WO2017053574A1 - Cannabinoid glycoside prodrugs and methods of synthesis - Google Patents
Cannabinoid glycoside prodrugs and methods of synthesis Download PDFInfo
- Publication number
- WO2017053574A1 WO2017053574A1 PCT/US2016/053122 US2016053122W WO2017053574A1 WO 2017053574 A1 WO2017053574 A1 WO 2017053574A1 US 2016053122 W US2016053122 W US 2016053122W WO 2017053574 A1 WO2017053574 A1 WO 2017053574A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cannabinoid
- ugt76g1
- glycoside
- udp
- glucosyltransferase
- Prior art date
Links
- 0 CCC(C(C)C(*1*2=C*2)C=*)C(*)C1*=C Chemical compound CCC(C(C)C(*1*2=C*2)C=*)C(*)C1*=C 0.000 description 15
- LPHNJQQKXSRZIG-MQWKRIRWSA-N CC(C)O[C@@](C)(C1)C1N=C Chemical compound CC(C)O[C@@](C)(C1)C1N=C LPHNJQQKXSRZIG-MQWKRIRWSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/549—Sugars, nucleosides, nucleotides or nucleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7012—Compounds having a free or esterified carboxyl group attached, directly or through a carbon chain, to a carbon atom of the saccharide radical, e.g. glucuronic acid, neuraminic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7016—Disaccharides, e.g. lactose, lactulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7028—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages
- A61K31/7034—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/10—Laxatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H15/00—Compounds containing hydrocarbon or substituted hydrocarbon radicals directly attached to hetero atoms of saccharide radicals
- C07H15/02—Acyclic radicals, not substituted by cyclic structures
- C07H15/04—Acyclic radicals, not substituted by cyclic structures attached to an oxygen atom of the saccharide radical
- C07H15/10—Acyclic radicals, not substituted by cyclic structures attached to an oxygen atom of the saccharide radical containing unsaturated carbon-to-carbon bonds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H15/00—Compounds containing hydrocarbon or substituted hydrocarbon radicals directly attached to hetero atoms of saccharide radicals
- C07H15/20—Carbocyclic rings
- C07H15/203—Monocyclic carbocyclic rings other than cyclohexane rings; Bicyclic carbocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
Definitions
- the present invention pertains to the field of drug development and in particular to novel cannabinoid glycoside prodrugs and methods for their production by enzyme-mediated carbohydrate transfer.
- CBD cannabidiol
- Cannabinoids are extremely hydrophobic in nature, complicating their use in drug formulations.
- Non-covalent methods have been found to improve the solubility of cannabinoids by utilizing carrier carbohydrates such as cyclized maltodextrins (Jarho 1998).
- Covalent chemical manipulations have produced novel CBD prodrugs with improved solubility (WO2009018389, WO 201201 1 1 12).
- Even fluorine substituted CBD compounds have been created through synthetic chemical manipulations in an effort to functionalize CBD (WO2014108899).
- the aforementioned strategies were somewhat successful in improving the solubility of CBD, but they create unnatural compositions which alter the composition and will release the unnatural prodrug moieties upon hydrolysis.
- glycosides are capable of acting as prodrugs and also to have direct therapeutic effects.
- Glycoside prodrugs may enable improved drug bioavailability or improved drug pharmacokinetics including more site-specific or tissue- specific drug delivery, more consistent levels of drug in the plasma, and sustained or delayed release of the drug.
- Site-specific delivery of steroid glycosides to the colon has previously been demonstrated (Friend 1985, Friend 1984), and could enable treatment of local disorders such as inflammatory bowel disease.
- Glycosylation of steroids enabled survival of stable bioactive molecules in the acidic stomach environment and delivery into the large intestine, where the aglycones were liberated by glycosidases produced by colonic bacteria, and then absorbed into the systemic circulation.
- glycosidesidases are also present universally in different tissues (Conchie 1959), so delivery of glycosides by methods that bypass the digestive tract and colon, such as intravenous delivery, will enable targeted delivery to other cells and tissues that have increased expression of glycosidases.
- delivery of alpha-glycosidase and beta- glycosidase enzymes differ throughout the intestinal tract and other tissues, and different forms of glycosides may therefore provide unique pharmacokinetic profiles, including formulations that target delivery of specific diseased areas, or targeted release at locations that can promote or restrict systemic absorption of the cannabinoids and other compounds described herein.
- glycosides including members of classes of compounds such as hormones, antibiotics, sweeteners, alkaloids, and flavonoids. While it is generally accepted that glycosides will be more water-soluble than the aglycones, literature reviews have analyzed structure-activity relationships and determined that it is nearly impossible to define a general pattern for the biological activities of glycosides across different classes of compounds (Kren 2008).
- CBD is glucuronidated in humans by the liver glucosyltransferases, but to date only minor activity has been demonstrated with UGT1 A9 and UGT2B7 in in vitro assays (US Patent No. 8,410,064).
- in vitro assays showed that cannabinol (CBN) is efficiently glucuronidated by the Human UGT1 A10 ( US Patent No. 8,410,064).
- CBN cannabinol
- the glucuronidation of CBD is one mechanism to increase CBD solubility and facilitate removal and excretion through the kidneys.
- cannabinol was found to be glycosylated when incubated with in vitro cell culture of Pinellia ternata (Tanaka 1993). Similarly, cannabidiol was shown to be glycosylated when incubated with tissue cultures from Pinellia ternata and Datura inoxia, yielding CBD-6'-0 ⁇ -D- glucopyranoside and CBD-(2',6')-0 ⁇ -D-diglucopyranoside (Tanaka 1996).
- Cannabinoids contain a hydroxylated hydrophobic backbone, similar to the steviol backbone of steviol glycosides found in the Stevia rebaudiana plant.
- UGT76G1 is a glucosyltransferase from Stevia that is capable of transferring a secondary glucose to the 3C- hydroxyl of the primary glycosylation on both C13-OH and C19-COOH position of the steviol glycoside, and thus its substrates include steviolmonoside, stevioside, rubusoside, RebA, RebD, RebG, RebE, etc. (Richman et al. 2005, Stevia First Corp unpublished work).
- the substrate recognition site of UGT76G1 is capable of binding and glycosylating multiple steviol glycosides, but it was previously not known to have glycosylation activity towards any other glycosides, and there previously was no established activity of UGT76G1 towards any aglycone compounds at all.
- UGT76G1 is capable of glycosylating steviol glycosides on the primary sugar located on both C13 hydroxyl group and the C19 carboxyl group it demonstrates bi- functional glycosylation.
- Cyclodextrin glucanotransferase (CGTase, Toruzyme 3.0L, Novozymes Inc.) is a member of the amylase family of enzymes and is best known for its ability to cyclize maltodextrin chains. A lesser known activity of CGTase is disproportionation of linear maltodextrin chains and transfer to an acceptor sugar molecule (Li 2012).
- cannabinoid glycosides available as cannabinoid prodrugs.
- screening of glucosyltransferase enzymes from various organisms has been conducted to identify candidates for the glycosylation of cannabinoids, and to identify cannabinoid glycosides as potential prodrugs of cannabinoids, and as novel cannabinoid compositions with novel properties and functions.
- the present invention relates to novel cannabinoid glycoside prodrugs and methods for their production by enzyme-mediated carbohydrate transfer.
- An object of the present invention is to provide a cannabinoid glycoside prodrug.
- a cannabinoid glycoside prodrug compound having formula (I
- R is H, ⁇ -D-glucopyranosyl, or 3-0 ⁇ -D-glucopyranosyl ⁇ -D-glucopyranosyl;
- R' is H or ⁇ -D-glucopyranosyl, or 3-0- ⁇ -D-glucopyranosyl ⁇ -D-glucopyranosyl;
- A is an aglycone moiety formed through reaction of a hydroxyl group on a cannabinoid compound, an endocannabinoid compound, or a vanilloid compound, or a pharmaceutically compatible salt thereof.
- a method for the site-specific delivery of a cannabinoid drug to a subject comprising the step of administering a cannabinoid glycoside prodrug in accordance with the present invention to a subject in need thereof.
- a method of producing a cannabinoid glycoside comprising incubating a cannabinoid aglycone with one or more sugar donors in the presence of one or more glycosyltransferases.
- Figure 1 A illustrates aglycones employed in the glycosylation methods of the present invention.
- Figure 1 B illustrates the possible points of glycosylation on the aglycones.
- Figure 2 illustrates possible products of the glycosylation of cannabidiol (CBD).
- FIG 3 illustrates possible products of the glycosylation of cannabidivarin (CBDV).
- Figure 4 illustrates possible rotational products of the glycosylation of cannabidiol (CBD).
- Figure 5 illustrates possible rotational products of the glycosylation of cannabidivarin (CBDV).
- Figure 6 illustrates the proposed superpositioning of the substrate cannabidiol (CBD) in the catalytic site of UGT76G1 .
- CBD cannabidiol
- Figure 7 illustrates possible products of the glycosylation of tetrahydrocannabinol ( ⁇ 9- THC).
- Figure 8 illustrates possible products of the glycosylation of cannabinol (CBN).
- Figure 9 illustrates possible products of the glycosylation of arachidonoyi ethanolamide
- Figure 10 illustrates possible products of the glycosylation of 2-arachidonoyl ethanolamide (2-AG).
- Figure 1 1 illustrates possible products of the glycosylation of 1 -arachidonoyi ethanolamide (1 -AG).
- Figure 12 illustrates possible products of the glycosylation of N- docosahexaenoylethanolamine (DHEA).
- DHEA docosahexaenoylethanolamine
- Figure 13 illustrates possible products of the glycosylation of capsaicin.
- Figure 14 illustrates possible products of the glycosylation of vanillin.
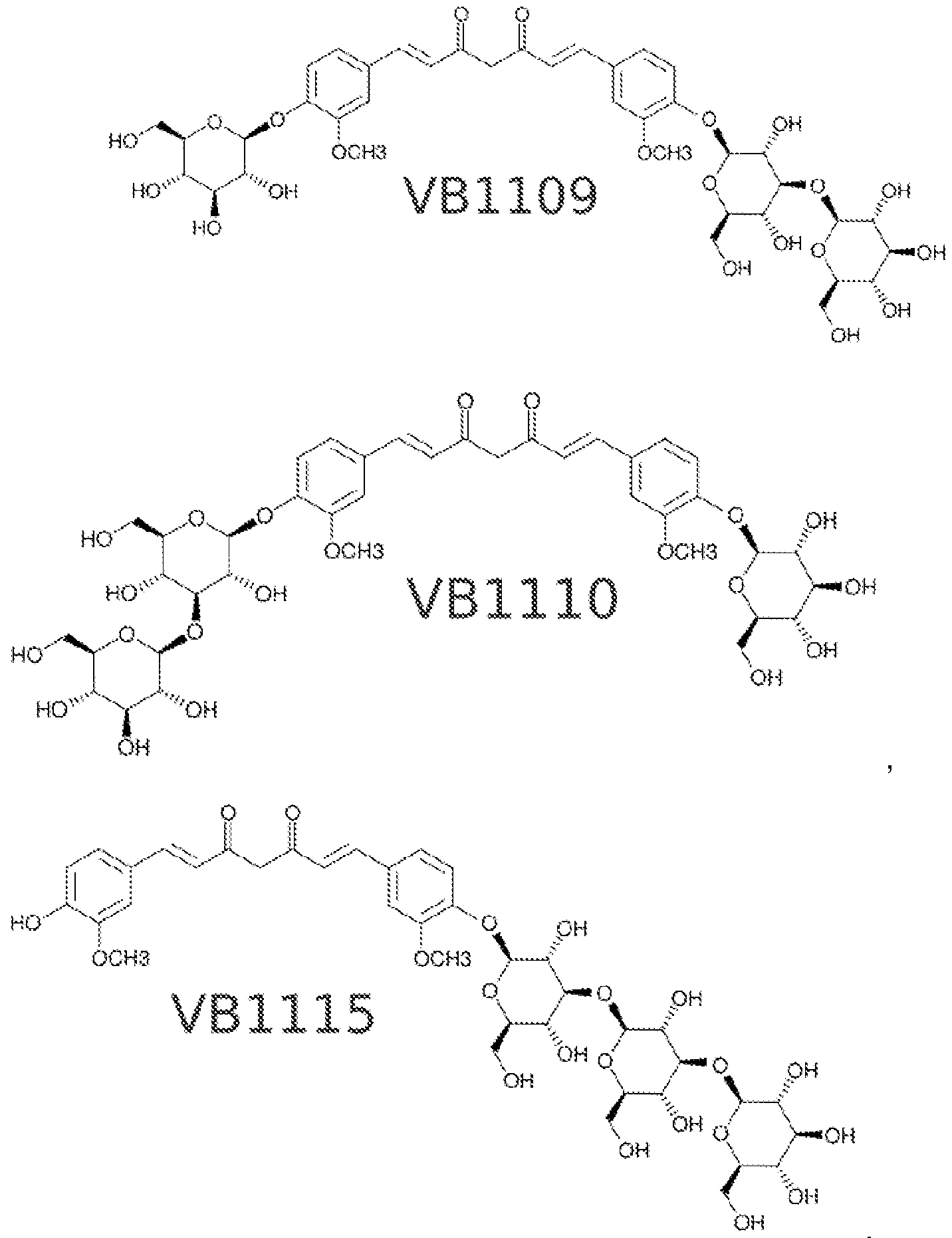
- Figures 15A and 15B illustrate possible products of the glycosylation of curcumin.
- Figure 16 is an HPLC linetrace of the reaction products of the glycosylation of CBD.
- Figure 17 is an HPLC linetrace of the reaction products of the glycosylation of CBDV.
- Figure 18 is an HPLC linetrace of the reaction products of the glycosylation of A9-THC.
- Figure 19 is an HPLC linetrace of the reaction products of the glycosylation of CBN.
- Figure 20 is an HPLC linetrace of the reaction products of the glycosylation of 1 -AG and 2-AG.
- Figure 21 is an HPLC linetrace of the reaction products of the glycosylation of synaptamide (DHEA).
- Figure 22 is an HPLC linetrace of the reaction products of the glycosylation of AEA.
- Figure 23 is an HPLC linetrace of the reaction products of the glycosylation of vanillin.
- Figure 24 is an HPLC linetrace of the reaction products of the glycosylation of capsaicin.
- Figure 25 is an HPLC linetrace of the reaction products of the glycosylation of CBDgl (VB104) with the glycosyltransf erase UGT76G1 .
- Figure 26 is an HPLC linetrace of the reaction products of the glycosylation of CBDgl (VB104) with the glycosyltransf erase Os03g0702000
- Figure 27 is a 1 NMR spectrum of an isolated product, VB104, of the glycosylation of CBD.
- Figure 28 is a 1 NMR spectrum of an isolated product, VB1 10 of the glycosylation of CBD.
- Figure 29 is a plot of C18 retention times vs cLogP values for selected cannabinoids and cannabinoid glycosides.
- Figure 30A is a graphical presentation of the results of the analysis of the small intestine extracts of a bioavailability assay.
- Figure 30B is a graphical presentation of the results of the analysis of the large intestine extracts of a bioavailability assay
- CBD Cannabidiol
- CBDV Cannabidivarin
- CBDA Cannabidiolic acid
- glucose is used for naming molecules and is shorthand for a ⁇ - D-glucose attached through the hydroxyl at the 1 -position (the anomeric carbon) of the glucose to the aglycone.
- glycoside compound is used in the present application to refer to the non-glycosidic portion of a glycoside compound.
- prodrug refers to a compound that, upon administration, must undergo a chemical conversion by metabolic processes before becoming an active pharmacological agent.
- cannabinoid glycoside prodrug refers generally to the glycosides of cannabinoid compounds, endocannabinoid compounds and vanilloid compounds.
- the cannabinoid glycoside prodrug undergoes hydrolysis of the glycosidic bond, typically by action of a glycosidase, to release the active cannabinoid, endocannabinoid or vanilloid compounds to a desired site in the body of the subject.
- the cannabinoid glycoside prodrug of the present invention may also be referred to using the term "cannaboside”.
- cannabinoid is used in the present application to refer generally to compounds found in cannabis and which act on cannabinoid receptors.
- Cannabinoid compounds include, but are not limited to, cannabidiol (CBD), cannabidivarin (CBDV), cannabigerol (CBG), tetrahydrocannabinol (A9-THC or THC), cannabinol (CBN), cannabidiolic acid (CBDA), and tetrahydrocannabivarin (THCV).
- CBD cannabidiol
- CBDV cannabidivarin
- CBG cannabigerol
- THC cannabinol
- CBDA9-THC or THC cannabinol
- CBDA9-THC or THC cannabinol
- CBDA9-THC or THC cannabinol
- CBDA9-THC or THC cannabinol
- endocannabinoid is used in the present application to refer to compounds including arachidonoyl ethanolamide (anandamide, AEA), 2-arachidonoyl ethanolamide (2-AG), 1 -arachidonoyl ethanolamide (1 -AG), and docosahexaenoyl ethanolamide (DHEA, synaptamide), oleoyl ethanolamide (OEA), eicsapentaenoyl ethanolamide, prostaglandin ethanolamide, docosahexaenoyl ethanolamide, linolenoyl ethanolamide, 5(Z),8(Z),1 1 (Z)- eicosatrienoic acid ethanolamide (mead acid ethanolamide), heptadecanoul ethanolamide, stearoyl ethanolamide, docosaenoyl ethanolamide, nervonoyl ethanolamide, tricosanoyl ethanolamide, lig
- vanilloid is used in the present application to refer to compounds comprising a vanillyl group and which act on vanilloid receptors like TRPV1 .
- vanilloid compounds include, but are not limited to, vanillin, capsaicin and curcumin.
- the term "about” refers to a +/-10% variation from the nominal value. It is to be understood that such a variation is always included in a given value provided herein, whether or not it is specifically referred to.
- subject or "patient” as used herein refers to an animal in need of treatment.
- the animal is a human.
- cannabinoids, endocannabinoids and vanilloids are employed as substrates for glucosyltransferases to which one or more sugar molecules are attached to create novel cannabinoid glycoside prodrugs.
- the resulting cannabinoid glycoside prodrugs demonstrate site-specific or tissue-specific delivery, improved aqueous solubility for improved pharmacological delivery, and/or sustained or delayed release of the cannabinoid, endocannabinoid and vanilloid drug molecules.
- the cannabinoid glycoside prodrugs are converted upon hydrolysis of the glycosidic bond to provide the active cannabinoid, endocannabinoid and vanilloid drug. Accordingly, the present invention has demonstrated that glycosides with a hydrophobic aglycone moiety undergo glucose hydrolysis in the gastrointestinal tract or in tissues having increased expression of glycosidases, yielding the hydrophobic cannabinoid compound in the targeted tissue or organ.
- glycosides are commonly acid-hydrolyzed in the stomach or cleaved by glycosidase enzymes in the intestinal tract, including by alpha-glycosidases and beta-glycosidases, which are expressed by intestinal microflora across different regions of the intestine. Accordingly, glycosides are hydrolyzed upon ingestion to release the desired compound into the intestines or target tissues.
- glycosylation of cannabinoid drugs provides cannabinoid glycoside prodrugs capable of persisting in the acidic stomach environment upon oral administration, thereby allowing delivery of the prodrug into the large intestine, where the cannabinoid aglycones can be liberated by glycosidases produced by colonic bacteria.
- glycosylation of cannabinoid drugs provides cannabinoid glycoside prodrugs suitable for targeted delivery to tissues having increased expression of glycosidases.
- the cannabinoid glycoside prodrug formulation Upon parenteral administration of the cannabinoid glycoside prodrug formulation to the subject, the cannabinoid aglycones are liberated by the glycosidases in the target tissues.
- the cannabinoid glycoside prodrug are also useful as pharmaceutical agents without glucose cleavage, where they exhibit novel pharmacodynamic properties compared to the parent compound alone.
- the increased aqueous solubility of the cannabinoid glycoside prodrugs of the present invention also enables new formulations for delivery in transdermal or aqueous formulations that would not have been achievable if formulating hydrophobic cannabinoid, endocannabinoid and vanilloid molecules.
- cannabinoid glycoside prodrug compounds having formula (I):
- R is H, ⁇ -D-glucopyranosyl, or 3-0- ⁇ - ⁇ - glucopyranosyl ⁇ -D-glucopyranosyl
- R' is H or ⁇ -D-glucopyranosyl, or 3-0 ⁇ -D-glucopyranosyl- ⁇ -D-glucopyranosyl
- A is an aglycone moiety formed through reaction of a hydroxyl group on a cannabinoid compound, an endocannabinoid compound, or a vanilloid compound.
- A is A', A" or A'";
- G is H, ⁇ -D-glucopyranosyl, 3-0 ⁇ -D-glucopyranosyl ⁇ -D-glucopyranosyl, or ⁇ -D- glucopyranosyl-(1 ⁇ 3) ⁇ -D-glucopyranosyl-(1 ⁇ 3)-D-glucopyranosyl; or a pharmaceutically compatible salt thereof.
- the cannabinoid glycoside prodrug is a glycoside of a cannabinoid, wherein the prodrug has the formula ( ⁇ ):
- R is H, ⁇ -D-glucopyranosyl, or 3-0- ⁇ -D-glucopyranosyl- ⁇ -D-glucopyranosyl;
- R' is H, ⁇ -D-glucopyranosyl, or 3-0- ⁇ -D-glucopyranosyl- ⁇ -D-glucopyranosyl;
- A' is:
- G is ⁇ -D-glucopyranosyl, 3-0 ⁇ -D-glucopyranosyl ⁇ -D-glucopyranosyl, or ⁇ -D- glucopyranosyl-(1 -3)- ⁇ -D-glucopyranosyl-(1 -3)-D-glucopyranosyl.
- Compounds of Formula ( ⁇ ) include the compounds listed in Tables 1 to 4.
- CBD-glycosides falling within the scope of Formula ( ⁇ ), produced by the glycosylation of CBD (VB101 ) in accordance with the present invention, include:
- CBDV cannabidivarin
- Exemplary tetrahydrocannabinol (A9-THC)-glycosides falling within the scope of Formula ( ⁇ ), produced by the glycosylation of ⁇ 9- ⁇ (VB301 ) in accordance with the present invention, include:
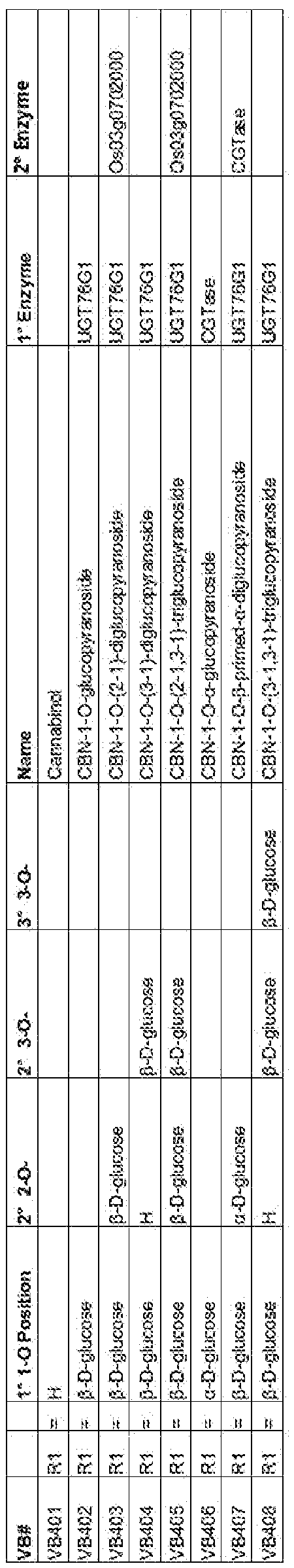
- Exemplary cannabinol (CBN)-glycosides falling within the scope of Formula ( ⁇ ), produced by the glycosylation of CBN (VB401 ) in accordance with the present invention, include:
- the cannabinoid glycoside prodrug is a glycoside of an endocannabinoid, the prodrug having the formula (I"):
- R is H, ⁇ -D-glucopyranosyl, or 3-0- ⁇ -D-glucopyranosyl- ⁇ -D-glucopyranosyl;
- R' is H, ⁇ -D-glucopyranosyl, or 3-0- ⁇ -D-glucopyranosyl- ⁇ -D-glucopyranosyl;
- Compounds of Formula (I") include the compounds listed in Tables 5 to 8.
- AEA-glycosides falling within the scope of Formula (I"), produced by the glycosylation of AEA (VB501 ) in accordance with the present invention, include:
- Exemplary 2-arachidonoyl ethanolamide (2-AG)-glycosides falling within the scope of Formula (I"), produced by the glycosylation of 2-AG (VB601 ) in accordance with the present invention, include:
- Exemplary 1 -arachidonoyi ethanolamide (l -AG)-glycosides falling within the scope of Formula (I"), produced by the glycosylation of 1 -AG (VB701 ) in accordance with the present invention, include:
- Exemplary N-docosahexaenoylethanolamine (DHEA)-glycosides falling within the scope of Formula (I"), produced by the glycosylation of DHEA (VB801 ) in accordance with the present invention, include:
- the cannabinoid glycoside prodrug is a glycoside of a vanilloid, the prodrug having the formula (!'"): wherein
- R is H, ⁇ -D-glucopyranosyl, or 3-0- ⁇ -D-glucopyranosyl- ⁇ -D-glucopyranosyl;
- R' is H or ⁇ -D-glucopyranosyl, or 3-0- ⁇ -D-glucopyranosyl- ⁇ -D-glucopyranosyl; and, wherein A'" is:
- Compounds of Formula (H) include the compounds listed in Tables 9 to 1 1 .
- Exemplary capsaicin-glycosides falling within the scope of Formula (H), produced by the glycosylation of capsaicin (VB901 ) in accordance with the present invention include:
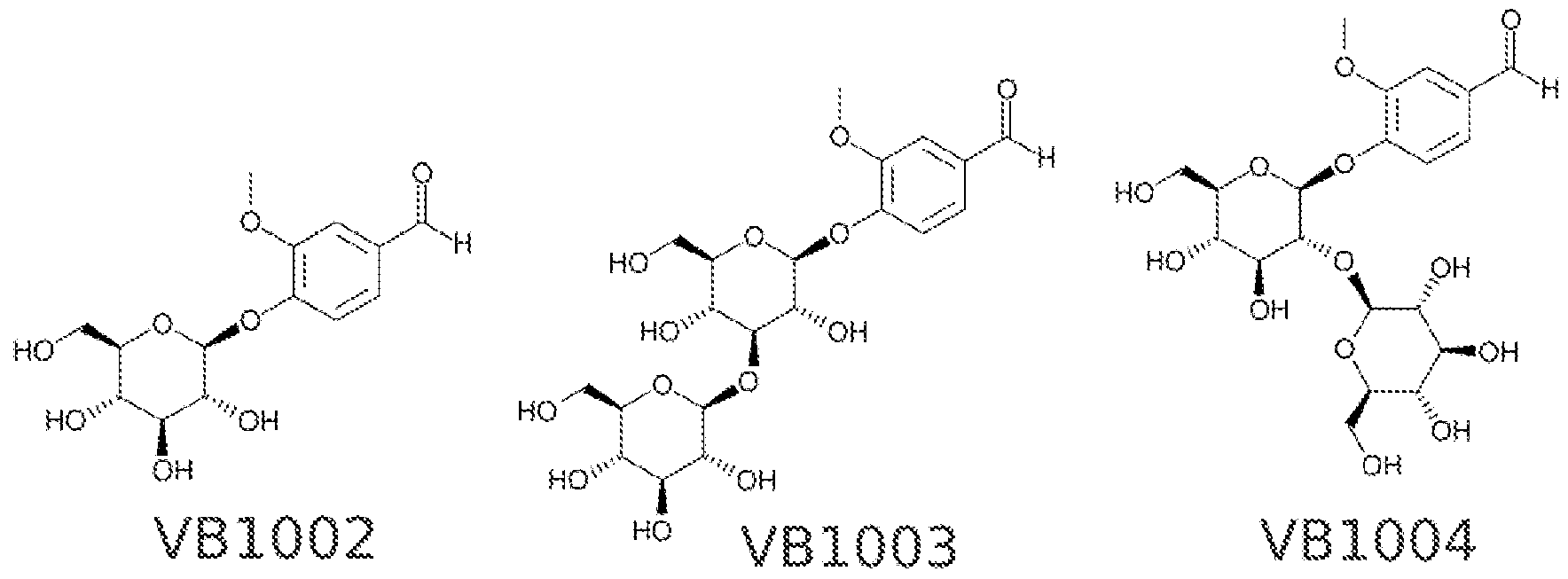
- Exemplary vanillin-glycosides falling within the scope of Formula (H), produced by the glycosylation of vanillin (VB1001 ) in accordance with the present invention include:
- curcumin-glycosides falling within the scope of Formula (H), produced by the glycosylation of curcumin (VB1 101 ) in accordance with the present invention include:
- a method for the site-specific delivery of a cannabinoid drug to a subject comprising the step of administering to a subject in need thereof one or more cannabinoid glycoside prodrugs in accordance with the present invention.
- the site of delivery is the large intestine.
- the site of delivery is the rectum.
- the site of delivery is the liver.
- the site of delivery is the skin.
- a method for facilitating the transport of a cannabinoid drug to the brain through intranasal, stereotactic, or intrathecal delivery, or delivery across the blood brain barrier of a subject comprising administering a cannabinoid glycoside prodrug in accordance with the present invention to a subject in need thereof.
- the cannabinoid glycoside prodrugs are useful in the treatment of conditions that benefit from or can be ameliorated with the administration of a cannabinoid drug.
- Conditions that can be treated or ameliorated through the administration of cannabinoid glycoside prodrugs of the present invention include but are not limited to, inflammatory bowel disease including induction of remission from Crohn's disease, and colitis and induction of remission from ulcerative colitis.
- cannabinoid glycoside prodrugs of the present invention are decreased inflammation of the intestines and rectum, decreased pain in the intestines, rectum, as well as decrease in neuropathic pain and abdominal pain, and inhibition of proliferation or cytotoxicity against colorectal cancer.
- Additional treatment indications, effects, or applications for cannabinoids or cannabinoid glycosides may include but are not limited to anorexia, nausea, emesis, pain, wasting syndrome, HIV-wasting, chemotherapy induced nausea and vomiting, epilepsy, schizophrenia, irritable bowel syndrome, cramping, spasticity, seizure disorders, alcohol use disorders, substance abuse disorders, addiction, cancer, amyotrophic lateral sclerosis, glioblastoma multiforme, glioma, increased intraocular pressure, glaucoma, cannabis use disorders, Tourette's syndrome, dystonia, multiple sclerosis, white matter disorders, demyelinating disorders, chronic traumatic encephalopathy, leukoencephalopathies, Guillain- Barre syndrome, inflammatory bowel disorders, gastrointestinal disorders, bacterial infections, MRSA, sepsis, septic shock, viral infections, arthritis, dermatitis, Rheumatoid arthritis, systemic lupus erythematosus, anti-
- the cannabinoid glycoside prodrug is administered in a pharmaceutical composition further comprising a pharmaceutically acceptable carrier, diluent, excipient, or adjuvant.
- the pharmaceutical compositions comprise one or more cannabinoid glycoside prodrugs and one or more pharmaceutically acceptable carriers, diluents, excipients and/or adjuvants.
- the pharmaceutical compositions can be formulated for administration by a variety of routes including but not limited to oral, topical, rectal, parenteral, and intranasal administration.
- compositions may comprise from about 1 % to about 95% of a cannabinoid glycoside prodrug of the invention.
- Compositions formulated for administration in a single dose form may comprise, for example, about 20% to about 90% of the cannabinoid glycoside prodrug of the invention, whereas compositions that are not in a single dose form may comprise, for example, from about 5% to about 20% of the cannabinoid glycoside prodrug of the invention.
- unit dose forms include tablets, ampoules, dragees, suppositories, and capsules.
- compositions are formulated for oral administration.
- Pharmaceutical compositions for oral administration can be formulated, for example, as tablets, troches, lozenges, aqueous or oily suspensions, dispersible powders or granules, emulsion hard or soft capsules, or syrups or elixirs.
- Such compositions can be prepared according to standard methods known in the art for the manufacture of pharmaceutical compositions and may contain one or more agents selected from the group of sweetening agents, flavouring agents, colouring agents and preserving agents in order to provide pharmaceutically elegant and palatable preparations.
- Tablets contain the active ingredient in admixture with suitable non-toxic pharmaceutically acceptable excipients including, for example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate; granulating and disintegrating agents, such as corn starch, or alginic acid; binding agents, such as starch, gelatine or acacia, and lubricating agents, such as magnesium stearate, stearic acid or talc.
- the tablets can be uncoated, or they may be coated by known techniques in order to delay disintegration and absorption in the gastrointestinal tract and thereby provide a sustained action over a longer period.
- a time delay material such as glyceryl monosterate or glyceryl distearate may be employed to further facilitate delivery of the drug compound to the desired location in the digestive tract.
- compositions for oral use can also be presented as hard gelatine capsules wherein the active ingredient is mixed with an inert solid diluent, for example, calcium carbonate, calcium phosphate or kaolin, or as soft gelatine capsules wherein the active ingredient is mixed with water or an oil medium such as peanut oil, liquid paraffin or olive oil.
- an inert solid diluent for example, calcium carbonate, calcium phosphate or kaolin
- an oil medium such as peanut oil, liquid paraffin or olive oil.
- compositions formulated as aqueous suspensions contain the active compound(s) in admixture with one or more suitable excipients, for example, with suspending agents, such as sodium carboxymethylcellulose, methyl cellulose, hydropropylmethylcellulose, sodium alginate, polyvinylpyrrolidone, hydroxypropyl ⁇ -cyclodextrin, gum tragacanth and gum acacia; dispersing or wetting agents such as a naturally-occurring phosphatide, for example, lecithin, or condensation products of an alkylene oxide with fatty acids, for example, polyoxyethyene stearate, or condensation products of ethylene oxide with long chain aliphatic alcohols, for example, hepta-decaethyleneoxycetanol, or condensation products of ethylene oxide with partial esters derived from fatty acids and a hexitol for example, polyoxyethylene sorbitol monooleate, or condensation products of ethylene oxide with partial esters derived from
- the aqueous suspensions may also contain one or more preservatives, for example ethyl, or n- propyl p-hydroxy-benzoate, one or more colouring agents, one or more flavouring agents or one or more sweetening agents, such as sucrose, stevia, or saccharin.
- preservatives for example ethyl, or n- propyl p-hydroxy-benzoate
- colouring agents for example ethyl, or n- propyl p-hydroxy-benzoate
- flavouring agents such as sucrose, stevia, or saccharin.
- sweetening agents such as sucrose, stevia, or saccharin.
- compositions can be formulated as oily suspensions by suspending the active compound(s) in a vegetable oil, for example, arachis oil, olive oil, sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
- the oily suspensions may contain a thickening agent, for example, beeswax, hard paraffin or cetyl alcohol.
- Sweetening agents such as those set forth above, and/or flavouring agents may be added to provide palatable oral preparations.
- These compositions can be preserved by the addition of an anti-oxidant such as ascorbic acid.
- the pharmaceutical compositions can be formulated as a dispersible powder or granules, which can subsequently be used to prepare an aqueous suspension by the addition of water.
- Such dispersible powders or granules provide the active ingredient in admixture with one or more dispersing or wetting agents, suspending agents and/or preservatives. Suitable dispersing or wetting agents and suspending agents are exemplified by those already mentioned above. Additional excipients, for example, sweetening, flavouring and colouring agents, can also be included in these compositions.
- compositions of the invention can also be formulated as oil-in-water emulsions.
- the oil phase can be a vegetable oil, for example, olive oil or arachis oil, or a mineral oil, for example, liquid paraffin, or it may be a mixture of these oils.
- Suitable emulsifying agents for inclusion in these compositions include naturally-occurring gums, for example, gum acacia or gum tragacanth; naturally-occurring phosphatides, for example, soy bean, lecithin; or esters or partial esters derived from fatty acids and hexitol, anhydrides, for example, sorbitan monoleate, and condensation products of the said partial esters with ethylene oxide, for example, polyoxyethylene sorbitan monoleate.
- the emulsions can also optionally contain sweetening and flavouring agents.
- compositions can be formulated as a syrup or elixir by combining the active ingredient(s) with one or more sweetening agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such formulations can also optionally contain one or more demulcents, preservatives, flavouring agents and/or colouring agents.
- sweetening agents for example glycerol, propylene glycol, sorbitol or sucrose.
- Such formulations can also optionally contain one or more demulcents, preservatives, flavouring agents and/or colouring agents.
- the glycoside prodrugs may be combined with other ingredients or substances that have glycosidase activity, or that may in other ways alter drug metabolism and pharmacokinetic profile of various compounds in vivo, including ones in purified form as well as such compounds found within food, beverages, and other products.
- the cannabinoid glycoside prodrug is administered in combination with, or formulated together with, substances that have direct glycosidase activity, or that contribute to modifications to the gut microflora that will alter the glycosidase activity in one or more regions of the intestines. Examples of such compositions include, but are not limited to, yogurt, prebiotics, probiotics, or fecal transplants.
- compositions are formulated for parenteral administration.
- parenteral as used herein includes subcutaneous injections, intravenous, intramuscular, intrathecal, intrasternal injection or infusion techniques.
- Parenteral pharmaceutical compositions can be formulated as a sterile injectable aqueous or oleaginous suspension according to methods known in the art and using one or more suitable dispersing or wetting agents and/or suspending agents, such as those mentioned above.
- the sterile injectable preparation can be a sterile injectable solution or suspension in a non-toxic parentally acceptable diluent or solvent, for example, as a solution in 1 ,3-butanediol.
- Acceptable vehicles and solvents that can be employed include, but are not limited to, water, Ringer's solution, lactated Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils which are conventionally employed as a solvent or suspending medium
- a variety of bland fixed oils including, for example, synthetic mono- or diglycerides.
- Fatty acids such as oleic acid can also be used in the preparation of injectables.
- cannabinoids Due to the highly lipophilic nature of cannabinoids, these molecules are typically poorly absorbed through membranes such as the skin of mammals, including humans, and the success of transdermally administering therapeutically effective quantities of cannabinoid to a subject in need thereof within a reasonable time frame and over a suitable surface area has been substantially limited. It is therefore proposed that the cannabinoid glycoside prodrugs of the present invention, through conjugation of the hydrophobic cannabinoid aglycone to the hydrophilic glycosidic moieties, provide a molecule having an amphiphilic character favourable for passive diffusion which should be more readily absorbed through the skin.
- the pharmaceutical compositions are formulated for topical administration.
- topical formulations may be presented as, for example, aerosol sprays, powders, sticks, granules, creams, liquid creams, pastes, gels, lotions, ointments, on sponges or cotton applicators, or as a solution or a suspension in an aqueous liquid, a nonaqueous liquid, an oil-in-water emulsion, or a water-in-oil liquid emulsion.
- Topical pharmaceutical compositions can be formulated with thickening (gelling) agents.
- the thickening agent used herein may include anionic polymers such as polyacrylic acid (CARBOPOL® by Noveon, Inc., Cleveland, Ohio), carboxypolymethylene, carboxymethylcellulose and the like, including derivatives of Carbopol® polymers, such as Carbopol® Ultrez 10, Carbopol® 940, Carbopol® 941 , Carbopol® 954, Carbopol® 980, Carbopol® 981 , Carbopol® ETD 2001 , Carbopol® EZ-2 and Carbopol® EZ-3, and other polymers such as Pemulen® polymeric emulsifiers, and Noveon® polycarbophils. Thickening agents or gelling agents are present in an amount sufficient to provide the desired rheological properties of the composition.
- Topical pharmaceutical compositions can be formulated with a penetration enhancer.
- penetration enhancing agents include C8-C22 fatty acids such as isostearic acid, octanoic acid, and oleic acid; C8-C22 fatty alcohols such as oleyl alcohol and lauryl alcohol; lower alkyl esters of C8-C22 fatty acids such as ethyl oleate, isopropyl myristate, butyl stearate, and methyl laurate; di(lower)alkyl esters of C6-C22 diacids such as diisopropyl adipate; monoglycerides of C8-C22 fatty acids such as glyceryl monolaurate; tetrahydrofurfuryl alcohol polyethylene glycol ether; polyethylene glycol, propylene glycol; 2-(2- ethoxyethoxyl)ethanol; diethylene glycol monomethyl ether; alkylaryl ethers
- the topical pharmaceutical compositions can further comprise wetting agents (surfactants), lubricants, emollients, antimicrobial preservatives, and emulsifying agents as are known in the art of pharmaceutical formations.
- wetting agents surfactants
- lubricants emollients
- antimicrobial preservatives emulsifying agents
- Transdermal delivery of the cannabinoid glycoside prodrug can be further facilitated through the use of a microneedle array drug delivery system.
- compositions and methods of preparing pharmaceutical compositions are known in the art and are described, for example, in “Remington: The Science and Practice of Pharmacy” (formerly “Remingtons Pharmaceutical Sciences”); Gennaro, A., Lippincott, Williams & Wilkins, Philadelphia, PA (2000).
- compositions of the present invention described above include one or more cannabinoid glycoside prodrugs of the invention in an amount effective to achieve the intended purpose.
- therapeutically effective dose refers to the amount of the cannabinoid glycoside prodrug that improves the status of the subject to be treated, for example, by ameliorating the symptoms of the disease or disorder to be treated, preventing the disease or disorder, or altering the pathology of the disease. Determination of a therapeutically effective dose of a compound is well within the capability of those skilled in the art.
- cannabinoid glycosides can be combined to enable simultaneous delivery of multiple cannabinoids in a site-specific manner, including THC and CBD, whose effects in some ways may be synergistic (Russo 2006).
- the pharmaceutical composition comprises one or more CBD-glycosides and one or more THC-glycosides formulated together in a single dosage form.
- the exact dosage to be administered to a subject can be determined by the practitioner, in light of factors related to the subject requiring treatment. Dosage and administration are adjusted to provide desired levels of the cannabinoid glycoside prodrug and/or the cannabinoid drug compound obtained upon hydrolysis of the prodrug. Factors which may be taken into account when determining an appropriate dosage include the severity of the disease state, general health of the subject, age, weight, and gender of the subject, diet, time and frequency of administration, drug combination(s), reaction sensitivities, and tolerance/response to therapy. Dosing regimens can be designed by the practitioner depending on the above factors as well as factors such as the half-life and clearance rate of the particular formulation.
- a method of producing a cannabinoid glycoside comprising incubating a cannabinoid aglycone with one or more sugar donors in the presence of one or more glycosyltransferases.
- the one or more glycosyltransferases is a UGT76G1 or UGT76G1 -like glucosyltransferase. In one embodiment, the one or more glycosyltransferases comprise a UGT76G1 or UGT76G1 -like glucosyltransferase and a Os03g0702000 or Os03g0702000-like glucosyltransferase.
- the one or more sugar donors are selected from the group consisting of UDP-glucose, UDP-glucuronic acid, UDP-mannose, UDP-fructose, UDP-xylose, UDP-rhamnose, UDP-fluoro-deoxyglucose, and combinations thereof.
- the sugar donor is UDP-glucose.
- the cannabinoid aglycone is a cannabinoid, an endocannabinoid, or a vanilloid.
- the cannabinoid glycoside prodrug produced by the methods of the present invention is a compound of the Formula (I).
- the method of producing a cannabinoid glycoside comprises incubating a cannabinoid aglycone with UDP-glucose, in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase under conditions that allow for glycosylation.
- the method of producing a cannabinoid glycoside comprises incubating a cannabinoid aglycone with one or more sugar donors in the presence of a first glycosyltransferase and a second glycosyltransferase under conditions which allow for glycosylation.
- sugar donor is UDP-glucose
- the first glycosyltransferase is a UGT76G1 or UGT76G1 -like glucosyltransferase
- the second glycosyltransferase is a Os03g0702000 or Os03g0702000-like glucosyltransferase.
- the method of producing a cannabinoid glycoside comprises incubating a cannabinoid aglycone with UDP-glucose in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase and Os03g0702000 or Os03g0702000-like glucosyltransferase under conditions which allow for glycosylation.
- the method of producing a cannabinoid glycoside comprises incubating a cannabinoid aglycone with maltodextrin, in the presence of a cyclodextrin glucanotransferase under conditions that allow for glycosylation.
- the method of producing a cannabinoid glycoside comprises incubating a cannabinoid aglycone with UDP-glucose and maltodextrin in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase and cyclodextrin glucanotransferase under conditions which allow for glycosylation.
- the glycosyltransferase employed in the methods of producing the cannabinoid glycoside is UGT76G1 or UGT76G1 -like glucosyltransferase.
- the UGT76G1 or UGT76G1 -like glucosyltransferase comprises the sequence as set forth in SEQ ID NO:1 , 3, 5 or 7.
- the glycosyltransferase employed in the methods of producing the cannabinoid glycoside is Os03g0702000 or Os03g0702000-like glucosyltransferase.
- the Os03g0702000 or Os03g0702000-like glucosyltransferase comprises the sequence as set forth in SEQ ID NO:9.
- the method of producing the cannabinoid glycoside further comprises incubating with sucrose synthase.
- the sucrose synthase comprises the sequence as set forth in SEQ ID NO: 15, 17, 19, 21 , 23 or 25.
- the method for the production of a cannabinoid glycoside prodrug comprises expressing one or more of the glycosyltransf erases in a cell or plant which produces the cannabinoid aglycone and isolating the cannabinoid glycoside prodrug.
- glycosylated cannabinoids can act as efficient prodrugs for selective delivery of cannabinoids to desired tissues where the glucose molecules can be hydrolyzed to release the aglycone cannabinoids. Additionally the glycosylations promote stability of CBD and CBDV by protecting them from oxidation and ring-closure of the C6'-hydroxyl group, which prevents degradation into A9-THC or A9-THCV, respectively, and subsequently into cannabinol (CBN) or cannabinavarin (CBNV), respectively
- the primary detoxification mechanism for cannabinoids in humans is CYP450 mediated hydroxylation of the C7 methyl group of CBD and CBDV, or the C1 1 methyl group of THC and CBN, glycosylation of the acceptor hydroxyl groups of the cannabinoid resorcinol ring may afford protection from C7/C1 1 hydroxylation and subsequent elimination from the body due to steric hindrance preventing the cannabinoid- glycoside from binding in the CYP450 active site.
- the hydroxyl groups of CBD are thought to facilitate the binding to the detoxification cytochrome P450 CYP3A4 in the epithelium of the small intestine (Yamaori 201 1 ). Reduced degradation or metabolism in the stomach and small intestine due to these effects could also lead to higher total bioavailability of any glycosylated product upon oral delivery.
- glycoside prodrugs may enable stable drug formulations that are resistant to abuse, due to the potential for their primary biological effects to only occur after oral ingestion.
- abuse-deterrent compounds are simply mixing or formulation based deterrents, they can still be compromised by simple physical and chemical methods.
- the beta-glycosides described herein will only release the aglycone upon the action of beta-glycosidase enzymes.
- Beta- glycosidases are known to be secreted by microbes that occupy the large intestines of mammals, therefore upon oral ingestion the glycoside prodrugs will remain glycosylated until they reach the large intestine.
- a similar approach may be used for abuse-resistant, abuse- deterrent, and site-specific delivery of other compounds through glycosylation. It has been found that the UGT76G1 enzyme (SEQ ID N0.1 ) from Stevia rebaudiana transfers a glucose molecule from the sugar donor UDP-glucose (UDPG) to the hydroxyl groups of CBD to create novel CBD-O-glycosides (Table 1 , Figures 2 & 4).
- the UDPG is inverted by UGT76G1 to produce ⁇ -D-glucose residues covalently linked through the to the hydroxyl acceptor sites on CBD.
- UGT76G1 open reading frame (ORF) codon optimization was performed (SEQ ID NOs. 4 and 6) for expression in Pichia pastoris. Similar to its activity towards steviol glycosides, UGT76G1 is highly productive and has an equilibrium constant (Keq) for CBD of -24. Through experimentation and analysis it was determined that UGT76G1 has the unique ability to apply multiple glucose moieties to the CBD molecule.
- CBD-glycoside product mobility groups also suggest that CBD can dock in the UGT76G1 active site both forwards and backwards creating a cis-like- conformation for the glycosylations relative to the cannabinoid backbone (mechanism depicted in Figure 3), or possibly the rotational freedom about the bond at C1 ' (C6 described by Mazur 2009) allows the hydroxyl group to rotate after glycosylation, placing the other hydroxyl group adjacent to the UDPG in the active site and creating a trans-like-conformation for the glycosylations on the cannabinoid backbone (mechanism depicted in Figure 4).
- Potential CBD molecular docking in the active site of UGT76G1 is depicted in Figure 6 where CBD is superpositioned over the bi-functional substrate for UGT76G1 , Rebaudioside E (RebE) ( Figure 6).
- CBDV was incubated with UGT76G1 and UDPG to test for glycosylation activity.
- CBDV depletion was observed upon HPLC analysis, in addition to the appearance of four additional product peak mobility groups, which were dependent on addition of both UGT76G1 and UDPG.
- the four new products formed displayed the same absorbance characteristics as CDBV and were determined to be the primary glycosides CBDV-2'-0-glucopyranosides, CBDV-6'-0-glucopyranosides, and the secondary glycosides CBDV-2'-0-(3-1 )-diglucopyranoside, and CBDV-6'-0-(3-1 )- diglucopyranoside (compounds VB202, VB206, VB204 and VB208, respectively, Table 2). With additional reaction time it was determined that higher order glycoside products were also formed. CBDV-glycoside production was similar to CBD-glycosides from UGT76G1 (Table 2), and proceeded to completion with a K eq -24. Given the number of CBDV-glycoside products, UGT76G1 transfers multiple glucose molecules onto CBDV on both C2' and C6' hydroxyl groups, as well as onto the primary and secondary glycosylations.
- the cannabinoid backbone is recognized in the active site of UGT76G1 with the A9-THC C1 hydroxyl group situated towards the UDPG sugar donor (pyran numbering, Figure 1 B).
- UGT76G1 demonstrated glycosylation activity for all other phytocannabinoids analyzed, it was also tested for glycosylation activity against cannabinol (CBN). Effective glycosylation of CBN by UGT76G1 was observed, in a similar pattern to A9-THC, as both share a single hydroxyl recipient group at the C1 position of the resorcinol ring. The activity seen with UGT76G1 is consistent with a broad recognition of cannabinoids by the enzyme active site.
- CBN cannabinol
- Alternative cannabinoid substrates may be inserted into this UGT76G1 glycosylation reaction infrastructure to generate novel cannabinoid-glycosides, given they possess hydroxyl groups in similar positions on the cannabinoid backbone.
- Ideal candidates are cannabigerol (CBG), cannabichromene (CBC), cannabidiol hydroxyquinone (CBDHQ), HU-331 , other isomers of A9-THC such as ⁇ -THC, etc., and synthetic analogues of A9-THC such as HU- 210.
- the UGT enzyme Os03g0702000 (SEQ ID NO.9) from Oryza sativa is also capable of transferring an additional glucose moiety from UDP- glucose onto the C2-hydroxyl of the primary sugar (Tables 1 - 1 1 , Figures 7- 9 & 12 - 14).
- This glycosylation activity is consistent with the activity of UGT Os03g0702000 towards steviol glycosides in establishing C2-hydroxyl secondary glycosylations (2 ⁇ 1 connectivity) on existing primary glucose residues.
- cyclodextrin- glucanotransferase (CGTase, Toruzyme 3.0L, trademark of Novozymes Inc.) is capable of transferring a short a-(1 -4)-maltodextrin chain onto the hydroxyl groups of cannabinoids.
- the CGTase is also capable of glycosylating primary and secondary glycosylations established by UGT76G1 and Os03g0702000, resulting in carbohydrate attachments that start with ⁇ -D- glucose molecules, but terminating in a-D-glucose molecules termed ⁇ -primed-a-glucosyl (Tables 1 -1 1 ).
- a-glycosylation by cyclodextrin glucanotransferase mediated maltodextrin transfer can occur on any of the hydroxyl groups of the primary or secondary sugars covalently linked to the cannabinoid.
- This makes possible any number of conformations of a-glycosyl chains linked to the glycosides listed in Tables 1 -1 1 .
- UGT76G1 and Os03g0702000 may be used to produce the same glycosylation of cannabinoids.
- Suitable enzymes for establishing the primary glycosylation similar to UGT76G1 are additional members of the UGT76 clade such as UGT76G2 or UGT76H1 .
- BLAST results with the UGT76G1 protein sequence yield a maximum homology of 49% identity, as much as 66% positives (similar identity). Ideal candidates may have low overall peptide identity or similarity, but will likely have conserved amino acids at the opening adjacent to the UDPG catalytic site.
- This sequence is exemplified by a leucine at position 379, and a broader peptide sequence of SDFGLDQ (AA's 375 to 381 of UGT76G1 ).
- Suitable enzymes for producing the secondary glycosylation of Os03g0702000 are members of the UGT91 clade, including UGT91 D1 and UGT91 D2.
- the glycosylation reactions performed herein included UDP-glucose as the nucleotide sugar donor, however there is some cross-reactivity amongst UGTs that allows for use of alternative nucleotide sugars such as UDP-glucuronic acid, etc.
- Glucuronic acid is the predominant nucleotide sugar utilized by phase-ll detoxification UGTs in the liver, and cannabinoid-glucuronides are a common detoxification product.
- nucleotide sugars which could be used to donate carbohydrate moieties to create novel glycosides with similar properties include UDP-glucuronic acid, UDP-mannose, UDP-fructose, UDP-xylose, UDP- rhamnose, UDP-fluorodeoxyglucose, etc.
- nucleotide sugars can also be used in combination to create glycosides that contain multiple types of residues on the same aglycone backbone.
- Alternative strategies to further improve the solubility and delivery of cannabinoids and other compounds described herein include their glycosylation and then functionalizing the sugar moieties with additional ligands or modifications. Examples of this include sulfation, myristoylation, phosphorylation, acetylation, etc.
- endocannabinoids such as AEA, 2-AG, 1 -AG, and synaptamide are glycosylated by UGT76G1 , it is hypothesized that similar endocannabinoids will also be suitable substrates for glycosylation by UGT76G1 .
- endocannabinoid candidates that are likely to be glycosylated by UGT76G1 include oleoyl ethanolamide (OEA), eicsapentaenoyl ethanolamide, prostaglandin ethanolamide, docosahexaenoyl ethanolamide, linolenoyl ethanolamide, 5(Z),8(Z),1 1 (Z)- eicosatrienoic acid ethanolamide (mead acid ethanolamide), heptadecanoul ethanolamide, stearoyl ethanolamide, docosaenoyl ethanolamide, nervonoyl ethanolamide, tricosanoyl ethanolamide, lignoceroyl ethanolamide, myristoyl ethanolamide, pentadecanoyl ethanolamide, palmitoleoyl ethanolamide, docosahexaenoic acid (DHA), and similar compounds.
- OOA oleoyl ethanolamide
- glycolipids may have a wide range of commercial uses, ranging from pharmaceutical use as a novel endocannabinoid drug with improved solubility and pharmacokinetic properties, to use as an antibacterial agent, to use as a detergent similar to other glycolipids, etc.
- TRPV1 toll-like vanilloid receptor type 1
- CBD cannabinoids and botanical extracts
- CBN cannabigerol
- CBG cannabigerol
- TRPs transient receptor potential channels
- TRPV1 vasodilation and inflammation
- capsaicin and its analogues act to desensitize the receptors to stimulants, and provide potent antiinflammatory effects (Bisogno 2001 ).
- Analogous effects may occur with TRPA1 in addition to other TRPs.
- CBD this may occur at concentrations that are lower than what is required for binding of cannabinoid receptors, and at concentrations that are within the range of those typically attained in human clinical testing and use.
- CBD has been shown to inhibit fatty acid amide hydroxylase (FAAH), the enzyme responsible for facilitating the metabolism of the endocannabinoid anandamide (Watanabe, 1998; DE e Petrocellis 2010).
- FAAH fatty acid amide hydroxylase
- UGT76G1 would be capable of glycosylating many different ligands of the same TRPs, including TRPM8, TRPV2, TRPA1 , and TRPV1 .
- Capsaicin is capable of contorting into a CBD-like structure (Bisogno 2001 ), therefore it was postulated that capsaicin was likely to be a suitable substrate for glycosylation by UGT76G1 .
- UGT76G1 is capable of glycosylating the vanilloid moiety of capsaicin in a structurally identical way to PaGT3 from Phytolacca americana (Noguchi 2009).
- the glycosylated structure of capsaicin is the vanilloid head, it was further hypothesized that UGT76G1 would be capable of glycosylation of the minimal vanilloid, i.e., vanillin, as well as many analogues.
- Cannabinoid glycosides may also have direct bioactive and therapeutic effects, beyond their utility a prodrug for their aglycone form.
- Quercetin is an antioxidant flavonoid that is ubiquitous in vegetables and often present both in its aglyone and glycosylated forms. It has been demonstrated through in vitro studies that quercetin glucuronides act as a bioactive agent as well as a precursor molecule to aglycone quercetin (Terao 201 1 ). In many cases, including with glycosides that exert antibacterial and antitumor effects, the glycosidic residues are crucial to activity (Kren & Rezanka 2008).
- Glycosides have also been demonstrated to receive facilitated transport across the blood brain barrier (BBB) by the glucose transporter GLUT1 .
- BBB blood brain barrier
- a prime example is the glycoside of ibuprofen achieving a significant increase of ibuprofen aglycone concentration in the brain (Chen 2009). Similar to these glycosides, glycosides of cannabinoids and other compounds described herein may benefit from enhanced facilitated transport across the BBB or other barriers.
- Glucose transporters are a wide group of membrane proteins encoded by the human genome and that are found not only in the BBB but across many different cells and tissues, including brain, erythrocytes, fat, muscle, kidney, liver, intestine, and pancreas, so glycosylation will be tailored to provide site-specific delivery to any of these tissues. Accordingly, in one embodiment, there is provided a method for facilitating the transport of a cannabinoid drug across the blood brain barrier of a subject comprising administering to the subject a cannabinoid glycoside prodrug in accordance with the present invention.
- cannabinoids and cannabidiol may be especially useful because of oligodendrocyte protective (oligoprotective) and general neuroprotective effects. It has been demonstrated that cannabinoid signaling is involved with both oligodendrocyte differentiation (Gomez 2010) and that cannabinoids promote oligodendrocyte progenitor survival (Molina-Holgado 2002). Drug formulations that include cannabidiol as a major ingredient have been approved to treat muscle spasticity and pain from multiple sclerosis, a neurodegenerative disorder that causes loss of myelin and oligodendrocyte progenitor cells.
- cannabidiol has been demonstrated to mediate oligoprotective effects through attenuation of endoplasmic reticulum stress pathways (Mecha 2012). Cannabidiol has also been studied extensively for its antipsychotic effects, however the exact role in protection of oligodendroctyes and promotion of remyelination has not yet been described (Zuardi 2012). Despite the correlation between the clinical symptoms of psychosis with neuropathological analysis that indicates dysmyelination is involved, the role of dysmyelination as a driver or cause of schizophrenia and other psychoses remains controversial (Mighdoll 2015). Remyelination has also been described as potentially useful for treatment of Alzheimer's disease and other forms of dementia (Bartzokis 2004).
- cannabinoids delivery of cannabinoids to the brain may be especially useful for its established neuroprotective and oligoprotective effects.
- Cannabinoid glycoside drug formulations co-administered in combination with other agents that influence other aspects of repair or regeneration, such as oligodendrocyte progenitor differentiation or remyelination, may also prove to be beneficial. This includes compounds such as anti-LINGO-1 monoclonal antibodies, guanabenz, sephinl , benzatropine, clemastine, polyunsaturated fatty acids, etc.
- UGT76G1 , Os03g0702000 and cyclodextrin glucanotransferase were capable of primary, secondary and tertiary glycosylations of steviol glycosides and aglycone products of diverse chemical structure, including cannabinoids, endocannabinoids, vanillin, curcumin, and capsaicin.
- UGT76G1 is an enzyme from the plant Stevia rebaudiana, it will be compatible with expression in the genus Cannabis.
- the ideal strategy for expression of UGT76G1 within the Cannabis plant is to genetically engineer the UGT76G1 open reading frame under a promoter element that is specific for the same tissue that cannabinoids are produced in, namely the secretory trichomes of the plant.
- Suitable promoter elements include the promoter for the cytosolic 0-acetylserine(thiol)lyase (OASA1 ) enzyme from Arabidopsis thaliana (Gutierrez-Alcala 2005).
- Candidates for transformation with UGT76G1 include Cannabis sativa, Cannabis indica, and Cannabis ruderalis.
- a similar approach may be used with UGT76G1 and similar enzymes for in planta production of glycosylated secondary metabolites within many other different plant species, and may be especially useful when plant species already produce large quantities of the desired aglycone product or known enzyme substrate.
- an antimicrobial agent comprising an effective amount of a cannabinoid glycoside prodrug in accordance with the present invention.
- a detersive agent comprising an effective amount of a cannabinoid glycoside prodrug in accordance with the present invention.
- the present invention provides for nucleic acids comprising nucleotide sequences encoding a glycosyltransferase.
- the glycosyltransf erases of the present invention are capable of primary, secondary, tertiary glycosylations or a combination thereof.
- the glycosyltransf erases are capable of primary, secondary and tertiary glycosylations.
- the glycosyltransf erases are capable of secondary and tertiary glycosylations.
- the nucleic acids encode a glucosyltransferase, including but not limited to a UDP-glucosyltransferase.
- the glucosyltransferases include but are not limited to a Stevia rebaudiana UDP-glucosyltransferase, such as UGT76G1 or UGT74G1 or an Oryza sativa glucosyltrasferase, such as Os03g0702000.
- the invention provides for nucleic acids comprising nucleotide sequences encoding a cyclodextrin glucanotransferase. Also provided are nucleic acids comprising nucleotide sequences that encode a sucrose synthase.
- Nucleic acids include, but are not limited to, genomic DNA, cDNA, RNA, fragments and modified versions, including but not limited to codon optimized versions thereof.
- the nucleotide sequences may be codon optimized for expression in Pichia pastoris or E. coli.
- the nucleic acids may include the coding sequence of the glycosyltransferase or sucrose synthase, in isolation, in combination with additional coding sequences (e.g., including but not limited to a purification tag).
- the nucleic acid comprises a sequence encoding UGT76G1 or UGT76G1 -like glucosyltransferase.
- UGT76G1 -like glucosyltransferase include for example, other members of the UGT76G1 clade such as UGT76G2 or UGT76H1 .
- the nucleic acid comprises a sequence encoding an UGT76G1 glucosyltransferase having the amino acid sequence as set forth in any one of SEQ ID NOs:1 , 3, 5 and 7 and listed below or fragments and variants thereof.
- SEQ ID NO:1 (UGT76G1 (native protein sequence)
- SEQ ID NO:3 (UGT76G1 with a 6x Histidine tag at the N-terminus)
- SEQ ID N0:5 (UGT76G1 with a 6x Histidine-Glutamine tag at the N-terminus)
- the nucleic acid comprises a sequence encoding UGT76G1 having the amino acid sequence as set forth in AAR06912.1 .
- the nucleic acid molecule comprises a sequence encoding UGT76G1 glucosyltransferase and comprising the nucleotide sequence as set forth in any one of SEQ ID NOs: 2, 4, 6 and 8 and listed below, or fragments and variants thereof.
- SEQ ID NO:2 (UGT76G1 native nucleic acid sequence)
- SEQ ID N0:4 (Sequence encoding SEQ ID NO:3 codon optimized for expression in Pichia pastoris)
- SEQ ID NO:6 (Sequence encoding SEQ ID NO:5 codon optimized for expression in Pichia pastoris)
- SEQ ID NO:8 (Sequence encoding SEQ ID NO:7 codon optimized for expression in Escherichia coli)
- the nucleic acid molecule encodes an UGT76G1 glucosyltransferase and comprises the nucleotide sequence as set forth in GenBank Accession number AY345974.1 or a variant or fragment thereof.
- the nucleic acid comprises a sequence encoding UGT76G2 glucosyltransferase.
- the nucleic acid comprises a sequence encoding UGT76G2 glucosyltransferase having the amino acid sequence as set forth in SEQ ID NO:27 and listed below or variants and fragments thereof.
- the nucleic acid comprises a sequence encoding UGT76G2 glucosyltransferase and having the nucleic acid sequence as set forth in SEQ ID NO:28 and listed below or variants and fragments thereof.
- the nucleic acid comprises a sequence encoding UGT76H1 glucosyltransferase.
- the nucleic acid comprises a sequence encoding UGT76H1 glucosyltransferase having the amino acid sequence as set forth in SEQ ID NO:29 and listed below or variants and fragments thereof.
- the nucleic acid comprises a sequence encoding UGT76H1 glucosyltransferase and having the nucleic acid sequence as set forth in SEQ ID NO:30 and listed below or variants and fragments thereof.
- the nucleic acid comprises a sequence encoding Oryza sativa Os03g0702000 or Os03g0702000-like glucosyltransferase.
- Os03g0702000-like glucosyltransferase include for example, other members of the UGT91 clade such as UGT91 D1 or UGT91 D2.
- the nucleic acid comprises a sequence encoding Os03g0702000 glucosyltransferase having the amino acid sequence as set forth in SEQ ID NO: 9 and listed below or a variant or fragment thereof.
- the nucleic acid molecule encodes Os03g0702000 glucosyltransferase and comprises a nucleotide sequence as set forth in SEQ ID NO: 10 and as detailed below or a variant or fragment thereof.
- the nucleic acid molecule encodes Os03g0702000 glucosyltransferase and comprises the sequence as set forth in GenBank Accession number XM 015773655 or a variant or fragment thereof.
- the nucleic acid comprises a sequence encoding UGT91 D1 glucosyltransferase. In certain embodiments, the nucleic acid comprises a sequence encoding UGT91 D1 glucosyltransferase having the amino acid sequence as set forth in SEQ ID NO:31 and listed below or a variant or fragment thereof.
- the nucleic acid molecule encodes UGT91 D1 glucosyltransferase and comprises a nucleotide sequence as set forth in SEQ ID NO: 32 and as detailed below or a variant or fragment thereof.
- SEQ ID NO:32 ATGTACAACGTTACTTATCATCAAAATTCAAAAGCAATGGCTACCAGTGACTCCATAGTTGA
- the nucleic acid comprises a sequence encoding UGT91 D2 glucosyltransferase. In certain embodiments, the nucleic acid comprises a sequence encoding UGT91 D2 glucosyltransferase having the amino acid sequence as set forth in SEQ ID NO: 33 and listed below or a variant or fragment thereof.
- the nucleic acid molecule encodes UGT91 D2 glucosyltransferase and comprises a nucleotide sequence as set forth in SEQ ID NO: 34 and as detailed below or a variant or fragment thereof.
- the nucleic acid comprises a sequence encoding Stevia rebaudiana UDP-glycosyltransferase 74G1 . In certain embodiments, the nucleic acid comprises a sequence encoding Stevia rebaudiana UDP-glycosyltransferase 74G1 which comprises the amino acid sequence as set forth in SEQ ID NO: 13 and as listed below or a variant or fragment thereof.
- the nucleic acid molecule encodes Stevia rebaudiana UDP-glycosyltransferase 74G1 and comprises a nucleotide sequence as set forth in SEQ ID NO: 14 and as listed below or a variant or fragment thereof. [00175] SEQ ID NO:14
- the nucleic acid molecule encodes Stevia rebaudiana UDP-glycosyltransferase 74G1 and comprises the sequence as set forth in GenBank Accession number AY345982 or a variant or fragment thereof.
- the invention provides for nucleic acids comprising nucleotide sequences encoding a cyclodextrin glucanotransferase (W01996033267; US6271010).
- nucleic acids comprising nucleotide sequences that encode a sucrose synthase. Accordingly, in certain embodiments, the nucleic acid comprises a sequence encoding sucrose synthase which comprises the amino acid sequence as set forth in SEQ ID NO: 15, 17, 19, 21 , 23 or 25 and listed below or a variant or fragment thereof.
- the nucleic acid molecule encodes sucrose synthase and comprises a nucleotide sequence as set forth in SEQ ID NO: 16,18, 20, 22, 24 or 26 and listed below or a fragment or variant thereof.
- SEQ ID NO:18 encodes SUS2 isoform
- nucleic acid comprising a sequence having at least 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99% or 100% identity to any one of the sequences set forth in SEQ ID NOs: 2, 4, 6, 8, 10, 14, 16,18, 20, 22, 24, 26, 28, 30, 32 and 34 and fragments thereof or the complement thereof.
- nucleic acid encoding a polypeptide comprising a sequence at least 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99% or 100% percent identity to any one of the sequences set forth in SEQ ID NOs: 1 , 3, 5, 7, 9, 13, 15, 17, 19, 21 , 23, 25, 27, 29, 30, 31 and 33 and fragments thereof.
- SEQ ID NOs: 1 3, 5, 7, 9, 13, 15, 17, 19, 21 , 23, 25, 27, 29, 30, 31 and 33 and fragments thereof.
- regions of the enzyme such as the catalytic site or areas adjacent to the catalytic site
- conserved amino acids at the opening adjacent to the UDPG catalytic site.
- the nucleic acid encodes an UDP- glucosyltransferase having the sequence SDFGLDQ at a position corresponding to amino acid residues 375 to 381 of the UGT76G1 set forth in SEQ ID NO:1 .
- fragments are at least 10, at least 20, at least 50 nucleotides in length.
- the fragments may be used, for example, as primers or probes.
- nucleic acids that hybridize to the nucleic acids of the present invention or the complement thereof.
- a nucleic acid that hybridizes to any one of the sequences set forth in SEQ ID NOs: 2, 4, 6, 8, 10, 14, 16, 18, 20, 22, 24,26, 28, 30, 32 and 34 or the complement thereof under conditions of low, moderate or high stringency.
- hybridization and the strength of hybridization i.e., the strength of the association between the nucleic acids is impacted by such factors as the degree of complementary between the nucleic acids, stringency of the conditions involved, the T m of the formed hybrid, and the G:C ratio within the nucleic acids.
- a non-limiting example of "high stringency conditions" when used in reference to nucleic acid hybridization comprise conditions equivalent to binding or hybridization at 42 °C in a solution consisting of 5XSSPE (43.8 g/l NaCI, 6.9 g/l NaH 2 P0 4 H 2 0 and 1 .85 g/l EDTA, pH adjusted to 7.4 with NaOH), 0.5% SDS, 5X Denhardt's reagent and 100 ⁇ g/ml denatured salmon sperm DNA followed by washing in a solution comprising 0.1 XSSPE, 1 .0% SDS at 42°C when a probe of about 500 nucleotides in length is employed.
- 5XSSPE 4.3.8 g/l NaCI, 6.9 g/l NaH 2 P0 4 H 2 0 and 1 .85 g/l EDTA, pH adjusted to 7.4 with NaOH
- SDS 5X Denhardt's reagent
- 100 ⁇ g/ml denatured salmon sperm DNA followed by washing
- a non-limiting example of "medium stringency conditions" when used in reference to nucleic acid hybridization comprise conditions equivalent to binding or hybridization at 42 °C in a solution consisting of 5XSSPE (43.8 g/l NaCI, 6.9 g/l NaH 2 P0 4 H 2 0 and 1 .85 g/l EDTA, pH adjusted to 7.4 with NaOH), 0.5% SDS, 5X Denhardt's reagent and 100 ⁇ g/ml denatured salmon sperm DNA followed by washing in a solution comprising 1 .0XSSPE, 1 .0% SDS at 42 °C when a probe of about 500 nucleotides in length is employed.
- Low stringency conditions when used in reference to nucleic acid hybridization comprise conditions equivalent to binding or hybridization at 42.degree. C. in a solution consisting of 5XSSPE (43.8 g/l NaCI, 6.9 g/l NaH 2 P0 4 H 2 0 and 1 .85 g/l EDTA, pH adjusted to 7.4 with NaOH), 0.5% SDS, 5X Denhardt's reagent and 100 ⁇ g/ml denatured salmon sperm DNA followed by washing in a solution comprising 5XSSPE, 0.1 % SDS at 42 °C when a probe of about 500 nucleotides in length is employed.
- 5XSSPE 43.8 g/l NaCI, 6.9 g/l NaH 2 P0 4 H 2 0 and 1 .85 g/l EDTA, pH adjusted to 7.4 with NaOH
- SDS 5X Denhardt's reagent
- 100 ⁇ g/ml denatured salmon sperm DNA followed by washing in a solution comprising 5
- the polynucleotides include the coding sequence polypeptide, in isolation, in combination with additional coding sequences (e.g., a purification tag, a localization signal, as a fusion-protein, as a pre-protein, or the like), in combination with non-coding sequences (e.g., introns or inteins, regulatory elements such as promoters (including inducible promoters, tissue- specific promoters (such as root-specific or leaf specific promoters), enhancers, terminators, and the like), and/or in a vector or host environment in which the polynucleotide encoding a transcription factor or transcription factor homologue polypeptide is an endogenous or exogenous gene.
- additional coding sequences e.g., a purification tag, a localization signal, as a fusion-protein, as a pre-protein, or the like
- non-coding sequences e.g., introns or inteins, regulatory elements such as promoters (including
- coding sequences e.g., a purification tag, a localization signal, as a fusion-protein, as a pre-protein, or the like
- non-coding sequences e.g. regulatory elements such as promoters (including inducible promoters, tissue-specific promoters (such as root-specific or leaf specific promoters), enhancers, terminators, and the like)
- vectors for use in prokaryotic such as E. coli and eukaryotic cells, including but not limited to yeast and plant cells are known in the art.
- the present invention provides for glycosyltransferases.
- the glycosyltransferases of the present invention are capable of primary, secondary and/or tertiary glycosylations.
- the glycosyltransferases are capable of primary, secondary and tertiary glycosylations.
- the glycosyltransferases are capable of secondary and/or tertiary glycosylations.
- the glycosyltransferases is a glucosyltransferase, including but not limited to a UDP- glycotransferase.
- the glucosyltransferases include but are not limited to a Stevia rebaudiana UDP-glucosyltransferase, such as UGT76G1 or UGT74G1 or an Oryza sativa glucosyltrasferase, such as Os03g0702000.
- the invention provides for a cyclodextrin glucanotransferase.
- sucrose synthases are also provided.
- an UGT76G1 or UGT76G1 -like glucosyltransferase include for example, other members of the UGT76G1 clade such as UGT76G2 or UGT76H1 . Accordingly, in certain embodiments, there is provided an UGT76G1 comprising the amino acid sequence as set forth in any one of SEQ ID NOs: 1 , 3, 5 and 7 or fragments and variants thereof. In certain embodiments, there is provided an UGT76G1 encoded by the nucleic acid molecule comprising the sequence as set forth in any one of SEQ ID NOs: 2, 4, 6 and 8.
- an UGT76G2 comprising the amino acid sequence as set forth in SEQ ID NO: 27 or fragments and variants thereof.
- an UGT76G1 encoded by the nucleic acid molecule comprising the sequence as set forth in SEQ ID NO: 28.
- an UGT76H1 comprising the amino acid sequence as set forth in SEQ ID NO: 29 or fragments and variants thereof.
- an UGT76G1 encoded by the nucleic acid molecule comprising the sequence as set forth in SEQ ID NO: 30.
- Os03g0702000 or Os03g0702000- like glucosyltransferase include for example, other members of the UGT91 clade such as UGT91 D1 or UGT91 D2. Accordingly, in certain embodiments, there is provided an Os03g0702000 comprising an amino acid sequence as set forth in SEQ ID NO: 9 or fragments and variants thereof. In certain embodiments, there is provided an Os03g0702000 encoded by the nucleic acid molecule comprising the sequence as set forth in SEQ ID NO: 10.
- an UGT91 D1 comprising the amino acid sequence as set forth in SEQ ID NO: 31 or fragments and variants thereof.
- an UGT91 D1 encoded by the nucleic acid molecule comprising the sequence as set forth in SEQ ID NO: 32.
- an UGT91 D2 comprising the amino acid sequence as set forth in SEQ ID NO: 33 or fragments and variants thereof.
- an UGT76G1 encoded by the nucleic acid molecule comprising the sequence as set forth in SEQ ID NO: 34.
- the UGT74G1 comprises the amino acid sequence as set forth in SEQ ID NO: 13 or fragments and variants thereof.
- the UGT74G1 is encoded by the nucleic acid molecule comprising the sequence as set forth in SEQ ID NO: 14.
- the invention provides for a cyclodextrin glucanotransferase.
- Cyclodextrin-glucanotransferase is commercially available (CGTase, Toruzyme 3.0L, trademark of Novozymes Inc.).
- sucrose synthase comprises the amino acid sequence as set forth in SEQ ID NO: 15, 17, 19, 21 , 23 or 25 or fragments and variants thereof.
- the polypeptide comprises an amino acid sequence encoded by the nucleic acid molecule comprises comprising the sequence as set forth in SEQ ID NO: 16,18, 20, 22, 24 or 26.
- polypeptide comprising a sequence at least 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99% or 100% percent identity to any one of the sequences set forth in SEQ ID NOs: 1 , 3, 5, 7, 9, 13, 15, 17, 19, 21 , 23, 25, 27, 29, 31 and 33 and fragments thereof.
- SEQ ID NOs: 1 3, 5, 7, 9, 13, 15, 17, 19, 21 , 23, 25, 27, 29, 31 and 33 and fragments thereof.
- the nucleic acid encodes an UDP-glucosyltransferase having the sequence SDFGLDQ at a position
- fragments are at least 10, at least 20, at least 50 amino acids in length.
- the polypeptide sequences contain heterologous sequences including but not limited to purification tags such as a HIS tag.
- a polypeptide comprising a 6X HIS tag at the N-terminus there is provided a polypeptide comprising a 6X HIS tag at the C-terminus.
- glycosyltransf erases including glucosyltransferases and cyclodextrin glucanotransferases are known in the art. As such, a worker skilled in the art could readily determine if the glycosyltransf erases are capable of primary, secondary and/or tertiary glycosylations (see, for example Dewitte et al., J Biotechnol. 2016 Sep 10;233:49-55. doi: 10.1016/j.jbiotec.2016.06.034; Grubb et al., Plant J. (2014) 79, 92- 105; Richman et al., Plant J.
- the present invention further provides cells and plants which express one or more of the polypeptides of the present invention.
- the cells and plants may naturally express one or more of the polypeptides of the present invention or have been modified to express one or more the polypeptides of the present invention.
- the cells may be prokaryotic or eukaryotic cells and include but are not limited to, E. coli, yeast such as Pichia pastoris, Stevia rebaudiana, Phytolacca Americana, Cannabis including but not limited to Cannabis sativa, Cannabis indica and Cannabis ruderalis.
- a cell which expresses an UGT76G1 or UGT76G1 -like glucosyltransferase (such as UGT76G2 and UGT76H1 ). Accordingly, in certain embodiments, there is provided a cell which expresses an UGT76G1 glucosyltransferase comprising a sequence encoding the amino acid sequence as set forth in any one of SEQ ID NOs: 1 , 3, 5 and 7. In certain embodiments, there is provided a cell which expresses an UGT76G1 -like glucosyltransferase comprising a sequence encoding the amino acid sequence as set forth in SEQ ID NO: 27 or 29.
- the cell may further express further glucosyltransferases, such as Os03g0702000 or Os03g0702000-like glucosyltransferase (such as UGT91 D1 and UGT91 D2) and/or a sucrose synthase, such as the sucrose synthase comprising the sequence as set forth in SEQ ID NO: 15, 17, 19, 21 , 23 or 25.
- glucosyltransferases such as Os03g0702000 or Os03g0702000-like glucosyltransferase (such as UGT91 D1 and UGT91 D2) and/or a sucrose synthase, such as the sucrose synthase comprising the sequence as set forth in SEQ ID NO: 15, 17, 19, 21 , 23 or 25.
- a cell which expresses UGT76G1 glucosyltransferase comprising a sequence encoding the amino acid sequence as set forth in any one of SEQ ID NOs: 1 , 3, 5 and 7 and Os03g0702000 glucosyltransferase comprising the sequence as set forth in SEQ ID NO:10.
- the cell may further express a sucrose synthase comprising the sequence as set forth in SEQ ID NO: 15, 17, 19, 21 , 23 or 25.
- a cell which expresses an Os03g0702000 or Os03g0702000-like glucosyltransferase expresses an Os03g0702000 or Os03g0702000-like glucosyltransferase. Accordingly, in certain embodiments, there is provided a cell which expresses Os03g0702000 glucosyltransferase comprising a sequence encoding the amino acid sequence as set forth in SEQ ID NO: 10. The cell may further express a sucrose synthase, such as the sucrose synthase comprising the sequence as set forth in SEQ ID NO: 15, 17, 19, 21 , 23 or 25.
- Transgenic cells and plants can be produced by a variety of well established techniques. Following construction of a vector, most typically an expression cassette, including a polynucleotide of the invention, standard techniques can be used to introduce the polynucleotide into cell or a plant. Optionally, the plant cell, explant or tissue can be regenerated to produce a transgenic plant.
- Cannabis plants genetically engineered to express one or more of the proteins of the invention are provided.
- a tissue specific promoter such as a secretory trichomes specific promoter may be used such that the proteins of the invention are expressed in the same tissue that cannabinoids are produced in, namely the secretory trichomes of the plant.
- Suitable promoter elements include the promoter for the cytosolic 0-acetylserine(thiol)lyase (OASA1 ) enzyme from Arabidopsis thaliana (Gutierrez-Alcala 2005).
- Transformation and regeneration of plant cells is now routine, and the selection of the most appropriate transformation technique will be determined by the practitioner. Suitable methods can include, but are not limited to: electroporation of plant protoplasts; liposome- mediated transformation; polyethylene glycol (PEG) mediated transformation; transformation using viruses; micro-injection of plant cells; micro-projectile bombardment of plant cells; vacuum infiltration; and Agrobacterium tumeficiens mediated transformation. Transformation means introducing a nucleotide sequence into a plant in a manner to cause stable or transient expression of the sequence.
- plants may be selected using a dominant selectable marker incorporated into the transformation vector.
- a dominant selectable marker will confer antibiotic or herbicide resistance on the transformed plants, and selection of transformants can be accomplished by exposing the plants to appropriate concentrations of the antibiotic or herbicide.
- the present invention further provides methods for the production of cannabinoid glycoside prodrugs and the cannabinoid glycosides prodrugs produced by the methods.
- the methods may be in vitro or in vivo (in a cell system or in planta).
- a method of producing cannabinoid glycoside prodrugs comprising incubating a cannabinoid aglycone with one or more sugar donors in the presence of one or more glycosyltransferases.
- the aglycones include but are not limited to: cannabinoids, including but not limited to cannabidiol, cannabidivarin, cannabigerol, tetrahydrocannabinol, cannabinol and cannabidiolic acid, endocannabinoids including but not limited to arachidonoylethanolamide (anandamide, AEA), 2-arachidonoylethanolamide (2-AG), 1 -arachidonoylethanolamide (1 -AG), and docosahexaenoyl ethanolamide (DHEA, synaptamide); and vanilloids including but not limited to vanillin, curcumin, and capsaicin.
- cannabinoids including but not limited to cannabidiol, cannabidivarin, cannabigerol, tetrahydrocannabinol, cannabinol and cannabidiolic acid
- endocannabinoids including but not limited to arachi
- the one or more sugar donors will be dependent on the one or more glycosyltransf erases used in the method and/or the desired end products.
- the sugar donors include but are not limited to UDP-glucose, UDP-glucuronic acid, UDP-mannose, UDP-fructose, UDP-xylose, UDP-fluorodeoxyglucose, and UDP-rhamnose.
- the sugar donor includes maltodextrin.
- a method of producing a cannabinoid glycoside comprising incubating an aglycone with a sugar donor in the presence of a glycosyltransferase. Also provided are the cannabinoid glycosides produced by the above method. In specific embodiments, there is provided a method of producing a cannabinoid glycoside, said method comprising incubating an aglycone with UDP-glucose, in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase under conditions that allow for glycosylation.
- a method of producing a glycoside prodrug comprising incubating an aglycone with maltodextrin, in the presence of a cyclodextrin glucanotransferase under conditions that allow for glycosylation.
- An exemplary method for producing cannabinoid-glycosides comprises incubating a cannabinoid, with UDP-glucose in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase under conditions which allow for glycosylation. Also provided are cannabinoid-glycosides produced by the above method.
- a further exemplary method for producing cannabinoid-glycosides comprises incubating a cannabinoid with maltodextrin in the presence of a cyclodextrin glucanotransferase under conditions which allow for glycosylation. Also provided are cannabinoid-glycosides produced by the above method.
- a method of producing a cannabinoid glycoside comprising incubating an aglycone with one or more sugar donors in the presence of a first glycosyltransf erase and a second glycosyltransferase under conditions which allow for glycosylation. Also provided are cannabinoid glycosides produced by the above method.
- first glycosyltransferase and a second glycosyltransferase may be provided concurrently or added sequentially.
- sugar donors may be provided concurrently or added sequentially.
- the structure of the resulting cannabinoid glycoside may be dependent on the order the glycosyltransferases are provided.
- the ratio of first to second glycosyltransferase may impact the resulting products.
- the activity levels of the glycosyltransferases may dictate the ratios and the ratios could be readily determined by a worker skilled in the art.
- the ratios first to second glycosyltransferase include but are not limited to 1 :1 , 1 :2, 1 :10, 1 :50 and vice versa.
- a method of producing a cannabinoid glycoside comprising incubating an aglycone with UDP-glucose in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase and Os03g0702000 or Os03g0702000-like glucosyltransferase under conditions which allow for glycosylation.
- a method of producing a cannabinoid glycoside comprising incubating an aglycone with UDP-glucose and maltodextrin in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase and cyclodextrin glucanotransferase under conditions which allow for glycosylation.
- cannabinoid glycosides produced by the above methods.
- An exemplary method for producing cannabinoid-glycosides comprises incubating cannabinoid, including but not limited to cannabidiol, cannabidivarin, canabigerol, tetrahydrocannabinol, cannabinol and cannabidiolic acid, with UDP-glucose in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase and Os03g0702000 or Os03g0702000-like glucosyltransferase under conditions which allow for glycosylation. Also provided are cannabinoid-glycosides produced by the above method.
- a further exemplary method for producing cannabinoid-glycosides comprises incubating cannabinoids with UDP-glucose and maltodextrin in the presence of a UGT76G1 or UGT76G1 -like glucosyltransferase and and cyclodextrin glucanotransferase under conditions which allow for glycosylation. Also provided are cannabinoid-glycosides produced by the above method.
- each of the above described glycosylation methods may be applied to a lower order cannabinoid glycoside to form a higher order cannabinoid glycoside.
- a cannabinoid monoglycoside may be glycosylated using any of the glycosylation methods of the present invention to form a diglycoside, or a cannabinoid diglycoside may be glycosylated to form a triglycoside, etc.
- Methods of purifying the cannabinoid glycosides include for example solid phase extraction, such as column purification.
- the invention also provides cell culture and in planta methods for the production of cannabinoid glycosides.
- the methods comprise expressing one or more of the glycosyltransferases in a cell or plant which produces the aglycone and isolating the cannabinoid glycosides.
- one or more sucrose synthases are also expressed.
- Appropriate vectors and genetic engineering methods are known in the art.
- the invention also provides methods for the conversion of UDP to UDPG utilizing the sucrose synthases of the present invention. Accordingly, in certain embodiments of the methods of producing cannabinoid glycosides which utilize UDP-glucose as a sugar donor, the methods further comprise the use of sucrose synthase to recycle UDP. In certain embodiments, there is provided a method of producing a cannabinoid glycoside, said method comprising incubating aglycone with UDP-glucose, in the presence of a UGT76G1 glucosyltransferase and a sucrose synthase under conditions that allow for glycosylation.
- Example 1 Conversion of cannabinoids to cannabinoid glycoside prodrugs
- Glycosylation reactions consisted of 50mM KP0 4 pH 7.2, 3mM MgCI 2 , 0.005% CBD, 2.5% UGT76G1 purified enzyme preparation, and 2.5mM UDP-glucose. Buffers were degassed and tubes were purged with nitrogen, reactions were protected from light and incubated at 28°C with 180rpm agitation for 18 hours. Reactions were then extracted 3x with an equal volume of ethyl acetate, evaporated to dryness, and dissolved in a half volume of HPLC grade methanol. 50 microliters was injected on a reverse phase C18 column and eluted with a gradient of acetonitrile starting at 10% and increasing to 99%.
- UGT76G1 was produced through expression in Pichia pastoris and purified through standard molecular biology techniques.
- the UGT76G1 enzyme was found to glycosylate CBD in a UDP-glucose dependent manner. This activity was also proportional to the amount of UDP-glucose present. Incubation temperature was 28 °C, and an acceptable range would be 20 °C to 30 °C as high temperatures can cause significant degradation of CBD. Reactions were carried out in the dark to prevent photo- degradation of the substrates. Gentle agitation from 120 to 200rpm were used to mix the reactions in an inert atmosphere.
- Substrate CBD in the reactions was replaced with A9THC and CBDV and performed in an identical fashion with similar results. Enzyme combinations needed to create various products are listed in Table 4 for CBD-glycosides, Table 5 for CBDV-glycosides, and Table 6 for A9THC-glycosides.
- Enzymatic reactions are performed as described in Example 1 but with the inclusion of recombinant Os03g0702000 enzyme at a 1 :2 ratio relative to UGT76G1 .
- Samples were extracted and analyzed as in Example 1 .
- Recombinant Os03g0702000 enzyme was codon optimized and expressed in E. coli BL21 -DE3 cells and purified by immobilized metal ion chromatography.
- Example 3 Conversion of CBD to alpha-glycoside linked CBD compounds.
- the resulting products contain a ⁇ -glycosylation on the cannabinoid backbone, and a-glycosylations emanating from the primary sugar.
- This additional treatment created a new category of compounds termed ⁇ -primed, a-glycosylated cannabinoids.
- Glycoside products were generated through the aforementioned biocatalytic reactions and purified to homogeneity by C18 solid phase extraction.
- 100mg Hypersep C18 columns (Thermo) were hydrated in methanol, rinsed with 50% methanol in water, rinsed with water, glycosylation reaction passed through the column, washed with water, washed with 10%, 20%, and 30% methanol, and the glycoside products were eluted with 45 and 60% methanol in water. Eluates were dried and extracted with ethyl acetate, and dried to completion to yield >95% pure cannabinoid -glycosides for further analysis and testing.
- HPLC linetraces of the reaction products of glycosylation reactions of the cannabinoid aglycones CBD, CBDV, A9-THC, CBN, 1 -AG and 2-AG, DHEA, AEA, capsaicin, and vanillin, are provided in Figures 16 to 24, respectively. Enzymatic reactions were performed as described in Example 1 .
- the solid lines indicate the elution profile of the starting aglycone and the dashed lines indicate the elution profile of the glycosylation reaction product mixture.
- the CBD aglycone retention time is 13.65 minutes, and product peaks are observed at 8.87, 9.02, 9.97, 10.33, and 10.37 min.
- the CBDV aglycone retention time is 12.75 minutes, and product peaks are observed at 8.53, 9.70, and 10.01 min.
- the THC aglycone retention time is 14.45 minutes, and product peaks are observed at 9.46, 10.67, 10.97, 1 1 .28, 1 1 .67, and 12.49 min.
- the CBN aglycone retention time is 14.32 minutes, and product peaks are observed at 10.87, 1 1 .50, and 12.25 min.
- the 1 -AG aglycone retention time is 14.18 minutes and the 2-AG aglycone retention time is 14.32 minutes, and product peaks are observed at 1 1 .40, 1 1 .78, 1 1 .83, 1 1 .97, 12.53, 12.92, 13.07, and 13.35 min.
- the vanillin aglycone retention time is 1 .95 minutes and product peaks are observed from 1 .25 to 1 .35 min.
- the capsaicin aglycone retention time is 1 1 .73 minutes, and product peaks are observed at 10.23 min.
- Example 6A LCMS analysis of CBD glycosides
- the LC column used was a Silia Chrom XDB C18 5um, 150A, 4.6X50 mm. The method was 12 min 5 to 95 H 2 0:ACN gradient .
- electrospray ionization ESI was performed in positive mode.
- Example 6B LCMS analysis of ⁇ -THC glycosides
- Figure 27 depicts the 1 NMR spectra of isolated VB104 and Figure 28 depicts the 1 H MR spectra of isolated VB1 10. Each of these products was isolated from the reaction mixture produced by the glycosylation reaction of CBD. The 1 H NMR spectra of 10 mg/ml solutions of each compound prepared in CD 3 OD were obtained on a Bruker Avance II 400 MHz instrument using TopSpin acquisition and processing software.
- Example 8 Solubility Analysis [00264] C18 retention times were empirically determined on a linear ramp of increasing acetonitrile on a Phenomenex Kinetex 2.6u 100A C18 column, on a Dionex HPLC equipped with Diode Array Detector. CLogP values in Table A were predicted by ChemDraw (CambridgeSoft). Reference cannabinoids were analyzed by HPLC and established logP values (http://pubchem.ncbi.nlm.nih.gov/) and used to create a calibration line as depicted in Figure 29. The predicted cLogP values correlated with the reference calibration line. C18 reverse phase HPLC retention times were plotted against the cLogP values presented in Table A, as depicted in Figure 29.
- VB1 10 was administered to three mice by oral gavage and the animals sacrificed at 30, 60, and 90 minutes. Eight week old male Swiss mice were fasted for 12 hours prior to administration of 120mg/kg VB1 10 in 10% Ethanol USP, 10% Propylene Glycol USP, 0.05% Sodium Deoxycholate USP, 79.95% Saline USP. Following termination and tissue harvest, the intestinal contents were then extracted and analyzed by C18 reverse phase HPLC. As shown in Figure 30A, the small intestinal contents showed intact VB1 10, but no decoupled CBD. As shown in Figure 30B, the large intestinal contents contained both VB1 10 and CBD in the 60 and 90 minute time points. This decoupling of VB1 10 is consistent with the large intestinal decoupling seen for sennoside beta-glycosides, and is the result of secreted beta-glycosidases from the large intestinal microflora.
- Example 10 Analysis of Large Intestine Contents Upon Administration of CBD and CBD Glycosides
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Rheumatology (AREA)
- Pain & Pain Management (AREA)
- Crystallography & Structural Chemistry (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Saccharide Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Abstract
Description
Claims
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201680060327.5A CN108136208B (en) | 2015-09-22 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis |
| EP16849598.4A EP3352855A4 (en) | 2015-09-22 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis |
| JP2018516020A JP2018528977A (en) | 2015-09-22 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis |
| BR112018005639A BR112018005639A2 (en) | 2015-09-22 | 2016-09-22 | cannabinoid glycoside prodrugs and synthesis methods |
| MX2018004848A MX2018004848A (en) | 2015-10-23 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis. |
| US15/762,180 US11207414B2 (en) | 2015-09-22 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis |
| CA2999764A CA2999764A1 (en) | 2015-09-22 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis |
| AU2016326518A AU2016326518B2 (en) | 2015-09-22 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis |
| US17/527,685 US20220168428A1 (en) | 2015-09-22 | 2021-11-16 | Cannabinoid glycoside prodrugs and methods of synthesis |
| US18/182,618 US20230346952A1 (en) | 2015-09-22 | 2023-03-13 | Cannabinoid glycoside prodrugs and methods of synthesis |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562222144P | 2015-09-22 | 2015-09-22 | |
| US62/222,144 | 2015-09-22 | ||
| US201562245928P | 2015-10-23 | 2015-10-23 | |
| US62/245,928 | 2015-10-23 | ||
| US201662363808P | 2016-07-18 | 2016-07-18 | |
| US62/363,808 | 2016-07-18 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/762,180 A-371-Of-International US11207414B2 (en) | 2015-09-22 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis |
| US17/527,685 Division US20220168428A1 (en) | 2015-09-22 | 2021-11-16 | Cannabinoid glycoside prodrugs and methods of synthesis |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017053574A1 true WO2017053574A1 (en) | 2017-03-30 |
Family
ID=58387412
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2016/053122 WO2017053574A1 (en) | 2015-09-22 | 2016-09-22 | Cannabinoid glycoside prodrugs and methods of synthesis |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US11207414B2 (en) |
| EP (1) | EP3352855A4 (en) |
| JP (1) | JP2018528977A (en) |
| CN (1) | CN108136208B (en) |
| AU (1) | AU2016326518B2 (en) |
| BR (1) | BR112018005639A2 (en) |
| CA (1) | CA2999764A1 (en) |
| WO (1) | WO2017053574A1 (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018208875A1 (en) | 2017-05-09 | 2018-11-15 | Vitality Biopharma, Inc. | Antimicrobial compositions comprising cannabinoids and methods of using the same |
| WO2019082171A1 (en) * | 2017-10-27 | 2019-05-02 | Alvit Pharma | Oral cannabinoid compositions with improved bioavailability |
| US10378020B2 (en) | 2017-03-24 | 2019-08-13 | Trait Biosciences, Inc. | High level in vivo biosynthesis and isolation of water-soluble cannabinoids in plant systems |
| US20190316144A1 (en) * | 2017-07-11 | 2019-10-17 | Trait Biosciences Inc. | In vivo generation of water-soluble cannabinoids in plant cell suspension cultures |
| WO2020061687A1 (en) * | 2018-09-28 | 2020-04-02 | Visceral Therapeutics Inc. | Pharmaceutically active cannabis-based compositions and methods of use for treating gastrointestinal conditions |
| CN111712507A (en) * | 2017-12-21 | 2020-09-25 | 药物有限公司 | Method for improving oral bioavailability of drugs |
| WO2020239784A1 (en) | 2019-05-27 | 2020-12-03 | Octarine Bio Ivs | Genetically modified host cells producing glycosylated cannabinoids. |
| WO2021146687A1 (en) * | 2020-01-16 | 2021-07-22 | Cannabis Global, Inc. | A cannaboside composition and method to produce |
| WO2021173130A1 (en) | 2020-02-26 | 2021-09-02 | Vitality Biopharma, Inc. | Novel cannabinoid glycosides and uses thereof |
| US11207414B2 (en) | 2015-09-22 | 2021-12-28 | Graphium Biosciences, Inc. | Cannabinoid glycoside prodrugs and methods of synthesis |
| WO2022126028A1 (en) * | 2020-12-11 | 2022-06-16 | Graphium Biosciences, Inc. | Continuous enzymatic perfusion reactor system |
| US11466281B2 (en) | 2017-07-11 | 2022-10-11 | Trait Biosciences Inc. | Generation of water-soluble cannabinoid compounds in yeast and plant cell suspension cultures and compositions of matter |
| EP3923915A4 (en) * | 2019-02-11 | 2023-03-22 | John Robert Chancey | Methods of making and using phytocannabinoids complexed with a protein, peptide, amino acid, polysaccharide, disaccharide, ormonosaccharide |
| WO2023053134A1 (en) * | 2021-10-01 | 2023-04-06 | Council Of Scientific & Industrial Research | Cannabinoids c- and o-glycosides possessing anti-proliferative and anti-metastatic properties and process for preparation thereof |
| US11905543B2 (en) | 2017-07-11 | 2024-02-20 | Trait Biosceinces, Inc. | In vivo generation of water-soluble acetylated cannabinoid glycoside compounds in plant cell suspension cultures |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10239808B1 (en) | 2016-12-07 | 2019-03-26 | Canopy Holdings, LLC | Cannabis extracts |
| US11202771B2 (en) | 2018-01-31 | 2021-12-21 | Treehouse Biotech, Inc. | Hemp powder |
| CA3119729A1 (en) | 2018-10-10 | 2020-04-16 | Treehouse Biotech, Inc. | Synthesis of cannabigerol |
| CN109943547B (en) * | 2019-04-18 | 2022-07-19 | 安徽农业大学 | Tea tree sucrose synthase CsSUS587, preparation method and application |
| CN110201019A (en) * | 2019-07-17 | 2019-09-06 | 李卫 | It is a kind of for treating the composition and preparation method thereof containing cannabidiol of muscle cramp |
| CN110693027A (en) * | 2019-10-14 | 2020-01-17 | 桂林莱茵生物科技股份有限公司 | Water-dispersible cannabidiol product and preparation method thereof |
| CN112010924B (en) * | 2020-09-04 | 2022-09-09 | 中国药科大学 | Novel Nosiheptide glycosylated derivative and preparation method and application thereof |
| CA3197361A1 (en) * | 2020-11-07 | 2022-05-12 | Mathias Schuetz | Production of glycosylated cannabinoids |
| CN114573648A (en) * | 2020-11-30 | 2022-06-03 | 云南汉盟制药有限公司 | Cannabinoid glycosides and methods of making the same |
| WO2023240221A2 (en) * | 2022-06-11 | 2023-12-14 | Trait Biosciences, Inc. | System and methods for sequential desorption of cannabidiol (cbd) glycoside species |
| DE102022004596A1 (en) * | 2022-12-08 | 2024-06-13 | Biosynth Gmbh | Novel cannabinoid oligosaccharides |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5292899A (en) * | 1991-11-27 | 1994-03-08 | Synthetic Technology Corporation | Synthesis of 11-nor-Δ-9-tetrahydrocannabinol-9-carboxylic acid glucuronide |
| US5627270A (en) * | 1991-12-13 | 1997-05-06 | Trustees Of Princeton University | Glycosylated steroid derivatives for transport across biological membranes and process for making and using same |
| WO2009158499A2 (en) * | 2008-06-25 | 2009-12-30 | University Of North Texas Health Science Center At Fort Worth | Prevention of bacterial growth and biofilm formation by ligands that act on cannabinoidergic systems |
| US8410064B2 (en) * | 2009-08-24 | 2013-04-02 | The Board Of Trustees Of The University Of Arkansas | Classical cannabinoid metabolites and methods of use thereof |
| WO2014122227A2 (en) * | 2013-02-06 | 2014-08-14 | Evolva Sa | Methods for improved production of rebaudioside d and rebaudioside m |
| US20140298511A1 (en) * | 2013-03-15 | 2014-10-02 | Biotech Institute, Llc | Breeding, production, processing and use of medical cannabis |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009018389A1 (en) | 2007-07-30 | 2009-02-05 | Alltranz Inc. | Prodrugs of cannabidiol, compositions comprising prodrugs of cannabidiol and methods of using the same |
| JP5551604B2 (en) | 2007-11-30 | 2014-07-16 | オールトランツ インコーポレイティド | Tetrahydrocannabinol prodrug, composition comprising tetrahydrocannabinol prodrug, and method of using the same |
| MX2011011445A (en) | 2009-04-29 | 2011-11-18 | Univ Kentucky Res Found | Cannabinoid-containing compositions and methods for their use. |
| WO2012011112A1 (en) | 2010-07-22 | 2012-01-26 | Yissum Research Development Company Of The Hebrew University Of Jerusalem, Ltd. | Non psychoactive cannabinoids and uses thereof |
| IT1402018B1 (en) | 2010-10-11 | 2013-08-28 | Indena Spa | FORMULATIONS FOR THE TREATMENT OF THE AFFECTIONS OF THE FIRST RESPIRATORY ROUTES. |
| ES2690550T3 (en) | 2011-01-13 | 2018-11-21 | Austin Research Labs Corp. | High load dispersions for the treatment of infections |
| CN105209424B (en) | 2013-01-08 | 2019-12-13 | 耶路撒冷希伯来大学伊森姆研究发展有限公司 | Fluorinated CBD compounds, compositions and uses thereof |
| ES2784229T3 (en) * | 2013-11-20 | 2020-09-23 | Panag Pharma Inc | Compositions and procedures for the treatment of eye inflammation and pain |
| US9497299B2 (en) | 2014-09-18 | 2016-11-15 | Blackberry Limited | Configuring a discard timer |
| MA42268A (en) | 2015-06-23 | 2018-05-02 | Axim Biotechnologies Inc | ANTIMICROBIAL COMPOSITIONS CONTAINING CANNABINOIDS |
| US11207414B2 (en) | 2015-09-22 | 2021-12-28 | Graphium Biosciences, Inc. | Cannabinoid glycoside prodrugs and methods of synthesis |
| AU2017250303B2 (en) | 2016-04-15 | 2019-09-26 | Teewinot Technologies Limited | Biosynthesis of cannabinoid prodrugs |
| EP3484469B1 (en) | 2016-07-14 | 2024-05-22 | Scisparc Ltd | Compositions and methods of potentiating antimicrobials |
| WO2018176055A2 (en) | 2017-03-24 | 2018-09-27 | Trait Biosciences, Inc. | High level in vivo biosynthesis and isolation of water-soluble cannabinoids in plant systems |
| CA3063186A1 (en) | 2017-05-09 | 2018-11-15 | Vitality Biopharma, Inc. | Antimicrobial compositions comprising cannabinoids and methods of using the same |
| US20200046639A1 (en) | 2017-07-11 | 2020-02-13 | Trait Biosciences Inc. | Consumable water-soluble cannabinoid food and beverage additive having enhanced stability |
-
2016
- 2016-09-22 US US15/762,180 patent/US11207414B2/en active Active
- 2016-09-22 CN CN201680060327.5A patent/CN108136208B/en active Active
- 2016-09-22 AU AU2016326518A patent/AU2016326518B2/en active Active
- 2016-09-22 EP EP16849598.4A patent/EP3352855A4/en active Pending
- 2016-09-22 BR BR112018005639A patent/BR112018005639A2/en not_active IP Right Cessation
- 2016-09-22 JP JP2018516020A patent/JP2018528977A/en active Pending
- 2016-09-22 WO PCT/US2016/053122 patent/WO2017053574A1/en active Application Filing
- 2016-09-22 CA CA2999764A patent/CA2999764A1/en active Pending
-
2021
- 2021-11-16 US US17/527,685 patent/US20220168428A1/en active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5292899A (en) * | 1991-11-27 | 1994-03-08 | Synthetic Technology Corporation | Synthesis of 11-nor-Δ-9-tetrahydrocannabinol-9-carboxylic acid glucuronide |
| US5627270A (en) * | 1991-12-13 | 1997-05-06 | Trustees Of Princeton University | Glycosylated steroid derivatives for transport across biological membranes and process for making and using same |
| WO2009158499A2 (en) * | 2008-06-25 | 2009-12-30 | University Of North Texas Health Science Center At Fort Worth | Prevention of bacterial growth and biofilm formation by ligands that act on cannabinoidergic systems |
| US8410064B2 (en) * | 2009-08-24 | 2013-04-02 | The Board Of Trustees Of The University Of Arkansas | Classical cannabinoid metabolites and methods of use thereof |
| WO2014122227A2 (en) * | 2013-02-06 | 2014-08-14 | Evolva Sa | Methods for improved production of rebaudioside d and rebaudioside m |
| US20140298511A1 (en) * | 2013-03-15 | 2014-10-02 | Biotech Institute, Llc | Breeding, production, processing and use of medical cannabis |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3352855A4 * |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11207414B2 (en) | 2015-09-22 | 2021-12-28 | Graphium Biosciences, Inc. | Cannabinoid glycoside prodrugs and methods of synthesis |
| US11352634B2 (en) | 2017-03-24 | 2022-06-07 | Trait Biosciences, Inc. | Methods for increasing cannabinoid production in cannabis plants |
| US11339400B2 (en) | 2017-03-24 | 2022-05-24 | Trait Biosciences, Inc. | Systems, methods, and compositions for high-level production of water-soluble cannabinoids in a plant trichome |
| US10378020B2 (en) | 2017-03-24 | 2019-08-13 | Trait Biosciences, Inc. | High level in vivo biosynthesis and isolation of water-soluble cannabinoids in plant systems |
| US11773402B2 (en) | 2017-03-24 | 2023-10-03 | Trait Biosciences Inc. | High level in vivo biosynthesis and isolation of water-soluble cannabinoids in stably transformed plant systems |
| US11447788B2 (en) | 2017-03-24 | 2022-09-20 | Trait Biosceinces, Inc. | System, methods, and compositions for high-level production of water-soluble cannabinoids in a plant cell culture |
| WO2018208875A1 (en) | 2017-05-09 | 2018-11-15 | Vitality Biopharma, Inc. | Antimicrobial compositions comprising cannabinoids and methods of using the same |
| US11207291B2 (en) | 2017-05-09 | 2021-12-28 | Graphium Biosciences, Inc. | Antimicrobial compositions comprising cannabinoids and methods of using the same |
| US20220071950A1 (en) * | 2017-05-09 | 2022-03-10 | Graphium Biosciences, Inc. | Antimicrobial compositions comprising cannabinoids and methods of using the same |
| US11946059B2 (en) | 2017-07-11 | 2024-04-02 | Trait Biosciences, Inc. | In vivo generation of water-soluble cannabinoids in plant cell suspension cultures |
| US11905543B2 (en) | 2017-07-11 | 2024-02-20 | Trait Biosceinces, Inc. | In vivo generation of water-soluble acetylated cannabinoid glycoside compounds in plant cell suspension cultures |
| US11466281B2 (en) | 2017-07-11 | 2022-10-11 | Trait Biosciences Inc. | Generation of water-soluble cannabinoid compounds in yeast and plant cell suspension cultures and compositions of matter |
| US20190316144A1 (en) * | 2017-07-11 | 2019-10-17 | Trait Biosciences Inc. | In vivo generation of water-soluble cannabinoids in plant cell suspension cultures |
| WO2019082171A1 (en) * | 2017-10-27 | 2019-05-02 | Alvit Pharma | Oral cannabinoid compositions with improved bioavailability |
| CN111712507B (en) * | 2017-12-21 | 2024-02-06 | 药物有限公司 | Method for improving oral bioavailability of drugs |
| US11633484B2 (en) * | 2017-12-21 | 2023-04-25 | Pharmacytics B.V. | Method for improving the oral bioavailability of a drug |
| CN111712507A (en) * | 2017-12-21 | 2020-09-25 | 药物有限公司 | Method for improving oral bioavailability of drugs |
| WO2020061687A1 (en) * | 2018-09-28 | 2020-04-02 | Visceral Therapeutics Inc. | Pharmaceutically active cannabis-based compositions and methods of use for treating gastrointestinal conditions |
| EP3923915A4 (en) * | 2019-02-11 | 2023-03-22 | John Robert Chancey | Methods of making and using phytocannabinoids complexed with a protein, peptide, amino acid, polysaccharide, disaccharide, ormonosaccharide |
| WO2020239784A1 (en) | 2019-05-27 | 2020-12-03 | Octarine Bio Ivs | Genetically modified host cells producing glycosylated cannabinoids. |
| CN114207108A (en) * | 2019-05-27 | 2022-03-18 | 奥克塔林生物制剂公司 | Genetically modified host cells producing glycosylated cannabinoids |
| WO2021146687A1 (en) * | 2020-01-16 | 2021-07-22 | Cannabis Global, Inc. | A cannaboside composition and method to produce |
| US20220227726A1 (en) * | 2020-02-26 | 2022-07-21 | Graphium Biosciences, Inc. | Cannabinoid glycosides and uses thereof |
| WO2021173130A1 (en) | 2020-02-26 | 2021-09-02 | Vitality Biopharma, Inc. | Novel cannabinoid glycosides and uses thereof |
| WO2022126028A1 (en) * | 2020-12-11 | 2022-06-16 | Graphium Biosciences, Inc. | Continuous enzymatic perfusion reactor system |
| WO2023053134A1 (en) * | 2021-10-01 | 2023-04-06 | Council Of Scientific & Industrial Research | Cannabinoids c- and o-glycosides possessing anti-proliferative and anti-metastatic properties and process for preparation thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2016326518B2 (en) | 2022-03-24 |
| US20220168428A1 (en) | 2022-06-02 |
| US20180264122A1 (en) | 2018-09-20 |
| CN108136208B (en) | 2022-07-15 |
| BR112018005639A2 (en) | 2018-10-09 |
| AU2016326518A1 (en) | 2018-04-26 |
| EP3352855A4 (en) | 2019-05-22 |
| CN108136208A (en) | 2018-06-08 |
| JP2018528977A (en) | 2018-10-04 |
| EP3352855A1 (en) | 2018-08-01 |
| US11207414B2 (en) | 2021-12-28 |
| CA2999764A1 (en) | 2017-03-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2016326518B2 (en) | Cannabinoid glycoside prodrugs and methods of synthesis | |
| EP3184537B1 (en) | Glycosylated indolocarbazoles, method for obtaining same and uses thereof | |
| Hardman et al. | Cannabinoid glycosides: In vitro production of a new class of cannabinoids with improved physicochemical properties | |
| US20220227726A1 (en) | Cannabinoid glycosides and uses thereof | |
| Shen et al. | Structural and pharmacological diversity of 1, 4-naphthoquinone glycosides in recent 20 years | |
| Parajuli et al. | Regiospecific biosynthesis of tamarixetin derivatives in Escherichia coli | |
| Wu et al. | Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of glycoconjugates of phenanthroindolizidines alkaloids | |
| KR101379978B1 (en) | New polyene compound, preparation method thereof and antifungal agent comprising the same | |
| Shimoda et al. | Glycosylation of daidzein by the Eucalyptus cell cultures | |
| US20230346952A1 (en) | Cannabinoid glycoside prodrugs and methods of synthesis | |
| Hoang et al. | Biochemical characterization of recombinant UDP-glucose: sterol 3-O-glycosyltransferase from Micromonospora rhodorangea ATCC 31603 and enzymatic biosynthesis of sterol-3-O-β-glucosides | |
| US20230192748A1 (en) | Etoposide Glycosides, Methods Of Making, And Uses Thereof As An Anti-Cancer Drug | |
| KR101575295B1 (en) | Novel Epothilone Glycosides and Method for Preparing the Same | |
| EP2627661B1 (en) | Glycosylated aminocoumarins and methods of preparing and uses of same | |
| CN113444135A (en) | Antrodia camphorata tetracyclic triterpene glycoside, and enzymatic preparation method and application thereof | |
| KR101704706B1 (en) | Method for Preparing Sterol Glucosides Using Sterol Glucosyltransferase | |
| KR101621426B1 (en) | Novel Alpha-Mangostin Glycosides Derivates and Method for Preparing the Same | |
| KR102141656B1 (en) | Method for bioconversion of corticosterone by glucosyltransferase and corticosterone glucoside produced thereby | |
| KR101966882B1 (en) | New Glycoside Derivatives of Genistein, Pharmaceutical Compositions for Treating Cancer Diseases Containing The Same and Method for Preparing the Same | |
| KR100548743B1 (en) | -3-2-5 Novel Inhibitor F-3-2-5 against Cell Cycle Regulating Factor Preparing Method Thereof and Use for Anti-Cancer Agent | |
| CN111138444A (en) | Epothilone B glucoside compounds and enzymatic preparation and application thereof | |
| Bader et al. | Sandacrabins-Antiviral and Insecticidal Farnesylated Benzimidazoles Produced by a Rare Myxobacterium | |
| KR101666667B1 (en) | Sugar-attached phenoxodiol compounds, preparing method thereof and pharmaceutical compositions containing the same as active ingredients | |
| Hamada et al. | Optical Resolution of (RS)-Denopamine to (R)-Denopamine β-D-Glucoside by Glucosyltransferase from Phytolacca americana Expressed in Recombinant Escherichia coli | |
| KR101414724B1 (en) | Method for preparing ansamycin glycoside using glycosyltranferase |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16849598 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2999764 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15762180 Country of ref document: US Ref document number: 2018516020 Country of ref document: JP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112018005639 Country of ref document: BR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2018/004848 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2016849598 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2016326518 Country of ref document: AU Date of ref document: 20160922 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 112018005639 Country of ref document: BR Kind code of ref document: A2 Effective date: 20180321 |