EP0061696A2 - Process for improving the flow of mineral oils - Google Patents
Process for improving the flow of mineral oils Download PDFInfo
- Publication number
- EP0061696A2 EP0061696A2 EP82102388A EP82102388A EP0061696A2 EP 0061696 A2 EP0061696 A2 EP 0061696A2 EP 82102388 A EP82102388 A EP 82102388A EP 82102388 A EP82102388 A EP 82102388A EP 0061696 A2 EP0061696 A2 EP 0061696A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- ethylene
- weight
- copolymer
- vinyl
- middle distillate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000002480 mineral oil Substances 0.000 title claims abstract description 17
- 229920001577 copolymer Polymers 0.000 claims abstract description 34
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims abstract description 22
- 239000005977 Ethylene Substances 0.000 claims abstract description 22
- 229920002554 vinyl polymer Polymers 0.000 claims abstract description 16
- -1 vinyl acid amide Chemical class 0.000 claims abstract description 12
- 235000010446 mineral oil Nutrition 0.000 claims description 9
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 claims description 9
- 229920001038 ethylene copolymer Polymers 0.000 claims description 5
- 239000005038 ethylene vinyl acetate Substances 0.000 claims description 3
- 239000000178 monomer Substances 0.000 claims description 3
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 claims description 3
- 239000002253 acid Substances 0.000 claims 4
- 239000000243 solution Substances 0.000 description 9
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 7
- 239000008096 xylene Substances 0.000 description 7
- PNLUGRYDUHRLOF-UHFFFAOYSA-N n-ethenyl-n-methylacetamide Chemical compound C=CN(C)C(C)=O PNLUGRYDUHRLOF-UHFFFAOYSA-N 0.000 description 6
- 238000006116 polymerization reaction Methods 0.000 description 6
- 238000009835 boiling Methods 0.000 description 5
- 239000012188 paraffin wax Substances 0.000 description 5
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 4
- 239000000654 additive Substances 0.000 description 3
- 239000010779 crude oil Substances 0.000 description 3
- 239000003999 initiator Substances 0.000 description 3
- 239000000155 melt Substances 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- BAPJBEWLBFYGME-UHFFFAOYSA-N Methyl acrylate Chemical compound COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- RAHZWNYVWXNFOC-UHFFFAOYSA-N Sulphur dioxide Chemical compound O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- LRSCEVQEEBMAHN-UHFFFAOYSA-N n-methylbut-3-enamide Chemical compound CNC(=O)CC=C LRSCEVQEEBMAHN-UHFFFAOYSA-N 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- ISXSCDLOGDJUNJ-UHFFFAOYSA-N tert-butyl prop-2-enoate Chemical compound CC(C)(C)OC(=O)C=C ISXSCDLOGDJUNJ-UHFFFAOYSA-N 0.000 description 2
- BQCIDUSAKPWEOX-UHFFFAOYSA-N 1,1-Difluoroethene Chemical compound FC(F)=C BQCIDUSAKPWEOX-UHFFFAOYSA-N 0.000 description 1
- JWYVGKFDLWWQJX-UHFFFAOYSA-N 1-ethenylazepan-2-one Chemical compound C=CN1CCCCCC1=O JWYVGKFDLWWQJX-UHFFFAOYSA-N 0.000 description 1
- GOXQRTZXKQZDDN-UHFFFAOYSA-N 2-Ethylhexyl acrylate Chemical compound CCCCC(CC)COC(=O)C=C GOXQRTZXKQZDDN-UHFFFAOYSA-N 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- YIVJZNGAASQVEM-UHFFFAOYSA-N Lauroyl peroxide Chemical compound CCCCCCCCCCCC(=O)OOC(=O)CCCCCCCCCCC YIVJZNGAASQVEM-UHFFFAOYSA-N 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- 229920002367 Polyisobutene Polymers 0.000 description 1
- 125000005396 acrylic acid ester group Chemical group 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- DQXBYHZEEUGOBF-UHFFFAOYSA-N but-3-enoic acid;ethene Chemical compound C=C.OC(=O)CC=C DQXBYHZEEUGOBF-UHFFFAOYSA-N 0.000 description 1
- CQEYYJKEWSMYFG-UHFFFAOYSA-N butyl acrylate Chemical compound CCCCOC(=O)C=C CQEYYJKEWSMYFG-UHFFFAOYSA-N 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 150000001728 carbonyl compounds Chemical class 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 150000008280 chlorinated hydrocarbons Chemical class 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- LSXWFXONGKSEMY-UHFFFAOYSA-N di-tert-butyl peroxide Chemical compound CC(C)(C)OOC(C)(C)C LSXWFXONGKSEMY-UHFFFAOYSA-N 0.000 description 1
- 239000002283 diesel fuel Substances 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 229920006226 ethylene-acrylic acid Polymers 0.000 description 1
- XUCNUKMRBVNAPB-UHFFFAOYSA-N fluoroethene Chemical compound FC=C XUCNUKMRBVNAPB-UHFFFAOYSA-N 0.000 description 1
- 239000003502 gasoline Substances 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- FQPSGWSUVKBHSU-UHFFFAOYSA-N methacrylamide Chemical class CC(=C)C(N)=O FQPSGWSUVKBHSU-UHFFFAOYSA-N 0.000 description 1
- RQAKESSLMFZVMC-UHFFFAOYSA-N n-ethenylacetamide Chemical compound CC(=O)NC=C RQAKESSLMFZVMC-UHFFFAOYSA-N 0.000 description 1
- ZQXSMRAEXCEDJD-UHFFFAOYSA-N n-ethenylformamide Chemical compound C=CNC=O ZQXSMRAEXCEDJD-UHFFFAOYSA-N 0.000 description 1
- IUWVWLRMZQHYHL-UHFFFAOYSA-N n-ethenylpropanamide Chemical compound CCC(=O)NC=C IUWVWLRMZQHYHL-UHFFFAOYSA-N 0.000 description 1
- KKFHAJHLJHVUDM-UHFFFAOYSA-N n-vinylcarbazole Chemical compound C1=CC=C2N(C=C)C3=CC=CC=C3C2=C1 KKFHAJHLJHVUDM-UHFFFAOYSA-N 0.000 description 1
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000011045 prefiltration Methods 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 229920006300 shrink film Polymers 0.000 description 1
- 239000010802 sludge Substances 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- GJBRNHKUVLOCEB-UHFFFAOYSA-N tert-butyl benzenecarboperoxoate Chemical compound CC(C)(C)OOC(=O)C1=CC=CC=C1 GJBRNHKUVLOCEB-UHFFFAOYSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229930195735 unsaturated hydrocarbon Natural products 0.000 description 1
- 229920001567 vinyl ester resin Polymers 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/236—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof
- C10L1/2362—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof homo- or copolymers derived from unsaturated compounds containing nitrile groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/236—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof
- C10L1/2364—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof homo- or copolymers derived from unsaturated compounds containing amide and/or imide groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/236—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof
- C10L1/2368—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof homo- or copolymers derived from unsaturated compounds containing heterocyclic compounds containing nitrogen in the ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/24—Organic compounds containing sulfur, selenium and/or tellurium
- C10L1/2462—Organic compounds containing sulfur, selenium and/or tellurium macromolecular compounds
- C10L1/2468—Organic compounds containing sulfur, selenium and/or tellurium macromolecular compounds obtained by reactions involving only carbon to carbon unsaturated bonds; derivatives thereof
Definitions
- copolymers are obtained by high-pressure polymerization in the presence of radical-forming compounds at pressures of about 1000 to 8000, preferably 1500 to 2500 bar, temperatures of 100 to 350, preferably 200 to 350 ° C. and an average residence time of up to 150 seconds.
- the ethylene used for the polymerization is used in the purity customary for polymerization reactions of at least 99.9%.
- suitable vinyl acid amides are vinylformamide, vinyl acetamide, vinyl N-methylacetamide and vinyl propionamide.
- the share of Vinyl acid amide in the copolymer is 0.1 to 40% by weight and, accordingly, the proportion of ethylene is 99.9 to 60% by weight.
- Vinyl-N-methylacetamide is preferably copolymerized with ethylene in amounts of 0.5 to 30% by weight.
- the copolymer can contain up to 40% by weight of further monomers copolymerizable with ethylene, in particular acrylic acid esters and vinyl esters such as, for example, methyl acrylate, ethyl acrylate, butyl acrylate, 2-ethylhexyl acrylate or vinyl acetate.
- acrylic acid esters and vinyl esters such as, for example, methyl acrylate, ethyl acrylate, butyl acrylate, 2-ethylhexyl acrylate or vinyl acetate.
- the polymerization is carried out under the conditions indicated above in the presence of catalytic amounts of free radical initiators, for example with 2 to 250 mol ppm of oxygen, based on the ethylene.
- free radical initiators for example with 2 to 250 mol ppm of oxygen, based on the ethylene.
- peroxides such as tert-butyl perbenzoate, dilauroyl peroxide, di-tert-butyl peroxide or azo-butyronitrile in amounts of 2 to 200 mol ppm, based on ethylene, can also be used as initiator.
- the molecular weight is adjusted by adding moderators in amounts of 2 to 25% by volume, depending on the desired molecular weight. Low molecular weight copolymers with molecular weights of 500 to 10,000 are sought, determined according to K. Rast, Ber. 550, 1922 pp. 1051 u. 3727.
- Aliphatic alcohols can serve as moderators and carbonyl compounds, saturated and unsaturated
- copolymers of ethylene and vinyl acid amide obtained in this way show an improvement in the flow properties of mineral oils such as middle distillates from crude oil distillation and also of the crude oil itself, because they influence the crystal growth of the paraffin which precipitates in the cold in such a way that the paraffin crystals remain small and do not agglomerate that they can pass through the filters.
- These copolymers are normally added to the mineral oil in the form of approx. 40-45% solutions in an aromatic hydrocarbon.
- the amount of copolymer based on the mineral oil should be 0.001 to 2, preferably 0.005 to 0.5% by weight.
- these copolymers can be used alone or together with other oil additives, such as, for example, with other pour point depressants or dewaxing aids, corrosion inhibitors, antioxidants or sludge inhibitors.
- the N-ethylene-vinyl acid amide copolymers are also suitable as adhesives, as coating materials, for the production of stretch, skin and shrink films, for injection molding and for pipe and cable sheathing.
- a reaction mixture consisting of 98.7% by weight of ethylene and 1.3% by weight of vinyl-N-methylacetamide (VIMA) is compressed to 2000 bar.
- the polymerization is initiated with 30 ppm butyl peroctoate (in the form of a gasoline solution).
- the reaction temperature is 218 ° C.
- the melt index of the copolymer obtained is 2.7 g / 10 min, the density is 0.927 g / cm 3 . It contains 0.9% by weight of VIMA in polymer-bound form.
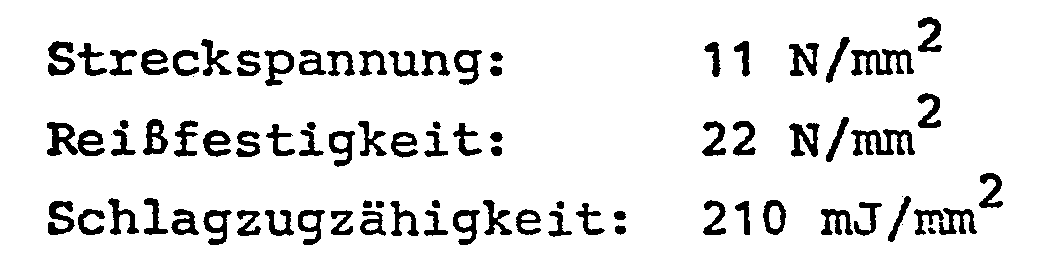
- the mechanical values of the copolymer are:
- a reaction mixture consisting of 94.3% by weight of ethylene and 5.7% by weight of VIMA is compressed to 2100 bar.
- the polymerization is initiated with 35 ppm t-butyl peroctoate.
- the reaction temperature is 210 ° C. 5.7% by weight of VIMA are incorporated.
- the melt index of the copolymer obtained is 1.8 g / 10 min. the density is 0.92 9 g / cm 3 .
- the mechanical values of the copolymer are:
- a reaction mixture consisting of 88.8% by weight of ethylene and 11.2% by weight of VIMA is compressed to 2100 bar and polymerized at a temperature around 210 ° C. using 40 ppm butyl peroctoate as the initiator.
- a copolymer with 10.3% by weight of VIMA, a melt index of 4.3 g / 10 min and a density of 0.931 g / cm 3 is obtained .
- the mechanical values of the copolymer are:
- the yield stress and tear strength were determined in accordance with DIN 53 455 and the impact strength in accordance with DIN 53 448.
- a middle distillate with an initial boiling point of 178 ° C., 5% point of 201 ° C., 95% point of 359 ° C., end of boiling point 376 ° C. and cloud point of -1 ° C. was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 95% by weight. -% ethylene and 5 wt .-% vinyl methylacetamide with a viscosity of 600 mPas.
- the middle distillate treated in this way had a CFPP value of -11 ° C.
- a middle distillate according to Example 4 was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 70% by weight of ethylene, 25% by weight of t-butyl acrylate and 5% by weight of vinyl methylacetamide with a viscosity of 600 mPas.
- the middle distillate treated in this way had a CFPP value of -13 ° C.
- a paraffin-rich middle distillate with a start of boiling 172 ° C, 5% point 190 ° C , 95% point 359 ° C, end of boiling 384 ° C and a cloud point of + 8 ° C was mixed with 300 ppm of a 45% solution in xylene of a copolymer 90% by weight of ethylene and 10% by weight of vinyl methylacetamide with a viscosity of 600 mPas.
- the middle distillate treated in this way had a CFPP value of -6 ° C.
- a middle distillate according to Example 6 was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 86.8% by weight of ethylene, 6.6% by weight of vinyl acetate and 6.6% by weight of vinyl methylacetamide with a viscosity of 600 mPas.
- the middle distillate treated in this way had a CFPP value of -6 ° C.
- a middle distillate with an initial boiling point of 167 ° C., 5% point 175 ° C., 95% point 372 ° C. and cloud point + 5 ° C. was used sets with 300 ppm of a 45% solution in xylene of a copolymer of 68% by weight of ethylene and 32% by weight of vinyl acetate with a viscosity of 900 mPas.
- the middle distillate thus treated showed a CFPP value of -8 ° C.
- a middle distillate according to Example 4 was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 70% by weight of ethylene and 30% by weight of t-butyl acrylate with a viscosity of 600 mPas.
- the middle distillate treated in this way had a CFPP value of -7 ° C.
- the middle distillate according to Example 6 was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 85% by weight of ethylene and 15% by weight of vinyl acetate with a viscosity of 1500 mPas.
- the middle distillate treated in this way showed a CFPP value of + 1 ° C.
- the viscosity in the above examples was measured at 140 ° C. in a rotary viscometer (Rotovisko).
- the CFPP value is the cold filter clogging point and indicates the temperature at which the oil in the test apparatus stops flowing. This test is described in "Journal of the Institute of Petroleum", Vol. 52, No. 510, June 1966, pp. 173-185 and in DIN 51 428.
- the copolymer to be used according to the invention not only brings about a significant improvement in the flow properties of mineral oils and mineral oil products when it is used alone, but moreover also shows a pronounced synergistic effect when copolymers other than pour point depressants are also used. This is clear from Example 8, where a significantly better effect was measured for the mixture of ethylene-vinyl acetate copolymer and ethylene-vinyl methylacetamide copolymer than for the same amount of each of these two copolymers alone.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Liquid Carbonaceous Fuels (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
Abstract
Verfahren zur Verbesserung der Fließfähigkeit von Mineralölen durch Zusatz eines Copolymers aus 66 bis 99,9 Gew.-% Ethylen und 0,1 bis 40 Gew.-% Vinylsäureamid sowie gegebenenfalls weiteren Comonomeren.Process for improving the flowability of mineral oils by adding a copolymer of 66 to 99.9% by weight of ethylene and 0.1 to 40% by weight of vinyl acid amide and optionally further comonomers.
Description
Es ist bekannt, daß in der Kälte aus Mineralölen, wie zum Beispiel Rohöl, Dieselkraftstoff oder Heizöl das darin enthaltene Paraffin auskristallisiert. Dies führt zu störenden Ablagerungen in den ölfeldeinrichtungen oder zur Verstopfung von Vorfiltern von Dieselmotoren und Feuerungsanlagen, so daß es in den Wintermonaten zum Ausfall dieser Anlagen kommen kann. Um dies zu vermei-' den, setzt man als Mineralöladditive Ethylen-Vinylacetat Copolymerwachse, Ethylen- Acrylsäureester-Copolymere oder Polyisobutylen zu. Diese Produkte haben jedoch nur eine geringe Löslichkeit und sind daher in ihrer Wirksamkeit nicht befriedigend. Es stellte sich daher die Aufgabe, wirksamere Additive zu finden, die ein derartiges Auskristallisieren von Paraffin verhindern und so die Fließfähigkeit von Mineralölen verbessern.It is known that in the cold from mineral oils, such as crude oil, diesel fuel or heating oil, the paraffin contained therein crystallizes out. This leads to disturbing deposits in the oilfield facilities or to the clogging of pre-filters of diesel engines and combustion systems, so that these systems can fail in the winter months. In order to avoid this, ethylene-vinyl acetate, copolymer waxes, ethylene-acrylic acid ester copolymers or polyisobutylene are added as mineral oil additives. However, these products have a low solubility and are therefore unsatisfactory in their effectiveness. It was therefore the task to find more effective additives which prevent such a crystallization of paraffin and thus improve the flowability of mineral oils.
Eswurde nun gefunden, daß man die Fließfähigkeit von Mineralölen verbessern kann, indem man dem Mineralöl ein Vinylsäureamid-Ethylen Copolymerisat zugibt.It has now been found that the fluidity of mineral oils can be improved by adding a vinyl acid amide / ethylene copolymer to the mineral oil.
Diese Copolymerisate werden erhalten durch Hochdruckpolymerisation in Gegenwart von Radikale bildenden Verbindungen bei Drücken von ca. 1000 bis 8000,vorzugsweise 1500 bis 2500 bar, Temperaturen von 100 bis 350, vorzugsweise 200 bis 350°C und einer mittleren Verweilzeit bis maximal 150 Sekunden. Das für die Polymerisation verwendete Ethylen wird in der für Polymerisationsreaktionen üblichen Reinheit von mindestens 99,9 % eingesetzt'. Als Vinylsäureamide kommen beispielsweise Vinylformamid, Vinylacetamid, Vinyl-N-methylacetamid und Vinylpropionamid in Frage. Der Anteil des Vinylsäureamids in dem Copolymeren beträgt 0,1 bis 40 Gew.-% und dementsprechend der Anteil des Ethylens 99,9 bis 60 Gew.-%.These copolymers are obtained by high-pressure polymerization in the presence of radical-forming compounds at pressures of about 1000 to 8000, preferably 1500 to 2500 bar, temperatures of 100 to 350, preferably 200 to 350 ° C. and an average residence time of up to 150 seconds. The ethylene used for the polymerization is used in the purity customary for polymerization reactions of at least 99.9%. Examples of suitable vinyl acid amides are vinylformamide, vinyl acetamide, vinyl N-methylacetamide and vinyl propionamide. The share of Vinyl acid amide in the copolymer is 0.1 to 40% by weight and, accordingly, the proportion of ethylene is 99.9 to 60% by weight.
Vorzugsweise wird Vinyl-N-methylacetamid in Mengen von 0,5 bis 30 Gew.-% mit Ethylen mischpolymerisiert.Vinyl-N-methylacetamide is preferably copolymerized with ethylene in amounts of 0.5 to 30% by weight.
Daneben kann das Copolymerisat noch bis zu 40 Gew.-% weitere, mit Ethylen copolymerisierbare Monomere enthalten, insbesondere Acrylsäureester und Vinylester wie beispielsweise Methylacrylat, Ethylacrylat, Butylacrylat, 2-Ethyl-hexylacrylat oder Essigsäurevinylester. Außerdem kommen andere mit Ethylen copolymerisierbare Monomere in Frage wie zum Beispiel C3- bis Cg-Alkene, Vinyl- und Alkenyläther, Vinyl- und Alkenylalkohole, N-Vinyl- und N-Alkenylverbindungen, wie N-Vinylpyrrolidon, N-Vinylcarbazol, N-Vinylcaprolactam, Acryl- und Methacrylamide, Acryl- und Methacrylnitrile, Alkenylhalogenide wie Vinylfluorid und Vinylidenfluorid, Vinyl- und Alkenylketone, Vinyl- und Alkenylsulfone und -sulfonate und Styrol. Außer ethylenisch ungesättigten Verbindungen können auch Kohlenmonoxid und Schwefeldioxid mit einpolymerisiert werden.In addition, the copolymer can contain up to 40% by weight of further monomers copolymerizable with ethylene, in particular acrylic acid esters and vinyl esters such as, for example, methyl acrylate, ethyl acrylate, butyl acrylate, 2-ethylhexyl acrylate or vinyl acetate. In addition, other monomers copolymerizable with ethylene are possible, such as, for example, C 3 -C 6 -alkenes, vinyl and alkenyl ethers, vinyl and alkenyl alcohols, N-vinyl and N-alkenyl compounds, such as N-vinylpyrrolidone, N-vinylcarbazole, N- Vinyl caprolactam, acrylic and methacrylamides, acrylic and methacrylonitriles, alkenyl halides such as vinyl fluoride and vinylidene fluoride, vinyl and alkenyl ketones, vinyl and alkenyl sulfones and sulfonates and styrene. In addition to ethylenically unsaturated compounds, carbon monoxide and sulfur dioxide can also be copolymerized.
Die Polymerisation erfolgt unter den oben angegebenen Bedingungen in Gegenwart katalytischer Mengen von Radikale bildenden Initiatoren, etwa mit 2 bis 250 Mol-ppm Sauerstoff, bezogen auf das Ethylen. Neben Sauerstoff können als Initiator auch Peroxide wie tert.-Butylperbenzoat Dilauroylperoxid, Di-tert.-Butylperoxid oder Azo-buttersäuredinitril in Mengen von 2 bis 200 Mol-ppm, bezogen auf Ethylen, verwendet werden. Die Einstellung des Molekulargewichts erfolgt durch Zugabe von Moderatoren in Mengen von 2 bis 25 Vol-% in Abhängigkeit von dem gewünschten Molekulargewicht. Angestrebt werden niedermolekulare Copolymerisate mit Molgewichten von 500 bis 10 000, bestimmt nach K. Rast, Ber. 550, 1922 S. 1051 u. 3727. Als Moderatoren können dienen aliphatische Alkohole und Carbonylverbindungen, gesättigte und ungesättigte Kohlenwasserstoffe, chlorierte Kohlenwasserstoffe und Wasserstoff.The polymerization is carried out under the conditions indicated above in the presence of catalytic amounts of free radical initiators, for example with 2 to 250 mol ppm of oxygen, based on the ethylene. In addition to oxygen, peroxides such as tert-butyl perbenzoate, dilauroyl peroxide, di-tert-butyl peroxide or azo-butyronitrile in amounts of 2 to 200 mol ppm, based on ethylene, can also be used as initiator. The molecular weight is adjusted by adding moderators in amounts of 2 to 25% by volume, depending on the desired molecular weight. Low molecular weight copolymers with molecular weights of 500 to 10,000 are sought, determined according to K. Rast, Ber. 550, 1922 pp. 1051 u. 3727. Aliphatic alcohols can serve as moderators and carbonyl compounds, saturated and unsaturated hydrocarbons, chlorinated hydrocarbons and hydrogen.
Die so erhaltenen Copolymere aus Ethylen und Vinylsäureamid zeigen eine Verbesserung der Fließeigenschaften bei Mineralölen wie Mitteldestillaten der Rohöldestillation und auch beim Rohöl selbst, weil sie das Kristallwachstum des in der Kälte ausfallenden Paraffins beeinflussen in der Weise, daß die Paraffinkristalle klein bleiben und nicht agglomerieren, so daß sie die Filter passieren können. Man gibt diese Copolymerisate dem Mineralöl normalerweise zu in Form von ca. 40 - 45 %igen Lösungen in einem aromatischen Kohlenwasserstoff. Die Menge an Copolymerisat bezogen auf das Mineralöl soll 0,001 bis 2, vorzugsweise 0,005 bis 0,5 Gew.-% betragen. Es versteht sich, daß man diese Copolymeren allein oder auch zusammen mit anderen öladditiven verwenden kann, wie beispielsweise mit anderen Stockpunkterniedrigern oder Entwachsungshilfsmitteln, Korrosionsinhibitoren, Antioxidantien oder Schlamminhibitoren. Darüberhinaus eignen sich die N-Ethylen-Vinylsäureamid-Copolymere auch als Kleber, als Beschichtungsmaterialien, zur Herstellung von Stretch-, Skin- und Schrumpffolien, zum Spritzgießen sowie zur Rohr- und Kabelummantelung.The copolymers of ethylene and vinyl acid amide obtained in this way show an improvement in the flow properties of mineral oils such as middle distillates from crude oil distillation and also of the crude oil itself, because they influence the crystal growth of the paraffin which precipitates in the cold in such a way that the paraffin crystals remain small and do not agglomerate that they can pass through the filters. These copolymers are normally added to the mineral oil in the form of approx. 40-45% solutions in an aromatic hydrocarbon. The amount of copolymer based on the mineral oil should be 0.001 to 2, preferably 0.005 to 0.5% by weight. It goes without saying that these copolymers can be used alone or together with other oil additives, such as, for example, with other pour point depressants or dewaxing aids, corrosion inhibitors, antioxidants or sludge inhibitors. In addition, the N-ethylene-vinyl acid amide copolymers are also suitable as adhesives, as coating materials, for the production of stretch, skin and shrink films, for injection molding and for pipe and cable sheathing.
Ein Reaktionsgemisch bestehend aus 98,7 Gew.-% Ethylen und 1,3 Gew.-% Vinyl-N-methylacetamid (VIMA) wird auf 2000 bar komprimiert. Die Polymerisation wird mit 30 ppm Butylperoktoat (in Form einer Benzinlösung) initiiert. Die Reaktionstemperatur beträgt 218°C. Der Schmelzindex des erhaltenen Copolymers liegt bei 2,7 g/10 min, die Dichte beträgt 0,927 g/cm3. Es enthält 0,9 Gew.-% VIMA in polymer gebundener Form.A reaction mixture consisting of 98.7% by weight of ethylene and 1.3% by weight of vinyl-N-methylacetamide (VIMA) is compressed to 2000 bar. The polymerization is initiated with 30 ppm butyl peroctoate (in the form of a gasoline solution). The reaction temperature is 218 ° C. The melt index of the copolymer obtained is 2.7 g / 10 min, the density is 0.927 g / cm 3 . It contains 0.9% by weight of VIMA in polymer-bound form.
Die mechanischen Werte des Copolymers betragen:
Ein Reaktionsgemisch bestehend aus 94,3 Gew.-% Ethylen und 5,7 Gew.-% VIMA wird auf 2100 bar verdichtet. Die Polymerisation wird mit 35 ppm t-Butylperoktoat initiiert. Die Reaktionstemperatur beträgt 210°C. Es werden 5,7 Gew.-% VIMA eingebaut. Der Schmelzindex des erhaltenen Copolymers liegt bei 1,8 g/10 min. die Dichte beträgt 0,929 g/cm3.A reaction mixture consisting of 94.3% by weight of ethylene and 5.7% by weight of VIMA is compressed to 2100 bar. The polymerization is initiated with 35 ppm t-butyl peroctoate. The reaction temperature is 210 ° C. 5.7% by weight of VIMA are incorporated. The melt index of the copolymer obtained is 1.8 g / 10 min. the density is 0.92 9 g / cm 3 .
Die mechanischen Werte des Copolymers betragen:
Ein Reaktionsgemisch bestehend aus 88,8 Gew.-% Ethylen und 11,2 Gew.-% VIMA wird auf 2100 bar verdichtet und bei einer Temperatur um 210°C mit Hilfe von 40 ppm Butylperoktoat als Initiator polymerisiert. Man erhält ein Mischpolymerisat mit 10,3 Gew.-% VIMA, einem Schmelzindex von 4,3 g/10 min und einer Dichte von 0,931 g/cm3. Die mechanischen Werte des Copolymers betragen:
Die Streckspannung und die Reißfestigkeit wurden nach DIN 53 455 und die Schlagzugzähigkeit nach DIN 53 448 bestimmt.The yield stress and tear strength were determined in accordance with DIN 53 455 and the impact strength in accordance with DIN 53 448.
Ein Mitteldestillat mit Siedebeginn 178°C, 5 % Punkt 201°C, 95 % Punkt 359°C, Siedeende 376°C und Cloudpunkt -1°C wurde versetzt mit 300 ppm einer 45 %igen Lösung in Xylol eines Copolymers aus 95 Gew.-% Ethylen und 5 Gew.-% Vinylmethylacetamid mit einer Viskosität von 600 mPas. Das so behandelte Mitteldestillat zeigte einen CFPP-Wert von -11°C.A middle distillate with an initial boiling point of 178 ° C., 5% point of 201 ° C., 95% point of 359 ° C., end of boiling point 376 ° C. and cloud point of -1 ° C. was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 95% by weight. -% ethylene and 5 wt .-% vinyl methylacetamide with a viscosity of 600 mPas. The middle distillate treated in this way had a CFPP value of -11 ° C.
Ein Mitteldestillat gemäß Beispiel 4 wurde versetzt mit 300 ppm einer 45 %igen Lösung in Xylol eines Copolymers aus 70 Gew.-% Ethylen, 25 Gew.-% t-Butylacrylat und 5 Gew.-% Vinylmethylacetamid mit einer Viskosität von 600 mPas. Das so behandelte Mitteldestillat zeigte einen CFPP-Wert von -13°C.A middle distillate according to Example 4 was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 70% by weight of ethylene, 25% by weight of t-butyl acrylate and 5% by weight of vinyl methylacetamide with a viscosity of 600 mPas. The middle distillate treated in this way had a CFPP value of -13 ° C.
Ein paraffinreiches Mitteldestillat mit Siedebeginn 172°C, 5 % Punkt 190°C, 95 % Punkt 359°C, Siedeende 384°C und einem Cloudpunkt von + 8°C wurde versetzt mit 300 ppm einer 45 %igen Lösung in Xylol eines Copolymers aus 90 Gew.-% Ethylen und 10 Gew.-% Vinylmethylacetamid mit einer Viskosität von 600 mPas. Das so behandelte Mitteldestillat zeigte einen CFPP-Wert von -6°C.A paraffin-rich middle distillate with a start of boiling 172 ° C, 5% point 190 ° C , 95% point 359 ° C, end of boiling 384 ° C and a cloud point of + 8 ° C was mixed with 300 ppm of a 45% solution in xylene of a copolymer 90% by weight of ethylene and 10% by weight of vinyl methylacetamide with a viscosity of 600 mPas. The middle distillate treated in this way had a CFPP value of -6 ° C.
Ein Mitteldestillat nach Beispiel 6 wurde versetzt mit 300 ppm einer 45 %igen Lösung in Xylol eines Copolymers aus 86,8 Gew.-% Ethylen, 6,6 Gew.-% Vinylacetat und 6,6 Gew.-% Vinylmethylacetamid mit einer Viskosität von 600 mPas. Das so behandelte Mitteldestillat hatte einen CFPP-Wert von -6°C.A middle distillate according to Example 6 was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 86.8% by weight of ethylene, 6.6% by weight of vinyl acetate and 6.6% by weight of vinyl methylacetamide with a viscosity of 600 mPas. The middle distillate treated in this way had a CFPP value of -6 ° C.
Ein Mitteldestillat mit Siedebeginn 167°C, 5'% Punkt 175°C, 95 % Punkt 372°C und Cloudpunkt + 5°C wurde versetzt mit 300 ppm einer 45 %igen Lösung in Xylol eines Copolymers aus 68 Gew.-% Ethylen und 32 Gew.-% Vinylacetat mit einer Viskosität von 900 mPas. Das-so behandelte Mitteldestillat zeigte einen CFPP-Wert von -8°C.A middle distillate with an initial boiling point of 167 ° C., 5% point 175 ° C., 95% point 372 ° C. and cloud point + 5 ° C. was used sets with 300 ppm of a 45% solution in xylene of a copolymer of 68% by weight of ethylene and 32% by weight of vinyl acetate with a viscosity of 900 mPas. The middle distillate thus treated showed a CFPP value of -8 ° C.
Gibt man zu diesem Mitteldestillat die gleiche Menge eines Copolymers aus 75 Gew.-% Ethylen und 25 Gew.-% Vinylmethylacetamid mit einer Viskosität von 500 mPas so wurde ein CFPP-Wert von -11°C gemessen.If the same amount of a copolymer of 75% by weight of ethylene and 25% by weight of vinyl methylacetamide with a viscosity of 500 mPas was added to this middle distillate, a CFPP value of -11 ° C. was measured.
Gibt man zu dem Mitteldestillat die gleiche Menge eines Gemischs der zuvor beschriebenen Copolymerisate im Verhältnis 1:1 so erhält man für das Mitteldestillat einen CFPP-Wert von -16°CIf the same amount of a mixture of the copolymers described above is added to the middle distillate in a ratio of 1: 1, a CFPP value of -16 ° C. is obtained for the middle distillate
Ein Mitteldestillat gemäß Beispiel 4 wurde versetzt mit 300 ppm einer 45 %igen Lösung in Xylol eines Copolymers aus 70 Gew.-% Ethylen und 30 Gew.-% t-Butylacrylat mit einer Viskosität von 600 mPas. Das so behandelte Mitteldestillat zeigte einen CFPP-Wert von -7°C.A middle distillate according to Example 4 was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 70% by weight of ethylene and 30% by weight of t-butyl acrylate with a viscosity of 600 mPas. The middle distillate treated in this way had a CFPP value of -7 ° C.
Das Mitteldestillat nach Beispiel 6 wurde versetzt mit 300 ppm einer 45 %igen Lösung in Xylol eines Copolymers aus 85 Gew.-% Ethylen und 15 Gew.-% Vinylacetat mit einer Viskosität von 1500 mPas. Das so behandelte Mitteldestillat zeigte einen CFPP-Wert von + 1°C.The middle distillate according to Example 6 was mixed with 300 ppm of a 45% solution in xylene of a copolymer of 85% by weight of ethylene and 15% by weight of vinyl acetate with a viscosity of 1500 mPas. The middle distillate treated in this way showed a CFPP value of + 1 ° C.
Die Viskosität in den obigen Beispielen wurde bei 140°C im Rotationsviskosimeter (Rotovisko) gemessen. Der CFPP-Wert ist der Kalt-Filterverstopfungspunkt und gibt die Temperatur an, bei der das öl in der Testapparatur nicht mehr fließt. Dieser Test ist beschrieben in "Journal of the Institute of Petroleum", Bd. 52, Nr. 510, Juni 1966, S. 173 - 185 sowie in DIN 51 428.The viscosity in the above examples was measured at 140 ° C. in a rotary viscometer (Rotovisko). The CFPP value is the cold filter clogging point and indicates the temperature at which the oil in the test apparatus stops flowing. This test is described in "Journal of the Institute of Petroleum", Vol. 52, No. 510, June 1966, pp. 173-185 and in DIN 51 428.
Das erfindungsgemäß zu verwendende Copolymer bringt nicht nur dann eine wesentliche Verbesserung der Fließeigenschaften bei Mineralölen und Mineralölprodukten mit sich wenn es allein eingesetzt wird, sondern zeigt darüberhinaus auch einen ausgeprägten synergistischen Effekt bei der Mitverwendung anderer Copolymere als Stockpunkterniedriger. Dies wird aus dem Beispiel 8 deutlich, wo für das Gemisch aus Ethylen-Vinylacetat-Copolymer und Ethylen-Vinylmethylacetamid-Copolymer ein deutlich besserer Effekt gemessen wurde als für die gleiche Menge jedes dieser beiden Copolymere allein.The copolymer to be used according to the invention not only brings about a significant improvement in the flow properties of mineral oils and mineral oil products when it is used alone, but moreover also shows a pronounced synergistic effect when copolymers other than pour point depressants are also used. This is clear from Example 8, where a significantly better effect was measured for the mixture of ethylene-vinyl acetate copolymer and ethylene-vinyl methylacetamide copolymer than for the same amount of each of these two copolymers alone.
Claims (6)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3112456 | 1981-03-28 | ||
| DE19813112456 DE3112456A1 (en) | 1981-03-28 | 1981-03-28 | "METHOD FOR IMPROVING THE FLOWABILITY OF MINERAL OILS" |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0061696A2 true EP0061696A2 (en) | 1982-10-06 |
| EP0061696A3 EP0061696A3 (en) | 1983-01-26 |
| EP0061696B1 EP0061696B1 (en) | 1985-05-02 |
Family
ID=6128660
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP82102388A Expired EP0061696B1 (en) | 1981-03-28 | 1982-03-23 | Process for improving the flow of mineral oils |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US4556499A (en) |
| EP (1) | EP0061696B1 (en) |
| JP (1) | JPS57170994A (en) |
| CA (1) | CA1191834A (en) |
| DE (2) | DE3112456A1 (en) |
| ZA (1) | ZA822078B (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0309897A2 (en) * | 1987-09-29 | 1989-04-05 | Hoechst Aktiengesellschaft | Copolymers of ethylene and methoxy acetic acid vinyl ester and their use as additives for mineral oil distillates |
| EP0345008A1 (en) * | 1988-06-02 | 1989-12-06 | E.I. Du Pont De Nemours And Company | Low pour crude oil compositions |
| EP0405270A1 (en) * | 1989-06-29 | 1991-01-02 | Hoechst Aktiengesellschaft | Process to improve mineral oil and mineral oil distillate flowability |
| WO1994012761A1 (en) * | 1992-11-20 | 1994-06-09 | Colorado School Of Mines | Method for controlling clathrate hydrates in fluid systems |
| US5814110A (en) * | 1986-09-24 | 1998-09-29 | Exxon Chemical Patents Inc. | Chemical compositions and use as fuel additives |
| DE19816797A1 (en) * | 1998-04-16 | 1999-10-21 | Clariant Gmbh | Process for improving lubricity of fuel oils |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4769043A (en) * | 1984-08-20 | 1988-09-06 | Texaco Inc. | Oil containing dispersant VII olefin copolymer |
| JP2508783B2 (en) * | 1988-01-26 | 1996-06-19 | 日本油脂株式会社 | Fluidity improver for fuel oil |
| US5078917A (en) * | 1989-11-01 | 1992-01-07 | Functional Products Incorporated | White oil pour point depressants |
| US5420370A (en) * | 1992-11-20 | 1995-05-30 | Colorado School Of Mines | Method for controlling clathrate hydrates in fluid systems |
| DE19739271A1 (en) * | 1997-09-08 | 1999-03-11 | Clariant Gmbh | Additive to improve the flowability of mineral oils and mineral oil distillates |
| US6495495B1 (en) | 1999-08-20 | 2002-12-17 | The Lubrizol Corporation | Filterability improver |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1266384A (en) * | 1960-09-01 | 1961-07-07 | Exxon Research Engineering Co | Fuels for internal combustion engines |
| FR1514629A (en) * | 1966-03-17 | 1968-02-23 | Shell Int Research | Lower Creep Point Fuel Composition |
| GB1266037A (en) * | 1968-09-17 | 1972-03-08 | ||
| FR96138E (en) * | 1967-11-30 | 1972-05-19 | Exxon Research Engineering Co | Copolymeric compositions for lowering the viscosity of petroleum products. |
| FR2307032A1 (en) * | 1975-04-07 | 1976-11-05 | Exxon Research Engineering Co | POLYMERIC ADDITIVES IMPROVING THE PROPERTIES OF HYDROCARBON OILS |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL97884C (en) * | 1954-07-12 | |||
| US3089832A (en) * | 1955-12-01 | 1963-05-14 | Exxon Research Engineering Co | Polymeric lubricating oil additives |
| US2958590A (en) * | 1957-10-15 | 1960-11-01 | Exxon Research Engineering Co | Stabilized hydrocarbon fuel oil composition |
| NL238305A (en) * | 1958-04-21 | |||
| US3015546A (en) * | 1958-10-28 | 1962-01-02 | Exxon Research Engineering Co | Gasolines inhibited against the formation of deposits, sludge and varnish |
| US3037850A (en) * | 1960-10-18 | 1962-06-05 | Exxon Research Engineering Co | Middle distillate pour point depressants |
| BE637124A (en) * | 1962-09-07 | |||
| FR96140E (en) * | 1967-11-30 | 1972-05-19 | ||
| US3658493A (en) * | 1969-09-15 | 1972-04-25 | Exxon Research Engineering Co | Distillate fuel oil containing nitrogen-containing salts or amides as was crystal modifiers |
| US3966428A (en) * | 1973-10-31 | 1976-06-29 | Exxon Research And Engineering Company | Ethylene backbone polymers in combination with ester polymers having long alkyl side chains are low viscosity distillate fuel cold flow improvers |
| US4211534A (en) * | 1978-05-25 | 1980-07-08 | Exxon Research & Engineering Co. | Combination of ethylene polymer, polymer having alkyl side chains, and nitrogen containing compound to improve cold flow properties of distillate fuel oils |
| US4261703A (en) * | 1978-05-25 | 1981-04-14 | Exxon Research & Engineering Co. | Additive combinations and fuels containing them |
-
1981
- 1981-03-28 DE DE19813112456 patent/DE3112456A1/en not_active Withdrawn
-
1982
- 1982-03-23 EP EP82102388A patent/EP0061696B1/en not_active Expired
- 1982-03-23 DE DE8282102388T patent/DE3263354D1/en not_active Expired
- 1982-03-26 JP JP57047517A patent/JPS57170994A/en active Pending
- 1982-03-26 CA CA000399461A patent/CA1191834A/en not_active Expired
- 1982-03-26 ZA ZA822078A patent/ZA822078B/en unknown
-
1983
- 1983-12-08 US US06/559,836 patent/US4556499A/en not_active Expired - Fee Related
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1266384A (en) * | 1960-09-01 | 1961-07-07 | Exxon Research Engineering Co | Fuels for internal combustion engines |
| FR1514629A (en) * | 1966-03-17 | 1968-02-23 | Shell Int Research | Lower Creep Point Fuel Composition |
| FR96138E (en) * | 1967-11-30 | 1972-05-19 | Exxon Research Engineering Co | Copolymeric compositions for lowering the viscosity of petroleum products. |
| GB1266037A (en) * | 1968-09-17 | 1972-03-08 | ||
| FR2307032A1 (en) * | 1975-04-07 | 1976-11-05 | Exxon Research Engineering Co | POLYMERIC ADDITIVES IMPROVING THE PROPERTIES OF HYDROCARBON OILS |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5814110A (en) * | 1986-09-24 | 1998-09-29 | Exxon Chemical Patents Inc. | Chemical compositions and use as fuel additives |
| EP0309897A2 (en) * | 1987-09-29 | 1989-04-05 | Hoechst Aktiengesellschaft | Copolymers of ethylene and methoxy acetic acid vinyl ester and their use as additives for mineral oil distillates |
| EP0309897A3 (en) * | 1987-09-29 | 1991-05-15 | Hoechst Aktiengesellschaft | Copolymers of ethylene and methoxy acetic acid vinyl ester and their use as additives for mineral oil distillates |
| EP0345008A1 (en) * | 1988-06-02 | 1989-12-06 | E.I. Du Pont De Nemours And Company | Low pour crude oil compositions |
| EP0405270A1 (en) * | 1989-06-29 | 1991-01-02 | Hoechst Aktiengesellschaft | Process to improve mineral oil and mineral oil distillate flowability |
| WO1994012761A1 (en) * | 1992-11-20 | 1994-06-09 | Colorado School Of Mines | Method for controlling clathrate hydrates in fluid systems |
| DE19816797A1 (en) * | 1998-04-16 | 1999-10-21 | Clariant Gmbh | Process for improving lubricity of fuel oils |
| EP0964052A1 (en) * | 1998-04-16 | 1999-12-15 | Clariant GmbH | Use of nitrogen-containing ethylene copolymers for producing fuel oils with improved lubricating activity |
| DE19816797C2 (en) * | 1998-04-16 | 2001-08-02 | Clariant Gmbh | Use of nitrogen-containing ethylene copolymers for the production of fuel oils with improved lubrication |
Also Published As
| Publication number | Publication date |
|---|---|
| DE3263354D1 (en) | 1985-06-05 |
| DE3112456A1 (en) | 1982-10-07 |
| EP0061696A3 (en) | 1983-01-26 |
| CA1191834A (en) | 1985-08-13 |
| US4556499A (en) | 1985-12-03 |
| EP0061696B1 (en) | 1985-05-02 |
| JPS57170994A (en) | 1982-10-21 |
| ZA822078B (en) | 1983-02-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0493769B1 (en) | Terpolymers of ethylene, their preparation and their use as additives for mineral oil distillates | |
| EP0271738B1 (en) | Process for the manufacture of copolymers of ethylene and their use as additives to mineral oil and mineral oil fractions | |
| EP0807642B1 (en) | Ethylene terpolymers, their preparation and their use as additives for mineral oil distillates | |
| EP2935346B1 (en) | Polymer compositions of ethylene-vinyl ester copolymers and alkyl(meth)acrylates, method for the production thereof and use thereof as pour-point depressants for crude oils, mineral oils or mineral oil products | |
| EP0922716B1 (en) | Process for the manufacture of terpolymers of ethylene and their use as additives to mineral oil and mineral oil distillates | |
| EP0485773B1 (en) | Petroleum middle distillate with improved cold flow characteristics | |
| EP0203554B1 (en) | Use of ethylene terpolymers as additives for mineral distillates oils and mineral oil | |
| DE4020640A1 (en) | TERPOLYMERISATES OF ETHYLENE, THEIR PRODUCTION AND THEIR USE AS ADDITIVES FOR MINERAL OIL DISTILLATES | |
| EP0332002B2 (en) | Use of selected acrylic and/or methacrylic acid ester copolymers as flow enhancers in paraffin-rich mineral oils and mineral-oil fractions (II) | |
| EP0254284B1 (en) | Process to improve the flowability of mineral oils and mineral oil distillates | |
| EP0184083A2 (en) | Terpolymers of ethylene, process for their manufacture and their use | |
| EP0061696B1 (en) | Process for improving the flow of mineral oils | |
| DE2557793A1 (en) | MIDDLE DISTILLATE FUEL COMPOSITIONS WITH IMPROVED COLD FLOW PROPERTIES AND ADDITIVES IMPROVING THESE PROPERTIES | |
| EP0258572A1 (en) | Process to improve the flowability of mineral oils and mineral oil distillates | |
| DE69415095T2 (en) | Polymer flow additives | |
| EP3394122B1 (en) | Polymer compositions allowing easier handling | |
| EP0486836B1 (en) | Petroleum middle distillate with improved cold flow characteristics | |
| EP0603573A2 (en) | Graft copolymer, the preparation thereof and its use as pour point depressant and flow improver in crude oils, residual oils and middle distillates | |
| EP0190553B1 (en) | Process to improve the viscosity of mineral oils and of the distillates of mineral oils | |
| DE2515805A1 (en) | ETHYLENE CO-POLYMERS, PROCESS FOR THEIR MANUFACTURING AND DISTILLED OILS CONTAINING THE SAME | |
| EP0890633B1 (en) | Use of copolymers of ethylene and unsaturated carboxylic esters in middle distillates to improve cold flow properties | |
| EP0111883B2 (en) | Use of ethylene copolymers as mineral oil additives | |
| EP0309897B1 (en) | Copolymers of ethylene and methoxy acetic acid vinyl ester and their use as additives for mineral oil distillates | |
| EP0964052B1 (en) | Use of nitrogen-containing ethylene copolymers for producing fuel oils with improved lubricating activity | |
| EP0792892B1 (en) | Use of ethylene/vinylformiate copolymers as flow improvers and fuel compositions containing them |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT NL |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19830420 |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT NL |
|
| REF | Corresponds to: |
Ref document number: 3263354 Country of ref document: DE Date of ref document: 19850605 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19860331 Year of fee payment: 5 |
|
| 26N | No opposition filed | ||
| BERE | Be: lapsed |
Owner name: HOECHST A.G. Effective date: 19870331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19871001 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19871130 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19881121 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19890331 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19920513 Year of fee payment: 11 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19931201 |