EP0564037B1 - Abrasion resistant stopper to prevent generation of particles by piercing - Google Patents
Abrasion resistant stopper to prevent generation of particles by piercing Download PDFInfo
- Publication number
- EP0564037B1 EP0564037B1 EP93200885A EP93200885A EP0564037B1 EP 0564037 B1 EP0564037 B1 EP 0564037B1 EP 93200885 A EP93200885 A EP 93200885A EP 93200885 A EP93200885 A EP 93200885A EP 0564037 B1 EP0564037 B1 EP 0564037B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- stopper
- spike
- abrasion
- fluid
- elastomeric
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000005299 abrasion Methods 0.000 title claims abstract description 29
- 239000002245 particle Substances 0.000 title claims abstract description 12
- 239000013536 elastomeric material Substances 0.000 claims abstract description 13
- 239000012530 fluid Substances 0.000 claims abstract description 10
- -1 polytetrafluoroethylene Polymers 0.000 claims abstract description 8
- 238000001802 infusion Methods 0.000 claims description 18
- 238000000034 method Methods 0.000 claims description 6
- 239000004800 polyvinyl chloride Substances 0.000 claims description 6
- 229920000915 polyvinyl chloride Polymers 0.000 claims description 5
- 229920002292 Nylon 6 Polymers 0.000 claims description 4
- 230000004888 barrier function Effects 0.000 claims description 4
- 229920000139 polyethylene terephthalate Polymers 0.000 claims description 4
- 239000005020 polyethylene terephthalate Substances 0.000 claims description 4
- 239000005033 polyvinylidene chloride Substances 0.000 claims description 4
- 229920001634 Copolyester Polymers 0.000 claims description 3
- 239000004721 Polyphenylene oxide Substances 0.000 claims description 3
- 239000004793 Polystyrene Substances 0.000 claims description 3
- 229920001328 Polyvinylidene chloride Polymers 0.000 claims description 3
- 229920001577 copolymer Polymers 0.000 claims description 3
- 229920000515 polycarbonate Polymers 0.000 claims description 3
- 239000004417 polycarbonate Substances 0.000 claims description 3
- 229920006380 polyphenylene oxide Polymers 0.000 claims description 3
- 229920002223 polystyrene Polymers 0.000 claims description 3
- 239000011118 polyvinyl acetate Substances 0.000 claims description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 claims description 2
- 229920012287 polyphenylene sulfone Polymers 0.000 claims description 2
- 229920002689 polyvinyl acetate Polymers 0.000 claims description 2
- 239000011248 coating agent Substances 0.000 abstract description 12
- 238000000576 coating method Methods 0.000 abstract description 12
- 229920001343 polytetrafluoroethylene Polymers 0.000 abstract description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 abstract 1
- 238000007789 sealing Methods 0.000 abstract 1
- 230000035515 penetration Effects 0.000 description 8
- 239000000463 material Substances 0.000 description 4
- 239000013618 particulate matter Substances 0.000 description 4
- 239000012634 fragment Substances 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 239000000314 lubricant Substances 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 229920001296 polysiloxane Polymers 0.000 description 3
- 229920001971 elastomer Polymers 0.000 description 2
- 239000003182 parenteral nutrition solution Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000012791 spiking procedure Methods 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 229920001169 thermoplastic Polymers 0.000 description 2
- 239000004416 thermosoftening plastic Substances 0.000 description 2
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- 229920000265 Polyparaphenylene Polymers 0.000 description 1
- 208000034809 Product contamination Diseases 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229920005556 chlorobutyl Polymers 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical compound FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000012633 leachable Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229920003052 natural elastomer Polymers 0.000 description 1
- 229920001194 natural rubber Polymers 0.000 description 1
- 239000011236 particulate material Substances 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 229920002545 silicone oil Polymers 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000012421 spiking Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 125000001174 sulfone group Chemical group 0.000 description 1
- 229920003051 synthetic elastomer Polymers 0.000 description 1
- 239000005061 synthetic rubber Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D51/00—Closures not otherwise provided for
- B65D51/002—Closures to be pierced by an extracting-device for the contents and fixed on the container by separate retaining means
Definitions
- This invention relates to a stopper for a container and, more particularly, to an improved stopper for a container of parenteral solutions which is suitable for infusion spike penetration without producing unacceptable amounts of particulate matter.
- Stopper systems for vials, bottles and the like are made of materials that are resistant to chemicals and pharmaceuticals such as corrosive materials, reagents, parenteral solutions and solid formulations reconstitutable with a solvent prior to use.
- the most commonly used stopper system for such products has been glass or plastic bottles and vials equipped with rubber stoppers made of elastomeric materials. The system appears to provide for good hermetical seal, safe storage and easy access to the content through the elastomeric stopper via the use of an infusion spike when withdrawal of the content is desired.
- the elastomeric stopper used comprises an elastomeric base, such as natural or synthetic rubber and an inert coating covering at least some portions of the stopper.
- the coating used heretofore includes chlorobutyl rubber, polymeric fluorocarbon resins such as polytetrafluoroethylene (TEFLON) and various thermoplastic films.
- the coating is intended to insulate the elastomeric stopper base from the content of the container in order to prevent contact and possible chemical reactions therebetween.
- untreated elastomeric stoppers offer a high degree of resistance against the exterior surface of the spike as the spike is being pushed into the penetration area.
- stopper fragments are generated, they are the result of the elastomeric portion of the stopper being abraded off the upper surface of the stopper as it conforms to the shape of the penetrating spike. The fragments are then transported into the interior wall of the vial as the spike rolls and drags the fragments during penetration.
- an abrasion resistant stopper for a medical vial containing a fluid therein, the stopper being adapted to be pierced by an infusion spike and comprising a stopper body of an elastomeric material having a head portion and a fluid contacting leg portion, said leg portion being adapted to be inserted into said medical vial to hermetically seal said fluid therein, said head portion having a bottom, fluid-contacting surface and a top having a central pierceable portion, said central portion having a spike-receiving surface.
- Silicone lubricant applied to the top of the stopper body head portion provides abrasion resistance.
- silicone does reduce particle generation from the spiking procedure it also increases the risk of product contamination from its own composition.
- the present invention addresses the need to eliminate or at least greatly reduce the particle generation from surface erosion of the stopper during spike penetration.
- the invention reduces the risk of the push-through and blow-out tendency by minimizing frictional drag and residual elastic tension during spike penetration.
- the present invention is characterised in that said spike-receiving surface is coated with an abrasion resistant film, said film being adapted to conform to the edges of a hole created by an infusion spike upon said spike piercing the stopper and providing a barrier between the spike and the elastomeric material, thereby preventing mechanical contact between the spike and the elastomeric material and the consequent generation of elastomeric particles by abrasion, said abrasion-resistant film being selected from the group consisting of polystyrene, polyvinyl acetate, polyvinyl chloride, polyvinylidene chloride, copolymer of polyvinyl chloride and polyvinylidine chloride, polymethylene oxide, polyphenylene oxide, polyphenylene sulfone, polyethylene terephthalate, polycarbonate, copolyesters and polycaprolactam.
- the top of the head portion has a region surrounding the coated central portion which is not coated.
- the coating on the top surface of the stopper conforms to the deformation of the stopper caused by the spike penetration procedure. It appears that, upon piercing, the spike is not in contact with the elastomeric stopper body but only with the abrasion-resistant coating thereby circumventing abrasion and eliminating the formation of elastomeric particulate materials.
- the present invention provides a method of preventing the generation of elastomeric particles by abrasion between an infusion spike and an abrasion resistant stopper as defined above, wherein during piercing of the stopper by the infusion spike the abrasion resistant film of the stopper provides a barrier between the infusion spike and the elastomeric material of the stopper thereby preventing mechanical contact between the infusion spike and the elastomeric material and the consequent generation of elastomeric particles by abrasion.
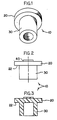
- numeral 10 shows one embodiment of the stopper of the present invention comprising: a head portion 20 and a leg portion 30.
- Head portion 20 comprises a flange 22 which is adapted to cover a corresponding planar, circular mouth portion of a medical vial, while leg portion 30 is adapted for insertion into the neck of the vial to tightly seal the content therein.
- Numeral 40 shows an abrasion-resistant film mounted on the centre part of the head portion 20 which serves as the piercing area for insertion and withdrawal of a spike.
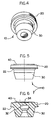
- numeral 10 shows another embodiment of the stopper of the present invention comprising: a head portion 20 and a leg portion 30.
- Head portion 20 comprises a flange 22 which is adapted to cover a corresponding planar, circular mouth portion of a medical vial, while leg portion 30 is adapted for insertion into the neck of the vial to tightly seal the content therein.
- Numeral 40 shows an abrasion-resistant film mounted on the top part of the head portion 20.
- recess 32 extends toward the top surface of the head portion 20 forming a thin portion 34 in head portion 20 for facilitating piercing of the stopper by a spike.
- FIG. 7 illustrates a stopper 10 having an abrasion-resistant film 40 covering vial 1.
- Vial 1 containing an injectable fluid 5, is sealed by a stopper (which could be the stopper of FIGS. 1-3 or that of FIGS. 4-6) by inserting leg portion 30 of the stopper into the neck 7 of the vial 1.
- Flange portion 22 of head portion 20 tightly seals the mouth 8 of vial 1.
- a thin metal foil 9 is crimped over head portion 20 and flange portion 22 of the stopper to tightly seal and securely hold the stopper in vial 1.
- the elastomeric material of the stopper body must be a fluid-impervious, resilient and inert material without leachable additives therein in order to prevent any alteration of the product contained in the vial. It may be of a single component or a blend of components.
- the elastomeric material may be made into the desired stopper configuration by known methods. Such methods conventionally include the use of a curing agent, a stabilizer and a filler and comprise a primary and secondary curing step at elevated temperatures.
- the abrasion-resistant coating for covering the top portion of the stopper, but at least the centre, pierceable portion thereof, may be: polystyrene, polyvinyl acetate (PVA), polyvinyl chloride (PVC), polyvinylidene chloride (PVDC), a copolymer of PVC and PVDC, polymethylene oxide, polyphenylene oxide, polyphenylene sulphone, polyethylene terephthalate (PET), polycarbonate, copolyesters and polycaprolactam (Nylon 6).
- the coating thickness will be in the range of about 0.002 to 1.0 mm, and preferably about 0.02 to 0.5 mm.
- the coating may be applied or bonded to the stopper body in any suitable manner known in the art, such as, but not limited to, by the use of adhesives, solvents, spray applications, radio waves, infrared, microwaves, ultrasonics and heat or a combination thereof.
Landscapes
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Closures For Containers (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Radiation-Therapy Devices (AREA)
- Drilling Tools (AREA)
- Polishing Bodies And Polishing Tools (AREA)
- Heating, Cooling, Or Curing Plastics Or The Like In General (AREA)
- Physical Or Chemical Processes And Apparatus (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Glass Compositions (AREA)
- Tires In General (AREA)
- Materials For Medical Uses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Wrappers (AREA)
- Transition And Organic Metals Composition Catalysts For Addition Polymerization (AREA)
- Polysaccharides And Polysaccharide Derivatives (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Arc Welding In General (AREA)
- Control And Other Processes For Unpacking Of Materials (AREA)
- Passenger Equipment (AREA)
Abstract
Description
- This invention relates to a stopper for a container and, more particularly, to an improved stopper for a container of parenteral solutions which is suitable for infusion spike penetration without producing unacceptable amounts of particulate matter.
- Stopper systems for vials, bottles and the like are made of materials that are resistant to chemicals and pharmaceuticals such as corrosive materials, reagents, parenteral solutions and solid formulations reconstitutable with a solvent prior to use. The most commonly used stopper system for such products has been glass or plastic bottles and vials equipped with rubber stoppers made of elastomeric materials. The system appears to provide for good hermetical seal, safe storage and easy access to the content through the elastomeric stopper via the use of an infusion spike when withdrawal of the content is desired. The elastomeric stopper used comprises an elastomeric base, such as natural or synthetic rubber and an inert coating covering at least some portions of the stopper. The coating used heretofore includes chlorobutyl rubber, polymeric fluorocarbon resins such as polytetrafluoroethylene (TEFLON) and various thermoplastic films. The coating is intended to insulate the elastomeric stopper base from the content of the container in order to prevent contact and possible chemical reactions therebetween.
- One of the major concerns in all products, and especially pharmaceutical parenteral products, is the generation of particulate foreign matter which may contaminate such products. In order to eliminate macroscopic and microscopic particulates, elaborate measures have been taken to remove them, such as filtration of the product and special washing and drying of the stopper system components. These steps help assure that the products meet the requirements and guidelines of the pharmaceutical industry, such as compendia guidelines, when the products reach the point of use. However, at the point of use, such as in the case of a parenteral product, new particulate matter is frequently generated by the practitioner when the stopper is penetrated by a spike of an infusion set or an infusion spike. During such penetration a combination of elastic and plastic deformation of the stopper target area increases the stopper contact surface with the infusion spike as it is pressed into the stopper. Typically, untreated elastomeric stoppers offer a high degree of resistance against the exterior surface of the spike as the spike is being pushed into the penetration area. Most frequently, when stopper fragments are generated, they are the result of the elastomeric portion of the stopper being abraded off the upper surface of the stopper as it conforms to the shape of the penetrating spike. The fragments are then transported into the interior wall of the vial as the spike rolls and drags the fragments during penetration.
- In addition to the problem of particulate matter produced and carried into the vial during the spiking procedure, there are two other, although less frequently occurring, anomalies: stopper push-through into the vial and spike blow-out caused by residual elastic tension of the stopper against the spike which urges the spike outward.
- The most common solution to these problems has been the application of silicone lubricant to the stopper and/or the spike to reduce the frictional drag between the stopper and the spike. It is thus known to provide an abrasion resistant stopper for a medical vial containing a fluid therein, the stopper being adapted to be pierced by an infusion spike and comprising a stopper body of an elastomeric material having a head portion and a fluid contacting leg portion, said leg portion being adapted to be inserted into said medical vial to hermetically seal said fluid therein, said head portion having a bottom, fluid-contacting surface and a top having a central pierceable portion, said central portion having a spike-receiving surface. Silicone lubricant applied to the top of the stopper body head portion provides abrasion resistance. However, while silicone does reduce particle generation from the spiking procedure it also increases the risk of product contamination from its own composition.
- Another approach proposed in the prior art to reduce the tendency of the stopper to generate particulate matter during manufacturing and storage is to coat the elastomeric core of the stopper with a thermoplastic film on the fluid-contacting side thereof. We have found, however, that the use of such construction is less than satisfactory to solve the problem.
- The present invention addresses the need to eliminate or at least greatly reduce the particle generation from surface erosion of the stopper during spike penetration. In addition, the invention reduces the risk of the push-through and blow-out tendency by minimizing frictional drag and residual elastic tension during spike penetration. These advantages are achieved without the use of a lubricant, such as silicone oil, which could contaminate the product contained in the vial or bottle.
- We have found surprisingly that if a non-reactive, inert, coating which is highly resistant to abrasion is applied to the upper surface of an elastomeric stopper where spike penetration will take place, particle generation during spiking is all but eliminated and the tendency of push-through as well as blow-out of the spike is greatly reduced.
- It is known from EP-A-0 294 127 to provide a fluorine resin film on the top of an elastomeric stopper, and from US-A-4 499 148 to provide a polyolefin film on the top of an elastomeric stopper. In both cases, the film is intended to reduce the occurrence of elastomeric particles due to a coring process when a hypodermic needle pierces the elastomer, resulting in the cutting out of a cylindrical elastomeric portion which may then undergo fragmentation into smaller pieces. The documents are not concerned with the problem of abrasion between the outer surface of an infusion spike and the surface of a hole which it creates.
- Viewed from one aspect, the present invention is characterised in that said spike-receiving surface is coated with an abrasion resistant film, said film being adapted to conform to the edges of a hole created by an infusion spike upon said spike piercing the stopper and providing a barrier between the spike and the elastomeric material, thereby preventing mechanical contact between the spike and the elastomeric material and the consequent generation of elastomeric particles by abrasion, said abrasion-resistant film being selected from the group consisting of polystyrene, polyvinyl acetate, polyvinyl chloride, polyvinylidene chloride, copolymer of polyvinyl chloride and polyvinylidine chloride, polymethylene oxide, polyphenylene oxide, polyphenylene sulfone, polyethylene terephthalate, polycarbonate, copolyesters and polycaprolactam.
- Preferably the top of the head portion has a region surrounding the coated central portion which is not coated.
- In use, the coating on the top surface of the stopper conforms to the deformation of the stopper caused by the spike penetration procedure. It appears that, upon piercing, the spike is not in contact with the elastomeric stopper body but only with the abrasion-resistant coating thereby circumventing abrasion and eliminating the formation of elastomeric particulate materials.
- Viewed from another aspect, therefore, the present invention provides a method of preventing the generation of elastomeric particles by abrasion between an infusion spike and an abrasion resistant stopper as defined above, wherein during piercing of the stopper by the infusion spike the abrasion resistant film of the stopper provides a barrier between the infusion spike and the elastomeric material of the stopper thereby preventing mechanical contact between the infusion spike and the elastomeric material and the consequent generation of elastomeric particles by abrasion.
- Certain preferred embodiments of the invention will now be described by way of example and with reference to the following drawings in which
- FIG. 1 is a perspective view of one embodiment of the stopper of the present invention;
- FIG. 2 is a plan view of the stopper shown in FIG. 1;
- FIG. 3 is a cross-sectional view of the stopper shown in FIG. 2 taken along the line a-a;
- FIG. 4 is a perspective view of another embodiment of the stopper of the present invention;
- FIG. 5 is a plan view of the stopper shown in FlG.4;
- FIG. 6 is a cross-sectional view of the stopper shown in FIG. 2 taken-along the line b-b; and
- FIG. 7 is a cross-section of a vial containing an injectable liquid closed with the stopper of the present invention.
- Referring to FIGS. 1, 2 and 3,
numeral 10 shows one embodiment of the stopper of the present invention comprising: ahead portion 20 and aleg portion 30.Head portion 20 comprises aflange 22 which is adapted to cover a corresponding planar, circular mouth portion of a medical vial, whileleg portion 30 is adapted for insertion into the neck of the vial to tightly seal the content therein. Numeral 40 shows an abrasion-resistant film mounted on the centre part of thehead portion 20 which serves as the piercing area for insertion and withdrawal of a spike. - Referring to FIGS. 4, 5 and 6,
numeral 10 shows another embodiment of the stopper of the present invention comprising: ahead portion 20 and aleg portion 30.Head portion 20 comprises aflange 22 which is adapted to cover a corresponding planar, circular mouth portion of a medical vial, whileleg portion 30 is adapted for insertion into the neck of the vial to tightly seal the content therein. Numeral 40 shows an abrasion-resistant film mounted on the top part of thehead portion 20. In thisembodiment recess 32 extends toward the top surface of thehead portion 20 forming athin portion 34 inhead portion 20 for facilitating piercing of the stopper by a spike. - FIG. 7 illustrates a
stopper 10 having an abrasion-resistant film 40 covering vial 1. Vial 1, containing aninjectable fluid 5, is sealed by a stopper (which could be the stopper of FIGS. 1-3 or that of FIGS. 4-6) by insertingleg portion 30 of the stopper into theneck 7 of the vial 1.Flange portion 22 ofhead portion 20 tightly seals themouth 8 of vial 1. Athin metal foil 9 is crimped overhead portion 20 andflange portion 22 of the stopper to tightly seal and securely hold the stopper in vial 1. - The elastomeric material of the stopper body must be a fluid-impervious, resilient and inert material without leachable additives therein in order to prevent any alteration of the product contained in the vial. It may be of a single component or a blend of components.
- The elastomeric material may be made into the desired stopper configuration by known methods. Such methods conventionally include the use of a curing agent, a stabilizer and a filler and comprise a primary and secondary curing step at elevated temperatures.
- The abrasion-resistant coating for covering the top portion of the stopper, but at least the centre, pierceable portion thereof, may be: polystyrene, polyvinyl acetate (PVA), polyvinyl chloride (PVC), polyvinylidene chloride (PVDC), a copolymer of PVC and PVDC, polymethylene oxide, polyphenylene oxide, polyphenylene sulphone, polyethylene terephthalate (PET), polycarbonate, copolyesters and polycaprolactam (Nylon 6).
- The coating thickness will be in the range of about 0.002 to 1.0 mm, and preferably about 0.02 to 0.5 mm. The coating may be applied or bonded to the stopper body in any suitable manner known in the art, such as, but not limited to, by the use of adhesives, solvents, spray applications, radio waves, infrared, microwaves, ultrasonics and heat or a combination thereof.
- The present invention has been described in connection with the preferred embodiments shown in the drawings but is in no way limited thereto. It is to be noted that various changes and modifications of the invention as claimed are apparent to those skilled in the art.
Claims (3)
- An abrasion resistant stopper (10) for a medical vial (1) containing a fluid therein, the stopper being adapted to be pierced by an infusion spike and comprising a stopper body of an elastomeric material having a head portion (20) and a fluid contacting leg portion (30), said leg portion being adapted to be inserted into said medical vial to hermetically seal said fluid therein, said head portion having a bottom, fluid-contacting surface and a top having a central pierceable portion, said central portion having a spike-receiving surface, characterised in that said spike-receiving surface is coated with an abrasion resistant film (40), said film being adapted to conform to the edges of a hole created by an infusion spike upon said spike piercing the stopper and providing a barrier between the spike and the elastomeric material, thereby preventing mechanical contact between the spike and the elastomeric material and the consequent generation of elastomeric particles by abrasion, said abrasion-resistant film being selected from the group consisting of polystyrene, polyvinyl acetate, polyvinyl chloride, polyvinylidene chloride, copolymer of polyvinyl chloride and polyvinylidine chloride, polymethylene oxide, polyphenylene oxide, polyphenylene sulfone, polyethylene terephthalate, polycarbonate, copolyesters and polycaprolactam.
- An abrasion resistant stopper as claimed in claim 1, wherein the top of the head portion (20) has a region surrounding the coated central portion which is not coated.
- A method of preventing the generation of elastomeric particles by abrasion between an infusion spike and an abrasion resistant stopper (10) as claimed in claim 1 or 2, wherein during piercing of the stopper by the infusion spike the abrasion resistant film (40) of the stopper provides a barrier between the infusion spike and the elastomeric material of the stopper thereby preventing mechanical contact between the infusion spike and the elastomeric material and the consequent generation of elastomeric particles by abrasion.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US862120 | 1986-05-12 | ||
| US86212092A | 1992-04-02 | 1992-04-02 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0564037A1 EP0564037A1 (en) | 1993-10-06 |
| EP0564037B1 true EP0564037B1 (en) | 1997-07-30 |
Family
ID=25337719
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP93200885A Expired - Lifetime EP0564037B1 (en) | 1992-04-02 | 1993-03-27 | Abrasion resistant stopper to prevent generation of particles by piercing |
Country Status (20)
| Country | Link |
|---|---|
| EP (1) | EP0564037B1 (en) |
| JP (1) | JPH0639017A (en) |
| KR (1) | KR930021174A (en) |
| AT (1) | ATE156088T1 (en) |
| AU (1) | AU666910B2 (en) |
| BR (1) | BR9301388A (en) |
| CA (1) | CA2091020C (en) |
| CZ (1) | CZ54393A3 (en) |
| DE (1) | DE69312545T2 (en) |
| DK (1) | DK0564037T3 (en) |
| ES (1) | ES2108205T3 (en) |
| FI (1) | FI931501A (en) |
| HU (1) | HUT67955A (en) |
| IL (1) | IL105247A (en) |
| MX (1) | MX9301044A (en) |
| MY (1) | MY131318A (en) |
| NO (1) | NO931288L (en) |
| NZ (1) | NZ247323A (en) |
| SK (1) | SK27493A3 (en) |
| TW (1) | TW227523B (en) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6386392B1 (en) | 1999-11-02 | 2002-05-14 | The Procter & Gamble Company | Reservoirs for use with cleaning devices |
| EP1078880B1 (en) | 1999-08-05 | 2003-03-19 | The Procter & Gamble Company | Dispensing device comprising a reservoir and attachment means provided with protected piercing means |
| US6321941B1 (en) | 2000-04-20 | 2001-11-27 | The Procter & Gamble Company | Consumer safe fitment for connecting a reservoir to a dispensing appliance |
| JP4601127B2 (en) * | 2000-06-06 | 2010-12-22 | 住友ゴム工業株式会社 | Medical rubber stopper |
| TWI303565B (en) | 2002-08-16 | 2008-12-01 | Glaxosmithkline Biolog Sa | Closure system,vial having the closure system,method of closing a vial,method of filling a pharmaceutical vial, and vial closure |
| EP2546152A3 (en) | 2002-09-03 | 2014-03-26 | Medical Instill Technologies, Inc. | Sealed containers and methods of making and filling same |
| US20090196798A1 (en) * | 2008-02-06 | 2009-08-06 | Robert Sassa | Barrier with Low Extractables and Resealing Properties |
| CN102283776B (en) * | 2011-07-07 | 2013-09-04 | 海南卫康制药(潜山)有限公司 | Puncture-free bottle cork and application method thereof |
| DE102012101509A1 (en) * | 2012-02-24 | 2013-08-29 | Krones Aktiengesellschaft | Pierceable plastic closure for sealing containers |
| USD713931S1 (en) | 2013-01-09 | 2014-09-23 | Central Garden & Pet Company | Sprayer |
| CN113474083A (en) * | 2019-01-04 | 2021-10-01 | 仪器实验室公司 | Container stopper for high puncture count applications |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3628681A (en) * | 1969-10-06 | 1971-12-21 | Plastics Consulting And Mfg Co | Stopper |
| US4499148A (en) * | 1983-01-10 | 1985-02-12 | Canton Bio-Medical Products, Inc. | Composite materials of silicone elastomers and polyolefin films, and method of making |
| JP2545540B2 (en) * | 1987-05-29 | 1996-10-23 | 株式会社 大協精工 | Double-sided laminated rubber stopper |
| DE8906346U1 (en) * | 1989-05-23 | 1989-09-14 | Pharma-Gummi Wimmer West Gmbh, 5180 Eschweiler | Closure for medicine bottles |
-
1993
- 1993-02-04 AU AU32805/93A patent/AU666910B2/en not_active Ceased
- 1993-02-22 TW TW082101220A patent/TW227523B/zh active
- 1993-02-25 MX MX9301044A patent/MX9301044A/en unknown
- 1993-03-04 CA CA002091020A patent/CA2091020C/en not_active Expired - Lifetime
- 1993-03-27 AT AT93200885T patent/ATE156088T1/en not_active IP Right Cessation
- 1993-03-27 ES ES93200885T patent/ES2108205T3/en not_active Expired - Lifetime
- 1993-03-27 DK DK93200885.7T patent/DK0564037T3/en active
- 1993-03-27 DE DE69312545T patent/DE69312545T2/en not_active Expired - Lifetime
- 1993-03-27 EP EP93200885A patent/EP0564037B1/en not_active Expired - Lifetime
- 1993-03-29 JP JP5069697A patent/JPH0639017A/en active Pending
- 1993-03-31 KR KR1019930005347A patent/KR930021174A/en not_active Application Discontinuation
- 1993-03-31 CZ CZ93543A patent/CZ54393A3/en unknown
- 1993-03-31 BR BR9301388A patent/BR9301388A/en not_active Application Discontinuation
- 1993-04-01 IL IL105247A patent/IL105247A/en not_active IP Right Cessation
- 1993-04-02 FI FI931501A patent/FI931501A/en not_active Application Discontinuation
- 1993-04-02 NO NO93931288A patent/NO931288L/en unknown
- 1993-04-02 NZ NZ247323A patent/NZ247323A/en unknown
- 1993-04-02 MY MYPI93000586A patent/MY131318A/en unknown
- 1993-04-02 HU HU9300971A patent/HUT67955A/en unknown
- 1993-04-05 SK SK274-93A patent/SK27493A3/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| IL105247A (en) | 1997-04-15 |
| TW227523B (en) | 1994-08-01 |
| BR9301388A (en) | 1993-10-13 |
| JPH0639017A (en) | 1994-02-15 |
| FI931501A0 (en) | 1993-04-02 |
| AU666910B2 (en) | 1996-02-29 |
| SK27493A3 (en) | 1993-11-10 |
| AU3280593A (en) | 1993-10-07 |
| NZ247323A (en) | 1996-03-26 |
| CZ54393A3 (en) | 1995-11-15 |
| MX9301044A (en) | 1993-10-01 |
| NO931288L (en) | 1993-10-04 |
| ES2108205T3 (en) | 1997-12-16 |
| HU9300971D0 (en) | 1993-06-28 |
| FI931501A (en) | 1993-10-03 |
| KR930021174A (en) | 1993-11-22 |
| HUT67955A (en) | 1995-05-29 |
| NO931288D0 (en) | 1993-04-02 |
| CA2091020A1 (en) | 1993-10-03 |
| EP0564037A1 (en) | 1993-10-06 |
| IL105247A0 (en) | 1993-08-18 |
| DE69312545D1 (en) | 1997-09-04 |
| ATE156088T1 (en) | 1997-08-15 |
| DE69312545T2 (en) | 1998-02-19 |
| DK0564037T3 (en) | 1997-09-29 |
| CA2091020C (en) | 2005-01-25 |
| MY131318A (en) | 2007-08-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5219083A (en) | Stopper for reduction of particulate matter | |
| AU753628B2 (en) | Closure system for containers | |
| EP0573102B1 (en) | Double-seal elastomeric stopper | |
| EP0564037B1 (en) | Abrasion resistant stopper to prevent generation of particles by piercing | |
| US10246232B2 (en) | Septa | |
| EP0960616A2 (en) | Multiple use universal stopper | |
| EP1008337B1 (en) | Medicament container closure with recessed integral spike access means | |
| EP1221923B1 (en) | Transfer set for vials and other medical containers | |
| US7615271B2 (en) | Method of fusing a component to a medical storage or transfer device and container assembly | |
| EP0956849A2 (en) | Universal stopper | |
| EP0172613A2 (en) | Resin-laminated rubber plug | |
| US3198368A (en) | Container closure | |
| US5794804A (en) | Pharmaceutical container with metal closures having reduced particulates | |
| US6682518B1 (en) | Injectable micro-glass vial | |
| US6461345B1 (en) | Cannula operated pinch valve |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL PT SE |
|
| 17P | Request for examination filed |
Effective date: 19940316 |
|
| 17Q | First examination report despatched |
Effective date: 19950413 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: NYCOMED IMAGING AS |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL PT SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19970730 Ref country code: LI Effective date: 19970730 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19970730 Ref country code: CH Effective date: 19970730 Ref country code: BE Effective date: 19970730 Ref country code: AT Effective date: 19970730 |
|
| REF | Corresponds to: |
Ref document number: 156088 Country of ref document: AT Date of ref document: 19970815 Kind code of ref document: T |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REF | Corresponds to: |
Ref document number: 69312545 Country of ref document: DE Date of ref document: 19970904 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 |
|
| ITF | It: translation for a ep patent filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19971030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Effective date: 19971105 |
|
| ET | Fr: translation filed | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2108205 Country of ref document: ES Kind code of ref document: T3 |
|
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980327 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980331 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980930 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20120326 Year of fee payment: 20 Ref country code: FR Payment date: 20120406 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20120326 Year of fee payment: 20 Ref country code: IT Payment date: 20120327 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20120328 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 69312545 Country of ref document: DE Representative=s name: J D REYNOLDS & CO., GB |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 69312545 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20130326 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20130326 Ref country code: DE Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20130328 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20120326 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20130705 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20130328 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20130327 |