EP0441663A1 - 1,1-Dichloro-1-fluoroethane and methyl formate based cleaning composition - Google Patents
1,1-Dichloro-1-fluoroethane and methyl formate based cleaning composition Download PDFInfo
- Publication number
- EP0441663A1 EP0441663A1 EP19910400008 EP91400008A EP0441663A1 EP 0441663 A1 EP0441663 A1 EP 0441663A1 EP 19910400008 EP19910400008 EP 19910400008 EP 91400008 A EP91400008 A EP 91400008A EP 0441663 A1 EP0441663 A1 EP 0441663A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- methyl formate
- fluoroethane
- dichloro
- composition
- composition according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 38
- TZIHFWKZFHZASV-UHFFFAOYSA-N methyl formate Chemical compound COC=O TZIHFWKZFHZASV-UHFFFAOYSA-N 0.000 title claims abstract description 33
- 238000004140 cleaning Methods 0.000 title claims abstract description 13
- FRCHKSNAZZFGCA-UHFFFAOYSA-N 1,1-dichloro-1-fluoroethane Chemical compound CC(F)(Cl)Cl FRCHKSNAZZFGCA-UHFFFAOYSA-N 0.000 title claims abstract description 6
- 238000005238 degreasing Methods 0.000 claims abstract description 6
- 150000001875 compounds Chemical group 0.000 claims abstract description 4
- 239000007787 solid Substances 0.000 claims abstract description 4
- 238000009835 boiling Methods 0.000 claims description 6
- 239000003381 stabilizer Substances 0.000 claims description 6
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 claims description 5
- LYGJENNIWJXYER-BJUDXGSMSA-N nitromethane Chemical group [11CH3][N+]([O-])=O LYGJENNIWJXYER-BJUDXGSMSA-N 0.000 claims 1
- AJDIZQLSFPQPEY-UHFFFAOYSA-N 1,1,2-Trichlorotrifluoroethane Chemical compound FC(F)(Cl)C(F)(Cl)Cl AJDIZQLSFPQPEY-UHFFFAOYSA-N 0.000 abstract description 2
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 7
- 230000004907 flux Effects 0.000 description 6
- LYGJENNIWJXYER-UHFFFAOYSA-N nitromethane Chemical compound C[N+]([O-])=O LYGJENNIWJXYER-UHFFFAOYSA-N 0.000 description 6
- 239000012808 vapor phase Substances 0.000 description 5
- 238000010992 reflux Methods 0.000 description 4
- 229910000679 solder Inorganic materials 0.000 description 4
- 238000004821 distillation Methods 0.000 description 3
- 238000004817 gas chromatography Methods 0.000 description 3
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- 239000007791 liquid phase Substances 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- 238000007605 air drying Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000001066 destructive effect Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 238000009304 pastoral farming Methods 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 238000004506 ultrasonic cleaning Methods 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
- C11D7/50—Solvents
- C11D7/5004—Organic solvents
- C11D7/5018—Halogenated solvents
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
- C11D7/50—Solvents
- C11D7/5036—Azeotropic mixtures containing halogenated solvents
- C11D7/5068—Mixtures of halogenated and non-halogenated solvents
- C11D7/5077—Mixtures of only oxygen-containing solvents
- C11D7/5086—Mixtures of only oxygen-containing solvents the oxygen-containing solvents being different from alcohols, e.g. mixtures of water and ethers
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23G—CLEANING OR DE-GREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS
- C23G5/00—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents
- C23G5/02—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents
- C23G5/028—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents containing halogenated hydrocarbons
- C23G5/02809—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents containing halogenated hydrocarbons containing chlorine and fluorine
- C23G5/02825—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents containing halogenated hydrocarbons containing chlorine and fluorine containing hydrogen
- C23G5/02829—Ethanes
- C23G5/02832—C2H3Cl2F
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
- C11D7/22—Organic compounds
- C11D7/26—Organic compounds containing oxygen
- C11D7/266—Esters or carbonates
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
- C11D7/22—Organic compounds
- C11D7/28—Organic compounds containing halogen
Definitions
- the present invention relates to the field of chlorofluorinated hydrocarbons and more particularly relates to a new composition having an azeotrope and usable as an agent for cleaning and degreasing solid surfaces, in particular in defluxing and cold cleaning of printed circuits.
- 1,1,2-Trichloro-1,2,2-trifluoroethane (known in the art under the designation F113) is widely used in the industry for cleaning and degreasing solid surfaces.
- F113 1,1,2-Trichloro-1,2,2-trifluoroethane
- F113 1,1,2-Trichloro-1,2,2-trifluoroethane
- F113 is most often combined with other organic solvents (for example methanol), preferably in the form of azeotropic or pseudo-azeotropic mixtures which do not demix and which, when used at reflux, have substantially the same composition in the vapor phase as in the liquid phase.
- organic solvents for example methanol
- F113 is one of the fully halogenated chlorofluorocarbons that are currently suspected of attacking or degrading stratospheric ozone.
- the present invention proposes to replace the compositions based on F113 with a new composition based on methyl formate and 1,1-dichloro-1-fluoroethane.
- the latter compound known in the art under the designation F141b, is practically devoid of destructive effect with respect to ozone.
- the composition to be used according to the invention comprises from 55 to 80% by weight of F141b and from 20 to 45% of methyl formate.
- F141b azeotrope whose boiling point is 28.4 ° C at normal atmospheric pressure (1.013 bar) and the composition according to the invention has a pseudo-azeotropic behavior, that is to say that the composition of the vapor and liquid phases is substantially the same, which is particularly advantageous for the intended applications.
- the content of F141b is chosen between 61 and 65% by weight and that of methyl formate between 35 and 39% by weight.
- the azeotrope F141b / methyl formate is a positive azeotrope since its boiling point (28.4 ° C) is lower than that of the two constituents (F141b: 32 ° C; methyl formate: 31.7 ° C).
- the composition according to the invention can be advantageously stabilized against hydrolysis and / or free radical attacks likely to occur in the cleaning processes, by adding to it a usual stabilizer such as, for example , nitromethane, propylene oxide or a mixture of these compounds, the proportion of stabilizer possibly ranging from 0.01 to 5% relative to the total weight: F141b - methyl formate.
- a usual stabilizer such as, for example , nitromethane, propylene oxide or a mixture of these compounds, the proportion of stabilizer possibly ranging from 0.01 to 5% relative to the total weight: F141b - methyl formate.
- composition according to the invention can be used in the same applications and according to the same techniques as the previous compositions based on F113.
- This azeotrope used for cleaning solder flux or degreasing mechanical parts, gives as good results as compositions based on F113 and methanol.
- Example 3 is repeated using 0.1% nitromethane and 0.1% propylene oxide. The following results are obtained:
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Detergent Compositions (AREA)
- Cleaning And De-Greasing Of Metallic Materials By Chemical Methods (AREA)
- Manufacturing Of Printed Wiring (AREA)
Abstract
Description
La présente invention concerne le domaine des hydrocarbures chlorofluorés et a plus particulièrement pour objet une nouvelle composition présentant un azéotrope et utilisable comme agent de nettoyage et de dégraissage de surfaces solides, en particulier dans le défluxage et le nettoyage à froid de circuits imprimés.The present invention relates to the field of chlorofluorinated hydrocarbons and more particularly relates to a new composition having an azeotrope and usable as an agent for cleaning and degreasing solid surfaces, in particular in defluxing and cold cleaning of printed circuits.
Le 1,1,2-trichloro-1,2,2-trifluoroéthane (connu dans le métier sous la désignation F113) est largement utilisé dans l'industrie pour le nettoyage et le dégraissage des surfaces solides. Outre son application en électronique au nettoyage des flux de soudure pour éliminer le flux décapant qui adhère aux circuits imprimés, on peut mentionner ses applications au dégraissage de pièces métalliques lourdes et au nettoyage de pièces mécaniques de haute qualité et de grande précision comme, par exemple, les gyroscopes et le matériel militaire ou aérospatial. Dans ses diverses applications, le F113 est le plus souvent associé à d'autres solvants organiques (par exemple le méthanol), de préférence sous forme de mélanges azéotropiques ou pseudo-azéotropiques qui ne démixent pas et qui, employés au reflux, ont sensiblement la même composition dans la phase vapeur que dans la phase liquide.1,1,2-Trichloro-1,2,2-trifluoroethane (known in the art under the designation F113) is widely used in the industry for cleaning and degreasing solid surfaces. In addition to its application in electronics to the cleaning of solder fluxes to eliminate the flux flux which adheres to printed circuits, one can mention its applications to the degreasing of heavy metal parts and to the cleaning of high quality and high precision mechanical parts such as, for example , gyroscopes and military or aerospace equipment. In its various applications, F113 is most often combined with other organic solvents (for example methanol), preferably in the form of azeotropic or pseudo-azeotropic mixtures which do not demix and which, when used at reflux, have substantially the same composition in the vapor phase as in the liquid phase.
Cependant, le F113 fait partie des chlorofluorocarbures complètement halogénés qui sont actuellement suspectés d'attaquer ou de dégrader l'ozone stratosphérique.However, F113 is one of the fully halogenated chlorofluorocarbons that are currently suspected of attacking or degrading stratospheric ozone.
Pour contribuer à résoudre ce problème, la présente invention propose de remplacer les compositions à base de F113 par une nouvelle composition à base de formiate de méthyle et de 1,1-dichloro-1-fluoroéthane. Ce dernier composé, connu dans le métier sous la désignation F141b, est pratiquement dépourvu d'effet destructeur vis-à-vis de l'ozone.To contribute to solving this problem, the present invention proposes to replace the compositions based on F113 with a new composition based on methyl formate and 1,1-dichloro-1-fluoroethane. The latter compound, known in the art under the designation F141b, is practically devoid of destructive effect with respect to ozone.
La composition à utiliser selon l'invention comprend de 55 à 80 % en poids de F141b et de 20 à 45 % de formiate de méthyle. Dans ce domaine, il existe un azéotrope dont la température d'ébullition est de 28,4°C à la pression atmosphérique normale (1,013 bar) et la composition selon l'invention a un comportement pseudo-azéotropique, c'est-à-dire que la composition des phases vapeur et liquide est sensiblement la même, ce qui est particulièrement avantageux pour les applications visées. De préférence, la teneur en F141b est choisie entre 61 et 65 % en poids et celle de formiate de méthyle entre 35 et 39 % en poids.The composition to be used according to the invention comprises from 55 to 80% by weight of F141b and from 20 to 45% of methyl formate. In this field, there is an azeotrope whose boiling point is 28.4 ° C at normal atmospheric pressure (1.013 bar) and the composition according to the invention has a pseudo-azeotropic behavior, that is to say that the composition of the vapor and liquid phases is substantially the same, which is particularly advantageous for the intended applications. Preferably, the content of F141b is chosen between 61 and 65% by weight and that of methyl formate between 35 and 39% by weight.
L'azéotrope F141b/formiate de méthyle est un azéotrope positif puisque son point d'ébullition (28,4°C) est inférieur à ceux des deux constituants (F141b : 32°C ; formiate de méthyle : 31,7°C).The azeotrope F141b / methyl formate is a positive azeotrope since its boiling point (28.4 ° C) is lower than that of the two constituents (F141b: 32 ° C; methyl formate: 31.7 ° C).
Comme dans les compositions connues à base de F113, la composition selon l'invention peut être avantageusement stabilisée contre l'hydrolyse et/ou les attaques radicalaires susceptibles de survenir dans les processus de nettoyage, en y ajoutant un stabilisant usuel tel que, par exemple, le nitrométhane, l'oxyde de propylène ou un mélange de ces composés, la proportion de stabilisant pouvant aller de 0,01 à 5 % par rapport au poids total : F141b - formiate de méthyle.As in the known compositions based on F113, the composition according to the invention can be advantageously stabilized against hydrolysis and / or free radical attacks likely to occur in the cleaning processes, by adding to it a usual stabilizer such as, for example , nitromethane, propylene oxide or a mixture of these compounds, the proportion of stabilizer possibly ranging from 0.01 to 5% relative to the total weight: F141b - methyl formate.
La composition selon l'invention peut être utilisée dans les mêmes applications et selon les mêmes techniques que les compositions antérieures à base de F113.The composition according to the invention can be used in the same applications and according to the same techniques as the previous compositions based on F113.
Les exemples suivants illustrent l'invention sans la limiter.The following examples illustrate the invention without limiting it.
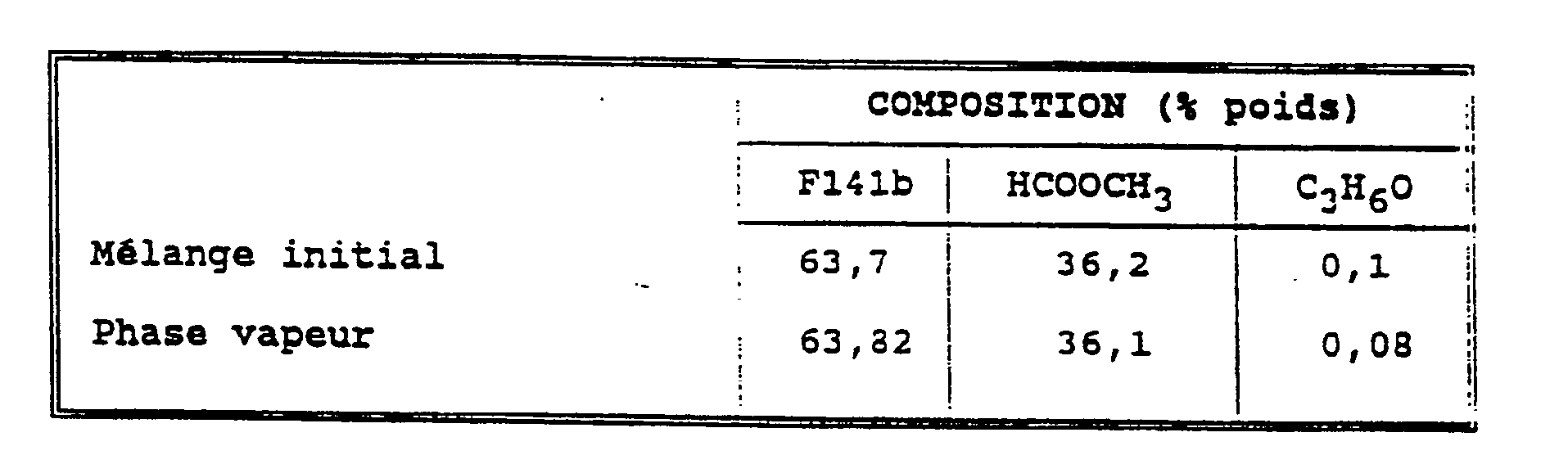
Dans le bouilleur d'une colonne à distiller (30 plateaux), on introduit 100 g de formiate de méthyle et 100 g de F141b. Le mélange est ensuite mis à reflux total pendant une heure pour amener le système à l'équilibre. Au palier de température (28,4°C), on prélève une fraction (environ 50 g) et on l'analyse par chromatographie en phase gazeuse.100 g of methyl formate and 100 g of F141b are introduced into the distiller of a distillation column (30 trays). The mixture is then put under total reflux for one hour to bring the system to equilibrium. At the temperature level (28.4 ° C), a fraction (about 50 g) is taken and analyzed by gas chromatography.
L'examen des résultats, consignés dans le tableau suivant, indique la présence d'un azéotrope F141b/formiate de méthyle.
Dans le bouilleur d'une colonne à distiller adiabatique (30 plateaux), on introduit 200 g d'un mélange comprenant 64 % en poids de F141b et 36 % en poids de formiate de méthyle. Le mélange est ensuite porté à reflux pendant une heure pour amener le système à l'équilibre, puis on soutire une fraction d'environ 50 g et on procède à son analyse par chromatographie en phase gazeuse ainsi que celle du pied de distillation. Les résultats consignés dans le tableau suivant montrent la présence d'un azéotrope positif puisque son point d'ébullition est inférieur à ceux des deux constituants purs : F141b et formiate de méthyle.
Cet azéotrope, employé pour le nettoyage de flux de soudure ou en dégraissage de pièces mécaniques, donne d'aussi bons résultats que les compositions à base de F113 et de méthanol.This azeotrope, used for cleaning solder flux or degreasing mechanical parts, gives as good results as compositions based on F113 and methanol.
Dans une cuve de nettoyage à ultra-sons, on introduit 150 g d'un mélange contenant en poids 64 % de F141b, 35,92 % de formiate de méthyle, et 0,08 % de nitrométhane comme stabilisant. Après avoir mis le système à reflux pendant une heure, on prélève un aliquat de la phase vapeur. Son analyse par chromatographie en phase gazeuse montre la présence de nitrométhane ce qui indique que le mélange est stabilisé dans la phase vapeur.
Si on répète l'exemple 3 en remplaçant le nitrométhane par l'oxyde de propylène, on obtient les résultats suivants :
On répète l'exemple 3 en utilisant 0,1 % de nitrométhane et 0,1 % d'oxyde de propylène. On obtient les résultats suivants :
Dans une cuve à ultra-sons Annemasse, on introduit 200 g de la composition azéotropique F141b/formiate de méthyle, puis on porte le mélange à la température d'ébullition.200 g of the azeotropic composition F141b / methyl formate are introduced into an Annemasse ultrasonic tank, then the mixture is brought to the boiling temperature.
Des plaques de verre, enduites de flux de soudure et recuites à l'étuve pendant 30 secondes à 220°C, sont plongées durant 3 minutes dans le liquide à l'ébullition sous ultra-sons, puis rincées dans la phase vapeur pendant 3 minutes.Glass plates, coated with solder flux and annealed in an oven for 30 seconds at 220 ° C., are immersed for 3 minutes in the boiling liquid under ultrasound, then rinsed in the vapor phase for 3 minutes .
Après séchage à l'air, une visualisation en lumière rasante revèle l'absence totale de résidu de flux de soudure. On a ainsi obtenu le même résultat qu'avec une composition F113-méthanol (93,7 %-6,3 %).After air drying, viewing in grazing light reveals the total absence of solder flux residue. The same result was thus obtained as with an F113-methanol composition (93.7% -6.3%).
Claims (8)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR9001395A FR2657876B1 (en) | 1990-02-07 | 1990-02-07 | CLEANING COMPOSITION BASED ON 1,1-DICHLORO-1-FLUOROETHANE AND METHYL FORMIATE. |
| FR9001395 | 1990-02-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0441663A1 true EP0441663A1 (en) | 1991-08-14 |
Family
ID=9393453
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19910400008 Withdrawn EP0441663A1 (en) | 1990-02-07 | 1991-01-03 | 1,1-Dichloro-1-fluoroethane and methyl formate based cleaning composition |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US5308528A (en) |
| EP (1) | EP0441663A1 (en) |
| JP (1) | JPH06207197A (en) |
| CA (1) | CA2035363A1 (en) |
| FR (1) | FR2657876B1 (en) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8309619B2 (en) | 2004-09-03 | 2012-11-13 | Pactiv LLC | Reduced-VOC and non-VOC blowing agents for making expanded and extruded thermoplastic foams |
| US20060052465A1 (en) * | 2004-09-03 | 2006-03-09 | Handa Yash P | Thermoplastic foams made with methyl formate-based blowing agents |
| US20060052466A1 (en) * | 2004-09-03 | 2006-03-09 | Handa Yash P | Expanded and extruded thermoplastic foams made with methyl formate-based blowing agents |
| WO2007101034A2 (en) * | 2006-02-22 | 2007-09-07 | Pactiv Corporation | Polyolefin foams made with methyl formate-based blowing agents |
| US7977397B2 (en) * | 2006-12-14 | 2011-07-12 | Pactiv Corporation | Polymer blends of biodegradable or bio-based and synthetic polymers and foams thereof |
| PL2089460T3 (en) * | 2006-12-14 | 2012-02-29 | Pactiv Corp | Expanded and extruded biodegradable and reduced emission foams made with methyl formate-based blowing agents |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3914191A (en) * | 1974-07-31 | 1975-10-21 | Union Carbide Corp | Methyl format E-trichloromonofluoromethane blowing agent for polystyrene |
| EP0116343A2 (en) * | 1983-02-14 | 1984-08-22 | The Dow Chemical Company | Photoresist stripper composition and method of use |
| US4816174A (en) * | 1988-05-03 | 1989-03-28 | Allied-Signal Inc. | Azeotrope-like compositions of 1,1-dichloro-1-fluoroethane, methanol and nitromethane |
| EP0325265A1 (en) * | 1988-01-20 | 1989-07-26 | E.I. Du Pont De Nemours And Company | Azeotropic compositions of 1,1-Dichloro-1-Fluoroethane and Methanol/Ethanol |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2101993A (en) * | 1932-04-30 | 1937-12-14 | Gen Motors Corp | Refrigerant mixture and the method of using the same |
| US4483917A (en) * | 1983-02-14 | 1984-11-20 | The Dow Chemical Company | Photoresist stripper composition and method of use |
| JPH01132539A (en) * | 1987-11-18 | 1989-05-25 | Asahi Glass Co Ltd | Method for stabilizing azeotropic compositions |
| JP2550622B2 (en) * | 1987-11-19 | 1996-11-06 | 旭硝子株式会社 | Cleaning agent for dry cleaning |
| JPH01139780A (en) * | 1987-11-27 | 1989-06-01 | Asahi Glass Co Ltd | Cleaner for buffed article |
| US4842764A (en) * | 1988-05-03 | 1989-06-27 | Allied-Signal Inc. | Azeotrope-like compositions of 1,1-dichloro-1-fluoroethane and methanol |
| US4804493A (en) * | 1988-05-24 | 1989-02-14 | E. I. Du Pont De Nemours And Company | Stabilized azeotrope or azeotrope-like composition of 1,1,2-trichloro-1,2,2-trifluoroethane and trans-1,2-dichloroethylene |
| US4836947A (en) * | 1988-06-09 | 1989-06-06 | Allied-Signal Inc. | Azeotrope-like compositions of 1,1-dichloro-1-fluoroethane and ethanol |
| US4960804A (en) * | 1989-03-09 | 1990-10-02 | Mobay Corporation | Rigid foams using blends of chlorofluorocarbons and alkyl alkanoates as blowing agent |
| US4945119A (en) * | 1989-05-10 | 1990-07-31 | The Dow Chemical Company | Foaming system for rigid urethane and isocyanurate foams |
| US5049301A (en) * | 1989-12-20 | 1991-09-17 | Allied-Signal Inc. | Azeotrope-like compositions of 1,1-dichloro-1-fluoroethane; dichlorotrifluoroethane; and methyl formate |
| FR2657877B1 (en) * | 1990-02-07 | 1992-05-15 | Atochem | CLEANING COMPOSITION BASED ON 1,1-DICHLORO-1-FLUOROETHANE, METHYL FORMATE AND METHANOL. |
| WO1991013966A1 (en) * | 1990-03-12 | 1991-09-19 | E.I. Du Pont De Nemours And Company | Binary azeotropes of hydrogen-containing halocarbons with methyl formate |
-
1990
- 1990-02-07 FR FR9001395A patent/FR2657876B1/en not_active Expired - Lifetime
-
1991
- 1991-01-03 EP EP19910400008 patent/EP0441663A1/en not_active Withdrawn
- 1991-01-31 CA CA002035363A patent/CA2035363A1/en not_active Abandoned
- 1991-02-05 JP JP3014463A patent/JPH06207197A/en active Pending
- 1991-02-07 US US07/651,813 patent/US5308528A/en not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3914191A (en) * | 1974-07-31 | 1975-10-21 | Union Carbide Corp | Methyl format E-trichloromonofluoromethane blowing agent for polystyrene |
| EP0116343A2 (en) * | 1983-02-14 | 1984-08-22 | The Dow Chemical Company | Photoresist stripper composition and method of use |
| EP0325265A1 (en) * | 1988-01-20 | 1989-07-26 | E.I. Du Pont De Nemours And Company | Azeotropic compositions of 1,1-Dichloro-1-Fluoroethane and Methanol/Ethanol |
| US4816174A (en) * | 1988-05-03 | 1989-03-28 | Allied-Signal Inc. | Azeotrope-like compositions of 1,1-dichloro-1-fluoroethane, methanol and nitromethane |
Also Published As
| Publication number | Publication date |
|---|---|
| US5308528A (en) | 1994-05-03 |
| CA2035363A1 (en) | 1991-08-08 |
| FR2657876A1 (en) | 1991-08-09 |
| FR2657876B1 (en) | 1992-05-15 |
| JPH06207197A (en) | 1994-07-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0512885B1 (en) | Composition based on 1,1,1,3,3-pentafluorobutane and methanol for cleaning and/or drying of hard surfaces | |
| EP0512884B1 (en) | Composition based on 1,1-dichloro-1-fluoroethane, 1,1,1,3,3-pentafluorobutane and methanol used for cleaning and/or drying of hard surfaces | |
| EP0653484B1 (en) | Compositions containing pentafluorobutane and use thereof | |
| EP0443911B1 (en) | Use of (perfluoroalkyl)-ethylenes as cleaning or drying agents | |
| FR2694943A1 (en) | Composition based on 1,1,1,3,3-pentafluorobutane, methylene chloride and methanol, for cleaning and / or drying solid surfaces. | |
| FR2694942A1 (en) | Composition based on 1,1,1,3,3-pentafluorobutane and methylene chloride, for cleaning and / or drying solid surfaces. | |
| EP0731162A1 (en) | Use of hydrofluoroalkenes as cleaning agents and compositions containing them | |
| EP0456551A1 (en) | Cleaning composition based on 1,1,1,2,2-pentafluoro-3,3-dichloro-propane, and methyl ter butyl ether | |
| EP0974642B1 (en) | 1,1,1,2,3,4,4,5,5,5-decafluoropentane and 1,1,1,3,3-pentafluorobutane-based cleaning and drying compositions | |
| EP0441663A1 (en) | 1,1-Dichloro-1-fluoroethane and methyl formate based cleaning composition | |
| EP0856578B1 (en) | Cleaning or drying compositions containing 1,1,1,2,3,4,4,5,5,6-decafluoropentane | |
| EP0441664A1 (en) | Cleaning composition based on 1,1-dichloro-1-fluoroethane, methylformiate and methanol | |
| EP0525266B1 (en) | Composition based on n-perfluorobutyl-ethylene for cleaning solid surfaces | |
| FR2656328A1 (en) | Cleaning composition based on 1,1-dichloro-2,2,2-trifluoroethane, methyl formate and methanol | |
| FR2656327A1 (en) | Cleaning composition based on 1,1-dichloro-2,2,2-trifluoroethane and methyl formate | |
| EP0609125B1 (en) | Stabilized cleaning composition based on 1,1-dichloro-1-fluoroethane and methanol | |
| EP0474528A1 (en) | Cleaning composition based on 1,1-dichloro-1-fluoroethane, methylene chloride and methanol | |
| EP0771865A1 (en) | Cleaning composition based on 1,1,1,2,2,4,4-heptafluorobutane and alcohols | |
| FR2766837A1 (en) | New azeotropic compositions based on (n-perfluorohexyl)ethylene and an organic solvent | |
| FR2741354A1 (en) | New composition comprising 1,1-di-chloro-1-fluoroethane | |
| WO2003025109A1 (en) | Cleaning or drying compositions based on n-perfluorobutyl-ethylene and hfc 365mfc |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19910111 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE DE ES FR GB IT NL |
|
| RAP3 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ELF ATOCHEM S.A. |
|
| K1C3 | Correction of patent application (complete document) published |
Effective date: 19910814 |
|
| 17Q | First examination report despatched |
Effective date: 19940520 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19951014 |