EP3013213B1 - Dispositif de mesure des propriétés de la peau et dispositif de traitement non invasif - Google Patents
Dispositif de mesure des propriétés de la peau et dispositif de traitement non invasif Download PDFInfo
- Publication number
- EP3013213B1 EP3013213B1 EP14732228.3A EP14732228A EP3013213B1 EP 3013213 B1 EP3013213 B1 EP 3013213B1 EP 14732228 A EP14732228 A EP 14732228A EP 3013213 B1 EP3013213 B1 EP 3013213B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- probe
- axis
- treatment
- skin
- optical
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Not-in-force
Links
- 238000011282 treatment Methods 0.000 title claims description 213
- 238000005259 measurement Methods 0.000 title claims description 74
- 239000000523 sample Substances 0.000 claims description 202
- 230000003287 optical effect Effects 0.000 claims description 172
- 238000003384 imaging method Methods 0.000 claims description 69
- 230000005855 radiation Effects 0.000 claims description 65
- 238000000034 method Methods 0.000 claims description 18
- 238000007493 shaping process Methods 0.000 claims description 14
- 238000001514 detection method Methods 0.000 claims description 11
- 238000012545 processing Methods 0.000 claims description 7
- 238000000691 measurement method Methods 0.000 claims description 4
- 239000011159 matrix material Substances 0.000 claims description 2
- 210000003491 skin Anatomy 0.000 description 132
- 102000008186 Collagen Human genes 0.000 description 11
- 108010035532 Collagen Proteins 0.000 description 11
- 229920001436 collagen Polymers 0.000 description 11
- 210000001519 tissue Anatomy 0.000 description 11
- 210000004207 dermis Anatomy 0.000 description 8
- 230000004075 alteration Effects 0.000 description 5
- 238000004925 denaturation Methods 0.000 description 5
- 230000036425 denaturation Effects 0.000 description 5
- 230000002500 effect on skin Effects 0.000 description 5
- 210000002615 epidermis Anatomy 0.000 description 5
- 238000005286 illumination Methods 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 4
- 230000005670 electromagnetic radiation Effects 0.000 description 4
- 230000005540 biological transmission Effects 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 210000002752 melanocyte Anatomy 0.000 description 3
- 210000000434 stratum corneum Anatomy 0.000 description 3
- 238000012800 visualization Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 230000037303 wrinkles Effects 0.000 description 3
- 208000002874 Acne Vulgaris Diseases 0.000 description 2
- 238000012935 Averaging Methods 0.000 description 2
- 102000012422 Collagen Type I Human genes 0.000 description 2
- 108010022452 Collagen Type I Proteins 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 206010000496 acne Diseases 0.000 description 2
- 238000005345 coagulation Methods 0.000 description 2
- 230000015271 coagulation Effects 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 230000005284 excitation Effects 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 238000013532 laser treatment Methods 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 230000037368 penetrate the skin Effects 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 230000003595 spectral effect Effects 0.000 description 2
- 208000035484 Cellulite Diseases 0.000 description 1
- 102000016942 Elastin Human genes 0.000 description 1
- 108010014258 Elastin Proteins 0.000 description 1
- 102000011782 Keratins Human genes 0.000 description 1
- 108010076876 Keratins Proteins 0.000 description 1
- 206010049752 Peau d'orange Diseases 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- 208000009621 actinic keratosis Diseases 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 210000000270 basal cell Anatomy 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000036232 cellulite Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 229940096422 collagen type i Drugs 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 229920002549 elastin Polymers 0.000 description 1
- 210000001339 epidermal cell Anatomy 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000000649 photocoagulation Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000003716 rejuvenation Effects 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000003685 thermal hair damage Effects 0.000 description 1
- 238000001149 thermolysis Methods 0.000 description 1
- 231100000216 vascular lesion Toxicity 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/18—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves
- A61B18/20—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser
- A61B18/203—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by applying electromagnetic radiation, e.g. microwaves using laser applying laser energy to the outside of the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/0059—Measuring for diagnostic purposes; Identification of persons using light, e.g. diagnosis by transillumination, diascopy, fluorescence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/44—Detecting, measuring or recording for evaluating the integumentary system, e.g. skin, hair or nails
- A61B5/441—Skin evaluation, e.g. for skin disorder diagnosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N5/0613—Apparatus adapted for a specific treatment
- A61N5/0616—Skin treatment other than tanning
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00452—Skin
- A61B2018/00458—Deeper parts of the skin, e.g. treatment of vascular disorders or port wine stains
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00452—Skin
- A61B2018/0047—Upper parts of the skin, e.g. skin peeling or treatment of wrinkles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00636—Sensing and controlling the application of energy
- A61B2018/00773—Sensed parameters
- A61B2018/00779—Power or energy
- A61B2018/00785—Reflected power
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N2005/0626—Monitoring, verifying, controlling systems and methods
- A61N2005/0627—Dose monitoring systems and methods
- A61N2005/0628—Dose monitoring systems and methods including a radiation sensor
Definitions
- the invention relates generally to the measurement of skin properties, in particular properties relevant for the treatment of skin using electromagnetic treatment radiation, such as laser light. It relates even more particularly to a non-invasive device for skin treatment in which these measurements are made and used to modify or control the skin treatment.
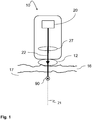
- FIG. 1 schematically shows a skin treatment device 10 known in the art, comprising a radiation source 20, and beam shaping and directing components 27.
- the radiation source 20 provides an incident radiation beam 22 suitable for treating human or animal skin.
- the radiation used may be any type of electromagnetic or thermal radiation which provides a beneficial effect in the skin.
- the skin treatment device 10 may comprise a pulsed laser light source 20 such as a Nd:YAG laser with emission at 1064 nm and 1-1000 ps pulse duration.
- the beam shaping and directing components 27 receive the radiation beam 22 from the radiation source 20, and create a radiation beam 22 with the desired properties which exits the device 10 along a treatment axis 21.
- these beam shaping and directing components 27 may be optical elements, such as mirrors, lenses, beam splitters, prisms etc, for directing the laser light beam 22 to exit the device along a treatment axis 21, and for focusing the light beam 22 inside the skin at a treatment location 90 on the treatment axis 21.
- these beam shaping and directing components 27 may be waveguides, apertures, reflectors etc. for directing the radio-frequency beam 22 to exit the device along a treatment axis 21.
- the skin comprises multiple layers with different radiation transmission and absorption properties.
- the epidermis 16 is composed of the outermost layers and forms a waterproof protective barrier.
- the outermost layer of the epidermis is the stratum corneum which, due to its microscopic fluctuations in roughness, impedes the coupling of radiation, in particular light, between the device 10 and the skin.
- a radiation coupler 12 is used between the device 10 where the radiation beam exits and the skin surface where the radiation enters into the skin. This optimizes the penetration of the treatment radiation beam 22 into the skin.
- an optical coupler 12 may be used which comprises lenses, mirrors, prisms, an index-matching fluid or a combination thereof.
- the dermis 17 is situated which is the region at which many of the skin treatments are directed.
- the treatment location 90 is in the collagen of the dermis 17 in order to create microscopic lesions at the treatment location, which results in new collagen formation.
- the laser light treatment devices 10 use the fact that the skin transmits electromagnetic radiation that is to be focused to a very small focal spot in the dermis 17.
- the wavelength of the laser light is between 800 and 1100 nm. In this range, transmission is high and scattering and linear absorption are low.
- phenomena exploited using a skin treatment such as photothermolysis or laser induced optical breakdown (LIOB), may be achieved easily, accurately (i.e. very locally) and efficiently. It is however not excluded to use other wavelengths.
- LIOB laser induced optical breakdown
- US 2005/0154382 A1 discloses a handheld dermatological device for visualizing a skin treatment region prior to, during, or after therapeutic treatment with therapeutic energy.
- This known device comprises an illumination source for illuminating a treatment area of the skin and an image capture device.
- the device further comprises an optical system to direct radiation, emanating from the treatment area in response to the illumination by the illumination source, onto the image capture device, which can form an image of the treatment area.
- the device is used for visualization of superficial targets, for the visualization of targets at depths up to about 1 millimeter below the skin, or for visualization of deeper targets up to about 3 millimeters beneath the skin surface.
- the optical axis of the illumination source and the common optical axis of the image capture device and the optical system mutually enclose an angle. During use, these optical axes have an oblique orientation relative to the skin surface.
- An object of the invention is to provide a non-invasive measurement device and a method of measuring skin properties which are relevant for skin treatments.
- the object is achieved according to the invention by means of a non-invasive measurement device for the measurement of skin properties using laser light, the device comprising a probe module and an imaging module, wherein:
- the object of the invention is also achieved by providing a method for non-invasive measurement of skin properties using a device generating laser light, the device comprising a probe module and an imaging module, the method comprising:
- the invention is based on the insight that skin measurement devices known in the art are inherently limited because they only measure specific parameters of the skin at the treatment location during the application of the treatment radiation.
- the invention provides a probe light beam that enters the skin along the probe axis.
- the probe light beam is a radiation beam separate from the treatment radiation beam (which may be used before, during or after the measurement), so that the characteristics of the probe light beam maybe predetermined before the measurement.
- the probe module may therefore be optimized for the measurement.
- a second insight is that, although the properties of the treatment location are important, the treatment radiation beam passes through the skin between the outer layer of the skin and the treatment location and the energy directed to the treatment location will spread to surrounding tissue. Consequently, the invention provides a more reliable skin measurement system, because it measures a plurality of positions along the probe axis, within a probe region. As the treatment location is (or will be after the measurement) comprised within this probe region, the invention measures the skin properties at the treatment location and the surrounding points along the probe axis.
- Known measurement devices such as described in US 2005/0154382 , US 2007/0252997 , and US 2010/0130969 do not image a plurality of points along the probe axis.
- a third insight is that a more reliable measurement is provided when the plurality of positions are imaged by an imaging module.
- Many measurement devices known in the art such as the device described in US 2008/0215038 , simply make an image of the top surface of the skin and try to interpret the skin properties from this image.
- the invention comprises an imaging module and a probe module, the angle between the probe axis and the imaging optical axis is predetermined, and the angle between the probe axis and the outer layer of the skin is also predetermined.
- the second optical system is configured and arranged such that the plurality of points lie in an object plane of the second optical system, and such that the optical detector array is disposed in the image plane of the second optical system. This means that a plurality of points are imaged at the same time using, respectively, a plurality of light detection elements comprised in the detector array.
- the measurement device provides an optical depth profile of the section of probe axis comprising the plurality of points - these are the positions in the skin that are relevant for the treatment - by measuring either during treatment, or before treatment, or after treatment.
- the skin parameters measured may then be used to set or modify the treatment parameters, or to indicate that no further treatment is required.
- this measurement device and method are fast, accurate and can provide appropriate skin physiological information which is relevant for the optimisation of the radiation treatment.
- the detector axis is comprised in a plane which comprises the probe axis and the imaging optical axis. This may result in fewer aberrations in the image on the optical detector array, reducing the need to correct the image to compensate for the detector axis not being in the same plane.
- the measurement device may also be advantageous to configure and arrange the measurement device such that the angle enclosed by the probe axis and the imaging optical axis is in a range of 20 to 90 degrees.
- the use of an imaging module means that there is considerable flexibility in the position of each component within the measurement device, allowing the device dimensions to be minimized or to be defined to make operation simpler. This is particularly advantageous when the measurement device is used by a consumer.
- the measurement device may also be advantageous to configure and arrange the measurement device such that, during operation of the measurement device, an angle enclosed by the probe axis and the skin outer surface is in a range of 45 to 90 degrees.
- the probe light beam must penetrate the skin to the desired positions of the treatment location, so that an angle greater than 45 degrees is preferred.
- the image of the plurality of probe positions may be affected by the distance and tissue type between each probe position and the outer surface of the skin. Therefore, it is preferable to dispose the imaging optical axis such that the distance between the outer surface of the skin and each of the probe positions is as similar as possible.
- the measurement device may be even more advantageous to configure and arrange the measurement device such that the detector axis and the probe axis are parallel to each other. This may be achieved by predefining the lens planes of the second optical system so as to be parallel to the probe axis. Although the optical detector array may be tilted from this parallel position to compensate for any deviation from parallel of the lens planes, the parallel detector axis configuration is expected to provide the simplest and fastest measurement system.
- the skin properties measured by the device may be immediately used to improve or modify the measurement. For example, if the average intensity is too low, the operating parameters of the probe laser light source maybe adapted to provide more energy.
- the object of the invention is also achieved by providing a non-invasive treatment device for treatment of skin using electromagnetic treatment radiation, the device comprising a measurement device according to the invention, and the treatment device further comprising a treatment module, wherein:
- the measurement method according to the invention may be used in a method of non-invasive skin treatment using electromagnetic treatment radiation, the method of treatment further comprising:
- a treatment device By including the treatment module into the measurement device, a treatment device is provided.
- the angle between the probe axis and the treatment axis may be predetermined, thus enabling the treatment location to be at a desired position within the probe region. This increases the reproducibility of the measurement, because the differences in position between the probe region and the treatment location are limited.
- the skin properties measured by the device can be immediately used to improve or modify the treatment. For example, if the skin properties indicate that the presence of collagen is not detected, the position of the treatment location may be changed.
- the treatment radiation is laser light
- Figure 2 illustrates a first embodiment 30 of the invention. It depicts a non-invasive measurement device 30 for the measurement of skin properties using laser light.
- the measurement device comprises a probe module 70 and an imaging module 50 with a fixed relative disposition.
- the probe module comprises a laser light source 80 and a first optical system 87 to receive the laser light from the source 80, and to direct the laser light beam 82 to an aperture in the measurement device 30.
- the probe module 70 is configured and arranged such that, in use, the probe light beam 82 exits the device 30 along a probe axis 71 and impinges on an outer surface of the skin to be treated.
- the first optical system 87 is configured and arranged to direct, in use, the probe light beam 82 to a probe region 95 inside the skin.
- the probe laser light source 80 is selected to provide laser light which penetrates the skin to a sufficient depth, with the appropriate properties depending on the anticipated physiological changes expected in the probe region 95 of the skin during radiation treatment, and the method is selected to measure a related skin property.
- the device 30 may also comprise an optical coupler 12 to optimize the energy delivery to the treatment location 90.
- Any suitable optical coupler 12, such as those known in the art, may be used.
- the imaging module 50 comprises a second optical system 67 and an optical detector array 60, the optical detector array 60 being disposed along a detector axis 61 comprised in the image plane of the second optical system 67.
- the second optical system 67 is configured and arranged such that, in use, an image on a plurality of optical detectors comprised in the optical detector array 60 of, respectively, a plurality of probe positions distributed along the probe axis 71 within the probe region 95 is obtained.
- the imaging optical axis 51 of the optical system 67 intersects with the probe axis 71 within the probe region 95 due to the fixed relative disposition of the probe 70 and imaging module 50 within the device 30.
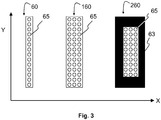
- the optical detector array 60, 160, 260 may be a linear array of individual light sensors 65, as depicted in Figure 3 - in other words, a plurality of light sensors disposed at equal distances from each other along a longitudinal axis.
- the light collected by the optical detector array 60, 160, 260 is converted into an electrical signal via a circuit that may be built into the array substrate.
- Each of the plurality of probe positions is imaged onto the detector array onto one or more of the optical detectors.
- the detector array 60 needs to be rectangular - that is, the longitudinal axis of the array 60 is disposed along the detector axis 61.
- the detector during use, comprises a matrix of Y light detection elements 65 in the direction extending along the detector axis 61 times X light detection elements 65 in the direction extending along a further axis perpendicular to the detector axis 61, then Y will be larger than X.
- Y is the direction of the longitudinal axis of the detector array 60.
- X is 1 and Y is 14. This makes it suitable for measuring 14 probe positions along the probe axis 71.
- This may be, for example, a linear CCD detector.
- X is 3 and Y is 14.
- Y is 14.
- This also makes it suitable for measuring 14 probe positions along the probe axis 71.
- light sensors 65 in Y direction may also be combined in some ways, for example, to measure 7 probe positions along the probe axis 71. If multiple light sensors 65 in Y direction are used, substantially increased spatial information on the treatment profile can be obtained, e.g. lesion width, and greater flexibility in laser treatment efficacy improvement is achieved.
- a linear array is formed using a large number of light sensors 65 extending in both X and Y directions, such as a CCD array, but light sensors that are not required are covered with a mask 63.
- the electrical signals from light sensors 65 that are not required may simply be ignored when the signals are processed, or these signals may not be used.
- a Y to X ratio of light detection elements is preferably greater than or equal to 5 to 1.
- detectors are known in the art, such as in published US patent 6,413,257 .
- This patent discloses the use of a plurality of detectors to monitor the energy characteristics of skin tissue during treatment.
- Two to four infrared detectors are disposed such that they measure the radial dependence of the diffuse radiation emitted from the treatment location.
- the treatment radiation enters the skin at 90 degrees, and the detectors are disposed at different distances from the entry point into the skin.
- the detectors disclosed are simply infrared intensity detectors - no image is formed of the treatment location.
- the imaging module 50 may then be further configured to detect the desired optical signal from the skin, by additionally providing it with optical components such as wavelength filters and/or by appropriate processing 40 of the signals from the optical detector array 60.
- optical components such as wavelength filters and/or by appropriate processing 40 of the signals from the optical detector array 60.
- the configuration required depends upon the type of treatment that the measured skin properties are to be used for, and the position of the treatment location 90 in the skin.
- the optical signal is a Second-Harmonic Generation (SHG) signal, which may indicate the presence of collagen.
- the probe laser light source 80 may then be pulsed (femtosecond to nanosecond range) at a wavelength in the range of 700 to 2200nm.
- the optical signal measured will depend on the related skin properties, namely the collagen denaturation state, the dermal 17 depth and the epidermal 16 thickness.
- the optical signal is 1-photon and 2-photon excited autofluorescence, which may indicate the presence of stratum corneum (namely keratin and lipids), epidermal cells, NAD(P)H, collagen, elastin, hair.
- the probe laser light source 80 for 1-photon excitation may be a continuous wave laser light source operating at a wavelength in the range of 300 to 500 nm, or for 2-photon excitation it may be pulsed (femtosecond to nanosecond range) operating at a wavelength in the range of 600 to 1000 nm.
- the optical signal measured will depend on the related skin properties, namely strateum corneum thickness, epidermal 16 thickness, dermal 17 depth, hair thickness, hair depth, melanin concentration, basal layer depth and melanocyte depth.
- the optical signal is Rayleigh-scattering, which may indicate the presence of scattering centers.
- the probe laser light source 80 may then be a continuous wave or pulsed laser light source operating at a wavelength in the range of 350 to 1100nm.
- the optical signal measured will depend on the related skin properties, namely melanocyte depth, basal layer depth, epidermal thickness 16, and tissue coagulation state.
- the optical signal is Raman-scattering, which may indicate the presence of lipids, water or collagen.
- the probe laser light source 80 may then be a continuous wave or pulsed nanosecond range laser light source operating at a wavelength in the range of 350 to 1100nm.
- the optical signal measured will depend on the related skin properties, namely strateum corneum thickness, water concentration and collagen denaturation state.
- the optical signal is a second- or third-harmonic generation signal, which may indicate the presence of tissue interfaces or membranes.
- the probe laser light source 80 may then be pulsed (femtosecond to nanosecond range) at a wavelength in the range of 1050-3300 nm.
- the optical signal measured will depend on the related skin properties, namely strateum corneum thickness, epidermal 16 thickness, and dermal 17 depth.
- the optical signal is infrared thermal radiation, which may indicate the presence of heated tissue.
- the probe laser light source 80 may then be a continuous wave or pulsed laser light source operating at a wavelength in the range of 350 to 1100nm.
- the optical signal measured will depend on the related skin properties, namely temperature.
- the skilled person will be able to configure the measurement device 30 to perform the measurement required. This may be done using simulation calculations, or based upon trial and error.
- the measurement device may comprise a plurality of probe 70 and imaging 50 modules, each one configured to perform the measurement of one or more optical properties.
- Each module may also be configured to perform different measurements by using a laser source 80 operating at a variable wavelength and/or an optical detector array 60 sensitive to selectable wavelengths.
- the device is configured such that the probe axis 71 makes an angle 112 of about 45 degrees with the axis coinciding with the outer layer of skin 11.
- the device is further configured such that the imaging optical axis 51 makes an angle 111 of about 90 degrees with the probe axis 71. This configuration may result in a reduced amount of aberration in the measurement because the distances between the outer skin surface and the probe positions are of a similar order of magnitude.
- the angle of about 90 degrees between the probe axis 71 and the imaging optical axis 51 means that the lens planes of the second optical system 67 are approximately parallel to the probe axis 71 - in other words, the plurality of probe positions along the probe axis 71 are located in the object plane and the optical detector array is located in the image plane of the second optical system 67.
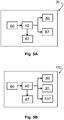
- the measurement device may further comprise a processor 40 for processing the signals from the optical detector array 60 to determine the required skin properties.
- the processor 40 may also be electrically connected to adjustable components of the second optical system 67 to optimise imaging.
- the processor 40 may also be electrically connected to the probe laser light source 80 and/or adjustable components of the first optical system 87. This may be used to optimize the probe module 70 by adjusting, for example, the probe laser intensity, pulse rate, focus, and the position of the probe axis 71.
- the skin properties measured by the invention may be used to determine the parameters for a subsequent treatment using electromagnetic radiation, or to indicate that a treatment currently in progress should be modified or even stopped, or to indicate that no further treatment is required.
- the measurement device may further comprise an indication system to make these results known to the user, such as a green and red led or an audible warning.
- the measurement device and method may be used to characterize the skin within a zone of the body. These measurements may be converted into a map of specific positions, or combined in some way, such as averaging, to determine a single set of skin properties for the whole zone. The outcome may then be made known to the user, or provided in some way to the subsequent treatment device.
- the characterization may be repeated to monitor the progress of the treatment, and to prevent excess treatment.
- the invention may also be used to create look-up tables for multiple body zones and a multiplicity of individuals, so that typical treatment settings may be provided for different treatment devices.
- a treatment module 110 may also be comprised in the measurement device of Figures 2 and 3 . This may also be described as a treatment device 130 comprising a measurement device 30.
- the probe module 70 and imaging module 50 are the same as described in relation to Figures 2 and 3 .
- the functionality of the treatment module 110 is similar to the functionality of the treatment device 10 depicted in Figure 1 .
- the treatment module 110 comprises a treatment radiation source 20 for providing a treatment radiation beam 22 and beam-shaping and directing components 137, the treatment module 110 being configured and arranged such that, in use, the treatment radiation beam 22 exits the device through an aperture 130 along a treatment axis 21 and impinges on an outer surface of the skin to be treated; the beam-shaping and directing components 137 being configured and arranged to direct, in use, the radiation treatment beam 22 to a treatment location 90 disposed within the probe region 95.
- the imaging module 50 may further comprise a confocal slit disposed at the conjugate plane of the second optical system 67 to minimize out-of-focus signals.
- the treatment axis 21 may be arranged to be disposed proximate and parallel to the probe axis 71. This may be advantageous if the treatment module 110 is configured to cause tissue ablation, and the separation of the probe axis 71 and the treatment axis 21 is arranged such that the probe positions to be imaged are not ablated, but only heated by the treatment radiation.
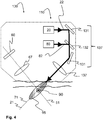
- Fig. 4 further depicts the second embodiment 130 of a non-invasive measurement device for measurement of skin properties using laser light, which comprises a laser beam probe module 70, an imaging module 50 and a laser beam treatment module 110.
- the treatment module 110 comprises a treatment laser radiation source 20 for providing a treatment light beam 22 and a third optical system 137, the treatment module 110 being configured and arranged such that, in use, the treatment light beam 22 exits the device 130 through an aperture along a treatment axis 21 and impinges on an outer surface of the skin to be treated, and optical system 137 being configured and arranged to direct, in use, the light treatment beam 22 to a treatment location 90 disposed within the probe region 95.
- the optical elements 131, 132 found in the optical system 137 may comprise one or more lenses for converging and/or diverging the light beam 21, and one or more mirrors 131 for deflecting the light beam in a desired direction.
- the exact position and/or orientation of the optical elements may be adjustable using techniques known in the art to adapt the position and quality of the light beam 22 such that the beam is focused at the treatment location 90.
- Focus control may be provided by adjusting the position of one or more of the lenses and/or rotating one or more of the mirrors.
- the number and positions of lenses and mirrors 131 are determined by the disposition of the components within the third optical system 137 and the desired degrees of adjustment that the skilled person wishes to provide.
- the treatment laser source 20 may be a pulsed Nd:YAG laser at 1064 nm with sufficient pulse energy in the laser treatment beam 22 to heat the treatment location 90 and cause thermal damage, e.g. photocoagulation.
- the probe laser beam 82 may be configured to have sufficient peak intensity to induce SHG in collagen in the dermis 17. It is known from Tian, L., H. Wei, et al. (2011), "Backward emission angle of microscopic second-harmonic generation from crystallized type I collagen fiber.”, Journal of Biomedical Optics 16(7): 075001-075001 that the SHG radiation pattern in collagen Type I is characterized by both forward and backward propagated light, as well as non-axial side lobes. It is estimated that this, together with the anisotropic nature of skin tissues, may allow a significant amount of SHG signals to propagate through the tissue in a direction perpendicular to the treatment axis 21.
- the imaging module's optical system 67 and the optical detector array may be configured to have a maximum spectral transmission and efficiency, respectively, for optimum sensitivity to the SHG signal. Typically, this is achieved by using an appropriate spectral filter, such as one having a narrow bandwidth centered around the half wavelength of the probe laser source 80.
- the wavelength of the SHG signal is 532 nm for a 1064 nm laser source.
- the information that is obtained from the SHG depth profile includes depth of dermis 17, epidermal 16 thickness, and collagen denaturation state.
- the treatment module 110 may be configured and arranged to provide the treatment axis 21 along the probe axis 71. Such an arrangement may be advantageous because the probe positions being imaged coincide with the region being treated and such an arrangement is expected to give more accurate measurement results to optimize the treatment.
- the third optical system 137 may be configured and arranged to direct both the treatment laser beam 22 and the probe light beam 82, as depicted in Figure 4 .
- the functions performed by the probe module 70 and the treatment module 110 may be implemented in completely separate hardware, the only requirement being that the treatment 110 and probe 70 modules have a fixed relative disposition -in other words, the relationship between the treatment axis 21, the probe axis 71, the treatment location 90 and the probe region 95 must be known, so that the measurements being made can be related to the skin treatment.
- the way in which the treatment 110 and probe 70 modules are implemented depends mainly on the type of radiation used for the treatment beam 21.
- the treatment device 130 may further comprise a processor 40 for processing the signals from the optical detector array 60 to determine the required skin properties. As depicted in Figure 5B , the processor 40 may also be electrically connected to adjustable components of the second optical system 67 to optimize imaging.
- the processor 40 may also be electrically connected to the probe laser light source 80 and/or adjustable components of the first optical system 87. This may be used to optimize the probe module 70 by adjusting, for example, the probe laser intensity, pulse rate, focus, and the position of the probe axis 71.
- the processor 40 may also be electrically connected to the treatment radiation source 80 and/or adjustable components of the third optical system 87. This may be used to optimize the treatment module 110 by adjusting, for example, the treatment radiation fluence, the pulse duration, pulse rate, the focus, and the position of the treatment location 90.
- the treatment parameters implemented in the treatment module 110 may be based on the desired nature and level of treatment and on the measurement of skin properties by means of the invention.
- the skin properties that may be used include:
- the treatment device may further comprise a skin optical coupler 12, configured to optically couple both the treatment laser beam 22, the probe laser beam 82 and the imaging module optical system 67 to the skin.
- the coupler 12 may comprise at least one optical element having at least three planar sides, the first side facing the treatment beam 22, the second side facing the probe beam 82 and the third side facing the imaging module optical system 67.
- the optical coupler 12 may be made of a material having a refractive index in the range of 1.36 to 1.46.
- the skilled person may wish to adapt the angle 112 between the outer layer of the skin axis 11 and the probe axis 71. It may be advantageous to configure and arrange the measurement device 30, 130, 230, 330, 430 so that this angle 112 is in the range of 45 degrees up to and including 90 degrees. By changing this angle 112, the penetration depth of the probe light beam 82 into the skin can be adjusted.
- Figs. 6A to 6D illustrate schematically the relative angles between the probe axis 71, the imaging optical axis 51 and the outer surface of the skin axis 11 for respectively the first 30 and second 130 embodiment, the third embodiment 230, the fourth embodiment 330 and the fifth embodiment 430.
- Figure 6A is included, so that the angles 111, 112 of the different embodiments may be easily compared.
- the angles depicted are the same as in Figures 2 and 4 - the angle 111 between the probe axis 71 and the imaging optical axis 51 is about 90 degrees, and the angle 112 between the probe axis 71 and the outer skin layer axis 11 is about 45 degrees.
- Figure 6B illustrates the third embodiment 230, in which the angle 111 between the probe axis 71 and the imaging optical axis 51 is about 45 degrees, and the angle 112 between the probe axis 71 and the outer skin layer axis 11 is about 45 degrees.
- the image on the optical detector array 60 may have more aberrations than the configuration of Figure 6A , the device 230 may be more compact. Additionally, the processor 40 may be configured to correct for the aberrations. As the imaging optical axis 51 is no longer perpendicular to the probe axis 71, one or more lens planes in the imaging optical system 67 will not be parallel to the probe axis 71.
- the detector axis 61 will typically need to be arranged at an angle to the imaging optical axis 51 that is equal to the angle 111, which is about 45 degrees in this case. This correction to the detector axis 61 is calculated according to the Scheimpflug principle.
- Figure 6C illustrates a fourth embodiment 330, in which the angle 111 between the probe axis 71 and the imaging optical axis 51 is about 20 degrees, and the angle 112 between the probe axis 71 and the outer skin layer axis 11 is about 45 degrees.
- the processor 40 may be configured to correct for additional aberrations, and the detector axis must be corrected, in this case also to an angle of 20 degrees, with respect to the imaging optical axis 51.
- Figure 6D illustrates a fifth embodiment 430 in which the angles are identical to those indicated in the fourth embodiment 330, but additional mirrors are used to reduce the height of the device 430, defined as the dimension in the direction perpendicular to the outer skin axis, and to increase the length of the device 430, defined as the dimension in the direction of the outer skin axis 11.
- the invention provides a non-invasive measurement device 30 and a method for the measurement of skin properties using laser light, the device comprising a probe module 70 and an imaging module 50.
- the invention also provides a non-invasive treatment device 130, 230, 330, 430 comprising the measurement device/method.
- the probe module 70 provides a probe light beam 82 that enters the skin along the probe axis 71.
- the probe light beam 82 is separate from any treatment radiation beam 22, so that the probe light beam 82 can be optimized for the measurement.
- a more reliable skin measurement system is provided because it measures a plurality of positions along the probe axis 71, within a probe region 95.
- the invention measures the skin properties at the treatment location 90 and the surrounding points along the probe axis 71.
- the imaging module 50 is configured and arranged such that the plurality of points are imaged on an optical detector array 60, so that all the points are measured at the same time.
- the measurement device 30, 130, 230, 330, 430 measures the optical depth profile of the section of probe axis 71 comprising the plurality of points.
- the skin parameters measured may then be used to set or modify the treatment parameters, or to indicate that no further treatment is required.
- the measurement device and method may also comprise a treatment module 110 or treatment step.
- the skin parameters measured may then be directly used to control the treatment parameters. This provides a skin treatment device 10 that is both effective and delivers reproducible results.
- any reference signs placed between parentheses shall not be construed as limiting the claim.
- Use of the verb "comprise” and its conjugations does not exclude the presence of elements or steps other than those stated in a claim.
- the article “a” or “an” preceding an element does not exclude the presence of a plurality of such elements.
- the invention may be implemented by means of hardware comprising several distinct elements, and by means of a suitably programmed computer.
- module should not be interpreted to mean that the functionality and hardware are distinguishable in the device. It is used to indicate a functionality which the device comprises, and in practice different “modules” may use in part or entirely the same hardware and optical components.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Pathology (AREA)
- Optics & Photonics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Dermatology (AREA)
- Electromagnetism (AREA)
- Otolaryngology (AREA)
- Radiology & Medical Imaging (AREA)
- Radiation-Therapy Devices (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Measuring And Recording Apparatus For Diagnosis (AREA)
- Investigating Or Analysing Materials By Optical Means (AREA)
Claims (11)
- Dispositif de mesure (30) non invasif destiné à la mesure des propriétés cutanées à l'aide d'une lumière laser, ledit dispositif comprenant un module de sonde (70) et un module d'imagerie (50), dans lequel :- le module de sonde (70) comprend un premier système optique (87) et une source lumineuse laser (80) pour générer un faisceau lumineux de sonde (82), le module de sonde (70) étant conçu et agencé de telle sorte que, lors de l'utilisation, le faisceau lumineux de sonde (82) sort du dispositif (30) le long d'un axe de sonde (71) et frappe une surface externe de la peau à traiter ; le premier système optique (87) étant conçu et agencé pour diriger, lors de l'utilisation, le faisceau lumineux de sonde (82) vers une zone de sonde (95) à l'intérieur de la peau ;- le module d'imagerie (50) comprend un second système optique (67) et un réseau de détecteurs optiques (60), ledit réseau de détecteurs optiques (60) étant disposé le long d'un axe de détecteur (61) compris dans un plan d'image du second système optique (67), dans lequel le second système optique (67) comporte un axe optique d'imagerie (51), lequel coupe l'axe de sonde (71),caractérisé en ce que le second système optique (67) est conçu et agencé pour former, lors de l'utilisation, une image sur une pluralité d'éléments de détection de lumière (65) compris dans le réseau de détecteurs optiques (60) d'une pluralité de positions de sonde, respectivement, répartis le long de l'axe de sonde (71) dans la zone de sonde (95), dans lequel ladite pluralité de positions de sonde se trouve dans un plan d'objet du second système optique (67), et dans lequel un angle (111) entre l'axe de sonde (71) et l'axe optique d'imagerie (51) est égal à un angle entre l'axe optique d'imagerie (51) et l'axe du détecteur (61).
- Dispositif de mesure selon la revendication 1, dans lequel l'axe de détecteur (61) est compris dans un plan, lequel comprend l'axe de sonde (71) et l'axe optique d'imagerie (51).
- Dispositif de mesure selon la revendication 1, dans lequel l'angle (111) enfermé par l'axe de sonde (71) et l'axe optique d'imagerie (51) est dans une plage comprise entre 20 et 90 degrés.
- Dispositif de mesure selon la revendication 1, dans lequel pendant le fonctionnement du dispositif de mesure, un angle (112) enfermé par l'axe de sonde (71) et la surface externe de la peau (11) est dans une plage comprise entre 45 et 90 degrés.
- Dispositif de mesure selon la revendication 1, dans lequel l'axe de détecteur (61) et l'axe de sonde (71) sont parallèles l'un à l'autre ou l'angle inclus par l'axe de détecteur (61) et l'axe optique d'imagerie (51) est corrigé selon la condition de Scheimpflug.
- Dispositif de mesure selon la revendication 1, dans lequel le réseau de détecteurs optiques (60) comprend une matrice de Y éléments de détection de lumière (65) s'étendant le long de l'axe de détecteur (61) fois X éléments de détection de lumière (65) s'étendant le long d'un autre axe perpendiculaire à l'axe de détecteur (61), un rapport Y à X étant supérieur à 5 à 1.
- Dispositif de traitement (130) non invasif destiné au traitement de la peau à l'aide d'un rayonnement de traitement électromagnétique, ledit dispositif comprenant un dispositif de mesure selon l'une quelconque des revendications 1 à 6, ledit dispositif de traitement comprenant en outre un module de traitement (110), dans lequel :- le module de traitement (110) comprend une source de rayonnement de traitement (20) pour fournir un faisceau de rayonnement de traitement (22) et des composants de formation et de direction du faisceau (137), le module de traitement (110) étant conçu et agencé de telle sorte que, lors de l'utilisation, le faisceau de rayonnement de traitement (22) sort du dispositif (130) le long d'un axe de traitement (21) et frappe une surface externe de la peau à traiter ; les composants de formation et de direction du faisceau (137) étant conçus et agencés pour diriger, lors de l'utilisation, le faisceau de traitement par rayonnement (22) vers un emplacement de traitement (90) disposé à l'intérieur de la zone de sonde (95).
- Dispositif de traitement (130) selon la revendication 7, dans lequel le rayonnement de traitement est une lumière laser, et le module de traitement (110) est conçu et agencé de telle sorte que l'axe de traitement (21) coïncide avec l'axe de sonde (71).
- Procédé de mesure non invasive des propriétés cutanées à l'aide d'un dispositif (30) générant la lumière laser, le dispositif comprenant un module de sonde (70) et un module d'imagerie (50),
ledit procédé comprenant :la fourniture d'un module de sonde (70) comprenant un premier système optique (87) et une source lumineuse laser (80) pour générer un faisceau lumineux de sonde (82) ;- la conception et l'agencement du module de sonde (70) de telle sorte que, lors de l'utilisation, le faisceau lumineux de sonde (82) sort du dispositif (30) le long d'un axe de sonde (71) et frappe une surface externe de la peau à traiter ;- la conception et l'agencement du premier système optique (87) pour diriger, lors de l'utilisation, le faisceau lumineux de sonde (82) vers une zone de sonde (95) à l'intérieur de la peau ;- la fourniture d'un module d'imagerie (50) comprenant un second système optique (67) et un réseau de détecteurs optiques (60), le réseau de détecteurs optiques (60) étant disposé le long d'un axe de détecteur (61) compris dans un plan d'image du second système optique (67), et le second système optique (67) comportant un axe optique d'imagerie (51), lequel coupe l'axe de sonde (71) ;caractérisé en ce que le second système optique (67) est conçu et agencé pour former, lors de l'utilisation, une image sur une pluralité d'éléments de détection de lumière (65) compris dans le réseau de détecteurs optiques (60) d'une pluralité de positions de sonde, respectivement, répartis le long de l'axe de sonde (71) dans la zone de sonde (95), dans lequel ladite pluralité de positions de sonde se trouve dans un plan d'objet du second système optique (67), et dans lequel un angle (111) entre l'axe de sonde (71) et l'axe optique d'imagerie (51) est égal à un angle entre l'axe optique d'imagerie (51) et l'axe de détecteur (61). - Procédé de mesure selon la revendication 9, dans lequel l'axe de détecteur (61) est compris dans un plan, lequel comprend l'axe de sonde (71) et l'axe optique d'imagerie (51).
- Procédé de mesure selon la revendication 9, dans lequel ledit procédé comprend en outre :- le traitement (40) de l'image détectée par le réseau de détecteurs optiques (60) pour générer au moins un paramètre de commande ;- l'application de l'au moins un paramètre de commande pour déterminer les paramètres de fonctionnement de la source lumineuse laser de sonde (80) et/ou du premier système optique (87).
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14732228.3A EP3013213B1 (fr) | 2013-06-25 | 2014-06-25 | Dispositif de mesure des propriétés de la peau et dispositif de traitement non invasif |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP13173476 | 2013-06-25 | ||
| EP14732228.3A EP3013213B1 (fr) | 2013-06-25 | 2014-06-25 | Dispositif de mesure des propriétés de la peau et dispositif de traitement non invasif |
| PCT/EP2014/063323 WO2014207003A1 (fr) | 2013-06-25 | 2014-06-25 | Dispositif de mesure des propriétés de la peau et dispositif de traitement non invasif |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3013213A1 EP3013213A1 (fr) | 2016-05-04 |
| EP3013213B1 true EP3013213B1 (fr) | 2020-09-23 |
Family
ID=48782163
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP14732228.3A Not-in-force EP3013213B1 (fr) | 2013-06-25 | 2014-06-25 | Dispositif de mesure des propriétés de la peau et dispositif de traitement non invasif |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US10448997B2 (fr) |
| EP (1) | EP3013213B1 (fr) |
| JP (1) | JP6509829B2 (fr) |
| CN (2) | CN112914515A (fr) |
| WO (1) | WO2014207003A1 (fr) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105852808B (zh) * | 2015-05-08 | 2019-08-30 | 江苏坤辉生物科技有限公司 | 一种活体无创检测紫外光诱导皮肤损伤的方法及其检测设备 |
| CN105769127B (zh) * | 2016-05-05 | 2019-03-29 | 中国科学院苏州生物医学工程技术研究所 | 一种基于共聚焦的诊疗设备及其控制方法 |
| EP3281598A1 (fr) * | 2016-08-09 | 2018-02-14 | Koninklijke Philips N.V. | Dispositif et procédé de traitement cutané àbase de lumière |
| WO2018073266A1 (fr) | 2016-10-18 | 2018-04-26 | Koninklijke Philips N.V. | Dispositif d'accessoire, dispositif d'imagerie et procédé de détermination d'un paramètre de peau d'un sujet |
| GB201702098D0 (en) | 2017-02-08 | 2017-03-22 | Michelson Diagnostics Ltd | Processing optical coherence tomography (OCT) scans |
| WO2020053810A1 (fr) * | 2018-09-12 | 2020-03-19 | Anupam Lavania | Dispositif et procédé d'émission contrôlée de rayonnement |
| DE102018221524A1 (de) * | 2018-12-12 | 2020-06-18 | Robert Bosch Gmbh | Vorrichtung und Verfahren zum Zusammenstellen eines Pflegeprodukts sowie zur Ermittlung wenigstens eines Hautparameters |
| USD935030S1 (en) * | 2019-04-15 | 2021-11-02 | Koninklijke Philips N.V. | Skin measurement device |
| USD942020S1 (en) * | 2019-10-15 | 2022-01-25 | Koninklijke Philips N.V. | Skin measurement device |
| JP7503216B2 (ja) | 2020-12-31 | 2024-06-19 | ルミナス ビーイー リミテッド | 美容レーザによる審美的皮膚処置手順の実時間モニタリングのための方法およびシステム |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5071417A (en) | 1990-06-15 | 1991-12-10 | Rare Earth Medical Lasers, Inc. | Laser fusion of biological materials |
| JP2763823B2 (ja) * | 1990-09-25 | 1998-06-11 | 花王株式会社 | 角層の厚さ測定方法及びその測定装置 |
| AU658669B2 (en) * | 1991-09-06 | 1995-04-27 | Commonwealth Scientific And Industrial Research Organisation | Measurement method and apparatus |
| US5334191A (en) | 1992-05-21 | 1994-08-02 | Dix Phillip Poppas | Laser tissue welding control system |
| US6015404A (en) | 1996-12-02 | 2000-01-18 | Palomar Medical Technologies, Inc. | Laser dermatology with feedback control |
| US5810801A (en) | 1997-02-05 | 1998-09-22 | Candela Corporation | Method and apparatus for treating wrinkles in skin using radiation |
| US6413257B1 (en) | 1997-05-15 | 2002-07-02 | Surgical Dynamics, Inc. | Clamping connector for spinal fixation systems |
| US6190377B1 (en) | 1999-05-05 | 2001-02-20 | James A. Kuzdrall | Method and apparatus for predictive beam energy control in laser surgery |
| US6413267B1 (en) | 1999-08-09 | 2002-07-02 | Theralase, Inc. | Therapeutic laser device and method including noninvasive subsurface monitoring and controlling means |
| JP3188437B2 (ja) * | 1999-12-08 | 2001-07-16 | ヤーマン株式会社 | レーザ光照射プローブ |
| JP2002011106A (ja) * | 2000-06-28 | 2002-01-15 | Nidek Co Ltd | レーザ治療装置 |
| JP4105554B2 (ja) * | 2003-01-16 | 2008-06-25 | 株式会社資生堂 | 皮膚の透明感評価方法 |
| US7562025B2 (en) * | 2003-09-19 | 2009-07-14 | Vesta Medical, Llc | Waste sorting system with query function, and method thereof |
| US7309335B2 (en) * | 2003-12-31 | 2007-12-18 | Palomar Medical Technologies, Inc. | Dermatological treatment with visualization |
| WO2005099607A1 (fr) * | 2004-04-15 | 2005-10-27 | Koninklijke Philips Electronics N.V. | Dispositif pour le traitement de la peau par un faisceau de rayonnement |
| EP1740090B1 (fr) | 2004-04-20 | 2014-06-25 | Koninklijke Philips N.V. | Dispositif de detection de poils |
| US20050254381A1 (en) | 2004-04-28 | 2005-11-17 | Desormeaux Joseph Jr | System and method for detecting faulty media in a media player |
| US9283038B2 (en) | 2005-07-26 | 2016-03-15 | Koninklijke Philips N.V. | Hair removing system |
| EP1931263A2 (fr) | 2005-08-29 | 2008-06-18 | Reliant Technologies, Inc. | Procede et appareil pour la surveillance et la regulation de traitement tissulaire induit thermiquement |
| US8346347B2 (en) * | 2005-09-15 | 2013-01-01 | Palomar Medical Technologies, Inc. | Skin optical characterization device |
| US20070260230A1 (en) | 2006-05-04 | 2007-11-08 | Reliant Technologies, Inc. | Opto-mechanical Apparatus and Method for Dermatological Treatment |
| WO2008001284A2 (fr) * | 2006-06-26 | 2008-01-03 | Koninklijke Philips Electronics N.V. | Dispositif et procédé pour le traitement de la peau, et utilisation du dispositif |
| ES2636973T3 (es) | 2007-03-02 | 2017-10-10 | Candela Corporation | Calentamiento de la piel a profundidad variable con láseres |
| WO2009115947A1 (fr) * | 2008-03-18 | 2009-09-24 | Koninklijke Philips Electronics N.V. | Appareil d'imagerie de peau, système d'analyse de peau |
| US20100114080A1 (en) | 2008-11-05 | 2010-05-06 | Theriault Richard H | Apparatus, system and method for medical treatment |
| US20100130969A1 (en) * | 2008-11-25 | 2010-05-27 | Apogen Technologies, Inc. | System and method for dermatological treatment |
| JP2011133593A (ja) * | 2009-12-24 | 2011-07-07 | Kyocera Corp | 撮像装置 |
| FR2954690A1 (fr) | 2009-12-29 | 2011-07-01 | Ekkyo | Dispositif de traitement dermatologique par faisceau lumineux |
| US20120283712A1 (en) | 2011-02-03 | 2012-11-08 | TRIA Beauty | Devices and Methods for Radiation-Based Dermatological Treatments |
| JP2012186612A (ja) * | 2011-03-04 | 2012-09-27 | Olympus Corp | 撮像装置 |
| JP5662223B2 (ja) * | 2011-03-31 | 2015-01-28 | 株式会社ミツトヨ | 形状測定装置 |
| US9037204B2 (en) * | 2011-09-07 | 2015-05-19 | Covidien Lp | Filtered detector array for optical patient sensors |
-
2014
- 2014-06-25 CN CN202110301010.9A patent/CN112914515A/zh active Pending
- 2014-06-25 CN CN201480036135.1A patent/CN105338888A/zh active Pending
- 2014-06-25 US US14/896,760 patent/US10448997B2/en not_active Expired - Fee Related
- 2014-06-25 WO PCT/EP2014/063323 patent/WO2014207003A1/fr active Application Filing
- 2014-06-25 JP JP2016520541A patent/JP6509829B2/ja not_active Expired - Fee Related
- 2014-06-25 EP EP14732228.3A patent/EP3013213B1/fr not_active Not-in-force
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2016526408A (ja) | 2016-09-05 |
| CN112914515A (zh) | 2021-06-08 |
| US20160120604A1 (en) | 2016-05-05 |
| JP6509829B2 (ja) | 2019-05-08 |
| WO2014207003A1 (fr) | 2014-12-31 |
| EP3013213A1 (fr) | 2016-05-04 |
| US10448997B2 (en) | 2019-10-22 |
| CN105338888A (zh) | 2016-02-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3013213B1 (fr) | Dispositif de mesure des propriétés de la peau et dispositif de traitement non invasif | |
| US11826096B2 (en) | Method and apparatus for selective treatment of biological tissue | |
| JP5458250B2 (ja) | 皮膚の療法emr治療を行う方法及び装置 | |
| JP5986586B2 (ja) | 放射線ベースの皮膚科治療のデバイスおよび方法 | |
| US20060253176A1 (en) | Dermatological treatment device with deflector optic | |
| KR20160042069A (ko) | 진피 기미의 치료를 위한 방법 및 장치 | |
| RU2679295C2 (ru) | Неинвазивное устройство для лечения кожи лазерным светом | |
| JP7408906B2 (ja) | Emrを用いた組織治療のための回折光学 | |
| Sebern et al. | Tissue modification with feedback: the smart scalpel | |
| WO2006030622A1 (fr) | Dispositif thérapeutique à laser |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20160125 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAX | Request for extension of the european patent (deleted) | ||
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: KONINKLIJKE PHILIPS N.V. |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20200415 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602014070468 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1315532 Country of ref document: AT Kind code of ref document: T Effective date: 20201015 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201224 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201223 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201223 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1315532 Country of ref document: AT Kind code of ref document: T Effective date: 20200923 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20200923 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210125 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210123 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602014070468 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20210625 Year of fee payment: 8 Ref country code: DE Payment date: 20210628 Year of fee payment: 8 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20210615 Year of fee payment: 8 Ref country code: GB Payment date: 20210625 Year of fee payment: 8 |
|
| 26N | No opposition filed |
Effective date: 20210624 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20210630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210625 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210630 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210625 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210630 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602014070468 Country of ref document: DE |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20220625 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20140625 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220625 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220625 Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200923 |