EP1571237A1 - Treating fluid for surface treatment of metal and method for surface treatment - Google Patents
Treating fluid for surface treatment of metal and method for surface treatment Download PDFInfo
- Publication number
- EP1571237A1 EP1571237A1 EP03780727A EP03780727A EP1571237A1 EP 1571237 A1 EP1571237 A1 EP 1571237A1 EP 03780727 A EP03780727 A EP 03780727A EP 03780727 A EP03780727 A EP 03780727A EP 1571237 A1 EP1571237 A1 EP 1571237A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- metal
- surface treatment
- treating solution
- compound
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 229910052751 metal Inorganic materials 0.000 title claims abstract description 155
- 238000004381 surface treatment Methods 0.000 title claims abstract description 154
- 239000002184 metal Substances 0.000 title claims abstract description 149
- 238000000034 method Methods 0.000 title claims description 56
- 239000012530 fluid Substances 0.000 title 1
- 239000000463 material Substances 0.000 claims abstract description 106
- 239000007769 metal material Substances 0.000 claims abstract description 106
- 150000001875 compounds Chemical class 0.000 claims abstract description 61
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 claims abstract description 52
- 229920000642 polymer Polymers 0.000 claims abstract description 24
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical group O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 claims abstract description 21
- 239000002253 acid Substances 0.000 claims abstract description 20
- 150000003839 salts Chemical class 0.000 claims abstract description 20
- 239000004094 surface-active agent Substances 0.000 claims abstract description 18
- 150000003755 zirconium compounds Chemical class 0.000 claims abstract description 18
- 150000003609 titanium compounds Chemical class 0.000 claims abstract description 16
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims abstract description 12
- 229940043430 calcium compound Drugs 0.000 claims abstract description 12
- 150000001674 calcium compounds Chemical class 0.000 claims abstract description 12
- 239000001301 oxygen Substances 0.000 claims abstract description 12
- 229910052760 oxygen Inorganic materials 0.000 claims abstract description 12
- 150000003438 strontium compounds Chemical class 0.000 claims abstract description 12
- 150000002681 magnesium compounds Chemical class 0.000 claims abstract description 10
- 239000000243 solution Substances 0.000 claims description 126
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 72
- 239000007864 aqueous solution Substances 0.000 claims description 40
- 229910052726 zirconium Inorganic materials 0.000 claims description 28
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 claims description 26
- 229910052719 titanium Inorganic materials 0.000 claims description 26
- 239000010936 titanium Substances 0.000 claims description 26
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 24
- 229910052725 zinc Inorganic materials 0.000 claims description 21
- 239000011701 zinc Substances 0.000 claims description 21
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 claims description 20
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 16
- 239000011777 magnesium Substances 0.000 claims description 14
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 claims description 13
- 229910052749 magnesium Inorganic materials 0.000 claims description 12
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 11
- 229920003169 water-soluble polymer Polymers 0.000 claims description 11
- 229910017052 cobalt Inorganic materials 0.000 claims description 9
- 239000010941 cobalt Substances 0.000 claims description 9
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims description 9
- 125000002091 cationic group Chemical group 0.000 claims description 8
- 229910052759 nickel Inorganic materials 0.000 claims description 8
- 229910017604 nitric acid Inorganic materials 0.000 claims description 8
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 7
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 claims description 7
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 7
- 229910052802 copper Inorganic materials 0.000 claims description 7
- 239000010949 copper Substances 0.000 claims description 7
- 229910052718 tin Inorganic materials 0.000 claims description 7
- 239000011135 tin Substances 0.000 claims description 7
- 238000005406 washing Methods 0.000 claims description 7
- NHNBFGGVMKEFGY-UHFFFAOYSA-N nitrate group Chemical group [N+](=O)([O-])[O-] NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 claims description 5
- IOVCWXUNBOPUCH-UHFFFAOYSA-N Nitrous acid Chemical compound ON=O IOVCWXUNBOPUCH-UHFFFAOYSA-N 0.000 claims description 4
- 230000002378 acidificating effect Effects 0.000 claims description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 4
- 125000000129 anionic group Chemical group 0.000 claims description 4
- XTEGARKTQYYJKE-UHFFFAOYSA-N chloric acid Chemical compound OCl(=O)=O XTEGARKTQYYJKE-UHFFFAOYSA-N 0.000 claims description 4
- 229910003562 H2MoO4 Inorganic materials 0.000 claims description 3
- 229910003893 H2WO4 Inorganic materials 0.000 claims description 3
- SXDBWCPKPHAZSM-UHFFFAOYSA-N bromic acid Chemical compound OBr(=O)=O SXDBWCPKPHAZSM-UHFFFAOYSA-N 0.000 claims description 3
- VLAPMBHFAWRUQP-UHFFFAOYSA-L molybdic acid Chemical compound O[Mo](O)(=O)=O VLAPMBHFAWRUQP-UHFFFAOYSA-L 0.000 claims description 3
- CMPGARWFYBADJI-UHFFFAOYSA-L tungstic acid Chemical compound O[W](O)(=O)=O CMPGARWFYBADJI-UHFFFAOYSA-L 0.000 claims description 3
- 239000010408 film Substances 0.000 description 104
- 230000000052 comparative effect Effects 0.000 description 57
- 238000011282 treatment Methods 0.000 description 37
- 230000007797 corrosion Effects 0.000 description 36
- 238000005260 corrosion Methods 0.000 description 36
- 239000011248 coating agent Substances 0.000 description 35
- 238000000576 coating method Methods 0.000 description 34
- 239000003153 chemical reaction reagent Substances 0.000 description 29
- 238000005238 degreasing Methods 0.000 description 21
- 239000000047 product Substances 0.000 description 21
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 20
- 229910000838 Al alloy Inorganic materials 0.000 description 18
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 15
- 238000004070 electrodeposition Methods 0.000 description 14
- 229910000831 Steel Inorganic materials 0.000 description 13
- 229910052731 fluorine Inorganic materials 0.000 description 13
- 239000010959 steel Substances 0.000 description 13
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- 238000011156 evaluation Methods 0.000 description 12
- 239000011737 fluorine Substances 0.000 description 12
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 11
- LRXTYHSAJDENHV-UHFFFAOYSA-H zinc phosphate Chemical compound [Zn+2].[Zn+2].[Zn+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O LRXTYHSAJDENHV-UHFFFAOYSA-H 0.000 description 11
- 229910000165 zinc phosphate Inorganic materials 0.000 description 11
- 239000010960 cold rolled steel Substances 0.000 description 10
- 230000000694 effects Effects 0.000 description 10
- 238000010438 heat treatment Methods 0.000 description 10
- 238000005304 joining Methods 0.000 description 10
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 9
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 9
- 239000003513 alkali Substances 0.000 description 9
- 238000001035 drying Methods 0.000 description 9
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 8
- 229910052782 aluminium Inorganic materials 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 8
- YIXJRHPUWRPCBB-UHFFFAOYSA-N magnesium nitrate Chemical compound [Mg+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O YIXJRHPUWRPCBB-UHFFFAOYSA-N 0.000 description 8
- 239000010802 sludge Substances 0.000 description 8
- 238000003466 welding Methods 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 7
- 238000010276 construction Methods 0.000 description 7
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- -1 ZrOSO4 Chemical compound 0.000 description 6
- ZCDOYSPFYFSLEW-UHFFFAOYSA-N chromate(2-) Chemical compound [O-][Cr]([O-])(=O)=O ZCDOYSPFYFSLEW-UHFFFAOYSA-N 0.000 description 6
- 239000011651 chromium Substances 0.000 description 6
- 229920001577 copolymer Polymers 0.000 description 6
- 238000007747 plating Methods 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000009257 reactivity Effects 0.000 description 6
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 5
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 5
- 229910000861 Mg alloy Inorganic materials 0.000 description 5
- 235000011114 ammonium hydroxide Nutrition 0.000 description 5
- 229910052791 calcium Inorganic materials 0.000 description 5
- 239000011575 calcium Substances 0.000 description 5
- 238000000151 deposition Methods 0.000 description 5
- UJVRJBAUJYZFIX-UHFFFAOYSA-N nitric acid;oxozirconium Chemical compound [Zr]=O.O[N+]([O-])=O.O[N+]([O-])=O UJVRJBAUJYZFIX-UHFFFAOYSA-N 0.000 description 5
- 238000005507 spraying Methods 0.000 description 5
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 4
- 239000005977 Ethylene Substances 0.000 description 4
- 229910003708 H2TiF6 Inorganic materials 0.000 description 4
- 229910003899 H2ZrF6 Inorganic materials 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 239000003929 acidic solution Substances 0.000 description 4
- 230000008021 deposition Effects 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- DHEQXMRUPNDRPG-UHFFFAOYSA-N strontium nitrate Chemical compound [Sr+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O DHEQXMRUPNDRPG-UHFFFAOYSA-N 0.000 description 4
- DXIGZHYPWYIZLM-UHFFFAOYSA-J tetrafluorozirconium;dihydrofluoride Chemical compound F.F.F[Zr](F)(F)F DXIGZHYPWYIZLM-UHFFFAOYSA-J 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 3
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 3
- 229910002651 NO3 Inorganic materials 0.000 description 3
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 230000003750 conditioning effect Effects 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 238000005868 electrolysis reaction Methods 0.000 description 3
- 229910052742 iron Inorganic materials 0.000 description 3
- 239000007800 oxidant agent Substances 0.000 description 3
- DCKVFVYPWDKYDN-UHFFFAOYSA-L oxygen(2-);titanium(4+);sulfate Chemical compound [O-2].[Ti+4].[O-]S([O-])(=O)=O DCKVFVYPWDKYDN-UHFFFAOYSA-L 0.000 description 3
- 229910052712 strontium Inorganic materials 0.000 description 3
- CIOAGBVUUVVLOB-UHFFFAOYSA-N strontium atom Chemical compound [Sr] CIOAGBVUUVVLOB-UHFFFAOYSA-N 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N titanium dioxide Inorganic materials O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 3
- 229910000348 titanium sulfate Inorganic materials 0.000 description 3
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 2
- 229910001018 Cast iron Inorganic materials 0.000 description 2
- 229920000298 Cellophane Polymers 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- 229910003638 H2SiF6 Inorganic materials 0.000 description 2
- 229910004039 HBF4 Inorganic materials 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 2
- 239000004372 Polyvinyl alcohol Substances 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- 239000006227 byproduct Substances 0.000 description 2
- ZCCIPPOKBCJFDN-UHFFFAOYSA-N calcium nitrate Chemical compound [Ca+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O ZCCIPPOKBCJFDN-UHFFFAOYSA-N 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 239000013527 degreasing agent Substances 0.000 description 2
- 238000005237 degreasing agent Methods 0.000 description 2
- 239000003822 epoxy resin Substances 0.000 description 2
- 150000002222 fluorine compounds Chemical class 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 239000005011 phenolic resin Substances 0.000 description 2
- 150000002989 phenols Chemical class 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 2
- 230000000704 physical effect Effects 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 229920000647 polyepoxide Polymers 0.000 description 2
- 239000004645 polyester resin Substances 0.000 description 2
- 229920001225 polyester resin Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 2
- BFXAWOHHDUIALU-UHFFFAOYSA-M sodium;hydron;difluoride Chemical compound F.[F-].[Na+] BFXAWOHHDUIALU-UHFFFAOYSA-M 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- ZEFWRWWINDLIIV-UHFFFAOYSA-N tetrafluorosilane;dihydrofluoride Chemical compound F.F.F[Si](F)(F)F ZEFWRWWINDLIIV-UHFFFAOYSA-N 0.000 description 2
- POILWHVDKZOXJZ-ARJAWSKDSA-M (z)-4-oxopent-2-en-2-olate Chemical compound C\C([O-])=C\C(C)=O POILWHVDKZOXJZ-ARJAWSKDSA-M 0.000 description 1
- TUSDEZXZIZRFGC-UHFFFAOYSA-N 1-O-galloyl-3,6-(R)-HHDP-beta-D-glucose Natural products OC1C(O2)COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC1C(O)C2OC(=O)C1=CC(O)=C(O)C(O)=C1 TUSDEZXZIZRFGC-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 239000001263 FEMA 3042 Substances 0.000 description 1
- MGKOSOIDPUKBRW-UHFFFAOYSA-H F[Ti](F)(F)(F)(F)F Chemical compound F[Ti](F)(F)(F)(F)F MGKOSOIDPUKBRW-UHFFFAOYSA-H 0.000 description 1
- IMQLKJBTEOYOSI-GPIVLXJGSA-N Inositol-hexakisphosphate Chemical compound OP(O)(=O)O[C@H]1[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@@H]1OP(O)(O)=O IMQLKJBTEOYOSI-GPIVLXJGSA-N 0.000 description 1
- 229910017665 NH4HF2 Inorganic materials 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- LRBQNJMCXXYXIU-PPKXGCFTSA-N Penta-digallate-beta-D-glucose Natural products OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-PPKXGCFTSA-N 0.000 description 1
- IMQLKJBTEOYOSI-UHFFFAOYSA-N Phytic acid Natural products OP(O)(=O)OC1C(OP(O)(O)=O)C(OP(O)(O)=O)C(OP(O)(O)=O)C(OP(O)(O)=O)C1OP(O)(O)=O IMQLKJBTEOYOSI-UHFFFAOYSA-N 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229910011006 Ti(SO4)2 Inorganic materials 0.000 description 1
- 229910003074 TiCl4 Inorganic materials 0.000 description 1
- 229910010342 TiF4 Inorganic materials 0.000 description 1
- 229910010298 TiOSO4 Inorganic materials 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 229910001297 Zn alloy Inorganic materials 0.000 description 1
- 229910008159 Zr(SO4)2 Inorganic materials 0.000 description 1
- 229910007932 ZrCl4 Inorganic materials 0.000 description 1
- 229910007998 ZrF4 Inorganic materials 0.000 description 1
- 229910008334 ZrO(NO3)2 Inorganic materials 0.000 description 1
- 229910006213 ZrOCl2 Inorganic materials 0.000 description 1
- ANFIEGWCRSNVFS-UHFFFAOYSA-N [Na].OCl(=O)=O Chemical compound [Na].OCl(=O)=O ANFIEGWCRSNVFS-UHFFFAOYSA-N 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- 239000012445 acidic reagent Substances 0.000 description 1
- 239000002390 adhesive tape Substances 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 239000012670 alkaline solution Substances 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- LDDQLRUQCUTJBB-UHFFFAOYSA-N ammonium fluoride Chemical compound [NH4+].[F-] LDDQLRUQCUTJBB-UHFFFAOYSA-N 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 229910052787 antimony Inorganic materials 0.000 description 1
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 239000012267 brine Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- ABXXWVKOBZHNNF-UHFFFAOYSA-N chromium(3+);dioxido(dioxo)chromium Chemical compound [Cr+3].[Cr+3].[O-][Cr]([O-])(=O)=O.[O-][Cr]([O-])(=O)=O.[O-][Cr]([O-])(=O)=O ABXXWVKOBZHNNF-UHFFFAOYSA-N 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- UFMZWBIQTDUYBN-UHFFFAOYSA-N cobalt dinitrate Chemical compound [Co+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O UFMZWBIQTDUYBN-UHFFFAOYSA-N 0.000 description 1
- 229910001981 cobalt nitrate Inorganic materials 0.000 description 1
- 238000004512 die casting Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 238000005246 galvanizing Methods 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910000398 iron phosphate Inorganic materials 0.000 description 1
- WBJZTOZJJYAKHQ-UHFFFAOYSA-K iron(3+) phosphate Chemical compound [Fe+3].[O-]P([O-])([O-])=O WBJZTOZJJYAKHQ-UHFFFAOYSA-K 0.000 description 1
- 239000011133 lead Substances 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 150000002736 metal compounds Chemical class 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- YOYLLRBMGQRFTN-SMCOLXIQSA-N norbuprenorphine Chemical compound C([C@@H](NCC1)[C@]23CC[C@]4([C@H](C3)C(C)(O)C(C)(C)C)OC)C3=CC=C(O)C5=C3[C@@]21[C@H]4O5 YOYLLRBMGQRFTN-SMCOLXIQSA-N 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- KADRTWZQWGIUGO-UHFFFAOYSA-L oxotitanium(2+);sulfate Chemical compound [Ti+2]=O.[O-]S([O-])(=O)=O KADRTWZQWGIUGO-UHFFFAOYSA-L 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000000467 phytic acid Substances 0.000 description 1
- 235000002949 phytic acid Nutrition 0.000 description 1
- 229940068041 phytic acid Drugs 0.000 description 1
- 239000012286 potassium permanganate Substances 0.000 description 1
- VBKNTGMWIPUCRF-UHFFFAOYSA-M potassium;fluoride;hydrofluoride Chemical compound F.[F-].[K+] VBKNTGMWIPUCRF-UHFFFAOYSA-M 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000007420 reactivation Effects 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 235000002639 sodium chloride Nutrition 0.000 description 1
- 235000010288 sodium nitrite Nutrition 0.000 description 1
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 1
- 229920002258 tannic acid Polymers 0.000 description 1
- LRBQNJMCXXYXIU-NRMVVENXSA-N tannic acid Chemical compound OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-NRMVVENXSA-N 0.000 description 1
- 229940033123 tannic acid Drugs 0.000 description 1
- 235000015523 tannic acid Nutrition 0.000 description 1
- 229920001864 tannin Polymers 0.000 description 1
- 239000001648 tannin Substances 0.000 description 1
- 235000018553 tannin Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- QDZRBIRIPNZRSG-UHFFFAOYSA-N titanium nitrate Inorganic materials [O-][N+](=O)O[Ti](O[N+]([O-])=O)(O[N+]([O-])=O)O[N+]([O-])=O QDZRBIRIPNZRSG-UHFFFAOYSA-N 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- XJDNKRIXUMDJCW-UHFFFAOYSA-J titanium tetrachloride Chemical compound Cl[Ti](Cl)(Cl)Cl XJDNKRIXUMDJCW-UHFFFAOYSA-J 0.000 description 1
- XROWMBWRMNHXMF-UHFFFAOYSA-J titanium tetrafluoride Chemical compound [F-].[F-].[F-].[F-].[Ti+4] XROWMBWRMNHXMF-UHFFFAOYSA-J 0.000 description 1
- HDUMBHAAKGUHAR-UHFFFAOYSA-J titanium(4+);disulfate Chemical compound [Ti+4].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O HDUMBHAAKGUHAR-UHFFFAOYSA-J 0.000 description 1
- CENHPXAQKISCGD-UHFFFAOYSA-N trioxathietane 4,4-dioxide Chemical compound O=S1(=O)OOO1 CENHPXAQKISCGD-UHFFFAOYSA-N 0.000 description 1
- 229960004418 trolamine Drugs 0.000 description 1
- 238000007740 vapor deposition Methods 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 239000010930 yellow gold Substances 0.000 description 1
- 229910001097 yellow gold Inorganic materials 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
- OERNJTNJEZOPIA-UHFFFAOYSA-N zirconium nitrate Inorganic materials [Zr+4].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O OERNJTNJEZOPIA-UHFFFAOYSA-N 0.000 description 1
- DUNKXUFBGCUVQW-UHFFFAOYSA-J zirconium tetrachloride Chemical compound Cl[Zr](Cl)(Cl)Cl DUNKXUFBGCUVQW-UHFFFAOYSA-J 0.000 description 1
- OMQSJNWFFJOIMO-UHFFFAOYSA-J zirconium tetrafluoride Chemical compound F[Zr](F)(F)F OMQSJNWFFJOIMO-UHFFFAOYSA-J 0.000 description 1
- LBVWQMVSUSYKGQ-UHFFFAOYSA-J zirconium(4+) tetranitrite Chemical compound [Zr+4].[O-]N=O.[O-]N=O.[O-]N=O.[O-]N=O LBVWQMVSUSYKGQ-UHFFFAOYSA-J 0.000 description 1
- ZXAUZSQITFJWPS-UHFFFAOYSA-J zirconium(4+);disulfate Chemical compound [Zr+4].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O ZXAUZSQITFJWPS-UHFFFAOYSA-J 0.000 description 1
- IPCAPQRVQMIMAN-UHFFFAOYSA-L zirconyl chloride Chemical compound Cl[Zr](Cl)=O IPCAPQRVQMIMAN-UHFFFAOYSA-L 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/05—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions
- C23C22/06—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6
- C23C22/34—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing fluorides or complex fluorides
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/05—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions
- C23C22/06—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6
- C23C22/40—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing molybdates, tungstates or vanadates
- C23C22/44—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing molybdates, tungstates or vanadates containing also fluorides or complex fluorides
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/73—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals characterised by the process
- C23C22/76—Applying the liquid by spraying
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/82—After-treatment
- C23C22/83—Chemical after-treatment
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D9/00—Electrolytic coating other than with metals
- C25D9/04—Electrolytic coating other than with metals with inorganic materials
- C25D9/08—Electrolytic coating other than with metals with inorganic materials by cathodic processes
Definitions
- the present invention relates to the treating solution for surface treatment of metal which may deposit a surface treated film having excellent corrosion resistance after being coated on the surface of a metal material of a structural construction such as car body consisting of a single material or two to four materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material independently or simultaneously.
- a zinc phosphate treatment and a chromate treatment are currently used as ordinary methods.
- the zinc phosphate treatment it is possible to deposit a film having excellent corrosion resistance on the surface of steel such as cold rolled steel plate, zinc plated steel plate and some of aluminum alloys.
- the generation of sludge, which is the byproduct of the reaction can not be avoided, and depending on the kind of aluminum alloy, the sufficient corrosion resistance after coated can not be obtained.
- the chromate treatment which contains harmful hexavalent chrome in the treating solution is more likely to be avoided.
- various methods have been proposed as described below.
- JP 2000-204485 A a compound containing nitrogen atom having a lone electron-pair and a non-chrome coating agent for metal surface treatment containing said compound and zirconium compound are suggested.
- This method may obtain a surface treated film which is excellent in corrosion resistance and adhesiveness after being coated, and yet does not contain harmful hexavalent chrome by coating above mentioned coating agent.
- the metal material which can be treated is limited to aluminum alloys only, and, it is difficult to be applied to a structural construction having complex structure such as car body, because the surface treated film is formed by coating and drying.
- metal materials to be treated may include magnesium, magnesium alloy, zinc and zinc plated alloy other than aluminum alloy.
- a method for metal surface treatment by chrome free coating acid composition by coating aqueous solution containing component which can be a film having excellent corrosion resistance over the surface of metal, then baking and drying without rinsing with water so as the film to be fixed (see JP 5-195244 A).
- This method does not involve any chemical reaction to form a film, so this method may form a film on the surface of metal such as zinc plated steel plate, cold rolled steel plate or aluminum alloy.
- JP 2000-204485 A since the film is formed by coating and drying, it is difficult to form a uniform film on the surface of a structural construction having complex structure such as car body.
- the object of the present invention is to provide a treating solution for surface treatment of metal to form a surface treated film having excellent corrosion resistance after coated on the surface of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material, which does not contain harmful component to the environment and does not generate sludge to be wasted, which was not accomplished by the prior arts.
- the object of the present invention is to provide a treating solution for metal surface treatment to form a surface treated film of a uniform component having excellent corrosion resistance after coated on the surface of a metal material composing a structural construction such as car body consisting of two to four materials selecting from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material by same component simultaneously under a uniform condition.
- a metal material composing a structural construction such as car body consisting of two to four materials selecting from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material by same component simultaneously under a uniform condition.
- another object of the present invention is to provide a method for treatment using the treating solution.
- the inventors of the present invention have conducted intensive study to dissolve the above mentioned problem and have accomplished a treating solution for surface treatment of metal and a method for surface treatment which were not provided by the prior art.
- the present invention is the treating solution for surface treatment of metal, which is aqueous surface treating solution to treat independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material, the treating solution containing 5 to 5000 ppm of at least one compound selected from the group consisting of zirconium compound and titanium compound calculated as metal element, and 0.1 to 100 ppm of free fluorine ion, and having pH 2 to 6.
- the treating solution for surface treatment of metal may further contain at least one compound selected from the group consisting of calcium compound, magnesium compound and strontium compound, wherein concentration of the compound calculated as metal element is 5 to 100 ppm in the case of the calcium compound, 10 to 5000 ppm in the case of the magnesium and 10 to 5000 ppm in the case of the strontium compound. It is desirable that the treating solution for surface treatment of metal further contains 1000 to 50000 ppm of nitrate group.
- the treating solution for surface treatment of metal further contains at least one oxygen acid and/or salt of oxygen acid selected from the group consisting of HClO 3 , HBrO 3 , HNO 2 , HNO 3 , HMnO 4 , HVO 3 , H 2 O 2 , H 2 WO 4 , H 2 MoO 4 and salts thereof.
- the treating solution for surface treatment of metal may further contain at least one polymer compound selected from the group consisting of water soluble polymer compounds and water dispersible polymer compounds, and may further contain at least one surface active agent selected from the group consisting of nonionic surface active agents, anionic surface active agents and cationic surface active agents.
- the present invention is the method for surface treatment of metal comprising, contacting independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material with the treating solution for surface treatment.
- the metal material or the two or more metal materials after contacting with the treating solution for surface treatment, it is possible to further contact the metal material or the two or more metal materials with acidic aqueous solution of compound containing at least one element selected from the group consisting of cobalt, nickel, tin, copper, titanium and zirconium, with or without washing by water, or it is possible to further contact the metal material or the two or more metal materials with treating solution containing at least one polymer compound selected from water soluble polymer compounds and water dispersible polymer compounds.
- the present invention is the method for surface treatment of metal comprising, electrolytic treating in the treating solution for surface treatment, wherein independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material are a cathode.
- the metal material or the two or more metal materials after electrolytic treating in the treating solution for surface treatment, it is possible to further contact the metal material or the two or more metal materials with acidic aqueous solution of compound containing at least one element selected from the group consisting of cobalt, nickel, tin, copper, titanium and zirconium, with or without washing by water, or it is possible to further contact the metal material or the two or more metal materials with treating solution containing at least one polymer compound selected from water soluble polymer compounds and water dispersible polymer compounds, with or without washing by water.
- the present invention is the method for surface treatment of metal comprising, contacting independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material whose surfaces are not degreased and cleaned, with the treating solution for surface treatment containing at least one surface active agent selected from the group consisting of the above described nonionic surface active agents, anionic surface active agents and cationic surface active agents.
- the present invention is the metal material having a surface treated film containing at least one metal element selected from the group consisting of titanium and zirconium formed on a surface of iron metal material by the method for surface treatment, wherein an adhesion amount of the surface treated film calculated as the metal element is 30 mg/m 2 or more; in the case where formed on a surface of zinc metal material, an adhesion amount of the surface treated film calculated as the metal element is 20mg/m 2 or more; in the case where formed on a surface of aluminum metal material, an adhesion amount of the surface treated film calculated as the metal element is 10mg/m 2 or more; and in the case where formed on a surface of magnesium metal material, an adhesion amount of the surface treated film calculated as the metal element is 10mg/m 2 or more.
- the present invention relates to the art characterizing to deposit a surface treated film having excellent corrosion resistance after coated, by surface treatment on independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material.
- ferriferous material is an iron metal such as cold rolled steel plate, hot rolled steel plate, cast iron or sintered steel.
- Zinciferous material is a die casting zinc or a zinc contaiing plating.
- This zinc containing plating means a metal plating with zinc or zinc alloy composed of zinc and other metals (for example, at least one metal selected from the group consisting of nickel, iron, aluminum, manganese, chromium, magnesium, cobalt, lead or antimony) and inevitable impurities, and the methods for such plating includes hot galvanizing, electric plating and vapor deposition plating, and are not limited to these methods.
- the aluminiferous material is an aluminum alloy board such as JIS 5000 series aluminum alloy or JIS 6000 series aluminum alloy, or an aluminum alloy die cast represented by ADC-12. Still more, the magnesiferous material is a metal board or a die cast made of magnesium alloy.
- the present invention can be applied to the structural construction which contains one metal material mentioned above alone in the composing parts or to the structural construction which contains two to four metal materials mentioned above in the composing parts. And, in the case to apply the present invention to the structural construction which contains two to four metal materials mentioned above, it is possible to treat the surfaces of two to four metal materials at the same time. In the case to treat the surfaces of two to four metal materials at the same time, the different metals can be in the condition not contacting each other or in the condition being joined and contacted by means of joining method such as welding, adhesion or riveting.
- the treating solution for surface treatment of metal of the present invention contains 5 to 5000 ppm of at least one compound selected from the group consisting of zirconium compound and titanium compound calculated as the metal element, and 0.1 to 100 ppm of free fluorine ion, further having pH of 2 to 6.
- zirconium compound used in the present invention ZrCl 4 , ZrOCl 2 , Zr(SO 4 ) 2 , ZrOSO 4 , Zr(NO 3 ) 4 , ZrO(NO 3 ) 2 , H 2 ZrF 6 , salt of H 2 ZrF 6 , ZrO 2 , ZrOBr 2 and ZrF 4 can be mentioned.
- titanium compound TiCl 4 , Ti(SO 4 ) 2 , TiOSO 4 , Ti(NO 3 ) 4 , TiO(NO 3 ) 2 , TiO 2 OC 2 O 4 , H 2 TiF 6 , salt of H 2 TiF 6 , TiO 2 and TiF 4 can be mentioned.
- zirconium compound is desirably used.
- the desirable concentration of at least one compound selected from the group consisting of zirconium compound and titanium compound is 5 to 5000 ppm calculated as the metal element (that is, as zirconium and/or titanium), and the more desirable concentration is 10 to 3000 ppm.
- the film obtained by using the treating solution for surface treatment of metal and the method for surface treatment of the present invention is oxide or hydroxide of zirconium or titanium. Therefore, when the concentration of the compound selected from the group consisting of zirconium compound and titanium compound calculated as zirconium and/or titanium is smaller than 5 ppm, it is difficult to obtain sufficient adhesion amount to attain corrosion resistance in a practical period of time for treating, because the concentration of main component of film is too low. On the contrary, when the concentration is larger than 5000 ppm, the sufficient adhesion amount can be obtained, but it is not effective to improve the corrosion resistance and is disadvantageous from the economical view point.
- the zirconium compound and the titanium compound can be easily dissolved in the acidic solution, but are not stable in the alkaline solution, and easily deposit as the oxide or the hydroxide of zirconium or titanium.
- the desirable pH of the treating solution for surface treatment of metal of the present invention is pH 2 to 6, more desirably pH 3 to 6.
- the dissolving reaction of the metal material to be treated occurs.
- the pH becomes higher at the surface of the metal material to be treated, and the oxide or the hydroxide of zirconium or titanium deposits as a film on the surface of the metal material to be treated.
- the treating solution for surface treatment of metal of the present invention has free fluorine ion existing therein.
- the fluorine compound is added into the treating solution for surface treatment of metal.
- hydrofluoric acid, H 2 ZrF 6 and salt of H 2 ZrF 6 , H 2 TiF 6 , salt of H 2 TiF 6 , H 2 SiF 6 , salt of H 2 SiF 6 , HBF 4 and salt of HBF 4 , NaHF 2 , KHF 2 , NH 4 HF 2 , NaF, KF and NH 4 F can be mentioned.
- the free fluorine ion has an effect to improve the stability of the zirconium compound and the titanium compound in the treating solution for surface treatment of metal. Further, the free fluorine ion has the function to promote the dissolving reaction of any of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material which are the metal materials to be surface treated in the present invention. Therefore, by allowing free fluorine ion to exist therein by adding fluorine compound, the stability of the treating solution for surface treatment of metal of the present invention is improved, and further the reactivity to the metal material to be treated can be improved.
- composition for surface treatment and treating solution for surface treatment of metal containing at least one of iron and zinc in WO02/103080 as follows. That is, the composition for surface treatment and treating solution for surface treatment of metal use titanium compound or zirconium compound and fluorine containing compound, wherein the ratio A/B is set within the specific range from 0.06 to 0.18, where A refers to the total mole weight of metal elements in the composition for surface treatment and treating solution for surface treatment of metal and B refers to the mole weight which when total fluorine atom in fluorine containing compound is calculated as HF.
- the present invention it is possible to perform surface treatment on independently one metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material, even if out of above mentioned specific range of the ratio, by regulating the concentration of metal element in the titanium compound and zirconium compound, pH and the concentration of free fluorine ion.
- the concentration of free fluorine ion means the concentration of fluorine ion measured by an ion electrode which is on the market.
- the concentration of free fluorine ion in the treating solution for surface treatment of metal of the present invention is desirably 0.1 to 100 ppm, and more desirably 2 to 70 ppm.
- the concentration of free fluorine ion is higher than 100 ppm, the dissolving reaction of the metal material to be treated is promoted.
- zirconium compound and titanium compound in the treating solution for surface treatment of metal are very stable, even if the pH of the surface of metal material to be treated increases, it becomes difficult to deposit as a film.
- the concentration of free fluorine ion is lower than 0.1 ppm, the effect for the improvement of the stability of the treating solution for surface treatment of metal and the reactivity thereof is small, and thus, it is no longer advantageous for the treating solution to contain free fluorine ion.
- the free fluorine ion of the present invention has a role to keep the eluted component by dissolution of the metal material to be treated stable in the treating solution for surface treatment of metal.
- sludge generates, because, for example, iron ion eluted from iron metal material reacts with phosphoric acid and forms iron phosphate which is an insoluble salt.
- the treating solution for surface treatment of metal of the present invention may also contain phosphoric acid group, but, if the concentration of phosphoric acid group excesses 1.0g/L, sludge can be generated.
- one or more compounds selected from the group, for example, consisting of inorganic acid such as sulfuric acid or hydrochloric; organic acid such as acetic acid, oxalic acid, tartaric acid, citric acid, succinic acid, gluconic acid or phthalic acid; and chelating agent which can chelete eluted component, may be added in the treating solution to thereby solubilize the eluted component.
- the treating solution for surface treatment of metal in the present invention may contain at least one compound selected from the group consisting of calcium compound, magnesium compound and strontium compound.
- the present invention realizes to perform surface treatment on each metal material independently or two to four materials simultaneously selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material by regulating the concentration of free fluorine ion in the aqueous solution containing zirconium compound and titanium compound of specific concentration within the specified range.
- the metal elements (calcium, magnesium or strontium) contained in above mentioned calcium compound, magnesium compound or strontium compound have a function to maintain the concentration of free fluorine ion in aqueous solution to a certain value by generating salt of fluorine and fluorinated compound in the aqueous solution. Due to the function, when the surface of various kinds of metal materials are treated at the same time, the optimum deposit amount of film can be obtained on each metal material to be treated, because certain concentration of free fluorine ion can be maintained regardless of the ratio among the materials used.
- calcium compound, magnesium compound or strontium compound which can be used in the present invention for example, oxide, hydroxide, chloride, sulfate, nitrate and carbonate of these metal elements can be mentioned. Further, besides calcium compound, magnesium compound and strontium compound, the compound which has a function to maintain the concentration of free fluorine ion constant can be used regardless of whether an organic compound or an inorganic compound.
- the concentration of the magnesium compound or the strontium compound which can be used in the present invention is desirably 10 to 5000 ppm as the metal element, and more desirably is 100 to 3000 ppm.
- the desirable concentration as calcium is 5 to 100 ppm and more desirable concentration is 5 to 50 ppm, because the solubility of calcium is remarkably small.
- the concentration of these compounds is higher than the upper limit, the stability of the treating solution for surface treatment of metal may decrease, and the continuous treatment is interrupted. And, when the concentration of these compounds is lower than the lower limit, the deposit amount of film particularly on ferriferous material decreases.
- Nitric acid group acts as an oxidizing agent, and has a function to promote film depositing reaction of the present invention and a function to improve the solubility of above mentioned calcium compound, magnesium compound or strontium compound in the treating solution for surface treatment of metal. Therefore, even if the concentration of nitric acid group is lower than 1000 ppm, the film having excellent corrosion resistance can be deposited. However, in the case where the concentration of above mentioned calcium compound, magnesium compound or strontium compound is high, the stability of the treating solution for surface treatment of metal may decrease. The concentration of nitric acid group of 50000 ppm is sufficient, and it is disadvantageous to add more nitric acid group from the economical view point.
- At least one oxygen acid and/or salt of oxygen acid selected from the group consisting of HClO 3 , HBrO 3 , HNO 3 , HNO 2 , HMnO 4 , HVO 3 , H 2 O 2 , H 2 WO 4 , H 2 MoO 4 .
- Oxygen acid or salt thereof acts as oxidizing agent to the materials to be treated, and promotes the film forming reaction in the present invention.
- the concentration of these oxygen acid or salts thereof to be added is not restricted, but adding an amount to 10 to 5000 ppm exhibits sufficient effect as the oxidizing agent.
- At least one polymer compound selected from the group consisting of water soluble polymer compounds and water dispersible polymer compounds may be added.
- the metal element whose surface is treated by using the treating solution for surface treatment of metal of the present invention has an enough corrosion resistance, but, if additional function such as lubricity is required, it is possible to improve the physical property of the film by adding preferably selected polymer according to the desired function.
- polymer compounds which are generally used for the surface treatment of metal such as polyvinyl alcohol, poly(metha)acrylic acid, copolymer of acrylic acid and methacrylic acid, copolymer of ethylene with acrylic monomer such as (metha)acrylic acid or (metha)acrylate, copolymer of ethylene and vinyl acetate, polyurethane, amino-modified phenol resin, polyester resin and epoxy resin can be used.
- metal such as polyvinyl alcohol, poly(metha)acrylic acid, copolymer of acrylic acid and methacrylic acid, copolymer of ethylene with acrylic monomer such as (metha)acrylic acid or (metha)acrylate, copolymer of ethylene and vinyl acetate, polyurethane, amino-modified phenol resin, polyester resin and epoxy resin
- metal such as polyvinyl alcohol, poly(metha)acrylic acid, copolymer of acrylic acid and methacrylic acid, copolymer of ethylene with acrylic monomer such as (metha)acrylic acid
- the method for surface treatment of the present invention can be illustrated as follows. Namely, the surface is merely treated by degreasing treatment according to an ordinary method, and the cleaned metal material to be treated is brought into contact with the treating solution for surface treatment of metal. Accordingly, the film composed of oxide and/or hydroxide of a metal element selected from the group consisting of zirconium and titanium is deposited and the surface treated film layer having good adhesiveness and corrosion resistance is formed.
- any kind of treatment e.g., spraying treatment, immersion treatment or pouring treatment can be used, and the properties of the product will not be influenced by the treating method.
- the structure of the surface treated layer of the present invention is considered to be a state where oxide and hydroxide are mixed when dried at an ordinary temperature or at a low temperature after surface treatment. And, when dried at a high temperature after surface treatment, the structure of the surface treated layer is considered to be a state of oxide alone or oxide rich.
- the condition to use the treating solution for surface treatment of metal is not restricted.
- the reactivity of the treating solution for surface treatment of metal of the present invention can be voluntarily regulated by changing the concentration of zirconium compound or titanium compound and the concentration of free fluorine ion in the treating solution for surface treatment of metal. Therefore, the treating temperature and treating period of time can be changed voluntarily in combination of the reactivity of the treating bath.
- At least one surface active agent selected from the group consisting of nonionic surface active agent, anionic surface active agent and cationic surface active agent can be added to the treating solution for surface treatment of metal.
- this treating solution for surface treatment of a metal can be used also as a surface treating agent and a degreasing agent.
- the method to carry out the electrolysis in the treating solution for surface treatment of metal having a metal material to be treated as a cathode When the electrolysis treatment is carried out using the metal material to be treated as a cathode, the reduction occurs at the surface of the cathode and the pH goes up. Along with the elevation of the pH, the stability of zirconium compound and/or titanium compound at the surface of cathode is deteriorated, and the surface treated film is deposited as an oxide or a hydroxide containing water.
- the effect of the present invention can be improved when, after contacting with treating solution for surface treatment of metal, or after being electrolyzed in the treating solution for surface treatment of metal with or without washed by water, the metal material is brought into contact with the acidic solution of the compound containing at least one element selected from the group consisting of cobalt, nickel, tin, copper, titanium and zirconium, or with the treating solution containing at least one polymer compound selected from the group consisting of water soluble polymer compound and water dispersible polymer.
- the surface treated film layer obtained by the present invention is characterized in a thin film and exhibits excellent coating property, but depending on the surface condition of the metal material to be treated, sometimes tiny defects may be formed on the surface treated film layer.
- the layer in contact with the acidic solution of the compound containing at least one element selected from the group consisting of cobalt, nickel, tin, copper, titanium and zirconium or the treating solution containing at least one polymer compound selected from the group consisting of water soluble polymer compound and water dispersible polymer the tiny defects are covered and the corrosion resistance can be further improved.
- the compound containing at least one element selected from the group consisting of cobalt, nickel, tin, copper, titanium and zirconium is not restricted, and, it is possible to use oxide, hydroxide, fluoride, complex fluoride, chloride, nitrate, oxynitrate, sulfate, oxysulfate, carbonate, oxycarbonate, phosphate, oxyphosphate, oxalate, oxyoxalate, and organic metal compounds and the like.
- the pH of acidic solution containing the metal element is 2 to 6, and can be adjusted with acid such as phosphoric acid, nitric acid, sulfuric acid, hydrofluoric acid, hydrochloric acid and organic acid or alkali such as sodium hydroxide, potassium hydroxide, lithium hydroxide, salts of alkali metal, ammonium salt or amines.
- acid such as phosphoric acid, nitric acid, sulfuric acid, hydrofluoric acid, hydrochloric acid and organic acid or alkali such as sodium hydroxide, potassium hydroxide, lithium hydroxide, salts of alkali metal, ammonium salt or amines.
- At least one polymer compound selected from above mentioned water soluble polymer compound or water dispersible polymer compound for example, polyvinyl alcohol, poly(metha)acrylic acid, copolymer of acrylic acid and methacrylic acid, copolymer of ethylene with acrylic monomer such as (metha)acrylic acid or (metha)acrylate, copolymer of ethylene and vinyl acetate, polyurethane, amino-modified phenol resin, polyester resin or epoxy resin, tannin and tannic acid and salts thereof, and phytic acid can be used.
- the present invention may remarkably improve the corrosion resistance of metal material by providing a surface treated film layer composed of oxide and/or hydroxide of metal elements selected from zirconium and/or titanium on the surface of metal material to be treated.
- the oxide and hydroxide of above mentioned metal elements have a physical property characterized not to be damaged by acid or alkali, and chemically stabilized.
- the pH becomes lower, while, at the cathode where reduction occurs, the pH becomes higher. Therefore, the surface treated film of less resistant to acid and alkali may be dissolved under the corrosive environment and its effect would be lost. Since the main component of the surface treated film layer of the present invention is resistive to acid or alkali, the excellent effect can be maintained under the corrosive environment.
- the oxide and hydroxide of above mentioned metal elements form a network structure mediated by metal and oxide, it becomes a very good barrier film.
- the corrosion of metal material which can be varied depending on the environment for use, generally, is oxygen demanding type corrosion in the atmosphere in which water and oxygen exist, and the speed of corrosion is promoted by the presence of the components such as chloride. Having a barrier effect against water, an acid and a corrosion promoting component, the surface treated film layer of the present invention may exhibit excellent corrosion resistance.
- the adhesion amount over 30 mg/m 2 calculated as the metal element is necessary, desirably over 40 mg/m 2 and more desirably over 50 mg/m 2 .
- the adhesion amount over 20 mg/m 2 calculated as the metal element is necessary, desirably over 30 mg/m 2 .
- the adhesion amount over than 10 mg/m 2 calculated as the metal element is necessary, desirably over 20 mg/m 2 .
- the adhesion amount over than 10 mg/m 2 calculated as the metal element is necessary, desirably over than 20 mg/m 2 .
- the adhesion amount there is no upper limit.
- the desirable upper limit of adhesion amount is 1 g/m 2 , more desirably 800 mg/m 2 .
- test plates cold rolled steel plates, hot-dip zinc-coated steel plates, aluminum alloy plates and magnesium alloy plates are used in the Examples and Comparative Examples.
- the abbreviations and specifications of these test plates are shown below.
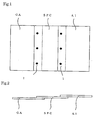
- the test plate prepared by joining three metal materials of SPC, GA and Al by a spot welding was used.
- each test plate of SPC, GA, Al and Mg, and the test plate prepared by joining three metal materials of SPC, GA and Al by a spot welding were used.
- the coating property the test plate prepared by joining three metal materials of SPC, GA and Al by a spot welding was used and the test from surface treatment, coating and evaluation of coating property were carried out in series.
- Fig.1 is the plane view of the test plate prepared by joining three metal materials of SPC, GA and Al by a spot welding
- Fig.2 is an elevation view of it.
- the numeral 1 indicates a spot welded portion.
- alkali degreasing was carried out as follows. That is, Fine Cleaner L4460 (Trade Mark: Product of Nihon Parkerizing) was diluted to 2% concentration by city water, and was sprayed to a plate to be treated at 40 °C for 120 sec. Rinsing by water and rinsing by pure water after film formation treatment were performed by spraying water and pure water on the plate to be treated at a room temperature for 30 sec both in Examples and Comparative Examples.
- Aqueous solution of zirconium with concentration of 200 ppm was prepared using zirconium oxynitrate reagent and nitric acid. After heating the aqueous solution to 45°C, the pH was adjusted to 3.0 using sodium hydroxide reagent and hydrofluoric acid, and the concentration of free fluorine ion measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 1 ppm, thus obtaining the treating solution for surface treatment of metal. The total fluorine concentration in the treating solution for surface treatment of metal after adjusting free fluorine ion was 50 ppm.
- test plate rinsed by water after degreasing was immersed into the treating solution for surface treatment of metal for 120 seconds so as to carry out the surface treatment.
- Aqueous solution of zirconium with concentration of 100 ppm, magnesium with concentration of 5000 ppm, strontium with concentration of 2000 ppm and nitric acid group with concentration of 28470 ppm was prepared using zirconium oxynitrate reagent, magnesium nitrate reagent and strontium nitrate reagent. After heating the aqueous solution to 50°C, the pH was adjusted to 4.0 using ammonium water reagent and hydrofluoric acid, and the concentration of free fluorine ion measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 80 ppm, thus obtaining the treating solution for surface treatment of metal. The total fluorine concentration in the treating solution for surface treatment of metal after adjusting free fluorine ion was 2000 ppm.
- IM-55G fluorine ion meter
- test plate rinsed by water after degreasing was immersed into the treating solution for surface treatment of metal for 60 seconds so as to carry out the surface treatment.
- Aqueous solution of zirconium with concentration of 1000 ppm, titanium with concentration of 2000 ppm, calcium with concentration of 5 ppm and nitric acid group with concentration of 1000 ppm was prepared using aqueous solution of hexafluorozirconic acid (IV), aqueous solution of titanium sulfate (IV) and calcium sulfate reagent.
- the pH was adjusted to 5.0 using potassium hydroxide reagent and hydrofluoric acid, and the concentration of free fluorine ion measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 25 ppm, thus obtaining the treating solution for surface treatment of metal.
- IM-55G fluorine ion meter
- the total fluorine concentration in the treating solution for surface treatment of metal after adjusting free fluorine ion was 2250 ppm.
- test plate rinsed by water after degreasing was immersed into the treating solution for surface treatment of metal for 90 seconds so as to carry out the surface treatment.
- Aqueous solution of titanium with concentration of 5000 ppm, strontium with concentration of 5000 ppm, nitric acid group with concentration of 7080 ppm and nitrous acid group with concentration of 40 ppm was prepared using aqueous solution of hexafluorotitanium acid (IV), strontium nitrate reagent, and sodium nitrite reagent.
- the pH was adjusted to 4.0 using triethanol amine reagent and hydrofluoric acid, and the concentration of free fluorine ion measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 10 ppm, thus obtaining the treating solution for surface treatment of metal.
- the total fluorine concentration in the treating solution for surface treatment of metal after adjusting free fluorine ion was 11900 ppm.
- test plate was rinsed by water after degreasing, then the obtained treating solution for surface treatment of metal was sprayed to the surface thereof for 120 sec., thus carrying out the surface treatment.
- Aqueous solution of zirconium with concentration of 5 ppm, titanium with concentration of 5 ppm, magnesium with concentration of 100 ppm, nitric acid group with concentration of 30520 ppm and chloric acid group with concentration of 100 ppm was prepared using zirconium oxynitrate reagent, aqueous solution of hexafluorotitanic acid (IV), magnesium nitrate reagent, nitric acid and sodium chloric acid reagent.
- the pH was adjusted to 6.0 using ammonia water reagent and hydrofluoric acid, and the concentration of free fluorine ion measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 0.5 ppm, thus obtaining the treating solution for surface treatment of metal.
- IM-55G fluorine ion meter
- the total fluorine concentration in the treating solution for surface treatment of metal after adjusting free fluorine ion was 12 ppm.
- test plate was electrolyzed in the treating solution for surface treatment of metal for 5 seconds under the condition of 5A/dm 2 , thus carrying out the surface treatment.
- Aqueous solution of zirconium with concentration of 150 ppm, magnesium with concentration of 10 ppm, nitric acid group with concentration of 5200 ppm and hydrogen peroxide concentration of 10 ppm was prepared using zirconium oxynitrate reagent, magnesium oxide reagent, nitric acid, and hydrogen peroxide reagent.
- the pH was adjusted to 5.0 using ammonia water reagent and hydrofluoric acid, the concentration of free fluorine ion measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 50 ppm and 2 g/L of polyoxyethylenenonylphenylether (ethylene oxide addition mole number: 12 mol), which is nonionic surface active agent, was added, thus obtaining the treating solution for surface treatment of metal.
- the total fluorine concentration in the treating solution for surface treatment of metal after adjusting free fluorine ion was 170 ppm.
- Aqueous solution of titanium with concentration of 100 ppm, calcium with concentration of 50 ppm, magnesium with concentration of 5000 ppm, nitric acid group with concentration of 25660 ppm and permanganate with concentration of 10 ppm was prepared using aqueous solution of titanium sulfate (IV), calcium nitrate reagent, magnesium nitrate reagent and potassium permanganate reagent.
- Water soluble acrylic polymer compound (Jurymer AC-10L: product of Nihon Junyaku Co., Ltd.) was added in the aqueous solution so as the concentration of solid to be 1%, then the aqueous solution was heated to 50°C.
- the pH was adjusted to 3.0 using sodium hydroxide reagent and hydrofluoric acid, and the total free fluorine ion concentration in the aqueous solution to be measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 95 ppm, thus obtaining the treating solution for surface treatment of metal.
- IM-55G fluorine ion meter
- the total fluorine concentration in the treating solution for surface treatment of metal was 2000 ppm.
- test plate rinsed by water after degreasing was immersed into the treating solution for surface treatment of metal for 60 seconds so as to carry out the surface treatment.
- the aqueous solution with 1% of water soluble acrylic polymer compound (Jurymer AC-10L: product of Nihon Junyaku Co., Ltd.) in solid concentration and 2g/L of phosphoric acid reagent as phosphoric acid group was prepared.
- This aqueous solution was heated to 40°C, then the pH was adjusted to 4.5 using ammonia water reagent, thus obtaining the after treating solution.
- the test plate on which film formation was carried out by the surface treatment of Example 5 and rinsed by water was dipped into the above mentioned after treating solution for 30 seconds so as to carry out the after treatment.
- the aqueous solution of zirconium with concentration of 50 ppm and cobalt with concentration of 50 ppm was prepared using aqueous solution of hexafluorozirconic acid (IV) and cobalt nitrate reagent. After heating the aqueous solution to 40°C, the pH was adjusted to 5.0 with ammonia water reagent, thus obtaining the after treating solution.
- the test plate on which film formation was carried out by the surface treatment of Example 6 and rinsed by water was immersed into the above mentioned after treating solution for 30 seconds so as to carry out the after treatment.
- the aqueous solution of zirconium with concentration of 500 ppm, magnesium with concentration of 1000 ppm and nitric acid group with concentration of 6780 ppm was prepared using zirconium oxynitrate reagent, magnesium nitrate and nitric acid. After heating the aqueous solution to 45°C, the pH was adjusted to 4.0 with sodium hydroxide solution, thus obtaining the treating solution for surface treatment of metal.
- the free fluorine ion concentration of the treating solution for surface treatment of metal was measured by a fluorine ion meter on the market (IM-55G; product of Toa Denpa Industries Co., Ltd), and the result was 0 ppm.
- test plate which was rinsed by water after degreasing was immersed into the above mentioned treating solution for surface treatment of metal for 120 seconds so as to carry out the surface treatment.
- the aqueous solution of titanium with concentration of 2000 ppm was prepared by using aqueous solution of titanium sulfate (IV). After heating the aqueous solution to 50°C, the pH was adjusted to 3.5 using ammonia water reagent and hydrofluoric acid, and the concentration of free fluorine ion measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 400 ppm, thus obtaining the treating solution for surface treatment of metal.
- IM-55G fluorine ion meter
- test plate which was rinsed by water after degreasing was immersed into the above mentioned treating solution for surface treatment of metal for 90 seconds so as to carry out the surface treatment.
- Alchrom 713 (Trade Mark, product of Nihon Parkerizing Co., Ltd.), which is the chromic chromate treating agent on the market, was diluted by city water to the concentration of 3.6%, then total acidity and free acid acidity were adjusted to the center value described in the brochure.
- test plate was rinsed by water after degreasing, then immersed into the chromate treating solution heated to the temperature of 35°C and chromate treatment was carried out for 60 sec.
- Palcoat 3756 (Trade Mark, product of Nihon Parkerizing Co., Ltd.), which is the chrome free treating agent on the market, was diluted by city water to the concentration of 2%, then total acidity and free acid acidity were adjusted to the center value described in the brochure. The test plate was rinsed by water after degreasing, then immersed into the chrome free treating solution heated to the temperature of 40°C and chrome free treatment was carried out for 60 sec.
- the test plate was rinsed by water after degreasing, then the solution prepared by diluting Prepalene ZN (Trade Mark, product of Nihon Pakerizing Co., Ltd.), which is a surface conditioning agent, with city water to the concentration of 0.1% was sprayed thereon at the room temperature for 30 sec.
- Palbond L3020 (Trade Mark, product of Nihon Parkerizing Co., Ltd.) was diluted to the concentration of 4.8% with city water.
- sodium hydrogen fluoride reagent as fluorine was added into the solution to 200 ppm, and then, total acidity and free acid acidity thereof were adjusted to the center value described in the brochure.
- the zinc phosphate treating solution was prepared. Above mentioned test plate was immersed into the zinc phosphate chemical treating solution heated to the temperature of 42°C, and zinc phosphate film was deposited.
- Example 1 uniform interference color uniform dark black uniform white
- Example 2 uniform interference color uniform dark black uniform white
- Example 3 uniform interference color uniform dark black uniform white
- Example 4 uniform interference color uniform dark black uniform white
- Example 5 uniform interference color uniform dark black uniform white
- Example 6 uniform interference color uniform dark black uniform white
- Example 7 uniform interference color uniform dark black uniform white Comparative Example 1 film not deposited film not deposited uneven white Comparative Example 2 pale yellow uneven gray uneven white Comparative Example 3 film not deposited slightly turned to yellow gold Comparative Example 4 film not deposited film not deposited uniform white Comparative Example 5 material partially exposed uniform gray uneven white
- coating was carried out by the following process: cationic electrodeposition coating ⁇ rinsing with pure water ⁇ baking ⁇ surfacer ⁇ baking ⁇ top coating ⁇ baking.
- the coating property in the Examples and Comparative Examples was evaluated and the results thereof are shown in Table 4 and Table 5. Items evaluated and the abbreviations are described below.
- the coated film after electrodeposition coating process is called as electrodeposition coated film and the coated film after top coating is called as 3-coats film.
- Example 4 The results for evaluation of coating property of the electrodeposition coated film are summarized in Table 4. Examples showed good corrosive resistance on all test plates. On the contrary, in Comparative Example 1, since free fluorine ions were not contained in the treating solution for surface treatment of metal at all, the deposition of surface treated film was not sufficient and thus the corrosion resistance was not so good. Further, in Comparative Example 2, since the concentration of free fluorine ion in the treating solution for surface treatment of metal was high, especially, the adhesion amount of film on SPC was small and the corrosion resistance was not so good. The coating properties of Examples 5 and 6 were superior to those of Comparative Examples, but when compared with other Examples, corrosive resistances after electrodeposition coating was inferior to those of other Examples. However, as shown in Examples 8 and 9, the corrosive resistance was further improved by carrying out the after treatment.
- Comparative Example 3 Because in Comparative Example 3, a chromate treating agent for aluminum alloy was used and in Comparative Example 4, a chrome free treating agent for aluminum alloy was used, the corrosion resistance of Al was good, but the corrosion resistance of other test plates were obviously inferior to those of Examples.
- Comparative Example 5 a zinc phosphate treating agent, which is now usually used as the base for coating was used. However, Comparative Example 5, in the condition where each of the test plates was joined by welding, showed the test results inferior to those of Examples.
- Table 5 shows the evaluation results of adhesion of a 3-coats plate. Examples showed good adhesion to all test plates. Regarding to 1st ADH, good results were obtained in all Comparative Examples. However, regarding to 2nd ADH, Comparative Examples did not show the good level of adhesion to all test plates same as the corrosive resistance of the electrodeposition coating. Further, in Comparative Example 5, the generation of sludge, which is the by-product of zinc phosphate treatment, was observed in the treating bath after surface treatment. However, in Examples of the present invention, the generation of sludge was not observed.
- the treating solution for metal surface treatment and the method for surface treatment of the present invention it is possible to deposit a surface treated film having excellent corrosion resistance after coating on the surface of a metal made of two or more, or each of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material in the treating bath containing no harmful component to the environment and without generating sludge, which have never been achieved in the prior art. Further, since the present invention does not need a process for surface conditioning on the metal material to be treated, it is possible to shorten the treatment time and to reduce space for the treatment.
Abstract
Description
- SPC: cold rolled steel plate (JIS-G-3141)
- GA: both-side alloyed hot-dip zinc-coated steel plate (45 g/m2)
- Al: aluminum alloy plate (6000 series aluminum alloy)
- Mg: magnesium alloy plate (JIS-H-4201)
Thereafter, the pH was adjusted to 3.0 using sodium hydroxide reagent and hydrofluoric acid, and the total free fluorine ion concentration in the aqueous solution to be measured by a fluorine ion meter (IM-55G; product of Toa Denpa Industries Co., Ltd) was adjusted to 95 ppm, thus obtaining the treating solution for surface treatment of metal. After adjusting the free fluorine ion concentration, the total fluorine concentration in the treating solution for surface treatment of metal was 2000 ppm.
| appearance after surface treatment | |||
| on SPC | on GA | on Al | |
| Example 1 | uniform interference color | uniform dark black | uniform white |
| Example 2 | uniform interference color | uniform dark black | uniform white |
| Example 3 | uniform interference color | uniform dark black | uniform white |
| Example 4 | uniform interference color | uniform dark black | uniform white |
| Example 5 | uniform interference color | uniform dark black | uniform white |
| Example 6 | uniform interference color | uniform dark black | uniform white |
| Example 7 | uniform interference color | uniform dark black | uniform white |
| Comparative Example 1 | film not deposited | film not deposited | uneven white |
| Comparative Example 2 | pale yellow | uneven gray | uneven white |
| Comparative Example 3 | film not deposited | slightly turned to yellow | gold |
| Comparative Example 4 | film not deposited | film not deposited | uniform white |
| Comparative Example 5 | material partially exposed | uniform gray | uneven white |
| adhesion amount of surface treated film layer (without joining) (total adhesion amount of Zr and Ti: mg/m2) | ||||
| on SPC | on GA | on Al | on Mg | |
| Example 1 | 122 | 67 | 48 | 45 |
| Example 2 | 108 | 66 | 49 | 41 |
| Example 3 | 61 | 58 | 42 | 38 |
| Example 4 | 73 | 59 | 14 | 12 |
| Example 5 | 41 | 52 | 38 | 26 |
| Example 6 | 35 | 38 | 25 | 19 |
| Example 7 | 31 | 29 | 24 | 18 |
| Comparative Example 1 | trace | trace | trace | trace |
| Comparative Example 2 | 25 | 15 | 15 | 10 |
| Comparative Example 3 | trace | Cr 33 | Cr 95 | Cr 75 |
| Comparative Example 4 | trace | trace | 25 | 15 |
| Comparative Example 5 | weight of film 2.5g/m2 | weight of film 4.5g/m2 | weight of film 1.2g/m2 | weight of film 0.5g/m2 |
| Adhesion amount of surface treated film layer (with joining) (total adhesion amount of Zr and Ti: mg/m2) | |||
| on SPC | on GA | on Al | |
| Example 1 | 125 | 67 | 48 |
| Example 2 | 118 | 66 | 49 |
| Example 3 | 65 | 58 | 42 |
| Example 4 | 72 | 59 | 14 |
| Example 5 | 45 | 52 | 38 |
| Example 6 | 38 | 38 | 25 |
| Example 7 | 32 | 29 | 24 |
| Comparative Example 1 | trace | trace | trace |
| Comparative Example 2 | 28 | 17 | 12 |
| Comparative Example 3 | trace | Cr 35 | Cr 85 |
| Comparative Example 4 | trace | trace | 21 |
| Comparative Example 5 | weight of film 2.8 g/m2 | weight of film 4.7 g/m2 | Weight of film 0.7 g/m2 |
- SST: Cross cut line is notched using a sharp knife on the electrodeposition coated plate, and 5% brine is sprayed to the plate for 840 hours (according to JIS-Z-2371). After spraying, maximum blister widths at both sides from the cross cut line were measured.
- SDT: The electrodeposition coated plate was soaked into aqueous solution of 5 wt% of NaCl at 50°C for 840 hours. After soaking, the test plate washed with city water and dried at the room temperature. The whole surface of the test plate was peeled off using an adhesive tape, and the removed area of coated film on each metal material was evaluated by inspector's eye.
- 1st ADH: Checker lines of 100 squares with 2 mm intervals were marked using a sharpened knife on a 3-coats film. The squares in the checker were peeled using a cellophane tape, and numbers of peeled squares were counted.
- 2nd ADH: A 3-coats film was soaked in pure water of 40
°C for 240 hours. Then, 100 checker squares with 2mm
interval were marked using a sharpened knife on it. The
checker squares were peeled using a cellophane tape, and
numbers of peeled squares were counted.
coating property of electro deposition film SST max blister widths at both sides (mm) SDT removed area (%) on SPC on GA on Al on SPC on GA on Al Example 1 2.0 0.5 0.5 5> 5> 5> Example 2 2.0 0.5 0.5 5> 5> 5> Example 3 3.0 0.5 0.5 5> 5> 5> Example 4 3.0 0.5 0.5 5> 5> 5> Example 5 3.0 0.5 0.5 5 5> 5> Example 6 3.5 1.0 0.5 10 5> 5> Example 7 3.5 1.0 0.5 10 5> 5> Example 8 2.5 0.5 0.5 5> 5> 5> Example 9 2.5 0.5 0.5 5> 5> 5> Comparative Example 1 6.5 3.5 3.0 70 40 15 Comparative Example 2 4.5 2.0 0.5 30 10 5 Comparative Example 3 10.0 5.0 0.5 80 40 5> Comparative Example. 4 10.0 5.0 1.0 90 50 10 Comparative Example 5 5.0 2.0 2.0 40 10 20
| coating properties of 3-coats film | ||||||
| 1st ADH | 2nd ADH | |||||
| on SPC | on GA | on Al | on SPC | on GA | on Al | |
| Example 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Example 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Example 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Example 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Example 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Example 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Example 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Example 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Example 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Example 1 | 0 | 0 | 0 | 5 | 8 | 0 |
| Comparative Example 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Example 3 | 6 | 0 | 0 | 17 | 3 | 0 |
| Comparative Example 4 | 0 | 0 | 0 | 5 | 0 | 0 |
| Comparative Example 5 | 5 | 0 | 0 | 8 | 0 | 6 |
Claims (17)
- Treating solution for surface treatment of metal, which is aqueous surface treating solution to treat independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material, the treating solution containing 5 to 5000 ppm of at least one compound selected from the group consisting of zirconium compound and titanium compound calculated as metal element, and 0.1 to 100 ppm of free fluorine ion, and having pH 2 to 6.
- The treating solution for surface treatment of metal according to claim 1, further containing at least one compound selected from the group consisting of calcium compound, magnesium compound and strontium compound, wherein concentration of the compound calculated as metal element is 5 to 100 ppm in the case of the calcium compound, 10 to 5000 ppm in the case of the magnesium and 10 to 5000 ppm in the case of the strontium compound.
- The treating solution for surface treatment of metal according to claim 1 or 2, further containing 1000 to 50000 ppm of nitrate group.
- The treating solution for surface treatment of metal according to any one of claims 1 to 3, further containing at least one oxygen acid and/or salt of oxygen acid selected from the group consisting of HClO3, HBrO3, HNO2, HNO3, HMnO4, HVO3, H2O2, H2WO4, H2MoO4 and salts thereof.
- The treating solution for surface treatment of metal according to any one of claims 1 to 4, further containing at least one polymer compound selected from the group consisting of water soluble polymer compounds and water dispersible polymer compounds.
- The treating solution for surface treatment of metal according to any one of claims 1 to 5, further containing at least one surface active agent selected from the group consisting of nonionic surface active agents, anionic surface active agents and cationic surface active agents.
- A method for surface treatment of metal comprising, contacting independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material with the treating solution for surface treatment according to any one of claims 1 to 6.
- The method for surface treatment of metal according to claim 7, comprising, further contacting the metal material or the two or more metal materials with acidic aqueous solution of compound containing at least one element selected from the group consisting of cobalt, nickel, tin, copper, titanium and zirconium, after contacting with the treating solution for surface treatment, with or without washing by water.
- The method for surface treatment of metal according to claim 7, comprising, further contacting the metal material or the two or more metal materials with treating solution containing at least one polymer compound selected from water soluble polymer compounds and water dispersible polymer compounds, after contacting with the treating solution for surface treatment, with or without washing by water.
- A method for surface treatment of metal comprising, electrolytic treating in the treating solution for surface treatment according to any one of claims 1 to 6, wherein independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material are a cathode.
- The method for surface treatment of metal according to claim 10, comprising, further contacting the metal material or the two or more metal materials with acidic aqueous solution of compound containing at least one element selected from the group consisting of cobalt, nickel, tin, copper, titanium and zirconium, after electrolytic treating in the treating solution for surface treatment, with or without washing by water.
- The method for surface treatment of metal according to claim 10, comprising, further contacting the metal material or the two or more metal materials with treating solution containing at least one polymer compound selected from water soluble polymer compounds and water dispersible polymer compounds, after electrolytic treating in the treating solution for surface treatment, with or without washing by water.
- A method for surface treatment of metal comprising, contacting independently each metal material or simultaneously two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material, whose surface is not degreased and cleaned with the treating solution for surface treatment according to claim 6.
- A metal material having a surface treated film containing at least one metal element selected from the group consisting of titanium and zirconium formed on a surface of iron metal material by the method for surface treatment according to any one of claims 7 to 13, wherein an adhesion amount of the surface treated film calculated as the metal element is 30mg/m2 or more.
- A metal material having a surface treated film containing at least one metal element selected from the group consisting of titanium and zirconium formed on a surface of zinc metal material by the method for surface treatment according to any one of claims 7 to 13, wherein an adhesion amount of the surface treated film calculated as the metal element is 20mg/m2 or more.
- A metal material having a surface treated film containing at least one metal element selected from the group consisting of titanium and zirconium formed on a surface of aluminum metal material by the method for surface treatment according to any one of claims 7 to 13, wherein an adhesion amount of the surface treated film calculated as the metal element is 10mg/m2 or more.
- A metal material having a surface treated film containing at least one metal element selected from the group consisting of titanium and zirconium formed on a surface of magnesium metal material by the method for surface treatment according to any one of claims 7 to 13, wherein an adhesion amount of the surface treated film calculated as the metal element is 10mg/m2 or more.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002362640 | 2002-12-13 | ||
| JP2002362640A JP4205939B2 (en) | 2002-12-13 | 2002-12-13 | Metal surface treatment method |
| PCT/JP2003/015868 WO2004055237A1 (en) | 2002-12-13 | 2003-12-11 | Treating fluid for surface treatment of metal and method for surface treatment |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP1571237A1 true EP1571237A1 (en) | 2005-09-07 |
| EP1571237A4 EP1571237A4 (en) | 2007-11-21 |
| EP1571237B1 EP1571237B1 (en) | 2019-03-20 |
Family
ID=32588165
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP03780727.8A Expired - Lifetime EP1571237B1 (en) | 2002-12-13 | 2003-12-11 | Treating fluid for surface treatment of metal and method for surface treatment |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US20060185769A1 (en) |
| EP (1) | EP1571237B1 (en) |
| JP (1) | JP4205939B2 (en) |
| KR (1) | KR100674778B1 (en) |
| CN (2) | CN101487115B (en) |
| AU (1) | AU2003289323A1 (en) |
| CA (1) | CA2509772A1 (en) |
| ES (1) | ES2730576T3 (en) |
| MX (1) | MXPA05006156A (en) |
| TW (1) | TW200416300A (en) |
| WO (1) | WO2004055237A1 (en) |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1612294A1 (en) * | 2004-07-02 | 2006-01-04 | Italtecno S.R.L. | Bath and associated method for the conversion coating of articles made of aluminium and alloys thereof |
| WO2008029926A1 (en) | 2006-09-08 | 2008-03-13 | Nippon Paint Co., Ltd. | Method of treating surface of metal base, metallic material treated by the surface treatment method, and method of coating the metallic material |
| WO2008110195A1 (en) * | 2007-03-15 | 2008-09-18 | Basf Coatings Ag | Method for the anti-corrosive treatment of metal substrates |
| WO2009045872A1 (en) * | 2007-09-28 | 2009-04-09 | Ppg Industries Ohio, Inc. | Methods for treating a ferrous metal substrate |
| WO2009117397A1 (en) | 2008-03-17 | 2009-09-24 | Henkel Corporation | Metal treatment coating compositions, methods of treating metals therewith and coated metals prepared using the same |
| DE102008014465A1 (en) | 2008-03-17 | 2009-09-24 | Henkel Ag & Co. Kgaa | Optimized passivation on Ti / Zr-BAsis for metal surfaces |
| FR2948690A1 (en) * | 2009-07-30 | 2011-02-04 | Snecma | PIECE COMPRISING A SUBSTRATE CARRYING A CERAMIC COATING LAYER |
| US8399061B2 (en) | 2006-11-13 | 2013-03-19 | Basf Coatings Gmbh | Anti-corrosion agent forming a coating film with good adhesion and method for nongalvanic application thereof |
| US8475883B2 (en) | 2005-05-23 | 2013-07-02 | Basf Coatings Gmbh | Corrosion-protection agent forming a layer of paint and method for current-free application thereof |
| EP2767615A1 (en) | 2005-12-09 | 2014-08-20 | Henkel AG&Co. KGAA | Wet-on-wet method and chromium free acidic solution for the corrosion treatment of steel surfaces |
| EP2862957A1 (en) | 2013-10-16 | 2015-04-22 | Coatings Foreign IP Co. LLC | Process for producing a multilayer coating |
| EP2459770B1 (en) | 2009-07-27 | 2015-05-13 | Henkel AG & Co. KGaA | Multi-stage method for the treatment of metal surfaces prior to electrodeposition |
| EP2876186A1 (en) * | 2013-11-21 | 2015-05-27 | Samsung Electronics Co., Ltd | Composition for forming film of metal object, film formed using the same, and method of forming film |
| US9273399B2 (en) | 2013-03-15 | 2016-03-01 | Ppg Industries Ohio, Inc. | Pretreatment compositions and methods for coating a battery electrode |
| EP3031951A1 (en) | 2014-12-12 | 2016-06-15 | Henkel AG & Co. KGaA | Optimized process control in the pretreatment of metals to protect against corrosion on the basis of baths containing fluoride |
| WO2016091703A1 (en) * | 2014-12-09 | 2016-06-16 | Henkel Ag & Co. Kgaa | Integration of light metals into steel pickling and pretreating processes |
| EP2578727A4 (en) * | 2010-05-28 | 2016-07-20 | Toyo Seikan Kaisha Ltd | Surface treatment bath, method of manufacturing surface-treated steel plate using said surface treatment bath, and surface-treated steel plate formed with said manufacturing method |
| US9428410B2 (en) | 2007-09-28 | 2016-08-30 | Ppg Industries Ohio, Inc. | Methods for treating a ferrous metal substrate |
| US9631281B2 (en) | 2014-12-04 | 2017-04-25 | Axalta Coating Systems Ip Co., Llc | Processes for producing a multilayer coating |
| US9970115B2 (en) | 2009-12-28 | 2018-05-15 | Henkel Ag & Co. Kgaa | Metal pretreatment composition containing zirconium, copper, zinc, and nitrate and related coatings on metal substrates |
| US10125424B2 (en) | 2012-08-29 | 2018-11-13 | Ppg Industries Ohio, Inc. | Zirconium pretreatment compositions containing molybdenum, associated methods for treating metal substrates, and related coated metal substrates |
| US10137476B2 (en) | 2009-02-05 | 2018-11-27 | Basf Coatings Gmbh | Coating agent for corrosion-resistant coatings |
| US10400337B2 (en) | 2012-08-29 | 2019-09-03 | Ppg Industries Ohio, Inc. | Zirconium pretreatment compositions containing lithium, associated methods for treating metal substrates, and related coated metal substrates |
| WO2020167758A1 (en) * | 2019-02-11 | 2020-08-20 | Ppg Industries Ohio, Inc. | Systems for treating a metal substrate |
Families Citing this family (74)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7569132B2 (en) | 2001-10-02 | 2009-08-04 | Henkel Kgaa | Process for anodically coating an aluminum substrate with ceramic oxides prior to polytetrafluoroethylene or silicone coating |
| US7452454B2 (en) * | 2001-10-02 | 2008-11-18 | Henkel Kgaa | Anodized coating over aluminum and aluminum alloy coated substrates |
| US7578921B2 (en) | 2001-10-02 | 2009-08-25 | Henkel Kgaa | Process for anodically coating aluminum and/or titanium with ceramic oxides |
| JP4205939B2 (en) * | 2002-12-13 | 2009-01-07 | 日本パーカライジング株式会社 | Metal surface treatment method |
| JP4067103B2 (en) * | 2002-12-24 | 2008-03-26 | 日本ペイント株式会社 | Degreasing and chemical conversion treatment agent and surface-treated metal |
| CA2454029A1 (en) * | 2002-12-24 | 2004-06-24 | Nippon Paint Co., Ltd. | Chemical conversion coating agent and surface-treated metal |
| EP1836330A1 (en) * | 2005-01-14 | 2007-09-26 | Henkel Kommanditgesellschaft Auf Aktien | Stable, non-chrome, thin-film organic passivates |
| DE102005005858A1 (en) * | 2005-02-08 | 2006-08-17 | Henkel Kgaa | Process for coating metal sheet, in particular zinc sheet |
| JP2006241579A (en) * | 2005-03-07 | 2006-09-14 | Nippon Paint Co Ltd | Chemical conversion treatment agent and surface-treated metal |
| WO2006098359A1 (en) * | 2005-03-16 | 2006-09-21 | Nihon Parkerizing Co., Ltd. | Surface-treated metallic material |
| JP4748384B2 (en) * | 2005-09-21 | 2011-08-17 | 日立金属株式会社 | Saddle type fitting |
| US7815751B2 (en) * | 2005-09-28 | 2010-10-19 | Coral Chemical Company | Zirconium-vanadium conversion coating compositions for ferrous metals and a method for providing conversion coatings |
| JP5023468B2 (en) * | 2005-10-28 | 2012-09-12 | Jfeスチール株式会社 | Surface treatment metal plate for can or can lid and method for producing the same, resin-coated metal plate for can or can lid, metal can and can lid |
| WO2007061011A1 (en) * | 2005-11-22 | 2007-05-31 | Nihon Parkerizing Co., Ltd. | Chemical conversion coated metal plate and method for producing same |
| JP2007182626A (en) * | 2005-12-06 | 2007-07-19 | Nippon Steel Corp | Composite coated metal sheet, treatment agent for composite coating, and method of manufacturing composite coated metal sheet |
| TWI340770B (en) | 2005-12-06 | 2011-04-21 | Nippon Steel Corp | Composite coated metal sheet, treatment agent and method of manufacturing composite coated metal sheet |
| JP2007262577A (en) * | 2006-03-01 | 2007-10-11 | Nippon Paint Co Ltd | Composition for metal surface treatment, metal surface treatment method, and metallic material |
| JP5092332B2 (en) * | 2006-03-22 | 2012-12-05 | Jfeスチール株式会社 | Surface-treated steel sheet and manufacturing method thereof |
| BRPI0711143B1 (en) * | 2006-05-02 | 2018-01-30 | Jfe Steel Corporation | METHOD FOR MANUFACTURING HOT DIP GALVANIZED STEEL PLATE AND HOT DIP GALVANIZED STEEL PLATE |
| MY148014A (en) * | 2006-09-07 | 2013-02-28 | Jfe Steel Corp | Surface-treated steel shee t |
| EP2067881B1 (en) | 2006-09-08 | 2017-11-15 | Chemetall GmbH | Method of treating surface of metal base, metallic material treated by the surface treatment method, and method of coating the metallic material |
| CN101631895B (en) * | 2007-02-12 | 2013-05-08 | 汉高股份及两合公司 | Process for treating metal surfaces |
| EP1978131B2 (en) * | 2007-03-29 | 2019-03-06 | ATOTECH Deutschland GmbH | Means for manufacturing corrosion protection coats on metal surfaces |
| JP5571277B2 (en) | 2007-04-13 | 2014-08-13 | 日本パーカライジング株式会社 | Surface treatment liquid for zinc-based metal material and surface treatment method for zinc-based metal material |
| US8673091B2 (en) * | 2007-08-03 | 2014-03-18 | Ppg Industries Ohio, Inc | Pretreatment compositions and methods for coating a metal substrate |
| US9574093B2 (en) * | 2007-09-28 | 2017-02-21 | Ppg Industries Ohio, Inc. | Methods for coating a metal substrate and related coated metal substrates |
| JP5470751B2 (en) * | 2008-02-13 | 2014-04-16 | Tdk株式会社 | Active material and electrode manufacturing method, active material and electrode |
| KR20110020237A (en) * | 2008-04-25 | 2011-03-02 | 헨켈 아게 운트 코 카게아아 | Trichrome passivates for treating galvanized steel |
| CN101629299B (en) * | 2008-07-16 | 2011-03-30 | 宝山钢铁股份有限公司 | Electroplating degreasing agent for secondary cold rolled material |
| JP5338195B2 (en) * | 2008-08-20 | 2013-11-13 | 新日鐵住金株式会社 | Surface-treated galvanized steel sheet and method for producing the same |
| JP5463652B2 (en) * | 2008-11-14 | 2014-04-09 | Tdk株式会社 | Active material and electrode manufacturing method |
| EP2186928A1 (en) * | 2008-11-14 | 2010-05-19 | Enthone, Inc. | Method for the post-treatment of metal layers |
| JP5407062B2 (en) * | 2008-11-17 | 2014-02-05 | Tdk株式会社 | Active material and electrode manufacturing method, active material, electrode and lithium ion secondary battery |
| US8282801B2 (en) * | 2008-12-18 | 2012-10-09 | Ppg Industries Ohio, Inc. | Methods for passivating a metal substrate and related coated metal substrates |
| JP5347522B2 (en) * | 2009-01-20 | 2013-11-20 | Tdk株式会社 | Active material and electrode manufacturing method, active material and electrode |
| US9701177B2 (en) | 2009-04-02 | 2017-07-11 | Henkel Ag & Co. Kgaa | Ceramic coated automotive heat exchanger components |
| JP5434278B2 (en) | 2009-05-29 | 2014-03-05 | Tdk株式会社 | Active material and electrode manufacturing method, active material and electrode |
| US20100316881A1 (en) * | 2009-06-16 | 2010-12-16 | Kaylo Alan J | Method of reducing mapping of an electrodepositable coating layer |
| WO2011002040A1 (en) * | 2009-07-02 | 2011-01-06 | 日本パーカライジング株式会社 | Chromium- and fluorine-free chemical conversion treatment solution for metal surfaces, metal surface treatment method, and metal surface coating method |
| CN101603174B (en) * | 2009-07-28 | 2010-12-08 | 武汉钢铁(集团)公司 | Non-chromium pretreating agent for color coated steel plate |
| JP4920800B2 (en) * | 2010-03-23 | 2012-04-18 | 新日本製鐵株式会社 | Manufacturing method of steel plate for containers |
| TW201139736A (en) * | 2010-05-13 | 2011-11-16 | Zen Material Technologies Inc | Color whitening processing method for stainless steel surface and processing liquid used thereby |
| JP5861249B2 (en) * | 2010-09-15 | 2016-02-16 | Jfeスチール株式会社 | Manufacturing method of steel plate for containers |
| PL2673394T3 (en) * | 2011-02-08 | 2020-05-18 | Henkel Ag & Co. Kgaa | Processes and compositions for improving corrosion performance of zirconium oxide pretreated zinc surfaces |
| JP5892619B2 (en) * | 2011-03-25 | 2016-03-23 | 日本ペイント・サーフケミカルズ株式会社 | Surface treatment agent composition, method for producing surface-treated steel sheet, surface-treated steel sheet, organic-coated surface-treated steel sheet, can lid, can body and seamless can |
| JP5894576B2 (en) | 2011-03-25 | 2016-03-30 | 日本ペイント・サーフケミカルズ株式会社 | Surface treatment composition for tin-plated steel and surface-treated tin-plated steel |
| JP5750013B2 (en) * | 2011-09-07 | 2015-07-15 | 日本ペイント株式会社 | Electrodeposition coating composition and method for forming an electrocoating film on an object to be coated |
| JP6055263B2 (en) * | 2011-10-14 | 2016-12-27 | 日本ペイント・サーフケミカルズ株式会社 | Manufacturing method of automobile parts |
| CA2857436C (en) * | 2011-11-30 | 2015-02-24 | Nihon Parkerizing Co., Ltd. | Replenisher and method for producing surface-treated steel sheet |
| WO2013089292A1 (en) * | 2011-12-15 | 2013-06-20 | 대영엔지니어링 주식회사 | Electro-deposition coating method for magnesium steel material |
| TR201910649T4 (en) | 2013-09-25 | 2019-08-21 | Toyo Kohan Co Ltd | SURFACE TREATED STEEL SHEET PRODUCTION METHOD |
| CN103526250A (en) * | 2013-09-27 | 2014-01-22 | 宁波金恒机械制造有限公司 | Cast iron surface treatment agent and treatment method |
| CN103540918A (en) * | 2013-09-27 | 2014-01-29 | 宁波金恒机械制造有限公司 | Cast iron surface anti-corrosion treatment agent |
| CN103540919A (en) * | 2013-09-27 | 2014-01-29 | 宁波金恒机械制造有限公司 | Cast iron surface anti-corrosion treatment agent and cast iron surface anti-corrosion treatment method |
| JP6220226B2 (en) | 2013-10-31 | 2017-10-25 | 東洋鋼鈑株式会社 | Method for producing surface-treated steel sheet, surface-treated steel sheet, and organic resin-coated metal container |
| JP6530885B2 (en) | 2013-12-18 | 2019-06-12 | 東洋製罐株式会社 | Surface-treated steel sheet, organic resin-coated metal container, and method for producing surface-treated steel sheet |
| JP5886919B1 (en) | 2014-09-12 | 2016-03-16 | 東洋製罐株式会社 | Surface-treated steel sheet, method for producing the same, and resin-coated surface-treated steel sheet |
| CN104532224A (en) * | 2014-12-15 | 2015-04-22 | 镁联科技(芜湖)有限公司 | Chromium-free aluminum alloy passivator, preparation method thereof and aluminum alloy passivating method |
| CN104532225A (en) * | 2014-12-15 | 2015-04-22 | 镁联科技(芜湖)有限公司 | Aluminum alloy passivator, preparation method thereof and aluminum alloy passivating method |
| CN104532221B (en) * | 2014-12-15 | 2017-10-17 | 镁联科技(芜湖)有限公司 | Passivating method without Cr-Al alloy passivator and preparation method thereof He aluminium alloy |
| JP6495702B2 (en) * | 2015-03-19 | 2019-04-03 | 株式会社神戸製鋼所 | Surface treatment method and surface treatment apparatus |
| WO2018039462A1 (en) | 2016-08-24 | 2018-03-01 | Ppg Industries Ohio, Inc. | Alkaline composition for treating metal substartes |
| EP3715504B1 (en) * | 2017-11-24 | 2022-06-22 | Nippon Steel Corporation | Method for producing conversion-treated alloy material and device for regenerating conversion treatment solution used in method for producing conversion-treated alloy material |
| EP3749797A1 (en) * | 2018-02-09 | 2020-12-16 | PPG Industries Ohio Inc. | System for treating a metal substrate |
| CN108796578A (en) * | 2018-05-30 | 2018-11-13 | 江苏寅凯照明科技有限公司 | A kind of formula and technique substituting aluminium alloy light pole anodic oxidation |
| CN110424039B (en) * | 2019-09-16 | 2021-08-10 | 东北大学 | Preparation method of magnesium alloy corrosion-resistant self-repairing micro-arc oxidation coating based on hydrotalcite nano corrosion-inhibiting microcapsule |
| TW202134364A (en) * | 2020-01-31 | 2021-09-16 | 美商恩特葛瑞斯股份有限公司 | Cmp composition for polishing hard materials |
| KR102176895B1 (en) | 2020-07-28 | 2020-11-10 | 주식회사 성진케미칼 | Chemical composition for forming metal oxide layer wiht excellent paint adhesion on metal surface and using method thereof |
| JP7438078B2 (en) | 2020-10-20 | 2024-02-26 | 日本ペイント・サーフケミカルズ株式会社 | Water-based coating agent for steel materials, film, coating method for steel materials, and steel materials |
| CN112899664B (en) * | 2021-01-27 | 2022-08-02 | 太原科技大学 | Magnesium alloy surface zirconia-based film and preparation method thereof |
| CN113046813B (en) * | 2021-02-19 | 2022-04-19 | 赣州有色冶金研究所有限公司 | Magnesium alloy material, preparation method and welding method thereof |
| KR20230052550A (en) | 2021-10-13 | 2023-04-20 | 주식회사 성진케미칼 | Chemical composition for forming metal oxide layer wiht excellent paint adhesion on metal surface and using method thereof |
| TWI812437B (en) * | 2022-08-30 | 2023-08-11 | 中國鋼鐵股份有限公司 | Method for rapidly evaluating surface discoloration of hot-dip galvanized steel |
| CN116791072B (en) * | 2023-08-14 | 2024-02-23 | 广东宏泰节能环保工程有限公司 | Metal surface treatment passivating agent and preparation method and application thereof |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4338140A (en) * | 1978-02-21 | 1982-07-06 | Hooker Chemicals & Plastics Corp. | Coating composition and method |
| US4457790A (en) * | 1983-05-09 | 1984-07-03 | Parker Chemical Company | Treatment of metal with group IV B metal ion and derivative of polyalkenylphenol |
| GB2165165A (en) * | 1984-10-09 | 1986-04-09 | Parker Chemical Co | Coating process for aluminium |
| EP0337075A2 (en) * | 1988-02-15 | 1989-10-18 | Nippon Paint Co., Ltd. | Surface treatment composition and surface treatment bath for aluminium and aluminium alloys |
| EP0411606A2 (en) * | 1989-08-01 | 1991-02-06 | Nippon Paint Co., Ltd. | Surface treatment chemicals and bath for aluminum or its alloy and surface treatment method |
| EP0411609A2 (en) * | 1989-08-01 | 1991-02-06 | Nippon Paint Co., Ltd. | Surface treatment chemicals and bath for aluminum or its alloy and surface treatment method |
| US5380374A (en) * | 1993-10-15 | 1995-01-10 | Circle-Prosco, Inc. | Conversion coatings for metal surfaces |
| EP0774535A1 (en) * | 1995-11-20 | 1997-05-21 | Nippon Paint Co., Ltd. | Surface treatment composition, surface treatment solution and surface treatment method for aluminium and its alloys |
| WO2002103080A1 (en) * | 2001-06-15 | 2002-12-27 | Nihon Parkerizing Co., Ltd. | Treating solution for surface treatment of metal and surface treatment method |
| US20030196728A1 (en) * | 2002-04-23 | 2003-10-23 | Satoshi Nishimura | Nonchromate metallic surface-treating agent, nonchromate metallic surface-treating method, and aluminum or aluminum alloy |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1586975A (en) * | 1976-07-05 | 1981-03-25 | Kansai Paint Co Ltd | Surface treatment of metals |
| US4313769A (en) * | 1980-07-03 | 1982-02-02 | Amchem Products, Inc. | Coating solution for metal surfaces |
| US4273592A (en) * | 1979-12-26 | 1981-06-16 | Amchem Products, Inc. | Coating solution for metal surfaces |
| CA2113453C (en) * | 1991-08-30 | 2003-04-29 | Shawn E. Dolan | Process for treating metal with aqueous acidic composition that is substantially free from chromium (vi) |
| US5143562A (en) * | 1991-11-01 | 1992-09-01 | Henkel Corporation | Broadly applicable phosphate conversion coating composition and process |
| JP3105322B2 (en) * | 1991-12-27 | 2000-10-30 | 日産自動車株式会社 | Method for forming colorless chromate film on glittering aluminum wheels |
| DE4317217A1 (en) * | 1993-05-24 | 1994-12-01 | Henkel Kgaa | Chrome-free conversion treatment of aluminum |
| DE4401566A1 (en) * | 1994-01-20 | 1995-07-27 | Henkel Kgaa | Process for the common pretreatment of steel, galvanized steel, magnesium and aluminum before joining with rubber |
| US6059896A (en) * | 1995-07-21 | 2000-05-09 | Henkel Corporation | Composition and process for treating the surface of aluminiferous metals |
| JPH0982495A (en) * | 1995-09-18 | 1997-03-28 | Toshiba Corp | Plasma producing device and method |
| US5935348A (en) * | 1995-11-14 | 1999-08-10 | Henkel Kommanditgesellschaft Auf Aktien | Composition and process for preventing corrosion and reducing friction on metallic surfaces |
| US6361833B1 (en) * | 1998-10-28 | 2002-03-26 | Henkel Corporation | Composition and process for treating metal surfaces |
| DE19857799A1 (en) * | 1998-12-15 | 2000-06-21 | Henkel Kgaa | Method of controlling a treatment line |
| JP4099307B2 (en) * | 2000-04-20 | 2008-06-11 | 日本ペイント株式会社 | Non-chromium anti-rust treatment agent for aluminum, anti-rust treatment method and anti-rust treated aluminum products |
| WO2002024344A2 (en) * | 2000-09-25 | 2002-03-28 | Chemetall Gmbh | Method for pretreating and coating metal surfaces, prior to forming, with a paint-like coating and use of substrates so coated |
| JP3820165B2 (en) * | 2002-03-04 | 2006-09-13 | 日本ペイント株式会社 | Metal surface treatment composition |
| JP4205939B2 (en) * | 2002-12-13 | 2009-01-07 | 日本パーカライジング株式会社 | Metal surface treatment method |
-
2002
- 2002-12-13 JP JP2002362640A patent/JP4205939B2/en not_active Expired - Lifetime
-
2003
- 2003-12-03 TW TW092133808A patent/TW200416300A/en unknown
- 2003-12-11 MX MXPA05006156A patent/MXPA05006156A/en active IP Right Grant
- 2003-12-11 CN CN2008101849496A patent/CN101487115B/en not_active Expired - Lifetime
- 2003-12-11 ES ES03780727T patent/ES2730576T3/en not_active Expired - Lifetime
- 2003-12-11 CA CA002509772A patent/CA2509772A1/en not_active Abandoned
- 2003-12-11 KR KR1020057010530A patent/KR100674778B1/en active IP Right Grant
- 2003-12-11 WO PCT/JP2003/015868 patent/WO2004055237A1/en active IP Right Grant
- 2003-12-11 CN CNB2003801059479A patent/CN100537845C/en not_active Expired - Lifetime
- 2003-12-11 AU AU2003289323A patent/AU2003289323A1/en not_active Abandoned
- 2003-12-11 EP EP03780727.8A patent/EP1571237B1/en not_active Expired - Lifetime
- 2003-12-11 US US10/537,329 patent/US20060185769A1/en not_active Abandoned
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4338140A (en) * | 1978-02-21 | 1982-07-06 | Hooker Chemicals & Plastics Corp. | Coating composition and method |
| US4457790A (en) * | 1983-05-09 | 1984-07-03 | Parker Chemical Company | Treatment of metal with group IV B metal ion and derivative of polyalkenylphenol |
| GB2165165A (en) * | 1984-10-09 | 1986-04-09 | Parker Chemical Co | Coating process for aluminium |
| EP0337075A2 (en) * | 1988-02-15 | 1989-10-18 | Nippon Paint Co., Ltd. | Surface treatment composition and surface treatment bath for aluminium and aluminium alloys |
| EP0411606A2 (en) * | 1989-08-01 | 1991-02-06 | Nippon Paint Co., Ltd. | Surface treatment chemicals and bath for aluminum or its alloy and surface treatment method |
| EP0411609A2 (en) * | 1989-08-01 | 1991-02-06 | Nippon Paint Co., Ltd. | Surface treatment chemicals and bath for aluminum or its alloy and surface treatment method |
| US5380374A (en) * | 1993-10-15 | 1995-01-10 | Circle-Prosco, Inc. | Conversion coatings for metal surfaces |
| EP0774535A1 (en) * | 1995-11-20 | 1997-05-21 | Nippon Paint Co., Ltd. | Surface treatment composition, surface treatment solution and surface treatment method for aluminium and its alloys |
| WO2002103080A1 (en) * | 2001-06-15 | 2002-12-27 | Nihon Parkerizing Co., Ltd. | Treating solution for surface treatment of metal and surface treatment method |
| US20030196728A1 (en) * | 2002-04-23 | 2003-10-23 | Satoshi Nishimura | Nonchromate metallic surface-treating agent, nonchromate metallic surface-treating method, and aluminum or aluminum alloy |
Non-Patent Citations (1)
| Title |
|---|
| See also references of WO2004055237A1 * |
Cited By (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1612294A1 (en) * | 2004-07-02 | 2006-01-04 | Italtecno S.R.L. | Bath and associated method for the conversion coating of articles made of aluminium and alloys thereof |
| US8475883B2 (en) | 2005-05-23 | 2013-07-02 | Basf Coatings Gmbh | Corrosion-protection agent forming a layer of paint and method for current-free application thereof |
| DE102005059314B4 (en) | 2005-12-09 | 2018-11-22 | Henkel Ag & Co. Kgaa | Acid, chromium-free aqueous solution, its concentrate, and a process for the corrosion protection treatment of metal surfaces |
| EP2767615A1 (en) | 2005-12-09 | 2014-08-20 | Henkel AG&Co. KGAA | Wet-on-wet method and chromium free acidic solution for the corrosion treatment of steel surfaces |
| WO2008029926A1 (en) | 2006-09-08 | 2008-03-13 | Nippon Paint Co., Ltd. | Method of treating surface of metal base, metallic material treated by the surface treatment method, and method of coating the metallic material |
| EP2067882A1 (en) * | 2006-09-08 | 2009-06-10 | Nippon Paint Co., Ltd. | Method of treating surface of metal base, metallic material treated by the surface treatment method, and method of coating the metallic material |
| EP2067882A4 (en) * | 2006-09-08 | 2010-12-29 | Nippon Paint Co Ltd | Method of treating surface of metal base, metallic material treated by the surface treatment method, and method of coating the metallic material |
| US8399061B2 (en) | 2006-11-13 | 2013-03-19 | Basf Coatings Gmbh | Anti-corrosion agent forming a coating film with good adhesion and method for nongalvanic application thereof |
| WO2008110195A1 (en) * | 2007-03-15 | 2008-09-18 | Basf Coatings Ag | Method for the anti-corrosive treatment of metal substrates |
| US8652270B2 (en) | 2007-09-28 | 2014-02-18 | Ppg Industries Ohio, Inc. | Methods for treating a ferrous metal substrate |
| US8097093B2 (en) | 2007-09-28 | 2012-01-17 | Ppg Industries Ohio, Inc | Methods for treating a ferrous metal substrate |
| US9428410B2 (en) | 2007-09-28 | 2016-08-30 | Ppg Industries Ohio, Inc. | Methods for treating a ferrous metal substrate |
| WO2009045872A1 (en) * | 2007-09-28 | 2009-04-09 | Ppg Industries Ohio, Inc. | Methods for treating a ferrous metal substrate |
| US10422042B2 (en) | 2008-03-17 | 2019-09-24 | Henkel Ag & Co. Kgaa | Metal treatment coating compositions, methods of treating metals therewith and coated metals prepared using the same |
| DE102008014465A1 (en) | 2008-03-17 | 2009-09-24 | Henkel Ag & Co. Kgaa | Optimized passivation on Ti / Zr-BAsis for metal surfaces |
| US8815021B2 (en) | 2008-03-17 | 2014-08-26 | Henkel Ag & Co. Kgaa | Optimized passivation on Ti/Zr-basis for metal surfaces |
| EP2265741A4 (en) * | 2008-03-17 | 2014-10-08 | Henkel Ag & Co Kgaa | Metal treatment coating compositions, methods of treating metals therewith and coated metals prepared using the same |
| WO2009117397A1 (en) | 2008-03-17 | 2009-09-24 | Henkel Corporation | Metal treatment coating compositions, methods of treating metals therewith and coated metals prepared using the same |
| US10137476B2 (en) | 2009-02-05 | 2018-11-27 | Basf Coatings Gmbh | Coating agent for corrosion-resistant coatings |
| EP2459770B1 (en) | 2009-07-27 | 2015-05-13 | Henkel AG & Co. KGaA | Multi-stage method for the treatment of metal surfaces prior to electrodeposition |
| FR2948690A1 (en) * | 2009-07-30 | 2011-02-04 | Snecma | PIECE COMPRISING A SUBSTRATE CARRYING A CERAMIC COATING LAYER |
| US11131027B2 (en) | 2009-12-28 | 2021-09-28 | Henkel Ag & Co. Kgaa | Metal pretreatment composition containing zirconium, copper, zinc and nitrate and related coatings on metal substrates |
| US9970115B2 (en) | 2009-12-28 | 2018-05-15 | Henkel Ag & Co. Kgaa | Metal pretreatment composition containing zirconium, copper, zinc, and nitrate and related coatings on metal substrates |
| EP2578727A4 (en) * | 2010-05-28 | 2016-07-20 | Toyo Seikan Kaisha Ltd | Surface treatment bath, method of manufacturing surface-treated steel plate using said surface treatment bath, and surface-treated steel plate formed with said manufacturing method |
| US10920324B2 (en) | 2012-08-29 | 2021-02-16 | Ppg Industries Ohio, Inc. | Zirconium pretreatment compositions containing molybdenum, associated methods for treating metal substrates, and related coated metal substrates |
| US10400337B2 (en) | 2012-08-29 | 2019-09-03 | Ppg Industries Ohio, Inc. | Zirconium pretreatment compositions containing lithium, associated methods for treating metal substrates, and related coated metal substrates |
| US10125424B2 (en) | 2012-08-29 | 2018-11-13 | Ppg Industries Ohio, Inc. | Zirconium pretreatment compositions containing molybdenum, associated methods for treating metal substrates, and related coated metal substrates |
| US9273399B2 (en) | 2013-03-15 | 2016-03-01 | Ppg Industries Ohio, Inc. | Pretreatment compositions and methods for coating a battery electrode |
| EP2862957A1 (en) | 2013-10-16 | 2015-04-22 | Coatings Foreign IP Co. LLC | Process for producing a multilayer coating |
| EP2876186A1 (en) * | 2013-11-21 | 2015-05-27 | Samsung Electronics Co., Ltd | Composition for forming film of metal object, film formed using the same, and method of forming film |
| US9631281B2 (en) | 2014-12-04 | 2017-04-25 | Axalta Coating Systems Ip Co., Llc | Processes for producing a multilayer coating |
| WO2016091703A1 (en) * | 2014-12-09 | 2016-06-16 | Henkel Ag & Co. Kgaa | Integration of light metals into steel pickling and pretreating processes |
| CN107002245A (en) * | 2014-12-12 | 2017-08-01 | 汉高股份有限及两合公司 | The process control of optimization in the corrosion-resistant metal pretreatment of tank liquor based on fluoride |
| CN107002245B (en) * | 2014-12-12 | 2019-04-09 | 汉高股份有限及两合公司 | The process control of optimization in the corrosion-resistant metal pretreatment of tank liquor based on fluoride |
| US10458022B2 (en) | 2014-12-12 | 2019-10-29 | Henkel Ag & Co. Kgaa | Optimized process control in the anti-corrosive metal pretreatment based on fluoride-containing baths |
| TWI678434B (en) * | 2014-12-12 | 2019-12-01 | 德商漢高股份有限及兩合公司 | Method for a serial corrosion protection treatment of components having metallic surfaces of zinic and/or iron |
| WO2016091713A1 (en) | 2014-12-12 | 2016-06-16 | Henkel Ag & Co. Kgaa | Optimised operation in anti-corrosion pretreatment of metal using fluoride baths |
| EP3031951A1 (en) | 2014-12-12 | 2016-06-15 | Henkel AG & Co. KGaA | Optimized process control in the pretreatment of metals to protect against corrosion on the basis of baths containing fluoride |
| WO2020167758A1 (en) * | 2019-02-11 | 2020-08-20 | Ppg Industries Ohio, Inc. | Systems for treating a metal substrate |
| CN113631759A (en) * | 2019-02-11 | 2021-11-09 | Ppg工业俄亥俄公司 | System for treating metal substrates |
| CN113631759B (en) * | 2019-02-11 | 2023-10-20 | Ppg工业俄亥俄公司 | System for processing metal substrates |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2509772A1 (en) | 2004-07-01 |
| CN100537845C (en) | 2009-09-09 |
| US20060185769A1 (en) | 2006-08-24 |
| CN1726304A (en) | 2006-01-25 |
| KR100674778B1 (en) | 2007-01-25 |
| EP1571237A4 (en) | 2007-11-21 |
| TW200416300A (en) | 2004-09-01 |
| JP4205939B2 (en) | 2009-01-07 |
| WO2004055237A1 (en) | 2004-07-01 |
| MXPA05006156A (en) | 2005-12-05 |
| EP1571237B1 (en) | 2019-03-20 |
| ES2730576T3 (en) | 2019-11-12 |
| AU2003289323A8 (en) | 2004-07-09 |
| CN101487115B (en) | 2013-05-22 |
| JP2004190121A (en) | 2004-07-08 |
| CN101487115A (en) | 2009-07-22 |
| KR20050097916A (en) | 2005-10-10 |
| AU2003289323A1 (en) | 2004-07-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1571237B1 (en) | Treating fluid for surface treatment of metal and method for surface treatment | |
| KR100839744B1 (en) | Treating solution for metal surface treatment and a method for surface treatment | |
| JP4402991B2 (en) | Metal surface treatment composition, metal surface treatment liquid, metal surface treatment method and metal material | |
| EP1489198B1 (en) | Treating liquid for surface treatment of aluminum or magnesium based metal and method of surface treatment | |
| JP4242827B2 (en) | Metal surface treatment composition, surface treatment liquid, surface treatment method, and surface-treated metal material | |
| JP5215043B2 (en) | Metal surface treatment liquid and surface treatment method | |
| EP1859930B1 (en) | Surface-treated metallic material | |
| US7575644B2 (en) | Solution for treating metal surface, surface treating method, and surface treated material | |
| MX2007011230A (en) | Surface-treated metallic material. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20050602 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK |
|
| DAX | Request for extension of the european patent (deleted) | ||
| A4 | Supplementary search report drawn up and despatched |
Effective date: 20071022 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C25D 11/00 20060101ALI20071016BHEP Ipc: C23C 22/83 20060101ALI20071016BHEP Ipc: C23C 22/44 20060101AFI20071016BHEP |
|
| 17Q | First examination report despatched |
Effective date: 20101207 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: TOYOTA JIDOSHA KABUSHIKI KAISHA Owner name: NIHON PARKERIZING CO., LTD. |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: NIHON PARKERIZING CO., LTD. Owner name: TOYOTA JIDOSHA KABUSHIKI KAISHA |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20181019 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C23C 22/83 20060101ALI20071016BHEP Ipc: C25D 11/00 20060101ALI20071016BHEP Ipc: C23C 22/44 20060101AFI20071016BHEP |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R081 Ref document number: 60351884 Country of ref document: DE Owner name: TOYOTA JIDOSHA KABUSHIKI KAISHA, TOYOTA-SHI, JP Free format text: FORMER OWNERS: NIHON PARKERIZING CO., LTD., TOKIO/TOKYO, JP; TOYOTA JIDOSHA KABUSHIKI KAISHA, TOYOTA-SHI, AICHI-KEN, JP; DAIHATSU MOTOR CO., LTD., IKEDA, OSAKA, JP Ref country code: DE Ref legal event code: R081 Ref document number: 60351884 Country of ref document: DE Owner name: NIHON PARKERIZING CO., LTD., JP Free format text: FORMER OWNERS: NIHON PARKERIZING CO., LTD., TOKIO/TOKYO, JP; TOYOTA JIDOSHA KABUSHIKI KAISHA, TOYOTA-SHI, AICHI-KEN, JP; DAIHATSU MOTOR CO., LTD., IKEDA, OSAKA, JP |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 60351884 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1110617 Country of ref document: AT Kind code of ref document: T Effective date: 20190415 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20190320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190621 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190620 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1110617 Country of ref document: AT Kind code of ref document: T Effective date: 20190320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190720 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2730576 Country of ref document: ES Kind code of ref document: T3 Effective date: 20191112 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 60351884 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 |
|
| 26N | No opposition filed |
Effective date: 20200102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20191231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191211 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191211 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191231 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191231 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190320 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20031211 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20221027 Year of fee payment: 20 Ref country code: FR Payment date: 20221110 Year of fee payment: 20 Ref country code: DE Payment date: 20221102 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20230109 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 60351884 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20231227 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20231210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20231210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20231212 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20231210 Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20231212 |