EP0017618B1 - Process for optically brightening polyester fibres by the exhaust process - Google Patents
Process for optically brightening polyester fibres by the exhaust process Download PDFInfo
- Publication number
- EP0017618B1 EP0017618B1 EP80810098A EP80810098A EP0017618B1 EP 0017618 B1 EP0017618 B1 EP 0017618B1 EP 80810098 A EP80810098 A EP 80810098A EP 80810098 A EP80810098 A EP 80810098A EP 0017618 B1 EP0017618 B1 EP 0017618B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- dispersion
- process according
- fluorescent whitening
- whitening agent
- dye
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 238000000034 method Methods 0.000 title claims description 32
- 229920000728 polyester Polymers 0.000 title claims description 25
- 238000005282 brightening Methods 0.000 title description 10
- 239000006185 dispersion Substances 0.000 claims description 32
- 239000000975 dye Substances 0.000 claims description 31
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 18
- 239000002270 dispersing agent Substances 0.000 claims description 11
- 238000004043 dyeing Methods 0.000 claims description 10
- 239000000203 mixture Substances 0.000 claims description 7
- 239000000986 disperse dye Substances 0.000 claims description 5
- 239000000463 material Substances 0.000 claims description 4
- BCMCBBGGLRIHSE-UHFFFAOYSA-N 1,3-benzoxazole Chemical compound C1=CC=C2OC=NC2=C1 BCMCBBGGLRIHSE-UHFFFAOYSA-N 0.000 claims description 3
- XJHABGPPCLHLLV-UHFFFAOYSA-N benzo[de]isoquinoline-1,3-dione Chemical compound C1=CC(C(=O)NC2=O)=C3C2=CC=CC3=C1 XJHABGPPCLHLLV-UHFFFAOYSA-N 0.000 claims description 3
- 239000002245 particle Substances 0.000 claims description 3
- 239000003381 stabilizer Substances 0.000 claims description 2
- 239000000758 substrate Substances 0.000 claims description 2
- 239000000080 wetting agent Substances 0.000 claims description 2
- 239000006081 fluorescent whitening agent Substances 0.000 claims 8
- NGQSLSMAEVWNPU-UHFFFAOYSA-N 1,2-bis(2-phenylethenyl)benzene Chemical compound C=1C=CC=CC=1C=CC1=CC=CC=C1C=CC1=CC=CC=C1 NGQSLSMAEVWNPU-UHFFFAOYSA-N 0.000 claims 1
- GLIKXZUJKIVGIE-UHFFFAOYSA-N 2-[2-(2-phenylethenyl)phenyl]-1,3-benzoxazole Chemical compound C=1C=CC=C(C=2OC3=CC=CC=C3N=2)C=1C=CC1=CC=CC=C1 GLIKXZUJKIVGIE-UHFFFAOYSA-N 0.000 claims 1
- UKLNMMHNWFDKNT-UHFFFAOYSA-M sodium chlorite Chemical compound [Na+].[O-]Cl=O UKLNMMHNWFDKNT-UHFFFAOYSA-M 0.000 claims 1
- 229960002218 sodium chlorite Drugs 0.000 claims 1
- 230000002087 whitening effect Effects 0.000 claims 1
- 239000004744 fabric Substances 0.000 description 18
- 239000000835 fiber Substances 0.000 description 15
- -1 aromatic halogenated hydrocarbons Chemical class 0.000 description 10
- 230000003287 optical effect Effects 0.000 description 10
- 125000000217 alkyl group Chemical group 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical group C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 5
- 125000003545 alkoxy group Chemical group 0.000 description 5
- 238000004061 bleaching Methods 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- PJANXHGTPQOBST-VAWYXSNFSA-N Stilbene Natural products C=1C=CC=CC=1/C=C/C1=CC=CC=C1 PJANXHGTPQOBST-VAWYXSNFSA-N 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 4
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical compound C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 4
- 235000021286 stilbenes Nutrition 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 150000002170 ethers Chemical class 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- RPACBEVZENYWOL-XFULWGLBSA-M sodium;(2r)-2-[6-(4-chlorophenoxy)hexyl]oxirane-2-carboxylate Chemical compound [Na+].C=1C=C(Cl)C=CC=1OCCCCCC[C@]1(C(=O)[O-])CO1 RPACBEVZENYWOL-XFULWGLBSA-M 0.000 description 3
- WBIQQQGBSDOWNP-UHFFFAOYSA-N 2-dodecylbenzenesulfonic acid Chemical compound CCCCCCCCCCCCC1=CC=CC=C1S(O)(=O)=O WBIQQQGBSDOWNP-UHFFFAOYSA-N 0.000 description 2
- IGFHQQFPSIBGKE-UHFFFAOYSA-N 4-nonylphenol Chemical compound CCCCCCCCCC1=CC=C(O)C=C1 IGFHQQFPSIBGKE-UHFFFAOYSA-N 0.000 description 2
- 229920000742 Cotton Polymers 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 2
- ZUBJEHHGZYTRPH-KTKRTIGZSA-N [(z)-octadec-9-enyl] hydrogen sulfate Chemical compound CCCCCCCC\C=C/CCCCCCCCOS(O)(=O)=O ZUBJEHHGZYTRPH-KTKRTIGZSA-N 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 229910052783 alkali metal Inorganic materials 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 239000007859 condensation product Substances 0.000 description 2
- 229940060296 dodecylbenzenesulfonic acid Drugs 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 150000002191 fatty alcohols Chemical class 0.000 description 2
- 239000002657 fibrous material Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 239000011976 maleic acid Substances 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 2
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 2
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 2
- 229920000151 polyglycol Polymers 0.000 description 2
- 239000010695 polyglycol Substances 0.000 description 2
- 239000010453 quartz Substances 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- QIVUCLWGARAQIO-OLIXTKCUSA-N (3s)-n-[(3s,5s,6r)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl]-2-oxospiro[1h-pyrrolo[2,3-b]pyridine-3,6'-5,7-dihydrocyclopenta[b]pyridine]-3'-carboxamide Chemical compound C1([C@H]2[C@H](N(C(=O)[C@@H](NC(=O)C=3C=C4C[C@]5(CC4=NC=3)C3=CC=CN=C3NC5=O)C2)CC(F)(F)F)C)=C(F)C=CC(F)=C1F QIVUCLWGARAQIO-OLIXTKCUSA-N 0.000 description 1
- RELMFMZEBKVZJC-UHFFFAOYSA-N 1,2,3-trichlorobenzene Chemical class ClC1=CC=CC(Cl)=C1Cl RELMFMZEBKVZJC-UHFFFAOYSA-N 0.000 description 1
- DQBNLFRFALCILS-UHFFFAOYSA-N 1-[(2-sulfonaphthalen-1-yl)methyl]naphthalene-2-sulfonic acid Chemical compound C1=CC=C2C(CC3=C4C=CC=CC4=CC=C3S(=O)(=O)O)=C(S(O)(=O)=O)C=CC2=C1 DQBNLFRFALCILS-UHFFFAOYSA-N 0.000 description 1
- LWEAHXKXKDCSIE-UHFFFAOYSA-N 2,3-di(propan-2-yl)naphthalene-1-sulfonic acid Chemical compound C1=CC=C2C(S(O)(=O)=O)=C(C(C)C)C(C(C)C)=CC2=C1 LWEAHXKXKDCSIE-UHFFFAOYSA-N 0.000 description 1
- QZEDXQFZACVDJE-UHFFFAOYSA-N 2,3-dibutylnaphthalene-1-sulfonic acid Chemical compound C1=CC=C2C(S(O)(=O)=O)=C(CCCC)C(CCCC)=CC2=C1 QZEDXQFZACVDJE-UHFFFAOYSA-N 0.000 description 1
- CHZLVSBMXZSPNN-UHFFFAOYSA-N 2,4-dimethylbenzenesulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C(C)=C1 CHZLVSBMXZSPNN-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- RBABXJPJIHMBBP-WXUKJITCSA-N 2-[(e)-2-[4-[(e)-2-(2-cyanophenyl)ethenyl]phenyl]ethenyl]benzonitrile Chemical compound N#CC1=CC=CC=C1\C=C\C(C=C1)=CC=C1\C=C\C1=CC=CC=C1C#N RBABXJPJIHMBBP-WXUKJITCSA-N 0.000 description 1
- WFYSPVCBIJCZPX-UHFFFAOYSA-N 2-[4-(1,3-benzoxazol-2-yl)naphthalen-1-yl]-1,3-benzoxazole Chemical compound C12=CC=CC=C2C(C=2OC3=CC=CC=C3N=2)=CC=C1C1=NC2=CC=CC=C2O1 WFYSPVCBIJCZPX-UHFFFAOYSA-N 0.000 description 1
- UGFSLKRMHPGLFU-UHFFFAOYSA-N 2-[5-(1,3-benzoxazol-2-yl)thiophen-2-yl]-1,3-benzoxazole Chemical compound C1=CC=C2OC(C3=CC=C(S3)C=3OC4=CC=CC=C4N=3)=NC2=C1 UGFSLKRMHPGLFU-UHFFFAOYSA-N 0.000 description 1
- CBCZQJLYYRPMRI-UHFFFAOYSA-N 2-benzylnaphthalene-1-sulfonic acid Chemical compound C1=CC2=CC=CC=C2C(S(=O)(=O)O)=C1CC1=CC=CC=C1 CBCZQJLYYRPMRI-UHFFFAOYSA-N 0.000 description 1
- CYEJMVLDXAUOPN-UHFFFAOYSA-N 2-dodecylphenol Chemical compound CCCCCCCCCCCCC1=CC=CC=C1O CYEJMVLDXAUOPN-UHFFFAOYSA-N 0.000 description 1
- XNOVXSVWOMRJCP-UHFFFAOYSA-N 2-hexyl-3-propylnaphthalene-1-sulfonic acid Chemical compound C1=CC=CC2=C(S(O)(=O)=O)C(CCCCCC)=C(CCC)C=C21 XNOVXSVWOMRJCP-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- DLHGMUSMPXAUDH-UHFFFAOYSA-N 4-[2-(5,6-dimethyl-1,3-benzoxazol-2-yl)ethenyl]benzonitrile Chemical compound O1C=2C=C(C)C(C)=CC=2N=C1C=CC1=CC=C(C#N)C=C1 DLHGMUSMPXAUDH-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 125000006267 biphenyl group Chemical group 0.000 description 1
- 239000001045 blue dye Substances 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229910001919 chlorite Inorganic materials 0.000 description 1
- 229910052619 chlorite group Inorganic materials 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- QBWCMBCROVPCKQ-UHFFFAOYSA-N chlorous acid Chemical compound OCl=O QBWCMBCROVPCKQ-UHFFFAOYSA-N 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 150000004816 dichlorobenzenes Chemical class 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000007730 finishing process Methods 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 238000010327 methods by industry Methods 0.000 description 1
- QLAXRNXBCBELEB-UHFFFAOYSA-N methyl 4-[2-(5,6-dimethyl-1,3-benzoxazol-2-yl)ethenyl]benzoate Chemical compound C1=CC(C(=O)OC)=CC=C1C=CC1=NC2=CC(C)=C(C)C=C2O1 QLAXRNXBCBELEB-UHFFFAOYSA-N 0.000 description 1
- PSZYNBSKGUBXEH-UHFFFAOYSA-N naphthalene-1-sulfonic acid Chemical compound C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-N 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- HVAMZGADVCBITI-UHFFFAOYSA-M pent-4-enoate Chemical compound [O-]C(=O)CCC=C HVAMZGADVCBITI-UHFFFAOYSA-M 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N phenylbenzene Natural products C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000001057 purple pigment Substances 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- GIPRGFRQMWSHAK-UHFFFAOYSA-M sodium;2-propan-2-ylbenzenesulfonate Chemical compound [Na+].CC(C)C1=CC=CC=C1S([O-])(=O)=O GIPRGFRQMWSHAK-UHFFFAOYSA-M 0.000 description 1
- KVCGISUBCHHTDD-UHFFFAOYSA-M sodium;4-methylbenzenesulfonate Chemical compound [Na+].CC1=CC=C(S([O-])(=O)=O)C=C1 KVCGISUBCHHTDD-UHFFFAOYSA-M 0.000 description 1
- MZSDGDXXBZSFTG-UHFFFAOYSA-M sodium;benzenesulfonate Chemical compound [Na+].[O-]S(=O)(=O)C1=CC=CC=C1 MZSDGDXXBZSFTG-UHFFFAOYSA-M 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 125000005504 styryl group Chemical group 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000004753 textile Substances 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/34—Material containing ester groups
- D06P3/52—Polyesters
- D06P3/54—Polyesters using dispersed dyestuffs
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/40—Dyes ; Pigments
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/40—Dyes ; Pigments
- C11D3/42—Brightening agents ; Blueing agents
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06L—DRY-CLEANING, WASHING OR BLEACHING FIBRES, FILAMENTS, THREADS, YARNS, FABRICS, FEATHERS OR MADE-UP FIBROUS GOODS; BLEACHING LEATHER OR FURS
- D06L4/00—Bleaching fibres, filaments, threads, yarns, fabrics, feathers or made-up fibrous goods; Bleaching leather or furs
- D06L4/60—Optical bleaching or brightening
- D06L4/686—Fugitive optical brightening; Discharge of optical brighteners in discharge paste; Blueing; Differential optical brightening
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S8/00—Bleaching and dyeing; fluid treatment and chemical modification of textiles and fibers
- Y10S8/92—Synthetic fiber dyeing
- Y10S8/922—Polyester fiber
Definitions
- the present invention relates to a new, improved process for the optical brightening of polyester fibers in the exhaust process with the aid of conventional polyester brighteners with the use of shading dyes.
- the process according to the invention for the optical brightening of polyester fibers in the drawing-out process by treating the fibers in an aqueous polyester brightener which is stable under the application conditions and which absorbs onto the substrate and a small amount of a blue to violet disperse dye or mixtures of such dyes as shading dye is thereby characterized in that the treatment in said dispersion is carried out at a pH of more than 9.
- the dispersion has a pH between 11 and 14, e.g. B. between 11 and 13.5, in particular between 11 and 12.5.
- the pH is adjusted using a suitable alkaline substance, preferably using alkali metal hydroxides, in particular using KOH, but especially NaOH.
- the treatment is carried out in the customary manner at a temperature between room temperature and 140 ° C., in particular between 50 ° and 130 ° C.
- the fiber material is advantageously used at a lower temperature (for example 50 ° C.), whereupon the temperature is increased (e.g. to 120 ° C).
- HT high temperature
- carrier is added to the treatment bath that is common in dyeing practice.
- carrier addition it is also possible to achieve very good results at lower temperatures, for example below 100 ° C.
- carrier addition can also be used under HT conditions.
- Suitable carriers are those customary in dyeing practice, e.g. B. aromatic hydrocarbons, aromatic halogenated hydrocarbons and esters and ethers of aromatic carboxylic acids.
- Preferred carriers are dichlorobenzenes and trichlorobenzenes, optionally also diphenyl and mixtures of the substances mentioned.
- the duration of the treatment of the textiles in the brightener dispersion can vary within wide limits, but an application period of at least 20 to 30 ° minutes is advantageous.
- the amount of brightener (pure substance) in the dispersion can, depending on the brightener used, vary between 0.002 and 0.5%, based on the material to be lightened.
- the amount of shading dye is, depending on the dye and the desired shade, about 0.0025-2.5%, preferably 0.025-1.25%, based on the amount of brightener (pure substance) used.
- polyester brighteners are used as brighteners, which in practice are applied together with shading dyes. These are mostly benzoxazole, stilbene and naphthalimide brighteners.
- rings A, B and C may also contain simple radicals, such as lower alkyl or alkoxy groups or chlorine atoms, especially 2,5-bis-benzoxazol-2-yl-thiophene, 2,5-bis- (5- Methyl-benzoxazol-2-yl) ethylene and 1,4-bis-benzoxazol-2-yl-naphthalene; or the formula where n is 0 or 1 and rings A and B may be further substituted, e.g. B.
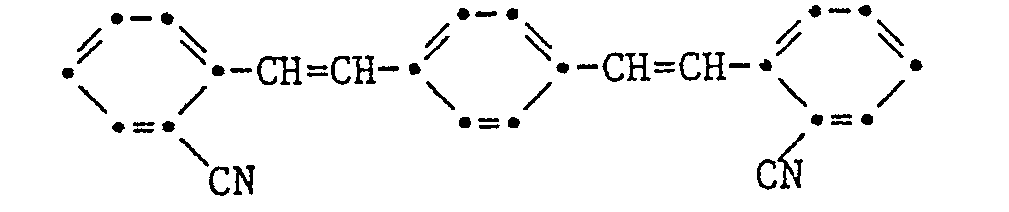
- stilbene brighteners are those of the formulas respectively. wherein the rings A, B and C can carry different substituents, such as. As alkyl, alkoxy, chlorine, cyano, carboxy and its derivatives, etc., to mention, in particular the compounds 2-cyano-4- (naphtho (1,2-d) v-triazol-2-yl) -4'- chlorstilbene, 4- (naphtho [1,2-dlv-triazol-2-yl) -4'-methoxycarbonylstilbene, and 1,4-bis- (2-cyanostyryl) benzene.
- substituents such as alkyl, alkoxy, chlorine, cyano, carboxy and its derivatives, etc.
- naphthalimide brighteners are those of the formula in which R i and R 2 are hydrogen or alkoxy, especially those in which R 1 is hydrogen and R 2 is methoxy or in which R 1 and R 2 are each ethoxy.
- Blue to violet disperse dyes are used as shading dyes, which of course have to be suitable for treatment in an alkaline liquor.
- Acylaminoanthraquinone dyes are preferably used, in particular those of the formula wherein the benzene rings A may be optionally substituted, e.g. B. by alkyl, alkoxy, halogen, etc.
- Dyes whose benzene rings A are unsubstituted or substituted in the para position with chlorine or methoxy are particularly suitable.
- the aqueous dispersion advantageously also contains one or more dispersants and, if appropriate, wetting agents, stabilizers and / or other customary dyeing auxiliaries.
- Suitable dispersants include: alkali metal salts, especially sodium salts of alkyl or alkylarylsulfonic acids and carboxylic acids, alkali metal salts, especially sodium salts of condensation products of arylsulfonic acids with formaldehyde, macromolecular substances which are suitable for liquefaction and dispersion, carboxylates of the polymerized maleic acid type polymerized acrylic acid and copolymers of maleic acid with allyl acetate.
- Examples of such dispersants are: lauryl sulfate sodium salt, oleyl sulfate sodium salt, oleyl sulfate diethanolamine salt, benzylnaphthalenesulfonic acid Na, di- (2-sulfo-1-naphthyl) methane di-Na salt, m-xylenesulfonic acid Na, dodecylbenzenesulfonic acid Na Na salt, dodecylbenzenesulfonic acid, diethanolamine, diisopropylnaphthalenesulfonic acid Na, di-n-butylnaphthalenesulfonic acid Na, n-propyl-n-hexylnaphthalenesulfonic acid Na, N-oleylmethyl taurine Na salt, Na salt of the condensation product of naphthalenesulfonic acid and formaldehyde acid formaldehyde , Benzenesulfonic acid sodium salt, cum
- particularly preferred dispersants are nonionic, water-soluble ethoxylated or propoxylated fatty alcohols and alkylphenols and fatty alcohol polyglycol ethers, for example alkanols, alkenols (C 8 -C 22 ) with different amounts of ethyleneoxy or propyleneoxy groups, alkyl or arylpolyglycol ethers with up to 50 ethyleneoxy or propyleneoxy groups, such as octyl, nonyl or dodecylphenol polyglycol ether.
- the individual components can be introduced separately into the treatment bath, which is already alkaline or is only then adjusted to the desired pH.
- Such dispersions contain brightener and dye in the desired ratio. They are made by adding brightener and dye, preferably made together with a dispersant in a small amount of water. It is advantageous if this dispersion is subjected to grinding (for example in a ball mill) in order to obtain particle sizes of less than 10 ⁇ m, preferably less than 2 ⁇ m.
- this stock dispersion can then be introduced (calculated on the desired amount of brightener in the bath) into the treatment bath, which may also contain a dispersant and / or other auxiliaries.
- the treatment bath which may also contain a dispersant and / or other auxiliaries.
- the bleaching bath can then be bleached directly.
- Sodium chloride is preferably added to the liquor, the alkaline bath is acidified and then heated to about boiling temperature.
- polyester fibers naturally also include polyester fibers in blended fabrics, eg. B. in mixed fabrics polyester / cotton.
- the nuanced lightening of such blended fabrics by the process according to the invention can also be advantageous with the lightening of the cotton content, with the bleaching (e.g. with peroxide) and / or the various conventional finishing and finishing processes (e.g. crease-resistant, wash and wear, soft grip and other equipment) can be combined.
- 0.4 g of the stock dispersion described under a) and 0.2 g of a dispersant are processed into a dispersion with 400 ml of water and the latter is made alkaline with 0.8 ml of NaOH 30%.
- polyester staple fabric (washed and heat-set at 1800 C for 20 seconds) are wound evenly onto bobbin tubes as a fabric tape of approx. 250 x 12 cm and treated with the dispersion just described in an HT dyeing machine.
- the fleet had an initial pH of 11.5.
- the temperature is 50 ° C, after that heated to 130 ° C within 30 minutes and then left at this temperature for 30 minutes.
- the mixture is then cooled to 70 ° C., the liquor is drained off, the fabric is rinsed twice warm and dried in a drying cabinet at 80 ° C. for 5 minutes.
- the lightened fabric has a high degree of whiteness with perfect levelness.
- Example 1b As described in Example 1b), 0.4 g of this stock dispersion is processed into a liquor (pH approx. 11.5) and thus 40 g of polyester staple fabric is lightened in the manner given there.
- the treated fabric also has a high degree of whiteness with perfect levelness.
- polyester brightener used is that of the formula a, you also get a highly lightened polyester fabric of excellent levelness.
- Example 1 a 0.4 g of the stock dispersion described in Example 1 a) and 0.2 g of a dispersant (adduct of 35 ethylene oxide groups with stearyl alcohol) are processed into a dispersion with 400 ml of water and the latter made alkaline with 0.8 ml of NaOH 30%.
- a dispersant adduct of 35 ethylene oxide groups with stearyl alcohol
- polyester staple fabric (washed and heat-set at 180 ° C for 30 seconds) are evenly wound onto bobbin tubes as a fabric tape of approx. 250x12 cm and treated with the dispersion just described in an HT dyeing machine.
- the fleet had an initial pH of 11.5.
- the fabric is introduced at 50 ° C. and the liquor is heated to 120 ° C. in 30 minutes, treated at this temperature for 15 minutes, cooled to 70 ° C., 0.8 g of sodium chloride 80% is added and the liquor is made up with 1 , 4 ml of formic acid 85% to a pH of 3-4.
- the mixture is heated to about 100 ° C. in 20 minutes, treated at this temperature for 30 minutes, then the liquor is drained off, rinsed and dried.
- the piece of fabric is immaculate and has a high degree of whiteness.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Coloring (AREA)
Description
Die vorliegende Erfindung betrifft ein neues, verbessertes Verfahren zum optischen Aufhellen von Polyesterfasern im Ausziehverfahren mit Hilfe von üblichen Polyesteraufhellern unter Mitverwendung von Nuancierfarbstoffen.The present invention relates to a new, improved process for the optical brightening of polyester fibers in the exhaust process with the aid of conventional polyester brighteners with the use of shading dyes.
Beim Bleichen und optischen Aufhellen ist es eine oft geübte Methode, zur Verbesserung des Bleich- bzw. Aufhelleffektes Violett- oder Blaufarbstoffe mitzuverwenden. Wird ein derartiger Farbstoff gemeinsam mit einem optischen Aufheller appliziert, kann dies zwei verschiedenen Zwecken dienen: einerseits kann eine Weißgradsteigerung durch Kompensation des Gelbanteiles der Faser angestrebt werden, wobei der durch den Aufheller auf der Faser erzeugte Farbton weitgehend beibehalten wird. Andererseits kann mit besagtem Farbstoff eine Änderung der Nuance des durch den Aufheller auf der Faser erzeugten Farbtons angestrebt werden, wobei auch hier versucht wird, zusätzlich noch eine Weißgradsteigerung zu erreichen. Man kann damit die jeweils gewünschte Nuance des Weißes einstellen.In bleaching and optical brightening, it is an often practiced method to use violet or blue dyes to improve the bleaching or lightening effect. If such a dye is applied together with an optical brightener, this can serve two different purposes: on the one hand, an increase in the degree of whiteness can be sought by compensating for the yellow portion of the fiber, the color tone produced by the brightener on the fiber being largely retained. On the other hand, a change in the shade of the color tone produced by the brightener on the fiber can be aimed for with said dye, and here too an attempt is made to additionally increase the degree of whiteness. You can use it to set the desired shade of white.
Besonders interessante Weißeffekte sind so auf Fasermaterialien mit hohem Grundweiß zu erreichen, bei denen die Erreichung eines geringen Zuwachses an Helligkeit (Weißgrad) einen erheblichen Mehraufwand im Bleich- bzw. Aufhellprozeß bedeutet. Man setzt zur Nuancierung kleine Mengen an Farbstoff, in der Regel etwa 0,0025―2,5%, vorzugsweise 0,025-1,25%, bezogen auf die eingesetzte Menge an Aufheller, zu. Verfahrenstechnisch bereitet die Nuancierung von kontinuierlich herstellbaren optischen Aufhellungen (z. B. im Foulardthermverfahren) im allgemeinen keine Schwierigkeiten. Die Nuancierung, z. B. mit Dispersionsfarbstoffen, von optischen Aufhellungen nach dem Ausziehverfahren ist hingegen häufig mit erheblichen Egalitätsproblemen behaftet. Als nahezu unmöglich erwies sich bisher die Nuancierung von optischen Aufhellungen mit Hilfe des Ausziehverfahrens auf Polyesterfasern, wenn dem Echtheitsstandard des Aufhellers angepaßte Dispersionsfarbstoffe als Nuancierfarbstoffe verwendet wurden. Die übliche Aufhellung, aber auch das Färben von Polyesterfasern nach dem Ausziehverfahren wird üblicherweise aus schwach sauren bis neutralen Bädern vorgenommen. Auf diese Weise erhält man, wenn neben dem Aufheller ein Nuancierfarbstoff mitverwendet wird, stark unegale Aufhellungen, da der Farbstoff sich rasch auf der Faser ablagert und so das Gewebe an bestimmten Stellen zu stark anfärbt. Es wurde auch schon versucht, diesem Nachteil durch Zusatz von Egalisiermitteln zu begegnen. Dadurch konnte aber einerseits keine vollständige Behebung des Problems erreicht werden, andererseits wird das Verfahren durch die Notwendigkeit des Einsatzes von teuren Egalisiermitteln unwirtschaftlicher.Particularly interesting white effects can be achieved in this way on fiber materials with a high basic white, in which the achievement of a small increase in brightness (degree of whiteness) means a considerable additional effort in the bleaching or lightening process. Small amounts of dye, generally about 0.0025-2.5%, preferably 0.025-1.25%, based on the amount of brightener used, are added for shading. In terms of process engineering, the nuance of continuously producible optical brightenings (for example in the padding thermal process) generally presents no difficulties. The shading, e.g. B. with disperse dyes, of optical brightening after the exhaust process, however, is often associated with significant equal problems. Up to now, the shading of optical brightenings with the aid of the pull-out process on polyester fibers has proven to be almost impossible if disperse dyes adapted to the authenticity standard of the brightener were used as shading dyes. The usual lightening, but also the dyeing of polyester fibers after the exhaust process is usually carried out from weakly acidic to neutral baths. In this way, if a shading dye is used in addition to the brightener, very uneven lightenings are obtained, since the dye quickly deposits on the fiber and so the fabric stains too strongly at certain points. Attempts have already been made to counter this disadvantage by adding leveling agents. On the one hand, this did not, however, completely solve the problem, and on the other hand, the process became more uneconomical due to the need to use expensive leveling agents.
Es wurde nun ein neues, verbessertes Verfahren gefunden, das die eben beschriebenen Nachteile der bekannten Verfahren auf einfache Art vollständig beseitigt.A new, improved method has now been found which completely eliminates the disadvantages of the known methods just described.
Das erfindungsgemäße Verfahren zum optischen Aufhellen von Polyesterfasern im Ausziehverfahren durch Behandlung der Fasern in einer wässerigen, einen oder mehrere unter den Applikationsbedingungen beständige und auf das Substrat aufziehende Polyesteraufheller und eine kleine Menge eines blauen bis violetten Dispersionsfarbstoffes oder Mischungen solcher Farbstoffe als Nuancierfarbstoff enthaltenden Dispersion ist dadurch gekennzeichnet, daß man die Behandlung in besagter Dispersion bei einem pH-Wert von über 9 vornimmt.The process according to the invention for the optical brightening of polyester fibers in the drawing-out process by treating the fibers in an aqueous polyester brightener which is stable under the application conditions and which absorbs onto the substrate and a small amount of a blue to violet disperse dye or mixtures of such dyes as shading dye is thereby characterized in that the treatment in said dispersion is carried out at a pH of more than 9.
Prinzipiell ist keine Beschränkung des pH-Wertes nach oben nötig. Auch Alkalimengen, die zu einem pH-Wert von über 14 in der Dispersion führen, beeinträchtigen die Egalität der Aufhellung nicht. Eine obere Grenze ist allenfalls aus wirtschaftlichen Überlegungen heraus gegeben. Eine obere Grenze der Alkalität wäre beispielsweise durch die Verfahrensbedingungen des »Schälprozesses« des Polyestermaterials gegeben (beispielsweise bei einer Konzentration von 10-20 g/I NaOH).In principle, there is no need to limit the pH upwards. Even amounts of alkali that lead to a pH of over 14 in the dispersion do not affect the levelness of the brightening. An upper limit is at best given for economic reasons. An upper limit of the alkalinity would be given, for example, by the process conditions of the »peeling process« of the polyester material (for example at a concentration of 10-20 g / l NaOH).
Vorzugsweise weist die Dispersion einen pH-Wert zwischen 11 und 14, z. B. zwischen 11 und 13,5, insbesondere zwischen 11 und 12,5, auf.Preferably the dispersion has a pH between 11 and 14, e.g. B. between 11 and 13.5, in particular between 11 and 12.5.
Der pH-Wert wird durch eine geeignete alkalische Substanz, vorzugsweise durch Alkalihydroxide, insbesondere durch KOH, vor allem aber NaOH eingestellt.The pH is adjusted using a suitable alkaline substance, preferably using alkali metal hydroxides, in particular using KOH, but especially NaOH.
Die Behandlung erfolgt in der üblichen Weise bei einer Temperatur zwischen Raumtemperatur und 140° C, insbesondere zwischen 50° und 130° C. Vorteilhaft wird mit dem Fasermaterial bei tieferer Temperatur (z. B. 50° C) eingegangen, woraufhin die Temperatur gesteigert wird (z. B. auf 120° C).The treatment is carried out in the customary manner at a temperature between room temperature and 140 ° C., in particular between 50 ° and 130 ° C. The fiber material is advantageously used at a lower temperature (for example 50 ° C.), whereupon the temperature is increased (e.g. to 120 ° C).
Um ein optimales Aufziehen des Aufhellers auf die Faser zu gewährleisten, wird in der Praxis vorzugsweise unter Hochtemperatur (HT)-Bedingungen gearbeitet, das heißt, die Behandlung der Fasern erfolgt in einem üblichen HT-Färbeapparat oberhalb von 100°C, beispielsweise zwischen 100 und 130° C, z. B. bei 120° C.In order to ensure that the brightener is optimally drawn onto the fiber, work is preferably carried out in practice under high temperature (HT) conditions, that is to say the fibers are treated in a conventional HT dyeing machine above 100 ° C., for example between 100 and 130 ° C, e.g. B. at 120 ° C.
Eine weitere Möglichkeit, das Aufziehen des Aufhellers auf die Faser zu erleichtern, ist der Zusatz eines in der Färbereipraxis üblichen Carriers zum Behandlungsbad. Bei Carrierzusatz ist es auch möglich, bei tieferen Temperaturen sehr gute Resultate zu erzielen, beispielsweise unter 100° C. Aber auch mit Carrier-Zusatz kann unter HT-Bedingungen gearbeitet werden. Als Carrier kommen die in der Färbereipraxis üblichen in Betracht, z. B. aromatische Kohlenwasserstoffe, aromatische Halogenkohlenwasserstoffe sowie Ester und Äther von aromatischen Carbonsäuren. Bevorzugte Carrier sind Dichlor- und Trichlorbenzole, gegebenenfalls auch Diphenyl sowie Mischungen der genannten Substanzen.Another way to make it easier to attach the brightener to the fiber is to add a carrier to the treatment bath that is common in dyeing practice. With carrier addition, it is also possible to achieve very good results at lower temperatures, for example below 100 ° C. However, carrier addition can also be used under HT conditions. Suitable carriers are those customary in dyeing practice, e.g. B. aromatic hydrocarbons, aromatic halogenated hydrocarbons and esters and ethers of aromatic carboxylic acids. Preferred carriers are dichlorobenzenes and trichlorobenzenes, optionally also diphenyl and mixtures of the substances mentioned.
Die Dauer der Behandlung der Textilien in der Aufhellerdispersion kann in weiten Grenzen schwanken, jedoch ist eine Applikationsdauer von mindestens 20 bis 30° Minuten vorteilhaft.The duration of the treatment of the textiles in the brightener dispersion can vary within wide limits, but an application period of at least 20 to 30 ° minutes is advantageous.
Die Menge an Aufheller (Reinsubstanz) in der Dispersion kann, je nach verwendetem Aufheller, zwischen 0,002 und 0,5%, bezogen auf das aufzuhellende Material, schwanken.The amount of brightener (pure substance) in the dispersion can, depending on the brightener used, vary between 0.002 and 0.5%, based on the material to be lightened.
Die Menge an Nuancierfarbstoff (reiner Farbstoff) beträgt, je nach Farbstoff und der gewünschten Nuance, etwa 0,0025-2,5%, vorzugsweise 0,025-1,25%, bezogen auf die eingesetzte Menge an Aufheller (Reinsubstanz).The amount of shading dye (pure dye) is, depending on the dye and the desired shade, about 0.0025-2.5%, preferably 0.025-1.25%, based on the amount of brightener (pure substance) used.
Als Aufheller werden die üblichen Polyesteraufheller verwendet, die in der Praxis gemeinsam mit Nuancierfarbstoffen appliziert werden. Es sind dies meist Benzoxazol-, Stilben- und Naphthalimid-Aufheller.The usual polyester brighteners are used as brighteners, which in practice are applied together with shading dyes. These are mostly benzoxazole, stilbene and naphthalimide brighteners.
Von den Benzoxazol-Aufhellern sind z. B. bis-Benzoxazol-, Styryl- oder Stilbenyl-benzoxyzoltypen zu erwähnen, besonders Verbindungen der Formeln
Von den Stilben-Aufhellern seien solche der Formeln
Von den Naphthalimid-Aufhellern sind solche der Formel
Als Nuancierfarbstoffe werden blaue bis violette Dispersionsfarbstoffe verwendet, die natürlich für eine Behandlung in einer alkalischen Flotte geeignet sein müssen. Bevorzugt werden Acylaminoanthrachinonfarbstoffe verwendet, insbesondere solche der Formel
Die wässerige Dispersion enthält neben dem (den) optischen Aufheller(n) und dem (den) Nuancierfarbstoff(en) vorteilhaft zusätzlich einen oder mehrere Dispergatoren und gegebenenfalls noch Netz-, Stabilisier- und/oder weitere übliche Färbereihilfsmittel.In addition to the optical brightener (s) and the shading dye (s), the aqueous dispersion advantageously also contains one or more dispersants and, if appropriate, wetting agents, stabilizers and / or other customary dyeing auxiliaries.
Als Dispergatoren kommen unter anderen in Betracht: Alkalimetallsalze, besonders Natriumsalze von Alkyl- oder Alkylarylsulfonsäuren und -carbonsäuren, Alkalimetallsalze, besonders Natriumsalze von Kondensationsprodukten aus Arylsulfonsäuren mit Formaldehyd, makromolekulare Stoffe, welche sich zum Verflüssigen und Dispergieren eignen, Carboxylate vom Typ der polymerisierten Maleinsäure oder polymerisierten Acrylsäure und Copolymerisate aus Maleinsäure mit Allylacetat. Als Beispiele solcher Dispergatoren sind zu erwähnen: Laurylsulfat Na-Salz, Oleylsulfat Na-Salz, Oleylsulfat Diäthanolaminsalz, Benzylnaphthalinsulfosaures Na, Di-(2-sulfo-1-naphthyl)-methan Di-Na-Salz, m-Xylolsulfosaures Na, Dodecylbenzolsulfosaures-Na-Salz, Dodecylbenzolsulfosaures Diäthanolamin, Diisopropylnaphthalinsulfosaures Na, Di-n-butylnaphthalinsulfosaures Na, n-Propyl-n-hexyl-naphthalinsulfosaures Na, N-Oleylmethyltaurin Na-Salz, Na-Salz des Kondensationsproduktes aus Naphthalinsulfosäure und Formaldehyd, Sulfanilsäure-Na-Salz, Benzolsulfosäure-Na-Salz, Cumolsulfosäure-Na-Salz, Toluolsulfosäure-Na-Salz, oxäthylierte Harzkörper, N-Polyvinylpyrrolidon, Sulfitcelluloseablauge (CaO-frei), Stärkeäther und Polysaccharide. Besonders bevorzugt als Dispergatoren sind aber nichtionogene, wasserlösliche äthoxylierte bzw. propoxylierte Fettalkohole und Alkylphenole sowie Fettalkoholpolyglykoläther, z.B. Alkanole, Alkenole (C8-C22) mit verschiedenen Mengen an Äthylenoxy- bzw. Propylenoxygruppen, Alkyl- oder Arylpolyglykoläther mit bis zu 50 Äthylenoxy- bzw. Propylenoxygruppen, etwa Octyl-, Nonyl- oder Dodecylphenolpolyglykol- äther.Suitable dispersants include: alkali metal salts, especially sodium salts of alkyl or alkylarylsulfonic acids and carboxylic acids, alkali metal salts, especially sodium salts of condensation products of arylsulfonic acids with formaldehyde, macromolecular substances which are suitable for liquefaction and dispersion, carboxylates of the polymerized maleic acid type polymerized acrylic acid and copolymers of maleic acid with allyl acetate. Examples of such dispersants are: lauryl sulfate sodium salt, oleyl sulfate sodium salt, oleyl sulfate diethanolamine salt, benzylnaphthalenesulfonic acid Na, di- (2-sulfo-1-naphthyl) methane di-Na salt, m-xylenesulfonic acid Na, dodecylbenzenesulfonic acid Na Na salt, dodecylbenzenesulfonic acid, diethanolamine, diisopropylnaphthalenesulfonic acid Na, di-n-butylnaphthalenesulfonic acid Na, n-propyl-n-hexylnaphthalenesulfonic acid Na, N-oleylmethyl taurine Na salt, Na salt of the condensation product of naphthalenesulfonic acid and formaldehyde acid formaldehyde , Benzenesulfonic acid sodium salt, cumene sulfonic acid sodium salt, toluenesulfonic acid sodium salt, oxyethylated resin bodies, N-polyvinylpyrrolidone, sulfite cellulose waste liquor (CaO-free), starch ether and polysaccharides. However, particularly preferred dispersants are nonionic, water-soluble ethoxylated or propoxylated fatty alcohols and alkylphenols and fatty alcohol polyglycol ethers, for example alkanols, alkenols (C 8 -C 22 ) with different amounts of ethyleneoxy or propyleneoxy groups, alkyl or arylpolyglycol ethers with up to 50 ethyleneoxy or propyleneoxy groups, such as octyl, nonyl or dodecylphenol polyglycol ether.
Die einzelnen Bestandteile können getrennt in das Behandlungsbad eingebracht werden, welches bereits alkalisch ist oder erst anschließend auf dem gewünschten pH-Wert eingestellt wird.The individual components can be introduced separately into the treatment bath, which is already alkaline or is only then adjusted to the desired pH.
Vorzugsweise liegt jedoch eine konzentrierte, lagerstabile Stammdispersion des (der) optischen Aufheller(s) und des (der) Nuancierfarbstoffe(s) vor. Derartige Dispersionen enthalten Aufheller und Farbstoff im gewünschten Verhältnis. Sie werden durch Einbringen von Aufheller und Farbstoff, vorzugsweise gemeinsam mit einem Dispergator, in eine kleine Menge Wasser hergestellt. Vorteilhaft ist es, wenn man diese Dispersion noch einer Mahlung unterwirft (z. B. in einer Kugelmühle), um Teilchengrößen von kleiner als 10 µm, vorzugsweise kleiner als 2 µm, zu erhalten.However, there is preferably a concentrated, storage-stable stock dispersion of the optical brightener (s) and the shading dye (s). Such dispersions contain brightener and dye in the desired ratio. They are made by adding brightener and dye, preferably made together with a dispersant in a small amount of water. It is advantageous if this dispersion is subjected to grinding (for example in a ball mill) in order to obtain particle sizes of less than 10 μm, preferably less than 2 μm.
Von dieser Stammdispersion kann dann ein Teil (berechnet auf die gewünschte Aufhellermenge im Bad) in das Behandlungsbad eingebracht werden, das gegebenenfalls noch einen Dispergator und/oder andere Hilfsmittel enthält. Nach Einstellung des gewünschten pH-Wertes können Polyesterfasern in Form von Garn, Gewebe usw. in den entsprechenden dafür geeigneten Färbeapparaturen mit der so erhaltenen Dispersion behandelt werden.A part of this stock dispersion can then be introduced (calculated on the desired amount of brightener in the bath) into the treatment bath, which may also contain a dispersant and / or other auxiliaries. After the desired pH has been set, polyester fibers in the form of yarn, woven fabric, etc. can be treated with the dispersion thus obtained in the corresponding dyeing apparatus.
Sofern ein chloritbeständiger Nuancierfarbstoff verwendet wurde, kann im Aufhellungsbad anschließend direkt gebleicht werden. Vorzugsweise gibt man hierzu der Flotte Natriumchlorid bei, säuert das alkalische Bad an und erhitzt anschließend etwa auf Kochtemperatur.If a chlorite-resistant shading dye was used, the bleaching bath can then be bleached directly. Sodium chloride is preferably added to the liquor, the alkaline bath is acidified and then heated to about boiling temperature.
Unter »Polyesterfasern« sind in der vorliegenden Anmeldung selbstverständlich auch Polyesterfasern in Mischgeweben, z. B. in Mischgeweben Polyester/Baumwolle zu verstehen. Die nuancierte Aufhellung von solchen Mischgeweben nach dem erfindungsgemäßen Verfahren kann auch vorteilhaft mit dem Aufhellen des Baumwollanteiles, mit dem Bleichen (z. B. mit Peroxid) und/oder den verschiedenen üblichen Ausrüstungs- und Veredelungsprozessen (z. B. Knitterfest-, wash and wear-, Weichgriff- und anderen Ausrüstungen) kombiniert werden.In the present application, "polyester fibers" naturally also include polyester fibers in blended fabrics, eg. B. in mixed fabrics polyester / cotton. The nuanced lightening of such blended fabrics by the process according to the invention can also be advantageous with the lightening of the cotton content, with the bleaching (e.g. with peroxide) and / or the various conventional finishing and finishing processes (e.g. crease-resistant, wash and wear, soft grip and other equipment) can be combined.
Die folgenden Beispiele dienen der näheren Erläuterung des erfindungsgemäßen Verfahrens, ohne es jedoch darauf zu beschränken.The following examples serve to explain the process according to the invention in more detail, but without restricting it to them.
20 g des optischen Aufhellers der Formel
0,4 g der unter a) beschriebenen Stammdispersion und 0,2 g eines Dispergators (Anlagerungsprodukt von 35 Äthylenoxidgruppen an Stearylalkohol) werden mit 400 ml Wasser zu einer Dispersion verarbeitet und letztere mit 0,8 ml NaOH 30% alkalisch gemacht.0.4 g of the stock dispersion described under a) and 0.2 g of a dispersant (adduct of 35 ethylene oxide groups with stearyl alcohol) are processed into a dispersion with 400 ml of water and the latter is made alkaline with 0.8 ml of NaOH 30%.
40 g Polyester-Stapelgewebe (gewaschen und 20 Sekunden thermofixiert bei 1800 C) werden als Gewebeband von ca. 250 x 12 cm gleichmäßig auf Spulenhülsen gewickelt und in einem HT-Färbeapparat mit der eben beschriebenen Dispersion behandelt. Die Flotte hatte dabei einen Anfangs-pH-Wert von 11,5. Zu Beginn der Behandlung beträgt die Temperatur 50°C, danach wird innerhalb von 30 Minuten auf 130°C erhitzt und anschließend 30 Minuten bei dieser Temperatur belassen. Hierauf wird auf 70° C abgekühlt, die Flotte abgelassen, das Gewebe zweimal warm gespült und in einem Trockenschrank bei 80° C 5 Minuten lang getrocknet.40 g of polyester staple fabric (washed and heat-set at 1800 C for 20 seconds) are wound evenly onto bobbin tubes as a fabric tape of approx. 250 x 12 cm and treated with the dispersion just described in an HT dyeing machine. The fleet had an initial pH of 11.5. At the beginning of the treatment the temperature is 50 ° C, after that heated to 130 ° C within 30 minutes and then left at this temperature for 30 minutes. The mixture is then cooled to 70 ° C., the liquor is drained off, the fabric is rinsed twice warm and dried in a drying cabinet at 80 ° C. for 5 minutes.
Das aufgehellte Gewebe weist einen hohen Weißgrad von einwandfreier Egalität auf.The lightened fabric has a high degree of whiteness with perfect levelness.
Zu Vergleichszwecken wird der Versuch wiederholt, jedoch werden der Dispersion nicht 0,8 ml NaOH 30%, sondern 0,2 ml Essigsäure 80% beigegeben (pH-Wert der Flotte: 5,5). Auf diese Weise wird die Aufhellung nach der herkömmlichen Methode durchgeführt. Man erhält nach dem Trocknen ein Gewebe, das eine sehr starke Unegalität aufweist und daher unbrauchbar ist. Der Nuancierfarbstoff ist weitgehend im Innenteil des Stoffbandes abgelagert.For comparison purposes, the experiment is repeated, but not 0.8 ml of NaOH 30%, but 0.2 ml of acetic acid 80% are added to the dispersion (pH of the liquor: 5.5). In this way, the brightening is carried out according to the conventional method. After drying, a fabric is obtained which is very uneven and therefore unusable. The shading dye is largely deposited in the inner part of the ribbon.
Analog Beispiel 1 a) wird aus 20 g des Aufhellers der Formel
0,4 g dieser Stammdispersion werden, wie in Beispiel 1 b) beschrieben, zu einer Flotte verarbeitet (pH-Wert ca. 11,5) und damit in der dort angegebenen Weise 40 g Polyester-Stapelgewebe aufgehellt. Das behandelte Gewebe weist ebenfalls einen hohen Weißgrad von einwandfreier Egalität auf.As described in Example 1b), 0.4 g of this stock dispersion is processed into a liquor (pH approx. 11.5) and thus 40 g of polyester staple fabric is lightened in the manner given there. The treated fabric also has a high degree of whiteness with perfect levelness.
Verfährt man völlig analog zu Beispiel 2, verwendet jedoch als Aufheller eine Mischung der beiden Verbindungen der Formeln
Wiederholt man Beispiel 2, setzt jedoch als Polyesteraufheller jenen der Formel
0,4 g der in Beispiel 1 a) beschriebenen Stammdispersion und 0,2 g eines Dispergators (Anlagerungsprodukt von 35 Athylenoxidgruppen an Stearylalkohol) werden mit 400 ml Wasser zu einer Dispersion verarbeitet und letztere mit 0,8 ml NaOH 30% alkalisch gemacht.0.4 g of the stock dispersion described in Example 1 a) and 0.2 g of a dispersant (adduct of 35 ethylene oxide groups with stearyl alcohol) are processed into a dispersion with 400 ml of water and the latter made alkaline with 0.8 ml of NaOH 30%.
40 g Polyester-Stapelgewebe (gewaschen und 30 Sekunden thermofixiert bei 180°C) werden als Gewebeband von ca. 250x12 cm gleichmäßig auf Spulenhülsen gewickelt und in einem HT-Färbeapparat mit der eben beschriebenen Dispersion behandelt. Die Flotte hatte dabei einen Anfangs-pH-Wert von 11,5. Man gibt das Gewebe bei 50°C ein und erhitzt die Flotte in 30 Minuten auf 120° C, behandelt 15 Minuten bei dieser Temperatur, kühlt auf 70°C ab, setzt 0,8 g Natriumchlorid 80% zu und stellt die Flotte mit 1,4 ml Ameisensäure 85% auf einen pH-Wert von 3 -4 ein. Man erhitzt in 20 Minuten auf ca. 100°C, behandelt 30 Minuten bei dieser Temperatur, läßt anschließend die Flotte ab, spült und trocknet. Das Gewebestück ist einwandfrei egal und weist einen hohen Weißgrad auf.40 g of polyester staple fabric (washed and heat-set at 180 ° C for 30 seconds) are evenly wound onto bobbin tubes as a fabric tape of approx. 250x12 cm and treated with the dispersion just described in an HT dyeing machine. The fleet had an initial pH of 11.5. The fabric is introduced at 50 ° C. and the liquor is heated to 120 ° C. in 30 minutes, treated at this temperature for 15 minutes, cooled to 70 ° C., 0.8 g of sodium chloride 80% is added and the liquor is made up with 1 , 4 ml of formic acid 85% to a pH of 3-4. The mixture is heated to about 100 ° C. in 20 minutes, treated at this temperature for 30 minutes, then the liquor is drained off, rinsed and dried. The piece of fabric is immaculate and has a high degree of whiteness.
Ersetzt man in den Beispielen 1 bis 5 den jeweiligen Farbstoff durch die gleiche Menge des Farbstoffes der Formel
Claims (10)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH2919/79 | 1979-03-29 | ||
| CH291979 | 1979-03-29 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0017618A1 EP0017618A1 (en) | 1980-10-15 |
| EP0017618B1 true EP0017618B1 (en) | 1983-03-02 |
Family
ID=4244560
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP80810098A Expired EP0017618B1 (en) | 1979-03-29 | 1980-03-24 | Process for optically brightening polyester fibres by the exhaust process |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US4283197A (en) |
| EP (1) | EP0017618B1 (en) |
| DE (1) | DE3062164D1 (en) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE4426004A1 (en) * | 1994-07-22 | 1996-01-25 | Basf Ag | Process for the optical brightening of polyamides |
| US8268016B2 (en) | 2004-09-23 | 2012-09-18 | The Sun Products Corporation | Laundry treatment compositions |
| PL1794275T3 (en) * | 2004-09-23 | 2009-12-31 | Unilever Nv | Laundry treatment compositions |
| GB0421145D0 (en) * | 2004-09-23 | 2004-10-27 | Unilever Plc | Laundry treatment compositions |
| DE102004051174A1 (en) * | 2004-10-20 | 2006-05-04 | BSH Bosch und Siemens Hausgeräte GmbH | Lighting device for a water-conducting household appliance |
| CN101370923B (en) * | 2006-01-18 | 2012-01-11 | 西巴控股公司 | Process for the treatment of fiber materials |
| AU2007207050A1 (en) * | 2006-01-18 | 2007-07-26 | Basf Se | Process for the treatment of fiber materials |
| US20080177089A1 (en) | 2007-01-19 | 2008-07-24 | Eugene Steven Sadlowski | Novel whitening agents for cellulosic substrates |
| WO2009074488A1 (en) * | 2007-12-10 | 2009-06-18 | Basf Se | Dye formulation and process for the treatment of fiber materials |
| US20110275551A1 (en) * | 2009-01-26 | 2011-11-10 | Stephen Norman Batchelor | Incorporation of dye into granular laundry detergent |
| US8715368B2 (en) | 2010-11-12 | 2014-05-06 | The Procter & Gamble Company | Thiophene azo dyes and laundry care compositions containing the same |
| EP2714878B2 (en) | 2011-05-26 | 2021-06-02 | Unilever PLC, a company registered in England and Wales under company no. 41424 | Liquid laundry composition |
| EP3097175B1 (en) * | 2014-01-22 | 2018-10-17 | The Procter and Gamble Company | Fabric treatment composition |
| CN112853526A (en) * | 2020-12-30 | 2021-05-28 | 湖北鸿鑫化工有限公司 | Method for improving whiteness of PP flat filament |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1072348B (en) * | 1956-10-01 | 1960-06-02 | The Procter & Gamble Company, Cincinnati, Ohio (V. St. A.) | laundry detergent |
| US3755201A (en) * | 1971-07-26 | 1973-08-28 | Colgate Palmolive Co | Laundry product containing mixed dye bluing agents |
| DE2727112C3 (en) | 1977-06-16 | 1981-06-04 | Öffentliche Prüfstelle und Textilinstitut für Vertragsforschung e.V., 4150 Krefeld | Process for pre-cleaning and dyeing textile materials |
-
1980
- 1980-03-17 US US06/130,949 patent/US4283197A/en not_active Expired - Lifetime
- 1980-03-24 EP EP80810098A patent/EP0017618B1/en not_active Expired
- 1980-03-24 DE DE8080810098T patent/DE3062164D1/en not_active Expired
Also Published As
| Publication number | Publication date |
|---|---|
| EP0017618A1 (en) | 1980-10-15 |
| US4283197A (en) | 1981-08-11 |
| DE3062164D1 (en) | 1983-04-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0017618B1 (en) | Process for optically brightening polyester fibres by the exhaust process | |
| EP0417040A1 (en) | Dyeing process for wool | |
| DE2444823B2 (en) | Process for dyeing cellulose fibers | |
| DE3614377C2 (en) | Process for quenching fluorescence from optical brighteners | |
| EP1504149A2 (en) | Method for brightening textile materials | |
| AT393846B (en) | METHOD FOR TEXTILE TREATMENT | |
| DE2329991C2 (en) | 3-triazolyl- (4) -coumarin derivatives | |
| DE2459393C3 (en) | Preparations for making synthetic fiber material made of polyester or polyamide flame resistant | |
| DE2727112C3 (en) | Process for pre-cleaning and dyeing textile materials | |
| EP0147783A2 (en) | Mixtures of blue disperse azo dyes for the dyeing of synthetic fibres | |
| EP0497172B1 (en) | Dispersing agent | |
| DE2500915C3 (en) | Process for tinting textile fibers made of polyesters or mixed fibers of polyesters and cellulose or wool white | |
| DE1955310A1 (en) | Optical brightener mixtures of benzoxazole - and phenyloxazole derivs | |
| DE1029792B (en) | Optical brighteners | |
| EP1674608A1 (en) | Dispersing agent for polyester oligomers | |
| DE2521106C3 (en) | Process for dyeing materials containing synthetic fibers | |
| AT162913B (en) | Process for finishing fiber materials | |
| EP0474594A1 (en) | Process for dyeing of wool and mixtures thereof with other fibres with reactive dyestuffs | |
| EP0278440A2 (en) | Use of sulphurous acid cyclic esters in dyeing polyamide textile materials, and dyeing process | |
| DE2442553C2 (en) | Dye preparations for the production of deep yellow tones with reactive dyes, their production and their use | |
| EP0183969A1 (en) | Process for dyeing wool with 1:1 metal complex dyes | |
| EP0641837A1 (en) | Process for dyeing polyester and polyester-containing textile materials | |
| DE1220381B (en) | Process for lightening polyester fiber material | |
| DE19638566A1 (en) | Use of aryloxypolyglycol ethers as leveling and dispersing agents | |
| WO1990013703A1 (en) | A process for dyeing cellulose fibre textiles |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed | ||
| AK | Designated contracting states |
Designated state(s): CH DE FR GB |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): CH DE FR GB |
|
| ET | Fr: translation filed | ||
| REF | Corresponds to: |
Ref document number: 3062164 Country of ref document: DE Date of ref document: 19830407 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19840210 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19840217 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19840326 Year of fee payment: 5 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Effective date: 19880331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19881118 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19881130 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19881201 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |