EP1178106A1 - Aqueous cleaning agent concentrates for rough preferably profiled tiles and plates - Google Patents
Aqueous cleaning agent concentrates for rough preferably profiled tiles and plates Download PDFInfo
- Publication number
- EP1178106A1 EP1178106A1 EP01117825A EP01117825A EP1178106A1 EP 1178106 A1 EP1178106 A1 EP 1178106A1 EP 01117825 A EP01117825 A EP 01117825A EP 01117825 A EP01117825 A EP 01117825A EP 1178106 A1 EP1178106 A1 EP 1178106A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cleaning
- cleaning agent
- weight
- aqueous cleaning
- parts
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000012141 concentrate Substances 0.000 title claims abstract description 15
- 239000012459 cleaning agent Substances 0.000 title claims description 17
- 238000004140 cleaning Methods 0.000 claims abstract description 35
- -1 amine compounds Chemical class 0.000 claims abstract description 21
- 239000000654 additive Substances 0.000 claims abstract description 9
- 125000000129 anionic group Chemical group 0.000 claims abstract description 9
- 239000003623 enhancer Substances 0.000 claims abstract description 9
- 239000002280 amphoteric surfactant Substances 0.000 claims abstract description 8
- 239000003945 anionic surfactant Substances 0.000 claims abstract description 7
- 239000002736 nonionic surfactant Substances 0.000 claims abstract description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 17
- 229910052572 stoneware Inorganic materials 0.000 claims description 16
- 229910052573 porcelain Inorganic materials 0.000 claims description 12
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 9
- 239000000194 fatty acid Substances 0.000 claims description 9
- 229930195729 fatty acid Natural products 0.000 claims description 9
- 150000004665 fatty acids Chemical class 0.000 claims description 8
- 239000000203 mixture Substances 0.000 claims description 8
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 claims description 6
- KIWBPDUYBMNFTB-UHFFFAOYSA-N Ethyl hydrogen sulfate Chemical compound CCOS(O)(=O)=O KIWBPDUYBMNFTB-UHFFFAOYSA-N 0.000 claims description 6
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 claims description 5
- 125000004432 carbon atom Chemical group C* 0.000 claims description 5
- KPPVNWGJXFMGAM-UUILKARUSA-N (e)-2-methyl-1-(6-methyl-3,4-dihydro-2h-quinolin-1-yl)but-2-en-1-one Chemical compound CC1=CC=C2N(C(=O)C(/C)=C/C)CCCC2=C1 KPPVNWGJXFMGAM-UUILKARUSA-N 0.000 claims description 4
- QLAJNZSPVITUCQ-UHFFFAOYSA-N 1,3,2-dioxathietane 2,2-dioxide Chemical compound O=S1(=O)OCO1 QLAJNZSPVITUCQ-UHFFFAOYSA-N 0.000 claims description 4
- JZMJDSHXVKJFKW-UHFFFAOYSA-M methyl sulfate(1-) Chemical compound COS([O-])(=O)=O JZMJDSHXVKJFKW-UHFFFAOYSA-M 0.000 claims description 4
- 239000003760 tallow Substances 0.000 claims description 4
- 239000002888 zwitterionic surfactant Substances 0.000 claims description 4
- 229910019142 PO4 Inorganic materials 0.000 claims description 3
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 claims description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 3
- 239000010452 phosphate Substances 0.000 claims description 3
- 235000013162 Cocos nucifera Nutrition 0.000 claims description 2
- 244000060011 Cocos nucifera Species 0.000 claims description 2
- 239000003599 detergent Substances 0.000 abstract description 9
- 239000000243 solution Substances 0.000 description 13
- 150000001875 compounds Chemical class 0.000 description 8
- 239000000344 soap Substances 0.000 description 8
- 239000004094 surface-active agent Substances 0.000 description 8
- 230000001804 emulsifying effect Effects 0.000 description 7
- BAVYZALUXZFZLV-UHFFFAOYSA-O Methylammonium ion Chemical compound [NH3+]C BAVYZALUXZFZLV-UHFFFAOYSA-O 0.000 description 6
- 239000000839 emulsion Substances 0.000 description 6
- 239000003921 oil Substances 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- 235000019198 oils Nutrition 0.000 description 5
- 238000003892 spreading Methods 0.000 description 5
- ZPFAVCIQZKRBGF-UHFFFAOYSA-N 1,3,2-dioxathiolane 2,2-dioxide Chemical compound O=S1(=O)OCCO1 ZPFAVCIQZKRBGF-UHFFFAOYSA-N 0.000 description 4
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 4
- 235000011941 Tilia x europaea Nutrition 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- 239000004571 lime Substances 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- 239000012085 test solution Substances 0.000 description 4
- BCKXLBQYZLBQEK-KVVVOXFISA-M Sodium oleate Chemical compound [Na+].CCCCCCCC\C=C/CCCCCCCC([O-])=O BCKXLBQYZLBQEK-KVVVOXFISA-M 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- NQMRYBIKMRVZLB-UHFFFAOYSA-N methylamine hydrochloride Chemical compound [Cl-].[NH3+]C NQMRYBIKMRVZLB-UHFFFAOYSA-N 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 2
- 239000003082 abrasive agent Substances 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 239000007844 bleaching agent Substances 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 239000008139 complexing agent Substances 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 238000006073 displacement reaction Methods 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 230000003670 easy-to-clean Effects 0.000 description 2
- 150000002191 fatty alcohols Chemical class 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- JZMJDSHXVKJFKW-UHFFFAOYSA-N methyl sulfate Chemical compound COS(O)(=O)=O JZMJDSHXVKJFKW-UHFFFAOYSA-N 0.000 description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-M oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC([O-])=O ZQPPMHVWECSIRJ-KTKRTIGZSA-M 0.000 description 2
- 229940049964 oleate Drugs 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- VJWGHGJYLCJIEK-UHFFFAOYSA-N 1,4-bis(6-methylheptoxy)-1,4-dioxobutane-2-sulfonic acid Chemical compound CC(C)CCCCCOC(=O)CC(S(O)(=O)=O)C(=O)OCCCCCC(C)C VJWGHGJYLCJIEK-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 241001136792 Alle Species 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 206010017577 Gait disturbance Diseases 0.000 description 1
- RVRHBLSINNOLPI-UHFFFAOYSA-N Lythridin Natural products COc1ccc(cc1OC)C2CC(CC3CCCCN23)OC(=O)CC(O)c4ccc(O)cc4 RVRHBLSINNOLPI-UHFFFAOYSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 229920001410 Microfiber Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-N Propionic acid Chemical class CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 1
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- ULUAUXLGCMPNKK-UHFFFAOYSA-N Sulfobutanedioic acid Chemical class OC(=O)CC(C(O)=O)S(O)(=O)=O ULUAUXLGCMPNKK-UHFFFAOYSA-N 0.000 description 1
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 1
- WFZSPNBFCBYLJU-UHFFFAOYSA-L [Na+].[Na+].[O-]C(=O)CC(=N)C([O-])=O Chemical compound [Na+].[Na+].[O-]C(=O)CC(=N)C([O-])=O WFZSPNBFCBYLJU-UHFFFAOYSA-L 0.000 description 1
- 238000005299 abrasion Methods 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 150000001449 anionic compounds Chemical class 0.000 description 1
- 239000003637 basic solution Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000000536 complexating effect Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- LTNZEXKYNRNOGT-UHFFFAOYSA-N dequalinium chloride Chemical compound [Cl-].[Cl-].C1=CC=C2[N+](CCCCCCCCCC[N+]3=C4C=CC=CC4=C(N)C=C3C)=C(C)C=C(N)C2=C1 LTNZEXKYNRNOGT-UHFFFAOYSA-N 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- DUYCTCQXNHFCSJ-UHFFFAOYSA-N dtpmp Chemical compound OP(=O)(O)CN(CP(O)(O)=O)CCN(CP(O)(=O)O)CCN(CP(O)(O)=O)CP(O)(O)=O DUYCTCQXNHFCSJ-UHFFFAOYSA-N 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000003912 environmental pollution Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 238000009408 flooring Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000008233 hard water Substances 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000003658 microfiber Substances 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- DGSDBJMBHCQYGN-UHFFFAOYSA-M sodium;2-ethylhexyl sulfate Chemical compound [Na+].CCCCC(CC)COS([O-])(=O)=O DGSDBJMBHCQYGN-UHFFFAOYSA-M 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 230000003746 surface roughness Effects 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 235000019871 vegetable fat Nutrition 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000004711 α-olefin Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/65—Mixtures of anionic with cationic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/835—Mixtures of non-ionic with cationic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/86—Mixtures of anionic, cationic, and non-ionic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/88—Ampholytes; Electroneutral compounds
- C11D1/94—Mixtures with anionic, cationic or non-ionic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/62—Quaternary ammonium compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D2111/00—Cleaning compositions characterised by the objects to be cleaned; Cleaning compositions characterised by non-standard cleaning or washing processes
- C11D2111/10—Objects to be cleaned
- C11D2111/14—Hard surfaces
- C11D2111/24—Mineral surfaces, e.g. stones, frescoes, plasters, walls or concretes
Definitions
- the invention relates to aqueous cleaning agent concentrates for rough, especially profiled stoneware tiles and Non-ionic, anionic or amphoteric plates
- Surfactants which are quaternary as cleaning enhancers contain alkoxylated amine compounds.
- stoneware stands for hard-fired tiles and Plates with or without a burned-in ceramic surface coating, can be glazed or unglazed. According to DIN norms are subdivided into water absorption capacity and manufacturing method. DIN EN 176 defines stoneware tiles and porcelain stoneware tiles.
- the displacement space (V) according to DIN 51 130 is also given for profiled slabs and tiles.
- Tiles are generally considered to be easy to care for and easy to clean. This applies without restriction to level, smooth and hard substrates of R classes 9 to 11. With increasing R-Class surfaces are becoming increasingly rougher. Fine-rough substrates of class R 12 and in particular profiled tiles of the classes R 12 to 13 and V 8 to 10, so-called porcelain stoneware tiles / -plates.
- the cleaning effort increases with increasing roughness / unevenness of the subsurface considerably, especially if additional surface enlargement due to porosity given is.
- the object of the present invention was therefore to achieve this Disadvantages of the known commercial cleaning agents overcome and improved cleaning supplies available too ask which at the same or reduced application concentrations reliable cleaning of rough and profiled Ensure tiles and so-called porcelain stoneware.
- the present invention therefore relates to aqueous Detergent concentrates for rough, in particular profiled tiles based on non-ionic, anionic, amphoteric surfactants, optionally with concomitant use of usual auxiliaries and additives, which thereby are marked as cleaning enhancers contain quaternary alkoxylated amine compounds.
- Another object of the invention is the use of aqueous detergent concentrates according to one or more of claims for cleaning porcelain stoneware tiles.
- aqueous detergent concentrates according to the present Invention contain water in the range of about 45 to about 95 parts by weight, preferably about 70 to about 90 parts by weight. You can use it with additional water to the desired one or usual or required application concentration of approx. 0.5 parts by weight to approx. 10 parts by weight.
- coconut fatty acid, palm fatty acid, Tallow fatty acid which is a chain distribution from approx. 6 to approx. 20, mainly approx. 8 to approx. 18, Have carbon atoms and both saturated and unsaturated could be.
- the known methods can Double bonds are hydrogenated in whole or in part, so that the iodine numbers in the range from about 0 to about 50, especially in the Range from about 15 to about 40.
- Suitable alkoxylating agents are ethylene oxide, propylene oxide or butylene oxide alone or as copolymers with both statistical and blockwise distribution.
- These compounds are made up of cleaning formulations from mixtures of one or more surfactants from the Group of anionic, nonionic, amphoteric compounds and, if appropriate, customary auxiliaries and additives, such as alkalis, Complexing agent, solubilizer, chlorine bleach additive and mild abrasives in amounts of approx. 0.1 to 5, in particular 0.5 to 3 parts by weight are added.
- surfactants from the Group of anionic, nonionic, amphoteric compounds and, if appropriate, customary auxiliaries and additives, such as alkalis, Complexing agent, solubilizer, chlorine bleach additive and mild abrasives in amounts of approx. 0.1 to 5, in particular 0.5 to 3 parts by weight are added.
- the cationic cleaning boosters show not in the presence of anionic compounds the tendency to form insoluble complexes and fail.
- the surface-active compounds which can also be used according to the invention are the anionic, nonionic, amphoteric / zwitterionic surfactants for manufacturing of cleaning agents for household and industry.
- the compounds can be used individually or as mixtures are and are, for example, anionic, nonionic and amphoteric surfactants such as alkali, ammonium or magnesium alkyl sulfates or alkyl ether sulfates, secondary alkane sulfonates, alkali ⁇ -olefin sulfonates, Sulfosuccinates, acyl isethionates, sarcosides, Taurides, alkyl polyglucosides, ether citrates, carboxylates, Ether carboxylates, alkyl amide ether sulfates, and ethoxylates of Fatty alcohols, glycerides, oils, fatty acids but also fatty acid esters, Amine oxides, alkyl betaines, alkyl amido betaines, propionates, Glycinates, acetates and sulfobetaines and sodium, potassium or triethanolamine soap.
- composition of commercially available cleaning agents surfactants
- Trial product D (base D) Wt .-% TEGOTENS EC 11 (decyl ethoxylate, end-capped) 10 REWOPOL D 510 (sodium isoctyl sulfate) 9 Na stearate 1.2 TEGOTENS DO (decamine oxide) 4.35 triethanolamine 2.0 IDS (30%, sodium iminosuccinate) 0.9 water
- Trial product E (base E) Wt .-% REWOPOL D 510 (sodium isooctyl sulfate) 4.8
- REWOPOL SBDO 75 (diisooctyl sulfosuccinate) 0.9 Na phosphonate DTPMP 0.32 water
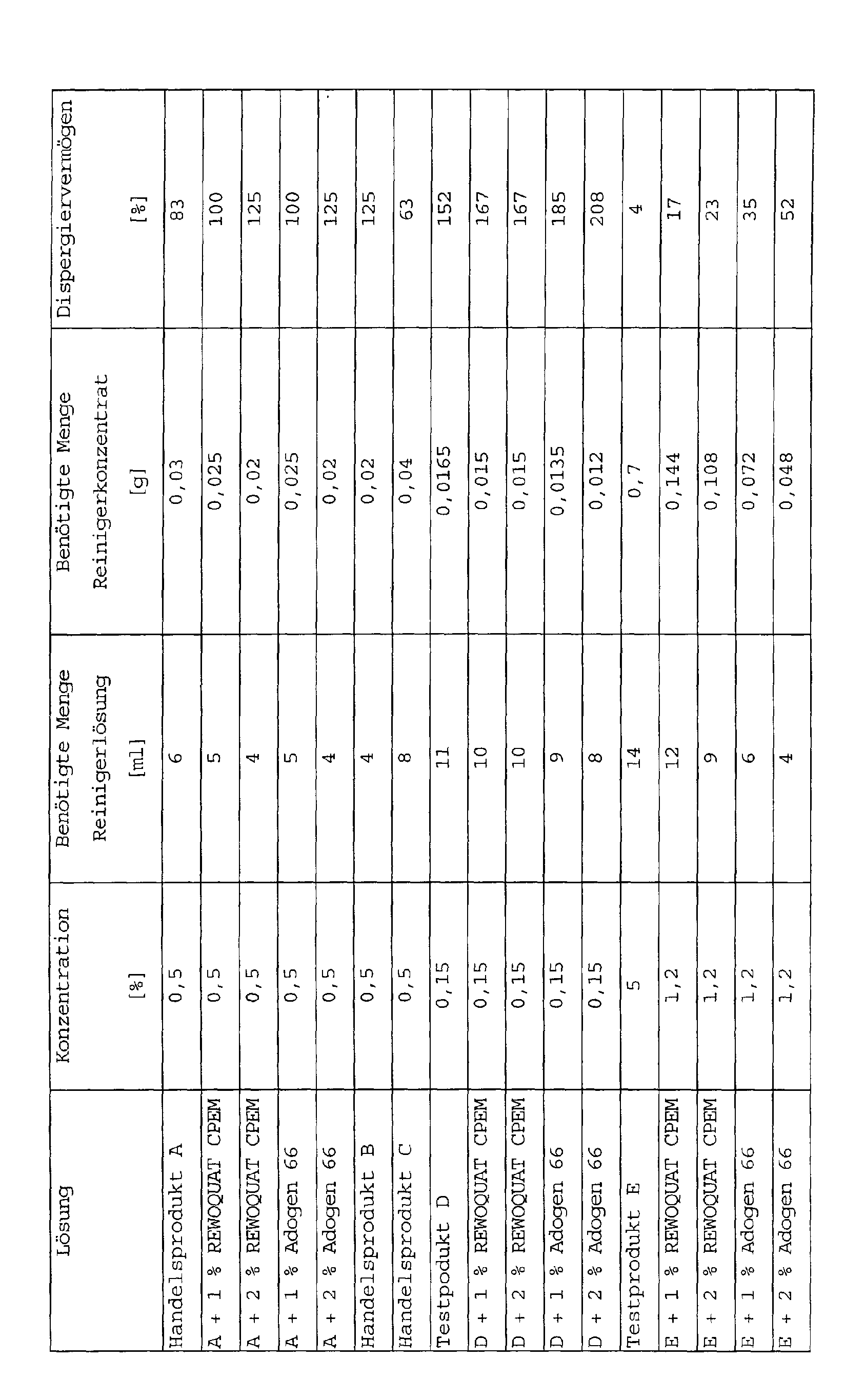
- Typical usage dilutions of floor cleaners A 3.0% Especially for professional porcelain stoneware cleaning B 0.9% Normal household all-purpose floor cleaner C 0.9% Normal household all-purpose floor cleaner Base D 0.3% Highly concentrated floor cleaner Base E 1.2% Floor cleaner with base silk
- the cleaning solution was with tap water, the city of Essen (Water hardness ⁇ 10), to the respective application concentration diluted.
- the pipette tip was used for the test (Rainin (2.5 ml) EPD 2) 0.3 mm over the tile positioned.
- the porcelain stoneware tiles were first in the dishwasher washed at 70 ° C without surfactant.
- the spreading area in length and width was determined using a slide gauge. Each solution was repeated at least five times to correct the error. The area of the drop is calculated using the formula for an ellipse.
- the properties of the basic solution are considered as blank values. After adding the cleaning booster, a comparison is made in relation to the blank value.
- Spreading properties of commercial products Diameter of the drop (0.05 ml) on porcelain stoneware tiles original + 1% REWOQUAT CPEM + 1% ADOGEN 66 A in 3.0% solution 5.2 cm 2 52.8 cm 2 32.1 cm 2 Increase [%] 1023% 622% B in 0.9% solution 55.2 cm 2 57.9 cm 2 55.7 cm 2 Increase [%] 105% 101% C in 0.9% solution 70.2 cm 2 78.7 cm 2 79.5 cm 2 Increase [%] 112% 113%
- test solution E contains only short-chain anionic surfactants, is one of such surfactant systems Weakness in emulsifying behavior known.
- the cleaning booster according to the invention the emulsifying behavior of weakly emulsifying, Short chain anionic surfactant solutions greatly optimized. If the Emulsion breaks down, water instead of oil. This settling water does not affect the cleaning power or the dirt-carrying capacity during cleaning.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Detergent Compositions (AREA)
Abstract
Description
Gegenstand der Erfindung sind wässrige Reinigungsmittelkonzentrate für raue, insbesondere profilierte Steinzeugfliesen und Platten auf Basis von nichtionischen, anionischen, oder amphoteren Tensiden, welche als Reinigungsverstärker quartäre alkoxylierte Aminverbindungen enthalten.The invention relates to aqueous cleaning agent concentrates for rough, especially profiled stoneware tiles and Non-ionic, anionic or amphoteric plates Surfactants, which are quaternary as cleaning enhancers contain alkoxylated amine compounds.
Aus optischen und hygienischen Gründen werden sowohl in gewerblich genutzten als auch in öffentlichen Gebäuden und Einkaufszentren die Arbeitsbereiche, Verkehrswege und Treppen zunehmend mit Natur- und Steinzeug-Fliesen und Platten ausgelegt.For optical and hygienic reasons, both in commercial used as well as in public buildings and shopping centers the work areas, traffic routes and stairs increasingly designed with natural and stoneware tiles.
Zur Vermeidung von Unfällen durch Stolpern und Rutschen müssen diese Fußbodenbeläge gewisse Anforderungen hinsichtlich ihrer Trittsicherheit erfüllen, so müssen sie gemäß den gesetzlichen Vorgaben (Richtlinien der Arbeitsstättenverordnung) eben, rutschhemmend und leicht zu reinigen sein.To avoid accidents caused by stumbling and sliding these floor coverings have certain requirements with regard to their Sure-footedness, they must comply with the legal Specifications (guidelines of the Workplace Ordinance), anti-slip and easy to clean.
Der Begriff Steinzeug steht für hart gebrannte Fliesen und Platten, welche mit oder ohne eingebrannter keramischer Oberflächenvergütung, glasiert oder unglasiert sein können. Gemäß DIN-Norm erfolgt eine Unterteilung über Wasseraufnahmevermögen und Fertigungsart. Die DIN EN 176 definiert Steinzeugfliesen und Feinsteinzeugfliesen.The term stoneware stands for hard-fired tiles and Plates with or without a burned-in ceramic surface coating, can be glazed or unglazed. According to DIN norms are subdivided into water absorption capacity and manufacturing method. DIN EN 176 defines stoneware tiles and porcelain stoneware tiles.
Für die Rutschsicherheit ist jedoch die Oberflächenrauhigkeit entscheidend. Daher werden in den Merkblättern der Berufsgenossenschaft (ZH 1/571) bzw. der Gemeindeunfallversicherer (GUV 26.17; 26.18) für die diversen Anwendungsbereiche genau bestimmte Rutschhemmklassen (R-Klasse) vorgegeben, wobei höhere R-Werte für eine stärkere Rutschhemmung stehen.However, the surface roughness is important for slip resistance crucial. Therefore, in the leaflets of the professional association (ZH 1/571) or the municipal accident insurer (GUV 26.17; 26.18) for the various areas of application certain anti-slip classes (R-class) are given, higher ones R values stand for greater slip resistance.
Die Bestimmung erfolgt gemäß DIN 51 130 im Versuch an der schiefen Ebene und wird in Neigungswinkelbereichen angegeben: R 9 = 3°-10° Neigungswinkel; R 10 = 10°-19° Neigungswinkel; R 11 = 19°-27° Neigungswinkel; R 12 = 27°-35° Neigungswinkel; R 13 = > 35° Neigungswinkel.The determination is made according to DIN 51 130 in an experiment on the inclined plane and is specified in inclination angle ranges: R 9 = 3 ° -10 ° inclination angle; R 10 = 10 ° -19 ° inclination angle; R 11 = 19 ° -27 ° inclination angle; R 12 = 27 ° -35 ° inclination angle; R 13 => 35 ° angle of inclination.
Zusätzlich wird für profilierte Platten und Fliesen auch der Verdrängungsraum (V) entsprechend DIN 51 130 angegeben. Der Verdrängungsraum gibt das Volumen zwischen der oberen Gehebene und der unteren Entwässerungsebene an. Er liegt zwischen V 4 (= 4 cm3/dm2) und V 10 (= 10 cm3/dm2).In addition, the displacement space (V) according to DIN 51 130 is also given for profiled slabs and tiles. The displacement space indicates the volume between the upper level and the lower drainage level. It lies between V 4 (= 4 cm 3 / dm 2 ) and V 10 (= 10 cm 3 / dm 2 ).
Für Bereiche mit erhöhtem Ausrutschrisiko, das sind Bereiche in denen Fußböden und Treppenstufen mit reibungsmindernden, und/oder gleitfördernden Medien wie Wasser, Abfallresten, stärkehaltigen Rückständen, tierischen und pflanzlichen Fetten oder Ölen, mineralischen Fetten oder Ölen, Seifen, Pigmentschmutz, Gummiabrieb, Silikonen in Kontakt kommen, sind die Bewertungsgruppen > R 10, insbesondere R 12 und R 13 angebracht.For areas with increased risk of slipping, these are areas in which floors and stairs with friction-reducing, and / or slippery media such as water, waste residues, starchy residues, animal and vegetable fats or oils, mineral fats or oils, soaps, pigment dirt, Rubber abrasion, silicones come into contact are the assessment groups > R 10, in particular R 12 and R 13 attached.
Fliesen und Platten gelten im allgemeinen als pflegeleicht und reinigungsfreundlich. Dies gilt uneingeschränkt für ebene, glatte und harte Untergründe der R-Klassen 9 bis 11. Mit steigender R-Klasse werden die Oberflächen jedoch zunehmend rauer. Problematisch gelten feinraue Untergründe der Klasse R 12 und insbesondere profilierte Fliesen und Platten der Klassen R 12 bis 13 und V 8 bis 10, sogenannte Feinsteinzeugfliesen/ -platten.Tiles are generally considered to be easy to care for and easy to clean. This applies without restriction to level, smooth and hard substrates of R classes 9 to 11. With increasing R-Class surfaces are becoming increasingly rougher. Fine-rough substrates of class R 12 and in particular profiled tiles of the classes R 12 to 13 and V 8 to 10, so-called porcelain stoneware tiles / -plates.
Der Reinigungsaufwand vergrößert sich bei zunehmender Rauhigkeit/Unebenheit des Untergrundes erheblich, insbesondere wenn aufgrund von Porösizität zusätzliche Oberflächenvergrößerung gegeben ist.The cleaning effort increases with increasing roughness / unevenness of the subsurface considerably, especially if additional surface enlargement due to porosity given is.
Es hat daher in der Vergangenheit nicht an Versuchen gemangelt, neben speziellen mechanisch wirkenden Reinigungsmaschinen auch neue Reinigungsverfahren zu entwickeln und zusätzlich durch universell anwendbaren Allzweckreiniger insbesondere genau auf den jeweiligen Bodenbelagstyp und Verschmutzungsart abgestimmte Reinigungsmittel den Reinigungsaufwand zu minimieren und den Reinigungseffekt zu optimieren.There has been no shortage of attempts in the past in addition to special mechanical cleaning machines to develop new cleaning processes and additionally through universally applicable all-purpose cleaner in particular the respective flooring type and type of soiling Cleaning agents to minimize the cleaning effort and Optimize cleaning effect.
Handelsübliche Reinigungsmittel für die manuelle und maschinelle Reinigung sind in der Regel komplexe Mischungen aus anionischen, nichtionischen und amphoteren/zwitterionischen Tensiden, welche übliche Hilfs- und Zusatzstoffe wie Alkalien, Komplexbildner, Lösungsvermittler, Chlorbleichlaugezusatz und gegebenenfalls milde Scheuermittel enthalten. Sie kommen in Konzentrationen von ca. 0,5 bis 10 Gew.-% zur Anwendung.Commercial cleaning agents for manual and machine Cleaning tend to be complex mixtures anionic, nonionic and amphoteric / zwitterionic Surfactants, which are conventional auxiliaries and additives such as alkalis, Complexing agents, solubilizers, chlorine bleach additive and possibly contain mild abrasives. You come in Concentrations of approx. 0.5 to 10% by weight are used.
In der Praxis hat sich jedoch gezeigt, dass auch bei großer mechanischer Unterstützung durch Einsatz von Mikrofaserpads, Schrubber, oder Hochdruckgeräten, Scheuersaug- oder Bürstenwalzen und zusätzlichen intensiven Nachspülgang mit Wasser bei den problematischen Untergründen aus Feinsteinzeug der Schmutz oftmals nicht vollständig im ersten Arbeitsgang entfernt werden konnte. Zusätzliche Reinigungsgänge waren erforderlich.In practice, however, it has been shown that even with large mechanical Support through the use of microfiber pads, Scrubbers, or high pressure devices, scrubber driers or brush rollers and an additional intensive rinse with water for the problematic surfaces from porcelain stoneware the dirt often cannot be completely removed in the first step could. Additional cleaning cycles were required.
Abgesehen von dem erheblichen personellen, maschinellen Mehraufwand und zusätzlicher Umweltbelastung durch Reinigungsmittel wird durch die Abrasivwirkung der mechanischen Belastung die Trittsicherung des Bodenbelages schneller gemindert.Apart from the considerable additional human, mechanical effort and additional environmental pollution from cleaning agents is the abrasive effect of the mechanical load Securing of the floor covering is reduced more quickly.
Aufgabe der vorliegenden Erfindung war es daher, diese Nachteile der bekannten handelsüblichen Reinigungsmittel zu überwinden und verbesserte Reinigungsmittel zur Verfügung zu stellen, welche bei gleichen oder verminderten Anwendungskonzentrationen eine zuverlässige Reinigung von rauen und profilierten Fliesen und Platten, sogenanntem Feinsteinzeug, gewährleisten.The object of the present invention was therefore to achieve this Disadvantages of the known commercial cleaning agents overcome and improved cleaning supplies available too ask which at the same or reduced application concentrations reliable cleaning of rough and profiled Ensure tiles and so-called porcelain stoneware.
Diese Aufgabe wird gelöst durch Reinigungsmittel auf Basis von Tensiden, welche als Reinigungsverstärker quartäre alkoxylierte Aminverbindungen enthalten.This task is solved by cleaning agents based on Surfactants which act as quaternary alkoxylated cleaning enhancers Contain amine compounds.
Gegenstand der vorliegenden Erfindung sind daher wässrige Reinigungsmittelkonzentrate für raue, insbesondere profilierte Fliesen und Platten auf Basis von nichtionischen, anionischen, amphoteren Tensiden, gegebenenfalls unter Mitverwendung von üblichen Hilfs- und Zusatzstoffen, welche dadurch gekennzeichnet sind, dass sie als Reinigungsverstärker quartäre alkoxylierte Aminverbindungen enthalten.The present invention therefore relates to aqueous Detergent concentrates for rough, in particular profiled tiles based on non-ionic, anionic, amphoteric surfactants, optionally with concomitant use of usual auxiliaries and additives, which thereby are marked as cleaning enhancers contain quaternary alkoxylated amine compounds.
Ein weiterer Gegenstand der Erfindung sind wässrige Reinigungsmittelkonzentrate,
welche, bezogen auf die Gesamtmischung,
außer Wasser im wesentlichen
- R1 =
- ein geradkettiger, gegebenenfalls verzweigter, gegebenenfalls Mehrfachbindungen enthaltender Alkylrest mit 8 bis 22, insbesondere 8 bis 18 C-Atomen,
- R2 =
- -(CH2CHR5O)n-R6 mit R5 = H, -CH3, -C2H5; R6 = H, -CH3, -C2H5 -C3H7 oder -C4H9 und n = 1 bis 25, insbesondere 2 bis 15,
- R3 =
- R1 oder R2,
- R4 =
- -CH3 oder -C2H5 und
- X- =
- anionischer Rest, insbesondere Methylsulfat, Ethylsulfat, Phosphat, Chlorid, Bromid, Jodid
- R 1 =
- a straight-chain, optionally branched, optionally containing multiple bonds alkyl radical having 8 to 22, in particular 8 to 18, carbon atoms,
- R 2 =
- - (CH 2 CHR 5 O) n -R 6 with R 5 = H, -CH 3 , -C 2 H 5 ; R 6 = H, -CH 3 , -C 2 H 5 -C 3 H 7 or -C 4 H 9 and n = 1 to 25, in particular 2 to 15,
- R 3 =
- R 1 or R 2 ,
- R 4 =
- -CH 3 or -C 2 H 5 and
- X - =
- anionic radical, in particular methyl sulfate, ethyl sulfate, phosphate, chloride, bromide, iodide
Ein weiterer Gegenstand der Erfindung ist die Verwendung der wässrigen Reinigungsmittelkonzentrate gemäß einem oder mehreren der Ansprüche zur Reinigung von Feinsteinzeugfliesen.Another object of the invention is the use of aqueous detergent concentrates according to one or more of claims for cleaning porcelain stoneware tiles.
Weitere Gegenstände der Erfindung sind gekennzeichnet durch die Ansprüche.Further objects of the invention are characterized by the Expectations.
Die wässrigen Reinigungsmittelkonzentrate gemäß der vorliegenden Erfindung enthalten, bezogen auf die Gesamtmischung, Wasser im Bereich von ca. 45 bis ca. 95 Gew.-Teile, vorzugsweise ca. 70 bis ca. 90 Gew.-Teile. Sie können im Anwendungsfall mit zusätzlichem Wasser auf die jeweils gewünschte bzw. übliche oder erforderliche Anwendungskonzentration von ca. 0,5 Gew.-Teile bis ca. 10 Gew.-Teile verdünnt werden.The aqueous detergent concentrates according to the present Invention, based on the total mixture, contain water in the range of about 45 to about 95 parts by weight, preferably about 70 to about 90 parts by weight. You can use it with additional water to the desired one or usual or required application concentration of approx. 0.5 parts by weight to approx. 10 parts by weight.
Die erfindungsgemäß mitverwendeten Reinigungsverstärker sind
alkoxylierte Aminverbindungen der allgemeinen Formel
- R1 =
- ein geradkettiger, gegebenenfalls verzweigter, gegebenenfalls Mehrfachbindungen enthaltender Alkylrest mit 8 bis 22 C-Atomen,
- R2 =
- -(CH2CHR5O)n-R6 mit R5 = H, -CH3, -C2H5; R6 = H, -CH3, -C2H5, -C3H7 oder -C4H9 und n = 1 bis 25, insbesondere 2 bis 15,
- R3 =
- R1 oder R2,
- R4 =
- -CH3 oder -C2H5 und
- X- =
- anionischer Rest, insbesondere Methylsulfat, Ethylsulfat, Phosphat, Chlorid, Bromid, Jodid.
- R 1 =
- a straight-chain, optionally branched, optionally containing multiple bonds alkyl radical having 8 to 22 carbon atoms,
- R 2 =
- - (CH 2 CHR 5 O) n -R 6 with R 5 = H, -CH 3 , -C 2 H 5 ; R 6 = H, -CH 3 , -C 2 H 5 , -C 3 H 7 or -C 4 H 9 and n = 1 to 25, in particular 2 to 15,
- R 3 =
- R 1 or R 2 ,
- R 4 =
- -CH 3 or -C 2 H 5 and
- X - =
- anionic radical, in particular methyl sulfate, ethyl sulfate, phosphate, chloride, bromide, iodide.
Erfindungsgemäß bevorzugt werden Verbindungen mit R1 auf Basis von Fettaminen, hergestellt nach den bekannten Verfahren durch Umsetzung von natürlichen Fettsäuren mit Ammoniak und anschließender Hydrierung, eingesetzt.According to the invention, preference is given to using compounds with R 1 based on fatty amines, prepared by the known processes by reacting natural fatty acids with ammonia and subsequent hydrogenation.
Als Fettsäuren kommen hier insbesondere Kokosfettsäure, Palmfettsäure, Talgfettsäure in Betracht, welche eine Kettenverteilung von ca. 6 bis ca. 20, hauptsächlich ca. 8 bis ca. 18, C-Atomen aufweisen und sowohl gesättigt als auch ungesättigt sein können. Durch die bekannten Verfahren können die Doppelbindungen ganz oder teilweise hydriert werden, so dass die Jodzahlen im Bereich von ca. 0 bis ca. 50, insbesondere im Bereich von ca. 15 bis ca. 40 liegen.In particular, coconut fatty acid, palm fatty acid, Tallow fatty acid, which is a chain distribution from approx. 6 to approx. 20, mainly approx. 8 to approx. 18, Have carbon atoms and both saturated and unsaturated could be. The known methods can Double bonds are hydrogenated in whole or in part, so that the iodine numbers in the range from about 0 to about 50, especially in the Range from about 15 to about 40.
Als Alkoxilierungsmittel kommen Ethylenoxid, Propylenoxid oder Butylenoxid allein oder als Copolymere mit sowohl statistischer als auch blockweiser Verteilung in Betracht. Der Alkoxilierungsgrad wird mitbestimmt durch die angestrebte Hydrophilie der Verbindung. Er liegt im Mittel zwischen 1 bis 25, insbesondere 2 bis 15. Im Falle R3 = R2 gilt dieser Wert für beide Reste zusammen. Erfindungsgemäß besonders bevorzugt sind ethoxilierte Verbindungen mit einem Alkoxilierungsgrad um ca. 5 bis 10.Suitable alkoxylating agents are ethylene oxide, propylene oxide or butylene oxide alone or as copolymers with both statistical and blockwise distribution. The degree of alkoxylation is determined by the desired hydrophilicity of the compound. It is on average between 1 to 25, in particular 2 to 15. In the case R 3 = R 2 , this value applies to both radicals together. According to the invention, particular preference is given to ethoxylated compounds with a degree of alkoxylation of approximately 5 to 10.
Verbindungen dieser Art sind handelsübliche Produkte und werden beispielsweise von den Firmen Goldschmidt Rewo und Goldschmidt Chemical Corporation unter den Markennamen REWOQUAT® oder ADOGEN® angeboten, wie REWOQUAT® CPEM oder ADOGEN® 66.Compounds of this type are commercially available products and are for example from the companies Goldschmidt Rewo and Goldschmidt Chemical Corporation under the brand names REWOQUAT® or ADOGEN® are offered, such as REWOQUAT® CPEM or ADOGEN® 66.
Diese Verbindungen werden Reinigungsformulierungen bestehend aus Mischungen ein oder mehrerer Tenside aus der Gruppe anionischer, nichtionischer, amphoterer Verbindungen und gegebenenfalls üblicher Hilfs- und Zusatzstoffe wie Alkalien, Komplexbildner, Lösungsvermittler, Chlorbleichlaugezusatz und milden Scheuermitteln in Mengen von ca. 0,1 bis 5, insbesondere 0,5 bis 3 Gew.-Teile zugesetzt.These compounds are made up of cleaning formulations from mixtures of one or more surfactants from the Group of anionic, nonionic, amphoteric compounds and, if appropriate, customary auxiliaries and additives, such as alkalis, Complexing agent, solubilizer, chlorine bleach additive and mild abrasives in amounts of approx. 0.1 to 5, in particular 0.5 to 3 parts by weight are added.
Überraschenderweise zeigen die kationischen Reinigungsverstärker in Anwesenheit von anionischen Verbindungen nicht die Tendenz, unlösliche Komplexe zu bilden und auszufallen.Surprisingly, the cationic cleaning boosters show not in the presence of anionic compounds the tendency to form insoluble complexes and fail.
Sie verbessern nicht die Werte eines einzelnen technischen Effektes zu Lasten der anderen Eigenschaften sondern heben insgesamt das allgemeine Niveau:
- Besseres Spreiten auf porösen Oberflächen, z.B. Feinsteinzeugfliesen: (optimiertes Benetzungsverhalten führt gerade auf porösen Oberflächen - hier entspricht die reale Oberfläche einem Vielfachen der eigentlichen Grundfläche - zu einem besseren Ablösverhalten des Schmutzes. Auch die Schattenbereiche der rutschhemmenden Erhöhungen werden ausreichend benetzt und gereinigt.)
- Besseres Kalkseifendispergiervermögen (optimiertes Dispergiervermögen: für anorganische nichtlösliche Verschmutzungen verhindert das Absetzen solcher Verschmutzungen. Dies ist besonders wichtig auf porösen Oberflächen, da diese Rückstände sonst nicht entfernt werden können).
- Besseres Emulgiervermögen für einfache kostengünstige Tensidsysteme: (optimierte Emulgierfähigkeit, da das gesamte Öl kontinuierlich in der Emulsion vorliegt. Bei dem Absetzen von Wasser kommt es nicht zu einer Wiederanschmutzung durch eine brechende Emulsion). Um die rutschhemmenden Eigenschaften nicht zu reduzieren ist es hier besonders wichtig alle Rückstände vollständig zu entfernen, damit sich die benötigten Poren nicht zusetzen.
- Better spreading on porous surfaces, e.g. porcelain stoneware tiles: (Optimized wetting behavior leads especially to porous surfaces - here the real surface corresponds to a multiple of the actual base area - to better dirt removal behavior. The shadow areas of the anti-slip ridges are also adequately wetted and cleaned.)
- Better lime soap dispersing capacity (optimized dispersing capacity: for inorganic insoluble soiling prevents such soiling from settling. This is particularly important on porous surfaces, since these residues cannot otherwise be removed).
- Better emulsifying capacity for simple, inexpensive surfactant systems: (Optimized emulsifying capacity, since all of the oil is continuously present in the emulsion. When water is deposited, there is no contamination by a breaking emulsion). In order not to reduce the anti-slip properties, it is particularly important here to completely remove all residues so that the required pores do not become clogged.
Die erfindungsgemäß mitverwendbaren oberflächenaktiven Verbindungen sind die auf diesem Gebiet üblichen anionischen, nichtionischen, amphoteren/zwitterionischen Tenside zur Herstellung von Reinigungsmitteln für Haushalt und Industrie.The surface-active compounds which can also be used according to the invention are the anionic, nonionic, amphoteric / zwitterionic surfactants for manufacturing of cleaning agents for household and industry.
Die Verbindungen können einzeln oder als Mischungen verwendet werden und sind beispielsweise anionische, nichtionische und amphotere Tenside wie Alkali-, Ammonium- oder Magnesium-Alkylsulfate bzw. Alkylethersulfate, sekundäre Alkansulfonate, Alkali-α-Olefinsulfonate, Sulfosuccinate, Acylisethionate, Sarkoside, Tauride, Alkylpolyglukoside, Ethercitrate, Carboxylate, Ethercarboxylate, Alkylamidethersulfate, sowie Ethoxilate von Fettalkoholen, Glyceriden, Ölen, Fettsäuren aber auch Fettsäureestern, Aminoxide, Alkylbetaine, Alkylamidobetaine, Propionate, Glycinate, Acetate und Sulfobetaine und Natrium-, Kalium- oder Triethanolaminseife. The compounds can be used individually or as mixtures are and are, for example, anionic, nonionic and amphoteric surfactants such as alkali, ammonium or magnesium alkyl sulfates or alkyl ether sulfates, secondary alkane sulfonates, alkali α-olefin sulfonates, Sulfosuccinates, acyl isethionates, sarcosides, Taurides, alkyl polyglucosides, ether citrates, carboxylates, Ether carboxylates, alkyl amide ether sulfates, and ethoxylates of Fatty alcohols, glycerides, oils, fatty acids but also fatty acid esters, Amine oxides, alkyl betaines, alkyl amido betaines, propionates, Glycinates, acetates and sulfobetaines and sodium, potassium or triethanolamine soap.
Die auf diesem Gebiet einsetzbaren und üblichen bzw. möglichen Verbindungen der einzelnen Gruppen gehören zum Allgemeinwissen des Fachmanns und können außerdem bei Bedarf in der einschlägigen Fachliteratur sowie in den Rezepturempfehlungen der Hersteller der jeweiligen Tensidklassen nachgelesen werden.The usual and possible to be used in this area Connections of the individual groups belong to general knowledge of the expert and can also in if necessary the relevant specialist literature and in the recipe recommendations the manufacturer of the respective surfactant classes become.
REWOQUAT® CPEM Coco Pentaethoxy Methylammonium MethosulfateTrial product 1
REWOQUAT® CPEM Coco Pentaethoxy Methylammonium Methosulfate
ADOGEN® 66 Ethyl-Bis (Polyethoxy Ethanol) Tallow Ammonium EthosulfateTrial product 2
ADOGEN® 66 Ethyl-Bis (Polyethoxy Ethanol) Tallow Ammonium Ethosulfate
Capryl Bis-Polyethoxy Methylammonium ChloridTrial product 3
Capryl bis polyethoxy methylammonium chloride
Capryl Bis-Polyethoxy Methylammonium EthosulfateTrial product 4
Capryl bis-polyethoxy methyl ammonium ethosulfate
Coco Bis-Polyethoxy Methylammonium ChloridTrial product 5
Coco Bis-Polyethoxy Methylammonium Chloride
Coco Bis-Polyethoxy Methylammonium MethosulfateTrial product 6
Coco Bis-Polyethoxy Methylammonium Methosulfate
Coco Bis-Polyethoxy Methylammonium EthosulfateTrial product 7
Coco Bis-Polyethoxy Methylammonium Ethosulfate
Coco Pentaethoxy Methylammonium EthosulfateTrial product 8
Coco Pentaethoxy Methylammonium Ethosulfate
Tallow Bis-Polyethoxy Methylammonium Methosulfate Trial product 9
Tallow Bis-Polyethoxy Methylammonium Methosulfate
REWOQUAT® CQ 100 Coco Bis-Polyethoxy Methylammonium Chlorid (and) FettalkoholethoxylateTrial product 10
REWOQUAT® CQ 100 Coco Bis-Polyethoxy Methylammonium Chloride (and) fatty alcohol ethoxylates
Güteklasse: R 13 V 10, Hersteller Villeroy & Boch AG Fliesen mit verschiedener Testoberfläche: Bright porcelain stoneware tiles Art-Nr: 2292,
Quality class: R 13 V 10, manufacturer Villeroy & Boch AG tiles with different test surfaces:
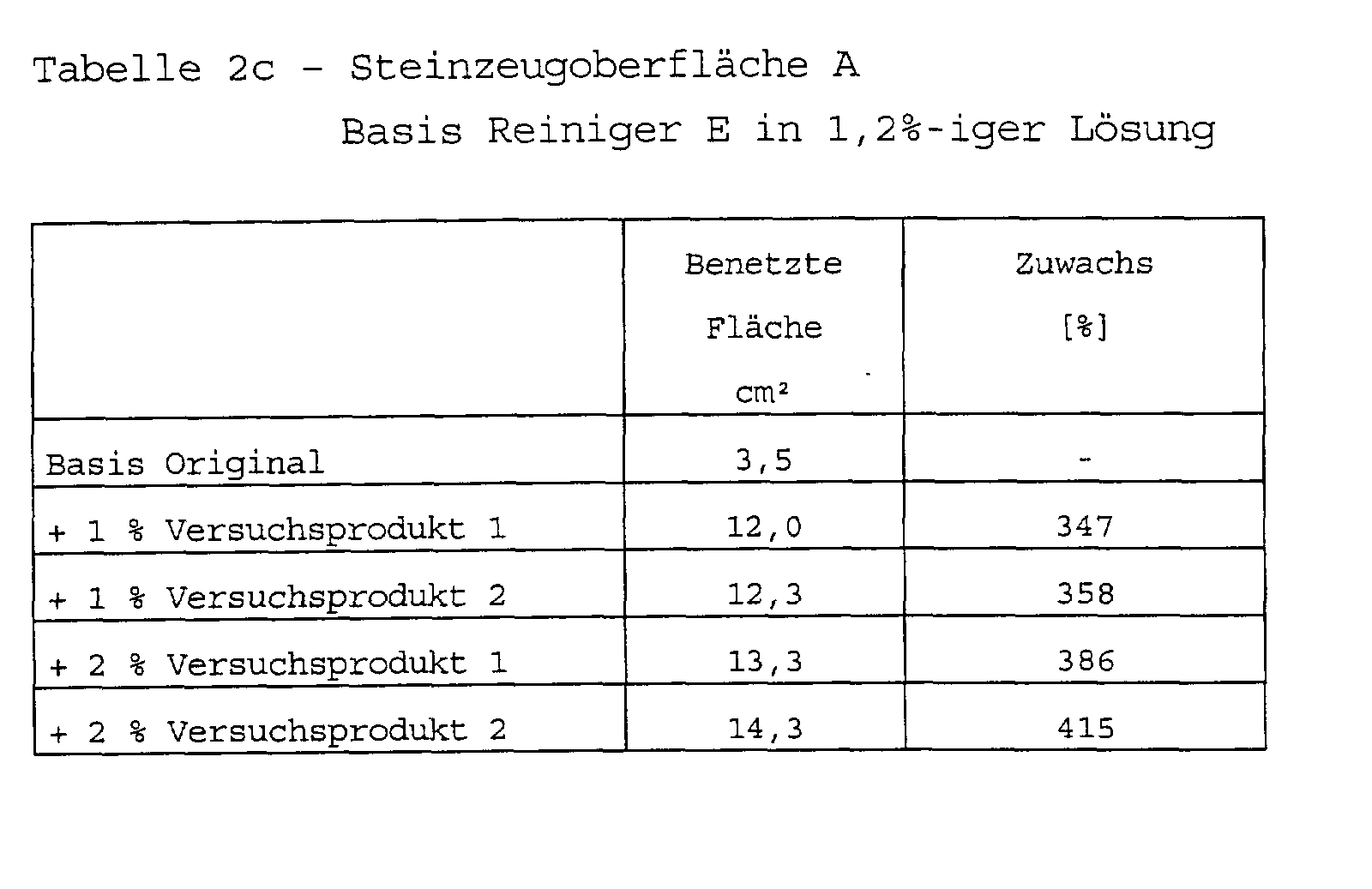
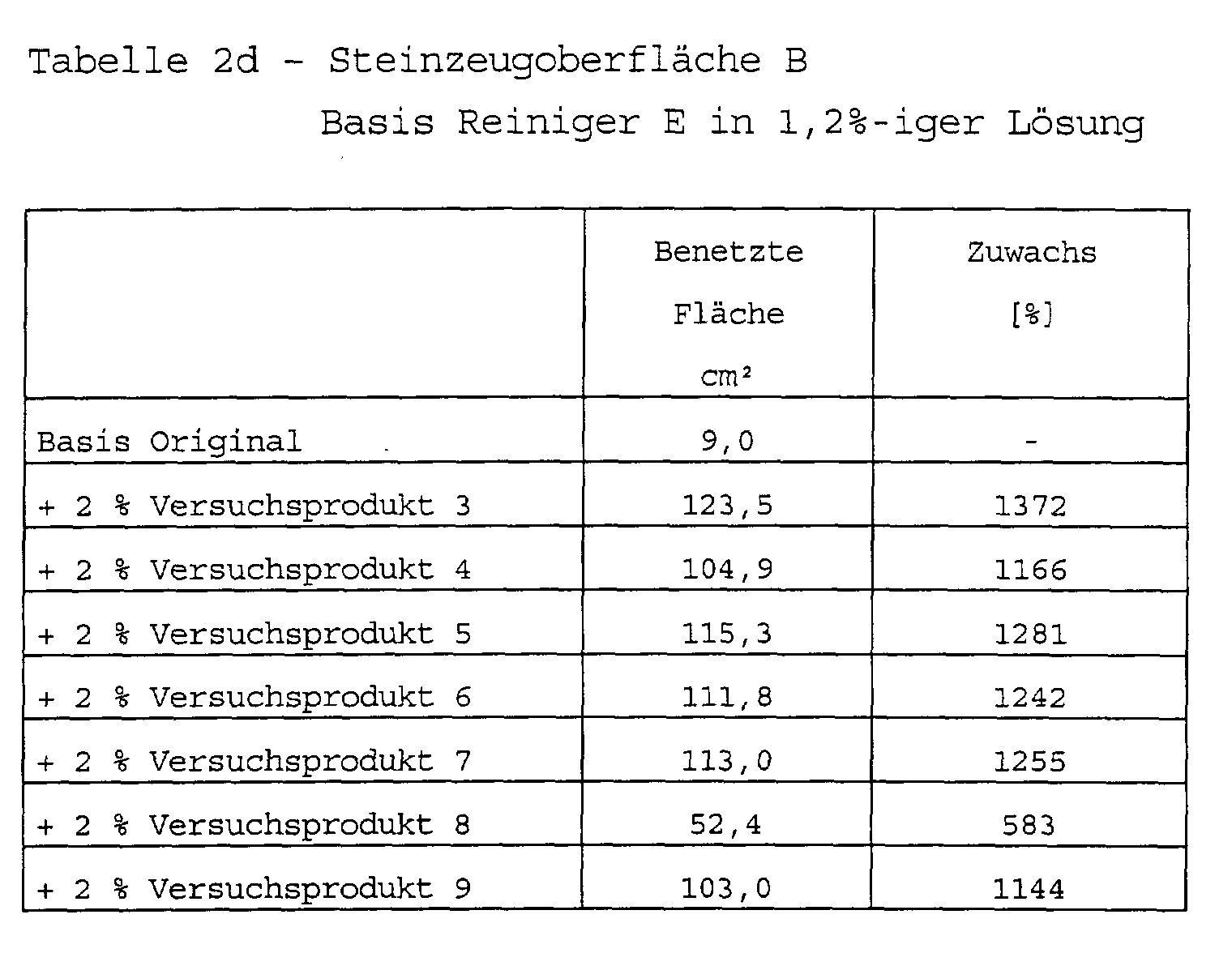
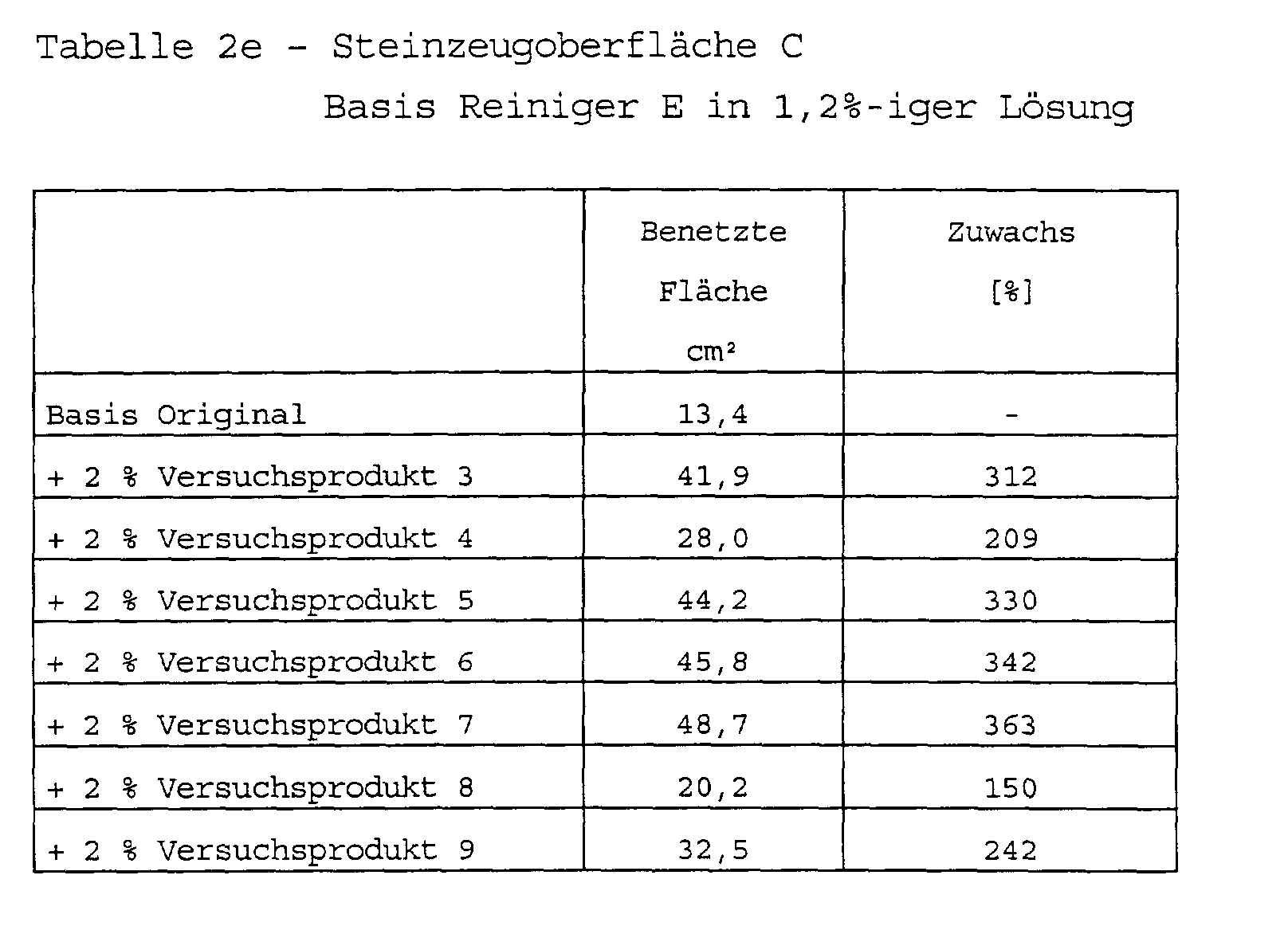
Zusammensetzung handelsüblicher Reinigungsmittel
Typische Gebrauchsverdünnungen der Bodenreiniger
Die Reinigungslösung wurde mit Leitungswasser, der Stadt Essen (Wasserhärte < 10), auf die jeweilige Anwendungskonzentration verdünnt. Für den Test wurde die Pipettenspitze (Fa. Rainin (2,5 ml) EPD 2) 0.3 mm über die Fliese positioniert.The cleaning solution was with tap water, the city of Essen (Water hardness <10), to the respective application concentration diluted. The pipette tip was used for the test (Rainin (2.5 ml) EPD 2) 0.3 mm over the tile positioned.
Die Feinsteinzeugfliesen wurden zuerst in der Geschirrspülmaschine bei 70°C ohne Tensid gewaschen.The porcelain stoneware tiles were first in the dishwasher washed at 70 ° C without surfactant.
Für den Spreitungstest wurde 0,05 ml Reiniger-Anwendungslösung auf die Fliese pipettiert.For the spreading test, 0.05 ml of cleaning solution was used pipetted onto the tile.
Nach 60 Sekunden wurde die Spreitfläche in Länge und Breite mit einer Schieblehre bestimmt. Zur Fehlerkorrektur wurde jede Lösung mindestens fünfmal wiederholt. Die Fläche des Tropfens wird berechnet durch die Formel für eine Ellipse. After 60 seconds, the spreading area in length and width was determined using a slide gauge. Each solution was repeated at least five times to correct the error. The area of the drop is calculated using the formula for an ellipse.
Die Eigenschaften der Basislösung werden als Blindwert betrachtet.
Nach Zugabe der Reinigungsverstärker wird im Verhältnis zu
dem Blindwert verglichen.
Test in Anlehnung an die Veröffentlichung von: Lime Soap Dispersion Test, Journal of American Oil Chemists' Society, Volume 27, March 1950, p 90 H.C. Boghetty & C.A Bergman Organic Chemicals Division General Dyestuff, N.Y.C.Test based on the publication of: Lime Soap Dispersion Test, Journal of American Oil Chemists' Society, Volume 27, March 1950, p 90 H.C. Boghetty & C.A Bergman Organic Chemicals Division General Dyestuff, N.Y.C.
Bestimmt wird die Fähigkeit von Reiniger-Anwendungslösungen, schwerlösliche Metallsalze zu dispergieren.The ability of cleaner application solutions is determined to disperse poorly soluble metal salts.
5 ml Natriumoleat (1) werden mit X ml einer Reiniger-Anwendungslösung (3) sowie 10 ml hartem Wasser (2) zusammen pipettiert und dann mit demineralisiertem Wasser auf 30 ml aufgefüllt. Die Testlösung wird zwanzigmal gedreht und dann nach 30 Sekunden optisch begutachtet. Bei nicht ausreichendem Dispergiermittel fällt die sich bildende Kalkseife als Wolken in der Lösung aus. Der Endpunkt ist erreicht wenn die Seife dispergiert ist.5 ml of sodium oleate (1) are mixed with X ml of a cleaning solution (3) and 10 ml of hard water (2) are pipetted together and then made up to 30 ml with demineralized water. The test solution is rotated twenty times and then after Visually inspected for 30 seconds. If not enough The lime soap that forms is dispersed as clouds in the solution. The end point is reached when the soap is dispersed.
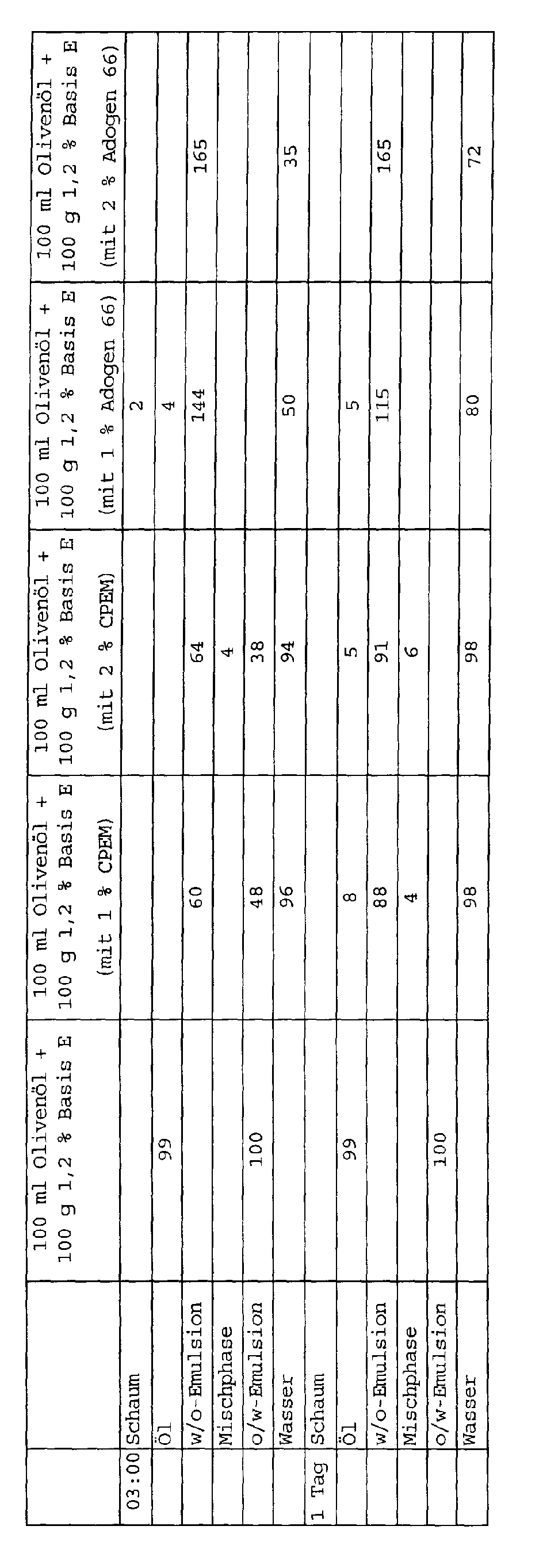
Untersucht wurde hier nur das Verhalten der Testlösung E, da man bei den anderen Reinigern durch den hohen Anteil nichtionischer Tenside keine deutliche Steigerung des Emulgierverhaltens erwarten konnte. Testlösung E enthält nur kurzkettige anionische Tenside, bei solchen Tensidsystemen ist eine Schwäche im Emulgierverhalten bekannt.Only the behavior of test solution E was examined here, since with the other cleaners due to the high proportion of non-ionic Surfactants no significant increase in emulsifying behavior could expect. Test solution E contains only short-chain anionic surfactants, is one of such surfactant systems Weakness in emulsifying behavior known.
100 g Anwendungslösung eines Reinigers wurden mit 90 g (= 100 ml) Ölivenöl gemischt. Dann 30 sec. mit 8000 U/min im "Ultra-Turrax T25" emulgiert. Diese Emulsion wird in einen 250 ml Standzylinder gegeben. Nach 15, 30, 60, 120, 180 Minuten bzw. nach 24 Stunden wird das Mengenverhältnis der einzelnen Phasen abgelesen. 100 g application solution of a cleaner were mixed with 90 g (= 100 ml) olive oil. Then emulsified at 8000 rpm in the "Ultra-Turrax T25" for 30 seconds. This emulsion is placed in a 250 ml standing cylinder. After 15, 30, 60, 120, 180 minutes or after 24 hours the quantitative ratio of the individual phases is read off.
Wie ersichtlich, wird durch Zusatz der erfindungsgemäßen Reinigungsverstärker das Emulgierverhalten von schwach-emulgierenden, kurzkettigen Aniontensidlösungen stark optimiert. Wenn die Emulsion bricht setzt sich nun Wasser anstelle des Öls ab. Dieses sich absetzende Wasser beeinträchtigt nicht die Reinigungskraft bzw. das Schmutztragevermögen während einer Reinigung.As can be seen, by adding the cleaning booster according to the invention the emulsifying behavior of weakly emulsifying, Short chain anionic surfactant solutions greatly optimized. If the Emulsion breaks down, water instead of oil. This settling water does not affect the cleaning power or the dirt-carrying capacity during cleaning.
Claims (6)
- R1 =
- ein geradkettiger, gegebenenfalls verzweigter, gegebenenfalls Mehrfachbindungen enthaltender Alkylrest mit 8 bis 22 C-Atomen,
- R2 =
- - (CH2CHR5O)n-R6 mit R5 = H, -CH3, -C2H5; R6 = H, -CH3, -C2H5, -C3H7 oder -C4H9 und n = 1-25,
- R3 =
- R1 oder R2,
- R4 =
- -CH3 oder -C2H5 und
- X- =
- anionischer Rest, insbesondere Methylsulfat, Ethylsulfat, Phosphat, Chlorid, Bromid, Jodid
- R 1 =
- a straight-chain, optionally branched, optionally containing multiple bonds alkyl radical having 8 to 22 carbon atoms,
- R 2 =
- - (CH 2 CHR 5 O) n -R 6 with R 5 = H, -CH 3 , -C 2 H 5 ; R 6 = H, -CH 3 , -C 2 H 5, -C 3 H 7 or -C 4 H 9 and n = 1-25,
- R 3 =
- R 1 or R 2 ,
- R 4 =
- -CH 3 or -C 2 H 5 and
- X - =
- anionic radical, in particular methyl sulfate, ethyl sulfate, phosphate, chloride, bromide, iodide
- R1 =
- ein geradkettiger, Mehrfachbindungen enthaltender Alkylrest mit 12 bis 18 C-Atomen ist,
- R2, R3 =
- - (CH2CHR5O)n-R6 mit R5 = H; R6 = H, und die Summe aller n insgesamt = 5 - 20 ist,
- R4 =
- -CH3 und
- X- =
- Methylsulfat, Ethylsulfat, Chlorid sind.
- R 1 =
- is a straight-chain alkyl radical containing multiple bonds with 12 to 18 carbon atoms,
- R 2 , R 3 =
- - (CH 2 CHR 5 O) n -R 6 with R 5 = H; R 6 = H, and the sum of all n in total = 5 - 20,
- R 4 =
- -CH 3 and
- X - =
- Are methyl sulfate, ethyl sulfate, chloride.
enthalten, in denen R1 = die Reste der Kokosfettsäure, Palmfettsäure, Talgfettsäure sein können. Aqueous cleaning agent concentrates according to claim 3, characterized in that they serve as cleaning enhancers quaternary alkoxylated amine compounds of the general formula [R 1 , R 2 , R 3 , R 4 N] + X -
contain, in which R 1 = the residues of coconut fatty acid, palm fatty acid, tallow fatty acid can be.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE10038198 | 2000-08-04 | ||
| DE10038198A DE10038198A1 (en) | 2000-08-04 | 2000-08-04 | Aqueous cleaning agent concentrates for rough, especially profiled tiles |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1178106A1 true EP1178106A1 (en) | 2002-02-06 |
| EP1178106B1 EP1178106B1 (en) | 2004-04-28 |
Family
ID=7651394
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP01117825A Expired - Lifetime EP1178106B1 (en) | 2000-08-04 | 2001-07-21 | Use of aqueous cleaning agent concentrates as detergency boosters for cleaning fine-stoneware tiles |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US6544350B2 (en) |
| EP (1) | EP1178106B1 (en) |
| JP (1) | JP2002097491A (en) |
| AT (1) | ATE265515T1 (en) |
| CA (1) | CA2352865A1 (en) |
| DE (2) | DE10038198A1 (en) |
| ES (1) | ES2220633T3 (en) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102004036067A1 (en) | 2004-07-24 | 2006-02-16 | Goldschmidt Gmbh | Aqueous cleaning agent concentrates for rough, in particular profiled tiles |
| US7332463B2 (en) * | 2005-04-22 | 2008-02-19 | On Legal Grounds, Inc. | Colloidal cleaning system comprising a saponified fatty acid and an anionic/nonionic surfactant mixture |
| DE102009001748A1 (en) | 2009-03-23 | 2010-09-30 | Evonik Goldschmidt Gmbh | Formulations containing sorbitan carboxylic acid ester |
| DE102014207386A1 (en) * | 2014-04-17 | 2015-10-22 | Evonik Degussa Gmbh | Surfactant compositions and high oily formulations containing these |
| US9920284B2 (en) | 2015-04-22 | 2018-03-20 | S. C. Johnson & Son, Inc. | Cleaning composition with a polypropdxylated 2-(trialkylammonio)ethanol ionic liquid |
| FR3047488B1 (en) * | 2016-02-05 | 2020-02-28 | Laboratoires Anios | DETERGENT COMPOSITIONS FOR CLEANING IN THE COSMETIC AND PHARMACEUTICAL INDUSTRY. |
| EP3532586B1 (en) | 2016-10-26 | 2022-05-18 | S.C. Johnson & Son, Inc. | Disinfectant cleaning composition with quaternary ammonium hydroxycarboxylate salt |
| EP3532584A1 (en) | 2016-10-26 | 2019-09-04 | S.C. Johnson & Son, Inc. | Disinfectant cleaning composition with quaternary amine ionic liquid |
| WO2018080836A1 (en) | 2016-10-26 | 2018-05-03 | S. C. Johnson & Son, Inc. | Disinfectant cleaning composition with quaternary ammonium hydroxycarboxylate salt |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4284435A (en) * | 1979-11-28 | 1981-08-18 | S. C. Johnson & Son, Inc. | Method for spray cleaning painted surfaces |
| EP0928829A1 (en) * | 1998-01-08 | 1999-07-14 | HENKEL-ECOLAB GmbH & CO. OHG | Cleaning of hard surfaces using rheopexic aqueous cleaning agents |
| WO1999035120A1 (en) * | 1998-01-09 | 1999-07-15 | Witco Corporation | Novel quaternary ammonium compounds, compositions containing them, and uses thereof |
| GB2334723A (en) * | 1998-02-26 | 1999-09-01 | Reckitt & Colmann Prod Ltd | Hard surface cleaner |
| US6017874A (en) * | 1995-09-29 | 2000-01-25 | The Procter & Gamble Company | Liquid laundry detergents containing selected quaternary ammonium compounds |
| WO2000049127A1 (en) * | 1999-02-17 | 2000-08-24 | Reckitt Benckiser, Inc. | Hard surface cleaning and disinfecting compositions |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2329901A (en) * | 1997-09-30 | 1999-04-07 | Reckitt & Colman Inc | Acidic hard surface cleaning and disinfecting compositions |
| GB2336370B (en) * | 1998-04-14 | 2002-09-04 | Reckitt & Colman Inc | Improvements in or relating to organic compositions |
| GB2336369B (en) * | 1998-04-14 | 2002-06-19 | Reckitt & Colman Inc | Improvements in or relating to organic compositions |
| GB9817457D0 (en) * | 1998-08-12 | 1998-10-07 | Reckitt & Colman Inc | Improvements in or related to organic compositions |
| US6465411B2 (en) * | 2000-12-21 | 2002-10-15 | Clariant International Ltd. | Pine oil cleaning composition |
-
2000
- 2000-08-04 DE DE10038198A patent/DE10038198A1/en not_active Withdrawn
-
2001
- 2001-07-11 CA CA002352865A patent/CA2352865A1/en not_active Abandoned
- 2001-07-19 US US09/909,308 patent/US6544350B2/en not_active Expired - Lifetime
- 2001-07-21 DE DE50102104T patent/DE50102104D1/en not_active Expired - Fee Related
- 2001-07-21 ES ES01117825T patent/ES2220633T3/en not_active Expired - Lifetime
- 2001-07-21 EP EP01117825A patent/EP1178106B1/en not_active Expired - Lifetime
- 2001-07-21 AT AT01117825T patent/ATE265515T1/en not_active IP Right Cessation
- 2001-08-06 JP JP2001237725A patent/JP2002097491A/en active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4284435A (en) * | 1979-11-28 | 1981-08-18 | S. C. Johnson & Son, Inc. | Method for spray cleaning painted surfaces |
| US6017874A (en) * | 1995-09-29 | 2000-01-25 | The Procter & Gamble Company | Liquid laundry detergents containing selected quaternary ammonium compounds |
| EP0928829A1 (en) * | 1998-01-08 | 1999-07-14 | HENKEL-ECOLAB GmbH & CO. OHG | Cleaning of hard surfaces using rheopexic aqueous cleaning agents |
| WO1999035120A1 (en) * | 1998-01-09 | 1999-07-15 | Witco Corporation | Novel quaternary ammonium compounds, compositions containing them, and uses thereof |
| GB2334723A (en) * | 1998-02-26 | 1999-09-01 | Reckitt & Colmann Prod Ltd | Hard surface cleaner |
| WO2000049127A1 (en) * | 1999-02-17 | 2000-08-24 | Reckitt Benckiser, Inc. | Hard surface cleaning and disinfecting compositions |
Also Published As
| Publication number | Publication date |
|---|---|
| DE50102104D1 (en) | 2004-06-03 |
| DE10038198A1 (en) | 2002-02-21 |
| US6544350B2 (en) | 2003-04-08 |
| JP2002097491A (en) | 2002-04-02 |
| CA2352865A1 (en) | 2002-02-04 |
| ATE265515T1 (en) | 2004-05-15 |
| US20020169093A1 (en) | 2002-11-14 |
| EP1178106B1 (en) | 2004-04-28 |
| ES2220633T3 (en) | 2004-12-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE69734427T2 (en) | HYDROTROP CONTAINING CLEANERS FOR HARD SURFACES WITH REDUCED RESIDUE CONSTRUCTION | |
| DE60117809T2 (en) | CLEANING COMPOSITION FOR CHEMICAL-MECHANICAL PLANARIZATION | |
| EP1178106B1 (en) | Use of aqueous cleaning agent concentrates as detergency boosters for cleaning fine-stoneware tiles | |
| EP2804937B1 (en) | Washing, cleaning or pre-treatment agent with increased cleaning power | |
| DE60018795T2 (en) | STAIN REMOVAL AND DIRT DETECTION IN TEXTILE WASH | |
| EP1619237B1 (en) | Aqueous cleaning concentrates for rough preferably profiled tiles and plates | |
| DE69321562T2 (en) | Detergent compositions with a combination of highly hydrophilic and highly hydrophobic nonionic surfactants | |
| DE10055555A1 (en) | Treatment of surfaces to temporarily improve the dirt release behavior | |
| EP0916717B1 (en) | Hard surface cleaning agent | |
| DE2211576A1 (en) | Textile treatment agents | |
| EP0928829B1 (en) | Cleaning of hard surfaces using rheopexic aqueous cleaning agents | |
| EP1137748A1 (en) | Method for machining and cleaning metal | |
| EP2843034A1 (en) | Cleaning agent with decalcification effect | |
| DE4243468A1 (en) | Neutral liquid detergent (I) | |
| EP0944712B1 (en) | Detergents | |
| DE3144470A1 (en) | FOAM CONTROLLED DETERGENTS | |
| EP1232236B1 (en) | Use of formulations for treating surfaces for temporarily improving dirt-removing behavior | |
| DE102004010152A1 (en) | Aqueous detergent or cleansing agent concentrates based on nonionic and anionic surfactants for cleaning tiles comprises a microemulsion free of ethoxylated quaternary compounds | |
| DE4032126C2 (en) | Aqueous cleaners for hard surfaces | |
| DE202013103933U1 (en) | Detergent with descaling effect | |
| WO2006094328A1 (en) | Cleaning and impregnating product designed for acid-resistant natural and artificial stones, and method for cleaning and simultaneously impregnating natural and artificial stones | |
| EP1147170B1 (en) | Agent for maintaining water-resistant surfaces | |
| DE2731152A1 (en) | CLEANING AGENTS FOR OBJECTS WITH A HARD SURFACE | |
| DE3328639A1 (en) | CLEANING AGENT FOR MECHANICALLY PROCESSED SEMICONDUCTOR MATERIALS | |
| WO2003010269A1 (en) | Acid aqueous detergent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20010728 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| AKX | Designation fees paid |
Free format text: AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| 17Q | First examination report despatched |
Effective date: 20030708 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RTI1 | Title (correction) |
Free format text: USE OF AQUEOUS CLEANING AGENT CONCENTRATES AS DETERGENCY BOOSTERS FOR CLEANING FINE-STONEWARE TILES |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20040428 Ref country code: IE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20040428 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20040428 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20040428 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: GERMAN |
|
| REF | Corresponds to: |
Ref document number: 50102104 Country of ref document: DE Date of ref document: 20040603 Kind code of ref document: P |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040721 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040721 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20040728 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20040728 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20040728 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040731 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20040812 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FD4D |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2220633 Country of ref document: ES Kind code of ref document: T3 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: GOLDSCHMIDT GMBH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Owner name: GOLDSCHMIDT GMBH Free format text: GOLDSCHMIDT AG#GOLDSCHMIDTSTRASSE 100#45127 ESSEN (DE) -TRANSFER TO- GOLDSCHMIDT GMBH#GOLDSCHMIDTSTRASSE 100#45127 ESSEN (DE) |
|
| 26N | No opposition filed |
Effective date: 20050131 |
|
| NLT2 | Nl: modifications (of names), taken from the european patent patent bulletin |
Owner name: GOLDSCHMIDT GMBH |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20050714 Year of fee payment: 5 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CD Ref country code: FR Ref legal event code: CJ |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20060714 Year of fee payment: 6 Ref country code: NL Payment date: 20060714 Year of fee payment: 6 Ref country code: DE Payment date: 20060714 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20060720 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20060728 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20060731 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20060731 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20060731 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20060822 Year of fee payment: 6 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040928 |
|
| BERE | Be: lapsed |
Owner name: *GOLDSCHMIDT G.M.B.H. Effective date: 20070731 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20070721 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20080201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080201 Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070721 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20080331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070731 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070731 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20070723 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070723 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070721 |