WO2019220676A1 - Electrode mixture, electrode mixture production method, electrode structure, electrode structure production method, and secondary battery - Google Patents
Electrode mixture, electrode mixture production method, electrode structure, electrode structure production method, and secondary battery Download PDFInfo
- Publication number
- WO2019220676A1 WO2019220676A1 PCT/JP2018/047007 JP2018047007W WO2019220676A1 WO 2019220676 A1 WO2019220676 A1 WO 2019220676A1 JP 2018047007 W JP2018047007 W JP 2018047007W WO 2019220676 A1 WO2019220676 A1 WO 2019220676A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- electrode mixture
- electrode
- vinylidene fluoride
- active material
- current collector
- Prior art date
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
- H01M4/0402—Methods of deposition of the material
- H01M4/0404—Methods of deposition of the material by coating on electrode collectors
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
- H01M4/0471—Processes of manufacture in general involving thermal treatment, e.g. firing, sintering, backing particulate active material, thermal decomposition, pyrolysis
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/131—Electrodes based on mixed oxides or hydroxides, or on mixtures of oxides or hydroxides, e.g. LiCoOx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/139—Processes of manufacture
- H01M4/1391—Processes of manufacture of electrodes based on mixed oxides or hydroxides, or on mixtures of oxides or hydroxides, e.g. LiCoOx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/52—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron
- H01M4/525—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron of mixed oxides or hydroxides containing iron, cobalt or nickel for inserting or intercalating light metals, e.g. LiNiO2, LiCoO2 or LiCoOxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/621—Binders
- H01M4/622—Binders being polymers
- H01M4/623—Binders being polymers fluorinated polymers
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to an electrode mixture, and more particularly to an electrode mixture for a lithium ion secondary battery.
- the electrode of the lithium ion secondary battery can be obtained, for example, as follows. First, a binder (binder) is mixed with an electrode active material and a powdered electrode material such as a conductive auxiliary agent added as necessary, and dissolved or dispersed in an appropriate solvent to form a slurry electrode mixture (hereinafter, (Also referred to as electrode mixture slurry). Subsequently, an electrode of a lithium ion secondary battery can be obtained by applying the obtained electrode mixture slurry on a current collector and evaporating the solvent to form a structure retained as an electrode mixture layer. .
- a technique for increasing the energy density in a lithium ion secondary battery a technique for increasing the charge / discharge capacity of the positive electrode active material itself in the electrode is used.
- a technique for increasing the charge / discharge capacity of the positive electrode active material for example, it is known to use a nickel-containing compound as the positive electrode active material. Further, it is known that the discharge capacity can be improved by using an electrode active material having a high nickel ratio.
- an electrode mixture for the purpose of suppressing gelation of the electrode mixture slurry has been developed so far.
- a negative electrode mixture for a non-aqueous electrolyte secondary battery containing a polar group-containing vinylidene fluoride polymer, a chlorine atom-containing vinylidene fluoride polymer, an electrode active material and an organic solvent.
- Patent Document 1 a lithium-containing composite oxide with a specific composition containing nickel as the positive electrode active material, and a polyvinylidene fluoride and vinylidene fluoride-chlorotrifluoroethylene copolymer as the positive electrode binder.
- Patent Document 2 a lithium-containing composite oxide with a specific composition containing nickel as the positive electrode active material, and a polyvinylidene fluoride and vinylidene fluoride-chlorotrifluoroethylene copolymer as the positive electrode binder.
- a vinylidene fluoride polymer containing a chlorine atom is used as a binder composition.
- chlorine compounds are chemically stable, if they are not treated properly, they have a large impact on the environmental impact such as dioxins. Therefore, in recent years, material designs that do not contain chlorine atoms have been required in various industries, and materials that do not contain chlorine atoms are also required in lithium ion secondary batteries.
- the present invention has been made in view of the above problems, and its purpose is to provide a novel electrode mixture in which gelation of slurry is suppressed even when an electrode active material having a high nickel content is used. There is to do.
- an electrode mixture according to the present invention contains an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector.
- the binder composition includes vinylidene fluoride and the following formula (1):
- R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms.
- Containing a copolymer with a monomer represented by The electrode active material has the following formula (2) Li 1 + x MO 2 (2) (X is a number satisfying ⁇ 0.15 ⁇ X ⁇ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
- the pH of the water when the lithium metal oxide is extracted with water is greater than 11.3.
- the method for producing an electrode mixture according to the present invention includes vinylidene fluoride and the following formula (1):
- R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms.
- the electrode active material has the following formula (2) Li 1 + x MO 2 (2) (X is a number satisfying ⁇ 0.15 ⁇ X ⁇ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
- the lithium metal oxide has a configuration in which the pH of the water when extracted with water is greater than 11.3.
- an aspect of the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector.
- the layer has a configuration that is a layer formed using the above-mentioned electrode mixture.
- the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector in order to solve the above-described problems.
- the electrode mixture layer is a layer containing a binder composition and an electrode active material, and the binder composition includes vinylidene fluoride and the following formula (1):
- the electrode active material contains a copolymer with a monomer represented by the following formula (2): Li 1 + x MO 2 (2) (X is a number satisfying ⁇ 0.15 ⁇ X ⁇ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.) And the pH of the water when the electrode mixture layer is extracted with water is greater than 11.3.
- the electrode mixture according to the present invention can provide a novel electrode mixture in which gelation of the slurry during storage is suppressed even when an electrode active material having a high nickel content is used.
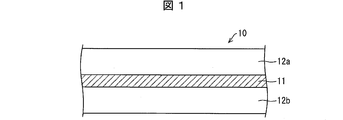
- FIG. 1 is an exploded perspective view of a secondary battery according to an embodiment of the present invention.
- the electrode mixture comprises an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector,
- the binder composition contains a specific vinylidene fluoride copolymer.
- the electrode active material includes a lithium metal oxide represented by the following formula (2), Li 1 + x MO 2 (2) (X is a number satisfying ⁇ 0.15 ⁇ X ⁇ 0.15.
- M is Ni or two or more element groups including Ni, and two or more element groups including Ni. In this case, Ni contains 55 mol% or more.)
- the pH of water when the lithium metal oxide is extracted with water is higher than 11.3.
- the binder composition in the present embodiment is used as a binder for binding an electrode active material onto a current collector.
- the binder composition contains a vinylidene fluoride copolymer that is a copolymer of vinylidene fluoride and a monomer represented by the following formula (1).
- R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms. Considering the influence on the environmental load, a hydrogen atom or an alkyl group having 1 to 6 carbon atoms is preferable. Further, from the viewpoint of the polymerization reaction, R 1 , R 2 or R 3 is desirably a substituent having a small steric hindrance, preferably hydrogen or an alkyl group having 1 to 3 carbon atoms, and preferably hydrogen or a methyl group. More preferred.
- the structural unit derived from the monomer represented by the formula (1) is preferably 0.40 to 10.00 mol%, and 0.50 to 7.00 mol. % Is more preferable, and 0.60 to 4.00 mol% is particularly preferable.
- the structural unit derived from vinylidene fluoride is preferably 90.0 to 99.6 mol%, more preferably 93.0 to 99.5 mol%, and 96.0 to 99.5 mol%. Particularly preferred.
- the structural unit derived from the monomer represented by the formula (1) is 0.40 mol% or more, it occupies in the electrode mixture slurry of the structural unit derived from the monomer represented by the formula (1).
- the ratio does not become too small, and the effect of suppressing the gelation of the electrode mixture slurry can be obtained.
- the structural unit derived from the monomer represented by the formula (1) is 10.00 mol% or less, the viscosity of the electrode mixture slurry does not become too high, and it becomes difficult to apply the electrode mixture slurry. Can be prevented.
- the vinylidene fluoride copolymer of the present embodiment it is possible to obtain an effect of suppressing gelation of the electrode mixture slurry even when stored for a longer time.
- the amount of the vinylidene fluoride unit of the vinylidene fluoride copolymer and the amount of the monomer unit represented by the formula (1) can be determined by 1 H NMR spectrum of the copolymer or neutralization titration. it can.
- the vinylidene fluoride copolymer in the present embodiment may have components of monomers other than vinylidene fluoride and the monomer represented by the formula (1).
- examples thereof include a fluorine monomer copolymerizable with vinylidene fluoride or a hydrocarbon monomer such as ethylene and propylene, or a monomer copolymerizable with the formula (1).
- examples of the fluorine-based monomer copolymerizable with vinylidene fluoride include perfluoroalkyl vinyl ethers typified by vinyl fluoride, trifluoroethylene, tetrafluoroethylene, chlorotrifluoroethylene, hexafluoropropylene, and perfluoromethyl vinyl ether. be able to.
- Examples of the monomer copolymerizable with the formula (1) include (meth) acrylic acid and alkyl (meth) acrylates represented by methyl (meth) acrylate.
- another monomer may be used individually by 1 type and may use 2 or more types.
- the vinylidene fluoride copolymer has the other monomer described above, it preferably has another monomer unit of 0.01 to 10 mol%.
- the vinylidene fluoride copolymer in the present embodiment can be obtained by polymerizing vinylidene fluoride and a monomer represented by the formula (1) by a conventionally known method.
- a conventionally known method for example, methods, such as suspension polymerization, emulsion polymerization, and solution polymerization, can be mentioned.
- the polymerization method is preferably aqueous suspension polymerization or emulsion polymerization in view of ease of post-treatment.
- the vinylidene fluoride and the monomer represented by the formula (1) used for the polymerization are already well-known compounds, and general commercial products may be used.
- the vinylidene fluoride copolymer in the present embodiment has a weight average molecular weight determined by measurement by GPC (gel permeation chromatography) in the range of 50,000 to 1,500,000.
- the inherent viscosity ⁇ i of the vinylidene fluoride copolymer in the present embodiment is preferably 0.5 dl / g to 5.0 dl / g, more preferably 1.0 dl / g to 4.5 dl / g. Preferably, it is 1.5 dl / g to 4.0 dl / g. If the inherent viscosity is within the above range, the deterioration of productivity due to a decrease in the electrode mixture slurry solid content is prevented, and the electrode can be easily produced without causing unevenness of the electrode thickness when the electrode mixture is applied. It is preferable in that it can be performed.
- the inherent viscosity ⁇ i can be obtained from the following equation by dissolving 80 mg of the polymer in 20 ml of N, N-dimethylformamide and using an Ubbelohde viscometer in a constant temperature bath at 30 ° C.
- ⁇ i (1 / C) ⁇ ln ( ⁇ / ⁇ 0 )
- ⁇ is the viscosity of the polymer solution
- ⁇ 0 is the viscosity of N, N-dimethylformamide as a solvent
- C is 0.4 g / dl.
- the binder composition according to the present embodiment may contain another vinylidene fluoride polymer as long as the required effect is not impaired.
- Other vinylidene fluoride-based polymers that can be included in the binder composition include vinylidene fluoride homopolymers, and fluoropolymers obtained by polymerizing vinylidene fluoride and other monomers copolymerizable with vinylidene fluoride. And vinylidene chloride copolymer.
- the other monomer here is a monomer not included in the monomer represented by the above formula (1).
- Examples of such other monomers include fluorine monomers copolymerizable with the above-mentioned vinylidene fluoride, hydrocarbon monomers such as ethylene and propylene, and alkyl (meth) acrylate compounds. It is done.
- a polar group containing compound may be sufficient.
- the compound containing a carboxyl group, an epoxy group, or a sulfonic acid group etc. is mentioned, for example, It is preferable that it is a compound containing a carboxyl group especially. Specific examples include 2-carboxyethyl acrylate, 2-carboxyethyl methacrylate, acryloyloxyethyl acrylic acid, and acryloyloxypropyl succinic acid.
- the proportion of the vinylidene fluoride copolymer containing the structural unit derived from the monomer represented by formula (1) in the entire polymer contained in the binder composition Is preferably 10% by weight or more, and more preferably 30% by weight or more. Further, the content of the structural unit derived from the monomer represented by the formula (1) in the whole polymer contained in the binder composition is preferably 0.10 mol% or more, and 0.20 mol% or more. More preferably, it is more preferably 0.30 mol% or more.
- the binder composition according to the present embodiment has been developed so that gelation of the electrode mixture slurry is suppressed even when a polymer that does not contain chlorine atoms is used in consideration of environmental load. It is. Therefore, it is desirable that the amount of chlorine in the binder composition in this embodiment is small, specifically, it is preferably 1000 ppm or less, more preferably 500 ppm or less, and particularly preferably 300 ppm.
- the amount of chlorine in the binder composition is in accordance with JIS K 7229.
- the binder composition is burned in an oxygen atmosphere in the flask, and the generated combustion gas is absorbed into the absorption liquid. It can be obtained by calculating the chlorine concentration by the method.
- the electrode active material in the present embodiment includes a lithium metal oxide represented by the following formula (2). Li 1 + x MO 2 (2) In the formula (2), X is a number satisfying ⁇ 0.15 ⁇ X ⁇ 0.15.
- M is Ni or two or more element groups including Ni.
- elements other than Ni included in M include, for example, Co, Mn, Ti, Cr, Fe, Cu, Zn, Al, Ge, Sn, Examples thereof include Mg, Ag, Ta, Nb, B, P, Zr, Ca, Sr and Ba. Of these, Co, Mn and Al are preferable.
- the element other than Ni included in M may be only one of these, or two or more. It is preferable that the lithium metal oxide contains Ni in that the capacity of the secondary battery can be increased by increasing the capacity density. Further, it is preferable that the lithium metal oxide further contains Co or the like in addition to Ni in that stable cycle characteristics are exhibited by suppressing changes in the crystal structure during the charge / discharge process.
- Ni in the lithium metal oxide represented by the formula (2) is a component that contributes to an increase in capacity in the electrode active material. Therefore, in the case where M is a group of two or more elements including Ni, when the total number of elements constituting M is 100 mol%, the proportion of Ni is preferably 55 mol% or more, and 60 mol% or more. Is preferable, and it is more preferable that it is 70 mol% or more.
- lithium metal oxide in the present embodiment for example, the following formula (3) LiNi Y1 N1 Y2 O 2 (3) (Wherein N1 represents Co or Mn, and 0.55 ⁇ Y1 ⁇ 1, 0 ⁇ Y2 ⁇ 0.55), or a binary lithium metal oxide represented by the following formula (4) LiNi Y1 Co Y2 N2 Y3 O 2 (4) (Wherein N2 represents Mn or Al, 0.55 ⁇ Y1 ⁇ 1, 0 ⁇ Y2 ⁇ 0.55, 0 ⁇ Y3 ⁇ 0.55, and Y1 / (Y1 + Y2 + Y3) ⁇ 0.55)
- the ternary lithium metal oxide is particularly preferably used as an electrode active material in this embodiment because it has a high charge potential and excellent cycle characteristics.
- composition of the binary lithium metal oxide in the present embodiment is not particularly limited, and examples thereof include Li 1.0 Ni 0.8 Co 0.2 O 2 .
- composition of the ternary lithium metal oxide in the present embodiment is not particularly limited, and for example, Li 1.00 Ni 0.6 Co 0.2 Mn 0.2 O 2 (NCM622) , Li 1.00 Ni 0.83 Co 0.12 Mn 0.05 O 2 (NCM811), and Li 1.00 Ni 0.85 Co 0.15 Al 0.05 O 2 (NCA811). .
- the electrode active material in the present embodiment may include a plurality of different types of lithium metal oxides.
- the electrode active material may include a plurality of LiNi Y1 Co Y2 Mn Y3 O 2 having different compositions, or LiNi Y1. Co Y2 Mn Y3 O 2 and LiNi Y1 Co Y2 Al Y3 O 2 and may contain.
- the lithium metal oxide in the present embodiment is prepared by adding 49 g of ultrapure water to 1 g of lithium metal oxide, stirring for 10 minutes, and then measuring the pH of the water to 11.3. Is more than The upper limit value of the pH of the water is not particularly limited. Generally, an alkaline substance adheres to the electrode active material added to the electrode mixture, and when the amount increases, the resulting electrode mixture slurry tends to gel. And the more the nickel content in the electrode active material, the more alkaline material that adheres. That is, the pH when extracted with water increases. Therefore, if a lithium metal oxide having a high nickel content is used as an electrode active material in order to increase the discharge capacity, the electrode mixture slurry is likely to gel. In the electrode mixture of the present embodiment, gelation of the electrode mixture slurry is suppressed even when such an electrode active material is used.
- the electrode active material may contain, for example, impurities and additives in addition to the lithium metal oxide represented by the formula (2). Also, the types of impurities and additives contained in the electrode active material are not particularly limited.
- the electrode mixture in the present embodiment may contain a solvent.
- the solvent may be water or a non-aqueous solvent.
- the non-aqueous solvent include N-methyl-2-pyrrolidone (NMP), dimethylformamide, N-dimethylformamide, N, N-dimethylacetamide, dimethyl sulfoxide, N, N-dimethyl sulfoxide, hexamethylphosphoamide, Examples include dioxane, tetrahydrofuran, tetramethylurea, triethyl phosphate, trimethyl phosphate, acetone, cyclohexane, methyl ethyl ketone, and tetrahydrofuran.
- One or more of these solvents may be contained in the electrode mixture.
- the solvent may be added to the binder composition, or may be added separately from the binder composition.
- the electrode mixture in the present embodiment may contain other components as necessary.
- other components include a conductive aid and a pigment dispersant.
- the conductive auxiliary agent is added for the purpose of improving the conductivity of the electrode mixture layer formed using the electrode mixture.
- the conductive assistant include graphites such as natural graphite (eg, scaly graphite), artificial graphite, graphite fine powder and graphite fiber, carbon blacks such as acetylene black, ketjen black, channel black and furnace black, carbon Examples include fibers and carbon materials such as carbon nano-nanotubes.
- conductive fibers such as metal fibers such as Ni and Al, metal powders, conductive metal oxides, and organic conductive materials are also included.
- examples of the pigment dispersant include polyvinyl pyrrolidone.
- the other components described above are 0 to 10 parts by weight, preferably 0 to 5 parts by weight with respect to 100 parts by weight of the electrode mixture.
- the electrode mixture of the present embodiment uses a vinylidene fluoride copolymer that does not contain chlorine atoms, the burden on the environment is reduced.
- gelation of the electrode mixture slurry can be suppressed even when an electrode active material having a high nickel content is used.
- gelation of the electrode mixture slurry is suppressed even when stored for a relatively long time.
- it can suppress that solid content, such as an electrode active material, settles and accumulates during a storage period. By suppressing sedimentation during the storage period, it is possible to prevent the solid content concentration from changing, thereby preventing an increase in the viscosity of the electrode mixture. As a result, it is possible to prevent the handling property when the electrode structure is manufactured from being lowered.
- the electrode mixture in the present embodiment is obtained by kneading vinylidene fluoride, a vinylidene fluoride copolymer obtained by copolymerizing the monomer represented by the formula (1), and an electrode active material. Can be manufactured.
- a solvent and other components may be kneaded as necessary, and the method is not particularly limited.
- the order of addition of various components at the time of kneading is not particularly limited.
- the electrode active material and the solvent may be first stirred and mixed, and then the vinylidene fluoride copolymer may be added.
- the electrode mixture in the present embodiment is manufactured by using a lithium metal oxide whose pH when extracted with water is greater than 11.3 as the lithium metal oxide contained in the electrode active material. It is what is done.
- the degree to which gelation of the electrode mixture slurry can be suppressed when the electrode mixture of the present invention is used can be determined by the slurry viscosity of the electrode mixture.
- “gelation” means, for example, when the electrode mixture slurry is stored at 40 ° C. in a nitrogen atmosphere for 96 hours, and then the electrode mixture slurry is stirred for 30 seconds using a mixer. The electrode mixture slurry does not become a uniform paste, and a solid is present, so that the slurry viscosity cannot be measured.
- a solid substance refers to the thing which passes a slurry through a mesh with an opening of 2.36 mm, and is left on the mesh after being left for 1 hour.
- the mixer is not particularly limited, and for example, Shintaro Awatori Nertaro ARE310 (autorotation 800 rpm, revolution 2000 rpm) manufactured by Shinky Co., Ltd. can be used.
- FIG. 1 is a cross-sectional view of an electrode structure according to an embodiment of the present invention.
- the electrode structure 10 includes a current collector 11 and electrode mixture layers 12a and 12b.
- the current collector 11 is a base material for the electrode structure 10 and is a terminal for taking out electricity.
- Examples of the material of the current collector 11 include iron, stainless steel, steel, aluminum, nickel, and titanium.
- the shape of the current collector 11 is preferably a foil or a net. In the present embodiment, the current collector 11 is preferably an aluminum foil.
- the thickness of the current collector 11 is preferably 5 to 100 ⁇ m, and more preferably 5 to 20 ⁇ m.
- the electrode mixture layers 12a and 12b are layers made of the electrode mixture of the present embodiment.
- the thickness of the electrode mixture layers 12a and 12b is 10 ⁇ m to 1000 ⁇ m, more preferably 20 ⁇ m to 250 ⁇ m, and still more preferably 20 ⁇ m to 150 ⁇ m.
- the electrode mixture layer in this embodiment is formed using the electrode mixture described above. Therefore, when the electrode mixture layer in this embodiment is extracted at room temperature (25 ° C.) by the extraction method specified in JIS K 5101-16-2, the pH of the water exceeds 11.3. ing. Specifically, the pH is measured by the same method as the above-described method for measuring the pH of a lithium metal oxide, except that the electrode mixture layer is peeled off from the current collector foil and used as a sample. It is.
- the electrode mixture layers 12 a and 12 b are formed on the upper and lower surfaces of the current collector 11 as shown in FIG. 1, but the present invention is not limited to this. What has the electrode mixture layer formed in any one surface, ie, the electrode structure in which any one of the electrode mixture layers 12a and 12b was formed may be sufficient.
- the electrode structure 10 can be used as a positive electrode of a lithium secondary battery, for example.
- a slurry-like electrode mixture (electrode mixture slurry) containing a vinylidene fluoride copolymer, a lithium metal oxide, and a solvent is applied to the surface of the current collector 11 and dried. By making it, it can obtain by passing through the process of forming a coating film in the collector 11 surface, and the process of heat-processing a coating film.
- a method for applying the electrode mixture slurry a known method can be used, and a method using a bar coater, a die coater, a comma coater (registered trademark), or the like can be given.
- the drying temperature for drying the electrode mixture slurry applied to the upper and lower surfaces of the current collector 11 can be 50 to 170 ° C., preferably 50 to 150 ° C.
- this embodiment demonstrated the method of forming an electrode mixture layer by apply
- the manufacturing method of the electrode structure of this embodiment is to this.
- the electrode mixture may be applied to at least one surface of the current collector.
- the secondary battery of the present embodiment is a nonaqueous electrolyte secondary battery including the electrode structure of the present embodiment.
- the secondary battery of this embodiment will be described with reference to FIG.
- FIG. 2 is an exploded perspective view of the secondary battery according to the present embodiment.
- the secondary battery 100 has a structure in which a power generating element in which a separator 3 is disposed and laminated between a positive electrode 1 and a negative electrode 2 and wound in a spiral shape is housed in a metal casing 5.
- the positive electrode 1 corresponds to the electrode structure 10 in FIG.
- a known material such as a porous film of a polymer material such as polypropylene and polyethylene may be used.
- a known material such as a porous film of a polymer material such as polypropylene and polyethylene may be used.
- the members used in the secondary battery 100 those normally used in this field can be appropriately used.
- the secondary battery 100 is a cylindrical battery, but of course, the secondary battery 100 in the present invention is not limited to this, and may be a secondary battery having another shape such as a coin shape, a square shape, or a paper shape. There may be.
- the electrode mixture of the present invention contains an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector.
- a copolymer of vinylidene fluoride and the monomer represented by the above formula (1) Contains a copolymer of vinylidene fluoride and the monomer represented by the above formula (1), and the electrode active material has the following formula (2) Li 1 + x MO 2 (2) (X is a number satisfying ⁇ 0.15 ⁇ X ⁇ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
- the pH of the water when the lithium metal oxide is extracted with water is greater than 11.3.
- the chlorine content in the binder composition is preferably 1000 ppm or less.
- the constituent unit derived from the monomer represented by the formula (1) in the copolymer is preferably 0.40 mol% or more.
- the binder composition includes a vinylidene fluoride polymer different from the copolymer.
- the electrode mixture of the present invention preferably contains a solvent.
- the method for producing an electrode mixture according to the present invention includes a step of kneading a copolymer of vinylidene fluoride, a monomer represented by the above formula (1), and an electrode active material,
- the active material contains a lithium metal oxide represented by the above formula (2), and the pH of the lithium metal oxide when extracted with water is greater than 11.3.
- One aspect of the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector, and the electrode mixture layer uses the electrode mixture described above. It is a layer formed.
- Another aspect of the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector, and the electrode mixture layer includes a binder composition and an electrode active layer.
- the binder composition contains a copolymer of vinylidene fluoride and a monomer represented by the above formula (1), and the electrode active material has the above formula ( The lithium metal oxide represented by 2) is included, and the pH of the water when the electrode mixture layer is extracted with water is greater than 11.3.
- the method for producing an electrode structure according to the present invention includes a step of forming a coating film on a surface of the current collector by applying the electrode mixture to the surface of the current collector and drying, and a heat treatment on the coating film. The process of giving.
- the secondary battery according to the present invention includes the electrode structure described above.
- an electrode mixture and an electrode structure were prepared using various binder compositions, and a confirmation test of viscosity change and solid content concentration change of the electrode mixture slurry was performed.
- a confirmation test of viscosity change and solid content concentration change of the electrode mixture slurry was performed.
- each method of measurement of pH of lithium metal oxide, calculation of slurry viscosity, and solid content concentration change test will be described.
- the pH of the lithium metal oxide as the electrode active material was the pH of water when the lithium metal oxide was extracted with water at room temperature (25 ° C.).
- the extraction of lithium metal oxide into water was performed by the extraction method defined in JIS K 5101-16-2. Specifically, the lithium metal oxide is put into ultrapure water 50 times the weight of the lithium metal oxide, and stirred with a magnetic stirrer at a rotation speed of 600 rpm for 10 minutes.
- the pH was measured using a pH meter MODEL: F-21 manufactured by Seisakusho.
- ⁇ i (1 / C) ⁇ ln ( ⁇ / ⁇ 0 )
- ⁇ is the viscosity of the polymer solution
- ⁇ 0 is the viscosity of the solvent N, N-dimethylformamide
- C is 0.4 g / dl.
- the slurry viscosity of the electrode mixture was measured at 25 ° C. and a shear rate of 2 s ⁇ 1 using an E-type viscometer RE-550 MODEL: R, RC-550 manufactured by Toki Sangyo Co., Ltd.
- the viscosity was measured by waiting 60 seconds after the slurry was charged into the measuring apparatus and then rotating the rotor. The value after 300 seconds from the start of rotation of the rotor was taken as the initial slurry viscosity.
- the slurry viscosity after being allowed to stand for a predetermined time (24 hours or 120 hours) in a nitrogen atmosphere at 25 ° C. was measured and used as the slurry viscosity after storage.
- the prepared electrode mixture slurry was poured into a polypropylene tube ( ⁇ 12 ⁇ 75 mm) to a height of 5 cm from the bottom of the tube, and stored in an environment of 25 ° C. and 20% RH for 24 hours. After storage, an electrode mixture slurry having a height of 1 cm from the bottom of the tube was collected, weighed in an aluminum cup, and the weight of the collected electrode mixture slurry was measured. The aluminum cup was heated at 110 ° C. for 2 hours to remove the solvent, and then weighed to measure the solid content of the collected electrode mixture slurry. It was calculated lower solids concentration (NV A) from the weight before and after drying obtained here.
- the ratio of the lower solid content concentration (NV A ) to the initial solid content concentration (NV B ) of the charged electrode mixture slurry (NV A / NV B ) was calculated as an index. This indicates that the larger the value of NV A / NV B, the easier the electrode active material sinks and accumulates in the lower part of the container during storage.
- Apparatus manufactured by Bruker. AVANCE AC 400FT NMR spectrum meter Measurement conditions Frequency: 400 MHz Measuring solvent: DMSO-d6 Measurement temperature: 25 ° C
- the amount of the structural unit derived from the vinylidene fluoride of the polymer and the amount of the structural unit derived from the comonomer were calculated from the 1 H NMR spectrum. Specifically, it was calculated based on the integrated intensity of signals mainly derived from comonomer and signals observed at 2.24 ppm and 2.87 ppm mainly derived from vinylidene fluoride.
- a comonomer having a structure derived from acrylic acid When a comonomer having a structure derived from acrylic acid is used as a comonomer, the amount of the structural unit containing a structure derived from acrylic acid in the polymer is neutralized with a sodium hydroxide aqueous solution of 0.03 mol / l. Determined by More specifically, a solution to be titrated was prepared by dissolving 0.3 g of a polymer in 9.7 g of acetone at about 80 ° C. and then adding 3 g of pure water. As an indicator, phenolphthalein was used, and neutralization titration was performed using a 0.03 mol / l aqueous sodium hydroxide solution at room temperature.
- Example 1 (Preparation of binder composition) Into an autoclave with an internal volume of 2 liters, each amount of 900 g of ion exchange water, 0.4 g of hydroxypropyl methylcellulose, 2 g of butyl peroxypivalate, 396 g of vinylidene fluoride, and 0.2 g of an initial addition amount of acrylic acid was charged to 50 ° C. Heated. A 1% by weight aqueous acrylic acid solution containing acrylic acid was continuously fed to the reaction vessel under the condition that the pressure was kept constant during the polymerization. The obtained polymer slurry was dehydrated and dried to obtain a vinylidene fluoride copolymer (VDF / AA). A total of 4 g of acrylic acid was added, including the amount added initially.
- VDF / AA vinylidene fluoride copolymer
- the vinylidene fluoride solution was prepared so that the solid content concentration was 84.2% by weight in the measurement of the solid content concentration change, and the solid content concentration was 81.5% by weight in the measurement of the viscosity change of the slurry.
- the mixture was added and primary kneading was performed at 2000 rpm for 2 minutes.
- the remaining vinylidene fluoride solution is added so that the solid content concentration is 72.0% by weight in the measurement of the solid content concentration change, and the solid content concentration is 75% by weight in the measurement of the viscosity change of the slurry.
- secondary kneading at 2000 rpm for 3 minutes to obtain an electrode mixture.
- the weight ratio of the electrode active material, carbon black, and vinylidene fluoride copolymer in the obtained electrode mixture was 100: 2: 1 in this order when measuring the solid content concentration change, and this ratio was measured when measuring the viscosity change of the slurry. In order, it is 100: 2: 2.
- Table 1 shows the composition of the electrode mixture.
- the obtained electrode mixture was applied on an aluminum foil having a thickness of 15 ⁇ m with a bar coater, and this was heat-dried at 110 ° C. for 30 minutes and further at 130 ° C. for 2 hours. An electrode structure of approximately 200 g / m 2 was produced.
- Example 2 A vinylidene fluoride copolymer (VDF / AA), a vinylidene fluoride copolymer (VDF / AA) which is a copolymer of vinylidene fluoride and acrylic acid, vinylidene fluoride and acryloyloxypropyl succinic acid, An electrode mixture was prepared in the same manner as in Example 1 except that it was changed to a blend with a vinylidene fluoride copolymer (VDF / APS) which is a copolymer of the above.
- the weight ratio of the vinylidene fluoride copolymer (VDF / AA) and the vinylidene fluoride copolymer (VDF / APS) is 5: 5.
- Example 2 The same vinylidene fluoride copolymer (VDF / AA) as in Example 1 was used as the vinylidene fluoride copolymer (VDF / AA) in this example.
- VDF / APS A vinylidene fluoride copolymer (VDF / APS) was prepared as follows. In an autoclave having an internal volume of 2 liters, 1096 g of ion-exchanged water, 0.2 g of Metroze 90SH-100 (manufactured by Shin-Etsu Chemical Co., Ltd.), 2.2 g of a 50 wt% diisopropyl peroxydicarbonate-fluorocarbon 225 cb, 426 g of vinylidene fluoride, and Each amount of 0.2 g of initial addition amount of acryloyloxypropyl succinic acid was charged, and the temperature was raised to 26 ° C. over 1 hour.
- the obtained electrode mixture was measured for slurry viscosity and solid content concentration change. The results are shown in Table 2.
- Example 3 Except that the vinylidene fluoride copolymer (VDF / AA) was changed to a blend of the vinylidene fluoride copolymer (VDF / AA) of Example 1 and a vinylidene fluoride homopolymer (PVDF), Example In the same manner as in Example 1, an electrode mixture was prepared to produce an electrode structure.
- the weight ratio of the vinylidene fluoride copolymer (VDF / AA) to the vinylidene fluoride homopolymer (PVDF) is 5: 5.
- the obtained electrode mixture was measured for slurry viscosity and solid content concentration change. The results are shown in Table 2.
- Example 1 An electrode mixture was prepared in the same manner as in Example 1 except that the vinylidene fluoride copolymer (VDF / AA) was changed to the vinylidene fluoride copolymer (VDF / APS) of Example 2, and an electrode structure was prepared. The body was made.
- the obtained electrode mixture was measured for slurry viscosity and solid content concentration change. The results are shown in Table 2.
- Example 2 The vinylidene fluoride copolymer (VDF / AA) was changed to the vinylidene fluoride copolymer (VDF / APS) of Example 2, and the electrode active material was Li 1.00 Ni 0.52 Co 0.20 Mn 0 An electrode mixture was prepared in the same manner as in Example 1 except that the material was changed to .30 O 2 (NCM523).
- VDF / CTFE A vinylidene fluoride copolymer (VDF / CTFE) was prepared as follows. An autoclave with an internal volume of 2 liters was charged with 1040 g of ion-exchanged water, 0.4 g of methylcellulose, 1.6 g of diisopropyl peroxydicarbonate, 2 g of ethyl acetate, g372 of vinylidene fluoride and 28 g of chlorotrifluoroethylene and suspended at 28 ° C. Polymerization was performed. After the polymerization is completed, the polymer slurry is dehydrated, the dehydrated polymer slurry is washed with water, the polymer slurry is dehydrated again, and then dried at 80 ° C. for 20 hours to obtain a vinylidene fluoride copolymer (VDF / CTFE). It was.
- the present invention can be used for a lithium ion secondary battery.
Abstract
Provided is an electrode mixture that is capable of minimizing gelling thereof in a slurry form. The electrode mixture according to the present invention comprises an electrode active material and a binder composition, wherein the binder composition comprises a copolymer between vinylidene fluoride and a monomer represented by formula (1), the electrode active material comprises a lithium metal oxide represented by Li1+xMO2, and, when water is used to extract the lithium metal oxide, the pH of said water is 11.3 or higher.
Description
本発明は、電極合剤に関し、さらに詳細には、リチウムイオン二次電池用の電極合剤に関する。
The present invention relates to an electrode mixture, and more particularly to an electrode mixture for a lithium ion secondary battery.
近年、電子技術の発展はめざましく、小型携帯機器の高機能化が進み、これらに使用される電源には小型および軽量化(高エネルギー密度化)が求められている。高いエネルギー密度を有する電池として、リチウムイオン二次電池等に代表される非水電解質二次電池が広く使用されている。
In recent years, the development of electronic technology has been remarkable, and advanced functions of small portable devices have progressed, and power sources used for these devices are required to be small and light (high energy density). As a battery having a high energy density, a nonaqueous electrolyte secondary battery represented by a lithium ion secondary battery or the like is widely used.
リチウムイオン二次電池の電極は、例えば、次のようにして得ることができる。まず、電極活物質および必要に応じて加えられる導電助剤等の粉末状電極材料にバインダー(結着剤)を混合し、適当な溶媒に溶解ないし分散してスラリー状の電極合剤(以下、電極合剤スラリーともいう)を得る。続いて、得られた電極合剤スラリーを集電体上に塗布し、溶媒を揮散して電極合剤層として保持された構造を形成することによりリチウムイオン二次電池の電極を得ることができる。
The electrode of the lithium ion secondary battery can be obtained, for example, as follows. First, a binder (binder) is mixed with an electrode active material and a powdered electrode material such as a conductive auxiliary agent added as necessary, and dissolved or dispersed in an appropriate solvent to form a slurry electrode mixture (hereinafter, (Also referred to as electrode mixture slurry). Subsequently, an electrode of a lithium ion secondary battery can be obtained by applying the obtained electrode mixture slurry on a current collector and evaporating the solvent to form a structure retained as an electrode mixture layer. .
リチウムイオン二次電池における高エネルギー密度化の手法として、電極における正極活物質自身の充放電容量を高める手法が用いられている。正極活物質の充放電容量を高める手法としては、例えば、正極活物質としてニッケル含有化合物を用いることが知られている。また、ニッケル比率を高めた電極活物質を用いることで、放電容量を向上させることができることが知られている。
As a technique for increasing the energy density in a lithium ion secondary battery, a technique for increasing the charge / discharge capacity of the positive electrode active material itself in the electrode is used. As a technique for increasing the charge / discharge capacity of the positive electrode active material, for example, it is known to use a nickel-containing compound as the positive electrode active material. Further, it is known that the discharge capacity can be improved by using an electrode active material having a high nickel ratio.
しかしながら電極活物質中のニッケル比率が高まると、電極合剤スラリーがゲル化しやすくなるという問題がある。
However, when the nickel ratio in the electrode active material is increased, there is a problem that the electrode mixture slurry is easily gelled.
そこで、電極合剤スラリーのゲル化の抑制を目的とした電極合剤がこれまでに開発されている。このような電極合剤の一例として、極性基含有フッ化ビニリデン系重合体、塩素原子含有フッ化ビニリデン系重合体、電極活物質および有機溶剤を含有する非水電解質二次電池用負極合剤が知られている(特許文献1)。また、正極活物質として、ニッケルを含有する特定組成のリチウム含有複合酸化物を使用し、正極のバインダーとして、ポリフッ化ビニリデンとフッ化ビニリデン-クロロトリフルオロエチレン共重合体とを含有するものも提案されている(例えば、特許文献2)。
Therefore, an electrode mixture for the purpose of suppressing gelation of the electrode mixture slurry has been developed so far. As an example of such an electrode mixture, there is a negative electrode mixture for a non-aqueous electrolyte secondary battery containing a polar group-containing vinylidene fluoride polymer, a chlorine atom-containing vinylidene fluoride polymer, an electrode active material and an organic solvent. Known (Patent Document 1). Also proposed is a lithium-containing composite oxide with a specific composition containing nickel as the positive electrode active material, and a polyvinylidene fluoride and vinylidene fluoride-chlorotrifluoroethylene copolymer as the positive electrode binder. (For example, Patent Document 2).
特許文献1および2に記載の何れの技術も、バインダー組成物として、塩素原子を含むフッ化ビニリデン系重合体を用いている。塩素化合物は化学的に安定ではあるものの、適切な処理がなされないとダイオキシン等、環境負荷への影響が大きい。そのため、近年、各種産業界において塩素原子を含まないような材料設計が求められており、リチウムイオン二次電池においても、塩素原子を含まない材料が求められる。
In any of the techniques described in Patent Documents 1 and 2, a vinylidene fluoride polymer containing a chlorine atom is used as a binder composition. Although chlorine compounds are chemically stable, if they are not treated properly, they have a large impact on the environmental impact such as dioxins. Therefore, in recent years, material designs that do not contain chlorine atoms have been required in various industries, and materials that do not contain chlorine atoms are also required in lithium ion secondary batteries.
そこで、本発明は上記の問題点に鑑みてなされたものであり、その目的は、ニッケル含量の高い電極活物質を用いていてもスラリーのゲル化が抑制された、新規な電極合剤を提供することにある。
Therefore, the present invention has been made in view of the above problems, and its purpose is to provide a novel electrode mixture in which gelation of slurry is suppressed even when an electrode active material having a high nickel content is used. There is to do.
本発明に係る電極合剤は、上記課題を解決するために、集電体上に設けられる電極活物質と、該電極活物質を該集電体に結着するためのバインダー組成物とを含有し、
上記バインダー組成物は、フッ化ビニリデンと、下記式(1) In order to solve the above problems, an electrode mixture according to the present invention contains an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector. And
The binder composition includes vinylidene fluoride and the following formula (1):
上記バインダー組成物は、フッ化ビニリデンと、下記式(1) In order to solve the above problems, an electrode mixture according to the present invention contains an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector. And
The binder composition includes vinylidene fluoride and the following formula (1):
(式中、R1、R2およびR3は、それぞれ独立に水素原子、塩素原子、フッ素原子、炭素数1~6のアルキル基または炭素原子1~6のフッ素置換アルキル基である。)
で表される単量体との共重合体を含有し、
上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、上記リチウム金属酸化物を水で抽出した際の該水のpHが11.3よりも大きいという構成を有している。 (Wherein R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms.)
Containing a copolymer with a monomer represented by
The electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
The pH of the water when the lithium metal oxide is extracted with water is greater than 11.3.
で表される単量体との共重合体を含有し、
上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、上記リチウム金属酸化物を水で抽出した際の該水のpHが11.3よりも大きいという構成を有している。 (Wherein R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms.)
Containing a copolymer with a monomer represented by
The electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
The pH of the water when the lithium metal oxide is extracted with water is greater than 11.3.
また、本発明に係る電極合剤の製造方法は、上記課題を解決するために、フッ化ビニリデンと、下記式(1)
In addition, in order to solve the above problems, the method for producing an electrode mixture according to the present invention includes vinylidene fluoride and the following formula (1):
(式中、R1、R2およびR3は、それぞれ独立に水素原子、塩素原子、フッ素原子、炭素数1~6のアルキル基または炭素原子1~6のフッ素置換アルキル基である。)
で表される単量体との共重合体と、電極活物質とを混練する工程を含み、
上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、上記リチウム金属酸化物は、水で抽出した際の該水のpHが11.3よりも大きいものであるという構成を有している。 (Wherein R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms.)
A step of kneading a copolymer with a monomer represented by: and an electrode active material,
The electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
The lithium metal oxide has a configuration in which the pH of the water when extracted with water is greater than 11.3.
で表される単量体との共重合体と、電極活物質とを混練する工程を含み、
上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、上記リチウム金属酸化物は、水で抽出した際の該水のpHが11.3よりも大きいものであるという構成を有している。 (Wherein R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms.)
A step of kneading a copolymer with a monomer represented by: and an electrode active material,
The electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
The lithium metal oxide has a configuration in which the pH of the water when extracted with water is greater than 11.3.
また、本発明に係る電極構造体の一態様は、上記課題を解決するために、集電体と、該集電体上に設けられた電極合剤層とを備えており、上記電極合剤層は、上述の電極合剤を用いて形成された層であるという構成を有している。
Moreover, in order to solve the above problems, an aspect of the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector. The layer has a configuration that is a layer formed using the above-mentioned electrode mixture.
また、本発明に係る電極構造体の別の態様は、上記課題を解決するために、集電体と、該集電体上に設けられた電極合剤層とを備えており、
上記電極合剤層は、バインダー組成物および電極活物質を含む層であり、上記バインダー組成物は、フッ化ビニリデンと、下記式(1) Further, another aspect of the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector in order to solve the above-described problems.
The electrode mixture layer is a layer containing a binder composition and an electrode active material, and the binder composition includes vinylidene fluoride and the following formula (1):
上記電極合剤層は、バインダー組成物および電極活物質を含む層であり、上記バインダー組成物は、フッ化ビニリデンと、下記式(1) Further, another aspect of the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector in order to solve the above-described problems.
The electrode mixture layer is a layer containing a binder composition and an electrode active material, and the binder composition includes vinylidene fluoride and the following formula (1):
(式中、R1、R2およびR3は、それぞれ独立に水素原子、塩素原子、フッ素原子、炭素数1~6のアルキル基または炭素原子1~6のフッ素置換アルキル基である。)
で表される単量体との共重合体を含有し、上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、上記電極合剤層を水で抽出した際の該水のpHが11.3よりも大きいという構成を有している。 (Wherein R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms.)
The electrode active material contains a copolymer with a monomer represented by the following formula (2):
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
And the pH of the water when the electrode mixture layer is extracted with water is greater than 11.3.
で表される単量体との共重合体を含有し、上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、上記電極合剤層を水で抽出した際の該水のpHが11.3よりも大きいという構成を有している。 (Wherein R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms.)
The electrode active material contains a copolymer with a monomer represented by the following formula (2):
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
And the pH of the water when the electrode mixture layer is extracted with water is greater than 11.3.
本発明に係る電極合剤によれば、ニッケル含量の高い電極活物質を用いていても保存時のスラリーのゲル化が抑制された、新規な電極合剤を提供することができる。
The electrode mixture according to the present invention can provide a novel electrode mixture in which gelation of the slurry during storage is suppressed even when an electrode active material having a high nickel content is used.
本発明に係る電極合剤、およびその利用の一実施形態について説明する。なお、本明細書において「~」とは、その前後に記載される数値を下限値および上限値として含む意味で使用される。
The electrode mixture according to the present invention and an embodiment thereof will be described. In the present specification, “to” is used to mean that the numerical values described before and after it are included as a lower limit value and an upper limit value.
〔電極合剤〕
本実施形態に係る電極合剤は、集電体上に設けられる電極活物質と、該電極活物質を該集電体に結着するためのバインダー組成物とを含有してなるものであり、バインダー組成物は、特定のフッ化ビニリデン共重合体を含有する。また、電極活物質は、下記式(2)で表されるリチウム金属酸化物を含むものであり、
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)当該リチウム金属酸化物を水で抽出した際の水のpHが11.3よりも大きいものである。 [Electrode mixture]
The electrode mixture according to the present embodiment comprises an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector, The binder composition contains a specific vinylidene fluoride copolymer. The electrode active material includes a lithium metal oxide represented by the following formula (2),
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is Ni or two or more element groups including Ni, and two or more element groups including Ni. In this case, Ni contains 55 mol% or more.) The pH of water when the lithium metal oxide is extracted with water is higher than 11.3.
本実施形態に係る電極合剤は、集電体上に設けられる電極活物質と、該電極活物質を該集電体に結着するためのバインダー組成物とを含有してなるものであり、バインダー組成物は、特定のフッ化ビニリデン共重合体を含有する。また、電極活物質は、下記式(2)で表されるリチウム金属酸化物を含むものであり、
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)当該リチウム金属酸化物を水で抽出した際の水のpHが11.3よりも大きいものである。 [Electrode mixture]
The electrode mixture according to the present embodiment comprises an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector, The binder composition contains a specific vinylidene fluoride copolymer. The electrode active material includes a lithium metal oxide represented by the following formula (2),
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is Ni or two or more element groups including Ni, and two or more element groups including Ni. In this case, Ni contains 55 mol% or more.) The pH of water when the lithium metal oxide is extracted with water is higher than 11.3.
(バインダー組成物)
本実施形態におけるバインダー組成物は、電極活物質を集電体上に結着するための結着剤として用いられるものである。バインダー組成物は、フッ化ビニリデンと、下記式(1)で表される単量体との共重合体であるフッ化ビニリデン共重合体を含有するものである。 (Binder composition)
The binder composition in the present embodiment is used as a binder for binding an electrode active material onto a current collector. The binder composition contains a vinylidene fluoride copolymer that is a copolymer of vinylidene fluoride and a monomer represented by the following formula (1).
本実施形態におけるバインダー組成物は、電極活物質を集電体上に結着するための結着剤として用いられるものである。バインダー組成物は、フッ化ビニリデンと、下記式(1)で表される単量体との共重合体であるフッ化ビニリデン共重合体を含有するものである。 (Binder composition)
The binder composition in the present embodiment is used as a binder for binding an electrode active material onto a current collector. The binder composition contains a vinylidene fluoride copolymer that is a copolymer of vinylidene fluoride and a monomer represented by the following formula (1).
式(1)において、R1、R2およびR3は、それぞれ独立に水素原子、塩素原子、フッ素原子、炭素数1~6のアルキル基または炭素原子1~6のフッ素置換アルキル基である。環境負荷への影響を考慮すれば、水素原子または炭素数1~6のアルキル基であることが好ましい。さらに重合反応の観点から、R1、R2またはR3は立体障害の小さな置換基であることが望まれ、水素または炭素数1~3のアルキル基が好ましく、水素またはメチル基であることがより好ましい。
In the formula (1), R 1 , R 2 and R 3 are each independently a hydrogen atom, a chlorine atom, a fluorine atom, an alkyl group having 1 to 6 carbon atoms or a fluorine-substituted alkyl group having 1 to 6 carbon atoms. Considering the influence on the environmental load, a hydrogen atom or an alkyl group having 1 to 6 carbon atoms is preferable. Further, from the viewpoint of the polymerization reaction, R 1 , R 2 or R 3 is desirably a substituent having a small steric hindrance, preferably hydrogen or an alkyl group having 1 to 3 carbon atoms, and preferably hydrogen or a methyl group. More preferred.
本実施形態におけるフッ化ビニリデン共重合体は、式(1)で表される単量体に由来する構成単位が0.40~10.00mol%であることが好ましく、0.50~7.00mol%であることがより好ましく、0.60~4.00mol%であることが特に好ましい。また、フッ化ビニリデンに由来する構成単位を、90.0~99.6mol%有することが好ましく、93.0~99.5mol%有することがより好ましく、96.0~99.5mol%有することが特に好ましい。式(1)で表される単量体に由来する構成単位が0.40mol%以上である場合、式(1)で表される単量体に由来する構成単位の電極合剤スラリー内で占める割合が小さくなり過ぎず、電極合剤スラリーのゲル化を抑制する効果を得ることができる。また、式(1)で表される単量体に由来する構成単位が10.00mol%以下である場合、電極合剤スラリーの粘度が高くなり過ぎず、電極合剤スラリーの塗工が困難になることを防ぐことができる。とりわけ、本実施形態のフッ化ビニリデン共重合体を用いることにより、より長い時間保存していた場合でも、電極合剤スラリーのゲル化を抑制する効果を得ることができる。
In the vinylidene fluoride copolymer in the present embodiment, the structural unit derived from the monomer represented by the formula (1) is preferably 0.40 to 10.00 mol%, and 0.50 to 7.00 mol. % Is more preferable, and 0.60 to 4.00 mol% is particularly preferable. In addition, the structural unit derived from vinylidene fluoride is preferably 90.0 to 99.6 mol%, more preferably 93.0 to 99.5 mol%, and 96.0 to 99.5 mol%. Particularly preferred. When the structural unit derived from the monomer represented by the formula (1) is 0.40 mol% or more, it occupies in the electrode mixture slurry of the structural unit derived from the monomer represented by the formula (1). The ratio does not become too small, and the effect of suppressing the gelation of the electrode mixture slurry can be obtained. Moreover, when the structural unit derived from the monomer represented by the formula (1) is 10.00 mol% or less, the viscosity of the electrode mixture slurry does not become too high, and it becomes difficult to apply the electrode mixture slurry. Can be prevented. In particular, by using the vinylidene fluoride copolymer of the present embodiment, it is possible to obtain an effect of suppressing gelation of the electrode mixture slurry even when stored for a longer time.
なお、フッ化ビニリデン共重合体のフッ化ビニリデン単位の量、および式(1)で表される単量体単位の量は、共重合体の1H NMRスペクトル、または中和滴定により求めることができる。
The amount of the vinylidene fluoride unit of the vinylidene fluoride copolymer and the amount of the monomer unit represented by the formula (1) can be determined by 1 H NMR spectrum of the copolymer or neutralization titration. it can.
本実施形態におけるフッ化ビニリデン共重合体は、フッ化ビニリデンおよび式(1)で表される単量体以外の他の単量体の成分を有していてもよい。例えば、フッ化ビニリデンと共重合可能なフッ素系単量体もしくはエチレンおよびプロピレン等の炭化水素系単量体、または、式(1)と共重合可能な単量体が挙げられる。フッ化ビニリデンと共重合可能なフッ素系単量体としては、フッ化ビニル、トリフルオロエチレン、テトラフルオロエチレン、クロロトリフルオロエチレン、ヘキサフルオロプロピレン、ペルフルオロメチルビニルエーテルに代表されるペルフルオロアルキルビニルエーテル等を挙げることができる。式(1)と共重合可能な単量体としては、(メタ)アクリル酸および(メタ)アクリル酸メチルに代表される(メタ)アクリル酸アルキル化合物等が挙げられる。なお、他の単量体は、1種単独で用いてもよく、2種以上を用いてもよい。
The vinylidene fluoride copolymer in the present embodiment may have components of monomers other than vinylidene fluoride and the monomer represented by the formula (1). Examples thereof include a fluorine monomer copolymerizable with vinylidene fluoride or a hydrocarbon monomer such as ethylene and propylene, or a monomer copolymerizable with the formula (1). Examples of the fluorine-based monomer copolymerizable with vinylidene fluoride include perfluoroalkyl vinyl ethers typified by vinyl fluoride, trifluoroethylene, tetrafluoroethylene, chlorotrifluoroethylene, hexafluoropropylene, and perfluoromethyl vinyl ether. be able to. Examples of the monomer copolymerizable with the formula (1) include (meth) acrylic acid and alkyl (meth) acrylates represented by methyl (meth) acrylate. In addition, another monomer may be used individually by 1 type and may use 2 or more types.
フッ化ビニリデン共重合体が上述した他の単量体を有する場合には、他の単量体単位を0.01~10mol%有することが好ましい。
When the vinylidene fluoride copolymer has the other monomer described above, it preferably has another monomer unit of 0.01 to 10 mol%.
本実施形態におけるフッ化ビニリデン共重合体は、フッ化ビニリデンと、式(1)で表される単量体とを従来公知の方法で重合させることによって得ることができる。重合方法については、特に限定されるものではないが、例えば、懸濁重合、乳化重合および溶液重合等の方法を挙げることができる。これらの中でも、後処理の容易さ等から、重合方法は、水系の懸濁重合または乳化重合であることが好ましい。また、重合に用いるフッ化ビニリデンおよび式(1)で表される単量体は、それぞれ既に周知の化合物であり、一般の市販品を用いてもよい。
The vinylidene fluoride copolymer in the present embodiment can be obtained by polymerizing vinylidene fluoride and a monomer represented by the formula (1) by a conventionally known method. Although it does not specifically limit about the polymerization method, For example, methods, such as suspension polymerization, emulsion polymerization, and solution polymerization, can be mentioned. Among these, the polymerization method is preferably aqueous suspension polymerization or emulsion polymerization in view of ease of post-treatment. The vinylidene fluoride and the monomer represented by the formula (1) used for the polymerization are already well-known compounds, and general commercial products may be used.
本実施形態におけるフッ化ビニリデン共重合体は、GPC(ゲルパミエーションクロマトグラフィー)で測定して求めた重量平均分子量が、5万から150万の範囲内である。
The vinylidene fluoride copolymer in the present embodiment has a weight average molecular weight determined by measurement by GPC (gel permeation chromatography) in the range of 50,000 to 1,500,000.
本実施形態におけるフッ化ビニリデン共重合体のインヘレント粘度ηiは、0.5dl/g~5.0dl/gであることが好ましく、1.0dl/g~4.5dl/gであることがより好ましく、1.5dl/g~4.0dl/gであることがさらに好ましい。インヘレント粘度が上記の範囲内であれば、電極合剤スラリー固形分低下による生産性の悪化を防ぎ、電極合剤を塗工する際に電極の厚みムラを発生させることなく、電極作製を容易に行える点で好ましい。インヘレント粘度ηiは、重合体80mgを20mlのN,N-ジメチルホルムアミドに溶解して、30℃の恒温槽内でウベローデ粘度計を用いて次式により求めることができる。
The inherent viscosity η i of the vinylidene fluoride copolymer in the present embodiment is preferably 0.5 dl / g to 5.0 dl / g, more preferably 1.0 dl / g to 4.5 dl / g. Preferably, it is 1.5 dl / g to 4.0 dl / g. If the inherent viscosity is within the above range, the deterioration of productivity due to a decrease in the electrode mixture slurry solid content is prevented, and the electrode can be easily produced without causing unevenness of the electrode thickness when the electrode mixture is applied. It is preferable in that it can be performed. The inherent viscosity η i can be obtained from the following equation by dissolving 80 mg of the polymer in 20 ml of N, N-dimethylformamide and using an Ubbelohde viscometer in a constant temperature bath at 30 ° C.
ηi=(1/C)・ln(η/η0)
上記式においてηは重合体溶液の粘度、η0は溶媒であるN,N-ジメチルホルムアミドの粘度、Cは0.4g/dlである。 η i = (1 / C) · ln (η / η 0 )
In the above formula, η is the viscosity of the polymer solution, η 0 is the viscosity of N, N-dimethylformamide as a solvent, and C is 0.4 g / dl.
上記式においてηは重合体溶液の粘度、η0は溶媒であるN,N-ジメチルホルムアミドの粘度、Cは0.4g/dlである。 η i = (1 / C) · ln (η / η 0 )
In the above formula, η is the viscosity of the polymer solution, η 0 is the viscosity of N, N-dimethylformamide as a solvent, and C is 0.4 g / dl.
本実施形態に係るバインダー組成物は、求められる効果を損なわない限り、他のフッ化ビニリデン系重合体を含んでいてもよい。バインダー組成物に含め得る他のフッ化ビニリデン系重合体としては、フッ化ビニリデン単独重合体、およびフッ化ビニリデンと、フッ化ビニリデンと共重合可能な他の単量体と、を重合させたフッ化ビニリデン共重合体が挙げられる。なお、ここでの他の単量体とは、上記式(1)で表される単量体には包含されない単量体である。このような他の単量体としては、上述したフッ化ビニリデンと共重合可能なフッ素系単量体、エチレンおよびプロピレン等の炭化水素系単量体、および(メタ)アクリル酸アルキル化合物等が挙げられる。また、他の単量体としては、極性基含有化合物であってもよい。極性基含有化合物としては、例えば、カルボキシル基、エポキシ基、またはスルホン酸基等を含む化合物が挙げられ、中でもカルボキシル基を含む化合物であることが好ましい。具体的には、例えば、2-カルボキシエチルアクリレート、2-カルボキシエチルメタクリレート、アクリロイロキシエチルアクリル酸、およびアクリロイロキシプロピルコハク酸等を挙げることができる。
The binder composition according to the present embodiment may contain another vinylidene fluoride polymer as long as the required effect is not impaired. Other vinylidene fluoride-based polymers that can be included in the binder composition include vinylidene fluoride homopolymers, and fluoropolymers obtained by polymerizing vinylidene fluoride and other monomers copolymerizable with vinylidene fluoride. And vinylidene chloride copolymer. In addition, the other monomer here is a monomer not included in the monomer represented by the above formula (1). Examples of such other monomers include fluorine monomers copolymerizable with the above-mentioned vinylidene fluoride, hydrocarbon monomers such as ethylene and propylene, and alkyl (meth) acrylate compounds. It is done. Moreover, as another monomer, a polar group containing compound may be sufficient. As a polar group containing compound, the compound containing a carboxyl group, an epoxy group, or a sulfonic acid group etc. is mentioned, for example, It is preferable that it is a compound containing a carboxyl group especially. Specific examples include 2-carboxyethyl acrylate, 2-carboxyethyl methacrylate, acryloyloxyethyl acrylic acid, and acryloyloxypropyl succinic acid.
他のフッ化ビニリデン系重合体を含む場合、バインダー組成物に含まれる重合体全体に占める、式(1)で表される単量体に由来する構成単位を含むフッ化ビニリデン共重合体の割合は、10重量%以上であることが好ましく、30重量%以上であることがより好ましい。また、バインダー組成物に含まれる重合体全体における、式(1)で表される単量体に由来する構成単位の含有量が0.10mol%以上であることが好ましく、0.20mol%以上であることがより好ましく、0.30mol%以上であることがさらに好ましい。バインダー組成物における、式(1)で表される単量体に由来する構成単位を含むフッ化ビニリデン共重合体の含有量、および、バインダー組成物における、式(1)で表される単量体に由来する構成単位の含有量を上記の範囲内とすることにより、電極合剤スラリーのゲル化を抑制する効果を好適に得ることができる。
When other vinylidene fluoride polymer is included, the proportion of the vinylidene fluoride copolymer containing the structural unit derived from the monomer represented by formula (1) in the entire polymer contained in the binder composition Is preferably 10% by weight or more, and more preferably 30% by weight or more. Further, the content of the structural unit derived from the monomer represented by the formula (1) in the whole polymer contained in the binder composition is preferably 0.10 mol% or more, and 0.20 mol% or more. More preferably, it is more preferably 0.30 mol% or more. Content of the vinylidene fluoride copolymer containing the structural unit derived from the monomer represented by Formula (1) in the binder composition, and the single amount represented by Formula (1) in the binder composition By making content of the structural unit derived from a body into said range, the effect which suppresses gelatinization of an electrode mixture slurry can be acquired suitably.
また、本実施形態に係るバインダー組成物は、環境への負荷を考慮して、塩素原子を含んでいない重合体を用いても電極合剤スラリーのゲル化が抑制されるように開発されたものである。したがって、本実施形態におけるバインダー組成物中の塩素量は、少ないことが望ましく、具体的には、1000ppm以下であることが好ましく、500ppm以下であることがより好ましく、300ppmであることが特に好ましい。
In addition, the binder composition according to the present embodiment has been developed so that gelation of the electrode mixture slurry is suppressed even when a polymer that does not contain chlorine atoms is used in consideration of environmental load. It is. Therefore, it is desirable that the amount of chlorine in the binder composition in this embodiment is small, specifically, it is preferably 1000 ppm or less, more preferably 500 ppm or less, and particularly preferably 300 ppm.
バインダー組成物中の塩素量は、JIS K 7229に準拠し、バインダー組成物をフラスコ中の酸素雰囲気下で燃焼させ、発生した燃焼ガスを吸収液に吸収させ、この液をイオンクロマトグラフにかけて検量線法によって塩素濃度を算出することにより、求めることができる。
The amount of chlorine in the binder composition is in accordance with JIS K 7229. The binder composition is burned in an oxygen atmosphere in the flask, and the generated combustion gas is absorbed into the absorption liquid. It can be obtained by calculating the chlorine concentration by the method.
(電極活物質)
本実施形態における電極活物質は、下記式(2)で表されるリチウム金属酸化物を含むものである。
Li1+xMO2 ・・・(2)
式(2)において、Xは、-0.15<X≦0.15を満たす数である。 (Electrode active material)
The electrode active material in the present embodiment includes a lithium metal oxide represented by the following formula (2).
Li 1 + x MO 2 (2)
In the formula (2), X is a number satisfying −0.15 <X ≦ 0.15.
本実施形態における電極活物質は、下記式(2)で表されるリチウム金属酸化物を含むものである。
Li1+xMO2 ・・・(2)
式(2)において、Xは、-0.15<X≦0.15を満たす数である。 (Electrode active material)
The electrode active material in the present embodiment includes a lithium metal oxide represented by the following formula (2).
Li 1 + x MO 2 (2)
In the formula (2), X is a number satisfying −0.15 <X ≦ 0.15.
Mは、NiまたはNiを含む2種以上の元素群である。MがNiを含む2種以上の元素群である場合には、Mに含まれるNi以外の元素としては、例えば、Co、Mn、Ti、Cr、Fe、Cu、Zn、Al、Ge、Sn、Mg、Ag、Ta、Nb、B、P、Zr、Ca、SrおよびBaなどが挙げられる。なかでも、Co、MnおよびAlが好ましい。MがNiを含む2種以上の元素群である場合に、Mに含まれるNi以外の元素は、これらのうちの1種のみであってもよく、2種以上であってもよい。リチウム金属酸化物がNiを含有することは、容量密度を高めることによって二次電池を高容量化できる点において好ましい。またリチウム金属酸化物が、Niに加えて、さらにCo等を含有することは、充放電過程での結晶構造変化が抑えられることによって安定なサイクル特性を示す点において好ましい。
M is Ni or two or more element groups including Ni. When M is a group of two or more elements including Ni, examples of elements other than Ni included in M include, for example, Co, Mn, Ti, Cr, Fe, Cu, Zn, Al, Ge, Sn, Examples thereof include Mg, Ag, Ta, Nb, B, P, Zr, Ca, Sr and Ba. Of these, Co, Mn and Al are preferable. In the case where M is a group of two or more elements including Ni, the element other than Ni included in M may be only one of these, or two or more. It is preferable that the lithium metal oxide contains Ni in that the capacity of the secondary battery can be increased by increasing the capacity density. Further, it is preferable that the lithium metal oxide further contains Co or the like in addition to Ni in that stable cycle characteristics are exhibited by suppressing changes in the crystal structure during the charge / discharge process.
式(2)で表されるリチウム金属酸化物におけるNiは、電極活物質において容量向上に寄与する成分である。よって、MがNiを含む2種以上の元素群である場合には、Mを構成する全元素数を100mol%としたとき、Niの割合は、55mol%以上が好ましく、60mol%以上であることが好ましく、70mol%以上であることがより好ましい。
Ni in the lithium metal oxide represented by the formula (2) is a component that contributes to an increase in capacity in the electrode active material. Therefore, in the case where M is a group of two or more elements including Ni, when the total number of elements constituting M is 100 mol%, the proportion of Ni is preferably 55 mol% or more, and 60 mol% or more. Is preferable, and it is more preferable that it is 70 mol% or more.
本実施形態における特に好ましいリチウム金属酸化物として、例えば下記式(3)
LiNiY1N1Y2O2・・・(3)
(式中、N1は、CoまたはMnを示し、0.55≦Y1<1、0<Y2<0.55である。)で表される二元リチウム金属酸化物、または、下記式(4)
LiNiY1CoY2N2Y3O2・・・(4)
(式中、N2は、MnまたはAlを示し、0.55≦Y1<1、0<Y2<0.55、0<Y3<0.55であり、かつY1/(Y1+Y2+Y3)≧0.55である。)で表される三元リチウム金属酸化物が挙げられる。 As a particularly preferable lithium metal oxide in the present embodiment, for example, the following formula (3)
LiNi Y1 N1 Y2 O 2 (3)
(Wherein N1 represents Co or Mn, and 0.55 ≦ Y1 <1, 0 <Y2 <0.55), or a binary lithium metal oxide represented by the following formula (4)
LiNi Y1 Co Y2 N2 Y3 O 2 (4)
(Wherein N2 represents Mn or Al, 0.55 ≦ Y1 <1, 0 <Y2 <0.55, 0 <Y3 <0.55, and Y1 / (Y1 + Y2 + Y3) ≧ 0.55) There is a ternary lithium metal oxide represented by:
LiNiY1N1Y2O2・・・(3)
(式中、N1は、CoまたはMnを示し、0.55≦Y1<1、0<Y2<0.55である。)で表される二元リチウム金属酸化物、または、下記式(4)
LiNiY1CoY2N2Y3O2・・・(4)
(式中、N2は、MnまたはAlを示し、0.55≦Y1<1、0<Y2<0.55、0<Y3<0.55であり、かつY1/(Y1+Y2+Y3)≧0.55である。)で表される三元リチウム金属酸化物が挙げられる。 As a particularly preferable lithium metal oxide in the present embodiment, for example, the following formula (3)
LiNi Y1 N1 Y2 O 2 (3)
(Wherein N1 represents Co or Mn, and 0.55 ≦ Y1 <1, 0 <Y2 <0.55), or a binary lithium metal oxide represented by the following formula (4)
LiNi Y1 Co Y2 N2 Y3 O 2 (4)
(Wherein N2 represents Mn or Al, 0.55 ≦ Y1 <1, 0 <Y2 <0.55, 0 <Y3 <0.55, and Y1 / (Y1 + Y2 + Y3) ≧ 0.55) There is a ternary lithium metal oxide represented by:
三元リチウム金属酸化物は、充電電位が高く、かつ優れたサイクル特性を有することから、本実施形態における電極活物質として特に好ましく用いられる。
The ternary lithium metal oxide is particularly preferably used as an electrode active material in this embodiment because it has a high charge potential and excellent cycle characteristics.
本実施形態における二元リチウム金属酸化物の組成は特に限定されるものではなく、その組成として、例えば、Li1.0Ni0.8Co0.2O2を挙げることができる。
The composition of the binary lithium metal oxide in the present embodiment is not particularly limited, and examples thereof include Li 1.0 Ni 0.8 Co 0.2 O 2 .
また、本実施形態における三元リチウム金属酸化物の組成は特に限定されるものではなく、その組成として、例えば、Li1.00Ni0.6Co0.2Mn0.2O2(NCM622)、Li1.00Ni0.83Co0.12Mn0.05O2(NCM811)、およびLi1.00Ni0.85Co0.15Al0.05O2(NCA811)を挙げることができる。
Further, the composition of the ternary lithium metal oxide in the present embodiment is not particularly limited, and for example, Li 1.00 Ni 0.6 Co 0.2 Mn 0.2 O 2 (NCM622) , Li 1.00 Ni 0.83 Co 0.12 Mn 0.05 O 2 (NCM811), and Li 1.00 Ni 0.85 Co 0.15 Al 0.05 O 2 (NCA811). .
さらに、本実施形態における電極活物質は、異なる複数種類のリチウム金属酸化物を含んでいてもよく、例えば、組成の異なるLiNiY1CoY2MnY3O2を複数含んでいてもよいし、LiNiY1CoY2MnY3O2とLiNiY1CoY2AlY3O2とを含んでいてもよい。
Furthermore, the electrode active material in the present embodiment may include a plurality of different types of lithium metal oxides. For example, the electrode active material may include a plurality of LiNi Y1 Co Y2 Mn Y3 O 2 having different compositions, or LiNi Y1. Co Y2 Mn Y3 O 2 and LiNi Y1 Co Y2 Al Y3 O 2 and may contain.
さらに、本実施形態におけるリチウム金属酸化物は、リチウム金属酸化物1gに対し、超純水49gを加え、10分間撹拌したのち、当該水のpHを測定した際の当該水のpHが11.3を超えるものである。当該水のpHの上限値は特に限定されない。一般に、電極合剤に添加される電極活物質にはアルカリ性物質が付着しており、その量が多くなると、得られる電極合剤スラリーはゲル化する傾向にある。そして、電極活物質中のニッケル含量が多くなるほど、付着するアルカリ性物質は多くなる。すなわち、水で抽出した際のpHが高くなる。そのため、放電容量を高めるために電極活物質としてニッケル含量の大きなリチウム金属酸化物を用いようとすると、電極合剤スラリーがゲル化しやすくなってしまう。本実施形態の電極合剤では、このような電極活物質を用いた場合であっても、電極合剤スラリーのゲル化が抑制されている。
Furthermore, the lithium metal oxide in the present embodiment is prepared by adding 49 g of ultrapure water to 1 g of lithium metal oxide, stirring for 10 minutes, and then measuring the pH of the water to 11.3. Is more than The upper limit value of the pH of the water is not particularly limited. Generally, an alkaline substance adheres to the electrode active material added to the electrode mixture, and when the amount increases, the resulting electrode mixture slurry tends to gel. And the more the nickel content in the electrode active material, the more alkaline material that adheres. That is, the pH when extracted with water increases. Therefore, if a lithium metal oxide having a high nickel content is used as an electrode active material in order to increase the discharge capacity, the electrode mixture slurry is likely to gel. In the electrode mixture of the present embodiment, gelation of the electrode mixture slurry is suppressed even when such an electrode active material is used.
本実施形態において、電極活物質は、式(2)で表されるリチウム金属酸化物の他に、例えば不純物および添加剤等を含んでいてもよい。また、電極活物質に含まれる不純物および添加剤等の種類は特に限定されるものではない。
In this embodiment, the electrode active material may contain, for example, impurities and additives in addition to the lithium metal oxide represented by the formula (2). Also, the types of impurities and additives contained in the electrode active material are not particularly limited.
(溶媒)
本実施形態における電極合剤は、溶媒を含んでいてもよい。溶媒は水であってもよく、非水溶媒であってもよい。非水溶媒としては、例えば、N-メチル-2-ピロリドン(NMP)、ジメチルホルムアミド、N-ジメチルホルムアミド、N,N-ジメチルアセトアミド、ジメチルスルホキシド、N,N-ジメチルスルホキシド、ヘキサメチルフォスフォアミド、ジオキサン、テトラヒドロフラン、テトラメチルウレア、トリエチルホスフェイト、トリメチルフォスフェイト、アセトン、シクロヘキサン、メチルエチルケトン、およびテトラヒドロフラン等が挙げられる。これらの溶媒は電極合剤に1種または2種以上含まれていてもよい。溶媒は、バインダー組成物に添加されていてもよく、バインダー組成物とは別に添加されたものであってもよい。 (solvent)
The electrode mixture in the present embodiment may contain a solvent. The solvent may be water or a non-aqueous solvent. Examples of the non-aqueous solvent include N-methyl-2-pyrrolidone (NMP), dimethylformamide, N-dimethylformamide, N, N-dimethylacetamide, dimethyl sulfoxide, N, N-dimethyl sulfoxide, hexamethylphosphoamide, Examples include dioxane, tetrahydrofuran, tetramethylurea, triethyl phosphate, trimethyl phosphate, acetone, cyclohexane, methyl ethyl ketone, and tetrahydrofuran. One or more of these solvents may be contained in the electrode mixture. The solvent may be added to the binder composition, or may be added separately from the binder composition.
本実施形態における電極合剤は、溶媒を含んでいてもよい。溶媒は水であってもよく、非水溶媒であってもよい。非水溶媒としては、例えば、N-メチル-2-ピロリドン(NMP)、ジメチルホルムアミド、N-ジメチルホルムアミド、N,N-ジメチルアセトアミド、ジメチルスルホキシド、N,N-ジメチルスルホキシド、ヘキサメチルフォスフォアミド、ジオキサン、テトラヒドロフラン、テトラメチルウレア、トリエチルホスフェイト、トリメチルフォスフェイト、アセトン、シクロヘキサン、メチルエチルケトン、およびテトラヒドロフラン等が挙げられる。これらの溶媒は電極合剤に1種または2種以上含まれていてもよい。溶媒は、バインダー組成物に添加されていてもよく、バインダー組成物とは別に添加されたものであってもよい。 (solvent)
The electrode mixture in the present embodiment may contain a solvent. The solvent may be water or a non-aqueous solvent. Examples of the non-aqueous solvent include N-methyl-2-pyrrolidone (NMP), dimethylformamide, N-dimethylformamide, N, N-dimethylacetamide, dimethyl sulfoxide, N, N-dimethyl sulfoxide, hexamethylphosphoamide, Examples include dioxane, tetrahydrofuran, tetramethylurea, triethyl phosphate, trimethyl phosphate, acetone, cyclohexane, methyl ethyl ketone, and tetrahydrofuran. One or more of these solvents may be contained in the electrode mixture. The solvent may be added to the binder composition, or may be added separately from the binder composition.
(他の成分)
本実施形態における電極合剤には、必要に応じて他の成分が含まれていてもよい。他の成分としては、例えば、導電助剤および顔料分散剤等が挙げられる。 (Other ingredients)
The electrode mixture in the present embodiment may contain other components as necessary. Examples of other components include a conductive aid and a pigment dispersant.
本実施形態における電極合剤には、必要に応じて他の成分が含まれていてもよい。他の成分としては、例えば、導電助剤および顔料分散剤等が挙げられる。 (Other ingredients)
The electrode mixture in the present embodiment may contain other components as necessary. Examples of other components include a conductive aid and a pigment dispersant.
導電助剤は、電極合剤を用いて形成される電極合剤層の導電性を向上させる目的で添加するものである。導電助剤としては、例えば、天然黒鉛(鱗片状黒鉛等)、人造黒鉛、黒鉛微粉末および黒鉛繊維等のグラファイト類、アセチレンブラック、ケッチェンブラック、チャンネルブラック、ファーネスブラック等のカーボンブラック類、炭素繊維、およびカーボンナノナノチューブ等の炭素材料が挙げられる。また、NiおよびAl等の金属繊維等の導電性繊維類、金属粉末類、導電性金属酸化物、ならびに有機導電性材料等も挙げられる。
The conductive auxiliary agent is added for the purpose of improving the conductivity of the electrode mixture layer formed using the electrode mixture. Examples of the conductive assistant include graphites such as natural graphite (eg, scaly graphite), artificial graphite, graphite fine powder and graphite fiber, carbon blacks such as acetylene black, ketjen black, channel black and furnace black, carbon Examples include fibers and carbon materials such as carbon nano-nanotubes. In addition, conductive fibers such as metal fibers such as Ni and Al, metal powders, conductive metal oxides, and organic conductive materials are also included.
また、顔料分散剤としては、例えば、ポリビニルピロリドン等を挙げることができる。
In addition, examples of the pigment dispersant include polyvinyl pyrrolidone.
本実施形態において、上述した他の成分は、電極合剤100重量部に対して、0~10重量部、好ましくは0~5重量部である。
In the present embodiment, the other components described above are 0 to 10 parts by weight, preferably 0 to 5 parts by weight with respect to 100 parts by weight of the electrode mixture.
以上のように、本実施形態の電極合剤は、塩素原子を含んでいないフッ化ビニリデン共重合体を用いているため、環境への負荷が低減している。そのうえで、ニッケルの含量の多い電極活物質を用いた場合であっても、電極合剤スラリーのゲル化を抑制することができる。とりわけ、比較的長い時間保存した場合であっても、電極合剤スラリーのゲル化が抑制されている。さらに、本実施形態の電極合剤によれば、電極活物質等の固形分が保存期間中に沈降して堆積することを抑制することができる。保存期間中の沈降が抑制されることで、固形分濃度が変化することを防ぐことができ、これにより、電極合剤の粘度上昇を防ぐことができる。その結果、電極構造体を作製する際のハンドリング性が低下することを防ぐことできる。
As described above, since the electrode mixture of the present embodiment uses a vinylidene fluoride copolymer that does not contain chlorine atoms, the burden on the environment is reduced. In addition, gelation of the electrode mixture slurry can be suppressed even when an electrode active material having a high nickel content is used. In particular, gelation of the electrode mixture slurry is suppressed even when stored for a relatively long time. Furthermore, according to the electrode mixture of this embodiment, it can suppress that solid content, such as an electrode active material, settles and accumulates during a storage period. By suppressing sedimentation during the storage period, it is possible to prevent the solid content concentration from changing, thereby preventing an increase in the viscosity of the electrode mixture. As a result, it is possible to prevent the handling property when the electrode structure is manufactured from being lowered.
(電極合剤の製造方法)
本実施形態における電極合剤は、フッ化ビニリデンと、式(1)で表される単量体とを共重合して得られるフッ化ビニリデン共重合体と、電極活物質とを混練することにより製造することができる。電極合剤の製造において、必要に応じて溶媒および他の成分を混練してもよく、その方法は特に限定されるものではない。また、混練する際の各種成分の添加の順序は特に限定されるものではない。さらに、溶媒を添加する場合には、先に電極活物質および溶媒を撹拌混合し、それからフッ化ビニリデン共重合体を加えてもよい。 (Method for producing electrode mixture)
The electrode mixture in the present embodiment is obtained by kneading vinylidene fluoride, a vinylidene fluoride copolymer obtained by copolymerizing the monomer represented by the formula (1), and an electrode active material. Can be manufactured. In the production of the electrode mixture, a solvent and other components may be kneaded as necessary, and the method is not particularly limited. Moreover, the order of addition of various components at the time of kneading is not particularly limited. Further, when a solvent is added, the electrode active material and the solvent may be first stirred and mixed, and then the vinylidene fluoride copolymer may be added.
本実施形態における電極合剤は、フッ化ビニリデンと、式(1)で表される単量体とを共重合して得られるフッ化ビニリデン共重合体と、電極活物質とを混練することにより製造することができる。電極合剤の製造において、必要に応じて溶媒および他の成分を混練してもよく、その方法は特に限定されるものではない。また、混練する際の各種成分の添加の順序は特に限定されるものではない。さらに、溶媒を添加する場合には、先に電極活物質および溶媒を撹拌混合し、それからフッ化ビニリデン共重合体を加えてもよい。 (Method for producing electrode mixture)
The electrode mixture in the present embodiment is obtained by kneading vinylidene fluoride, a vinylidene fluoride copolymer obtained by copolymerizing the monomer represented by the formula (1), and an electrode active material. Can be manufactured. In the production of the electrode mixture, a solvent and other components may be kneaded as necessary, and the method is not particularly limited. Moreover, the order of addition of various components at the time of kneading is not particularly limited. Further, when a solvent is added, the electrode active material and the solvent may be first stirred and mixed, and then the vinylidene fluoride copolymer may be added.
本実施形態における電極合剤は、電極活物質に含まれるリチウム金属酸化物として、水で抽出した際の水のpHが11.3よりも大きいものであるリチウム金属酸化物を用いることにより、製造されるものである。
The electrode mixture in the present embodiment is manufactured by using a lithium metal oxide whose pH when extracted with water is greater than 11.3 as the lithium metal oxide contained in the electrode active material. It is what is done.
(電極合剤のスラリー粘度)
本発明の電極合剤を用いた場合に、電極合剤スラリーのゲル化をどの程度抑制できるかについては、電極合剤のスラリー粘度によって判別することができる。なお、本明細書において、「ゲル化」とは、例えば、電極合剤スラリーを40℃、窒素雰囲気下、96時間保存した後に、当該電極合剤スラリーをミキサーを用いて30秒間撹拌した際に、電極合剤スラリーが均一なペースト状にならず、固形物が存在しているために、スラリー粘度が測定不可能な状態を指す。なお、固形物とは、目開き2.36mmのメッシュにスラリーを通し、1時間放置後、メッシュ上部に残るものを指す。また、ミキサーは特に限定されず、例えば(株)シンキー製 あわとり練太郎 ARE310(自転800rpm、公転2000rpm)を用いることができる。 (Slurry viscosity of electrode mixture)
The degree to which gelation of the electrode mixture slurry can be suppressed when the electrode mixture of the present invention is used can be determined by the slurry viscosity of the electrode mixture. In this specification, “gelation” means, for example, when the electrode mixture slurry is stored at 40 ° C. in a nitrogen atmosphere for 96 hours, and then the electrode mixture slurry is stirred for 30 seconds using a mixer. The electrode mixture slurry does not become a uniform paste, and a solid is present, so that the slurry viscosity cannot be measured. In addition, a solid substance refers to the thing which passes a slurry through a mesh with an opening of 2.36 mm, and is left on the mesh after being left for 1 hour. Further, the mixer is not particularly limited, and for example, Shintaro Awatori Nertaro ARE310 (autorotation 800 rpm, revolution 2000 rpm) manufactured by Shinky Co., Ltd. can be used.
本発明の電極合剤を用いた場合に、電極合剤スラリーのゲル化をどの程度抑制できるかについては、電極合剤のスラリー粘度によって判別することができる。なお、本明細書において、「ゲル化」とは、例えば、電極合剤スラリーを40℃、窒素雰囲気下、96時間保存した後に、当該電極合剤スラリーをミキサーを用いて30秒間撹拌した際に、電極合剤スラリーが均一なペースト状にならず、固形物が存在しているために、スラリー粘度が測定不可能な状態を指す。なお、固形物とは、目開き2.36mmのメッシュにスラリーを通し、1時間放置後、メッシュ上部に残るものを指す。また、ミキサーは特に限定されず、例えば(株)シンキー製 あわとり練太郎 ARE310(自転800rpm、公転2000rpm)を用いることができる。 (Slurry viscosity of electrode mixture)
The degree to which gelation of the electrode mixture slurry can be suppressed when the electrode mixture of the present invention is used can be determined by the slurry viscosity of the electrode mixture. In this specification, “gelation” means, for example, when the electrode mixture slurry is stored at 40 ° C. in a nitrogen atmosphere for 96 hours, and then the electrode mixture slurry is stirred for 30 seconds using a mixer. The electrode mixture slurry does not become a uniform paste, and a solid is present, so that the slurry viscosity cannot be measured. In addition, a solid substance refers to the thing which passes a slurry through a mesh with an opening of 2.36 mm, and is left on the mesh after being left for 1 hour. Further, the mixer is not particularly limited, and for example, Shintaro Awatori Nertaro ARE310 (autorotation 800 rpm, revolution 2000 rpm) manufactured by Shinky Co., Ltd. can be used.
〔電極構造体〕
続いて、本実施形態の電極合剤を用いて形成される電極構造体の一実施形態について、図1を参照しながら説明する。図1は、本発明の一実施形態に係る電極構造体の断面図である。 (Electrode structure)
Subsequently, an embodiment of an electrode structure formed using the electrode mixture of the present embodiment will be described with reference to FIG. FIG. 1 is a cross-sectional view of an electrode structure according to an embodiment of the present invention.
続いて、本実施形態の電極合剤を用いて形成される電極構造体の一実施形態について、図1を参照しながら説明する。図1は、本発明の一実施形態に係る電極構造体の断面図である。 (Electrode structure)
Subsequently, an embodiment of an electrode structure formed using the electrode mixture of the present embodiment will be described with reference to FIG. FIG. 1 is a cross-sectional view of an electrode structure according to an embodiment of the present invention.
図1に示すように、電極構造体10は、集電体11、電極合剤層12aおよび12bを有する。
As shown in FIG. 1, the electrode structure 10 includes a current collector 11 and electrode mixture layers 12a and 12b.
集電体11は、電極構造体10の基材であり、電気を取り出すための端子である。集電体11の材質としては、鉄、ステンレス鋼、鋼、アルミニウム、ニッケル、およびチタン等を挙げることができる。集電体11の形状は、箔または網であることが好ましい。本実施形態において、集電体11としては、アルミニウム箔とすることが好ましい。
The current collector 11 is a base material for the electrode structure 10 and is a terminal for taking out electricity. Examples of the material of the current collector 11 include iron, stainless steel, steel, aluminum, nickel, and titanium. The shape of the current collector 11 is preferably a foil or a net. In the present embodiment, the current collector 11 is preferably an aluminum foil.
集電体11の厚みは、5~100μmであることが好ましく、5~20μmであることが好ましい。
The thickness of the current collector 11 is preferably 5 to 100 μm, and more preferably 5 to 20 μm.
電極合剤層12aおよび12bは、本実施形態の電極合剤からなる層である。電極合剤層12aおよび12bの厚みは、10μm~1000μm、より好ましくは20μm~250μm、さらに好ましくは20μm~150μmである。
The electrode mixture layers 12a and 12b are layers made of the electrode mixture of the present embodiment. The thickness of the electrode mixture layers 12a and 12b is 10 μm to 1000 μm, more preferably 20 μm to 250 μm, and still more preferably 20 μm to 150 μm.
本実施形態における電極合剤層は、上述の電極合剤を用いて形成される。そのため、本実施形態における電極合剤層についても、JIS K 5101-16-2に規定される抽出方法で常温(25℃)抽出した際に、当該水のpHが11.3を超えるものとなっている。pHの測定方法は、具体的には、電極合剤層を集電箔から剥がし、それを試料とする以外は、上述したリチウム金属酸化物のpHの測定方法と同様の方法で測定を行うものである。
The electrode mixture layer in this embodiment is formed using the electrode mixture described above. Therefore, when the electrode mixture layer in this embodiment is extracted at room temperature (25 ° C.) by the extraction method specified in JIS K 5101-16-2, the pH of the water exceeds 11.3. ing. Specifically, the pH is measured by the same method as the above-described method for measuring the pH of a lithium metal oxide, except that the electrode mixture layer is peeled off from the current collector foil and used as a sample. It is.
なお、電極構造体10は、図1に示すように集電体11の上下面に電極合剤層12aおよび12bが形成されているが、これに限定されるものではなく、集電体11のいずれか一方の面に電極合剤層が形成されているもの、すなわち、電極合剤層12aおよび12bのいずれか一方が形成された電極構造体であってもよい。
In the electrode structure 10, the electrode mixture layers 12 a and 12 b are formed on the upper and lower surfaces of the current collector 11 as shown in FIG. 1, but the present invention is not limited to this. What has the electrode mixture layer formed in any one surface, ie, the electrode structure in which any one of the electrode mixture layers 12a and 12b was formed may be sufficient.
電極構造体10は、例えばリチウム二次電池の正極として用いることができる。
The electrode structure 10 can be used as a positive electrode of a lithium secondary battery, for example.
次に、電極構造体の製造方法について説明する。
Next, a method for manufacturing the electrode structure will be described.
本実施形態の電極構造体10は、フッ化ビニリデン共重合体とリチウム金属酸化物と溶媒とを含むスラリー状の電極合剤(電極合剤スラリー)を集電体11の表面に塗布して乾燥させることによって、集電体11表面に塗膜を形成する工程と、塗膜に熱処理を施す工程とを経ることによって得ることができる。
In the electrode structure 10 of the present embodiment, a slurry-like electrode mixture (electrode mixture slurry) containing a vinylidene fluoride copolymer, a lithium metal oxide, and a solvent is applied to the surface of the current collector 11 and dried. By making it, it can obtain by passing through the process of forming a coating film in the collector 11 surface, and the process of heat-processing a coating film.
電極合剤スラリーの塗布方法としては、公知の方法を用いることができ、バーコーター、ダイコーターまたはコンマコーター(登録商標)等を用いる方法を挙げることができる。
As a method for applying the electrode mixture slurry, a known method can be used, and a method using a bar coater, a die coater, a comma coater (registered trademark), or the like can be given.
集電体11の上下面に塗布された電極合剤スラリーを乾燥させる際の乾燥温度としては、50~170℃、好ましくは50~150℃とすることができる。
The drying temperature for drying the electrode mixture slurry applied to the upper and lower surfaces of the current collector 11 can be 50 to 170 ° C., preferably 50 to 150 ° C.
なお、本実施形態では、電極合剤スラリーを集電体11の上下面に塗布することで電極合剤層を形成する方法について説明したが、本実施形態の電極構造体の製造方法はこれに限定されず、電極合剤を集電体の少なくとも一方の面に塗布すればよい。
In addition, although this embodiment demonstrated the method of forming an electrode mixture layer by apply | coating an electrode mixture slurry to the upper and lower surfaces of the electrical power collector 11, the manufacturing method of the electrode structure of this embodiment is to this. Without limitation, the electrode mixture may be applied to at least one surface of the current collector.
〔二次電池〕
本実施形態の二次電池は、本実施形態の電極構造体を含む非水電解質二次電池である。本実施形態の二次電池について、図2を参照しながら説明する。図2は、本実施形態に係る二次電池の分解斜視図である。 [Secondary battery]
The secondary battery of the present embodiment is a nonaqueous electrolyte secondary battery including the electrode structure of the present embodiment. The secondary battery of this embodiment will be described with reference to FIG. FIG. 2 is an exploded perspective view of the secondary battery according to the present embodiment.
本実施形態の二次電池は、本実施形態の電極構造体を含む非水電解質二次電池である。本実施形態の二次電池について、図2を参照しながら説明する。図2は、本実施形態に係る二次電池の分解斜視図である。 [Secondary battery]
The secondary battery of the present embodiment is a nonaqueous electrolyte secondary battery including the electrode structure of the present embodiment. The secondary battery of this embodiment will be described with reference to FIG. FIG. 2 is an exploded perspective view of the secondary battery according to the present embodiment.
図2に示すように、二次電池100は、正極1および負極2の間にセパレータ3を配置積層したものを渦巻き状に巻き回した発電素子が、金属ケーシング5中に収容された構造を有する。正極1は、図1における電極構造体10に対応する。
As shown in FIG. 2, the secondary battery 100 has a structure in which a power generating element in which a separator 3 is disposed and laminated between a positive electrode 1 and a negative electrode 2 and wound in a spiral shape is housed in a metal casing 5. . The positive electrode 1 corresponds to the electrode structure 10 in FIG.
セパレータ3としては、ポリプロピレン、およびポリエチレン等の高分子物質の多孔性膜等の公知の材料を用いればよい。この他、二次電池100において用いられる部材は、本分野において通常用いられるものを適宜用いることができる。
As the separator 3, a known material such as a porous film of a polymer material such as polypropylene and polyethylene may be used. In addition, as the members used in the secondary battery 100, those normally used in this field can be appropriately used.
なお、二次電池100は円筒形電池であるが、もちろん本発明における二次電池100はこれに限定されるものではなく、例えばコイン形、角形またはペーパー形等の他の形状の二次電池であってもよい。
The secondary battery 100 is a cylindrical battery, but of course, the secondary battery 100 in the present invention is not limited to this, and may be a secondary battery having another shape such as a coin shape, a square shape, or a paper shape. There may be.
〔まとめ〕
以上のとおり、本発明の電極合剤は、集電体上に設けられる電極活物質と、該電極活物質を該集電体に結着するためのバインダー組成物とを含有し、バインダー組成物は、フッ化ビニリデンと、上述の式(1)で表される単量体との共重合体を含有し、上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、上記リチウム金属酸化物を水で抽出した際の該水のpHが11.3よりも大きい構成である。 [Summary]
As described above, the electrode mixture of the present invention contains an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector. Contains a copolymer of vinylidene fluoride and the monomer represented by the above formula (1), and the electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
The pH of the water when the lithium metal oxide is extracted with water is greater than 11.3.
以上のとおり、本発明の電極合剤は、集電体上に設けられる電極活物質と、該電極活物質を該集電体に結着するためのバインダー組成物とを含有し、バインダー組成物は、フッ化ビニリデンと、上述の式(1)で表される単量体との共重合体を含有し、上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、上記リチウム金属酸化物を水で抽出した際の該水のpHが11.3よりも大きい構成である。 [Summary]
As described above, the electrode mixture of the present invention contains an electrode active material provided on a current collector and a binder composition for binding the electrode active material to the current collector. Contains a copolymer of vinylidene fluoride and the monomer represented by the above formula (1), and the electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
The pH of the water when the lithium metal oxide is extracted with water is greater than 11.3.
また、本発明の電極合剤は、上記バインダー組成物中の塩素量が1000ppm以下であることが好ましい。
Further, in the electrode mixture of the present invention, the chlorine content in the binder composition is preferably 1000 ppm or less.
また、本発明の電極合剤は、上記共重合体における、上記式(1)で表される単量体に由来する構成単位が0.40mol%以上であることが好ましい。
Further, in the electrode mixture of the present invention, the constituent unit derived from the monomer represented by the formula (1) in the copolymer is preferably 0.40 mol% or more.
また、本発明の電極合剤の一態様では、上記バインダー組成物は、上記共重合体とは異なるフッ化ビニリデン系重合体を含む構成である。
Further, in one aspect of the electrode mixture of the present invention, the binder composition includes a vinylidene fluoride polymer different from the copolymer.
また、本発明の電極合剤は、溶媒を含んでいることが好ましい。
Further, the electrode mixture of the present invention preferably contains a solvent.
本発明に係る電極合剤の製造方法は、フッ化ビニリデンと、上述の式(1)で表される単量体との共重合体と、電極活物質とを混練する工程を含み、上記電極活物質は、上述の式(2)で表されるリチウム金属酸化物を含み、上記リチウム金属酸化物は、水で抽出した際の該水のpHが11.3よりも大きいものである。
The method for producing an electrode mixture according to the present invention includes a step of kneading a copolymer of vinylidene fluoride, a monomer represented by the above formula (1), and an electrode active material, The active material contains a lithium metal oxide represented by the above formula (2), and the pH of the lithium metal oxide when extracted with water is greater than 11.3.
本発明に係る電極構造体の一態様は、集電体と、該集電体上に設けられた電極合剤層とを備えており、上記電極合剤層は、上述の電極合剤を用いて形成された層である。
One aspect of the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector, and the electrode mixture layer uses the electrode mixture described above. It is a layer formed.
本発明に係る電極構造体の別の態様は、集電体と、該集電体上に設けられた電極合剤層とを備えており、上記電極合剤層は、バインダー組成物および電極活物質を含む層であり、上記バインダー組成物は、フッ化ビニリデンと、上述の式(1)で表される単量体との共重合体を含有し、上記電極活物質は、上述の式(2)で表されるリチウム金属酸化物を含み、上記電極合剤層を水で抽出した際の該水のpHが11.3よりも大きい構成である。
Another aspect of the electrode structure according to the present invention includes a current collector and an electrode mixture layer provided on the current collector, and the electrode mixture layer includes a binder composition and an electrode active layer. The binder composition contains a copolymer of vinylidene fluoride and a monomer represented by the above formula (1), and the electrode active material has the above formula ( The lithium metal oxide represented by 2) is included, and the pH of the water when the electrode mixture layer is extracted with water is greater than 11.3.
本発明に係る電極構造体の製造方法は、上述の電極合剤を集電体表面に塗布して乾燥させることによって該集電体表面上に塗膜を形成する工程と、上記塗膜に熱処理を施す工程とを含む。
The method for producing an electrode structure according to the present invention includes a step of forming a coating film on a surface of the current collector by applying the electrode mixture to the surface of the current collector and drying, and a heat treatment on the coating film. The process of giving.
本発明に係る二次電池は、上述の電極構造体を備えている。
The secondary battery according to the present invention includes the electrode structure described above.
以下に実施例を示し、本発明の実施の形態についてさらに詳しく説明する。もちろん、本発明は以下の実施例に限定されるものではなく、細部については様々な態様が可能であることはいうまでもない。さらに、本発明は上述した実施形態に限定されるものではなく、請求項に示した範囲で種々の変更が可能であり、それぞれ開示された技術的手段を適宜組み合わせて得られる実施形態についても本発明の技術的範囲に含まれる。また、本明細書中に記載された文献の全てが参考として援用される。
Examples will be shown below, and the embodiments of the present invention will be described in more detail. Of course, the present invention is not limited to the following examples, and it goes without saying that various aspects are possible in detail. Further, the present invention is not limited to the above-described embodiments, and various modifications can be made within the scope shown in the claims, and the present invention is also applied to the embodiments obtained by appropriately combining the disclosed technical means. It is included in the technical scope of the invention. Moreover, all the literatures described in this specification are used as reference.
以下に示すように、様々なバインダー組成物を用いて、電極合剤および電極構造体を作製し、電極合剤スラリーの粘度変化、および固形分濃度変化の確認試験を行った。具体的な実施例の説明の前に、リチウム金属酸化物のpHの測定、スラリー粘度の算出、および固形分濃度変化試験の各方法について説明する。
As shown below, an electrode mixture and an electrode structure were prepared using various binder compositions, and a confirmation test of viscosity change and solid content concentration change of the electrode mixture slurry was performed. Prior to the description of specific examples, each method of measurement of pH of lithium metal oxide, calculation of slurry viscosity, and solid content concentration change test will be described.
(リチウム金属酸化物のpH測定)
電極活物質であるリチウム金属酸化物のpHは、リチウム金属酸化物を水で常温(25℃)抽出したときの水のpHとした。リチウム金属酸化物の水への抽出はJIS K 5101-16-2に規定される抽出方法で行った。具体的には、リチウム金属酸化物の重量の50倍量の超純水にリチウム金属酸化物を入れ、マグネチックスターラーにて回転数:600rpmで10分間撹拌を行い、その溶液を(株)堀場製作所製pHメーターMODEL:F-21を用いてpH測定を行った。 (PH measurement of lithium metal oxide)
The pH of the lithium metal oxide as the electrode active material was the pH of water when the lithium metal oxide was extracted with water at room temperature (25 ° C.). The extraction of lithium metal oxide into water was performed by the extraction method defined in JIS K 5101-16-2. Specifically, the lithium metal oxide is put into ultrapure water 50 times the weight of the lithium metal oxide, and stirred with a magnetic stirrer at a rotation speed of 600 rpm for 10 minutes. The pH was measured using a pH meter MODEL: F-21 manufactured by Seisakusho.
電極活物質であるリチウム金属酸化物のpHは、リチウム金属酸化物を水で常温(25℃)抽出したときの水のpHとした。リチウム金属酸化物の水への抽出はJIS K 5101-16-2に規定される抽出方法で行った。具体的には、リチウム金属酸化物の重量の50倍量の超純水にリチウム金属酸化物を入れ、マグネチックスターラーにて回転数:600rpmで10分間撹拌を行い、その溶液を(株)堀場製作所製pHメーターMODEL:F-21を用いてpH測定を行った。 (PH measurement of lithium metal oxide)
The pH of the lithium metal oxide as the electrode active material was the pH of water when the lithium metal oxide was extracted with water at room temperature (25 ° C.). The extraction of lithium metal oxide into water was performed by the extraction method defined in JIS K 5101-16-2. Specifically, the lithium metal oxide is put into ultrapure water 50 times the weight of the lithium metal oxide, and stirred with a magnetic stirrer at a rotation speed of 600 rpm for 10 minutes. The pH was measured using a pH meter MODEL: F-21 manufactured by Seisakusho.
(インヘレント粘度ηiの算出)
インヘレント粘度ηiを算出するために、重合体80mgを20mlのN,N-ジメチルホルムアミドに溶解することによって重合体溶液を作製した。この重合体溶液の粘度ηを、30℃の恒温槽内においてウベローデ粘度計を用いて測定した。そして、インヘレント粘度ηiを、当該粘度ηを用いて下記式によって求めた。 (Calculation of inherent viscosity η i )
In order to calculate the inherent viscosity η i , a polymer solution was prepared by dissolving 80 mg of the polymer in 20 ml of N, N-dimethylformamide. The viscosity η of this polymer solution was measured using a Ubbelohde viscometer in a constant temperature bath at 30 ° C. And inherent viscosity (eta) i was calculated | required by the following formula using the said viscosity (eta).
インヘレント粘度ηiを算出するために、重合体80mgを20mlのN,N-ジメチルホルムアミドに溶解することによって重合体溶液を作製した。この重合体溶液の粘度ηを、30℃の恒温槽内においてウベローデ粘度計を用いて測定した。そして、インヘレント粘度ηiを、当該粘度ηを用いて下記式によって求めた。 (Calculation of inherent viscosity η i )
In order to calculate the inherent viscosity η i , a polymer solution was prepared by dissolving 80 mg of the polymer in 20 ml of N, N-dimethylformamide. The viscosity η of this polymer solution was measured using a Ubbelohde viscometer in a constant temperature bath at 30 ° C. And inherent viscosity (eta) i was calculated | required by the following formula using the said viscosity (eta).
ηi=(1/C)・ln(η/η0)
上記式においてηは重合体溶液の粘度、η0は溶媒のN,N-ジメチルホルムアミドの粘度、Cは0.4g/dlである。 η i = (1 / C) · ln (η / η 0 )
In the above formula, η is the viscosity of the polymer solution, η 0 is the viscosity of the solvent N, N-dimethylformamide, and C is 0.4 g / dl.
上記式においてηは重合体溶液の粘度、η0は溶媒のN,N-ジメチルホルムアミドの粘度、Cは0.4g/dlである。 η i = (1 / C) · ln (η / η 0 )
In the above formula, η is the viscosity of the polymer solution, η 0 is the viscosity of the solvent N, N-dimethylformamide, and C is 0.4 g / dl.
(スラリー粘度の測定)
電極合剤のスラリー粘度は、東機産業(株)製 E型粘度計 RE-550 MODEL:R、RC-550を用いて、25℃、せん断速度2s-1で測定を行った。なお、粘度は、スラリーを測定装置に仕込んでから60秒待機し、その後ローターを回転させることで測定を行った。また、ローターの回転開始から300秒後の値を初期スラリー粘度とした。25℃、窒素雰囲気下、所定時間(24時間または120時間)放置後のスラリー粘度を測定し、保存後スラリー粘度とした。 (Measurement of slurry viscosity)
The slurry viscosity of the electrode mixture was measured at 25 ° C. and a shear rate of 2 s −1 using an E-type viscometer RE-550 MODEL: R, RC-550 manufactured by Toki Sangyo Co., Ltd. The viscosity was measured by waiting 60 seconds after the slurry was charged into the measuring apparatus and then rotating the rotor. The value after 300 seconds from the start of rotation of the rotor was taken as the initial slurry viscosity. The slurry viscosity after being allowed to stand for a predetermined time (24 hours or 120 hours) in a nitrogen atmosphere at 25 ° C. was measured and used as the slurry viscosity after storage.
電極合剤のスラリー粘度は、東機産業(株)製 E型粘度計 RE-550 MODEL:R、RC-550を用いて、25℃、せん断速度2s-1で測定を行った。なお、粘度は、スラリーを測定装置に仕込んでから60秒待機し、その後ローターを回転させることで測定を行った。また、ローターの回転開始から300秒後の値を初期スラリー粘度とした。25℃、窒素雰囲気下、所定時間(24時間または120時間)放置後のスラリー粘度を測定し、保存後スラリー粘度とした。 (Measurement of slurry viscosity)
The slurry viscosity of the electrode mixture was measured at 25 ° C. and a shear rate of 2 s −1 using an E-type viscometer RE-550 MODEL: R, RC-550 manufactured by Toki Sangyo Co., Ltd. The viscosity was measured by waiting 60 seconds after the slurry was charged into the measuring apparatus and then rotating the rotor. The value after 300 seconds from the start of rotation of the rotor was taken as the initial slurry viscosity. The slurry viscosity after being allowed to stand for a predetermined time (24 hours or 120 hours) in a nitrogen atmosphere at 25 ° C. was measured and used as the slurry viscosity after storage.
(固形分濃度変化の測定)
作製した電極合剤スラリーをポリプロピレンチューブ(φ12×75mm)に、チューブ下部から5cmの高さまで流し込み、25℃20%RH環境下で24時間保管した。保管後、チューブ下部から高さ1cmまでの電極合剤スラリーを採取し、アルミカップに入れて秤量し、採取した電極合剤スラリーの重量を測定した。そのアルミカップを110℃、2時間加熱して溶媒を除去した後、秤量することで、採取した電極合剤スラリーの固形分量を測定した。ここで得られた乾燥前後の重量から下部固形分濃度(NVA)を算出した。仕込電極合剤スラリーの初期固形分濃度(NVB)に対する下部固形分濃度(NVA)の割合(NVA/NVB)を、指標として算出した。NVA/NVBの値が大きい程、保存中に電極活物質が容器下部に沈み、堆積しやすいことを表している。 (Measurement of solid content concentration change)
The prepared electrode mixture slurry was poured into a polypropylene tube (φ12 × 75 mm) to a height of 5 cm from the bottom of the tube, and stored in an environment of 25 ° C. and 20% RH for 24 hours. After storage, an electrode mixture slurry having a height of 1 cm from the bottom of the tube was collected, weighed in an aluminum cup, and the weight of the collected electrode mixture slurry was measured. The aluminum cup was heated at 110 ° C. for 2 hours to remove the solvent, and then weighed to measure the solid content of the collected electrode mixture slurry. It was calculated lower solids concentration (NV A) from the weight before and after drying obtained here. The ratio of the lower solid content concentration (NV A ) to the initial solid content concentration (NV B ) of the charged electrode mixture slurry (NV A / NV B ) was calculated as an index. This indicates that the larger the value of NV A / NV B, the easier the electrode active material sinks and accumulates in the lower part of the container during storage.
作製した電極合剤スラリーをポリプロピレンチューブ(φ12×75mm)に、チューブ下部から5cmの高さまで流し込み、25℃20%RH環境下で24時間保管した。保管後、チューブ下部から高さ1cmまでの電極合剤スラリーを採取し、アルミカップに入れて秤量し、採取した電極合剤スラリーの重量を測定した。そのアルミカップを110℃、2時間加熱して溶媒を除去した後、秤量することで、採取した電極合剤スラリーの固形分量を測定した。ここで得られた乾燥前後の重量から下部固形分濃度(NVA)を算出した。仕込電極合剤スラリーの初期固形分濃度(NVB)に対する下部固形分濃度(NVA)の割合(NVA/NVB)を、指標として算出した。NVA/NVBの値が大きい程、保存中に電極活物質が容器下部に沈み、堆積しやすいことを表している。 (Measurement of solid content concentration change)
The prepared electrode mixture slurry was poured into a polypropylene tube (φ12 × 75 mm) to a height of 5 cm from the bottom of the tube, and stored in an environment of 25 ° C. and 20% RH for 24 hours. After storage, an electrode mixture slurry having a height of 1 cm from the bottom of the tube was collected, weighed in an aluminum cup, and the weight of the collected electrode mixture slurry was measured. The aluminum cup was heated at 110 ° C. for 2 hours to remove the solvent, and then weighed to measure the solid content of the collected electrode mixture slurry. It was calculated lower solids concentration (NV A) from the weight before and after drying obtained here. The ratio of the lower solid content concentration (NV A ) to the initial solid content concentration (NV B ) of the charged electrode mixture slurry (NV A / NV B ) was calculated as an index. This indicates that the larger the value of NV A / NV B, the easier the electrode active material sinks and accumulates in the lower part of the container during storage.
(フッ化ビニリデンおよびコモノマーの構成単位量)
重合体粉末の1H NMRスペクトルを下記条件で求めた。 (Constitutional unit amount of vinylidene fluoride and comonomer)
The 1 H NMR spectrum of the polymer powder was determined under the following conditions.
重合体粉末の1H NMRスペクトルを下記条件で求めた。 (Constitutional unit amount of vinylidene fluoride and comonomer)
The 1 H NMR spectrum of the polymer powder was determined under the following conditions.
装置:Bruker社製。AVANCE AC 400FT NMRスペクトルメーター
測定条件
周波数:400MHz
測定溶媒:DMSO-d6
測定温度:25℃
重合体のフッ化ビニリデンに由来する構成単位の量、およびコモノマーに由来する構成単位の量を、1H NMRスペクトルから算出した。具体的には、主としてコモノマーに由来するシグナルと、主としてフッ化ビニリデンに由来する2.24ppmおよび2.87ppmに観察されるシグナルとの積分強度に基づき算出した。 Apparatus: manufactured by Bruker. AVANCE AC 400FT NMR spectrum meter Measurement conditions Frequency: 400 MHz
Measuring solvent: DMSO-d6
Measurement temperature: 25 ° C
The amount of the structural unit derived from the vinylidene fluoride of the polymer and the amount of the structural unit derived from the comonomer were calculated from the 1 H NMR spectrum. Specifically, it was calculated based on the integrated intensity of signals mainly derived from comonomer and signals observed at 2.24 ppm and 2.87 ppm mainly derived from vinylidene fluoride.
測定条件
周波数:400MHz
測定溶媒:DMSO-d6
測定温度:25℃
重合体のフッ化ビニリデンに由来する構成単位の量、およびコモノマーに由来する構成単位の量を、1H NMRスペクトルから算出した。具体的には、主としてコモノマーに由来するシグナルと、主としてフッ化ビニリデンに由来する2.24ppmおよび2.87ppmに観察されるシグナルとの積分強度に基づき算出した。 Apparatus: manufactured by Bruker. AVANCE AC 400FT NMR spectrum meter Measurement conditions Frequency: 400 MHz
Measuring solvent: DMSO-d6
Measurement temperature: 25 ° C
The amount of the structural unit derived from the vinylidene fluoride of the polymer and the amount of the structural unit derived from the comonomer were calculated from the 1 H NMR spectrum. Specifically, it was calculated based on the integrated intensity of signals mainly derived from comonomer and signals observed at 2.24 ppm and 2.87 ppm mainly derived from vinylidene fluoride.
コモノマーとしてアクリル酸に由来する構造を有するコモノマーを用いた場合には、重合体におけるアクリル酸に由来する構造を含む構成単位の量を0.03mol/lの水酸化ナトリウム水溶液を用いた中和滴定により求めた。より具体的には、重合体0.3gをアセトン9.7gに約80℃で溶解した後、3gの純水を加えることで被滴定溶液を調製した。指示薬として、フェノールフタレインを用い、室温下、0.03mol/lの水酸化ナトリウム水溶液を用いて中和滴定を行った。
When a comonomer having a structure derived from acrylic acid is used as a comonomer, the amount of the structural unit containing a structure derived from acrylic acid in the polymer is neutralized with a sodium hydroxide aqueous solution of 0.03 mol / l. Determined by More specifically, a solution to be titrated was prepared by dissolving 0.3 g of a polymer in 9.7 g of acetone at about 80 ° C. and then adding 3 g of pure water. As an indicator, phenolphthalein was used, and neutralization titration was performed using a 0.03 mol / l aqueous sodium hydroxide solution at room temperature.
〔実施例1〕
(バインダー組成物の調製)
内容量2リットルのオートクレーブに、イオン交換水900g、ヒドロキシプロピルメチルセルロース0.4g、ブチルペルオキシピバレート2g、フッ化ビニリデン396g、およびアクリル酸の初期添加量0.2gの各量を仕込み、50℃に加熱した。重合中に圧力を一定に保つ条件で、アクリル酸を含む1重量%アクリル酸水溶液を反応容器に連続的に供給した。得られた重合体スラリーを脱水、乾燥してフッ化ビニリデン共重合体(VDF/AA)を得た。アクリル酸は、初期に添加した量を含め、全量4gを添加した。 [Example 1]
(Preparation of binder composition)
Into an autoclave with an internal volume of 2 liters, each amount of 900 g of ion exchange water, 0.4 g of hydroxypropyl methylcellulose, 2 g of butyl peroxypivalate, 396 g of vinylidene fluoride, and 0.2 g of an initial addition amount of acrylic acid was charged to 50 ° C. Heated. A 1% by weight aqueous acrylic acid solution containing acrylic acid was continuously fed to the reaction vessel under the condition that the pressure was kept constant during the polymerization. The obtained polymer slurry was dehydrated and dried to obtain a vinylidene fluoride copolymer (VDF / AA). A total of 4 g of acrylic acid was added, including the amount added initially.
(バインダー組成物の調製)
内容量2リットルのオートクレーブに、イオン交換水900g、ヒドロキシプロピルメチルセルロース0.4g、ブチルペルオキシピバレート2g、フッ化ビニリデン396g、およびアクリル酸の初期添加量0.2gの各量を仕込み、50℃に加熱した。重合中に圧力を一定に保つ条件で、アクリル酸を含む1重量%アクリル酸水溶液を反応容器に連続的に供給した。得られた重合体スラリーを脱水、乾燥してフッ化ビニリデン共重合体(VDF/AA)を得た。アクリル酸は、初期に添加した量を含め、全量4gを添加した。 [Example 1]
(Preparation of binder composition)
Into an autoclave with an internal volume of 2 liters, each amount of 900 g of ion exchange water, 0.4 g of hydroxypropyl methylcellulose, 2 g of butyl peroxypivalate, 396 g of vinylidene fluoride, and 0.2 g of an initial addition amount of acrylic acid was charged to 50 ° C. Heated. A 1% by weight aqueous acrylic acid solution containing acrylic acid was continuously fed to the reaction vessel under the condition that the pressure was kept constant during the polymerization. The obtained polymer slurry was dehydrated and dried to obtain a vinylidene fluoride copolymer (VDF / AA). A total of 4 g of acrylic acid was added, including the amount added initially.
(電極合剤の調製)
電極活物質としてのNCA811に導電助剤としてカーボンブラック(SP:Timcal Japan社製 SuperP(登録商標)、平均粒子径:40nm、比表面積:60m2/g)を加え、粉体混合を行った。一方、フッ化ビニリデン共重合体(VDF/AA)をN-メチル-2ピロリドン(以下、NMP)に溶解し、5重量%濃度のフッ化ビニリデン溶液を作製した。NCA811およびカーボンブラックの混合物に対し、フッ化ビニリデン溶液を2回に分けて添加し、混練をおこなった。具体的には、固形分濃度変化の測定では固形分濃度が84.2重量%となるように、スラリーの粘度変化測定では固形分濃度が81.5重量%となるようにフッ化ビニリデン溶液を添加し、2000rpmで2分間1次混練を行った。次いで、残りのフッ化ビニリデン溶液を添加して、固形分濃度変化の測定では固形分濃度が72.0重量%となるように、スラリーの粘度変化測定では固形分濃度が75重量%となるようにし、2000rpmで3分間2次混練を行うことで、電極合剤を得た。得られた電極合剤における電極活物質、カーボンブラック、およびフッ化ビニリデン共重合体の重量比は、固形分濃度変化の測定ではこの順で、100:2:1、スラリーの粘度変化測定ではこの順で、100:2:2である。電極合剤の組成を表1に示す。 (Preparation of electrode mixture)
Carbon black (SP: SuperP (registered trademark) manufactured by Timcal Japan, average particle size: 40 nm, specific surface area: 60 m 2 / g) was added to NCA811 as an electrode active material, and powder mixing was performed. On the other hand, a vinylidene fluoride copolymer (VDF / AA) was dissolved in N-methyl-2pyrrolidone (hereinafter referred to as NMP) to prepare a 5 wt% vinylidene fluoride solution. To the mixture of NCA811 and carbon black, the vinylidene fluoride solution was added in two portions and kneaded. Specifically, the vinylidene fluoride solution was prepared so that the solid content concentration was 84.2% by weight in the measurement of the solid content concentration change, and the solid content concentration was 81.5% by weight in the measurement of the viscosity change of the slurry. The mixture was added and primary kneading was performed at 2000 rpm for 2 minutes. Then, the remaining vinylidene fluoride solution is added so that the solid content concentration is 72.0% by weight in the measurement of the solid content concentration change, and the solid content concentration is 75% by weight in the measurement of the viscosity change of the slurry. And secondary kneading at 2000 rpm for 3 minutes to obtain an electrode mixture. The weight ratio of the electrode active material, carbon black, and vinylidene fluoride copolymer in the obtained electrode mixture was 100: 2: 1 in this order when measuring the solid content concentration change, and this ratio was measured when measuring the viscosity change of the slurry. In order, it is 100: 2: 2. Table 1 shows the composition of the electrode mixture.
電極活物質としてのNCA811に導電助剤としてカーボンブラック(SP:Timcal Japan社製 SuperP(登録商標)、平均粒子径:40nm、比表面積:60m2/g)を加え、粉体混合を行った。一方、フッ化ビニリデン共重合体(VDF/AA)をN-メチル-2ピロリドン(以下、NMP)に溶解し、5重量%濃度のフッ化ビニリデン溶液を作製した。NCA811およびカーボンブラックの混合物に対し、フッ化ビニリデン溶液を2回に分けて添加し、混練をおこなった。具体的には、固形分濃度変化の測定では固形分濃度が84.2重量%となるように、スラリーの粘度変化測定では固形分濃度が81.5重量%となるようにフッ化ビニリデン溶液を添加し、2000rpmで2分間1次混練を行った。次いで、残りのフッ化ビニリデン溶液を添加して、固形分濃度変化の測定では固形分濃度が72.0重量%となるように、スラリーの粘度変化測定では固形分濃度が75重量%となるようにし、2000rpmで3分間2次混練を行うことで、電極合剤を得た。得られた電極合剤における電極活物質、カーボンブラック、およびフッ化ビニリデン共重合体の重量比は、固形分濃度変化の測定ではこの順で、100:2:1、スラリーの粘度変化測定ではこの順で、100:2:2である。電極合剤の組成を表1に示す。 (Preparation of electrode mixture)
Carbon black (SP: SuperP (registered trademark) manufactured by Timcal Japan, average particle size: 40 nm, specific surface area: 60 m 2 / g) was added to NCA811 as an electrode active material, and powder mixing was performed. On the other hand, a vinylidene fluoride copolymer (VDF / AA) was dissolved in N-methyl-2pyrrolidone (hereinafter referred to as NMP) to prepare a 5 wt% vinylidene fluoride solution. To the mixture of NCA811 and carbon black, the vinylidene fluoride solution was added in two portions and kneaded. Specifically, the vinylidene fluoride solution was prepared so that the solid content concentration was 84.2% by weight in the measurement of the solid content concentration change, and the solid content concentration was 81.5% by weight in the measurement of the viscosity change of the slurry. The mixture was added and primary kneading was performed at 2000 rpm for 2 minutes. Then, the remaining vinylidene fluoride solution is added so that the solid content concentration is 72.0% by weight in the measurement of the solid content concentration change, and the solid content concentration is 75% by weight in the measurement of the viscosity change of the slurry. And secondary kneading at 2000 rpm for 3 minutes to obtain an electrode mixture. The weight ratio of the electrode active material, carbon black, and vinylidene fluoride copolymer in the obtained electrode mixture was 100: 2: 1 in this order when measuring the solid content concentration change, and this ratio was measured when measuring the viscosity change of the slurry. In order, it is 100: 2: 2. Table 1 shows the composition of the electrode mixture.
(電極構造体の作製)
得られた電極合剤を、厚み15μmのアルミ箔上にバーコーターで塗布し、これを110℃で30分間、さらに130℃で2時間加熱乾燥して、乾燥状態における電極合剤の目付け量がおよそ200g/m2の電極構造体を作製した。 (Production of electrode structure)
The obtained electrode mixture was applied on an aluminum foil having a thickness of 15 μm with a bar coater, and this was heat-dried at 110 ° C. for 30 minutes and further at 130 ° C. for 2 hours. An electrode structure of approximately 200 g / m 2 was produced.
得られた電極合剤を、厚み15μmのアルミ箔上にバーコーターで塗布し、これを110℃で30分間、さらに130℃で2時間加熱乾燥して、乾燥状態における電極合剤の目付け量がおよそ200g/m2の電極構造体を作製した。 (Production of electrode structure)
The obtained electrode mixture was applied on an aluminum foil having a thickness of 15 μm with a bar coater, and this was heat-dried at 110 ° C. for 30 minutes and further at 130 ° C. for 2 hours. An electrode structure of approximately 200 g / m 2 was produced.
(スラリー粘度変化および固形分濃度変化の測定)
得られた電極合剤に関し、スラリー粘度および固形分濃度変化について測定を行った。結果を表2に示す。 (Measurement of slurry viscosity change and solid content concentration change)
The obtained electrode mixture was measured for slurry viscosity and solid content concentration change. The results are shown in Table 2.
得られた電極合剤に関し、スラリー粘度および固形分濃度変化について測定を行った。結果を表2に示す。 (Measurement of slurry viscosity change and solid content concentration change)
The obtained electrode mixture was measured for slurry viscosity and solid content concentration change. The results are shown in Table 2.
〔実施例2〕
フッ化ビニリデン共重合体(VDF/AA)を、フッ化ビニリデンとアクリル酸との共重合体であるフッ化ビニリデン共重合体(VDF/AA)と、フッ化ビニリデンとアクリロイロキシプロピルコハク酸との共重合体であるフッ化ビニリデン共重合体(VDF/APS)とのブレンドに変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。フッ化ビニリデン共重合体(VDF/AA)と、フッ化ビニリデン共重合体(VDF/APS)との重量比は、5:5である。 [Example 2]
A vinylidene fluoride copolymer (VDF / AA), a vinylidene fluoride copolymer (VDF / AA) which is a copolymer of vinylidene fluoride and acrylic acid, vinylidene fluoride and acryloyloxypropyl succinic acid, An electrode mixture was prepared in the same manner as in Example 1 except that it was changed to a blend with a vinylidene fluoride copolymer (VDF / APS) which is a copolymer of the above. The weight ratio of the vinylidene fluoride copolymer (VDF / AA) and the vinylidene fluoride copolymer (VDF / APS) is 5: 5.
フッ化ビニリデン共重合体(VDF/AA)を、フッ化ビニリデンとアクリル酸との共重合体であるフッ化ビニリデン共重合体(VDF/AA)と、フッ化ビニリデンとアクリロイロキシプロピルコハク酸との共重合体であるフッ化ビニリデン共重合体(VDF/APS)とのブレンドに変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。フッ化ビニリデン共重合体(VDF/AA)と、フッ化ビニリデン共重合体(VDF/APS)との重量比は、5:5である。 [Example 2]
A vinylidene fluoride copolymer (VDF / AA), a vinylidene fluoride copolymer (VDF / AA) which is a copolymer of vinylidene fluoride and acrylic acid, vinylidene fluoride and acryloyloxypropyl succinic acid, An electrode mixture was prepared in the same manner as in Example 1 except that it was changed to a blend with a vinylidene fluoride copolymer (VDF / APS) which is a copolymer of the above. The weight ratio of the vinylidene fluoride copolymer (VDF / AA) and the vinylidene fluoride copolymer (VDF / APS) is 5: 5.
本実施例におけるフッ化ビニリデン共重合体(VDF/AA)は、実施例1におけるフッ化ビニリデン共重合体(VDF/AA)と同様のものを使用した。
The same vinylidene fluoride copolymer (VDF / AA) as in Example 1 was used as the vinylidene fluoride copolymer (VDF / AA) in this example.
フッ化ビニリデン共重合体(VDF/APS)は、次のように調製した。内容量2リットルのオートクレーブに、イオン交換水1096g、メトローズ90SH-100(信越化学工業(株)製)0.2g、50wt%ジイソプロピルペルオキシジカーボネート-フロン225cb溶液2.2g、フッ化ビニリデン426g、およびアクリロイロキシプロピルコハク酸の初期添加量0.2gの各量を仕込み、26℃まで1時間で昇温した。その後、26℃を維持し、6wt%アクリロイロキシプロピルコハク酸水溶液を0.5g/minの速度で徐々に添加した。得られた重合体スラリーを脱水、乾燥してフッ化ビニリデン共重合体(VDF/APS)を得た。アクリロイロキシプロピルコハク酸は、初期に添加した量を含め、全量4gを添加した。
A vinylidene fluoride copolymer (VDF / APS) was prepared as follows. In an autoclave having an internal volume of 2 liters, 1096 g of ion-exchanged water, 0.2 g of Metroze 90SH-100 (manufactured by Shin-Etsu Chemical Co., Ltd.), 2.2 g of a 50 wt% diisopropyl peroxydicarbonate-fluorocarbon 225 cb, 426 g of vinylidene fluoride, and Each amount of 0.2 g of initial addition amount of acryloyloxypropyl succinic acid was charged, and the temperature was raised to 26 ° C. over 1 hour. Thereafter, the temperature was maintained at 26 ° C., and a 6 wt% acryloyloxypropyl succinic acid aqueous solution was gradually added at a rate of 0.5 g / min. The obtained polymer slurry was dehydrated and dried to obtain a vinylidene fluoride copolymer (VDF / APS). A total of 4 g of acryloyloxypropyl succinic acid was added, including the amount added initially.
得られた電極合剤に関し、スラリー粘度および固形分濃度変化について測定を行った。結果を表2に示す。
The obtained electrode mixture was measured for slurry viscosity and solid content concentration change. The results are shown in Table 2.
〔実施例3〕
フッ化ビニリデン共重合体(VDF/AA)を、実施例1のフッ化ビニリデン共重合体(VDF/AA)と、フッ化ビニリデン単独重合体(PVDF)とのブレンドに変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。フッ化ビニリデン単独重合体(PVDF)としては、株式会社クレハ製のKF#7200を用いた。フッ化ビニリデン共重合体(VDF/AA)と、フッ化ビニリデン単独重合体(PVDF)との重量比は、5:5である。 Example 3
Except that the vinylidene fluoride copolymer (VDF / AA) was changed to a blend of the vinylidene fluoride copolymer (VDF / AA) of Example 1 and a vinylidene fluoride homopolymer (PVDF), Example In the same manner as in Example 1, an electrode mixture was prepared to produce an electrode structure. As the vinylidene fluoride homopolymer (PVDF), KF # 7200 manufactured by Kureha Co., Ltd. was used. The weight ratio of the vinylidene fluoride copolymer (VDF / AA) to the vinylidene fluoride homopolymer (PVDF) is 5: 5.
フッ化ビニリデン共重合体(VDF/AA)を、実施例1のフッ化ビニリデン共重合体(VDF/AA)と、フッ化ビニリデン単独重合体(PVDF)とのブレンドに変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。フッ化ビニリデン単独重合体(PVDF)としては、株式会社クレハ製のKF#7200を用いた。フッ化ビニリデン共重合体(VDF/AA)と、フッ化ビニリデン単独重合体(PVDF)との重量比は、5:5である。 Example 3
Except that the vinylidene fluoride copolymer (VDF / AA) was changed to a blend of the vinylidene fluoride copolymer (VDF / AA) of Example 1 and a vinylidene fluoride homopolymer (PVDF), Example In the same manner as in Example 1, an electrode mixture was prepared to produce an electrode structure. As the vinylidene fluoride homopolymer (PVDF), KF # 7200 manufactured by Kureha Co., Ltd. was used. The weight ratio of the vinylidene fluoride copolymer (VDF / AA) to the vinylidene fluoride homopolymer (PVDF) is 5: 5.
得られた電極合剤に関し、スラリー粘度および固形分濃度変化について測定を行った。結果を表2に示す。
The obtained electrode mixture was measured for slurry viscosity and solid content concentration change. The results are shown in Table 2.
〔比較例1〕
フッ化ビニリデン共重合体(VDF/AA)を、実施例2のフッ化ビニリデン共重合体(VDF/APS)に変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。 [Comparative Example 1]
An electrode mixture was prepared in the same manner as in Example 1 except that the vinylidene fluoride copolymer (VDF / AA) was changed to the vinylidene fluoride copolymer (VDF / APS) of Example 2, and an electrode structure was prepared. The body was made.
フッ化ビニリデン共重合体(VDF/AA)を、実施例2のフッ化ビニリデン共重合体(VDF/APS)に変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。 [Comparative Example 1]
An electrode mixture was prepared in the same manner as in Example 1 except that the vinylidene fluoride copolymer (VDF / AA) was changed to the vinylidene fluoride copolymer (VDF / APS) of Example 2, and an electrode structure was prepared. The body was made.
得られた電極合剤に関し、スラリー粘度および固形分濃度変化について測定を行った。結果を表2に示す。
The obtained electrode mixture was measured for slurry viscosity and solid content concentration change. The results are shown in Table 2.
〔比較例2〕
フッ化ビニリデン共重合体(VDF/AA)を、実施例2のフッ化ビニリデン共重合体(VDF/APS)に変更し、電極活物資をLi1.00Ni0.52Co0.20Mn0.30O2(NCM523)に変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。 [Comparative Example 2]
The vinylidene fluoride copolymer (VDF / AA) was changed to the vinylidene fluoride copolymer (VDF / APS) of Example 2, and the electrode active material was Li 1.00 Ni 0.52 Co 0.20 Mn 0 An electrode mixture was prepared in the same manner as in Example 1 except that the material was changed to .30 O 2 (NCM523).
フッ化ビニリデン共重合体(VDF/AA)を、実施例2のフッ化ビニリデン共重合体(VDF/APS)に変更し、電極活物資をLi1.00Ni0.52Co0.20Mn0.30O2(NCM523)に変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。 [Comparative Example 2]
The vinylidene fluoride copolymer (VDF / AA) was changed to the vinylidene fluoride copolymer (VDF / APS) of Example 2, and the electrode active material was Li 1.00 Ni 0.52 Co 0.20 Mn 0 An electrode mixture was prepared in the same manner as in Example 1 except that the material was changed to .30 O 2 (NCM523).
得られた電極合剤に関し、スラリー粘度について測定を行った。結果を表2に示す。
The slurry viscosity was measured for the obtained electrode mixture. The results are shown in Table 2.
〔比較例3〕
フッ化ビニリデン共重合体(VDF/AA)を、実施例2のフッ化ビニリデン共重合体(VDF/APS)と、フッ化ビニリデンとクロロトリフルオロエチレンとの共重合体であるフッ化ビニリデン共重合体(VDF/CTFE)とのブレンドに変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。フッ化ビニリデン共重合体(VDF/APS)と、フッ化ビニリデン共重合体(VDF/CTFE)との重量比は、5:5である。 [Comparative Example 3]
The vinylidene fluoride copolymer (VDF / AA) was converted into the vinylidene fluoride copolymer (VDF / APS) of Example 2 and a copolymer of vinylidene fluoride and chlorotrifluoroethylene. An electrode mixture was prepared in the same manner as in Example 1, except that the blend was changed to a blend (VDF / CTFE). The weight ratio of the vinylidene fluoride copolymer (VDF / APS) and the vinylidene fluoride copolymer (VDF / CTFE) is 5: 5.
フッ化ビニリデン共重合体(VDF/AA)を、実施例2のフッ化ビニリデン共重合体(VDF/APS)と、フッ化ビニリデンとクロロトリフルオロエチレンとの共重合体であるフッ化ビニリデン共重合体(VDF/CTFE)とのブレンドに変更した以外は、実施例1と同様にして電極合剤を調製し、電極構造体を作製した。フッ化ビニリデン共重合体(VDF/APS)と、フッ化ビニリデン共重合体(VDF/CTFE)との重量比は、5:5である。 [Comparative Example 3]
The vinylidene fluoride copolymer (VDF / AA) was converted into the vinylidene fluoride copolymer (VDF / APS) of Example 2 and a copolymer of vinylidene fluoride and chlorotrifluoroethylene. An electrode mixture was prepared in the same manner as in Example 1, except that the blend was changed to a blend (VDF / CTFE). The weight ratio of the vinylidene fluoride copolymer (VDF / APS) and the vinylidene fluoride copolymer (VDF / CTFE) is 5: 5.
フッ化ビニリデン共重合体(VDF/CTFE)は、次のように調製した。内容量2リットルのオートクレーブに、イオン交換水1040g、メチルセルロース0.4g、ジイソプロピルパーオキシジカーボネート1.6g、酢酸エチル2g、フッ化ビニリデンg372、およびクロロトリフルオロエチレン28gを仕込み、28℃で懸濁重合を行った。重合完了後、重合体スラリーを脱水し、脱水した重合体スラリーを水洗し、再度重合体スラリーを脱水した後に、80℃で20時間乾燥してフッ化ビニリデン共重合体(VDF/CTFE)を得た。
A vinylidene fluoride copolymer (VDF / CTFE) was prepared as follows. An autoclave with an internal volume of 2 liters was charged with 1040 g of ion-exchanged water, 0.4 g of methylcellulose, 1.6 g of diisopropyl peroxydicarbonate, 2 g of ethyl acetate, g372 of vinylidene fluoride and 28 g of chlorotrifluoroethylene and suspended at 28 ° C. Polymerization was performed. After the polymerization is completed, the polymer slurry is dehydrated, the dehydrated polymer slurry is washed with water, the polymer slurry is dehydrated again, and then dried at 80 ° C. for 20 hours to obtain a vinylidene fluoride copolymer (VDF / CTFE). It was.
得られた電極合剤に関し、スラリー粘度について測定を行った。結果を表2に示す。
The slurry viscosity was measured for the obtained electrode mixture. The results are shown in Table 2.
表2に示されるように、バインダー組成部にフッ化ビニリデン共重合体(VDF/AA)を用いた電極合剤では、5日保存した後であっても、電極合剤スラリー粘度の増加が抑えられていた。一方、バインダー組成部にフッ化ビニリデン共重合体(VDF/APS)を用いた電極合剤では、短い期間(1日間)の保存であれば、電極合剤スラリー粘度の増加は抑えられていたが、より長い期間(5日間)保存した場合には、スラリー粘度の増加が観察された。
As shown in Table 2, in the electrode mixture using vinylidene fluoride copolymer (VDF / AA) in the binder composition part, the increase in the electrode mixture slurry viscosity is suppressed even after storage for 5 days. It was done. On the other hand, in the electrode mixture using the vinylidene fluoride copolymer (VDF / APS) in the binder composition part, the increase in the electrode mixture slurry viscosity was suppressed if stored for a short period (one day). When stored for a longer period (5 days), an increase in slurry viscosity was observed.
また、同じ電極活物質(NCA811)を用いた場合、フッ化ビニリデン共重合体(VDF/AA)を用いた電極合剤では、一定時間保存後の固形分濃度変化が抑えられていることが観察された。
In addition, when the same electrode active material (NCA811) was used, it was observed that the change in solid content concentration after storage for a certain time was suppressed in the electrode mixture using vinylidene fluoride copolymer (VDF / AA). It was done.
本発明は、リチウムイオン二次電池に利用することができる。
The present invention can be used for a lithium ion secondary battery.
1 正極
2 負極
3 セパレータ
5 金属ケーシング
10 電極構造体
11 集電体
12a,12b 電極合剤層 DESCRIPTION OF SYMBOLS 1Positive electrode 2 Negative electrode 3 Separator 5 Metal casing 10 Electrode structure 11 Current collector 12a, 12b Electrode mixture layer
2 負極
3 セパレータ
5 金属ケーシング
10 電極構造体
11 集電体
12a,12b 電極合剤層 DESCRIPTION OF SYMBOLS 1
Claims (10)
- 集電体上に設けられる電極活物質と、該電極活物質を該集電体に結着するためのバインダー組成物とを含有し、
上記バインダー組成物は、フッ化ビニリデンと、下記式(1)
で表される単量体との共重合体を含有し、
上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、
上記リチウム金属酸化物を水で抽出した際の該水のpHが11.3よりも大きいことを特徴とする電極合剤。 An electrode active material provided on the current collector, and a binder composition for binding the electrode active material to the current collector,
The binder composition includes vinylidene fluoride and the following formula (1):
Containing a copolymer with a monomer represented by
The electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
A lithium metal oxide represented by
An electrode mixture, wherein the pH of the lithium metal oxide when extracted with water is greater than 11.3. - 上記バインダー組成物中の塩素量が1000ppm以下であることを特徴とする請求項1に記載の電極合剤。 The electrode mixture according to claim 1, wherein the amount of chlorine in the binder composition is 1000 ppm or less.
- 上記共重合体における、上記式(1)で表される単量体に由来する構成単位が0.40mol%以上であることを特徴とする請求項1または2に記載の電極合剤。 The electrode mixture according to claim 1 or 2, wherein the constituent unit derived from the monomer represented by the formula (1) in the copolymer is 0.40 mol% or more.
- 上記バインダー組成物は、上記共重合体とは異なるフッ化ビニリデン系重合体を含むことを特徴とする請求項1~3のいずれか1項に記載の電極合剤。 The electrode mixture according to any one of claims 1 to 3, wherein the binder composition contains a vinylidene fluoride polymer different from the copolymer.
- 溶媒を含んでいる、請求項1~4のいずれか1項に記載の電極合剤。 The electrode mixture according to any one of claims 1 to 4, comprising a solvent.
- フッ化ビニリデンと、下記式(1)
で表される単量体との共重合体と、電極活物質とを混練する工程を含み、
上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、
上記リチウム金属酸化物は、水で抽出した際の該水のpHが11.3よりも大きいものであることを特徴とする電極合剤の製造方法。 Vinylidene fluoride and the following formula (1)
A step of kneading a copolymer with a monomer represented by: and an electrode active material,
The electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
A lithium metal oxide represented by
The method for producing an electrode mixture, wherein the lithium metal oxide has a pH higher than 11.3 when extracted with water. - 集電体と、該集電体上に設けられた電極合剤層とを備えており、
上記電極合剤層は、請求項1~5のいずれか1項に記載の電極合剤を用いて形成された層である、電極構造体。 A current collector, and an electrode mixture layer provided on the current collector,
6. The electrode structure according to claim 1, wherein the electrode mixture layer is a layer formed using the electrode mixture according to any one of claims 1 to 5. - 集電体と、該集電体上に設けられた電極合剤層とを備えており、
上記電極合剤層は、バインダー組成物および電極活物質を含む層であり、
上記バインダー組成物は、フッ化ビニリデンと、下記式(1)
で表される単量体との共重合体を含有し、
上記電極活物質は、下記式(2)
Li1+xMO2 ・・・(2)
(Xは、-0.15<X≦0.15を満たす数である。Mは、NiまたはNiを含む2種以上の元素群であって、Niを含む2種以上の元素群である場合には、Niを55mol%以上含む。)
で表されるリチウム金属酸化物を含み、
上記電極合剤層を水で抽出した際の該水のpHが11.3よりも大きいことを特徴とする電極構造体。 A current collector, and an electrode mixture layer provided on the current collector,
The electrode mixture layer is a layer containing a binder composition and an electrode active material,
The binder composition includes vinylidene fluoride and the following formula (1):
Containing a copolymer with a monomer represented by
The electrode active material has the following formula (2)
Li 1 + x MO 2 (2)
(X is a number satisfying −0.15 <X ≦ 0.15. M is a group of two or more elements including Ni or Ni and two or more elements including Ni. Includes 55 mol% or more of Ni.)
A lithium metal oxide represented by
An electrode structure, wherein the pH of the water when the electrode mixture layer is extracted with water is greater than 11.3. - 請求項5に記載の電極合剤を集電体表面に塗布して乾燥させることによって該集電体表面上に塗膜を形成する工程と、
上記塗膜に熱処理を施す工程とを含むことを特徴とする電極構造体の製造方法。 Forming a coating film on the current collector surface by applying the electrode mixture according to claim 5 to the current collector surface and drying;
And a step of heat-treating the coating film. - 請求項7または8に記載の電極構造体を備えていることを特徴とする二次電池。 A secondary battery comprising the electrode structure according to claim 7 or 8.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201880092521.0A CN112005406B (en) | 2018-05-15 | 2018-12-20 | Electrode mixture, method for producing electrode mixture, electrode structure, method for producing electrode structure, and secondary battery |
| KR1020207033834A KR102561871B1 (en) | 2018-05-15 | 2018-12-20 | Electrode mixture, method for manufacturing electrode mixture, electrode structure, method for manufacturing electrode structure, and secondary battery |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018-094081 | 2018-05-15 | ||
| JP2018094081A JP7017468B2 (en) | 2018-05-15 | 2018-05-15 | Electrode mixture, method for manufacturing electrode mixture, electrode structure, method for manufacturing electrode structure and secondary battery |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019220676A1 true WO2019220676A1 (en) | 2019-11-21 |
Family
ID=68539992
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/047007 WO2019220676A1 (en) | 2018-05-15 | 2018-12-20 | Electrode mixture, electrode mixture production method, electrode structure, electrode structure production method, and secondary battery |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP7017468B2 (en) |
| KR (1) | KR102561871B1 (en) |
| CN (1) | CN112005406B (en) |
| WO (1) | WO2019220676A1 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20220154689A (en) | 2020-03-17 | 2022-11-22 | 주식회사 쿠라레 | Additive for electrochemical device positive electrode, composition for electrochemical device positive electrode containing the same, and electrochemical device |
| TW202314738A (en) * | 2021-06-29 | 2023-04-01 | 日商大金工業股份有限公司 | Composition for forming electrode, electrode and secondary battery |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11195419A (en) * | 1997-12-26 | 1999-07-21 | Kureha Chem Ind Co Ltd | Depolarizing mix for nonaqueous battery and nonaqueous battery |
| WO2012090876A1 (en) * | 2010-12-28 | 2012-07-05 | 株式会社クレハ | Vinylidene-fluoride-based copolymer and application of said copolymer |
| JP2014502650A (en) * | 2010-12-22 | 2014-02-03 | ソルヴェイ・スペシャルティ・ポリマーズ・イタリー・エッセ・ピ・ア | Vinylidene fluoride copolymer |
| WO2018003448A1 (en) * | 2016-06-30 | 2018-01-04 | 日立金属株式会社 | Positive electrode material for lithium secondary cell, and positive electrode for lithium secondary cell and lithium secondary cell in which said positive electrode material for lithium secondary cell is used |
| JP2018073562A (en) * | 2016-10-27 | 2018-05-10 | 住友金属鉱山株式会社 | Positive electrode material for nonaqueous electrolyte secondary battery, nonaqueous electrolyte secondary battery arranged by use thereof, and method for manufacturing positive electrode material for nonaqueous electrolyte secondary battery |
| WO2018092675A1 (en) * | 2016-11-15 | 2018-05-24 | 株式会社クレハ | Vinylidene fluoride copolymer, binder composition, electrode mix, electrode, and nonaqueous-electrolyte secondary battery |
| WO2018092677A1 (en) * | 2016-11-15 | 2018-05-24 | 株式会社クレハ | Electrode mix, method for producing electrode mix, electrode structure, method for producing electrode structure, and secondary battery |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002249589A (en) * | 2001-02-23 | 2002-09-06 | Nippon Mektron Ltd | Gel composition |
| TW200410439A (en) * | 2002-11-22 | 2004-06-16 | Kureha Chemical Ind Co Ltd | Binder composition for electrode of nonaqueous electrolyte battery, and use thereof |
| JP2010074041A (en) | 2008-09-22 | 2010-04-02 | Victor Co Of Japan Ltd | Photodiode and integrated light receiving device with the same |
| JP2014007088A (en) | 2012-06-26 | 2014-01-16 | Hitachi Maxell Ltd | Nonaqueous electrolytic secondary battery and method for manufacturing the same |
| JPWO2017056974A1 (en) * | 2015-09-30 | 2018-03-29 | 株式会社クレハ | Binder composition, electrode for non-aqueous electrolyte secondary battery, and non-aqueous electrolyte secondary battery |
| JP2019140914A (en) * | 2016-06-27 | 2019-08-29 | 公立大学法人大阪府立大学 | Heat transfer plate, well plate unit and cell peeling device |
-
2018
- 2018-05-15 JP JP2018094081A patent/JP7017468B2/en active Active
- 2018-12-20 CN CN201880092521.0A patent/CN112005406B/en active Active
- 2018-12-20 KR KR1020207033834A patent/KR102561871B1/en active IP Right Grant
- 2018-12-20 WO PCT/JP2018/047007 patent/WO2019220676A1/en active Application Filing
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11195419A (en) * | 1997-12-26 | 1999-07-21 | Kureha Chem Ind Co Ltd | Depolarizing mix for nonaqueous battery and nonaqueous battery |
| JP2014502650A (en) * | 2010-12-22 | 2014-02-03 | ソルヴェイ・スペシャルティ・ポリマーズ・イタリー・エッセ・ピ・ア | Vinylidene fluoride copolymer |
| WO2012090876A1 (en) * | 2010-12-28 | 2012-07-05 | 株式会社クレハ | Vinylidene-fluoride-based copolymer and application of said copolymer |
| WO2018003448A1 (en) * | 2016-06-30 | 2018-01-04 | 日立金属株式会社 | Positive electrode material for lithium secondary cell, and positive electrode for lithium secondary cell and lithium secondary cell in which said positive electrode material for lithium secondary cell is used |
| JP2018073562A (en) * | 2016-10-27 | 2018-05-10 | 住友金属鉱山株式会社 | Positive electrode material for nonaqueous electrolyte secondary battery, nonaqueous electrolyte secondary battery arranged by use thereof, and method for manufacturing positive electrode material for nonaqueous electrolyte secondary battery |
| WO2018092675A1 (en) * | 2016-11-15 | 2018-05-24 | 株式会社クレハ | Vinylidene fluoride copolymer, binder composition, electrode mix, electrode, and nonaqueous-electrolyte secondary battery |
| WO2018092677A1 (en) * | 2016-11-15 | 2018-05-24 | 株式会社クレハ | Electrode mix, method for producing electrode mix, electrode structure, method for producing electrode structure, and secondary battery |
Also Published As
| Publication number | Publication date |
|---|---|
| CN112005406A (en) | 2020-11-27 |
| JP7017468B2 (en) | 2022-02-08 |
| CN112005406B (en) | 2024-02-09 |
| KR102561871B1 (en) | 2023-07-31 |
| KR20210002633A (en) | 2021-01-08 |
| JP2019200894A (en) | 2019-11-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5912814B2 (en) | Aqueous electrode binder for secondary battery | |
| WO2018092675A1 (en) | Vinylidene fluoride copolymer, binder composition, electrode mix, electrode, and nonaqueous-electrolyte secondary battery | |
| TW201041992A (en) | Aqueous polyvinylidene fluoride composition | |
| JP7160696B2 (en) | Electrodes for non-aqueous electrolyte secondary batteries | |
| JP6931658B2 (en) | Electrode mixture, electrode mixture manufacturing method, electrode structure, electrode structure manufacturing method and secondary battery | |
| JP2005310747A (en) | Binder for forming nonaqueous electrochemical element electrode, electrode mix, electrode structure, and electrochemical element | |
| WO2019239781A1 (en) | Binder composition, electrode mixture, electrode structure, method for manufacturing electrode structure, and secondary cell | |
| KR20230091957A (en) | Method for manufacturing an electrode for an all-solid-state battery | |
| WO2019220676A1 (en) | Electrode mixture, electrode mixture production method, electrode structure, electrode structure production method, and secondary battery | |
| JP7060405B2 (en) | Binder composition, electrode mixture and non-aqueous electrolyte secondary battery | |
| WO2019220677A1 (en) | Electrode mixture, electrode mixture production method, electrode structure, electrode structure production method, and secondary battery | |
| WO2018092676A1 (en) | Electrode mixture, electrode mixture production method, electrode structure body, electrode structure body production method and secondary battery | |
| JP2013178926A (en) | Positive electrode mixture for nonaqueous secondary battery | |
| JP5400058B2 (en) | Non-aqueous electrolyte secondary battery negative electrode mixture, non-aqueous electrolyte secondary battery negative electrode and non-aqueous electrolyte secondary battery | |
| JP7209420B2 (en) | Electrodes for non-aqueous electrolyte secondary batteries | |
| JP2021504882A (en) | PVDF binder for graphite / silicon anode |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18919220 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20207033834 Country of ref document: KR Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18919220 Country of ref document: EP Kind code of ref document: A1 |