EP0135535B1 - Niedrige energie aufweisende aluminium-reduktionszelle mit induzierter badströmung - Google Patents
Niedrige energie aufweisende aluminium-reduktionszelle mit induzierter badströmung Download PDFInfo
- Publication number
- EP0135535B1 EP0135535B1 EP84900797A EP84900797A EP0135535B1 EP 0135535 B1 EP0135535 B1 EP 0135535B1 EP 84900797 A EP84900797 A EP 84900797A EP 84900797 A EP84900797 A EP 84900797A EP 0135535 B1 EP0135535 B1 EP 0135535B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cathode
- anode
- cell
- bath
- cell according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25C—PROCESSES FOR THE ELECTROLYTIC PRODUCTION, RECOVERY OR REFINING OF METALS; APPARATUS THEREFOR
- C25C3/00—Electrolytic production, recovery or refining of metals by electrolysis of melts
- C25C3/06—Electrolytic production, recovery or refining of metals by electrolysis of melts of aluminium
- C25C3/08—Cell construction, e.g. bottoms, walls, cathodes
Definitions
- This invention relates to a novel electrolytic cell for the manufacture of aluminum from alumina, and to the operation of such a cell.
- the invention provides an aluminum producing electrolysis cell using a bath electrolyte based on sodium cryolite, wherein the problems resulting from reduced anode to cathode gap distances achieved in previous drained cathode cells are overcome by inducing a particular manner of bath flow in the anode to cathode gap, thus facilitating alumina feeding, removal of gaseous products, and enhanced drainage of product metal. Operation of a test electrolysis cell has demonstrated the ability to provide a plentiful supply of dissolved alumina to the electrolysis zone even at very narrow anode to cathode spacings.
- a commonly utilized electrolytic cell for the manufacture of aluminum is of the classic Hall-Heroult design, utilizing carbon anodes and a substantially flat carbon-lined bottom which functions as part of the cathode system.
- An electrolyte is used in the production of aluminum by electrolytic reduction of alumina, which electrolyte consists primarily of molten cryolite with dissolved alumina, and which may contain other materials such as fluorspar, aluminum fluoride, and other metal fluoride salts.
- Molten aluminum resulting from the reduction of alumina is most frequently permitted to accumulate in the bottom of the receptacle forming the electrolytic cell, as a molten metal pad or pool over the carbon-lined bottom, thus forming a liquid metal cathode.
- Carbon anodes extending into the receptacle, and contacting the molten electrolyte, are adjusted relative to the liquid metal cathode.
- Current collector bars, such as steel, are frequently embedded in the carbon-lined cell bottom, and complete the connection to the cathodic system.
- Hall-Heroult electrolytic cells vary, all have a relatively low energy efficiency, ranging from about 35 to 45 percent, dependent upon cell geometry and mode of operation.
- the theoretical power requirement to produce one pound of aluminum is about 2.85 kilowatt hours (kWh)
- in practice power usage ranges from 6 to 8.5 kWh/lb, with an industry average of about 7.5 kWh/lb.
- a large proportion of this discrepancy from theoretical energy consumption is the result of the voltage drop of the electrolyte between the anode and cathode.
- ACD anode-cathode distance
- the molten aluminum pad which serves as the cell cathode can become irregular and variable in thickness due to electromagnetic effects and bath circulation, past practice has required that the ACD be kept at a safe 3.5 to 6 cm to ensure relatively high current efficiencies and to prevent direct shorting between the anode and the metal pad.
- Such gap distances result in voltage drops from 1.4 to 2.7 volts, which is in addition to the energy required for the electrochemical reaction itself (2.1 volts, based upon enthalpy and free energy calculations). Accordingly, much effort has been directed to developing a more stable aluminum pad, so as to reduce the ACD to less than 3.5 cm, with attendant energy savings.
- Refractory hard materials such as titanium diboride
- RHM Refractory hard materials
- titanium diboride has been under study for quite some time for use as cathode surfaces in the form of tiles, but until recently, adherent RHM tiles or surface coatings have not been available.
- Titanium diboride is known to be conductive, as well as possessing the unique characteristic of being wetted by molten aluminum, thus permitting formation of very thin aluminum films.
- the use of a very thin aluminum film draining down an inclined cathode covered with an RHM surface, to replace the unstable molten aluminum pad of the prior art, has been suggested as a means to reduce the ACD, thus improving efficiency, and reducing voltage drop.
- GB-A-2024864 describes electrolytic cells for the production of aluminum having wettable cathodes comprising individual exchangeable elements each supplied with electrical power.
- the electrolyte between the anode and cathode is subjected to a magneto-hydrodynamic pumping effect and thus led through channels in the cathode elements.
- US 4333813 describes drained cathodes for alumina reduction cells comprising a carbonaceous bed, preferably of graphite, and a composite tile. This tile is characterised by a layer of a RHM bonded to a base layer of graphitic material. The RHM layer provides a refractory surface for contacting molten aluminum in the cell.
- US 4341611 similarly describes alumina reduction cells in which a carbonaceous cathode includes RHM tiles. Intermediate tiles are provided to separate the RHM tiles from the carbonaceous cathode to prevent reaction therebetween in the presence of molten aluminum.
- a drained cathode cell for the electrolytic reduction of alumina to aluminum in a cryolite-based bath containing alumina, comprising:
- the invention provides an improved aluminum electrolysis cell, utilizing a bath electrolyte based on sodium cryolite, and having improved electrical efficiencies.
- the cell may be used in a single cell system, or in a multiple cell system, to achieve construction and operating economies.
- the cell design according to the invention generally provides a narrower ACD than the prior art, and hence improved current efficiencies and voltage drops, and which may be retrofitted to existing Hall cell installations.
- an electrolysis cell may have an ACD of less than 3 cm, while an adequate alumina supply to the anode-cathode gap is assured and without requiring overfeeding.
- a sloped solid cathode is utilized to shape the anode, which may be either Soderberg or pre-baked, which in concert with the proper choice of other parameters defined herein induces such a bath flow through the anode-cathode gap as to provide the proper alumina supply to the anode, without adverse impact on the drainage of aluminum product into a collection sump.
- gas pumping action under the anode causes the bath to flow through the cell, into a cell feeding chamber, out of the cell feeding chamber, and back to the opposite side of the cell.
- Multiple individual cells of this nature may be combined in numerous configurations to structure the system according to the physical and economic restrictions of any plant site.
- the present invention relates to an electrolytic cell in which the cathode is so arranged and inclined that the anode gases induce a plentiful and sufficient flow of enriched materials between the anode and cathode, without impeding the drainage of molten aluminum to a collection sump, or disrupting the surface of the draining aluminum so as to cause lower current efficiences.
- the electrolyte bath is induced to flow from an enriched bath zone to a zone where the bath is somewhat depleted in alumina content.
- the bath may be supplied with an automatic feed of alumina in a controlled amount, to prevent either mucking or anode effects.
- the present invention utilizes a sloped, drained cathode, having a surface of titanium diboride or other similar refractory hard material, in which the slope or inclination from the horizontal is selected so as to contribute to a controlled bath motion, which bath motion is hydrodynamically dependent upon specifically identified critical parameters.
- the bath motion is so controlled as to induce sufficient alumina-rich bath to flow into the interelectrode gap in the space immediately beneath the anode face to avoid any anode effects, while not interfering with the drainage of aluminum.
- these anode gases will drive, by a well known action also utilized in the design of gas lifts and gas jet pumps, as well as bubble columns, the bath near the anode surface in the same desired direction.
- This drive may be supplemented and enhanced (if necessary) by other pumping action, e.g. a suitably designed pump.
- the flow of a fluid system is established by a balance between the fluid drive and the resistance to flow within the components of the system, and that, dependent upon the configuration, the velocity within local regions flow may be in the same direction but may sometimes be in the direction opposite to the direction of the fluid drive. It is a principle of the present invention to so arrange the slope, or slopes, so as to achieve that balance between buoyancy-generated bubble forces from the inclination and those forces which drive bubbles sideways beneath horizontal anodes on the one hand, and the flow resistance on the other hand, to give a net motion of the bath to provide the required alumina supply.
- the local bath velocity near the anode surface is in the desired direction, i.e. the direction in which the driving gas bubbles move.
- the configuration is so positioned to provide bath velocities near the cathode surface in the same direction as those near the anode, yet not so large as to interfere with the drainage of aluminum at the cathode, which aluminum drainage is opposite in direction to the flow of the gas bubbles driving the bath.
- a bath flow into the space immediately beneath the anode face is achieved when this side of the anode is low with an upward slope away from this side. This slope must be sufficiently large to overcome, and reverse, the flow of gas which would otherwise be toward this anode edge from the inner parts of the anode.
- the precise configuration and arrangement of inclinations may vary with the location of the alumina-rich bath supply. This may be on one side, on both sides, or in the center of the cell, depending upon the size of the cell and the type of anodes used (prebaked or Soderberg).
- the cathode (and therefore anode) inclination is uniform over the width of the cell, or in which the slope is variable, in a variety of forms. These may include, for example, unequal but constant inclinations in the same directions in the two halves of a transverse cross-section of a cell, and also equal but opposite inclinations in these two halves (e.g. a double slope, inverted V configuration).
- the concept of this invention can be applied to cells of both prebaked and/or Soderberg anode design.
- inverted V configuration may be most suitable for cells whose width is covered by two prebaked anode blocks
- such a configuration can also be applied to a vertical stud Soderberg anode, vented in the center.
- An inverted V configuration would be applicable to feeding from both sides, while a V configuration would be suitable for center feeding.
- a design with the slope in one direction only may be preferentially applied, but the invention is not restricted to a single monolithic unvented Soderberg anode fed from one side.
- cathode slope for a particular application must be made compatible with other governing parameters to achieve the desired hydrodynamic characteristics. These parameters are the ACD gap (i.e. the ACD spacing), anode current density, (current and anode face dimensions), and bath return resistance (i.e. the bath return channel or passageway length, depth, width, and wall material).
- the controlled bath flow ensures a sufficient supply of alumina-rich electrolyte to the anode face for the prevention of excessive anode effects. Additional benefits are the avoidance of excessive gas accumulation in the ACD, and reduction of anode and cathode overvoltages, and minimizing of disruption of flow of aluminum product.
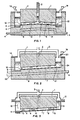
- FIG. 1 An end view of a vertical stud Soderberg reduction cell with side feed, utilizing an inverted V cathode design with center gas exit, and collector bar, is shown in Figure 1.
- the cathode surface 6, is sloped upwardly from the cell sides toward the center of the anode, 1, where anode gas is vented through the gas vent, 2, to a collection system (not shown).
- Collector bars, 4, are illustrated as closely parallel to the cathode surface, 6, and being offset to clear aluminum drain sump 5.
- the angle of the cathode surface 6 from the horizontal should be large enough to induce the desired bath flow; but not so large as to cause excessive deformation of the bubbles, or bath turbulence, in the anode-cathode space, 7, and in accordance with the limitations disclosed hereinafter. It has now been observed, for example, that the angle of inclination has a pronounced, and previously unreported, effect upon bubble configuration and travel. When the anode surface is tilted from the horizontal, the bubbles exhibit a more pronounced tendency towards an elongated oval shape, the long axis of which is perpendicular to the direction of travel of the bubble up the anode slope. The leading edge of the bubble forms a brow, or increased thickness, as the relative speed between bubble and liquid increases.
- the increasingly thick leading edge may result in decreased bubble driving action, due to increased resistance resulting from the effects of interfacial distortion and friction, over some range of inclination.
- large bubbles on the anode surface are subject to considerable distortion and resistance at an anode inclination of about 15° from the horizontal.
- Suitable angles of inclination have been found to range from about 2 to about 15 degrees, although slightly larger or smaller angles may be acceptable for given cell conditions.
- a preferred slope is from about 5 degrees to about 10 degrees from the horizontal, with a more preferred slope of from about 6 to about 8 degrees.
- the most preferred cathode slope has been found to be about 8 degrees.
- the cathode surface, 6 comprises an electrically conducting and aluminum wetted material, such as TiB 2' to facilitate the formation of a thin film of aluminum (or aluminum alloy) on the cathode surface.
- an electrically conducting and aluminum wetted material such as TiB 2' to facilitate the formation of a thin film of aluminum (or aluminum alloy) on the cathode surface.
- TiB 2' an electrically conducting and aluminum wetted material
- TiB 2' to facilitate the formation of a thin film of aluminum (or aluminum alloy) on the cathode surface.
- titanium diboride containing surface is to be preferred, the use of other aluminum-wettable refractory hard materials (RHM), such as titanium carbide, zirconium carbide, zirconium diboride, or mixtures thereof, is also contemplated.
- RHM aluminum-wettable refractory hard materials
- Suitable cathode surfaces, and coating compositions for providing such cathode surfaces are set forth in WO 83/00338 and EP-A
- the aluminum drains from the sloping surface of the cathode into a side collection drain sump 5, thus minimizing the likelihood of back reaction, which would reduce current efficiency, and expediting tapping procedures, and making them independent of other cell operations.
- This thin film of draining aluminum is insensitive to induced magnetic fields, a major improvement over the use of conventional molten aluminum pads. Further, the controlled bath motion, as described, does not interfere with its drainage.
- Venting may be done through a slot or slots or a pattern of vent tubes, channels, holes, etc., in the anode. Venting holes may be made by inserting pipes just through the paste or plastic zone of a Soderberg anode, or baked into a prebaked carbon anode. It may be noted that a non-uniform distribution of vents in the anode will redistribute the bath flow in the cell to compensate for bath flow non-uniformities in the cell.
- Alumina may be added to the bath at hopper 19, to replenish the depleted alumina bath. Of course, other bath make-up materials may also be added simultaneously.
- the arrows, 10, indicate flow of the alumina rich bath through the narrow anode to cathode space, 7, induced by gas bubbles 8, while arrow 13 indicates the flow of aluminum metal, as a thin film, to drain sump 5.
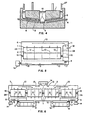
- Figure 2 represents an aluminum reduction cell utilizing a single sloped cathode, in end view.
- the single slope cathode surface 6, enables the use of a straight collector bar, 4, permitting maintenance of a constant distance between the upper cathode surface and the collector bar. While there are some voltage reduction advantages if the collector bar extends from both sides of the cell 22, this is not necessary if a deeper aluminum drain sump, 5, is more advantageous.
- the configuration of this cell permits the use of more-or-less conventional construction and installation techniques for collector bar placement.
- the inclination of the cathode surface, 6, must be sufficient to efficiently move gas bubbles up along the shaped anode face, and to allow aluminum to drain down the cathode surface.
- excess anode gas bubbles, 8, which might otherwise cause excessive distortion of the metal film flowing down the cathode face, may be vented through the gas vents in the anode, 2, to a gas collection system 3.
- Use of a mechanical bath cover, 18, ensures complete control of bath atmosphere and reduces emissions to the atmosphere.
- Alumina is added to the bath at the interelectrode gap outlet, 21, by means of hopper 19.
- An automatic control, 20, may be utilized to accurately replenish the bath. Addition of the alumina, and other bath make-up materials, in this fashion has several advantages. First, after exiting the electrolysis zone, the bath is partially depleted of dissolved alumina, and thus provides the most ideal environment for rapid alumina dissolution.
- the absence of a metal pad or metal sump to cover the settled alumina muck will facilitate its dissolution into the flowing bath. Further, the return path for the bath provides extra dissolution time and acts as a trap for any undissolved alumina in order to ensure that only muck-free bath is circulated into the ACD gap. Whereas addition of alumina to the metal drain sump side, 5, of the cell could result in the formation of muck beneath the metal in the sump, thus causing a sump overflow and a possible direct metal electrical short between anode and cathode, such is avoided in the present arrangement.
- FIG. 3 An end view of a flow-through single-slope cathode aluminum reduction cell is given in Figure 3.
- the cathode angle as illustrated, is sufficient to efficiently move anode gas bubbles, 8, up along the face of the shaped anode, 1, and to allow aluminum to drain down the cathode surface, 6, to the aluminum drain sump, 5, as indicated by arrow 13.
- Gas vents, 2, which may be required, are illustrated in the anode, and may be a slot or a pattern of vent holes.
- the anode itself may be of the Soderberg or prebake carbon variety.
- the aluminum drain sump, 5, preferably leads to a continuous tapping system, which minimizes disturbances to the cell, such as variations in pad thickness.
- the gas vents, 2, and unoccupied bath cavities may advantageously be connected to a gas collection system, 3.
- the -electrolysis bath is replenished externally, and flows through bath inlet 9, in the path shown by arrows 10, through the anode-to-cathode space 7, to the bath outlet 11, at which point the electrolyte has been partially depleted in alumina by the electrolysis.
- FIG 4 a dual sloped drained cathode, or V-shaped cathode, is illustrated.
- replenishment is provided by point feeders 27, which are shown as being located at the higher end of the cathode slope, although replenishment may also occur between the illustrated prebaked anodes, 1.
- Flow of the aluminum metal, 13, is down the cathode surface 6 to the drain sump 5, advantageously connected to a continuous tapping system.
- Anode gas bubbles 8 induce bath material flow 10 along the bottom face of the anodes.
- Figure 5 illustrates a plan view of a single cell, incorporating a cell feeding and mixing zone.
- the anode 1 is illustrated as having a slot gas vent, 2.
- the cathode surface, 6, is illustrated with the aluminum drain sump, 5, feeding a continuous metal tapping system, 14.
- the direction of net flow of aluminum metal is shown by arrow 13, while the direction of net flow of the cryolite bath is illustrated by arrows 10.
- the partially depleted bath flows from outlet 11 to a cell feeding and mixing area or zone, 16, wherein alumina is replenished, 12.
- the cryolite bath is recirculated in the electrolytic cell in the direction indicated by arrows 10.
- the drawing illustrates the use of an optional magnetic bath circulation pump, 17, adjacentto bath inlet 9, although such a pump is not considered necessary under most conditions.
- a barrier, 15, may be positioned in return channels 28 to prevent bath backflow and disruption of circulation in designs incorporating a cell feeding and replenishment zone (16).
- FIG. 6 illustrates a plan view of a multiple cell system, comprising four individual electrolytic cells operating in tandem.
- This system as illustrated incorporates a continuous aluminum tapping system, 14, providing a constant aluminum level, and thus resulting in a more steady heat balance and bath level.
- Conventional discontinuous metal tapping could be used, but provision would have to be made to compensate for resulting changes in bath level. Such changes would necessarily include a bath surge tank, a lower level in the aluminum feeding/mixing tank, and heat balance adjustment means.
- alumina and other make-up materials may be added, 12, to a cell feeding and mixing zone, 16, and circulated through the anode-cathode gap in the direction indicated by arrows 10.
- the use of a magnetic circulation pump, 17, is optional.
- the aluminum formed drains into the aluminum drain sumps, 5, and passes in the direction indicated by arrows 13 to the continuous metal tapping system 14. Back flow of depleted bath is prevented by the presence of barriers 15.
- An alumina feeding and dissolution system may be readily incorporated into either a single cell system or a more economical multiple cell system.
- a feature of the present system which enables it to be run under a controlled atmosphere is a sealed cell top, thus reducing reactor materials problems, emission control problems, and heat losses, although a sealed cell top is not required.
- the addition of a non-consumable anode surface to this design results in a self-contained, unattended, multiple electrolysis unit in a single vessel.
- Figure 7 represents an aluminum reduction cell, utilizing a single slope cathode. As illustrated, the figure shows a continuous aluminum tapping system 14, to remove aluminum from the cell by way of drain sump 5. This acts to provide a constant aluminum level in the cell and also results in a more steady heat balance and bath level in the cell.
- An adjustable partial barrier, 25, is used to restrict, if required, the overall bath circulation velocity within the cell in return channel 28. The desired restriction can be achieved by means of barrier plates composed of a non-corrodable insulator, such as, for instance, silicon nitride. Necessary replenishment means, while not illustrated, may be of any suitable type as previously discussed.
- a dual sloping cathode cell could alternatively be utilized within the scope of the present invention, having, for example, an inverted V configuration, as shown in Figure 1, or a V shape, as shown in Figure 4, whereby metal aluminum would flow downwardly to aluminum drain sumps.
- the single sloped cathode, inclined upward towards the alumina feeding side of the cell, as illustrated in Figure 2 may be considered a preferred embodiment, since this arrangement makes cleaning and other pot room servicing of the cell much simpler, as well as cell construction per se.
- FIG 8 illustrating a plan view of an aluminum reduction cell utilizing dual single slope cathodes, illustrates another alternative form of the present invention.
- the flow of electrolyte is illustrated by arrows 10, indicating a circulation between the anode 1, and the cathode surface, 6, through a zone where replenishment, 12, takes place, and through the ACD of the adjacent anode-cathode pair, sloping in the opposite direction.
- the center line of the cell, 26, is conveniently the demarcation point of the two oppositely sloped cathodes.
- barriers 15 and partial barriers 25 are present to prevent bath backflow, and to control net bath circulation flow rate, respectively. All other elements of the drawing are as previously discussed.
- this configuration represents an alternative to the previously suggested V shape and inverted V configurations, and could suitably be used with prebaked anodes rather than Soderberg.
- Still another configuration, particularly suitable for prebaked anodes has the angle of the inclination for adjacent cathodes (and anode surfaces) in the same direction although at different values.
- the resulting bath circulation was found to be controlled by the balance between the pumping efficiency of the gas bubbles in the ACD gap and the back pressure, or flow resistance, to bath circulation through the return channels. Further results and data were obtained from a 1:20 scale aluminum electrolysis cell and a hydraulic analog model.
- the hydraulic analog model was constructed to simulate a gas evolving anode surface (about 1.11 square meters) located above a sloping solid cathode surface. This arrangement simulated a typical "drained cathode" aluminum reduction cell design in which the working anode is located above a sloping, drained TiB 2 cathode surface.
- the water model studies were performed in room temperature water. The flow patterns and velocities observed in the water model were similar to those anticipated in a full scale cell, since the observed flows had Reynolds numbers in the turbulent regime (>5000). In this regime the flow is primarily controlled by the physical dimensions of the flow channels and not the fluid properties (e.g. viscosity).
- An air pressurized chamber with a bottom constructed from Alundum (trade mark) porous plates (about 20 micron pore diameter) was used to simulate a working anode evolving gas (e.g. CO2), A gas velocity of 0.1778 cm/second through the porous anode plate was used to simulate an anode current density of 0.68 amps/cm 2 (the gas velocity was corrected for differences in temperature and hydrostatic pressure). Simulated currents up to about 1.4 amps/cm 2 were tested in the model.
- a working anode evolving gas e.g. CO2
- a gas velocity of 0.1778 cm/second through the porous anode plate was used to simulate an anode current density of 0.68 amps/cm 2 (the gas velocity was corrected for differences in temperature and hydrostatic pressure). Simulated currents up to about 1.4 amps/cm 2 were tested in the model.
- the model design a side elevation view of which is illustrated by Figure 9(a), simulated one half of the cell shown in Figure 1.
- the model simulated the cell shown in Figure 7.
- the end wall at the "upper" end of the cathode corresponded to a vertical plane passing through the center slot or gas vent, 2.
- the figure illustrates the relationship of such parameters as ACD, BFL, cp, h, ho, lower channel width, and upper channel width (hereinafter defined), to the anode (1), the cathode surface (6), and the bath (29).
- the desired bath flow typically passes through four different types of channels or passageways, namely:
- the bath flow rate, Q, for the model and full-scale cells is defined as the total volumetric rate of bath flow entering the ACD gap from the lower channel.
- the water model was capable of being altered in various ways. Provision was made in the model to simulate the adjustable partial barriers (number 25 in Figure 7). The slope of the cathode and anode in the model was varied from 0 to 15 degrees to determine the effect of cathode slope on bath flow in the ACD gap. The ACD gap was varied from 1 to 5 cm in the water model studies. Gas flow was variable to simulate different current densities. Fluid flow in the model was observed and measured, using injections of colored dye in the ACD. gap and in the return channel.
- Figure 9(b) illustrates the desired flow of gas bubbles, 8, and direction of bath flow, 10, in the ACD. It is clear from this figure that too large a velocity of bath at the cathode surface, in the same direction as at the anode surface, may interfere with drainage of metal on the cathode. Thus, while unidirectional flow is preferable, excessive flow velocity at the cathode surface is to be avoided. As demonstrated in the water model, the water model studies revealed that under certain design conditions three different undesired phenomena can occur in the ACD; specifically:
- Figures 10, 11 and 12 illustrate the effect of cathode slope, ACD, and the flow resistance in the return channel, R f , on the net bath flow in the ACD gap at a simulated anode current density of 0.68 amp/cm 2 .
- the observation of reversed flow at the cathode surface is indicated by dashes. In all cases this is associated with significantly reduced net bath flow, becoming more severe as the cathode slope is reduced.
- Flow resistance is related to return channel width, for particular operating conditions.
- a cross-plot, at a constant flow rate, of data from figures corresponding to Figures 10, 11, and 12 leads to a relationship between cathode slope and ACD which must be satisfied for a given anode current density in order to achieve a flow rate selected to be adequate to supply the cell, as calculated by methods defined hereinafter.
- This relationship between cathode slope and ACD is represented by the curved lines in Figure 15. For each return channel width, therefore, these curved lines represent a condition at which adequate alumina supply will be achieved. These lines are limited by boundary conditions defined by undesirable hydrodynamic conditions, as previously described.
- a region forbidden by reason of excessive bubble thickness bounds the operating region at lower ACD dimensions, i.e. at the left of the parameter diagram ( Figure 15).
- a preferred operating region is shown in Figure 15 as one somewhat above the appropriate curved line representative of the selected return channel width, so as to minimize the ACD while being compatible with the region marked "bubble thickness restriction". Operating within this region simultaneously assures an adequate alumina supply, through a sufficiently large bathflow rate, and avoids an excessively large velocity of bath at the cathode surface (and thus interference with aluminum drainage), as well as excessive bubble thickness and attendant losses in current efficiency.
- the following examples will illustrate the construction and use of such a parameter diagram.
- the bath flow rate, Q, (cm 3 /second) must be sufficient to supply the alumina required to maintain the electrolysis reaction in order to prevent anode effects in an operating cell.
- Q the required minimum flow rate, Q, for a working aluminum reduction cell is given by the equation where A wt.% A1 2 0 3 is the difference in the wt.% A1 2 0 3 in the bath entering and exiting the ACD gap, or, A alumina.
- the 0.008 constant is derived from the Faraday equation:
- Q may also be expressed in terms of bath volume per second per unit of anode area.
- Awt.% AI 2 0 3 5
- the calculated minimum bath flow rates are 202, 4000 and 12000 cm 3 /second for the water model cell and example cells I and II, respectively. While the minimum Q bath flows are theoretically sufficient, in practice such low values should be exceeded to prevent operating problems (e.g., excessive anode effects, high effective bath resistance and overvoltages due to excessive bubble volume in the ACD gap). Cell operating conditions will modify the bath flow to a degree (e.g., due to ledging, crusting, etc.) and hence could lead to less than theoretical bath flow rates.
- the water model data demonstrate that the flow resistance properties of the return channel are a critical component of this invention. As the return channel becomes more restrictive (a result in attempting to maximize the anode area in the cell) the bath flow becomes increasingly sensitive to changes in the flow resistance properties of the return channel. Since there are many cell designs with equivalent effective flow resistance properties in the return channel, it is beneficial to construct a simplified hydraulic model to provide a generalized design criteria for the return channel.
- the hydraulic head loss, h i due to the flow resistance in the return channel is given by the well-known equation:
- the friction may be a composite value to reflect differences in the bottom and side surfaces of the channel.
- volume bath flow which is independent of changing channel dimensions.
- the volume bath flow, Q is given by velocity times the channel cross section area, or hence
- the Q value used in these equations represents the net effective average Q for the channel as determined by timing the period required for injected, highly colored dye to be carried through the channel.
- the hydraulic head loss can be written as: where R f represents a flow resistance geometry term, dependent upon the physical dimensions of the return channel, and K f is a fluid/materials properties coefficient which is less dependent upon physical scale up and can be determined in practice: and Since the value of h varies in an inclined return channel, R f is actually calculated as an integral function of h over the channel length L, with the width w generally remaining constant.
- R f represents a flow resistance geometry term, dependent upon the physical dimensions of the return channel
- K f is a fluid/materials properties coefficient which is less dependent upon physical scale up and can be determined in practice: and Since the value of h varies in an inclined return channel, R f is actually calculated as an integral function of h over the channel length L, with the width w generally remaining constant.
- the cell design in this invention is analogous to a pumped fluid loop where the gas bubbles in the ACD gap perform the pumping action to drive the bath around the ACD gap-return channel circuit.
- An analogous pump efficiency diagram as shown in Figure 13 is used to describe the circulation properties of the "drained cathode" cell. Pump efficiency increases through curves 1, 2, and 3, reflecting increasing cathode slope and/or decreasing ACD. Flow resistance increases through slopes a, b, and c, reflecting increased length and/or decreased cross sectional area of the return channel. The observed flow rate through the ACD is determined by the intersection of the appropriate pump efficiency and flow resistance curves.
- the low flow rate (Q) illustrates that a resistance factor of 2.7x10 -1 cm- 4 is outside of limits of this invention under the stated conditions. This is confirmed by the observed dominant reverse flow condition in the ACD. For the same stated conditions, an R f value of 2.7x10- 2 cm- 4 is within the limits of this invention, but is too large to achieve the preferred flow of 890 cm 3 /second for the water model, resulting in some (slight) reverse flow. It is to be noted that slight reverse flow may, in some instances, be advantageous since it tends to improve, rather than hinder, drainage of aluminum down the cathode surface.
- R f (cell) may be calculated from the equation
- Equation XV R,(model) is a geometric resistance factor (X) having a value of from about 230x10 -3 /cm 4 to about 0.2x10- 3 / cm 4 .
- An alternative method to reduce the calculated w (cell channel) value to 33 cm, without sacrificing the bath flow design factor, is to increase the anode immersion depth by 8 cm (e.g., h o 18 cm).
- Figure 14 presents the R f (model) values (as a function of cathode slope and ACD) required to obtain the preferred Q value of 890 cm 3 /second for the model.
- R f values for other Q values can be extracted from the water model data such as shown in Figures 10, 11 and 12.
- the measured maximum bubble thickness decreased from about 1.0 cm to about 0.5 cm as the cathode slope increased from 2° to 5°, with a corresponding increase in net liquid flow rate. As the slope was increased beyond 5°, the bubble thickness was observed to remain fairly constant, and the bubbles were observed to assume the characteristic shape previously described, and to move more slowly. Changing the ACD was determined to have little effect on the observed bubble thickness. If the bubble protrudes across a major portion of the ACD, the current efficiency will be seriously degraded by the back reaction of the C0 2 with the aluminum metal film on the cathode and the bath electrical resistivity will be greatly increased. For these reasons and practical considerations, the preferred ACD is from about 2 to about 4 cm, with a more preferred range of from about 2 to about 3 cm.
- the bath flow, Q exceeds the preferred 890 cm 3 /second value at cathode slopes greater than or equal to 5 degrees.
- Increasing the cathode slope helps overcome excessive reverse flow and air locking (excessively large stagnant gas bubbles) problems in the ACD gap.
- too steep an angle may disrupt the flow of molten metal on the cathode surface.
- Back pressure due to restrictions in the return channel can also be offset by increasing the cathode slope.
- the preferred cathode slope is in the range of from about 5° to about 11°, with about 8° being the most preferred slope.
- the criteria of this invention may be applied to several types of cells, such as those shown in Figures 1 to 7.
- the cell may consist of an anode which traverses the width of the cell as one continuous mass.
- An example of this anode is shown in Figure 2 with the center vent (2) being absent and hence, the Bubble Flow Length (BFL) is approximately equal to the total anode width.
- BFL Bubble Flow Length
- FIG. 2 Another type of cell would be as shown in Figure 2 where the center vent (2) is included in the design.
- the center vent exhausts the bubbles accumulated under the first half of the anode traversed by the bath flow.
- the BFL is defined as half of the total anode width. Venting of the anode gas is necessary to prevent excessive bubble volume accumulation in the ACD gap which can increase the voltage losses and decrease the current efficiency.
- the total working anode width for the most preferred case would be about 244 cm, which is a more practical cell width.
- the preferred BFL should be decreased from 122 to 61 cm.
- the preferred cell design for the lower anode current densities is shown in Figure 1.
- the concept of a vent to release the anode gas may be expanded to multiple vents to further increase the width of the anode.
- the vent may be present as a slot between the adjacent anode masses or a row of suitably spaced holes through the anode mass.
- the center channel serves multiple purposes, such as gas venting, and as the upper channel to convey the bath exiting the ACD gap to the return channel or channels.
- the BFL is defined as half the total working anode width, which yields a more practical size commercial cell. It is also understood that the cathode slopes shown in Figure 1 could be reversed with a central metal collection trough or well. In this latter case, the upper channels would be located along the exterior sides of the cell, as illustrated in Figure 4.
- the dimensions of the return channel or channels would be calculated according to the teachings given in Example 1.
- the initial step is to select the desired bath flow, Q, based on the scale of the cell being designed, and then use the developed relationships to determine the appropriate flow resistance term, R f .
- the hydraulic head loss equations may then be applied under the conditions stated to calculate a set of preferred return channel design options. Heat balance and other cell design and operation criteria are then used to select one of the hydraulically equivalent return channel designs. This final selection of return channel design can now be done with regard to impact on the critical bath flow.
- Test data from a laboratory scale aluminum reduction cell with a sloping TiB 2 cathode substantiates the use of a water model to simulate the hydrodynamics of a commercial aluminum reduction cell.
- Automatic AI 2 0 3 feed and anode lowering equipment permitted continuous electrolysis tests for up to 3 weeks without any interruption for anode replacement.
- Cell voltage data were measured as a function of current density, ACD value and cathode slope in the electrolysis test cell described in Example 5.
- Reference voltage data for a conventional metal pad cathode were obtained by replacing the sloping TiB 2 cathode with a 3 cm deep aluminum pad in the electrolysis test cell.
- Figure 17 illustrates the dramatic reduction in cell voltage noise achieved by replacing an unstable horizontal aluminum pad with a sloping TiB 2 cathode in an aluminum reduction cell.
- the drained cathode test cell demonstrated the following properties.
- Figure 18 shows the measured anode-cathode polarization voltage as a function of the TiB 2 cathode slope in the test electrolysis cell.
- the test data indicates an approximate 45% reduction in the anode-cathode polarization voltage, or an approximate 0.22 volt saving in the cell voltage in addition to that achieved by reducing the ACD.
- the electrolysis test data for a sloping TiB 2 cathode cell compared to a horizontal metal pad cathode cell have demonstrated improved cell stability (e.g., improved current efficiency and reduced anode effects), at reduced ACD values (i.e., to reduce cell voltage), improved cell response to changes in ACD and current (e.g., ability to use simpler and more reliable cell control automation), more localized anode gas venting (e.g., simpler fume control system), and reduced anode-cathode polarization voltages.
- the asymptotic portion of the anode-cathode polarization curve at cathode slopes greater than 5 degrees in Figure 18 is consistent with the preferred cathode slope range indicated in the water model studies. It is understood that the foregoing description of the present invention is susceptible to various modifications, changes, and adaptations by those skilled in the art, and that the same are intended to be considered to be within the scope of the present invention.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Electrolytic Production Of Metals (AREA)
- Water Treatment By Electricity Or Magnetism (AREA)
- Battery Electrode And Active Subsutance (AREA)
Claims (25)
wobei der mindestens eine Rückströmkanal Abmessungen h, w und L (h ist die Tiefe in cm an irgendeiner gegebenen Stelle, w ist die Breite des Rückströmkanals in cm und L ist die Länge des Rückströmkanals in cm) aufweist, die durch die Beziehung
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US46757083A | 1983-02-17 | 1983-02-17 | |
| US467570 | 1983-02-17 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0135535A1 EP0135535A1 (de) | 1985-04-03 |
| EP0135535B1 true EP0135535B1 (de) | 1987-12-23 |
Family
ID=23856249
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP84900797A Expired EP0135535B1 (de) | 1983-02-17 | 1984-01-20 | Niedrige energie aufweisende aluminium-reduktionszelle mit induzierter badströmung |
Country Status (11)
| Country | Link |
|---|---|
| EP (1) | EP0135535B1 (de) |
| JP (1) | JPS60500541A (de) |
| AU (1) | AU578410B2 (de) |
| BR (1) | BR8405353A (de) |
| CA (1) | CA1254855A (de) |
| DE (1) | DE3468238D1 (de) |
| ES (1) | ES529828A0 (de) |
| IS (1) | IS1296B6 (de) |
| NZ (1) | NZ207174A (de) |
| SU (1) | SU1542420A3 (de) |
| WO (1) | WO1984003308A1 (de) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11242604B2 (en) | 2016-07-26 | 2022-02-08 | Cobex Gmbh | Cathode assembly for the production of aluminum |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0393816B1 (de) * | 1989-02-20 | 1994-04-27 | Comalco Aluminium, Ltd. | Zelle zur schmelzflusselektrolytischen Gewinnung von Aluminium |
| NZ505730A (en) * | 1998-02-11 | 2002-05-31 | Moltech Invent Sa | Drained cathode aluminium electrowinning cell having v-shaped sloped anode faces that cover recessed grooves or channels along the cathode faces |
| JP4830275B2 (ja) * | 2004-07-22 | 2011-12-07 | ソニー株式会社 | 記憶素子 |
| WO2015017925A1 (fr) * | 2013-08-09 | 2015-02-12 | Rio Tinto Alcan International Limited | Cuve d'électrolyse à plancher crénelé |
| JP7076296B2 (ja) * | 2018-06-19 | 2022-05-27 | 東邦チタニウム株式会社 | 溶融金属の製造方法および、溶融塩電解槽 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3582483A (en) * | 1962-06-29 | 1971-06-01 | Elektrokemisk As | Process for electrolytically producing aluminum |
| US4093524A (en) * | 1976-12-10 | 1978-06-06 | Kaiser Aluminum & Chemical Corporation | Bonding of refractory hard metal |
| FR2409326A1 (fr) * | 1977-11-18 | 1979-06-15 | Nippon Light Metal Co | Cellule d'electrolyse d'aluminium |
| US4333813A (en) * | 1980-03-03 | 1982-06-08 | Reynolds Metals Company | Cathodes for alumina reduction cells |
| GB2084864A (en) * | 1980-10-06 | 1982-04-21 | Brookes Nigel Terence | Visor wiper |
| US4341611A (en) * | 1980-12-18 | 1982-07-27 | Reynolds Metals Company | Alumina reduction cell |
| AU2713684A (en) * | 1983-04-26 | 1984-11-01 | Aluminium Company Of America | Electrolytic cell |
-
1984
- 1984-01-20 WO PCT/US1984/000075 patent/WO1984003308A1/en active IP Right Grant
- 1984-01-20 JP JP59500833A patent/JPS60500541A/ja active Granted
- 1984-01-20 AU AU24929/84A patent/AU578410B2/en not_active Expired
- 1984-01-20 EP EP84900797A patent/EP0135535B1/de not_active Expired
- 1984-01-20 DE DE8484900797T patent/DE3468238D1/de not_active Expired
- 1984-01-20 BR BR8405353A patent/BR8405353A/pt not_active IP Right Cessation
- 1984-02-09 IS IS2881A patent/IS1296B6/is unknown
- 1984-02-13 CA CA000447259A patent/CA1254855A/en not_active Expired
- 1984-02-16 NZ NZ207174A patent/NZ207174A/en unknown
- 1984-02-17 ES ES529828A patent/ES529828A0/es active Granted
- 1984-10-16 SU SU843806469A patent/SU1542420A3/ru active
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11242604B2 (en) | 2016-07-26 | 2022-02-08 | Cobex Gmbh | Cathode assembly for the production of aluminum |
Also Published As
| Publication number | Publication date |
|---|---|
| BR8405353A (pt) | 1985-02-12 |
| AU578410B2 (en) | 1988-10-27 |
| IS1296B6 (is) | 1987-07-07 |
| NZ207174A (en) | 1987-03-31 |
| AU2492984A (en) | 1984-09-10 |
| SU1542420A3 (ru) | 1990-02-07 |
| WO1984003308A1 (en) | 1984-08-30 |
| IS2881A7 (is) | 1984-08-18 |
| JPS60500541A (ja) | 1985-04-18 |
| ES8501809A1 (es) | 1984-12-01 |
| EP0135535A1 (de) | 1985-04-03 |
| CA1254855A (en) | 1989-05-30 |
| JPH0310715B2 (de) | 1991-02-14 |
| DE3468238D1 (en) | 1988-02-04 |
| ES529828A0 (es) | 1984-12-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4602990A (en) | Low energy aluminum reduction cell with induced bath flow | |
| AU2002236366B2 (en) | A method and an electrowinning cell for production of metal | |
| EP0560814B1 (de) | Elektrodenzusammenstellung und multimonopolare zellen für die aluminiumelektrogewinnung | |
| AU2008291662B2 (en) | Method for operating copper electrolysis cells | |
| EP0096990B1 (de) | Metallherstellung durch Schmelzelektrolyse | |
| US4282082A (en) | Slurry electrowinning apparatus | |
| EP0135535B1 (de) | Niedrige energie aufweisende aluminium-reduktionszelle mit induzierter badströmung | |
| CA1072055A (en) | Electrolytic cell and circulating method for electrolyte | |
| US7470354B2 (en) | Utilisation of oxygen evolving anode for Hall-Hèroult cells and design thereof | |
| EP0393816B1 (de) | Zelle zur schmelzflusselektrolytischen Gewinnung von Aluminium | |
| CA1337059C (en) | Electrolytic cell for recovery of metal | |
| US2468022A (en) | Electrolytic apparatus for producing magnesium | |
| US3178363A (en) | Apparatus and process for production of aluminum and other metals by fused bath electrolysis | |
| NO168432B (no) | Dreneringskatodecelle for elektrolytisk reduksjon av aluminiumoksyd til aluminium | |
| WO2002031225A2 (en) | Electrode assembly for aluminium production cell with at least one anode having a sloped lower surface | |
| RU2482224C2 (ru) | Катодное устройство алюминиевого электролизера с рельефной подиной | |
| JPS6033904B2 (ja) | 電解還元槽 | |
| Phillips | The interaction of design and operation for optimized aluminum reduction | |
| EP0604664A1 (de) | Verfahren zur gewinnung von aluminium und anderen metallen | |
| Dorward et al. | Design, operation and electrochemical aspects of Hall-Héroult cells containing solid wetted cathodes: a 30-year chronicle | |
| JP2020128580A (ja) | 電解精製用電解槽への電解液の給液方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19841006 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: COMMONWEALTH ALUMINIUM CORPORATION |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: COMMONWEALTH ALUMINUM CORPORATION |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB |
|
| REF | Corresponds to: |
Ref document number: 3468238 Country of ref document: DE Date of ref document: 19880204 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: TP |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19930113 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19941001 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20030110 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20030115 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20040119 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 |