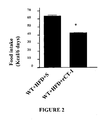
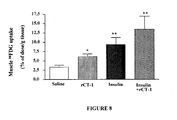
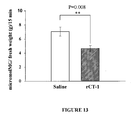
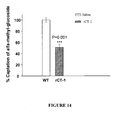
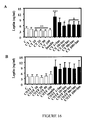
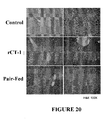
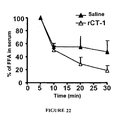
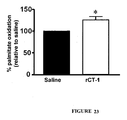
EP2397152B1 - Use of cardiotrophin- 1 for the treatment of metabolic diseases - Google Patents
Use of cardiotrophin- 1 for the treatment of metabolic diseases Download PDFInfo
- Publication number
- EP2397152B1 EP2397152B1 EP10710061.2A EP10710061A EP2397152B1 EP 2397152 B1 EP2397152 B1 EP 2397152B1 EP 10710061 A EP10710061 A EP 10710061A EP 2397152 B1 EP2397152 B1 EP 2397152B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- insulin
- sequence
- cardiotrophin
- treatment
- identity
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 102100028892 Cardiotrophin-1 Human genes 0.000 title claims description 145
- 108010041776 cardiotrophin 1 Proteins 0.000 title claims description 145
- 238000011282 treatment Methods 0.000 title claims description 86
- 208000030159 metabolic disease Diseases 0.000 title claims description 28
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 claims description 222
- 102000004877 Insulin Human genes 0.000 claims description 116
- 108090001061 Insulin Proteins 0.000 claims description 116
- 229940125396 insulin Drugs 0.000 claims description 111
- 230000000694 effects Effects 0.000 claims description 66
- 239000000203 mixture Substances 0.000 claims description 55
- 150000001875 compounds Chemical class 0.000 claims description 54
- 108091033319 polynucleotide Proteins 0.000 claims description 40
- 239000002157 polynucleotide Substances 0.000 claims description 40
- 102000040430 polynucleotide Human genes 0.000 claims description 40
- 238000000034 method Methods 0.000 claims description 35
- 208000008589 Obesity Diseases 0.000 claims description 34
- 235000020824 obesity Nutrition 0.000 claims description 32
- 230000001684 chronic effect Effects 0.000 claims description 31
- 239000003795 chemical substances by application Substances 0.000 claims description 27
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims description 24
- 208000016097 disease of metabolism Diseases 0.000 claims description 23
- 230000001154 acute effect Effects 0.000 claims description 19
- 239000013598 vector Substances 0.000 claims description 17
- 230000002218 hypoglycaemic effect Effects 0.000 claims description 16
- 206010022489 Insulin Resistance Diseases 0.000 claims description 15
- 239000002537 cosmetic Substances 0.000 claims description 14
- 239000003814 drug Substances 0.000 claims description 13
- 239000003472 antidiabetic agent Substances 0.000 claims description 10
- 229940079593 drug Drugs 0.000 claims description 10
- 201000001421 hyperglycemia Diseases 0.000 claims description 10
- 230000003178 anti-diabetic effect Effects 0.000 claims description 8
- 230000003248 secreting effect Effects 0.000 claims description 8
- 238000002360 preparation method Methods 0.000 claims description 7
- 206010033307 Overweight Diseases 0.000 claims description 5
- 230000001235 sensitizing effect Effects 0.000 claims description 5
- 101000916283 Homo sapiens Cardiotrophin-1 Proteins 0.000 claims 12
- 208000032928 Dyslipidaemia Diseases 0.000 claims 3
- 208000017170 Lipid metabolism disease Diseases 0.000 claims 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 45
- 239000008103 glucose Substances 0.000 description 45
- 241000699670 Mus sp. Species 0.000 description 39
- 210000004027 cell Anatomy 0.000 description 35
- -1 poly(amino)-p-xylylene Polymers 0.000 description 31
- 235000009200 high fat diet Nutrition 0.000 description 29
- 210000003205 muscle Anatomy 0.000 description 29
- 210000004369 blood Anatomy 0.000 description 28
- 239000008280 blood Substances 0.000 description 28
- 210000002966 serum Anatomy 0.000 description 27
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical group [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 26
- 241001465754 Metazoa Species 0.000 description 24
- 230000014509 gene expression Effects 0.000 description 21
- 239000011780 sodium chloride Substances 0.000 description 21
- 108010005939 Ciliary Neurotrophic Factor Proteins 0.000 description 20
- 102100031614 Ciliary neurotrophic factor Human genes 0.000 description 20
- 230000000968 intestinal effect Effects 0.000 description 20
- 230000001939 inductive effect Effects 0.000 description 18
- 102000016267 Leptin Human genes 0.000 description 17
- 108010092277 Leptin Proteins 0.000 description 17
- 210000001789 adipocyte Anatomy 0.000 description 17
- 230000037396 body weight Effects 0.000 description 17
- NRYBAZVQPHGZNS-ZSOCWYAHSA-N leptin Chemical compound O=C([C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)CCSC)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CS)C(O)=O NRYBAZVQPHGZNS-ZSOCWYAHSA-N 0.000 description 17
- 235000000346 sugar Nutrition 0.000 description 17
- 238000011740 C57BL/6 mouse Methods 0.000 description 16
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 16
- 229940039781 leptin Drugs 0.000 description 16
- 238000001990 intravenous administration Methods 0.000 description 15
- 108090000623 proteins and genes Proteins 0.000 description 15
- 150000001413 amino acids Chemical group 0.000 description 14
- 150000007523 nucleic acids Chemical group 0.000 description 14
- 239000003925 fat Substances 0.000 description 13
- 108090000695 Cytokines Proteins 0.000 description 12
- 102000004127 Cytokines Human genes 0.000 description 12
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 12
- 230000002891 anorexigenic effect Effects 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 11
- 235000021588 free fatty acids Nutrition 0.000 description 11
- 239000003112 inhibitor Substances 0.000 description 11
- 108090000765 processed proteins & peptides Proteins 0.000 description 11
- 230000001225 therapeutic effect Effects 0.000 description 11
- 230000009471 action Effects 0.000 description 10
- 229940024606 amino acid Drugs 0.000 description 10
- 235000001014 amino acid Nutrition 0.000 description 10
- 238000009472 formulation Methods 0.000 description 10
- 238000001727 in vivo Methods 0.000 description 10
- 238000007254 oxidation reaction Methods 0.000 description 10
- 102000004196 processed proteins & peptides Human genes 0.000 description 10
- 210000002027 skeletal muscle Anatomy 0.000 description 10
- 230000032258 transport Effects 0.000 description 10
- 241000282414 Homo sapiens Species 0.000 description 9
- 108091028043 Nucleic acid sequence Proteins 0.000 description 9
- 208000035475 disorder Diseases 0.000 description 9
- 238000000338 in vitro Methods 0.000 description 9
- 230000003647 oxidation Effects 0.000 description 9
- 229920001184 polypeptide Polymers 0.000 description 9
- 150000003626 triacylglycerols Chemical class 0.000 description 9
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 8
- 230000007423 decrease Effects 0.000 description 8
- 230000003247 decreasing effect Effects 0.000 description 8
- 238000002347 injection Methods 0.000 description 8
- 239000007924 injection Substances 0.000 description 8
- HOVAGTYPODGVJG-ZFYZTMLRSA-N methyl alpha-D-glucopyranoside Chemical compound CO[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O HOVAGTYPODGVJG-ZFYZTMLRSA-N 0.000 description 8
- DTHNMHAUYICORS-KTKZVXAJSA-N Glucagon-like peptide 1 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 DTHNMHAUYICORS-KTKZVXAJSA-N 0.000 description 7
- 241000700159 Rattus Species 0.000 description 7
- 235000005911 diet Nutrition 0.000 description 7
- 201000010099 disease Diseases 0.000 description 7
- 230000001965 increasing effect Effects 0.000 description 7
- 230000002401 inhibitory effect Effects 0.000 description 7
- 230000026731 phosphorylation Effects 0.000 description 7
- 238000006366 phosphorylation reaction Methods 0.000 description 7
- 235000018102 proteins Nutrition 0.000 description 7
- 102000004169 proteins and genes Human genes 0.000 description 7
- 108010065920 Insulin Lispro Proteins 0.000 description 6
- 241000699666 Mus <mouse, genus> Species 0.000 description 6
- 239000000556 agonist Substances 0.000 description 6
- 230000006378 damage Effects 0.000 description 6
- 206010012601 diabetes mellitus Diseases 0.000 description 6
- 235000014113 dietary fatty acids Nutrition 0.000 description 6
- 239000000194 fatty acid Substances 0.000 description 6
- 229930195729 fatty acid Natural products 0.000 description 6
- 150000004665 fatty acids Chemical class 0.000 description 6
- 230000006377 glucose transport Effects 0.000 description 6
- WNRQPCUGRUFHED-DETKDSODSA-N humalog Chemical compound C([C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O)C1=CC=C(O)C=C1.C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CS)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 WNRQPCUGRUFHED-DETKDSODSA-N 0.000 description 6
- 239000000411 inducer Substances 0.000 description 6
- 210000004185 liver Anatomy 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 102400000322 Glucagon-like peptide 1 Human genes 0.000 description 5
- ZSJLQEPLLKMAKR-UHFFFAOYSA-N Streptozotocin Natural products O=NN(C)C(=O)NC1C(O)OC(CO)C(O)C1O ZSJLQEPLLKMAKR-UHFFFAOYSA-N 0.000 description 5
- LEMUFSYUPGXXCM-JNEQYSBXSA-N caninsulin Chemical compound [Zn].C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC3N=CN=C3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1C=NC=N1 LEMUFSYUPGXXCM-JNEQYSBXSA-N 0.000 description 5
- 230000037213 diet Effects 0.000 description 5
- 230000002708 enhancing effect Effects 0.000 description 5
- 239000013604 expression vector Substances 0.000 description 5
- 235000013305 food Nutrition 0.000 description 5
- 235000021070 high sugar diet Nutrition 0.000 description 5
- 210000004379 membrane Anatomy 0.000 description 5
- 239000012528 membrane Substances 0.000 description 5
- 239000011859 microparticle Substances 0.000 description 5
- 102000039446 nucleic acids Human genes 0.000 description 5
- 108020004707 nucleic acids Proteins 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 230000001105 regulatory effect Effects 0.000 description 5
- 230000028327 secretion Effects 0.000 description 5
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 5
- 229960001052 streptozocin Drugs 0.000 description 5
- 101710198884 GATA-type zinc finger protein 1 Proteins 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 235000012000 cholesterol Nutrition 0.000 description 4
- 230000009699 differential effect Effects 0.000 description 4
- 230000008030 elimination Effects 0.000 description 4
- 238000003379 elimination reaction Methods 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 230000004130 lipolysis Effects 0.000 description 4
- 230000004060 metabolic process Effects 0.000 description 4
- 210000003098 myoblast Anatomy 0.000 description 4
- 235000021590 normal diet Nutrition 0.000 description 4
- 239000002773 nucleotide Substances 0.000 description 4
- 125000003729 nucleotide group Chemical group 0.000 description 4
- 239000003921 oil Substances 0.000 description 4
- 235000019198 oils Nutrition 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 230000011664 signaling Effects 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 239000003981 vehicle Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- 230000004580 weight loss Effects 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 101000976075 Homo sapiens Insulin Proteins 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- 102000004889 Interleukin-6 Human genes 0.000 description 3
- 108090001005 Interleukin-6 Proteins 0.000 description 3
- 108010041872 Islet Amyloid Polypeptide Proteins 0.000 description 3
- 102000036770 Islet Amyloid Polypeptide Human genes 0.000 description 3
- 108010081368 Isophane Insulin Proteins 0.000 description 3
- 102000005237 Isophane Insulin Human genes 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 229940123464 Thiazolidinedione Drugs 0.000 description 3
- 210000003486 adipose tissue brown Anatomy 0.000 description 3
- 239000005557 antagonist Substances 0.000 description 3
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 230000000747 cardiac effect Effects 0.000 description 3
- 210000003169 central nervous system Anatomy 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 210000001842 enterocyte Anatomy 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- 230000002496 gastric effect Effects 0.000 description 3
- 238000003304 gavage Methods 0.000 description 3
- 244000144993 groups of animals Species 0.000 description 3
- 229940038661 humalog Drugs 0.000 description 3
- 238000009396 hybridization Methods 0.000 description 3
- 239000000017 hydrogel Substances 0.000 description 3
- 230000002757 inflammatory effect Effects 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- PBGKTOXHQIOBKM-FHFVDXKLSA-N insulin (human) Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3NC=NC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 PBGKTOXHQIOBKM-FHFVDXKLSA-N 0.000 description 3
- 229960002068 insulin lispro Drugs 0.000 description 3
- 230000037356 lipid metabolism Effects 0.000 description 3
- 150000002632 lipids Chemical class 0.000 description 3
- 238000013116 obese mouse model Methods 0.000 description 3
- 230000002093 peripheral effect Effects 0.000 description 3
- 239000008194 pharmaceutical composition Substances 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- 239000000018 receptor agonist Substances 0.000 description 3
- 229940044601 receptor agonist Drugs 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- 150000001467 thiazolidinediones Chemical class 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- VRYALKFFQXWPIH-PBXRRBTRSA-N (3r,4s,5r)-3,4,5,6-tetrahydroxyhexanal Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)CC=O VRYALKFFQXWPIH-PBXRRBTRSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 2
- 102000007469 Actins Human genes 0.000 description 2
- 108010085238 Actins Proteins 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- 101710161573 Cardiotrophin-2 Proteins 0.000 description 2
- 241000701022 Cytomegalovirus Species 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 2
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 2
- 208000031226 Hyperlipidaemia Diseases 0.000 description 2
- 108010089308 Insulin Detemir Proteins 0.000 description 2
- 229930195714 L-glutamate Natural products 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 108090000362 Lymphotoxin-beta Proteins 0.000 description 2
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 2
- 102000015494 Mitochondrial Uncoupling Proteins Human genes 0.000 description 2
- 108010050258 Mitochondrial Uncoupling Proteins Proteins 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 102100027467 Pro-opiomelanocortin Human genes 0.000 description 2
- 102000007327 Protamines Human genes 0.000 description 2
- 108010007568 Protamines Proteins 0.000 description 2
- 108010029485 Protein Isoforms Proteins 0.000 description 2
- 102000001708 Protein Isoforms Human genes 0.000 description 2
- 206010037660 Pyrexia Diseases 0.000 description 2
- 241000700157 Rattus norvegicus Species 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- YASAKCUCGLMORW-UHFFFAOYSA-N Rosiglitazone Chemical compound C=1C=CC=NC=1N(C)CCOC(C=C1)=CC=C1CC1SC(=O)NC1=O YASAKCUCGLMORW-UHFFFAOYSA-N 0.000 description 2
- 210000001744 T-lymphocyte Anatomy 0.000 description 2
- 102000013534 Troponin C Human genes 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- PMMURAAUARKVCB-UHFFFAOYSA-N alpha-D-ara-dHexp Natural products OCC1OC(O)CC(O)C1O PMMURAAUARKVCB-UHFFFAOYSA-N 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000003243 anti-lipolytic effect Effects 0.000 description 2
- 230000003579 anti-obesity Effects 0.000 description 2
- 229940125708 antidiabetic agent Drugs 0.000 description 2
- 235000020827 calorie restriction Nutrition 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 2
- 208000012696 congenital leptin deficiency Diseases 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000010217 densitometric analysis Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000000378 dietary effect Effects 0.000 description 2
- 229960004580 glibenclamide Drugs 0.000 description 2
- NSJYMFYVNWVGON-UHFFFAOYSA-N glisentide Chemical compound COC1=CC=CC=C1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)NC2CCCC2)C=C1 NSJYMFYVNWVGON-UHFFFAOYSA-N 0.000 description 2
- 229950008402 glisentide Drugs 0.000 description 2
- 230000009229 glucose formation Effects 0.000 description 2
- 230000004190 glucose uptake Effects 0.000 description 2
- ZNNLBTZKUZBEKO-UHFFFAOYSA-N glyburide Chemical compound COC1=CC=C(Cl)C=C1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)NC2CCCCC2)C=C1 ZNNLBTZKUZBEKO-UHFFFAOYSA-N 0.000 description 2
- 210000005260 human cell Anatomy 0.000 description 2
- 230000002396 hypoinsulinemic effect Effects 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000008595 infiltration Effects 0.000 description 2
- 238000001764 infiltration Methods 0.000 description 2
- 239000004026 insulin derivative Substances 0.000 description 2
- 210000000936 intestine Anatomy 0.000 description 2
- 229940028435 intralipid Drugs 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 102000005861 leptin receptors Human genes 0.000 description 2
- 108010019813 leptin receptors Proteins 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- UGOZVNFCFYTPAZ-IOXYNQHNSA-N levemir Chemical compound CCCCCCCCCCCCCC(=O)NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H]1NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=2N=CNC=2)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=2N=CNC=2)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=2C=CC=CC=2)C(C)C)CSSC[C@@H]2NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)C(C)C)CSSC[C@H](NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC2=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H](CSSC1)C(=O)N[C@@H](CC(N)=O)C(O)=O)CC1=CC=C(O)C=C1 UGOZVNFCFYTPAZ-IOXYNQHNSA-N 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 208000001022 morbid obesity Diseases 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- AHLBNYSZXLDEJQ-FWEHEUNISA-N orlistat Chemical compound CCCCCCCCCCC[C@H](OC(=O)[C@H](CC(C)C)NC=O)C[C@@H]1OC(=O)[C@H]1CCCCCC AHLBNYSZXLDEJQ-FWEHEUNISA-N 0.000 description 2
- 210000000496 pancreas Anatomy 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- HYAFETHFCAUJAY-UHFFFAOYSA-N pioglitazone Chemical compound N1=CC(CC)=CC=C1CCOC(C=C1)=CC=C1CC1C(=O)NC(=O)S1 HYAFETHFCAUJAY-UHFFFAOYSA-N 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 108010066381 preproinsulin Proteins 0.000 description 2
- 229940048914 protamine Drugs 0.000 description 2
- 238000003753 real-time PCR Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000000580 secretagogue effect Effects 0.000 description 2
- 210000003491 skin Anatomy 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 229920001169 thermoplastic Polymers 0.000 description 2
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 239000013603 viral vector Substances 0.000 description 2
- SFLSHLFXELFNJZ-QMMMGPOBSA-N (-)-norepinephrine Chemical compound NC[C@H](O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-QMMMGPOBSA-N 0.000 description 1
- DIGQNXIGRZPYDK-WKSCXVIASA-N (2R)-6-amino-2-[[2-[[(2S)-2-[[2-[[(2R)-2-[[(2S)-2-[[(2R,3S)-2-[[2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S,3S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2R)-2-[[2-[[2-[[2-[(2-amino-1-hydroxyethylidene)amino]-3-carboxy-1-hydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1,5-dihydroxy-5-iminopentylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]hexanoic acid Chemical compound C[C@@H]([C@@H](C(=N[C@@H](CS)C(=N[C@@H](C)C(=N[C@@H](CO)C(=NCC(=N[C@@H](CCC(=N)O)C(=NC(CS)C(=N[C@H]([C@H](C)O)C(=N[C@H](CS)C(=N[C@H](CO)C(=NCC(=N[C@H](CS)C(=NCC(=N[C@H](CCCCN)C(=O)O)O)O)O)O)O)O)O)O)O)O)O)O)O)N=C([C@H](CS)N=C([C@H](CO)N=C([C@H](CO)N=C([C@H](C)N=C(CN=C([C@H](CO)N=C([C@H](CS)N=C(CN=C(C(CS)N=C(C(CC(=O)O)N=C(CN)O)O)O)O)O)O)O)O)O)O)O)O DIGQNXIGRZPYDK-WKSCXVIASA-N 0.000 description 1
- XUFXOAAUWZOOIT-SXARVLRPSA-N (2R,3R,4R,5S,6R)-5-[[(2R,3R,4R,5S,6R)-5-[[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)-1-cyclohex-2-enyl]amino]-2-oxanyl]oxy]-3,4-dihydroxy-6-(hydroxymethyl)-2-oxanyl]oxy]-6-(hydroxymethyl)oxane-2,3,4-triol Chemical compound O([C@H]1O[C@H](CO)[C@H]([C@@H]([C@H]1O)O)O[C@H]1O[C@@H]([C@H]([C@H](O)[C@H]1O)N[C@@H]1[C@@H]([C@@H](O)[C@H](O)C(CO)=C1)O)C)[C@@H]1[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]1O XUFXOAAUWZOOIT-SXARVLRPSA-N 0.000 description 1
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- IAZGSEXFFGHKDF-VIFPVBQESA-N (2r)-2-azaniumyl-3-phenylmethoxycarbonylsulfanylpropanoate Chemical compound OC(=O)[C@@H](N)CSC(=O)OCC1=CC=CC=C1 IAZGSEXFFGHKDF-VIFPVBQESA-N 0.000 description 1
- VSWSDTLXDWESGZ-AWEZNQCLSA-N (2s)-3-[4-(4-hydroxyphenoxy)phenyl]-2-(iodoamino)propanoic acid Chemical compound C1=CC(C[C@@H](C(=O)O)NI)=CC=C1OC1=CC=C(O)C=C1 VSWSDTLXDWESGZ-AWEZNQCLSA-N 0.000 description 1
- KWTSXDURSIMDCE-QMMMGPOBSA-N (S)-amphetamine Chemical compound C[C@H](N)CC1=CC=CC=C1 KWTSXDURSIMDCE-QMMMGPOBSA-N 0.000 description 1
- LLJFMFZYVVLQKT-UHFFFAOYSA-N 1-cyclohexyl-3-[4-[2-(7-methoxy-4,4-dimethyl-1,3-dioxo-2-isoquinolinyl)ethyl]phenyl]sulfonylurea Chemical compound C=1C(OC)=CC=C(C(C2=O)(C)C)C=1C(=O)N2CCC(C=C1)=CC=C1S(=O)(=O)NC(=O)NC1CCCCC1 LLJFMFZYVVLQKT-UHFFFAOYSA-N 0.000 description 1
- NVSWJKWHLUTHLP-CPJSRVTESA-N 11-[(2s)-2-[[2-[(2s)-2-cyanopyrrolidin-1-yl]-2-oxoethyl]amino]propyl]-3-n,3-n,8-n,8-n-tetramethyl-11-(2h-tetrazol-5-yl)-5,6-dihydrodibenzo[1,3-a:1',3'-e][7]annulene-3,8-dicarboxamide Chemical compound C([C@H](C)NCC(=O)N1[C@@H](CCC1)C#N)C1(C2=CC=C(C=C2CCC2=CC(=CC=C21)C(=O)N(C)C)C(=O)N(C)C)C=1N=NNN=1 NVSWJKWHLUTHLP-CPJSRVTESA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- QDGAVODICPCDMU-UHFFFAOYSA-N 2-amino-3-[3-[bis(2-chloroethyl)amino]phenyl]propanoic acid Chemical compound OC(=O)C(N)CC1=CC=CC(N(CCCl)CCCl)=C1 QDGAVODICPCDMU-UHFFFAOYSA-N 0.000 description 1
- QLIBJPGWWSHWBF-UHFFFAOYSA-N 2-aminoethyl methacrylate Chemical compound CC(=C)C(=O)OCCN QLIBJPGWWSHWBF-UHFFFAOYSA-N 0.000 description 1
- AOYNUTHNTBLRMT-SLPGGIOYSA-N 2-deoxy-2-fluoro-aldehydo-D-glucose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](F)C=O AOYNUTHNTBLRMT-SLPGGIOYSA-N 0.000 description 1
- WIGIZIANZCJQQY-UHFFFAOYSA-N 4-ethyl-3-methyl-N-[2-[4-[[[(4-methylcyclohexyl)amino]-oxomethyl]sulfamoyl]phenyl]ethyl]-5-oxo-2H-pyrrole-1-carboxamide Chemical compound O=C1C(CC)=C(C)CN1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)NC2CCC(C)CC2)C=C1 WIGIZIANZCJQQY-UHFFFAOYSA-N 0.000 description 1
- 102100036009 5'-AMP-activated protein kinase catalytic subunit alpha-2 Human genes 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- 206010050013 Abulia Diseases 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 102000006822 Agouti Signaling Protein Human genes 0.000 description 1
- 108010072151 Agouti Signaling Protein Proteins 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 229940077274 Alpha glucosidase inhibitor Drugs 0.000 description 1
- 229940127438 Amylin Agonists Drugs 0.000 description 1
- 108700001281 BIM 51077 Proteins 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 229940123208 Biguanide Drugs 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 108010055448 CJC 1131 Proteins 0.000 description 1
- 102000005367 Carboxypeptidases Human genes 0.000 description 1
- 108010006303 Carboxypeptidases Proteins 0.000 description 1
- RKWGIWYCVPQPMF-UHFFFAOYSA-N Chloropropamide Chemical compound CCCNC(=O)NS(=O)(=O)C1=CC=C(Cl)C=C1 RKWGIWYCVPQPMF-UHFFFAOYSA-N 0.000 description 1
- 101800001982 Cholecystokinin Proteins 0.000 description 1
- 102100025841 Cholecystokinin Human genes 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- 102000015771 Ciliary Neurotrophic Factor Receptor alpha Subunit Human genes 0.000 description 1
- 108010010079 Ciliary Neurotrophic Factor Receptor alpha Subunit Proteins 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000031288 Combined hyperlipidaemia Diseases 0.000 description 1
- 101150073133 Cpt1a gene Proteins 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- 241000484025 Cuniculus Species 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- KDXKERNSBIXSRK-RXMQYKEDSA-N D-lysine Chemical compound NCCCC[C@@H](N)C(O)=O KDXKERNSBIXSRK-RXMQYKEDSA-N 0.000 description 1
- JVHXJTBJCFBINQ-ADAARDCZSA-N Dapagliflozin Chemical compound C1=CC(OCC)=CC=C1CC1=CC([C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)=CC=C1Cl JVHXJTBJCFBINQ-ADAARDCZSA-N 0.000 description 1
- URRAHSMDPCMOTH-LNLFQRSKSA-N Denagliptin Chemical compound C=1C=C(F)C=CC=1C([C@H](N)C(=O)N1[C@@H](C[C@H](F)C1)C#N)C1=CC=C(F)C=C1 URRAHSMDPCMOTH-LNLFQRSKSA-N 0.000 description 1
- 108010067722 Dipeptidyl Peptidase 4 Proteins 0.000 description 1
- 102100025012 Dipeptidyl peptidase 4 Human genes 0.000 description 1
- 108090000194 Dipeptidyl-peptidases and tripeptidyl-peptidases Proteins 0.000 description 1
- 102000003779 Dipeptidyl-peptidases and tripeptidyl-peptidases Human genes 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- UPEZCKBFRMILAV-JNEQICEOSA-N Ecdysone Natural products O=C1[C@H]2[C@@](C)([C@@H]3C([C@@]4(O)[C@@](C)([C@H]([C@H]([C@@H](O)CCC(O)(C)C)C)CC4)CC3)=C1)C[C@H](O)[C@H](O)C2 UPEZCKBFRMILAV-JNEQICEOSA-N 0.000 description 1
- 241000206602 Eukaryota Species 0.000 description 1
- 208000004930 Fatty Liver Diseases 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- 108010022355 Fibroins Proteins 0.000 description 1
- 239000001828 Gelatine Substances 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 101800000224 Glucagon-like peptide 1 Proteins 0.000 description 1
- FAEKWTJYAYMJKF-QHCPKHFHSA-N GlucoNorm Chemical compound C1=C(C(O)=O)C(OCC)=CC(CC(=O)N[C@@H](CC(C)C)C=2C(=CC=CC=2)N2CCCCC2)=C1 FAEKWTJYAYMJKF-QHCPKHFHSA-N 0.000 description 1
- 102000030595 Glucokinase Human genes 0.000 description 1
- 108010021582 Glucokinase Proteins 0.000 description 1
- 229920002527 Glycogen Polymers 0.000 description 1
- 108010023302 HDL Cholesterol Proteins 0.000 description 1
- 108010010234 HDL Lipoproteins Proteins 0.000 description 1
- 102000015779 HDL Lipoproteins Human genes 0.000 description 1
- 206010019708 Hepatic steatosis Diseases 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 108091016366 Histone-lysine N-methyltransferase EHMT1 Proteins 0.000 description 1
- 101000783681 Homo sapiens 5'-AMP-activated protein kinase catalytic subunit alpha-2 Proteins 0.000 description 1
- 101001050288 Homo sapiens Transcription factor Jun Proteins 0.000 description 1
- 108010090613 Human Regular Insulin Proteins 0.000 description 1
- 102000013266 Human Regular Insulin Human genes 0.000 description 1
- WOBHKFSMXKNTIM-UHFFFAOYSA-N Hydroxyethyl methacrylate Chemical compound CC(=C)C(=O)OCCO WOBHKFSMXKNTIM-UHFFFAOYSA-N 0.000 description 1
- 208000035150 Hypercholesterolemia Diseases 0.000 description 1
- 206010020606 Hyperchylomicronaemia Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 208000013016 Hypoglycemia Diseases 0.000 description 1
- 238000012404 In vitro experiment Methods 0.000 description 1
- 108010073961 Insulin Aspart Proteins 0.000 description 1
- 108010057186 Insulin Glargine Proteins 0.000 description 1
- COCFEDIXXNGUNL-RFKWWTKHSA-N Insulin glargine Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3NC=NC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(=O)NCC(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 COCFEDIXXNGUNL-RFKWWTKHSA-N 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000011845 Iodide peroxidase Human genes 0.000 description 1
- 108010036012 Iodide peroxidase Proteins 0.000 description 1
- 102000004310 Ion Channels Human genes 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- AGPKZVBTJJNPAG-UHNVWZDZSA-N L-allo-Isoleucine Chemical compound CC[C@@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-UHNVWZDZSA-N 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- 108010028554 LDL Cholesterol Proteins 0.000 description 1
- 238000008214 LDL Cholesterol Methods 0.000 description 1
- 108010018112 LY 315902 Proteins 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 102000004882 Lipase Human genes 0.000 description 1
- 108090001060 Lipase Proteins 0.000 description 1
- 239000004367 Lipase Substances 0.000 description 1
- 229940127470 Lipase Inhibitors Drugs 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102000001796 Melanocortin 4 receptors Human genes 0.000 description 1
- 101800001751 Melanocyte-stimulating hormone alpha Proteins 0.000 description 1
- 101710151321 Melanostatin Proteins 0.000 description 1
- 102000003792 Metallothionein Human genes 0.000 description 1
- 108090000157 Metallothionein Proteins 0.000 description 1
- IBAQFPQHRJAVAV-ULAWRXDQSA-N Miglitol Chemical compound OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO IBAQFPQHRJAVAV-ULAWRXDQSA-N 0.000 description 1
- 102100029820 Mitochondrial brown fat uncoupling protein 1 Human genes 0.000 description 1
- 108050002686 Mitochondrial brown fat uncoupling protein 1 Proteins 0.000 description 1
- GXCLVBGFBYZDAG-UHFFFAOYSA-N N-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amine Chemical compound CN(CCC1=CNC2=C1C=CC=C2)CC=C GXCLVBGFBYZDAG-UHFFFAOYSA-N 0.000 description 1
- 150000001200 N-acyl ethanolamides Chemical class 0.000 description 1
- JVEUTCWLEJSEDI-JHJMLUEUSA-N N[C@H](CCCC(N)C(=O)OCc1ccccc1)C(O)=O Chemical compound N[C@H](CCCC(N)C(=O)OCc1ccccc1)C(O)=O JVEUTCWLEJSEDI-JHJMLUEUSA-N 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 102400000064 Neuropeptide Y Human genes 0.000 description 1
- 108010015847 Non-Receptor Type 1 Protein Tyrosine Phosphatase Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 208000031964 Other metabolic disease Diseases 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 206010034759 Petit mal epilepsy Diseases 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 108010069820 Pro-Opiomelanocortin Proteins 0.000 description 1
- 239000000683 Pro-Opiomelanocortin Substances 0.000 description 1
- 102100040918 Pro-glucagon Human genes 0.000 description 1
- 108010076181 Proinsulin Proteins 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- 241000714474 Rous sarcoma virus Species 0.000 description 1
- 108091006277 SLC5A1 Proteins 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 108010086019 Secretin Proteins 0.000 description 1
- 102100037505 Secretin Human genes 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 102000058090 Sodium-Glucose Transporter 1 Human genes 0.000 description 1
- 229940123518 Sodium/glucose cotransporter 2 inhibitor Drugs 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 102000005157 Somatostatin Human genes 0.000 description 1
- 108010056088 Somatostatin Proteins 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229940100389 Sulfonylurea Drugs 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- JLRGJRBPOGGCBT-UHFFFAOYSA-N Tolbutamide Chemical compound CCCCNC(=O)NS(=O)(=O)C1=CC=C(C)C=C1 JLRGJRBPOGGCBT-UHFFFAOYSA-N 0.000 description 1
- 102100023132 Transcription factor Jun Human genes 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102100040247 Tumor necrosis factor Human genes 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 108010021436 Type 4 Melanocortin Receptor Proteins 0.000 description 1
- 102100033001 Tyrosine-protein phosphatase non-receptor type 1 Human genes 0.000 description 1
- 239000004699 Ultra-high molecular weight polyethylene Substances 0.000 description 1
- 101710137651 Vasoactive intestinal polypeptide receptor 2 Proteins 0.000 description 1
- 102100038286 Vasoactive intestinal polypeptide receptor 2 Human genes 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- FZNCGRZWXLXZSZ-CIQUZCHMSA-N Voglibose Chemical compound OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O FZNCGRZWXLXZSZ-CIQUZCHMSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 229960002632 acarbose Drugs 0.000 description 1
- XUFXOAAUWZOOIT-UHFFFAOYSA-N acarviostatin I01 Natural products OC1C(O)C(NC2C(C(O)C(O)C(CO)=C2)O)C(C)OC1OC(C(C1O)O)C(CO)OC1OC1C(CO)OC(O)C(O)C1O XUFXOAAUWZOOIT-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 210000000593 adipose tissue white Anatomy 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- ZSBOMTDTBDDKMP-OAHLLOKOSA-N alogliptin Chemical compound C=1C=CC=C(C#N)C=1CN1C(=O)N(C)C(=O)C=C1N1CCC[C@@H](N)C1 ZSBOMTDTBDDKMP-OAHLLOKOSA-N 0.000 description 1
- 239000003888 alpha glucosidase inhibitor Substances 0.000 description 1
- UPEZCKBFRMILAV-UHFFFAOYSA-N alpha-Ecdysone Natural products C1C(O)C(O)CC2(C)C(CCC3(C(C(C(O)CCC(C)(C)O)C)CCC33O)C)C3=CC(=O)C21 UPEZCKBFRMILAV-UHFFFAOYSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 229950000562 amlintide Drugs 0.000 description 1
- PLOPBXQQPZYQFA-AXPWDRQUSA-N amlintide Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H]1NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)CSSC1)[C@@H](C)O)C(C)C)C1=CC=CC=C1 PLOPBXQQPZYQFA-AXPWDRQUSA-N 0.000 description 1
- 229940025084 amphetamine Drugs 0.000 description 1
- 230000003444 anaesthetic effect Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 230000001430 anti-depressive effect Effects 0.000 description 1
- 239000000935 antidepressant agent Substances 0.000 description 1
- 229940005513 antidepressants Drugs 0.000 description 1
- 229940112930 apidra Drugs 0.000 description 1
- RCHHVVGSTHAVPF-ZPHPLDECSA-N apidra Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3N=CNC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CNC=N1 RCHHVVGSTHAVPF-ZPHPLDECSA-N 0.000 description 1
- 235000021229 appetite regulation Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 210000000227 basophil cell of anterior lobe of hypophysis Anatomy 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 150000004283 biguanides Chemical class 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- OZVBMTJYIDMWIL-AYFBDAFISA-N bromocriptine Chemical compound C1=CC(C=2[C@H](N(C)C[C@@H](C=2)C(=O)N[C@]2(C(=O)N3[C@H](C(N4CCC[C@H]4[C@]3(O)O2)=O)CC(C)C)C(C)C)C2)=C3C2=C(Br)NC3=C1 OZVBMTJYIDMWIL-AYFBDAFISA-N 0.000 description 1
- 229960002802 bromocriptine Drugs 0.000 description 1
- 229960004111 buformin Drugs 0.000 description 1
- XSEUMFJMFFMCIU-UHFFFAOYSA-N buformin Chemical compound CCCC\N=C(/N)N=C(N)N XSEUMFJMFFMCIU-UHFFFAOYSA-N 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- BBBFJLBPOGFECG-VJVYQDLKSA-N calcitonin Chemical compound N([C@H](C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(N)=O)C(C)C)C(=O)[C@@H]1CSSC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 BBBFJLBPOGFECG-VJVYQDLKSA-N 0.000 description 1
- 229960003773 calcitonin (salmon synthetic) Drugs 0.000 description 1
- 235000019577 caloric intake Nutrition 0.000 description 1
- 239000003554 cannabinoid 1 receptor agonist Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 210000004413 cardiac myocyte Anatomy 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 241000902900 cellular organisms Species 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 229960001761 chlorpropamide Drugs 0.000 description 1
- 229940107137 cholecystokinin Drugs 0.000 description 1
- 210000001612 chondrocyte Anatomy 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- PMMYEEVYMWASQN-IMJSIDKUSA-N cis-4-Hydroxy-L-proline Chemical compound O[C@@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-IMJSIDKUSA-N 0.000 description 1
- 229960003920 cocaine Drugs 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 229960005188 collagen Drugs 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 210000001608 connective tissue cell Anatomy 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 102000003675 cytokine receptors Human genes 0.000 description 1
- 108010057085 cytokine receptors Proteins 0.000 description 1
- 229960003834 dapagliflozin Drugs 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 229950010300 denagliptin Drugs 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000003210 dopamine receptor blocking agent Substances 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- UPEZCKBFRMILAV-JMZLNJERSA-N ecdysone Chemical compound C1[C@@H](O)[C@@H](O)C[C@]2(C)[C@@H](CC[C@@]3([C@@H]([C@@H]([C@H](O)CCC(C)(C)O)C)CC[C@]33O)C)C3=CC(=O)[C@@H]21 UPEZCKBFRMILAV-JMZLNJERSA-N 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 230000008918 emotional behaviour Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000002621 endocannabinoid Substances 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 201000011110 familial lipoprotein lipase deficiency Diseases 0.000 description 1
- 239000003188 fatty acid synthesis inhibitor Substances 0.000 description 1
- 208000010706 fatty liver disease Diseases 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 230000003328 fibroblastic effect Effects 0.000 description 1
- 235000013312 flour Nutrition 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000012631 food intake Nutrition 0.000 description 1
- AIWAEWBZDJARBJ-PXUUZXDZSA-N fz7co35x2s Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCNC(=O)COCCOCCNC(=O)CCN1C(C=CC1=O)=O)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 AIWAEWBZDJARBJ-PXUUZXDZSA-N 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 229960004346 glimepiride Drugs 0.000 description 1
- WIGIZIANZCJQQY-RUCARUNLSA-N glimepiride Chemical compound O=C1C(CC)=C(C)CN1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)N[C@@H]2CC[C@@H](C)CC2)C=C1 WIGIZIANZCJQQY-RUCARUNLSA-N 0.000 description 1
- 229960001381 glipizide Drugs 0.000 description 1
- ZJJXGWJIGJFDTL-UHFFFAOYSA-N glipizide Chemical compound C1=NC(C)=CN=C1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)NC2CCCCC2)C=C1 ZJJXGWJIGJFDTL-UHFFFAOYSA-N 0.000 description 1
- 229960003468 gliquidone Drugs 0.000 description 1
- 108010063245 glucagon-like peptide 1 (7-36)amide Proteins 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 229940096919 glycogen Drugs 0.000 description 1
- 108091005608 glycosylated proteins Proteins 0.000 description 1
- 102000035122 glycosylated proteins Human genes 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 210000001624 hip Anatomy 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 229960002885 histidine Drugs 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940103471 humulin Drugs 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 208000020346 hyperlipoproteinemia Diseases 0.000 description 1
- 208000006575 hypertriglyceridemia Diseases 0.000 description 1
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229960004717 insulin aspart Drugs 0.000 description 1
- 229960003948 insulin detemir Drugs 0.000 description 1
- 229960002869 insulin glargine Drugs 0.000 description 1
- 108700039926 insulin glulisine Proteins 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- MDXYQVPFSHFPHS-CVYXXLPWSA-N irp peptide Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)C(C)C)NC(=O)[C@H](CCC(=O)OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(=O)OC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)NC(=O)[C@@H](N)CCCCN)C1=CC=CC=C1 MDXYQVPFSHFPHS-CVYXXLPWSA-N 0.000 description 1
- 210000004153 islets of langerhan Anatomy 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- 230000000155 isotopic effect Effects 0.000 description 1
- 210000001630 jejunum Anatomy 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 229940102988 levemir Drugs 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 235000019421 lipase Nutrition 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000037323 metabolic rate Effects 0.000 description 1
- XZWYZXLIPXDOLR-UHFFFAOYSA-N metformin Chemical compound CN(C)C(=N)NC(N)=N XZWYZXLIPXDOLR-UHFFFAOYSA-N 0.000 description 1
- 229960003105 metformin Drugs 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 229960001110 miglitol Drugs 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 229960003365 mitiglinide Drugs 0.000 description 1
- WPGGHFDDFPHPOB-BBWFWOEESA-N mitiglinide Chemical compound C([C@@H](CC(=O)N1C[C@@H]2CCCC[C@@H]2C1)C(=O)O)C1=CC=CC=C1 WPGGHFDDFPHPOB-BBWFWOEESA-N 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 230000003387 muscular Effects 0.000 description 1
- QNILTEGFHQSKFF-UHFFFAOYSA-N n-propan-2-ylprop-2-enamide Chemical compound CC(C)NC(=O)C=C QNILTEGFHQSKFF-UHFFFAOYSA-N 0.000 description 1
- OELFLUMRDSZNSF-BRWVUGGUSA-N nateglinide Chemical compound C1C[C@@H](C(C)C)CC[C@@H]1C(=O)N[C@@H](C(O)=O)CC1=CC=CC=C1 OELFLUMRDSZNSF-BRWVUGGUSA-N 0.000 description 1
- 229960000698 nateglinide Drugs 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 239000002858 neurotransmitter agent Substances 0.000 description 1
- 229960002748 norepinephrine Drugs 0.000 description 1
- SFLSHLFXELFNJZ-UHFFFAOYSA-N norepinephrine Natural products NCC(O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-UHFFFAOYSA-N 0.000 description 1
- VOMXSOIBEJBQNF-UTTRGDHVSA-N novorapid Chemical compound C([C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@H](CO)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O)C1=CC=C(O)C=C1.C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CS)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 VOMXSOIBEJBQNF-UTTRGDHVSA-N 0.000 description 1
- URPYMXQQVHTUDU-OFGSCBOVSA-N nucleopeptide y Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(N)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 URPYMXQQVHTUDU-OFGSCBOVSA-N 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 229960001243 orlistat Drugs 0.000 description 1
- 210000002997 osteoclast Anatomy 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 229960000292 pectin Drugs 0.000 description 1
- 210000004197 pelvis Anatomy 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 210000002824 peroxisome Anatomy 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- DCWXELXMIBXGTH-QMMMGPOBSA-N phosphonotyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(OP(O)(O)=O)C=C1 DCWXELXMIBXGTH-QMMMGPOBSA-N 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 229960005095 pioglitazone Drugs 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 229920000724 poly(L-arginine) polymer Polymers 0.000 description 1
- 229920000729 poly(L-lysine) polymer Polymers 0.000 description 1
- 229920000962 poly(amidoamine) Polymers 0.000 description 1
- 229920001308 poly(aminoacid) Polymers 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 108010060272 poly-S-benzylcysteine Proteins 0.000 description 1
- 108700024573 poly-gamma-benzyl-L-glutamate Proteins 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 108010094020 polyglycine Proteins 0.000 description 1
- 229920000232 polyglycine polymer Polymers 0.000 description 1
- 229920002338 polyhydroxyethylmethacrylate Polymers 0.000 description 1
- 108010050934 polyleucine Proteins 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 108010055896 polyornithine Proteins 0.000 description 1
- 229920001299 polypropylene fumarate Polymers 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229920000128 polypyrrole Polymers 0.000 description 1
- 108010000222 polyserine Proteins 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000000291 postprandial effect Effects 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 229960003611 pramlintide Drugs 0.000 description 1
- 108010029667 pramlintide Proteins 0.000 description 1
- NRKVKVQDUCJPIZ-MKAGXXMWSA-N pramlintide acetate Chemical compound C([C@@H](C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CCCCN)[C@@H](C)O)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 NRKVKVQDUCJPIZ-MKAGXXMWSA-N 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- GCYXWQUSHADNBF-AAEALURTSA-N preproglucagon 78-108 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 GCYXWQUSHADNBF-AAEALURTSA-N 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 229960002429 proline Drugs 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000003127 radioimmunoassay Methods 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 231100000272 reduced body weight Toxicity 0.000 description 1
- 229960002354 repaglinide Drugs 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- JZCPYUJPEARBJL-UHFFFAOYSA-N rimonabant Chemical compound CC=1C(C(=O)NN2CCCCC2)=NN(C=2C(=CC(Cl)=CC=2)Cl)C=1C1=CC=C(Cl)C=C1 JZCPYUJPEARBJL-UHFFFAOYSA-N 0.000 description 1
- 229960003015 rimonabant Drugs 0.000 description 1
- 229960004586 rosiglitazone Drugs 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 108010068072 salmon calcitonin Proteins 0.000 description 1
- QGJUIPDUBHWZPV-SGTAVMJGSA-N saxagliptin Chemical compound C1C(C2)CC(C3)CC2(O)CC13[C@H](N)C(=O)N1[C@H](C#N)C[C@@H]2C[C@@H]21 QGJUIPDUBHWZPV-SGTAVMJGSA-N 0.000 description 1
- 229960004937 saxagliptin Drugs 0.000 description 1
- 108010033693 saxagliptin Proteins 0.000 description 1
- 229960002101 secretin Drugs 0.000 description 1
- OWMZNFCDEHGFEP-NFBCVYDUSA-N secretin human Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(N)=O)[C@@H](C)O)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)C1=CC=CC=C1 OWMZNFCDEHGFEP-NFBCVYDUSA-N 0.000 description 1
- 230000000276 sedentary effect Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 229940126842 sergliflozin Drugs 0.000 description 1
- HFLCZNNDZKKXCS-OUUBHVDSSA-N sergliflozin Chemical compound C1=CC(OC)=CC=C1CC1=CC=CC=C1O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HFLCZNNDZKKXCS-OUUBHVDSSA-N 0.000 description 1
- 229960001153 serine Drugs 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- UNAANXDKBXWMLN-UHFFFAOYSA-N sibutramine Chemical compound C=1C=C(Cl)C=CC=1C1(C(N(C)C)CC(C)C)CCC1 UNAANXDKBXWMLN-UHFFFAOYSA-N 0.000 description 1
- 229960004425 sibutramine Drugs 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- IZTQOLKUZKXIRV-YRVFCXMDSA-N sincalide Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)NCC(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](N)CC(O)=O)C1=CC=C(OS(O)(=O)=O)C=C1 IZTQOLKUZKXIRV-YRVFCXMDSA-N 0.000 description 1
- 229960004034 sitagliptin Drugs 0.000 description 1
- MFFMDFFZMYYVKS-SECBINFHSA-N sitagliptin Chemical compound C([C@H](CC(=O)N1CC=2N(C(=NN=2)C(F)(F)F)CC1)N)C1=CC(F)=C(F)C=C1F MFFMDFFZMYYVKS-SECBINFHSA-N 0.000 description 1
- 235000020183 skimmed milk Nutrition 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- RYYKJJJTJZKILX-UHFFFAOYSA-M sodium octadecanoate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC([O-])=O RYYKJJJTJZKILX-UHFFFAOYSA-M 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- NHXLMOGPVYXJNR-ATOGVRKGSA-N somatostatin Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CSSC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(=O)N1)[C@@H](C)O)NC(=O)CNC(=O)[C@H](C)N)C(O)=O)=O)[C@H](O)C)C1=CC=CC=C1 NHXLMOGPVYXJNR-ATOGVRKGSA-N 0.000 description 1
- 229960000553 somatostatin Drugs 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 229940032147 starch Drugs 0.000 description 1
- 231100000240 steatosis hepatitis Toxicity 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- WRGVLTAWMNZWGT-VQSPYGJZSA-N taspoglutide Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NC(C)(C)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 WRGVLTAWMNZWGT-VQSPYGJZSA-N 0.000 description 1
- 229950007151 taspoglutide Drugs 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 229960002898 threonine Drugs 0.000 description 1
- 102000004217 thyroid hormone receptors Human genes 0.000 description 1
- 108090000721 thyroid hormone receptors Proteins 0.000 description 1
- 229960005371 tolbutamide Drugs 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N tryptophan Chemical compound C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 210000004026 tunica intima Anatomy 0.000 description 1
- 238000007492 two-way ANOVA Methods 0.000 description 1
- 229920000785 ultra high molecular weight polyethylene Polymers 0.000 description 1
- 210000003954 umbilical cord Anatomy 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- SYOKIDBDQMKNDQ-XWTIBIIYSA-N vildagliptin Chemical compound C1C(O)(C2)CC(C3)CC1CC32NCC(=O)N1CCC[C@H]1C#N SYOKIDBDQMKNDQ-XWTIBIIYSA-N 0.000 description 1
- 229960001254 vildagliptin Drugs 0.000 description 1
- 229960001729 voglibose Drugs 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 238000004260 weight control Methods 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000013585 weight reducing agent Substances 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229940002552 xenical Drugs 0.000 description 1
- WHNFPRLDDSXQCL-UAZQEYIDSA-N α-msh Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(N)=O)NC(=O)[C@H](CO)NC(C)=O)C1=CC=C(O)C=C1 WHNFPRLDDSXQCL-UAZQEYIDSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/20—Interleukins [IL]
- A61K38/204—IL-6
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/28—Insulins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
Definitions
- the invention is related to the use of cardiotrophin-1 (CT-1) for the treatment of obesity and associated disorders: hyperglycaemias, insulin resistance, development of type 2 diabetes and dyslipemias and given its anorexigenic role, fat oxidation stimulant, hypoglycaemic, sensitizing agent of the action of insulin on a skeletal muscle level and inhibitor of the intestinal transport of glucose by enterocytes.
- CT-1 cardiotrophin-1
- Obesity is a serious public-health problem which has reached epidemic proportions in many developed countries ( Bellanger and Bray, 2005, J The State Med Soc;157:S42-49 ; quiz 49).
- the increased food intake, unhealthy diet habits, and sedentary lifestyle in our developed countries have undoubtedly contributed to the obesity boom ( Stein and Colditz et al., 2004, J. Clin. Endocrinol. Metab. 89:2522-5 ).
- Many studies have shown that the alarming increase in the prevalence of obesity and its metabolic disorders is associated to insulin resistance, dyslipemias, and eventual failure of the beta cells in the pancreas, leading inexorably to type 2 diabetes with all the consequences this disease entails ( Rana et al., 2007, Diabetes Obes. Metab., 9:218-232 ).
- Xenical is an inhibitor of gastrointestinal lipase which produces a moderate weight loss but has the main problem of adverse gastrointestinal effects, including colon cancer.
- Sibutramine is a monoamine-capturing inhibitor which produces a greater weight loss than the previous treatment, but is associated to an increase in blood pressure and an increase in cardiac output.
- Rimonabant which is approved in Europe (though not by the FDA), and is an endocannabinoid receptor antagonist, but which has the problem of causing alterations in emotional behaviour such as depression, for which reason it has been withdrawn from the market.
- thiazolidinediones are used in combination with other therapies.
- the problem with these drugs is weight gain, especially when administered together with insulin. From all of this it can be gathered that an agent capable of sensitizing or increasing insulin action in addition to reducing body weight may be of great interest in these pathologies.
- treatments with peripheral actions such as intestinal glucose inhibition could be of great interest in obesity and diabetes mellitus.
- leptin at the end of 1994 opened up new horizons in the study of the factors secreted by the adipocyte in regulating energy balance.
- This protein is mainly produced and secreted by the adipocyte proportionally to the fat mass.
- This protein was identified as a central nervous system modulator for appetite regulation, in addition to peripheral actions increasing fat oxidation and glucose capture by muscles.
- obesity was related to high leptin levels and that endogenous leptin was not effective, for which reason the majority of obese humans and rodents were leptin-resistant, therefore this therapeutic weapon would be limited to individuals with disorders that result in leptin deficiency (a low percentage of obese people).
- leptin is structurally similar to the cytokines of the gp-130 family and the leptin receptor (LRb) is structurally similar to the gp130 cytokine receptor known as gp-130Rbeta
- LRb leptin receptor
- CNTF gp-130Rbeta
- the CNTF cytokine has a possible therapeutic potential in mice suffering from obesity, insulin resistance, and a fatty liver, as it improves all these parameters ( Sleeman et al., 2003, Proc Natl Acad. Sci. USA, 100:14297-14302 ).
- the anorexigenic role of CNTF (Stephens TW et al., 1995), the capacity of this cytokine to revert insulin resistance through the AMPK activation capacity ( Watt et al., 2006, Nat.
- the invention is related to a compound that induces cardiotrophin 1 (CT-1) activity for the treatment of a metabolic disease, as well as to the use of a compound that induces cardiotrophin 1 (CT-1) activity for the preparation of a drug for the treatment of a metabolic disease according to the claims.
- CT-1 cardiotrophin 1
- the invention is related to cosmetic methods which comprise administering the patient a pharmaceutically active quantity of a compound that induces cardiotrophin 1 activity according to the claims.
- the invention is related to a composition that comprises, together or separately, a compound that induces cardiotrophin activity and an anti-diabetic compound.
- the invention is related to a composition in accordance with the invention for the treatment of a metabolic disease, wherein the composition is administered simultaneously, separately or sequentially, as well as to the use of a composition in accordance with the invention for the preparation of a drug for the treatment of a metabolic disease, wherein the composition is administered simultaneously, separately or sequentially.
- the invention is related to cosmetics methods that comprise administering the patient a pharmaceutically active quantity of a composition in accordance with the invention.
- CT-1 is capable of causing weight reduction in mice fed a normal diet as well as in mice fed a high-fat diet. Said effect depends, at least in part, on the anorexigenic effect of CT-1 ( figure 2 ) as well as on the capacity of CT-1 of decreasing the increase in plasma lipids following a high-fat diet ( figures 20 and 21 ), of decreasing blood sugar after a high sugar diet ( figures 5-6 ), of causing an increase in glucose consumption by muscles ( figure 8 ) and of decreasing glucose capture by the intestine ( figures 11-14 ).
- CT-1 is able to inhibit basal and insulin-stimulated leptin secretion in adipocytes, as well as to induce the release of glycerol and the inhibition of the anti-lipolytic activity of insulin ( figures 16 and 17 ).
- these effects are not observed when CNTF is used. While not adhering to any particular theory, it is thought that this differential effect might give the use of CT-1 an advantage, since said effect would be added to the action that this molecule has on muscle (stimulation of glucose capture with the consequent hypoglycaemia), causing a decrease in the glucose available for the adipocyte to turn into triglycerides.
- the invention is related to a compound that induces cardiotrophin 1 (CT-1) activity for the treatment of a metabolic disease. Additionally, the invention is related to the use of a compound that induces cardiotrophin-1 activity for the preparation of a drug for the treatment of a metabolic disease as well as with a method of treatment of a metabolic disease which comprises administration to a subject of a compound that induces CT-1 activity.
- CT-1 cardiotrophin 1
- the term "compound that induces cardiotrophin-1 activity”, as used here, is understood as any compound administration of which causes an increase in cardiotrophin-1 activity (CT-1) irrespective of whether said increase is caused by an increase in the specific activity of the pre-existing CT-1 or by an increase in the synthesis of CT-1 or analogs thereof that substantially share the same function as cardiotrophin.
- CT-1 cardiotrophin-1 activity
- the agent that induces CT-1 activity is CT-1 itself.
- CT-1 is understood as the protein defined by the sequence described in the UniProtKB database by access number CTF1_HUMAN or Q16619 in version 62 dated 16-DEC-2008, corresponding to isoform 1 of human cardiotrophin, or access number Q5U5Y7 in its version 1 dated 20.01.2009, corresponding to isoform 2 of human cardiotrophin, as well as orthologues in other species, such as mouse (sequences defined in the UniProtKB database with access numbers NP_031821 or Q60753 in version 50 dated 16.12.2008, and NP_942155 or P83714 in version 42 dated 16.12.2008), rat (sequences defined in the UniProtKB database with access numbers NP_058825 or Q63086 in version 50 dated 04.11.2008, and NP_001129272 or Q6R2R3 in version 30 dated 20.01.2009) and chimpanzee (sequence defined in the UniProtKB database with access number NP_00100
- the agent that induces CT-1 activity is a functionally equivalent variant of CT-1.
- the expression "functionally equivalent variant of CT-1", as used here, is understood as any molecule which shares with CT-1 one or more of the functions described in the present invention associated to CT-1, both in vitro and in vivo and which has a minimum of 90% identity in the amino acid sequence.
- variants of CT-1 suitable for their use in the present invention are derived from the aforementioned sequences by insertion, substitution or deletion of one or more amino acids and include natural alleles, variants resulting from alternative processing and secreted and truncated forms which appear naturally.
- the variants of CT-1 show an identity of sequence with CT-1 of at least 90%, at least 91%, at least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at least 97%, at least 98% or at least 99%.
- the degree of identity is determined using methods very well known to those skilled in the art.
- the identity between two amino acid sequences is preferably determined using the BLASTP algorithm [ BLASTManual, Altschul, S., et al., NCBI NLM NIH Bethesda, Md. 20894 , Altschul, S., et al., J. Mol. Biol. 215: 403-410 (1990 )] preferably using the default parameters.
- the variants of CT-1 envisaged show at least one of the functions of CT-1 such as, without limitation:
- the agent capable of inducing CT-1 activity is a polynucleotide that codes for CT-1 or a functionally equivalent variant thereof.
- polynucleotide as used in the present invention, relates to a polymeric form of nucleotides of any length and formed by ribonucleotides and/or deoxyribonucleotides.
- the term includes both single-chain and double-chain polynucleotides, as well as modified polynucleotides (methylated, protected and such like).
- Polynucleotides suitable for use as agents capable of inducing CT-1 activity include, without limitation, the polynucleotides the sequences of which correspond to those described in the GenEMBL database with access numbers BC064416 in version 9 with date 15.10.2008, corresponding to variant 1 of the transcript of human cardiotrophin, BC036787 in version 9 with date 12.08.2009, corresponding to variant 1 of the transcript of human cardiotrophin, the sequence described in the GenEMBL database with access number D78591, in its version 4 of 12.01.2009, corresponding to the polynucleotide that codes for cardiotrophin 1 of Rattus norvegicus (rat), the sequence described in the GenEMBL database with access number AY518205, in its version 3 of 12.01.2009, corresponding to the polynucleotide that codes for cardiotrophin 2 of Rattus norvegicus (rat), the sequence described in the GenEMBL database with access number U18366, in its version 4 of 12.01.2009, corresponding to the polynucleotide that
- the agents capable of inducing CT-1 activity include functionally equivalent variants of the polynucleotides previously defined by means of their specific sequences.
- “Functionally equivalent polynucleotide” is understood, in the context of the present invention, as all those polynucleotides capable of coding for a polypeptide with CT-1 activity, as previously defined, and which result from the aforementioned polynucleotides by means of the insertion, deletion or substitution of one or several nucleotides with respect to the aforementioned sequences.
- the variant polynucleotides of the present invention are polynucleotides the sequence of which allows them to hybridize in highly restrictive conditions with the aforementioned polynucleotides.
- Typical conditions of highly restrictive hybridization include incubation in 6 X SSC (1 X SSC: 0.15 M NaCl, 0.015 M sodium citrate) and 40% formamide at 42 °C for 141 hours, followed by one or several cycles of washing using 0.5 X SSC, 0.1% SDS at 60°C.
- highly restrictive conditions include those which comprise hybridization at an approximate temperature of 50°-55° C in 6X SSC and a washing final at a temperature of 68° C in 1-3 X SSC.
- the moderate restrictive conditions comprise hybridization at a temperature of approximately 50° C until 65° C in 0.2 or 0.3 M NaCl, followed by washing at approximately 50° C until 55° C in 0.2X SSC, 0.1% SDS (sodium dodecyl sulfate).
- the agent capable of inducing CT-1 activity is a polynucleotide
- this is operationally associated to a regulatory region of the expression.
- the regulatory sequences used in the present invention can be nuclear promoter sequences or, alternatively, enhancer sequences and/or other regulatory sequences that increase the expression of the heterologous nucleic acid sequence.
- the promoter can be constitutive or inducible. If one wants a constant expression of the heterologous nucleic acid sequence, then a constitutive promoter is used. Examples of well-known constitutive promoters include the cytomegalovirus (CMV) immediate-early promoter, Rous sarcoma virus promoter and such like.
- CMV cytomegalovirus
- constitutive promoters are well known in the state of the art and can be used in the practice of the invention. If the controlled expression of the heterologous nucleic acid sequence is desired, then an inducible promoter should be used. In a non-induced state, the inducible promoter is "silent". "Silent" means that in the absence of an inducer little or no expression of the heterologous nucleic acid sequence is detected; in the presence of an inducer, however, the expression occurs of the heterologous nucleic acid sequence. Frequently, it is possible to control the expression level by varying the inducer concentration.
- Controlling the expression for example varying the concentration of the inducer so that an inducible promoter is more strongly or more weakly stimulated, it is possible to affect the concentration of the transcript product of the heterologous nucleic acid sequence.
- the heterologous nucleic acid sequence codes for a gene, it is possible to control the quantity of synthesized protein. In this way, it is possible to vary the concentration of the therapeutic product.
- inducible promoters are: an estrogen or androgen promoter, a metallothionein promoter, or a promoter that responds to ecdysone. Many other examples are well known in the state of the art and can be used in the practice of the invention.
- tissue promoters can be used to achieve expression of the heterologous sequence of specific nucleic acid in cells or tissues.
- specific tissue promoters include different specific muscle promoters including: skeletal ⁇ -actin promoter, cardiac actin promoter, skeletal troponin C promoter, cardiac troponin C promoter and of slow contraction, and the creatine kinase promoter/enhancer.
- muscle promoters which are well known in the state of the art and which can be used in the practice of the invention (for a review of specific muscle promoters, see Miller et al., (1993) Bioessays 15: 191-196 ).
- the agent capable of inducing CT-1 activity is a vector that comprises a polynucleotide as previously defined, i.e. that codes for CT-1 or a functionally equivalent variant thereof.
- Suitable vectors for the insertion of said polynucleotides are vectors derived from expression vectors in prokaryotes such as pUC18, pUC19, pBluescript and their derivatives, mp18, mp19, pBR322, pMB9, ColE1, pCR1 , RP4, phages and "shuttle" vectors such as pSA3 and pAT28, yeast expression vectors such as vectors of the 2 micron plasmid type, integration plasmids, YEP vectors, centromeric plasmids and such like, expression vectors in insect cells such as the vectors of the pAC series and of the pVL series, expression vectors in plants such as vectors of the pIBI, pEarleyGate, pAV
- the agent capable of causing an increase in CT-1 activity is a cell capable of secreting cardiotrophin-1 or a functionally equivalent variant thereof into the medium.
- Suitable cells for the expression of cardiotrophin-1 or of the functionally equivalent variant thereof include, without limitation, cardiomyocytes, adipocytes, endothelial cells, epithelial cells, lymphocytes (B and T cells), mastocytes, eosinophils, cells of the vascular intima, primary culture of cells isolated from different organs, preferably from cells isolated from islets of Langerhans, hepatocytes, leukocytes, including mononuclear, mesenchymal, umbilical cord or adult leukocytes (from the skin, lungs, kidney and liver), osteoclasts, chondrocytes and other connective tissue cells.
- Established cell lines such as Jurkat t cells, NIH-3T3, CHO, Cos, VERO, BHK, HeLa, COS, MDCK, 293, 3T3 cells, C2
- Suitable materials for forming the microparticles that are an object of the invention include any biocompatible polymeric material that allows the continuous secretion of therapeutic products, acting as cell support.
- said biocompatible polymeric material can be, for example, thermoplastic polymers or hydrogel polymers.
- Thermoplastic polymers include acrylic acid, acrylamide, 2-aminoethyl methacrylate, poly(tetrafluoroethylene-cohexafluoropropylene), methacrylic-(7-cumaroxy) ethyl ester acid, N-isopropyl acrylamide, polyacrylic acid, polyacrylamide, polyamidoamine, poly(amino)-p-xylylene, poly(chloroethyl vinyl ether), polycaprolactone, poly(caprolactone-co-trimethylene carbonate), poly(carbonate urea) urethane, poly(carbonate) urethane, polyethylene, polyethylene copolymer and acrylamide, polyethylene glycol, polyethylene glycol methacrylate, poly(ethylene terephthalate), poly(4-hydroxybutyl acrylate), poly(hydroxyethyl methacrylate), poly(N-2-hydroxypropyl methacrylate), poly(lactic acid-glycolic acid), poly
- the polymers of hydrogel type include natural materials of alginate, agarose, collagen, starch, hyaluronic acid, bovine serum albumin, cellulose and its derivatives, pectin, chondroitin sulfate, fibrin and fibroin type as well as synthetic hydrogels such as sefarose and sefadex.
- the microparticle of the invention can be surrounded by a semi-permeable membrane which gives stability to the particles, forming a barrier impermeable to antibodies.
- Semipermeable membrane means a membrane that allows the entry of all those solutes necessary for cell viability and allows the exit of therapeutic proteins produced by the cells contained in the microparticle, but which is substantially impermeable to antibodies, so that the cells are protected from the immune response produced by the organism housing the microparticle.
- Suitable materials for forming the semipermeable membrane are materials insoluble in biological fluids, preferably polyamino acids, such as, for example poly-L-lysine, poly-L-ornithine, poly-L-arginine, poly-L-asparagine, poly-L-aspartic, poly benzyl-L-aspartate, poly-S-benzyl-L-cysteine, poly-gamma-benzyl-L-glutamate, poly-S-CBZ-L-cysteine, poly- ⁇ -CBZ-D-lysine, poly- ⁇ -CBZ-DL-ornithine, poly-O-CBZ-L-serine, poly-O-CBZ-D-thyrosine, poly( ⁇ -ethyl-L-glutamate), poly-D-glutamic, polyglycine, poly- ⁇ -N-hexyl L-glutamate, poly-L-histidine, poly( ⁇ ,
- metabolic disease is understood as all types of disorders that lead to errors and imbalances in the metabolism as well as to metabolic processes taking place in a sub-optimal form.
- the expression also relates to disorders that can be treated through metabolism modulation, although the disease in itself may not have been caused by a metabolic disorder.
- the metabolic disease is selected from the set of obesity, hyperglycaemias, insulin resistance, type 2 diabetes, and dyslipemias.
- the term "obesity”, as used in the present invention, relates to the definition of obesity provided by the WHO based on the body mass index (BMI), which consists of the ratio between the weight of a person (in kg) and the square of their height in metres.
- BMI body mass index
- a BMI lower than 18.5 kg/m 2 is considered as insufficient weight or thinness
- a BMI of 18.5-24.9 kg/m 2 is considered a normal weight
- a BMI of 25.0-29.9 kg/m 2 is considered grade 1 of overweight
- a BMI of 30.0-39.0 kg/m 2 is considered a grade 2 of overweight
- a BMI greater than or equal to 40.0 kg/m 2 is considered morbid obesity.
- an individual's degree of obesity such as the diameter of the waist measured at the midpoint between the lower limit of the ribs and the upper limit of the pelvis (in cm), the thickness of skin folds, and bioimpedance, based on the principle that a lean mass transmits electricity better than a fatty mass.
- hyperglycaemia relates to a state where abnormally high blood glucose levels appear in relation to the fasting baseline levels.
- hyperglycaemia is understood to take place when fasting blood glucose levels are consistently higher than 126 mg/dL, the postprandial glucose levels are higher than 140 mg/dL, and/or the glucose levels in venous plasma 2 hours after administration of a dose of glucose of 1.75 grams for each kilogram of body weight is over 200 mg/dL.
- insulin resistance relates to a disorder wherein the cells do not respond correctly to insulin. As a result, the body produces more insulin in response to high blood glucose levels. Patients with insulin resistance frequently display high glucose levels and high circulating insulin levels. Insulin resistance is frequently linked to obesity, hypertension, and hyperlipidemia. Additionally, insulin resistance frequently appears in patients with type 2 diabetes.
- type 2 diabetes relates to a disease characterized by an inappropriate increase in blood glucose levels, which generates chronic complications as it affects large and small vessels and nerves.
- the underlying disorder in this disease is the difficulty for insulin action (in the form of a loss of tissue sensitivity to this hormone), which is called insulin resistance, and an inadequate secretion of insulin by the cells responsible for their production in the pancreas.
- insulin resistance in the form of a loss of tissue sensitivity to this hormone
- insulin resistance an inadequate secretion of insulin by the cells responsible for their production in the pancreas.
- faulty insulin action frequently translates into an increase in cholesterol and/or triglyceride levels.
- Dyslipemia relates to any pathological condition characterized by a disorder in the lipid metabolism, with a consequent disorder in lipid concentration (cholesterol, triglycerides and such like) and lipoproteins (high density lipoproteins) in the blood.
- Dyslipemias that can be treated with the methods of the present invention include, without limitation, hypercholesterolemia, hypertriglyceridemia, hyperlipoproteinemia of type I, IIa, IIb, III, IV, V, hyperchylomicronemia, combined hyperlipidemia, etc.
- compositions of the invention are useful both for the treatment of morbid obesity and for the treatment of grade 1 or grade 2 of overweight, in which case the methods of the invention have a cosmetic purpose. Therefore, in another aspect, the invention is related to a cosmetic method for the treatment of obesity which comprises administering to a patient a compound that induces cardiotrophin 1 activity.
- the patients with excess weight in the form of fat and who can be treated by the cosmetic method of the present invention are identified visually or by having a BMI higher than or equal to 25 kg/m 2 , preferably between 25 and 30. These individuals are considered obese, and needing weight control for cosmetic reasons.
- the compounds of the invention can be administered both for acute and for chronic treatment.
- chronic administration as used in the present invention, relates to a method of administration wherein the compound is administered to the patient continuously for extended periods of time in order to maintain the therapeutic form during said period.
- forms of chronic administration include the administration of multiple doses of the compound daily, twice daily, thrice daily or with more or less frequency.
- Chronic administration can be carried out by means of different intravenous injections administered periodically throughout a single day.
- chronic administration involves the administration in the form of bolus or by continuous transfusion which can be carried out daily, every two days, every 3 to 15 days, every 10 days or more.
- chronic administration is maintained for at least 72 hours, at least 96 hours, at least 120 hours, at least 144 hours, at least 1 week, at least 2 weeks, at least 3 weeks, at least 4 weeks, at least 5 weeks, at least 6 weeks, at least 7 weeks, at least 8 weeks, at least 9 weeks, at least 10 weeks, at least 11 weeks, at least 12 weeks, at least 4 months, at least 5 months, at least 6 months, at least 9 months, at least one year, at least 2 years or more.
- acute administration relates to a method of administration wherein the patient is exposed to a single dose of the compound or several doses but over a reduced time period, such as, for example, 1, 2, 4, 6, 8, 12 or 24 hours or 2, 3, or 4 days.
- Therapeutically effective quantity is understood as the quantity of compound that makes it possible to totally or partially alleviate the symptoms associated with a metabolic disease or which prevents the progression or worsening the symptoms or which prevents the appearance of the disease in a subject at risk from suffering the disease.
- chronic administration of the compound of the invention can be administered in a sustained release composition such as that disclosed in documents US5672659 , US5595760 , US5821221 , US5916883 and WO9938536 . Otherwise, if acute administration is desired, a treatment with immediate release will be preferred. Irrespective of the type of administration, the quantity of the dose and the interval can be individually adjusted to provide plasma levels of the compounds sufficient for maintaining the therapeutic effect. Someone with normal experience in the state of the art will be capable of optimizing the therapeutically effective local doses without too much experimentation.
- compositions in the practice of the method of the invention include a therapeutically effective quantity of an active agent, and pharmaceutically acceptable carrier.
- pharmaceutically acceptable means approved by a regulatory agency of the Federal government or of a state or included in the USA Pharmacopoeia or another generally recognized pharmacopoeia, for use in animals, and more particularly in humans.
- carrier relates to a diluent, coadjuvent, excipient, or vehicle whereby the therapeutic compound is administered.
- Said pharmaceutical carriers can be sterile liquids, such as water and oils, including petroleum, animal or plant origin or synthetic oils, such a peanut oil, soy oil, mineral oil, sesame oil and such like.
- Suitable pharmaceutical excipients include starch, glucose, lactose, sucrose, gelatine, malt, rice, flour, chalk, silica gel, sodium stearate, glycerol monostearate, talc, sodium chloride, powdered skimmed milk, glycerol, propylene glycol, water, ethanol and such like.
- the composition if desired, can also contain smaller quantities of wetting or emulsifying agents, or pH buffering agents.
- compositions may take the form of solutions, suspensions, tablets, pills, capsules, powder, prolonged release formulations and such like.
- the composition may be formulated as a suppository, with binders and traditional carriers such as triglycerides.
- the oral formulation can include standard carriers such as pharmaceutical types of mannitol, lactose, starch, magnesium stearate, sodium saccharin, cellulose, magnesium carbonate, etc. Examples of suitable pharmaceutical carriers are described in "Remington's Pharmaceutical Sciences” by E.W. Martin.
- the composition can be formulated in accordance with routine procedures as a pharmaceutical composition adapted for intravenous, subcutaneous, or intramuscular administration to human beings.
- the composition may also include a solubilising agent and a local anaesthetic such as lidocaine to relieve pain at the injection site.
- a solubilising agent such as lidocaine to relieve pain at the injection site.
- a local anaesthetic such as lidocaine to relieve pain at the injection site.
- the composition When the composition is going to be administered by infiltration, it can be dispensed with an infiltration bottle which contains water or saline solution of pharmaceutical quality.
- a water vial can be provided for injection or sterile saline solution, so that the ingredients can be mixed before administration.
- the quantity of CT-1 activity inducing compound that will be effective in the treatment of metabolic disease can be determined by standard clinical techniques based on the present description. Furthermore, in vitro tests can also be optionally used to help identify optimum dosage ranges.
- the precise dose to use in the formulation will depend on the administration route, and the severity of the condition, and it should be decided at the doctor's judgement and depending on each patient's circumstances.
- the dosage ranges suitable for intravenous administration are generally 50-5000 micrograms of active compound per kilogram of body weight.
- the dosage ranges suitable for intranasal administration are generally approximately of 0.01 pg/kg of body weight to 1 mg/kg of body weight.
- the effective doses can be extrapolated from a pair of response curves to doses derived from model in vitro assay systems or in animals.
- a therapeutically effective dose can be initially estimated from in vitro assays.
- a dose can be formulated in animal models to achieve a circulating concentration range which includes the IC50 which has been determined in cell culture. Said information can be used to precisely determine useful doses in humans.
- the initial doses can also be estimated from in vivo data, e.g. animal models, using techniques well known in the state of the art. Someone with normal experience in the state of the art can easily optimize administration to humans based on the data in animals.
- the invention envisages the combined use of the agent capable of inducing CT-1 activity with one or several compounds capable of reducing the availability of nutrients in the organism, which have an anorexigenic effect or which have an anti-obesity effect.
- the metabolic disease treatment methods in accordance with the invention envisage the joint, sequential or separate administration of the compounds with capacity for inducing CT-1 activity with one or several compounds selected from the group of amylin, amylin agonists, calcitonin salmon, cholecystokinin or an agonist thereof, leptin (OB protein), exendin or an exendin analog, GLP1 or an agonist thereof, polypeptides (PYY) or agonists thereof, agents that affect neurotransmitters or neuronal ion channels such as anti-depressants, noradrenaline capture inhibitors, serotinin 2c receptor agonists, some dopaminergic antagonists, cannabinoid 1 receptor agonists, agents which modulate the route of leptin/insulin
- compositions of the invention are provided.
- figure 7 shows how the treatment of mice subjected to the experimental destruction of beta pancreatic cells with streptozotocin with a combination of CT-1 and insulin results in a hypoglycaemic effect higher than that observed after administration of each one of the components separately.
- figure 8 shows how the combination of CT-1 and insulin causes an increase in glucose capture by muscles higher than that observed with any of the compounds administered separately.
- the invention is related to a composition (hereinafter, composition of the invention) which comprises, together or separately, a compound that induces cardiotrophin activity and an anti-diabetic compound.
- anti-diabetic compound is understood as an agent which, irrespective of its action mechanism, is capable of at least partially compensating for the symptoms of diabetes, including hyperglycaemia.
- the anti-diabetic compound is a compound that induces insulin activity or a compound that induces hypoglycaemic activity of insulin or sensitizing agent to insulin activity.
- the anti-diabetic compounds capable of inducing insulin activity include, without limitation,
- insulin as used in the present invention, relates to insulin of any species (primate, rodent or rabbit), but preferably of human origin and more preferably insulin of native sequence, which comprises a polypeptide which has the same sequence of amino acids as the insulin derived from nature. Said insulin polypeptides of native sequence can be isolated from nature or can be produced by recombinant and/or synthetic medium.
- native sequence insulin specifically covers the truncated or secreted forms and allelic variants that occur naturally.
- the native sequence human insulin is a mature native sequence insulin or of a complete length which comprises an alpha or A chain, corresponding to amino acids 90 to 110 of the sequence of human preproinsulin (access number P01308 in the UniProtKB database in version 126 with date 20.01.2009) and a beta or B chain, corresponding to amino acids 25 to 54 (access number P01308 in the NCBI database in version 126 with date 20.01.2009).
- “Functionally equivalent variant of insulin” is understood as all those polypeptides resulting from the elimination, insertion or modification of at least one amino acid with respect to the insulin sequence and which substantially maintains the same properties as the insulin it comes from. Insulin activity can be determined by methods widely known by those skilled in the art such as normoglycaemic clamping or the measurement of glycosylated proteins in serum ( Bunn et al., Diabetes, 1981, 30:613-617 ).
- Functionally equivalent variants of insulin include, without limitation, the des-pentapeptide (B26-B30)-Phe B21 - ⁇ -carboxamide]insulin, Asp B10 insulin (disclosed in US4992417 ), Lys B28 -Pro B29 insulin Lys B28 -Pro B29 and the hexameric variant thereof (disclosed in US5474978 and US5514646 ), formulations of insulin and protamine ( US5650486 ), acylated Lys B28 -Pro B29 insulin ( US5922675 ), and compositions of stabilized insulin such as those disclosed in US5952297 , US6034054 and US6211144 , superactive analogs of insulin, monomeric insulins, hepatospecific insulins, insulin lispro (Humalog®), insulin lispro formulated with insulin lispro protamine (marketed as Humalog ®50/50 TM , Humalog® 75/25 TM ), NPH insulin or insulin isophan
- functionally equivalent variants of insulin include polypeptides that show at least approximately 80% of identity of amino acid sequence with the sequence of amino acids of: (a) residues 1 to 21 of the high-fat diet A chain in combination with residues 1 to 30 of the high-fat diet B chain, or (b) another fragment specifically derived from said sequences.
- the secondary structure of the insulin maintained by disulfide bonds between cysteine residues A6-Al1, A7-B7 and A20-B 19 seems necessary for the activity, for which reason the functionally equivalent variants preferably preserve said secondary structure as much as possible.
- Functionally equivalent variants of insulin include, for example, the polypeptides wherein one or more amino acid residues are added, or deleted, at the N- and/or C-terminal ends, as well as within one or more of the internal domains, of the A and B chains of insulin.
- a polypeptide variant of insulin will have at least approximately an identity of amino acids sequence with the sequence formed by residues 1 to 21 of the A chain of the high-fat diet in combination with residues 1 to 30 of the B chain of the high-fat diet or with another fragment specifically derived from said sequences of amino acids of at least 80%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98% or 99% of identity of amino acids sequence.
- polypeptide variants will have a sequence length of at least 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 or 35 of the A chain and at least 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 27, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 or 50 residues of the B chain.
- Polynucleotides which code for insulin include both the polynucleotide that codes for human insulin as well as the precursors thereof (preproinsulin and proinsulin), as well as the orthologs of other species.
- Polynucleotide that codes for a functionally equivalent variant of insulin indicates (a) a nucleic acid that codes for a polypeptide of active insulin as defined above, and which has at least approximately 80% identity of nucleic acid sequence with a nucleic acid sequence that codes for: (a) residues 1 to 21 of the A chain of the high-fat diet defined above in combination with residues 1 to 30 of the B chain of the human insulin polypeptide defined above, or (b) one of nucleic acid that codes for another fragment specifically derived from the aforementioned sequences of amino acids which shows at least 80%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91% 92%, 93%, 94%, 95%, 96%, 97%, 98% or 99% of identity of amino acid sequence with said sequences.
- the polynucleotides which code for a variant of insulin contain a nucleic acid that codes for an A chain of insulin of at least approximately 45, 48, 51, 54, 57, 60, 63, 66, 69, 72, 75, 81, 87, 105 nucleotides and/or a nucleic acid that codes for a B chain of insulin of at least approximately 75, 78, 81, 84, 87, 90, 93, 96, 99, 102, 105, 108, 111, 114, 117, 120, 123, 126, 129, 132, 135 or 150 nucleotides.
- Said polynucleotides may appear isolated or be found as part of a vector.
- Suitable vectors for the expression of the polynucleotides that code for insulin or the functionally equivalent variant thereof are essentially the same as those previously described in relation to the composition comprised by CT-1.
- the anti-diabetic compounds that can be used in the context of the present invention include compounds which that antiglycaemic activity of the insulin or sensitizing agents of insulin activity and include, without limitation, secretagogues of insulin such as the sulfonylureas (tolbutamide, chlorpropamide, glipicide, glibenclamide, glicazide, glipentide, glimepiride, glibenclamide, glipizide, gliquidone, glisentide, glimepride and such like) and metiglinides (repaglinide, nateglinide, mitiglinide and such like), reducing agents of liver glucose production (biguanides and, in particular, metformin and buformin), agents which cause carbohydrate decrease such as ⁇ -glucosidase inhibitors (acarbose, miglitol or voglibose), agents that increase peripheral use of glucose such as thiazolidinediones (rosi)
- compositions of the invention can be used for the treatment of metabolic disease given their hypoglycaemic effects and their capacity to increase glucose capture by muscles (see example 2).
- Said compositions can be used as preparations of the different compounds for their administration simultaneously, separately or sequentially.
- the invention is related to a composition of the invention for the treatment of a metabolic disease wherein the composition is administered simultaneously, separately or sequentially.
- the invention is related to the use of a composition of the invention for the preparation of a drug for the treatment of a metabolic disease wherein the composition is administered simultaneously, separately or sequentially.
- the invention is related to a method for the treatment of a metabolic disease which comprises the simultaneous, separate or sequential administration of a composition of the invention.
- compositions of the invention in particular, the CT-1 activity inducer agent and the anti-diabetic agent, can be presented as a single formulation (for example, as a tablet or a capsule comprising a fixed quantity of each one of the components) or can, on the other hand, be presented as separate formulations to be later combined for joint, sequential, or separate administration.
- compositions of the invention also include the formulation as a kit-of-parts wherein the components are formulated separately but are packaged in the same container.
- the formulation of the first and second components of the compositions of the invention may be similar, in other words, similarly formulated (in tablets or pills), which allows their administration by the same route.
- the two components can be presented in a blister.
- Each blister contains the drugs that must be consumed during the day. If the drugs must be administered several times a day, the drugs corresponding to each administration can be placed in different sections of the blister, preferably recording in each section of the blister the time of day when they should be administered.
- the components of the composition of the invention can be formulated differently so that the different components are differently administered.
- the first component is formulated as a tablet or capsule for its oral administration and the second component is formulated for its intravenous administration.
- compositions of the invention are administered by the methods known to those skilled in the art, including, but without limitation, intravenous, oral, nasal, parenteral, topical, transdermal, rectal, and such like.
- the ratio between the components that are part of the compositions of the invention shall depend on the CT-1 activity inducer agent and the anti-diabetic agent used in each particular case, as well as of the desired indication.
- the invention envisages compositions wherein the ratio between the quantities of the two components can range from 50:1 to 1:50, in particular from 20:1 to 1:20, from 1:10 to 10:1, or from 5:1 to 1:5.
- the metabolic diseases that can be treated with the compositions of the invention include, without limitation, obesity, insulin resistance, hyperglycaemia, dyslipemia, and type 2 diabetes, as has been previously described in relation to the first aspect of the invention (therapeutic and cosmetic uses of CT-1).
- the invention is related to a cosmetic method for the treatment of obesity which comprises administering a patient a composition in accordance with the invention, wherein those patients candidate to an anti-obesity treatment for purely cosmetic reasons are identified as has been described in the first aspect of the invention (therapeutic and cosmetic uses of CT-1).
- Obese mice were obtained by the intake of a high-fat diet (HFD, 60% fat) during 3 months.
- HFD high-fat diet
- the intravenous administration for 6 consecutive days of rCT-1 caused a reduction in the body weight of C57BL/6 mice (5 months of age) with high-fat diet (HFD) ( Fig. 1 ).
- the dose used in this experiment did not produce a feverish reaction (rectal temperature was measured during all days of treatment).
- the figure shows the kilocalories ingested during the 6 days of treatment of the group treated with rCT-1 and its corresponding control group treated with physiological serum intravenously (S).
- rCT-1 0.2mg/kg/day
- NCD normal diet
- the first group received a physiological serum intravenously (the same volume as that used for the administration of rCT-1); the second group (rCT-1 group) received rCT-1 intravenously at the aforementioned dose (0.2mg/kg/day) and the third group (Pair-Fed group) received the same quantity of food daily as that ingested by the mice treated with rCT-1 and which were injected with serum during the same experimental period.
- mice treated with rCT-1 lost more weight than the pair-fed group, despite the energy intake being identical in both groups, indicating that in addition to the previously observed anorexigenic effect, rCT-1 would have other metabolic effects responsible for weight loss.
- mice C57BL/6, 3 months of age
- gastric gavage in a volume 1% body weight
- the intravenous administration of the same volume of saline serum was given (Saline) or rCT-1 (10 ⁇ g). Blood was taken at the hours indicated in the graphic and blood sugar was measured observing that no increase in blood sugar occurred in the animals treated with rCT-1 ( Fig. 6 ).
- mice C57BL/6, 3 months of age
- streptozotocin 200mg/kg
- the first group (saline group) was treated with treated with saline serum; the second group (Insulin group) was treated with 0.75U/kg insulin, the third group (rCT-1 group) was treated with 10 ⁇ g of rCT-1 and the fourth group (Insulin group + rCT-1) was treated with 0.75U/kg of insulin and 10 ⁇ g of rCT-1.
- the different treatments have been administered, blood sugar was measured after 15, 30, 60 and 120 min. As observed in figure 7 , rCT-1 significantly decreased blood sugar 30 min after its administration and enhanced the effects of insulin in all blood extraction points analysed.
- mice In order to determine if the hypoglycaemic effect resulting from acute treatment with rCT-1 is due to an increase in glucose capture by muscles, muscular capture of 18FDG (18 FluoroDeoxyGlucose) was determined by gamma counter in different groups of mice. To perform this experiment, the mice (C57BL/6, 3 months of age) were divided into 4 groups as follows: Saline group (treated with saline serum); Insulin group (treated with 0.75U/kg insulin), rCT-1 group (treated with 10 ⁇ g of rCT-1) and Insulin group + rCT-1 (treated with 0.75U/kg of insulin + 10 ⁇ g of rCT-1.
- rCT-1 may increase glucose consumption by muscles and enhance (although not significantly with respect to those observed with insulin).
- hypoglycaemic and hypoinsulinemic effects of chronic treatment with rCT-1 were determined in C57BL/6 mice (5 months of age) with obesity induced by the intake of a high-fat diet (HFD, 60% fat) for 3 months.
- HFD high-fat diet
- the mice were treated for 6 consecutive days (chronic treatment) with rCT-1 (0.2mg/kg/day) administered endovenously.
- rCT-1 0.2mg/kg/day
- the hypoglycaemic and hypoinsulinemic effects of chronic treatment with rCT-1 were compared with their corresponding pair-fed (PF) group.
- the rCT-1 group received rCT-1 by intravenous route at the aforementioned dose (0.2mg/kg/day) for 6 days; the Pair-Fed group received the same quantity of daily food as that ingested by the mice treated with rCT-1 and serum was injected into them for the same experimental period.
- the intestinal transport of glucose was determined by measuring the capture of ⁇ -methylglucoside (1 mM) by everted jejunum rings for 15 minutes of incubation in the absence or presence of r-CT-1 at 37° C.
- the ⁇ -methylglucoside is an analog of the glucose which only crosses the apical membrane of the enterocyte by the SGLT1 sodium-sugar co-transcarrier, which means that the changes in their transport should be attributed to changes in the activity of said transcarrier.
- Figure 11 shows that CT-1 is capable of inhibiting the absorption of glucose in the intestine although the effect observed is not dose-dependent.
- Figure 16 shows the results of an experiment carried out on primary cultures of rat adipocytes to determine the capacity of rCT-1 and CNTF to modulate leptin secretion by adipocytes.
- Figure 16A shows how treatment with rCT-1 (72 h) inhibits both baseline secretion of leptin and that stimulated by insulin (1.6 nM). However, these effects were not observed with similar concentrations of CNTF ( figure 16B ).
- Figure 17 shows the results of experiments made in primary cultures of rat adipocytes to determine the ability of CT-1 and CNTF to modulate lipolysis in the absence or presence of insulin.
- CT-1 is able to induce the release of glycerol (lipolysis measurement) and also inhibits the anti-lipolytic activity of insulin, however CNTF is not able.
- CT-1 is able to induce typical genes of brown adipose tissue in adipocytes (deiodinase iodothyronine type II, Dio-2 and uncoupling protein-1, UCP-1) while CNTF is not able ( figure 18 ).
- adipocytes deiodinase iodothyronine type II, Dio-2 and uncoupling protein-1, UCP-1
- CNTF uncoupling protein-1
- histological cuts of liver were performed in order to detect by microscopy the appearance of symptoms of inflammation.
- the results show images representative of histological cuts of liver (H&E 100X) in 3 groups of mice (C57BL/6, 4 months of age): Control group: which received physiological serum by intravenous route (the same volume as that used for the administration of rCT-1); rCT-1 group: which received rCT-1 by intravenous route at the aforementioned dose (0.2mg/kg/day) for 6 consecutive days; Pair-Fed group: which received the same quantity of daily food as that the mice treated with rCT-1 would ingest and into which serum was injected during the same experimental period.
- Control group which received physiological serum by intravenous route (the same volume as that used for the administration of rCT-1)
- rCT-1 group which received rCT-1 by intravenous route at the aforementioned dose (0.2mg/kg/day) for 6 consecutive days
- Pair-Fed group which received the same quantity of daily food as that the mice
- rCT-1 accelerated the elimination of free fatty acids in serum after an intralipid injection.
- the control animals received saline.
- the concentration of free fatty acids in serum was determined after 5 minutes.
- the elimination values of the free fatty acids taken at different times were normalized with respect to the FFA values 5 minutes after the injection of intralipids (100%).
- the abulia kinetics of the FFA significantly differered between the mice treated with saline and with CT-1 ( P ⁇ 0.05 by two-way ANOVA with Bonferroni analysis post hoc ).
- Skeletal muscle is the most sensitive tissue to insulin action and provides in healthy individuals for 90% of glucose uptake stimulated by insulin.
- CT-1 enhances insulin signaling in muscle " in vitro " and "in vivo ".
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Diabetes (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Obesity (AREA)
- Gastroenterology & Hepatology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Hematology (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Endocrinology (AREA)
- Emergency Medicine (AREA)
- Child & Adolescent Psychology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines Containing Plant Substances (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES200900396 | 2009-02-12 | ||
| PCT/ES2010/070072 WO2010092218A2 (es) | 2009-02-12 | 2010-02-10 | Uso de la cardiotrofina-1 para el tratamiento de enfermedades metabólicas |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2397152A2 EP2397152A2 (en) | 2011-12-21 |
| EP2397152B1 true EP2397152B1 (en) | 2014-05-28 |
Family
ID=42562119
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP10710061.2A Active EP2397152B1 (en) | 2009-02-12 | 2010-02-10 | Use of cardiotrophin- 1 for the treatment of metabolic diseases |
Country Status (11)
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11419916B2 (en) | 2012-09-11 | 2022-08-23 | Energesis Pharmaceuticals, Inc. | Methods and compositions for inducing differentiation of human brown adipocyte progenitors |
| US10405961B2 (en) | 2013-03-14 | 2019-09-10 | Cell and Molecular Tissue Engineering, LLC | Coated surgical mesh, and corresponding systems and methods |
| US10130288B2 (en) | 2013-03-14 | 2018-11-20 | Cell and Molecular Tissue Engineering, LLC | Coated sensors, and corresponding systems and methods |
| CN106255748A (zh) * | 2014-02-24 | 2016-12-21 | 释放能量医药股份有限公司 | 诱导人褐色脂肪细胞祖细胞分化的方法和组合物 |
Family Cites Families (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL68769A (en) | 1983-05-23 | 1986-02-28 | Hadassah Med Org | Pharmaceutical compositions containing insulin for oral administration |
| US4849405A (en) | 1984-05-09 | 1989-07-18 | Synthetic Blood Corporation | Oral insulin and a method of making the same |
| EP0179904A1 (en) | 1984-05-09 | 1986-05-07 | Medaphore Inc. | Oral insulin and a method of making the same |
| US4963526A (en) | 1984-05-09 | 1990-10-16 | Synthetic Blood Corporation | Oral insulin and a method of making the same |
| US5614492A (en) | 1986-05-05 | 1997-03-25 | The General Hospital Corporation | Insulinotropic hormone GLP-1 (7-36) and uses thereof |
| US4992417A (en) | 1987-07-17 | 1991-02-12 | Mount Sinai School Of Medicine | Superactive human insulin analogues |
| US5514646A (en) | 1989-02-09 | 1996-05-07 | Chance; Ronald E. | Insulin analogs modified at position 29 of the B chain |
| US5642868A (en) | 1990-05-02 | 1997-07-01 | The United States Of America As Represented By The Secretary Of The Navy | Ceramic material |
| IL99699A (en) | 1990-10-10 | 2002-04-21 | Autoimmune Inc | Drug with the option of oral, intra-intestinal, or inhaled dosing for suppression of autoimmune response associated with type I diabetes |
| US5672659A (en) | 1993-01-06 | 1997-09-30 | Kinerton Limited | Ionic molecular conjugates of biodegradable polyesters and bioactive polypeptides |
| US6191105B1 (en) | 1993-05-10 | 2001-02-20 | Protein Delivery, Inc. | Hydrophilic and lipophilic balanced microemulsion formulations of free-form and/or conjugation-stabilized therapeutic agents such as insulin |
| US5474978A (en) | 1994-06-16 | 1995-12-12 | Eli Lilly And Company | Insulin analog formulations |
| US5461031A (en) | 1994-06-16 | 1995-10-24 | Eli Lilly And Company | Monomeric insulin analog formulations |
| US5595760A (en) | 1994-09-02 | 1997-01-21 | Delab | Sustained release of peptides from pharmaceutical compositions |
| US5547929A (en) | 1994-09-12 | 1996-08-20 | Eli Lilly And Company | Insulin analog formulations |
| US5693609A (en) | 1994-11-17 | 1997-12-02 | Eli Lilly And Company | Acylated insulin analogs |
| US5843866A (en) | 1994-12-30 | 1998-12-01 | Hampshire Chemical Corp. | Pesticidal compositions comprising solutions of polyurea and/or polyurethane |
| YU18596A (sh) | 1995-03-31 | 1998-07-10 | Eli Lilly And Company | Analogne formulacije monomernog insulina |
| US5824638A (en) | 1995-05-22 | 1998-10-20 | Shire Laboratories, Inc. | Oral insulin delivery |
| US5665702A (en) | 1995-06-06 | 1997-09-09 | Biomeasure Incorporated | Ionic molecular conjugates of N-acylated derivatives of poly(2-amino-2-deoxy-D-glucose) and polypeptides |
| US5916883A (en) | 1996-11-01 | 1999-06-29 | Poly-Med, Inc. | Acylated cyclodextrin derivatives |
| US6153632A (en) | 1997-02-24 | 2000-11-28 | Rieveley; Robert B. | Method and composition for the treatment of diabetes |
| CO4750643A1 (es) | 1997-06-13 | 1999-03-31 | Lilly Co Eli | Formulacion estable de la insulina que contiene l-arginina y protamina |
| DE69916031T2 (de) | 1998-01-29 | 2005-02-17 | Poly-Med Inc. | Asorbierbare mikropartikel |
| ATE251465T1 (de) | 1998-07-31 | 2003-10-15 | Novo Nordisk As | In-vitro stimulation von beta zellen vermehrung |
| US6211144B1 (en) | 1998-10-16 | 2001-04-03 | Novo Nordisk A/S | Stable concentrated insulin preparations for pulmonary delivery |
| CA2494040A1 (en) * | 2002-07-29 | 2004-02-05 | Es Cell International Pte Ltd. | Multi-step method for the differentiation of insulin positive, glucose |
| WO2005053728A2 (de) * | 2003-12-01 | 2005-06-16 | Xantos Biomedicine Ag | Mit adipositas assoziierte proteine und deren verwendung in therapie und diagnostik |
| GT200600046A (es) * | 2005-02-09 | 2006-09-25 | Terapia de combinacion | |
| WO2006097526A1 (en) * | 2005-03-17 | 2006-09-21 | Novo Nordisk A/S | Compounds for use in the treatment of obesity |
| TW200800920A (en) | 2006-02-15 | 2008-01-01 | Sanofi Aventis | Novel azacyclyl-substituted arylthienopyrimidinones, process for their preparation and their use as medicaments |
-
2010
- 2010-02-10 CA CA2750074A patent/CA2750074A1/en not_active Abandoned
- 2010-02-10 MX MX2011008562A patent/MX2011008562A/es not_active Application Discontinuation
- 2010-02-10 US US13/201,431 patent/US20110293562A1/en not_active Abandoned
- 2010-02-10 WO PCT/ES2010/070072 patent/WO2010092218A2/es active Application Filing
- 2010-02-10 ES ES10710061.2T patent/ES2493618T3/es active Active
- 2010-02-10 AU AU2010212794A patent/AU2010212794A1/en not_active Abandoned
- 2010-02-10 EP EP10710061.2A patent/EP2397152B1/en active Active
- 2010-02-10 CN CN201080009256.9A patent/CN102316892B/zh not_active Expired - Fee Related
- 2010-02-10 JP JP2011549615A patent/JP2012517459A/ja active Pending
- 2010-02-10 BR BRPI1008415-0A patent/BRPI1008415A2/pt not_active IP Right Cessation
- 2010-02-10 RU RU2011137413/15A patent/RU2011137413A/ru unknown
Non-Patent Citations (4)
| Title |
|---|
| HONGKUI J ET AL: "In vivo effects of cardiotrophin-1", CYTOKINE, ACADEMIC PRESS LTD, PHILADELPHIA, PA, US, vol. 8, no. 12, 1 December 1996 (1996-12-01), pages 920 - 926, XP002966035, ISSN: 1043-4666, DOI: 10.1006/CYTO.1996.0123 * |
| NATAL CRISTINA ET AL: "Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome.", January 2008, AMERICAN JOURNAL OF PHYSIOLOGY. ENDOCRINOLOGY AND METABOLISM JAN 2008 LNKD- PUBMED:17940213, VOL. 294, NR. 1, PAGE(S) E52 - E60, ISSN: 0193-1849 * |
| PATEL SANJEEV B ET AL: "Leptin: linking obesity, the metabolic syndrome, and cardiovascular disease.", April 2008, CURRENT HYPERTENSION REPORTS APR 2008 LNKD- PUBMED:18474180, VOL. 10, NR. 2, PAGE(S) 131 - 137, ISSN: 1534-3111 * |
| WHITE URSULA A ET AL: "Neuropoietin attenuates adipogenesis and induces insulin resistance in adipocytes.", 15 August 2008, THE JOURNAL OF BIOLOGICAL CHEMISTRY 15 AUG 2008 LNKD- PUBMED:18562323, VOL. 283, NR. 33, PAGE(S) 22505 - 22512, ISSN: 0021-9258 * |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010092218A3 (es) | 2010-11-18 |
| CN102316892A (zh) | 2012-01-11 |
| CA2750074A1 (en) | 2010-08-19 |
| CN102316892B (zh) | 2014-06-04 |
| EP2397152A2 (en) | 2011-12-21 |
| US20110293562A1 (en) | 2011-12-01 |
| JP2012517459A (ja) | 2012-08-02 |
| AU2010212794A1 (en) | 2011-08-11 |
| RU2011137413A (ru) | 2013-03-20 |
| WO2010092218A2 (es) | 2010-08-19 |
| MX2011008562A (es) | 2011-09-09 |
| ES2493618T3 (es) | 2014-09-12 |
| BRPI1008415A2 (pt) | 2018-02-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4959005B2 (ja) | 毎日の注射頻度より少ないインスリン注射での真性糖尿病の治療 | |
| US20130331320A1 (en) | Fast-acting insulin in combination with long-acting insulin | |
| CN102389413A (zh) | 用于治疗糖尿病的组合物及其应用 | |
| Jayakrishnapillai et al. | Current trend in drug delivery considerations for subcutaneous insulin depots to treat diabetes | |
| RU2670106C2 (ru) | Фармацевтическая композиция | |
| EP2397152B1 (en) | Use of cardiotrophin- 1 for the treatment of metabolic diseases | |
| KR20160075794A (ko) | 질환 및 질병 치료용 칼시토닌 모방체 | |
| KR20200067745A (ko) | Fas 신호전달 억제용 펩티드를 포함하는 비만, 지방간 또는 지방간염의 예방 또는 치료용 약학적 조성물 | |
| EP3845550A1 (en) | Composition for accelerating cell proliferation comprising erythropoietin-derived peptide | |
| US11344607B2 (en) | Treatment of disease with relaxin | |
| CN114129711A (zh) | 度拉糖肽在制备抗肿瘤药物中的应用 | |
| Chen et al. | An overview of hypoglycemic biological drugs | |
| US20230158116A1 (en) | Use of human amylin analog polypeptides for providing superior glycemic control to type 1 diabetics | |
| US20040151693A1 (en) | Use of mullerian inhibiting substance and interferon for treating tumors | |
| US20180099031A1 (en) | Diabetes treatment | |
| US20040033954A1 (en) | Therapeutic method for combined use of modified CNTF and thiadolidinedione | |
| Jayakrishnapillai et al. | Current trend in drug delivery considerations for | |
| Zhang | Cytokines and skeletal muscle wasting. | |
| CN101219208A (zh) | 白介素-22的医药用途 | |
| CN1410128A (zh) | 一种用于直接肌肉注射治疗糖尿病的质粒 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20110907 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA RS |
|
| 17Q | First examination report despatched |
Effective date: 20120808 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: UNIVERSIDAD DE NAVARRA Owner name: PROYECTO DE BIOMEDICINA CIMA, S.L. |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20131122 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: UNIVERSIDAD DE NAVARRA Owner name: PROYECTO DE BIOMEDICINA CIMA, S.L. |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA RS |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 670195 Country of ref document: AT Kind code of ref document: T Effective date: 20140615 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602010016346 Country of ref document: DE Effective date: 20140710 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2493618 Country of ref document: ES Kind code of ref document: T3 Effective date: 20140912 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 670195 Country of ref document: AT Kind code of ref document: T Effective date: 20140528 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20140528 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140829 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140828 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140929 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: BE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602010016346 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20150303 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602010016346 Country of ref document: DE Effective date: 20150303 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602010016346 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20150210 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20150210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150228 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150228 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20151030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150210 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150210 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150901 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150302 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20160329 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150211 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20100210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20140528 |