WO2017170516A1 - Flexible polarizing film, manufacturing method for same and image display device - Google Patents
Flexible polarizing film, manufacturing method for same and image display device Download PDFInfo
- Publication number
- WO2017170516A1 WO2017170516A1 PCT/JP2017/012588 JP2017012588W WO2017170516A1 WO 2017170516 A1 WO2017170516 A1 WO 2017170516A1 JP 2017012588 W JP2017012588 W JP 2017012588W WO 2017170516 A1 WO2017170516 A1 WO 2017170516A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- polyvinyl alcohol
- polarizing film
- meth
- resin
- flexible polarizing
- Prior art date
Links
Images
Classifications
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
- G02B5/3025—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state
- G02B5/3033—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state in the form of a thin sheet or foil, e.g. Polaroid
- G02B5/3041—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state in the form of a thin sheet or foil, e.g. Polaroid comprising multiple thin layers, e.g. multilayer stacks
- G02B5/305—Polarisers, i.e. arrangements capable of producing a definite output polarisation state from an unpolarised input state in the form of a thin sheet or foil, e.g. Polaroid comprising multiple thin layers, e.g. multilayer stacks including organic materials, e.g. polymeric layers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/042—Coating with two or more layers, where at least one layer of a composition contains a polymer binder
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C55/00—Shaping by stretching, e.g. drawing through a die; Apparatus therefor
- B29C55/02—Shaping by stretching, e.g. drawing through a die; Apparatus therefor of plates or sheets
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/30—Layered products comprising a layer of synthetic resin comprising vinyl (co)polymers; comprising acrylic (co)polymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/30—Layered products comprising a layer of synthetic resin comprising vinyl (co)polymers; comprising acrylic (co)polymers
- B32B27/306—Layered products comprising a layer of synthetic resin comprising vinyl (co)polymers; comprising acrylic (co)polymers comprising vinyl acetate or vinyl alcohol (co)polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/18—Manufacture of films or sheets
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L29/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an alcohol, ether, aldehydo, ketonic, acetal or ketal radical; Compositions of hydrolysed polymers of esters of unsaturated alcohols with saturated carboxylic acids; Compositions of derivatives of such polymers
- C08L29/02—Homopolymers or copolymers of unsaturated alcohols
- C08L29/04—Polyvinyl alcohol; Partially hydrolysed homopolymers or copolymers of esters of unsaturated alcohols with saturated carboxylic acids
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
- G02F1/1335—Structural association of cells with optical devices, e.g. polarisers or reflectors
-
- G—PHYSICS
- G09—EDUCATION; CRYPTOGRAPHY; DISPLAY; ADVERTISING; SEALS
- G09F—DISPLAYING; ADVERTISING; SIGNS; LABELS OR NAME-PLATES; SEALS
- G09F9/00—Indicating arrangements for variable information in which the information is built-up on a support by selection or combination of individual elements
-
- G—PHYSICS
- G09—EDUCATION; CRYPTOGRAPHY; DISPLAY; ADVERTISING; SEALS
- G09F—DISPLAYING; ADVERTISING; SIGNS; LABELS OR NAME-PLATES; SEALS
- G09F9/00—Indicating arrangements for variable information in which the information is built-up on a support by selection or combination of individual elements
- G09F9/30—Indicating arrangements for variable information in which the information is built-up on a support by selection or combination of individual elements in which the desired character or characters are formed by combining individual elements
- G09F9/301—Indicating arrangements for variable information in which the information is built-up on a support by selection or combination of individual elements in which the desired character or characters are formed by combining individual elements flexible foldable or roll-able electronic displays, e.g. thin LCD, OLED
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/02—Details
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2329/00—Characterised by the use of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an alcohol, ether, aldehydo, ketonic, acetal, or ketal radical; Hydrolysed polymers of esters of unsaturated alcohols with saturated carboxylic acids; Derivatives of such polymer
- C08J2329/02—Homopolymers or copolymers of unsaturated alcohols
- C08J2329/04—Polyvinyl alcohol; Partially hydrolysed homopolymers or copolymers of esters of unsaturated alcohols with saturated carboxylic acids
Definitions
- the present invention relates to a flexible polarizing film and a manufacturing method thereof.
- the flexible polarizing film can form an image display device such as a liquid crystal display device (LCD) or an organic EL display device alone or as an optical film obtained by laminating the flexible polarizing film.
- LCD liquid crystal display device
- organic EL display device alone or as an optical film obtained by laminating the flexible polarizing film.
- a polarizer In a liquid crystal display device, it is indispensable to dispose a polarizer on both sides of a glass substrate that forms the surface of a liquid crystal panel because of its image forming method.
- the polarizer generally used is a polyvinyl alcohol polarizer in which a polyvinyl alcohol resin is oriented in one direction and iodine or a dichroic dye is adsorbed and oriented on the polyvinyl alcohol resin.
- polyvinyl alcohol polarizers have the problem that they are brittle and easy to tear.
- a polyvinyl alcohol-based polarizer is easy to tear parallel to the direction in which the polyvinyl alcohol-based resin is oriented (absorption axis direction). For example, when an external force that contracts in the absorption axis direction is applied, it is easily broken. .
- the polyvinyl alcohol polarizer is used as a polarizing film in which a transparent protective film is bonded to one or both sides.
- a general polarizing film using a polyvinyl alcohol-based polarizer has rigidity (rigidity) because a transparent protective film is bonded, and lacks flexibility (flexibility). Therefore, when twisting is applied to the polarizing film, the entire film may be cracked, broken marks (folded marks) may remain, or light leakage may occur in the polarizer. It was constrained.
- the grid polarizer has flexibility but lacks versatility.
- An object of the present invention is to provide a flexible polarizing film having a high degree of flexibility despite the use of a polyvinyl alcohol polarizer and a method for producing the same.
- Another object of the present invention is to provide an image display device having the flexible polarizing film.
- the present invention comprises a polyvinyl alcohol polarizer having a thickness of 10 ⁇ m or less, wherein the polyvinyl alcohol resin is oriented in one direction and iodine or a dichroic dye is adsorbed and oriented on the polyvinyl alcohol resin, and
- the present invention relates to a flexible polarizing film characterized by being free from cracks, folds, and light leakage after being subjected to a twist test in which the polyvinyl alcohol resin is twisted in the direction in which the polyvinyl alcohol resin is oriented.
- the flexible polarizing film after performing a U-shaped expansion / contraction test that repeatedly expands and contracts in a U-shape in an alignment direction in which the polyvinyl alcohol-based resin is aligned and in a direction orthogonal to the alignment direction, cracks occur in any direction. It is preferable that there are no folds and no light leakage.
- the bent shape is maintained in any direction. In addition, it is preferable that no cracks occur.
- the bending resistance (mm) is preferably 60 mm or less in the bending test in the alignment direction in which the polyvinyl alcohol-based resin is aligned and in the direction orthogonal to the alignment direction.
- the tensile strength in the direction orthogonal to the orientation direction in which the polyvinyl alcohol resin is oriented in a tensile test is 5 N / 10 mm or more.
- the polyvinyl alcohol polarizer has an optical property represented by the following formula: P> ⁇ (10 0.929T-42.4 ⁇ 1) ⁇ 100 However, T ⁇ 42.3) or It is preferable that the lens is configured to satisfy the condition of P ⁇ 99.9 (however, T ⁇ 42.3).
- the flexible polarizing film preferably has a reinforcing film in close contact with the polyvinyl alcohol polarizer on at least one surface of the polyvinyl alcohol polarizer.
- the thickness of the reinforcing film is preferably 15 ⁇ m or less.
- the flexible polarizing film preferably has a first reinforcing film having a thickness of 15 ⁇ m or less on the first side of the polyvinyl alcohol-based polarizer and a second reinforcing film having a thickness of 15 ⁇ m or less on the other second side. .
- the thickness difference between the first reinforcing film and the second reinforcing film is preferably 10 ⁇ m or less.
- the ratio of the thickness of the reinforcing film to the thickness of the polyvinyl alcohol polarizer is preferably 0.4 or more.
- the reinforcing film preferably has a compressive elastic modulus at 23 ° C. of 1 MPa or more.
- the reinforcing film that is not substantially oriented can be used.
- a resin film can be used as the reinforcing film.
- the resin film is preferably a thermosetting resin or an active energy ray curable resin.
- the present invention is a method for producing the flexible polarizing film, A step (1) of preparing a polyvinyl alcohol polarizer having a thickness of 10 ⁇ m or less, wherein the polyvinyl alcohol resin is oriented in one direction and iodine or a dichroic dye is adsorbed and oriented on the polyvinyl alcohol resin; By applying a liquid material containing a curable component capable of constituting a resin component or a resin film on at least one surface of the polyvinyl alcohol polarizer, and then solidifying or curing the liquid material, a reinforcing film
- the manufacturing method of the flexible polarizing film characterized by including the process (2) of forming.
- the present invention also relates to an image display device having the flexible polarizing film.
- the flexible polarizing film of the present invention uses a polyvinyl alcohol polarizer and can satisfy general versatility. Moreover, the polyvinyl alcohol-type polarizer is 10 micrometers or less in thickness, and is suitable also at the point currently made thin.
- the flexible polarizing film of the present invention has a high degree of flexibility despite the fact that it uses a polyvinyl alcohol polarizer that is brittle and easy to tear, and has deformed its shape by twisting. Even in this case, there is no occurrence of cracks in the entire film, no broken marks (fold marks), and no light leakage from the polarizer.
- the flexible polarizing film of the present invention can have flexibility against various deformations such as expansion and contraction. As described above, the flexible polarizing film of the present invention itself has flexibility, and a normal polyvinyl alcohol-based polarizer alone has a remarkably small tensile breaking stress, so that it is substantially impossible to handle. On the other hand, it is thin and easy to handle.
- the flexible polarizing film of the present invention has flexibility even when used in combination with other members or by using the flexibility, and can suppress cracks in the polarizer. Yes, it can be used for various purposes. For this reason, the use of the flexible polarizing film is greatly expanded by expanding the application of the flexible polarizing film alone or expanding the tolerance in the process. Therefore, the flexible polarizing film of the present invention can be used as an alternative to a polarizer, for example, a design that could not be applied due to the brittleness and tearing of conventional polarizers, and a polarizing film (transparent protection to the polarizer). As an alternative to the one provided with a film), for example, it is possible to deal with various deformed shapes that could not be applied due to the rigidity of the conventional polarizing film, and the application development can be expanded.
- the flexible polarizing film of the present invention has a polyvinyl alcohol polarizer.
- the polyvinyl alcohol-based polarizer is obtained by aligning a polyvinyl alcohol-based resin in one direction (absorption axis direction) and adsorbing or aligning iodine or a dichroic dye on the polyvinyl alcohol-based resin.
- the polyvinyl alcohol-based resin include polyvinyl alcohol, partially formalized polyvinyl alcohol, ethylene / vinyl acetate copolymer partially saponified products, and the like.
- a polarizer can be obtained by adsorbing a dichroic dye such as iodine or a dichroic dye to a polyvinyl alcohol film using the polyvinyl alcohol resin and stretching the film uniaxially.
- a polarizer obtained by dying a polyvinyl alcohol film with iodine or a dichroic dye and uniaxially stretching it is prepared, for example, by immersing polyvinyl alcohol in an aqueous solution of iodine and stretching it 3 to 7 times the original length. can do. If necessary, it may contain boric acid, zinc sulfate, zinc chloride, or the like, or may be immersed in an aqueous solution such as potassium iodide. Further, if necessary, the polyvinyl alcohol film may be immersed in water and washed before dyeing.
- Stretching may be performed after dyeing with iodine or a dichroic dye, may be performed while dyeing, or may be dyed with iodine or a dichroic dye after stretching.
- the film can be stretched even in an aqueous solution such as boric acid or potassium iodide or in a water bath.
- the polyvinyl alcohol polarizer preferably contains boric acid from the viewpoint of stretching stability and optical durability.
- the boric acid content contained in the polarizer is preferably 25% by weight or less, more preferably 20% by weight or less, based on the total amount of the polarizer, from the viewpoint of suppressing the occurrence of light leakage. Further, it is preferably 18% by weight or less, more preferably 16% by weight or less.
- the boric acid content with respect to the total amount of the polarizer is preferably 10% by weight or more, and more preferably 12% by weight or more.
- a polyvinyl alcohol polarizer having a thickness of 10 ⁇ m or less is used.
- the thickness of the polarizer is preferably 8 ⁇ m or less, more preferably 7 ⁇ m or less, and further preferably 6 ⁇ m or less from the viewpoints of thinning and flexibility.
- the thickness of the polarizer is preferably 2 ⁇ m or more, and more preferably 3 ⁇ m or more.
- Such a thin polarizer has less thickness unevenness, excellent visibility, and less dimensional change, and therefore excellent durability against thermal shock.
- Patent No. 4751486 Japanese Patent No. 4751481, Patent No. 4815544, Patent No. 5048120, International Publication No. 2014/077599 pamphlet, International Publication No. 2014/077636 Pamphlet, And the thin polarizers obtained from the production methods described therein.
- the polarizer has an optical characteristic expressed by a single transmittance T and a polarization degree P of the following formula P> ⁇ (10 0.929T-42.4 ⁇ 1) ⁇ 100 (where T ⁇ 42.3), Or It is configured to satisfy the condition of P ⁇ 99.9 (however, T ⁇ 42.3).
- a polarizer configured so as to satisfy the above-described conditions uniquely has performance required as a display for a liquid crystal television using a large display element. Specifically, the contrast ratio is 1000: 1 or more and the maximum luminance is 500 cd / m 2 or more. As other uses, for example, it is bonded to the viewing side of the organic EL display device.
- a polarizer configured to satisfy the above conditions has a high orientation of a polymer (for example, a polyvinyl alcohol-based molecule), so that the thickness of the polarizer is 10 ⁇ m or less.
- the tensile rupture stress in the direction orthogonal to the absorption axis direction (transmission axis direction) is significantly reduced.
- the flexible polarizing film of the present invention has excellent flexibility in spite of the use of such a polyvinyl alcohol-based polarizer that is weak against impact of 10 ⁇ m or less.
- Patent No. 4751486, Patent in that it can be stretched at a high magnification and the polarization performance can be improved.
- stretching in a boric-acid aqueous solution as described in the 4751481 specification and the patent 4815544 specification is preferable, and it describes especially in the patent 4751481 specification and the patent 4815544 specification.
- stretching in the boric-acid aqueous solution which has this is preferable.
- These thin polarizers can be obtained by a production method including a step of stretching a polyvinyl alcohol-based resin (hereinafter also referred to as PVA-based resin) layer and a stretching resin base material in a laminated state and a step of dyeing.
- PVA-based resin polyvinyl alcohol-based resin
- a stretching resin base material in a laminated state
- dyeing a step of dyeing
- the flexible polarizing film of the present invention is characterized in that after the twist test shown in the examples is performed, the polyvinyl alcohol-based polarizer is free from cracks, generation of fold marks, and light leakage.
- the twist test is an index indicating the flexibility in the twisted state in the orientation direction (absorption axis direction) in which the polyvinyl alcohol-based resin that is likely to generate cracks, fold marks, and light leakage is oriented. It can be seen that the flexible polarizing film of the present invention has excellent flexibility in a twisted state in the twisting test without occurrence of cracks, folds and light leakage.
- the flexible polarizing film of the present invention is preferably capable of suppressing the generation of cracks, creases and light leakage after the U-shaped expansion / contraction test shown in the examples.
- the U-shaped expansion / contraction test is an index indicating the flexibility related to unfolded bending in a U-shape in the alignment direction (absorption axis direction) in which the polyvinyl alcohol-based resin is aligned and in the orthogonal direction (transmission axis direction). .
- the flexible polarizing film of the present invention has excellent unfolded bendability in both the absorption axis direction and the transmission axis direction by suppressing the generation of cracks, fold marks and light leakage in the U-shaped stretch test. It can be seen that it has the flexibility involved.
- the flexible polarizing film of the present invention preferably retains the bent shape and suppresses cracking after the bending holding test shown in the examples.
- the flexible polarizing film maintains its original shape even when it is bent and bent in the orientation direction (absorption axis direction) in which the polyvinyl alcohol resin is oriented and in the orthogonal direction (transmission axis direction). It is an index indicating the flexibility related to the retention that can be performed. It can be seen that the flexible polarizing film of the present invention has excellent holding properties in both the absorption axis direction and the transmission axis direction by holding the bent shape and suppressing cracking in the bending holding test.
- the flexible polarizing film of the present invention preferably satisfies the bending resistance (mm) of 60 mm or less in the bending resistance test specifically shown in the examples.
- the bending resistance test is an index indicating flexibility related to the bending followability (low resistance to bending) in the orientation direction (absorption axis direction) in which the polyvinyl alcohol-based resin is oriented and in the orthogonal direction (transmission axis direction).
- the flexible polarizing film of the present invention has a bending flexibility (low resistance to bending) having a bending resistance of 60 mm or less.
- the index indicating the flexibility of the bending resistance (mm) is preferably 50 mm or less, and more preferably 40 mm or less.
- the flexible polarizing film of the present invention preferably satisfies a tensile strength of 5 N / 10 mm or more in the tensile test specifically shown in the examples.
- the tensile test is an index indicating the strength in the direction (transmission axis direction) orthogonal to the orientation direction (absorption axis direction) in which the polyvinyl alcohol-based resin is oriented. It can be seen that the flexible polarizing film of the present invention has strength in the transmission axis direction when the tensile strength satisfies 5 N / 10 mm or more.
- the tensile strength is preferably 7 N / 10 mm or more, more preferably 10 N / 10 mm or more from the viewpoint of strength.
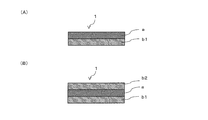
- the flexible polarizing film 1 in FIG. 1A is a case where the first reinforcing film b1 is provided only on the first one surface of the polyvinyl alcohol polarizer a.
- the flexible polarizing film 1 of FIG. 1 (B) has the 1st reinforcement film
- the first reinforcing film b1 and / or the second reinforcing film b2 are provided directly on the polyvinyl alcohol polarizer 1.
- the flexible polarizing film 1 of the present invention has flexibility in the twist test, and has a transparent protective film or the like on one side or both sides of the polyvinyl alcohol-based polarizer a, and cannot satisfy the flexibility. This is clearly distinguished from a normal polarizing film.
- the thickness of the reinforcing membrane is preferably 15 ⁇ m or less from the viewpoint of thinning and flexibility, more preferably 10 ⁇ m or less, and further preferably 7 ⁇ m or less.
- the thickness of the reinforcing membrane is preferably 1 ⁇ m or more, more preferably 3 ⁇ m or more, and further preferably 5 ⁇ m or more from the viewpoint of flexibility and strength.
- the ratio (t2 / t1) of the thickness (t2) of the reinforcing film to the thickness (t1) of the polyvinyl alcohol polarizer is preferably 0.4 or more from the viewpoint of flexibility and strength, and more preferably 0. .6 or more is preferable, and further 0.8 or more is preferable.
- the ratio (t2 / t1) is preferably 2.0 or less, more preferably 1.5 or less, and further preferably 1.2 or less from the viewpoint of thinning. preferable.
- the reinforcing film has a polyvinyl alcohol polarizer as shown in FIG. 1 (B), rather than having the first reinforcing film b1 only on one side of the polyvinyl alcohol polarizer 1 as shown in FIG. 1 (A).
- 1 having both the first reinforcing film b1 and the second reinforcing film b2 can satisfy the flexibility in the twist test, the U-shaped stretch test, and the like, and can satisfy the strength in the tensile test. It is preferable from the point.
- each reinforcing film may be the same or different.
- the thickness difference between the first reinforcing film b1 and the second reinforcing film b2 is good, it is normal (a state where the flexible polarizing film is left alone) and a flexibility test (various tests such as a twist test). Is preferably 10 ⁇ m or less, more preferably 7 ⁇ m or less, and even more preferably 5 ⁇ m or less, because the stress in the flexible polarizing film is made uniform (symmetric) and strength and flexibility are easily secured. It is preferable that they have the same thickness.
- the reinforcing membrane preferably has a compressive elastic modulus at 23 ° C. of 1 MPa or more from the viewpoint of strength. Furthermore, the compressive elastic modulus of the reinforcing membrane is preferably 10 MPa or more, and more preferably 100 MPa or more. On the other hand, when the compressive elastic modulus of the reinforcing membrane layer increases, the compressive elastic modulus is preferably 10 GPa or less, and more preferably 1 GPa or less, because it tends to be too hard and poor flexibility.
- the reinforcing film can be formed from various forming materials.
- the reinforcing film can be formed, for example, by applying a resin material to a polyvinyl alcohol polarizer, or by depositing an inorganic oxide such as SiO 2 on the polyvinyl alcohol polarizer by a sputtering method or the like. You can also
- the reinforcing film is preferably a resin film formed from a resin material from the viewpoint of simple formation.
- the reinforcing film may or may not be oriented, and any of them can be used.
- the reinforcing film When the reinforcing film is oriented, a phase difference occurs and the optical characteristics of the polyvinyl alcohol polarizer change. Therefore, when maintaining the optical characteristics of the polarizer, the reinforcing film that is not substantially oriented.
- “Substantially not oriented” refers to a state in which the treatment for positively orienting the reinforcing film is not performed although the orientation exists inside the reinforcing film due to the orientation of the polarizer.
- a reinforcing film that is not practically oriented can be formed by, for example, applying a resin film forming material to a polyvinyl alcohol polarizer.
- an oriented film can be used as the reinforcing film.
- the oriented reinforcing film expresses a phase difference and can be used as an optical compensation film or the like.
- a material for forming the reinforcing film is not particularly limited as long as it is a material that can be adhered to a polyvinyl alcohol polarizer.
- a polyester resin, a polyether resin, a polycarbonate resin, a polyurethane resin, a silicone resin examples thereof include resins, polyamide resins, polyimide resins, PVA resins, acrylic resins, and epoxy resins. These resin materials can be used singly or in combination of two or more, and among these, one or more selected from the group consisting of polyurethane resins, PVA resins, acrylic resins, and epoxy resins Are preferred, and polyurethane resins and acrylic resins are more preferred.
- the reinforcing film is formed by applying a liquid material containing a curable component capable of constituting the surface of the polarizer, the resin component or the resin, and then solidifying or curing the liquid material. Can do.
- the form of the liquid coating liquid is not particularly limited as long as it exhibits a liquid state, and may be any of water-based, water-dispersed, solvent-based, and solvent-free.
- the forming material may contain an additive as long as the function of the reinforcing film is not impaired.
- silane coupling agents polyalkylene glycol polyether compounds such as polypropylene glycol
- powders such as colorants and pigments, dyes, surfactants, plasticizers, tackifiers, antistatic agents, surface lubricants,
- leveling agents softeners, antioxidants, antioxidants, light stabilizers, UV absorbers, polymerization inhibitors, inorganic or organic fillers, metal powders, particles, foils, etc. It can be added as appropriate.
- the liquid (coating liquid) has a lower viscosity.
- the viscosity measured at 25 ° C. is preferably 2000 mPa ⁇ s or less, more preferably 1000 mPa ⁇ s or less, further preferably 500 mPa ⁇ s or less, and further 100 mPa ⁇ s. It is preferable that:
- the resin component is solidified according to the type.
- the liquid substance containing the resin component is a solution obtained by dissolving or dispersing the resin component in a solvent, and is used as, for example, an aqueous solution, an aqueous dispersion, or a solvent solution.
- the solidification means forming a resin layer by removing a solvent from the liquid material.
- An aqueous resin emulsion can be used as the aqueous dispersion.
- the aqueous resin emulsion contains emulsion resin particles emulsified in water (dispersion medium).
- the reinforcing film of the present invention can be formed by directly applying a forming material containing the aqueous resin emulsion to a polarizer and drying it.
- the resin constituting the emulsion resin is not particularly limited, and examples thereof include acrylic resins, silicone resins, polyurethane resins, and fluorine resins. Among these, in the present invention, polyurethane resins and acrylic resins are preferable because they are excellent in optical transparency and weather resistance and heat resistance.
- water-based resin emulsion examples include trade name: SE-2915E (acrylic emulsion containing UV absorber) manufactured by Taisei Fine Chemical Co., Ltd., trade names: Aron A-104 and Aron A-106 manufactured by Toagosei Co., Ltd. It is done.
- SE-2915E acrylic emulsion containing UV absorber
- the curable component forms a resin according to the type of the curable component.
- the liquid material containing a curable component that can constitute the resin can be used in a solventless system as long as the curable component exhibits a liquid material.
- the liquid material may be a solution in which the curable component is dissolved in a solvent.
- the said curable component exhibits a liquid substance, it can be used as a solution.
- the solvent can be appropriately selected according to the curable component to be used.
- the liquid material containing the curable component is irradiated with active energy rays (ultraviolet rays). Curing by irradiation) or the like can be performed.
- a curable forming material containing a curable component capable of constituting a resin will be described.
- the curable component can be roughly classified into an active energy ray curable type such as an electron beam curable type, an ultraviolet ray curable type, and a visible light curable type, and a thermosetting type.
- the ultraviolet curable type and the visible light curable type can be classified into a radical polymerization curable type and a cationic polymerization curable type.
- an active energy ray having a wavelength range of 10 nm to less than 380 nm is expressed as ultraviolet light
- an active energy ray having a wavelength range of 380 nm to 800 nm is expressed as visible light.
- the radical polymerization curable component can be used as a thermosetting curable component.
- the curable component examples include a radical polymerizable compound.
- the radical polymerizable compound examples include compounds having a radical polymerizable functional group of a carbon-carbon double bond such as a (meth) acryloyl group and a vinyl group.
- these curable components either a monofunctional radical polymerizable compound or a bifunctional or higher polyfunctional radical polymerizable compound can be used.
- these radically polymerizable compounds can be used individually by 1 type or in combination of 2 or more types.
- compounds having a (meth) acryloyl group are suitable.
- (meth) acryloyl means an acryloyl group and / or methacryloyl group, and “(meth)” has the same meaning hereinafter.
- Examples of the monofunctional radical polymerizable compound include (meth) acrylamide derivatives having a (meth) acrylamide group.
- the (meth) acrylamide derivative is preferable in terms of ensuring adhesion with the polarizer and having a high polymerization rate and excellent productivity.
- (meth) acrylamide derivatives include, for example, N-methyl (meth) acrylamide, N, N-dimethyl (meth) acrylamide, N, N-diethyl (meth) acrylamide, N-isopropyl (meth) acrylamide, N N-alkyl group-containing (meth) acrylamide derivatives such as butyl (meth) acrylamide and N-hexyl (meth) acrylamide; N-methylol (meth) acrylamide, N-hydroxyethyl (meth) acrylamide, N-methylol-N— N-hydroxyalkyl group-containing (meth) acrylamide derivatives such as propane (meth) acrylamide; N-aminoalkyl group-containing (meth) acrylamide derivatives such as aminomethyl (meth) acrylamide and aminoethyl (meth) acrylamide; N-methoxymethyl N-alkoxy group-containing (meth) acrylamide derivatives such as
- heterocyclic-containing (meth) acrylamide derivative in which the nitrogen atom of the (meth) acrylamide group forms a heterocyclic ring examples include, for example, N-acryloylmorpholine, N-acryloylpiperidine, N-methacryloylpiperidine, N-acryloylpyrrolidine. Etc.
- an N-hydroxyalkyl group-containing (meth) acrylamide derivative is preferable from the viewpoint of adhesion to a polarizer, and N-hydroxyethyl (meth) acrylamide is particularly preferable.
- examples of the monofunctional radical polymerizable compound include various (meth) acrylic acid derivatives having a (meth) acryloyloxy group. Specifically, for example, methyl (meth) acrylate, ethyl (meth) acrylate, n-propyl (meth) acrylate, isopropyl (meth) acrylate, 2-methyl-2-nitropropyl (meth) acrylate, n-butyl ( (Meth) acrylate, isobutyl (meth) acrylate, s-butyl (meth) acrylate, t-butyl (meth) acrylate, n-pentyl (meth) acrylate, t-pentyl (meth) acrylate, 3-pentyl (meth) acrylate, 2,2-dimethylbutyl (meth) acrylate, n-hexyl (meth) acrylate, cetyl (meth) acrylate, n-
- Examples of the (meth) acrylic acid derivative include cycloalkyl (meth) acrylates such as cyclohexyl (meth) acrylate and cyclopentyl (meth) acrylate; Aralkyl (meth) acrylates such as benzyl (meth) acrylate; 2-isobornyl (meth) acrylate, 2-norbornylmethyl (meth) acrylate, 5-norbornen-2-yl-methyl (meth) acrylate, 3-methyl-2-norbornylmethyl (meth) acrylate, dicyclo Polycyclic (meth) acrylates such as pentenyl (meth) acrylate, dicyclopentenyloxyethyl (meth) acrylate, dicyclopentanyl (meth) acrylate, and the like; 2-methoxyethyl (meth) acrylate, 2-ethoxyethyl (meth) acrylate, 2-methoxymethoxyethyl (
- Examples of the (meth) acrylic acid derivative include 2-hydroxyethyl (meth) acrylate, 2-hydroxypropyl (meth) acrylate, 3-hydroxypropyl (meth) acrylate, 2-hydroxybutyl (meth) acrylate, 4- Hydroxyalkyl (meth) acrylates such as hydroxybutyl (meth) acrylate, 6-hydroxyhexyl (meth) acrylate, 8-hydroxyoctyl (meth) acrylate, 10-hydroxydecyl (meth) acrylate, 12-hydroxylauryl (meth) acrylate, etc.
- hydroxyl groups such as [4- (hydroxymethyl) cyclohexyl] methyl acrylate, cyclohexanedimethanol mono (meth) acrylate, 2-hydroxy-3-phenoxypropyl (meth) acrylate, etc.
- Meth) acrylate Epoxy group-containing (meth) acrylates such as glycidyl (meth) acrylate and 4-hydroxybutyl (meth) acrylate glycidyl ether; 2,2,2-trifluoroethyl (meth) acrylate, 2,2,2-trifluoroethylethyl (meth) acrylate, tetrafluoropropyl (meth) acrylate, hexafluoropropyl (meth) acrylate, octafluoropentyl (meth) ) Halogen-containing (meth) acrylates such as acrylate, heptadecafluorodecyl (meth) acrylate, 3-chloro-2-hydroxypropyl (meth) acrylate; Alkylaminoalkyl (meth) acrylates such as dimethylaminoethyl (meth) acrylate; 3-Oxetanylmethyl (meth) acrylate
- examples of the monofunctional radically polymerizable compound include carboxyl group-containing monomers such as (meth) acrylic acid, carboxyethyl acrylate, carboxypentyl acrylate, itaconic acid, maleic acid, fumaric acid, crotonic acid, and isocrotonic acid.
- carboxyl group-containing monomers such as (meth) acrylic acid, carboxyethyl acrylate, carboxypentyl acrylate, itaconic acid, maleic acid, fumaric acid, crotonic acid, and isocrotonic acid.
- Examples of the monofunctional radical polymerizable compound include lactam vinyl monomers such as N-vinylpyrrolidone, N-vinyl- ⁇ -caprolactam, and methylvinylpyrrolidone; vinylpyridine, vinylpiperidone, vinylpyrimidine, vinylpiperazine, vinylpyrazine, Examples thereof include vinyl monomers having a nitrogen-containing heterocyclic ring such as vinyl pyrrole, vinyl imidazole, vinyl oxazole and vinyl morpholine.
- lactam vinyl monomers such as N-vinylpyrrolidone, N-vinyl- ⁇ -caprolactam, and methylvinylpyrrolidone
- vinylpyridine vinylpiperidone
- vinylpyrimidine vinylpiperazine
- vinylpyrazine examples thereof include vinyl monomers having a nitrogen-containing heterocyclic ring such as vinyl pyrrole, vinyl imidazole, vinyl oxazole and vinyl morpholine.
- a radically polymerizable compound having an active methylene group can be used as the monofunctional radically polymerizable compound.
- the radical polymerizable compound having an active methylene group is a compound having an active methylene group having an active double bond group such as a (meth) acryl group at the terminal or in the molecule.
- the active methylene group include an acetoacetyl group, an alkoxymalonyl group, and a cyanoacetyl group.
- the active methylene group is preferably an acetoacetyl group.
- radical polymerizable compound having an active methylene group examples include 2-acetoacetoxyethyl (meth) acrylate, 2-acetoacetoxypropyl (meth) acrylate, 2-acetoacetoxy-1-methylethyl (meth) acrylate, and the like.
- Examples include acrylamide, N- (4-acetoacetoxymethylbenzyl) acrylamide, and N- (2-acetoacetylaminoethyl) acrylamide.
- the radical polymerizable compound having an active methylene group is preferably acetoacetoxyalkyl (meth) acrylate.
- Examples of the bifunctional or higher polyfunctional radical polymerizable compound include tripropylene glycol di (meth) acrylate, tetraethylene glycol di (meth) acrylate, 1,6-hexanediol di (meth) acrylate, 1,9 -Nonanediol di (meth) acrylate, 1,10-decanediol diacrylate, 2-ethyl-2-butylpropanediol di (meth) acrylate, bisphenol A di (meth) acrylate, bisphenol A ethylene oxide adduct di (meth) ) Acrylate, bisphenol A propylene oxide adduct di (meth) acrylate, bisphenol A diglycidyl ether di (meth) acrylate, neopentyl glycol di (meth) acrylate, tricyclodecane dimethanol di (meth) Acryte, cyclic trimethylol
- Aronix M-220, M-306 manufactured by Toagosei Co., Ltd.
- light acrylate 1,9ND-A manufactured by Kyoeisha Chemical Co., Ltd.
- light acrylate DGE-4A manufactured by Kyoeisha Chemical Co., Ltd.
- light acrylate DCP- A manufactured by Kyoeisha Chemical Co., Ltd.
- SR-531 manufactured by Sartomer
- CD-536 manufactured by Sartomer
- various epoxy (meth) acrylates, urethane (meth) acrylates, polyester (meth) acrylates, various (meth) acrylate monomers, and the like are included as necessary.
- the radical polymerizable compound is preferably used in combination with a monofunctional radical polymerizable compound and a polyfunctional radical polymerizable compound from the viewpoint of achieving both adhesion to the polarizer and optical durability.
- the radical polymerization curable forming material can be used as an active energy ray curable forming material or a thermosetting forming material.
- the active energy ray curable forming material does not need to contain a photopolymerization initiator, but when using ultraviolet rays or visible light for the active energy ray, It preferably contains a photopolymerization initiator.
- the curable component when used as a thermosetting component, the forming material preferably contains a thermal polymerization initiator.
- the photopolymerization initiator in the case of using the radical polymerizable compound is appropriately selected depending on the active energy ray.
- a photopolymerization initiator for ultraviolet light or visible light cleavage is used.
- photopolymerization initiator examples include benzophenone compounds such as benzyl, benzophenone, benzoylbenzoic acid, 3,3′-dimethyl-4-methoxybenzophenone; 4- (2-hydroxyethoxy) phenyl (2-hydroxy-2 -Propyl) ketone, aromatic ketone compounds such as ⁇ -hydroxy- ⁇ , ⁇ '-dimethylacetophenone, 2-methyl-2-hydroxypropiophenone, ⁇ -hydroxycyclohexyl phenyl ketone; methoxyacetophenone, 2,2-dimethoxy- Acetophenone compounds such as 2-phenylacetophenone, 2,2-diethoxyacetophenone, 2-methyl-1- [4- (methylthio) -phenyl] -2-morpholinopropane-1; benzoin methyl ether; Benzoin ethyl ether, benzoin Benzoin ether compounds such as isopropyl ether, benzoin butyl ether and ani
- the blending amount of the photopolymerization initiator is 20 parts by weight or less with respect to 100 parts by weight of the total amount of the curable component (radical polymerizable compound).
- the blending amount of the photopolymerization initiator is preferably 0.01 to 20 parts by weight, more preferably 0.05 to 10 parts by weight, and further preferably 0.1 to 5 parts by weight.
- a photopolymerization initiator that is particularly sensitive to light of 380 nm or more.
- a photopolymerization initiator that is highly sensitive to light of 380 nm or more will be described later.
- the compound represented by following General formula (1) (Wherein R 1 and R 2 represent —H, —CH 2 CH 3 , —iPr or Cl, and R 1 and R 2 may be the same or different), respectively, or a general formula ( It is preferable to use together the compound represented by 1) and a photopolymerization initiator that is highly sensitive to light of 380 nm or more, which will be described later.
- the adhesion is excellent as compared with the case where a photopolymerization initiator having high sensitivity to light of 380 nm or more is used alone.
- diethylthioxanthone in which R 1 and R 2 are —CH 2 CH 3 is particularly preferable.
- the composition ratio of the compound represented by the general formula (1) in the forming material is preferably 0.1 to 5 parts by weight with respect to 100 parts by weight of the total amount of the curable component, and preferably 0.5 to 4 parts. More preferred are parts by weight, and even more preferred is 0.9 to 3 parts by weight.
- polymerization initiators include triethylamine, diethylamine, N-methyldiethanolamine, ethanolamine, 4-dimethylaminobenzoic acid, methyl 4-dimethylaminobenzoate, ethyl 4-dimethylaminobenzoate, isoamyl 4-dimethylaminobenzoate, etc. Among them, ethyl 4-dimethylaminobenzoate is particularly preferable.
- a polymerization initiation assistant When a polymerization initiation assistant is used, its addition amount is usually 0 to 5 parts by weight, preferably 0 to 4 parts by weight, most preferably 0 to 3 parts by weight, based on 100 parts by weight of the total amount of the curable component. is there.
- a known photopolymerization initiator can be used in combination as necessary.
- the photopolymerization initiator it is preferable to use a photopolymerization initiator that is highly sensitive to light of 380 nm or more.
- a photopolymerization initiator in addition to the photopolymerization initiator of the general formula (1), a compound represented by the following general formula (2); Wherein R 3 , R 4 and R 5 represent —H, —CH 3 , —CH 2 CH 3 , —iPr or Cl, and R 3 , R 4 and R 5 may be the same or different. It is preferable to use it.
- the compound represented by the general formula (2) 2-methyl-1- (4-methylthiophenyl) -2-morpholinopropan-1-one (trade name: IRGACURE907 manufacturer: BASF) which is also a commercial product is suitable. Can be used.
- 2-benzyl-2-dimethylamino-1- (4-morpholinophenyl) -butanone-1 (trade name: IRGACURE369 manufacturer: BASF)
- 2- (dimethylamino) -2-[(4-methylphenyl) Methyl] -1- [4- (4-morpholinyl) phenyl] -1-butanone (trade name: IRGACURE379 manufacturer: BASF) is preferred because of its high sensitivity.
- a radical polymerizable compound having an active methylene group when used as the radical polymerizable compound, it is preferably used in combination with a radical polymerization initiator having a hydrogen abstraction function.
- radical polymerization initiator having a hydrogen abstracting action examples include thioxanthone radical polymerization initiators and benzophenone radical polymerization initiators.
- the radical polymerization initiator is preferably a thioxanthone radical polymerization initiator.
- examples of the thioxanthone radical polymerization initiator include compounds represented by the above general formula (1).
- Specific examples of the compound represented by the general formula (1) include thioxanthone, dimethylthioxanthone, diethylthioxanthone, isopropylthioxanthone, and chlorothioxanthone.
- diethylthioxanthone in which R 1 and R 2 are —CH 2 CH 3 is particularly preferable.
- the radical polymerizable compound having an active methylene group and a radical polymerization initiator having a hydrogen abstraction function when the total amount of the curable component is 100% by weight, It is preferable to contain 1 to 50% by weight of the radical polymerizable compound having an active methylene group and 0.1 to 10 parts by weight of the radical polymerization initiator with respect to 100 parts by weight of the total amount of the curable component.
- thermal polymerization initiator those in which polymerization does not start by thermal cleavage when the reinforcing film is formed are preferable.
- thermal polymerization initiator those having a 10-hour half-life temperature of 65 ° C. or higher, more preferably 75 to 90 ° C. are preferable.
- the half-life is an index representing the decomposition rate of the polymerization initiator and refers to the time until the remaining amount of the polymerization initiator is halved.
- the decomposition temperature for obtaining a half-life at an arbitrary time and the half-life time at an arbitrary temperature are described in the manufacturer catalog, for example, “Organic peroxide catalog 9th edition by Nippon Oil & Fats Co., Ltd.” (May 2003) ".
- thermal polymerization initiator examples include lauroyl peroxide (10 hour half-life temperature: 64 ° C.), benzoyl peroxide (10 hour half-life temperature: 73 ° C.), 1,1-bis (t-butylperoxy) -3.
- thermal polymerization initiator examples include 2,2′-azobisisobutyronitrile (10 hour half-life temperature: 67 ° C.), 2,2′-azobis (2-methylbutyronitrile) (10 hours). And azo compounds such as 1,1-azobis-cyclohexane-1-carbonitrile (10 hour half-life temperature: 87 ° C.).
- the blending amount of the thermal polymerization initiator is 0.01 to 20 parts by weight with respect to 100 parts by weight of the total amount of the curable component (radical polymerizable compound).
- the blending amount of the thermal polymerization initiator is preferably 0.05 to 10 parts by weight, more preferably 0.1 to 3 parts by weight.
- Examples of the curable component of the cationic polymerization curable forming material include compounds having an epoxy group or an oxetanyl group.
- the compound having an epoxy group is not particularly limited as long as it has at least two epoxy groups in the molecule, and various generally known curable epoxy compounds can be used.
- a preferable epoxy compound a compound having at least two epoxy groups and at least one aromatic ring in the molecule (aromatic epoxy compound), or at least two epoxy groups in the molecule, at least one of them. Examples thereof include a compound (alicyclic epoxy compound) formed between two adjacent carbon atoms constituting an alicyclic ring.
- the cationic polymerization curable forming material contains the epoxy compound and the oxetane compound described above as the curable component, and both of these are cured by cationic polymerization, and therefore, a photocationic polymerization initiator is blended therein.
- This cationic photopolymerization initiator generates a cationic species or a Lewis acid by irradiation with active energy rays such as visible light, ultraviolet rays, X-rays, and electron beams, and starts a polymerization reaction of an epoxy group or an oxetanyl group.
- the curable forming material according to the present invention preferably contains the following components.
- the active energy ray-curable forming material according to the present invention can contain an acrylic oligomer obtained by polymerizing a (meth) acrylic monomer, in addition to the curable component related to the radical polymerizable compound.
- an acrylic oligomer in the active energy ray-curable forming material curing shrinkage when irradiating and curing the active energy ray to the reinforcing film is reduced, and the interfacial stress between the reinforcing film and the polarizer is reduced. can do.
- the content of the acrylic oligomer is preferably 20 parts by weight or less and more preferably 15 parts by weight or less with respect to 100 parts by weight of the total amount of the curable component.
- the acrylic oligomer is preferably contained in an amount of 3 parts by weight or more, more preferably 5 parts by weight or more, based on 100 parts by weight of the total amount of the curable component.
- the active energy ray-curable forming material preferably has a low viscosity in consideration of workability and uniformity during coating, and thus an acrylic oligomer obtained by polymerizing a (meth) acrylic monomer also has a low viscosity. It is preferable.
- the acrylic oligomer having a low viscosity and capable of preventing curing shrinkage of the reinforcing film preferably has a weight average molecular weight (Mw) of 15000 or less, more preferably 10,000 or less, and particularly preferably 5000 or less. .
- the weight average molecular weight (Mw) of the acrylic oligomer is preferably 500 or more, more preferably 1000 or more, and preferably 1500 or more. Particularly preferred.
- the (meth) acrylic monomer constituting the acrylic oligomer include, for example, methyl (meth) acrylate, ethyl (meth) acrylate, n-propyl (meth) acrylate, isopropyl (meth) acrylate, 2-methyl- 2-nitropropyl (meth) acrylate, n-butyl (meth) acrylate, isobutyl (meth) acrylate, S-butyl (meth) acrylate, t-butyl (meth) acrylate, n-pentyl (meth) acrylate, t-pentyl (Meth) acrylate, 3-pentyl (meth) acrylate, 2,2-dimethylbutyl (meth) acrylate, n-hexyl (meth) acrylate, cetyl (meth) acrylate, n-octyl (meth) acrylate, 2-ethylhexyl (
- acrylic oligomer examples include “ARUFON” manufactured by Toagosei Co., Ltd., “Act Flow” manufactured by Soken Chemical Co., Ltd., “JONCRYL” manufactured by BASF Japan.
- the active energy ray-curable forming material may contain a photoacid generator.
- the active energy ray-curable forming material contains a photoacid generator, the water resistance and durability of the reinforcing film can be dramatically improved as compared with a case where no photoacid generator is contained.
- the photoacid generator can be represented by the following general formula (3).
- onium salts constituting the photoacid generator include PF 6 ⁇ , SbF 6 ⁇ , AsF 6 ⁇ , SbCl 6 ⁇ , BiCl 5 ⁇ , SnCl 6 ⁇ , ClO 4 ⁇ , dithiocarbamate anion, SCN ⁇ . It is an onium salt comprising an anion selected from more.
- the content of the photoacid generator is 10 parts by weight or less, preferably 0.01 to 10 parts by weight, and preferably 0.05 to 5 parts by weight with respect to 100 parts by weight of the total amount of the curable component. More preferred is 0.1 to 3 parts by weight.
- the formation of the reinforcing film by the curable forming material is performed by coating the curable forming material on the surface of the polarizer and then curing.
- the polarizer may be subjected to a surface modification treatment before coating the curable forming material.
- Specific examples of the treatment include corona treatment, plasma treatment, and saponification treatment.
- the coating method of the curable forming material is appropriately selected depending on the viscosity of the curable forming material and the target thickness.
- coating methods include reverse coaters, gravure coaters (direct, reverse and offset), bar reverse coaters, roll coaters, die coaters, bar coaters, rod coaters and the like.
- a method such as a dapping method can be appropriately used.
- the curable forming material is used as an active energy ray curable forming material or a thermosetting forming material.
- the active energy ray curable forming material can be used in an electron beam curable type, an ultraviolet curable type, or a visible light curable type.
- active energy ray curing type In the active energy ray curable forming material, after applying the active energy ray curable forming material to the polarizer, the active energy ray (electron beam, ultraviolet ray, visible light, etc.) is irradiated, and the active energy ray curable forming material is applied. Curing to form a reinforcing film.
- the irradiation direction of active energy rays can be irradiated from any appropriate direction. Preferably, irradiation is performed from the reinforcing film side.
- the acceleration voltage is preferably 5 kV to 300 kV, and more preferably 10 kV to 250 kV. If the acceleration voltage is less than 5 kV, the electron beam may not reach the deepest part of the reinforcing film and may be insufficiently cured. If the acceleration voltage exceeds 300 kV, the penetrating force through the sample is too strong and damages the polarizer. There is a fear.
- the irradiation dose is 5 to 100 kGy, more preferably 10 to 75 kGy.
- the adhesive is insufficiently cured, and when it exceeds 100 kGy, the polarizer is damaged, resulting in a decrease in mechanical strength and yellowing, and the predetermined optical characteristics cannot be obtained.
- the electron beam irradiation is usually performed in an inert gas, but if necessary, it may be performed in the atmosphere or under a condition where a little oxygen is introduced.
- an active energy ray containing visible light having a wavelength range of 380 nm to 450 nm particularly an active energy ray having the largest irradiation amount of visible light having a wavelength range of 380 nm to 450 nm.
- the active energy ray a gallium-filled metal halide lamp and an LED light source that emits light in the wavelength range of 380 to 440 nm are preferable.
- low pressure mercury lamp medium pressure mercury lamp, high pressure mercury lamp, ultra high pressure mercury lamp, incandescent lamp, xenon lamp, halogen lamp, carbon arc lamp, metal halide lamp, fluorescent lamp, tungsten lamp, gallium lamp, excimer laser or sunlight
- a light source including visible light can be used, and ultraviolet light having a wavelength shorter than 380 nm can be blocked using a band pass filter.
- thermosetting type forming material after applying to a polarizer, by heating, polymerization is started by a thermal polymerization initiator to form a cured product layer (reinforcing film).
- the heating temperature is set according to the thermal polymerization initiator, but is about 60 to 200 ° C., preferably 80 to 150 ° C.
- a material for forming the reinforcing film for example, a cyanoacrylate-based forming material, an epoxy-based forming material, or an isocyanate-based forming material can be used.
- Examples of the cyanoacrylate-based forming material include alkyl- ⁇ -cyanoacrylates such as methyl- ⁇ -cyanoacrylate, ethyl- ⁇ -cyanoacrylate, butyl- ⁇ -cyanoacrylate, octyl- ⁇ -cyanoacrylate, and cyclohexyl- ⁇ -. And cyanoacrylate and methoxy- ⁇ -cyanoacrylate.
- alkyl- ⁇ -cyanoacrylates such as methyl- ⁇ -cyanoacrylate, ethyl- ⁇ -cyanoacrylate, butyl- ⁇ -cyanoacrylate, octyl- ⁇ -cyanoacrylate, and cyclohexyl- ⁇ -.
- cyanoacrylate and methoxy- ⁇ -cyanoacrylate methoxy- ⁇ -cyanoacrylate.
- those used as a cyanoacrylate-based adhesive can be used as the cyanoacrylate-based adhesive can be used.
- the epoxy-based forming material may be used alone as an epoxy resin or may contain an epoxy curing agent. When the epoxy resin is used alone, it is cured by adding a photopolymerization initiator and irradiating active energy rays. When an epoxy curing agent is added as an epoxy-based forming material, for example, those used as an epoxy-based adhesive can be used.
- the usage form of the epoxy-based forming material can be used as a one-component type containing an epoxy resin and its curing agent, but it is used as a two-component type in which a curing agent is blended with the epoxy resin.
- Epoxy-based forming materials are usually used as solutions.
- the solution may be a solvent system or an aqueous system such as an emulsion, a colloidal dispersion, or an aqueous solution.
- the epoxy resin examples include various compounds containing two or more epoxy groups in the molecule.
- the epoxy resin examples include various compounds containing two or more epoxy groups in the molecule.
- bisphenol type epoxy resin aliphatic type epoxy resin, aromatic type epoxy resin, halogenated bisphenol type epoxy resin, biphenyl And epoxy resin.
- an epoxy resin can be suitably determined according to an epoxy equivalent and the number of functional groups, the epoxy equivalent of 500 or less is used suitably from a durable viewpoint.
- the curing agent for the epoxy resin is not particularly limited, and various types such as phenol resin type, acid anhydride type, carboxylic acid type, and polyamine type can be used.
- phenol resin-based curing agent for example, phenol novolak resin, bisphenol novolak resin, xylylene phenol resin, cresol novolak resin, or the like is used.
- acid anhydride-based curing agents include: maleic anhydride, tetrahydrophthalic anhydride, hexahydrophthalic anhydride, succinic anhydride, and the like.
- carboxylic acid-based curing agents include carboxylic acids such as pyromellitic acid and trimellitic acid.
- Examples thereof include block carboxylic acids added with acids and vinyl ether.
- an epoxy-type two-component formation material what consists of two liquids of an epoxy resin and a polythiol, what consists of two liquids of an epoxy resin and polyamide, etc. can be used, for example.
- the blending amount of the curing agent varies depending on the equivalent to the epoxy resin, but is preferably 30 to 70 parts by weight, more preferably 40 to 60 parts by weight with respect to 100 parts by weight of the epoxy resin.
- curing accelerators can be used for the epoxy-based forming material.
- the curing accelerator include various imidazole compounds and derivatives thereof, dicyandiamide, and the like.
- Examples of the isocyanate-based forming material include those used as a crosslinking agent in the formation of the pressure-sensitive adhesive layer.
- As the isocyanate-based crosslinking agent a compound having at least two isocyanate groups can be used.
- the polyisocyanate compound can be used as an isocyanate-based forming material.
- Examples include those reacted with polyhydric alcohols and polyhydric amines.
- isocyanate-based crosslinking agent those having three or more isocyanate groups such as isocyanuric acid tris (6-inocyanate hexyl) are preferable.
- isocyanate type formation material what is used as an isocyanate type adhesive agent is mention
- the isocyanate-based forming materials in the present invention, it is preferable to use those having a rigid structure in which a cyclic structure (benzene ring, cyanurate ring, isocyanurate ring, etc.) accounts for a large proportion in the structure.
- a cyclic structure benzene ring, cyanurate ring, isocyanurate ring, etc.
- the isocyanate-based forming material for example, trimethylolpropane-tri-tolylene isocyanate, tris (hexamethylene isocyanate) isocyanurate and the like are preferably used.
- the said isocyanate type crosslinking agent can also use what provided the protective group to the terminal isocyanate group.
- Protecting groups include oximes and lactams. In the case where the isocyanate group is protected, the protecting group is dissociated from the isocyanate group by heating, and the isocyanate group reacts.
- a reaction catalyst can be used to increase the reactivity of the isocyanate group.
- the reaction catalyst is not particularly limited, but a tin-based catalyst or an amine-based catalyst is suitable.
- the reaction catalyst can use 1 type (s) or 2 or more types.
- the amount of the reaction catalyst used is usually 5 parts by weight or less with respect to 100 parts by weight of the isocyanate-based crosslinking agent. When the amount of the reaction catalyst is large, the crosslinking reaction rate increases and foaming of the forming material occurs. Even if the forming material after foaming is used, sufficient adhesion cannot be obtained.
- a reaction catalyst it is preferably 0.01 to 5 parts by weight, more preferably 0.05 to 4 parts by weight.
- the tin-based catalyst both inorganic and organic catalysts can be used, but an organic catalyst is preferred.
- the inorganic tin-based catalyst include stannous chloride and stannic chloride.
- the organic tin-based catalyst is preferably one having at least one organic group such as an aliphatic group or alicyclic group having a skeleton such as a methyl group, an ethyl group, an ether group or an ester group. Examples include tetra-n-butyltin, tri-n-butyltin acetate, n-butyltin trichloride, trimethyltin hydroxide, dimethyltin dichloride, dibutyltin dilaurate, and the like.
- the amine catalyst is not particularly limited. For example, those having at least one organic group such as an alicyclic group such as quinoclidine, amidine, and diazabicycloundecene are preferable.
- examples of the amine catalyst include triethylamine.
- reaction catalysts other than the above include cobalt naphthenate and benzyltrimethylammonium hydroxide.
- the isocyanate-based forming material is usually used as a solution.
- the solution may be a solvent system or an aqueous system such as an emulsion, a colloidal dispersion, or an aqueous solution.
- the organic solvent is not particularly limited as long as the components constituting the forming material are uniformly dissolved. Examples of the organic solvent include toluene, methyl ethyl ketone, ethyl acetate and the like.
- alcohols such as n-butyl alcohol and isopropyl alcohol and ketones such as acetone can be blended.
- a dispersant is used, or an isocyanate-based crosslinking agent, a functional group having low reactivity with an isocyanate group such as a carboxylate, a sulfonate, or a quaternary ammonium salt, or an aqueous dispersion such as polyethylene glycol. It can carry out by introduce
- the formation (curing) of the reinforcing film by the cyanoacrylate-based forming material, the epoxy-based forming material, or the isocyanate-based forming material can be appropriately selected depending on the type of the forming material, but is usually 30 to 100 ° C. It is carried out by drying at a temperature of preferably about 50 to 80 ° C. for about 0.5 to 15 minutes. In the case of a cyanoacrylate-based forming material, since the curing is fast, the reinforcing film can be formed in a time shorter than the above time.
- polyurethane can be used as a material for forming the reinforcing film.
- polyurethane it is preferable to use a reaction product of a high-molecular polyol compound and / or a low-molecular polyol and an isocyanate compound and an isocyanate compound.
- the polyurethane can be further reacted with a low molecular polyamino compound and / or a polyol compound as a chain extender.
- the polymer polyol compound preferably has a weight average molecular weight of 100 to 4000 and has two or more hydroxyl groups in one molecule, and polyether polyol, polyester polyol, polycarbonate polyol and the like are used.
- the polymer polyol compound preferably has a weight average molecular weight of 500 to 4000, more preferably 600 to 3500, and even more preferably 1000 to 3000.
- polyether polyols examples include aliphatic polyether polyols and aromatic polyether polyols. More specifically, for example, low molecular polyols such as dihydric alcohols such as ethylene glycol, diethylene glycol, propylene glycol, butylene glycol and hexamethylene glycol, and trihydric alcohols such as trimethylolpropane, glycerin and pentaerythritol, ethylene oxide , Polyether obtained by addition polymerization of propylene oxide, tetrahydrofuran, or the like is used. These may be used singly or in combination of two or more.
- low molecular polyols such as dihydric alcohols such as ethylene glycol, diethylene glycol, propylene glycol, butylene glycol and hexamethylene glycol
- trihydric alcohols such as trimethylolpropane, glycerin and pentaerythritol, ethylene oxide
- polyester polyols examples include aliphatic polyester polyols and aromatic polyester polyols. More specifically, alcohols such as the above dihydric alcohols, dipropylene glycol, 1,4-butanediol, 1,6-hexanediol and neopentyl glycol, and two bases such as adipic acid, azelaic acid and sebacic acid Polyester composed of a polycondensate with an acid is used. These may be used singly or in combination of two or more.
- polydiene-based polyols such as polybutadiene, butadiene-acrylonitrile copolymer, polyisoprene having hydroxyl groups at both ends of the molecule, polybutadiene hydrogenated products, polyisoprene hydrogenated products having hydroxyl groups at both ends of the molecules, polyisoprene
- polyolefin polyols such as isobutylene.
- examples of the polyamino compound used as the chain extender include aliphatic polyamino compounds and aromatic polyamino compounds. More specifically, for example, ethylenediamine, 3,3′-dichloro-4,4′-diaminodiphenylmethane (MOCA), diethyltoluenediamine (DETDA), 44′-bis- (sec-butyl) diphenylmethane, 2,4 -Tolylenediamine, 2,6-tolylenediamine, xylylenediamine, hexanediamine, isophoronediamine and the like. Of these, ethylenediamine and the like are preferable. These may be used singly or in combination of two or more.
- MOCA 3,3′-dichloro-4,4′-diaminodiphenylmethane
- DETDA diethyltoluenediamine
- 44′-bis- (sec-butyl) diphenylmethane 2,4 -To
- examples of the low molecular polyol and the polyol compound used as the chain extender include low molecular polyols exemplified in polyether polyol and polyester polyol.
- the chain extender is preferably used in an amount of 0.1 to 10 parts by weight, more preferably 0.5 to 7 parts by weight, more preferably 1 to 5 parts by weight, based on 100 parts by weight of the polymer polyol compound. More preferably, The use of a chain extender can sufficiently increase the molecular weight and improve the durability.
- the isocyanate compound is a polyisocyanate (isocyanate compound) having two or more isocyanate groups, and the same as the isocyanate-based forming material can be used.
- the isocyanate compound can also be used as a urethane prepolymer obtained by reacting the low-molecular polyol exemplified in the polyester polyol and the exemplified isocyanate compound in advance.
- the formation (curing) of the reinforcing film with polyurethane may be usually performed by applying a liquid material (coating solution) of polyurethane prepared in advance to the polarizer, or contains the polymer polyol compound and the isocyanate compound. After applying the composition to the polarizer, a reinforcing film may be formed of polyurethane as a cured reaction product.
- the reinforcing membrane can be formed usually by drying at about 30 to 100 ° C., preferably 50 to 80 ° C., for about 0.5 to 15 minutes.
- an annealing treatment may be performed.
- Annealing treatment promotes the reaction especially when isocyanate is not sufficiently reacted even after the initial curing in the isocyanate-based forming agent, polyurethane-based forming material, etc. (reactive group remains in the reinforcing film).
- the annealing treatment can be performed in any atmosphere of a dry condition or a humidified condition.
- the annealing temperature is about 30 to 100 ° C., preferably 50 to 80 ° C., similarly to the conditions for the initial curing. There is no particular limitation on the annealing time.
- the weight average molecular weight of polyurethane is preferably 30,000 to 200,000, more preferably 40 to 150,000, and still more preferably 50,000 to 130,000.
- the weight average molecular weight of the polymer polyol compound and polyurethane was measured under the following conditions of GPC (gel permeation chromatography).
- Analyzing apparatus HLC-8120GPC manufactured by Tosoh Corporation. Column: manufactured by Tosoh Corporation, G7000HXL + GMHXL + GMHXL. Column size: 7.8 mm ⁇ ⁇ 30 cm each 90 cm in total. Column temperature: 40 ° C. Flow rate: 0.8 ml / min. Injection volume: 100 ⁇ l.
- Eluent tetrahydrofuran.
- Detector Suggested refractometer. Standard sample: polystyrene.
- the reinforcing film has a functional group capable of forming a covalent bond with PVA such as an isocyanate group or an epoxy group, and has a carbon-carbon double bond such as a (meth) acryloyl group or a vinyl group.
- a compound having a radical polymerizable functional group can be used. Examples of the compound include 2-isocyanatoethyl acrylate (manufactured by Showa Denko, product name Karenz AOI), 1,1- (bisacryloyloxymethyl) ethyl isocyanate (manufactured by Showa Denko, Karenz BEI), and the like. It is done.
- a reaction product of a polymer containing a diisocyanate compound as a constituent and a hydroxyl group-containing hydroxyl group-containing (meth) acrylate, or the like can be used as the compound.
- a reaction product of 2-hydroxyethyl acrylate and a polymer containing 1,6-diisocyanatohexane BASF, product name Ralomer LR9000.
- the compound has a functional group such as an isocyanate group or an epoxy group, a reinforcing film can be formed by thermosetting in the same manner as the isocyanate-based forming agent, and further, an annealing treatment can be performed.
- the said compound since the said compound has a radically polymerizable functional group, it can be used as an active energy ray hardening type or thermosetting type forming material concerning a radical polymerization hardening type forming material.
- the compound can be used in combination with other radical polymerizable compounds.
- the reinforcing film may be formed from a forming material that does not contain a curable component, for example, a forming material that contains the polyvinyl alcohol-based resin as a main component.
- the polyvinyl alcohol resin forming the reinforcing film may be the same as or different from the polyvinyl alcohol resin contained in the polarizer as long as it is a “polyvinyl alcohol resin”.
- polyvinyl alcohol resin examples include polyvinyl alcohol.
- Polyvinyl alcohol is obtained by saponifying polyvinyl acetate.
- polyvinyl alcohol-based resin examples include a saponified product of a copolymer of vinyl acetate and a monomer having copolymerizability.
- the copolymerizable monomer is ethylene
- an ethylene-vinyl alcohol copolymer is obtained.
- the copolymerizable monomer include unsaturated carboxylic acids such as (anhydrous) maleic acid, fumaric acid, crotonic acid, itaconic acid, (meth) acrylic acid, and esters thereof; ethylene, propylene, etc.
- ⁇ -olefin (meth) allylsulfonic acid (soda), sulfonic acid soda (monoalkylmalate), disulfonic acid soda alkylmalate, N-methylolacrylamide, acrylamide alkylsulfonic acid alkali salt, N-vinylpyrrolidone, N- Examples include vinyl pyrrolidone derivatives.
- These polyvinyl alcohol resins can be used alone or in combination of two or more.
- Polyvinyl alcohol obtained by saponifying polyvinyl acetate is preferable from the viewpoint of satisfying moisture heat resistance and water resistance by controlling the heat of crystal fusion of the reinforcing film to 30 mj / mg or more.
- the saponification degree of the polyvinyl alcohol-based resin can be, for example, 95% or more, but the heat of crystal fusion of the reinforcing film is controlled to 30 mj / mg or more to satisfy the heat and moisture resistance and water resistance. From the viewpoint, the degree of saponification is preferably 99.0% or more, and more preferably 99.7% or more.
- the degree of saponification represents the proportion of units that are actually saponified to vinyl alcohol units among the units that can be converted to vinyl alcohol units by saponification, and the residue is a vinyl ester unit.
- the degree of saponification can be determined according to JIS K 6726-1994.
- the average degree of polymerization of the polyvinyl alcohol-based resin can be, for example, 500 or more, but the viewpoint of satisfying moisture heat resistance and water resistance by controlling the heat of crystal fusion of the reinforcing film to 30 mj / mg or more. Therefore, the average degree of polymerization is preferably 1000 or more, more preferably 1500 or more, and further preferably 2000 or more.
- the average degree of polymerization of the polyvinyl alcohol resin is measured according to JIS-K6726.
- a modified polyvinyl alcohol resin having a hydrophilic functional group in the side chain of the polyvinyl alcohol or a copolymer thereof can be used.
- the hydrophilic functional group include an acetoacetyl group and a carbonyl group.
- modified polyvinyl alcohol obtained by acetalization, urethanization, etherification, grafting, phosphoric esterification or the like of a polyvinyl alcohol resin can be used.
- the forming material containing the polyvinyl alcohol-based resin as a main component can contain a curable component (crosslinking agent) and the like.
- the proportion of the polyvinyl alcohol resin in the reinforcing film or the forming material (solid content) is preferably 80% by weight or more, more preferably 90% by weight or more, and further preferably 95% by weight or more.
- the forming material does not contain a curable component (crosslinking agent) from the viewpoint of easily controlling the heat of crystal fusion of the reinforcing film to 30 mj / mg or more.
- a compound having at least two functional groups having reactivity with the polyvinyl alcohol resin can be used.
- alkylenediamine having two alkylene groups and two amino groups such as ethylenediamine, triethylenediamine, hexamethylenediamine; tolylene diisocyanate, hydrogenated tolylene diisocyanate, trimethylolpropane tolylene diisocyanate adduct, triphenylmethane triisocyanate, methylene bis (4-Phenylmethane triisocyanate, isophorone diisocyanate and isocyanates such as ketoxime block product or phenol block product thereof; ethylene glycol diglycidyl ether, polyethylene glycol diglycidyl ether, glycerin di or triglycidyl ether, 1,6-hexane Diol diglycidyl ether, trimethylolpropane triglycidyl ether, di Epoxies
- amino-formaldehyde resins and water-soluble dihydrazine are preferred, and the amino-formaldehyde resin is preferably a compound having a methylol group, particularly methylol melamine, which is a compound having a methylol group.
- hardenable component crosslinking agent
- the ratio is 20 weight part or less, 10 weight part or less, 5 weight part with respect to 100 weight part of polyvinyl alcohol-type resin. It is preferable that:
- the forming material is prepared as a solution in which the polyvinyl alcohol resin is dissolved in a solvent.
- the solvent include water, dimethyl sulfoxide, dimethylformamide, dimethylacetamide N-methylpyrrolidone, various glycols, polyhydric alcohols such as alcohols, and amines such as ethylenediamine and diethylenetriamine. These may be used alone or in combination of two or more. Among these, it is preferable to use it as an aqueous solution using water as a solvent.
- the concentration of the polyvinyl alcohol-based resin in the forming material is not particularly limited, but is 0.1 to 15% by weight, preferably 0.5%, in consideration of coating properties and storage stability. ⁇ 10% by weight.
- a plasticizer examples include polyhydric alcohols such as ethylene glycol and glycerin.
- the surfactant examples include nonionic surfactants.
- the reinforcing film can be formed by applying the forming material to a polarizer and drying it.
- the application operation is not particularly limited, and any appropriate method can be adopted.
- various means such as a roll coating method, a spin coating method, a wire bar coating method, a dip coating method, a die coating method, a curtain coating method, a spray coating method, a knife coating method (comma coating method, etc.) can be employed.
- a surface treatment layer can be provided on one side or both sides of the flexible polarizing film of the present invention.
- the surface treatment layer include a hard coat layer, an antiglare treatment layer, an antireflection layer, and an antisticking layer.
- the surface treatment layer is preferably a hard coat layer.
- a material for forming the hard coat layer for example, a thermoplastic resin or a material that is cured by heat or radiation can be used.
- the material include radiation curable resins such as thermosetting resins, ultraviolet curable resins, and electron beam curable resins.
- an ultraviolet curable resin that can efficiently form a cured resin layer by a simple processing operation by a curing treatment by ultraviolet irradiation is preferable.
- these curable resins include polyesters, acrylics, urethanes, amides, silicones, epoxies, melamines, and the like, and these monomers, oligomers, polymers, and the like are included.
- an antiglare treatment layer or an antireflection layer for the purpose of improving visibility can be provided.
- An antiglare treatment layer or an antireflection layer can be provided on the hard coat layer.
- the constituent material of the antiglare layer is not particularly limited, and for example, a radiation curable resin, a thermosetting resin, a thermoplastic resin, or the like can be used.
- As the antireflection layer titanium oxide, zirconium oxide, silicon oxide, magnesium fluoride, or the like is used.
- the antireflection layer can be provided with a plurality of layers.
- examples of the surface treatment layer include a sticking prevention layer.
- the thickness of the surface treatment layer can be appropriately set depending on the type of the surface treatment layer, but is generally preferably 0.1 to 100 ⁇ m.
- the thickness of the hard coat layer is preferably 0.5 to 20 ⁇ m.
- An adhesive layer can be provided on one side or both sides of the flexible polarizing film of the present invention.
- An appropriate pressure-sensitive adhesive can be used for forming the pressure-sensitive adhesive layer, and the type thereof is not particularly limited.
- Adhesives include rubber adhesives, acrylic adhesives, silicone adhesives, urethane adhesives, vinyl alkyl ether adhesives, polyvinyl alcohol adhesives, polyvinyl pyrrolidone adhesives, polyacrylamide adhesives, Examples thereof include cellulose-based pressure-sensitive adhesives.
- pressure-sensitive adhesives those having excellent optical transparency, suitable wettability, cohesiveness, and adhesive pressure characteristics, and excellent weather resistance and heat resistance are preferably used.
- An acrylic pressure-sensitive adhesive is preferably used as one exhibiting such characteristics.
- the pressure-sensitive adhesive layer for example, a method in which the pressure-sensitive adhesive is applied to a peeled separator, the polymerization solvent is dried and removed to form a pressure-sensitive adhesive layer, and then transferred to a flexible polarizing film, or flexible
- the pressure-sensitive adhesive is produced by a method of applying the pressure-sensitive adhesive to the polarizing film, drying and removing the polymerization solvent, and forming a pressure-sensitive adhesive layer on the polarizer.
- one or more solvents other than the polymerization solvent may be added as appropriate.
- the thickness of the pressure-sensitive adhesive layer is not particularly limited, and is, for example, about 1 to 100 ⁇ m.
- the thickness is preferably 2 to 50 ⁇ m, more preferably 2 to 40 ⁇ m, and still more preferably 5 to 35 ⁇ m.
- the pressure-sensitive adhesive layer When the pressure-sensitive adhesive layer is exposed, the pressure-sensitive adhesive layer may be protected with a peeled sheet (separator) until practical use.
- the flexible polarizing film of the present invention can be provided with a surface protective film.
- the surface protective film usually has a base film and an adhesive layer, and protects the flexible polarizing film via the adhesive layer.
- a film material having isotropic property or close to isotropic property is selected from the viewpoints of inspection property and manageability.
- film materials include polyester resins such as polyethylene terephthalate film, cellulose resins, acetate resins, polyether sulfone resins, polycarbonate resins, polyamide resins, polyimide resins, polyolefin resins, acrylic resins, and the like. Examples thereof include transparent polymers such as resins. Of these, polyester resins are preferred.
- the base film can be used as a laminate of one kind or two or more kinds of film materials, and a stretched product of the film can also be used.
- the thickness of the base film is generally 500 ⁇ m or less, preferably 10 to 200 ⁇ m.
- the pressure-sensitive adhesive that forms the pressure-sensitive adhesive layer of the surface protective film includes a (meth) acrylic polymer, a silicone-based polymer, a polyester, a polyurethane, a polyamide, a polyether, a fluorine-based or a rubber-based pressure-sensitive adhesive. Can be appropriately selected and used. From the viewpoints of transparency, weather resistance, heat resistance and the like, an acrylic pressure-sensitive adhesive having an acrylic polymer as a base polymer is preferable.
- the thickness (dry film thickness) of the pressure-sensitive adhesive layer is determined according to the required adhesive force. Usually, it is about 1 to 100 ⁇ m, preferably 5 to 50 ⁇ m.
- the surface protective film can be provided with a release treatment layer on the surface opposite to the surface on which the pressure-sensitive adhesive layer is provided on the base film, using a low adhesion material such as silicone treatment, long-chain alkyl treatment, or fluorine treatment. .
- the flexible polarizing film of the present invention is intended to expand various applications that cannot be applied to conventional polarizers and polarizing films as an alternative to polarizers or polarizing films (those provided with a transparent protective film on the polarizer). For example, it can be used as an optical film laminated with another optical layer.
- the optical layer is not particularly limited.
- a protective film used for protecting the polarizer.
- One optical layer or two or more optical layers that may be used for forming an image display device such as a liquid crystal display device or an organic EL display device can be used.
- the optical layer and the flexible polarizing film can be laminated via an intervening layer such as an adhesive layer, a pressure-sensitive adhesive layer, or an undercoat layer (primer layer). At this time, it is desirable that the both are laminated without an air gap by an intervening layer.
- an intervening layer such as an adhesive layer, a pressure-sensitive adhesive layer, or an undercoat layer (primer layer).
- the flexible polarizing film of the present invention or an optical film using the same can be preferably used for forming various image display devices such as liquid crystal display devices and organic EL display devices.
- the liquid crystal display device can be formed according to the conventional method. That is, a liquid crystal display device is generally formed by appropriately assembling components such as a liquid crystal cell, a flexible polarizing film of the present invention or an optical film using the same, and an illumination system as required, and incorporating a drive circuit.
- the flexible polarizing film of the present invention is used, and the conventional method can be applied.
- As the liquid crystal cell an arbitrary type such as an IPS type or a VA type can be used, but is particularly suitable for the IPS type.
- ⁇ Preparation of polarizer A0> One side of an amorphous isophthalic acid copolymerized polyethylene terephthalate (IPA copolymerized PET) film (thickness: 100 ⁇ m) having a water absorption of 0.75% and Tg of 75 ° C. is subjected to corona treatment.
- Alcohol polymerization degree 4200, saponification degree 99.2 mol%) and acetoacetyl-modified PVA (polymerization degree 1200, acetoacetyl modification degree 4.6%, saponification degree 99.0 mol% or more, manufactured by Nippon Synthetic Chemical Industry Co., Ltd.
- aqueous solution containing 9: 1 ratio of the trade name “Gosefimer Z200”) was applied and dried at 25 ° C. to form a PVA-based resin layer having a thickness of 11 ⁇ m, thereby preparing a laminate.
- the obtained laminate was uniaxially stretched in the longitudinal direction (longitudinal direction) 2.0 times between rolls having different peripheral speeds in an oven at 120 ° C. (air-assisted stretching process).
- the laminate was immersed in an insolubilization bath (a boric acid aqueous solution obtained by blending 4 parts by weight of boric acid with respect to 100 parts by weight of water) for 30 seconds (insolubilization treatment).
- boric acid aqueous solution obtained by blending 3 parts by weight of potassium iodide and 3 parts by weight of boric acid with respect to 100 parts by weight of water.
- Crosslinking treatment Thereafter, the laminate was immersed in a boric acid aqueous solution (an aqueous solution obtained by blending 4 parts by weight of boric acid and 5 parts by weight of potassium iodide with respect to 100 parts by weight of water) at a liquid temperature of 70 ° C.
- uniaxial stretching was performed between rolls having different peripheral speeds in the longitudinal direction (longitudinal direction) so that the total stretching ratio was 5.5 times (in-water stretching treatment).
- the laminate was immersed in a cleaning bath (an aqueous solution obtained by blending 4 parts by weight of potassium iodide with respect to 100 parts by weight of water) at a liquid temperature of 30 ° C. (cleaning treatment).
- a cleaning bath an aqueous solution obtained by blending 4 parts by weight of potassium iodide with respect to 100 parts by weight of water
- cleaning treatment a liquid temperature of 30 ° C.
- Polarizers A1 and A2 were produced in the same manner as in the production of the polarizer A0 except that the production conditions for the polarizer A0 were changed as shown in Table 1.
- Table 1 shows the thickness, optical characteristics (single transmittance, polarization degree), and boric acid concentration of the polarizers A0 to A2.
- ⁇ Preparation of Polarizer B1 (12 ⁇ m Thick Polarizer)>
- a polyvinyl alcohol film having an average polymerization degree of 2400 and a saponification degree of 99.9 mol% and a thickness of 30 ⁇ m was immersed in warm water at 30 ° C. for 60 seconds to swell.
- the thickness of the obtained polarizer B1 was 12 ⁇ m.
- the optical properties of the obtained polarizer B1 were a transmittance of 42.8% and a polarization degree of 99.99%.
- Polyurethane material 50 parts of urethane prepolymer consisting of tolylene diisocyanate and trimethylolpropane (manufactured by Tosoh Corporation, Coronate L), 50 parts of polycarbonate polyol (manufactured by Sumika Bayer Urethane Co., Ltd., Desmophen C3100XP), dioctyltin dilaurate system 0.1 parts of a catalyst (manufactured by Tokyo Fine Chemical Co., Ltd., ENBOLiser OL-1) was added, and methyl isobutyl ketone was used as a solvent to obtain a coating solution adjusted to a solid content concentration of 35%.
- urethane prepolymer consisting of tolylene diisocyanate and trimethylolpropane
- polycarbonate polyol manufactured by Sumika Bayer Urethane Co., Ltd., Desmophen C3100XP
- dioctyltin dilaurate system
- UV curable material Reaction product of 2-hydroxyethyl acrylate and a polymer containing 1,6-diisocyanatohexane as a component in 50 parts of urethane acrylate oligomer (manufactured by Nippon Synthetic Chemical Co., Ltd., purple light UV1700TL) BASF, product name Laromar LR9000 (50 parts) and photoinitiator (BASF, IRGACURE907) (3 parts) were added, and methyl isobutyl ketone was used as a solvent to obtain a coating solution adjusted to a solid content concentration of 35%. .
- HEAA N-hydroxyethylacrylamide
- ACMO acryloylmorpholine
- UVGACURE 819 photoinitiator
- Example 1 Preparation of flexible polarizing film
- the polyurethane-based material (1) of the reinforcing film is cured on the polarizer (first surface) of the polarizer A0 (optical film laminate including a polarizer having a thickness of 5 ⁇ m) obtained above.
- initial curing is performed under conditions of 60 ° C. and 10 minutes, and then a first reinforcing film is formed by performing annealing treatment under conditions of 60 ° C. and 12 hours. did.
- the amorphous PET substrate of the optical film laminate was peeled off. Thereafter, the polyurethane-based material (1) of the reinforcing film was applied to a polarizer (second surface as a peeling surface) using a wire bar coater so that the thickness after curing was 5 ⁇ m, and then 60 ° C.
- the second reinforcing film was formed by initial curing under conditions of 10 minutes, and then annealing treatment at 60 ° C. for 12 hours. Then, the flexible polarizing film which has a reinforcement film on both surfaces of a polarizer was obtained by removing the surface protection film bonded on the 1st reinforcement film.
- Example 1 a flexible polarizing film having a reinforcing film on both sides of the polarizer was prepared in the same manner as in Example 1 except that the type of polarizer and the thickness of the reinforcing film were changed as shown in Table 2. .
- Example 5 Preparation of flexible polarizing film
- the UV curable material (2) of the reinforcing film is applied to the polarizer (first surface) of the polarizer A0 (optical film laminate including a polarizer having a thickness of 5 ⁇ m) obtained above.
- the coating was heated at 60 ° C. for 1 minute.
- a first reinforcing film was formed by irradiating the heated coating film with ultraviolet light having an integrated light quantity of 300 mJ / cm 2 with a high-pressure mercury lamp.
- the amorphous PET substrate of the optical film laminate was peeled off.
- the UV curable material (2) of the reinforcing film was applied to a polarizer (second surface as a peeling surface) using a wire bar coater so that the thickness after curing was 5 ⁇ m, and then 60 Heated at 0 ° C. for 1 minute.
- a second reinforcing film was formed by irradiating the heated coating film with ultraviolet light having an integrated light quantity of 300 mJ / cm 2 using a high-pressure mercury lamp.
- the flexible polarizing film which has a reinforcement film on both surfaces of a polarizer was obtained by removing the surface protection film bonded on the 1st reinforcement film.
- Example 6 Preparation of flexible polarizing film
- the aqueous resin emulsion material (3) of the reinforcing film is used with a wire bar coater. After coating so that the thickness after curing was 5 ⁇ m, the coating was cured under the conditions of 80 ° C. for 5 minutes to form a first reinforcing film.
- a surface protective film manufactured by Nitto Denko, RP301
- the amorphous PET substrate of the optical film laminate was peeled off.
- Example 7 In Example 1, a flexible polarizing film having a second reinforcing film only on one side of the polarizer was produced in the same manner as in Example 1 except that the first reinforcing film was not provided.
- Example 8 In Example 1, a flexible polarizing film having the first reinforcing film only on one surface of the polarizer was produced in the same manner as in Example 1 except that the second reinforcing film was not provided.
- Comparative Examples 1 to 3 A polarizing film having a reinforcing film having reinforcing films on both sides of the polarizer in the same manner as in Example 1 except that the type of polarizer and the thickness of the reinforcing film were changed as shown in Table 2 in Example 1. Was made.
- Comparative Example 4 (Production of a piece protective polarizing film) On the polarizer (first surface) of the polarizer A0 (optical film laminate including a polarizer having a thickness of 5 ⁇ m) obtained above, the thickness of the adhesive layer after curing the ultraviolet curable adhesive becomes 1 ⁇ m. Then, the protective film (acrylic) was applied, and ultraviolet rays were applied as active energy rays to cure the adhesive. Ultraviolet irradiation is performed using a gallium-encapsulated metal halide lamp, an irradiation device: Fusion UV Systems, Inc.
- Light HAMMER 10, Inc. bulb: V bulb, peak illuminance: 1600 mW / cm 2 , integrated irradiation amount 1000 / mJ / cm 2 (wavelength 380 to 440 nm) ), And the illuminance of ultraviolet rays was measured using a Sola-Check system manufactured by Solatell. Subsequently, the amorphous PET base material of the optical film laminate was peeled off to obtain a piece protective polarizing film.
- ⁇ Single transmittance T and polarization degree P of polarizer> The single transmittance T and the polarization degree P of the obtained polarizing film were measured using a spectral transmittance measuring device with an integrating sphere (Dot-3c of Murakami Color Research Laboratory).
- the degree of polarization P is the transmittance when two identical polarizing films are overlapped so that their transmission axes are parallel (parallel transmittance: Tp), and overlapped so that their transmission axes are orthogonal to each other. It is calculated
- Polarization degree P (%) ⁇ (Tp ⁇ Tc) / (Tp + Tc) ⁇ 1/2 ⁇ 100
- Each transmittance is indicated by a Y value obtained by correcting visibility with a two-degree field of view (C light source) of JIS Z8701 with 100% of the completely polarized light obtained through the Granteller prism.
- TI900 TriboIndenter manufactured by Hystron was used for the measurement of compression modulus.
- the obtained polarizer with a reinforcing film (polarizing film) was cut into a size of 10 mm ⁇ 10 mm, fixed to a support equipped with a TriboIndenter, and the compression modulus was measured by a nanoindentation method. At that time, the position was adjusted so that the used indenter pushed in the vicinity of the center of the transparent layer.
- the measurement conditions are shown below.
- Working indenter Berkovich (triangular pyramid type) Measuring method: Single indentation measurement Measuring temperature: 23 ° C Indentation depth setting: 100 nm
- ⁇ Torsion test> It was performed using a planar body no-load twist tester (product name: main body TCDM111LH and jig: planar body no-load twist test jig) manufactured by Yuasa System Equipment Co., Ltd. After sandwiching and fixing both short sides of the rectangular object 1 (sample) of 100 mm (absorption axis direction) ⁇ 150 mm (transmission axis direction) with the twisting clips 11 and 12 of the tester, one short side is the clip 12. The clip 11 on the other short side was twisted under the following conditions while being fixed at. The state of twisting is shown in FIG.
- Twist speed 10rpm Twist angle: 45 degrees Twist number: 100 times
- ⁇ No cracking or light leakage occurred. And there were no creases left.
- ⁇ No cracking or light leakage occurred. However, fold marks remained.
- X Cracks and light leakage occurred. And there were creases left.
- the bending by the expansion and contraction was performed in the same manner as described above so that the other one side (second surface) of the rectangular object was inwardly U-shaped (bending on the second surface side in the transmission axis direction). ). Further, the bending by the expansion and contraction is performed so that the first surface and the second surface are inwardly U-shaped with respect to a rectangular object (sample) of 50 mm (transmission axis direction) ⁇ 100 mm (absorption axis direction). The same was done (bending on the first surface side and the second surface side in the absorption axis direction).
- Criteria for cracks, creases and light leakage in the twist test and U-shaped stretch test are as follows. “Crack” means that cracks or cracks have occurred through the sample (a flexible polarizing film, a polarizing film having a reinforcing film, or all or part of a layer constituting a single protective polarizing film). Show. “Fold marks” are applied to the sample (flexible polarizing film, polarizing film having a reinforcing film, or all or part of a layer constituting a single protective polarizing film) even after the load is removed by bending or local load. It shows that the remaining visible trace has occurred. “Light loss” indicates that the polarizer itself is torn or cracked.
- Confirmation of light leakage is performed by using a sample after testing (flexible polarizing film, polarizing film having a reinforcing film, or all layers constituting a piece of protective polarizing film) and other normal polarizing films on both sides of the glass in a crossed Nicol state. It was carried out by pasting light such as a backlight.
- a rectangular object 1 (sample) of 200 mm (absorption axis direction) ⁇ 300 mm (transmission axis direction) was placed on a horizontal base 31.
- the rectangular object 1 was folded in three in the transmission axis direction (major axis direction), and then a load 32 of 100 g was applied so as to apply a load to the entire uppermost surface, and left for 5 minutes. The state of bending is shown in FIG. Thereafter, the load 32 was removed.
- the same operation (however, the sample was folded in three in the absorption axis direction (major axis direction)) was performed on a rectangular object (sample) of 200 mm (transmission axis direction) ⁇ 300 mm (absorption axis direction). . After the bending holding test, it is visually confirmed whether the sample is held in a tri-folded state, and when the tri-folded shape is maintained, the sample is returned to the original state (planar) and visually checked as follows. Evaluated by criteria. The evaluation was performed three times for each sample of the long side in the transmission axis direction and the long side in the absorption axis direction.
- the sample was gently slid and moved to the slope side at an extrusion speed of 10 mm / sec (1).
- the sample stopped moving at the point where the tip of the sample first contacted the slope (2).
- the distance L (mm) by which the sample moved while the top was flat was measured.
- a rectangular object (flexible polarizing film sample) of 50 mm (transmission axis direction) ⁇ 150 mm (absorption axis direction) the same operation as described above (however, the sample was set to have a slope in the absorption axis direction) was performed. .
- the bending resistance (mm) is the shortest linear distance for each of the two patterns with the first surface on the upper side and the second surface on the upper side for the samples with the transmission axis direction long side and the absorption axis direction long side, respectively.
- L (mm) was measured (12 samples in total) and used as the arithmetic average value thereof. Further, in any one or more measurements of 12 samples, when there was a sample that could not be measured due to deformation or curl of the sample, it was determined that the sample was not measurable.
- ⁇ Tensile test> A sample (flexible polarizing film sample) was prepared by cutting into a predetermined shape of 10 mm (absorption axis direction) ⁇ 150 mm (transmission axis direction) with a multipurpose test piece cutter (dumbbell). About the said sample, the tension test was implemented by autograph AG-IS by Shimadzu Corporation at the tension
Abstract
Description
P>-(100.929T-42.4-1)×100(ただし、T<42.3)、又は、
P≧99.9(ただし、T≧42.3)の条件を満足するように構成されたものであることが好ましい。 In the flexible polarizing film, the polyvinyl alcohol polarizer has an optical property represented by the following formula: P> − (10 0.929T-42.4 −1) × 100 However, T <42.3) or
It is preferable that the lens is configured to satisfy the condition of P ≧ 99.9 (however, T ≧ 42.3).
ポリビニルアルコール系樹脂が一方向に配向し、かつ前記ポリビニルアルコール系樹脂にヨウ素又は二色性色素が吸着配向してなる厚み10μm以下のポリビニルアルコール系偏光子を準備する工程(1)、
前記ポリビニルアルコール系偏光子の少なくとも片面に、樹脂成分または樹脂膜を構成することができる硬化性成分を含む液状物を塗工し、その後に、当該液状物を固化または硬化することにより、補強膜を形成する工程(2)を含むことを特徴とするフレキシブル偏光膜の製造方法、に関する。 Further, the present invention is a method for producing the flexible polarizing film,
A step (1) of preparing a polyvinyl alcohol polarizer having a thickness of 10 μm or less, wherein the polyvinyl alcohol resin is oriented in one direction and iodine or a dichroic dye is adsorbed and oriented on the polyvinyl alcohol resin;
By applying a liquid material containing a curable component capable of constituting a resin component or a resin film on at least one surface of the polyvinyl alcohol polarizer, and then solidifying or curing the liquid material, a reinforcing film The manufacturing method of the flexible polarizing film characterized by including the process (2) of forming.
ポリビニルアルコール系偏光子は、ポリビニルアルコール系樹脂が一方向(吸収軸方向)に配向し、かつ前記ポリビニルアルコール系樹脂にヨウ素又は二色性色素が吸着配向してなるものである。ポリビニルアルコール系樹脂としては、例えば、ポリビニルアルコール、部分ホルマール化ポリビニルアルコール、エチレン・酢酸ビニル共重合体系部分ケン化物等が挙げられる。偏光子は、前記ポリビニルアルコール系樹脂を用いたポリビニルアルコール系フィルムに、ヨウ素や二色性染料の二色性色素を吸着させて一軸延伸することにより得ることができる。 <Polyvinyl alcohol polarizer>
The polyvinyl alcohol-based polarizer is obtained by aligning a polyvinyl alcohol-based resin in one direction (absorption axis direction) and adsorbing or aligning iodine or a dichroic dye on the polyvinyl alcohol-based resin. Examples of the polyvinyl alcohol-based resin include polyvinyl alcohol, partially formalized polyvinyl alcohol, ethylene / vinyl acetate copolymer partially saponified products, and the like. A polarizer can be obtained by adsorbing a dichroic dye such as iodine or a dichroic dye to a polyvinyl alcohol film using the polyvinyl alcohol resin and stretching the film uniaxially.
特許第4751486号明細書、
特許第4751481号明細書、
特許第4815544号明細書、
特許第5048120号明細書、
国際公開第2014/077599号パンフレット、
国際公開第2014/077636号パンフレット、
等に記載されている薄型偏光子またはこれらに記載の製造方法から得られる薄型偏光子を挙げることができる。 As a thin polarizer, typically,
Patent No. 4751486,
Japanese Patent No. 4751481,
Patent No. 4815544,
Patent No. 5048120,
International Publication No. 2014/077599 pamphlet,
International Publication No. 2014/077636 Pamphlet,
And the thin polarizers obtained from the production methods described therein.
P>-(100.929T-42.4-1)×100(ただし、T<42.3)、又は、
P≧99.9(ただし、T≧42.3)の条件を満足するように構成されている。前記条件を満足するように構成された偏光子は、一義的には、大型表示素子を用いた液晶テレビ用のディスプレイとして求められる性能を有する。具体的にはコントラスト比1000:1以上かつ最大輝度500cd/m2以上である。他の用途としては、例えば有機EL表示装置の視認側に貼り合される。 The polarizer has an optical characteristic expressed by a single transmittance T and a polarization degree P of the following formula P> − (10 0.929T-42.4 −1) × 100 (where T <42.3), Or
It is configured to satisfy the condition of P ≧ 99.9 (however, T ≧ 42.3). A polarizer configured so as to satisfy the above-described conditions uniquely has performance required as a display for a liquid crystal television using a large display element. Specifically, the contrast ratio is 1000: 1 or more and the maximum luminance is 500 cd / m 2 or more. As other uses, for example, it is bonded to the viewing side of the organic EL display device.
本発明のフレキシブル偏光膜は、例えば、ポリビニルアルコール系偏光子の少なくとも片面に補強膜を有するものを用いることができる。図1(A)のフレキシブル偏光膜1は、ポリビニルアルコール系偏光子aの第1の片面にのみ第1補強膜b1を有する場合である。また、図1(B)のフレキシブル偏光膜1は、ポリビニルアルコール系偏光子1の第1の片面に第1補強膜b1を有し、他方の第2の片面には第2補強膜b2を有する場合である。本発明のフレキシブル偏光膜1において、第1補強膜b1および/または第2補強膜b2は、前記ポリビニルアルコール系偏光子1に、直接、設けられている。 Hereinafter, an example of the flexible polarizing film of the present invention will be described with reference to FIG.
As the flexible polarizing film of the present invention, for example, a polyvinyl alcohol polarizer having a reinforcing film on at least one surface can be used. The flexible
補強膜の厚さは、薄層化および柔軟性の観点から15μm以下であるのが好ましく、さらには10μm以下であるのが好ましく、さらには7μm以下であるのが好ましい。一方、補強膜の厚さは、柔軟性および強度の観点から、1μm以上であるのが好ましく、さらには3μm以上が好ましく、さらには5μm以上であるのが好ましい。 <Reinforcing membrane>
The thickness of the reinforcing membrane is preferably 15 μm or less from the viewpoint of thinning and flexibility, more preferably 10 μm or less, and further preferably 7 μm or less. On the other hand, the thickness of the reinforcing membrane is preferably 1 μm or more, more preferably 3 μm or more, and further preferably 5 μm or more from the viewpoint of flexibility and strength.
前記硬化性成分としては、例えば、ラジカル重合性化合物が挙げられる。ラジカル重合性化合物は、(メタ)アクリロイル基、ビニル基等の炭素-炭素二重結合のラジカル重合性の官能基を有する化合物が挙げられる。これら硬化性成分は、単官能ラジカル重合性化合物または二官能以上の多官能ラジカル重合性化合物のいずれも用いることができる。また、これらラジカル重合性化合物は、1種を単独で、または2種以上を組み合わせて用いることができる。これらラジカル重合性化合物としては、例えば、(メタ)アクリロイル基を有する化合物が好適である。なお、本発明において、(メタ)アクリロイルとは、アクリロイル基および/またはメタクリロイル基を意味し、「(メタ)」は以下同様の意味である。 ≪Radical polymerization curable forming material≫
Examples of the curable component include a radical polymerizable compound. Examples of the radical polymerizable compound include compounds having a radical polymerizable functional group of a carbon-carbon double bond such as a (meth) acryloyl group and a vinyl group. As these curable components, either a monofunctional radical polymerizable compound or a bifunctional or higher polyfunctional radical polymerizable compound can be used. Moreover, these radically polymerizable compounds can be used individually by 1 type or in combination of 2 or more types. As these radically polymerizable compounds, for example, compounds having a (meth) acryloyl group are suitable. In the present invention, (meth) acryloyl means an acryloyl group and / or methacryloyl group, and “(meth)” has the same meaning hereinafter.
単官能ラジカル重合性化合物としては、例えば、(メタ)アクリルアミド基を有する(メタ)アクリルアミド誘導体が挙げられる。(メタ)アクリルアミド誘導体は、偏光子との密着性を確保するうえで、また、重合速度が速く生産性に優れる点で好ましい。(メタ)アクリルアミド誘導体の具体例としては、例えば、N-メチル(メタ)アクリルアミド、N,N-ジメチル(メタ)アクリルアミド、N,N-ジエチル(メタ)アクリルアミド、N-イソプロピル(メタ)アクリルアミド、N-ブチル(メタ)アクリルアミド、N-ヘキシル(メタ)アクリルアミド等のN-アルキル基含有(メタ)アクリルアミド誘導体;N-メチロール(メタ)アクリルアミド、N-ヒドロキシエチル(メタ)アクリルアミド、N-メチロール-N-プロパン(メタ)アクリルアミド等のN-ヒドロキシアルキル基含有(メタ)アクリルアミド誘導体;アミノメチル(メタ)アクリルアミド、アミノエチル(メタ)アクリルアミド等のN-アミノアルキル基含有(メタ)アクリルアミド誘導体;N-メトキシメチルアクリルアミド、N-エトキシメチルアクリルアミド等のN-アルコキシ基含有(メタ)アクリルアミド誘導体;メルカプトメチル(メタ)アクリルアミド、メルカプトエチル(メタ)アクリルアミド等のN-メルカプトアルキル基含有(メタ)アクリルアミド誘導体;などが挙げられる。また、(メタ)アクリルアミド基の窒素原子が複素環を形成している複素環含有(メタ)アクリルアミド誘導体としては、例えば、N-アクリロイルモルホリン、N-アクリロイルピペリジン、N-メタクリロイルピペリジン、N-アクリロイルピロリジン等があげられる。 ≪Monofunctional radical polymerizable compound≫
Examples of the monofunctional radical polymerizable compound include (meth) acrylamide derivatives having a (meth) acrylamide group. The (meth) acrylamide derivative is preferable in terms of ensuring adhesion with the polarizer and having a high polymerization rate and excellent productivity. Specific examples of (meth) acrylamide derivatives include, for example, N-methyl (meth) acrylamide, N, N-dimethyl (meth) acrylamide, N, N-diethyl (meth) acrylamide, N-isopropyl (meth) acrylamide, N N-alkyl group-containing (meth) acrylamide derivatives such as butyl (meth) acrylamide and N-hexyl (meth) acrylamide; N-methylol (meth) acrylamide, N-hydroxyethyl (meth) acrylamide, N-methylol-N— N-hydroxyalkyl group-containing (meth) acrylamide derivatives such as propane (meth) acrylamide; N-aminoalkyl group-containing (meth) acrylamide derivatives such as aminomethyl (meth) acrylamide and aminoethyl (meth) acrylamide; N-methoxymethyl N-alkoxy group-containing (meth) acrylamide derivatives such as acrylamide and N-ethoxymethylacrylamide; N-mercaptoalkyl group-containing (meth) acrylamide derivatives such as mercaptomethyl (meth) acrylamide and mercaptoethyl (meth) acrylamide; It is done. Examples of the heterocyclic-containing (meth) acrylamide derivative in which the nitrogen atom of the (meth) acrylamide group forms a heterocyclic ring include, for example, N-acryloylmorpholine, N-acryloylpiperidine, N-methacryloylpiperidine, N-acryloylpyrrolidine. Etc.
ベンジル(メタ)アクリレート等のアラルキル(メタ)アクリレート;
2-イソボルニル(メタ)アクリレート、2-ノルボルニルメチル(メタ)アクリレート、5-ノルボルネン-2-イル-メチル(メタ)アクリレート、3-メチル-2-ノルボルニルメチル(メタ)アクリレート、ジシクロペンテニル(メタ)アクリレ-ト、ジシクロペンテニルオキシエチル(メタ)アクリレ-ト、ジシクロペンタニル(メタ)アクリレ-ト、等の多環式(メタ)アクリレート;
2-メトキシエチル(メタ)アクリレート、2-エトキシエチル(メタ)アクリレート、2-メトキシメトキシエチル(メタ)アクリレート、3-メトキシブチル(メタ)アクリレート、エチルカルビトール(メタ)アクリレート、フェノキシエチル(メタ)アクリレート、アルキルフェノキシポリエチレングリコール(メタ)アクリレート等のアルコキシ基またはフェノキシ基含有(メタ)アクリレート;等が挙げられる。 Examples of the (meth) acrylic acid derivative include cycloalkyl (meth) acrylates such as cyclohexyl (meth) acrylate and cyclopentyl (meth) acrylate;
Aralkyl (meth) acrylates such as benzyl (meth) acrylate;
2-isobornyl (meth) acrylate, 2-norbornylmethyl (meth) acrylate, 5-norbornen-2-yl-methyl (meth) acrylate, 3-methyl-2-norbornylmethyl (meth) acrylate, dicyclo Polycyclic (meth) acrylates such as pentenyl (meth) acrylate, dicyclopentenyloxyethyl (meth) acrylate, dicyclopentanyl (meth) acrylate, and the like;
2-methoxyethyl (meth) acrylate, 2-ethoxyethyl (meth) acrylate, 2-methoxymethoxyethyl (meth) acrylate, 3-methoxybutyl (meth) acrylate, ethyl carbitol (meth) acrylate, phenoxyethyl (meth) Examples thereof include alkoxy groups such as acrylates and alkylphenoxypolyethylene glycol (meth) acrylates or phenoxy group-containing (meth) acrylates.
グリシジル(メタ)アクリレート、4-ヒドロキシブチル(メタ)アクリレートグリシジルエーテル等のエポキシ基含有(メタ)アクリレート;
2,2,2-トリフルオロエチル(メタ)アクリレート、2,2,2-トリフルオロエチルエチル(メタ)アクリレート、テトラフルオロプロピル(メタ)アクリレート、ヘキサフルオロプロピル(メタ)アクリレート、オクタフルオロペンチル(メタ)アクリレート、ヘプタデカフルオロデシル(メタ)アクリレート、3-クロロ-2-ヒドロキシプロピル(メタ)アクリレート等のハロゲン含有(メタ)アクリレート;
ジメチルアミノエチル(メタ)アクリレート等のアルキルアミノアルキル(メタ)アクリレート;
3-オキセタニルメチル(メタ)アクリレート、3-メチルーオキセタニルメチル(メタ)アクリレート、3-エチルーオキセタニルメチル(メタ)アクリレート、3-ブチルーオキセタニルメチル(メタ)アクリレート、3-ヘキシルーオキセタニルメチル(メタ)アクリレート等のオキセタン基含有(メタ)アクリレート;
テトラヒドロフルフリル(メタ)アクリレート、ブチロラクトン(メタ)アクリレート、などの複素環を有する(メタ)アクリレートや、ヒドロキシピバリン酸ネオペンチルグリコール(メタ)アクリル酸付加物、p-フェニルフェノール(メタ)アクリレート等が挙げられる。 Examples of the (meth) acrylic acid derivative include 2-hydroxyethyl (meth) acrylate, 2-hydroxypropyl (meth) acrylate, 3-hydroxypropyl (meth) acrylate, 2-hydroxybutyl (meth) acrylate, 4- Hydroxyalkyl (meth) acrylates such as hydroxybutyl (meth) acrylate, 6-hydroxyhexyl (meth) acrylate, 8-hydroxyoctyl (meth) acrylate, 10-hydroxydecyl (meth) acrylate, 12-hydroxylauryl (meth) acrylate, etc. And hydroxyl groups such as [4- (hydroxymethyl) cyclohexyl] methyl acrylate, cyclohexanedimethanol mono (meth) acrylate, 2-hydroxy-3-phenoxypropyl (meth) acrylate, etc. Meth) acrylate;
Epoxy group-containing (meth) acrylates such as glycidyl (meth) acrylate and 4-hydroxybutyl (meth) acrylate glycidyl ether;
2,2,2-trifluoroethyl (meth) acrylate, 2,2,2-trifluoroethylethyl (meth) acrylate, tetrafluoropropyl (meth) acrylate, hexafluoropropyl (meth) acrylate, octafluoropentyl (meth) ) Halogen-containing (meth) acrylates such as acrylate, heptadecafluorodecyl (meth) acrylate, 3-chloro-2-hydroxypropyl (meth) acrylate;
Alkylaminoalkyl (meth) acrylates such as dimethylaminoethyl (meth) acrylate;
3-Oxetanylmethyl (meth) acrylate, 3-methyl-oxetanylmethyl (meth) acrylate, 3-ethyl-oxetanylmethyl (meth) acrylate, 3-butyl-oxetanylmethyl (meth) acrylate, 3-hexyloxetanylmethyl (meta) ) Oxetane group-containing (meth) acrylates such as acrylates;
(Meth) acrylates having a heterocyclic ring such as tetrahydrofurfuryl (meth) acrylate, butyrolactone (meth) acrylate, neopentyl glycol (meth) acrylic acid adducts such as hydroxypivalate, p-phenylphenol (meth) acrylate, etc. Can be mentioned.
また、二官能以上の多官能ラジカル重合性化合物としては、例えば、トリプロピレングリコールジ(メタ)アクリレート、テトラエチレングリコールジ(メタ)アクリレート、1,6-ヘキサンジオールジ(メタ)アクリレート、1,9-ノナンジオールジ(メタ)アクリレート、1,10-デカンジオールジアクリレート、2-エチル-2-ブチルプロパンジオールジ(メタ)アクリレート、ビスフェノールAジ(メタ)アクリレート、ビスフェノールAエチレンオキサイド付加物ジ(メタ)アクリレート、ビスフェノールAプロピレンオキサイド付加物ジ(メタ)アクリレート、ビスフェノールAジグリシジルエーテルジ(メタ)アクリレート、ネオぺンチルグリコールジ(メタ)アクリレート、トリシクロデカンジメタノールジ(メタ)アクリート、環状トリメチロールプロパンフォルマル(メタ)アクリレート、ジオキサングリコールジ(メタ)アクリレート、トリメチロールプロパントリ(メタ)アクリレート、ペンタエリスリトールトリ(メタ)アクリレート、ペンタエリスリトールテトラ(メタ)アクリレート、ジペンタエリスリトールペンタ(メタ)アクリレート、ジペンタエリスリトールヘキサ(メタ)アクリレート、EO変性ジグリセリンテトラ(メタ)アクリレート等の(メタ)アクリル酸と多価アルコールとのエステル化物、9,9-ビス[4-(2-(メタ)アクリロイルオキシエトキシ)フェニル]フルオレンがあげられる。具体例としては、アロニックスM-220、M-306(東亞合成社製)、ライトアクリレート1,9ND-A(共栄社化学社製)、ライトアクリレートDGE-4A(共栄社化学社製)、ライトアクリレートDCP-A(共栄社化学社製)、SR-531(Sartomer社製)、CD-536(Sartomer社製)等が挙げられる。また必要に応じて、各種のエポキシ(メタ)アクリレート、ウレタン(メタ)アクリレート、ポリエステル(メタ)アクリレートや、各種の(メタ)アクリレート系モノマー等が挙げられる。 ≪Polyfunctional radical polymerizable compound≫
Examples of the bifunctional or higher polyfunctional radical polymerizable compound include tripropylene glycol di (meth) acrylate, tetraethylene glycol di (meth) acrylate, 1,6-hexanediol di (meth) acrylate, 1,9 -Nonanediol di (meth) acrylate, 1,10-decanediol diacrylate, 2-ethyl-2-butylpropanediol di (meth) acrylate, bisphenol A di (meth) acrylate, bisphenol A ethylene oxide adduct di (meth) ) Acrylate, bisphenol A propylene oxide adduct di (meth) acrylate, bisphenol A diglycidyl ether di (meth) acrylate, neopentyl glycol di (meth) acrylate, tricyclodecane dimethanol di (meth) Acryte, cyclic trimethylolpropane formal (meth) acrylate, dioxane glycol di (meth) acrylate, trimethylolpropane tri (meth) acrylate, pentaerythritol tri (meth) acrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol penta Esterified products of (meth) acrylic acid and polyhydric alcohols such as (meth) acrylate, dipentaerythritol hexa (meth) acrylate, EO-modified diglycerin tetra (meth) acrylate, 9,9-bis [4- (2- (Meth) acryloyloxyethoxy) phenyl] fluorene. Specific examples include Aronix M-220, M-306 (manufactured by Toagosei Co., Ltd.),
ラジカル重合硬化型形成材は、活性エネルギー線硬化型または熱硬化型の形成材として用いることができる。活性エネルギー線に電子線等を用いる場合には、当該活性エネルギー線硬化型形成材は光重合開始剤を含有することは必要ではないが、活性エネルギー線に紫外線または可視光線を用いる場合には、光重合開始剤を含有するのが好ましい。一方、前記硬化性成分を熱硬化性成分として用いる場合には、当該形成材は熱重合開始剤を含有するのが好ましい。 ≪Aspects of radical polymerization curable forming material≫
The radical polymerization curable forming material can be used as an active energy ray curable forming material or a thermosetting forming material. When using an electron beam or the like for the active energy ray, the active energy ray curable forming material does not need to contain a photopolymerization initiator, but when using ultraviolet rays or visible light for the active energy ray, It preferably contains a photopolymerization initiator. On the other hand, when the curable component is used as a thermosetting component, the forming material preferably contains a thermal polymerization initiator.
ラジカル重合性化合物を用いる場合の光重合開始剤は、活性エネルギー線によって適宜に選択される。紫外線または可視光線により硬化させる場合には紫外線または可視光線開裂の光重合開始剤が用いられる。前記光重合開始剤としては、例えば、ベンジル、ベンゾフェノン、ベンゾイル安息香酸、3,3′-ジメチル-4-メトキシベンゾフェノンなどのベンゾフェノン系化合物;4-(2-ヒドロキシエトキシ)フェニル(2-ヒドロキシ-2-プロピル)ケトン、α-ヒドロキシ-α,α´-ジメチルアセトフェノン、2-メチル-2-ヒドロキシプロピオフェノン、α-ヒドロキシシクロヘキシルフェニルケトンなどの芳香族ケトン化合物;メトキシアセトフェノン、2,2-ジメトキシ-2-フェニルアセトフエノン、2,2-ジエトキシアセトフェノン、2-メチル-1-[4-(メチルチオ)-フェニル]-2-モルホリノプロパン-1などのアセトフェノン系化合物;べンゾインメチルエーテル、べンゾインエチルエーテル、ベンゾインイソプロピルエーテル、べンゾインブチルエーテル、アニソインメチルエーテルなどのベンゾインエーテル系化合物;ベンジルジメチルケタールなどの芳香族ケタール系化合物;2-ナフタレンスルホニルクロリドなどの芳香族スルホニルクロリド系化合物;1-フェノン-1,1―プロパンジオン-2-(o-エトキシカルボニル)オキシムなどの光活性オキシム系化合物;チオキサンソン、2-クロロチオキサンソン、2-メチルチオキサンソン、2,4-ジメチルチオキサンソン、イソプロピルチオキサンソン、2,4-ジクロロチオキサンソン、2,4-ジエチルチオキサンソン、2,4-ジイソプロピルチオキサンソン、ドデシルチオキサントンなどのチオキサンソン系化合物;カンファーキノン;ハロゲン化ケトン;アシルホスフィノキシド;アシルホスフォナートなどがあげられる。 ≪Photopolymerization initiator≫
The photopolymerization initiator in the case of using the radical polymerizable compound is appropriately selected depending on the active energy ray. In the case of curing by ultraviolet light or visible light, a photopolymerization initiator for ultraviolet light or visible light cleavage is used. Examples of the photopolymerization initiator include benzophenone compounds such as benzyl, benzophenone, benzoylbenzoic acid, 3,3′-dimethyl-4-methoxybenzophenone; 4- (2-hydroxyethoxy) phenyl (2-hydroxy-2 -Propyl) ketone, aromatic ketone compounds such as α-hydroxy-α, α'-dimethylacetophenone, 2-methyl-2-hydroxypropiophenone, α-hydroxycyclohexyl phenyl ketone; methoxyacetophenone, 2,2-dimethoxy- Acetophenone compounds such as 2-phenylacetophenone, 2,2-diethoxyacetophenone, 2-methyl-1- [4- (methylthio) -phenyl] -2-morpholinopropane-1; benzoin methyl ether; Benzoin ethyl ether, benzoin Benzoin ether compounds such as isopropyl ether, benzoin butyl ether and anisoin methyl ether; aromatic ketal compounds such as benzyldimethyl ketal; aromatic sulfonyl chloride compounds such as 2-naphthalenesulfonyl chloride; 1-phenone-1 , 1-propanedione-2- (o-ethoxycarbonyl) oxime, etc .; thioxanthone, 2-chlorothioxanthone, 2-methylthioxanthone, 2,4-dimethylthioxanthone, isopropylthioxanthone Thioxanthone compounds such as Son, 2,4-dichlorothioxanthone, 2,4-diethylthioxanthone, 2,4-diisopropylthioxanthone, dodecylthioxanthone; camphorquinone; halogenated ketone; Inokishido; and acyl phospholipase diisocyanate, and the like.
上記活性エネルギー線硬化型形成材において、ラジカル重合性化合物として、活性メチレン基を有するラジカル重合性化合物を用いる場合には、水素引き抜き作用のあるラジカル重合開始剤と組み合わせて用いるのが好ましい。 <Radical polymerizable compound having active methylene group and radical polymerization initiator having hydrogen abstraction action>
In the active energy ray curable forming material, when a radical polymerizable compound having an active methylene group is used as the radical polymerizable compound, it is preferably used in combination with a radical polymerization initiator having a hydrogen abstraction function.
熱重合開始剤としては、補強膜の形成の際には熱開裂によって重合が開始しないものが好ましい。例えば、熱重合開始剤としては、10時間半減期温度が65℃以上、さらには75~90℃であるものが好ましい。なお、この半減期とは、重合開始剤の分解速度を表す指標であり、重合開始剤の残存量が半分になるまでの時間をいう。任意の時間で半減期を得るための分解温度や、任意の温度での半減期時間に関しては、メーカーカタログなどに記載されており、たとえば、日本油脂株式会社の「有機過酸化物カタログ第9版(2003年5月)」などに記載されている。 ≪Thermal polymerization initiator≫
As the thermal polymerization initiator, those in which polymerization does not start by thermal cleavage when the reinforcing film is formed are preferable. For example, as the thermal polymerization initiator, those having a 10-hour half-life temperature of 65 ° C. or higher, more preferably 75 to 90 ° C. are preferable. The half-life is an index representing the decomposition rate of the polymerization initiator and refers to the time until the remaining amount of the polymerization initiator is halved. The decomposition temperature for obtaining a half-life at an arbitrary time and the half-life time at an arbitrary temperature are described in the manufacturer catalog, for example, “Organic peroxide catalog 9th edition by Nippon Oil & Fats Co., Ltd.” (May 2003) ".
カチオン重合硬化型形成材の硬化性成分としては、エポキシ基やオキセタニル基を有する化合物が挙げられる。エポキシ基を有する化合物は、分子内に少なくとも2個のエポキシ基を有するものであれば特に限定されず、一般に知られている各種の硬化性エポキシ化合物を用いることができる。好ましいエポキシ化合物として、分子内に少なくとも2個のエポキシ基と少なくとも1個の芳香環を有する化合物(芳香族系エポキシ化合物)や、分子内に少なくとも2個のエポキシ基を有し、そのうちの少なくとも1個は脂環式環を構成する隣り合う2個の炭素原子との間で形成されている化合物(脂環式エポキシ化合物)等が例として挙げられる。 ≪Cation polymerization curable forming material≫
Examples of the curable component of the cationic polymerization curable forming material include compounds having an epoxy group or an oxetanyl group. The compound having an epoxy group is not particularly limited as long as it has at least two epoxy groups in the molecule, and various generally known curable epoxy compounds can be used. As a preferable epoxy compound, a compound having at least two epoxy groups and at least one aromatic ring in the molecule (aromatic epoxy compound), or at least two epoxy groups in the molecule, at least one of them. Examples thereof include a compound (alicyclic epoxy compound) formed between two adjacent carbon atoms constituting an alicyclic ring.
カチオン重合硬化型形成材は、硬化性成分として以上説明したエポキシ化合物及びオキセタン化合物を含有し、これらはいずれもカチオン重合により硬化するものであることから、光カチオン重合開始剤が配合される。この光カチオン重合開始剤は、可視光線、紫外線、X線、電子線等の活性エネルギー線の照射によって、カチオン種又はルイス酸を発生し、エポキシ基やオキセタニル基の重合反応を開始する。 ≪Photo cationic polymerization initiator≫
The cationic polymerization curable forming material contains the epoxy compound and the oxetane compound described above as the curable component, and both of these are cured by cationic polymerization, and therefore, a photocationic polymerization initiator is blended therein. This cationic photopolymerization initiator generates a cationic species or a Lewis acid by irradiation with active energy rays such as visible light, ultraviolet rays, X-rays, and electron beams, and starts a polymerization reaction of an epoxy group or an oxetanyl group.
本発明に係る硬化型形成材は、下記成分を含有することが好ましい。 <Other ingredients>
The curable forming material according to the present invention preferably contains the following components.
本発明に係る活性エネルギー線硬化型形成材は、前記ラジカル重合性化合物に係る硬化性成分に加えて、(メタ)アクリルモノマーを重合してなるアクリル系オリゴマーを含有することができる。活性エネルギー線硬化型形成材中にアクリル系オリゴマーを含有することで、前記補強膜に活性エネルギー線を照射・硬化させる際の硬化収縮を低減し、補強膜と、偏光子との界面応力を低減することができる。硬化収縮を十分に抑制するためには、硬化性成分の全量100重量部に対して、アクリル系オリゴマーの含有量は、20重量部以下であることが好ましく、15重量部以下であることがより好ましい。形成材中のアクリル系オリゴマーの含有量が多すぎると、該形成材に活性エネルギー線を照射した際の反応速度の低下が激しく、硬化不良となる場合がある。一方、硬化性成分の全量100重量部に対して、アクリル系オリゴマーを3重量部以上含有することが好ましく、5重量部以上含有することがより好ましい。 <Acrylic oligomer>
The active energy ray-curable forming material according to the present invention can contain an acrylic oligomer obtained by polymerizing a (meth) acrylic monomer, in addition to the curable component related to the radical polymerizable compound. By containing an acrylic oligomer in the active energy ray-curable forming material, curing shrinkage when irradiating and curing the active energy ray to the reinforcing film is reduced, and the interfacial stress between the reinforcing film and the polarizer is reduced. can do. In order to sufficiently suppress the curing shrinkage, the content of the acrylic oligomer is preferably 20 parts by weight or less and more preferably 15 parts by weight or less with respect to 100 parts by weight of the total amount of the curable component. preferable. When the content of the acrylic oligomer in the forming material is too large, the reaction rate when the forming material is irradiated with active energy rays is drastically reduced, which may result in poor curing. On the other hand, the acrylic oligomer is preferably contained in an amount of 3 parts by weight or more, more preferably 5 parts by weight or more, based on 100 parts by weight of the total amount of the curable component.
上記活性エネルギー線硬化型形成材において、光酸発生剤を含有することができる。上記活性エネルギー線硬化型形成材に、光酸発生剤を含有する場合、光酸発生剤を含有しない場合に比べて、補強膜の耐水性および耐久性を飛躍的に向上することができる。光酸発生剤は、下記一般式(3)で表すことができる。 <Photo acid generator>
The active energy ray-curable forming material may contain a photoacid generator. When the active energy ray-curable forming material contains a photoacid generator, the water resistance and durability of the reinforcing film can be dramatically improved as compared with a case where no photoacid generator is contained. The photoacid generator can be represented by the following general formula (3).
前記硬化型形成材は、活性エネルギー線硬化型形成材または熱硬化型形成材として用いられる。活性エネルギー線硬化型形成材では、電子線硬化型、紫外線硬化型、可視光線硬化型の態様で用いることができる。 <Curing of forming material>
The curable forming material is used as an active energy ray curable forming material or a thermosetting forming material. The active energy ray curable forming material can be used in an electron beam curable type, an ultraviolet curable type, or a visible light curable type.
活性エネルギー線硬化型形成材では、偏光子に活性エネルギー線硬化型形成材を塗工した後、活性エネルギー線(電子線、紫外線、可視光線など)を照射し、活性エネルギー線硬化型形成材を硬化して補強膜を形成する。活性エネルギー線(電子線、紫外線、可視光線など)の照射方向は、任意の適切な方向から照射することができる。好ましくは、補強膜側から照射する。 ≪Active energy ray curing type≫
In the active energy ray curable forming material, after applying the active energy ray curable forming material to the polarizer, the active energy ray (electron beam, ultraviolet ray, visible light, etc.) is irradiated, and the active energy ray curable forming material is applied. Curing to form a reinforcing film. The irradiation direction of active energy rays (electron beam, ultraviolet ray, visible light, etc.) can be irradiated from any appropriate direction. Preferably, irradiation is performed from the reinforcing film side.
電子線硬化型において、電子線の照射条件は、上記活性エネルギー線硬化型形成材を硬化しうる条件であれば、任意の適切な条件を採用できる。例えば、電子線照射は、加速電圧が好ましくは5kV~300kVであり、さらに好ましくは10kV~250kVである。加速電圧が5kV未満の場合、電子線が補強膜最深部まで届かず硬化不足となるおそれがあり、加速電圧が300kVを超えると、試料を通る浸透力が強すぎて、偏光子にダメージを与えるおそれがある。照射線量としては、5~100kGy、さらに好ましくは10~75kGyである。照射線量が5kGy未満の場合は、接着剤が硬化不足となり、100kGyを超えると、偏光子にダメージを与え、機械的強度の低下や黄変を生じ、所定の光学特性を得ることができない。 ≪Electron beam curing type≫
In the electron beam curable type, any appropriate condition can be adopted as the electron beam irradiation condition as long as the active energy ray curable forming material can be cured. For example, in the electron beam irradiation, the acceleration voltage is preferably 5 kV to 300 kV, and more preferably 10 kV to 250 kV. If the acceleration voltage is less than 5 kV, the electron beam may not reach the deepest part of the reinforcing film and may be insufficiently cured. If the acceleration voltage exceeds 300 kV, the penetrating force through the sample is too strong and damages the polarizer. There is a fear. The irradiation dose is 5 to 100 kGy, more preferably 10 to 75 kGy. When the irradiation dose is less than 5 kGy, the adhesive is insufficiently cured, and when it exceeds 100 kGy, the polarizer is damaged, resulting in a decrease in mechanical strength and yellowing, and the predetermined optical characteristics cannot be obtained.
活性エネルギー線として、波長範囲380nm~450nmの可視光線を含むもの、特には波長範囲380nm~450nmの可視光線の照射量が最も多い活性エネルギー線を使用することが好ましい。活性エネルギー線としては、ガリウム封入メタルハライドランプ、波長範囲380~440nmを発光するLED光源が好ましい。あるいは、低圧水銀灯、中圧水銀灯、高圧水銀灯、超高圧水銀灯、白熱電球、キセノンランプ、ハロゲンランプ、カーボンアーク灯、メタルハライドランプ、蛍光灯、タングステンランプ、ガリウムランプ、エキシマレーザーまたは太陽光などの紫外線と可視光線を含む光源を使用することができ、バンドパスフィルターを用いて380nmより短波長の紫外線を遮断して用いることもできる。 ≪Ultraviolet curing type, visible light curing type≫
It is preferable to use an active energy ray containing visible light having a wavelength range of 380 nm to 450 nm, particularly an active energy ray having the largest irradiation amount of visible light having a wavelength range of 380 nm to 450 nm. As the active energy ray, a gallium-filled metal halide lamp and an LED light source that emits light in the wavelength range of 380 to 440 nm are preferable. Or low pressure mercury lamp, medium pressure mercury lamp, high pressure mercury lamp, ultra high pressure mercury lamp, incandescent lamp, xenon lamp, halogen lamp, carbon arc lamp, metal halide lamp, fluorescent lamp, tungsten lamp, gallium lamp, excimer laser or sunlight A light source including visible light can be used, and ultraviolet light having a wavelength shorter than 380 nm can be blocked using a band pass filter.
一方、熱硬化型形成材では、偏光子に塗布した後に、加熱することにより、熱重合開始剤により重合を開始して、硬化物層(補強膜)を形成する。加熱温度は、熱重合開始剤に応じて設定されるが、60~200℃程度、好ましくは80~150℃である。 ≪Thermosetting type≫
On the other hand, in a thermosetting type forming material, after applying to a polarizer, by heating, polymerization is started by a thermal polymerization initiator to form a cured product layer (reinforcing film). The heating temperature is set according to the thermal polymerization initiator, but is about 60 to 200 ° C., preferably 80 to 150 ° C.
高分子ポリオール化合物、ポリウレタンの重量平均分子量は、GPC(ゲルパーミエーションクロマトグラフィー)方の下記条件にて測定した。
分析装置:東ソー製、HLC-8120GPC。
カラム:東ソー製、G7000HXL+GMHXL+GMHXL。
カラムサイズ:各7.8mmφ×30cm 計90cm。
カラム温度:40℃。
流速:0.8ml/min。
注入量:100μl。
溶離液:テトラヒドロフラン。
検出器:示唆屈折計。
標準試料:ポリスチレン。 (Measurement of weight average molecular weight)
The weight average molecular weight of the polymer polyol compound and polyurethane was measured under the following conditions of GPC (gel permeation chromatography).
Analyzing apparatus: HLC-8120GPC manufactured by Tosoh Corporation.
Column: manufactured by Tosoh Corporation, G7000HXL + GMHXL + GMHXL.
Column size: 7.8 mmφ × 30 cm each 90 cm in total.
Column temperature: 40 ° C.
Flow rate: 0.8 ml / min.
Injection volume: 100 μl.
Eluent: tetrahydrofuran.
Detector: Suggested refractometer.
Standard sample: polystyrene.
本発明のフレキシブル偏光膜の片面または両面には、表面処理層を設けることができる。前記表面処理層としては、ハードコート層、防眩処理層、反射防止層、スティッキング防止層などが挙がられる。前記表面処理層としては、ハードコート層であることが好ましい。ハードコート層の形成材料としては、例えば、熱可塑性樹脂、熱または放射線により硬化する材料を用いることができる。前記材料としては、熱硬化型樹脂や紫外線硬化型樹脂、電子線硬化型樹脂等の放射線硬化性樹脂があげられる。これらのなかでも、紫外線照射による硬化処理にて、簡単な加工操作にて効率よく硬化樹脂層を形成することができる紫外線硬化型樹脂が好適である。これら硬化型樹脂としては、ポリエステル系、アクリル系、ウレタン系、アミド系、シリコーン系、エポキシ系、メラミン系等の各種のものがあげられ、これらのモノマー、オリゴマー、ポリマー等が含まれる。 <Surface treatment layer>
A surface treatment layer can be provided on one side or both sides of the flexible polarizing film of the present invention. Examples of the surface treatment layer include a hard coat layer, an antiglare treatment layer, an antireflection layer, and an antisticking layer. The surface treatment layer is preferably a hard coat layer. As a material for forming the hard coat layer, for example, a thermoplastic resin or a material that is cured by heat or radiation can be used. Examples of the material include radiation curable resins such as thermosetting resins, ultraviolet curable resins, and electron beam curable resins. Among these, an ultraviolet curable resin that can efficiently form a cured resin layer by a simple processing operation by a curing treatment by ultraviolet irradiation is preferable. Examples of these curable resins include polyesters, acrylics, urethanes, amides, silicones, epoxies, melamines, and the like, and these monomers, oligomers, polymers, and the like are included.
本発明のフレキシブル偏光膜の片面または両面には粘着剤層を設けることができる。粘着剤層の形成には、適宜な粘着剤を用いることができ、その種類について特に制限はない。粘着剤としては、ゴム系粘着剤、アクリル系粘着剤、シリコーン系粘着剤、ウレタン系粘着剤、ビニルアルキルエーテル系粘着剤、ポリビニルアルコール系粘着剤、ポリビニルピロリドン系粘着剤、ポリアクリルアミド系粘着剤、セルロース系粘着剤などがあげられる。 <Adhesive layer>
An adhesive layer can be provided on one side or both sides of the flexible polarizing film of the present invention. An appropriate pressure-sensitive adhesive can be used for forming the pressure-sensitive adhesive layer, and the type thereof is not particularly limited. Adhesives include rubber adhesives, acrylic adhesives, silicone adhesives, urethane adhesives, vinyl alkyl ether adhesives, polyvinyl alcohol adhesives, polyvinyl pyrrolidone adhesives, polyacrylamide adhesives, Examples thereof include cellulose-based pressure-sensitive adhesives.
また、本発明のフレキシブル偏光膜には、表面保護フィルムを設けることができる。表面保護フィルムは、通常、基材フィルムおよび粘着剤層を有し、当該粘着剤層を介してフレキシブル偏光膜を保護する。 <Surface protection film>
The flexible polarizing film of the present invention can be provided with a surface protective film. The surface protective film usually has a base film and an adhesive layer, and protects the flexible polarizing film via the adhesive layer.
本発明のフレキシブル偏光膜は、偏光子又は偏光フィルム(偏光子に透明保護フィルムを設けたもの)の代替として、従来の偏光子や偏光フィルムでは適用できなかった種々の用途展開の拡大を図ることができ、例えば他の光学層と積層した光学フィルムとしても用いることができる。その光学層については特に限定はないが、例えば、本発明のフレキシブル偏光膜を、偏光子の代替として用いる場合には、偏光子の保護に用いられる保護フィルム(位相差フィルム、輝度向上フィルム、拡散フィルム等も含む)を用いることができ、偏光フィルムの代替として用いる場合には、反射板や半透過板、位相差フィルム(1/2や1/4などの波長板を含む)、視角補償フィルムなど、液晶表示装置や有機EL表示装置といった画像表示装置などの形成に用いられることのある光学層を1層または2層以上用いることができる。 <Other optical layers>
The flexible polarizing film of the present invention is intended to expand various applications that cannot be applied to conventional polarizers and polarizing films as an alternative to polarizers or polarizing films (those provided with a transparent protective film on the polarizer). For example, it can be used as an optical film laminated with another optical layer. The optical layer is not particularly limited. For example, when the flexible polarizing film of the present invention is used as a substitute for a polarizer, a protective film (retardation film, brightness enhancement film, diffusion) used for protecting the polarizer. (Including films, etc.), and when used as an alternative to polarizing films, reflectors, transflective plates, retardation films (including wavelength plates such as 1/2 and 1/4), viewing angle compensation films One optical layer or two or more optical layers that may be used for forming an image display device such as a liquid crystal display device or an organic EL display device can be used.
前記光学層とフレキシブル偏光膜は接着剤層、粘着剤層、下塗り層(プライマー層)などの介在層を介して積層することができる。この際、介在層により両者を空気間隙なく積層することが望ましい。 <Intervening layer>
The optical layer and the flexible polarizing film can be laminated via an intervening layer such as an adhesive layer, a pressure-sensitive adhesive layer, or an undercoat layer (primer layer). At this time, it is desirable that the both are laminated without an air gap by an intervening layer.
吸水率0.75%、Tg75℃の非晶質のイソフタル酸共重合ポリエチレンテレフタレート(IPA共重合PET)フィルム(厚み:100μm)基材の片面に、コロナ処理を施し、このコロナ処理面に、ポリビニルアルコール(重合度4200、ケン化度99.2モル%)およびアセトアセチル変性PVA(重合度1200、アセトアセチル変性度4.6%、ケン化度99.0モル%以上、日本合成化学工業社製、商品名「ゴーセファイマーZ200」)を9:1の比で含む水溶液を25℃で塗布および乾燥して、厚み11μmのPVA系樹脂層を形成し、積層体を作製した。
得られた積層体を、120℃のオーブン内で周速の異なるロール間で縦方向(長手方向)に2.0倍に自由端一軸延伸した(空中補助延伸処理)。
次いで、積層体を、液温30℃の不溶化浴(水100重量部に対して、ホウ酸を4重量部配合して得られたホウ酸水溶液)に30秒間浸漬させた(不溶化処理)。
次いで、液温30℃の染色浴に、偏光板が所定の透過率となるようにヨウ素濃度、浸漬時間を調整しながら浸漬させた。本実施例では、水100重量部に対して、ヨウ素を0.2重量部配合し、ヨウ化カリウムを1.0重量部配合して得られたヨウ素水溶液に60秒間浸漬させた(染色処理)。
次いで、液温30℃の架橋浴(水100重量部に対して、ヨウ化カリウムを3重量部配合し、ホウ酸を3重量部配合して得られたホウ酸水溶液)に30秒間浸漬させた(架橋処理)。
その後、積層体を、液温70℃のホウ酸水溶液(水100重量部に対して、ホウ酸を4重量部配合し、ヨウ化カリウムを5重量部配合して得られた水溶液)に浸漬させながら、周速の異なるロール間で縦方向(長手方向)に総延伸倍率が5.5倍となるように一軸延伸を行った(水中延伸処理)。
その後、積層体を液温30℃の洗浄浴(水100重量部に対して、ヨウ化カリウムを4重量部配合して得られた水溶液)に浸漬させた(洗浄処理)。
以上により、厚み5μmの偏光子を含む光学フィルム積層体を得た。 <Preparation of polarizer A0>
One side of an amorphous isophthalic acid copolymerized polyethylene terephthalate (IPA copolymerized PET) film (thickness: 100 μm) having a water absorption of 0.75% and Tg of 75 ° C. is subjected to corona treatment. Alcohol (polymerization degree 4200, saponification degree 99.2 mol%) and acetoacetyl-modified PVA (polymerization degree 1200, acetoacetyl modification degree 4.6%, saponification degree 99.0 mol% or more, manufactured by Nippon Synthetic Chemical Industry Co., Ltd. An aqueous solution containing 9: 1 ratio of the trade name “Gosefimer Z200”) was applied and dried at 25 ° C. to form a PVA-based resin layer having a thickness of 11 μm, thereby preparing a laminate.
The obtained laminate was uniaxially stretched in the longitudinal direction (longitudinal direction) 2.0 times between rolls having different peripheral speeds in an oven at 120 ° C. (air-assisted stretching process).
Next, the laminate was immersed in an insolubilization bath (a boric acid aqueous solution obtained by blending 4 parts by weight of boric acid with respect to 100 parts by weight of water) for 30 seconds (insolubilization treatment).
Subsequently, it was immersed in a dyeing bath having a liquid temperature of 30 ° C. while adjusting the iodine concentration and the immersion time so that the polarizing plate had a predetermined transmittance. In this example, 0.2 parts by weight of iodine was blended with 100 parts by weight of water, and immersed in an aqueous iodine solution obtained by blending 1.0 part by weight of potassium iodide (dyeing treatment). .
Subsequently, it was immersed for 30 seconds in a crosslinking bath having a liquid temperature of 30 ° C. (a boric acid aqueous solution obtained by blending 3 parts by weight of potassium iodide and 3 parts by weight of boric acid with respect to 100 parts by weight of water). (Crosslinking treatment).
Thereafter, the laminate was immersed in a boric acid aqueous solution (an aqueous solution obtained by blending 4 parts by weight of boric acid and 5 parts by weight of potassium iodide with respect to 100 parts by weight of water) at a liquid temperature of 70 ° C. However, uniaxial stretching was performed between rolls having different peripheral speeds in the longitudinal direction (longitudinal direction) so that the total stretching ratio was 5.5 times (in-water stretching treatment).
Thereafter, the laminate was immersed in a cleaning bath (an aqueous solution obtained by blending 4 parts by weight of potassium iodide with respect to 100 parts by weight of water) at a liquid temperature of 30 ° C. (cleaning treatment).
As a result, an optical film laminate including a polarizer having a thickness of 5 μm was obtained.
上記の偏光子A0の作製において、製造条件を表1に示すように変えたこと以外は偏光子A0の作製と同様にして、偏光子A1~A2を作製した。偏光子A0~A2の厚み、光学特性(単体透過率、偏光度)、ホウ酸濃度を表1に示す。 <Preparation of polarizers A1 and A2>
Polarizers A1 and A2 were produced in the same manner as in the production of the polarizer A0 except that the production conditions for the polarizer A0 were changed as shown in Table 1. Table 1 shows the thickness, optical characteristics (single transmittance, polarization degree), and boric acid concentration of the polarizers A0 to A2.
平均重合度2400、ケン化度99.9モル%の厚み30μmのポリビニルアルコールフィルムを、30℃の温水中に60秒間浸漬し膨潤させた。次いで、ヨウ素/ヨウ化カリウム(重量比=0.5/8)の濃度0.3%の水溶液に浸漬し、3.5倍まで延伸させながらフィルムを染色した。その後、65℃のホウ酸エステル水溶液中で、総延伸倍率が6倍となるように延伸を行った。延伸後に、40℃のオーブンにて3分間乾燥を行い、偏光子B1を得た。得られた偏光子B1の厚みは12μmであった。得られた偏光子B1の光学特性は、透過率42.8%、偏光度99.99%であった。 <Preparation of Polarizer B1 (12 μm Thick Polarizer)>
A polyvinyl alcohol film having an average polymerization degree of 2400 and a saponification degree of 99.9 mol% and a thickness of 30 μm was immersed in warm water at 30 ° C. for 60 seconds to swell. Next, the film was dyed while being immersed in an aqueous solution of 0.3% concentration of iodine / potassium iodide (weight ratio = 0.5 / 8) and stretched to 3.5 times. Then, it extended | stretched so that the total draw ratio might be 6 time in 65 degreeC borate ester aqueous solution. After extending | stretching, it dried for 3 minutes in 40 degreeC oven, and obtained polarizer B1. The thickness of the obtained polarizer B1 was 12 μm. The optical properties of the obtained polarizer B1 were a transmittance of 42.8% and a polarization degree of 99.99%.
平均重合度2400、ケン化度99.9モル%の厚み75μmのポリビニルアルコールフィルムを、30℃の温水中に60秒間浸漬し膨潤させた。次いで、ヨウ素/ヨウ化カリウム(重量比=0.5/8)の濃度0.3%の水溶液に浸漬し、3.5倍まで延伸させながらフィルムを染色した。その後、65℃のホウ酸エステル水溶液中で、トータルの延伸倍率が6倍となるように延伸を行った。延伸後に、40℃のオーブンにて3分間乾燥を行い、偏光子B2を得た。得られた偏光子B2の厚みは23μmであった。得られた偏光子B2の光学特性は、透過率42.8%、偏光度99.99%であった。 <Preparation of Polarizer B2 (23 μm Thick Polarizer)>
A polyvinyl alcohol film having an average polymerization degree of 2400 and a saponification degree of 99.9 mol% and a thickness of 75 μm was immersed in warm water at 30 ° C. for 60 seconds to swell. Next, the film was dyed while being immersed in an aqueous solution of 0.3% concentration of iodine / potassium iodide (weight ratio = 0.5 / 8) and stretched to 3.5 times. Then, it extended | stretched so that the total draw ratio might be 6 times in 65 degreeC borate ester aqueous solution. After extending | stretching, it dried for 3 minutes in 40 degreeC oven, and obtained polarizer B2. The thickness of the obtained polarizer B2 was 23 μm. The optical properties of the obtained polarizer B2 were a transmittance of 42.8% and a polarization degree of 99.99%.
(1)ポリウレタン系材料
トリレンジイソシアネートとトリメチロールプロパンよりなるウレタンプレポリマー(東ソー社製,コロネートL)50部に、ポリカーボネートポリオール(住化バイエルウレタン社製,デスモフェンC3100XP)50部、ジオクチルスズジラウレート系触媒(東京ファインケミカル社製,エンビライザーOL-1)0.1部を加え、溶媒としてメチルイソブチルケトンを用いて、固形分濃度35%に調整した塗工液を得た。 <Reinforcing film forming material>
(1) Polyurethane material 50 parts of urethane prepolymer consisting of tolylene diisocyanate and trimethylolpropane (manufactured by Tosoh Corporation, Coronate L), 50 parts of polycarbonate polyol (manufactured by Sumika Bayer Urethane Co., Ltd., Desmophen C3100XP), dioctyltin dilaurate system 0.1 parts of a catalyst (manufactured by Tokyo Fine Chemical Co., Ltd., ENBOLiser OL-1) was added, and methyl isobutyl ketone was used as a solvent to obtain a coating solution adjusted to a solid content concentration of 35%.
ウレタンアクリレートオリゴマー(日本合成化学社製,紫光UV1700TL)50部に、2-ヒドロキシエチルアクリレートと1,6-ジイソシアナトヘキサンを構成成分とする重合物との反応物(BASF社製,製品名ラロマーLR9000)50部、光開始剤(BASF社製,IRGACURE907)3部を加え、溶媒としてメチルイソブチルケトンを用いて、固形分濃度35%に調整した塗工液を得た。 (2) UV curable material Reaction product of 2-hydroxyethyl acrylate and a polymer containing 1,6-diisocyanatohexane as a component in 50 parts of urethane acrylate oligomer (manufactured by Nippon Synthetic Chemical Co., Ltd., purple light UV1700TL) ( BASF, product name Laromar LR9000 (50 parts) and photoinitiator (BASF, IRGACURE907) (3 parts) were added, and methyl isobutyl ketone was used as a solvent to obtain a coating solution adjusted to a solid content concentration of 35%. .
アクリルエマルション(大成ファインケミカル社製の商品名:SE‐2915E)に、溶媒として純水を加えて固形分濃度30%に調整した塗工液を得た。 (3) Water-based resin emulsion material A coating liquid prepared by adding pure water as a solvent to an acrylic emulsion (trade name: SE-2915E manufactured by Taisei Fine Chemical Co., Ltd.) to a solid content concentration of 30% was obtained.
厚み20μmのラクトン環構造を有する(メタ)アクリル樹脂フィルムの易接着処理面にコロナ処理を施して用いた。 <Protective film (acrylic) and adhesive>
A (meth) acrylic resin film having a lactone ring structure with a thickness of 20 μm was subjected to corona treatment on the easy adhesion treated surface.
上記で得られた偏光子A0(厚み5μmの偏光子を含む光学フィルム積層体)の偏光子(第1面)に、上記補強膜のポリウレタン系材料(1)をワイヤーバーコーターを用いて、硬化後の厚みが5μmになるように塗工した後、60℃、10分の条件下で初期硬化させ、その後さらに60℃、12時間の条件下でアニール処理を施すことで第1補強膜を形成した。次いで、前記第1補強膜上に、表面保護フィルム(日東電工製,RP301)を貼り合せた後に、前記光学フィルム積層体の非晶性PET基材を剥離した。その後、偏光子(剥離面である第2面)に、上記補強膜のポリウレタン系材料(1)をワイヤーバーコーターを用いて、硬化後の厚みが5μmになるように塗工した後、60℃、10分の条件下で初期硬化させ、その後さらに60℃、12時間の条件下でアニール処理を施すことで、第2補強膜を形成した。その後、第1補強膜上に貼り合せていた表面保護フィルム取り外すことで、偏光子の両面に補強膜を有するフレキシブル偏光膜を得た。 Example 1 (Preparation of flexible polarizing film)
Using the wire bar coater, the polyurethane-based material (1) of the reinforcing film is cured on the polarizer (first surface) of the polarizer A0 (optical film laminate including a polarizer having a thickness of 5 μm) obtained above. After coating to a subsequent thickness of 5 μm, initial curing is performed under conditions of 60 ° C. and 10 minutes, and then a first reinforcing film is formed by performing annealing treatment under conditions of 60 ° C. and 12 hours. did. Next, after a surface protective film (manufactured by Nitto Denko, RP301) was bonded onto the first reinforcing film, the amorphous PET substrate of the optical film laminate was peeled off. Thereafter, the polyurethane-based material (1) of the reinforcing film was applied to a polarizer (second surface as a peeling surface) using a wire bar coater so that the thickness after curing was 5 μm, and then 60 ° C. The second reinforcing film was formed by initial curing under conditions of 10 minutes, and then annealing treatment at 60 ° C. for 12 hours. Then, the flexible polarizing film which has a reinforcement film on both surfaces of a polarizer was obtained by removing the surface protection film bonded on the 1st reinforcement film.
実施例1において、偏光子の種類、補強膜の厚みを表2に示すように変えたこと以外は、実施例1と同様にして、偏光子の両面に補強膜を有するフレキシブル偏光膜を作製した。 Examples 2-4
In Example 1, a flexible polarizing film having a reinforcing film on both sides of the polarizer was prepared in the same manner as in Example 1 except that the type of polarizer and the thickness of the reinforcing film were changed as shown in Table 2. .
上記で得られた偏光子A0(厚み5μmの偏光子を含む光学フィルム積層体)の偏光子(第1面)に、上記補強膜のUV硬化系材料(2)をワイヤーバーコーターを用いて、硬化後の厚みが5μmになるように塗工した後、60℃で1分加熱した。加熱後の塗布膜に高圧水銀ランプにて積算光量300mJ/cm2の紫外線を照射して、第1補強膜を形成した。次いで、前記第1補強膜上に表面保護フィルム(日東電工製,RP301)を貼り合せた後に、前記光学フィルム積層体の非晶性PET基材を剥離した。その後、偏光子(剥離面である第2面)に、上記補強膜のUV硬化系材料(2)をワイヤーバーコーターを用いて、硬化後の厚みが5μmになるように塗工した後、60℃で1分加熱した。加熱後の塗布膜に高圧水銀ランプにて積算光量300mJ/cm2の紫外線を照射することで、第2補強膜を形成した。その後、第1補強膜上に貼り合せていた表面保護フィルム取り外すことで、偏光子の両面に補強膜を有するフレキシブル偏光膜を得た。 Example 5 (Preparation of flexible polarizing film)
Using a wire bar coater, the UV curable material (2) of the reinforcing film is applied to the polarizer (first surface) of the polarizer A0 (optical film laminate including a polarizer having a thickness of 5 μm) obtained above. After coating so that the thickness after curing was 5 μm, the coating was heated at 60 ° C. for 1 minute. A first reinforcing film was formed by irradiating the heated coating film with ultraviolet light having an integrated light quantity of 300 mJ / cm 2 with a high-pressure mercury lamp. Next, after a surface protective film (manufactured by Nitto Denko, RP301) was bonded onto the first reinforcing film, the amorphous PET substrate of the optical film laminate was peeled off. Thereafter, the UV curable material (2) of the reinforcing film was applied to a polarizer (second surface as a peeling surface) using a wire bar coater so that the thickness after curing was 5 μm, and then 60 Heated at 0 ° C. for 1 minute. A second reinforcing film was formed by irradiating the heated coating film with ultraviolet light having an integrated light quantity of 300 mJ / cm 2 using a high-pressure mercury lamp. Then, the flexible polarizing film which has a reinforcement film on both surfaces of a polarizer was obtained by removing the surface protection film bonded on the 1st reinforcement film.
上記で得られた偏光子A0(厚み5μmの偏光子を含む光学フィルム積層体)の偏光子(第1面)に、上記補強膜の水系樹脂エマルション材料(3)をワイヤーバーコーターを用いて、硬化後の厚みが5μmになるように塗工した後、80℃、5分の条件下で硬化させ、第1補強膜を形成した。次いで、前記第1補強膜上に、表面保護フィルム(日東電工製,RP301)を貼り合せた後に、前記光学フィルム積層体の非晶性PET基材を剥離した。その後、偏光子(剥離面である第2面)に、上記補強膜の水系樹脂エマルション材料(3)をワイヤーバーコーターを用いて、硬化後の厚みが5μmになるように塗工した後、80℃、5分の条件下で硬化させ、第2補強膜を形成した。その後、第1補強膜上に貼り合せていた表面保護フィルム取り外すことで、偏光子の両面に補強膜を有するフレキシブル偏光膜を得た。 Example 6 (Preparation of flexible polarizing film)
To the polarizer (first surface) of the polarizer A0 (optical film laminate including a polarizer having a thickness of 5 μm) obtained above, the aqueous resin emulsion material (3) of the reinforcing film is used with a wire bar coater. After coating so that the thickness after curing was 5 μm, the coating was cured under the conditions of 80 ° C. for 5 minutes to form a first reinforcing film. Next, after a surface protective film (manufactured by Nitto Denko, RP301) was bonded onto the first reinforcing film, the amorphous PET substrate of the optical film laminate was peeled off. Then, after applying the water-based resin emulsion material (3) of the reinforcing film to a polarizer (second surface as a peeling surface) using a wire bar coater so that the thickness after curing becomes 5 μm, 80 Curing was carried out at 5 ° C. for 5 minutes to form a second reinforcing film. Then, the flexible polarizing film which has a reinforcement film on both surfaces of a polarizer was obtained by removing the surface protection film bonded on the 1st reinforcement film.
実施例1において、第1補強膜を設けなかったこと以外は、実施例1と同様にして、偏光子の片面にのみ第2補強膜を有するフレキシブル偏光膜を作製した。 Example 7
In Example 1, a flexible polarizing film having a second reinforcing film only on one side of the polarizer was produced in the same manner as in Example 1 except that the first reinforcing film was not provided.
実施例1において、第2補強膜を設けなかったこと以外は、実施例1と同様にして、偏光子の片面にのみ第1補強膜を有するフレキシブル偏光膜を作製した。 Example 8
In Example 1, a flexible polarizing film having the first reinforcing film only on one surface of the polarizer was produced in the same manner as in Example 1 except that the second reinforcing film was not provided.
実施例1において、偏光子の種類、補強膜の厚みを表2に示すように変えたこと以外は、実施例1と同様にして、偏光子の両面に補強膜を有する補強膜を有する偏光膜を作製した。 Comparative Examples 1 to 3
A polarizing film having a reinforcing film having reinforcing films on both sides of the polarizer in the same manner as in Example 1 except that the type of polarizer and the thickness of the reinforcing film were changed as shown in Table 2 in Example 1. Was made.
上記で得られた偏光子A0(厚み5μmの偏光子を含む光学フィルム積層体)の偏光子(第1面)に、上記紫外線硬化型接着剤を硬化後の接着剤層の厚みが1μmになるように塗布し、上記保護フィルム(アクリル)を貼合せたのち、活性エネルギー線として、紫外線を照射し、接着剤を硬化させた。紫外線照射は、ガリウム封入メタルハライドランプ、照射装置:Fusion UV Systems,Inc社製のLight HAMMER10、バルブ:Vバルブ、ピーク照度:1600mW/cm2、積算照射量1000/mJ/cm2(波長380~440nm)を使用し、紫外線の照度は、Solatell社製のSola-Checkシステムを使用して測定した。次いで、光学フィルム積層体の非晶性PET基材を剥離して、片保護偏光フィルムを得た。 Comparative Example 4 (Production of a piece protective polarizing film)
On the polarizer (first surface) of the polarizer A0 (optical film laminate including a polarizer having a thickness of 5 μm) obtained above, the thickness of the adhesive layer after curing the ultraviolet curable adhesive becomes 1 μm. Then, the protective film (acrylic) was applied, and ultraviolet rays were applied as active energy rays to cure the adhesive. Ultraviolet irradiation is performed using a gallium-encapsulated metal halide lamp, an irradiation device: Fusion UV Systems, Inc. Light HAMMER 10, Inc., bulb: V bulb, peak illuminance: 1600 mW / cm 2 , integrated irradiation amount 1000 / mJ / cm 2 (wavelength 380 to 440 nm) ), And the illuminance of ultraviolet rays was measured using a Sola-Check system manufactured by Solatell. Subsequently, the amorphous PET base material of the optical film laminate was peeled off to obtain a piece protective polarizing film.
得られた偏光膜の単体透過率Tおよび偏光度Pを、積分球付き分光透過率測定器(村上色彩技術研究所のDot-3c)を用いて測定した。
なお、偏光度Pは、2枚の同じ偏光フィルムを両者の透過軸が平行となるように重ね合わせた場合の透過率(平行透過率:Tp)および、両者の透過軸が直交するように重ね合わせた場合の透過率(直交透過率:Tc)を以下の式に適用することにより求められるものである。偏光度P(%)={(Tp-Tc)/(Tp+Tc)}1/2×100
各透過率は、グランテラープリズムを通して得られた完全偏光を100%として、JIS Z8701の2度視野(C光源)により視感度補整したY値で示したものである。 <Single transmittance T and polarization degree P of polarizer>
The single transmittance T and the polarization degree P of the obtained polarizing film were measured using a spectral transmittance measuring device with an integrating sphere (Dot-3c of Murakami Color Research Laboratory).
The degree of polarization P is the transmittance when two identical polarizing films are overlapped so that their transmission axes are parallel (parallel transmittance: Tp), and overlapped so that their transmission axes are orthogonal to each other. It is calculated | required by applying the transmittance | permeability (orthogonal transmittance | permeability: Tc) at the time of combining to the following formula | equation. Polarization degree P (%) = {(Tp−Tc) / (Tp + Tc)} 1/2 × 100
Each transmittance is indicated by a Y value obtained by correcting visibility with a two-degree field of view (C light source) of JIS Z8701 with 100% of the completely polarized light obtained through the Granteller prism.
圧縮弾性率の測定には、TI900 TriboIndenter(Hysitron社製)を使用した。得られた補強膜付き偏光子(偏光膜)を10mm×10mmのサイズに裁断しTriboIndenter備付の支持体に固定し、ナノインデンテーション法により圧縮弾性率の測定を行った。その際、使用圧子が透明層の中心部付近を押し込むように位置を調整した。測定条件を以下に示す。
使用圧子:Berkovich(三角錐型)
測定方法:単一押し込み測定
測定温度:23℃
押し込み深さ設定:100nm <Measurement of compression modulus>
TI900 TriboIndenter (manufactured by Hystron) was used for the measurement of compression modulus. The obtained polarizer with a reinforcing film (polarizing film) was cut into a size of 10 mm × 10 mm, fixed to a support equipped with a TriboIndenter, and the compression modulus was measured by a nanoindentation method. At that time, the position was adjusted so that the used indenter pushed in the vicinity of the center of the transparent layer. The measurement conditions are shown below.
Working indenter: Berkovich (triangular pyramid type)
Measuring method: Single indentation measurement Measuring temperature: 23 ° C
Indentation depth setting: 100 nm
ユアサシステム機器社製の面状体無負荷捻回試験機(製品名:本体TCDM111LH及び治具:面状体無負荷捻回試験治具)を用いて行った。
100mm(吸収軸方向)×150mm(透過軸方向)の矩形物1(サンプル)の両短辺を、前記試験機の捻回用クリップ11、12で挟み固定した後、一方の短辺はクリップ12で固定したまま、もう一方の短辺側のクリップ11を下記条件で捻回した。捻回の状態を図2に示す。
捻回速度:10rpm
捻回角度:45度
捻回回数:100回
捻回試験後のサンプルの状態を、目視により下記基準で評価した。
〇:割れおよび光抜けは発生しなかった。かつ、折れ跡の残りはなかった。
△:割れおよび光抜けは発生しなかった。しかし折れ跡が残っていた
×:割れおよび光抜けが発生した。かつ、折れ跡が残っていた。 <Torsion test>
It was performed using a planar body no-load twist tester (product name: main body TCDM111LH and jig: planar body no-load twist test jig) manufactured by Yuasa System Equipment Co., Ltd.
After sandwiching and fixing both short sides of the rectangular object 1 (sample) of 100 mm (absorption axis direction) × 150 mm (transmission axis direction) with the twisting clips 11 and 12 of the tester, one short side is the
Twist speed: 10rpm
Twist angle: 45 degrees Twist number: 100 times The state of the sample after the twist test was visually evaluated according to the following criteria.
◯: No cracking or light leakage occurred. And there were no creases left.
Δ: No cracking or light leakage occurred. However, fold marks remained. X: Cracks and light leakage occurred. And there were creases left.
ユアサシステム機器社製の面状体無負荷U字伸縮試験機(製品名:本体DLDM111LH及び治具:面状体無負荷U字伸縮試験治具)を用いて行った。
50mm(吸収軸方向)×100mm(透過軸方向)の矩形物1(サンプル)の両端部x、y(50mm)を、前記試験機の支持部21、22に両面テープ(図示せず)で固定した後、前記矩形物1の片面側(第1面)が内側にU字状になるような伸縮を下記条件で行い、前記矩形物を折り曲げた(透過軸方向の第1面側の折り曲げ)。U字伸縮の一過程の状態を図3に示す。U字伸縮では、折り曲げR(曲げ半径)が0になるように設定し、平面の状態から、矩形物1(サンプル)が二つ折り状態で接触するまで折り曲げた。前記折り曲げは、両端部x、yを支持部の作動により両端部x、yの接触を行うとともに、矩形物1(サンプル)の他の部分は別途設置されている板部23、24により両外側から無負荷で挟み込むようにして接触させた。
また、前記伸縮による折り曲げは、前記矩形物の他の片面側(第2面)についても内側にU字状になるような伸縮を前記同様に行った(透過軸方向の第2面側の折り曲げ)。
さらに、前記伸縮による折り曲げは、50mm(透過軸方向)×100mm(吸収軸方向)の矩形物(サンプル)についても、第1面および第2面が内側にU字状になるような伸縮を前記同様に行った(吸収軸方向の第1面側および第2面側の折り曲げ)。
伸縮速度 :30rpm
折り曲げR:0
伸縮回数 :100回
U字伸縮試験のサンプルの状態を、目視により下記基準で評価した。
〇:前記透過軸方向の第1面側および第2面側の折り曲げ、吸収軸方向の第1面側および第2面側の折り曲げの、いずれの折り曲げにおいても、割れおよび光抜けは発生しなかった。かつ、折れ跡の残りはなかった。
×:前記透過軸方向の第1面側および第2面側の折り曲げ、吸収軸方向の第1面側および第2面側の折り曲げの、いずれかの折り曲げにおいて、割れもしくは光抜けが発生した。または、折れ跡が確認された。 <U-shaped stretch test>
It was carried out using a planar body unloaded U-shaped expansion tester (product name: main body DLDM111LH and jig: planar body unloaded U-shaped expansion test jig) manufactured by Yuasa System Equipment Co., Ltd.
Both ends x and y (50 mm) of a rectangular object 1 (sample) of 50 mm (absorption axis direction) × 100 mm (transmission axis direction) are fixed to the
Further, the bending by the expansion and contraction was performed in the same manner as described above so that the other one side (second surface) of the rectangular object was inwardly U-shaped (bending on the second surface side in the transmission axis direction). ).
Further, the bending by the expansion and contraction is performed so that the first surface and the second surface are inwardly U-shaped with respect to a rectangular object (sample) of 50 mm (transmission axis direction) × 100 mm (absorption axis direction). The same was done (bending on the first surface side and the second surface side in the absorption axis direction).
Telescopic speed: 30rpm
Bending R: 0
Number of expansions / contractions: 100 times The state of the sample of the U-shaped expansion / contraction test was visually evaluated according to the following criteria.
◯: No cracking or light leakage occurred in any of the bending on the first surface side and the second surface side in the transmission axis direction and the bending on the first surface side and the second surface side in the absorption axis direction. It was. And there were no creases left.
X: Cracking or light leakage occurred in any of the bending of the first surface side and the second surface side in the transmission axis direction and the bending of the first surface side and the second surface side in the absorption axis direction. Or a crease was confirmed.
“割れ”は、サンプル(フレキシブル偏光膜、補強膜を有する偏光膜、または片保護偏光フィルムを構成する全層又はその一部の層)に貫通して、裂けまたはクラックが発生していることを示す。
“折れ跡”は、サンプル(フレキシブル偏光膜、補強膜を有する偏光膜、または片保護偏光フィルムを構成する全層又はその一部の層)に、折り曲げや局所的な負荷により、負荷除去後も残留する視認可能な跡が発生していることを示す。
“光抜け”は、偏光子自体に裂けまたはクラックが発生していることを示す。光抜けの確認は、試験後のサンプル(フレキシブル偏光膜、補強膜を有する偏光膜、または片保護偏光フィルムを構成する全層)と他の通常の偏光フィルムを、クロスニコルの状態でガラスの両面に貼り合せ、バックライト等の光を透かすことで行った。 Criteria for cracks, creases and light leakage in the twist test and U-shaped stretch test are as follows.
“Crack” means that cracks or cracks have occurred through the sample (a flexible polarizing film, a polarizing film having a reinforcing film, or all or part of a layer constituting a single protective polarizing film). Show.
“Fold marks” are applied to the sample (flexible polarizing film, polarizing film having a reinforcing film, or all or part of a layer constituting a single protective polarizing film) even after the load is removed by bending or local load. It shows that the remaining visible trace has occurred.
“Light loss” indicates that the polarizer itself is torn or cracked. Confirmation of light leakage is performed by using a sample after testing (flexible polarizing film, polarizing film having a reinforcing film, or all layers constituting a piece of protective polarizing film) and other normal polarizing films on both sides of the glass in a crossed Nicol state. It was carried out by pasting light such as a backlight.
200mm(吸収軸方向)×300mm(透過軸方向)の矩形物1(サンプル)を水平な台31に設置した。次いで、前記矩形物1を透過軸方向(長軸方向)に3つ折りとした後、最上部の全面に荷重がかかるように100gの荷重32を掛けて、5分間放置した。折り曲げの状態を図4に示す。その後に、前記荷重32を除いた。
また、200mm(透過軸方向)×300mm(吸収軸方向)の矩形物(サンプル)についても、前記同様の操作(但し、サンプルは吸収軸方向(長軸方向)に3つ折りとした)を行った。
折り曲げ保持試験後にサンプルが3つ折り状態を保持しているかを目視で確認すると共に、3つ折り形状が維持されていた場合には、サンプルを元の状態(平面)の状態に戻して、目視により下記基準で評価した。評価は透過軸方向長辺および吸収軸方向長辺のサンプルについてそれぞれ3回行った。
〇:いずれのサンプルでも3つ折り形状が保持されると共に割れはなく元の状態が維持された(保持性有り)。
×:いずれかのサンプルで3つ折り形状が保持されなかった。または、3つ折り形状は保持されていたが、荷重時に割れが生じた(保持性なし)。
なお、“割れ”の判断は上記捻回試験、U字伸縮試験と同じである。 <Bending test>
A rectangular object 1 (sample) of 200 mm (absorption axis direction) × 300 mm (transmission axis direction) was placed on a
In addition, the same operation (however, the sample was folded in three in the absorption axis direction (major axis direction)) was performed on a rectangular object (sample) of 200 mm (transmission axis direction) × 300 mm (absorption axis direction). .
After the bending holding test, it is visually confirmed whether the sample is held in a tri-folded state, and when the tri-folded shape is maintained, the sample is returned to the original state (planar) and visually checked as follows. Evaluated by criteria. The evaluation was performed three times for each sample of the long side in the transmission axis direction and the long side in the absorption axis direction.
◯: In any sample, the tri-fold shape was maintained and the original state was maintained without cracking (with retaining property).
X: Trifold shape was not hold | maintained by any sample. Alternatively, the tri-fold shape was retained, but cracking occurred during loading (no retainability).
The determination of “cracking” is the same as in the above twist test and U-shaped stretch test.
安田精機製作所製のNo.476のカンチレバー型柔軟度試験機を用いた。また、本試験では静電気の影響を排除するため、試験に用いるサンプル等を適切に除電して行った。
50mm(吸収軸方向)×150mm(透過軸方向)の矩形物1(サンプル)を、頂部が平面(50mm×150mm:サンプルと同じサイズ)で、長辺の一端に45°の斜面を持ち、断面が台形の滑らかなSUS板台41の頂面に、収まるように設置した(図5参照)。サンプルの設置は透過軸方向に斜面があるように行った。
前記サンプルを、押し出し速度10mm/secで、斜面側に静かに滑らせ移動させた(1)。サンプルの先端が斜面に初めて接した箇所でサンプルの移動を止めた(2)。頂部が平面においてサンプルが移動した距離L(mm)を測定した。
50mm(透過軸方向)×150mm(吸収軸方向)の矩形物(フレキシブル偏光膜のサンプル)について、前記同様の操作(但し、サンプルの設置は吸収軸方向に斜面があるようにした)を行った。
剛軟度(mm)は、透過軸方向長辺および吸収軸方向長辺のサンプルについてそれぞれ第1面を上側の場合および第2面を上側とした場合の2パターンについてそれぞれ3回、最短直線距離L(mm)を測定(合計12サンプル)し、それらの算術平均値とした。
また12サンプルのいずれか1回以上の測定において、サンプルの変形やカールにより測定不可能なサンプルがあった場合には、そのサンプルは測定不可と判定とした。 <Flexibility test>
No. made by Yasuda Seiki Seisakusho. A 476 cantilever type flexibility tester was used. Moreover, in this test, in order to eliminate the influence of static electricity, the sample used for the test was appropriately discharged.
A rectangular object 1 (sample) of 50 mm (absorption axis direction) x 150 mm (transmission axis direction), the top is flat (50 mm x 150 mm: the same size as the sample), and has a 45 ° slope at one end of the long side. Was placed on the top surface of the trapezoid smooth SUS plate base 41 (see FIG. 5). The sample was placed so that there was a slope in the direction of the transmission axis.
The sample was gently slid and moved to the slope side at an extrusion speed of 10 mm / sec (1). The sample stopped moving at the point where the tip of the sample first contacted the slope (2). The distance L (mm) by which the sample moved while the top was flat was measured.
For a rectangular object (flexible polarizing film sample) of 50 mm (transmission axis direction) × 150 mm (absorption axis direction), the same operation as described above (however, the sample was set to have a slope in the absorption axis direction) was performed. .
The bending resistance (mm) is the shortest linear distance for each of the two patterns with the first surface on the upper side and the second surface on the upper side for the samples with the transmission axis direction long side and the absorption axis direction long side, respectively. L (mm) was measured (12 samples in total) and used as the arithmetic average value thereof.
Further, in any one or more measurements of 12 samples, when there was a sample that could not be measured due to deformation or curl of the sample, it was determined that the sample was not measurable.
多目的試験片裁断機(ダンベル)にて、10mm(吸収軸方向)×150mm(透過軸方向)の所定形状に裁断してサンプル(フレキシブル偏光膜のサンプル)を作成した。
前記サンプルについて、島津製作所製のオートグラフAG‐ISを使用して、引張速度:50mm/minで、引張試験を実施した。
前記サンプルは、透過軸方向の両辺の側をクランプ51、52に固定し(初期クランプ間の距離53は50mm)、一方のクランプ52側を引張ることにより行った(図6参照)。サンプルが破断または塑性変形を開始した点を“引張強度”として評価した。
引張強度は3回測定した結果の算術平均値とした。 <Tensile test>
A sample (flexible polarizing film sample) was prepared by cutting into a predetermined shape of 10 mm (absorption axis direction) × 150 mm (transmission axis direction) with a multipurpose test piece cutter (dumbbell).
About the said sample, the tension test was implemented by autograph AG-IS by Shimadzu Corporation at the tension | pulling speed: 50 mm / min.
The sample was obtained by fixing both sides in the transmission axis direction to the
The tensile strength was an arithmetic average value obtained by measuring three times.
a 透明保護フィルム
b1 第1補強膜
b2 第2補強膜 DESCRIPTION OF
Claims (17)
- ポリビニルアルコール系樹脂が一方向に配向し、かつ前記ポリビニルアルコール系樹脂にヨウ素又は二色性色素が吸着配向してなる厚み10μm以下のポリビニルアルコール系偏光子を有し、かつ、前記ポリビニルアルコール系樹脂が配向する方向に捻回させる捻回試験を施した後において、割れ、折れ跡及び光抜けがないことを特徴とするフレキシブル偏光膜。 The polyvinyl alcohol resin has a polyvinyl alcohol polarizer having a thickness of 10 μm or less, in which the polyvinyl alcohol resin is oriented in one direction and iodine or a dichroic dye is adsorbed and oriented on the polyvinyl alcohol resin, and the polyvinyl alcohol resin. A flexible polarizing film characterized by being free from cracks, folds and light leakage after being subjected to a twisting test in which the film is twisted in the direction in which it is oriented.
- 前記ポリビニルアルコール系樹脂が配向する配向方向および前記配向方向に対して直交する方向にU字状に伸縮を繰り返すU字伸縮試験を施した後において、いずれの方向にも割れ、折れ跡及び光抜けがないことを特徴とする請求項1記載のフレキシブル偏光膜。 After conducting a U-shaped expansion / contraction test in which the polyvinyl alcohol-based resin is repeatedly aligned and expanded in a U-shape in a direction perpendicular to the alignment direction, cracks, fold marks and light leakage are observed in either direction. The flexible polarizing film according to claim 1, wherein the flexible polarizing film is not present.
- 前記ポリビニルアルコール系樹脂が配向する配向方向および前記配向方向に対して直交する方向に折り曲げて保持する折り曲げ保持試験を施した後において、いずれの方向にも折り曲げ形状が保持されると共に、割れが生じないことを特徴とする請求項1または2記載のフレキシブル偏光膜。 After performing a bending holding test in which the polyvinyl alcohol-based resin is bent and held in a direction perpendicular to the alignment direction, the bent shape is maintained in both directions and cracks are generated. The flexible polarizing film according to claim 1, wherein the flexible polarizing film is not present.
- 前記ポリビニルアルコール系樹脂が配向する配向方向および前記配向方向に対して直交する方向への剛軟性試験において、剛軟度(mm)が60mm以下であることを特徴とする1~3のいずれかに記載のフレキシブル偏光膜。 In any one of 1 to 3, wherein the bending resistance (mm) is 60 mm or less in a bending test in an alignment direction in which the polyvinyl alcohol-based resin is aligned and in a direction orthogonal to the alignment direction. The flexible polarizing film as described.
- 引張試験において、前記ポリビニルアルコール系樹脂が配向する配向方向に対して直交する方向の引張強度が5N/10mm以上であることを特徴とする1~4のいずれかに記載のフレキシブル偏光膜。 5. The flexible polarizing film according to any one of 1 to 4, wherein, in a tensile test, the tensile strength in a direction orthogonal to the orientation direction in which the polyvinyl alcohol-based resin is oriented is 5 N / 10 mm or more.
- 前記ポリビニルアルコール系偏光子は、単体透過率T及び偏光度Pによって表される光学特性が、下記式
P>-(100.929T-42.4-1)×100(ただし、T<42.3)、又は、
P≧99.9(ただし、T≧42.3)の条件を満足するように構成されたものであることを特徴とする請求項1~5のいずれかに記載のフレキシブル偏光膜。 The polyvinyl alcohol-based polarizer has an optical characteristic expressed by a single transmittance T and a polarization degree P of the following formula: P> − (10 0.929T-42.4 −1) × 100 (where T <42. 3) or
6. The flexible polarizing film according to claim 1, wherein the flexible polarizing film is configured to satisfy a condition of P ≧ 99.9 (however, T ≧ 42.3). - 前記ポリビニルアルコール系偏光子の少なくとも片面に、前記ポリビニルアルコール系偏光子に密着した補強膜を有することを特徴とする請求項1~6のいずれかに記載のフレキシブル偏光膜。 The flexible polarizing film according to any one of claims 1 to 6, further comprising a reinforcing film in close contact with the polyvinyl alcohol polarizer on at least one surface of the polyvinyl alcohol polarizer.
- 前記補強膜の厚みが15μm以下であることを特徴とする請求項7記載のフレキシブル偏光膜。 The flexible polarizing film according to claim 7, wherein the reinforcing film has a thickness of 15 μm or less.
- 前記ポリビニルアルコール系偏光子の第1の片面に厚み15μm以下の第1補強膜を有し、他方の第2の片面に厚み15μm以下の第2補強膜を有することを特徴とする請求項8記載のフレキシブル偏光膜。 The first reinforcing film having a thickness of 15 μm or less is provided on a first surface of the polyvinyl alcohol polarizer, and a second reinforcing film having a thickness of 15 μm or less is provided on the other second surface. Flexible polarizing film.
- 前記第1補強膜と第2補強膜の厚み差が10μm以下であることを特徴とする請求項9記載のフレキシブル偏光膜。 The flexible polarizing film according to claim 9, wherein a thickness difference between the first reinforcing film and the second reinforcing film is 10 μm or less.
- 前記ポリビニルアルコール系偏光子の厚みに対する、補強膜の厚みの比は0.4以上であることを特徴とする請求項7~10のいずれかに記載のフレキシブル偏光膜。 The flexible polarizing film according to any one of claims 7 to 10, wherein the ratio of the thickness of the reinforcing film to the thickness of the polyvinyl alcohol-based polarizer is 0.4 or more.
- 前記補強膜は、23℃における圧縮弾性率が1MPa以上であることを特徴とする請求項7~11のいずれかに記載のフレキシブル偏光膜。 12. The flexible polarizing film according to claim 7, wherein the reinforcing film has a compressive elastic modulus at 23 ° C. of 1 MPa or more.
- 前記補強膜は、実質的に配向していないことを特徴とする請求項7~12のいずれかに記載のフレキシブル偏光膜。 The flexible polarizing film according to any one of claims 7 to 12, wherein the reinforcing film is not substantially oriented.
- 前記補強膜が、樹脂膜であることを特徴とする請求項7~13のいずれかに記載のフレキシブル偏光膜。 The flexible polarizing film according to any one of claims 7 to 13, wherein the reinforcing film is a resin film.
- 前記樹脂膜は、熱硬化型樹脂または活性エネルギー線硬化型樹脂の形成物であることを特徴とする請求項14記載のフレキシブル偏光膜。 15. The flexible polarizing film according to claim 14, wherein the resin film is a thermosetting resin or an active energy ray curable resin.
- 請求項14または15記載のフレキシブル偏光膜の製造方法であって、
ポリビニルアルコール系樹脂が一方向に配向し、かつ前記ポリビニルアルコール系樹脂にヨウ素又は二色性色素が吸着配向してなる厚み10μm以下のポリビニルアルコール系偏光子を準備する工程(1)、
前記ポリビニルアルコール系偏光子の少なくとも片面に、樹脂成分または樹脂膜を構成することができる硬化性成分を含む液状物を塗工し、その後に、当該液状物を固化または硬化することにより、補強膜を形成する工程(2)を含むことを特徴とするフレキシブル偏光膜の製造方法。 It is a manufacturing method of the flexible polarizing film according to claim 14 or 15,
A step (1) of preparing a polyvinyl alcohol polarizer having a thickness of 10 μm or less, wherein the polyvinyl alcohol resin is oriented in one direction and iodine or a dichroic dye is adsorbed and oriented on the polyvinyl alcohol resin;
By applying a liquid material containing a curable component capable of constituting a resin component or a resin film on at least one surface of the polyvinyl alcohol polarizer, and then solidifying or curing the liquid material, a reinforcing film The manufacturing method of the flexible polarizing film characterized by including the process (2) of forming. - 請求項1~15のいずれかに記載のフレキシブル偏光膜を有する画像表示装置。 An image display device comprising the flexible polarizing film according to any one of claims 1 to 15.
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020187028928A KR102219925B1 (en) | 2016-03-29 | 2017-03-28 | Flexible polarizing film, manufacturing method thereof, and image display device |
| CN201780020448.1A CN108885295A (en) | 2016-03-29 | 2017-03-28 | Flexible polarizing coating, its manufacturing method and image display device |
| JP2018508051A JPWO2017170516A1 (en) | 2016-03-29 | 2017-03-28 | Flexible polarizing film, manufacturing method thereof, and image display device |
| TW106110465A TWI722157B (en) | 2016-03-29 | 2017-03-29 | Flexible polarizing film, its manufacturing method and image display device |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-066377 | 2016-03-29 | ||
| JP2016066377 | 2016-03-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017170516A1 true WO2017170516A1 (en) | 2017-10-05 |
Family
ID=59964608
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/012588 WO2017170516A1 (en) | 2016-03-29 | 2017-03-28 | Flexible polarizing film, manufacturing method for same and image display device |
Country Status (5)
| Country | Link |
|---|---|
| JP (2) | JPWO2017170516A1 (en) |
| KR (1) | KR102219925B1 (en) |
| CN (1) | CN108885295A (en) |
| TW (1) | TWI722157B (en) |
| WO (1) | WO2017170516A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2017170525A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
| JPWO2017170522A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
| JPWO2017170527A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
| EP3858931A4 (en) * | 2018-09-26 | 2022-06-15 | Mitsui Chemicals, Inc. | Functional layer including adhesive layer, laminate, and use thereof |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11183726A (en) * | 1997-12-18 | 1999-07-09 | Nippon Synthetic Chem Ind Co Ltd:The | Polarizing film and polarizing plate using the same |
| JP2000249833A (en) * | 1999-03-01 | 2000-09-14 | Nitto Denko Corp | Polarizing film, optical member and liquid crystal display device |
| JP2006291173A (en) * | 2005-03-16 | 2006-10-26 | Nippon Synthetic Chem Ind Co Ltd:The | Polyvinyl alcohol-based film and manufacturing method thereof |
| JP2007009056A (en) * | 2005-06-30 | 2007-01-18 | Nippon Synthetic Chem Ind Co Ltd:The | Polyvinyl alcohol film for optical film, its manufacturing method and polarizing film and polarizing plate obtained using the same |
| JP2013125077A (en) * | 2011-12-13 | 2013-06-24 | Nitto Denko Corp | Adhesive for polarizing plate, polarizing plate, manufacturing method of the same, optical film, and image display device |
| JP2016033215A (en) * | 2013-06-21 | 2016-03-10 | 日東電工株式会社 | Adhesive layer with separator and method for producing the same, polarization film having adhesive layer with separator and method for producing the same, and image display device |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4306269B2 (en) * | 2003-02-12 | 2009-07-29 | 住友化学株式会社 | Polarizing plate, manufacturing method thereof, optical member, and liquid crystal display device |
| KR100632510B1 (en) | 2004-04-30 | 2006-10-09 | 엘지전자 주식회사 | Wire grid polarizer and its manufacturing method |
| JP2007232792A (en) | 2006-02-27 | 2007-09-13 | Nippon Zeon Co Ltd | Method of manufacturing grid polarizing film |
| JP2010133987A (en) * | 2007-03-12 | 2010-06-17 | Toagosei Co Ltd | Optical film laminate and display device using the same |
| JP2010107969A (en) * | 2008-10-02 | 2010-05-13 | Dainippon Printing Co Ltd | Optical film for protecting polarizer, polarizing plate and image display device |
| JP4691205B1 (en) * | 2010-09-03 | 2011-06-01 | 日東電工株式会社 | Method for producing optical film laminate including thin high-performance polarizing film |
| JP5983098B2 (en) * | 2012-06-29 | 2016-08-31 | 東亞合成株式会社 | Polarizer |
| JP2014206725A (en) * | 2013-03-19 | 2014-10-30 | 富士フイルム株式会社 | Polarizing plate and liquid crystal display device |
| WO2014175040A1 (en) * | 2013-04-26 | 2014-10-30 | コニカミノルタ株式会社 | Polarizing plate, method for producing same and liquid crystal display device |
| JP6378525B2 (en) * | 2014-04-17 | 2018-08-22 | 日東電工株式会社 | Organic electroluminescence display device |
| JPWO2017170527A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
| JPWO2017170522A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
| JPWO2017170525A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
-
2017
- 2017-03-28 WO PCT/JP2017/012588 patent/WO2017170516A1/en active Application Filing
- 2017-03-28 KR KR1020187028928A patent/KR102219925B1/en active IP Right Grant
- 2017-03-28 CN CN201780020448.1A patent/CN108885295A/en active Pending
- 2017-03-28 JP JP2018508051A patent/JPWO2017170516A1/en active Pending
- 2017-03-29 TW TW106110465A patent/TWI722157B/en not_active IP Right Cessation
-
2020
- 2020-06-08 JP JP2020099412A patent/JP2020160460A/en not_active Withdrawn
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11183726A (en) * | 1997-12-18 | 1999-07-09 | Nippon Synthetic Chem Ind Co Ltd:The | Polarizing film and polarizing plate using the same |
| JP2000249833A (en) * | 1999-03-01 | 2000-09-14 | Nitto Denko Corp | Polarizing film, optical member and liquid crystal display device |
| JP2006291173A (en) * | 2005-03-16 | 2006-10-26 | Nippon Synthetic Chem Ind Co Ltd:The | Polyvinyl alcohol-based film and manufacturing method thereof |
| JP2007009056A (en) * | 2005-06-30 | 2007-01-18 | Nippon Synthetic Chem Ind Co Ltd:The | Polyvinyl alcohol film for optical film, its manufacturing method and polarizing film and polarizing plate obtained using the same |
| JP2013125077A (en) * | 2011-12-13 | 2013-06-24 | Nitto Denko Corp | Adhesive for polarizing plate, polarizing plate, manufacturing method of the same, optical film, and image display device |
| JP2016033215A (en) * | 2013-06-21 | 2016-03-10 | 日東電工株式会社 | Adhesive layer with separator and method for producing the same, polarization film having adhesive layer with separator and method for producing the same, and image display device |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2017170525A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
| JPWO2017170522A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
| JPWO2017170527A1 (en) * | 2016-03-29 | 2018-10-18 | 日東電工株式会社 | Flexible polarizing film, manufacturing method thereof, and image display device |
| EP3858931A4 (en) * | 2018-09-26 | 2022-06-15 | Mitsui Chemicals, Inc. | Functional layer including adhesive layer, laminate, and use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| TWI722157B (en) | 2021-03-21 |
| KR102219925B1 (en) | 2021-02-25 |
| KR20180120744A (en) | 2018-11-06 |
| CN108885295A (en) | 2018-11-23 |
| JP2020160460A (en) | 2020-10-01 |
| JPWO2017170516A1 (en) | 2018-10-18 |
| TW201738092A (en) | 2017-11-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2017170527A1 (en) | Flexible polarizing film, manufacturing method for same and image display device | |
| JP6077619B2 (en) | Single protective polarizing film, polarizing film with pressure-sensitive adhesive layer, image display device, and continuous production method thereof | |
| JP6077618B2 (en) | Single protective polarizing film, polarizing film with pressure-sensitive adhesive layer, image display device, and continuous production method thereof | |
| WO2017170522A1 (en) | Flexible polarizing film, manufacturing method for same and image display device | |
| KR102567394B1 (en) | One-side-protected polarizing film, adhesive-layer-equipped polarizing film, image display device, and method for continuously producing same | |
| WO2017170525A1 (en) | Flexible polarizing film, manufacturing method for same and image display device | |
| JP2020160460A (en) | Flexible polarizing film, manufacturing method therefor, and image display device | |
| WO2016052549A1 (en) | One-side-protected polarizing film, adhesive-layer-equipped polarizing film, image display device, and method for continuously producing same | |
| US20140315019A1 (en) | Adhesive film having a phase difference, method for manufacturing same, and optical member including same | |
| WO2016052531A1 (en) | One-side-protected polarizing film, adhesive-layer-equipped polarizing film, image display device, and method for continuously producing same | |
| JP7178776B2 (en) | Method for manufacturing polarizing film | |
| JP2017045023A (en) | Production method of polarizing film | |
| TWI683142B (en) | Single-sided protective polarizing film, polarizing film with adhesive layer, image display device and continuous manufacturing method thereof | |
| JP6931518B2 (en) | Single-sided protective polarizing film, polarizing film with adhesive layer, image display device and its continuous manufacturing method | |
| JP2019053210A (en) | Polarization film and picture display unit | |
| WO2016052534A1 (en) | Polarizing film protected on one side, polarizing film with pressure-sensitive adhesive layer, image display device, and continuous production process therefor | |
| CN114341682A (en) | Polarizing film laminate with adhesive layer, and optical display panel using same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ENP | Entry into the national phase |
Ref document number: 2018508051 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20187028928 Country of ref document: KR Kind code of ref document: A |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17775053 Country of ref document: EP Kind code of ref document: A1 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17775053 Country of ref document: EP Kind code of ref document: A1 |