EP0041732B1 - Electrolytic cell having an improved ion exchange membrane and process for operating - Google Patents
Electrolytic cell having an improved ion exchange membrane and process for operating Download PDFInfo
- Publication number
- EP0041732B1 EP0041732B1 EP81104462A EP81104462A EP0041732B1 EP 0041732 B1 EP0041732 B1 EP 0041732B1 EP 81104462 A EP81104462 A EP 81104462A EP 81104462 A EP81104462 A EP 81104462A EP 0041732 B1 EP0041732 B1 EP 0041732B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- membrane
- ion exchange
- cell
- pendant
- membranes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 239000003014 ion exchange membrane Substances 0.000 title claims description 9
- 238000000034 method Methods 0.000 title abstract description 12
- 239000012528 membrane Substances 0.000 claims abstract description 119
- 238000005342 ion exchange Methods 0.000 claims abstract description 32
- 229910052801 chlorine Inorganic materials 0.000 claims abstract description 17
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 claims abstract description 16
- 229910052731 fluorine Inorganic materials 0.000 claims abstract description 11
- QLOAVXSYZAJECW-UHFFFAOYSA-N methane;molecular fluorine Chemical group C.FF QLOAVXSYZAJECW-UHFFFAOYSA-N 0.000 claims abstract description 11
- 229920002313 fluoropolymer Polymers 0.000 claims abstract description 8
- 229910001508 alkali metal halide Inorganic materials 0.000 claims abstract 2
- 150000008045 alkali metal halides Chemical class 0.000 claims abstract 2
- 239000000460 chlorine Substances 0.000 claims description 18
- 229920001577 copolymer Polymers 0.000 claims description 14
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 8
- 229910052739 hydrogen Inorganic materials 0.000 claims description 6
- 239000001257 hydrogen Substances 0.000 claims description 4
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 2
- 125000003709 fluoroalkyl group Chemical group 0.000 claims 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims 1
- 229910052783 alkali metal Chemical group 0.000 claims 1
- 150000001340 alkali metals Chemical group 0.000 claims 1
- 125000000217 alkyl group Chemical group 0.000 claims 1
- 239000011737 fluorine Substances 0.000 claims 1
- 238000000926 separation method Methods 0.000 claims 1
- 238000005868 electrolysis reaction Methods 0.000 abstract description 4
- 239000003518 caustics Substances 0.000 description 34
- 229920000642 polymer Polymers 0.000 description 27
- 239000000178 monomer Substances 0.000 description 18
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical group FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 description 16
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 15
- -1 trifluoroethylene, chlorotrifluoroethylene Chemical group 0.000 description 11
- 239000000243 solution Substances 0.000 description 10
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 8
- 239000003513 alkali Substances 0.000 description 8
- 150000001735 carboxylic acids Chemical class 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 239000000126 substance Substances 0.000 description 7
- 125000000542 sulfonic acid group Chemical group 0.000 description 7
- 238000011156 evaluation Methods 0.000 description 6
- 125000000524 functional group Chemical group 0.000 description 6
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 6
- 229920001897 terpolymer Polymers 0.000 description 6
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 5
- 239000012267 brine Substances 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 230000000704 physical effect Effects 0.000 description 5
- 229910005143 FSO2 Inorganic materials 0.000 description 4
- 229940124530 sulfonamide Drugs 0.000 description 4
- 150000003456 sulfonamides Chemical class 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- 229910000831 Steel Inorganic materials 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 238000003843 chloralkali process Methods 0.000 description 3
- 239000004811 fluoropolymer Substances 0.000 description 3
- 239000010959 steel Substances 0.000 description 3
- 150000003460 sulfonic acids Chemical class 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 2
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical compound C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000001125 extrusion Methods 0.000 description 2
- 229920001519 homopolymer Polymers 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 2
- BQCIDUSAKPWEOX-UHFFFAOYSA-N 1,1-Difluoroethene Chemical compound FC(F)=C BQCIDUSAKPWEOX-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- 241000047703 Nonion Species 0.000 description 1
- 238000002479 acid--base titration Methods 0.000 description 1
- 239000010425 asbestos Substances 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 238000010504 bond cleavage reaction Methods 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- 238000010349 cathodic reaction Methods 0.000 description 1
- UUAGAQFQZIEFAH-UHFFFAOYSA-N chlorotrifluoroethylene Chemical group FC(F)=C(F)Cl UUAGAQFQZIEFAH-UHFFFAOYSA-N 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011033 desalting Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 229940021013 electrolyte solution Drugs 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 239000002360 explosive Substances 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- XUCNUKMRBVNAPB-UHFFFAOYSA-N fluoroethene Chemical compound FC=C XUCNUKMRBVNAPB-UHFFFAOYSA-N 0.000 description 1
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical group FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- HCDGVLDPFQMKDK-UHFFFAOYSA-N hexafluoropropylene Chemical group FC(F)=C(F)C(F)(F)F HCDGVLDPFQMKDK-UHFFFAOYSA-N 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000011244 liquid electrolyte Substances 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229920000620 organic polymer Polymers 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 125000005010 perfluoroalkyl group Chemical group 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 229910052895 riebeckite Inorganic materials 0.000 description 1
- 238000009938 salting Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000009528 severe injury Effects 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B13/00—Diaphragms; Spacing elements
- C25B13/04—Diaphragms; Spacing elements characterised by the material
- C25B13/08—Diaphragms; Spacing elements characterised by the material based on organic materials
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/34—Simultaneous production of alkali metal hydroxides and chlorine, oxyacids or salts of chlorine, e.g. by chlor-alkali electrolysis
- C25B1/46—Simultaneous production of alkali metal hydroxides and chlorine, oxyacids or salts of chlorine, e.g. by chlor-alkali electrolysis in diaphragm cells
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/20—Manufacture of shaped structures of ion-exchange resins
- C08J5/22—Films, membranes or diaphragms
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/20—Manufacture of shaped structures of ion-exchange resins
- C08J5/22—Films, membranes or diaphragms
- C08J5/2206—Films, membranes or diaphragms based on organic and/or inorganic macromolecular compounds
- C08J5/2218—Synthetic macromolecular compounds
- C08J5/2231—Synthetic macromolecular compounds based on macromolecular compounds obtained by reactions involving unsaturated carbon-to-carbon bonds
- C08J5/2237—Synthetic macromolecular compounds based on macromolecular compounds obtained by reactions involving unsaturated carbon-to-carbon bonds containing fluorine
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2327/00—Characterised by the use of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a halogen; Derivatives of such polymers
- C08J2327/02—Characterised by the use of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a halogen; Derivatives of such polymers not modified by chemical after-treatment
- C08J2327/12—Characterised by the use of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a halogen; Derivatives of such polymers not modified by chemical after-treatment containing fluorine atoms
- C08J2327/18—Homopolymers or copolymers of tetrafluoroethylene
Definitions
- Membranes have been developed for that purpose which are substantially hydraulically-impermeable, but which will permit hydrated Na + ions to be transported from the anolyte portion to the catholyte portions, while substantially preventing transport of CI- ions.
- Such cells are operated by flowing a brine solution into the anolyte portion and by providing salt-free water to the catholyte portion to serve as the caustic medium.
- the anodic reactions and cathodic reactions are the same regardless of whether a membrane cell or a diaphragm cell is employed.
- KWH kilowatt hours
- the polymers used in the prior art as membranes are generally copolymers formed by copolymerizing a monomer chosen from the group of fluorinated vinyl compounds composed of vinyl fluoride, hexafluoropropylene, vinylidene fluoride, trifluoroethylene, chlorotrifluoroethylene, perfluoroalkyl vinyl ether and tetrafluoroethylene with an ion exchange functional (or group easily converted to ion exchange functional) vinyl ether monomer.
- the carboxylic acid monomers are represented by similar structures where the sulfonyl group has been replaced with either a carboxylic acid or a group such as that is easily converted to a carboxylic acid (U.S. Patent 4,065,366, Brit. 1,497,748; 1,497,749; 1,518,387). In one case (U.S.
- the membrane is composed of a terpolymer made by selecting one monomer from the group of perfluorovinyl compounds listed above and the other two from different carboxylic acid functional monomers.
- One is chosen from a group represented by where A represents a carboxylic acid or derivative and the other from a group represented by where A' is defined as A above.
- Two different functional monomers were used in the above case to achieve desirable physical properties of the polymers.
- U.S. Patents 4,025,405 and 4,192,725 and GB-A-1184321 show electrolytic cells having a stable, hydrated, selectively permeable, electrically conductive membrane.
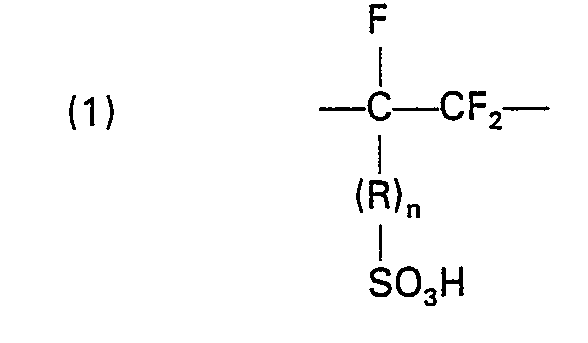
- the membrane is a film of fluorinated copolymer having pendant sulfonic acid groups containing recurring structural units of the formula: and where R is in which R' is F or perfluoroalkyl of 1 to 10 carbon atoms; Y is F or CF 3 ; m is 1, 2 or 3; n is 0 or 1; X is F, Cl, H, CF 3 ; X' and X are CF 3 - (CF 2 ) z wherein Z is 0-5; the units of formula (1) being present in an amount of from 3-20 mole percent.
- An electrolytic cell and a process to use the cell are provided.
- the electrolytic cell is separated into an anode compartment and a cathode compartment by a fluorinated polymer membrane; wherein the membrane has:
- Any holes or tears that develop in the film lead to contamination of the caustic product in the catholyte with salt from the anolyte and even worse, can lead to explosive mixtures of hydrogen in chlorine when cathodes are used that produce hydrogen along with attendant production of chlorine on the anode.
- fluoropolymers in general, meet the chemical requirements of the chlor-alkali cell. These fluoropolymers can be substituted with other halogen atoms such as chlorine or bromine that are not reactive in the cell environment, but, although contrary to some teachings, these polymers should not contain hydrogen atoms on carbons that make up the main polymer backbone. Carbon- hydrogen bonds are chemically attacked by both oxidation from the anolyte components and caustic in the catholyte. Chemical attack on the polymer backbone can lead to reduced molecular weight by carbon-carbon bond cleavage and thus to severe damage to film physical properties.
- halogen atoms such as chlorine or bromine
- a highly crystalline fluoropolymer made from simple, unsubstituted monomers such as tetrafluoroethylene is tough, but has extremely high melting or softening temperatures. Fabrication is difficult or near impossible by simple techniques such as melt extrusion. Homopolymers of long chain, terminal fluorocarbon olefins which result in polymers having many pendant groups are difficult to prepare because of having a relatively unreactive olefin site and when formed are often low molecular weight, waxy, amorphous solids having little, if any, plastic quality. Materials of this nature are useless as films. Copolymers of the two type monomers described above often have properties, better than the homopolymers.
- Copolymers of tetrafluoroethylene and perfluoroalkyl vinyl ethers (US Patent 3,896,179) and halofluoroalkyl vinyl ethers have excellent physical properties and can be conveniently melt fabricated into films.
- polymers with a limited number of pendant groups can maintain most of the favorable physical characteristics of the parent (no long pendant groups) polymer and also lend itself to simple fabrication.
- a simple fluorocarbon olefin such as tetrafluoroethylene or chlorotrifluoroethylene
- a monomer having a halofluoro or perfluoro chain having no ion exchange functionality attached to the olefin function.
- polymers are selected from the above class of polymers that contain a sufficient (greater than 60 mole percent) amount of the simple, non pendant group originating, monomer such as tetrafluoroethylene, tough, easily fabricated films result that give outstanding performance when used as membranes in chlor-alkali cells. These films may or may not be supported by materials such as polytetrafluoroethylene scrim.
- the concentration of the sulfonate ion exchange functional group in the polymers is also critical to the performance of the materials as membranes in electrolytic cells. Concentration of the functional group in the dry polymer is expressed herein as equivalent weight, which is defined as the formula weight of the polymer containing one equivalent of the functional group. It can be defined and conveniently determined, by standard acid-base titration, as the weight of the polymer, having the functional group, the sulfonic acid group in the present invention, in the acid form, required to neutralize one equivalent of base.
- equivalent weight which is defined as the formula weight of the polymer containing one equivalent of the functional group. It can be defined and conveniently determined, by standard acid-base titration, as the weight of the polymer, having the functional group, the sulfonic acid group in the present invention, in the acid form, required to neutralize one equivalent of base.
- equivalent weight which is defined as the formula weight of the polymer containing one equivalent of the functional group. It can be defined and conveniently determined, by standard acid-base
- Sulfonic acid membranes having lower equivalent weight allow excessive migration of hydroxide ions from the catholyte to the anolyte portion of the cell and thus result in excessively low current efficiency. It has been found that equivalent weights of at least part of the membranes of the current invention can be substantially less than 1100 and still be useful in chloralkali cells. This is particularly true when the pendant group having the ion exchange functional group is short. In fact, particularly preferred polymers of the present invention are made using the ion exchange functional monomer as opposed to the functional monomer of the prior art. One terpolymer having an equivalent weight of 900 is shown in the examples to perform substantially better than an 1100 equivalent weight copolymer of the prior art. It is thought that equivalent weights as low as 600, when the pendant group having the sulfonic acid functionality is short, should be operable in chlor-alkali cells.
- the Figure shows the results of using various ion exchange membranes in an electrolytic cell for the electrolysis of a NaCl brine solution. For each membrane tested, the figure shows its operational voltage, its current efficiency and the concentration of the caustic produced in the cell.
- a series of ion exchange membranes were individually tested in an electrolytic test cell.
- the cell had an anode and a cathode with the ion exchange membrane being evaluated positioned therebetween, thus separating the cell into an anode chamber and a cathode chamber.
- Each electrode had a square shape and an area of 8.63 square inches (55,68 cm 2 ).
- Each electrode had a solid, metal stud welded to it.
- Each stud passed through a wall of the cell and was provided with leak proof seals. Both studs were connected to a power supply.
- the stud connected to the anode was constructed of titanium, while the stud connected to the cathode was constructed of steel.
- the anode, itself, was an expanded titanium mesh screen coated with a RuO z - TiO z mixture, while the cathode was constructed from woven steel wires.
- the anode chamber was filled with a saturated NaCl brine solution (approximately 25 weight percent NaCI) and catholyte chamber was filled with a caustic solution having approximately 12 weight percent NaOH concentration.
- the cell was energized by applying a. constant current of approximately 8.63 amps, to give a current density of 1.0 amps per square inch (0,155 amps per cm 2 ) of electrode area.
- a saturated brine solution (approximately 25 weight percent NaCI) was flowed into the anode chamber at a rate sufficient to maintain the concentration of the anolyte leaving the cell at approximately 17-20 weight percent NaCI.
- Deionized water was flowed into the catholyte chamber, in a similar manner, at a rate sufficient to maintain the catholyte leaving the cell at a desired NaOH concentration.
- the NaOH concentration- was varied in order to determine the cell operation over a range of caustic concentrations.
- the temperature of the cell was controlled throughout each evaluation at about 80°C by means of an immersion heater connected to a thermocouple inserted into the anolyte chamber.
- the cell voltage was constantly monitored by measuring the difference in voltage potential between the anode stud and the cathode stud.
- the cell was operated for several days to reach equilibrium. Then current efficiency was determined by collecting the catholyte leaving the cell for a given period of time, usually 16 hours, and determining the amount of NaOH actually produced, as compared to the amount theoretically produced at the applied current.
- This membrane was received from E. I. DuPont in the acid form and was treated in the TEA solution as received.
- the figure shows the results obtained from the evaluation of various membranes in the above described cell, except for membranes designated as F and G.
- the data shown for these latter two membranes was obtained from published literature and is inserted for comparative purposes.
- the figure shows the relationship of the current efficiency of the cell to the caustic concentration of the catholyte as it leaves the cell.
- the numbers in parenthesis beside each curve represent average cell operating voltages over the range of caustic concentrations under which each membrane was tested.
- Membranes designated as B and C are copolymers differing only in the relative amounts of TFE and the functional monomer. Thus, membrane C has more ion exchange functionality than membrane B, which is reflected in their 860 and 1375 eq. wts. respectively.
- Membranes designated as D, E and H are all membranes which have pendant sulfonyl ion exchange groups and have pendant, substantially fluorinated carbon groups which have no ion exchange functionality. As shown by their respective equivalent weights of 1240, 900 and 1350, they have differing amounts of ion exchange functionality.
- a direct comparison of cells having membrane B with cells having membrane E shows that where the two types of membranes operate at essentially equal current efficiencies, the cell containing the membrane having pendant, substantially fluorinated carbon groups which have no ion exchange functionality, as well as containing pendant sulfonyl ion exchange groups, operates at substantially (14%) lower voltage than the cell having a membrane which does not have a pendant, substantially fluorinated carbon group which has no ion exchange functionality, but has only the sulfonyl containing pendant group.
- a comparison of the cell containing membrane C with a cell containing membrane E demonstrates that while the cells operate at approximately the same voltage, the cell having a membrane which has pendant substantially fluorinated carbon groups not having ion exchange functionality, as well as containing pendant sulfonyl containing groups, operates at a substantially higher current efficiency than the cell containing the membrane which has only pendant sulfonyl containing groups.
- the table shows a comparison of polymers A, B, C, D, E and H where power consumption per metric ton of caustic has been calculated with all cells operating at 12% caustic.
- the table clearly demonstrates the superiority of the cells which have membranes (D, E and H) having two pendant groups; one pendant group having sulfonyl ion exchange groups and one pendant, substantially fluorinated carbon group which has no ion exchange groups, as compared to cells which have membranes (B and C) having only one pendant group, a sulfonyl containing group.
- This comparison between the cells containing membranes D, E and H as opposed to cells containing membranes B and C is made where the membrane thicknesses are similar.
- the table shows that even when the thickness of membrane A (the prior art membrane having only sulfonyl containing pendant groups) is only one-fourth (1/4) that of membranes D, E and H (which have two types of pendant groups) the latter perform equally (D) or better (E).
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Electrochemistry (AREA)
- Metallurgy (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Electrolytic Production Of Non-Metals, Compounds, Apparatuses Therefor (AREA)
- Hybrid Cells (AREA)
Abstract
Description
- The electrolytic production of chlorine and caustic by the electrolysis of brine has been well known for many years. Historically, diaphragm cells using a hydraulically-permeable asbestos diaphragm, vacuum-deposited onto foraminous steel cathodes, have been widely commercialized. Such diaphragm cells, employing permeable diaphragms, produce NaCI-containing NaOH catholytes because NaCl passes through the diaphragm from the anolyte to the catholyte. Such NaCI-containing caustic generally requires a de-salting process to obtain a low-salt caustic for industrial purposes.
- In recent years, the chlor-alkali industry has focused much of its attention on developing membrane cells to produce low-salt or salt-free caustic in order to improve quality and avoid the costly desalting processes. Membranes have been developed for that purpose which are substantially hydraulically-impermeable, but which will permit hydrated Na+ ions to be transported from the anolyte portion to the catholyte portions, while substantially preventing transport of CI- ions. Such cells are operated by flowing a brine solution into the anolyte portion and by providing salt-free water to the catholyte portion to serve as the caustic medium. The anodic reactions and cathodic reactions are the same regardless of whether a membrane cell or a diaphragm cell is employed.
- Since the disclosure of fluorocarbon polymers containing sulfonic acid functional groups on pendant fluorocarbon chains was first disclosed by Connolly (3,282,875), a great deal of work has been done on using these and similar materials as ion exchange membranes in chloralkali cells.
- It has been stated that because of excessive hydration, sulfonic acid membranes are not useful, particularly at cell conditions where the caustic strength in the operating cell exceeds 18% (Maomi Seko, Commercial Operation of the Ion Exchange Membrane Chlor-Alkali Process, The American Chemical Society Meeting, April, 1976, New York, N. Y.). Because of the problems encountered with sulfonic acid substituted membranes, considerable work has been directed at finding a suitable substitute for the sulfonic acid. Carboxylic acid functional membranes have been reported to operate at considerably higher caustic strengths than sulfonic acid membranes. (M. Seko above ref., U.S. 4,065,366, Brit. Pats. 1,497,748; 1,497,749; 1,518,387). Membranes with at least part of the sulfonic acid groups converted to sulfonamide have also been reported to operate at higher caustic strengths than membranes with only sulfonic acid functional groups (3,784,399; 3,969,285). The incentive for striving for high caustic strength in the cell lies in the fact that most commercial caustic is sold as a 50% solution. Thus, higher strengths achieved in the cell results in less water that must be evaporated to reach the commercial 50% level. This results in savings of "evaporation energy", the energy required to evaporate the solution.
- In addition to the caustic strength being important, two other criteria of the operating cell must also be considered for a complete energy view of the overall process. One is current efficiency, which is the ability of the membrane to prevent migration of the caustic produced at the cathode into the anode compartment and the second is the voltage at which the cell operates, which is partly determined by the electrical resistance of the membrane. Power efficiency is often used as one term that considers both the current efficiency and cell voltage. It is defined as the product of the theoretical voltage divided by the actual voltage multiplied by the actual caustic produced divided by the theoretical caustic that could have been produced at a given current. Thus, it is apparent that power efficiency is reduced by higher cell voltage or lower current efficiency. The membrane has a direct effect on both. The most common method of comparing cells is to express the operation as kilowatt hours (KWH) of power consumed per metric ton (mt) of product produced. This expression also considers both voltage, higher voltage increasing the quantity KWH, and current efficiency, lower efficiency decreasing the quantity of product produced (mt). Thus, the lower the value KWH/mt, the better the performance of the cell.
- In general, the changes that have been made in membranes to increase the caustic strength in the cell have resulted in at least partially offsetting increases in the quantity KWH/mt. It has been reported that even though a carboxylic acid membrane was capable of producing greater than 30% caustic at above 90% current efficiency, the most economical operation was at 21-25% caustic because of lower cell voltage (M.Seko, "The Asahi Chemical Membrane Chlor-Alkali Process", The Chlorine Institute, Inc, 20th Chlorine Plant Managers Seminar, New Orleans, February, 1977). In addition to problems of increasing cell voltage caused by membranes capable of higher caustic strength operation, these types of membranes, when compared to sulfonic acids, do not last as long in service. This is at least in part caused by greater sensitivity than the sulfonic acids to impurities found in brine feed. It has been reported that the useful operating life of sulfonamide membranes is only about one year (D.R. Pulver, presented at the Chlorine Institute's 21 st Plant Managers Seminar, Houston, Texas, Feb., 1978). Sulfonic acid membranes have operated up to three years in chlor-alkali cells. A great deal of expense is incurred by shortened membrane life because of having to replace the expensive membrane materials. Also the loss of production and labor in having to remove cells from service, disassemble, assemble and put them back in service is costly.
- The polymers used in the prior art as membranes are generally copolymers formed by copolymerizing a monomer chosen from the group of fluorinated vinyl compounds composed of vinyl fluoride, hexafluoropropylene, vinylidene fluoride, trifluoroethylene, chlorotrifluoroethylene, perfluoroalkyl vinyl ether and tetrafluoroethylene with an ion exchange functional (or group easily converted to ion exchange functional) vinyl ether monomer. The functional monomers for the sulfonic acids of the prior art are represented by U.S. Patent 3,282,875)
- The sulfonamides of the prior art are represented by the general structure
- In addition to work described above where changes in functional groups have been used as a means of achieving higher caustic strength in operating cells, methods of operating the celis. themselves that lead to increased caustic strength have been described. Series catholyte flow (U.S. 1,284,618) and counter-current series anolyte and catholyte flow (U.S. 4,197,179) lead to increased caustic strength without sacrificing either current efficiency or cell voltage. These techniques are also useful. because caustic strengths approaching those obtained with carboxylic acid and sulfonamide membranes can be attained using sulfonic acid membranes with their inherently longer service life.
- U.S. Patents 4,025,405 and 4,192,725 and GB-A-1184321 show electrolytic cells having a stable, hydrated, selectively permeable, electrically conductive membrane. The membrane is a film of fluorinated copolymer having pendant sulfonic acid groups containing recurring structural units of the formula:
- An electrolytic cell and a process to use the cell are provided. The electrolytic cell is separated into an anode compartment and a cathode compartment by a fluorinated polymer membrane; wherein the membrane has:
- (a) at least 60 mole percent [CFX-CF2], where X = F or Cl;
- (b) an ion exchange equivalent weight of at least 600;
- (c) pendant sulfonyl ion exchange groups; and
- (d) pendant substantially fluorinated carbon. groups which have no ion exchange functionality.
- It has been discovered that certain sulfonic acid membranes perform in electrolytic chlor- alkali cells better than those of the prior art. It has been found that non exchange pendant groups attached to the polymer backbone cause surprising and unexpected reductions in the electrical resistance of the membrane. It is known to those skilled in the art of polymer science that pendant groups, whether chemically inert or active, act as internal plasticizers and render polymers more pliable and easier to fabricate than similar polymers not having the pendant groups. In many cases this technique is used to reduce the crystallinity of polymer structures. It is not known in the prior art that introduction of inert pendant groups to polymers used as membranes in electrolytic cells would beneficially affect the performance of the cell.
- Several criteria, aside from the criteria of cell performance, are necessary for use of polymers as membranes in electrolytic cells. When the polymers are used as films, which are conveniently made by melt extrusion or the like, on a commercial scale, the physical and chemical properties of the film must withstand the environment of the cell. This severely restricts the materials useful in the harsh environment of a chlor-alkali cell. The cell is divided by the membrane into two compartments, an anolyte compartment wherein chlorine gas is made and constantly evolved from an anode and a catholyte compartment wherein caustic is produced at a cathode. These cells normally operate at temperatures of from about 70°C up to temperatures of about 100°C and are expected to continuously operate at these conditions for many months and even years. This chemical environment of strong, hot caustic on one side and a highly oxidative environment on the other virtually eliminates the use of most organic polymers or membranes. The constant churning of gas being evolved through the liquid electrolyte solutions in the cell severely limits the physical properties that a film must have in order to meet the lifetime requirements of the cell. It is known to physically support polymer films on such materials as polytetrafluoroethylene scrim to aid in meeting the life requirements, but even then, the film must be physically sound to a large degree. Any holes or tears that develop in the film lead to contamination of the caustic product in the catholyte with salt from the anolyte and even worse, can lead to explosive mixtures of hydrogen in chlorine when cathodes are used that produce hydrogen along with attendant production of chlorine on the anode.
- It is known in the art that fluoropolymers, in general, meet the chemical requirements of the chlor-alkali cell. These fluoropolymers can be substituted with other halogen atoms such as chlorine or bromine that are not reactive in the cell environment, but, although contrary to some teachings, these polymers should not contain hydrogen atoms on carbons that make up the main polymer backbone. Carbon- hydrogen bonds are chemically attacked by both oxidation from the anolyte components and caustic in the catholyte. Chemical attack on the polymer backbone can lead to reduced molecular weight by carbon-carbon bond cleavage and thus to severe damage to film physical properties.
- Physical properties of a polymer are dependent on polymer structure. A highly crystalline fluoropolymer made from simple, unsubstituted monomers such as tetrafluoroethylene is tough, but has extremely high melting or softening temperatures. Fabrication is difficult or near impossible by simple techniques such as melt extrusion. Homopolymers of long chain, terminal fluorocarbon olefins which result in polymers having many pendant groups are difficult to prepare because of having a relatively unreactive olefin site and when formed are often low molecular weight, waxy, amorphous solids having little, if any, plastic quality. Materials of this nature are useless as films. Copolymers of the two type monomers described above often have properties, better than the homopolymers. Copolymers of tetrafluoroethylene and perfluoroalkyl vinyl ethers (US Patent 3,896,179) and halofluoroalkyl vinyl ethers have excellent physical properties and can be conveniently melt fabricated into films. Thus, polymers with a limited number of pendant groups can maintain most of the favorable physical characteristics of the parent (no long pendant groups) polymer and also lend itself to simple fabrication.
- Membranes of the present invention are conveniently made from polymers prepared by copolymerizing at least three monomers where one is a simple fluorocarbon olefin such as tetrafluoroethylene or chlorotrifluoroethylene, another is a monomer having potential sulfonate ion exchange functionality such as
- The concentration of the sulfonate ion exchange functional group in the polymers is also critical to the performance of the materials as membranes in electrolytic cells. Concentration of the functional group in the dry polymer is expressed herein as equivalent weight, which is defined as the formula weight of the polymer containing one equivalent of the functional group. It can be defined and conveniently determined, by standard acid-base titration, as the weight of the polymer, having the functional group, the sulfonic acid group in the present invention, in the acid form, required to neutralize one equivalent of base. The prior art teaches and demonstrates that sulfonic acid membranes of the prior art should have equivalent weights of at least about 1100 to be useful in chlor-alkali cells. Sulfonic acid membranes having lower equivalent weight allow excessive migration of hydroxide ions from the catholyte to the anolyte portion of the cell and thus result in excessively low current efficiency. It has been found that equivalent weights of at least part of the membranes of the current invention can be substantially less than 1100 and still be useful in chloralkali cells. This is particularly true when the pendant group having the ion exchange functional group is short. In fact, particularly preferred polymers of the present invention are made using the ion exchange functional monomer
- The beneficial effects of the terpolymers, having the non ion exchange pendant groups, are apparent when cells, in the examples, containing these materials as membranes are compared to cells containing the copolymers of the prior art as membranes and to cells containing copolymers of tetrafluoroethylene and the short functional monomer shown above as membranes.
- The Figure shows the results of using various ion exchange membranes in an electrolytic cell for the electrolysis of a NaCl brine solution. For each membrane tested, the figure shows its operational voltage, its current efficiency and the concentration of the caustic produced in the cell.
- A series of ion exchange membranes were individually tested in an electrolytic test cell. The cell had an anode and a cathode with the ion exchange membrane being evaluated positioned therebetween, thus separating the cell into an anode chamber and a cathode chamber. Each electrode had a square shape and an area of 8.63 square inches (55,68 cm2). Each electrode had a solid, metal stud welded to it. Each stud passed through a wall of the cell and was provided with leak proof seals. Both studs were connected to a power supply. The stud connected to the anode was constructed of titanium, while the stud connected to the cathode was constructed of steel. The anode, itself, was an expanded titanium mesh screen coated with a RuOz- TiOz mixture, while the cathode was constructed from woven steel wires.
- The anode chamber was filled with a saturated NaCl brine solution (approximately 25 weight percent NaCI) and catholyte chamber was filled with a caustic solution having approximately 12 weight percent NaOH concentration. The cell was energized by applying a. constant current of approximately 8.63 amps, to give a current density of 1.0 amps per square inch (0,155 amps per cm2) of electrode area. A saturated brine solution (approximately 25 weight percent NaCI) was flowed into the anode chamber at a rate sufficient to maintain the concentration of the anolyte leaving the cell at approximately 17-20 weight percent NaCI. Deionized water was flowed into the catholyte chamber, in a similar manner, at a rate sufficient to maintain the catholyte leaving the cell at a desired NaOH concentration. During the evaluation of each membrane, the NaOH concentration- was varied in order to determine the cell operation over a range of caustic concentrations.
- The temperature of the cell was controlled throughout each evaluation at about 80°C by means of an immersion heater connected to a thermocouple inserted into the anolyte chamber. During the evaluation of each membrane the cell voltage was constantly monitored by measuring the difference in voltage potential between the anode stud and the cathode stud. For each evaluation, the cell was operated for several days to reach equilibrium. Then current efficiency was determined by collecting the catholyte leaving the cell for a given period of time, usually 16 hours, and determining the amount of NaOH actually produced, as compared to the amount theoretically produced at the applied current.
- Before the evaluation of each membrane, the following preparatory procedures were followed:
- (1) the acid form of each membrane was dried in an oven and then equilibrated at ambient conditions;
- (2) the membrane was soaked in a 30 weight % solution of triethanolamine in water for 30 minutes at 25°C;
- (3) the membrane was removed from the solution and air dried; and
- (4) the membrane was installed in the above- described electrolytic cell.
- In the above manner, the following membranes were evaluated in actual cell operation.
- A.* Hydrolyzed 1500 eq. wt., 0.05 mm (.002 inches) thick copolymer of tetrafluoroethylene (TFE) and
- B. Hydrolyzed 1375 eq. wt., 0.18mm thick copolymer of TFE and FSO2CF2CF2OCF = CF2.
- C. Hydrolyzed 860 eq. wt., 0.19 mm thick copolymer of TFE and FSO2CF2CF2OCF = CF2.
- D. Hydrolyzed 1240 eq. wt., 0.20 mm thick terpolymer of TFE and a mixture of
- FS02CF2CF20CF = CF2 and CICF2CF2CF20CF = CF2
in a ratio of 8:1. - E. Hydrolyzed 900 eq. wt., 0.20 mm thick terpolymer of TFE and a mixture of
- FS02CF2CF20CF = CF2 and ClCF2CF2CF2OCF=CF2
in a ratio of 8:1.- FEtG. Literature performance for hydrolyzed 1100 and 1200 eq. wt. copolymers of TFE and
- M Seko, "Commercial Operation of the Ion Exchange Membrane Chlor-Alkali Process", The American Chemical Society, Centennial Meeting, New York, April, 1976.
- H. Hydrolyzed 1350 eq. wt., 0.24 mm thick terpolymer of TFE and a mixture of FSO2CF2CF2OCF = CF2 and
- *This membrane was received from E. I. DuPont in the acid form and was treated in the TEA solution as received.
- The figure shows the results obtained from the evaluation of various membranes in the above described cell, except for membranes designated as F and G. The data shown for these latter two membranes was obtained from published literature and is inserted for comparative purposes.
- The figure shows the relationship of the current efficiency of the cell to the caustic concentration of the catholyte as it leaves the cell. The numbers in parenthesis beside each curve represent average cell operating voltages over the range of caustic concentrations under which each membrane was tested.
- All membranes showed that current efficiency is indirectly proportional to the caustic concentration of the catholyte. As has been discussed earlier, it is beneficial to maximise the current efficiency and minimize the voltage.
- Certain comparisons have been made between the performance of the various membranes.
- Comparison of membranes designated as B, C, D, H and E show the beneficial effects caused by incorporation of pendant, substantially fluorinated carbon groups having no ion exchange functionality into copolymers of TFE and the same functional monomer, FSO2CF2CF2OCF = CF2.
- Membranes designated as B and C are copolymers differing only in the relative amounts of TFE and the functional monomer. Thus, membrane C has more ion exchange functionality than membrane B, which is reflected in their 860 and 1375 eq. wts. respectively.
- Membranes designated as D, E and H are all membranes which have pendant sulfonyl ion exchange groups and have pendant, substantially fluorinated carbon groups which have no ion exchange functionality. As shown by their respective equivalent weights of 1240, 900 and 1350, they have differing amounts of ion exchange functionality.
- A direct comparison of cells having membrane B with cells having membrane E shows that where the two types of membranes operate at essentially equal current efficiencies, the cell containing the membrane having pendant, substantially fluorinated carbon groups which have no ion exchange functionality, as well as containing pendant sulfonyl ion exchange groups, operates at substantially (14%) lower voltage than the cell having a membrane which does not have a pendant, substantially fluorinated carbon group which has no ion exchange functionality, but has only the sulfonyl containing pendant group.
- A comparison of the cell containing membrane C with a cell containing membrane E demonstrates that while the cells operate at approximately the same voltage, the cell having a membrane which has pendant substantially fluorinated carbon groups not having ion exchange functionality, as well as containing pendant sulfonyl containing groups, operates at a substantially higher current efficiency than the cell containing the membrane which has only pendant sulfonyl containing groups.
- Comparison of cells which have membrane B to cells having membranes D or H, shows that cells operate at a substantially higher current efficiency if the membrane contains pendant, substantially fluorinated carbon groups and pendant, sulfonyl groups (membranes D and H) as compared to cells which have membranes containing pendant sulfonyl containing groups only.
- Comparison of the cell containing membrane D with the cell containing membrane A, demonstrates the clear superiority of the membrane which has both types of pendant groups as compared to the membrane of the prior art which has only pendant groups which contain sulfonyl groups. Even though membrane D is four (4) times as thick as membrane A, the cell voltages in both cells are approximately the same. It has been calculated that if membrane A were as thick as membrane D, the cell containing membrane A would operate at well above four (4) volts. As shown in the figure, the cell containing the membrane of the prior art (A) does not operate as efficiently as membranes of the present invention, such as membrane D. The cell containing membrane D operates at a higher current efficiency than the cell having membrane A, while at the same time having less electrical resistance per unit of thickness. The table shows a comparison of polymers A, B, C, D, E and H where power consumption per metric ton of caustic has been calculated with all cells operating at 12% caustic. The table clearly demonstrates the superiority of the cells which have membranes (D, E and H) having two pendant groups; one pendant group having sulfonyl ion exchange groups and one pendant, substantially fluorinated carbon group which has no ion exchange groups, as compared to cells which have membranes (B and C) having only one pendant group, a sulfonyl containing group. This comparison between the cells containing membranes D, E and H as opposed to cells containing membranes B and C is made where the membrane thicknesses are similar. Additionally, the table shows that even when the thickness of membrane A (the prior art membrane having only sulfonyl containing pendant groups) is only one-fourth (1/4) that of membranes D, E and H (which have two types of pendant groups) the latter perform equally (D) or better (E).
-
Claims (13)
characterized in that it comprises (d) additional pendant, substantially fluorinated carbon groups which have no ion exchange functionality.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT81104462T ATE9721T1 (en) | 1980-06-11 | 1981-06-10 | ELECTROLYTIC CELL WITH IMPROVED ION EXCHANGE MEMBRANE AND METHOD OF OPERATING SUCH CELL. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US158429 | 1980-06-11 | ||
| US06/158,429 US4470889A (en) | 1980-06-11 | 1980-06-11 | Electrolytic cell having an improved ion exchange membrane and process for operating |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0041732A1 EP0041732A1 (en) | 1981-12-16 |
| EP0041732B1 true EP0041732B1 (en) | 1984-10-03 |
Family
ID=22568081
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP81104462A Expired EP0041732B1 (en) | 1980-06-11 | 1981-06-10 | Electrolytic cell having an improved ion exchange membrane and process for operating |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US4470889A (en) |
| EP (1) | EP0041732B1 (en) |
| JP (1) | JPS5779184A (en) |
| KR (1) | KR850000101B1 (en) |
| AT (1) | ATE9721T1 (en) |
| AU (1) | AU547061B2 (en) |
| BR (1) | BR8103718A (en) |
| CA (1) | CA1185921A (en) |
| DE (1) | DE3166440D1 (en) |
| ZA (1) | ZA813902B (en) |
Families Citing this family (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4804727A (en) * | 1980-06-11 | 1989-02-14 | The Dow Chemical Company | Process to produce novel fluorocarbon vinyl ethers and resulting polymers |
| US4834922A (en) * | 1982-02-04 | 1989-05-30 | The Dow Chemical Company | Process to produce novel fluorocarbon vinyl ethers and resulting polymers |
| US4871703A (en) * | 1983-05-31 | 1989-10-03 | The Dow Chemical Company | Process for preparation of an electrocatalyst |
| US4784900A (en) * | 1985-05-31 | 1988-11-15 | University Of Bath | Method for sizing polytretrafluoroethylene fabrics |
| US5110385A (en) * | 1985-05-31 | 1992-05-05 | The Dow Chemical Company | Method for forming polymer composite films using a removable substrate |
| US4650551A (en) * | 1985-05-31 | 1987-03-17 | The Dow Chemical Company | Supported ion exchange membrane films |
| US4650711A (en) * | 1985-05-31 | 1987-03-17 | The Dow Chemical Company | Method for sizing polytetrafluoroethylene fabrics |
| US5114515A (en) * | 1985-05-31 | 1992-05-19 | The Dow Chemical Company | Method for forming polymer composite films using removable substrates |
| US4784882A (en) * | 1985-05-31 | 1988-11-15 | The Dow Chemical Company | Method for forming composite polymer films |
| US4610762A (en) * | 1985-05-31 | 1986-09-09 | The Dow Chemical Company | Method for forming polymer films having bubble release surfaces |
| US4698243A (en) * | 1986-06-20 | 1987-10-06 | The Dow Chemical Company | Method for sizing and hydrolyzing polytetrafluoroethylene fabrics, fibers, yarns, or threads |
| US4778723A (en) * | 1986-06-20 | 1988-10-18 | The Dow Chemical Company | Method for sizing polytetrafluoroethylene fibers, yarn, or threads |
| US4731263A (en) * | 1986-09-26 | 1988-03-15 | The Dow Chemical Company | Method for the preparation of ionomer films |
| EP0326632A1 (en) * | 1988-02-02 | 1989-08-09 | The Dow Chemical Company | Method for the preparation of perfluorosulfonate ionomer films |
| US4752370A (en) * | 1986-12-19 | 1988-06-21 | The Dow Chemical Company | Supported membrane/electrode structure combination wherein catalytically active particles are coated onto the membrane |
| US4738741A (en) * | 1986-12-19 | 1988-04-19 | The Dow Chemical Company | Method for forming an improved membrane/electrode combination having interconnected roadways of catalytically active particles |
| US4889577A (en) * | 1986-12-19 | 1989-12-26 | The Dow Chemical Company | Method for making an improved supported membrane/electrode structure combination wherein catalytically active particles are coated onto the membrane |
| US5039389A (en) * | 1986-12-19 | 1991-08-13 | The Dow Chemical Company | Membrane/electrode combination having interconnected roadways of catalytically active particles |
| JPS63107618U (en) * | 1986-12-27 | 1988-07-11 | ||
| US4940525A (en) * | 1987-05-08 | 1990-07-10 | The Dow Chemical Company | Low equivalent weight sulfonic fluoropolymers |
| US4859745A (en) * | 1987-12-22 | 1989-08-22 | The Dow Chemical Company | Stratified fibrous fluoropolymer compositions and process for forming such fluoropolymers |
| JPH05509414A (en) * | 1990-03-06 | 1993-12-22 | ザ ダウ ケミカル カンパニー | electrochromic device |
| ES2097357T3 (en) * | 1990-06-11 | 1997-04-01 | Dow Chemical Co | IONIC EXCHANGE MEMBRANE THAT HAS IMPROVED EFFECTIVENESS IN PROTON EXCHANGE PROCESSES. |
| US5164060A (en) * | 1990-06-11 | 1992-11-17 | The Dow Chemical Company | Ion exchange membrane having increased efficiency in proton exchange processes |
| US5433861A (en) * | 1993-09-17 | 1995-07-18 | The Dow Chemical Company | Permanent deformation and use of sulfonated halopolymer articles |
| US5654109A (en) * | 1995-06-30 | 1997-08-05 | The Dow Chemical Company | Composite fuel cell membranes |
| US5882810A (en) * | 1996-03-08 | 1999-03-16 | The Dow Chemicalcompany | Active layer for membrane electrode assembly |
| US7326736B2 (en) * | 2002-11-04 | 2008-02-05 | Giner Electrochemical Systems, Llc | Composite proton exchange membrane and method of manufacturing the same |
| US7071271B2 (en) * | 2003-10-30 | 2006-07-04 | 3M Innovative Properties Company | Aqueous emulsion polymerization of functionalized fluoromonomers |
| US7074841B2 (en) * | 2003-11-13 | 2006-07-11 | Yandrasits Michael A | Polymer electrolyte membranes crosslinked by nitrile trimerization |
| US7265162B2 (en) * | 2003-11-13 | 2007-09-04 | 3M Innovative Properties Company | Bromine, chlorine or iodine functional polymer electrolytes crosslinked by e-beam |
| US7179847B2 (en) | 2003-11-13 | 2007-02-20 | 3M Innovative Properties Company | Polymer electrolytes crosslinked by e-beam |
| US7259208B2 (en) * | 2003-11-13 | 2007-08-21 | 3M Innovative Properties Company | Reinforced polymer electrolyte membrane |
| US7060756B2 (en) | 2003-11-24 | 2006-06-13 | 3M Innovative Properties Company | Polymer electrolyte with aromatic sulfone crosslinking |
| US7112614B2 (en) * | 2003-12-08 | 2006-09-26 | 3M Innovative Properties Company | Crosslinked polymer |
| US7060738B2 (en) * | 2003-12-11 | 2006-06-13 | 3M Innovative Properties Company | Polymer electrolytes crosslinked by ultraviolet radiation |
| US7173067B2 (en) * | 2003-12-17 | 2007-02-06 | 3M Innovative Properties Company | Polymer electrolyte membranes crosslinked by direct fluorination |
| US7807063B2 (en) * | 2004-09-28 | 2010-10-05 | Giner Electrochemical Systems, Llc | Solid polymer electrolyte composite membrane comprising plasma etched porous support |
| US7867669B2 (en) * | 2004-09-28 | 2011-01-11 | Giner Electrochemical Systems, Llc | Solid polymer electrolyte composite membrane comprising laser micromachined porous support |
| US8962132B2 (en) | 2004-09-28 | 2015-02-24 | Giner, Inc. | Solid polymer electrolyte composite membrane comprising a porous support and a solid polymer electrolyte including a dispersed reduced noble metal or noble metal oxide |
| US7947405B2 (en) * | 2004-09-29 | 2011-05-24 | Giner Electrochemical Systems, Llc | Solid polymer electrolyte composite membrane comprising porous ceramic support |
| WO2009079006A1 (en) | 2007-12-17 | 2009-06-25 | Giner Electrochemical Systems, Llc | Electrochemical device comprising composite bipolar plate and method of using the same |
| WO2012067650A1 (en) | 2010-11-16 | 2012-05-24 | Giner Electrochemical Systems, Llc | Electrochemical device comprising an electrically-conductive, selectively-permeable membrane |
| US9728802B2 (en) | 2013-05-14 | 2017-08-08 | Giner, Inc. | Micromold methods for fabricating perforated substrates and for preparing solid polymer electrolyte composite membranes |
| WO2017053563A1 (en) * | 2015-09-23 | 2017-03-30 | 3M Innovative Properties Company | Method of making a copolymer of tetrafluoroethylene having sulfonyl pendant groups |
| CN110139683B (en) | 2016-11-15 | 2022-08-30 | 吉纳生命科学公司 | Self-regulating electrolytic gas generator and implant system including the same |
| WO2019222704A1 (en) | 2018-05-17 | 2019-11-21 | Giner Life Sciences, Inc. | Electrolytic gas generator with combined lead and gas port terminals |
| BR112022019801A2 (en) | 2020-03-31 | 2022-11-16 | Plug Power Inc | METHOD AND SYSTEM FOR ELECTROCHEMICAL COMPRESSION OF GAS HYDROGEN |
| US11978912B2 (en) | 2020-11-19 | 2024-05-07 | The Research Foundation For The State University Of New York | Atomically dispersed platinum-group metal-free catalysts and method for synthesis of the same |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1184321A (en) * | 1968-05-15 | 1970-03-11 | Du Pont | Electrochemical Cells |
Family Cites Families (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3114778A (en) * | 1963-12-17 | Fluorinated vinyl ethers and their | ||
| US2393967A (en) * | 1942-12-24 | 1946-02-05 | Du Pont | Process for polymerizing tetrafluoroethylene |
| US2554752A (en) * | 1947-07-31 | 1951-05-29 | Nat Broach & Mach | Method of shaving gears |
| US2593583A (en) * | 1951-03-14 | 1952-04-22 | Du Pont | Method for coagulating aqueous dispersions of polytetrafluoroethylene |
| US3041317A (en) * | 1960-05-02 | 1962-06-26 | Du Pont | Fluorocarbon sulfonyl fluorides |
| US3242218A (en) * | 1961-03-29 | 1966-03-22 | Du Pont | Process for preparing fluorocarbon polyethers |
| DE1249247B (en) * | 1961-04-25 | 1967-09-07 | E. I. Du Pont De Nemours And Company, Wilmington, Del. (V. St. A.) | Process for the preparation of perfluoroolefin polyethers |
| US3250806A (en) * | 1962-04-05 | 1966-05-10 | Du Pont | Fluorocarbon ethers of tetrafluoroethylene epoxide |
| US3450684A (en) * | 1963-07-24 | 1969-06-17 | Du Pont | Fluorocarbon polyethers |
| US3301893A (en) * | 1963-08-05 | 1967-01-31 | Du Pont | Fluorocarbon ethers containing sulfonyl groups |
| US3282875A (en) * | 1964-07-22 | 1966-11-01 | Du Pont | Fluorocarbon vinyl ether polymers |
| US3536733A (en) * | 1967-08-10 | 1970-10-27 | Du Pont | Method for the preparation of halogenated epoxides |
| US3560568A (en) * | 1968-11-26 | 1971-02-02 | Du Pont | Preparation of sulfonic acid containing fluorocarbon vinyl ethers |
| US3784399A (en) * | 1971-09-08 | 1974-01-08 | Du Pont | Films of fluorinated polymer containing sulfonyl groups with one surface in the sulfonamide or sulfonamide salt form and a process for preparing such |
| BE788557A (en) * | 1971-09-09 | 1973-03-08 | Ppg Industries Inc | DIAPHRAGMS FOR ELECTROLYTIC CELLS |
| BE790369A (en) * | 1971-10-21 | 1973-04-20 | Diamond Shamrock Corp | METHOD AND APPARATUS FOR THE PREPARATION OF HYDROXIDES FROM HIGH PURE ALKALINE METALS IN AN ELECTROLYTIC TANK. |
| US4192725A (en) * | 1971-10-21 | 1980-03-11 | Diamond Shamrock Corporation | Electrolytic production of high purity alkali metal hydroxide |
| US3976549A (en) * | 1973-02-26 | 1976-08-24 | Hooker Chemicals & Plastics Corporation | Electrolysis method |
| US4035254A (en) * | 1973-05-18 | 1977-07-12 | Gerhard Gritzner | Operation of a cation exchange membrane electrolytic cell for producing chlorine including feeding an oxidizing gas having a regulated moisture content to the cathode |
| US4035255A (en) * | 1973-05-18 | 1977-07-12 | Gerhard Gritzner | Operation of a diaphragm electrolylytic cell for producing chlorine including feeding an oxidizing gas having a regulated moisture content to the cathode |
| DE2437395C3 (en) * | 1973-10-15 | 1979-02-08 | E.I. Du Pont De Nemours And Co., Wilmington, Del. (V.St.A.) | Film made from fluorine-containing polymers with side chains containing sulfonyl groups |
| US3969285A (en) * | 1973-12-17 | 1976-07-13 | E. I. Du Pont De Nemours And Company | Heat-treated fluorocarbon sulfonylamine cation permselectivity |
| JPS551351B2 (en) * | 1974-03-07 | 1980-01-12 | ||
| US3909378A (en) * | 1974-06-21 | 1975-09-30 | Du Pont | Composite cation exchange membrane and use thereof in electrolysis of an alkali metal halide |
| US4151053A (en) * | 1975-07-09 | 1979-04-24 | Asahi Kasei Kogyo Kabushiki Kaisha | Cation exchange membrane preparation and use thereof |
| GB1518387A (en) * | 1975-08-29 | 1978-07-19 | Asahi Glass Co Ltd | Fluorinated cation exchange membrane and use thereof in electrolysis of an alkali metal halide |
| NL7610922A (en) * | 1975-10-06 | 1977-04-12 | Basf Wyandotte Corp | PROCEDURE TO IMPROVE THE SELECTIVITY OF MEMBRANES FOR USE IN CHLORALKALI CELLS. |
| JPS5248598A (en) * | 1975-10-17 | 1977-04-18 | Asahi Glass Co Ltd | Method for producing alkali hydroxide |
| US4126588A (en) * | 1975-12-30 | 1978-11-21 | Asahi Glass Company Ltd. | Fluorinated cation exchange membrane and use thereof in electrolysis of alkali metal halide |
| DE2618457A1 (en) * | 1976-03-31 | 1977-10-13 | Champion Spark Plug Co | SEMI-CONDUCTIVE CERAMIC BODY |
| MX145160A (en) * | 1976-05-21 | 1982-01-12 | Diamond Shamrock Corp | METHOD FOR FORMING A MEMBRANE ON A NORMAL DIAPHRAGM CELL FORAMINOUS ELECTRODE |
| US4131740A (en) * | 1977-04-20 | 1978-12-26 | E. I. Du Pont De Nemours And Company | Alkyl perfluoro-ω-fluoroformyl esters and their preparation |
| US4197179A (en) * | 1978-07-13 | 1980-04-08 | The Dow Chemical Company | Electrolyte series flow in electrolytic chlor-alkali cells |
-
1980
- 1980-06-11 US US06/158,429 patent/US4470889A/en not_active Expired - Lifetime
-
1981
- 1981-06-10 EP EP81104462A patent/EP0041732B1/en not_active Expired
- 1981-06-10 AT AT81104462T patent/ATE9721T1/en not_active IP Right Cessation
- 1981-06-10 CA CA000379414A patent/CA1185921A/en not_active Expired
- 1981-06-10 KR KR1019810002082A patent/KR850000101B1/en active
- 1981-06-10 AU AU71605/81A patent/AU547061B2/en not_active Ceased
- 1981-06-10 JP JP56088306A patent/JPS5779184A/en active Granted
- 1981-06-10 ZA ZA00813902A patent/ZA813902B/en unknown
- 1981-06-10 BR BR8103718A patent/BR8103718A/en unknown
- 1981-06-10 DE DE8181104462T patent/DE3166440D1/en not_active Expired
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1184321A (en) * | 1968-05-15 | 1970-03-11 | Du Pont | Electrochemical Cells |
Also Published As
| Publication number | Publication date |
|---|---|
| AU7160581A (en) | 1981-12-17 |
| EP0041732A1 (en) | 1981-12-16 |
| BR8103718A (en) | 1982-03-02 |
| ATE9721T1 (en) | 1984-10-15 |
| JPS5779184A (en) | 1982-05-18 |
| KR830006473A (en) | 1983-09-24 |
| DE3166440D1 (en) | 1984-11-08 |
| CA1185921A (en) | 1985-04-23 |
| JPS6123933B2 (en) | 1986-06-09 |
| US4470889A (en) | 1984-09-11 |
| AU547061B2 (en) | 1985-10-03 |
| ZA813902B (en) | 1983-01-26 |
| KR850000101B1 (en) | 1985-02-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0041732B1 (en) | Electrolytic cell having an improved ion exchange membrane and process for operating | |
| EP0041733B1 (en) | Improved sulfonic acid electrolytic cell membranes | |
| US4478695A (en) | Sulfonic acid electrolytic cell membranes and use thereof in the electrolysis of sodium chloride | |
| KR840001538B1 (en) | Improved composite ion exchange memhraneo | |
| US4417969A (en) | Sulfonic acid electrolytic cell membranes | |
| US4462877A (en) | Composite ion exchange membranes | |
| US4683040A (en) | Process for electrolysis of sodium chloride | |
| US4085071A (en) | Ion exchange polymer film, consisting of fluorinated polymer with N-monosubstituted sulfonamido groups method and apparatus for electrolysis of alkali or alkaline earth metal halide | |
| US4062753A (en) | Electrolysis method and apparatus | |
| US3899403A (en) | Electrolytic method of making concentrated hydroxide solutions by sequential use of 3-compartment and 2-compartment electrolytic cells having separating compartment walls of particular cation-active permselective membranes | |
| US4053376A (en) | Electrolytic production of hydrogen iodide | |
| JPS6356257B2 (en) | ||
| US5716504A (en) | Cation exchange membrane for electrolysis and process for producing potassium hydroxide of high purity | |
| US4584071A (en) | Process for electrolysis of brine with iodide impurities | |
| US4487668A (en) | Fluorinated ion exchange polymer containing carboxylic groups, and film and membrane thereof | |
| US4217198A (en) | Coated perfluorosulfonic acid resin membranes and a method for their preparation | |
| US4144227A (en) | Novel copolymers and diaphragms made therefrom | |
| US4113585A (en) | Method and apparatus for electrolysis of alkali or alkaline earth metal halide | |
| US4147601A (en) | Electrolytic production of hydrobromic acid | |
| US4182661A (en) | Electrochemical production of available chlorine containing organic compounds in a divided cell | |
| US4060473A (en) | Novel copolymers and diaphragms made therefrom | |
| EP0039189B1 (en) | Process for producing alkali metal hydroxide | |
| EP0044157B1 (en) | Method of installation of membrane to electrolytic cell | |
| US4055475A (en) | Method for operating electrolytic diaphragm cells | |
| CA1203509A (en) | Composite ion exchange membranes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI NL SE |
|
| 17P | Request for examination filed |
Effective date: 19820615 |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI NL SE |
|
| REF | Corresponds to: |
Ref document number: 9721 Country of ref document: AT Date of ref document: 19841015 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3166440 Country of ref document: DE Date of ref document: 19841108 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19910417 Year of fee payment: 11 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19920610 |
|
| EAL | Se: european patent in force in sweden |
Ref document number: 81104462.7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19970221 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19970224 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19970228 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19970310 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19970314 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19970401 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19970422 Year of fee payment: 17 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980610 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980611 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980630 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980630 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980630 |
|
| BERE | Be: lapsed |
Owner name: THE DOW CHEMICAL CY Effective date: 19980630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990101 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19980610 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990226 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 81104462.7 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 19990101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990401 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |