block copolymers thereof, and blends thereof.
- Amphiphilic polymers can be polymers containing a hydrophobic polymer block and a hydrophilic polymer block.
- the hydrophobic polymer block can contain one or more of the hydrophobic polymers above or a derivative or copolymer thereof.
- the hydrophilic polymer block can contain one or more of the hydrophilic polymers above or a derivative or copolymer thereof.
- the microparticle contains biodegradable polyesters or polyanhydrides such as poly(lactic acid), poly(glycolic acid), and poly(lactic-co-glycolic acid).
- the microparticles can contain one more of the following polyesters: homopolymers including glycolic acid units, referred to herein as "PGA,” and lactic acid units, such as poly-L-lactic acid, poly-D-lactic acid, poly-D,L-lactic acid, poly-L-lactide, poly-D-lactide, and poly-D,L-lactide, collectively referred to herein as "PLA,” and caprolactone units, such as poly(s-caprolactone), collectively referred to herein as "PCL;” and copolymers including lactic acid and glycolic acid units, such as various forms of poly(lactic acid-co-glycolic acid) and poly(lactide-co-glycolide) characterized by the ratio of lactic acid:glycolic acid, collectively referred to herein as
- Exemplary polymers also include copolymers of polyethylene glycol (PEG) and the aforementioned polyesters, such as various forms of PLGA-PEG or PLA-PEG copolymers, collectively referred to herein as "PEGylated polymers.”
- PEG polyethylene glycol
- the PEG region can be covalently associated with polymer to yield "PEGylated polymers" by a cleavable linker.
- the formulation can contain one or a mixture of two or more polymers.
- the microparticles may contain other entities such as stabilizers, surfactants, or lipids.
- Solvents must be biocompatible, since some residue will always be present in the polymeric formulations.
- Representative polymer solvents include organic solvents such as chloroform, dichloromethane, tetrafluoroethylene, and acyl acetate.
- the antigen can be dissolved in aqueous or aqueous miscible solvents such as acetone, ethanol, methanol, isopropyl alcohol, and mixtures thereof.
- Antigens for delivery are killed or attenuated infectious agents such as bacteria such as Clostridia tetani, viruses such as hepatitis, influenza, and polio, and protozoans such as Plasmodium (malaria) and Leishmania.
- Table 2 lists some vaccines the antigens of which can be used in the disclosed formulations.
- Other antigens are antigenic proteins or haptens such as carbohydrate or sugar antigens effective as antigens for these infectious agents, as cancer antigens, or as immunostimulants.
- Poliomyelitis is a highly contagious viral disease that invades the nervous system and can cause total paralysis in a matter of hours.
- One in 200 infections leads to irreversible paralysis, which is usually confined to the legs. Among those paralyzed, 5% to 10% die due to paralysis of the diaphragm. There is no cure for polio. However, it can be prevented by vaccination.
- Polio cases have decreased by over 99% since 1988, from an estimated 350,000 cases in more than 125 endemic countries to only 223 reported cases in 2012. As of early 2013, only three countries (Afghanistan, Nigeria, and Pakistan) in the world were endemic for the disease. Despite aggressive vaccination efforts, polio has not been completely eradicated and outbreaks still occur, particularly in developing countries.
- IPV Inactivated Polio Vaccine
- OOV Oral Polio Vaccine
- Any protein produced in a tumor cell that has an abnormal structure due to mutation can act as a tumor antigen.
- Such abnormal proteins are produced due to mutation of the concerned gene. Mutation of protooncogenes and tumor suppressors which lead to abnormal protein production are the cause of the tumor and thus such abnormal proteins are called tumor-specific antigens. Examples of tumor-specific antigens include the abnormal products of ras and p53 genes. In contrast, mutation of other genes unrelated to the tumor formation may lead to synthesis of abnormal proteins which are called tumor-associated antigens.
- An example of such a protein is the enzyme tyrosinase, which is required for melanin production. Normally tyrosinase is produced in minute quantities but its levels are very much elevated in melanoma cells.
- Oncofetal antigens are another important class of tumor antigens. Examples are alphafetoproteins (AFP) and carcinoembryonic antigen (CEA). These proteins are normally produced in the early stages of embryonic development and disappear by the time the immune system is fully developed. Thus self-tolerance does not develop against these antigens.
- AFP alphafetoproteins
- CEA carcinoembryonic antigen
- Abnormal proteins are also produced by cells infected with oncoviruses, e.g., Epstein Barr Virus ("EBV”) and Human Papillomavirus (“HPV”). Cells infected by these viruses contain latent viral DNA which is transcribed and the resulting protein produces an immune response. In addition to proteins, other substances like cell surface glycolipids and glycoproteins may also have an abnormal structure in tumor cells and could thus be targets of the immune system.
- EBV Epstein Barr Virus
- HPV Human Papillomavirus
- tumor antigens have the potential to be effective as tumor vaccines.
- alpha fetoprotein germ cell tumors, hepatocellular carcinoma
- carcinoembryonic antigen bowel, lung, breast cancers
- tumor antigens include CA-125 (ovarian cancer), MUC-1 (breast cancer, epithelial tumor antigen (breast cancer), and melanoma-associated antigen (malignant melanoma).
- Antigen stability is defined as the maintenance of antigen structure during formation of the vaccine formulation and at body temperature. As discussed below, the polymer composition, selection of solvent, and processing conditions are critical to maintain antigen stability.
- Stabilizing agents may also be added.
- Sugars are a typical group of stabilizing agents for proteins. Examples include simple sugars such as sucrose, fructose, mannitol, glucose, and trehalose as well as more complex sugars. See Alcock et al, Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Science Translational Medicine, 2(19):19-19ral2 (2010 ).
- Stabilization of the antigen can be determined by antigen specific ELISA in vitro and by measuring the immune response (e.g., IgGs) in animals in vivo. Stability is evaluated during each step of the encapsulation and/or manufacturing process, during storage (at 25°C, room temp, high humidity/ high temp conditions, under physiological conditions (pH 7.2, 37°C) and in vivo (animal models).
- the immune response e.g., IgGs
- Stability is evaluated during each step of the encapsulation and/or manufacturing process, during storage (at 25°C, room temp, high humidity/ high temp conditions, under physiological conditions (pH 7.2, 37°C) and in vivo (animal models).
- Gas-generated burst-release systems may allow for instantaneous release of encapsulated antigen. Pore forming agents which are removed by leaching or lyophilization may also be utilized.
- Post-formulation sterilization can typically be accomplished through a combination of sterile manufacturing conditions in combination with methods such as gamma irradiation.
- Microparticles can be made using standard techniques.
- a preferred technique is emulsification of a polymer solution in an organic solvent with an aqueous solution. Addition of organic phase to a large volume of non-solvent phase forms a spontaneous single emulsion and the resulting solution is stirred continuously for solvent evaporation. Immediate formation of microspheres occurs. After stirring, microspheres are washed and then dried.
- the examples demonstrate formation of microparticles using emulsification of polymer and antigen, alone or in combination with stabilizers such as trehalose and sucrose.
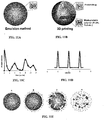
- 3D printing could increase consistency of microspheres, allowing for more uniform release, as well as provide a means for making more complex devices such as 'Micro-rods', having increased carrying capacity, that could eliminate the need for simultaneous release from multiple microspheres, as well as facilitate scale up.
- Three-dimensional (3D) printing is a process of making 3D objects from a digital model.
- 3D printing is an additive process, where successive layers of material are laid down in different shapes. After each layer is added the "ink” is polymerized, typically by photopolymerization, and the process repeated until a 3D object is created.
- the recent commercial availability and reduced cost makes 3D printing of biomolecules, including vaccines and pharmaceuticals, attractive for distribution of these compounds to developing countries. This would negate the need to ship a finished product into the country. Instead, a 3D printer at the point of care can print out the required biomolecule from a simple computer program, which can come from anywhere in the world.
- the 3D printing workflow can be described in 3 sequential steps: 1) the powder supply system platform is lifted and the fabrication platform is lowered one layer; 2) a roller spreads the polymer powder into a thin layer; . 3) a print-head prints a liquid binder that bonds the adjacent powder particles together.
- Billiet et al Biomaterials, 33 :6020-6041 (2012 ).
- Two kinds of 3D printing techniques are mostly adopted for nanobiomaterial fabrication.
- One is inkjet printing with the typical printers.
- Marizza et al Microelectrionic Engin. 111:391-395 (2013 ).
- the other is nanoimprint lithography.
- Nanoimprint lithography is a fast and cost-efficient technique for fabricating nanostructures.
- the procedure of NIL is to stack multiple layers of such structures on top of each other; that is, a finished double -layer of structures is covered with a spacer-layer which is planarized using the chemical-mechanical polishing so that a second layer can be processed on top.
- Ink-Jet printing has been used to produce monodisperse PLGA particles.
- Bohmer et ah Colloids and Surfaces A: Physiochem. Eng. Aspects, 289: 96-104 (2006 ).
- droplets of a PLGA solution are printed with the ink-jet nozzle submerged into an aqueous phase.
- This method produces microspheres at predictable and controllable sizes.
- This technique has been used to created Paclitaxel-loaded monodisperse microspheres.
- Radulecu et ah Digital Fabrication Sep: 18-21. (2005 ).Variation of this technology has been used to create multilayer monodisperse microspheres. See Kim and Pack, BioMEMS and Biomedical Nanotechnology, 1:19-50 (2006 ).
- microcapsule shell thickness can be varied from less than 2 microns to tens of microns while maintaining complete and well-centered core encapsulation for microcapsules near 50 microns in overall diameter.
- Drug delivery rates from microspheres have been varied by providing uniform monodisperse microparticles, mixtures of microparticles of varying sizes, and microparticles having different degradable layers. See Kim and Pack, BioMEMS and Biomedical Nanotechnology, 1:19-50 (2006 ).
- Waveform was optimized for jetting monodisperse ink droplets consistently.
- the applied voltage, the duration of the applied voltage, and the change in voltage over time (slope) are all parameters that must be optimized to jet high quality ink drops.
- the waveform was optimized for a solution of 5% w/v 31k (average molecular weight) PLGA in 1,4-dioxane.
- Waveforms were optimized with the JefXpert imaging system, and the ink and waveform and then transferred to the multi-material inkjet 3D-printer. JetXpert imaging was done with a constant pressure waveform.
- Figures 11A and 11B are schematics of particles made by emulsion ( Figure 11A ) and by 3D printing ( Figure 11B ), and the release of protein from the emulsion particle ( Figure 11C ) and in an ideal case the 3DP particle ( Figure 11D ). These show the differences in the resulting structure, which also changes the release kinetics.
- Figures 12A-12D are schematics of the 3D printing process: Creating the structure of PLGA particles ( Figures 12A and 24A ), filling drugs or proteins into the particles ( Figures 12B and 24B ), drying the drugs or proteins ( Figure 12C ), and encapsulation of the particles ( Figures 12D and 24C ).
- the schematic shows a cube shaped structure filled with vaccine, but it is understood that a mold may be utilized to provide any shaped, and that the mold may be filled in layers or more complex patterns.
- Table 1 Solvents for 3DP.
- Waveform parameters can be optimized to produce uniform single droplets of "ink” (polymer solution) during printing from a piezoelectric nozzle jet ( Figure 13 ).
- Drop size is a function of various parameters which are optimized, including applied voltage, slope of voltage, polymer and solvent selection and concentration.
- micromolds were filled with polymer microparticles, to produce microstructures composed of multiple materials, having complex geometries, and made using mild processing conditions. These microparticles are typically prepared using an oil-water, double-emulsion system; spray drying methods; supercritical conditioning methods; and milling methods.
- micromolds can be prepared by photolithographically creating a female master mold made of photoresist, molding a male master structure out of polydimethylsiloxane (PDMS) from the female master mold and) molding a female replicate mold out of PDMS from the male master structure.
- Polymer microparticles can be micromolded using temperature/press methods and/or from solvent.
- Polymeric microparticles of 1 to 30 ⁇ n in size were made from PLA, PGA and PLGA using spray drying and emulsion techniques. These polymer microparticles were filled into PDMS micromolds at room temperature and melted or bonded together, for example, by ultrasonically welding microparticles together in the mold while maintaining the voids inherent in their packing structure. Multi-layered microstructures were fabricated to have different compositions of polymers and encapsulated compounds located in different regions of the microstructures. Molds were filled with solid polymer microparticles instead of a polymer melt to copy microstructures with complex geometries and composed of multiple materials using mild processing conditions.
- Microparticles can flow easily into the cavities of micromolds at room temperature and low pressure, which facilitates making microstructures with high aspect ratios.
- polymer microparticles can encapsulate chemical compounds, such as drugs, and can be filled into molds in sequential layers to accommodate multiple material compositions. After filling the mold, the final microstructures can be created by welding the microparticles within the mold by plastic welding methods, including thermal and ultrasonic welding as well as solvent and gas based welding.
- the vaccine formulations are administered to an individual in need of vaccination. These are administered as a dosage formulation including an effective amount of one or more antigens released in a schedule that elicits a protective effect against the source of the antigen.
- Microparticles or microcapsules can be administered by injection, preferably subcutaneously or intramuscularly, for example, under the skin of the back of the upper arm, or to a mucosal surface (orally, intranasally, via the pulmonary route, or other orifice), although injection is preferred if release is to occur over a prolonged period of time of more than a few days.
- the dosage form is designed to release a bolus of antigen at the time of administration. This may be achieved by administering a solution or dispersion of antigen in combination with the vaccine formulation providing multiple releases at subsequent times, or the device may be formulated to provide an initial bolus as well as subsequent release(s).
- Additional vaccines of great interest in third world countries include polio and smallpox.
- the following uses polio vaccine as an exemplary vaccine for this application.
- IPV Vaccine SSI is an inactivated vaccine used for prophylactic vaccination against paralytic poliomyelitis.
- IPV Vaccine SSI contains inactivated poliovirus type 1, 2 and 3, propagated in Vero cells.
- IPV Vaccine SSI contains trace amounts of residual formaldehyde. It is manufactured in Denmark by Statens Serum Institut.
- IPV Vaccine SSI is a solution for injection distributed in single-dose vials. For primary vaccination a series of three doses of 0.5 ml is administered.
- the vaccine should be administered intramuscularly or subcutaneously.
- the vaccine must not be administered intravascular.
- the age at the first dose should be at least 6 weeks, and the primary vaccination series should include at least three immunizations, with an interval of at least four weeks. Most countries give IPV using the same schedule as DPT vaccine (typically 2 months, 4 months, and 6 months of age).
- IPV Vaccine SSI The immunogenicity and safety of IPV Vaccine SSI has been investigated in several clinical trials, including clinical trials with combined vaccines for pediatric use. Apart from IPV these trials included vaccine antigens against tetanus, diphtheria, pertussis and Haemophilus influenzae type b.
- IPV Vaccine SSI can be used for revaccination in infants, pre-school aged children and adults primary immunized with IPV or OPV.
- IPV Vaccine SSI can be used in mixed IPV/OPV schedules, using one to three doses of IPV followed by one to three doses of OPV. It is recommended to administer IPV before the first dose of OPV. In a mixed IPV/OPV schedule, persistence of protective antibodies after primary vaccination has been shown to last at least 20 years. In an IPV only schedule the persistence of protective SSI recommended dose:
- the examples below describe different polymer-antigen formulations for controlled release of the antigen.
- the controlled release relies on polymer degradation to achieve multiple bursts of antigen release over time following single injection.
- Immunogenicity of the formulations following polymer degradation-based bursts of antigen release and sustained increase in anti-antigen antibody titer are also presented.
- Methods of making the formulations which include incorporation of the antigen into the polymer matrix via spontaneous emulsion, or encapsulation of the antigen within a polymer shell via 3D printing or micromolding, are also described.
- Studies on improving the stability of the antigen, the stability of the polymer matrix, and the stability of the formulations, so that formulations with desired release characteristics and immunogenicity can be obtained, are also presented.
- Example 1 Selection of Polymers and Solvents for Discrete Release of Vaccine (Comparative Example)
- PVA poly(vinyl alcohol)
- BSA bovine serum albumin
- IPV inactivated polio virus
- Figure 21A is a graph of the release of a model protein, bovine serum albumin ("BSA") over time (weeks) for 5% BSA, 3% BSA, and 0.5% BSA from PLGA (50:50), 20 kD, derivatized with a carboxylic group.
- BSA bovine serum albumin
- Figure 21D is a graph of the release of bovine serum albumin over time (weeks) for 5% BSA, 3% BSA, and 0.5% BSA from PLGA (50:50), 9.5 kD, derivatized with a carboxylic group.
- Figures 3A and 3B are graphs of the release of bovine serum albumin over time (weeks) for 5% BSA and 0.5% BSA from PLLA, 50 kD.
- Figures 4A and 4B are graphs of the release of bovine serum albumin over time (weeks) for 5% BSA and 0.5% BSA from PLLA, 100 kD.
- Figures 5A and 5B are graphs of the release of bovine serum albumin over time (weeks) for 5% BSA and 0.5% BSA from PLLA, 300 kD.
- Figures 6A and 6B are graphs of the release of bovine serum albumin over time (weeks) for 5% BSA and 0.5% BSA from P(d,l)LA, 20 kD. The results are similar to Figures 3-5 and 21 .
- Figures 8A and 8B are graphs of the release of 0.5% of bovine serum albumin compared to release of 0.5% of ovalbumin over time (weeks) from PLGA (50:50), 20 kD, derivatized with a carboxylic group.
- Figures 9A and 9B are graphs of the release of 5% of bovine serum albumin compared to release of 5% of ovalbumin over time (weeks) from PLGA (50:50), 31 kD, derivatized with a carboxylic group.
- Figures 10A and 10B are graphs of the release of 0.5% of bovine serum albumin compared to release of 0.5% of ovalbumin over time (weeks) from PLGA (50:50), 31 kD, derivatized with a carboxylic group.
- Example 2 Predicted Release Profiles from Microparticles made by Emulsion Compared to Microparticles made by 3DP
- Particles fabricated by computer-controlled inkjet 3D-printing can be made with identical micrometer-scale dimensions, drug loadings, and spatial locations of drugs within the polymer microstructures.
- the main difference between the two methods is that emulsion based particles (left) are matrix based and the drug is homogeneously distributed throughout the particle.
- the 3D or micromolded particle is shown on the right.
- the vaccine and polymer are distinctly separated, where the vaccine is in the core and the polymer is only in the shell.
- the release graph of Figure 11C is based on the data in Example 1.
- the drug or the vaccine is encapsulated in polymer microspheres and dispersed throughout the polymer matrix (A). Once hydrated, any drug on the surface is immediately released (B) thus causing the initial burst. Following this event, the microspheres have remnant pores through which drug can slowly diffuse causing the secondary burst (C). Finally, when the polymer bulk erodes, due to significant Mw degradation, the reminder of the drug is released in a third burst (D).
- Z number ranges can be defined for determining ink printability with a specific waveform.
- Solvents are chosen based on PLGA solubility and physical properties (i.e. vapor pressure and boiling point).
- Example 4 Studies to minimize loss of IPV D-antigen and increase stability during lyophilization and encapsulation
- IPV stability in 3D-printed substrates was tested in a similar manner. IPV solutions containing co-dissolved sugars (sucrose and trehalose) were deposited onto a scaled-up PLA structure printed using the MakerBot Replicator 2. Drying of.the IPV/sugar solutions was done at ambient humidity (-20% humidity), which was higher than that used by Alcock et al (-10%).
- Figures 14A, 14B and 14C are graphs of the percent D-antigen (IPV type I, II, and III) retained after lyophilizing with sugar excipients 1 M trehalose, 1 M sucrose, and 3 M sucrose, then incubating at 4°C, 25°C or 37°C.
- IPV type I, II, and III percent D-antigen
- Figures 15A and 15B are graphs of the percent D-antigen (IPV type I, II, and III) retained after lyophilizing with 0, 0.5, 0.75, 1 or 1.25 M trehalose, 1 then incubating at 4°C ( Figure 15A ) or 25 °C ( Figure 15B ). The best results were obtained with 0.5 M and 0.75 M trehalose.
- Figures 16A and 16B are graphs of the percent D-antigen (IPV type I, II, and III) retained after mixing with solvents until solvent evaporation, with solvents tetrafluoroethylene (“TFE”), dichloromethane (“DCM”), or TFE and DCM, without sugar excipient ( Figure 16A ) or with 0.75 M trehalose as excipient ( Figure 16B ). DCM was statistically significantly better. IPV was not stable when exposed to the organic solvents during the encapsulation process, even when trehalose was used as an excipient.
- Figure 17A is a graph of the percent D-antigen (IPV type I, II, and III) retained after IVP with 0 M sugar, 0.5 M trehalose, or 0.5 M trehalose-sucrose is printed onto 3D-printed PLA substrate and dried at 25°C, 22% RH.
- Figure 17B is a graph of the percent D-antigen (IPV type I, II, and III) retained after IVP with 0.25 M trehalose or 0.5 M trehalose-sucrose is printed onto 3D-printed PLA substrate and dried at 25°C, 10.7% RH.
- Figures 18A and 18B are graphs of the percent D-antigen retained after lyophilization with 0.5 or 0.75 M trehalose then incubating with 25°C ( Figure 18A ) or after lyophilization with 0.5 M trehalose-sucrose, pipetted, or 0.5 M trehalose-sucrose, jetted, then drying at 25°C, 10.7% RH ( Figure 18B ).
- formulations F3, F5 and F7 presented in Table 3 above and Table 4 below.
- Formulations F4 and F8 are blank microspheres (no drug), used as negative controls (see Table 4).
- Poly(D,L-lactic-co-glycolic acid) (PLGA Resomer® RG 502 H, RG 503 H, RG 504 H, and RG 752 H) and BSA were purchased from Sigma-Aldrich (St. Louis, MO).
- Microsphere size distribution was determined using a Multisizer 3 Coulter Counter. Histograms were created using a bin size of 0.39 ⁇ m and smoothed using central moving average with a window size of ⁇ 5 bins. Scanning electron microscope (SEM) images were collected using a JSM-5600LV SEM (JEOL, Tokyo, Japan) at an acceleration voltage of 5kV. Prior to imaging, samples were coated with Au/Pd using a Hummer 6.2 Sputtering System (Anatech, Battle Creek, MI) to prevent surface charging.
- SEM scanning electron microscope
- mice between 6 and 8 weeks of age received injections of (1) BSA-loaded microspheres, (2) unloaded microspheres, (3) bolus BSA, or (4) saline-only. While mice in the first two groups received only one injection, those receiving a bolus BSA or saline-only were injected again at 4 and 8 weeks to match the amount and timing of BSA release from PLGA microspheres in vitro. Samples were dissolved or suspended (when applicable) in 200 ⁇ of saline and injected subcutaneously into each hind limb for a total of 400 ⁇ l . Table 5 contains the exact dosing regimen for each group.
- BSA Group First Dose Second Dose Third Dose Total Formulation C (0.5% BSA, 7-17 kDaPLGA)* 22 20 22 64 Formulation G (0.5% BSA, 24-38 kDa PLGA)* 23 14 34 71 Low Dose Bolus BSA 22 22 22 66 Formulation E (5% BSA, 24-38 kDa PLGA)* 298 68 65 431 High Dose Bolus BSA 298 68 65 431 Unloaded PLGA microspheres, 7-17 kDa 0 0 0 0 Unloaded PLGA microspheres, 24-38 kDa 0 0 0 0 Saline 0 0 0 0 0 All PLGA used in vivo was 50:50. indicates theoretical dose based on in vitro results.
- Serum antibody titers against BSA were determined using an endpoint enzyme-linked immunosorbent assay (ELISA).
- ELISA enzyme-linked immunosorbent assay
- 96-well Maxisorp ELISA plates (Thermo Fisher Scientific, Waltham, MA) were coated overnight at 4°C with 100 ⁇ L of a 100 ( ⁇ g/m solution of BSA in 0.1M sodium bicarbonate. Plates were then washed three times in PBS containing 0.05% Tween 20 (PBST) and then incubated in 5% non-fat milk in PBST for 2 hours at 37°C as a blocking agent. Following another series of three washes with PBST, mouse serum samples were added in four-fold serial dilutions and incubated for 2 hours at 37°C.
- PBST PBS containing 0.05% Tween 20
- Formulations G and E demonstrated similar characteristics with particle diameters of 12.1 ⁇ 8.2 and 8.6 ⁇ 6.7 ⁇ respectively, yet with 90% of particle volume contained in particles larger than 23.1 and 21.4 ⁇ respectively. Histograms of microsphere diameter and volume distribution can be seen in Figure 20A-20F . Particles at the large end of the distribution also contributed substantially to surface area effects as 50% of the cumulative particle surface area was present on particles larger than 23.67, 29.54, and 27.58 ⁇ in diameter for Formulations C, G, and E respectively.
- Low molecular weight (7-17 kDa) PLGA released in three distinct bursts over the course of 8 weeks and completely degraded by week 14 as seen in Figure 21A .
- the medium molecular weight (24-38 kDa) PLGA also displayed three bursts spread over 9-12 weeks depending on BSA loading and degraded by week 14 ( Figure 21B ).
- the highest molecular weight (38-54 kDa) PLGA released BSA over 9-12 weeks with prominent bursts at day 1 and week 8, but with more continuous release kinetics in between from the 3 and 5% BSA loaded microspheres ( Figure 21C ).
- Formulations C, G, and E were chosen for the subsequent in vivo study based on their in vitro release kinetics.
- Formulation C microspheres were made using low molecular weight PLGA (7-17 kDa) loaded with 0.5% BSA, Formulation G with slightly higher molecular weight PLGA (24-38 kDa) and 0.5% BSA, and Formulation E with the same molecular weight PLGA as Formulation G, but with higher (5%) BSA loading.
- BSA release from Formulation C was characterized by three distinct peaks at day 1 when 33.4 ⁇ 5.1% of total BSA was released, at week 4 when 27.2 ⁇ 4.3 % was released, and across the week 6 and week 7 time points during which 32.1 ⁇ 5.2% was released respectively ( Figure 21A ). Minimal BSA release was observed at weeks 1, 2, 4, and 5, or after week 7 and microspheres were completely degraded by week 10 as evidenced by complete dissolution of the particles.
- Formulation G microspheres were also characterized by BSA release in three bursts ( Figure 21B ), but spread out over a longer timeframe. In addition, total microsphere degradation was observed after 14 weeks rather than 10 weeks for Formulation C. The first BSA burst from Formulation G was observed at the 1-day time-point, releasing 28.0 ⁇ 5.7% of total BSA. This was followed by a second burst of 12.9 ⁇ 2.9% at week 4, and an elongated burst at weeks 8 through 11 during which a cumulative 43.8 ⁇ 2.0% of BSA was released. Little-to-no BSA was observed in the release media at any other time points through complete degradation of the particles.
- Formulation E microspheres released 63.7 ⁇ 7.3% of its BSA at day 1 which was the largest initial burst in terms of both total quantity and percentage of any of the formulations ( Figure 21C ). The only time points with substantial BSA release following this initial burst were weeks 3 and 8 when 8.9 ⁇ 0.9% and 8.8 ⁇ 2.1%> of the total load was released respectively. Similar to Formulation G, which used PLGA at the same molecular weight (24-38 kDa), Formulation E degraded completely at 14 weeks.
- mice receiving Formulation G showed a significant increase in titer at weeks 1, 2, and 4 (p ⁇ 0.05, pO.001, and pO.O1 respectively), remained steady at week 6, and then fell significantly by week 8 (p ⁇ 0.05) before stabilizing again at week 10.
- Formulation E induced a similar response as antibody titers increased significantly at weeks 1, 2, and 4 (p ⁇ 0.001 for all), leveled off at week 6, and then decreased through the end of the study (p ⁇ 0.05).
- the immune response to all three microparticle formulations demonstrated a similar progression over time as titers rose over the first 4 weeks then slowly decreased through week 10 ( Figure 22 ). However, the magnitude of antibody titers appeared highly dependent on BSA loading.
- Formulation E released approximately 13 times more BSA than Formulations C at the earliest time point (1 day) due to a large initial burst and induced antibody titers that was 13-fold higher as well. This trend was also observed at the end of the study as the antibody titer induced by Formulation E was 8-fold higher than those associated with Formulation C after releasing 7 times as much BSA.
- Antibody titers from animals receiving any of the BSA-loaded microsphere formulations were significantly higher than those from animals receiving dose-matched bolus injections after 2 and 4 weeks (p ⁇ 0.05 and p ⁇ 0.01 respectively). However, after boosting with a second bolus injection at week 4, titers were statistically similar between formulations and their dose-matched bolus group at 6 and 8 weeks. Then at week 10, following administration of the third bolus, animals receiving bolus BSA showed another spike in titer resulting in significantly higher antibody titers compared to all dose-matched microsphere groups at that time point (pO.001).
- Antibody titers for Formulations C, G, and E peaked at 13.9 ⁇ 1.3, 13.7 ⁇ 2.2, and 16.1 ⁇ 2.1 on a log2 scale respectively 4 weeks after microsphere administration, whereas the small and large dose-matched boluses peaked after 10 weeks at 15.5 ⁇ 1.5 and 17.7 ⁇ 0.8 log2 titer respectively ( Figure 23 ).
- Example 7 PLGA micromolded particles for drug delivery
- the shell microfabrication may use mask fabrication (lithography), 0 fluoro-mold fabrication (UV core) and heat pressing of PLGA (120°C for 3 min).
- the step of drug filling may be carried out by the Biodot robot, filling the core with 1.5 nL volume of the drug.
- the sealing is achieved by placing a PLGA cap on the shell and then sealing the particles at 37°C for 5 min and acetone vapor for 5 min.
- FIGS 12A-12D and 24A-24C The schematic of the process is presented in Figures 12A-12D and 24A-24C. These microparticles may be manufactured as stacks, sealing each one with another at 37°C for 5 min.
- the micromolded particle shells have an x, y, and z dimensions of 450 x 450 x 300 ⁇ with a core of 100 x 100 x 100 ⁇ (cap dimensions are 450 x 450 x 150 ⁇ m), or 200 x 200 x 150 ⁇ ⁇ , with a core of 100 x 100 x 100 ⁇ . These dimensions allow the particles to be delivered with a 21 gauge needle (inner diameter 514 ⁇ m) or with a 23 gauge needle (inner diameter 337 ⁇ ).
- Example 8 Single PLA particles for depositing drug/sugar solution (comparative example)
- IPV in 0.5 M trehalose and 0.5 M sucrose solution was deposited onto PLA particles then dried in the stem cell culture hood at room temperature.
- PLA particles may be 3D-printed using a Makerbot printer.
- Single PLA particles may serve as a useful platform for drying onto and delivering drugs and antigens, as this method allows to deposit drug or antigen in a sugar solution onto a substrate such that the drug or antigen is stabilized within a sugar glass.
- Studies on the stability of IPV antigens on PLA particles are presented in Examples below.
- IPV microsphere formation presents challenges for IPV stability during the manufacturing process. These challenges with a summary of the findings that overcome the challenges are presented in Table 6. Table 6. Summary of obstacles to IPV stability, approaches taken to overcome the obstacles and findings. Obstacle Approach Findings IPV is not stable in organic solvents or at organic/aqueous interface Double emulsion method keeps IPV in an aqueous "bubble.” Minimal direct contact with organics - Double emulsionis effective - Vortexing and sonication parameters can be tuned to maximize IPV recovery Co-encapsulate IPV with BSA or mild surfactant to reduce IPV exposure to aqueous/organic interface during emulsification - Excipients improve recovery - Gelatin IPV may not survive the physical mixing processes that form the emulsion Use lower-energy mixing: homogenization, vortexing, low-amplitude sonication, etc.
- IPV is not stable after drying Change drying method (lyophilization, airdrying, vacuum) - Microspheres dried at room temp for 1 hr under vacuum show high IPV recovery Add excipients during drying - Sorbitol/MSG/MgCl 2 improves recovery during drying Co-encapsulate with excipients - Sorbitol/MSG/MgCl 2 can be co-encapsulated in w/o/o microspheres IPV is not stable in microsphere matrix in hydrated, 37C environment Co-encapsulate IPV with excipients to help maintain stability - Gelatin and sorbitol/MSG/MgCl 2 improve stability over time IPV is not stable in polymer degradation products Co-encapsulate IPV with basic excipients to buffer acidic byproducts - IPV is not stable ⁇ pH 6 or >pH 8 - Mg(OH) 2 buffers acidic byproducts Microspheres do not show bursts of release at
- Example 10 Co-encapsulation of IPV with gelatin or mild surfactant and sonication increase IPV stability during emulsification
- IPV was mixed with water and/or excipients at 100: 1 w/w ratio. Excipients used were gelatin, maltodextrin, puUulan, myristic acid, and Tween80. IPV with excipients was then added to DCM, or PLGA/DCM. The mixture was vortexed for 10 sec on highest setting, or was sonicated 10 sec at 25% amplitude. ELISA buffer (1% BSA, 1% Triton-X 100 in PBS) was added, the mixture was vortexed for 10 sec on high setting, and layers were separated by centrifugation. The aqueous layer was removed and tested by ELISA.
- Type I and III IPV generally the most sensitive to damage, showed best recovery with sonication at 20% amplitude for 30 sec ( Figure 26 ).
- Example 11 Drying of IPV and excipients with Genevac improves IPV recovery
- IPV was concentrated 26x and mixed with pullulan, pullulan/BSA, or sorbitol/MSG/MgC12 at 100:1, 350:1, or 700:1 mass ratio.
- IPV with excipient was dried by lyophilizatioh (overnight) or using Genevac (1 hr, 30-35°C). Recovery was tested by ELISA after resuspending the dried IPV with excipients.
- Example 12 Lyophilized IPV shows long-term stability at 37°C
- IPV was mixed with excipients 10% sorbitol, 8.5% MSG, and 8.5% MgCl 2 at 40,000:1, and lyophilized in plastic tube.
- the lyophilized IPV with excipients was stored in a plastic tube in a pouch with desiccant at humidified atmosphere and 37°C for up to 30 days. At various time points, the lyophilized IPV was resuspended and tested for recovery by ELISA.
- Types I, II and III IPV in lyophilized form with excipients showed long-term stability when stored at 37°C ( Figure 2 ).
- Example 13 Changes in pH of release medium by PLGA particles
- PLGA degrades by bulk erosion leading to acid buildup inside microspheres.
- finite volume of buffer may cause acid build up in tubes, which may lead to particle erosion and acid build up inside the microspheres.
- the buffer in local environment is constantly replenished with minimal acid build up outside of the particles. Because PLGA degrades by bulk erosion, acid build up inside the microspheres may be a problem. Therefore, several PLGA molecules were tested for changes in the pH of the release medium over time, when the buffer was changed every 2-3 days, or every 7 days.
- Example 14 Excipients to buffer the acidic products of PLGA microspheres
- Type I IPV was relatively stable at pH 7.4 at 37°C
- type II and type III IPV were stable at pH 6-8 at 37°C. Therefore, several excipients were tested for their buffering capacity to buffer the acidic products of PLGA microspheres.
- Buffering agents such as Mg(OH) 2 , Al(OH) 3 , and myristic acid, were incorporated into PLGA microspheres to minimize changes in pH of release medium. Also, Mg(OH) 2 has little to no solubility in water, it remains distributed throughout the polymer matrix and can potentially buffer the internal compartment. Al(OH) 3 is a known adjuvant and can increase immunogenicity
- Example 15 IPV microspheres incorporating stabilizing excipients
- PLGA microspheres incorporating IPV and various stabilizing additives were generated. These formulations are presented in Table 7 below, and were made with PLGA 502H. Other formulations with higher MW polymers, like PLGA 503H, are also contemplated. Table 7. IPV microsphere formulations incorporating stabilizing additives for polymer degradation-based burstsof drug/antigen release.
- S/M/M refers to 10% sorbitol, 8.5% MSG, 8.5% MgCl 2 mg polymer (MW) Approx DUIPV loaded (Type I Type II Type III) Additives to IPV, phase Additives to PLGA phase Outer stirring phase (2 nd emulsion) Mixing (1 st emulsion) Miking speed time Drying 20 (12k) 21662/187 4mg gelatin None Oil: mineral oil + 8 pan 80 Sonicator 20% amplitude, 30 sec 22 mg S/M/M 20 (12k) 21662/187 4 mg gelatin 10%myristic acid Aqueous: 1% PVA, 5%sucrose Sonicator 20% amplitude, 30 sec 22 mg S/M/M 20 (12k) 21662/187 4 mg gelatin 10% Al(OH) 3 Aqueous: 1% PVA, 5%sucrose Sonicator 20 % amplitude, 30 sec 22 mg S/M/M 20 (12k) 21662/187 865 mg S/M/M None Oil: mineral oil + Span 80
- Example 17 Other formulations of concentrated IPV mixed with excipients and dried in a PLA cube
Claims (16)
- Polymerformulierung, umfassend eine biokompatible polymere Hülle und einen Kern, der ein Agens enthält, das ein Antigen ist, wobei die Hülle, der Kern oder beide mehrschichtige Strukturen sind, die durch dreidimensionales Drucken oder Mikroformen hergestellt sind, und wobei das Antigen in zwei oder mehr Stößen freigesetzt wird und wobei die Formulierung zur Verabreichung an eine Person geeignet ist, wobei die Formulierung gegebenenfalls einen Stabilisator umfasst.
- Formulierung nach Anspruch 1, wobei die Formulierung durch dreidimensionales Drucken hergestellt wird.
- Formulierung nach Anspruch 1 oder Anspruch 2, ferner umfassend eine wirksame Menge eines Antigens zur Auslösung einer Immunantwort in vivo, das im Exzipienten, auf der Oberfläche der Formulierung oder gemischt mit der Formulierung zum Zeitpunkt der Verabreichung vorhanden ist.
- Formulierung nach einem der Ansprüche 1 bis 3, wobei die Formulierung ein Stabilisierungsmittel umfasst und wobei das Stabilisierungsmittel eine Verbindung umfasst, ausgewählt aus der Gruppe bestehend aus Zuckern, Ölen, Lipiden und Kohlenhydraten, vorzugsweise Zucker, am meisten bevorzugt umfassend einen Zucker, ausgewählt aus der Gruppe bestehend aus Saccharose, Trehalose und Kombinationen davon, gegebenenfalls in Form eines Zuckerglases.
- Formulierung nach Anspruch 1, wobei das Antigen eine Immunantwort auf ein infektiöses Agens oder einen Tumor hervorruft;
optionala) wobei das infektiöse Agens ein Virus, ein Bakterium, ein Pilz oder ein Protozoon ist; wobei das Virus gegebenenfalls ausgewählt ist aus der Gruppe bestehend aus Polio, Influenza, Hepatitis, Rotavirus, Masern, Mumps, Röteln und Varizellen; oder wobei das Bakterium ausgewählt ist aus der Gruppe bestehend aus Corynebacterium diphtheriae, Bordetella pertussis, Clostridium tetani, Streptococcus pneumoniae (Pneumokokken) und Neisseria meningitidis (Meningokokken); oderb) wobei das Antigen ein Tumorantigen ist, das selektiv eine T-Zell-Antwort auf einen Tumor hervorruft. - Formulierung nach einem der Ansprüche 1 bis 5, wobei das Polymer durch Hydrolyse biologisch abbaubar ist, vorzugsweise ein Polyester, am meisten bevorzugt ausgewählt aus der Gruppe bestehend aus Poly(milchsäure), Poly(glykolsäure) und Copolymeren davon.
- Formulierung nach einem der Ansprüche 1 bis 6, die eine Freigabe in Abständen von zehn bis neunzig Tagen vorsieht, vorzugsweise in Abständen von 30 bis 60 Tagen.
- Formulierung nach einem der Ansprüche 1 bis 7, die in Form von Mikropartikeln, Mikrokapseln oder Mikrokugeln vorliegt.
- Formulierung nach einem der Ansprüche 1 bis 8, wobei die Formulierung:injizierbar ist; oderimplantierbar ist; oderwobei die Formulierung auf eine Schleimhautoberfläche aufgetragen werden kann, die ausgewählt ist aus der Gruppe bestehend aus nasalen, pulmonalen, oralen, vaginalen und rektalen Oberflächen.
- Formulierung nach einem der Ansprüche 1 bis 9, wobei die Formulierung das Polymer Poly(milch-co-glykolsäure) (PLGA) umfasst, und wobei die Formulierung gegebenenfalls ferner ein Puffermittel umfasst, wobei das Puffermittel gegebenenfalls ausgewählt ist aus der Gruppe bestehend aus Magnesiumhydroxid, Aluminiumhydroxid und Myristinsäure.
- Formulierung nach einem der Ansprüche 1 bis 10, wobei die Formulierung eine polymere Hülle und ein darin eingekapseltes Antigen umfasst.
- Formulierung nach Anspruch 1, wobei die Formulierung durch Mikroformen gebildet wird und wobei das Agens in Mikropartikeln eingekapselt ist.
- Formulierung nach einem der Ansprüche 1 bis 12, wobei das Agens ein Antigen ist, das zum Zeitpunkt der Verabreichung im Exzipienten oder auf der Oberfläche der Formulierung zur sofortigen Freisetzung vorhanden ist und/oder in der Formulierung zur Freisetzung zehn bis 45 Tage nach der anfänglichen Freisetzung des Antigens eingearbeitet ist, wobei das Antigen gegebenenfalls zehn bis neunzig Tage nach der anfänglichen Freisetzung des Antigens freigesetzt wird.
- Formulierung nach einem der Ansprüche 1 bis 13 zur Verwendung in einem therapeutischen Verfahren zur Verabreichung des Mittels an eine Person, die es benötigt.
- Formulierung zur Verwendung nach Anspruch 14, wobei das Antigen in zwei oder mehr monatlichen Abständen freigesetzt wird und wobei das Antigen ein Polio- oder eine Mischung aus Diphtherie-, Keuchhusten- und Tetanus-Antigen(en) (DPT) ist.
- Formulierung zur Verwendung nach Anspruch 14, wobei das Antigen Polio ist und wobei das Antigen zum Zeitpunkt der Verabreichung, sowie zwei, vier und sechs Monate danach freigesetzt wird.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP20177222.5A EP3763383A1 (de) | 2013-12-16 | 2014-12-16 | Mikrogeformte oder 3d-gedruckte impfstoffformulierungen mit pulsierender freisetzung |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361916555P | 2013-12-16 | 2013-12-16 | |
| PCT/US2014/070664 WO2015095230A1 (en) | 2013-12-16 | 2014-12-16 | Micromolded or 3-d printed pulsatile release vaccine formulations |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP20177222.5A Division EP3763383A1 (de) | 2013-12-16 | 2014-12-16 | Mikrogeformte oder 3d-gedruckte impfstoffformulierungen mit pulsierender freisetzung |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3082852A1 EP3082852A1 (de) | 2016-10-26 |
| EP3082852B1 true EP3082852B1 (de) | 2020-06-17 |
Family
ID=52396804
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP20177222.5A Pending EP3763383A1 (de) | 2013-12-16 | 2014-12-16 | Mikrogeformte oder 3d-gedruckte impfstoffformulierungen mit pulsierender freisetzung |
| EP14830756.4A Active EP3082852B1 (de) | 2013-12-16 | 2014-12-16 | Mikrogeformte oder 3d-gedruckte impstoff-formulierungen mit rhythmischer freisetzung |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP20177222.5A Pending EP3763383A1 (de) | 2013-12-16 | 2014-12-16 | Mikrogeformte oder 3d-gedruckte impfstoffformulierungen mit pulsierender freisetzung |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US10300136B2 (de) |
| EP (2) | EP3763383A1 (de) |
| JP (4) | JP2017507165A (de) |
| AU (1) | AU2014364930B2 (de) |
| CA (1) | CA2933867A1 (de) |
| WO (1) | WO2015095230A1 (de) |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017507165A (ja) * | 2013-12-16 | 2017-03-16 | マサチューセッツ インスティテュート オブ テクノロジー | マイクロモールド成形された、または3次元印刷されたパルス放出ワクチン製剤 |
| AU2016272089B2 (en) | 2015-06-03 | 2021-02-18 | Triastek, Inc. | Dosage forms and use thereof |
| WO2018053300A1 (en) * | 2016-09-16 | 2018-03-22 | Auburn University | Biodegradable polymeric particles encapsulating an active agent, pharmaceutical compositions and uses thereof |
| US10150282B2 (en) | 2016-10-14 | 2018-12-11 | Xerox Corporation | System and method for additive manufacture of chemical delivery devices using halftone screening |
| US10933579B2 (en) | 2017-03-10 | 2021-03-02 | Prellis Biologics, Inc. | Methods and systems for printing biological material |
| US11085018B2 (en) | 2017-03-10 | 2021-08-10 | Prellis Biologics, Inc. | Three-dimensional printed organs, devices, and matrices |
| US11666239B2 (en) | 2017-03-14 | 2023-06-06 | University Of Connecticut | Biodegradable pressure sensor |
| JP2020524483A (ja) | 2017-05-25 | 2020-08-20 | プレリス バイオロジクス,インク. | 三次元印刷された器官、デバイス、およびマトリックス |
| JP7358340B2 (ja) | 2017-05-26 | 2023-10-10 | インフィニット・マテリアル・ソリューションズ,エルエルシー | 水溶性ポリマー組成物 |
| US20190076631A1 (en) * | 2017-09-13 | 2019-03-14 | Massachusetts Institute Of Technology | Microdevices with complex geometries |
| CN116270513A (zh) | 2018-01-09 | 2023-06-23 | 南京三迭纪医药科技有限公司 | 一种包含固定剂量adhd非兴奋剂和adhd兴奋剂的复方口服药物剂型 |
| WO2019173402A1 (en) | 2018-03-05 | 2019-09-12 | University Of Connecticut | Core-shell microneedle platform for transdermal and pulsatile drug/vaccine delivery and method of manufacturing the same |
| US20210259960A1 (en) * | 2018-06-29 | 2021-08-26 | The Doshisha | Formulation containing emricasan |
| EP3831879B1 (de) * | 2018-07-30 | 2023-07-12 | Mitsubishi Chemical Corporation | Material für generative fertigung vom schmelzschichtungsmodellierungstyp |
| GB2592486B (en) * | 2018-07-31 | 2022-09-14 | Prellis Biologics Inc | Optically-induced auto-encapsulation |
| US11678989B2 (en) | 2019-03-01 | 2023-06-20 | University Of Connecticut | Biodegradable piezoelectric nanofiber scaffold for bone or tissue regeneration |
| US11826495B2 (en) | 2019-03-01 | 2023-11-28 | University Of Connecticut | Biodegradable piezoelectric ultrasonic transducer system |
| CN110420324B (zh) * | 2019-08-01 | 2022-07-12 | 临沂大学 | 含兔葡萄球菌抗原的免疫原性组合物及其制备方法和应用 |
| WO2021183626A1 (en) | 2020-03-10 | 2021-09-16 | University Of Connecticut | Therapeutic bandage |
| CN112370531B (zh) * | 2020-11-18 | 2022-09-23 | 金宇保灵生物药品有限公司 | 一种犬瘟热、犬细小、犬腺病毒、犬副流感四联活疫苗耐热保护剂及其制备方法和应用 |
| WO2022192361A2 (en) * | 2021-03-09 | 2022-09-15 | Board Of Regents Of The University Of Nebraska | Microparticle compositions and methods use thereof |
| CA3223912A1 (en) * | 2021-06-21 | 2022-12-29 | Kevin Mchugh | High-throughput preparation of microparticles with pulsatile release |
| CN113521263A (zh) * | 2021-08-03 | 2021-10-22 | 苏州大学 | 一种3d打印的肿瘤疫苗组合物及其制备方法与应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040241236A1 (en) * | 2001-09-28 | 2004-12-02 | Shun-Por Li | Modified release dosage forms |
| US7122143B2 (en) * | 2001-09-28 | 2006-10-17 | Mcneil-Ppc, Inc. | Methods for manufacturing dosage forms |
| US20080026040A1 (en) * | 2006-07-31 | 2008-01-31 | Isaac Farr | Active agent-releasing dosage forms |
| CN103251941A (zh) * | 2012-02-16 | 2013-08-21 | 海南大学 | 动物病毒性疫苗脉冲释放系统、其制备方法及用途 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5490962A (en) | 1993-10-18 | 1996-02-13 | Massachusetts Institute Of Technology | Preparation of medical devices by solid free-form fabrication methods |
| WO1995011010A1 (en) | 1993-10-22 | 1995-04-27 | Genentech, Inc. | Methods and compositions for microencapsulation of antigens for use as vaccines |
| AU8015300A (en) | 1999-10-12 | 2001-04-23 | Ohio State University, The | Reactive polymeric valve, dispensing devices and methods using same |
| US20040033241A1 (en) * | 2000-06-02 | 2004-02-19 | Allergan, Inc. | Controlled release botulinum toxin system |
| ES2310201T3 (es) | 2001-02-13 | 2009-01-01 | Government Of The United States Of America, As Represented By The Secretary Of The Army | Vacuna para inmunizacion transcutanea contra la diarrea de los viajeros. |
| US7285289B2 (en) | 2002-04-12 | 2007-10-23 | Nagy Jon O | Nanoparticle vaccines |
| AU2003232078A1 (en) | 2002-05-06 | 2003-11-17 | Massachusetts Institute Of Technology | Diffusion-controlled dosage form and method of fabrication including three dimensional printing |
| CA2551644C (en) | 2003-12-24 | 2014-03-04 | Cerus Corporation | Recombinant nucleic acid molecules encoding fusion proteins comprising antigens and bacterial secretory signal polypeptides, expression cassettes, and bacteria, and methods of usethereof |
| JP5532280B2 (ja) * | 2003-12-24 | 2014-06-25 | アドゥロ バイオテック | 抗原および細菌分泌シグナルポリペプチドを含む融合タンパク質をコードする組換え核酸分子、発現カセット、および細菌、ならびにこれらを使用する方法 |
| WO2007001448A2 (en) | 2004-11-04 | 2007-01-04 | Massachusetts Institute Of Technology | Coated controlled release polymer particles as efficient oral delivery vehicles for biopharmaceuticals |
| US9180102B2 (en) | 2005-05-06 | 2015-11-10 | Board Of Regents, The University Of Texas System | Methods for fabricating nano and microparticles for drug delivery |
| CA2613474A1 (en) * | 2005-06-20 | 2007-03-08 | Elan Pharma International Limited | Nanoparticulate and controlled release compositions comprising aryl-heterocyclic compounds |
| ES2627233T3 (es) | 2007-10-12 | 2017-07-27 | Massachusetts Institute Of Technology | Nanotecnología de vacunas |
| CN102014873A (zh) | 2008-02-25 | 2011-04-13 | 诺瓦瓦克斯股份有限公司 | 糖玻璃化的病毒样颗粒 |
| EA201390810A1 (ru) * | 2010-12-02 | 2013-09-30 | Онколитикс Байотек Инк. | Жидкие вирусные составы |
| CA2875146C (en) | 2012-05-30 | 2022-03-22 | The Regents Of The University Of California | Bioactive agent delivery devices and methods of making and using the same |
| JP2017507165A (ja) * | 2013-12-16 | 2017-03-16 | マサチューセッツ インスティテュート オブ テクノロジー | マイクロモールド成形された、または3次元印刷されたパルス放出ワクチン製剤 |
-
2014
- 2014-12-16 JP JP2016558550A patent/JP2017507165A/ja not_active Withdrawn
- 2014-12-16 WO PCT/US2014/070664 patent/WO2015095230A1/en active Application Filing
- 2014-12-16 EP EP20177222.5A patent/EP3763383A1/de active Pending
- 2014-12-16 CA CA2933867A patent/CA2933867A1/en active Pending
- 2014-12-16 EP EP14830756.4A patent/EP3082852B1/de active Active
- 2014-12-16 US US14/572,631 patent/US10300136B2/en active Active
- 2014-12-16 AU AU2014364930A patent/AU2014364930B2/en active Active
-
2019
- 2019-05-02 US US16/401,476 patent/US10960073B2/en active Active
- 2019-11-18 JP JP2019208044A patent/JP2020055818A/ja active Pending
-
2021
- 2021-01-07 US US17/143,871 patent/US20210205444A1/en active Pending
- 2021-05-21 JP JP2021085890A patent/JP2021120413A/ja active Pending
-
2023
- 2023-03-17 JP JP2023042995A patent/JP2023075311A/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040241236A1 (en) * | 2001-09-28 | 2004-12-02 | Shun-Por Li | Modified release dosage forms |
| US7122143B2 (en) * | 2001-09-28 | 2006-10-17 | Mcneil-Ppc, Inc. | Methods for manufacturing dosage forms |
| US20080026040A1 (en) * | 2006-07-31 | 2008-01-31 | Isaac Farr | Active agent-releasing dosage forms |
| CN103251941A (zh) * | 2012-02-16 | 2013-08-21 | 海南大学 | 动物病毒性疫苗脉冲释放系统、其制备方法及用途 |
Non-Patent Citations (6)
| Title |
|---|
| AUDRAN REGINE ET AL: "Encapsulation of peptides in biodegradable microspheres prolongs their MHC class-I presentation by dendritic cells and macrophages in vitro.", VACCINE, vol. 21, no. 11-12, 7 March 2003 (2003-03-07), pages 1250 - 1255, ISSN: 0264-410X * |
| GALLOWAY ASHLEY L ET AL: "Development of a nanoparticle-based influenza vaccine using the PRINT technology.", NANOMEDICINE : NANOTECHNOLOGY, BIOLOGY, AND MEDICINE MAY 2013, vol. 9, no. 4, May 2013 (2013-05-01), pages 523 - 531, ISSN: 1549-9642 * |
| JOHANSEN PAL ET AL: "Improving stability and release kinetics of microencapsulated tetanus toxoid by co-encapsulation of additives", PHARMACEUTICAL RESEARCH (NEW YORK), vol. 15, no. 7, July 1998 (1998-07-01), pages 1103 - 1110, ISSN: 0724-8741 * |
| KEVIN MCHUGH ET AL.: "Novel pulsatile-release microparticles for single-injection vaccination", ECI SYMPOSIUM SERIES. VACCINE TECHNOLOGY, June 2016 (2016-06-01), Retrieved from the Internet <URL:http://dc.engconfintl.org/vaccine_vi/68/> * |
| MURILLO M ET AL: "Influence of the co-encapsulation of different excipients on the properties of polyester microparticle-based vaccine against brucellosis.", INTERNATIONAL JOURNAL OF PHARMACEUTICS (KIDLINGTON), vol. 271, no. 1-2, 1 March 2004 (2004-03-01), pages 125 - 135, ISSN: 0378-5173 * |
| TZENG STEPHANY Y ET AL: "Thermostabilization of inactivated polio vaccine in PLGA-based microspheres for pulsatile release.", JOURNAL OF CONTROLLED RELEASE : OFFICIAL JOURNAL OF THE CONTROLLED RELEASE SOCIETY 10 JUL 2016, vol. 233, 10 July 2016 (2016-07-10), pages 101 - 113, ISSN: 1873-4995 * |
Also Published As
| Publication number | Publication date |
|---|---|
| US20190328871A1 (en) | 2019-10-31 |
| JP2021120413A (ja) | 2021-08-19 |
| US20210205444A1 (en) | 2021-07-08 |
| CA2933867A1 (en) | 2015-06-25 |
| JP2023075311A (ja) | 2023-05-30 |
| JP2020055818A (ja) | 2020-04-09 |
| US10960073B2 (en) | 2021-03-30 |
| AU2014364930A1 (en) | 2016-07-14 |
| JP2017507165A (ja) | 2017-03-16 |
| US10300136B2 (en) | 2019-05-28 |
| US20150165020A1 (en) | 2015-06-18 |
| EP3763383A1 (de) | 2021-01-13 |
| EP3082852A1 (de) | 2016-10-26 |
| AU2014364930B2 (en) | 2017-06-15 |
| WO2015095230A1 (en) | 2015-06-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10960073B2 (en) | Micromolded or 3-D printed pulsatile release vaccine formulations | |
| JP6235063B2 (ja) | ポリマーマトリックスおよび油相を含んで成る免疫調節組成物 | |
| Sosnik et al. | Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers | |
| Irache et al. | Nanomedicine: novel approaches in human and veterinary therapeutics | |
| Lamprecht | Nanotherapeutics: drug delivery concepts in nanoscience | |
| US20210290921A1 (en) | Microdevices with complex geometries | |
| JP6835733B2 (ja) | 医薬組成物、その調製法及び使用 | |
| Mao et al. | Recent advances in polymeric microspheres for parenteral drug delivery—part 2 | |
| Diesner et al. | Use of lectin-functionalized particles for oral immunotherapy | |
| D’Mello et al. | Polymeric nanoparticles for small-molecule drugs: biodegradation of polymers and fabrication of nanoparticles | |
| Johansen et al. | Diphtheria and tetanus toxoid microencapsulation into conventional and end-group alkylated PLA/PLGAs | |
| Chavda et al. | Advanced particulate carrier-mediated technologies for nasal drug delivery | |
| Kersten et al. | 3.2 Biodegradable Microspheres as Vehicles for Antigens | |
| JP2002538195A (ja) | 生物活性化合物の持続放出用の無針注射器を使用する微粒子製剤の送達 | |
| Wang et al. | Recent advances in micro-and nano-scale carrier systems for controlled delivery of vaccines | |
| Takada | Microfabrication derived DDS: From batch to individual production | |
| Sailaja et al. | Review on microspheres as a drug delivery carrier | |
| Nechaeva | Development of oral microencapsulated forms for delivering viral vaccines | |
| Xia et al. | Uniform biodegradable microparticle systems for controlled release | |
| Schiller | Scalable processes to manufacture nanoparticulate dosage forms for oral vaccination | |
| Lin et al. | The preparation and characteristic of poly (lactide co–glycolide) microspheres as novel antigen delivery systems | |
| Sinha | Use of biodegradable micro and nano-particles in vaccine delivery | |
| as Promising | Biolological Medicinal Chemistry | |
| Johansen et al. | End-Group Alkylated PLA/PLGAs | |
| Jang | Development of a lower intestine targeting mucoadhesive platform of oral drug delivery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20160708 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: GATES, WILLIAM Inventor name: NIKOLIC, BORIS Inventor name: JAKLENEC, ANA Inventor name: ECKHOFF, PHILIP, A. Inventor name: WOOD, LOWELL, L. Inventor name: LANGER, ROBERT, S. Inventor name: MCHUGH, KEVIN Inventor name: NGUYEN, THANH DUC Inventor name: TZENG, STEPHANY YI Inventor name: NORMAN, JAMES J |
|
| 17Q | First examination report despatched |
Effective date: 20170713 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1230497 Country of ref document: HK |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: NORMAN, JAMES J Inventor name: LANGER, ROBERT, S. Inventor name: WELKHOFF, PHILIP, A. Inventor name: NGUYEN, THANH DUC Inventor name: MCHUGH, KEVIN Inventor name: GATES, WILLIAM Inventor name: TZENG, STEPHANY YI Inventor name: NIKOLIC, BORIS Inventor name: WOOD, LOWELL, L. Inventor name: JAKLENEC, ANA |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20200114 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602014066798 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1280526 Country of ref document: AT Kind code of ref document: T Effective date: 20200715 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: ISLER AND PEDRAZZINI AG, CH |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200918 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200917 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20200617 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200917 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1280526 Country of ref document: AT Kind code of ref document: T Effective date: 20200617 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201019 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201017 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602014066798 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| 26N | No opposition filed |
Effective date: 20210318 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201216 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200617 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20221227 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20230109 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20221228 Year of fee payment: 9 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230528 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: WD Ref document number: 1230497 Country of ref document: HK |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20231227 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20231227 Year of fee payment: 10 Ref country code: IE Payment date: 20231227 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20231227 Year of fee payment: 10 |