EP0219367B1 - Organic electrolysis cell with a consumable electrode - Google Patents
Organic electrolysis cell with a consumable electrode Download PDFInfo
- Publication number
- EP0219367B1 EP0219367B1 EP86401895A EP86401895A EP0219367B1 EP 0219367 B1 EP0219367 B1 EP 0219367B1 EP 86401895 A EP86401895 A EP 86401895A EP 86401895 A EP86401895 A EP 86401895A EP 0219367 B1 EP0219367 B1 EP 0219367B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- electrode
- electrolysis cell
- organic
- electrosynthesis
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000005868 electrolysis reaction Methods 0.000 title claims description 53
- 229910052751 metal Inorganic materials 0.000 claims abstract description 32
- 239000002184 metal Substances 0.000 claims abstract description 32
- 239000011810 insulating material Substances 0.000 claims abstract description 9
- 239000007787 solid Substances 0.000 claims abstract description 9
- 150000002894 organic compounds Chemical class 0.000 claims abstract description 7
- 238000003487 electrochemical reaction Methods 0.000 claims abstract description 5
- 150000002902 organometallic compounds Chemical class 0.000 claims abstract description 5
- 239000008151 electrolyte solution Substances 0.000 claims abstract 2
- 150000004820 halides Chemical class 0.000 claims description 17
- 229910052782 aluminium Inorganic materials 0.000 claims description 15
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 15
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 claims description 14
- 239000011777 magnesium Substances 0.000 claims description 14
- 229910052749 magnesium Inorganic materials 0.000 claims description 14
- 229910045601 alloy Inorganic materials 0.000 claims description 13
- 239000000956 alloy Substances 0.000 claims description 13
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 claims description 11
- 229910052725 zinc Inorganic materials 0.000 claims description 11
- 239000011701 zinc Substances 0.000 claims description 11
- 150000001735 carboxylic acids Chemical class 0.000 claims description 10
- 150000002576 ketones Chemical class 0.000 claims description 10
- 239000000463 material Substances 0.000 claims description 9
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 8
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 8
- 150000001299 aldehydes Chemical class 0.000 claims description 8
- 239000004033 plastic Substances 0.000 claims description 8
- 229920003023 plastic Polymers 0.000 claims description 8
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims description 7
- 150000001298 alcohols Chemical class 0.000 claims description 6
- 229910052799 carbon Inorganic materials 0.000 claims description 6
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims description 6
- 239000003960 organic solvent Substances 0.000 claims description 6
- 239000010935 stainless steel Substances 0.000 claims description 5
- 229910001220 stainless steel Inorganic materials 0.000 claims description 5
- 125000000524 functional group Chemical group 0.000 claims description 4
- 229910052759 nickel Inorganic materials 0.000 claims description 4
- 150000007524 organic acids Chemical class 0.000 claims description 4
- 229910002092 carbon dioxide Inorganic materials 0.000 claims description 3
- 239000001569 carbon dioxide Substances 0.000 claims description 3
- 239000004020 conductor Substances 0.000 claims description 2
- 239000004411 aluminium Substances 0.000 claims 3
- 230000005611 electricity Effects 0.000 claims 2
- 239000013543 active substance Substances 0.000 abstract 1
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 15
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 14
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 13
- 239000000243 solution Substances 0.000 description 12
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 10
- 125000003118 aryl group Chemical group 0.000 description 10
- 125000001931 aliphatic group Chemical group 0.000 description 9
- 230000000694 effects Effects 0.000 description 8
- -1 Pb0 2 and Ni0 2 Chemical class 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 6
- 239000002609 medium Substances 0.000 description 6
- 229920006395 saturated elastomer Polymers 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 5
- 150000008064 anhydrides Chemical class 0.000 description 5
- 229910052736 halogen Inorganic materials 0.000 description 5
- 150000002367 halogens Chemical class 0.000 description 5
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 5
- 238000009434 installation Methods 0.000 description 5
- 150000002739 metals Chemical class 0.000 description 5
- 229930192474 thiophene Natural products 0.000 description 5
- RIWRBSMFKVOJMN-UHFFFAOYSA-N 2-methyl-1-phenylpropan-2-ol Chemical compound CC(C)(O)CC1=CC=CC=C1 RIWRBSMFKVOJMN-UHFFFAOYSA-N 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 4
- MRMOZBOQVYRSEM-UHFFFAOYSA-N tetraethyllead Chemical compound CC[Pb](CC)(CC)CC MRMOZBOQVYRSEM-UHFFFAOYSA-N 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 125000004429 atom Chemical group 0.000 description 3
- KCXMKQUNVWSEMD-UHFFFAOYSA-N benzyl chloride Chemical compound ClCC1=CC=CC=C1 KCXMKQUNVWSEMD-UHFFFAOYSA-N 0.000 description 3
- 229940073608 benzyl chloride Drugs 0.000 description 3
- 239000003792 electrolyte Substances 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 229910002804 graphite Inorganic materials 0.000 description 3
- 239000010439 graphite Substances 0.000 description 3
- 238000003754 machining Methods 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Natural products CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical compound BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 2
- GZUXJHMPEANEGY-UHFFFAOYSA-N bromomethane Chemical compound BrC GZUXJHMPEANEGY-UHFFFAOYSA-N 0.000 description 2
- 150000007942 carboxylates Chemical class 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000010924 continuous production Methods 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 230000005484 gravity Effects 0.000 description 2
- GNOIPBMMFNIUFM-UHFFFAOYSA-N hexamethylphosphoric triamide Chemical compound CN(C)P(=O)(N(C)C)N(C)C GNOIPBMMFNIUFM-UHFFFAOYSA-N 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- LGRFSURHDFAFJT-UHFFFAOYSA-N phthalic anhydride Chemical compound C1=CC=C2C(=O)OC(=O)C2=C1 LGRFSURHDFAFJT-UHFFFAOYSA-N 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 2
- RLUFBDIRFJGKLY-UHFFFAOYSA-N (2,3-dichlorophenyl)-phenylmethanone Chemical compound ClC1=CC=CC(C(=O)C=2C=CC=CC=2)=C1Cl RLUFBDIRFJGKLY-UHFFFAOYSA-N 0.000 description 1
- YTKRILODNOEEPX-UHFFFAOYSA-N 1-chlorobut-2-ene Chemical compound CC=CCCl YTKRILODNOEEPX-UHFFFAOYSA-N 0.000 description 1
- GTLWADFFABIGAE-UHFFFAOYSA-N 1-chloroethylbenzene Chemical compound CC(Cl)C1=CC=CC=C1 GTLWADFFABIGAE-UHFFFAOYSA-N 0.000 description 1
- MZMVVHAHSRJOEO-UHFFFAOYSA-N 1-chloropropylbenzene Chemical compound CCC(Cl)C1=CC=CC=C1 MZMVVHAHSRJOEO-UHFFFAOYSA-N 0.000 description 1
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- OHXAOPZTJOUYKM-UHFFFAOYSA-N 3-Chloro-2-methylpropene Chemical compound CC(=C)CCl OHXAOPZTJOUYKM-UHFFFAOYSA-N 0.000 description 1
- VZGLVCFVUREVDP-UHFFFAOYSA-N 3-chlorobut-1-ene Chemical compound CC(Cl)C=C VZGLVCFVUREVDP-UHFFFAOYSA-N 0.000 description 1
- IWTYTFSSTWXZFU-UHFFFAOYSA-N 3-chloroprop-1-enylbenzene Chemical compound ClCC=CC1=CC=CC=C1 IWTYTFSSTWXZFU-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- OSDWBNJEKMUWAV-UHFFFAOYSA-N Allyl chloride Chemical compound ClCC=C OSDWBNJEKMUWAV-UHFFFAOYSA-N 0.000 description 1
- CAHQGWAXKLQREW-UHFFFAOYSA-N Benzal chloride Chemical compound ClC(Cl)C1=CC=CC=C1 CAHQGWAXKLQREW-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 241000287107 Passer Species 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical group [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- IKHGUXGNUITLKF-XPULMUKRSA-N acetaldehyde Chemical compound [14CH]([14CH3])=O IKHGUXGNUITLKF-XPULMUKRSA-N 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 125000000746 allylic group Chemical group 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 1
- 239000012965 benzophenone Substances 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- JHIWVOJDXOSYLW-UHFFFAOYSA-N butyl 2,2-difluorocyclopropane-1-carboxylate Chemical compound CCCCOC(=O)C1CC1(F)F JHIWVOJDXOSYLW-UHFFFAOYSA-N 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- IBAHLNWTOIHLKE-UHFFFAOYSA-N cyano cyanate Chemical compound N#COC#N IBAHLNWTOIHLKE-UHFFFAOYSA-N 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 230000005518 electrochemistry Effects 0.000 description 1
- 229940082150 encore Drugs 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 239000011133 lead Substances 0.000 description 1
- MHCFAGZWMAWTNR-UHFFFAOYSA-M lithium perchlorate Chemical compound [Li+].[O-]Cl(=O)(=O)=O MHCFAGZWMAWTNR-UHFFFAOYSA-M 0.000 description 1
- 229910001486 lithium perchlorate Inorganic materials 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 229940102396 methyl bromide Drugs 0.000 description 1
- 125000002560 nitrile group Chemical group 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000000615 nonconductor Substances 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 125000002524 organometallic group Chemical group 0.000 description 1
- 238000013021 overheating Methods 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Chemical group 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Chemical group 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 239000010802 sludge Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 229940014800 succinic anhydride Drugs 0.000 description 1
- 229910052717 sulfur Chemical group 0.000 description 1
- 239000011593 sulfur Chemical group 0.000 description 1
- 150000003509 tertiary alcohols Chemical class 0.000 description 1
- DPKBAXPHAYBPRL-UHFFFAOYSA-M tetrabutylazanium;iodide Chemical compound [I-].CCCC[N+](CCCC)(CCCC)CCCC DPKBAXPHAYBPRL-UHFFFAOYSA-M 0.000 description 1
- XOOGZRUBTYCLHG-UHFFFAOYSA-N tetramethyllead Chemical compound C[Pb](C)(C)C XOOGZRUBTYCLHG-UHFFFAOYSA-N 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/02—Electrodes; Manufacture thereof not otherwise provided for characterised by shape or form
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B3/00—Electrolytic production of organic compounds
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B9/00—Cells or assemblies of cells; Constructional parts of cells; Assemblies of constructional parts, e.g. electrode-diaphragm assemblies; Process-related cell features
- C25B9/30—Cells comprising movable electrodes, e.g. rotary electrodes; Assemblies of constructional parts thereof
Definitions
- the present invention relates to an electrolysis cell for electrosynthesis, in an organic medium, of organic or organometallic compounds, comprising two electrodes, one and only one of which is consumed during electrosynthesis by the electrochemical reaction of which it is the seat.
- South African patent No. 6806413 describes the synthesis of tetraethyl lead in an electrolysis cell comprising a consumable anode in the form of a metallic strip which passes between two cathodes in the form of discs.
- This system has a number of drawbacks.
- the thickness of the anode must in particular be small so that the interelectrode space remains constant; the speed of advance of the anode must therefore be rapid, and, to avoid rupture of the ribbon, the device requires a relatively complicated mechanical system.
- FR-1 412 239 describes an electrolysis cell designed to operate in an aqueous medium and comprising 2 compartments, cathodic and anodic, separated by a diaphragm.
- the wedge-shaped anode is made of graphite.
- SU-1,046,022 describes an anode device for obtaining metal powders by electrolysis of aqueous solutions using consumable anodes, comprising an anode chamber in the form of a triangular prism and soluble anode elements, in the form of plates arranged horizontally.
- the wall of the chamber oriented towards the cathode is in the form of a grid composed of insulated fins and inclined with respect to the horizontal plane.
- German patent 2107305 describes for example such a device.
- Electrolysis cells comprising a consumable anode have already been described for the electrosynthesis of oxalic acid from carbon dioxide, on the one hand with aluminum in Chim. lnd. (Milan) 55. (1973) 156 and on the other hand with zinc in J. Appl. Electrochem. 11 (1981) 743, for the electrocarboxylation of ethylene (Tetrahedron Lett. 1973,3025) and for that of thioethers (Patent of the Democratic Republic of Germany No. 203537).
- central electrode acts as a consumable anode (metal bar for example); in others, it acts as a cathode (graphite for example).
- cathode graphite for example
- the present invention aims to provide an electrolysis cell allowing simple continuous industrial use, having the advantages of the above-mentioned industrial cells, namely in particular maintaining a constant gap between the electrodes, without having the disadvantages.
- the inclination, at a point of a surface, with respect to the vertical is conventionally considered to be the angle formed by the plane of tangency to the surface at this point and by the vertical passing through this point.
- the cell according to the invention has many advantages. First of all, it allows a constant and preferably small gap (less than 5 mm) to be maintained between the two electrodes during the entire duration of the electrolysis, which is very important in an organic conductive medium, in order to avoid excessive power consumption and overheating by Joule effect.
- One of the two electrodes being progressively consumed during the electrochemical reaction a means is necessary necessarily allowing the distance between the two electrodes to be kept constant, which is obtained in the context of this invention, thanks to the particular design and geometry of the cell. It is also necessary to be able to easily replace the consumable electrode as soon as it is completely consumed, or better, for continuous processes, as it is consumed, without stopping and disturbing the electrolysis.
- the cell according to the invention allows very easy replacement of the consumable electrode, without stopping the electrolysis, by superposition of one (or more) another block on the solid metal block (s) constituting the consumable electrode, which is a considerable advantage when implementing continuous processes. In addition, the entire electrode is consumed, without falling or loss.
- the cell according to the invention also allows the use of massive consumable electrodes, therefore not very bulky for a given mass, and of various shapes. This fact is economically very interesting.
- Another advantage is the fact that, taking into account the geometry of the cell and in particular the inclination of the non-consumable electrode, the space requirement on the ground is very reduced, which allows a saving of space which is economically very appreciable.
- the consumable electrode is the anode (anodic oxidation) as for the examples which follow but sometimes the consumable electrode is the cathode as is the case for the electrosynthesis of tetramethyl lead in acetonitrile medium from methyl bromide with lead cathode according to HE. Ulery JECS 116, 1201, 1969:
- the consumable electrode consists of at least one solid metal block.
- the metal is chosen from the group consisting of magnesium, aluminum, zinc and their alloys, namely any alloy containing at least one of the three aforementioned metals.
- Many other metals are also suitable, such as copper, nickel, and lead.
- the choice of metal depends inter alia on the compound which one wants to synthesize.
- the consumable electrode is for example constituted by the corresponding metal or an alloy based on this metal.
- magnesium is preferred.
- a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys.
- the solid metal blocks can be, for example, ingots whose cross section is square, or rectangular, or trapezoidal, or circular, or in any other form. They can optionally be machined before use so that their geometry is adapted to that of the non-consumable electrode. Preferably, but without this having an imperative character, such machining is carried out to facilitate the start of electrolysis.
- the consumable electrode consists of solid metal blocks stacked, each layer of the stack comprising only one block.
- at least one layer of the stack comprises several blocks arranged side by side.
- the consumable electrode is applied under the effect of its own weight, by gravity, against the other non-consumable electrode.
- the consumable electrode is applied against the other electrode under the sole effect of its own weight.
- the consumable electrode is applied against the other electrode under the effect, in addition to its own weight, of that of an inert charge resting on the consumable electrode.
- the inert charge is electrically conductive and also serves to ensure the electrical supply of the consumable electrode.
- the consumable electrode is applied against the other electrode under the effect, in addition to its own weight, of the force produced by a compressed spring between the upper part of the consumable electrode and a wall of the cell.
- the non-consumable electrode is made of a conductive material.
- a conductive material such as iron, aluminum and nickel, alloys such as stainless steel, metal oxides such as Pb0 2 and Ni0 2 , graphite.
- metals such as iron, aluminum and nickel, alloys such as stainless steel, metal oxides such as Pb0 2 and Ni0 2 , graphite.
- it is made of a metal chosen from the group consisting of nickel and stainless steel.
- the distance between the active surfaces of the two electrodes is less than 5 mm. This distance is conventionally measured on a common perpendicular, between the two parallel surfaces;
- the two electrodes are separated by an electrical insulating material allowing the electrolysis solution to pass and whose shape and dimensions allow the active surfaces of the 2 electrodes to remain parallel during electrosynthesis.
- this electrical insulating material must have sufficient mechanical strength to support the consumable electrode which rests on this material.
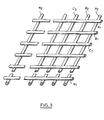
- the electrical insulating material is a plastic material in the form of a grid whose thickness is less than 5 mm and whose mesh consists of two networks of parallel wires, these two networks being superimposed, crossed, fixed one on the other at the contact points of the wires, the thickness of the wires of each network being the same.
- the two networks are fixed to each other by welding and the wires of the two networks have the same thickness.
- the distance between the wires of each network is between a few millimeters and a few centimeters.
- the wires in each network may not be parallel; their thickness may not be constant provided that after assembling the networks, the mesh has a constant maximum thickness at several points, less than about 5 mm.
- the cross section of the wires can be arbitrary, for example square, rectangular, circular, elliptical, trapezoidal.
- the plastic material can be, for example, polypropylene, polyethylene or polytetrafluoroethylene.
- Such plastic gratings have on the one hand a high vacuum rate, which allows easy circulation of the electrolysis solution between the two electrodes and on the other hand a relatively small contact surface with the electrodes, which avoids an excessive reduction in their active surface.
- a fabric As other materials separating the two electrodes, it is possible to use, within the framework of the present invention, a fabric, a canvas or a porous material of constant thickness such as for example a ceramic or a felt.
- the renewal of the electrolysis solution between the electrodes can, for example, be ensured by mechanical agitation or by forced circulation using a pump, for example.
- the active surface of the consumable electrode opposite the active surface of the other electrode dissolves.
- the consumable electrode therefore descends gradually, by gravity, under the simple effect of its own weight.
- the dissolution being stronger at the locations closest to the non-consumable electrode, the consumable electrode tends to conform as best as possible to the shape of the non-consumable electrode, which limits the risks of irregular dissolution.
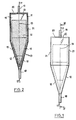
- the electrolysis cell shown in Figures 1 and 2 comprises a tank whose bottom wall is formed by the cathode 12, which is not consumable.
- the active surface of the non-consumable electrode 12 is conical, the tip of the cone being directed downwards. This active surface has at all points a constant inclination of 15 degrees relative to the direction 19 which is that of the axis of the cone. For the cell shown in Figures 1 and 2 this axis is vertical.
- the upper wall 21 of the tank is cylindrical and extends the cone so that the cylinder and the cone have the same axis, the diameter of the cylinder being the same as that of the base circle of the cone.
- the walls 20 and 21 are made of an electrical insulating material or internally covered with an electrical insulator 11, for example a paint or any other electrically insulating coating.
- the anode 14 consists of a stack of cylindrical solid metal ingots whose diameter is slightly less than that of the cylindrical wall 21 of the tank. It is applied under the sole effect of its own weight against the cathode 12 which, alone, ensures the maintenance of the anode 14.
- the anode 14 and the cathode 12 are separated by a plastic material 15 in the form of a mesh such as that shown in FIG. 3.
- the mesh consists of two networks of parallel wires A 1 B 1 C 1 ... N 1 of part and A 2 8 2 C Z ... N 2 on the other hand.
- the wires of these two networks are cylindrical, with a diameter of 1 mm. The distance between the wires is 1 cm.
- the two networks are superimposed, crossed at right angles and welded to the contact points of the wires.
- the electrolysis solution 16 circulates from bottom to top in the cell. Pipes 18 allow the arrival and the exit of this solution 16, in the direction of the arrows 17.
- the inlet pipe extends the tip of the cathode 12 along the axis of the cell.
- the electrodes 12 and 14 are supplied with electric current by a DC voltage source, not shown in FIGS. 1 and 2.
- the active surface of the non-consumable electrode 12 When the axis of the cell is rotated by an alpha angle around the tip of the cone, the active surface of the non-consumable electrode 12 always has at all its points a constant inclination of 15 degrees relative to the direction D represented by the axis of the cell and any straight line of direction D passing through any point of the consumable anode 14 crosses the active surface of the non-consumable cathode 12.
- First of all alpha must be less than 45 degrees in the context of the present invention.
- the inclination of the active surface of the non-consumable electrode 12 is between (15 + alpha) and 115-alphal.
- the inclination relative to the vertical must be less than 45 degrees, that is to say for this particular embodiment, that alpha must be less than 30 degrees. Otherwise, there may be significant anomalies in the functioning of the cell.
- the upper wall 20 of the electrolysis cell according to the invention is removable or has a part removable so as to allow the introduction of massive metal blocks.
- FIG. 4 A complete installation allowing the continuous electrolysis of a solution is schematically represented in FIG. 4.
- It consists of a closed circuit comprising a jacketed reactor 51 allowing the loading and recovery of the products, an electrolysis cell 52 and a pump 53 allowing the circulation of the electrolysis solution in the circuit.
- the lower part of the reactor 51 is connected to the lower part (inlet) of the cell 52 and the outlet of the cell 52 is connected to the upper part of the reactor 51.
- the jacketed reactor 51 is cooled by a circulation of water, symbolized by the arrows 54.
- the cell 52 shown diagrammatically in FIG. 4 is that shown in FIGS. 1 and 2.
- the present invention also relates to the use of the new electrolysis cells described above, provided with an anode consumable in a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys for electrosynthesis. in an organic solvent medium of organic compounds chosen from the group consisting of carboxylic acids, alcohols, ketones and aldehydes by electrochemical reduction of organic halides.
- an electrolysis cell is used provided with an anode consumable in a metal chosen from the group formed by magnesium and its alloys for the electrosynthesis of carboxylic acids by electrochemical reduction of organic halides in the presence of carbon dioxide.
- aromatic chains mention may, for example, be made of substituted or unsubstituted phenyl, thiophene, furan and pyridine rings.
- the carboxyl group can as well be linked to an aliphatic carbon as to a carbon of an aromatic ring.
- the use of a magnesium anode provides the best results. In particular, tests have been carried out with an anode either of aluminum or of zinc, all other conditions identical elsewhere. The yields are then lower than those obtained with the magnesium anode.
- the organic solvents used are the low protein solvents usually used in organic electrochemistry, such as hexamethylphosphorotriamide (HMPT) letetrahydrofuran (THF), N-methylpyrolidone (NMP), dimethylformamide (DMF).
- the organic solvent conventionally contains an indifferent electrolyte such as tetrabutylammonium tetrafluoroborate (BF 4 NBu 4 ) or lithium perchlorate.
- an indifferent electrolyte such as tetrabutylammonium tetrafluoroborate (BF 4 NBu 4 ) or lithium perchlorate.
- the yields obtained in the carboxylate formed are high, very often greater than 99%.
- the yields of isolated carboxylic acid vary from 70 to 90% of the yield of carboxylate formed.
- an electrolysis cell is used provided with an anode consumable in a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys for the electrosynthesis of alcohols, by electrochemical reduction. of organic halides having an atom or a functional group stabilizing carbanions attached to the carbon carrying the halogen, in the presence of carbonyl derivatives.
- the latter can be aldehydes as well as ketones; the yields are high and the implementation relatively simple.
- the organic halides have at least one atom or a functional group stabilizing carbanions, attached to the carbon carrying the halogen, that is to say located in the alpha position relative to the halogen.
- atoms and functional groups which stabilize carbanions are well known to those skilled in the art. Mention may be made, for example, of halogens, ester, ketone, allyl, benzene, alkoxy or nitrile groups.
- benzyl chloride By way of illustration and without limitation, mention may be made, for example, of benzyl chloride, benzyl bromide, allyl chloride, 3-chloro 2 methyl propene, 3-chloro 1 butene, 1-chloro 1-methyl ethyl acetate, carbon tetrachloride, dichlorophenylmethane, 1-phenyl 3-chloro propene and 1-methyl 3-chloropropene.
- R, and R 2 together with the carbon atom to which they are attached, form a ring, saturated or unsaturated, substituted or unsubstituted, optionally comprising one or more heteroatoms such as nitrogen, oxygen , phosphorus or sulfur.
- heteroatoms such as nitrogen, oxygen , phosphorus or sulfur.
- the alcohols obtained according to the process which is the subject of the present invention correspond to the general formula wherein R, R, and R 2 have the above meaning.
- organic solvents and the indifferent electrolytes used are the same as those mentioned above for the synthesis of carboxylic acids.
- DMF is used as solvent and the electrolysis is carried out at a temperature between -20 ° C. and + 30 ° C.
- an electrolysis cell is used provided with an anode consumable in a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys for the electrosynthesis of ketones and aldehydes by electrochemical reduction of organic halides in the presence of organic acid anhydrides.
- a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys for the electrosynthesis of ketones and aldehydes by electrochemical reduction of organic halides in the presence of organic acid anhydrides.
- the implementation is simple and the mass and faradaic yields high.
- R 3 has an aliphatic chain substituted with at least one aromatic group, for example in benzyl chloride, benzyl bromide, 1-phenyl 1-chloro ethane and 1-phenyl 1-chloro propane.
- R 3 can carry non-electro-reducible functions or more difficult to reduce than the R 3 -X bond, under the experimental conditions of electrosynthesis.
- non-electroreducible functions are, for example, the cyano, ether, sulfide or ester functions.
- R s represents a group OR s
- the corresponding anhydrides are then mixed anhydrides of carboxylic acids and carbonic acid. In the other cases, they are anhydrous carboxylic acids.
- R 4 and R 5 can carry non-electro-reducible functions, or more difficult to reduce than the bond R 3 ⁇ X, under the experimental conditions of electrosynthesis, and none of the functions carried by R 3 or R 4 does must be more electrophilic than the anhydride function itself.
- R 4 and R 5 represent a linear or branched alkyl chain.
- R 4 and R s are identical.
- R 4 and R s are identical and represent an alkyl chain, linear or branched, as is the case for example for acetic anhydride.
- organic solvents and the indifferent electrolytes used are the same as those mentioned above for the synthesis of carboxylic acids.
- DMF is used as solvent.
- the direction D is to preferably the vertical direction.
- the cathode 12 made of stainless steel, is a cone with a height of 100 mm and a base diameter of 53 mm.
- the other walls of the tank are made of stainless steel and are internally covered with an inert electrical insulating coating 11.
- the anode 14 consists of a stack of cylindrical aluminum blocks with a diameter of 50 mm and a height of 100 mm.
- the plastic material 15 in the form of a mesh is polypropylene. This mesh is just placed on the active surface of the cathode 12, the shape of which it matches, before the introduction of the anode 14.
- the lower aluminum block was machined so that it is approximately in the form of a cone with a height of 100 mm and a base diameter of 50 mm, which is easily achieved from of a cylindrical block having these dimensions.
- the machined block which introduces the shape of the cathode is introduced, then several other blocks are stacked on this lower block up to the top of the cell.
- the dimethylbenzylcarbinol formed is isolated, and identified according to the usual methods, well known to those skilled in the art.
- the alcohol formed was isolated after hydrolysis of the solution using an aqueous solution of ammonium chloride and extraction with ether. After evaporation of the ether, the crude alcohol was purified by distillation. The pure alcohol thus isolated (purity verified by CPG) is identified by its NMR and IR spectra.
- the yield of distilled dimethylbenzylcarbinol obtained is 60% (purity greater than 95%).
- the intensity of the current is fixed at the start at 2.5 A since the optimal operating conditions are then already met, the anode being in the optimal position relative to the cathode.
- Example 1 The same test is carried out as that of Example 1 but without machining the lower block before the first electrolysis. The same result is obtained but the operating equilibrium is much longer to reach.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Electrolytic Production Of Non-Metals, Compounds, Apparatuses Therefor (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
Abstract
Description
La présente invention concerne une cellule d'électrolyse pour l'électrosynthèse, en milieu organique, de composés organiques ou organométalliques, comportant deux électrodes dont l'une et une seulement est consommée au cours de l'électrosynthèse par la réaction électrochimique dont elle est le siège.The present invention relates to an electrolysis cell for electrosynthesis, in an organic medium, of organic or organometallic compounds, comprising two electrodes, one and only one of which is consumed during electrosynthesis by the electrochemical reaction of which it is the seat.
Les brevets US-3 573 178 et US-3 141 841 décrivent la synthèse du plomb tétraéthyle dans une cellule d'électrolyse comportant une anode constituée par des billes de plomb séparée de la cathode cylindrique par une paroi poreuse isolante. Des billes sont rajoutées en cours d'électrolyse pour remplacer celles qui sont consommées. Toutefois ce dispositif fonctionne mal pour les métaux très réducteurs comme le magnésium, l'aluminium, le zinc, le titane, qui sont recouverts d'une couche d'oxyde isolant qui augmente considérablement la résistance de contact entre grains. Par ailleurs, la présentation en granulés de ces métaux est parfois coûteuse. De plus il se forme souvent des boues et des poussières métalliques, ce qui perturbe le fonctionnement.The patents US-3,573,178 and US-3,141,841 describe the synthesis of tetraethyl lead in an electrolysis cell comprising an anode constituted by lead balls separated from the cylindrical cathode by a porous insulating wall. Balls are added during electrolysis to replace those which are consumed. However, this device does not work well for very reducing metals such as magnesium, aluminum, zinc, titanium, which are covered with an insulating oxide layer which considerably increases the contact resistance between grains. Furthermore, the presentation in granules of these metals is sometimes expensive. In addition, metallic sludge and dust often form, which disrupts operation.
Le brevet sud africain n° 6806413 décrit la synthèse du plomb tétraéthyle dans une cellule d'électrolyse comportant une anode consommable se présentant sous forme d'un ruban métallique qui défile entre deux cathodes en forme de disques. Ce système présente un certain nombre d'inconvénients. L'épaisseur de l'anode doit notamment être faible pour que l'espace interélectrodes reste constant; la vitesse d'avance de l'anode doit donc être rapide, et, pour éviter la rupture du ruban, le dispositif nécessite un système mécanique relativement compliqué.South African patent No. 6806413 describes the synthesis of tetraethyl lead in an electrolysis cell comprising a consumable anode in the form of a metallic strip which passes between two cathodes in the form of discs. This system has a number of drawbacks. The thickness of the anode must in particular be small so that the interelectrode space remains constant; the speed of advance of the anode must therefore be rapid, and, to avoid rupture of the ribbon, the device requires a relatively complicated mechanical system.
FR-1 412 239 décrit une cellule d'électrolyse conçue pour fonctionner en milieu aqueux et comportant 2 compartiments, cathodique et anodique, séparés par un diaphragme. L'anode, en forme de coin, est en graphite.FR-1 412 239 describes an electrolysis cell designed to operate in an aqueous medium and comprising 2 compartments, cathodic and anodic, separated by a diaphragm. The wedge-shaped anode is made of graphite.
SU-1 046 022 décrit un dispositif anodique pour l'obtention des poudres métalliques par électrolyse de solutions aqueuses à l'aide d'anodes consommables, comprenant une chambre anodique en forme de prisme triangulaire et des éléments anodiques solubles, se présentant sous forme de plaques disposées horizontalement. La paroi de la chambre orientée vers la cathode se présente sous la forme d'une grille composée d'ailettes isolées et inclinées par rapport au plan horizontal. Ce dispositif permet de maintenir un écart constant entre les électrodes en cours d'électrolyse.SU-1,046,022 describes an anode device for obtaining metal powders by electrolysis of aqueous solutions using consumable anodes, comprising an anode chamber in the form of a triangular prism and soluble anode elements, in the form of plates arranged horizontally. The wall of the chamber oriented towards the cathode is in the form of a grid composed of insulated fins and inclined with respect to the horizontal plane. This device makes it possible to maintain a constant distance between the electrodes during electrolysis.
Sont connus, par ailleurs, plusieurs dispositifs mécaniques, souvent très compliqués, permettant de contrôler la distance entre les électrodes afin de la maintenir constante ou permettant le remplacement des anodes usées. Le brevet allemand 2107305 décrit par exemple un tel dispositif.Also known are several mechanical devices, often very complicated, allowing the distance between the electrodes to be controlled in order to keep it constant or allowing replacement of the used anodes. German patent 2107305 describes for example such a device.
Des cellules d'électrolyse comportant une anode consommable ont déjà été décrites pour l'électrosynthèse de l'acide oxalique à partir de gaz carbonique, d'une part avec l'aluminium dans Chim. lnd. (Milan) 55. (1973) 156 et d'autre part avec le zinc dans J. Appl. Electrochem. 11 (1981) 743, pour l'électrocarboxylation de l'éthylène (Tetrahedron Lett. 1973,3025) et pour celle de thioéthers (brevet de la République Démocratique d'Allemagne n° 203537).Electrolysis cells comprising a consumable anode have already been described for the electrosynthesis of oxalic acid from carbon dioxide, on the one hand with aluminum in Chim. lnd. (Milan) 55. (1973) 156 and on the other hand with zinc in J. Appl. Electrochem. 11 (1981) 743, for the electrocarboxylation of ethylene (Tetrahedron Lett. 1973,3025) and for that of thioethers (Patent of the Democratic Republic of Germany No. 203537).
Ces cellules sont sans diaphragme et présentent généralement une symétrie cylindrique coaxiale. Dans certains cas l'électrode centrale fait fonction d'anode consommable (barre métallique par exemple); dans d'autres, elle fait fonction de cathode (graphite par exemple). Ces cellules de laboratoire se prêtent mal à une utilisation industrielle, notamment en continu, car d'une part elles nécessitent un renouvellement fréquent et peu commode de l'anode et d'autre part la distance entre les 2 électrodes varie au cours du temps.These cells are without diaphragm and generally have a coaxial cylindrical symmetry. In some cases the central electrode acts as a consumable anode (metal bar for example); in others, it acts as a cathode (graphite for example). These laboratory cells do not lend themselves to industrial use, in particular continuously, because on the one hand they require frequent and inconvenient renewal of the anode and on the other hand the distance between the 2 electrodes varies over time.
La présente invention a pour but de fournir une cellule d'électrolyse permettant une utilisation industrielle en continu simple, ayant les avantages des cellules industrielles précitées à savoir notamment le maintien d'un écart constant entre les électrodes, sans en avoir les inconvénients.The present invention aims to provide an electrolysis cell allowing simple continuous industrial use, having the advantages of the above-mentioned industrial cells, namely in particular maintaining a constant gap between the electrodes, without having the disadvantages.
La cellule d'électrolyse selon l'invention, pour l'électrosynthèse en milieu organique, de composés organiques ou organométalliques, comportant deux électrodes dont seule l'une est consommée au cours de l'électrosynthèse par la réaction électrochimique dont elle est le siège, est caractérisée en ce que:
- - l'électrode consommable est constituée d'au moins un bloc métallique massif et s'applique, sous l'effet de son propre poids, contre l'autre électrode dont elle est séparée par un matériau isolant électrique laissant passer la solution d'électrolyse et dont la forme et les dimensions permettent aux surfaces actives des deux électrodes de rester parallèles au cours de l'électrosynthèse,
- -et en ce que la surface active de l'électrode non consommable est conique et présente en tous ses points une inclinaison inférieure à 45 degrés par rapport à la verticale.
- - the consumable electrode consists of at least one solid metal block and is applied, under the effect of its own weight, against the other electrode from which it is separated by an electrical insulating material allowing the electrolysis solution to pass and whose shape and dimensions allow the active surfaces of the two electrodes to remain parallel during electrosynthesis,
- -and in that the active surface of the non-consumable electrode is conical and has at all points an inclination less than 45 degrees relative to the vertical.
L'inclinaison, en un point d'une surface, par rapport à la verticale est classiquement considérée comme étant l'angle formé par le plan de tangence à la surface en ce point et par la verticale passant par ce point.The inclination, at a point of a surface, with respect to the vertical is conventionally considered to be the angle formed by the plane of tangency to the surface at this point and by the vertical passing through this point.
La cellule selon l'invention présente de nombreux avantages. Elle permet tout d'abord de maintenir un écart constant et de préférence faible (inférieur à 5 mm) entre les deux électrodes pendant toute la durée de l'électrolyse, ce qui est très important en milieu organique peu conducteur, afin d'éviter une consommation électrique et un échauffement par effet Joule excessifs.The cell according to the invention has many advantages. First of all, it allows a constant and preferably small gap (less than 5 mm) to be maintained between the two electrodes during the entire duration of the electrolysis, which is very important in an organic conductive medium, in order to avoid excessive power consumption and overheating by Joule effect.
Une des deux électrodes étant progressivement consommée lors de la réaction électrochimique, il faut nécessairement un moyen permettant de maintenir constant la distance entre les deux électrodes, ce qui est obtenu dans le cadre de cette invention, grâce à la conception et à la géométrie particulières de la cellule. Il faut de plus pouvoir remplacer aisément l'électrode consommable dès qu'elle est complètement consommée, ou mieux, pour les procédés en continu, au fur et à mesure de sa consommation, sans arrêter et perturber l'électrolyse.One of the two electrodes being progressively consumed during the electrochemical reaction, a means is necessary necessarily allowing the distance between the two electrodes to be kept constant, which is obtained in the context of this invention, thanks to the particular design and geometry of the cell. It is also necessary to be able to easily replace the consumable electrode as soon as it is completely consumed, or better, for continuous processes, as it is consumed, without stopping and disturbing the electrolysis.
La cellule selon l'invention permet un remplacement très facile de l'électrode consommable, sans arrêt de l'électrolyse, par superposition d'un (ou plusieurs) autre bloc sur le (ou les) bloc métallique massif constituant l'électrode consommable, ce qui est un avantage considérable lors de la mise en oeuvre de procédés en continu. Par ailleurs toute l'électrode est consommée, sans chute ni perte. La cellule selon l'invention permet aussi d'utiliser des électrodes consommables massives, donc peu volumineuses pour une masse donnée, et de formes variées. Ce fait est économiquement très intéressant.The cell according to the invention allows very easy replacement of the consumable electrode, without stopping the electrolysis, by superposition of one (or more) another block on the solid metal block (s) constituting the consumable electrode, which is a considerable advantage when implementing continuous processes. In addition, the entire electrode is consumed, without falling or loss. The cell according to the invention also allows the use of massive consumable electrodes, therefore not very bulky for a given mass, and of various shapes. This fact is economically very interesting.
Un autre avantage est le fait que, compte tenu de la géométrie de la cellule et notamment de l'inclinaison de l'électrode non consommable, l'encombrement au sol est très réduit ce qui permet un gain de place éconimiquement fort appréciable.Another advantage is the fact that, taking into account the geometry of the cell and in particular the inclination of the non-consumable electrode, the space requirement on the ground is very reduced, which allows a saving of space which is economically very appreciable.
Dans le nombreux cas l'électrode consommable est l'anode (oxydation anodique) comme pour les exemples qui vont suivre mais parfois l'électrode consommable est la cathode comme c'est le cas pour l'électrosynthèse du plomb tétraméthyle en milieu acétonitrile à partir de bromure de méthyle avec cathode en plomb selon HE. Ulery JECS 116, 1201, 1969:
L'électrode consommable est constituée d'au moins un bloc métallique massif. De façon préférée le métal est choisi dans le groupe constitué par le magnésium, l'aluminium, le zinc et leurs alliages, à savoir tout alliage contenant au moins un des trois métaux précités. De nombreux autres métaux conviennent également, comme notamment le cuivre, le nickel, et le plomb. Le choix du métal dépend entre autres du composé que l'on veut synthétiser. Dans le cas d'électrosynthèse de dérivés organométalliques l'électrode consommable est par exemple constituée par le métal correspondant ou un alliage à base de ce métal.The consumable electrode consists of at least one solid metal block. Preferably, the metal is chosen from the group consisting of magnesium, aluminum, zinc and their alloys, namely any alloy containing at least one of the three aforementioned metals. Many other metals are also suitable, such as copper, nickel, and lead. The choice of metal depends inter alia on the compound which one wants to synthesize. In the case of electrosynthesis of organometallic derivatives, the consumable electrode is for example constituted by the corresponding metal or an alloy based on this metal.
Dans le cas de l'électrosynthèse d'acides carboxyliques par réduction d'halogénures organiques en présence de C02 on préférera le magnésium. Pour l'électrosynthèse d'alcools par réduction électrochimique d'halogénures organiques en présence de dérivés carboxylés ainsi que pour l'électrosynthèse de cétones et d'aldéhydes par réduction électrochimique d'halogénures organiques en présence d'anhydrides d'acides organiques on préférera un métal choisi dans le groupe formé par le magnésium, le zinc, l'aluminium et leurs alliages.In the case of electrosynthesis of carboxylic acids by reduction of organic halides in the presence of C0 2, magnesium is preferred. For the electrosynthesis of alcohols by electrochemical reduction of organic halides in the presence of carboxylated derivatives as well as for the electrosynthesis of ketones and aldehydes by electrochemical reduction of organic halides in the presence of organic acid anhydrides, a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys.
Les blocs métalliques massifs peuvent être par exemple des lingots de coulée dont la section droite est carrée, ou rectangulaire, ou trapézoïdale, ou circulaire, ou sous toute autre forme. Ils peuvent éventuellement être usinés avant utilisation de façon à ce que leur géométrie soit adaptée à celle de l'électrode non consommable. De façon préférée, mais sans que cela ait un caractère impératif, on effectue un tel usinage pour faciliter le démarrage de l'électrolyse.The solid metal blocks can be, for example, ingots whose cross section is square, or rectangular, or trapezoidal, or circular, or in any other form. They can optionally be machined before use so that their geometry is adapted to that of the non-consumable electrode. Preferably, but without this having an imperative character, such machining is carried out to facilitate the start of electrolysis.
Selon une variante préférée, l'électrode consommable est constituée de blocs métalliques massifs empilés, chaque couche de l'empilement ne comprenant qu'un seul bloc. Selon une autre variante, au moins une couche de l'empilement comprend plusieurs blocs disposés côte à côte.According to a preferred variant, the consumable electrode consists of solid metal blocks stacked, each layer of the stack comprising only one block. According to another variant, at least one layer of the stack comprises several blocks arranged side by side.
L'électrode consommable s'applique sous l'effet de son propre poids, par gravité, contre l'autre électrode, non consommable. Selon une variante préférée, l'électrode consommable s'applique contre l'autre électrode sous le seul effet de son propre poids. Selon une autre variante, l'électrode consommable s'applique contre l'autre électrode sous l'effet, outre de son propre poids, de celui d'une charge inerte reposant sur l'électrode consommable. De préférence, la charge inerte est conductrice de l'électricité et sert également à assurer l'alimentation électrique de l'électrode consommable.The consumable electrode is applied under the effect of its own weight, by gravity, against the other non-consumable electrode. According to a preferred variant, the consumable electrode is applied against the other electrode under the sole effect of its own weight. According to another variant, the consumable electrode is applied against the other electrode under the effect, in addition to its own weight, of that of an inert charge resting on the consumable electrode. Preferably, the inert charge is electrically conductive and also serves to ensure the electrical supply of the consumable electrode.
Selon une autre variante, l'électrode consommable s'applique contre l'autre électrode sous l'effet, outre de son propre poids, de la force produite par un ressort comprimé entre la partie supérieure de l'électrode consommable et une paroi de la cellule.According to another variant, the consumable electrode is applied against the other electrode under the effect, in addition to its own weight, of the force produced by a compressed spring between the upper part of the consumable electrode and a wall of the cell.
L'électrode non consommable est réalisée en un matériau conducteur. De façon non limitative on peut citer les métaux tels que le fer, l'aluminium et le nickel, les alliages tel que l'acier inoxydable, les oxydes métalliques tels que Pb02 et Ni02, le graphite. De façon préférée elle est en un métal choisi dans le groupe constitué par le nickel et l'acier inoxydable.The non-consumable electrode is made of a conductive material. Without limitation, mention may be made of metals such as iron, aluminum and nickel, alloys such as stainless steel, metal oxides such as Pb0 2 and Ni0 2 , graphite. Preferably, it is made of a metal chosen from the group consisting of nickel and stainless steel.
De façon préférée, la distance entre les surfaces actives des deux électrodes est inférieure à 5 mm. Cette distance est classiquement mesurée sur une perpendiculaire commune, entre les deux surfaces parallèles;Preferably, the distance between the active surfaces of the two electrodes is less than 5 mm. This distance is conventionally measured on a common perpendicular, between the two parallel surfaces;
Les deux électrodes sont séparées par un matériau isolant électrique laissant passer la solution d'électrolyse et dont la forme et les dimensions permettent aux surfaces actives des 2 électrodes de rester parallèles au cours de l'électrosynthèse. Bien entendu, ce matériau isolant électrique doit avoir une résistance mécanique suffisante pour supporter l'électrode consommable qui repose sur ce matériau.The two electrodes are separated by an electrical insulating material allowing the electrolysis solution to pass and whose shape and dimensions allow the active surfaces of the 2 electrodes to remain parallel during electrosynthesis. Of course, this electrical insulating material must have sufficient mechanical strength to support the consumable electrode which rests on this material.
De façon préférée, le matériau isolant électrique est une matière plastique en forme de grillage dont l'épaisseur est inférieure à 5 mm et dont le maillage est constitué de deux réseaux de fils parallèles, ces deux réseaux étant superposés, croisés, fixés l'un sur l'autre aux points de contact des fils, l'épaisseur des fils de chaque réseau étant la même. En général les deux réseaux sont fixés l'un sur l'autre par soudure et les fils des deux réseaux ont la même épaisseur.Preferably, the electrical insulating material is a plastic material in the form of a grid whose thickness is less than 5 mm and whose mesh consists of two networks of parallel wires, these two networks being superimposed, crossed, fixed one on the other at the contact points of the wires, the thickness of the wires of each network being the same. In general, the two networks are fixed to each other by welding and the wires of the two networks have the same thickness.
A titre indicatif, la distance entre les fils de chaque réseau est comprise entre quelques millimètres et quelques centimètres.As an indication, the distance between the wires of each network is between a few millimeters and a few centimeters.
Les fils de chaque réseau peuvent ne pas être parallèles; leur épaisseur peut ne pas être constante pourvu qu'après assemblage des réseaux, le grillage présente une épaisseur maximale constante en plusieurs points, inférieure à environ 5 mm.The wires in each network may not be parallel; their thickness may not be constant provided that after assembling the networks, the mesh has a constant maximum thickness at several points, less than about 5 mm.
La section des fils peut être quelconque, par exemple carrée, rectangulaire, circulaire, elliptique, trapézoïdale.The cross section of the wires can be arbitrary, for example square, rectangular, circular, elliptical, trapezoidal.
La matière plastique peut être par exemple en polypropylène, en polyéthylène ou en polytétrafluoroéthylène.The plastic material can be, for example, polypropylene, polyethylene or polytetrafluoroethylene.
De tels grillages en matière plastique présentent d'une part un taux de vide élevé, ce qui permet une circulation aisée de la solution d'électrolyse entre les deux électrodes et d'autre part une relativement faible surface de contact avec les électrodes, ce qui évite une trop forte diminution de leur surface active.Such plastic gratings have on the one hand a high vacuum rate, which allows easy circulation of the electrolysis solution between the two electrodes and on the other hand a relatively small contact surface with the electrodes, which avoids an excessive reduction in their active surface.
Comme autres matériaux séparant les deux électrodes, on peut utiliser, dans le cadre de la présente invention, un tissu, une toile ou un matériau poreux d'épaisseur constante comme par exemple une céramique ou un feutre.As other materials separating the two electrodes, it is possible to use, within the framework of the present invention, a fabric, a canvas or a porous material of constant thickness such as for example a ceramic or a felt.
Le renouvellement de la solution d'électrolyse entre les électrodes peut être, par exemple, assuré par agitation mécanique ou par circulation forçée à l'aide d'une pompe, par exemple.The renewal of the electrolysis solution between the electrodes can, for example, be ensured by mechanical agitation or by forced circulation using a pump, for example.
En cours d'électrolyse, la surface active de l'électrode consommable en regard de la surface active de l'autre électrode se dissout. L'électrode consommable descend donc progressivement, par gravité, sous le simple effet de son propre poids. Par ailleurs, la dissolution étant plus forte aux endroits les plus proches de l'électrode non consommable, l'électrode consommable a tendance à épouser au mieux la forme de l'électrode non consommable, ce qui limite les risques de dissolution irrégulière.During electrolysis, the active surface of the consumable electrode opposite the active surface of the other electrode dissolves. The consumable electrode therefore descends gradually, by gravity, under the simple effect of its own weight. Furthermore, the dissolution being stronger at the locations closest to the non-consumable electrode, the consumable electrode tends to conform as best as possible to the shape of the non-consumable electrode, which limits the risks of irregular dissolution.
La description suivante d'un mode particulier de réalisation de l'invention illustre l'invention, sans la limiter.
- - La figure 1 représente une vue de face d'un mode de réalisation d'une cellule d'électrolyse selon l'invention,
- - La figure 2 représente, en coupe droite selon IV-IV, la cellule représentée figure 1.
- - La figure 3 représente une vue en perspective d'une matière plastique en forme de grillage utilisable comme matériau isolant électrique entre les deux électrodes, et
- - La figure 4 représente un schéma synoptique d'une installation complète d'électrolyse.
- FIG. 1 represents a front view of an embodiment of an electrolysis cell according to the invention,
- - Figure 2 shows, in cross section along IV-IV, the cell shown in Figure 1.
- FIG. 3 represents a perspective view of a plastic material in the form of a grid usable as an electrical insulating material between the two electrodes, and
- - Figure 4 shows a block diagram of a complete electrolysis installation.
La cellule d'électrolyse représentée figures 1 et 2 comprend une cuve dont la paroi inférieure est constituée par la cathode 12, non consommable. La surface active de l'électrode non consommable 12 est conique, la pointe du cône étant dirigée vers le bas. Cette surface active présente en tous ses points une inclinaison constante de 15 degrés par rapport à la direction 19 qui est celle de l'axe du cône. Pour la cellule représentée figures 1 et 2 cet axe est vertical.The electrolysis cell shown in Figures 1 and 2 comprises a tank whose bottom wall is formed by the
La paroi supérieure 21 de la cuve est cylindrique et prolonge le cône de façon telle que le cylindre et le cône ont le même axe, le diamètre du cylindre étant le même que celui du cercle de base du cône.The
Une paroi horizontale 20 circulaire, de diamètre égal à celui du cylindre, ferme la cuve à sa partie supérieure.A horizontal
Les parois 20 et 21 sont en un matériau isolant électrique ou intérieurement recouvertes d'un isolant électrique 11 par exemple une peinture ou tout autre revêtement électriquement isolant.The
L'anode 14 est constituée d'un empilement de lingots métalliques massifs cylindriques dont le diamètre est légèrement inférieur à celui de la paroi cylindrique 21 de la cuve. Elle s'applique sous le seul effet de son propre poids contre la cathode 12 qui, seule, assure le maintien de l'anode 14.The
Toute droite de direction 19 passant par un point quelconque de l'anode 14 consommable traverse la surface active de la cathode 12 non consommable.Any
L'anode 14 et la cathode 12 sont séparées par une matière plastique 15 en forme de grillage tel que celui représenté figure 3. Le grillage est constitué de deux réseaux de fils parallèles A1B1C1...N1 d'une part et A2 82 CZ...N2 d'autre part. Les fils de ces deux réseaux sont cylindriques, de diamètre 1 mm. La distance entre les fils est de 1 cm. Les deux réseaux sont surposés, croisés à angle droit et soudés aux points de contact des fils.The
La solution 16 d'électrolyse circule de bas en haut dans la cellule. Des canalisations 18 permettent l'arrivée et la sortie de cette solution 16, dans le sens des flèches 17.The
La canalisation d'arrivée prolonge la pointe de la cathode 12 selon l'axe de la cellule. Les électrodes 12 et 14 sont alimentées en courant électrique par une source de tension continue, non représentée aux figures 1 et 2.The inlet pipe extends the tip of the
Lorsque l'on fait pivoter l'axe de la cellule d'un angle alpha autour de la pointe du cône, la surface active de l'électrode non consommable 12 présente toujours en tous ses points une inclinaison constante de 15 degrés par rapport à la direction D représentée par l'axe de la cellule et toute droite de direction D passant par un point quelconque de l'anode 14 consommable traverse la surface active de la cathode 12 non consommable. Tout d'abord alpha doit être inférieur à 45 degrés dans le cadre de la présente invention. Par ailleurs, par rapport à la verticale, l'inclinaison de la surface active de l'électrode non consommable 12 est comprise entre (15+alpha) et 115-alphal.When the axis of the cell is rotated by an alpha angle around the tip of the cone, the active surface of the
Dans le cadre de la présente invention l'inclinaison par rapport à la verticale doit être inférieure à 45 degrés, c'est-à-dire pour ce mode particulier de réalisation, que alpha doit être inférieur à 30 degrés. Dans le cas contraire, on peut constater des anomalies importantes de fonctionnement de la cellule.In the context of the present invention, the inclination relative to the vertical must be less than 45 degrees, that is to say for this particular embodiment, that alpha must be less than 30 degrees. Otherwise, there may be significant anomalies in the functioning of the cell.
La paroi supérieure 20 de la cellule d'électrolyse selon l'invention est amovible ou présente une partie amovible de façon à permettre l'introduction des blocs métalliques massifs.The
Une installation complète permettant l'électrolyse en continu d'une solution est schématiquement représentée à la figure 4.A complete installation allowing the continuous electrolysis of a solution is schematically represented in FIG. 4.
Elle est constituée d'un circuit fermé comprenant un réacteur 51 à double enveloppe permettant le chargement et la récupération des produits, une cellule d'électrolyse 52 et une pompe 53 permettant la circulation de la solution d'électrolyse dans le circuit. La partie inférieure du réacteur 51 est reliée à la partie inférieure (entrée) de la cellule 52 et la sortie de la cellule 52 est reliée à la partie supérieure du réacteur 51.It consists of a closed circuit comprising a jacketed
Le réacteur 51 à double enveloppe est refroidi par une circulation d'eau, symbolisée par les flèches 54.The jacketed
Le sens de circulation de la solution d'électrolyse précédemment défini est symbolisé par les flèches 55.The direction of circulation of the electrolysis solution previously defined is symbolized by the
La cellule 52 schématisée figure 4 est celle représentée aux figures 1 et 2.The
La présente invention est également relative à l'utilisation des nouvelles cellules d'électrolyse précédemment décrites, pourvues d'une anode consommable en un métal choisi dans le groupe formé par le magnésium, le zinc, l'aluminium et leurs alliages pour l'électrosynthèse en milieu solvant organique de composés organiques choisis dans le groupe constitué par les acides carboxyliques, les alcools, les cétones et les aldéhydes par réduction électrochimique d'halogénures organiques.The present invention also relates to the use of the new electrolysis cells described above, provided with an anode consumable in a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys for electrosynthesis. in an organic solvent medium of organic compounds chosen from the group consisting of carboxylic acids, alcohols, ketones and aldehydes by electrochemical reduction of organic halides.
Selon une première variante, on utilise une cellule d'électrolyse pourvue d'une anode consommable en un métal choisi dans le groupe formé par le magnésium et ses alliages pour l'électrosynthèse d'acides carboxyliques par réduction électrochimique d'halogénures organiques en présence de gaz carbonique.According to a first variant, an electrolysis cell is used provided with an anode consumable in a metal chosen from the group formed by magnesium and its alloys for the electrosynthesis of carboxylic acids by electrochemical reduction of organic halides in the presence of carbon dioxide.
On a constaté que de façon totalement inattendue, on obtient des rendements très élevés avec peu ou pas de sous-produits alors que la mise en oeuvre est très simple et ne fait pas appel à un ou des catalyseurs. Cette utilisation particulière s'applique à l'électrosynthèse de très nombreux acides carboxyliques, aussi bien aliphatiques qu'aromatiques. Comme chaîne aliphatique, on peut par exemple citer les chaînes alkyles ou cycloalkyles, substituées ou non, insaturées ou non, comportant de 1 à 21 atomes de carbone.It has been found that, completely unexpectedly, very high yields are obtained with little or no by-products, while the implementation is very simple and does not require one or more catalysts. This particular use applies to the electrosynthesis of a very large number of carboxylic acids, both aliphatic and aromatic. As the aliphatic chain, mention may, for example, be made of alkyl or cycloalkyl chains, substituted or not, unsaturated or not, containing from 1 to 21 carbon atoms.
Comme chaînes aromatiques, on peut par exemple citer les noyaux phényle, thiophène, furanne et pyridine, substitués ou non substitués. Le groupement carboxyle peut aussi bien être relié à un carbone aliphatique qu'à un carbone d'un cycle aromatique.As aromatic chains, mention may, for example, be made of substituted or unsubstituted phenyl, thiophene, furan and pyridine rings. The carboxyl group can as well be linked to an aliphatic carbon as to a carbon of an aromatic ring.
L'utilisation d'une anode en magnésium permet d'obtenir les meilleurs résultats. En particulier des essais ont été réalisés avec une anode soit en aluminium soit en zinc, toutes autres conditions identiques par ailleurs. Les rendements sont alors inférieurs à ceux obtenus avec l'anode en magnésium. Les solvants organiques utilisés sont les solvants peu protiques usuellement utilisés en électrochimie organique, comme l'hexaméthylphosphorotriamide (HMPT) letétrahydrofuranne (THF), la N-méthylpyrolidone (NMP), le diméthylformamide (DMF).The use of a magnesium anode provides the best results. In particular, tests have been carried out with an anode either of aluminum or of zinc, all other conditions identical elsewhere. The yields are then lower than those obtained with the magnesium anode. The organic solvents used are the low protein solvents usually used in organic electrochemistry, such as hexamethylphosphorotriamide (HMPT) letetrahydrofuran (THF), N-methylpyrolidone (NMP), dimethylformamide (DMF).
Le solvant organique contient classiquement un électrolyte indifférent comme le tétrafluoroborate de tétrabutylammonium (BF4NBu4) ou le perchlorate de lithium.The organic solvent conventionally contains an indifferent electrolyte such as tetrabutylammonium tetrafluoroborate (BF 4 NBu 4 ) or lithium perchlorate.
Les rendements obtenus en carboxylate formé sont élevés, très souvent supérieurs à 99%. Les rendements en acide carboxylique isolé varient de 70 à 90% du rendement en carboxylate formé.The yields obtained in the carboxylate formed are high, very often greater than 99%. The yields of isolated carboxylic acid vary from 70 to 90% of the yield of carboxylate formed.
Selon une deuxième variante, on utilise une cellule d'électrolyse pourvue d'une anode consommable en un métal choisi dans le groupe formé par le magnésium, le zinc, l'aluminium et leurs alliages pour l'électrosynthèse d'alcools, par réduction électrochimique d'halogénures organiques présentant un atome ou un groupement fonctionnel stabilisateur de carbanions fixé au carbone porteur de l'halogène, en présence de dérivés carbonylés.According to a second variant, an electrolysis cell is used provided with an anode consumable in a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys for the electrosynthesis of alcohols, by electrochemical reduction. of organic halides having an atom or a functional group stabilizing carbanions attached to the carbon carrying the halogen, in the presence of carbonyl derivatives.
Ces derniers peuvent aussi bien être des aldéhydes que des cétones; les rendements sont élevés et la mise en oeuvre relativement simple.The latter can be aldehydes as well as ketones; the yields are high and the implementation relatively simple.
Les halogénures organiques présentent au moins un atome ou un groupement fonctionnel stabilisateur de carbanions, fixé au carbone porteur de l'halogène c'est-à-dire situé en position alpha par rapport à l'halogène.The organic halides have at least one atom or a functional group stabilizing carbanions, attached to the carbon carrying the halogen, that is to say located in the alpha position relative to the halogen.
Les atomes et groupements fonctionnels qui stabilisent les carbanions sont bien connus de l'homme de métier. On peut citer par exemple les halogènes, les groupements esters, cétoniques, allyliques, benzéniques, alcoxy, nitrile.The atoms and functional groups which stabilize carbanions are well known to those skilled in the art. Mention may be made, for example, of halogens, ester, ketone, allyl, benzene, alkoxy or nitrile groups.
De façon préférée, les halogénures organiques utilisables dans le cadre de la présente invention répondent à la formule générale RX dans laquelle X représente un atome d'halogène et R représente:
- - un groupement benzylique, substitué ou non substitué
- - un groupement allylique, substitué ou non substitué
- - un groupement alpha monohalogéné (-C-X), alpha dihalogéné
- - un groupement alpha ester
- - un groupement alpha cétonique
- - a benzyl group, substituted or unsubstituted
- - an allylic group, substituted or unsubstituted
- - an alpha monohalogenated group (-CX), alpha dihalogenated
- - an alpha ester group
- - an alpha ketone group
A titre illustratif et non limitatif, on peut citer par exemple le chlorure de benzyle, le bromure de benzyle, le chlorure d'allyle, le 3-chloro 2 méthyl propène, le 3-chloro 1 butène, le 1-chloro 1-méthyl acétate d'éthyle, le tétrachlorure de carbone, le dichlorophényl-méthane, le 1-phényl 3-chloro propène et le 1-méthyl 3-chloropropène.By way of illustration and without limitation, mention may be made, for example, of benzyl chloride, benzyl bromide, allyl chloride, 3-chloro 2 methyl propene, 3-chloro 1 butene, 1-chloro 1-methyl ethyl acetate, carbon tetrachloride, dichlorophenylmethane, 1-phenyl 3-chloro propene and 1-methyl 3-chloropropene.
Selon un mode particulier de réalisation les dérivés carbonylés répondent à la formule générale
- - un atome d'hydrogène,
- - une chaîne aliphatique ou cycloaliphatique, substituée ou non substituée, saturée ou non saturée,
- - un groupement aryle, substitué ou non substitué,
- - a hydrogen atom,
- - an aliphatic or cycloaliphatic chain, substituted or unsubstituted, saturated or unsaturated,
- - an aryl group, substituted or unsubstituted,
ou bien encore, R, et R2, conjointement avec l'atome de carbone auxquels il sont attachés, forment un cycle, saturé ou non saturé, substitué ou non substitué, comportant éventuellement un ou plusieurs hétéroatomes comme l'azote, l'oxygène, le phosphore ou le soufre. A titre illustratif et non limitatif, on peut citer par exemple l'acétone, la cyclohexanone, la méthyléthylcétone, l'acétaldehyde, la benzophénone et la dichlorobenzophénone.or alternatively, R, and R 2 , together with the carbon atom to which they are attached, form a ring, saturated or unsaturated, substituted or unsubstituted, optionally comprising one or more heteroatoms such as nitrogen, oxygen , phosphorus or sulfur. By way of illustration and without limitation, mention may, for example, be made of acetone, cyclohexanone, methyl ethyl ketone, acetaldehyde, benzophenone and dichlorobenzophenone.
Selon une variante préférée, les alcools obtenus selon le procédé objet de la présente invention répondent à la formule générale
De façon particulièrement préférée, lorsque les dérivés carbonylés sont des cétones, c'est-à-dire lorsque R, et R2 sont différents de l'hydrogène, on obtient des alcools tertiaires.Particularly preferably, when the carbonyl derivatives are ketones, that is to say when R 1 and R 2 are different from hydrogen, tertiary alcohols are obtained.
En règle générale, pour réaliser la présente invention, il est bien évident pour l'homme de métier que le dérivé carbonylé doit être plus difficilement réductible que l'halogénure organique et aucun des substituants portés par R, et R2 ne doit être plus électrophile que le groupement carbonylé lui-même.In general, to carry out the present invention, it is quite obvious to the skilled person that the carbonyl derivative must be more difficult to reduce than the organic halide and none of the substituents carried by R, and R 2 must not be more electrophilic. than the carbonyl group itself.
Les solvants organiques et les électrolytes indifférents utilisés sont les mêmes que ceux précités pour la synthèse d'acides carboxyliques. De façon préférée on utilise le DMF comme solvant et l'électrolyse est conduite à une température comprise entre -20°C et +30°C.The organic solvents and the indifferent electrolytes used are the same as those mentioned above for the synthesis of carboxylic acids. Preferably, DMF is used as solvent and the electrolysis is carried out at a temperature between -20 ° C. and + 30 ° C.
Selon une troisième variante, on utilise une cellule d'électrolyse pourvue d'une anode consommable en un métal choisi dans le groupe formé par le magnésium, le zinc, l'aluminium et leurs alliages pour l'électrosynthèse de cétones et d'aldéhydes par réduction électrochimique d'halogénures organiques en présence d'anhydrides d'acides organiques. La mise en oeuvre est simple et les rendements massique et faradique élevés.According to a third variant, an electrolysis cell is used provided with an anode consumable in a metal chosen from the group formed by magnesium, zinc, aluminum and their alloys for the electrosynthesis of ketones and aldehydes by electrochemical reduction of organic halides in the presence of organic acid anhydrides. The implementation is simple and the mass and faradaic yields high.
Selon un mode particulier de réalisation, les halogénures organiques répondent à la formule générale R3X dans laquelle X représente un halogène choisi dans le groupe constitué par le chlore, le brome et l'iode et R3 représente:
- - une chaîne aliphatique ou cycloaliphatique, substituée ou non substituée, saturée ou non saturée,
- - un groupement aryle, substitué ou non substitué,
- - un hétérocycle aromatique, substitué ou non substitué, comme par exemple le cycle thiophène, furanne ou pyridine.
- - an aliphatic or cycloaliphatic chain, substituted or unsubstituted, saturated or unsaturated,
- - an aryl group, substituted or unsubstituted,
- - an aromatic heterocycle, substituted or unsubstituted, such as for example the thiophene, furan or pyridine ring.
De façon préférée, R3 eprésente une chaîne aliphatique substituée par au moins un groupement aromatique comme par exemple dans le chlorure de benzyle, le bromure de benzyle, le 1-phényl 1-chloro éthane et l 1-phényl 1-chloro propane.Preferably, R 3 has an aliphatic chain substituted with at least one aromatic group, for example in benzyl chloride, benzyl bromide, 1-phenyl 1-chloro ethane and 1-phenyl 1-chloro propane.
De façon générale R3 peut être porteur de fonctions non électroréductibles ou plus difficilement réductibles que la liaison R3-X, dans les conditions expérimentales de l'électrosynthèse. De telles fonctions non électroréductibles sont par exemple les fonctions cyano, éther, sulfure ou ester.In general, R 3 can carry non-electro-reducible functions or more difficult to reduce than the R 3 -X bond, under the experimental conditions of electrosynthesis. Such non-electroreducible functions are, for example, the cyano, ether, sulfide or ester functions.
Selon un autre mode particulier de réalisation, les anhydrides d'acides organiques répondent à la formule générale dans laquelle, R4 représente:
- - un atome d'hydrogène,
- - une chaîne aliphatique ou cycloaliphatique, substituée ou non substituée, saturée ou non saturée,
- - un groupement aryle, substitué ou non substitué, ou
- - un hétérocycle aromatique, substitué ou non substitué, comme par exemple le cycle furanne, thiophène ou pyridine,
et Rs représente: - - une chaîne aliphatique ou cycloaliphatique, substituée ou non substituée, saturée ou non saturée,
- - un groupement aryle substitué ou non substitué,
- - un hétérocycle aromatique, substitué ou non substitué, comme par exemple le cycle furanne, thiophène ou pyridine, ou
- - un groupement OR6 dans lequel R6 représente:
- * une chaîne aliphatique ou cycloaliphatique, substituée ou non substituée, saturée ou non saturée,
- * un groupement aryle, substitué ou non substitué,
- * un hétérocycle aromatique, substitué ou non substitué, comme par exemple le cycle furanne, thiophène ou pyridine,
ou bien encore R4 et Rs forment au moins un cycle, substitué ou non substitué, comme c'est le cas par exemple pour l'anhydride phtalique ou l'anhydride succinique.
- - a hydrogen atom,
- - an aliphatic or cycloaliphatic chain, substituted or unsubstituted, saturated or unsaturated,
- - an aryl group, substituted or unsubstituted, or
- - an aromatic heterocycle, substituted or unsubstituted, such as for example the furan, thiophene or pyridine ring,
and R s represents: - - an aliphatic or cycloaliphatic chain, substituted or unsubstituted, saturated or unsaturated,
- - a substituted or unsubstituted aryl group,
- - an aromatic heterocycle, substituted or unsubstituted, such as for example the furan, thiophene or pyridine ring, or
- - an OR 6 group in which R 6 represents:
- * an aliphatic or cycloaliphatic chain, substituted or unsubstituted, saturated or unsaturated,
- * an aryl group, substituted or unsubstituted,
- * an aromatic heterocycle, substituted or unsubstituted, such as for example the furan, thiophene or pyridine ring,
or alternatively R 4 and R s form at least one ring, substituted or unsubstituted, as is the case for example for phthalic anhydride or succinic anhydride.
Lorsque Rs représente un groupement ORs, les anhydrides correspondants sont alors des anhydrides mixtes d'acides carboxyliques et d'acide carbonique. Dans les autres cas, ce sont des anhydres d'acides carboxyliques.When R s represents a group OR s , the corresponding anhydrides are then mixed anhydrides of carboxylic acids and carbonic acid. In the other cases, they are anhydrous carboxylic acids.
Lorsque R4 représente un atome d'hydrogène, on obtient des aldéhydes. Dans ce cas, lorsque les halogénures organiques répondent à la formule générale R3X précédemment définie, les aldéhydes obtenus répondent à la formule générale R3CHO. Dans les autres cas, lorsque R4 ne représente pas un atome d'hydrogène, on obtient des cétones. Ces cétones répondent à la formule générale
De façon générale, R4 et R5 peuvent être porteurs de fonctions non électroréductibles, ou plus difficilement réductibles que la liaison R3―X, dans les conditions expérimentales de l'électrosynthèse, et aucune des fonctions portées par R3 ou R4 ne doit être plus électrophile que la fonction anhydride elle- mème.In general, R 4 and R 5 can carry non-electro-reducible functions, or more difficult to reduce than the bond R 3― X, under the experimental conditions of electrosynthesis, and none of the functions carried by R 3 or R 4 does must be more electrophilic than the anhydride function itself.
De façon préférée, R4 et R5 représentent une chaîne alkyle linéaire ou ramifiée.Preferably, R 4 and R 5 represent a linear or branched alkyl chain.
De façon également préférée, R4 et Rs sont identiques.Also preferably, R 4 and R s are identical.
De façon particulièrement préférée, R4 et Rs sont identiques et représentent une chaîne alkyle, linéaire ou ramifiée, comme c'est le cas par exemple pour l'anhydride acétique.In a particularly preferred manner, R 4 and R s are identical and represent an alkyl chain, linear or branched, as is the case for example for acetic anhydride.
Les solvants organiques et les électrolytes indifférents utilisés sont les mêmes que ceux précités pour la synthèse d'acides carboxyliques. De façon préférée, on utilise le DMF comme solvant.The organic solvents and the indifferent electrolytes used are the same as those mentioned above for the synthesis of carboxylic acids. Preferably, DMF is used as solvent.
En règle générale, lors de l'utilisation d'une cellule d'électrolyse selon l'invention pour l'électrosynthèse, en milieu organique, de composés organiques ou organométalliques et notamment pour l'électrosynthèse des dérivés organiques précités, la direction D est de façon préférée la direction verticale. Les exemples suivants illustrent l'invention sans la limiter.As a general rule, when using an electrolysis cell according to the invention for electrosynthesis, in organic medium, of organic or organometallic compounds and in particular for the electrosynthesis of the abovementioned organic derivatives, the direction D is to preferably the vertical direction. The following examples illustrate the invention without limiting it.
On utilise une cellule d'électrolyse telle que celle représentée aux figures 1 et 2. La cathode 12, en acier inoxydable, est un cône de hauteur 100 mm et de diamètre de base 53 mm. Les autres parois de la cuve sont en acier inoxydable et sont intérieurement recouvertes d'un revêtement 11 inerte et isolant électrique.An electrolysis cell such as that shown in FIGS. 1 and 2 is used. The
L'anode 14 est constituée d'un empilement de blocs cylindriques en aluminium de diamètre 50 mm et de hauteur 100 mm.The
La matière plastique 15 en forme de grillage est un polypropylène. Ce grillage est juste posé sur la surface active de la cathode 12 dont il épouse la forme, avant l'introduction de l'anode 14.The
L'installation complète est telle que celle schématisée à la figure 4.The complete installation is as shown in Figure 4.
Pour la première électrolyse, on a usiné le bloc inférieur d'aluminium de façon à ce qu'il se présente approximativement sous la forme d'un cône de hauteur 100 mm et de diamètre de base 50 mm, ce qui se réalise facilement à partir d'un bloc cylindrique ayant ces dimensions.For the first electrolysis, the lower aluminum block was machined so that it is approximately in the form of a cone with a height of 100 mm and a base diameter of 50 mm, which is easily achieved from of a cylindrical block having these dimensions.
Après positionnement de la matière plastique 15 en forme de grillage sur la surface active de la cathode, on introduit le bloc usiné qui épouse la forme de la cathode, puis on empile sur ce bloc inférieur plusieurs autres blocs jusqu'au sommet de la cellule.After positioning the
Après avoir mélangé dans le réacteur 200 g de chlorure de benzyle (1,58 mol), 20 g d'iodure de tétrabutylammonium, 280 g de DMF et 1500 g d'acétone, on fait circuler la solution ainsi obtenue dans l'installation.After having mixed 200 g of benzyl chloride (1.58 mol), 20 g of tetrabutylammonium iodide, 280 g of DMF and 1500 g of acetone in the reactor, the solution thus obtained is circulated in the installation.
Pour la première électrolyse, on opère tout d'abord à intensité constante de 1A. Dès que le bloc d'aluminium inférieur atteint le fond de la cellule, on maintient une intensité constante de 2,5 A. La tension d'électrolyse reste alors stable, à 15 V environ, ce qui prouve le bon fonctionnement de la cellule. Elle se stabilise très rapidement, du fait de l'usinage du premier bloc. On stoppe l'électrolyse après 42 h.For the first electrolysis, we first operate at a constant intensity of 1A. As soon as the lower aluminum block reaches the bottom of the cell, a constant intensity of 2.5 A is maintained. The electrolysis voltage then remains stable, at around 15 V, which proves the proper functioning of the cell. It stabilizes very quickly, due to the machining of the first block. The electrolysis is stopped after 42 h.
Après l'arrêt de l'électrolyse, le diméthylbenzylcarbinol formé est isolé, et identifié selon les méthodes habituelles, bien connues de l'homme de métier. L'alcool formé a été isolé après hydrolyse de la solution à l'aide d'une solution aqueuse de chlorure d'ammonium et extraction à l'éther. Après évaporation de l'éther, l'alcool brut a été purifié par distillation. L'alcool pur ainsi isolé (pureté vérifiée par CPG) est identifié par ses spectres RMN et IR.After stopping the electrolysis, the dimethylbenzylcarbinol formed is isolated, and identified according to the usual methods, well known to those skilled in the art. The alcohol formed was isolated after hydrolysis of the solution using an aqueous solution of ammonium chloride and extraction with ether. After evaporation of the ether, the crude alcohol was purified by distillation. The pure alcohol thus isolated (purity verified by CPG) is identified by its NMR and IR spectra.
Le rendement en diméthylbenzylcarbinol distillé obtenu est de 60% (pureté supérieure à 95%).The yield of distilled dimethylbenzylcarbinol obtained is 60% (purity greater than 95%).
Pour réaliser ensuite d'autres électrolyses, l'intensité du courant est dès le départ fixée à 2,5 A puisque les conditions optimales de fonctionnement sont alors déjà réunies, l'anode étant en position optimale par rapport à la cathode.To then carry out other electrolyses, the intensity of the current is fixed at the start at 2.5 A since the optimal operating conditions are then already met, the anode being in the optimal position relative to the cathode.
On réalise le même essai que celui de l'exemple 1 mais sans usiner le bloc inférieur avant la première électrolyse. Le même résultat est obtenu mais l'équilibre de fonctionnement est beaucoup plus long à atteindre.The same test is carried out as that of Example 1 but without machining the lower block before the first electrolysis. The same result is obtained but the operating equilibrium is much longer to reach.
Claims (12)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT86401895T ATE54472T1 (en) | 1985-09-05 | 1986-08-29 | ORGANIC ELECTROLYTIC CELL WITH CONSUMABLE ELECTRODE. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8513188 | 1985-09-05 | ||
| FR8513188A FR2586710B1 (en) | 1985-09-05 | 1985-09-05 | ORGANIC ELECTROLYSIS CELL WITH CONSUMABLE ELECTRODE |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0219367A1 EP0219367A1 (en) | 1987-04-22 |
| EP0219367B1 true EP0219367B1 (en) | 1990-07-11 |
Family
ID=9322650
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP86401895A Expired - Lifetime EP0219367B1 (en) | 1985-09-05 | 1986-08-29 | Organic electrolysis cell with a consumable electrode |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US4686018A (en) |
| EP (1) | EP0219367B1 (en) |
| JP (1) | JPH07122155B2 (en) |
| AT (1) | ATE54472T1 (en) |
| DE (1) | DE3672556D1 (en) |
| FR (1) | FR2586710B1 (en) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IT1203373B (en) * | 1987-03-18 | 1989-02-15 | Silvestri Silvestri | DEVICE FOR THE TRANSFORMATION OF ELECTROLYTIC CELLS OF THE FILTER TYPE PRESS IN CELLS WITH CONTINUOUS RENEWABLE SACRIFICAL ELECTRODES |
| FR2617197B1 (en) * | 1987-06-25 | 1991-07-12 | Poudres & Explosifs Ste Nale | ELECTROLYSIS CELL WITH CONSUMABLE BIPOLAR ELECTRODES |
| FR2624884B1 (en) * | 1987-12-18 | 1990-04-20 | Poudres & Explosifs Ste Nale | METHOD FOR THE ELECTROCHEMICAL SYNTHESIS OF SATURATED ALPHA KETONES |
| US4834858A (en) * | 1988-03-23 | 1989-05-30 | Montvale Process Company, Inc. | Electrolytic reactor |
| DE3813017A1 (en) * | 1988-04-19 | 1989-11-02 | Wiederaufarbeitung Von Kernbre | DEVICE FOR THE ELECTROCHEMICAL TREATMENT OF RADIOACTIVE FUEL SOLUTIONS |
| FR2639364B1 (en) * | 1988-11-23 | 1990-12-28 | Poudres & Explosifs Ste Nale | ELECTROSYNTHESIS OF ALDEHYDES |
| FR2646441B1 (en) * | 1989-04-28 | 1991-07-12 | Poudres & Explosifs Ste Nale | ELECTROSYNTHESIS OF AN ESTER BETA GAMMA UNSATURE |
| FR2688519A1 (en) * | 1992-03-12 | 1993-09-17 | Poudres & Explosifs Ste Nale | Process for electrosynthesis of symmetric fluorobiphenyls |
| HU226037B1 (en) | 1993-06-25 | 2008-03-28 | Aventis Inc | Process for producing antihistaminic 4-diphenylmethyl/diphenylmethoxy piperidine derivatives and novel intermediates |
| US6147216A (en) * | 1993-06-25 | 2000-11-14 | Merrell Pharmaceuticals Inc. | Intermediates useful for the preparation of antihistaminic piperidine derivatives |
| DE4429354A1 (en) | 1994-08-18 | 1996-02-22 | Hoechst Ag | Electrolytic cell with consumption anodes |
| US6683094B2 (en) | 1998-07-02 | 2004-01-27 | Aventis Pharmaceuticals Inc. | Antihistaminic piperidine derivatives and intermediates for the preparation thereof |
| US6700012B2 (en) | 1998-07-02 | 2004-03-02 | Aventis Pharmaceuticals Inc. | Antihistaminic piperidine derivatives and intermediates for the preparation thereof |
| EP1046616A3 (en) * | 1999-02-06 | 2001-03-21 | Vallendar, Hubertus | Arrangement of electrodes for galvanic treatment of flowing media |
| FR2795749B1 (en) * | 1999-07-02 | 2001-10-05 | Electricite De France | ELECTROCHEMICAL REACTOR WITH ROTARY CONSUMABLE ELECTRODE |
| US20080116144A1 (en) | 2006-10-10 | 2008-05-22 | Spicer Randolph, Llc | Methods and compositions for reducing chlorine demand, decreasing disinfection by-products and controlling deposits in drinking water distribution systems |
| WO2012053736A2 (en) * | 2010-10-22 | 2012-04-26 | Kim Tae Gyo | Metal ion sterilization device |
| KR101239206B1 (en) * | 2011-05-06 | 2013-03-05 | 김태규 | Water cleaning apparatus using metal ion |
| US8617403B1 (en) | 2013-06-25 | 2013-12-31 | Blue Earth Labs, Llc | Methods and stabilized compositions for reducing deposits in water systems |
| DE102016109822A1 (en) * | 2016-05-27 | 2017-11-30 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Electrolytic reactor |
| US10837116B2 (en) * | 2017-11-27 | 2020-11-17 | Fraunhofer-Gesellschaft Zur Foerderung Der Angewandten Forschung E.V. | Electrolytic reactor |
| CN113278996A (en) * | 2021-04-01 | 2021-08-20 | 安徽海康药业有限责任公司 | Preparation method of 2, 4, 5-trifluorophenylacetic acid |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US982037A (en) * | 1909-09-30 | 1911-01-17 | Mcdonald Electrolytic Company | Electrolytic cell. |
| US1278723A (en) * | 1914-08-19 | 1918-09-10 | Frank H Nickle | Electrolytic cell. |
| BE606111A (en) * | 1960-07-13 | |||
| FR1412239A (en) * | 1963-10-23 | 1965-09-24 | Chimica Dell Aniene S P A Soc | Adjustable multiple electrode electrolysis cell |
| SU1046022A1 (en) * | 1982-06-07 | 1983-10-07 | Новочеркасский Ордена Трудового Красного Знамени Политехнический Институт Им.Серго Орджоникидзе | Anode apparatus for producing metal powder |
-
1985
- 1985-09-05 FR FR8513188A patent/FR2586710B1/en not_active Expired - Lifetime
-
1986
- 1986-08-29 DE DE8686401895T patent/DE3672556D1/en not_active Expired - Lifetime
- 1986-08-29 EP EP86401895A patent/EP0219367B1/en not_active Expired - Lifetime
- 1986-08-29 AT AT86401895T patent/ATE54472T1/en not_active IP Right Cessation
- 1986-09-02 US US06/904,025 patent/US4686018A/en not_active Expired - Lifetime
- 1986-09-05 JP JP61208089A patent/JPH07122155B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| FR2586710B1 (en) | 1990-03-30 |
| JPH07122155B2 (en) | 1995-12-25 |
| FR2586710A1 (en) | 1987-03-06 |
| DE3672556D1 (en) | 1990-08-16 |
| US4686018A (en) | 1987-08-11 |
| ATE54472T1 (en) | 1990-07-15 |
| EP0219367A1 (en) | 1987-04-22 |
| JPS6256589A (en) | 1987-03-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0219367B1 (en) | Organic electrolysis cell with a consumable electrode | |
| FR2810996A1 (en) | ELECTROLYSIS PROCESS | |
| EP0277048A1 (en) | Process for the electrochemical manufacture of carboxylic acids | |
| CA1251160A (en) | Process for the production of a metal by way of halogen electrolysis in a molten salt bath, including double simultaneous and continuous deposition, and devices used in said process | |
| FR2646441A1 (en) | METHOD FOR ELECTROSYNTHESIS OF A BETA GAMMA UNSATURATED ESTER | |
| CA2254788C (en) | Process for preparing phthalides | |
| CH664979A5 (en) | PROCESS FOR THE ELECTROSYNTHESIS OF CARBOXYLIC ACIDS. | |
| FR2617197A1 (en) | Electrolysis cell with sacrificial bipolar electrodes | |
| EP0370866B1 (en) | Aldehydes electrosynthesis process | |
| FR2546910A1 (en) | PROCESS FOR THE PREPARATION OF SQUARIC ACID BY ELECTROLYTICALLY TETRAMERIZING CARBON MONOXIDE IN ANHYDROUS ALIPHATIC NITRILE AS A SOLVENT | |
| FR2579626A1 (en) | METHOD FOR ELECTROSYNTHESIS OF KETONES AND ALDEHYDES | |
| EP0201365B1 (en) | Process for the electrosynthesis of alcohols and epoxide compounds | |
| US4022672A (en) | Electrochemical synthesis of insecticide intermediates | |
| EP0230171B1 (en) | Process and apparatus for the continuous manufacture of lithium by electrolysis of lithium chloride | |
| FR2795749A1 (en) | Electrochemical reactor has large cylindrical rotating consumable electrode kept at constant distance from fixed counter electrode by resting on insulating layers on counter electrode | |
| US4659441A (en) | Process for preparing tetraalkyl 1,1,2,2-ethene-tetracarboxylate | |
| EP0003446B1 (en) | Electrochemical process for producing amino-2, ethyl-2, thiophene | |
| FR2532332A1 (en) | PROCESS FOR THE CONTINUOUS PREPARATION OF LITHIUM BY ELECTROLYSIS OF LITHIUM CHLORIDE IN A MIXTURE OF MOLTEN SALTS AND APPARATUS FOR CARRYING OUT SAID PROCESS | |
| FR2753726A1 (en) | New electrochemical reactor based on consumable anodes | |
| US5567299A (en) | Process for the electrochemical oxidation of arylketones | |
| JP4154707B2 (en) | Method for producing difluorodienone compound | |
| EP0394154B1 (en) | Apparatus for the continuous production of a multivalent metal by electrolysis of a halide thereof dissolved in a molten bath | |
| FR2656631A1 (en) | Electrochemical process for the preparation of especially aromatic chalcogen compounds, electrode used in this process and new chalcogen compounds thus obtained | |
| FR2629474A1 (en) | Process for electrosynthesis of benzyl ketones | |
| KR20010024139A (en) | Method for Producing Phthalides |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19871012 |
|
| 17Q | First examination report despatched |
Effective date: 19890508 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 54472 Country of ref document: AT Date of ref document: 19900715 Kind code of ref document: T |
|
| ITF | It: translation for a ep patent filed | ||
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) | ||
| REF | Corresponds to: |
Ref document number: 3672556 Country of ref document: DE Date of ref document: 19900816 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| ITTA | It: last paid annual fee | ||
| EPTA | Lu: last paid annual fee | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 86401895.7 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20030528 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20030710 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20030806 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 20030811 Year of fee payment: 18 Ref country code: AT Payment date: 20030811 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20030827 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20030831 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20031030 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20031104 Year of fee payment: 18 |
|
| NLT1 | Nl: modifications of names registered in virtue of documents presented to the patent office pursuant to art. 16 a, paragraph 1 |
Owner name: SNPE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040829 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040829 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040829 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040830 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040831 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040831 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040831 |
|
| BERE | Be: lapsed |
Owner name: *SNPE Effective date: 20040831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050301 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050301 |
|
| EUG | Se: european patent has lapsed | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20040829 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050429 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20050301 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050829 |
|
| BERE | Be: lapsed |
Owner name: *SNPE Effective date: 20040831 |