EP0245970A1 - Dithiocarbamate compostions having increased rain fastness - Google Patents
Dithiocarbamate compostions having increased rain fastness Download PDFInfo
- Publication number
- EP0245970A1 EP0245970A1 EP87303373A EP87303373A EP0245970A1 EP 0245970 A1 EP0245970 A1 EP 0245970A1 EP 87303373 A EP87303373 A EP 87303373A EP 87303373 A EP87303373 A EP 87303373A EP 0245970 A1 EP0245970 A1 EP 0245970A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- molecular weight
- poly
- polymer
- dithiocarbamate
- cellulose
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000012990 dithiocarbamate Substances 0.000 title claims abstract description 57
- DKVNPHBNOWQYFE-UHFFFAOYSA-N carbamodithioic acid Chemical compound NC(S)=S DKVNPHBNOWQYFE-UHFFFAOYSA-N 0.000 title claims abstract description 44
- 239000000203 mixture Substances 0.000 claims abstract description 49
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims abstract description 13
- 229920002451 polyvinyl alcohol Polymers 0.000 claims abstract description 13
- 229920013820 alkyl cellulose Polymers 0.000 claims abstract description 11
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims abstract description 11
- 229920002401 polyacrylamide Polymers 0.000 claims abstract description 11
- 229920002432 poly(vinyl methyl ether) polymer Polymers 0.000 claims abstract description 10
- 239000004372 Polyvinyl alcohol Substances 0.000 claims abstract description 5
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims abstract description 4
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims abstract description 4
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims abstract description 4
- -1 poly(ethylene oxide) Polymers 0.000 claims description 66
- 229920000642 polymer Polymers 0.000 claims description 52
- 239000005802 Mancozeb Substances 0.000 claims description 21
- 230000000855 fungicidal effect Effects 0.000 claims description 17
- 241000233866 Fungi Species 0.000 claims description 13
- 238000000034 method Methods 0.000 claims description 12
- 239000002689 soil Substances 0.000 claims description 9
- 125000006531 (C2-C5) alkyl group Chemical group 0.000 claims description 8
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 claims description 7
- 239000004354 Hydroxyethyl cellulose Substances 0.000 claims description 7
- 239000004480 active ingredient Substances 0.000 claims description 7
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 claims description 7
- YKSNLCVSTHTHJA-UHFFFAOYSA-L maneb Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S YKSNLCVSTHTHJA-UHFFFAOYSA-L 0.000 claims description 7
- 229920000940 maneb Polymers 0.000 claims description 6
- 230000003032 phytopathogenic effect Effects 0.000 claims description 6
- 239000000428 dust Substances 0.000 claims description 5
- 230000000694 effects Effects 0.000 claims description 5
- 238000007127 saponification reaction Methods 0.000 claims description 5
- 230000002411 adverse Effects 0.000 claims description 4
- 239000008187 granular material Substances 0.000 claims description 4
- 239000007788 liquid Substances 0.000 claims description 4
- RPZANUYHRMRTTE-UHFFFAOYSA-N 2,3,4-trimethoxy-6-(methoxymethyl)-5-[3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxyoxane;1-[[3,4,5-tris(2-hydroxybutoxy)-6-[4,5,6-tris(2-hydroxybutoxy)-2-(2-hydroxybutoxymethyl)oxan-3-yl]oxyoxan-2-yl]methoxy]butan-2-ol Chemical compound COC1C(OC)C(OC)C(COC)OC1OC1C(OC)C(OC)C(OC)OC1COC.CCC(O)COC1C(OCC(O)CC)C(OCC(O)CC)C(COCC(O)CC)OC1OC1C(OCC(O)CC)C(OCC(O)CC)C(OCC(O)CC)OC1COCC(O)CC RPZANUYHRMRTTE-UHFFFAOYSA-N 0.000 claims description 3
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 claims description 3
- 230000012010 growth Effects 0.000 claims description 3
- 239000001863 hydroxypropyl cellulose Substances 0.000 claims description 3
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 claims description 3
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 claims description 3
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 3
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 claims description 3
- 150000001875 compounds Chemical class 0.000 claims description 2
- 244000038559 crop plants Species 0.000 claims description 2
- 238000003958 fumigation Methods 0.000 claims description 2
- 239000001963 growth medium Substances 0.000 claims description 2
- QRNATDQRFAUDKF-UHFFFAOYSA-N 2-carbamothioylsulfanylethyl carbamodithioate Chemical compound NC(=S)SCCSC(N)=S QRNATDQRFAUDKF-UHFFFAOYSA-N 0.000 claims 4
- 238000010297 mechanical methods and process Methods 0.000 claims 1
- 230000005226 mechanical processes and functions Effects 0.000 claims 1
- 150000004659 dithiocarbamates Chemical class 0.000 abstract description 14
- 238000009472 formulation Methods 0.000 abstract description 7
- 229920003169 water-soluble polymer Polymers 0.000 abstract description 2
- 239000000417 fungicide Substances 0.000 description 18
- 241000196324 Embryophyta Species 0.000 description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 239000004563 wettable powder Substances 0.000 description 9
- 239000007921 spray Substances 0.000 description 7
- 239000000575 pesticide Substances 0.000 description 6
- 230000009969 flowable effect Effects 0.000 description 4
- 229920000831 ionic polymer Polymers 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 239000001768 carboxy methyl cellulose Substances 0.000 description 3
- 239000012141 concentrate Substances 0.000 description 3
- WHDGWKAJBYRJJL-UHFFFAOYSA-K ferbam Chemical compound [Fe+3].CN(C)C([S-])=S.CN(C)C([S-])=S.CN(C)C([S-])=S WHDGWKAJBYRJJL-UHFFFAOYSA-K 0.000 description 3
- AFCCDDWKHLHPDF-UHFFFAOYSA-M metam-sodium Chemical compound [Na+].CNC([S-])=S AFCCDDWKHLHPDF-UHFFFAOYSA-M 0.000 description 3
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 3
- KKMLIVYBGSAJPM-UHFFFAOYSA-L propineb Chemical compound [Zn+2].[S-]C(=S)NC(C)CNC([S-])=S KKMLIVYBGSAJPM-UHFFFAOYSA-L 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- KUAZQDVKQLNFPE-UHFFFAOYSA-N thiram Chemical compound CN(C)C(=S)SSC(=S)N(C)C KUAZQDVKQLNFPE-UHFFFAOYSA-N 0.000 description 3
- 229960002447 thiram Drugs 0.000 description 3
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- 240000008067 Cucumis sativus Species 0.000 description 2
- 235000009849 Cucumis sativus Nutrition 0.000 description 2
- 239000005644 Dazomet Substances 0.000 description 2
- 239000005823 Propineb Substances 0.000 description 2
- 239000005843 Thiram Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 2
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- QAYICIQNSGETAS-UHFFFAOYSA-N dazomet Chemical compound CN1CSC(=S)N(C)C1 QAYICIQNSGETAS-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 239000002917 insecticide Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- GEPDYQSQVLXLEU-AATRIKPKSA-N methyl (e)-3-dimethoxyphosphoryloxybut-2-enoate Chemical compound COC(=O)\C=C(/C)OP(=O)(OC)OC GEPDYQSQVLXLEU-AATRIKPKSA-N 0.000 description 2
- 229920000058 polyacrylate Chemical class 0.000 description 2
- ZEVCJZRMCOYJSP-UHFFFAOYSA-N sodium;2-(dithiocarboxyamino)ethylcarbamodithioic acid Chemical compound [Na+].SC(=S)NCCNC(S)=S ZEVCJZRMCOYJSP-UHFFFAOYSA-N 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- DUBNHZYBDBBJHD-UHFFFAOYSA-L ziram Chemical compound [Zn+2].CN(C)C([S-])=S.CN(C)C([S-])=S DUBNHZYBDBBJHD-UHFFFAOYSA-L 0.000 description 2
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical class O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical group CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 1
- 241000218631 Coniferophyta Species 0.000 description 1
- ZAKOWWREFLAJOT-CEFNRUSXSA-N D-alpha-tocopherylacetate Chemical compound CC(=O)OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C ZAKOWWREFLAJOT-CEFNRUSXSA-N 0.000 description 1
- 229920001479 Hydroxyethyl methyl cellulose Polymers 0.000 description 1
- 229920001732 Lignosulfonate Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 241000235004 Saccharomycopsis fibuligera Species 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 1
- PTFCDOFLOPIGGS-UHFFFAOYSA-N Zinc dication Chemical compound [Zn+2] PTFCDOFLOPIGGS-UHFFFAOYSA-N 0.000 description 1
- 239000005870 Ziram Substances 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical class [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 229920006322 acrylamide copolymer Polymers 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000011717 all-trans-retinol Substances 0.000 description 1
- 235000019169 all-trans-retinol Nutrition 0.000 description 1
- 229920006318 anionic polymer Polymers 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000003899 bactericide agent Substances 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- AWYFNIZYMPNGAI-UHFFFAOYSA-N ethylenebis(dithiocarbamic acid) Chemical class SC(=S)NCCNC(S)=S AWYFNIZYMPNGAI-UHFFFAOYSA-N 0.000 description 1
- 244000037666 field crops Species 0.000 description 1
- 238000005189 flocculation Methods 0.000 description 1
- 230000016615 flocculation Effects 0.000 description 1
- 235000013312 flour Nutrition 0.000 description 1
- 229920006158 high molecular weight polymer Polymers 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 229910052806 inorganic carbonate Inorganic materials 0.000 description 1
- 229910052909 inorganic silicate Inorganic materials 0.000 description 1
- 230000000749 insecticidal effect Effects 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 235000014571 nuts Nutrition 0.000 description 1
- 239000003090 pesticide formulation Substances 0.000 description 1
- 239000005648 plant growth regulator Substances 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 239000006283 soil fumigant Substances 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- AMHNZOICSMBGDH-UHFFFAOYSA-L zineb Chemical compound [Zn+2].[S-]C(=S)NCCNC([S-])=S AMHNZOICSMBGDH-UHFFFAOYSA-L 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/24—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests containing ingredients to enhance the sticking of the active ingredients
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/08—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests containing solids as carriers or diluents
- A01N25/10—Macromolecular compounds
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/12—Powders or granules
- A01N25/14—Powders or granules wettable
Definitions
- the present invention is concerned with dithiocarbamate compositions of enhanced rain fastness. These compositions are useful in the control of phytopathogenic fungi.
- Dithiocarbamates and derivatives thereof are a class of fungicides useful in the control of phytopathogenic fungi.
- Examples of dithiocarbamate fungicides include mancozeb, maneb, zineb, propineb, ferbam, ziram, metham, thiram and dazomet.
- Dithiocarbamates like most fungicides and other pesticides, tend to be washed off plants by rain.
- Dithiocarbamate salts have been combined with a larger amount (1:1 to 1:30) of a water-insoluble vinyl acetate polymer to increase the resistance of the dithiocarbamate to removal by weathering (rain).
- a water-insoluble vinyl acetate polymer to increase the resistance of the dithiocarbamate to removal by weathering (rain).
- Central Patents Index Basic Abstracts Journal, C03-54361Y/31 Derwent Publications Ltd., London (1977).
- the polyvinyl acetate forms a film, it is not conducive for formulating the dithiocarbamate in a dry or powder form.
- due to the polymer's insolubility the equipment used to apply such pesticides cannot be readily cleaned with water.
- Chemical Abstracts: 72, 11637a (1970) discloses the addition of polyvinyl alcohol to fungicides to increase the affinity of the fungicide to foliage.
- the polyvinyl alcohols have a molecular weight of about 150,000 to about 500,000 and a saponification index of 30 or less which are only readily soluble in water having a temperature of above about 100°F.
- Polyoxyalkylenepolyols having a molecular weight of 200-25,000 increase the adhesion of finely granulated pesticides. These are acting as surfactants and in the presence of rain the pesticide is readily washed off the plant, Central Patents Index, Basic Abstracts Journal, C03-38356U-AC, Derwent Publications L td., London (1973). It has also been recognized that the residual insecticidal activity (in the absence of rain) of phosdrin may be increased by the addition of polyethylene, hydroxyethyl cellulose and methyl cellulose polymers, but that conventional stickers and/or spreaders do not enhance the effectiveness of phosdrin's residues, Aller, et al., J. Econ. Ent., 54 (3), pp 508-510 (1961) .
- non-ionic polymers are useful for improving the rain fastness of certain form of dithiocarbamates.
- the nonionic polymers used in the dithiocarbamate compositions of the present invention are not prone to give the flocculation problems as a result of high molecular weight ionic polymers interacting with the divalent cations of dithiocarbamates.
- the high molecular weight polymers utilized in the present invention have no, or minimal, surfactant properties so they are less apt to be washed off a plant by rain.
- the polymers used in the present invention dissolve readily in water at the ambient temperatures at which fungicides are applied to plants, seeds or plant habitats, e.g., fields.
- compositions comprising dry form dithiocarbamate and water-soluble, nonionic polymer which comprises hydroxy(C 2 -C 5 )alkyl cellulose, poly(ethylene oxide), poly(vinyl alcohol) having a saponification index of from about 45 to about 150, poly(vinyl pyrrolidone), poly(acrylamide) and/or poly(vinylmethylether), in an amount sufficient to increase the rain fastness of the dithiocarbamate.
- Rain fastness relates to the ability of the dithiocarbamate to remain on foliage, seed or soil after rain or a heavy dew.
- the use of the dithiocarbamate composition of the invention as an agricultural fungicide, wherein dithiocarbamate is less readily removed from the plant, seed or soil by heavy dew or rain, may give a longer lasting activity of the dithiocarbamate under adverse weather conditions such as rain or wind, may result in a lower rate of use under adverse weather conditions and/or may allow for a longer period of time between applications of the fungicide.
- the dithiocarbamates which are used in this invention comprise a chemical class of fungicides, including their derivatives, which have activity against phytopathogenic fungi.
- dithiocarbamates include maneb (manganese ethylenebis- dithiocarbamate), mancozeb (the zinc ion coordination product of maneb), zineb (zinc ethylenebisdithio- carbamate), propineb (zinc propylenebisdithiocarbamate), metham (sodium methyldithiocarbamate), ferbam (ferric dimethyldithiocarbamate), thiram (tetra- methylthiuram disulfide), ziram (zinc dimethyldithiocarbamate) and dazomet (3,5-dimethyl-l,3,5-2H-tetrahydrothiadiazine-2-thione) .
- Preferred dithiocarbamates are ethylenebisdithiocarbamates and their metal salts and/or metal coordination products, more preferred dithiocarbamates are maneb and mancozeb; and mancozeb is the most preferred dithiocarbamate.
- dry form any dry form of dithiocarbamate including dry powder, wettable powder, dry flowable, dust, granules, dispersable granules and a diluted dry form includes a dry form which has been made into an aqueous form in preparation for application of the dithiocarbamate. Dry form does not include liquid flowable emulsions, liquid flowable emulsion concentrates or liquid suspension concentrates.
- the polymer used in the composition of the present invention is nonionic, water-soluble and has a molecular weight sufficient to increase the rain fastness of the dithiocarbamate.
- Preferred polymers are hydroxy(C 2 -C 5 )alkyl cellulose having a molecular weight of at least about 80,000, more preferably from about 600,000 to about 1,000,000; poly(ethyleneoxide) (pEO) having a molecular weight of at least about 80,000, more preferably from about 100,000 to about 500,000; poly(vinyl alcohol) having a molecular weight of at least about 80,000 and a saponification index of from about 45 to about 150, more preferably a molecular weight from about 95,000 to about 300,000; poly(vinyl pyrrolidone) having a molecular weight of at least about 40,000, more preferably from about 300,000 to about 600,000; poly(acrylamide) having a molecular weight of at least about 500,000, more preferably from about 700,000 to about 1,500,000;
- Preferred polymers are hydroxy(C 2 -C 5 )-alkyl cellulose, particularly hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose and hydroxybutylmethyl cellulose; poly(ethyleneoxide); poly(vinyl alcohol); and poly(vinyl pyrrolidone) all as previously described.
- Most preferred polymers are poly(ethylene oxide) having a molecular weight from about 100,000 to about 500,000, and most particularly, hydroxyethyl cellulose having a molecular weight of from about 600,000 to about 1,000,000.
- the upper limit of the molecular weight of the polymer is not critical and will be governed primarily by its viscoelastic properties it imparts to the aqueous mixture of the polymer and dithiocarbamate. Generally, the molecular weight of the polymer will not exceed about 1 million or about 1.5 million for a polyacrylamide polymer. Molecular weights, unless otherwise specified are viscosity average molecular weights.
- the amount of polymer added to enhance rain fastness will be from about 0.1 to about 5 weight percent based on the weight of dithiocarbamate, preferably from about 0.2 to about 1 weight percent by weight of the dithiocarbamate. If the molecular weight of the polymer is high enough, then less than about 0.1% may be used. At greater than about 5% weight percent of the polymer, the solution of the dithiocarbamate and polymer may be too viscous to allow for its uniform application. Additionally, as the amount of polymer increases and the rain fastness increases, then the ability of the dithiocarbamate to redistribute on the plant surface in the presence of water decreases.
- the polymer can be blended directly with the dry form of the dithiocarbamate, for example by the use of mixing equipment.
- the polymer can be added to the dry dithiocarbamate after the dithiocarbamate has been formulated with water, for example, added to a tank mix of the dithiocarbamate.
- the polymer may be spray dried with sodium sulfate or sodium benzoate (for example a ratio of 2:1 based on weight) aqueous solution, and then the spray-dried material blended with the dry form of the dithiocarbamate or added to a tank mix with the dithiocarbamate.
- the last technique is particularly useful if the polymer is soft, i.e., has a low melting point. Additionally, the spray-dried polymer may dissolve more readily in a tank mix of the dithiocarbamate.
- a second polymer for example, a lower molecular weight water-soluble polymer having a molecular weight of from about 10,000 to about 150,000, which is either an ionic or, preferably, a nonionic polymer.
- suitable ionic polymers include sodium carboxymethyl cellulose, polyacrylate salts, salts of polyacrylate copolymers, lignosulfonates and alginate salts.
- nonionic polymers examples include hydroxy(C 1 -C 5 )alkyl cellulose, polyethylene oxide, polyvinyl alcohol, polyvinyl pyrrolidone, polyacrylamide and polyvinylmethylether.
- the addition of the lower molecular weight polymer improves the redistribution properties of the dithiocarbamate; that is the ability of the dithiocarbamate to respread itself on a leaf surface after a dew or light rain.
- a balance of rain fastness and redistribution properties may be achieved from a mixture of about 0.5 weight percent of a 900,000 molecular weight hydroxyethyl cellulose and about 0.25 weight percent of a 125,000 molecular weight hydroxyethyl cellulose combined with a wettable powder form of a dithiocarbamate, e.g., mancozeb. Since dithiocarbamates are contact fungicides, the ability of the dithiocarbamate to redistribute itself is desirable as it will allow the fungicide to move to areas of the leaf surface not previously covered by the fungicide but which have been exposed to the fungus.
- a dithiocarbamate e.g., mancozeb
- compositions of the present invention are useful as agricultural fungicides and as such can be applied to various loci such as the seed, the soil or the foliage.
- these compositions can be used in the technical or pure form as prepared, as solutions or as formulations.
- the compositions are usually taken up in a carrier or are formulated so as to render them suitable for subsequent dissemination as fungicides.
- these compositions can be formulated as wettable powders, dusts, dry flowables, or granular formulations. In such formulations, the compositions can be extended with a solid carrier and, when desired, suitable surfactants are incorporated.
- adjuvants such as wetting agents, spreading agents or dispersing agents, in accordance with agricultural practices.
- adjuvants commonly used in the art can be found in the John W. McCutcheon, Inc. publication "Detergents and Emulsifiers, Annual".
- the concentration of the dithiocarbamate in the formulations for use may vary from about 1% to about 99% with a preferred range being from about 5% to about 90%.
- Wettable powders suitable for spraying can be prepared by admixing the composition of the present invention with a finely divided solid, such as clays, inorganic silicates and carbonates, and silicas and incorporating wetting agents, and/or dispersing agents in such mixtures.
- concentration of active ingredients in such formulations is usually in the range of from about 20% to about 98%, preferably from about 40% to about 85%.
- a typical wettable powder is made by blending 91 parts of mancozeb (about 88% pure), 6.5 parts of a clay or talc and 0.5 parts of a polymer described herein.
- Dusts are prepared by mixing the composition of the present invention with finely divided inert solids which can be organic or inorganic in nature.
- Materials useful for this purpose include botanical flours, silicas, silicates, carbonates and clays.
- One convenient method of preparing a dust is to dilute a wettable powder with a finely divided carrier. Dust concentrates containing from about 20% to about 80% of the active ingredient are commonly made and are subsequently diluted to from about 1% to about 10% use concentration.
- compositions of the present invention can be applied as fungicidal sprays by methods commonly employed, such as conventional high-gallonage hydraulic sprays, low-gallonage sprays, air-blast spray, aerial sprays and dusts.
- Dithiocarbamates have been used for years in a variety of applications, for example, vegetables, fruits and nuts, field crops, ornamentals, turf, conifers, seed profectant and soil fumigant. Consequently, the dilution and rate of application can be readily determined by one in the art depending upon the type of equipment used, the desired method, timing and frequency of applications, plants, seeds or soil to be treated and diseases to be controlled.
- the dithiocarbamates of the present invention will be applied in an amount of from about 0.25 to 28 kg/ha (0.25 to 25 lb/acre) of active ingredient when applied foliarly, from about 1.1 to 3.4 kg/ha (1 to 3 lb/acre) of active ingredient when applied to soil and from about 0.626 to 10 g of active ingredient per kg of seed (1 to 16 oz/100 lb).
- compositions of the present invention can be applied sequentially or in combination with other fungicides.
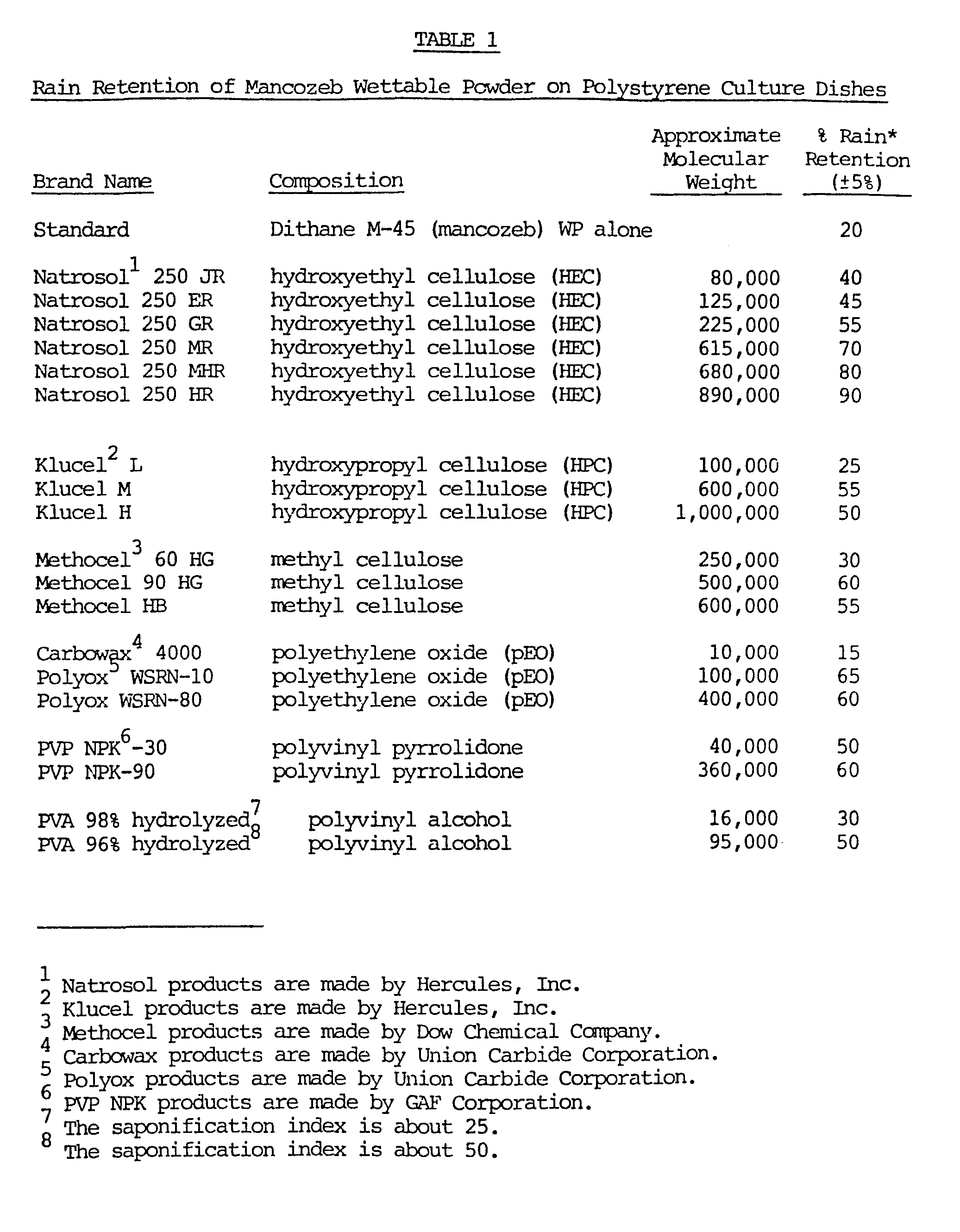
- the polymer-mancozeb compositions were prepared by first dissolving the polymer in water (room temperature) then adding the mancozeb wettable powder to the polymer solution. Several tests were conducted and each standard and polymer-mancozeb compositions of each test were run with from five to ten replicates. The replicates of each standard and composition were averaged and the results from each test were linearly normalized. (The value of the standard of each test was adjusted to a value of 20% and the results of the polymer-mancozeb compositions of the same test were adjusted by the same amount as the standard was). The normalized results are reported in Table 1.
- the effect of adding polymers to mancozeb wettable powder was also measured by spraying cucumbers with 4.48 kg pounds of the mancozeb per hectare (4 lb/acre) of cucumbers.
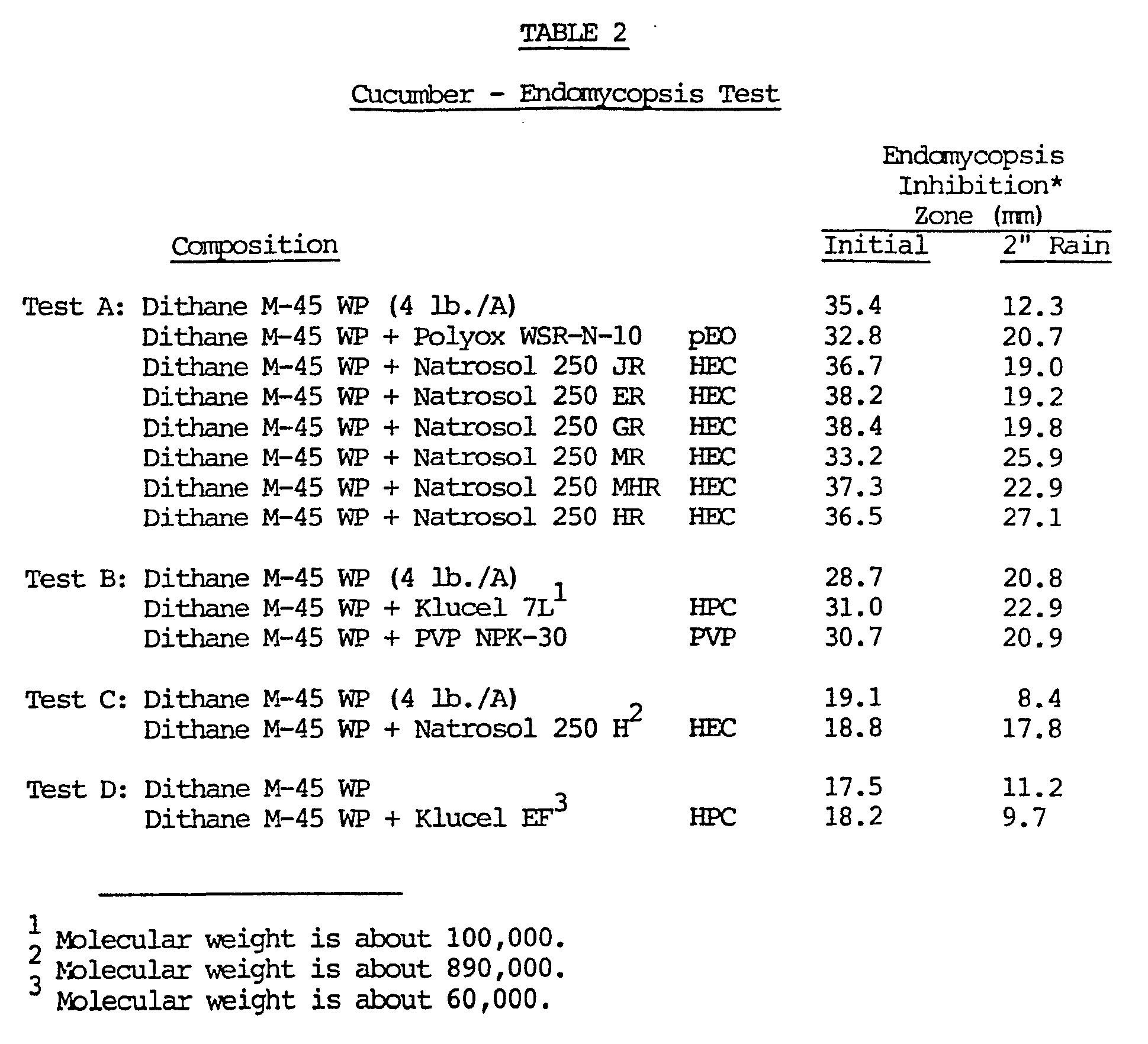
- the initial activity of the mancozeb and its activity after 5.1 cm (2 inches) of water were sprayed on the plants was determined by placing leaf plugs from the sprayed plants on growth mediums of Endomycopsis yeast, specifically Saccharomycopsis fibuligera. The zone of inhibition around each plug was measured. The results are listed in Table 2 grouped by individual tests. The larger the inhibition zone, the greater the fungicidal activity.
- the polymers were each added in a weight amount equal to 1% of the weight of the mancozeb by simply physically mixing the dry polymer with the dry mancozeb wettable powder then diluting with the appropriate amount of water (room temperature) .
- a process of the invention for improving the commercial value and/or profitability of vendible crops from plants whose growth is affected or likely to be affected by fungi comprises (1) charging to a container, fumigation device or mechanical dissemination device a fungicidal composition of the invention as hereinbefore described (2) using the container, fumigator or mechanical dissemination device to apply the fungicidal composition in the form of granules, dust, vapour or diluted dry form to growing plants or to the seeds thereof or to a growth medium where the plants are growing or are to be grown, or to the fungus itself, (3) controlling the dose of the active ingredient during this application step so that the rate of application of active fungicidal compound is sufficient to combat the fungi but is insufficient to cause an unacceptably adverse effect on the crop plants growing or to be grown in the treated area.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Health & Medical Sciences (AREA)
- Toxicology (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Agronomy & Crop Science (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
The rain fastness of dithiocarbamates in dry form is enhanced by formulation with nonionic water-soluble polymer comprising hydroxy(C2-Cs) alkyl cellulose, polyethylene oxide, polyvinyl alcohol, polyvinyl pyrrolidone, polyacrylamide and/or polyvinylmethylether in an amount of from about 0.1 to about 5 weight percent based on the weight of the dithiocarbamate.
Description
- The present invention is concerned with dithiocarbamate compositions of enhanced rain fastness. These compositions are useful in the control of phytopathogenic fungi.
- Dithiocarbamates and derivatives thereof are a class of fungicides useful in the control of phytopathogenic fungi. Examples of dithiocarbamate fungicides include mancozeb, maneb, zineb, propineb, ferbam, ziram, metham, thiram and dazomet. Dithiocarbamates, like most fungicides and other pesticides, tend to be washed off plants by rain.
- The literature contains several suggestions for use of polymers with pesticides, for example, to increase the adhesion of the pesticide or to increase its resistance to rain. Dithiocarbamate salts have been combined with a larger amount (1:1 to 1:30) of a water-insoluble vinyl acetate polymer to increase the resistance of the dithiocarbamate to removal by weathering (rain). Central Patents Index, Basic Abstracts Journal, C03-54361Y/31 Derwent Publications Ltd., London (1977). However, because the polyvinyl acetate forms a film, it is not conducive for formulating the dithiocarbamate in a dry or powder form. Moreover, due to the polymer's insolubility, the equipment used to apply such pesticides cannot be readily cleaned with water. Chemical Abstracts: 72, 11637a (1970) discloses the addition of polyvinyl alcohol to fungicides to increase the affinity of the fungicide to foliage. The polyvinyl alcohols have a molecular weight of about 150,000 to about 500,000 and a saponification index of 30 or less which are only readily soluble in water having a temperature of above about 100°F.
- The use of ionic polymers as additives has also been suggested. U.S. 2,236,545 suggests the use of carboxymethyl cellulose as a sticking agent for insecticides. Carboxymethyl cellulose has also been used as a binder in granular pesticide formulations which utilize mono-, di- and poly-saccarides as stickers, Chemical Abstracts: 87, 147082h (1977). Ionic water-soluble acrylamide copolymer resins have been suggested for enhancing the initial activity of bactericides, fungicides, insecticides and plant growth regulators, Chemical Abstracts: 80 141786z (1974).
- Polyoxyalkylenepolyols having a molecular weight of 200-25,000 increase the adhesion of finely granulated pesticides. These are acting as surfactants and in the presence of rain the pesticide is readily washed off the plant, Central Patents Index, Basic Abstracts Journal, C03-38356U-AC, Derwent Publications Ltd., London (1973). It has also been recognized that the residual insecticidal activity (in the absence of rain) of phosdrin may be increased by the addition of polyethylene, hydroxyethyl cellulose and methyl cellulose polymers, but that conventional stickers and/or spreaders do not enhance the effectiveness of phosdrin's residues, Aller, et al., J. Econ. Ent., 54 (3), pp 508-510 (1961) .
- We have now surprisingly found that certain non-ionic polymers are useful for improving the rain fastness of certain form of dithiocarbamates. Unlike the prior art anionic polymers, the nonionic polymers used in the dithiocarbamate compositions of the present invention are not prone to give the flocculation problems as a result of high molecular weight ionic polymers interacting with the divalent cations of dithiocarbamates. The high molecular weight polymers utilized in the present invention have no, or minimal, surfactant properties so they are less apt to be washed off a plant by rain. Moreover, the polymers used in the present invention dissolve readily in water at the ambient temperatures at which fungicides are applied to plants, seeds or plant habitats, e.g., fields.
- The present invention provides compositions comprising dry form dithiocarbamate and water-soluble, nonionic polymer which comprises hydroxy(C2-C5)alkyl cellulose, poly(ethylene oxide), poly(vinyl alcohol) having a saponification index of from about 45 to about 150, poly(vinyl pyrrolidone), poly(acrylamide) and/or poly(vinylmethylether), in an amount sufficient to increase the rain fastness of the dithiocarbamate.
- Rain fastness relates to the ability of the dithiocarbamate to remain on foliage, seed or soil after rain or a heavy dew. The use of the dithiocarbamate composition of the invention as an agricultural fungicide, wherein dithiocarbamate is less readily removed from the plant, seed or soil by heavy dew or rain, may give a longer lasting activity of the dithiocarbamate under adverse weather conditions such as rain or wind, may result in a lower rate of use under adverse weather conditions and/or may allow for a longer period of time between applications of the fungicide.
- The dithiocarbamates which are used in this invention comprise a chemical class of fungicides, including their derivatives, which have activity against phytopathogenic fungi. Examples of such dithiocarbamates include maneb (manganese ethylenebis- dithiocarbamate), mancozeb (the zinc ion coordination product of maneb), zineb (zinc ethylenebisdithio- carbamate), propineb (zinc propylenebisdithiocarbamate), metham (sodium methyldithiocarbamate), ferbam (ferric dimethyldithiocarbamate), thiram (tetra- methylthiuram disulfide), ziram (zinc dimethyldithiocarbamate) and dazomet (3,5-dimethyl-l,3,5-2H-tetrahydrothiadiazine-2-thione) . Preferred dithiocarbamates are ethylenebisdithiocarbamates and their metal salts and/or metal coordination products, more preferred dithiocarbamates are maneb and mancozeb; and mancozeb is the most preferred dithiocarbamate.
- To make the compositions, the polymers are added to dithiocarbamate in dry form which may however have been diluted for use. By "dry form" is meant any dry form of dithiocarbamate including dry powder, wettable powder, dry flowable, dust, granules, dispersable granules and a diluted dry form includes a dry form which has been made into an aqueous form in preparation for application of the dithiocarbamate. Dry form does not include liquid flowable emulsions, liquid flowable emulsion concentrates or liquid suspension concentrates.
- The polymer used in the composition of the present invention is nonionic, water-soluble and has a molecular weight sufficient to increase the rain fastness of the dithiocarbamate. Preferred polymers are hydroxy(C2-C5)alkyl cellulose having a molecular weight of at least about 80,000, more preferably from about 600,000 to about 1,000,000; poly(ethyleneoxide) (pEO) having a molecular weight of at least about 80,000, more preferably from about 100,000 to about 500,000; poly(vinyl alcohol) having a molecular weight of at least about 80,000 and a saponification index of from about 45 to about 150, more preferably a molecular weight from about 95,000 to about 300,000; poly(vinyl pyrrolidone) having a molecular weight of at least about 40,000, more preferably from about 300,000 to about 600,000; poly(acrylamide) having a molecular weight of at least about 500,000, more preferably from about 700,000 to about 1,500,000; and poly(vinylmethylether) having a molecular weight of at least about 100,000, more preferably from about 200,000 to about 500,000. Preferred polymers are hydroxy(C2-C5)-alkyl cellulose, particularly hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose and hydroxybutylmethyl cellulose; poly(ethyleneoxide); poly(vinyl alcohol); and poly(vinyl pyrrolidone) all as previously described. Most preferred polymers are poly(ethylene oxide) having a molecular weight from about 100,000 to about 500,000, and most particularly, hydroxyethyl cellulose having a molecular weight of from about 600,000 to about 1,000,000.
- The upper limit of the molecular weight of the polymer is not critical and will be governed primarily by its viscoelastic properties it imparts to the aqueous mixture of the polymer and dithiocarbamate. Generally, the molecular weight of the polymer will not exceed about 1 million or about 1.5 million for a polyacrylamide polymer. Molecular weights, unless otherwise specified are viscosity average molecular weights.
- Generally, the amount of polymer added to enhance rain fastness will be from about 0.1 to about 5 weight percent based on the weight of dithiocarbamate, preferably from about 0.2 to about 1 weight percent by weight of the dithiocarbamate. If the molecular weight of the polymer is high enough, then less than about 0.1% may be used. At greater than about 5% weight percent of the polymer, the solution of the dithiocarbamate and polymer may be too viscous to allow for its uniform application. Additionally, as the amount of polymer increases and the rain fastness increases, then the ability of the dithiocarbamate to redistribute on the plant surface in the presence of water decreases.
- The polymer can be blended directly with the dry form of the dithiocarbamate, for example by the use of mixing equipment. The polymer can be added to the dry dithiocarbamate after the dithiocarbamate has been formulated with water, for example, added to a tank mix of the dithiocarbamate. The polymer may be spray dried with sodium sulfate or sodium benzoate (for example a ratio of 2:1 based on weight) aqueous solution, and then the spray-dried material blended with the dry form of the dithiocarbamate or added to a tank mix with the dithiocarbamate. The last technique is particularly useful if the polymer is soft, i.e., has a low melting point. Additionally, the spray-dried polymer may dissolve more readily in a tank mix of the dithiocarbamate.
- Generally, the higher the molecular weight of the polymer, the greater the adhesion of the dithiocarbamate has to the plant. Consequently, it may be desirable in some instances to add a second polymer, for example, a lower molecular weight water-soluble polymer having a molecular weight of from about 10,000 to about 150,000, which is either an ionic or, preferably, a nonionic polymer. Examples of suitable ionic polymers include sodium carboxymethyl cellulose, polyacrylate salts, salts of polyacrylate copolymers, lignosulfonates and alginate salts. Examples of suitable nonionic polymers include hydroxy(C1-C5)alkyl cellulose, polyethylene oxide, polyvinyl alcohol, polyvinyl pyrrolidone, polyacrylamide and polyvinylmethylether. The addition of the lower molecular weight polymer improves the redistribution properties of the dithiocarbamate; that is the ability of the dithiocarbamate to respread itself on a leaf surface after a dew or light rain. As an example, a balance of rain fastness and redistribution properties may be achieved from a mixture of about 0.5 weight percent of a 900,000 molecular weight hydroxyethyl cellulose and about 0.25 weight percent of a 125,000 molecular weight hydroxyethyl cellulose combined with a wettable powder form of a dithiocarbamate, e.g., mancozeb. Since dithiocarbamates are contact fungicides, the ability of the dithiocarbamate to redistribute itself is desirable as it will allow the fungicide to move to areas of the leaf surface not previously covered by the fungicide but which have been exposed to the fungus.
- The polymer-containing dithiocarbamate compositions of the present invention are useful as agricultural fungicides and as such can be applied to various loci such as the seed, the soil or the foliage. For such purposes these compositions can be used in the technical or pure form as prepared, as solutions or as formulations. The compositions are usually taken up in a carrier or are formulated so as to render them suitable for subsequent dissemination as fungicides. For example, these compositions can be formulated as wettable powders, dusts, dry flowables, or granular formulations. In such formulations, the compositions can be extended with a solid carrier and, when desired, suitable surfactants are incorporated.
- It may be desirable, particularly in the case of foliar spray formulations, to include adjuvants, such as wetting agents, spreading agents or dispersing agents, in accordance with agricultural practices. Such adjuvants commonly used in the art can be found in the John W. McCutcheon, Inc. publication "Detergents and Emulsifiers, Annual".
- The concentration of the dithiocarbamate in the formulations for use may vary from about 1% to about 99% with a preferred range being from about 5% to about 90%.
- Wettable powders suitable for spraying, can be prepared by admixing the composition of the present invention with a finely divided solid, such as clays, inorganic silicates and carbonates, and silicas and incorporating wetting agents, and/or dispersing agents in such mixtures. The concentration of active ingredients in such formulations is usually in the range of from about 20% to about 98%, preferably from about 40% to about 85%. A typical wettable powder is made by blending 91 parts of mancozeb (about 88% pure), 6.5 parts of a clay or talc and 0.5 parts of a polymer described herein.
- Dusts are prepared by mixing the composition of the present invention with finely divided inert solids which can be organic or inorganic in nature. Materials useful for this purpose include botanical flours, silicas, silicates, carbonates and clays. One convenient method of preparing a dust is to dilute a wettable powder with a finely divided carrier. Dust concentrates containing from about 20% to about 80% of the active ingredient are commonly made and are subsequently diluted to from about 1% to about 10% use concentration.
- The compositions of the present invention can be applied as fungicidal sprays by methods commonly employed, such as conventional high-gallonage hydraulic sprays, low-gallonage sprays, air-blast spray, aerial sprays and dusts. Dithiocarbamates have been used for years in a variety of applications, for example, vegetables, fruits and nuts, field crops, ornamentals, turf, conifers, seed profectant and soil fumigant. Consequently, the dilution and rate of application can be readily determined by one in the art depending upon the type of equipment used, the desired method, timing and frequency of applications, plants, seeds or soil to be treated and diseases to be controlled. Generally, however, the dithiocarbamates of the present invention will be applied in an amount of from about 0.25 to 28 kg/ha (0.25 to 25 lb/acre) of active ingredient when applied foliarly, from about 1.1 to 3.4 kg/ha (1 to 3 lb/acre) of active ingredient when applied to soil and from about 0.626 to 10 g of active ingredient per kg of seed (1 to 16 oz/100 lb).
- The compositions of the present invention can be applied sequentially or in combination with other fungicides.
- The following examples are illustrative of the invention.
- To test the rain fastness of various polymers an in vitro test was utilized. Polystyrene culture dishes were sprayed with the equivalent of 4.48 kg/ha; 234 1/ha of diluted product (41b/acre; 25 gals/acre) of Dithane M-45 WP fungicide (a wettable powder form of mancozeb sold by Rohm and Haas Company) plus 1% by weight of the polymer based on the weight of the mancozeb, allowing the dishes to dry, spraying (raining) 5-7.6 cm (2-3 inches) of water on them and then visually estimating the amount of mancozeb remaining on the dishes as compared to the amount on the dish prior to the rain. Standards of the mancozeb wettable powder were also run in the same manner. The polymer-mancozeb compositions were prepared by first dissolving the polymer in water (room temperature) then adding the mancozeb wettable powder to the polymer solution. Several tests were conducted and each standard and polymer-mancozeb compositions of each test were run with from five to ten replicates. The replicates of each standard and composition were averaged and the results from each test were linearly normalized. (The value of the standard of each test was adjusted to a value of 20% and the results of the polymer-mancozeb compositions of the same test were adjusted by the same amount as the standard was). The normalized results are reported in Table 1.
- The effect of adding polymers to mancozeb wettable powder (Dithane M-45 WP fungicide from Rohm and Haas Company) was also measured by spraying cucumbers with 4.48 kg pounds of the mancozeb per hectare (4 lb/acre) of cucumbers. The initial activity of the mancozeb and its activity after 5.1 cm (2 inches) of water were sprayed on the plants was determined by placing leaf plugs from the sprayed plants on growth mediums of Endomycopsis yeast, specifically Saccharomycopsis fibuligera. The zone of inhibition around each plug was measured. The results are listed in Table 2 grouped by individual tests. The larger the inhibition zone, the greater the fungicidal activity. The polymers were each added in a weight amount equal to 1% of the weight of the mancozeb by simply physically mixing the dry polymer with the dry mancozeb wettable powder then diluting with the appropriate amount of water (room temperature) .
- In its mechanical aspects therefore a process of the invention for improving the commercial value and/or profitability of vendible crops from plants whose growth is affected or likely to be affected by fungi comprises (1) charging to a container, fumigation device or mechanical dissemination device a fungicidal composition of the invention as hereinbefore described (2) using the container, fumigator or mechanical dissemination device to apply the fungicidal composition in the form of granules, dust, vapour or diluted dry form to growing plants or to the seeds thereof or to a growth medium where the plants are growing or are to be grown, or to the fungus itself, (3) controlling the dose of the active ingredient during this application step so that the rate of application of active fungicidal compound is sufficient to combat the fungi but is insufficient to cause an unacceptably adverse effect on the crop plants growing or to be grown in the treated area.
Claims (18)
1. A composition comprising dithiocarbamate in dry form, which may optionally have been diluted with liquid for use, and water-soluble nonionic polymer in an amount and having a molecular weight sufficient to increase the rain fastness of the dithiocarbamate when applied to foliage, seed or soil wherein the polymer comprises hydroxy(C2-C5)alkyl cellulose, polyethylene oxide, polyvinyl alcohol having a saponification index of from 45 to 150, polyvinyl pyrrolidone, polyacrylamide and/or polyvinylmethylether.
2. A composition according to claim 1 wherein the polymer: if hydroxy(C2-C5)alkyl cellulose or poly(ethylene oxide) or poly(vinylalcohol) has a molecular weight of at least about 80,000; if poly(vinyl pyrrolidone), has a molecular weight of at least about 40,000; if poly(acrylamide), has a molecular weight of at least 500,000; and if poly(vinylmethylether) has a molecular weight of at least about 100,000.
3. A composition according to claim 2 containing the polymer in an amount of 0.1 to 5.0 weight percent based on the weight of the dithiocarbamate.
4. A composition according to any preceding claim wherein the polymer: if hydroxy(C2-C5)alkyl cellulose, has a molecular weight of from 600,000 to 1,000,000; if poly(ethylene oxide), has a molecular weight of from 100,000 to 500,000; if poly(vinyl alcohol), has a molecular weight of from 95,000 to 300,000; if poly(vinyl pyrrolidone), has a molecular weight of from 300,000 to 600,000; if poly(acrylamide), has a molecular weight of from 700,000 to 1,500,000, and if poly(vinylmethylether), has a molecular weight of from 200,000 to 500,000.
5. A composition according to any preceding claim wherein the polymer is hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose or hydroxybutylmethyl cellulose.
6. A composition according to any preceding claim wherein the dithiocarbamate is an ethylene bisdithiocarbamate.
7. A composition according to claim 6 wherein the ethylene bisdithiocarbamate comprises mancozeb and/or maneb.
8. A method for combatting phytopathogenic fungi comprising applying to a plant, its habitat, plant seed or soil a fungicidally-effective amount of a composition according to any preceding claim.
1. The use of (a) dithiocarbamate in dry form, which may optionally have been diluted with liquid for use, together with (b) water-soluble nonionic polymer in an amount and having a molecular weight sufficient to increase the rain fastness of the dithiocarbamate when applied to foliage, seed or soil wherein the polymer comprises hydroxy(C2-C5)alkyl cellulose, polyethylene oxide, polyvinyl alcohol having a saponification index of from 45 to 150, polyvinyl pyrrolidone, polyacrylamide and/or polyvinylmethylether, to make a rain-resistant fungicidal composition.
2. The use of a rain-resistant fungicidal composition made in accordance with claim 1 for combatting phytopathogenic fungi.
3. A mechanical process for improving the commercial value and/or profitability of vendible crops from plants whose growth is affected or likely to be affected by fungi comprises (1) charging to a container, fumigation device or mechanical dissemination device a rain-resistant fungicidal composition made in accordance with claim 1, (2) using the container, fumigator or mechanical dissemination device to apply the composition, in the form of granules, dust, vapour or diluted dry form to growing plants or to the seeds thereof or to a growth medium where the plants are growing or are to be grown, or to the fungus itself, (3) controlling the dose of the active ingredient during this application step so that the rate of application of active fungicidal compound is sufficient to combat the fungi but is insufficient to cause an unacceptably adverse effect on the crop plants growing or to be grown in the treated area.
4. The use or process according to claim 1, 2 or 3 wherein the polymer: if hydroxy(C2-CS)alkyl cellulose or poly(ethylene oxide) or poly(vinylalcohol) has a molecular weight of at least about 80,000; if poly(vinyl pyrrolidone), has a molecular weight of at least about 40,000; if poly(acrylamide), has a molecular weight of at least 500,000; and if poly(vinylmethylether) has a molecular weight of at least about 100,000.
5. The use or process according to any preceding claim wherein the composition contains the polymer in an amount of 0.1 to 5.0 weight percent based on the weight of the dithiocarbamate.
6. The use or process according to any preceding claim wherein the polymer: if hydroxy(C2-C5)alkyl cellulose, has a molecular weight of from 600,000 to 1,000,000; if poly(ethylene oxide), has a molecular weight of from 100,000 to 500,000; if poly(vinyl alcohol), has a molecular weight of from 95,000 to 300,000; if poly(vinyl pyrrolidone), has a molecular weight of from 300,000 to 600,000; if poly(acrylamide), has a molecular weight of from 700,000 to 1,500,000, and if poly(vinylmethylether), has a molecular weight of from 200,000 to 500,000.
7. The use or process according to any preceding claim wherein the polymer is hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose or hydroxybutylmethyl cellulose.
8. The use or process according to any preceding claim wherein the dithiocarbamate is an ethylene bisdithiocarbamate.
9. The use or process according to claim 8 wherein the ethylene bisdithiocarbamate comprises mancozeb and/or maneb.
10. The use according to any of claims 2 and 4 to 9, for combatting phytopathogenic fungi, of the rain-resistant fungicidal composition by applying it to a plant, its habitat, plant seed, or soil in a fungicidally-effective amount.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US85608086A | 1986-04-25 | 1986-04-25 | |
| US856080 | 2004-05-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0245970A1 true EP0245970A1 (en) | 1987-11-19 |
Family
ID=25322815
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP87303373A Withdrawn EP0245970A1 (en) | 1986-04-25 | 1987-04-16 | Dithiocarbamate compostions having increased rain fastness |
Country Status (12)
| Country | Link |
|---|---|
| EP (1) | EP0245970A1 (en) |
| JP (1) | JPS62258307A (en) |
| KR (1) | KR950012755B1 (en) |
| AU (1) | AU601179B2 (en) |
| BR (1) | BR8701910A (en) |
| CA (1) | CA1328599C (en) |
| HU (1) | HU202716B (en) |
| IL (1) | IL82286A (en) |
| NZ (1) | NZ220049A (en) |
| PH (1) | PH22421A (en) |
| PT (1) | PT84761B (en) |
| ZA (1) | ZA872917B (en) |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0506313A1 (en) * | 1991-03-26 | 1992-09-30 | Ciba Specialty Chemicals Water Treatments Limited | Sprayable agricultural compositions |
| ES2042418A1 (en) * | 1992-05-18 | 1993-12-01 | Tecno Holding S A | External treatment of vegetable crops |
| US5529975A (en) * | 1990-03-26 | 1996-06-25 | Allied Colloids Limited | Sprayable agricultural compositions |
| WO1997040668A1 (en) * | 1996-05-02 | 1997-11-06 | Uniroyal Chemical Company, Inc. | Powder formulation useful for seed treatment and foliar treatment of plants |
| EP0862856A1 (en) * | 1997-03-03 | 1998-09-09 | Rohm And Haas Company | Pesticide compositions |
| EP0950354A1 (en) * | 1998-04-17 | 1999-10-20 | Rohm And Haas Company | Dithiocarbamate fungicide compositions |
| EP0981957A1 (en) * | 1998-08-11 | 2000-03-01 | American Cyanamid Company | Method for the enhancement of the residual activity of pesticide formulations |
| WO2001015527A1 (en) * | 1999-09-02 | 2001-03-08 | Forrest, Jack | Biocidal adjuvant |
| US6214771B1 (en) * | 1995-11-06 | 2001-04-10 | American Cyanamid Company | Aqueous spray compositions |
| US6753003B1 (en) | 1999-05-31 | 2004-06-22 | Basf Aktiengesellschaft | Dithiocarbamate liquid formulations |
| US10694741B2 (en) | 2013-12-05 | 2020-06-30 | Upl Limited | Agrochemical compositions having increased rainfastness |
| WO2023288294A1 (en) | 2021-07-16 | 2023-01-19 | Novozymes A/S | Compositions and methods for improving the rainfastness of proteins on plant surfaces |
| WO2023225459A2 (en) | 2022-05-14 | 2023-11-23 | Novozymes A/S | Compositions and methods for preventing, treating, supressing and/or eliminating phytopathogenic infestations and infections |
| WO2025131903A1 (en) | 2023-12-21 | 2025-06-26 | Chr. Hansen A/S | Priestia megaterium and uses thereof |
| WO2025157988A1 (en) | 2024-01-26 | 2025-07-31 | Chr. Hansen A/S | Phytoprotective combinations of bacilli that thrive under low temperature conditions |
| WO2025217017A1 (en) | 2024-04-08 | 2025-10-16 | Novozymes A/S | Compositions and methods for increasing phosphorous availability |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08217604A (en) * | 1995-02-17 | 1996-08-27 | Agurosu:Kk | Fixable composition of agrochemical active component |
| CA2894706A1 (en) * | 2012-12-31 | 2014-07-03 | Dow Agrosciences Llc | Compositions and methods to modulate the rate of ebis production from dithiocarbamate fungicides |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1296118A (en) * | 1961-05-09 | 1962-06-15 | Sipcam Spa | Process for imparting marked adhesive properties to polymeric resins of vinyl alcohol |
| GB1056887A (en) * | 1963-11-13 | 1967-02-01 | Zimmie A G | Aqueous powder mixture |
| FR1493069A (en) * | 1966-07-13 | 1967-08-25 | Rhone Poulenc Sa | Process for increasing the potency of pesticides |
| DE1642122B1 (en) * | 1967-04-15 | 1970-07-30 | Riedel De Haen Ag | Process for the production of granules for the fight against damage |
| GB1209996A (en) * | 1968-05-16 | 1970-10-28 | Ici Ltd | Wettable powders |
| US4447413A (en) * | 1980-05-08 | 1984-05-08 | Aviation Chemical, Inc. | Drift influencing composition |
-
1987
- 1987-04-01 PH PH35096A patent/PH22421A/en unknown
- 1987-04-10 CA CA000534374A patent/CA1328599C/en not_active Expired - Fee Related
- 1987-04-16 EP EP87303373A patent/EP0245970A1/en not_active Withdrawn
- 1987-04-22 IL IL8228687A patent/IL82286A/en not_active IP Right Cessation
- 1987-04-22 NZ NZ220049A patent/NZ220049A/en unknown
- 1987-04-22 BR BR8701910A patent/BR8701910A/en not_active Application Discontinuation
- 1987-04-23 AU AU71902/87A patent/AU601179B2/en not_active Ceased
- 1987-04-24 KR KR1019870003942A patent/KR950012755B1/en not_active Expired - Fee Related
- 1987-04-24 JP JP62101712A patent/JPS62258307A/en active Pending
- 1987-04-24 ZA ZA872917A patent/ZA872917B/en unknown
- 1987-04-24 HU HU871799A patent/HU202716B/en not_active IP Right Cessation
- 1987-04-24 PT PT84761A patent/PT84761B/en not_active IP Right Cessation
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1296118A (en) * | 1961-05-09 | 1962-06-15 | Sipcam Spa | Process for imparting marked adhesive properties to polymeric resins of vinyl alcohol |
| GB1056887A (en) * | 1963-11-13 | 1967-02-01 | Zimmie A G | Aqueous powder mixture |
| FR1493069A (en) * | 1966-07-13 | 1967-08-25 | Rhone Poulenc Sa | Process for increasing the potency of pesticides |
| DE1642122B1 (en) * | 1967-04-15 | 1970-07-30 | Riedel De Haen Ag | Process for the production of granules for the fight against damage |
| GB1209996A (en) * | 1968-05-16 | 1970-10-28 | Ici Ltd | Wettable powders |
| US4447413A (en) * | 1980-05-08 | 1984-05-08 | Aviation Chemical, Inc. | Drift influencing composition |
Non-Patent Citations (1)
| Title |
|---|
| JOURNAL OF ECONOMIC ENTOMOLOGY, vol. 54, no. 3, June 1961, pages 508-510, College Park, Maryland, US; H.E. ALLER et al.: "Adjuvants increasing the residual activity of phosdrin" * |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5529975A (en) * | 1990-03-26 | 1996-06-25 | Allied Colloids Limited | Sprayable agricultural compositions |
| US5525575A (en) * | 1991-03-26 | 1996-06-11 | Allied Colloids Limited | Sprayable agricultural compositions |
| EP0506313A1 (en) * | 1991-03-26 | 1992-09-30 | Ciba Specialty Chemicals Water Treatments Limited | Sprayable agricultural compositions |
| ES2042418A1 (en) * | 1992-05-18 | 1993-12-01 | Tecno Holding S A | External treatment of vegetable crops |
| US6214771B1 (en) * | 1995-11-06 | 2001-04-10 | American Cyanamid Company | Aqueous spray compositions |
| WO1997040668A1 (en) * | 1996-05-02 | 1997-11-06 | Uniroyal Chemical Company, Inc. | Powder formulation useful for seed treatment and foliar treatment of plants |
| EP0862856A1 (en) * | 1997-03-03 | 1998-09-09 | Rohm And Haas Company | Pesticide compositions |
| EP0950354A1 (en) * | 1998-04-17 | 1999-10-20 | Rohm And Haas Company | Dithiocarbamate fungicide compositions |
| US6004570A (en) * | 1998-04-17 | 1999-12-21 | Rohm And Haas Company | Dithiocarbamate fungicide compositions with improved properties |
| AU749554B2 (en) * | 1998-04-17 | 2002-06-27 | Dow Agrosciences Llc | Dithiocarbanate fungicide compositions with improved properties |
| EP0981957A1 (en) * | 1998-08-11 | 2000-03-01 | American Cyanamid Company | Method for the enhancement of the residual activity of pesticide formulations |
| US6753003B1 (en) | 1999-05-31 | 2004-06-22 | Basf Aktiengesellschaft | Dithiocarbamate liquid formulations |
| WO2001015527A1 (en) * | 1999-09-02 | 2001-03-08 | Forrest, Jack | Biocidal adjuvant |
| US10694741B2 (en) | 2013-12-05 | 2020-06-30 | Upl Limited | Agrochemical compositions having increased rainfastness |
| WO2023288294A1 (en) | 2021-07-16 | 2023-01-19 | Novozymes A/S | Compositions and methods for improving the rainfastness of proteins on plant surfaces |
| WO2023225459A2 (en) | 2022-05-14 | 2023-11-23 | Novozymes A/S | Compositions and methods for preventing, treating, supressing and/or eliminating phytopathogenic infestations and infections |
| WO2025131903A1 (en) | 2023-12-21 | 2025-06-26 | Chr. Hansen A/S | Priestia megaterium and uses thereof |
| WO2025157988A1 (en) | 2024-01-26 | 2025-07-31 | Chr. Hansen A/S | Phytoprotective combinations of bacilli that thrive under low temperature conditions |
| WO2025217017A1 (en) | 2024-04-08 | 2025-10-16 | Novozymes A/S | Compositions and methods for increasing phosphorous availability |
Also Published As
| Publication number | Publication date |
|---|---|
| HUT46504A (en) | 1988-11-28 |
| AU7190287A (en) | 1987-10-29 |
| JPS62258307A (en) | 1987-11-10 |
| PH22421A (en) | 1988-09-12 |
| CA1328599C (en) | 1994-04-19 |
| IL82286A (en) | 1994-02-27 |
| KR870009644A (en) | 1987-11-30 |
| PT84761B (en) | 1995-07-03 |
| ZA872917B (en) | 1987-12-30 |
| KR950012755B1 (en) | 1995-10-21 |
| IL82286A0 (en) | 1987-10-30 |
| AU601179B2 (en) | 1990-09-06 |
| BR8701910A (en) | 1988-02-02 |
| PT84761A (en) | 1988-04-21 |
| NZ220049A (en) | 1990-10-26 |
| HU202716B (en) | 1991-04-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA3101800C (en) | Composition for fortification and nutrition of crops comprising elemental sulphur and manganese salt, complexes, derivatives thereof | |
| EP0245970A1 (en) | Dithiocarbamate compostions having increased rain fastness | |
| US5443835A (en) | Bicarbonate salt pesticide composition containing a clathrate spreader-sticker ingredient | |
| RU2662289C2 (en) | Method of controlling strobilurine resistant septoria tritici | |
| CN101433199A (en) | Bactericidal composition | |
| CN110352967A (en) | A kind of composition, preparation and its application, preparation method | |
| WO2003094614A1 (en) | Auxiliary compositions | |
| WO2010015578A1 (en) | Method for controlling fungal diseases in legumes | |
| JPS6337762B2 (en) | ||
| CN105532657B (en) | It is a kind of for Herbicidal combinations of monocot crops and application thereof | |
| JPH0768087B2 (en) | Herbicide composition | |
| EA000532B1 (en) | Herbicidal composition containing 4-benzoylisoxazoles and aclonifen | |
| US5834403A (en) | Low rate application of inhibitors of ethylene biosynthesis or action | |
| KR101208335B1 (en) | Synergistic herbicidal compositions comprising Metamifop | |
| SU1034596A3 (en) | Granulated fungicidal composition for rice plant protection | |
| JP2003104820A (en) | Pest control agent | |
| JP4212194B2 (en) | 5-methylisoxazole composition with enhanced efficacy | |
| WO2025173008A1 (en) | Solid formulation | |
| WO2025052472A1 (en) | Novel fungicidal composition | |
| KR920001507B1 (en) | An agricultural and horticultural fungicidal composition | |
| JPH0761924B2 (en) | Herbicide composition | |
| JPS59108702A (en) | Agent for preventing spoilage by eating by birds | |
| EP0202294B1 (en) | Synergistic herbicidal compositions | |
| CN116584496A (en) | Agricultural composition containing fenoxycarb and lufenuron | |
| JPS5948807B2 (en) | Acaricide, insecticidal composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19870629 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE ES FR GB GR IT LI LU NL SE |
|
| 17Q | First examination report despatched |
Effective date: 19890317 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19941102 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: KOSTANSEK, EDWARD CHARLES |