EP0103466B1 - Oven cleaner - Google Patents
Oven cleaner Download PDFInfo
- Publication number
- EP0103466B1 EP0103466B1 EP83305244A EP83305244A EP0103466B1 EP 0103466 B1 EP0103466 B1 EP 0103466B1 EP 83305244 A EP83305244 A EP 83305244A EP 83305244 A EP83305244 A EP 83305244A EP 0103466 B1 EP0103466 B1 EP 0103466B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- oven cleaner
- oven
- cleaner
- alkali metal
- polyhydric alcohol
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims abstract description 24
- 239000000203 mixture Substances 0.000 claims abstract description 16
- 229910052783 alkali metal Inorganic materials 0.000 claims abstract description 15
- -1 alkali metal salt Chemical class 0.000 claims abstract description 15
- 150000005846 sugar alcohols Polymers 0.000 claims abstract description 15
- 238000004140 cleaning Methods 0.000 claims abstract description 13
- 239000003518 caustics Substances 0.000 claims abstract description 8
- 238000000034 method Methods 0.000 claims abstract description 5
- 229910000288 alkali metal carbonate Inorganic materials 0.000 claims abstract description 4
- 150000008041 alkali metal carbonates Chemical class 0.000 claims abstract description 4
- 238000005406 washing Methods 0.000 claims abstract description 4
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 claims abstract description 3
- 238000010438 heat treatment Methods 0.000 claims abstract description 3
- 239000007788 liquid Substances 0.000 claims abstract description 3
- 229940071207 sesquicarbonate Drugs 0.000 claims abstract description 3
- 238000010411 cooking Methods 0.000 claims description 12
- 239000003925 fat Substances 0.000 claims description 10
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 claims description 6
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 claims description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 5
- 239000004615 ingredient Substances 0.000 claims description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical group C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 3
- 150000007524 organic acids Chemical class 0.000 claims description 3
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical group [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 claims description 3
- ZWVMLYRJXORSEP-UHFFFAOYSA-N 1,2,6-Hexanetriol Chemical compound OCCCCC(O)CO ZWVMLYRJXORSEP-UHFFFAOYSA-N 0.000 claims description 2
- 239000002202 Polyethylene glycol Substances 0.000 claims description 2
- SZXQTJUDPRGNJN-UHFFFAOYSA-N dipropylene glycol Chemical compound OCCCOCCCO SZXQTJUDPRGNJN-UHFFFAOYSA-N 0.000 claims description 2
- 239000003205 fragrance Substances 0.000 claims description 2
- 239000003550 marker Substances 0.000 claims description 2
- 235000005985 organic acids Nutrition 0.000 claims description 2
- 239000000049 pigment Substances 0.000 claims description 2
- 229920001223 polyethylene glycol Polymers 0.000 claims description 2
- 229920001451 polypropylene glycol Polymers 0.000 claims description 2
- 239000003380 propellant Substances 0.000 claims description 2
- 229910052708 sodium Inorganic materials 0.000 claims description 2
- 239000011734 sodium Substances 0.000 claims description 2
- 239000004094 surface-active agent Substances 0.000 claims description 2
- 239000002562 thickening agent Substances 0.000 claims description 2
- ZIBGPFATKBEMQZ-UHFFFAOYSA-N triethylene glycol Chemical compound OCCOCCOCCO ZIBGPFATKBEMQZ-UHFFFAOYSA-N 0.000 claims description 2
- 239000004480 active ingredient Substances 0.000 abstract 1
- 235000014593 oils and fats Nutrition 0.000 abstract 1
- 238000012360 testing method Methods 0.000 description 10
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- UIIMBOGNXHQVGW-UHFFFAOYSA-M sodium bicarbonate Substances [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 7
- 238000009472 formulation Methods 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 4
- 235000017557 sodium bicarbonate Nutrition 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 159000000011 group IA salts Chemical class 0.000 description 3
- 235000011121 sodium hydroxide Nutrition 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 159000000021 acetate salts Chemical class 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 159000000001 potassium salts Chemical class 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- YIWGJFPJRAEKMK-UHFFFAOYSA-N 1-(2H-benzotriazol-5-yl)-3-methyl-8-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidine-5-carbonyl]-1,3,8-triazaspiro[4.5]decane-2,4-dione Chemical compound CN1C(=O)N(c2ccc3n[nH]nc3c2)C2(CCN(CC2)C(=O)c2cnc(NCc3cccc(OC(F)(F)F)c3)nc2)C1=O YIWGJFPJRAEKMK-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 150000007824 aliphatic compounds Chemical class 0.000 description 1
- 150000001447 alkali salts Chemical class 0.000 description 1
- 239000004411 aluminium Substances 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 235000021168 barbecue Nutrition 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 210000003298 dental enamel Anatomy 0.000 description 1
- TUCSOESCAKHLJM-UHFFFAOYSA-L dipotassium carbonic acid carbonate Chemical compound [K+].[K+].OC(O)=O.OC(O)=O.[O-]C([O-])=O TUCSOESCAKHLJM-UHFFFAOYSA-L 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 231100001261 hazardous Toxicity 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 239000002574 poison Substances 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229910052573 porcelain Inorganic materials 0.000 description 1
- 229910000028 potassium bicarbonate Inorganic materials 0.000 description 1
- 235000015497 potassium bicarbonate Nutrition 0.000 description 1
- 239000011736 potassium bicarbonate Substances 0.000 description 1
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910052572 stoneware Inorganic materials 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000000037 vitreous enamel Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
- C11D7/50—Solvents
- C11D7/5004—Organic solvents
- C11D7/5022—Organic solvents containing oxygen
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/0005—Other compounding ingredients characterised by their effect
- C11D3/0057—Oven-cleaning compositions
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
- C11D7/02—Inorganic compounds
- C11D7/04—Water-soluble compounds
- C11D7/10—Salts
- C11D7/12—Carbonates bicarbonates
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
- C11D7/22—Organic compounds
- C11D7/26—Organic compounds containing oxygen
- C11D7/261—Alcohols; Phenols
Definitions
- the present invention relates to an improved oven cleaner and to a method for removing baked on fats and greases from cooking surfaces.
- Oven cleaners are compositions of matter used to remove baked on fats and greases from cooking surfaces. Such compositions are commonly used for cleaning ovens as the name implies but may also be used to clean other cooking surfaces on which there has been a build up of baked on fats and greases. Such surfaces include fry pans, kitchenware, barbecue equipment, cooking utensils and the like. These surfaces may be of bare metal, metal coated as with baked enamel, glazed stoneware, porcelain, glass or the like.
- oven cleaners are based on caustic soda. It has generally been considered necessary to use a caustic alkali in order to effectively saponify the fats in the baked on material in order to enable its removal.
- a few oven cleaners have been based on solvents, acetate salts, amines or ammonia.
- the known caustic based systems suffer from the disadvantage of being quite hazardous and/or require the consumer to wear gloves during usage. These known caustic-based systems are required to carry poisons schedule labelling and warning statements.
- the systems based on acetate salts have the disadvantage that they require temperatures of 250°C or above to activate them.
- GB-A-1 478 482 and GB-A-1 478 481 both provide oven cleaning compositions which include, as essential ingredients, an aliphatic compound such as glycerol having two or more hydroxy groups, and up to 2% by weight of an alkaline-acting catalyst such as an alkali metal carbonate.
- an alkaline-acting catalyst such as an alkali metal carbonate.
- oven cleaners effective at temperatures of at least 121°C (250°F) are provided.
- GB-A-1576454 provides a similar oven cleaner utilizing an alkali metal bicarbonate, but also essentially requiring an alkali metal salt of a weak organic acid to provide a suitable oven cleaner effective at temperatures of at least 121°C (250°F).
- an oven cleaner comprising an effective amount of a polyhydric alcohol which is a liquid and non-volatile at a temperature of at least 125°C at atmospheric pressure, and an alkali metal salt soluble therein at said temperature, said oven cleaner not containing alkali metal salts of weak organic acids and wherein the alkali metal salt is non-caustic and selected from an alkali metal carbonate or bicarbonate or sesquicarbonate, or mixtures thereof in an amount of from more than 2% by weight of the cleaner.
- the present invention further consists of applying the oven cleaner according to this invention to a cooking surface carrying baked on fats or greases, heating the cooking surface at atmospheric pressure to at least 125°C, and preferably from 125 to 250°C, for a period of at least five minutes and washing and/or wiping the saponified fats or greases from the cooking surface.
- the oven cleaner according to the present invention may have a pH as low as 9 or less which allows its use without rubber gloves and like protective clothing. These oven cleaners also have the advantage that surfaces of stainless steel, aluminium, and some other metals will be left with a shiny surface after cleaning rather than being left with a dull, oxidised surface as is the case with many of the prior art oven cleaning compositions.
- the non-caustic alkali metal salt is most preferably sodium or potassium bicarbonate.
- alkali salts which can be advantageously used include sodium or potassium sesquicarbonate.
- the alkaline salts preferably comprise from more than 2 to 20% by weight of the product.
- the pH of the product is preferably below 11 and more preferably below 10 and most preferably 9 or below.
- the polyhydric alcohol used in the present invention preferably have the general formula:
- the alcohol is most preferably glycerol or includes glycerol.
- Other compounds in this group which may be used include mannitol, ethylene glycol and sorbitol.
- the salt is a potassium salt
- the polyhydric alcohol may with equal effectiveness be an alcohol falling outside the above general formula. This is believed to be due to the higher solubility of the potassium salts in the polyols as compared with the corresponding sodium salt.
- Polyhydric alcohols which work efficiently with the potassium salts include various grades of propylene glycol, diethylene glycol, polyethylene glycol, dipropylene glycol, polypropylene glycol, triethylene glycol, and 1,2,6-hexanetriol.
- the polyhydric alcohol preferably comprises from 1 to 50% by weight of the product.
- the oven cleaner according to this invention preferably includes water.
- the water is preferably present in an amount of at least 35% by weight.
- the oven cleaner is preferably water based and preferably contains a thickener to prevent it running off vertical surfaces.
- Other ingredients may include a surfactant or soap, a fragrance, a pigment marker and a propellant.
- the oven cleaner according to this invention may be applied in any suitable manner. These include an aerosol, a trigger or pump spray, a brush or pad.
- the oven cleaner is preferably applied to a surface to be cleaned and heated to a temperature of from 125°C to 250°C for a time of from 5 minutes to 2 hours.
- oven cleaners according to this invention are effective, despite their limited alkalinity and the evaporation of the water from the cleaner, due to the continued action of the alkaline salt dissolved in the non-volatile polyhydric alcohol.
- the alkaline salt is maintained in a condition in which it is available for reaction with the baked on fats and greases at the required elevated temperature by being dissolved in the polyhydric alcohol. For this reason the polyhydric alcohol is required to be substantially non-volatile at the cleaning temperature.
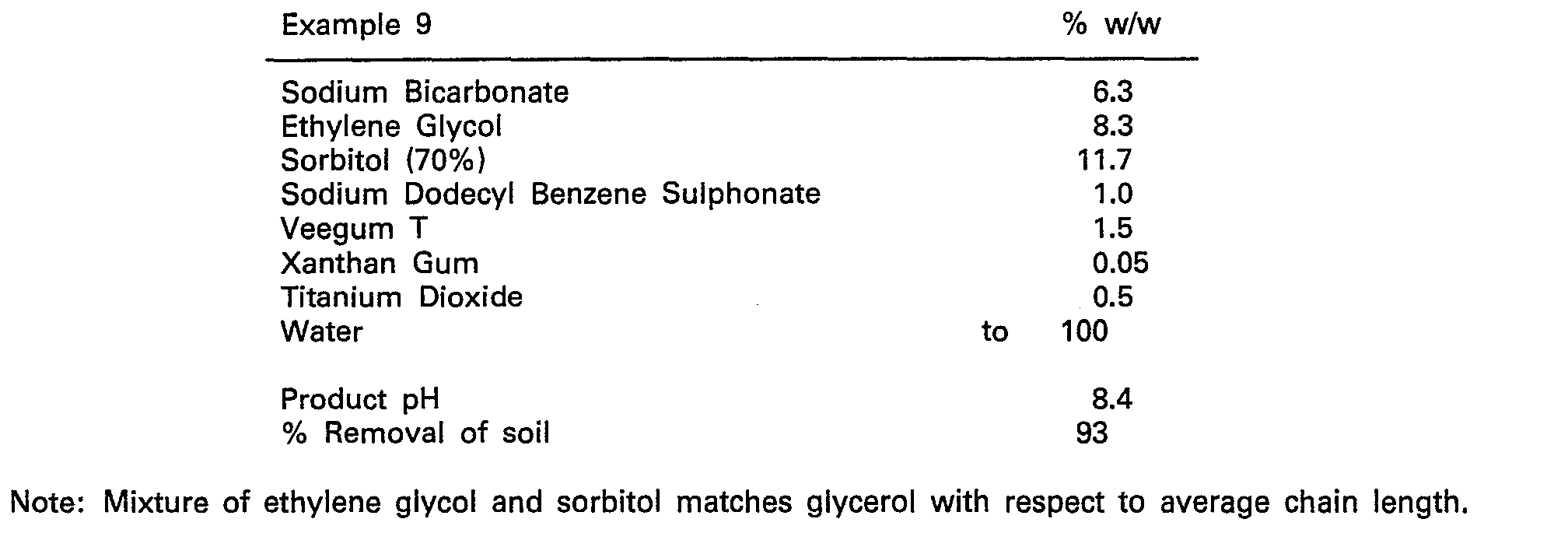
- Test surfaces were prepared by baking smeared dripping onto white vitreous enamel metal plates for 1 1/2 hours at 250°C. The baked-on dripping could not be removed at all by washing or wiping without a scourer.
- Cleaners consisting of the formulations given below were applied to the test surfaces to be cleaned from a trigger pack after shaking well.
- test plates were then heated in an oven for 30 minutes to a final temperature of 150°C.
- test plates were then either rinsed under a fast running tap or wiped wiwh a damp sponge and the percentage removal of baked-on dripping recorded.
- a cleaner consisting of the following formulation was applied to a test surface to be cleaned, prepared as for Examples 1-9 from an aerosol pack after shaking well. The test was then carried out in the same manner as for Examples 1-9.
- a cleaner consisting of the following formulation was brushed onto a test surface to be cleaned, prepared as for Examples 1-8. The test was then carried out in the same manner as for Example 1-9.
- composition according to Examples for instance, 1 to 5 was equal to that of a molar equivalent active level of caustic soda in the same formulation base.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Inorganic Chemistry (AREA)
- Detergent Compositions (AREA)
- Iron Core Of Rotating Electric Machines (AREA)
Abstract
Description
- The present invention relates to an improved oven cleaner and to a method for removing baked on fats and greases from cooking surfaces.
- Oven cleaners are compositions of matter used to remove baked on fats and greases from cooking surfaces. Such compositions are commonly used for cleaning ovens as the name implies but may also be used to clean other cooking surfaces on which there has been a build up of baked on fats and greases. Such surfaces include fry pans, kitchenware, barbecue equipment, cooking utensils and the like. These surfaces may be of bare metal, metal coated as with baked enamel, glazed stoneware, porcelain, glass or the like.
- Conventional oven cleaners are based on caustic soda. It has generally been considered necessary to use a caustic alkali in order to effectively saponify the fats in the baked on material in order to enable its removal. A few oven cleaners have been based on solvents, acetate salts, amines or ammonia. The known caustic based systems suffer from the disadvantage of being quite hazardous and/or require the consumer to wear gloves during usage. These known caustic-based systems are required to carry poisons schedule labelling and warning statements. The systems based on acetate salts have the disadvantage that they require temperatures of 250°C or above to activate them.
- It has been proposed in the past to individually add organic solvents and inorganic builder salts to oven cleaning compositions based on alkali or nitrogen containing active cleaning ingredients. Such additions are for instance disclosed in U.S. Patent Specifications 3,829,387; 3,813,343; and 3,658,711. It has also been known to use aqueous solutions of alkali metal salts to form non-stick coatings on ovens; see U.K. Patent Specifications 2,019,876, 1,523,491 and 1,576,454 and Australian Patent Specification 453,537.
- GB-A-1 478 482 and GB-A-1 478 481 both provide oven cleaning compositions which include, as essential ingredients, an aliphatic compound such as glycerol having two or more hydroxy groups, and up to 2% by weight of an alkaline-acting catalyst such as an alkali metal carbonate. By incorporation of up to 2% of the alkaline acting catalyst, oven cleaners effective at temperatures of at least 121°C (250°F) are provided. Further GB-A-1576454 provides a similar oven cleaner utilizing an alkali metal bicarbonate, but also essentially requiring an alkali metal salt of a weak organic acid to provide a suitable oven cleaner effective at temperatures of at least 121°C (250°F).
- According to the present invention, therefore, there is provided an oven cleaner comprising an effective amount of a polyhydric alcohol which is a liquid and non-volatile at a temperature of at least 125°C at atmospheric pressure, and an alkali metal salt soluble therein at said temperature, said oven cleaner not containing alkali metal salts of weak organic acids and wherein the alkali metal salt is non-caustic and selected from an alkali metal carbonate or bicarbonate or sesquicarbonate, or mixtures thereof in an amount of from more than 2% by weight of the cleaner.
- The present invention further consists of applying the oven cleaner according to this invention to a cooking surface carrying baked on fats or greases, heating the cooking surface at atmospheric pressure to at least 125°C, and preferably from 125 to 250°C, for a period of at least five minutes and washing and/or wiping the saponified fats or greases from the cooking surface.
- The oven cleaner according to the present invention may have a pH as low as 9 or less which allows its use without rubber gloves and like protective clothing. These oven cleaners also have the advantage that surfaces of stainless steel, aluminium, and some other metals will be left with a shiny surface after cleaning rather than being left with a dull, oxidised surface as is the case with many of the prior art oven cleaning compositions.
- The non-caustic alkali metal salt is most preferably sodium or potassium bicarbonate.
- Other alkali salts which can be advantageously used include sodium or potassium sesquicarbonate. The alkaline salts preferably comprise from more than 2 to 20% by weight of the product.
- The pH of the product is preferably below 11 and more preferably below 10 and most preferably 9 or below.
- The polyhydric alcohol used in the present invention preferably have the general formula:
- The oven cleaner according to this invention preferably includes water. The water is preferably present in an amount of at least 35% by weight.
- The oven cleaner is preferably water based and preferably contains a thickener to prevent it running off vertical surfaces. Other ingredients may include a surfactant or soap, a fragrance, a pigment marker and a propellant.
- The oven cleaner according to this invention may be applied in any suitable manner. These include an aerosol, a trigger or pump spray, a brush or pad.
- In carrying out the method according to this invention the oven cleaner is preferably applied to a surface to be cleaned and heated to a temperature of from 125°C to 250°C for a time of from 5 minutes to 2 hours.
- It is believed that oven cleaners according to this invention are effective, despite their limited alkalinity and the evaporation of the water from the cleaner, due to the continued action of the alkaline salt dissolved in the non-volatile polyhydric alcohol. The alkaline salt is maintained in a condition in which it is available for reaction with the baked on fats and greases at the required elevated temperature by being dissolved in the polyhydric alcohol. For this reason the polyhydric alcohol is required to be substantially non-volatile at the cleaning temperature.
- Hereinafter given by way of example are preferred embodiments of the present invention.
- Test surfaces were prepared by baking smeared dripping onto white vitreous enamel metal plates for 1 1/2 hours at 250°C. The baked-on dripping could not be removed at all by washing or wiping without a scourer.
- Cleaners consisting of the formulations given below were applied to the test surfaces to be cleaned from a trigger pack after shaking well.
- The test plates were then heated in an oven for 30 minutes to a final temperature of 150°C.
-
-
-
- The performance of the composition according to Examples, for instance, 1 to 5 was equal to that of a molar equivalent active level of caustic soda in the same formulation base.
- Caustic soda based products on the market when tested under identical conditions removed only 80-95% of the baked-on dripping.
- A synergistic effect between the polyhydric alcohol(s) and the non-caustic alkali metal salt(s) has been demonstrated by testing glycerol and sodium bicarbonate separately alongside a mixture of both on the same prepared test plate.
- Sodium bicarbonate on its own removed only 50% of the baked-on dripping and glycerol on its own removed only 30% of the baked-on dripping whereas the mixture of sodium bicarbonate and glycerol removed 100% of the baked-on dripping.
Claims (14)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT83305244T ATE27716T1 (en) | 1982-09-09 | 1983-09-08 | OVEN CLEANING AGENT. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AUPF582082 | 1982-09-09 | ||
| AU5820/82 | 1982-09-09 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0103466A1 EP0103466A1 (en) | 1984-03-21 |
| EP0103466B1 true EP0103466B1 (en) | 1987-06-10 |
Family
ID=3769740
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP83305244A Expired EP0103466B1 (en) | 1982-09-09 | 1983-09-08 | Oven cleaner |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP0103466B1 (en) |
| AT (1) | ATE27716T1 (en) |
| DE (1) | DE3372000D1 (en) |
| ES (1) | ES8507603A1 (en) |
| MY (1) | MY102376A (en) |
| NZ (1) | NZ205464A (en) |
| ZA (1) | ZA836720B (en) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5380454A (en) * | 1993-07-09 | 1995-01-10 | Reckitt & Colman Inc. | Low temperature non-caustic oven cleaning composition |
| JP3683600B2 (en) * | 1994-06-30 | 2005-08-17 | ミネソタ マイニング アンド マニュファクチャリング カンパニー | Cleaning composition |
| US7135446B1 (en) * | 2002-01-28 | 2006-11-14 | Diamondite, L.L.C. | System for cleaning and protecting windshields |
| US20050059565A1 (en) * | 2003-09-03 | 2005-03-17 | Sutton David C. | Cleaning composition |
| JP7312431B2 (en) * | 2019-05-30 | 2023-07-21 | 株式会社ニイタカ | LIQUID CLEANING COMPOSITION FOR COOKING EQUIPMENT AND METHOD FOR CLEANING COOKING EQUIPMENT |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3881948A (en) * | 1973-07-20 | 1975-05-06 | Church & Dwight Co Inc | Method for removing organic acid soil from surfaces |
| US4193886A (en) * | 1976-04-22 | 1980-03-18 | Church & Dwight Co., Inc. | Novel low temperature cleaner |
| DE3124348A1 (en) * | 1980-07-02 | 1982-04-01 | Metallgesellschaft Ag, 6000 Frankfurt | Process for the removal of phosphate and/or reaction lubricant layers |
-
1983
- 1983-09-02 NZ NZ205464A patent/NZ205464A/en unknown
- 1983-09-08 AT AT83305244T patent/ATE27716T1/en not_active IP Right Cessation
- 1983-09-08 EP EP83305244A patent/EP0103466B1/en not_active Expired
- 1983-09-08 ES ES525816A patent/ES8507603A1/en not_active Expired
- 1983-09-08 DE DE8383305244T patent/DE3372000D1/en not_active Expired
- 1983-09-09 ZA ZA836720A patent/ZA836720B/en unknown
-
1987
- 1987-09-29 MY MYPI87002161A patent/MY102376A/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| MY102376A (en) | 1992-06-17 |
| ZA836720B (en) | 1984-06-27 |
| ATE27716T1 (en) | 1987-06-15 |
| ES525816A0 (en) | 1985-10-01 |
| DE3372000D1 (en) | 1987-07-16 |
| ES8507603A1 (en) | 1985-10-01 |
| NZ205464A (en) | 1986-08-08 |
| EP0103466A1 (en) | 1984-03-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US3829387A (en) | Caustic cleaner composition | |
| US5102573A (en) | Detergent composition | |
| US3650831A (en) | Method of cleaning surfaces | |
| AU633814B2 (en) | Heavy duty hard surface liquid detergent | |
| CA2380491C (en) | Detergent for vitroceramic surfaces | |
| US4214915A (en) | Method and composition for cleaning ovens | |
| JPH02289697A (en) | Composition for cleaning rigid surface | |
| EP0708820B1 (en) | Low temperature non-caustic oven cleaning composition | |
| US4372788A (en) | Grill and oven cleaner | |
| CA1306921C (en) | Detergent composition | |
| CA1100394A (en) | Low temperature cleaner | |
| US4116848A (en) | Novel cleaning method and compositions | |
| US3658711A (en) | Caustic alkali free oven cleaning composition | |
| EP0103466B1 (en) | Oven cleaner | |
| US4135947A (en) | Method of cleaning surfaces with CO2 -neutralized amine compositions | |
| US4236935A (en) | Method for removing organic acid soil from surfaces | |
| US3881948A (en) | Method for removing organic acid soil from surfaces | |
| US20050059565A1 (en) | Cleaning composition | |
| CA1211674A (en) | Oven cleaner | |
| CA3168855A1 (en) | Oven cleaning compositions and methods of making and using same | |
| JPH09151395A (en) | Detergent composition | |
| JPS63309594A (en) | Household cleaner composition | |
| KR20050102312A (en) | Aqueous composition for glass cleaner | |
| JP3207432B2 (en) | CLEANING COMPOSITION COMPRISING A Saturated Dialkylcationic Surfactant | |
| JPS62185796A (en) | Detergent composition for vehicle |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19840912 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: R & C PRODUCTS PTY. LIMITED |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 27716 Country of ref document: AT Date of ref document: 19870615 Kind code of ref document: T |
|
| ITF | It: translation for a ep patent filed | ||
| REF | Corresponds to: |
Ref document number: 3372000 Country of ref document: DE Date of ref document: 19870716 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19920811 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19930908 |
|
| ITTA | It: last paid annual fee | ||
| EPTA | Lu: last paid annual fee | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 83305244.2 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19960813 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19960814 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19960827 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19960830 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19960901 Year of fee payment: 14 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970908 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970909 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970930 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970930 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970930 |
|
| BERE | Be: lapsed |
Owner name: R & C PRODUCTS PTY. LTD Effective date: 19970930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980401 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 19980401 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 83305244.2 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: TP |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20010813 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20010817 Year of fee payment: 19 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20010820 Year of fee payment: 19 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: TP |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020908 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030401 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20020908 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030603 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |