EP0613670B1 - Verfahren zur Herstellung eines Klebeverbandes - Google Patents
Verfahren zur Herstellung eines Klebeverbandes Download PDFInfo
- Publication number
- EP0613670B1 EP0613670B1 EP94301407A EP94301407A EP0613670B1 EP 0613670 B1 EP0613670 B1 EP 0613670B1 EP 94301407 A EP94301407 A EP 94301407A EP 94301407 A EP94301407 A EP 94301407A EP 0613670 B1 EP0613670 B1 EP 0613670B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- adhesive
- strip
- zone
- pad
- process according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0276—Apparatus or processes for manufacturing adhesive dressings or bandages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00089—Wound bandages
- A61F2013/00119—Wound bandages elastic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00544—Plasters form or structure
- A61F2013/00604—Multilayer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00655—Plasters adhesive
- A61F2013/00659—Plasters adhesive polymeric base
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00727—Plasters means for wound humidity control
- A61F2013/00731—Plasters means for wound humidity control with absorbing pads
- A61F2013/0074—Plasters means for wound humidity control with absorbing pads containing foams
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00727—Plasters means for wound humidity control
- A61F2013/00731—Plasters means for wound humidity control with absorbing pads
- A61F2013/00744—Plasters means for wound humidity control with absorbing pads containing non-woven
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00727—Plasters means for wound humidity control
- A61F2013/00748—Plasters means for wound humidity control with hydrocolloids or superabsorbers
- A61F2013/00753—Plasters means for wound humidity control with hydrocolloids or superabsorbers superabsorbent fabric of cloth
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00902—Plasters containing means
- A61F2013/0091—Plasters containing means with disinfecting or anaesthetics means, e.g. anti-mycrobic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/15577—Apparatus or processes for manufacturing
- A61F2013/15821—Apparatus or processes for manufacturing characterized by the apparatus for manufacturing
- A61F2013/15861—Apparatus or processes for manufacturing characterized by the apparatus for manufacturing for bonding
- A61F2013/1591—Apparatus or processes for manufacturing characterized by the apparatus for manufacturing for bonding via adhesive
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/15577—Apparatus or processes for manufacturing
- A61F2013/15821—Apparatus or processes for manufacturing characterized by the apparatus for manufacturing
- A61F2013/15918—Apparatus or processes for manufacturing characterized by the apparatus for manufacturing for die cutting
Definitions
- This invention relates to a process for making an adhesive dressing.
- CH-A-676197 discloses a process for making an adhesive bandage, in which adhesive is continuously applied to a backing strip and a release paper is then applied to cover the adhesive. In a subsequent operation, spots of adhesive are applied to a different region of the backing strip, and absorbent pads are then adhered to such spots of adhesive.
- US-A-2862846 discloses the manufacture of adhesive bandages from a laminate comprising a carrier paper, a plastic film, an adhesive layer on the plastic film, and a silicone coated release paper.
- an adhesive dressing which comprises
- the process of the invention is thus characterized thereby that it is continuous, ie the strip passes continuously and directly from one zone to the next, without intermediate taking up thereof into a roll.
- the adhesive may be a pressure sensitive adhesive.
- the adhesive may be a curable adhesive such as a solvent or emulsion based adhesive. Curing of the adhesive may then take place in the adhesive conditioning zone.
- the pad may then be of curable foam material, as hereinbefore described, with the adhesive coated strip passing through the pad application zone before passing through the conditioning or curing zone, so that curing of the foam material is also effected in the conditioning or curing zone.
- the foam material may be applied continuously to the backing strip, eg so that a single pad extending along the strip is thereby provided.
- the foam material may be applied intermittently to the backing strip, eg so that a plurality of pads spaced longitudinally and/or laterally on the strip are thereby provided.
- the pad(s) may be of fabric.
- the fabric may be applied as a continuous strip to the adhesive coated strip.
- individual fabric pads may be applied intermittently to the adhesive coated strip.
- the fabric, when used as the pad material, is preferably, but not necessarily always, absorbent.
- the pad(s) can instead be of any other suitable material such as fibrous material or a gel.
- the adhesive coated strip may pass through the curing zone before application of the pad(s) thereto.
- the adhesive may be a hot-melt adhesive applied to the backing strip at elevated temperature. Cooling of the adhesive may then be effected in the conditioning zone.
- the pad(s) may then, irrespective of whether they are in the form of a curable foam material, or a non-curable fabric, as hereinbefore described, preferably be applied to the adhesive coated strip after the adhesive has been cooled. Howver, when the pad(s) is/are of curable foam material, the process may include passing the padded strip through a curing stage prior to entering the release paper application zone.
- the process may include passing the continuous adhesive dressing from the release paper application zone to a cutting zone, and cutting it into individual dressings of a desired shape and size in the cutting zone.
- the cutting may be effected by means of a die cutting arrangement, eg by cutting a plurality of dressings side-by-side from the strip.
- the backing strip or layer may be cellulose based, may be in the form of a polymeric film, may be in the form of either a knitted or a non-woven fabric, may be of stretch or non-stretch form, or may be combinations thereof, as desired.
- a foam material is used for the pad(s), it is preferably a SBR styrene butadiene rubber or a polyurethane foam.
- any other foam which can be coated as a relatively thin layer can be used.
- the foam may contain absorbency enhancing materials, such as acrylic superabsorbent powder. It may also contain a medication such as silver sulphur diazine and/or a facing layer such as a plastic net or a non-woven fabric.
- the pad can thus be continuous, ie extending along the strip with the adhesive coated zone(s) located alongside it. Instead, individual pads, spaced from one another along and across the strip, can be provided.
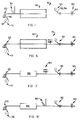
- reference numeral 10 generally indicates a process for making an adhesive dressing, according to a first embodiment of the invention.
- the process 10 includes a backing strip feed zone 12 which contains a roll 14 of backing strip 16.
- the backing strip 16 can be cellulose based, can be in the form of a polymeric strip, can be knitted or non-woven, can be stretch or non-stretch, or can be combinations thereof.
- feed means (not shown) for continuously feeding the backing strip 16 from the roll 14 to an adhesive application zone, generally indicated by reference numeral 20.
- a curable adhesive such as a solvent or emulsion based adhesive, is applied as a layer 22 to one side of the backing strip 16, as the backing strip 16 passes continuously through the zone 20.
- the adhesive coated backing layer passes to a foam coating zone 30 where a foam material, such as polyurethane foam containing absorbency enhancing material such as acrylic superabsorbent powder is applied as a pad 32 to the adhesive coated side of the backing strip or layer 16.
- a foam material such as polyurethane foam containing absorbency enhancing material such as acrylic superabsorbent powder is applied as a pad 32 to the adhesive coated side of the backing strip or layer 16.
- the pad 32 can be applied intermittently, or continuously, along the strip 16, depending on the eventual dressing required.
- the strip passes to a curing/drying zone 40 where both the adhesive and the foam are cured/dried at elevated temperature.
- the strip 16 then passes to a release paper application or laminating zone 50, where overlapping release paper layers 52, 54 are applied over the pad 32 and the adhesive layer or coating 22, while the strip 16 passes continuously therethrough.
- the continuous adhesive dressing strip thus formed passes to a cutting zone 60 where it is cut, by means of a rotating cylindrical die 62, having raised cutting edges 64 defining desired dressing shapes and sizes on its outer surface, for cutting the strip into individual dressings of said desired shape and size.
- dressing strips 70 as indicated in Figure 2, can be formed.
- the dressing strips 70 thus comprise the backing strip 16, the adhesive layer 22 and the foam pad 32.
- a number of dressings can be formed side-by-side across the width of the strip 16, as indicated in Figure 3.
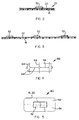
- reference numeral 70 generally indicates a process according to a second embodiment of the invention for making an adhesive dressing.
- the foam coating zone 30 is dispensed with. Instead, individual absorbent fibrous material pads 32 are applied intermittently in a pad application zone 72 located after the curing/drying zone 40.
- reference numeral 80 generally indicates a process according to a third embodiment of the invention for making an adhesive dressing.
- a hot melt adhesive is applied, in the zone 20, to the backing strip 16 at elevated temperature.
- the zones 30, 40 are then dispensed with. Instead, the adhesive coated strip passes through a cooling zone or stage 82 where the adhesive is cooled, whereafter individual absorbent fibrous material pads are applied thereto in the zone 72.
- reference numeral 90 generally indicates a process according to a fourth embodiment of the invention for making an adhesive dressing.
- a hot melt adhesive is also used, with the drying/curing stage 40 of Figure 1 being replaced by the cooling stage 82.
- the foam coating zone 30 of Figure 1 located after the cooling stage 82 and ahead of the release paper application zone 50, is provided.
- curing/drying stage (not shown), similar to the stage 40, can be provided after the zone 30 and ahead of the zone 50.
Landscapes
- Engineering & Computer Science (AREA)
- Health & Medical Sciences (AREA)
- Manufacturing & Machinery (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Adhesive Tapes (AREA)
Claims (12)
- Verfahren zum Herstellen eines Klebeverbandes, welches umfaßt:kontinuierliches Leiten eines Verstärkungsstreifens (16) durch eine Klebstoffauftragezone (20);Auftragen eines Klebstoffes auf eine Seite des Verstärkungsstreifens (16), während er diese Zone (20) kontinuierlich durchläuft, um einen mit Klebstoff beschichteten Streifen zu bilden;
dadurch gekennzeichnet, daß es umfaßt:kontinuierliches Leiten des mit Klebstoff beschichteten Streifens von der Klebstoffauftragezone (20) durch eine Klebstoffkonditionierzone (40), um den Klebstoff zu konditionieren;kontinuierliches Leiten des mit Klebstoff beschichteten Streifens vor oder nach der Klebstoffkonditionierzone durch eine Auflagen-Aufbringzone (30), in der mindestens eine Auflage (32) auf die mit Klebstoff beschichtete Seite des Verstärkungsstreifens derart aufgebracht wird, daß Abschnitte des Klebstoffes nicht durch die Auflage bedeckt werden, wodurch ein mit einer oder mehreren Auflagen versehener Streifen gebildet wird; undkontinuierliches und direktes Leiten des mit Auflagen versehenen Streifens zu einer Schutzpapier-Aufbringzone (50), in der Schutzpapier (52, 54) zumindest über dem Klebstoff aufgebracht wird, um einen Endlos-Klebeverband zu erzeugen. - Verfahren nach Anspruch 1, dadurch gekennzeichnet, daß der Klebstoff ein selbstklebender aushärtbarer Klebstoff ist, wobei das Aushärten des Klebstoffes dann in der Klebstoffkonditionierzone (40) stattfindet.
- Verfahren nach Anspruch 2, dadurch gekennzeichnet, daß die Auflage (32) aus einem aushärtbaren Schaummaterial besteht, wobei der mit Klebstoff beschichtete Streifen die Auflagen-Aufbringzone (30) durchläuft, bevor er die Konditionierzone (40) durchläuft, so daß auch das Aushärten des Schaummaterials in der Konditionierzone (40) erfolgt.
- Verfahren nach Anspruch 3, dadurch gekennzeichnet, daß das Schaummaterial kontinuierlich auf den Verstärkungsstreifen (16) aufgebracht wird.
- Verfahren nach Anspruch 3, dadurch gekennzeichnet, daß das Schaummaterial mit Unterbrechungen auf den Verstärkungsstreifen (16) aufgebracht wird.
- Verfahren nach Anspruch 2, dadurch gekennzeichnet, daß die Auflage (32) aus absorbierendem Stoff besteht und als ein kontinuierlicher Streifen auf den mit Klebstoff beschichteten Streifen (16) aufgebracht wird.
- Verfahren nach Anspruch 2, dadurch gekennzeichnet, daß einzelne Auflagen (32) aus absorbierendem Stoff mit Abstand voneinander auf den mit Klebstoff beschichteten Streifen (16) aufgebracht werden.
- Verfahren nach Anspruch 6 oder 7, dadurch gekennzeichnet, daß der mit Klebstoff beschichtete Streifen die Aushärtzone (40) vor dem Aufbringen der Auflage(n) (32) durchläuft.
- Verfahren nach Anspruch 1, dadurch gekennzeichnet, daß der Klebstoff ein heißschmelzender Klebstoff ist, der bei einer erhöhten Temperatur auf den Verstärkungsstreifen (16) aufgetragen wird, wobei das Abkühlen des Klebstoffes in der Konditionierzone (40) erfolgt und das Aufbringen der Auflage (32) auf den mit Klebstoffbeschichteten Streifen erfolgt, nachdem der Klebstoff abgekühlt worden ist.
- Verfahren nach Anspruch 9, dadurch gekennzeichnet, daß die Auflage (32) aus einem aushärtbaren Schaummaterial besteht, wobei das Verfahren das Leiten des mit einer oder mehreren Auflagen versehenen Streifens durch eine Aushärtstufe (40) vor dem Eintritt in die Schutzpapier-Aufbringzone (50) umfaßt.
- Verfahren nach einem der Ansprüche 1 bis 10, dadurch gekennzeichnet, daß es das Leiten des endlosen Klebeverbandes von der Schutzpapier-Aufbringzone (50) zu einer Schneidzone (60) und das Schneiden zu Einzelverbänden einer gewünschten Form und Größe in der Schneidzone umfaßt.
- Verfahren nach Anspruch 11, dadurch gekennzeichnet, daß das Schneiden durch eine Schneidstempelanordnung (62) erfolgt.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ZA931440 | 1993-03-01 | ||
| ZA931440 | 1993-03-01 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0613670A1 EP0613670A1 (de) | 1994-09-07 |
| EP0613670B1 true EP0613670B1 (de) | 2000-05-31 |
Family
ID=25582613
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP94301407A Expired - Lifetime EP0613670B1 (de) | 1993-03-01 | 1994-02-28 | Verfahren zur Herstellung eines Klebeverbandes |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP0613670B1 (de) |
| BR (1) | BR9400769A (de) |
| CA (1) | CA2116645A1 (de) |
| DE (1) | DE69424698T2 (de) |
| GR (1) | GR1002976B (de) |
| ZA (1) | ZA943224B (de) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU4391497A (en) * | 1996-09-27 | 1998-04-17 | Weston Medical Limited | Needleless injector accessory |
| GB9724814D0 (en) * | 1997-11-25 | 1998-01-21 | Rhodes Harry G | Filtration assembly |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2633128A (en) * | 1949-08-26 | 1953-03-31 | Johnson & Johnson | Pad construction and process |
| US2862846A (en) * | 1953-11-23 | 1958-12-02 | Johnson & Johnson | Method of making plastic strip adhesive bandages |
| CH676197A5 (en) * | 1988-10-21 | 1990-12-28 | Flawa Schweiz Verband Wattefab | Variable pressure bandage - has cushioning pad at one end and system of fastening at other which allows pressure on wound to be changed as often as desired |
-
1994
- 1994-02-28 EP EP94301407A patent/EP0613670B1/de not_active Expired - Lifetime
- 1994-02-28 CA CA002116645A patent/CA2116645A1/en not_active Abandoned
- 1994-02-28 DE DE69424698T patent/DE69424698T2/de not_active Expired - Lifetime
- 1994-03-01 GR GR940100106A patent/GR1002976B/el not_active IP Right Cessation
- 1994-03-01 BR BR9400769A patent/BR9400769A/pt not_active IP Right Cessation
- 1994-05-09 ZA ZA943224A patent/ZA943224B/xx unknown
Also Published As
| Publication number | Publication date |
|---|---|
| DE69424698D1 (de) | 2000-07-06 |
| CA2116645A1 (en) | 1994-09-02 |
| BR9400769A (pt) | 1994-10-11 |
| GR1002976B (el) | 1998-09-23 |
| ZA943224B (en) | 1995-01-18 |
| EP0613670A1 (de) | 1994-09-07 |
| DE69424698T2 (de) | 2001-02-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US3285245A (en) | Absorbent wound dressing | |
| US2896618A (en) | Corrugated dressing | |
| US3331728A (en) | Perforate film-fiber laminate | |
| US6765123B2 (en) | Process for the manufacture of multilayered wound dressings | |
| US3183116A (en) | Method of making perforated adhesive tapes | |
| US6254582B1 (en) | Absorbent product provided in roll form | |
| CA2073274C (en) | Hydrogel bandage | |
| US6566577B1 (en) | Wound dressings having low adherency | |
| US4397704A (en) | Method and apparatus for applying discrete lengths of elastic strip material to a continuously moving web | |
| US4269885A (en) | Laminated material and method of forming | |
| US2862846A (en) | Method of making plastic strip adhesive bandages | |
| US3434472A (en) | Surgical dressings | |
| ES284727Y (es) | Vendaje para heridas externas | |
| RU2000117283A (ru) | Абсорбирующее изделие | |
| US20020111599A1 (en) | Method of manufacturing a disposable absorbent article having a reinforced fastening tape landing zone | |
| EP0613670B1 (de) | Verfahren zur Herstellung eines Klebeverbandes | |
| EP0613669A2 (de) | Verfahren zur Herstellung eines Klebeverbandes | |
| EP1458568B1 (de) | Vefahren zur herstellung von mehrlagigen wundverbänden | |
| MXPA99011483A (es) | Almohadilla, metodo de produccion para la misma y vendaje adhesivo con el uso de dicha almohadilla. | |
| EP4678149A1 (de) | Verbesserte wundverbände und integriertes verfahren zur herstellung von wundverbänden | |
| JP2016537039A (ja) | 医療用接着性材料の製造方法 | |
| JP2006523168A (ja) | 弾性要素を材料ウェブに適用するための配置及び方法 | |
| KR102622473B1 (ko) | 의료용 드레싱 밴드 제조방법 | |
| EP0183460A2 (de) | Wegwerfbare Windel | |
| NL8003586A (nl) | Chirurgisch steriliseerbaar tampon alsmede inrichting voor het continu vervaardigen van een dergelijk tampon. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE FR IT |
|
| 17P | Request for examination filed |
Effective date: 19950209 |
|
| 17Q | First examination report despatched |
Effective date: 19960430 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR IT |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: CHARLTON, DOUGLAS JOHN Inventor name: SPERINCK, PETER GEORGE |
|
| REF | Corresponds to: |
Ref document number: 69424698 Country of ref document: DE Date of ref document: 20000706 |
|
| ET | Fr: translation filed | ||
| ITF | It: translation for a ep patent filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20120220 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20130301 Year of fee payment: 20 Ref country code: DE Payment date: 20130220 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 69424698 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20140301 |