EP0037232A2 - Feueranzünder - Google Patents
Feueranzünder Download PDFInfo
- Publication number
- EP0037232A2 EP0037232A2 EP81301249A EP81301249A EP0037232A2 EP 0037232 A2 EP0037232 A2 EP 0037232A2 EP 81301249 A EP81301249 A EP 81301249A EP 81301249 A EP81301249 A EP 81301249A EP 0037232 A2 EP0037232 A2 EP 0037232A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- firelighter
- weight
- kerosene
- perlite
- stearine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L11/00—Fire-lighters
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L7/00—Fuels produced by solidifying fluid fuels
- C10L7/02—Fuels produced by solidifying fluid fuels liquid fuels
Definitions
- the invention relates to firelighters.
- a well known firelighter in general domestic use is the so-called white firelighter which comprises a solid block and which is based on a kerosene/urea- formaldehyde resin system. It was developed during the 1950's out of wartime work carried out at the Fuel Research Station.
- the firelighter is white because this colour is produced in cross-linking of the resin to afford a solid matrix.
- the white firelighter has been accorded certain advantages such as a clean, aesthetically pleasing appearance, with low odour and good tactile properties.
- the material is easily cut and it provides a block which is readily visible for ignition among the darker fuel used in a domestic fireplace such as coal or coke.
- the known white firelighter does however present some disadvantages. Its manufacture depends on the emulsification of kerosene into the resin base and subsequent hardening of the resin. Poor emulsification and/or poor hardening can lead .to products which exude kerosene, or water, or both. These effects, in turn, can lead to such problems as staining of packs, poor appearance, poor smell, condensation within film wrappings, difficulty of ignition, poor candle-like flames and general inferior quality. Such problems may be exacerbated by attempts to conserve or reduce the kerosene contents of firelighters, a desirable requirement in view of the present problems with oil supplies.
- a method of manufacturing a firelighter comprises the combining of kerosene, a binder in the form of a soap system, and perlite, a volcanic glass.
- Perlite is a white material which takes up some of the liquids in the composition. It fills and thickens the composition and helps to promote long, controlled but effective burning. Perlite is also a low density material which helps to lighten the product. This may be advantageous in transporting the product in bulk and certainly to the housewife carrying the product home.
- a firelighter according to the invention is relatively easy to light, burns well, and may afford significant kerosene economies.
- the soap system is formed by reacting stearine with caustic soda.
- kerosene and stearine are first mixed together, for example in a steam jacketed vessel at 70° to 75 0 C and are then mixed with caustic-soda at a similar temperature, for example in a further steam jacketed vessel.
- the perlite is added subsequently.
- the firelighter may contain from 35 to 80% (e.g. 50 to 70%) kerosene by weight.
- the firelighter may contain from 1 to 10% (e.g. 5 to 8%) stearine by weight.
- the firelighter may contain from 1 to 5% (e.g. 1 to 3%) solid caustic soda by weight.
- the firelighter may contain from 5 to 15% (e.g. 6 to 10%) water by weight.
- the firelighter may contain from 1 to 25% (e.g. 1 to 20% or 2, to 15%) perlite by weight.
- sodium silicate may be used.
- the firelighter may contain from 2 to 10% (e.g. 4 to 7%) by weight of a sodium silicate solution containing 25 to 55% total solids.
- the firelighter may include one or more of the following additives: liquid fuels or waxy materials; solids which have an intrinsic fuel value; solids which have no fuel value.but which may act as modifiers of appearance, consistency, burning properties, or which may function solely as fillers.
- Additives may include whiting (Snowcal), Kaolin, Kiesselguhr, mica, calcite, vermiculite, fillite, flyash, pumice, talc, Bentonite, alumina, sodium carboxymethyl cellulose and related compounds, starch, sodium carbonate, sodium bicarbonate, and foamed-or expanded plastics.
- the invention includes a firelighter manufactured by the method according to the invention.
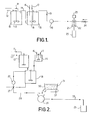
- kerosene and stearine are fed to a first steam jacketed vessel 10 via pipes 11 and 12 respectively. They are mixed in this vessel by means of a stirrer 13 and the mixture overflows via pipe 14 to the bottom of a second steam jacketed vessel 15.
- Caustic soda and water are fed into the vessel 15 through pipes 16 and 17 respectively and mixing again takes place using a stirrer 18.
- a soap forms into which the kerosene is bound.
- the hot mixture overflows from the steam jacketed vessel 15 through a pipe 19 and is pumped by a pump 20 to a mixing device 21.
- the mixing device comprises a downwardly directed tube containing paddles 22 driven by a motor 23.
- Fillers are fed to the mixing device 21 through a screw feed device 24. The fillers are mixed into the hot composition as the composition travels down the tube 21 and the final composition passes into a mould device 25 where the composition cools and sets.
- the caustic soda and water are first mixed together in an unheated vessel 26 before they join the kerosene and stearine mixture in the steam jacketed vessel 18.
- the output.from the pump 20 leads to a two-way valve 27.so that the hot mixture can either be recycled to the vessel 18 or can be ; pumped via pipe 28 to the inlet of a wide throat monopump 29.
- the fillers 31 are fed from a hopper 30 to the pump 29 by a moving belt conveyor 32 which forms the base of the hopper 30.
- the hot composition and the fillers are mixed together by the pump 29 and are fed via pipe 33 to the mould device 25 for cooling and setting.
- the kerosene and stearine were first mixed together at a temperature of from 70° to 75°C. This mixture was then reacted with the caustic soda and water at the same temperature. The perlite was then subsequently mixed in and the composition was allowed to set.
- the set composition was cut into rectangular blocks weighing'25 grams and it was found that one 25 gram block burnt for 19 minutes and 44 seconds.
- the finished composition set to give a non-gritty solid which had dry surfaces even when cut.
- the kerosene, stearine, caustic soda and water were mixed as before.
- the snowcal was then added and mixed in, the perlite was then added and the mixture was allowed to set. It was cut into blocks of 25 grams each and one block was found to burn for 26 minutes and 45 seconds.
- Example 1 and Example 2 were produced by a batch process, the following Example was produced by a continuous process, the proportions of the ingredients being given as a percentage of the total mixture by weight.
- the final mixture was moulded by a continuous process into blocks, each weighing 29 grams in weight. It was found that a 29 gram block burned for 22 minutes and 41 seconds.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Fireproofing Substances (AREA)
- Physical Or Chemical Processes And Apparatus (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB8010091 | 1980-03-26 | ||
| GB8010091 | 1980-03-26 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0037232A2 true EP0037232A2 (de) | 1981-10-07 |
| EP0037232A3 EP0037232A3 (de) | 1982-02-03 |
Family
ID=10512382
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP81301249A Withdrawn EP0037232A3 (de) | 1980-03-26 | 1981-03-24 | Feueranzünder |
Country Status (1)
| Country | Link |
|---|---|
| EP (1) | EP0037232A3 (de) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0047123A3 (en) * | 1980-09-01 | 1982-09-15 | Reckitt And Colman Products Limited | Combustible compositions, firelighters, barbeque starters and firelogs |
| EP0047124A3 (en) * | 1980-09-01 | 1982-09-15 | Reckitt And Colman Products Limited | Combustible compositions and methods for producing them |
| WO2013138872A1 (en) | 2012-03-23 | 2013-09-26 | BRANKOVIĆ, Vladimir | Waterproof firelighter based on polymethylmethacrylate |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1444335A (en) * | 1974-03-28 | 1976-07-28 | Ciba Geigy Ag | Fire-lighters |
| US4165968A (en) * | 1978-05-09 | 1979-08-28 | Duncan Norman B | Composition for coating charcoal briquettes |
| IE50033B1 (en) * | 1979-08-02 | 1986-02-05 | Reckitt & Colmann Prod Ltd | Combustible compositions and processes for their production |
-
1981
- 1981-03-24 EP EP81301249A patent/EP0037232A3/de not_active Withdrawn
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0047123A3 (en) * | 1980-09-01 | 1982-09-15 | Reckitt And Colman Products Limited | Combustible compositions, firelighters, barbeque starters and firelogs |
| EP0047124A3 (en) * | 1980-09-01 | 1982-09-15 | Reckitt And Colman Products Limited | Combustible compositions and methods for producing them |
| EP0047125A3 (en) * | 1980-09-01 | 1982-09-22 | Reckitt And Colman Products Limited | Combustible compositions, firelighters, barbeque starters and firelogs |
| WO2013138872A1 (en) | 2012-03-23 | 2013-09-26 | BRANKOVIĆ, Vladimir | Waterproof firelighter based on polymethylmethacrylate |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0037232A3 (de) | 1982-02-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4518652A (en) | Method for forming a lightweight cementitious structural product and product formed thereby | |
| CA1142361A (en) | Combustible briquettes | |
| KR890011986A (ko) | 방수성괴상연료와 그 제조방법 및 이에 사용된 조성물 | |
| EP0037232A2 (de) | Feueranzünder | |
| US4756719A (en) | Portable fuel composition | |
| BG97606A (bg) | Хомогенен състав от цимент и смола и метод за формоване на фасонни изделия от него | |
| US4282037A (en) | Gilsonite emulsion compositions | |
| US5755836A (en) | Process for manufacturing a composite fire log and product resulting therefrom | |
| RU2015161C1 (ru) | Способ получения кальциевой соли жирной кислоты | |
| EP0036783A2 (de) | Feueranzünder | |
| US3829297A (en) | Pulp bound compacted fuels | |
| GB2351295A (en) | Synthetic fire logs | |
| SU1611898A1 (ru) | Способ приготовлени легкобетонной смеси | |
| RU95108209A (ru) | Сырьевая смесь для изготовления строительных изделий и способ ее получения | |
| US3026189A (en) | Preparation of fuel briquettes | |
| RU2146694C1 (ru) | Способ получения топливной композиции | |
| KR840002293B1 (ko) | 착화탄용 점결제의 제조방법 | |
| JP3408314B2 (ja) | 成形物及びその製造方法 | |
| SU1541194A1 (ru) | Способ изготовлени гипсокартонных листов | |
| SU1527215A1 (ru) | Сырьева смесь дл изготовлени теплоизол ционного материала | |
| CA1175653A (en) | Composite fluid fuel made of natural cellulose materials and a process of preparation thereof | |
| GB2084183A (en) | Solid foam fuels | |
| EP0047125B1 (de) | Brennbare Mischungen, Feueranzünder, Barbecueanzünder und Brennblocks | |
| US313086A (en) | Artificial fuel | |
| RU2100417C1 (ru) | Состав для брикетированного топлива |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LU NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19820727 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19830621 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: SMITH, THOMAS FLETCHER Inventor name: RYAN, DAVID EDWARD |