EP0065322A1 - Gold coloured alloy for coins - Google Patents
Gold coloured alloy for coins Download PDFInfo
- Publication number
- EP0065322A1 EP0065322A1 EP82200368A EP82200368A EP0065322A1 EP 0065322 A1 EP0065322 A1 EP 0065322A1 EP 82200368 A EP82200368 A EP 82200368A EP 82200368 A EP82200368 A EP 82200368A EP 0065322 A1 EP0065322 A1 EP 0065322A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- coins
- copper
- aluminum
- tin
- gold
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 229910045601 alloy Inorganic materials 0.000 title claims abstract description 14
- 239000000956 alloy Substances 0.000 title claims abstract description 14
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 title abstract description 7
- 239000010931 gold Substances 0.000 title abstract description 7
- 229910052737 gold Inorganic materials 0.000 title abstract description 7
- 229910052782 aluminium Inorganic materials 0.000 claims abstract description 19
- 229910052718 tin Inorganic materials 0.000 claims abstract description 17
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims abstract description 13
- 229910052802 copper Inorganic materials 0.000 claims abstract description 13
- 239000010949 copper Substances 0.000 claims abstract description 13
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims abstract description 12
- 239000000463 material Substances 0.000 claims abstract description 11
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims abstract description 10
- 239000012535 impurity Substances 0.000 claims abstract description 4
- 238000004519 manufacturing process Methods 0.000 claims description 7
- 229910000881 Cu alloy Inorganic materials 0.000 claims description 6
- -1 unavoidable Chemical compound 0.000 claims description 3
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 10
- 229910052759 nickel Inorganic materials 0.000 description 5
- 239000003086 colorant Substances 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- 206010013786 Dry skin Diseases 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 238000004049 embossing Methods 0.000 description 1
- 238000009499 grossing Methods 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 238000005554 pickling Methods 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 239000010939 rose gold Substances 0.000 description 1
- 229910001112 rose gold Inorganic materials 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
- C22C9/01—Alloys based on copper with aluminium as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
- C22C9/02—Alloys based on copper with tin as the next major constituent
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12493—Composite; i.e., plural, adjacent, spatially distinct metal components [e.g., layers, joint, etc.]
- Y10T428/12771—Transition metal-base component
- Y10T428/12861—Group VIII or IB metal-base component
- Y10T428/12903—Cu-base component
- Y10T428/1291—Next to Co-, Cu-, or Ni-base component
Definitions
- the invention relates to the use of a copper-based alloy with 1 to 7% tin and 1 to 7% aluminum, but the sum of the percentages of aluminum and tin does not exceed 10%, the rest of copper including unavoidable, production-related impurities as a material for the production of coins or the like, which must have a golden hue and high tarnish resistance.
- the coin authorities are therefore considering switching to newer, higher value coins from smaller pieces and distinguishing them from the existing highest value coins of the system by a different coin color.
- Gold shades are particularly suitable for this because they combine certain values and most of the coins in circulation - at least the higher-quality ones - have silver shades.
- Coin materials with gold-like colors are known and some have already been used. Almost without exception, these are copper-based alloys that e.g. are alloyed with 25% zinc, with 20% zinc and 1% nickel, with 5 to 6% aluminum and 2% nickel or with 2% aluminum and 6% nickel.
- all these materials have the disadvantage that they lose their originally brilliant appearance relatively quickly in use and assume a matt and more brownish color. This disadvantage is accepted in the case of coins of low value. For higher-value coins, such a discoloration, which soon arises when used, is not acceptable. For this reason, silver-colored materials, preferably nickel or alloys with a relatively high nickel content, have been used for higher-value coins.
- this object can be achieved if as a material for the production of coins or the like, a copper-based alloy with 1 to 7% tin and 1 to 7% aluminum, but the sum of the percentages of aluminum and tin does not exceed 10%, the rest of copper including unavoidable, production-related impurities are used.
- the sum of the percentages of tin and aluminum expediently does not exceed 9%.
- a copper alloy with 5 to 7% Sn and 1 to 3% Al is particularly suitable.
- a copper alloy with 2.5 to 3.5% Sn and 1.5 to 5.5% Al can be used for the purpose according to the invention.
- This pretreatment was necessary in order to be able to check the minted or non-minted coin blanks for tarnish resistance in the state in which they are also available in practice.
- the coin blanks were also degreased to remove any fingerprints before the start of the start-up test.
- Atmospheres of different aggressiveness were used as test media, namely:
- test duration was 20 days.
- the samples were then removed and assessed individually and separately for each test medium visually according to a point system with the marks 1 to 5, 1 meaning very good tarnish resistance and 5 very poor tarnish resistance with a strongly tarnished surface.
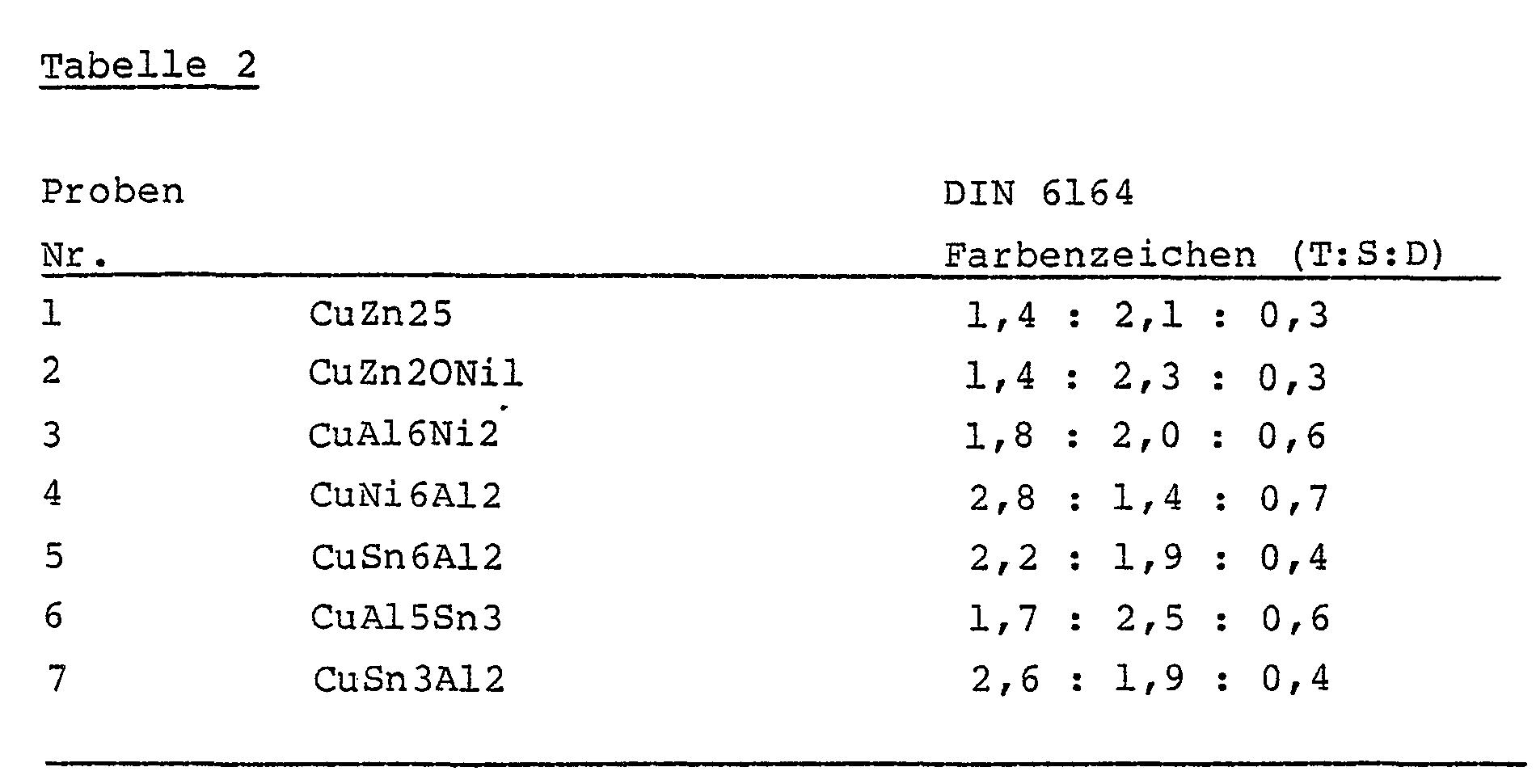
- the gold shades were determined by determining color measures according to DIN 5033 and measures according to the color system DIN 6164 from the spectral reflectance.
- the hue T, the saturation level S and the dark level D in the form of the "color symbol" (T: S: D) as a measure according to the color system DIN 6164 for the copper alloys examined are summarized in Table 2:
- samples 5 and 7 have almost the same color saturation as the well-known coin alloy CuA16Ni2, but that their color is closer to rose gold, which gives the impression of a warmer gold tone, which is preferred for coins.
- the sample 6 is more in the light yellow area, but in view of its good tarnish resistance it is still much more suitable as a coin material than the comparison materials.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Adornments (AREA)
- Gloves (AREA)
- Detergent Compositions (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Control Of Vending Devices And Auxiliary Devices For Vending Devices (AREA)
- Auxiliary Devices For And Details Of Packaging Control (AREA)
- Seeds, Soups, And Other Foods (AREA)
- Electroplating And Plating Baths Therefor (AREA)
- Chemically Coating (AREA)
- Crucibles And Fluidized-Bed Furnaces (AREA)
Abstract
Description
Die Erfindung bezieht sich auf die Verwendung einer Kupferbasislegierung mit 1 bis 7 % Zinn und 1 bis 7 % Aluminium, wobei jedoch die Summe der Prozentgehalte an Aluminium und Zinn 10 % nicht übersteigt, Rest Kupfer einschließlich unvermeidlicher, herstellungsbedingter Verunreinigungen als Werkstoff zur Herstellung von Münzen oder dergleichen, die einen goldenden Farbton und eine hohe Anlaufbeständigkeit aufweisen müssen.The invention relates to the use of a copper-based alloy with 1 to 7% tin and 1 to 7% aluminum, but the sum of the percentages of aluminum and tin does not exceed 10%, the rest of copper including unavoidable, production-related impurities as a material for the production of coins or the like, which must have a golden hue and high tarnish resistance.
Wegen der seit Jahren anhaltenden, weltweit mehr oder weniger starken Inflatiönstendenz und der erheblichen Zunahme der Geschäfte über Waren- und Dienstleistungsautomaten, ist ein Bedarf an höherwertigen Münzen entstanden. So wird beispielsweise in Deutschland schon einige Zeit die Einführung eines 10 DM-Stückes erwogen. Für höherwertige Münzen kommt in den meisten bestehenden Münzsystemen eine Unterscheidung von den vorhandenen höchsten Münzwerten durch entsprechende Vergrößerung der Münzstücke nicht in Betracht. Das höhere Gewicht und das größere Volumen würden nicht nur die Handhabung im Wortsinne "erschweren", sondern auch mit einem erheblichen Metallbedarf verbunden sein, wobei zu berücksichtigen ist, daß bei steigenden Preisen für die für Münzen geeigneten Metalle die Spanne zwischen Metallwert und Prägewert immer kleiner wird.Because of the persistent, more or less strong inflation trend worldwide and the considerable increase in business via vending and service machines, there has been a need for higher-value coins. For example, the introduction of a DM 10 piece has been considered for some time in Germany. In most existing coin systems, a distinction from the existing highest coin values by correspondingly enlarging the coin pieces is not considered for higher-value coins. The higher weight and the larger volume would not only "difficult" the handling in the literal sense, but would also be connected with a considerable metal requirement, whereby it must be taken into account that with increasing prices for the metals suitable for coins the range between metal value and minting value becomes smaller and smaller becomes.
Die Münzbehörden erwägen daher, bei neuen, höherwertigen Münzen auf kleinere Stücke überzugehen und diese von den vorhandenen höchstwertigen Münzen des Systems durch eine andere Münzfarbe zu unterscheiden. Hierfür eignen sich insbesondere Goldfarbtöne, weil damit bestimmte Wertvorstellungen verbunden werden und die meisten im Umlauf befindlichen Münzen - jedenfalls die höherwertigen - silberne Farbtöne aufweisen.The coin authorities are therefore considering switching to newer, higher value coins from smaller pieces and distinguishing them from the existing highest value coins of the system by a different coin color. Gold shades are particularly suitable for this because they combine certain values and most of the coins in circulation - at least the higher-quality ones - have silver shades.
Münzwerkstoffe mit goldähnlichen Farbtönen sind bekannt und teilweise auch schon benutzt worden. Dabei handelt es sich fast ausnahmslos um Kupferbasislegierungen, die z.B. mit 25 % Zink, mit 20 % Zink und 1 % Nickel, mit 5 bis 6 % Aluminium und 2 % Nickel oder mit 2 % Aluminium und 6 % Nickel legiert sind. Alle diese Werkstoffe haben jedoch den Nachteil, daß sie im Gebrauch verhältnismäßig rasch ihr ursprünglich brillantes Aussehen verlieren und einen matten und mehr ins Bräunliche gehenden Farbton annehmen. Dieser Nachteil wird im Falle von Münzen mit geringem Wert in Kauf genommen. Für höherwertige Münzen ist eine derartige, sich bei Gebrauch alsbald einstellende Verfärbung nicht akzeptabel. Deshalb hat man auch bisher für höherwertige Münzen silberfarbene Werkstoffe, vorzugsweise Nickel oder Legierungen mit einem verhältnismäßig hohen Nickelanteil, verwendet.Coin materials with gold-like colors are known and some have already been used. Almost without exception, these are copper-based alloys that e.g. are alloyed with 25% zinc, with 20% zinc and 1% nickel, with 5 to 6% aluminum and 2% nickel or with 2% aluminum and 6% nickel. However, all these materials have the disadvantage that they lose their originally brilliant appearance relatively quickly in use and assume a matt and more brownish color. This disadvantage is accepted in the case of coins of low value. For higher-value coins, such a discoloration, which soon arises when used, is not acceptable. For this reason, silver-colored materials, preferably nickel or alloys with a relatively high nickel content, have been used for higher-value coins.
Es besteht somit die Aufgabe, einen goldfarbenen Werkstoff für die Herstellung von Münzen oder dergleichen vorzuschlagen, der sich einerseits durch Gießen, Walzen und Prägen gut zu Münzen verarbeiten läßt und andererseits den ursprünglich vorhandenen goldendenen Farbton möglichst lange behält, d.h., der eine hohe Anlaufbeständigkeit aufweist.It is therefore the task of proposing a gold-colored material for the production of coins or the like, which can be processed well into coins on the one hand by casting, rolling and minting and on the other hand retains the originally existing gold-colored hue as long as possible, ie which has a high tarnish resistance .
Überraschenderweise hat sich herausgestellt, daß diese Aufgabe gelöst werden kann, wenn als Werkstoff zur Herstellung von Münzen oder dergleichen eine Kupferbasislegierung mit 1 bis 7 % Zinn und 1 bis 7 % Aluminium, wobei jedoch die Summe der Prozentgehalte an Aluminium und Zinn 10 % nicht übersteigt, Rest Kupfer einschließlich unvermeidlicher, herstellungsbedingter Verunreinigungen verwendet wird. Die Summe der Prozentgehalte an Zinn und Aluminium übersteigt zweckmäßigerweise nicht 9 %. Besonders geeignet ist eine Kupferlegierung mit 5 bis 7 % Sn und 1 bis 3 % Al. Ferner kann für den erfindungsgemäßen Zweck eine Kupferlegierung mit 2,5 bis 3,5 % Sn und 1,5 bis 5,5 % Al verwendet werden.Surprisingly, it has been found that this object can be achieved if as a material for the production of coins or the like, a copper-based alloy with 1 to 7% tin and 1 to 7% aluminum, but the sum of the percentages of aluminum and tin does not exceed 10%, the rest of copper including unavoidable, production-related impurities are used. The sum of the percentages of tin and aluminum expediently does not exceed 9%. A copper alloy with 5 to 7% Sn and 1 to 3% Al is particularly suitable. Furthermore, a copper alloy with 2.5 to 3.5% Sn and 1.5 to 5.5% Al can be used for the purpose according to the invention.
Zur Prüfung der Anlaufbeständigkeit wurden gestanzte Münzrohlinge aus der erfindungsgemäß zu verwendenden Kupferbasislegierung (Probe 5: 6 % Sn, 2 % Al; Probe 6: 5 % Al, 3 % Sn; Probe 7: 2 % Al, 3 % Sn), mit oder ohne Prägung zunächst folgender Vorbehandlung unterzogen:
- Blankbeizen,
- in Wasser Spülen,
- mit einem Glättmittel behandeln,
- ohne Abspülen in Reisschrot trocknen,
- gegebenenfalls Prägen ohne weiteres Schmiermittel.
- Bright pickling,
- rinse in water,
- treat with a smoothing agent,
- dry in rice grist without rinsing,
- if necessary, stamp without further lubricant.
Diese Vorbehandlung war erforderlich, um die geprägten oder ungeprägten Münzrohlinge in dem Zustand auf Anlaufbeständigkeit prüfen zu können, in dem sie auch in der Praxis vorliegen. Um evtl. Fingerabdrücke vor Beginn des Anlauf testes zu entfernen, wurden die Münzrohlinge außerdem noch entfettet.This pretreatment was necessary in order to be able to check the minted or non-minted coin blanks for tarnish resistance in the state in which they are also available in practice. The coin blanks were also degreased to remove any fingerprints before the start of the start-up test.
Als Prüfmedien wurden Atmosphären verschiedener Aggressivität verwendet, und zwar:
Die Prüfdauer betrug 20 Tage. Danach wurden die Proben entnommen und einzeln und getrennt für jedes Prüfmedium visuell nach einem Punktsystem mit den Noten 1 bis 5 beurteilt, wobei 1 sehr gute Anlaufbeständigkeit und 5 sehr schlechte Anlaufbeständigkeit mit stark angelaufener Oberfläche bedeuten.The test duration was 20 days. The samples were then removed and assessed individually and separately for each test medium visually according to a point system with the
In gleicher Weise wurden Münzrohlinge aus den bereits genannten, einschlägig verwendeten Kupferbasislegierungen (Proben 1 bis 4) vorbehandelt und getestet. Die Ergebnisse sind in Tabelle 1 zusammengefaßt.
Die Goldfarbtöne wurden durch Bestimmung von Farbmaßzahlen nach DIN 5033 und Maßzahlen nach dem Farbsystem DIN 6164 aus dem spektralen Reflexionsgrad ermittelt. Der Buntton T, die Sättigungsstufe S und die Dunkelstufe D in Form des "Farbzeichens" (T:S:D) als Maßzahl nach dem Farbsystem DIN 6164 für die untersuchten Kupferlegierungen sind in Tabelle 2 zusammengestellt:
Man erkennt, daß die Proben 5 und 7 fast die gleiche Farbsättigung wie die bekannte Münzlegierung CuA16Ni2 aufweisen, daß sie im Farbton aber näher am Rose-Gold liegt, was den Eindruck eines wärmeren Goldtones vermittelt, der für Münzen bevorzugt wird. Die Probe 6 liegt zwar mehr im hellgelben Bereich, ist im Hinblick auf ihre gute Anlaufbeständigkeit aber immer noch wesentlich besser als Münzwerkstoff geeignet, als die Vergleichswerkstoffe.It can be seen that
Claims (4)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT82200368T ATE10952T1 (en) | 1981-04-23 | 1982-03-25 | GOLD COLORED COIN MATERIAL. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3116125 | 1981-04-23 | ||
| DE3116125A DE3116125C2 (en) | 1981-04-23 | 1981-04-23 | Use of a copper alloy as a material for gold-colored coins |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0065322A1 true EP0065322A1 (en) | 1982-11-24 |
| EP0065322B1 EP0065322B1 (en) | 1984-12-27 |
Family
ID=6130658
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP82200368A Expired EP0065322B1 (en) | 1981-04-23 | 1982-03-25 | Gold coloured alloy for coins |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US4436790A (en) |
| EP (1) | EP0065322B1 (en) |
| JP (1) | JPS57181350A (en) |
| KR (1) | KR830010215A (en) |
| AT (1) | ATE10952T1 (en) |
| CA (1) | CA1209829A (en) |
| DE (2) | DE3116125C2 (en) |
| DK (1) | DK179382A (en) |
| ES (1) | ES511622A0 (en) |
| FI (1) | FI69873C (en) |
| NO (1) | NO155398C (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1984003522A1 (en) * | 1983-03-01 | 1984-09-13 | Imi Kynoch Plc | Alloy for coins and the like |
| EP0450883A2 (en) * | 1990-04-02 | 1991-10-09 | Sherritt Inc. | Electroplated Blank for Coins, Medallions, Tokens or Tags |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA1219708A (en) * | 1984-05-01 | 1987-03-31 | Michael J.H. Ruscoe | Aureate coins, medallions and tokens |
| DE3428951A1 (en) * | 1984-08-06 | 1986-02-13 | Leybold-Heraeus GmbH, 5000 Köln | WITH A COATING LAYER FROM GOLD OR A GOLD-CONTAINING MATERIAL-COVERED DECORATIVE USED ITEM AND METHOD FOR THE PRODUCTION THEREOF |
| USRE35624E (en) * | 1990-01-05 | 1997-10-07 | Kiilunen; David D. | Wires made of copper-based alloy compositions |
| US5312696A (en) * | 1991-09-16 | 1994-05-17 | United Technologies Corporation | Method for reducing fretting wear between contacting surfaces |
| US5472796A (en) * | 1995-01-13 | 1995-12-05 | Olin Corporation | Copper alloy clad for coinage |
| US6089828A (en) * | 1998-02-26 | 2000-07-18 | United Technologies Corporation | Coated article and method for inhibiting frictional wear between mating titanium alloy substrates in a gas turbine engine |
| JP4424810B2 (en) * | 2000-03-27 | 2010-03-03 | 株式会社小松製作所 | Sintered material |
| US6656606B1 (en) | 2000-08-17 | 2003-12-02 | The Westaim Corporation | Electroplated aluminum parts and process of production |
| US6737175B2 (en) | 2001-08-03 | 2004-05-18 | Exxonmobil Research And Engineering Company | Metal dusting resistant copper based alloy surfaces |
| SE525460C2 (en) * | 2002-02-28 | 2005-02-22 | Sandvik Ab | Use of a copper alloy in carburizing environments |
| US7891898B2 (en) * | 2005-01-28 | 2011-02-22 | S.C. Johnson & Son, Inc. | Cleaning pad for wet, damp or dry cleaning |
| AU2009202339C1 (en) | 2008-06-13 | 2012-03-22 | Monnaie Royale Canadienne/ Royal Canadian Mint | Control of electromagnetic signals of coins by multi-ply plating technology |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR530585A (en) * | 1921-02-07 | 1921-12-26 | Mitsubishi Zosen Kaisha Ltd | Alloy enhancements |
| GB268654A (en) * | 1926-04-15 | 1927-04-07 | Metallbank & Metallurg Ges Ag | Copper-aluminium-alloys |
| DE1216547B (en) * | 1955-08-08 | 1966-05-12 | Ver Deutsche Metallwerke Ag | Tin bronzes containing aluminum |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1630999A (en) | 1926-01-28 | 1927-05-31 | American Brass Co | Wrought-metal article |
| US1881257A (en) | 1932-08-16 | 1932-10-04 | American Brass Co | Wrought metal article |
| US2133845A (en) | 1936-03-30 | 1938-10-18 | Chase Brass & Copper Co | Corrosion resistant tubular article |
| US2231940A (en) | 1939-12-28 | 1941-02-18 | Nylander Charles Victor | Alloy |
| US4292377A (en) | 1980-01-25 | 1981-09-29 | The International Nickel Co., Inc. | Gold colored laminated composite material having magnetic properties |
| US4330599A (en) | 1980-06-09 | 1982-05-18 | Olin Corporation | Composite material |
-
1981
- 1981-04-23 DE DE3116125A patent/DE3116125C2/en not_active Expired
-
1982
- 1982-03-25 EP EP82200368A patent/EP0065322B1/en not_active Expired
- 1982-03-25 DE DE8282200368T patent/DE3261673D1/en not_active Expired
- 1982-03-25 AT AT82200368T patent/ATE10952T1/en not_active IP Right Cessation
- 1982-04-06 FI FI821220A patent/FI69873C/en not_active IP Right Cessation
- 1982-04-15 NO NO821238A patent/NO155398C/en unknown
- 1982-04-22 ES ES511622A patent/ES511622A0/en active Granted
- 1982-04-22 CA CA000401462A patent/CA1209829A/en not_active Expired
- 1982-04-22 DK DK179382A patent/DK179382A/en not_active Application Discontinuation
- 1982-04-22 KR KR1019820001778A patent/KR830010215A/en unknown
- 1982-04-22 US US06/370,692 patent/US4436790A/en not_active Expired - Fee Related
- 1982-04-23 JP JP57069440A patent/JPS57181350A/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR530585A (en) * | 1921-02-07 | 1921-12-26 | Mitsubishi Zosen Kaisha Ltd | Alloy enhancements |
| GB268654A (en) * | 1926-04-15 | 1927-04-07 | Metallbank & Metallurg Ges Ag | Copper-aluminium-alloys |
| DE1216547B (en) * | 1955-08-08 | 1966-05-12 | Ver Deutsche Metallwerke Ag | Tin bronzes containing aluminum |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1984003522A1 (en) * | 1983-03-01 | 1984-09-13 | Imi Kynoch Plc | Alloy for coins and the like |
| EP0450883A2 (en) * | 1990-04-02 | 1991-10-09 | Sherritt Inc. | Electroplated Blank for Coins, Medallions, Tokens or Tags |
| EP0450883A3 (en) * | 1990-04-02 | 1992-12-30 | Sherritt Gordon Limited | Electroplated blank for coins, medallions and tokens |
Also Published As
| Publication number | Publication date |
|---|---|
| KR830010215A (en) | 1983-12-26 |
| DK179382A (en) | 1982-10-24 |
| US4436790A (en) | 1984-03-13 |
| DE3116125A1 (en) | 1982-11-25 |
| CA1209829A (en) | 1986-08-19 |
| EP0065322B1 (en) | 1984-12-27 |
| DE3261673D1 (en) | 1985-02-07 |
| ATE10952T1 (en) | 1985-01-15 |
| DE3116125C2 (en) | 1983-02-10 |
| NO155398B (en) | 1986-12-15 |
| NO821238L (en) | 1982-10-25 |
| FI69873B (en) | 1985-12-31 |
| ES8400495A1 (en) | 1983-10-16 |
| FI821220L (en) | 1982-10-24 |
| NO155398C (en) | 1987-03-25 |
| JPS57181350A (en) | 1982-11-08 |
| ES511622A0 (en) | 1983-10-16 |
| FI69873C (en) | 1986-05-26 |
| FI821220A0 (en) | 1982-04-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE3116135C2 (en) | Use of a copper alloy as a material for gold-colored coins | |
| EP0065322B1 (en) | Gold coloured alloy for coins | |
| DE60003555T2 (en) | COPPER ALLOY WITH GOLDEN APPEARANCE | |
| DE4213897B4 (en) | Use of a silver alloy for the production of goldsmith, jeweler, coin or medal work pieces | |
| EP0457478A1 (en) | Machinable lead-free wrought copper-based alloys | |
| CH655130A5 (en) | NEW PALLADIUM ALLOY AND THESE DENTAL RESTORATIONS. | |
| DE19958800A1 (en) | White gold jewelry alloy for all jewelry purposes contains alloying additions of silver and iron | |
| EP1798298A1 (en) | Use of a low-migration copper alloy and parts made of such alloy | |
| EP0135547B1 (en) | Alloy for coins and the like | |
| DE2251058A1 (en) | METAL AMINATE COMPOSITE MATERIAL AND ITS USE FOR THE MANUFACTURE OF COINS | |
| DE2908203A1 (en) | GOLD-SILVER ALLOYS WITH GOOD STARTING RESISTANCE FOR DENTAL TECHNOLOGY | |
| DE60030849T2 (en) | JEWELRY ALLOY COMPOSITION | |
| DE3235832A1 (en) | Use of a gold-coloured copper alloy for the production of fittings and the like | |
| DE3522656A1 (en) | Production of coin blanks | |
| DE3819904C1 (en) | ||
| DE3235833A1 (en) | Use of a gold-coloured copper alloy for the production of fittings and the like | |
| DE2620733C2 (en) | Use of copper-based alloys for dental purposes | |
| EP0931843A1 (en) | Copper-Nickel-Zinc-Manganese-Aluminium alloy and its use | |
| DE2909542A1 (en) | USE OF A COPPER-TITANIUM FINE-INCLINING ALLOY | |
| DE3307182A1 (en) | Alloy for electrical contacts and use for such an alloy | |
| DE19646657C1 (en) | Copper-containing stainless steel clad laminate | |
| DE29605756U1 (en) | Autograph cards made of precious metal | |
| DE202007006994U1 (en) | Platinum alloy and a piece of jewelry made from the platinum alloy, in particular a wedding ring | |
| DE29517278U1 (en) | Precious metal phone cards | |
| DE29610045U1 (en) | Annual pocket calendar made of precious metal |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT SE |
|
| 17P | Request for examination filed |
Effective date: 19830301 |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI SE |
|
| REF | Corresponds to: |
Ref document number: 10952 Country of ref document: AT Date of ref document: 19850115 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3261673 Country of ref document: DE Date of ref document: 19850207 |
|
| ET | Fr: translation filed | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: KABEL- UND METALLWERKE GUTEHOFFNUNGSHUETTE AKTIEN Effective date: 19850921 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: KABEL- UND METALLWERKE GUTEHOFFNUNGSHUETTE AKTIEN Effective date: 19850921 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: KABEL- UND METALLWERKE GUTEHOFFNUNGSHUETTE AKTIEN Effective date: 19850921 |
|
| PLBN | Opposition rejected |
Free format text: ORIGINAL CODE: 0009273 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: OPPOSITION REJECTED |
|
| 27O | Opposition rejected |
Effective date: 19871026 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19890209 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19890325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19890326 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19890331 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19890331 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19890412 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19890608 Year of fee payment: 8 |
|
| BERE | Be: lapsed |
Owner name: VEREINIGTE DEUTSCHE METALLWERKE A.G. Effective date: 19890331 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19900325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Effective date: 19900331 Ref country code: LI Effective date: 19900331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19901130 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19901201 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| EUG | Se: european patent has lapsed |
Ref document number: 82200368.7 Effective date: 19900125 |