WO2023188482A1 - Adhesive sheet - Google Patents
Adhesive sheet Download PDFInfo
- Publication number
- WO2023188482A1 WO2023188482A1 PCT/JP2022/038804 JP2022038804W WO2023188482A1 WO 2023188482 A1 WO2023188482 A1 WO 2023188482A1 JP 2022038804 W JP2022038804 W JP 2022038804W WO 2023188482 A1 WO2023188482 A1 WO 2023188482A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- less
- adhesive
- adhesive sheet
- adhesive layer
- Prior art date
Links
- 230000001070 adhesive effect Effects 0.000 title claims abstract description 306
- 239000000853 adhesive Substances 0.000 title claims abstract description 298
- 239000000178 monomer Substances 0.000 claims abstract description 165
- 229920000058 polyacrylate Polymers 0.000 claims abstract description 120
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims abstract description 42
- ZWEDFBKLJILTMC-UHFFFAOYSA-N ethyl 4,4,4-trifluoro-3-hydroxybutanoate Chemical compound CCOC(=O)CC(O)C(F)(F)F ZWEDFBKLJILTMC-UHFFFAOYSA-N 0.000 claims abstract description 35
- 229920000642 polymer Polymers 0.000 claims abstract description 23
- 229920005989 resin Polymers 0.000 claims description 241
- 239000011347 resin Substances 0.000 claims description 241
- 239000012790 adhesive layer Substances 0.000 claims description 136
- 239000004820 Pressure-sensitive adhesive Substances 0.000 claims description 98
- 239000003431 cross linking reagent Substances 0.000 claims description 85
- 239000000203 mixture Substances 0.000 claims description 54
- -1 polypropylene Polymers 0.000 claims description 46
- 239000010410 layer Substances 0.000 claims description 45
- 239000004743 Polypropylene Substances 0.000 claims description 32
- 229920001155 polypropylene Polymers 0.000 claims description 32
- 239000004698 Polyethylene Substances 0.000 claims description 31
- 229920000573 polyethylene Polymers 0.000 claims description 31
- 238000012360 testing method Methods 0.000 claims description 12
- 229910001220 stainless steel Inorganic materials 0.000 claims description 7
- 239000010935 stainless steel Substances 0.000 claims description 7
- 238000006073 displacement reaction Methods 0.000 claims description 4
- 239000000463 material Substances 0.000 abstract description 192
- RSWGJHLUYNHPMX-UHFFFAOYSA-N Abietic-Saeure Natural products C12CCC(C(C)C)=CC2=CCC2C1(C)CCCC2(C)C(O)=O RSWGJHLUYNHPMX-UHFFFAOYSA-N 0.000 description 104
- KHPCPRHQVVSZAH-HUOMCSJISA-N Rosin Natural products O(C/C=C/c1ccccc1)[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 KHPCPRHQVVSZAH-HUOMCSJISA-N 0.000 description 104
- KHPCPRHQVVSZAH-UHFFFAOYSA-N trans-cinnamyl beta-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OCC=CC1=CC=CC=C1 KHPCPRHQVVSZAH-UHFFFAOYSA-N 0.000 description 104
- 239000002585 base Substances 0.000 description 78
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 75
- 239000002028 Biomass Substances 0.000 description 74
- 239000010408 film Substances 0.000 description 60
- 150000003505 terpenes Chemical class 0.000 description 47
- 235000007586 terpenes Nutrition 0.000 description 46
- 150000002148 esters Chemical class 0.000 description 40
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 38
- 239000004593 Epoxy Substances 0.000 description 35
- 238000000034 method Methods 0.000 description 31
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 30
- 125000000217 alkyl group Chemical group 0.000 description 30
- 229910052799 carbon Inorganic materials 0.000 description 30
- 239000005011 phenolic resin Substances 0.000 description 28
- 239000012948 isocyanate Substances 0.000 description 24
- 150000002513 isocyanates Chemical class 0.000 description 24
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 23
- 238000005516 engineering process Methods 0.000 description 23
- 239000000047 product Substances 0.000 description 23
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 21
- 238000005259 measurement Methods 0.000 description 21
- 125000000524 functional group Chemical group 0.000 description 19
- 230000000694 effects Effects 0.000 description 18
- 239000000243 solution Substances 0.000 description 14
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 13
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 12
- 230000001747 exhibiting effect Effects 0.000 description 12
- 239000002904 solvent Substances 0.000 description 12
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 11
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerol Natural products OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 11
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 10
- 229910052751 metal Inorganic materials 0.000 description 10
- 239000002184 metal Chemical class 0.000 description 10
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 9
- 230000000052 comparative effect Effects 0.000 description 9
- 238000006116 polymerization reaction Methods 0.000 description 9
- 238000011282 treatment Methods 0.000 description 9
- 229920002799 BoPET Polymers 0.000 description 8
- 239000005057 Hexamethylene diisocyanate Substances 0.000 description 8
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 8
- 238000004132 cross linking Methods 0.000 description 8
- 239000003208 petroleum Substances 0.000 description 8
- 239000005056 polyisocyanate Substances 0.000 description 8
- 229920001228 polyisocyanate Polymers 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- 238000003786 synthesis reaction Methods 0.000 description 8
- GOXQRTZXKQZDDN-UHFFFAOYSA-N 2-Ethylhexyl acrylate Chemical compound CCCCC(CC)COC(=O)C=C GOXQRTZXKQZDDN-UHFFFAOYSA-N 0.000 description 7
- 125000003118 aryl group Chemical group 0.000 description 7
- CQEYYJKEWSMYFG-UHFFFAOYSA-N butyl acrylate Chemical compound CCCCOC(=O)C=C CQEYYJKEWSMYFG-UHFFFAOYSA-N 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 229920001971 elastomer Polymers 0.000 description 7
- 235000011187 glycerol Nutrition 0.000 description 7
- 239000000123 paper Substances 0.000 description 7
- 239000003505 polymerization initiator Substances 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 6
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 6
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 6
- 125000004432 carbon atom Chemical group C* 0.000 description 6
- 229920001577 copolymer Polymers 0.000 description 6
- 238000007334 copolymerization reaction Methods 0.000 description 6
- 238000010528 free radical solution polymerization reaction Methods 0.000 description 6
- 238000010438 heat treatment Methods 0.000 description 6
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 6
- YLLIGHVCTUPGEH-UHFFFAOYSA-M potassium;ethanol;hydroxide Chemical compound [OH-].[K+].CCO YLLIGHVCTUPGEH-UHFFFAOYSA-M 0.000 description 6
- 235000013311 vegetables Nutrition 0.000 description 6
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 5
- 125000003647 acryloyl group Chemical group O=C([*])C([H])=C([H])[H] 0.000 description 5
- 239000000654 additive Substances 0.000 description 5
- 150000001298 alcohols Chemical class 0.000 description 5
- 125000001931 aliphatic group Chemical group 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 239000000835 fiber Substances 0.000 description 5
- 229910052731 fluorine Inorganic materials 0.000 description 5
- 239000011737 fluorine Substances 0.000 description 5
- 229920000728 polyester Polymers 0.000 description 5
- 229920000139 polyethylene terephthalate Polymers 0.000 description 5
- 239000005020 polyethylene terephthalate Substances 0.000 description 5
- 229920000098 polyolefin Polymers 0.000 description 5
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 5
- 239000004810 polytetrafluoroethylene Substances 0.000 description 5
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 5
- 239000005060 rubber Substances 0.000 description 5
- 238000003756 stirring Methods 0.000 description 5
- GRWFGVWFFZKLTI-IUCAKERBSA-N (-)-α-pinene Chemical compound CC1=CC[C@@H]2C(C)(C)[C@H]1C2 GRWFGVWFFZKLTI-IUCAKERBSA-N 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 4
- 206010040844 Skin exfoliation Diseases 0.000 description 4
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 238000005227 gel permeation chromatography Methods 0.000 description 4
- RRAMGCGOFNQTLD-UHFFFAOYSA-N hexamethylene diisocyanate Chemical compound O=C=NCCCCCCN=C=O RRAMGCGOFNQTLD-UHFFFAOYSA-N 0.000 description 4
- 229930195733 hydrocarbon Natural products 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 230000014759 maintenance of location Effects 0.000 description 4
- 239000003960 organic solvent Substances 0.000 description 4
- DVKJHBMWWAPEIU-UHFFFAOYSA-N toluene 2,4-diisocyanate Chemical class CC1=CC=C(N=C=O)C=C1N=C=O DVKJHBMWWAPEIU-UHFFFAOYSA-N 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- KXGFMDJXCMQABM-UHFFFAOYSA-N 2-methoxy-6-methylphenol Chemical compound [CH]OC1=CC=CC([CH])=C1O KXGFMDJXCMQABM-UHFFFAOYSA-N 0.000 description 3
- 239000004215 Carbon black (E152) Substances 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 239000004793 Polystyrene Substances 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 3
- 125000002723 alicyclic group Chemical group 0.000 description 3
- 239000007795 chemical reaction product Substances 0.000 description 3
- 230000006835 compression Effects 0.000 description 3
- 238000007906 compression Methods 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 125000003700 epoxy group Chemical group 0.000 description 3
- 239000006260 foam Substances 0.000 description 3
- 239000011888 foil Substances 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 150000002430 hydrocarbons Chemical class 0.000 description 3
- 239000003999 initiator Substances 0.000 description 3
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate group Chemical group [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 150000004702 methyl esters Chemical class 0.000 description 3
- 238000000465 moulding Methods 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 239000004745 nonwoven fabric Substances 0.000 description 3
- 150000002978 peroxides Chemical class 0.000 description 3
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N phenol group Chemical group C1(=CC=CC=C1)O ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 3
- 229920001568 phenolic resin Polymers 0.000 description 3
- 229920006267 polyester film Polymers 0.000 description 3
- 229920001225 polyester resin Polymers 0.000 description 3
- 239000004645 polyester resin Substances 0.000 description 3
- 229920005672 polyolefin resin Polymers 0.000 description 3
- 229920002223 polystyrene Polymers 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 3
- 239000013638 trimer Substances 0.000 description 3
- 239000002759 woven fabric Substances 0.000 description 3
- WTARULDDTDQWMU-RKDXNWHRSA-N (+)-β-pinene Chemical compound C1[C@H]2C(C)(C)[C@@H]1CCC2=C WTARULDDTDQWMU-RKDXNWHRSA-N 0.000 description 2
- WTARULDDTDQWMU-IUCAKERBSA-N (-)-Nopinene Natural products C1[C@@H]2C(C)(C)[C@H]1CCC2=C WTARULDDTDQWMU-IUCAKERBSA-N 0.000 description 2
- HASUCEDGKYJBDC-UHFFFAOYSA-N 1-[3-[[bis(oxiran-2-ylmethyl)amino]methyl]cyclohexyl]-n,n-bis(oxiran-2-ylmethyl)methanamine Chemical compound C1OC1CN(CC1CC(CN(CC2OC2)CC2OC2)CCC1)CC1CO1 HASUCEDGKYJBDC-UHFFFAOYSA-N 0.000 description 2
- BBMCTIGTTCKYKF-UHFFFAOYSA-N 1-heptanol Chemical compound CCCCCCCO BBMCTIGTTCKYKF-UHFFFAOYSA-N 0.000 description 2
- IMSODMZESSGVBE-UHFFFAOYSA-N 2-Oxazoline Chemical compound C1CN=CO1 IMSODMZESSGVBE-UHFFFAOYSA-N 0.000 description 2
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 2
- UPMLOUAZCHDJJD-UHFFFAOYSA-N 4,4'-Diphenylmethane Diisocyanate Chemical compound C1=CC(N=C=O)=CC=C1CC1=CC=C(N=C=O)C=C1 UPMLOUAZCHDJJD-UHFFFAOYSA-N 0.000 description 2
- OZAIFHULBGXAKX-VAWYXSNFSA-N AIBN Substances N#CC(C)(C)\N=N\C(C)(C)C#N OZAIFHULBGXAKX-VAWYXSNFSA-N 0.000 description 2
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 2
- 239000004342 Benzoyl peroxide Substances 0.000 description 2
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229920000298 Cellophane Polymers 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 244000043261 Hevea brasiliensis Species 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 229920000877 Melamine resin Polymers 0.000 description 2
- WTARULDDTDQWMU-UHFFFAOYSA-N Pseudopinene Natural products C1C2C(C)(C)C1CCC2=C WTARULDDTDQWMU-UHFFFAOYSA-N 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 2
- 230000021736 acetylation Effects 0.000 description 2
- 238000006640 acetylation reaction Methods 0.000 description 2
- 239000003522 acrylic cement Substances 0.000 description 2
- 125000005250 alkyl acrylate group Chemical group 0.000 description 2
- XCPQUQHBVVXMRQ-UHFFFAOYSA-N alpha-Fenchene Natural products C1CC2C(=C)CC1C2(C)C XCPQUQHBVVXMRQ-UHFFFAOYSA-N 0.000 description 2
- MVNCAPSFBDBCGF-UHFFFAOYSA-N alpha-pinene Natural products CC1=CCC23C1CC2C3(C)C MVNCAPSFBDBCGF-UHFFFAOYSA-N 0.000 description 2
- 230000003712 anti-aging effect Effects 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 239000002216 antistatic agent Substances 0.000 description 2
- 229920005601 base polymer Polymers 0.000 description 2
- 235000019400 benzoyl peroxide Nutrition 0.000 description 2
- 229930006722 beta-pinene Natural products 0.000 description 2
- 229920005549 butyl rubber Polymers 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 229910001873 dinitrogen Inorganic materials 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- FJKIXWOMBXYWOQ-UHFFFAOYSA-N ethenoxyethane Chemical compound CCOC=C FJKIXWOMBXYWOQ-UHFFFAOYSA-N 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000004744 fabric Substances 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- LCWMKIHBLJLORW-UHFFFAOYSA-N gamma-carene Natural products C1CC(=C)CC2C(C)(C)C21 LCWMKIHBLJLORW-UHFFFAOYSA-N 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- ZFSLODLOARCGLH-UHFFFAOYSA-N isocyanuric acid Chemical group OC1=NC(O)=NC(O)=N1 ZFSLODLOARCGLH-UHFFFAOYSA-N 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- JDSHMPZPIAZGSV-UHFFFAOYSA-N melamine Chemical compound NC1=NC(N)=NC(N)=N1 JDSHMPZPIAZGSV-UHFFFAOYSA-N 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 229920003052 natural elastomer Polymers 0.000 description 2
- 229920001194 natural rubber Polymers 0.000 description 2
- 239000004014 plasticizer Substances 0.000 description 2
- 229920002647 polyamide Polymers 0.000 description 2
- 229920006122 polyamide resin Polymers 0.000 description 2
- 229920001707 polybutylene terephthalate Polymers 0.000 description 2
- 229920001721 polyimide Polymers 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 238000003918 potentiometric titration Methods 0.000 description 2
- GRWFGVWFFZKLTI-UHFFFAOYSA-N rac-alpha-Pinene Natural products CC1=CCC2C(C)(C)C1C2 GRWFGVWFFZKLTI-UHFFFAOYSA-N 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 238000004381 surface treatment Methods 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- LDHQCZJRKDOVOX-UHFFFAOYSA-N trans-crotonic acid Natural products CC=CC(O)=O LDHQCZJRKDOVOX-UHFFFAOYSA-N 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 235000021122 unsaturated fatty acids Nutrition 0.000 description 2
- 150000004670 unsaturated fatty acids Chemical group 0.000 description 2
- DTGKSKDOIYIVQL-WEDXCCLWSA-N (+)-borneol Chemical group C1C[C@@]2(C)[C@@H](O)C[C@@H]1C2(C)C DTGKSKDOIYIVQL-WEDXCCLWSA-N 0.000 description 1
- FKTHNVSLHLHISI-UHFFFAOYSA-N 1,2-bis(isocyanatomethyl)benzene Chemical class O=C=NCC1=CC=CC=C1CN=C=O FKTHNVSLHLHISI-UHFFFAOYSA-N 0.000 description 1
- ZTNJGMFHJYGMDR-UHFFFAOYSA-N 1,2-diisocyanatoethane Chemical compound O=C=NCCN=C=O ZTNJGMFHJYGMDR-UHFFFAOYSA-N 0.000 description 1
- VGHSXKTVMPXHNG-UHFFFAOYSA-N 1,3-diisocyanatobenzene Chemical compound O=C=NC1=CC=CC(N=C=O)=C1 VGHSXKTVMPXHNG-UHFFFAOYSA-N 0.000 description 1
- 239000005059 1,4-Cyclohexyldiisocyanate Substances 0.000 description 1
- ALQLPWJFHRMHIU-UHFFFAOYSA-N 1,4-diisocyanatobenzene Chemical compound O=C=NC1=CC=C(N=C=O)C=C1 ALQLPWJFHRMHIU-UHFFFAOYSA-N 0.000 description 1
- OVBFMUAFNIIQAL-UHFFFAOYSA-N 1,4-diisocyanatobutane Chemical class O=C=NCCCCN=C=O OVBFMUAFNIIQAL-UHFFFAOYSA-N 0.000 description 1
- 229940008841 1,6-hexamethylene diisocyanate Drugs 0.000 description 1
- IANQTJSKSUMEQM-UHFFFAOYSA-N 1-benzofuran Chemical compound C1=CC=C2OC=CC2=C1 IANQTJSKSUMEQM-UHFFFAOYSA-N 0.000 description 1
- LFSYUSUFCBOHGU-UHFFFAOYSA-N 1-isocyanato-2-[(4-isocyanatophenyl)methyl]benzene Chemical compound C1=CC(N=C=O)=CC=C1CC1=CC=CC=C1N=C=O LFSYUSUFCBOHGU-UHFFFAOYSA-N 0.000 description 1
- DTZHXCBUWSTOPO-UHFFFAOYSA-N 1-isocyanato-4-[(4-isocyanato-3-methylphenyl)methyl]-2-methylbenzene Chemical compound C1=C(N=C=O)C(C)=CC(CC=2C=C(C)C(N=C=O)=CC=2)=C1 DTZHXCBUWSTOPO-UHFFFAOYSA-N 0.000 description 1
- XLPJNCYCZORXHG-UHFFFAOYSA-N 1-morpholin-4-ylprop-2-en-1-one Chemical compound C=CC(=O)N1CCOCC1 XLPJNCYCZORXHG-UHFFFAOYSA-N 0.000 description 1
- KQSMCAVKSJWMSI-UHFFFAOYSA-N 2,4-dimethyl-1-n,1-n,3-n,3-n-tetrakis(oxiran-2-ylmethyl)benzene-1,3-diamine Chemical compound CC1=C(N(CC2OC2)CC2OC2)C(C)=CC=C1N(CC1OC1)CC1CO1 KQSMCAVKSJWMSI-UHFFFAOYSA-N 0.000 description 1
- HIXDQWDOVZUNNA-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-hydroxy-7-methoxychromen-4-one Chemical compound C=1C(OC)=CC(O)=C(C(C=2)=O)C=1OC=2C1=CC=C(OC)C(OC)=C1 HIXDQWDOVZUNNA-UHFFFAOYSA-N 0.000 description 1
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 description 1
- AOBIOSPNXBMOAT-UHFFFAOYSA-N 2-[2-(oxiran-2-ylmethoxy)ethoxymethyl]oxirane Chemical compound C1OC1COCCOCC1CO1 AOBIOSPNXBMOAT-UHFFFAOYSA-N 0.000 description 1
- WTYYGFLRBWMFRY-UHFFFAOYSA-N 2-[6-(oxiran-2-ylmethoxy)hexoxymethyl]oxirane Chemical compound C1OC1COCCCCCCOCC1CO1 WTYYGFLRBWMFRY-UHFFFAOYSA-N 0.000 description 1
- TXBCBTDQIULDIA-UHFFFAOYSA-N 2-[[3-hydroxy-2,2-bis(hydroxymethyl)propoxy]methyl]-2-(hydroxymethyl)propane-1,3-diol Chemical compound OCC(CO)(CO)COCC(CO)(CO)CO TXBCBTDQIULDIA-UHFFFAOYSA-N 0.000 description 1
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 1
- DPNXHTDWGGVXID-UHFFFAOYSA-N 2-isocyanatoethyl prop-2-enoate Chemical compound C=CC(=O)OCCN=C=O DPNXHTDWGGVXID-UHFFFAOYSA-N 0.000 description 1
- QOXOZONBQWIKDA-UHFFFAOYSA-N 3-hydroxypropyl Chemical group [CH2]CCO QOXOZONBQWIKDA-UHFFFAOYSA-N 0.000 description 1
- SXIFAEWFOJETOA-UHFFFAOYSA-N 4-hydroxy-butyl Chemical group [CH2]CCCO SXIFAEWFOJETOA-UHFFFAOYSA-N 0.000 description 1
- 229920001342 Bakelite® Polymers 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical class CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- 239000004831 Hot glue Substances 0.000 description 1
- 239000013032 Hydrocarbon resin Substances 0.000 description 1
- 239000005058 Isophorone diisocyanate Substances 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 1
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 1
- 240000000907 Musa textilis Species 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- IIGAAOXXRKTFAM-UHFFFAOYSA-N N=C=O.N=C=O.CC1=C(C)C(C)=C(C)C(C)=C1C Chemical class N=C=O.N=C=O.CC1=C(C)C(C)=C(C)C(C)=C1C IIGAAOXXRKTFAM-UHFFFAOYSA-N 0.000 description 1
- 241000238413 Octopus Species 0.000 description 1
- 235000019482 Palm oil Nutrition 0.000 description 1
- 229930040373 Paraformaldehyde Natural products 0.000 description 1
- 229920000538 Poly[(phenyl isocyanate)-co-formaldehyde] Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 229920000297 Rayon Polymers 0.000 description 1
- 239000006087 Silane Coupling Agent Substances 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical compound C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 229920006221 acetate fiber Polymers 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 125000004018 acid anhydride group Chemical group 0.000 description 1
- 239000003377 acid catalyst Substances 0.000 description 1
- 238000010306 acid treatment Methods 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 238000007259 addition reaction Methods 0.000 description 1
- 239000002313 adhesive film Substances 0.000 description 1
- 239000002390 adhesive tape Substances 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 150000004703 alkoxides Chemical class 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 125000005370 alkoxysilyl group Chemical group 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 125000003368 amide group Chemical group 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 125000001204 arachidyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 125000005160 aryl oxy alkyl group Chemical group 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 239000007869 azo polymerization initiator Substances 0.000 description 1
- 239000004637 bakelite Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- HIFVAOIJYDXIJG-UHFFFAOYSA-N benzylbenzene;isocyanic acid Chemical class N=C=O.N=C=O.C=1C=CC=CC=1CC1=CC=CC=C1 HIFVAOIJYDXIJG-UHFFFAOYSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 238000012662 bulk polymerization Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 229920006026 co-polymeric resin Polymers 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 239000011889 copper foil Substances 0.000 description 1
- 238000003851 corona treatment Methods 0.000 description 1
- LDHQCZJRKDOVOX-NSCUHMNNSA-N crotonic acid Chemical compound C\C=C\C(O)=O LDHQCZJRKDOVOX-NSCUHMNNSA-N 0.000 description 1
- 238000007766 curtain coating Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- BRYSLIOQYFVAJD-UHFFFAOYSA-N cyclohexane;1,2-dichloroethane Chemical compound ClCCCl.C1CCCCC1 BRYSLIOQYFVAJD-UHFFFAOYSA-N 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000004512 die casting Methods 0.000 description 1
- 125000005442 diisocyanate group Chemical group 0.000 description 1
- KIQKWYUGPPFMBV-UHFFFAOYSA-N diisocyanatomethane Chemical compound O=C=NCN=C=O KIQKWYUGPPFMBV-UHFFFAOYSA-N 0.000 description 1
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Natural products C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 238000007720 emulsion polymerization reaction Methods 0.000 description 1
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 125000005448 ethoxyethyl group Chemical group [H]C([H])([H])C([H])([H])OC([H])([H])C([H])([H])* 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000002657 fibrous material Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 239000011086 glassine Substances 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- MDNFYIAABKQDML-UHFFFAOYSA-N heptyl 2-methylprop-2-enoate Chemical compound CCCCCCCOC(=O)C(C)=C MDNFYIAABKQDML-UHFFFAOYSA-N 0.000 description 1
- XXMIOPMDWAUFGU-UHFFFAOYSA-N hexane-1,6-diol Chemical compound OCCCCCCO XXMIOPMDWAUFGU-UHFFFAOYSA-N 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229920006270 hydrocarbon resin Polymers 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 125000005462 imide group Chemical group 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 239000011256 inorganic filler Substances 0.000 description 1
- 229910003475 inorganic filler Inorganic materials 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- LDHQCZJRKDOVOX-IHWYPQMZSA-N isocrotonic acid Chemical compound C\C=C/C(O)=O LDHQCZJRKDOVOX-IHWYPQMZSA-N 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- NIMLQBUJDJZYEJ-UHFFFAOYSA-N isophorone diisocyanate Chemical compound CC1(C)CC(N=C=O)CC(C)(CN=C=O)C1 NIMLQBUJDJZYEJ-UHFFFAOYSA-N 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- 239000002655 kraft paper Substances 0.000 description 1
- 239000004611 light stabiliser Substances 0.000 description 1
- 235000001510 limonene Nutrition 0.000 description 1
- 229940087305 limonene Drugs 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 125000002960 margaryl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 125000005395 methacrylic acid group Chemical group 0.000 description 1
- AYLRODJJLADBOB-QMMMGPOBSA-N methyl (2s)-2,6-diisocyanatohexanoate Chemical compound COC(=O)[C@@H](N=C=O)CCCCN=C=O AYLRODJJLADBOB-QMMMGPOBSA-N 0.000 description 1
- XJRBAMWJDBPFIM-UHFFFAOYSA-N methyl vinyl ether Chemical compound COC=C XJRBAMWJDBPFIM-UHFFFAOYSA-N 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000005065 mining Methods 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- CWQXQMHSOZUFJS-UHFFFAOYSA-N molybdenum disulfide Chemical compound S=[Mo]=S CWQXQMHSOZUFJS-UHFFFAOYSA-N 0.000 description 1
- 229930003658 monoterpene Natural products 0.000 description 1
- 150000002773 monoterpene derivatives Chemical class 0.000 description 1
- 235000002577 monoterpenes Nutrition 0.000 description 1
- 125000001421 myristyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000003136 n-heptyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- SLCVBVWXLSEKPL-UHFFFAOYSA-N neopentyl glycol Chemical compound OCC(C)(C)CO SLCVBVWXLSEKPL-UHFFFAOYSA-N 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 125000001196 nonadecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000012766 organic filler Substances 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000003346 palm kernel oil Substances 0.000 description 1
- 235000019865 palm kernel oil Nutrition 0.000 description 1
- 239000002540 palm oil Substances 0.000 description 1
- 125000000913 palmityl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000002958 pentadecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L persulfate group Chemical group S(=O)(=O)([O-])OOS(=O)(=O)[O-] JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 230000029553 photosynthesis Effects 0.000 description 1
- 238000010672 photosynthesis Methods 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 238000009832 plasma treatment Methods 0.000 description 1
- 229920001084 poly(chloroprene) Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000011112 polyethylene naphthalate Substances 0.000 description 1
- 229920000223 polyglycerol Polymers 0.000 description 1
- 239000009719 polyimide resin Substances 0.000 description 1
- 230000000379 polymerizing effect Effects 0.000 description 1
- 229920006324 polyoxymethylene Polymers 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- USHAGKDGDHPEEY-UHFFFAOYSA-L potassium persulfate Chemical compound [K+].[K+].[O-]S(=O)(=O)OOS([O-])(=O)=O USHAGKDGDHPEEY-UHFFFAOYSA-L 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002250 progressing effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000002964 rayon Substances 0.000 description 1
- 239000012966 redox initiator Substances 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000006557 surface reaction Methods 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000010557 suspension polymerization reaction Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 239000003784 tall oil Substances 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- 238000000954 titration curve Methods 0.000 description 1
- RUELTTOHQODFPA-UHFFFAOYSA-N toluene 2,6-diisocyanate Chemical compound CC1=C(N=C=O)C=CC=C1N=C=O RUELTTOHQODFPA-UHFFFAOYSA-N 0.000 description 1
- 125000002889 tridecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000002948 undecyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 229920001567 vinyl ester resin Polymers 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 238000010792 warming Methods 0.000 description 1
- 239000013585 weight reducing agent Substances 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- 210000000707 wrist Anatomy 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J11/00—Features of adhesives not provided for in group C09J9/00, e.g. additives
- C09J11/02—Non-macromolecular additives
- C09J11/06—Non-macromolecular additives organic
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J11/00—Features of adhesives not provided for in group C09J9/00, e.g. additives
- C09J11/08—Macromolecular additives
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J133/00—Adhesives based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Adhesives based on derivatives of such polymers
- C09J133/02—Homopolymers or copolymers of acids; Metal or ammonium salts thereof
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J133/00—Adhesives based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Adhesives based on derivatives of such polymers
- C09J133/04—Homopolymers or copolymers of esters
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J133/00—Adhesives based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Adhesives based on derivatives of such polymers
- C09J133/04—Homopolymers or copolymers of esters
- C09J133/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, the oxygen atom being present only as part of the carboxyl radical
- C09J133/08—Homopolymers or copolymers of acrylic acid esters
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J7/00—Adhesives in the form of films or foils
- C09J7/30—Adhesives in the form of films or foils characterised by the adhesive composition
- C09J7/38—Pressure-sensitive adhesives [PSA]
Definitions
- the present invention relates to an adhesive sheet.
- This application claims priority based on Japanese Patent Application No. 2022-54421 filed on March 29, 2022 and Japanese Patent Application No. 2022-164655 filed on October 13, 2022. , the entire contents of those applications are incorporated herein by reference.
- adhesives also referred to as pressure-sensitive adhesives, hereinafter the same
- adhesives exhibit a soft solid (viscoelastic) state in a temperature range around room temperature, and have the property of adhering to adherends under pressure.
- adhesives are widely used in various industrial fields such as home appliances, automobiles, and OA equipment, typically in the form of adhesive sheets containing an adhesive layer, for purposes such as bonding parts and protecting surfaces. It's being used.
- Technical documents related to adhesive sheets include Patent Documents 1 and 2.
- Patent Documents 1 and 2 describe adhesives containing acrylic polymers polymerized using n-heptyl acrylate as a monomer component.
- pressure-sensitive adhesive sheets are required to have good adhesion performance to various materials.
- one of the objects to be bonded is made of a highly polar material such as a stainless steel plate, and the other is made of a low polar material such as polyolefin.
- the ability to reliably fix a member whose surface is composed of a highly polar material and a low polar material may be required.
- a soft adhesive whose storage modulus is limited to a predetermined value or less, adhesiveness to different types of materials such as high polarity materials and low polarity materials can be easily obtained.
- such adhesives generally tend to have reduced holding power.
- an object of the present invention is to provide a pressure-sensitive adhesive sheet that can achieve both high levels of adhesive strength for highly polar materials, adhesive strength for low polar materials, and holding power.
- a pressure-sensitive adhesive sheet having a pressure-sensitive adhesive layer containing an acrylic polymer is provided.
- the acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer. Furthermore, the monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer. Furthermore, the gel fraction of the adhesive layer is less than 70%. The weight average molecular weight of the acrylic polymer is greater than 600,000.
- Patent Document 1 the adhesive strength and holding power for polypropylene (40 ° C., adhesive area 25 mm ⁇ 25 mm, load 1 kg) are evaluated, and in Patent Document 2, the holding force (80 ° C., adhesive area 25 mm ⁇ 25 mm,
- the acrylic polymers used either have a low Mw or have a low copolymerization ratio of carboxyl group-containing monomers, so the retention will be evaluated in the examples below. (80° C., adhesion area 10 mm x 20 mm, load 1.5 kg) was not obtained, and the adhesion force to high polarity materials, the adhesion force to low polarity materials, and the holding force were not fully satisfied.
- the adhesive composition for forming the adhesive layer includes a crosslinking agent.
- a crosslinking agent By using a crosslinking agent, the cohesive force of the adhesive increases, and sufficient holding power can be suitably achieved.
- the adhesive layer includes a tackifying resin.
- a tackifying resin By using a tackifying resin, the gel fraction of the adhesive layer can be adjusted to an appropriate range, and the adhesive strength to high polarity materials and low polarity materials can be improved.
- the thickness of the adhesive layer is 0.1 to 500 ⁇ m.
- the technology disclosed herein can be implemented with a configuration including an adhesive layer having the above thickness.
- the adhesive layer has a gel fraction of 20% or more and less than 70%.
- the adhesive sheet according to some preferred embodiments has a 180 degree peel strength against a stainless steel plate (adhesive strength against SUS) of 15 N/25 mm or more.
- the pressure-sensitive adhesive sheet having the adhesive strength against SUS can exhibit excellent adhesive strength against highly polar materials.
- Adhesive sheets according to some preferred embodiments have a 180 degree peel strength against polypropylene (adhesive strength against PP) of 10 N/25 mm or more.
- the pressure-sensitive adhesive sheet having the above-mentioned adhesive strength to PP can have sufficient adhesion reliability to low polarity materials.
- Adhesive sheets according to some preferred embodiments have a 180 degree peel strength against polyethylene (adhesion strength against PE) of 5 N/25 mm or more.
- the pressure-sensitive adhesive sheet having the above-mentioned adhesive strength to PE can have sufficient adhesion reliability to low polarity materials.
- Adhesive sheets according to some preferred embodiments have a displacement distance of 10 mm or less in a holding force test conducted at 80° C., adhesive area 10 mm x 20 mm, load 1.5 kg, and for 1 hour.
- a pressure-sensitive adhesive sheet that does not easily shift in the above-mentioned holding power test can have sufficient holding power (specifically, high temperature holding power).
- the adhesive sheet disclosed herein has both adhesion and holding power to different materials, so it can be preferably used for adhesion to highly polar or low polar materials, or for applications that require long-term adhesion reliability. can be done. For example, it is suitable for fixing members in electronic devices including home appliances, office automation equipment, and portable electronic devices such as smartphones. As described above, this specification provides an electronic device using any of the adhesive sheets disclosed herein, in other words, an electronic device including the adhesive sheet.
- FIG. 1 is a cross-sectional view schematically showing the configuration of a pressure-sensitive adhesive sheet according to an embodiment.
- FIG. 3 is a cross-sectional view schematically showing the configuration of a pressure-sensitive adhesive sheet according to another embodiment.
- FIG. 3 is a cross-sectional view schematically showing the configuration of a pressure-sensitive adhesive sheet according to another embodiment.
- FIG. 1 is a front view schematically showing an example of a portable electronic device including an adhesive sheet.
- the term "adhesive” as used herein refers to a material that exhibits a soft solid (viscoelastic) state in the temperature range around room temperature and has the property of easily adhering to an adherend under pressure. .
- the adhesive referred to here generally has a complex tensile modulus E * (1Hz) as defined in "C. A. Dahlquist, “Adhesion: Fundamentals and Practice", McLaren & Sons, (1966) P. 143". ⁇ 10 7 dyne/cm 2 (typically, a material having the above properties at 25° C.).
- biomass-derived carbon means carbon derived from biomass materials, that is, materials derived from renewable organic resources (renewable carbon).
- biomass materials are typically materials derived from biological resources (typically plants that perform photosynthesis) that can be reproduced sustainably in the presence of sunlight, water, and carbon dioxide. means. Therefore, materials derived from fossil resources that are depleted through use after mining (fossil resource-based materials) are excluded from the concept of biomass materials here.
- the biomass carbon ratio of the adhesive layer and the adhesive sheet that is, the proportion of biomass-derived carbon in the total carbon contained in the adhesive layer and the adhesive sheet, is the carbon isotope content with a mass number of 14 measured in accordance with ASTM D6866. It can be estimated from the amount.
- the adhesive sheet disclosed herein includes an adhesive layer.
- the above-mentioned pressure-sensitive adhesive sheet is, for example, a base material-less double-sided pressure-sensitive adhesive sheet comprising a first pressure-sensitive adhesive surface formed by one surface of the pressure-sensitive adhesive layer, and a second pressure-sensitive adhesive surface formed by the other surface of the pressure-sensitive adhesive layer.
- the pressure-sensitive adhesive sheet disclosed herein may be in the form of a pressure-sensitive adhesive sheet with a base material, in which the pressure-sensitive adhesive layer is laminated on one or both sides of a support base material.
- the supporting base material may be simply referred to as "base material”.
- adhesive sheet here may include what is called an adhesive tape, an adhesive label, an adhesive film, and the like.
- the pressure-sensitive adhesive sheet disclosed herein may be in the form of a roll or a sheet. Alternatively, the adhesive sheet may be further processed into various shapes.
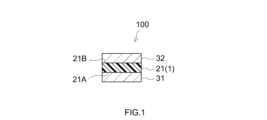
- FIG. 1 The structure of a pressure-sensitive adhesive sheet according to one embodiment is schematically shown in FIG.
- This adhesive sheet 1 is configured as a double-sided adhesive sheet without a base material, which includes an adhesive layer 21.
- the adhesive sheet 1 has a first adhesive surface 21A constituted by one surface (first surface) of the adhesive layer 21 and a second adhesive surface constituted by the other surface (second surface) of the adhesive layer 21. 21B are attached to different parts of the adherend.
- the locations on which the adhesive surfaces 21A and 21B are attached may be on different members, or may be on different locations within a single member. As shown in FIG.
- the adhesive sheet 1 before use (that is, before being attached to an adherend) has a first adhesive surface 21A and a second adhesive surface 21B that are peeled off at least on the side facing the adhesive layer 21. It may be a component of the adhesive sheet 100 with a release liner that is protected by the release liners 31 and 32 serving as surfaces.
- the release liners 31 and 32 it is preferable to use, for example, a sheet-like base material (liner base material) that is constructed by providing a release layer made of a release treatment agent on one side so that one side becomes a release surface. obtain.
- the release liner 32 may be omitted and a release liner 31 having release surfaces on both sides may be used, and this and the adhesive sheet 1 may be overlapped and spirally wound so that the second adhesive surface 21B is on the release liner 31.
- the pressure-sensitive adhesive sheet with a release liner may be in a protected form (roll form) in contact with the back surface of the adhesive sheet.
- FIG. 2 The structure of a pressure-sensitive adhesive sheet according to another embodiment is schematically shown in FIG. 2.
- This adhesive sheet 2 is a base material comprising a sheet-shaped support base material (for example, a resin film) 10 having a first surface 10A and a second surface 10B, and an adhesive layer 21 provided on the first surface 10A side. It is constructed as a single-sided adhesive sheet.
- the adhesive layer 21 is fixedly provided on the first surface 10A side of the support base material 10, that is, without the intention of separating the adhesive layer 21 from the support base material 10. As shown in FIG.
- the pressure-sensitive adhesive sheet 2 before use has a surface (adhesive surface) 21A of the pressure-sensitive adhesive layer 21 protected by a release liner 31 having a release surface at least on the side facing the pressure-sensitive adhesive layer 21. It may be a component of the pressure-sensitive adhesive sheet 200 with a release liner. Alternatively, the release liner 31 may be omitted, the second surface 10B may be the release surface, and the adhesive sheet 2 may be wound so that the adhesive surface 21A is the second surface (back surface) of the support substrate 10. ) 10B may be in a protected form (roll form).
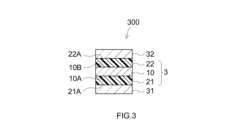
- FIG. 3 schematically shows the structure of a pressure-sensitive adhesive sheet according to yet another embodiment.
- This adhesive sheet 3 includes a sheet-shaped support base material (for example, a resin film) 10 having a first surface 10A and a second surface 10B, and a first adhesive layer 21 fixedly provided on the first surface 10A side. and a second adhesive layer 22 fixedly provided on the second surface 10B side.
- 32 may be a component of the adhesive sheet 300 with a release liner.
- the release liner 32 may be omitted and a release liner 31 having release surfaces on both sides may be used, and this and the adhesive sheet 3 may be overlapped and spirally wound so that the second adhesive surface 22A is on the release liner 31.
- the pressure-sensitive adhesive sheet with a release liner may be in a protected form (roll form) in contact with the back surface of the adhesive sheet.
- the first pressure-sensitive adhesive layer and the second pressure-sensitive adhesive layer may be the pressure-sensitive adhesive layer described below.
- the other adhesive layer (for example, the second adhesive layer) may be the adhesive layer disclosed herein, and the adhesive layer disclosed herein (specifically, the one adhesive layer described above) may be the adhesive layer disclosed herein.
- the adhesive layer may have a composition different from that of the adhesive layer (for example, the first adhesive layer).
- the other pressure-sensitive adhesive layer may be formed from a known or commonly used pressure-sensitive adhesive, for example.
- the adhesive layer constituting the adhesive sheet disclosed herein contains an acrylic polymer.
- the pressure-sensitive adhesive layer is typically a pressure-sensitive adhesive layer containing an acrylic polymer as a base polymer.
- Such an adhesive layer is also referred to as an acrylic adhesive layer.
- the base polymer refers to the main component of a rubbery polymer (a polymer that exhibits rubber elasticity in a temperature range around room temperature) contained in the adhesive layer.
- the term "main component” refers to a component contained in an amount exceeding 50% by weight, unless otherwise specified.
- the following description regarding the adhesive and the components that can be included in the adhesive layer is also applicable to the adhesive composition used to form the adhesive (layer) unless otherwise specified.
- the term "acrylic polymer” refers to a polymer containing monomer units derived from a monomer having at least one (meth)acryloyl group in one molecule, as monomer units constituting the polymer. .
- a monomer having at least one (meth)acryloyl group in one molecule will also be referred to as an "acrylic monomer.”
- an acrylic polymer in this specification is defined as a polymer containing monomer units derived from acrylic monomers.
- (meth)acryloyl” refers comprehensively to acryloyl and methacryloyl.
- (meth)acrylate” comprehensively refers to acrylate and methacrylate
- (meth)acrylic” comprehensively refers to acrylic and methacrylic.
- acrylic polymer As the acrylic polymer used in the technique disclosed herein, a polymer of monomer components containing n-heptyl acrylate is used. Acrylic polymers polymerized using monomer components containing n-heptyl acrylate have superior flexibility than polymers of other alkyl acrylates such as n-butyl acrylate (BA) and 2-ethylhexyl acrylate (2EHA). It can exhibit superior adhesive strength to both high polarity materials and low polarity materials. The reason for this is not particularly limited, but is because polymers containing n-heptyl acrylate as a monomer unit have relatively long linear side chains in addition to having a low glass transition temperature. This is thought to be due to the relatively large space between the main chains within the adhesive.
- BA n-butyl acrylate
- 2EHA 2-ethylhexyl acrylate
- the proportion of n-heptyl acrylate in the monomer components of the acrylic polymer is 50% by weight or more (for example, more than 50% by weight), preferably 70% by weight or more, more preferably 80% by weight. % or more, more preferably 85% by weight or more, particularly preferably 90% by weight or more, may be 92% by weight or more, may be 94% by weight or more, or may be 96% by weight or more.
- the proportion of n-heptyl acrylate in the monomer components is less than 97% by weight, and in some embodiments is 95% by weight or less.
- the content may be 93% by weight or less, or may be 91% by weight or less.
- the acrylic polymer may be copolymerized with an alkyl (meth)acrylate (hereinafter also referred to as "optional alkyl (meth)acrylate”) other than n-heptyl acrylate.
- an alkyl (meth)acrylate hereinafter also referred to as "optional alkyl (meth)acrylate
- optional alkyl (meth)acrylate for example, a compound represented by the following formula (1) can be suitably used.
- CH 2 C(R 1 )COOR 2 (1)
- R 1 in the above formula (1) is a hydrogen atom or a methyl group.
- R 2 is a chain alkyl group having 1 to 20 carbon atoms (excluding n-heptyl group).
- optional alkyl (meth)acrylate examples include methyl (meth)acrylate, ethyl (meth)acrylate, propyl (meth)acrylate, isopropyl (meth)acrylate, n-butyl (meth)acrylate, isobutyl (meth)acrylate, s -Butyl (meth)acrylate, pentyl (meth)acrylate, isopentyl (meth)acrylate, hexyl (meth)acrylate, heptyl methacrylate, 2-ethylhexyl (meth)acrylate, octyl (meth)acrylate, isooctyl (meth)acrylate, nonyl ( meth)acrylate, isononyl(meth)acrylate, decyl(meth)acrylate, isodecyl(meth)acrylate, undecyl(meth)acrylate, lauryl(meth)acryl
- optional alkyl (meth)acrylates can be used alone or in combination of two or more.
- Optional alkyl (meth)acrylates that may be preferably used include n-butyl acrylate (BA) and 2-ethylhexyl acrylate (2EHA).
- the proportion of optional alkyl (meth)acrylate included in the monomer component is, for example, in some embodiments less than 47% by weight, may be less than 45% by weight, may be less than 30% by weight, and may be less than 10% by weight. It may be less than 5% by weight, or less than 1% by weight.
- the technology disclosed herein can be preferably carried out in an embodiment in which the monomer component does not substantially contain any alkyl (meth)acrylate.
- the monomer component does not substantially contain monomer A (for example, the above-mentioned optional alkyl (meth)acrylate), it means that the monomer A is not used, at least intentionally, and the monomer component is For example, unintentional inclusion of about 0.01% by weight or less is acceptable.
- monomer A for example, the above-mentioned optional alkyl (meth)acrylate
- the monomer component may include an alkyl (meth)acrylate having a biomass-derived alkyl group at the ester end (hereinafter also referred to as "biomass alkyl (meth)acrylate”).
- biomass alkyl (meth)acrylate having a biomass-derived alkyl group at the ester end
- biomass alkyl (meth)acrylate it is possible to suitably realize an acrylic pressure-sensitive adhesive that is designed to reduce dependence on fossil resource-based materials.
- the biomass alkyl (meth)acrylate is not particularly limited, and is, for example, an ester of a biomass-derived alkanol and a biomass-derived or non-biomass-derived (meth)acrylic acid.
- alkanols derived from biomass include biomass ethanol, alkanols derived from plant materials such as palm oil, palm kernel oil, coconut oil, and castor oil.
- the biomass-derived alkanol has three or more carbon atoms, the alkanol may be linear or branched.
- an ester of a biomass-derived alkanol and a non-biomass-derived (meth)acrylic acid is used as the biomass alkyl (meth)acrylate used in the synthesis of the acrylic polymer.
- biomass alkyl (meth)acrylate In such a biomass alkyl (meth)acrylate, the greater the number of carbon atoms in the alkanol, the greater the number ratio of biomass-derived carbon to the total number of carbons contained in the biomass alkyl (meth)acrylate, that is, the biomass carbon ratio of the alkyl (meth)acrylate. becomes higher. Therefore, in the above-mentioned biomass alkyl (meth)acrylate, it is desirable that the alkyl group derived from biomass has a large number of carbon atoms in order to reduce dependence on fossil resource materials.
- biomass-derived n-heptyl acrylate (biomass n-heptyl acrylate) is used as n-heptyl acrylate.
- biomass n-heptyl acrylate is an ester of a biomass-derived alkanol and a biomass-derived or non-biomass-derived acrylic acid.
- an ester of a biomass-derived alkanol and a non-biomass-derived acrylic acid can be used. In such compounds, only the linear heptyl groups are derived from biomass.
- the proportion of biomass alkyl (meth)acrylate (preferably biomass n-heptyl acrylate) in the monomer components of the acrylic polymer is, for example, 50% by weight or more (for example, more than 50% by weight) in some embodiments, Preferably 70% by weight or more, more preferably 80% by weight or more, further preferably 85% by weight or more, particularly preferably 90% by weight or more, may be 92% by weight or more, may be 94% by weight or more, and may be 96% by weight. The above is fine.
- the proportion of biomass alkyl (meth)acrylate (preferably biomass n-heptyl acrylate) among the monomer components is less than 97% by weight, and in some embodiments may be 95% by weight or less, and in some embodiments may be 93% by weight or less. % or less, or 91% by weight or less. In some other embodiments, the proportion of biomass alkyl (meth)acrylate in the monomer component may be 90% by weight or less, 70% by weight or less, 50% by weight or less, 30% by weight or less, It may be 10% by weight or less, or 1% by weight or less.
- the monomer component of the acrylic polymer contains more than 3% by weight of a carboxyl group-containing monomer.
- Carboxy group-containing monomers can exhibit improved cohesiveness based on their polarity.
- a crosslinking agent such as an isocyanate type crosslinking agent or an epoxy type crosslinking agent
- the carboxy group can serve as a crosslinking point of the acrylic polymer.
- carboxy group-containing monomers examples include acrylic acid (AA), methacrylic acid (MAA), carboxyethyl (meth)acrylate, carboxypentyl (meth)acrylate, itaconic acid, maleic acid, fumaric acid, crotonic acid, isocrotonic acid, etc. be done.
- preferred carboxy group-containing monomers include AA and MAA.
- AA is particularly preferred.
- Carboxy group-containing monomers can be used singly or in combination of two or more.
- the proportion of the carboxy group-containing monomer in the monomer component of the acrylic polymer is more than 3% by weight (specifically more than 3.0% by weight), preferably 4.0% by weight or more, more preferably 4% by weight or more.
- the content is .5% by weight or more, more preferably 5.0% by weight or more (for example, more than 5.0% by weight), particularly preferably 5.5% by weight or more, and may be 6.0% by weight or more.
- the proportion of the carboxy group-containing monomer in the monomer component may be 7.0% by weight or more, 8.0% by weight or more (for example, more than 8.0% by weight), and 9. It may be 0% by weight or more.
- the amount of the carboxy group-containing monomer is, for example, suitably 20% by weight or less of the total monomer components, preferably 15% by weight or less, and more preferably 12% by weight or less. In some embodiments, the amount of the carboxy group-containing monomer may be less than 10% by weight, may be less than 8% by weight, may be less than 6% by weight, and may be less than 5% by weight.
- the acrylic polymer may be copolymerized with a functional group-containing monomer (any functional group-containing monomer) other than the carboxy group-containing monomer.
- a functional group-containing monomer any functional group-containing monomer
- optional functional group-containing monomers that can introduce functional groups that can serve as crosslinking base points into acrylic polymers or contribute to improving adhesive strength include hydroxyl group (OH group)-containing monomers (2-hydroxyethyl (meth)acrylate, 2-hydroxyethyl (meth)acrylate, 2- Hydroxyalkyl (meth)acrylates such as hydroxypropyl (meth)acrylate, 3-hydroxypropyl (meth)acrylate, 2-hydroxybutyl (meth)acrylate, 4-hydroxybutyl (meth)acrylate; polypropylene glycol mono(meth)acrylate, etc.
- OH group hydroxyl group
- 2-hydroxyethyl (meth)acrylate 2-hydroxyethyl (meth)acrylate
- acid anhydride group-containing monomers acid anhydride group-containing monomers, amide group-containing monomers ((meth)acrylamide, N,N-dimethyl(meth)acrylamide, etc.), amino group-containing monomers (aminoethyl (meth)acrylate, N,N-dimethylaminoethyl (meth)acrylate, etc.), epoxy group-containing monomers, cyano group-containing monomers, keto group-containing monomers, monomers with nitrogen atom-containing rings (N-vinyl-2-pyrrolidone, N-(meth)acryloylmorpholine, etc.), alkoxysilyl Examples include group-containing monomers, imide group-containing monomers, and the like. The above arbitrary functional group-containing monomers can be used alone or in combination of two or more.
- the content of the optional functional group-containing monomer in the monomer component is not particularly limited.
- the content of the optional functional group-containing monomer in the monomer component can be, for example, 0.1% by weight or more, and 0.5% by weight or more. It is appropriate that the amount is 1% by weight or more.
- the content of the arbitrary functional group-containing monomer in the monomer component is 40% by weight or less.
- the content of optional functional group-containing monomers in the monomer component is, for example, less than 3% by weight, may be less than 1% by weight, may be less than 0.5% by weight, and may be less than 0.3% by weight. % or less than 0.1% by weight.
- the technique disclosed herein can be preferably carried out in an embodiment in which the monomer component of the acrylic polymer does not substantially contain any functional group-containing monomer.
- the content thereof may generally be approximately 0.001% by weight or more of the total monomer components, or approximately 0.01% by weight or more, It may be approximately 0.02% by weight or more.
- the content of the hydroxyl group-containing monomer is suitably about 10% by weight or less, preferably about 5% by weight or less, more preferably about 2% by weight or less, based on the total monomer components.
- the content of hydroxyl group-containing monomer in the monomer component may be less than 1% by weight, may be less than 0.5% by weight, may be less than 0.3% by weight, and may be less than 0.1% by weight. % or less than 0.01% by weight.
- the monomer component of the acrylic polymer may be substantially free of hydroxyl group-containing monomers. According to the technology disclosed herein, desired effects can be achieved without relying on hydroxyl group-containing monomers.
- the ratio of the carboxy group-containing monomer to the total functional group-containing monomers (total functional group-containing monomers including the carboxy group-containing monomer) used as a copolymerization component of the acrylic polymer is determined by the effect of copolymerizing the carboxy group-containing monomer.
- the content is suitably 30% by weight or more, preferably 50% by weight or more, more preferably 70% by weight or more, further preferably 80% by weight or more, particularly preferably 90% by weight or more, such as It may be 95% by weight or more, 97% by weight or more, 98% by weight or more, or 99% by weight or more (for example, 99.9% by weight or more).
- the upper limit of the proportion of the carboxy group-containing monomer to the total of the functional group-containing monomers is 100% by weight, and may be, for example, 95% by weight or less.
- the monomer components constituting the acrylic polymer may contain copolymerization components other than the above-mentioned functional group-containing monomers for the purpose of improving cohesive force and the like.
- copolymerization components include vinyl ester monomers such as vinyl acetate; aromatic vinyl compounds such as styrene; cycloalkyl (meth)acrylates such as cyclohexyl (meth)acrylate, cyclopentyl (meth)acrylate, and isobornyl ) acrylates; aryl (meth)acrylates (e.g. phenyl (meth)acrylate), aryloxyalkyl (meth)acrylates (e.g.
- Aromatic ring-containing (meth)acrylate Olefinic monomer; Chlorine-containing monomer; Isocyanate group-containing monomer such as 2-(meth)acryloyloxyethyl isocyanate; Alkoxy such as methoxyethyl (meth)acrylate and ethoxyethyl (meth)acrylate Group-containing monomers; vinyl ether monomers such as methyl vinyl ether and ethyl vinyl ether; and the like.
- the other copolymerization components mentioned above can be used alone or in combination of two or more.
- the amount of such other copolymerized components is not particularly limited as long as it can be selected as appropriate depending on the purpose and use, but from the viewpoint of appropriately exhibiting the effects of use, it is appropriate to set it to 0.05% by weight or more. , 0.5% by weight or more.
- the content of other copolymer components in the monomer components is 20% by weight or less, so that the adhesive properties based on the essential monomer components can be suitably exhibited. From this point of view, it is preferably 10% by weight or less, more preferably 8% by weight or less, still more preferably less than 5% by weight, for example, it may be less than 3% by weight, and may be less than 1% by weight.
- the technology disclosed herein can also be preferably practiced in an embodiment in which the monomer component does not substantially contain other copolymer components.
- Acrylic polymers are polyfunctional polymers that have at least two polymerizable functional groups (typically radically polymerizable functional groups) having unsaturated double bonds, such as (meth)acryloyl groups and vinyl groups, as other monomer components. It may also contain monomers. By using a polyfunctional monomer as a monomer component, the cohesive force of the adhesive layer can be increased. Polyfunctional monomers can be used as crosslinking agents.
- the polyfunctional monomer is not particularly limited, and includes, for example, 1,6-hexanediol di(meth)acrylate, trimethylolpropane tri(meth)acrylate, dipentaerythritol hexa(meth)acrylate, and neopentyl glycol di(meth)acrylate. etc.
- One type of polyfunctional monomer can be used alone or two or more types can be used in combination.
- the amount of the polyfunctional monomer used is not particularly limited, and can be appropriately set so that the intended use of the polyfunctional monomer is achieved.
- the amount of the polyfunctional monomer used can be about 3% by weight or less of the monomer components, preferably about 2% by weight or less, and more preferably about 1% by weight or less (for example, about 0.5% by weight or less).
- the lower limit of the amount used when using a polyfunctional monomer is not particularly limited, as long as it is greater than 0% by weight.
- the effect of using the polyfunctional monomer can be appropriately exhibited by setting the amount of the polyfunctional monomer to be approximately 0.001% by weight or more (for example, approximately 0.01% by weight or more) of the monomer components.
- biomass carbon ratio of the monomer component constituting the acrylic polymer may be, for example, 1% or more, suitably 10% or more, preferably 30% or more, and more preferably is 50% or more (for example, more than 50%), may be 70% or more, may be 80% or more, or may be 90% to 100%.
- the method for obtaining the acrylic polymer is not particularly limited, and various polymerization methods known as methods for synthesizing acrylic polymers, such as solution polymerization, emulsion polymerization, bulk polymerization, suspension polymerization, and photopolymerization, can be used. may be adopted as appropriate.
- a solution polymerization method can be preferably employed.
- a monomer supply method when performing solution polymerization a batch charging method in which all monomer raw materials are supplied at once, a continuous supply (dropping) method, a divided supply (dropping) method, etc. can be appropriately adopted.
- the polymerization temperature can be selected as appropriate depending on the type of monomer and solvent used, the type of polymerization initiator, etc., and is, for example, about 20°C to 170°C (typically about 40°C to 140°C). I can do it.
- the solvent (polymerization solvent) used for solution polymerization can be appropriately selected from conventionally known organic solvents.
- aromatic compounds such as toluene (typically aromatic hydrocarbons); acetate esters such as ethyl acetate; aliphatic or alicyclic hydrocarbons such as hexane and cyclohexane; 1,2-dichloroethane, etc. halogenated alkanes; lower alcohols such as isopropyl alcohol (for example, monohydric alcohols having 1 to 4 carbon atoms); ethers such as tert-butyl methyl ether; ketones such as methyl ethyl ketone; etc. Any one type of solvent or a mixed solvent of two or more types can be used.
- the initiator used for polymerization can be appropriately selected from conventionally known polymerization initiators depending on the type of polymerization method.
- one or more azo polymerization initiators such as 2,2'-azobisisobutyronitrile (AIBN) can be preferably used.
- Other examples of polymerization initiators include persulfates such as potassium persulfate; peroxide initiators such as benzoyl peroxide (BPO) and hydrogen peroxide; substituted ethane initiators such as phenyl-substituted ethane; aromatic carbonyl compounds; and the like.
- Still another example of the polymerization initiator is a redox initiator using a combination of a peroxide and a reducing agent.
- Such polymerization initiators can be used alone or in combination of two or more.
- the amount of the polymerization initiator used may be any normal amount, for example, approximately 0.005 to 1 part by weight (typically approximately 0.01 to 1 part by weight) per 100 parts by weight of all monomer components. degree).
- the acrylic polymer an acrylic polymer having a weight average molecular weight (Mw) of more than 600,000 is used.
- Mw weight average molecular weight
- the Mw of the acrylic polymer is preferably 650,000 or more.
- the Mw of the acrylic polymer is suitably 700,000 or more, preferably 750,000 or more, more preferably 800,000 or more, even more preferably 850,000 or more, particularly preferably 900,000 or more (e.g. (over 900,000).
- the Mw of the acrylic polymer By setting the Mw of the acrylic polymer high to increase the cohesive force of the adhesive, it is possible to adopt, for example, a monomer composition that emphasizes adhesive force to different materials.
- the Mw of the acrylic polymer is usually about 3 million or less, preferably 2 million or less, more preferably 1.5 million or less, More preferably, it is 1.2 million or less, particularly preferably 1,000,000 or less.
- the Mw of the acrylic polymer may be 900,000 or less, 850,000 or less, 800,000 or less, 750,000 or less, 700,000 or less, It may be less than 10,000.
- the Mw of the acrylic polymer can be measured by gel permeation chromatography (GPC) and determined as a value in terms of standard polystyrene. Specifically, it can be determined by measuring under the following conditions using a GPC measurement device with the trade name "HLC-8220GPC" (manufactured by Tosoh Corporation). The same applies to the embodiments described later.
- GPC gel permeation chromatography
- the adhesive layer includes a tackifying resin.
- a tackifying resin By using a tackifying resin, it is possible to improve adhesion to high polarity materials and low polarity materials. Furthermore, by using an appropriate amount of tackifier resin, the gel fraction of the adhesive layer can be adjusted to an appropriate range.
- the tackifier resin is not particularly limited and includes, for example, rosin-based tackifier resin, terpene-based tackifier resin, hydrocarbon-based tackifier resin, epoxy-based tackifier resin, polyamide-based tackifier resin, elastomer-based tackifier resin, Various tackifying resins such as phenolic tackifying resins and ketone tackifying resins can be used. Such tackifying resins can be used alone or in combination of two or more.
- rosin-based tackifying resins include unmodified rosin (raw rosin) such as gum rosin, wood rosin, and tall oil rosin; Hydrogenated rosin, disproportionated rosin, polymerized rosin, other chemically modified rosin, etc. (the same applies hereinafter); and other various rosin derivatives.
- unmodified rosin raw rosin
- Hydrogenated rosin disproportionated rosin
- polymerized rosin other chemically modified rosin, etc.
- other various rosin derivatives include rosins such as those obtained by esterifying unmodified rosin with alcohols (i.e., esterified products of rosin), and those obtained by esterifying modified rosin with alcohols (i.e., esterified products of modified rosin).
- Esters Unsaturated fatty acid-modified rosins, which are unmodified rosin or modified rosin modified with unsaturated fatty acids; Unsaturated fatty acid-modified rosin esters, which are rosin esters modified with unsaturated fatty acids; Unmodified rosin, modified rosin, unsaturated Rosin alcohols obtained by reducing the carboxyl group in fatty acid-modified rosins or unsaturated fatty acid-modified rosin esters; metal salts of rosins (especially rosin esters) such as unmodified rosin, modified rosin, and various rosin derivatives; rosins; Examples include rosin phenol resins obtained by adding phenol to (unmodified rosin, modified rosin, various rosin derivatives, etc.) with an acid catalyst and thermally polymerizing them. Among them, rosin ester is preferred.
- rosin esters include esters of unmodified rosin or modified rosin (hydrogenated rosin, disproportionated rosin, polymerized rosin, etc.), such as methyl ester, triethylene glycol ester, glycerin ester. , pentaerythritol ester and the like.
- terpene-based tackifying resins examples include terpene resins such as ⁇ -pinene polymer, ⁇ -pinene polymer, and dipentene polymer; Modified terpene resins (modified etc.); etc.
- modified terpene resin is terpene phenol resin.
- Terpene phenol resin refers to a polymer containing terpene residues and phenol residues, and includes copolymers of terpenes and phenol compounds (terpene-phenol copolymer resins), and homopolymers or copolymers of terpenes. This concept includes both phenol-modified products (phenol-modified terpene resins). Specific examples of terpenes constituting such terpene phenol resin include monoterpenes such as ⁇ -pinene, ⁇ -pinene, and limonene (including d-form, l-form, and d/l-form (dipentene)); can be mentioned.
- the hydrogenated terpene phenol resin refers to a hydrogenated terpene phenol resin having a structure obtained by hydrogenating such a terpene phenol resin. Sometimes called hydrogenated terpene phenolic resin.
- hydrocarbon-based tackifying resins examples include aliphatic (C5-based) petroleum resins, aromatic (C9-based) petroleum resins, aliphatic/aromatic copolymerized (C5/C9-based) petroleum resins, and Hydrogenated substances (e.g., alicyclic petroleum resins obtained by hydrogenating aromatic petroleum resins), various modified products thereof (e.g., maleic anhydride modified products), coumaron-based resins, coumaron-indene-based resins Examples include various hydrocarbon resins such as.
- rosin-based tackifying resins and terpene-based tackifying resins include embodiments in which the tackifying resin contains only rosin-based tackifying resins, and embodiments in which the tackifying resin contains terpene-based tackifying resins (e.g., terpene-based tackifying resins).
- the tackifying resin contains only a phenolic resin
- the tackifying resin includes a rosin-based tackifying resin and a terpene-based tackifying resin.
- the total proportion of the rosin-based tackifying resin and the terpene-based tackifying resin in the entire tackifying resin contained in the adhesive layer is, for example, approximately more than 50% by weight (more than 50% by weight and not more than 100% by weight). ), and may be about 70% by weight or more, about 80% by weight or more, about 90% by weight or more, about 95% by weight or more, or about 99% by weight or more.
- a rosin-based tackifier resin as the tackifier resin.
- the adhesive strength to high polarity materials and low polarity materials can be preferably improved.
- rosin ester is preferred.
- the proportion of the rosin-based tackifying resin in the entire tackifying resin contained in the adhesive layer can be, for example, about 50% by weight or more, about 70% by weight or more, or about 80% by weight or more. .
- the technology disclosed herein is preferably in an embodiment in which substantially all of the tackifier resin (for example, approximately 97% by weight or more, or 99% by weight or more, and may be 100% by weight) is a rosin-based tackifier resin. can be implemented.
- the content ratio of the tackifying resin other than the rosin-based tackifying resin (non-rosin-based tackifying resin) in the adhesive layer is, for example, based on 100 parts by weight of the acrylic polymer. It is appropriate that the amount is 40 parts by weight or less. Thereby, the effect of including rosin ester is suitably exhibited.
- the amount of the non-rosin tackifier resin used is preferably about 20 parts by weight or less (for example, less than 20 parts by weight), and may be about 10 parts by weight or less, based on 100 parts by weight of the acrylic polymer. The amount may be 5 parts by weight or less, or approximately 1 part by weight or less.
- the content of the terpene phenol resin as the tackifier resin in the adhesive layer is, for example, 40 parts by weight or less, and 35 parts by weight or less, based on 100 parts by weight of the acrylic polymer. It may be 30 parts by weight or less, 20 parts by weight or less, 15 parts by weight or less, 10 parts by weight or less, or 5 parts by weight or less.
- the content of the terpene phenol resin being X parts by weight or less based on 100 parts by weight of the acrylic polymer means that the adhesive layer does not contain the terpene phenol resin and that the terpene phenol resin is not contained in the acrylic polymer.
- the term is used to include both the inclusion of X parts by weight or less per 100 parts by weight.
- the content of the terpene phenol resin in the adhesive layer may be 3 parts by weight or less, and 1 part by weight or less (for example, 0 to 0.1 parts by weight) based on 100 parts by weight of the acrylic polymer.
- the content of the terpene phenol resin in the adhesive layer may be, for example, 1 part by weight or more, 3 parts by weight or more, 5 parts by weight or more, based on 100 parts by weight of the acrylic polymer. It may be at least 7 parts by weight, or at least 9 parts by weight.
- the content of the terpene phenol resin in the adhesive layer is 10 parts by weight or more, and 12 parts by weight or more based on 100 parts by weight of the acrylic polymer. The amount may be 14 parts by weight or more.
- terpene phenol resin By using an appropriate amount of terpene phenol resin, it is possible to improve the adhesive strength to high polarity materials while maintaining the adhesive strength to low polarity materials.
- the amount of terpene phenol resin used in the above range applies to either the embodiment in which only terpene phenol resin is used as the tackifier resin, or the embodiment in which terpene phenol resin and non-terpene phenol resin (preferably rosin-based tackifier resin) are used in combination. is also applicable.
- the amount of terpene phenol resin used within the above range may be preferably employed, for example, in embodiments in which the terpene phenol resin is used in combination with a non-terpene phenol resin (preferably a rosin-based tackifying resin).
- the tackifying resin is a tackifying resin T L having a softening point of less than 150°C.
- the softening point of the tackifier resin T L may be lower than 140°C or lower than 130°C from the viewpoint of improving adhesive strength to high polarity materials and low polarity materials.
- the softening point of the tackifier resin T L is less than 120°C, suitably less than 110°C, preferably about 105°C or less, more preferably about 100°C or less, More preferably, the temperature is about 95°C or less (for example, less than 95°C), particularly preferably about 90°C or less (for example, about 85°C or less).
- the lower limit of the softening point of the tackifier resin T L is not particularly limited. In some embodiments, the softening point of the tackifier resin T L may be approximately 50° C. or higher, approximately 60° C. or higher, or approximately 65° C. or higher, for example, from the viewpoint of exhibiting appropriate cohesive force. The temperature may be approximately 70°C or higher. In some other embodiments, the softening point of the tackifier resin T L may be, for example, about 80°C or higher, about 90°C or higher, about 100°C or higher, or about 110°C or higher.
- the tackifier resin T L one type suitably selected from among the tackifier resins exemplified above having a softening point of less than 150°C can be used alone or in combination of two or more types.
- the tackifying resin T L preferably includes at least one selected from rosin-based tackifying resins and terpene-based tackifying resins, and more preferably includes rosin-based tackifying resins.
- the tackifying resin T L may contain one type of rosin-based tackifying resin alone, or may contain a combination of two or more types of rosin-based tackifying resin.
- examples of rosin-based tackifier resins that can be preferably employed as the tackifier resin T L include rosin esters such as unmodified rosin esters and modified rosin esters.
- a suitable example of the modified rosin ester is a hydrogenated rosin ester.
- esters of unmodified rosin or modified rosin eg, hydrogenated rosin
- rosin esters such as methyl ester, glycerin ester, etc.
- the tackifier resin T L includes a hydrogenated rosin ester.
- the tackifier resin T L may also include a non-hydrogenated rosin ester.
- non-hydrogenated rosin ester is a concept that comprehensively refers to rosin esters other than hydrogenated rosin esters mentioned above. Examples of non-hydrogenated rosin esters include unmodified rosin esters, disproportionated rosin esters and polymerized rosin esters.
- the tackifier resin T L may contain a combination of a hydrogenated rosin ester and a non-hydrogenated rosin ester as rosin esters, or may contain only one or more hydrogenated rosin esters. It may contain only one species or two or more non-hydrogenated rosin esters.
- the adhesive layer according to some preferred embodiments contains only one or more hydrogenated rosin esters as the rosin esters contained in the tackifier resin T L.
- the tackifying resin T L may contain other tackifying resins in addition to the rosin-based tackifying resin.
- the other tackifier resin one type suitably selected from the tackifier resins exemplified above having a softening point of less than 150°C can be used alone or in combination of two or more types.
- the proportion of the rosin-based tackifier resin in the entire tackifier resin T L can be, for example, more than about 50% by weight, may be about 65% by weight or more, and may be about 75% by weight or more. Good too.
- the technology disclosed herein is an embodiment in which substantially all of the tackifier resin T L (for example, approximately 97% by weight or more, or 99% by weight or more, and may be 100% by weight) is a rosin-based tackifier resin. It can be preferably carried out.
- tackifying resin T L for example, a tackifying resin having a softening point of less than 50°C, more preferably approximately 40°C or less (typically a rosin-based, terpene-based, hydrocarbon-based, etc. tackifying resin, e.g. Hydrogenated rosin methyl ester, etc.) may or may not be included.
- a low softening point tackifier resin may be a liquid tackifier resin that exhibits a liquid state at 30°C.
- the liquid tackifying resin can be used alone or in combination of two or more.
- the content of the liquid tackifying resin can be approximately 30% by weight or less of the entire tackifier resin T L from the viewpoint of cohesive force etc., and should be approximately 10% by weight or less (for example, 0 to 10% by weight). is suitable, and may be approximately 2% by weight or less (0.5 to 2% by weight), and may be less than 1% by weight.
- the content of the tackifying resin T L (the total amount when two or more types of tackifying resin T L are included) is not particularly limited, but is about 100 parts by weight or less (for example, 100 parts by weight) based on 100 parts by weight of the acrylic polymer. It is appropriate to set it as less than By limiting the amount of the tackifying resin T L used to a predetermined amount or less, it is possible to improve adhesive strength to high polarity materials and low polarity materials while maintaining sufficient holding power. In some preferred embodiments, the amount of the tackifier resin T L used is suitably 90 parts by weight or less, preferably 80 parts by weight, based on 100 parts by weight of the acrylic polymer, from the viewpoint of holding power etc.
- the amount of the tackifier resin T L to be used may be 35 parts by weight or less, 30 parts by weight or less, or 25 parts by weight or less based on 100 parts by weight of the acrylic polymer. In most cases, the amount may be 20 parts by weight or less (for example, less than 20 parts by weight). In some embodiments, from the viewpoint of improving adhesive strength, the amount of the tackifying resin T L used is, for example, more than 10 parts by weight, and not less than 12 parts by weight, based on 100 parts by weight of the acrylic polymer.
- the amount may be 14 parts by weight or more, it may be more than 15 parts by weight, suitably 20 parts by weight or more, preferably 30 parts by weight or more, more preferably 35 parts by weight or more, still more preferably 38 parts by weight or more. , may be 45 parts by weight or more, 50 parts by weight or more (for example, more than 50 parts by weight), 55 parts by weight or more, 60 parts by weight or more, 65 parts by weight or more, 70 parts by weight or more. Often, the amount may be 75 parts by weight or more.
- the acrylic polymer containing n-heptyl acrylate as a monomer unit used in the technology disclosed herein has good compatibility with the tackifier resin, so it can be used to maintain retention power by incorporating a larger amount of the tackifier resin. , it is possible to improve adhesion to different materials.
- the adhesive layer may include a combination of a tackifying resin T L and a tackifying resin T H having a softening point of 150° C. or higher (eg, 150° C. to 200° C.).
- a tackifying resin T H having a softening point of 150° C. or higher (eg, 150° C. to 200° C.).
- the tackifier resin T H one kind or a combination of two or more kinds of tackifier resins having a softening point of 150° C. or more among the tackifier resins exemplified above can be used.
- the softening point of the tackifier resin in this specification is defined as a value measured based on the softening point test method (ring and ball method) specified in JIS K5902 and JIS K2207. Specifically, the sample is melted as quickly as possible at the lowest possible temperature, and the sample is carefully filled into a ring placed on a flat metal plate, taking care not to form bubbles. After it has cooled down, use a slightly heated knife to cut off the raised part from the plane including the top of the ring.
- a supporter (ring stand) is placed in a glass container (heating bath) with a diameter of 85 mm or more and a height of 127 mm or more, and glycerin is poured into the container to a depth of 90 mm or more.
- the steel ball (diameter 9.5 mm, weight 3.5 g) and the ring filled with the sample were immersed in glycerin without coming into contact with each other, and the temperature of the glycerin was maintained at 20°C plus or minus 5°C for 15 minutes. .
- a steel ball is then placed in the center of the surface of the sample in the ring and placed in position on the support.
- thermometer place a thermometer, set the center of the mercury bulb of the thermometer at the same height as the center of the ring, and heat the container.
- the flame of the Bunsen burner used for heating should be halfway between the center of the bottom of the container and the edge to ensure even heating. Note that the rate at which the bath temperature increases after heating starts and reaches 40°C must be 5.0 plus or minus 0.5°C per minute.
- the sample gradually softens and flows down from the ring, and the temperature at which it finally touches the bottom plate is read, and this is taken as the softening point.
- the softening point is measured at two or more points at the same time, and the average value is used.
- the tackifier resin T L accounts for more than 50% by weight of the total amount of tackifier resins included in the adhesive layer. Thereby, the effect of containing the tackifying resin TL tends to be effectively expressed.
- the proportion of the tackifying resin T L in the total amount of the tackifying resin contained in the adhesive layer is preferably 60% by weight or more, more preferably The content is 70% by weight or more, more preferably 80% by weight or more, particularly preferably 90% by weight or more, may be 95% by weight or more, or may be 98% by weight or more.
- the tackifier resin contained in the adhesive layer consists essentially only of tackifier resin TL . In this embodiment, the proportion of the tackifying resin T L in the total amount of tackifying resin contained in the adhesive layer is in the range of 99 to 100% by weight.
- the tackifying resin may include a tackifying resin having a hydroxyl value of less than 70 mgKOH/g.
- tackifier resins with a hydroxyl value of less than 60 mgKOH/g (more preferably less than 50 mgKOH/g, still more preferably less than 45 mgKOH/g) are preferred.
- a tackifier resin having a hydroxyl value of less than 70 mgKOH/g may be referred to as a "low hydroxyl value resin".
- an adhesive layer having high adhesive strength to both high polarity materials and low polarity materials can be preferably realized.
- the lower limit of the hydroxyl value of the low hydroxyl value resin is 0 mgKOH/g or more, may be about 10 mgKOH/g or more, or may be about 15 mgKOH/g or more.
- One type of low hydroxyl value resin can be used alone or two or more types can be used in combination.
- As the low hydroxyl value resin one type suitably selected from the tackifying resins exemplified above having a hydroxyl value of less than 70 mgKOH/g can be used alone or in combination of two or more types.
- the low hydroxyl value resin preferably includes at least one selected from rosin-based tackifier resins and terpene-based tackifier resins, and more preferably includes rosin-based tackifier resins.
- the low hydroxyl value resin may contain one type of rosin-based tackifier resin alone, or may contain a combination of two or more types of rosin-based tackifier resin.
- the low hydroxyl value resin may be the above-mentioned tackifier resin T L or tackifier resin T H , and it is preferable that the tackifier resin T L is a low hydroxyl value resin.
- the low hydroxyl value resin preferably accounts for more than 50% by weight of the total amount of tackifying resin contained in the adhesive layer. Thereby, it is possible to preferably improve the adhesion strength to low polarity materials.
- the proportion of the low hydroxyl value resin in the total amount of tackifier resin contained in the adhesive layer is preferably 60% by weight from the viewpoint of more effectively exhibiting the effect of using the low hydroxyl value resin.
- the content is more preferably 70% by weight or more, further preferably 80% by weight or more, particularly preferably 90% by weight or more, may be 95% by weight or more, or may be 98% by weight or more.
- the tackifying resin contained in the adhesive layer consists essentially of a low hydroxyl value resin.
- the proportion of the low hydroxyl value resin in the total amount of tackifier resin contained in the adhesive layer is in the range of 99 to 100% by weight.
- the adhesive layer disclosed herein has a content of a tackifying resin having a hydroxyl value of 70 mgKOH/g or more (hereinafter also referred to as "high hydroxyl value resin”) as a tackifying resin. is preferably less than 5 parts by weight based on 100 parts by weight of the acrylic polymer.
- the content of the high hydroxyl value resin being less than 5 parts by weight based on 100 parts by weight of the acrylic polymer means that the adhesive layer does not contain the high hydroxyl value resin and that the adhesive layer does not contain the high hydroxyl value resin.
- the content of the high hydroxyl value resin in the adhesive layer is preferably less than 3 parts by weight based on 100 parts by weight of the acrylic polymer, and is in the range of 1 part by weight or less (for example, 0 to 0.1 parts by weight). It is more preferable that there be.
- a value measured by the potentiometric titration method specified in JIS K0070:1992 can be adopted.
- the specific measurement method is as shown below.
- [Method for measuring hydroxyl value] 1.
- Reagent (1) As the acetylation reagent, take about 12.5 g (about 11.8 mL) of acetic anhydride, add pyridine to make a total volume of 50 mL, and stir thoroughly. Alternatively, take about 25 g (about 23.5 mL) of acetic anhydride, add pyridine to make a total volume of 100 mL, stir thoroughly, and use.
- (2) A 0.5 mol/L potassium hydroxide ethanol solution is used as the measurement reagent.
- the adhesive layer disclosed herein includes a tackifier resin
- a tackifier resin derived from plants is preferably used as the tackifier resin. It can work.
- the vegetable tackifier resin include the above-mentioned rosin-based tackifier resin and terpene-based tackifier resin.
- the vegetable tackifying resins can be used alone or in combination of two or more.
- the proportion of the vegetable tackifying resin in the total amount of the tackifying resin is 30% by weight or more (for example, 50% by weight or more, typically 80% by weight). above) is preferable.
- the proportion of vegetable tackifying resin in the total amount of tackifying resin is 90% by weight or more (eg, 95% by weight or more, typically 99-100% by weight).
- the technology disclosed herein can be preferably implemented in an embodiment that does not substantially contain tackifying resins other than vegetable tackifying resins.
- the content of the tackifying resin (the total amount when two or more tackifying resins are included) is not particularly limited, but is about 100 parts by weight or less (for example, less than 100 parts by weight) based on 100 parts by weight of the acrylic polymer. It is appropriate to do so.
- the amount of tackifying resin used is suitably 90 parts by weight or less, preferably 80 parts by weight or less, based on 100 parts by weight of the acrylic polymer, from the viewpoint of holding power etc.
- the amount may be 60 parts by weight or less, 55 parts by weight or less, 50 parts by weight or less, or 45 parts by weight or less. In some other embodiments, the amount of the tackifying resin used may be 35 parts by weight or less, 30 parts by weight or less, or 25 parts by weight or less, based on 100 parts by weight of the acrylic polymer. It may be 20 parts by weight or less (for example, less than 20 parts by weight). Further, in some embodiments, from the viewpoint of improving adhesive strength, the amount of the tackifying resin used is, for example, more than 10 parts by weight, and may be 12 parts by weight or more, based on 100 parts by weight of the acrylic polymer.
- the acrylic polymer containing n-heptyl acrylate as a monomer unit used in the technology disclosed herein has good compatibility with the tackifier resin, so it can be used to maintain retention power by incorporating a larger amount of the tackifier resin. , it is possible to improve adhesion to different materials.
- the total content of the acrylic polymer and tackifying resin in the adhesive layer is appropriately set and limited to a specific range so that the effects of the technology disclosed herein are exhibited. It's not something you can do.
- the total amount (total amount) of the acrylic polymer and tackifier resin contained in the adhesive layer is more than 50% by weight from the viewpoint of preferably exhibiting the effects of the technology disclosed herein. is appropriate, preferably about 70% by weight or more, more preferably about 90% by weight or more, even more preferably 95% by weight or more (for example, 95% by weight or more and 100% by weight or less, or less than 100% by weight), and 97% by weight. % or more.
- the adhesive composition used to form the adhesive layer may contain a crosslinking agent as necessary.
- the type of crosslinking agent is not particularly limited, and examples include isocyanate crosslinking agents, epoxy crosslinking agents, oxazoline crosslinking agents, aziridine crosslinking agents, melamine crosslinking agents, peroxide crosslinking agents, urea crosslinking agents, and metals. Examples include alkoxide crosslinking agents, metal chelate crosslinking agents, metal salt crosslinking agents, carbodiimide crosslinking agents, hydrazine crosslinking agents, amine crosslinking agents, and silane coupling agents.
- crosslinking agent can be used alone or two or more types can be used in combination. Among these, isocyanate crosslinking agents, epoxy crosslinking agents, oxazoline crosslinking agents, aziridine crosslinking agents, and melamine crosslinking agents are preferred, and isocyanate crosslinking agents and epoxy crosslinking agents are more preferred.
- the pressure-sensitive adhesive layer can obtain cohesive force, and can preferably achieve both adhesion and holding power to different materials.
- the adhesive layer in the technology disclosed herein may contain the crosslinking agent in a form after a crosslinking reaction, a form before a crosslinking reaction, a partially crosslinked form, an intermediate or composite form thereof, etc. May contain.
- the crosslinking agent is typically contained in the adhesive layer exclusively in the form after crosslinking reaction.
- polyfunctional isocyanates referring to compounds having an average of two or more isocyanate groups per molecule, including those having an isocyanurate structure
- the isocyanate crosslinking agents can be used alone or in combination of two or more.
- polyfunctional isocyanates include aliphatic polyisocyanates, alicyclic polyisocyanates, aromatic polyisocyanates, and the like.
- aliphatic polyisocyanates include 1,2-ethylene diisocyanate; tetramethylene diisocyanates such as 1,2-tetramethylene diisocyanate, 1,3-tetramethylene diisocyanate, and 1,4-tetramethylene diisocyanate; 1,2-tetramethylene diisocyanate; - hexamethylene diisocyanate such as hexamethylene diisocyanate, 1,3-hexamethylene diisocyanate, 1,4-hexamethylene diisocyanate, 1,5-hexamethylene diisocyanate, 1,6-hexamethylene diisocyanate, 2,5-hexamethylene diisocyanate; Examples include 2-methyl-1,5-pentane diisocyanate, 3-methyl-1,5-pentane diisocyanate, ly
- alicyclic polyisocyanates include isophorone diisocyanate; cyclohexyl diisocyanates such as 1,2-cyclohexyl diisocyanate, 1,3-cyclohexyl diisocyanate, and 1,4-cyclohexyl diisocyanate; 1,2-cyclopentyl diisocyanate, and 1,3-cyclohexyl diisocyanate; -Cyclopentyl diisocyanates such as cyclopentyl diisocyanate; hydrogenated xylylene diisocyanate, hydrogenated tolylene diisocyanate, hydrogenated diphenylmethane diisocyanate, hydrogenated tetramethylxylene diisocyanate, 4,4'-dicyclohexylmethane diisocyanate, and the like.
- aromatic polyisocyanates include 2,4-tolylene diisocyanate, 2,6-tolylene diisocyanate, 4,4'-diphenylmethane diisocyanate, 2,4'-diphenylmethane diisocyanate, and 2,2'-diphenylmethane diisocyanate.
- polyfunctional isocyanates examples include polyfunctional isocyanates having an average of three or more isocyanate groups per molecule.
- Such trifunctional or higher functional isocyanates are polymers (typically dimers or trimers) of bifunctional or trifunctional or higher functional isocyanates, derivatives (for example, a combination of a polyhydric alcohol and two or more molecules of polyfunctional isocyanate). addition reaction products), polymers, etc.
- dimers and trimers of diphenylmethane diisocyanate dimers and trimers of diphenylmethane diisocyanate, isocyanurates of hexamethylene diisocyanate (trimeric adducts of isocyanurate structures), reaction products of trimethylolpropane and tolylene diisocyanate, and products of the reaction between trimethylolpropane and hexamethylene diisocyanate.
- polyfunctional isocyanates such as reaction products with methylene diisocyanate, polymethylene polyphenylisocyanate, polyether polyisocyanate, and polyester polyisocyanate.
- the amount of the isocyanate crosslinking agent used is not particularly limited.
- the amount can be approximately 0.1 part by weight or more per 100 parts by weight of the acrylic polymer.
- the amount of the isocyanate crosslinking agent to be used per 100 parts by weight of the acrylic polymer can be, for example, 0.5 parts by weight or more, and preferably 1.0 parts by weight or more. and preferably 1.5 parts by weight or more.
- the amount of the isocyanate crosslinking agent used is suitably 10 parts by weight or less per 100 parts by weight of the acrylic polymer, preferably less than 5 parts by weight, more preferably less than 4.0 parts by weight, More preferably, it is less than 3.0 parts by weight (for example, 2.5 parts by weight or less).
- epoxy crosslinking agent any compound having two or more epoxy groups in one molecule can be used without particular limitation. Epoxy crosslinking agents having 3 to 5 epoxy groups in one molecule are preferred. The epoxy crosslinking agents can be used alone or in combination of two or more.
- epoxy crosslinking agents include N,N,N',N'-tetraglycidyl-m-xylene diamine, 1,3-bis(N,N-diglycidylaminomethyl ) cyclohexane, 1,6-hexanediol diglycidyl ether, polyethylene glycol diglycidyl ether, polyglycerol polyglycidyl ether and the like.
- epoxy crosslinking agents include Mitsubishi Gas Chemical's product names “TETRAD-C” and “TETRAD-X”, DIC's product name “Epicron CR-5L”, and Nagase ChemteX's product name Examples include the product name “Denacol EX-512” manufactured by Nissan Chemical Industries, Ltd. and the product name "TEPIC-G” manufactured by Nissan Chemical Industries, Ltd.
- the amount of the epoxy crosslinking agent used is not particularly limited.
- the amount of the epoxy crosslinking agent used may be, for example, more than 0 parts by weight and no more than about 1 part by weight (typically about 0.001 to 1 part by weight) per 100 parts by weight of the acrylic polymer. can. From the viewpoint of suitably exhibiting the effect of improving cohesive force, it is appropriate that the amount of the epoxy crosslinking agent used is approximately 0.005 parts by weight or more, and approximately 0.01 parts by weight, based on 100 parts by weight of the acrylic polymer.
- the amount is preferably at least 0.02 parts by weight, more preferably about 0.02 parts by weight or more.
- the amount of the epoxy crosslinking agent used is approximately 0.5 parts by weight or less per 100 parts by weight of the acrylic polymer, and approximately 0.2 parts by weight. It is preferably at most 0.1 part by weight (for example less than 0.1 part by weight), more preferably at most 0.07 part by weight, and may be at most 0.04 part by weight.
- an epoxy crosslinking agent and at least one crosslinking agent having a different type of crosslinkable functional group from the epoxy crosslinking agent are used in combination.
- a crosslinking agent other than an epoxy crosslinking agent that is, a crosslinking agent having a different type of crosslinkable reactive group from an epoxy crosslinking agent; hereinafter also referred to as a "non-epoxy crosslinking agent"
- an epoxy crosslinking agent By using it in combination with an epoxy crosslinking agent, it is possible to suitably achieve both adhesion to different materials and high holding power.
- non-epoxy crosslinking agent that can be used in combination with the epoxy crosslinking agent is not particularly limited, and can be appropriately selected from the above-mentioned crosslinking agents.
- the non-epoxy crosslinking agents can be used alone or in combination of two or more.
- an isocyanate crosslinking agent can be employed as the non-epoxy crosslinking agent.
- an epoxy crosslinking agent and an isocyanate crosslinking agent in combination, better adhesive properties can be achieved.
- the relationship between the content of the epoxy crosslinking agent and the content of the non-epoxy crosslinking agent (preferably an isocyanate crosslinking agent) is not particularly limited.
- the content of the epoxy crosslinking agent can be, for example, about 1/10 or less of the content of the non-epoxy crosslinking agent (preferably an isocyanate crosslinking agent).
- the content of the epoxy crosslinking agent is approximately 1/30 or less of the content of the non-epoxy crosslinking agent, It is preferably about 1/50 or less, more preferably about 1/75 or less, and may be about 1/90 or less.
- the content of the epoxy crosslinking agent is usually adjusted to It is appropriate to set the content to about 1/1000 or more, for example about 1/500 or more, preferably about 1/300 or more, more preferably 1/150 or more, still more preferably 1/120 or more of the content of the system crosslinking agent. It is.
- the total amount of the crosslinking agent used is not particularly limited, and for example, approximately 0.005 parts by weight or more (for example, 0.01 parts by weight or more, typically 0.1 parts by weight or more) based on 100 parts by weight of the acrylic polymer.
- the amount can be selected from a range of about 10 parts by weight or less (for example, about 8 parts by weight or less, preferably about 5 parts by weight or less).
- the adhesive composition may contain a leveling agent, a crosslinking aid, a plasticizer, a softener, a filler, a coloring agent (pigment, dye, etc.), an antistatic agent, and an antiaging agent, as necessary.
- a leveling agent e.g., a plasticizer, a softener, a filler, a coloring agent (pigment, dye, etc.), an antistatic agent, and an antiaging agent, as necessary.
- ultraviolet absorbers, antioxidants, light stabilizers, and other additives commonly used in the adhesive field e.g., ultraviolet absorbers, antioxidants, light stabilizers, and other additives commonly used in the adhesive field.
- conventionally known ones can be used in a conventional manner, and since they do not particularly characterize the present invention, detailed explanations will be omitted.
- the adhesive layer (layer consisting of an adhesive) disclosed herein is formed from a water-based adhesive composition, a solvent-based adhesive composition, a hot-melt adhesive composition, or an active energy ray-curable adhesive composition. It may be an adhesive layer.
- the aqueous adhesive composition refers to an adhesive composition containing an adhesive (adhesive layer forming component) in a water-based solvent (aqueous solvent), and is typically a water-based adhesive composition. This includes what is called a type adhesive composition (a composition in which at least a portion of an adhesive is dispersed in water).
- a solvent-based adhesive composition refers to an adhesive composition containing an adhesive in an organic solvent.
- organic solvent contained in the solvent-based adhesive composition one or more of the organic solvents (toluene, ethyl acetate, etc.) that can be used in the above-mentioned solution polymerization can be used without particular limitation.
- the technology disclosed herein can be preferably implemented in an embodiment including an adhesive layer formed from a solvent-based adhesive composition from the viewpoint of adhesive properties and the like.
- the adhesive layer disclosed herein can be formed by a conventionally known method.
- a method can be adopted in which a pressure-sensitive adhesive layer is formed by applying a pressure-sensitive adhesive composition to a surface having peelability (peelability surface) or a non-peelability surface and drying it.
- a method directly method of forming an adhesive layer by directly applying (typically coating) an adhesive composition to the base material and drying is adopted.
- a method transfer method in which an adhesive composition is applied to a surface that has releasability (release surface) and dried to form an adhesive layer on the surface, and the adhesive layer is transferred to a base material. may be adopted.
- the transfer method is preferred.
- the release surface the surface of a release liner, the back surface of a release-treated base material, etc. can be used.
- the adhesive layer disclosed herein is typically formed continuously, it is not limited to this form, and may be formed, for example, in a regular or random pattern such as dots or stripes. It may also be a formed adhesive layer.
- the adhesive composition can be applied using a conventionally known coater such as a gravure roll coater, die coater, or bar coater. Alternatively, the adhesive composition may be applied by impregnation, curtain coating, or the like. From the viewpoint of promoting crosslinking reaction, improving production efficiency, etc., it is preferable to dry the adhesive composition under heating.
- the drying temperature can be, for example, about 40 to 150°C, and usually preferably about 60 to 130°C.
- aging may be performed for the purpose of adjusting component migration within the pressure-sensitive adhesive layer, progressing the crosslinking reaction, alleviating distortion that may exist within the pressure-sensitive adhesive layer, and the like.
- the thickness of the adhesive layer is not particularly limited, and a configuration having an adhesive layer having an appropriate thickness in the range of, for example, 0.1 to 500 ⁇ m may be adopted depending on the use and purpose of use.
- the thickness of the adhesive layer is usually approximately 100 ⁇ m or less, preferably approximately 70 ⁇ m or less, more preferably approximately 60 ⁇ m or less, More preferably, it is approximately 50 ⁇ m or less.
- the thickness of the adhesive layer can be approximately 35 ⁇ m or less, and may be approximately 30 ⁇ m or less, for example.
- An adhesive layer with a limited thickness can meet the demands for thinning and weight reduction.
- the lower limit of the thickness of the adhesive layer is suitably about 0.5 ⁇ m or more, from the viewpoint of adhesion to the adherend, it may be about 1 ⁇ m or more, and it may be about 3 ⁇ m or more.
- it is approximately 10 ⁇ m or more, more preferably approximately 12 ⁇ m or more (eg greater than 12 ⁇ m), still more preferably approximately 15 ⁇ m or more, and may for example be approximately 18 ⁇ m or more.
- the thickness of the adhesive layer is greater than 20 ⁇ m, may be greater than or equal to 24 ⁇ m, and may be greater than or equal to 27 ⁇ m.
- the pressure-sensitive adhesive sheet disclosed herein may be a pressure-sensitive adhesive sheet having pressure-sensitive adhesive layers having the above-mentioned thickness on both sides of a base material.
- the first adhesive layer and the second adhesive layer have the same thickness. They may have different thicknesses.
- the adhesive layer disclosed herein has a gel fraction of less than 70% (by weight). By using an adhesive with a gel fraction of less than 70%, it is possible to exhibit sufficient adhesive strength not only for highly polar materials but also for low polar materials.
- the gel fraction of the adhesive layer is preferably less than 65%, may be less than 60%, may be less than 55%, may be less than 50%, may be less than 45%, may be less than 40%. It may be less than 35%. Further, from the viewpoint of obtaining sufficient holding power, in some preferred embodiments, the gel fraction of the adhesive layer is 20% or more, more preferably 25% or more, still more preferably 30% or more, and 35% or more.
- the gel fraction of the adhesive layer may be more than 40%, it may be more than 45%, it may be more than 50%, it may be more than 55%, it may be more than 60%.
- the adhesive layer may include a biomass-derived material, and the biomass carbon ratio may be greater than or equal to a predetermined value.
- the biomass carbon ratio of the adhesive layer is, for example, 1% or more, and may be 10% or more, preferably 30% or more, and more preferably 50% or more.
- a high biomass carbon ratio in the adhesive means that less fossil resource-based materials, such as petroleum, are used. From this point of view, the higher the biomass carbon ratio of the adhesive, the more preferable.
- the biomass carbon ratio of the adhesive layer may be 55% or more, 60% or more, 70% or more, 75% or more, 80% or more, or more than 80%. good.
- the upper limit of the biomass carbon ratio is 100% by definition, and may be 99% or less, and from the viewpoint of material availability, it may be 95% or less, or 90% or less. In some embodiments, from the viewpoint of facilitating good adhesive performance, the biomass carbon ratio of the adhesive layer may be, for example, 90% or less, 85% or less, or 80% or less.
- the base material that supports (backs) the adhesive layer may be a resin film, paper, cloth, or rubber. Sheets, foam sheets, metal foils, composites thereof, etc. can be used.
- resin films include polyolefin films such as polyethylene (PE), polypropylene (PP), and ethylene-propylene copolymers; polyester films such as polyethylene terephthalate (PET); vinyl chloride resin films; vinyl acetate resin films; polyimide Examples include resin film; polyamide resin film; fluororesin film; cellophane, and the like.
- Examples of paper include Japanese paper, kraft paper, glassine paper, high quality paper, synthetic paper, top coated paper, and the like.
- Examples of the fabric include woven fabrics and nonwoven fabrics made of various fibrous substances alone or in combination.
- Examples of the above-mentioned fibrous materials include cotton, staple fiber, Manila hemp, pulp, rayon, acetate fiber, polyester fiber, polyvinyl alcohol fiber, polyamide fiber, and polyolefin fiber.
- Examples of rubber sheets include natural rubber sheets, butyl rubber sheets, and the like.
- Examples of foam sheets include foamed polyolefin sheets, foamed polyurethane sheets, foamed polychloroprene rubber sheets, and the like.
- Examples of metal foil include aluminum foil, copper foil, and the like. Note that the base material that supports the adhesive layer is also referred to as a base material layer in the adhesive sheet.
- the base material may be formed from a biomass-derived material or a non-biomass-derived material. From the viewpoint of producing a pressure-sensitive adhesive sheet in consideration of reducing dependence on fossil resource-based materials, biomass-derived base materials (typically resin films) are preferably used.
- the base material may be formed using a recyclable material or a recycled material (also referred to as recycled material).
- a resin film is preferably used. Resin films (for example, polyester films such as PET films) can be recycled, so whether or not they are made from plant-based materials, reusing the used resin film allows for sustainable reproduction. It is possible to reduce the environmental burden.
- a recyclable resin film or a recycled resin film is also referred to as a recycled film.
- the recycled material (for example, recycled film) may be formed from a biomass-derived material or a non-biomass-derived material.
- the base material constituting the base material-attached pressure-sensitive adhesive sheet one containing a resin film as a base film can be preferably used.
- the base film is typically an independently shape-maintainable (independent) member.
- the base material in the technology disclosed herein may be substantially composed of such a base film.
- the base material may include an auxiliary layer in addition to the base film. Examples of the auxiliary layer include a colored layer, a reflective layer, an undercoat layer, an antistatic layer, etc. provided on the surface of the base film.
- the resin film is a film whose main component is a resin material (for example, a component contained in the resin film in an amount exceeding 50% by weight).
- resin films include polyolefin resin films such as polyethylene (PE), polypropylene (PP), and ethylene-propylene copolymers; polyethylene terephthalate (PET), polybutylene terephthalate (PBT), polyethylene naphthalate (PEN), etc.
- PET polyethylene terephthalate
- PBT polybutylene terephthalate
- PEN polyethylene naphthalate
- polyester resin film vinyl chloride resin film; vinyl acetate resin film; polyimide resin film; polyamide resin film; fluororesin film; cellophane; and the like.
- the resin film may be a rubber film such as a natural rubber film or a butyl rubber film. Among these, polyester films are preferred from the viewpoint of handling and processability, and among these, PET films are particularly preferred.
- resin film is typically a non-porous sheet, and is a concept that is distinguished from so-called non-woven fabrics and woven fabrics (in other words, a concept excluding non-woven fabrics and woven fabrics).
- the resin film may be an unstretched film, a uniaxially stretched film, or a biaxially stretched film.
- such resin films may be non-foamed.
- non-foamed resin film refers to a resin film that has not been intentionally processed to form a foam.
- the non-foamed resin film may be a resin film with an expansion ratio of less than 1.1 times (for example, less than 1.05 times, typically less than 1.01 times).
- the above base material may contain fillers (inorganic fillers, organic fillers, etc.), colorants, dispersants (surfactants, etc.), anti-aging agents, antioxidants, ultraviolet rays, etc., as necessary.
- Various additives such as an absorbent, an antistatic agent, a lubricant, and a plasticizer may be blended.
- the blending ratio of various additives is about less than 30% by weight (for example, less than 20% by weight, typically less than 10% by weight).
- the base material may have a single layer structure, or may have a multilayer structure of two layers, three layers, or more. From the viewpoint of shape stability, the base material preferably has a single-layer structure. In the case of a multilayer structure, at least one layer (preferably all layers) is preferably a layer having a continuous structure of the above resin (for example, polyester resin).
- the method for manufacturing the base material is not particularly limited, and any conventionally known method may be appropriately adopted. For example, conventionally known general film forming methods such as extrusion molding, inflation molding, T-die casting molding, and calender roll molding can be appropriately employed.
- the surface of the base material may be subjected to conventionally known surface treatments such as corona discharge treatment, plasma treatment, ultraviolet irradiation treatment, acid treatment, alkali treatment, and application of an undercoat.
- Such surface treatment may be a treatment for improving the adhesion between the base material and the adhesive layer, in other words, the ability of the adhesive layer to anchor to the base material.
- the back surface of the base material may be subjected to a peeling treatment as necessary.
- the peeling treatment is performed by applying a general silicone-based, long-chain alkyl-based, or fluorine-based peeling agent to a thin film, typically about 0.01 ⁇ m to 1 ⁇ m (for example, 0.01 ⁇ m to 0.1 ⁇ m). It can be a process to add. By performing such a peeling treatment, effects such as facilitating the unwinding of a roll-shaped adhesive sheet can be obtained.
- the thickness of the base material is not particularly limited. From the viewpoint of preventing the pressure-sensitive adhesive sheet from becoming too thick, the thickness of the base material can be, for example, approximately 200 ⁇ m or less, preferably approximately 150 ⁇ m or less, and more preferably approximately 100 ⁇ m or less.
- the thickness of the base material may be approximately 70 ⁇ m or less, approximately 50 ⁇ m or less, or approximately 30 ⁇ m or less (for example, approximately 25 ⁇ m or less) depending on the purpose and manner of use of the adhesive sheet. In some embodiments, the thickness of the base film layer may be about 20 ⁇ m or less, about 15 ⁇ m or less, about 10 ⁇ m or less (eg, about 5 ⁇ m or less).
- the thickness of the adhesive layer can be increased even if the total thickness of the adhesive sheet is the same. This can be advantageous from the viewpoint of improving adhesion to adherends and base materials.
- the lower limit of the base material is not particularly limited. From the viewpoint of handleability and processability of the pressure-sensitive adhesive sheet, the thickness of the base material is usually about 0.5 ⁇ m or more (for example, 1 ⁇ m or more), preferably about 2 ⁇ m or more, for example about 6 ⁇ m or more. In some embodiments, the thickness of the substrate can be about 15 ⁇ m or more, and can be about 25 ⁇ m or more.
- a release liner can be used during formation of the adhesive layer, production of the adhesive sheet, storage of the adhesive sheet before use, distribution, shape processing, etc.
- the release liner is not particularly limited, and for example, a release liner having a release treatment layer on the surface of a liner base material such as a resin film or paper, a release liner made of a fluorine-based polymer (polytetrafluoroethylene, etc.), etc. may be used. be able to.
- the release treatment layer may be formed by surface-treating the liner base material with a release agent such as a silicone-based, long-chain alkyl-based, fluorine-based, or molybdenum sulfide release agent.
- a release agent such as a silicone-based, long-chain alkyl-based, fluorine-based, or molybdenum sulfide release agent.
- the liner base material like the base material of the above-mentioned adhesive sheet, one formed using a biomass-derived material or a recycled material (recycled film, etc.) can be preferably used.
- the total thickness of the adhesive sheet disclosed herein (which includes an adhesive layer and may further include a base layer, but does not include a release liner) is not particularly limited.
- the total thickness of the adhesive sheet is, for example, approximately 1 mm or less, may be approximately 500 ⁇ m or less, and may be approximately 300 ⁇ m or less, and from the viewpoint of thinning, approximately 200 ⁇ m or less is appropriate, and approximately 150 ⁇ m or less. (For example, approximately 100 ⁇ m or less).
- the thickness of the pressure-sensitive adhesive sheet can be approximately 50 ⁇ m or less, for example, approximately 35 ⁇ m or less.
- the lower limit of the thickness of the adhesive sheet is, for example, 0.1 ⁇ m or more (for example, 0.5 ⁇ m or more), suitably about 3 ⁇ m or more, preferably about 10 ⁇ m or more, more preferably about 15 ⁇ m or more, It may be about 50 ⁇ m or more, or about 100 ⁇ m or more.
- a pressure-sensitive adhesive sheet having a thickness of a predetermined value or more tends to have good adhesion to an adherend and also tends to have excellent handling properties.
- the thickness of the adhesive layer becomes the total thickness of the adhesive sheet.
- the adhesive sheet preferably has a 180 degree peel strength against a stainless steel plate (adhesive strength against SUS) of approximately 15 N/25 mm or more (for example, 17 N/25 mm or more).
- a pressure-sensitive adhesive sheet exhibiting such adhesive strength to SUS can exhibit excellent adhesive strength to highly polar materials.
- the adhesive force to SUS is more preferably about 20 N/25 mm or more, still more preferably about 25 N/25 mm or more, particularly preferably 30 N/25 mm or more, and may be 32 N/25 mm or more.
- the upper limit of the adhesive strength to SUS is not particularly limited, but from the viewpoint of coexistence with other adhesive properties such as holding power, it may usually be about 50 N/25 mm or less, for example.
- the adhesive strength to SUS is measured using an SUS plate as an adherend under the conditions of a tensile speed of 300 mm/min and a peel angle of 180 degrees in a measurement environment of 23° C. and 50% RH. More specifically, it is measured by the method described in Examples below.
- the adhesive sheet disclosed herein preferably has a 180 degree peel strength against polypropylene (adhesive strength against PP) of about 10 N/25 mm or more.
- a pressure-sensitive adhesive sheet exhibiting such adhesion to PP can adhere well to low polarity materials and exhibit high adhesion reliability to the above-mentioned adherends.
- the adhesive force to PP is more preferably about 12 N/25 mm or more, still more preferably about 14 N/25 mm or more, particularly preferably about 16 N/25 mm or more, and about 18 N/25 mm or more (for example, about 20 N/25 mm or more). There may be.
- the upper limit of the adhesive force to PP is not particularly limited, but from the viewpoint of coexistence with other adhesive properties such as holding power, it is usually about 40 N/25 mm or less, and may be 30 N/25 mm or less.
- the adhesive strength to PP is measured using PP as an adherend in a measurement environment of 23° C. and 50% RH at a tensile rate of 300 mm/min and a peel angle of 180 degrees. More specifically, it is measured by the method described in Examples below.
- the adhesive sheet disclosed herein preferably has a 180 degree peel strength against polyethylene (adhesive strength against PE) of approximately 5 N/25 mm or more.
- a pressure-sensitive adhesive sheet exhibiting such adhesive strength to PE can exhibit sufficient adhesion reliability to low polarity materials.
- the adhesive strength to PE is more preferably about 7 N/25 mm or more, still more preferably about 10 N/25 mm or more, particularly preferably about 12 N/25 mm or more, and 14 N/25 mm or more (for example, about 15 N/25 mm or more). It's okay.
- the upper limit of the adhesive force to PE is not particularly limited, but from the viewpoint of coexistence with other adhesive properties such as holding power, it is usually about 30 N/25 mm or less, and may be 25 N/25 mm or less.
- the above-mentioned adhesive strength to PE is measured using PE as an adherend in a measurement environment of 23° C. and 50% RH at a tensile rate of 300 mm/min and a peel angle of 180 degrees. More specifically, it is measured by the method described in Examples below.
- the adhesive sheet disclosed herein may exhibit adhesive strength of a predetermined value or more to PP and PE.
- Adhesive sheets exhibiting the above-mentioned adhesive strength to PP and adhesive strength to PE can have stable and sufficient adhesion reliability to different materials including various low polarity materials, and therefore are useful in a wide range of applications. .
- the pressure-sensitive adhesive sheet disclosed herein has a holding power test conducted under the conditions of 80° C., adhesion area 10 mm x 20 mm, load 1.5 kg, and 1 hour.
- the deviation distance may be 10 mm or less.
- Such a pressure-sensitive adhesive sheet has high cohesive force and sufficient holding power.
- the displacement distance in the holding force test is preferably 5 mm or less, more preferably 3 mm or less, and even more preferably 1 mm or less (0 to 0.1 mm). More specifically, the above-mentioned holding power test is carried out by the method described in the Examples below.
- the adhesive sheet may include a biomass-derived material, and the biomass carbon ratio may be greater than or equal to a predetermined value.
- the biomass carbon ratio of the adhesive sheet is, for example, 1% or more, and may be 10% or more, preferably 30% or more, and more preferably 50% or more.
- a high biomass carbon ratio in the adhesive sheet means that less fossil resource-based materials, such as petroleum, are used. From this point of view, the higher the biomass carbon ratio of the pressure-sensitive adhesive sheet, the more preferable it is.
- the biomass carbon ratio of the adhesive sheet may be 55% or more, 60% or more, 70% or more, 75% or more, 80% or more, or more than 80%. .
- the upper limit of the biomass carbon ratio is 100% by definition, and may be 99% or less, and from the viewpoint of material availability, it may be 95% or less, or 90% or less. In some embodiments, from the viewpoint of facilitating good adhesive performance, the biomass carbon ratio of the adhesive sheet may be, for example, 90% or less, 85% or less, or 80% or less.
- the use of the pressure-sensitive adhesive sheet disclosed herein is not particularly limited, and can be used for various purposes.
- the pressure-sensitive adhesive sheet disclosed herein exhibits high adhesion to both high-polar and low-polar materials, and has sufficient holding power, so it is suitable for use with members made of high-polar materials including metal members, , suitable for adhesively fixing members made of low polarity materials.
- members made of high-polar materials including metal members, , suitable for adhesively fixing members made of low polarity materials.
- the component to be bonded has a high polarity surface or a low polarity surface and long-term bonding reliability is required.
- Examples of materials constituting such a highly polar surface include metal materials such as stainless steel, glass materials, and polyester resin members such as PET.
- materials constituting the low polarity surface include polyolefin resins such as polyethylene (PE) and polypropylene (PP), which are generally known as materials with low surface free energy, fluorine-based polymers (such as polytetrafluoroethylene), Examples include polystyrene, polyoxymethylene, polyvinyl acetate, and the like.
- the pressure-sensitive adhesive sheet disclosed herein is preferably used for adhesively fixing an adherend having a surface made of a material containing a polyolefin such as PE or PP, or a fluorine-based polymer.
- Suitable applications for the adhesive sheet disclosed herein include fixing members in electronic devices such as home appliances, office automation equipment, and portable electronic devices.
- the members constituting the above-mentioned electronic devices can be made of highly polar materials such as metals, or low polar materials such as PE, PP, or fluororesin. It is meaningful to realize adhesive fixation with excellent adhesive reliability for various dissimilar materials including those with low polarity.
- Examples of the electronic devices include various home appliances, personal computers (desktop type, notebook type, tablet type, etc.), and the like.
- Non-limiting examples of the above-mentioned portable electronic devices include mobile phones, smartphones, tablet computers, notebook computers, and various wearable devices (e.g., wrist-wear type that is worn on the wrist like a wristwatch, and Modular type that is attached to a part of the body, eyewear type that includes glasses type (monocular type and binocular type, including head-mounted type), clothing type that is attached to shirts, socks, hats, etc. in the form of accessories, and earphones. digital cameras, digital video cameras, audio equipment (portable music players, IC recorders, etc.), calculators (calculators, etc.), portable game devices, electronic dictionaries, electronic notebooks, electronic books, and in-vehicle devices.
- wearable devices e.g., wrist-wear type that is worn on the wrist like a wristwatch, and Modular type that is attached to a part of the body
- eyewear type that includes glasses type (monocular type and binocular type, including head-mounted type)
- clothing type that is attached
- portable does not mean that it is sufficient to simply be able to carry it; it also means that it has a level of portability that allows an individual (standard adult) to carry it relatively easily. shall mean.
- FIG. 4 is an example schematically showing a portable electronic device (smartphone) using the adhesive sheet disclosed herein.
- a battery (heat generating element) 540 is built inside the casing 520 of the portable electronic device 500.
- the portable electronic device 500 is configured to include an adhesive sheet 550.
- the adhesive sheet 550 has the form of a double-sided adhesive sheet (double-sided adhesive sheet) that fixes members constituting the portable electronic device 500.
- the portable electronic device 500 includes a touch panel 570 whose display section also functions as an input section.
- the adhesive sheet disclosed herein is preferably used as a component (member joining means) of the above-mentioned portable electronic devices.
- the adhesive sheet disclosed herein may have an adhesive layer containing an acrylic polymer with a high biomass carbon ratio in some embodiments, conventional general acrylic adhesives (i.e. By being used as a substitute for acrylic adhesives in various applications where acrylic adhesives (acrylic adhesives with a low biomass carbon ratio) are used, it can contribute to reducing dependence on fossil resource-based materials.
- the adhesive sheet disclosed herein can be preferably used as an adhesive sheet with reduced dependence on fossil resource materials.
- the matters disclosed by this specification include the following.
- An adhesive sheet is bonded to the member constituting the electronic device,
- the adhesive sheet has an adhesive layer containing an acrylic polymer,
- the acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer,
- the monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer,
- the adhesive layer has a gel fraction of less than 70%,
- the weight average molecular weight of the acrylic polymer is greater than 600,000, an electronic device.
- the electronic device according to [1] above, wherein the surface of the member is made of a material selected from metal, polyolefin resin, and fluororesin.
- the adhesive layer has a gel fraction of 20% or more and less than 70%.
- the adhesive sheet is a base material-less double-sided adhesive sheet consisting only of the adhesive layer.
- the adhesive sheet has a base material and the adhesive layer provided on at least one surface of the base material.
- the acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer, The monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer, The adhesive layer has a gel fraction of less than 70%, A pressure-sensitive adhesive sheet, wherein the acrylic polymer has a weight average molecular weight of more than 600,000. [12] The pressure-sensitive adhesive sheet according to [11] above, wherein the pressure-sensitive adhesive composition for forming the pressure-sensitive adhesive layer contains a crosslinking agent.
- the pressure-sensitive adhesive sheet according to any one of [11] to [19] above which is a base material-less double-sided pressure-sensitive adhesive sheet consisting only of the pressure-sensitive adhesive layer.
- the pressure-sensitive adhesive sheet according to any one of [11] to [19] above comprising a base material and the pressure-sensitive adhesive layer provided on at least one surface of the base material.
- the adhesive sheet according to any one of [11] to [21] above which is used for fixing members in electronic devices.
- An electronic device comprising the adhesive sheet according to any one of [11] to [21] above.
- the above-mentioned porous polytetrafluoroethylene (PTFE) membrane is available from Nitto Denko under the trade name "Nitoflon (registered trademark) NTF1122" (average pore diameter 0.2 ⁇ m, porosity 75%, thickness 85 ⁇ m) or its equivalent. use the product.
- Adhesive strength to PE Under a measurement environment of 23°C and 50% RH, one adhesive side of an adhesive sheet (double-sided adhesive sheet) was lined with a PET film with a thickness of 50 ⁇ m, and cut into a size of 25 mm in width and 100 mm in length. Prepare a measurement sample. In an environment of 23° C. and 50% RH, the other adhesive surface of the measurement sample was pressed onto the surface of a polyethylene board (PE board) washed with ethanol by making one reciprocation with a 2 kg roller. After leaving it in the same environment for 72 hours, the peel strength (relative to PE Adhesive force) [N/25mm] is measured.
- the PE board for example, the product name "Kobe Polysheet EL-N-AN" (thickness: 2 mm) manufactured by Showa Denko Materials Co., Ltd. is used.
- the universal tensile compression tester used is Minebea's "Tensile Compression Tester, TG-1kN" or its equivalent. Note that when performing the above peel strength measurement on a single-sided adhesive sheet, backing with a PET film is not necessary.
- the base material thickness is thin (for example, when the base material thickness is 25 ⁇ m or less), it may be lined with a PET film.
- a load of 5 kg is applied to the product, and the product is left in an environment of 80° C. for 1 hour in accordance with JIS Z0237.
- n-HpA is a compound having a biomass-derived heptyl group at the ester end, which was synthesized using biomass-derived heptyl alcohol.
- the obtained adhesive composition was applied to the release surface of a polyester release film (trade name “Diafoil MRF”, manufactured by Mitsubishi Chemical Corporation) with a thickness of 38 ⁇ m, and dried at 100° C. for 2 minutes to form a film with a thickness of 30 ⁇ m.
- An adhesive layer was formed.
- a release surface of a 25 ⁇ m thick polyester release film (trade name “Diafoil MRF”, 25 ⁇ m thick, manufactured by Mitsubishi Chemical Corporation) was attached to this adhesive layer. In this way, a substrate-less double-sided pressure-sensitive adhesive sheet having a thickness of 30 ⁇ m and having both sides protected by the two polyester release films was obtained.
- Example 2 In the preparation of the adhesive composition in Example 1, an epoxy crosslinking agent (trade name "TETRAD-C", manufactured by Mitsubishi Gas Chemical Co., Ltd., 1,3-bis(N , N-diglycidylaminomethyl)cyclohexane) was further added and mixed with stirring to prepare a pressure-sensitive adhesive composition according to this example.
- a substrate-less double-sided pressure-sensitive adhesive sheet according to this example was produced in the same manner as in Example 1 except that the obtained pressure-sensitive adhesive composition was used.
- Examples 3 to 5> A solution of acrylic polymer (A2) was obtained in the same manner as the synthesis of acrylic polymer (A1), except that the monomer composition was changed to 94 parts of n-HpA and 6 parts of AA, and the concentration of monomer components during polymerization was adjusted. .
- adhesive compositions according to each example were prepared in the same manner as in Example 2 except that the composition was changed to that shown in Table 1.
- a base material-less double-sided adhesive sheet according to the example was produced.
- Example 6 A solution of acrylic polymer (A3) was obtained in the same manner as in the synthesis of acrylic polymer (A1) except that the monomer composition was changed to 96 parts of n-HpA and 4 parts of AA.
- Adhesive compositions according to this example were prepared in the same manner as in Example 2 except that the above acrylic polymer (A3) was used, and the adhesive compositions were used to form a base material-less double-sided adhesive sheet according to this example. was created.
- Example 7 Adhesive compositions according to each example were prepared in the same manner as in Example 6, except that the type of tackifier resin was changed as shown in Table 1, and the adhesive composition was used to prepare a base material according to each example.
- a double-sided pressure-sensitive adhesive sheet was prepared. Specifically, in Example 7, 40 parts of the rosin-based tackifier resin of Example 6 was replaced with 25 parts of the rosin-based tackifier resin and a terpene-based tackifier resin (product name "YS In Example 8, the above rosin of Example 6 was used as the tackifying resin. In Example 9, the amount of the rosin tackifier resin used was changed from 40 parts in Example 6 to 15 parts.
- a solution of an acrylic polymer (A4) having a lower molecular weight than the acrylic polymer (A3) was obtained in the same manner as the synthesis of the acrylic polymer (A3) except that the concentration of monomer components during polymerization was adjusted.
- a pressure-sensitive adhesive composition according to this example was prepared in the same manner as in Example 6 except that the above acrylic polymer (A4) was used, and a base material-less double-sided pressure-sensitive adhesive sheet according to this example was prepared using the pressure-sensitive adhesive composition. Created.
- the acrylic polymer was synthesized basically in the same manner as the acrylic polymer (A1) except that the monomer composition was changed to 95 parts of n-butyl acrylate (BA) or 95 parts of 2-ethylhexyl acrylate (2EHA) and 5 parts of AA. Solutions of (A6) and (A7) were obtained. Adhesive compositions according to each example were prepared in the same manner as in Example 2 except that the above acrylic polymer (A6) or (A7) was used, and the adhesive composition was used to create a base material according to each example. A double-sided pressure-sensitive adhesive sheet was prepared.
- Table 1 shows the outline and evaluation results of the pressure-sensitive adhesive sheets of each example.
- the adhesives according to Examples 1 to 9 contain n-heptyl acrylate as a monomer component, and contain an acrylic polymer having a Mw of more than 600,000 and containing more than 3% by weight of a carboxyl group-containing monomer, The gel fraction was less than 70%.
- the adhesives according to these examples have adhesive strength to SUS of 15 N/25 mm or more, PP adhesive strength of 10 N/25 mm or more, and PE adhesive strength of 5 N/25 mm or more, and also pass the holding power test (specifically In general, the deviation distance was 1 mm or less).
- Examples 1 and 2 in which the amount of carboxyl group-containing monomer used was the largest, had high adhesive strength to low polar materials (PP and PE) and particularly excellent adhesive strength to SUS. was. Furthermore, the results of Examples 3 to 5 show that by increasing the amount of tackifier resin, the adhesive strength to SUS, the adhesive strength to PP, and the adhesive strength to PE can be improved. The results of these examples also show that as the amount of tackifier resin increases, the gel fraction of the adhesive layer tends to decrease and the adhesive strength tends to increase. On the other hand, in Comparative Example 1 in which an acrylic polymer having an Mw of 600,000 was used, the holding power test result was a failure.
- Comparative Example 2 in which an acrylic polymer with a copolymerization ratio of a carboxyl group-containing monomer (specifically, AA) was 3% also failed in the holding power test.
- Comparative Example 3 in which the amount of crosslinking agent was increased compared to Comparative Example 2, the holding power was improved, but the adhesive strength to SUS, the adhesive strength to PP, and the adhesive strength to PE all decreased significantly. Furthermore, from the results of Comparative Examples 2 and 3, it can be seen that when the gel fraction becomes too high, it becomes difficult to satisfy the adhesive properties. Furthermore, in Comparative Examples 4 to 5 in which an alkyl acrylate (BA or 2EHA) other than n-heptyl acrylate was used, adhesive strength at the level of Examples 1 to 9 could not be obtained.
- BA or 2EHA alkyl acrylate
- the acrylic polymer a polymer of a monomer component containing n-heptyl acrylate and more than 3% by weight of a carboxyl group-containing monomer is used, the gel fraction of the adhesive layer is less than 70%, and the acrylic polymer It can be seen that adhesives with Mw greater than 600,000 can achieve both high levels of adhesion to highly polar materials, adhesion to low polar materials, and holding power.
- Adhesive sheet Supporting base material 10A First side 10B Second side (back side) 21 Adhesive layer (first adhesive layer) 21A Adhesive surface (first adhesive surface) 21B Second adhesive surface 22 Adhesive layer (second adhesive layer) 22A Adhesive surface (second adhesive surface) 31, 32 Release liner 100, 200, 300 Adhesive sheet with release liner
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Adhesives Or Adhesive Processes (AREA)
- Adhesive Tapes (AREA)
Abstract
Provided is an adhesive sheet capable of achieving a high level of adhesion to high-polarity materials, adhesion to low-polarity materials, and holding power. The adhesive sheet has an adhesive agent layer containing an acrylic polymer. The acrylic polymer is a polymer of a monomer component containing n-heptyl acrylate and a carboxy-group-containing monomer. The monomer component of the acrylic polymer contains more than 3 wt% of the carboxy-group-containing monomer. Furthermore, the gel fraction of the adhesive agent layer is less than 70%. The weight-average molecular weight of the acrylic polymer is greater than 600,000.
Description
本発明は、粘着シートに関する。
本出願は、2022年3月29日に出願された日本国特許出願2022-54421号および2022年10月13日に出願された日本国特許出願2022-164655号に基づく優先権を主張しており、それらの出願の全内容は本明細書中に参照として組み入れられている。 The present invention relates to an adhesive sheet.
This application claims priority based on Japanese Patent Application No. 2022-54421 filed on March 29, 2022 and Japanese Patent Application No. 2022-164655 filed on October 13, 2022. , the entire contents of those applications are incorporated herein by reference.
本出願は、2022年3月29日に出願された日本国特許出願2022-54421号および2022年10月13日に出願された日本国特許出願2022-164655号に基づく優先権を主張しており、それらの出願の全内容は本明細書中に参照として組み入れられている。 The present invention relates to an adhesive sheet.
This application claims priority based on Japanese Patent Application No. 2022-54421 filed on March 29, 2022 and Japanese Patent Application No. 2022-164655 filed on October 13, 2022. , the entire contents of those applications are incorporated herein by reference.
一般に粘着剤(感圧接着剤ともいう。以下同じ。)は、室温付近の温度域において柔らかい固体(粘弾性体)の状態を呈し、圧力により被着体に接着する性質を有する。かかる性質を活かして、粘着剤は、家電製品から自動車、OA機器等の各種産業分野において、典型的には粘着剤層を含む粘着シートの形態で、部品の接合や表面保護等の目的で広く利用されている。粘着シートに関する技術文献として特許文献1,2が挙げられる。特許文献1,2には、n-ヘプチルアクリレートをモノマー成分として用いて重合されたアクリル系ポリマーを含む粘着剤が記載されている。
In general, adhesives (also referred to as pressure-sensitive adhesives, hereinafter the same) exhibit a soft solid (viscoelastic) state in a temperature range around room temperature, and have the property of adhering to adherends under pressure. Taking advantage of these properties, adhesives are widely used in various industrial fields such as home appliances, automobiles, and OA equipment, typically in the form of adhesive sheets containing an adhesive layer, for purposes such as bonding parts and protecting surfaces. It's being used. Technical documents related to adhesive sheets include Patent Documents 1 and 2. Patent Documents 1 and 2 describe adhesives containing acrylic polymers polymerized using n-heptyl acrylate as a monomer component.
一般に、粘着シートには、種々の材料に対して良好に接着する性能が求められる。例えば、上記家電製品等の電子機器を構成する部材の固定に用いられる粘着剤には、接着対象の一方がステンレス鋼板等の高極性材料製の部材であり、他方がポリオレフィン等の低極性材料製の部材であったり、表面が高極性材料と低極性材料とから構成される部材等を信頼性よく固定する性能が求められ得る。通常、貯蔵弾性率が所定値以下に制限された柔らかい粘着剤を使用することにより、高極性材料や低極性材料など異種の材料に対する接着性は得られやすい。しかし、そのような粘着剤は、概して保持力が低下してしまう傾向がある。異種材料に対する接着性と保持力とはトレードオフの関係にある。特に近年、上記電子機器等の小型化、軽量化、精密化の要請から、粘着剤の接着面積は小さくなる傾向がある。かかる用途においては、高極性材料に対する接着力、低極性材料に対する接着力、および、保持力を高いレベルで実現することはより困難な傾向にある。
In general, pressure-sensitive adhesive sheets are required to have good adhesion performance to various materials. For example, with adhesives used to fix components that make up electronic devices such as the above-mentioned home appliances, one of the objects to be bonded is made of a highly polar material such as a stainless steel plate, and the other is made of a low polar material such as polyolefin. The ability to reliably fix a member whose surface is composed of a highly polar material and a low polar material may be required. Generally, by using a soft adhesive whose storage modulus is limited to a predetermined value or less, adhesiveness to different types of materials such as high polarity materials and low polarity materials can be easily obtained. However, such adhesives generally tend to have reduced holding power. There is a trade-off relationship between adhesion and holding power to different materials. Particularly in recent years, the adhesive area of adhesives has tended to become smaller due to the demand for smaller, lighter, and more precise electronic devices. In such applications, it tends to be more difficult to achieve high levels of adhesion to highly polar materials, adhesion to low polar materials, and retention.
本発明者らは鋭意検討の結果、n-ヘプチルアクリレートをモノマー成分として含むアクリル系ポリマーを用いる構成で、高極性材料、低極性材料のいずれに対しても高い接着力を発揮し、かつ、十分な保持力を有する粘着剤が得られることを見出し、本発明を完成するに至った。すなわち、本発明は、高極性材料に対する接着力、低極性材料に対する接着力、および、保持力を高いレベルで両立し得る粘着シートを提供することを目的とする。
As a result of extensive studies, the present inventors found that a structure using an acrylic polymer containing n-heptyl acrylate as a monomer component exhibits high adhesion to both high and low polarity materials, and has a sufficient adhesive strength. The present inventors have discovered that it is possible to obtain a pressure-sensitive adhesive having a strong holding power, and have completed the present invention. That is, an object of the present invention is to provide a pressure-sensitive adhesive sheet that can achieve both high levels of adhesive strength for highly polar materials, adhesive strength for low polar materials, and holding power.
この明細書によると、アクリル系ポリマーを含む粘着剤層を有する粘着シートが提供される。前記アクリル系ポリマーは、n-ヘプチルアクリレートおよびカルボキシ基含有モノマーを含むモノマー成分の重合物である。また、前記アクリル系ポリマーのモノマー成分は、前記カルボキシ基含有モノマーを3重量%よりも多く含む。さらに、前記粘着剤層のゲル分率は70%未満である。そして、前記アクリル系ポリマーの重量平均分子量は60万よりも大きい。モノマー成分としてn-ヘプチルアクリレートを含むアクリル系ポリマーを含み、ゲル分率70%未満の粘着剤を用いることで、高極性材料だけでなく、低極性材料に対しても十分な接着力を発揮することができる。また、上記アクリル系ポリマーにカルボキシ基含有モノマーを3重量%超の割合で共重合し、重量平均分子量(Mw)を60万よりも大きくなるよう設計することで、異種材料に対して高い接着力を有しつつ、保持力を向上することができる。上記カルボキシ基含有モノマーの使用により、高極性材料に対する接着性も改善される。すなわち、上記構成によると、高極性材料に対する接着力、低極性材料に対する接着力、および、保持力を高いレベルで両立することができる。なお、特許文献1では、ポリプロピレンに対する粘着力や保持力(40℃、接着面積25mm×25mm、荷重1kg)が評価されており、特許文献2では、保持力(80℃、接着面積25mm×25mm、荷重1kg)が評価されているが、用いられているアクリル系ポリマーはいずれも、Mwが低いか、あるいはカルボキシ基含有モノマーの共重合割合が低い範囲にあるため、後述の実施例で評価する保持力(80℃、接着面積10mm×20mm、荷重1.5kg)は得られず、高極性材料に対する接着力、低極性材料に対する接着力、および、保持力を十分に満足するものではない。
According to this specification, a pressure-sensitive adhesive sheet having a pressure-sensitive adhesive layer containing an acrylic polymer is provided. The acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer. Furthermore, the monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer. Furthermore, the gel fraction of the adhesive layer is less than 70%. The weight average molecular weight of the acrylic polymer is greater than 600,000. By using an adhesive that contains an acrylic polymer containing n-heptyl acrylate as a monomer component and has a gel fraction of less than 70%, it exhibits sufficient adhesive strength not only for highly polar materials but also for low polar materials. be able to. In addition, by copolymerizing the above acrylic polymer with a monomer containing a carboxyl group at a ratio of more than 3% by weight and designing the weight average molecular weight (Mw) to be greater than 600,000, we have achieved high adhesive strength to different materials. It is possible to improve the holding force while having the following properties. The use of the carboxy group-containing monomer also improves adhesion to highly polar materials. That is, according to the above configuration, it is possible to achieve both high levels of adhesive force for high polar materials, adhesive force for low polar materials, and holding force. In addition, in Patent Document 1, the adhesive strength and holding power for polypropylene (40 ° C., adhesive area 25 mm × 25 mm, load 1 kg) are evaluated, and in Patent Document 2, the holding force (80 ° C., adhesive area 25 mm × 25 mm, However, all of the acrylic polymers used either have a low Mw or have a low copolymerization ratio of carboxyl group-containing monomers, so the retention will be evaluated in the examples below. (80° C., adhesion area 10 mm x 20 mm, load 1.5 kg) was not obtained, and the adhesion force to high polarity materials, the adhesion force to low polarity materials, and the holding force were not fully satisfied.
いくつかの好ましい態様において、前記粘着剤層を形成するための粘着剤組成物は架橋剤を含む。架橋剤を用いることで、粘着剤の凝集力が高まり、十分な保持力を好適に実現することができる。
In some preferred embodiments, the adhesive composition for forming the adhesive layer includes a crosslinking agent. By using a crosslinking agent, the cohesive force of the adhesive increases, and sufficient holding power can be suitably achieved.
いくつかの好ましい態様において、前記粘着剤層は粘着付与樹脂を含む。粘着付与樹脂を用いることで、粘着剤層のゲル分率は適度な範囲に調整され、高極性材料および低極性材料に対する接着力を向上することができる。
In some preferred embodiments, the adhesive layer includes a tackifying resin. By using a tackifying resin, the gel fraction of the adhesive layer can be adjusted to an appropriate range, and the adhesive strength to high polarity materials and low polarity materials can be improved.
いくつかの好ましい態様において、前記粘着剤層の厚さは0.1~500μmである。ここに開示される技術は、上記厚さの粘着剤層を備える構成で実施され得る。
In some preferred embodiments, the thickness of the adhesive layer is 0.1 to 500 μm. The technology disclosed herein can be implemented with a configuration including an adhesive layer having the above thickness.
いくつかの好ましい態様において、前記粘着剤層のゲル分率は20%以上70%未満である。粘着剤層のゲル分率を上記の範囲内とすることにより、接着力と保持力とが好適に両立され得る。
In some preferred embodiments, the adhesive layer has a gel fraction of 20% or more and less than 70%. By setting the gel fraction of the pressure-sensitive adhesive layer within the above range, both adhesive strength and holding power can be suitably achieved.
いくつかの好ましい態様に係る粘着シートは、ステンレス鋼板に対する180度剥離強度(対SUS粘着力)が15N/25mm以上である。上記対SUS粘着力を有する粘着シートは、高極性材料に対して優れた接着力を発揮し得る。
The adhesive sheet according to some preferred embodiments has a 180 degree peel strength against a stainless steel plate (adhesive strength against SUS) of 15 N/25 mm or more. The pressure-sensitive adhesive sheet having the adhesive strength against SUS can exhibit excellent adhesive strength against highly polar materials.
いくつかの好ましい態様に係る粘着シートは、ポリプロピレンに対する180度剥離強度(対PP粘着力)が10N/25mm以上である。上記対PP粘着力を有する粘着シートは、低極性材料に対して十分な接着信頼性を有するものとなり得る。
Adhesive sheets according to some preferred embodiments have a 180 degree peel strength against polypropylene (adhesive strength against PP) of 10 N/25 mm or more. The pressure-sensitive adhesive sheet having the above-mentioned adhesive strength to PP can have sufficient adhesion reliability to low polarity materials.
いくつかの好ましい態様に係る粘着シートは、ポリエチレンに対する180度剥離強度(対PE粘着力)が5N/25mm以上である。上記対PE粘着力を有する粘着シートは、低極性材料に対して十分な接着信頼性を有するものとなり得る。
Adhesive sheets according to some preferred embodiments have a 180 degree peel strength against polyethylene (adhesion strength against PE) of 5 N/25 mm or more. The pressure-sensitive adhesive sheet having the above-mentioned adhesive strength to PE can have sufficient adhesion reliability to low polarity materials.
いくつかの好ましい態様に係る粘着シートは、80℃、接着面積10mm×20mm、荷重1.5kg、1時間の条件で実施される保持力試験におけるズレ距離が10mm以下である。上記保持力試験においてズレにくい粘着シートは、十分な保持力(具体的には高温保持力)を有するものとなり得る。
Adhesive sheets according to some preferred embodiments have a displacement distance of 10 mm or less in a holding force test conducted at 80° C., adhesive area 10 mm x 20 mm, load 1.5 kg, and for 1 hour. A pressure-sensitive adhesive sheet that does not easily shift in the above-mentioned holding power test can have sufficient holding power (specifically, high temperature holding power).
ここに開示される粘着シートによると、異種材料に対する接着性と保持力とが両立されるので、高極性材料や低極性材料に接着したり、長期に亘る接着信頼性が求められる用途に好ましく利用され得る。例えば、家電製品や、OA機器、スマートフォン等の携帯電子機器を含む電子機器における部材の固定に好適である。上記より、この明細書によると、ここに開示されるいずれかの粘着シートが用いられた電子機器、換言すると、当該粘着シートを含む電子機器が提供される。
The adhesive sheet disclosed herein has both adhesion and holding power to different materials, so it can be preferably used for adhesion to highly polar or low polar materials, or for applications that require long-term adhesion reliability. can be done. For example, it is suitable for fixing members in electronic devices including home appliances, office automation equipment, and portable electronic devices such as smartphones. As described above, this specification provides an electronic device using any of the adhesive sheets disclosed herein, in other words, an electronic device including the adhesive sheet.
以下、本発明の好適な実施形態を説明する。なお、本明細書において特に言及している事項以外の事柄であって本発明の実施に必要な事柄は、本明細書に記載された発明の実施についての教示と出願時の技術常識とに基づいて当業者に理解され得る。本発明は、本明細書に開示されている内容と当該分野における技術常識とに基づいて実施することができる。また、以下の図面において、同じ作用を奏する部材・部位には同じ符号を付して説明することがあり、重複する説明は省略または簡略化することがある。また、図面に記載の実施形態は、本発明を明瞭に説明するために模式化されており、製品として実際に提供される本発明の粘着シートのサイズや縮尺を必ずしも正確に表したものではない。
Hereinafter, preferred embodiments of the present invention will be described. Matters other than those specifically mentioned in this specification that are necessary for carrying out the present invention are based on the teachings regarding carrying out the invention described in this specification and the common general knowledge at the time of filing. can be understood by those skilled in the art. The present invention can be implemented based on the content disclosed in this specification and the common general knowledge in the field. Furthermore, in the following drawings, members and portions that have the same function may be described with the same reference numerals, and overlapping descriptions may be omitted or simplified. Furthermore, the embodiments shown in the drawings are schematic for clearly explaining the present invention, and do not necessarily accurately represent the size or scale of the adhesive sheet of the present invention that is actually provided as a product. .
本明細書において「粘着剤」とは、前述のように、室温付近の温度域において柔らかい固体(粘弾性体)の状態を呈し、圧力により簡単に被着体に接着する性質を有する材料をいう。ここでいう粘着剤は、「C. A. Dahlquist, “Adhesion : Fundamentals and Practice”, McLaren & Sons, (1966) P. 143」に定義されているとおり、一般的に、複素引張弾性率E*(1Hz)<107dyne/cm2を満たす性質を有する材料(典型的には、25℃において上記性質を有する材料)であり得る。
As mentioned above, the term "adhesive" as used herein refers to a material that exhibits a soft solid (viscoelastic) state in the temperature range around room temperature and has the property of easily adhering to an adherend under pressure. . The adhesive referred to here generally has a complex tensile modulus E * (1Hz) as defined in "C. A. Dahlquist, "Adhesion: Fundamentals and Practice", McLaren & Sons, (1966) P. 143". <10 7 dyne/cm 2 (typically, a material having the above properties at 25° C.).
この明細書において、バイオマス由来の炭素とは、バイオマス材料、すなわち再生可能な有機資源に由来する材料に由来する炭素(再生可能炭素)を意味する。上記バイオマス材料とは、典型的には、太陽光と水と二酸化炭素とが存在すれば持続的な再生産が可能な生物資源(典型的には、光合成を行う植物)に由来する材料のことをいう。したがって、採掘後の使用によって枯渇する化石資源に由来する材料(化石資源系材料)は、ここでいうバイオマス材料の概念から除かれる。粘着剤層および粘着シートのバイオマス炭素比、すなわち該粘着剤層および粘着シートに含まれる全炭素に占めるバイオマス由来炭素の割合は、ASTM D6866に準拠して測定される質量数14の炭素同位体含有量から見積もることができる。
In this specification, biomass-derived carbon means carbon derived from biomass materials, that is, materials derived from renewable organic resources (renewable carbon). The above-mentioned biomass materials are typically materials derived from biological resources (typically plants that perform photosynthesis) that can be reproduced sustainably in the presence of sunlight, water, and carbon dioxide. means. Therefore, materials derived from fossil resources that are depleted through use after mining (fossil resource-based materials) are excluded from the concept of biomass materials here. The biomass carbon ratio of the adhesive layer and the adhesive sheet, that is, the proportion of biomass-derived carbon in the total carbon contained in the adhesive layer and the adhesive sheet, is the carbon isotope content with a mass number of 14 measured in accordance with ASTM D6866. It can be estimated from the amount.
<粘着シートの構成>
ここに開示される粘着シートは、粘着剤層を含んで構成されている。上記粘着シートは、例えば、粘着剤層の一方の表面により構成された第一粘着面と、該粘着剤層の他方の表面により構成された第二粘着面と、を備える基材レス両面粘着シートの形態であり得る。あるいは、ここに開示される粘着シートは、上記粘着剤層が支持基材の片面または両面に積層された基材付き粘着シートの形態であってもよい。以下、支持基材のことを単に「基材」ということもある。なお、ここでいう粘着シートの概念には、粘着テープ、粘着ラベル、粘着フィルム等と称されるものが包含され得る。なお、ここに開示される粘着シートは、ロール状であってもよく、枚葉状であってもよい。あるいは、さらに種々の形状に加工された形態の粘着シートであってもよい。 <Configuration of adhesive sheet>
The adhesive sheet disclosed herein includes an adhesive layer. The above-mentioned pressure-sensitive adhesive sheet is, for example, a base material-less double-sided pressure-sensitive adhesive sheet comprising a first pressure-sensitive adhesive surface formed by one surface of the pressure-sensitive adhesive layer, and a second pressure-sensitive adhesive surface formed by the other surface of the pressure-sensitive adhesive layer. It can be in the form of Alternatively, the pressure-sensitive adhesive sheet disclosed herein may be in the form of a pressure-sensitive adhesive sheet with a base material, in which the pressure-sensitive adhesive layer is laminated on one or both sides of a support base material. Hereinafter, the supporting base material may be simply referred to as "base material". Note that the concept of adhesive sheet here may include what is called an adhesive tape, an adhesive label, an adhesive film, and the like. Note that the pressure-sensitive adhesive sheet disclosed herein may be in the form of a roll or a sheet. Alternatively, the adhesive sheet may be further processed into various shapes.
ここに開示される粘着シートは、粘着剤層を含んで構成されている。上記粘着シートは、例えば、粘着剤層の一方の表面により構成された第一粘着面と、該粘着剤層の他方の表面により構成された第二粘着面と、を備える基材レス両面粘着シートの形態であり得る。あるいは、ここに開示される粘着シートは、上記粘着剤層が支持基材の片面または両面に積層された基材付き粘着シートの形態であってもよい。以下、支持基材のことを単に「基材」ということもある。なお、ここでいう粘着シートの概念には、粘着テープ、粘着ラベル、粘着フィルム等と称されるものが包含され得る。なお、ここに開示される粘着シートは、ロール状であってもよく、枚葉状であってもよい。あるいは、さらに種々の形状に加工された形態の粘着シートであってもよい。 <Configuration of adhesive sheet>
The adhesive sheet disclosed herein includes an adhesive layer. The above-mentioned pressure-sensitive adhesive sheet is, for example, a base material-less double-sided pressure-sensitive adhesive sheet comprising a first pressure-sensitive adhesive surface formed by one surface of the pressure-sensitive adhesive layer, and a second pressure-sensitive adhesive surface formed by the other surface of the pressure-sensitive adhesive layer. It can be in the form of Alternatively, the pressure-sensitive adhesive sheet disclosed herein may be in the form of a pressure-sensitive adhesive sheet with a base material, in which the pressure-sensitive adhesive layer is laminated on one or both sides of a support base material. Hereinafter, the supporting base material may be simply referred to as "base material". Note that the concept of adhesive sheet here may include what is called an adhesive tape, an adhesive label, an adhesive film, and the like. Note that the pressure-sensitive adhesive sheet disclosed herein may be in the form of a roll or a sheet. Alternatively, the adhesive sheet may be further processed into various shapes.
一実施形態に係る粘着シートの構造を図1に模式的に示す。この粘着シート1は、粘着剤層21からなる基材レスの両面粘着シートとして構成されている。粘着シート1は、粘着剤層21の一方の表面(第一面)により構成された第一粘着面21Aと、粘着剤層21の他方の表面(第二面)により構成された第二粘着面21Bとを、被着体の異なる箇所に貼り付けて用いられる。粘着面21A,21Bが貼り付けられる箇所は、異なる部材のそれぞれの箇所であってもよく、単一の部材内の異なる箇所であってもよい。使用前(すなわち、被着体への貼付け前)の粘着シート1は、図1に示すように、第一粘着面21Aおよび第二粘着面21Bが、少なくとも粘着剤層21に対向する側がそれぞれ剥離面となっている剥離ライナー31,32によって保護された形態の剥離ライナー付き粘着シート100の構成要素であり得る。剥離ライナー31,32としては、例えば、シート状の基材(ライナー基材)の片面に剥離処理剤による剥離層を設けることで該片面が剥離面となるように構成されたものを好ましく使用し得る。あるいは、剥離ライナー32を省略し、両面が剥離面となっている剥離ライナー31を用い、これと粘着シート1とを重ね合わせて渦巻き状に巻回することにより第二粘着面21Bが剥離ライナー31の背面に当接して保護された形態(ロール形態)の剥離ライナー付き粘着シートを構成していてもよい。
The structure of a pressure-sensitive adhesive sheet according to one embodiment is schematically shown in FIG. This adhesive sheet 1 is configured as a double-sided adhesive sheet without a base material, which includes an adhesive layer 21. The adhesive sheet 1 has a first adhesive surface 21A constituted by one surface (first surface) of the adhesive layer 21 and a second adhesive surface constituted by the other surface (second surface) of the adhesive layer 21. 21B are attached to different parts of the adherend. The locations on which the adhesive surfaces 21A and 21B are attached may be on different members, or may be on different locations within a single member. As shown in FIG. 1, the adhesive sheet 1 before use (that is, before being attached to an adherend) has a first adhesive surface 21A and a second adhesive surface 21B that are peeled off at least on the side facing the adhesive layer 21. It may be a component of the adhesive sheet 100 with a release liner that is protected by the release liners 31 and 32 serving as surfaces. As the release liners 31 and 32, it is preferable to use, for example, a sheet-like base material (liner base material) that is constructed by providing a release layer made of a release treatment agent on one side so that one side becomes a release surface. obtain. Alternatively, the release liner 32 may be omitted and a release liner 31 having release surfaces on both sides may be used, and this and the adhesive sheet 1 may be overlapped and spirally wound so that the second adhesive surface 21B is on the release liner 31. The pressure-sensitive adhesive sheet with a release liner may be in a protected form (roll form) in contact with the back surface of the adhesive sheet.
他の一実施形態に係る粘着シートの構造を図2に模式的に示す。この粘着シート2は、第一面10Aおよび第二面10Bを有するシート状の支持基材(例えば樹脂フィルム)10と、その第一面10A側に設けられた粘着剤層21とを備える基材付き片面粘着シートとして構成されている。粘着剤層21は、支持基材10の第一面10A側に固定的に、すなわち当該支持基材10から粘着剤層21を分離する意図なく、設けられている。使用前の粘着シート2は、図2に示すように、粘着剤層21の表面(粘着面)21Aが、少なくとも粘着剤層21に対向する側が剥離面となっている剥離ライナー31によって保護された形態の剥離ライナー付き粘着シート200の構成要素であり得る。あるいは、剥離ライナー31を省略し、第二面10Bが剥離面となっている支持基材10を用い、粘着シート2を巻回することにより粘着面21Aが支持基材10の第二面(背面)10Bに当接して保護された形態(ロール形態)であってもよい。
The structure of a pressure-sensitive adhesive sheet according to another embodiment is schematically shown in FIG. 2. This adhesive sheet 2 is a base material comprising a sheet-shaped support base material (for example, a resin film) 10 having a first surface 10A and a second surface 10B, and an adhesive layer 21 provided on the first surface 10A side. It is constructed as a single-sided adhesive sheet. The adhesive layer 21 is fixedly provided on the first surface 10A side of the support base material 10, that is, without the intention of separating the adhesive layer 21 from the support base material 10. As shown in FIG. 2, the pressure-sensitive adhesive sheet 2 before use has a surface (adhesive surface) 21A of the pressure-sensitive adhesive layer 21 protected by a release liner 31 having a release surface at least on the side facing the pressure-sensitive adhesive layer 21. It may be a component of the pressure-sensitive adhesive sheet 200 with a release liner. Alternatively, the release liner 31 may be omitted, the second surface 10B may be the release surface, and the adhesive sheet 2 may be wound so that the adhesive surface 21A is the second surface (back surface) of the support substrate 10. ) 10B may be in a protected form (roll form).
さらに他の一実施形態に係る粘着シートの構造を図3に模式的に示す。この粘着シート3は、第一面10Aおよび第二面10Bを有するシート状の支持基材(例えば樹脂フィルム)10と、その第一面10A側に固定的に設けられた第一粘着剤層21と、第二面10B側に固定的に設けられた第二粘着剤層22と、を備える基材付き両面粘着シートとして構成されている。使用前の粘着シート3は、図3に示すように、第一粘着剤層21の表面(第一粘着面)21Aおよび第二粘着剤層22の表面(第二粘着面)22Aが剥離ライナー31,32によって保護された形態の剥離ライナー付き粘着シート300の構成要素であり得る。あるいは、剥離ライナー32を省略し、両面が剥離面となっている剥離ライナー31を用い、これと粘着シート3とを重ね合わせて渦巻き状に巻回することにより第二粘着面22Aが剥離ライナー31の背面に当接して保護された形態(ロール形態)の剥離ライナー付き粘着シートを構成していてもよい。
FIG. 3 schematically shows the structure of a pressure-sensitive adhesive sheet according to yet another embodiment. This adhesive sheet 3 includes a sheet-shaped support base material (for example, a resin film) 10 having a first surface 10A and a second surface 10B, and a first adhesive layer 21 fixedly provided on the first surface 10A side. and a second adhesive layer 22 fixedly provided on the second surface 10B side. In the adhesive sheet 3 before use, as shown in FIG. , 32 may be a component of the adhesive sheet 300 with a release liner. Alternatively, the release liner 32 may be omitted and a release liner 31 having release surfaces on both sides may be used, and this and the adhesive sheet 3 may be overlapped and spirally wound so that the second adhesive surface 22A is on the release liner 31. The pressure-sensitive adhesive sheet with a release liner may be in a protected form (roll form) in contact with the back surface of the adhesive sheet.
なお、上記基材付き両面粘着シートにおいては、第一粘着剤層および第二粘着剤層の少なくとも一方の粘着剤層(例えば第一粘着剤層)が、以下で説明される粘着剤層であればよく、他方の粘着剤層(例えば第二粘着剤層)は、ここに開示される粘着剤層であってもよく、ここに開示される粘着剤層(具体的には、上記一方の粘着剤層。例えば第一粘着剤層)とは異なる組成を有する粘着剤層であってもよい。そのような他方の粘着剤層は、例えば、公知ないし慣用の粘着剤から形成されたものであり得る。
In addition, in the double-sided pressure-sensitive adhesive sheet with a base material, at least one of the first pressure-sensitive adhesive layer and the second pressure-sensitive adhesive layer (for example, the first pressure-sensitive adhesive layer) may be the pressure-sensitive adhesive layer described below. The other adhesive layer (for example, the second adhesive layer) may be the adhesive layer disclosed herein, and the adhesive layer disclosed herein (specifically, the one adhesive layer described above) may be the adhesive layer disclosed herein. The adhesive layer may have a composition different from that of the adhesive layer (for example, the first adhesive layer). The other pressure-sensitive adhesive layer may be formed from a known or commonly used pressure-sensitive adhesive, for example.
<粘着剤層>
ここに開示される粘着シートを構成する粘着剤層はアクリル系ポリマーを含む。上記粘着剤層は、典型的にはアクリル系ポリマーをベースポリマーとする粘着剤層である。そのような粘着剤層は、アクリル系粘着剤層ともいう。なお、ベースポリマーとは、粘着剤層に含まれるゴム状ポリマー(室温付近の温度域においてゴム弾性を示すポリマー)の主成分をいう。また、この明細書において「主成分」とは、特記しない場合、50重量%を超えて含まれる成分を指す。また、粘着剤および粘着剤層に含まれ得る成分に関する下記の説明は、特に断りがないかぎり粘着剤(層)を形成するために用いられる粘着剤組成物にも適用可能である。 <Adhesive layer>
The adhesive layer constituting the adhesive sheet disclosed herein contains an acrylic polymer. The pressure-sensitive adhesive layer is typically a pressure-sensitive adhesive layer containing an acrylic polymer as a base polymer. Such an adhesive layer is also referred to as an acrylic adhesive layer. Note that the base polymer refers to the main component of a rubbery polymer (a polymer that exhibits rubber elasticity in a temperature range around room temperature) contained in the adhesive layer. Furthermore, in this specification, the term "main component" refers to a component contained in an amount exceeding 50% by weight, unless otherwise specified. Further, the following description regarding the adhesive and the components that can be included in the adhesive layer is also applicable to the adhesive composition used to form the adhesive (layer) unless otherwise specified.
ここに開示される粘着シートを構成する粘着剤層はアクリル系ポリマーを含む。上記粘着剤層は、典型的にはアクリル系ポリマーをベースポリマーとする粘着剤層である。そのような粘着剤層は、アクリル系粘着剤層ともいう。なお、ベースポリマーとは、粘着剤層に含まれるゴム状ポリマー(室温付近の温度域においてゴム弾性を示すポリマー)の主成分をいう。また、この明細書において「主成分」とは、特記しない場合、50重量%を超えて含まれる成分を指す。また、粘着剤および粘着剤層に含まれ得る成分に関する下記の説明は、特に断りがないかぎり粘着剤(層)を形成するために用いられる粘着剤組成物にも適用可能である。 <Adhesive layer>
The adhesive layer constituting the adhesive sheet disclosed herein contains an acrylic polymer. The pressure-sensitive adhesive layer is typically a pressure-sensitive adhesive layer containing an acrylic polymer as a base polymer. Such an adhesive layer is also referred to as an acrylic adhesive layer. Note that the base polymer refers to the main component of a rubbery polymer (a polymer that exhibits rubber elasticity in a temperature range around room temperature) contained in the adhesive layer. Furthermore, in this specification, the term "main component" refers to a component contained in an amount exceeding 50% by weight, unless otherwise specified. Further, the following description regarding the adhesive and the components that can be included in the adhesive layer is also applicable to the adhesive composition used to form the adhesive (layer) unless otherwise specified.
また、本明細書において、「アクリル系ポリマー」とは、該ポリマーを構成するモノマー単位として、1分子中に少なくとも1つの(メタ)アクリロイル基を有するモノマーに由来するモノマー単位を含む重合物をいう。以下、1分子中に少なくとも1つの(メタ)アクリロイル基を有するモノマーを「アクリル系モノマー」ともいう。したがって、この明細書におけるアクリル系ポリマーは、アクリル系モノマーに由来するモノマー単位を含むポリマーとして定義される。なお、この明細書において「(メタ)アクリロイル」とは、アクリロイルおよびメタクリロイルを包括的に指す意味である。同様に、「(メタ)アクリレート」とはアクリレートおよびメタクリレートを、「(メタ)アクリル」とはアクリルおよびメタクリルを、それぞれ包括的に指す意味である。
In addition, as used herein, the term "acrylic polymer" refers to a polymer containing monomer units derived from a monomer having at least one (meth)acryloyl group in one molecule, as monomer units constituting the polymer. . Hereinafter, a monomer having at least one (meth)acryloyl group in one molecule will also be referred to as an "acrylic monomer." Accordingly, an acrylic polymer in this specification is defined as a polymer containing monomer units derived from acrylic monomers. In addition, in this specification, "(meth)acryloyl" refers comprehensively to acryloyl and methacryloyl. Similarly, "(meth)acrylate" comprehensively refers to acrylate and methacrylate, and "(meth)acrylic" comprehensively refers to acrylic and methacrylic.
(アクリル系ポリマー)
ここに開示される技術で用いられるアクリル系ポリマーとしては、n-ヘプチルアクリレートを含むモノマー成分の重合物が用いられる。n-ヘプチルアクリレートを含むモノマー成分を用いて重合されたアクリル系ポリマーは、n-ブチルアクリレート(BA)や2-エチルヘキシルアクリレート(2EHA)等の他のアルキルアクリレートの重合物よりも柔軟性に優れ、高極性材料、低極性材料のいずれに対してもより優れた接着力を発揮し得る。その理由は、特に限定的に解釈されるものではないが、n-ヘプチルアクリレートをモノマー単位として含むポリマーは、ガラス転移温度が低いことに加え、比較的長い直鎖状の側鎖を有することから、粘着剤内において主鎖間の空間が相対的に大きいためと考えられる。 (acrylic polymer)
As the acrylic polymer used in the technique disclosed herein, a polymer of monomer components containing n-heptyl acrylate is used. Acrylic polymers polymerized using monomer components containing n-heptyl acrylate have superior flexibility than polymers of other alkyl acrylates such as n-butyl acrylate (BA) and 2-ethylhexyl acrylate (2EHA). It can exhibit superior adhesive strength to both high polarity materials and low polarity materials. The reason for this is not particularly limited, but is because polymers containing n-heptyl acrylate as a monomer unit have relatively long linear side chains in addition to having a low glass transition temperature. This is thought to be due to the relatively large space between the main chains within the adhesive.
ここに開示される技術で用いられるアクリル系ポリマーとしては、n-ヘプチルアクリレートを含むモノマー成分の重合物が用いられる。n-ヘプチルアクリレートを含むモノマー成分を用いて重合されたアクリル系ポリマーは、n-ブチルアクリレート(BA)や2-エチルヘキシルアクリレート(2EHA)等の他のアルキルアクリレートの重合物よりも柔軟性に優れ、高極性材料、低極性材料のいずれに対してもより優れた接着力を発揮し得る。その理由は、特に限定的に解釈されるものではないが、n-ヘプチルアクリレートをモノマー単位として含むポリマーは、ガラス転移温度が低いことに加え、比較的長い直鎖状の側鎖を有することから、粘着剤内において主鎖間の空間が相対的に大きいためと考えられる。 (acrylic polymer)
As the acrylic polymer used in the technique disclosed herein, a polymer of monomer components containing n-heptyl acrylate is used. Acrylic polymers polymerized using monomer components containing n-heptyl acrylate have superior flexibility than polymers of other alkyl acrylates such as n-butyl acrylate (BA) and 2-ethylhexyl acrylate (2EHA). It can exhibit superior adhesive strength to both high polarity materials and low polarity materials. The reason for this is not particularly limited, but is because polymers containing n-heptyl acrylate as a monomer unit have relatively long linear side chains in addition to having a low glass transition temperature. This is thought to be due to the relatively large space between the main chains within the adhesive.
アクリル系ポリマーのモノマー成分に占めるn-ヘプチルアクリレートの割合は、例えば、いくつかの態様において、50重量%以上(例えば50重量%超)であり、好ましくは70重量%以上、より好ましくは80重量%以上、さらに好ましくは85重量%以上、特に好ましくは90重量%以上であり、92重量%以上でもよく、94重量%以上でもよく、96重量%以上でもよい。n-ヘプチルアクリレートの使用量を増大することにより、その使用効果を効果的に発現させることができる。一方、カルボキシ基含有モノマーや、その他のモノマーを共重合する観点から、モノマー成分中のn-ヘプチルアクリレートの割合は、97重量%未満であり、いくつかの態様において、95重量%以下であってもよく、93重量%以下でもよく、91重量%以下でもよい。
For example, in some embodiments, the proportion of n-heptyl acrylate in the monomer components of the acrylic polymer is 50% by weight or more (for example, more than 50% by weight), preferably 70% by weight or more, more preferably 80% by weight. % or more, more preferably 85% by weight or more, particularly preferably 90% by weight or more, may be 92% by weight or more, may be 94% by weight or more, or may be 96% by weight or more. By increasing the amount of n-heptyl acrylate used, the effects of its use can be effectively expressed. On the other hand, from the viewpoint of copolymerizing carboxyl group-containing monomers and other monomers, the proportion of n-heptyl acrylate in the monomer components is less than 97% by weight, and in some embodiments is 95% by weight or less. The content may be 93% by weight or less, or may be 91% by weight or less.
アクリル系ポリマーには、n-ヘプチルアクリレート以外のアルキル(メタ)アクリレート(以下、「任意アルキル(メタ)アクリレート」ともいう。)が共重合されていてもよい。任意アルキル(メタ)アクリレートとしては、例えば下記式(1)で表される化合物を好適に用いることができる。
CH2=C(R1)COOR2 (1)
ここで、上記式(1)中のR1は水素原子またはメチル基である。また、R2は炭素原子数1~20の鎖状アルキル基(ただし、n-ヘプチル基を除く。)である。 The acrylic polymer may be copolymerized with an alkyl (meth)acrylate (hereinafter also referred to as "optional alkyl (meth)acrylate") other than n-heptyl acrylate. As the optional alkyl (meth)acrylate, for example, a compound represented by the following formula (1) can be suitably used.
CH 2 =C(R 1 )COOR 2 (1)
Here, R 1 in the above formula (1) is a hydrogen atom or a methyl group. Further, R 2 is a chain alkyl group having 1 to 20 carbon atoms (excluding n-heptyl group).
CH2=C(R1)COOR2 (1)
ここで、上記式(1)中のR1は水素原子またはメチル基である。また、R2は炭素原子数1~20の鎖状アルキル基(ただし、n-ヘプチル基を除く。)である。 The acrylic polymer may be copolymerized with an alkyl (meth)acrylate (hereinafter also referred to as "optional alkyl (meth)acrylate") other than n-heptyl acrylate. As the optional alkyl (meth)acrylate, for example, a compound represented by the following formula (1) can be suitably used.
CH 2 =C(R 1 )COOR 2 (1)
Here, R 1 in the above formula (1) is a hydrogen atom or a methyl group. Further, R 2 is a chain alkyl group having 1 to 20 carbon atoms (excluding n-heptyl group).
上記任意アルキル(メタ)アクリレートとしては、例えばメチル(メタ)アクリレート、エチル(メタ)アクリレート、プロピル(メタ)アクリレート、イソプロピル(メタ)アクリレート、n-ブチル(メタ)アクリレート、イソブチル(メタ)アクリレート、s-ブチル(メタ)アクリレート、ペンチル(メタ)アクリレート、イソペンチル(メタ)アクリレート、ヘキシル(メタ)アクリレート、ヘプチルメタクリレート、2-エチルヘキシル(メタ)アクリレート、オクチル(メタ)アクリレート、イソオクチル(メタ)アクリレート、ノニル(メタ)アクリレート、イソノニル(メタ)アクリレート、デシル(メタ)アクリレート、イソデシル(メタ)アクリレート、ウンデシル(メタ)アクリレート、ラウリル(メタ)アクリレート、トリデシル(メタ)アクリレート、テトラデシル(メタ)アクリレート、ペンタデシル(メタ)アクリレート、ヘキサデシル(メタ)アクリレート、ヘプタデシル(メタ)アクリレート、オクタデシル(メタ)アクリレート、ノナデシル(メタ)アクリレート、エイコシル(メタ)アクリレート等が挙げられる。これら任意アルキル(メタ)アクリレートは、1種を単独でまたは2種以上を組み合わせて用いることができる。好ましく使用し得る任意アルキル(メタ)アクリレートとして、n-ブチルアクリレート(BA)および2-エチルヘキシルアクリレート(2EHA)が挙げられる。
Examples of the above-mentioned optional alkyl (meth)acrylate include methyl (meth)acrylate, ethyl (meth)acrylate, propyl (meth)acrylate, isopropyl (meth)acrylate, n-butyl (meth)acrylate, isobutyl (meth)acrylate, s -Butyl (meth)acrylate, pentyl (meth)acrylate, isopentyl (meth)acrylate, hexyl (meth)acrylate, heptyl methacrylate, 2-ethylhexyl (meth)acrylate, octyl (meth)acrylate, isooctyl (meth)acrylate, nonyl ( meth)acrylate, isononyl(meth)acrylate, decyl(meth)acrylate, isodecyl(meth)acrylate, undecyl(meth)acrylate, lauryl(meth)acrylate, tridecyl(meth)acrylate, tetradecyl(meth)acrylate, pentadecyl(meth)acrylate Acrylate, hexadecyl (meth)acrylate, heptadecyl (meth)acrylate, octadecyl (meth)acrylate, nonadecyl (meth)acrylate, eicosyl (meth)acrylate, and the like. These optional alkyl (meth)acrylates can be used alone or in combination of two or more. Optional alkyl (meth)acrylates that may be preferably used include n-butyl acrylate (BA) and 2-ethylhexyl acrylate (2EHA).
モノマー成分に含まれる任意アルキル(メタ)アクリレートの割合は、例えば、いくつかの態様において、47重量%未満であり、45重量%以下であってもよく、30重量%以下でもよく、10重量%以下でもよく、5重量%以下でもよく、1重量%以下でもよい。ここに開示される技術は、モノマー成分が任意アルキル(メタ)アクリレートを実質的に含まない態様で好ましく実施され得る。
The proportion of optional alkyl (meth)acrylate included in the monomer component is, for example, in some embodiments less than 47% by weight, may be less than 45% by weight, may be less than 30% by weight, and may be less than 10% by weight. It may be less than 5% by weight, or less than 1% by weight. The technology disclosed herein can be preferably carried out in an embodiment in which the monomer component does not substantially contain any alkyl (meth)acrylate.
なお、本明細書において、モノマー成分がモノマーA(例えば上記任意アルキル(メタ)アクリレート)を実質的に含まないとは、少なくとも意図的には当該モノマーAを用いないことをいい、当該モノマーAが例えば0.01重量%以下程度、非意図的に含まれることは許容され得る。
In addition, in this specification, when the monomer component does not substantially contain monomer A (for example, the above-mentioned optional alkyl (meth)acrylate), it means that the monomer A is not used, at least intentionally, and the monomer component is For example, unintentional inclusion of about 0.01% by weight or less is acceptable.
いくつかの態様において、上記モノマー成分は、バイオマス由来のアルキル基をエステル末端に有するアルキル(メタ)アクリレート(以下「バイオマスアルキル(メタ)アクリレート」ともいう。)を含み得る。近年、地球温暖化等の環境問題が重視されるようになり、石油等の化石資源系材料の使用量を低減することが望まれている。このような状況下、粘着剤の分野においても化石資源系材料の使用量を低減することが求められている。バイオマスアルキル(メタ)アクリレートを用いることにより、化石資源系材料への依存抑制に配慮されたアクリル系粘着剤を好適に実現することができる。
In some embodiments, the monomer component may include an alkyl (meth)acrylate having a biomass-derived alkyl group at the ester end (hereinafter also referred to as "biomass alkyl (meth)acrylate"). In recent years, environmental issues such as global warming have become more important, and it is desired to reduce the amount of fossil resource-based materials such as petroleum used. Under these circumstances, there is a need to reduce the amount of fossil resource-based materials used in the adhesive field as well. By using biomass alkyl (meth)acrylate, it is possible to suitably realize an acrylic pressure-sensitive adhesive that is designed to reduce dependence on fossil resource-based materials.
バイオマスアルキル(メタ)アクリレートは、特に限定されず、例えば、バイオマス由来のアルカノールと、バイオマス由来または非バイオマス由来の(メタ)アクリル酸とのエステルである。バイオマス由来のアルカノールの例には、バイオマスエタノール、パーム油やパーム核油、ヤシ油、ヒマシ油等の植物原料に由来するアルカノール、等が含まれる。バイオマス由来のアルカノールの炭素原子数が3以上である場合、該アルカノールは、直鎖状であってもよく、分岐を有していてもよい。いくつかの態様において、アクリル系ポリマーの合成に用いられるバイオマスアルキル(メタ)アクリレートとして、バイオマス由来のアルカノールと、非バイオマス由来の(メタ)アクリル酸とのエステルが用いられる。かかるバイオマスアルキル(メタ)アクリレートでは、アルカノールの炭素原子数が多いほど、該バイオマスアルキル(メタ)アクリレートに含まれる総炭素数に占めるバイオマス由来炭素の個数割合、すなわちアルキル(メタ)アクリレートのバイオマス炭素比が高くなる。したがって、上記のバイオマスアルキル(メタ)アクリレートでは、バイオマス由来となるアルキル基の炭素数が多いことが、化石資源系材料への依存度低減の点で望ましい。その一方で、アルキル(メタ)アクリレートを構成するアルキル基の炭素数が多すぎると、接着力等の粘着特性が得られにくくなる傾向があり、また合成や取扱い性、コストなど生産性の点でも不利になり得る。バイオマスアルキル(メタ)アクリレートとして、バイオマス由来のアルカノールと、非バイオマス由来の(メタ)アクリル酸とのエステルを用いる態様では、粘着特性と、化石資源系材料への依存度低減(より具体的には上記アルキル(メタ)アクリレートのバイオマス炭素比)とをバランスよく両立する材料を用いることが望ましい。
The biomass alkyl (meth)acrylate is not particularly limited, and is, for example, an ester of a biomass-derived alkanol and a biomass-derived or non-biomass-derived (meth)acrylic acid. Examples of alkanols derived from biomass include biomass ethanol, alkanols derived from plant materials such as palm oil, palm kernel oil, coconut oil, and castor oil. When the biomass-derived alkanol has three or more carbon atoms, the alkanol may be linear or branched. In some embodiments, an ester of a biomass-derived alkanol and a non-biomass-derived (meth)acrylic acid is used as the biomass alkyl (meth)acrylate used in the synthesis of the acrylic polymer. In such a biomass alkyl (meth)acrylate, the greater the number of carbon atoms in the alkanol, the greater the number ratio of biomass-derived carbon to the total number of carbons contained in the biomass alkyl (meth)acrylate, that is, the biomass carbon ratio of the alkyl (meth)acrylate. becomes higher. Therefore, in the above-mentioned biomass alkyl (meth)acrylate, it is desirable that the alkyl group derived from biomass has a large number of carbon atoms in order to reduce dependence on fossil resource materials. On the other hand, if the number of carbon atoms in the alkyl group constituting the alkyl (meth)acrylate is too large, it tends to be difficult to obtain adhesive properties such as adhesive strength, and it also has a negative impact on productivity such as synthesis, ease of handling, and cost. It can be disadvantageous. In an embodiment in which an ester of biomass-derived alkanol and non-biomass-derived (meth)acrylic acid is used as the biomass alkyl (meth)acrylate, adhesive properties and reduced dependence on fossil resource materials (more specifically, It is desirable to use a material that is compatible with the above-mentioned alkyl (meth)acrylate biomass carbon ratio) in a well-balanced manner.
いくつかの好ましい態様において、n-ヘプチルアクリレートとして、バイオマス由来のn-ヘプチルアクリレート(バイオマスn-ヘプチルアクリレート)が用いられる。バイオマスn-ヘプチルアクリレートを用いることにより、化石資源系材料への依存度を低減しつつ、ここに開示される技術による効果を実現することができる。上記バイオマスn-ヘプチルアクリレートは、バイオマス由来のアルカノールと、バイオマス由来または非バイオマス由来のアクリル酸とのエステルであり、例えば、バイオマス由来のアルカノールと非バイオマス由来のアクリル酸とのエステルが用いられ得る。かかる化合物では、直鎖ヘプチル基のみがバイオマス由来となる。
In some preferred embodiments, biomass-derived n-heptyl acrylate (biomass n-heptyl acrylate) is used as n-heptyl acrylate. By using biomass n-heptyl acrylate, the effects of the technology disclosed herein can be achieved while reducing dependence on fossil resource materials. The biomass n-heptyl acrylate is an ester of a biomass-derived alkanol and a biomass-derived or non-biomass-derived acrylic acid. For example, an ester of a biomass-derived alkanol and a non-biomass-derived acrylic acid can be used. In such compounds, only the linear heptyl groups are derived from biomass.
上記アクリル系ポリマーのモノマー成分に占めるバイオマスアルキル(メタ)アクリレート(好ましくはバイオマスn-ヘプチルアクリレート)の割合は、例えば、いくつかの態様において、50重量%以上(例えば50重量%超)であり、好ましくは70重量%以上、より好ましくは80重量%以上、さらに好ましくは85重量%以上、特に好ましくは90重量%以上であり、92重量%以上でもよく、94重量%以上でもよく、96重量%以上でもよい。また、モノマー成分のうちバイオマスアルキル(メタ)アクリレート(好ましくはバイオマスn-ヘプチルアクリレート)の割合は、97重量%未満であり、いくつかの態様において、95重量%以下であってもよく、93重量%以下でもよく、91重量%以下でもよい。他のいくつかの態様において、モノマー成分に占めるバイオマスアルキル(メタ)アクリレートの割合は、90重量%以下でもよく、70重量%以下でもよく、50重量%以下でもよく、30重量%以下でもよく、10重量%以下でもよく、1重量%以下でもよい。
The proportion of biomass alkyl (meth)acrylate (preferably biomass n-heptyl acrylate) in the monomer components of the acrylic polymer is, for example, 50% by weight or more (for example, more than 50% by weight) in some embodiments, Preferably 70% by weight or more, more preferably 80% by weight or more, further preferably 85% by weight or more, particularly preferably 90% by weight or more, may be 92% by weight or more, may be 94% by weight or more, and may be 96% by weight. The above is fine. Further, the proportion of biomass alkyl (meth)acrylate (preferably biomass n-heptyl acrylate) among the monomer components is less than 97% by weight, and in some embodiments may be 95% by weight or less, and in some embodiments may be 93% by weight or less. % or less, or 91% by weight or less. In some other embodiments, the proportion of biomass alkyl (meth)acrylate in the monomer component may be 90% by weight or less, 70% by weight or less, 50% by weight or less, 30% by weight or less, It may be 10% by weight or less, or 1% by weight or less.
また、アクリル系ポリマーのモノマー成分は、カルボキシ基含有モノマーを3重量%よりも多く含む。カルボキシ基含有モノマーは、その極性に基づく凝集性向上を発揮することができる。また、イソシアネート系、エポキシ系架橋剤等の架橋剤を使用する場合には、当該カルボキシ基がアクリル系ポリマーの架橋点となり得る。カルボキシ基含有モノマーを3重量%超使用することで、異種材料に対して高い接着力を有しつつ、十分な保持力を得ることができる。また、カルボキシ基含有モノマーの使用により、高極性材料に対して、より優れた接着性を得ることができる。
Furthermore, the monomer component of the acrylic polymer contains more than 3% by weight of a carboxyl group-containing monomer. Carboxy group-containing monomers can exhibit improved cohesiveness based on their polarity. Further, when using a crosslinking agent such as an isocyanate type crosslinking agent or an epoxy type crosslinking agent, the carboxy group can serve as a crosslinking point of the acrylic polymer. By using more than 3% by weight of the carboxy group-containing monomer, it is possible to obtain sufficient holding power while having high adhesive strength to different materials. Further, by using a carboxy group-containing monomer, better adhesion to highly polar materials can be obtained.
カルボキシ基含有モノマーとしては、アクリル酸(AA)、メタクリル酸(MAA)、カルボキシエチル(メタ)アクリレート、カルボキシペンチル(メタ)アクリレート、イタコン酸、マレイン酸、フマル酸、クロトン酸、イソクロトン酸等が例示される。なかでも好ましいカルボキシ基含有モノマーとして、AAおよびMAAが挙げられる。AAが特に好ましい。カルボキシ基含有モノマーは、1種を単独でまたは2種以上を組み合わせて用いることができる。
Examples of carboxy group-containing monomers include acrylic acid (AA), methacrylic acid (MAA), carboxyethyl (meth)acrylate, carboxypentyl (meth)acrylate, itaconic acid, maleic acid, fumaric acid, crotonic acid, isocrotonic acid, etc. be done. Among these, preferred carboxy group-containing monomers include AA and MAA. AA is particularly preferred. Carboxy group-containing monomers can be used singly or in combination of two or more.
アクリル系ポリマーのモノマー成分中のカルボキシ基含有モノマーの割合は、3重量%よりも多く(具体的には3.0重量%超)、好ましくは4.0重量%以上であり、より好ましくは4.5重量%以上、さらに好ましくは5.0重量%以上(例えば5.0重量%超)、特に好ましくは5.5重量%以上であり、6.0重量%以上であってもよい。いくつかの態様において、モノマー成分に占めるカルボキシ基含有モノマーの割合は、7.0重量%以上であってもよく、8.0重量%以上(例えば8.0重量%超)でもよく、9.0重量%以上でもよい。カルボキシ基含有モノマーの使用量を多くすることで、カルボキシ基含有モノマーの作用に基づき粘着剤層の凝集力が向上するので、アクリル系ポリマーのMwを適度に低く設計し、接着力を高めることが可能となる。また、カルボキシ基含有モノマーの量は、例えば、全モノマー成分の20重量%以下とすることが適当であり、好ましくは15重量%以下、より好ましくは12重量%以下である。いくつかの態様において、上記カルボキシ基含有モノマーの量は、10重量%未満であってもよく、8重量%未満でもよく、6重量%未満でもよく、5重量%未満でもよい。カルボキシ基含有モノマーの使用量を上記範囲内で適切に調節することにより、高極性材料に対する接着力、低極性材料に対する接着力および保持力をバランスよく両立することができる。
The proportion of the carboxy group-containing monomer in the monomer component of the acrylic polymer is more than 3% by weight (specifically more than 3.0% by weight), preferably 4.0% by weight or more, more preferably 4% by weight or more. The content is .5% by weight or more, more preferably 5.0% by weight or more (for example, more than 5.0% by weight), particularly preferably 5.5% by weight or more, and may be 6.0% by weight or more. In some embodiments, the proportion of the carboxy group-containing monomer in the monomer component may be 7.0% by weight or more, 8.0% by weight or more (for example, more than 8.0% by weight), and 9. It may be 0% by weight or more. By increasing the amount of the carboxyl group-containing monomer used, the cohesive force of the adhesive layer will improve based on the action of the carboxyl group-containing monomer, so it is possible to design the Mw of the acrylic polymer to be appropriately low and increase the adhesive force. It becomes possible. Further, the amount of the carboxy group-containing monomer is, for example, suitably 20% by weight or less of the total monomer components, preferably 15% by weight or less, and more preferably 12% by weight or less. In some embodiments, the amount of the carboxy group-containing monomer may be less than 10% by weight, may be less than 8% by weight, may be less than 6% by weight, and may be less than 5% by weight. By appropriately adjusting the amount of the carboxyl group-containing monomer within the above range, it is possible to achieve a good balance between adhesion to highly polar materials, adhesion to low polar materials, and holding power.
アクリル系ポリマーには、カルボキシ基含有モノマー以外の官能基含有モノマー(任意官能基含有モノマー)が共重合されていてもよい。アクリル系ポリマーに架橋基点となり得る官能基を導入し、あるいは接着力の向上に寄与し得る任意官能基含有モノマーとしては、水酸基(OH基)含有モノマー(2-ヒドロキシエチル(メタ)アクリレート、2-ヒドロキシプロピル(メタ)アクリレート、3-ヒドロキシプロピル(メタ)アクリレート、2-ヒドロキシブチル(メタ)アクリレート、4-ヒドロキシブチル(メタ)アクリレート等のヒドロキシアルキル(メタ)アクリレート;ポリプロピレングリコールモノ(メタ)アクリレート等)、酸無水物基含有モノマー、アミド基含有モノマー((メタ)アクリルアミド、N,N-ジメチル(メタ)アクリルアミド等)、アミノ基含有モノマー(アミノエチル(メタ)アクリレート、N,N-ジメチルアミノエチル(メタ)アクリレート等)、エポキシ基含有モノマー、シアノ基含有モノマー、ケト基含有モノマー、窒素原子含有環を有するモノマー(N-ビニル-2-ピロリドン、N-(メタ)アクリロイルモルホリン等)、アルコキシシリル基含有モノマー、イミド基含有モノマー類等が挙げられる。上記任意官能基含有モノマーは、1種を単独でまたは2種以上を組み合わせて用いることができる。
The acrylic polymer may be copolymerized with a functional group-containing monomer (any functional group-containing monomer) other than the carboxy group-containing monomer. Examples of optional functional group-containing monomers that can introduce functional groups that can serve as crosslinking base points into acrylic polymers or contribute to improving adhesive strength include hydroxyl group (OH group)-containing monomers (2-hydroxyethyl (meth)acrylate, 2-hydroxyethyl (meth)acrylate, 2- Hydroxyalkyl (meth)acrylates such as hydroxypropyl (meth)acrylate, 3-hydroxypropyl (meth)acrylate, 2-hydroxybutyl (meth)acrylate, 4-hydroxybutyl (meth)acrylate; polypropylene glycol mono(meth)acrylate, etc. ), acid anhydride group-containing monomers, amide group-containing monomers ((meth)acrylamide, N,N-dimethyl(meth)acrylamide, etc.), amino group-containing monomers (aminoethyl (meth)acrylate, N,N-dimethylaminoethyl (meth)acrylate, etc.), epoxy group-containing monomers, cyano group-containing monomers, keto group-containing monomers, monomers with nitrogen atom-containing rings (N-vinyl-2-pyrrolidone, N-(meth)acryloylmorpholine, etc.), alkoxysilyl Examples include group-containing monomers, imide group-containing monomers, and the like. The above arbitrary functional group-containing monomers can be used alone or in combination of two or more.
アクリル系ポリマーを構成するモノマー成分が上述の任意官能基含有モノマーを含む場合、該モノマー成分における任意官能基含有モノマーの含有量は特に限定されない。任意官能基含有モノマーの使用による効果を適切に発揮する観点から、モノマー成分における任意官能基含有モノマーの含有量は、例えば0.1重量%以上とすることができ、0.5重量%以上とすることが適当であり、1重量%以上としてもよい。また、n-ヘプチルアクリレートおよびカルボキシ基含有モノマーとの関係で粘着性能のバランスをとりやすくする観点から、モノマー成分における任意官能基含有モノマーの含有量は、40重量%以下とすることが適当であり、20重量%以下とすることが好ましく、10重量%以下(例えば5重量%以下)としてもよい。いくつかの態様において、モノマー成分における任意官能基含有モノマーの含有量は、例えば3重量%未満であり、1重量%未満であってもよく、0.5重量%未満でもよく、0.3重量%未満でもよく、0.1重量%未満でもよい。ここに開示される技術は、アクリル系ポリマーのモノマー成分が任意官能基含有モノマーを実質的に含まない態様で好ましく実施され得る。
When the monomer component constituting the acrylic polymer contains the above-mentioned optional functional group-containing monomer, the content of the optional functional group-containing monomer in the monomer component is not particularly limited. From the viewpoint of appropriately exhibiting the effect of using the optional functional group-containing monomer, the content of the optional functional group-containing monomer in the monomer component can be, for example, 0.1% by weight or more, and 0.5% by weight or more. It is appropriate that the amount is 1% by weight or more. In addition, from the viewpoint of making it easier to balance the adhesive performance in relation to n-heptyl acrylate and the carboxyl group-containing monomer, it is appropriate that the content of the arbitrary functional group-containing monomer in the monomer component is 40% by weight or less. , is preferably 20% by weight or less, and may be 10% by weight or less (for example, 5% by weight or less). In some embodiments, the content of optional functional group-containing monomers in the monomer component is, for example, less than 3% by weight, may be less than 1% by weight, may be less than 0.5% by weight, and may be less than 0.3% by weight. % or less than 0.1% by weight. The technique disclosed herein can be preferably carried out in an embodiment in which the monomer component of the acrylic polymer does not substantially contain any functional group-containing monomer.
また、上記任意官能基含有モノマーとして水酸基含有モノマーを用いる場合、その含有量は、通常、全モノマー成分の凡そ0.001重量%以上であってもよく、凡そ0.01重量%以上でもよく、凡そ0.02重量%以上でもよい。また、水酸基含有モノマーの含有量は、全モノマー成分中、凡そ10重量%以下とすることが適当であり、好ましくは凡そ5重量%以下、より好ましくは凡そ2重量%以下である。いくつかの態様において、モノマー成分における水酸基含有モノマーの含有量は、例えば1重量%未満であってもよく、0.5重量%未満でもよく、0.3重量%未満でもよく、0.1重量%未満でもよく、0.01重量%未満でもよい。アクリル系ポリマーのモノマー成分は水酸基含有モノマーを実質的に含まなくてもよい。ここに開示される技術によると、水酸基含有モノマーに頼ることなく、所望の効果を実現することができる。
Further, when a hydroxyl group-containing monomer is used as the above-mentioned optional functional group-containing monomer, the content thereof may generally be approximately 0.001% by weight or more of the total monomer components, or approximately 0.01% by weight or more, It may be approximately 0.02% by weight or more. The content of the hydroxyl group-containing monomer is suitably about 10% by weight or less, preferably about 5% by weight or less, more preferably about 2% by weight or less, based on the total monomer components. In some embodiments, the content of hydroxyl group-containing monomer in the monomer component may be less than 1% by weight, may be less than 0.5% by weight, may be less than 0.3% by weight, and may be less than 0.1% by weight. % or less than 0.01% by weight. The monomer component of the acrylic polymer may be substantially free of hydroxyl group-containing monomers. According to the technology disclosed herein, desired effects can be achieved without relying on hydroxyl group-containing monomers.
アクリル系ポリマーの共重合成分として使用される官能基含有モノマー全体(カルボキシ基含有モノマーを含む官能基含有モノマー全体)に占めるカルボキシ基含有モノマーの割合は、カルボキシ基含有モノマーを共重合する効果を効果的に発揮させる観点から、30重量%以上が適当であり、好ましくは50重量%以上、より好ましくは70重量%以上、さらに好ましくは80重量%以上、特に好ましくは90重量%以上であり、例えば95重量%以上であってもよく、97重量%以上であってもよく、98重量%以上でもよく、99重量%以上(例えば99.9重量%以上)でもよい。上記官能基含有モノマー全体に占めるカルボキシ基含有モノマーの割合の上限は100重量%であり、例えば95重量%以下であってもよい。
The ratio of the carboxy group-containing monomer to the total functional group-containing monomers (total functional group-containing monomers including the carboxy group-containing monomer) used as a copolymerization component of the acrylic polymer is determined by the effect of copolymerizing the carboxy group-containing monomer. From the viewpoint of achieving the desired performance, the content is suitably 30% by weight or more, preferably 50% by weight or more, more preferably 70% by weight or more, further preferably 80% by weight or more, particularly preferably 90% by weight or more, such as It may be 95% by weight or more, 97% by weight or more, 98% by weight or more, or 99% by weight or more (for example, 99.9% by weight or more). The upper limit of the proportion of the carboxy group-containing monomer to the total of the functional group-containing monomers is 100% by weight, and may be, for example, 95% by weight or less.
アクリル系ポリマーを構成するモノマー成分は、凝集力向上等の目的で、上述した官能基含有モノマー以外の他の共重合成分を含んでいてもよい。他の共重合成分の例としては、酢酸ビニル等のビニルエステル系モノマー;スチレン等の芳香族ビニル化合物;シクロヘキシル(メタ)アクリレート、シクロペンチル(メタ)アクリレート、イソボルニル(メタ)アクリレート等のシクロアルキル(メタ)アクリレート;アリール(メタ)アクリレート(例えばフェニル(メタ)アクリレート)、アリールオキシアルキル(メタ)アクリレート(例えばフェノキシエチル(メタ)アクリレート)、アリールアルキル(メタ)アクリレート(例えばベンジル(メタ)アクリレート)等の芳香族性環含有(メタ)アクリレート;オレフィン系モノマー;塩素含有モノマー;2-(メタ)アクリロイルオキシエチルイソシアネート等のイソシアネート基含有モノマー;メトキシエチル(メタ)アクリレート、エトキシエチル(メタ)アクリレート等のアルコキシ基含有モノマー;メチルビニルエーテル、エチルビニルエーテル等のビニルエーテル系モノマー;等が挙げられる。上記他の共重合成分は、1種を単独でまたは2種以上を組み合わせて用いることができる。
The monomer components constituting the acrylic polymer may contain copolymerization components other than the above-mentioned functional group-containing monomers for the purpose of improving cohesive force and the like. Examples of other copolymerization components include vinyl ester monomers such as vinyl acetate; aromatic vinyl compounds such as styrene; cycloalkyl (meth)acrylates such as cyclohexyl (meth)acrylate, cyclopentyl (meth)acrylate, and isobornyl ) acrylates; aryl (meth)acrylates (e.g. phenyl (meth)acrylate), aryloxyalkyl (meth)acrylates (e.g. phenoxyethyl (meth)acrylate), arylalkyl (meth)acrylates (e.g. benzyl (meth)acrylate), etc. Aromatic ring-containing (meth)acrylate; Olefinic monomer; Chlorine-containing monomer; Isocyanate group-containing monomer such as 2-(meth)acryloyloxyethyl isocyanate; Alkoxy such as methoxyethyl (meth)acrylate and ethoxyethyl (meth)acrylate Group-containing monomers; vinyl ether monomers such as methyl vinyl ether and ethyl vinyl ether; and the like. The other copolymerization components mentioned above can be used alone or in combination of two or more.
かかる他の共重合成分の量は、目的および用途に応じて適宜選択すればよく特に限定されないが、使用による効果を適切に発揮する観点から、0.05重量%以上とすることが適当であり、0.5重量%以上としてもよい。また、粘着性能のバランスをとりやすくする観点から、モノマー成分における他の共重合成分の含有量は、20重量%以下とすることが適当であり、必須モノマー成分に基づく粘着特性を好適に発揮させる観点から、好ましくは10重量%以下、より好ましくは8重量%以下、さらに好ましくは5重量%未満であり、例えば3重量%未満であってもよく、1重量%未満でもよい。ここに開示される技術は、モノマー成分が他の共重合成分を実質的に含まない態様でも好ましく実施され得る。
The amount of such other copolymerized components is not particularly limited as long as it can be selected as appropriate depending on the purpose and use, but from the viewpoint of appropriately exhibiting the effects of use, it is appropriate to set it to 0.05% by weight or more. , 0.5% by weight or more. In addition, from the viewpoint of making it easier to balance the adhesive performance, it is appropriate that the content of other copolymer components in the monomer components is 20% by weight or less, so that the adhesive properties based on the essential monomer components can be suitably exhibited. From this point of view, it is preferably 10% by weight or less, more preferably 8% by weight or less, still more preferably less than 5% by weight, for example, it may be less than 3% by weight, and may be less than 1% by weight. The technology disclosed herein can also be preferably practiced in an embodiment in which the monomer component does not substantially contain other copolymer components.
アクリル系ポリマーは、他のモノマー成分として、(メタ)アクリロイル基やビニル基等の不飽和二重結合を有する重合性官能基(典型的にはラジカル重合性官能基)を少なくとも2つ有する多官能モノマーを含んでもよい。モノマー成分として、多官能モノマーを用いることにより、粘着剤層の凝集力を高めることができる。多官能モノマーは、架橋剤として用いることができる。多官能モノマーとしては、特に限定されず、例えば1,6-ヘキサンジオールジ(メタ)アクリレート、トリメチロールプロパントリ(メタ)アクリレート、ジペンタエリスリトールヘキサ(メタ)アクリレート、ネオペンチルグリコールジ(メタ)アクリレート等が挙げられる。多官能モノマーは、1種を単独でまたは2種以上を組み合わせて使用することができる。
Acrylic polymers are polyfunctional polymers that have at least two polymerizable functional groups (typically radically polymerizable functional groups) having unsaturated double bonds, such as (meth)acryloyl groups and vinyl groups, as other monomer components. It may also contain monomers. By using a polyfunctional monomer as a monomer component, the cohesive force of the adhesive layer can be increased. Polyfunctional monomers can be used as crosslinking agents. The polyfunctional monomer is not particularly limited, and includes, for example, 1,6-hexanediol di(meth)acrylate, trimethylolpropane tri(meth)acrylate, dipentaerythritol hexa(meth)acrylate, and neopentyl glycol di(meth)acrylate. etc. One type of polyfunctional monomer can be used alone or two or more types can be used in combination.
多官能モノマーの使用量は特に限定されず、該多官能モノマーの使用目的が達成されるように適切に設定することができる。多官能モノマーの使用量は、上記モノマー成分の凡そ3重量%以下とすることができ、凡そ2重量%以下が好ましく、凡そ1重量%以下(例えば凡そ0.5重量%以下)がより好ましい。多官能モノマーを使用する場合における使用量の下限は、0重量%より大きければよく、特に限定されない。通常は、多官能モノマーの使用量をモノマー成分の凡そ0.001重量%以上(例えば凡そ0.01重量%以上)とすることにより、該多官能モノマーの使用効果が適切に発揮され得る。
The amount of the polyfunctional monomer used is not particularly limited, and can be appropriately set so that the intended use of the polyfunctional monomer is achieved. The amount of the polyfunctional monomer used can be about 3% by weight or less of the monomer components, preferably about 2% by weight or less, and more preferably about 1% by weight or less (for example, about 0.5% by weight or less). The lower limit of the amount used when using a polyfunctional monomer is not particularly limited, as long as it is greater than 0% by weight. Usually, the effect of using the polyfunctional monomer can be appropriately exhibited by setting the amount of the polyfunctional monomer to be approximately 0.001% by weight or more (for example, approximately 0.01% by weight or more) of the monomer components.
上記アクリル系ポリマーを構成するモノマー成分のバイオマス炭素比(アクリル系ポリマーのバイオマス炭素比)は、例えば1%以上であってもよく、10%以上が適当であり、好ましくは30%以上、より好ましくは50%以上(例えば50%超)であり、70%以上でもよく、80%以上でもよく、90%~100%でもよい。このように設計することにより、化石資源系材料への依存抑制に配慮したアクリル系粘着剤が得られる。
The biomass carbon ratio of the monomer component constituting the acrylic polymer (biomass carbon ratio of the acrylic polymer) may be, for example, 1% or more, suitably 10% or more, preferably 30% or more, and more preferably is 50% or more (for example, more than 50%), may be 70% or more, may be 80% or more, or may be 90% to 100%. By designing in this way, an acrylic pressure-sensitive adhesive can be obtained that takes into account the suppression of dependence on fossil resource-based materials.
アクリル系ポリマーを得る方法は特に限定されず、溶液重合法、エマルション重合法、バルク重合法、懸濁重合法、光重合法等の、アクリル系ポリマーの合成手法として知られている各種の重合方法を適宜採用することができる。例えば、溶液重合法を好ましく採用し得る。溶液重合を行う際のモノマー供給方法としては、全モノマー原料を一度に供給する一括仕込み方式、連続供給(滴下)方式、分割供給(滴下)方式等を適宜採用することができる。重合温度は、使用するモノマーおよび溶媒の種類、重合開始剤の種類等に応じて適宜選択することができ、例えば20℃~170℃程度(典型的には40℃~140℃程度)とすることができる。
The method for obtaining the acrylic polymer is not particularly limited, and various polymerization methods known as methods for synthesizing acrylic polymers, such as solution polymerization, emulsion polymerization, bulk polymerization, suspension polymerization, and photopolymerization, can be used. may be adopted as appropriate. For example, a solution polymerization method can be preferably employed. As a monomer supply method when performing solution polymerization, a batch charging method in which all monomer raw materials are supplied at once, a continuous supply (dropping) method, a divided supply (dropping) method, etc. can be appropriately adopted. The polymerization temperature can be selected as appropriate depending on the type of monomer and solvent used, the type of polymerization initiator, etc., and is, for example, about 20°C to 170°C (typically about 40°C to 140°C). I can do it.
溶液重合に用いる溶媒(重合溶媒)は、従来公知の有機溶媒から適宜選択することができる。例えば、トルエン等の芳香族化合物類(典型的には芳香族炭化水素類);酢酸エチル等の酢酸エステル類;ヘキサンやシクロヘキサン等の脂肪族または脂環式炭化水素類;1,2-ジクロロエタン等のハロゲン化アルカン類;イソプロピルアルコール等の低級アルコール類(例えば、炭素原子数1~4の一価アルコール類);tert-ブチルメチルエーテル等のエーテル類;メチルエチルケトン等のケトン類;等から選択されるいずれか1種の溶媒、または2種以上の混合溶媒を用いることができる。
The solvent (polymerization solvent) used for solution polymerization can be appropriately selected from conventionally known organic solvents. For example, aromatic compounds such as toluene (typically aromatic hydrocarbons); acetate esters such as ethyl acetate; aliphatic or alicyclic hydrocarbons such as hexane and cyclohexane; 1,2-dichloroethane, etc. halogenated alkanes; lower alcohols such as isopropyl alcohol (for example, monohydric alcohols having 1 to 4 carbon atoms); ethers such as tert-butyl methyl ether; ketones such as methyl ethyl ketone; etc. Any one type of solvent or a mixed solvent of two or more types can be used.
重合に用いる開始剤は、重合方法の種類に応じて、従来公知の重合開始剤から適宜選択することができる。例えば、2,2’-アゾビスイソブチロニトリル(AIBN)等のアゾ系重合開始剤の1種または2種以上を好ましく使用し得る。重合開始剤の他の例としては、過硫酸カリウム等の過硫酸塩;ベンゾイルパーオキサイド(BPO)、過酸化水素等の過酸化物系開始剤;フェニル置換エタン等の置換エタン系開始剤;芳香族カルボニル化合物;等が挙げられる。重合開始剤のさらに他の例として、過酸化物と還元剤との組み合わせによるレドックス系開始剤が挙げられる。このような重合開始剤は、1種を単独でまたは2種以上を組み合わせて使用することができる。重合開始剤の使用量は、通常の使用量であればよく、例えば、全モノマー成分100重量部に対して凡そ0.005~1重量部程度(典型的には凡そ0.01~1重量部程度)の範囲から選択することができる。
The initiator used for polymerization can be appropriately selected from conventionally known polymerization initiators depending on the type of polymerization method. For example, one or more azo polymerization initiators such as 2,2'-azobisisobutyronitrile (AIBN) can be preferably used. Other examples of polymerization initiators include persulfates such as potassium persulfate; peroxide initiators such as benzoyl peroxide (BPO) and hydrogen peroxide; substituted ethane initiators such as phenyl-substituted ethane; aromatic carbonyl compounds; and the like. Still another example of the polymerization initiator is a redox initiator using a combination of a peroxide and a reducing agent. Such polymerization initiators can be used alone or in combination of two or more. The amount of the polymerization initiator used may be any normal amount, for example, approximately 0.005 to 1 part by weight (typically approximately 0.01 to 1 part by weight) per 100 parts by weight of all monomer components. degree).
アクリル系ポリマーとしては、重量平均分子量(Mw)が60万よりも大きいアクリル系ポリマーを使用する。これにより、良好な凝集力を示す粘着剤が得られ、異種材料に対して高い接着力を有しつつ、十分な保持力が得られる。アクリル系ポリマーのMwは、65万以上とすることが好ましい。いくつかの態様において、アクリル系ポリマーのMwは、例えば70万以上が適当であり、好ましくは75万以上、より好ましくは80万以上、さらに好ましくは85万以上、特に好ましくは90万以上(例えば90万超)である。アクリル系ポリマーのMwを高く設定して粘着剤の凝集力を高めることで、例えばモノマー組成については、異種材料に対する接着力をより重視した組成を採用し得る。また、異種材料に対する接着力、合成容易性等の観点から、アクリル系ポリマーのMwは、通常、凡そ300万以下であることが適当であり、好ましくは200万以下、より好ましくは150万以下、さらに好ましくは120万以下、特に好ましくは100万以下である。いくつかの態様において、異種材料に対する接着力向上の観点から、アクリル系ポリマーのMwは90万以下であってもよく、85万以下でもよく、80万以下でもよく、75万以下でもよく、70万以下でもよい。
As the acrylic polymer, an acrylic polymer having a weight average molecular weight (Mw) of more than 600,000 is used. As a result, a pressure-sensitive adhesive exhibiting good cohesive force can be obtained, and sufficient holding power can be obtained while having high adhesive strength to different materials. The Mw of the acrylic polymer is preferably 650,000 or more. In some embodiments, the Mw of the acrylic polymer is suitably 700,000 or more, preferably 750,000 or more, more preferably 800,000 or more, even more preferably 850,000 or more, particularly preferably 900,000 or more (e.g. (over 900,000). By setting the Mw of the acrylic polymer high to increase the cohesive force of the adhesive, it is possible to adopt, for example, a monomer composition that emphasizes adhesive force to different materials. In addition, from the viewpoint of adhesive strength to different materials, ease of synthesis, etc., it is appropriate that the Mw of the acrylic polymer is usually about 3 million or less, preferably 2 million or less, more preferably 1.5 million or less, More preferably, it is 1.2 million or less, particularly preferably 1,000,000 or less. In some embodiments, from the viewpoint of improving adhesive strength to different materials, the Mw of the acrylic polymer may be 900,000 or less, 850,000 or less, 800,000 or less, 750,000 or less, 700,000 or less, It may be less than 10,000.
アクリル系ポリマーのMwは、ゲルパーミエーションクロマトグラフィ(GPC)により測定し、標準ポリスチレン換算の値として求めることができる。具体的には、GPC測定装置として商品名「HLC-8220GPC」(東ソー社製)を用いて、下記の条件で測定して求めることができる。後述の実施例においても同様である。
[GPCの測定条件]
サンプル濃度:0.2重量%(テトラヒドロフラン溶液)
サンプル注入量:10μL
溶離液:テトラヒドロフラン(THF)
流量(流速):0.6mL/分
カラム温度(測定温度):40℃
カラム:
サンプルカラム:商品名「TSKguardcolumn SuperHZ-H」1本+商品名「TSKgel SuperHZM-H」2本」(東ソー社製)
リファレンスカラム:商品名「TSKgel SuperH-RC」1本(東ソー社製)
検出器:示差屈折計(RI)
標準試料:ポリスチレン The Mw of the acrylic polymer can be measured by gel permeation chromatography (GPC) and determined as a value in terms of standard polystyrene. Specifically, it can be determined by measuring under the following conditions using a GPC measurement device with the trade name "HLC-8220GPC" (manufactured by Tosoh Corporation). The same applies to the embodiments described later.
[GPC measurement conditions]
Sample concentration: 0.2% by weight (tetrahydrofuran solution)
Sample injection volume: 10μL
Eluent: Tetrahydrofuran (THF)
Flow rate (flow rate): 0.6 mL/min Column temperature (measurement temperature): 40°C
column:
Sample column: 1 product name “TSKguardcolumn SuperHZ-H” + 2 product name “TSKgel SuperHZM-H” (manufactured by Tosoh Corporation)
Reference column: 1 piece of product name “TSKgel SuperH-RC” (manufactured by Tosoh Corporation)
Detector: Differential refractometer (RI)
Standard sample: polystyrene
[GPCの測定条件]
サンプル濃度:0.2重量%(テトラヒドロフラン溶液)
サンプル注入量:10μL
溶離液:テトラヒドロフラン(THF)
流量(流速):0.6mL/分
カラム温度(測定温度):40℃
カラム:
サンプルカラム:商品名「TSKguardcolumn SuperHZ-H」1本+商品名「TSKgel SuperHZM-H」2本」(東ソー社製)
リファレンスカラム:商品名「TSKgel SuperH-RC」1本(東ソー社製)
検出器:示差屈折計(RI)
標準試料:ポリスチレン The Mw of the acrylic polymer can be measured by gel permeation chromatography (GPC) and determined as a value in terms of standard polystyrene. Specifically, it can be determined by measuring under the following conditions using a GPC measurement device with the trade name "HLC-8220GPC" (manufactured by Tosoh Corporation). The same applies to the embodiments described later.
[GPC measurement conditions]
Sample concentration: 0.2% by weight (tetrahydrofuran solution)
Sample injection volume: 10μL
Eluent: Tetrahydrofuran (THF)
Flow rate (flow rate): 0.6 mL/min Column temperature (measurement temperature): 40°C
column:
Sample column: 1 product name “TSKguardcolumn SuperHZ-H” + 2 product name “TSKgel SuperHZM-H” (manufactured by Tosoh Corporation)
Reference column: 1 piece of product name “TSKgel SuperH-RC” (manufactured by Tosoh Corporation)
Detector: Differential refractometer (RI)
Standard sample: polystyrene
(粘着付与樹脂)
いくつかの好ましい態様において、粘着剤層は粘着付与樹脂を含む。粘着付与樹脂を用いることで、高極性材料および低極性材料に対する接着力を向上することができる。また、粘着付与樹脂を適当量使用することで、粘着剤層のゲル分率は適度な範囲に調整され得る。粘着付与樹脂としては、特に制限されず、例えば、ロジン系粘着付与樹脂、テルペン系粘着付与樹脂、炭化水素系粘着付与樹脂、エポキシ系粘着付与樹脂、ポリアミド系粘着付与樹脂、エラストマー系粘着付与樹脂、フェノール系粘着付与樹脂、ケトン系粘着付与樹脂等の各種粘着付与樹脂を用いることができる。このような粘着付与樹脂は、1種を単独でまたは2種以上を組み合わせて使用することができる。 (tackifying resin)
In some preferred embodiments, the adhesive layer includes a tackifying resin. By using a tackifying resin, it is possible to improve adhesion to high polarity materials and low polarity materials. Furthermore, by using an appropriate amount of tackifier resin, the gel fraction of the adhesive layer can be adjusted to an appropriate range. The tackifier resin is not particularly limited and includes, for example, rosin-based tackifier resin, terpene-based tackifier resin, hydrocarbon-based tackifier resin, epoxy-based tackifier resin, polyamide-based tackifier resin, elastomer-based tackifier resin, Various tackifying resins such as phenolic tackifying resins and ketone tackifying resins can be used. Such tackifying resins can be used alone or in combination of two or more.
いくつかの好ましい態様において、粘着剤層は粘着付与樹脂を含む。粘着付与樹脂を用いることで、高極性材料および低極性材料に対する接着力を向上することができる。また、粘着付与樹脂を適当量使用することで、粘着剤層のゲル分率は適度な範囲に調整され得る。粘着付与樹脂としては、特に制限されず、例えば、ロジン系粘着付与樹脂、テルペン系粘着付与樹脂、炭化水素系粘着付与樹脂、エポキシ系粘着付与樹脂、ポリアミド系粘着付与樹脂、エラストマー系粘着付与樹脂、フェノール系粘着付与樹脂、ケトン系粘着付与樹脂等の各種粘着付与樹脂を用いることができる。このような粘着付与樹脂は、1種を単独でまたは2種以上を組み合わせて使用することができる。 (tackifying resin)
In some preferred embodiments, the adhesive layer includes a tackifying resin. By using a tackifying resin, it is possible to improve adhesion to high polarity materials and low polarity materials. Furthermore, by using an appropriate amount of tackifier resin, the gel fraction of the adhesive layer can be adjusted to an appropriate range. The tackifier resin is not particularly limited and includes, for example, rosin-based tackifier resin, terpene-based tackifier resin, hydrocarbon-based tackifier resin, epoxy-based tackifier resin, polyamide-based tackifier resin, elastomer-based tackifier resin, Various tackifying resins such as phenolic tackifying resins and ketone tackifying resins can be used. Such tackifying resins can be used alone or in combination of two or more.
ロジン系粘着付与樹脂の具体例としては、ガムロジン、ウッドロジン、トール油ロジン等の未変性ロジン(生ロジン);これらの未変性ロジンを水添化、不均化、重合等により変性した変性ロジン(水添ロジン、不均化ロジン、重合ロジン、その他の化学的に修飾されたロジン等。以下同じ。);その他の各種ロジン誘導体;等が挙げられる。上記ロジン誘導体の例としては、未変性ロジンをアルコール類によりエステル化したもの(すなわち、ロジンのエステル化物)、変性ロジンをアルコール類によりエステル化したもの(すなわち、変性ロジンのエステル化物)等のロジンエステル類;未変性ロジンや変性ロジンを不飽和脂肪酸で変性した不飽和脂肪酸変性ロジン類;ロジンエステル類を不飽和脂肪酸で変性した不飽和脂肪酸変性ロジンエステル類;未変性ロジン、変性ロジン、不飽和脂肪酸変性ロジン類または不飽和脂肪酸変性ロジンエステル類におけるカルボキシ基を還元処理したロジンアルコール類;未変性ロジン、変性ロジン、各種ロジン誘導体等のロジン類(特に、ロジンエステル類)の金属塩;ロジン類(未変性ロジン、変性ロジン、各種ロジン誘導体等)にフェノールを酸触媒で付加させ熱重合することにより得られるロジンフェノール樹脂;等が挙げられる。なかでも、ロジンエステルが好ましい。
Specific examples of rosin-based tackifying resins include unmodified rosin (raw rosin) such as gum rosin, wood rosin, and tall oil rosin; Hydrogenated rosin, disproportionated rosin, polymerized rosin, other chemically modified rosin, etc. (the same applies hereinafter); and other various rosin derivatives. Examples of the above-mentioned rosin derivatives include rosins such as those obtained by esterifying unmodified rosin with alcohols (i.e., esterified products of rosin), and those obtained by esterifying modified rosin with alcohols (i.e., esterified products of modified rosin). Esters: Unsaturated fatty acid-modified rosins, which are unmodified rosin or modified rosin modified with unsaturated fatty acids; Unsaturated fatty acid-modified rosin esters, which are rosin esters modified with unsaturated fatty acids; Unmodified rosin, modified rosin, unsaturated Rosin alcohols obtained by reducing the carboxyl group in fatty acid-modified rosins or unsaturated fatty acid-modified rosin esters; metal salts of rosins (especially rosin esters) such as unmodified rosin, modified rosin, and various rosin derivatives; rosins; Examples include rosin phenol resins obtained by adding phenol to (unmodified rosin, modified rosin, various rosin derivatives, etc.) with an acid catalyst and thermally polymerizing them. Among them, rosin ester is preferred.
特に限定するものではないが、ロジンエステル類の具体例として、未変性ロジンまたは変性ロジン(水素添加ロジン、不均化ロジン、重合ロジン等)のエステル、例えばメチルエステル、トリエチレングリコールエステル、グリセリンエステル、ペンタエリスリトールエステル等が挙げられる。
Although not particularly limited, specific examples of rosin esters include esters of unmodified rosin or modified rosin (hydrogenated rosin, disproportionated rosin, polymerized rosin, etc.), such as methyl ester, triethylene glycol ester, glycerin ester. , pentaerythritol ester and the like.
テルペン系粘着付与樹脂の例としては、α-ピネン重合体、β-ピネン重合体、ジペンテン重合体等のテルペン樹脂;これらのテルペン樹脂を変性(フェノール変性、芳香族変性、水素添加変性、炭化水素変性等)した変性テルペン樹脂;等が挙げられる。上記変性テルペン樹脂の一例としてテルペンフェノール樹脂が挙げられる。
Examples of terpene-based tackifying resins include terpene resins such as α-pinene polymer, β-pinene polymer, and dipentene polymer; Modified terpene resins (modified etc.); etc. An example of the above-mentioned modified terpene resin is terpene phenol resin.
テルペンフェノール樹脂とは、テルペン残基およびフェノール残基を含むポリマーを指し、テルペン類とフェノール化合物との共重合体(テルペン-フェノール共重合体樹脂)と、テルペン類の単独重合体または共重合体をフェノール変性したもの(フェノール変性テルペン樹脂)との双方を包含する概念である。このようなテルペンフェノール樹脂を構成するテルペン類の具体例としては、α-ピネン、β-ピネン、リモネン(d体、l体およびd/l体(ジペンテン)を包含する。)等のモノテルペン類が挙げられる。水素添加テルペンフェノール樹脂とは、このようなテルペンフェノール樹脂を水素化した構造を有する水素添加テルペンフェノール樹脂をいう。水添テルペンフェノール樹脂と称されることもある。
Terpene phenol resin refers to a polymer containing terpene residues and phenol residues, and includes copolymers of terpenes and phenol compounds (terpene-phenol copolymer resins), and homopolymers or copolymers of terpenes. This concept includes both phenol-modified products (phenol-modified terpene resins). Specific examples of terpenes constituting such terpene phenol resin include monoterpenes such as α-pinene, β-pinene, and limonene (including d-form, l-form, and d/l-form (dipentene)); can be mentioned. The hydrogenated terpene phenol resin refers to a hydrogenated terpene phenol resin having a structure obtained by hydrogenating such a terpene phenol resin. Sometimes called hydrogenated terpene phenolic resin.
炭化水素系粘着付与樹脂の例としては、脂肪族系(C5系)石油樹脂、芳香族系(C9系)石油樹脂、脂肪族/芳香族共重合系(C5/C9系)石油樹脂、これらの水素添加物(例えば、芳香族系石油樹脂に水素添加して得られる脂環族系石油樹脂)、これらの各種変性物(例えば、無水マレイン酸変性物)、クマロン系樹脂、クマロンインデン系樹脂等の、各種の炭化水素系の樹脂が挙げられる。
Examples of hydrocarbon-based tackifying resins include aliphatic (C5-based) petroleum resins, aromatic (C9-based) petroleum resins, aliphatic/aromatic copolymerized (C5/C9-based) petroleum resins, and Hydrogenated substances (e.g., alicyclic petroleum resins obtained by hydrogenating aromatic petroleum resins), various modified products thereof (e.g., maleic anhydride modified products), coumaron-based resins, coumaron-indene-based resins Examples include various hydrocarbon resins such as.
いくつかの態様において、粘着付与樹脂として、ロジン系粘着付与樹脂およびテルペン系粘着付与樹脂から選択される少なくとも1種を用いることが好ましい。ロジン系粘着付与樹脂およびテルペン系粘着付与樹脂から選択される少なくとも1種を用いる態様には、粘着付与樹脂がロジン系粘着付与樹脂のみを含む態様、粘着付与樹脂がテルペン系粘着付与樹脂(例えばテルペンフェノール樹脂)のみを含む態様、粘着付与樹脂がロジン系粘着付与樹脂およびテルペン系粘着付与樹脂を含む態様が包含される。ロジン系粘着付与樹脂および/またはテルペン系粘着付与樹脂をアクリル系粘着剤に含有させることで、接着力など優れた粘着特性が得られやすい。いくつかの好ましい態様において、粘着剤層に含まれる粘着付与樹脂全体に占めるロジン系粘着付与樹脂およびテルペン系粘着付与樹脂の合計割合は、例えば凡そ50重量%超(50重量%超100重量%以下)とすることができ、凡そ70重量%以上としてもよく、凡そ80重量%以上としてもよく、凡そ90重量%以上としてもよく、95重量%以上としてもよく、99重量%以上としてもよい。
In some embodiments, it is preferable to use at least one selected from rosin-based tackifying resins and terpene-based tackifying resins as the tackifying resin. Examples of embodiments using at least one selected from rosin-based tackifying resins and terpene-based tackifying resins include embodiments in which the tackifying resin contains only rosin-based tackifying resins, and embodiments in which the tackifying resin contains terpene-based tackifying resins (e.g., terpene-based tackifying resins). Examples include embodiments in which the tackifying resin contains only a phenolic resin) and embodiments in which the tackifying resin includes a rosin-based tackifying resin and a terpene-based tackifying resin. By incorporating a rosin-based tackifying resin and/or a terpene-based tackifying resin into an acrylic adhesive, excellent adhesive properties such as adhesive strength can be easily obtained. In some preferred embodiments, the total proportion of the rosin-based tackifying resin and the terpene-based tackifying resin in the entire tackifying resin contained in the adhesive layer is, for example, approximately more than 50% by weight (more than 50% by weight and not more than 100% by weight). ), and may be about 70% by weight or more, about 80% by weight or more, about 90% by weight or more, about 95% by weight or more, or about 99% by weight or more.
いくつかの態様において、粘着付与樹脂として、ロジン系粘着付与樹脂を用いることがより好ましい。ロジン系粘着付与樹脂を粘着剤に含有させることで、高極性材料および低極性材料に対する接着力を好ましく向上させることができる。なかでも、ロジンエステルが好ましい。粘着剤層に含まれる粘着付与樹脂全体に占めるロジン系粘着付与樹脂の割合は、例えば凡そ50重量%超とすることができ、凡そ70重量%以上としてもよく、凡そ80重量%以上としてもよい。ここに開示される技術は、粘着付与樹脂の実質的に全部(例えば凡そ97重量%以上、または99重量%以上であり、100重量%でもよい。)がロジン系粘着付与樹脂である態様で好ましく実施され得る。
In some embodiments, it is more preferable to use a rosin-based tackifier resin as the tackifier resin. By containing a rosin-based tackifying resin in the adhesive, the adhesive strength to high polarity materials and low polarity materials can be preferably improved. Among them, rosin ester is preferred. The proportion of the rosin-based tackifying resin in the entire tackifying resin contained in the adhesive layer can be, for example, about 50% by weight or more, about 70% by weight or more, or about 80% by weight or more. . The technology disclosed herein is preferably in an embodiment in which substantially all of the tackifier resin (for example, approximately 97% by weight or more, or 99% by weight or more, and may be 100% by weight) is a rosin-based tackifier resin. can be implemented.
粘着付与樹脂としてロジン系粘着付与樹脂が用いられる場合、粘着剤層中のロジン系粘着付与樹脂以外の粘着付与樹脂(非ロジン系粘着付与樹脂)の含有割合は、例えばアクリルポリマー100重量部に対して40重量部以下とすることが適当である。これにより、ロジンエステルを含ませる効果が好適に発揮される。非ロジン系粘着付与樹脂の使用量は、アクリル系ポリマー100重量部に対して、好ましくは凡そ20重量部以下(例えば20重量部未満)であり、凡そ10重量部以下であってもよく、凡そ5重量部以下でもよく、凡そ1重量部以下でもよい。
When a rosin-based tackifying resin is used as the tackifying resin, the content ratio of the tackifying resin other than the rosin-based tackifying resin (non-rosin-based tackifying resin) in the adhesive layer is, for example, based on 100 parts by weight of the acrylic polymer. It is appropriate that the amount is 40 parts by weight or less. Thereby, the effect of including rosin ester is suitably exhibited. The amount of the non-rosin tackifier resin used is preferably about 20 parts by weight or less (for example, less than 20 parts by weight), and may be about 10 parts by weight or less, based on 100 parts by weight of the acrylic polymer. The amount may be 5 parts by weight or less, or approximately 1 part by weight or less.
いくつかの好ましい態様において、粘着剤層中、粘着付与樹脂としてのテルペンフェノール樹脂の含有量は、アクリル系ポリマー100重量部に対して、例えば40重量部以下であり、35重量部以下であってもよく、30重量部以下でもよく、20重量部以下でもよく、15重量部以下でもよく、10重量部以下でもよく、5重量部以下でもよい。このようなテルペンフェノール樹脂の使用量を採用することにより、低極性材料に対する接着力改善効果が得られやすい。ここで、テルペンフェノール樹脂の含有量がアクリル系ポリマー100重量部に対してX重量部以下であるとは、粘着剤層が、テルペンフェノール樹脂を含まないこと、および、テルペンフェノール樹脂をアクリル系ポリマー100重量部に対してX重量部以下の割合で含むことの両方を包含する意味で用いられる。いくつかの態様において、粘着剤層中のテルペンフェノール樹脂の含有量は、アクリル系ポリマー100重量部に対して3重量部以下であってもよく、1重量部以下(例えば0~0.1重量部)の範囲でもよい。他のいくつかの態様において、粘着剤層中のテルペンフェノール樹脂の含有量は、アクリル系ポリマー100重量部に対して、例えば1重量部以上であってもよく、3重量部以上でもよく、5重量部以上でもよく、7重量部以上でもよく、9重量部以上でもよい。上記範囲でテルペンフェノール樹脂を適量使用することにより、ここに開示される技術による効果は好ましく実現され得る。テルペンフェノール樹脂を使用する他のいくつかの好ましい態様において、粘着剤層中のテルペンフェノール樹脂の含有量は、アクリル系ポリマー100重量部に対して、10重量部以上であり、12重量部以上であってもよく、14重量部以上でもよい。適当量のテルペンフェノール樹脂を使用することで、低極性材料に対する接着力を有しつつ、高極性材料に対する接着力を向上することができる。上記範囲のテルペンフェノール樹脂使用量は、粘着付与樹脂としてテルペンフェノール樹脂のみを使用する態様、テルペンフェノール樹脂と非テルペンフェノール樹脂(好ましくはロジン系粘着付与樹脂)とを併用する態様のいずれの態様にも適用可能である。いくつかの態様において、上記範囲のテルペンフェノール樹脂使用量は、例えば、非テルペンフェノール樹脂(好ましくはロジン系粘着付与樹脂)と併用する態様において、好ましく採用され得る。
In some preferred embodiments, the content of the terpene phenol resin as the tackifier resin in the adhesive layer is, for example, 40 parts by weight or less, and 35 parts by weight or less, based on 100 parts by weight of the acrylic polymer. It may be 30 parts by weight or less, 20 parts by weight or less, 15 parts by weight or less, 10 parts by weight or less, or 5 parts by weight or less. By employing such an amount of terpene phenol resin, it is easy to obtain an effect of improving adhesive strength to low polarity materials. Here, the content of the terpene phenol resin being X parts by weight or less based on 100 parts by weight of the acrylic polymer means that the adhesive layer does not contain the terpene phenol resin and that the terpene phenol resin is not contained in the acrylic polymer. The term is used to include both the inclusion of X parts by weight or less per 100 parts by weight. In some embodiments, the content of the terpene phenol resin in the adhesive layer may be 3 parts by weight or less, and 1 part by weight or less (for example, 0 to 0.1 parts by weight) based on 100 parts by weight of the acrylic polymer. It may be within the range of In some other embodiments, the content of the terpene phenol resin in the adhesive layer may be, for example, 1 part by weight or more, 3 parts by weight or more, 5 parts by weight or more, based on 100 parts by weight of the acrylic polymer. It may be at least 7 parts by weight, or at least 9 parts by weight. By using an appropriate amount of terpene phenol resin within the above range, the effects of the technology disclosed herein can be preferably achieved. In some other preferred embodiments using a terpene phenol resin, the content of the terpene phenol resin in the adhesive layer is 10 parts by weight or more, and 12 parts by weight or more based on 100 parts by weight of the acrylic polymer. The amount may be 14 parts by weight or more. By using an appropriate amount of terpene phenol resin, it is possible to improve the adhesive strength to high polarity materials while maintaining the adhesive strength to low polarity materials. The amount of terpene phenol resin used in the above range applies to either the embodiment in which only terpene phenol resin is used as the tackifier resin, or the embodiment in which terpene phenol resin and non-terpene phenol resin (preferably rosin-based tackifier resin) are used in combination. is also applicable. In some embodiments, the amount of terpene phenol resin used within the above range may be preferably employed, for example, in embodiments in which the terpene phenol resin is used in combination with a non-terpene phenol resin (preferably a rosin-based tackifying resin).
いくつかの態様において、粘着付与樹脂として、軟化点が150℃未満の粘着付与樹脂TLが用いられる。粘着付与樹脂TLを用いることにより、高極性材料および低極性材料の両方に対してより高い接着力を得ることができる。上記粘着付与樹脂TLの軟化点は、高極性材料、低極性材料に対する接着力向上の観点から、140℃未満であってもよく、130℃未満でもよい。いくつかの好ましい態様において、上記粘着付与樹脂TLの軟化点は、120℃未満であり、110℃未満であることが適当であり、好ましくは凡そ105℃以下、より好ましくは凡そ100℃以下、さらに好ましくは凡そ95℃以下(例えば95℃未満)、特に好ましくは凡そ90℃以下(例えば凡そ85℃以下)である。粘着付与樹脂TLの軟化点の下限は特に制限されない。いくつかの態様において、粘着付与樹脂TLの軟化点は、適度な凝集力を発揮させる観点から、例えば凡そ50℃以上であってよく、凡そ60℃以上でもよく、凡そ65℃以上でもよく、凡そ70℃以上でもよい。他のいくつかの態様において、粘着付与樹脂TLの軟化点は、例えば凡そ80℃以上であってよく、凡そ90℃以上でもよく、凡そ100℃以上でもよく、凡そ110℃以上でもよい。
In some embodiments, the tackifying resin is a tackifying resin T L having a softening point of less than 150°C. By using the tackifier resin T L , higher adhesion strength can be obtained for both high and low polarity materials. The softening point of the tackifier resin T L may be lower than 140°C or lower than 130°C from the viewpoint of improving adhesive strength to high polarity materials and low polarity materials. In some preferred embodiments, the softening point of the tackifier resin T L is less than 120°C, suitably less than 110°C, preferably about 105°C or less, more preferably about 100°C or less, More preferably, the temperature is about 95°C or less (for example, less than 95°C), particularly preferably about 90°C or less (for example, about 85°C or less). The lower limit of the softening point of the tackifier resin T L is not particularly limited. In some embodiments, the softening point of the tackifier resin T L may be approximately 50° C. or higher, approximately 60° C. or higher, or approximately 65° C. or higher, for example, from the viewpoint of exhibiting appropriate cohesive force. The temperature may be approximately 70°C or higher. In some other embodiments, the softening point of the tackifier resin T L may be, for example, about 80°C or higher, about 90°C or higher, about 100°C or higher, or about 110°C or higher.
粘着付与樹脂TLとしては、上記で例示した粘着付与樹脂のうち軟化点が150℃未満のものから適宜選択される1種を単独でまたは2種以上を組み合わせて用いることができる。いくつかの態様において、粘着付与樹脂TLは、ロジン系粘着付与樹脂およびテルペン系粘着付与樹脂から選択される少なくとも1種を含むことが好ましく、より好ましくはロジン系粘着付与樹脂を含む。粘着付与樹脂TLは、1種のロジン系粘着付与樹脂を単独で含んでもよく、2種以上のロジン系粘着付与樹脂を組み合わせて含んでもよい。
As the tackifier resin T L , one type suitably selected from among the tackifier resins exemplified above having a softening point of less than 150°C can be used alone or in combination of two or more types. In some embodiments, the tackifying resin T L preferably includes at least one selected from rosin-based tackifying resins and terpene-based tackifying resins, and more preferably includes rosin-based tackifying resins. The tackifying resin T L may contain one type of rosin-based tackifying resin alone, or may contain a combination of two or more types of rosin-based tackifying resin.
特に限定するものではないが、粘着付与樹脂TLとして好ましく採用し得るロジン系粘着付与樹脂の例として、未変性ロジンエステルおよび変性ロジンエステル等のロジンエステル類が挙げられる。変性ロジンエステルの好適例として水素添加ロジンエステルが挙げられる。例えば、未変性ロジンまたは変性ロジン(例えば水素添加ロジン)のエステル、例えばメチルエステル、グリセリンエステル等のロジンエステル類を、粘着付与樹脂TLとして用いることができる。
Although not particularly limited, examples of rosin-based tackifier resins that can be preferably employed as the tackifier resin T L include rosin esters such as unmodified rosin esters and modified rosin esters. A suitable example of the modified rosin ester is a hydrogenated rosin ester. For example, esters of unmodified rosin or modified rosin (eg, hydrogenated rosin), such as rosin esters such as methyl ester, glycerin ester, etc., can be used as the tackifying resin T L.
いくつかの好ましい態様に係る粘着剤層は、粘着付与樹脂TLが水素添加ロジンエステルを含む。また、粘着付与樹脂TLは、非水素添加ロジンエステルを含んでもよい。ここで非水素添加ロジンエステルとは、上述したロジンエステル類のうち水素添加ロジンエステル以外のものを包括的に指す概念である。非水素添加ロジンエステルの例には、未変性ロジンエステル、不均化ロジンエステルおよび重合ロジンエステルが含まれる。粘着付与樹脂TLは、ロジンエステル類として、水素添加ロジンエステルと非水素添加ロジンエステルとを組み合わせて含んでもよく、1種または2種以上の水素添加ロジンエステルのみを含んでいてもよく、1種または2種以上の非水素添加ロジンエステルのみを含んでいてもよい。いくつかの好ましい態様に係る粘着剤層は、粘着付与樹脂TLに含まれるロジンエステル類として、1種または2種以上の水素添加ロジンエステルのみを含む。
In the adhesive layer according to some preferred embodiments, the tackifier resin T L includes a hydrogenated rosin ester. The tackifier resin T L may also include a non-hydrogenated rosin ester. Here, the term "non-hydrogenated rosin ester" is a concept that comprehensively refers to rosin esters other than hydrogenated rosin esters mentioned above. Examples of non-hydrogenated rosin esters include unmodified rosin esters, disproportionated rosin esters and polymerized rosin esters. The tackifier resin T L may contain a combination of a hydrogenated rosin ester and a non-hydrogenated rosin ester as rosin esters, or may contain only one or more hydrogenated rosin esters. It may contain only one species or two or more non-hydrogenated rosin esters. The adhesive layer according to some preferred embodiments contains only one or more hydrogenated rosin esters as the rosin esters contained in the tackifier resin T L.
粘着付与樹脂TLは、ロジン系粘着付与樹脂に加えて他の粘着付与樹脂を含んでいてもよい。上記他の粘着付与樹脂としては、上記で例示した粘着付与樹脂のうち軟化点が150℃未満のものから適宜選択される1種を単独でまたは2種以上を組み合わせて用いることができる。
The tackifying resin T L may contain other tackifying resins in addition to the rosin-based tackifying resin. As the other tackifier resin, one type suitably selected from the tackifier resins exemplified above having a softening point of less than 150°C can be used alone or in combination of two or more types.
いくつかの態様において、粘着付与樹脂TL全体に占めるロジン系粘着付与樹脂の割合は、例えば凡そ50重量%超とすることができ、凡そ65重量%以上としてもよく、凡そ75重量%以上としてもよい。ここに開示される技術は、粘着付与樹脂TLの実質的に全部(例えば凡そ97重量%以上、または99重量%以上であり、100重量%でもよい。)がロジン系粘着付与樹脂である態様で好ましく実施され得る。
In some embodiments, the proportion of the rosin-based tackifier resin in the entire tackifier resin T L can be, for example, more than about 50% by weight, may be about 65% by weight or more, and may be about 75% by weight or more. Good too. The technology disclosed herein is an embodiment in which substantially all of the tackifier resin T L (for example, approximately 97% by weight or more, or 99% by weight or more, and may be 100% by weight) is a rosin-based tackifier resin. It can be preferably carried out.
また、粘着付与樹脂TLとして、例えば、軟化点が50℃未満、より好ましくは凡そ40℃以下の粘着付与樹脂(典型的にはロジン系、テルペン系、炭化水素系等の粘着付与樹脂、例えば水添ロジンメチルエステル等)を含んでもよく、含まなくてもよい。このような低軟化点粘着付与樹脂は、30℃において液状を呈する液状粘着付与樹脂であり得る。液状粘着付与樹脂は、1種を単独でまたは2種以上を組み合わせて用いることができる。液状粘着付与樹脂の含有量は、凝集力等の観点から、粘着付与樹脂TL全体の凡そ30重量%以下とすることができ、凡そ10重量%以下(例えば0~10重量%)とすることが適当であり、凡そ2重量%以下(0.5~2重量%)であってもよく、1重量%未満でもよい。
In addition, as the tackifying resin T L , for example, a tackifying resin having a softening point of less than 50°C, more preferably approximately 40°C or less (typically a rosin-based, terpene-based, hydrocarbon-based, etc. tackifying resin, e.g. Hydrogenated rosin methyl ester, etc.) may or may not be included. Such a low softening point tackifier resin may be a liquid tackifier resin that exhibits a liquid state at 30°C. The liquid tackifying resin can be used alone or in combination of two or more. The content of the liquid tackifying resin can be approximately 30% by weight or less of the entire tackifier resin T L from the viewpoint of cohesive force etc., and should be approximately 10% by weight or less (for example, 0 to 10% by weight). is suitable, and may be approximately 2% by weight or less (0.5 to 2% by weight), and may be less than 1% by weight.
粘着付与樹脂TLの含有量(2種以上の粘着付与樹脂TLを含む場合はその合計量)は、特に限定されないが、アクリルポリマー100重量部に対して100重量部以下程度(例えば100重量部未満)とすることが適当である。粘着付与樹脂TLの使用量を所定量以下に制限することにより、十分な保持力を有しつつ、高極性材料および低極性材料に対する接着力を向上することができる。いくつかの好ましい態様において、粘着付与樹脂TLの使用量は、保持力等の観点から、アクリル系ポリマー100重量部に対して、90重量部以下であることが適当であり、好ましくは80重量部以下であり、60重量部以下であってもよく、55重量部以下でもよく、50重量部以下でもよく、45重量部以下でもよい。他のいくつかの態様において、粘着付与樹脂TLの使用量は、アクリル系ポリマー100重量部に対して、35重量部以下であってもよく、30重量部以下でもよく、25重量部以下でもよく、20重量部以下(例えば20重量部未満)でもよい。また、いくつかの態様において、接着力向上の観点から、粘着付与樹脂TLの使用量は、アクリル系ポリマー100重量部に対して、例えば10重量部超であり、12重量部以上であってもよく、14重量部以上でもよく、15重量部超でもよく、20重量部以上が適当であり、好ましくは30重量部以上、より好ましくは35重量部以上、さらに好ましくは38重量部以上であり、45重量部以上であってよく、50重量部以上(例えば50重量部超)でもよく、55重量部以上でもよく、60重量部以上でもよく、65重量部以上でもよく、70重量部以上でもよく、75重量部以上でもよい。ここに開示される技術において用いられるn-ヘプチルアクリレートをモノマー単位として含むアクリル系ポリマーは、粘着付与樹脂との相溶性がよいので、粘着付与樹脂をより多く含ませて、保持力を維持しつつ、異種材料に対する接着力を向上させることができる。
The content of the tackifying resin T L (the total amount when two or more types of tackifying resin T L are included) is not particularly limited, but is about 100 parts by weight or less (for example, 100 parts by weight) based on 100 parts by weight of the acrylic polymer. It is appropriate to set it as less than By limiting the amount of the tackifying resin T L used to a predetermined amount or less, it is possible to improve adhesive strength to high polarity materials and low polarity materials while maintaining sufficient holding power. In some preferred embodiments, the amount of the tackifier resin T L used is suitably 90 parts by weight or less, preferably 80 parts by weight, based on 100 parts by weight of the acrylic polymer, from the viewpoint of holding power etc. 60 parts by weight or less, 55 parts by weight or less, 50 parts by weight or less, or 45 parts by weight or less. In some other embodiments, the amount of the tackifier resin T L to be used may be 35 parts by weight or less, 30 parts by weight or less, or 25 parts by weight or less based on 100 parts by weight of the acrylic polymer. In most cases, the amount may be 20 parts by weight or less (for example, less than 20 parts by weight). In some embodiments, from the viewpoint of improving adhesive strength, the amount of the tackifying resin T L used is, for example, more than 10 parts by weight, and not less than 12 parts by weight, based on 100 parts by weight of the acrylic polymer. The amount may be 14 parts by weight or more, it may be more than 15 parts by weight, suitably 20 parts by weight or more, preferably 30 parts by weight or more, more preferably 35 parts by weight or more, still more preferably 38 parts by weight or more. , may be 45 parts by weight or more, 50 parts by weight or more (for example, more than 50 parts by weight), 55 parts by weight or more, 60 parts by weight or more, 65 parts by weight or more, 70 parts by weight or more. Often, the amount may be 75 parts by weight or more. The acrylic polymer containing n-heptyl acrylate as a monomer unit used in the technology disclosed herein has good compatibility with the tackifier resin, so it can be used to maintain retention power by incorporating a larger amount of the tackifier resin. , it is possible to improve adhesion to different materials.
いくつかの態様において、上記粘着剤層は、粘着付与樹脂TLと、軟化点が150℃以上(例えば150℃~200℃)の粘着付与樹脂THを組み合わせて含んでもよい。粘着付与樹脂THとしては、上記で例示した粘着付与樹脂のうち軟化点が150℃以上のものから1種を単独でまたは2種以上を組み合わせて用いることができる。
In some embodiments, the adhesive layer may include a combination of a tackifying resin T L and a tackifying resin T H having a softening point of 150° C. or higher (eg, 150° C. to 200° C.). As the tackifier resin T H , one kind or a combination of two or more kinds of tackifier resins having a softening point of 150° C. or more among the tackifier resins exemplified above can be used.
なお、本明細書における粘着付与樹脂の軟化点は、JIS K5902およびJIS K2207に規定する軟化点試験方法(環球法)に基づいて測定された値として定義される。具体的には、試料をできるだけ低温ですみやかに融解し、これを平らな金属板の上に置いた環の中に、泡ができないように注意して満たす。冷えたのち、少し加熱した小刀で環の上端を含む平面から盛り上がった部分を切り去る。つぎに、径85mm以上、高さ127mm以上のガラス容器(加熱浴)の中に支持器(環台)を入れ、グリセリンを深さ90mm以上となるまで注ぐ。つぎに、鋼球(径9.5mm、重量3.5g)と、試料を満たした環とを互いに接触しないようにしてグリセリン中に浸し、グリセリンの温度を20℃プラスマイナス5℃に15分間保つ。つぎに、環中の試料の表面の中央に鋼球をのせ、これを支持器の上の定位置に置く。つぎに、環の上端からグリセリン面までの距離を50mmに保ち、温度計を置き、温度計の水銀球の中心の位置を環の中心と同じ高さとし、容器を加熱する。加熱に用いるブンゼンバーナーの炎は、容器の底の中心と縁との中間にあたるようにし、加熱を均等にする。なお、加熱が始まってから40℃に達したのちの浴温の上昇する割合は、毎分5.0プラスマイナス0.5℃でなければならない。試料がしだいに軟化して環から流れ落ち、ついに底板に接触したときの温度を読み、これを軟化点とする。軟化点の測定は、同時に2個以上行い、その平均値を採用する。
Note that the softening point of the tackifier resin in this specification is defined as a value measured based on the softening point test method (ring and ball method) specified in JIS K5902 and JIS K2207. Specifically, the sample is melted as quickly as possible at the lowest possible temperature, and the sample is carefully filled into a ring placed on a flat metal plate, taking care not to form bubbles. After it has cooled down, use a slightly heated knife to cut off the raised part from the plane including the top of the ring. Next, a supporter (ring stand) is placed in a glass container (heating bath) with a diameter of 85 mm or more and a height of 127 mm or more, and glycerin is poured into the container to a depth of 90 mm or more. Next, the steel ball (diameter 9.5 mm, weight 3.5 g) and the ring filled with the sample were immersed in glycerin without coming into contact with each other, and the temperature of the glycerin was maintained at 20°C plus or minus 5°C for 15 minutes. . A steel ball is then placed in the center of the surface of the sample in the ring and placed in position on the support. Next, keeping the distance from the top of the ring to the glycerin surface at 50 mm, place a thermometer, set the center of the mercury bulb of the thermometer at the same height as the center of the ring, and heat the container. The flame of the Bunsen burner used for heating should be halfway between the center of the bottom of the container and the edge to ensure even heating. Note that the rate at which the bath temperature increases after heating starts and reaches 40°C must be 5.0 plus or minus 0.5°C per minute. The sample gradually softens and flows down from the ring, and the temperature at which it finally touches the bottom plate is read, and this is taken as the softening point. The softening point is measured at two or more points at the same time, and the average value is used.
いくつかの態様において、粘着付与樹脂TLは、粘着剤層に含まれる粘着付与樹脂の総量の50重量%超を占めることが好ましい。これにより、粘着付与樹脂TL含有の効果が効果的に発現しやすい。粘着剤層に含まれる粘着付与樹脂の総量に占める粘着付与樹脂TLの割合は、粘着付与樹脂TLの使用効果をより効果的に発揮する観点から、好ましくは60重量%以上、より好ましくは70重量%以上、さらに好ましくは80重量%以上、特に好ましくは90重量%以上であり、95重量%以上であってもよく、98重量%以上でもよい。いくつかの好ましい態様において、粘着剤層に含まれる粘着付与樹脂は、実質的に粘着付与樹脂TLのみからなる。かかる態様において、粘着剤層に含まれる粘着付与樹脂の総量に占める粘着付与樹脂TLの割合は99~100重量%の範囲である。
In some embodiments, it is preferred that the tackifier resin T L accounts for more than 50% by weight of the total amount of tackifier resins included in the adhesive layer. Thereby, the effect of containing the tackifying resin TL tends to be effectively expressed. The proportion of the tackifying resin T L in the total amount of the tackifying resin contained in the adhesive layer is preferably 60% by weight or more, more preferably The content is 70% by weight or more, more preferably 80% by weight or more, particularly preferably 90% by weight or more, may be 95% by weight or more, or may be 98% by weight or more. In some preferred embodiments, the tackifier resin contained in the adhesive layer consists essentially only of tackifier resin TL . In this embodiment, the proportion of the tackifying resin T L in the total amount of tackifying resin contained in the adhesive layer is in the range of 99 to 100% by weight.
特に限定するものではないが、いくつかの態様において、上記粘着付与樹脂は、水酸基価が70mgKOH/g未満の粘着付与樹脂を含み得る。なかでも水酸基価が60mgKOH/g未満(より好ましくは50mgKOH/g未満、さらに好ましくは45mgKOH/g未満)の粘着付与樹脂が好ましい。以下、水酸基価が70mgKOH/g未満の粘着付与樹脂を「低水酸基価樹脂」ということがある。このような低水酸基価樹脂を含む粘着付与樹脂によると、高極性材料、低極性材料のいずれに対しても高い接着力を有する粘着剤層が好ましく実現され得る。低水酸基価樹脂の水酸基価の下限は、0mgKOH/g以上であり、凡そ10mgKOH/g以上であってもよく、凡そ15mgKOH/g以上でもよい。低水酸基価樹脂は、1種を単独でまたは2種以上を組み合わせて用いることができる。低水酸基価樹脂としては、上記で例示した粘着付与樹脂のうち水酸基価が70mgKOH/g未満のものから適宜選択される1種を単独でまたは2種以上を組み合わせて用いることができる。いくつかの態様において、低水酸基価樹脂は、好ましくはロジン系粘着付与樹脂およびテルペン系粘着付与樹脂から選択される少なくとも1種を含み、より好ましくはロジン系粘着付与樹脂を含む。低水酸基価樹脂は、1種のロジン系粘着付与樹脂を単独で含んでもよく、2種以上のロジン系粘着付与樹脂を組み合わせて含んでもよい。また、低水酸基価樹脂は、上述の粘着付与樹脂TLであってもよく、粘着付与樹脂THであってもよく、粘着付与樹脂TLが低水酸基価樹脂であることが好ましい。
Although not particularly limited, in some embodiments, the tackifying resin may include a tackifying resin having a hydroxyl value of less than 70 mgKOH/g. Among these, tackifier resins with a hydroxyl value of less than 60 mgKOH/g (more preferably less than 50 mgKOH/g, still more preferably less than 45 mgKOH/g) are preferred. Hereinafter, a tackifier resin having a hydroxyl value of less than 70 mgKOH/g may be referred to as a "low hydroxyl value resin". According to the tackifying resin containing such a low hydroxyl value resin, an adhesive layer having high adhesive strength to both high polarity materials and low polarity materials can be preferably realized. The lower limit of the hydroxyl value of the low hydroxyl value resin is 0 mgKOH/g or more, may be about 10 mgKOH/g or more, or may be about 15 mgKOH/g or more. One type of low hydroxyl value resin can be used alone or two or more types can be used in combination. As the low hydroxyl value resin, one type suitably selected from the tackifying resins exemplified above having a hydroxyl value of less than 70 mgKOH/g can be used alone or in combination of two or more types. In some embodiments, the low hydroxyl value resin preferably includes at least one selected from rosin-based tackifier resins and terpene-based tackifier resins, and more preferably includes rosin-based tackifier resins. The low hydroxyl value resin may contain one type of rosin-based tackifier resin alone, or may contain a combination of two or more types of rosin-based tackifier resin. Moreover, the low hydroxyl value resin may be the above-mentioned tackifier resin T L or tackifier resin T H , and it is preferable that the tackifier resin T L is a low hydroxyl value resin.
いくつかの態様において、低水酸基価樹脂は、粘着剤層に含まれる粘着付与樹脂の総量の50重量%超を占めることが好ましい。これにより、低極性材料に対する接着力向上を好ましく実現することができる。いくつかの好ましい態様では、粘着剤層に含まれる粘着付与樹脂の総量に占める低水酸基価樹脂の割合は、低水酸基価樹脂の使用効果をより効果的に発揮する観点から、好ましくは60重量%以上、より好ましくは70重量%以上、さらに好ましくは80重量%以上、特に好ましくは90重量%以上であり、95重量%以上であってもよく、98重量%以上でもよい。いくつかの好ましい態様において、粘着剤層に含まれる粘着付与樹脂は、実質的に低水酸基価樹脂のみからなる。かかる態様において、粘着剤層に含まれる粘着付与樹脂の総量に占める低水酸基価樹脂の割合は99~100重量%の範囲である。
In some embodiments, the low hydroxyl value resin preferably accounts for more than 50% by weight of the total amount of tackifying resin contained in the adhesive layer. Thereby, it is possible to preferably improve the adhesion strength to low polarity materials. In some preferred embodiments, the proportion of the low hydroxyl value resin in the total amount of tackifier resin contained in the adhesive layer is preferably 60% by weight from the viewpoint of more effectively exhibiting the effect of using the low hydroxyl value resin. The content is more preferably 70% by weight or more, further preferably 80% by weight or more, particularly preferably 90% by weight or more, may be 95% by weight or more, or may be 98% by weight or more. In some preferred embodiments, the tackifying resin contained in the adhesive layer consists essentially of a low hydroxyl value resin. In this embodiment, the proportion of the low hydroxyl value resin in the total amount of tackifier resin contained in the adhesive layer is in the range of 99 to 100% by weight.
特に限定するものではないが、ここに開示される粘着剤層は、粘着付与樹脂として、水酸基価が70mgKOH/g以上の粘着付与樹脂(以下、「高水酸基価樹脂」ともいう。)の含有量がアクリル系ポリマー100重量部に対して5重量部未満であることが好ましい。このように高水酸基価樹脂の使用量を制限することにより、低極性材料に対する接着力改善効果が得られやすい。ここで、高水酸基価樹脂の含有量がアクリル系ポリマー100重量部に対して5重量部未満であるとは、粘着剤層が、高水酸基価樹脂を含まないこと、および、高水酸基価樹脂をアクリル系ポリマー100重量部に対して5重量部未満の割合で含むことの両方を包含する意味で用いられる。粘着剤層中の高水酸基価樹脂の含有量は、アクリル系ポリマー100重量部に対して3重量部未満であることが好ましく、1重量部以下(例えば0~0.1重量部)の範囲であることがより好ましい。
Although not particularly limited, the adhesive layer disclosed herein has a content of a tackifying resin having a hydroxyl value of 70 mgKOH/g or more (hereinafter also referred to as "high hydroxyl value resin") as a tackifying resin. is preferably less than 5 parts by weight based on 100 parts by weight of the acrylic polymer. By limiting the amount of high hydroxyl value resin used in this way, the effect of improving adhesive strength to low polarity materials can be easily obtained. Here, the content of the high hydroxyl value resin being less than 5 parts by weight based on 100 parts by weight of the acrylic polymer means that the adhesive layer does not contain the high hydroxyl value resin and that the adhesive layer does not contain the high hydroxyl value resin. It is used in the sense that it is included in a proportion of less than 5 parts by weight based on 100 parts by weight of the acrylic polymer. The content of the high hydroxyl value resin in the adhesive layer is preferably less than 3 parts by weight based on 100 parts by weight of the acrylic polymer, and is in the range of 1 part by weight or less (for example, 0 to 0.1 parts by weight). It is more preferable that there be.
ここで、上記水酸基価の値としては、JIS K0070:1992に規定する電位差滴定法により測定される値を採用することができる。具体的な測定方法は以下に示すとおりである。
[水酸基価の測定方法]
1.試薬
(1)アセチル化試薬としては、無水酢酸約12.5g(約11.8mL)を取り、これにピリジンを加えて全量を50mLにし、充分に攪拌したものを使用する。または、無水酢酸約25g(約23.5mL)を取り、これにピリジンを加えて全量を100mLにし、充分に攪拌したものを使用する。
(2)測定試薬としては、0.5mol/L水酸化カリウムエタノール溶液を使用する。
(3)その他、トルエン、ピリジン、エタノールおよび蒸留水を準備する。
2.操作
(1)平底フラスコに試料約2gを精秤採取し、アセチル化試薬5mLおよびピリジン10mLを加え、空気冷却管を装着する。
(2)上記フラスコを100℃の浴中で70分間加熱した後、放冷し、冷却管の上部から溶剤としてトルエン35mLを加えて攪拌した後、蒸留水1mLを加えて攪拌することにより無水酢酸を分解する。分解を完全にするため再度浴中で10分間加熱し、放冷する。
(3)エタノール5mLで冷却管を洗い、取り外す。次いで、溶剤としてピリジン50mLを加えて攪拌する。
(4)0.5mol/L水酸化カリウムエタノール溶液を、ホールピペットを用いて25mL加える。
(5)0.5mol/L水酸化カリウムエタノール溶液で電位差滴定を行う。得られた滴定曲線の変曲点を終点とする。
(6)空試験は、試料を入れないで上記(1)~(5)を行う。
3.計算
以下の式により水酸基価を算出する。
水酸基価(mgKOH/g)=[(B-C)×f×28.05]/S+D
ここで、
B: 空試験に用いた0.5mol/L水酸化カリウムエタノール溶液の量(mL)、
C: 試料に用いた0.5mol/L水酸化カリウムエタノール溶液の量(mL)、
f: 0.5mol/L水酸化カリウムエタノール溶液のファクター、
S: 試料の重量(g)、
D: 酸価、
28.05: 水酸化カリウムの分子量56.11の1/2、
である。 Here, as the value of the hydroxyl value, a value measured by the potentiometric titration method specified in JIS K0070:1992 can be adopted. The specific measurement method is as shown below.
[Method for measuring hydroxyl value]
1. Reagent (1) As the acetylation reagent, take about 12.5 g (about 11.8 mL) of acetic anhydride, add pyridine to make a total volume of 50 mL, and stir thoroughly. Alternatively, take about 25 g (about 23.5 mL) of acetic anhydride, add pyridine to make a total volume of 100 mL, stir thoroughly, and use.
(2) A 0.5 mol/L potassium hydroxide ethanol solution is used as the measurement reagent.
(3) Additionally, prepare toluene, pyridine, ethanol, and distilled water.
2. Procedure (1) Accurately weigh about 2 g of sample into a flat bottom flask, add 5 mL of acetylation reagent and 10 mL of pyridine, and attach an air cooling tube.
(2) After heating the above flask in a bath at 100°C for 70 minutes, let it cool, add 35 mL of toluene as a solvent from the upper part of the cooling tube and stir, and then add 1 mL of distilled water and stir to remove acetic anhydride. Disassemble. To complete decomposition, heat again in the bath for 10 minutes and allow to cool.
(3) Wash the cooling tube with 5 mL of ethanol and remove it. Next, 50 mL of pyridine is added as a solvent and stirred.
(4) Add 25 mL of 0.5 mol/L potassium hydroxide ethanol solution using a whole pipette.
(5) Perform potentiometric titration with a 0.5 mol/L potassium hydroxide ethanol solution. The inflection point of the obtained titration curve is defined as the end point.
(6) For a blank test, perform (1) to (5) above without adding a sample.
3. Calculation Calculate the hydroxyl value using the following formula.
Hydroxyl value (mgKOH/g)=[(B-C)×f×28.05]/S+D
here,
B: Amount (mL) of 0.5 mol/L potassium hydroxide ethanol solution used in the blank test,
C: Amount (mL) of 0.5 mol/L potassium hydroxide ethanol solution used in the sample,
f: factor of 0.5 mol/L potassium hydroxide ethanol solution,
S: weight of sample (g),
D: acid value,
28.05: 1/2 of the molecular weight of potassium hydroxide, 56.11,
It is.
[水酸基価の測定方法]
1.試薬
(1)アセチル化試薬としては、無水酢酸約12.5g(約11.8mL)を取り、これにピリジンを加えて全量を50mLにし、充分に攪拌したものを使用する。または、無水酢酸約25g(約23.5mL)を取り、これにピリジンを加えて全量を100mLにし、充分に攪拌したものを使用する。
(2)測定試薬としては、0.5mol/L水酸化カリウムエタノール溶液を使用する。
(3)その他、トルエン、ピリジン、エタノールおよび蒸留水を準備する。
2.操作
(1)平底フラスコに試料約2gを精秤採取し、アセチル化試薬5mLおよびピリジン10mLを加え、空気冷却管を装着する。
(2)上記フラスコを100℃の浴中で70分間加熱した後、放冷し、冷却管の上部から溶剤としてトルエン35mLを加えて攪拌した後、蒸留水1mLを加えて攪拌することにより無水酢酸を分解する。分解を完全にするため再度浴中で10分間加熱し、放冷する。
(3)エタノール5mLで冷却管を洗い、取り外す。次いで、溶剤としてピリジン50mLを加えて攪拌する。
(4)0.5mol/L水酸化カリウムエタノール溶液を、ホールピペットを用いて25mL加える。
(5)0.5mol/L水酸化カリウムエタノール溶液で電位差滴定を行う。得られた滴定曲線の変曲点を終点とする。
(6)空試験は、試料を入れないで上記(1)~(5)を行う。
3.計算
以下の式により水酸基価を算出する。
水酸基価(mgKOH/g)=[(B-C)×f×28.05]/S+D
ここで、
B: 空試験に用いた0.5mol/L水酸化カリウムエタノール溶液の量(mL)、
C: 試料に用いた0.5mol/L水酸化カリウムエタノール溶液の量(mL)、
f: 0.5mol/L水酸化カリウムエタノール溶液のファクター、
S: 試料の重量(g)、
D: 酸価、
28.05: 水酸化カリウムの分子量56.11の1/2、
である。 Here, as the value of the hydroxyl value, a value measured by the potentiometric titration method specified in JIS K0070:1992 can be adopted. The specific measurement method is as shown below.
[Method for measuring hydroxyl value]
1. Reagent (1) As the acetylation reagent, take about 12.5 g (about 11.8 mL) of acetic anhydride, add pyridine to make a total volume of 50 mL, and stir thoroughly. Alternatively, take about 25 g (about 23.5 mL) of acetic anhydride, add pyridine to make a total volume of 100 mL, stir thoroughly, and use.
(2) A 0.5 mol/L potassium hydroxide ethanol solution is used as the measurement reagent.
(3) Additionally, prepare toluene, pyridine, ethanol, and distilled water.
2. Procedure (1) Accurately weigh about 2 g of sample into a flat bottom flask, add 5 mL of acetylation reagent and 10 mL of pyridine, and attach an air cooling tube.
(2) After heating the above flask in a bath at 100°C for 70 minutes, let it cool, add 35 mL of toluene as a solvent from the upper part of the cooling tube and stir, and then add 1 mL of distilled water and stir to remove acetic anhydride. Disassemble. To complete decomposition, heat again in the bath for 10 minutes and allow to cool.
(3) Wash the cooling tube with 5 mL of ethanol and remove it. Next, 50 mL of pyridine is added as a solvent and stirred.
(4) Add 25 mL of 0.5 mol/L potassium hydroxide ethanol solution using a whole pipette.
(5) Perform potentiometric titration with a 0.5 mol/L potassium hydroxide ethanol solution. The inflection point of the obtained titration curve is defined as the end point.
(6) For a blank test, perform (1) to (5) above without adding a sample.
3. Calculation Calculate the hydroxyl value using the following formula.
Hydroxyl value (mgKOH/g)=[(B-C)×f×28.05]/S+D
here,
B: Amount (mL) of 0.5 mol/L potassium hydroxide ethanol solution used in the blank test,
C: Amount (mL) of 0.5 mol/L potassium hydroxide ethanol solution used in the sample,
f: factor of 0.5 mol/L potassium hydroxide ethanol solution,
S: weight of sample (g),
D: acid value,
28.05: 1/2 of the molecular weight of potassium hydroxide, 56.11,
It is.
ここに開示される粘着剤層が粘着付与樹脂を含む場合、粘着付与樹脂としては、粘着剤層のバイオマス炭素比向上の観点から、植物に由来する粘着付与樹脂(植物性粘着付与樹脂)を好ましく作用し得る。植物性粘着付与樹脂の例としては、例えば上述のロジン系粘着付与樹脂、テルペン系粘着付与樹脂が挙げられる。植物性粘着付与樹脂は、1種を単独でまたは2種以上を組み合わせて用いることができる。ここに開示される粘着剤層が粘着付与樹脂を含む場合、粘着付与樹脂の総量に占める植物性粘着付与樹脂の割合は、30重量%以上(例えば50重量%以上、典型的には80重量%以上)とすることが好ましい。いくつかの態様において、粘着付与樹脂の総量に占める植物性粘着付与樹脂の割合は、90重量%以上(例えば95重量%以上、典型的には99~100重量%)である。ここに開示される技術は、植物性粘着付与樹脂以外の粘着付与樹脂を実質的に含まない態様で好ましく実施され得る。
When the adhesive layer disclosed herein includes a tackifier resin, from the viewpoint of improving the biomass carbon ratio of the adhesive layer, a tackifier resin derived from plants (vegetable tackifier resin) is preferably used as the tackifier resin. It can work. Examples of the vegetable tackifier resin include the above-mentioned rosin-based tackifier resin and terpene-based tackifier resin. The vegetable tackifying resins can be used alone or in combination of two or more. When the adhesive layer disclosed herein includes a tackifying resin, the proportion of the vegetable tackifying resin in the total amount of the tackifying resin is 30% by weight or more (for example, 50% by weight or more, typically 80% by weight). above) is preferable. In some embodiments, the proportion of vegetable tackifying resin in the total amount of tackifying resin is 90% by weight or more (eg, 95% by weight or more, typically 99-100% by weight). The technology disclosed herein can be preferably implemented in an embodiment that does not substantially contain tackifying resins other than vegetable tackifying resins.
粘着付与樹脂の含有量(2種以上の粘着付与樹脂を含む場合はその合計量)は、特に限定されないが、アクリルポリマー100重量部に対して100重量部以下程度(例えば100重量部未満)とすることが適当である。粘着付与樹脂の使用量を所定量以下に制限することにより、十分な保持力を有しつつ、高極性材料および低極性材料に対する接着力を向上することができる。いくつかの好ましい態様において、粘着付与樹脂の使用量は、保持力等の観点から、アクリル系ポリマー100重量部に対して、90重量部以下であることが適当であり、好ましくは80重量部以下であり、60重量部以下であってもよく、55重量部以下でもよく、50重量部以下でもよく、45重量部以下でもよい。他のいくつかの態様において、粘着付与樹脂の使用量は、アクリル系ポリマー100重量部に対して、35重量部以下であってもよく、30重量部以下でもよく、25重量部以下でもよく、20重量部以下(例えば20重量部未満)でもよい。また、いくつかの態様において、接着力向上の観点から、粘着付与樹脂の使用量は、アクリル系ポリマー100重量部に対して、例えば10重量部超であり、12重量部以上であってもよく、14重量部以上でもよく、15重量部超でもよく、20重量部以上が適当であり、好ましくは30重量部以上、より好ましくは35重量部以上、さらに好ましくは38重量部以上であり、45重量部以上であってよく、50重量部以上(例えば50重量部超)でもよく、55重量部以上でもよく、60重量部以上でもよく、65重量部以上でもよく、70重量部以上でもよく、75重量部以上でもよい。ここに開示される技術において用いられるn-ヘプチルアクリレートをモノマー単位として含むアクリル系ポリマーは、粘着付与樹脂との相溶性がよいので、粘着付与樹脂をより多く含ませて、保持力を維持しつつ、異種材料に対する接着力を向上させることができる。
The content of the tackifying resin (the total amount when two or more tackifying resins are included) is not particularly limited, but is about 100 parts by weight or less (for example, less than 100 parts by weight) based on 100 parts by weight of the acrylic polymer. It is appropriate to do so. By limiting the amount of tackifying resin used to a predetermined amount or less, it is possible to improve adhesive strength to high polarity materials and low polarity materials while maintaining sufficient holding power. In some preferred embodiments, the amount of the tackifying resin used is suitably 90 parts by weight or less, preferably 80 parts by weight or less, based on 100 parts by weight of the acrylic polymer, from the viewpoint of holding power etc. The amount may be 60 parts by weight or less, 55 parts by weight or less, 50 parts by weight or less, or 45 parts by weight or less. In some other embodiments, the amount of the tackifying resin used may be 35 parts by weight or less, 30 parts by weight or less, or 25 parts by weight or less, based on 100 parts by weight of the acrylic polymer. It may be 20 parts by weight or less (for example, less than 20 parts by weight). Further, in some embodiments, from the viewpoint of improving adhesive strength, the amount of the tackifying resin used is, for example, more than 10 parts by weight, and may be 12 parts by weight or more, based on 100 parts by weight of the acrylic polymer. , may be 14 parts by weight or more, may be more than 15 parts by weight, suitably 20 parts by weight or more, preferably 30 parts by weight or more, more preferably 35 parts by weight or more, still more preferably 38 parts by weight or more, 45 parts by weight or more. It may be at least 50 parts by weight (for example, more than 50 parts by weight), at least 55 parts by weight, at least 60 parts by weight, at least 65 parts by weight, at least 70 parts by weight, The amount may be 75 parts by weight or more. The acrylic polymer containing n-heptyl acrylate as a monomer unit used in the technology disclosed herein has good compatibility with the tackifier resin, so it can be used to maintain retention power by incorporating a larger amount of the tackifier resin. , it is possible to improve adhesion to different materials.
ここに開示される技術において、粘着剤層中のアクリル系ポリマーおよび粘着付与樹脂の合計含有量は、ここに開示される技術による効果が発揮されるよう適切に設定され、特定の範囲に限定されるものではない。いくつかの好ましい態様に係る粘着剤層に含まれるアクリル系ポリマーおよび粘着付与樹脂の合計量(総量)は、ここに開示される技術による効果を好ましく発揮する観点から、50重量%超であることが適当であり、好ましくは凡そ70重量%以上、より好ましくは凡そ90重量%以上、さらに好ましくは95重量%以上(例えば95重量%以上100重量%以下あるいは100重量%未満)であり、97重量%以上であってもよい。
In the technology disclosed herein, the total content of the acrylic polymer and tackifying resin in the adhesive layer is appropriately set and limited to a specific range so that the effects of the technology disclosed herein are exhibited. It's not something you can do. The total amount (total amount) of the acrylic polymer and tackifier resin contained in the adhesive layer according to some preferred embodiments is more than 50% by weight from the viewpoint of preferably exhibiting the effects of the technology disclosed herein. is appropriate, preferably about 70% by weight or more, more preferably about 90% by weight or more, even more preferably 95% by weight or more (for example, 95% by weight or more and 100% by weight or less, or less than 100% by weight), and 97% by weight. % or more.
(架橋剤)
ここに開示される技術において、粘着剤層の形成に用いられる粘着剤組成物は、必要に応じて架橋剤を含んでもよい。架橋剤の種類は特に制限されず、例えば、イソシアネート系架橋剤、エポキシ系架橋剤、オキサゾリン系架橋剤、アジリジン系架橋剤、メラミン系架橋剤、過酸化物系架橋剤、尿素系架橋剤、金属アルコキシド系架橋剤、金属キレート系架橋剤、金属塩系架橋剤、カルボジイミド系架橋剤、ヒドラジン系架橋剤、アミン系架橋剤、シランカップリング剤等が挙げられる。架橋剤は、1種を単独で、または2種以上を組み合わせて用いることができる。なかでも、イソシアネート系架橋剤、エポキシ系架橋剤、オキサゾリン系架橋剤、アジリジン系架橋剤、メラミン系架橋剤が好ましく、イソシアネート系架橋剤、エポキシ系架橋剤がより好ましい。架橋剤を適切に選定して使用することにより、粘着剤層は凝集力を得て、異種材料に対する接着力および保持力を好ましく両立することができる。なお、ここに開示される技術における粘着剤層は、上記架橋剤を、架橋反応後の形態、架橋反応前の形態、部分的に架橋反応した形態、これらの中間的または複合的な形態等で含有し得る。上記架橋剤は、典型的には、専ら架橋反応後の形態で粘着剤層に含まれている。 (Crosslinking agent)
In the technology disclosed herein, the adhesive composition used to form the adhesive layer may contain a crosslinking agent as necessary. The type of crosslinking agent is not particularly limited, and examples include isocyanate crosslinking agents, epoxy crosslinking agents, oxazoline crosslinking agents, aziridine crosslinking agents, melamine crosslinking agents, peroxide crosslinking agents, urea crosslinking agents, and metals. Examples include alkoxide crosslinking agents, metal chelate crosslinking agents, metal salt crosslinking agents, carbodiimide crosslinking agents, hydrazine crosslinking agents, amine crosslinking agents, and silane coupling agents. One type of crosslinking agent can be used alone or two or more types can be used in combination. Among these, isocyanate crosslinking agents, epoxy crosslinking agents, oxazoline crosslinking agents, aziridine crosslinking agents, and melamine crosslinking agents are preferred, and isocyanate crosslinking agents and epoxy crosslinking agents are more preferred. By appropriately selecting and using a crosslinking agent, the pressure-sensitive adhesive layer can obtain cohesive force, and can preferably achieve both adhesion and holding power to different materials. The adhesive layer in the technology disclosed herein may contain the crosslinking agent in a form after a crosslinking reaction, a form before a crosslinking reaction, a partially crosslinked form, an intermediate or composite form thereof, etc. May contain. The crosslinking agent is typically contained in the adhesive layer exclusively in the form after crosslinking reaction.
ここに開示される技術において、粘着剤層の形成に用いられる粘着剤組成物は、必要に応じて架橋剤を含んでもよい。架橋剤の種類は特に制限されず、例えば、イソシアネート系架橋剤、エポキシ系架橋剤、オキサゾリン系架橋剤、アジリジン系架橋剤、メラミン系架橋剤、過酸化物系架橋剤、尿素系架橋剤、金属アルコキシド系架橋剤、金属キレート系架橋剤、金属塩系架橋剤、カルボジイミド系架橋剤、ヒドラジン系架橋剤、アミン系架橋剤、シランカップリング剤等が挙げられる。架橋剤は、1種を単独で、または2種以上を組み合わせて用いることができる。なかでも、イソシアネート系架橋剤、エポキシ系架橋剤、オキサゾリン系架橋剤、アジリジン系架橋剤、メラミン系架橋剤が好ましく、イソシアネート系架橋剤、エポキシ系架橋剤がより好ましい。架橋剤を適切に選定して使用することにより、粘着剤層は凝集力を得て、異種材料に対する接着力および保持力を好ましく両立することができる。なお、ここに開示される技術における粘着剤層は、上記架橋剤を、架橋反応後の形態、架橋反応前の形態、部分的に架橋反応した形態、これらの中間的または複合的な形態等で含有し得る。上記架橋剤は、典型的には、専ら架橋反応後の形態で粘着剤層に含まれている。 (Crosslinking agent)
In the technology disclosed herein, the adhesive composition used to form the adhesive layer may contain a crosslinking agent as necessary. The type of crosslinking agent is not particularly limited, and examples include isocyanate crosslinking agents, epoxy crosslinking agents, oxazoline crosslinking agents, aziridine crosslinking agents, melamine crosslinking agents, peroxide crosslinking agents, urea crosslinking agents, and metals. Examples include alkoxide crosslinking agents, metal chelate crosslinking agents, metal salt crosslinking agents, carbodiimide crosslinking agents, hydrazine crosslinking agents, amine crosslinking agents, and silane coupling agents. One type of crosslinking agent can be used alone or two or more types can be used in combination. Among these, isocyanate crosslinking agents, epoxy crosslinking agents, oxazoline crosslinking agents, aziridine crosslinking agents, and melamine crosslinking agents are preferred, and isocyanate crosslinking agents and epoxy crosslinking agents are more preferred. By appropriately selecting and using a crosslinking agent, the pressure-sensitive adhesive layer can obtain cohesive force, and can preferably achieve both adhesion and holding power to different materials. The adhesive layer in the technology disclosed herein may contain the crosslinking agent in a form after a crosslinking reaction, a form before a crosslinking reaction, a partially crosslinked form, an intermediate or composite form thereof, etc. May contain. The crosslinking agent is typically contained in the adhesive layer exclusively in the form after crosslinking reaction.
イソシアネート系架橋剤としては、多官能イソシアネート(1分子当たり平均2個以上のイソシアネート基を有する化合物をいい、イソシアヌレート構造を有するものを包含する。)が好ましく使用され得る。イソシアネート系架橋剤は、1種を単独でまたは2種以上を組み合わせて用いることができる。
As the isocyanate-based crosslinking agent, polyfunctional isocyanates (referring to compounds having an average of two or more isocyanate groups per molecule, including those having an isocyanurate structure) can be preferably used. The isocyanate crosslinking agents can be used alone or in combination of two or more.
多官能イソシアネートの例として、脂肪族ポリイソシアネート類、脂環族ポリイソシアネート類、芳香族ポリイソシアネート類等が挙げられる。
脂肪族ポリイソシアネート類の具体例としては、1,2-エチレンジイソシアネート;1,2-テトラメチレンジイソシアネート、1,3-テトラメチレンジイソシアネート、1,4-テトラメチレンジイソシアネート等のテトラメチレンジイソシアネート;1,2-ヘキサメチレンジイソシアネート、1,3-ヘキサメチレンジイソシアネート、1,4-ヘキサメチレンジイソシアネート、1,5-ヘキサメチレンジイソシアネート、1,6-ヘキサメチレンジイソシアネート、2,5-ヘキサメチレンジイソシアネート等のヘキサメチレンジイソシアネート;2-メチル-1,5-ペンタンジイソシアネート、3-メチル-1,5-ペンタンジイソシアネート、リジンジイソシアネート、等が挙げられる。 Examples of polyfunctional isocyanates include aliphatic polyisocyanates, alicyclic polyisocyanates, aromatic polyisocyanates, and the like.
Specific examples of aliphatic polyisocyanates include 1,2-ethylene diisocyanate; tetramethylene diisocyanates such as 1,2-tetramethylene diisocyanate, 1,3-tetramethylene diisocyanate, and 1,4-tetramethylene diisocyanate; 1,2-tetramethylene diisocyanate; - hexamethylene diisocyanate such as hexamethylene diisocyanate, 1,3-hexamethylene diisocyanate, 1,4-hexamethylene diisocyanate, 1,5-hexamethylene diisocyanate, 1,6-hexamethylene diisocyanate, 2,5-hexamethylene diisocyanate; Examples include 2-methyl-1,5-pentane diisocyanate, 3-methyl-1,5-pentane diisocyanate, lysine diisocyanate, and the like.
脂肪族ポリイソシアネート類の具体例としては、1,2-エチレンジイソシアネート;1,2-テトラメチレンジイソシアネート、1,3-テトラメチレンジイソシアネート、1,4-テトラメチレンジイソシアネート等のテトラメチレンジイソシアネート;1,2-ヘキサメチレンジイソシアネート、1,3-ヘキサメチレンジイソシアネート、1,4-ヘキサメチレンジイソシアネート、1,5-ヘキサメチレンジイソシアネート、1,6-ヘキサメチレンジイソシアネート、2,5-ヘキサメチレンジイソシアネート等のヘキサメチレンジイソシアネート;2-メチル-1,5-ペンタンジイソシアネート、3-メチル-1,5-ペンタンジイソシアネート、リジンジイソシアネート、等が挙げられる。 Examples of polyfunctional isocyanates include aliphatic polyisocyanates, alicyclic polyisocyanates, aromatic polyisocyanates, and the like.
Specific examples of aliphatic polyisocyanates include 1,2-ethylene diisocyanate; tetramethylene diisocyanates such as 1,2-tetramethylene diisocyanate, 1,3-tetramethylene diisocyanate, and 1,4-tetramethylene diisocyanate; 1,2-tetramethylene diisocyanate; - hexamethylene diisocyanate such as hexamethylene diisocyanate, 1,3-hexamethylene diisocyanate, 1,4-hexamethylene diisocyanate, 1,5-hexamethylene diisocyanate, 1,6-hexamethylene diisocyanate, 2,5-hexamethylene diisocyanate; Examples include 2-methyl-1,5-pentane diisocyanate, 3-methyl-1,5-pentane diisocyanate, lysine diisocyanate, and the like.
脂環族ポリイソシアネート類の具体例としては、イソホロンジイソシアネート;1,2-シクロヘキシルジイソシアネート、1,3-シクロヘキシルジイソシアネート、1,4-シクロヘキシルジイソシアネート等のシクロヘキシルジイソシアネート;1,2-シクロペンチルジイソシアネート、1,3-シクロペンチルジイソシアネート等のシクロペンチルジイソシアネート;水素添加キシリレンジイソシアネート、水素添加トリレンジイソシアネート、水素添加ジフェニルメタンジイソシアネート、水素添加テトラメチルキシレンジイソシアネート、4,4’-ジシクロヘキシルメタンジイソシアネート、等が挙げられる。
Specific examples of alicyclic polyisocyanates include isophorone diisocyanate; cyclohexyl diisocyanates such as 1,2-cyclohexyl diisocyanate, 1,3-cyclohexyl diisocyanate, and 1,4-cyclohexyl diisocyanate; 1,2-cyclopentyl diisocyanate, and 1,3-cyclohexyl diisocyanate; -Cyclopentyl diisocyanates such as cyclopentyl diisocyanate; hydrogenated xylylene diisocyanate, hydrogenated tolylene diisocyanate, hydrogenated diphenylmethane diisocyanate, hydrogenated tetramethylxylene diisocyanate, 4,4'-dicyclohexylmethane diisocyanate, and the like.
芳香族ポリイソシアネート類の具体例としては、2,4-トリレンジイソシアネート、2,6-トリレンジイソシアネート、4,4’-ジフェニルメタンジイソシアネート、2,4’-ジフェニルメタンジイソシアネート、2,2’-ジフェニルメタンジイソシアネート、4,4’-ジフェニルエーテルジイソシアネート、2-ニトロジフェニル-4,4’-ジイソシアネート、2,2’-ジフェニルプロパン-4,4’-ジイソシアネート、3,3’-ジメチルジフェニルメタン-4,4’-ジイソシアネート、4,4’-ジフェニルプロパンジイソシアネート、m-フェニレンジイソシアネート、p-フェニレンジイソシアネート、ナフチレン-1,4-ジイソシアネート、ナフチレン-1,5-ジイソシアネート、3,3’-ジメトキシジフェニル-4,4’-ジイソシアネート、キシリレン-1,4-ジイソシアネート、キシリレン-1,3-ジイソシアネート等が挙げられる。
Specific examples of aromatic polyisocyanates include 2,4-tolylene diisocyanate, 2,6-tolylene diisocyanate, 4,4'-diphenylmethane diisocyanate, 2,4'-diphenylmethane diisocyanate, and 2,2'-diphenylmethane diisocyanate. , 4,4'-diphenyl ether diisocyanate, 2-nitrodiphenyl-4,4'-diisocyanate, 2,2'-diphenylpropane-4,4'-diisocyanate, 3,3'-dimethyldiphenylmethane-4,4'-diisocyanate , 4,4'-diphenylpropane diisocyanate, m-phenylene diisocyanate, p-phenylene diisocyanate, naphthylene-1,4-diisocyanate, naphthylene-1,5-diisocyanate, 3,3'-dimethoxydiphenyl-4,4'-diisocyanate , xylylene-1,4-diisocyanate, xylylene-1,3-diisocyanate, and the like.
好ましい多官能イソシアネートとして、1分子当たり平均して3個以上のイソシアネート基を有する多官能イソシアネートが例示される。かかる3官能以上のイソシアネートは、2官能または3官能以上のイソシアネートの多量体(典型的には2量体または3量体)、誘導体(例えば、多価アルコールと2分子以上の多官能イソシアネートとの付加反応生成物)、重合物等であり得る。例えば、ジフェニルメタンジイソシアネートの2量体や3量体、ヘキサメチレンジイソシアネートのイソシアヌレート体(イソシアヌレート構造の3量体付加物)、トリメチロールプロパンとトリレンジイソシアネートとの反応生成物、トリメチロールプロパンとヘキサメチレンジイソシアネートとの反応生成物、ポリメチレンポリフェニルイソシアネート、ポリエーテルポリイソシアネート、ポリエステルポリイソシアネート、等の多官能イソシアネートが挙げられる。かかる多官能イソシアネートの市販品としては、旭化成ケミカルズ社製の商品名「デュラネートTPA-100」、東ソー社製の商品名「コロネートL」、同「コロネートHL」、同「コロネートHK」、同「コロネートHX」、同「コロネート2096」等が挙げられる。
Examples of preferred polyfunctional isocyanates include polyfunctional isocyanates having an average of three or more isocyanate groups per molecule. Such trifunctional or higher functional isocyanates are polymers (typically dimers or trimers) of bifunctional or trifunctional or higher functional isocyanates, derivatives (for example, a combination of a polyhydric alcohol and two or more molecules of polyfunctional isocyanate). addition reaction products), polymers, etc. For example, dimers and trimers of diphenylmethane diisocyanate, isocyanurates of hexamethylene diisocyanate (trimeric adducts of isocyanurate structures), reaction products of trimethylolpropane and tolylene diisocyanate, and products of the reaction between trimethylolpropane and hexamethylene diisocyanate. Examples include polyfunctional isocyanates such as reaction products with methylene diisocyanate, polymethylene polyphenylisocyanate, polyether polyisocyanate, and polyester polyisocyanate. Commercial products of such polyfunctional isocyanates include "Duranate TPA-100" manufactured by Asahi Kasei Chemicals, "Coronate L", "Coronate HL", "Coronate HK", and "Coronate HL" manufactured by Tosoh Corporation. Examples include "Coronate 2096" and "Coronate 2096".
イソシアネート系架橋剤の使用量は特に限定されない。例えば、アクリル系ポリマー100重量部に対して、凡そ0.1重量部以上とすることができる。凝集力と密着性との両立等の観点から、アクリル系ポリマー100重量部に対するイソシアネート系架橋剤の使用量は、例えば0.5重量部以上とすることができ、1.0重量部以上が適当であり、好ましくは1.5重量部以上である。また、上記イソシアネート系架橋剤の使用量は、アクリル系ポリマー100重量部に対して10重量部以下とすることが適当であり、好ましくは5重量部未満、より好ましくは4.0重量部未満、さらに好ましくは3.0重量部未満(例えば2.5重量部以下)である。
The amount of the isocyanate crosslinking agent used is not particularly limited. For example, the amount can be approximately 0.1 part by weight or more per 100 parts by weight of the acrylic polymer. From the viewpoint of achieving both cohesive force and adhesion, the amount of the isocyanate crosslinking agent to be used per 100 parts by weight of the acrylic polymer can be, for example, 0.5 parts by weight or more, and preferably 1.0 parts by weight or more. and preferably 1.5 parts by weight or more. Further, the amount of the isocyanate crosslinking agent used is suitably 10 parts by weight or less per 100 parts by weight of the acrylic polymer, preferably less than 5 parts by weight, more preferably less than 4.0 parts by weight, More preferably, it is less than 3.0 parts by weight (for example, 2.5 parts by weight or less).
エポキシ系架橋剤としては、1分子中に2個以上のエポキシ基を有する化合物を特に制限なく用いることができる。1分子中に3~5個のエポキシ基を有するエポキシ系架橋剤が好ましい。エポキシ系架橋剤は、1種を単独でまたは2種以上を組み合わせて用いることができる。
As the epoxy crosslinking agent, any compound having two or more epoxy groups in one molecule can be used without particular limitation. Epoxy crosslinking agents having 3 to 5 epoxy groups in one molecule are preferred. The epoxy crosslinking agents can be used alone or in combination of two or more.
特に限定するものではないが、エポキシ系架橋剤の具体例として、例えばN,N,N’,N’-テトラグリシジル-m-キシレンジアミン、1,3-ビス(N,N-ジグリシジルアミノメチル)シクロヘキサン、1,6-ヘキサンジオールジグリシジルエーテル、ポリエチレングリコールジグリシジルエーテル、ポリグリセロールポリグリシジルエーテル等が挙げられる。エポキシ系架橋剤の市販品としては、三菱ガス化学社製の商品名「TETRAD-C」および商品名「TETRAD-X」、DIC社製の商品名「エピクロンCR-5L」、ナガセケムテックス社製の商品名「デナコールEX-512」、日産化学工業社製の商品名「TEPIC-G」等が挙げられる。
Although not particularly limited, specific examples of epoxy crosslinking agents include N,N,N',N'-tetraglycidyl-m-xylene diamine, 1,3-bis(N,N-diglycidylaminomethyl ) cyclohexane, 1,6-hexanediol diglycidyl ether, polyethylene glycol diglycidyl ether, polyglycerol polyglycidyl ether and the like. Commercially available epoxy crosslinking agents include Mitsubishi Gas Chemical's product names "TETRAD-C" and "TETRAD-X", DIC's product name "Epicron CR-5L", and Nagase ChemteX's product name Examples include the product name "Denacol EX-512" manufactured by Nissan Chemical Industries, Ltd. and the product name "TEPIC-G" manufactured by Nissan Chemical Industries, Ltd.
エポキシ系架橋剤の使用量は特に限定されない。エポキシ系架橋剤の使用量は、例えば、アクリル系ポリマー100重量部に対して、0重量部を超えて凡そ1重量部以下(典型的には凡そ0.001~1重量部)とすることができる。凝集力の向上効果を好適に発揮する観点から、エポキシ系架橋剤の使用量は、アクリル系ポリマー100重量部に対して凡そ0.005重量部以上とすることが適当であり、凡そ0.01重量部以上が好ましく、凡そ0.02重量部以上がより好ましい。また、被着体に対する密着性向上の観点から、エポキシ系架橋剤の使用量は、アクリル系ポリマー100重量部に対して凡そ0.5重量部以下とすることが適当であり、凡そ0.2重量部以下とすることが好ましく、凡そ0.1重量部以下(例えば0.1重量部未満)がより好ましく、0.07重量部以下であってもよく、0.04重量部以下でもよい。
The amount of the epoxy crosslinking agent used is not particularly limited. The amount of the epoxy crosslinking agent used may be, for example, more than 0 parts by weight and no more than about 1 part by weight (typically about 0.001 to 1 part by weight) per 100 parts by weight of the acrylic polymer. can. From the viewpoint of suitably exhibiting the effect of improving cohesive force, it is appropriate that the amount of the epoxy crosslinking agent used is approximately 0.005 parts by weight or more, and approximately 0.01 parts by weight, based on 100 parts by weight of the acrylic polymer. The amount is preferably at least 0.02 parts by weight, more preferably about 0.02 parts by weight or more. In addition, from the viewpoint of improving adhesion to the adherend, the amount of the epoxy crosslinking agent used is approximately 0.5 parts by weight or less per 100 parts by weight of the acrylic polymer, and approximately 0.2 parts by weight. It is preferably at most 0.1 part by weight (for example less than 0.1 part by weight), more preferably at most 0.07 part by weight, and may be at most 0.04 part by weight.
いくつかの好ましい態様において、架橋剤として、エポキシ系架橋剤と、該エポキシ系架橋剤とは架橋性官能基の種類が異なる少なくとも一種の架橋剤とが組み合わせて用いられる。ここに開示される技術によると、エポキシ系架橋剤以外の架橋剤(すなわち、エポキシ系架橋剤とは架橋性反応基の種類の異なる架橋剤。以下「非エポキシ系架橋剤」ともいう。)とエポキシ系架橋剤とを組み合わせて用いることにより、異種材料に対する接着力と高い保持力とを好適に両立することができる。
In some preferred embodiments, as the crosslinking agent, an epoxy crosslinking agent and at least one crosslinking agent having a different type of crosslinkable functional group from the epoxy crosslinking agent are used in combination. According to the technology disclosed herein, a crosslinking agent other than an epoxy crosslinking agent (that is, a crosslinking agent having a different type of crosslinkable reactive group from an epoxy crosslinking agent; hereinafter also referred to as a "non-epoxy crosslinking agent") By using it in combination with an epoxy crosslinking agent, it is possible to suitably achieve both adhesion to different materials and high holding power.
エポキシ系架橋剤と組み合わせて用いられ得る非エポキシ系架橋剤の種類は特に制限されず、上述の架橋剤から適宜選択して用いることができる。非エポキシ系架橋剤は、1種を単独でまたは2種以上を組み合わせて用いることができる。
The type of non-epoxy crosslinking agent that can be used in combination with the epoxy crosslinking agent is not particularly limited, and can be appropriately selected from the above-mentioned crosslinking agents. The non-epoxy crosslinking agents can be used alone or in combination of two or more.
いくつかの好ましい態様において、非エポキシ系架橋剤としてイソシアネート系架橋剤を採用することができる。例えば、エポキシ系架橋剤とイソシアネート系架橋剤とを併用することにより、より優れた粘着特性を実現することができる。エポキシ系架橋剤の含有量と非エポキシ系架橋剤(好適にはイソシアネート系架橋剤)の含有量との関係は特に限定されない。エポキシ系架橋剤の含有量は、例えば、非エポキシ系架橋剤(好適にはイソシアネート系架橋剤)の含有量の凡そ1/10以下とすることができる。被着体に対する密着性と凝集力とをより好適に両立する観点から、エポキシ系架橋剤の含有量は、非エポキシ系架橋剤の含有量の凡そ1/30以下とすることが適当であり、凡そ1/50以下とすることが好ましく、凡そ1/75以下とすることがより好ましく、凡そ1/90以下であってもよい。また、エポキシ系架橋剤と非エポキシ系架橋剤(好適にはイソシアネート系架橋剤)とを組み合わせて用いることによる効果を好適に発揮する観点から、通常、エポキシ系架橋剤の含有量は、非エポキシ系架橋剤の含有量の凡そ1/1000以上、例えば凡そ1/500以上とすることが適当であり、好ましくは凡そ1/300以上、より好ましくは1/150以上、さらに好ましくは1/120以上である。
In some preferred embodiments, an isocyanate crosslinking agent can be employed as the non-epoxy crosslinking agent. For example, by using an epoxy crosslinking agent and an isocyanate crosslinking agent in combination, better adhesive properties can be achieved. The relationship between the content of the epoxy crosslinking agent and the content of the non-epoxy crosslinking agent (preferably an isocyanate crosslinking agent) is not particularly limited. The content of the epoxy crosslinking agent can be, for example, about 1/10 or less of the content of the non-epoxy crosslinking agent (preferably an isocyanate crosslinking agent). From the viewpoint of achieving both adhesion to the adherend and cohesive force, it is appropriate that the content of the epoxy crosslinking agent is approximately 1/30 or less of the content of the non-epoxy crosslinking agent, It is preferably about 1/50 or less, more preferably about 1/75 or less, and may be about 1/90 or less. In addition, from the viewpoint of suitably exhibiting the effect of using an epoxy crosslinking agent and a non-epoxy crosslinking agent (preferably an isocyanate crosslinking agent) in combination, the content of the epoxy crosslinking agent is usually adjusted to It is appropriate to set the content to about 1/1000 or more, for example about 1/500 or more, preferably about 1/300 or more, more preferably 1/150 or more, still more preferably 1/120 or more of the content of the system crosslinking agent. It is.
架橋剤の総使用量は特に制限されず、例えば、アクリル系ポリマー100重量部に対して凡そ0.005重量部以上(例えば0.01重量部以上、典型的には0.1重量部以上)程度、凡そ10重量部以下(例えば凡そ8重量部以下、好ましくは凡そ5重量部以下)程度の範囲から選択することができる。
The total amount of the crosslinking agent used is not particularly limited, and for example, approximately 0.005 parts by weight or more (for example, 0.01 parts by weight or more, typically 0.1 parts by weight or more) based on 100 parts by weight of the acrylic polymer. The amount can be selected from a range of about 10 parts by weight or less (for example, about 8 parts by weight or less, preferably about 5 parts by weight or less).
(その他の添加剤)
粘着剤組成物には、上述した各成分以外に、必要に応じてレベリング剤、架橋助剤、可塑剤、軟化剤、充填剤、着色剤(顔料、染料等)、帯電防止剤、老化防止剤、紫外線吸収剤、酸化防止剤、光安定剤等の、粘着剤の分野において一般的な各種の添加剤が含まれていてもよい。このような各種添加剤については、従来公知のものを常法により使用することができ、特に本発明を特徴づけるものではないので、詳細な説明は省略する。 (Other additives)
In addition to the above-mentioned components, the adhesive composition may contain a leveling agent, a crosslinking aid, a plasticizer, a softener, a filler, a coloring agent (pigment, dye, etc.), an antistatic agent, and an antiaging agent, as necessary. , ultraviolet absorbers, antioxidants, light stabilizers, and other additives commonly used in the adhesive field. Regarding such various additives, conventionally known ones can be used in a conventional manner, and since they do not particularly characterize the present invention, detailed explanations will be omitted.
粘着剤組成物には、上述した各成分以外に、必要に応じてレベリング剤、架橋助剤、可塑剤、軟化剤、充填剤、着色剤(顔料、染料等)、帯電防止剤、老化防止剤、紫外線吸収剤、酸化防止剤、光安定剤等の、粘着剤の分野において一般的な各種の添加剤が含まれていてもよい。このような各種添加剤については、従来公知のものを常法により使用することができ、特に本発明を特徴づけるものではないので、詳細な説明は省略する。 (Other additives)
In addition to the above-mentioned components, the adhesive composition may contain a leveling agent, a crosslinking aid, a plasticizer, a softener, a filler, a coloring agent (pigment, dye, etc.), an antistatic agent, and an antiaging agent, as necessary. , ultraviolet absorbers, antioxidants, light stabilizers, and other additives commonly used in the adhesive field. Regarding such various additives, conventionally known ones can be used in a conventional manner, and since they do not particularly characterize the present invention, detailed explanations will be omitted.
ここに開示される粘着剤層(粘着剤からなる層)は、水系粘着剤組成物、溶剤型粘着剤組成物、ホットメルト型粘着剤組成物、活性エネルギー線硬化型粘着剤組成物から形成された粘着剤層であり得る。水系粘着剤組成物とは、水を主成分とする溶媒(水系溶媒)中に粘着剤(粘着剤層形成成分)を含む形態の粘着剤組成物のことをいい、典型的には、水分散型粘着剤組成物(粘着剤の少なくとも一部が水に分散した形態の組成物)等と称されるものが含まれる。また、溶剤型粘着剤組成物とは、有機溶媒中に粘着剤を含む形態の粘着剤組成物のことをいう。溶剤型粘着剤組成物に含まれる有機溶媒としては、上述の溶液重合で用いられ得る有機溶媒(トルエンや酢酸エチル等)として例示した1種または2種以上を特に制限なく用いることができる。ここに開示される技術は、粘着特性等の観点から、溶剤型粘着剤組成物から形成された粘着剤層を備える態様で好ましく実施され得る。
The adhesive layer (layer consisting of an adhesive) disclosed herein is formed from a water-based adhesive composition, a solvent-based adhesive composition, a hot-melt adhesive composition, or an active energy ray-curable adhesive composition. It may be an adhesive layer. The aqueous adhesive composition refers to an adhesive composition containing an adhesive (adhesive layer forming component) in a water-based solvent (aqueous solvent), and is typically a water-based adhesive composition. This includes what is called a type adhesive composition (a composition in which at least a portion of an adhesive is dispersed in water). Moreover, a solvent-based adhesive composition refers to an adhesive composition containing an adhesive in an organic solvent. As the organic solvent contained in the solvent-based adhesive composition, one or more of the organic solvents (toluene, ethyl acetate, etc.) that can be used in the above-mentioned solution polymerization can be used without particular limitation. The technology disclosed herein can be preferably implemented in an embodiment including an adhesive layer formed from a solvent-based adhesive composition from the viewpoint of adhesive properties and the like.
ここに開示される粘着剤層は、従来公知の方法によって形成することができる。例えば、剥離性を有する表面(剥離面)または非剥離性の表面に粘着剤組成物を付与して乾燥させることにより粘着剤層を形成する方法を採用することができる。基材を有する構成の粘着シートでは、例えば、該基材に粘着剤組成物を直接付与(典型的には塗布)して乾燥させることにより粘着剤層を形成する方法(直接法)を採用することができる。また、剥離性を有する表面(剥離面)に粘着剤組成物を付与して乾燥させることにより該表面上に粘着剤層を形成し、その粘着剤層を基材に転写する方法(転写法)を採用してもよい。生産性の観点から、転写法が好ましい。上記剥離面としては、剥離ライナーの表面や、剥離処理された基材背面等を利用し得る。なお、ここに開示される粘着剤層は典型的には連続的に形成されるが、このような形態に限定されるものではなく、例えば点状、ストライプ状等の規則的あるいはランダムなパターンに形成された粘着剤層であってもよい。
The adhesive layer disclosed herein can be formed by a conventionally known method. For example, a method can be adopted in which a pressure-sensitive adhesive layer is formed by applying a pressure-sensitive adhesive composition to a surface having peelability (peelability surface) or a non-peelability surface and drying it. For an adhesive sheet having a base material, for example, a method (direct method) of forming an adhesive layer by directly applying (typically coating) an adhesive composition to the base material and drying is adopted. be able to. In addition, a method (transfer method) in which an adhesive composition is applied to a surface that has releasability (release surface) and dried to form an adhesive layer on the surface, and the adhesive layer is transferred to a base material. may be adopted. From the viewpoint of productivity, the transfer method is preferred. As the release surface, the surface of a release liner, the back surface of a release-treated base material, etc. can be used. Note that although the adhesive layer disclosed herein is typically formed continuously, it is not limited to this form, and may be formed, for example, in a regular or random pattern such as dots or stripes. It may also be a formed adhesive layer.
粘着剤組成物の塗布は、例えば、グラビアロールコーター、ダイコーター、バーコーター等の、従来公知のコーターを用いて行うことができる。あるいは、含浸やカーテンコート法等により粘着剤組成物を塗布してもよい。
架橋反応の促進、製造効率向上等の観点から、粘着剤組成物の乾燥は加熱下で行うことが好ましい。乾燥温度は、例えば40~150℃程度とすることができ、通常は60~130℃程度とすることが好ましい。粘着剤組成物を乾燥させた後、さらに、粘着剤層内における成分移行の調整、架橋反応の進行、粘着剤層内に存在し得る歪の緩和等を目的としてエージングを行ってもよい。 The adhesive composition can be applied using a conventionally known coater such as a gravure roll coater, die coater, or bar coater. Alternatively, the adhesive composition may be applied by impregnation, curtain coating, or the like.
From the viewpoint of promoting crosslinking reaction, improving production efficiency, etc., it is preferable to dry the adhesive composition under heating. The drying temperature can be, for example, about 40 to 150°C, and usually preferably about 60 to 130°C. After drying the pressure-sensitive adhesive composition, aging may be performed for the purpose of adjusting component migration within the pressure-sensitive adhesive layer, progressing the crosslinking reaction, alleviating distortion that may exist within the pressure-sensitive adhesive layer, and the like.
架橋反応の促進、製造効率向上等の観点から、粘着剤組成物の乾燥は加熱下で行うことが好ましい。乾燥温度は、例えば40~150℃程度とすることができ、通常は60~130℃程度とすることが好ましい。粘着剤組成物を乾燥させた後、さらに、粘着剤層内における成分移行の調整、架橋反応の進行、粘着剤層内に存在し得る歪の緩和等を目的としてエージングを行ってもよい。 The adhesive composition can be applied using a conventionally known coater such as a gravure roll coater, die coater, or bar coater. Alternatively, the adhesive composition may be applied by impregnation, curtain coating, or the like.
From the viewpoint of promoting crosslinking reaction, improving production efficiency, etc., it is preferable to dry the adhesive composition under heating. The drying temperature can be, for example, about 40 to 150°C, and usually preferably about 60 to 130°C. After drying the pressure-sensitive adhesive composition, aging may be performed for the purpose of adjusting component migration within the pressure-sensitive adhesive layer, progressing the crosslinking reaction, alleviating distortion that may exist within the pressure-sensitive adhesive layer, and the like.
(厚さ)
粘着剤層の厚さは特に制限されず、用途や使用目的等に応じて、例えば0.1~500μmの範囲で適当な厚さを有する粘着剤層を有する構成が採用され得る。いくつかの態様において、粘着シートが過度に厚くなることを避ける観点から、粘着剤層の厚さは、通常、凡そ100μm以下が適当であり、好ましくは凡そ70μm以下、より好ましくは凡そ60μm以下、さらに好ましくは凡そ50μm以下である。粘着剤層の厚さは凡そ35μm以下とすることができ、例えば凡そ30μm以下であってもよい。厚さの制限された粘着剤層は、薄厚化、軽量化の要請によく対応したものとなり得る。また一般に、粘着剤層の厚さが小さくなると、被着体に対する密着性は低下しやすい傾向にあるが、ここに開示される技術によると、制限された厚さの粘着剤層を有する構成で、高極性材料、低極性材料のいずれに対しても十分な接着力を実現することができる。粘着剤層の厚さの下限は、被着体に対する密着性の観点からは、いくつかの態様において、凡そ0.5μm以上が適当であり、凡そ1μm以上であってもよく、凡そ3μm以上とすることが有利であり、好ましくは凡そ10μm以上、より好ましくは凡そ12μm以上(例えば12μm超)、さらに好ましくは凡そ15μm以上であり、例えば凡そ18μm以上であってもよい。いくつかの好ましい態様において、粘着剤層の厚さは20μm超であり、24μm以上であってもよく、27μm以上でもよい。粘着剤層の厚さが大きいほど、目標とする接着力を実現しやすい傾向がある。ここに開示される粘着シートは、上記厚さの粘着剤層を基材の両面に有する粘着シートであり得る。また、基材の各面に第1粘着剤層と第2粘着剤層とをそれぞれ有する基材付き両面粘着シートにおいては、第1粘着剤層と第2粘着剤層とは同一の厚さであってもよく、相互に異なる厚さであってもよい。 (thickness)
The thickness of the adhesive layer is not particularly limited, and a configuration having an adhesive layer having an appropriate thickness in the range of, for example, 0.1 to 500 μm may be adopted depending on the use and purpose of use. In some embodiments, from the viewpoint of preventing the adhesive sheet from becoming excessively thick, the thickness of the adhesive layer is usually approximately 100 μm or less, preferably approximately 70 μm or less, more preferably approximately 60 μm or less, More preferably, it is approximately 50 μm or less. The thickness of the adhesive layer can be approximately 35 μm or less, and may be approximately 30 μm or less, for example. An adhesive layer with a limited thickness can meet the demands for thinning and weight reduction. Additionally, in general, as the thickness of the adhesive layer becomes smaller, the adhesion to the adherend tends to decrease, but according to the technology disclosed herein, it is possible to , it is possible to achieve sufficient adhesion to both high-polar and low-polar materials. In some embodiments, the lower limit of the thickness of the adhesive layer is suitably about 0.5 μm or more, from the viewpoint of adhesion to the adherend, it may be about 1 μm or more, and it may be about 3 μm or more. Advantageously, it is approximately 10 μm or more, more preferably approximately 12 μm or more (eg greater than 12 μm), still more preferably approximately 15 μm or more, and may for example be approximately 18 μm or more. In some preferred embodiments, the thickness of the adhesive layer is greater than 20 μm, may be greater than or equal to 24 μm, and may be greater than or equal to 27 μm. The thicker the adhesive layer is, the easier it is to achieve the target adhesive force. The pressure-sensitive adhesive sheet disclosed herein may be a pressure-sensitive adhesive sheet having pressure-sensitive adhesive layers having the above-mentioned thickness on both sides of a base material. In addition, in a double-sided adhesive sheet with a base material that has a first adhesive layer and a second adhesive layer on each side of the base material, the first adhesive layer and the second adhesive layer have the same thickness. They may have different thicknesses.
粘着剤層の厚さは特に制限されず、用途や使用目的等に応じて、例えば0.1~500μmの範囲で適当な厚さを有する粘着剤層を有する構成が採用され得る。いくつかの態様において、粘着シートが過度に厚くなることを避ける観点から、粘着剤層の厚さは、通常、凡そ100μm以下が適当であり、好ましくは凡そ70μm以下、より好ましくは凡そ60μm以下、さらに好ましくは凡そ50μm以下である。粘着剤層の厚さは凡そ35μm以下とすることができ、例えば凡そ30μm以下であってもよい。厚さの制限された粘着剤層は、薄厚化、軽量化の要請によく対応したものとなり得る。また一般に、粘着剤層の厚さが小さくなると、被着体に対する密着性は低下しやすい傾向にあるが、ここに開示される技術によると、制限された厚さの粘着剤層を有する構成で、高極性材料、低極性材料のいずれに対しても十分な接着力を実現することができる。粘着剤層の厚さの下限は、被着体に対する密着性の観点からは、いくつかの態様において、凡そ0.5μm以上が適当であり、凡そ1μm以上であってもよく、凡そ3μm以上とすることが有利であり、好ましくは凡そ10μm以上、より好ましくは凡そ12μm以上(例えば12μm超)、さらに好ましくは凡そ15μm以上であり、例えば凡そ18μm以上であってもよい。いくつかの好ましい態様において、粘着剤層の厚さは20μm超であり、24μm以上であってもよく、27μm以上でもよい。粘着剤層の厚さが大きいほど、目標とする接着力を実現しやすい傾向がある。ここに開示される粘着シートは、上記厚さの粘着剤層を基材の両面に有する粘着シートであり得る。また、基材の各面に第1粘着剤層と第2粘着剤層とをそれぞれ有する基材付き両面粘着シートにおいては、第1粘着剤層と第2粘着剤層とは同一の厚さであってもよく、相互に異なる厚さであってもよい。 (thickness)
The thickness of the adhesive layer is not particularly limited, and a configuration having an adhesive layer having an appropriate thickness in the range of, for example, 0.1 to 500 μm may be adopted depending on the use and purpose of use. In some embodiments, from the viewpoint of preventing the adhesive sheet from becoming excessively thick, the thickness of the adhesive layer is usually approximately 100 μm or less, preferably approximately 70 μm or less, more preferably approximately 60 μm or less, More preferably, it is approximately 50 μm or less. The thickness of the adhesive layer can be approximately 35 μm or less, and may be approximately 30 μm or less, for example. An adhesive layer with a limited thickness can meet the demands for thinning and weight reduction. Additionally, in general, as the thickness of the adhesive layer becomes smaller, the adhesion to the adherend tends to decrease, but according to the technology disclosed herein, it is possible to , it is possible to achieve sufficient adhesion to both high-polar and low-polar materials. In some embodiments, the lower limit of the thickness of the adhesive layer is suitably about 0.5 μm or more, from the viewpoint of adhesion to the adherend, it may be about 1 μm or more, and it may be about 3 μm or more. Advantageously, it is approximately 10 μm or more, more preferably approximately 12 μm or more (eg greater than 12 μm), still more preferably approximately 15 μm or more, and may for example be approximately 18 μm or more. In some preferred embodiments, the thickness of the adhesive layer is greater than 20 μm, may be greater than or equal to 24 μm, and may be greater than or equal to 27 μm. The thicker the adhesive layer is, the easier it is to achieve the target adhesive force. The pressure-sensitive adhesive sheet disclosed herein may be a pressure-sensitive adhesive sheet having pressure-sensitive adhesive layers having the above-mentioned thickness on both sides of a base material. In addition, in a double-sided adhesive sheet with a base material that has a first adhesive layer and a second adhesive layer on each side of the base material, the first adhesive layer and the second adhesive layer have the same thickness. They may have different thicknesses.
(ゲル分率)
ここに開示される粘着剤層のゲル分率は70%未満(重量基準)である。ゲル分率70%未満の粘着剤を用いることで、高極性材料だけでなく、低極性材料に対しても十分な接着力を発揮することができる。いくつかの態様において、粘着剤層のゲル分率は、65%未満が好ましく、60%未満であってもよく、55%未満でもよく、50%未満でもよく、45%未満でもよく、40%未満でもよく、35%未満でもよい。また、十分な保持力を得る観点から、いくつかの好ましい態様において、粘着剤層のゲル分率は20%以上であり、より好ましくは25%以上、さらに好ましくは30%以上であり、35%以上であってもよく、40%以上でもよく、45%以上でもよく、50%以上でもよく、55%以上でもよく、60%以上でもよい。アクリル系ポリマーのモノマー組成、Mw、粘着剤組成に応じて、粘着剤層のゲル分率を上記の範囲内で調節することにより、接着力と保持力とが好適に両立され得る。上記ゲル分率は、具体的には、後述の実施例に記載の方法で測定される。 (gel fraction)
The adhesive layer disclosed herein has a gel fraction of less than 70% (by weight). By using an adhesive with a gel fraction of less than 70%, it is possible to exhibit sufficient adhesive strength not only for highly polar materials but also for low polar materials. In some embodiments, the gel fraction of the adhesive layer is preferably less than 65%, may be less than 60%, may be less than 55%, may be less than 50%, may be less than 45%, may be less than 40%. It may be less than 35%. Further, from the viewpoint of obtaining sufficient holding power, in some preferred embodiments, the gel fraction of the adhesive layer is 20% or more, more preferably 25% or more, still more preferably 30% or more, and 35% or more. It may be more than 40%, it may be more than 45%, it may be more than 50%, it may be more than 55%, it may be more than 60%. By adjusting the gel fraction of the adhesive layer within the above range depending on the monomer composition of the acrylic polymer, Mw, and adhesive composition, both adhesive strength and holding power can be suitably achieved. Specifically, the gel fraction is measured by the method described in Examples below.
ここに開示される粘着剤層のゲル分率は70%未満(重量基準)である。ゲル分率70%未満の粘着剤を用いることで、高極性材料だけでなく、低極性材料に対しても十分な接着力を発揮することができる。いくつかの態様において、粘着剤層のゲル分率は、65%未満が好ましく、60%未満であってもよく、55%未満でもよく、50%未満でもよく、45%未満でもよく、40%未満でもよく、35%未満でもよい。また、十分な保持力を得る観点から、いくつかの好ましい態様において、粘着剤層のゲル分率は20%以上であり、より好ましくは25%以上、さらに好ましくは30%以上であり、35%以上であってもよく、40%以上でもよく、45%以上でもよく、50%以上でもよく、55%以上でもよく、60%以上でもよい。アクリル系ポリマーのモノマー組成、Mw、粘着剤組成に応じて、粘着剤層のゲル分率を上記の範囲内で調節することにより、接着力と保持力とが好適に両立され得る。上記ゲル分率は、具体的には、後述の実施例に記載の方法で測定される。 (gel fraction)
The adhesive layer disclosed herein has a gel fraction of less than 70% (by weight). By using an adhesive with a gel fraction of less than 70%, it is possible to exhibit sufficient adhesive strength not only for highly polar materials but also for low polar materials. In some embodiments, the gel fraction of the adhesive layer is preferably less than 65%, may be less than 60%, may be less than 55%, may be less than 50%, may be less than 45%, may be less than 40%. It may be less than 35%. Further, from the viewpoint of obtaining sufficient holding power, in some preferred embodiments, the gel fraction of the adhesive layer is 20% or more, more preferably 25% or more, still more preferably 30% or more, and 35% or more. It may be more than 40%, it may be more than 45%, it may be more than 50%, it may be more than 55%, it may be more than 60%. By adjusting the gel fraction of the adhesive layer within the above range depending on the monomer composition of the acrylic polymer, Mw, and adhesive composition, both adhesive strength and holding power can be suitably achieved. Specifically, the gel fraction is measured by the method described in Examples below.
(バイオマス炭素比)
いくつかの態様において、粘着剤層はバイオマス由来材料を含み、そのバイオマス炭素比が所定値以上であり得る。粘着剤層のバイオマス炭素比は、例えば1%以上であり、10%以上であってもよく、好ましくは30%以上、より好ましくは50%以上である。粘着剤のバイオマス炭素比が高いことは、石油等に代表される化石資源系材料の使用量が少ないことを意味する。かかる観点において、粘着剤のバイオマス炭素比は高いほど好ましい。例えば、粘着剤層のバイオマス炭素比は、55%以上であってよく、60%以上であってもよく、70%以上でもよく、75%以上でもよく、80%以上でもよく、80%超でもよい。バイオマス炭素比の上限は、定義上100%であり、99%以下であってもよく、材料の入手容易性の観点から、95%以下でもよく、90%以下でもよい。良好な粘着性能を発揮しやすくする観点から、いくつかの態様において、粘着剤層のバイオマス炭素比は、例えば90%以下であってよく、85%以下でもよく、80%以下でもよい。 (Biomass carbon ratio)
In some embodiments, the adhesive layer may include a biomass-derived material, and the biomass carbon ratio may be greater than or equal to a predetermined value. The biomass carbon ratio of the adhesive layer is, for example, 1% or more, and may be 10% or more, preferably 30% or more, and more preferably 50% or more. A high biomass carbon ratio in the adhesive means that less fossil resource-based materials, such as petroleum, are used. From this point of view, the higher the biomass carbon ratio of the adhesive, the more preferable. For example, the biomass carbon ratio of the adhesive layer may be 55% or more, 60% or more, 70% or more, 75% or more, 80% or more, or more than 80%. good. The upper limit of the biomass carbon ratio is 100% by definition, and may be 99% or less, and from the viewpoint of material availability, it may be 95% or less, or 90% or less. In some embodiments, from the viewpoint of facilitating good adhesive performance, the biomass carbon ratio of the adhesive layer may be, for example, 90% or less, 85% or less, or 80% or less.
いくつかの態様において、粘着剤層はバイオマス由来材料を含み、そのバイオマス炭素比が所定値以上であり得る。粘着剤層のバイオマス炭素比は、例えば1%以上であり、10%以上であってもよく、好ましくは30%以上、より好ましくは50%以上である。粘着剤のバイオマス炭素比が高いことは、石油等に代表される化石資源系材料の使用量が少ないことを意味する。かかる観点において、粘着剤のバイオマス炭素比は高いほど好ましい。例えば、粘着剤層のバイオマス炭素比は、55%以上であってよく、60%以上であってもよく、70%以上でもよく、75%以上でもよく、80%以上でもよく、80%超でもよい。バイオマス炭素比の上限は、定義上100%であり、99%以下であってもよく、材料の入手容易性の観点から、95%以下でもよく、90%以下でもよい。良好な粘着性能を発揮しやすくする観点から、いくつかの態様において、粘着剤層のバイオマス炭素比は、例えば90%以下であってよく、85%以下でもよく、80%以下でもよい。 (Biomass carbon ratio)
In some embodiments, the adhesive layer may include a biomass-derived material, and the biomass carbon ratio may be greater than or equal to a predetermined value. The biomass carbon ratio of the adhesive layer is, for example, 1% or more, and may be 10% or more, preferably 30% or more, and more preferably 50% or more. A high biomass carbon ratio in the adhesive means that less fossil resource-based materials, such as petroleum, are used. From this point of view, the higher the biomass carbon ratio of the adhesive, the more preferable. For example, the biomass carbon ratio of the adhesive layer may be 55% or more, 60% or more, 70% or more, 75% or more, 80% or more, or more than 80%. good. The upper limit of the biomass carbon ratio is 100% by definition, and may be 99% or less, and from the viewpoint of material availability, it may be 95% or less, or 90% or less. In some embodiments, from the viewpoint of facilitating good adhesive performance, the biomass carbon ratio of the adhesive layer may be, for example, 90% or less, 85% or less, or 80% or less.
<基材>
ここに開示される粘着シートが片面粘着タイプまたは両面粘着タイプの基材付き粘着シートの形態である態様において、粘着剤層を支持(裏打ち)する基材としては、樹脂フィルム、紙、布、ゴムシート、発泡体シート、金属箔、これらの複合体等を用いることができる。樹脂フィルムの例としては、ポリエチレン(PE)、ポリプロピレン(PP)、エチレン・プロピレン共重合体等のポリオレフィン製フィルム;ポリエチレンテレフタレート(PET)等のポリエステルフィルム;塩化ビニル樹脂フィルム;酢酸ビニル樹脂フィルム;ポリイミド樹脂フィルム;ポリアミド樹脂フィルム;フッ素樹脂フィルム;セロハン等が挙げられる。紙の例としては、和紙、クラフト紙、グラシン紙、上質紙、合成紙、トップコート紙等が挙げられる。布の例としては、各種繊維状物質の単独または混紡等による織布や不織布等が挙げられる。上記繊維状物質としては、綿、スフ、マニラ麻、パルプ、レーヨン、アセテート繊維、ポリエステル繊維、ポリビニルアルコール繊維、ポリアミド繊維、ポリオレフィン繊維等が例示される。ゴムシートの例としては、天然ゴムシート、ブチルゴムシート等が挙げられる。発泡体シートの例としては、発泡ポリオレフィンシート、発泡ポリウレタンシート、発泡ポリクロロプレンゴムシート等が挙げられる。金属箔の例としては、アルミニウム箔、銅箔等が挙げられる。なお、粘着剤層を支持する基材は、粘着シートにおいて基材層ともいう。 <Base material>
In an embodiment in which the adhesive sheet disclosed herein is in the form of a single-sided adhesive type or double-sided adhesive type adhesive sheet with a base material, the base material that supports (backs) the adhesive layer may be a resin film, paper, cloth, or rubber. Sheets, foam sheets, metal foils, composites thereof, etc. can be used. Examples of resin films include polyolefin films such as polyethylene (PE), polypropylene (PP), and ethylene-propylene copolymers; polyester films such as polyethylene terephthalate (PET); vinyl chloride resin films; vinyl acetate resin films; polyimide Examples include resin film; polyamide resin film; fluororesin film; cellophane, and the like. Examples of paper include Japanese paper, kraft paper, glassine paper, high quality paper, synthetic paper, top coated paper, and the like. Examples of the fabric include woven fabrics and nonwoven fabrics made of various fibrous substances alone or in combination. Examples of the above-mentioned fibrous materials include cotton, staple fiber, Manila hemp, pulp, rayon, acetate fiber, polyester fiber, polyvinyl alcohol fiber, polyamide fiber, and polyolefin fiber. Examples of rubber sheets include natural rubber sheets, butyl rubber sheets, and the like. Examples of foam sheets include foamed polyolefin sheets, foamed polyurethane sheets, foamed polychloroprene rubber sheets, and the like. Examples of metal foil include aluminum foil, copper foil, and the like. Note that the base material that supports the adhesive layer is also referred to as a base material layer in the adhesive sheet.
ここに開示される粘着シートが片面粘着タイプまたは両面粘着タイプの基材付き粘着シートの形態である態様において、粘着剤層を支持(裏打ち)する基材としては、樹脂フィルム、紙、布、ゴムシート、発泡体シート、金属箔、これらの複合体等を用いることができる。樹脂フィルムの例としては、ポリエチレン(PE)、ポリプロピレン(PP)、エチレン・プロピレン共重合体等のポリオレフィン製フィルム;ポリエチレンテレフタレート(PET)等のポリエステルフィルム;塩化ビニル樹脂フィルム;酢酸ビニル樹脂フィルム;ポリイミド樹脂フィルム;ポリアミド樹脂フィルム;フッ素樹脂フィルム;セロハン等が挙げられる。紙の例としては、和紙、クラフト紙、グラシン紙、上質紙、合成紙、トップコート紙等が挙げられる。布の例としては、各種繊維状物質の単独または混紡等による織布や不織布等が挙げられる。上記繊維状物質としては、綿、スフ、マニラ麻、パルプ、レーヨン、アセテート繊維、ポリエステル繊維、ポリビニルアルコール繊維、ポリアミド繊維、ポリオレフィン繊維等が例示される。ゴムシートの例としては、天然ゴムシート、ブチルゴムシート等が挙げられる。発泡体シートの例としては、発泡ポリオレフィンシート、発泡ポリウレタンシート、発泡ポリクロロプレンゴムシート等が挙げられる。金属箔の例としては、アルミニウム箔、銅箔等が挙げられる。なお、粘着剤層を支持する基材は、粘着シートにおいて基材層ともいう。 <Base material>
In an embodiment in which the adhesive sheet disclosed herein is in the form of a single-sided adhesive type or double-sided adhesive type adhesive sheet with a base material, the base material that supports (backs) the adhesive layer may be a resin film, paper, cloth, or rubber. Sheets, foam sheets, metal foils, composites thereof, etc. can be used. Examples of resin films include polyolefin films such as polyethylene (PE), polypropylene (PP), and ethylene-propylene copolymers; polyester films such as polyethylene terephthalate (PET); vinyl chloride resin films; vinyl acetate resin films; polyimide Examples include resin film; polyamide resin film; fluororesin film; cellophane, and the like. Examples of paper include Japanese paper, kraft paper, glassine paper, high quality paper, synthetic paper, top coated paper, and the like. Examples of the fabric include woven fabrics and nonwoven fabrics made of various fibrous substances alone or in combination. Examples of the above-mentioned fibrous materials include cotton, staple fiber, Manila hemp, pulp, rayon, acetate fiber, polyester fiber, polyvinyl alcohol fiber, polyamide fiber, and polyolefin fiber. Examples of rubber sheets include natural rubber sheets, butyl rubber sheets, and the like. Examples of foam sheets include foamed polyolefin sheets, foamed polyurethane sheets, foamed polychloroprene rubber sheets, and the like. Examples of metal foil include aluminum foil, copper foil, and the like. Note that the base material that supports the adhesive layer is also referred to as a base material layer in the adhesive sheet.
基材は、バイオマス由来の材料から形成されたものであってもよく、非バイオマス由来の材料から形成されたものであってもよい。化石資源系材料への依存抑制に配慮した粘着シート作製の観点から、バイオマス由来の基材材料(典型的には樹脂フィルム)が好ましく使用される。
The base material may be formed from a biomass-derived material or a non-biomass-derived material. From the viewpoint of producing a pressure-sensitive adhesive sheet in consideration of reducing dependence on fossil resource-based materials, biomass-derived base materials (typically resin films) are preferably used.
また、基材は、リサイクル可能な材料やリサイクルされた材料(リサイクル材料ともいう。)を用いて形成されたものであってもよい。かかるリサイクル材料としては、樹脂フィルムが好ましく用いられる。樹脂フィルム(例えばPETフィルム等のポリエステルフィルム)はリサイクルが可能であるので、植物由来の材料を用いているか否かにかかわらず、使用後の樹脂フィルムを再利用することで、持続的な再生産が可能であり、環境負荷を低減することができる。このような、リサイクル可能な樹脂フィルムや、リサイクルされた樹脂フィルムは、リサイクルフィルムともいう。上記リサイクル材料(例えばリサイクルフィルム)は、バイオマス由来の材料から形成されたものであってもよく、非バイオマス由来の材料から形成されたものであってもよい。
Furthermore, the base material may be formed using a recyclable material or a recycled material (also referred to as recycled material). As such a recycled material, a resin film is preferably used. Resin films (for example, polyester films such as PET films) can be recycled, so whether or not they are made from plant-based materials, reusing the used resin film allows for sustainable reproduction. It is possible to reduce the environmental burden. Such a recyclable resin film or a recycled resin film is also referred to as a recycled film. The recycled material (for example, recycled film) may be formed from a biomass-derived material or a non-biomass-derived material.
基材付き粘着シートを構成する基材としては、ベースフィルムとして樹脂フィルムを含むものを好ましく用いることができる。上記ベースフィルムは、典型的には、独立して形状維持可能な(非依存性の)部材である。ここに開示される技術における基材は、このようなベースフィルムから実質的に構成されたものであり得る。あるいは、上記基材は、上記ベースフィルムの他に、補助的な層を含むものであってもよい。上記補助的な層の例としては、上記ベースフィルムの表面に設けられた着色層、反射層、下塗り層、帯電防止層等が挙げられる。
As the base material constituting the base material-attached pressure-sensitive adhesive sheet, one containing a resin film as a base film can be preferably used. The base film is typically an independently shape-maintainable (independent) member. The base material in the technology disclosed herein may be substantially composed of such a base film. Alternatively, the base material may include an auxiliary layer in addition to the base film. Examples of the auxiliary layer include a colored layer, a reflective layer, an undercoat layer, an antistatic layer, etc. provided on the surface of the base film.
上記樹脂フィルムは、樹脂材料を主成分(例えば、当該樹脂フィルム中に50重量%を超えて含まれる成分)とするフィルムである。樹脂フィルムの例としては、ポリエチレン(PE)、ポリプロピレン(PP)、エチレン・プロピレン共重合体等のポリオレフィン系樹脂フィルム;ポリエチレンテレフタレート(PET)、ポリブチレンテレフタレート(PBT)、ポリエチレンナフタレート(PEN)等のポリエステル系樹脂フィルム;塩化ビニル系樹脂フィルム;酢酸ビニル系樹脂フィルム;ポリイミド系樹脂フィルム;ポリアミド系樹脂フィルム;フッ素樹脂フィルム;セロハン;等が挙げられる。樹脂フィルムは、天然ゴムフィルム、ブチルゴムフィルム等のゴム系フィルムであってもよい。なかでも、ハンドリング性、加工性の観点から、ポリエステルフィルムが好ましく、そのなかでもPETフィルムが特に好ましい。
The resin film is a film whose main component is a resin material (for example, a component contained in the resin film in an amount exceeding 50% by weight). Examples of resin films include polyolefin resin films such as polyethylene (PE), polypropylene (PP), and ethylene-propylene copolymers; polyethylene terephthalate (PET), polybutylene terephthalate (PBT), polyethylene naphthalate (PEN), etc. Examples include polyester resin film; vinyl chloride resin film; vinyl acetate resin film; polyimide resin film; polyamide resin film; fluororesin film; cellophane; and the like. The resin film may be a rubber film such as a natural rubber film or a butyl rubber film. Among these, polyester films are preferred from the viewpoint of handling and processability, and among these, PET films are particularly preferred.
なお、本明細書において「樹脂フィルム」とは、典型的には非多孔質のシートであって、いわゆる不織布や織布とは区別される概念(換言すると、不織布や織布を除く概念)である。上記樹脂フィルムは、無延伸フィルム、一軸延伸フィルム、二軸延伸フィルムのいずれであってもよい。また、そのような樹脂フィルムは非発泡であり得る。ここで非発泡の樹脂フィルムとは、発泡体とするための意図的な処理を行っていない樹脂フィルムのことを指す。非発泡の樹脂フィルムは、具体的には、発泡倍率が1.1倍未満(例えば1.05倍未満、典型的には1.01倍未満)の樹脂フィルムであり得る。
In addition, in this specification, "resin film" is typically a non-porous sheet, and is a concept that is distinguished from so-called non-woven fabrics and woven fabrics (in other words, a concept excluding non-woven fabrics and woven fabrics). be. The resin film may be an unstretched film, a uniaxially stretched film, or a biaxially stretched film. Also, such resin films may be non-foamed. Here, the term "non-foamed resin film" refers to a resin film that has not been intentionally processed to form a foam. Specifically, the non-foamed resin film may be a resin film with an expansion ratio of less than 1.1 times (for example, less than 1.05 times, typically less than 1.01 times).
上記基材(例えば樹脂フィルム)には、必要に応じて、充填剤(無機充填剤、有機充填剤等)、着色剤、分散剤(界面活性剤等)、老化防止剤、酸化防止剤、紫外線吸収剤、帯電防止剤、滑剤、可塑剤等の各種添加剤が配合されていてもよい。各種添加剤の配合割合は、30重量%未満(例えば20重量%未満、典型的には10重量%未満)程度である。
The above base material (e.g. resin film) may contain fillers (inorganic fillers, organic fillers, etc.), colorants, dispersants (surfactants, etc.), anti-aging agents, antioxidants, ultraviolet rays, etc., as necessary. Various additives such as an absorbent, an antistatic agent, a lubricant, and a plasticizer may be blended. The blending ratio of various additives is about less than 30% by weight (for example, less than 20% by weight, typically less than 10% by weight).
上記基材(例えば樹脂フィルム)は、単層構造であってもよく、2層、3層またはそれ以上の多層構造を有するものであってもよい。形状安定性の観点から、基材は単層構造であることが好ましい。多層構造の場合、少なくとも一つの層(好ましくは全ての層)は上記樹脂(例えばポリエステル系樹脂)の連続構造を有する層であることが好ましい。基材(典型的には樹脂フィルム)の製造方法は、従来公知の方法を適宜採用すればよく、特に限定されない。例えば、押出成形、インフレーション成形、Tダイキャスト成形、カレンダーロール成形等の従来公知の一般的なフィルム成形方法を適宜採用することができる。
The base material (for example, a resin film) may have a single layer structure, or may have a multilayer structure of two layers, three layers, or more. From the viewpoint of shape stability, the base material preferably has a single-layer structure. In the case of a multilayer structure, at least one layer (preferably all layers) is preferably a layer having a continuous structure of the above resin (for example, polyester resin). The method for manufacturing the base material (typically a resin film) is not particularly limited, and any conventionally known method may be appropriately adopted. For example, conventionally known general film forming methods such as extrusion molding, inflation molding, T-die casting molding, and calender roll molding can be appropriately employed.
基材の表面には、コロナ放電処理、プラズマ処理、紫外線照射処理、酸処理、アルカリ処理、下塗り剤の塗布等の、従来公知の表面処理が施されていてもよい。このような表面処理は、基材と粘着剤層との密着性、言い換えると粘着剤層の基材への投錨性を向上させるための処理であり得る。
The surface of the base material may be subjected to conventionally known surface treatments such as corona discharge treatment, plasma treatment, ultraviolet irradiation treatment, acid treatment, alkali treatment, and application of an undercoat. Such surface treatment may be a treatment for improving the adhesion between the base material and the adhesive layer, in other words, the ability of the adhesive layer to anchor to the base material.
また、ここに開示される技術が、基材付き片面粘着シートの形態で実施される場合、基材の背面に、必要に応じて剥離処理が施されていてもよい。剥離処理は、例えば、一般的なシリコーン系、長鎖アルキル系、フッ素系等の剥離処理剤を、典型的には0.01μm~1μm(例えば0.01μm~0.1μm)程度の薄膜状に付与する処理であり得る。かかる剥離処理を施すことにより、粘着シートをロール状に巻回した巻回体の巻き戻しを容易にする等の効果が得られる。
Furthermore, when the technology disclosed herein is implemented in the form of a single-sided pressure-sensitive adhesive sheet with a base material, the back surface of the base material may be subjected to a peeling treatment as necessary. For example, the peeling treatment is performed by applying a general silicone-based, long-chain alkyl-based, or fluorine-based peeling agent to a thin film, typically about 0.01 μm to 1 μm (for example, 0.01 μm to 0.1 μm). It can be a process to add. By performing such a peeling treatment, effects such as facilitating the unwinding of a roll-shaped adhesive sheet can be obtained.
基材を含む態様の粘着シートにおいて、該基材の厚さは特に限定されない。粘着シートが過度に厚くなることを避ける観点から、基材の厚さは、例えば凡そ200μm以下、好ましくは凡そ150μm以下、より好ましくは凡そ100μm以下とすることができる。粘着シートの使用目的や使用態様に応じて、基材の厚さは、凡そ70μm以下であってよく、凡そ50μm以下でもよく、凡そ30μm以下(例えば凡そ25μm以下)でもよい。いくつかの態様において、基材フィルム層の厚さは、凡そ20μm以下であってよく、凡そ15μm以下でもよく、凡そ10μm以下(例えば凡そ5μm以下)でもよい。基材の厚さを小さくすることにより、粘着シートの総厚さが同じであっても粘着剤層の厚さをより大きくすることができる。被着体や基材との密着性向上の観点から有利となり得る。基材の下限は特に制限されない。粘着シートの取扱い性(ハンドリング性)や加工性等の観点から、基材の厚さは、通常は凡そ0.5μm以上(例えば1μm以上)、好ましくは凡そ2μm以上、例えば凡そ6μm以上である。いくつかの態様において、基材の厚さは、凡そ15μm以上とすることができ、凡そ25μm以上でもよい。
In the pressure-sensitive adhesive sheet including a base material, the thickness of the base material is not particularly limited. From the viewpoint of preventing the pressure-sensitive adhesive sheet from becoming too thick, the thickness of the base material can be, for example, approximately 200 μm or less, preferably approximately 150 μm or less, and more preferably approximately 100 μm or less. The thickness of the base material may be approximately 70 μm or less, approximately 50 μm or less, or approximately 30 μm or less (for example, approximately 25 μm or less) depending on the purpose and manner of use of the adhesive sheet. In some embodiments, the thickness of the base film layer may be about 20 μm or less, about 15 μm or less, about 10 μm or less (eg, about 5 μm or less). By reducing the thickness of the base material, the thickness of the adhesive layer can be increased even if the total thickness of the adhesive sheet is the same. This can be advantageous from the viewpoint of improving adhesion to adherends and base materials. The lower limit of the base material is not particularly limited. From the viewpoint of handleability and processability of the pressure-sensitive adhesive sheet, the thickness of the base material is usually about 0.5 μm or more (for example, 1 μm or more), preferably about 2 μm or more, for example about 6 μm or more. In some embodiments, the thickness of the substrate can be about 15 μm or more, and can be about 25 μm or more.
<剥離ライナー>
ここに開示される技術において、粘着剤層の形成、粘着シートの作製、使用前の粘着シートの保存、流通、形状加工等の際に、剥離ライナーを用いることができる。剥離ライナーとしては、特に限定されず、例えば、樹脂フィルムや紙等のライナー基材の表面に剥離処理層を有する剥離ライナーや、フッ素系ポリマー(ポリテトラフルオロエチレン等)からなる剥離ライナー等を用いることができる。上記剥離処理層は、例えば、シリコーン系、長鎖アルキル系、フッ素系、硫化モリブデン等の剥離処理剤により上記ライナー基材を表面処理して形成されたものであり得る。ライナー基材としては、前述の粘着シートの基材と同様、バイオマス由来の材料を用いて形成されたものや、リサイクル材料(リサイクルフィルム等)が好ましく用いられ得る。 <Release liner>
In the technology disclosed herein, a release liner can be used during formation of the adhesive layer, production of the adhesive sheet, storage of the adhesive sheet before use, distribution, shape processing, etc. The release liner is not particularly limited, and for example, a release liner having a release treatment layer on the surface of a liner base material such as a resin film or paper, a release liner made of a fluorine-based polymer (polytetrafluoroethylene, etc.), etc. may be used. be able to. The release treatment layer may be formed by surface-treating the liner base material with a release agent such as a silicone-based, long-chain alkyl-based, fluorine-based, or molybdenum sulfide release agent. As the liner base material, like the base material of the above-mentioned adhesive sheet, one formed using a biomass-derived material or a recycled material (recycled film, etc.) can be preferably used.
ここに開示される技術において、粘着剤層の形成、粘着シートの作製、使用前の粘着シートの保存、流通、形状加工等の際に、剥離ライナーを用いることができる。剥離ライナーとしては、特に限定されず、例えば、樹脂フィルムや紙等のライナー基材の表面に剥離処理層を有する剥離ライナーや、フッ素系ポリマー(ポリテトラフルオロエチレン等)からなる剥離ライナー等を用いることができる。上記剥離処理層は、例えば、シリコーン系、長鎖アルキル系、フッ素系、硫化モリブデン等の剥離処理剤により上記ライナー基材を表面処理して形成されたものであり得る。ライナー基材としては、前述の粘着シートの基材と同様、バイオマス由来の材料を用いて形成されたものや、リサイクル材料(リサイクルフィルム等)が好ましく用いられ得る。 <Release liner>
In the technology disclosed herein, a release liner can be used during formation of the adhesive layer, production of the adhesive sheet, storage of the adhesive sheet before use, distribution, shape processing, etc. The release liner is not particularly limited, and for example, a release liner having a release treatment layer on the surface of a liner base material such as a resin film or paper, a release liner made of a fluorine-based polymer (polytetrafluoroethylene, etc.), etc. may be used. be able to. The release treatment layer may be formed by surface-treating the liner base material with a release agent such as a silicone-based, long-chain alkyl-based, fluorine-based, or molybdenum sulfide release agent. As the liner base material, like the base material of the above-mentioned adhesive sheet, one formed using a biomass-derived material or a recycled material (recycled film, etc.) can be preferably used.
<粘着シートの総厚>
ここに開示される粘着シート(粘着剤層を含み、基材層をさらに含み得るが、剥離ライナーは含まない。)の総厚さは特に限定されない。粘着シートの総厚さは、例えば凡そ1mm以下であり、凡そ500μm以下であってもよく、凡そ300μm以下とすることができ、薄型化の観点から、凡そ200μm以下が適当であり、凡そ150μm以下(例えば凡そ100μm以下)であってもよい。いくつかの好ましい態様では、粘着シートの厚さは凡そ50μm以下とすることができ、例えば凡そ35μm以下であってもよい。粘着シートの厚さの下限は、例えば0.1μm以上(例えば0.5μm以上)であり、凡そ3μm以上とすることが適当であり、好ましくは凡そ10μm以上、より好ましくは凡そ15μm以上であり、凡そ50μm以上であってもよく、凡そ100μm以上でもよい。所定値以上の厚さを有する粘着シートは、被着体への密着性が得られやすく、また、取扱い性にも優れる傾向がある。なお、基材レスの粘着シートでは、粘着剤層の厚さが粘着シートの総厚さとなる。 <Total thickness of adhesive sheet>
The total thickness of the adhesive sheet disclosed herein (which includes an adhesive layer and may further include a base layer, but does not include a release liner) is not particularly limited. The total thickness of the adhesive sheet is, for example, approximately 1 mm or less, may be approximately 500 μm or less, and may be approximately 300 μm or less, and from the viewpoint of thinning, approximately 200 μm or less is appropriate, and approximately 150 μm or less. (For example, approximately 100 μm or less). In some preferred embodiments, the thickness of the pressure-sensitive adhesive sheet can be approximately 50 μm or less, for example, approximately 35 μm or less. The lower limit of the thickness of the adhesive sheet is, for example, 0.1 μm or more (for example, 0.5 μm or more), suitably about 3 μm or more, preferably about 10 μm or more, more preferably about 15 μm or more, It may be about 50 μm or more, or about 100 μm or more. A pressure-sensitive adhesive sheet having a thickness of a predetermined value or more tends to have good adhesion to an adherend and also tends to have excellent handling properties. In addition, in the adhesive sheet without a base material, the thickness of the adhesive layer becomes the total thickness of the adhesive sheet.
ここに開示される粘着シート(粘着剤層を含み、基材層をさらに含み得るが、剥離ライナーは含まない。)の総厚さは特に限定されない。粘着シートの総厚さは、例えば凡そ1mm以下であり、凡そ500μm以下であってもよく、凡そ300μm以下とすることができ、薄型化の観点から、凡そ200μm以下が適当であり、凡そ150μm以下(例えば凡そ100μm以下)であってもよい。いくつかの好ましい態様では、粘着シートの厚さは凡そ50μm以下とすることができ、例えば凡そ35μm以下であってもよい。粘着シートの厚さの下限は、例えば0.1μm以上(例えば0.5μm以上)であり、凡そ3μm以上とすることが適当であり、好ましくは凡そ10μm以上、より好ましくは凡そ15μm以上であり、凡そ50μm以上であってもよく、凡そ100μm以上でもよい。所定値以上の厚さを有する粘着シートは、被着体への密着性が得られやすく、また、取扱い性にも優れる傾向がある。なお、基材レスの粘着シートでは、粘着剤層の厚さが粘着シートの総厚さとなる。 <Total thickness of adhesive sheet>
The total thickness of the adhesive sheet disclosed herein (which includes an adhesive layer and may further include a base layer, but does not include a release liner) is not particularly limited. The total thickness of the adhesive sheet is, for example, approximately 1 mm or less, may be approximately 500 μm or less, and may be approximately 300 μm or less, and from the viewpoint of thinning, approximately 200 μm or less is appropriate, and approximately 150 μm or less. (For example, approximately 100 μm or less). In some preferred embodiments, the thickness of the pressure-sensitive adhesive sheet can be approximately 50 μm or less, for example, approximately 35 μm or less. The lower limit of the thickness of the adhesive sheet is, for example, 0.1 μm or more (for example, 0.5 μm or more), suitably about 3 μm or more, preferably about 10 μm or more, more preferably about 15 μm or more, It may be about 50 μm or more, or about 100 μm or more. A pressure-sensitive adhesive sheet having a thickness of a predetermined value or more tends to have good adhesion to an adherend and also tends to have excellent handling properties. In addition, in the adhesive sheet without a base material, the thickness of the adhesive layer becomes the total thickness of the adhesive sheet.
<粘着シートの特性>
いくつかの態様において、粘着シートは、ステンレス鋼板に対する180度剥離強度(対SUS粘着力)が凡そ15N/25mm以上(例えば17N/25mm以上)であることが好ましい。このような対SUS粘着力を示す粘着シートは、高極性材料に対して優れた接着力を発揮し得る。上記対SUS粘着力は、より好ましくは凡そ20N/25mm以上、さらに好ましくは凡そ25N/25mm以上、特に好ましくは30N/25mm以上であり、32N/25mm以上であってもよい。上記対SUS粘着力の上限は特に制限されないが、保持力等の他の粘着特性との両立の観点から、通常は例えば凡そ50N/25mm以下であってもよい。上記対SUS粘着力は、被着体としてSUS板を用いて、23℃、50%RHの測定環境下において、引張速度300mm/分、剥離角度180度の条件で測定される。より具体的には、後述の実施例に記載の方法で測定される。 <Characteristics of adhesive sheet>
In some embodiments, the adhesive sheet preferably has a 180 degree peel strength against a stainless steel plate (adhesive strength against SUS) of approximately 15 N/25 mm or more (for example, 17 N/25 mm or more). A pressure-sensitive adhesive sheet exhibiting such adhesive strength to SUS can exhibit excellent adhesive strength to highly polar materials. The adhesive force to SUS is more preferably about 20 N/25 mm or more, still more preferably about 25 N/25 mm or more, particularly preferably 30 N/25 mm or more, and may be 32 N/25 mm or more. The upper limit of the adhesive strength to SUS is not particularly limited, but from the viewpoint of coexistence with other adhesive properties such as holding power, it may usually be about 50 N/25 mm or less, for example. The adhesive strength to SUS is measured using an SUS plate as an adherend under the conditions of a tensile speed of 300 mm/min and a peel angle of 180 degrees in a measurement environment of 23° C. and 50% RH. More specifically, it is measured by the method described in Examples below.
いくつかの態様において、粘着シートは、ステンレス鋼板に対する180度剥離強度(対SUS粘着力)が凡そ15N/25mm以上(例えば17N/25mm以上)であることが好ましい。このような対SUS粘着力を示す粘着シートは、高極性材料に対して優れた接着力を発揮し得る。上記対SUS粘着力は、より好ましくは凡そ20N/25mm以上、さらに好ましくは凡そ25N/25mm以上、特に好ましくは30N/25mm以上であり、32N/25mm以上であってもよい。上記対SUS粘着力の上限は特に制限されないが、保持力等の他の粘着特性との両立の観点から、通常は例えば凡そ50N/25mm以下であってもよい。上記対SUS粘着力は、被着体としてSUS板を用いて、23℃、50%RHの測定環境下において、引張速度300mm/分、剥離角度180度の条件で測定される。より具体的には、後述の実施例に記載の方法で測定される。 <Characteristics of adhesive sheet>
In some embodiments, the adhesive sheet preferably has a 180 degree peel strength against a stainless steel plate (adhesive strength against SUS) of approximately 15 N/25 mm or more (for example, 17 N/25 mm or more). A pressure-sensitive adhesive sheet exhibiting such adhesive strength to SUS can exhibit excellent adhesive strength to highly polar materials. The adhesive force to SUS is more preferably about 20 N/25 mm or more, still more preferably about 25 N/25 mm or more, particularly preferably 30 N/25 mm or more, and may be 32 N/25 mm or more. The upper limit of the adhesive strength to SUS is not particularly limited, but from the viewpoint of coexistence with other adhesive properties such as holding power, it may usually be about 50 N/25 mm or less, for example. The adhesive strength to SUS is measured using an SUS plate as an adherend under the conditions of a tensile speed of 300 mm/min and a peel angle of 180 degrees in a measurement environment of 23° C. and 50% RH. More specifically, it is measured by the method described in Examples below.
ここに開示される粘着シートは、ポリプロピレンに対する180度剥離強度(対PP粘着力)が凡そ10N/25mm以上であることが好ましい。このような対PP粘着力を示す粘着シートは、低極性材料によく接着し、上記被着体に対して高い接着信頼性を発揮し得る。上記対PP粘着力は、より好ましくは凡そ12N/25mm以上、さらに好ましくは凡そ14N/25mm以上、特に好ましくは凡そ16N/25mm以上であり、凡そ18N/25mm以上(例えば凡そ20N/25mm以上)であってもよい。上記対PP粘着力の上限は特に制限されないが、保持力等の他の粘着特性との両立の観点から、通常は例えば凡そ40N/25mm以下であり、30N/25mm以下であってもよい。上記対PP粘着力は、被着体としてPPを用いて、23℃、50%RHの測定環境下において、引張速度300mm/分、剥離角度180度の条件で測定される。より具体的には、後述の実施例に記載の方法で測定される。
The adhesive sheet disclosed herein preferably has a 180 degree peel strength against polypropylene (adhesive strength against PP) of about 10 N/25 mm or more. A pressure-sensitive adhesive sheet exhibiting such adhesion to PP can adhere well to low polarity materials and exhibit high adhesion reliability to the above-mentioned adherends. The adhesive force to PP is more preferably about 12 N/25 mm or more, still more preferably about 14 N/25 mm or more, particularly preferably about 16 N/25 mm or more, and about 18 N/25 mm or more (for example, about 20 N/25 mm or more). There may be. The upper limit of the adhesive force to PP is not particularly limited, but from the viewpoint of coexistence with other adhesive properties such as holding power, it is usually about 40 N/25 mm or less, and may be 30 N/25 mm or less. The adhesive strength to PP is measured using PP as an adherend in a measurement environment of 23° C. and 50% RH at a tensile rate of 300 mm/min and a peel angle of 180 degrees. More specifically, it is measured by the method described in Examples below.
ここに開示される粘着シートは、ポリエチレンに対する180度剥離強度(対PE粘着力)が凡そ5N/25mm以上であることが好ましい。このような対PE粘着力を示す粘着シートは、低極性材料に対して十分な接着信頼性を発揮し得る。上記対PE粘着力は、より好ましくは凡そ7N/25mm以上、さらに好ましくは凡そ10N/25mm以上、特に好ましくは凡そ12N/25mm以上であり、14N/25mm以上(例えば凡そ15N/25mm以上)であってもよい。上記対PE粘着力の上限は特に制限されないが、保持力等の他の粘着特性との両立の観点から、通常は例えば凡そ30N/25mm以下であり、25N/25mm以下であってもよい。上記対PE粘着力は、被着体としてPEを用いて、23℃、50%RHの測定環境下において、引張速度300mm/分、剥離角度180度の条件で測定される。より具体的には、後述の実施例に記載の方法で測定される。
The adhesive sheet disclosed herein preferably has a 180 degree peel strength against polyethylene (adhesive strength against PE) of approximately 5 N/25 mm or more. A pressure-sensitive adhesive sheet exhibiting such adhesive strength to PE can exhibit sufficient adhesion reliability to low polarity materials. The adhesive strength to PE is more preferably about 7 N/25 mm or more, still more preferably about 10 N/25 mm or more, particularly preferably about 12 N/25 mm or more, and 14 N/25 mm or more (for example, about 15 N/25 mm or more). It's okay. The upper limit of the adhesive force to PE is not particularly limited, but from the viewpoint of coexistence with other adhesive properties such as holding power, it is usually about 30 N/25 mm or less, and may be 25 N/25 mm or less. The above-mentioned adhesive strength to PE is measured using PE as an adherend in a measurement environment of 23° C. and 50% RH at a tensile rate of 300 mm/min and a peel angle of 180 degrees. More specifically, it is measured by the method described in Examples below.
ここに開示される粘着シートは、上記のように、PPおよびPEに対して所定値以上の粘着力を示すものであり得る。上記対PP粘着力および対PE粘着力を示す粘着シートは、各種低極性材料を含む異種材料に対して安定的に十分な接着信頼性を有するものとなり得るので、その利用範囲が広く有用である。
As mentioned above, the adhesive sheet disclosed herein may exhibit adhesive strength of a predetermined value or more to PP and PE. Adhesive sheets exhibiting the above-mentioned adhesive strength to PP and adhesive strength to PE can have stable and sufficient adhesion reliability to different materials including various low polarity materials, and therefore are useful in a wide range of applications. .
ここに開示される粘着シートは、80℃、接着面積10mm×20mm、荷重1.5kg、1時間の条件で実施される保持力試験において、保持力試験開始から1時間後の被着体からのズレ距離が10mm以下であり得る。このような粘着シートは、高い凝集力を有し、十分な保持力を有する。上記保持力試験における上記ズレ距離は、5mm以下であることが好ましく、3mm以下がより好ましく、1mm以下(0~0.1mm)がさらに好ましい。上記保持力試験は、より具体的には、後述の実施例に記載の方法で実施される。
The pressure-sensitive adhesive sheet disclosed herein has a holding power test conducted under the conditions of 80° C., adhesion area 10 mm x 20 mm, load 1.5 kg, and 1 hour. The deviation distance may be 10 mm or less. Such a pressure-sensitive adhesive sheet has high cohesive force and sufficient holding power. The displacement distance in the holding force test is preferably 5 mm or less, more preferably 3 mm or less, and even more preferably 1 mm or less (0 to 0.1 mm). More specifically, the above-mentioned holding power test is carried out by the method described in the Examples below.
いくつかの態様において、粘着シートはバイオマス由来材料を含み、そのバイオマス炭素比が所定値以上であり得る。粘着シートのバイオマス炭素比は、例えば1%以上であり、10%以上であってもよく、好ましくは30%以上、より好ましくは50%以上である。粘着シートのバイオマス炭素比が高いことは、石油等に代表される化石資源系材料の使用量が少ないことを意味する。かかる観点において、粘着シートのバイオマス炭素比は高いほど好ましい。例えば、粘着シートのバイオマス炭素比は、55%以上であってよく、60%以上であってもよく、70%以上でもよく、75%以上でもよく、80%以上でもよく、80%超でもよい。バイオマス炭素比の上限は、定義上100%であり、99%以下であってもよく、材料の入手容易性の観点から、95%以下でもよく、90%以下でもよい。良好な粘着性能を発揮しやすくする観点から、いくつかの態様において、粘着シートのバイオマス炭素比は、例えば90%以下であってよく、85%以下でもよく、80%以下でもよい。
In some embodiments, the adhesive sheet may include a biomass-derived material, and the biomass carbon ratio may be greater than or equal to a predetermined value. The biomass carbon ratio of the adhesive sheet is, for example, 1% or more, and may be 10% or more, preferably 30% or more, and more preferably 50% or more. A high biomass carbon ratio in the adhesive sheet means that less fossil resource-based materials, such as petroleum, are used. From this point of view, the higher the biomass carbon ratio of the pressure-sensitive adhesive sheet, the more preferable it is. For example, the biomass carbon ratio of the adhesive sheet may be 55% or more, 60% or more, 70% or more, 75% or more, 80% or more, or more than 80%. . The upper limit of the biomass carbon ratio is 100% by definition, and may be 99% or less, and from the viewpoint of material availability, it may be 95% or less, or 90% or less. In some embodiments, from the viewpoint of facilitating good adhesive performance, the biomass carbon ratio of the adhesive sheet may be, for example, 90% or less, 85% or less, or 80% or less.
<用途>
ここに開示される粘着シートの用途は特に限定されず、各種用途に用いられ得る。ここに開示される粘着シートは、高極性材料、低極性材料のいずれに対しても高い接着力を発揮し、かつ、十分な保持力を有するので、金属部材を含む高極性材料製の部材や、低極性材料製の部材の接着固定に好適である。例えば、接着対象となる構成部材が高極性面や低極性面を有し、長期に亘る接着信頼性が求められる用途に好適である。そのような高極性面を構成する材料としては、例えば、ステンレス鋼等の金属材料やガラス材料、PET等のポリエステル樹脂製部材が挙げられる。また、低極性面を構成する材料としては、一般に表面自由エネルギーが低い材料として知られているポリエチレン(PE)、ポリプロピレン(PP)等のポリオレフィン樹脂や、フッ素系ポリマー(ポリテトラフルオロエチレン等)、ポリスチレン、ポリオキシメチレン、ポリ酢酸ビニル等が挙げられる。なかでも、PE、PP等のポリオレフィン、フッ素系ポリマーを含む材料から構成された表面を有する被着体の接着固定に、ここに開示される粘着シートは好ましく用いられる。 <Application>
The use of the pressure-sensitive adhesive sheet disclosed herein is not particularly limited, and can be used for various purposes. The pressure-sensitive adhesive sheet disclosed herein exhibits high adhesion to both high-polar and low-polar materials, and has sufficient holding power, so it is suitable for use with members made of high-polar materials including metal members, , suitable for adhesively fixing members made of low polarity materials. For example, it is suitable for applications where the component to be bonded has a high polarity surface or a low polarity surface and long-term bonding reliability is required. Examples of materials constituting such a highly polar surface include metal materials such as stainless steel, glass materials, and polyester resin members such as PET. In addition, materials constituting the low polarity surface include polyolefin resins such as polyethylene (PE) and polypropylene (PP), which are generally known as materials with low surface free energy, fluorine-based polymers (such as polytetrafluoroethylene), Examples include polystyrene, polyoxymethylene, polyvinyl acetate, and the like. Among these, the pressure-sensitive adhesive sheet disclosed herein is preferably used for adhesively fixing an adherend having a surface made of a material containing a polyolefin such as PE or PP, or a fluorine-based polymer.
ここに開示される粘着シートの用途は特に限定されず、各種用途に用いられ得る。ここに開示される粘着シートは、高極性材料、低極性材料のいずれに対しても高い接着力を発揮し、かつ、十分な保持力を有するので、金属部材を含む高極性材料製の部材や、低極性材料製の部材の接着固定に好適である。例えば、接着対象となる構成部材が高極性面や低極性面を有し、長期に亘る接着信頼性が求められる用途に好適である。そのような高極性面を構成する材料としては、例えば、ステンレス鋼等の金属材料やガラス材料、PET等のポリエステル樹脂製部材が挙げられる。また、低極性面を構成する材料としては、一般に表面自由エネルギーが低い材料として知られているポリエチレン(PE)、ポリプロピレン(PP)等のポリオレフィン樹脂や、フッ素系ポリマー(ポリテトラフルオロエチレン等)、ポリスチレン、ポリオキシメチレン、ポリ酢酸ビニル等が挙げられる。なかでも、PE、PP等のポリオレフィン、フッ素系ポリマーを含む材料から構成された表面を有する被着体の接着固定に、ここに開示される粘着シートは好ましく用いられる。 <Application>
The use of the pressure-sensitive adhesive sheet disclosed herein is not particularly limited, and can be used for various purposes. The pressure-sensitive adhesive sheet disclosed herein exhibits high adhesion to both high-polar and low-polar materials, and has sufficient holding power, so it is suitable for use with members made of high-polar materials including metal members, , suitable for adhesively fixing members made of low polarity materials. For example, it is suitable for applications where the component to be bonded has a high polarity surface or a low polarity surface and long-term bonding reliability is required. Examples of materials constituting such a highly polar surface include metal materials such as stainless steel, glass materials, and polyester resin members such as PET. In addition, materials constituting the low polarity surface include polyolefin resins such as polyethylene (PE) and polypropylene (PP), which are generally known as materials with low surface free energy, fluorine-based polymers (such as polytetrafluoroethylene), Examples include polystyrene, polyoxymethylene, polyvinyl acetate, and the like. Among these, the pressure-sensitive adhesive sheet disclosed herein is preferably used for adhesively fixing an adherend having a surface made of a material containing a polyolefin such as PE or PP, or a fluorine-based polymer.
ここに開示される粘着シートの好適な適用対象としては、家電製品やOA機器、携帯電子機器等の電子機器における部材の固定が挙げられる。上記電子機器を構成する部材には、金属等の高極性材料や、PEやPP、フッ素樹脂等の低極性材料が用いられ得るため、ここに開示される粘着シートを適用して、高極性および低極性を含む種々の異種材料に対して接着信頼性に優れた接着固定を実現することが有意義である。上記電子機器の例としては、各種家電製品、パソコン(デスクトップ型、ノート型、タブレット型等)等が挙げられる。上記携帯電子機器の非限定的な例には、携帯電話、スマートフォン、タブレット型パソコン、ノート型パソコン、各種ウェアラブル機器(例えば、腕時計のように手首に装着するリストウェア型、クリップやストラップ等で体の一部に装着するモジュラー型、メガネ型(単眼型や両眼型。ヘッドマウント型も含む。)を包含するアイウェア型、シャツや靴下、帽子等に例えばアクセサリの形態で取り付ける衣服型、イヤホンのように耳に取り付けるイヤウェア型等)、デジタルカメラ、デジタルビデオカメラ、音響機器(携帯音楽プレーヤー、ICレコーダー等)、計算機(電卓等)、携帯ゲーム機器、電子辞書、電子手帳、電子書籍、車載用情報機器、携帯ラジオ、携帯テレビ、携帯プリンター、携帯スキャナ、携帯モデム等が含まれる。なお、この明細書において「携帯」とは、単に携帯することが可能であるだけでは充分ではなく、個人(標準的な成人)が相対的に容易に持ち運び可能なレベルの携帯性を有することを意味するものとする。
Suitable applications for the adhesive sheet disclosed herein include fixing members in electronic devices such as home appliances, office automation equipment, and portable electronic devices. The members constituting the above-mentioned electronic devices can be made of highly polar materials such as metals, or low polar materials such as PE, PP, or fluororesin. It is meaningful to realize adhesive fixation with excellent adhesive reliability for various dissimilar materials including those with low polarity. Examples of the electronic devices include various home appliances, personal computers (desktop type, notebook type, tablet type, etc.), and the like. Non-limiting examples of the above-mentioned portable electronic devices include mobile phones, smartphones, tablet computers, notebook computers, and various wearable devices (e.g., wrist-wear type that is worn on the wrist like a wristwatch, and Modular type that is attached to a part of the body, eyewear type that includes glasses type (monocular type and binocular type, including head-mounted type), clothing type that is attached to shirts, socks, hats, etc. in the form of accessories, and earphones. digital cameras, digital video cameras, audio equipment (portable music players, IC recorders, etc.), calculators (calculators, etc.), portable game devices, electronic dictionaries, electronic notebooks, electronic books, and in-vehicle devices. This includes information equipment, portable radios, portable televisions, portable printers, portable scanners, portable modems, etc. Note that in this specification, "portable" does not mean that it is sufficient to simply be able to carry it; it also means that it has a level of portability that allows an individual (standard adult) to carry it relatively easily. shall mean.
図4は、ここに開示される粘着シートが用いられた携帯電子機器(スマートフォン)を模式的に示す一例である。図4に示すように、携帯電子機器500の筐体520の内部には、バッテリー(発熱要素)540が内蔵されている。また、携帯電子機器500は、粘着シート550を含んで構成されている。この構成例では、粘着シート550は、携帯電子機器500を構成する部材を固定する両面接着性のシート(両面粘着シート)の形態を有する。なお、携帯電子機器500は、表示部が入力部としても機能するタッチパネル570を備えている。ここに開示される粘着シートは、上記のような携帯電子機器の構成要素(部材接合手段)として好ましく用いられる。
FIG. 4 is an example schematically showing a portable electronic device (smartphone) using the adhesive sheet disclosed herein. As shown in FIG. 4, a battery (heat generating element) 540 is built inside the casing 520 of the portable electronic device 500. Moreover, the portable electronic device 500 is configured to include an adhesive sheet 550. In this configuration example, the adhesive sheet 550 has the form of a double-sided adhesive sheet (double-sided adhesive sheet) that fixes members constituting the portable electronic device 500. Note that the portable electronic device 500 includes a touch panel 570 whose display section also functions as an input section. The adhesive sheet disclosed herein is preferably used as a component (member joining means) of the above-mentioned portable electronic devices.
また、ここに開示される粘着シートは、いくつかの態様において、バイオマス炭素比の高いアクリル系ポリマーを含む粘着剤層を有するものであり得ることから、従来の一般的なアクリル系粘着剤(すなわち、バイオマス炭素比の低いアクリル系粘着剤)が使用されている各種の用途において該アクリル系粘着剤の代替として用いられることで、化石資源系材料の依存抑制に貢献することができる。ここに開示される粘着シートは、化石資源系材料への依存度が低減された粘着シートとして好ましく利用され得る。
Furthermore, since the adhesive sheet disclosed herein may have an adhesive layer containing an acrylic polymer with a high biomass carbon ratio in some embodiments, conventional general acrylic adhesives (i.e. By being used as a substitute for acrylic adhesives in various applications where acrylic adhesives (acrylic adhesives with a low biomass carbon ratio) are used, it can contribute to reducing dependence on fossil resource-based materials. The adhesive sheet disclosed herein can be preferably used as an adhesive sheet with reduced dependence on fossil resource materials.
この明細書により開示される事項には以下のものが含まれる。
〔1〕 電子機器であって、
前記電子機器を構成する部材には、粘着シートが接合されており、
前記粘着シートは、アクリル系ポリマーを含む粘着剤層を有しており、
前記アクリル系ポリマーは、n-ヘプチルアクリレートおよびカルボキシ基含有モノマーを含むモノマー成分の重合物であり、
前記アクリル系ポリマーのモノマー成分は、前記カルボキシ基含有モノマーを3重量%よりも多く含み、
前記粘着剤層のゲル分率は70%未満であり、
前記アクリル系ポリマーの重量平均分子量は60万よりも大きい、電子機器。
〔2〕 前記部材の表面は、金属、ポリオレフィン樹脂およびフッ素樹脂から選択される材料から構成されている、上記〔1〕に記載の電子機器。
〔3〕 前記電子機器は家電製品である、上記〔1〕または〔2〕に記載の電子機器。
〔4〕 前記電子機器は携帯電子機器である、上記〔1〕または〔2〕に記載の電子機器。
〔5〕 前記粘着剤層を形成するための粘着剤組成物は架橋剤を含む、上記〔1〕~〔4〕のいずれかに記載の電子機器。
〔6〕 前記粘着剤層は粘着付与樹脂を含む、上記〔1〕~〔5〕のいずれかに記載の電子機器。
〔7〕 前記粘着剤層の厚さは0.1~500μmである、上記〔1〕~〔6〕のいずれかに記載の電子機器。
〔8〕 前記粘着剤層のゲル分率は20%以上70%未満である、上記〔1〕~〔7〕のいずれかに記載の電子機器。
〔9〕 前記粘着シートは、前記粘着剤層のみからなる基材レス両面粘着シートである、上記〔1〕~〔8〕のいずれかに記載の電子機器。
〔10〕 前記粘着シートは、基材と、該基材の少なくとも一方の表面に設けられた前記粘着剤層と、を有する、上記〔1〕~〔8〕のいずれかに記載の電子機器。 The matters disclosed by this specification include the following.
[1] Electronic equipment,
An adhesive sheet is bonded to the member constituting the electronic device,
The adhesive sheet has an adhesive layer containing an acrylic polymer,
The acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer,
The monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer,
The adhesive layer has a gel fraction of less than 70%,
The weight average molecular weight of the acrylic polymer is greater than 600,000, an electronic device.
[2] The electronic device according to [1] above, wherein the surface of the member is made of a material selected from metal, polyolefin resin, and fluororesin.
[3] The electronic device according to [1] or [2] above, wherein the electronic device is a home appliance.
[4] The electronic device according to [1] or [2] above, wherein the electronic device is a portable electronic device.
[5] The electronic device according to any one of [1] to [4] above, wherein the adhesive composition for forming the adhesive layer contains a crosslinking agent.
[6] The electronic device according to any one of [1] to [5] above, wherein the adhesive layer contains a tackifier resin.
[7] The electronic device according to any one of [1] to [6] above, wherein the adhesive layer has a thickness of 0.1 to 500 μm.
[8] The electronic device according to any one of [1] to [7] above, wherein the adhesive layer has a gel fraction of 20% or more and less than 70%.
[9] The electronic device according to any one of [1] to [8] above, wherein the adhesive sheet is a base material-less double-sided adhesive sheet consisting only of the adhesive layer.
[10] The electronic device according to any one of [1] to [8] above, wherein the adhesive sheet has a base material and the adhesive layer provided on at least one surface of the base material.
〔1〕 電子機器であって、
前記電子機器を構成する部材には、粘着シートが接合されており、
前記粘着シートは、アクリル系ポリマーを含む粘着剤層を有しており、
前記アクリル系ポリマーは、n-ヘプチルアクリレートおよびカルボキシ基含有モノマーを含むモノマー成分の重合物であり、
前記アクリル系ポリマーのモノマー成分は、前記カルボキシ基含有モノマーを3重量%よりも多く含み、
前記粘着剤層のゲル分率は70%未満であり、
前記アクリル系ポリマーの重量平均分子量は60万よりも大きい、電子機器。
〔2〕 前記部材の表面は、金属、ポリオレフィン樹脂およびフッ素樹脂から選択される材料から構成されている、上記〔1〕に記載の電子機器。
〔3〕 前記電子機器は家電製品である、上記〔1〕または〔2〕に記載の電子機器。
〔4〕 前記電子機器は携帯電子機器である、上記〔1〕または〔2〕に記載の電子機器。
〔5〕 前記粘着剤層を形成するための粘着剤組成物は架橋剤を含む、上記〔1〕~〔4〕のいずれかに記載の電子機器。
〔6〕 前記粘着剤層は粘着付与樹脂を含む、上記〔1〕~〔5〕のいずれかに記載の電子機器。
〔7〕 前記粘着剤層の厚さは0.1~500μmである、上記〔1〕~〔6〕のいずれかに記載の電子機器。
〔8〕 前記粘着剤層のゲル分率は20%以上70%未満である、上記〔1〕~〔7〕のいずれかに記載の電子機器。
〔9〕 前記粘着シートは、前記粘着剤層のみからなる基材レス両面粘着シートである、上記〔1〕~〔8〕のいずれかに記載の電子機器。
〔10〕 前記粘着シートは、基材と、該基材の少なくとも一方の表面に設けられた前記粘着剤層と、を有する、上記〔1〕~〔8〕のいずれかに記載の電子機器。 The matters disclosed by this specification include the following.
[1] Electronic equipment,
An adhesive sheet is bonded to the member constituting the electronic device,
The adhesive sheet has an adhesive layer containing an acrylic polymer,
The acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer,
The monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer,
The adhesive layer has a gel fraction of less than 70%,
The weight average molecular weight of the acrylic polymer is greater than 600,000, an electronic device.
[2] The electronic device according to [1] above, wherein the surface of the member is made of a material selected from metal, polyolefin resin, and fluororesin.
[3] The electronic device according to [1] or [2] above, wherein the electronic device is a home appliance.
[4] The electronic device according to [1] or [2] above, wherein the electronic device is a portable electronic device.
[5] The electronic device according to any one of [1] to [4] above, wherein the adhesive composition for forming the adhesive layer contains a crosslinking agent.
[6] The electronic device according to any one of [1] to [5] above, wherein the adhesive layer contains a tackifier resin.
[7] The electronic device according to any one of [1] to [6] above, wherein the adhesive layer has a thickness of 0.1 to 500 μm.
[8] The electronic device according to any one of [1] to [7] above, wherein the adhesive layer has a gel fraction of 20% or more and less than 70%.
[9] The electronic device according to any one of [1] to [8] above, wherein the adhesive sheet is a base material-less double-sided adhesive sheet consisting only of the adhesive layer.
[10] The electronic device according to any one of [1] to [8] above, wherein the adhesive sheet has a base material and the adhesive layer provided on at least one surface of the base material.
〔11〕 アクリル系ポリマーを含む粘着剤層を有し、
前記アクリル系ポリマーは、n-ヘプチルアクリレートおよびカルボキシ基含有モノマーを含むモノマー成分の重合物であり、
前記アクリル系ポリマーのモノマー成分は、前記カルボキシ基含有モノマーを3重量%よりも多く含み、
前記粘着剤層のゲル分率は70%未満であり、
前記アクリル系ポリマーの重量平均分子量は60万よりも大きい、粘着シート。
〔12〕 前記粘着剤層を形成するための粘着剤組成物は架橋剤を含む、上記〔11〕に記載の粘着シート。
〔13〕 前記粘着剤層は粘着付与樹脂を含む、上記〔11〕または〔12〕に記載の粘着シート。
〔14〕 前記粘着剤層の厚さは0.1~500μmである、上記〔11〕~〔13〕のいずれかに記載の粘着シート。
〔15〕 前記粘着剤層のゲル分率は20%以上70%未満である、上記〔11〕~〔14〕のいずれかに記載の粘着シート。
〔16〕 ステンレス鋼板に対する180度剥離強度が15N/25mm以上である、上記〔11〕~〔15〕のいずれかに記載の粘着シート。
〔17〕 ポリプロピレンに対する180度剥離強度が10N/25mm以上である、上記〔11〕~〔16〕のいずれかに記載の粘着シート。
〔18〕 ポリエチレンに対する180度剥離強度が5N/25mm以上である、上記〔11〕~〔17〕のいずれかに記載の粘着シート。
〔19〕 80℃、接着面積10mm×20mm、荷重1.5kg、1時間の条件で実施される保持力試験におけるズレ距離が10mm以下である、上記〔11〕~〔18〕のいずれかに記載の粘着シート。
〔20〕 前記粘着剤層のみからなる基材レス両面粘着シートである、上記〔11〕~〔19〕のいずれかに記載の粘着シート。
〔21〕 基材と、該基材の少なくとも一方の表面に設けられた前記粘着剤層と、を有する、上記〔11〕~〔19〕のいずれかに記載の粘着シート。
〔22〕 電子機器において部材の固定に用いられる、上記〔11〕~〔21〕のいずれかに記載の粘着シート。
〔23〕 上記〔11〕~〔21〕のいずれかに記載の粘着シートを含む電子機器。 [11] Has an adhesive layer containing an acrylic polymer,
The acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer,
The monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer,
The adhesive layer has a gel fraction of less than 70%,
A pressure-sensitive adhesive sheet, wherein the acrylic polymer has a weight average molecular weight of more than 600,000.
[12] The pressure-sensitive adhesive sheet according to [11] above, wherein the pressure-sensitive adhesive composition for forming the pressure-sensitive adhesive layer contains a crosslinking agent.
[13] The pressure-sensitive adhesive sheet according to [11] or [12] above, wherein the pressure-sensitive adhesive layer contains a tackifying resin.
[14] The pressure-sensitive adhesive sheet according to any one of [11] to [13] above, wherein the pressure-sensitive adhesive layer has a thickness of 0.1 to 500 μm.
[15] The adhesive sheet according to any one of [11] to [14] above, wherein the adhesive layer has a gel fraction of 20% or more and less than 70%.
[16] The adhesive sheet according to any one of [11] to [15] above, which has a 180 degree peel strength against a stainless steel plate of 15 N/25 mm or more.
[17] The adhesive sheet according to any one of [11] to [16] above, which has a 180 degree peel strength against polypropylene of 10 N/25 mm or more.
[18] The adhesive sheet according to any one of [11] to [17] above, which has a 180 degree peel strength against polyethylene of 5 N/25 mm or more.
[19] Any one of [11] to [18] above, wherein the displacement distance in a holding force test conducted at 80° C.,adhesive area 10 mm x 20 mm, load 1.5 kg, and 1 hour is 10 mm or less. adhesive sheet.
[20] The pressure-sensitive adhesive sheet according to any one of [11] to [19] above, which is a base material-less double-sided pressure-sensitive adhesive sheet consisting only of the pressure-sensitive adhesive layer.
[21] The pressure-sensitive adhesive sheet according to any one of [11] to [19] above, comprising a base material and the pressure-sensitive adhesive layer provided on at least one surface of the base material.
[22] The adhesive sheet according to any one of [11] to [21] above, which is used for fixing members in electronic devices.
[23] An electronic device comprising the adhesive sheet according to any one of [11] to [21] above.
前記アクリル系ポリマーは、n-ヘプチルアクリレートおよびカルボキシ基含有モノマーを含むモノマー成分の重合物であり、
前記アクリル系ポリマーのモノマー成分は、前記カルボキシ基含有モノマーを3重量%よりも多く含み、
前記粘着剤層のゲル分率は70%未満であり、
前記アクリル系ポリマーの重量平均分子量は60万よりも大きい、粘着シート。
〔12〕 前記粘着剤層を形成するための粘着剤組成物は架橋剤を含む、上記〔11〕に記載の粘着シート。
〔13〕 前記粘着剤層は粘着付与樹脂を含む、上記〔11〕または〔12〕に記載の粘着シート。
〔14〕 前記粘着剤層の厚さは0.1~500μmである、上記〔11〕~〔13〕のいずれかに記載の粘着シート。
〔15〕 前記粘着剤層のゲル分率は20%以上70%未満である、上記〔11〕~〔14〕のいずれかに記載の粘着シート。
〔16〕 ステンレス鋼板に対する180度剥離強度が15N/25mm以上である、上記〔11〕~〔15〕のいずれかに記載の粘着シート。
〔17〕 ポリプロピレンに対する180度剥離強度が10N/25mm以上である、上記〔11〕~〔16〕のいずれかに記載の粘着シート。
〔18〕 ポリエチレンに対する180度剥離強度が5N/25mm以上である、上記〔11〕~〔17〕のいずれかに記載の粘着シート。
〔19〕 80℃、接着面積10mm×20mm、荷重1.5kg、1時間の条件で実施される保持力試験におけるズレ距離が10mm以下である、上記〔11〕~〔18〕のいずれかに記載の粘着シート。
〔20〕 前記粘着剤層のみからなる基材レス両面粘着シートである、上記〔11〕~〔19〕のいずれかに記載の粘着シート。
〔21〕 基材と、該基材の少なくとも一方の表面に設けられた前記粘着剤層と、を有する、上記〔11〕~〔19〕のいずれかに記載の粘着シート。
〔22〕 電子機器において部材の固定に用いられる、上記〔11〕~〔21〕のいずれかに記載の粘着シート。
〔23〕 上記〔11〕~〔21〕のいずれかに記載の粘着シートを含む電子機器。 [11] Has an adhesive layer containing an acrylic polymer,
The acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer,
The monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer,
The adhesive layer has a gel fraction of less than 70%,
A pressure-sensitive adhesive sheet, wherein the acrylic polymer has a weight average molecular weight of more than 600,000.
[12] The pressure-sensitive adhesive sheet according to [11] above, wherein the pressure-sensitive adhesive composition for forming the pressure-sensitive adhesive layer contains a crosslinking agent.
[13] The pressure-sensitive adhesive sheet according to [11] or [12] above, wherein the pressure-sensitive adhesive layer contains a tackifying resin.
[14] The pressure-sensitive adhesive sheet according to any one of [11] to [13] above, wherein the pressure-sensitive adhesive layer has a thickness of 0.1 to 500 μm.
[15] The adhesive sheet according to any one of [11] to [14] above, wherein the adhesive layer has a gel fraction of 20% or more and less than 70%.
[16] The adhesive sheet according to any one of [11] to [15] above, which has a 180 degree peel strength against a stainless steel plate of 15 N/25 mm or more.
[17] The adhesive sheet according to any one of [11] to [16] above, which has a 180 degree peel strength against polypropylene of 10 N/25 mm or more.
[18] The adhesive sheet according to any one of [11] to [17] above, which has a 180 degree peel strength against polyethylene of 5 N/25 mm or more.
[19] Any one of [11] to [18] above, wherein the displacement distance in a holding force test conducted at 80° C.,
[20] The pressure-sensitive adhesive sheet according to any one of [11] to [19] above, which is a base material-less double-sided pressure-sensitive adhesive sheet consisting only of the pressure-sensitive adhesive layer.
[21] The pressure-sensitive adhesive sheet according to any one of [11] to [19] above, comprising a base material and the pressure-sensitive adhesive layer provided on at least one surface of the base material.
[22] The adhesive sheet according to any one of [11] to [21] above, which is used for fixing members in electronic devices.
[23] An electronic device comprising the adhesive sheet according to any one of [11] to [21] above.
以下、本発明に関するいくつかの実施例を説明するが、本発明をかかる実施例に示すものに限定することを意図したものではない。なお、以下の説明において「部」および「%」は、特に断りがない限り重量基準である。
Hereinafter, some examples relating to the present invention will be described, but the present invention is not intended to be limited to what is shown in these examples. In the following description, "parts" and "%" are based on weight unless otherwise specified.
<評価方法>
[ゲル分率]
約0.1gの粘着剤サンプル(重量Wg1)を平均孔径0.2μmの多孔質ポリテトラフルオロエチレン膜(重量Wg2)で巾着状に包み、口をタコ糸(重量Wg3)で縛る。上記多孔質ポリテトラフルオロエチレン(PTFE)膜としては、日東電工社から入手可能な商品名「ニトフロン(登録商標)NTF1122」(平均孔径0.2μm、気孔率75%、厚さ85μm)またはその相当品を使用する。
この包みを酢酸エチル50mLに浸し、室温(約23℃)で7日間保持して粘着剤層中のゾル成分のみを上記膜外に溶出させた後、上記包みを取り出して外表面に付着している酢酸エチルを拭き取り、該包みを130℃で2時間乾燥させ、該包みの重量(Wg4)を測定する。粘着剤層のゲル分率は、各値を以下の式に代入することにより求められる。
ゲル分率(%)=[(Wg4-Wg2-Wg3)/Wg1]×100 <Evaluation method>
[Gel fraction]
Approximately 0.1 g of an adhesive sample (weight Wg 1 ) is wrapped in a purse-like shape with a porous polytetrafluoroethylene membrane (weight Wg 2 ) having an average pore diameter of 0.2 μm, and the opening is tied with an octopus string (weight Wg 3 ). The above-mentioned porous polytetrafluoroethylene (PTFE) membrane is available from Nitto Denko under the trade name "Nitoflon (registered trademark) NTF1122" (average pore diameter 0.2 μm, porosity 75%, thickness 85 μm) or its equivalent. use the product.
This package was immersed in 50 mL of ethyl acetate and kept at room temperature (approximately 23°C) for 7 days to allow only the sol component in the adhesive layer to elute out of the membrane.The package was then taken out and attached to the outer surface. The remaining ethyl acetate is wiped off, the package is dried at 130° C. for 2 hours, and the weight (Wg 4 ) of the package is measured. The gel fraction of the adhesive layer is determined by substituting each value into the following formula.
Gel fraction (%) = [(Wg 4 - Wg 2 - Wg 3 )/Wg 1 ] x 100
[ゲル分率]
約0.1gの粘着剤サンプル(重量Wg1)を平均孔径0.2μmの多孔質ポリテトラフルオロエチレン膜(重量Wg2)で巾着状に包み、口をタコ糸(重量Wg3)で縛る。上記多孔質ポリテトラフルオロエチレン(PTFE)膜としては、日東電工社から入手可能な商品名「ニトフロン(登録商標)NTF1122」(平均孔径0.2μm、気孔率75%、厚さ85μm)またはその相当品を使用する。
この包みを酢酸エチル50mLに浸し、室温(約23℃)で7日間保持して粘着剤層中のゾル成分のみを上記膜外に溶出させた後、上記包みを取り出して外表面に付着している酢酸エチルを拭き取り、該包みを130℃で2時間乾燥させ、該包みの重量(Wg4)を測定する。粘着剤層のゲル分率は、各値を以下の式に代入することにより求められる。
ゲル分率(%)=[(Wg4-Wg2-Wg3)/Wg1]×100 <Evaluation method>
[Gel fraction]
Approximately 0.1 g of an adhesive sample (weight Wg 1 ) is wrapped in a purse-like shape with a porous polytetrafluoroethylene membrane (weight Wg 2 ) having an average pore diameter of 0.2 μm, and the opening is tied with an octopus string (weight Wg 3 ). The above-mentioned porous polytetrafluoroethylene (PTFE) membrane is available from Nitto Denko under the trade name "Nitoflon (registered trademark) NTF1122" (average pore diameter 0.2 μm, porosity 75%, thickness 85 μm) or its equivalent. use the product.
This package was immersed in 50 mL of ethyl acetate and kept at room temperature (approximately 23°C) for 7 days to allow only the sol component in the adhesive layer to elute out of the membrane.The package was then taken out and attached to the outer surface. The remaining ethyl acetate is wiped off, the package is dried at 130° C. for 2 hours, and the weight (Wg 4 ) of the package is measured. The gel fraction of the adhesive layer is determined by substituting each value into the following formula.
Gel fraction (%) = [(Wg 4 - Wg 2 - Wg 3 )/Wg 1 ] x 100
[対SUS粘着力]
23℃、50%RHの測定環境下において、粘着シート(両面粘着シート)の一方の粘着面に厚さ50μmのPETフィルムを貼り付けて裏打ちし、幅25mm、長さ100mmのサイズにカットして測定サンプルを作製する。23℃、50%RHの環境下にて、上記測定サンプルの他方の粘着面を、酢酸エチルで洗浄したステンレス鋼板(SUS304BA板)の表面に、2kgのローラを1往復させて圧着する。これを同環境下に72時間放置した後、万能引張圧縮試験機を使用して、JIS Z 0237:2000に準じて、引張速度300mm/分、剥離角度180度の条件で、剥離強度(対SUS粘着力)[N/25mm]を測定する。 [Adhesive strength to SUS]
Under a measurement environment of 23°C and 50% RH, one adhesive side of an adhesive sheet (double-sided adhesive sheet) was lined with a PET film with a thickness of 50 μm, and cut into a size of 25 mm in width and 100 mm in length. Prepare a measurement sample. In an environment of 23° C. and 50% RH, the other adhesive surface of the measurement sample is pressed onto the surface of a stainless steel plate (SUS304BA plate) that has been cleaned with ethyl acetate by making one reciprocation with a 2 kg roller. After leaving it in the same environment for 72 hours, using a universal tensile compression tester, the peel strength (relative to SUS Adhesive force) [N/25mm] is measured.
23℃、50%RHの測定環境下において、粘着シート(両面粘着シート)の一方の粘着面に厚さ50μmのPETフィルムを貼り付けて裏打ちし、幅25mm、長さ100mmのサイズにカットして測定サンプルを作製する。23℃、50%RHの環境下にて、上記測定サンプルの他方の粘着面を、酢酸エチルで洗浄したステンレス鋼板(SUS304BA板)の表面に、2kgのローラを1往復させて圧着する。これを同環境下に72時間放置した後、万能引張圧縮試験機を使用して、JIS Z 0237:2000に準じて、引張速度300mm/分、剥離角度180度の条件で、剥離強度(対SUS粘着力)[N/25mm]を測定する。 [Adhesive strength to SUS]
Under a measurement environment of 23°C and 50% RH, one adhesive side of an adhesive sheet (double-sided adhesive sheet) was lined with a PET film with a thickness of 50 μm, and cut into a size of 25 mm in width and 100 mm in length. Prepare a measurement sample. In an environment of 23° C. and 50% RH, the other adhesive surface of the measurement sample is pressed onto the surface of a stainless steel plate (SUS304BA plate) that has been cleaned with ethyl acetate by making one reciprocation with a 2 kg roller. After leaving it in the same environment for 72 hours, using a universal tensile compression tester, the peel strength (relative to SUS Adhesive force) [N/25mm] is measured.
[対PP粘着力]
23℃、50%RHの測定環境下において、粘着シート(両面粘着シート)の一方の粘着面に厚さ50μmのPETフィルムを貼り付けて裏打ちし、幅25mm、長さ100mmのサイズにカットして測定サンプルを作製する。23℃、50%RHの環境下にて、上記測定サンプルの他方の粘着面を、エタノールで洗浄したポリプロピレン板(PP板)の表面に、2kgのローラを1往復させて圧着する。これを同環境下に72時間放置した後、万能引張圧縮試験機を使用して、JIS Z 0237:2000に準じて、引張速度300mm/分、剥離角度180度の条件で、剥離強度(対PP粘着力)[N/25mm]を測定する。PP板としては、例えば昭和電工マテリアルズ社製の製品名「コウベポリシート PP-N-AN」(厚さ2mm)が用いられる。 [Adhesion to PP]
Under a measurement environment of 23°C and 50% RH, one adhesive side of an adhesive sheet (double-sided adhesive sheet) was lined with a PET film with a thickness of 50 μm, and cut into a size of 25 mm in width and 100 mm in length. Prepare a measurement sample. In an environment of 23° C. and 50% RH, the other adhesive surface of the measurement sample was pressed onto the surface of a polypropylene plate (PP plate) washed with ethanol by making one reciprocation with a 2 kg roller. After leaving it in the same environment for 72 hours, the peel strength (relative to PP Adhesive force) [N/25mm] is measured. As the PP board, for example, the product name "Kobe Polysheet PP-N-AN" (thickness: 2 mm) manufactured by Showa Denko Materials Co., Ltd. is used.
23℃、50%RHの測定環境下において、粘着シート(両面粘着シート)の一方の粘着面に厚さ50μmのPETフィルムを貼り付けて裏打ちし、幅25mm、長さ100mmのサイズにカットして測定サンプルを作製する。23℃、50%RHの環境下にて、上記測定サンプルの他方の粘着面を、エタノールで洗浄したポリプロピレン板(PP板)の表面に、2kgのローラを1往復させて圧着する。これを同環境下に72時間放置した後、万能引張圧縮試験機を使用して、JIS Z 0237:2000に準じて、引張速度300mm/分、剥離角度180度の条件で、剥離強度(対PP粘着力)[N/25mm]を測定する。PP板としては、例えば昭和電工マテリアルズ社製の製品名「コウベポリシート PP-N-AN」(厚さ2mm)が用いられる。 [Adhesion to PP]
Under a measurement environment of 23°C and 50% RH, one adhesive side of an adhesive sheet (double-sided adhesive sheet) was lined with a PET film with a thickness of 50 μm, and cut into a size of 25 mm in width and 100 mm in length. Prepare a measurement sample. In an environment of 23° C. and 50% RH, the other adhesive surface of the measurement sample was pressed onto the surface of a polypropylene plate (PP plate) washed with ethanol by making one reciprocation with a 2 kg roller. After leaving it in the same environment for 72 hours, the peel strength (relative to PP Adhesive force) [N/25mm] is measured. As the PP board, for example, the product name "Kobe Polysheet PP-N-AN" (thickness: 2 mm) manufactured by Showa Denko Materials Co., Ltd. is used.
[対PE粘着力]
23℃、50%RHの測定環境下において、粘着シート(両面粘着シート)の一方の粘着面に厚さ50μmのPETフィルムを貼り付けて裏打ちし、幅25mm、長さ100mmのサイズにカットして測定サンプルを作製する。23℃、50%RHの環境下にて、上記測定サンプルの他方の粘着面を、エタノールで洗浄したポリエチレン板(PE板)の表面に、2kgのローラを1往復させて圧着する。これを同環境下に72時間放置した後、万能引張圧縮試験機を使用して、JIS Z 0237:2000に準じて、引張速度300mm/分、剥離角度180度の条件で、剥離強度(対PE粘着力)[N/25mm]を測定する。PE板としては、例えば昭和電工マテリアルズ社製の製品名「コウベポリシート EL-N-AN」(厚さ2mm)が用いられる。 [Adhesive strength to PE]
Under a measurement environment of 23°C and 50% RH, one adhesive side of an adhesive sheet (double-sided adhesive sheet) was lined with a PET film with a thickness of 50 μm, and cut into a size of 25 mm in width and 100 mm in length. Prepare a measurement sample. In an environment of 23° C. and 50% RH, the other adhesive surface of the measurement sample was pressed onto the surface of a polyethylene board (PE board) washed with ethanol by making one reciprocation with a 2 kg roller. After leaving it in the same environment for 72 hours, the peel strength (relative to PE Adhesive force) [N/25mm] is measured. As the PE board, for example, the product name "Kobe Polysheet EL-N-AN" (thickness: 2 mm) manufactured by Showa Denko Materials Co., Ltd. is used.
23℃、50%RHの測定環境下において、粘着シート(両面粘着シート)の一方の粘着面に厚さ50μmのPETフィルムを貼り付けて裏打ちし、幅25mm、長さ100mmのサイズにカットして測定サンプルを作製する。23℃、50%RHの環境下にて、上記測定サンプルの他方の粘着面を、エタノールで洗浄したポリエチレン板(PE板)の表面に、2kgのローラを1往復させて圧着する。これを同環境下に72時間放置した後、万能引張圧縮試験機を使用して、JIS Z 0237:2000に準じて、引張速度300mm/分、剥離角度180度の条件で、剥離強度(対PE粘着力)[N/25mm]を測定する。PE板としては、例えば昭和電工マテリアルズ社製の製品名「コウベポリシート EL-N-AN」(厚さ2mm)が用いられる。 [Adhesive strength to PE]
Under a measurement environment of 23°C and 50% RH, one adhesive side of an adhesive sheet (double-sided adhesive sheet) was lined with a PET film with a thickness of 50 μm, and cut into a size of 25 mm in width and 100 mm in length. Prepare a measurement sample. In an environment of 23° C. and 50% RH, the other adhesive surface of the measurement sample was pressed onto the surface of a polyethylene board (PE board) washed with ethanol by making one reciprocation with a 2 kg roller. After leaving it in the same environment for 72 hours, the peel strength (relative to PE Adhesive force) [N/25mm] is measured. As the PE board, for example, the product name "Kobe Polysheet EL-N-AN" (thickness: 2 mm) manufactured by Showa Denko Materials Co., Ltd. is used.
上記各剥離強度の測定において、万能引張圧縮試験機としては、ミネベア社製の「引張圧縮試験機、TG-1kN」またはその相当品が用いられる。なお、片面粘着シートについて上記剥離強度測定を実施する場合、PETフィルムの裏打ちは不要である。基材厚さが薄い場合(例えば基材厚さ25μm以下の場合)は、PETフィルムの裏打ちをしてもよい。
In the above-mentioned peel strength measurements, the universal tensile compression tester used is Minebea's "Tensile Compression Tester, TG-1kN" or its equivalent. Note that when performing the above peel strength measurement on a single-sided adhesive sheet, backing with a PET film is not necessary. When the base material thickness is thin (for example, when the base material thickness is 25 μm or less), it may be lined with a PET film.
[保持力]
23℃、50%RHの環境下において、粘着シート(両面粘着シート)の一方の粘着面に厚さ50μmのPETフィルムを貼り付けて裏打ちし、幅10mm長さにカットして測定サンプルを作製する。その測定サンプルの他方の粘着面を、被着体としてのベークライト板(フェノール樹脂板)に、幅10mm、長さ20mmの貼付け面積(接着面積200mm2)にて、2kgのローラーを1往復させて圧着する。このようにして被着体に貼り付けたサンプルを同環境下に30分間放置した後、サンプルの長さ方向が鉛直方向となるように被着体を垂下し、該サンプルの自由端に1.5kgの荷重を付与し、JIS Z0237に準じて、該荷重が付与された状態で80℃の環境下に1時間放置する。1時間経過後、サンプルの上端の最初の貼付け位置からのズレ距離[mm]を測定する(1時間後のズレ距離)。測定は、各粘着シートにつき3つのサンプルを用いて行い(すなわちN=3)、それらの算術平均値を求める。上記ズレ距離が10mm以下であれば、合格と判定され、上記ズレ距離が10mm超あるいは1時間以内にサンプルが落下した場合は不合格と判定される。 [Holding power]
Under an environment of 23°C and 50% RH, a 50 μm thick PET film is pasted on one adhesive side of an adhesive sheet (double-sided adhesive sheet) for lining, and a measurement sample is prepared by cutting it into a length of 10 mm in width. . The other adhesive side of the measurement sample was attached to a Bakelite plate (phenolic resin plate) as an adherend, with a pasting area of 10 mm width and 20 mm length (adhesion area 200 mm 2 ), and a 2 kg roller was moved back and forth once. Crimp. After leaving the sample affixed to the adherend in this way in the same environment for 30 minutes, the adherend was hung so that the length direction of the sample was in the vertical direction, and 1. A load of 5 kg is applied to the product, and the product is left in an environment of 80° C. for 1 hour in accordance with JIS Z0237. After 1 hour has passed, the deviation distance [mm] of the upper end of the sample from the initial pasting position is measured (deviation distance after 1 hour). Measurements are performed using three samples for each adhesive sheet (ie, N=3), and their arithmetic mean value is determined. If the deviation distance is 10 mm or less, it is determined to pass, and if the deviation distance exceeds 10 mm or the sample falls within 1 hour, it is determined to be rejected.
23℃、50%RHの環境下において、粘着シート(両面粘着シート)の一方の粘着面に厚さ50μmのPETフィルムを貼り付けて裏打ちし、幅10mm長さにカットして測定サンプルを作製する。その測定サンプルの他方の粘着面を、被着体としてのベークライト板(フェノール樹脂板)に、幅10mm、長さ20mmの貼付け面積(接着面積200mm2)にて、2kgのローラーを1往復させて圧着する。このようにして被着体に貼り付けたサンプルを同環境下に30分間放置した後、サンプルの長さ方向が鉛直方向となるように被着体を垂下し、該サンプルの自由端に1.5kgの荷重を付与し、JIS Z0237に準じて、該荷重が付与された状態で80℃の環境下に1時間放置する。1時間経過後、サンプルの上端の最初の貼付け位置からのズレ距離[mm]を測定する(1時間後のズレ距離)。測定は、各粘着シートにつき3つのサンプルを用いて行い(すなわちN=3)、それらの算術平均値を求める。上記ズレ距離が10mm以下であれば、合格と判定され、上記ズレ距離が10mm超あるいは1時間以内にサンプルが落下した場合は不合格と判定される。 [Holding power]
Under an environment of 23°C and 50% RH, a 50 μm thick PET film is pasted on one adhesive side of an adhesive sheet (double-sided adhesive sheet) for lining, and a measurement sample is prepared by cutting it into a length of 10 mm in width. . The other adhesive side of the measurement sample was attached to a Bakelite plate (phenolic resin plate) as an adherend, with a pasting area of 10 mm width and 20 mm length (
<実施例1>
(アクリル系ポリマーの合成)
攪拌機、温度計、窒素ガス導入管、還流冷却器および滴下ロートを備えた反応容器に、モノマー成分としてのn-ヘプチルアクリレート(n-HpA)90部およびアクリル酸(AA)10部と、重合溶媒としての酢酸エチルとを仕込み、窒素ガスを導入しながら2時間撹拌した。このようにして重合系内の酸素を除去した後、重合開始剤として2,2’-アゾビスイソブチロニトリル(AIBN)0.2部を加え、60℃~70℃で8時間溶液重合してアクリル系ポリマー(A1)の溶液を得た。アクリル系ポリマー(A1)の重量平均分子量(Mw)は67万であった。なお、上記n-HpAは、バイオマス由来のヘプチルアルコールを用いて合成された、バイオマス由来のヘプチル基をエステル末端に有する化合物である。 <Example 1>
(Synthesis of acrylic polymer)
In a reaction vessel equipped with a stirrer, a thermometer, a nitrogen gas inlet tube, a reflux condenser, and a dropping funnel, 90 parts of n-heptyl acrylate (n-HpA) and 10 parts of acrylic acid (AA) as monomer components, and a polymerization solvent were added. and ethyl acetate were added thereto, and the mixture was stirred for 2 hours while introducing nitrogen gas. After removing oxygen in the polymerization system in this way, 0.2 part of 2,2'-azobisisobutyronitrile (AIBN) was added as a polymerization initiator, and solution polymerization was carried out at 60°C to 70°C for 8 hours. A solution of acrylic polymer (A1) was obtained. The weight average molecular weight (Mw) of the acrylic polymer (A1) was 670,000. Note that the above n-HpA is a compound having a biomass-derived heptyl group at the ester end, which was synthesized using biomass-derived heptyl alcohol.
(アクリル系ポリマーの合成)
攪拌機、温度計、窒素ガス導入管、還流冷却器および滴下ロートを備えた反応容器に、モノマー成分としてのn-ヘプチルアクリレート(n-HpA)90部およびアクリル酸(AA)10部と、重合溶媒としての酢酸エチルとを仕込み、窒素ガスを導入しながら2時間撹拌した。このようにして重合系内の酸素を除去した後、重合開始剤として2,2’-アゾビスイソブチロニトリル(AIBN)0.2部を加え、60℃~70℃で8時間溶液重合してアクリル系ポリマー(A1)の溶液を得た。アクリル系ポリマー(A1)の重量平均分子量(Mw)は67万であった。なお、上記n-HpAは、バイオマス由来のヘプチルアルコールを用いて合成された、バイオマス由来のヘプチル基をエステル末端に有する化合物である。 <Example 1>
(Synthesis of acrylic polymer)
In a reaction vessel equipped with a stirrer, a thermometer, a nitrogen gas inlet tube, a reflux condenser, and a dropping funnel, 90 parts of n-heptyl acrylate (n-HpA) and 10 parts of acrylic acid (AA) as monomer components, and a polymerization solvent were added. and ethyl acetate were added thereto, and the mixture was stirred for 2 hours while introducing nitrogen gas. After removing oxygen in the polymerization system in this way, 0.2 part of 2,2'-azobisisobutyronitrile (AIBN) was added as a polymerization initiator, and solution polymerization was carried out at 60°C to 70°C for 8 hours. A solution of acrylic polymer (A1) was obtained. The weight average molecular weight (Mw) of the acrylic polymer (A1) was 670,000. Note that the above n-HpA is a compound having a biomass-derived heptyl group at the ester end, which was synthesized using biomass-derived heptyl alcohol.
(粘着剤組成物の調製)
上記アクリル系ポリマー(A1)100部、ロジン系粘着付与樹脂(製品名「ハリタック SE10」、ハリマ化成社製、水添ロジングリセリンエステル、軟化点75~85℃、水酸基価25~40mgKOH/g)40部、イソシアネート系架橋剤(商品名「コロネートL」、トリメチロールプロパン/トリレンジイソシアネート3量体付加物の75%酢酸エチル溶液、東ソー社製)2部を撹拌混合して、本例に係る粘着剤組成物を調製した。 (Preparation of adhesive composition)
100 parts of the above acrylic polymer (A1), rosin tackifier resin (product name "Haritac SE10", manufactured by Harima Kasei Co., Ltd., hydrogenated rosin glycerin ester, softening point 75-85°C, hydroxyl value 25-40 mgKOH/g) 40 1 part, and 2 parts of an isocyanate crosslinking agent (trade name "Coronate L", 75% ethyl acetate solution of trimethylolpropane/tolylene diisocyanate trimer adduct, manufactured by Tosoh Corporation) were stirred and mixed to obtain the adhesive according to this example. A drug composition was prepared.
上記アクリル系ポリマー(A1)100部、ロジン系粘着付与樹脂(製品名「ハリタック SE10」、ハリマ化成社製、水添ロジングリセリンエステル、軟化点75~85℃、水酸基価25~40mgKOH/g)40部、イソシアネート系架橋剤(商品名「コロネートL」、トリメチロールプロパン/トリレンジイソシアネート3量体付加物の75%酢酸エチル溶液、東ソー社製)2部を撹拌混合して、本例に係る粘着剤組成物を調製した。 (Preparation of adhesive composition)
100 parts of the above acrylic polymer (A1), rosin tackifier resin (product name "Haritac SE10", manufactured by Harima Kasei Co., Ltd., hydrogenated rosin glycerin ester, softening point 75-85°C, hydroxyl value 25-40 mgKOH/g) 40 1 part, and 2 parts of an isocyanate crosslinking agent (trade name "Coronate L", 75% ethyl acetate solution of trimethylolpropane/tolylene diisocyanate trimer adduct, manufactured by Tosoh Corporation) were stirred and mixed to obtain the adhesive according to this example. A drug composition was prepared.
(粘着シートの作製)
得られた粘着剤組成物を、厚さ38μmのポリエステル製剥離フィルム(商品名「ダイアホイルMRF」、三菱ケミカル社製)の剥離面に塗布し、100℃で2分間乾燥させて、厚さ30μmの粘着剤層を形成した。この粘着剤層に、厚さ25μmのポリエステル製剥離フィルム(商品名「ダイアホイルMRF」、厚さ25μm、三菱ケミカル社製)の剥離面を貼り合わせた。このようにして、両面が上記2枚のポリエステル製剥離フィルムで保護された厚さ30μmの基材レス両面粘着シートを得た。 (Preparation of adhesive sheet)
The obtained adhesive composition was applied to the release surface of a polyester release film (trade name "Diafoil MRF", manufactured by Mitsubishi Chemical Corporation) with a thickness of 38 μm, and dried at 100° C. for 2 minutes to form a film with a thickness of 30 μm. An adhesive layer was formed. A release surface of a 25 μm thick polyester release film (trade name “Diafoil MRF”, 25 μm thick, manufactured by Mitsubishi Chemical Corporation) was attached to this adhesive layer. In this way, a substrate-less double-sided pressure-sensitive adhesive sheet having a thickness of 30 μm and having both sides protected by the two polyester release films was obtained.
得られた粘着剤組成物を、厚さ38μmのポリエステル製剥離フィルム(商品名「ダイアホイルMRF」、三菱ケミカル社製)の剥離面に塗布し、100℃で2分間乾燥させて、厚さ30μmの粘着剤層を形成した。この粘着剤層に、厚さ25μmのポリエステル製剥離フィルム(商品名「ダイアホイルMRF」、厚さ25μm、三菱ケミカル社製)の剥離面を貼り合わせた。このようにして、両面が上記2枚のポリエステル製剥離フィルムで保護された厚さ30μmの基材レス両面粘着シートを得た。 (Preparation of adhesive sheet)
The obtained adhesive composition was applied to the release surface of a polyester release film (trade name "Diafoil MRF", manufactured by Mitsubishi Chemical Corporation) with a thickness of 38 μm, and dried at 100° C. for 2 minutes to form a film with a thickness of 30 μm. An adhesive layer was formed. A release surface of a 25 μm thick polyester release film (trade name “Diafoil MRF”, 25 μm thick, manufactured by Mitsubishi Chemical Corporation) was attached to this adhesive layer. In this way, a substrate-less double-sided pressure-sensitive adhesive sheet having a thickness of 30 μm and having both sides protected by the two polyester release films was obtained.
<実施例2>
実施例1における粘着剤組成物の調製において、アクリル系ポリマー(A1)100部に対して、エポキシ系架橋剤(商品名「TETRAD-C」、三菱ガス化学社製、1,3-ビス(N,N-ジグリシジルアミノメチル)シクロへキサン)0.02部をさらに添加し、撹拌混合して、本例に係る粘着剤組成物を調製した。得られた粘着剤組成物を用いた他は実施例1と同様にして、本例に係る基材レス両面粘着シートを作製した。 <Example 2>
In the preparation of the adhesive composition in Example 1, an epoxy crosslinking agent (trade name "TETRAD-C", manufactured by Mitsubishi Gas Chemical Co., Ltd., 1,3-bis(N , N-diglycidylaminomethyl)cyclohexane) was further added and mixed with stirring to prepare a pressure-sensitive adhesive composition according to this example. A substrate-less double-sided pressure-sensitive adhesive sheet according to this example was produced in the same manner as in Example 1 except that the obtained pressure-sensitive adhesive composition was used.
実施例1における粘着剤組成物の調製において、アクリル系ポリマー(A1)100部に対して、エポキシ系架橋剤(商品名「TETRAD-C」、三菱ガス化学社製、1,3-ビス(N,N-ジグリシジルアミノメチル)シクロへキサン)0.02部をさらに添加し、撹拌混合して、本例に係る粘着剤組成物を調製した。得られた粘着剤組成物を用いた他は実施例1と同様にして、本例に係る基材レス両面粘着シートを作製した。 <Example 2>
In the preparation of the adhesive composition in Example 1, an epoxy crosslinking agent (trade name "TETRAD-C", manufactured by Mitsubishi Gas Chemical Co., Ltd., 1,3-bis(N , N-diglycidylaminomethyl)cyclohexane) was further added and mixed with stirring to prepare a pressure-sensitive adhesive composition according to this example. A substrate-less double-sided pressure-sensitive adhesive sheet according to this example was produced in the same manner as in Example 1 except that the obtained pressure-sensitive adhesive composition was used.
<実施例3~5>
モノマー組成をn-HpA94部およびAA6部に変更し、重合時のモノマー成分の濃度を調節した他はアクリル系ポリマー(A1)の合成と同様にして、アクリル系ポリマー(A2)の溶液を得た。上記アクリル系ポリマー(A2)を用いて、表1に示す組成に変更した他は実施例2と同様にして各例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて各例に係る基材レス両面粘着シートを作製した。 <Examples 3 to 5>
A solution of acrylic polymer (A2) was obtained in the same manner as the synthesis of acrylic polymer (A1), except that the monomer composition was changed to 94 parts of n-HpA and 6 parts of AA, and the concentration of monomer components during polymerization was adjusted. . Using the above acrylic polymer (A2), adhesive compositions according to each example were prepared in the same manner as in Example 2 except that the composition was changed to that shown in Table 1. A base material-less double-sided adhesive sheet according to the example was produced.
モノマー組成をn-HpA94部およびAA6部に変更し、重合時のモノマー成分の濃度を調節した他はアクリル系ポリマー(A1)の合成と同様にして、アクリル系ポリマー(A2)の溶液を得た。上記アクリル系ポリマー(A2)を用いて、表1に示す組成に変更した他は実施例2と同様にして各例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて各例に係る基材レス両面粘着シートを作製した。 <Examples 3 to 5>
A solution of acrylic polymer (A2) was obtained in the same manner as the synthesis of acrylic polymer (A1), except that the monomer composition was changed to 94 parts of n-HpA and 6 parts of AA, and the concentration of monomer components during polymerization was adjusted. . Using the above acrylic polymer (A2), adhesive compositions according to each example were prepared in the same manner as in Example 2 except that the composition was changed to that shown in Table 1. A base material-less double-sided adhesive sheet according to the example was produced.
<実施例6>
モノマー組成をn-HpA96部およびAA4部に変更した他はアクリル系ポリマー(A1)の合成と同様にして、アクリル系ポリマー(A3)の溶液を得た。上記アクリル系ポリマー(A3)を用いた他は実施例2と同様にして本例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて本例に係る基材レス両面粘着シートを作製した。 <Example 6>
A solution of acrylic polymer (A3) was obtained in the same manner as in the synthesis of acrylic polymer (A1) except that the monomer composition was changed to 96 parts of n-HpA and 4 parts of AA. Adhesive compositions according to this example were prepared in the same manner as in Example 2 except that the above acrylic polymer (A3) was used, and the adhesive compositions were used to form a base material-less double-sided adhesive sheet according to this example. was created.
モノマー組成をn-HpA96部およびAA4部に変更した他はアクリル系ポリマー(A1)の合成と同様にして、アクリル系ポリマー(A3)の溶液を得た。上記アクリル系ポリマー(A3)を用いた他は実施例2と同様にして本例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて本例に係る基材レス両面粘着シートを作製した。 <Example 6>
A solution of acrylic polymer (A3) was obtained in the same manner as in the synthesis of acrylic polymer (A1) except that the monomer composition was changed to 96 parts of n-HpA and 4 parts of AA. Adhesive compositions according to this example were prepared in the same manner as in Example 2 except that the above acrylic polymer (A3) was used, and the adhesive compositions were used to form a base material-less double-sided adhesive sheet according to this example. was created.
<実施例7~9>
粘着付与樹脂の種類を表1に示すように変更した他は実施例6と同様にして各例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて各例に係る基材レス両面粘着シートを作製した。具体的には、実施例7では、粘着付与樹脂として、実施例6の上記ロジン系粘着付与樹脂40部に替えて、上記ロジン系粘着付与樹脂25部およびテルペン系粘着付与樹脂(製品名「YSポリスターT-115」、ヤスハラケミカル社製、テルペンフェノール樹脂、軟化点約115℃、水酸基価30~60mgKOH/g)15部を使用し、実施例8では、粘着付与樹脂として、実施例6の上記ロジン系粘着付与樹脂40部に替えて、上記テルペン系粘着付与樹脂15部を使用し、実施例9では、上記ロジン系粘着付与樹脂の使用量を実施例6の40部から15部に変更した。 <Examples 7 to 9>
Adhesive compositions according to each example were prepared in the same manner as in Example 6, except that the type of tackifier resin was changed as shown in Table 1, and the adhesive composition was used to prepare a base material according to each example. A double-sided pressure-sensitive adhesive sheet was prepared. Specifically, in Example 7, 40 parts of the rosin-based tackifier resin of Example 6 was replaced with 25 parts of the rosin-based tackifier resin and a terpene-based tackifier resin (product name "YS In Example 8, the above rosin of Example 6 was used as the tackifying resin. In Example 9, the amount of the rosin tackifier resin used was changed from 40 parts in Example 6 to 15 parts.
粘着付与樹脂の種類を表1に示すように変更した他は実施例6と同様にして各例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて各例に係る基材レス両面粘着シートを作製した。具体的には、実施例7では、粘着付与樹脂として、実施例6の上記ロジン系粘着付与樹脂40部に替えて、上記ロジン系粘着付与樹脂25部およびテルペン系粘着付与樹脂(製品名「YSポリスターT-115」、ヤスハラケミカル社製、テルペンフェノール樹脂、軟化点約115℃、水酸基価30~60mgKOH/g)15部を使用し、実施例8では、粘着付与樹脂として、実施例6の上記ロジン系粘着付与樹脂40部に替えて、上記テルペン系粘着付与樹脂15部を使用し、実施例9では、上記ロジン系粘着付与樹脂の使用量を実施例6の40部から15部に変更した。 <Examples 7 to 9>
Adhesive compositions according to each example were prepared in the same manner as in Example 6, except that the type of tackifier resin was changed as shown in Table 1, and the adhesive composition was used to prepare a base material according to each example. A double-sided pressure-sensitive adhesive sheet was prepared. Specifically, in Example 7, 40 parts of the rosin-based tackifier resin of Example 6 was replaced with 25 parts of the rosin-based tackifier resin and a terpene-based tackifier resin (product name "YS In Example 8, the above rosin of Example 6 was used as the tackifying resin. In Example 9, the amount of the rosin tackifier resin used was changed from 40 parts in Example 6 to 15 parts.
<比較例1>
重合時のモノマー成分の濃度を調節した他はアクリル系ポリマー(A3)の合成と同様にして、アクリル系ポリマー(A3)よりも低分子量のアクリル系ポリマー(A4)の溶液を得た。上記アクリル系ポリマー(A4)を用いた他は実施例6と同様にして本例に係る粘着剤組成物を調製し、該粘着剤組成物を用いて本例に係る基材レス両面粘着シートを作製した。 <Comparative example 1>
A solution of an acrylic polymer (A4) having a lower molecular weight than the acrylic polymer (A3) was obtained in the same manner as the synthesis of the acrylic polymer (A3) except that the concentration of monomer components during polymerization was adjusted. A pressure-sensitive adhesive composition according to this example was prepared in the same manner as in Example 6 except that the above acrylic polymer (A4) was used, and a base material-less double-sided pressure-sensitive adhesive sheet according to this example was prepared using the pressure-sensitive adhesive composition. Created.
重合時のモノマー成分の濃度を調節した他はアクリル系ポリマー(A3)の合成と同様にして、アクリル系ポリマー(A3)よりも低分子量のアクリル系ポリマー(A4)の溶液を得た。上記アクリル系ポリマー(A4)を用いた他は実施例6と同様にして本例に係る粘着剤組成物を調製し、該粘着剤組成物を用いて本例に係る基材レス両面粘着シートを作製した。 <Comparative example 1>
A solution of an acrylic polymer (A4) having a lower molecular weight than the acrylic polymer (A3) was obtained in the same manner as the synthesis of the acrylic polymer (A3) except that the concentration of monomer components during polymerization was adjusted. A pressure-sensitive adhesive composition according to this example was prepared in the same manner as in Example 6 except that the above acrylic polymer (A4) was used, and a base material-less double-sided pressure-sensitive adhesive sheet according to this example was prepared using the pressure-sensitive adhesive composition. Created.
<比較例2~3>
モノマー組成をn-HpA97部およびAA3部に変更した他はアクリル系ポリマー(A1)の合成と同様にして、アクリル系ポリマー(A5)の溶液を得た。上記アクリル系ポリマー(A5)を用いて、表1に示す組成に変更した他は実施例2と同様にして各例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて各例に係る基材レス両面粘着シートを作製した。 <Comparative Examples 2-3>
A solution of acrylic polymer (A5) was obtained in the same manner as in the synthesis of acrylic polymer (A1) except that the monomer composition was changed to 97 parts of n-HpA and 3 parts of AA. Using the above acrylic polymer (A5), adhesive compositions according to each example were prepared in the same manner as in Example 2 except that the composition was changed to that shown in Table 1. A base material-less double-sided adhesive sheet according to the example was produced.
モノマー組成をn-HpA97部およびAA3部に変更した他はアクリル系ポリマー(A1)の合成と同様にして、アクリル系ポリマー(A5)の溶液を得た。上記アクリル系ポリマー(A5)を用いて、表1に示す組成に変更した他は実施例2と同様にして各例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて各例に係る基材レス両面粘着シートを作製した。 <Comparative Examples 2-3>
A solution of acrylic polymer (A5) was obtained in the same manner as in the synthesis of acrylic polymer (A1) except that the monomer composition was changed to 97 parts of n-HpA and 3 parts of AA. Using the above acrylic polymer (A5), adhesive compositions according to each example were prepared in the same manner as in Example 2 except that the composition was changed to that shown in Table 1. A base material-less double-sided adhesive sheet according to the example was produced.
<比較例4~5>
モノマー組成をn-ブチルアクリレート(BA)95部もしくは2-エチルヘキシルアクリレート(2EHA)95部およびAA5部に変更した他は基本的にアクリル系ポリマー(A1)の合成とそれぞれ同様にして、アクリル系ポリマー(A6)および(A7)の溶液を得た。上記アクリル系ポリマー(A6)または(A7)を用いた他は実施例2と同様にして各例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて各例に係る基材レス両面粘着シートを作製した。 <Comparative Examples 4-5>
The acrylic polymer was synthesized basically in the same manner as the acrylic polymer (A1) except that the monomer composition was changed to 95 parts of n-butyl acrylate (BA) or 95 parts of 2-ethylhexyl acrylate (2EHA) and 5 parts of AA. Solutions of (A6) and (A7) were obtained. Adhesive compositions according to each example were prepared in the same manner as in Example 2 except that the above acrylic polymer (A6) or (A7) was used, and the adhesive composition was used to create a base material according to each example. A double-sided pressure-sensitive adhesive sheet was prepared.
モノマー組成をn-ブチルアクリレート(BA)95部もしくは2-エチルヘキシルアクリレート(2EHA)95部およびAA5部に変更した他は基本的にアクリル系ポリマー(A1)の合成とそれぞれ同様にして、アクリル系ポリマー(A6)および(A7)の溶液を得た。上記アクリル系ポリマー(A6)または(A7)を用いた他は実施例2と同様にして各例に係る粘着剤組成物をそれぞれ調製し、該粘着剤組成物を用いて各例に係る基材レス両面粘着シートを作製した。 <Comparative Examples 4-5>
The acrylic polymer was synthesized basically in the same manner as the acrylic polymer (A1) except that the monomer composition was changed to 95 parts of n-butyl acrylate (BA) or 95 parts of 2-ethylhexyl acrylate (2EHA) and 5 parts of AA. Solutions of (A6) and (A7) were obtained. Adhesive compositions according to each example were prepared in the same manner as in Example 2 except that the above acrylic polymer (A6) or (A7) was used, and the adhesive composition was used to create a base material according to each example. A double-sided pressure-sensitive adhesive sheet was prepared.
各例の粘着シートの概要および評価結果を表1に示す。
Table 1 shows the outline and evaluation results of the pressure-sensitive adhesive sheets of each example.
表1に示されるように、実施例1~9に係る粘着剤は、モノマー成分としてn-ヘプチルアクリレートを含み、カルボキシ基含有モノマーを3重量%超含むMw60万超のアクリル系ポリマーを含有し、ゲル分率が70%未満であった。これらの例に係る粘着剤は、対SUS粘着力が15N/25mm以上、対PP粘着力が10N/25mm以上、対PE粘着力が5N/25mm以上であり、保持力試験の結果も合格(具体的にはズレ距離1mm以下)であった。より具体的には、カルボキシ基含有モノマー使用量が最も多かった実施例1~2では、低極性材料(PPおよびPE)に対して高い接着力を有し、かつ、対SUS粘着力が特に優れていた。また、実施例3~5の結果から、粘着付与樹脂を増量することにより、対SUS粘着力、対PP粘着力、対PE粘着力が改善され得ることがわかる。これらの例の結果から、粘着付与樹脂量が多くなるほど、粘着剤層のゲル分率は低下し、接着力が高くなる傾向にあることもわかる。一方、Mwが60万のアクリル系ポリマーを使用した比較例1では、保持力試験の結果が不合格であった。また、カルボキシ基含有モノマー(具体的にはAA)の共重合割合が3%のアクリル系ポリマーを使用した比較例2でも、保持力試験の結果が不合格であった。比較例2に対して架橋剤を増量した比較例3では、保持力を改善できたが、対SUS粘着力、対PP粘着力、対PE粘着力がいずれも大きく低下する結果となった。また、比較例2~3の結果から、ゲル分率が高くなりすぎると、粘着力特性を満足しにくくなることがわかる。さらに、n-ヘプチルアクリレート以外のアルキルアクリレート(BAまたは2EHA)を使用した比較例4~5では、実施例1~9レベルの粘着力を得ることができなかった。
As shown in Table 1, the adhesives according to Examples 1 to 9 contain n-heptyl acrylate as a monomer component, and contain an acrylic polymer having a Mw of more than 600,000 and containing more than 3% by weight of a carboxyl group-containing monomer, The gel fraction was less than 70%. The adhesives according to these examples have adhesive strength to SUS of 15 N/25 mm or more, PP adhesive strength of 10 N/25 mm or more, and PE adhesive strength of 5 N/25 mm or more, and also pass the holding power test (specifically In general, the deviation distance was 1 mm or less). More specifically, Examples 1 and 2, in which the amount of carboxyl group-containing monomer used was the largest, had high adhesive strength to low polar materials (PP and PE) and particularly excellent adhesive strength to SUS. was. Furthermore, the results of Examples 3 to 5 show that by increasing the amount of tackifier resin, the adhesive strength to SUS, the adhesive strength to PP, and the adhesive strength to PE can be improved. The results of these examples also show that as the amount of tackifier resin increases, the gel fraction of the adhesive layer tends to decrease and the adhesive strength tends to increase. On the other hand, in Comparative Example 1 in which an acrylic polymer having an Mw of 600,000 was used, the holding power test result was a failure. Furthermore, Comparative Example 2 in which an acrylic polymer with a copolymerization ratio of a carboxyl group-containing monomer (specifically, AA) was 3% also failed in the holding power test. In Comparative Example 3, in which the amount of crosslinking agent was increased compared to Comparative Example 2, the holding power was improved, but the adhesive strength to SUS, the adhesive strength to PP, and the adhesive strength to PE all decreased significantly. Furthermore, from the results of Comparative Examples 2 and 3, it can be seen that when the gel fraction becomes too high, it becomes difficult to satisfy the adhesive properties. Furthermore, in Comparative Examples 4 to 5 in which an alkyl acrylate (BA or 2EHA) other than n-heptyl acrylate was used, adhesive strength at the level of Examples 1 to 9 could not be obtained.
上記の結果から、モノマー成分としてn-ヘプチルアクリレートを含むアクリル系ポリマーを含み、ゲル分率70%未満の粘着剤を用いることで、高極性材料だけでなく、低極性材料に対しても十分な接着力が得られることがわかる。また、上記アクリル系ポリマーにカルボキシ基含有モノマーを3重量%超の割合で共重合し、Mwが60万よりも大きくなるよう設計することで、異種材料に対して高い接着力を有しつつ、保持力を向上できることがわかる。上記カルボキシ基含有モノマーの使用により、高極性材料に対する接着性も改善できることがわかる。すなわち、アクリル系ポリマーとして、n-ヘプチルアクリレートを含み、カルボキシ基含有モノマーを3重量%超含むモノマー成分の重合物を使用し、粘着剤層のゲル分率が70%未満であり、アクリル系ポリマーのMwが60万よりも大きい粘着剤によると、高極性材料に対する接着力、低極性材料に対する接着力、および、保持力を高いレベルで両立し得ることがわかる。
From the above results, we found that by using an adhesive that contains an acrylic polymer containing n-heptyl acrylate as a monomer component and has a gel fraction of less than 70%, it is effective against not only highly polar materials but also low polar materials. It can be seen that adhesive strength is obtained. In addition, by copolymerizing the above acrylic polymer with a carboxyl group-containing monomer at a ratio of more than 3% by weight and designing the Mw to be greater than 600,000, it has high adhesive strength to different materials. It can be seen that the holding force can be improved. It can be seen that the adhesion to highly polar materials can also be improved by using the above carboxy group-containing monomer. That is, as the acrylic polymer, a polymer of a monomer component containing n-heptyl acrylate and more than 3% by weight of a carboxyl group-containing monomer is used, the gel fraction of the adhesive layer is less than 70%, and the acrylic polymer It can be seen that adhesives with Mw greater than 600,000 can achieve both high levels of adhesion to highly polar materials, adhesion to low polar materials, and holding power.
以上、本発明の具体例を詳細に説明したが、これらは例示にすぎず、請求の範囲を限定するものではない。請求の範囲に記載の技術には、以上に例示した具体例を様々に変形、変更したものが含まれる。
Although specific examples of the present invention have been described above in detail, these are merely illustrative and do not limit the scope of the claims. The technology described in the claims includes various modifications and changes to the specific examples illustrated above.
1,2,3 粘着シート
10 支持基材
10A 第一面
10B 第二面(背面)
21 粘着剤層(第一粘着剤層)
21A 粘着面(第一粘着面)
21B 第二粘着面
22 粘着剤層(第二粘着剤層)
22A 粘着面(第二粘着面)
31,32 剥離ライナー
100,200,300 剥離ライナー付き粘着シート 1, 2, 3Adhesive sheet 10 Supporting base material 10A First side 10B Second side (back side)
21 Adhesive layer (first adhesive layer)
21A Adhesive surface (first adhesive surface)
21B Second adhesive surface 22 Adhesive layer (second adhesive layer)
22A Adhesive surface (second adhesive surface)
31, 32 Release liner 100, 200, 300 Adhesive sheet with release liner
10 支持基材
10A 第一面
10B 第二面(背面)
21 粘着剤層(第一粘着剤層)
21A 粘着面(第一粘着面)
21B 第二粘着面
22 粘着剤層(第二粘着剤層)
22A 粘着面(第二粘着面)
31,32 剥離ライナー
100,200,300 剥離ライナー付き粘着シート 1, 2, 3
21 Adhesive layer (first adhesive layer)
21A Adhesive surface (first adhesive surface)
21B Second adhesive surface 22 Adhesive layer (second adhesive layer)
22A Adhesive surface (second adhesive surface)
31, 32
Claims (10)
- アクリル系ポリマーを含む粘着剤層を有し、
前記アクリル系ポリマーは、n-ヘプチルアクリレートおよびカルボキシ基含有モノマーを含むモノマー成分の重合物であり、
前記アクリル系ポリマーのモノマー成分は、前記カルボキシ基含有モノマーを3重量%よりも多く含み、
前記粘着剤層のゲル分率は70%未満であり、
前記アクリル系ポリマーの重量平均分子量は60万よりも大きい、粘着シート。 Has an adhesive layer containing an acrylic polymer,
The acrylic polymer is a polymer of monomer components including n-heptyl acrylate and a carboxyl group-containing monomer,
The monomer component of the acrylic polymer contains more than 3% by weight of the carboxy group-containing monomer,
The adhesive layer has a gel fraction of less than 70%,
A pressure-sensitive adhesive sheet, wherein the acrylic polymer has a weight average molecular weight of more than 600,000. - 前記粘着剤層を形成するための粘着剤組成物は架橋剤を含む、請求項1に記載の粘着シート。 The adhesive sheet according to claim 1, wherein the adhesive composition for forming the adhesive layer contains a crosslinking agent.
- 前記粘着剤層は粘着付与樹脂を含む、請求項1または2に記載の粘着シート。 The pressure-sensitive adhesive sheet according to claim 1 or 2, wherein the pressure-sensitive adhesive layer contains a tackifying resin.
- 前記粘着剤層の厚さは0.1~500μmである、請求項1または2に記載の粘着シート。 The adhesive sheet according to claim 1 or 2, wherein the adhesive layer has a thickness of 0.1 to 500 μm.
- 前記粘着剤層のゲル分率は20%以上70%未満である、請求項1または2に記載の粘着シート。 The adhesive sheet according to claim 1 or 2, wherein the adhesive layer has a gel fraction of 20% or more and less than 70%.
- ステンレス鋼板に対する180度剥離強度が15N/25mm以上である、請求項1または2に記載の粘着シート。 The adhesive sheet according to claim 1 or 2, having a 180 degree peel strength of 15 N/25 mm or more against a stainless steel plate.
- ポリプロピレンに対する180度剥離強度が10N/25mm以上である、請求項1または2に記載の粘着シート。 The adhesive sheet according to claim 1 or 2, having a 180 degree peel strength against polypropylene of 10 N/25 mm or more.
- ポリエチレンに対する180度剥離強度が5N/25mm以上である、請求項1または2に記載の粘着シート。 The pressure-sensitive adhesive sheet according to claim 1 or 2, having a 180 degree peel strength against polyethylene of 5 N/25 mm or more.
- 80℃、接着面積10mm×20mm、荷重1.5kg、1時間の条件で実施される保持力試験におけるズレ距離が10mm以下である、請求項1または2に記載の粘着シート。 The adhesive sheet according to claim 1 or 2, wherein the adhesive sheet has a displacement distance of 10 mm or less in a holding force test conducted at 80° C., adhesive area 10 mm x 20 mm, load 1.5 kg, and for 1 hour.
- 電子機器において部材の固定に用いられる、請求項1または2に記載の粘着シート。 The adhesive sheet according to claim 1 or 2, which is used for fixing members in electronic equipment.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022-054421 | 2022-03-29 | ||
| JP2022054421 | 2022-03-29 | ||
| JP2022164655A JP7311695B1 (en) | 2022-03-29 | 2022-10-13 | Adhesive sheet |
| JP2022-164655 | 2022-10-13 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2023188482A1 true WO2023188482A1 (en) | 2023-10-05 |
Family
ID=87201300
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2022/038804 WO2023188482A1 (en) | 2022-03-29 | 2022-10-18 | Adhesive sheet |
Country Status (3)
| Country | Link |
|---|---|
| JP (2) | JP7311695B1 (en) |
| TW (1) | TW202346513A (en) |
| WO (1) | WO2023188482A1 (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020164844A (en) * | 2019-03-28 | 2020-10-08 | 積水化学工業株式会社 | Pressure-sensitive adhesive composition and adhesive tape |
| JP2020176202A (en) * | 2019-04-18 | 2020-10-29 | 東洋インキScホールディングス株式会社 | Solvent type pressure sensitive adhesive composition and pressure sensitive adhesive sheet |
| WO2020246351A1 (en) * | 2019-06-04 | 2020-12-10 | Dic株式会社 | Double-sided adhesive tape and electronic device |
| WO2021125278A1 (en) * | 2019-12-18 | 2021-06-24 | 積水化学工業株式会社 | Adhesive agent composition, adhesive tape, affixing method for electronic device component or in-vehicle component, and production method for electronic device component or in-vehicle component |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015003959A (en) * | 2013-06-19 | 2015-01-08 | 株式会社日本触媒 | Optical adhesive composition and optical adhesive product |
| JP6171673B2 (en) | 2013-07-25 | 2017-08-02 | Dic株式会社 | Adhesive sheet and speaker |
| JP7346887B2 (en) * | 2019-04-15 | 2023-09-20 | 三菱ケミカル株式会社 | Adhesive composition, adhesive, adhesive sheet, and laminate |
-
2022
- 2022-10-13 JP JP2022164655A patent/JP7311695B1/en active Active
- 2022-10-18 WO PCT/JP2022/038804 patent/WO2023188482A1/en unknown
-
2023
- 2023-03-09 TW TW112108785A patent/TW202346513A/en unknown
- 2023-05-15 JP JP2023079914A patent/JP2023147288A/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020164844A (en) * | 2019-03-28 | 2020-10-08 | 積水化学工業株式会社 | Pressure-sensitive adhesive composition and adhesive tape |
| JP2020176202A (en) * | 2019-04-18 | 2020-10-29 | 東洋インキScホールディングス株式会社 | Solvent type pressure sensitive adhesive composition and pressure sensitive adhesive sheet |
| WO2020246351A1 (en) * | 2019-06-04 | 2020-12-10 | Dic株式会社 | Double-sided adhesive tape and electronic device |
| WO2021125278A1 (en) * | 2019-12-18 | 2021-06-24 | 積水化学工業株式会社 | Adhesive agent composition, adhesive tape, affixing method for electronic device component or in-vehicle component, and production method for electronic device component or in-vehicle component |
Also Published As
| Publication number | Publication date |
|---|---|
| JP7311695B1 (en) | 2023-07-19 |
| JP2023147288A (en) | 2023-10-12 |
| JP2023147166A (en) | 2023-10-12 |
| TW202346513A (en) | 2023-12-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7175622B2 (en) | Acrylic pressure-sensitive adhesive composition and pressure-sensitive adhesive sheet | |
| JPWO2018092904A1 (en) | Adhesive sheet | |
| JP6849533B2 (en) | Adhesive sheet | |
| JPWO2020013168A1 (en) | Adhesive sheet | |
| JP7166052B2 (en) | Adhesive sheet | |
| WO2023188482A1 (en) | Adhesive sheet | |
| WO2024009529A1 (en) | Adhesive sheet | |
| JP7176153B2 (en) | Adhesive sheet | |
| JP7114685B2 (en) | Adhesive sheet | |
| JP7387824B1 (en) | adhesive sheet | |
| WO2024009531A1 (en) | Adhesive sheet | |
| WO2024057561A1 (en) | Adhesive sheet | |
| JP7321343B1 (en) | double-sided adhesive sheet | |
| WO2022054789A1 (en) | Adhesive sheet | |
| JP7321344B1 (en) | Adhesive sheet | |
| WO2024057558A1 (en) | Double-sided sticky sheet with release liners | |
| WO2023027091A1 (en) | Multilayer body | |
| CN118103470A (en) | Pressure-sensitive adhesive sheet | |
| JP2019070101A (en) | Acrylic adhesive composition and adhesive sheet |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22935613 Country of ref document: EP Kind code of ref document: A1 |