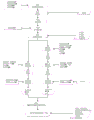
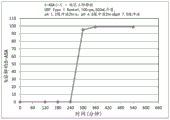
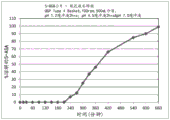
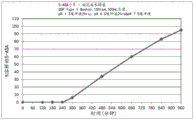
CN109310642B - 美沙拉嗪的口服药物组合物 - Google Patents
美沙拉嗪的口服药物组合物 Download PDFInfo
- Publication number
- CN109310642B CN109310642B CN201780031230.6A CN201780031230A CN109310642B CN 109310642 B CN109310642 B CN 109310642B CN 201780031230 A CN201780031230 A CN 201780031230A CN 109310642 B CN109310642 B CN 109310642B
- Authority
- CN
- China
- Prior art keywords
- mesalamine
- unit dose
- drug product
- release
- dose drug
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2077—Tablets comprising drug-containing microparticles in a substantial amount of supporting matrix; Multiparticulate tablets
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/60—Salicylic acid; Derivatives thereof
- A61K31/606—Salicylic acid; Derivatives thereof having amino groups
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/2027—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/284—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone
- A61K9/2846—Poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/286—Polysaccharides, e.g. gums; Cyclodextrin
- A61K9/2866—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2886—Dragees; Coated pills or tablets, e.g. with film or compression coating having two or more different drug-free coatings; Tablets of the type inert core-drug layer-inactive layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4808—Preparations in capsules, e.g. of gelatin, of chocolate characterised by the form of the capsule or the structure of the filling; Capsules containing small tablets; Capsules with outer layer for immediate drug release
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2095—Tabletting processes; Dosage units made by direct compression of powders or specially processed granules, by eliminating solvents, by melt-extrusion, by injection molding, by 3D printing
Landscapes
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
- Immunology (AREA)
- Rheumatology (AREA)
- Pain & Pain Management (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662324416P | 2016-04-19 | 2016-04-19 | |
| US62/324416 | 2016-04-19 | ||
| PCT/US2017/028067 WO2017184566A1 (en) | 2016-04-19 | 2017-04-18 | Oral pharmaceutical compositions of mesalazine |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN109310642A CN109310642A (zh) | 2019-02-05 |
| CN109310642B true CN109310642B (zh) | 2021-07-20 |
Family
ID=59298513
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201780031230.6A Expired - Fee Related CN109310642B (zh) | 2016-04-19 | 2017-04-18 | 美沙拉嗪的口服药物组合物 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US10874617B2 (enExample) |
| EP (1) | EP3445340A1 (enExample) |
| JP (1) | JP2019513800A (enExample) |
| KR (1) | KR102381586B1 (enExample) |
| CN (1) | CN109310642B (enExample) |
| AR (1) | AR108231A1 (enExample) |
| AU (1) | AU2017252410A1 (enExample) |
| BR (1) | BR112018071370A2 (enExample) |
| CA (1) | CA3021071A1 (enExample) |
| MX (1) | MX387731B (enExample) |
| RU (1) | RU2744576C2 (enExample) |
| TW (1) | TW201740932A (enExample) |
| WO (1) | WO2017184566A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ITUA20162293A1 (it) * | 2016-04-05 | 2017-10-05 | Sofar Spa | Processo per formulazioni solide di mesalazina |
| CA3021066C (en) | 2016-04-19 | 2024-04-09 | Ferring B.V. | Oral pharmaceutical compositions of nicotinamide |
| TR201722039A2 (tr) * | 2017-12-27 | 2019-07-22 | Sanovel Ilac Sanayi Ve Ticaret Anonim Sirketi | Mesalazi̇ni̇n kati oral farmasöti̇k kompozi̇syonlari |
| IT201800011120A1 (it) | 2018-12-14 | 2020-06-14 | Dpl Pharma S P A | Composizioni farmaceutiche orali solide per la somministrazione di mesalazina o suoi derivati |
| CA3136081A1 (en) * | 2019-05-03 | 2020-11-12 | Azora Therapeutics, Inc. | Compositions comprising indigo and/or an indigo derivative and methods of use thereof |
| US20210236519A1 (en) * | 2020-01-30 | 2021-08-05 | Atoz Pharmaceuticals Pvt Ltd | Controlled release dosage forms of 5-aminosalicylic acid and process thereof |
| US20220125733A1 (en) * | 2020-10-23 | 2022-04-28 | Atoz Pharmaceuticals Pvt Ltd | Nongranulated compressed tablets of mesalamine, and process of preparation thereof |
| EP4382099A1 (en) * | 2022-12-05 | 2024-06-12 | ADD Advanced Drug Delivery Technologies, Ltd. | Drug loaded modified release pellets |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020098235A1 (en) * | 2000-11-20 | 2002-07-25 | Dittmar Gregory Paul | Pharmaceutical dosage form with multiple coatings for reduced impact of coating fractures |
| US20030152627A1 (en) * | 2001-01-31 | 2003-08-14 | Thomas Beckert | Multi-particulate form of medicament, comprising at least two differently coated forms of pellet |
| CN102579388A (zh) * | 2012-03-10 | 2012-07-18 | 吉林化工学院 | 美沙拉秦结肠定位给药缓释片的制备 |
| WO2013134348A1 (en) * | 2012-03-07 | 2013-09-12 | Santarus, Inc. | Controlled-release solid dosage forms of mesalamine |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IT1246382B (it) * | 1990-04-17 | 1994-11-18 | Eurand Int | Metodo per la cessione mirata e controllata di farmaci nell'intestino e particolarmente nel colon |
| US6458383B2 (en) | 1999-08-17 | 2002-10-01 | Lipocine, Inc. | Pharmaceutical dosage form for oral administration of hydrophilic drugs, particularly low molecular weight heparin |
| IT1318625B1 (it) | 2000-07-14 | 2003-08-27 | Roberto Valducci | Formulazioni farmaceutiche solide orali a rilascio multifasicoph-dipendente. |
| US8048924B2 (en) | 2001-08-29 | 2011-11-01 | Biocon Limited | Methods and compositions employing 4-aminophenylacetic acid compounds |
| US20040185097A1 (en) | 2003-01-31 | 2004-09-23 | Glenmark Pharmaceuticals Ltd. | Controlled release modifying complex and pharmaceutical compositions thereof |
| US20080286344A1 (en) | 2007-05-16 | 2008-11-20 | Olivia Darmuzey | Solid form |
| CA2746767A1 (en) | 2008-12-17 | 2010-07-08 | Altheus Therapeutics, Inc. | Oral formulations |
| GB201003734D0 (en) | 2010-03-05 | 2010-04-21 | Univ Strathclyde | Delayed prolonged drug delivery |
| GB201003731D0 (en) | 2010-03-05 | 2010-04-21 | Univ Strathclyde | Immediate/delayed drug delivery |
| EP2468264A1 (en) * | 2010-12-27 | 2012-06-27 | Laboratorios Liconsa, S.A. | Oral pharmaceutical tablet for controled release of mesalazine and process for obtaining it |
| WO2013144176A1 (en) | 2012-03-30 | 2013-10-03 | Laboratorios Del Dr. Esteve, S.A. | Controlled release formulatin comprising mesalamine |
| CA2876540C (en) | 2012-06-15 | 2022-11-29 | Conaris Research Institute Ag | A pharmaceutical composition containing nicotinic acid and/or nicotinamide and/or tryptophan for positively influencing the intestinal microbiota |
| US20140178468A1 (en) | 2012-12-24 | 2014-06-26 | Ranbaxy Laboratories Limited | Multiparticulate extended-release composition of mesalamine |
| US9763887B2 (en) * | 2013-03-15 | 2017-09-19 | Allergan Pharmaceuticals International Limited | Mesalamine pharmaceutical composition with multiple dosage elements for reduced delivery variability |
| ES2788866T3 (es) | 2013-12-13 | 2020-10-23 | Conaris Res Institute Ag | Una composición farmacéutica que contiene ácido nicotínico y/o nicotinamida para su uso en influir beneficiosamente en los niveles de lípidos en sangre mediante la modificación de la microbiota intestinal |
| CA2932504A1 (en) | 2013-12-13 | 2015-06-18 | Conaris Research Institute Ag | A pharmaceutical composition containing combinations of nicotinamide and 5-aminosalicylic acid for beneficially influencing the intestinal microbiota and/or treating gastrointestinal inflammation |
| US20160045442A1 (en) * | 2014-08-13 | 2016-02-18 | Cadila Healthcare Limited | Stable pharmaceutical compositions of mesalamine |
| CN104257669A (zh) | 2014-09-25 | 2015-01-07 | 合肥平光制药有限公司 | 一种用于向肠道递送水杨酸类药物的口服给药组合物 |
| CN105687158B (zh) * | 2016-01-21 | 2019-06-07 | 贝沃特医药技术(上海)有限公司 | 一种时间依赖释放机制的美沙拉嗪微粒制剂及其制备方法 |
-
2017
- 2017-04-18 CA CA3021071A patent/CA3021071A1/en active Pending
- 2017-04-18 TW TW106112907A patent/TW201740932A/zh unknown
- 2017-04-18 AR ARP170100993A patent/AR108231A1/es unknown
- 2017-04-18 JP JP2018554489A patent/JP2019513800A/ja active Pending
- 2017-04-18 CN CN201780031230.6A patent/CN109310642B/zh not_active Expired - Fee Related
- 2017-04-18 KR KR1020187033227A patent/KR102381586B1/ko active Active
- 2017-04-18 US US16/094,644 patent/US10874617B2/en not_active Expired - Fee Related
- 2017-04-18 RU RU2018137359A patent/RU2744576C2/ru active
- 2017-04-18 WO PCT/US2017/028067 patent/WO2017184566A1/en not_active Ceased
- 2017-04-18 EP EP17737401.4A patent/EP3445340A1/en not_active Withdrawn
- 2017-04-18 MX MX2018012618A patent/MX387731B/es unknown
- 2017-04-18 AU AU2017252410A patent/AU2017252410A1/en not_active Abandoned
- 2017-04-18 BR BR112018071370A patent/BR112018071370A2/pt not_active IP Right Cessation
-
2020
- 2020-12-23 US US17/132,577 patent/US20210220281A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020098235A1 (en) * | 2000-11-20 | 2002-07-25 | Dittmar Gregory Paul | Pharmaceutical dosage form with multiple coatings for reduced impact of coating fractures |
| US20030152627A1 (en) * | 2001-01-31 | 2003-08-14 | Thomas Beckert | Multi-particulate form of medicament, comprising at least two differently coated forms of pellet |
| WO2013134348A1 (en) * | 2012-03-07 | 2013-09-12 | Santarus, Inc. | Controlled-release solid dosage forms of mesalamine |
| CN102579388A (zh) * | 2012-03-10 | 2012-07-18 | 吉林化工学院 | 美沙拉秦结肠定位给药缓释片的制备 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20190105275A1 (en) | 2019-04-11 |
| CN109310642A (zh) | 2019-02-05 |
| BR112018071370A2 (pt) | 2019-02-05 |
| EP3445340A1 (en) | 2019-02-27 |
| RU2744576C2 (ru) | 2021-03-11 |
| CA3021071A1 (en) | 2017-10-26 |
| KR20180133503A (ko) | 2018-12-14 |
| US20210220281A1 (en) | 2021-07-22 |
| RU2018137359A (ru) | 2020-05-19 |
| US10874617B2 (en) | 2020-12-29 |
| MX387731B (es) | 2025-03-18 |
| KR102381586B1 (ko) | 2022-04-04 |
| MX2018012618A (es) | 2019-06-24 |
| WO2017184566A1 (en) | 2017-10-26 |
| AU2017252410A1 (en) | 2018-11-01 |
| AR108231A1 (es) | 2018-08-01 |
| TW201740932A (zh) | 2017-12-01 |
| RU2018137359A3 (enExample) | 2020-07-03 |
| JP2019513800A (ja) | 2019-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109310642B (zh) | 美沙拉嗪的口服药物组合物 | |
| JP6434429B2 (ja) | デフェラシロクスの経口製剤 | |
| US20130011476A1 (en) | Stable compositions of famotidine and ibuprofen | |
| CN109152772B (zh) | 烟酰胺的口服药物组合物 | |
| JP2008534681A (ja) | ジピリダモール持続放出製剤及びそれを調製のための方法 | |
| JP6902043B2 (ja) | フマル酸ジメチルを含む医薬ビーズ製剤 | |
| CN105025883A (zh) | 右哌甲酯或其盐的调节释放的药物组合物 | |
| WO2020101586A1 (en) | Controlled release propiverine formulations | |
| US20150224056A1 (en) | Pharmaceutical compositions of ibuprofen and famotidine | |
| HK40005191A (en) | Oral pharmaceutical compositions of nicotinamide | |
| HK40005191B (en) | Oral pharmaceutical compositions of nicotinamide | |
| WO2025215658A1 (en) | Pharmaceutical compositions of ketotifen | |
| WO2014167439A1 (en) | Modified release pharmaceutical compositions of topiramate or salts thereof | |
| US20130236538A1 (en) | Pharmaceutical compositions of ibuprofen and famotidine | |
| HK40000470A (en) | Extended release dosage forms of pregabalin |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20210720 |
|
| CF01 | Termination of patent right due to non-payment of annual fee |