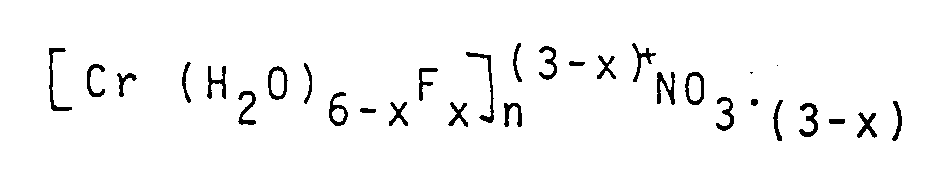EP0337411B1 - Process for preparing an acidic passivating bath for zinc, zinc alloys an cadmium surfaces, containing chromium III and fluoride - Google Patents
Process for preparing an acidic passivating bath for zinc, zinc alloys an cadmium surfaces, containing chromium III and fluoride Download PDFInfo
- Publication number
- EP0337411B1 EP0337411B1 EP89106447A EP89106447A EP0337411B1 EP 0337411 B1 EP0337411 B1 EP 0337411B1 EP 89106447 A EP89106447 A EP 89106447A EP 89106447 A EP89106447 A EP 89106447A EP 0337411 B1 EP0337411 B1 EP 0337411B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fluoride
- zinc
- chromium
- iii
- acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/05—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions
- C23C22/06—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6
- C23C22/34—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing fluorides or complex fluorides
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C2222/00—Aspects relating to chemical surface treatment of metallic material by reaction of the surface with a reactive medium
- C23C2222/10—Use of solutions containing trivalent chromium but free of hexavalent chromium
Definitions
- the invention relates to a method for producing an acidic chromium-containing and fluoride-containing passivation bath for zinc or cadmium surfaces.
- an acidic chromium-containing passivation bath for zinc or cadmium surfaces is known from US Pat. No. 4,263,059 and DE-OS 30 38 699, which, in addition to a "blue solution" of trivalent chromium and an acid, such as formic, acetic or propionic acid or nitric, sulfuric, hydrochloric and hydrofluoric acid contains a fluoride with a "green solution" of hexavalent chromium, for example Chromium trioxide, alkali metal chromate and dichromate and a reducing agent such as an aldehyde or alcohol or an alkali metal sulfite, bisulfite, metabisulfite, iodide, hydrogen peroxide, sulfur dioxide or an iron (II) salt is formed.
- a fluoride with a "green solution” of hexavalent chromium for example Chromium trioxide, alkali metal chromate and dichromate and a reducing
- surfaces made of zinc, zinc alloys, galvanically or by hot-dip galvanizing on iron or steel or zinc layers or cadmium surfaces are made more corrosion-resistant, which is indicated by a coloration that ranges from blue to black and yellow to olive and is also used for decorative purposes.
- a passivation for example, the so-called blue passivation, ie Generation of a light blue, very thin passivation layer with a good decorative appearance, formation of zinc corrosion products also called "white rust", greatly delayed.
- the salt spray test according to DIN 50021 provides a measure of the corrosion protection of these chromates.
- a zinc-coated and passivated part is exposed to a 5% sodium chloride mist at 40 ° C and 100% humidity. The time required to oxidize 5 - 10% of the surface to white rust is then given.
- the corrosion protection according to DIN 50021 should be 20 to 24 hours.
- Chromium (III) fluorine complexes of chromium (III) are known, the formation of which is accelerated by zinc, but there is no evidence of the blue passivation of zinc or galvanized in this investigation of equilibrium constants Iron surfaces, on where there is no chrome metal deposition, but a polymeric compound with divalent chromium is deposited.
- This object is achieved according to the invention by a process for producing an acidic, chromium (III) -containing and fluoride-containing passivation bath for surfaces made of zinc, zinc alloys and cadmium by mixing 20 to 200 g / l of a soluble chromium (111) compound 20 to 600 g / l of a soluble nitrate (total nitrate) 5 to 100 g / l of a fluoride and hydrochloric or nitric acid to a pH of 1.8 to 2.2 and an anion selected from the group consisting of sulfate, phosphate, chloride, bromide, fluoride and iodide, the soluble nitrate in greater concentration than the chromium (III ) Concentration (in g / l) is present, and the mixture is either heated to 60 ° C.
- the concentrate obtained is optionally brought to an application concentration of 2 to 20% by weight in water , dissolved, which is characterized in that it additionally contains an acidic ion exchanger loaded with Cr3+ and / or an agent for the insolubilization of Fe3+ ions in an amount of 0.1 to 100 g / l.
- a catalyst in particular with activated carbon at 15 ° C. or above, for example at room temperature (20-25 ° C.) or brief heating, for example 30 seconds to 15 minutes. to at least 60 to 80 ° C, seems to promote the formation of the more stable chromium (III) complexes, which is surprising is because only the hexahydrate complexes of chromium are stable at room temperature, but they are unusable for passivation.
- Passivation baths preferred according to the invention contain the following amounts in a concentrate which is usually used in a concentration of 2 to 20% by weight in water: 20-200 g / l chromium (III) compound, e.g. as chromium chloride or chromium nitrate, 20 - 600 g / l soluble nitrate, such as sodium, potassium or ammonium nitrate, 5 - 100 g / l fluoride, e.g. sodium, potassium, ammonium fluoride Hydrochloric or nitric acid up to a pH of 1.8 to 2.2.
- a concentrate which is usually used in a concentration of 2 to 20% by weight in water: 20-200 g / l chromium (III) compound, e.g. as chromium chloride or chromium nitrate, 20 - 600 g / l soluble nitrate, such as sodium, potassium or ammonium nitrate, 5 - 100
- Passivation baths made from this concentrate show corrosion protection values of a blue passivation according to DIN 50021 from 44 to 50 h, the layers can be colored and good organic polymers are applied to the layers.
- a concentrate of the following composition was produced: 50 g / l chromium (III) chloride 125 g / l sodium nitrate 50 g / l sodium fluoride
- the concentrate was heated to 65 ° C. It contained complexes of the formula
- the bath was mixed with water in a weight ratio of 1:10 and adjusted to pH 2.0 with nitric acid. With this bath, hot-dip galvanized iron bars of 2 cm in diameter and 20 cm in length were blue passivated. After 3 seconds, the rods were removed from the bath and checked for corrosion behavior in accordance with DIN 50021. The corrosion protection value was 48 hours.
- the durability of the bath was extended by a factor of 10 by adding 50 g / l of acetylacetone.
- a bath was out 60 g / l Cr (NO3) 3.9H2O 100 g / l NaNO3 40 g / l NaF HNO3 ad pH 2.1 and without heating, but prepared by treating with granular activated carbon in a porous bag for 10 minutes and, after mixing with water in a weight ratio of 1:12, used for the same experiment. It contained complexes of the formula The same corrosion protection value was obtained.
- the following results were obtained in a comparison test on normal blue chromating (commercially available): with Cr VI (commercially available): 15 mg Cr / m2 zinc; 24 h corrosion protection Cr III (according to the invention: 30 mg Cr / m2 zinc; 48 h corrosion protection.
- the durability of the bath was extended by a factor of 5 by adding 20 g / l salicylic acid.
- the bath with the ion exchanger showed a perfect blue passivation, while the comparison bath was unusable after a short time.
- the addition of 10 g / l fumaric acid increases the life of the bath by a factor of 5.
Landscapes
- Chemical & Material Sciences (AREA)
- Metallurgy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Treatment Of Metals (AREA)
- Chemically Coating (AREA)
- Preventing Corrosion Or Incrustation Of Metals (AREA)
- Cleaning And De-Greasing Of Metallic Materials By Chemical Methods (AREA)
- Glass Compositions (AREA)
- Electroplating And Plating Baths Therefor (AREA)
Abstract
Description
Die Erfindung betrifft ein Verfahren zur Herstellung eines sauren chromhaltigen und fluoridhaltigen Passivierungsbades für Zink- oder Cadmiumoberflächen.The invention relates to a method for producing an acidic chromium-containing and fluoride-containing passivation bath for zinc or cadmium surfaces.
Es ist z.B. aus der US-PS 4 263 059 bzw. der DE-OS 30 38 699 ein saures chromhaltiges Passivierungsbad für Zink- oder Cadmiumoberflächen bekannt, das neben einer "blauen Lösung" aus dreiwertigem Chrom und einer Säure, wie Ameisen-, Essig- oder Propionsäure oder Salpeter-, Schwefel-, Salz- und Fluorwasserstoffsäure ein Fluorid enthält mit einer "grünen Lösung" aus sechswertigem Chrom, z.B. Chromtrioxid, Alkalimetallchromat und -dichromat und einem Reduktionsmittel, wie ein Aldehyd oder Alkohol oder einem Alkalimetallsulfit, -bisulfit, -metabisulfit, -jodid, Wasserstoffperoxid, Schwefeldioxid oder einem Eisen-II-Salz gebildet wird.It is e.g. an acidic chromium-containing passivation bath for zinc or cadmium surfaces is known from US Pat. No. 4,263,059 and DE-OS 30 38 699, which, in addition to a "blue solution" of trivalent chromium and an acid, such as formic, acetic or propionic acid or nitric, sulfuric, hydrochloric and hydrofluoric acid contains a fluoride with a "green solution" of hexavalent chromium, for example Chromium trioxide, alkali metal chromate and dichromate and a reducing agent such as an aldehyde or alcohol or an alkali metal sulfite, bisulfite, metabisulfite, iodide, hydrogen peroxide, sulfur dioxide or an iron (II) salt is formed.
Hierdurch werden Oberflächen aus Zink, Zinklegierungen, galvanisch oder durch Feuerverzinkung auf Eisen oder Stahl aufgebrachte Zinkschichten oder Cadmiumoberflächen korrosionsfester gemacht, was durch eine Färbung angezeigt wird, die von blau über schwarz und gelb bis zu oliv reicht und auch für Dekorationszwecke Anwendung findet. Insbesondere wird durch eine solche Passivierung z.B. die sogenannte Blaupassivierung, d.h. die Erzeugung einer leicht blauen, sehr dünnen Passivierungsschicht mit gutem dekorativen Aussehen, eine Bildung der Zinkkorrosionsprodukte auch "Weißrost" genannt, stark verzögert.As a result, surfaces made of zinc, zinc alloys, galvanically or by hot-dip galvanizing on iron or steel or zinc layers or cadmium surfaces are made more corrosion-resistant, which is indicated by a coloration that ranges from blue to black and yellow to olive and is also used for decorative purposes. In particular, such a passivation, for example, the so-called blue passivation, ie Generation of a light blue, very thin passivation layer with a good decorative appearance, formation of zinc corrosion products also called "white rust", greatly delayed.
Ein Maß für den Korrosionsschutz dieser Chromatierungen liefert der Salzsprühtest nach DIN 50021. Dabei wird ein zinkbeschichtetes und passiviertes Teil bei 40°C und 100 % Luftfeuchtigkeit einem 5%igen Natriumchloridnebel ausgesetzt. Angegeben wird dann die Zeit, die benötigt wird, 5 - 10% der Fläche zu Weißrost zu oxidieren.The salt spray test according to DIN 50021 provides a measure of the corrosion protection of these chromates. A zinc-coated and passivated part is exposed to a 5% sodium chloride mist at 40 ° C and 100% humidity. The time required to oxidize 5 - 10% of the surface to white rust is then given.
Bei der Blaupassivierung sollte der Korrosionsschutz nach DIN 50021 bei 20 bis 24 h liegen.In the case of blue passivation, the corrosion protection according to DIN 50021 should be 20 to 24 hours.
Durch das Vorhandensein von giftigen Chrom-(IV)-Verbindungen in den Passivierungslösungen des Standes der Technik ist es jedoch möglich, dass Chrom-(VI) in die Passivierungsschicht eingebaut wird, was insbesondere bei Verwendung solcher Schichten zu Dermatitiserkrankungen führen kann und auch aus diesem Grunde in Geräten der Nahrungsmittelindustrie bedenklich ist. Die Abwasserbehandlung zur Entgiftung von Passivierungslösungsresten bzw. bei Spülwässern bietet Probleme und die Lösungen verbrauchen sich schnell und können nur begrenzt nachgeschärft (regeneriert) werden, müssen vielmehr bald entsorgt werden. Vielmehr läßt der Korrosionsschutz nach kurzer Zeit, oft schon nach einem Tag, nach. Andere saure Lösungen, die Chrom-(III)-Ionen enthalten und neben diesen auch Oxidationsmittel (US-PS 4 171 231) und weitere Zusätze wie Silikate und/oder andere Metallionen (Us-PS 4 384 902, 4 359 347, 4 367 099) oder Organophosphorverbindungen (US-PS 4 359 348) bzw. Carbonsäuren (US-PS 4 349 392) enthalten, haben zwar die Eigenschaft, dekorative Blau- bzw. Gelbpassivierungen zu bilden, die aber nicht nachträglich einfärbbar sind und deren Korrosionsverhalten in der Größenordnung von maximal 6 h auf 10% Weißrost nach DIN 50021 lag. Wegen des Vorhandenseins von Oxidationsmitteln sind die Schichten nicht frei von Chrom-(VI)-Verbindungen, die insbesondere auch bei der pH-Wert-Erhöhung im Sedimentationsbecken der Abwasserbehandlung entstehen und die Entgiftung erschweren.However, due to the presence of toxic chromium (IV) compounds in the passivation solutions of the prior art, it is possible for chromium (VI) to be incorporated into the passivation layer, which can lead to and also from dermatitis diseases, particularly when such layers are used Reasonable concern in food industry equipment. Wastewater treatment for the detoxification of passivation solution residues or rinsing water offers problems and the solutions consume quickly and can only be re-sharpened (regenerated) to a limited extent, but must soon be disposed of. Rather, the corrosion protection wears off after a short time, often after just one day. Other acidic solutions which contain chromium (III) ions and in addition to these also oxidizing agents (US Pat. No. 4,171,231) and further additives such as silicates and / or other metal ions (US Pat. No. 4,384,902, 4,359,347, 4,367 099) or organophosphorus compounds (US Pat. No. 4,359,348) or carboxylic acids (US Pat. No. 4,349,392) do have the property of forming decorative blue or yellow passivations, but they cannot be subsequently colored and their corrosion behavior in the Order of magnitude of a maximum of 6 hours on 10% white rust according to DIN 50021. Because of the presence of oxidizing agents, the layers are not free of chromium (VI) compounds, which also occur when the pH is increased in the sedimentation basin of wastewater treatment and make detoxification more difficult.
Zwar sind aus J. Am. Chem. Soc. 74(1952) Seiten 3509 - 3512 Chrom-(III)-Fluor-Komplexe von Chrom-(III) bekannt, deren Bildung durch Zink beschleunigt wird, jedoch findet sich bei dieser Untersuchung von Gleichgewichtskonstanten kein Hinweis auf die Blaupassivierung von Zink bzw. verzinkten Eisenoberflächen, auf denen keine Chrommetallabscheidung erfolgt, sondern eine polymere Verbindung mit zweiwertigem Chrom abgeschieden wird.Although from J. Am. Chem. Soc. 74 (1952) Pages 3509 - 3512 Chromium (III) fluorine complexes of chromium (III) are known, the formation of which is accelerated by zinc, but there is no evidence of the blue passivation of zinc or galvanized in this investigation of equilibrium constants Iron surfaces, on where there is no chrome metal deposition, but a polymeric compound with divalent chromium is deposited.
Ferner wird durch saure Chromatierungslösungen bei verzinkten Eisengegenständen eine gewisse Menge Eisen der Lösung gelöst, wodurch nach einer gewissen Zeit derartige Lösungen , insbesondere wenn sie Cr³⁺ enthalten, unbrauchbar werden. Insbesondere ist dies der Fall bei Gegenständen, die aus geometrischen Gründen keine ganz geschlossene Zinkschicht aufweisen.In addition, a certain amount of iron is dissolved in the solution by means of acidic chromating solutions in the case of galvanized iron objects, as a result of which such solutions become unusable after a certain time, in particular if they contain Cr³⁺. This is particularly the case for objects that do not have a completely closed zinc layer for geometric reasons.
Ein saures chromhaltiges Passivierungsbad, das nur Chrom-III-Verbindungen, aber keine Oxidationsmittel und keine starken Komplexbildner enthält ist aus der US-4.705.576 bekannt. Solche Bäder werden aber durch einen Eisengehalt, wie er Zwangsläufig z.B. bei der Behandlung verzinkter Einsenrohre entsteht, beeinträchtigt. Es wurde nun ein Verfahren zur Herstellung eines Passivierungsbades gefunden, das eine lange Standzeit hat, einfärbbare Passivierungen erzeugt und erlaubt, organische Polymere auf erzeugte Passivierungsschicht besser adsorbieren zu lassen sowie nicht durch eventuell im Bad gelöstes Eisen unbrauchbar wird.An acidic chromium-containing passivation bath which contains only chromium III compounds but no oxidizing agents and no strong complexing agents is known from US Pat. No. 4,705,576. However, such baths are characterized by an iron content such as is inevitably e.g. arises during the treatment of galvanized inner tubes. A process has now been found for producing a passivation bath which has a long service life, produces passivations which can be colored and allows organic polymers to be better adsorbed onto the passivation layer produced and does not become unusable due to iron which may have been dissolved in the bath.
Diese Aufgabe wird erfindungsgemäß durch ein Verfahren zur Herstellung eines sauren, chrom-(III)-haltigen und fluoridhaltigen Passivierungsbades für Oberflächen aus Zink, Zinklegierungnen und Cadmium durch Mischen von
20 bis 200 g/l einer löslichen Chrom-(111)-Verbindung
20 bis 600 g/l eines löslichen Nitrats (Gesamtnitrat)
5 bis 100 g/l eines Fluorids
und Salz- oder Salpetersäure bie zu einem pH-Wert 1,8 bis 2,2 sowie einem Anion ausgewählt aus der Gruppe Sulfat, Phosphat, Chlorid, Bromid, Fluorid und Jodid, wobei das lösliche Nitrat in größerer Konzentration als die Chrom-(III)-Konzentration (in g/l) vorliegt, sowie das Gemisch entweder auf 60°C erwärmt oder mit einem Katalysator oberhalb 15°C behandelt wird und das erhaltene Konzentrat gegebenenfalls auf eine Anwedungskonzentration von 2 bis 20 Gew.-% in Wasser gebracht wird, gelöst, das dadurch gekennzeichnet ist, daß es zusätzlich einen sauren, mit Cr³⁺ beladenen Jonenaustauscher und/oder ein Mittel zum Unlöslichmachen von Fe³⁺-Jonen in einer Menge von 0,1 bis 100 g/l enthält.This object is achieved according to the invention by a process for producing an acidic, chromium (III) -containing and fluoride-containing passivation bath for surfaces made of zinc, zinc alloys and cadmium by mixing
20 to 200 g / l of a soluble chromium (111) compound
20 to 600 g / l of a soluble nitrate (total nitrate)
5 to 100 g / l of a fluoride
and hydrochloric or nitric acid to a pH of 1.8 to 2.2 and an anion selected from the group consisting of sulfate, phosphate, chloride, bromide, fluoride and iodide, the soluble nitrate in greater concentration than the chromium (III ) Concentration (in g / l) is present, and the mixture is either heated to 60 ° C. or treated with a catalyst above 15 ° C. and the concentrate obtained is optionally brought to an application concentration of 2 to 20% by weight in water , dissolved, which is characterized in that it additionally contains an acidic ion exchanger loaded with Cr³⁺ and / or an agent for the insolubilization of Fe³⁺ ions in an amount of 0.1 to 100 g / l.
Die Behandlung mit einem Katalysator, insbesondere mit Aktivkohle bei 15°C oder darüber, z.B. bei Raumtemperatur (20 - 25°C) oder das kurzzeitige Erhitzen z.B. 30 sec. bis 15 min. auf mindestens 60 bis 80°C, scheint die Bildung der stabileren Chrom-(III)-komplexe zu fördern, was überraschend ist, da bei Raumtemperatur lediglich die Hexahydrat-komplexe des Chroms stabil sind, diese aber zur Passivierung unbrauchbar sind.Treatment with a catalyst, in particular with activated carbon at 15 ° C. or above, for example at room temperature (20-25 ° C.) or brief heating, for example 30 seconds to 15 minutes. to at least 60 to 80 ° C, seems to promote the formation of the more stable chromium (III) complexes, which is surprising is because only the hexahydrate complexes of chromium are stable at room temperature, but they are unusable for passivation.
In den erfindungsgemäß hergestellten Passivierungsbädern liegen Komplexe der allgemeinen Formel
vor, worin x = 1 - 3 (Anzahl der Fluoridionen) n = Wertigkeit des Anions A und A = Nitrat, Sulfat, Phosphat, Chlorid, Bromid, Fluorid und Jodid, was durch Leitfähigkeitsmessungen an der Fluorid-Elektrode bestimmt werden kann.The passivation baths produced according to the invention contain complexes of the general formula
before, where x = 1-3 (number of fluoride ions) n = valence of the anion A and A = nitrate, sulfate, phosphate, chloride, bromide, fluoride and iodide, which can be determined by conductivity measurements at the fluoride electrode.
Erfindungsgemäß bevorzugte Passivierungsbäder enthalten folgenden Mengen in einem Konzentrat, das üblicherweise in einer Konzentration von 2 bis 20 Gew.-% in Wasser angewendet wird:
20 - 200 g/l Chrom-(III)-verbindung, z.B. als Chromchlorid oder Chromnitrat,
20 - 600 g/l lösliches Nitrat, wie Natrium, Kalium- oder Ammoniumnitrat,
5 - 100 g/l Fluorid, z.B. Natrium, Kalium-, Ammoniumfluorid
Salz- oder Salpetersäure bis zu einem pH-Wert 1,8 bis 2,2.Passivation baths preferred according to the invention contain the following amounts in a concentrate which is usually used in a concentration of 2 to 20% by weight in water:
20-200 g / l chromium (III) compound, e.g. as chromium chloride or chromium nitrate,
20 - 600 g / l soluble nitrate, such as sodium, potassium or ammonium nitrate,
5 - 100 g / l fluoride, e.g. sodium, potassium, ammonium fluoride
Hydrochloric or nitric acid up to a pH of 1.8 to 2.2.
Aus diesem Konzentrat hergestellte Passivierungsbäder zeigen Korrosionsschutzwerte einer Blaupassivierung nach DIN 50021 von 44 bis 50 h, eine Einfärbbarkeit der Schichten und ein gutes Aufziehen organischer Polymere auf die Schichten.Passivation baths made from this concentrate show corrosion protection values of a blue passivation according to DIN 50021 from 44 to 50 h, the layers can be colored and good organic polymers are applied to the layers.
Es wurde ein Konzentrat folgender Zusammensetzung hergestellt:
50 g/l Chrom-(III)-chlorid
125 g/l Natriumnitrat
50 g/l Natriumfluorid
Das Konzentrat wurde auf 65°C erwärmt. Es enthielt Komplexe der Formel
Das Bad wurde mit Wasser im Gew.-Verhältnis 1 : 10 gemischt und mit Salpetersäure auf den pH-Wert 2,0 eingestellt. Mit diesem Bad wurden feuerverzinkte Eisenstangen von 2 cm Durchmesser und 20 cm Länge blaupassiviert. Nach 3 sec wurden die Stangen aus dem Bad genommen und auf Korrosionsverhalten nach DIN 50021 geprüft. Der Korrosionsschutzwert betrug 48 h.A concentrate of the following composition was produced:
50 g / l chromium (III) chloride
125 g / l sodium nitrate
50 g / l sodium fluoride
The concentrate was heated to 65 ° C. It contained complexes of the formula
The bath was mixed with water in a weight ratio of 1:10 and adjusted to pH 2.0 with nitric acid. With this bath, hot-dip galvanized iron bars of 2 cm in diameter and 20 cm in length were blue passivated. After 3 seconds, the rods were removed from the bath and checked for corrosion behavior in accordance with DIN 50021. The corrosion protection value was 48 hours.
Durch Zugabe von 50 g/l Acetylaceton wurde die Haltbarkeit des Bades um den Faktor 10 verlängert.The durability of the bath was extended by a factor of 10 by adding 50 g / l of acetylacetone.
Ein Bad wurde aus
60 g/l Cr(NO₃)₃ .9H₂O
100 g/l NaNO₃
40 g/l NaF HNO₃ ad pH 2,1
und ohne Erhitzen, aber durch Behandeln 10 min mit gekörnter Aktivkohle in einem porösen Säckchen hergestellt und nach Mischen mit Wasser im Gew.-Verhältnis 1 : 12 für den gleichen Versuch verwendet. Es enthielt Komplexe der Formel
Es wurde der gleiche Korrosionsschutzwert erhalten. Im Vergleichsversuch auf einer normalen Blauchromatierung (handelsüblich) wurden folgende Ergebnisse erhalten: mit
Cr VI (handelsüblich) : 15 mg Cr/m² Zink; 24h Korrosionsschutz
Cr III (erfindungsgemäß : 30 mg Cr/m² Zink; 48h Korrosionsschutz.A bath was out
60 g / l Cr (NO₃) ₃.9H₂O
100 g / l NaNO₃
40 g / l NaF HNO₃ ad pH 2.1
and without heating, but prepared by treating with granular activated carbon in a porous bag for 10 minutes and, after mixing with water in a weight ratio of 1:12, used for the same experiment. It contained complexes of the formula
The same corrosion protection value was obtained. The following results were obtained in a comparison test on normal blue chromating (commercially available): with
Cr VI (commercially available): 15 mg Cr / m² zinc; 24 h corrosion protection
Cr III (according to the invention: 30 mg Cr / m² zinc; 48 h corrosion protection.
Die Haltbarkeit des Bades wurde um den Faktor 5 durch Zugabe von 20 g/l Salicylsäure verlängert.The durability of the bath was extended by a factor of 5 by adding 20 g / l salicylic acid.
Es wurde ein Bad gemäß Beispiel 1 mit einem stark sauren Ionenaustauscher,
der mit einer 3 M Chrom-(III)-Nitratlösung beladen worden war, in einem Bad mit 1,0 g/l Fe³⁺-Gehalt eingesetzt. Zum Vergleich wurde das gleiche Bad ohne Ionenaustauscher verwendet.There was a bath according to Example 1 with a strongly acidic ion exchanger,
which had been loaded with a 3 M chromium (III) nitrate solution, used in a bath with 1.0 g / l Fe³⁺ content. The same bath without ion exchanger was used for comparison.
Das Bad mit dem Jonenaustauscher ergab eine einwandfreie Blaupassivierung,während das Vergleichsbad nach kurzer Zeit unbrauchbar war. Eine Zugabe von 10 g/l Fumarsäure erhöht die Lebensdauer des Bades um Faktor 5.The bath with the ion exchanger showed a perfect blue passivation, while the comparison bath was unusable after a short time. The addition of 10 g / l fumaric acid increases the life of the bath by a factor of 5.
Es wurden vier Lösungen aus je 100 ml einer 10%igen Passivierungslösung nach Beispiel 1 angesetzt (pH 1,6) und mit den in der Tabelle angegebenen Mengen eines Gemisches aus Alkylaminen und Amiden mit 5 - 12 C-Atomen versetzt. Es wurde 1mm dickes Eisenblech mit einer Fläche von 1 dm² eingelegt. Nach 12 Stunden wurden die Eisengehalte der Lösungen analytisch durch Atomabsorptionspektrometrie bestimmt. Es wurden folgende Ergebnisse erhalten, die die Abhängigkeit des Eisengehalts der Passivierungslösung von der Menge zugesetzten Inhibitor, d.h. Mittel zum Unlöslichmachen der gelösten Fe³⁺-Jonen zeigen. Der Zusatz von 1 g/l Inhibitor ergab praktisch einen nur geringen Unterschied hinsichtlich Farbe und Korrosionsschutz gegenüber der Passivierungslösung ohne Inhibitor.
Claims (2)
- Process for preparing an acid chromium (III)- and fluoride-containing passivation bath for surfaces of zinc, zinc alloys and cadmium by mixing of
20-200 g/l of a soluble chromium (III) compound;
20-600 g/l of a soluble nitrate (total nitrate);
5-100 g/l of a fluoride,
and hydrochloric or nitric acid up to pH value 1.8-2.2 as well as an anion selected from the group consisting of sulphate, phosphate, chloride, bromide, fluoride and iodide, in which the soluble nitrate is present in a higher concentration than the chromium (III) concentration (expressed in g/l), and the mixture is heated either to 60°C or is treated with a catalyst at a temperature above 15°C and the concentrate obtained is optionally brought to a use concentration of 2-20 wt.% in water, characterized in that further an acid ion exchanger loaded with Cr³⁺ and/or an insolubilizer for Fe³⁺ ions are added in an amount of 0.1-100 g/l. - Process according to Claim 1, characterized in that the insolubiliser for Fe³⁺ ions is selected from the group of acetylacetone, fumaric acid, gluconic acid, oxalic acid, lactic acid, cyclic and alicyclic, primary or secondary amine, amino acids and amides having 3-16 carbon atoms, which form compounds with Fe³⁺ ions in water that are barely soluble, respectively insoluble.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE8916143U DE8916143U1 (en) | 1988-04-12 | 1989-04-11 | Acidic chromium (III) -containing and fluoride-containing passivation bath concentrate for surfaces made of zinc, zinc alloys and cadmium |
| AT89106447T ATE97700T1 (en) | 1988-04-12 | 1989-04-11 | PROCESS FOR THE MANUFACTURE OF AN ACIDIC CHROMIUM(III) AND FLUORIDE PASSIVATION BATH FOR ZINC, ZINC ALLOYS AND CADMIUM SURFACES. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3812076A DE3812076A1 (en) | 1988-04-12 | 1988-04-12 | ACID CHROMIUM (III) AND FLUORIDE-BASED PASSIVATION BATH FOR SURFACES OF ZINC, ZINC ALLOYS AND CADMIUM |
| DE3812076 | 1988-04-12 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0337411A2 EP0337411A2 (en) | 1989-10-18 |
| EP0337411A3 EP0337411A3 (en) | 1990-05-09 |
| EP0337411B1 true EP0337411B1 (en) | 1993-11-24 |
Family
ID=6351774
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP89106447A Expired - Lifetime EP0337411B1 (en) | 1988-04-12 | 1989-04-11 | Process for preparing an acidic passivating bath for zinc, zinc alloys an cadmium surfaces, containing chromium III and fluoride |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP0337411B1 (en) |
| AT (1) | ATE97700T1 (en) |
| DD (1) | DD280557A5 (en) |
| DE (2) | DE3812076A1 (en) |
| YU (1) | YU71689A (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6946201B2 (en) | 1996-04-19 | 2005-09-20 | Surtec International Gmbh | Chromium (VI)-free conversion layer and method for producing it |
| CN102912337A (en) * | 2012-10-30 | 2013-02-06 | 四川华丰企业集团有限公司 | Process for producing green passivation coating of cadmium-plated workpiece |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE4135524C2 (en) * | 1991-10-28 | 1995-01-26 | Gc Galvano Consult Gmbh | Method and means for chromating surfaces made of zinc or cadmium or alloys thereof |
| US7314671B1 (en) | 1996-04-19 | 2008-01-01 | Surtec International Gmbh | Chromium(VI)-free conversion layer and method for producing it |
| JP4145016B2 (en) * | 2001-01-31 | 2008-09-03 | 日本パーカライジング株式会社 | Rust preventive agent for galvanized steel sheet and galvanized steel sheet |
| US6761774B2 (en) * | 2001-05-24 | 2004-07-13 | Basf Corporation | Composition and method for the in situ removal scale from a substrate |
| US7101469B2 (en) | 2004-11-10 | 2006-09-05 | Atotech Deutschland Gmbh | Metal pieces and articles having improved corrosion resistance |
| DE102009042861B4 (en) | 2009-09-24 | 2020-08-20 | AnJo Oberflächentechnik GmbH | Composition, application solution and process for passivation of zinc and its alloys |
| WO2011127473A1 (en) * | 2010-04-09 | 2011-10-13 | Enthone Inc. | Passivation treatment of zinc-based coatings |
| DE102011013319B4 (en) | 2011-03-07 | 2018-06-14 | AnJo Oberflächentechnik GmbH | Composition and application solution for passivating zinc and its alloys |
| EP2708620A1 (en) | 2012-09-12 | 2014-03-19 | Anjo Oberflächentechnik GmbH | Composition and solution to use for passivating zinc and its alloys |
| CN103215580B (en) * | 2013-03-25 | 2016-01-27 | 沈阳帕卡濑精有限总公司 | There is for surface of steel plate fast filming the trivalent chromium passivator of high anti-corrosion |
| CN103215581B (en) * | 2013-03-25 | 2015-06-03 | 沈阳帕卡濑精有限总公司 | Passivation enclosing integrated trivalent chromium passivating agent for fast processing steel plate surface |
| EP2907894B1 (en) * | 2014-02-13 | 2019-04-10 | Ewald Dörken Ag | Method for production of a substrate with a chromium VI free and cobalt-free passivation |
| NL2017398B1 (en) * | 2016-08-31 | 2018-03-08 | Ad Productions B V | Method of treating metal surfaces with an aqueous composition and aqueous composition |
| WO2018209348A1 (en) | 2017-05-12 | 2018-11-15 | Chemeon Surface Technology, Llc | pH STABLE TRIVALENT CHROMIUM COATING SOLUTIONS |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2166737A1 (en) * | 1970-04-02 | 1975-06-05 | Du Pont | Complex chromium salt metal surface primer in aqs soln |
| US4171231A (en) * | 1978-04-27 | 1979-10-16 | R. O. Hull & Company, Inc. | Coating solutions of trivalent chromium for coating zinc surfaces |
| US4298404A (en) * | 1979-09-06 | 1981-11-03 | Richardson Chemical Company | Chromium-free or low-chromium metal surface passivation |
| US4263059A (en) * | 1979-12-21 | 1981-04-21 | Rohco, Inc. | Coating solutions of trivalent chromium for coating zinc and cadmium surfaces |
| US4359347A (en) * | 1981-04-16 | 1982-11-16 | Occidental Chemical Corporation | Chromium-free passivate solution and process |
| US4349392A (en) * | 1981-05-20 | 1982-09-14 | Occidental Chemical Corporation | Trivalent chromium passivate solution and process |
| US4384902A (en) * | 1981-06-15 | 1983-05-24 | Occidental Chemical Corporation | Trivalent chromium passivate composition and process |
| US4367099A (en) * | 1981-06-15 | 1983-01-04 | Occidental Chemical Corporation | Trivalent chromium passivate process |
| CA1228000A (en) * | 1981-04-16 | 1987-10-13 | David E. Crotty | Chromium appearance passivate solution and process |
| US4539348A (en) * | 1982-12-16 | 1985-09-03 | Celanese Corporation | Water-swellable crosslinked polymeric microgel particles and aqueous dispersions of organic film-forming resins containing the same |
| US4578122A (en) * | 1984-11-14 | 1986-03-25 | Omi International Corporation | Non-peroxide trivalent chromium passivate composition and process |
| NO168953C (en) * | 1986-08-27 | 1992-04-22 | Elektro Brite Gmbh | ACID CHROME PASSIVATOR FOR ZINC OR CADMIUM SURFACES |
-
1988
- 1988-04-12 DE DE3812076A patent/DE3812076A1/en active Granted
-
1989
- 1989-04-10 DD DD89327449A patent/DD280557A5/en not_active IP Right Cessation
- 1989-04-10 YU YU00716/89A patent/YU71689A/en unknown
- 1989-04-11 DE DE89106447T patent/DE58906227D1/en not_active Expired - Fee Related
- 1989-04-11 AT AT89106447T patent/ATE97700T1/en not_active IP Right Cessation
- 1989-04-11 EP EP89106447A patent/EP0337411B1/en not_active Expired - Lifetime
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6946201B2 (en) | 1996-04-19 | 2005-09-20 | Surtec International Gmbh | Chromium (VI)-free conversion layer and method for producing it |
| CN102912337A (en) * | 2012-10-30 | 2013-02-06 | 四川华丰企业集团有限公司 | Process for producing green passivation coating of cadmium-plated workpiece |
| CN102912337B (en) * | 2012-10-30 | 2015-01-21 | 四川华丰企业集团有限公司 | Process for producing green passivation coating of cadmium-plated workpiece |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE97700T1 (en) | 1993-12-15 |
| DE3812076C2 (en) | 1990-09-13 |
| DE3812076A1 (en) | 1989-10-26 |
| EP0337411A3 (en) | 1990-05-09 |
| YU71689A (en) | 1990-12-31 |
| EP0337411A2 (en) | 1989-10-18 |
| DD280557A5 (en) | 1990-07-11 |
| DE58906227D1 (en) | 1994-01-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0337411B1 (en) | Process for preparing an acidic passivating bath for zinc, zinc alloys an cadmium surfaces, containing chromium III and fluoride | |
| DE3213384C2 (en) | ||
| DE3004927C2 (en) | ||
| DE977586C (en) | Process for the production of coatings on aluminum and its alloys | |
| DE60213124T2 (en) | AFTER-TREATMENT FOR METAL-COATED SUBSTRATE | |
| DE2715292C2 (en) | ||
| DE69514107T2 (en) | IMPROVED NON-CHROMED OXIDE COATING FOR ALUMINUM SUBSTRATES | |
| DE2904402A1 (en) | PHOSPHATING AGENTS | |
| DE3038699A1 (en) | AQUEOUS ACID CHROMATE COATING SOLUTION, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE FOR COATING ZINC, ZINC ALLOY AND CADIMIUM SURFACES | |
| EP0454211B1 (en) | Process for applying phosphate coatings on metal surfaces | |
| EP0187917A1 (en) | Process for improving the protection against corrosion of resin layers autophoretically deposited on metal surfaces | |
| EP0328908B1 (en) | Process for applying conversion coatings | |
| EP0183161A2 (en) | Process for improving the corrosion protection of resin layers autophoretically deposited on metal surfaces | |
| DE4214954A1 (en) | METHOD OF SEALING CHROMAT CONVERSION OVERLAYS ON GALVANICALLY SEPARATED ZINC | |
| DE974713C (en) | Process for the production of coatings on metals | |
| DE69326021T2 (en) | IN ESSENTIAL NICKEL-FREE PHOSPHATE CONVERSION COATING COMPOSITION AND METHOD | |
| DE3780078T2 (en) | CORROSION-RESISTANT COATING. | |
| DE4135524A1 (en) | Chrome plating of zinc@, cadmium@ and their alloys - using aq. soln. of chromium (III) oxalate complex, at acidic pH to form blue corrosion-resistant coating | |
| DE1152591B (en) | Process for the production of coatings on zinc and zinc alloys and concentrate for the implementation of the process | |
| WO2003054249A1 (en) | Black passivation method | |
| DE10305450A1 (en) | Material mixture for blackening passivating drums and frames comprises an aqueous acidic solution containing chromium (III) ions | |
| DE102011013319B4 (en) | Composition and application solution for passivating zinc and its alloys | |
| DE1198171B (en) | Aqueous acidic solutions and processes for the production of chemical coatings on aluminum and its alloys | |
| DE8916143U1 (en) | Acidic chromium (III) -containing and fluoride-containing passivation bath concentrate for surfaces made of zinc, zinc alloys and cadmium | |
| DE584411C (en) | Process for the production of corrosion-resistant coatings on iron and steel |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE CH DE ES FR GB GR IT LI LU NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE ES FR GB GR IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19900518 |
|
| 17Q | First examination report despatched |
Effective date: 19920218 |
|
| RAP3 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: UNILEVER PLC Owner name: UNILEVER N.V. |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: UNILEVER N.V. Owner name: UNILEVER PLC |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: SURTEC GMBH |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE ES FR GB GR IT LI LU NL SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 19931124 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19931124 Ref country code: ES Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19931124 |
|
| REF | Corresponds to: |
Ref document number: 97700 Country of ref document: AT Date of ref document: 19931215 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 58906227 Country of ref document: DE Date of ref document: 19940105 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19940302 |
|
| ET | Fr: translation filed | ||
| EPTA | Lu: last paid annual fee | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 89106447.9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19950401 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19950410 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19950419 Year of fee payment: 7 Ref country code: FR Payment date: 19950419 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19950424 Year of fee payment: 7 Ref country code: SE Payment date: 19950424 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19950428 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19950430 Year of fee payment: 7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19950612 Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19960411 Ref country code: AT Effective date: 19960411 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19960411 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19960412 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19960430 Ref country code: CH Effective date: 19960430 Ref country code: LI Effective date: 19960430 |
|
| BERE | Be: lapsed |
Owner name: SURTEC G.M.B.H. Effective date: 19960430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19961101 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19960411 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19961227 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19970101 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 19961101 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 89106447.9 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |