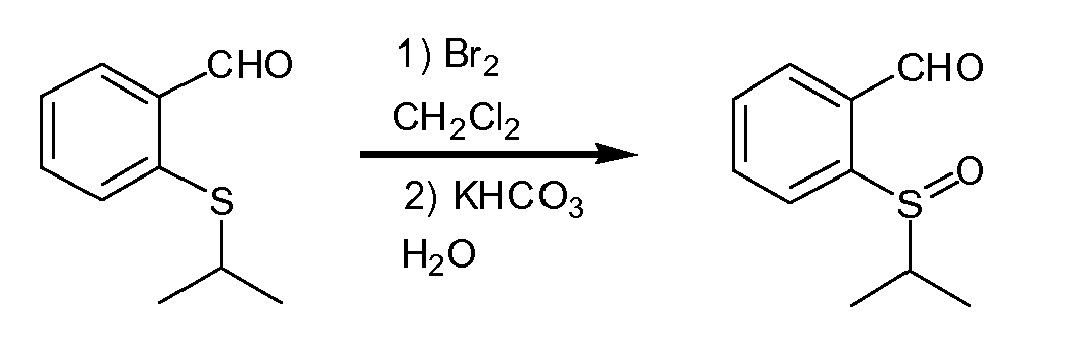EP1990348A2 - Metathesis catalysts containing sulphur - Google Patents
Metathesis catalysts containing sulphur Download PDFInfo
- Publication number
- EP1990348A2 EP1990348A2 EP08103339A EP08103339A EP1990348A2 EP 1990348 A2 EP1990348 A2 EP 1990348A2 EP 08103339 A EP08103339 A EP 08103339A EP 08103339 A EP08103339 A EP 08103339A EP 1990348 A2 EP1990348 A2 EP 1990348A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- compounds
- sulfur
- alkyl
- ligand
- radicals
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F15/00—Compounds containing elements of Groups 8, 9, 10 or 18 of the Periodic System
- C07F15/0006—Compounds containing elements of Groups 8, 9, 10 or 18 of the Periodic System compounds of the platinum group
- C07F15/0046—Ruthenium compounds
Definitions
- the present invention relates to novel transition metal complexes of the formula (I) a process for the preparation of these transition metal complexes, and the use of the transition metal complexes as catalysts in metathesis reactions.
- Metathesis is a chemical reaction in which formal substituents are exchanged at double or triple bonds.
- the metathesis reactions include the oligomerization and polymerization of acyclic dienes (ADMET) or the polymerization of cyclic olefins (ROMP) as well as the synthesis of cyclic compounds of different sizes by ring-closing metathesis (RCM).
- ADMET oligomerization and polymerization of acyclic dienes
- RPM cyclic olefins
- RCM ring-closing metathesis
- CM cross-metatheses of different alkenes
- Enyne metathesis metathesis of alkenes with alkynes
- M is Ru or Os, preferably Ru, L and L ', independently of one another or different, represent a neutral electron donor
- X1 and X2 are the same or different and represent an anionic ligand
- R is hydrogen, cyclic, straight-chain or branched-chain alkyl radicals, or represents optionally substituted aryl radicals
- Z represents a sulfur-containing unit coordinating directly with the metal
- A is a bridge which covalently connects the unit Z to the carbene carbon
- n is the number 0 or 1, preferably 0.
- the olefin metathesis with the complex compounds of the invention is characterized by low activity at room temperature and in particular rapid increase in activity with increase in temperature.
- the compounds of the invention can thereby be used as thermoschaltbare catalysts.
- the complex compounds of the invention are air-stable compounds and have remarkable thermal stability, with respect to the catalysts of the prior art, not significantly lower activity.
- the complex compounds according to the invention for L have saturated or unsaturated NHC (N-heterocyclic carbenes) as ligands.
- NHC N-heterocyclic carbenes
- Such ligands are exemplified in the following literature ( DE 19815275 and T. Weskamp, WC Shadowman, M. Spiegler, WA Herrmann Angew. Chem. 1998, 110, 263-2633 ; DE 19902439 and T. Weskamp, FJ Kohl, W. Hieringer, D. Gleich, WA Herrmann Angew. Chem., 1999, 111, 2573-2576 ; EP1180108 ).
- the above-mentioned sulfur-containing unit Z are preferably radicals from the series: thiol, thioether, thioacetals, disulfides, dithiocarboxylic acids, thioesters, thioketones, thioaldehydes, thiocarbamates, thiourethanes, phosphine sulfides, Thiophosphates, thiophosphonates, sulfonates, sulfones, sulfonamides or sulfur-containing heterocycles, wherein it must be ensured that the compound ZM, preferably Z-Ru, is formed via the sulfur atom or an oxygen atom located on the sulfur. This will preferably be the case when the coordinating ring closure between the sulfur or the oxygen atom and the metal atom forms a 5-, 6- or 7-membered ring.
- bridging moiety A the person skilled in the art can in principle use a radical which is suitable for the present purpose, a carbon skeleton which consists of 2 to 4 carbon atoms is preferred, a C 2 bridge is particularly preferred, where appropriate both Atoms may have an sp 2 hybridization and the residue advantageously forms part of a 3-, 4-, 5-, 6-, 7- or 8-membered ring system.
- the ring systems just mentioned may optionally have one or more heteroatoms. As such, in particular oxygen, sulfur or nitrogen atoms.
- they may be further unsaturated and may also be of an aromatic nature.
- X1 and X2 are preferably inorganic or organic anions from the group of halides, especially F -, Cl -, Br - (, pseudohalides, hydroxides, alkoxides or amides RO -, R 2 N - ), Phenols, thiols, thiophenols, carboxylates, carbonates, sulfonates, sulfates, phosphates and phosphonates, allyl, and cyclopentadienyl, wherein among the pseudohalides preferably cyanide, rhodanide, cyanate, isocyanate, thiocyanate and isothiocyanate is understood, wherein the radicals R is the meet below definition.
- Y, R, R 'and R 1 to R 4 are independently selectable radicals from the group consisting of hydrogen, (C 1 -C 8 ) -alkyl, (C 1 -C 8 ) -alkoxy, (C 6 -C 18 ) - Aryloxy, HO- (C 1 -C 8 ) -alkyl, (C 2 -C 8 ) -alkoxyalkyl, (C 6 -C 18 ) -aryl, (C 7 -C 19 ) -aralkyl, (C 3 -C 18 ) Heteroaryl, (C 4 -C 19 ) heteroaralkyl, (C 1 -C 8 ) alkyl (C 6 -C 18 ) aryl, (C
- radicals R 'and R 1 to R 4 can independently of one another mean: (Cyclo) alkylthio, (hetero) arylthio, alkyl / arylsulfonylalkyl / arylsulfinyl, each optionally substituted with (C 1 -C 8 ) alkyl, (C 1 -C 8 ) alkoxy, (C 6 -C 18 ) aryloxy , HO- (C 1 -C 8 ) -alkyl, (C 2 -C 8 ) -alkoxyalkyl, (C 6 -C 18 ) -aryl, perfluoroalkyl, Halogen, (C 1 -C 8 ) -acyloxy, (C 1 -C 8 ) -acyl (C 1 -C 8 ) -alkoxycarbonyl, (C 1 -C 8 ) -alkylsulfonyl or (C 1 -C 8
- R 1 to R 4 may also represent a nitro group, sulfate, amine, ammonium salt, phosphate and phosphonium salt.
- the radicals R ' can be linked to one another or more of the radicals R 1 to R 4 in cyclic compounds.
- the radical R 1 can also be linked to the radical Y to form a (hetero) cyclic compound.
- the compounds according to the invention are preferably prepared by exchange reaction of the phosphine ligand in compounds of the formula (IV) by ligands of the formula (V) wherein the radicals and indices have the abovementioned meanings and PR 3 is a phosphine ligand, preferably tricyclohexylphosphane.
- the compounds according to the invention in particular those of the formula (I) and (II), of compounds of the formula (VI) are preferably prepared in a solvent, particularly preferably in toluene, benzene, tetrahydrofuran or dichloromethane, very particularly preferably in dichloromethane.
- the reaction preferably takes place in the presence of compounds capable of scavenging phosphines, more preferably in the presence of CuCl 2 and CuCl, most preferably in the presence of CuCl. In this case, it is preferable to work in the presence of equimolar amounts or of an excess of phosphine scavenger, based on compounds of the formula (IV).
- CuCl is used as the phosphine scavenger, it is particularly preferable to use from 1 to 1.5 equivalents. It preferably 0.9 to 3 equivalents of the compounds of the formula (V), based on compounds of the formula (IV) are used, more preferably 1 to 2 equivalents.
- the reaction is preferably carried out at temperatures of 20 to 80 ° C, more preferably at temperatures of 30 to 50 ° C.
- the reaction is preferably carried out under an inert gas such as nitrogen or argon.
- the compounds (I) according to the invention in particular those of the formula (II) and (III) can be used as catalysts in metathesis reactions. They can be used, for example, in ring-closing metathesis. Most preferably, they are used in ROMP and ADMET polymerization reactions.
- alkyl radicals are (C 1 -C 8 ) -alkyl radicals, such as, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, hexyl, heptyl or octyl, including all their binding isomers.
- the radical (C 1 -C 8 ) -alkoxy corresponds to the radical (C 1 -C 8 ) -alkyl, with the proviso that it is bonded to the molecule via an oxygen atom.
- (C 2 -C 8 ) alkoxyalkyl residues in which the alkyl chain is interrupted by at least one oxygen function, whereby two oxygen atoms can not be linked together.
- the number of carbon atoms indicates the total number of carbon atoms contained in the radical.
- a (C 3 -C 5 ) -alkylene bridge is a carbon chain with three to five carbon atoms, which chain is bound to the molecule under consideration via two different carbon atoms.
- the radicals described in the preceding paragraphs may be monosubstituted or polysubstituted by halogens and / or N, O, P, S, Si atom-containing radicals. These are in particular alkyl radicals of the abovementioned type which have one or more of these heteroatoms in their chain or which are bonded to the molecule via one of these heteroatoms.
- (C 3 -C 8 ) -cycloalkyl is meant cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl radicals, etc. These may be substituted with one or more halogens and / or N, O, P, S, Si atom-containing radicals and / or N, O, P, S atoms in the ring such.
- a (C 3 -C 8 ) -cycloalkyl (C 1 -C 8 ) -alkyl radical denotes a cycloalkyl radical as described above which is bonded to the molecule via an alkyl radical as indicated above.
- (C 1 -C 8 ) -Acyloxy in the context of the invention means an alkyl radical as defined above with max. 8 C atoms, which is bound to the molecule via a COO function.
- (C 1 -C 8 ) acyl means in the context of the invention an alkyl radical as defined above with max. 8 C atoms, which is bound to the molecule via a CO function.
- an aryl radical is meant in particular a (C 6 -C 18 ) -aryl radical which is an aromatic radical having 6 to 18 C atoms.
- these include compounds such as phenyl, naphthyl, anthryl, phenanthryl, biphenyl radicals or systems of the type described above, such as, for example, indenyl systems which are optionally halogenated with (C 1 -C 8 ) -alkyl, ( C 1 -C 8 ) -alkoxy, NH 2 , NH (C 1 -C 8 ) -alkyl, N ((C 1 -C 8 ) -alkyl) 2 , OH, CF 3 , NH (C 1 -C 8 ) Acyl, N ((C 1 -C 8 ) -acyl) 2 , (C 1 -C 8 ) -acyl, (C 1 -C 8 ) -acyloxy.
- a (C 7 -C 19 ) -aralkyl radical is a (C 6 -C 18 ) -aryl radical bonded to the molecule via a (C 1 -C 8 ) -alkyl radical.
- a (C 3 -C 18 ) -heteroaryl radical denotes a five-, six- or seven-membered aromatic ring system comprising 3 to 18 C atoms, which heteroatoms such.
- radicals are considered, such as 1-, 2-, 3-furyl, such as 1-, 2-, 3-pyrrolyl, 1-, 2-, 3-thienyl, 2-, 3-, 4-pyridyl, 2-, 3-, 4-, 5-, 6-, 7-indolyl, 3-, 4-, 5-pyrazolyl, 2-, 4-, 5-imidazolyl, acridinyl, quinolinyl, phenanthridinyl, 2-, 4- , 5-, 6-pyrimidinyl.
- the radical may be substituted by the same radicals as the abovementioned aryl radical.
- heteroaralkyl is meant a heteroaromatic system corresponding to the (C 7 -C 19 ) aralkyl radical.
- Suitable halogens are fluorine, chlorine, bromine and iodine.
- N, N-diallyl-p-toluenesulfonamide (0.350 mmol, 84 mg) in 17.5 ml of toluene was mixed with 5 mol% of the catalyst (0.018 mmol) SR1 from Example 5a under argon and stirred at 80 ° C. 200 ⁇ L of an aliquot of reaction solution was added to 500 ⁇ L of 2M ethyl vinyl ether solution in methylene chloride and analyzed by GC. After 24 h, 51% conversion to the desired Np-toluenesulfonyl-2,5-dihydropyrrole was found.
- Figure 2 RCM of N, N-diallyl-p-toluenesulfonamide in the presence of 5 mol% complex SOR1 in methylene chloride at room temperature.
Abstract
Description
Die vorliegende Erfindung betrifft neue Übergangsmetallkomplexe der Formel (I),
Unter Metathese versteht man eine chemische Reaktion bei der formal Substituenten an Doppel- bzw. Dreifachbindungen ausgetauscht werden. Zu den Metathesereaktionen zählen die Oligomerisation und Polymerisation von acyclischen Dienen (ADMET) oder Polymerisation von zyklischen Olefinen (ROMP) sowie die Synthese von zyklischen Verbindungen unterschiedlicher Größen durch Ringschlussmetathese (RCM). Darüber hinaus sind gekreuzte Metathesen unterschiedlicher Alkene (CM) und Metathese von Alkenen mit Alkinen (Enyne Metathese) bekannt. Zahlreiche, grundlegende Arbeiten haben wesentlich zum Verständnis dieser übergangsmetallkatalysierten Reaktion beigetragen (Übersicht siehe:
Für die Olefin-Metathese steht eine Vielzahl an Katalysatorsystemen zur Verfügung. Speziell durch Arbeiten von Schrock wurden Alkylidenkomplexe des Molybdäns und Wolframs als erste wohldefinierte Katalysatoren eingeführt. (
For the olefin metathesis a variety of catalyst systems is available. Specially by Schrock's work, alkylidene complexes of molybdenum and tungsten were the first well-defined catalysts to be introduced. (
In
Das Patent
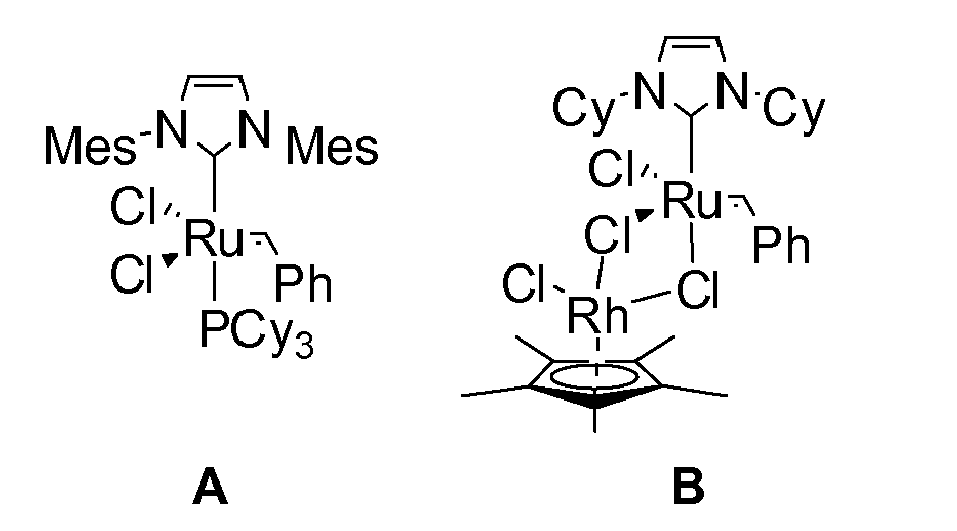
Es sind einige Beispiele für Metathesekatalysatoren mit schwefelhaltigen Einheiten in der Seitenkette in der Literatur beschrieben worden (Komplex H in
Nichtsdestotrotz bestand weiterhin Bedarf an neuen Katalysatorsystemen für die Olefin-Metathese, welche stabil sind und zudem eine hohe ggf. steuerbare Aktivität zeigen und als alternative zu den existierenden Katalysatoren benutzt werden können. Insbesondere sollten die Katalysatoren ganzheitlich betrachtet den Katalysatoren des Standes der Technik vom ökonomischen und/oder ökologischen Standpunkt aus betrachtet überlegen sein.Nonetheless, there has been a continuing need for new olefin metathesis catalyst systems that are stable and also exhibit high, possibly controllable, activity and can be used as alternatives to existing catalysts. In particular, the catalysts should be holistically superior to the catalysts of the prior art from the economic and / or ecological point of view.
Überraschenderweise wurden nun Verbindungen der Formel(I) gefunden
M für Ru oder Os steht, vorzugsweise für Ru,
L und L' unabhängig voneinander gleich oder verschieden für einen neutralen Elektronendonor stehen,
X1 und X2 gleich oder verschieden sind und für einen anionischen Liganden stehen,
R für Wasserstoff, cyclische, geradkettige oder verzweigtkettige Alkylreste, oder für ggf. substituierte Arylreste steht,
Z für eine schwefelhaltige direkt am Metall koordinierende Einheit steht, und
A eine Brücke, welche die Einheit Z mit dem Carben-Kohlenstoff kovalent verbindet, und n die Zahl 0 oder 1, bevorzugt 0 bedeutet.
Die Olefin-Metathese mit den erfindungsgemäßen Komplexverbindungen zeichnet sich durch geringe Aktivität bei Raumtemperatur und insbesondere schnelle Aktivitätssteigerung mit Erhöhung der Temperatur aus. Die erfindungsgemäßen Verbindungen können dadurch als thermoschaltbare Katalysatoren verwendet werden. Darüber hinaus sind die erfindungsgemäßen Komplexverbindungen luftstabile Verbindungen und besitzen bemerkenswerte Thermostabilität, bei gegenüber den Katalysatoren des Standes der Technik nicht wesentlich geringerer Aktivität.Surprisingly, compounds of the formula (I) have now been found
M is Ru or Os, preferably Ru,
L and L ', independently of one another or different, represent a neutral electron donor,
X1 and X2 are the same or different and represent an anionic ligand,
R is hydrogen, cyclic, straight-chain or branched-chain alkyl radicals, or represents optionally substituted aryl radicals,
Z represents a sulfur-containing unit coordinating directly with the metal, and
A is a bridge which covalently connects the unit Z to the carbene carbon, and n is the
The olefin metathesis with the complex compounds of the invention is characterized by low activity at room temperature and in particular rapid increase in activity with increase in temperature. The compounds of the invention can thereby be used as thermoschaltbare catalysts. In addition, the complex compounds of the invention are air-stable compounds and have remarkable thermal stability, with respect to the catalysts of the prior art, not significantly lower activity.
Durch Variation der neutralen Elektronendonorliganden L und L' kann die Aktivität und Selektivität der Komplexe weiterhin gezielt gesteuert werden. Vorzugsweise besitzen die erfindungsgemäßen Komplexverbindungen für L gesättigte oder ungesättigte NHC (N-heterocyclische Carbene) als Liganden. Diese zeichnen sich neben ihrer Variationsvielfalt zur Modellierung der Ligandensphäre insbesondere durch Bereitstellung von hohen Katalysatoraktivitäten aus. Derartige Liganden sind in folgender Literatur beispielhaft erwähnt (
In den erfindungsgemäßen Komplexverbindungen handelt es sich bei der oben genannten schwefelhaltigen Einheit Z vorzugsweise um Reste aus der Reihe: Thiol, Thioether, Thioacetale, Disulfide, Dithiocarbonsäuren, Thioester, Thioketone, Thioaldehyde, Thiocarbamate, Thiourethane, Phosphinsulfide, Thiophosphate, Thiophosphonate, Sulfonate, Sulfone, Sulfonamide oder schwefelhaltige Heterocyclen, wobei gewährleistet sein muss, dass die Verbindung Z-M, vorzugsweise Z-Ru, über das Schwefelatom oder ein am Schwefel befindliches Sauerstoffatom gebildet wird. Das wird vorzugsweise dann der Fall sein, wenn der koordinierende Ringschluss zwischen dem Schwefel oder dem Sauerstoffatom und dem Metallatom einen 5-, 6- oder 7-Ring bildet.In the complex compounds according to the invention, the above-mentioned sulfur-containing unit Z are preferably radicals from the series: thiol, thioether, thioacetals, disulfides, dithiocarboxylic acids, thioesters, thioketones, thioaldehydes, thiocarbamates, thiourethanes, phosphine sulfides, Thiophosphates, thiophosphonates, sulfonates, sulfones, sulfonamides or sulfur-containing heterocycles, wherein it must be ensured that the compound ZM, preferably Z-Ru, is formed via the sulfur atom or an oxygen atom located on the sulfur. This will preferably be the case when the coordinating ring closure between the sulfur or the oxygen atom and the metal atom forms a 5-, 6- or 7-membered ring.
Für den verbrückenden Molekülteil A kann der Fachmann im Prinzip einen ihm für den vorliegenden Zweck in Frage kommenden Rest verwenden, bevorzugt ist ein Kohlenstoffgerüst, welches aus 2 bis 4 Kohlenstoffatomen besteht, besonders bevorzugt ist eine C2-Brücke, wobei ggf. beide C-Atome eine sp2-Hybridisierung aufweisen können und der Rest vorteilhafterweise einen Teil eines 3-, 4-, 5-, 6-, 7- oder 8 gliedrigen Ringsystems bildet. Die eben angesprochenen Ringsysteme können gegebenenfalls ein oder mehrere Heteroatome aufweisend. Als solche kommen insbesondere Sauerstoff-, Schwefel- oder Stickstoff-Atome. Sie können über die oben beschriebene sp2-Hybridisierung hinaus weiter ungesättigt sowie gegebenenfalls aromatischer Natur sein. Sie können einfach oder mehrfach mit weiteren Resten, insbesondere solchen ausgewählt aus der Gruppe bestehend aus (C1-C8)-Alkyl, (C1-C8) -Alkoxy, (C6-C18)-Aryloxy, HO-(C1-C8)-Alkyl, (C2-C8)-Alkoxyalkyl, (C6-C18)-Aryl, (C7-C19)-Aralkyl, (C3-C18)-Heteroaryl, (C4-C19)-Heteroaralkyl, (C1-C8) -Alkyl- (C6-C18) -Aryl, (C1-C8) -Alkyl- (C3-C18) -Heteroaryl, (C3-C8)-Cycloalkyl, (C1-C8)-Alkyl-(C3-C8)-Cycloalkyl, (C3-C8)-Cycloalkyl-(C1-C8)-Alkyl substituiert sein.
Darüber hinaus können die Ringsysteme auch einen oder mehrere Substituenten aufweisen, insbesondere solchen ausgewählt aus der Gruppe bestehend aus Halogen, Hydroxy, Carbonsäuren, Ester, Silylether, Thioether, Thioacetale, Imine, Silylenolether, Ammoniumsalze, Amide, Nitrile, Perfluoralkyl-Gruppen, Ketone, Aldehyde, Carbamate, Carbonate, Urethane, Sulfonate, Sulfone, Sulfonamide, Nitro-Gruppen, Organosilan-Einheiten " Phosphon- und Phosphat-Gruppe, und Phosphoniumsalze.For the bridging moiety A, the person skilled in the art can in principle use a radical which is suitable for the present purpose, a carbon skeleton which consists of 2 to 4 carbon atoms is preferred, a C 2 bridge is particularly preferred, where appropriate both Atoms may have an sp 2 hybridization and the residue advantageously forms part of a 3-, 4-, 5-, 6-, 7- or 8-membered ring system. The ring systems just mentioned may optionally have one or more heteroatoms. As such, in particular oxygen, sulfur or nitrogen atoms. In addition to the above-described sp 2 hybridization, they may be further unsaturated and may also be of an aromatic nature. They may be monosubstituted or polysubstituted by further radicals, in particular those selected from the group consisting of (C 1 -C 8 ) -alkyl, (C 1 -C 8 ) -alkoxy, (C 6 -C 18 ) -aryloxy, HO- ( C 1 -C 8 ) -alkyl, (C 2 -C 8 ) -alkoxyalkyl, (C 6 -C 18 ) -aryl, (C 7 -C 19 ) -aralkyl, (C 3 -C 18 ) -heteroaryl, ( C 4 -C 19 ) heteroaralkyl, (C 1 -C 8 ) -alkyl (C 6 -C 18 ) -aryl, (C 1 -C 8 ) -alkyl (C 3 -C 18 ) -heteroaryl, ( C 3 -C 8 ) cycloalkyl, (C 1 -C 8 ) alkyl (C 3 -C 8 ) cycloalkyl, (C 3 -C 8 ) cycloalkyl (C 1 -C 8 ) alkyl ,
In addition, the ring systems may also have one or more substituents, in particular those selected from among halogen, hydroxy, carboxylic acids, esters, silyl ethers, thioethers, thioacetals, imines, silyl enol ethers, ammonium salts, amides, nitriles, perfluoroalkyl groups, ketones, Aldehydes, carbamates, carbonates, urethanes, sulfonates, sulfones, sulfonamides, nitro groups, organosilane units "phosphonic and phosphate groups, and phosphonium salts.
In den erfindungsgemäßen Komplexverbindungen handelt es sich bei den anionischen Liganden X1 und X2 vorzugsweise um anorganischen oder organischen Anionen aus der Reihe Halogenide, insbesondere F-, Cl-, Br-, Pseudohalogenide, Hydroxide, Alkoxide oder Amide (RO-, R2N-), Phenole, Thiole, Thiophenole, Carboxylate, Carbonate, Sulfonate, Sulfate, Phosphate und Phosphonate, Allyl, und Cyclopentadienyl stehen, wobei unter den Pseudohalogeniden vorzugsweise Cyanid, Rhodanid, Cyanat, Isocyanat, Thiocyanat und Isothiocyanat verstanden wird, wobei die Reste R die unten genannte Definition erfüllen.In the inventive complex compounds are in the anionic ligands X1 and X2 are preferably inorganic or organic anions from the group of halides, especially F -, Cl -, Br - (, pseudohalides, hydroxides, alkoxides or amides RO -, R 2 N - ), Phenols, thiols, thiophenols, carboxylates, carbonates, sulfonates, sulfates, phosphates and phosphonates, allyl, and cyclopentadienyl, wherein among the pseudohalides preferably cyanide, rhodanide, cyanate, isocyanate, thiocyanate and isothiocyanate is understood, wherein the radicals R is the meet below definition.
Ganz besonders bevorzugt sind Komplexverbindungen der allgemeinen Formel (II) und (III).
X1 und X2 nehmen die oben angegebenen Reste an,
Y, R, R' und R1 bis R4 sind unabhängig voneinander wählbare Reste aus der Gruppe Wasserstoff, (C1-C8) -Alkyl, (C1-C8) -Alkoxy, (C6-C18) -Aryloxy, HO- (C1-C8) -Alkyl, (C2-C8) -Alkoxyalkyl, (C6-C18) -Aryl, (C7-C19) -Aralkyl, (C3-C18) -Heteroaryl, (C4-C19) -Heteroaralkyl, (C1-C8) -Alkyl- (C6-C18) -Aryl, (C1-C8) -Alkyl- (C3-C18) -Heteroaryl, (C3-C8) -Cycloalkyl, (C1-C8) -Alkyl- (C3-C8) -Cycloalkyl, (C3-C8) -Cycloalkyl- (C1-C8) -Alkyl . Außerdem können die Reste R' und R1 bis R4 unabhängig voneinander bedeuten:
(Cyclo)Alkylthio, (Hetero)Arylthio, Alkyl/Arylsulfonyl Alkyl/Arylsulfinyl, jeweils wahlweise substituiert mit (C1-C8) -Alkyl, (C1-C8) -Alkoxy, (C6-C18) -Aryloxy, HO- (C1-C8) - Alkyl, (C2-C8) -Alkoxyalkyl, (C6-C18)-Aryl, Perfluoralkyl, Halogen, (C1-C8) -Acyloxy, (C1-C8) -Acyl (C1-C8) -Alkoxycarbonyl, (C1-C8) -Alkylsulfonyl oder (C1-C8) -Alkylsulfinyl, (C6-C18) -Arylsulfonyl oder (C6-C18)-Arylsulfinyl.
R1 bis R4 können ebenfalls eine Nitro-Gruppe, Sulfat, Amin, Ammoniumsalz, Phospat und Phosphoniumsalz bedeuten.
Die Reste R' können mit einem oder mehreren der Reste R1 bis R4 in cyclischen Verbindungen miteinander verknüpft vorliegen. Auch der Rest R1 kann mit dem Rest Y unter Ausbildung einer (hetero)cyclischen Verbindung miteinander verknüpft sein.Very particular preference is given to complex compounds of the general formula (II) and (III).
X1 and X2 adopt the radicals indicated above,
Y, R, R 'and R 1 to R 4 are independently selectable radicals from the group consisting of hydrogen, (C 1 -C 8 ) -alkyl, (C 1 -C 8 ) -alkoxy, (C 6 -C 18 ) - Aryloxy, HO- (C 1 -C 8 ) -alkyl, (C 2 -C 8 ) -alkoxyalkyl, (C 6 -C 18 ) -aryl, (C 7 -C 19 ) -aralkyl, (C 3 -C 18 ) Heteroaryl, (C 4 -C 19 ) heteroaralkyl, (C 1 -C 8 ) alkyl (C 6 -C 18 ) aryl, (C 1 -C 8 ) alkyl (C 3 -C 18 ) Heteroaryl, (C 3 -C 8 ) -cycloalkyl, (C 1 -C 8 ) -alkyl- (C 3 -C 8 ) -cycloalkyl, (C 3 -C 8 ) -cycloalkyl- (C 1 -C 8 ) -Alkyl. In addition, the radicals R 'and R 1 to R 4 can independently of one another mean:
(Cyclo) alkylthio, (hetero) arylthio, alkyl / arylsulfonylalkyl / arylsulfinyl, each optionally substituted with (C 1 -C 8 ) alkyl, (C 1 -C 8 ) alkoxy, (C 6 -C 18 ) aryloxy , HO- (C 1 -C 8 ) -alkyl, (C 2 -C 8 ) -alkoxyalkyl, (C 6 -C 18 ) -aryl, perfluoroalkyl, Halogen, (C 1 -C 8 ) -acyloxy, (C 1 -C 8 ) -acyl (C 1 -C 8 ) -alkoxycarbonyl, (C 1 -C 8 ) -alkylsulfonyl or (C 1 -C 8 ) -alkylsulfinyl , (C 6 -C 18 ) arylsulfonyl or (C 6 -C 18 ) arylsulfinyl.
R 1 to R 4 may also represent a nitro group, sulfate, amine, ammonium salt, phosphate and phosphonium salt.
The radicals R 'can be linked to one another or more of the radicals R 1 to R 4 in cyclic compounds. The radical R 1 can also be linked to the radical Y to form a (hetero) cyclic compound.
Die erfindungsgemäßen Verbindungen, insbesondere die der Formel (I) und(II) werden vorzugsweise durch Austauschreaktion des Phosphanliganden in Verbindungen der Formel (IV) durch Liganden der Formel (V) hergestellt
Die Herstellung der erfindungsgemäßen Verbindungen, insbesondere die der Formel (I) und (II) aus Verbindungen der Formel(VI) erfolgt vorzugsweise in einem Lösungsmittel, besonders bevorzugt in Toluol, Benzol, Tetrahydrofuran oder Dichlormethan, ganz besonders bevorzugt in Dichlormethan. Die Umsetzung findet vorzugsweise in Gegenwart von Verbindungen statt, die in der Lage sind, Phosphane abzufangen, besonders bevorzugt in Gegenwart von CuCl2 und CuCl, ganz besonders bevorzugt in Gegenwart von CuCl. Dabei wird vorzugsweise in Gegenwart von äquimolaren Mengen bzw. eines Überschusses an Phosphanfänger, bezogen auf Verbindungen der Formel (IV), gearbeitet. Wird als Phosphanfänger CuCl eingesetzt, so werden besonders bevorzugt 1 bis 1,5 Äquivalente eingesetzt. Es werden vorzugsweise 0,9 bis 3 Äquivalente der Verbindungen der Formel(V), bezogen auf Verbindungen der Formel(IV) eingesetzt, besonders bevorzugt 1 bis 2 Äquivalente. Die Umsetzung erfolgt vorzugsweise bei Temperaturen von 20 bis 80°C, besonders bevorzugt bei Temperaturen von 30 bis 50°C. Die Umsetzung wird vorzugsweise unter Inertgas wie beispielsweise Stickstoff oder Argon durchgeführt.The compounds according to the invention, in particular those of the formula (I) and (II), of compounds of the formula (VI) are preferably prepared in a solvent, particularly preferably in toluene, benzene, tetrahydrofuran or dichloromethane, very particularly preferably in dichloromethane. The reaction preferably takes place in the presence of compounds capable of scavenging phosphines, more preferably in the presence of CuCl 2 and CuCl, most preferably in the presence of CuCl. In this case, it is preferable to work in the presence of equimolar amounts or of an excess of phosphine scavenger, based on compounds of the formula (IV). If CuCl is used as the phosphine scavenger, it is particularly preferable to use from 1 to 1.5 equivalents. It preferably 0.9 to 3 equivalents of the compounds of the formula (V), based on compounds of the formula (IV) are used, more preferably 1 to 2 equivalents. The reaction is preferably carried out at temperatures of 20 to 80 ° C, more preferably at temperatures of 30 to 50 ° C. The reaction is preferably carried out under an inert gas such as nitrogen or argon.
Die erfindungsgemäßen Verbindungen (I), insbesondere die der Formel (II) und (III) können als Katalysatoren in Metathesereaktionen eingesetzt werden. Sie können beispielsweise in Ringschluss-Metathesen eingesetzt werden. Besonders bevorzugt werden sie in ROMP und ADMET-Polymerisationsreaktionen eingesetzt.The compounds (I) according to the invention, in particular those of the formula (II) and (III) can be used as catalysts in metathesis reactions. They can be used, for example, in ring-closing metathesis. Most preferably, they are used in ROMP and ADMET polymerization reactions.
Als Alkylreste sind anzusehen insbesondere (C1-C8)-Alkylreste, wie z.B. Methyl, Ethyl, n-Propyl, Isopropyl, n-Butyl, Isobutyl, sec-Butyl, tert-Butyl, Pentyl, Hexyl, Heptyl oder Octyl samt aller ihrer Bindungsisomeren.
Der Rest (C1-C8) -Alkoxy entspricht dem Rest (C1-C8) -Alkyl mit der Maßgabe, dass dieser über ein Sauerstoffatom an das Molekül gebunden ist.
Als (C2-C8)-Alkoxyalkyl sind Reste gemeint, bei denen die Alkylkette durch mindestens eine Sauerstoffunktion unterbrochen ist, wobei nicht zwei Sauerstoffatome miteinander verbunden sein können. Die Anzahl der Kohlenstoffatome gibt die Gesamtzahl der im Rest enthaltenen Kohlenstoffatome an. Eine (C3-C5)-Alkylenbrücke ist eine Kohlenstoffkette mit drei bis fünf C-Atomen, wobei diese Kette über zwei verschiedene C-Atome an das betrachtete Molekül gebunden ist.
Die in den vorangehenden Absätzen beschriebenen Reste können einfach oder mehrfach mit Halogenen und/oder N-, O-, P-, S-, Si-atomhaltigen Resten substituiert sein. Dies sind insbesondere Alkylreste der oben genannten Art, welche eines oder mehrere dieser Heteroatome in ihrer Kette aufweisen bzw. welche über eines dieser Heteroatome an das Molekül gebunden sind.Particularly suitable alkyl radicals are (C 1 -C 8 ) -alkyl radicals, such as, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, hexyl, heptyl or octyl, including all their binding isomers.
The radical (C 1 -C 8 ) -alkoxy corresponds to the radical (C 1 -C 8 ) -alkyl, with the proviso that it is bonded to the molecule via an oxygen atom.
By (C 2 -C 8 ) alkoxyalkyl are meant residues in which the alkyl chain is interrupted by at least one oxygen function, whereby two oxygen atoms can not be linked together. The number of carbon atoms indicates the total number of carbon atoms contained in the radical. A (C 3 -C 5 ) -alkylene bridge is a carbon chain with three to five carbon atoms, which chain is bound to the molecule under consideration via two different carbon atoms.
The radicals described in the preceding paragraphs may be monosubstituted or polysubstituted by halogens and / or N, O, P, S, Si atom-containing radicals. These are in particular alkyl radicals of the abovementioned type which have one or more of these heteroatoms in their chain or which are bonded to the molecule via one of these heteroatoms.
Unter (C3-C8)-Cycloalkyl versteht man Cyclopropyl, Cyclobutyl, Cyclopentyl, Cyclohexyl bzw. Cycloheptylreste etc. Diese können mit einem oder mehreren Halogenen und/oder N-, O-, P-, S-, Si-atomhaltige Reste substituiert sein und/oder N-, O-, P-, S-Atome im Ring aufweisen, wie z. B. 1-, 2-, 3-, 4-Piperidyl, 1-, 2-, 3-Pyrrolidinyl, 2-, 3-Tetrahydrofuryl, 2-, 3-, 4-Morpholinyl.By (C 3 -C 8 ) -cycloalkyl is meant cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl radicals, etc. These may be substituted with one or more halogens and / or N, O, P, S, Si atom-containing radicals and / or N, O, P, S atoms in the ring such. 1-, 2-, 3-, 4-piperidyl, 1-, 2-, 3-pyrrolidinyl, 2-, 3-tetrahydrofuryl, 2-, 3-, 4-morpholinyl.
Ein (C3-C8)-Cycloalkyl-(C1-C8)-Alkylrest bezeichnet einen wie oben dargestellten Cycloalkylrest, welcher über einen wie oben angegebenen Alkylrest an das Molekül gebunden ist.A (C 3 -C 8 ) -cycloalkyl (C 1 -C 8 ) -alkyl radical denotes a cycloalkyl radical as described above which is bonded to the molecule via an alkyl radical as indicated above.
(C1-C8)-Acyloxy bedeutet im Rahmen der Erfindung einen wie oben definierten Alkylrest mit max. 8 C-Atomen, welcher über eine COO-Funktion an das Molekül gebunden ist.(C 1 -C 8 ) -Acyloxy in the context of the invention means an alkyl radical as defined above with max. 8 C atoms, which is bound to the molecule via a COO function.
(C1-C8)-Acyl bedeutet im Rahmen der Erfindung einen wie oben definierten Alkylrest mit max. 8 C-Atomen, welcher über eine CO-Funktion an das Molekül gebunden ist.(C 1 -C 8 ) acyl means in the context of the invention an alkyl radical as defined above with max. 8 C atoms, which is bound to the molecule via a CO function.
Unter einem Arylrest wird insbesondere ein (C6-C18)-Arylrest verstanden, welcher ein aromatischer Rest mit 6 bis 18 C-Atomen ist. Insbesondere zählen hierzu Verbindungen wie Phenyl-, Naphthyl-, Anthryl-, Phenanthryl-, Biphenylreste oder an das betreffende Molekül annelierte Systeme der vorbeschriebenen Art, wie z.B. Indenylsysteme, welche ggf. mit Halogen, (C1-C8) -Alkyl, (C1-C8) -Alkoxy, NH2, NH (C1-C8) -Alkyl, N((C1-C8)-Alkyl)2, OH, CF3, NH (C1-C8) -Acyl, N ((C1-C8) -Acyl)2, (C1-C8) -Acyl, (C1-C8) -Acyloxy substituiert sein können.By an aryl radical is meant in particular a (C 6 -C 18 ) -aryl radical which is an aromatic radical having 6 to 18 C atoms. In particular, these include compounds such as phenyl, naphthyl, anthryl, phenanthryl, biphenyl radicals or systems of the type described above, such as, for example, indenyl systems which are optionally halogenated with (C 1 -C 8 ) -alkyl, ( C 1 -C 8 ) -alkoxy, NH 2 , NH (C 1 -C 8 ) -alkyl, N ((C 1 -C 8 ) -alkyl) 2 , OH, CF 3 , NH (C 1 -C 8 ) Acyl, N ((C 1 -C 8 ) -acyl) 2 , (C 1 -C 8 ) -acyl, (C 1 -C 8 ) -acyloxy.
Ein (C7-C19)-Aralkylrest ist ein über einen (C1-C8) -Alkylrest an das Molekül gebundener (C6-C18)-Arylrest.A (C 7 -C 19 ) -aralkyl radical is a (C 6 -C 18 ) -aryl radical bonded to the molecule via a (C 1 -C 8 ) -alkyl radical.
Ein (C3-C18)-Heteroarylrest bezeichnet im Rahmen der Erfindung ein fünf-, sechs- oder siebengliedriges aromatisches Ringsystem aus 3 bis 18 C-Atomen, welches Heteroatome wie z. B. Stickstoff, Sauerstoff oder Schwefel im Ring aufweist. Als solche Heteroaromaten werden insbesondere Reste angesehen, wie 1-, 2-, 3-Furyl, wie 1-, 2-, 3-Pyrrolyl, 1-, 2-, 3-Thienyl, 2-, 3-, 4-Pyridyl, 2-, 3-, 4-, 5-, 6-, 7-Indolyl, 3-, 4-, 5-Pyrazolyl, 2-, 4-, 5-Imidazolyl, Acridinyl, Chinolinyl, Phenanthridinyl, 2-, 4-, 5-, 6-Pyrimidinyl. Dieser Rest kann mit den gleichen Resten substituiert sein wie der oben genannte Arylrest.In the context of the invention, a (C 3 -C 18 ) -heteroaryl radical denotes a five-, six- or seven-membered aromatic ring system comprising 3 to 18 C atoms, which heteroatoms such. B. nitrogen, oxygen or sulfur in the ring. As such heteroaromatics, especially radicals are considered, such as 1-, 2-, 3-furyl, such as 1-, 2-, 3-pyrrolyl, 1-, 2-, 3-thienyl, 2-, 3-, 4-pyridyl, 2-, 3-, 4-, 5-, 6-, 7-indolyl, 3-, 4-, 5-pyrazolyl, 2-, 4-, 5-imidazolyl, acridinyl, quinolinyl, phenanthridinyl, 2-, 4- , 5-, 6-pyrimidinyl. This The radical may be substituted by the same radicals as the abovementioned aryl radical.
Unter einem (C4-C19) -Heteroaralkyl wird ein dem (C7-C19)-Aralkylrest entsprechendes heteroaromatisches System verstanden.By (C 4 -C 19 ) heteroaralkyl is meant a heteroaromatic system corresponding to the (C 7 -C 19 ) aralkyl radical.
Als Halogene (Hal) kommen Fluor, Chlor, Brom und Iod in Frage.Suitable halogens (Hal) are fluorine, chlorine, bromine and iodine.
Zu einer Suspension von Thiosalicylsäure (16.7 g; 109 mmol; Fluka) und Isopropylbromid (20.0 g; 163 mmol) in Ethanol (125 mL) wurden KOH-Plätzchen (17.3 g; 433 mmol) unter kräftigem Rühren langsam zugegeben. Nach 6 h Rühren wurde die Reaktionsmischung in Wasser-Eis Mischung (1200 mL) gegossen und mit konzentrierte Salzsäure (ca. 50 ml) sauer gestellt. Das ausgefallene Produkt wurde abfiltriert, mit 50% wässrigem Ethanol (2 x 100 mL) gewaschen und im Vakuum getrocknet. Ausbeute 6.4 g (30 %).
IR (film): ν 3084, 3058, 2962, 2924, 2865, 1828, 1625, 1588, 1463, 1365, 1243, 1197, 1155, 1049, 991, 912, 769, 746 cm-1; 1H NMR (200 MHz, DMSO-d6) δ 1.74-1.82 (d, 6H, J = 2.2 Hz), 4.02-4.12 (septet, 1H, J = 6.6 Hz), 7.70 (m, 1H), 7.66-7.72 (m, 1H), 7.95-8.00 (m, 2H), 8.36-8.42 (m, 1H); 13C NMR (50 MHz, DMSO-d6) δ 22.4, 35.3, 124.6, 128.3, 131.1, 132.1, 140.3, 167.2, 205.6; MS (EI) m/z (rel intensity) 196 (21, [M]+.), 154(13), 137(11), 136(100), 108(21), 69(8), 43(13), 41(13), 39(12) ; HRMS (EI): calcd for [M]+. (C7H12O2S): 196. 05580; found 196.05604.To a suspension of thiosalicylic acid (16.7 g, 109 mmol, Fluka) and isopropyl bromide (20.0 g, 163 mmol) in ethanol (125 mL) were slowly added KOH pellets (17.3 g, 433 mmol) with vigorous stirring. After stirring for 6 h, the reaction mixture was poured into water-ice mixture (1200 mL) and acidified with concentrated hydrochloric acid (ca 50 mL). The precipitated product was filtered off, washed with 50% aqueous ethanol (2 × 100 ml) and dried in vacuo. Yield 6.4 g (30%).
IR (film): ν 3084, 3058, 2962, 2924, 2865, 1828, 1625, 1588, 1463, 1365, 1243, 1197, 1155, 1049, 991, 912, 769, 746 cm -1 ; 1 H NMR (200 MHz, DMSO-d 6 ) δ 1.74-1.82 (d, 6H, J = 2.2 Hz), 4.02-4.12 (septet, 1H, J = 6.6 Hz), 7.70 (m, 1H), 7.66- 7.72 (m, 1H), 7.95-8.00 (m, 2H), 8.36-8.42 (m, 1H); 13 C NMR (50 MHz, DMSO-d 6 ) δ 22.4, 35.3, 124.6, 128.3, 131.1, 132.1, 140.3, 167.2, 205.6; MS (EI) m / z (rel intensity) 196 (21, [M] +. ), 154 (13), 137 (11), 136 (100), 108 (21), 69 (8), 43 (13 ), 41 (13), 39 (12); HRMS (EI): calcd for [M] +. (C 7 H 12 O 2 S): 196. 05580; found 196.05604.
Zu einer Lösung von 2-(Isopropylthio)benzoesäure (0.98 g, 5 mmol) in THF (10 mL) wurde Boran-Dimethylsulfid Komplex (0.8 mL, 8 mmol) bei 3 °C unter Argon und kräftigem rühren zugetropft. Nach 30 min Nachrühren im Eis-Bad wurde die Reaktion für weitere 24 h bei RT stehen gelassen. Methanol (1.5 mL) wurde vorsichtig zugegeben und die Reaktionslösung im Vakuum eingeengt. Der Rückstand wurde in Diethylether (50 ml) aufgenommen, mit gesättigter K2CO3-Lösung und NaCl-Lösung gewaschen. Wässrige Lösungen wurden zusätzlich mit Ether extrahiert, die vereinigten organischen Phasen über MgSO4 getrocknet, filtriert und das Lösungsmittel im Vakuum entfernt. Der Rückstand wurde in Dichlormethan (30 ml) aufgenommen, mit PCC (1.20 g, 5.6 mmol) vorsichtig versetzt und bei Raumtemperatur 36 h gerührt. Der Rückstand nach Lösungsmittel Entfernung wurde durch Säulenchromatography an Kieselgel (Hexan : Ethylacetat (10:1) gereinigt. Ausbeute 0.69 g. (76%) .
IR (film): ν 3686, 3601, 3362, 3063, 2968, 2929, 2867, 2744, 1950, 1692, 1648, 1587, 1559, 1460, 1442, 1383, 1368, 1287, 1242, 1195, 1156, 1128, 1073, 1062, 1052, 1039, 931, 879, 845, 825, 777, 679, 660, 635, 509 cm-1; 1H NMR (200 MHz, CDCl3) δ 1.30-1.40 (d, 6H, J = 6.6 Hz), 3.38-3.48 (septet, 1H, J = 6.8 Hz), 7.25-7.60 (m, 3H), 7.84-7.92 (m, 1H), 10.54 (d, 1H, J = 0.63 Hz); 13C NMR (50 MHz, CDCl3) δ 22.9, 38.8, 126.7, 130.1, 132.4, 133.8, 135.8, 140.3, 191.9; MS (EI) m/z (rel intensity) 180(66, [M]+.), 165(18), 138(49), 137(100), 110 (35), 109(41), 104 (57), 69 (11), 66 (11), 65 (26), 43 (25), 41 (19), 39 (17) ; HRMS (EI): calcd for [M]+. (C10H120S): 180. 07122; found 180.07148.To a solution of 2- (isopropylthio) benzoic acid (0.98 g, 5 mmol) in THF (10 mL) was added dropwise borane-dimethyl sulfide complex (0.8 mL, 8 mmol) at 3 ° C under argon and vigorous stirring. After stirring for 30 min in the ice bath was the Reaction allowed to stand at RT for a further 24 h. Methanol (1.5 mL) was added cautiously and the reaction solution was concentrated in vacuo. The residue was taken up in diethyl ether (50 ml), washed with saturated K 2 CO 3 solution and NaCl solution. Aqueous solutions were additionally extracted with ether, the combined organic phases dried over M g SO 4 , filtered and the solvent removed in vacuo. The residue was taken up in dichloromethane (30 ml), cautiously treated with PCC (1.20 g, 5.6 mmol) and stirred at room temperature for 36 h. The residue after solvent removal was purified by column chromatography on silica gel (hexane: ethyl acetate (10: 1). Yield 0.69 g (76%).
IR (film): ν 3686, 3601, 3362, 3063, 2968, 2929, 2867, 2744, 1950, 1692, 1648, 1587, 1559, 1460, 1442, 1383, 1368, 1287, 1242, 1195, 1156, 1128, 1073, 1062, 1052, 1039, 931, 879, 845, 825, 777, 679, 660, 635, 509 cm-1; 1H NMR (200 MHz, CDCl3) δ 1.30-1.40 (d, 6H, J = 6.6 Hz), 3.38-3.48 (septet, 1H, J = 6.8 Hz), 7.25-7.60 (m, 3H), 7.84-7.92 ( m, 1H), 10.54 (d, 1H, J = 0.63 Hz); 13 C NMR (50 MHz, CDCl3) δ 22.9, 38.8, 126.7, 130.1, 132.4, 133.8, 135.8, 140.3, 191.9; MS (EI) m / z (rel intensity) 180 (66, [M] +.), 165 (18), 138 (49), 137 (100), 110 (35), 109 (41), 104 (57 69 (11), 66 (11), 65 (26), 43 (25), 41 (19), 39 (17); HRMS (EI): calcd for [M] +. (C10H120S): 180. 07122; found 180.07148.
Eine Lösung von 2-(Isopropylthio)benzaldehyd (0.33 g, 1.83 mmol) in Dichloromethan (10 mL) wurde mit wässrige KHCO3-Lösung (1.18g in 10 mL H2O) versetzt. Unter kräftigem Rühren wurde eine Lösung von Brom (0.310g, 1.93 mmol) in Dichloromethan (1.5 mL) zugetropft. Nach 20 min Nachrühren wurde eine Spatelspitze von Na2SO3 zugegeben und die organisch Phase abgetrennt, mit gesättigter NaCl-Lösung gewaschen und über M9SO4 getrocknet, filtriert und das Lösungsmittel im Vakuum entfernt. Der Rückstand wurde durch Säulenchromatographie an Kieselgel (Hexan : Ethylacetat (3:1 bis 1:1) gereinigt. Das Produkt fiel als gelbliches Öl an. Ausbeute 0.304 g, (85%).
IR (film): ν 3447, 2963, 2917, 2864, 2738, 1702, 1628, 1607, 1482, 1439, 1380, 1264, 1227, 1183, 1158, 1121, 1105, 1037, 992, 950, 932, 852, 803, 751, 697, 635, 593, 578, 535, 506, 446, 418 cm-1; 1H NMR (200 MHz, CDCl3) δ 0.86-0.94 (d, 3H, J = 6.8 Hz), 1.50-1.56 (d, 3H, J = 7.1 Hz), 2.90-3.10 (septet, 1H, J = 6.8 Hz), 7.60-8.00 (m, 1H), 8.16-8.22 (m, 1H), 10.01-10.06 (d, 1H J = 0.63 Hz); 13C NMR (50 MHz, CDCl3) δ 21.7, 38.2, 124.4, 130.5, 133.9, 135.2, 144.9, 198.5; MS (EI) m/z (rel intensity) 180(66, [M]+.), 165(18), 138(49), 137(100), 110 (35), 109(41), 104(57), 69(11), 66(11), 65(26), 43(25), 41 (19), 39 (17) ; HRMS (EI): calcd for [M] +. (C7H1002S): 178. 08162; found 178.08148.A solution of 2- (isopropylthio) benzaldehyde (0.33 g, 1.83 mmol) in dichloromethane (10 mL) was treated with aqueous KHCO 3 solution (1.18 g in 10 mL H 2 O). With vigorous stirring, a solution of bromine (0.310 g, 1.93 mmol) in dichloromethane (1.5 mL) was added dropwise. After stirring for 20 minutes, a spatula tip of Na 2 SO 3 was added and the organic phase separated, washed with saturated NaCl solution and over Dried M 9 SO 4 , filtered and the solvent removed in vacuo. The residue was purified by column chromatography on silica gel (hexane: ethyl acetate (3: 1 to 1: 1) to give the product as a yellowish oil, yield 0.304 g, (85%).
IR (film): ν 3447, 2963, 2917, 2864, 2738, 1702, 1628, 1607, 1482, 1439, 1380, 1264, 1227, 1183, 1158, 1121, 1105, 1037, 992, 950, 932, 852, 803, 751, 697, 635, 593, 578, 535, 506, 446, 418 cm-1; 1H NMR (200 MHz, CDCl3) δ 0.86-0.94 (d, 3H, J = 6.8 Hz), 1.50-1.56 (d, 3H, J = 7.1 Hz), 2.90-3.10 (septet, 1H, J = 6.8 Hz) , 7.60-8.00 (m, 1H), 8.16-8.22 (m, 1H), 10.01-10.06 (d, 1H J = 0.63 Hz); 13C NMR (50 MHz, CDCl3) δ 21.7, 38.2, 124.4, 130.5, 133.9, 135.2, 144.9, 198.5; MS (EI) m / z (rel intensity) 180 (66, [M] +.), 165 (18), 138 (49), 137 (100), 110 (35), 109 (41), 104 (57 69 (11), 66 (11), 65 (26), 43 (25), 41 (19), 39 (17); HRMS (EI): calcd for [M] +. (C7H1002S): 178. 08162; found 178.08148.
Zu einer Suspension von Methyltriphenylphosphonium Bromid (0.690 g, 1.93 mmol, Aldrich) in 8 mL THF wurde n-BuLi (1.5 M, 1.4 mL, 2.07 mmol) bei -78 °C unter einer Argonatmosphäre zugetropft. Die gelbe Reaktionslösung wurde innerhalb von 1 h auf Raumtemperatur erwärmt. Nach erneutem Abkühlen auf -78°C wurde eine Lösung des entsprechenden Aldehyds (1.39 mmol) in THF (5 mL) gegeben, danach langsam auf Raumtemperatur erwärmt und 1 h bei dieser Temperatur nachgerührt. Nach Zugabe einer gesättigten NH4Cl-Lösung wurde die wässrige Phase mit Ethylacetat extrahiert (4 x 20 mL). Die vereinigten organischen Phasen wurden über MgSO4 getrocknet und das Lösungsmittel im Vakuum entfernt. Der Rückstand wurde durch Säulenchromatographie an Kieselgel (Cyclohexan : Ethylacetat (2:8) gereinigt.To a suspension of methyltriphenylphosphonium bromide (0.690 g, 1.93 mmol, Aldrich) in 8 mL THF was added dropwise n-BuLi (1.5 M, 1.4 mL, 2.07 mmol) at -78 ° C under an argon atmosphere. The yellow reaction solution was warmed to room temperature over 1 h. After renewed cooling to -78 ° C, a solution of the corresponding aldehyde (1.39 mmol) in THF (5 mL) was added, then slowly warmed to room temperature and stirred for 1 h at this temperature. After adding a saturated NH 4 Cl solution, the aqueous phase was extracted with ethyl acetate (4 × 20 ml). The combined organic phases were dried over MgSO 4 and the solvent removed in vacuo. The residue was purified by column chromatography on silica gel (cyclohexane: ethyl acetate (2: 8).
4a. 2-Isopropylthio-1-vinylbenzol
IR (film): ν 3084, 3058, 2962, 2924, 2865, 1828, 1625, 1588, 1463, 1365, 1243, 1197, 1155, 1049, 991, 912, 769, 746 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.26 (d, 6H, J = 6.7 Hz), 3.24-3.33 (septet, 1H, J = 6.7 Hz), 5.30 (dd, 1H, J = 1.2, 11 Hz), 5. 67 (dd, 1H, J = 1.2, 17.5 Hz), 7.18-7.27 (m, 2H), 7.35 (dd, 1H, J = 11, 17.5 Hz), 7.42-7.47 (m, 1H), 7. 52-7. 57 (m, 1H); 13C NMR (125 MHz, CD3CCD3) δ 23.1, 38.8, 115.3, 125.9, 127.5, 127.9, 133.8, 133.9, 135.3, 140.2; MS (EI) m/z (rel intensity) 178 (9, [M]+·) , 136(10), 135(100), 134(14), 91(18), 77(2), 65 (1), 43 (2) ; HRMS (EI): calcd for [M]+· (C11H14S) : 178. 08162; found 178.08148.4a. 2-isopropylthio-1-vinyl-benzene
IR (film): ν 3084, 3058, 2962, 2924, 2865, 1828, 1625, 1588, 1463, 1365, 1243, 1197, 1155, 1049, 991, 912, 769, 746 cm -1 ; 1 H NMR (500 MHz, CDCl3) δ 1.26 (d, 6H, J = 6.7 Hz), 3:24 to 3:33 (septet, 1H, J = 6.7 Hz), 5.30 (dd, 1H, J = 1.2, 11 Hz) , 5.67 (dd, 1H, J = 1.2, 17.5 Hz), 7.18-7.27 (m, 2H), 7.35 (dd, 1H, J = 11, 17.5 Hz), 7.42-7.47 (m, 1H), 7 52-7. 57 (m, 1H); 13 C NMR (125 MHz, CD 3 CCD 3 ) δ 23.1, 38.8, 115.3, 125.9, 127.5, 127.9, 133.8, 133.9, 135.3, 140.2; MS (EI) m / z (rel intensity) 178 (9, [M] + ·), 136 (10), 135 (100), 134 (14), 91 (18), 77 (2), 65 (1 ), 43 (2); HRMS (EI): calcd for [M] + (C 11 H 14 S): 178. 08162; found 178.08148.
IR (film): ν 3686, 3603, 3411, 2982, 2933, 2870, 2543, 2049, 1969, 1940, 1856, 1727, 1628, 1606, 1562, 1465, 1440, 1414, 1384, 1366, 1193, 1158, 1125, 1069, 1024, 990, 956, 926, 891, 875, 638, 582, 549, 506 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.06 (d, 3H, J = 7.0 Hz), 1.3 (d, 3H, J = 7.0 Hz), 2.83-2.93 (septet, 1H, J = 7.1 Hz), 5.41 (dd, 1H, J = 0.83, 11 Hz), 5. 77 (dd, 1H, J = 0.96, 17.3 Hz), 6.93-7.02 (q, 1H, J = 11 Hz), 7.44-7.58 (m, 2H), 7.86-7.89 (m, 1H, J = 11, 17.5 Hz); 13C NMR (100 MHz, CDCl3) δ 13.1, 17.1, 53.2, 117.8, 124.8, 125.7, 128.3, 130.7, 131.6, 135.9, 139.8; MS (EI) m/z (rel intensity) 194 (4, [M]+.), 152(13), 137(10), 136(11), 135(100), 134(11), 91(21), 77(11), 51(7), 45(8), 43(11), 41(9), 39(8); HRMS (EI): calcd for [M]+. (C11H14OS): 194. 07654; found 194.07703.
IR (film): ν 3686, 3603, 3411, 2982, 2933, 2870, 2543, 2049, 1969, 1940, 1856, 1727, 1628, 1606, 1562, 1465, 1440, 1414, 1384, 1366, 1193, 1158, 1125, 1069, 1024, 990, 956, 926, 891, 875, 638, 582, 549, 506 cm -1 ; 1 H NMR (400 MHz, CDCl 3) δ 1:06 (d, 3H, J = 7.0 Hz), 1.3 (d, 3H, J = 7.0 Hz), 2.83-2.93 (septet, 1H, J = 7.1 Hz), 5:41 (dd, 1H, J = 0.83, 11 Hz), 5.77 (dd, 1H, J = 0.96, 17.3 Hz), 6.93-7.02 (q, 1H, J = 11 Hz), 7.44-7.58 (m, 2H ), 7.86-7.89 (m, 1H, J = 11, 17.5 Hz); 13 C NMR (100 MHz, CDCl 3) δ 13.1, 17.1, 53.2, 117.8, 124.8, 125.7, 128.3, 130.7, 131.6, 135.9, 139.8; MS (EI) m / z (rel intensity) 194 (4, [M] +. ), 152 (13), 137 (10), 136 (11), 135 (100), 134 (11), 91 (21 ), 77 (11), 51 (7), 45 (8), 43 (11), 41 (9), 39 (8); HRMS (EI): calcd for [M] +. (C 11 H 14 OS): 194. 07654; found 194.07703.
Zu einer Suspension von Kupfer(I)chlorid (13 mg, 0.12 mmol) und Tricyclohexylphosphan[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydrimidazol-2-yliden][benzyliden]ruthenium(IV)dichlorid (102 mg; 0.12 mmol) in 2 mL Dichlormethan wurde eine Lösung des entsprechenden Styrolderivats (0.132 mmol) in 3 mL Dichlormethan gelöst zugegeben. Nach 20 min Rühren bei 40°C wurde die Reaktionslösung im Vakuum eingeengt. Der Rückstand wurde in 20 ml Ethylacetat aufgenommen und über eine Pasteurpipette mit Kieselgel filtriert. Das Filtrat wurde wieder im Vakuum eingeengt und der Rückstand mit sehr wenig Ethylacetat und kaltem Pentan gewaschen.To a suspension of copper (I) chloride (13 mg, 0.12 mmol) and tricyclohexylphosphine [1,3-bis (2,4,6-trimethylphenyl) -4,5-dihydrimidazol-2-ylidene] [benzylidene] ruthenium (IV ) dichloride (102 mg, 0.12 mmol) in 2 mL dichloromethane was added a solution of the corresponding styrene derivative (0.132 mmol) dissolved in 3 mL dichloromethane. After stirring for 20 minutes at 40 ° C, the reaction solution was concentrated in vacuo. The residue was taken up in 20 ml of ethyl acetate and filtered through a pasteur pipette with silica gel. The filtrate was again concentrated in vacuo and the residue washed with very little ethyl acetate and cold pentane.
Grünes mikrokristallines Feststoff, Ausbeute 86 %.
IR (film): ν 2952, 2908, 2862, 1606, 1479, 1420, 1382, 1262, 1154, 1055, 1033, 863, 843, 798, 742 cm-1; 1H NMR (500 MHz, CDCl3) δ 0. 99 (d, 6H, J = 6. Hz), 2.37 (s, 6H), 2.48 (s, 12H), 3. 18 (septet, 1H, J = 6.6 Hz), 4.14 (s, 4H), 6.73 (d, 1H, J = 7.5 Hz), 7.03 (s, 4H), 7.11-7.20 (m, 1H); 7.40-7.52 (m, 2H), 17.33 (s, 1H); 13C NMR (125 MHz, CD3COCD3) δ 19.5, 21.0, 51.7, 121.8, 123.3, 129.3, 129.4, 133.6, 136.4, 138.1, 138.6, 140.0, 140.3, 156.2, 162.0, 210.0, 285.7;
MS (EI) m/z (rel intensity) 642 (8, [M]+·) , 530 (10), 528 (18), 527(14), 526(13), 525(10), 305(40), 304(100), 303(91), 289 (19), 287(10), 166 (25), 163(12), 159(12), 158(22), 149 (19), 146(12), 145 (14), 144 (13), 135(14), 124(32), 91(45), 77(15), 71(11), 57(14), 55(12), 45(10), 44(46), 43(33), 42(12), 41 (35), 40 (31), 39 (20), 38 (15), 36 (46) ; HRMS (EI) : calcd for [M]+· (C31 H38N2 35Cl2 S 102Ru): 642. 11762; found 642.11634.
IR (film): ν 2952, 2908, 2862, 1606, 1479, 1420, 1382, 1262, 1154, 1055, 1033, 863, 843, 798, 742 cm -1 ; 1 H NMR (500 MHz, CDCl 3 ) δ 0.99 (d, 6H, J = 6 Hz), 2.37 (s, 6H), 2.48 (s, 12H), 3. 18 (septet, 1H, J = 6.6 Hz), 4.14 (s, 4H), 6.73 (d, 1H, J = 7.5 Hz), 7.03 (s, 4H), 7.11-7.20 (m, 1H); 7.40-7.52 (m, 2H), 17.33 (s, 1H); 13 C NMR (125 MHz, CD 3 COCD 3 ) δ 19.5, 21.0, 51.7, 121.8, 123.3, 129.3, 129.4, 133.6, 136.4, 138.1, 138.6, 140.0, 140.3, 156.2, 162.0, 210.0, 285.7;
MS (EI) m / z (rel intensity) 642 (8, [M] + ·), 530 (10), 528 (18), 527 (14), 526 (13), 525 (10), 305 (40 304 (100), 303 (91), 289 (19), 287 (10), 166 (25), 163 (12), 159 (12), 158 (22), 149 (19), 146 (12), 145 (14), 144 (13), 135 (14), 124 (32), 91 (45), 77 (15), 71 (11), 57 (14), 55 (12), 45 (10), 44 (46), 43 (33), 42 (12), 41 (35), 40 (31), 39 (20), 38 (15), 36 (46); HRMS (EI): calcd for [M] + · (C 31 H 38 N 2 35 Cl 2 S 102 Ru): 642. 11762; found 642.11634.
Hell-grünes mikrokristallines Feststoff, Ausbeute 72 %:
IR (film): ν 3447, 2963, 2917, 2738, 1702, 1628, 1607, 1482, 1439, 1380, 1264, 1227, 1183, 1158, 1121, 1105, 1037, 992, 950, 932, 852, 803, 751, 697, 635, 593, 578, 535, 506, 446, 418 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.05 (q, 6H, J = 6.7 Hz), 2.29 (m, 3H), 2.35-2.45 (m, 12H), 2.55 (s, 3H), 3.61 (septet, 1H, J = 6.7 Hz), 4.15 (s, 4H), 6.74 (d, 1H, J = 7.6 Hz), 6.95-7.05 (m, 4H), 7.34 (m, 1H), 7. 65 (m, 1H) 7.72-7.78 (m, 1H), 16.81 s, 1H); 13C NMR (125 MHz, CDCl3) δ 21.0, 51.7, 121.0, 127.5, 128.9, 129.4, 129.6, 129.7, 133.9, 135.4, 138.1, 138.5, 138.9, 139.0, 156.2, 207.2, 301.3; MS (ESI; m/z): 658 [M - Cl + CH3CN]+.Bright green microcrystalline solid, yield 72%:
IR (film): ν 3447, 2963, 2917, 2738, 1702, 1628, 1607, 1482, 1439, 1380, 1264, 1227, 1183, 1158, 1121, 1105, 1037, 992, 950, 932, 852, 803, 751, 697, 635, 593, 578, 535, 506, 446, 418 cm -1 ; 1 H NMR (500 MHz, CDCl 3 ) δ 1.05 (q, 6H, J = 6.7 Hz), 2.29 (m, 3H), 2.35-2.45 (m, 12H), 2.55 (s, 3H), 3.61 (sept. 1H, J = 6.7 Hz), 4.15 (s, 4H), 6.74 (d, 1H, J = 7.6 Hz), 6.95-7.05 (m, 4H), 7.34 (m, 1H), 7. 65 (m, 1H ) 7.72-7.78 (m, 1H), 16.81 s, 1H); 13 C NMR (125 MHz, CDCl 3) δ 21.0, 51.7, 121.0, 127.5, 128.9, 129.4, 129.6, 129.7, 133.9, 135.4, 138.1, 138.5, 138.9, 139.0, 156.2, 207.2, 301.3; MS (ESI, m / z): 658 [M-Cl + CH 3 CN] + .
Eine Lösung von N,N-Diallyl-p-toluolsulfonamid (0.350 mmol, 84 mg) in 17.5 ml Toluol wurde mit 5 Mol% der Katalysator (0.018 mmol) SR1 aus der Beispiel 5a unter Argon versetzt und bei 80°C gerührt. 200 µL einer Aliquote Reaktionslösung wurden zu 500 µL von 2M Ethyl-vinyl-ether Lösung in Methylenchlorid zugegen und Mittels GC analysiert. Nach 24 h wurde 51% Umsatz zum gewünschten N-p-Toluolsulfonyl-2,5-dihydropyrrol festgestellt.A solution of N, N-diallyl-p-toluenesulfonamide (0.350 mmol, 84 mg) in 17.5 ml of toluene was mixed with 5 mol% of the catalyst (0.018 mmol) SR1 from Example 5a under argon and stirred at 80 ° C. 200 μL of an aliquot of reaction solution was added to 500 μL of 2M ethyl vinyl ether solution in methylene chloride and analyzed by GC. After 24 h, 51% conversion to the desired Np-toluenesulfonyl-2,5-dihydropyrrole was found.
Eine Lösung von N,N-Diallyl-p-toluolsulfonamid (0.350 mmol, 84 mg) in 17.5 ml Dichlormethan wurde mit 5 Mol% der Katalysator (0.018 mmol) SOR1 aus der Beispiel 5b unter Argon versetzt und bei Raumtemperatur gerührt. 200 µL einer Aliquote Reaktionslösung wurden zu 500 µL von 2M Ethyl-vinyl-ether Lösung in Methylenchlorid zugegen und Mittels GC analysiert. Der Verlauf der Reaktion ist in der
Eine Lösung von Diethyl-2-allyl-2-(2-methylallyl)malonat (0.350 mmol) in 17.5 ml Toluol wurde mit 1 Mol% des Katalysator (0.0035 mmol) SOR1 aus Beispiel 5b unter Argon versetzt und bei 80°C gerührt. 200 µL der Reaktionslösung wurden zu 500 µL von 2M Ethyl-vinyl-ether Lösung in Methylenchlorid zugegen und Mittels GC analysiert. Nach 1 h wurde 99% Umsatz zum gewünschten 4,4-Bis(ethoxycarbonyl)-1-methylcyclopenten festgestellt.A solution of diethyl 2-allyl-2- (2-methylallyl) malonate (0.350 mmol) in 17.5 ml of toluene was treated with 1 mol% of the catalyst (0.0035 mmol) SOR1 from Example 5b under argon and stirred at 80 ° C. 200 μL of the reaction solution was added to 500 μL of 2M ethyl vinyl ether solution in methylene chloride and analyzed by GC. After 1 h 99% conversion to the desired 4,4-bis (ethoxycarbonyl) -1-methylcyclopentene was found.
Eine Lösung von 3-Allyloxy-3,3-diphenylpropin (0.350 mmol) in 17.5 ml Toluol wurde mit 5 Mol% der Katalysator (0.018 mmol) SR1 aus der Beispiel 5a unter Argon versetzt und bei 80°C gerührt. 200 µL der Reaktionslösung wurden zu 500 µL von 2M Ethyl-vinyl-ether Lösung in Methylenchlorid zugegeben und Mittels GC analysiert. Nach 1 h wurde 99% Umsatz zum gewünschten 3-Ethenyl-2,5-dihydro-2,2-diphenylfuran festgestellt.A solution of 3-allyloxy-3,3-diphenylpropyne (0.350 mmol) in 17.5 ml of toluene was treated with 5 mol% of the catalyst (0.018 mmol) SR1 from Example 5a under argon and stirred at 80 ° C. 200 μL of the reaction solution was added to 500 μL of 2M ethyl vinyl ether solution in methylene chloride and analyzed by GC. After 1 h, 99% conversion to the desired 3-ethenyl-2,5-dihydro-2,2-diphenylfuran was found.
Claims (7)
M für Ru, Os steht
L und L' unabhängig voneinander gleich oder verschieden für einen neutralen Elektronendonor stehen,
X1 und X2 gleich oder verschieden sind und für einen anionischen Liganden stehen,
R für Wasserstoff, einen cyclischen, geradkettigen oder verzweigtkettigen Alkyl- oder ggf. substituierten Arylrest steht,
Z für eine schwefelhaltige direkt am Metall koordinierende Einheit steht, und
A eine Brücke, welche die Einheit Z mit dem Carben-Kohlenstoff kovalent verbindet, und n die Zahl 0 oder 1, bevorzugt 0 bedeutet.Compounds of the formulas (I),
M stands for Ru, Os
L and L ', independently of one another or different, represent a neutral electron donor,
X1 and X2 are the same or different and represent an anionic ligand,
R is hydrogen, a cyclic, straight-chain or branched-chain alkyl or optionally substituted aryl radical,
Z represents a sulfur-containing unit coordinating directly with the metal, and
A is a bridge which covalently connects the unit Z to the carbene carbon, and n is the number 0 or 1, preferably 0.
dadurch gekennzeichnet, dass
L ein gesättigter oder ungesättigter NHC-Ligand ist.Compounds according to claim 1,
characterized in that
L is a saturated or unsaturated NHC ligand.
dadurch gekennzeichnet, dass
Z eine Einheit enthaltend Thiol, Thioether, Thioacetale, Disulfide, Dithiocarbonsäuren, Thioester, Thioketone, Thioaldehyde, Thiocarbamate, Thiourethane, Phosphinsulfide, Thiophosphate, Thiophosphonate, Sulfonate, Sulfone, Sulfonamide oder schwefelhaltige Heterocyclen ist, wobei gewährleistet sein muss, dass die Verbindung Z-Ru über das Schwefelatom oder ein am Schwefel befindliches Sauerstoffatom gebildet wird.Compounds according to claim 1 and / or 2,
characterized in that
Z is a moiety containing thiol, thioether, thioacetals, disulfides, dithiocarboxylic acids, thioesters, thioketones, thioaldehydes, thiocarbamates, thiourethanes, phosphine sulfides, thiophosphates, thiophosphonates, sulfonates, sulfones, sulfonamides or sulfur-containing heterocycles, it being necessary to ensure that the compound Z is Ru is formed via the sulfur atom or an oxygen atom located on the sulfur.
dadurch gekennzeichnet, dass
der Molekülteil A eine C2-Brücke bildet, in der beide C-Atome eine sp2-Hybridisierung aufweisen.Connection according to one of the preceding claims,
characterized in that
the moiety A forms a C 2 bridge in which both C atoms have an sp 2 hybridization.
dadurch gekennzeichnet, dass
es sich bei X1 und X2 um anorganische oder organische Anionen aus der Reihe Halogenide, insbesondere F-, Cl-, Br-, Pseudohalogenide, Hydroxide, Alkoxide oder Amide, Phenole, Thiole, Thiophenole, Carboxylate, Carbonate, Sulfonate, Sulfate, Phosphate und Phosphonate, Allyl-, und Cyclopentadienyl- stehen, wobei unter den Pseudohalogeniden vorzugsweise Cyanid, Rhodanid, Cyanat, Isocyanat, Thiocyanat und Isothiocyanat verstanden wird.Compound according to one or more of the preceding claims,
characterized in that
X1 and X2 are inorganic or organic anions from the series halides, in particular F - , Cl - , Br - , pseudohalides, hydroxides, alkoxides or amides, phenols, thiols, thiophenols, carboxylates, carbonates, sulfonates, sulfates, phosphates and Phosphonates, allyl - , and cyclopentadienyl - are, among the pseudohalides preferably cyanide, rhodanide, cyanate, isocyanate, thiocyanate and isothiocyanate is understood.
dadurch gekennzeichnet, dass
man die Verbindungen durch Austauschreaktion des Phosphanliganden in Verbindungen der Formel (IV) durch Liganden der Formel (V) hergestellt
characterized in that
the compounds are prepared by exchange reaction of the phosphine ligand in compounds of formula (IV) by ligands of formula (V)
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102007020694A DE102007020694A1 (en) | 2007-05-03 | 2007-05-03 | Sulfur-containing metathesis catalysts |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1990348A2 true EP1990348A2 (en) | 2008-11-12 |
| EP1990348A3 EP1990348A3 (en) | 2008-11-26 |
Family
ID=39717576
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP08103339A Withdrawn EP1990348A3 (en) | 2007-05-03 | 2008-04-02 | Metathesis catalysts containing sulphur |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8183382B2 (en) |
| EP (1) | EP1990348A3 (en) |
| JP (1) | JP2008273971A (en) |
| CN (1) | CN101298462B (en) |
| DE (1) | DE102007020694A1 (en) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8513151B2 (en) | 2007-09-20 | 2013-08-20 | Ben-Gurion University Of The Negev Research And Development Authority | Sulfur chelated ruthenium compounds useful as olefin metathesis catalysts |
| EP2260047B2 (en) | 2008-04-08 | 2016-07-20 | Evonik Degussa GmbH | Method for manufacturing ruthenium carbene complexes |
| EP2280033B1 (en) * | 2008-05-22 | 2014-03-12 | Limited Liability Company "United Research and Development Centre" | Dicyclopentadiene metathesis polymerisation catalyst |
| ES2705496T3 (en) * | 2010-12-16 | 2019-03-25 | Hoffmann La Roche | Process for the preparation of aromatic thiol derivatives by hydrogenation of disulfides |
| WO2012102247A1 (en) * | 2011-01-24 | 2012-08-02 | 国立大学法人名古屋大学 | Ruthenium complex-containing catalyst for hydrogen transfer reaction and method for producing hydrogen transfer reaction product |
| PL216649B1 (en) * | 2011-06-06 | 2014-04-30 | Univ Warszawski | New ruthenium complexes, process for the preparation thereof and the use thereof in olefin metathesis reactions |
| KR102049820B1 (en) | 2012-03-16 | 2020-01-22 | 제온 코포레이션 | Method for producing ring-opening metathesis polymer hydride, and resin composition |
| MX2018006241A (en) | 2015-11-18 | 2018-11-09 | Provivi Inc | Production of fatty olefin derivatives via olefin metathesis. |
| EP3376859B1 (en) | 2015-11-18 | 2021-01-06 | Provivi, Inc. | Microorganisms for the production of insect pheromones and related compounds |
| CN109890832B (en) | 2016-06-06 | 2022-08-12 | 普罗维维股份有限公司 | Semi-biosynthetic production of fatty alcohols and fatty aldehydes |
| WO2018191373A1 (en) * | 2017-04-12 | 2018-10-18 | Materia, Inc. | Synthesis and characterization of metathesis catalysts |
| MX2019013671A (en) | 2017-05-17 | 2020-01-15 | Provivi Inc | Microorganisms for the production of insect pheromones and related compounds. |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999000397A1 (en) | 1997-06-27 | 1999-01-07 | Ciba Specialty Chemicals Holding Inc. | Ruthenium and osmium catalysts |
| DE19815275A1 (en) | 1998-04-06 | 1999-10-07 | Aventis Res & Tech Gmbh & Co | Alkylidene complexes of ruthenium with N-heterocyclic carbene ligands and their use as highly active, selective catalysts for olefin metathesis |
| DE19902439A1 (en) | 1999-01-22 | 2000-08-03 | Aventis Res & Tech Gmbh & Co | Homo- and heterobimetallic alkylidene complexes of ruthenium with N-heterocyclic carbene ligands and their use as highly active, selective catalysts for olefin metathesis |
| EP1180108A2 (en) | 1999-05-24 | 2002-02-20 | California Institute Of Technology | Imidazolidine-based metal carbene metathesis catalysts |
| WO2005094345A2 (en) | 2004-03-29 | 2005-10-13 | California Institute Of Technology | Latent, high-activity olefin metathesis catalysts containing an n-heterocyclic carbene ligand |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE60140455D1 (en) * | 2000-08-10 | 2009-12-24 | Trustees Boston College | REUSABLE METHATHESIS CATALYSTS |
| WO2005003843A1 (en) | 2003-07-08 | 2005-01-13 | Koninklijke Philips Electronics N.V. | Sunglasses with adaptable transmissivity |
| EP1543875A1 (en) * | 2003-12-04 | 2005-06-22 | Boehringer Ingelheim Pharma GmbH & Co. KG | Novel metathesis ruthenium catalyst |
| CN1907992B (en) | 2005-07-04 | 2012-03-28 | 赞南科技(上海)有限公司 | Ruthenium complex compound ligand, ruthenium complex compound, solid carrying ruthenium complex catalyst and preparation method and use thereof |
-
2007
- 2007-05-03 DE DE102007020694A patent/DE102007020694A1/en not_active Withdrawn
-
2008
- 2008-04-02 EP EP08103339A patent/EP1990348A3/en not_active Withdrawn
- 2008-04-24 US US12/109,092 patent/US8183382B2/en not_active Expired - Fee Related
- 2008-04-29 CN CN2008100956077A patent/CN101298462B/en not_active Expired - Fee Related
- 2008-04-30 JP JP2008118437A patent/JP2008273971A/en active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999000397A1 (en) | 1997-06-27 | 1999-01-07 | Ciba Specialty Chemicals Holding Inc. | Ruthenium and osmium catalysts |
| DE19815275A1 (en) | 1998-04-06 | 1999-10-07 | Aventis Res & Tech Gmbh & Co | Alkylidene complexes of ruthenium with N-heterocyclic carbene ligands and their use as highly active, selective catalysts for olefin metathesis |
| WO1999051344A1 (en) | 1998-04-06 | 1999-10-14 | Aventis Research & Technologies Gmbh & Co. Kg | Alkylidene complexes of ruthenium with n-heterocyclic carbene ligands and their use as highly active, selective catalysts for olefin metathesis |
| DE19902439A1 (en) | 1999-01-22 | 2000-08-03 | Aventis Res & Tech Gmbh & Co | Homo- and heterobimetallic alkylidene complexes of ruthenium with N-heterocyclic carbene ligands and their use as highly active, selective catalysts for olefin metathesis |
| EP1180108A2 (en) | 1999-05-24 | 2002-02-20 | California Institute Of Technology | Imidazolidine-based metal carbene metathesis catalysts |
| WO2005094345A2 (en) | 2004-03-29 | 2005-10-13 | California Institute Of Technology | Latent, high-activity olefin metathesis catalysts containing an n-heterocyclic carbene ligand |
Non-Patent Citations (9)
| Title |
|---|
| "Handbook of Metathesis", 2003, WILEY-VCH |
| GARBER ET AL., J. AM. CHEM. SOC., vol. 122, 2000, pages 8168 - 8179 |
| GESSLER ET AL., TETRAHEDRON LETT., vol. 41, 2000, pages 9973 - 9976 |
| J.S. MURDZEK; R.R. SCHROCK, ORGANOMETALLICS, vol. 6, 1987, pages 1373 - 1374 |
| M. BIENIEK ET AL., J. ORGANOMET. CHEM., vol. 691, 2006, pages 5289 |
| P. A. VAN DER SCHAAF ET AL., J. ORGANOMETALLIC CHEM., vol. 606, 2000, pages 65 - 74 |
| P. SCHWAB ET AL., ANGEW. CHEM. INT. ED. ENGL., vol. 34, 1995, pages 2039 - 2041 |
| T. WESKAMP ET AL., ANGEW. CHEM., vol. 110, 1998, pages 263 - 2633 |
| T. WESKAMP ET AL., ANGEW. CHEM., vol. 111, 1999, pages 2573 - 2576 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2008273971A (en) | 2008-11-13 |
| US8183382B2 (en) | 2012-05-22 |
| US20080275247A1 (en) | 2008-11-06 |
| CN101298462B (en) | 2012-10-31 |
| CN101298462A (en) | 2008-11-05 |
| EP1990348A3 (en) | 2008-11-26 |
| DE102007020694A1 (en) | 2008-11-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1990348A2 (en) | Metathesis catalysts containing sulphur | |
| EP2260047B2 (en) | Method for manufacturing ruthenium carbene complexes | |
| DE60306795T2 (en) | RUTHENIUM COMPLEXES AS (PRE) CATALYSTS FOR METATHERE REACTIONS | |
| EP1037897B1 (en) | Highly active cationic ruthenium and osmium complexes for olefin metathesis reactions | |
| EP1075482B1 (en) | Cationic ruthenium complexes, their production and their use | |
| EP2550284A1 (en) | Ruthenium complexes for use in olefin metathesis | |
| CN102834175A (en) | Process for preparation of ruthenium-based carbene catalysts with chelating alkylidene ligands | |
| WO1993015089A1 (en) | Diphosphine ligands | |
| CN101479283A (en) | New ruthenium complexes as catalysts for metahesis reactions | |
| EP1414833B1 (en) | Novel transition-metal complexes and use thereof in transition-metal catalysed reactions | |
| CN104689849B (en) | One class phosphamide (primary) secondary amine bifunctional catalyst and its synthetic method | |
| CA2478482A1 (en) | Metalorganic catalysts for chemo-, regio- and stereoselective reactions, and corresponding precursors | |
| EP0345653B1 (en) | Transition metal complex | |
| Nejman et al. | The preparation and characterisation of rhodium (III) and Iridium (III) half sandwich complexes with napthalene-1, 8-dithiolate, acenaphthene-5, 6-dithiolate and biphenyl-2, 2′-dithiolate | |
| Durap et al. | A new efficient bis (phosphinite)-ruthenium (II) catalyst system for the asymmetric transfer hydrogenation of aromatic ketones | |
| EP1035093B1 (en) | Metathesis in the presence of ionic liquids | |
| EP1371657A1 (en) | Transition-metal complexes and use thereof in transition-metal catalysed reactions.. | |
| DE60206262T2 (en) | NEW METHOD FOR THE PRODUCTION OF METAL CARBIDE COMPLEXES | |
| EP1394190B1 (en) | Metathesis catalysts | |
| EP0915076B1 (en) | Process for the preparation of trans-(R,R)-actinol | |
| DE102004039277A1 (en) | At the central atom substituted immoblisierbaren ruthenium (II) catalysts, processes for their preparation and use | |
| DE60214689T2 (en) | OPTICALLY ACTIVE ALKENYLPHOSPHINIC ACID ESTERS AND PROCESS FOR THEIR PREPARATION | |
| EP2403842A1 (en) | Chiral disulfonimides | |
| DE102007018148A1 (en) | Preparation of alkene, which is a polymer compound, preferably homopolymer, copolymer or block-copolymer, comprises metathesis reaction of unsaturated compound containing an alkene or alkyne unit in the presence of an active catalyst | |
| WO2011042675A1 (en) | Novel configurationally stable, bicyclic p-chiral phosphines, preparation method thereof and use of same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| 17P | Request for examination filed |
Effective date: 20080402 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA MK RS |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA MK RS |
|
| 17Q | First examination report despatched |
Effective date: 20090415 |
|
| AKX | Designation fees paid |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 20140207 |