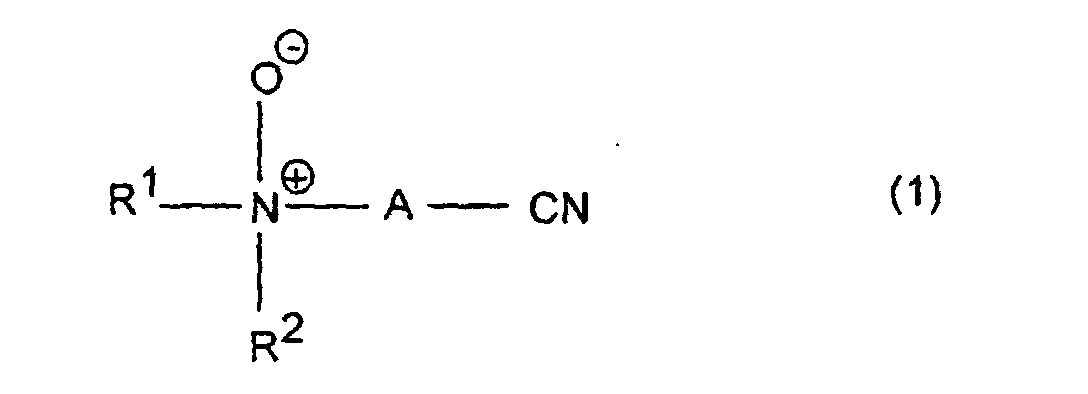EP0909810B1 - Use of aminonitrile-N-oxides as bleach activators - Google Patents
Use of aminonitrile-N-oxides as bleach activators Download PDFInfo
- Publication number
- EP0909810B1 EP0909810B1 EP98117813A EP98117813A EP0909810B1 EP 0909810 B1 EP0909810 B1 EP 0909810B1 EP 98117813 A EP98117813 A EP 98117813A EP 98117813 A EP98117813 A EP 98117813A EP 0909810 B1 EP0909810 B1 EP 0909810B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- aminonitrile
- formula
- oxide
- alkyl
- detergent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/39—Organic or inorganic per-compounds
- C11D3/3902—Organic or inorganic per-compounds combined with specific additives
- C11D3/3905—Bleach activators or bleach catalysts
- C11D3/3907—Organic compounds
- C11D3/3917—Nitrogen-containing compounds
- C11D3/3925—Nitriles; Isocyanates or quarternary ammonium nitriles
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06L—DRY-CLEANING, WASHING OR BLEACHING FIBRES, FILAMENTS, THREADS, YARNS, FABRICS, FEATHERS OR MADE-UP FIBROUS GOODS; BLEACHING LEATHER OR FURS
- D06L4/00—Bleaching fibres, filaments, threads, yarns, fabrics, feathers or made-up fibrous goods; Bleaching leather or furs
- D06L4/10—Bleaching fibres, filaments, threads, yarns, fabrics, feathers or made-up fibrous goods; Bleaching leather or furs using agents which develop oxygen
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H21/00—Non-fibrous material added to the pulp, characterised by its function, form or properties; Paper-impregnating or coating material, characterised by its function, form or properties
- D21H21/14—Non-fibrous material added to the pulp, characterised by its function, form or properties; Paper-impregnating or coating material, characterised by its function, form or properties characterised by function or properties in or on the paper
- D21H21/32—Bleaching agents
Definitions
- peroxidic bleaching agents such as perborates, Percarbonates, persilicates and perphosphates
- Bleach activators are called.
- nitrile bleach activators and their use as bleach activators in Bleaching agents are described, for example, in EP 303 520, GB 802 035, US 4,883,917, US 5 478 356, US 5 591 378, WO 9 606 912 and WO 9 640 661.
- aminonitrile N-oxides or thereof derived salts have a better bleaching effect than bleach activators according to the state of the art.
- Aminonitrile N-oxides or their salts of the formula 1 are preferred, in which R 1 and R 2 are C 1 -C 4 -alkyl, in particular methyl, and A is phenylene.
- the aminonitrile N-oxides or the salts derived therefrom are easily accessible by reacting aminonitriles with oxidizing agents; Such reactions are described, for example, in J. Backes "Amine", Methods of Organic Chemistry (Houben-Weyl), D. Klamann (ed.) Vol. E 16d (1992), pp. 1235-1329 and the literature cited therein.
- the invention also relates to the use of these bleach activators in bleaching detergents and cleaning agents as well as in paper and textile bleaching.
- the detergents and cleaning agents contain a peroxide compound and the bleach activator usually also surface-active compounds and other known ingredients.
- Bleach activators and other suitable bleach activators e.g. TAED, Tetraacetylglycoluril, glucose pentaacetate, sodium nonanoyloxybenzenesulfonate, Benzoylcaprolactam or nitrile activators may be included.
- TAED Tetraacetylglycoluril
- glucose pentaacetate glucose pentaacetate
- sodium nonanoyloxybenzenesulfonate Benzoylcaprolactam or nitrile activators
- Benzoylcaprolactam or nitrile activators may be included.
- These additional Bleach activators can be present in an amount of 1 to 10% by weight.
- the surfactant can be derived from natural products such as Soap, or is a synthetic compound from the group of anionic, non-ionic, amphoteric, zwitterionic or cationic surface-active Substances, or mixtures of these.
- suitable substances commercially available, and are described in the literature, for example in "Surface active agents and detergents", Vol. 1 and 2, by Schwartz, Perry and Berch.
- the total proportion of surface-active compounds can be up to 50 % By weight, preferably 1% by weight to 40% by weight, in particular 4% by weight up to 25% by weight.
- Synthetic anionic surfactants are common water-soluble alkali metal salts of organic sulfates and sulfonates with alkyl radicals from about 8 to 22 carbon atoms, the term "alkyl” being the Includes alkyl substituents of higher aryl groups.
- Examples include sodium and ammonium alkyl sulfates, especially the sulfates obtained by sulfating higher (C 8 -C 18 ) alcohols; Sodium and ammonium alkylbenzenesulfonates with an alkyl radical from C 9 to C 20 , in particular linear secondary sodium alkylbenzenesulfonates with an alkyl radical from C 10 to C 15 ; Sodium alkyl glycerol ether sulfates, especially the esters of the higher alcohols derived from tallow and coconut oil; the sodium sulfates and sulfonates of the coconut fatty acid monoglycerides; Sodium and ammonium salts of the sulfuric acid esters of higher (C 9 to C 18 ) oxalkylated, especially the fatty alcohols alkoxylated with ethylene oxide; the reaction products of the esterification of fatty acids with isethionic acid and subsequent neutralization with sodium hydroxide; Sodium and ammonium salts of the
- nonionic surface-active compounds which are preferably used together with anionic surface-active compounds, are in particular the reaction products of alkylene oxides (usually ethylene oxide) with alkylphenols (alkyl radicals from C 5 to C 22 ), the reaction products generally 5 to 25 ethylene oxide (EO ) Units contained in the molecule; the reaction products of aliphatic (C 8 to C 18 ) primary or secondary, linear or branched alcohols with ethylene oxide, generally with 6 to 30 EO, and the addition products of ethylene oxide with reaction products of propylene oxide and ethylenediamine.
- Other nonionic surface active compounds are alkyl polyglycosides, long chain tertiary amine oxides, long chain tertiary phosphine oxides and dialkyl sulfoxides.
- Amphoteric or zwitterionic surfactants can also be used can be used in the compositions according to the invention, but what is usually not desired because of its high cost. If amphoteric or zwitterionic compounds are used, so this usually happens in small amounts in compositions that are mainly anionic and contain nonionic surfactants.
- the detergents and cleaning agents generally also contain a builder.
- Builders come into consideration: calcium binding substances, precipitants, calcium-specific Ion exchangers and their mixtures.
- calcium binders include alkali metal polyphosphates such as sodium tripolyphosphate; Nitrilotriacetic acid and its water-soluble salts; the alkali metal salts of Carboxymethyloxy succinic acid, ethylenediaminetetraacetic acid, Oxydisuccinic acid, mellitic acid, benzene polycarboxylic acids, citric acid and Polyacetal carboxylates as described in U.S. Pat. 4,144,226 and 4,146,495.
- precipitants are sodium orthophosphate, sodium carbonate and Long chain fatty acid soaps.
- ion exchangers specific for calcium are various types of water-insoluble, crystalline or amorphous Aluminum silicates, of which the zeolites are the best known representatives.
- the washing and Detergents contain each of the conventional additives in amounts that one usually finds in such means.
- additives are Foaming agents, such as alkanolamides, especially the monoethanolamides Palm kernel oil fatty acids and coconut fatty acids; foam-preventing substances such as alkyl phosphates and silicones; Graying inhibitors and the like Auxiliaries such as sodium carboxymethyl cellulose and alkyl or substituted alkyl cellulose ethers; Stabilizers such as ethylenediaminetetraacetic acid; softener for textiles; inorganic salts such as sodium sulfate; and, usually small Quantities, fluorescent substances, perfumes, enzymes such as proteases, cellulases, Lipases and amylases, disinfectants and dyes.
- the bleach activators of this invention can be used in a variety of laundry and Detergents are used. These include laundry detergents, Textile bleach, surface cleaners, toilet cleaners, Dishwasher cleaner, and also denture cleaner.
- the detergents can be in solid or liquid form. It is for the sake of stability and Manageability advantageous to the bleach activators in the form of granules use that contain a binder in addition to the bleaching catalyst.
- Various methods for producing such granules are in the patent literature described, for example, in Canada Pat. No.
- the granules containing the bleach activators are generally the Detergent composition along with the other dry Components such as enzymes, inorganic peroxide bleach added.
- the detergent composition to which the catalyst granules are added can be obtained in several ways, such as Dry mixing, extruding, spray drying.
- the bleach activators according to the invention are Particularly suitable for non-aqueous, liquid detergents, together with a bleaching peroxide compound, such as sodium perborate, around the detergent great cleaning ability for fabrics and textiles.
- aqueous, liquid detergents, pasty and gelatinous Including detergent compositions are well known in the art are known, and are described, for example, in US 2,864,770, US 2,940,938, US 4,772,412, US 3 368 977, GB-A-1 205 711 GB-A-1 370 377, GB-A-1 270 040, GB-A-1 292 352, GB-A-2 194 536, DE-A-2 233 771, EP-A-0 028 849.
- compositions in the form of a non-aqueous, liquid medium in which a solid phase can be dispersed can be a liquid, surface-active substance, preferably a nonionic surfactant; a non polar liquid medium such as liquid paraffin; a polar solvent such as Polyols, for example glycerin, sorbitol, ethylene glycol, possibly in combination with low molecular weight monohydric alcohols such as ethanol or isopropanol; or Mixtures of these.
- the solid phase can consist of builder substances, alkalis, abrasive substances, Polymers and other solid ionic surfactants, bleaches fluorescent substances and other common solid ingredients.
- a lead composition was prepared by combining 200 ml of an aqueous solution of reference detergent WMP (laundry research Krefeld, 5 g / l in water with 15 ° dH) solution, 150 mg of sodium perborate monohydrate and 50 mg of the respective activator.
- WMP laundry research Krefeld, 5 g / l in water with 15 ° dH
- tissue pieces soiled with the standard soiling BC-1 tea were subjected to a treatment at a temperature of 40 ° C. under isothermal washing conditions in a Linitest device (Heraeus).
- Connections 1-5 are: 1 2 3 4 5 ⁇ R 1.0 0.7 1.2 0.3 0.1 mCBA: meta-chlorobenzoic acid salt
- the washing experiments show that the aminonitrile N-oxides according to the invention have good bleaching performance. Further advantageous properties of the complexes described are low color damage and low fiber damage.
Description
Es ist bekannt, daß das Bleichvermögen peroxidischer Bleichmittel, wie Perborate, Percarbonate, Persilicate und Perphosphate, verbessert werden kann, so daß die Bleichwirkung bei niedrigeren Temperaturen einsetzt, etwa bei oder unter 60°C, indem man die Vorstufen von bleichenden Peroxysäuren zusetzt, die oft als Bleichaktivatoren bezeichnet werden.It is known that the bleaching power of peroxidic bleaching agents, such as perborates, Percarbonates, persilicates and perphosphates can be improved so that the Uses bleaching action at lower temperatures, for example at or below 60 ° C, by adding the precursors of bleaching peroxyacids, often called Bleach activators are called.
Viele Substanzen sind nach dem Stand der Technik als Bleichaktivatoren bekannt. Gewöhnlich handelt es sich dabei um reaktive organische Verbindungen mit einer O-Acyl- oder N-Acyl-Gruppe, die in alkalischer Lösung zusammen mit einer Quelle für Wasserstoffperoxid die entsprechenden Peroxysäuren bilden.Many substances are known in the art as bleach activators. Usually these are reactive organic compounds with a O-acyl or N-acyl group in alkaline solution together with a source form the corresponding peroxyacids for hydrogen peroxide.
Repräsentative Beispiele für Bleichaktivatoren sind etwa N,N,N',N'-Tetraacetylethylendiamin (TAED), Glucosepentaacetat (GPA), Xylosetetraacetat (TAX), Natrium-4-benzoyloxybenzolsulfonat (SBOBS), Natriumtrimethylhexanoyloxybenzolsulfonat (STHOBS), Tetraacetylglucoluril (TAGU), Tetraacetylcyansäure (TACA), Di-N-acetyldimethylglyoxin (ADMG) und 1-Phenyl-3-acetylhydantoin (PAH). Es sei beispielsweise auf GB-A-836 988, GB-A-907 356, EP-A-0 098 129 und EP-A-0 120 591 verwiesen.Representative examples of bleach activators are, for example N, N, N ', N'-tetraacetylethylenediamine (TAED), glucose pentaacetate (GPA), Xylose tetraacetate (TAX), sodium 4-benzoyloxybenzenesulfonate (SBOBS), Sodium trimethylhexanoyloxybenzenesulfonate (STHOBS), tetraacetylglucoluril (TAGU), tetraacetylcyanoic acid (TACA), di-N-acetyldimethylglyoxin (ADMG) and 1-phenyl-3-acetylhydantoin (PAH). For example, on GB-A-836 988, GB-A-907 356, EP-A-0 098 129 and EP-A-0 120 591.
Mittlerweise haben nitrilische Bleichaktivatoren an Bedeutung gewonnen, da sie sich als außerordentlich bleichaktiv erweisen. Wahrscheinlich bilden diese Verbindungen bei der Perhydrolyse eine Peroxyimidsäure, welche das bleichende Agens ist.In the meantime, nitrile bleach activators have become more important because they are turn out to be extraordinarily bleaching active. These are probably connections in perhydrolysis a peroxyimidic acid, which is the bleaching agent.
Solche nitrilischen Bleichaktivatoren und deren Verwendung als Bleichaktivator in Bleichmitteln sind beispielsweise in EP 303 520, GB 802 035, US 4 883 917, US 5 478 356, US 5 591 378, WO 9 606 912 und WO 9 640 661 beschrieben.Such nitrile bleach activators and their use as bleach activators in Bleaching agents are described, for example, in EP 303 520, GB 802 035, US 4,883,917, US 5 478 356, US 5 591 378, WO 9 606 912 and WO 9 640 661.
Überraschenderweise wurde nun gefunden, daß Aminonitril-N-oxide bzw. davon abgeleitete Salze eine bessere Bleichwirkung aufweisen als Bleichaktivatoren gemäß dem Stand der Technik.Surprisingly, it has now been found that aminonitrile N-oxides or thereof derived salts have a better bleaching effect than bleach activators according to the state of the art.
Gegenstand der Erfindung ist somit die Verwendung von Aminonitril-N-oxiden der allgemeinen Formel (1) oder deren Salzen, worin
- R1, R2
- unabhängig voneinander substituierte oder unsubstituierte C1-C15-Alkyl-, Cycloalkyl- oder Arylreste bedeuten, die durch Fluor, Chlor, Brom, C1-C5-Alkoxy, C1-C5-Alkoxycarbonyl, Amino, Ammonium, Carboxyl, Cyano oder Cyanamino substituiert sein können, oder zusammen mit dem Stickstoffatom, an das sie gebunden sind, einen Ring mit 4 bis 6 Kohlenstoffatomen bilden, der durch C1-C5-Alkyl, C1-C5-Alkoxy, C1-C5-Alkanoyl, Phenyl, Amino, Ammonium, Cyano, Cyanamino, Chlor oder Brom substituiert sein kann und wobei dieser Ring zusätzlich zum Stickstoffatom anstelle von -CH2-Gruppen ein oder zwei Sauerstoffatome oder eine Gruppe enthalten kann, worin R3 Wasserstoff, C1-C7-Alkyl oder Cycloalkyl, Phenyl oder C7-C9-Alkylaryl ist, und
- A
- für einen C1-C5-Alkylen-, einen C5-C10-Cycloalkylen- oder einen Arylenrest steht
- R 1 , R 2
- independently substituted or unsubstituted C 1 -C 15 alkyl, cycloalkyl or aryl radicals which are substituted by fluorine, chlorine, bromine, C 1 -C 5 alkoxy, C 1 -C 5 alkoxycarbonyl, amino, ammonium, carboxyl, Cyano or cyanamino can be substituted, or together with the nitrogen atom to which they are attached, form a ring with 4 to 6 carbon atoms, which is represented by C 1 -C 5 alkyl, C 1 -C 5 alkoxy, C 1 -C 5 -alkanoyl, phenyl, amino, ammonium, cyano, cyanamino, chlorine or bromine may be substituted and this ring in addition to the nitrogen atom instead of -CH 2 groups, one or two oxygen atoms or a group may contain, wherein R 3 is hydrogen, C 1 -C 7 alkyl or cycloalkyl, phenyl or C 7 -C 9 alkylaryl, and
- A
- represents a C 1 -C 5 alkylene, a C 5 -C 10 cycloalkylene or an arylene radical
Die erfindungsgemäß zu verwendenden Aminonitril-N-oxide umfassen auch deren Salze, beispielsweise solche Salze, die z.B. durch Umsetzung des entsprechenden Aminonitril-N-oxids mit Säuren wie insbesondere HCl, HBr, HF, H2SO4, H3PO4 und andere saure Phosphate, pyro-, meta- und poly-Phosphorsäure, HBF4, HPF6, H2CO3, HNO3-, Zitronensäure, Ameisensäure, R4SO4H, R4SO3H, R4COOH, wobei R4 für einen substituierten oder unsubstituierten C1-C21-Alkyl- oder Arylrest steht, erhalten werden.The aminonitrile-N-oxides to be used according to the invention also include their salts, for example those salts which are obtained, for example, by reacting the corresponding aminonitrile-N-oxide with acids such as, in particular, HCl, HBr, HF, H 2 SO 4 , H 3 PO 4 and others acidic phosphates, pyro-, meta- and poly-phosphoric acid, HBF 4 , HPF 6 , H 2 CO 3 , HNO 3 -, citric acid, formic acid, R 4 SO 4 H, R 4 SO 3 H, R 4 COOH, where R 4 represents a substituted or unsubstituted C 1 -C 21 alkyl or aryl radical can be obtained.
Bevorzugt sind Aminonitril-N-oxide oder deren Salze der Formel 1, worin R1 und R2 C1-C4-Alkyl, insbesondere Methyl, und A Phenylen bedeutet. Die Aminonitril-N-oxide bzw. die davon abgeleiteten Salze sind durch Umsetzung von Aminonitrilen mit Oxidationsmitteln leicht zugänglich; derartige Reaktionen sind z.B. in J. Backes "Amine", Methoden der Organischen Chemie (Houben-Weyl), D. Klamann (Hrsg.) Bd. E 16d (1992), S. 1235-1329 und der dort zitierten Literatur beschrieben.Aminonitrile N-oxides or their salts of the formula 1 are preferred, in which R 1 and R 2 are C 1 -C 4 -alkyl, in particular methyl, and A is phenylene. The aminonitrile N-oxides or the salts derived therefrom are easily accessible by reacting aminonitriles with oxidizing agents; Such reactions are described, for example, in J. Backes "Amine", Methods of Organic Chemistry (Houben-Weyl), D. Klamann (ed.) Vol. E 16d (1992), pp. 1235-1329 and the literature cited therein.
Gegenstand der Erfindung ist auch die Verwendung dieser Bleichaktivatoren in bleichenden Wasch- und Reinigungsmitteln sowie in der Papier- und Textilbleiche. Die Wasch- und Reinigungsmittel enthalten neben einer Peroxidverbindung und dem Bleichaktivator üblicherweise auch oberflächenaktive Verbindungen und weitere bekannte Inhaltsstoffe.The invention also relates to the use of these bleach activators in bleaching detergents and cleaning agents as well as in paper and textile bleaching. The detergents and cleaning agents contain a peroxide compound and the bleach activator usually also surface-active compounds and other known ingredients.
Geeignete peroxidische Bleichmittel sind Alkaliperoxide, organische Peroxide wie Harnstoffperoxid, und anorganische Persalze, wie die Alkaliperborate, -percarbonate, -perphosphate, -persilicate und -persulfate. Mischungen aus zwei oder mehreren dieser Verbindungen sind ebenfalls geeignet. Besonders bevorzugt sind Natriumperborat-Tetrahydrat und insbesondere Natriumperborat-Monohydrat. Natriumperborat-Monohydrat ist wegen seiner guten Lagerbeständigkeit und seiner guten Löslichkeit in Wasser bevorzugt. Natriumpercarbonat kann aus Umweltschutzgründen bevorzugt sein. Alkylhydroperoxide sind eine weitere geeignete Gruppe von Peroxidverbindungen. Beispiele für diese Stoffe sind Cumolhydroperoxid und tert.-Butylhydroperoxid.Suitable peroxidic bleaching agents are alkali peroxides, organic peroxides such as Urea peroxide, and inorganic persalts, such as the alkali perborates, -percarbonates, -perphosphates, -persilicates and -persulfate. Mixtures of two or more of these compounds are also suitable. Particularly preferred are sodium perborate tetrahydrate and especially sodium perborate monohydrate. Sodium perborate monohydrate is because of its good shelf life and its good solubility in water preferred. Sodium percarbonate can be made Environmental reasons may be preferred. Alkyl hydroperoxides are another suitable group of peroxide compounds. Examples of these substances are Cumene hydroperoxide and tert-butyl hydroperoxide.
In den Wasch- und Reinigungsmitteln kann der erfindungsgemäße nitrilische Bleichaktivator mit einem Gewichtsanteil von etwa 0,05 % bis 20 %, bevorzugt von 0,5 % bis 10 %, insbesondere von 1 % bis 7,5 % vorhanden sein, zusammen mit einer Peroxidverbindung. Der Gewichtsanteil dieser Peroxidverbindungen beträgt gewöhnlich von 1 % bis 60 %, bevorzugt von 4 % bis 30 %, insbesondere von 10 % bis 25 %.The nitrile agent according to the invention can be used in the washing and cleaning agents Bleach activator with a weight fraction of about 0.05% to 20%, preferably of 0.5% to 10%, especially 1% to 7.5%, be present together with a peroxide compound. The proportion by weight of these peroxide compounds is usually from 1% to 60%, preferably from 4% to 30%, in particular from 10% up to 25%.
In den Wasch- und Reinigungsmitteln können neben den erfindungsgemäßen Bleichaktivatoren noch andere geeignete Bleichaktivatoren, wie z.B. TAED, Tetraacetylglykoluril, Glucosepentaacetat, Natriumnonanoyloxybenzolsulfonat, Benzoylcaprolactam oder nitrilische Aktivatoren enthalten sein. Diese zusätzlichen Bleichaktivatoren können in einer Menge von 1 bis 10 Gew.-% vorhanden sein.In the detergents and cleaning agents, in addition to those according to the invention Bleach activators and other suitable bleach activators, e.g. TAED, Tetraacetylglycoluril, glucose pentaacetate, sodium nonanoyloxybenzenesulfonate, Benzoylcaprolactam or nitrile activators may be included. These additional Bleach activators can be present in an amount of 1 to 10% by weight.
Die oberflächenaktive Substanz kann von Naturprodukten abgeleitet sein, wie etwa Seife, oder ist eine synthetische Verbindung aus der Gruppe der anionischen, nichtionischen, amphoteren, zwitterionischen oder kationischen oberflächenaktiven Substanzen, oder Mischungen aus diesen. Viele geeignete Substanzen sind kommerziell erhältlich, und sind in der Literatur beschrieben, beispielsweise in "Surface active agents and detergents", Vol. 1 und 2, von Schwartz, Perry und Berch. Der Gesamtanteil der oberflächenaktiven Verbindungen kann bis zu 50 Gew.-% betragen, vorzugsweise 1 Gew.-% bis 40 Gew.-%, insbesondere 4 Gew.-% bis 25 Gew.-%.The surfactant can be derived from natural products such as Soap, or is a synthetic compound from the group of anionic, non-ionic, amphoteric, zwitterionic or cationic surface-active Substances, or mixtures of these. There are many suitable substances commercially available, and are described in the literature, for example in "Surface active agents and detergents", Vol. 1 and 2, by Schwartz, Perry and Berch. The total proportion of surface-active compounds can be up to 50 % By weight, preferably 1% by weight to 40% by weight, in particular 4% by weight up to 25% by weight.
Synthetische anionische oberflächenaktive Substanzen sind üblicherweise wasserlösliche Alkalimetallsalze organischer Sulfate und Sulfonate mit Alkylresten von etwa 8 bis 22 Kohlenstoffatomen, wobei der Ausdruck "Alkyl" die Alkylsubstituenten höherer Arylreste einschließt. Synthetic anionic surfactants are common water-soluble alkali metal salts of organic sulfates and sulfonates with alkyl radicals from about 8 to 22 carbon atoms, the term "alkyl" being the Includes alkyl substituents of higher aryl groups.
Beispiele hierfür sind Natrium- und Ammoniumalkylsulfate, speziell die durch Sulfatierung höherer (C8-C18)-Alkohole erhaltenen Sulfate; Natrium- und Ammoniumalkylbenzolsulfonate mit einem Alkylrest von C9 bis C20, insbesondere lineare sekundäre Natriumalkylbenzolsulfonate mit einem Alkylrest von C10 bis C15; Natriumalkylglycerinethersulfate, besonders die Ester der höheren, von Talg- und Kokosnußöl abgeleiteten Alkohole; die Natriumsulfate und -sulfonate der Kokosfettsäuremonoglyceride; Natrium- und Ammoniumsalze der Schwefelsäureester höherer (C9 bis C18)oxalkylierter, insbesondere der mit Ethylenoxid oxalkylierten Fettalkohole; die Reaktionsprodukte der Veresterung von Fettsäuren mit Isethionsäure und nachfolgender Neutralisierung mit Natriumhydroxid; Natrium- und Ammoniumsalze der Fettsäureamide des Methyltaurins; Alkan- Monosulfonate wie diejenigen aus der Reaktion von α-Olefinen (C8-C20) mit Natriumbisulfit und diejenigen aus der Reaktion von Paraffinen mit SO2 und Cl2 mit anschließender basischer Hydrolyse, wobei ein Gemisch verschiedener Sulfonate entsteht; Natrium- und Ammoniumdialkylsulfosuccinate mit Alkylresten von C7 bis C12; und Olefinsulfonate, die bei der Reaktion von Olefinen, insbesondere C10- bis C20-α-Olefinen, mit SO3 und nachfolgender Hydrolyse der Reaktionsprodukte entstehen. Die bevorzugten anionischen Detergentien sind Natriumalkylbenzolsulfonate mit Alkylresten von C15 bis C18, und Natriumalkylethersulfate mit Alkylresten von C16 bis C18.Examples include sodium and ammonium alkyl sulfates, especially the sulfates obtained by sulfating higher (C 8 -C 18 ) alcohols; Sodium and ammonium alkylbenzenesulfonates with an alkyl radical from C 9 to C 20 , in particular linear secondary sodium alkylbenzenesulfonates with an alkyl radical from C 10 to C 15 ; Sodium alkyl glycerol ether sulfates, especially the esters of the higher alcohols derived from tallow and coconut oil; the sodium sulfates and sulfonates of the coconut fatty acid monoglycerides; Sodium and ammonium salts of the sulfuric acid esters of higher (C 9 to C 18 ) oxalkylated, especially the fatty alcohols alkoxylated with ethylene oxide; the reaction products of the esterification of fatty acids with isethionic acid and subsequent neutralization with sodium hydroxide; Sodium and ammonium salts of the fatty acid amides of methyl taurine; Alkane monosulfonates such as those from the reaction of α-olefins (C 8 -C 20 ) with sodium bisulfite and those from the reaction of paraffins with SO 2 and Cl 2 with subsequent basic hydrolysis, giving a mixture of different sulfonates; Sodium and ammonium dialkyl sulfosuccinates with alkyl radicals from C 7 to C 12 ; and olefin sulfonates which are formed in the reaction of olefins, in particular C 10 - to C 20 -α-olefins, with SO 3 and subsequent hydrolysis of the reaction products. The preferred anionic detergents are sodium alkylbenzenesulfonates with alkyl radicals from C 15 to C 18 , and sodium alkyl ether sulfates with alkyl radicals from C 16 to C 18 .
Beispiele für geeignete nichtionische oberflächenaktive Verbindungen, die bevorzugt zusammen mit anionischen oberflächenaktiven Verbindungen benutzt werden, sind insbesondere die Reaktionsprodukte von Alkylenoxiden (gewöhnlich Ethylenoxid) mit Alkylphenolen (Alkylreste von C5 bis C22), wobei die Reaktionsprodukte im allgemeinen 5 bis 25 Ethylenoxid(EO)-Einheiten im Molekül enthalten; die Reaktionsprodukte aliphatischer (C8 bis C18) primärer oder sekundärer, linearer oder verzweigter Alkohole mit Ethylenoxid, mit im allgemeinen 6 bis 30 EO, und die Additionsprodukte von Ethylenoxid an Reaktionsprodukte aus Propylenoxid und Ethylendiamin. Andere nichtionische oberflächenaktive Verbindungen sind Alkylpolyglycoside, langkettige tertiäre Aminoxide, langkettige tertiäre Phosphinoxide und Dialkylsulfoxide.Examples of suitable nonionic surface-active compounds, which are preferably used together with anionic surface-active compounds, are in particular the reaction products of alkylene oxides (usually ethylene oxide) with alkylphenols (alkyl radicals from C 5 to C 22 ), the reaction products generally 5 to 25 ethylene oxide (EO ) Units contained in the molecule; the reaction products of aliphatic (C 8 to C 18 ) primary or secondary, linear or branched alcohols with ethylene oxide, generally with 6 to 30 EO, and the addition products of ethylene oxide with reaction products of propylene oxide and ethylenediamine. Other nonionic surface active compounds are alkyl polyglycosides, long chain tertiary amine oxides, long chain tertiary phosphine oxides and dialkyl sulfoxides.
Amphotere oder zwitterionische oberflächenaktive Verbindungen können ebenfalls in den erfindunsgemäßen Zusammensetzungen verwendet werden, was aber wegen deren hohen Kosten meistens nicht erwünscht ist. Wenn amphotere oder zwitterionische Verbindungen verwendet werden, so geschieht das in der Regel in kleinen Mengen in Zusammensetzungen, die hauptsächlich anionische und nichtionische Tenside enthalten.Amphoteric or zwitterionic surfactants can also be used can be used in the compositions according to the invention, but what is usually not desired because of its high cost. If amphoteric or zwitterionic compounds are used, so this usually happens in small amounts in compositions that are mainly anionic and contain nonionic surfactants.
Auch Seifen können in den erfindungsgemäßen Zusammensetzungen verwendet werden, vorzugsweise mit einem Anteil von weniger als 25 Gew.-%. Sie sind besonders geeignet in geringen Mengen in binären (Seife/anionisches Tensid) oder in ternären Mischungen zusammen mit nichtionischen oder gemischten synthetischen anionischen und nichtionischen Tensiden. Die verwendeten Seifen sind bevorzugt die Natriumsalze, und weniger bevorzugt die Kaliumsalze gesättigter oder ungesättigter C10-C24-Fettsäuren, oder deren Mischungen. Die Anteile solcher Seifen können von 0,5 Gew.-% bis 25 Gew.-% betragen, geringere Mengen von 0,5 Gew.-% bis 5 Gew.-% sind im allgemeinen ausreichend zur Schaumkontrolle. Seifenanteile zwischen etwa 2 % und etwa 20 %, besonders zwischen etwa 5 % und etwa 10 %, haben einen positiven Effekt. Dies ist besonders der Fall in hartem Wasser, wo die Seife als zusätzliche Buildersubstanz dient.Soaps can also be used in the compositions according to the invention, preferably in a proportion of less than 25% by weight. They are particularly suitable in small amounts in binary (soap / anionic surfactant) or in ternary mixtures together with nonionic or mixed synthetic anionic and nonionic surfactants. The soaps used are preferably the sodium salts, and less preferably the potassium salts of saturated or unsaturated C 10 -C 24 fatty acids, or mixtures thereof. The proportions of such soaps can be from 0.5% by weight to 25% by weight, smaller amounts from 0.5% by weight to 5% by weight are generally sufficient for foam control. Soap contents between about 2% and about 20%, especially between about 5% and about 10%, have a positive effect. This is especially the case in hard water, where the soap serves as an additional builder.
Die Wasch- und Reinigungsmittel enthalten im allgemeinen auch einen Builder. Als Builder kommen in Betracht: Calcium bindende Stoffe, Fällungsmittel, Calciumspezifische Ionenaustauscher und deren Mischungen. Beispiele für Calcium bindende Stoffe umfassen Alkalimetallpolyphosphate, wie Natriumtripolyphosphat; Nitrilotriessigsäure und ihre wasserlöslichen Salze; die Alkalimetallsalze der Carboxymethyloxybernsteinsäure, Ethylendiamintetraessigsäure, Oxydibernsteinsäure, Mellithsäure, Benzolpolycarbonsäuren, Zitronensäure und Polyacetalcarboxylate, wie in U.S. Pat. 4 144 226 und 4 146 495 offenbart. The detergents and cleaning agents generally also contain a builder. As Builders come into consideration: calcium binding substances, precipitants, calcium-specific Ion exchangers and their mixtures. Examples of calcium binders include alkali metal polyphosphates such as sodium tripolyphosphate; Nitrilotriacetic acid and its water-soluble salts; the alkali metal salts of Carboxymethyloxy succinic acid, ethylenediaminetetraacetic acid, Oxydisuccinic acid, mellitic acid, benzene polycarboxylic acids, citric acid and Polyacetal carboxylates as described in U.S. Pat. 4,144,226 and 4,146,495.
Beispiele von Fällungsmitteln sind Natriumorthophosphat, Natriumcarbonat und Seifen aus langkettigen Fettsäuren.Examples of precipitants are sodium orthophosphate, sodium carbonate and Long chain fatty acid soaps.
Beispiele von Ionenaustauschern, die für Calcium spezifisch sind, sind die verschiedenen Arten wasserunlöslicher, kristalliner oder amorpher Aluminiumsilicate, von denen die Zeolithe die bekanntesten Vertreter sind.Examples of ion exchangers specific for calcium are various types of water-insoluble, crystalline or amorphous Aluminum silicates, of which the zeolites are the best known representatives.
Diese Buildersubstanzen können von 5 Gew.-% bis 80 Gew.-% vorhanden sein, bevorzugt ist ein Anteil von 10 Gew.-% bis 60 Gew.-%.These builder substances can be present from 5% by weight to 80% by weight, a proportion of 10% by weight to 60% by weight is preferred.
Neben den bereits erwähnten Inhaltsstoffen können die Wasch- und Reinigungsmittel jeden der konventionellen Zusatzstoffen in Mengen enthalten, die man üblicherweise in solchen Mitteln vorfindet. Beispiele für solche Zusatzstoffe sind Schaumbildner, wie etwa Alkanolamide, besonders die Monoethanolamide aus Palmkernöl-Fettsäuren und Kokosnuß-Fettsäuren; schaumverhindernde Substanzen , wie etwa Alkylphopsphate und -silicone; Vergrauungsinhibitoren und ähnliche Hilfsmittel, wie etwa Natriumcarboxymethylcellulose und Alkyl- oder substituierte Alkylcelluloseether; Stabilisatoren, wie Ethylendiamintetraessigsäure; Weichmacher für Textilien; anorganische Salze, wie Natriumsulfat; und, in üblicherweise kleinen Mengen, fluoreszierende Stoffe, Parfüme, Enzyme wie Proteasen, Cellulasen, Lipasen und Amylasen, Desinfektionsmittel und Farbstoffe.In addition to the ingredients already mentioned, the washing and Detergents contain each of the conventional additives in amounts that one usually finds in such means. Examples of such additives are Foaming agents, such as alkanolamides, especially the monoethanolamides Palm kernel oil fatty acids and coconut fatty acids; foam-preventing substances such as alkyl phosphates and silicones; Graying inhibitors and the like Auxiliaries such as sodium carboxymethyl cellulose and alkyl or substituted alkyl cellulose ethers; Stabilizers such as ethylenediaminetetraacetic acid; softener for textiles; inorganic salts such as sodium sulfate; and, usually small Quantities, fluorescent substances, perfumes, enzymes such as proteases, cellulases, Lipases and amylases, disinfectants and dyes.
Die Bleichaktivatoren dieser Erfindung können in einer Vielzahl von Wasch- und Reinigungsmitteln eingesetzt werden. Diese umfassen Textilwaschmittel, Textilbleichmittel, Oberflächenreiniger, Toilettenreiniger, Geschirrspülmaschinenreiniger, und auch Gebißreiniger. Die Waschmittel können in fester Form oder flüssiger Form vorliegen. Es ist aus Gründen der Stabilität und Handhabbarkeit vorteilhaft, die Bleichaktivatoren in Form von Granulaten zu verwenden, die neben dem Bleichkatalysator ein Bindemittel enthalten. Verschiedene Methoden, solche Granulate herzustellen, sind in der Patentliteratur beschrieben, so beispielsweise in Kanada Pat. Nr. 1 102 966, GB-1 561 333, US-4 087 369, EP-A-0 240 057, EP-A-0 241 962, EP-A-0 101 634 und EP-A-0 062 523. Jede dieser Methoden ist für die erfindungsgemäß zu verwendenden Aminonitril-N-oxide anwendbar.The bleach activators of this invention can be used in a variety of laundry and Detergents are used. These include laundry detergents, Textile bleach, surface cleaners, toilet cleaners, Dishwasher cleaner, and also denture cleaner. The detergents can be in solid or liquid form. It is for the sake of stability and Manageability advantageous to the bleach activators in the form of granules use that contain a binder in addition to the bleaching catalyst. Various methods for producing such granules are in the patent literature described, for example, in Canada Pat. No. 1 102 966, GB-1 561 333, US-4 087 369, EP-A-0 240 057, EP-A-0 241 962, EP-A-0 101 634 and EP-A-0 062 523. Each of these methods is suitable for those according to the invention using aminonitrile-N-oxides applicable.
Die die Bleichaktivatoren enthaltenden Granulate werden im allgemeinen der Waschmittelzusammensetzung zusammen mit den anderen trockenen Bestandteilen wie etwa Enzymen, anorganischen Peroxidbleichmitteln zugesetzt. Die Waschmittelzusammensetzung, zu der die Katalysatorgranulate zugegeben werden, kann auf verschiedenen Wegen erhalten werden, wie etwa Trockenmischen, Extrudieren, Sprühtrocknung.The granules containing the bleach activators are generally the Detergent composition along with the other dry Components such as enzymes, inorganic peroxide bleach added. The detergent composition to which the catalyst granules are added can be obtained in several ways, such as Dry mixing, extruding, spray drying.
In einer weiteren Ausführungsform sind die erfindungsgemäßen Bleichaktivatoren besonders geeignet für nicht wäßrige, flüssige Waschmittel, zusammen mit einer bleichenden Peroxidverbindung, etwa Natriumperborat, um dem Waschmittel ein großes Reinigungsvermögen für Gewebe und Textilien zu verleihen. Derartige nicht wäßrige, flüssige Waschmittel, die pastöse und gelatinöse Detergentienzusammensetzungen mit einschließen, sind im Stand der Technik bekannt, und sind beispielsweise in US 2 864 770, US 2 940 938, US 4 772 412, US 3 368 977, GB-A-1 205 711 GB-A-1 370 377, GB-A-1 270 040, GB-A-1 292 352, GB-A-2 194 536, DE-A-2 233 771, EP-A-0 028 849 beschrieben.In a further embodiment, the bleach activators according to the invention are Particularly suitable for non-aqueous, liquid detergents, together with a bleaching peroxide compound, such as sodium perborate, around the detergent great cleaning ability for fabrics and textiles. Not that aqueous, liquid detergents, pasty and gelatinous Including detergent compositions are well known in the art are known, and are described, for example, in US 2,864,770, US 2,940,938, US 4,772,412, US 3 368 977, GB-A-1 205 711 GB-A-1 370 377, GB-A-1 270 040, GB-A-1 292 352, GB-A-2 194 536, DE-A-2 233 771, EP-A-0 028 849.
Es handelt sich dabei um Zusammensetzungen in Form eines nichtwäßrigen, flüssigen Mediums, in dem eine feste Phase dispergiert sein kann. Das nicht wäßrige, flüssige Medium kann eine flüssige, oberflächenaktive Substanz sein, vorzugsweise eine nichtionische oberflächenaktive Substanz; ein nicht polares flüssiges Medium wie etwa flüssiges Paraffin; ein polares Lösungsmittel, wie etwa Polyole, zum Beispiel Glycerin, Sorbitol, Ethylenglycol, eventuell in Verbindung mit niedermolekularen einwertigen Alkoholen wie Ethanol oder Isopropanol; oder Mischungen daraus. These are compositions in the form of a non-aqueous, liquid medium in which a solid phase can be dispersed. Not that one aqueous, liquid medium can be a liquid, surface-active substance, preferably a nonionic surfactant; a non polar liquid medium such as liquid paraffin; a polar solvent such as Polyols, for example glycerin, sorbitol, ethylene glycol, possibly in combination with low molecular weight monohydric alcohols such as ethanol or isopropanol; or Mixtures of these.
Die feste Phase kann aus Buildersubstanzen, Alkalien, abrasiven Stoffen, Polymeren und anderen festen ionischen oberflächenaktiven Stoffen, Bleichmitteln fluoreszierenden Stoffen und anderen üblichen festen Inhaltsstoffen bestehen.The solid phase can consist of builder substances, alkalis, abrasive substances, Polymers and other solid ionic surfactants, bleaches fluorescent substances and other common solid ingredients.
Die folgenden, nicht abschließenden Beispiele sollen einen Überblick über die Ausführungsformen der Erfindung geben.The following non-exhaustive examples are intended to provide an overview of the Embodiments of the invention.
Zur Lösung von 11,7 g para-Dimethylaminobenzonitril in 80 ml Methylenchlorid wurden bei einer Temperatur von 0°C bis -5°C innerhalb von zwei Stunden eine Lösung von 13,8 g meta-Chlorperoxybenzoesäure in 200 ml Methylenchlorid zugetropft. Nachdem kein Peroxid mehr nachweisbar war (negativer KI-Test) wurde das Solvens am Rotationsverdampfer evaporiert. Erhalten wurden 25,7 g para-Dimethylaminobenzonitril-N-oxid meta-Chlorbenzoesäuresalz. Nach Chromatographie an Aluminiumoxid wurde reines para-Dimethylaminobenzonitril-N-oxid isoliert.To dissolve 11.7 g para-dimethylaminobenzonitrile in 80 ml methylene chloride were at a temperature of 0 ° C to -5 ° C within two hours Solution of 13.8 g meta-chloroperoxybenzoic acid in 200 ml methylene chloride dropwise. After peroxide was no longer detectable (negative KI test) the solvent evaporated on a rotary evaporator. 25.7 g of para-dimethylaminobenzonitrile-N-oxide were obtained meta-Chlorbenzoesäuresalz. To Chromatography on alumina became pure para-dimethylaminobenzonitrile-N-oxide isolated.
Durch Zusammengeben von 200 ml einer wäßrigen Lösung Referenzwaschmittel
WMP (Wäschereiforschung Krefeld, 5 g/l in Wasser mit 15° dH) Lösung, 150 mg
natriumperborat-Monohydrat und 50 mg des jeweiligen Aktivators wurde eine
Bleimittelzusammensetzung hergestellt. Mit dieser Zusammensetzung wurden mit
der Standardanschmutzung BC-1-Tee verschmutzte Gewebestücke (auf Baumwolle,
Wäschereiforschung Krefeld) in einem Linitest-Gerät (Heraeus) einer Behandlung
bei einer Temperatur von 40°C unter isothermen Waschbedingungen unterworfen.
Nach einer dreißigminütigen Waschzeit wurden die Gewebestücke mit Wasser
gespült, getrocknet und gebügelt, anschließend wurde die Bleichwirkung durch eine
Bestimmung der Differenzen ΔR(AKT) der Remission vor und nach dem Bleichen
mittels eines Weißgrad-Meßgerätes ELREPHO 2000 (Firma Datacolor) quantifiziert.
Aus diesen ΔR(AKT)-Werten und den in Kontrollversuchen ohne Bleichaktivator
ermittelten ΔR0-Werten wurden die in Tabelle 1 aufgelisteten ΔΔR-Werte berechnet,
die einen direkten Maßstab für die durch den Zusatz an Aktivator hervorgerufene
Verbesserung der Bleichwirkung darstellen:
Die Verbindungen 1 - 5 sind:
Die Waschexperimente zeigen, daß die erfindungsgemäßen Aminonitril-N-oxide
über eine gute Bleichleistung verfügen.
Weitere vorteilhafte Eigenschaften der beschriebenen Komplexe sind geringe
Farbschädigung und geringe Faserschädigung.The washing experiments show that the aminonitrile N-oxides according to the invention have good bleaching performance.
Further advantageous properties of the complexes described are low color damage and low fiber damage.
Claims (8)
- The use of aminonitrile N-oxides of the formula (1) in which
- R1 and R2
- independently of one another are substituted or unsubstituted C1-C15-alkyl, cycloalkyl or aryl radicals which may be substituted by fluorine, chlorine, bromine, C1-C5-alkoxy, C1-C5-alkoxycarbonyl, amino, ammonium, carboxyl, cyano or cyanamino, or together with the nitrogen atom to which they are bonded form a ring having from 4 to 6 carbon atoms which may be substituted by C1-C5-alkyl, C1-C5-alkoxy, C1-C5-alkanoyl, phenyl, amino, ammonium, cyano, cyanamino, chlorine or bromine, which ring can contain, in addition to the nitrogen atom and instead of -CH2- groups, one or two oxygen atoms or a group in which R3 is hydrogen, C1-C7-alkyl or cycloalkyl, phenyl or C7-C9-alkylaryl,
- A
- is a C1-C5-alkylene, a C5-C10-cycloalkylene or an arylene radical,
- The use of aminonitrile N-oxides or salts thereof of the formula (1) as claimed in claim 1, wherein a compound of the formula (1) in which R1 and R2 are C1-C4-alkyl, in particular methyl, and A is phenylene is used.
- A detergent or cleaner comprising a peroxide compound and an aminonitrile N-oxide of the formula (1) as claimed in claim 1.
- A detergent or cleaner comprising a peroxide compound and from 0.05 to 20% by weight of an aminonitrile N-oxide of the formula 1 as claimed in claim 1.
- A detergent or cleaner comprising from 1 to 60% by weight of a peroxide compound and an aminonitrile N-oxide of the formula (1) as claimed in claim 1.
- A detergent or cleaner comprising an aminonitrile N-oxide of the formula (1) as claimed in claim 1 and a perborate, percarbonate, perphosphate, persilicate, monopersulfate, urea peroxide, cumene hydroperoxide or tert-butyl hydroperoxide.
- A detergent or cleaner comprising a peroxide compound, an aminonitrile N-oxide of the formula (1) as claimed in claim 1 and a bleach activator which is not an aminonitrile N-oxide.
- A detergent or cleaner comprising a peroxide compound, an aminonitrile N-oxide of the formula (1) as claimed in claim 1 and surface-active compounds.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19746290 | 1997-10-20 | ||
| DE19746290A DE19746290A1 (en) | 1997-10-20 | 1997-10-20 | Use of aminonitrile-N-oxides as bleach activators |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0909810A1 EP0909810A1 (en) | 1999-04-21 |
| EP0909810B1 true EP0909810B1 (en) | 2004-12-22 |
Family
ID=7846054
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP98117813A Expired - Lifetime EP0909810B1 (en) | 1997-10-20 | 1998-09-19 | Use of aminonitrile-N-oxides as bleach activators |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US6007583A (en) |
| EP (1) | EP0909810B1 (en) |
| JP (1) | JP4021075B2 (en) |
| BR (1) | BR9804057A (en) |
| DE (2) | DE19746290A1 (en) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6903060B1 (en) | 1999-08-27 | 2005-06-07 | Procter & Gamble Company | Stable formulation components, compositions and laundry methods employing same |
| US7109156B1 (en) | 1999-08-27 | 2006-09-19 | Procter & Gamble Company | Controlled availability of formulation components, compositions and laundry methods employing same |
| US6818607B1 (en) * | 1999-08-27 | 2004-11-16 | Procter & Gamble Company | Bleach boosting components, compositions and laundry methods |
| AU6935800A (en) * | 1999-08-27 | 2001-03-26 | Procter & Gamble Company, The | Controlled availability of formulation components, compositions and laundry methods employing same |
| MXPA02002124A (en) * | 1999-08-27 | 2002-09-18 | Procter & Gamble | Stability enhancing formulation components, compositions and laundry methods employing same. |
| WO2001016274A1 (en) * | 1999-08-27 | 2001-03-08 | The Procter & Gamble Company | Stable formulation components, compositions and laundry methods employing same |
| US6825160B1 (en) | 1999-08-27 | 2004-11-30 | Procter & Gamble Company | Color safe laundry methods employing cationic formulation components |
| EP1206520A1 (en) * | 1999-08-27 | 2002-05-22 | The Procter & Gamble Company | Fast-acting formulation components, compositions and laundry methods employing same |
| US6821935B1 (en) | 1999-08-27 | 2004-11-23 | Procter & Gamble Company | Color safe laundry methods employing zwitterionic formulation components |
| DE10064636A1 (en) * | 2000-12-22 | 2002-07-04 | Henkel Kgaa | Liquid washing and / or cleaning agent |
| US7557076B2 (en) * | 2002-06-06 | 2009-07-07 | The Procter & Gamble Company | Organic catalyst with enhanced enzyme compatibility |
| US7169744B2 (en) | 2002-06-06 | 2007-01-30 | Procter & Gamble Company | Organic catalyst with enhanced solubility |
| US20050113246A1 (en) * | 2003-11-06 | 2005-05-26 | The Procter & Gamble Company | Process of producing an organic catalyst |
| AR051659A1 (en) * | 2005-06-17 | 2007-01-31 | Procter & Gamble | A COMPOSITION THAT INCLUDES AN ORGANIC CATALYST WITH IMPROVED ENZYMATIC COMPATIBILITY |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL208181A (en) * | 1955-07-08 | |||
| DE2346504A1 (en) * | 1973-09-15 | 1975-04-24 | Hoechst Ag | PROCESS FOR DYING TEXTILE MATERIAL MADE FROM POLYESTER FIBER / CELLULOSE BLENDS |
| US4883917A (en) * | 1985-10-01 | 1989-11-28 | Ethyl Corporation | Quaternary ammonium compounds |
| US4915863A (en) * | 1987-08-14 | 1990-04-10 | Kao Corporation | Bleaching composition |
| GB9011618D0 (en) * | 1990-05-24 | 1990-07-11 | Unilever Plc | Bleaching composition |
| US5478356B1 (en) * | 1994-05-10 | 1997-11-18 | Clorox Co | Cyanoimides and compositions useful for bleaching |
| US5591378A (en) * | 1994-07-06 | 1997-01-07 | The Clorox Company | Substituted benzonitriles and compositions useful for bleaching |
| JP3811508B2 (en) * | 1994-08-31 | 2006-08-23 | ジョンソン株式会社 | Method for activating peroxide and composition thereof |
| US5739327A (en) * | 1995-06-07 | 1998-04-14 | The Clorox Company | N-alkyl ammonium acetonitrile bleach activators |
| DE19605526A1 (en) * | 1996-02-15 | 1997-08-21 | Hoechst Ag | Ammonium nitriles and their use as bleach activators |
| US5739096A (en) * | 1996-05-06 | 1998-04-14 | S. C. Johnson & Son, Inc. | Cyanopyridine N-oxide peroxide bleach activators |
-
1997
- 1997-10-20 DE DE19746290A patent/DE19746290A1/en not_active Withdrawn
-
1998
- 1998-09-19 EP EP98117813A patent/EP0909810B1/en not_active Expired - Lifetime
- 1998-09-19 DE DE59812405T patent/DE59812405D1/en not_active Expired - Fee Related
- 1998-10-19 JP JP29697798A patent/JP4021075B2/en not_active Expired - Fee Related
- 1998-10-19 BR BR9804057-0A patent/BR9804057A/en not_active Application Discontinuation
- 1998-10-19 US US09/174,841 patent/US6007583A/en not_active Expired - Fee Related
-
1999
- 1999-10-06 US US09/413,831 patent/US6120557A/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| US6120557A (en) | 2000-09-19 |
| DE59812405D1 (en) | 2005-01-27 |
| JP4021075B2 (en) | 2007-12-12 |
| US6007583A (en) | 1999-12-28 |
| BR9804057A (en) | 1999-12-07 |
| JPH11199894A (en) | 1999-07-27 |
| DE19746290A1 (en) | 1999-04-22 |
| EP0909810A1 (en) | 1999-04-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE19605526A1 (en) | Ammonium nitriles and their use as bleach activators | |
| CA2016030C (en) | Bleach activation and bleaching compositions | |
| EP0869171B1 (en) | Metal complexes as bleach activators | |
| DE69825166T2 (en) | Washing and bleaching compositions | |
| EP0909810B1 (en) | Use of aminonitrile-N-oxides as bleach activators | |
| DE2060762A1 (en) | Preparations for the production of cold bleach liquors, in particular washing liquors with a cold bleaching effect | |
| DE19633305A1 (en) | Sulphonylimine derivatives as bleaching catalysts | |
| EP0877078B1 (en) | Bleach activating metal complex | |
| CH642677A5 (en) | Bleach and detergent. | |
| DE10211389A1 (en) | Ammonium nitrile compounds, used as activator for peroxide bleach in laundry, dishwasher and other detergents and disinfectants and in bleaching textile, paper and wood are new | |
| EP0816336A1 (en) | Quaternary ammonium compounds as bleach activators and their preparation | |
| EP0889050A2 (en) | Metal complexes as bleach activators | |
| EP0930358B1 (en) | Use of formamidinium salts as bleach activators | |
| DE19629159A1 (en) | Bleach activators for peroxy compounds in detergents | |
| DE19738274A1 (en) | Cyanopyridine-N-oxides and salts | |
| EP0806473A2 (en) | Bleach activating cyanopyridinium compounds | |
| DE19629162A1 (en) | Salts of cyanamides as bleach activators | |
| EP0698661A2 (en) | Activators for inorganic peroxygen compounds and product containing them | |
| DE19614822A1 (en) | Use of isocyanuric acid, aliphatic or aromatic mono or di:isocyanate | |
| DE102005037761A1 (en) | Detergents and cleaning agents containing 1,3,5-triacetyl-2,4-dioxo-1,3,5-hexahydrotriazine as bleach activator | |
| DE102004041760A1 (en) | Diethylmethyl ammonium nitriles and detergents and cleaners containing these ammonium nitriles | |
| DE102005009135A1 (en) | Ammonium nitriles and their use as bleach activators |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB IT LI NL PT SE |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| 17P | Request for examination filed |
Effective date: 19991021 |
|
| AKX | Designation fees paid |
Free format text: AT BE CH DE DK ES FR GB IT LI NL PT SE |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): DE FR GB IT |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB IT |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REF | Corresponds to: |
Ref document number: 59812405 Country of ref document: DE Date of ref document: 20050127 Kind code of ref document: P |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20050309 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20050923 |
|
| ET | Fr: translation filed | ||
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CD |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20080826 Year of fee payment: 11 Ref country code: FR Payment date: 20080813 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20080915 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20080808 Year of fee payment: 11 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20090919 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20100531 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090930 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100401 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090919 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090919 |