WO2018026971A1 - Symmetric or semi-symmetric compounds useful as immunomodulators - Google Patents
Symmetric or semi-symmetric compounds useful as immunomodulators Download PDFInfo
- Publication number
- WO2018026971A1 WO2018026971A1 PCT/US2017/045185 US2017045185W WO2018026971A1 WO 2018026971 A1 WO2018026971 A1 WO 2018026971A1 US 2017045185 W US2017045185 W US 2017045185W WO 2018026971 A1 WO2018026971 A1 WO 2018026971A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- amino
- biphenyl
- methoxy
- hydroxyethyl
- Prior art date
Links
- 0 C1IC*IC1 Chemical compound C1IC*IC1 0.000 description 9
- ZNAJUTSDMWGQTK-UHFFFAOYSA-N Bc1c[s]c(C(O)=O)c1C Chemical compound Bc1c[s]c(C(O)=O)c1C ZNAJUTSDMWGQTK-UHFFFAOYSA-N 0.000 description 1
- ZJOKBALCQWVEIM-UHFFFAOYSA-N Bc1c[s]c(CO)c1C Chemical compound Bc1c[s]c(CO)c1C ZJOKBALCQWVEIM-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N C1CCCCC1 Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- HJDVELGCILNENX-FPLPWBNLSA-N COc1cc(OC(C/C=C\C=C)Oc2cc(OC)c(C=O)c(OC)c2)cc(OC)c1C=O Chemical compound COc1cc(OC(C/C=C\C=C)Oc2cc(OC)c(C=O)c(OC)c2)cc(OC)c1C=O HJDVELGCILNENX-FPLPWBNLSA-N 0.000 description 1
- NWAJHVJGMVOKKY-UHFFFAOYSA-N Cc(c(-c1c(C)c(-c2c[n](cc(C=O)cc3)c3n2)ccc1)ccc1)c1-c1c[n](cc(C=O)cc2)c2n1 Chemical compound Cc(c(-c1c(C)c(-c2c[n](cc(C=O)cc3)c3n2)ccc1)ccc1)c1-c1c[n](cc(C=O)cc2)c2n1 NWAJHVJGMVOKKY-UHFFFAOYSA-N 0.000 description 1
- GJPJZXDIJPHRQE-UHFFFAOYSA-N Cc(c(-c1c(C)c(-c2c[n](cc(CNCCO)cc3)c3n2)ccc1)ccc1)c1-c1c[n](cc(CNCCO)cc2)c2n1 Chemical compound Cc(c(-c1c(C)c(-c2c[n](cc(CNCCO)cc3)c3n2)ccc1)ccc1)c1-c1c[n](cc(CNCCO)cc2)c2n1 GJPJZXDIJPHRQE-UHFFFAOYSA-N 0.000 description 1
- XSOBGBMZWVMOPG-UHFFFAOYSA-N Cc(c(-c1c[n](cc(cc2)I)c2n1)ccc1)c1-c1cccc(-c2c[n](cc(cc3)I)c3n2)c1C Chemical compound Cc(c(-c1c[n](cc(cc2)I)c2n1)ccc1)c1-c1cccc(-c2c[n](cc(cc3)I)c3n2)c1C XSOBGBMZWVMOPG-UHFFFAOYSA-N 0.000 description 1
- QGUQZBUGTYGYIR-UHFFFAOYSA-N Cc(c(-c1cccc(-c2c[n](cc(cc3)C#N)c3n2)c1C)ccc1)c1-c1c[n](cc(cc2)C#N)c2n1 Chemical compound Cc(c(-c1cccc(-c2c[n](cc(cc3)C#N)c3n2)c1C)ccc1)c1-c1c[n](cc(cc2)C#N)c2n1 QGUQZBUGTYGYIR-UHFFFAOYSA-N 0.000 description 1
- PUOCKKZSAZMBRZ-UHFFFAOYSA-N Cc1c(CO)[s]cc1-c1c(C)c(CO)ccc1 Chemical compound Cc1c(CO)[s]cc1-c1c(C)c(CO)ccc1 PUOCKKZSAZMBRZ-UHFFFAOYSA-N 0.000 description 1
- IQODZKORUMDYEX-UHFFFAOYSA-N Cc1c(COc2cc(OC)c(CNCCO)c(OC)c2)cccc1-c1cccc(COc2cc(OC)c(CNCCO)c(OC)c2)c1C Chemical compound Cc1c(COc2cc(OC)c(CNCCO)c(OC)c2)cccc1-c1cccc(COc2cc(OC)c(CNCCO)c(OC)c2)c1C IQODZKORUMDYEX-UHFFFAOYSA-N 0.000 description 1
- IVILGUFRMDBUEQ-UHFFFAOYSA-N Nc(cc1)ncc1I Chemical compound Nc(cc1)ncc1I IVILGUFRMDBUEQ-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D205/00—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom
- C07D205/02—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom not condensed with other rings
- C07D205/04—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/10—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/12—Oxygen or sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/10—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/16—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/30—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members
- C07D207/32—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms
- C07D207/33—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having two double bonds between ring members or between ring members and non-ring members with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to ring carbon atoms with substituted hydrocarbon radicals, directly attached to ring carbon atoms
- C07D207/333—Radicals substituted by oxygen or sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/04—Indoles; Hydrogenated indoles
- C07D209/08—Indoles; Hydrogenated indoles with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, directly attached to carbon atoms of the hetero ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/44—Iso-indoles; Hydrogenated iso-indoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/36—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D211/60—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/24—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D213/28—Radicals substituted by singly-bound oxygen or sulphur atoms
- C07D213/30—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/62—Oxygen or sulfur atoms
- C07D213/63—One oxygen atom
- C07D213/64—One oxygen atom attached in position 2 or 6
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/62—Oxygen or sulfur atoms
- C07D213/69—Two or more oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/84—Nitriles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/84—Nitriles
- C07D213/85—Nitriles in position 3
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/54—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings condensed with carbocyclic rings or ring systems
- C07D231/56—Benzopyrazoles; Hydrogenated benzopyrazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D263/00—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings
- C07D263/52—Heterocyclic compounds containing 1,3-oxazole or hydrogenated 1,3-oxazole rings condensed with carbocyclic rings or ring systems
- C07D263/54—Benzoxazoles; Hydrogenated benzoxazoles
- C07D263/56—Benzoxazoles; Hydrogenated benzoxazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached in position 2
- C07D263/57—Aryl or substituted aryl radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/60—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings condensed with carbocyclic rings or ring systems
- C07D277/62—Benzothiazoles
- C07D277/64—Benzothiazoles with only hydrocarbon or substituted hydrocarbon radicals attached in position 2
- C07D277/66—Benzothiazoles with only hydrocarbon or substituted hydrocarbon radicals attached in position 2 with aromatic rings or ring systems directly attached in position 2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/14—Radicals substituted by singly bound hetero atoms other than halogen
- C07D333/16—Radicals substituted by singly bound hetero atoms other than halogen by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D519/00—Heterocyclic compounds containing more than one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system not provided for in groups C07D453/00 or C07D455/00
Definitions
- the present invention generally relates to compounds useful as inhibitors of the PD- 1/PD-Ll protein/protein and CD80/PD-L1 protein/protein interactions.
- Programmed cell death- 1 (PD-1, CD279) protein is a member of the CD28 superfamily that can suppress activation signals upon interaction with either of its natural ligands, program cell death-ligand 1 (PD-Ll, CD274, B7-H1) and PD-L2 (CD273, B7-DC).
- PD-1 and its ligands are broadly expressed in immune systems and play critical roles in immune modulation including T cell activation and tolerance (Sharpe et. al., Nat. Imm. 2007).
- T cells When PD-Ll expressing cells contact T cells that express PD-1, it results in attenuation of T cell activities in cytokine secretion, cytolytic activity and proliferation, in response to antigenic stimulation.
- T cell exhaustion a biological significance of PD-1/PD-L1 interaction in attenuating infectious immunity, tumor immunity and in facilitating chronic infection and tumor progression.
- PD-Ll has also been shown to interact with CD80.
- the interaction between PD-Ll and CD80 on immune cells has also been shown to inhibit immune response (Deng et. al., J Immunol. 2015).
- Monoclonal antibodies that block the PD-1 -mediated immune checkpoint pathway can prevent T cell down regulation and promote immune responses against cancer and infectious diseases (Harvey et. al., Clin Pharmacol Ther 2014). Ample clinical and preclinical evidence has demonstrated that inhibition of PD-1/PD-L1 interaction with PD-l-or PD-L1- specific antibodies can restore immune response to diverse forms of cancer and infectious diseases (Dong et. al., Nat. Med. 2002; Dong et. al., J Mol. Med. 2003).
- PD- 1/PD-Ll -targeting antibodies including pembrolizumab, nivolumab and atezolizumab
- IO immuno-oncology
- studies have demonstrated that interference with PD-l/PD- Ll interaction can also enhance T cell activity in chronic infection systems, thus expected to function against viral infections, like HIV (Attanasio et. al., Immunity. 2016; Porichis et. al., Curr HIV/ AIDS Rep. 2012).
- blockage of the PD-l/PD-Ll pathway can also enhance responses to vaccination, including therapeutic vaccination in the context of viral infection (Ha et. al., J. Exp. Med. 2008). More recent discoveries also suggested therapeutic value of disrupting PD1/PD-L1 interaction for patients with certain inflammation and neurodegenerative diseases like Alzheimer's disease (Bodhankar et. al., Stroke 2015; Baruch et. al., Nat Med. 2016).
- agents that block the interaction of PD-Ll with either PD-1 or CD80 are considered beneficial for diverse therapeutic areas, including but not limited to cancer, viral infection and vaccination.
- PD-l/PD-Ll -targeting antibodies there is still great demand for more potent, better selective and more easily administered therapeutics against PD-l/PD-Ll and/or CD80/PD-L1 protein-protein interactions.
- the elucidation of structural details of the human PD-l/PD-Ll complex has provided unique insights into the molecular mechanism of PD-l/PD-Ll protein-protein interaction (Zak et. al., Oncotarget 2016; Zak et. al., Structure 2015). Such has afforded an opportunity for structural- based design of small molecule inhibitors to target PD-l/PD-Ll and/or CD80/PD-L1 interaction.
- potent small molecules that can have activity as inhibitors of the interaction of PD-Ll with PD-1 and/or CD80, and thus may be useful for therapeutic administration to enhance immunity against cancer and/or infectious diseases.
- These small molecules are expected to be useful as pharmaceuticals with desirable stability, bioavailability, therapeutic index, and toxicity values that are crucial to become efficient medicines to promote human health.
- inhibitors of the PD-l/PD-Ll protein/protein and CD80/PD-L1 protein/protein are the inhibitors of the PD-l/PD-Ll protein/protein and CD80/PD-L1 protein/protein
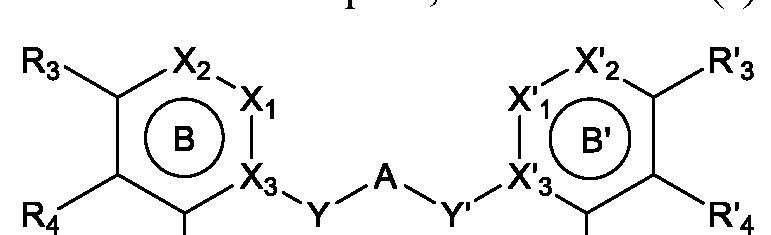
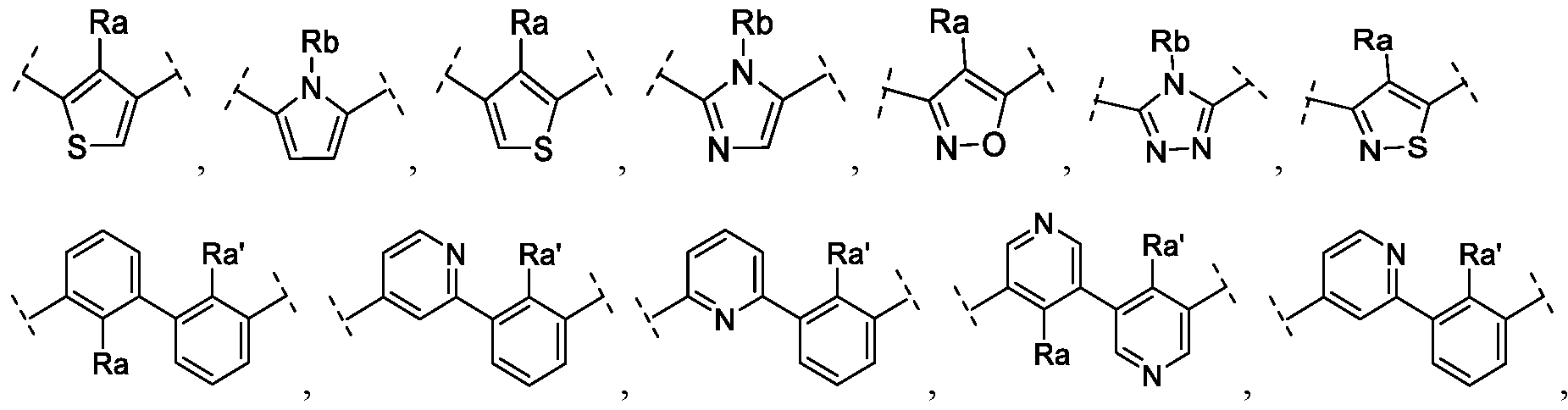
- A is a bivalent arene or a bivalent heteroarene
- Ring B and Ring B' are independently a 6-membered aromatic hydrocarbon ring, a 6-membered heterocyclic ring, a 9- to 10-membered aromatic hydrocarbon ring, or a 9- to 10-membered heterocyclic ring
- Y and Y' are independently, null (direct bond), -CHRi-, -CH2-CH2-, - Ri-, -0-, -OCH2-, -CH2O-, -SCH2-, -CH 2 S-, -SOCH2-, -CH2SO- or -S0 2 CH 2 -
- Ri is H, C 1-6 alkyl, or C3-6 cycloalkyl
- R 3 and R' 3 are independently H, SO 2 H 2 ,
- R 5 and Rg are independently H, C 1-6 alkyl, C 3-8 cycloalkyl, or heteroaryl or R 5 and 5 form a C 3 - 8 cycloalkyl, heterocyclyl, or heteroaryl ring
- R 7 is H, aryl, heteroaryl, acetyl, CH 2 CH 2 OH, CH 2 CH 2 HCOCH 3 , C 3 -C 8 alkyl carboxylic acid, C 3 -C 8 alkyl amide, C 3 -C 8 alkyl alcohol, - CH 2 -Ar, or -CH 2 -heterocyclyl
- R 4 , R' 4 , Z, and Z' are independently H, halogen, CHF 2 , CF 3 , CN, Ci-6 alkyl, C 1-6 alkoxy, aryl, or heteroaryl.
- Formula (I) is represented by following Formula (II):
- Ring B and Ring B' are independently a 6- membered aromatic hydrocarbon ring or a 6-membered heterocyclic ring;
- X 3 , and X' 3 are
- Y and Y' are independently -CHRi-, -CH 2 -CH 2 -, -NRi-, -0-, -OCH 2 -, -CH 2 O-, -SCH 2 -, -CH 2 S-, -SOCH 2 -, -CH 2 SO- or -SO 2 CH 2 -, and Ri is H, Ci -6 alkyl, or C 3-6 cycloalkyl;
- Formula (I) is represented by following Formula (III):
- Ring B and Ring B' are independently a 9- to 10-membered aromatic hydrocarbon ring or a 9- to 10-membered heterocyclic ring;
- X 2 , X' 2, X 3 , and X' 3 are independently C or N;
- U and U' are independently C and N;
- Y and Y' are independently, null (direct bond), -CHRi- , -CH 2 -CH 2 -, - Ri-, -0-, -OCH 2 -, -CH 2
- R a ' are independently COR', OMe, halogen, C 1-6 alkyl, C 2-6 alkynyl, C 1-6 cycloalkyl, CN, CF 3; CH 2 CF 3 ;
- R' is C 1-6 alkyl;
- R and R b ' are independently C 1-6 alkyl, C 2 - 6 alkynyl, C 1-6 cycloalkyl or alkyl halogen, and
- R 3 and R' 3 are the same, R4 and R' 4 are the same, Y and Y' are the same, Z and Z' are the same, or Ring B and Ring B' are the same.
- R 3 and R' 3 are the same, R4 and R' 4 are the same, Y and Y' are the same, Z and Z' are the same, and Ring B and Ring B' are the same.
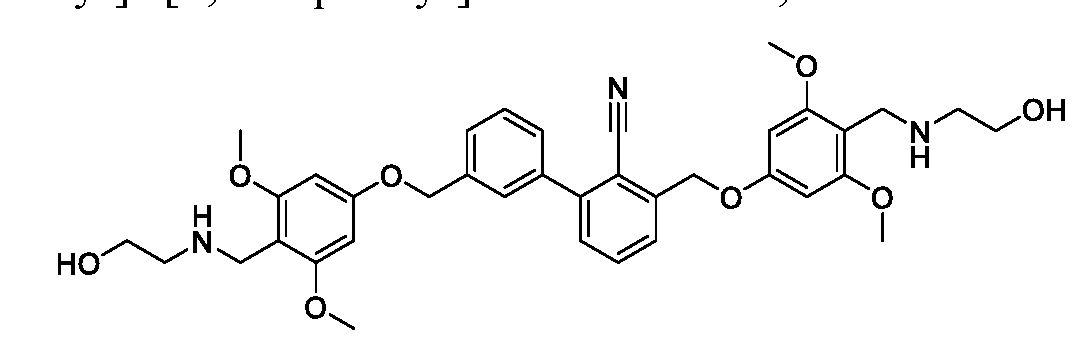
- the inhibitors of the PD-l/PD-Ll protein/protein and CD80/PD-L1 protein/protein interactions are 2-( ⁇ [4-( ⁇ 3'-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -2- methylphenoxy)methyl]-2,2'-dimethyl-[ 1 , 1 '-biphenyl]-3 -yl ⁇ methoxy)-3 - methylphenyl]methyl ⁇ amino)ethan-l-ol, 2- ⁇ [(4- ⁇ [3-( ⁇ 4-[(azetidin-l-yl)methyl]-2- methylphenoxy ⁇ methyl)-2-chlorophenyl]methoxy ⁇ -3 -methylphenyl)m ethyl] amino ⁇ ethan- 1 - ol, l-[(4- ⁇ [3-( ⁇ 4-[(azetidin-l-yl)methyl]-2-methylphenoxy ⁇ methyl)-2-
- described herein is a method of treating a disease associated with the modulation of PD-1/PD-L1 or CD80/PD-L1 interaction comprising administering to a patient the compound of Formula (I) or a pharmaceutically acceptable salt thereof.
- the disease is an infection, inflammation cancer, or neurodegenerative disorders like Alzheimer's disease.
- Figure 1 shows the docking pose of compound 1-1 in PD-L1 dimer.
- Figure 2 shows the docking pose of compound I- 10 in PD-L1 dimer.
- Prodrugs mean any compound which releases an active parent drug according to Formula (I) in vivo when such prodrug is administered to a mammalian subject.
- Prodrugs of a compound of Formula (I) are prepared by modifying functional groups present in the compound of Formula (I) in such a way that the modifications may be cleaved in vivo to release the parent compound.
- Prodrugs may be prepared by modifying functional groups present in the compounds in such a way that the modifications are cleaved, either in routine manipulation or in vivo, to the parent compounds.
- Tautomers mean compounds produced by the phenomenon wherein a proton of one atom of a molecule shifts to another atom. Tautomers also refer to one of two or more structural isomers that exist in equilibrium and are readily converted from one isomeric form to another. One of ordinary skill in the art would recognize that other tautomeric ring atom arrangements are possible. All such isomeric forms of these compounds are expressly included in the present disclosure.
- Isomers mean compounds having identical molecular formulae but differ in the nature or sequence of bonding of their atoms or in the arrangement of their atoms in space. Isomers that differ in the arrangement of their atoms in space are termed stereoisomers. Stereoisomers that are not mirror images of one another are termed diastereomers, and those that are non- superimposable mirror images of each other are termed enantiomers. When a compound has an asymmetric center, for example, it is bonded to four different groups, a pair of enantiomers is possible. A chiral compound can exist as either individual enantiomer or as a mixture thereof. Unless otherwise indicated, the description is intended to include individual stereoisomers as well as mixtures.
- Solvates refer to a complex formed by combination of solvent molecules with the compound of Formula (I).
- the solvent can be an organic compound, an inorganic compound, or a mixture thereof.
- Pharmaceutically acceptable salts represent those salts which are, within the scope of medical judgement, suitable for use in contact for the tissues of humans and lower animals without undue toxicity, irritation, allergic response and the like, and are commensurate with a reasonable benefit/risk ratio. They may be obtained during the final isolation and purification of the compounds of the invention, or separately by reacting the free base function with a suitable mineral acid such as hydrochloric acid, phosphoric acid, or sulfuric acid, or with an organic acid such as for example ascorbic acid, citric acid, tartaric acid, lactic acid, maleic acid, malonic acid, fumaric acid, glycolic acid, succinic acid, propionic acid, acetic acid, methanesulfonic acid, and the like.
- the acid function can be reacted with an organic or a mineral base, like sodium hydroxide, potassium hydroxide or lithium hydroxide.
- Therapeutically effective amount means an amount of compound or a composition of the present invention effective in inhibiting the PD-1/PD-L1 protein/protein and CD80/PD- Ll protein/protein interactions, and thus producing the desired therapeutic effect.
- alkyl refers to a monovalent straight or branched chain, saturated aliphatic hydrocarbon radical having a number of carbon atoms in the specified range.
- C 1-6 alkyl refers to any of the hexyl alkyl and pentyl alkyl isomers as well as n-, iso-, sec- and t-butyl, n- and iso-propyl, ethyl and methyl.
- Alkyl also includes saturated aliphatic hydrocarbon radicals wherein one or more hydrogen atoms are replaced with deuterium, for example, CD 3 .
- branched alkyl refers to an alkyl group as defined above except that straight chain alkyl groups in the specified range are excluded.
- branched alkyl includes alkyl groups in which the alkyl is attached to the rest of the compound via a secondary or tertiary carbon.
- isopropyl is a branched alkyl group.
- cycloalkyl refers to any monocyclic ring of an alkane having a number of carbon atoms in the specified range.
- C 3 - 6 cycloalkyl refers to cyclopropyl, cyclobutyl,cyclopentyl, and cyclohexyl.
- halogen refers to fluorine, chlorine, bromine and iodine (alternatively referred to as fluoro, chloro, bromo, and iodo).
- haloalkyl refers to an alkyl group as defined above in which one or more of the hydrogen atoms have been replaced with a halogen (i.e., F, CI, Br and/or I).
- a halogen i.e., F, CI, Br and/or I.
- Ci-6 haloalkyl refers to a Ci to C 6 linear or branched alkyl group as defined above with one or more halogen substituents.
- fluoroalkyl has an analogous meaning except that the halogen substituents are restricted to fluoro. Suitable fluoroalkyls include the series (CH 2 ) 0- 4 CF 3 .
- C(O) or CO refers to carbonyl.
- S(0) 2 or S0 2 refers to sulfonyl.
- S(O) or SO refers to sulfinyl.
- aromatic hydrocarbons refers to hydrocarbons with sigma bonds and delocalized pi electrons between carbon atoms forming rings.
- aryl refers to phenyl, naphthyl, tetrahydronaphthyl, idenyl, dihydroindenyl and the like.
- An aryl of particular interest is phenyl.
- heterocyclic refers to heteroaryl, saturated heterocyclic, and unsaturated heterocyclic with a double bond.
- heteroaryl refers to (i) a 5- or 6-membered heteroaromatic ring containing from 1 to 4 heteroatoms independently selected from N, O and S, or (ii) is a heterobicyclic ring selected from quinolinyl, isoquinolinyl, and quinoxalinyl.
- Suitable 5- and 6-membered heteroaromatic rings include, for example, pyridyl (also referred to as pyridinyl), pyrrolyl, pyrazinyl, pyrimidinyl, pyridazinyl, triazinyl, thienyl, furanyl, imidazolyl, pyrazolyl, triazolyl, tetrazolyl, oxazolyl, isooxazolyl, oxadiazolyl, oxatriazolyl, thiazolyl, isothiazolyl, and thiadiazolyl.
- a class of heteroaryls of interest consists of (i) 5- and 6-membered heteroaromatic rings containing from 1 to 3 heteroatoms independently selected from N, O and S, and (ii) heterobicyclic rings selected from quinolinyl, isoquinolinyl, and quinoxalinyl.
- Heteroaryls of particular interest are pyrrolyl, imidazolyl, pyridyl, pyrazinyl, quinolinyl (or quinolyl), isoquinolinyl (or isoquinolyl), and quinoxalinyl.
- Examples of 4- to 7-membered, saturated heterocyclic rings within the scope of this invention include, for example, azetidinyl, piperidinyl, morpholinyl, thiomorpholinyl, thiazolidinyl, isothiazolidinyl, oxazolidinyl, isoxazolidinyl, pyrrolidinyl, imidazolidinyl, piperazinyl, tetrahydrofuranyl, tetrahydrothienyl, pyrazolidinyl, hexahydropyrimidinyl, thiazinanyl, thiazepanyl, azepanyl, diazepanyl, tetrahydropyranyl, tetrahydrothiopyranyl, and dioxanyl.
- Examples of 4- to 7-membered, unsaturated heterocyclic rings within the scope of this invention include mono-unsaturated heterocyclic rings corresponding to the saturated heterocyclic rings listed in the preceding sentence in which a single bond is replaced with a double bond (e.g., a carbon-carbon single bond is replaced with a carbon-carbon double bond).
- A is a bivalent bi-aryl or bi-heteroaryl core
- a symmetric or semi-symmetric compound of Formula (I) can be synthesized via Suzuki palladium catalyzed cross-coupling of aryl/heteroaryl halide (II) and aryl/heteroaryl boronic acid/ester (III) as shown in Scheme 1-3.
- A is Xi-3, ⁇ -3 , Y, Y', Z and Z' are defined as in Formula (I).
- A is , R 3 -R4, R 3 '-R 4 ', X1-3, X' 1 -3 , Y, Y', Z and Z' are defined as in Formula (I).
- a semi-symmetric compound of Formula (I) can be synthesized via Chan-Lam copper catalyzed C-N coupling reaction of aryl/heteroaryl boronic acid (III) with N-H containing aryl/heteroaryl (V) as shown in Scheme 3.
- A is R3-R4, R3 -R4' , X1-3, X' 1-3, Y, Y', Z and Z' are defined as in Formula (I).
- A is a bivalent aryl or heteroaryl core
- a symmetric or semi-symmetric compound of Formula (I) can be synthesized via Mitsunobu reaction of aryl/heteroaryl alcohol (VI) and aryl/heteroaryl phenol (VII).
- a compound of formula (I) can also be prepared by O-alkylation or N-alkylation of aryl/heteroaryl (VII or IX) with aryl/heteroaryl halides (VIII) under basic condition (Scheme 4 to Scheme6).
- A is -R4 R3 -R4', X1-3, X' 1-3, Y, Y', Z and Z' are defined as in Formula (I).
- IP A isopropyl alcohol
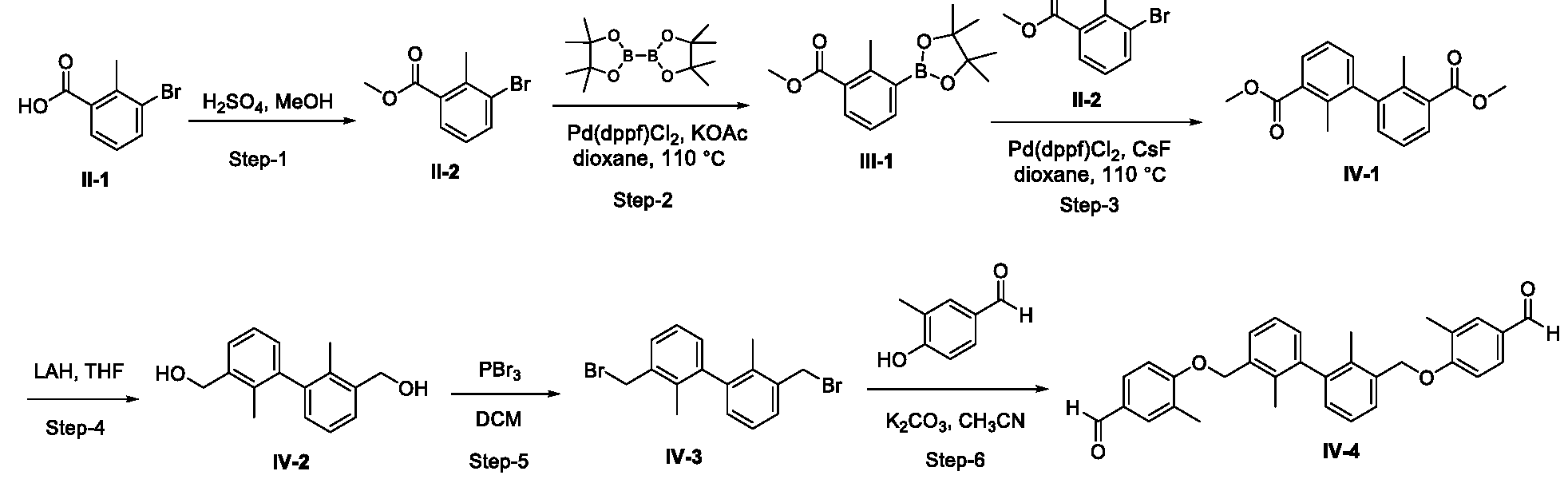
- Example 1 2-( ⁇ [4-( ⁇ 3'-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -2-methylphenoxy) methyl]-2,2'-dimethyl-[l, l'-biphenyl]-3-yl ⁇ methoxy)-3-methylphenyl]methyl ⁇ amino)ethan- l-ol (1-1).
- Example 1-1 The compound of Example 1 was synthesized via the route shown in the scheme below.
- Step-2 2-methyl-3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl) benzoate ( ⁇ -1)
- Step-3 dimethyl 2,2'-dimethyl-[l, r-biphenyl]-3,3'-dicarboxylate (IV-1)
- 2-methyl-3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl) benzoate III-l 1.5 g, 5.4 mmol
- methyl-3-bromo-2-methylbenzoate II-2 1.1 g, 4.9 mmol
- CsF 2.2 g, 14.7 mmol
- Pd(dppf)Cl 2 250 mg, 0.07 mmol
- Step-4 (2,2'-dimethyl-[l, r-biphenyl]-3,3'-diyl)dimethanol (IV-2)
- Step-6 4,4'-(((2,2'-dimethyl-[l,r-biphenyl]-3,3'-diyl)bis(methylene))bis(oxy))bis(3- methyl benzaldehyde), IV-4
- Step-7 2-( ⁇ [4-( ⁇ 3'-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -2-methylphenoxy)methyl]- 2,2'-dimethyl-[l, l'-biphenyl]-3-yl ⁇ methoxy)-3-methylphenyl]methyl ⁇ amino)ethan-l-ol, 1-1.
- Example 2 2- ⁇ [(4- ⁇ [3-( ⁇ 4-[(azetidin-l-yl)methyl]-2-methylphenoxy ⁇ methyl)-2- chlorophenyl]methoxy ⁇ -3-m hylphenyl)methyl]amino ⁇ ethan-l-ol (1-2).
- Example 2 The compound of Example 2 was synthesized via the route shown in the scheme below.
- Step-1 synthesis of l,3-bis(bromomethyl)-2-chlorobenzene
- Step-2 synthesis of 4,4'-(2-chloro-l,3-phenylene)bis(methylene)bis(oxy)bis(3-methyl benzaldehyde) (IV-5)
- Step-3 synthesis of 4-(3-((4-(azetidin-l-ylmethyl)-2-methylphenoxy)methyl)-2- chlorobenzyloxy)-3-methylbenzaldehyde (IV-6) and l-[(4- ⁇ [3-( ⁇ 4-[(azetidin-l-yl)methyl]-2- methylphenoxy ⁇ methyl)-2-chlorophenyl]methoxy ⁇ -3-methylphenyl)methyl]azetidine (1-3)
- Step-4 synthesis of 2- ⁇ [(4- ⁇ [3-( ⁇ 4-[(azetidin-l-yl)methyl]-2- methylphenoxy ⁇ methyl)-2-chlorophenyl] methoxy ⁇ -3 -methylphenyl)methyl] amino ⁇ ethan- 1 - ol (1-2)
- Example 3 The compound of Example 3 was synthesized via the route shown in the scheme below.
- Step-1 4,4'-(2,2'-dimethylbiphenyl-3,3'-diyl)bis(methylene)bis(oxy)bis(2,6- dimethoxybenzaldehyde) (IV-7)
- Step-2 (S)-l-(4-((3'-((4-formyl-3,5-dimethoxyphenoxy)methyl)-2,2'- dimethylbiphenyl-3-yl)methoxy)-2,6-dimethoxybenzyl)piperidine-2-carboxylic acid (IV-8) & (2S)-l- ⁇ [4-( ⁇ 3'-[(4- ⁇ [(2S)-2-carboxypiperidin-l-yl]methyl ⁇ -3,5-dimethoxyphenoxy)methyl]- 2,2'-dimethyl-[l, -biphenyl]-3-yl ⁇ methoxy)-2,6-dimethoxyphenyl]methyl ⁇ piperidine-2- carboxylic acid (1-4)
- Example 4 (2 S)- 1 - ⁇ [4-( ⁇ 3 ' -[(4- ⁇ [(2-hy droxy ethyl)amino]methyl ⁇ -3 , 5 - dimethoxyphenoxy)methyl]-2,2'-dimethyl-[l, l'-biphenyl]-3-yl ⁇ methoxy)-2,6- dimethoxyphenyl]methyl ⁇ piperidine-2-carboxylic acid (1-5).
- Example 6 l- ⁇ [4-( ⁇ 3'-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -2-methyl phenoxy)methyl]-2,2'-dimethyl-[l, l'-biphenyl]-3-yl ⁇ methoxy)-2- methoxyphenyl]methyl ⁇ azetidin-3-ol (1-7).
- Step-1 4-((3-bromo-2-methylbenzyl)oxy)-2-methoxybenzaldehyde ( ⁇ -4)
- Step-2 l-(4-((3-bro -2-methylbenzyl)oxy)-2-methoxybenzyl)azetidin-3-ol(II-5)
- Step-1 4-((3'-((4-((3-hydroxyazetidin-l-yl)methyl)-3-methoxy phenoxy)methyl)-2,2' dimethyl-[ 1 , 1 '-biphenyl]-3 -yl)methoxy)benzaldehyde
- Tetrahydrofuran solvent and aqueous 0.5M potassium tribasic phosphate solutions were sparged with nitrogen for 15 minutes prior to dispensing for use.
- reaction product is purified by silica gel chromatography eluting with an appropriate gradient of ethyl acetate/hexanes to yield a pure title compound.
- Step-2 l- ⁇ [4-( ⁇ 3'-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ phenoxy)methyl]-2,2'- dimethyl-[l,r-biphenyl]-3-yl ⁇ methoxy)-2-methoxyphenyl]methyl ⁇ azetidin-3-ol (1-7)
- Tetrahydrofuran solvent and aqueous 0.5M potassium tribasic phosphate solutions were sparged with nitrogen for 15 minutes prior to dispensing for use.
- a round-bottom flask charge (2-methyl-3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)phenyl)methanol III-2 (1.2 mmol), (4-bromo-3-methylthiophen-2-yl)methanol ( ⁇ -6) (1.0 mmol) and 2G palladium Xphos precatalyst (0.03 mmol)(CAS number 1310584- 14-5), add previously deoxygenated tetrahydrofuran (5 mL) and 0.5 M aq potassium phosphate, tribasic solution (5 mL, 2.5 mmol), place under nitrogen and sparged with additional nitrogen for 10 minutes.
- reaction product is purified by silica gel chromatography eluting with an appropriate gradient of ethyl acetate/hexanes to yield a pure title compound.
- Step-2 6- ⁇ [3-(5- ⁇ [(5-formyl-6-methoxypyridin-2-yl)oxy]methyl ⁇ -4- methylthiophen-3-yl)-2-methylphenyl]methoxy ⁇ -2-methoxypyridine-3-carbaldehyde
- Cesium carbonate (4.0 mmol, 2 eqv.), palladium(II) acetate (0.2 mmol, 0.1 eqv.), 2-di-tert-butylphosphino-2',4',6'-triisopropylbiphenyl (t-butyl Xphos) (0.4 mmol, 0.2 eqv.), 6-chloro-2-methoxynicotinaldehyde (2.6 mmol, 1.3 eqv.), and (4-(3-(hydroxymethyl)-2- methylphenyl)-3-methylthiophen-2-yl)methanol (1.0 mmol, 2N) are combined in a 25 mL round bottom flask equipped with a stir bar.
- Step-3 2- ⁇ [(6- ⁇ [3-(5- ⁇ [(5- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -6-methoxypyridin- 2-yl)oxy ] methyl ⁇ -4-methylthiophen-3 -yl)-2-methylphenyl]methoxy ⁇ -2- methoxypyridin-3-yl)methyl]amino ⁇ ethan-l-ol (1-8)
- Example 8 2-( ⁇ [4-( ⁇ 4-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -3-methoxy phenoxy) methyl]-3-methylthiophen-2-yl ⁇ methoxy)-2-methoxyphenyl]methyl ⁇ amino)ethan-l-ol (1-9)
- Step-2 4-( ⁇ 4-[(4-formyl-3-methoxyphenoxy)methyl]-3-methylthiophen-2- yl ⁇ methoxy)-2-methoxybenzaldehyde
- Step-3 2-( ⁇ [4-( ⁇ 4-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -3-methoxyphenoxy) methyl]-3-methylthiophen-2-yl ⁇ methoxy)-2-methoxyphenyl]methyl ⁇ amino)ethan-l-ol (1-9)
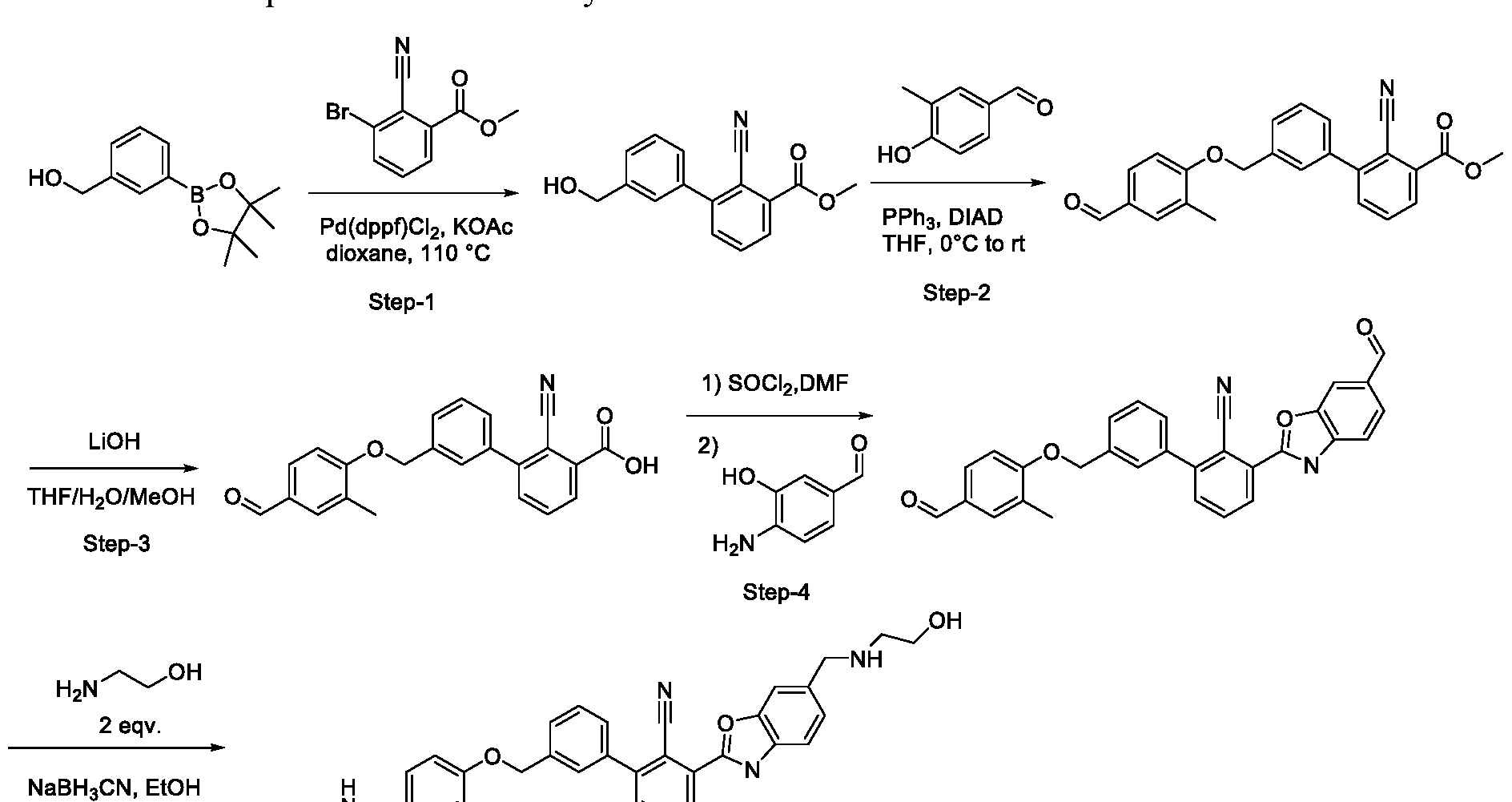
- Step-l methyl 2-cyano-3- -formyl-2-methylphenoxy)methyl)benzoate
- Step-2 methyl 3-((4-(azetidin-l-ylmethyl)-2-methylphenoxy)methyl)-2- cyanobenzoate
- Step-3 2-((4-(azetidin- 1 -ylmethyl)-2-methylphenoxy)methyl)-6-
- saturated aqueous ammonium chloride is charged to a multineck round- bottom flask and cooled to - 5 °C (inner temperature) to which is added the crude reaction mixture slowly over 15 minutes. After addition is complete the temperature is maintained at - 5 °C for 20 minutes.
- the reaction is diluted with dichloromethane (15 ml ) and the layers are separated.
- the aqueous layer is extracted with dichloromethane (1 x 15 ml) and the combined organic portions are washed with 1.5 N aqueous hydrochloric acid (1 x 1.3 ml ), saturated aqueous sodium chloride ( 1 x 1.3 ml ) and dried over sodium sulfate.
- Step-4 2-((4-(azetidin- 1 -ylmethyl)-2-methylphenoxy)methyl)-6-((4- formylphenoxy) methyl nzonitrile ( ⁇ -5)
- Step-5 2-( ⁇ 4-[(azetidin-l-yl)methyl]-2-methylphenoxy ⁇ methyl)-6- ⁇ [4-( ⁇ [(4- oxoazetidin-2-yl)methyl ]amino ⁇ methyl)phenoxy]methyl ⁇ benzonitrile (1-10)
- Product was purified via preparative HPLC with the following conditions: Column: XBridge CI 8, 19 x 200 mm, 5- ⁇ particles; Mobile Phase A: 5:95 acetonitrile: water with 10-mM ammonium acetate; Mobile Phase B: 95:5 acetonitrile: water with 10-mM ammonium acetate; Gradient: 20-60% B over 30 minutes, then a 5- minute hold at 100% B; Flow: 20 mL/min. Fractions containing the desired product were combined and dried via centrifugal evaporation.
- Compound 1-11 is prepared in the same manner as the general procedure described above using aldehyde ( ⁇ -5) and N-(2-aminoethyl)acetamide.
- Compound 1-12 is prepared in the same manner as the general procedure described above using aldehyde ( ⁇ -5) and 2-aminoethan-l-ol.
- Compound 1-13 is prepared in the same manner as the general procedure described above using aldehyde ( ⁇ -5) and azetidine hydrochloride salt.
- Step-1 4-((lH-indol-4-yl)methoxy)-2,6-dimethoxybenzaldehyde
- Step-2 4-((l-(4-formylbenzyl)-lH-indol-4-yl)methoxy)-2,6-dimethoxy benzaldehyde
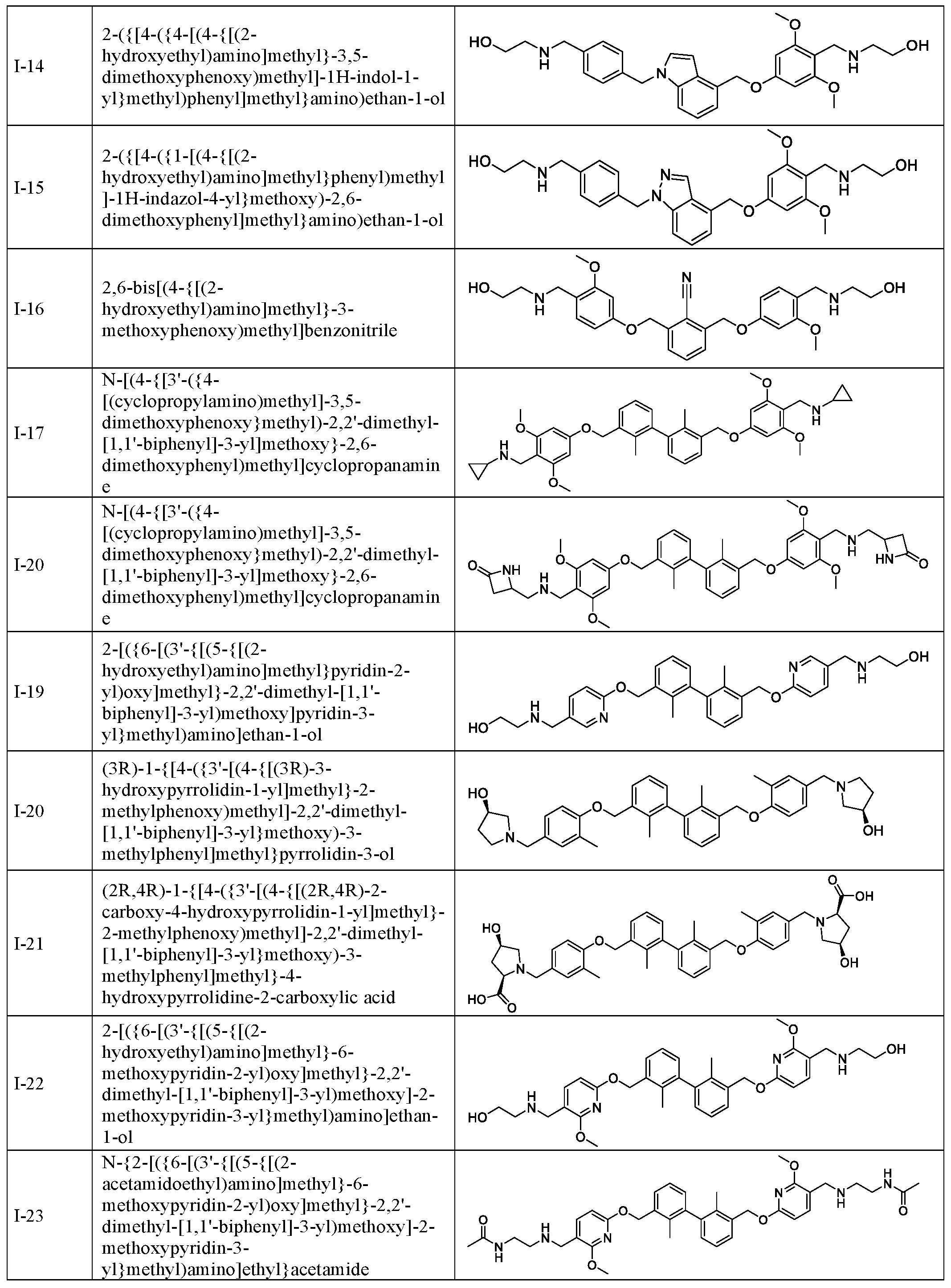
- Step-3 2-( ⁇ [4-( ⁇ 4-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -3,5- dimethoxy phenoxy )methy 1] - 1 H-indol- 1 -y 1 ⁇ methy l)pheny ljmethy 1 ⁇ amino)ethan- 1 -ol (I- 14)
- Example-14 2-( ⁇ [4-( ⁇ l-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ phenyl)methyl]- lH-indazol-4- l methox -2 6-dimethox hen l meth l amino ethan-l-ol (1-15)
- Compound 1-15 is prepared in the same manner as the procedure described for I- 14 except using ( lH-indazol-4-yl)methanol (III-6) and azetidine hydrochloride salt.
- step 1-3 The detailed procedures for step 1-3 are described in Example 9.
- Example-16 preparation of compound 1-17, 1-18, 1-19, 1-20 and 1-21.
- Cesium carbonate (4.0 mmol, 2 eqv.), palladium(II) acetate (0.2 mmol, 0.1 eqv.), 2-di-tert-butylphosphino-2',4',6'-triisopropylbiphenyl (t-butyl Xphos) (0.4 mmol, 0.2 eqv.), 6-chloro-2-methoxynicotinaldehyde (2.6 mmol, 1.3 eqv.), and (2,2'-dimethyl-[l, - biphenyl]-3,3'-diyi)dimethanol IV-2 (1.0 mmol, 2N) are combined in a 25 mL round bottom flask equipped with a stir bar.
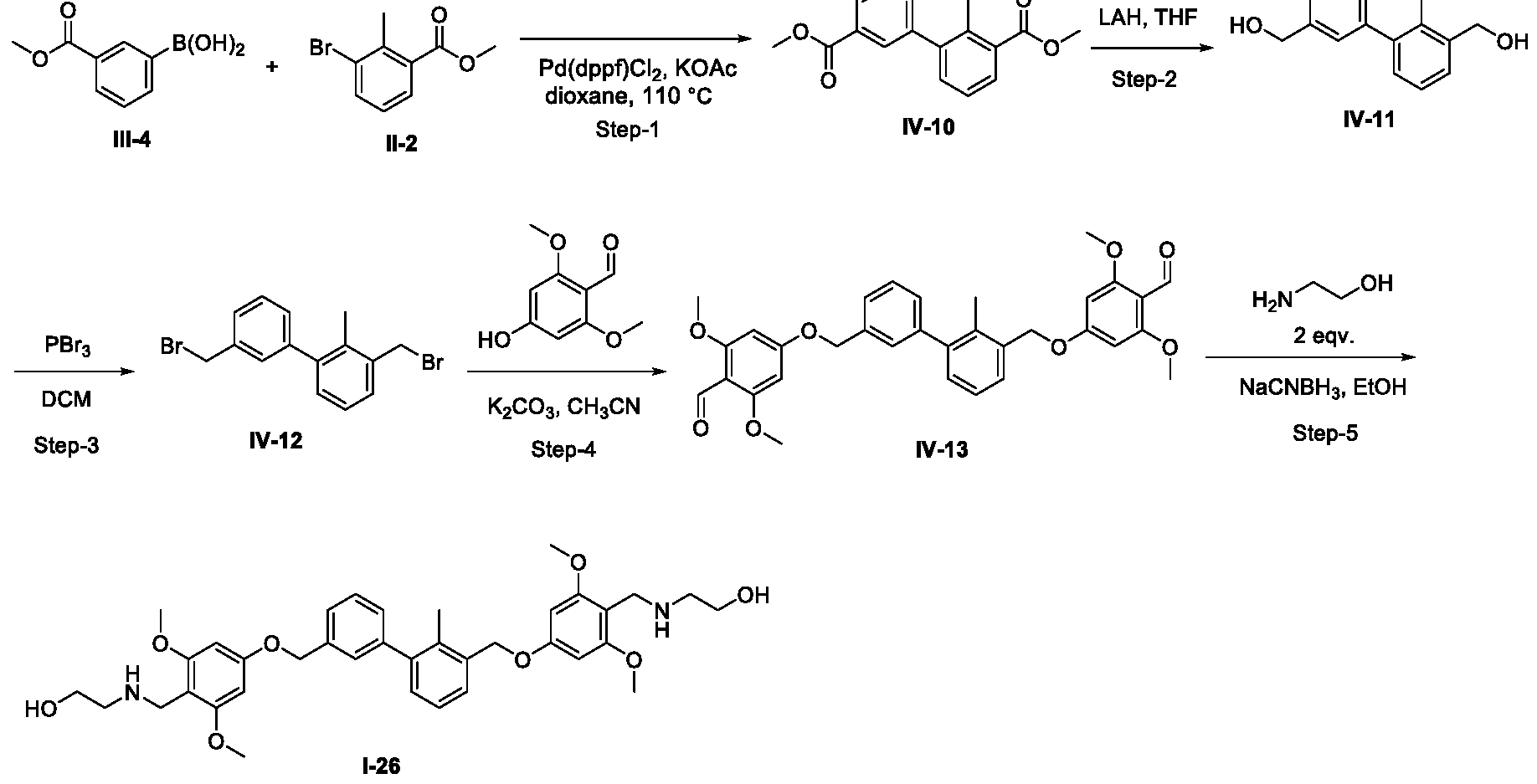
- Example-18 2-( ⁇ [4-( ⁇ 3'-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -3,5- dimethoxyphenoxy) methyl]-2'-methyl-[l, -biphenyl]-3-yl ⁇ methoxy)-2,6- dimethoxyphenyl]methyl ⁇ amino) ethan-l-ol, 1-26.
- Step-1 dimethyl 2-methyl-[l, -biphenyl]-3,3'-dicarboxylate (IV-10)
- Step-2 (2-methyl-[l, -biphenyl]-3,3'-diyl)dimethanol (IV-11)
- Step-3 3,3'-bis(bromomethyl)-2-methyl-l, 1 '-biphenyl, IV-12
- Example-19 3,3'-bis( ⁇ [(5- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -6- methoxypyridin-2-yl)oxy]methyl ⁇ )-[l, -biphenyl]-2-carbonitrile, 1-30.
- Step-1 dimethyl 2-cyano-[l, -biphenyl]-3,3 ! -dicarboxyiate (IV-14)
- Step-3 3,3 ! -bis(((5-formyl-6-methoxypyridin-2-yi)oxy)methyl)-[l, -biphenyi]-2- carbonitrile, IV-16
- Cesium carbonate (4.0 mmol, 2 eqv.), palladium(II) acetate (0.2 mmol, 0.1 eqv.), 2-di-tert-butylphosphino-2',4',6'-triisopropylbiphenyl (t-butyl Xphos) (0.4 mmol, 0.2 eqv.), 6-chloro-2-methoxynicotinaldehyde (2.6 mmol, 1.3 eqv.), and 3,3'-bis(hydroxymethyl)- [l, -biphenyl]-2-carbonitrile IV-15 (1.0 mmol, 2N) are combined in a 25 mL round bottom flask equipped with a stir bar.
- Step-4 follow the standard reductive amination procedures described for Example- 16 to yield the title compound 1-30.
- Example-20 3,3'-bis[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -3,5- dimethoxyphenoxy)methyl] -[l,l '-biphenyl]-2-carbonitrile, 1-31.
- Step-1 3,3 ! -bis(bromomethyl)-[l, -biphenyi]-2-carboiiitrile, IV-17
- Step-2 3,3'-bis((4-formyl-3,5-dimethoxyphenoxy)methyl)-[l, -biphenyl]-2- carbonitrile, IV-18
- Step-3 3,3'-bis[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -3,5- dimethoxyphenoxy)methyl] -[l,l '-biphenyl]-2-carbonitrile, 1-31.
- Step-1 4,4'-(((2,2'-dimethyl-[l, -biphenyl]-3,3 , -diyl)bis(methylene))bis(oxy))bis(2- hydroxy-5 -methyl benzaldehyde), I V- 1 .
- Step-2 5,5 , -((((((((2,2'-dimethyi-[l ,r )iphenyi]-3,3 , -diyi) ⁇
- Step-3 5-[(5- ⁇ [3'-( ⁇ 5-[(5-cyanopyridin-3-yl)methoxy]-4- ⁇ [(2-hydroxyethyl)amino]- methyl ⁇ -2-methylphenoxy ⁇ methyl)-2,2'-dimethyl-[l,r-biphenyl]-3-yl]methoxy ⁇ -2- ⁇ [(2- hydroxy ethyl) amino]methyl ⁇ -4-methylphenoxy)methyl]pyridine-3-carbonitrile, 1-50.
- Example-22 5-[(5- ⁇ [3'-( ⁇ 5-[(5-cyanopyridin-3-yl)methoxy]-4-(hydroxymethyl)-2- methylphenoxy ⁇ methyl)-2,2'-dimethyl-[l, l'-biphenyl]-3-yl]methoxy ⁇ -2-(hydroxymethyl)-4- methylphenoxy)methyl]pyridine-3-carbonitrile, 1-54.
- the ethanol is removed by evaporation, the aqueous phase is extracted with ethyl acetate three times and the combined organic phase was washed with NaOH (1.0 M, 15 mL), water (15 mL) and brine (15 mL) and dried over anhydrous Na 2 S0 4 , filtered and concentrated.
- the residue was purified by a silica gel column chromatography (eluent: ethyl acetate/petroleum ether) to yield the title molecule 1-54.
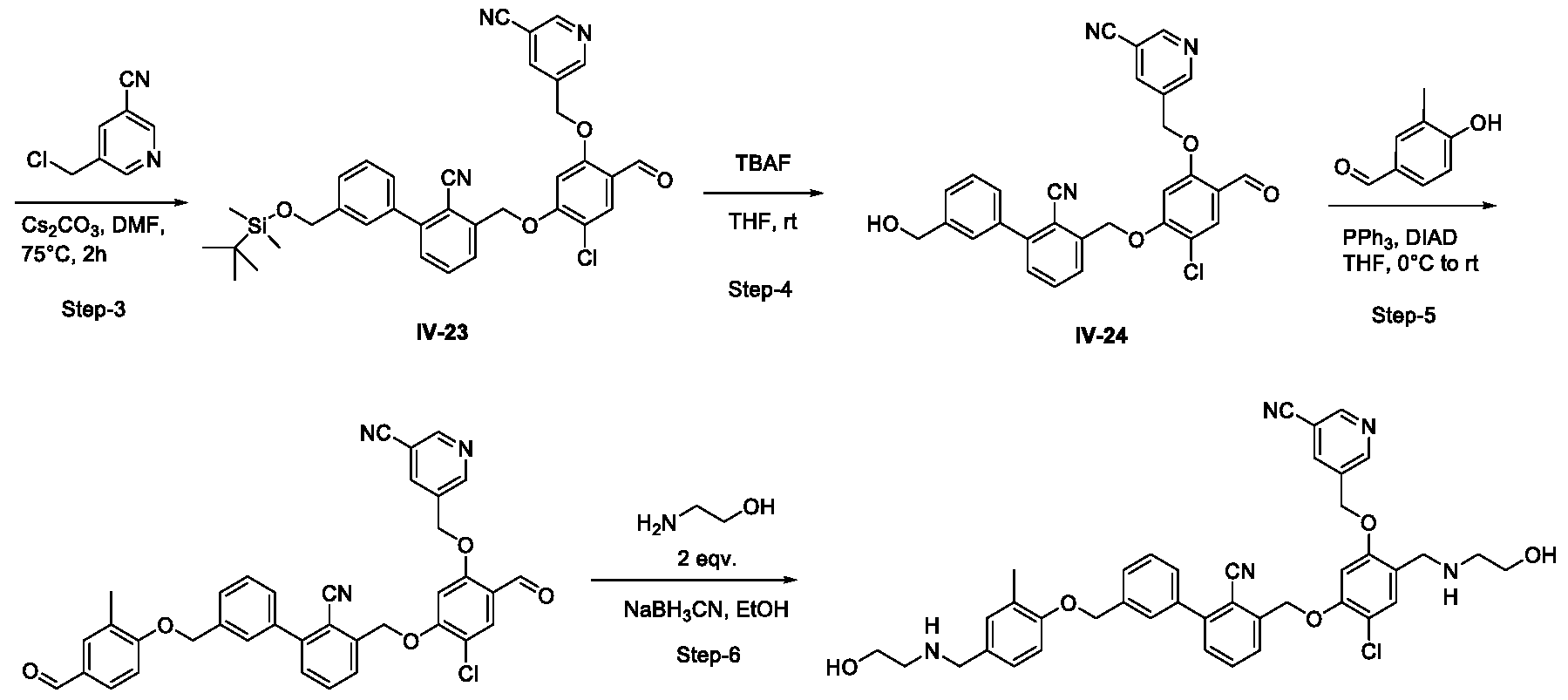
- Example-23 5- ⁇ [4-chloro-5-( ⁇ 2-cyano-3'-[(4- ⁇ [(2-hydroxyethyl)amino] methyl ⁇ - 2-methylphenoxy)methyl]-[l, l'-biphenyl]-3-yl ⁇ methoxy)-2- ⁇ [(2-hydroxy ethyl)
- Step- J 3'-(((tert-butyldimethylsilyl)oxy)methyl)-3-(hydroxymethyi)-[l,r- biphenyl]-2-carbonitriie, 11-10.
- Step-2 3'-(((tert-butyldimethylsilyl)oxy)methyl)-3-((2-chloro-4-formyl-5- hydroxyphenoxy) rnethyl)-[ 1 , 1 ! -biphenyl]-2-carbonitrile, IV-22.
- Step-3 5-((5-((3'-(((tert-butyldimethylsilyl)oxy)methyl)-2-cyano-[l, l '-biphenyl]-3- yl) methoxy)-4-chloro-2-formylphenoxy)methyl)nicotinonitrile, IV-23.
- Step-4 5-((4-chloro-5-((2-cyano-3'-(hydroxymethyl)-[l, -biphenyl]-3- yl)methoxy)-2-formyl phenoxy)methyl)nicotinonitrile, IV-24.
- Step-5 5-((4-chloro-5-((2-cyano-3'-((4-formyl-2-methylphenoxy)methyl)-[l,r- biphenyl]-3-yl)methoxy)-2-formylphenoxy)methyl)nicotinonitrile, IV-25.
- Example 24 5-[(4-chloro-2- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -5-( ⁇ 3'-[(4- ⁇ [(2- hydroxy ethyl) amino]methyl ⁇ -3,5-dimethoxyphenoxy)methyl]-2-methyl-[l, l'-biphenyl]-3- yl ⁇ methoxy) phenoxy)methyl]pyridine-3-carbonitrile, 1-69.
- the compound 1-69 can be synthesized by Method A shown below: :
- Step- 1-6 in Method A follow the stardard procedures described for Example-23 to yield the tiltle compound 1-69.
- the compound 1-69 can be synthesized by Method B shown below:
- the compounds listed in Table 2 are prepared using methods similar to those described for the preparation of 1-50, 1-54, 1-59 and 1-69.
- Example 25 2-[( ⁇ 2-[3'-(6- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -[l,2,4]triazolo[l,5- a]pyridin-2-yl)-2,2'-dimethyl-[ 1 , 1 '-biphenyl]-3 -yl]-[ 1 ,2,4]triazolo[ 1 ,5-a]pyridin-6- yl ⁇ methyl)amino]ethan- 1 -ol, 1-73.
- the compound 1-73 can be synthesized via the route shown in the scheme below.
- Example 26 2-[( ⁇ 2-[3'-(6- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ imidazo[l,2-a]pyridin- 2-yl)-2,2'-dimethyl-[ 1 , 1 '-biphenyl]-3 -yl]imidazo[ 1 ,2-a]pyridin-6-yl ⁇ methyl) amino]ethan- 1 - ol 1-74.
- the compound 1-74 can be synthesized via the route shown in the scheme below.
- Example 27 2-[( ⁇ 2-[3'-(6- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -l,3-benzoxazol-2-yl)- 2,2'-dimethyl-[l, l'-biphenyl]-3-yl]-l,3-benzoxazol-6-yl ⁇ methyl)amino]ethan-l-ol, 1-75.
- the compound 1-75 can be synthesized via the route shown in the scheme below.
- Example 28 3-(5- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -l,3-benzoxazol-2-yl)-3'-[(4- ⁇ [(2-hydroxyethyl)amino]methyl ⁇ -2-methylphenoxy)methyl]-[ 1 , 1 '-biphenyl]-2-carbonitrile, 1-87
- the compound 1-87 can be synthesized via the route shown in the scheme below:
- FIG. 1 shows docking pose of compound I-l in PD-Ll dimer (Panel A).
- Figure 2 shows docking pose of compound 1-10 in PD-Ll dimer (Panel B)
- compounds 1-1 and 1-5 dock well with PD-L1 dimer. In both cases, hydrophobic channel accommodates the designed novel core scaffold in the center; two pseudo symmetrical side chains attached to the core are extended to either side of the dimer interface. It is believed that such designed inhibitors can effectively induce/stabilize PD-L1 dimer formation, therefore potently disrupting PD-1/PD-L1 protein- protein and CD80/PD-L1 protein-protein interaction. Other compounds disclosed herein are believed to exhibit the same properties in docking experiments. Thus, these compounds can also be potent and selective inhibitors of the PD-1/PD-L1 protein/protein and CD80/PD-L1 protein/protein interactions.
- the activity of the compounds of Formula (I) to inhibit PD-1/PD-L1 protein-protein interaction can be readily investigated using biochemical and cellular assays well accepted in the field.
- HTRF Homogenous time-resolved fluorescence
- HTRF binding assay The ability of the designed compounds to physically disrupt PD-1/PD-L1 interaction was measured by HTRF binding assay.
- the tag is the Fc portion of immunoglobulin (PD-l-Ig).
- PD-L1 it is the 6 histidine motif (PD-Ll-His). All required fusion proteins with desired tags were obtained from commercial sources.
- HTRF assay buffer consists of lxPBS supplemented with 0.1% (w/v) bovine serum albumin and 0.05% (v/v) Tween-20.
- PD-Ll-His (30 nM final) and PD- l-Ig (10 nM final) in HTRF assay buffer were pre-incubated at RT for 30 min, followed by addition of inhibitors and incubated for another 30 min.
- HTRF detection was achieved using Tb cryptate-labeled anti-Ig antibody (1 nM final) and d2-labeled anti-His antibody (20 nM final).
- Antibodies were diluted in HTRF assay buffer and dispensed on top of the binding reaction. The reaction mixture was allowed to equilibrate for 60 min at RT before the resulting signal (665nm/620nm ratio) was obtained using an En Vision fluorometer.
- DMSO concentration in the solution is 0.2%.
- Additional binding assays can be established between PD- 1-Ig and PD-L2-His or CD80-His/PD-Ll -Ig, in a similar format.
- the IC 50 of the designed compounds in disrupting PD-1/PD-L1 interaction will be expected to fall in the range of 0.01 nM to 100 uM, depending on the corresponding structure-activity relationship.
- IC 50 determination was performed by fitting the curve of percent control activity versus the log of the inhibitor concentration using the GraphPad Prism 5.0 software.
- T-cell activation assay Human peripheral blood mononuclear cells (PBMC) can be isolated from blood buffy coats by commercial kits available.
- PBMC peripheral blood mononuclear cells
- CD4+ T cells can be isolated with CD4 enrichment kit as per the manufacturer's instructions.
- Mouse Ig capture beads can be coated with anti-CD3, anti-CD28 and PD-Ll Fc fusion by incubation under rotation at 4°C.
- CD4+ T cells can be cultured in 96-well plates together with coated beads, with or without designed compounds at varying concentrations for 3 days at 37°C in RPMI1640 Glutamax I supplemented with 4% human AB serum.
- Culture supernatant can be removed to measure cytokine expression (e.g. IFNs, IL-2) by ELISA, DELFIA or Luminex technology.
- the amount of cytokine can be determined by comparing with a standard curve of known amounts of human cytokines.
- the remaining T cells can be quantified by standard cell proliferation/survival assays (e.g. Thymidine incorporation, CellTiter-Glo) according to manufacturer's instructions. Potent inhibitor compounds will disrupt PD-Ll protein binding to PD-1 on the T cell surface, thus resulting in enhanced cytokine expression and promotion of T cell proliferation/activity.
- the ability of the designed compounds to functionally inhibit endogenous PD-l/PD- Ll interaction and promote T cell activity can be measured by mixed lymphocyte reaction assay.
- Human PBMCs can be isolated from leukapheresis packs using Ficoll-Paque Plus as per the manufacturer's instructions. Cells can be cultured in serum-free RPMI 1640 for short period at 37°C. After removal of nonadherent cells, remaining monocytes can be cultured in RPMI 1640 supplemented with 5% human AB serum, 2 ng/mL GM-CSF, and 10 ng/mL IL4. Fresh media with cytokine supplements can be added every 2 to 3 days.
- Mature dendritic cells can be induced by addition of TNFa on day 6 and culture for 24 hours.
- CD4+ T cells can be isolated from PBMCs using magnetic beads as per the manufacturer's instructions.
- CD4+ T cells can be cultured in 96 well-flat bottom plates together with allogeneic dendritic cells at an optimal ratio (e.g. 1 :2.5), using RPMI 1640 supplemented with 10% human AB serum.
- Dendritic cells can be treated with 100 mg/mL of mitomycin C before addition. Designed compounds or DMSO can be added as desired. Cytokine expression and T cell proliferation/activity can be measured as indicated above according to manufacturer's instructions. Potent inhibitor compound is expected to promote cytokine expression and T cell proliferation/activity.
- the compounds of Formula (I) possess activity as inhibitors of the PD-l/PD-Ll interaction, and therefore, may be used in the treatment of diseases dependent on or associated with the PD-l/PD-Ll interaction.
- the compounds of the present disclosure may be utilized to treat infectious diseases such as Hepatitis C, as well as multiple forms of cancer.
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2017305399A AU2017305399A1 (en) | 2016-08-03 | 2017-08-02 | Symmetric or semi-symmetric compounds useful as immunomodulators |
| CA3030773A CA3030773A1 (en) | 2016-08-03 | 2017-08-02 | Symmetric or semi-symmetric compounds useful as immunomodulators |
| JP2019527771A JP2019530732A (en) | 2016-08-03 | 2017-08-02 | Symmetric or semi-symmetrical compounds useful as immune modulators |
| EP17837640.6A EP3493804A1 (en) | 2016-08-03 | 2017-08-02 | Symmetric or semi-symmetric compounds useful as immunomodulators |
| CN201780024052.4A CN109195602B (en) | 2016-08-03 | 2017-08-02 | Symmetrical or semi-symmetrical compounds useful as immunomodulators |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662370679P | 2016-08-03 | 2016-08-03 | |
| US62/370,679 | 2016-08-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018026971A1 true WO2018026971A1 (en) | 2018-02-08 |
Family
ID=61073835
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2017/045185 WO2018026971A1 (en) | 2016-08-03 | 2017-08-02 | Symmetric or semi-symmetric compounds useful as immunomodulators |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP3493804A1 (en) |
| JP (1) | JP2019530732A (en) |
| CN (1) | CN109195602B (en) |
| AU (1) | AU2017305399A1 (en) |
| CA (1) | CA3030773A1 (en) |
| WO (1) | WO2018026971A1 (en) |
Cited By (85)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018119236A1 (en) * | 2016-12-22 | 2018-06-28 | Incyte Corporation | Triazolo[1,5-a]pyridine derivatives as immunomodulators |
| WO2018119266A1 (en) * | 2016-12-22 | 2018-06-28 | Incyte Corporation | Benzooxazole derivatives as immunomodulators |
| WO2018195321A1 (en) | 2017-04-20 | 2018-10-25 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019034172A1 (en) * | 2017-08-18 | 2019-02-21 | 上海轶诺药业有限公司 | Compound having pd-l1 inhibitory activity, preparation method therefor and use thereof |
| WO2019160882A1 (en) | 2018-02-13 | 2019-08-22 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019165374A1 (en) | 2018-02-26 | 2019-08-29 | Gilead Sciences, Inc. | Substituted pyrrolizine compounds as hbv replication inhibitors |
| WO2019175897A1 (en) * | 2018-03-13 | 2019-09-19 | Jubilant Biosys Limited | Bicyclic compounds as inhibitors of pd1/pd-l1 interaction/activation |
| WO2019191707A1 (en) * | 2018-03-30 | 2019-10-03 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2019193542A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides |
| WO2019193543A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides |
| WO2019195181A1 (en) | 2018-04-05 | 2019-10-10 | Gilead Sciences, Inc. | Antibodies and fragments thereof that bind hepatitis b virus protein x |
| WO2019193533A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'2'-cyclic dinucleotides |
| WO2019200247A1 (en) | 2018-04-12 | 2019-10-17 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| WO2019204609A1 (en) | 2018-04-19 | 2019-10-24 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| JP2019531274A (en) * | 2016-09-01 | 2019-10-31 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | Biaryl compounds useful as immunomodulators |
| WO2019211799A1 (en) | 2018-05-03 | 2019-11-07 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide |
| WO2019217821A1 (en) * | 2018-05-11 | 2019-11-14 | Incyte Corporation | Tetrahydro-imidazo[4,5-c]pyridine derivatives as pd-l1 immunomodulators |
| WO2019232319A1 (en) | 2018-05-31 | 2019-12-05 | Peloton Therapeutics, Inc. | Compositions and methods for inhibiting cd73 |
| WO2020014643A1 (en) | 2018-07-13 | 2020-01-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020011246A1 (en) * | 2018-07-13 | 2020-01-16 | 广州丹康医药生物有限公司 | Benzene ring-containing compound, preparation method therefor and application thereof |
| WO2020028097A1 (en) | 2018-08-01 | 2020-02-06 | Gilead Sciences, Inc. | Solid forms of (r)-11-(methoxymethyl)-12-(3-methoxypropoxy)-3,3-dimethyl-8-0x0-2,3,8,13b-tetrahydro-1h-pyrido[2,1-a]pyrrolo[1,2-c] phthalazine-7-c arboxylic acid |
| WO2020024997A1 (en) * | 2018-08-01 | 2020-02-06 | 上海轶诺药业有限公司 | Preparation and application of class of n-containing heterocyclic compounds having immunoregulatory function |
| WO2020052650A1 (en) * | 2018-09-13 | 2020-03-19 | Betta Pharmaceuticals Co., Ltd | Immunomodulators, compositions and methods there of |
| WO2020086556A1 (en) | 2018-10-24 | 2020-04-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020092528A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds having hpk1 inhibitory activity |
| WO2020092621A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| US10662416B2 (en) | 2016-10-14 | 2020-05-26 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the hepatitis B virus genome |
| WO2020123674A1 (en) | 2018-12-12 | 2020-06-18 | Arbutus Biopharma Corporation | Substituted arylmethylureas and heteroarylmethylureas, analogues thereof, and methods using same |
| WO2020156323A1 (en) * | 2019-01-31 | 2020-08-06 | Betta Pharmaceuticals Co., Ltd | Immunomodulators, compositions and methods thereof |
| WO2020178769A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020178770A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020178768A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020192570A1 (en) | 2019-03-22 | 2020-10-01 | 上海再极医药科技有限公司 | Small-molecule inhibitor of pd-1/pd-l1, pharmaceutical composition thereof with pd-l1 antibody, and application of same |
| US10806785B2 (en) | 2016-12-22 | 2020-10-20 | Incyte Corporation | Immunomodulator compounds and methods of use |
| WO2020214663A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214652A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020237025A1 (en) | 2019-05-23 | 2020-11-26 | Gilead Sciences, Inc. | Substituted exo-methylene-oxindoles which are hpk1/map4k1 inhibitors |
| CN112028870A (en) * | 2019-06-04 | 2020-12-04 | 中国科学院上海药物研究所 | Compound with benzyloxy aromatic ring structure, preparation method and application thereof |
| WO2020255021A1 (en) * | 2019-06-18 | 2020-12-24 | Janssen Sciences Ireland Unlimited Company | Combination of hepatitis b virus (hbv) vaccines and small molecule pdl1 or pd1 inhibitor |
| WO2021011891A1 (en) | 2019-07-18 | 2021-01-21 | Gilead Sciences, Inc. | Long-acting formulations of tenofovir alafenamide |
| WO2021034804A1 (en) | 2019-08-19 | 2021-02-25 | Gilead Sciences, Inc. | Pharmaceutical formulations of tenofovir alafenamide |
| US10966999B2 (en) | 2017-12-20 | 2021-04-06 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| WO2021067181A1 (en) | 2019-09-30 | 2021-04-08 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| CN112638899A (en) * | 2018-08-01 | 2021-04-09 | 上海轶诺药业有限公司 | Preparation and application of aromatic compound with immunoregulation function |
| WO2021113765A1 (en) | 2019-12-06 | 2021-06-10 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| JP2021520342A (en) * | 2018-04-03 | 2021-08-19 | ベータ ファーマシューティカルズ カンパニー リミテッド | Immunomodulators, compositions and methods thereof |
| WO2021188959A1 (en) | 2020-03-20 | 2021-09-23 | Gilead Sciences, Inc. | Prodrugs of 4'-c-substituted-2-halo-2'-deoxyadenosine nucleosides and methods of making and using the same |
| US11130740B2 (en) | 2017-04-25 | 2021-09-28 | Arbutus Biopharma Corporation | Substituted 2,3-dihydro-1H-indene analogs and methods using same |
| WO2021226206A2 (en) | 2020-05-05 | 2021-11-11 | Teon Therapeutics, Inc. | Cannabinoid receptor type 2 (cb2) modulators and uses thereof |
| US11203610B2 (en) | 2017-12-20 | 2021-12-21 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| US11304949B2 (en) | 2018-03-30 | 2022-04-19 | Incyte Corporation | Treatment of hidradenitis suppurativa using JAK inhibitors |
| WO2022147302A1 (en) * | 2020-12-30 | 2022-07-07 | Chulalongkorn University | 4-phenyl-indole derivatives and related uses |
| US11401279B2 (en) | 2019-09-30 | 2022-08-02 | Incyte Corporation | Pyrido[3,2-d]pyrimidine compounds as immunomodulators |
| US11407749B2 (en) | 2015-10-19 | 2022-08-09 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11426412B2 (en) | 2017-10-18 | 2022-08-30 | Jubilant Epipad LLC | Imidazo-pyridine compounds as PAD inhibitors |
| US11459338B2 (en) | 2017-11-24 | 2022-10-04 | Jubilant Episcribe Llc | Heterocyclic compounds as PRMT5 inhibitors |
| US11465981B2 (en) | 2016-12-22 | 2022-10-11 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2022241134A1 (en) | 2021-05-13 | 2022-11-17 | Gilead Sciences, Inc. | COMBINATION OF A TLR8 MODULATING COMPOUND AND ANTI-HBV siRNA THERAPEUTICS |
| WO2022261310A1 (en) | 2021-06-11 | 2022-12-15 | Gilead Sciences, Inc. | Combination mcl-1 inhibitors with anti-body drug conjugates |
| WO2022261301A1 (en) | 2021-06-11 | 2022-12-15 | Gilead Sciences, Inc. | Combination mcl-1 inhibitors with anti-cancer agents |
| US11535615B2 (en) | 2015-12-22 | 2022-12-27 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2022271677A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271684A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271650A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271659A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| US11572366B2 (en) | 2015-11-19 | 2023-02-07 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2023034530A1 (en) | 2021-09-02 | 2023-03-09 | Teon Therapeutics, Inc. | Methods of improving growth and function of immune cells |
| US11608337B2 (en) | 2016-05-06 | 2023-03-21 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11613536B2 (en) | 2016-08-29 | 2023-03-28 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11629135B2 (en) | 2017-11-06 | 2023-04-18 | Jubilant Prodell Llc | Pyrimidine derivatives as inhibitors of PD1/PD-L1 activation |
| WO2023081730A1 (en) | 2021-11-03 | 2023-05-11 | Teon Therapeutics, Inc. | 4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide derivatives as cannabinoid cb2 receptor modulators for the treatment of cancer |
| WO2023097211A1 (en) | 2021-11-24 | 2023-06-01 | The University Of Southern California | Methods for enhancing immune checkpoint inhibitor therapy |
| US11673883B2 (en) | 2016-05-26 | 2023-06-13 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11718605B2 (en) | 2016-07-14 | 2023-08-08 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11753406B2 (en) | 2019-08-09 | 2023-09-12 | Incyte Corporation | Salts of a PD-1/PD-L1 inhibitor |
| US11760764B2 (en) | 2020-05-22 | 2023-09-19 | Aligos Therapeutics, Inc. | Methods and compositions for targeting PD-L1 |
| US11760756B2 (en) | 2020-11-06 | 2023-09-19 | Incyte Corporation | Crystalline form of a PD-1/PD-L1 inhibitor |
| US11780836B2 (en) | 2020-11-06 | 2023-10-10 | Incyte Corporation | Process of preparing a PD-1/PD-L1 inhibitor |
| US11833156B2 (en) | 2017-09-22 | 2023-12-05 | Jubilant Epipad LLC | Heterocyclic compounds as pad inhibitors |
| US11866434B2 (en) | 2020-11-06 | 2024-01-09 | Incyte Corporation | Process for making a PD-1/PD-L1 inhibitor and salts and crystalline forms thereof |
| US11866451B2 (en) | 2019-11-11 | 2024-01-09 | Incyte Corporation | Salts and crystalline forms of a PD-1/PD-L1 inhibitor |
| US11873309B2 (en) | 2016-06-20 | 2024-01-16 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2024015372A1 (en) | 2022-07-14 | 2024-01-18 | Teon Therapeutics, Inc. | Adenosine receptor antagonists and uses thereof |
| WO2024019957A1 (en) * | 2022-07-18 | 2024-01-25 | Celgene Corporation | Compounds for the treatment of neurodegenerative diseases |
| EP4100381A4 (en) * | 2020-02-03 | 2024-04-03 | Arbutus Biopharma Corp | Substituted 1,1'-biphenyl compounds and methods using same |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11459339B2 (en) * | 2018-02-05 | 2022-10-04 | Abbisko Therapeutics Co., Ltd. | Biaryl derivative, preparation method thereof and pharmaceutical application thereof |
| CN109897036B (en) * | 2019-03-15 | 2021-07-30 | 沈阳药科大学 | Triazolopyridine compound and preparation method and application thereof |
| CN112979641A (en) * | 2019-12-17 | 2021-06-18 | 上海轶诺药业有限公司 | Preparation and application of N-containing heterocyclic compound with immunoregulation function |
| CN113248492B (en) * | 2020-02-10 | 2022-11-08 | 上海海雁医药科技有限公司 | Heterocycle substituted nitrogen-containing six-membered heterocyclic derivative, preparation method and medical application thereof |
| EP4313946A1 (en) * | 2021-03-26 | 2024-02-07 | Jacobio Pharmaceuticals Co., Ltd. | Novel compounds useful as sting agonists and uses thereof |
| CN113135895A (en) * | 2021-04-30 | 2021-07-20 | 中国药科大学 | Novel biphenyl derivative, preparation method and medical application thereof |
| CN113321575B (en) * | 2021-06-15 | 2023-05-26 | 南开大学 | Preparation method of benzyl aryl ether and application of benzyl aryl ether in synthesis |
| WO2024032782A1 (en) * | 2022-08-12 | 2024-02-15 | Jacobio Pharmaceuticals Co.Ltd. | Vaccine adjuvants and uses thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20130022629A1 (en) * | 2010-01-04 | 2013-01-24 | Sharpe Arlene H | Modulators of Immunoinhibitory Receptor PD-1, and Methods of Use Thereof |
| US20130217664A1 (en) * | 2012-02-10 | 2013-08-22 | Christel Jeanne Marie Menet | Novel compounds useful for the treatment of degenerative and inflammatory diseases |
| WO2015034820A1 (en) * | 2013-09-04 | 2015-03-12 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| US20150291549A1 (en) * | 2014-04-14 | 2015-10-15 | Bristol-Myers Squibb Company | Compounds Useful as Immunomodulators |
| US20150352206A1 (en) * | 2012-10-26 | 2015-12-10 | The University Of Chicago | Synergistic combination of immunologic inhibitors for the treatment of cancer |
-
2017
- 2017-08-02 JP JP2019527771A patent/JP2019530732A/en active Pending
- 2017-08-02 WO PCT/US2017/045185 patent/WO2018026971A1/en unknown
- 2017-08-02 AU AU2017305399A patent/AU2017305399A1/en not_active Abandoned
- 2017-08-02 CN CN201780024052.4A patent/CN109195602B/en active Active
- 2017-08-02 CA CA3030773A patent/CA3030773A1/en not_active Abandoned
- 2017-08-02 EP EP17837640.6A patent/EP3493804A1/en not_active Withdrawn
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20130022629A1 (en) * | 2010-01-04 | 2013-01-24 | Sharpe Arlene H | Modulators of Immunoinhibitory Receptor PD-1, and Methods of Use Thereof |
| US20130217664A1 (en) * | 2012-02-10 | 2013-08-22 | Christel Jeanne Marie Menet | Novel compounds useful for the treatment of degenerative and inflammatory diseases |
| US20150352206A1 (en) * | 2012-10-26 | 2015-12-10 | The University Of Chicago | Synergistic combination of immunologic inhibitors for the treatment of cancer |
| WO2015034820A1 (en) * | 2013-09-04 | 2015-03-12 | Bristol-Myers Squibb Company | Compounds useful as immunomodulators |
| US20150291549A1 (en) * | 2014-04-14 | 2015-10-15 | Bristol-Myers Squibb Company | Compounds Useful as Immunomodulators |
Cited By (155)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11407749B2 (en) | 2015-10-19 | 2022-08-09 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11572366B2 (en) | 2015-11-19 | 2023-02-07 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11535615B2 (en) | 2015-12-22 | 2022-12-27 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11866435B2 (en) | 2015-12-22 | 2024-01-09 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11608337B2 (en) | 2016-05-06 | 2023-03-21 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11673883B2 (en) | 2016-05-26 | 2023-06-13 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11873309B2 (en) | 2016-06-20 | 2024-01-16 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11718605B2 (en) | 2016-07-14 | 2023-08-08 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11613536B2 (en) | 2016-08-29 | 2023-03-28 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| JP2019531274A (en) * | 2016-09-01 | 2019-10-31 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | Biaryl compounds useful as immunomodulators |
| EP3507284B1 (en) * | 2016-09-01 | 2022-05-25 | Bristol-Myers Squibb Company | Biaryl compounds useful as immunomodulators |
| JP7155110B2 (en) | 2016-09-01 | 2022-10-18 | ブリストル-マイヤーズ スクイブ カンパニー | Biaryl compounds useful as immunomodulators |
| US10662416B2 (en) | 2016-10-14 | 2020-05-26 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the hepatitis B virus genome |
| US11274285B2 (en) | 2016-10-14 | 2022-03-15 | Precision Biosciences, Inc. | Engineered meganucleases specific for recognition sequences in the Hepatitis B virus genome |
| US11787793B2 (en) | 2016-12-22 | 2023-10-17 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| EP4223369A1 (en) * | 2016-12-22 | 2023-08-09 | Incyte Corporation | Immunomodulator compounds and methods of use |
| US11339149B2 (en) | 2016-12-22 | 2022-05-24 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11465981B2 (en) | 2016-12-22 | 2022-10-11 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2018119266A1 (en) * | 2016-12-22 | 2018-06-28 | Incyte Corporation | Benzooxazole derivatives as immunomodulators |
| US10308644B2 (en) | 2016-12-22 | 2019-06-04 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11566026B2 (en) | 2016-12-22 | 2023-01-31 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US10793565B2 (en) | 2016-12-22 | 2020-10-06 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US10806785B2 (en) | 2016-12-22 | 2020-10-20 | Incyte Corporation | Immunomodulator compounds and methods of use |
| WO2018119236A1 (en) * | 2016-12-22 | 2018-06-28 | Incyte Corporation | Triazolo[1,5-a]pyridine derivatives as immunomodulators |
| US10800768B2 (en) | 2016-12-22 | 2020-10-13 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2018195321A1 (en) | 2017-04-20 | 2018-10-25 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| KR102522886B1 (en) | 2017-04-20 | 2023-04-19 | 길리애드 사이언시즈, 인코포레이티드 | Pd-1/pd-l1 inhibitors |
| EP4026835A2 (en) | 2017-04-20 | 2022-07-13 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| KR20220030318A (en) * | 2017-04-20 | 2022-03-10 | 길리애드 사이언시즈, 인코포레이티드 | Pd-1/pd-l1 inhibitors |
| US11130740B2 (en) | 2017-04-25 | 2021-09-28 | Arbutus Biopharma Corporation | Substituted 2,3-dihydro-1H-indene analogs and methods using same |
| WO2019034172A1 (en) * | 2017-08-18 | 2019-02-21 | 上海轶诺药业有限公司 | Compound having pd-l1 inhibitory activity, preparation method therefor and use thereof |
| US11787766B2 (en) | 2017-08-18 | 2023-10-17 | Shanghai Ennovabio Pharmaceuticals Co., Ltd. | Compound having PD-L1 inhibitory activity, preparation method therefor and use thereof |
| US20200392083A1 (en) * | 2017-08-18 | 2020-12-17 | Shanghai Ennovabio Pharmaceuticals Co., Ltd. | Compound having pd-l1 inhibitory activity, preparation method therefor and use thereof |
| US11833156B2 (en) | 2017-09-22 | 2023-12-05 | Jubilant Epipad LLC | Heterocyclic compounds as pad inhibitors |
| US11426412B2 (en) | 2017-10-18 | 2022-08-30 | Jubilant Epipad LLC | Imidazo-pyridine compounds as PAD inhibitors |
| US11629135B2 (en) | 2017-11-06 | 2023-04-18 | Jubilant Prodell Llc | Pyrimidine derivatives as inhibitors of PD1/PD-L1 activation |
| US11459338B2 (en) | 2017-11-24 | 2022-10-04 | Jubilant Episcribe Llc | Heterocyclic compounds as PRMT5 inhibitors |
| US11203610B2 (en) | 2017-12-20 | 2021-12-21 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| US10966999B2 (en) | 2017-12-20 | 2021-04-06 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′ cyclic dinucleotides with phosphonate bond activating the sting adaptor protein |
| KR20200121323A (en) * | 2018-02-13 | 2020-10-23 | 길리애드 사이언시즈, 인코포레이티드 | PD-1/PD-L1 inhibitor |
| CN111712494A (en) * | 2018-02-13 | 2020-09-25 | 吉利德科学公司 | PD-1/PD-L1 inhibitors |
| EP4227302A1 (en) | 2018-02-13 | 2023-08-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| JP2021513561A (en) * | 2018-02-13 | 2021-05-27 | ギリアード サイエンシーズ, インコーポレイテッド | PD-1 / PD-L1 inhibitor |
| AU2019222644B2 (en) * | 2018-02-13 | 2021-04-01 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| JP7062792B2 (en) | 2018-02-13 | 2022-05-06 | ギリアード サイエンシーズ, インコーポレイテッド | PD-1 / PD-L1 inhibitor |
| WO2019160882A1 (en) | 2018-02-13 | 2019-08-22 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US11555029B2 (en) | 2018-02-13 | 2023-01-17 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| KR102445054B1 (en) | 2018-02-13 | 2022-09-22 | 길리애드 사이언시즈, 인코포레이티드 | PD-1/PD-L1 inhibitors |
| US10710986B2 (en) | 2018-02-13 | 2020-07-14 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| AU2021204222B2 (en) * | 2018-02-13 | 2023-02-16 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2019165374A1 (en) | 2018-02-26 | 2019-08-29 | Gilead Sciences, Inc. | Substituted pyrrolizine compounds as hbv replication inhibitors |
| WO2019175897A1 (en) * | 2018-03-13 | 2019-09-19 | Jubilant Biosys Limited | Bicyclic compounds as inhibitors of pd1/pd-l1 interaction/activation |
| CN112105610A (en) * | 2018-03-13 | 2020-12-18 | 朱比连特普罗德尔有限责任公司 | Bicyclic compounds as inhibitors of PD1/PD-L1 interaction/activation |
| CN112105610B (en) * | 2018-03-13 | 2024-01-26 | 朱比连特普罗德尔有限责任公司 | Bicyclic compounds as inhibitors of PD1/PD-L1 interaction/activation |
| US11529341B2 (en) | 2018-03-13 | 2022-12-20 | Jubilant Prodel LLC | Bicyclic compounds as inhibitors of PD1/PD-L1 interaction/activation |
| CN112135824A (en) * | 2018-03-30 | 2020-12-25 | 因赛特公司 | Heterocyclic compounds as immunomodulators |
| AU2019245288B2 (en) * | 2018-03-30 | 2023-10-19 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11304949B2 (en) | 2018-03-30 | 2022-04-19 | Incyte Corporation | Treatment of hidradenitis suppurativa using JAK inhibitors |
| JP2021519779A (en) * | 2018-03-30 | 2021-08-12 | インサイト・コーポレイションIncyte Corporation | Heterocyclic compounds as immunomodulators |
| US10669271B2 (en) | 2018-03-30 | 2020-06-02 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| EP4212529A1 (en) * | 2018-03-30 | 2023-07-19 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2019191707A1 (en) * | 2018-03-30 | 2019-10-03 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11124511B2 (en) | 2018-03-30 | 2021-09-21 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| JP7372255B2 (en) | 2018-03-30 | 2023-10-31 | インサイト・コーポレイション | Heterocyclic compounds as immunomodulators |
| JP2021520342A (en) * | 2018-04-03 | 2021-08-19 | ベータ ファーマシューティカルズ カンパニー リミテッド | Immunomodulators, compositions and methods thereof |
| WO2019195181A1 (en) | 2018-04-05 | 2019-10-10 | Gilead Sciences, Inc. | Antibodies and fragments thereof that bind hepatitis b virus protein x |
| US11149052B2 (en) | 2018-04-06 | 2021-10-19 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2′3′-cyclic dinucleotides |
| US11292812B2 (en) | 2018-04-06 | 2022-04-05 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′-cyclic dinucleotides |
| WO2019193533A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'2'-cyclic dinucleotides |
| WO2019193542A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides |
| WO2019193543A1 (en) | 2018-04-06 | 2019-10-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides |
| US11142750B2 (en) | 2018-04-12 | 2021-10-12 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the Hepatitis B virus genome |
| US11788077B2 (en) | 2018-04-12 | 2023-10-17 | Precision Biosciences, Inc. | Polynucleotides encoding optimized engineered meganucleases having specificity for a recognition sequence in the Hepatitis B virus genome |
| WO2019200247A1 (en) | 2018-04-12 | 2019-10-17 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| CN112041311A (en) * | 2018-04-19 | 2020-12-04 | 吉利德科学公司 | PD-1/PD-L1 inhibitors |
| TWI712412B (en) * | 2018-04-19 | 2020-12-11 | 美商基利科學股份有限公司 | Pd-1/pd-l1 inhibitors |
| KR20200145834A (en) * | 2018-04-19 | 2020-12-30 | 길리애드 사이언시즈, 인코포레이티드 | PD-1/PD-L1 inhibitor |
| CN112041311B (en) * | 2018-04-19 | 2023-10-03 | 吉利德科学公司 | PD-1/PD-L1 inhibitors |
| KR102591947B1 (en) * | 2018-04-19 | 2023-10-25 | 길리애드 사이언시즈, 인코포레이티드 | PD-1/PD-L1 inhibitors |
| JP2021522168A (en) * | 2018-04-19 | 2021-08-30 | ギリアード サイエンシーズ, インコーポレイテッド | PD-1 / PD-L1 inhibitor |
| JP7242702B2 (en) | 2018-04-19 | 2023-03-20 | ギリアード サイエンシーズ, インコーポレイテッド | PD-1/PD-L1 inhibitor |
| US10899735B2 (en) | 2018-04-19 | 2021-01-26 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| WO2019204609A1 (en) | 2018-04-19 | 2019-10-24 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2019211799A1 (en) | 2018-05-03 | 2019-11-07 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide |
| EP4219492A1 (en) * | 2018-05-11 | 2023-08-02 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| WO2019217821A1 (en) * | 2018-05-11 | 2019-11-14 | Incyte Corporation | Tetrahydro-imidazo[4,5-c]pyridine derivatives as pd-l1 immunomodulators |
| US10618916B2 (en) | 2018-05-11 | 2020-04-14 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| TWI821288B (en) * | 2018-05-11 | 2023-11-11 | 美商英塞特公司 | Heterocyclic compounds as immunomodulators |
| US10906920B2 (en) | 2018-05-11 | 2021-02-02 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| US11414433B2 (en) | 2018-05-11 | 2022-08-16 | Incyte Corporation | Heterocyclic compounds as immunomodulators |
| JP7328995B2 (en) | 2018-05-11 | 2023-08-17 | インサイト・コーポレイション | Tetrahydro-imidazo[4,5-C]pyridine derivatives as PD-L1 immunomodulators |
| AU2019267824B2 (en) * | 2018-05-11 | 2023-10-05 | Incyte Corporation | Tetrahydro-imidazo(4,5-C)pyridine derivatives as PD-L1 immunomodulators |
| CN112752756A (en) * | 2018-05-11 | 2021-05-04 | 因赛特公司 | Tetrahydro-imidazo [4,5-c ] pyridine derivatives as PD-L1 immunomodulators |
| WO2019232319A1 (en) | 2018-05-31 | 2019-12-05 | Peloton Therapeutics, Inc. | Compositions and methods for inhibiting cd73 |
| WO2020011246A1 (en) * | 2018-07-13 | 2020-01-16 | 广州丹康医药生物有限公司 | Benzene ring-containing compound, preparation method therefor and application thereof |
| US10774071B2 (en) | 2018-07-13 | 2020-09-15 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| CN112424185A (en) * | 2018-07-13 | 2021-02-26 | 广州丹康医药生物有限公司 | Compound containing benzene ring, preparation method and application thereof |
| EP4234030A2 (en) | 2018-07-13 | 2023-08-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| WO2020014643A1 (en) | 2018-07-13 | 2020-01-16 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| CN112638900A (en) * | 2018-08-01 | 2021-04-09 | 上海轶诺药业有限公司 | Preparation and application of N-containing heterocyclic compound with immunoregulation function |
| CN112638899A (en) * | 2018-08-01 | 2021-04-09 | 上海轶诺药业有限公司 | Preparation and application of aromatic compound with immunoregulation function |
| US20220056023A1 (en) * | 2018-08-01 | 2022-02-24 | Shanghai Ennovabio Pharmaceuticals Co., Ltd. | Preparation and application of class of n-containing heterocyclic compounds having immunoregulatory function |
| CN112638899B (en) * | 2018-08-01 | 2023-09-05 | 上海轶诺药业有限公司 | Preparation and application of aromatic compound with immunoregulation function |
| WO2020024997A1 (en) * | 2018-08-01 | 2020-02-06 | 上海轶诺药业有限公司 | Preparation and application of class of n-containing heterocyclic compounds having immunoregulatory function |
| EP3831823A4 (en) * | 2018-08-01 | 2022-04-27 | Shanghai Ennovabio Pharmaceuticals Co., Ltd. | Preparation and application of aromatic compound having immunoregulatory function |
| WO2020028097A1 (en) | 2018-08-01 | 2020-02-06 | Gilead Sciences, Inc. | Solid forms of (r)-11-(methoxymethyl)-12-(3-methoxypropoxy)-3,3-dimethyl-8-0x0-2,3,8,13b-tetrahydro-1h-pyrido[2,1-a]pyrrolo[1,2-c] phthalazine-7-c arboxylic acid |
| WO2020052650A1 (en) * | 2018-09-13 | 2020-03-19 | Betta Pharmaceuticals Co., Ltd | Immunomodulators, compositions and methods there of |
| CN112654617A (en) * | 2018-09-13 | 2021-04-13 | 贝达药业股份有限公司 | Immunomodulator, composition and preparation method thereof |
| WO2020086556A1 (en) | 2018-10-24 | 2020-04-30 | Gilead Sciences, Inc. | Pd-1/pd-l1 inhibitors |
| US11236085B2 (en) | 2018-10-24 | 2022-02-01 | Gilead Sciences, Inc. | PD-1/PD-L1 inhibitors |
| CN112955435A (en) * | 2018-10-24 | 2021-06-11 | 吉利德科学公司 | PD-1/PD-L1 inhibitors |
| WO2020092621A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds as hpk1 inhibitors |
| WO2020092528A1 (en) | 2018-10-31 | 2020-05-07 | Gilead Sciences, Inc. | Substituted 6-azabenzimidazole compounds having hpk1 inhibitory activity |
| WO2020123674A1 (en) | 2018-12-12 | 2020-06-18 | Arbutus Biopharma Corporation | Substituted arylmethylureas and heteroarylmethylureas, analogues thereof, and methods using same |
| WO2020156323A1 (en) * | 2019-01-31 | 2020-08-06 | Betta Pharmaceuticals Co., Ltd | Immunomodulators, compositions and methods thereof |
| WO2020178768A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020178769A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 2'3'-cyclic dinucleotides and prodrugs thereof |
| WO2020178770A1 (en) | 2019-03-07 | 2020-09-10 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3'3'-cyclic dinucleotides and prodrugs thereof |
| US11766447B2 (en) | 2019-03-07 | 2023-09-26 | Institute Of Organic Chemistry And Biochemistry Ascr, V.V.I. | 3′3′-cyclic dinucleotide analogue comprising a cyclopentanyl modified nucleotide as sting modulator |
| WO2020192570A1 (en) | 2019-03-22 | 2020-10-01 | 上海再极医药科技有限公司 | Small-molecule inhibitor of pd-1/pd-l1, pharmaceutical composition thereof with pd-l1 antibody, and application of same |
| WO2020214663A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020214652A1 (en) | 2019-04-17 | 2020-10-22 | Gilead Sciences, Inc. | Solid forms of a toll-like receptor modulator |
| WO2020237025A1 (en) | 2019-05-23 | 2020-11-26 | Gilead Sciences, Inc. | Substituted exo-methylene-oxindoles which are hpk1/map4k1 inhibitors |
| CN112028870A (en) * | 2019-06-04 | 2020-12-04 | 中国科学院上海药物研究所 | Compound with benzyloxy aromatic ring structure, preparation method and application thereof |
| CN112028870B (en) * | 2019-06-04 | 2021-11-05 | 中国科学院上海药物研究所 | Compound with benzyloxy aromatic ring structure, preparation method and application thereof |
| WO2020255021A1 (en) * | 2019-06-18 | 2020-12-24 | Janssen Sciences Ireland Unlimited Company | Combination of hepatitis b virus (hbv) vaccines and small molecule pdl1 or pd1 inhibitor |
| WO2021011891A1 (en) | 2019-07-18 | 2021-01-21 | Gilead Sciences, Inc. | Long-acting formulations of tenofovir alafenamide |
| US11753406B2 (en) | 2019-08-09 | 2023-09-12 | Incyte Corporation | Salts of a PD-1/PD-L1 inhibitor |
| WO2021034804A1 (en) | 2019-08-19 | 2021-02-25 | Gilead Sciences, Inc. | Pharmaceutical formulations of tenofovir alafenamide |
| US11401279B2 (en) | 2019-09-30 | 2022-08-02 | Incyte Corporation | Pyrido[3,2-d]pyrimidine compounds as immunomodulators |
| WO2021067181A1 (en) | 2019-09-30 | 2021-04-08 | Gilead Sciences, Inc. | Hbv vaccines and methods treating hbv |
| US11866451B2 (en) | 2019-11-11 | 2024-01-09 | Incyte Corporation | Salts and crystalline forms of a PD-1/PD-L1 inhibitor |
| WO2021113765A1 (en) | 2019-12-06 | 2021-06-10 | Precision Biosciences, Inc. | Optimized engineered meganucleases having specificity for a recognition sequence in the hepatitis b virus genome |
| EP4100381A4 (en) * | 2020-02-03 | 2024-04-03 | Arbutus Biopharma Corp | Substituted 1,1'-biphenyl compounds and methods using same |
| WO2021188959A1 (en) | 2020-03-20 | 2021-09-23 | Gilead Sciences, Inc. | Prodrugs of 4'-c-substituted-2-halo-2'-deoxyadenosine nucleosides and methods of making and using the same |
| WO2021226206A2 (en) | 2020-05-05 | 2021-11-11 | Teon Therapeutics, Inc. | Cannabinoid receptor type 2 (cb2) modulators and uses thereof |
| US11760764B2 (en) | 2020-05-22 | 2023-09-19 | Aligos Therapeutics, Inc. | Methods and compositions for targeting PD-L1 |
| US11780836B2 (en) | 2020-11-06 | 2023-10-10 | Incyte Corporation | Process of preparing a PD-1/PD-L1 inhibitor |
| US11866434B2 (en) | 2020-11-06 | 2024-01-09 | Incyte Corporation | Process for making a PD-1/PD-L1 inhibitor and salts and crystalline forms thereof |
| US11760756B2 (en) | 2020-11-06 | 2023-09-19 | Incyte Corporation | Crystalline form of a PD-1/PD-L1 inhibitor |
| WO2022147302A1 (en) * | 2020-12-30 | 2022-07-07 | Chulalongkorn University | 4-phenyl-indole derivatives and related uses |
| WO2022241134A1 (en) | 2021-05-13 | 2022-11-17 | Gilead Sciences, Inc. | COMBINATION OF A TLR8 MODULATING COMPOUND AND ANTI-HBV siRNA THERAPEUTICS |
| WO2022261301A1 (en) | 2021-06-11 | 2022-12-15 | Gilead Sciences, Inc. | Combination mcl-1 inhibitors with anti-cancer agents |
| WO2022261310A1 (en) | 2021-06-11 | 2022-12-15 | Gilead Sciences, Inc. | Combination mcl-1 inhibitors with anti-body drug conjugates |
| US11931424B2 (en) | 2021-06-11 | 2024-03-19 | Gilead Sciences, Inc. | Combination MCL-1 inhibitors with anti-body drug conjugates |
| US11957693B2 (en) | 2021-06-11 | 2024-04-16 | Gilead Sciences, Inc. | Combination MCL-1 inhibitors with anti-cancer agents |
| WO2022271659A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271677A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271684A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2022271650A1 (en) | 2021-06-23 | 2022-12-29 | Gilead Sciences, Inc. | Diacylglyercol kinase modulating compounds |
| WO2023034530A1 (en) | 2021-09-02 | 2023-03-09 | Teon Therapeutics, Inc. | Methods of improving growth and function of immune cells |
| WO2023081730A1 (en) | 2021-11-03 | 2023-05-11 | Teon Therapeutics, Inc. | 4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide derivatives as cannabinoid cb2 receptor modulators for the treatment of cancer |
| WO2023097211A1 (en) | 2021-11-24 | 2023-06-01 | The University Of Southern California | Methods for enhancing immune checkpoint inhibitor therapy |
| WO2024015372A1 (en) | 2022-07-14 | 2024-01-18 | Teon Therapeutics, Inc. | Adenosine receptor antagonists and uses thereof |
| WO2024019957A1 (en) * | 2022-07-18 | 2024-01-25 | Celgene Corporation | Compounds for the treatment of neurodegenerative diseases |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3493804A1 (en) | 2019-06-12 |
| CN109195602A (en) | 2019-01-11 |
| CN109195602B (en) | 2022-01-07 |
| AU2017305399A1 (en) | 2019-01-31 |
| CA3030773A1 (en) | 2018-02-08 |
| JP2019530732A (en) | 2019-10-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3493804A1 (en) | Symmetric or semi-symmetric compounds useful as immunomodulators | |
| JP7005582B2 (en) | Multifluoro-substituted compound as Bruton's tyrosine kinase (BTK) inhibitor | |
| TWI641600B (en) | Piperidinyl-indole derivatives complement factor b inhibitors and uses thereof | |
| RU2674701C2 (en) | Aminopiridazinove compounds as proteinkinase inhibitors | |
| EP2920162B1 (en) | Inhibitors of bruton's tyrosine kinase | |
| US9586948B2 (en) | Inhibitors of IRAK4 activity | |
| KR101358532B1 (en) | Novel phenylimidazopyrazines | |
| WO2020114482A1 (en) | Isoindoline compound, and preparation method, pharmaceutical composition, and application of isoindoline compound | |
| US11066392B2 (en) | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use | |
| US20180208577A1 (en) | Compounds and Methods of Use | |
| JP2018508554A (en) | Tricyclic heterocyclic compounds useful as TNF inhibitors | |
| US20190040012A1 (en) | Ror gamma (rory) modulators | |
| JP2024505732A (en) | Pyridopyrimidinone derivatives and their production methods and uses | |
| KR20170129192A (en) | A heterocyclic compound useful as an inhibitor of TNF | |
| TWI804266B (en) | Tyk2 inhibitors and use thereof | |
| JP6477484B2 (en) | Sulfur-containing bicyclic compound | |
| TW202332436A (en) | Therapeutic compounds | |
| CN107849044B (en) | Triaza-spirodecanones as DDR1 inhibitors | |
| CN117800904A (en) | ROR gamma inhibitors containing sulfonyl structures | |
| CA2893637A1 (en) | Thiazole derivatives as inhibitors of bruton's tyrosine kinase | |
| KR20230134500A (en) | Imidazo[1,2-A]pyridine derivatives as IRAK4 inhibitors and their use in the treatment of diseases | |
| JP2024516194A (en) | Compounds as PD1/PD-L1 inhibitors and methods thereof | |
| AU2022318751A1 (en) | Rock2 inhibitors and uses thereof | |
| WO2023250316A1 (en) | Compounds for treating spinocerebellar ataxia type 3 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17837640 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3030773 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2017305399 Country of ref document: AU Date of ref document: 20170802 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2019527771 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2017837640 Country of ref document: EP Effective date: 20190304 |