EP2245047B1 - Peptides issus de pres modifiés hydrophobes du virus de l'hépatite b (vhb) et leur utilisation en tant que véhicules pour l'administration spécifique de composés au foie - Google Patents
Peptides issus de pres modifiés hydrophobes du virus de l'hépatite b (vhb) et leur utilisation en tant que véhicules pour l'administration spécifique de composés au foie Download PDFInfo
- Publication number
- EP2245047B1 EP2245047B1 EP09704030.7A EP09704030A EP2245047B1 EP 2245047 B1 EP2245047 B1 EP 2245047B1 EP 09704030 A EP09704030 A EP 09704030A EP 2245047 B1 EP2245047 B1 EP 2245047B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- liver
- hbv
- hydrophobic modified
- derived peptide
- pres
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims description 147
- 241000700721 Hepatitis B virus Species 0.000 title claims description 128
- 210000004185 liver Anatomy 0.000 title claims description 113
- 230000002209 hydrophobic effect Effects 0.000 title claims description 103
- 150000001875 compounds Chemical class 0.000 title claims description 66
- 102000004196 processed proteins & peptides Human genes 0.000 title description 65
- -1 cyclic amino acid Chemical class 0.000 claims description 62
- 239000003814 drug Substances 0.000 claims description 44
- 210000003494 hepatocyte Anatomy 0.000 claims description 40
- 229940079593 drug Drugs 0.000 claims description 38
- 208000015181 infectious disease Diseases 0.000 claims description 34
- 208000019423 liver disease Diseases 0.000 claims description 31
- 238000000034 method Methods 0.000 claims description 27
- 230000004048 modification Effects 0.000 claims description 25
- 238000012986 modification Methods 0.000 claims description 25
- 108090000623 proteins and genes Proteins 0.000 claims description 25
- 150000001413 amino acids Chemical group 0.000 claims description 22
- 102000004169 proteins and genes Human genes 0.000 claims description 21
- 241000700605 Viruses Species 0.000 claims description 20
- 210000004899 c-terminal region Anatomy 0.000 claims description 17
- 239000003112 inhibitor Substances 0.000 claims description 17
- 206010073071 hepatocellular carcinoma Diseases 0.000 claims description 15
- 230000015572 biosynthetic process Effects 0.000 claims description 14
- 125000001419 myristoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 14
- 125000003696 stearoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 14
- 238000011282 treatment Methods 0.000 claims description 14
- 231100000844 hepatocellular carcinoma Toxicity 0.000 claims description 13
- 230000002265 prevention Effects 0.000 claims description 12
- 229940002612 prodrug Drugs 0.000 claims description 12
- 239000000651 prodrug Substances 0.000 claims description 12
- 208000002672 hepatitis B Diseases 0.000 claims description 11
- 201000004792 malaria Diseases 0.000 claims description 11
- 239000008194 pharmaceutical composition Substances 0.000 claims description 11
- 241000282414 Homo sapiens Species 0.000 claims description 10
- 108010091867 peptide P Proteins 0.000 claims description 10
- 239000003981 vehicle Substances 0.000 claims description 10
- 230000003612 virological effect Effects 0.000 claims description 10
- 241001465754 Metazoa Species 0.000 claims description 9
- 208000006454 hepatitis Diseases 0.000 claims description 9
- 231100000283 hepatitis Toxicity 0.000 claims description 9
- 208000005331 Hepatitis D Diseases 0.000 claims description 8
- 125000006850 spacer group Chemical group 0.000 claims description 8
- 230000010933 acylation Effects 0.000 claims description 7
- 238000005917 acylation reaction Methods 0.000 claims description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 7
- 229940079322 interferon Drugs 0.000 claims description 7
- 238000011191 terminal modification Methods 0.000 claims description 7
- 150000008574 D-amino acids Chemical class 0.000 claims description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 6
- 108020004459 Small interfering RNA Proteins 0.000 claims description 6
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 6
- 201000010099 disease Diseases 0.000 claims description 6
- 201000004409 schistosomiasis Diseases 0.000 claims description 6
- 108010050904 Interferons Proteins 0.000 claims description 5
- 102000014150 Interferons Human genes 0.000 claims description 5
- 102000035195 Peptidases Human genes 0.000 claims description 5
- 108091005804 Peptidases Proteins 0.000 claims description 5
- 150000001408 amides Chemical class 0.000 claims description 5
- 239000003937 drug carrier Substances 0.000 claims description 5
- 229960005486 vaccine Drugs 0.000 claims description 5
- 244000052613 viral pathogen Species 0.000 claims description 5
- 102000007698 Alcohol dehydrogenase Human genes 0.000 claims description 4
- 108010021809 Alcohol dehydrogenase Proteins 0.000 claims description 4
- 229940124226 Farnesyltransferase inhibitor Drugs 0.000 claims description 4
- 206010016654 Fibrosis Diseases 0.000 claims description 4
- 208000018565 Hemochromatosis Diseases 0.000 claims description 4
- 208000004554 Leishmaniasis Diseases 0.000 claims description 4
- 241000124008 Mammalia Species 0.000 claims description 4
- 230000004913 activation Effects 0.000 claims description 4
- 230000033228 biological regulation Effects 0.000 claims description 4
- 230000015556 catabolic process Effects 0.000 claims description 4
- 230000007882 cirrhosis Effects 0.000 claims description 4
- 208000019425 cirrhosis of liver Diseases 0.000 claims description 4
- 238000006731 degradation reaction Methods 0.000 claims description 4
- 230000003908 liver function Effects 0.000 claims description 4
- 208000014018 liver neoplasm Diseases 0.000 claims description 4
- 208000030159 metabolic disease Diseases 0.000 claims description 4
- 208000008338 non-alcoholic fatty liver disease Diseases 0.000 claims description 4
- 206010053219 non-alcoholic steatohepatitis Diseases 0.000 claims description 4
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 4
- 239000003528 protein farnesyltransferase inhibitor Substances 0.000 claims description 4
- 208000037972 tropical disease Diseases 0.000 claims description 4
- 102000018832 Cytochromes Human genes 0.000 claims description 3
- 108010052832 Cytochromes Proteins 0.000 claims description 3
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 claims description 3
- 208000005176 Hepatitis C Diseases 0.000 claims description 3
- 206010019771 Hepatitis F Diseases 0.000 claims description 3
- 206010019773 Hepatitis G Diseases 0.000 claims description 3
- 206010019776 Hepatitis H Diseases 0.000 claims description 3
- 208000001145 Metabolic Syndrome Diseases 0.000 claims description 3
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 claims description 3
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 claims description 3
- 235000012000 cholesterol Nutrition 0.000 claims description 3
- 230000009918 complex formation Effects 0.000 claims description 3
- 150000002148 esters Chemical class 0.000 claims description 3
- 208000005252 hepatitis A Diseases 0.000 claims description 3
- 201000010284 hepatitis E Diseases 0.000 claims description 3
- 230000001404 mediated effect Effects 0.000 claims description 3
- 239000000137 peptide hydrolase inhibitor Substances 0.000 claims description 3
- 239000003419 rna directed dna polymerase inhibitor Substances 0.000 claims description 3
- 102100027211 Albumin Human genes 0.000 claims description 2
- 108010088751 Albumins Chemical class 0.000 claims description 2
- 206010019695 Hepatic neoplasm Diseases 0.000 claims description 2
- 108090001074 Nucleocapsid Proteins Proteins 0.000 claims description 2
- 208000008589 Obesity Diseases 0.000 claims description 2
- 229940123690 Raf kinase inhibitor Drugs 0.000 claims description 2
- 229940122423 Viral RNA polymerase inhibitor Drugs 0.000 claims description 2
- 239000012190 activator Substances 0.000 claims description 2
- 230000030741 antigen processing and presentation Effects 0.000 claims description 2
- 208000016097 disease of metabolism Diseases 0.000 claims description 2
- 230000006126 farnesylation Effects 0.000 claims description 2
- 239000012634 fragment Substances 0.000 claims description 2
- 230000028993 immune response Effects 0.000 claims description 2
- 229940043355 kinase inhibitor Drugs 0.000 claims description 2
- 229920005615 natural polymer Chemical class 0.000 claims description 2
- 229940127073 nucleoside analogue Drugs 0.000 claims description 2
- 235000020824 obesity Nutrition 0.000 claims description 2
- 102000051624 phosphatidylethanolamine binding protein Human genes 0.000 claims description 2
- 108700021017 phosphatidylethanolamine binding protein Proteins 0.000 claims description 2
- 239000003757 phosphotransferase inhibitor Substances 0.000 claims description 2
- 239000002243 precursor Substances 0.000 claims description 2
- 229920001059 synthetic polymer Chemical class 0.000 claims description 2
- 150000007970 thio esters Chemical class 0.000 claims description 2
- 150000003573 thiols Chemical class 0.000 claims description 2
- 230000003115 biocidal effect Effects 0.000 claims 1
- 101150115433 SLC26A5 gene Proteins 0.000 description 78
- 210000004027 cell Anatomy 0.000 description 41
- 125000000539 amino acid group Chemical group 0.000 description 36
- 125000003275 alpha amino acid group Chemical group 0.000 description 31
- 102220600635 DIS3-like exonuclease 1_Q46K_mutation Human genes 0.000 description 20
- 230000008685 targeting Effects 0.000 description 17
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 16
- 108091035707 Consensus sequence Proteins 0.000 description 14
- 241000699670 Mus sp. Species 0.000 description 14
- 241000285424 HBV genotype C Species 0.000 description 13
- 210000001519 tissue Anatomy 0.000 description 13
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 12
- 230000027455 binding Effects 0.000 description 12
- 230000010415 tropism Effects 0.000 description 11
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 10
- 238000001727 in vivo Methods 0.000 description 10
- 210000005229 liver cell Anatomy 0.000 description 10
- 244000045947 parasite Species 0.000 description 10
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- 230000035508 accumulation Effects 0.000 description 9
- 238000009825 accumulation Methods 0.000 description 9
- 102000005962 receptors Human genes 0.000 description 9
- 108020003175 receptors Proteins 0.000 description 9
- 208000037262 Hepatitis delta Diseases 0.000 description 8
- 241000724709 Hepatitis delta virus Species 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 230000014509 gene expression Effects 0.000 description 8
- 238000003786 synthesis reaction Methods 0.000 description 8
- 108010028921 Lipopeptides Proteins 0.000 description 7
- 208000029570 hepatitis D virus infection Diseases 0.000 description 7
- 238000006467 substitution reaction Methods 0.000 description 7
- 101001065501 Escherichia phage MS2 Lysis protein Proteins 0.000 description 6
- 208000026350 Inborn Genetic disease Diseases 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 6
- 238000007920 subcutaneous administration Methods 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- 241000701447 unidentified baculovirus Species 0.000 description 6
- 239000013598 vector Substances 0.000 description 6
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 5
- 241000699666 Mus <mouse, genus> Species 0.000 description 5
- 238000011785 NMRI mouse Methods 0.000 description 5
- 102000037865 fusion proteins Human genes 0.000 description 5
- 108020001507 fusion proteins Proteins 0.000 description 5
- 210000002216 heart Anatomy 0.000 description 5
- 238000010166 immunofluorescence Methods 0.000 description 5
- 230000002458 infectious effect Effects 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 230000007498 myristoylation Effects 0.000 description 5
- 150000007523 nucleic acids Chemical group 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 5
- 239000011347 resin Substances 0.000 description 5
- 229920005989 resin Polymers 0.000 description 5
- 241000894007 species Species 0.000 description 5
- 238000010254 subcutaneous injection Methods 0.000 description 5
- 239000007929 subcutaneous injection Substances 0.000 description 5
- 239000013603 viral vector Substances 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 4
- BPYKTIZUTYGOLE-IFADSCNNSA-N Bilirubin Chemical compound N1C(=O)C(C)=C(C=C)\C1=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(C(C)=C(\C=C/3C(=C(C=C)C(=O)N\3)C)N2)CCC(O)=O)N1 BPYKTIZUTYGOLE-IFADSCNNSA-N 0.000 description 4
- 101100505076 Caenorhabditis elegans gly-2 gene Proteins 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 102220625993 HMG box transcription factor BBX_D20A_mutation Human genes 0.000 description 4
- 208000028782 Hereditary disease Diseases 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 101001115218 Homo sapiens Ubiquitin-40S ribosomal protein S27a Proteins 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- 102220475968 Keratin, type I cytoskeletal 10_N29A_mutation Human genes 0.000 description 4
- 241000282675 Lagothrix Species 0.000 description 4
- 239000004472 Lysine Substances 0.000 description 4
- 208000024556 Mendelian disease Diseases 0.000 description 4
- 102100023341 Ubiquitin-40S ribosomal protein S27a Human genes 0.000 description 4
- 210000000013 bile duct Anatomy 0.000 description 4
- 230000037396 body weight Effects 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 238000004113 cell culture Methods 0.000 description 4
- 210000000170 cell membrane Anatomy 0.000 description 4
- 230000008878 coupling Effects 0.000 description 4
- 238000010168 coupling process Methods 0.000 description 4
- 238000005859 coupling reaction Methods 0.000 description 4
- 239000012228 culture supernatant Substances 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 238000001415 gene therapy Methods 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 210000003734 kidney Anatomy 0.000 description 4
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 4
- 108700029353 mouse Ifna Proteins 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 239000002953 phosphate buffered saline Substances 0.000 description 4
- 229920001184 polypeptide Polymers 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 3
- ROHFNLRQFUQHCH-RXMQYKEDSA-N D-leucine Chemical compound CC(C)C[C@@H](N)C(O)=O ROHFNLRQFUQHCH-RXMQYKEDSA-N 0.000 description 3
- COLNVLDHVKWLRT-MRVPVSSYSA-N D-phenylalanine Chemical compound OC(=O)[C@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-MRVPVSSYSA-N 0.000 description 3
- 229920002527 Glycogen Polymers 0.000 description 3
- 241000238631 Hexapoda Species 0.000 description 3
- 102100034349 Integrase Human genes 0.000 description 3
- 102000018697 Membrane Proteins Human genes 0.000 description 3
- 108010052285 Membrane Proteins Proteins 0.000 description 3
- 241000224016 Plasmodium Species 0.000 description 3
- 241000242594 Platyhelminthes Species 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 238000011210 chromatographic step Methods 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 238000003745 diagnosis Methods 0.000 description 3
- 230000004069 differentiation Effects 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- 238000010828 elution Methods 0.000 description 3
- 239000012894 fetal calf serum Substances 0.000 description 3
- 230000004927 fusion Effects 0.000 description 3
- 238000010353 genetic engineering Methods 0.000 description 3
- 229940096919 glycogen Drugs 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 239000002054 inoculum Substances 0.000 description 3
- 210000004072 lung Anatomy 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- 210000003205 muscle Anatomy 0.000 description 3
- 108020004707 nucleic acids Proteins 0.000 description 3
- 102000039446 nucleic acids Human genes 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 239000012071 phase Substances 0.000 description 3
- 239000007790 solid phase Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 210000000952 spleen Anatomy 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- BHNHHSOHWZKFOX-UHFFFAOYSA-N 2-methyl-1H-indole Chemical compound C1=CC=C2NC(C)=CC2=C1 BHNHHSOHWZKFOX-UHFFFAOYSA-N 0.000 description 2
- 201000011374 Alagille syndrome Diseases 0.000 description 2
- 208000007848 Alcoholism Diseases 0.000 description 2
- 102000005427 Asialoglycoprotein Receptor Human genes 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 108090001008 Avidin Proteins 0.000 description 2
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 description 2
- 208000033222 Biliary cirrhosis primary Diseases 0.000 description 2
- 208000007257 Budd-Chiari syndrome Diseases 0.000 description 2
- 206010008609 Cholangitis sclerosing Diseases 0.000 description 2
- 206010010317 Congenital absence of bile ducts Diseases 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 241000255925 Diptera Species 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 208000009139 Gilbert Disease Diseases 0.000 description 2
- 208000022412 Gilbert syndrome Diseases 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 208000032007 Glycogen storage disease due to acid maltase deficiency Diseases 0.000 description 2
- 206010053185 Glycogen storage disease type II Diseases 0.000 description 2
- 206010019799 Hepatitis viral Diseases 0.000 description 2
- 208000002972 Hepatolenticular Degeneration Diseases 0.000 description 2
- 241000701085 Human alphaherpesvirus 3 Species 0.000 description 2
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 2
- HHZQLQREDATOBM-CODXZCKSSA-M Hydrocortisone Sodium Succinate Chemical compound [Na+].O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)COC(=O)CCC([O-])=O)[C@@H]4[C@@H]3CCC2=C1 HHZQLQREDATOBM-CODXZCKSSA-M 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- 206010067125 Liver injury Diseases 0.000 description 2
- 101100170937 Mus musculus Dnmt1 gene Proteins 0.000 description 2
- 208000010428 Muscle Weakness Diseases 0.000 description 2
- 208000021642 Muscular disease Diseases 0.000 description 2
- 206010028372 Muscular weakness Diseases 0.000 description 2
- 201000009623 Myopathy Diseases 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 241000223960 Plasmodium falciparum Species 0.000 description 2
- 241000223801 Plasmodium knowlesi Species 0.000 description 2
- 241000223821 Plasmodium malariae Species 0.000 description 2
- 241001505293 Plasmodium ovale Species 0.000 description 2
- 241000223810 Plasmodium vivax Species 0.000 description 2
- 229920002594 Polyethylene Glycol 8000 Polymers 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 208000012654 Primary biliary cholangitis Diseases 0.000 description 2
- 201000002150 Progressive familial intrahepatic cholestasis Diseases 0.000 description 2
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 2
- 241000242678 Schistosoma Species 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 208000018839 Wilson disease Diseases 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 229960001997 adefovir Drugs 0.000 description 2
- WOZSCQDILHKSGG-UHFFFAOYSA-N adefovir depivoxil Chemical compound N1=CN=C2N(CCOCP(=O)(OCOC(=O)C(C)(C)C)OCOC(=O)C(C)(C)C)C=NC2=C1N WOZSCQDILHKSGG-UHFFFAOYSA-N 0.000 description 2
- 201000007930 alcohol dependence Diseases 0.000 description 2
- 208000006682 alpha 1-Antitrypsin Deficiency Diseases 0.000 description 2
- 108010006523 asialoglycoprotein receptor Proteins 0.000 description 2
- 230000001363 autoimmune Effects 0.000 description 2
- 230000005784 autoimmunity Effects 0.000 description 2
- 210000000941 bile Anatomy 0.000 description 2
- 201000005271 biliary atresia Diseases 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 208000006990 cholangiocarcinoma Diseases 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000001784 detoxification Methods 0.000 description 2
- PEASPLKKXBYDKL-FXEVSJAOSA-N enfuvirtide Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(C)=O)[C@@H](C)O)[C@@H](C)CC)C1=CN=CN1 PEASPLKKXBYDKL-FXEVSJAOSA-N 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 210000003743 erythrocyte Anatomy 0.000 description 2
- 108010042430 galactose receptor Proteins 0.000 description 2
- 210000001035 gastrointestinal tract Anatomy 0.000 description 2
- 238000001641 gel filtration chromatography Methods 0.000 description 2
- 208000016361 genetic disease Diseases 0.000 description 2
- 201000004502 glycogen storage disease II Diseases 0.000 description 2
- YMAWOPBAYDPSLA-UHFFFAOYSA-N glycylglycine Chemical compound [NH3+]CC(=O)NCC([O-])=O YMAWOPBAYDPSLA-UHFFFAOYSA-N 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 231100000234 hepatic damage Toxicity 0.000 description 2
- 210000002989 hepatic vein Anatomy 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 238000000265 homogenisation Methods 0.000 description 2
- 229950006240 hydrocortisone succinate Drugs 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 230000008818 liver damage Effects 0.000 description 2
- 210000005228 liver tissue Anatomy 0.000 description 2
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 description 2
- 208000037819 metastatic cancer Diseases 0.000 description 2
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 108091005601 modified peptides Proteins 0.000 description 2
- 210000000865 mononuclear phagocyte system Anatomy 0.000 description 2
- 210000000653 nervous system Anatomy 0.000 description 2
- 229940118768 plasmodium malariae Drugs 0.000 description 2
- 239000002574 poison Substances 0.000 description 2
- 231100000614 poison Toxicity 0.000 description 2
- 229920000724 poly(L-arginine) polymer Polymers 0.000 description 2
- 108010011110 polyarginine Proteins 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- INDBQLZJXZLFIT-UHFFFAOYSA-N primaquine Chemical compound N1=CC=CC2=CC(OC)=CC(NC(C)CCCN)=C21 INDBQLZJXZLFIT-UHFFFAOYSA-N 0.000 description 2
- 229960005179 primaquine Drugs 0.000 description 2
- 201000000742 primary sclerosing cholangitis Diseases 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 230000006337 proteolytic cleavage Effects 0.000 description 2
- 244000000040 protozoan parasite Species 0.000 description 2
- 238000004007 reversed phase HPLC Methods 0.000 description 2
- 102200006618 rs727503094 Human genes 0.000 description 2
- 208000010157 sclerosing cholangitis Diseases 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 210000002027 skeletal muscle Anatomy 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 239000003440 toxic substance Substances 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 230000006648 viral gene expression Effects 0.000 description 2
- 201000001862 viral hepatitis Diseases 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- DHBXNPKRAUYBTH-UHFFFAOYSA-N 1,1-ethanedithiol Chemical compound CC(S)S DHBXNPKRAUYBTH-UHFFFAOYSA-N 0.000 description 1
- JMTMSDXUXJISAY-UHFFFAOYSA-N 2H-benzotriazol-4-ol Chemical compound OC1=CC=CC2=C1N=NN2 JMTMSDXUXJISAY-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 241000256186 Anopheles <genus> Species 0.000 description 1
- 241000711404 Avian avulavirus 1 Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 239000005996 Blood meal Substances 0.000 description 1
- 210000003771 C cell Anatomy 0.000 description 1
- 108010075254 C-Peptide Proteins 0.000 description 1
- 101710132601 Capsid protein Proteins 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- 102000005367 Carboxypeptidases Human genes 0.000 description 1
- 108010006303 Carboxypeptidases Proteins 0.000 description 1
- 241000238008 Cerithidea rhizophorarum Species 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 208000000419 Chronic Hepatitis B Diseases 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 102100031673 Corneodesmosin Human genes 0.000 description 1
- 241000709687 Coxsackievirus Species 0.000 description 1
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 description 1
- 102000003849 Cytochrome P450 Human genes 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 241000450599 DNA viruses Species 0.000 description 1
- 241000725619 Dengue virus Species 0.000 description 1
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 1
- 108010032976 Enfuvirtide Proteins 0.000 description 1
- 101710091045 Envelope protein Proteins 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 206010015866 Extravasation Diseases 0.000 description 1
- 108010016306 Glycylpeptide N-tetradecanoyltransferase Proteins 0.000 description 1
- 241001197893 Glyptemys herpesvirus Species 0.000 description 1
- 241000282575 Gorilla Species 0.000 description 1
- 229940118186 HBV capsid assembly inhibitor Drugs 0.000 description 1
- 241000285387 HBV genotype A Species 0.000 description 1
- 241000285452 HBV genotype B Species 0.000 description 1
- 241000285366 HBV genotype D Species 0.000 description 1
- 241000285370 HBV genotype E Species 0.000 description 1
- 241000285563 HBV genotype F Species 0.000 description 1
- 241000285576 HBV genotype G Species 0.000 description 1
- 241000285579 HBV genotype H Species 0.000 description 1
- 229940124771 HCV-NS3 protease inhibitor Drugs 0.000 description 1
- 229940124772 HCV-NS5B polymerase inhibitor Drugs 0.000 description 1
- 241000700586 Herpesviridae Species 0.000 description 1
- 208000029433 Herpesviridae infectious disease Diseases 0.000 description 1
- 208000031226 Hyperlipidaemia Diseases 0.000 description 1
- 102100023915 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 108010078049 Interferon alpha-2 Proteins 0.000 description 1
- 102100039350 Interferon alpha-7 Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 101710192606 Latent membrane protein 2 Proteins 0.000 description 1
- 241000222722 Leishmania <genus> Species 0.000 description 1
- 239000000232 Lipid Bilayer Substances 0.000 description 1
- 241000255134 Lutzomyia <genus> Species 0.000 description 1
- 101710085938 Matrix protein Proteins 0.000 description 1
- 101710127721 Membrane protein Proteins 0.000 description 1
- 102000034452 Methionyl aminopeptidases Human genes 0.000 description 1
- 108090000192 Methionyl aminopeptidases Proteins 0.000 description 1
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 description 1
- 102100032965 Myomesin-2 Human genes 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 208000030852 Parasitic disease Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 241000722350 Phlebotomus <genus> Species 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 101710188315 Protein X Proteins 0.000 description 1
- 241000242683 Schistosoma haematobium Species 0.000 description 1
- 241000242687 Schistosoma intercalatum Species 0.000 description 1
- 241000242677 Schistosoma japonicum Species 0.000 description 1
- 241000242680 Schistosoma mansoni Species 0.000 description 1
- 241001520868 Schistosoma mekongi Species 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 101710109576 Terminal protein Proteins 0.000 description 1
- 201000005485 Toxoplasmosis Diseases 0.000 description 1
- 108010032099 V(D)J recombination activating protein 2 Proteins 0.000 description 1
- 102100029591 V(D)J recombination-activating protein 2 Human genes 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 229940118555 Viral entry inhibitor Drugs 0.000 description 1
- 108010031318 Vitronectin Proteins 0.000 description 1
- 241000972172 Woolly monkey hepatitis B Virus Species 0.000 description 1
- 241000710772 Yellow fever virus Species 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- MKUXAQIIEYXACX-UHFFFAOYSA-N aciclovir Chemical compound N1C(N)=NC(=O)C2=C1N(COCCO)C=N2 MKUXAQIIEYXACX-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- RDOXTESZEPMUJZ-UHFFFAOYSA-N anisole Chemical compound COC1=CC=CC=C1 RDOXTESZEPMUJZ-UHFFFAOYSA-N 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- JPIYZTWMUGTEHX-UHFFFAOYSA-N auramine O free base Chemical compound C1=CC(N(C)C)=CC=C1C(=N)C1=CC=C(N(C)C)C=C1 JPIYZTWMUGTEHX-UHFFFAOYSA-N 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 239000003833 bile salt Substances 0.000 description 1
- 229940093761 bile salts Drugs 0.000 description 1
- 238000005842 biochemical reaction Methods 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000004700 cellular uptake Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- VDQQXEISLMTGAB-UHFFFAOYSA-N chloramine T Chemical compound [Na+].CC1=CC=C(S(=O)(=O)[N-]Cl)C=C1 VDQQXEISLMTGAB-UHFFFAOYSA-N 0.000 description 1
- 238000011097 chromatography purification Methods 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- PJZPDFUUXKKDNB-KNINVFKUSA-N ciluprevir Chemical compound N([C@@H]1C(=O)N2[C@H](C(N[C@@]3(C[C@H]3\C=C/CCCCC1)C(O)=O)=O)C[C@H](C2)OC=1C2=CC=C(C=C2N=C(C=1)C=1N=C(NC(C)C)SC=1)OC)C(=O)OC1CCCC1 PJZPDFUUXKKDNB-KNINVFKUSA-N 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- MKRTXPORKIRPDG-UHFFFAOYSA-N diphenylphosphoryl azide Chemical compound C=1C=CC=CC=1P(=O)(N=[N+]=[N-])C1=CC=CC=C1 MKRTXPORKIRPDG-UHFFFAOYSA-N 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 229960000980 entecavir Drugs 0.000 description 1
- YXPVEXCTPGULBZ-WQYNNSOESA-N entecavir hydrate Chemical compound O.C1=NC=2C(=O)NC(N)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)C1=C YXPVEXCTPGULBZ-WQYNNSOESA-N 0.000 description 1
- 230000007515 enzymatic degradation Effects 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 230000036251 extravasation Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 238000001215 fluorescent labelling Methods 0.000 description 1
- 239000013505 freshwater Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 229940099052 fuzeon Drugs 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- 210000005161 hepatic lobe Anatomy 0.000 description 1
- 230000001553 hepatotropic effect Effects 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 1
- 201000001421 hyperglycemia Diseases 0.000 description 1
- 230000037451 immune surveillance Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000001524 infective effect Effects 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000011488 interferon-alpha production Effects 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000006192 iodination reaction Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 229960001627 lamivudine Drugs 0.000 description 1
- JTEGQNOMFQHVDC-NKWVEPMBSA-N lamivudine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)SC1 JTEGQNOMFQHVDC-NKWVEPMBSA-N 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 238000012317 liver biopsy Methods 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 229960004844 lovastatin Drugs 0.000 description 1
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 210000003936 merozoite Anatomy 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- DUAFKXOFBZQTQE-IXZVNPRYSA-N myristoyl-coa Chemical compound O[C@@H]1[C@@H](OP(O)(O)=O)[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OCC(C)(C)[C@H](O)C(=O)NCCC(=O)NCCSC(=O)CCCCCCCCCCCCC)O[C@H]1N1C2=NC=NC(N)=C2N=C1 DUAFKXOFBZQTQE-IXZVNPRYSA-N 0.000 description 1
- OHDXDNUPVVYWOV-UHFFFAOYSA-N n-methyl-1-(2-naphthalen-1-ylsulfanylphenyl)methanamine Chemical compound CNCC1=CC=CC=C1SC1=CC=CC2=CC=CC=C12 OHDXDNUPVVYWOV-UHFFFAOYSA-N 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 210000000822 natural killer cell Anatomy 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 230000026792 palmitoylation Effects 0.000 description 1
- 230000003071 parasitic effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 108010054442 polyalanine Proteins 0.000 description 1
- 229920000232 polyglycine polymer Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000009465 prokaryotic expression Effects 0.000 description 1
- 239000013636 protein dimer Substances 0.000 description 1
- 108020001580 protein domains Proteins 0.000 description 1
- 238000001243 protein synthesis Methods 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 150000003254 radicals Chemical group 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- IVDHYUQIDRJSTI-UHFFFAOYSA-N sorafenib tosylate Chemical compound [H+].CC1=CC=C(S([O-])(=O)=O)C=C1.C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 IVDHYUQIDRJSTI-UHFFFAOYSA-N 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 210000003046 sporozoite Anatomy 0.000 description 1
- 238000013097 stability assessment Methods 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 229960001479 tosylchloramide sodium Drugs 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- 229920003169 water-soluble polymer Polymers 0.000 description 1
- 229940051021 yellow-fever virus Drugs 0.000 description 1
- 229940107931 zovirax Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/162—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/60—Medicinal preparations containing antigens or antibodies characteristics by the carrier linked to the antigen
- A61K2039/6031—Proteins
- A61K2039/6075—Viral proteins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/033—Fusion polypeptide containing a localisation/targetting motif containing a motif for targeting to the internal surface of the plasma membrane, e.g. containing a myristoylation motif
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2730/00—Reverse transcribing DNA viruses
- C12N2730/00011—Details
- C12N2730/10011—Hepadnaviridae
- C12N2730/10111—Orthohepadnavirus, e.g. hepatitis B virus
- C12N2730/10122—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2810/00—Vectors comprising a targeting moiety
- C12N2810/50—Vectors comprising as targeting moiety peptide derived from defined protein
- C12N2810/60—Vectors comprising as targeting moiety peptide derived from defined protein from viruses
- C12N2810/6036—DNA rev transcr viruses
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- the present invention relates to hydrophobic modified preS-derived peptides of hepatitis B virus (HBV) which are versatile vehicles for the specific delivery of compounds to the liver, preferably to hepatocytes, in vitro as well as in vivo.
- HBV hepatitis B virus
- This liver targeting can further be used for the targeted treatment of liver diseases or disorders, such as hepatitis, malaria, hepatocellular carcinoma (HCC), as well as for the prevention of HBV and/or HDV infection.
- liver diseases or disorders such as hepatitis, malaria, hepatocellular carcinoma (HCC), as well as for the prevention of HBV and/or HDV infection.
- the liver an organ which is present in vertebrates and other animals, plays a major role in the metabolism and has a number of functions in the body, including glycogen storage, decomposition of red blood cells, synthesis of plasma proteins, and detoxification.
- the liver also is the largest gland in the human body. It lies below the diaphragm in the thoracic region of the abdomen. It produces bile, an alkaline compound which aids in digestion, via the emulsification of lipids. It also performs and regulates a wide variety of high-volume biochemical reactions requiring specialized tissues.
- Hepatocytes make up 70 to 80% of the cytoplasmic mass of the liver. Hepatocytes are involved in protein synthesis, protein storage and transformation of carbohydrates, synthesis of cholesterol, bile salts and phospholipids, and detoxification, modification and excretion of exogenous and endogenous substances. The hepatocyte also initiates the formation and secretion of bile.
- liver hematoma liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver hematoma, liver
- liver disease such as biliary atresia, alpha-1-antitrypsin deficiency, alagille syndrome, and progressive familial intrahepatic cholestasis; as well as metabolic diseases.
- Malaria which is one of the most common infectious diseases and an enormous public-health problem. Malaria is caused by protozoan parasites of the genus Plasmodium. The most serious forms of the disease are caused by Plasmodium falciparum and Plasmodium vivax, but other related species ( Plasmodium ovale, Plasmodium malariae, and sometimes Plasmodium knowlesi ) can also infect humans. This group of human-pathogenic Plasmodium species is usually referred to as malaria parasites. Malaria parasites are transmitted by female Anopheles mosquitoes. Malaria in humans develops via two phases: an exoerythrocytic (hepatic phase or "liver stage") and an erythrocytic phase.
- the parasite is relatively protected from attack by the body's immune system because for most of its human life cycle it resides within the liver and blood cells and is relatively invisible to immune surveillance.
- drugs that specifically address the liver specific stages of malaria parasites (e.g. primaquin) in order to obviate the development of blood stages.
- schistosomiasis or bilharziosis, which is a parasitic disease caused by several species of flatworm. Although it has a low mortality rate, schistosomiasis can be very debilitating. It is an often chronic illness that results from infection of the blood with a parasitic flatworm (schistosome). It causes debilitation and causes liver and intestinal damage. It is most commonly found in Asia, Africa, and South America, especially in areas with water that is contaminated with fresh water snails, which contain the parasite.
- Hepatitis B virus the cause for hepatitis B, is the prototype of a family of small, enveloped DNA viruses of mammals and birds (1).
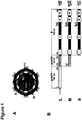
- the HBV envelope encloses three proteins termed L-(large), M-(middle) and S-(small) ( Figure 1A ). They share the C-terminal S-domain with four transmembrane regions.
- the M- and L-protein carry additional N-terminal extensions of 55 and, genotype-dependent, 107 or 118 aa (preS2- and preS1) ( Figure 1B ).
- the stoichiometric ratio of L, M and S is about 1:1:4, while the more abundantly secreted non-infectious subviral particles (SVPs) contain almost exclusively Sand only traces of L-protein (2).
- SVPs non-infectious subviral particles
- the preS1-domain of L is myristoylated and at some point of the HBV life cycle translocated through the ER-derived membrane. This modification is essential for infectivity (3,4).
- HBV as well as the other hepatotropic viruses have a liver tropism, i.e. the liver is the tissue which specifically supports the growth of HBV.
- drug targeting should fulfill the following criteria: 1) exclusive transfer of the drug to the required site of action; 2) a minimum of effects for the remaining organism; 3) use of a pharmacologically inactive vector.
- tissue-specific enzymes for example the use of prodrugs, from which the pharmacologically active part is released in the target tissue by tissue-specific enzymes.
- a further possibility is to couple effective, non-tissue-specific drugs to tissue-specific, but pharmacologically inert carrier systems like receptor-affine peptides or colloidal particles.
- liver targeting of drugs has been used to enhance liver targeting of drugs.
- a straightforward approach is based on the active phagocytosis of the reticuloendothelial system in the liver by delivering drugs in particular carriers, such as liposomes and microspheres.
- particulate carriers incorporating a drug are mainly captured by the reticuloendothelial system in the liver, resulting in drug targeting of the liver (5).
- liver targeting of drugs with positively charged, water-soluble polymers is based on free extravasation of most water-soluble substances from the vascular system of the liver as well as on negative charges on the liver cell surface (6).
- polymers have been used as the carrier to allow drugs to target to the liver based on such anatomical and biochemical characteristics of the liver. More specific drug targeting of the liver has been attempted by using asialoglycoprotein receptors of liver cells.
- the asialoglycoprotein receptor (galactose receptor) is present on hepatocytes with high density.
- the ligand-receptor complex is internalized which allows the cellular uptake of galactosylated ligands (7).
- HBV vectors derived from hepatitis B virus (HBV), which can be used for gene therapy, such as the delivery of therapeutic genes to liver cells and the expression of heterologous genes in liver cells.
- HBV vectors have a limited capacity for packaging genes.
- CN 101 045 156 A discloses "a targeting medicine" with the general formula X-L-Y, wherein X is an amino acid sequence, containing (a) an amino acid sequence derived from a protein of an infective virus with liver specificity, (b) a sequence of (a) generated by permutation, depletion, insertion or accession of one or more amino acid residues; L is an optional linker between X and Y; and Y is a medicine or radical group.
- X can contain a polypeptide of protein pre-S1 of HBV or a variant thereof.
- the present invention aims to provide means and methods for the effective, prevention and/or treatment of liver diseases and the regulation of the liver function.
- this object is solved by providing a hydrophobic modified preS-derived peptide of HBV as vehicle for the specific delivery of compound(s) to the liver for use as a medicament.
- Said hydrophobic modified preS-derived peptide has the general formula: [H m -P-R n ]A 0 .
- P is said preS-derived peptide consisting of an amino acid sequence selected from SEQ ID NOs. 11, 12, 13, 16, 23, and 26.
- H is said hydrophobic modification of the preS-derived peptide P, which is N-terminal of P and selected from acylation with myristoyl (C14) or stearoyl (C18); wherein m is 1.
- R is an optional C-terminal modification (i.e. n is 0 or at least 1) of said preS-derived peptide P.
- A is an optional anchor group (i.e. o is 0 or at least 1), which is suitable for the covalent attachment of the compound(s) and is linked to H, P and/or R.
- Said hydrophobic modified preS-derived peptide further comprises compound(s) selected from a drug; a prodrug; a carrier or depot for a drug, or prodrug and wherein the compound(s) and the hydrophobic peptide form a conjugate.
- this object is furthermore solved by providing the hydrophobic modified preS-derived peptide(s) of HBV of the invention for the treatment of a liver disease or disorder or for the regulation of liver function.
- the present invention provides hydrophobic modified preS-derived peptides of hepatitis B virus (HBV) as vehicle for the specific delivery of compound(s) to the liver for use as a medicament.
- HBV hepatitis B virus
- HBV The envelope of HBV encloses three proteins termed L (large), M (middle) and S (small) (see Figure 1A ). They share the C-terminal S-domain with four transmembrane regions.
- L- and M-protein carry additional N-terminal extensions of 55 and, genotype-dependent, 107 or 118 amino acids (preS2- and preS1) (see Figure 1B ).
- preS-derived peptide of HBV refers to a peptide with an amino acid sequence that corresponds to the N-terminal extensions of the L-protein of HBV, preS1, preferably to a consensus sequence of the species and genotypes A to H as well as of woolly monkey (WMHBV), chimpanzee and gorilla hepatitis B viruses, but it also refers to variants thereof, preferably N-terminally and/or C-terminally truncated variants, amino acid substitution variants.
- SEQ ID NO. 1 shows the HBV preS consensus amino acid sequence of the species and genotypes A to H as well as of woolly monkey (WMHBV).
- HBV preS consensus sequence Consensus
- A-H the eight HBV genotypes
- WHBV woolly monkey HBV
- HBV genotype C refers to an artificial sequence, which corresponds to or is identical to the HBV Genotype C, as e.g. shown in Genbank ABV02850.1, except that position 46 (according to the numbering as described below) is Lys (K) in the genotype C of the present invention instead of Gln (Q) as in the Genbank sequence; the HBV genotype C sequence of this application can also be referred to as "HBV genotype C Q46K". See also SEQ ID NOs. 4, 12, 21-27.
- Figure 2 also shows the numbering of the amino acid residues of the HBV preS consensus sequence, which will be referred to throughout this specification: Amino acid residue number 1 is the methionine (Met1) of genotype D (formerly described as subtype ayw, see also SEQ ID NO. 5), whereas amino acid residue number (-11) is the methionine (Met(-11)) of genotype C (SEQ ID NO. 4).
- Met1 or Met(-11), respectively is cleaved off by a cellular methionyl aminopeptidase and modified by a subsequent transfer of a myristoyl residue from Myristoyl-CoA to amino acid residue number 2 glycine (Gly2) or amino acid residue number (-10) glycine (Gly(-10)), respectively, by N-myristoyl transferase.
- the N-terminal amino acid residue of genotype D is the natural amino acid Glycin (Gly2) and is numbered 2 according to the respective numbering from the codons of the underlying open reading frame of L (or e.g. Gly(-10) for genotype C).
- the HBV preS consensus sequence also comprises the additional N-terminal amino acids of genotypes A, B, C, E, F, G and H (designated at "-" positions). Thus, in total the HBV preS consensus sequence encompasses positions (-11) to 48. Thus, there is a difference between the above described numbering and the actual listing of amino acids in SEQ ID NO. 1, e.g.
- a hydrophobic modified preS-derived peptide of HBV provided for the use according to the present invention has the formula [H m -P-R n ]A 0 wherein
- n and o are 0.
- P does not comprise amino acid substitutions and/or deletions at residues 9 to 15 of SEQ ID NO. 1.
- sequence motif NPLGFFP (corresponding to residues 9 to 15 of SEQ ID NO. 1) is not interrupted or modified, such as by replacing residues 11-15 by D-amino acids.
- the inventors have identified these amino acid residues to be important for the liver tropism of the hydrophobic modified preS-derived peptides of HBV, as described below.
- P consists of an amino acid sequence selected from amino acid residues 2 to 48 of the consensus sequence [SEQ ID NO. 11]; amino acid residues 2 to 21 of the consensus sequence [SEQ ID NO. 12]; amino acid residues 2 to 48 of genotype C [SEQ ID NO. 13]; amino acid residues 2 to 21 of genotype C [SEQ ID NO. 16]; amino acid residues 2 to 48 of genotype D [SEQ ID NO. 23]; and amino acid residues 2 to 21 of genotype D [SEQ ID NO. 26].
- P does not comprise amino acid substitutions and/or deletions at residues 9 to 15 of SEQ ID NO. 1 in order to maintain the liver targeting/tropism.
- P comprises an amino acid sequence selected from SEQ ID NO. 11 (residues 2 to 48 of the consensus sequence), SEQ ID NO. 12 (residues 2 to 21 of the consensus sequence), SEQ ID NO. 13 (residues 2 to 48 of genotype C), SEQ ID NO. 16 (residues 2 to 21 of genotype C), SEQ ID NO. 23 (residues 2 to 48 of genotype D), and SEQ ID NO. 26 (residues 2 to 21 of genotype D),
- the hydrophobic modification (H) of the preS-derived peptide P is N-terminal of P.
- N-terminal refers to the hydrophobic modification at the N-terminus, i.e. the respective first amino acid residue (e.g. Gly 2), but comprises also the hydrophobic modification in close proximity to the N-terminus, such as respective amino acid residues (-4), (-3), (-2), (-1), 1, 2 or 3 or 4.
- the hydrophobic modification can furthermore be obtained by an attachment of a hydrophobic moiety at a site close to the N-terminus of P.
- hydrophobic modification of said preS-derived peptide of HBV adds a hydrophobic moiety to the peptide.
- m is 1, i.e. modification with one hydrophobic moiety or group.
- hydrophobic modification of said preS-derived peptide of HBV provided for the use according to the present invention is selected from:
- Modification by myristoylation is preferred in in vivo and medicinal applications due to its higher safety, e.g. not showing the adverse effects of the stearoyl group (innate immune response etc).
- hydrophobic moieties is prefereably by covalent binding, which can be achieved via carbamate, amide, ether, disulfide or any other linkage that is within the skill of the person skilled in the art.
- hydrophobic modified, namely acylated preS-derived peptides of this invention are preferably lipopeptides due to their N-terminal lipophilic or hydrophobic group/moiety.
- the C-terminal modification (R) of said preS-derived peptide P is preferably a modification with a moiety that protects from degradation, such as in vivo degradation.
- C-terminal refers to the modification at the C-terminus, i.e. the respective last amino acid residue, but comprises also the modification in close proximity to the C-terminus, such as the last but one amino acid residue, the last but two amino acid residue or more amino acid residues (e.g. introduction of one D-amino acid that protects the carrier from enzymatic degradation e.g. by the action of carboxypeptidases).
- Preferred moieties that protect from degradation are selected from amides, D-amino acids, modified amino acids, cyclic amino acids, albumin, natural and synthetic polymers, such as PEG, glycane.
- n is 0 or at least 1, i.e. the C-terminal modification (R) is optional.
- n 1
- n is 1, 2, 3, 4 or more. That is, the C-terminus of P or its proximity can be modified with more than one moiety or group, such as 2.
- the moieties or groups can be the same or different to each other.
- H and/or R are linked to P via a linker or spacer.
- Linker or spacer are known to the skilled artisan, such as polyalanine, polyglycin, carbohydrates, (CH 2 ) n groups.
- the anchor group (A) serves as a point of attachment for a compound, a tag, a label.
- the anchor group is "C-terminal" of P, wherein “C-terminal” refers to the modification at the C-terminus, i.e. the respective last amino acid residue, but comprises also the close proximity of the C-terminus, such as the last but one amino acid residue, the last but two amino acid residue or more amino acid residues.
- n can be 0, thus there is no other C-terminal modification R.
- hydrophobic modified preS-derived peptide of HBV can have the following general formula
- the anchor group A can be at an amino acid side chain of the preS-derived peptide P or can be the amino acid side chain of the preS-derived peptide P itself, i.e. A can be a side chain itself or a modified side chain.
- the anchor group can also be a modified amino acid residue which was introduced into the amino acid sequence of P to serve as an anchor group.
- anchor group A is attached to the hydrophobic modification H and/or the C-terminal modification R.
- Preferred anchor groups are selected from ester, ether, disulfide, amide, thiol, thioester.
- the anchor group can furthermore be suitable for attaching a complex-forming component, such as of the biotin/avidin, polyarginine/oligonucleotide (e.g. siRNA) complex.
- a complex-forming component such as of the biotin/avidin, polyarginine/oligonucleotide (e.g. siRNA) complex.
- o is 0 or at least 1, i.e. the anchor group (R) is optional.
- o is 1.
- o is 1, 2, 3, 4 or more. That is, there are more than one anchor group, such as 2.
- the anchor groups can be the same or different to each other, allowing the attachment of several compounds, such as a drug and a label or different drugs.
- hydrophobic modified preS-derived peptides provided for the use of the invention are the following:
- the more preferred hydrophobic modified preS-derived peptides of the invention are: Designation of peptide Amino acid sequence Hydrophobic modification HBVpreS/2-48 stearoyl (consensus) SEQ ID NO. 11 Stearoyl (C18) HBVpreS/2-48 stearoyl (consensus) SEQ ID NO. 12 Stearoyl (C18) HBVpreS/2-48 stearoyl (C) SEQ ID NO. 13 Stearoyl (C18) HBVpreS/2-48 stearoyl (C) SEQ ID NO. 16 Stearoyl (C18) HBVpreS/2-48 myr (C) SEQ ID NO. 13 Myristoyl (C14) HBVpreS/2-48 myr (D) SEQ ID NO. 23 Myristoyl (C14) HBVpreS/2-21 stearoyl (D) SEQ ID NO. 26 Stearoyl (C18)
- peptides of this invention can be prepared by a variety of procedures readily known to those skilled in the art, in general by synthetic chemical procedures and/or genetic engineering procedures.
- Synthetic chemical procedures include more particularly the solid phase sequential and block synthesis (Erickson and Merrifield, 1976).
- the solid phase sequential procedure can be performed using established automated methods such as by use of an automated peptide synthesizer.
- an ⁇ -amino protected amino acid is bound to a resin support.
- the resin support employed can be any suitable resin conventionally employed in the art for the solid phase preparation of (poly)peptides, preferably polystyrene which has been copolymerized with polyoxyethylen to provide sites for ester formation with the initially introduced o-amino protected amino acid.
- This optimized method applied by the inventors, has been explicitly described (see e.g. 12).
- the amino acids are introduced one by one (stepwise).
- Each synthesis cycle corresponding to the introduction of one amino acid includes a deprotection step, successive washing steps, a coupling step with activation of the amino acid, and subsequent washing steps. Each of these steps is followed by a filtration.
- the reactive agents for coupling are the classical reactive agents for (poly)peptide synthesis such as dicyclohexylcarbodiimide, hydroxybenzotriazole, benzotriazil-1-yl-oxytris (dimethylamino) phosphonium hexafluorophosphate, and diphenylphosphorylazide.
- the polypeptide is separated from the resin by a treatment with a strong acid such as trifluoroacetic acid in the presence of anisol, ethanedithiol or 2-methylindole.
- a strong acid such as trifluoroacetic acid in the presence of anisol, ethanedithiol or 2-methylindole.
- the compound is then purified by the classical techniques of purification, in particular by means of HPLC.
- the peptides of the present invention may also be obtained by coupling (poly)peptide fragments that are selectively protected, this coupling being effected e.g. in a solution.
- the peptides can further be produced by genetic engineering techniques as known to the skilled artisan.
- An eukaryotic expression system such as the baculovirus system, is particularly suitable. According to this procedure proteins are expressed in insect cells infected with a recombinant baculovirus containing a nucleic acid sequence encoding a heterologous protein and regulating nucleic acid sequences, such as a promoter.
- a recombinant baculovirus containing a nucleic acid sequence encoding a heterologous protein and regulating nucleic acid sequences, such as a promoter.
- Several cell-lines are available for infection with recombinant baculovirus, such as cell line Sf-9, available from the American Type Culture Collection (CRL 1711).
- Expression in prokaryotic expression system such as E. coli, is also particularly suitable.
- hydrophobic moiety to the peptide can be accomplished by a variety of procedures readily known to those skilled in the art, including synthetic and genetic engineering approaches.
- the peptides and/or fusion peptides can be produced by stably transfected eukaryotic cell lines, like CHO and other cell lines which are known in the art and usually used for generating vaccines and the like. Due to the intrinsic property that the N-terminal 47-preS1 amino acids promote secretion of a myristoylated protein/peptide, the biologically active hydrophobic modified peptide can be extracted from cell culture supernatants.
- the present invention provides the hydrophobic modified preS-derived peptides of HBV as vehicle or shuttle for the specific delivery of a compound to the liver for use as a medicament.
- “Vehicle” or “shuttle” for the specific delivery of a compound to the liver according to the present invention refers to the liver tropism or hepatotropism of the hydrophobic modified preS-derived peptides of HBV as found by the inventors and described herein, i.e. to their capacity to selectively accumulate in the liver, preferably to selectively accumulate at the plasma membrane of hepatocytes as well as to selectively enter into hepatocytes.
- the invention is based on the finding of a highly specific liver accumulation and on the identification of the determinants of the liver tropism of HBV in the preS 1 sequence of HBV by the inventors.
- the invention uses the knowledge about the determinants of the liver tropism for the design of universal vehicles or shuttles for specific liver targeting or delivery, respectively.
- hydrophobic modified preS-derived peptides of the present invention are versatile vehicles or shuttles for specifically delivering compound(s) to the liver.
- the specific delivery of a compound to the liver is the specific delivery of the compound to hepatocytes.
- the compound can specifically be delivered to hepatocytes in vitro as well as in vivo.
- the compound is preferably specifically delivered to the liver of an animal, preferably mammal or human, or a bird.
- the "compound” to be specifically delivered to the liver according to this invention are selected from drugs; prodrugs; a carrier or depot for a drug, or prodrug.
- Drugs can be in form of prodrugs or preprodrugs.
- the compound to be specifically delivered to hepatocytes is a drug (or a drug in form of a prodrug) and is preferably selected from Drug class Examples interferon IFN ⁇ viral reverse transcriptase inhibitor Lamivudine, Adefovir, Entecavir viral RNA polymerase inhibitor HCV NS5B polymerase inhibitor viral core assembly inhibitor or for hepatropic viruses viral nucleocapsid inhibitor e.g.
- dihydroarylpyrimidine-derived HBV capsid assembly inhibitors kinase inhibitor Raf kinase inhibitor Sorafenib (BAY 43-9006) nucleoside analogue protease inhibitor HCV NS3 protease inhibitor, BILN-2061, VX950 - viral or general protease inhibitor proteasom inhibitor MG132, bortezomib antibody or fragment thereof Neutralizing anti-HCV E2 antibodies siRNA or precursor thereof HBV, HCV, HDV-specific siRNAs and modifications thereof; siRNAs targeting other sequences, including cellular sequences farnesylation inhibitor; such as farnesyl transferase inhibitor (FTI) alcohol dehydrogenase (ADH) or activator thereof cholesterol biosynthesis inhibitor, such as HMD-CoA-reductase inhibitors Mevinacor/Lovastatin inhibitor of the liver stage of a virus or a non-viral pathogen, such as malaria, schistosomiasis (bilharzio
- the compound is preferably selected from a clinically approved drug.
- the compound to be delivered is IFN-alpha.
- P preferably comprises an amino acid sequence of SEQ ID NO. 23 and/or H is a hydrophobic modification with myristoyl (C14).
- the hydrophobic modified preS1-derived peptide(s) used in such an embodiment can be HBVpreS/2- 20 myr (D), HBVpreS/2-48 myr (D), HBVpreS/2-20 stearoyl (D), HBVpreS/2-48 stearoyl (D) (see also Figure 14 ).
- a fusion protein or construct with mouse interferon-alpha was obtained by recombinant expression in insect cells using the baculovirus expression system. See also Figures 14 and 15 as well as Examples.
- the compound is interferon, such as IFN ⁇
- P preferably comprises an amino acid sequence of SEQ ID NO. 23 and/or H is a hydrophobic modification with myristoyl (C14)
- the compound is labelled, i.e. carries a label as defined herein.
- the compounds are in form of depots or carriers, which e.g. carry a drug, prodrug.
- depots or carriers are known in the art, such as nanoparticles, liposomes, microbubbles, gas emulsions.
- the compound and the hydrophobic modified preS-derived peptide of HBV form a conjugate.
- the conjugate of compound and hydrophobic modified preS-derived peptide is formed by covalent attachment or by complex formation.
- the compound is covalently attached to the hydrophobic modified preS-derived peptide, preferably by attaching the compound to an anchor group A.
- the form of attachment depends on the type of compound. The person of skill in the art will be able to determine suitable anchor groups for forming suitable conjugates.
- A furthermore comprises a spacer or linker.
- the spacer or linker preferably comprises a recognition site for hepatocyte specific activation, which is preferably recognized by a liver protein.
- the recognition site is preferably a proteolytic cleavage site.
- the liver protein is, thus, preferably a hepatocellular protein, more preferably a hepatocellular proteolytic enzyme.
- the conjugate can be administered to a subject and will be transported through the body, such as in the bodily fluids, without being cleaved. However, as soon as the conjugate reaches its target, the liver or the hepatocytes, respectively, the liver protein, such as a hepatocellular proteolytic enzyme will cleave the proteolytic cleavage site and release the compound from its shuttle, i.e. the hydrophobic modified preS-derived peptide.
- liver proteins are cytochromes, such as cytochrome P450.
- the HepDirect® technology (of Metabasis Technologies, Inc.) as used in Adefovir or Pradevofir, is also suitable for the present invention.
- the conjugate of compound and hydrophobic modified preS-derived peptide is formed by complex formation.
- Preferred complexes useful in the invention are biotin/avidin, polyarginine/oligonucleotide (e.g. siRNA). The skilled artisan will be able to determine suitable complex components and to design the compound and hydrophobic modified preS-derived peptide accordingly.
- the hydrophobic modified preS-derived peptide in particular the conjugates of the present invention, are preferably used to enrich a compound, that is shuttled to the liver, in the liver.
- the compound is cleaved of the conjugate with the hydrophobic modified preS-derived peptide of HBV by a liver protein, preferably a hepatocellular proteolytic enzyme, in particular in vivo in the liver.
- the above hydrophobic modified preS-derived peptides in particular their conjugates with compounds, are provided for the treatment of a liver disease or disorder.
- the respective compound is selected and selectively, specifically delivered to the liver.
- liver disease or a “liver disorder” according to the present invention refers to any disease or disorder that has an effect on or involves the organ liver, liver tissue or hepatocytes.
- liver diseases are:
- liver diseases of animals such as pets or livestock
- diseases that can be transmitted to humans such as toxoplasmosis
- the liver disease or disorder to be treated is preferably selected from hepatitis, cirrhosis, haemochromatosis, preferably hepatitis caused by hepatitis A, B, C, D, E, F, G and H virus.
- the liver disease or disorder to be treated can also be concomitant hepatitis caused by viruses, such as viruses of the family Herpesviridae, e.g. herpes virus, cytomegalie virus (CMV) but also varicella zoster virus (VZV), Epstein Barr virus (EBV), coxsackie viruses, yellow fever virus, Dengue virus.
- viruses of the family Herpesviridae e.g. herpes virus, cytomegalie virus (CMV) but also varicella zoster virus (VZV), Epstein Barr virus (EBV), coxsackie viruses, yellow fever virus, Dengue virus.
- the liver disease or disorder to be treated can also be a disease which involves a liver stadium of a virus or a non-viral pathogen, such as in many tropical diseases. Since the liver stadium of some pathogens is an early stadium, the respective infection can be selectively, specifically treated in such an early stadium.
- viruses are hepatitis A, B, C, D, E, F, G and H virus, herpes viruses.
- Such non-viral pathogens are bacteria, parasites and/or worms.
- Parasites are for example protozoan parasites of the genus Plasmodium that cause malaria, such as Plasmodium falciparum, Plasmodium vivax, and related species (e.g. Plasmodium ovale, Plasmodium malariae, Plasmodium knowlesi ).
- Such worms are for example flatworms of the genus Schistosoma that cause schistosomiasis or bilharziosis, such as Schistosoma mansoni, Schistosoma intercalatum, Schistosoma haematobium, Schistosoma japonicum and Schistosoma mekongi.
- Such parasites are also for example Leishmania trypanosome protozoa of the genus Phlebotomus and Lutzomyia which are responsible for the disease leishmaniasis.
- malaria malaria, schistosomiasis (bilharziosis), and/or leishmaniasis can be treated by the means of this invention.
- liver disease or disorder to be treated are preferably liver tumors, preferably hepatocellular carcinoma (HCC).
- HCC hepatocellular carcinoma
- the liver disease or disorder to be treated can also be a metabolic disease, such as diabetes, hyperlipidemia, metabolic syndrome and obesity, chronic hyperglycemia, metabolic syndrome, non-alcoholic steatohepatitis (NASH) (see also (9)).
- a metabolic disease such as diabetes, hyperlipidemia, metabolic syndrome and obesity, chronic hyperglycemia, metabolic syndrome, non-alcoholic steatohepatitis (NASH) (see also (9)).
- NASH non-alcoholic steatohepatitis
- the above hydrophobic modified preS-derived peptides in particular their conjugates with compounds, are provided for the regulation of liver function.
- the compound to be delivered to the liver is preferably an immunogenic epitope.
- the above described hydrophobic modified preS-derived peptides are also suitable for the prevention of hepatitis B and/or D infection.
- This is possible due to the property of the above described hydrophobic modified preS-derived peptides to target not only the liver (liver tropism) but furthermore act as viral entry inhibitors of HBV and/or HDV, which can be seen e.g. in Figure 9 .
- the above described hydrophobic modified preS-derived peptides are suitable HBV and/or HDV vaccines.
- acylated preS-derived peptides in particular their conjugates with compounds, can be provided for the combined treatment of a liver disease or disorder and the prevention of hepatitis B and/or D infection.
- the use according of the invention comprises the use of a pharmaceutical composition comprising at least one hydrophobic modified preS-derived peptide of HBV as defined herein and at least a compound to be specifically delivered to the liver as defined herein and optionally a pharmaceutically acceptable carrier and/or excipient.
- the pharmaceutical composition according to the present invention comprises:
- compositions according to the present invention are very well suited for all the uses and methods described herein.
- a “pharmaceutically acceptable carrier or excipient” refers to any vehicle wherein or with which the pharmaceutical or vaccine compositions according to the invention may be formulated. It includes a saline solution such as phosphate buffer saline. In general, a diluent or carrier is selected on the basis of the mode and route of administration, and standard pharmaceutical practice.
- the present disclosure describes methods for the diagnosis, prevention and/or treatment of a liver disease or disorder by utilizing the hydrophobic modified preS-derived peptide(s) of HBV or the pharmaceutical composition(s) of the invention.
- the present disclosure also describes a method for the combined diagnosis, prevention and/or treatment of a liver disease or disorder and the prevention of HBV and/or HDV infection by administering to a subject a conjugate as defined herein, which comprises a hydrophobic modified preS-derived peptide of HBV and a compound, or a pharmaceutical composition as defined herein.
- the method for the combined diagnosis, prevention and/or treatment of a liver disease or disorder and the prevention of hepatitis B and/or D infection can comprise administering to a subject in a therapeutically effective amount
- the route of administration of the conjugates or pharmaceutical compositions of the present invention is selected from subcutaneous, intravenous, oral, nasal, intramuscular, transdermal, inhalative, by suppository.
- a preferred embodiment for nasal administration or application is a nasal spray.
- a “therapeutically effective amount” of a conjugate or a pharmaceutical composition of this invention refers to the amount that is sufficient to treat the respective liver disease or disorder and/or to prevent hepatitis B and/or D infection.
- a preferred therapeutically effective amount is in the range of 10 ⁇ g to 1 mg per kg body weight.
- the preferred therapeutically effective amount is in the range of 10 ⁇ g to 1 mg per kg body weight.
- a preferred therapeutically effective amount is about 100 ⁇ g per kg body weight or in the range of 1 to 5 mg per patient.
- the preferred therapeutically effective amount depends on the respective compound that is to be delivered and its respective therapeutically effective amount.
- the disclosure also describes the preS-derived peptides, i.e. nucleic acids encoding them, in gene therapy approaches.
- a viral vector comprises a nucleic acid encoding a preS-derived peptide P as defined herein for the gene therapy of a liver disease or disorder.
- the nucleic acid encodes the amino acid sequence of the preS-derived peptide P, which is necessary and sufficient to accomplish liver targeting.
- the viral vector is replication defective, preferably an adeno-associated viral vector.
- the viral vector can further comprise a heterologous sequence to be expressed in the liver.
- the viral vector can be a gene therapeutically suitable vector, which are known to the skilled person.
- the disclosure further describes a method for in vitro and/or in vivo identification of a hepatocyte receptor involved in the attachment and/or penetration of HBV and/or quantitation of the expression of said receptor that comprises using a hydrophobic modified preS-derived peptide as described above.
- Said hepatocyte receptor can be identified in mammals or respective animal models, such as mouse or human.
- said method comprises the steps comprising:
- the method for the identification of a HBV receptor comprises the use of
- Enzyme labels comprise conjugation of an enzyme to a molecule of interest, e. g. a polypeptide, and can be detected by any of colorimetric, spectrophotometric, or fluorospectrophotometric techniques.
- HBV Hepatitis B Virus
- Hallmarks of HBV infection are a remarkable in vivo efficacy and a pronounced liver tropism. The latter is probably the result of a specific interaction of one of the three HBV surface proteins with liver associated receptors or liver-targeting factors in the circulation system.
- the present invention led to the development of a new class of peptide-drug conjugates to target liver diseases, such as HCC, and optionally to interfere with HBV and HDV infection.
- HBV-preS1-surface protein-derived lipopeptides that efficiently block HBV entry in vitro and in vivo.
- Biodistribution studies of the present invention on these inhibitory peptides revealed that they selectively accumulate in the liver were they bind to and presumably enter into hepatocytes.
- This hepatotropism requires N-terminal acylation of the peptide and depends on a certain HBV preS-sequence motif within the N-terminal 47 preS1 amino acids, i.e. within the amino acid residues 2 to 21 or amino acid residues 2 to 20 (or preferably the minimal sequence of residues 9 to 15).
- this peptide sequence additionally bears a membrane translocation signal which facilitates the transport of even complete fusion proteins across plasma membranes (unpublished results) opens the possibility of specifically delivering any kind of drug to the plasma membrane of hepatocytes or selectively even into this cell.
- HBV preS1-derived lipopeptides are capable to completely prevent HBV infection in a transplanted uPA-RAG-1 mouse model at very low doses.
- Pharmakokinetic studies on these HBVpreS-derived entry inhibitors indicated a remarkable hepatotropism combined with an extraordinary high serum stability (t 1/2 ca. 60 h) and a long half life time in the target organ (t 1/2 ca. 24 h).
- Both N-terminal acylation as well as the integrity of the certain amino acid sequence of the peptides are mandatory (i.e. within the amino acid residues 2 to 21 (or preferably the minimal sequence of residues 9 to 15)).
- the peptides can be used as versatile vectors for liver specific drug targeting to conquer infections of hepatocytes or to treat hepatocellular carcinoma.
- the inventors have furthermore proven the principle that WMHBV infection can be efficiently blocked through subcutaneous application of HBV envelope protein-derived peptides in vivo. This opens new perspectives for the prevention of acute HBV-infection and therapeutic options for chronic hepatitis B. Since the uPA/RAG-2/Pfp mice used lack B cells, T cells, and NK cells, a direct inhibitory effect of the peptides on susceptible hepatocytes is assumed. This is supported by the efficient accumulation of acylated preS-derived peptides in the liver, followed by a slow clearance possibly via the biliary route. Both properties permit subcutaneous application at very low doses and low frequencies.
- the biodistribution of the hydrophobic modified preS-derived peptides was studied in male NMRI mice. All experiments were performed in compliance with German laws.
- the peptides, containing an additional Tyr-residue at the C-terminal end were labelled with 131 I (Amersham Biosciences, Freiburg, Germany) by the chloramine-T method and purified by HPLC.
- the labelled peptides were subcutaneously administered by injection of a solution in 50% DMSO.
- mice were sacrificed and the radioactivity contained in the blood, heart, lung, spleen, liver, kidney, muscle and brain was measured in a ⁇ -counter (Canberra Packard, Rüsselsheim, Germany) and expressed as a percentage of injected dose per gram of tissue (%ID/g).
- I labelled HBVpreS/2-48 myr D was extracted from one liver lobe 24 h post subcutaneous injection. To that aim, 1 ml water per gram frozen liver tissue was added to the sample. After homogenization an equal volume of acetonitrile was added and the homogenization was repeated. After centrifugation (2 x 10 min at 4000 x g) this solution was separated on a reverse phase HPLC column and the radioactivity of each fraction was quantified in a gamma counter.
- HepaRG cells were grown in William's E medium supplemented with 10% fetal calf serum (FCS), 100 units/ml penicillin, 100 ⁇ g/ml streptomycin, 5 ⁇ g/ml insulin and 5x10 -5 M hydrocortisone hemisuccinate (10). Cells were passaged 1/5 every two weeks by trypsination. Two to three weeks before infection cell differentiation was induced by adding 2% DMSO into the maintenance medium. The medium was exchanged every 2-3 days.
- FCS fetal calf serum
- HepG2 clone 2.2.15 (11) cells As an infectious inoculum, a 50-fold concentrated culture supernatant of HepG2 clone 2.2.15 (11) cells was used, because of an unlimited supply and a constant quality. It was prepared from freshly collected supernatants by precipitating viral particles in the presence of 6% polyethylene glycol (PEG) 8000. The pellet was resuspended in phosphate buffered saline (PBS) containing 25% FCS. Aliquots were stored at -80°C. Differentiated HepaRG cells or PHH were incubated with the concentrated infectious source, 10-fold diluted in culture medium supplemented with 4% PEG 8000 (Sigma), for 20 h at 37°C. At the end of the incubation, cells were washed three times with the culture medium and maintained in the presence of 2% DMSO and 5x10 -5 M hydrocortisone hemisuccinate and harvested at indicated times.
- PEG polyethylene glycol
- HepaRG cells were infected either in absence (0 nM) or in the presence of 1, 5, 25, 100 and 2000 nM of the respective peptides of the invention.
- the infectious inoculum (HBV of genotype D) and the peptides were incubated overnight. After washing, cells were maintained for another 12 days to allow viral gene expression.
- Cell culture supernatants from day 8-12 were collected and analyzed for secreted HBSAg using a quantitative commercially available ELISA.
- HBsAg values from the respective uncompleted infection were set to 100 % and the degree of infection inhibition is given in % of the uncompleted infection.
- Cryo-preserved primary hepatocytes were thawed and washed with serum-free medium.
- 4 x 10 5 cells/ml were incubated with the respective peptide at a concentration of 200 nM in serum-free cell culture medium. The staining was performed for 30 minutes at room temperature with frequent mixing. Subsequently, cells were washed extensively and resuspended in PBS to proceed with the FACS analysis.
- HBVpreS1-peptide - mouse interferon alpha fusion proteins were expressed in Hi5-insect cells using the baculovirus expression system.
- the fusion proteins secreted in the cell supernatants were harvested on day 5 post infection and purified in a first step by an affinity chromatography for the His-tag fused C-terminally to the preS1-interferon.
- the activity of the preS1-interferon proteins in the elution fractions was measured by their ability to inhibit Newcastle disease virus mediated cell death.
- the elution fractions containing functional preS1-interferon proteins were further purified by gel filtration chromatography on a S75-sepharose column with a 1M urea-PBS buffer (see Figure 15 ).
Claims (14)
- Peptide issu de préS modifié hydrophobe du virus de l'hépatite B (VHB) possédant la formule
[Hm-P-Rn]A0
en tant que véhicule pour l'administration spécifique de composé(s) au foie, destiné à être utilisé en tant que médicament,
dans laquelleP est ledit peptide issu de préS consistant en une séquence d'acides aminés sélectionnée parmi SEQ ID NO: 11, 12, 13, 16, 23, et 26 ;H est ladite modification hydrophobe du peptide P issu de préS, qui est l'extrémité N-terminale de P et sélectionnée parmi une acylation avec un stéaroyle (C18) ou un myristoyle (C14),m est 1,R est une modification C-terminale dudit peptide P issu de préS,
qui est de préférence un fragment qui confère une protection contre la dégradation, sélectionné parmi un amide, un acide D-aminé, un acide aminé modifié, un acide aminé cyclique, l'albumine, un polymère naturel et synthétique, tel que le PEG, un glycane,n est 0 ou au moins 1,A est un groupe d'ancrage,
de préférence sélectionné parmi un ester, un éther, un disulfure, un amide, un thiol, un thioester,o est 0 ou au moins 1,comprenant en outre un/des composé(s) sélectionné(s) parmi un médicament ; un promédicament ; un véhicule ou un dépôt pour un médicament, ou un promédicament, et où le(s) composé(s) et le peptide hydrophobe forment un conjugué. - Peptide issu de préS modifié hydrophobe destiné à être utilisé selon la revendication 1, dans lequel H et/ou R sont liés à P via un segment de liaison ou un espaceur.
- Peptide issu de préS modifié hydrophobe destiné à être utilisé selon la revendication 1, dans lequel le composé est sélectionné parmi un médicament, de préférence
un interféron, tel que l'IFNα ;
un inhibiteur de transcriptase inverse virale ;
un inhibiteur d'ARN polymérase virale ;
un inhibiteur d'assemblage du noyau viral ou un inhibiteur de nucléocapside virale ;
un inhibiteur de kinase, tel qu'un inhibiteur de kinase Raf ;
un analogue de nucléoside ;
un inhibiteur de protéase ;
un inhibiteur du protéasome ;
un anticorps ou un fragment de celui-ci ;
un ARNsi ou un précurseur de celui-ci ;
un inhibiteur de farnésylation ; tel qu'un inhibiteur de la farnésyltransférase (FTI) ; l'alcool déshydrogénase (ADH) ou un activateur de celle-ci ;
un inhibiteur de la biosynthèse du cholestérol ;
un inhibiteur du stade hépatique d'un virus ou d'un agent pathogène non viral ;
un antibiotique,
où, de préférence, le médicament est sous la forme d'un promédicament. - Peptide issu de préS modifié hydrophobe destiné à être utilisé selon la revendication 3, dans lequel le composé est un interféron, tel que l'IFNα, et P comprend de préférence une séquence d'acides aminés de SEQ ID NO: 23 et/ou H est une modification hydrophobe avec un myristoyle (C14).
- Peptide issu de préS modifié hydrophobe destiné à être utilisé selon l'une quelconque des revendications 1 à 4, dans lequel le conjugué est formé par un attachement covalent ou par la formation d'un complexe, de manière davantage préférée par un attachement covalent d'un composé à un groupe d'ancrage A.
- Peptide issu de préS modifié hydrophobe destiné à être utilisé selon l'une quelconque des revendications 1 à 5, dans lequel le groupe d'ancrage A comprend en outre un espaceur ou un segment de liaison,
dans lequel l'espaceur ou le segment de liaison comprend de préférence un site de reconnaissance, qui est de préférence reconnu par une protéine hépatique, de préférence une protéine hépatocellulaire, de manière davantage préférée une enzyme protéolytique hépatocellulaire ou un cytochrome. - Peptide issu de préS modifié hydrophobe destiné à être utilisé selon l'une quelconque des revendications 1 à 6, comprenant l'utilisation d'une composition pharmaceutique comprenant :au moins un peptide issu de préS modifié hydrophobe du VHB selon la revendication 1 ou 2 ;au moins un composé devant être spécifiquement administré au foie, de préférence à des hépatocytes, de préférence tel que défini dans l'une quelconque des revendications 1, 3 à 4 ;ou un conjugué tel que défini dans la revendication 5 ; etfacultativement un véhicule et/ou excipient pharmaceutiquement acceptable.
- Peptide issu de préS modifié hydrophobe destiné à être utilisé selon l'une quelconque des revendications 1 à 7, dans lequel l'administration spécifique d'un composé au foie est de préférence l'administration spécifique du composé à des hépatocytes.
- Peptide issu de préS modifié hydrophobe destiné à être utilisé selon la revendication 8, dans lequel le composé est spécifiquement administré au foie d'un animal, de préférence un mammifère ou un humain, ou un oiseau,
et/ou dans lequel le composé est de préférence enrichi dans le foie,
et/ou dans lequel le composé est de préférence clivé du conjugué covalent avec le peptide issu de préS modifié hydrophobe du VHB par une protéine hépatique, de préférence une protéine hépatocellulaire, de manière davantage préférée une enzyme protéolytique hépatocellulaire ou un cytochrome. - Peptide issu de préS modifié hydrophobe destiné à être utilisé selon l'une quelconque des revendications 1 à 9, destiné à être utilisé dans un procédé de traitement d'une maladie ou d'un trouble hépatique.
- Peptide issu de préS modifié hydrophobe destiné à être utilisé selon la revendication 10, dans lequel la maladie ou le trouble hépatique est sélectionné(e) parmi l'hépatite, la cirrhose, l'hémochromatose, de préférence l'hépatite provoquée par le virus de l'hépatite A, B, C, D, E, F, G et H ou une hépatite concomitante provoquée par des virus,
et/ou dans lequel la maladie ou le trouble hépatique est une maladie qui implique un stade hépatique d'un virus ou d'un agent pathogène non viral, de préférence une maladie tropicale, la malaria, la schistosomiase, la leishmaniose. - Peptide issu de préS modifié hydrophobe destiné à être utilisé selon la revendication 10, dans lequel la maladie ou le trouble hépatique est une tumeur hépatique, de préférence un carcinome hépatocellulaire (CHC),
ou dans lequel la maladie ou le trouble hépatique est une maladie métabolique, de préférence l'obésité, le syndrome métabolique, la stéatohépatite non alcoolique (NASH). - Peptide issu de préS modifié hydrophobe destiné à être utilisé selon la revendication 10, destiné à être utilisé dans la régulation de la fonction hépatique et/ou destiné à être utilisé dans la présentation d'un antigène médiée par un hépatocyte et l'activation de réponses immunologiques dirigées vers le foie.
- Peptide issu de préS modifié hydrophobe destiné à être utilisé selon la revendication 10 dans la prévention d'une infection par l'hépatite B et/ou D en tant que vaccin.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US6234708P | 2008-01-25 | 2008-01-25 | |
| PCT/EP2009/000477 WO2009092612A1 (fr) | 2008-01-25 | 2009-01-26 | Peptides issus de pres modifiés hydrophobes du virus de l'hépatite b (vhb) et leur utilisation en tant que véhicules pour l'administration spécifique de composés au foie |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2245047A1 EP2245047A1 (fr) | 2010-11-03 |
| EP2245047B1 true EP2245047B1 (fr) | 2020-11-18 |
Family
ID=40521848
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09704030.7A Active EP2245047B1 (fr) | 2008-01-25 | 2009-01-26 | Peptides issus de pres modifiés hydrophobes du virus de l'hépatite b (vhb) et leur utilisation en tant que véhicules pour l'administration spécifique de composés au foie |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US9868768B2 (fr) |
| EP (1) | EP2245047B1 (fr) |
| JP (1) | JP5686468B2 (fr) |
| CN (1) | CN102015753A (fr) |
| ES (1) | ES2847288T3 (fr) |
| WO (1) | WO2009092612A1 (fr) |
Families Citing this family (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1281761A1 (fr) | 2001-07-27 | 2003-02-05 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Polypeptides synthetiques derivées du proteine pre-S1 de Hepatitis B virus et utilisations associées |
| WO2008098182A1 (fr) | 2007-02-08 | 2008-08-14 | University Of Utah Research Foundation | Procédés et compositions apparentés à l'inhibition d'une entrée virale |
| US8875361B2 (en) | 2008-05-21 | 2014-11-04 | Wirtz Manufacturing Co., Inc. | Reformed battery grids |
| KR20120052352A (ko) * | 2009-08-07 | 2012-05-23 | 트랜스진 에스.에이. | Hbv 감염을 치료하는 조성물 |
| CN102241744B (zh) * | 2010-05-14 | 2015-03-04 | 上海贺普药业股份有限公司 | 一种病毒感染阻断剂、其药物组合物及其应用 |
| WO2012106403A2 (fr) * | 2011-02-01 | 2012-08-09 | Uab Research Foundation | Procédés et compositions pour alphavirus pseudo-infectieux |
| JP6486003B2 (ja) * | 2011-02-10 | 2019-03-20 | ルプレヒト−カールス−ウニヴェルジテート ハイデルベルクRuprecht−Karls−Universitaet Heidelberg | 肝臓特異的診断のための疎水性修飾ペプチド |
| JP6486004B2 (ja) * | 2011-02-10 | 2019-03-20 | ルプレヒト−カールス−ウニヴェルジテート ハイデルベルクRuprecht−Karls−Universitaet Heidelberg | 疎水性修飾ペプチドおよび肝臓特異的標的化のためのその使用 |
| CA2868735C (fr) | 2011-03-28 | 2020-02-25 | University Of Utah Research Foundation | Conjugues de peptide d renfemant une membrane localisant une molecule cargo ameliorant le potentiel en vue d'empecher l'entree du vih |
| CN109354623B (zh) * | 2012-04-25 | 2022-06-24 | 华辉安健(北京)生物科技有限公司 | 乙肝肝炎病毒功能性受体的组成以及相关应用 |
| EP2703001A1 (fr) | 2012-08-26 | 2014-03-05 | XAX Kft. | Vaccination anti-tumorale |
| CN103665095A (zh) * | 2012-09-11 | 2014-03-26 | 中国人民解放军军事医学科学院卫生学环境医学研究所 | 一种疏水性多肽修饰抗体的合成方法 |
| US8961995B2 (en) * | 2012-09-20 | 2015-02-24 | Uab Research Foundation | Methods and compositions for alphavirus replicons |
| CN104781274B (zh) * | 2012-11-12 | 2020-03-06 | 海德堡吕布莱希特-卡尔斯大学 | 用于治疗肝脏疾病和心血管疾病的脂肽 |
| US20180228804A1 (en) * | 2014-10-07 | 2018-08-16 | Myr Gmbh | Combination therapy of hbv and hdv infection |
| EP3138579A1 (fr) * | 2015-09-05 | 2017-03-08 | Biomay Ag | Protéine de fusion pour son utilisation dans le traitement d'une infection par le virus de l'hépatite b |
| EP3181146A1 (fr) * | 2015-12-16 | 2017-06-21 | Ruprecht-Karls-Universität Heidelberg | Peptides ciblant les ntcp cycliques et leurs utilisations en tant qu'inhibiteurs d'entrées |
| EP3189850A1 (fr) * | 2015-12-16 | 2017-07-12 | Ruprecht-Karls-Universität Heidelberg | Ciblage hépatique de peptides issus de pres cyclique de hbv |
| US10487121B2 (en) | 2016-01-07 | 2019-11-26 | Navigen, Inc. | D-peptide inhibitors of HIV entry and methods of use |
| WO2018054891A1 (fr) | 2016-09-20 | 2018-03-29 | Ruprecht-Karls-Universität | Composés et leurs combinaisons de prévention et/ou de traitement d'infections par le hbv et/ou le hdv |
| EP3392267A1 (fr) * | 2017-04-18 | 2018-10-24 | Myr GmbH | Traitement de l'athérosclérose, de la cirrhose biliaire primaire et des maladies associées à l'inflammasome nlrp3 par des inhibiteurs de htcp |
| CN109718364A (zh) * | 2017-10-27 | 2019-05-07 | 上海贺普药业股份有限公司 | 充分剂量条件下治疗乙肝病毒相关肝病的药物和方法 |
| EP3804750A1 (fr) | 2019-10-10 | 2021-04-14 | Universität Heidelberg | Composés conjugués pour la prévention et/ou le traitement des infections par le vhb et/ou le vhd, des maladies du foie et pour le ciblage des ntcp |
| KR20240052981A (ko) | 2021-09-14 | 2024-04-23 | 어드바팜 게엠베하 | 신규 지질펩티드 제형 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5204096A (en) * | 1984-03-07 | 1993-04-20 | New York Blood Center, Inc. | Pre-S gene coded peptide hepatitis B immunogens, vaccines, diagnostics, and synthetic lipid vesicle carriers |
| EP1281761A1 (fr) * | 2001-07-27 | 2003-02-05 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Polypeptides synthetiques derivées du proteine pre-S1 de Hepatitis B virus et utilisations associées |
| CN101045156B (zh) * | 2006-03-29 | 2012-05-02 | 刘宏利 | 特异靶向性药物及其用途 |
| US8748373B2 (en) * | 2007-02-21 | 2014-06-10 | Fox Chase Cancer Center | Hepatitis B virus compositions and methods of use |
-
2009
- 2009-01-26 ES ES09704030T patent/ES2847288T3/es active Active
- 2009-01-26 JP JP2010543441A patent/JP5686468B2/ja not_active Expired - Fee Related
- 2009-01-26 US US12/864,197 patent/US9868768B2/en active Active
- 2009-01-26 CN CN2009801125069A patent/CN102015753A/zh active Pending
- 2009-01-26 WO PCT/EP2009/000477 patent/WO2009092612A1/fr active Application Filing
- 2009-01-26 EP EP09704030.7A patent/EP2245047B1/fr active Active
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2847288T3 (es) | 2021-08-02 |
| JP2011512123A (ja) | 2011-04-21 |
| WO2009092612A1 (fr) | 2009-07-30 |
| CN102015753A (zh) | 2011-04-13 |
| EP2245047A1 (fr) | 2010-11-03 |
| US9868768B2 (en) | 2018-01-16 |
| JP5686468B2 (ja) | 2015-03-18 |
| US20110027183A1 (en) | 2011-02-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2245047B1 (fr) | Peptides issus de pres modifiés hydrophobes du virus de l'hépatite b (vhb) et leur utilisation en tant que véhicules pour l'administration spécifique de composés au foie | |
| EP2247605B1 (fr) | Peptides dérivés de préS modifiés hydrophobes du virus de l'hépatite B (VHB) et leur utilisation en tant qu'inhibiteurs de la pénétration du VHB et du VHD | |
| EP2673004B1 (fr) | Peptides modifiés hydrophobes et leur utilisation pour un ciblage hépatique spécifique | |
| US10363323B2 (en) | Hydrophobic modified peptides for liver specific diagnosis | |
| EP3189850A1 (fr) | Ciblage hépatique de peptides issus de pres cyclique de hbv |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20100805 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA RS |
|
| DAX | Request for extension of the european patent (deleted) | ||
| 17Q | First examination report despatched |
Effective date: 20130614 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20200707 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602009063066 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1335646 Country of ref document: AT Kind code of ref document: T Effective date: 20201215 Ref country code: CH Ref legal event code: NV Representative=s name: E. BLUM AND CO. AG PATENT- UND MARKENANWAELTE , CH |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1335646 Country of ref document: AT Kind code of ref document: T Effective date: 20201118 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20201118 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210218 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210318 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210219 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210218 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210318 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2847288 Country of ref document: ES Kind code of ref document: T3 Effective date: 20210802 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602009063066 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210126 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20210131 |
|
| 26N | No opposition filed |
Effective date: 20210819 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210126 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20220125 Year of fee payment: 14 Ref country code: DE Payment date: 20220120 Year of fee payment: 14 Ref country code: CH Payment date: 20220125 Year of fee payment: 14 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210318 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20220124 Year of fee payment: 14 Ref country code: FR Payment date: 20220120 Year of fee payment: 14 Ref country code: ES Payment date: 20220216 Year of fee payment: 14 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20090126 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602009063066 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20230126 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230131 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230126 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230801 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230126 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20240402 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230127 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201118 Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230127 |