EP0502397A2 - Preparation process for soft magnetic Fe-containing material with high saturation magnetisation and ultrafine structure - Google Patents
Preparation process for soft magnetic Fe-containing material with high saturation magnetisation and ultrafine structure Download PDFInfo
- Publication number
- EP0502397A2 EP0502397A2 EP92103081A EP92103081A EP0502397A2 EP 0502397 A2 EP0502397 A2 EP 0502397A2 EP 92103081 A EP92103081 A EP 92103081A EP 92103081 A EP92103081 A EP 92103081A EP 0502397 A2 EP0502397 A2 EP 0502397A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- main component
- powder
- component
- heat treatment
- starting powder
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/03—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity
- H01F1/12—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials
- H01F1/14—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys
- H01F1/20—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys in the form of particles, e.g. powder
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/02—Making metallic powder or suspensions thereof using physical processes
- B22F9/04—Making metallic powder or suspensions thereof using physical processes starting from solid material, e.g. by crushing, grinding or milling
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C33/00—Making ferrous alloys
- C22C33/02—Making ferrous alloys by powder metallurgy
- C22C33/0257—Making ferrous alloys by powder metallurgy characterised by the range of the alloying elements
- C22C33/0278—Making ferrous alloys by powder metallurgy characterised by the range of the alloying elements with at least one alloying element having a minimum content above 5%
- C22C33/0285—Making ferrous alloys by powder metallurgy characterised by the range of the alloying elements with at least one alloying element having a minimum content above 5% with Cr, Co, or Ni having a minimum content higher than 5%
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/03—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity
- H01F1/12—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials
- H01F1/14—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys
- H01F1/147—Alloys characterised by their composition
- H01F1/153—Amorphous metallic alloys, e.g. glassy metals
- H01F1/15333—Amorphous metallic alloys, e.g. glassy metals containing nanocrystallites, e.g. obtained by annealing
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/03—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity
- H01F1/12—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials
- H01F1/14—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys
- H01F1/147—Alloys characterised by their composition
- H01F1/153—Amorphous metallic alloys, e.g. glassy metals
- H01F1/15341—Preparation processes therefor
- H01F1/1535—Preparation processes therefor by powder metallurgy, e.g. spark erosion
Definitions
- the invention relates to a method for producing a soft magnetic, Fe-containing material with high saturation magnetization and ultra-fine structure. Such a method is e.g. from "J. Appl. Phys.”, Vol. 64, No. 10, Nov. 1988, pages 6044 to 6046.
- Soft-magnetic alloys based on Fe with an ultra-fine structure can be provided in particular for magnetic components in which minimal hysteresis losses and / or low eddy current losses are important in high-frequency applications.
- Nanocrystalline Fe-Si-Nb-Cu-B alloys with average grain sizes of about 10 to 20 nm are known, which have excellent soft magnetic properties, ie a small coercive force H c of less than 0.01 A / cm and a high permeability.
- the known alloy is obtained from rapidly solidified strips, which are initially amorphous and in which the nanocrystalline Fe-Si phase is eliminated as the main constituent by subsequent heat treatment above the crystallization temperature.
- the glass former boron cannot be dispensed with in the production of such rapidly solidified amorphous ribbons. This limits the saturation magnetization of the soft magnetic material to values of approximately 1.2 to 1.3 T.
- This object is achieved in that a starting powder from an Fe-containing main component of the material is milled so long and with such intensity that a pulverulent ground material is obtained from powder particles of the main component with an average grain size between 5 and 50 nm, and then the regrind is subjected to a heat treatment at a temperature below 600 ° C for a maximum of one hour.
- the invention is based on the finding that numerous stresses and defects are introduced into the material by a grinding process, which causes an intensive cold deformation of the Fe-containing main component, which are due to magnetostrictive Effects act as Blochwand pinning centers and magnetically harden the material.
- a heat treatment of the mechanically alloyed material is now carried out according to the invention at a temperature at which these tensions and defects heal, but excessive grain growth does not yet occur.
- the advantages associated with this can be seen in particular in the fact that a nanocrystalline material of extremely small coercive field strength and high saturation magnetization can be obtained.
- a starting powder can be formed particularly advantageously from the Fe-containing main component of the material and 0.1 to 10 atom% of an Fe-free additional component, which shows practically no solubility in the main component in thermodynamic equilibrium.
- This starting powder is then to be ground until a mixed powder of powder particles having the predetermined mean grain size and consisting of the main component with the additional component embedded therein has formed as the millbase.
- Such an additional component in the regrind of the main component advantageously supports the healing process with regard to the stresses and defects which are undesirably introduced into the material by the grinding. This is done in that this additional component preferably precipitates at the grain boundaries in the final heat treatment and thus stabilizes the individual grains with regard to undesired grain growth, i.e. hindered this growth.
- the invention is further explained below using an exemplary embodiment, reference being made to the drawing.
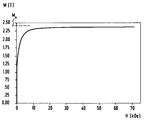
- the figure shows a diagram of the saturation magnetization of a material produced according to the invention.
- Common soft magnetic materials which have a saturation magnetization M s of at least 1.3 T are preferably alloys which contain at least one further component in addition to the component Fe. At least one element from the group of the elements Co, Si, Al, Ni is preferably considered as a further component.
- the percentage composition of the individual components is chosen from the point of view of the highest possible saturation magnetization and a small magnetocrystalline anisotropy and magnetostriction constants. From this point of view, for example, an approximate composition of the Fe component with approximately 42 atomic% Co, or approximately 22 atomic% Si, or approximately 70 atomic% Ni or approximately 25 atomic% Al can be regarded as favorable. This Fe component and the at least one further component form the main component of the material to be produced.
- the main component can also be three-component or even higher component, whereby slight impurities of the elements with a respective proportion below 0.1 atom% should always be included.
- at least one element from the group of elements Ti, V, Nb, Ta, Cr, Mo, W, Mn, Al, Sb, Ge, Sn, Zr, Hf can be provided as the third component.
- This third component can be selected, for example, from the point of view of the material's resistance to corrosion. In general, their proportion within the main component is less than 20 atom%, in particular less than 10 atom%.
- an additional component with a proportion within the material of between 0.1 atom% and 10 atom% is provided for the production of the soft magnetic material.
- the choice of material for this additional component is to be carried out such that, on the one hand, the additional component in the thermodynamic equilibrium of the overall system consisting of the main component and the additional component has practically no solubility in the Main component shows during the individual process steps.
- the material of the additional component during heat treatment after the step of mechanically alloying the main and additional components on the surface of the individual grains of the main component is said to settle in a quasi-precipitation reaction.
- the material of the additional component is in particular an element from the first or second group of the periodic table (Li, Na, K, Rb, Cs or Ca, Sr, Ba, Mg) or one of the elements Pb, Bi, In, Cu, Ag, Sn, Cd or Hg in question. Pb or Bi are particularly suitable.
- the material system Fe-Co should be selected as the main component.
- the zero crossing of the magnetocrystalline anisotropy constant K 1, which must be as small as possible in order to achieve small H c values, is in this composition range (at approximately 42 atomic% Co).
- elemental Fe and Co powders or powders made of an Fe-Co master alloy with a composition in the range of around 60 atomic% Fe and 40 atomic% Co are added as the main component of the material to be produced, together with an addition of some % By weight of Pb or Bi powder weighed out as Fe-free additional component.
- the proportion of this additional component within the powder mixture thus composed should in particular be between 2 and 5 atomic%.
- the individual powders of the constituents of the powder mixture involved should be sufficiently pure and in particular each have a purity of at least 99.5%.
- This powder mixture forming the starting powder with predetermined, Generally customary particle sizes of their powdery constituents in the order of magnitude of the largest diameters between 1 ⁇ m and 1 mm are now placed in a suitable grinding device, as is known in principle from processes of "mechanical alloying" (cf., for example, "Metal. Trans.” , Vol. 5, Aug. 1974, pages 1929 to 1934).
- the starting powder is then subjected to the grinding process, for example in a planetary ball mill, with the aid of hardened steel balls in a container made of hardened steel, for example filled with H2 or Ar.
- the grinding time t m of the grinding process depends in particular on the grinding parameters. Important parameters are the ball diameter, the number of balls and the materials used for the grinding device.
- the grinding speed and the ratio of the steel balls to the amount of powder are further parameters that determine the necessary grinding time.
- the spherical mass can be, for example, approximately 10 times the powder mass.
- the finely crystalline regrind to be obtained in this way is finally subjected to a special heat treatment under protective gas such as Ar or in a vacuum.
- protective gas such as Ar or in a vacuum.
- the temperature must not be too high and the heat treatment time must not be too long to avoid excessive grain growth. Therefore, the temperature must in any case be below 600 ° C, preferably below 400 ° C and in particular between about 150 ° C and 250 ° C.
- the duration of the heat treatment must not be longer than one hour, although longer times are permissible at relatively low temperatures than at higher temperatures. The exact duration can be determined experimentally depending on the specified temperature by observing the grain growth.
- an additional component which precipitates on the individual grains during the final heat treatment can optionally be dispensed with and a significant reduction in the coercive field strength of the ground material can nevertheless be achieved by means of this heat treatment.
- the magnetization M (in T) as a function of the applied field strength H (in kOe) is plotted in the diagram of the drawing.
- a saturation magnetization M s of approximately 2.35 T can be achieved for this material.
- the material is to be regarded as soft magnetic, since its coercive field strength is well below 10 A / cm.
- the soft magnetic materials produced according to the invention can be processed further in a known manner. For example, compact the powder into a shaped body with a desired shape.
- a plastic-bonded magnetic body can also be produced from the powder by casting with a plastic without a special compacting step.
Abstract
Description
Die Erfindung bezieht sich auf ein Verfahren zur Herstellung eines weichmagnetischen, Fe-haltigen Werkstoffes mit hoher Sättigungsmagnetisierung und ultrafeiner Konstruktur. Ein derartiges Verfahren geht z.B. aus "J. Appl. Phys.", Vol. 64, No. 10, Nov. 1988, Seiten 6044 bis 6046 hervor.The invention relates to a method for producing a soft magnetic, Fe-containing material with high saturation magnetization and ultra-fine structure. Such a method is e.g. from "J. Appl. Phys.", Vol. 64, No. 10, Nov. 1988, pages 6044 to 6046.
Weichmagnetische Legierungen auf Fe-Basis mit ultrafeiner Konstruktur können insbesondere für magnetische Bauteile vorgesehen werden, bei denen es auf minimale Hysteresis-Verluste und/oder geringe Wirbelstromverluste bei hochfrequenten Anwendungen ankommt. So sind z.B. aus der eingangs genannten Veröffentlichung "J. Appl. Phys." nanokristalline Fe-Si-Nb-Cu-B-Legierungen mit mittleren Korngrößen von etwa 10 bis 20 nm bekannt, welche hervorragende weichmagnetische Eigenschaften, d.h. eine kleine Koerzitivfeldstärke Hc von unter 0,01 A/cm und eine hohe Permeabilität, besitzen. Die bekannte Legierung wird aus rascherstarrten Bändern gewonnen, die zunächst amorph sind und in denen sich durch eine nachträgliche Wärmebehandlung oberhalb der Kristallisationstemperatur die nanokristalline Fe-Si-Phase als Hauptbestandteil ausscheidet. Bei der Herstellung solcher rascherstarrten amorphen Bänder kann jedoch auf den Glasbildner Bor nicht verzichtet werden. Dieser begrenzt die Sättigungsmagnetisierung des weichmagnetischen Materials auf Werte von etwa 1,2 bis 1,3 T.Soft-magnetic alloys based on Fe with an ultra-fine structure can be provided in particular for magnetic components in which minimal hysteresis losses and / or low eddy current losses are important in high-frequency applications. For example, from the publication "J. Appl. Phys." Nanocrystalline Fe-Si-Nb-Cu-B alloys with average grain sizes of about 10 to 20 nm are known, which have excellent soft magnetic properties, ie a small coercive force H c of less than 0.01 A / cm and a high permeability. The known alloy is obtained from rapidly solidified strips, which are initially amorphous and in which the nanocrystalline Fe-Si phase is eliminated as the main constituent by subsequent heat treatment above the crystallization temperature. However, the glass former boron cannot be dispensed with in the production of such rapidly solidified amorphous ribbons. This limits the saturation magnetization of the soft magnetic material to values of approximately 1.2 to 1.3 T.
Zwar können auch durch intensives Mahlen, wie es aus der Technik des "Mechanischen Legierens" her bekannt ist, nanokristalline Metallpartikel mit kleinsten erreichbaren Korngrößen von etwa 10 nm hergestellt werden. So ist z.B. in "Metall. Trans. A", Vol. 21 A, Sept. 1990, Seiten 2333 bis 2337 ein entsprechendes Mahlen von reinen Metallen wie z.B. von Fe beschrieben. Diese Technik wurde auch zur Herstellung der intermetallischen Verbindung AlRu untersucht (vgl. "J. Appl. Phys.", Vol. 65, No. 1, Jan. 1989, Seiten 305 bis 310). Will man jedoch auf diesem Wege an sich bekannte Fe-haltige Werkstoffe mit hoher Sättigungsmagnetisierung herstellen, so hat sich gezeigt, daß nur pulverförmige Materialien mit verhältnismäßig hohen Koerzitivfeldstärken von deutlich über 0,1 A/cm, beispielsweise über 5 bis 10 A/cm zu erhalten sind. Aus diesem Grunde wurde von einem Einsatz entsprechender Mahlverfahren zur Herstellung weichmagnetischer Werkstoffe bisher abgesehen.By intensive grinding, as is known from the technique of "mechanical alloying", nanocrystalline metal particles with the smallest achievable grain sizes of about 10 nm can be produced. For example, in "Metall. Trans. A", Vol. 21 A, Sept. 1990, pages 2333 to 2337, a corresponding grinding of pure metals such as Fe is described. This technique was also investigated for the production of the intermetallic compound AlRu (cf. "J. Appl. Phys.", Vol. 65, No. 1, Jan. 1989, pages 305 to 310). However, if you want to produce Fe-containing materials known per se with high saturation magnetization in this way, it has been shown that only powdery materials with relatively high coercive field strengths of well over 0.1 A / cm, for example over 5 to 10 A / cm, increase are preserved. For this reason, the use of appropriate grinding processes for the production of soft magnetic materials has so far been avoided.
Aufgabe der vorliegenden Erfindung ist es nun, das Verfahren mit den eingangs genannten Merkmalen dahingehend auszubilden, daß sich ein nanokristalliner, Fe-haltiger Werkstoff herstellen läßt, der gute weichmagnetische Eigenschaften wie das über eine Rascherstarrungstechnik gewonnene Material aufweist und der dennoch eine hohe Sättigungsmagnetisierung von mindestens 1,3 T, insbesondere mindestens 1,5 T, besitzt.It is an object of the present invention to develop the method with the features mentioned at the outset in such a way that a nanocrystalline, Fe-containing material can be produced which has good soft magnetic properties such as the material obtained via rapid solidification technology and which nevertheless has a high saturation magnetization of at least 1.3 T, in particular at least 1.5 T, has.
Diese Aufgabe wird erfindungsgemäß dadurch gelöst, daß ein Ausgangspulver aus einer Fe-haltigen Hauptkomponente des Werkstoffes so lange und mit solcher Intensität gemahlen wird, bis ein pulverförmiges Mahlgut aus Pulverpartikeln der Hauptkomponente mit einer mittleren Korngröße zwischen 5 und 50 nm erhalten wird, und daß anschließend das Mahlgut einer Wärmebehandlung bei einer Temperatur unter 600 °C während höchstens einer Stunde unterzogen wird.This object is achieved in that a starting powder from an Fe-containing main component of the material is milled so long and with such intensity that a pulverulent ground material is obtained from powder particles of the main component with an average grain size between 5 and 50 nm, and then the regrind is subjected to a heat treatment at a temperature below 600 ° C for a maximum of one hour.
Die Erfindung basiert dabei auf der Erkenntnis, daß durch einen Mahlvorgang, der eine intensive Kaltverformung der Fe-haltigen Hauptkomponente bewirkt, zahlreiche Spannungen und Defekte in das Material eingebracht werden, die aufgrund magnetostriktiver Effekte als Blochwand-Pinningzentren wirken und das Material magnetisch härten. Zur Lösung dieses Problems wird nun erfindungsgemäß eine Wärmebehandlung des mechanisch legierten Materials bei einer Temperatur durchgeführt, bei der diese Spannungen und Defekte ausheilen, aber noch kein exzessives Kornwachstum auftritt. Die damit verbundenen Vorteile sind insbesondere darin zu sehen, daß sich ein nanokristalliner Werkstoff äußerst kleiner Koerzitivfeldstärke und hoher Sättigungsmagnetisierung erhalten läßt.The invention is based on the finding that numerous stresses and defects are introduced into the material by a grinding process, which causes an intensive cold deformation of the Fe-containing main component, which are due to magnetostrictive Effects act as Blochwand pinning centers and magnetically harden the material. To solve this problem, a heat treatment of the mechanically alloyed material is now carried out according to the invention at a temperature at which these tensions and defects heal, but excessive grain growth does not yet occur. The advantages associated with this can be seen in particular in the fact that a nanocrystalline material of extremely small coercive field strength and high saturation magnetization can be obtained.
Besonders vorteilhaft kann zunächst ein Ausgangspulver aus der Fe-haltigen Hauptkomponente des Werkstoffes und 0,1 bis 10 Atom-% einer Fe-freien Zusatzkomponente gebildet werden, welche im thermodynamischen Gleichgewicht praktisch keine Löslichkeit in der Hauptkomponente zeigt. Dieses Ausgangspulver soll dann gemahlen werden, bis als Mahlgut ein Mischpulver aus Pulverpartikeln, die die vorbestimmte mittlere Korngröße aufweisen und aus der Hauptkomponente mit der in sie eingelagerten Zusatzkomponente bestehen, entstanden ist. Mit einer solchen Zusatzkomonente im Mahlgut der Hauptkomponente wird vorteilhaft der Ausheilprozeß bezüglich der durch das Mahlen in das Material unerwünscht eingebrachten Spannungen und Defekte unterstützt. Dies geschieht dadurch, daß sich diese Zusatzkomponente bei der abschließenden Wärmebehandlung bevorzugt an den Korngrenzen ausscheidet und so die einzelnen Körner bezüglich eines unerwünschten Kornwachstums stabilisiert, d.h. dieses Wachstum behindert.A starting powder can be formed particularly advantageously from the Fe-containing main component of the material and 0.1 to 10 atom% of an Fe-free additional component, which shows practically no solubility in the main component in thermodynamic equilibrium. This starting powder is then to be ground until a mixed powder of powder particles having the predetermined mean grain size and consisting of the main component with the additional component embedded therein has formed as the millbase. Such an additional component in the regrind of the main component advantageously supports the healing process with regard to the stresses and defects which are undesirably introduced into the material by the grinding. This is done in that this additional component preferably precipitates at the grain boundaries in the final heat treatment and thus stabilizes the individual grains with regard to undesired grain growth, i.e. hindered this growth.
Weitere vorteilhafte Ausgestaltungen des Verfahrens nach der Erfindung gehen aus den übrigen Unteransprüchen hervor.Further advantageous embodiments of the method according to the invention emerge from the remaining subclaims.
Die Erfindung wird nachfolgend anhand eines Ausführungsbeispiels noch weiter erläutert, wobei auf die Zeichnung Bezug genommen wird. Dabei zeigt die Figur als Diagramm die Sättigungsmagnetisierung eines erfindungsgemäß hergestellten Werkstoffes.The invention is further explained below using an exemplary embodiment, reference being made to the drawing. The figure shows a diagram of the saturation magnetization of a material produced according to the invention.
Gebräuchliche weichmagnetische Werkstoffe, die eine Sättigungsmagnetisierung Ms von mindestens 1,3 T aufweisen, sind bevorzugt Legierungen, die neben der Komponente Fe noch mindestens eine weitere Komponente enthalten. Als weitere Komponente kommt dabei vorzugsweise mindestens ein Element aus der Gruppe der Elemente Co, Si, Al, Ni in Frage. Die prozentuale Zusammensetzung der einzelnen Komponenten wird dabei unter dem Gesichtspunkt einer möglichst hohen Sättigungsmagnetisierung sowie einer kleinen magnetokristallinen Anisotropie- und Magnetostriktionskonstanten gewählt. Beispielsweise sind unter diesem Gesichtspunkten eine ungefähre Zusammensetzung der Fe-Komponente mit etwa 42 Atom-% Co, oder etwa 22 Atom-% Si, oder etwa 70 Atom-% Ni oder etwa 25 Atom-% Al als günstig anzusehen. Diese Fe-Komponente und die mindestens eine weitere Komponente bilden die Hauptkomponente des herzustellenden Werkstoffes. Selbstverständlich kann die Hauptkomponente auch drei-komponentig oder noch höherkomponentig sein, wobei geringfügige Verunreinigungen der Elemente mit einem jeweiligen Anteil unter 0,1 Atom-% stets mit eingeschlossen sein sollen. Als dritte Komponente kann z.B. mindestens ein Element aus der Gruppe der Elemente Ti, V, Nb, Ta, Cr, Mo, W, Mn, Al, Sb, Ge, Sn, Zr, Hf vorgesehen sein. Diese dritte Komponente läßt sich beispielsweise unter dem Gesichtspunkt einer Korrosionsfestigkeit des Materials auswählen. Im allgemeinen liegt ihr Anteil innerhalb der Hauptkomponente unter 20 Atom-%, insbesondere unter 10 Atom-%.Common soft magnetic materials which have a saturation magnetization M s of at least 1.3 T are preferably alloys which contain at least one further component in addition to the component Fe. At least one element from the group of the elements Co, Si, Al, Ni is preferably considered as a further component. The percentage composition of the individual components is chosen from the point of view of the highest possible saturation magnetization and a small magnetocrystalline anisotropy and magnetostriction constants. From this point of view, for example, an approximate composition of the Fe component with approximately 42 atomic% Co, or approximately 22 atomic% Si, or approximately 70 atomic% Ni or approximately 25 atomic% Al can be regarded as favorable. This Fe component and the at least one further component form the main component of the material to be produced. Of course, the main component can also be three-component or even higher component, whereby slight impurities of the elements with a respective proportion below 0.1 atom% should always be included. For example, at least one element from the group of elements Ti, V, Nb, Ta, Cr, Mo, W, Mn, Al, Sb, Ge, Sn, Zr, Hf can be provided as the third component. This third component can be selected, for example, from the point of view of the material's resistance to corrosion. In general, their proportion within the main component is less than 20 atom%, in particular less than 10 atom%.
Für das erfindungsgemäße Verfahren kann es gegebenenfalls von Vorteil sein, wenn man zur Herstellung des weichmagnetischen Werkstoffes noch eine Zusatzkomponente mit einem Anteil innerhalb des Werkstoffes zwischen 0,1 Atom-% und 10 Atom-% vorsieht. Die Materialwahl für diese Zusatzkomponente ist dabei so vorzunehmen, daß zum einen die Zusatzkomponente im thermodynamischen Gleichgewicht des Gesamtsystems aus Hauptkomponente und Zusatzkomponente praktisch keine Löslichkeit in der Hauptkomponente während der einzelnen Verfahrensschritte zeigt. Zum anderen soll sich das Material der Zusatzkomponente bei einer Wärmebehandlung nach dem Schritt des mechanischen Legierens der Haupt- und Zusatzkomponente an der Oberfläche der einzelnen Körner der Hauptkomponente quasi in einer Ausscheidungsreaktion absetzen. Für die genannten Fe-Legierungen als Hauptkomponente kommt als Material der Zusatzkomponente insbesondere ein Element aus der ersten oder zweiten Gruppe des Periodensystems (Li, Na, K, Rb, Cs bzw. Ca, Sr, Ba, Mg) oder eines der Elemente Pb, Bi, In, Cu, Ag, Sn, Cd oder Hg in Frage. Pb oder Bi sind besonders geeignet.For the method according to the invention it may be advantageous if an additional component with a proportion within the material of between 0.1 atom% and 10 atom% is provided for the production of the soft magnetic material. The choice of material for this additional component is to be carried out such that, on the one hand, the additional component in the thermodynamic equilibrium of the overall system consisting of the main component and the additional component has practically no solubility in the Main component shows during the individual process steps. On the other hand, the material of the additional component during heat treatment after the step of mechanically alloying the main and additional components on the surface of the individual grains of the main component is said to settle in a quasi-precipitation reaction. For the Fe alloys mentioned as the main component, the material of the additional component is in particular an element from the first or second group of the periodic table (Li, Na, K, Rb, Cs or Ca, Sr, Ba, Mg) or one of the elements Pb, Bi, In, Cu, Ag, Sn, Cd or Hg in question. Pb or Bi are particularly suitable.
Für das Ausführungsbeispiel sei als Hauptkomponente das Stoffsystem Fe-Co ausgewählt. Eine entsprechende Legierung Fe1-xCox weist im Bereich 0,3 = x = 0,5 die höchsten Werte der Sättigungsmagnetisierung Ms von bis zu etwa 2,4 T auf. Das heißt, die Zusammensetzung der einzelnen Elemente der Hauptkomponente wird unter dem Gesichtspunkt einer möglichst hohen Sättigungsmagnetisierung vorgenommen. Außerdem liegt in diesem Zusammensetzungsbereich (bei etwa 42 Atom-% Co) der Nulldurchgang der magnetokristallinen Anisotropiekonstanten K₁, die zur Erzielung kleiner Hc-Werte möglichst klein sein muß.For the exemplary embodiment, the material system Fe-Co should be selected as the main component. A corresponding alloy Fe 1-x Co x has the highest values of the saturation magnetization M s of up to about 2.4 T in the range 0.3 = x = 0.5. This means that the composition of the individual elements of the main component is carried out from the point of view of the highest possible saturation magnetization. In addition, the zero crossing of the magnetocrystalline anisotropy constant K 1, which must be as small as possible in order to achieve small H c values, is in this composition range (at approximately 42 atomic% Co).
Gemäß dem ausgewählten Ausführungsbeispiel werden als Hauptkomponente des herzustellenden Werkstoffes elementare Fe- und Co-Pulver oder Pulver aus einer Fe-Co-Vorlegierung mit einer Zusammensetzung im Bereich um etwa 60 Atom-% Fe und 40 Atom-% Co gemeinsam mit einem Zusatz von einigen Gew.-% Pb- oder Bi-Pulver als Fe-freie Zusatzkomponente eingewogen. Der Anteil dieser Zusatzkomponente innerhalb der so zusammengestellten Pulvermischung soll dabei insbesondere zwischen 2 und 5 Atom-% liegen. Die einzelnen Pulver der beteiligten Bestandteile der Pulvermischung sollen hinreichend rein sein und insbesondere jeweils eine Reinheit von mindestens 99,5 % aufweisen. Diese das Ausgangspulver bildende Pulvermischung mit vorbestimmten, allgemein üblichen Partikelgrößen ihrer pulverförmigen Bestandteile in der Größenordnung der jeweils größten Durchmesser zwischen 1 µm und 1 mm wird nun in eine geeignete Mahlvorrichtung gegeben, wie sie von Verfahren des "Mechanischen Legierens" her prinzipiell bekannt ist (vgl. z.B. "Metall. Trans.", Vol. 5, Aug. 1974, Seiten 1929 bis 1934). Das Ausgangspulver wird dann z.B. in einer Planetenkugelmühle dem Mahlprozeß mit Hilfe von gehärteten Stahlkugeln in einem z.B. mit H₂ oder Ar gefüllten Behälter aus gehärtetem Stahl unterzogen. Die Mahldauer tm des Mahlprozesses hängt insbesondere von den Mahlparametern ab. Wichtige Parameter sind der Kugeldurchmesser, die Kugelanzahl sowie die verwendeten Materialien der Mahlvorrichtung. Auch die Mahlgeschwindigkeit und das Verhältnis der Stahlkugeln zu der Pulvermenge sind weitere Parameter, welche die notwendige Mahldauer bestimmen. Die Kugelmasse kann beispielsweise etwa das 10-fache der Pulvermasse betragen. Mit dem Mahlprozeß, der vorteilhaft mit hoher Intensität, d.h. mit hoher Mahlgeschwindigkeit bzw. Drehzahl der Kugeln durchgeführt wird, wird fortschreitend das Ausgangspulver in ein Mischpulver mit Partikeln überführt, die aus einer Legierung der Hauptkomponente Fe-Co mit an deren Gitterplätzen statistisch verteilt angeordneter Zusatzkomponente Pb oder Bi bestehen. Um solche Partikel aus der Fe-Co-Hauptkomponente mit eingelagerter Pb- oder Bi-Zusatzkomponente zu erhalten, deren mittlere Korngrößen (Korndurchmesser) zwischen 10 und 50 nm liegen, ist im allgemeinen eine Mahldauer von mehreren Stunden, vielfach über 10 Stunden, beispielsweise von mindestens 60 Stunden, erforderlich.According to the selected embodiment, elemental Fe and Co powders or powders made of an Fe-Co master alloy with a composition in the range of around 60 atomic% Fe and 40 atomic% Co are added as the main component of the material to be produced, together with an addition of some % By weight of Pb or Bi powder weighed out as Fe-free additional component. The proportion of this additional component within the powder mixture thus composed should in particular be between 2 and 5 atomic%. The individual powders of the constituents of the powder mixture involved should be sufficiently pure and in particular each have a purity of at least 99.5%. This powder mixture forming the starting powder with predetermined, Generally customary particle sizes of their powdery constituents in the order of magnitude of the largest diameters between 1 μm and 1 mm are now placed in a suitable grinding device, as is known in principle from processes of "mechanical alloying" (cf., for example, "Metal. Trans." , Vol. 5, Aug. 1974, pages 1929 to 1934). The starting powder is then subjected to the grinding process, for example in a planetary ball mill, with the aid of hardened steel balls in a container made of hardened steel, for example filled with H₂ or Ar. The grinding time t m of the grinding process depends in particular on the grinding parameters. Important parameters are the ball diameter, the number of balls and the materials used for the grinding device. The grinding speed and the ratio of the steel balls to the amount of powder are further parameters that determine the necessary grinding time. The spherical mass can be, for example, approximately 10 times the powder mass. With the grinding process, which is advantageously carried out with high intensity, ie with high grinding speed or rotational speed of the balls, the starting powder is progressively converted into a mixed powder with particles consisting of an alloy of the main component Fe-Co with additional components arranged statistically distributed at their lattice sites Pb or Bi exist. In order to obtain such particles from the main Fe-Co component with an incorporated Pb or Bi additional component, whose average grain sizes (grain diameter) are between 10 and 50 nm, a grinding time of several hours, in general over 10 hours, for example of at least 60 hours, required.
Das so zu gewinnende feinkristalline Mahlgut wird schließlich einer besonderen Wärmebehandlung unter Schutzgas wie z.B. Ar oder im Vakuum unterzogen. Mit dieser Maßnahme sollen die während des Mahlprozesses in die Pulverpartikel des Mischpulvers eingebrachten zahlreichen Spannungen und Defekte zumindest großenteils wieder ausgeheilt und eine Ausscheidung der Zusatzkomponente an der Oberfläche der jeweiligen Pulverpartikel bewirkt werden. Andererseits darf die Temperatur nicht zu hoch und die Wärmebehandlungsdauer nicht zu lang sein, um ein exzessives Kornwachstum zu vermeiden. Deshalb muß die Temperatur auf jeden Fall unter 600 °C, vorzugsweise unter 400 °C und insbesondere zwischen etwa 150 °C und 250 °C liegen. Die Dauer der Wärmebehandlung darf nicht länger als eine Stunde betragen, wobei bei verhältnismäßig niedrigen Temperaturen längere Zeiten als bei höheren Temperaturen zulässig sind. Die genaue Dauer läßt sich in Abhängigkeit von der vorgegebenen Temperatur durch Beobachtung des Kornwachstums experimentell bestimmen.The finely crystalline regrind to be obtained in this way is finally subjected to a special heat treatment under protective gas such as Ar or in a vacuum. With this measure, the numerous tensions and defects introduced into the powder particles of the mixed powder during the grinding process are at least to a large extent remedied and an excretion of the additional component can be effected on the surface of the respective powder particles. On the other hand, the temperature must not be too high and the heat treatment time must not be too long to avoid excessive grain growth. Therefore, the temperature must in any case be below 600 ° C, preferably below 400 ° C and in particular between about 150 ° C and 250 ° C. The duration of the heat treatment must not be longer than one hour, although longer times are permissible at relatively low temperatures than at higher temperatures. The exact duration can be determined experimentally depending on the specified temperature by observing the grain growth.
Für das erfindungsgemäße Verfahren kann gegebenenfalls auf einen Zusatz einer sich bei der abschließenden Wärmebehandlung an den einzelnen Körnern ausscheidenden Zusatzkomponente verzichtet und dennoch eine deutliche Absenkung der Koerzitivfeldstärke des Mahlgutes mittels dieser Wärmebehandlung erreicht werden. Für einen entsprechenden, d.h. ohne eine derartige Zusatzkomponente hergestellten Werkstoff der Zusammensetzung Fe₇₀Co₃₀ ist in dem Diagramm der Zeichnung die Magnetisierung M (in T) in Abhängigkeit von der angelegten Feldstärke H (in kOe) aufgetragen. Für diesen Werkstoff läßt sich eine Sättigungsmagnetisierung Ms von etwa 2,35 T erreichen. Der Werkstoff ist dabei als weichmagnetisch anzusehen, da seine Koerzitivfeldstärke deutlich unter 10 A/cm liegt.For the process according to the invention, an additional component which precipitates on the individual grains during the final heat treatment can optionally be dispensed with and a significant reduction in the coercive field strength of the ground material can nevertheless be achieved by means of this heat treatment. For a corresponding material, that is to say produced without such an additional component, of the composition Fe₇₀Co₃₀, the magnetization M (in T) as a function of the applied field strength H (in kOe) is plotted in the diagram of the drawing. A saturation magnetization M s of approximately 2.35 T can be achieved for this material. The material is to be regarded as soft magnetic, since its coercive field strength is well below 10 A / cm.
Die erfindungsgemäß hergestellten weichmagnetischen Werkstoffe können in bekannter Weise weiter verarbeitet werden. So läßt sich z.B. das Pulver zu einem Formkörper mit einer gewünschten Gestalt kompaktieren. Daneben kann auch ohne besonderen Kompaktierungsschritt aus dem Pulver durch Verguß mit einem Kunststoff ein kunststoffgebundener Magnetkörper hergestellt werden.The soft magnetic materials produced according to the invention can be processed further in a known manner. For example, compact the powder into a shaped body with a desired shape. In addition, a plastic-bonded magnetic body can also be produced from the powder by casting with a plastic without a special compacting step.
Claims (9)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE4107192 | 1991-03-06 | ||
| DE4107192 | 1991-03-06 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0502397A2 true EP0502397A2 (en) | 1992-09-09 |
| EP0502397A3 EP0502397A3 (en) | 1992-11-25 |
| EP0502397B1 EP0502397B1 (en) | 1995-05-03 |
Family
ID=6426621
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP92103081A Expired - Lifetime EP0502397B1 (en) | 1991-03-06 | 1992-02-24 | Preparation process for soft magnetic Fe-containing material with high saturation magnetisation and ultrafine structure |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP0502397B1 (en) |
| DE (1) | DE59202056D1 (en) |
| ES (1) | ES2071361T3 (en) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19610196A1 (en) * | 1996-03-15 | 1997-09-18 | Horst Dr Kleine | Magnetically soft cores of iron-silicon mixture manufacture e.g. for LF applications |
| DE102006028389A1 (en) * | 2006-06-19 | 2007-12-27 | Vacuumschmelze Gmbh & Co. Kg | Magnetic core, formed from a combination of a powder nanocrystalline or amorphous particle and a press additive and portion of other particle surfaces is smooth section or fracture surface without deformations |
| US7964043B2 (en) | 2001-07-13 | 2011-06-21 | Vacuumschmelze Gmbh & Co. Kg | Method for producing nanocrystalline magnet cores, and device for carrying out said method |
| US8287664B2 (en) | 2006-07-12 | 2012-10-16 | Vacuumschmelze Gmbh & Co. Kg | Method for the production of magnet cores, magnet core and inductive component with a magnet core |
| US8298352B2 (en) | 2007-07-24 | 2012-10-30 | Vacuumschmelze Gmbh & Co. Kg | Method for the production of magnet cores, magnet core and inductive component with a magnet core |
| US8327524B2 (en) | 2000-05-19 | 2012-12-11 | Vacuumscmelze Gmbh & Co. Kg | Inductive component and method for the production thereof |
| US9057115B2 (en) | 2007-07-27 | 2015-06-16 | Vacuumschmelze Gmbh & Co. Kg | Soft magnetic iron-cobalt-based alloy and process for manufacturing it |
| EP3613872A1 (en) * | 2018-08-21 | 2020-02-26 | Siemens Aktiengesellschaft | Method for producing a component for an electric or electronic component and component |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB466048A (en) * | 1935-01-31 | 1937-05-21 | Hans Vogt | A process for making magnetic powder |
-
1992
- 1992-02-24 ES ES92103081T patent/ES2071361T3/en not_active Expired - Lifetime
- 1992-02-24 DE DE59202056T patent/DE59202056D1/en not_active Expired - Fee Related
- 1992-02-24 EP EP92103081A patent/EP0502397B1/en not_active Expired - Lifetime
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB466048A (en) * | 1935-01-31 | 1937-05-21 | Hans Vogt | A process for making magnetic powder |
Non-Patent Citations (2)
| Title |
|---|
| APPLIED PHYSICS LETTERS Bd. 58, Nr. 2, 14. Januar 1991, NEW YORK US Seiten 119 - 121 A.CALKA ET AL * |
| IEEE TRANSACTIONS ON MAGNETICS. Bd. 26, Nr. 5, September 1990, NEW YORK US Seiten 1840 - 1842 W.A.KACZMAREK * |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19610196A1 (en) * | 1996-03-15 | 1997-09-18 | Horst Dr Kleine | Magnetically soft cores of iron-silicon mixture manufacture e.g. for LF applications |
| US8327524B2 (en) | 2000-05-19 | 2012-12-11 | Vacuumscmelze Gmbh & Co. Kg | Inductive component and method for the production thereof |
| US7964043B2 (en) | 2001-07-13 | 2011-06-21 | Vacuumschmelze Gmbh & Co. Kg | Method for producing nanocrystalline magnet cores, and device for carrying out said method |
| DE102006028389A1 (en) * | 2006-06-19 | 2007-12-27 | Vacuumschmelze Gmbh & Co. Kg | Magnetic core, formed from a combination of a powder nanocrystalline or amorphous particle and a press additive and portion of other particle surfaces is smooth section or fracture surface without deformations |
| US8372218B2 (en) | 2006-06-19 | 2013-02-12 | Vacuumschmelze Gmbh & Co. Kg | Magnet core and method for its production |
| US8287664B2 (en) | 2006-07-12 | 2012-10-16 | Vacuumschmelze Gmbh & Co. Kg | Method for the production of magnet cores, magnet core and inductive component with a magnet core |
| US8298352B2 (en) | 2007-07-24 | 2012-10-30 | Vacuumschmelze Gmbh & Co. Kg | Method for the production of magnet cores, magnet core and inductive component with a magnet core |
| US9057115B2 (en) | 2007-07-27 | 2015-06-16 | Vacuumschmelze Gmbh & Co. Kg | Soft magnetic iron-cobalt-based alloy and process for manufacturing it |
| EP3613872A1 (en) * | 2018-08-21 | 2020-02-26 | Siemens Aktiengesellschaft | Method for producing a component for an electric or electronic component and component |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2071361T3 (en) | 1995-06-16 |
| EP0502397B1 (en) | 1995-05-03 |
| EP0502397A3 (en) | 1992-11-25 |
| DE59202056D1 (en) | 1995-06-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0021101B1 (en) | Amorphous soft magnetic alloy | |
| EP1208244B1 (en) | Nickel-based metallic material and method for producing same | |
| DE112013005109T5 (en) | Sintered rare earth magnet and method of making the same | |
| DE60311421T2 (en) | RARE TERMINAL PERMANENT MAGNET ON R-T-B BASE | |
| DE4408114B4 (en) | Magnetic material | |
| DE602005003599T2 (en) | Rare earth permanent magnet | |
| DE10296960T5 (en) | Rare earth metal magnet and process for its manufacture | |
| DE102019129302A1 (en) | SOFT MAGNETIC ALLOY POWDER, DUST CORE, MAGNETIC COMPONENT AND ELECTRONIC DEVICE | |
| EP0200079B1 (en) | Method of manufacturing a metallic article from an amorphous alloy | |
| EP0502397B1 (en) | Preparation process for soft magnetic Fe-containing material with high saturation magnetisation and ultrafine structure | |
| DE69503957T3 (en) | SE-Fe-B magnets and their manufacturing processes | |
| EP0232772B1 (en) | Process for preparing a pulverulent amorphous material by way of a milling process | |
| DE19739959C2 (en) | Hard magnetic material | |
| DE69815479T2 (en) | Rare earth permanent magnet material and manufacturing process | |
| CH638566A5 (en) | MATERIAL FOR PERMANENT MAGNETS AND METHOD FOR THE PRODUCTION THEREOF. | |
| DE60311960T2 (en) | METHOD FOR THE PRODUCTION OF R-T-B BASED RARE-ELEMENT PERMANENT MAGNETS | |
| DE3841748C2 (en) | ||
| EP0243641B1 (en) | Process for manufacturing a permanent-magnet material from powder | |
| DE4126893A1 (en) | Permanent magnetic material based on samarium, iron@ and nitrogen - formed by nitriding alloy of the 2 metals in suitable ambient at high temp. to greatly increase energy prod. and raise the Curie-temp. | |
| DE3709138C2 (en) | Process for the production of a magnetic material from powdery starting components | |
| EP0468317B1 (en) | Method for the preparation of magnetic material based an the Sm-Fe-N substance system | |
| DE1029845B (en) | Process for the production of cube texture in the manufacture of objects from iron-silicon alloys | |
| DE4134245C2 (en) | Process for the production of magnetic material based on the Sm-Fe-C material system | |
| DE4116857A1 (en) | Magnetic material based on thorium-dodeca:manganese crystal structure - with interstitial nitrogen, carbon or hydrogen atmos. obtd. by heat-treatment in suitable atmos. | |
| DE3832472A1 (en) | METHOD FOR PRODUCING A MATERIAL WITH A HARD MAGNETIC PHASE FROM POWDER-BASED STARTING COMPONENTS |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): BE DE ES FR GB IT NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): BE DE ES FR GB IT NL SE |
|
| 17P | Request for examination filed |
Effective date: 19930519 |
|
| 17Q | First examination report despatched |
Effective date: 19940719 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE DE ES FR GB IT NL SE |
|
| REF | Corresponds to: |
Ref document number: 59202056 Country of ref document: DE Date of ref document: 19950608 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2071361 Country of ref document: ES Kind code of ref document: T3 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19950605 |
|
| ET | Fr: translation filed | ||
| ITF | It: translation for a ep patent filed |
Owner name: STUDIO JAUMANN |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 19980211 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19980212 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19980218 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19980223 Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990225 Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990225 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990228 |
|
| BERE | Be: lapsed |
Owner name: SIEMENS A.G. Effective date: 19990228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990901 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 92103081.3 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20010503 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20040202 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20040217 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20040318 Year of fee payment: 13 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050224 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050224 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050901 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20050223 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20051031 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20051031 |