CN212348296U - System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration - Google Patents
System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration Download PDFInfo
- Publication number
- CN212348296U CN212348296U CN202020294195.6U CN202020294195U CN212348296U CN 212348296 U CN212348296 U CN 212348296U CN 202020294195 U CN202020294195 U CN 202020294195U CN 212348296 U CN212348296 U CN 212348296U
- Authority
- CN
- China
- Prior art keywords
- chlorine dioxide
- flue gas
- turbulent ball
- tower
- turbulent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Landscapes
- Treating Waste Gases (AREA)
Abstract
The utility model provides an utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration's system, including turbulent ball tower, chlorine dioxide supply system, soluble alkali lye supply system carries soluble alkali lye to turbulent ball district through the feed liquor pipe, still sets up chlorine dioxide distributor in the turbulent ball tower, chlorine dioxide distributor below blowout chlorine dioxide, the flue gas is earlier from the flue gas import of turbulent ball tower and is entered into the tower in, NO in the chlorine dioxide oxidation flue gas forms high valence state nitrogen oxide, in turbulent ball district, soluble alkali lye absorbs the high valence state's in the flue gas nitrogen oxide. The utility model provides a problem that denitration efficiency is not high among the current oxidation technology to combine chlorine dioxide to carry out the denitration with the turbulent ball tower, have unexpected technological effect.
Description
Technical Field
The invention relates to the field of flue gas denitration, in particular to a system for flue gas denitration by using chlorine dioxide and a turbulent ball tower.
Background
Coal-fired power generation, steel mill steelmaking and other processes taking coal and petroleum as heat sources are one of the main sources of nitrogen oxide increase in the environment. In order to reduce the influence on the environment, various manufacturers adopt various methods to reduce the emission of nitrogen oxides in the combustion tail gas (the flue gas), namely, the flue gas is subjected to denitration treatment. In general flue gas, NO usually accounts for about 90% of the total amount of nitrogen oxides, but NO is difficult to dissolve in water, so that the NO is difficult to remove by water washing or alkali liquor neutralization, which is also the difficulty of the current flue gas denitration. The existing flue gas denitration technology mainly comprises two main types, namely a catalytic reduction method and an oxidation method. Compared with a catalytic reduction method, the oxidation method theoretically has the advantages of simple process, low cost and the like, so that the method becomes the research and research direction in the field of flue gas denitration at present. ClO2 is a green oxidant with strong oxidizability and low cost, so that the application of ClO2 in an oxidation absorption method for denitration is a better choice. Publication No. CN109718653A entitled "a flue gas desulfurization and denitration apparatus and method" discloses a technology for denitration by using chlorine dioxide, and also discloses patents such as publication nos. CN110624385A, CN106975337A, CN105771577A, and CN105169913A, and also discloses technologies for denitration by using chlorine dioxide, respectively. In the mentioned patent technology, the technical idea of denitration adopts the technical route of "oxidizing NO in flue gas by chlorine dioxide into high-valence nitrogen oxide which is easy to react with alkali liquor, and then washing and absorbing by alkaline solution". However, in actual production, the denitration effect of the technical route is not very good. Analyzing the reason, the technical route has an oxidation space and an absorption space in the process, but the existing process does not pay attention to the connection between the two spaces, so that the removal effect of nitrogen oxides in the flue gas is poor.
Disclosure of Invention
Different from the prior art route, the invention provides a system for flue gas denitration by using chlorine dioxide and a turbulent ball tower, and the system can solve the technical problems of poor denitration effect and high cost of the chlorine dioxide in the prior art. The content is as follows:
a system for denitrating flue gas by using chlorine dioxide and a turbulent ball tower is characterized in that the system comprises the turbulent ball tower, a chlorine dioxide supply system and a soluble alkali liquid supply system, wherein the soluble alkali liquid supply system conveys the soluble alkali liquid to a turbulent ball area through a liquid inlet pipe, a chlorine dioxide distributor is also arranged in the turbulent ball tower and is positioned at the lower part of a turbulent ball supporting plate, a flue gas inlet is arranged above the chlorine dioxide distributor, the chlorine dioxide supply system conveys the chlorine dioxide to the chlorine dioxide distributor through a chlorine inlet pipe, the chlorine dioxide distributor sprays chlorine dioxide downwards, the flue gas firstly enters the tower from the flue gas inlet of the turbulent ball tower and meets the chlorine dioxide sprayed by the chlorine dioxide distributor in the rising process, NO in the flue gas reacts with the chlorine dioxide to form high-valence nitrogen oxides, the high-valence nitrogen oxides and other gases continuously rise along with the flue gas and enter the turbulent ball area from the gap of the turbulent ball supporting plate, in the turbulent ball zone, soluble alkali liquor absorbs high-valence nitrogen oxides in the flue gas, then gas-liquid separation is carried out, the gas continuously rises out of the turbulent ball zone and flows out of the turbulent ball tower from a flue gas outlet, and meanwhile, part of liquid in the turbulent ball zone flows down from gaps of the supporting plates and is collected into an alkali liquor tank at the bottom of the turbulent ball tower.
Preferably wherein the chlorine dioxide distributor is a gas distributor.
Preferably wherein the chlorine dioxide distributor is a liquid atomizer.
Preferably, wherein the distance between the chlorine dioxide distributor and the turbulent ball support plate is less than 0.5 m.
Preferably, wherein the distance between the chlorine dioxide distributor and the turbulent ball support plate is less than 0.2 m.
The content of the invention is specifically described as follows:
theoretical basis of the invention
1. The existing oxidation denitration technology has the poor denitration effect because:
(1) the applicant believes that in the gaseous state, NO is in the direction of NO2There is an equilibrium relationship upon transformation:
2NO2 ↔ O2 + 2NO ①
at higher temperatures, lower pressures and lower concentrations of NO, NO is not readily converted to NO2Or NO after conversion2And immediately converted to NO. Thus, even if NO in the flue gas can be oxidized by an oxidant such as chlorine dioxide, since the concentration of NO in the flue gas is low, the formula (i) proceeds to the right, and a large proportion of NO finally exists even if NO in the flue gas is oxidized.
2ClO2+4NO →4NO2+Cl 2 ②
2NO2→ O2 + 2NO ③
According to the equilibrium relation (formula I), if the concentration of NO in the flue gas is low (the actual NO in the flue gas generally does not exceed 300 mg/m)3) Even if NO is oxidized to higher valence NO2(formula II), but NO according to the formula III2And back to NO, a large proportion of NO will also be present in the final flue gas. This is also the reason why the denitration effect of the oxidant (including chlorine dioxide) is not high in the existing oxidation denitration technology. Of course, the prior art can improve the denitration effect by increasing the input amount of the oxidant, which inevitably increases the cost greatly.
(2) In the prior art, the NO oxidation process in the flue gas and the oxidized high-valence nitrogen oxide absorption process are separated and independent, so that the distance between the oxidation space and the absorption space is large, even if NO in the flue gas is oxidized in the oxidation link, due to the analysis reason, the oxidation product NO2 is converted into NO in the process from the oxidation process to the absorption process of the flue gas. Therefore, in the absorption process, the absorption liquid can not completely remove the nitrogen oxides in the flue gas, so that the denitration effect is poor. The reaction in the absorption process is:
3NO2 +2 OH- → NO3 - + NO + H2O ④
NO2 + NO + 2OH- → 2NO2 - + H2 O ⑤
2. the difference between the present invention and the prior art lies in:
compared with the prior art, the invention puts the oxidation process and the absorption of the flue gas into a tower and a space. In a preferable scheme, the oxidation position is required to be close to the absorption position, such as less than 0.5 meter, even less than 0.2 meter, so that NO in the flue gas can immediately enter the absorption link after being oxidized, the time and space for converting NO2 into NO are reduced, and the denitration effect of the flue gas can be improved.
Preparation of (di) chlorine dioxide
The preparation of chlorine dioxide is a matter of prior art. As disclosed in patent publication No. CN 209362207U: the chlorine dioxide generator for sterilizing drinking water and sewage is mainly a chlorine dioxide preparation device using sodium chlorate and hydrochloric acid as raw materials, when the equipment is operated, the sodium chlorate solution and hydrochloric acid solution are fed into the chlorine dioxide generator according to a certain proportion, and chemical reaction is produced in the equipment to produce reaction products of chlorine dioxide, chlorine, sodium chloride and water, etc. The raw materials for producing chlorine dioxide are sodium chlorate and hydrochloric acid; publication No. CN110624385A also discloses a production method of chlorine dioxide denitration: a low-temperature denitration method for a sodium chlorite solution is characterized in that a 25% sodium chlorite aqueous solution is adopted as an oxidant, an acid solution is adopted as an activator, the acid solution is continuously added into the sodium chlorite solution when the denitration device is used, the sodium chlorite is conveyed to a flue gas inlet pipe through a conveying pump, the oxidant is sprayed into flue gas through an atomizing spray gun, chlorine dioxide is generated by the sodium chlorite through reaction with the acid solution, the nitrogen monoxide is oxidized into nitrogen dioxide by the chlorine dioxide generated after the heat of the flue gas is evaporated, and the oxidized nitrogen dioxide is absorbed by sodium hydroxide sprayed by an alkaline tower. The raw materials are sodium chlorite and acid; publication No. CN105771577A discloses an improved process for the preparation of chlorine dioxide: the method for preparing chlorine dioxide and using the chlorine dioxide in the flue gas denitration device is carried out according to the following steps:
(1) taking a sodium chlorate solution with the mass concentration of 15-18% and industrial hydrochloric acid with the mass concentration of 31% as raw materials, preheating the raw materials to 55-70 ℃, and then adopting a metering pump to mix the raw materials according to the volume ratio of the industrial hydrochloric acid: the sodium chlorate solution =1 (1-1.2) is prepared by respectively feeding the sodium chlorate solution and industrial hydrochloric acid into two V-shaped pipes with an included angle of 55-65 degrees of a three-way pipeline, and carrying out mixing reaction in a third pipeline; (2) the method comprises the steps of obtaining a mixture containing strong oxidant ClO2 after mixing reaction in a pipeline, uniformly distributing the mixture through a distribution spraying device at the outlet end of the pipeline, dispersing the gas-liquid mixture into fine mist, directly extending a spray head of the distribution spraying device into a flue gas pipeline, uniformly mixing the mixture with flue gas, carrying out redox reaction on low-valence Nitrogen Oxides (NO) in the flue gas and ClO2, oxidizing the low-valence Nitrogen Oxides (NO) into high-valence nitrogen oxides (NO 2), and absorbing the oxidized product by alkali liquor. Sodium chlorate and hydrochloric acid are also used as raw materials.
Large scale production of chlorine dioxide (both gas and liquid) is generally carried out by electrolysis of a brine solution under specific conditions to obtain sodium chlorate, which is then reacted with hydrochloric acid.
The chlorine dioxide can be chlorine dioxide aqueous solution, chlorine dioxide gas, or a mixture of the chlorine dioxide aqueous solution and the chlorine dioxide gas, and chlorine dioxide-containing liquid and chlorine dioxide-containing gas. Patent CN110624385A discloses a method for low-temperature denitration of sodium chlorite solution, in which an oxidant is 25% sodium chlorite aqueous solution, an acid solution is used as an activator, the acid solution is continuously added into the sodium chlorite solution when in use, the sodium chlorite solution is delivered into a flue gas inlet pipe through a delivery pump, and the oxidant is sprayed into the flue gas through an atomizing spray gun; CN105169913A also uses chlorine dioxide in liquid phase, which discloses: the oxidizing circulation pump 18 draws the oxidizing agent solution of ClO2 from the oxidizing agent circulation tank 17, and sends the oxidizing agent solution to the spray pipe 20 through the oxidizing agent delivery pipe 19, and the waste liquid collected by the liquid collector 21 after the oxidizing absorption of NO flows back to the oxidizing agent circulation tank 17 through the oxidizing agent return pipe 22 for recycling. CN105771577A uses chlorine dioxide in a mixture of liquid and gas phases. Patent CN106237814A discloses an apparatus and process for gas phase chlorine dioxide oxidation of NO in flue gas.
In summary, the technical means of preparing chlorine dioxide in the prior art can be applied to the present invention as a technical feature of the present invention.
Chlorine dioxide generators are available directly on the market for use in the production of chlorine dioxide according to the invention.
(III) chlorine dioxide supply System
The chlorine dioxide supply system comprises a chlorine dioxide generator, a booster pump, a valve, a chlorine inlet pipe, a chlorine dioxide distributor and the like. The function is to supply chlorine dioxide to the turbulent ball tower and distribute the chlorine dioxide in the flue gas.
The chlorine dioxide distributor is a device for uniformly distributing chlorine dioxide generated by a chlorine dioxide generator in smoke flowing through a turbulent ball tower. When chlorine dioxide is supplied in the system as a liquid or a mixture of gas and liquid, various liquid spray heads, liquid atomizers, and dedicated liquid distributors disclosed in the foregoing patents, etc. may be used. In a preferred embodiment of the invention, an atomization device is used. The aim is that the atomization device can obtain the chlorine dioxide liquid drops with smaller size, so that the chlorine dioxide liquid drops are contacted and mixed with the flue gas more fully. Moreover, the liquid drop is small, the weight is light, and the liquid drop can be utilized to a greater extent so as to prevent a great amount of liquid from entering the lye tank at the bottom of the turbulent ball tower, thereby causing the loss of the chlorine dioxide.
When chlorine dioxide is supplied as a gas, gas distributors, gas nozzles, etc. as disclosed in the prior art may be used, and a preferred form of the invention employs a spiral coil with small holes.
The amount of chlorine dioxide used in the process is determined according to the amount of NO in the flue gas. According to the formula (II), 1 mol of chlorine dioxide can oxidize 2 mol of NO, so that theoretically, the ratio of the input amount of chlorine dioxide in unit time to the amount of NO in unit time of the smoke is 1: 2. according to the formula, soluble alkali liquor can play a role in assisting oxidation, the input amount of chlorine dioxide can be smaller, but in order to remove nitrogen oxide in flue gas in a larger amount, the ratio of the input amount of pure chlorine dioxide in unit time to the amount of NO in unit time of flue gas is 1: 1 or greater than 1: 1.
(IV) soluble alkali liquor and soluble alkali liquor supply system
The soluble alkali is a substance capable of ionizing hydroxide ions in water, and specifically includes substances capable of dissolving in water and ionizing hydroxide ions in water, such as sodium hydroxide, potassium hydroxide, calcium hydroxide, ammonia, alcohol amine, sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate, and urea.
The soluble alkali liquor refers to an aqueous solution of soluble alkali or a mixture of the aqueous solution and part of soluble alkali.
The soluble alkali liquor spraying environment refers to an environment formed by spraying soluble alkali liquor through a sprayer and being coated by spraying liquor. In the environment, the flue gas can contact the soluble alkali liquor instantly and at any time.
The soluble alkali liquor supply system comprises an alkali liquor tank, a pressure pump, a valve, an alkali liquor inlet pipe and the like. The function is to convey the soluble alkali liquor to the turbulent ball region of the turbulent ball tower. The alkali liquor can be directly introduced into the turbulent ball region through the inlet pipe for conveying, and can also be conveyed above the turbulent ball region, and the better mode is that the alkali liquor is conveyed above the turbulent ball region through the liquid distributor. The lye flowing out of the turbulent ball area to the lye tank also has a larger pH value, so the lye can be recycled. If the pH is lowered, a soluble base may be added for adjustment. The absorption product may be periodically withdrawn from the lye tank.
The dosage of the soluble alkali liquor is determined according to the amount of nitrogen oxides in the flue gas, the concentration value of the alkali liquor and other factors. Experiments show that when the pH value reaches 9, the nitrogen oxide in the flue gas can be oxidized and absorbed, and under the condition that other conditions are not changed, the larger the pH value is, the better the removal effect of the nitrogen oxide is. The pH value is preferably in the range of 12 to 14 from the viewpoint of cost.
In practical application, the unit input amount of the soluble alkali can be 10-100 times of the corresponding nitrogen oxide amount, and the height requirement of the turbulent spherical zone formed in operation is taken as the standard.
(V) turbulent ball tower
The turbulent ball tower is a place for realizing the technical idea in the denitration system. Except the chlorine dioxide conveying system, the other structures of the turbulent ball tower are the prior art and comprise a tower shell, a support plate (grid plate), light small balls (turbulent balls), a baffle net, a demister and the like. The support plate is a circular disc structure with voids, the area occupied by the voids is expressed by the void fraction and is generally 0.2 to 0.6. The working principle is as follows: the support plate (grid plate) is arranged in the tower shell, a certain amount of turbulent balls are placed on the support plate, a blocking net is arranged at a certain position above the support plate, and the space between the blocking net and the support plate is a turbulent ball area. Under the general condition, flue gas enters the turbulent ball region from the lower part through the supporting plate, soluble alkali liquor enters the turbulent ball region from the upper part or the side part, and the turbulent ball is suspended to form turbulent rotation and mutual collision under the interaction of various forces such as impulsive force of high-speed ascending air flow, buoyancy of liquid, self gravity and the like, so that the gas and the liquid are closely contacted, and the effects of mass transfer, reaction and the like are effectively carried out. The turbulent ball tower has the advantages of high gas speed, great treating capacity, homogeneous gas-liquid distribution, simple structure and less jamming.
The turbulent ball is a light small ball with the diameter of 20-38 mm, is made of plastics such as PVC, PE, PP and the like, and is hollow or solid. Stainless steel hollow spheres are also sometimes used. When the turbulent ball tower runs, the turbulent ball is in a motion state.
The invention has the beneficial effects that:
1. the problem of low denitration efficiency in the prior oxidation technology is solved, and the cost is saved;
2. the turbulent ball tower is combined with chlorine dioxide for denitration, so that the method has great economic and social benefits.
Drawings
FIG. 1: a schematic of the denitrification system of example 1.
FIG. 2: a denitration system schematic of example 2.
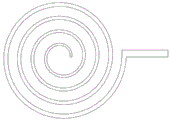
FIG. 3: schematic diagram of a chlorine dioxide distributor as in example 1.
Detailed Description
Example 1
The invention is explained by combining the attached drawings 1 and 3:
the turbulent ball tower is a cylinder with the diameter of 0.8m, the height of the tower is 5m, the wall thickness is 8mm, and the turbulent ball tower is made of 304L stainless steel. The support plate 1 is mounted 2.5 meters from the bottom of the tower housing. The support plate 1 is a circular grid plate structure, the width of the grid plate is 20mm, the gap spacing is 15mm, and the material is 304 stainless steel. A baffle net 2 is arranged 1.5m above a support plate 1, the diameter of a hole of the baffle net 2 is 20mm, the space between the support plate 1 and the baffle net 2 is a turbulent ball region 3, a turbulent ball 4 is placed in the turbulent ball region 3, the turbulent ball 4 is a 34mm hollow PE ball, and the height of a turbulent ball layer is about 0.4m when the turbulent ball is in no-load state. The flue gas inlet 5 is arranged at a position 1M away from the bottom of the turbulent ball tower, the flue gas outlet 6 is arranged at the top of the tower shell, the bottom space of the turbulent ball tower is used as a lye tank 7, and the molar concentration of the soluble lye solution put in before the tower starts to run is 1M. The chlorine dioxide inlet pipe 8 is connected with a chlorine dioxide generator 9 outside the tower, a gas booster pump 10 and a flow regulating valve 11 are further arranged on the pipeline, the other end of the chlorine inlet pipe 8 is connected with a chlorine dioxide distributor 12 inside the tower, the chlorine dioxide distributor 12 is located 0.2m below the support plate 1, the chlorine dioxide distributor 12 is a planar spiral coil, and one surface of the coil, which is deviated from the turbulent ball area 3 and is opposite to the incoming direction of flue gas, is uniformly provided with a plurality of small holes of 1 mm. One end of a liquid inlet pipe 13 of the soluble alkali liquor is connected with the alkali liquor tank 7, the other end of the liquid inlet pipe is connected with a shower nozzle 16 in the tower, the shower nozzle 16 is positioned at the position of 0.5m above the baffle net 2, the shower nozzle 16 sprays the soluble alkali liquor to the turbulent ball area 3, and a booster pump 14 and a flow regulating valve 15 are also arranged on a pipeline of the liquid inlet pipe 13.
The flue gas is the sintering flue gas of a sintering workshop of a certain steel mill. The initial flue gas parameters are: the temperature of the flue gas is 135-137 ℃, the humidity of the flue gas is 0.01-0.03%, and the NO content is 174-183mg/m3The flue gas flow is 904m3/h。
The chlorine dioxide generator 9 is QKJ-2000 model chlorine dioxide generator of Jinan Qili environmental protection science and technology Limited, when in use, the generator is modified (the water injector is disconnected) to only output gases such as chlorine dioxide, the raw materials are sodium chlorite and hydrochloric acid, and the unit chlorine dioxide yield is 2000 g/h.
The flue gas enters the turbulent ball tower through the flue gas inlet 5 and rises in the tower; opening a flow regulating valve 11 of a chlorine dioxide pipeline, starting a booster pump 10, conveying chlorine dioxide gas generated in a chlorine dioxide generator 9 to a chlorine dioxide distributor 12 through a chlorine inlet pipe 8, spraying the chlorine dioxide gas through small holes, enabling the sprayed chlorine dioxide gas to meet with flue gas and oxidize NO in the flue gas, and regulating the flow of the chlorine dioxide through the regulating valve 11 to enable the input amount of pure chlorine dioxide per hour to be not less than 400 g; the oxidation products continuously rise to the bearing plate 1 along with the flue gas and pass through the gap of the bearing plate 1 to enter the turbulent ball zone 3; meanwhile, an adjusting valve 15 and a booster pump 14 of the soluble alkali liquor input and circulation system are opened, the alkali liquor in the alkali liquor tank 7 is conveyed to the turbulent ball region 3 through the liquor inlet pipe 12, and is mixed with the flue gas under the assistance of the turbulent ball, and the soluble alkali liquor absorbs oxidation products in the turbulent ball region 3; finally, gas-liquid separation is carried out, and the flue gas flows out from the flue gas outlet 6; part of the liquid in the turbulent ball region 3 will flow downwards through the gaps of the support plate 1 under the action of gravity and finally collect in the lye tank 7 at the bottom of the tower.
Measured at the flue gas outlet 6 of the rotating turbulent ball tower, the measured values are 33, 39, 45, 46 and the like, and the NO content is 35-50mg/m3。
Example 2
The invention is described with reference to fig. 2:
the turbulent ball tower is a cylinder with the diameter of 0.8m, the height of the tower is 5m, the wall thickness is 8mm, and the turbulent ball tower is made of 304L stainless steel. The support plate 1 is mounted 2.5 meters from the bottom of the tower housing. The support plate 1 is a circular grid plate structure, the width of the grid plate is 20mm, the gap spacing is 15mm, and the material is 304 stainless steel. A baffle net 2 is arranged 1.5m above a support plate 1, the diameter of a hole of the baffle net 2 is 20mm, the space between the support plate 1 and the baffle net 2 is a turbulent ball region 3, a turbulent ball 4 is placed in the turbulent ball region 3, the turbulent ball 4 is a 34mm hollow PE ball, and the height of a turbulent ball layer is about 0.4m when the turbulent ball is in no-load state. The flue gas inlet 5 is arranged at a position 1M away from the bottom of the turbulent ball tower, the flue gas outlet 6 is arranged at the top of the tower shell, the bottom space of the turbulent ball tower is used as a lye tank 7, and the molar concentration of the soluble lye solution put in before the tower starts to run is 1M. The chlorine dioxide inlet pipe 8 is connected with a chlorine dioxide generator 9 outside the tower, a gas booster pump 10 and a flow regulating valve 11 are also arranged on the pipeline, the other end of the chlorine inlet pipe 8 is connected with a chlorine dioxide distributor 12 inside the tower, the chlorine dioxide distributor 12 is positioned at a position 0.2m below the cyclone disk 1, the chlorine dioxide distributor 12 is an atomizing nozzle, and the direction of the nozzle is downward. One end of a liquid inlet pipe 13 of the soluble alkali liquor is connected with the alkali liquor tank 7, the other end of the liquid inlet pipe is connected with a shower nozzle 16 in the tower, the shower nozzle 16 is positioned at the position of 0.5m above the baffle net 2, the shower nozzle 16 sprays the soluble alkali liquor to the turbulent ball area 3, and a booster pump 14 and a flow regulating valve 15 are also arranged on a pipeline of the liquid inlet pipe 13.
The flue gas is the sintering flue gas of a sintering workshop of a certain steel mill. The initial flue gas parameters are: the temperature of the flue gas is 135-137 ℃, the humidity of the flue gas is 0.01-0.03%, and the NO content is 174-183mg/m3The flue gas flow is 904m3/h。
The chlorine dioxide generator 9 is QKJ-2000 type chlorine dioxide generator of Jinan Qili environmental protection science and technology Limited, the raw materials are sodium chlorite and hydrochloric acid, the output is a gas-liquid mixture, and the unit yield of chlorine dioxide is 2000 g/h.
The flue gas enters the turbulent ball tower through the flue gas inlet 5 and rises in the tower; opening a flow regulating valve 11 of a chlorine dioxide pipeline, starting a booster pump 10, conveying chlorine dioxide generated in a chlorine dioxide generator 9 to a chlorine dioxide distributor 12 through a chlorine inlet pipe 8, spraying the chlorine dioxide through an atomizing nozzle, enabling atomized chlorine dioxide-containing liquid to meet with flue gas and oxidize NO in the flue gas, and regulating the flow of the chlorine dioxide through the regulating valve 11 to enable the input amount of pure chlorine dioxide per hour to be not less than 400 g; the oxidation products continuously rise to the bearing plate 1 along with the flue gas and pass through the gap of the bearing plate 1 to enter the turbulent ball zone 3; meanwhile, an adjusting valve 15 and a booster pump 14 of the soluble alkali liquor input and circulation system are opened, the alkali liquor in the alkali liquor tank 7 is conveyed to the turbulent ball region 3 through the liquor inlet pipe 12, and is mixed with the flue gas under the assistance of the turbulent ball, and the soluble alkali liquor absorbs oxidation products in the turbulent ball region 3; finally, gas-liquid separation is carried out, and the flue gas flows out from the flue gas outlet 6; part of the liquid in the turbulent ball region 3 will flow downwards through the gaps of the support plate 1 under the action of gravity and finally collect in the lye tank 7 at the bottom of the tower.
Measured at the flue gas outlet 6 of the rotating turbulent ball tower, the measured values are 33, 36, 42, 44 and the like, and the NO content is 35-45mg/m3。
Claims (5)
1. The utility model provides an utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration's system, its characterized in that, the system includes turbulent ball tower, chlorine dioxide feed system, soluble alkali lye feed system carries soluble alkali lye to turbulent ball district through the feed liquor pipe, still sets up chlorine dioxide distributor in the turbulent ball tower, chlorine dioxide distributor is located the lower part of turbulent ball bearing plate, the top of flue gas import, chlorine dioxide feed system carries chlorine dioxide to chlorine dioxide distributor through the chlorine inlet pipe, chlorine dioxide distributor spouts chlorine dioxide downwards.
2. The system for flue gas denitration using chlorine dioxide and a turbulent ball tower of claim 1, wherein the chlorine dioxide distributor is a gas distributor.
3. The system for flue gas denitration using chlorine dioxide and a turbulent ball tower of claim 1, wherein the chlorine dioxide distributor is a liquid atomizer.
4. The system for flue gas denitration using chlorine dioxide and a turbulent ball tower of claim 1, wherein the distance between the chlorine dioxide distributor and the turbulent ball support plate is less than 0.5 m.
5. The system for flue gas denitration using chlorine dioxide and a turbulent ball tower of claim 1, wherein the distance between the chlorine dioxide distributor and the turbulent ball support plate is less than 0.2 m.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202020294195.6U CN212348296U (en) | 2020-03-11 | 2020-03-11 | System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202020294195.6U CN212348296U (en) | 2020-03-11 | 2020-03-11 | System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN212348296U true CN212348296U (en) | 2021-01-15 |
Family
ID=74145681
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202020294195.6U Active CN212348296U (en) | 2020-03-11 | 2020-03-11 | System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN212348296U (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111228994A (en) * | 2020-03-11 | 2020-06-05 | 山东师范大学 | System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration |
-
2020
- 2020-03-11 CN CN202020294195.6U patent/CN212348296U/en active Active
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111228994A (en) * | 2020-03-11 | 2020-06-05 | 山东师范大学 | System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN105833695B (en) | A kind of fire coal boiler fume wet desulphurization denitration and wastewater treatment equipment and technique | |
| CN102772990B (en) | Denitration process and device of gas-phase oxidation and wet-process absorption | |
| CN207042222U (en) | Chlorine dioxide gaseous oxidation denitration integrated device | |
| EP2762221B1 (en) | Dual-chamber multi-absorption wet flue desulfurization device | |
| CN106166434A (en) | A kind of ozone oxidation double tower ammonia process of desulfurization denitrating technique and system thereof | |
| CN204710082U (en) | A kind of low-temperature denitration of flue gas system | |
| Cai et al. | Simultaneous removal of SO2 and NO using a spray dryer absorption (SDA) method combined with O3 oxidation for sintering/pelleting flue gas | |
| CN101862587A (en) | Wet flue-gas desulfurization tower based on baffle air distribution | |
| CN212348296U (en) | System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration | |
| CN105381699B (en) | A kind of hydrogen peroxide oxidation joint amino wet desulphurization method of denitration and its device | |
| CN211435768U (en) | Flue gas treatment device for semi-dry desulfurization and low-temperature SCR denitration of carbide slag | |
| CN101696023A (en) | Method and device for preparing raw material liquid of sodium nitrate and sodium nitrite by using reformed gas | |
| CN205796943U (en) | Ozone oxidation double tower ammonia process of desulfurization denitrating system | |
| CN111228994A (en) | System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration | |
| CN212348295U (en) | System for utilize chlorine dioxide and whirl dish tower to carry out flue gas denitration | |
| CN212068310U (en) | System for utilize chlorine dioxide and turbulent ball tower to carry out flue gas denitration | |
| WO2015161676A1 (en) | Method for removing nitrogen oxides in flue gas and nano flue gas denitration system | |
| CN202387362U (en) | Semi-dry low temperature flue gas denitrification system | |
| CN213132611U (en) | Denitration system for flue gas denitration by using chlorine dioxide and packed tower | |
| CN103482649B (en) | Urea solution hydrolysis reactor | |
| CN111228993A (en) | Method and system for flue gas denitration by using chlorine dioxide and spray tower | |
| CN111228991A (en) | Denitration system for flue gas denitration by using chlorine dioxide and packed tower | |
| CN107096378A (en) | The flue gas desulphurization system and sulfur method of a kind of floatation glass production line | |
| CN212348293U (en) | Denitration system for flue gas denitration by using ozone and spray tower | |
| CN212701281U (en) | Denitration system for flue gas denitration by utilizing ozone and turbulent ball tower |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| GR01 | Patent grant | ||
| GR01 | Patent grant |