EP0075369A1 - Verfahren und Einrichtung zum Konstanthalten der Heizbelastung einer gasgefeuerten Anlage - Google Patents
Verfahren und Einrichtung zum Konstanthalten der Heizbelastung einer gasgefeuerten Anlage Download PDFInfo
- Publication number
- EP0075369A1 EP0075369A1 EP82201152A EP82201152A EP0075369A1 EP 0075369 A1 EP0075369 A1 EP 0075369A1 EP 82201152 A EP82201152 A EP 82201152A EP 82201152 A EP82201152 A EP 82201152A EP 0075369 A1 EP0075369 A1 EP 0075369A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- gas
- pressure
- oxygen content
- volume
- controlled
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F23—COMBUSTION APPARATUS; COMBUSTION PROCESSES
- F23N—REGULATING OR CONTROLLING COMBUSTION
- F23N5/00—Systems for controlling combustion
- F23N5/003—Systems for controlling combustion using detectors sensitive to combustion gas properties
- F23N5/006—Systems for controlling combustion using detectors sensitive to combustion gas properties the detector being sensitive to oxygen
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F23—COMBUSTION APPARATUS; COMBUSTION PROCESSES
- F23N—REGULATING OR CONTROLLING COMBUSTION
- F23N2221/00—Pretreatment or prehandling
- F23N2221/10—Analysing fuel properties, e.g. density, calorific
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F23—COMBUSTION APPARATUS; COMBUSTION PROCESSES
- F23N—REGULATING OR CONTROLLING COMBUSTION
- F23N2223/00—Signal processing; Details thereof
- F23N2223/08—Microprocessor; Microcomputer
Definitions
- the invention relates to a method of keeping the heat load on gas-fired equipment connected to a gas distribution grid constant by withdrawing a volume-controlled sample stream from the fuel gas supplied completely combusting the flow of sample gas in a combustion chamber with a volume-controlled excess flow of combustion air, measuring the oxygen content of the combustion gases and, on the basis of the oxygen content measured, controlling a property of the gas in such a manner as to keep said heat load substantially constant.
- the heat load (hereinafter to be referred to as simply 'load') of gas-fired equipment is understood to be the amount of gas, by volume, burnt per unit of time (reduced to normalized pressure and temperature), multiplied by the calorific value of the gas.
- 'load' The heat load of gas-fired equipment is understood to be the amount of gas, by volume, burnt per unit of time (reduced to normalized pressure and temperature), multiplied by the calorific value of the gas.
- the Wobbe index is an important quantity in combustion engineering. According to formula (3), when the gas pressure is constant, the load on gas-fired equipment is constant if the Wobbe index of the fuel gas is constant, even if the composition of the fuel gas is variable.
- a method as described in the preamble is known from Dutch Patent Application No. 7808476 by Applicant, laid open for public inspection.
- the property of the gas which is controlled in the known method is the Wobbe index.
- use is made of the fact that, under suitably chosen measuring conditions, there is a good correlation between the measured oxygen content in the combustion gases from the combustion chamber and the Wobbe index of the fuel gas, if the combustible part of said gas consists of lower hydrocarbons, as is the case with natural gas.
- a fuel gas of substantially constant Wobbe index is obtained by mixing gases of different origin and composition; the ratio in which the gases are mixed is controlled in such a manner that the measured oxygen content is constant. If then, besides the Wobbe index, also the gas pressure is constant, the heat load on the connected gas-fired equipment is constant.
- the known method has the drawback that it cannot be used when mixing various suitable gases is impossible, for example because only one fuel gas with varying heating characteristics is available.
- the object of the invention is to provide a method which does not have this drawback.
- the density of the supplied gas relative to air is measured, and from the oxygen content measured and the relative density measured the Wobbe index W as well as the calorific value H is determined; the relation is given by equation (2) above. If in addition the volume of gas consumed is measured, from the outcome of the summation, over time, of the momentary values of the product of the volume of gas consumed per unit of time and the calorific value the amount of heat energy supplied with the gas can be determined.
- gas of variable quality it is not the number of cubic metres of gas supplied but the heat energy supplied in the form of that gas that is to be charged.
- the volume-controlled flows of gas and combustion air are preferably fed to the combustion chamber by means of two volumetric pumps operating synchronously, for example positive-displacement pumps, the pressure of the sample gas fed to the sample-gas pump being controlled such as to equal the pressure of the air supplied to the combustion-air pump.
- the oxygen content of the air supplied is introduced into the calculation. Usually, this can be assumed to be the oxygen content of the ambient air (20.95 %). However, if the oxygen content of the combustion air may vary, this content is preferably measured. If the sample gas may have an oxygen content of some significance, e.g. more than 1.5 per cent by volume, this too is preferably measured; the value for the oxygen content in air is then increased by a correction factor calculated from the oxygen content in the gas.
- the corrected oxygen content 10 2] a is calculated by: where:
- the calorific value H and the Wobbe index W can thus be determined from the oxygen contents measured, the relative gas density d and the air and gas flows L and G, according to equations (5), (7) and (2).
- the measurements can be carried out continuously as well as periodically. In the latter case, the flows of gas and air are fed to the combustion chamber periodically. After an equilibrium has established itself, the supply of gas and air and the discharge of combustion gases are blocked. After the gas mixture confined in the combustion chamber has been combusted completely, the oxygen content of the combustion gases is measured.
- the gas consumption of the device itself is thus limited; the frequency at which the measurements are repeated is chosen in dependence on the rate at which the properties of the gas change.
- the invention relates also to a device for the realization of the method according to the invention for keeping the heat load of gas-fired equipment connected to a gas distribution grid constant, which device is provided with a combustion chamber, means to withdraw a volume-controlled sample flow from the fuel gas supplied and to feed this sample to the combustion chamber, means to add a volume-controlled flow of combustion air to the sample flow, means in the combustion chamber to enable complete combustion of the gas-air mixture, an oxygen meter to measure the oxygen content of the combustion gases and means to control a property of the gas in such a manner that said heat load is kept substantially constant.
- the device is provided with a pressure-controlling device for controlling the pressure of the gas supplied to the gas-fired equipment and with calculating means which from the oxygen content measured can calculate a setting signal and feed it to the pressure controlling device, such that at all times the following equation is substantially met: where:
- the connected gas-fired equipment may be gas-fired equipment with a combustion chamber as well as open gas-fired equipment, such as cooking apparatus.
- the means to add the volume-cotrolled flow of combustion air to the sample flow comprise a first volumetric pump and the means to withdraw a sample stream from the fuel gas supplied comprise a second volumetric pump in synchronous action with the first volumetric pump and a pressure-controlling device which can control the pressure of the gas fed to the second pump in such a manner that this ' pressure equals the pressure of the combustion air fed to the first pump.
- the pumps may be any suitable type of volumetric pump, for example positive-displacement pumps.
- the device When it is to be expected that the device will be used for fuel gases with an oxygen content of some significance, for example more than 1.5 per cent by volume, the device is preferably provided with an oxygen meter in order to measure this oxygen content in the flow of sample gas and feed a measuring signal to said calculating means. If the oxygen content of the combustion air may vary, the device is preferably also provided with an oxygen meter to measure this oxygen content as well and provide said calculating means with a measuring signal.
- the device is preferably provided with a density meter for measuring the density relative to air of the sample gas which can feed a measuring signal to said calculating means. From the oxygen contents measured and the density, the calculating means can calculate the Wobbe index and the calorific value of the gas.
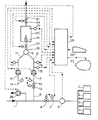
- a fuel gas for example natural gas
- a number of gas-fired equipment items 2 for example a battery of gas-fired industrial furnaces
- the pressure of the gas supplied to the gas-fired equipment items 2 is controlled by a pressure-controlling device 3, which controls a reducing valve 4; the consumed volume of gas is measured with a gas meter 5.
- a sample flow is withdrawn through a sample conduit 6 with the aid of a volumetric positive-displacement pump 7, which feeds the sample gas to a mixing chamber 9 through a conduit 8.
- a flow of combustion air is fed to the mixing chamber 9 through a conduit 11.
- a reducing valve 12 is incorporated which is controlled by a pressure-controlling device 13 which controls the pressure of the gas fed to pump 7 in such a way that this pressure equals the pressure of the combustion air fed to pump 10; to this end, the pressure-controlling device 13 receives a setting signal from the pressure meter 14 which measures the pressure of the air supplied to pump 10.
- the positive-displacement pumps 7 an 10 run synchronously, so that the ratio between the volumes of gas and air fed to the mixing chamber 9 is constant; in dependence on the average gas composition to be expected, the air volume: gas volume ratio is set at a value of between, for example, 11 and 16.
- the density relative to air of the gas flowing through conduit 8 can be measured with a gas density meter 15; the oxygen content of this gas can be measured with an oxygen meter 16 and the oxygen content of the combustion air flowing through conduit 11 can be measured with an oxygen meter 17.
- the gas-air mixture obtained is supplied to a burner 19 in a combustion chamber 20 through a conduit 18.
- the burner 19 is provided with an electric ignition (not shown).
- the combustion gases can be discharged through a discharge conduit 21.
- the oxygen content of the combustion gases can be measured with an oxygen meter 22.
- Conduits 18 and 21 can be closed with shut-off valves 23 and 24 respectively.
- the measuring data from the density meter 15, the oxygen meters 16, 17 and 22 and the gas meter 5 are fed to a microprocessor calculating unit 25, which from the measuring data generates numerical values for the calorific value and the Wobbe index of the gas supplied to the gas-fired equipment items 2 and for the amounts of gas heat energy consumed. These numerical values can be shown on a display 27.
- the calculating unit 25 generates a signal for the pressure setting of the pressure-controlling device 3, in such a way that the load on the gas-fired equipment 2 remains constant, as discussed above.
- a keyboard 26 the commands for controlling the complete installation can be given.
- the schema indicates the possibility to supply a correcting gas through conduit 28, controlled by calculating unit 25.
- a correcting gas may for example be desirable if the pressure to be set at pressure-controlling device 3 moves outside the desired control range, which might happen when the Wobbe index of the gas is temporarily considerably higher than it is on average. The pressure to be set might then become so low that the gas-fired equipment items 2 would no longer function reliably.
- a low-calorific or inert gas e.g. air
- the Wobbe index can then be lowered.
- the Wobbe index can be raised by addition of a high-calorific gas, if it is too low.
- the device according to the schema can be operated not only continuously but also discontinuously. Pumps 7 and 10 are then taken into operation periodically, after valves 23 and 24 have been opened. After and equilibrium has estabilished itself, valves 23 and 24 are closed and pumps 7 and 10 are stopped.
- the oxygen meter 22 is preferably provided with an electrochemical oxygen sensor (a so- called zirconium oxide sensor) with operates at high temperatures (approx. 800 °C), ensuring complete combustion of the gas mixture confined in the combustion chamber 20; the oxygen measurement takes place when this complete combustion has taken place.
- an electrochemical oxygen sensor a so- called zirconium oxide sensor
- the oxygen meter 16 must not be a meter operating at high temperatures, because at a high temperature any oxygen present in the fuel gas would react with the combustible components.
- Another type of oxygen meter should be used, for example a meter based on the paramagnetic properties of oxygen. If the oxygen content to be expected is negligible, the oxygen meter 16 will be dispensed with.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Combustion & Propulsion (AREA)
- Mechanical Engineering (AREA)
- General Engineering & Computer Science (AREA)
- Investigating Or Analyzing Materials Using Thermal Means (AREA)
- Separation By Low-Temperature Treatments (AREA)
- Devices And Processes Conducted In The Presence Of Fluids And Solid Particles (AREA)
- Bakery Products And Manufacturing Methods Therefor (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| NL8104308A NL8104308A (nl) | 1981-09-18 | 1981-09-18 | Werkwijze en inrichting voor het constant houden van de kalorische belasting van gastoestellen. |
| NL8104308 | 1981-09-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0075369A1 true EP0075369A1 (de) | 1983-03-30 |
Family
ID=19838089
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP82201152A Withdrawn EP0075369A1 (de) | 1981-09-18 | 1982-09-17 | Verfahren und Einrichtung zum Konstanthalten der Heizbelastung einer gasgefeuerten Anlage |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP0075369A1 (de) |
| DK (1) | DK415882A (de) |
| ES (1) | ES515781A0 (de) |
| NL (1) | NL8104308A (de) |
| NO (1) | NO156426C (de) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0156200A1 (de) * | 1984-03-08 | 1985-10-02 | Ruhrgas Aktiengesellschaft | Verfahren und Anordnung zur Bestimmung des Mischungsverhältnisses eines ein Sauerstoffträgergas und einen Brennstoff enthaltenden Gemisches |
| EP1022514A1 (de) * | 1999-01-22 | 2000-07-26 | Saint-Gobain Vitrage | Verfahren und Vorrichtung zur Regelung des gasförmigen Brennstoffstromes |
| WO2002077528A1 (de) * | 2001-03-23 | 2002-10-03 | Gvp Gesellschaft Zur Vermarktung Der Porenbrennertechnik Mbh | Verfahren und vorrichtung zur einstellung der luftzahl |
| CZ300482B6 (cs) * | 2003-08-27 | 2009-05-27 | Zpusob a zarízení pro regulaci výhrevnosti topného plynu | |
| ITMI20090153A1 (it) * | 2009-02-06 | 2010-08-07 | Ansaldo Energia Spa | Dispositivo e metodo per regolare l'alimentazione di gas ad una camera di combustione e impianto a turbina a gas comprendente tale dispositivo |
| WO2018054582A1 (de) * | 2016-09-23 | 2018-03-29 | Bosch Termotecnologia S.A. | Gasbereitungsvorrichtung und verfahren zur bereitstellung eines brenngasgemischs |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1016884B (de) * | 1955-02-14 | 1957-10-03 | Keram Ind Bedarfs K G | Vorrichtung zur Beurteilung der Ofenatmosphaere fuer Brennoefen, insbesondere Tunneloefen |
| US2829954A (en) * | 1954-11-30 | 1958-04-08 | Surface Combustion Corp | Apparatus for analyzing gas |
| GB1565310A (en) * | 1977-12-01 | 1980-04-16 | Battelle Development Corp | Method and apparatus for controlling fuel to oxidant ratioof a burner |
| GB2036290A (en) * | 1978-11-22 | 1980-06-25 | Hamworthy Engineering | Fuel sampling system |
| GB2080512A (en) * | 1980-07-04 | 1982-02-03 | Snam Spa | Heat load control of a plant fed with combustible gas |
-
1981
- 1981-09-18 NL NL8104308A patent/NL8104308A/nl not_active Application Discontinuation
-
1982
- 1982-09-17 EP EP82201152A patent/EP0075369A1/de not_active Withdrawn
- 1982-09-17 ES ES515781A patent/ES515781A0/es active Granted
- 1982-09-17 NO NO823164A patent/NO156426C/no unknown
- 1982-09-17 DK DK415882A patent/DK415882A/da not_active Application Discontinuation
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2829954A (en) * | 1954-11-30 | 1958-04-08 | Surface Combustion Corp | Apparatus for analyzing gas |
| DE1016884B (de) * | 1955-02-14 | 1957-10-03 | Keram Ind Bedarfs K G | Vorrichtung zur Beurteilung der Ofenatmosphaere fuer Brennoefen, insbesondere Tunneloefen |
| GB1565310A (en) * | 1977-12-01 | 1980-04-16 | Battelle Development Corp | Method and apparatus for controlling fuel to oxidant ratioof a burner |
| GB2036290A (en) * | 1978-11-22 | 1980-06-25 | Hamworthy Engineering | Fuel sampling system |
| GB2080512A (en) * | 1980-07-04 | 1982-02-03 | Snam Spa | Heat load control of a plant fed with combustible gas |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0156200A1 (de) * | 1984-03-08 | 1985-10-02 | Ruhrgas Aktiengesellschaft | Verfahren und Anordnung zur Bestimmung des Mischungsverhältnisses eines ein Sauerstoffträgergas und einen Brennstoff enthaltenden Gemisches |
| EP1022514A1 (de) * | 1999-01-22 | 2000-07-26 | Saint-Gobain Vitrage | Verfahren und Vorrichtung zur Regelung des gasförmigen Brennstoffstromes |
| FR2788839A1 (fr) * | 1999-01-22 | 2000-07-28 | Saint Gobain Vitrage | Procede et dispositif de regulation d'un courant de combustible gazeux |
| US6495731B1 (en) | 1999-01-22 | 2002-12-17 | Saint-Gobain Glass France | Method and apparatus for regulating a stream of gaseous fuel |
| CZ297204B6 (cs) * | 1999-01-22 | 2006-10-11 | Saint-Gobain Vitrage | Zpusob a zarízení pro regulaci proudu plynného paliva a sklárská pec vyuzívající tento zpusob a/nebo toto zarízení |
| WO2002077528A1 (de) * | 2001-03-23 | 2002-10-03 | Gvp Gesellschaft Zur Vermarktung Der Porenbrennertechnik Mbh | Verfahren und vorrichtung zur einstellung der luftzahl |
| US6939127B2 (en) | 2001-03-23 | 2005-09-06 | Gvp Gesellschaft Zur Vermarktung Der Porenbrennertechnik Mbh | Method and device for adjusting air ratio |
| US7223094B2 (en) | 2001-03-23 | 2007-05-29 | Emb-Papst Landshut Gmbh | Blower for combustion air |
| CZ300482B6 (cs) * | 2003-08-27 | 2009-05-27 | Zpusob a zarízení pro regulaci výhrevnosti topného plynu | |
| ITMI20090153A1 (it) * | 2009-02-06 | 2010-08-07 | Ansaldo Energia Spa | Dispositivo e metodo per regolare l'alimentazione di gas ad una camera di combustione e impianto a turbina a gas comprendente tale dispositivo |
| WO2018054582A1 (de) * | 2016-09-23 | 2018-03-29 | Bosch Termotecnologia S.A. | Gasbereitungsvorrichtung und verfahren zur bereitstellung eines brenngasgemischs |
Also Published As
| Publication number | Publication date |
|---|---|
| ES8400584A1 (es) | 1983-10-16 |
| ES515781A0 (es) | 1983-10-16 |
| NL8104308A (nl) | 1983-04-18 |
| DK415882A (da) | 1983-03-19 |
| NO823164L (no) | 1983-03-21 |
| NO156426B (no) | 1987-06-09 |
| NO156426C (no) | 1987-09-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5816705A (en) | Measuring heating value of a gas using flameless combustion | |
| EP0498809B1 (de) | Verbrennungsregelung | |
| EP2241811A1 (de) | Kraftstoffzuführungsvorrichtung | |
| CN108458486A (zh) | 一种热风炉燃烧自动控制系统及其控制方法 | |
| EP2241810A1 (de) | Durchflussregler | |
| US3734675A (en) | Burner controlling apparatus and method | |
| US4351614A (en) | Method of and apparatus for continually monitoring the heating value of a fuel gas using a combustibility meter | |
| CN100570218C (zh) | 带虚拟λ传感器的燃料控制系统 | |
| CN208108511U (zh) | 一种热风炉燃烧自动控制系统 | |
| EP0075369A1 (de) | Verfahren und Einrichtung zum Konstanthalten der Heizbelastung einer gasgefeuerten Anlage | |
| US6431457B1 (en) | Air heater control | |
| EP0060681B1 (de) | Analysator für brennbares Gas | |
| US6371147B1 (en) | Evaluation and regulation of the thermal power of a flow of combustible gas; characterization of a thermal mass flowmeter | |
| NL7808476A (nl) | Inrichting voor het bepalen van een aan de wobbe-index van een gas of gasmengsel gecorreleerde grootheid, als- mede werkwijze voor het toepassen van deze inrichting. | |
| US5748492A (en) | Measuring heating value using catalytic combustion | |
| US6129542A (en) | Dual mode pilot burner | |
| JP3453899B2 (ja) | 空燃比センサの特性評価方法及び評価装置 | |
| EP0323658A2 (de) | Verfahren zum Bestimmen der Wobbezahl eines Gasgemisches | |
| CA1168062A (en) | Method and apparatus for heat flow measurement | |
| EP0608736A2 (de) | Volumendurchflusskorrektor mit einem Densitometer | |
| KR20030052912A (ko) | 다종연료 연소시의 산소농도 제어방법 | |
| US20220282866A1 (en) | Power Output Determination by Way of a Fuel Parameter | |
| SU1244455A1 (ru) | Способ управлени сжиганием колошникового газа и устройство дл его осуществлени | |
| JPS6280222A (ja) | 多数バ−ナ炉における燃焼制御装置 | |
| JPS6361565B2 (de) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19830802 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN WITHDRAWN |
|
| 18W | Application withdrawn |
Withdrawal date: 19870518 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: BERGMAN, ALBERT PIETER |