CN111747871A - Production process of surfactant dioctyl sodium sulfosuccinate - Google Patents
Production process of surfactant dioctyl sodium sulfosuccinate Download PDFInfo
- Publication number
- CN111747871A CN111747871A CN202010761829.9A CN202010761829A CN111747871A CN 111747871 A CN111747871 A CN 111747871A CN 202010761829 A CN202010761829 A CN 202010761829A CN 111747871 A CN111747871 A CN 111747871A
- Authority
- CN
- China
- Prior art keywords
- dioctyl
- dioctyl sodium
- sodium sulfosuccinate
- sulfosuccinate
- octanol
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C303/00—Preparation of esters or amides of sulfuric acids; Preparation of sulfonic acids or of their esters, halides, anhydrides or amides
- C07C303/32—Preparation of esters or amides of sulfuric acids; Preparation of sulfonic acids or of their esters, halides, anhydrides or amides of salts of sulfonic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C303/00—Preparation of esters or amides of sulfuric acids; Preparation of sulfonic acids or of their esters, halides, anhydrides or amides
- C07C303/42—Separation; Purification; Stabilisation; Use of additives
- C07C303/44—Separation; Purification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/08—Preparation of carboxylic acid esters by reacting carboxylic acids or symmetrical anhydrides with the hydroxy or O-metal group of organic compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K23/00—Use of substances as emulsifying, wetting, dispersing, or foam-producing agents
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
The invention provides a production process of a surfactant dioctyl sodium sulfosuccinate, which comprises the following steps: 1) esterification reaction: heating octanol, maleic anhydride and a catalyst under the stirring action to raise the temperature, starting vacuumizing and dehydrating after reaching a certain temperature, stirring and cooling after dehydration is finished, and adding alkali to neutralize the catalyst to obtain an esterified dioctyl maleate; 2) addition reaction: the maleic dioctyl phthalate reacts with sodium bisulfite under heating to obtain maleic dioctyl sodium sulfonate, namely sodium dioctyl sulfosuccinate; 3) precipitation and filtration: adding organic solvent into the dioctyl sodium sulfosuccinate after dehydration to dissolve, precipitate and filter; 4) desolventizing and refining: and heating the supernatant part in the precipitation tank to remove the organic solvent to obtain the anhydrous salt-free high-purity dioctyl sodium sulfosuccinate. The production process of the surfactant dioctyl sodium sulfosuccinate provided by the invention is simple, the prepared product has good quality, and the cost is saved.
Description
Technical Field
The invention relates to the field of production of a surfactant dioctyl sodium sulfosuccinate, in particular to a production process of the surfactant dioctyl sodium sulfosuccinate.
Background
Dioctyl sodium sulfosuccinate is a chemical substance, colorless, pale yellow to brown viscous oily liquid. Molecular weight 444.25. Dissolving in water and various organic solvents. The pH value of the 1% aqueous solution is 6.0-7.0. It is not resistant to strong acid, strong alkali, reducing agent and heavy metal salt. Has high penetrability and rapid and uniform penetrability. The lubricating property, emulsifying property and foaming property are all good. Is widely used as an emulsifier and a detergent for shampoos, detergents, toothpastes and the like. Has excellent foaming property, wetting property and cleaning property. Has no toxicity and little irritation to skin.
At present, 2-ethylhexanol or sec-octanol is mainly used as alcohol for esterification reaction when sodium dioctyl sulfosuccinate is produced, but the sec-octanol is low in price but difficult to add, and the obtained product has low sulfonation degree and poor performance.
Disclosure of Invention
The invention aims to solve the problems in the prior art and provides a production process of a surfactant dioctyl sodium sulfosuccinate, which is simple in preparation method and can be used for obtaining a product with high cost performance and high quality by matching three kinds of alcohols.
In order to solve the technical problems, the technical scheme provided by the invention is as follows:
a production process of a surfactant dioctyl sodium sulfosuccinate comprises the following steps:
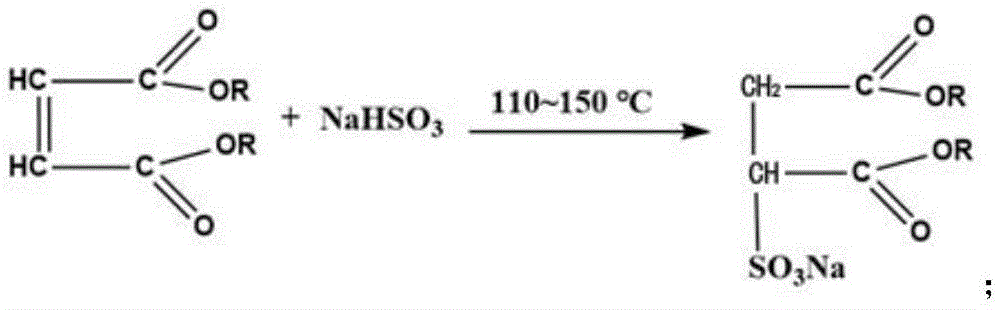
1) esterification reaction: heating octanol, maleic anhydride and a catalyst under the stirring action to raise the temperature, starting vacuumizing dehydration after reaching a certain temperature, stirring to lower the temperature after the dehydration is finished, adding alkali to neutralize the catalyst to obtain an esterified substance of dioctyl maleate, wherein the chemical reaction formula of the esterification reaction is as follows:
2) addition reaction: the reaction between maleic dioctyl phthalate and sodium hydrogen sulfite under heating to obtain maleic dioctyl sodium sulfonate, i.e. sodium dioctyl sulfosuccinate, the addition reaction has the following chemical formula:
3) precipitation and filtration: dehydrating the dioctyl sodium sulfosuccinate crude product obtained in the step 2), adding an organic solvent into the anhydrous dioctyl sodium sulfosuccinate after dehydration to dissolve and precipitate to separate out excessive sulfonating agent sodium bisulfite and inorganic salt;
4) desolventizing and refining: and heating the supernatant part in the precipitation tank to remove the organic solvent part to obtain the anhydrous salt-free high-purity dioctyl sodium sulfosuccinate.
As a refinement, the octanol is two or three mixed alcohols of n-octanol, isomeric 2-ethylhexanol and sec-octanol.
As an improvement, the catalyst in the esterification reaction is concentrated sulfuric acid, p-toluenesulfonic acid or dodecylbenzene sulfonic acid.
As an improvement, a fatty alcohol-polyoxyethylene ether wetting agent is added in the dehydration process of the dioctyl sodium sulfosuccinate crude product.
The improvement is that the solvent for precipitation is one of methanol, ethanol, propanol, isopropanol, butanol, isobutanol and ethylene glycol or propylene glycol.
The invention has the beneficial effects that:
according to the invention, two or three of n-octanol, isomeric 2-ethylhexanol and sec-octanol are mixed, so that the manufacturing cost is reduced while a high-quality product is prepared, the viscosity of the fatty alcohol-polyoxyethylene ether can be reduced during dehydration, the smooth dehydration is ensured, and the product quality is ensured.
Detailed Description
The invention is illustrated below by means of specific examples, without being restricted thereto.
Examples
The method comprises the following steps of putting 700 kg of n-octanol, isomeric 2-ethylhexanol and sec-octanol mixed solution, 200 kg of maleic anhydride and 5-15 kg of concentrated sulfuric acid into a reaction kettle in sequence, stirring and heating, continuously distilling off water along with the rise of temperature until the distilled water amount reaches about 36 kg, cooling, adding alkali (sodium hydroxide) for neutralization, and then transferring the materials into an addition kettle (or called a sulfonation kettle). Dissolving 150-225 kg of sodium bisulfite (or sodium pyrosulfite) in 550 kg of water 300-550 kg to prepare an aqueous solution of sodium bisulfite (or sodium pyrosulfite), adding the aqueous solution into a preparation kettle (or sulfonation kettle), adding 50-200 kg of aqueous dioctyl sodium sulfosuccinate, stirring and heating the mixture, and reacting for 3-6 hours under the pressure of 0.1-0.25 MPa. And (4) after the reaction is finished, starting dehydration, separating and metering the distilled water by an oil-water separator, and stopping dehydration until the residual water in the product meets the requirement. Cooling to below 50 ℃, adding 1000-1500 kg of absolute ethyl alcohol into the addition kettle, uniformly stirring, discharging into a precipitation tank, standing and precipitating for 24 hours, taking a supernatant part for filtering, pumping a filtrate part into a refining kettle, heating to 100-150 ℃ for removing the solvent ethyl alcohol, and removing the solvent ethyl alcohol to obtain the anhydrous salt-free refined Dioctyl Sodium Sulfosuccinate (DSS).
The reaction principle is as follows:
wherein ROH refers to octanol of eight carbons, n-octanol of eight carbons and the three isomeric forms of isomeric 2-ethylhexanol, 2-octanol (sec-octanol). Different alcohols are adopted as raw materials, and Dioctyl Sodium Sulfosuccinate (DSS) with different structures can be obtained. The DSS with different structures can be synthesized by using single three different alcohols, and can also be obtained by mixing and matching the three alcohols. DSS synthesized with either 2-ethylhexanol alone or octanol alone are traditionally referred to as the fast penetrant OT and the fast penetrant T, respectively. The ease of addition of sodium bisulfite varies due to the different structures of the three alcohols. N-octanol is the most readily added, 2-ethylhexanol is the least frequently added, and sec-octanol is the most difficult to add. The resulting DSS thus had a different degree of sulfonation. N-octanol is easy to add, and the obtained product has high sulfonation degree and good performance, but is expensive; the sec-octanol is cheap but difficult to add, and the obtained product has low sulfonation degree and poor performance. The factors such as price and performance are comprehensively balanced, and three kinds of alcohol are matched in different ways so as to obtain a product with high cost performance.
The above description is only for the preferred embodiment of the present invention, but the scope of the present invention is not limited thereto, and any person skilled in the art should be considered to be within the technical scope of the present invention, and the technical solutions and the inventive concepts thereof according to the present invention should be equivalent or changed within the scope of the present invention.
Claims (5)
1. A production process of a surfactant dioctyl sodium sulfosuccinate is characterized by comprising the following steps:
1) esterification reaction: heating octanol, maleic anhydride and a catalyst under the stirring action to raise the temperature, starting vacuumizing dehydration after reaching a certain temperature, stirring to lower the temperature after the dehydration is finished, adding alkali to neutralize the catalyst to obtain an esterified substance of dioctyl maleate, wherein the chemical reaction formula of the esterification reaction is as follows:
2) addition reaction: the reaction between maleic dioctyl phthalate and sodium hydrogen sulfite under heating to obtain maleic dioctyl sodium sulfonate, i.e. sodium dioctyl sulfosuccinate, the addition reaction has the following chemical formula:
3) precipitation and filtration: dehydrating the dioctyl sodium sulfosuccinate crude product obtained in the step 2), adding an organic solvent into the anhydrous dioctyl sodium sulfosuccinate after dehydration to dissolve and precipitate to separate out excessive sulfonating agent sodium bisulfite and inorganic salt;
4) desolventizing and refining: and heating the supernatant part in the precipitation tank to remove the organic solvent part to obtain the anhydrous salt-free high-purity dioctyl sodium sulfosuccinate.
2. The process of claim 1, wherein said octanol is a mixture of two or three of n-octanol, isomeric 2-ethylhexanol, and sec-octanol.
3. The process for producing dioctyl sodium sulfosuccinate as claimed in claim 1, wherein the catalyst used in the esterification reaction is concentrated sulfuric acid, p-toluenesulfonic acid or dodecylbenzenesulfonic acid.
4. The process for producing the surfactant dioctyl sodium sulfosuccinate according to claim 1, wherein the fatty alcohol-polyoxyethylene ether wetting agent is added during the dehydration of the crude dioctyl sodium sulfosuccinate.
5. The process of claim 1, wherein the solvent for precipitation is one of methanol, ethanol, propanol, isopropanol, butanol, isobutanol, and ethylene glycol or propylene glycol.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010761829.9A CN111747871A (en) | 2020-07-31 | 2020-07-31 | Production process of surfactant dioctyl sodium sulfosuccinate |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202010761829.9A CN111747871A (en) | 2020-07-31 | 2020-07-31 | Production process of surfactant dioctyl sodium sulfosuccinate |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111747871A true CN111747871A (en) | 2020-10-09 |
Family
ID=72712652
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010761829.9A Pending CN111747871A (en) | 2020-07-31 | 2020-07-31 | Production process of surfactant dioctyl sodium sulfosuccinate |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN111747871A (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113582888A (en) * | 2021-08-30 | 2021-11-02 | 陕西科技大学 | Acrylate sulfonate gemini surfactant as well as preparation method and application thereof |
| CN116082198A (en) * | 2023-02-07 | 2023-05-09 | 河北新启元能源技术开发股份有限公司 | Synthesis method of diisooctyl succinate sodium sulfonate |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1160710A (en) * | 1996-03-25 | 1997-10-01 | 南京理工大学 | Preparation process of diisooctyl sodium sulfosuccinate |
| CN1185429A (en) * | 1996-12-17 | 1998-06-24 | 南京理工大学 | Process for preparing di-n-octyl succinate sodium sulfonate |
| CN1361099A (en) * | 2000-12-26 | 2002-07-31 | 南京理工大学 | Prepn of dihexyl sodium sulfosuccinate |
| CN101015778A (en) * | 2006-12-25 | 2007-08-15 | 南通大学 | Sodium polyoxyethylene fatty alcohol ether (9) octyl sulfosuccinate and its preparing process |
| CN103709078A (en) * | 2013-12-31 | 2014-04-09 | 广州星业科技股份有限公司 | Preparation method of dioctyl sodium sulfosuccinate |
| CN104356031A (en) * | 2014-10-30 | 2015-02-18 | 青岛科技大学 | Di-n-octyl sodium sulfonate itaconate and preparation method thereof |
| CN110467547A (en) * | 2019-09-11 | 2019-11-19 | 广州方中化工有限公司 | A kind of anhydrous succinic acid di-isooctyl sulfonate sodium and preparation method thereof |
-
2020
- 2020-07-31 CN CN202010761829.9A patent/CN111747871A/en active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1160710A (en) * | 1996-03-25 | 1997-10-01 | 南京理工大学 | Preparation process of diisooctyl sodium sulfosuccinate |
| CN1185429A (en) * | 1996-12-17 | 1998-06-24 | 南京理工大学 | Process for preparing di-n-octyl succinate sodium sulfonate |
| CN1361099A (en) * | 2000-12-26 | 2002-07-31 | 南京理工大学 | Prepn of dihexyl sodium sulfosuccinate |
| CN101015778A (en) * | 2006-12-25 | 2007-08-15 | 南通大学 | Sodium polyoxyethylene fatty alcohol ether (9) octyl sulfosuccinate and its preparing process |
| CN103709078A (en) * | 2013-12-31 | 2014-04-09 | 广州星业科技股份有限公司 | Preparation method of dioctyl sodium sulfosuccinate |
| CN104356031A (en) * | 2014-10-30 | 2015-02-18 | 青岛科技大学 | Di-n-octyl sodium sulfonate itaconate and preparation method thereof |
| CN110467547A (en) * | 2019-09-11 | 2019-11-19 | 广州方中化工有限公司 | A kind of anhydrous succinic acid di-isooctyl sulfonate sodium and preparation method thereof |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113582888A (en) * | 2021-08-30 | 2021-11-02 | 陕西科技大学 | Acrylate sulfonate gemini surfactant as well as preparation method and application thereof |
| CN116082198A (en) * | 2023-02-07 | 2023-05-09 | 河北新启元能源技术开发股份有限公司 | Synthesis method of diisooctyl succinate sodium sulfonate |
| CN116082198B (en) * | 2023-02-07 | 2024-04-26 | 河北新启元能源技术开发股份有限公司 | Synthesis method of diisooctyl succinate sodium sulfonate |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN111747871A (en) | Production process of surfactant dioctyl sodium sulfosuccinate | |
| CN105330836B (en) | A kind of synthetic method of epoxy terminated allyl alcohol polyethenoxy ether | |
| CN112264090B (en) | Double-acid ionic liquid catalyst and preparation method and application thereof | |
| CA1095929A (en) | Process for making ether sulfonates | |
| US9133089B2 (en) | Process for preparing a cross linking catalyst from cashew nut shell liquid | |
| CN109134713B (en) | Preparation method of polyvinyl butyral resin | |
| CN105985224A (en) | Synthetic method of semi-hindered bisphenol antioxidant | |
| CN110054549A (en) | A kind of preparation method of p-methyl anisole | |
| CN101445570A (en) | Method for synthesizing ionic polyvinyl butyral latex | |
| CN101508678B (en) | Process for preparing 2-methyl-4-amino-5-acetyl aminomethyl pyrimidine | |
| CN1562945A (en) | Method for synthesizing isomeric alcohol polyoxyethylene ether carboxylate | |
| CN113387844B (en) | Preparation method of dialkyl azodicarbonate | |
| EP0209060B1 (en) | Process for the preparation of diols and tetrols containing fluorine | |
| CN108546232B (en) | Preparation method of mono-substituted or di-substituted benzoate compound | |
| CN106800498A (en) | A kind of process units of neopentyl glycol | |
| KR101558881B1 (en) | Method for manufacture of a solid acid catalyst of sulfonated carbon using sulfonated lignin and for preparation of organic compounds using the catalyst made with the same method | |
| DE102009045669A1 (en) | Process for the preparation of organooligosilsesquioxanes | |
| CN109761949B (en) | Industrial preparation method of 2, 2-dimethyl-4-hydroxymethyl-1, 3-dioxolane | |
| CN110746297B (en) | Preparation method of 2,2, 4-trimethyl-3-hydroxy methyl valerate, water-based industrial paint for steel structure and preparation method of water-based industrial paint | |
| CN110790666B (en) | Preparation method of ethyl 2,2, 4-trimethyl-3-hydroxypentanoate, water-based paint for containers and preparation method of water-based paint | |
| RU2187501C1 (en) | Dicyclohexyldisulfide production process | |
| CN111333084B (en) | Method for recycling caustic alkali in sulfonated alkali fusion process | |
| CN116981654A (en) | Purification of aliphatic taurine amides | |
| US20240158342A1 (en) | Purification of aliphatic taurate amide | |
| RU527064C (en) | Trisodium salts of sulfonates of monoethers of disulfomaleic acids as surface-active substances and method of obtaining the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20201009 |