EP2004758B1 - Färbezusammensetzung mit einem thiol/disulfid- leuchtfarbstoff mit einem heterozyklus und einer internen kationischen ladung sowie verfahren zur aufhellung von keratinmaterial mit diesem farbstoff - Google Patents
Färbezusammensetzung mit einem thiol/disulfid- leuchtfarbstoff mit einem heterozyklus und einer internen kationischen ladung sowie verfahren zur aufhellung von keratinmaterial mit diesem farbstoff Download PDFInfo
- Publication number
- EP2004758B1 EP2004758B1 EP07731817A EP07731817A EP2004758B1 EP 2004758 B1 EP2004758 B1 EP 2004758B1 EP 07731817 A EP07731817 A EP 07731817A EP 07731817 A EP07731817 A EP 07731817A EP 2004758 B1 EP2004758 B1 EP 2004758B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- alkyl
- group
- formula
- different
- radical
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Not-in-force
Links
- ODNNLRLQUNAVHB-UHFFFAOYSA-N Cc1cc[n+](CCSSCC[n+]2ccc(C)cc2)cc1 Chemical compound Cc1cc[n+](CCSSCC[n+]2ccc(C)cc2)cc1 ODNNLRLQUNAVHB-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B23/00—Methine or polymethine dyes, e.g. cyanine dyes
- C09B23/14—Styryl dyes
- C09B23/145—Styryl dyes the ethylene chain carrying an heterocyclic residue, e.g. heterocycle-CH=CH-C6H5
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/06—Preparations for styling the hair, e.g. by temporary shaping or colouring
- A61Q5/065—Preparations for temporary colouring the hair, e.g. direct dyes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/46—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing sulfur
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/4906—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with one nitrogen as the only hetero atom
- A61K8/4933—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with one nitrogen as the only hetero atom having sulfur as an exocyclic substituent, e.g. pyridinethione
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/494—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/494—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom
- A61K8/4946—Imidazoles or their condensed derivatives, e.g. benzimidazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/10—Preparations for permanently dyeing the hair
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/36—Radicals substituted by singly-bound nitrogen atoms
- C07D213/38—Radicals substituted by singly-bound nitrogen atoms having only hydrogen or hydrocarbon radicals attached to the substituent nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/10—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B49/00—Sulfur dyes
- C09B49/12—Sulfur dyes from other compounds, e.g. other heterocyclic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/42—Colour properties
- A61K2800/43—Pigments; Dyes
- A61K2800/432—Direct dyes
- A61K2800/4322—Direct dyes in preparations for temporarily coloring the hair further containing an oxidizing agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/42—Colour properties
- A61K2800/43—Pigments; Dyes
- A61K2800/434—Luminescent, Fluorescent; Optical brighteners; Photosensitizers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/80—Process related aspects concerning the preparation of the cosmetic composition or the storage or application thereof
- A61K2800/88—Two- or multipart kits
Definitions
- the invention relates to the dyeing of keratin materials using fluorescent dyes thiols or disulfides comprising a heterocycle.
- the direct dyes which are conventionally used are, for example, dyes of the nitrobenzene type, anthraquinone dyes, nitropyridines, dyes of the azo, xanthene, acridine, azine or triarylmethane type.
- the colorations that result from the use of direct dyes are temporary or semi-permanent dyes because the nature of the interactions that bind the direct dyes to the keratinous fiber, and their desorption of the surface and / or the core of the fiber are responsible their low dyeing power and their poor resistance to washes or perspiration.
- a chemical bleaching process consists in treating keratin materials such as keratinous fibers, in particular the hair, by a strong oxidizing system, generally consisting of hydrogen peroxide associated or not with persalts, most often in an alkaline medium.
- This bleaching system has the drawback of degrading keratin materials, in particular keratinous fibers, especially human fibers such as the hair, and of altering their cosmetic properties. Fibers indeed tend to become rough, more difficult to disentangle and more fragile. Finally, the lightening or the bleaching of keratinous fibers by oxidizing agents is incompatible with the treatments for modifying the shape of said fibers, particularly in the straightening treatments.
- Another lightening technique consists in applying fluorescent direct dyes to dark hair. This technique described in particular in the documents FR 2830189 and WO 2004/091473 makes it possible to respect the quality of the keratinous fiber during the treatment, but the fluorescent dyes used do not have satisfactory resistance to shampoos.
- direct dyes To increase the tenacity of the direct dyes, it is known to fix direct dyes by covalent bonding to the hair. For example, it is known to react reactive group dyes with the numerous cystine or cysteine residues in keratin fibers, for example see, for example Journal of the Society of Dyers and Colourists, Guise and Stapleton, 91, 259-264 (1975) ); Journal of Cosmetic Chemistry, 42 1-17 (1991) ); CA 2024509 ;
- disulfide dyes known for dyeing keratinous fibers are disulfide derivatives of aminothiophenol derivatives. Such dyes are described for example in the patent FR 1156407 . These dyes can be used under relatively mild conditions, in the presence of a slightly reducing medium or after a reducing pretreatment of the hair. However, these dyes may cause color turns during application.
- the object of the present invention is to provide novel systems for dyeing keratin materials, in particular human keratinous fibers, in particular the hair, which do not have the drawbacks of existing bleaching processes.
- one of the aims of the present invention is to provide direct dyeing systems which make it possible to obtain lightening effects, in particular on naturally or artificially dark keratin fibers, which are stubborn in the face of successive shampoos, which do not degrade the fibers. keratinous and which do not alter their cosmetic properties.
- Another subject of the invention is a dye composition
- a dye composition comprising, in a suitable cosmetic medium at least one fluorescent dye of formula (I) or (II) as defined above, and optionally a reducing agent.
- the invention also relates to new fluorescent dyes of formula (I) or (II) as defined above.
- the dyeing process according to the invention makes it possible to visibly stain dark keratin materials, in particular dark human keratin fibers, especially dark hair.
- the process of the invention makes it possible to obtain a coloration of the keratin materials, in particular human keratinous fibers, in particular the hair, without degrading said material, which is persistent with respect to shampoos, of the common aggressions (sun, perspiration). , and other hair treatments.
- the process of the invention also makes it possible to obtain a lightening of keratin materials such as keratinous fibers, particularly dark keratinous fibers and more particularly dark hair.
- the term naturally or artificially dark hair hair whose pitch is less than or equal to 6 (dark blonde) and preferably less than or equal to 4 (chestnut).
- the lightening of the hair is evaluated by the variation of "pitch" before and after application of the compound of formula (I) or (II).
- tone is based on the classification of natural nuances, a tone separating each nuance from that which follows or immediately precedes it. This definition and the classification of natural shades is well known to hair professionals and published in the book “Science of hair treatment” Charles ZVIAK 1988, Ed Masson, p. 215 and 278 .
- Heights range from 1 (black) to 10 (very light blond), a unit corresponding to a tone; the higher the number, the clearer the nuance.
- An artificially colored hair is a hair whose color has been modified by a coloring treatment, for example a coloration with direct dyes or oxidation dyes.
- the wavelength where the difference is greatest between the reflectance curve of the treated hair and that of the untreated hair is in the wavelength range of 500 to 650 nanometers, and preferably in the the wavelength range from 550 to 620 nanometers.
- the fluorescent dyes of formula (1) or (II) are compounds capable of absorbing in the UV or visible radiation at a wavelength ⁇ abs included between 250 and 800 nm and capable of reemitting in the visible range at an emission wavelength ⁇ em between 400 and 800 nm.
- the fluorescent compounds of the invention are dyes capable of absorbing in the visible ⁇ abs between 400 and 800 nm and reemitting in the visible ⁇ em between 400 and 800 nm.
- the heterocyclic group fluorescent dyes of formula (I) or (II) are dyes capable of absorbing at an ⁇ abs of between 420 nm and 550 nm and of reemitting in the visible at an ⁇ em between 470 and 600 nm. nm.
- Fluorescent compounds of formula (II) which contain a SY function which can. be in the covalent -SY or ionic -S - Y + form depending on the nature of Y and the pH of the medium.
- One particular embodiment relates to thiols fluorescent dyes having a group of formula (II) with a SY function, where Y represents a hydrogen atom or an alkali metal.
- Y represents a hydrogen atom.
- Y is a protective group known to those skilled in the art such as those described in the books “Protective Groups in Organic Synthesis”, TW Greene, John Willey & Sons ed., NY, 1981, pp.193-217 ; “Protecting Groups”, P. Kocienski, Thieme, 3rd ed., 2005, chap. 5 .
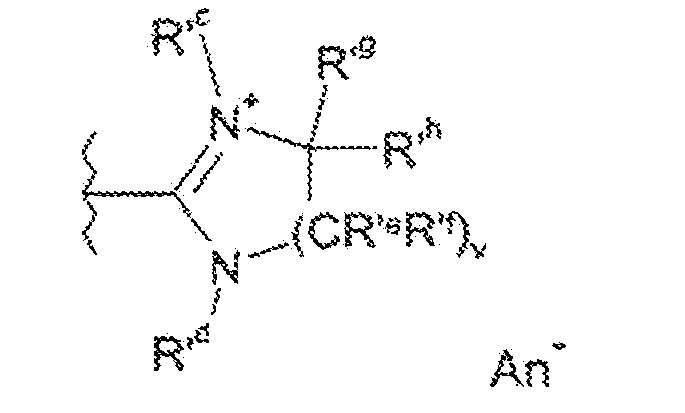
- the heterocyclic protected thiol protected fluorescent dyes of formula (II) contain a cationic aromatic 5- or 6-membered monocyclic moiety Y i) heteroaryl comprising from 1 to 4 heteroatoms chosen from oxygen, sulfur and nitrogen, such as oxazolium, isoxazolium, thiazolium, isothiazolium, 1,2,4-triazolium, 1,2,3-triazolium, 1,2,4-oxazolium, 1,2,4-thiadiazolium, pyrylium, pyridinium, pyrimidinium, pyrazinyl, pyrazinium, pyridazinium, triazinium, tetrazinium, oxazepinium, thiepinyl, thiepinium, imidazolium; ii) cationic 8 to 11-membered bicyclic heteroaryl such as indolinium, benzoimidazolium, benzoxazolium,
- Y represents a group chosen from oxazolium, isoxazolium, thiazolium, isothiazolium, 1,2,4-triazolium, 1,2,3-triazolium, 1,2,4-oxazolium, 1,2,4-thiadiazolium, pyrylium, pyridinium , pyrimidinium, pyrazinium, pyridazinium, triazinium and imidazolium, benzoimidazolium, benzoxazolium, benzothiazolium, these groups being optionally substituted by one or more (C 1 -C 4 ) alkyl groups including methyl.
- the dyes of formula (Ia) and (IIa) have the moiety and in the para position of the phenyl linked to the ethylene ie in the 1 'position of the phenyl radical, the ethylene being connected to the same phenyl radical at the 4' position.
- thiol fluorescent dyes By way of example of thiol fluorescent dyes, mention may be made in particular of the following compounds: with Me representing anionic counter-ion.
- the protected thiol dyes of formula (II ") can be synthesized in two steps: the first step of preparing the unprotected thiol dye (II ') according to the methods known to those skilled in the art, for example Thiols and organic Sulfides, Thiocyanates and Isothiocyanates, organic, Ullmann's Encyclopedia, Wiley-VCH, Weinheim, 2005 . And the second step of protecting the thiol function according to conventional methods known to those skilled in the art to lead to the protected thiol dyes of formula (II ").
- Met * represents an alkali metal or an alkaline earth metal, particularly sodium or potassium, it being understood that when the metal is an alkaline earth metal 2 thiolate-functional chromophores-S- may be associated with 1 Metal 2+ .
- R 1 , R 2 , R 3 , R 4 , R g , R ' g , R h , R' h , R 1 , R ' 1 , m, n, het and M' are as defined above;
- Y ' represents a protecting group of thiol function;
- R represents a leaving group nucleofuge, such as, for example, mesylate, tosylate, triflate or halide.
- a protected thiol compound (b) with a protective group Y 'as defined previously prepared according to one of the procedures described in the works cited above, said protected thiol compound comprising at least one nucleophilic function with a sufficient, preferably equimolar, amount of a "reactive fluorescent chromophore” or a compound comprising such a "reactive fluorescent chromophore” ( a ) .
- ( a ) comprises an electrophilic function to form a covalent bond ⁇ as can be schematized below in the preparation of fluorescent dyes of formula (II "'): with R 1 , R 2 , R 3 , R 4 , R g , R ' g , R h , R' h , R 1 , R ' 1 , m, n, het, Y' and M 'are as defined previously ; Nude representing a nucleophilic group; E representing an electrophilic group; and ⁇ the bond generated after nucleophilic attack on the electrophile.
- the covalent bonds that can be generated are listed in the table below from the condensation of electrophiles with nucleophiles: Electrophiles E Nucleophiles Naked Covalent bonds ⁇ Esters activated * amines carboxamides Acyl azides ** amines carboxamides Acyl halogenides amines carboxamides Acyl halogenides alcohols esters Acyl cyanides alcohols esters Acyl cyanides amines carboxamides Alkali halides amines alkylamines Alkali halides Carboxylic acids esters Alkali halides thiols thioester Alkali halides alcohols ethers Sulphonic acids and their salts thiols thioethers Sulphonic acids and their salts Carboxylic acids esters Sulphonic acids and their salts alcohols ethers anhydrides alcohols esters anhydrides amines .Carboxamides Aryl halides thiols thiols
- thiol reagent Y'-SH comprising a group Y 'as defined previously, the nucleophilic function SH of which can react on the carbon atom in alpha of the halogen atom carried by the fluorescent chromophore ( a ').
- S C (NRR) NRR
- the reaction scheme is as follows: with R ' c.
- R d, R g, R 'g, R h, R' h, R i, R 'i, m, n, Het, Hal, An - and M' are as defined above.
- an imidazoline intermediate from the halide comprising the fluorescent chromophore ( a ' ) and a thioimidazoline (b') to conduct after alkylation with a reagent R-Gp with R representing an alkyl group and Gp a leaving group such as a halogen such as chlorine, bromine, iodine, or a group mesylate or tosylate.
- certain protected thiols fluorescent dyes can be obtained by reacting a protected thiol compound with a compound carrying two carboxylic acid functions activated by conventional methods (for example reaction with a carbodiimide or with the thionyl chloride)
- the resulting product ( d ) is then reacted with a fluorescent chromophore carrying a nucleophilic function ( c ), for example of the primary or secondary amine type, or of the aliphatic alcohol type.
- a variant of synthesis is to combine with the first channel the previous channel ie . from two equivalents of the nucleophilic reagent ( c ) with a disulfide dielectrophile reagent ( i ) it is possible to generate after condensation dichromophoric disulfide dye (I "), the latter can be reduced to form the heterocyclic fluorescent thiol dye which has its turn can either be protected to form the protected thiol fluorescent dye (II "') or be metallized with an alkali metal to lead to the metallized heterocyclic thiol fluorescent dye (II"' Metal) :
- the protected thiol fluorescent dyes of formula (II "') can be obtained by reaction of a compound comprising a thiol group protected by a Y' group, and a previously activated hydroxy group in leaving group nucleofuge (d). '), such as mesylate, tosylate, triflate or halide with a chromophore styrylpyridine (c').
- R 1 ' R 2 , R 3 , R 4 , R g , R' g , R h , R ' h , R 1 , R' 1 , het, T a , Y ', m, n, (II "' ) and E are as defined above.
- a compound containing a protected thiol group contains a leaving group R, such as, for example, mesylate, tosylate, or triflate, which can undergo the nucleophilic attack of the amine carried by the styryl fluorescent chromophore:
- halides as a nucleofugal leaving group on a thiol compound which may be substituted by a primary amine function, for example carried by a styryl fluorescent chromophore:
- the thiol fluorescent dyes of formula (I) according to the invention can be obtained by reaction of a compound comprising a thiol group Y as defined above and an electrophilic group (f) with a pyridinium compound comprising a nucleophilic group.
- a compound comprising a thiol group Y as defined above and an electrophilic group (f) with a pyridinium compound comprising a nucleophilic group.
- This reaction is commonly known as "Knoevenagel" condensation.
- activated methylenes are meant those which preferably comprises, in position 2 or 4 of the pyridinium group, a methylene group R 1 -CH 2 -: with R 1 , R 2 , R 3 , R 4 , R a , R b , R g , R ' g , R h , R' h , R i , R ' i , T a , h, m, n, Y and M 'as defined above and G representing an oxygen or sulfur atom.
- the thiol fluorescent dyes formed can be converted to protected thiol stain -SY 'by protecting the thiol -SH using conventional protecting groups.
- the thiol fluorescent dyes are metallated using also the conventional methods known to those skilled in the art such as those described in US Pat. Advanced Organic Chemistry, "Reactions, Mechanisms and Structures," J. March, 4th Ed, John Willey & Sons, NY, 1992 .
- Protected thiol dyes can be deprotected by conventional routes such as those described in the literature "Protective Groups in Organic Synthesis,” TW Greene, John Willey & Sons ed., NY, 1981 ; “Protecting Groups”, P. Kocienski, Thieme, 3rd ed., 2005 .
- the starting reagents are commercial or accessible by conventional methods known to those skilled in the art.
- Gp representing a leaving group nucleofuge, such as mesylate, tosylate, triflate or halide.
- the counterions Gp - compounds (I '), above may be replaced by counterions M' of other types from methods known to those skilled in the art including ion exchange resin.
- the dissymmetrical disulfide dyes of formula (I) may be synthesized in one step by reacting an unprotected thiol fluorescent dye with a Y'-protected thiol fluorescent dye to form the disulfide dye of formula (I).
- R g, R 'g, R "g, R”' g, R h, R 'h, R “h, R"' h, R i, R 'i, R "i, R” i, R 1 , R ' 1 , R 2, R' 2 , R 3 , R ' 3 , R 4 , R' 4 , m, m ', n, n', T a , T b , het, het 'and M' are as defined above; Y 'represents a protecting group of thiol function.
- the thiol fluorescent dyes formed can be converted into dyes protected thiols fluorescents -SY 'by protecting the thiol -SH using conventional protecting groups.
- the thiol fluorescent dyes are metallated using also the conventional methods known to those skilled in the art such as those described in US Pat. Advanced Organic Chemistry, "Reactions, Mechanisms and Structures," J. March, 4th Ed, John Willey & Sons, NY, 1992 .
- Protected thiol dyes can be deprotected by conventional routes such as those described in the literature "Protective Groups in Organic Synthesis,” TW Greene, John Willey & Sons ed., NY, 1981 ; “Protecting Groups”, P. Kocienski, Thieme, 3rd ed., 2005 .
- the composition of the invention contains at least one fluorescent dye of formula ( I ) or ( II ).
- the composition of the invention may also contain a reducing agent.
- This reducing agent may be chosen from thiols, for example cysteine, homocysteine, thiolactic acid, the salts of these thiols, phosphines, bisulfite, sulphites, thioglycolic acid and its esters, especially the glycerol monothioglycolate, and thioglycerol.
- This reducing agent may also be chosen from borohydrides and their derivatives, such as, for example, borohydride, cyanoborohydride, triacetoxyborohydride or trimethoxyborohydride salts: sodium, lithium, potassium, calcium, quaternary ammonium salts (tetramethylammonium, tetraethylammonium, tetra butylammonium, benzyltriethylammonium); catechol borane.
- borohydrides and their derivatives such as, for example, borohydride, cyanoborohydride, triacetoxyborohydride or trimethoxyborohydride salts: sodium, lithium, potassium, calcium, quaternary ammonium salts (tetramethylammonium, tetraethylammonium, tetra butylammonium, benzyltriethylammonium); catechol borane.
- the dye composition useful in the invention generally contains a quantity of fluorescent dye of formula ( I ) or ( II ) of between 0.001 and 50% relative to the total weight of the composition. Preferably, this amount is between 0.005 and 20% by weight and even more preferably between 0.01 and 5% by weight relative to the total weight of the composition.
- the dye composition may further contain additional direct dyes.
- These direct dyes are, for example, chosen from neutral, acidic or cationic nitro-benzene direct dyes, neutral azo, acid or cationic direct dyes, tetraazapentamethine dyes, neutral or acidic or cationic quinone dyes, in particular neutral anthraquinone dyes and direct azine dyes. , triarylmethane direct dyes, indoamine direct dyes and natural direct dyes.
- the dye composition may contain one or more oxidation bases and / or one or more couplers conventionally used for dyeing keratinous fibers.
- oxidation bases mention may be made of para-phenylenediamines, bis-phenylalkylenediamines, para-aminophenols, bis-para-aminophenols, ortho-aminophenols, heterocyclic bases and their addition salts.
- couplers there may be mentioned meta-phenylenediamines, meta-aminophenols, meta-diphenols, naphthalenic couplers, heterocyclic couplers and their addition salts.
- the coupler or couplers are each generally present in an amount of between 0.001 and 10% by weight of the total weight of the dye composition, preferably between 0.005 and 6%.
- the oxidation base (s) present in the dyeing composition are in general each present in an amount of between 0.001% and 10% by weight relative to the total weight of the dyeing composition, preferably between 0.005% and 6% by weight.
- addition salts of the oxidation bases and couplers that can be used in the context of the invention are chosen especially from the addition salts with an acid such as hydrochlorides, hydrobromides, sulphates, citrates, succinates, tartrates, lactates, tosylates, benzenesulfonates, phosphates and acetates and addition salts with a base such as alkali metal hydroxides such as sodium hydroxide, potassium hydroxide, ammonia, amines or alkanolamines.
- an acid such as hydrochlorides, hydrobromides, sulphates, citrates, succinates, tartrates, lactates, tosylates, benzenesulfonates, phosphates and acetates
- a base such as alkali metal hydroxides such as sodium hydroxide, potassium hydroxide, ammonia, amines or alkanolamines.
- the medium suitable for dyeing is a cosmetic medium generally consisting of water or a mixture of water and at least one organic solvent.
- organic solvents that may be mentioned are lower C 1 -C 4 alkanols, such as ethanol and isopropanol; polyols and polyol ethers such as 2-butoxyethanol, propylene glycol, propylene glycol monomethyl ether, diethylene glycol monoethyl ether and monomethyl ether, as well as aromatic alcohols such as benzyl alcohol or phenoxyethanol, and mixtures thereof.
- the solvents when present are preferably present in proportions preferably of between 1 and 40% by weight approximately relative to the total weight of the dye composition, and even more preferably between 5 and 30% by weight approximately.
- the invention contains a reducing agent capable of reducing the disulfide bonds of keratin and / or of the fluorescent dye of formula ( I ).
- This reducing agent is as defined above.
- the dye composition may also contain various adjuvants conventionally used in compositions for dyeing hair, such as anionic, cationic, nonionic, amphoteric, zwitterionic surfactants or mixtures thereof, anionic, cationic, nonionic, amphoteric and zwitterionic polymers.
- adjuvants conventionally used in compositions for dyeing hair such as anionic, cationic, nonionic, amphoteric, zwitterionic surfactants or mixtures thereof, anionic, cationic, nonionic, amphoteric and zwitterionic polymers.
- inorganic or organic thickeners and in particular anionic, cationic, nonionic and amphoteric polymeric associative thickeners, antioxidants, penetrating agents, sequestering agents, perfumes, buffers, dispersing agents, conditioning agents such as, for example, volatile or non-volatile silicones, modified or otherwise, such as aminosilicones, film-forming agents, ceramides, preserving agents, opacifying agents, conductive polymers.
- the adjuvants above are generally present in an amount for each of them between 0.01 and 20% by weight relative to the weight of the composition.
- the pH of the dyeing composition is generally between 3 and 14 approximately, and preferably between 5 and 11 approximately. It can be adjusted to the desired value by means of acidifying or alkalizing agents usually used for dyeing keratin fibers or else using conventional buffer systems.
- acidifying agents mention may be made, by way of example, of mineral or organic acids such as hydrochloric acid, orthophosphoric acid, sulfuric acid, carboxylic acids such as acetic acid, tartaric acid, citric acid, lactic acid, sulphonic acids.
- mineral or organic acids such as hydrochloric acid, orthophosphoric acid, sulfuric acid, carboxylic acids such as acetic acid, tartaric acid, citric acid, lactic acid, sulphonic acids.
- alkalinizing agents that may be mentioned, for example, ammonia, alkaline carbonates, alkanolamines such as mono-, di- and triethanolamines as well as their derivatives, the hydroxides of sodium or of potassium and the compounds of formula ( ⁇ ) below: in which W a is a propylene residue optionally substituted by a hydroxyl group or a C 1 -C 4 alkyl radical; R a1, R a2, R a3 and R a4, identical or different, represent a hydrogen atom, an alkyl radical in C 1 -C 4 hydroxyalkyl or C 1 -C 4.
- the dye composition may be in various forms, such as in the form of liquid, cream, gel, or in any other form suitable for dyeing keratinous fibers, and in particular hair.
- a reducing agent may be applied pre-treatment before the application of the composition containing at least one heterocyclic fluorescent dye of formula ( I ) or ( II ).
- This reducing agent may be chosen from thiols, for example cysteine, homocysteine, thiolactic acid, the salts of these thiols, phosphines, bisulfite, sulphites, thioglycolic acid and its esters, especially the glycerol monothioglycolate, and thioglycerol.
- thiols for example cysteine, homocysteine, thiolactic acid, the salts of these thiols, phosphines, bisulfite, sulphites, thioglycolic acid and its esters, especially the glycerol monothioglycolate, and thioglycerol.
- This reducing agent may also be chosen from borohydrides and their derivatives, such as, for example, borohydride, cyanoborohydride, triacetoxyborohydride or trimethoxyborohydride salts: sodium, lithium, potassium, calcium, quaternary ammonium salts (tetramethylammonium, tetraethylammonium, tetra butylammonium, benzyltriethylammonium); catecholborane.
- borohydrides and their derivatives such as, for example, borohydride, cyanoborohydride, triacetoxyborohydride or trimethoxyborohydride salts: sodium, lithium, potassium, calcium, quaternary ammonium salts (tetramethylammonium, tetraethylammonium, tetra butylammonium, benzyltriethylammonium); catecholborane.
- This pretreatment may be of short duration, in particular from 0.1 second to 30 minutes, preferably from 1 minute to 15 minutes with a reducing agent as mentioned above.
- the composition comprising at least one fluorescent dye of formula ( I ) or ( II ) also contains at least one reducing agent as defined above. This composition is then applied to the hair.
- the reducing agent is applied in post-treatment, after the application of the composition containing at least one thiol fluorescent dye (I).
- the duration of the post-treatment with the reducing agent may be short, for example 0.1 second at 30 minutes preferably from 1 minute to 15 minutes, with a reducing agent as described above.
- the reducing agent is a thiol or borohydride type agent as described above.
- the process of the invention may be preceded by a deprotection step aimed at rendering the SH function in situ .
- Y protective group protection alkylcarbonyl, pH> 9 arylcarbonyl, pH> 9 alkoxycarbonyl, pH> 9 aryloxycarbonyl, pH> 9 aralkoxycarbonyl pH> 9 (Di) (alkyl) aminocarbonyl, pH> 9 (Alkyl) arylaminocarbonyl pH> 9 optionally substituted aryl such as phenyl, pH> 9 monocyclic 5-, 6- or 7-membered heteroaryl such as oxazolium; pH> 9 8 to 11-membered bicyclic heteroaryl such as benzoimidazolium, or benzoxazolium pH> 9
- the deprotection step may also be carried out during a hair pretreatment step such as, for example, the hair reduction pretreatment.
- the reducing agent is added to the dyeing composition containing at least one fluorescent dye of formula ( I ) or ( II ) at the time of use.
- a particular embodiment of the invention relates to a method in which the fluorescent dye of formula ( I ) or ( II ) can be applied directly to hair without reducing agents, free of pre or post-treatment reducers.
- a treatment with an oxidizing agent may optionally be associated.
- Any type of conventional oxidizing agent may be used in the art. Thus, it can be chosen from hydrogen peroxide, urea peroxide, bromates of alkali metals, persalts such as perborates and persulfates, and enzymes including peroxidases, 2-electron oxidoreductases such as uricases and 4-electron oxygenases such as laccases. The use of hydrogen peroxide is particularly preferred.
- This oxidizing agent may be applied to the fibers before or after the application of the composition containing at least one fluorescent dye of formula ( I ) or ( II ).
- the application of the dye composition according to the invention is generally carried out at room temperature. It can however be carried out at temperatures ranging from 20 to 180 ° C.
- the invention also relates to a multi-compartment device or "kit" of dyeing in which a first compartment contains a dye composition comprising at least one fluorescent dye of formula ( I ) or ( II ) and a second compartment contains a reducing agent capable of reducing the disulfide functions of keratin materials and / or the fluorescent dye of formula ( I ).
- One of these compartments may further contain one or more other dyes of direct dye type or oxidation dye.
- a multi-compartment device in which a first compartment contains a dye composition comprising at least one fluorescent dye of formula ( I ) or ( II ); a second compartment contains a reducing agent capable of reducing the disulfide bond of the keratin materials and / or the fluorescent dye of formula ( I ); a third compartment contains an oxidizing agent.
- the dyeing device contains a first compartment containing a dye composition which comprises at least one protected thiol fluorescent dye of formula ( II ) and a second compartment containing an agent capable of deprotecting the protected thiol to release the thiol.
- Each of the devices mentioned above may be equipped with a means for delivering the desired mixture onto the hair, for example such as the devices described in the patent. FR2 586 913 .
- the organic phases are extracted with 100 ml of ice water, 100 ml of water, 3 times 50 ml of a saturated solution of sodium hydrogencarbonate (NaHCO 3 ), with 2 times 20 ml of saturated sodium chloride solution. (NaCl), then dried over anhydrous sodium sulfate (Na 2 SO 4 ). AcOEt is evaporated, and 17.49 g pale yellow translucent oil is collected and stored at -25 ° C. The analyzes indicate that the product is compliant and pure.
- Step 2 Synthesis of 1,1 '- (disulfanediyldiethane-2,1-diyl) bis (4-methylpyridinium) Dimethhanesulfonate
- Step 3 Synthesis of 1,1 '- (disulfanediyldiethane-2,1-diyl) -bis ⁇ 4 - [(E) -2- (4-pyrrolidin-1-ylphenyl) vinyl] pyridinium] dimethane sulfonate [1]
- Step 1 Synthesis of 1,1 '- (disulfanediyldiethane-2,1-diyl) bis (2-methylpyridinium) dibromide
- Step 2 Synthesis of 1,1 '- (disulfanediyldiethane-2,1-diyl) bis (2 - [(E) -2- (4-pyrrolidin-1-ylphenyl) vinyl] pyridinium dibromide [3]
- Step 1 Synthesis of N, N '- (disulfanediyldiethane-2,1-diyl) bis (2-chloroacetamide)
- cystamine dihydrochloride 40.3 g are dissolved in 100 ml of water, 32 ml of 35% sodium hydroxide are added (pH 9.7) and the temperature is lowered to 5 ° C. 33.5 mL of chloroacetyl chloride are added dropwise, maintaining the temperature below 10 ° C and maintaining the pH between 7.9 and 9.3 by addition of sodium hydroxide. The medium is kept stirred at ambient temperature for 2 hours. The precipitate is filtered, washed with 5 ⁇ 150 mL of water and then dried under vacuum in the presence of P 2 O 5 . 35.3 g of white powder are collected. the analyzes indicate that the product is compliant.
- Step 2 Synthesis of 1,1 '- ⁇ disulfanediylbis (ethane-2,1-diylimino (2-oxoethane-2,1-diyl)] ⁇ bis (4-methylpyridinium) dichloride
- Step 3 Synthesis of 1,1 '- ⁇ disulfanediylbis [ethane-2,1-diylimino (2-oxoethane-2,1-diyl)] ⁇ bis ⁇ 4 - [(E) -2- (4-) dichloride) pyrrolidin-1-ylphenyl) vinyl] pyridinium) [4]
- compositions A (9 ml) and B (1 ml) are mixed, and then the mixture obtained is applied to a lock of 1 g of dark hair (pitch 4) for 30 minutes at room temperature. (The locks are returned and re-impregnated after 15 minutes).
- the locks are then rinsed with running water and dried.
- the locks thus treated are divided in two, one half is subjected to 5 successive shampoos in a cycle which comprises wetting the locks with water, washing with a conventional shampoo, rinsing with water followed by drying.
- L * a * b * L * represents the intensity of the color
- a * indicates the green / red color axis
- b * the blue / yellow color axis.
- the higher the L value the lighter or less intense the color.
- the lower the value of L the darker or more intense the color.
- the higher the value of a * the higher the shade is red and the higher the value of b * the hue is yellow.
- L *, a * and b * represent the measured values before staining and L 0 *, a 0 * and b 0 * represent the values measured after treatment (staining, or staining and successive washes).
- compositions according to the invention were expressed as a function of the reflectance of the hair. These reflectances are compared to the reflectance of an untreated strand of HT4 pitch hair.
- the reflectance is measured using a KONIKA-MINOLTA® spectrophotocolorimeter, CM 2600d and after irradiation of hair with visible light in the wavelength range of 400 to 700 nanometers.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Cosmetics (AREA)
Claims (16)
- Fluoressenzfarbstoff der Formel (I) oder (II):
wobei in den Formeln (I) und (II):➢ - Fluoreszenzfarbstoff der Formel (II) nach dem vorhergehenden Anspruch, worin Y für ein Wasserstoffatom oder ein Alkalimetall steht.
- Fluoressenzfarbstoff der Formel (II) nach Anspruch 1, worin Y für eine Schutzgruppe steht.
- Fluoreszenzfarbstoff der Formel (II) nach dem vorhergehenden Anspruch, worin Y für eine unter den folgenden Resten ausgewählte Schutzgruppe steht:• (C1-C4)Alkylcarbonyl ;• (C1-C4)Alkylthiocarbonyl ;• (C1-C4)Alkoxycarbonyl ;• (C1-C4)Alkoxythiocarbonyl ;• (C1-C4)Alkylthiothiocarbonyl ;• (Di)(C1-C4)(alkyl)aminocarbonyl ;• (Di)(C1-C4)(alkyl)aminothiocarbonyl ;• Arylcarbonyl ;• Aryloxycarbonyl;• Aryl(C1-C4)alkoxycarbonyl;• (Di)(C1-C4)(alkyl)aminocarbcnyl;• (C1-C4)(Alkyl)arylaminocarbonyl;• Carboxyl;• SO3 -; M+, wobei M+ für ein Alkalimetall steht oder M' der Formel (II) und M+ fehlen;• gegebenenfalls substituiertes Aryl;• gegebenenfalls substituiertes Heteroaryl;• gegebenenfalls kationisches, gegebenenfalls substituiertes Heterocycloalkyl;• den folgenden Heterocyclus:
ausgewählte Gruppe stehen;• eine sterisch gehinderte cyclische Gruppe und• gegebenenfalls substituiertes Alkoxy(C1-C4)-alkyl. - Fluoreszenzfarbstoff der Formel (II) nach einem der vorhergehenden Ansprüche, worin Y für ein Alkalimetall oder eine unter:➢ (C1-C4)Alkylcarbonyl;➢ Arylcarbonyl;➢ (C1-C4)Alkoxycarbonyl;➢ Aryloxycarbonyl;➢ Aryl(C1-C4)alkoxycarbonyl;➢ (Di)(C1-C4)(alkyl)aminocarbonyl;➢ (C1-C4)(Alkyl)arylaminocarbonyl;➢ gegebenenfalls substituiertes Aryl;➢ 5- oder 5-gliedriges kationisches monocyclisches Heteroaryl;➢ 8- bis 11-gliedriges kationisches bicyclisches Heteroaryl;➢ Isothiouronium -C(NH2)=N+H2; An-;➢ Isothioharnstoff -C(NH2)=NH und➢ SO3 -; M+, wobei M+ für ein Alkalimetall steht
oder M+ der Formel (II) und M+ fehlen;
ausgewählte Schutzgruppe steht. - Fluoressensfarbstoff nach einem der vorhergehenden Ansprüche der Formel (Ia) oder (IIa) mit der Ethylengruppe, die den Pyridiniumteil mit dem Phenyl verbindet, in ortho- oder para-Position, d. h. in den Positionen 4-4', 4-2' oder 2-4':
sind und für eine monocylische, gesättigte oder ungesättigte 5- bis 7-gliedrige heterocyclische Gruppe mit 1 oder zwei unter Stickstoff-, Sauerstoff- und Schwefelatomen ausgewählten Heteroatomen stehen;➢ Ri, R'i, R"i, und R"'i gleich oder verschieden sind und für ein Wasserstoffatom oder eine (C1-C4)-Alkylgruppe stehen;➢ Rg, R'g, R"g, R"'g; Rh, R'h, R"h und R"'h gleich oder verschieden sind und für ein Wasserstoffatom oder eine (C1-C4)-Alkylgruppe stehen;➢ Ta und Tb für eine kovalente o-Bindung oder eine unter -NR2-, -C(O)-N(R)-, -N(R)-C(O)-, -O-C(O)-(R)-, -C(O)-O- und -N+(R)(R°)-, ausgewählte Gruppe stehen, wobei R und R° gleich oder verschieden sind und für ein Wasserstoffatom oder eine C1-C4-Alkylgruppe stehen;➢ m, m', n und n' gleich oder verschieden sind und für eine ganze Zahl zwischen 0 und 6 einschließlich stehen, wobei m+n=m' +n' = eine ganze Zahl zwischen 2 und 4 einschließlich ergeben;➢ M' für ein anionisches Gegenion steht und➢ Y die in einem der vorhergehenden Ansprüche
angegebene Bedeutung besitzt;
mit der Maßgabe, daß:- die Verbindung der Formel (Ia) oder (IIa) dann, wenn sie andere kationische Teile enthält, mit einem oder mehreren anionischen Gegenionen assoziiert ist, so daß die Elektroneutralität der Formel (1a) oder (IIa) gewährleistet ist. - Kosmetische Färbezusammensetzung, die in einem geeigneten kosmetischen Medium einen Fluoreszenzfarbstoff der Formel (I) oder (II) gemäß einem der Ansprüche 1 bis 8 umfaßt.
- Kosmetische Färbezusammensetzung, die in einem geeigneten kosmetischen Medium einen Fluoreszenzfarbstoff der Formel (I) oder (II) gemäß einem der Ansprüche 1 bis 8 und mindestens ein Reduktionsmittel umfaßt.
- Zusammensetzung nach den Ansprüchen 9 oder 10, in der der Fluoreszenzfarbstoff der Formel (I) oder (II) in einer Menge zwischen 0,001 und 50 Gew.-%, bezogen auf das Gesamtgewicht der Zusammensetzung, vorliegt.
- Verfahren zum Färben von Keratinmaterialien, bei dem man auf die Materialien eine Färbezusammensetzung gemäß einem der Ansprüche 9 bis 11, die in einem geeigneten kosmetischen Medium mindestens einen Fluoreszenzfarbstoff der Formel (I) oder (II) gemäß einem der Ansprüche 1 bis 8 umfaßt, aufbringt, gegebenenfalls in Gegenwart eines Reduktionsmittels.
- Verfahren zum Färben von Keratinmaterialien nach Anspruch 12, dadurch gekennzeichnet, daß es sich bei den Keratinmaterialien um dunkle Keratinfasern mit einer Tonhöhe kleiner gleich 6 handelt.
- Verfahren nach den Ansprüchen 12 oder 13, bei dem man zusätzlich auf die Keratinfasern ein Oxidationsmittel aufbringt.
- Vorrichtung mit mehreren Kompartimenten, in der ein erstes Kompartiment eine Färbezusammensetzung, die einen Fluoreszenzfarbstoff der Formel (I) oder (II) gemäß einem der Ansprüche 1 bis 8 umfaßt, enthält und ein zweites Kompartiment ein Reduktionsmittel enthält.
- Verwendung der Fluoreszenzfarbstoffe gemäß einem der Ansprüche 1 bis 8 zur Aufhellung von dunklen Keratinfasern mit einer Tonhöhe kleiner 6,
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0651035A FR2898903B1 (fr) | 2006-03-24 | 2006-03-24 | Composition de teinture comprenant un colorant disulfure fluorescent, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
| US79294106P | 2006-04-19 | 2006-04-19 | |
| FR0753072A FR2912140B1 (fr) | 2007-02-05 | 2007-02-05 | Composition de teinture comprenant un colorant fluorescent thiol a heterocycle et a charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
| US90036307P | 2007-02-09 | 2007-02-09 | |
| PCT/FR2007/051005 WO2007110539A2 (fr) | 2006-03-24 | 2007-03-23 | Composition de teinture comprenant un colorant fluorescent thiol/disulfure a heterocycle et a charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2004758A2 EP2004758A2 (de) | 2008-12-24 |
| EP2004758B1 true EP2004758B1 (de) | 2011-08-03 |
Family
ID=38541471
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP07731817A Not-in-force EP2004758B1 (de) | 2006-03-24 | 2007-03-23 | Färbezusammensetzung mit einem thiol/disulfid- leuchtfarbstoff mit einem heterozyklus und einer internen kationischen ladung sowie verfahren zur aufhellung von keratinmaterial mit diesem farbstoff |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP2004758B1 (de) |
| KR (1) | KR101088333B1 (de) |
| WO (1) | WO2007110539A2 (de) |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2898903B1 (fr) | 2006-03-24 | 2012-08-31 | Oreal | Composition de teinture comprenant un colorant disulfure fluorescent, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
| US8070830B2 (en) | 2006-03-24 | 2011-12-06 | L'oreal S.A. | Fluorescent entity, dyeing composition containing at least one fluorescent entity comprising at least one heterocycle, with at least one internal cationic charge, and method for lightening keratin materials using said at least one fluorescent entity |
| KR101088335B1 (ko) | 2006-03-24 | 2011-11-30 | 로레알 | 티올/디술피드 나프틸이미드 형광 염료를 함유하는 염색 조성물, 및 상기 색소를 이용한 케라틴 물질의 미백 방법 |
| FR2921376B1 (fr) | 2007-09-21 | 2009-10-30 | Oreal | Compose styryl tetrahydroquinolinium thiol/disulfure, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
| FR2921373B1 (fr) | 2007-09-21 | 2009-10-30 | Oreal | Colorant derive d'indole styryle a linker alkylene, composition tinctoriale comprenant ce colorant, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
| FR2921377B1 (fr) | 2007-09-21 | 2009-10-30 | Oreal | Compose styryl a motif hydroxy(cyclo)alkylamino thiol/disulfure, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
| FR2921381B1 (fr) | 2007-09-21 | 2009-10-30 | Oreal | Colorant hemicyanine styryle thiol/disulfure, composition tinctoriale comprenant ce colorant, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
| FR2968954B1 (fr) | 2010-12-15 | 2012-12-21 | Oreal | Procede de coloration de fibres keratiniques mettant en oeuvre un colorant direct a fonction disulfure/thiol/thiol protege et de la vapeur d'eau |
| FR2971938B1 (fr) | 2011-02-25 | 2013-08-02 | Oreal | Composition pour colorer les fibres keratiniques comprenant un colorant direct a fonction disulfure/thiol, un alcool gras faiblement ou non ethoxyle, un tensioactif cationique, un agent alcalin, et un agent reducteur |
| FR2971935B1 (fr) | 2011-02-25 | 2013-02-15 | Oreal | Composition pour colorer les fibres keratiniques comprenant un colorant direct a fonction disulfure/thiol, un polymere epaississant, un tensioactif non ionique, un agent alcalin, et un agent reducteur |
| FR2971936B1 (fr) | 2011-02-25 | 2013-02-15 | Oreal | Composition pour colorer les fibres keratiniques comprenant un colorant direct a fonction disulfure/thiol, un tensioactif non ionique, un tensioactif amphotere, un alcool gras ethoxyle, un agent alcalin, et un agent reducteur |
| EP2677996B1 (de) | 2011-02-25 | 2017-11-29 | L'Oréal | Zusammensetzung zur färbung von keratinfasern mit einem direktfarbstoff mit einer disulfid/thiol-funktionsgruppe, einem verdickungspolymer, einem ethoxylierten fettalkohol und/oder einem nichtionischen tensid, einem alkalisierungsmittel und einem reduktionsmittel |
| FR2971937B1 (fr) | 2011-02-25 | 2013-02-15 | Oreal | Composition pour colorer les fibres keratiniques comprenant un colorant direct a fonction disulfure/thiol, un polymere epaississant non cellulosique, un agent alcalin, et un agent reducteur |
| FR2971939B1 (fr) | 2011-02-25 | 2013-02-15 | Oreal | Composition pour colorer les fibres keratiniques comprenant un colorant direct a fonction disulfure/thiol, un corps gras, un agent alcalin, et un agent reducteur |
| FR2990944A1 (fr) | 2012-05-23 | 2013-11-29 | Oreal | Procede de coloration des fibres keratiniques comprenant un colorant /pigment, un compose photoactif, et une source lumineuse |
| WO2015059368A1 (fr) | 2013-09-02 | 2015-04-30 | L'oreal | Procede de coloration des fibres keratiniques a partir de colorants cationiques styryles disulfures, et composition comprenant lesdits colorants |
| FR3043551B1 (fr) | 2015-11-12 | 2017-11-24 | Oreal | Colorant direct cationique a chaine aliphatique et a fonction disulfure/thiol/thiol protege pour colorer les fibres keratiniques |
| FR3066108B1 (fr) | 2017-05-10 | 2020-10-30 | Oreal | Colorant direct fluorescent a chaine aliphatique et a fonction disulfure/thiol/thiol protege pour colorer les matieres keratiniques |
| FR3067599B1 (fr) | 2017-06-16 | 2020-09-04 | Oreal | Procede de coloration des matieres keratiniques mettant en oeuvre au moins un colorant bleu, violet ou vert et au moins un colorant fluorescent disulfure, thiol ou thiol protege |
| FR3067597B1 (fr) | 2017-06-16 | 2020-09-04 | Oreal | Procede de coloration des fibres keratiniques mettant en œuvre au moins un colorant direct et au moins un colorant fluorescent disulfure, thiol ou thiol protege |
| FR3067598B1 (fr) | 2017-06-16 | 2020-09-11 | Oreal | Procede de coloration des fibres keratiniques mettant en œuvre au moins un colorant fluorescent disulfure, thiol ou thiol protege et au moins un activateur comprenant un reducteur et moins deux agents alcalins differents |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL105936C (de) * | 1956-07-17 | |||
| FR2586913B1 (fr) * | 1985-09-10 | 1990-08-03 | Oreal | Procede pour former in situ une composition constituee de deux parties conditionnees separement et ensemble distributeur pour la mise en oeuvre de ce procede |
| FR2830189B1 (fr) * | 2001-09-28 | 2004-10-01 | Oreal | Composition de teinture a effet eclaircissant pour fibres keratiniques humaines |
| FR2853229B1 (fr) * | 2003-04-01 | 2009-06-12 | Oreal | Procede de coloration pour matieres keratiniques humaines avec effet eclaircissant, compose fluorescent particulier et composition le comprenant |
| FR2857258B1 (fr) * | 2003-07-09 | 2007-08-17 | Oreal | Composition comprenant au moins un derive substitue de 2-[2-(4-aminophenyl)ethenyl]-1-pyridinium, procede de traitement des fibres keratiniques la mettant en oeuvre, dispositif et utilisation |
| BRPI0508391B1 (pt) | 2004-04-08 | 2017-10-10 | Ciba Specialty Chemicals Holding Inc | Method of dyeing fibers containing keratin, and composition |
| FR2876576B1 (fr) * | 2004-10-14 | 2006-12-08 | Oreal | Composition de teinture comprenant un colorant disulfure particulier et procede de coloration des fibres keratiniques humaines a partir de ce colorant |
| ES2328068T3 (es) * | 2005-06-16 | 2009-11-06 | Basf Se | Colorantes del tipo esteril sulfuro. |
| EP1937780B1 (de) * | 2005-10-11 | 2013-02-13 | Basf Se | Sulfidfarbstoffgemisch |
-
2007
- 2007-03-23 WO PCT/FR2007/051005 patent/WO2007110539A2/fr not_active Ceased
- 2007-03-23 EP EP07731817A patent/EP2004758B1/de not_active Not-in-force
- 2007-03-23 KR KR1020087023326A patent/KR101088333B1/ko not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| EP2004758A2 (de) | 2008-12-24 |
| WO2007110539A3 (fr) | 2008-06-19 |
| WO2007110539A2 (fr) | 2007-10-04 |
| KR101088333B1 (ko) | 2011-11-30 |
| KR20080102235A (ko) | 2008-11-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2004758B1 (de) | Färbezusammensetzung mit einem thiol/disulfid- leuchtfarbstoff mit einem heterozyklus und einer internen kationischen ladung sowie verfahren zur aufhellung von keratinmaterial mit diesem farbstoff | |
| EP2004757B1 (de) | Färbezusammensetzung mit einem thiol/disulfid- leuchtfarbstoff mit amingruppen und einer internen kationischen ladung sowie verfahren zur aufhellung von keratinmaterial mit diesem farbstoff | |
| EP2004754B1 (de) | Färbezusammensetzung mit einem thiol/disulfid- leuchtfarbstoff mit einem heterozyklus und einer externen kationischen ladung sowie verfahren zur aufhellung von keratinmaterial mit diesem farbstoff | |
| EP2024444B1 (de) | Färbezusammensetzung mit einem thiol/disulfid-naphthylimid-leuchtfarbstoff und verfahren zur aufhellung von keratinmaterial mit diesem farbstoff | |
| EP2001960B1 (de) | Färbemittel mit einem thiol/disulphid-fluoreszenzfarbstoff mit einer ortho-pyridiniumgruppe mit unterbrochener alkylenkette und einer externen kationischen ladung sowie davon gebrauch machendes verfahren zum aufhellen von keratinmaterialien | |
| EP2075289B1 (de) | Styryltetrahydrochinolinium-Thiol/Disulfidverbindung, Verfahren zum Aufhellen keratinischer Materialien unter Verwendung dieses Farbstoffs | |
| EP2004756B1 (de) | Färbezusammensetzung mit einem thiol/disulfid- leuchtfarbstoff mit einer externen kationischen ladung sowie verfahren zur aufhellung von keratinmaterial mit diesem farbstoff | |
| EP2004755B1 (de) | Färbezusammensetzung mit einem thiol/disulfid- leuchtfarbstoff mit einer externen kationischen ladung und einer unterbrochenen alkylenkette sowie verfahren zur aufhellung von keratinmaterial mit diesem farbstoff | |
| EP2070988B1 (de) | Styrylverbindung mit einer Hydroxy(cyclo)alkylamino- sowie Thiol/Disulfideinheit, Verfahren zum Aufhellen keratinischer Materialien ausgehend von diesem Farbmittel | |
| EP2062945B1 (de) | Hemicyaninstyryl-thiol/disulphid-Farbstoff, Farbstoffzusammensetzung enthaltend diesen Farbstoff, Verfahren zur Aufhellung keratinischen Materialien unter Verwendung dieses Farbstoffs | |
| WO2007110541A2 (fr) | Composition de teinture comprenant un colorant fluorescent thiol/disulfure a cycle condense et charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| WO2007110540A2 (fr) | Composition de teinture comprenant un colorant fluorescent thiol/disulfure a groupement alcoxy/hydroxy et a charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| WO2007110538A2 (fr) | Composition de teinture comprenant un colorant fluorescent thiol masque a groupe dimethylamino, a charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant et de colorant disufure | |
| WO2007110536A2 (fr) | Composition de teinture comprenant un colorant fluorescent thiol/disulfure a groupe ortho-pyridinium, a chaine alkylene non interrompue et a charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2921382A1 (fr) | Colorant derive de phenyl-pyridol[1,2-a]indolium thiol-disulfure, composition tinctoriale comprenant ce colorant, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2921379A1 (fr) | Colorant derive d'indole styryle thiol/disulfure, composition tinctoriale comprenant ce colorant, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2921380A1 (fr) | Colorant derive de pyridocycloalkyle styryle thiol/disulfure, composition tinctoriale comprenant ce colorant, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2921374A1 (fr) | Colorant derive d'indolinium thiol/disulfure, composition tinctoriale comprenant ce colorant, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2921378A1 (fr) | COLORANT DICETORPYRROLO[3,4-c]PYRROLE THIOL/DISULFURE, COMPOSITION TINCTORIALE COMPRENANT CE COLORANT, PROCEDE D'ECLAIRCISSEMENT DES MATIERES KERATINIQUES A PARTIR DE CE COLORANT | |
| FR2912140A1 (fr) | Composition de teinture comprenant un colorant fluorescent thiol a heterocycle et a charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2912137A1 (fr) | Composition tinctoriale comprenant un colorant fluorescent thiol/disulfure a groupe pyridinium et chaine alkylene interrompue,procede d'eclaircissement a partir de la composition | |
| FR2912143A1 (fr) | Composition de teinture comprenant un colorant fluorescent thiol, a charge cationique externe, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2912141A1 (fr) | Composition de teinture comprenant un colorant fluorescent thiol a heterocycle et a charge cationique externe, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2912138A1 (fr) | Composition de teinture comprenant un colorant fluosrescent thiol a groupes amines et a charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant | |
| FR2912134A1 (fr) | Composition de teinture comprenant un colorant fluorescent thiol a groupement alcoxy/hydroxy et a charge cationique interne, procede d'eclaircissement des matieres keratiniques a partir de ce colorant |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA HR MK RS |
|
| 17P | Request for examination filed |
Effective date: 20081219 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: GREAVES, ANDREW Inventor name: DAUBRESSE, NICOLAS |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| DAX | Request for extension of the european patent (deleted) | ||
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602007016277 Country of ref document: DE Effective date: 20111006 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2367592 Country of ref document: ES Kind code of ref document: T3 Effective date: 20111104 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20110803 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20110803 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111205 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111203 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 518916 Country of ref document: AT Kind code of ref document: T Effective date: 20110803 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111104 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FD4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 |
|
| 26N | No opposition filed |
Effective date: 20120504 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602007016277 Country of ref document: DE Effective date: 20120504 |
|
| BERE | Be: lapsed |
Owner name: L'OREAL Effective date: 20120331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120331 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120331 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120331 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110803 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070323 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 10 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20210210 Year of fee payment: 15 Ref country code: IT Payment date: 20210211 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20210310 Year of fee payment: 15 Ref country code: DE Payment date: 20210310 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20210406 Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602007016277 Country of ref document: DE |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220323 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220331 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20221001 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20230504 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220323 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20220324 |