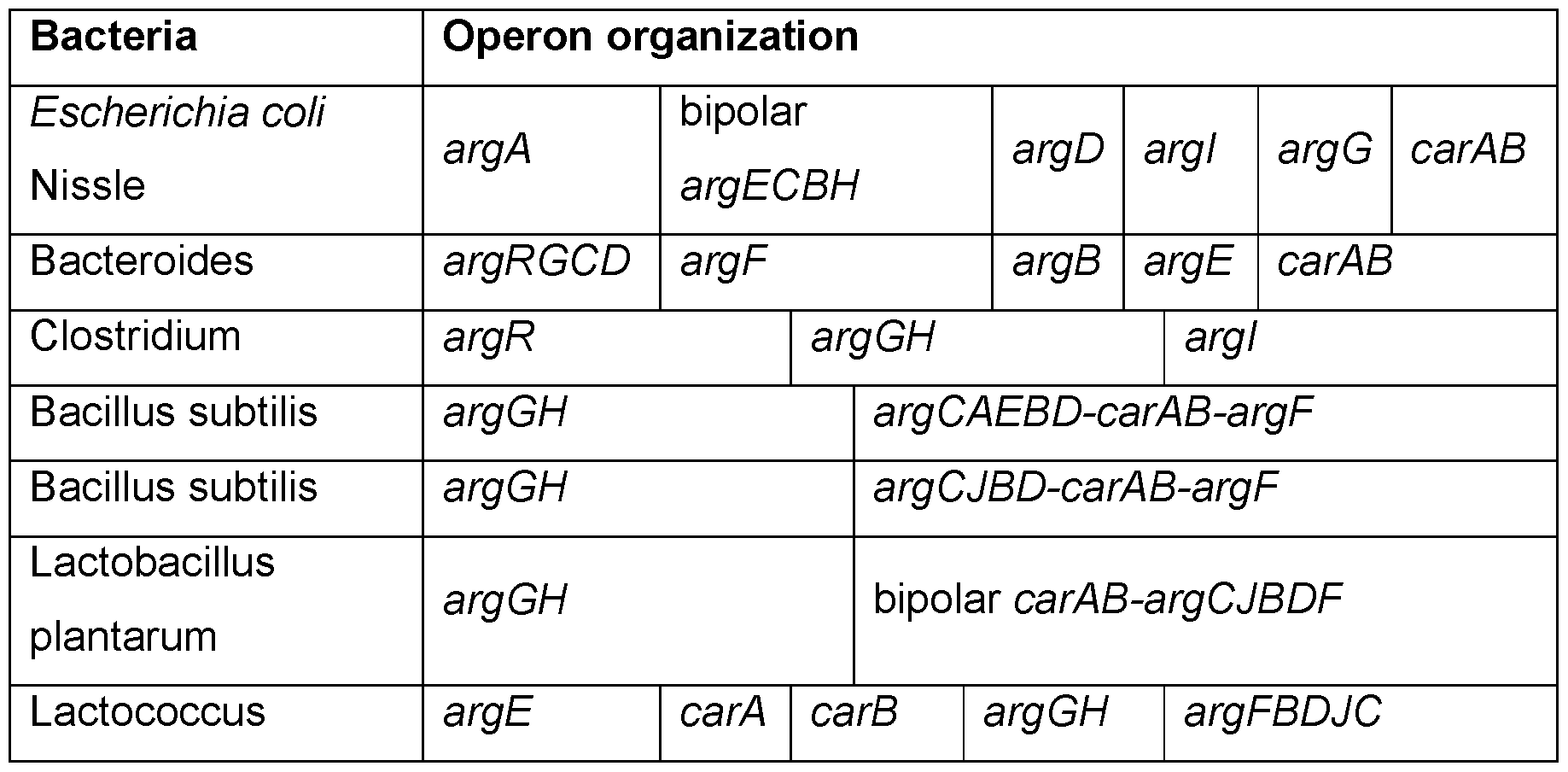
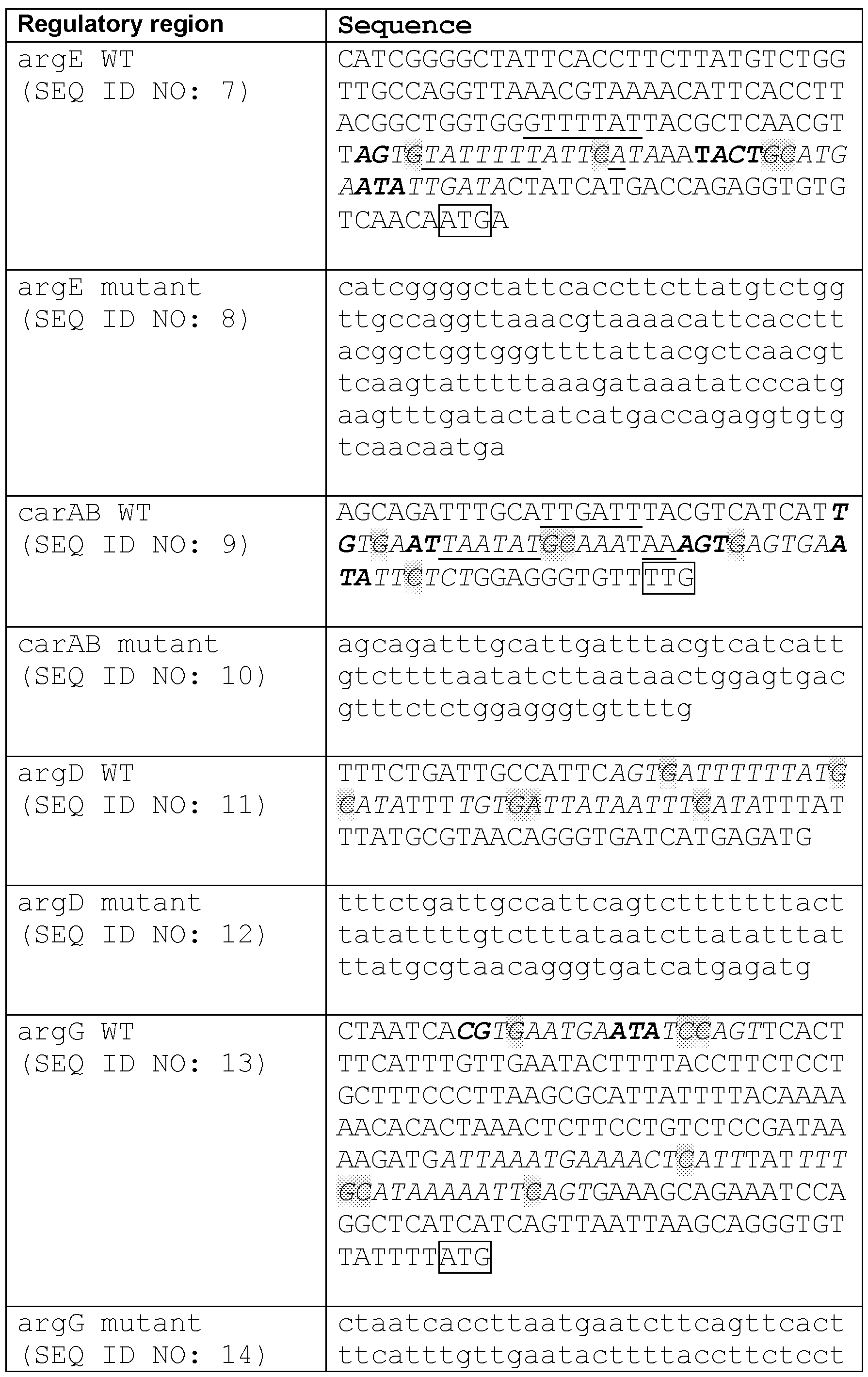
Bacteria Engineered to Treat Diseases Associated with Hyperammonemia
Background
[0001]This application is a continuation-in-part of U.S. Application No. 14/960,333, filed December 4, 2015; a continuation-in-part of PCT Application No. PCT/US2016/020530, filed March 2, 2016; and claims the benefit of U.S. Provisional Application No. 62/173,710, filed June 10, 2015; U.S. Provisional Application No. 62/173,706, filed June 10, 2015; U.S. Provisional Application No. 62/183,935, filed June 24, 2015; U.S. Provisional Application No.
62/184,811 , filed June 25, 2015; U.S. Provisional Application No. 62/184,770, filed June 25, 2015; U.S. Provisional Application No. 62/248,805, filed October 30, 2015; U.S. Provisional Application No. 62/256,041 , filed November 16, 2015; U.S. Provisional Application No. 62/256,039, filed November 16, 2015; U.S. Provisional Application No. 62/256,048, filed November 16, 2015; U.S. Provisional Application No. 62/263,329, filed December 4, 2015; U.S.
Provisional Application No. 62/291 ,468, filed February 4, 2016; and U.S.
Provisional Application No. 62/293,749, filed February 10, 2016; which are incorporated herein by reference in their entirety to provide continuity of disclosure.
[0002]Ammonia is highly toxic and generated during metabolism in all organs (Walker, 2012). In mammals, the healthy liver protects the body from ammonia by converting ammonia to non-toxic molecules, e.g., urea or glutamine, and preventing excess amounts of ammonia from entering the systemic circulation. Hyperammonemia is characterized by the decreased detoxification and/or increased production of ammonia. In mammals, the urea cycle detoxifies ammonia by enzymatically converting ammonia into urea, which is then removed in the urine. Decreased ammonia detoxification may be caused by urea cycle disorders (UCDs) in which urea cycle enzymes are defective, such as argininosuccinic aciduria, arginase deficiency,
carbamoylphosphate synthetase deficiency, citrullinemia, N-acetylglutamate synthetase deficiency, and ornithine transcarbamylase deficiency (Haberle et al., 2012). The National Urea Cycle Disorders Foundation estimates that the prevalence of UCDs is 1 in 8,500 births. In addition, several non-UCD disorders, such as hepatic encephalopathy, portosystemic shunting, and
organic acid disorders, can also cause hyperammonemia. Hyperammonemia can produce neurological manifestations, e.g., seizures, ataxia, stroke-like lesions, coma, psychosis, vision loss, acute encephalopathy, cerebral edema, as well as vomiting, respiratory alkalosis, hypothermia, or death (Haberle et al., 2012; Haberle et al., 2013).
[0003]Ammonia is also a source of nitrogen for amino acids, which are synthesized by various biosynthesis pathways. For example, arginine biosynthesis converts glutamate, which comprises one nitrogen atom, to arginine, which comprises four nitrogen atoms. Intermediate metabolites formed in the arginine biosynthesis pathway, such as citrulline, also incorporate nitrogen. Thus, enhancement of arginine biosynthesis may be used to incorporate excess nitrogen in the body into non-toxic molecules in order to modulate or treat conditions associated with hyperammonemia. Likewise, histidine biosynthesis, methionine biosynthesis, lysine biosynthesis, asparagine biosynthesis, glutamine biosynthesis, and tryptophan biosynthesis are also capable of incorporating excess nitrogen, and enhancement of those pathways may be used to modulate or treat conditions associated with hyperammonemia.
[0004] Current therapies for hyperammonemia and UCDs aim to reduce ammonia excess, but are widely regarded as suboptimal (Nagamani et al., 2012; Hoffmann et al., 2013; Torres-Vega et al., 2014). Most UCD patients require substantially modified diets consisting of protein restriction. However, a low-protein diet must be carefully monitored; when protein intake is too restrictive, the body breaks down muscle and consequently produces ammonia. In addition, many patients require supplementation with ammonia scavenging drugs, such as sodium phenylbutyrate, sodium benzoate, and glycerol phenylbutyrate, and one or more of these drugs must be administered three to four times per day (Leonard, 2006; Diaz et al., 2013). Side effects of these drugs include nausea, vomiting, irritability, anorexia, and menstrual disturbance in females (Leonard, 2006). In children, the delivery of food and medication may require a gastrostomy tube surgically implanted in the stomach or a nasogastric tube manually inserted through the nose into the stomach. When these treatment options fail, a liver transplant may be required (National Urea Cycle Disorders Foundation). Thus, there is significant unmet need for
effective, reliable, and/or long-term treatment for disorders associated with hyperammonemia, including urea cycle disorders.
[0005] The liver plays a central role in amino acid metabolism and protein synthesis and breakdown, as well as in several detoxification processes, notably those of end-products of intestinal metabolism, like ammonia. Liver dysfunction, resulting in hyperammonemia, may cause hepatic encephalopathy (HE), which disorder encompassesa spectrum of potentially reversible neuropsychiatric abnormalities observed in patients with liver dysfunction (after exclusion of unrelated neurologic and/or metabolic abnormalities). In HE, severe liver failure (e.g., cirrhosis) and/or portosystemic shunting of blood around the liver permit elevated arterial levels of ammonia to permeate the blood-brain barrier (Williams, 2006), resulting in altered brain function.
[0006]Ammonia accumulation in the brain leads to cognitive and motor disturbances, reduced cerebral perfusion, as well as oxidative stress-mediated injury to astrocytes, the brain cells capable of metabolizing ammonia. There is evidence to suggest that excess ammonia in the brain disrupts
neurotransmission by altering levels of the predominant inhibitory
neurotransmitter, γ-aminobutyric acid (GABA) (Ahboucha and Butterworth, 2004). Elevated cerebral manganese concentrations and manganese
deposition have also been reported in the basal ganglia of cirrhosis patients, and are suspected to contribute to the clinical presentation of HE (Cash et al., 2010; Rivera-Mancia et al., 2012). General neurological manifestations of hyperammonemia include seizures, ataxia, stroke-like lesions, Parkinsonian symptoms (such as tremors), coma, psychosis, vision loss, acute
encephalopathy, cerebral edema, as well as vomiting, respiratory alkalosis, hypothermia, or death (Haberle et al., 2012; Haberle et al., 2013).
[0007]Ammonia dysmetabolism cannot solely explain all the neurological changes that are seen in patients with HE. Sepsis is a well-known precipitating factor for HE. The systemic inflammatory response syndrome (SIRS) results from the release and circulation of proinflammatory cytokines and mediators. In patients with cirrhosis, SIRS may exacerbate the symptoms of HE, both in patients with minimal and overt HE in a process likely mediated by tumor necrosis factor (TNF) and interleukin- 6 (IL6). Notably, enhanced production of
reactive nitrogen species (RNS) and reactive oxygen species (ROS) occurs in cultured astrocytes that are exposed to ammonia, inflammatory cytokines, hyponatremia or benzodiazepines.
[0008] Hyperammonemia is also a prominent feature of Huntington's disease, an autosomal dominant disorder characterized by
intranuclear/cytoplasm ic aggregates and cell death in the brain (Chen et al., 2015; Chiang et al., 2007). In fact, hyperammonemia is a feature of several other disorders, as discussed herein, all of which can be treated by reducing the levels of ammonia.
[0009] Current therapies for hepatic encephalopathy, Huntington's disease, and other diseases and disorders associated with excess ammonia levels, are insufficient (Cash et al., 2010; Cordoba and Minguez, 2008;
Shannon and Fraint, 2015). In Huntington's disease, the side effects of antipsychotic drugs (e.g., haloperidol, risperidone, quetiapine) and drugs administered to suppress involuntary movements (e.g., tetrabenazine, amantadine, levetiracetam, clonazepam) may worsen muscle rigidity and cognitive decline in patients (Mayo Clinic). Antibiotics directed to urease- producing bacteria were shown to have severe secondary effects, such as nephrotoxicity, especially if administered for long periods (Blanc et al., 1992; Berk and Chalmers, 1970). Protein restriction is also no longer a mainstay therapy, as it can favor protein degradation and poor nutritional status, and has been associated with increased mortality (Kondrup and MCiller, 1997; Vaqero et al., 2003). Protein restriction is only appropriate for one third of cirrhotic patients with HE (Nguyen and Morgan, 2014). Thus, there is significant unmet need for effective, reliable, and/or long-term treatment for hepatic
encephalopathy and Huntington's disease.
Summary
[0010] The disclosure provides genetically engineered bacteria that are capable of reducing excess ammonia and converting ammonia and/or nitrogen into alternate byproducts. In certain embodiments, the genetically engineered bacteria reduce excess ammonia and convert ammonia and/or nitrogen into alternate byproducts. In certain embodiments, the genetically engineered bacteria are non-pathogenic and may be introduced into the gut in order to
reduce toxic ammonia. As much as 70% of excess ammonia in a hyperammonemic patient accumulates in the gastrointestinal tract. Another aspect of the invention provides methods for selecting or targeting genetically engineered bacteria based on increased levels of ammonia and/or nitrogen consumption, or production of a non-toxic byproduct, e.g., arginine or citrulline. The invention also provides pharmaceutical compositions comprising the genetically engineered bacteria, and methods of modulating and treating disorders associated with hyperammonemia, e.g., urea cycle disorders and hepatic encephalopathy.
[0011] The disclosure also provides genetically engineered bacteria that are capable of reducing excess ammonia and other deleterious molecules, e.g., GABA, manganese. In certain embodiments, the genetically engineered bacteria reduce excess ammonia and convert ammonia and/or nitrogen into alternate byproducts. In certain embodiments, the genetically engineered bacteria are non-pathogenic and may be introduced into the gut in order to reduce toxic ammonia. In certain embodiments, the genetically engineered bacteria are capable of reducing excess ammonia and other deleterious molecules, e.g., GABA, manganese, and are also capable of producing one or more gut barrier enhancer molecules, e.g., one or more short chain fatty acid(s), such as butyrate. Another aspect of the disclosure provides methods for selecting or targeting genetically engineered bacteria based on increased levels of ammonia and/or nitrogen consumption, or production of a non-toxic
byproduct, e.g., arginine or citrulline. The invention also provides
pharmaceutical compositions comprising the genetically engineered bacteria, and methods of modulating and treating disorders associated with excess ammonia, including, for example, hepatic encephalopathy and Huntington's disease.
[0012] In some embodiments, the genetically engineered bacteria comprise one or more gene(s) or gene cassette(s) or circuit(s), containing one or more native or non-native component(s), which mediate one or more mechanisms of action. Additionally, one or more endogenous genes or regulatory regions within the bacterial chromosome may be mutated or deleted. The genetically engineered bacteria harbor these genes or gene cassettes or
circuits on a plasm id or, alternatively, the genes/gene cassettes have been inserted into the chromosome at certain regions, where they do not interfere with essential gene expression.
[0013] These gene(s)/gene cassette(s) may be under the control of constitutive or inducible promoters. Exemplary inducible promoters described herein include oxygen level-dependent promoters (e.g., FNR-inducible promoter), promoters induced by HE-specific molecules or metabolites indicative of liver damage (e.g., bilirubin), promoters induced by inflammation or an inflammatory response (RNS, ROS promoters), and promoters induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose and tetracycline.
[0014] In addition, the engineered bacteria may further comprise one or more of more of the following: (1 ) one or more auxotrophies, such as any auxotrophies known in the art and provided herein, e.g., thyA auxotrophy, (2) one or more kill switch circuits, such as any of the kill-switches described herein or otherwise known in the art, (3) one or more antibiotic resistance circuits, (4) one or more transporters for importing biological molecules or substrates, such any of the transporters described herein or otherwise known in the art, (5) one or more secretion circuits, such as any of the secretion circuits described herein and otherwise known in the art, and (6) combinations of one or more of such additional circuits.
Brief Description of the Figures
[0015] Figs. 1A and 1B depict the state of the arginine regulon in one embodiment of an ArgR deletion bacterium of the invention under non-inducing (Fig. 1A) and inducing (Fig. 1 B) conditions. Fig. 1A depicts relatively low arginine production under aerobic conditions due to arginine ("Arg" in oval) interacting with ArgA (squiggle ) to inhibit (indicated by "X") ArgA activity, while oxygen (02) prevents (indicated by "X") FNR (dotted boxed FNR) from fbr dimerizing and activating the FNR promoter (grey FNR box) and the argA gene under its control. Fig. 1 B depicts up-regulated arginine production under anaerobic conditions due to FNR dimerizing (two dotted boxed FNRs) and
inducing FNR promoter (grey FNR box)-mediated expression of ArgA
fbr
(squiggle »above argA ), which is resistant to inhibition by arginine. This overcomes (curved arrow) the inhibition of the wild-type ArgA caused by arginine ("Arg" in oval) interacting with ArgA (squiggle above box depicting argA). Each gene in the arginine regulon is depicted by a rectangle containing the name of the gene. Each arrow adjacent to one or a cluster of rectangles depict the promoter responsible for driving transcription, in the direction of the arrow, of such gene(s). Heavier lines adjacent one or a series of rectangles depict ArgR binding sites, which are not utilized because of the ArgR deletion in this bacterium. Arrows above each rectangle depict the expression product of each gene.
[0016] Figs. 2A and 2B depict an alternate exemplary embodiment of the present invention. Fig. 2A depicts the embodiment under aerobic conditions where, in the presence of oxygen, the FNR proteins (FNR boxes) remain as monomers and are unable to bind to and activate the FNR promoter ("FNR") which drives expression of the arginine feedback resistant argAmr gene. The wild-type ArgA protein is functional, but is susceptible to negative feedback inhibition by binding to arginine, thus keeping arginine levels at or below normal. All of the arginine repressor (ArgR) binding sites in the promoter regions of each arginine biosynthesis gene {argA, argE, argC, argB, argH, argD, argl, argG, carA, and carB) have been mutated (black bars; black "X") to reduce or eliminate binding to ArgR. Fig. 2B depicts the same embodiment under anaerobic conditions where, in the absence of oxygen the FNR protein (FNR boxes) dimerizes and binds to and activates the FNR promoter ("FNR"). This drives expression of the arginine feedback resistant argAfbr gene (black squiggle ( ) = argAmr gene expression product), which is resistant to feedback inhibition by arginine ("Arg" in ovals). All of the arginine repressor (ArgR) binding sites in the promoter regions of each arginine biosynthetic gene {argA, argE, argC, argB, argH, argD, argl, argG, car A, and carB) have been mutated (black bars) to reduce or eliminate binding to ArgR (black "X"), thus preventing inhibition by an arginine-ArgR complex. This allows high level production of arginine.
[0017] Fig. 3 depicts another embodiment of the invention. In this embodiment, a construct comprising an ArgR binding site (black bar) in a
promoter driving expression of the Tet repressor (TetR) from the tetR gene is linked to a second promoter comprising a TetR binding site (black bar between TetR and X) that drives expression of gene X. Under low arginine
concentrations, TetR is expressed and inhibits the expression of gene X. At high arginine concentrations, ArgR associates with arginine and binds to the ArgR binding site, thereby inhibiting expression of TetR from the tetR gene. This, in turn, removes the inhibition by TetR allowing gene X expression (black squiggle ( )).
[0018] Fig. 4 depicts another embodiment of the invention. In this embodiment, a construct comprising an ArgR binding site (black bar) in a promoter driving expression of the Tet repressor (TetR) from the tetR gene is linked to a second promoter comprising a TetR binding site (black bar bound to TetR oval) that drives expression of green fluorescent protein ("GFP"). Under low arginine concentrations, TetR is expressed and inhibits the expression of GFP. At high arginine concentrations, ArgR associates with arginine and binds to the ArgR binding site, thereby inhibiting expression of TetR from the tetR gene. This, in turn, removes the inhibition by TetR allowing GFP expression. By mutating a host containing this construct, high arginine producers can be selected on the basis of GFP expression using fluorescence-activated cell sorting ("FACS").
[0019] Fig. 5 depicts another embodiment of the invention. In this embodiment, a construct comprising an ArgR binding site (black bar bound by the ArgR-Arg complex) in a promoter driving expression of the Tet repressor (not shown) from the tetR gene is linked to a second promoter comprising a TetR binding site (black bar) that drives expression of an auxotrophic protein necessary for host survival ("AUX"). Under high arginine concentrations, the ArgR-arginine complex binds to the ArgR binding site, thereby inhibiting expression of TetR from the tetR gene. This, in turn, allows expression of AUX, allowing the host to survive. Under low arginine concentrations, TetR is expressed from the tetR gene and inhibits the expression of AUX, thus killing the host. The construct in Fig. 5 enforces high arginine ("Arg") production by making it necessary for host cell survival through its control of AUX expression.
[0020] Fig. 6 depicts a schematic diagram of the argA gene under the control of an exemplary FNR promoter (fnrS) fused to a strong ribosome binding site.
[0021] Fig. 7 depicts another schematic diagram of the argAmr gene under the control of an exemplary FNR promoter (nirB) fused to a strong ribosome binding site. Other regulatory elements may also be present.
[0022] Fig. 8 depicts a schematic diagram of the argAmr gene under the control of an exemplary FNR promoter (nirB) fused to a weak ribosome binding site.
[0023] Figs. 9A and 9B depict exemplary embodiments of a FNR- responsive promoter fused to a CRP binding site. Fig. 9A depicts a map of the FNR-CRP promoter region, with restriction sites shown in bold. Fig. 9B depicts a schematic diagram of the argAmr gene under the control of an exemplary FNR promoter (nirB promoter), fused to both a CRP binding site and a ribosome binding site. Other regulatory elements may also be present.
[0024] Figs. 10A and 10B depict alternate exemplary embodiments of a FNR-responsive promoter fused to a CRP binding site. Fig. 10A depicts a map of the FNR-CRP promoter region, with restriction shown in bold. Fig. 10B depicts a schematic diagram of the argAmr gene under the control of an exemplary FNR promoter (fnrS promoter), fused to both a CRP binding site and a ribosome binding site.
[0025] Fig. 11 depicts an exemplary embodiment of a constitutively expressed argG construct in E. coli Nissle. The constitutive promoter is
BBa_J23100, boxed in gray. Restriction sites for use in cloning are in bold.
[0026] Fig. 12 depicts a map of the wild-type argG operon E. coli Nissle, and a constitutively expressing mutant thereof. ARG boxes are present in the wild-type operon, but absent from the mutant. ArgG is constitutively expressed under the control of the BBa_J23100 promoter.
[0027] Fig. 13 depicts a schematic diagram of an exemplary BAD
fbr fbr
promoter-driven argA construct. In this embodiment, the argA gene is
fbr
inserted between the araC and araD genes. ArgA is flanked by a ribosome binding site, a FRT site, and one or more transcription terminator sequences.
[0028] Fig. 14 depicts an exemplary embodiment of an engineered bacterial strain deleted for the argR gene and expressing the feedback-resistant argA mr gene. In some embodiments, this strain further comprises one or more auxotrophic modifications on the chromosome. This strain is useful for the consumption of ammonia and the production of arginine.
[0029] Fig. 15 depicts an exemplary embodiment of an engineered bacterial strain deleted for the argR and argG genes, and expressing the feedback-resistant argAmr gene. In some embodiments, this strain further comprises one or more auxotrophic modifications on the chromosome. This strain is useful for the consumption of ammonia and the production of citrulline.
[0030] Fig. 16 depicts an exemplary embodiment of an engineered bacterial strain which lacks ArgR binding sites and expresses the feedback- resistant argAfbr gene. In some embodiments, this strain further comprises one or more auxotrophic modifications on the chromosome. This strain is useful for the consumption of ammonia and the production of arginine.
[0031] Fig. 17 depicts an exemplary embodiment of an engineered bacterial strain which lacks ArgR binding sites in all of the arginine biosynthesis operons except for argG, and expresses the feedback-resistant argAmr gene. In some embodiments, this strain further comprises one or more auxotrophic modifications on the chromosome. This strain is useful for the consumption of ammonia and the production of citrulline.
[0032] Fig. 18 depicts a map of exemplary integration sites within the E. coli 1917 Nissle chromosome. These sites indicate regions where circuit components may be inserted into the chromosome without interfering with essential gene expression. Backslashes (/) are used to show that the insertion will occur between divergently or convergently expressed genes. Insertions within biosynthetic genes, such as thyA, can be useful for creating nutrient auxotrophies. In some embodiments, an individual circuit component is inserted into more than one of the indicated sites. The malE/K site is circled. In some embodiments of the disclosure, FNR-ArgAfbr is inserted at the maIEK locus.
[0033] Fig. 19 depicts three bacterial strains which constitutively express red fluorescent protein (RFP). In strains 1 -3, the rfp gene has been inserted
into different sites within the bacterial chromosome, and results in varying degrees of brightness under fluorescent light. Unmodified E. coli Nissle (strain 4) is non-fluorescent.
[0034] Fig. 20 depicts the gene organization of exemplary contructs of the disclosure. Non-limiting examples of strains comprising such a construct include SYN-UCD301 and SYN-UCD302. SYN-UCD301 comprises AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, Wild type ThyA, and Chloramphenicol resistance.
[0035] Fig. 21 depicts the gene organization of an exemplary construct of the disclosure. Non-limiting examples of strains comprising such a construct include SYN-UCD303, SYN-UCD306, SYN-UCD307, and SYN-UCD309. For example, SYN-UCD303 comprises AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and kanamycin resistance.
[0036] Fig. 22 depicts the gene organization of exemplary constructs of the disclosure. Non-limiting examples of strains comprising such a construct include SYN-UCD304, SYN-UCD305, SYN-UCD308, and SYN-UCD310. For example, SYN-UCD304 comprises AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, wild type ThyA, and no antibiotic resistance. SYN-UCD305 comprises AArgR, PfnrS- ArgAfbr integrated into the
chromosome at the maIEK locus, AThyA, and no antibiotic resistance.
[0037] Fig. 23 depicts a bar graph of in vitro arginine levels produced by streptomycin-resistant control Nissle (SYN-UCD103), SYN-UCD201 , SYN- UCD202, and SYN-UCD203 under inducing (+ATC) and non-inducing (-ATC) fbr
conditions. SYN-UCD201 comprises AArgR and no argA . SYN-UCD202
fbr
comprises AArgR and tetracycline-inducible argA on a high-copy
fbr plasmid. SYN-UCD203 comprises AArgR and tetracycline-driven argA on a low-copy plasmid.
[0038] Fig. 24 depicts a bar graph of in vitro levels of arginine and citrulline produced by streptomycin-resistant control Nissle (SYN-UCD103), SYN-UCD104, SYN-UCD204, and SYN-UCD105 under inducing conditions. SYN-UCD104 comprises wild-type ArgR, tetracycline-inducible argAmr on a low- copy plasmid, tetracycline-inducible argG, and mutations in each ARG box for
each arginine biosynthesis operon except for argG. SYN-UCD204 comprises AArgR and argAmr expressed under the control of a tetracycline-inducible promoter on a low-copy plasmid. SYN-UCD105 comprises wild-type ArgR, tetracycline-inducible argAfbr on a low-copy plasmid, constitutively expressed argG (BBa_J23100 constitutive promoter), and mutations in each ARG box for each arginine biosynthesis operon except for argG.
[0039] Fig. 25 depicts a bar graph of in vitro arginine levels produced by streptomycin-resistant Nissle (SYN-UCD103), SYN-UCD205, and SYN- UCD204 under inducing (+ATC) and non-inducing (-ATC) conditions, in the presence (+O2) or absence (-O2) of oxygen. SYN-UCD103 is a control Nissle construct. SYN-UCD205 comprises AArgR and argAfbr expressed under the control of a FNR-inducible promoter on a low-copy plasmid. SYN204 comprises AArgR and argAmr expressed under the control of a tetracycline-inducible promoter on a low-copy plasmid.
[0040] Fig. 26 depicts a bar graph of in vitro ammonia levels in culture media from SYN-UCD101 , SYN-UCD102, and blank controls at baseline, two hours, and four hours. Both SYN-UCD101 and SYN-UCD102 are capable of consuming ammonia in vitro. SYN-UCD101 comprises wild type ArgR, and wild type ThyA, and no ArgAFbr; SYN-UCD102 comprises wild type ArgR, tetracycline-inducible argAfbr on a low copy plasmid, and wild type ThyA.
[0041] Fig. 27 depicts a bar graph of in vitro ammonia levels in culture media from SYN-UCD202, SYN-UCD203, and blank controls at baseline, two hours, and four hours. Both SYN-UCD202 and SYN-UCD203 are capable of consuming ammonia in vitro. SYN-UCD202 and SYN-UCD203 both comprise AArgR, tetracycline-inducible argAfbr on a high-copy plasmid or low copy plasmid, respectively, Amp resistance, and wild type ThyA.
[0042] Figs. 28A, 28B, and 28C depict bar graphs of ammonia levels in hyperammonemic TAA mice. Fig. 28A depicts a bar graph of ammonia levels in hyperammonemic mice treated with unmodified control Nissle or SYN-UCD202, a genetically engineered strain in which the Arg repressor gene is deleted and fbr
the argA gene is under the control of a tetracycline-inducible promoter on a high-copy plasmid. A total of 96 mice were tested, and the error bars represent standard error. Blood ammonia (BA) levels in mice treated with SYN-UCD202
are lower than ammonia levels in mice treated with unmodified control Nissle at day 4 and day 5 (Nissle, BA = 220 mM; SYN-UCD202, BA = 105 mM; BANissie - BASYN-UCD202 = 5 mM; average blood volume = 1.5 ml_. Fig. 28B depicts a bar graph showing in vivo efficacy (ammonia consumption) of SYN-UCD204 in the TAA mouse model, relative to streptomycin-resistant control Nissle (SYN- UCD103) and vehicle-only controls. Fig. 28C depicts a bar graph of the percent change in blood ammonia concentration between 24-48 hours post-TAA treatment.
[0043] Fig. 29 depicts a bar graph of ammonia levels in
hyperammonemic spfash mice. Fifty-six spfash mice were separated into four groups. Group 1 was fed normal chow, and groups 2-4 were fed 70% protein chow following an initial blood draw. Groups were gavaged twice daily, with water, streptomycin-resistant Nissle control (SYN-UCD103), or SYN-UCD204, and blood was drawn 4 hours following the first gavage. SYN-UCD204, comprising AArgR and argAmr expressed under the control of a tetracycline- inducible promoter on a low-copy plasmid, significantly reduced blood ammonia to levels below the hyperammonemia threshold.
[0044] Fig. 30 depicts a bar graph of ammonia levels in
hyperammonemic spfash mice on a high protein diet. Mice were treated with SYN-UCD204 (comprising AArgR, PfnrS-ArgAfbr on a low-copy plasmid and wild type ThyA), SYN-UCD206 (comprising AArgR, PfnrS- ArgAfbr on a low- copy plasmid and AThyA) or water, then switched to high protein chow after 2 days. As seen in Fig. 30, at 48 hours after switch to high protein chow ammonia levels were reduced to a similar extent in both SYN-UCD205 and SYN- UCD206, indicating that ThyA auxotrophy does not have a significant effect on efficacy.
[0045] Figs. 31 A and 31 B depict bar graphs of ammonia levels in the media at various time points post anaerobic induction. Fig. 31A depicts a bar graph of the levels of arginine production of SYN-UCD205, SYN-UCD206, and SYN-UCD301 measured at 0, 30, 60, and 120 minutes. Fig. 31 B depicts a bar graph of the levels of arginine production of SYN-UCD204 (comprising AArgR, PfnrS-ArgAfbr on a low-copy plasmid and wild type ThyA), SYN-UCD301 , SYN- UCD302, and SYN-UCD303 (all three of which comprise an integrated FNR-
ArgAfbr construct; SYN UCD301 comprises AArgR, and wtThyA; SYN 303 comprises AArgR, and AThyA). Results indicate that chromosomal integration of FNR ArgA fbr results in similar levels of arginine production as seen with the low copy plasm id strains expressing the same construct.
[0046] Figs. 32A and 32B depicts a bar graph of ammonia levels and a survival curve for hyperammonemic spfash mice on a normal (NC) or high protein (HP) diet. Two strains with an integrated copy of FNR-ArgAfbr, one with (SYN-UCD303) and one without a ThyA deletion (SYN-UCD301 ) were compared. Fig. 32A depicts a bar graph of ammonia levels in hyperammonemic spfash mice on a normal (NC) or high protein (HP) diet. Ammonia levels of spfash mice in a high protein diet were reduced in the SYN-UCD301 and SYN- UCD303 groups as compared to the H20 high protein diet control group. The observed reduction in ammonia levels was similar in both SYN-UCD301 and SYN-UCD303, indicating that ThyA auxotrophy does not have a significant effect on efficacy of SYN-UCD303. Fig. 32B depicts a survival curve of hyperammonemic spfash mice on a normal (NC) or high protein (HP) diet and shows that SYN-UCD301 and SYN-UCD303 displayed prolonged survival as compared to controls.
[0047] Fig. 33 depicts a graph of bood ammonia levels in an
hyperammonemic spfash mice on a normal (NC) or high protein (HP) diet. For SYN-UCD303, doses of 1 X107, 1X108, 1X109 and 1 X1010 cells were administered daily over a time course of 12 days. Blood ammonia levels were measured on day 5. Both doses of 1X108 and 1X109were sufficient to result in a significant reduction of blood ammonia levels in this model. SYN-UCD303 comprises AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and Kanamycin resistance.
[0048] Fig. 34 depicts a graph of Nissle residence in vivo. Streptomycin- resistant Nissle was administered to mice via oral gavage without antibiotic pre- treatment. Fecal pellets from six total mice were monitored post-administration to determine the amount of administered Nissle still residing within the mouse gastrointestinal tract. The bars represent the number of bacteria administered to the mice. The line represents the number of Nissle recovered from the fecal samples each day for 10 consecutive days.
[0049] Figs. 35A, 35B, and 35C depict bar graphs of bacterial residence in various compartments of the intestinal tract at 1 , 4, 8, 12, 24, and 30 hours post gavage. Mice were treated with approximately 109 CFU, and at each timepoint, animals (n=4) were euthanized, and intestine, cecum, and colon were removed. The small intestine was cut into three sections, and the large intestine and colon each into two sections. Intestinal effluents gathered and CFUs in each compartment were determined by serial dilution plating. Fig. 35A depicts a bar graph of residence over time for SYN-UCD103 (streptomycin resistant Nissle). Fig. 35B depicts a bar graph residence over time for SYN-UCD106, comprising AArgR and AThyA and no ArgAfbr. Fig. 35C depicts a bar graph showing residence over time for SYN-UCD303, comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, and AThyA.
[0050] Figs. 36A, 36B, and 36C depict bar graphs of viable bacterial cells and arginine production. Cells were either incubated with 70% isopropanol or phosphate buffered saline (PBS) as a control for 1 hour with shaking. After treatment, the cells were mixed at specific ratios in M9 media supplemented with 0.5% glucose and 3mM thymidine and incubated with shaking at 37 C for 2 hours. As seen in Figs. 36A and 36B., a greater ratio of isopropanol treated cells to untreated in a culture results in fewer CFUs as determined by plating, and lower levels of arginine production. Arginine production relative to amount of bacteria present remained constant across the various cultures (Fig. 36C). These results indicate that only viable bacteria are contributing to arginine production.
[0051] Fig. 37. Depicts the number of bacteria quantified in fecal samples collected in a non-human primate toxicity study. Pharmacokinetics and pharmacodynamics resulting from administration of SYN-UCD107 (a kanamycin resistant Nissle) and SYN-UCD303 (comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and Kanamycin resistance) was compared over 50 days. Results indicate that under these dosing conditions, similar amounts of bacteria were recovered with the auxotroph SYN-UCD303 as control kanamycin resistant Nissle in the feces. Similar results have been observed in mice.
[0052] Fig. 38 depicts an exemplary synthetic genetic circuit for treating hepatic encephalopathy and other disorders characterized by
hyperammonemia. In the ammonia conversion circuit, ammonia is taken up by a bacterium (e.g., E. coli Nissle), converted to glutamate, and glutamate is subsequently metabolized to arginine. Arginine ultimately exits the bacterial cell.
[0053] Fig. 39 depicts one embodiment of the invention. In this embodiment, the genetically engineered bacteria comprise four exemplary circuits for the treatment of hepatic encephalopathy. In one circuit, ammonia is taken up by the bacterium, converted to glutamate, and glutamate is
subsequently metabolized to arginine. Arginine ultimately exits the bacterial cell. In a second circuit, the GABA membrane transport protein (GabP) is expressed by the gabP gene, and facilitates GABA transport into the cell. In a third circuit, the bacterial manganese transport protein (MntH) is expressed by the mntH gene, and facilitates manganese transport into the cell. In a fourth circuit, expression of a butyrate gene cassette results in the production of butyrate, and release of this gut barrier enhancer molecule outside of the cell. In some embodiments, all four circuits are each under the control of the same inducible promoter. In other embodiments, the four circuits may be under the control of different inducible promoters. Exemplary inducible promoters include oxygen level-dependent promoters (e.g., FNR-inducible promoter), promoters induced by HE-specific molecules or metabolites indicative of liver damage (e.g., bilirubin), promoters induced by inflammation or an inflammatory response, and promoters induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose.
[0054] Fig. 40 depicts one embodiment of the invention. In this embodiment, the genetically engineered bacteria comprise two exemplary circuits for the treatment of hepatic encephalopathy. In one circuit, ammonia is taken up by the bacterium, converted to glutamate, and glutamate is
subsequently metabolized to arginine. Arginine ultimately exits the bacterial cell. In a second circuit, the GABA membrane transport protein (GabP) is expressed by the gabP gene, and facilitates GABA transport into the cell. In some embodiments, both circuits are under the control of the same inducible
promoter. In other embodiments, the two circuits may each be under the control of a different inducible promoter. Exemplary inducible promoters include oxygen level-dependent promoters (e.g., FNR-inducible promoter), promoters induced by HE-specific molecules or metabolites indicative of liver damage (e.g., bilirubin), promoters induced by inflammation or an inflammatory response, and promoters induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose. In other embodiments, the genetically engineered bacteria may further comprise an additional circuit for reducing the level of GABA, e.g., a circuit for
metabolizing (catabolizing) GABA.
[0055] Fig. 41 depicts the catabolism of GABA following uptake into genetically engineered bacteria comprising synthetic genetic circuits. In Fig. 41A, upon entry into the cell, GABA is converted to succinyl semialdehyde by GABA a-ketoglutarate transaminase (GSST). Succinate-semialdehyde dehydrogenase (SSDH) then catalyzes the second and only other specific step in GABA catabolism, the oxidation of succinyl semialdehyde to succinate.
Ultimately, succinate becomes a substrate for the citric acid (TCA) cycle. GOT (glutamate oxaloacetate transaminase) converts alpha-ketoglutarate to glutamate. In certain embodiments, the genetically engineered bacteria of the disclosure comprise a GABA consuming circuit including, but not limited to, one or more of GSST, SSDH, and GOT. Fig. 41 B depicts a schematic
representation of the GABA utilization pathway in E. coli Nissle.
[0056] Fig. 42 depicts one embodiment of the invention. In this embodiment, the genetically engineered bacteria comprise two exemplary circuits for the treatment of hepatic encephalopathy. In one circuit, ammonia is taken up by the bacterium, converted to glutamate, and glutamate is
subsequently metabolized to arginine. Arginine ultimately exits the bacterial cell. In a second circuit, the bacterial manganese transport protein (MntH) is expressed by the mntH gene, and facilitates manganese transport into the cell. In some embodiments, both circuits are under the control of the same inducible promoter. In other embodiments, the two circuits may each be under the control of different inducible promoter. Exemplary inducible promoters include oxygen level-dependent promoters (e.g., FNR-inducible promoter), promoters
induced by HE-specific molecules or metabolites indicative of liver damage {e.g., bilirubin), promoters induced by inflammation or an inflammatory response, and promoters induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose. Fig. 43 depicts one embodiment of the invention. In this embodiment, the genetically engineered bacteria comprise two exemplary circuits for the treatment of hepatic encephalopathy. In one circuit, ammonia is taken up by the bacterium, converted to glutamate, and glutamate is subsequently metabolized to arginine. Arginine ultimately exits the bacterial cell. In a second circuit, expression of a butyrate gene cassette results in the production of butyrate, and release of this gut barrier enhancer molecule outside of the cell. In some embodiments, both circuits are under the control of the same inducible promoter. In other embodiments, the two circuits may each be under the control of different inducible promoter. Exemplary inducible promoters include oxygen level-dependent promoters (e.g., FNR-inducible promoter), promoters induced by HE-specific molecules or metabolites indicative of liver damage (e.g., bilirubin), promoters induced by inflammation or an inflammatory response, and promoters induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose. One or more of the butyrate cassettes described herein may be expressed by the genetically engineered bacteria comprising an arginine (and/or citrulline) producing circuit. Fig. 44 depicts an exemplary schematic of the E. coli 1917 Nissle chromosome comprising multiple mechanisms of action (MoAs).
[0057] Fig. 45 depicts an exemplary schematic of the E. coli 1917 Nissle chromosome comprising multiple MoAs. In some embodiments, an ammonia conversion circuit, a butyrate production circuit, a GABA transport and/or a GABA metabolic circuit, and a manganese transport circuit are inserted at four or more different chromosomal insertion sites
[0058] Fig. 46 depicts an exemplary schematic of the E. coli 1917 Nissle chromosome comprising multiple MoAs. In Fig. 46A, an ammonia conversion circuit, a butyrate production circuit, and a GABA transport and/or GABA metabolic circuit are inserted at three different chromosomal insertion sites. In Fig. 46B, an ammonia conversion circuit, a GABA transport and/or GABA
metabolic circuit, and a manganese transport circuit are inserted at three or more different chromosomal insertion sites.
[0059] Fig. 47 depicts an exemplary schematic of the E. coli 1917 Nissle chromosome comprising multiple MoAs. In Fig. 47A, an ammonia conversion circuit, and a manganese transport circuit are inserted at two different chromosomal insertion sites. In Fig. 47B, an ammonia conversion circuit, and a GABA transport and/or GABA metabolic circuit are inserted at two or more different chromosomal insertion sites.
[0060] Fig. 48A depicts a metabolic pathway for butyrate production
Figs. 48B and 48C depict two schematics of two different butyrate producing circuits (found in SYN-UCD503 and SYN-UCD504), both under the control of a tetracycline inducible promoter. Fig. 48D depicts a schematic of a third butyrate gene cassette (found in SYN-UCD505) under the control of a tetracycline inducible promoter. SYN-UCD503 comprises a bdc2 butyrate cassette under control of tet promoter on a plasm id. A "bdc2 cassette" or "bdc2 butyrate cassette" refres to a butyrate producing cassette that comprises at least the following genes: bcd2, etfB3, etfA3, hbd, crt2, pbt, and buk genes. SYN- UCD504 comprises a ter butyrate cassette (ter gene replaces the bcd2, etfB3, and etfA3 genes) under control of tet promoter on a plasm id. A "ter cassette" or "ter butyrate cassette" refers to a butyrate producing cassete that comprises at least the following genes: ter, thiA1 , hbd, crt2, pbt, buk. SYN-UCD505 comprises a tesB butyrate cassette (ter gene is present and tesB gene replaces the pbt gene and the buk gene) under control of tet promoter on a plasmid. A "tes or tesB cassette or "tes or tesB butyrate cassette" refers to a butyrate producing cassette that comprises at least ter, thiA1 , hbd, crt2, and tesB genes. An alternative butyrate cassette of the disclosure comprises at least bcd2, etfB3, etfA3, thiA1 , hbd, crt2, and tesB genes. In some embodiments, the tes or tesB cassette is under control of an inducible promoter other than tetracycline. Exemplary inducible promoters which may control the expression of the tesB cassette include oxygen level-dependent promoters (e.g., FNR-inducible promoter), promoters induced by HE-specific molecules or metabolites indicative of liver damage (e.g., bilirubin), promoters induced by inflammation or an inflammatory response (RNS, ROS promoters), and promoters induced by a
metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose and tetracycline.
[0061] Fig. 49 depicts the gene organization of exemplary engineered bacteria of the disclosure and their induction under anaerobic or inflammatory conditions for the production of butyrate. Figs. 49A and 49B depict the gene organization of an exemplary recombinant bacterium of the invention and its induction under low-oxygen conditions. Fig. 49A depicts relatively low butyrate production under aerobic conditions in which oxygen (02) prevents (indicated by "X") FNR (grey boxed "FNR") from dimerizing and activating the FNR- responsive promoter ("FNR promoter"). Therefore, none of the butyrate biosynthesis enzymes (bcd2, etfB3, etfA3, thiA1 , hbd, crt2, pbt, and buk; black boxes) is expressed. Fig. 49B depicts increased butyrate production under low-oxygen conditions due to FNR dimerizing (two grey boxed "FNR"s), binding to the FNR-responsive promoter, and inducing expression of the butyrate biosynthesis enzymes, which leads to the production of butyrate. Figs. 49C and 49D depict the gene organization of an exemplary recombinant bacterium of the invention and its derepression in the presence of nitric oxide (NO). In Fig. 49C, in the absence of NO, the NsrR transcription factor (gray circle, "NsrR") binds to and represses a corresponding regulatory region. Therefore, none of the butyrate biosynthesis enzymes (bcd2, etfB3, etfA3, thiA1 , hbd, crt2, pbt, buk; black boxes) is expressed. In Fig. 49D, in the presence of NO, the NsrR transcription factor interacts with NO, and no longer binds to or represses the regulatory sequence. This leads to expression of the butyrate biosynthesis enzymes (indicated by gray arrows and black squiggles) and ultimately to the production of butyrate. Figs. 49E and F depict the gene organization of an exemplary recombinant bacterium of the invention and its induction in the presence of H2O2. In Fig. 49E, in the absence of H2O2, the OxyR transcription factor (gray circle, "OxyR") binds to, but does not induce, the oxyS promoter. Therefore, none of the butyrate biosynthesis enzymes (bcd2, etfB3, etfA3, thiA1 , hbd, crt2, pbt, buk; black boxes) is expressed. In Fig. 49F, in the presence of H2O2, the OxyR transcription factor interacts with H2O2 and is then capable of inducing the oxyS promoter. This leads to expression of the
butyrate biosynthesis enzymes (indicated by gray arrows and black squiggles) and ultimately to the production of butyrate.
[0062] Fig. 50 depicts the gene organization of exemplary recombinant bacteria of the disclosure and their induction under anaerobic or inflammatory conditions for the production of butyrate. Figs. 50A and 50B depict the gene organization of an exemplary recombinant bacterium of the invention and its induction under low-oxygen conditions. Fig. 50A depicts relatively low butyrate production under aerobic conditions in which oxygen (O2) prevents (indicated by "X") FNR (grey boxed "FNR") from dimerizing and activating the FNR- responsive promoter ("FNR promoter"). Therefore, none of the butyrate biosynthesis enzymes (ter, thiA1, hbd, crt2, pbt, and buk; black boxes) is expressed. Fig. 50B depicts increased butyrate production under low-oxygen conditions due to FNR dimerizing (two grey boxed "FNR"s), binding to the FNR- responsive promoter, and inducing expression of the butyrate biosynthesis enzymes, which leads to the production of butyrate. Figs. 50C and 50D depict the gene organization of another exemplary recombinant bacterium of the invention and its derepression in the presence of NO. In Fig. 50C, in the absence of NO, the NsrR transcription factor (gray circle, "NsrR") binds to and represses a corresponding regulatory region. Therefore, none of the butyrate biosynthesis enzymes (ter, thiA1, hbd, crt2, pbt, buk; black boxes) is expressed. In Fig. 50D, in the presence of NO, the NsrR transcription factor interacts with NO, and no longer binds to or represses the regulatory sequence. This leads to expression of the butyrate biosynthesis enzymes (indicated by gray arrows and black squiggles) and ultimately to the production of butyrate. Figs. 50E and 50F depict the gene organization of another exemplary recombinant bacterium of the invention and its induction in the presence of H2O2. In Fig. 50E, in the absence of H2O2, the OxyR transcription factor (gray circle, OxyR") binds to, but does not induce, the oxyS promoter. Therefore, none of the butyrate biosynthesis enzymes (ter, thiA1, hbd, crt2, pbt, buk; black boxes) is expressed. In Figs. 50F, in the presence of H2O2, the OxyR transcription factor interacts with H2O2 and is then capable of inducing the oxyS promoter. This leads to expression of the butyrate biosynthesis enzymes (indicated by gray arrows and black squiggles) and ultimately to the production of butyrate.
[0063] Fig. 51 depicts a graph of butyrate production using the circuits (SYN-UCD-503, SYN-UCD-504, SYN-UCD-505) shown in Fig. 48. Cells were grown in M9 minimal media containing 0.2% glucose and induced with ATC at early log phase. As seen in Fig. 51 A, similar amounts of butyrate were produced for each construct under aerobic vs anaerobic conditions. The ter strain produces more butyrate overall. SYN-UCD503 comprises pl_ogic031 (bdc2 butyrate cassette under control of tet promoter on a plasmid) and SYN- UCD504 comprises pl_ogic046 (ter butyrate cassette under control of tet promoter on a plasmid). Fig. 51 B depicts butyrate production of SYN-UCD504 (pl_ogic046 (ter butyrate cassette under control of tet promoter on a plasmid)) and SYN-UCD505 (a Nissle strain comprising plasmid pl_OGIC046-delta pbt.buk/tesB+, an ATC-inducible ter-com prising butyrate construct with a deletion in the pbt-buk genes and their replacement with the tesB gene). The tesB construct results in greater butyrate production.
[0064] Fig. 52 depicts a graph of butyrate production using different butyrate-producing circuits comprising a nuoB gene deletion. Strains depicted are SYN-UCD503, SYN-UCD504, SYN-UCD510 (SYN-UCD510 is the same as SYN-UCD503 except that it further comprises a nuoB deletion), and SYN- UCD511 (SYN-UCD511 is the same as SYN-UCD504 except that it further comprises a nuoB deletion). The NuoB gene deletion results in greater levels of butyrate production as compared to a wild-type parent control in butyrate producing strains. NuoB is a main protein complex involved in the oxidation of NADH during respiratory growth. In some embodiments, preventing the coupling of NADH oxidation to electron transport increases the amount of NADH being used to support butyrate production.
[0065] Fig. 53A depicts a schematic of a butyrate producing circuit under the control of an FNR promoter. Fig. 53B depicts a bar graph of anaerobic induction of butyrate production. FNR-responsive promoters were fused to butyrate cassettes containing either the bed or ter circuits. Transformed cells were grown in LB to early log and placed in anaerobic chamber for 4 hours to induce expression of butyrate genes. Cells were washed and resuspended in minimal media w/ 0.5% glucose and incubated microaerobically to monitor butyrate production over time. SYN-UCD501 led to significant butyrate
production under anaerobic conditions. Fig. 53C depicts SYN-UCD501 in the presence and absence of glucose and oxygen in vitro. SYN-UCD501 comprises pSC101 PydfZ-ter butyrate plasmid; SYN-UCD500 comprises pSC101 PydfZ- bcd butyrate plasmid; SYN-UCD506 comprises pSC101 nirB-bcd butyrate plasmid. Fig. 53D depicts levels of mouse lipocalin 2 and calprotectin quantified by ELISA using the fecal samples in an in vivo model of HE. SYN-UCD501 reduces inflammation and/or protects gut barrier function as compared to control SYN-UCD103.
[0066] Fig. 54 depicts bar graphs showing in vitro arginine (Fig. 54A) and butyrate (Fig. 54B) production for (1 ) butyrate producing strain; (2) arginine producing strain (ammonia consuming strain), and (3) strain that produces butyrate and also consumes ammonia. SYN-UCD501 (butyrate producing strain comprising Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance)), and SYN-UCD305 (arginine producing/ammonia consuming strain comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, and AThyA, with no antibiotic resistance), and SYN-UCD601 (butyrate producing and arginine producing/ammonia consuming strain comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance)). The data show that SYN-UCD601 is able to produce similar levels of arginine as SYN-UCD305 and similar levels of butyrate as SYN-UCD501 in vitro.
[0067] Fig. 55 depicts the gene organization of an exemplary engineered bacterium of the invention and its induction under low-oxygen conditions for the production of propionate. Fig. 55A depicts relatively low propionate production under aerobic conditions in which oxygen (O2) prevents (indicated by "X") FNR (grey boxed "FNR") from dimerizing and activating the FNR-responsive promoter ("FNR promoter"). Therefore, none of the propionate biosynthesis enzymes (pet, IcdA, IcdB, IcdC, etfA, acrB, acrC; black boxes) are expressed. Fig. 55B depicts increased propionate production under low-oxygen conditions due to FNR dimerizing (two grey boxed "FNR"s), binding to the FNR-responsive promoter, and inducing expression of the propionate biosynthesis enzymes, which leads to the production of propionate.
[0068] Fig. 56 depicts an exemplary propionate biosynthesis gene cassette.
[0069] Figs. 57 A, 57B amd 57C depict the gene organization of an exemplary engineered bacterium and its induction under low-oxygen conditions for the production of propionate. Fig. 57A depicts relatively low propionate production under aerobic conditions in which oxygen (O2) prevents (indicated by "X") FNR (grey boxed "FNR") from dimerizing and activating the FNR- responsive promoter ("FNR promoter"). Therefore, none of the propionate biosynthesis enzymes (thrA, thrB, thrC, ilvA, aceE, aceF, Ipd; black boxes) are expressed. Fig. 57B depicts increased propionate production under low- oxygen conditions due to FNR dimerizing (two grey boxed "FNR"s), binding to the FNR-responsive promoter, and inducing expression of the propionate biosynthesis enzymes, which leads to the production of propionate. Fig. 57C depicts an exemplary propionate biosynthesis gene cassette.
[0070] Fig. 58 depicts the gene organization of an exemplary engineered bacterium of the invention and its induction under low-oxygen conditions for the production of propionate. Fig. 58A depicts relatively low propionate production under aerobic conditions in which oxygen (O2) prevents (indicated by "X") FNR (grey boxed "FNR") from dimerizing and activating the FNR-responsive promoter ("FNR promoter"). Therefore, none of the propionate biosynthesis enzymes (thrA, thrB, thrC, ilvA, aceE, aceF, Ipd, tesB; black boxes) are expressed. Fig. 58B depicts increased propionate production under low- oxygen conditions due to FNR dimerizing (two grey boxed "FNR"s), binding to the FNR-responsive promoter, and inducing expression of the propionate biosynthesis enzymes, which leads to the production of propionate.
[0071] Fig. 59 depicts an exemplary propionate biosynthesis gene cassette.
[0072] Figs. 60A and 60B depict diagrams of exemplary constructs which may be used to produce a positive feedback auxotroph and select for high arginine production. Fig. 60A depicts a map of the astC promoter driving expression of thy A. Fig. 60B depicts a schematic diagram of the thy A gene under the control of an astC promoter. The regulatory region comprises binding
sites for CRP, ArgR, and RNA polymerase (RNAP), and may also comprise additional regulatory elements.
[0073] Fig. 61 depicts another exemplary embodiment of an engineered bacterial strain to target urea cycle disorder (UCD), via the conversion of ammonia to desired products, such as citrulline or arginine. The strain is deleted for the argR gene and expressing the feedback-resistant argAfbr gene. In some embodiments, this strain further comprises one or more auxotrophic
modifications on the chromosome. The synthetic biotic engineered to target urea cycle disorder (UCD) also has the kill-switch embodiment described in Fig. 65. In this example, the Int recombinase and the Kid-Kis toxin-antitoxin system are used in a recombinant bacterial cell for treating UCD. The recombinant bacterial cell is engineered to consume excess ammonia to produce beneficial byproducts to improve patient outcomes. The recombinant bacterial cell also comprises a highly controllable kill switch to ensure safety. In response to a low oxygen environment (e.g., such as that found in the gut), the FNR promoter induces expression of the Int recombinase and also induces expression of the Kis anti-toxin. The Int recombinase causes the Kid toxin gene to flip into an activated conformation, but the presence of the accumulated Kis anti-toxin suppresses the activity of the expressed Kid toxin. In the presence of oxygen (e.g., outside the gut), expression of the anti-toxin is turned off. Since the toxin is constitutively expressed, it continues to accumulate and kills the bacterial cell.
[0074] Hyperammonemia can also contribute to other pathologies. Fig. 66A depicts another non-limiting embodiment of the disclosure, wherein the expression of a heterologous gene is activated by an exogenous environmental signal. In the absence of arabinose, the AraC transcription factor adopts a conformation that represses transcription. In the presence of arabinose, the AraC transcription factor undergoes a conformational change that allows it to bind to and activate the ParaBAD promoter (ParaBAD), which induces expression of the Tet repressor (TetR) and an anti-toxin. The anti-toxin builds up in the recombinant bacterial cell, while TetR prevents expression of a toxin (which is under the control of a promoter having a TetR binding site). However, when arabinose is not present, both the anti-toxin and TetR are not expressed. Since TetR is not present to repress expression of the toxin, the toxin is expressed
and kills the cell. Fig. 66A also depicts another non-limiting embodiment of the disclosure, wherein the expression of an essential gene not found in the recombinant bacteria is activated by an exogenous environmental signal. In the absence of arabinose, the AraC transcription factor adopts a conformation that represses transcription of the essential gene under the control of the araBAD promoter and the bacterial cell cannot survive. In the presence of arabinose, the AraC transcription factor undergoes a conformational change that allows it to bind to and activate the araBAD promoter, which induces expression of the essential gene and maintains viability of the bacterial cell. Fig. 66B depicts a non-limiting embodiment of the disclosure, where an anti-toxin is expressed from a constitutive promoter, and expression of a heterologous gene is activated by an exogenous environmental signal. In the absence of arabinose, the AraC transcription factor adopts a conformation that represses transcription. In the presence of arabinose, the AraC transcription factor undergoes a conformational change that allows it to bind to and activate the araBAD promoter, which induces expression of TetR, thus preventing expression of a toxin. However, when arabinose is not present, TetR is not expressed, and the toxin is expressed, eventually overcoming the anti-toxin and killing the cell. The constitutive promoter regulating expression of the anti-toxin should be a weaker promoter than the promoter driving expression of the toxin. The araC gene is under the control of a constitutive promoter in this circuit. Fig. 66C depicts another non-limiting embodiment of the disclosure, wherein the expression of a heterologous gene is activated by an exogenous environmental signal. In the absence of arabinose, the AraC transcription factor adopts a conformation that represses transcription. In the presence of arabinose, the AraC transcription factor undergoes a conformational change that allows it to bind to and activate the araBAD promoter, which induces expression of the Tet repressor (TetR) and an anti-toxin. The anti-toxin builds up in the recombinant bacterial cell, while TetR prevents expression of a toxin (which is under the control of a promoter having a TetR binding site). However, when arabinose is not present, both the anti-toxin and TetR are not expressed. Since TetR is not present to repress expression of the toxin, the toxin is expressed and kills the cell. The
araC gene is either under the control of a constitutive promoter or an inducible promoter (e.g., AraC promoter) in this circuit.
[0075] Fig. 67 depicts the use of GeneGuards as an engineered safety component. All engineered DNA is present on a plasmid which can be conditionally destroyed. See, e.g., Wright et al., "GeneGuard: A Modular Plasmid System Designed for Biosafety," ACS Synthetic Biology (2015) 4: 307- 316.
[0076] Fig. 68 depicts a one non-limiting embodiment of the disclosure, which comprises a plasmid stability system with a plasmid that produces both a short-lived anti-toxin and a long-lived toxin. When the cell loses the plasmid, the anti-toxin is no longer produced, and the toxin kills the cell. In one embodiment, the genetically engineered bacteria produce a equal amount of a Hok toxin and a short-lived Sok antitoxin. In the upper panel, the cell produces equal amounts of toxin and anti-toxin and is stable. In the center panel, the cell loses the plasmid and anti-toxin begins to decay. In the lower panel, the anti-toxin decays completely, and the cell dies.
[0077] Fig. 69 depicts a schematic of a secretion system based on the flagellar type III secretion in which an incomplete flagellum is used to secrete a therapeutic peptide of interest (star) by recombinantly fusing the peptide to an N-terminal flagellar secretion signal of a native flagellar component so that the intracellularly expressed chimeric peptide can be mobilized across the inner and outer membranes into the surrounding host environment.
[0078] Fig. 70 depicts a schematic of a type V secretion system for the extracellular production of recombinant proteins in which a therapeutic peptide (star) can be fused to an N-terminal secretion signal, a linker and the beta- domain of an autotransporter. In this system, the N-terminal signal sequence directs the protein to the SecA-YEG machinery which moves the protein across the inner membrane into the periplasm, followed by subsequent cleavage of the signal sequence. The beta-domain is recruited to the Bam complex where the beta-domain is folded and inserted into the outer membrane as a beta-barrel structure. The therapeutic peptide is then thread through the hollow pore of the beta-barrel structure ahead of the linker sequence. The therapeutic peptide is freed from the linker system by an autocatalytic cleavage or by targeting of a
membrane-associated peptidase (scissors) to a complementary protease cut site in the linker.
[0079] Fig. 71 depicts a schematic of a type I secretion system, which translocates a passenger peptide directly from the cytoplasm to the extracellular space using HlyB (an ATP-binding cassette transporter); HlyD (a membrane fusion protein); and TolC (an outer membrane protein) which form a channel through both the inner and outer membranes. The secretion signal-containing C-terminal portion of HlyA is fused to the C-terminal portion of a therapeutic peptide (star) to mediate secretion of this peptide.
[0080] Fig. 72 depicts a schematic of the outer and inner membranes of a gram-negative bacterium, and several deletion targets for generating a leaky or destabilized outer membrane, thereby facilitating the translocation of a therapeutic polypeptides to the extracellular space, e.g., therapeutic
polypeptides of eukaryotic origin containing disulphide bonds. Deactivating mutations of one or more genes encoding a protein that tethers the outer membrane to the peptidoglycan skeleton, e.g., Ipp, ompC, ompA, ompF, tolA, tolB, pal, and/or one or more genes encoding a periplasmic protease, e.g., degS, degP, nlpl, generates a leaky phenotype. Combinations of mutations may synergistically enhance the leaky phenotype.
[0081] Fig. 73 depicts a modified type 3 secretion system (T3SS) to allow the bacteria to inject secreted therapeutic proteins into the gut lumen. An inducible promoter (small arrow, top), e.g. a FNR-inducible promoter, drives expression of the T3 secretion system gene cassette (3 large arrows, top) that produces the apparatus that secretes tagged peptides out of the cell. An inducible promoter (small arrow, bottom), e.g. a FNR-inducible promoter, drives expression of a regulatory factor, e.g. T7 polymerase, that then activates the expression of the tagged therapeutic peptide (hexagons).
[0082] Fig. 74 depicts an exemplary L-homoserine and L-methionine biosynthesis pathway. Circles indicate genes repressed by MetJ, and deletion of metJ leads to constitutive expression of these genes and activation of the pathway.
[0083] Fig. 75 depicts an exemplary histidine biosynthesis pathway.
[0084] Fig. 76 depicts an exemplary lysine biosynthesis pathway.
[0085] Fig. 77 depicts an exemplary asparagine biosynthesis pathway.
[0086] Fig. 78 depicts an exemplary glutamine biosynthesis pathway.
[0087] Fig. 79 depicts an exemplary tryptophan biosynthesis pathway.
[0088] Fig. 80 depicts a schematic of non-limiting processes for designing and producing the genetically engineered bacteria of the present disclosure. The step of "defining" comprises 1. Identification of diverse candidate approaches based on microbial physiology and disease biology; 2. Use of bioinformatics to determine candidate metabolic pathways; the use of prospective tools to determine performance targets required of optimized engineered synthetic biotics. The step of "designing" comprises the use of 1. Cutting-edge DNA assembly to enable combinatorial testing of pathway organization; 2. Mathematical models to predict pathway efficiency; 3. Internal stable of proprietary switches and parts to permit control and tuning of engineered circuits. The step of "Bulling" comprises 1. Building core structures "chassies" 2. Stably integrating engineered circuits into optimal chromosomal locations for efficient expression; 3. Employing unique functional assays to assess genetic circuit fidelity and activity. The step of "integrating" comprises 1. Use of chromosomal markers, which enable monitoring of synthetic biotic localization and transit times in animal models; 2. Leveraging expert
microbiome network and bioinformatics support to expand understanding of how specific disease states affect Gl microbial flora and the behaviors of synthetic biotics in that environment; 3. Activating process development research and optimization in-house during the discovery phase, enabling rapid and seamless transition of development candidates to pre-clinical progression; Drawing upon extensive experience in specialized disease animal model refinement, which supports prudent, high quality testing of candidate synthetic biotics.
[0089] Figs. 81 A, B, C, D, and E depict a schematic of non-limiting manufacturing processes for upstream and downstream production of the genetically engineered bacteria of the present disclosure. Fig. 81 A depicts the parameters for starter culture 1 (SC1 ): loop full - glycerol stock, duration overnight, temperature 37° C, shaking at 250 rpm. Fig. 81 B depicts the parameters for starter culture 2 (SC2): 1/100 dilution from SC1 , duration 1.5
hours, temperature 37° C, shaking at 250 rpm. Fig. 81 C depicts the
parameters for the production bioreactor: inoculum - SC2, temperature 37° C, pH set point 7.00, pH dead band 0.05, dissolved oxygen set point 50%, dissolved oxygen cascade agitation/gas FLO, agitation limits 300-1200 rpm, gas FLO limits 0.5-20 standard liters per minute, duration 24 hours. Fig. 81 D depicts the parameters for harvest: centrifugation at speed 4000 rpm and duration 30 minutes, wash 1X 10% glycerol/PBS, centrifugation, re-suspension 10% glycerol/PBS. Fig. 81 E depicts the parameters for vial fill/storage: 1 -2 mL aliquots, -80° C.
[0090] Fig. 82 depicts ATC (Fig. 82A) or nitric oxide-inducible (Fig. 82B) reporter constructs. These constructs, when induced by their cognate inducer, lead to expression of GFP. Nissle cells harboring plasmids with either the control, ATC-inducible Ptet-GFP reporter construct or the nitric oxide inducible PnsrR-GFP reporter construct induced across a range of concentrations.
Promoter activity is expressed as relative florescence units. Fig. 82C depicts a schematic of the constructs.
[0091] Fig. 83 depicts a dot blot of bacteria harboring a plasmid expressing NsrR under control of a constitutive promoter and the reporter gene gfp (green fluorescent protein) under control of an NsrR-inducible promoter. DSS-treated mice serve as exemplary models for HE. As in HE subjects, the guts of mice are damaged by supplementing drinking water with 2-3% dextran sodium sulfate (DSS). Chemiluminescent is shown for NsrR-regulated promoters induced in DSS-treated mice.
[0092] Fig. 84 depicts butyrate production by genetically engineered Nissle comprising the pLogic031-nsrR-norB-butyrate construct (SYN-UCD507) or the pLogic046-nsrR-norB-butyrate construct (SYN-UCD508), which produce more butyrate as compared to wild-type Nissle.
Description of Embodiments
[0093] The invention includes genetically engineered bacteria, pharmaceutical compositions thereof, and methods of modulating or treating disorders associated with hyperammonemia, e.g., urea cycle disorders, hepatic encephalopathy and other disorders associated with excess ammonia or elevated ammonia levels. The genetically engineered bacteria are capable of
reducing excess ammonia, particularly under certain environmental conditions, such as those in the mammalian gut. In some embodiments, the genetically engineered bacteria reduce excess ammonia by incorporating excess nitrogen in the body into non-toxic molecules, e.g., arginine, citrulline, methionine, histidine, lysine, asparagine, glutamine, or tryptophan. In some embodiments, the genetically engineered bacteria reduce excess ammonia and also reduce one or more other toxic substances, e.g., GABA and/or manganese. In some embodiments, the genetically engineered bacteria reduce excess ammonia and also reduce GABA levels, e.g., by importing GABA and/or by metabolizing GABA. In some embodiments, the genetically engineered bacteria reduce excess ammonia and also reduce manganese levels, e.g., by importing manganese. The genetically engineered bacteria may additionally produce one or molecules that improve gut barrier function or otherwise alleviate a symptom of a disorder associated with elevated ammonia (e.g., UCDs, HE, etc). Thus, in any of the described embodiments, the genetically engineered bacteria may also produce one or molecules that improve gut barrier function or otherwise alleviate a symptom of a disorder associated with elevated ammonia. In some embodiments, the genetically engineered bacteria produce a short chain fatty acid, e.g., butyrate, propionate, and/or acetate. In some embodiments, the engineered bacteria reduce excess ammonia and produce one or molecules that improve gut barrier function or otherwise alleviate a symptom of a disorder associated with elevated ammonia, e.g., produce a short chain fatty acid, such as butyrate, propionate, and/or acetate. In some embodiments, the engineered bacteria reduce excess ammonia, reduce one or more other toxic substances, e.g., GABA and/or manganese, and produce one or molecules that improve gut barrier function or alleviate a symptom of a disorder associated with elevated ammonia, e.g., produce a short chain fatty acid, such as butyrate, propionate, and/or acetate. In some embodiments, the genetically engineered bacteria reduce excess ammonia, reduce GABA levels, e.g., by importing GABA and/or by metabolizing GABA, and produce one or molecules that improve gut barrier function or alleviate a symptom of a disorder associated with elevated ammonia, e.g., produce a short chain fatty acid, such as butyrate, propionate, and/or acetate. In some embodiments, the genetically engineered bacteria
reduce excess ammonia, reduce manganese levels, e.g., by importing manganese, and produce one or molecules that improve gut barrier function or alleviate a symptom of a disorder associated with elevated ammonia, e.g., produce a short chain fatty acid, such as butyrate, propionate, and/or acetate.
[0094] In any of the described embodiments, the engineered bacteria may further comprise one or more of more of the following: (1 ) one or more auxotrophies, such as any auxotrophies known in the art and provided herein, e.g., thyA auxotrophy, (2) one or more kill switch circuits, such as any of the kill- switches described herein or otherwise known in the art, (3) one or more antibiotic resistance circuits, (4) one or more transporters for importing biological molecules or substrates, such any of the transporters described herein or otherwise known in the art, (5) one or more secretion circuits, such as any of the secretion circuits described herein and otherwise known in the art, and (6) combinations of one or more of such additional circuits.
[0095] In some embodiments, any one or more of the payload or therapeutic circuits (e.g., ammonia consuming, GABA reducing, manganese reducing, short chain fatty acid producing circuits) and/or any one or more of the additional circuits (e.g., auxotrophies, kill switch circuits, antibiotic resistance circuits, transporters, and secretion circuits) may be regulated by a constitutive promoter. In some embodiments, any one or more of the payload or therapeutic circuits (e.g., ammonia consuming, GABA reducing, manganese reducing, short chain fatty acid producing circuits) and/or any one or more of the additional circuits (e.g., auxotrophies, kill switch circuits, antibiotic resistance circuits, transporters, and secretion circuits) may be regulated by a tissue-specific promoter. In some embodiments, any one or more of the payload or
therapeutic circuits (e.g., ammonia consuming, GABA reducing, manganese reducing, short chain fatty acid producing circuits) and/or any one or more of the additional circuits (e.g., auxotrophies, kill switch circuits, antibiotic resistance circuits, transporters, and secretion circuits) may be regulated by an inducible promoter. In some embodiments, any one or more of the payload or therapeutic circuits (e.g., ammonia consuming, GABA reducing, manganese reducing, short chain fatty acid producing circuits) and/or any one or more of the additional circuits (e.g., auxotrophies, kill switch circuits, antibiotic resistance circuits,
transporters, and secretion circuits) may be regulated by an inducible promoter that is responsive to environmental conditions, factors, or cues, e.g.,
environmental conditions, factors, or cues found in the mammalian gut.
Exemplary inducible promoters include oxygen level-dependent promoters (e.g., FNR-inducible promoter), promoters induced by HE-specific molecules or metabolites indicative of liver damage (e.g., bilirubin), promoters induced by inflammation or an inflammatory response (RNS, ROS promoters), and promoters induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose and tetracycline.
[0096] In some embodiments, any one or more of the payload or therapeutic circuits (e.g., ammonia consuming, GABA reducing, manganese reducing, short chain fatty acid producing circuits) and/or any one or more of the additional circuits (e.g., auxotrophies, kill switch circuits, antibiotic resistance circuits, transporters, and secretion circuits) may be present on one or more low copy or high copy plasmids. In some embodiments, any one or more of the payload or therapeutic circuits (e.g., ammonia consuming, GABA reducing, manganese reducing, short chain fatty acid producing circuits) and/or any one or more of the additional circuits (e.g., auxotrophies, kill switch circuits, antibiotic resistance circuits, transporters, and secretion circuits) may be integrated into the bacterial chromosome. In order that the disclosure may be more readily understood, certain terms are first defined. These definitions should be read in light of the remainder of the disclosure and as understood by a person of ordinary skill in the art. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by a person of ordinary skill in the art. Additional definitions are set forth throughout the detailed description.
[0097] "Hyperammonemia," "hyperammonemic," or "excess ammonia" is used to refer to increased concentrations of ammonia in the body.
Hyperammonemia is caused by decreased detoxification and/or increased production of ammonia. Decreased detoxification may result from urea cycle disorders (UCDs), such as argininosuccinic aciduria, arginase deficiency, carbamoylphosphate synthetase deficiency, citrullinemia, N-acetylglutamate synthetase deficiency, and ornithine transcarbamylase deficiency; or from
bypass of the liver, e.g., open ductus hepaticus; and/or deficiencies in glutamine synthetase (Hoffman et al., 2013; Haberle et al., 2013). Decreased
detoxification may also result from liver disorders such as hepatic
encephalopathy, acute liver failure, or chronic liver failure; and
neurodegenerative disorders such as Huntington's disease (Chen et al., 2015; Chiang et al., 2007). Increased production of ammonia may result from infections, drugs, neurogenic bladder, and intestinal bacterial overgrowth (Haberle et al., 2013). Other disorders and conditions associated with hyperammonemia include, but are not limited to, liver disorders such as hepatic encephalopathy, acute liver failure, or chronic liver failure; organic acid disorders; isovaleric aciduria; 3-methylcrotonylglycinuria; methylmalonic acidemia; propionic aciduria; fatty acid oxidation defects; carnitine cycle defects; carnitine deficiency; β-oxidation deficiency; lysinuric protein intolerance;
pyrroline-5-carboxylate synthetase deficiency; pyruvate carboxylase deficiency; ornithine aminotransferase deficiency; carbonic anhydrase deficiency;
hyperinsulinism-hyperammonemia syndrome; mitochondrial disorders;
valproate therapy; asparaginase therapy; total parenteral nutrition; cystoscopy with glycine-containing solutions; post-lung/bone marrow transplantation;
portosystemic shunting; urinary tract infections; ureter dilation; multiple myeloma; and chemotherapy (Hoffman et al., 2013; Haberle et al., 2013; Pham et al., 2013; Lazier et al., 2014). In healthy subjects, plasma ammonia concentrations are typically less than about 50 pmol/L (Leonard, 2006). In some embodiments, a diagnostic signal of hyperammonemia is a plasma ammonia concentration of at least about 50 pmol/L, at least about 80 pmol/L, at least about 150 pmol/L, at least about 180 pmol/L, or at least about 200 pmol/L (Leonard, 2006; Hoffman et al., 2013; Haberle et al., 2013).
[0098] "Ammonia" is used to refer to gaseous ammonia (NH3), ionic ammonia (NH4 +), or a mixture thereof. In bodily fluids, gaseous ammonia and ionic ammonium exist in equilibrium:
[0099] NH3 + H+ « NH4 +
[0100] Some clinical laboratory tests analyze total ammonia (NH3 + NH4 +) (Walker, 2012). In any embodiment of the invention, unless otherwise
indicated, "ammonia" may refer to gaseous ammonia, ionic ammonia, and/or total ammonia.
[0101] "Detoxification" of ammonia is used to refer to the process or processes, natural or synthetic, by which toxic ammonia is removed and/or converted into one or more non-toxic molecules, including but not limited to: arginine, citrulline, methionine, histidine, lysine, asparagine, glutamine, tryptophan, or urea. The urea cycle, for example, enzymatically converts ammonia into urea for removal from the body in the urine. Because ammonia is a source of nitrogen for many amino acids, which are synthesized via numerous biochemical pathways, enhancement of one or more of those amino acid biosynthesis pathways may be used to incorporate excess nitrogen into nontoxic molecules. For example, arginine biosynthesis converts glutamate, which comprises one nitrogen atom, to arginine, which comprises four nitrogen atoms, thereby incorporating excess nitrogen into non-toxic molecules. In humans, arginine is not reabsorbed from the large intestine, and as a result, excess arginine in the large intestine is not considered to be harmful. Likewise, citrulline is not reabsorbed from the large intestine, and as a result, excess citrulline in the large intestine is not considered to be harmful. Arginine biosynthesis may also be modified to produce citrulline as an end product;
citrulline comprises three nitrogen atoms and thus the modified pathway is also capable of incorporating excess nitrogen into non-toxic molecules.
[0102] "Arginine regulon," "arginine biosynthesis regulon," and "arg regulon" are used interchangeably to refer to the collection of operons in a given bacterial species that comprise the genes encoding the enzymes responsible for converting glutamate to arginine and/or intermediate metabolites, e.g., citrulline, in the arginine biosynthesis pathway. The arginine regulon also comprises operators, promoters, ARG boxes, and/or regulatory regions associated with those operons. The arginine regulon includes, but is not limited to, the operons encoding the arginine biosynthesis enzymes N-acetylglutamate synthetase, N-acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine aminotransferase, N-acetylornithinase, ornithine
transcarbamylase, argininosuccinate synthase, argininosuccinate lyase, carbamoylphosphate synthase, operators thereof, promoters thereof, ARG
boxes thereof, and/or regulatory regions thereof. In some embodiments, the arginine regulon comprises an operon encoding ornithine acetyltransferase and associated operators, promoters, ARG boxes, and/or regulatory regions, either in addition to or in lieu of N-acetylglutamate synthetase and/or N- acetylornithinase. In some embodiments, one or more operons or genes of the arginine regulon may be present on a plasmid in the bacterium. In some embodiments, a bacterium may comprise multiple copies of any gene or operon in the arginine regulon, wherein one or more copies may be mutated or otherwise altered as described herein.
[0103] One gene may encode one enzyme, e.g., N-acetylglutamate synthetase {argA). Two or more genes may encode distinct subunits of one enzyme, e.g., subunit A and subunit B of carbamoylphosphate synthase (carA and carB). In some bacteria, two or more genes may each independently encode the same enzyme, e.g., ornithine transcarbamylase {argF and argl). In some bacteria, the arginine regulon includes, but is not limited to, argA, encoding N-acetylglutamate synthetase; argB, encoding N-acetylglutamate kinase; argC, encoding N-acetylglutamylphosphate reductase; argD, encoding acetylornithine aminotransferase; argE, encoding N-acetylornithinase; argG, encoding argininosuccinate synthase; argH, encoding argininosuccinate lyase; one or both of argF and argl, each of which independently encodes ornithine transcarbamylase; car A, encoding the small subunit of carbamoylphosphate synthase; carB, encoding the large subunit of carbamoylphosphate synthase; operons thereof; operators thereof; promoters thereof; ARG boxes thereof; and/or regulatory regions thereof. In some embodiments, the arginine regulon comprises argJ, encoding ornithine acetyltransferase (either in addition to or in lieu of N-acetylglutamate synthetase and/or N-acetylornithinase), operons thereof, operators thereof, promoters thereof, ARG boxes thereof, and/or regulatory regions thereof.
[0104] "Arginine operon," "arginine biosynthesis operon," and "arg operon" are used interchangeably to refer to a cluster of one or more of the genes encoding arginine biosynthesis enzymes under the control of a shared regulatory region comprising at least one promoter and at least one ARG box. In some embodiments, the one or more genes are co-transcribed and/or co-
translated. Any combination of the genes encoding the enzymes responsible for arginine biosynthesis may be organized, naturally or synthetically, into an operon. For example, in B. subtilis, the genes encoding N- acetylglutamylphosphate reductase, N-acetylglutamate kinase, N- acetylornithinase, N-acetylglutamate kinase, acetylornithine aminotransferase, carbamoylphosphate synthase, and ornithine transcarbamylase are organized in a single operon, argCAEBD-carAB-argF (see, e.g., Table 2), under the control of a shared regulatory region comprising a promoter and ARG boxes. In E. coli K12 and Nissle, the genes encoding N-acetylornithinase, N- acetylglutamylphosphate reductase, N-acetylglutamate kinase, and
argininosuccinate lyase are organized in two bipolar operons, argECBH. The operons encoding the enzymes responsible for arginine biosynthesis may be distributed at different loci across the chromosome. In unmodified bacteria, each operon may be repressed by arginine via ArgR. In some embodiments, arginine and/or intermediate byproduct production may be altered in the genetically engineered bacteria of the invention by modifying the expression of the enzymes encoded by the arginine biosynthesis operons as provided herein. Each arginine operon may be present on a plasmid or bacterial chromosome. In addition, multiple copies of any arginine operon, or a gene or regulatory region within an arginine operon, may be present in the bacterium, wherein one or more copies of the operon or gene or regulatory region may be mutated or otherwise altered as described herein. In some embodiments, the genetically engineered bacteria are engineered to comprise multiple copies of the same product (e.g., operon or gene or regulatory region) to enhance copy number or to comprise multiple different components of an operon performing multiple different functions.
[0105] "ARG box consensus sequence" refers to an ARG box nucleic acid sequence, the nucleic acids of which are known to occur with high frequency in one or more of the regulatory regions of argR, argA, argB, argC, argD, argE, argF, argG, argH, argl, argJ, car A, and/or carB. As described above, each arg operon comprises a regulatory region comprising at least one 18-nucleotide imperfect palindromic sequence, called an ARG box, that overlaps with the promoter and to which the repressor protein binds (Tian et al.,
1992). The nucleotide sequences of the ARG boxes may vary for each operon, and the consensus ARG box sequence is A/T nTGAAT A/T A T/A T/A ATTCAn T/A (Maas, 1994). The arginine repressor binds to one or more ARG boxes to actively inhibit the transcription of the arginine biosynthesis enzyme(s) that are operably linked to that one or more ARG boxes.
[0106] "Mutant arginine regulon" or "mutated arginine regulon" is used to refer to an arginine regulon comprising one or more nucleic acid mutations that reduce or eliminate arginine-mediated repression of each of the operons that encode the enzymes responsible for converting glutamate to arginine and/or an intermediate byproduct, e.g., citrulline, in the arginine biosynthesis pathway, such that the mutant arginine regulon produces more arginine and/or
intermediate byproduct than an unmodified regulon from the same bacterial subtype under the same conditions. In some embodiments, the genetically engineered bacteria comprise an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmr, and a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for one or more of the operons that encode the arginine biosynthesis enzymes N-acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine
aminotransferase, N-acetylornithinase, ornithine transcarbamylase,
argininosuccinate synthase, argininosuccinate lyase, and carbamoylphosphate synthase, thereby derepressing the regulon and enhancing arginine and/or intermediate byproduct biosynthesis. In some embodiments, the genetically engineered bacteria comprise a mutant arginine repressor comprising one or more nucleic acid mutations such that arginine repressor function is decreased or inactive, or the genetically engineered bacteria do not have an arginine repressor (e.g., the arginine repressor gene has been deleted), resulting in derepression of the regulon and enhancement of arginine and/or intermediate byproduct biosynthesis. In some embodiments, the genetically engineered bacteria comprise an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmr, a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes, and/or a mutant or deleted arginine repressor. In some embodiments, the genetically engineered bacteria comprise an
arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argA and a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes. In some embodiments, the genetically engineered bacteria comprise an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmrand a mutant or deleted arginine repressor. In some embodiments, the mutant arginine regulon comprises an operon encoding wild- type N-acetylglutamate synthetase and one or more nucleic acid mutations in at least one ARG box for said operon. In some embodiments, the mutant arginine regulon comprises an operon encoding wild-type N-acetylglutamate synthetase and mutant or deleted arginine repressor. In some embodiments, the mutant arginine regulon comprises an operon encoding ornithine acetyltransferase (either in addition to or in lieu of N-acetylglutamate synthetase and/or N- acetylornithinase) and one or more nucleic acid mutations in at least one ARG box for said operon.
[0107] The ARG boxes overlap with the promoter in the regulatory region of each arginine biosynthesis operon. In the mutant arginine regulon, the regulatory region of one or more arginine biosynthesis operons is sufficiently mutated to disrupt the palindromic ARG box sequence and reduce ArgR binding, but still comprises sufficiently high homology to the promoter of the non-mutant regulatory region to be recognized as the native operon-specific promoter. The operon comprises at least one nucleic acid mutation in at least one ARG box such that ArgR binding to the ARG box and to the regulatory region of the operon is reduced or eliminated. In some embodiments, bases that are protected from DNA methylation and bases that are protected from hydroxyl radical attack during ArgR binding are the primary targets for mutations to disrupt ArgR binding (see, e.g., Table 3). The promoter of the mutated regulatory region retains sufficiently high homology to the promoter of the non- mutant regulatory region such that RNA polymerase binds to it with sufficient affinity to promote transcription of the operably linked arginine biosynthesis enzyme(s). In some embodiments, the G/C:A/T ratio of the promoter of the mutant differs by no more than 10% from the G/C:A/T ratio of the wild-type promoter.
[0108] In some embodiments, more than one ARG box may be present in a single operon. In one aspect of these embodiments, at least one of the ARG boxes in an operon is altered to produce the requisite reduced ArgR binding to the regulatory region of the operon. In an alternate aspect of these
embodiments, each of the ARG boxes in an operon is altered to produce the requisite reduced ArgR binding to the regulatory region of the operon.
[0109] "Reduced" ArgR binding is used to refer to a reduction in repressor binding to an ARG box in an operon or a reduction in the total repressor binding to the regulatory region of said operon, as compared to repressor binding to an unmodified ARG box and regulatory region in bacteria of the same subtype under the same conditions. In some embodiments, ArgR binding to a mutant ARG box and regulatory region of an operon is at least about 50% lower, at least about 60% lower, at least about 70% lower, at least about 80% lower, at least about 90% lower, or at least about 95% lower than ArgR binding to an unmodified ARG box and regulatory region in bacteria of the same subtype under the same conditions. In some embodiments, reduced ArgR binding to a mutant ARG box and regulatory region results in at least about 1.5-fold, at least about 2-fold, at least about 10-fold, at least about 15- fold, at least about 20-fold, at least about 30-fold, at least about 50-fold, at least about 100-fold, at least about 200-fold, at least about 300-fold, at least about 400-fold, at least about 500-fold, at least about 600-fold, at least about 700-fold, at least about 800-fold, at least about 900-fold, at least about 1 , 000-fold, or at least about 1 , 500-fold increased mRNA expression of the one or more genes in the operon.
[0110] "ArgR" or "arginine repressor" is used to refer to a protein that is capable of suppressing arginine biosynthesis by regulating the transcription of arginine biosynthesis genes in the arginine regulon. When expression of the gene that encodes for the arginine repressor protein ("argR") is increased in a wild-type bacterium, arginine biosynthesis is decreased. When expression of argR is decreased in a wild-type bacterium, or if argR is deleted or mutated to inactivate arginine repressor function, arginine biosynthesis is increased.
[0111] Bacteria that "lack any functional ArgR" and "ArgR deletion bacteria" are used to refer to bacteria in which each arginine repressor has
significantly reduced or eliminated activity as compared to unmodified arginine repressor from bacteria of the same subtype under the same conditions.
Reduced or eliminated arginine repressor activity can result in, for example, increased transcription of the arginine biosynthesis genes and/or increased concentrations of arginine and/or intermediate byproducts, e.g., citrulline. Bacteria in which arginine repressor activity is reduced or eliminated can be generated by modifying the bacterial argR gene or by modifying the
transcription of the argR gene. For example, the chromosomal argR gene can be deleted, can be mutated, or the argR gene can be replaced with an argR gene that does not exhibit wild-type repressor activity.
[0112]"Operably linked" refers a nucleic acid sequence, e.g., a gene encoding feedback resistant ArgA, that is joined to a regulatory region sequence in a manner which allows expression of the nucleic acid sequence, e.g., acts in cis. A regulatory region is a nucleic acid that can direct transcription of a gene of interest and may comprise promoter sequences, enhancer sequences, response elements, protein recognition sites, inducible elements, promoter control elements, protein binding sequences, 5' and 3' untranslated regions, transcriptional start sites, termination sequences, polyadenylation sequences, and introns.
An "inducible promoter" refers to a regulatory region that is operably linked to one or more genes, wherein expression of the gene(s) is increased in the presence of an inducer of said regulatory region. In some embodiments, the genetically engineered bacteria of the invention comprise an oxygen level- dependent promoter induced by low-oxygen, microaerobic, or anaerobic conditions. In some embodiments, the genetically engineered bacteria comprise a promoter induced by a molecule or metabolite, for example, a tissue-specific molecule or metabolite or a molecule or metabolite indicative of liver damage. In some embodiments, the metabolites may be gut specific. In some embodiments, the metabolite may be associated with hepatic
encephalopathy, e.g., bilirubin. Non-limiting examples of molecules or metabolites include, e.g., bilirubin, aspartate aminotransferase, alanine aminotransferase, blood coagulation factors II, VII, IX, and X, alkaline phosphatase, gamma glutamyl transferase, hepatitis antigens and antibodies,
alpha fetoprotein, anti-mitochondrial, smooth muscle, and anti-nuclear antibodies, iron, transferrin, ferritin, copper, ceruloplasmin, ammonia, and manganese in their blood and intestines. Promoters that respond to one of these molecules or their metabolites may be used in the genetically engineered bacteria provided herein. In some embodiments, the genetically engineered bacteria comprise a promoter induced by inflammation or an inflammatory response, e.g., RNS or ROS promoter. In some embodiments, the genetically engineered bacteria comprise a promoter induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose and tetracycline.
[0113] "Exogenous environmental condition(s)" refer to setting(s) or circumstance(s) under which the promoter described herein is induced. The phrase "exogenous environmental conditions" is meant to refer to the
environmental conditions external to the engineered micororganism, but endogenous or native to the host subject environment. Thus, "exogenous" and "endogenous" may be used interchangeably to refer to environmental conditions in which the environmental conditions are endogenous to a mammalian body, but external or exogenous to an intact microorganism cell. In some
embodiments, the exogenous environmental conditions are specific to the gut of a mammal. In some embodiments, the exogenous environmental conditions are specific to the upper gastrointestinal tract of a mammal. In some
embodiments, the exogenous environmental conditions are specific to the lower gastrointestinal tract of a mammal. In some embodiments, the exogenous environmental conditions are specific to the small intestine of a mammal. In some embodiments, exogenous environmental conditions refer to the presence of molecules or metabolites that are specific to the mammalian gut in a healthy or disease state (e.g., HE). In some embodiments, the exogenous
environmental conditions are low-oxygen, microaerobic, or anaerobic
conditions, such as the environment of the mammalian gut. In some
embodiments, exogenous environmental conditions are molecules or
metabolites that are specific to the mammalian gut, e.g., propionate. In some embodiments, the exogenous environmental condition is a tissue-specific or disease-specific metabolite or molecule(s). In some embodiments, the
exogenous environmental condition is a low-pH environment. In some embodiments, the genetically engineered microorganism of the disclosure comprises a pH-dependent promoter. In some embodiments, the genetically engineered microorganism of the diclosure comprise an oxygen level- dependent promoter. In some aspects, bacteria have evolved transcription factors that are capable of sensing oxygen levels. Different signaling pathways may be triggered by different oxygen levels and occur with different kinetics.
[0114] An "oxygen level-dependent promoter" or "oxygen level- dependent regulatory region" refers to a nucleic acid sequence to which one or more oxygen level-sensing transcription factors is capable of binding, wherein the binding and/or activation of the corresponding transcription factor activates downstream gene expression.
[0115] Examples of oxygen level-dependent transcription factors include, but are not limited to, FNR, ANR, and DNR. Corresponding FNR-responsive promoters, ANR-responsive promoters, and DNR-responsive promoters are known in the art (see, e.g., Castiglione et al., 2009; Eiglmeier et al., 1989;
Galimand et al., 1991 ; Hasegawa et al., 1998; Hoeren et al., 1993; Salmon et al., 2003), and non-limiting examples are shown in Table 1.
[0116] In a non-limiting example, a promoter (PfnrS) was derived from the E. coli Nissle fumarate and nitrate reductase gene S (fnrS) that is known to be highly expressed under conditions of low or no environmental oxygen (Durand and Storz, 2010; Boysen et al, 2010). The PfnrS promoter is activated under anaerobic conditions by the global transcriptional regulator FNR that is naturally found in Nissle. Under anaerobic conditions, FNR forms a dimer and binds to specific sequences in the promoters of specific genes under its control, thereby activating their expression. However, under aerobic conditions, oxygen reacts with iron-sulfur clusters in FNR dimers and converts them to an inactive form. In this way, the PfnrS inducible promoter is adopted to modulate the expression of proteins or RNA. PfnrS is used interchangeably in this application as FNRS, fnrs, FNR, P-FNRS promoter and other such related designations to indicate the promoter PfnrS.
Table 1. Examples of transcription factors and responsive genes and regulatory regions
Transcription Exemplary responsive genes, Factor promoters, and/or regulatory
regions:
FNR nirB, ydfZ, pdhR, focA, ndH, hlyE,
narK, narX, narG, yfi'D, tdcD
ANR arcDABC
DNR norb, norC
[0117] "Gut barrier function enhancer molecules" include, but are not limited to, short-chain fatty acids, butyrate, propionate, acetate, GLP-2, IL-10, IL-27, TGF-βΙ , TGF-P2, N-acylphosphatidylethanolamines (NAPEs), elafin (also called peptidase inhibitor 3 and SKALP), trefoil factor, melatonin, PGD2, kynurenic acid, and kynurenine. A gut barrier function enhancer molecule may be encoded by a single gene, e.g., elafin is encoded by the PI3 gene.
Alternatively, a gut barrier function enhancer molecule may be synthesized by a biosynthetic pathway requiring multiple genes, e.g., butyrate. These molecules may also be referred to as therapeutic molecules.
[0118] As used herein, a "gene cassette" or "operon" encoding a biosynthetic pathway refers to the two or more genes that are required to produce a gut barrier function enhancer molecule, e.g., butyrate, propionate. In addition to encoding a set of genes capable of producing said molecule, the gene cassette or operon may also comprise additional transcription and translation elements, e.g., a ribosome binding site.
[0119] A "butyrogenic gene cassette," "butyrate biosynthesis gene cassette," and "butyrate operon" are used interchangeably to refer to a set of genes capable of producing butyrate in a biosynthetic pathway. Unmodified bacteria that are capable of producing butyrate via an endogenous butyrate biosynthesis pathway include, but are not limited to, Clostridium,
Peptoclostridium, Fusobacterium, Butyrivibrio, Eubacterium, and Treponema, and these endogenous butyrate biosynthesis pathways may be a source of genes for the genetically engineered bacteria of the invention. The genetically engineered bacteria of the invention may comprise butyrate biosynthesis genes
from a different species, strain, or substrain of bacteria, or a combination of butyrate biosynthesis genes from different species, strains, and/or substrains of bacteria. A butyrogenic gene cassette may comprise, for example, the eight genes of the butyrate production pathway from Peptoclostridium difficile (also called Clostridium difficile): bcd2, etfB3, etfA3, thiA1, hbd, crt2, pbt, and buk, which encode butyryl-CoA dehydrogenase subunit, electron transfer
flavoprotein subunit beta, electron transfer flavoprotein subunit alpha, acetyl- CoA C-acetyltransferase, 3-hydroxybutyryl-CoA dehydrogenase, crotonase, phosphate butyryltransferase, and butyrate kinase, respectively (Aboulnaga et al., 2013). One or more of the butyrate biosynthesis genes may be functionally replaced or modified. Peptoclostridium difficile strain 630 and strain 1296 are both capable of producing butyrate, but comprise different nucleic acid sequences for etfA3, thiA1, hbd, crt2, pbt, and buk. A butyrogenic gene cassette may comprise bcd2, etfB3, etfA3, and thiA1 from Peptoclostridium difficile strain 630, and hbd, crt2, pbt, and buk from Peptoclostridium difficile strain 1296. Alternatively, a single gene from Treponema denticola (ter, encoding frans-2-enoynl-CoA reductase,) is capable of functionally replacing all three of the bcd2, etfB3, and etfA3 genes from Peptoclostridium difficile. Thus, a butyrogenic gene cassette may comprise thiA1, hbd, crt2, pbt, and buk from Peptoclostridium difficile and ferfrom Treponema denticola. The butyrogenic gene cassette may comprise genes for the aerobic biosynthesis of butyrate and/or genes for the anaerobic or microaerobic biosynthesis of butyrate.
[0120] Likewise, a "propionate gene cassette" or "propionate operon" refers to a set of genes capable of producing propionate in a biosynthetic pathway. Unmodified bacteria that are capable of producing propionate via an endogenous propionate biosynthesis pathway include, but are not limited to, Clostridium propionicum, Megasphaera elsdenii, and Prevotella ruminicola, and these endogenous propionate biosynthesis pathways may be a source of genes for the genetically engineered bacteria of the invention. The genetically engineered bacteria of the invention may comprise propionate biosynthesis genes from a different species, strain, or substrain of bacteria, or a combination of propionate biosynthesis genes from different species, strains, and/or substrains of bacteria. In some embodiments, the propionate gene cassette
comprises acrylate pathway propionate biosynthesis genes, e.g., pet, IcdA, IcdB, IcdC, etfA, acrB, and acrC, which encode propionate CoA-transferase, lactoyl-CoA dehydratase A, lactoyl-CoA dehydratase B, lactoyl-CoA
dehydratase C, electron transfer flavoprotein subunit A, acryloyl-CoA reductase B, and acryloyl-CoA reductase C, respectively (Hetzel et al., 2003, Selmer et al., 2002). In alternate embodiments, the propionate gene cassette comprises pyruvate pathway propionate biosynthesis genes (see, e.g., Tseng and Prather, 2012), e.g., thrA r, thrB, thrC, HvAfbr, aceE, aceF, and Ipd, which encode homoserine dehydrogenase 1 , homoserine kinase, L-threonine synthase, L- threonine dehydratase, pyruvate dehydrogenase, dihydrolipoamide
acetyltransferase, and dihydrolipoyl dehydrogenase, respectively. In some embodiments, the propionate gene cassette further comprises tesB, which encodes acyl-CoA thioesterase. The propionate gene cassette may comprise genes for the aerobic biosynthesis of propionate and/or genes for the anaerobic or microaerobic biosynthesis of propionate. One or more of the propionate biosynthesis genes may be functionally replaced or modified, e.g., codon optimized.
[0121] An "acetate gene cassette" or "acetate operon" refers to a set of genes capable of producing acetate in a biosynthetic pathway. Bacteria
"synthesize acetate from a number of carbon and energy sources," including a variety of substrates such as cellulose, lignin, and inorganic gases, and utilize different biosynthetic mechanisms and genes, which are known in the art (Ragsdale et al., 2008). Unmodified bacteria that are capable of producing acetate via an endogenous acetate biosynthesis pathway may be a source of acetate biosynthesis genes for the genetically engineered bacteria of the invention. The genetically engineered bacteria of the invention may comprise acetate biosynthesis genes from a different species, strain, or substrain of bacteria, or a combination of acetate biosynthesis genes from different species, strains, and/or substrains of bacteria. Escherichia coli are capable of consuming glucose and oxygen to produce acetate and carbon dioxide during aerobic growth (Kleman et al., 1994). Several bacteria, such as
Acetitomaculum, Acetoanaerobium, Acetohalobium, Acetonema, Balutia, Butyribacterium, Clostridium, Moorella, Oxobacter, Sporomusa, and
Thermoacetogenium, are acetogenic anaerobes that are capable of converting CO or CO2 + H2 into acetate, e.g., using the Wood-Ljungdahl pathway (Schiel- Bengelsdorf et al., 2012). Genes in the Wood-Ljungdahl pathway for various bacterial species are known in the art. The acetate gene cassette may comprise genes for the aerobic biosynthesis of acetate and/or genes for the anaerobic or microaerobic biosynthesis of acetate. One or more of the acetate biosynthesis genes may be functionally replaced or modified.
[0122] "GABA" and "γ-aminobutyric acid" are used to refer to the the predominant inhibitory neurotransmitter (C4H9NO2) in the mammalian central nervous system. In humans, GABA is also directly responsible for regulating muscle tone. GABA is capable of activating the GABAA receptor, which is part of a ligand-gated ion channel complex, as well as the GABAB metabotropic G protein-coupled receptor. Neurons that produce GABA are known as
"GABAergic" neurons, and activation of GABA receptors is described as
GABAergic tone (i.e., increased activation of GABA receptors refers to increased GABAergic tone).
[0123] "GABA transporter" and "GabP" are used to refer to a membrane transport protein that is capable of transporting GABA into bacterial cells (see, e.g., Li et al., 2001 ). In Escherichia coli, the gabP gene encodes a high-affinity GABA permease responsible for GABA transport (Li et al., 2001 ). In some embodiments, the GABA transporter is encoded by a gabP gene derived from a bacterial species, including but not limited to, Bacillus subtilis and Escherichia coli. These endogenous GABA transporter genes may be a source of genes for the genetically engineered bacteria of the invention. Any suitable gene(s) encoding a GABA transporter may be used.
[0124] "Manganese" refers to a chemical element with the symbol "Mn" and atomic number 25. In biological systems, manganese is an essential trace metal and plays an important role in enzyme-mediated catalysis, but can also have deleterious effects. Cells maintain manganese under tight homeostatic control in order to avoid toxicity. Some disorders associated with
hyperammonemia may also be characterized by elevated levels of manganese; manganese may contribute to disease pathogenesis (e.g., hepatic
encephalopathy) (Rivera-Mancia et al., 2012).
[0125] "Manganese transporter" and "MntH" refer to a membrane transport protein that is capable of transporting manganese into bacterial cells {see, e.g., Jensen and Jensen, 2014). In Escherichia coli, the mntH gene encodes a proton-stimulated, divalent metal cation uptake system involved in manganese transport (Porcheron et al., 2013). In some embodiments, the manganese transporter is encoded by a mntH gene derived from a bacterial species, including but not limited to, Salmonella typhimurium, Shigella flexneri, Yersinia pestis, and Escherichia coli. These endogenous manganese transporter genes may be a source of genes for the genetically engineered bacteria of the invention. Any suitable gene(s) encoding a manganese transporter may be used.
[0126] As used herein, a "non-native" nucleic acid sequence refers to a nucleic acid sequence not normally present in a bacterium, e.g., an extra copy of an endogenous sequence, or a heterologous sequence such as a sequence from a different species, strain, or substrain of bacteria, or a sequence that is modified and/or mutated as compared to the unmodified sequence from bacteria of the same subtype. In some embodiments, the non-native nucleic acid sequence is a synthetic, non-naturally occurring sequence (see, e.g., Purcell et al., 2013). The non-native nucleic acid sequence may be a regulatory region, a promoter, a gene, and/or one or more genes in gene cassette. In some embodiments, "non-native" refers to two or more nucleic acid sequences that are not found in the same relationship to each other in nature. The non- native nucleic acid sequence, e.g., gene or gene cassette, may be present on a plasmid or bacterial chromosome. In some embodiments, the genetically engineered bacteria of the invention comprise a gene cassette that is operably linked to a directly or indirectly inducible promoter that is not associated with said gene cassette in nature, e.g., a FNR-responsive promoter operably linked to a butyrogenic gene cassette, or an arginine production cassette. In addition, multiple copies of the gene, gene cassette, or regulatory region may be present in the bacterium, wherein one or more copies may be mutated or otherwise altered as described herein. In some embodiments, the genetically engineered bacteria are engineered to comprise multiple copies of the same non-native nucleic acid sequence, e.g., gene, gene cassette, or regulatory region, in order
to enhance copy number or to comprise multiple different components of a gene cassette performing multiple different functions.
[0127] "Constitutive promoter" refers to a promoter that is capable of facilitating continuous transcription of a coding sequence or gene under its control and/or to which it is operably linked. Constitutive promoters and variants are well known in the art and include, but are not limited to, BBa_J23100, a constitutive Escherichia coli os promoter (e.g. , an osmY promoter (International Genetically Engineered Machine (iGEM) Registry of Standard Biological Parts Name BBa_J45992; BBa_J45993)), a constitutive Escherichia coli o32 promoter (e.g. , htpG heat shock promoter (BBa_J45504)), a constitutive Escherichia coli o70 promoter (e.g. , lacq promoter (BBa_J54200; BBa_J56015), E. coli
CreABCD phosphate sensing operon promoter (BBa_J64951 ), GlnRS promoter (BBa_K088007), lacZ promoter (BBa_K119000; BBa_K119001 ); M13K07 gene I promoter (BBa_M13101 ); M13K07 gene II promoter (BBa_M 13102), M13K07 gene III promoter (BBa_M13103), M13K07 gene IV promoter (BBa_M13104), M13K07 gene V promoter (BBa_M13105), M13K07 gene VI promoter
(BBa_M13106), M13K07 gene VIII promoter (BBa_M13108), M13110
(BBa_M13110)), a constitutive Bacillus subtilis oA promoter (e.g. , promoter veg (BBa_K143013), promoter 43 (BBa_K143013), PNaG (BBa_K823000), P|epA (BBa_K823002), Pveg (BBa_K823003)), a constitutive Bacillus subtilis oB promoter (e.g. , promoter etc (BBa_K143010), promoter gsiB (BBa_K143011 )), a Salmonella promoter (e.g. , Pspv2 from Salmonella (BBa_K112706), Pspv from Salmonella (BBa_K112707)), a bacteriophage T7 promoter (e.g. , T7 promoter (BBa_l712074; BBa_l719005; BBa_J34814; BBa_J64997; BBa_K113010; BBa_K113011 ; BBa_K113012; BBa_R0085; BBa_R0180; BBa_R0181 ;
BBa_R0182; BBa_R0183; BBa_Z0251 ; BBa_Z0252; BBa_Z0253)), a
bacteriophage SP6 promoter (e.g. , SP6 promoter (BBa_J64998)), and functional fragments thereof.
[0128] As used herein, genetically engineered bacteria that
"overproduce" arginine or an intermediate byproduct, e.g., citrulline, refer to bacteria that comprise a mutant arginine regulon. For example, the engineered bacteria may comprise a feedback resistant form of ArgA, and when the arginine feedback resistant ArgA is expressed, are capable of producing more
arginine and/or intermediate byproduct than unmodified bacteria of the same subtype under the same conditions. The genetically engineered bacteria may alternatively or further comprise a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes. The genetically engineered bacteria may alternatively or further comprise a mutant or deleted arginine repressor. In some embodiments, the genetically engineered bacteria produce at least about 1.5-fold, at least about 2-fold, at least about 10-fold, at least about 15-fold, at least about 20-fold, at least about 30-fold, at least about 50-fold, at least about 100-fold, at least about 200-fold, at least about 300-fold, at least about 400-fold, at least about 500-fold, at least about 600-fold, at least about 700-fold, at least about 800-fold, at least about 900-fold, at least about 1 , 000- fold, or at least about 1 , 500-fold more arginine than unmodified bacteria of the same subtype under the same conditions. In some embodiments, the
genetically engineered bacteria produce at least about 1.5-fold, at least about 2- fold, at least about 10-fold, at least about 15-fold, at least about 20-fold, at least about 30-fold, at least about 50-fold, at least about 100-fold, at least about 200- fold, at least about 300-fold, at least about 400-fold, at least about 500-fold, at least about 600-fold, at least about 700-fold, at least about 800-fold, at least about 900-fold, at least about 1 , 000-fold, or at least about 1 , 500-fold more citrulline or other intermediate byproduct than unmodified bacteria of the same subtype under the same conditions. In some embodiments, the mRNA transcript levels of one or more of the arginine biosynthesis genes in the genetically engineered bacteria are at least about 1.5-fold, at least about 2-fold, at least about 10-fold, at least about 15-fold, at least about 20-fold, at least about 30-fold, at least about 50-fold, at least about 100-fold, at least about 200- fold, at least about 300-fold, at least about 400-fold, at least about 500-fold, at least about 600-fold, at least about 700-fold, at least about 800-fold, at least about 900-fold, at least about 1 , 000-fold, or at least about 1 , 500-fold higher than the mRNA transcript levels in unmodified bacteria of the same subtype under the same conditions. In certain embodiments, the unmodified bacteria will not have detectable levels of arginine, intermediate byproduct, and/or transcription of the gene(s) in such operons. However, protein and/or transcription levels of
arginine and/or intermediate byproduct will be detectable in the corresponding genetically engineered bacterium having the mutant arginine regulon.
Transcription levels may be detected by directly measuring mRNA levels of the genes. Methods of measuring arginine and/or intermediate byproduct levels, as well as the levels of transcript expressed from the arginine biosynthesis genes, are known in the art. Arginine and citrulline, for example, may be measured by mass spectrometry.
[0129] "Gut" refers to the organs, glands, tracts, and systems that are responsible for the transfer and digestion of food, absorption of nutrients, and excretion of waste. In humans, the gut comprises the gastrointestinal tract, which starts at the mouth and ends at the anus, and additionally comprises the esophagus, stomach, small intestine, and large intestine. The gut also comprises accessory organs and glands, such as the spleen, liver, gallbladder, and pancreas. The upper gastrointestinal tract comprises the esophagus, stomach, and duodenum of the small intestine. The lower gastrointestinal tract comprises the remainder of the small intestine, i.e., the jejunum and ileum, and all of the large intestine, i.e., the cecum, colon, rectum, and anal canal.
Bacteria can be found throughout the gut, e.g., in the gastrointestinal tract, and particularly in the intestines.
[0130] As used herein, the term "gene sequence" is meant to refer to a genetic sequence, e.g., a nucleic acid sequence. The gene sequence or genetic sequence is meant to include a complete gene sequence or a partial gene sequence. The gene sequence or genetic sequence is meant to include sequence that encodes a protein or polypeptide and is also menat to include genetic sequence that does not encode a protein or polypeptide, e.g., a regulatory sequence, leader sequence, signal sequence, or other non-protein coding sequence.
[0131] "Microorganism" refers to an organism or microbe of microscopic, submicroscopic, or ultramicroscopic size that typically consists of a single cell. Examples of microrganisms include bacteria, viruses, parasites, fungi, certain algae, and protozoa. In some aspects, the microorganism is engineered ("engineered microorganism") to produce one or more therapeutic molecules. In certain aspects, the microorganism is engineered to import and/or catabolize
certain toxic metabolites, substrates, or other compounds from its environment, e.g., the gut. In certain aspects, the microorganism is engineered to synthesize certain beneficial metabolites, molecules, or other compounds (synthetic or naturally occurring) and release them into its environment. In certain
embodiments, the engineered microorganism is an engineered bacterium. In certain embodiments, the engineered microorganism is an engineered virus.
[0132] "Non-pathogenic bacteria" refer to bacteria that are not capable of causing disease or harmful responses in a host. In some embodiments, nonpathogenic bacteria are commensal bacteria. Examples of non-pathogenic bacteria include, but are not limited to Bacillus, Bacteroides, Bifidobacterium, Brevi bacteria, Clostridium, Enterococcus, Escherichia coli, Lactobacillus, Lactococcus, Saccharomyces, and Staphylococcus, e.g., Bacillus coagulans, Bacillus subtilis, Bacteroides fragilis, Bacteroides subtilis, Bacteroides thetaiotaomicron, Bifidobacterium bifidum, Bifidobacterium infantis,
Bifidobacterium lactis, Bifidobacterium longum, Clostridium butyricum,
Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus johnsonii, Lactobacillus paracasei,
Lactobacillus plantarum, Lactobacillus reuteri, Lactobacillus rhamnosus, Lactococcus lactis, and Saccharomyces boulardii (Sonnenborn et al., 2009; Dinleyici et al., 2014; U.S. Patent No. 6,835,376; U.S. Patent No. 6,203,797; U.S. Patent No. 5,589,168; U.S. Patent No. 7,731 ,976). Naturally pathogenic bacteria may be genetically engineered to provide reduce or eliminate pathogenicity.
[0133] As used herein, "payload" refers to one or more polynucleotides and/or polypeptides of interest to be produced by a genetically engineered microorganism, such as a bacteria or a virus. In some embodiments, the payload is encoded by a gene or multiple genes or an operon. In some embodiments, the one or more genes and/or operon(s) comprising the payload are endogenous to the microorganism. In some embodiments, the one or more elements of the payload is derived from a different microorganism and/or organism. In some embodiments, the payload is a therapeutic payload. In some embodiments, the payload is encoded by genes for the biosynthesis of a molecule. In some embodiments, the payload is encoded by genes for the
metabolism, catabolism, or degradation of a molecule. In some embodiments, the payload is encoded by genes for the importation of a molecule. In some embodiments, the payload is encoded by genes for the exportation of a molecule. In some embodiments, the payload is a regulatory molecule(s), e.g., a transcriptional regulator such as FNR. In some embodiments, the payload comprises a regulatory element, such as a promoter or a repressor. In some embodiments, the payload comprises an inducible promoter, such as from FNRS. In some embodiments the payload comprises a repressor element, such as a kill switch. In alternate embodiments, the payload is produced by a biosynthetic or biochemical pathway, wherein the biosynthetic or biochemical pathway may optionally be endogenous to the microorganism. In some embodiments, the genetically engineered microorganism comprises two or more payloads. Non-limiting examples of payload(s) include one or more of the following: (1 ) ArgAfbr, (2) mutated Arg Boxes, (3) mutated ArgR, (4) mutated ArgG, (5) butyrate biosynthetic cassette, (6) proprionate biosynthetic cassette, (7) acetate biosynthetic cassette; (8) GABA-metabolizing cassette, (9) GABA- transporter, (10) Mn-transporter. Other exemplary payloads include GLP-2, IL- 10, IL-27, TGF-βΙ , TGF-P2, elafin (also known as peptidase inhibitor 3 or SKALP), trefoil factor, melatonin, PGD2, kynurenic acid, and kynurenine. Other exemplary payloads include mutated sequence(s) that result in an auxotrophy, e.g., thyA auxotrophy, kill switch circuit, antibiotic resistance circuits, transporter sequence for importing biological molecules or substrates, secretion circuit.
[0134]"Probiotic" is used to refer to live, non-pathogenic microorganisms, e.g., bacteria, which can confer health benefits to a host organism that contains an appropriate amount of the microorganism. In some embodiments, the host organism is a mammal. In some embodiments, the host organism is a human. Some species, strains, and/or subtypes of non-pathogenic bacteria are currently recognized as probiotic bacteria. Examples of probiotic bacteria include, but are not limited to, Bifidobacteria, Escherichia coli, Lactobacillus, and
Saccharomyces, e.g., Bifidobacterium bifidum, Enterococcus faecium,
Escherichia coli strain Nissle, Lactobacillus acidophilus, Lactobacillus
bulgaricus, Lactobacillus paracasei, Lactobacillus plantarum, and
Saccharomyces boulardii (Dinleyici et al., 2014; U.S. Patent No. 5,589,168;
U.S. Patent No. 6,203,797; U.S. Patent 6,835,376). The probiotic may be a variant or a mutant strain of bacterium (Arthur et al., 2012; Cuevas-Ramos et al., 2010; Olier et al., 2012; Nougayrede et al., 2006). Non-pathogenic bacteria may be genetically engineered to enhance or improve desired biological properties, e.g., survivability. Non-pathogenic bacteria may be genetically engineered to provide probiotic properties. Probiotic bacteria may be
genetically engineered to enhance or improve probiotic properties.
[0135]As used herein, "stably maintained" or "stable" bacterium is used to refer to a bacterial host cell carrying non-native genetic material, e.g., a feedback resistant argA gene, mutant arginine repressor, and/or other mutant arginine regulon that is incorporated into the host genome or propagated on a self-replicating extra-chromosomal plasm id, such that the non-native genetic material is retained, expressed, and propagated. The stable bacterium is capable of survival and/or growth in vitro, e.g., in medium, and/or in vivo, e.g., in the gut. For example, the stable bacterium may be a genetically engineered bacterium comprising an argAfbr gene, in which the plasmid or chromosome carrying the argAmr gene is stably maintained in the bacterium, such that argAmr can be expressed in the bacterium, and the bacterium is capable of survival and/or growth in vitro and/or in vivo.
[0136] As used herein, the terms "modulate" and "treat" and their cognates refer to an amelioration of a disease, disorder, and/or condition, or at least one discernible symptom thereof. In another embodiment, "modulate" and "treat" refer to an amelioration of at least one measurable physical parameter, not necessarily discernible by the patient. In another embodiment, "modulate" and "treat" refer to inhibiting the progression of a disease, disorder, and/or condition, either physically (e.g., stabilization of a discernible symptom), physiologically (e.g., stabilization of a physical parameter), or both. In another embodiment, "modulate" and "treat" refer to slowing the progression or reversing the progression of a disease, disorder, and/or condition. As used herein, "prevent" and its cognates refer to delaying the onset or reducing the risk of acquiring a given disease, disorder and/or condition or a symptom associated with such disease, disorder, and/or condition.
[0137] Those in need of treatment may include individuals already having a particular medical disorder, as well as those at risk of having, or who may ultimately acquire the disorder. The need for treatment is assessed, for example, by the presence of one or more risk factors associated with the development of a disorder, the presence or progression of a disorder, or likely receptiveness to treatment of a subject having the disorder. Primary
hyperammonemia is caused by UCDs, which are autosomal recessive or X- linked inborn errors of metabolism for which there are no known cures.
Hyperammonemia can also be secondary to other disruptions of the urea cycle, e.g., toxic metabolites, infections, and/or substrate deficiencies.
Hyperammonemia can also contribute to other pathologies. For example, Huntington's disease is an autosomal dominant disorder for which there are no known cures. Urea cycle abnormalities characterized by hyperammonemia, high blood citrulline, and suppression of urea cycle enzymes may contribute to the pathology of Huntington's disease, an autosomal dominant disorder for which there are no known cures. Treating hyperammonemia may encompass reducing or eliminating excess ammonia and/or associated symptoms, and does not necessarily encompass the elimination of the underlying
hyperammonemia-associated disorder.
[0138]As used herein a "pharmaceutical composition" refers to a preparation of genetically engineered bacteria of the invention with other components such as a physiologically suitable carrier and/or excipient.
[0139] The phrases "physiologically acceptable carrier" and
"pharmaceutically acceptable carrier" which may be used interchangeably refer to a carrier or a diluent that does not cause significant irritation to an organism and does not abrogate the biological activity and properties of the administered bacterial compound. An adjuvant is included under these phrases.
[0140] The term "excipient" refers to an inert substance added to a pharmaceutical composition to further facilitate administration of an active ingredient. Examples include, but are not limited to, calcium bicarbonate, calcium phosphate, various sugars and types of starch, cellulose derivatives, gelatin, vegetable oils, polyethylene glycols, and surfactants, including, for example, polysorbate 20.
[0141]The terms "therapeutically effective dose" and "therapeutically effective amount" are used to refer to an amount of a compound that results in prevention, delay of onset of symptoms, or amelioration of symptoms of a condition, e.g., hyperammonemia. A therapeutically effective amount may, for example, be sufficient to treat, prevent, reduce the severity, delay the onset, and/or reduce the risk of occurrence of one or more symptoms of a disorder associated with elevated ammonia concentrations. A therapeutically effective amount, as well as a therapeutically effective frequency of administration, can be determined by methods known in the art and discussed below.
[0142] As used herein, the term "polypeptide" includes "polypeptide" as well as "polypeptides," and refers to a molecule composed of amino acid monomers linearly linked by amide bonds (i.e., peptide bonds). The term
"polypeptide" refers to any chain or chains of two or more amino acids, and does not refer to a specific length of the product. Thus, "peptides," "dipeptides," "tripeptides, "oligopeptides," "protein," "amino acid chain," or any other term used to refer to a chain or chains of two or more amino acids, are included within the definition of "polypeptide," and the term "polypeptide" may be used instead of, or interchangeably with any of these terms. The term "polypeptide" is also intended to refer to the products of post-expression modifications of the polypeptide, including but not limited to glycosylation, acetylation,
phosphorylation, amidation, derivatization, proteolytic cleavage, or modification by non-naturally occurring amino acids. A polypeptide may be derived from a natural biological source or produced by recombinant technology. In other embodiments, the polypeptide is produced by the genetically engineered bacteria or virus of the current invention. A polypeptide of the invention may be of a size of about 3 or more, 5 or more, 10 or more, 20 or more, 25 or more, 50 or more, 75 or more, 100 or more, 200 or more, 500 or more, 1 ,000 or more, or 2,000 or more amino acids. Polypeptides may have a defined three-dimensional structure, although they do not necessarily have such structure. Polypeptides with a defined three-dimensional structure are referred to as folded, and polypeptides, which do not possess a defined three-dimensional structure, but rather can adopt a large number of different conformations, are referred to as unfolded. The term "peptide" or "polypeptide" may refer to an amino acid
sequence that corresponds to a protein or a portion of a protein or may refer to an amino acid sequence that corresponds with non-protein sequence, e.g., a sequence selected from a regulatory peptide sequence, leader peptide sequence, signal peptide sequence, linker peptide sequence, and other peptide sequence.
[0143]An "isolated" polypeptide or a fragment, variant, or derivative thereof refers to a polypeptide that is not in its natural milieu. No particular level of purification is required. Recombinantly produced polypeptides and proteins expressed in host cells, including but not limited to bacterial or mammalian cells, are considered isolated for purposed of the invention, as are native or recombinant polypeptides which have been separated, fractionated, or partially or substantially purified by any suitable technique. Recombinant peptides, polypeptides or proteins refer to peptides, polypeptides or proteins produced by recombinant DNA techniques, i.e. produced from cells, microbial or mammalian, transformed by an exogenous recombinant DNA expression construct encoding the polypeptide. Proteins or peptides expressed in most bacterial cultures will typically be free of glycan. Fragments, derivatives, analogs or variants of the foregoing polypeptides, and any combination thereof are also included as polypeptides. The terms "fragment," "variant," "derivative" and "analog" include polypeptides having an amino acid sequence sufficiently similar to the amino acid sequence of the original peptide and include any polypeptides, which retain at least one or more properties of the corresponding original polypeptide.
Fragments of polypeptides of the present invention include proteolytic
fragments, as well as deletion fragments. Fragments also include specific antibody or bioactive fragments or immunologically active fragments derived from any polypeptides described herein. Variants may occur naturally or be non-naturally occurring. Non-naturally occurring variants may be produced using mutagenesis methods known in the art. Variant polypeptides may comprise conservative or non-conservative amino acid substitutions, deletions or additions.
[0144] Polypeptides also include fusion proteins. As used herein, the term "variant" includes a fusion protein, which comprises a sequence of the original peptide or sufficiently similar to the original peptide. As used herein, the
term "fusion protein" refers to a chimeric protein comprising amino acid sequences of two or more different proteins. Typically, fusion proteins result from well known in vitro recombination techniques. Fusion proteins may have a similar structural function (but not necessarily to the same extent), and/or similar regulatory function (but not necessarily to the same extent), and/or similar biochemical function (but not necessarily to the same extent) and/or
immunological activity (but not necessarily to the same extent) as the individual original proteins which are the components of the fusion proteins. "Derivatives" include but are not limited to peptides, which contain one or more naturally occurring amino acid derivatives of the twenty standard amino acids. "Similarity" between two peptides is determined by comparing the amino acid sequence of one peptide to the sequence of a second peptide. An amino acid of one peptide is similar to the corresponding amino acid of a second peptide if it is identical or a conservative amino acid substitution. Conservative substitutions include those described in Dayhoff, M. 0., ed., The Atlas of Protein Sequence and Structure 5, National Biomedical Research Foundation, Washington, D.C. (1978), and in Argos, EMBO J. 8 (1989), 779-785. For example, amino acids belonging to one of the following groups represent conservative changes or substitutions: -Ala, Pro, Gly, Gin, Asn, Ser, Thr; -Cys, Ser, Tyr, Thr; -Val, lie, Leu, Met, Ala, Phe; - Lys, Arg, His; -Phe, Tyr, Trp, His; and -Asp, Glu.
[0145]As used herein, the term "sufficiently similar" means a first amino acid sequence that contains a sufficient or minimum number of identical or equivalent amino acid residues relative to a second amino acid sequence such that the first and second amino acid sequences have a common structural domain and/or common functional activity. For example, amino acid sequences that comprise a common structural domain that is at least about 45%, at least about 50%, at least about 55%, at least about 60%, at least about 65%, at least about 70%, at least about 75%, at least about 80%, at least about 85%, at least about 90%, at least about 91 %, at least about 92%, at least about 93%, at least about 94%, at least about 95%, at least about 96%, at least about 97%, at least about 98%, at least about 99%, or at least about 100%, identical are defined herein as sufficiently similar. Preferably, variants will be sufficiently similar to the amino acid sequence of the peptides of the invention. Such variants generally
retain the functional activity of the peptides of the present invention. Variants include peptides that differ in amino acid sequence from the native and wt peptide, respectively, by way of one or more amino acid deletion(s), addition(s), and/or substitution(s). These may be naturally occurring variants as well as artificially designed ones.
[0146]As used herein the term "linker", "linker peptide" or "peptide linkers" or "linker" refers to synthetic or non-native or non-naturally-occurring amino acid sequences that connect or link two polypeptide sequences, e.g., that link two polypeptide domains. As used herein the term "synthetic" refers to amino acid sequences that are not naturally occurring. Exemplary linkers are described herein. Additional exemplary linkers are provided in US
20140079701 , the contents of which are herein incorporated by reference in its entirety.
[0147] As used herein the term "codon-optimized sequence" refers to a sequence, which was modified from an existing coding sequence, or designed, for example, to improve translation in an expression host cell or organism of a transcript RNA molecule transcribed from the coding sequence, or to improve transcription of a coding sequence. Codon optimization includes, but is not limited to, processes including selecting codons for the coding sequence to suit the codon preference of the expression host organism.
[0148] Many organisms display a bias or preference for use of particular codons to code for insertion of a particular amino acid in a growing polypeptide chain. Codon preference or codon bias, differences in codon usage between organisms, is allowed by the degeneracy of the genetic code, and is well documented among many organisms. Codon bias often correlates with the efficiency of translation of messenger RNA (mRNA), which is in turn believed to be dependent on, inter alia, the properties of the codons being translated and the availability of particular transfer RNA (tRNA) molecules. The predominance of selected tRNAs in a cell is generally a reflection of the codons used most frequently in peptide synthesis. Accordingly, genes can be tailored for optimal gene expression in a given organism based on codon optimization.
[0149] As used herein, the terms "secretion system" or "secretion protein" refers to a native or non-native secretion mechanism capable of secreting or
exporting the protein of interest or therapeutic protein from the microbial, e.g., bacterial cytoplasm. The secretion system may comprise a single protein or may comprise two or more proteins assembled in a complex e.g. HlyBD. Non- limiting examples of secretion systems for gram negative bacteria include the modified type III flagellar, type I (e.g., hemolysin secretion system), type II, type IV, type V, type VI, and type VII secretion systems, resistance-nodulation- division (RND) multi-drug efflux pumps, various single membrane secretion systems. Non-liming examples of secretion systems for gram positive bacteria include Sec and TAT secretion systems. In some embodiments, the the protein(s) of interest or therapeutic protein(s) include a "secretion tag" of either RNA or peptide origin to direct the the protein(s) of interest or therapeutic protein(s) to specific secretion systems. In some embodiments, the secretion system is able to remove this tag before secreting the the protein(s) of interest or therapeutic protein(s) from the engineered bacteria. For example, in Type V auto-secretion-mediated secretion the N-terminal peptide secretion tag is removed upon translocation of the "passenger" peptide from the cytoplasm into the periplasmic compartment by the native Sec system. Further, once the auto- secretor is translocated across the outer membrane the C-terminal secretion tag can be removed by either an autocatalytic or protease-catalyzed e.g., OmpT cleavage thereby releasing the the protein(s) of interest or therapeutic protein(s) into the extracellular milieu.
[0150] As used herein, the term "transporter" is meant to refer to a mechanism, e.g., protein or proteins, for importing a molecule, e.g., amino acid, toxin, metabolite, substrate, etc. into the microorganism from the extracellular milieu.
[0151] The articles "a" and "an," as used herein, should be understood to mean "at least one," unless clearly indicated to the contrary.
[0152] The phrase "and/or," when used between elements in a list, is intended to mean either (1 ) that only a single listed element is present, or (2) that more than one element of the list is present. For example, "A, B, and/or C" indicates that the selection may be A alone; B alone; C alone; A and B; A and C; B and C; or A, B, and C. The phrase "and/or" may be used interchangeably with "at least one of" or "one or more of" the elements in a list.
Bacteria
[0153] The genetically engineered bacteria disclosed herein are capable of reducing excess ammonia and converting ammonia and/or nitrogen into alternate byproducts. In some embodiments, the genetically engineered bacteria are naturally non-pathogenic bacteria. In some embodiments, the genetically engineered bacteria are commensal bacteria. In some
embodiments, the genetically engineered bacteria are probiotic bacteria. In some embodiments, the genetically engineered bacteria are naturally pathogenic bacteria that are modified or mutated to reduce or eliminate pathogenicity. Exemplary bacteria include, but are not limited to Bacillus, Bacteroides, Bifidobacterium, Brevibacteria, Clostridium, Enterococcus, Escherichia coli, Lactobacillus, Lactococcus, Saccharomyces, and
Staphylococcus, e.g., Bacillus coagulans, Bacillus subtilis, Bacteroides fragilis, Bacteroides subtilis, Bacteroides thetaiotaomicron, Bifidobacterium bifidum, Bifidobacterium infantis, Bifidobacterium lactis, Bifidobacterium longum, Clostridium butyricum, Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus johnsonii,
Lactobacillus paracasei, Lactobacillus plantarum, Lactobacillus reuteri,
Lactobacillus rhamnosus, Lactococcus lactis, and Saccharomyces boulardii. In certain embodiments, the genetically engineered bacteria are selected from the group consisting of Bacteroides fragilis, Bacteroides thetaiotaomicron,
Bacteroides subtilis, Bifidobacterium bifidum, Bifidobacterium infantis,
Bifidobacterium lactis, Clostridium butyricum, Escherichia coli Nissle,
Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus reuteri, and Lactococcus lactis.
[0154] In some embodiments, the genetically engineered bacteria are Escherichia coli strain Nissle 1917 (E. coli Nissle), a Gram-negative bacterium of the Enterobacteriaceae family that has evolved into one of the best characterized probiotics (Ukena et al., 2007). The strain is characterized by its complete harmlessness (Schultz, 2008), and has GRAS (generally recognized as safe) status (Reister et al., 2014, emphasis added). Genomic sequencing confirmed that E. coli Nissle lacks prominent virulence factors (e.g., E coli a- hemolysin, P-fimbrial adhesins) (Schultz, 2008). In addition, it has been shown
that E. coli Nissle does not carry pathogenic adhesion factors, does not produce any enterotoxins or cytotoxins, is not invasive, and not uropathogenic
(Sonnenborn et al., 2009). As early as in 1917, E. coli Nissle was packaged into medicinal capsules, called Mutaflor, for therapeutic use. E. coli Nissle has since been used to treat ulcerative colitis in humans in vivo (Rembacken et al., 1999), to treat inflammatory bowel disease, Crohn's disease, and pouchitis in humans in vivo (Schultz, 2008), and to inhibit enteroinvasive Salmonella, Legionella, Yersinia, and Shigella in vitro (Altenhoefer et al., 2004). It is commonly accepted that E coli Nissle's therapeutic efficacy and safety have convincingly been proven (Ukena et al., 2007).
[0155] One of ordinary skill in the art would appreciate that the genetic modifications disclosed herein may be modified and adapted for other species, strains, and subtypes of bacteria. It is known, for example, that arginine- mediated regulation is remarkably well conserved in very divergent bacteria, i.e., Gram-negative bacteria, such as E coli, Salmonella enterica serovar Typhimurium, Thermotoga, and Moritella profunda, and Gram-positive bacteria, such as B. subtilis, Geobacillus stearothermophilus, and Streptomyces clavuligerus, as well as other bacteria (Nicoloff et al., 2004). Furthermore, the arginine repressor is universally conserved in bacterial genomes and that its recognition signal (the ARG box), a weak palindrome, is also conserved between genomes (Makarova et al., 2001 ).
[0156] Unmodified E coli Nissle and the genetically engineered bacteria of the invention may be destroyed, e.g., by defense factors in the gut or blood serum (Sonnenborn et al., 2009). The residence time of bacteria in vivo can be determined using the methods described herein. In some embodiments, the residence time is calculated for a human subject. A non-limiting example using a streptomycin-resistant E coli Nissle comprising a wild-type ArgR and a wild- type arginine regulon is provided herein. In some embodiments, residence time in vivo is calculated for the genetically engineered bacteria of the invention.
Reduction of Excess Ammonia
Arginine Biosynthesis Pathway
[0157] In bacteria such as Escherichia coli (E. coli), the arginine biosynthesis pathway is capable of converting glutamate to arginine in an eight- step enzymatic process involving the enzymes N-acetylglutamate synthetase, N-acetylglutamate kinase, N-acetylglutamate phosphate reductase,
acetylornithine aminotransferase, N-acetylornithinase, carbamoylphosphate synthase, ornithine transcarbamylase, argininosuccinate synthase, and argininosuccinate lyase (Cunin et al., 1986). The first five steps involve N- acetylation to generate an ornithine precursor. In the sixth step, ornithine transcarbamylase (also known as ornithine carbamoyltransferase) catalyzes the formation of citrulline. The final two steps involve carbamoylphosphate utilization to generate arginine from citrulline.
[0158] In some bacteria, e.g., Bacillus stearothermophilus and Neisseria gonorrhoeae, the first and fifth steps in arginine biosynthesis may be catalyzed by the bifunctional enzyme ornithine acetyltransferase. This bifunctionality was initially identified when ornithine acetyltransferase {argJ) was shown to complement both N-acetylglutamate synthetase (argA) and N-acetylornithinase {argE) auxotrophic gene mutations in E. coli (Mountain et al., 1984; Crabeel et al., 1997).
encodes N-acetylglutamate synthetase, argB encodes N- acetylglutamate kinase, argC encodes N-acetylglutamylphosphate reductase, argD encodes acetylornithine aminotransferase, argE encodes N- acetylornithinase, argF encodes ornithine transcarbamylase, argl also encodes ornithine transcarbamylase, argG encodes argininosuccinate synthase, argH encodes argininosuccinate lyase, and argJ encodes ornithine acetyltransferase. CarA encodes the small A subunit of carbamoylphosphate synthase having glutaminase activity, and carB encodes the large B subunit of
carbamoylphosphate synthase that catalyzes carbamoylphosphate synthesis from ammonia. Different combinations of one or more of these arginine biosynthesis genes (i.e., argA, argB, argC, argD, argE, argF, argG, argH, argl, argJ, car A, and carB) may be organized, naturally or synthetically, into one or
more operons, and such organization may vary between bacterial species, strains, and subtypes (see, e.g., Table 2). The regulatory region of each operon contains at least one ARG box, and the number of ARG boxes per regulatory region may vary between operons and bacteria.
[0160] All of the genes encoding these enzymes are subject to
repression by arginine via its interaction with ArgR to form a complex that binds to the regulatory region of each gene and inhibits transcription. N- acetylglutamate synthetase is also subject to allosteric feedback inhibition at the protein level by arginine alone (Tuchman et al., 1997; Caldara et al., 2006; Caldara et al., 2008; Caldovic et al., 2010).
[0161] The genes that regulate arginine biosynthesis in bacteria are scattered across the chromosome and organized into multiple operons that are controlled by a single repressor, which Maas and Clark (1964) termed a
"regulon." Each operon is regulated by a regulatory region comprising at least one 18-nucleotide imperfect palindromic sequence, called an ARG box, that overlaps with the promoter and to which the repressor protein binds (Tian et al., 1992; Tian et al., 1994). The argR gene encodes the repressor protein, which binds to one or more ARG boxes (Lim et al., 1987). Arginine functions as a corepressor that activates the arginine repressor. The ARG boxes that regulate each operon may be non-identical, and the consensus ARG box sequence is A/T nTGAAT A/T A/T T/A T/A ATTCAn T/A (Maas, 1994). In addition, the regulatory region of argR contains two promoters, one of which overlaps with two ARG boxes and is autoregulated.
[0162] In some embodiments, the genetically engineered bacteria comprise a mutant arginine regulon and produce more arginine and/or an intermediate byproduct, e.g., citrulline, than unmodified bacteria of the same subtype under the same conditions. The mutant arginine regulon comprises one or more nucleic acid mutations that reduce or prevent arginine-mediated repression - via ArgR binding to ARG boxes and/or arginine binding to N- acetylglutamate synthetase - of one or more of the operons that encode the enzymes responsible for converting glutamate to arginine in the arginine biosynthesis pathway, thereby enhancing arginine and/or intermediate byproduct biosynthesis.
[0163] In alternate embodiments, the bacteria are genetically engineered to consume excess ammonia via another metabolic pathway, e.g., a histidine biosynthesis pathway, a methionine biosynthesis pathway, a lysine biosynthesis pathway, an asparagine biosynthesis pathway, a glutamine biosynthesis pathway, and a tryptophan biosynthesis pathway. As used herein, "an ammonia conversion circuit" refers to a metabolic pathway by which excess ammonia may be consumed and/or reduced.
Histidine Biosynthesis Pathway
[0164] Histidine biosynthesis, for example, is carried out by eight genes located within a single operon in E. coli. Three of the eight genes of the operon (hisD, hisB, and hisl) encode bifunctional enzymes, and two (hisH and hisF) encode polypeptide chains which together form one enzyme to catalyze a single step, for a total of 10 enzymatic reactions (Alifano et al., 1996). The product of the hisG gene, ATP phosphoribosyltransferase, is inhibited at the protein level by histidine. In some embodiments, the genetically engineered bacteria of the invention comprise a feedback-resistant hisG. Bacteria may be mutagenized and/or screened for feedback-resistant hisG mutants using techniques known in the art. Bacteria engineered to comprise a feedback-resistant hisG would have elevated levels of histidine production, thus increasing ammonia consumption and reducing hyperammonemia. Alternatively, one or more genes required for histidine biosynthesis could be placed under the control of an inducible promoter, such as a FNR-inducible promoter, and allow for increased
production of rate-limiting enzymes. Any other suitable modification(s) to the histidine biosynthesis pathway may be used to increase ammonia consumption.
Methionine Biosynthesis Pathway
[0165] The bacterial methionine regulon controls the three-step synthesis of methionine from homoserine (i.e., acylation, sulfurylation, and methylation). The metJ gene encodes a regulatory protein that, when combined with methionine or a derivative thereof, causes repression of genes within the methionine regulon at the transcriptional level (Saint-Girons et al., 1984;
Shoeman et al., 1985). In some embodiments, the genetically engineered bacteria of the invention comprise deleted, disrupted, or mutated metJ.
Bacteria engineered to delete, disrupt, or mutate metJ would have elevated levels of methionine production, thus increasing ammonia consumption and reducing hyperammonemia. Any other suitable modification(s) to the
methionine biosynthesis pathway may be used to increase ammonia
consumption.
Lysine Biosynthesis Pathway
[0166] Microorganisms synthesize lysine by one of two pathways. The diaminopimelate (DAP) pathway is used to synthesize lysine from aspartate and pyruvate (Dogovski et al., 2012), and the aminoadipic acid pathway is used to synthesize lysine from alpha-ketoglutarate and acetyl coenzyme A. The dihydrodipicolinate synthase (DHDPS) enzyme catalyzes the first step of the DAP pathway, and is subject to feedback inhibition by lysine (Liu et al., 2010; Reboul et al., 2012). In some embodiments, the genetically engineered bacteria of the invention comprise a feedback-resistant DHDPS. Bacteria engineered to comprise a feedback-resistant DHDPS would have elevated levels of histidine production, thus increasing ammonia consumption and reducing hyperammonemia. Alternatively, lysine production could be optimized by placing one or more genes required for lysine biosynthesis under the control of an inducible promoter, such as a FNR-inducible promoter. Any other suitable modification(s) to the lysine biosynthesis pathway may be used to increase ammonia consumption.
Asparagine Biosynthesis Pathway
[0167]Asparagine is synthesized directly from oxaloacetate and aspartic acid via the oxaloacetate transaminase and asparagine synthetase enzymes, respectively. In the second step of this pathway, either L-glutamine or ammonia serves as the amino group donor. In some embodiments, the genetically engineered bacteria of the invention overproduce asparagine as compared to unmodified bacteria of the same subtype under the same conditions, thereby consuming excess ammonia and reducing hyperammonemia. Alternatively, asparagine synthesis may be optimized by placing one or both of these genes under the control of an inducible promoter, such as a FNR-inducible promoter. Any other suitable modification(s) to the asparagine biosynthesis pathway may be used to increase ammonia consumption.
Glutamine Biosynthesis Pathway
[0168] The synthesis of glutamine and glutamate from ammonia and oxoglutarate is tightly regulated by three enzymes. Glutamate dehydrogenase catalyzes the reductive amination of oxoglutarate to yield glutamate in a single step. Glutamine synthetase catalyzes the ATP-dependent condensation of glutamate and ammonia to form glutamine (Lodeiro et al., 2008). Glutamine synthetase also acts with glutamine-oxoglutarate amino transferase (also known as glutamate synthase) in a cyclic reaction to produce glutamate from glutamine and oxoglutarate. In some embodiments, the genetically engineered bacteria of the invention express glutamine synthetase at elevated levels as compared to unmodified bacteria of the same subtype under the same conditions. Bacteria engineered to have increased expression of glutamine synthetase would have elevated levels of glutamine production, thus increasing ammonia consumption and reducing hyperammonemia. Alternatively, expression of glutamate dehydrogenase and/or glutamine-oxoglutarate amino transferase could be modified to favor the consumption of ammonia. Since the production of glutamine synthetase is regulated at the transcriptional level by nitrogen (Feng et al., 1992; van Heeswijk et al., 2013), placing the glutamine synthetase gene under the control of different inducible promoter, such as a FNR-inducible promoter, may also be used to improve glutamine production. Any other suitable modification(s) to the glutamine and glutamate biosynthesis pathway may be used to increase ammonia consumption.
Tryptophan Biosynthesis Pathway
[0169] In most bacteria, the genes required for the synthesis of tryptophan from a chorismate precursor are organized as a single
transcriptional unit, the trp operon. The trp operon is under the control of a single promoter that is inhibited by the tryptophan repressor (TrpR) when high levels of tryptophan are present. Transcription of the trp operon may also be terminated in the presence of high levels of charged tryptophan tRNA. In some embodiments, the genetically engineered bacteria of the invention comprise a deleted, disrupted, or mutated trpR gene. The deletion, disruption, or mutation of the trpR gene, and consequent inactivation of TrpR function, would result in
elevated levels of both tryptophan production and ammonia consumption.
Alternatively, one or more enzymes required for tryptophan biosynthesis could be placed under the control of an inducible promoter, such as a FNR-inducible promoter. Any other suitable modification(s) to the tryptophan biosynthesis pathway may be used to increase ammonia consumption.
Engineered Bacteria Comprising a Mutant Arginine Regulon
[0170] In some embodiments, the genetically engineered bacteria comprise an arginine biosynthesis pathway and are capable of reducing excess ammonia. In a more specific aspect, the genetically engineered bacteria comprise a mutant arginine regulon in which one or more operons encoding arginine biosynthesis enzyme(s) is derepressed to produce more arginine or an intermediate byproduct, e.g., citrulline, than unmodified bacteria of the same subtype under the same conditions. In some embodiments, the genetically engineered bacteria overproduce arginine. In some embodiments, the genetically engineered bacteria overproduce citrulline; this may be additionally beneficial, because citrulline is currently used as a therapeutic for particular urea cycle disorders (National Urea Cycle Disorders Foundation). In some embodiments, the genetically engineered bacteria overproduce an alternate intermediate byproduct in the arginine biosynthesis pathway, such as any of the intermediates described herein. In some embodiments, the genetically engineered bacterium consumes excess ammonia by producing more arginine, citrulline, and/or other intermediate byproduct than an unmodified bacterium of the same bacterial subtype under the same conditions. Enhancement of arginine and/or intermediate byproduct biosynthesis may be used to incorporate excess nitrogen in the body into non-toxic molecules in order to treat conditions associated with hyperammonemia, including urea cycle disorders and hepatic encephalopathy.
[0171] One of skill in the art would appreciate that the organization of arginine biosynthesis genes within an operon varies across species, strains, and subtypes of bacteria, e.g., bipolar argECBH in E. coli K12, argCAEBD- carAB-argF in B. subtilis, and bipolar carAB-argCJBDF in L. plantarum. Non- limiting examples of operon organization from different bacteria are shown in Table 2 (in some instances, the genes are putative and/or identified by
sequence homology to known sequences in Escherichia coli; in some instances, not all of the genes in the arginine regulon are known and/or shown below). In certain instances, the arginine biosynthesis enzymes vary across species, strains, and subtypes of bacteria.
Table 2: Examples of arg operon organization
[0172] Each operon is regulated by a regulatory region comprising at least one promoter and at least one ARG box, which control repression and expression of the arginine biosynthesis genes in said operon.
[0173] In some embodiments, the genetically engineered bacteria of the invention comprise an arginine regulon comprising one or more nucleic acid mutations that reduce or eliminate arginine-mediated repression of one or more of the operons that encode the enzymes responsible for converting glutamate to arginine and/or an intermediate byproduct in the arginine biosynthesis pathway. Reducing or eliminating arginine-mediated repression may be achieved by reducing or eliminating ArgR repressor binding (e.g., by mutating or deleting the arginine repressor or by mutating at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes) and/or arginine binding to N- acetylglutamate synthetase (e.g., by mutating the N-acetylglutamate synthetase to produce an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argA r).
ARG Box
[0174] In some embodiments, the genetically engineered bacteria comprise a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for one or more of the operons that encode the arginine biosynthesis enzymes N-acetylglutamate kinase, N- acetylglutamylphosphate reductase, acetylornithine aminotransferase, N- acetylornithinase, ornithine transcarbamylase, argininosuccinate synthase, argininosuccinate lyase, and carbamoylphosphate synthase, thereby
derepressing the regulon and enhancing arginine and/or intermediate byproduct biosynthesis. In some embodiments, the genetically engineered bacteria comprise a mutant arginine repressor comprising one or more nucleic acid mutations such that arginine repressor function is decreased or inactive, or the genetically engineered bacteria do not have an arginine repressor (e.g., the arginine repressor gene has been deleted), resulting in derepression of the regulon and enhancement of arginine and/or intermediate byproduct
biosynthesis. In either of these embodiments, the genetically engineered bacteria may further comprise an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmr. Thus, in some embodiments, the genetically engineered bacteria comprise a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for one or more of the operons that encode the arginine biosynthesis enzymes and an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmr. In some embodiments, the genetically engineered bacteria comprise a mutant or deleted arginine repressor and an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmr. In some embodiments, the genetically
engineered bacteria comprise an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmr, a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes, and/or a mutant or deleted arginine repressor.
[0175] In some embodiments, the genetically engineered bacteria encode an arginine feedback resistant N-acetylglutamate synthase and further comprise a mutant arginine regulon comprising one or more nucleic acid
mutations in each ARG box for one or more of the operons that encode N- acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine aminotransferase, N-acetylornithinase, ornithine transcarbamylase,
argininosuccinate synthase, argininosuccinate lyase, carbamoylphosphate synthase, and wild-type N-acetylglutamate synthetase, such that ArgR binding is reduced or eliminated, thereby derepressing the regulon and enhancing arginine and/or intermediate byproduct biosynthesis.
[0176] In some embodiments, the ARG boxes for the operon encoding argininosuccinate synthase {argG) maintain the ability to bind to ArgR, thereby driving citrulline biosynthesis. For example, the regulatory region of the operon encoding argininosuccinate synthase {argG) may be a constitutive, thereby driving arginine biosynthesis. In alternate embodiments, the regulatory region of one or more alternate operons may be constitutive. In certain bacteria, however, genes encoding multiple enzymes may be organized in bipolar operons or under the control of a shared regulatory region; in these instances, the regulatory regions may need to be deconvoluted in order to engineer constitutively active regulatory regions. For example, in E. coli K12 and Nissle, argE and argCBH are organized in two bipolar operons, argECBH, and those regulatory regions may be deconvoluted in order to generate constitutive versions of argE and/or argCBH.
[0177] In some embodiments, all ARG boxes in one or more operons that comprise an arginine biosynthesis gene are mutated to reduce or eliminate ArgR binding. In some embodiments, all ARG boxes in one or more operons that encode an arginine biosynthesis enzyme are mutated to reduce or eliminate ArgR binding. In some embodiments, all ARG boxes in each operon that comprises an arginine biosynthesis gene are mutated to reduce or eliminate ArgR binding. In some embodiments, all ARG boxes in each operon that encodes an arginine biosynthesis enzyme are mutated to reduce or eliminate ArgR binding.
[0178] In some embodiments, the genetically engineered bacteria encode an arginine feedback resistant N-acetylglutamate synthase,
argininosuccinate synthase driven by a ArgR-repressible regulatory region, and further comprise a mutant arginine regulon comprising one or more nucleic acid
mutations in each ARG box for each of the operons that encode N- acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine aminotransferase, N-acetylornithinase, ornithine transcarbamylase,
argininosuccinate synthase, argininosuccinate lyase, carbamoylphosphate synthase, and optionally, wild-type N-acetylglutamate synthetase, such that ArgR binding is reduced or eliminated, thereby derepressing the regulon and enhancing citrulline biosynthesis. In some embodiments, the genetically engineered bacteria capable of producing citrulline is particularly advantageous, because citrulline further serves as a therapeutically effective supplement for the treatment of certain urea cycle disorders (National Urea Cycle Disorders Foundation).
[0179] In some embodiments, the genetically engineered bacteria encode an arginine feedback resistant N-acetylglutamate synthase,
argininosuccinate synthase driven by a constitutive promoter, and further comprise a mutant arginine regulon comprising one or more nucleic acid mutations in each ARG box for each of the operons that encode N- acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine aminotransferase, N-acetylornithinase, ornithine transcarbamylase,
argininosuccinate lyase, carbamoylphosphate synthase, and optionally, wild- type N-acetylglutamate synthetase, such that ArgR binding is reduced or eliminated, thereby derepressing the regulon and enhancing arginine
biosynthesis.
[0180] In some embodiments, the genetically engineered bacteria comprise a mutant arginine regulon and a feedback resistant ArgA, and when the arginine feedback resistant ArgA is expressed, are capable of producing more arginine and/or an intermediate byproduct than unmodified bacteria of the same subtype under the same conditions. In any of these embodiments, the mutant arginine regulon and/or a feedback resistant ArgA may be integrated into the bacterial chromosome at one or more integration sites or may be present on one or more plasm ids.
Arginine Repressor Binding Sites (ARG Boxes)
[0181] In some embodiments, the genetically engineered bacteria additionally comprise a mutant arginine regulon comprising one or more nucleic
acid mutations in at least one ARG box for one or more of the operons that encode the arginine biosynthesis enzymes N-acetylglutamate kinase, N- acetylglutamylphosphate reductase, acetylornithine aminotransferase, N- acetylornithinase, ornithine transcarbamylase, argininosuccinate synthase, argininosuccinate lyase, and carbamoylphosphate synthase, such that the arginine regulon is derepressed and biosynthesis of arginine and/or an intermediate byproduct, e.g., citrulline, is enhanced.
[0182] In some embodiments, the mutant arginine regulon comprises an operon encoding ornithine acetyltransferase and one or more nucleic acid mutations in at least one ARG box for said operon. The one or more nucleic acid mutations results in the disruption of the palindromic ARG box sequence, such that ArgR binding to that ARG box and to the regulatory region of the operon is reduced or eliminated, as compared to ArgR binding to an unmodified ARG box and regulatory region in bacteria of the same subtype under the same conditions. In some embodiments, nucleic acids that are protected from DNA methylation and hydroxyl radical attack during ArgR binding are the primary targets for mutations to disrupt ArgR binding. In some embodiments, the mutant arginine regulon comprises at least three nucleic acid mutations in one or more ARG boxes for each of the operons that encode the arginine
biosynthesis enzymes described above. The ARG box overlaps with the promoter, and in the mutant arginine regulon, the G/C:A/T ratio of the mutant promoter region differs by no more than 10% from the G/C:A/T ratio of the wild- type promoter region (Table 3). The promoter retains sufficiently high homology to the non-mutant promoter such that RNA polymerase binds with sufficient affinity to promote transcription.
[0183] The wild-type genomic sequences comprising ARG boxes and mutants thereof for each arginine biosynthesis operon in E. coli Nissle are shown in Table 3. For exemplary wild-type sequences, the ARG boxes are indicated in italics, and the start codon of each gene is |boxed|. The RNA polymerase binding sites are underlined (Cunin, 1983; Maas, 1994). In some embodiments, the underlined sequences are not altered. Bases that are protected from DNA methylation during ArgR binding are highlighted, and bases that are protected from hydroxyl radical attack during ArgR binding are bolded
(Charlier et al., 1992). The highlighted and bolded bases are the primary targets for mutations to disrupt ArgR binding.
Table 3
Regulatory region Sequence
gctttcccttaagcgcattattttacaaaa aacacactaaactcttcctgtctccgataa aagatgatcttatgaaaacctttttatttc ttataaaaatcttgtgaaagcagaaatcca ggctcatcatcagttaattaagcagggtgt tattttatg argG mutant cctgaaacgtggcaaattctactcgttttg (SEQ ID NO: 15) ggtaaaaaatgcaaatactgctgggatttg gtgtaccgagacgggacgtaaaatctgcag gcattatagtgatccacgccacattttgtc aacgtttattgctaatcattgacggctagc tcagtcctaggtacagtgctagcACCCGTT T GGGCTAGAAATAA G AACT TTAAGAAGGAGATATACATACCC
[0184] In some embodiments, more than one ARG box may be present in a single operon. In one aspect of these embodiments, at least one of the ARG boxes in an operon is mutated to produce the requisite reduced ArgR binding to the regulatory region of the operon. In an alternate aspect of these
embodiments, each of the ARG boxes in an operon is mutated to produce the requisite reduced ArgR binding to the regulatory region of the operon. One of skill in the art would appreciate that the number of ARG boxes per regulatory region may vary across bacteria, and the nucleotide sequences of the ARG boxes may vary for each operon. For example, the carAB operon in E. coli Nissle comprises two ARG boxes, and one or both ARG box sequences may be mutated. The argG operon in E. coli Nissle comprises three ARG boxes, and one, two, or three ARG box sequences may be mutated, disrupted, or deleted. In some embodiments, all three ARG box sequences are mutated, disrupted, or deleted, and a constitutive promoter, e.g., BBa_J23100, is inserted in the regulatory region of the argG operon. One of skill in the art would appreciate that the number of ARG boxes per regulatory region may vary across bacteria, and the nucleotide sequences of the ARG boxes may vary for each operon.
[0185] An exemplary embodiment of a constitutively expressed argG construct in E. coli Nissle is depicted in Table 4. Table 4 depicts the wild-type
genomic sequence of the regulatory region and 5' portion of the argG gene in E. coli Nissle, and a constitutive mutant thereof. The promoter region of each sequence is underlined, and a 5' portion of the argG gene is |boxed|. In the wild- type sequence, ArgR binding sites are in uppercase and underlined. In the mutant sequence, the 5' untranslated region is in uppercase and underlined. Bacteria expressing argG under the control of the constitutive promoter are capable of producing arginine. Bacteria expressing argG under the control of the wild-type, ArgR-repressible promoter are capable of producing citrulline. A map of the wild-type argG operon E. coli Nissle and a constitutively expressing mutant thereof is shown in Fig. 12.
Table 4
tccacggactccaacatgcttggtgcaacgcatgaagcgaagg atctggaatacctcaactccagcgtcaaaatcgtcaacccaat tatgggcgtgaagttttgggatgagagcgtgaaaatcccggca gaagaagtcacagtacgctttgagcaaggtcatccggtggcgc tgaacggtaaaacctttagcgacgacgtagaaatgatgctgga agctaaccgcatcggc
Constitutive ttgacggctagctcagtcctaggtacagtgctagcACCCGTTTT argG
TTTGGGCTAGAAATAATTTTGTTTAACTTTAAGAAGGAGATATA
(SEQ ID NO:
17) CATACCCatgacgacgattctcaagcatctcccggtaggtcaa cgtattggtatcgctttttccggcggtctggacaccagtgccg cactgctgtggatgcgacaaaagggagcggttccttatgcata tactgcaaacctgggccagccagacgaagaggattatgatgcg atccctcgtcgtgccatggaatacggcgcggagaacgcacgtc tgatcgactgccgcaaacaactggtggccgaaggtattgccgc tattcagtgtggcgcatttcataacaccactggtggactgacc tatttcaacacgacgccgctgggccgcgccgtgaccggcacca tgctggttgctgctatgaaagaagatggcgtgaatatctgggg tgacggcagcacctataaaggaaacgatatcgaacgtttctac cgttacggtctgctgaccaatgctgaactgcagatttacaaac cgtggcttgatactgactttattgatgaactgggtggccgtca tgagatgtctgaatttatgattgcctgcggtttcgactacaaa atgtctgtcgaaaaagcttactccacggactccaacatgcttg
|gtgcaacgcatgaagcgaaggatctggaatacctcaactccag
[0186] In some embodiments, the ArgR binding affinity to a mutant ARG box or regulatory region of an operon is at least about 50% lower, at least about 60% lower, at least about 70% lower, at least about 80% lower, at least about 90% lower, or at least about 95% lower than the ArgR binding affinity to an
unmodified ARG box and regulatory region in bacteria of the same subtype under the same conditions. In some embodiments, the reduced ArgR binding to a mutant ARG box and regulatory region increases mRNA expression of the gene(s) in the associated operon by at least about 1.5-fold, at least about 2-fold, at least about 10-fold, at least about 15-fold, at least about 20-fold, at least about 30-fold, at least about 50-fold, at least about 100-fold, at least about 200- fold, at least about 300-fold, at least about 400-fold, at least about 500-fold, at least about 600-fold, at least about 700-fold, at least about 800-fold, at least about 900-fold, at least about 1 , 000-fold, or at least about 1 , 500-fold.
[0187] In some embodiments, quantitative PCR (qPCR) is used to amplify, detect, and/or quantify mRNA expression levels of the arginine biosynthesis genes. Primers specific for arginine biosynthesis genes, e.g., argA, argB, argC, argD, argE, argF, argG, argH, argl, argJ, carA, and carB, may be designed and used to detect mRNA in a sample according to methods known in the art (Fraga et al., 2008). In some embodiments, a fluorophore is added to a sample reaction mixture that may contain arg mRNA, and a thermal cycler is used to illuminate the sample reaction mixture with a specific
wavelength of light and detect the subsequent emission by the fluorophore. The reaction mixture is heated and cooled to predetermined temperatures for predetermined time periods. In certain embodiments, the heating and cooling is repeated for a predetermined number of cycles. In some embodiments, the reaction mixture is heated and cooled to 90-100° C, 60-70° C, and 30-50° C for a predetermined number of cycles. In a certain embodiment, the reaction mixture is heated and cooled to 93-97° C, 55-65° C, and 35-45° C for a predetermined number of cycles. In some embodiments, the accumulating amplicon is quantified after each cycle of the qPCR. The number of cycles at which fluorescence exceeds the threshold is the threshold cycle (CT). At least one CT result for each sample is generated, and the CT result(s) may be used to determine mRNA expression levels of the arginine biosynthesis genes.
[0188] In some embodiments, the genetically engineered bacteria comprising one or more nucleic acid mutations in at least one ARG box for one or more of the operons that encode the arginine biosynthesis enzymes N- acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine
aminotransferase, N-acetylornithinase, ornithine transcarbamylase,
argininosuccinate synthase, argininosuccinate lyase, and carbamoylphosphate synthase additionally comprise an arginine feedback resistant N- acetylglutamate synthase mutant, e.g., argAmr.
[0189] In some embodiments, the genetically engineered bacteria comprise a feedback resistant form of ArgA, as well as one or more nucleic acid mutations in each ARG box of one or more of the operons that encode the arginine biosynthesis enzymes N-acetylglutamate kinase, N- acetylglutamylphosphate reductase, acetylornithine aminotransferase, N- acetylornithinase, ornithine transcarbamylase, argininosuccinate synthase, argininosuccinate lyase, ornithine acetyltransferase, and carbamoylphosphate synthase.
[0190] In some embodiments, the genetically engineered bacteria comprise a feedback resistant form of ArgA, argininosuccinate synthase driven by a ArgR-repressible regulatory region, as well as one or more nucleic acid mutations in each ARG box of each of the operons that encode the arginine biosynthesis enzymes N-acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine aminotransferase, N-acetylornithinase, ornithine transcarbamylase, argininosuccinate lyase, ornithine acetyltransferase, and carbamoylphosphate synthase. In these embodiments, the bacteria are capable of producing citrulline.
[0191] In some embodiments, the genetically engineered bacteria comprise a feedback resistant form of ArgA, argininosuccinate synthase expressed from a constitutive promoter, as well as one or more nucleic acid mutations in each ARG box of each of the operons that encode the arginine biosynthesis enzymes N-acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine aminotransferase, N-acetylornithinase, ornithine transcarbamylase, argininosuccinate synthase, argininosuccinate lyase, ornithine acetyltransferase, and carbamoylphosphate synthase. In these embodiments, the bacteria are capable of producing arginine.
[0192] Table 3 shows examples of mutant constructs in which one or more nucleic acid mutations reduce or eliminate arginine-mediated repression of each of the arginine operons. The mutant constructs comprise feedback
resistant form of ArgA driven by an oxygen level-dependent promoter, e.g., a FNR promoter. Each mutant arginine regulon comprises one or more nucleic acid mutations in at least one ARG box for one or more of the operons that encode N-acetylglutamate kinase, N-acetylglutamylphosphate reductase, acetylornithine aminotransferase, N-acetylornithinase, ornithine
transcarbamylase, argininosuccinate synthase, argininosuccinate lyase, carbamoylphosphate synthase, and wild-type N-acetylglutamate synthetase, such that ArgR binding is reduced or eliminated, thereby enhancing arginine and/or intermediate byproduct biosynthesis. Non-limiting examples of mutant arginine regulon constructs are shown in Table 5.
Table 5: Examples of ARG Box mutant constructs
[0193] The mutations may be present on a plasmid or chromosome. In some embodiments, the arginine regulon is regulated by a single repressor protein. In particular species, strains, and/or subtypes of bacteria, it has been proposed that the arginine regulon may be regulated by two putative repressors (Nicoloff et al., 2004). Thus, in certain embodiments, the arginine regulon of the invention is regulated by more than one repressor protein.
[0194] In certain embodiments, the mutant arginine regulon is expressed in one species, strain, or subtype of genetically engineered bacteria. In alternate embodiments, the mutant arginine regulon is expressed in two or more species, strains, and/or subtypes of genetically engineered bacteria.
Arginine Repressor (ArgR)
[0195] The genetically engineered bacteria of the invention comprise an arginine regulon comprising one or more nucleic acid mutations that reduce or eliminate arginine-mediated repression of one or more of the operons that encode the enzymes responsible for converting glutamate to arginine and/or an intermediate byproduct in the arginine biosynthesis pathway. In some embodiments, the reduction or elimination of arginine-mediated repression may be achieved by reducing or eliminating ArgR repressor binding, e.g., by mutating at least one ARG box for one or more of the operons that encode the arginine biosynthesis enzymes (as discussed above) or by mutating or deleting the arginine repressor (discussed here) and/or by reducing or eliminating arginine binding to N-acetylglutamate synthetase (e.g., by mutating the N- acetylglutamate synthetase to produce an arginine feedback resistant N- acetylglutamate synthase mutant, e.g., argAmr).
[0196] Thus, in some embodiments, the genetically engineered bacteria lack a functional ArgR repressor and therefore ArgR repressor-mediated transcriptional repression of each of the arginine biosynthesis operons is reduced or eliminated. In some embodiments, the engineered bacteria comprise a mutant arginine repressor comprising one or more nucleic acid mutations such that arginine repressor function is decreased or inactive. In some embodiments, the genetically engineered bacteria do not have an arginine repressor (e.g., the arginine repressor gene has been deleted), resulting in derepression of the regulon and enhancement of arginine and/or intermediate byproduct biosynthesis. In some embodiments, each copy of a functional argR gene normally present in a corresponding wild-type bacterium is independently deleted or rendered inactive by one or more nucleotide deletions, insertions, or substitutions. In some embodiments, each copy of the functional argR gene normally present in a corresponding wild-type bacterium is deleted.
[0197] In some embodiments, the arginine regulon is regulated by a single repressor protein. In particular species, strains, and/or subtypes of bacteria, it has been proposed that the arginine regulon may be regulated by two distinct putative repressors (Nicoloff et al., 2004). Thus, in certain
embodiments, two distinct ArgR proteins each comprising a different amino acid sequence are mutated or deleted in the genetically engineered bacteria.
[0198] In some embodiments, the genetically modified bacteria
comprising a mutant or deleted arginine repressor additionally comprise an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argA
fbr. In some embodiments, the genetically engineered bacteria comprise a feedback resistant form of ArgA, lack any functional arginine repressor, and are capable of producing arginine. In certain embodiments, the genetically engineered bacteria further lack functional ArgG and are capable of producing citrulline. In some embodiments, the argR gene is deleted in the genetically engineered bacteria. In some embodiments, the argR gene is mutated to inactivate ArgR function. In some embodiments, the argG gene is deleted in the genetically engineered bacteria. In some embodiments, the argG gene is mutated to inactivate ArgR function. In some embodiments, the genetically engineered bacteria comprise argA
mr and deleted ArgR. In some embodiments, the genetically engineered bacteria comprise argA
mr, deleted ArgR, and deleted argG. In some embodiments, the deleted ArgR and/or the deleted argG is deleted from the bacterial genome and the argA
mris present in a plasm id. In some embodiments, the deleted ArgR and/or the deleted argG is deleted from the bacterial genome and the argA
fbrs chromosomally integrated. In one specific embodiment, the genetically modified bacteria comprise chromosomally integrated argA
mr, deleted genomic ArgR, and deleted genomic argG. In another specific embodiment, the genetically modified bacteria comprise
on a plasmid, deleted genomic ArgR, and deleted genomic argG. In any of the embodiments in which argG is deleted, citrulline rather than arginine is produced
[0199] In some embodiments, under conditions where a feedback resistant form of ArgA is expressed, the genetically engineered bacteria of the invention produce at least about 1.5-fold, at least about 2-fold, at least about 10- fold, at least about 15-fold, at least about 20-fold, at least about 30-fold, at least about 50-fold, at least about 100-fold, at least about 200-fold, at least about 300-fold, at least about 400-fold, at least about 500-fold, at least about 600-fold, at least about 700-fold, at least about 800-fold, at least about 900-fold, at least
about 1 ,000-fold, or at least about 1 ,500-fold more arginine, citrulline, other intermediate byproduct, and/or transcript of the gene(s) in the operon as compared to unmodified bacteria of the same subtype under the same conditions.
[0200] In some embodiments, quantitative PCR (qPCR) is used to amplify, detect, and/or quantify mRNA expression levels of the arginine biosynthesis genes. Primers specific for arginine biosynthesis genes, e.g., argA, argB, argC, argD, argE, argF, argG, argH, argl, argJ, carA, and carB, may be designed and used to detect mRNA in a sample according to methods known in the art (Fraga et al., 2008). In some embodiments, a fluorophore is added to a sample reaction mixture that may contain arg mRNA, and a thermal cycler is used to illuminate the sample reaction mixture with a specific wavelength of light and detect the subsequent emission by the fluorophore. The reaction mixture is heated and cooled to predetermined temperatures for predetermined time periods. In certain embodiments, the heating and cooling is repeated for a predetermined number of cycles. In some embodiments, the reaction mixture is heated and cooled to 90-100° C, 60-70° C, and 30-50° C for a predetermined number of cycles. In a certain embodiment, the reaction mixture is heated and cooled to 93-97° C, 55-65° C, and 35-45° C for a predetermined number of cycles. In some embodiments, the accumulating amplicon is quantified after each cycle of the qPCR. The number of cycles at which fluorescence exceeds the threshold is the threshold cycle (CT). At least one CT result for each sample is generated, and the CT result(s) may be used to determine mRNA expression levels of the arginine biosynthesis genes.
[0201] In any of these embodiments in which the ArgR is mutated, the mutant ArgR and/or a feedback resistant ArgA may be integrated into the bacterial chromosome at one or more integration sites or may be present on one or more plasmids.
Feedback Resistant N-acetylglutamate Synthetase
[0202] In some embodiments, the genetically engineered bacteria comprise an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmr. In some embodiments, the genetically engineered bacteria comprise a mutant arginine regulon comprising an arginine feedback resistant
ArgA, and when the arginine feedback resistant ArgA is expressed, are capable of producing more arginine and/or an intermediate byproduct than unmodified bacteria of the same subtype under the same conditions. The arginine feedback resistant N-acetylglutamate synthetase protein (argAfbr) is significantly less sensitive to L-arginine than the enzyme from the feedback sensitive parent strain (see, e.g., Eckhardt et al., 1975; Rajagopal et al., 1998). The feedback resistant argA gene can be present on a plasm id or chromosome. In some embodiments, expression from the plasmid may be useful for increasing argAmr expression. In some embodiments, expression from the chromosome may be useful for increasing stability of argAfbr expression.
[0203] In some embodiments, any of the described mutant sequences involved in the arginine biosynthetic pathway (e.g., ArgR, argAmr , Arg Box sequence) are integrated into the bacterial chromosome at one or more integration sites. For example, one or more copies of the sequence encoding the arginine feedback resistant N-acetylglutamate synthase may be integrated into the bacterial chromosome. Having multiple copies of the arginine feedback resistant N-acetylglutamate synthase integrated into the chromosome allows for greater production of the N-acetylglutamate synthase and also permits fine- tuning of the level of expression. Alternatively, different circuits described herein, such as any of the transporter or kill-switch circuits, in addition to the arginine feedback resistant N-acetylglutamate synthase could be integrated into the bacterial chromosome at one or more different integration sites to perform multiple different functions. Multiple distinct feedback resistant N- acetylglutamate synthetase proteins are known in the art and may be combined in the genetically engineered bacteria. In some embodiments, the argAmr gene is expressed under the control of a constitutive promoter. In some
embodiments, the argAmr gene is expressed under the control of a promoter that is induced by exogenous environmental conditions. In some embodiments, the exogenous environmental conditions are specific to the gut of a mammal. In some embodiments, exogenous environmental conditions are molecules or metabolites that are specific to the mammalian gut in a healthy or disease state, e.g., propionate. In some embodiments, the exogenous environmental
conditions are low-oxygen or anaerobic conditions, such as the environment of the mammalian gut.
[0204] Bacteria have evolved transcription factors that are capable of sensing oxygen levels. Different signaling pathways may be triggered by different oxygen levels and occur with different kinetics. An oxygen level- dependent promoter is a nucleic acid sequence to which one or more oxygen level-sensing transcription factors is capable of binding, wherein the binding and/or activation of the corresponding transcription factor activates downstream gene expression. In one embodiment, the argAmr gene is under control of an oxygen level-dependent promoter. In a more specific aspect, the argAfbr gene is under control of an oxygen level-dependent promoter that is activated under low-oxygen or anaerobic environments, such as the environment of the mammalian gut.
[0205] In certain embodiments, the genetically engineered bacteria comprise argAfbr expressed under the control of the fumarate and nitrate reductase regulator (FNR) promoter. In E. coli, FNR is a major transcriptional activator that controls the switch from aerobic to anaerobic metabolism (Unden et al., 1997). In the anaerobic state, FNR dimerizes into an active DNA binding protein that activates hundreds of genes responsible for adapting to anaerobic growth. In the aerobic state, FNR is prevented from dimerizing by oxygen and is inactive. In alternate embodiments, the genetically engineered bacteria comprise argAfbr expressed under the control of an alternate oxygen level- dependent promoter, e.g., an anaerobic regulation of arginine deiminiase and nitrate reduction ANR promoter (Ray et al., 1997), a dissimilatory nitrate respiration regulator DNR promoter (Trunk et al., 2010). In these embodiments, the arginine biosynthesis pathway is particularly activated in a low-oxygen or anaerobic environment, such as in the gut.
[0206] In P. aeruginosa, the anaerobic regulation of arginine deiminiase and nitrate reduction (ANR) transcriptional regulator is "required for the expression of physiological functions which are inducible under oxygen-limiting or anaerobic conditions" (Winteler et al., 1996; Sawers 1991 ). P. aeruginosa ANR is homologous with E. coli FNR, and "the consensus FNR site (TTGAT— ATCAA) was recognized efficiently by ANR and FNR" (Winteler et al., 1996).
Like FNR, in the anaerobic state, ANR activates numerous genes responsible for adapting to anaerobic growth. In the aerobic state, ANR is inactive.
Pseudomonas fiuorescens, Pseudomonas putida, Pseudomonas syringae, and Pseudomonas mendocina all have functional analogs of ANR (Zimmermann et al., 1991 ). Promoters that are regulated by ANR are known in the art, e.g., the promoter of the arcDABC operon (see, e.g., Hasegawa et al., 1998).
[0207] The FNR family also includes the dissimilatory nitrate respiration regulator (DNR) (Arai et al., 1995), a transcriptional regulator that is required in conjunction with ANR for "anaerobic nitrate respiration of Pseudomonas aeruginosa" (Hasegawa et al., 1998). For certain genes, the FNR-binding motifs "are probably recognized only by DNR" (Hasegawa et al., 1998). Any suitable transcriptional regulator that is controlled by exogenous environmental conditions and corresponding regulatory region may be used. Non-limiting examples include ArcA/B, ResD/E, NreA/B/C, and AirSR, and others are known in the art.
[0208] FNR promoter sequences are known in the art, and any suitable FNR promoter sequence(s) may be used in the genetically engineered bacteria of the invention. Any suitable FNR promoter(s) may be combined with any suitable argAmr (e.g., the exemplary argAmr sequence shown in Table 7). Non- limiting FNR promoter sequences are provided in Table 6. Table 6 depicts the nucleic acid sequences of exemplary regulatory region sequences comprising a FNR-responsive promoter sequence. Underlined sequences are predicted ribosome binding sites, and bolded sequences are restriction sites used for cloning. In some embodiments, the genetically engineered bacteria of the invention comprise one or more of: SEQ ID NO: 18, SEQ ID NO: 19, nirB1 promoter (SEQ ID NO: 20), nirB2 promoter (SEQ ID NO: 21 ), nirB3 promoter (SEQ ID NO: 22), ydfZ promoter (SEQ ID NO: 23), nirB promoter fused to a strong ribosome binding site (SEQ ID NO: 24), ydfZ promoter fused to a strong ribosome binding site (SEQ ID NO: 25), fnrS, an anaerobically induced small RNA gene (fnrS1 promoter SEQ ID NO: 26 or fnrS2 promoter SEQ ID NO: 27), nirB promoter fused to a crp binding site (SEQ ID NO: 28), and fnrS fused to a crp binding site (SEQ ID NO: 29).
[0209] In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 18, 19, 20, 21 , 22, 23, 24, 25, 26, 27, 28, or 29, or a functional fragment thereof.
Table 6
ATTTCCTCTCATCCCATCCGGGGTGAGAGTCTTTTCCCCCGACTTATGGC ydfZ
TCATGCATGCATCAAAAAAGATGTGAGCTTGATCAAAAACAAAAAATATT SEQ ID NO:
TCACTCGACAGGAGTATTTATATTGCGCCCGTTACGTGGGCTTCGACTGT
23
AAATCAGAAAGGAGAAAACACCT
GTCAGCATAACACCCTGACCTCTCATTAATTGTTCATGCCGGGCGGCACT ATCGTCGTCCGGCCTTTTCCTCTCTTACTCTGCTACGTACATCTATTTCT
nirB+RBS ATAAATCCGTTCAATTTGTCTGTTTTTTGCACAAACATGAAATATCAGAC SEQ ID NO: AATTCCGTGACTTAAGAAAATTTATACAAATCAGCAATATACCCCTTAAG 24 GAGTATATAAAGGTGAATTTGATTTACATCAATAAGCGGGGTTGCTGAAT
CGTT AAGGATCCCTCTAGAAATAATTTTGTTTAACTTTAAGAAGGAGATA TACAT
CATTTCCTCTCATCCCATCCGGGGTGAGAGTCTTTTCCCCCGACTTATGG
ydfZ+RBS
CTCATGCATGCATCAAAAAAGATGTGAGCTTGATCAAAAACAAAAAATAT SEQ ID NO:
TTCACTCGACAGGAGTATTTATATTGCGCCC GGATCCCTCTAGAAATAAT
25
TTTGTTTAACTTTAAGAAGGAGATATACAT
AGTTGTTCTTATTGGTGGTGTTGCTTTATGGTTGCATCGTAGTAAATGGT
fnrSl
TGTAACAAAAGCAATTTTTCCGGCTGTCTGTATACAAAAACGCCGTAAAG SEQ ID NO:
TTTGAGCGAAGTCAATAAACTCTCTACCCATTCAGGGCAATATCTCTCTT
26
GGATCCCTCTAGAAATAATTTTGTTTAACTTTAAGAAGGAGATATACAT
AGTTGTTCTTATTGGTGGTGTTGCTTTATGGTTGCATCGTAGTAAATGGT
fnrS2 TGTAACAAAAGCAATTTTTCCGGCTGTCTGTATACAAAAACGCCGCAAAG SEQ ID NO: TTTGAGCGAAGTCAATAAACTCTCTACCCATTCAGGGCAATATCTCTCTT
27 GGATCCAAAGTGAACTCTAGAAATAATTTTGTTTAACTTTAAGAAGGAGA
TATACAT
TCGTCTTTGTGATGTGCTTCCTGTTAGGTTTCGTCAGCCGTCACCGTCAG CATAACACCCTGACCTCTCATTAATTGCTCATGCCGGACGGCACTATCGT CGTCCGGCCTTTTCCTCTCTTCCCCCGCTACGTGCATCTATTTCTATAAA
nirB+crp
CCCGCTCATTTTGTCTATTTTTTGCACAAACATGAAATATCAGACAATTC SEQ ID NO:
CGTGACTTAAGAAAATTTATACAAATCAGCAATATACCCATTAAGGAGTA
28
TATAAAGGTGAATTTGATTTACATCAATAAGCGGGGTTGCTGAATCGTTA AGGTAGaaatgtgatctagttcacatttGCGGTAATAGAAAAGAAATCGA GGCAAAAatgtttgtttaactttaagaaggagatatacat
AGTTGTTCTTATTGGTGGTGTTGCTTTATGGTTGCATCGTAGTAAATGGT
fnrS+crp
TGTAACAAAAGCAATTTTTCCGGCTGTCTGTATACAAAAACGCCGCAAAG SEQ ID NO:
TTTGAGCGAAGTCAATAAACTCTCTACCCATTCAGGGCAATATCTCTCaa 29
atgtgatctagttcacattt t ttgtttaactt taagaaggagatatacat
[0210] In other embodiments, argA is expressed under the control of an oxygen level-dependent promoter fused to a binding site for a transcriptional activator, e.g., CRP. CRP (cyclic AMP receptor protein or catabolite activator protein or CAP) plays a major regulatory role in bacteria by repressing genes responsible for the uptake, metabolism and assimilation of less favorable carbon sources when rapidly metabolizable carbohydrates, such as glucose, are present (Wu et al., 2015). This preference for glucose has been termed glucose repression, as well as carbon catabolite repression (Deutscher, 2008; Gorke and Stulke, 2008). In some embodiments, argAmr expression is controlled by an oxygen level-dependent promoter fused to a CRP binding site. In some embodiments, argAfbr expression is controlled by a FNR promoter fused to a CRP binding site. In these embodiments, cyclic AMP binds to CRP when no glucose is present in the environment. This binding causes a conformational change in CRP, and allows CRP to bind tightly to its binding site. CRP binding then activates transcription of the argAmr gene by recruiting RNA polymerase to the FNR promoter via direct protein-protein interactions. In the presence of glucose, cyclic AMP does not bind to CRP and argAmr gene transcription is repressed. In some embodiments, an oxygen level-dependent promoter (e.g., a FNR promoter) fused to a binding site for a transcriptional activator is used to ensure that argA^ is not expressed under anaerobic conditions when sufficient amounts of glucose are present, e.g., by adding glucose to growth media in vitro.
[0211] In some embodiments, argAfbr is expressed under the control of an inducible promoter that is responsive to specific molecules or metabolites in the environment, e.g., the mammalian gut. For example, the short-chain fatty acid propionate is a major microbial fermentation metabolite localized to the gut (Hosseini et al., 2011 ). In one embodiment, argAmr gene expression is under the control of a propionate-inducible promoter. In a more specific embodiment, argA mr gene expression is under the control of a propionate-inducible promoter that is activated by the presence of propionate in the mammalian gut. Any molecule or metabolite found in the mammalian gut, in a healthy and/or disease state, may be used to induce argAmr expression. Non-limiting examples include propionate, bilirubin, aspartate aminotransferase, alanine aminotransferase,
blood coagulation factors II, VII, IX, and X, alkaline phosphatase, gamma glutamyl transferase, hepatitis antigens and antibodies, alpha fetoprotein, anti- mitochondrial, smooth muscle, and anti-nuclear antibodies, iron, transferrin, ferritin, copper, ceruloplasmin, ammonia, and manganese. In alternate embodiments, argAmr gene expression is under the control of a ParaBAD promoter, which is activated in the presence of the sugar arabinose (see, e.g., Fig. 13)
[0212] Subjects with hepatic encephalopathy (HE) and other liver disease or disorders have chronic liver damage that results in high ammonia levels in their blood and intestines. In addition to ammonia, these patients also have elevated levels of bilirubin, aspartate aminotransferase, alanine
aminotransferase, blood coagulation factors II, VII, IX, and X, alkaline
phosphatase, gamma glutamyl transferase, hepatitis antigens and antibodies, alpha fetoprotein, anti-mitochondrial, smooth muscle, and anti-nuclear antibodies, iron, transferrin, ferritin, copper, ceruloplasmin, ammonia, and manganese in their blood and intestines. Promoters that respond to one of these HE - related molecules or their metabolites may be used in the
genetically engineered bacteria to induce expression of argAmr in the gut.
These promoters would not be expected to be induced in non-HE patients.
[0213] In some embodiments, the argAmr gene is expressed under the control of a promoter that is induced by exposure to tetracycline. In some embodiments, the argAmr gene is expressed under the control of a promoter that is induced by exposure to inflammation or an imflammatory response (e.g., RNS or ROS promoters). In some embodiments, the argAmr gene is expressed under the control of a promoter that is induced by exposure to a metabolite such as arabinose (e.g., AraBAD promoter).
[0214] In some embodiments, gene expression is further optimized by methods known in the art, e.g., by optimizing ribosomal binding sites, manipulating transcriptional regulators, and/or increasing mRNA stability. The fbr
nucleic acid sequence of an exemplary argA sequence is shown in Table 7.
fbr
The polypeptide sequence of an exemplary argA sequence is shown in Table 8
Table 7
Nucleotide sequence of exemplary argA sequence
(SEQ ID NO: 30)
ATGGTAAAGGAACGTAAAACCGAGTTGGTCGAGGGATTCCGCCATTCGGTTCCCTGTA TCAATACCCACCGGGGAAAAACGTTTGTCATCATGCTCGGCGGTGAAGCCATTGAGCA TGAGAATTTCTCCAGTATCGTTAATGATATCGGGTTGTTGCACAGCCTCGGCATCCGT CTGGTGGTGGTCTATGGCGCACGTCCGCAGATCGACGCAAATCTGGCTGCGCATCACC ACGAACCGCTGTATCACAAGAATATACGTGTGACCGACGCCAAAACACTGGAACTGGT GAAGCAGGCTGCGGGAACATTGCAACTGGATATTACTGCTCGCCTGTCGATGAGTCTC AATAACACGCCGCTGCAGGGCGCGCATATCAACGTCGTCAGTGGCAATTTTATTATTG CCCAGCCGCTGGGCGTCGATGACGGCGTGGATTACTGCCATAGCGGGCGTATCCGGCG GATTGATGAAGACGCGATCCATCGTCAACTGGACAGCGGTGCAATAGTGCTAATGGGG CCGGTCGCTGTTTCAGTCACTGGCGAGAGCTTTAACCTGACCTCGGAAGAGATTGCCA CTCAACTGGCCATCAAACTGAAAGCTGAAAAGATGATTGGTTTTTGCTCTTCCCAGGG CGTCACTAATGACGACGGTGATATTGTCTCCGAACTTTTCCCTAACGAAGCGCAAGCG CGGGTAGAAGCCCAGGAAGAGAAAGGCGATTACAACTCCGGTACGGTGCGCTTTTTGC GTGGCGCAGTGAAAGCCTGCCGCAGCGGCGTGCGTCGCTGTCATTTAATCAGTTATCA GGAAGATGGCGCGCTGTTGCAAGAGTTGTTCTCACGCGACGGTATCGGTACGCAGATT GTGATGGAAAGCGCCGAGCAGATTCGTCGCGCAACAATCAACGATATTGGCGGTATTC TGGAGTTGATTCGCCCACTGGAGCAGCAAGGTATTCTGGTACGCCGTTCTCGCGAGCA GCTGGAGATGGAAATCGACAAATTCACCATTATTCAGCGCGATAACACGACTATTGCC TGCGCCGCGCTCTATCCGTTCCCGGAAGAGAAGATTGGGGAAATGGCCTGTGTGGCAG TTCACCCGGATTACCGCAGTTCATCAAGGGGTGAAGTTCTGCTGGAACGCATTGCCGC TCAGGCTAAGCAGAGCGGCTTAAGCAAATTGTTTGTGCTGACCACGCGCAGTATTCAC TGGTTCCAGGAACGTGGATTTACCCCAGTGGATATTGATTTACTGCCCGAGAGCAAAA AGCAGTTGTACAACTACCAGCGTAAATCCAAAGTGTTGATGGCGGATTTAGGGTAA
Table 8
Polypeptide sequence of exemplary argA sequence
(SEQ ID NO: 31)
MVKERKTELVEGFRHSVPCINTHRGKTFVIMLGGEAIEHENFSSIVNDIGLLHSLGIRL\AA YGARPQIDANLAAHHHEPLYHKNIRVTDAKTLELVKQAAGTLQLDITARLSMSLNNTPLQGA HINVVSGNFI IAQPLGVDDGVDYCHSGRIRRIDEDAIHRQLDSGAIVLMGPVAVSVTGESFN LTSEEIATQLAIKLKAEKMIGFCSSQGVTNDDGDIVSELFPNEAQARVEAQEEKGDYNSGTV RFLRGAVKACRSGVRRCHLISYQEDGALLQELFSRDGIGTQIVMESAEQIRRATINDIGGIL ELIRPLEQQGILVRRSREQLEMEIDKFTI IQRDNTTIACAALYPFPEEKIGEMACVAVHPDY RSSSRGEVLLERIAAQAKQSGLSKLFVLTTRS IHWFQERGFTPVDIDLLPESKKQLYNYQRK SKVLMADLG
Bold underline: mutated amino acid resulting feedback resistance, (mutation is Y19C)
[0215] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 30 or a functional fragment
thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 30 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 30 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 30 or a functional fragment thereof.
[0216] In some embodiments, the genetically engineered bacteria encode a polypeptide sequence of SEQ ID NO: 31 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria encode a polypeptide sequence encodes a polypeptide, which contains one or more conservative amino acid substutions relative to SEQ ID NO: 31 or a functional fragment thereof. In some embodiments, genetically engineered bacteria encode a polypeptide sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 31 or a functional fragment thereof.
[0217] In some embodiments, arginine feedback inhibition of N- acetylglutamate synthetase is reduced by at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, or at least about 95% in the genetically engineered bacteria when the arginine feedback resistant N-acetylglutamate synthetase is active, as compared to a wild-type N- acetylglutamate synthetase from bacteria of the same subtype under the same conditions.
[0218] In some embodiments, the genetically engineered bacteria comprise a stably maintained plasm id or chromosome carrying the argAmr gene, such that argAfbr can be expressed in the host cell, and the host cell is capable of survival and/or growth in vitro, e.g., in medium, and/or in vivo, e.g., in the gut. In some embodiments, a bacterium may comprise multiple copies of the feedback resistant argA gene. In some embodiments, the feedback resistant argA gene is expressed on a low-copy plasmid. In some embodiments, the low- copy plasm id may be useful for increasing stability of expression. In some
embodiments, the low-copy plasm id may be useful for decreasing leaky expression under non-inducing conditions. In some embodiments, the feedback resistant argA gene is expressed on a high-copy plasmid. In some
embodiments, the high-copy plasmid may be useful for increasing argAmr expression. In some embodiments, the feedback resistant argA gene is expressed on a chromosome.
[0219] In some embodiments, the bacteria are genetically engineered to include multiple mechanisms of action (MOAs), e.g., circuits producing multiple copies of the same product or circuits performing multiple different functions. Examples of insertion sites include, but are not limited to, malE/K, insB/l, araC/BAD, lacZ, dapA, cea, and other shown in Fig. 18. For example, the genetically engineered bacteria may include four copies of argAmr inserted at four different insertion sites, e.g., malE/K, insB/l, araC/BAD, and lacZ.
Alternatively, the genetically engineered bacteria may include three copies of argAmr inserted at three different insertion sites, e.g., malE/K, insB/l, and lacZ, and three mutant arginine regulons, e.g., two producing citrulline and one producing arginine, inserted at three different insertion sites dapA, cea, and araC/BAD.
[0220] In some embodiments, the plasmid or chromosome also comprises wild-type ArgR binding sites, e.g., ARG boxes. In some instances, the presence and/or build-up of functional ArgR may result in off-target binding at sites other than the ARG boxes, which may cause off-target changes in gene expression. A plasmid or chromosome that further comprises functional ARG boxes may be used to reduce or eliminate off-target ArgR binding, i.e., by acting as an ArgR sink. In some embodiments, the plasmid or chromosome does not comprise functional ArgR binding sites, e.g., the plasmid or chromosome comprises modified ARG boxes or does not comprise ARG boxes.
[0221] In some embodiments, the feedback resistant argA gene is present on a plasmid and operably linked to a promoter that is induced under low-oxygen or anaerobic conditions. In some embodiments, the feedback resistant argA gene is present in the chromosome and operably linked to a promoter that is induced under low-oxygen or anaerobic conditions. In some embodiments, the feedback resistant argA gene is present on a plasmid and
operably linked to a promoter that is induced by molecules or metabolites that are specific to the mammalian gut. In some embodiments, the feedback resistant argA gene is present on a chromosome and operably linked to a promoter that is induced by molecules or metabolites that are specific to the mammalian gut. In some embodiments, the feedback resistant argA gene is present on a chromosome and operably linked to a promoter that is induced by exposure to tetracycline. In some embodiments, the feedback resistant argA gene is present on a plasm id and operably linked to a promoter that is induced by exposure to tetracycline.
[0222] In some embodiments, the genetically engineered bacteria comprise a variant or mutated oxygen level-dependent transcriptional regulator, e.g., FNR, ANR, or DNR, in addition to the corresponding oxygen level- dependent promoter. The variant or mutated oxygen level-dependent transcriptional regulator increases the transcription of operably linked genes in a low-oxygen or anaerobic environment. In some embodiments, the
corresponding wild-type transcriptional regulator retains wild-type activity. In alternate embodiments, the corresponding wild-type transcriptional regulator is deleted or mutated to reduce or eliminate wild-type activity. In certain embodiments, the mutant oxygen level-dependent transcriptional regulator is a FNR protein comprising amino acid substitutions that enhance dimerization and FNR activity (see, e.g., Moore et al., 2006).
[0223] In some embodiments, the genetically engineered bacteria comprise an oxygen level-dependent transcriptional regulator from a different bacterial species that reduces and/or consumes ammonia in low-oxygen or anaerobic environments. In certain embodiments, the mutant oxygen level- dependent transcriptional regulator is a FNR protein from N. gonorrhoeae (see, e.g., Isabella et al., 2011 ). In some embodiments, the corresponding wild-type transcriptional regulator is left intact and retains wild-type activity. In alternate embodiments, the corresponding wild-type transcriptional regulator is deleted or mutated to reduce or eliminate wild-type activity.
[0224] In some embodiments, the genetically engineered bacteria comprise argAfbr expressed under the control of an oxygen level-dependent promoter, e.g., a FNR promoter, as well as wild-type argA expressed under the
control of a mutant regulatory region comprising one or more ARG box mutations as discussed above. In certain embodiments, the genetically engineered bacteria comprise argAfbr expressed under the control of an oxygen level-dependent promoter, e.g., a FNR promoter and do not comprise wild-type argA. In still other embodiments, the mutant arginine regulon comprises argAmr expressed under the control of an oxygen level-dependent promoter, e.g., a FNR promoter, and further comprises wild-type argA without any ARG box mutations.
[0225] In some embodiments, the genetically engineered bacteria express argAmr from a plasmid and/or chromosome. In some embodiments, the argAmr gene is expressed under the control of a constitutive promoter. In some embodiments, the argAmr gene is expressed under the control of an inducible promoter. In one embodiment, argAmr is expressed under the control of an oxygen level-dependent promoter that is activated under low-oxygen or anaerobic environments, e.g., a FNR promoter. The nucleic acid sequence of fbr
an exemplary FNR promoter-driven argA sequence is shown in Table 9. The fbr
FNR promoter sequence is bolded and the argA sequence is |boxed|. The nucleic acid sequence of a FNR promoter-driven argAfbr plasmid is shown in Table 10, with the FNR promoter sequence bolded and argA mr sequence boxeq. Table 11 shows the nucleic acid sequence of an exemplary pSC101 plasmid. Any suitable FNR promoter(s) may be combined with any suitable feedback-resistant ArgA. Non-limiting FNR promoter sequences are provided in Table 6. In some embodiments, the genetically engineered bacteria of the invention comprise one or more of: SEQ ID NO: 16, SEQ ID NO: 17, nirB1 promoter (SEQ ID NO: 18), nirB2 promoter (SEQ ID NO: 19), nirB3 promoter (SEQ ID NO: 20), ydfZ promoter (SEQ ID NO: 21 ), nirB promoter fused to a strong ribosome binding site (SEQ ID NO: 22), ydfZ promoter fused to a strong ribosome binding site (SEQ ID NO: 23), fnrS, an anaerobically induced small RNA gene (fnrS1 promoter SEQ ID NO: 24 or fnrS2 promoter SEQ ID NO: 25), nirB promoter fused to a crp binding site (SEQ ID NO: 26), and fnrS fused to a crp binding site (SEQ ID NO: 27). Table 12 depicts the nucleic acid sequence fbr
of an exemplary fnrS promoter-driven argA sequence. The FNR promoter
fbr sequence is bolded, the ribosome binding site is highlighted, and the argA sequence is boxed
[0226] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 32 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 32. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 32, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 32.
[0227] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 33 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 33. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 33, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 33.
[0228] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 35 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 35. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 35, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 35.
Table 9
Exemplary FNR promoter-driven argAtbr sequence
(SEQ ID NO: 32)
AGTTGTTCTTATTGGTGGTGTTGCTTTATGGTTGCATCGTAGTAAATGGTTGTAACAA AAGCAATTTTTCCGGCTGTCTGTATACAAAAACGCCGCAAAGTTTGAGCGAAGTCAAT
AAACTCTCTACCCATTCAGGGCAATATCTCTCTTggatccaaagtgaactctagaaat aattttgtttaactttaagaaggagatatacatATGGTAAAGGAACGTAAAACCGAG
TTGGTCGAGGGATTCCGCCATTCGGTTCCCTGTATCAATACCCACCGGGGAAAAACG
TTTGTCATCATGCTCGGCGGTGAAGCCATTGAGCATGAGAATTTCTCCAGTATCGTT
AATGATATCGGGTTGTTGCACAGCCTCGGCATCCGTCTGGTGGTGGTCTATGGCGCA
CGTCCGCAGATCGACGCAAATCTGGCTGCGCATCACCACGAACCGCTGTATCACAAG
AATATACGTGTGACCGACGCCAAAACACTGGAACTGGTGAAGCAGGCTGCGGGAACA
TTGCAACTGGATATTACTGCTCGCCTGTCGATGAGTCTCAATAACACGCCGCTGCAG
GGCGCGCATATCAACGTCGTCAGTGGCAATTTTATTATTGCCCAGCCGCTGGGCGTC
GATGACGGCGTGGATTACTGCCATAGCGGGCGTATCCGGCGGATTGATGAAGACGCG
ATCCATCGTCAACTGGACAGCGGTGCAATAGTGCTAATGGGGCCGGTCGCTGTTTCA
GTCACTGGCGAGAGCTTTAACCTGACCTCGGAAGAGATTGCCACTCAACTGGCCATC
AAACTGAAAGCTGAAAAGATGATTGGTTTTTGCTCTTCCCAGGGCGTCACTAATGAC
GACGGTGATATTGTCTCCGAACTTTTCCCTAACGAAGCGCAAGCGCGGGTAGAAGCC
CAGGAAGAGAAAGGCGATTACAACTCCGGTACGGTGCGCTTTTTGCGTGGCGCAGTG
AAAGCCTGCCGCAGCGGCGTGCGTCGCTGTCATTTAATCAGTTATCAGGAAGATGGC
GCGCTGTTGCAAGAGTTGTTCTCACGCGACGGTATCGGTACGCAGATTGTGATGGAA
AGCGCCGAGCAGATTCGTCGCGCAACAATCAACGATATTGGCGGTATTCTGGAGTTG
ATTCGCCCACTGGAGCAGCAAGGTATTCTGGTACGCCGTTCTCGCGAGCAGCTGGAG
ATGGAAATCGACAAATTCACCATTATTCAGCGCGATAACACGACTATTGCCTGCGCC
GCGCTCTATCCGTTCCCGGAAGAGAAGATTGGGGAAATGGCCTGTGTGGCAGTTCAC
CCGGATTACCGCAGTTCATCAAGGGGTGAAGTTCTGCTGGAACGCATTGCCGCTCAG
GCTAAGCAGAGCGGCTTAAGCAAATTGTTTGTGCTGACCACGCGCAGTATTCACTGG
TTCCAGGAACGTGGATTTACCCCAGTGGATATTGATTTACTGCCCGAGAGCAAAAAG
CAGTTGTACAACTACCAGCGTAAATCCAAAGTGTTGATGGCGGATTTAGGGTAA
Table 10
GATGAAGACGCGATCCATCGTCAACTGGACAGCGGTGCAATAGTGCTAATGGGGCCGGTCGC TGTTTCAGTCACTGGCGAGAGCTTTAACCTGACCTCGGAAGAGATTGCCACTCAACTGGCCA
TCAAACTGAAAGCTGAAAAGATGATTGGTTTTTGCTCTTCCCAGGGCGTCACTAATGACGAC
GGTGATATTGTCTCCGAACTTTTCCCTAACGAAGCGCAAGCGCGGGTAGAAGCCCAGGAAGA
GAAAGGCGATTACAACTCCGGTACGGTGCGCTTTTTGCGTGGCGCAGTGAAAGCCTGCCGCA
GCGGCGTGCGTCGCTGTCATTTAATCAGTTATCAGGAAGATGGCGCGCTGTTGCAAGAGTTG
TTCTCACGCGACGGTATCGGTACGCAGATTGTGATGGAAAGCGCCGAGCAGATTCGTCGCGC
AACAATCAACGATATTGGCGGTATTCTGGAGTTGATTCGCCCACTGGAGCAGCAAGGTATTC
TGGTACGCCGTTCTCGCGAGCAGCTGGAGATGGAAATCGACAAATTCACCATTATTCAGCGC
GATAACACGACTATTGCCTGCGCCGCGCTCTATCCGTTCCCGGAAGAGAAGATTGGGGAAAT
GGCCTGTGTGGCAGTTCACCCGGATTACCGCAGTTCATCAAGGGGTGAAGTTCTGCTGGAAC
GCATTGCCGCTCAGGCTAAGCAGAGCGGCTTAAGCAAATTGTTTGTGCTGACCACGCGCAGT
ATTCACTGGTTCCAGGAACGTGGATTTACCCCAGTGGATATTGATTTACTGCCCGAGAGCAA
AAAG C AG T T G T AC AAC T AC C AG C G T AAAT C C AAAG T G T T GAT G G C G GAT T TAG G G T AAAC AG
AATAAAAATACAATAATTTCGAATAATCATGCAAAGCTTGGCGTAATCATGGTCATAGCTGT TTCCTGTGT GAAAT TGTTATCCGCT C AC AAT T C C AC AC AAC AT AC GAG C C G GAAG C AT G T AC GGGTTTTGCTGCCCGCAAACGGGCTGTTCTGGTGTTGCTAGTTTGTTATCAGAATCGCAGAT CCGGCTTCAGGTTTGCCGGCTGAAAGCGCTATTTCTTCCAGAATTGCCATGATTTTTTCCCC ACGGGAGGCGTCACTGGCTCCCGTGTTGTCGGCAGCTTTGATTCGATAAGCAGCATCGCCTG TTTCAGGCTGTCTATGTGTGACTGTTGAGCTGTAACAAGTTGTCTCAGGTGTTCAATTTCAT GTTCTAGTTGCTTTGTTTTACTGGTTTCACCTGTTCTATTAGGTGTTACATGCTGTTCATCT GTTACATTGTCGATCTGTTCATGGTGAACAGCTTTAAATGCACCAAAAACTCGTAAAAGCTC TGATGTATCTATCTTTTTTACACCGTTTTCATCTGTGCATATGGACAGTTTTCCCTTTGATA TCTAACGGTGAACAGTTGTTCTACTTTTGTTTGTTAGTCTTGATGCTTCACTGATAGATACA AGAGCCATAAGAACCTCAGATCCTTCCGTATTTAGCCAGTATGTTCTCTAGTGTGGTTCGTT GTTTTTGCGTGAGCCATGAGAACGAACCATTGAGATCATGCTTACTTTGCATGTCACTCAAA AATTTTGCCTCAAAACTGGTGAGCTGAATTTTTGCAGTTAAAGCATCGTGTAGTGTTTTTCT TAGTCCGTTACGTAGGTAGGAATCTGATGTAATGGTTGTTGGTATTTTGTCACCATTCATTT TTATCTGGTTGTTCTCAAGTTCGGTTACGAGATCCATTTGTCTATCTAGTTCAACTTGGAAA ATCAACGTATCAGTCGGGCGGCCTCGCTTATCAACCACCAATTTCATATTGCTGTAAGTGTT TAAATCTT TACT TAT TGGTTTCAAAACCCATTGGTTAAGCCTTTTAAACTCATGGTAGT TAT T T T CAAGCAT TAACAT GAAC T TAAAT T CAT CAAGGC TAAT C T C TAT AT T T GCC T T GT GAGT T TTCTTTTGTGTTAGTTCTTTTAATAACCACTCATAAATCCTCATAGAGTATTTGTTTTCAAA AGAC T TAACAT GT T CCAGAT TAT AT T T TAT GAAT T T T T T TAAC T GGAAAAGATAAGGCAATA TCTCTTCACTAAAAACTAATTCTAATTTTTCGCTTGAGAACTTGGCATAGTTTGTCCACTGG AAAATCTCAAAGCCTTTAACCAAAGGATTCCTGATTTCCACAGTTCTCGTCATCAGCTCTCT GGTTGCTTTAGC TAAT AC AC C AT AAG CATTTTCCCTACTGATGTTCATCATCT GAG C G T AT T GGTTATAAGTGAACGATACCGTCCGTTCTTTCCTTGTAGGGTTTTCAATCGTGGGGTTGAGT AG T G C C AC AC AG C AT AAAAT TAG CTTGGTTT CAT G C T C C G T T AAG T CAT AG C GAC TAAT C G C TAG T T CAT T T GC T T T GAAAACAAC TAAT T CAGACAT ACAT C T CAAT T GG T C T AGG T GAT T T T AAT C AC TAT AC CAAT T GAGAT G G G C T AG T CAAT GATAATTACTAGTCCTTTTCCTTT GAG T T GTGGGTATCTGTAAATTCTGCTAGACCTTTGCTGGAAAACTTGTAAATTCTGCTAGACCCTC TGTAAATTCCGCTAGACCTTTGTGTGTTTTTTTTGTTTATATTCAAGTGGTTATAATTTATA GAAT AAAGAAAGAAT AAAAAAAGAT AAAAAGAAT AGAT C C C AG C C C T G T G TAT AAC T C AC T A CTTTAGTCAGTTCCGCAGTATTACAAAAGGATGTCGCAAACGCTGTTTGCTCCTCTACAAAA CAGACCTTAAAACCCTAAAGGCTTAAGTAGCACCCTCGCAAGCTCGGGCAAATCGCTGAATA TTCCTTTTGTCTCCGACCATCAGGCACCTGAGTCGCTGTCTTTTTCGTGACATTCAGTTCGC TGCGCTCACGGCTCTGGCAGTGAATGGGGGTAAATGGCACTACAGGCGCCTTTTATGGATTC ATGCAAGGAAACTACCCATAATACAAGAAAAGCCCGTCACGGGCTTCTCAGGGCGTTTTATG
GCGGGTCTGCTATGTGGTGCTATCTGACTTTTTGCTGTTCAGCAGTTCCTGCCCTCTGATTT TCCAGTCTGACCACTTCGGATTATCCCGTGACAGGTCATTCAGACTGGCTAATGCACCCAGT AAGGCAGCGGTATCATCAACAGGCTTACCCGTCTTACTGTCTTTTCTACGGGGTCTGACGCT CAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCAC CTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTT GGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGATCTGTCTATTTCGT TCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATC TGGCCCCAGTGCTGCAATGATACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAA TAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATC CAGTCTATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAA CGTTGTTGCCATTGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCA GCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTT AGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGT TATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCGTAAGATGCTTTTCTGTGACTG GTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCG GCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGTGCTCATCATTGGAAA ACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCGATGTAAC CCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAGCA AAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACT CATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGAT ACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAA GTGCCACCTGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTAT CACGAGGCCCTTTCGTCTCGCGCGTTTCGGTGATGACGGTGAAAACCTCTGACACATGCAGC TCCCGGAGACGGTCACAGCTTGTCTGTAAGCGGATGCCGGGAGCAGACAAGCCCGTCAGGGC GCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCTTAACTATGCGGCATCAGAGCAGATTGT ACTGAGAGTGCACCATATGCGGTGTGAAATACCGCACAGATGCGTAAGGAGAAAATACCGCA TCAGGCGCCATTCGCCATTCAGGCTGCGCAACTGTTGGGAAGGGCGATCGGTGCGGGCCTCT TCGCTATTACGCCAGCTGGCGAAAGGGGGATGTGCTGCAAGGCGATTAAGTTGGGTAACGCC AGGGTTTTCCCAGTCACGACGTT
Table 11 Nucleic acid sequence of pSC101 plasmid (SEQ ID NO: 34)
ATTAAGTTGGGTAACGCCAGGGTTTTCCCAGTCACGACGTTATTGCGTTGCGCTCACTG CCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGAATCGGCCAACGCGC GGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTCGCTGC GCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAGTACGGGTT TTGCTGCCCGCAAACGGGCTGTTCTGGTGTTGCTAGTTTGTTATCAGAATCGCAGATCC GGCTTCAGGTTTGCCGGCTGAAAGCGCTATTTCTTCCAGAATTGCCATGATTTTTTCCC CACGGGAGGCGTCACTGGCTCCCGTGTTGTCGGCAGCTTTGATTCGATAAGCAGCATCG CCTGTTTCAGGCTGTCTATGTGTGACTGTTGAGCTGTAACAAGTTGTCTCAGGTGTTCA ATTTCATGTTCTAGTTGCTTTGTTTTACTGGTTTCACCTGTTCTATTAGGTGTTACATG CTGTTCATCTGTTACATTGTCGATCTGTTCATGGTGAACAGCTTTAAATGCACCAAAAA CTCGTAAAAGCTCTGATGTATCTATCTTTTTTACACCGTTTTCATCTGTGCATATGGAC AGTTTTCCCTTTGATATCTAACGGTGAACAGTTGTTCTACTTTTGTTTGTTAGTCTTGA TGCTTCACTGATAGATACAAGAGCCATAAGAACCTCAGATCCTTCCGTATTTAGCCAGT ATGTTCTCTAGTGTGGTTCGTTGTTTTTGCGTGAGCCATGAGAACGAACCATTGAGATC ATGCTTACTTTGCATGTCACTCAAAAATTTTGCCTCAAAACTGGTGAGCTGAATTTTTG CAGTTAAAGCATCGTGTAGTGTTTTTCTTAGTCCGTTACGTAGGTAGGAATCTGATGTA
ATGGTTGTTGGTATTTTGTCACCATTCATTTTTATCTGGTTGTTCTCAAGTTCGGTTAC GAGATCCATTTGTCTATCTAGTTCAACTTGGAAAATCAACGTATCAGTCGGGCGGCCTC GCTTATCAACCACCAATTTCATATTGCTGTAAGTGTTTAAATCTT TACT TAT TGGTTTC AAAAC C C AT T G G T T AAG C C T T T T AAAC TCATGGTAGTTATTTT C AAG CAT T AAC AT GAA CTTAAATTCATCAAGGCTAATCTCTATATTTGCCTTGTGAGTTTTCTTTTGTGTTAGTT C T T T T AAT AAC C AC T CAT AAAT C C T C AT AGAG TATTTGTTTT C AAAAGAC T T AAC AT G T TCCAGAT TAT AT T T TAT GAAT T T T T T TAAC T G GAAAAGAT AAG G C AAT AT C T C T T CAC T AAAAACTAATTCTAATTTTTCGCTTGAGAACTTGGCATAGTTTGTCCACTGGAAAATCT CAAAGCCTTTAACCAAAGGATTCCTGATTTCCACAGTTCTCGTCATCAGCTCTCTGGTT G C T T T AG C T AAT AC AC CAT AAG CATTTTCCCTACTGATGTTCATCATCT GAG C G T AT T G GTTATAAGTGAACGATACCGTCCGTTCTTTCCTTGTAGGGTTTTCAATCGTGGGGTTGA G TAG T G C CAC AC AG C AT AAAAT TAG CTTGGTTT CAT G C T C C G T T AAG T CAT AG C GAC T A AT C GC T AG T T CAT T T GC T T T GAAAACAAC T AAT T CAGACAT ACAT C T CAAT T GG T C TAG GTGATTTTAAT CAC TAT AC CAAT T GAGAT G G G C T AG T CAAT GAT AAT T AC TAG T C C T T T TCCTTTGAGTTGTGGGTATCTGTAAATTCTGCTAGACCTTTGCTGGAAAACTTGTAAAT TCTGCTAGACCCTCTGTAAATTCCGCTAGACCTTTGTGTGTTTTTTTTGTTTATATTCA AGTGGTTATAATT TAT AGAAT AAAGAAAGAAT AAAAAAAGAT AAAAAGAAT AGAT C C C A GCCCTGTG TAT AAC T CAC T AC T T T AG T C AG T T C C G C AG TAT T AC AAAAG GAT G T C G C AA ACGCTGTTTGCTCCTCTACAAAACAGACCTTAAAACCCTAAAGGCTTAAGTAGCACCCT CGCAAGCTCGGGCAAATCGCTGAATATTCCTTTTGTCTCCGACCATCAGGCACCTGAGT CGCTGTCTTTTTCGTGACATTCAGTTCGCTGCGCTCACGGCTCTGGCAGTGAATGGGGG T AAAT G G CAC T AC AG GCGCCTTTTATG GAT T CAT G C AAG GAAAC T AC C CAT AAT AC AAG AAAAGCCCGTCACGGGCTTCTCAGGGCGTTTTATGGCGGGTCTGCTATGTGGTGCTATC TGACTTTTTGCTGTTCAGCAGTTCCTGCCCTCTGATTTTCCAGTCTGACCACTTCGGAT TATCCCGT GAC AG G T CAT T C AGAC T G G C T AAT G CAC C C AG T AAG G C AG C G G T AT CAT C A ACAGGCTTACCCGTCTTACTGTCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAAC T CAC G T T AAG G GAT T T T G G T CAT GAGAT TAT CAAAAAG GAT C T T CAC C T AGAT C C T T T T AAAT T AAAAAT GAAG T T T T AAAT CAAT C T AAAG TAT AT AT GAG T AAAC T T G G T C T GAC A GTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGATCTGTCTATTTCGTTCATCC ATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATCTGG CCCCAGTGCTGCAATGATACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAA TAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCC ATCCAGTCTATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTT GCGCAACGTTGTTGCCATTGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGG CTTCATTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGC AAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGT GTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCGTAA GATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGG CGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAAC TTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTAC CGCTGTTGAGATCCAGTTCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCT TTTACTTTCACCAGCGTTTCTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGCAAAAAA G G GAAT AAG G G C GAC AC G GAAAT G T T GAAT AC T CAT AC TCTTCCTTTTT CAAT AT TAT T GAAG CAT T TAT CAGGGT TAT T GT C T CAT GAG CG GAT AC AT AT T T GAAT GTAT T TAGAAA AATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTAAGA AAC CATTATTATCAT GAC AT TAAC C TAT AAAAAT AG G C G T AT CAC GAG GCCCTTTCGTC TCGCGCGTTTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTC ACAGCTTGTCTGTAAGCGGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGG TGTTGGCGGGTGTCGGGGCTGGCTTAACTATGCGGCATCAGAGCAGATTGTACTGAGAG T G CAC CAT AT G C G G T G T GAAAT AC C G CAC AGAT G C G T AAG GAGAAAAT AC C G CAT C AG G
CGCCATTCGCCATTCAGGCTGCGCAACTGTTGGGAAGGGCGATCGGTGCGGGCCTCTTC GCTATTACGCCAGCTGGCGAAAGGGGGATGTGCTGCAAGGCG
Table 12
Nucleotide sequence of exemplary fnrS promoter-driven
fbr
argA pSC101 plasmid (SEQ ID NO: 35)
ggtaccAGTTGTTCTTATTGGTGGTGTTGCTTTATGGTTGCATCGTAGTAAATGGTTGT AACAAAAGCAATTTTTCCGGCTGTCTGTATACAAAAACGCCGCAAAGTTTGAGCGAAGT CAATAAACTCTCTACCCATTCAGGGCAATATCTCTCTTqgatccaaagtgaactctaga aalaa
AGTTGGTCGAGGGATTCCGCCATTCGGTTCCCTGTATCAATACCCACCGGGGAAAAAC
GTTTGTCATCATGCTCGGCGGTGAAGCCATTGAGCATGAGAATTTCTCCAGTATCGTT
AATGATATCGGGTTGTTGCACAGCCTCGGCATCCGTCTGGTGGTGGTCTATGGCGCAC
GTCCGCAGATCGACGCAAATCTGGCTGCGCATCACCACGAACCGCTGTATCACAAGAA
TATACGTGTGACCGACGCCAAAACACTGGAACTGGTGAAGCAGGCTGCGGGAACATTG
CAACTGGATATTACTGCTCGCCTGTCGATGAGTCTCAATAACACGCCGCTGCAGGGCG
CGCATATCAACGTCGTCAGTGGCAATTTTATTATTGCCCAGCCGCTGGGCGTCGATGA
CGGCGTGGATTACTGCCATAGCGGGCGTATCCGGCGGATTGATGAAGACGCGATCCAT
CGTCAACTGGACAGCGGTGCAATAGTGCTAATGGGGCCGGTCGCTGTTTCAGTCACTG
GCGAGAGCTTTAACCTGACCTCGGAAGAGATTGCCACTCAACTGGCCATCAAACTGAA
AGCTGAAAAGATGATTGGTTTTTGCTCTTCCCAGGGCGTCACTAATGACGACGGTGAT
ATTGTCTCCGAACTTTTCCCTAACGAAGCGCAAGCGCGGGTAGAAGCCCAGGAAGAGA
AAGGCGATTACAACTCCGGTACGGTGCGCTTTTTGCGTGGCGCAGTGAAAGCCTGCCG
CAGCGGCGTGCGTCGCTGTCATTTAATCAGTTATCAGGAAGATGGCGCGCTGTTGCAA
GAGTTGTTCTCACGCGACGGTATCGGTACGCAGATTGTGATGGAAAGCGCCGAGCAGA
TTCGTCGCGCAACAATCAACGATATTGGCGGTATTCTGGAGTTGATTCGCCCACTGGA
GCAGCAAGGTATTCTGGTACGCCGTTCTCGCGAGCAGCTGGAGATGGAAATCGACAAA
TTCACCATTATTCAGCGCGATAACACGACTATTGCCTGCGCCGCGCTCTATCCGTTCC
CGGAAGAGAAGATTGGGGAAATGGCCTGTGTGGCAGTTCACCCGGATTACCGCAGTTC
ATCAAGGGGTGAAGTTCTGCTGGAACGCATTGCCGCTCAGGCTAAGCAGAGCGGCTTA
AGCAAATTGTTTGTGCTGACCACGCGCAGTATTCACTGGTTCCAGGAACGTGGATTTA
CCCCAGTGGATATTGATTTACTGCCCGAGAGCAAAAAGCAGTTGTACAACTACCAGCG
TAAATCCAAAGTGTTGATGGCGGATTTAGGGTAAGGAAGTTTGTCTAGATCTCAGGCG
TGGATGGCTTGGCGTAATCATGGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTC ACAATTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATG AGTGAGCTAACTCACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACC TGTCGTGCCAGCTGCATTAATGAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATT GGGCGCTCTTCCGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCG AGCGGTATCAGCTCACTCAAAGGCGGTAGTACGGGTTTTGCTGCCCGCAAACGGGCTGT TCTGGTGTTGCTAGTTTGTTATCAGAATCGCAGATCCGGCTTCAGGTTTGCCGGCTGAA AGCGCTATTTCTTCCAGAATTGCCATGATTTTTTCCCCACGGGAGGCGTCACTGGCTCC CGTGTTGTCGGCAGCTTTGATTCGATAAGCAGCATCGCCTGTTTCAGGCTGTCTATGTG TGACTGTTGAGCTGTAACAAGTTGTCTCAGGTGTTCAATTTCATGTTCTAGTTGCTTTG TTTTACTGGTTTCACCTGTTCTATTAGGTGTTACATGCTGTTCATCTGTTACATTGTCG ATCTGTTCATGGTGAACAGCTTTAAATGCACCAAAAACTCGTAAAAGCTCTGATGTATC
TATCTTTTTTACACCGTTTTCATCTGTGCATATGGACAGTTTTCCCTTTGATATCTAAC GGTGAACAGTTGTTCTACTTTTGTTTGTTAGTCTTGATGCTTCACTGATAGATACAAGA GCCATAAGAACCTCAGATCCTTCCGTATTTAGCCAGTATGTTCTCTAGTGTGGTTCGTT GTTTTTGCGTGAGCCATGAGAACGAACCATTGAGATCATGCTTACTTTGCATGTCACTC AAAAATTTTGCCTCAAAACTGGTGAGCTGAATTTTTGCAGTTAAAGCATCGTGTAGTGT TTTTCTTAGTCCGTTACGTAGGTAGGAATCTGATGTAATGGTTGTTGGTATTTTGTCAC CATTCATTTTTATCTGGTTGTTCTCAAGTTCGGTTACGAGATCCATTTGTCTATCTAGT TCAACTTGGAAAATCAACGTATCAGTCGGGCGGCCTCGCTTATCAACCACCAATTTCAT ATTGCTGTAAGTGTTTAAATCTTTACTTATTGGTTTCAAAACCCATTGGTTAAGCCTTT TAAAC T CAT GGTAGT TAT T T T CAAGCAT TAACAT GAAC T TAAAT T CAT CAAGGC TAAT C TCTATATTTGCCTTGTGAGTTTTCTTTTGTGTTAGTTCTTTTAATAACCACTCATAAAT C C T C AT AGAG TATTTGTTTT C AAAAGAC T TAACAT G T T C C AGAT TATATTTTAT GAAT T T T T T TAAC T GGAAAAGATAAGGCAAT AT C T C T T CAC TAAAAAC TAAT T C TAAT T T T T CG CTTGAGAACTTGGCATAGTTTGTCCACTGGAAAATCTCAAAGCCTTTAACCAAAGGATT CCTGATTTCCACAGTTCTCGTCATCAGCTCTCTGGTTGCTTTAGCTAATACACCATAAG CATTTTCCCTACTGATGTTCATCATCTGAGCGTATTGGTTATAAGTGAACGATACCGTC CGTTCTTTCCTTGTAGGGTTTTCAATCGTGGGGTTGAGTAGTGCCACACAGCATAAAAT TAGCTTGGTTTCATGCTCCGTTAAGTCATAGCGACTAATCGCTAGTTCATTTGCTTTGA AAAC AAC T AAT T C AGAC AT AC AT C T C AAT TGGTCTAGGTGATTTTAAT CAC TAT AC C AA TTGAGATGGGCTAGTCAATGATAATTACTAGTCCTTTTCCTTTGAGTTGTGGGTATCTG TAAAT TCTGCTAGACCTTTGCTGGAAAACTTGTAAATTCTGCTAGACCCTCTGTAAATT CCGCTAGACCTTTGTGTGTTTTTTTTGTTTATATTCAAGTGGTTATAATTTATAGAATA AAGAAAGAAT AAAAAAAGAT AAAAAGAAT AGAT C C C AG C C C T G T G TAT AAC T CAC T AC T TTAGTCAGTTCCGCAGTATTACAAAAGGATGTCGCAAACGCTGTTTGCTCCTCTACAAA ACAGACCTTAAAACCCTAAAGGCTTAAGTAGCACCCTCGCAAGCTCGGGCAAATCGCTG AATATTCCTTTTGTCTCCGACCATCAGGCACCTGAGTCGCTGTCTTTTTCGTGACATTC AGTTCGCTGCGCTCACGGCTCTGGCAGTGAATGGGGGTAAATGGCACTACAGGCGCCTT T T AT G GAT T CAT G C AAG GAAAC T AC C C AT AAT AC AAGAAAAG C C C G T CAC GGGCTTCTC AGGGCGTTTTATGGCGGGTCTGCTATGTGGTGCTATCTGACTTTTTGCTGTTCAGCAGT TCCTGCCCTCTGATTTTCCAGTCTGACCACTTCGGATTATCCCGTGACAGGTCATTCAG ACTGGCTAATGCACCCAGTAAGGCAGCGGTATCATCAACAGGCTTACCCGTCTTACTGT CTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCA T GAGAT TAT CAAAAAG GAT C T T CAC C T AGAT C C T T T TAAAT TAAAAAT GAAG T T T T AAA T C AAT C TAAAG TAT AT AT GAG TAAAC T T G G T C T GAC AG T T AC C AAT G C T T AAT C AG T GA GGCACCTATCTCAGCGATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCG TGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCTGCAATGATACCG C GAGAC C CAC G C T CAC C G G C T C C AGAT T T AT C AG C AAT AAAC C AG C C AG C C G GAAG G G C CGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCTATTAATTGTTGCC GGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATTGCT ACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCA ACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCG GTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCA GCACTGCATAATTCTCTTACTGTCATGCCATCCGTAAGATGCTTTTCTGTGACTGGTGA GTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCGG C G T C AAT AC G G GAT AAT AC C G C G C CAC AT AG C AGAAC T T T AAAAG T G C T CAT CAT T G GA AAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCGAT GTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTG G G T GAG C AAAAAC AG GAAG G C AAAAT G C C G C AAAAAAG G GAAT AAG G G C GAC AC G GAAA TGTTGAATACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTG T C T CAT GAG C G GAT AC AT AT T T GAAT G T AT T T AGAAAAAT AAAC AAAT AG GGGTTCCGC
G C AC AT T T C C C C GAAAAG T G C C AC C T GAC G T C T AAGAAAC CATTATTATCAT GAC AT T A ACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGCGTTTCGGTGATGACGG TGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAAGCGGATG CCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGG C T T AAC T AT G C G G CAT C AGAG C AGAT T G T AC T GAGAG T G C AC CAT AT G C G G T G T GAAAT ACCGCACAGATGCGTAAGGAGAAAATACCGCATCAGGCGCCATTCGCCATTCAGGCTGC GCAACTGTTGGGAAGGGCGATCGGTGCGGGCCTCTTCGCTATTACGCCAGCTGGCGAAA GGGGGATGTGCTGCAAGGCG
[0229] n some embodiments, the genetically engineered bacteria comprise argA^ integrated into the chromosome. In some embodiments, the integrated fbrArgA is under the control of the fnrS promoter. In some
embodiments, an antibiotic resistance cassette is also present at the same site. In some embodiments, no antibiotic resistance cassette is present. In some embodiments, the antibiotic resistance is chloramphenicol. In some
embodiments, the antibiotic resistance is kanamycin. In some embodiments, the genetically engineered bacteria comprising argAmr integrated into the
chromosome is a thyA auxotroph. In some embodiments, the genetically engineered bacteria comprise argAmr integrated into the chromosome and also comprise an ArgR mutation or have ArgR deleted. In one specific embodiment, the genetically engineered bacteria comprise argA mr under the control of the fnrS promoter and integrated into the chromosome, comprise an ArgR mutation or have ArgR deleted, and comprise a thyA auxotrophy. In another specific embodiment, the genetically engineered bacteria comprise argAmr under the control of the fnrS promoter and integrated into the chromosome, comprise an ArgR mutation or have ArgR deleted, comprise a thyA auxotrophy, and comprise an antibiotic resistance cassette. In another specific embodiment, the genetically engineered bacteria comprise argA or under the control of the fnrS promoter and integrated into the chromosome, comprise an ArgR mutation or have ArgR deleted, comprise a thyA auxotrophy, and comprise a kanamycin resistance cassette. In one specific embodiment, the genetically engineered bacteria is SYN-UCD305. In another specific embodiment, the genetically engineered bacteria is SYN_UCD303.
[0230] Table 13 shows non-limiting examples of FNRS-fbrArgA constructs which are integrated into the chromosome.
[0231] SEQ ID NO: 36 comprises FNRS-fbrArgA and chloramphenicol resistance, e.g., as comprised in SYN-UCD301 , SYN-UCD302. SEQ ID NO: 37 comprises FNRS-fbrArgA and kanamycin resistance, e.g., as comprised inSYN- UCD303, SYN-UCD306, SYN-UCD307, and SYN-UCD309. SEQ ID NO: 38 FNRS-fbrArgA and no antibiotic resistance, e.g., as comprised in SYN-UCD305 SYN-UCD304, SYN-UCD308, SYNUCD310.
Table 13 of Integrated argA fbr sequences
TGATCTTCCGTCACAGGTAGGCGCGCCGAAGTTCCTATA
CTTTCTAGAGAATAGGAACTTCGGAATAGGAACTAAGGA
GGATATTCATATGGACCATGGCTAATTCCCAGGTACCAG TTGTTCTTATTGGTGGTGTTGCTTTATGGTTGCATCGTA
GTAAATGGTTGTAACAAAAGCAATTTTTCCGGCTGTCTG
TATACAAAAACGCCGTAAAGTTTGAGCGAAGTCAATAAA
CTCTCTACCCATTCAGGGCAATATCTCTCTTGGATCCaa
agtgaactctagaaataattttgtttaactttaagaagg agatatacatATGGTAAAGGAACGTAAAACCGAGTTGGT
CGAGGGATTCCGCCATTCGGTTCCCTGTATCAATACCCA CCGGGGAAAAACGTTTGTCATCATGCTCGGCGGTGAAGC CATTGAGCATGAGAATTTCTCCAGTATCGTTAATGATAT CGGGTTGTTGCACAGCCTCGGCATCCGTCTGGTGGTGGT CTATGGCGCACGTCCGCAGATCGACGCAAATCTGGCTGC GCATCACCACGAACCGCTGTATCACAAGAATATACGTGT GACCGACGCCAAAACACTGGAACTGGTGAAGCAGGCTGC GGGAACATTGCAACTGGATATTACTGCTCGCCTGTCGAT GAGTCTCAATAACACGCCGCTGCAGGGCGCGCATATCAA CGTCGTCAGTGGCAATTTTATTATTGCCCAGCCGCTGGG CGTCGATGACGGCGTGGATTACTGCCATAGCGGGCGTAT CCGGCGGATTGATGAAGACGCGATCCATCGTCAACTGGA CAGCGGTGCAATAGTGCTAATGGGGCCGGTCGCTGTTTC AGTCACTGGCGAGAGCTTTAACCTGACCTCGGAAGAGAT TGCCACTCAACTGGCCATCAAACTGAAAGCTGAAAAGAT GATTGGTTTTTGCTCTTCCCAGGGCGTCACTAATGACGA CGGTGATATTGTCTCCGAACTTTTCCCTAACGAAGCGCA AGCGCGGGTAGAAGCCCAGGAAGAGAAAGGCGATTACAA CTCCGGTACGGTGCGCTTTTTGCGTGGCGCAGTGAAAGC CTGCCGCAGCGGCGTGCGTCGCTGTCATTTAATCAGTTA TCAGGAAGATGGCGCGCTGTTGCAAGAGTTGTTCTCACG CGACGGTATCGGTACGCAGATTGTGATGGAAAGCGCCGA GCAGATTCGTCGCGCAACAATCAACGATATTGGCGGTAT TCTGGAGTTGATTCGCCCACTGGAGCAGCAAGGTATTCT GGTACGCCGTTCTCGCGAGCAGCTGGAGATGGAAATCGA CAAATTCACCATTATTCAGCGCGATAACACGACTATTGC CTGCGCCGCGCTCTATCCGTTCCCGGAAGAGAAGATTGG GGAAATGGCCTGTGTGGCAGTTCACCCGGATTACCGCAG TTCATCAAGGGGTGAAGTTCTGCTGGAACGCATTGCCGC TCAGGCTAAGCAGAGCGGCTTAAGCAAATTGTTTGTGCT GACCACGCGCAGTATTCACTGGTTCCAGGAACGTGGATT TACCCCAGTGGATATTGATTTACTGCCCGAGAGCAAAAA GCAGTTGTACAACTACCAGCGTAAATCCAAAGTGTTGAT GGCGGATTTAGGGTAAgtcgacgcatgcatcgataagcc gcgttctcatcctcccgcctcctcccccataaaaaagcc agggggtggaggatttaagccatctcctgatgac
FNRS-fbrArgA ctacgccccatcgttgctttgtgtgatctctgttacaga SEQ ID and attggcggtaatgtggagatgcgcacataaaatcgccat NO: 37 kanamycin gatttttgcaagcaacatcacgaaattccttacatgacc resistance, tcggtttagttcacaggacgtcccatggctcgagCATGC
e.g., SYN- GAGAGTAGGGAACTGCCAGGCATCAAATAAAATGAAAGG UCD303, SYN- CTCAGTCGAAAGACTGGGCCTTTCGTTTTATCTGTTGTT UCD306, SYN- TGTCGGTGAACGCTCTCCTGAGTAGGACAAATCCGCCGG UCD307, and GAGCGGATTTGAACGTTGCGAAGCAACGGCCCGGAGGGT SYN-UCD309; GGCGGGCAGGACGCCCGCCATAAACTGCCAGGCATCAAA a schematic TTAAGCAGAAGGCCATCCTGACGGATGGCCTTTTTGCGT of the GGCCAGTGCCAAGCTTGCATGCAGATTGCAGCATTACAC construct is GTCTTGAGCGATTGTGTAGGCTGGAGCTGCTTCGAAGTT depicted in CCTATACTTTCTAGAGAATAGGAACTTCGGAATAGGAAC Fig. 21 TTCaagatcccctcacgctgccgcaagcactcagggcgc
Lowercase aagggctgctaaaggaagcggaacacgtagaaagccagt underlined : ccgcagaaacggtgctgaccccggatgaatgtcagctac KanR tgggctatctggacaagggaaaacgcaagcgcaaagaga promoter; aagcaggtagcttgcagtgggcttacatggcgatagcta lowercase gactgggcggttttatggacagcaagcgaaccggaattg italic: KanR ccagctggggcgccctctggtaaggttgggaagccctgc gene ; aaagtaaactggatggctttcttgccgccaaggatctga UPPERCASE tggcgcaggggatcaagatctgatcaagagacaggatga underlined : ggatcgtttcgcatgattgaacaagatggattgcacgca fnrS ggttctccggccgcttgggtggagaggctattcggctat promoter; gactgggcacaacagacaatcggctgctctgatgccgcc
UPPERCASE gtgttccggctgtcagcgcaggggcgcccggttcttttt bold: gtcaagaccgacctgtccggtgccctgaatgaactgcag
ArgAfbr gacgaggcagcgcggctatcgtggctggccacgacgggc
UPPERCASE gttccttgcgcagctgtgctcgacgttgtcactgaagcg bold ggaagggactggctgctattgggcgaagtgccggggcag underline : gatctcctgtcatctcaccttgctcctgccgagaaagta terminator tcca teatggctgatgcaatgcggcggctgcatacgctt sequence ; gatccggctacctgcccattcgaccaccaagcgaaacat UPPERCASE cgcatcgagcgagcacgtactcggatggaagccggtctt italic bold: gtcgatcaggatgatctggacgaagagcatcaggggctc frt sites gcgccagccgaactgttcgccaggctcaaggcgcgcatg cccgacggcgaggatctcgtcgtgacccatggcgatgcc tgcttgccgaatatcatggtggaaaatggccgcttttct ggattcatcgactgtggccggctgggtgtggcggaccgc tatcaggacatagcgttggctacccgtgatattgctgaa gagcttggcggcgaatgggctgaccgcttcctcgtgctt tacggtatcgccgctcccgattcgcagcgcatcgccttc tatcgccttcttgacgagttcttctgaqcqqqactctgg ggttcgaaatgaccgaccaagcgacgcccaacctgccat cacgagatttcgattccaccgccgccttctatgaaaggt tgggcttcggaatcgttttccgggacgccggctggatga tcctccagcgcggggatctcatgctggagttcttcgccc accccagcttcaaaagcgctctGAAGTTCCTATACTTTC TAGAGAATAGGAACTTCGGAATAGGAACTAAGGAGGATA TTCATATGGACCATGGCTAATTCCCAGATATGGTACCAG TTGTTCTTATTGGTGGTGTTGCTTTATGGTTGCATCGTA GTAAATGGTTGTAACAAAAGCAATTTTTCCGGCTGTCTG TATACAAAAACGCCGTAAAGTTTGAGCGAAGTCAATAAA CTCTCTACCCATTCAGGGCAATATCTCTCTTGGATCCaa
agtgaactctagaaataattttgtttaactttaagaagg
agatatacatATGGTAAAGGAACGTAAAACCGAGTTGGT
CGAGGGATTCCGCCATTCGGTTCCCTGTATCAATACCCA CCGGGGAAAAACGTTTGTCATCATGCTCGGCGGTGAAGC CATTGAGCATGAGAATTTCTCCAGTATCGTTAATGATAT CGGGTTGTTGCACAGCCTCGGCATCCGTCTGGTGGTGGT CTATGGCGCACGTCCGCAGATCGACGCAAATCTGGCTGC GCATCACCACGAACCGCTGTATCACAAGAATATACGTGT GACCGACGCCAAAACACTGGAACTGGTGAAGCAGGCTGC GGGAACATTGCAACTGGATATTACTGCTCGCCTGTCGAT GAGTCTCAATAACACGCCGCTGCAGGGCGCGCATATCAA CGTCGTCAGTGGCAATTTTATTATTGCCCAGCCGCTGGG CGTCGATGACGGCGTGGATTACTGCCATAGCGGGCGTAT CCGGCGGATTGATGAAGACGCGATCCATCGTCAACTGGA CAGCGGTGCAATAGTGCTAATGGGGCCGGTCGCTGTTTC AGTCACTGGCGAGAGCTTTAACCTGACCTCGGAAGAGAT TGCCACTCAACTGGCCATCAAACTGAAAGCTGAAAAGAT GATTGGTTTTTGCTCTTCCCAGGGCGTCACTAATGACGA CGGTGATATTGTCTCCGAACTTTTCCCTAACGAAGCGCA AGCGCGGGTAGAAGCCCAGGAAGAGAAAGGCGATTACAA CTCCGGTACGGTGCGCTTTTTGCGTGGCGCAGTGAAAGC CTGCCGCAGCGGCGTGCGTCGCTGTCATTTAATCAGTTA TCAGGAAGATGGCGCGCTGTTGCAAGAGTTGTTCTCACG CGACGGTATCGGTACGCAGATTGTGATGGAAAGCGCCGA GCAGATTCGTCGCGCAACAATCAACGATATTGGCGGTAT TCTGGAGTTGATTCGCCCACTGGAGCAGCAAGGTATTCT GGTACGCCGTTCTCGCGAGCAGCTGGAGATGGAAATCGA CAAATTCACCATTATTCAGCGCGATAACACGACTATTGC CTGCGCCGCGCTCTATCCGTTCCCGGAAGAGAAGATTGG GGAAATGGCCTGTGTGGCAGTTCACCCGGATTACCGCAG TTCATCAAGGGGTGAAGTTCTGCTGGAACGCATTGCCGC TCAGGCTAAGCAGAGCGGCTTAAGCAAATTGTTTGTGCT GACCACGCGCAGTATTCACTGGTTCCAGGAACGTGGATT TACCCCAGTGGATATTGATTTACTGCCCGAGAGCAAAAA GCAGTTGTACAACTACCAGCGTAAATCCAAAGTGTTGAT GGCGGATTTAGGGTAAgtcgacgcatgcatcgataagcc gcgttctcatcctcccgcctcctcccccataaaaaagcc agggggtggaggatttaagccatctcctgatgac
FNRS-fbrArgA ctacgccccatcgttgctttgtgtgatctctgttacaga SEQID and no attggcggtaatgtggagatgcgcacataaaatcgccat NO: 38 antibiotic gatttttgcaagcaacatcacgaaattccttacatgacc resistance, tcggtttagttcacaggacgtcccatggctcgagCATGC e.g., SYN- GAGAGTAGGGAACTGCCAGGCATCAAATAAAATGAAAGG
UCD305 SYN- CTCAGTCGAAAGACTGGGCCTTTCGTTTTATCT
UCD304, SYN- GTTGTTTGTCGGTGAACGCTCTCCTGAGTAGGACAAATC
UCD308, CGCCGGGAGCGGATTTGAACGTTGCGAAGCAACGGCCCG
SYNUCD310; a GAGGGTGGCGGGCAGGACGCCCGCCATAAACTGCCAGGC schematic of ATCAAATTAAGCAGAAGGCCATCCTGACGGATGGCCTTT the TTGCGTGGCCAGTGCCAAGCTTGCATGCAGATTGCAGCA
construct is TTACACGTCTTGAGCGATTGTGTAGGCTGGAGCTGCTTC depicted in GAAGTTCCTATACTTTCTAGAGAATAGGAACTTCGGAAT Fig. 22; AGGAACTAAGGAGGATATTCATATGGACCATGGCTAATT
UPPERCASE CCCAGGTACCAGTTGTTCTTATTGGTGGTGTTGCTTTAT
underline : GGTTGCATCGTAGTAAATGGTTGTAACAAAAGCAATTTT fnrS TCCGGCTGTCTGTATACAAAAACGCCGTAAAGTTTGAGC promoter; GAAGTCAATAAACTCTCTACCCATTCAGGGCAATATCTC
UPPERCASE TCTTGGATCCaaagtgaactctagaaataattttgttta bold: actttaagaaggagatatacatATGGTAAAGGAACGTAA
ArgAfbr ; AACCGAGTTGGTCGAGGGATTCCGCCATTCGGTTCCCTG
UPPERCASE TATCAATACCCACCGGGGAAAAACGTTTGTCATCATGCT
bold CGGCGGTGAAGCCATTGAGCATGAGAATTTCTCCAGTAT
underline : CGTTAATGATATCGGGTTGTTGCACAGCCTCGGCATCCG terminator TCTGGTGGTGGTCTATGGCGCACGTCCGCAGATCGACGC sequence ; AAATCTGGCTGCGCATCACCACGAACCGCTGTATCACAA
UPPERCASE GAATATACGTGTGACCGACGCCAAAACACTGGAACTGGT
italic bold: GAAGCAGGCTGCGGGAACATTGCAACTGGATATTACTGC frt sites TCGCCTGTCGATGAGTCTCAATAACACGCCGCTGCAGGG
CGCGCATATCAACGTCGTCAGTGGCAATTTTATTATTGC CCAGCCGCTGGGCGTCGATGACGGCGTGGATTACTGCCA TAGCGGGCGTATCCGGCGGATTGATGAAGACGCGATCCA TCGTCAACTGGACAGCGGTGCAATAGTGCTAATGGGGCC GGTCGCTGTTTCAGTCACTGGCGAGAGCTTTAACCTGAC CTCGGAAGAGATTGCCACTCAACTGGCCATCAAACTGAA AGCTGAAAAGATGATTGGTTTTTGCTCTTCCCAGGGCGT CACTAATGACGACGGTGATATTGTCTCCGAACTTTTCCC TAACGAAGCGCAAGCGCGGGTAGAAGCCCAGGAAGAGAA AGGCGATTACAACTCCGGTACGGTGCGCTTTTTGCGTGG CGCAGTGAAAGCCTGCCGCAGCGGCGTGCGTCGCTGTCA TTTAATCAGTTATCAGGAAGATGGCGCGCTGTTGCAAGA GTTGTTCTCACGCGACGGTATCGGTACGCAGATTGTGAT GGAAAGCGCCGAGCAGATTCGTCGCGCAACAATCAACGA TATTGGCGGTATTCTGGAGTTGATTCGCCCACTGGAGCA GCAAGGTATTCTGGTACGCCGTTCTCGCGAGCAGCTGGA GATGGAAATCGACAAATTCACCATTATTCAGCGCGATAA CACGACTATTGCCTGCGCCGCGCTCTATCCGTTCCCGGA AGAGAAGATTGGGGAAATGGCCTGTGTGGCAGTTCACCC GGATTACCGCAGTTCATCAAGGGGTGAAGTTCTGCTGGA ACGCATTGCCGCTCAGGCTAAGCAGAGCGGCTTAAGCAA ATTGTTTGTGCTGACCACGCGCAGTATTCACTGGTTCCA GGAACGTGGATTTACCCCAGTGGATATTGATTTACTGCC CGAGAGCAAAAAGCAGTTGTACAACTACCAGCGTAAATC CAAAGTGTTGATGGCGGATTTAGGGTAAgtcgacgcatg catcgataagccgcgttctcatcctcccgcctcctcccc cataaaaaagccagggggtggaggatttaagccatctcc tgatgac
[0232] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 36 or a functional fragment
thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 36 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 36 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 37 or a functional fragment thereof.
[0233] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 37 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 37 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 37 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 37 or a functional fragment thereof.
[0234] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 38 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 38 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 38 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 38 or a functional fragment thereof.
-Ill-
Arginine Catabolism
[0235]An important consideration in practicing the invention is to ensure that ammonia is not overproduced as a byproduct of arginine and/or citrulline catabolism. In the final enzymatic step of the urea cycle, arginase catalyzes the hydrolytic cleavage of arginine into ornithine and urea (Cunin et al., 1986). Urease, which may be produced by gut bacteria, catalyzes the cleavage of urea into carbon dioxide and ammonia (Summerskill, 1966; Aoyagi et al., 1966; Cunin et al., 1986). Thus, urease activity may generate ammonia that can be toxic for human tissue (Konieczna et al., 2012). In some bacteria, including E. coli Nissle, the gene arcD encodes an arginine/ornithine antiporter, which may also liberate ammonia (Vander Wauven et al., 1984; Gamper et al., 1991 ; Meng et al., 1992).
[0236] AstA is an enzyme involved in the conversion of arginine to succinate, which liberates ammonia. SpeA is an enzyme involved in the conversion of arginine to agmatine, which can be further catabolized to produce ammonia. Thus, in some instances, it may be advantageous to prevent the breakdown of arginine. In some embodiments, the genetically engineered bacteria comprising a mutant arginine regulon additionally includes mutations that reduce or eliminate arginine catabolism, thereby reducing or eliminating further ammonia production. In some embodiments, the genetically engineered bacteria also comprise mutations that reduce or eliminate ArcD activity. In certain embodiments, ArcD is deleted. In some embodiments, the genetically engineered bacteria also comprise mutations that reduce or eliminate AstA activity. In certain embodiments, AstA is deleted. In some embodiments, the genetically engineered bacteria also comprise mutations that reduce or eliminate SpeA activity. In certain embodiments, SpeA is deleted. In some embodiments, the genetically engineered bacteria also comprise mutations that reduce or eliminate arginase activity. In certain embodiments, arginase is deleted. In some embodiments, the genetically engineered bacteria also comprise mutations that reduce or eliminate urease activity. In certain embodiments, urease is deleted. In some embodiments, one or more other genes involved in arginine catabolism are mutated or deleted.
Other Hyperammonemia Disorders
[0237] Hepatic encephalopathy (HE) is characterized by neurocognitive changes in patients and biochemical derangements have been implicated in pathogenesis. Specifically, elevated ammonia levels are suspected to partly contribute to disease pathophysiology. In addition to hyperammonemia, elevated levels of cerebral GABA and manganese levels have been noted and suspected to contribute to clinical presentation.
[0238] In some embodiments, the disclosure provides genetically engineered microorganisms, e.g., bacteria and virus, pharmaceutical compositions thereof, and methods of modulating or treating diseases or disorders associated with hyperammonemia, e.g., hepatic encephalopathy and Huntington's disease. The genetically engineered bacteria are capable of reducing excess ammonia in a mammal. In some embodiments, the genetically engineered bacteria reduce excess ammonia by incorporating excess nitrogen in the body into non-toxic molecules, e.g., arginine, citrulline, methionine, histidine, lysine, asparagine, glutamine, or tryptophan. In some embodiments, the genetically engineered bacteria further comprise one or more circuits (genetic sequence) to reduce the levels of other toxic or deleterious
molecule(s), e.g., GABA, manganese. In some embodiments, the genetically engineered bacteria further comprise one or more circuits to produce a gut barrier enhancer molecule, e.g., a short chain fatty acid such as butyrate, propionate, and acetate. This disclosure also provides compositions and therapeutic methods for reducing excess ammonia and other deleterious molecules, e.g., GABA and manganese. In certain aspects, the disclosure provides genetically engineered bacteria that are capable of reducing excess ammonia and other deleterious molecules. In certain embodiments, the disclosure provides genetically engineered bacteria that are capable of reducing excess ammonia and other deleterious molecules and further producing one or more therapeutic molecules, such as a gut barrier function enhancer molecule, e.g., butyrate. In some embodiments, the disclosure provides genetically engineered bacteria comprising one or more circuits for reducing excess ammonia in which the circuits are under the control of an inducible promoter. In some embodiments, the disclosure provides genetically engineered bacteria
comprising one or more circuits for reducing excess ammonia and one or more circuits for reducing other deleterious molecules in which one or more of the circuits are under the control of an inducible promoter. In some embodiments, the disclosure provides genetically engineered bacteria comprising one or more circuits for reducing excess ammonia and one or more circuits for reducing other deleterious molecules and further producing one or more therapeutic molecules, such as a gut barrier function enhancer molecule, e.g., butyrate in which one or more of the circuits and/or therapeutic molecule(s) are under the control of an inducible promoter. In certain aspects, the compositions and methods disclosed herein may be used for treating a disease or disorder associated with excess ammonia, for example, hepatic encephalopathy or Huntington's disease, and/or one or more symptoms associated with disease or disorder associated with excess ammonia, such as hepatic encephalopathy or Huntington's disease.
GABA Transport and Metabolism
[0239]Y-Aminobutyric acid (GABA) is the predominant inhibitory neurotransmitter in the mammalian central nervous system. In humans, GABA activates the post-synaptic GABAA receptor, which is part of a ligand-gated chloride-specific ion channel complex. Activation of this complex on a postsynaptic neuron allows chloride ions to enter the neuron and exert an inhibitory effect. Alterations of such GABAergic neurotransmission have been implicated in the pathophysiology of several neurological disorders, including epilepsy (Jones-Davis and MacDonald, 2003), Huntington's disease (Krogsgaard- Larsen, 1992), and hepatic encephalopathy (Jones and Basile, 1997).
[0240] Neurons in the brain that are modulated by GABA are said to be under inhibitory GABAergic tone. This inhibitory tone prevents neuronal firing until a sufficiently potent stimulatory stimulus is received, or until the inhibitory tone is otherwise released. Increased GABAergic tone in hepatic
encephalopathy was initially described in the early 1980s, based on a report of similar visual response patterns in rabbits with galactosamine-induced liver failure and rabbits treated with allosteric modulators of the GABAA receptor (e.g., pentobarbital, diazepam) (Jones and Basile, 1997). Clinical
improvements in HE patients treated with a highly selective benzodiazapene
antagonist at the GABAA receptor, flumazenil, further confirmed these observations (Banksy et al., 1985; Scollo-Lavizzari and Steinmann, 1985).
Increased GABAergic tone in HE has since been proposed as a consequence of one or more of the following: (1 ) increased GABA concentrations in the brain, (2) altered integrity of the GABAA receptor, and/or (3) increased concentrations of endogenous modulators of the GABAA receptor (Ahboucha and Butterworth, 2004).
[0241] GABA uptake in E. coli is driven by membrane potential and facilitated by the membrane transport protein, GabP (Li et al., 2001 ). GabP is a member of the amino acid/polymaine/organocation (APC) transporter superfamily, one of the two largest families of secondary active transporters (Jack et al., 2000). GabP protein, encoded by the gabP gene, consists of 466 amino acids and 12 transmembrane alpha-helices, wherein both N- and C- termini face the cytosol (Hu and King, 1998a). The GabP residue sequence also includes a consensus amphipathic region (CAR), which is conserved between members of the APC family from bacteria to mammals (Hu and King, 1998b). Upon entry into the cell, GABA is converted to succinyl semialdehyde (SSA) by GABA a-ketoglutarate transaminase (GSST). Succinate- semialdehyde dehydrogenase (SSDH) then catalyzes the second and only other specific step in GABA catabolism, the oxidation of succinyl semialdehyde to succinate (Dover and Halpern, 1972). Ultimately, succinate becomes a substrate for the citric acid (TCA) cycle.
[0242] In some embodiments, the bacteria are genetically engineered to consume excess ammonia via a metabolic pathway, e.g., an arginine
biosynthesis pathway, a histidine biosynthesis pathway, a methionine
biosynthesis pathway, a lysine biosynthesis pathway, an asparagine
biosynthesis pathway, a glutamine biosynthesis pathway, or a tryptophan biosynthesis pathway as described herein (an "ammonia conversion circuit"). In some embodiments, the genetically engineered bacteria comprise an arginine biosynthesis pathway and are capable of reducing excess ammonia. In some embodiments, the ammonia conversion circuit is under the control of an inducible promoter. In some embodiments, the ammonia conversion circuit is under the control of an an oxygen level-dependent promoter, e.g., an FNR-
inducible promoter. In some embodiments, the ammonia conversion circuit is under the control of a promoter induced by a molecule or metabolite associated with hepatic encephalopathy, e.g., bilirubin, aspartate aminotransferase, alanine aminotransferase, .transferase, hepatitis antigens and antibodies, alpha fetoprotein, anti-mitochondrial, smooth muscle, and anti-nuclear antibodies, iron, transferrin, ferritin, copper, ceruloplasmin, ammonia, or manganese
[0243] In some embodiments, the genetically engineered bacteria comprising an ammonia conversion circuit further comprise one or more circuits for producing one or more GABA membrane transport protein(s), e.g., GabP, and are capable of transporting GABA into the cell (a "GABA transport circuit") (Fig. 41).
[0244] In some embodiments, the genetically engineered bacteria comprising an ammonia conversion circuit further comprise one or more circuits for producing one or more GABA catabolism enzyme(s), e.g., GSST,SSDH, and/or COT (a "GABA metabolic circuit") (Fig. 49). In some embodiments, the genetically engineered bacteria comprising an ammonia conversion circuit further comprise one or more circuits for producing one or more GABA membrane transport protein(s), e.g., GabP, and one or more circuits for producing one or more GABA catabolism enzyme(s), e.g., GSST, SSDH, and/or COT (a "GABA metabolic circuit") (Fig. 41).
[0245] In a more specific aspect, the genetically engineered bacteria comprise an ammonia conversion circuit, a GABA transport circuit, and a GABA metabolic circuit. In some embodiments, the ammonia conversion circuit, GABA transport circuit, and GABA metabolic circuit are under the control of the same promoter. In alternate embodiments, the ammonia conversion circuit, GABA transport circuit, and GABA metabolic circuit are under the control of different promoters. Exemplary promoters include any of the promoters disclosed herein. For example, in some embodiments, the genetically
engineered bacteria of the invention comprise an oxygen level-dependent promoter induced by low-oxygen, microaerobic, or anaerobic conditions. In some embodiments, the genetically engineered bacteria comprise a promoter induced by a molecule or metabolite, for example, a tissue-specific molecule or metabolite or a molecule or metabolite indicative of liver damage. Non-limiting
examples of molecules or metabolites include, e.g., bilirubin, aspartate aminotransferase, alanine aminotransferase, blood coagulation factors II, VII, IX, and X, alkaline phosphatase, gamma glutamyl transferase, hepatitis antigens and antibodies, alpha fetoprotein, anti-mitochondrial, smooth muscle, and anti-nuclear antibodies, iron, transferrin, ferritin, copper, ceruloplasmin, ammonia, and manganese in their blood and intestines. In some embodiments, the genetically engineered bacteria comprise a promoter induced by
inflammation or an inflammatory response, e.g., RNS or ROS promoter. In some embodiments, the genetically engineered bacteria comprise a promoter induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose and tetracycline.
[0246] The amino acid sequence of an exemplary GabP transporter is shown in Table 42. In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 105 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 105 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino acid sequence of SEQ ID NO: 105 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 105 or a functional fragment thereof.
[0247]A non-limiting example of a polynucleotide sequence is shown in Table 43 (SEQ ID NO: 106).
Manganese Transport
[0248] Manganese is a biologically important trace metal and is required for the survival of most living organisms. In mammals, manganese is excreted in the bile, but its disposal is affected by the impaired flow of bile from the liver to the duodenum (i.e., cholestasis) that accompanies liver failure. Similar to ammonia, elevated concentrations of manganese play a role in the
development of hepatic encephalopathy (Rivera-Mancia et al., 2012).
Astrocytes in the brain which detoxify ammonia in a reaction catalyzed by
glutamine synthetase, require manganese as a cofactor and thus have a tendency to accumulate this metal (Aschner et al., 1999). In vitro studies have demonstrated that manganese can result in the inhibition of glutamate transport (Hazell and Norenberg, 1996), abnormalities in astrocyte morphology (Hazell et al., 2006), and increased cell volume (Rama Rao et al., 2007). Manganese and ammonia have also been shown to act synergistically in the pathogenesis of hepatic encephalopathy (Jayakumar et al., 2004).
[0249] Metal ion homeostasis in prokaryotic cells, which lack internal compartmentalization, is maintained by the tight regulation of metal ion flux across in cytoplasmic membrane (Jensen and Jensen, 2014). Manganese uptake in bacteria predominantly involves two major types of transporters:
proton-dependent Nramp-related transporters, and/or ATP-dependent ABC transporters. The Nramp (Natural resistance-associated macrophage protein) transporter family was first described in plants, animals, and yeasts (Cellier et al., 1996), but MntH has since been characterized in several bacterial species (Porcheron et al., 2013). Selectivity of the Nrampl transporter for manganese has been shown in metal accumulation studies, wherein overexpression of Staphylococcus aureus mntH resulted in increased levels of cell-associated manganese, but no accumulation of calcium, copper, iron, magnesium, or zinc (Horsburgh et al., 2002). Additionally, Bacillus subtllls strains comprising a mutation in the mntH gene exhibited impaired growth in metal-free medium that was rescued by the addition of manganese (Que and Helmann, 2000). The amino acid sequence of an exemplary MntH transporter is shown in Table 44. In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 107 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 36 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino acid sequence of SEQ ID NO: 107 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide
as SEQ ID NO: 107 or a functional fragment thereof. A non-limiting example of a polynucleotide sequence is shown in Table 45 (SEQ ID NO: 108).
[0250] High-affinity manganese uptake may also be mediated by ABC (ATP-binding cassette) transporters. Members of this transporter superfamily utilize the hydrolysis of ATP to fuel the import or export of diverse substrates, ranging from ions to macromolecules, and are well characterized for their role in multi-drug resistance in both prokaryotic and eukaryotic cells. Non-limiting examples of bacterial ABC transporters involved in manganese import include MntABCD (Bacilis subtilis, Staphylococcus aureus), SitABCD (Salmonella typhimurium, Shigella flexneri), PsaABCD (Streptococcus pneumoniae), and YfeABCD (Yersinia pestis) (Bearden and Perry, 1999; Kehres et al., 2002; McAllister et al., 2004; Zhou et al., 1999). The MntABCD transporter complex consists of three subunits, wherein MntC and MntD are integral membrane proteins that comprise the permease subunit mediate cation transport, MntB is the ATPase, and MntA binds and delivers manganese to the permease submit. Other ABC transporter operons, such as sitABCD, psaABCD, and yfeABCD, exhibit similar subunit organization and function (Higgins, 1992; Rees et al., 2009).
[0251] In some embodiments, the genetically engineered bacteria comprising an ammonia conversion circuit further comprise one or more circuits for producing a manganese membrane transport protein, e.g., MntH, and are capable of transporting manganese ions into the cell (a "manganese transport circuit") (Fig. 42).
[0252] In some embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a manganese transport circuit, and a GABA metabolic circuit. In some embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a manganese transport circuit, and a GABA transport circuit. In some embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a manganese transport circuit, a GABA transport circuit, and a GABA metabolic circuit. In some embodiments, the circuits are under the control of the same promoter. In alternate embodiments, the circuits are under the control of different promoters. In some embodiments, the genetically engineered bacteria of the invention
comprise an oxygen level-dependent promoter induced by low-oxygen, microaerobic, or anaerobic conditions. In some embodiments, the genetically engineered bacteria comprise a promoter induced by a molecule or metabolite, for example, a tissue-specific molecule or metabolite or a molecule or metabolite indicative of liver damage. Non-limiting examples of molecules or metabolites include, e.g., bilirubin, aspartate aminotransferase, alanine aminotransferase, blood coagulation factors II, VII, IX, and X, alkaline phosphatase, gamma glutamyl transferase, hepatitis antigens and antibodies, alpha fetoprotein, anti-mitochondrial, smooth muscle, and anti-nuclear antibodies, iron, transferrin, ferritin, copper, ceruloplasmin, ammonia, and manganese in their blood and intestines. In some embodiments, the genetically engineered bacteria comprise a promoter induced by inflammation or an inflammatory response, e.g., RNS or ROS promoter. In some embodiments, the genetically engineered bacteria comprise a promoter induced by a metabolite that may or may not be naturally present (e.g., can be exogenously added) in the gut, e.g., arabinose and tetracycline.
Production of Butyrate and Other Gut Barrier Function Enhancer
Molecules
[0253] In some embodiments, the genetically engineered bacteria of the invention further comprise a gene encoding a gut barrier function enhancer molecule, or a gene cassette encoding a biosynthetic pathway capable of producing a gut barrier function enhancer molecule. In some embodiments, the molecule is selected from the group consisting of a short-chain fatty acid, butyrate, propionate, acetate, GLP-2, IL-10, IL-27, TGF-βΙ , TGF-P2, elafin (also known as peptidase inhibitor 3 or SKALP), trefoil factor, melatonin, PGD2, kynurenic acid, and kynurenine.
[0254] In some embodiments, the genetically engineered bacteria of the invention express a gut barrier function enhancer molecule that is encoded by a single gene, e.g., the molecule is elafin and encoded by the PI3 gene. In alternate embodiments, the genetically engineered bacteria of the invention encode a gut barrier function enhancer molecule, e.g., butyrate or propionate, that is synthesized by a biosynthetic pathway requiring multiple genes.
[0255] The gene or gene cassette may be expressed on a high-copy plasmid, a low-copy plasmid, or a chromosome. In some embodiments, expression from the plasmid may be useful for increasing expression of the gut barrier function enhancer molecule. In some embodiments, expression from the chromosome may be useful for increasing stability of expression of the gut barrier function enhancer molecule. In some embodiments, the gene or gene cassette for producing the gut barrier function enhancer molecule is integrated into the bacterial chromosome at one or more integration sites in the genetically engineered bacteria. For example, one or more copies of the butyrate biosynthesis gene cassette may be integrated into the bacterial chromosome. In some embodiments, the gene or gene cassette for producing the gut barrier function enhancer molecule is expressed from a plasmid in the genetically engineered bacteria. In some embodiments, the gene or gene cassette for producing the gut barrier function enhancer molecule is inserted into the bacterial genome at one or more of the following insertion sites in E. coli Nissle: malE/K, araC/BAD, lacZ, thyA, malP/T. Any suitable insertion site may be used {see, e.g., Fig. 18). The insertion site may be anywhere in the genome, e.g., in a gene required for survival and/or growth, such as thyA (to create an auxotroph); in an active area of the genome, such as near the site of genome replication; and/or in between divergent promoters in order to reduce the risk of unintended transcription, such as between AraB and AraC of the arabinose operon.
[0256] In some embodiments, the genetically engineered bacteria of the invention comprise a butyrogenic gene cassette and are capable of producing butyrate. The genetically engineered bacteria may include any suitable set of butyrogenic genes (see, e.g., Table 14). Unmodified bacteria comprising butyrate biosynthesis genes are known and include, but are not limited to, Peptoclostridium, Clostridium, Fusobacterium, Butyrivibrio, Eubacterium, and Treponema, and these endogenous butyrate biosynthesis pathways may be a source of genes for the genetically engineered bacteria of the invention. In some embodiments, the genetically engineered bacteria of the invention comprise butyrate biosynthesis genes from a different species, strain, or substrain of bacteria. In some embodiments, the genetically engineered
bacteria comprise the eight genes of the butyrate biosynthesis pathway from Peptociostridium difficile, e.g., Peptociostridium difficile strain 630: bcd2, etfB3, etfA3, thiA1, hbd, crt2, pbt, and buk (Aboulnaga et al., 2013), and are capable of producing butyrate in low-oxygen conditions, in the presence of HE-specific molecules or metabolites, in the presence of molecules or metabolites associated with liver damage, inflammation or an inflammatory response, or in the presence of some other metabolite such as arabinose. Peptociostridium difficile strain 630 and strain 1296 are both capable of producing butyrate, but comprise different nucleic acid sequences for etfA3, thiA1, hbd, crt2, pbt, and buk. In some embodiments, the genetically engineered bacteria comprise a combination of butyrogenic genes from different species, strains, and/or substrains of bacteria, and are capable of producing butyrate in low-oxygen conditions or in the presence of HE-specific molecules or metabolites. For example, in some embodiments, the genetically engineered bacteria comprise bcd2, etfB3, etfA3, and thiA1 from Peptociostridium difficile strain 630, and hbd, crt2, pbt, and buk from Peptociostridium difficile strain 1296. In some embodiments, the genetically engineered bacteria are capable of expressing the butyrate biosynthesis cassette and producing butyrate in low-oxygen conditions or in the presence of HE-specific molecules or metabolites. The genes may be codon-optimized, and translational and transcriptional elements may be added. Table 14 depicts the nucleic acid sequences of exemplary genes in butyrate biosynthesis gene cassettes. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 39, 40, 41 , 42, 43, 44, 45, 46, 47, or 48 a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 39, 40, 41 , 42, 43, 44, 45, 46, 47, or 48 , or a functional fragment thereof.
Table 14
AT G G G T AAC G T T T T AG T AG T AAT AGAAC AAAGAGAAAAT G T AAT T CAAAC TGTTTCTT TAGAAT TAC TAGGAAAGGC TACAGAAATAGCAAAAGAT TAT G AT AC AAAAG TTTCTGCATTACTTTTAGGTAG T AAG G T AGAAG G T T T AAT A GAT ACAT TAGC AC AC T AT GG T GCAGAT GAGG T AAT AG TAG T AGAT GAT GA AGCTT TAG C AG T G TAT AC AAC T GAAC CAT AT AC AAAAG C AG C T TAT GAAG C AAT AAAAG C AG C T GAC CCTATAGTTGTATTATTTGGTG C AAC T T C AAT A G G T AGAGAT T TAG C G C C T AGAG T T T C T G C TAGAAT AC AT AC AG G T C T TAC T GC T GAC T GTACAGGT C T T GCAGTAGC T GAAGATACAAAAT TAT TAT TAA T GAC AAGAC CTGCCTTTGGTG GAAAT AT AAT G G C AAC AAT AG T T T G T AAA
etfA3 GAT T T CAGAC C T CAAAT G T C TAC AG T TAGAC C AG G G G T T AT GAAGAAAAA (SEQ ID NO: T GAAC C T GAT GAAAC T AAAGAAG C T G T AAT T AAC C G T T T C AAG G TAGAAT 41 ) TTAATGATGCT GAT AAAT T AG T T C AAG T T G TAC AAG T AAT AAAAGAAG C T
AAAAAACAAG T T AAAAT AGAAGAT GC T AAGAT AT TAG T T T C T GC T GGAC G T GGAAT GGGT GGAAAAGAAAAC T TAGACAT AC T T TAT GAAT TAGC T GAAA TTATAGGTGGAGAAGTTTCTGGTTCTCGTGCCACTATAGATGCAGGTTGG T T AGAT AAAG C AAGAC AAG T T G G T CAAAC T G G TAAAAC T G T AAGAC C AGA CC T T TATATAGCAT GT GGTATAT C T G GAG C AAT AC AAC AT AT AG C T GGTA T GGAAGAT GC T GAG T T TAT AG T T GC TAT AAAT AAAAAT C CAGAAGC T C CA AT AT T T AAAT AT GCTGATGTTGGTATAGTTG GAGAT G T T CAT AAAG T G C T T C C AGAAC T T AT C AG T C AG T T AAG T G T T G C AAAAGAAAAAG G T GAAG T T T TAGC TAAC TAA
AT GAGAGAAG TAG T AAT T G C C AG T G C AG C T AGAAC AG C AG TAG GAAG T T T T G GAG GAG CAT T T AAAT C AG T T T C AG C G G T AGAG T TAG G G G TAAC AG C AG C T AAAGAAG C T AT AAAAAGAG C TAAC AT AAC T CCAGATAT GAT AGAT GAA T C T C T T T TAG G G G GAG TAC T TAC AG C AG G T C T T G GAC AAAAT AT AG C AAG ACAAATAGCATTAGGAGCAGGAATACCAGTAGAAAAACCAGCTATGACTA TAAATATAGTTTGTGGTTCTGGATTAAGATCTGTTTCAATGGCATCTCAA C T TATAGCAT TAGGT GAT GC T GAT AT AAT GT TAGT T GGT GGAGC T GAAAA CAT GAG TATGTCTCCTTATTTAGTAC C AAG T G C GAGAT AT G G T G CAAGAA TGGGTGATGCTGCTTTTGTTGATTCAATGATAAAAGATGGATTATCAGAC AT AT T TAATAAC TAT CACAT GGGTAT TAC T GC T GAAAACATAGCAGAGCA AT GGAATATAAC TAGAGAAGAACAAGAT GAAT TAGC T C T T GCAAGT CAAA
thiA1
ATAAAGC T GAAAAAGC T CAAGCT GAAG GAAAAT T T GAT GAAGAAAT AG T T
(SEQ ID NO:
CCTGTTGT TAT AAAAG GAAGAAAAG G T GAC AC T G T AG T AGAT AAAGAT GA
42)
AT AT AT TAAGCC T GGCAC TAC AAT G GAGAAAC T T GC T AAG T T AAGAC C T G CAT T T AAAAAAGAT G GAAC AG T TAC T GC T GGTAAT GCAT C AG GAAT AAAT GAT GGT GC T GC TAT GT TAGTAGTAAT GGC TAAAGAAAAAGC T GAAGAAC T AGGAATAGAGCCTCTTGCAACTATAGTTTCTTATGGAACAGCTGGTGTTG AC C C T AAAAT AAT G G GAT AT G GAC C AG T T C C AG C AAC T AAAAAAG C T T T A GAAG C T G C T AAT AT GAC TAT T GAAGATATAGAT T TAGT T GAAG CT AAT GA GGCAT T T GC T GCCCAAT C T G TAG C T G T AAT AAGAGAC T TAAATATAGATA T GAAT AAAGT TAAT GT TAAT GGT GGAGCAATAGC TAT AGGACAT CCAATA GGAT GC T C AG GAG C AAGAAT AC T TAC TACAC T T T TAT AT GAAAT GAAGAG AAGAGATGCTAAAACTGGTCTTGCTACACTTTGTATAGGCGGTGGAATGG GAAC TAC T T TAAT AG T T AAGAGAT AG
Description Sequence
AT GAAAT TAG C T G T AAT AG G TAG T G GAAC T AT G G GAAG TGGTATTG T AC A AAC T T T T G C AAG T T G T G GAC AT GATGTATGTT TAAAGAG T AGAAC T C AAG G T G C TAT AGAT AAAT GTTTAGCTTTAT T AGAT AAAAAT T T AAC T AAG T T A GT TAC T AAG G GAAAAAT G GAT GAAG C T AC AAAAGC AGAAAT AT TAAGTCA T G T T AG T T C AAC TACTAATTAT GAAGAT T T AAAAGAT AT GGAT T TAATAA TAGAAG C AT C T G T AGAAGAC AT GAAT AT AAAGAAAGAT G T T T T C AAG T T A C TAGAT GAAT T AT G T AAAGAAGAT AC TAT C T T G G C AAC AAAT AC T T CAT C
hbd AT TAT C TAT AAC AGAAAT AG CTTCTTCTAC T AAG C G C C C AGAT AAAG T T A (SEQ ID NO: TAG GAAT G CAT T T C T T T AAT C C AG T T C C TAT GAT GAAAT TAG T T GAAG T T 43) AT AAG T G G T C AG T T AAC AT CAAAAG T T AC T T T T GAT AC AG T AT T T GAAT T
AT C TAAGAG TAT C AAT AAAG TAC C AG TAGAT G T AT C T GAAT CTCCTGGAT T T G T AG T AAAT AGAAT AC TTATACCTAT GAT AAAT GAAG CTGTTGGTATA TAT GCAGAT GGT GT T GCAAGTAAAGAAGAAAT AGAT GAAGC TAT GAAAT T AG GAG C AAAC CAT C C AAT G G GAC C AC TAG CAT TAG G T GAT T T AAT C G GAT TAGAT GTTGTTTTAGCTATAAT GAAC G T T T TAT AT AC T GAAT T T G GAGAT AC T AAAT AT AGAC C T CAT C CAC T T T TAGCTAAAATGGT T AGAGC T AAT CA AT TAGGAAGAAAAAC T AAGAT AG GAT T C TAT GAT TAT AAT AAAT AA
AT GAG TAC AAG T GAT G T T AAAG T T T AT GAGAAT GTAGCTGTT GAAG TAGA T G GAAAT AT AT G TAC AG T GAAAAT GAAT AGAC C T AAAG CCCTTAATG C AA T AAAT T CAAAGAC T T T AGAAGAAC T T T AT GAAG T AT T T G TAGAT AT T AAT AAT GAT GAAAC TAT T GAT GT T GTAATAT T GACAGGG GAAG GAAAGG CAT T T G TAG C T G GAG C AGAT AT T G CAT AC AT GAAAGAT T TAGAT G C T G TAG C T G C TAAAGAT T T TAG TAT C T TAG GAG CAAAAG C T T T T G GAGAAAT AGAAAAT AGTAAAAAAGTAGTGATAGCTGCTGTAAACGGATTTGCTTTAGGTGGAGG
crt2
AT GT GAAC T T G C AAT G G CAT G T GAT AT AAGAAT TGCAT C T GC TAAAGC TA
(SEQ ID NO:
AAT T T G G T C AG C C AGAAG T AAC T C T T G GAAT AAC T C C AG GAT AT G GAG GA
44)
AC T C AAAG G C T TAC AAGAT T G G T T G GAAT G G CAAAAG C AAAAGAAT T AAT C T T TACAGGT CAAGT TAT AAAAGC T GAT GAAGC T GAAAAAATAGGGC TAG T AAAT AGAG T C G T T GAG C C AGAC AT T T T AAT AGAAGAAG T T GAGAAAT T A GC T AAGAT AAT AGC T AAAAAT GC T CAGCT T GCAGT TAGAT AC T C TAAAGA AG C AAT AC AAC TTGGTGCT CAAAC T GAT AT AAAT AC T G GAAT AGAT AT AG AAT C T AAT T T AT T T G G T C T T T G T T T T T C AAC T AAAGAC C AAAAAGAAG GA AT G T CAGCT T T C G T T GAAAAGAGAGAAGC T AAC T T T AT AAAAGGG T AA
AT GAGAAG T T T T GAAGAAG T AAT T AAG T T T G C AAAAGAAAGAG GAC C T AA AAC TAT AT C AG TAGCATGTTGC C AAGAT AAAGAAG TTTTAATGG C AG T T G AAAT GGC TAGAAAAGAAAAAATAGCAAAT GCCAT T T TAG TAGGAGAT ATA GAAAAGAC TAAAGAAAT T GCAAAAAGCATAGACAT GGAT AT C GAAAAT TA T GAAC T GAT AGAT AT AAAAGAT T TAGCAGAAGCAT C T C TAAAAT C T GT T G AAT TAG T T T CAC AAG GAAAAG C C GAC AT G G T AAT GAAAG G C T TAG T AGAC
pbt AC AT C AAT AAT AC TAAAAG C AG T T T T AAAT AAAGAAG T AG G T C T T AGAAC (SEQ ID NO: T G GAAAT G T AT T AAG T CAC G T AG C AG TATTTGATG T AGAG G GAT AT GAT A 45) GATTATTTTTCG T AAC T GAC G C AG C T AT GAAC TTAGCTCCT GAT AC AAAT
AC TAAAAAGCAAAT CATAGAAAAT GC T T GCACAGTAGCACAT T CAT TAGA TAT AAG T GAAC CAAAAG T T G C T G C AAT AT G C G C AAAAGAAAAAG T AAAT C C AAAAAT GAAAGAT AC AG T T GAAG C T AAAGAAC T AGAAGAAAT G T AT GAA AGAGGAGAAATCAAAGGTTGTATGGTTGGTGGGCCTTTTGCAATTGATAA TGCAG TAT C T T T AGAAG C AGC T AAAC AT AAAG G TAT AAAT CAT C C T G TAG C AG GAC GAG C T GAT AT AT TAT TAG C C C C AGAT AT T GAAG G T G G T AAC AT A
Description Sequence
T TATATAAAGC T T TGGTAT T C T T C T CAAAAT CAAAAAAT GCAGGAGT TAT AGT T GGGGC TAAAGCACCAATAATAT TAAC T T C T AGAG C AGAC AG T GAAG AAAC TAAAC TAAAC T CAATAGC T T TAGGT GT T T TAAT GGCAGCAAAGGCA TAA
AT GAG CAAAAT AT T TAAAAT C T TAACAATAAAT CCTGGTTC GAC AT C AAC TAAAATAGCT G TAT T T GAT AAT GAGGAT T TAG TAT T T GAAAAAAC T T TAA GAC AT T C T T C AGAAGAAAT AG GAAAAT AT GAGAAG G T G T C T GAC C AAT T T GAAT T T C G TAAAC AAG T AAT AGAAGAAG C T C T AAAAGAAG G T G GAG T AAA AACAT C T GAAT T AGAT GC T G TAG T AGG T AGAGGAGGAC T T C T TAAAC C T A T AAAAGG T GG T AC T TAT T CAG T AAG T GC T GC T AT GAT T GAAGAT T TAAAA G T G G GAG T T T TAG GAGAAC AC G C T T C AAAC C TAG G T G GAAT AAT AG C AAA AC AAAT AG G T GAAGAAG TAAAT G T T C C T T CAT AC AT AG T AGAC C C T G T T G T T G T AGAT GAAT T AGAAGAT G T T G C TAGAAT TTCTGGTATGCCT GAAAT A AG T AGAG C AAG T G TAG T AC AT G C T T TAAAT C AAAAG G C AAT AG C AAGAAG
buk
AT AT GC T AGAGAAAT AAACAAGAAAT AT GAAGAT AT AAAT C T TAT AG T T G
(SEQ ID NO:
C AC AC AT G G G T G GAG GAG TTTCTGTTG GAG C T C AT AAAAAT G G TAAAAT A
46)
G T AGAT G T T G C AAAC G C AT T AGAT G GAGAAG GAC CTTTCTCTC CAGAAAG AAGTGGTGGACTACCAGTAGGTGCATTAGTAAAAATGTGCTTTAGTGGAA AAT AT AC T CAAGAT GAAAT TAAAAAGAAAAT AAAAG G TAAT G G C G GAC TA G T T GCAT AC T TAAACAC TAAT GAT GC T AGAGAAG T T GAAGAAAGAAT T GA AGC T GGT GAT GAAAAAGC TAAAT TAG TAT AT GAAG C T AT G G CAT AT CAAA T C T C TAAAGAAATAGGAGC TAGT GC T GCAGT T C T TAAGGGAGAT GTAAAA GCAATAT TAT TAAC T GGT GGAAT CGCATAT T C AAAAAT GT T TACAGAAAT GAT T G C AGAT AGAG T TAAAT T TAT AG C AGAT G T AAAAG T T T AT C CAG G T G AAGAT GAAAT GAT T GCAT TAGC T CAAGGT GGAC T TAGAGT T T TAAC T GGT GAAG AAG AG G C T CAAGTT TAT GAT AAC TAA
ATGATCGTAAAACCTATGGTACGCAACAATATCTGCCTGAACGCCCATCC T CAG G G C T G C AAGAAG G GAG T G GAAGAT C AGAT T GAAT AT AC C AAGAAAC GCATTACCGCAGAAGTCAAAGCTGGCGCAAAAGCTCCAAAAAACGTTCTG GTGCTTGGCTGCTCAAATGGTTACGGCCTGGCGAGCCGCATTACTGCTGC GTTCGGATACGGGGCTGCGACCATCGGCGTGTCCTTTGAAAAAGCGGGTT C AGAAAC C AAAT AT G G T AC AC C G G GAT G G T AC AAT AAT T T G G CAT T T GAT GAAGCGGCAAAACGCGAGGGTCTTTATAGCGTGACGATCGACGGCGATGC G T T T T C AGAC GAGAT C AAG G C C CAG G TAAT T GAG GAAG C C AAAAAAAAAG G T AT C AAAT TTGATCTGATCG TAT AC AG C T T G G C CAG C C CAG T AC G T AC T
ter GAT C C T GAT AC AG G TAT CAT G C AC AAAAG C G T T T T GAAAC C C T T T G GAAA
(SEQ ID NO: AAC G T T C AC AG G C AAAAC AG T AGAT C C G T T T AC T G G C GAG C T GAAG GAAA 47) TCTCCGCGGAACCAGCAAATGACGAGGAAGCAGCCGCCACTGTTAAAGTT
ATGGGGGGTGAAGATTGGGAACGTTGGATTAAGCAGCTGTCGAAGGAAGG CCTCTTAGAAGAAGGCTGTATTACCTTGGCCTATAGTTATATTGGCCCTG AAG C T AC C C AAG C T T T G T AC C G T AAAG G C AC AAT C G G C AAG G C C AAAGAA C AC C T G GAG G C C AC AG C AC AC C G T C T C AAC AAAGAGAAC C C G T C AAT C C G TGCCTTCGTGAGCGTGAATAAAGGCCTGGTAACCCGCGCAAGCGCCGTAA TCCCGGTAATCCCTCTGTATCTCGCCAGCTTGTTCAAAGTAATGAAAGAG AAGGGCAATCATGAAGGTTGTATTGAACAGATCACGCGTCTGTACGCCGA GCGCCTGTACCGTAAAGATGGTACAATTCCAGTTGATGAGGAAAATCGCA T T C G CAT T GAT GAT T G G GAG T T AGAAGAAGAC G T C CAGAAAG CGGTATCC
Description Sequence
GCGTTGATGGAGAAAGTCACGGGTGAAAACGCAGAATCTCTCACTGACTT AGCGGGGTACCGCCATGATTTCTTAGCTAGTAACGGCTTTGATGTAGAAG GTATTAATTATGAAGCGGAAGTTGAACGCTTCGACCGTATCTGA
AT GAGT CAGGCGC TAAAAAAT T TAC T GACAT TGTTAAAT C T GGAAAAAAT TGAGGAAGGACTCTTTCGCGGCCAGAGTGAAGATTTAGGTTTACGCCAGG TGTTTGGCGGCCAGGTCGTGGGTCAGGCCTTGTATGCTGCAAAAGAGACC GTCCCTGAAGAGCGGCTGGTACATTCGTTTCACAGCTACTTTCTTCGCCC TGGCGATAGTAAGAAGCCGATTATTTATGATGTCGAAACGCTGCGTGACG GTAACAGCTTCAGCGCCCGCCGGGTTGCTGCTATTCAAAACGGCAAACCG ATTTTTTATATGACTGCCTCTTTCCAGGCACCAGAAGCGGGTTTCGAACA TCAAAAAACAATGCCGTCCGCGCCAGCGCCTGATGGCCTCCCTTCGGAAA
tesB
CGCAAATCGCCCAATCGCTGGCGCACCTGCTGCCGCCAGTGCTGAAAGAT SEQ ID NO:
AAATTCATCTGCGATCGTCCGCTGGAAGTCCGTCCGGTGGAGTTTCATAA
48
CCCACTGAAAGGTCACGTCGCAGAACCACATCGTCAGGTGTGGATCCGCG CAAATGGTAGCGTGCCGGATGACCTGCGCGTTCATCAGTATCTGCTCGGT TACGCTTCTGATCTTAACTTCCTGCCGGTAGCTCTACAGCCGCACGGCAT CGGTTTTCTCGAACCGGGGATTCAGATTGCCACCATTGACCATTCCATGT GGTTCCATCGCCCGTTTAATTTGAATGAATGGCTGCTGTATAGCGTGGAG AGCACCTCGGCGTCCAGCGCACGTGGCTTTGTGCGCGGTGAGTTTTATAC CCAAGACGGCGTACTGGTTGCCTCGACCGTTCAGGAAGGGGTGATGCGTA AT CACAAT T AA
[0257] The gene products of the bcd2, etfA3, and etfB3 genes in
Clostridium difficile form a complex that converts crotonyl-CoA to butyryl-CoA, which may function as an oxygen-dependent co-oxidant. In some
embodiments, because the genetically engineered bacteria of the invention are designed to produce butyrate in a microaerobic or oxygen-limited environment, e.g., the mammalian gut, oxygen-dependence could have a negative effect on butyrate production in the gut. It has been shown that a single gene from
Treponema denticola {ter, encoding frans-2-enoynl-CoA reductase) can functionally replace this three-gene complex in an oxygen-independent manner. In some embodiments, the genetically engineered bacteria comprise a fer gene, e.g., from Treponema denticola, which can functionally replace all three of the bcd2, etfB3, and etfA3 genes, e.g., from Peptoclostridium difficile. In this embodiment, the genetically engineered bacteria comprise thiA1, hbd, crt2, pbt, and buk, e.g., from Peptoclostridium difficile, and ter, e.g., from Treponema denticola, and produce butyrate in low-oxygen conditions, in the presence of HE- specific molecules or metabolites, in the presence of molecules or metabolites
associated with liver damage, inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose. In some embodiments, the genetically engineered bacteria comprise genes for aerobic butyrate biosynthesis and/or genes for anaerobic or microaerobic butyrate biosynthesis. In some embodiments, the genetically engineered bacteria of the invention comprise thiA1, hbd, crt2, pbt, and buk, e.g., from Peptociostridium difficile; ter, e.g., from Treponema denticola; one or more of bcd2, etfB3, and etfA3, e.g., from Peptociostridium difficile; and produce butyrate in low-oxygen conditions or in the presence of HE-specific molecules or metabolites. In some embodiments, one or more of the butyrate biosynthesis genes is functionally replaced, modified, and/or mutated in order to enhance stability and/or increase butyrate production in low- oxygen conditions or in the presence of HE-specific molecules or metabolites, or molecules or metabolites associated with liver damage, or other condition(s) such as inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose. . In some embodiments, the local production of butyrate induces the
differentiation of regulatory T cells in the gut and/or promotes the barrier function of colonic epithelial cells.
[0258] The gene products of pbt and buk convert butyrylCoA to Butyrate. In some embodiments, the pbt and buk genes can be replaced by a tesB gene. tesB can be used to cleave off the CoA from butyryl-coA. In one embodiment, the genetically engineered bacteria comprise bcd2, etfB3, etfA3, thiA1, hbd, and crt2, e.g., from Peptociostridium difficile, and tesB from E. Coli and produce butyrate in low-oxygen conditions, in the presence of HE-specific molecules or metabolites, in the presence of molecules or metabolites associated with liver damage, inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose. In one embodiment, the genetically engineered bacteria comprise fer gene (encoding frans-2-enoynl-CoA reductase) e.g., from Treponema denticola, thiA1, hbd, crt2, pbt, and buk, e.g., from Peptociostridium difficile, and tesB from E. Coli , and produce butyrate in low-oxygen conditions, in the presence of HE- specific molecules or metabolites, in the presence of molecules or metabolites
associated with liver damage, inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose. In some embodiments, one or more of the butyrate biosynthesis genes is functionally replaced, modified, and/or mutated in order to enhance stability and/or increase butyrate production in low-oxygen conditions or in the presence of HE-specific molecules or metabolites, or molecules or metabolites associated with liver damage, or other condition(s) such as inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose. In some embodiments, the local production of butyrate induces the differentiation of regulatory T cells in the gut and/or promotes the barrier function of colonic epithelial cells.
[0259] In some embodiments, the genetically engineered bacteria of the invention comprise a propionate gene cassette and are capable of producing propionate in low-oxygen conditions or in the presence of HE-specific molecules or metabolites. The genetically engineered bacteria may express any suitable set of propionate biosynthesis genes (see, e.g., Table 15). Unmodified bacteria that are capable of producing propionate via an endogenous propionate biosynthesis pathway include, but are not limited to, Clostridium propionicum, Megasphaera elsdenii, and Prevotella ruminicola, and these endogenous propionate biosynthesis pathways may be a source of genes for the genetically engineered bacteria of the invention. In some embodiments, the genetically engineered bacteria of the invention comprise propionate biosynthesis genes from a different species, strain, or substrain of bacteria. In some embodiments, the genetically engineered bacteria comprise the genes pet, led, and acr from Clostridium propionicum. In some embodiments, the genetically engineered bacteria comprise acrylate pathway genes for propionate biosynthesis, e.g., pet, IcdA, IcdB, IcdC, etfA, acrB, and acrC. In alternate embodiments, the
genetically engineered bacteria comprise pyruvate pathway genes for propionate biosynthesis, e.g., thrAfbr, thrB, thrC, ilvA^, aceE, aceF, and Ipd, and optionally further comprise tesB. The genes may be codon-optimized, and translational and transcriptional elements may be added. Table 15 depicts the nucleic acid sequences of exemplary genes in the propionate biosynthesis gene
cassette. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 48, 49, 50, 51 , 52, 53, 54, 55, 56, 57, 58, 59, 60, 61 or 62 a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 48, 49, 50, 51 , 52, 53, 54, 55, 56, 57, 58, 59, 60, 61 or 62, or a functional fragment thereof.
Table 15
ATGAGCTTAACCCAAGGCATGAAAGCTAAACAACTGTTAGCATACTTTCA GGGTAAAGCCGATCAGGATGCACGTGAAGCGAAAGCCCGCGGTGAGCTGG TCTGCTGGTCGGCGTCAGTCGCGCCGCCGGAATTTTGCGTAACAATGGGC ATTGCCATGATCTACCCGGAGACTCATGCAGCGGGCATCGGTGCCCGCAA AGGTGCGATGGACATGCTGGAAGTTGCGGACCGCAAAGGCTACAACGTGG ATTGTTGTTCCTACGGCCGTGTAAATATGGGTTACATGGAATGTTTAAAA GAAGCCGCCATCACGGGCGTCAAGCCGGAAGTTTTGGTTAATTCCCCTGC TGCTGACGTTCCGCTTCCCGATTTGGTGATTACGTGTAATAATATCTGTA ACACGCTGCTGAAATGGTACGAAAACTTAGCAGCAGAACTCGATATTCCT TGCATCGTGATCGACGTACCGTTTAATCATACCATGCCGATTCCGGAATA TGCCAAGGCCTACATCGCGGACCAGTTCCGCAATGCAATTTCTCAGCTGG AAGTTATTTGTGGCCGTCCGTTCGATTGGAAGAAATTTAAGGAGGTCAAA
IcdA
GATCAGACCCAGCGTAGCGTATACCACTGGAACCGCATTGCCGAGATGGC SEQ ID NO:
GAAATACAAGCCTAGCCCGCTGAACGGCTTCGATCTGTTCAATTACATGG
50
CGTTAATCGTGGCGTGCCGCAGCCTGGATTATGCAGAAATTACCTTTAAA GCGTTCGCGGACGAATTAGAAGAGAATTTGAAGGCGGGTATCTACGCCTT TAAAGGTGCGGAAAAAACGCGCTTTCAATGGGAAGGTATCGCGGTGTGGC CACATTTAGGTCACACGTTTAAATCTATGAAGAATCTGAATTCGATTATG ACCGGTACGGCATACCCCGCCCTTTGGGACCTGCACTATGACGCTAACGA CGAATCTATGCACTCTATGGCTGAAGCGTACACCCGTATTTATATTAATA CTTGTCTGCAGAACAAAGTAGAGGTCCTGCTTGGGATCATGGAAAAAGGC CAGGTGGATGGTACCGTATATCATCTGAATCGCAGCTGCAAACTGATGAG TTTCCTGAACGTGGAAACGGCTGAAATTATTAAAGAGAAGAACGGTCTTC CTTACGTCTCCATTGATGGCGATCAGACCGATCCTCGCGTTTTTTCTCCG GCCCAGTTTGATACCCGTGTTCAGGCCCTGGTTGAGATGATGGAGGCCAA TATGGCGGCAGCGGAATAA
ATGTCACGCGTGGAGGCAATCCTGTCGCAGCTGAAAGATGTCGCCGCGAA TCCGAAAAAAGCCATGGATGACTATAAAGCTGAAACAGGTAAGGGCGCGG TTGGTATCATGCCGATCTACAGCCCCGAAGAAATGGTACACGCCGCTGGC TATTTGCCGATGGGAATCTGGGGCGCCCAGGGCAAAACGATTAGTAAAGC GCGCACCTATCTGCCTGCTTTTGCCTGCAGCGTAATGCAGCAGGTTATGG AATTACAGTGCGAGGGCGCGTATGATGACCTGTCCGCAGTTATTTTTAGC GTACCGTGCGACACTCTCAAATGTCTTAGCCAGAAATGGAAAGGTACGTC CCCAGTGATTGTATTTACGCATCCGCAGAACCGCGGATTAGAAGCGGCGA ACCAATTCTTGGTTACCGAGTATGAACTGGTAAAAGCACAACTGGAATCA GTTCTGGGTGTGAAAATTTCAAACGCCGCCCTGGAAAATTCGATTGCAAT
IcdB
TTATAACGAGAATCGTGCCGTGATGCGTGAGTTCGTGAAAGTGGCAGCGG SEQ ID NO:
ACTATCCTCAAGTCATTGACGCAGTGAGCCGCCACGCGGTTTTTAAAGCG
51
CGCCAGTTTATGCTTAAGGAAAAACATACCGCACTTGTGAAAGAACTGAT CGCTGAGATTAAAGCAACGCCAGTCCAGCCGTGGGACGGAAAAAAGGTTG TAGTGACGGGCATTCTGTTGGAACCGAATGAGTTATTAGATATCTTTAAT GAGTTTAAGATCGCGATTGTTGATGATGATTTAGCGCAGGAAAGCCGTCA GATCCGTGTTGACGTTCTGGACGGAGAAGGCGGACCGCTCTACCGTATGG CTAAAGCGTGGCAGCAAATGTATGGCTGCTCGCTGGCAACCGACACCAAG AAGGGTCGCGGCCGTATGTTAATTAACAAAACGATTCAGACCGGTGCGGA CGCTATCGTAGTTGCAATGATGAAGTTTTGCGACCCAGAAGAATGGGATT ATCCGGTAATGTACCGTGAATTTGAAGAAAAAGGGGTCAAATCACTTATG ATTGAGGTGGATCAGGAAGTATCGTCTTTCGAACAGATTAAAACCCGTCT
Description Sequence
GCAGTCATTCGTCGAAATGCTTTAA
ATGTATACCTTGGGGATTGATGTCGGTTCTGCCTCTAGTAAAGCGGTGAT TCTGAAAGATGGAAAAGATATTGTCGCTGCCGAGGTTGTCCAAGTCGGTA CCGGCTCCTCGGGTCCCCAACGCGCACTGGACAAAGCCTTTGAAGTCTCT G G C T T AAAAAAG GAAGAC AT C AG C T AC AC AG TAG C T AC GGGCTATGGGCG C T T C AAT T T TAG C GAC G C G GAT AAAC AGAT T T C G GAAAT TAG C T G T CAT G C C AAAG G CAT TTATTTCT TAG T AC C AAC T G C G C G C AC TAT TAT T GAC AT T GGCGGCCAAGATGCGAAAGCCATCCGCCTGGACGACAAGGGGGGTATTAA
IcdC
GCAATTCTTCATGAATGATAAATGCGCGGCGGGCACGGGGCGTTTCCTGG SEQ ID NO:
AAGTCATGGCTCGCGTACTTGAAACCACCCTGGATGAAATGGCTGAACTG
52
GATGAACAGGCGACTGACACCGCTCCCATTTCAAGCACCTGCACGGTTTT CGCCGAAAGCGAAGTAATTAGCCAATTGAGCAATGGTGTCTCACGCAACA ACATCATTAAAGGTGTCCATCTGAGCGTTGCGTCACGTGCGTGTGGTCTG GCGTATCGCGGCGGTTTGGAGAAAGATGTTGTTATGACAGGTGGCGTGGC AAAAAATGCAGGGGTGGTGCGCGCGGTGGCGGGCGTTCTGAAGACCGATG TTATCGTTGCTCCGAATCCTCAGACGACCGGTGCACTGGGGGCAGCGCTG TATGCTTAT GAG G C C G C C C AGAAGAAG T A
ATGGCCTTCAATAGCGCAGATATTAATTCTTTCCGCGATATTTGGGTGTT T T G T GAAC AG C G T GAG G G C AAAC T GAT T AAC AC C GAT T T C GAAT T AAT T A GCGAAGGTCGTAAACTGGCTGACGAACGCGGAAGCAAACTGGTTGGAATT TTGCTGGGGCACGAAGTTGAAGAAATCGCAAAAGAATTAGGCGGCTATGG TGCGGACAAGGTAATTGTGTGCGATCATCCGGAACTTAAATTTTACACTA CGGATGCTTATGCCAAAGTTTTATGTGACGTCGTGATGGAAGAGAAACCG GAGGTAATTTTGATCGGTGCCACCAACATTGGCCGTGATCTCGGACCGCG TTGTGCTGCACGCTTGCACACGGGGCTGACGGCTGATTGCACGCACCTGG ATATTGATAT GAAT AAAT AT G T G GAC T T T C T T AG C AC C AG TAG C AC C T T G GATATCTCGTCGAT GAC TTTCCCTATG GAAGAT AC AAAC C T TAAAAT GAC
etfA GCGCCCTGCATTTGGCGGACATCTGATGGCAACGATCATTTGTCCACGCT SEQ ID NO: TCCGTCCCTGTATGAGCACAGTGCGCCCCGGAGTGATGAAGAAAGCGGAG
53 TTCTCGCAGGAGATGGCGCAAGCATGTCAAGTAGTGACCCGTCACGTAAA
TTTGTCGGAT GAAGAC C T TAAAAC T AAAG T AAT T AAT AT C G T GAAG GAAA CGAAAAAGATTGTGGATCTGATCGGCGCAGAAATTATTGTGTCAGTTGGT CGTGGTATCTCGAAAGATGTCCAAGGTGGAATTGCACTGGCTGAAAAACT TGCGGACGCATTTGGTAACGGTGTCGTGGGCGGCTCGCGCGCAGTGATTG ATTCCGGCTGGTTACCTGCGGATCATCAGGTTGGACAAACCGGTAAGACC GTGCACCCGAAAGTCTACGTGGCGCTGGGTATTAGTGGGGCTATCCAGCA TAAGGC T GGGAT GCAAGAC T C T GAAC T GAT CAT T GCCGT CAACAAAGACG AAACGGCGCCTATCTTCGACTGCGCCGATTATGGCATCACCGGTGATTTA T T TAAAAT C G T AC C GAT GAT GAT C GAC G C GAT CAAAGAG G G TAAAAAC G C ATGA
Description Sequence
ATGCGCATCTATGTGTGTGTGAAACAAGTCCCAGATACGAGCGGCAAGGT GGCCGTTAACCCTGATGGGACCCTTAACCGTGCCTCAATGGCAGCGATTA T T AAC C C G GAC GATATGTCCGCGATC GAAC AG G C AT TAAAAC T GAAAGAT GAAACCGGATGCCAGGTTACGGCGCTTACGATGGGTCCTCCTCCTGCCGA GGGCATGTTGCGCGAAATTATTGCAATGGGGGCCGACGATGGTGTGCTGA TTTCGGCCCGTGAATTTGGGGGGTCCGATACCTTCGCAACCAGTCAAATT AT TAG C G C G G C AAT C C AT AAAT TAG G C T T AAG C AAT GAAGAC AT GAT C T T
acrB TTGCGGTCGTCAGGCCATTGACGGTGATACGGCCCAAGTCGGCCCTCAAA SEQ ID NO: TTGCCGAAAAACTGAGCATCCCACAGGTAACCTATGGCGCAGGAATCAAA
54 AAATCTGGTGATTTAGTGCTGGTGAAGCGTATGTTGGAGGATGGTTATAT
GAT GAT C GAAG T C GAAAC TCCATGTCTGATTACCTGCATT C AG GAT AAAG C G G TAAAAC C AC G T T AC AT GAC T C T C AAC GGTATTATG GAAT G C T AC T C C AAGCCGCTCCTCGTTCTCGATTACGAAGCACTGAAAGATGAACCGCTGAT CGAACTTGATACCATTGGGCTTAAAGGCTCCCCGACGAATATCTTTAAAT CGTTTACGCCGCCTCAGAAAGGCGTTGGTGTCATGCTCCAAGGCACCGAT AAG GAAAAAG T C GAG GAT C T G G T G GAT AAG C T GAT G C AGAAAC AT G T CAT CTAA
ATGTTCTTACT GAAGAT T AAAAAAGAAC G T AT GAAAC G C AT G GAC T T T AG TTTAACGCGTGAACAGGAGATGTTAAAAAAACTGGCGCGTCAGTTTGCTG AGATCGAGCTGGAACCGGTGGCCGAAGAGATTGATCGTGAGCACGTTTTT CCTGCAGAAAACTTTAAGAAGATGGCGGAAATTGGCTTAACCGGCATTGG TATCCCGAAAGAATTTGGTGGCTCCGGTGGAGGCACCCTGGAGAAGGTCA TTGCCGTGTCAGAATTCGGCAAAAAGTGTATGGCCTCAGCTTCCATTTTA AG CAT T CAT CTTATCGCGCCG C AG G C AAT C T AC AAAT AT G G GAC C AAAGA ACAGAAAGAGACGTACCTGCCGCGTCTTACCAAAGGTGGTGAACTGGGCG CCTTTGCGCTGACAGAACCAAACGCCGGAAGCGATGCCGGCGCGGTAAAA AC GAC C G C GAT T C T G GAC AG C C AGAC AAAC GAG T AC G T G C T GAAT G G C AC CAAATGCTTTATCAGCGGGGGCGGGCGCGCGGGTGTTCTTGTAATTTTTG
acrC
CGCTTACTGAACCGAAAAAAGGTCTGAAAGGGATGAGCGCGATTATCGTG SEQ ID NO:
GAGAAAGGGACCCCGGGCTTCAGCATCGGCAAGGTGGAGAGCAAGATGGG
55
GATCGCAGGTTCGGAAACCGCGGAACTTATCTTCGAAGATTGTCGCGTTC CGGCTGCCAACCTTTTAGGTAAAGAAGGCAAAGGCTTTAAAATTGCTATG GAAGCCCTGGATGGCGCCCGTATTGGCGTGGGCGCTCAAGCAATCGGAAT TGCCGAGGGGGCGATCGACCTGAGTGTGAAGTACGTTCACGAGCGCATTC AATTTGGTAAACCGATCGCGAATCTGCAGGGAATTCAATGGTATATCGCG GATATGGCGACCAAAACCGCCGCGGCACGCGCACTTGTTGAGTTTGCAGC GTATCTTGAAGACGCGGGTAAACCGTTCACAAAGGAATCTGCTATGTGCA AGCTGAACGCCTCCGAAAACGCGCGTTTTGTGACAAATTTAGCTCTGCAG ATTCACGGGGGTTACGGTTATATGAAAGATTATCCGTTAGAGCGTATGTA T C G C GAT G C T AAGAT T AC G GAAAT T T AC GAG G G GAC AT C AGAAAT C CAT A AGGTGGTGATTGCGCGTGAAGTAATGAAACGCTAA
ATGCGAGTGTTGAAGTTCGGCGGTACATCAGTGGCAAATGCAGAACGTTT TCTGCGTGTTGCCGATATTCTGGAAAGCAATGCCAGGCAGGGGCAGGTGG
thrA r CCACCGTCCTCTCTGCCCCCGCCAAAATCACCAACCACCTGGTGGCGATG SEQ ID NO: ATT GAAAAAAC CAT TAG C G G C C AG GAT G C T T T AC C C AAT AT C AG C GAT G C
56 CGAACGTATTTTTGCCGAACTTTTGACGGGACTCGCCGCCGCCCAGCCGG
GGTTCCCGCTGGCGCAATTGAAAACTTTCGTCGATCAGGAATTTGCCCAA ATAAAACATGTCCTGCATGGCATTAGTTTGTTGGGGCAGTGCCCGGATAG
Description Sequence
CATCAACGCTGCGCTGATTTGCCGTGGCGAGAAAATGTCGATCGCCATTA TGGCCGGCGTATTAGAAGCGCGCGGTCACAACGTTACTGTTATCGATCCG GTCGAAAAACTGCTGGCAGTGGGGCATTACCTCGAATCTACCGTCGATAT TGCTGAGTCCACCCGCCGTATTGCGGCAAGCCGCATTCCGGCTGATCACA TGGTGCTGATGGCAGGTTTCACCGCCGGTAATGAAAAAGGCGAACTGGTG GTGCTTGGACGCAACGGTTCCGACTACTCTGCTGCGGTGCTGGCTGCCTG TTTACGCGCCGATTGTTGCGAGATTTGGACGGACGTTGACGGGGTCTATA CCTGCGACCCGCGTCAGGTGCCCGATGCGAGGTTGTTGAAGTCGATGTCC TACCAGGAAGCGATGGAGCTTTCCTACTTCGGCGCTAAAGTTCTTCACCC CCGCACCATTACCCCCATCGCCCAGTTCCAGATCCCTTGCCTGATTAAAA ATACCGGAAATCCTCAAGCACCAGGTACGCTCATTGGTGCCAGCCGTGAT GAAGAC GAAT T AC C G G T C AAG G G CAT T T C C AAT C T GAAT AAC AT G G C AAT GTTCAGCGTTTCTGGTCCGGGGATGAAAGGGATGGTCGGCATGGCGGCGC GCGTCTTTGCAGCGATGTCACGCGCCCGTATTTCCGTGGTGCTGATTACG C AAT C AT C T T C C GAAT AC AG CAT C AG TTTCTGCGTTC C AC AAAG C GAC T G TGTGCGAGCTGAACGGGCAATGCAGGAAGAGTTCTACCTGGAACTGAAAG AAGGCTTACTGGAGCCGCTGGCAGTGACGGAACGGCTGGCCATTATCTCG GTGGTAGGTGATGGTATGCGCACCTTGCGTGGGATCTCGGCGAAATTCTT TGCCGCACTGGCCCGCGCCAATATCAACATTGTCGCCATTGCTCAGAGAT CTTCTGAACGCTCAATCTCTGTCGTGGTAAATAACGATGATGCGACCACT GGCGTGCGCGTTACTCATCAGATGCTGTTCAATACCGATCAGGTTATCGA AGTGTTTGTGATTGGCGTCGGTGGCGTTGGCGGTGCGCTGCTGGAGCAAC TGAAGCGTCAGCAAAGCTGGCTGAAGAATAAACATATCGACTTACGTGTC TGCGGTGTTGCCAACTCGAAGGCTCTGCTCACCAATGTACATGGCCTTAA TCTGGAAAACTGGCAGGAAGAACTGGCGCAAGCCAAAGAGCCGTTTAATC TCGGGCGCTTAATTCGCCTCGTGAAAGAATATCATCTGCTGAACCCGGTC ATTGTTGACTGCACTTCCAGCCAGGCAGTGGCGGATCAATATGCCGACTT CCTGCGCGAAGGTTTCCACGTTGTCACGCCGAACAAAAAGGCCAACACCT CGTCGATGGATTACTACCATCAGTTGCGTTATGCGGCGGAAAAATCGCGG CGTAAATTCCTCTATGACACCAACGTTGGGGCTGGATTACCGGTTATTGA GAACCTGCAAAATCTGCTCAATGCAGGTGATGAATTGATGAAGTTCTCCG GCATTCTTTCTGGTTCGCTTTCTTATATCTTCGGCAAGTTAGACGAAGGC ATGAGTTTCTCCGAGGCGACCACGCTGGCGCGGGAAATGGGTTATACCGA ACCGGACCCGCGAGATGATCTTTCTGGTATGGATGTGGCGCGTAAACTAT TGATTCTCGCTCGTGAAACGGGACGTGAACTGGAGCTGGCGGATATTGAA ATTGAACCTGTGCTGCCCGCAGAGTTTAACGCCGAGGGTGATGTTGCCGC TTTTATGGCGAATCTGTCACAACTCGACGATCTCTTTGCCGCGCGCGTGG CGAAGGCCCGTGATGAAGGAAAAGTTTTGCGCTATGTTGGCAATATTGAT GAAGATGGCGTCTGCCGCGTGAAGATTGCCGAAGTGGATGGTAATGATCC GCTGTTCAAAGTGAAAAATGGCGAAAACGCCCTGGCCTTCTATAGCCACT ATTATCAGCCGCTGCCGTTGGTACTGCGCGGATATGGTGCGGGCAATGAC GTTACAGCTGCCGGTGTCTTTGCTGATCTGCTACGTACCCTCTCATGGAA GTTAGGAGTCTGA
Description Sequence
ATGGTTAAAGTTTATGCCCCGGCTTCCAGTGCCAATATGAGCGTCGGGTT TGATGTGCTCGGGGCGGCGGTGACACCTGTTGATGGTGCATTGCTCGGAG ATGTAGTCACGGTT GAG G C G G C AG AG AC AT T C AG T C T C AAC AAC C T C G G A CGCTTTGCCGATAAGCTGCCGTCAGAACCACGGGAAAATATCGTTTATCA GTGCTGGGAGCGTTTTTGCCAGGAACTGGGTAAGCAAATTCCAGTGGCGA TGACCCTGGAAAAGAATATGCCGATCGGTTCGGGCTTAGGCTCCAGTGCC TGTTCGGTGGTCGCGGCGCTGATGGCGATGAATGAACACTGCGGCAAGCC GCTTAATGACACTCGTTTGCTGGCTTTGATGGGCGAGCTGGAAGGCCGTA
thrB TCTCCGGCAGCATTCATTACGACAACGTGGCACCGTGTTTTCTCGGTGGT SEQ ID NO: AT G C AG T T GAT GAT C GAAGAAAAC GAC AT CAT C AG C C AG C AAG T G C C AG G
57 GTTTGATGAGTGGCTGTGGGTGCTGGCGTATCCGGGGATTAAAGTCTCGA
CGGCAGAAGCCAGGGCTATTTTACCGGCGCAGTATCGCCGCCAGGATTGC ATTGCGCACGGGCGACATCTGGCAGGCTTCATTCACGCCTGCTATTCCCG TCAGCCTGAGCTTGCCGCGAAGCTGATGAAAGATGTTATCGCTGAACCCT ACCGTGAACGGTTACTGCCAGGCTTCCGGCAGGCGCGGCAGGCGGTCGCG GAAATCGGCGCGGTAGCGAGCGGTATCTCCGGCTCCGGCCCGACCTTGTT CGCTCTGTGTGACAAGCCGGAAACCGCCCAGCGCGTTGCCGACTGGTTGG GTAAGAACTACCTGCAAAATCAGGAAGGTTTTGTTCATATTTGCCGGCTG GATACGGCGGGCGCACGAGTACTGGAAAACTAA
AT GAAAC T C TACAAT C T GAAAGAT CACAACGAGCAGGT CAGC T T T GCGCA AGCCGTAACCCAGGGGTTGGGCAAAAATCAGGGGCTGTTTTTTCCGCACG ACCTGCCGGAATTCAGCCTGACTGAAATTGATGAGATGCTGAAGCTGGAT TTTGTCACCCGCAGTGCGAAGATCCTCTCGGCGTTTATTGGTGATGAAAT CCCACAGGAAATCCTGGAAGAGCGCGTGCGCGCGGCGTTTGCCTTCCCGG CTCCGGTCGCCAATGTTGAAAGCGATGTCGGTTGTCTGGAATTGTTCCAC GGGCCAACGCTGGCATTTAAAGATTTCGGCGGTCGCTTTATGGCACAAAT GCTGACCCATATTGCGGGTGATAAGCCAGTGACCATTCTGACCGCGACCT CCGGTGATACCGGAGCGGCAGTGGCTCATGCTTTCTACGGTTTACCGAAT GTGAAAGTGGTTATCCTCTATCCACGAGGCAAAATCAGTCCACTGCAAGA AAAACTGTTCTGTACATTGGGCGGCAATATCGAAACTGTTGCCATCGACG GCGATTTCGATGCCTGTCAGGCGCTGGTGAAGCAGGCGTTTGATGATGAA
thrC
GAACTGAAAGTGGCGCTAGGGTTAAACTCGGCTAACTCGATTAACATCAG SEQ ID NO:
CCGTTTGCTGGCGCAGATTTGCTACTACTTTGAAGCTGTTGCGCAGCTGC
58
CGCAGGAGACGCGCAACCAGCTGGTTGTCTCGGTGCCAAGCGGAAACTTC GGCGATTTGACGGCGGGTCTGCTGGCGAAGTCACTCGGTCTGCCGGTGAA ACGTTTTATTGCTGCGACCAACGTGAACGATACCGTGCCACGTTTCCTGC ACGACGGTCAGTGGTCACCCAAAGCGACTCAGGCGACGTTATCCAACGCG ATGGACGTGAGTCAGCCGAACAACTGGCCGCGTGTGGAAGAGTTGTTCCG CCGCAAAATCTGGCAACTGAAAGAGCTGGGTTATGCAGCCGTGGATGATG AAAC C AC G C AAC AGAC AAT G C G T GAG T T AAAAGAAC T G G G C T AC AC T T C G GAGCCGCACGCTGCCGTAGCTTATCGTGCGCTGCGTGATCAGTTGAATCC AGGCGAATATGGCTTGTTCCTCGGCACCGCGCATCCGGCGAAATTTAAAG AGAGCGTGGAAGCGATTCTCGGTGAAACGTTGGATCTGCCAAAAGAGCTG GCAGAACGTGCTGATTTACCCTTGCTTTCACATAATCTGCCCGCCGATTT TGCTGCGTTGCGTAAATTGATGATGAATCATCAGTAA
Description Sequence
ATGAGTGAAACATACGTGTCTGAGAAAAGTCCAGGAGTGATGGCTAGCGG AGCGGAGCTGATTCGTGCCGCCGACATTCAAACGGCGCAGGCACGAATTT CCTCCGTCATTGCACCAACTCCATTGCAGTATTGCCCTCGTCTTTCTGAG GAAACCGGAGCGGAAATCTACCTTAAGCGTGAGGATCTGCAGGATGTTCG TTCCTACAAGATCCGCGGTGCGCTGAACTCTGGAGCGCAGCTCACCCAAG AGCAGCGCGATGCAGGTATCGTTGCCGCATCTGCAGGTAACCATGCCCAG GGCGTGGCCTATGTGTGCAAGTCCTTGGGCGTTCAGGGACGCATCTATGT TCCTGTGCAGACTCCAAAGCAAAAGCGTGACCGCATCATGGTTCACGGCG GAGAGTTTGTCTCCTTGGTGGTCACTGGCAATAACTTCGACGAAGCATCG GCTGCAGCGCATGAAGATGCAGAGCGCACCGGCGCAACGCTGATCGAGCC TTTCGATGCTCGCAACACCGTCATCGGTCAGGGCACCGTGGCTGCTGAGA TCTTGTCGCAGCTGACTTCCATGGGCAAGAGTGCAGATCACGTGATGGTT
ilvA r CCAGTCGGCGGTGGCGGACTTCTTGCAGGTGTGGTCAGCTACATGGCTGA SEQ ID NO: TATGGCACCTCGCACTGCGATCGTTGGTATCGAACCAGCGGGAGCAGCAT
59 CCATGCAGGCTGCATTGCACAATGGTGGACCAATCACTTTGGAGACTGTT
GATCCCTTTGTGGACGGCGCAGCAGTCAAACGTGTCGGAGATCTCAACTA CACCATCGTGGAGAAGAACCAGGGTCGCGTGCACATGATGAGCGCGACCG AGGGCGCTGTGTGTACTGAGATGCTCGATCTTTACCAAAACGAAGGCATC ATCGCGGAGCCTGCTGGCGCGCTGTCTATCGCTGGGTTGAAGGAAATGTC CTTTGCACCTGGTTCTGCAGTGGTGTGCATCATCTCTGGTGGCAACAACG ATGTGCTGCGTTATGCGGAAATCGCTGAGCGCTCCTTGGTGCACCGCGGT TTGAAGCACTACTTCTTGGTGAACTTCCCGCAAAAGCCTGGTCAGTTGCG TCACTTCCTGGAAGATATCCTGGGACCGGATGATGACATCACGCTGTTTG AGTACCTCAAGCGCAACAACCGTGAGACCGGTACTGCGTTGGTGGGTATT CACTTGAGTGAAGCATCAGGATTGGATTCTTTGCTGGAACGTATGGAGGA ATCGGCAATTGATTCCCGTCGCCTCGAGCCGGGCACCCCTGAGTACGAAT ACTTGACCTAA
ATGTCAGAACGTTTCCCAAATGACGTGGATCCGATCGAAACTCGCGACTG GCTCCAGGCGATCGAATCGGTCATCCGTGAAGAAGGTGTTGAGCGTGCTC AGTATCTGATCGACCAACTGCTTGCTGAAGCCCGCAAAGGCGGTGTAAAC G TAG C C G C AG G C AC AG G T AT C AG C AAC T AC AT C AAC AC CAT C C C C G T T GA AGAACAACCGGAGTATCCGGGTAATCTGGAACTGGAACGCCGTATTCGTT CAGCTATCCGCTGGAACGCCATCATGACGGTGCTGCGTGCGTCGAAAAAA GACCTCGAACTGGGCGGCCATATGGCGTCCTTCCAGTCTTCCGCAACCAT TTATGATGTGTGCTTTAACCACTTCTTCCGTGCACGCAACGAGCAGGATG GCGGCGACCTGGTTTACTTCCAGGGCCACATCTCCCCGGGCGTGTACGCT
aceE CGTGCTTTCCTGGAAGGTCGTCTGACTCAGGAGCAGCTGGATAACTTCCG SEQ ID NO: TCAGGAAGTTCACGGCAATGGCCTCTCTTCCTATCCGCACCCGAAACTGA
60 TGCCGGAATTCTGGCAGTTCCCGACCGTATCTATGGGTCTGGGTCCGATT
GGTGCTATTTACCAGGCTAAATTCCTGAAATATCTGGAACACCGTGGCCT GAAAGATACCTCTAAACAAACCGTTTACGCGTTCCTCGGTGACGGTGAAA TGGACGAACCGGAATCCAAAGGTGCGATCACCATCGCTACCCGTGAAAAA CTGGATAACCTGGTCTTCGTTATCAACTGTAACCTGCAGCGTCTTGACGG CCCGGTCACCGGTAACGGCAAGATCATCAACGAACTGGAAGGCATCTTCG AAGGTGCTGGCTGGAACGTGATCAAAGTGATGTGGGGTAGCCGTTGGGAT GAACTGCTGCGTAAGGATACCAGCGGTAAACTGATCCAGCTGATGAACGA AACCGTTGACGGCGACTACCAGACCTTCAAATCGAAAGATGGTGCGTACG TTCGTGAACACTTCTTCGGTAAATATCCTGAAACCGCAGCACTGGTTGCA
Description Sequence
GACTGGACTGACGAGCAGATCTGGGCACTGAACCGTGGTGGTCACGATCC GAAGAAAATCTACGCTGCATTCAAGAAAGCGCAGGAAACCAAAGGCAAAG CGACAGTAATCCTTGCTCATACCATTAAAGGTTACGGCATGGGCGACGCG GCTGAAGGTAAAAACATCGCGCACCAGGTTAAGAAAATGAACATGGACGG TGTGCGTCATATCCGCGACCGTTTCAATGTGCCGGTGTCTGATGCAGATA TCGAAAAACTGCCGTACATCACCTTCCCGGAAGGTTCTGAAGAGCATACC TATCTGCACGCTCAGCGTCAGAAACTGCACGGTTATCTGCCAAGCCGTCA GCCGAACTTCACCGAGAAGCTTGAGCTGCCGAGCCTGCAAGACTTCGGCG CGCTGTTGGAAGAGCAGAGCAAAGAGATCTCTACCACTATCGCTTTCGTT CGTGCTCTGAACGTGATGCTGAAGAACAAGTCGATCAAAGATCGTCTGGT ACCGATCATCGCCGACGAAGCGCGTACTTTCGGTATGGAAGGTCTGTTCC GTCAGATTGGTATTTACAGCCCGAACGGTCAGCAGTACACCCCGCAGGAC CGCGAGCAGGTTGCTTACTATAAAGAAGACGAGAAAGGTCAGATTCTGCA GGAAGGGATCAACGAGCTGGGCGCAGGTTGTTCCTGGCTGGCAGCGGCGA CCTCTTACAGCACCAACAATCTGCCGATGATCCCGTTCTACATCTATTAC TCGATGTTCGGCTTCCAGCGTATTGGCGATCTGTGCTGGGCGGCTGGCGA CCAGCAAGCGCGTGGCTTCCTGATCGGCGGTACTTCCGGTCGTACCACCC TGAACGGCGAAGGTCTGCAGCACGAAGATGGTCACAGCCACATTCAGTCG CTGACTATCCCGAACTGTATCTCTTACGACCCGGCTTACGCTTACGAAGT TGCTGTCATCATGCATGACGGTCTGGAGCGTATGTACGGTGAAAAACAAG AGAACGTTTACTACTACATCACTACGCTGAACGAAAACTACCACATGCCG GCAATGCCGGAAGGTGCTGAGGAAGGTATCCGTAAAGGTATCTACAAACT CGAAACTATTGAAGGTAGCAAAGGTAAAGTTCAGCTGCTCGGCTCCGGTT CTATCCTGCGTCACGTCCGTGAAGCAGCTGAGATCCTGGCGAAAGATTAC GGCGTAGGTTCTGACGTTTATAGCGTGACCTCCTTCACCGAGCTGGCGCG TGATGGTCAGGATTGTGAACGCTGGAACATGCTGCACCCGCTGGAAACTC CGCGCGTTCCGTATATCGCTCAGGTGATGAACGACGCTCCGGCAGTGGCA TCTACCGACTATATGAAACTGTTCGCTGAGCAGGTCCGTACTTACGTACC GGCTGACGACTACCGCGTACTGGGTACTGATGGCTTCGGTCGTTCCGACA GCCGTGAGAACCTGCGTCACCACTTCGAAGTTGATGCTTCTTATGTCGTG GTTGCGGCGCTGGGCGAACTGGCTAAACGTGGCGAAATCGATAAGAAAGT GGTTGCTGACGCAATCGCCAAATTCAACATCGATGCAGATAAAGTTAACC CGCGTCTGGCGTAA
ATGGCTATCGAAATCAAAGTACCGGACATCGGGGCTGATGAAGTTGAAAT CACCGAGATCCTGGTCAAAGTGGGCGACAAAGTTGAAGCCGAACAGTCGC TGATCACCGTAGAAGGCGACAAAGCCTCTATGGAAGTTCCGTCTCCGCAG GCGGGTATCGTTAAAGAGATCAAAGTCTCTGTTGGCGATAAAACCCAGAC CGGCGCACTGATTATGATTTTCGATTCCGCCGACGGTGCAGCAGACGCTG CACCTGCTCAGGCAGAAGAGAAGAAAGAAGCAGCTCCGGCAGCAGCACCA
aceF GCGGCTGCGGCGGCAAAAGACGTTAACGTTCCGGATATCGGCAGCGACGA SEQ ID NO: AGTTGAAGTGACCGAAATCCTGGTGAAAGTTGGCGATAAAGTTGAAGCTG 61 AACAGTCGCTGATCACCGTAGAAGGCGACAAGGCTTCTATGGAAGTTCCG
GCTCCGTTTGCTGGCACCGTGAAAGAGATCAAAGTGAACGTGGGTGACAA AGTGTCTACCGGCTCGCTGATTATGGTCTTCGAAGTCGCGGGTGAAGCAG GCGCGGCAGCTCCGGCCGCTAAACAGGAAGCAGCTCCGGCAGCGGCCCCT GCACCAGCGGCTGGCGTGAAAGAAGTTAACGTTCCGGATATCGGCGGTGA CGAAGTTGAAGTGACTGAAGTGATGGTGAAAGTGGGCGACAAAGTTGCCG CTGAACAGTCACTGATCACCGTAGAAGGCGACAAAGCTTCTATGGAAGTT
Description Sequence
CCGGCGCCGTTTGCAGGCGTCGTGAAGGAACTGAAAGTCAACGTTGGCGA TAAAGTGAAAACTGGCTCGCTGATTATGATCTTCGAAGTTGAAGGCGCAG CGCCTGCGGCAGCTCCTGCGAAACAGGAAGCGGCAGCGCCGGCACCGGCA GCAAAAGCTGAAGCCCCGGCAGCAGCACCAGCTGCGAAAGCGGAAGGCAA ATCTGAATTTGCTGAAAACGACGCTTATGTTCACGCGACTCCGCTGATCC GCCGTCTGGCACGCGAGTTTGGTGTTAACCTTGCGAAAGTGAAGGGCACT GGCCGTAAAGGTCGTATCCTGCGCGAAGACGTTCAGGCTTACGTGAAAGA AGCTATCAAACGTGCAGAAGCAGCTCCGGCAGCGACTGGCGGTGGTATCC CTGGCATGCTGCCGTGGCCGAAGGTGGACTTCAGCAAGTTTGGTGAAATC GAAGAAGTGGAACTGGGCCGCATCCAGAAAATCTCTGGTGCGAACCTGAG CCGTAACTGGGTAATGATCCCGCATGTTACTCACTTCGACAAAACCGATA T C AC C GAG T T G G AAG C G T T C C G T AAAC AG C AG AAC G AAG AAG C G G C G AAA CGTAAGCTGGATGTGAAGATCACCCCGGTTGTCTTCATCATGAAAGCCGT TGCTGCAGCTCTTGAGCAGATGCCTCGCTTCAATAGTTCGCTGTCGGAAG ACGGTCAGCGTCTGACCCTGAAGAAATACATCAACATCGGTGTGGCGGTG GATACCCCGAACGGTCTGGTTGTTCCGGTATTCAAAGACGTCAACAAGAA AGGCATCATCGAGCTGTCTCGCGAGCTGATGACTATTTCTAAGAAAGCGC GTGACGGTAAGCTGACTGCGGGCGAAATGCAGGGCGGTTGCTTCACCATC TCCAGCATCGGCGGCCTGGGTACTACCCACTTCGCGCCGATTGTGAACGC GCCGGAAGTGGCTATCCTCGGCGTTTCCAAGTCCGCGATGGAGCCGGTGT GGAATGGTAAAGAGTTCGTGCCGCGTCTGATGCTGCCGATTTCTCTCTCC TTCGACCACCGCGTGATCGACGGTGCTGATGGTGCCCGTTTCATTACCAT CATTAACAACACGCTGTCTGACATTCGCCGTCTGGTGATGTAA
ATGAGTACTGAAATCAAAACTCAGGTCGTGGTACTTGGGGCAGGCCCCGC AGGTTACTCCGCTGCCTTCCGTTGCGCTGATTTAGGTCTGGAAACCGTAA TCGTAGAACGTTACAACACCCTTGGCGGTGTTTGCCTGAACGTCGGCTGT ATCCCTTCTAAAGCACTGCTGCACGTAGCAAAAGTTATCGAAGAAGCCAA AGCGCTGGCTGAACACGGTATCGTCTTCGGCGAACCGAAAACCGATATCG AC AAGAT TCGTACCTG GAAAGAGAAAG T GAT CAAT C AG C T GAC C G G T G G T CTGGCTGGTATGGCGAAAGGCCGCAAAGTCAAAGTGGTCAACGGTCTGGG TAAATTCACCGGGGCTAACACCCTGGAAGTTGAAGGTGAGAACGGCAAAA CCGTGATCAACTTCGACAACGCGATCATTGCAGCGGGTTCTCGCCCGATC CAACTGCCGTTTATTCCGCATGAAGATCCGCGTATCTGGGACTCCACTGA CGCGCTGGAACTGAAAGAAGTACCAGAACGCCTGCTGGTAATGGGTGGCG
Ipd GTATCATCGGTCTGGAAATGGGCACCGTTTACCACGCGCTGGGTTCACAG SEQ ID NO: ATTGACGTGGTTGAAATGTTCGACCAGGTTATCCCGGCAGCTGACAAAGA
62 C AT C G T TAAAG T C T T C AC C AAG C G T AT C AG C AAGAAAT T C AAC C T GAT G C
TGGAAACCAAAGTTACCGCCGTTGAAGCGAAAGAAGACGGCATTTATGTG ACGATGGAAGGCAAAAAAGCACCCGCTGAACCGCAGCGTTACGACGCCGT GCTGGTAGCGATTGGTCGTGTGCCGAACGGTAAAAACCTCGACGCAGGCA AAGCAGGCGTGGAAGTTGACGACCGTGGTTTCATCCGCGTTGACAAACAG CTGCGTACCAACGTACCGCACATCTTTGCTATCGGCGATATCGTCGGTCA ACCGATGCTGGCACACAAAGGTGTTCACGAAGGTCACGTTGCCGCTGAAG TTATCGCCGGTAAGAAACACTACTTCGATCCGAAAGTTATCCCGTCCATC GCCTATACCAAACCAGAAGTTGCATGGGTGGGTCTGACTGAGAAAGAAGC GAAAGAGAAAGGCATCAGCTATGAAACCGCCACCTTCCCGTGGGCTGCTT CTGGTCGTGCTATCGCTTCCGACTGCGCAGACGGTATGACCAAGCTGATT TTCGACAAAGAATCTCACCGTGTGATCGGTGGTGCGATTGTCGGTACTAA
Description Sequence
CGGCGGCGAGCTGCTGGGTGAAATCGGCCTGGCAATCGAAATGGGTTGTG ATGCTGAAGACATCGCACTGACCATCCACGCGCACCCGACTCTGCACGAG TCTGTGGGCCTGGCGGCAGAAGTGTTCGAAGGTAGCATTACCGACCTGCC GAAC C C GAAAG C GAAGAAGAAG T AA
AT GAGT CAGGCGC TAAAAAAT T TAC T GACAT TGTTAAAT C T GGAAAAAAT TGAGGAAGGACTCTTTCGCGGCCAGAGTGAAGATTTAGGTTTACGCCAGG TGTTTGGCGGCCAGGTCGTGGGTCAGGCCTTGTATGCTGCAAAAGAGACC GTCCCTGAAGAGCGGCTGGTACATTCGTTTCACAGCTACTTTCTTCGCCC TGGCGATAGTAAGAAGCCGATTATTTATGATGTCGAAACGCTGCGTGACG GTAACAGCTTCAGCGCCCGCCGGGTTGCTGCTATTCAAAACGGCAAACCG ATTTTTTATATGACTGCCTCTTTCCAGGCACCAGAAGCGGGTTTCGAACA TCAAAAAACAATGCCGTCCGCGCCAGCGCCTGATGGCCTCCCTTCGGAAA
tesB
CGCAAATCGCCCAATCGCTGGCGCACCTGCTGCCGCCAGTGCTGAAAGAT SEQ ID NO:
AAATTCATCTGCGATCGTCCGCTGGAAGTCCGTCCGGTGGAGTTTCATAA
48
CCCACTGAAAGGTCACGTCGCAGAACCACATCGTCAGGTGTGGATCCGCG CAAATGGTAGCGTGCCGGATGACCTGCGCGTTCATCAGTATCTGCTCGGT TACGCTTCTGATCTTAACTTCCTGCCGGTAGCTCTACAGCCGCACGGCAT CGGTTTTCTCGAACCGGGGATTCAGATTGCCACCATTGACCATTCCATGT GGTTCCATCGCCCGTTTAATTTGAATGAATGGCTGCTGTATAGCGTGGAG AGCACCTCGGCGTCCAGCGCACGTGGCTTTGTGCGCGGTGAGTTTTATAC CCAAGACGGCGTACTGGTTGCCTCGACCGTTCAGGAAGGGGTGATGCGTA AT CACAAT T AA
[0260] In some embodiments, one or more of the propionate biosynthesis genes is a synthetic propionate biosynthesis gene. In some embodiments, one or more of the propionate biosynthesis genes is an E. coli propionate
biosynthesis gene. In some embodiments, one or more of the propionate biosynthesis genes is a C. glutamicum propionate biosynthesis gene. In some embodiments, one or more of the propionate biosynthesis genes is a C.
propionicum propionate biosynthesis gene. The propionate gene cassette may comprise genes for the aerobic biosynthesis of propionate and/or genes for the anaerobic or microaerobic biosynthesis of propionate. One or more of the propionate biosynthesis genes may be functionally replaced or modified, e.g., codon optimized. In some embodiments, the genetically engineered bacteria comprise a combination of propionate biosynthesis genes from different species, strains, and/or substrains of bacteria, and are capable of producing
propionate in low-oxygen conditions, in the presence of HE-specific molecules or metabolites, in the presence of molecules or metabolites associated with liver damage, inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose. In some embodiments, one or more of the propionate biosynthesis genes is functionally replaced, modified, and/or mutated in order to enhance stability and/or increase propionate production in low-oxygen conditions, in the presence of HE-specific molecules or metabolites, in the presence of molecules or metabolites associated with liver damage, inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose. In some embodiments, the genetically engineered bacteria are capable of expressing the propionate biosynthesis cassette and producing propionate in low-oxygen conditions, in the presence of HE-specific molecules or metabolites, in the presence of molecules or metabolites associated with liver damage, inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose.
[0261] In some embodiments, the genetically engineered bacteria of the invention comprise an acetate gene cassette and produce acetate. The genetically engineered bacteria may include any suitable set of acetate biosynthesis genes. Unmodified bacteria comprising endogenous acetate biosynthesis genes are known in the art and are capable of consuming various substrates to produce acetate under aerobic and/or anaerobic conditions (see, e.g., Ragsdale et al., 2008). These endogenous acetate biosynthesis genes may be a source of genes for the genetically engineered bacteria of the invention. In some embodiments, the genetically engineered bacteria of the invention comprise acetate biosynthesis genes from a different species, strain, or substrain of bacteria. In some embodiments, the native acetate biosynthesis genes in the genetically engineered bacteria are enhanced. In some
embodiments, the genetically engineered bacteria comprise aerobic acetate biosynthesis genes, e.g., from Escherichia coli. In some embodiments, the genetically engineered bacteria comprise anaerobic acetate biosynthesis genes, e.g., from Acetitomaculum, Acetoanaerobium, Acetohalobium,
Acetonema, Balutia, Butyribacterium, Clostridium, Moorella, Oxobacter,
Sporomusa, and/or Thermoacetogenium. The genetically engineered bacteria may comprise genes for aerobic acetate biosynthesis or genes for anaerobic or microaerobic acetate biosynthesis. In some embodiments, the genetically engineered bacteria comprise both aerobic and anaerobic or microaerobic acetate biosynthesis genes. In some embodiments, the genetically engineered bacteria comprise a combination of acetate biosynthesis genes from different species, strains, and/or substrains of bacteria, and are capable of producing acetate. In some embodiments, one or more of the acetate biosynthesis genes is functionally replaced, modified, and/or mutated in order to enhance stability and/or acetate production. In some embodiments, the genetically engineered bacteria are capable of expressing the acetate biosynthesis cassette and producing acetate in low-oxygen conditions or in the presence of HE-specific molecules or metabolites. In some embodiments, the genetically engineered bacteria are capable of producing an alternate short-chain fatty acid.
[0262] One of skill in the art would appreciate that additional genes and gene cassettes capable of producing gut barrier function enhancer molecules are known in the art and may be expressed by the genetically engineered bacteria of the invention. In some embodiments, the gene or gene cassette for producing a therapeutic molecule also comprises additional transcription and translation elements, e.g., a ribosome binding site, to enhance expression of the therapeutic molecule.
[0263] In some embodiments, the genetically engineered bacteria produce two or more gut barrier function enhancer molecules. In certain embodiments, the two or more molecules behave synergistically to enhance gut barrier function. In certain embodiments, the genetically engineered bacteria express butyrate and propionate.
[0264] In some embodiments, the genetically engineered bacteria comprising an ammonia conversion circuit further comprise one or more circuits for producing a gut barrier enhancer molecule, e.g., butyrate (Fig. 43).
[0265] In some embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a GABA metabolic circuit, and one or more circuits for producing a gut barrier enhancer molecule, e.g., butyrate. In
some embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a GABA transport circuit, and one or more circuits for producing a gut barrier enhancer molecule, e.g., butyrate. In some
embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a GABA transport circuit, a GABA metabolic circuit, and one or more circuits for producing a gut barrier enhancer molecule, e.g., butyrate. In some embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a manganese transport circuit, a GABA metabolic circuit, and one or more circuits for producing a gut barrier enhancer molecule, e.g., butyrate. In some embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a manganese transport circuit, a GABA transport circuit, and one or more circuits for producing a gut barrier enhancer molecule, e.g., butyrate. In some embodiments, the genetically engineered bacteria comprise an ammonia conversion circuit, a manganese transport circuit, a GABA transport circuit, a GABA metabolic circuit, and one or more circuits for producing a gut barrier enhancer molecule, e.g., butyrate. In some embodiments, the circuits are under the control of the same promoter. In alternate embodiments, the circuits are under the control of different promoters.
[0266] In some embodiments, the genetically engineered bacteria are capable of expressing any one or more of the described circuits in low-oxygen conditions, in the presence of HE-specific molecules or metabolites, in the presence of molecules or metabolites associated with liver damage,
inflammation or an inflammatory response, or in the presence of some other metabolite that may or may not be present in the gut, such as arabinose. In some embodiments, any one or more of the described circuits are present on one or more plasmids (e.g., high copy or low copy) or are integrated into one or more sites in the bacterial chromosome. Also, in some embodiments, the genetically engineered bacteria are further capable of expressing any one or more of the described circuits and further comprise one or more of the following: (1 ) one or more auxotrophies, such as any auxotrophies known in the art and provided herein, e.g., thyA auxotrophy, (2) one or more kill switch circuits, such as any of the kill-switches described herein or otherwise known in the art, (3) one or more antibiotic resistance circuits, (4) one or more transporters for
importing biological molecules or substrates, such any of the transporters described herein or otherwise known in the art, (5) one or more secretion circuits, such as any of the secretion circuits described herein and otherwise known in the art, and (6) combinations of one or more of such additional circuits.
[0267] In any of the embodiments described herein, the genetically engineered bacteria may further comprise a resistance to rifaximin. Resistance to rifaximin is caused primarily by mutations in the rpoB gene. In some embodiments, the genetically engineered bacteria comprise a known rifaximin resistance mutation, e.g., in the rpoB gene. In other embodiments, a screen can be employed, exposing the genetically engineered bacteria to increasing amounts of rifaximin, to identify a useful mutation which confers rifaximin resistance.
Inducible Promoters
[0268] In some embodiments, the bacterial cell comprises a stably maintained plasmid or chromosome carrying the gene(s) encoding the payload (s), such that the payload(s) can be expressed in the host cell, and the host cell is capable of survival and/or growth in vitro, e.g., in medium, and/or in vivo, e.g., in the gut. In some embodiments, bacterial cell comprises two or more distinct payloads or operons, e.g., two or more payload genes. In some embodiments, bacterial cell comprises three or more distinct transporters or operons, e.g., three or more payload genes. In some embodiments, bacterial cell comprises 4, 5, 6, 7, 8, 9, 10, or more distinct payloads or operons, e.g., 4, 5, 6, 7, 8, 9, 10, or more payload genes.
[0269] In some embodiments, the genetically engineered bacteria comprise multiple copies of the same payload gene(s). In some embodiments, the gene encoding the payload is present on a plasmid and operably linked to a directly or indirectly inducible promoter. In some embodiments, the gene encoding the payload is present on a plasmid and operably linked to a promoter that is induced under low-oxygen or anaerobic conditions. In some
embodiments, the gene encoding the payload is present on a chromosome and operably linked to a directly or indirectly inducible promoter. In some
embodiments, the gene encoding the payload is present in the chromosome
and operably linked to a promoter that is induced under low-oxygen or anaerobic conditions. In some embodiments, the gene encoding the payload is present on a plasmid and operably linked to a promoter that is induced by exposure to tetracycline or arabinose.
[0270] In some embodiments, the promoter that is operably linked to the gene encoding the payload is directly induced by exogenous environmental conditions. In some embodiments, the promoter that is operably linked to the gene encoding the payload is indirectly induced by exogenous environmental conditions. In some embodiments, the promoter is directly or indirectly induced by exogenous environmental conditions specific to the gut of a mammal. In some embodiments, the promoter is directly or indirectly induced by exogenous environmental conditions specific to the small intestine of a mammal. In some embodiments, the promoter is directly or indirectly induced by low-oxygen or anaerobic conditions such as the environment of the mammalian gut. In some embodiments, the promoter is directly or indirectly induced by molecules or metabolites that are specific to the gut of a mammal. In some embodiments, the promoter is directly or indirectly induced by a molecule that is coadministered with the bacterial cell.
[0271] In certain embodiments, the bacterial cell comprises a gene encoding a payload expressed under the control of a fumarate and nitrate reductase regulator (FNR) responsive promoter. In E. coli, FNR is a major transcriptional activator that controls the switch from aerobic to anaerobic metabolism (Unden et al., 1997). In the anaerobic state, FNR dimerizes into an active DNA binding protein that activates hundreds of genes responsible for adapting to anaerobic growth. In the aerobic state, FNR is prevented from dimerizing by oxygen and is inactive. FNR responsive promoters include, but are not limited to, the FNR responsive promoters listed in the chart, below. Underlined sequences are predicted ribosome binding sites, and bolded sequences are restriction sites used for cloning.
[0272] FNR promoter sequences are known in the art, and any suitable FNR promoter sequence(s) may be used in the genetically engineered bacteria of the invention. Any suitable FNR promoter(s) may be combined with any suitable payload (e.g., the exemplary argAmr sequence shown in Table 7). Non-
limiting FNR promoter sequences are provided in Table 6. Table 6 depicts the nucleic acid sequences of exemplary regulatory region sequences comprising a FNR-responsive promoter sequence. Underlined sequences are predicted ribosome binding sites, and bolded sequences are restriction sites used for cloning. In some embodiments, the genetically engineered bacteria of the invention comprise one or more of: SEQ ID NO: 18, SEQ ID NO: 19, nirB1 promoter (SEQ ID NO: 20), nirB2 promoter (SEQ ID NO: 21 ), nirB3 promoter (SEQ ID NO: 22), ydfZ promoter (SEQ ID NO: 23), nirB promoter fused to a strong ribosome binding site (SEQ ID NO: 24), ydfZ promoter fused to a strong ribosome binding site (SEQ ID NO: 25), fnrS, an anaerobically induced small RNA gene (fnrS1 promoter SEQ ID NO: 26 or fnrS2 promoter SEQ ID NO: 27), nirB promoter fused to a crp binding site (SEQ ID NO: 28), and fnrS fused to a crp binding site (SEQ ID NO: 29).
[0273] In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 18, 19, 20, 21 , 22, 23, 24, 25, 26, 27, 28, or 29, or a functional fragment thereof.
[0274] In one embodiment, the FNR responsive promoter comprises SEQ ID NO:1. In another embodiment, the FNR responsive promoter comprises SEQ ID NO:2. In another embodiment, the FNR responsive promoter comprises SEQ ID NO:3. In another embodiment, the FNR
responsive promoter comprises SEQ ID NO:4. In yet another embodiment, the FNR responsive promoter comprises SEQ ID NO:5.
[0275] In some embodiments, multiple distinct FNR nucleic acid sequences are inserted in the genetically engineered bacteria. In alternate embodiments, the genetically engineered bacteria comprise a gene encoding a payload expressed under the control of an alternate oxygen level-dependent promoter, e.g., DNR (Trunk et al., 2010) or ANR (Ray et al., 1997). In these embodiments, expression of the payload gene is particularly activated in a low- oxygen or anaerobic environment, such as in the gut. In some embodiments, gene expression is further optimized by methods known in the art, e.g., by
optimizing ribosomal binding sites and/or increasing mRNA stability. In one embodiment, the mammalian gut is a human mammalian gut.
[0276] In some embodiments, the bacterial cell comprises an oxygen- level dependent transcriptional regulator, e.g., FNR, ANR, or DNR, and corresponding promoter from a different bacterial species. The heterologous oxygen-level dependent transcriptional regulator and promoter increase the transcription of genes operably linked to said promoter, e.g., the gene encoding the payload, in a low-oxygen or anaerobic environment, as compared to the native gene(s) and promoter in the bacteria under the same conditions. In certain embodiments, the non-native oxygen-level dependent transcriptional regulator is an FNR protein from N. gonorrhoeae (see, e.g., Isabella et al., 2011 ). In some embodiments, the corresponding wild-type transcriptional regulator is left intact and retains wild-type activity. In alternate embodiments, the corresponding wild-type transcriptional regulator is deleted or mutated to reduce or eliminate wild-type activity.
[0277] In some embodiments, the genetically engineered bacteria comprise a wild-type oxygen-level dependent transcriptional regulator, e.g., FNR, ANR, or DNR, and corresponding promoter that is mutated relative to the wild-type promoter from bacteria of the same subtype. The mutated promoter enhances binding to the wild-type transcriptional regulator and increases the transcription of genes operably linked to said promoter, e.g., the gene encoding the payload, in a low-oxygen or anaerobic environment, as compared to the wild-type promoter under the same conditions. In some embodiments, the genetically engineered bacteria comprise a wild-type oxygen-level dependent promoter, e.g., FNR, ANR, or DNR promoter, and corresponding transcriptional regulator that is mutated relative to the wild-type transcriptional regulator from bacteria of the same subtype. The mutated transcriptional regulator enhances binding to the wild-type promoter and increases the transcription of genes operably linked to said promoter, e.g., the gene encoding the payload, in a low- oxygen or anaerobic environment, as compared to the wild-type transcriptional regulator under the same conditions. In certain embodiments, the mutant oxygen-level dependent transcriptional regulator is an FNR protein comprising
amino acid substitutions that enhance dimerization and FNR activity (see, e.g., Moore et al., (2006).
[0278] In some embodiments, the bacterial cells comprise multiple copies of the endogenous gene encoding the oxygen level-sensing transcriptional regulator, e.g., the FNR gene. In some embodiments, the gene encoding the oxygen level-sensing transcriptional regulator is present on a plasm id. In some embodiments, the gene encoding the oxygen level-sensing transcriptional regulator and the gene encoding the payload are present on different plasm ids. In some embodiments, the gene encoding the oxygen level-sensing
transcriptional regulator and the gene encoding the payload are present on the same plasmid. In some embodiments, the gene encoding the oxygen level- sensing transcriptional regulator is present on a chromosome. In some embodiments, the gene encoding the oxygen level-sensing transcriptional regulator and the gene encoding the payload are present on different
chromosomes. In some embodiments, the gene encoding the oxygen level- sensing transcriptional regulator and the gene encoding the payload are present on the same chromosome. In some instances, it may be advantageous to express the oxygen level-sensing transcriptional regulator under the control of an inducible promoter in order to enhance expression stability. In some embodiments, expression of the transcriptional regulator is controlled by a different promoter than the promoter that controls expression of the gene encoding the payload. In some embodiments, expression of the transcriptional regulator is controlled by the same promoter that controls expression of the payload. In some embodiments, the transcriptional regulator and the payload are divergently transcribed from a promoter region.
RNS-dependent regulation
[0279] In some embodiments, the genetically engineered bacteria or genetically engineered virus comprise a gene encoding a payload that is expressed under the control of an inducible promoter. In some embodiments, the genetically engineered bacterium or genetically engineered virus that expresses a payload under the control of a promoter that is activated by inflammatory conditions. In one embodiment, the gene for producing the payload is expressed under the control of an inflammatory-dependent promoter
that is activated in inflammatory environments, e.g., a reactive nitrogen species or RNS promoter.
[0280]As used herein, "reactive nitrogen species" and "RNS" are used interchangeably to refer to highly active molecules, ions, and/or radicals derived from molecular nitrogen. RNS can cause deleterious cellular effects such as nitrosative stress. RNS includes, but is not limited to, nitric oxide (ΝΟ·), peroxynitrite or peroxynitrite anion (ONOO-), nitrogen dioxide (·Ν02), dinitrogen trioxide (N203), peroxynitrous acid (ONOOH), and nitroperoxycarbonate (ONOOC02-) (unpaired electrons denoted by ·). Bacteria have evolved transcription factors that are capable of sensing RNS levels. Different RNS signaling pathways are triggered by different RNS levels and occur with different kinetics.
[0281]As used herein, "RNS-inducible regulatory region" refers to a nucleic acid sequence to which one or more RNS-sensing transcription factors is capable of binding, wherein the binding and/or activation of the corresponding transcription factor activates downstream gene expression; in the presence of RNS, the transcription factor binds to and/or activates the regulatory region. In some embodiments, the RNS-inducible regulatory region comprises a promoter sequence. In some embodiments, the transcription factor senses RNS and subsequently binds to the RNS-inducible regulatory region, thereby activating downstream gene expression. In alternate embodiments, the transcription factor is bound to the RNS-inducible regulatory region in the absence of RNS; in the presence of RNS, the transcription factor undergoes a conformational change, thereby activating downstream gene expression. The RNS-inducible regulatory region may be operatively linked to a gene or genes, e.g., a payload gene sequence(s), e.g., any of the payloads described herein. For example, in the presence of RNS, a transcription factor senses RNS and activates a corresponding RNS-inducible regulatory region, thereby driving expression of an operatively linked gene sequence. Thus, RNS induces expression of the gene or gene sequences.
[0282]As used herein, "RNS-derepressible regulatory region" refers to a nucleic acid sequence to which one or more RNS-sensing transcription factors is capable of binding, wherein the binding of the corresponding transcription
factor represses downstream gene expression; in the presence of RNS, the transcription factor does not bind to and does not repress the regulatory region. In some embodiments, the RNS-derepressible regulatory region comprises a promoter sequence. The RNS-derepressible regulatory region may be operatively linked to a gene or genes, e.g., a payload gene sequence(s). For example, in the presence of RNS, a transcription factor senses RNS and no longer binds to and/or represses the regulatory region, thereby derepressing an operatively linked gene sequence or gene cassette. Thus, RNS derepresses expression of the gene or genes.
[0283] As used herein, "RNS-repressible regulatory region" refers to a nucleic acid sequence to which one or more RNS-sensing transcription factors is capable of binding, wherein the binding of the corresponding transcription factor represses downstream gene expression; in the presence of RNS, the transcription factor binds to and represses the regulatory region. In some embodiments, the RNS-repressible regulatory region comprises a promoter sequence. In some embodiments, the transcription factor that senses RNS is capable of binding to a regulatory region that overlaps with part of the promoter sequence. In alternate embodiments, the transcription factor that senses RNS is capable of binding to a regulatory region that is upstream or downstream of the promoter sequence. The RNS-repressible regulatory region may be operatively linked to a gene sequence or gene cassette. For example, in the presence of RNS, a transcription factor senses RNS and binds to a
corresponding RNS-repressible regulatory region, thereby blocking expression of an operatively linked gene sequence or gene sequences. Thus, RNS represses expression of the gene or gene sequences.
[0284]As used herein, a "RNS-responsive regulatory region" refers to a RNS-inducible regulatory region, a RNS-repressible regulatory region, and/or a RNS-derepressible regulatory region. In some embodiments, the RNS- responsive regulatory region comprises a promoter sequence. Each regulatory region is capable of binding at least one corresponding RNS-sensing
transcription factor. Examples of transcription factors that sense RNS and their corresponding RNS-responsive genes, promoters, and/or regulatory regions include, but are not limited to, those shown in Table A.
Table A. Examples of RNS-sensing transcription factors and RNS- responsive genes
[0285] In some embodiments, the genetically engineered bacteria of the invention comprise a tunable regulatory region that is directly or indirectly controlled by a transcription factor that is capable of sensing at least one reactive nitrogen species. The tunable regulatory region is operatively linked to a gene or genes capable of directly or indirectly driving the expression of a payload, thus controlling expression of the payload relative to RNS levels. For example, the tunable regulatory region is a RNS-inducible regulatory region, and the payload is a payload, such as any of the payloads provided herein; when RNS is present, e.g., in an inflamed tissue, a RNS-sensing transcription factor binds to and/or activates the regulatory region and drives expression of the payload gene or genes. Subsequently, when inflammation is ameliorated, RNS levels are reduced, and production of the payload is decreased or eliminated.
[0286] In some embodiments, the tunable regulatory region is a RNS- inducible regulatory region; in the presence of RNS, a transcription factor senses RNS and activates the RNS-inducible regulatory region, thereby driving expression of an operatively linked gene or genes. In some embodiments, the transcription factor senses RNS and subsequently binds to the RNS-inducible regulatory region, thereby activating downstream gene expression. In alternate embodiments, the transcription factor is bound to the RNS-inducible regulatory region in the absence of RNS; when the transcription factor senses RNS, it undergoes a conformational change, thereby inducing downstream gene expression.
[0287] In some embodiments, the tunable regulatory region is a RNS- inducible regulatory region, and the transcription factor that senses RNS is NorR. NorR "is an NO-responsive transcriptional activator that regulates expression of the norVW genes encoding flavorubredoxin and an associated flavoprotein, which reduce NO to nitrous oxide" (Spiro 2006). The genetically engineered bacteria of the invention may comprise any suitable RNS- responsive regulatory region from a gene that is activated by NorR. Genes that are capable of being activated by NorR are known in the art (see, e.g., Spiro 2006; Vine et al., 2011 ; Karlinsey et al., 2012). In certain embodiments, the genetically engineered bacteria of the invention comprise a RNS-inducible regulatory region from norVW that is operatively linked to a gene or genes, e.g., one or more payload gene sequence(s). In the presence of RNS, a NorR transcription factor senses RNS and activates to the norVW regulatory region, thereby driving expression of the operatively linked gene(s) and producing the payload.
[0288] In some embodiments, the tunable regulatory region is a RNS- inducible regulatory region, and the transcription factor that senses RNS is DNR. DNR (dissimilatory nitrate respiration regulator) "promotes the
expression of the nir, the nor and the nos genes" in the presence of nitric oxide (Castiglione et al., 2009). The genetically engineered bacteria of the invention may comprise any suitable RNS-responsive regulatory region from a gene that is activated by DNR. Genes that are capable of being activated by DNR are known in the art (see, e.g., Castiglione et al., 2009; Giardina et al., 2008). In certain embodiments, the genetically engineered bacteria of the invention comprise a RNS-inducible regulatory region from norCB that is operatively linked to a gene or gene cassette, e.g., a butyrogenic gene cassette. In the presence of RNS, a DNR transcription factor senses RNS and activates to the norCB regulatory region, thereby driving expression of the operatively linked gene or genes and producing one or more payloads. In some embodiments, the DNR is Pseudomonas aeruginosa DNR.
[0289] In some embodiments, the tunable regulatory region is a RNS- derepressible regulatory region, and binding of a corresponding transcription factor represses downstream gene expression; in the presence of RNS, the
transcription factor no longer binds to the regulatory region, thereby
derepressing the operatively linked gene or gene cassette.
[0290] In some embodiments, the tunable regulatory region is a RNS- derepressible regulatory region, and the transcription factor that senses RNS is NsrR. NsrR is "an Rrf2-type transcriptional repressor [that] can sense NO and control the expression of genes responsible for NO metabolism" (Isabella et al., 2009). The genetically engineered bacteria of the invention may comprise any suitable RNS-responsive regulatory region from a gene that is repressed by NsrR. In some embodiments, the NsrR is Neisseria gonorrhoeae NsrR. Genes that are capable of being repressed by NsrR are known in the art (see, e.g., Isabella et al., 2009; Dunn et al., 2010). In certain embodiments, the genetically engineered bacteria of the invention comprise a RNS-derepressible regulatory region from norB that is operatively linked to a gene or genes, e.g., a payload gene or genes. In the presence of RNS, an NsrR transcription factor senses RNS and no longer binds to the norB regulatory region, thereby derepressing the operatively linked a payload gene or genes and producing the encoding a payload(s).
[0291] In some embodiments, it is advantageous for the genetically engineered bacteria to express a RNS-sensing transcription factor that does not regulate the expression of a significant number of native genes in the bacteria. In some embodiments, the genetically engineered bacterium of the invention expresses a RNS-sensing transcription factor from a different species, strain, or substrain of bacteria, wherein the transcription factor does not bind to regulatory sequences in the genetically engineered bacterium of the invention. In some embodiments, the genetically engineered bacterium of the invention is
Escherichia coli, and the RNS-sensing transcription factor is NsrR, e.g., from is Neisseria gonorrhoeae, wherein the Escherichia coli does not comprise binding sites for said NsrR. In some embodiments, the heterologous transcription factor minimizes or eliminates off-target effects on endogenous regulatory regions and genes in the genetically engineered bacteria.
[0292] In some embodiments, the tunable regulatory region is a RNS- repressible regulatory region, and binding of a corresponding transcription factor represses downstream gene expression; in the presence of RNS, the
transcription factor senses RNS and binds to the RNS-repressible regulatory region, thereby repressing expression of the operatively linked gene or gene cassette. In some embodiments, the RNS-sensing transcription factor is capable of binding to a regulatory region that overlaps with part of the promoter sequence. In alternate embodiments, the RNS-sensing transcription factor is capable of binding to a regulatory region that is upstream or downstream of the promoter sequence.
[0293] In these embodiments, the genetically engineered bacteria may comprise a two repressor activation regulatory circuit, which is used to express a payload. The two repressor activation regulatory circuit comprises a first RNS-sensing repressor and a second repressor, which is operatively linked to a gene or gene cassette, e.g., encoding a payload. In one aspect of these embodiments, the RNS-sensing repressor inhibits transcription of the second repressor, which inhibits the transcription of the gene or gene cassette.
Examples of second repressors useful in these embodiments include, but are not limited to, TetR, C1 , and LexA. In the absence of binding by the first repressor (which occurs in the absence of RNS), the second repressor is transcribed, which represses expression of the gene or genes. In the presence of binding by the first repressor (which occurs in the presence of RNS), expression of the second repressor is repressed, and the gene or genes, e.g., a payload gene or genes is expressed.
[0294] A RNS-responsive transcription factor may induce, derepress, or repress gene expression depending upon the regulatory region sequence used in the genetically engineered bacteria. One or more types of RNS-sensing transcription factors and corresponding regulatory region sequences may be present in genetically engineered bacteria. In some embodiments, the genetically engineered bacteria comprise one type of RNS-sensing transcription factor, e.g., NsrR, and one corresponding regulatory region sequence, e.g., from norB. In some embodiments, the genetically engineered bacteria comprise one type of RNS-sensing transcription factor, e.g., NsrR, and two or more different corresponding regulatory region sequences, e.g., from norB and aniA. In some embodiments, the genetically engineered bacteria comprise two or more types of RNS-sensing transcription factors, e.g., NsrR and NorR, and
two or more corresponding regulatory region sequences, e.g., from norB and norR, respectively. One RNS-responsive regulatory region may be capable of binding more than one transcription factor. In some embodiments, the genetically engineered bacteria comprise two or more types of RNS-sensing transcription factors and one corresponding regulatory region sequence.
Nucleic acid sequences of several RNS-regulated regulatory regions are known in the art (see, e.g., Spiro 2006; Isabella et al., 2009; Dunn et al., 2010; Vine et al., 2011 ; Karlinsey et al., 2012).
[0295] In some embodiments, the genetically engineered bacteria of the invention comprise a gene encoding a RNS-sensing transcription factor, e.g., the nsrR gene, that is controlled by its native promoter, an inducible promoter, a promoter that is stronger than the native promoter, e.g., the GlnRS promoter or the P(Bla) promoter, or a constitutive promoter. In some instances, it may be advantageous to express the RNS-sensing transcription factor under the control of an inducible promoter in order to enhance expression stability. In some embodiments, expression of the RNS-sensing transcription factor is controlled by a different promoter than the promoter that controls expression of the therapeutic molecule. In some embodiments, expression of the RNS-sensing transcription factor is controlled by the same promoter that controls expression of the therapeutic molecule. In some embodiments, the RNS-sensing
transcription factor and therapeutic molecule are divergently transcribed from a promoter region.
[0296] In some embodiments, the genetically engineered bacteria of the invention comprise a gene for a RNS-sensing transcription factor from a different species, strain, or substrain of bacteria. In some embodiments, the genetically engineered bacteria comprise a RNS-responsive regulatory region from a different species, strain, or substrain of bacteria. In some embodiments, the genetically engineered bacteria comprise a RNS-sensing transcription factor and corresponding RNS-responsive regulatory region from a different species, strain, or substrain of bacteria. The heterologous RNS-sensing transcription factor and regulatory region may increase the transcription of genes operatively linked to said regulatory region in the presence of RNS, as compared to the
native transcription factor and regulatory region from bacteria of the same subtype under the same conditions.
[0297] In some embodiments, the genetically engineered bacteria comprise a RNS-sensing transcription factor, NsrR, and corresponding regulatory region, nsrR, from Neisseria gonorrhoeae. In some embodiments, the native RNS-sensing transcription factor, e.g., NsrR, is left intact and retains wild-type activity. In alternate embodiments, the native RNS-sensing
transcription factor, e.g., NsrR, is deleted or mutated to reduce or eliminate wild- type activity.
[0298] In some embodiments, the genetically engineered bacteria of the invention comprise multiple copies of the endogenous gene encoding the RNS- sensing transcription factor, e.g., the nsrR gene. In some embodiments, the gene encoding the RNS-sensing transcription factor is present on a plasmid. In some embodiments, the gene encoding the RNS-sensing transcription factor and the gene or gene cassette for producing the therapeutic molecule are present on different plasmids. In some embodiments, the gene encoding the RNS-sensing transcription factor and the gene or gene cassette for producing the therapeutic molecule are present on the same plasmid. In some
embodiments, the gene encoding the RNS-sensing transcription factor is present on a chromosome. In some embodiments, the gene encoding the RNS-sensing transcription factor and the gene or gene cassette for producing the therapeutic molecule are present on different chromosomes. In some embodiments, the gene encoding the RNS-sensing transcription factor and the gene or gene cassette for producing the therapeutic molecule are present on the same chromosome.
[0299] In some embodiments, the genetically engineered bacteria comprise a wild-type gene encoding a RNS-sensing transcription factor, e.g., the NsrR gene, and a corresponding regulatory region, e.g., a norB regulatory region, that is mutated relative to the wild-type regulatory region from bacteria of the same subtype. The mutated regulatory region increases the expression of the payload in the presence of RNS, as compared to the wild-type regulatory region under the same conditions. In some embodiments, the genetically engineered bacteria comprise a wild-type RNS-responsive regulatory region,
e.g., the norB regulatory region, and a corresponding transcription factor, e.g., NsrR, that is mutated relative to the wild-type transcription factor from bacteria of the same subtype. The mutant transcription factor increases the expression of the payload in the presence of RNS, as compared to the wild-type
transcription factor under the same conditions. In some embodiments, both the RNS-sensing transcription factor and corresponding regulatory region are mutated relative to the wild-type sequences from bacteria of the same subtype in order to increase expression of the payload in the presence of RNS.
[0300] In some embodiments, the gene or gene cassette for producing the anti-inflammation and/or gut barrier function enhancer molecule is present on a plasmid and operably linked to a promoter that is induced by RNS. In some embodiments, expression is further optimized by methods known in the art, e.g., by optimizing ribosomal binding sites, manipulating transcriptional regulators, and/or increasing mRNA stability.
[0301] In some embodiments, any of the gene(s) of the present disclosure may be integrated into the bacterial chromosome at one or more integration sites. For example, one or more copies of one or more encoding a payload gene(s) may be integrated into the bacterial chromosome. Having multiple copies of the gene or gen(s) integrated into the chromosome allows for greater production of the payload(s) and also permits fine-tuning of the level of expression. Alternatively, different circuits described herein, such as any of the secretion or exporter circuits, in addition to the therapeutic gene(s) or gene cassette(s) could be integrated into the bacterial chromosome at one or more different integration sites to perform multiple different functions.
ROS-dependent regulation
[0302] In some embodiments, the genetically engineered bacteria or genetically engineered virus comprise a gene for producing a payload that is expressed under the control of an inducible promoter. In some embodiments, the genetically engineered bacterium or genetically engineered virus that expresses a payload under the control of a promoter that is activated by conditions of cellular damage. In one embodiment, the gene for producing the payload is expressed under the control of an cellular damaged-dependent
promoter that is activated in environments in which there is cellular or tissue damage, e.g., a reactive oxygen species or ROS promoter.
[0303] As used herein, "reactive oxygen species" and "ROS" are used interchangeably to refer to highly active molecules, ions, and/or radicals derived from molecular oxygen. ROS can be produced as byproducts of aerobic respiration or metal-catalyzed oxidation and may cause deleterious cellular effects such as oxidative damage. ROS includes, but is not limited to, hydrogen peroxide (H2O2), organic peroxide (ROOH), hydroxyl ion (OH-), hydroxyl radical (·ΟΗ), superoxide or superoxide anion (Ό2-), singlet oxygen (1 Ο2), ozone (O3), carbonate radical, peroxide or peroxyl radical (·Ο2-2),
hypochlorous acid (HOCI), hypochlorite ion (OCI-), sodium hypochlorite
(NaOCI), nitric oxide (ΝΟ·), and peroxynitrite or peroxynitrite anion (ONOO-) (unpaired electrons denoted by ·). Bacteria have evolved transcription factors that are capable of sensing ROS levels. Different ROS signaling pathways are triggered by different ROS levels and occur with different kinetics (Marinho et al., 2014).
[0304]As used herein, "ROS-inducible regulatory region" refers to a nucleic acid sequence to which one or more ROS-sensing transcription factors is capable of binding, wherein the binding and/or activation of the corresponding transcription factor activates downstream gene expression; in the presence of ROS, the transcription factor binds to and/or activates the regulatory region. In some embodiments, the ROS-inducible regulatory region comprises a promoter sequence. In some embodiments, the transcription factor senses ROS and subsequently binds to the ROS-inducible regulatory region, thereby activating downstream gene expression. In alternate embodiments, the transcription factor is bound to the ROS-inducible regulatory region in the absence of ROS; in the presence of ROS, the transcription factor undergoes a conformational change, thereby activating downstream gene expression. The ROS-inducible regulatory region may be operatively linked to a gene sequence or gene sequence, e.g., a sequence or sequences encoding one or more payload(s). For example, in the presence of ROS, a transcription factor, e.g., OxyR, senses ROS and activates a corresponding ROS-inducible regulatory region, thereby
driving expression of an operatively linked gene sequence or gene sequences. Thus, ROS induces expression of the gene or genes.
[0305]As used herein, "ROS-derepressible regulatory region" refers to a nucleic acid sequence to which one or more ROS-sensing transcription factors is capable of binding, wherein the binding of the corresponding transcription factor represses downstream gene expression; in the presence of ROS, the transcription factor does not bind to and does not repress the regulatory region. In some embodiments, the ROS-derepressible regulatory region comprises a promoter sequence. The ROS-derepressible regulatory region may be operatively linked to a gene or genes, e.g., one or more genes encoding one or more payload(s). For example, in the presence of ROS, a transcription factor, e.g., OhrR, senses ROS and no longer binds to and/or represses the regulatory region, thereby derepressing an operatively linked gene sequence or gene cassette. Thus, ROS derepresses expression of the gene or gene cassette.
[0306]As used herein, "ROS-repressible regulatory region" refers to a nucleic acid sequence to which one or more ROS-sensing transcription factors is capable of binding, wherein the binding of the corresponding transcription factor represses downstream gene expression; in the presence of ROS, the transcription factor binds to and represses the regulatory region. In some embodiments, the ROS-repressible regulatory region comprises a promoter sequence. In some embodiments, the transcription factor that senses ROS is capable of binding to a regulatory region that overlaps with part of the promoter sequence. In alternate embodiments, the transcription factor that senses ROS is capable of binding to a regulatory region that is upstream or downstream of the promoter sequence. The ROS-repressible regulatory region may be operatively linked to a gene sequence or gene sequences. For example, in the presence of ROS, a transcription factor, e.g., PerR, senses ROS and binds to a corresponding ROS-repressible regulatory region, thereby blocking expression of an operatively linked gene sequence or gene sequences. Thus, ROS represses expression of the gene or genes.
[0307]As used herein, a "ROS-responsive regulatory region" refers to a ROS-inducible regulatory region, a ROS-repressible regulatory region, and/or a ROS-derepressible regulatory region. In some embodiments, the ROS-
responsive regulatory region comprises a promoter sequence. Each regulatory region is capable of binding at least one corresponding ROS-sensing
transcription factor. Examples of transcription factors that sense ROS and their corresponding ROS-responsive genes, promoters, and/or regulatory regions include, but are not limited to, those shown in Table B.
Table B. Examples of ROS-sensing transcription factors and ROS- responsive genes
[0308] In some embodiments, the genetically engineered bacteria comprise a tunable regulatory region that is directly or indirectly controlled by a transcription factor that is capable of sensing at least one reactive oxygen species. The tunable regulatory region is operatively linked to a gene or gene cassette capable of directly or indirectly driving the expression of a payload, thus controlling expression of the payload relative to ROS levels. For example, the tunable regulatory region is a ROS-inducible regulatory region, and the molecule is a payload; when ROS is present, e.g., in an inflamed tissue, a ROS- sensing transcription factor binds to and/or activates the regulatory region and drives expression of the gene sequence for the payload, thereby producing the
payload. Subsequently, when inflammation is ameliorated, ROS levels are reduced, and production of the payload is decreased or eliminated.
[0309] In some embodiments, the tunable regulatory region is a ROS- inducible regulatory region; in the presence of ROS, a transcription factor senses ROS and activates the ROS-inducible regulatory region, thereby driving expression of an operatively linked gene or gene cassette. In some
embodiments, the transcription factor senses ROS and subsequently binds to the ROS-inducible regulatory region, thereby activating downstream gene expression. In alternate embodiments, the transcription factor is bound to the ROS-inducible regulatory region in the absence of ROS; when the transcription factor senses ROS, it undergoes a conformational change, thereby inducing downstream gene expression.
[0310] In some embodiments, the tunable regulatory region is a ROS- inducible regulatory region, and the transcription factor that senses ROS is OxyR. OxyR "functions primarily as a global regulator of the peroxide stress response" and is capable of regulating dozens of genes, e.g., "genes involved in H2O2 detoxification (katE, ahpCF), heme biosynthesis (hemH), reductant supply (grxA, gor, trxC), thiol-disulfide isomerization (dsbG), Fe-S center repair (sufA-E, sufS), iron binding (yaaA), repression of iron import systems (fur)" and "OxyS, a small regulatory RNA" (Dubbs et al., 2012). The genetically
engineered bacteria may comprise any suitable ROS-responsive regulatory region from a gene that is activated by OxyR. Genes that are capable of being activated by OxyR are known in the art (see, e.g., Zheng et al., 2001 ; Dubbs et al., 2012). In certain embodiments, the genetically engineered bacteria of the invention comprise a ROS-inducible regulatory region from oxyS that is operatively linked to a gene, e.g., a payload gene. In the presence of ROS, e.g., H2O2, an OxyR transcription factor senses ROS and activates to the oxyS regulatory region, thereby driving expression of the operatively linked payload gene and producing the payload. In some embodiments, OxyR is encoded by an E. coli oxyR gene. In some embodiments, the oxyS regulatory region is an E. coli oxyS regulatory region. In some embodiments, the ROS-inducible regulatory region is selected from the regulatory region of katG, dps, and ahpC.
[0311] In alternate embodiments, the tunable regulatory region is a ROS- inducible regulatory region, and the corresponding transcription factor that senses ROS is SoxR. When SoxR is "activated by oxidation of its [2Fe-2S] cluster, it increases the synthesis of SoxS, which then activates its target gene expression" (Koo et al., 2003). "SoxR is known to respond primarily to superoxide and nitric oxide" (Koo et al., 2003), and is also capable of
responding to H2O2. The genetically engineered bacteria of the invention may comprise any suitable ROS-responsive regulatory region from a gene that is activated by SoxR. Genes that are capable of being activated by SoxR are known in the art (see, e.g., Koo et al., 2003). In certain embodiments, the genetically engineered bacteria of the invention comprise a ROS-inducible regulatory region from soxS that is operatively linked to a gene, e.g., a payload. In the presence of ROS, the SoxR transcription factor senses ROS and activates the soxS regulatory region, thereby driving expression of the operatively linked a payload gene and producing the a payload.
[0312] In some embodiments, the tunable regulatory region is a ROS- derepressible regulatory region, and binding of a corresponding transcription factor represses downstream gene expression; in the presence of ROS, the transcription factor no longer binds to the regulatory region, thereby
derepressing the operatively linked gene or gene cassette.
[0313] In some embodiments, the tunable regulatory region is a ROS- derepressible regulatory region, and the transcription factor that senses ROS is OhrR. OhrR "binds to a pair of inverted repeat DNA sequences overlapping the ohrA promoter site and thereby represses the transcription event," but oxidized OhrR is "unable to bind its DNA target" (Duarte et al., 2010). OhrR is a
"transcriptional repressor [that]... senses both organic peroxides and NaOCI" (Dubbs et al., 2012) and is "weakly activated by H2O2 but it shows much higher reactivity for organic hydroperoxides" (Duarte et al., 2010). The genetically engineered bacteria of the invention may comprise any suitable ROS- responsive regulatory region from a gene that is repressed by OhrR. Genes that are capable of being repressed by OhrR are known in the art (see, e.g., Dubbs et al., 2012). In certain embodiments, the genetically engineered bacteria of the invention comprise a ROS-derepressible regulatory region from
ohrA that is operatively linked to a gene or gene cassette, e.g., a payload gene. In the presence of ROS, e.g., NaOCI, an OhrR transcription factor senses ROS and no longer binds to the ohrA regulatory region, thereby derepressing the operatively linked payload gene and producing the a payload.
[0314] OhrR is a member of the MarR family of ROS-responsive regulators. "Most members of the MarR family are transcriptional repressors and often bind to the -10 or -35 region in the promoter causing a steric inhibition of RNA polymerase binding" (Bussmann et al., 2010). Other members of this family are known in the art and include, but are not limited to, OspR, MgrA, RosR, and SarZ. In some embodiments, the transcription factor that senses ROS is OspR, MgRA, RosR, and/or SarZ, and the genetically engineered bacteria of the invention comprises one or more corresponding regulatory region sequences from a gene that is repressed by OspR, MgRA, RosR, and/or SarZ. Genes that are capable of being repressed by OspR, MgRA, RosR, and/or SarZ are known in the art (see, e.g., Dubbs et al., 2012).
[0315] In some embodiments, the tunable regulatory region is a ROS- derepressible regulatory region, and the corresponding transcription factor that senses ROS is RosR. RosR is "a MarR-type transcriptional regulator" that binds to an "18-bp inverted repeat with the consensus sequence
TTGTTGAYRYRTCAACWA" and is "reversibly inhibited by the oxidant H2O2" (Bussmann et al., 2010). RosR is capable of repressing numerous genes and putative genes, including but not limited to "a putative polyisoprenoid-binding protein (cg1322, gene upstream of and divergent from rosR), a sensory histidine kinase (cgtS9), a putative transcriptional regulator of the Crp/FNR family (cg3291 ), a protein of the glutathione S-transferase family (cg1426), two putative FMN reductases (cg1150 and cg1850), and four putative
monooxygenases (cg0823, cg1848, cg2329, and cg3084)" (Bussmann et al., 2010). The genetically engineered bacteria of the invention may comprise any suitable ROS-responsive regulatory region from a gene that is repressed by RosR. Genes that are capable of being repressed by RosR are known in the art (see, e.g., Bussmann et al., 2010). In certain embodiments, the genetically engineered bacteria of the invention comprise a ROS-derepressible regulatory region from cgtS9 that is operatively linked to a gene or gene cassette, e.g., a
payload. In the presence of ROS, e.g., H2O2, a RosR transcription factor senses ROS and no longer binds to the cgtS9 regulatory region, thereby derepressing the operatively linked payload gene and producing the payload.
[0316] In some embodiments, it is advantageous for the genetically engineered bacteria to express a ROS-sensing transcription factor that does not regulate the expression of a significant number of native genes in the bacteria. In some embodiments, the genetically engineered bacterium of the invention expresses a ROS-sensing transcription factor from a different species, strain, or substrain of bacteria, wherein the transcription factor does not bind to regulatory sequences in the genetically engineered bacterium of the invention. In some embodiments, the genetically engineered bacterium of the invention is
Escherichia coli, and the ROS-sensing transcription factor is RosR, e.g., from Corynebacterium glutamicum, wherein the Escherichia coli does not comprise binding sites for said RosR. In some embodiments, the heterologous
transcription factor minimizes or eliminates off-target effects on endogenous regulatory regions and genes in the genetically engineered bacteria.
[0317] In some embodiments, the tunable regulatory region is a ROS- repressible regulatory region, and binding of a corresponding transcription factor represses downstream gene expression; in the presence of ROS, the transcription factor senses ROS and binds to the ROS-repressible regulatory region, thereby repressing expression of the operatively linked gene or gene cassette. In some embodiments, the ROS-sensing transcription factor is capable of binding to a regulatory region that overlaps with part of the promoter sequence. In alternate embodiments, the ROS-sensing transcription factor is capable of binding to a regulatory region that is upstream or downstream of the promoter sequence.
[0318] In some embodiments, the tunable regulatory region is a ROS- repressible regulatory region, and the transcription factor that senses ROS is PerR. In Bacillus subtilis, PerR "when bound to DNA, represses the genes coding for proteins involved in the oxidative stress response (katA, ahpC, and mrgA), metal homeostasis (hemAXCDBL, fur, and zoaA) and its own synthesis (perR)" (Marinho et al., 2014). PerR is a "global regulator that responds primarily to H2O2" (Dubbs et al., 2012) and "interacts with DNA at the per box,
a specific palindromic consensus sequence (TTATAATNATTATAA) residing within and near the promoter sequences of PerR-controlled genes" (Marinho et al., 2014). PerR is capable of binding a regulatory region that "overlaps part of the promoter or is immediately downstream from it" (Dubbs et al., 2012). The genetically engineered bacteria of the invention may comprise any suitable ROS-responsive regulatory region from a gene that is repressed by PerR.
Genes that are capable of being repressed by PerR are known in the art (see, e.g., Dubbs et al., 2012).
[0319] In these embodiments, the genetically engineered bacteria may comprise a two repressor activation regulatory circuit, which is used to express a payload. The two repressor activation regulatory circuit comprises a first ROS-sensing repressor, e.g., PerR, and a second repressor, e.g., TetR, which is operatively linked to a gene or gene cassette, e.g., a payload. In one aspect of these embodiments, the ROS-sensing repressor inhibits transcription of the second repressor, which inhibits the transcription of the gene or gene cassette. Examples of second repressors useful in these embodiments include, but are not limited to, TetR, C1 , and LexA. In some embodiments, the ROS-sensing repressor is PerR. In some embodiments, the second repressor is TetR. In this embodiment, a PerR-repressible regulatory region drives expression of TetR, and a TetR-repressible regulatory region drives expression of the gene or gene cassette, e.g., a payload. In the absence of PerR binding (which occurs in the absence of ROS), tetR is transcribed, and TetR represses expression of the gene or gene cassette, e.g., a payload. In the presence of PerR binding (which occurs in the presence of ROS), tetR expression is repressed, and the gene or gene cassette, e.g., a payload, is expressed.
[0320] A ROS-responsive transcription factor may induce, derepress, or repress gene expression depending upon the regulatory region sequence used in the genetically engineered bacteria. For example, although "OxyR is primarily thought of as a transcriptional activator under oxidizing conditions... OxyR can function as either a repressor or activator under both oxidizing and reducing conditions" (Dubbs et al., 2012), and OxyR "has been shown to be a repressor of its own expression as well as that of fhuF (encoding a ferric ion reductase) and flu (encoding the antigen 43 outer membrane protein)" (Zheng
et al., 2001 ). The genetically engineered bacteria of the invention may comprise any suitable ROS-responsive regulatory region from a gene that is repressed by OxyR. In some embodiments, OxyR is used in a two repressor activation regulatory circuit, as described above. Genes that are capable of being repressed by OxyR are known in the art (see, e.g., Zheng et al., 2001 ). Or, for example, although RosR is capable of repressing a number of genes, it is also capable of activating certain genes, e.g., the narKGHJI operon. In some embodiments, the genetically engineered bacteria comprise any suitable ROS- responsive regulatory region from a gene that is activated by RosR. In addition, "PerR-mediated positive regulation has also been observed... and appears to involve PerR binding to distant upstream sites" (Dubbs et al., 2012). In some embodiments, the genetically engineered bacteria comprise any suitable ROS- responsive regulatory region from a gene that is activated by PerR.
[0321] One or more types of ROS-sensing transcription factors and corresponding regulatory region sequences may be present in genetically engineered bacteria. For example, "OhrR is found in both Gram-positive and Gram-negative bacteria and can coreside with either OxyR or PerR or both" (Dubbs et al., 2012). In some embodiments, the genetically engineered bacteria comprise one type of ROS-sensing transcription factor, e.g., OxyR, and one corresponding regulatory region sequence, e.g., from oxyS. In some embodiments, the genetically engineered bacteria comprise one type of ROS- sensing transcription factor, e.g., OxyR, and two or more different
corresponding regulatory region sequences, e.g., from oxyS and katG. In some embodiments, the genetically engineered bacteria comprise two or more types of ROS-sensing transcription factors, e.g., OxyR and PerR, and two or more corresponding regulatory region sequences, e.g., from oxyS and katA, respectively. One ROS-responsive regulatory region may be capable of binding more than one transcription factor. In some embodiments, the genetically engineered bacteria comprise two or more types of ROS-sensing transcription factors and one corresponding regulatory region sequence.
[0322] Nucleic acid sequences of several exemplary OxyR-regulated regulatory regions are shown in Table C. OxyR binding sites are underlined and bolded. In some embodiments, genetically engineered bacteria comprise a
nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 63, 64, 65, or 66, or a functional fragment thereof.
Table C. Nucleotide sequences of exemplary OxyR-regulated regulatory regions
[0323] In some embodiments, the genetically engineered bacteria of the invention comprise a gene encoding a ROS-sensing transcription factor, e.g., the oxyR gene, that is controlled by its native promoter, an inducible promoter, a promoter that is stronger than the native promoter, e.g., the GInRS promoter or the P(Bla) promoter, or a constitutive promoter. In some instances, it may be advantageous to express the ROS-sensing transcription factor under the control of an inducible promoter in order to enhance expression stability. In some
embodiments, expression of the ROS-sensing transcription factor is controlled by a different promoter than the promoter that controls expression of the therapeutic molecule. In some embodiments, expression of the ROS-sensing transcription factor is controlled by the same promoter that controls expression of the therapeutic molecule. In some embodiments, the ROS-sensing
transcription factor and therapeutic molecule are divergently transcribed from a promoter region.
[0324] In some embodiments, the genetically engineered bacteria of the invention comprise a gene for a ROS-sensing transcription factor from a different species, strain, or substrain of bacteria. In some embodiments, the genetically engineered bacteria comprise a ROS-responsive regulatory region from a different species, strain, or substrain of bacteria. In some embodiments, the genetically engineered bacteria comprise a ROS-sensing transcription factor and corresponding ROS-responsive regulatory region from a different species, strain, or substrain of bacteria. The heterologous ROS-sensing transcription factor and regulatory region may increase the transcription of genes operatively linked to said regulatory region in the presence of ROS, as compared to the native transcription factor and regulatory region from bacteria of the same subtype under the same conditions.
[0325] In some embodiments, the genetically engineered bacteria comprise a ROS-sensing transcription factor, OxyR, and corresponding regulatory region, oxyS, from Escherichia coli. In some embodiments, the native ROS-sensing transcription factor, e.g., OxyR, is left intact and retains wild-type activity. In alternate embodiments, the native ROS-sensing
transcription factor, e.g., OxyR, is deleted or mutated to reduce or eliminate wild-type activity.
[0326] In some embodiments, the genetically engineered bacteria of the invention comprise multiple copies of the endogenous gene encoding the ROS- sensing transcription factor, e.g., the oxyR gene. In some embodiments, the gene encoding the ROS-sensing transcription factor is present on a plasm id. In some embodiments, the gene encoding the ROS-sensing transcription factor and the gene or gene cassette for producing the therapeutic molecule are present on different plasmids. In some embodiments, the gene encoding the
ROS-sensing transcription factor and the gene or gene cassette for producing the therapeutic molecule are present on the same. In some embodiments, the gene encoding the ROS-sensing transcription factor is present on a
chromosome. In some embodiments, the gene encoding the ROS-sensing transcription factor and the gene or gene cassette for producing the therapeutic molecule are present on different chromosomes. In some embodiments, the gene encoding the ROS-sensing transcription factor and the gene or gene cassette for producing the therapeutic molecule are present on the same chromosome.
[0327] In some embodiments, the genetically engineered bacteria comprise a wild-type gene encoding a ROS-sensing transcription factor, e.g., the soxR gene, and a corresponding regulatory region, e.g., a soxS regulatory region, that is mutated relative to the wild-type regulatory region from bacteria of the same subtype. The mutated regulatory region increases the expression of the payload in the presence of ROS, as compared to the wild-type regulatory region under the same conditions. In some embodiments, the genetically engineered bacteria comprise a wild-type ROS-responsive regulatory region, e.g., the oxyS regulatory region, and a corresponding transcription factor, e.g., OxyR, that is mutated relative to the wild-type transcription factor from bacteria of the same subtype. The mutant transcription factor increases the expression of the payload in the presence of ROS, as compared to the wild-type
transcription factor under the same conditions. In some embodiments, both the ROS-sensing transcription factor and corresponding regulatory region are mutated relative to the wild-type sequences from bacteria of the same subtype in order to increase expression of the payload in the presence of ROS.
[0328] In some embodiments, the gene or gene cassette for producing the payload is present on a plasm id and operably linked to a promoter that is induced by ROS. In some embodiments, the gene or gene cassette for producing the payload is present in the chromosome and operably linked to a promoter that is induced by ROS. In some embodiments, the gene or gene cassette for producing the payload is present on a chromosome and operably linked to a promoter that is induced by exposure to tetracycline. In some embodiments, the gene or gene cassette for producing the payload is present
on a plasmid and operably linked to a promoter that is induced by exposure to tetracycline. In some embodiments, expression is further optimized by methods known in the art, e.g., by optimizing ribosomal binding sites, manipulating transcriptional regulators, and/or increasing mRNA stability.
[0329] In some embodiments, the genetically engineered bacteria may comprise multiple copies of the gene(s) capable of producing a payload(s). In some embodiments, the gene(s) capable of producing a payload(s) is present on a plasmid and operatively linked to a ROS-responsive regulatory region. In some embodiments, the gene(s) capable of producing a payload is present in a chromosome and operatively linked to a ROS-responsive regulatory region.
[0330] Thus, in some embodiments, the genetically engineered bacteria or genetically engineered virus produce one or more payloads under the control of an oxygen level-dependent promoter, a reactive oxygen species (ROS)- dependent promoter, or a reactive nitrogen species (RNS)-dependent promoter, and a corresponding transcription factor.
[0331] In some embodiments, the genetically engineered bacteria comprise a stably maintained plasmid or chromosome carrying a gene for producing a payload, such that the payload can be expressed in the host cell, and the host cell is capable of survival and/or growth in vitro, e.g., in medium, and/or in vivo. In some embodiments, a bacterium may comprise multiple copies of the gene encoding the payload. In some embodiments, the gene encoding the payload is expressed on a low-copy plasmid. In some
embodiments, the low-copy plasmid may be useful for increasing stability of expression. In some embodiments, the low-copy plasmid may be useful for decreasing leaky expression under non-inducing conditions. In some
embodiments, the gene encoding the payload is expressed on a high-copy plasmid. In some embodiments, the high-copy plasmid may be useful for increasing expression of the payload. In some embodiments, the gene encoding the payload is expressed on a chromosome.
[0332] In some embodiments, the bacteria are genetically engineered to include multiple mechanisms of action (MOAs), e.g., circuits producing multiple copies of the same product (e.g., to enhance copy number) or circuits
performing multiple different functions. For example, the genetically engineered
bacteria may include four copies of the gene encoding a particular payload inserted at four different insertion sites. Alternatively, the genetically
engineered bacteria may include three copies of the gene encoding a particular payload inserted at three different insertion sites and three copies of the gene encoding a different payload inserted at three different insertion sites.
[0333] In some embodiments, under conditions where the payload is expressed, the genetically engineered bacteria of the disclosure produce at least about 1.5-fold, at least about 2-fold, at least about 10-fold, at least about 15-fold, at least about 20-fold, at least about 30-fold, at least about 50-fold, at least about 100-fold, at least about 200-fold, at least about 300-fold, at least about 400-fold, at least about 500-fold, at least about 600-fold, at least about 700-fold, at least about 800-fold, at least about 900-fold, at least about 1 , 000- fold, or at least about 1 , 500-fold more of the payload, and/or transcript of the gene(s) in the operon as compared to unmodified bacteria of the same subtype under the same conditions.
[0334] In some embodiments, quantitative PCR (qPCR) is used to amplify, detect, and/or quantify mRNA expression levels of the payload gene(s). Primers specific for payload the gene(s) may be designed and used to detect mRNA in a sample according to methods known in the art. In some
embodiments, a fluorophore is added to a sample reaction mixture that may contain payload mRNA, and a thermal cycler is used to illuminate the sample reaction mixture with a specific wavelength of light and detect the subsequent emission by the fluorophore. The reaction mixture is heated and cooled to predetermined temperatures for predetermined time periods. In certain embodiments, the heating and cooling is repeated for a predetermined number of cycles. In some embodiments, the reaction mixture is heated and cooled to 90-100° C, 60-70° C, and 30-50° C for a predetermined number of cycles. In a certain embodiment, the reaction mixture is heated and cooled to 93-97° C, 55- 65° C, and 35-45° C for a predetermined number of cycles. In some
embodiments, the accumulating amplicon is quantified after each cycle of the qPCR. The number of cycles at which fluorescence exceeds the threshold is the threshold cycle (CT). At least one CT result for each sample is generated,
and the CT result(s) may be used to determine mRNA expression levels of the payload gene(s).
[0335] In some embodiments, quantitative PCR (qPCR) is used to amplify, detect, and/or quantify mRNA expression levels of the payload gene(s). Primers specific for payload the gene(s) may be designed and used to detect mRNA in a sample according to methods known in the art. In some
embodiments, a fluorophore is added to a sample reaction mixture that may contain payload mRNA, and a thermal cycler is used to illuminate the sample reaction mixture with a specific wavelength of light and detect the subsequent emission by the fluorophore. The reaction mixture is heated and cooled to predetermined temperatures for predetermined time periods. In certain embodiments, the heating and cooling is repeated for a predetermined number of cycles. In some embodiments, the reaction mixture is heated and cooled to 90-100° C, 60-70° C, and 30-50° C for a predetermined number of cycles. In a certain embodiment, the reaction mixture is heated and cooled to 93-97° C, 55- 65° C, and 35-45° C for a predetermined number of cycles. In some
embodiments, the accumulating amplicon is quantified after each cycle of the qPCR. The number of cycles at which fluorescence exceeds the threshold is the threshold cycle (CT). At least one CT result for each sample is generated, and the CT result(s) may be used to determine mRNA expression levels of the payload gene(s).
Multiple Mechanisms of Action
[0336] In some embodiments, the bacteria are genetically engineered to include multiple mechanisms of action (MoAs), e.g., circuits producing multiple copies of the same product or circuits performing multiple different functions. Examples of insertion sites include, but are not limited to, malE/K, insB/l, araC/BAD, lacZ, dapA, cea, and other shown in Fig. 18. For example, the genetically engineered bacteria may include four copies of argAmr inserted at four different insertion sites, e.g., malE/K, insB/l, araC/BAD, and lacZ.
Alternatively, the genetically engineered bacteria may include three copies of argAmr inserted at three different insertion sites, e.g., malE/K, insB/l, and lacZ, and three mutant arginine regulons, e.g., two producing citrulline and one producing arginine, inserted at three different insertion sites dapA, cea, and
araC/BAD. In some embodiments, the genetically engineered bacteria may include one or more ammonia conversion circuit(s) inserted at one or more different insertion sites and one or more additional circuits inserted at one or more other insertion sites. For example, the genetically engineered bacteria may include one or more copies of argAmr (and/or other ammonia conversion circuit(s)) inserted at one or more different insertion sites, and one or more gut barrier enhancing circuits, e.g., one or more butyrate production circuit(s) (or other gut barrier enhancing circuit(s)) at other insertion sites. In other exemplary embodiments, the genetically engineered bacteria may include one or more copies of argA mr and/or other ammonia conversion circuit(s)) inserted at one or more different insertion sites, and one or more GABA reducing circuits, e.g., GABA transport and/or GABA metabolic circuit(s), inserted at other insertion site(s). In other exemplary embodiments, the genetically engineered bacteria may include one or more copies of argAmr (and/or other ammonia conversion circuit(s)) inserted at one or more different insertion sites, and one or more manganese transport circuit(s) inserted at other insertion sites (Fig. 47A). In some embodiments, one or more ammonia conversion circuit(s) (e.g., argAmr and/or other ammonia conversion circuit(s)), one or more gut barrier enhancing circuit (e.g., butyrate, propionate, acetate biosynthetic circuit(s)), one or more GABA reducing circuit(s) (e.g., GABA transport and/or GABA metabolic circuit), and one or more manganese transport circuit(s) are inserted at four or more different chromosomal insertion sites (e.g., Fig. 45). In some embodiments, an ammonia conversion circuit, a gut barrier enhancing circuit, a GABA transport and/or GABA metabolic circuit are inserted at three different chromosomal insertion sites. In some embodiments, an ammonia conversion circuit, a GABA transport/metabolic circuit, and a manganese transport circuit are inserted at three different chromosomal insertion sites (Fig. 46B). In other embodiments, an ammonia conversion circuit, and a manganese transport circuit are inserted at two different chromosomal insertion sites (Fig. 47A). In still other
embodiments, an ammonia conversion circuit, and a GABA transport and/or GABA metabolic circuit are inserted at two different chromosomal insertion sites. In still other embodiments, an ammonia conversion circuit, and a gut barrier enhancing circuit are inserted at two different chromosomal insertion
sites. In still other embodiments, an ammonia conversion circuit, and a manganese reducing circuit are inserted at two different chromosomal insertion sites.
Table 14 lists non-limiting examples of embodiments of the disclosure.
Table 14. Non-limiting examples of embodiments of the disclosure
SYN- Wild type AArgR tetracycline- Wld Amp none UCD203 ARG Box inducible type
argAfbr on a ThyA
low-copy
plasmid (Amp)
SYN- Wild type AArgR tet-ArgAfbr on Wld Amp none UCD204 ARG Box a low-copy type
plasmid (Amp) ThyA
SYN- Wild type AArgR PfnrS- ArgAfbr Wld Amp none UCD205 ARG Box on a low-copy type
plasmid (Amp) ThyA
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA Amp, none UCD206 ARG Box on a low-copy Cam
plasmid (Amp)
Integrated FNRS-argAfbr
SYN- Wild type AArgR PfnrS- ArgAfbr Wld Cam none UCD301 ARG Box integrated into type
the ThyA
chromosome
at the maIEK
locus
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA Cam none UCD302 ARG Box integrated into
the
chromosome
at the maIEK
locus
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA Kan none UCD303 ARG Box integrated into
the
chromosome
at the maIEK
locus
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA None none UCD305 ARG Box integrated into
the
chromosome
at the maIEK
locus
SYN- Wild type AArgR PfnrS- ArgAfbr Wld None none UCD304 ARG Box integrated into type
the ThyA
chromosome
at the maIEK
locus
SYN- Wild type AArgR PfnrS- ArgAfbr Wld Kan none UCD306 ARG Box integrated into type
the ThyA
chromosome
at the maIEK
locus
SYN- Wild type Wild type PfnrS- ArgAfbr AThyA Kan none UCD307 ARG Box ArgR integrated into
the
locus
under control
of tet
promoter on a plasmid)
SYN- Wild type Wld type none Wild None pLogic046 (ter UCD504 ARG Box ArgR type butyrate
ThyA cassette
under control of tet promoter on a plasmid)
SYN- Wild type Wld type none Wild Amp PLOGIC046- UCD505 ARG Box ArgR type delta.pbt- ThyA buk/tesB+
(tesB butyrate cassette under control of tet promoter on a plasmid)
SYN- Wld type Wld type none Wild Amp Logid 56 UCD506 ARG Box ArgR type (pSC101 nirB- ThyA Bcd butyrate plasmid; amp resistance)
SYN- Wld type Wld type none Wild Amp pLogic031- UCD507 ARG Box ArgR type nsrR-norB-
ThyA butyrate
construct
SYN- Wld type Wld type none Wild Amp pLogic046- UCD508 ARG Box ArgR type nsrR-norB-
ThyA butyrate
construct
SYN- Wld type Wld type none Wild Amp tesB-butyrate UCD509 ARG Box ArgR type cassette
ThyA integrated into chromosome under control of FNR promoter
SYN- Wld type Wld type none Wild None pLogic031 UCD510 ARG Box ArgR type (bdc2 butyrate
ThyA cassette
under control of tet promoter on a plasmid); nuoB deletion
SYN- Wld type Wld type none Wild None pLogic046 (ter UCD51 1 ARG Box ArgR type butyrate
ThyA cassette
under control of tet promoter on a plasmid); nuoB deletion
Butyrate and Ammonium Circuits
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA Amp Logid 56 UCD601 ARG Box integrated into (pSC101 the PydfZ-ter chromosome butyrate at the maIEK plasmid; amp locus resistance)
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA Kan PydfZ-ter UCD603 ARG Box integrated into butyrate the cassette chromosome integrated on at the maIEK the locus chromosome
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA None PydfZ-ter UCD605 ARG Box integrated into butyrate the cassette chromosome integrated on at the maIEK the locus chromosome
SYN- Wild type AArgR PfnrS- ArgAfbr Wld None PydfZ-ter UCD604 ARG Box integrated into type butyrate the ThyA cassette chromosome integrated on at the maIEK the locus chromosome
SYN- Wild type AArgR PfnrS- ArgAfbr Wld Kan PydfZ-ter UCD606 ARG Box integrated into type butyrate the ThyA cassette chromosome integrated on at the maIEK the locus chromosome
SYN- Wild type Wild type PfnrS- ArgAfbr AThyA Kan PydfZ-ter UCD607 ARG Box ArgR integrated into butyrate the cassette chromosome integrated on at the maIEK the locus chromosome
SYN- Wild type Wild type PfnrS- ArgAfbr AThyA none PydfZ-ter UCD608 ARG Box ArgR integrated into butyrate the cassette chromosome integrated on at the maIEK the locus chromosome
SYN- Wild type Wild type PfnrS- ArgAfbr Wld Kan PydfZ-ter UCD609 ARG Box ArgR integrated into type butyrate the ThyA cassette chromosome integrated on at the maIEK the locus chromosome
SYN- Wild type Wild type PfnrS- ArgAfbr Wld none PydfZ-ter UCD610 ARG Box ArgR integrated into type butyrate the ThyA cassette chromosome integrated on at the maIEK the locus chromosome
SYN- Wild type AArgR none Wld Kan PydfZ-ter UCD611 ARG Box type butyrate
ThyA cassette integrated on
the
chromosome
SYN- Wild type AArgR none Wld none PydfZ-ter UCD612 ARG Box type butyrate
ThyA cassette
integrated on the chromosome
SYN- Wild type AArgR none AThyA Kan PydfZ-ter UCD613 ARG Box butyrate
cassette integrated on the chromosome
SYN- Wild type AArgR none AThyA none PydfZ-ter UCD614 ARG Box butyrate
cassette integrated on the chromosome
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA Kan PydfZ-ter UCD703 ARG Box integrated into butyrate
the cassette chromosome integrated on at the maIEK the locus chromosome ;
Rifaximin resistance
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA None PydfZ-ter UCD705 ARG Box integrated into butyrate
the cassette chromosome integrated on at the maIEK the locus chromosome;
Rifaximin resistance
SYN- Wild type AArgR PfnrS- ArgAfbr Wld None PydfZ-ter UCD704 ARG Box integrated into type butyrate
the ThyA cassette chromosome integrated on at the maIEK the locus chromosome;
Rifaximin resistance
SYN- Wild type AArgR PfnrS- ArgAfbr Wld Kan PydfZ-ter UCD706 ARG Box integrated into type butyrate
the ThyA cassette chromosome integrated on at the maIEK the locus chromosome;
Rifaximin resistance
SYN- Wild type Wild type PfnrS- ArgAfbr AThyA Kan PydfZ-ter UCD707 ARG Box ArgR integrated into butyrate
the cassette chromosome integrated on at the maIEK the
locus chromosome;
Rifaximin resistance
SYN- Wild type Wild type PfnrS- ArgAfbr AThyA none PydfZ-ter UCD708 ARG Box ArgR integrated into butyrate the cassette chromosome integrated on at the maIEK the locus chromosome;
Rifaximin resistance
SYN- Wild type Wild type PfnrS- ArgAfbr Wld Kan PydfZ-ter UCD709 ARG Box ArgR integrated into type butyrate the ThyA cassette chromosome integrated on at the maIEK the locus chromosome;
Rifaximin resistance
SYN- Wild type Wild type PfnrS- ArgAfbr Wld none PydfZ-ter UCD710 ARG Box ArgR integrated into type butyrate the ThyA cassette chromosome integrated on at the maIEK the locus chromosome;
Rifaximin resistance
SYN- Wild type AArgR none Wld Kan PydfZ-ter UCD711 ARG Box type butyrate
ThyA cassette integrated on the chromosome;
Rifaximin resistance
SYN- Wild type AArgR none Wld none PydfZ-ter UCD712 ARG Box type butyrate
ThyA cassette integrated on the chromosome;
Rifaximin resistance
SYN- Wild type AArgR none AThyA Kan PydfZ-ter UCD713 ARG Box butyrate cassette integrated on the chromosome;
Rifaximin resistance
SYN- Wild type AArgR none AThyA none PydfZ-ter UCD714 ARG Box butyrate cassette integrated on the chromosome;
Rifaximin resistance
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA Kan tesB-butyrate UCD715 ARG Box integrated into cassette the integrated into chromosome chromosome at maIEK locus under control of FNR promoter
SYN- Wild type AArgR PfnrS- ArgAfbr AThyA None tesB-butyrate UCD716 ARG Box integrated into cassette the integrated into chromosome chromosome at maIEK locus under control of FNR promoter
SYN- Wld type AArgR PfnrS- ArgAfbr Wild None tesB-butyrate UCD717 ARG Box integrated into type cassette the ThyA integrated into chromosome chromosome at maIEK locus under control of FNR promoter
SYN- Wld type AArgR PfnrS- ArgAfbr Wild Kan tesB-butyrate UCD718 ARG Box integrated into type cassette the ThyA integrated into chromosome chromosome at maIEK locus under control of FNR promoter
SYN- Wld type Wld type PfnrS- ArgAfbr AThyA Kan tesB-butyrate UCD719 ARG Box ArgR integrated into cassette the integrated into chromosome chromosome at maIEK locus under control of FNR promoter
SYN- Wld type Wld type PfnrS- ArgAfbr AThyA none tesB-butyrate UCD720 ARG Box ArgR integrated into cassette the integrated into chromosome chromosome at maIEK locus under control of FNR promoter
SYN- Wld type Wld type PfnrS- ArgAfbr Wild Kan tesB-butyrate UCD721 ARG Box ArgR integrated into type cassette the ThyA integrated into chromosome chromosome at maIEK locus under control of FNR promoter
SYN- Wld type Wld type PfnrS- ArgAfbr Wild none tesB-butyrate UCD722 ARG Box ArgR integrated into type cassette the ThyA integrated into chromosome chromosome at maIEK locus under control of FNR promoter
SYN- Wld type AArgR none Wild Kan tesB-butyrate UCD723 ARG Box type cassette
ThyA integrated into
chromosome under control of FNR promoter
SYN- Wild type AArgR none Wild none tesB-butyrate UCD724 ARG Box type cassette
ThyA integrated into chromosome under control of FNR promoter
SYN- Wild type AArgR none AThyA Kan tesB-butyrate UCD725 ARG Box cassette integrated into chromosome under control of FNR promoter
SYN- Wld type AArgR none AThyA none tesB-butyrate UCD726 ARG Box cassette integrated into chromosome under control of FNR promoter
SYN- Wld type AArgR PfnrS- ArgAfbr AThyA Kan tesB-butyrate UCD727 ARG Box integrated into cassette the integrated into chromosome chromosome at maIEK locus under control of FNR promoter; Rifaximin resistance
SYN- Wld type AArgR PfnrS- ArgAfbr AThyA None tesB-butyrate UCD728 ARG Box integrated into cassette the integrated into chromosome chromosome at maIEK locus under control of FNR promoter; Rifaximin resistance
SYN- Wld type AArgR PfnrS- ArgAfbr Wild None tesB-butyrate UCD729 ARG Box integrated into type cassette the ThyA integrated into chromosome chromosome at maIEK locus under control of FNR promoter; Rifaximin resistance
SYN- Wld type AArgR PfnrS- ArgAfbr Wild Kan tesB-butyrate UCD730 ARG Box integrated into type cassette the ThyA integrated into chromosome chromosome at maIEK locus under control of FNR promoter;
Rifaximin resistance
SYN- Wild type Wld type PfnrS- ArgAfbr AThyA Kan tesB-butyrate UCD731 ARG Box ArgR integrated into cassette the integrated into chromosome chromosome at maIEK locus under control of FNR promoter; Rifaximin resistance
SYN- Wild type Wld type PfnrS- ArgAfbr AThyA none tesB-butyrate UCD732 ARG Box ArgR integrated into cassette the integrated into chromosome chromosome at maIEK locus under control of FNR promoter; Rifaximin resistance
SYN- Wld type Wld type PfnrS- ArgAfbr Wild Kan tesB-butyrate UCD733 ARG Box ArgR integrated into type cassette the ThyA integrated into chromosome chromosome at maIEK locus under control of FNR promoter; Rifaximin resistance
SYN- Wld type Wld type PfnrS- ArgAfbr Wild none tesB-butyrate UCD734 ARG Box ArgR integrated into type cassette the ThyA integrated into chromosome chromosome at maIEK locus under control of FNR promoter; Rifaximin resistance
SYN- Wld type AArgR none Wild Kan tesB-butyrate UCD735 ARG Box type cassette
ThyA integrated into chromosome under control of FNR promoter; Rifaximin resistance
SYN- Wld type AArgR none Wild none tesB-butyrate UCD736 ARG Box type cassette
ThyA integrated into chromosome under control of FNR promoter; Rifaximin resistance
SYN- Wld type AArgR none AThyA Kan tesB-butyrate UCD737 ARG Box cassette integrated into
chromosome under control of FNR promoter;
Rifaximin resistance
SYN- Wild type AArgR none AThyA none tesB-butyrate UCD38 ARG Box cassette
integrated into chromosome under control of FNR promoter;
Rifaximin resistance
[0337] In some embodiments, butyrate production by the genetically engineered bacteria can be further enhanced by additional modifications.
Butyrate production under anaerobic conditions depends on endogenous NADH pools. In some embodiments, the flux through the butyrate pathway may be enhanced by eliminating competing routes for NADH utilization. A non-limiting example is the mutation/deletion of frdA, which utilizes NADH to catalyze the conversion of phosphoenolpyruvate to succinate. In some embodiments, any of the genetically engineered bacteria described herein further comprise a mutation, which eliminates competing routes for NADH utilization, e.g., a mutation/deletion of frdA.
Secretion
[0338] In some embodiments, the genetically engineered bacteria further comprise a native secretion mechanism (e.g., gram positive bacteria) or non- native secretion mechanism (e.g., gram negative bacteria) that is capable of secreting the protein(s) of interest or therapeutic protein(s), from the bacterial cytoplasm. Many bacteria have evolved sophisticated secretion systems to transport substrates across the bacterial cell envelope. Substrates, such as small molecules, proteins, and DNA, may be released into the extracellular space or periplasm (such as the gut lumen or other space), injected into a target cell, or associated with the bacterial membrane.
[0339] In Gram-negative bacteria, secretion machineries may span one or both of the inner and outer membranes. In some embodiments, the
genetically engineered bacteria further comprise a non-native double
membrane-spanning secretion system. Double membrane-spanning secretion systems include, but are not limited to, the type I secretion system (T1 SS), the type II secretion system (T2SS), the type III secretion system (T3SS), the type IV secretion system (T4SS), the type VI secretion system (T6SS), and the resistance-nodulation-division (RND) family of multi-drug efflux pumps (Pugsley 1993; Gerlach et al., 2007; Collinson et al., 2015; Costa et al., 2015; Reeves et al., 2015; WO2014138324A1 , incorporated herein by reference). Examples of such secretion systems are shown in Figs. 69-73. Mycobacteria, which have a Gram-negative-like cell envelope, may also encode a type VII secretion system (T7SS) (Stanley et al., 2003). With the exception of the T2SS, double
membrane-spanning secretions generally transport substrates from the bacterial cytoplasm directly into the extracellular space or into the target cell. In contrast, the T2SS and secretion systems that span only the outer membrane may use a two-step mechanism, wherein substrates are first translocated to the periplasm by inner membrane-spanning transporters, and then transferred to the outer membrane or secreted into the extracellular space. Outer membrane- spanning secretion systems include, but are not limited to, the type V secretion or autotransporter system (T5SS), the curli secretion system, and the
chaperone-usher pathway for pili assembly (Saier, 2006; Costa et al., 2015).
[0340] In some embodiments, the genetically engineered bacteria of the invention further comprise a type III or a type Ill-like secretion system (T3SS) from Shigella, Salmonella, E. coli, Bivrio, Burkholderia, Yersinia, Chlamydia, or Pseudomonas. The T3SS is capable of transporting a protein from the bacterial cytoplasm to the host cytoplasm through a needle complex. The T3SS may be modified to secrete the molecule from the bacterial cytoplasm, but not inject the molecule into the host cytoplasm. Thus, the molecule is secreted into the gut lumen or other extracellular space. In some embodiments, the genetically engineered bacteria comprise said modified T3SS and are capable of secreting the protein(s) of interest or therapeutic protein(s) from the bacterial cytoplasm. In some embodiments, the secreted molecule, such as a heterologous protein or peptide, e.g., the protein of interest or therapeutic protein, comprises a type
III secretion sequence that allows the protein(s) of interest or therapeutic protein(s) to be secreted from the bacteria.
[0341] In some embodiments, a flagellar type III secretion pathway is used to secrete the molecule of interest. In some embodiments, an incomplete flagellum is used to secrete a therapeutic peptide of interest, by recombinantly fusing the peptide to an N-terminal flagellar secretion signal of a native flagellar component. In this manner, the intracellular^ expressed chimeric peptide can be mobilized across the inner and outer membranes into the surrounding host environment.
[0342] In some embodiments, a Type V Autotransporter Secretion System is used to secrete the therapeutic peptide. Due to the simplicity of the machinery and capacity to handle relatively large protein fluxes, the Type V secretion system is attractive for the extracellular production of recombinant proteins. As shown in Fig. 70, a therapeutic peptide (star) can be fused to an N-terminal secretion signal, a linker, and the beta-domain of an autotransporter. The N-terminal signal sequence directs the protein to the SecA-YEG machinery which moves the protein across the inner membrane into the periplasm, followed by subsequent cleavage of the signal sequence. The Beta-domain is recruited to the Bam complex ('Beta-barrel assembly machinery') where the beta-domain is folded and inserted into the outer membrane as a beta-barrel structure. The therapeutic peptide, is threaded through the hollow pore of the beta-barrel structure ahead of the linker sequence. Once exposed to the extracellular environment, the therapeutic peptide, can be freed from the linker system by an autocatalytic cleavage (left side of Bam complex) or by targeting of a membrane-associated peptidase (black scissors; right side of Bam complex) to a complimentary protease cut site in the linker. Thus, in some embodiments, the secreted molecule, such as a heterologous protein or peptide, e.g., the protein of interest or therapeutic protein, comprises an N- terminal secretion signal, a linker, and beta-domain of an autotransporter so as to allow the molecule to be secreted from the bacteria.
[0343] In some embodiments, a Hemolysin-based Secretion System is used to secrete the molecule of interest. Type I Secretion systems offer the advantage of translocating their passenger peptide directly from the cytoplasm
to the extracellular space, obviating the two-step process of other secretion types. Fig. 71 shows the alpha-hemolysin (HlyA) of uropathogenic Escherichia coli. This pathway uses HlyB, an ATP-binding cassette transporter; HlyD, a membrane fusion protein; and TolC, an outer membrane protein. The assembly of these three proteins forms a channel through both the inner and outer membranes. Natively, this channel is used to secrete HlyA, however, to secrete the therapeutic peptide of the present disclosure, the secretion signal-containing C-terminal portion of HlyA is fused to the C-terminal portion of a therapeutic peptide (star) to mediate secretion of this peptide.
[0344] In alternate embodiments, the genetically engineered bacteria further comprise a non-native single membrane-spanning secretion system. Single membrane-spanning exporters may act as a component of a secretion system, or may export substrates independently. Such exporters include, but are not limited to, ATP-binding cassette translocases, flagellum/virulence- related translocases, conjugation-related translocases, the general secretory system (e.g., the SecYEG complex in E. coli), the accessory secretory system in mycobacteria and several types of Gram-positive bacteria (e.g., Bacillus anthracis, Lactobacillus johnsonii, Cory nebacteri urn glutamicum, Streptococcus gordonii, Staphylococcus aureus), and the twin-arginine translocation (TAT) system (Saier, 2006; Rigel and Braunstein, 2008; Albiniak et al., 2013). It is known that the general secretory and TAT systems can both export substrates with cleavable N-terminal signal peptides into the periplasm, and have been explored in the context of biopharmaceutical production. The TAT system may offer particular advantages, however, in that it is able to transport folded substrates, thus eliminating the potential for premature or incorrect folding. In certain embodiments, the genetically engineered bacteria comprise a TAT or a TAT-like system and are capable of secreting the protein(s) of interest or therapeutic protein(s), from the bacterial cytoplasm. One of ordinary skill in the art would appreciate that the secretion systems disclosed herein may be modified to act in different species, strains, and subtypes of bacteria, and/or adapted to deliver different payloads.
[0345] In order to translocate a protein, e.g., therapeutic polypeptide, to the extracellular space, the polypeptide must first be translated intracellular^,
mobilized across the inner membrane and finally mobilized across the outer membrane. Many effector proteins (e.g., therapeutic polypeptides) - particularly those of eukaryotic origin - contain disulphide bonds to stabilize the tertiary and quaternary structures. While these bonds are capable of correctly forming in the oxidizing periplasmic compartment with the help of periplasmic chaperones, in order to translocate the polypeptide across the outer membrane the disulphide bonds must be reduced and the protein unfolded again.
[0346] One way to secrete properly folded proteins in gram-negative bacteria- particularly those requiring disulphide bonds - is to target the periplasm in a bacterium with a destabilized outer membrane. In this manner the protein is mobilized into the oxidizing environment and allowed to fold properly. In contrast to orchestrated extracellular secretion systems, the protein is then able to escape the periplasmic space in a correctly folded form by membrane leakage. These "leaky" gram-negative mutants are therefore capable of secreting bioactive, properly disulphide-bonded polypeptides. In some embodiments, the genetically engineered bacteria have a "leaky" or destabilized outer membrane. Destabilizing the bacterial outer membrane to induce leakiness can be accomplished by deleting or mutagenizing genes responsible for tethering the outer membrane to the rigid peptidoglycan skeleton, including for example, lpp, ompC, ompA, ompF, tolA, tolB, pal, degS, degP, and nlpl. Lpp is the most abundant polypeptide in the bacterial cell existing at ~500,000 copies per cell and functions as the primary 'staple' of the bacterial cell wall to the peptidoglycan. Silhavy, T. J., Kahne, D. & Walker, S. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2, a000414 (2010). TolA-PAL and OmpA complexes function similarly to Lpp and are other deletion targets to generate a leaky phenotype. Additionally, leaky phenotypes have been observed when periplasmic proteases are deactivated. The periplasm is very densely packed with protein and therefore encode several periplasmic proteins to facilitate protein turnover. Removal of periplasmic proteases such as degS, degP or nlpl can induce leaky phenotypes by promoting an excessive build-up of periplasmic protein. Mutation of the proteases can also preserve the effector polypeptide by preventing targeted degradation by these proteases. Moreover, a combination of these mutations may synergistically enhance the
leaky phenotype of the cell without major sacrifices in cell viability. Thus, in some embodiments, the engineered bacteria have one or more deleted or mutated membrane genes. In some embodiments, the engineered bacteria have a deleted or mutated Ipp gene. In some embodiments, the engineered bacteria have one or more deleted or mutated gene(s), selected from ompA, ompA, and ompF genes. In some embodiments, the engineered bacteria have one or more deleted or mutated gene(s), selected from tolA, tolB, and pal genes, in some embodiments, the engineered bacteria have one or more deleted or mutated periplasmic protease genes. In some embodiments, the engineered bacteria have one or more deleted or mutated periplasmic protease genes selected from degS, degP, and nlpl. In some embodiments, the engineered bacteria have one or more deleted or mutated gene(s), selected from Ipp, ompA, ompA, ompF, tolA, tolB, pal, degS, degP, and nlpl genes.
[0347]To minimize disturbances to cell viability, the leaky phenotype can be made inducible by placing one or more membrane or periplasmic protease genes, e.g., selected from Ipp, ompA, ompA, ompF, tolA, tolB, pal, degS, degP, and nlpl, under the control of an inducible promoter. For example, expression of Ipp or other cell wall stability protein or periplasmic protease can be repressed in conditions where the therapeutic polypeptide needs to be delivered
(secreted). For instance, under inducing conditions a transcriptional repressor protein or a designed antisense RNA can be expressed which reduces transcription or translation of a target membrane or periplasmic protease gene. Conversely, overexpression of certain peptides can result in a destabilized phenotype, e.g., overexpression of colicins or the third topological domain of TolA, wherein peptide overexpression can be induced in conditions in which the therapeutic polypeptide needs to be delivered (secreted). These sorts of strategies would decouple the fragile, leaky phenotypes from biomass production. Thus, in some embodiments, the engineered bacteria have one or more membrane and/or periplasmic protease genes under the control of an inducible promoter.
[0348] Table 15 and Table 16 list secretion systems for Gram positive bacteria and Gram negative bacteria. These can be used to secrete
polypeptides, proteins of interest or therapeutic protein(s) from the engineered
bacteria, which are reviewed in Milton H. Saier, Jr. Microbe / Volume 1 , Number 9, 2006 "Protein Secretion Systems in Gram-Negative Bacteria Gram-negative bacteria possess many protein secretion-membrane insertion systems that apparently evolved independently", the contents of which is herein incorporated by reference in its entirety.
Table 15. Secretion systems for gram positive bacteria
Table 16. Secretion Systems for Gram negative bacteria
[0349] In some embodiments, the genetically engineered bacterial comprise a native or non-native secretion system described herein for the secretion of therapeutic enzyme. In some embodiments, the secretion system is selected from the modified type III flagellar, type I (e.g., hemolysin secretion system), type II, type IV, type V, type VI, and type VII secretion systems, resistance-nodulation-division (RND) multi-drug efflux pumps, a single membrane secretion system, Sec and, TAT secretion systems.
[0350] In some embodiments, the therapeutic proteins secreted by the genetically engineered bacteria are modified to increase resistance to proteases, e.g. intestinal proteases.
[0351] In some embodiments, the one or more proteins of interest or therapeutic proteins for secretion are under the control of an inducible promoter, as described herein. In one example, the one or more proteins of interest or therapeutic proteins are under the control of the FNR promoter and are produced and secreted under anaerobic conditions. In some embodiments, the one or more proteins of interest or therapeutic proteins for secretion are under the control of a constitutive promoter.
[0352] In some embodiments in which the one or more proteins of interest or therapeutic proteins are secreted or exported from the
microorganism, the engineered microorganism comprises gene sequence(s) that includes a secretion tag. In some embodiments, the one or more proteins of interest or therapeutic proteins include a "secretion tag" of either RNA orpeptide origin to direct the one or more proteins of interest or therapeutic proteins to specific secretion systems. For example, a secretion tag for the Type I
Hemolysin secretion system is encoded in the C-terminal 53 amino acids of the alpha hemolysin protein (HlyA). HlyA secretion signal.
[0353] HlyB inserts into inner membrane to form a pore, HlyD aligns HlyB with TolC (outer membrane pore) thereby forming a channel through inner and outer membrane. The C-terminal secretion tag can be removed by either an autocatalytic or protease-catalyzed e.g., OmpT cleavage thereby releasing the one or more proteins of interest or therapeutic proteins into the extracellular milieu.
[0354]The Type V Auto-secretion System utilizes an N-terminal Sec- dependent peptide tag (inner membrane) and C-terminal tag (outer-membrane). This uses Sec-system to get from cytoplasm to periplasm. C-terminal tag then inserts into the outer membrane forming a pore through which the "passenger protein" threads through. Once across the outer membrane, the passenger (anti-cancer molecule) is released from the membrane-embedded C-terminal tag by either an autocatalytic, intein-like mechanism or via a membrane-bound protease (I.e., OmpT). The N-terminal tag is removed by the Sec system.
Thus, in some embodiments, the secretion system is able to remove this tag before secreting the one or more proteins of interest or therapeutic proteins, from the engineered bacteria. In the Type V auto-secretion-mediated secretion
the N-terminal peptide secretion tag is removed upon translocation of the "passenger" peptide from the cytoplasm into the periplasm ic compartment by the native Sec system. Further, once the auto-secretor is translocated across the outer membrane the C-terminal secretion tag can be removed by either an autocatalytic or protease-catalyzed e.g., OmpT cleavage thereby releasing the anti-cancer molecule(s) into the extracellular milieu.
[0355] In the Flagellar modified Type III Secretion, the tag is encoded in 5'untranslated region of the mRNA and thus there is no peptide tag to
cleave/remove. This modified system does not contain the "syringe" portion and instead uses the basal body of the flagella structure as the pore to translocate across both membranes and out through the forming flagella. If the fliC/fliD genes (encoding the flagella "tail'Vwhip) are disrupted the flagella cannot fully form and this promotes overall secretion. In some embodiments, the tail portion can be removed entirely. In the Type III traditional secretion system, the basal body closely resembles the flagella, however, instead of a "tail'Vwhip, the traditional T3SS has a syringe to inject the passenger proteins into host cells. The secretion tag is encoded by an N-terminal peptide (lengths vary and there are several different tags, see PCT/US 14/020972). The N-terminal tag is not removed from the polypeptides in this secretion system.
[0356] In some embodiments the one or more proteins of interest or therapeutic proteins contain expressed as fusion protein with the 53 amino acids of the C termini of alpha-hemolysin (hlyA) of E. coli CFT073 (C terminal secretion tag).
Essential Genes and Auxotrophs
[0357] As used herein, the term "essential gene" refers to a gene which is necessary to for cell growth and/or survival. Bacterial essential genes are well known to one of ordinary skill in the art, and can be identified by directed deletion of genes and/or random mutagenesis and screening (see, for example, Zhang and Lin, 2009, DEG 5.0, a database of essential genes in both
prokaryotes and eukaryotes, Nucl. Acids Res., 37:D455-D458 and Gerdes et al., Essential genes on metabolic maps, Curr. Opin. Biotechnol., 17(5):448-456, the entire contents of each of which are expressly incorporated herein by reference).
[0358] An "essential gene" may be dependent on the circumstances and environment in which an organism lives. For example, a mutation of, modification of, or excision of an essential gene may result in the recombinant bacteria of the disclosure becoming an auxotroph. An auxotrophic modification is intended to cause bacteria to die in the absence of an exogenously added nutrient essential for survival or growth because they lack the gene(s) necessary to produce that essential nutrient.
[0359] Exemplary bacterial genes which may be disrupted or deleted to produce an auxotrophic strain are shown below. These include, but are not limited to, genes required for oligonucleotide synthesis, amino acid synthesis, and cell wall synthesis.
[0360] n auxotrophic modification is intended to cause bacteria to die in the absence of an exogenously added nutrient essential for survival or growth because they lack the gene(s) necessary to produce that essential nutrient. In some embodiments, any of the genetically engineered bacteria described herein also comprise a deletion or mutation in a gene required for cell survival and/or growth. In one embodiment, the essential gene is a DNA synthesis gene, for example, thyA. In another embodiment, the essential gene is a cell wall synthesis gene, for example, dapA. In yet another embodiment, the essential gene is an amino acid gene, for example, serA or MetA. Any gene required for cell survival and/or growth may be targeted, including but not limited to, cysE, gin A, ilvD, leuB, lysA, serA, met A, glyA, hisB, ilvA, pheA, pro A, thrC, trpC, tyrA, thyA, uraA, dapA, dapB, dapD, dapE, dapF, flhD, metB, metC, proAB, and thi1, as long as the corresponding wild-type gene product is not produced in the bacteria. Table 17 lists depicts exemplary bacterial genes which may be disrupted or deleted to produce an auxotrophic strain. These include, but are not limited to, genes required for oligonucleotide synthesis, amino acid synthesis, and cell wall synthesis.
Table 17. Non-limiting Examples of Bacterial Genes Useful for Generation of an Auxotroph
ilvD dapD
leuB dapE lysA dapF
serA
metA
glyA
hisB
ilvA
pheA
pro A
thrC
trpC
tyrA
[0361]Table 18 shows the survival of various amino acid auxotrophs in the mouse gut, as detected 24 hrs and 48 hrs post-gavage. These auxotrophs were generated using BW25113, a non-Nissle strain of E. coli.
Table 18. Survival of amino acid auxotrophs in the mouse gut
ilvD Valine/lsoleucine/ Present Present Absent
Leucine
thyA Thiamine Present Absent Absent
uraA Uracil Present Absent Absent
flhD FlhD Present Present Present
[0362] For example, thymine is a nucleic acid that is required for bacterial cell growth; in its absence, bacteria undergo cell death. The thyA gene encodes thimidylate synthetase, an enzyme that catalyzes the first step in thymine synthesis by converting dUMP to dTMP (Sat et al., 2003). In some embodiments, the bacterial cell of the disclosure is a thyA auxotroph in which the thyA gene is deleted and/or replaced with an unrelated gene. A thyA auxotroph can grow only when sufficient amounts of thymine are present, e.g., by adding thymine to growth media in vitro, or in the presence of high thymine levels found naturally in the human gut in vivo. In some embodiments, the bacterial cell of the disclosure is auxotrophic in a gene that is complemented when the bacterium is present in the mammalian gut. Without sufficient amounts of thymine, the thyA auxotroph dies. In some embodiments, the auxotrophic modification is used to ensure that the bacterial cell does not survive in the absence of the auxotrophic gene product (e.g., outside of the gut).
[0363] Diaminopimelic acid (DAP) is an amino acid synthetized within the lysine biosynthetic pathway and is required for bacterial cell wall growth
(Meadow et al., 1959; Clarkson et al., 1971 ). In some embodiments, any of the genetically engineered bacteria described herein is a dapD auxotroph in which dapD is deleted and/or replaced with an unrelated gene. A dapD auxotroph can grow only when sufficient amounts of DAP are present, e.g., by adding DAP to growth media in vitro. Without sufficient amounts of DAP, the dapD auxotroph dies. In some embodiments, the auxotrophic modification is used to ensure that the bacterial cell does not survive in the absence of the auxotrophic gene product (e.g., outside of the gut).
[0364] In other embodiments, the genetically engineered bacterium of the present disclosure is a uraA auxotroph in which uraA is deleted and/or replaced with an unrelated gene. The uraA gene codes for UraA, a membrane-bound transporter that facilitates the uptake and subsequent metabolism of the
pyrimidine uracil (Andersen et al., 1995). A uraA auxotroph can grow only when sufficient amounts of uracil are present, e.g., by adding uracil to growth media in vitro. Without sufficient amounts of uracil, the uraA auxotroph dies. In some embodiments, auxotrophic modifications are used to ensure that the bacteria do not survive in the absence of the auxotrophic gene product (e.g., outside of the gut).
[0365] In complex communities, it is possible for bacteria to share DNA. In very rare circumstances, an auxotrophic bacterial strain may receive DNA from a non-auxotrophic strain, which repairs the genomic deletion and permanently rescues the auxotroph. Therefore, engineering a bacterial strain with more than one auxotroph may greatly decrease the probability that DNA transfer will occur enough times to rescue the auxotrophy. In some
embodiments, the genetically engineered bacteria of the invention comprise a deletion or mutation in two or more genes required for cell survival and/or growth.
[0366] Other examples of essential genes include, but are not limited to yhbV, yagG, hemB, secD, secF, ribD, ribE, thiL, dxs, ispA, dnaX, adk, hemH, IpxH, cysS, fold, rpIT, infC, thrS, nadE, gapA, yeaZ, aspS, argS, pgsA, yefM, metG, folE, yejM, gyrA, nrdA, nrdB, folC, accD, fabB, gltX, HgA, zipA, dapE, dapA, der, hisS, ispG, suhB, tadA, acpS, era, rnc, ftsB, eno, pyrG, chpR, Igt, fbaA, pgk, yqgD, metK, yqgF, plsC, ygiT, pare, ribB, cca, ygjD, tdcF, yraL, yihA, ftsN, murl, murB, birA, secE, nusG, rplJ, rpIL, rpoB, rpoC, ubiA, plsB, lexA, dnaB, ssb, alsK, groS, psd, orn, yjeE, rpsR, chpS, ppa, valS, yjgP, yjgQ, dnaC, ribF, IspA, ispH, dapB, folA, imp, yabQ, ftsL, ftsl, murE, murF, mraY, murD, ftsW, murG, murC, ftsQ, ftsA, ftsZ, IpxC, secM, secA, can, folK, hemL, yadR, dapD, map, rpsB, infB ,nusA, ftsH, obgE, rpmA, rplU, ispB, murA, yrbB, yrbK, yhbN, rpsl, rplM, degS, mreD, mreC, mreB, accB, accC, yrdC, def, fmt, rplQ, rpoA, rpsD, rpsK, rpsM, entD, mrdB, mrdA, nadD, hlepB, rpoE, pssA, yfiO, rpIS, trmD, rpsP, ffh, grpE, yfjB, csrA, ispF, ispD, rplW, rpID, rpIC, rpsJ, fusA, rpsG, rpsL, trpS, yrfF, asd, rpoH, ftsX, ftsE, ftsY, frr, dxr, ispU, rfaK, kdtA, coaD, rpmB, dfp, dut, gmk, spot, gyrB, dnaN, dnaA, rpmH, rnpA, yidC, tnaB, glmS, glmU, wzyE, hemD, hemC, yigP, ubiB, ubiD, hemG, secY, rpIO, rpmD, rpsE, rpIR, rplF, rpsH, rpsN, rplE, rplX, rpIN, rpsQ, rpmC, rpIP, rpsC, rpIV, rpsS, rplB, cdsA,
yaeL, yaeT, IpxD, fabZ, IpxA, IpxB, dnaE, accA, tilS, proS, yafF, tsf, pyrH, olA, rlpB, leuS, Int, glnS, fldA, cydA, infA, cydC, ftsK, lolA, serS, rpsA, msbA, IpxK, kdsB, mukF, mukE, mukB, asnS, fab A, mviN, me, yceQ, fabD, fabG, acpP, tmk, holB, lolC, lolD, lolE, purB, ymfK, minE, mind, pth, rsA, ispE, lolB, hemA, prfA, prmC, kdsA, topA, ribA, fabl, racR, dicA, ydfB, tyrS, ribC, ydiL, pheT, pheS, yhhQ, bcsB, glyQ, yibJ, and gpsA. Other essential genes are known to those of ordinary skill in the art.
[0367] In some embodiments, the genetically engineered bacterium of the present disclosure is a synthetic ligand-dependent essential gene (SLiDE) bacterial cell. SLiDE bacterial cells are synthetic auxotrophs with a mutation in one or more essential genes that only grow in the presence of a particular ligand (see Lopez and Anderson "Synthetic Auxotrophs with Ligand-Dependent Essential Genes for a BL21 (DE3 Biosafety Strain, "ACS Synthetic Biology (2015) DOI: 10.1021 /acssynbio.5b00085, the entire contents of which are expressly incorporated herein by reference).
[0368] In some embodiments, the SLiDE bacterial cell comprises a mutation in an essential gene. In some embodiments, the essential gene is selected from the group consisting of pheS, dnaN, tyrS, metG, and adk. In some embodiments, the essential gene is dnaN comprising one or more of the following mutations: H191 N, R240C, I317S, F319V, L340T, V347I, and S345C. In some embodiments, the essential gene is dnaN comprising the mutations H191 N, R240C, I317S, F319V, L340T, V347I, and S345C. In some
embodiments, the essential gene is pheS comprising one or more of the following mutations: F125G, P183T, P184A, R186A, and I188L. In some embodiments, the essential gene is pheS comprising the mutations F125G, P183T, P184A, R186A, and I188L. In some embodiments, the essential gene is tyrS comprising one or more of the following mutations: L36V, C38A, and F40G. In some embodiments, the essential gene is tyrS comprising the mutations L36V, C38A, and F40G. In some embodiments, the essential gene is metG comprising one or more of the following mutations: E45Q, N47R, I49G, and A51 C. In some embodiments, the essential gene is metG comprising the mutations E45Q, N47R, I49G, and A51 C. In some embodiments, the essential gene is adk comprising one or more of the following mutations: I4L, L5I, and
L6G. In some embodiments, the essential gene is adk comprising the mutations I4L, L5I, and L6G.
[0369] In some embodiments, the genetically engineered bacterium is complemented by a ligand. In some embodiments, the ligand is selected from the group consisting of benzothiazole, indole, 2-aminobenzothiazole, indole-3- butyric acid, indole-3-acetic acid, and L-histidine methyl ester. For example, bacterial cells comprising mutations in metG (E45Q, N47R, I49G, and A51 C) are complemented by benzothiazole, indole, 2-aminobenzothiazole, indole-3- butyric acid, indole-3-acetic acid or L-histidine methyl ester. Bacterial cells comprising mutations in dnaN (H191 N, R240C, I317S, F319V, L340T, V347I, and S345C) are complemented by benzothiazole, indole or 2- aminobenzothiazole. Bacterial cells comprising mutations in pheS (F125G, P183T, P184A, R186A, and I188L) are complemented by benzothiazole or 2- aminobenzothiazole. Bacterial cells comprising mutations in tyrS (L36V, C38A, and F40G) are complemented by benzothiazole or 2-aminobenzothiazole.
Bacterial cells comprising mutations in adk (I4L, L5I, and L6G) are
complemented by benzothiazole or indole.
[0370] In some embodiments, the genetically engineered bacterium comprises more than one mutant essential gene that renders it auxotrophic to a ligand. In some embodiments, the bacterial cell comprises mutations in two essential genes. For example, in some embodiments, the bacterial cell comprises mutations in tyrS (L36V, C38A, and F40G) and metG (E45Q, N47R, I49G, and A51 C). In other embodiments, the bacterial cell comprises mutations in three essential genes. For example, in some embodiments, the bacterial cell comprises mutations in tyrS (L36V, C38A, and F40G), metG (E45Q, N47R, I49G, and A51 C), and pheS (F125G, P183T, P184A, R186A, and I188L).
[0371] In some embodiments, the genetically engineered bacterium is a conditional auxotroph whose essential gene(s) is replaced using the arabinose system, e.g., as shown in Fig. 66.
[0372] In some embodiments, the genetically engineered bacterium of the disclosure is an auxotroph and also comprises kill-switch circuitry, such as any of the kill-switch components and systems described herein. For example, the recombinant bacteria may comprise a deletion or mutation in an essential
gene required for cell survival and/or growth, for example, in a DNA synthesis gene, for example, thyA, cell wall synthesis gene, for example, dapA and/or an amino acid gene, for example, serA or MetA and may also comprise a toxin gene that is regulated by one or more transcriptional activators that are expressed in response to an environmental condition(s) and/or signal(s) (such as the described arabinose system) or regulated by one or more recombinases that are expressed upon sensing an exogenous environmental condition(s) and/or signal(s) (such as the recombinase systems described herein and in Figs. 62-65. Other embodiments are described in Wright et al., "GeneGuard: A Modular Plasmid System Designed for Biosafety," ACS Synthetic Biology (2015) 4: 307-316, the entire contents of which are expressly incorporated herein by reference). In some embodiments, the genetically engineered bacterium of the disclosure is an auxotroph and also comprises kill-switch circuitry, such as any of the kill-switch components and systems described herein, as well as another biosecurity system, such a conditional origin of replication (Wright et al., 2015).
[0373] In other embodiments, auxotrophic modifications may also be used to screen for mutant bacteria that consume excess ammonia. In a more specific aspect, auxotrophic modifications may be used to screen for mutant bacteria that consume excess ammonia by overproducing arginine. As described herein, many genes involved in arginine metabolism are subject to repression by arginine via its interaction with ArgR. The astC gene promoter is unique in that the arginine-ArgR complex acts as a transcriptional activator, as opposed to a transcriptional repressor. AstC encodes succinylornithine aminotransferase, the third enzyme of the ammonia-producing arginine succinyltransferase (AST) pathway and the first of the astCADBE operon in E. coli (Schneider et al., 1998). In certain embodiments, the genetically
engineered bacteria are auxotrophic for a gene, and express the auxotrophic gene product under the control of an astC promoter. In these embodiments, the auxotrophy is subject to a positive feedback mechanism and used to select for mutant bacteria which consume excess ammonia by overproducing arginine. A non-limiting example of a positive feedback auxotroph is shown in Figs. 60A and 60B.
Genetic Regulatory Circuits
[0374] In some embodiments, the genetically engineered bacteria comprise multi-layered genetic regulatory circuits for expressing the constructs described herein (see, e.g., U.S. Provisional Application No. 62/184,811 , incorporated herein by reference in its entirety).
[0375] In certain embodiments, the invention provides methods for selecting genetically engineered bacteria that overproduce arginine. In some embodiments, the invention provides methods for selecting genetically engineered bacteria that consume excess ammonia via an alternative metabolic pathway, e.g., a histidine biosynthesis pathway, a methionine biosynthesis pathway, a lysine biosynthesis pathway, an asparagine biosynthesis pathway, a glutamine biosynthesis pathway, and a tryptophan biosynthesis pathway. In some embodiments, the invention provides genetically engineered bacteria comprising a mutant arginine regulon and an ArgR-regulated two-repressor activation genetic regulatory circuit. The two-repressor activation genetic regulatory circuit is useful to screen for mutant bacteria that reduce ammonia or rescue an auxotroph. In some constructs, high levels of arginine and the resultant activation of ArgR by arginine can cause expression of a detectable label or an essential gene that is required for cell survival.
[0376] The two-repressor activation regulatory circuit comprises a first ArgR and a second repressor, e.g., the Tet repressor. In one aspect of these embodiments, ArgR inhibits transcription of a second repressor, which inhibits the transcription of a particular gene of interest, e.g., a detectable product, which may be used to screen for mutants that consume excess ammonia, and/or an essential gene that is required for cell survival. Any detectable product may be used, including but not limited to, luciferase, β-galactosidase, and fluorescent proteins such as GFP. In some embodiments, the second repressor is a Tet repressor protein (TetR). In this embodiment, an ArgR- repressible promoter comprising wild-type ARG boxes drives the expression of TetR, and a TetR-repressible promoter drives the expression of at least one gene of interest, e.g., GFP. In the absence of ArgR binding (which occurs at low arginine concentrations), tetR is transcribed, and TetR represses GFP expression. In the presence of ArgR binding (which occurs at high arginine
concentrations), tetR expression is repressed, and GFP is generated.
Examples of other second repressors useful in these embodiments include, but are not limited to, ArsR, AscG, LacI, CscR, DeoR, DgoR, FruR, GaIR, GatR, CI, LexA, RafR, QacR, and PtxS (US20030166191 ). In some embodiments, the mutant arginine regulon comprising a switch is subjected to mutagenesis, and mutants that reduce ammonia by overproducing arginine are selected based upon the level of detectable product, e.g., by flow cytometry, fluorescence- activated cell sorting (FACS) when the detectable product fluoresces.
[0377] In some embodiments, the gene of interest is one required for survival and/or growth of the bacteria. Any such gene may be used, including but not limited to, cysE, gin A, ilvD, leuB, lysA, serA, metA, glyA, hisB, ilvA, pheA, proA, thrC, trpC, tyrA, thyA, uraA, dapA, dapB, dapD, dapE, dapF, flhD, metB, metC, proAB, and thi1, as long as the corresponding wild-type gene has been removed or mutated so as not to produce the gene product except under control of ArgR. In some embodiments, an ArgR-repressible promoter comprising wild-type ARG boxes drives the expression of a TetR protein, and a TetR-repressible promoter drives the expression of at least one gene required for survival and/or growth of the bacteria, e.g., thyA, uraA (Sat et al., 2003). In some embodiments, the genetically engineered bacterium is auxotrophic in a gene that is not complemented when the bacterium is present in the
mammalian gut, wherein said gene is complemented by a second inducible gene present in the bacterium; transcription of the second gene is ArgR- repressible and induced in the presence of sufficiently high concentrations of arginine (thus complementing the auxotrophic gene). In some embodiments, the mutant arginine regulon comprising a two-repressor activation circuit is subjected to mutagenesis, and mutants that reduce excess ammonia are selected by growth in the absence of the gene product required for survival and/or growth. In some embodiments, the mutant arginine regulon comprising a two-repressor activation circuit is used to ensure that the bacteria do not survive in the absence of high levels of arginine (e.g., outside of the gut).
Host-Plasmid Mutual Dependency
[0378] In some embodiments, the genetically engineered bacteria of the invention also comprise a plasm id that has been modified to create a host-
plasmid mutual dependency. In certain embodiments, the mutually dependent host-plasm id platform is GeneGuard (Wright et al., 2015). In some
embodiments, the GeneGuard plasmid comprises (i) a conditional origin of replication, in which the requisite replication initiator protein is provided in trans; (ii) an auxotrophic modification that is rescued by the host via genomic translocation and is also compatible for use in rich media; and/or (iii) a nucleic acid sequence which encodes a broad-spectrum toxin. The toxin gene may be used to select against plasmid spread by making the plasmid DNA itself disadvantageous for strains not expressing the anti-toxin (e.g., a wild-type bacterium). In some embodiments, the GeneGuard plasmid is stable for at least 100 generations without antibiotic selection. In some embodiments, the GeneGuard plasmid does not disrupt growth of the host. The GeneGuard plasmid is used to greatly reduce unintentional plasmid propagation in the genetically engineered bacteria of the invention.
[0379] The mutually dependent host-plasmid platform may be used alone or in combination with other biosafety mechanisms, such as those described herein (e.g., kill switches, auxotrophies). In some embodiments, the genetically engineered bacteria comprise a GeneGuard plasmid. In other embodiments, the genetically engineered bacteria comprise a GeneGuard plasmid and/or one or more kill switches. In other embodiments, the genetically engineered bacteria comprise a GeneGuard plasmid and/or one or more auxotrophies. In still other embodiments, the genetically engineered bacteria comprise a
GeneGuard plasmid, one or more kill switches, and/or one or more
auxotrophies.
[0380] Synthetic gene circuits express on plasmids may function well in the short term but lose ability and/or function in the long term (Danino et al., 2015). In some embodiments, the genetically engineered bacteria comprise stable circuits for expressing genes of interest over prolonged periods. In some embodiments, the genetically engineered bacteria are capable of producing a gut enhancer molecule and further comprise a toxin-anti-toxin system that simultaneously produces a toxin (hok) and a short-lived anti-toxin (sok), wherein loss of the plasmid causes the cell to be killed by the long-lived toxin (Danino et al., 2015; Fig. 68). In some embodiments, the genetically engineered bacteria
further comprise alp7 from B. subtilis plasmid pl_20 and produces filaments that are capable of pushing plasm ids to the poles of the cells in order to ensure equal segregation during cell division (Danino et al., 2015).
Kill Switch
[0381] In some embodiments, the genetically engineered bacteria of the invention also comprise a kill switch (see, e.g., U.S. Provisional Application Nos. 62/183,935, 62/263,329, and 62/277,654, each of which is incorporated herein by reference in their entireties). The kill switch is intended to actively kill engineered microbes in response to external stimuli. As opposed to an auxotrophic mutation where bacteria die because they lack an essential nutrient for survival, the kill switch is triggered by a particular factor in the environment that induces the production of toxic molecules within the microbe that cause cell death.
[0382] Bacteria engineered with kill switches have been engineered for in vitro research purposes, e.g., to limit the spread of a biofuel-producing microorganism outside of a laboratory environment. Bacteria engineered for in vivo administration to treat a disease or disorder may also be programmed to die at a specific time after the expression and delivery of a heterologous gene or genes, for example, a therapeutic gene(s) or after the subject has
experienced the therapeutic effect. For example, in some embodiments, the kill switch is activated to kill the bacteria after a period of time following oxygen level-dependent expression of argAfbr. In some embodiments, the kill switch is activated in a delayed fashion following oxygen level-dependent expression of argAfbr, for example, after the production of arginine or citrulline. Alternatively, the bacteria may be engineered to die after the bacteria has spread outside of a disease site. Specifically, it may be useful to prevent long-term colonization of subjects by the microorganism, spread of the microorganism outside the area of interest (for example, outside the gut) within the subject, or spread of the microorganism outside of the subject into the environment (for example, spread to the environment through the stool of the subject). Examples of such toxins that can be used in kill-switches include, but are not limited to, bacteriocins, lysins, and other molecules that cause cell death by lysing cell membranes, degrading cellular DNA, or other mechanisms. Such toxins can be used
individually or in combination. The switches that control their production can be based on, for example, transcriptional activation (toggle switches; see, e.g., Gardner et al., 2000), translation (riboregulators), or DNA recombination
(recombinase-based switches), and can sense environmental stimuli such as anaerobiosis or reactive oxygen species. These switches can be activated by a single environmental factor or may require several activators in AND, OR, NAND and NOR logic configurations to induce cell death. For example, an AND riboregulator switch is activated by tetracycline, isopropyl β-D-l - thiogalactopyranoside (IPTG), and arabinose to induce the expression of lysins, which permeabilize the cell membrane and kill the cell. IPTG induces the expression of the endolysin and holin mRNAs, which are then derepressed by the addition of arabinose and tetracycline. All three inducers must be present to cause cell death. Examples of kill switches are known in the art (Callura et al., 2010). In some embodiments, the kill switch is activated to kill the bacteria after a period of time following oxygen level-dependent expression of argAfbr. In some embodiments, the kill switch is activated in a delayed fashion following oxygen level-dependent expression of argAmr.
[0383] Kill-switches can be designed such that a toxin is produced in response to an environmental condition or external signal (e.g., the bacteria is killed in response to an external cue) or, alternatively designed such that a toxin is produced once an environmental condition no longer exists or an external signal is ceased.
[0384] Thus, in some embodiments, the genetically engineered bacteria of the disclosure are further programmed to die after sensing an exogenous environmental signal, for example, in a low-oxygen environment. In some embodiments, the genetically engineered bacteria of the present disclosure, e.g., bacteria expressing argAfbr and repressor ArgR, comprise one or more genes encoding one or more recombinase(s), whose expression is induced in response to an environmental condition or signal and causes one or more recombination events that ultimately leads to the expression of a toxin which kills the cell. In some embodiments, the at least one recombination event is the flipping of an inverted heterologous gene encoding a bacterial toxin which is then constitutively expressed after it is flipped by the first recombinase. In one
embodiment, constitutive expression of the bacterial toxin kills the genetically engineered bacterium. In these types of kill-switch systems once the
engineered bacterial cell senses the exogenous environmental condition and expresses the heterologous gene of interest, the recombinant bacterial cell is no longer viable.
[0385] In another embodiment in which the genetically engineered bacteria of the present disclosure, e.g., bacteria expressing argAmr and repressor ArgR, express one or more recombinase(s) in response to an environmental condition or signal causing at least one recombination event, the genetically engineered bacterium further expresses a heterologous gene encoding an anti-toxin in response to an exogenous environmental condition or signal. In one embodiment, the at least one recombination event is flipping of an inverted heterologous gene encoding a bacterial toxin by a first
recombinase. In one embodiment, the inverted heterologous gene encoding the bacterial toxin is located between a first forward recombinase recognition sequence and a first reverse recombinase recognition sequence. In one embodiment, the heterologous gene encoding the bacterial toxin is
constitutively expressed after it is flipped by the first recombinase. In one embodiment, the anti-toxin inhibits the activity of the toxin, thereby delaying death of the genetically engineered bacterium. In one embodiment, the genetically engineered bacterium is killed by the bacterial toxin when the heterologous gene encoding the anti-toxin is no longer expressed when the exogenous environmental condition is no longer present.
[0386] In another embodiment, the at least one recombination event is flipping of an inverted heterologous gene encoding a second recombinase by a first recombinase, followed by the flipping of an inverted heterologous gene encoding a bacterial toxin by the second recombinase. In one embodiment, the inverted heterologous gene encoding the second recombinase is located between a first forward recombinase recognition sequence and a first reverse recombinase recognition sequence. In one embodiment, the inverted heterologous gene encoding the bacterial toxin is located between a second forward recombinase recognition sequence and a second reverse recombinase recognition sequence. In one embodiment, the heterologous gene encoding the
second recombinase is constitutively expressed after it is flipped by the first recombinase. In one embodiment, the heterologous gene encoding the bacterial toxin is constitutively expressed after it is flipped by the second recombinase. In one embodiment, the genetically engineered bacterium is killed by the bacterial toxin. In one embodiment, the genetically engineered bacterium further expresses a heterologous gene encoding an anti-toxin in response to the exogenous environmental condition. In one embodiment, the anti-toxin inhibits the activity of the toxin when the exogenous environmental condition is present, thereby delaying death of the genetically engineered bacterium. In one embodiment, the genetically engineered bacterium is killed by the bacterial toxin when the heterologous gene encoding the anti-toxin is no longer expressed when the exogenous environmental condition is no longer present.
[0387] In one embodiment, the at least one recombination event is flipping of an inverted heterologous gene encoding a second recombinase by a first recombinase, followed by flipping of an inverted heterologous gene encoding a third recombinase by the second recombinase, followed by flipping of an inverted heterologous gene encoding a bacterial toxin by the third recombinase.
[0388] In one embodiment, the at least one recombination event is flipping of an inverted heterologous gene encoding a first excision enzyme by a first recombinase. In one embodiment, the inverted heterologous gene encoding the first excision enzyme is located between a first forward
recombinase recognition sequence and a first reverse recombinase recognition sequence. In one embodiment, the heterologous gene encoding the first excision enzyme is constitutively expressed after it is flipped by the first recombinase. In one embodiment, the first excision enzyme excises a first essential gene. In one embodiment, the programmed recombinant bacterial cell is not viable after the first essential gene is excised.
[0389] In one embodiment, the first recombinase further flips an inverted heterologous gene encoding a second excision enzyme. In one embodiment, the wherein the inverted heterologous gene encoding the second excision enzyme is located between a second forward recombinase recognition
sequence and a second reverse recombinase recognition sequence. In one embodiment, the heterologous gene encoding the second excision enzyme is constitutively expressed after it is flipped by the first recombinase. In one embodiment, the genetically engineered bacterium dies or is no longer viable when the first essential gene and the second essential gene are both excised. In one embodiment, the genetically engineered bacterium dies or is no longer viable when either the first essential gene is excised or the second essential gene is excised by the first recombinase.
[0390] In one embodiment, the genetically engineered bacterium dies after the at least one recombination event occurs. In another embodiment, the genetically engineered bacterium is no longer viable after the at least one recombination event occurs.
[0391] In any of these embodiment, the recombinase can be a
recombinase selected from the group consisting of: Bxbl, PhiC31 , TP901 , Bxbl, PhiC31 , TP901 , HK022, HP1 , R4, Int1 , Int2, Int3, Int4, Int5, Int6, Int7, Int8, Int9, Int10, Int11 , Int12, Int13, Int14, Int15, Int16, Int17, Int18, Int19, Int20, Int21 , Int22, Int23, Int24, Int25, Int26, Int27, Int28, Int29, Int30, Int31 , Int32, Int33, and Int34, or a biologically active fragment thereof.
[0392] In the above-described kill-switch circuits, a toxin is produced in the presence of an environmental factor or signal. In another aspect of kill- switch circuitry, a toxin may be repressed in the presence of an environmental factor (not produced) and then produced once the environmental condition or external signal is no longer present. An exemplary kill-switch in which the toxin is repressed in the presence of an external factor or signal (and activated once the external signal is removed) is shown in Figs. 66-68. The disclosure provides recombinant bacterial cells which express one or more heterologous gene(s) upon sensing arabinose or other sugar in the exogenous environment. In this aspect, the recombinant bacterial cells contain the araC gene, which encodes the AraC transcription factor, as well as one or more genes under the control of the araBAD promoter. In the absence of arabinose, the AraC transcription factor adopts a conformation that represses transcription of genes under the control of the araBAD promoter. In the presence of arabinose, the AraC transcription factor undergoes a conformational change that allows it to
bind to and activate the araBAD promoter, which induces expression of the desired gene, for example tetR, which represses expression of a toxin gene. In this embodiment, the toxin gene is repressed in the presence of arabinose or other sugar. In an environment where arabinose is not present, the tetR gene is not activated and the toxin is expressed, thereby killing the bacteria. The arabinose system can also be used to express an essential gene, in which the essential gene is only expressed in the presence of arabinose or other sugar and is not expressed when arabinose or other sugar is absent from the environment.
[0393] Thus, in some embodiments in which one or more heterologous gene(s) are expressed upon sensing arabinose in the exogenous environment, the one or more heterologous genes are directly or indirectly under the control of the araBAD promoter. In some embodiments, the expressed heterologous gene is selected from one or more of the following: a heterologous therapeutic gene, a heterologous gene encoding an anti-toxin, a heterologous gene encoding a repressor protein or polypeptide, for example, a TetR repressor, a heterologous gene encoding an essential protein not found in the bacterial cell, and/or a heterologous encoding a regulatory protein or polypeptide.
fbr
[0394] In some embodiments, the argA gene is directly or indirectly under the control of the araBAD promoter. Fig. 13 depicts a schematic diagram fbr
of an exemplary BAD promoter-driven argA construct. In this embodiment, fbr fbr the argA gene is inserted between the araC and araD genes. ArgA is flanked by a ribosome binding site, a FRT site, and one or more transcription terminator sequences. The nucleic acid sequence of an exemplary BAD promoter-driven argAfbr construct is shown in Table 19. All bolded sequences are Nissle genomic DNA. A portion of the araC gene is bolded and underlined, the argAmr gene is |boxed|, and the bolded sequence in between is the promoter that is activated by the presence of arabinose. The ribosome binding site is in italics, the terminator sequences are highlighted, and the FRT site is boxed . A portion of the araD gene is boxed; in dashes. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least
about 99% homologous to the BAD promoter sequence of SEQ ID NO: 67 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise the BAD promoter sequence of SEQ ID NO: 67 or a functional fragment thereof.
Table 19
GTGTGACCGACGCCAAAACACTGGAACTGGTGAAGCAGGCTGCGGGAACATTG
CAACTGGATATTACTGCTCGCCTGTCGATGAGTCTCAATAACACGCCGCTGCA
GGGCGCGCATATCAACGTCGTCAGTGGCAATTTTATTATTGCCCAGCCGCTGG
GCGTCGATGACGGCGTGGATTACTGCCATAGCGGGCGTATCCGGCGGATTGAT
GAAGACGCGATCCATCGTCAACTGGACAGCGGTGCAATAGTGCTAATGGGGCC
GGTCGCTGTTTCAGTCACTGGCGAGAGCTTTAACCTGACCTCGGAAGAGATTG
CCACTCAACTGGCCATCAAACTGAAAGCTGAAAAGATGATTGGTTTTTGCTCT
TCCCAGGGCGTCACTAATGACGACGGTGATATTGTCTCCGAACTTTTCCCTAA
CGAAGCGCAAGCGCGGGTAGAAGCCCAGGAAGAGAAAGGCGATTACAACTCCG
GTACGGTGCGCTTTTTGCGTGGCGCAGTGAAAGCCTGCCGCAGCGGCGTGCGT
CGCTGTCATTTAATCAGTTATCAGGAAGATGGCGCGCTGTTGCAAGAGTTGTT
CTCACGCGACGGTATCGGTACGCAGATTGTGATGGAAAGCGCCGAGCAGATTC
GTCGCGCAACAATCAACGATATTGGCGGTATTCTGGAGTTGATTCGCCCACTG
GAGCAGCAAGGTATTCTGGTACGCCGTTCTCGCGAGCAGCTGGAGATGGAAAT
CGACAAATTCACCATTATTCAGCGCGATAACACGACTATTGCCTGCGCCGCGC
TCTATCCGTTCCCGGAAGAGAAGATTGGGGAAATGGCCTGTGTGGCAGTTCAC
CCGGATTACCGCAGTTCATCAAGGGGTGAAGTTCTGCTGGAACGCATTGCCGC
TCAGGCTAAGCAGAGCGGCTTAAGCAAATTGTTTGTGCTGACCACGCGCAGTA
TTCACTGGTTCCAGGAACGTGGATTTACCCCAGTGGATATTGATTTACTGCCC
GAGAGCAAAAAGCAGTTGTACAACTACCAGCGTAAATCCAAAGTGTTGATGGC
GGATTTAGGGTAATGGGAATTAGCCATGGTCCATATGAATATCCTCCTTAGTT
CCTATTCC gaagttcctattccgaagttcctattctctagaaagtataggaac ■ ttcGAAGCAGCTCCAGCCTACACAATCGCTCAAGACGTGTAATGCTGCAATCT
GCATGCAAGCTTGGCACTGGCCACGCA^^^HH^B^^^^^^B^ I CTTAATTTGATGCCTGGCAGTTTATGGCGGGCGTCCTGCCCGCCACCCTCCG
||1|||1||||||||||ATGCCTGGCAGTTCCCTACTCTCGCATGctcgagcca tgggacgtccaggtattagaagccaacctggcgctgccaaaacacaacctggt cacgctcacctggggcaatgtcagcgccgttgatcgcgggcgcggcgtcctgg
tgatcaaaccttccggcgtcgactacagcatcatgaccgctgacgatatggtc gtggtcagcatcgaaaccggtgaagtggttgaaggtacgaaaaagccctcctc cgacacgccaactcaccggctgctctatcaggcattcccgtctattggcggca ttgtgcacacacactcgcgccacgccaccatctgggcgcaggcgggccagtcg attccagcagccggcaccacccacgccgactatttctacggcaccattccctg cacccgcaaaatgaccgacgcagaaatcaacggtgaatatgagtgggaaaccg gtaacgtcatcgtagaaaccttcgaaaaacagggtatcaatgcagcgcaaatg cccggcgtgctggtccattctcacggcccatttgcatggggaaaaaacgccga agatgcggtgcataacgccatcgtgctggaagaagtcgcttatatggggatat tctgccgtcagttagcgccgcagttaccggatatgcagcaaacgctgctggat aaacactatctgcgtaagcatggcgcgaaggcatattacgggcagtaa
[0395]Arabinose inducible promoters are known in the art, including Para, ParaB, Parac, and ParaBAD- In one embodiment, the arabinose inducible promoter is from E. coli. In some embodiments, the Parac promoter and the ParaBAD promoter operate as a bidirectional promoter, with the ParaBAD promoter controlling expression of a heterologous gene(s) in one direction, and the Parac (in close proximity to, and on the opposite strand from the ParaBAD promoter), controlling expression of a heterologous gene(s) in the other direction. In the presence of arabinose, transcription of both heterologous genes from both promoters is induced. However, in the absence of arabinose, transcription of both heterologous genes from both promoters is not induced.
[0396] In one exemplary embodiment of the disclosure, the engineered bacteria of the present disclosure contains a kill-switch having at least the following sequences: a ParaBAD promoter operably linked to a heterologous gene encoding a tetracycline repressor protein (TetR), a Parac promoter operably linked to a heterologous gene encoding AraC transcription factor, and a heterologous gene encoding a bacterial toxin operably linked to a promoter which is repressed by the tetracycline repressor protein (PietR)- In the presence of arabinose, the AraC transcription factor activates the ParaBAD promoter, which activates transcription of the TetR protein which, in turn, represses transcription of the toxin. In the absence of arabinose, however, AraC suppresses
transcription from the the ParaBAD promoter and no TetR protein is expressed. In this case, expression of the heterologous toxin gene is activated, and the toxin is expressed. The toxin builds up in the recombinant bacterial cell, and the recombinant bacterial cell is killed. In one embodiment, the araC gene encoding the AraC transcription factor is under the control of a constitutive promoter and is therefore constitutively expressed.
[0397] In one embodiment of the disclosure, the recombinant bacterial cell further comprises an anti-toxin under the control of a constitutive promoter. In this situation, in the presence of arabinose, the toxin is not expressed due to repression by TetR protein, and the anti-toxin protein builds-up in the cell.
However, in the absence of arabinose, TetR protein is not expressed, and expression of the toxin is induced. The toxin begins to build-up within the recombinant bacterial cell. The recombinant bacterial cell is no longer viable once the toxin protein is present at either equal or greater amounts than that of the anti-toxin protein in the cell, and the recombinant bacterial cell will be killed by the toxin.
[0398] In another embodiment of the disclosure, the recombinant bacterial cell further comprises an anti-toxin under the control of the ParaBAD promoter. In this situation, in the presence of arabinose, TetR and the anti-toxin are expressed, the anti-toxin builds up in the cell, and the toxin is not expressed due to repression by TetR protein. However, in the absence of arabinose, both the TetR protein and the anti-toxin are not expressed, and expression of the toxin is induced. The toxin begins to build-up within the recombinant bacterial cell. The recombinant bacterial cell is no longer viable once the toxin protein is expressed, and the recombinant bacterial cell will be killed by the toxin.
[0399] In another exemplary embodiment of the disclosure, the engineered bacteria of the present disclosure contain a kill-switch having at least the following sequences: a ParaBAD promoter operably linked to a heterologous gene encoding an essential polypeptide not found in the recombinant bacterial cell (and required for survival), and a Parac promoter operably linked to a heterologous gene encoding the AraC transcription factor. In the presence of arabinose, the AraC transcription factor activates the ParaBAD promoter, which activates transcription of the heterologous gene encoding the
essential polypeptide, allowing the recombinant bacterial cell to survive. In the absence of arabinose, however, AraC suppresses transcription from the the araBAD promoter and the essential protein required for survival is not expressed. In this case, the recombinant bacterial cell dies in the absence of arabinose. In some embodiments, the sequence of ParaBAD promoter operably linked to a heterologous gene encoding an essential polypeptide not found in the
recombinant bacterial cell can be present in the bacterial cell in conjunction with the TetR/toxin kill-switch system described directly above. In some
embodiments, the sequence of ParaBAD promoter operably linked to a
heterologous gene encoding an essential polypeptide not found in the
recombinant bacterial cell can be present in the bacterial cell in conjunction with the TetR/toxin/anti-toxin kill-switch system described directly above.
[0400] In yet other embodiments, the bacteria may comprise a plasmid stability system with a plasmid that produces both a short-lived anti-toxin and a long-lived toxin. In this system, the bacterial cell produces equal amounts of toxin and anti-toxin to neutralize the toxin. However, if/when the cell loses the plasmid, the short-lived anti-toxin begins to decay. When the anti-toxin decays completely the cell dies as a result of the longer-lived toxin killing it.
[0401] In some embodiments, the engineered bacteria of the present disclosure, for example, bacteria expressing argAmr and repressor ArgR further comprise the gene(s) encoding the components of any of the above-described kill-switch circuits.
[0402] In any of the above-described embodiments, the bacterial toxin is selected from the group consisting of a lysin, Hok, Fst, TisB, LdrD, Kid, SymE, MazF, FlmA, lbs, XCV2162, dinJ, CcdB, MazF, ParE, YafO, Zeta, hicB, relB, yhaV, yoeB, chpBK, hipA, microcin B, microcin B17, microcin C, microcin C7- C51 , microcin J25, microcin ColV, microcin 24, microcin L, microcin D93, microcin L, microcin E492, microcin H47, microcin I47, microcin M, colicin A, colicin E1 , colicin K, colicin N, colicin U, colicin B, colicin la, colicin lb, colicin 5, colicinI O, colicin S4, colicin Y, colicin E2, colicin E7, colicin E8, colicin E9, colicin E3, colicin E4, colicin E6; colicin E5, colicin D, colicin M, and cloacin DF13, or a biologically active fragment thereof.
[0403] In any of the above-described embodiments, the anti-toxin is selected from the group consisting of an anti-lysin, Sok, RNAII, IstR, RdID, Kis, SymR, MazE, FlmB, Sib, ptaRNAI , yafQ, CcdA, MazE, ParD, yafN, Epsilon, HicA, relE, prIF, yefM, chpBI, hipB, MccE, MccECTD, MccF, Cai, ImmEI , Cki, Cni, Cui, Cbi, lia, Imm, Cfi, Im10, Csi, Cyi, Im2, Im7, Im8, Im9, Im3, Im4, lmmE6, cloacin immunity protein (Cim), lmmE5, ImmD, and Cmi, or a
biologically active fragment thereof.
[0404] In one embodiment, the bacterial toxin is bactericidal to the genetically engineered bacterium. In one embodiment, the bacterial toxin is bacteriostatic to the genetically engineered bacterium.
[0405] In some embodiments, the engineered bacteria provided herein have an arginine regulon comprising one or more nucleic acid mutations that reduce or eliminate arginine-mediated repression of each of the operons that encode the enzymes responsible for converting glutamate to arginine and/or an intermediate byproduct, e.g., citrulline, in the arginine biosynthesis pathway, such that the mutant arginine regulon produces more arginine and/or
intermediate byproduct than an unmodified regulon from the same bacterial subtype under the same conditions. In some embodiments, the genetically engineered bacteria comprise an arginine feedback resistant N-acetylglutamate synthase mutant, e.g., argAmr. In some embodiments, the genetically
engineered bacteria comprise a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes N-acetylglutamate kinase, N- acetylglutamylphosphate reductase, acetylornithine aminotransferase, N- acetylornithinase, ornithine transcarbamylase, argininosuccinate synthase, argininosuccinate lyase, and carbamoylphosphate synthase, thereby
derepressing the regulon and enhancing arginine and/or intermediate byproduct biosynthesis. In some embodiments, the genetically engineered bacteria further comprise an arginine feedback resistant N-acetylglutamate synthase mutant. In some embodiments, the arginine feedback resistant N-acetylglutamate synthase mutant is controlled by an oxygen level-dependent promoter. In some embodiments, the arginine feedback resistant N-acetylglutamate synthase mutant is controlled by a promoter that is induced under low-oxygen or
anaerobic conditions. In some embodiments, the promoter is selected from the fumarate and nitrate reductase regulator (FNR) promoter, arginine deiminiase and nitrate reduction (ANR) promoter, and dissimilatory nitrate respiration regulator (DNR) promoter. In some embodiments, the arginine feedback resistant N-acetylglutamate synthase mutant is argAmr.
[0406] In some embodiments, the genetically engineered bacteria comprise a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes and an arginine feedback resistant N- acetylglutamate synthase mutant. In some embodiments, the genetically engineered bacteria comprise a mutant arginine regulon, wherein the bacterium comprises a gene encoding a functional N-acetylglutamate synthetase that is mutated to reduce arginine feedback inhibition as compared to a wild-type N- acetylglutamate synthetase from the same bacterial subtype under the same conditions, wherein expression of the gene encoding the mutated N- acetylglutamate synthetase is controlled by a promoter that is induced under low-oxygen or anaerobic conditions, wherein the mutant arginine regulon comprises one or more operons comprising genes that encode arginine biosynthesis enzymes N-acetylglutamate kinase, N-acetylglutamate phosphate reductase, acetylornithine aminotransferase, N-acetylornithinase,
carbamoylphosphate synthase, ornithine transcarbamylase, argininosuccinate synthase, and argininosuccinate lyase, and wherein each operon comprises one or more mutated ARG box(es) characterized by one or more nucleic acid mutations that reduces arginine-mediated repression of the operon via ArgR repressor binding, and retains RNA polymerase binding with sufficient affinity to promote transcription of the genes in the operon.
[0407] In some embodiments, the genetically engineered bacteria is an auxotroph comprising a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes and an arginine feedback resistant N- acetylglutamate synthase mutant. In one embodiment, the genetically engineered bacteria comprising a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons
that encode the arginine biosynthesis enzymes and an arginine feedback resistant N-acetylglutamate synthase mutant is an auxotroph selected from a cysE, glnA, ilvD, leuB, lysA, serA, metA, glyA, hisB, ilvA, pheA, proA, thrC, trpC, tyrA, thyA, uraA, dapA, dapB, dapD, dapE, dapF, flhD, metB, metC, proAB, and thi1 auxotroph. In some embodiments, the engineered bacteria have more than one auxotrophy, for example, they may be a MhyA and dapA auxotroph.
[0408] In some embodiments, the genetically engineered bacteria comprising a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes and an arginine feedback resistant N- acetylglutamate synthase mutant further comprises a kill-switch circuit, such as any of the kill-switch circuits provided herein. For example, in some
embodiments, the genetically engineered bacteria further comprise one or more genes encoding one or more recombinase(s) under the control of an inducible promoter and an inverted toxin sequence. In some embodiments, the
genetically engineered bacteria further comprise one or more genes encoding an anti-toxin. In some embodiments, the engineered bacteria further comprise one or more genes encoding one or more recombinase(s) under the control of an inducible promoter and one or more inverted excision genes, wherein the excision gene(s) encode an enzyme that deletes an essential gene. In some embodiments, the genetically engineered bacteria further comprise one or more genes encoding an anti-toxin. In some embodiments, the engineered bacteria further comprise one or more genes encoding a toxin under the control of a promoter having a TetR repressor binding site and a gene encoding the TetR under the control of an inducible promoter that is induced by arabinose, such as araBAD- In some embodiments, the genetically engineered bacteria further comprise one or more genes encoding an anti-toxin.
[0409] In some embodiments, the genetically engineered bacteria is an auxotroph comprising a mutant arginine regulon comprising one or more nucleic acid mutations in at least one ARG box for each of the operons that encode the arginine biosynthesis enzymes and an arginine feedback resistant N- acetylglutamate synthase mutant and further comprises a kill-switch circuit, such as any of the kill-switch circuits described herein.
[0410] In some embodiments of the above described genetically engineered bacteria, the gene encoding the arginine feedback resistant N- acetylglutamate synthetase is present on a plasmid in the bacterium and operatively linked on the plasmid to the promoter that is induced under low- oxygen or anaerobic conditions. In other embodiments, the gene encoding the arginine feedback resistant N-acetylglutamate synthetase is present in the bacterial chromosome and is operatively linked in the chromosome to the promoter that is induced under low-oxygen or anaerobic conditions.
[0411] In some embodiments, the genetically engineered bacteria comprise a mutant arginine repressor comprising one or more nucleic acid mutations such that arginine repressor function is decreased or inactive, or the genetically engineered bacteria do not have an arginine repressor (e.g., the arginine repressor gene has been deleted), resulting in derepression of the regulon and enhancement of arginine and/or intermediate byproduct
biosynthesis. In some embodiments, the genetically engineered bacteria further comprise an arginine feedback resistant N-acetylglutamate synthase mutant. In some embodiments, the arginine feedback resistant N-acetylglutamate synthase mutant is controlled by an oxygen level-dependent promoter. In some embodiments, the arginine feedback resistant N-acetylglutamate synthase mutant is controlled by a promoter that is induced under low-oxygen or anaerobic conditions. In some embodiments, the promoter is selected from the fumarate and nitrate reductase regulator (FNR) promoter, arginine deiminiase and nitrate reduction (ANR) promoter, and dissimilatory nitrate respiration regulator (DNR) promoter. In some embodiments, the arginine feedback resistant N-acetylglutamate synthase mutant is argAmr.
[0412] In some embodiments, the genetically engineered bacteria comprise a mutant or deleted arginine repressor and an arginine feedback resistant N-acetylglutamate synthase mutant. In some embodiments, the genetically engineered bacterium comprise an arginine regulon, wherein the bacterium comprises a gene encoding a functional N-acetylglutamate
synthetase with reduced arginine feedback inhibition as compared to a wild-type N-acetylglutamate synthetase from the same bacterial subtype under the same conditions, wherein expression of the gene encoding arginine feedback
resistant N-acetylglutamate synthetase is controlled by a promoter that is induced by exogenous environmental conditions and wherein the bacterium has been genetically engineered to lack a functional ArgR repressor.
[0413] In some embodiments, the genetically engineered bacteria comprising a mutant or deleted arginine repressor and an arginine feedback resistant N-acetylglutamate synthase mutant is an auxotroph. In one
embodiment, the genetically engineered bacteria comprising a mutant or deleted arginine repressor and an arginine feedback resistant N- acetylglutamate synthase mutant is an auxotroph selected from a cysE, gin A, HvD, leuB, lysA, serA, metA, glyA, hisB, HvA, pheA, proA, thrC, trpC, tyrA, thyA, uraA, dap A, dapB, dapD, dapE, dapF, flhD, metB, metC, proAB, and thi1 auxotroph. In some embodiments, the engineered bacteria have more than one auxotrophy, for example, they may be a thyA and dapA auxotroph.
[0414] In some embodiments, the genetically engineered bacteria comprising a mutant or deleted arginine repressor and an arginine feedback resistant N-acetylglutamate synthase mutant further comprise a kill-switch circuit, such as any of the kill-switch circuits provided herein. For example, in some embodiments, the genetically engineered bacteria further comprise one or more genes encoding one or more recombinase(s) under the control of an inducible promoter, and an inverted toxin sequence. In some embodiments, the genetically engineered bacteria further comprise one or more genes encoding an anti-toxin. In some embodiments, the engineered bacteria further comprise one or more genes encoding one or more recombinase(s) under the control of an inducible promoter and one or more inverted excision genes, wherein the excision gene(s) encode an enzyme that deletes an essential gene. In some embodiments, the genetically engineered bacteria further comprise one or more genes encoding an anti-toxin. In some embodiments, the engineered bacteria further comprise one or more genes encoding a toxin under the control of a promoter having a TetR repressor binding site and a gene encoding the TetR under the control of an inducible promoter that is induced by arabinose, such as araBAD- In some embodiments, the genetically engineered bacteria further comprise one or more genes encoding an anti-toxin.
[0415] In some embodiments, the genetically engineered bacterium is an auxotroph comprising a mutant or deleted arginine repressor and an arginine feedback resistant N-acetylglutamate synthase mutant and further comprises a kill-switch circuit, such as any of the kill-switch circuits described herein.
[0416] In some embodiments of the above described genetically engineered bacteria, the gene encoding the arginine feedback resistant N- acetylglutamate synthetase is present on a plasmid in the bacterium and operatively linked on the plasmid to the promoter that is induced under low- oxygen or anaerobic conditions. In other embodiments, the gene encoding the arginine feedback resistant N-acetylglutamate synthetase is present in the bacterial chromosome and is operatively linked in the chromosome to the promoter that is induced under low-oxygen or anaerobic conditions.
Ammonia Transport
[0417] Ammonia transporters may further be expressed or modified in the genetically engineered bacteria of the invention in order to enhance ammonia transport into the cell. AmtB is a membrane transport protein that transports ammonia into bacterial cells. In some embodiments, the genetically engineered bacteria of the invention also comprise multiple copies of the native amtB gene. In some embodiments, the genetically engineered bacteria of the invention also comprise an amtB gene from a different bacterial species. In some
embodiments, the genetically engineered bacteria of the invention comprise multiple copies of an amtB gene from a different bacterial species. In some embodiments, the native amtB gene in the genetically engineered bacteria of the invention is not modified. In some embodiments, the genetically engineered bacteria of the invention comprise an amtB gene that is controlled by its native promoter, an inducible promoter, or a promoter that is stronger than the native promoter, e.g., a GlnRS promoter, a P(Bla) promoter, or a constitutive promoter.
[0418] In some embodiments, the native amtB gene in the genetically engineered bacteria is not modified, and one or more additional copies of the native amtB gene are inserted into the genome under the control of the same inducible promoter that controls expression of argAmr, e.g., a FNR promoter, or a different inducible promoter than the one that controls expression of argAfbr or a constitutive promoter. In alternate embodiments, the native amtB gene is not
modified, and a copy of a non-native amtB gene from a different bacterial species is inserted into the genome under the control of the same inducible promoter that controls expression of argAfbr, e.g., a FNR promoter, or a different inducible promoter than the one that controls expression of argAfbr or a constitutive promoter.
[0419] In some embodiments, the native amtB gene in the genetically engineered bacteria is not modified, and one or more additional copies of the native amtB gene are present in the bacteria on a plasmid and under the control of the same inducible promoter that controls expression of argAmr, e.g., a FNR promoter, or a different inducible promoter than the one that controls expression of argAfbr or a constitutive promoter. In alternate embodiments, the native amtB gene is not modified, and a copy of a non-native amtB gene from a different bacterial species is present in the bacteria on a plasmid and under the control of the same inducible promoter that controls expression of argAmr, e.g., a FNR promoter, or a different inducible promoter than the one that controls expression of argAfbr or a constitutive promoter.
[0420] In some embodiments, the native amtB gene is mutagenized, the mutants exhibiting increased ammonia transport are selected, and the
mutagenized amtB gene is isolated and inserted into the genetically engineered bacteria. In some embodiments, the native amtB gene is mutagenized, mutants exhibiting increased ammonia transport are selected, and those mutants are used to produce the bacteria of the invention. The ammonia transporter modifications described herein may be present on a plasmid or chromosome.
[0421] In some embodiments, the genetically engineered bacterium is E. coli Nissle, and the native amtB gene in E. coli Nissle is not modified; one or more additional copies the native E. coli Nissle amtB genes are inserted into the E coli Nissle genome under the control of the same inducible promoter that controls expression of argAmr, e.g., a FNR promoter, or a different inducible promoter than the one that controls expression of argAmr or a constitutive promoter. In an alternate embodiment, the native amtB gene in E coli Nissle is not modified, and a copy of a non-native amtB gene from a different bacterium, e.g., Lactobacillus plantarum, is inserted into the E coli Nissle genome under the control of the same inducible promoter that controls expression of argAmr,
e.g., a FNR promoter, or a different inducible promoter than the one that controls expression of argAmr or a constitutive promoter.
[0422] In some embodiments, the genetically engineered bacterium is E. coli Nissle, and the native amtB gene in E. coli Nissle is not modified; one or more additional copies the native E. coli Nissle amtB genes are present in the bacterium on a plasmid and under the control of the same inducible promoter that controls expression of argAmr, e.g., a FNR promoter, or a different inducible promoter than the one that controls expression of argAmr, or a constitutive promoter. In an alternate embodiment, the native amtB gene in E. coli Nissle is not modified, and a copy of a non-native amtB gene from a different bacterium, e.g., Lactobacillus plantarum, are present in the bacterium on a plasmid and under the control of the same inducible promoter that controls expression of argAmr, e.g., a FNR promoter, or a different inducible promoter than the one that controls expression of argAmr, or a constitutive promoter.
Pharmaceutical Compositions and Formulations
[0423] Pharmaceutical compositions comprising the genetically
engineered bacteria described herein may be used to treat, manage,
ameliorate, and/or prevent a disorder associated with hyperammonemia or symptom(s) associated with hyperammonemia. Pharmaceutical compositions comprising one or more genetically engineered bacteria, alone or in
combination with prophylactic agents, therapeutic agents, and/or
pharmaceutically acceptable carriers are provided.
[0424] In certain embodiments, the pharmaceutical composition comprises one species, strain, or subtype of bacteria that are engineered to comprise the genetic modifications described herein. In alternate embodiments, the pharmaceutical composition comprises two or more species, strains, and/or subtypes of bacteria that are each engineered to comprise the genetic modifications described herein.
[0425] The pharmaceutical compositions described herein may be formulated in a conventional manner using one or more physiologically acceptable carriers comprising excipients and auxiliaries, which facilitate processing of the active ingredients into compositions for pharmaceutical use. Methods of formulating pharmaceutical compositions are known in the art (see,
e.g., "Remington's Pharmaceutical Sciences," Mack Publishing Co., Easton, PA). In some embodiments, the pharmaceutical compositions are subjected to tabletting, lyophilizing, direct compression, conventional mixing, dissolving, granulating, levigating, emulsifying, encapsulating, entrapping, or spray drying to form tablets, granulates, nanoparticles, nanocapsules, microcapsules, microtablets, pellets, or powders, which may be enterically coated or uncoated. Appropriate formulation depends on the route of administration.
[0426] The genetically engineered bacteria described herein may be formulated into pharmaceutical compositions in any suitable dosage form (e.g., liquids, capsules, sachet, hard capsules, soft capsules, tablets, enteric coated tablets, suspension powders, granules, or matrix sustained release formations for oral administration) and for any suitable type of administration (e.g., oral, topical, injectable, immediate-release, pulsatile-release, delayed-release, or sustained release). Suitable dosage amounts for the genetically engineered bacteria may range from about 105 to 1012 bacteria, e.g., approximately 105 bacteria, approximately 106 bacteria, approximately 107 bacteria, approximately 108 bacteria, approximately 109 bacteria, approximately 1010 bacteria, approximately 1011 bacteria, or approximately 1011 bacteria. The composition may be administered once or more daily, weekly, or monthly. The composition may be administered before, during, or following a meal. In one embodiment, the pharmaceutical composition is administered before the subject eats a meal. In one embodiment, the pharmaceutical composition is administered currently with a meal. In one embodiment, the pharmaceutical composition is
administered after the subject eats a meal.
[0427] The composition may be administered once or more daily, weekly, or monthly. The genetically engineered bacteria may be formulated into pharmaceutical compositions comprising one or more pharmaceutically acceptable carriers, thickeners, diluents, buffers, buffering agents, surface active agents, neutral or cationic lipids, lipid complexes, liposomes, penetration enhancers, carrier compounds, and other pharmaceutically acceptable carriers or agents. For example, the pharmaceutical composition may include, but is not limited to, the addition of calcium bicarbonate, sodium bicarbonate, calcium phosphate, various sugars and types of starch, cellulose derivatives, gelatin,
vegetable oils, polyethylene glycols, and surfactants, including, for example, polysorbate 20. In some embodiments, the genetically engineered bacteria of the invention may be formulated in a solution of sodium bicarbonate, e.g., 1 molar solution of sodium bicarbonate (to buffer an acidic cellular environment, such as the stomach, for example). The genetically engineered bacteria may be administered and formulated as neutral or salt forms. Pharmaceutically acceptable salts include those formed with anions such as those derived from hydrochloric, phosphoric, acetic, oxalic, tartaric acids, etc., and those formed with cations such as those derived from sodium, potassium, ammonium, calcium, ferric hydroxides, isopropylamine, triethylamine, 2-ethylamino ethanol, histidine, procaine, etc.
[0428] The genetically engineered bacteria disclosed herein may be administered topically and formulated in the form of an ointment, cream, transdermal patch, lotion, gel, shampoo, spray, aerosol, solution, emulsion, or other form well-known to one of skill in the art. See, e.g., "Remington's
Pharmaceutical Sciences," Mack Publishing Co., Easton, PA. In an
embodiment, for non-sprayable topical dosage forms, viscous to semi-solid or solid forms comprising a carrier or one or more excipients compatible with topical application and having a dynamic viscosity greater than water are employed. Suitable formulations include, but are not limited to, solutions, suspensions, emulsions, creams, ointments, powders, liniments, salves, etc., which may be sterilized or mixed with auxiliary agents (e.g., preservatives, stabilizers, wetting agents, buffers, or salts) for influencing various properties, e.g., osmotic pressure. Other suitable topical dosage forms include sprayable aerosol preparations wherein the active ingredient in combination with a solid or liquid inert carrier, is packaged in a mixture with a pressurized volatile (e.g., a gaseous propellant, such as freon) or in a squeeze bottle. Moisturizers or humectants can also be added to pharmaceutical compositions and dosage forms. Examples of such additional ingredients are well known in the art. In one embodiment, the pharmaceutical composition comprising the recombinant bacteria of the invention may be formulated as a hygiene product. For example, the hygiene product may be an antibacterial formulation, or a fermentation
product such as a fermentation broth. Hygiene products may be, for example, shampoos, conditioners, creams, pastes, lotions, and lip balms.
[0429] The genetically engineered bacteria disclosed herein may be administered orally and formulated as tablets, pills, dragees, capsules, liquids, gels, syrups, slurries, suspensions, etc. Pharmacological compositions for oral use can be made using a solid excipient, optionally grinding the resulting mixture, and processing the mixture of granules, after adding suitable auxiliaries if desired, to obtain tablets or dragee cores. Suitable excipients include, but are not limited to, fillers such as sugars, including lactose, sucrose, mannitol, or sorbitol; cellulose compositions such as maize starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth, methyl cellulose, hydroxypropylmethyl- cellulose, sodium carbomethylcellulose; and/or physiologically acceptable polymers such as polyvinylpyrrolidone (PVP) or polyethylene glycol (PEG). Disintegrating agents may also be added, such as cross-linked
polyvinylpyrrolidone, agar, alginic acid or a salt thereof such as sodium alginate.
[0430] Tablets or capsules can be prepared by conventional means with pharmaceutically acceptable excipients such as binding agents (e.g.,
pregelatinised maize starch, polyvinylpyrrolidone, hydroxypropyl
methylcellulose, carboxymethylcellulose, polyethylene glycol, sucrose, glucose, sorbitol, starch, gum, kaolin, and tragacanth); fillers (e.g., lactose,
microcrystalline cellulose, or calcium hydrogen phosphate); lubricants (e.g., calcium, aluminum, zinc, stearic acid, polyethylene glycol, sodium lauryl sulfate, starch, sodium benzoate, L-leucine, magnesium stearate, talc, or silica);
disintegrants (e.g., starch, potato starch, sodium starch glycolate, sugars, cellulose derivatives, silica powders); or wetting agents (e.g., sodium lauryl sulphate). The tablets may be coated by methods well known in the art. A coating shell may be present, and common membranes include, but are not limited to, polylactide, polyglycolic acid, polyanhydride, other biodegradable polymers, alginate-polylysine-alginate (APA), alginate-polymethylene-co- guanidine-alginate (A-PMCG-A), hydroymethylacrylate-methyl methacrylate (HEMA-MMA), multilayered HEMA-MMA-MAA, polyacrylonitrilevinylchloride (PAN-PVC), acrylonitrile/sodium methallylsulfonate (AN-69), polyethylene glycol/poly pentamethylcyclopentasiloxane/polydimethylsiloxane
(PEG/PD5/PDMS), poly Ν,Ν- dimethyl acrylamide (PDMAAm), siliceous encapsulates, cellulose sulphate/sodium alginate/polymethylene-co-guanidine (CS/A/PMCG), cellulose acetate phthalate, calcium alginate, k-carrageenan- locust bean gum gel beads, gellan-xanthan beads, poly(lactide-co-glycolides), carrageenan, starch poly-anhydrides, starch polymethacrylates, polyamino acids, and enteric coating polymers.
[0431] In some embodiments, the genetically engineered bacteria are enterically coated for release into the gut or a particular region of the gut, for example, the large intestine. The typical pH profile from the stomach to the colon is about 1-4 (stomach), 5.5-6 (duodenum), 7.3-8.0 (ileum), and 5.5-6.5 (colon). In some diseases, the pH profile may be modified. In some
embodiments, the coating is degraded in specific pH environments in order to specify the site of release. In some embodiments, at least two coatings are used. In some embodiments, the outside coating and the inside coating are degraded at different pH levels.
[0432] Liquid preparations for oral administration may take the form of solutions, syrups, suspensions, or a dry product for constitution with water or other suitable vehicle before use. Such liquid preparations may be prepared by conventional means with pharmaceutically acceptable agents such as suspending agents (e.g., sorbitol syrup, cellulose derivatives, or hydrogenated edible fats); emulsifying agents (e.g., lecithin or acacia); non-aqueous vehicles (e.g., almond oil, oily esters, ethyl alcohol, or fractionated vegetable oils); and preservatives (e.g., methyl or propyl-p-hydroxybenzoates or sorbic acid). The preparations may also contain buffer salts, flavoring, coloring, and sweetening agents as appropriate. Preparations for oral administration may be suitably formulated for slow release, controlled release, or sustained release of the genetically engineered bacteria described herein.
[0433] In one embodiment, the genetically engineered bacteria of the disclosure may be formulated in a composition suitable for administration to pediatric subjects. As is well known in the art, children differ from adults in many aspects, including different rates of gastric emptying, pH, gastrointestinal permeability, etc. (Ivanovska et al., 2014). Moreover, pediatric formulation acceptability and preferences, such as route of administration and taste
attributes, are critical for achieving acceptable pediatric compliance. Thus, in one embodiment, the composition suitable for administration to pediatric subjects may include easy-to-swallow or dissolvable dosage forms, or more palatable compositions, such as compositions with added flavors, sweeteners, or taste blockers. In one embodiment, a composition suitable for administration to pediatric subjects may also be suitable for administration to adults.
[0434] In one embodiment, the composition suitable for administration to pediatric subjects may include a solution, syrup, suspension, elixir, powder for reconstitution as suspension or solution, dispersible/effervescent tablet, chewable tablet, gummy candy, lollipop, freezer pop, troche, chewing gum, oral thin strip, orally disintegrating tablet, sachet, soft gelatin capsule, sprinkle oral powder, or granules. In one embodiment, the composition is a gummy candy, which is made from a gelatin base, giving the candy elasticity, desired chewy consistency, and longer shelf-life. In some embodiments, the gummy candy may also comprise sweeteners or flavors.
[0435] In one embodiment, the composition suitable for administration to pediatric subjects may include a flavor. As used herein, "flavor" is a substance (liquid or solid) that provides a distinct taste and aroma to the formulation.
Flavors also help to improve the palatability of the formulation. Flavors include, but are not limited to, strawberry, vanilla, lemon, grape, bubble gum, and cherry.
[0436] In certain embodiments, the genetically engineered bacteria may be orally administered, for example, with an inert diluent or an assimilable edible carrier. The compound may also be enclosed in a hard or soft shell gelatin capsule, compressed into tablets, or incorporated directly into the subject's diet. For oral therapeutic administration, the compounds may be incorporated with excipients and used in the form of ingestible tablets, buccal tablets, troches, capsules, elixirs, suspensions, syrups, wafers, and the like. To administer a compound by other than parenteral administration, it may be necessary to coat the compound with, or co-administer the compound with, a material to prevent its inactivation.
[0437] In another embodiment, the pharmaceutical composition
comprising the recombinant bacteria of the invention may be a comestible product, for example, a food product. In one embodiment, the food product is
milk, concentrated milk, fermented milk (yogurt, sour milk, frozen yogurt, lactic acid bacteria-fermented beverages), milk powder, ice cream, cream cheeses, dry cheeses, soybean milk, fermented soybean milk, vegetable-fruit juices, fruit juices, sports drinks, confectionery, candies, infant foods (such as infant cakes), nutritional food products, animal feeds, or dietary supplements. In one embodiment, the food product is a fermented food, such as a fermented dairy product. In one embodiment, the fermented dairy product is yogurt. In another embodiment, the fermented dairy product is cheese, milk, cream, ice cream, milk shake, or kefir. In another embodiment, the recombinant bacteria of the invention are combined in a preparation containing other live bacterial cells intended to serve as probiotics. In another embodiment, the food product is a beverage. In one embodiment, the beverage is a fruit juice-based beverage or a beverage containing plant or herbal extracts. In another embodiment, the food product is a jelly or a pudding. Other food products suitable for
administration of the recombinant bacteria of the invention are well known in the art. For example, see U.S. 2015/0359894 and US 2015/0238545, the entire contents of each of which are expressly incorporated herein by reference. In yet another embodiment, the pharmaceutical composition of the invention is injected into, sprayed onto, or sprinkled onto a food product, such as bread, yogurt, or cheese.
[0438] In some embodiments, the composition is formulated for intraintestinal administration, intrajejunal administration, intraduodenal administration, intraileal administration, gastric shunt administration, or intracolic administration, via nanoparticles, nanocapsules, microcapsules, or microtablets, which are enterically coated or uncoated. The pharmaceutical compositions may also be formulated in rectal compositions such as suppositories or retention enemas, using, e.g., conventional suppository bases such as cocoa butter or other glycerides. The compositions may be suspensions, solutions, or emulsions in oily or aqueous vehicles, and may contain suspending, stabilizing and/or dispersing agents.
[0439] The genetically engineered bacteria described herein may be administered intranasally, formulated in an aerosol form, spray, mist, or in the form of drops, and conveniently delivered in the form of an aerosol spray
presentation from pressurized packs or a nebuliser, with the use of a suitable propellant {e.g., dichlorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane, carbon dioxide or other suitable gas). Pressurized aerosol dosage units may be determined by providing a valve to deliver a metered amount. Capsules and cartridges {e.g., of gelatin) for use in an inhaler or insufflator may be formulated containing a powder mix of the compound and a suitable powder base such as lactose or starch.
[0440] The genetically engineered bacteria may be administered and formulated as depot preparations. Such long acting formulations may be administered by implantation or by injection, including intravenous injection, subcutaneous injection, local injection, direct injection, or infusion. For example, the compositions may be formulated with suitable polymeric or hydrophobic materials {e.g., as an emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble derivatives {e.g., as a sparingly soluble salt).
[0441] In some embodiments, disclosed herein are pharmaceutically acceptable compositions in single dosage forms. Single dosage forms may be in a liquid or a solid form. Single dosage forms may be administered directly to a patient without modification or may be diluted or reconstituted prior to administration. In certain embodiments, a single dosage form may be administered in bolus form, e.g., single injection, single oral dose, including an oral dose that comprises multiple tablets, capsule, pills, etc. In alternate embodiments, a single dosage form may be administered over a period of time, e.g., by infusion.
[0442] Single dosage forms of the pharmaceutical composition may be prepared by portioning the pharmaceutical composition into smaller aliquots, single dose containers, single dose liquid forms, or single dose solid forms, such as tablets, granulates, nanoparticles, nanocapsules, microcapsules, microtablets, pellets, or powders, which may be enterically coated or uncoated. A single dose in a solid form may be reconstituted by adding liquid, typically sterile water or saline solution, prior to administration to a patient.
[0443] In other embodiments, the composition can be delivered in a controlled release or sustained release system. In one embodiment, a pump
may be used to achieve controlled or sustained release. In another
embodiment, polymeric materials can be used to achieve controlled or sustained release of the therapies of the present disclosure (see, e.g., U.S. Patent No. 5,989,463). Examples of polymers used in sustained release formulations include, but are not limited to, poly(2-hydroxy ethyl methacrylate), poly(methyl methacrylate), poly(acrylic acid), poly(ethylene-co-vinyl acetate), poly(methacrylic acid), polyglycolides (PLG), polyanhydrides, poly(N- vinyl pyrrolidone), polyvinyl alcohol), polyacrylamide, poly(ethylene glycol), polylactides (PLA), poly(lactide-co-glycolides) (PLGA), and polyorthoesters. The polymer used in a sustained release formulation may be inert, free of leachable impurities, stable on storage, sterile, and biodegradable. In some embodiments, a controlled or sustained release system can be placed in proximity of the prophylactic or therapeutic target, thus requiring only a fraction of the systemic dose. Any suitable technique known to one of skill in the art may be used.
[0444] Dosage regimens may be adjusted to provide a therapeutic response. Dosing can depend on several factors, including severity and responsiveness of the disease, route of administration, time course of treatment (days to months to years), and time to amelioration of the disease. For example, a single bolus may be administered at one time, several divided doses may be administered over a predetermined period of time, or the dose may be reduced or increased as indicated by the therapeutic situation. The
specification for the dosage is dictated by the unique characteristics of the active compound and the particular therapeutic effect to be achieved. Dosage values may vary with the type and severity of the condition to be alleviated. For any particular subject, specific dosage regimens may be adjusted over time according to the individual need and the professional judgment of the treating clinician. Toxicity and therapeutic efficacy of compounds provided herein can be determined by standard pharmaceutical procedures in cell culture or animal models. For example, LD50, ED50, EC50, and IC50 may be determined, and the dose ratio between toxic and therapeutic effects (LD50/ED50) may be calculated as the therapeutic index. Compositions that exhibit toxic side effects may be used, with careful modifications to minimize potential damage to reduce side
effects. Dosing may be estimated initially from cell culture assays and animal models. The data obtained from in vitro and in vivo assays and animal studies can be used in formulating a range of dosage for use in humans.
[0445] The ingredients are supplied either separately or mixed together in unit dosage form, for example, as a dry lyophilized powder or water- free concentrate in a hermetically sealed container such as an ampoule or sachet indicating the quantity of active agent. If the mode of administration is by injection, an ampoule of sterile water for injection or saline can be provided so that the ingredients may be mixed prior to administration.
[0446] The pharmaceutical compositions may be packaged in a hermetically sealed container such as an ampoule or sachet indicating the quantity of the agent. In one embodiment, one or more of the pharmaceutical compositions is supplied as a dry sterilized lyophilized powder or water-free concentrate in a hermetically sealed container and can be reconstituted {e.g., with water or saline) to the appropriate concentration for administration to a subject. In an embodiment, one or more of the prophylactic or therapeutic agents or pharmaceutical compositions is supplied as a dry sterile lyophilized powder in a hermetically sealed container stored between 2° C and 8° C and administered within 1 hour, within 3 hours, within 5 hours, within 6 hours, within 12 hours, within 24 hours, within 48 hours, within 72 hours, or within one week after being reconstituted. Cryoprotectants can be included for a lyophilized dosage form, principally 0-10% sucrose (optimally 0.5-1.0%). Other suitable cryoprotectants include trehalose and lactose. Other suitable bulking agents include glycine and arginine, either of which can be included at a concentration of 0-0.05%, and polysorbate-80 (optimally included at a concentration of 0.005- 0.01 %). Additional surfactants include but are not limited to polysorbate 20 and BRIJ surfactants. The pharmaceutical composition may be prepared as an injectable solution and can further comprise an agent useful as an adjuvant, such as those used to increase absorption or dispersion, e.g., hyaluronidase.
Methods of Treatment
[0447] Another aspect of the invention provides methods of treating a disease or disorder associated with hyperammonemia. In some embodiments, the invention provides methods for reducing, ameliorating, or eliminating one or
more symptom(s) associated with these diseases or disorders. In some embodiments, the disorder is a urea cycle disorder such as argininosuccinic aciduria, arginase deficiency, carbamoylphosphate synthetase deficiency, citrullinemia, N-acetylglutamate synthetase deficiency, and ornithine
transcarbamylase deficiency. In alternate embodiments, the disorder is a liver disorder such as hepatic encephalopathy, acute liver failure, or chronic liver failure; organic acid disorders; isovaleric aciduria; 3-methylcrotonylglycinuria; methylmalonic acidemia; propionic aciduria; fatty acid oxidation defects;
carnitine cycle defects; carnitine deficiency; β-oxidation deficiency; lysinuric protein intolerance; pyrroline-5-carboxylate synthetase deficiency; pyruvate carboxylase deficiency; ornithine aminotransferase deficiency; carbonic anhydrase deficiency; hyperinsulinism-hyperammonemia syndrome;
mitochondrial disorders; valproate therapy; asparaginase therapy; total parenteral nutrition; cystoscopy with glycine-containing solutions; post- lung/bone marrow transplantation; portosystemic shunting; urinary tract infections; ureter dilation; multiple myeloma; chemotherapy; infection;
neurogenic bladder; or intestinal bacterial overgrowth. In some embodiments, the symptom(s) associated thereof include, but are not limited to, seizures, ataxia, stroke-like lesions, coma, psychosis, vision loss, acute encephalopathy, cerebral edema, as well as vomiting, respiratory alkalosis, and hypothermia.
[0448] The method may comprise preparing a pharmaceutical composition with at least one genetically engineered species, strain, or subtype of bacteria described herein, and administering the pharmaceutical composition to a subject in a therapeutically effective amount. In some embodiments, the genetically engineered bacteria of the invention are administered orally, e.g., in a liquid suspension. In some embodiments, the genetically engineered bacteria of the invention are lyophilized in a gel cap and administered orally. In some embodiments, the genetically engineered bacteria of the invention are administered via a feeding tube or gastric shunt. In some embodiments, the genetically engineered bacteria of the invention are administered rectally, e.g., by enema. In some embodiments, the genetically engineered bacteria of the invention are administered topically, intraintestinally, intrajejunally,
intraduodenally, intraileally, and/or intracolically.
[0449] In certain embodiments, administering the pharmaceutical composition to the subject reduces ammonia concentrations in a subject. In some embodiments, the methods of the present disclosure may reduce the ammonia concentration in a subject by at least about 10%, 20%, 25%, 30%, 40%, 50%, 60%, 70%, 75%, 80%, 85%, 90%, 95%, or more as compared to levels in an untreated or control subject. In some embodiments, reduction is measured by comparing the ammonia concentration in a subject before and after administration of the pharmaceutical composition. In some embodiments, the method of treating or ameliorating hyperammonemia allows one or more symptoms of the condition or disorder to improve by at least about 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, or more.
[0450] Before, during, and after the administration of the pharmaceutical composition, ammonia concentrations in the subject may be measured in a biological sample, such as blood, serum, plasma, urine, fecal matter, peritoneal fluid, intestinal mucosal scrapings, a sample collected from a tissue, and/or a sample collected from the contents of one or more of the following: the stomach, duodenum, jejunum, ileum, cecum, colon, rectum, and anal canal. In some embodiments, the methods may include administration of the
compositions of the invention to reduce ammonia concentrations in a subject to undetectable levels, or to less than about 1 %, 2%, 5%, 10%, 20%, 25%, 30%, 40%, 50%, 60%, 70%, 75%, or 80% of the subject's ammonia concentrations prior to treatment.
[0451] In certain embodiments, the genetically engineered bacteria comprising the mutant arginine regulon is E. coli Nissle. The genetically engineered bacteria may be destroyed, e.g., by defense factors in the gut or blood serum (Sonnenborn et al., 2009), or by activation of a kill switch, several hours or days after administration. Thus, the pharmaceutical composition comprising the mutant arginine regulon may be re-administered at a
therapeutically effective dose and frequency. Length of Nissle residence in vivo in mice is shown in Figs. 34 and 35. In alternate embodiments, the genetically engineered bacteria are not destroyed within hours or days after administration and may propagate and colonize the gut.
[0452] The pharmaceutical composition may be administered alone or in combination with one or more additional therapeutic agents, including but not limited to, sodium phenylbutyrate, sodium benzoate, and glycerol
phenylbutyrate. An important consideration in the selection of the one or more additional therapeutic agents is that the agent(s) should be compatible with the genetically engineered bacteria of the invention, e.g., the agent(s) must not kill the bacteria.
[0453] In one embodiment, the genetically engineered bacteria are administered for prevention, treatment or management of HE. In some embodiments, the genetically engineered bacteria are administered in combination with another therapeutic approach to prevent HE reoccurrence. In one embodiment, the genetically engineered bacteria are administered in combination with branched-chain amino acid supplementation. In one embodiment, the genetically engineered bacteria are administered in combination with acetyl-l-carnitine and/or sodium benzoate and/or zinc and/or acarbose and/or ornithine aspartate. In one embodiment, the genetically engineered bacteria are administered in combination with non-absorbable disaccharides, which are commonly applied to both treat and prevent HE in patients. In one embodiment, the genetically engineered bacteria are administered in combination with lactulose and/or lactitol.
[0454] In one embodiment, the genetically engineered bacteria are administered in combination with one or more antibiotics, for example for the treatment of HE. Examples of such antibiotics include, but are not limited to, non-absorbable antibiotics, such as aminoglicosides, e.g., neomycin and/or paramomycin. In one embodiment, the antibiotic is rifamycin. In one
embodiment, the antibiotic is a rifamycin derivative, e.g., a synthetic derivative, including but not limited to, rifaximin.
[0455] Rifaximin has been shown to significantly reduce the risk of an episode of hepatic encephalopathy, as compared with placebo, over a 6-month period (Bass et a., Rifaximin Treatment in Hepatic Encephalopathy; N Engl J Med 2010; 362:1071 -1081 ). Rifaximin is a semi-synthetic derivative of rifampin and acts by binding to the beta-subunit of bacterial DNA-dependent RNA
polymerase, and thereby blocking transcription. As a result, bacterial protein synthesis and growth is inhibited.
[0456] Rifaximin has been shown to be active against E. coli both in vitro and in clinical studies. It therefore is understood that, for a combination treatment with rifaximin to be effective, the genetically engineered bacteria must further comprise a rifaximin resistance.
[0457] Resistance to rifaximin is caused primarily by mutations in the rpoB gene. This changes the binding site on DNA dependent RNA polymerase and decreases rifaximin binding affinity, thereby reducing efficacy. In one embodiment, the rifaximin resistance is a mutation in the rpoB gene. Non- limiting examples of such mutations are described in e.g., Rodriguez-Verdugo, Evolution of Escherichia coli rifampicin resistance in an antibiotic-free
environment during thermal stress. BMC Evol Biol. 2013 Feb 22; 13:50. Of note, mutations in the same three codons of the rpoB consensus sequence occur repeatedly in unrelated rifaxim in-resistant clinical isolates of several different bacterial species (as reviewed in Goldstein, Resistance to rifampicin: a review; The Journal of Antibiotics (2014), 1-6, the contents of which is herein
incorporated by reference in its entirety. In some embodiments, the genetically engineered bacteria comprise a known rifaximin resistance mutation, e.g., in the rpoB gene. In other embodiments, a screen can be employed, exposing the genetically engineered bacteria to increasing amounts of rifaximin, to identify a useful mutation which confers rifaximin resistance.
[0458] In some embodiments, the pharmaceutical composition is administered with food. In alternate embodiments, the pharmaceutical composition is administered before or after eating food. The pharmaceutical composition may be administered in combination with one or more dietary modifications, e.g., low-protein diet and amino acid supplementation. The dosage of the pharmaceutical composition and the frequency of administration may be selected based on the severity of the symptoms and the progression of the disorder. The appropriate therapeutically effective dose and/or frequency of administration can be selected by a treating clinician.
[0459] Table 20 shows non-limiting examples of target degradation rates, based on levels of phenylalanine on average in ) in hyperammonemic patients (UCD<HE).
Table 20. Target Ammonia Degradation/Arginine Production Rates
Treatment In Vivo
[0460] The genetically engineered bacteria of the invention may be evaluated in vivo, e.g., in an animal model. Any suitable animal model of a disease or condition associated with hyperammonemia may be used (see, e.g., Deignan et al., 2008; Nicaise et al., 2008), for example, a mouse model of acute liver failure and hyperammonemia. This acute liver failure and
hyperammonemia may be induced by treatment with thiol acetamide (TAA) (Basile et al., 1990; Nicaise et al., 2008). Alternatively, liver damage may be modeled using physical bile duct ligation (Rivera-Mancia et al., 2012).
Hyperammonemia may also be induced by oral supplementation with
ammonium acetate and/or magnesium chloride (Azorin et al., 1989; Rivera- Mancia et al. , 2012).
[0461]Additionally, CCI4 is often used to induce hepaticfibrosis and cirrhosis in animals (Nhung et al., Establishment of a standardized mouse model of hepatic fibrosis for biomedical research; Biomedical Research and Therapy 2014, 1 (2):43-49).
[0462] The genetically engineered bacteria of the invention may be administered to the animal, e.g., by oral gavage, and treatment efficacy determined, e.g., by measuring ammonia in blood samples and/or arginine, citrulline, or other byproducts in fecal samples.
[0463] Full citations for the references cited throughout the specification include:
1. Alifano et al. Histidine biosynthetic pathway and genes: structure,
regulation, and evolution. Microbiol Rev. 1996 Mar;60(1 ):44-69. PMID: 8852895.
2. Altenhoefer et al. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol Med Microbiol. 2004 Apr 9;40(3):223- 229. PMID: 15039098.
3. Andersen et al. Uracil uptake in Escherichia coli K-12: isolation of uraA
mutants and cloning of the gene. J Bacteriol. 1995 Apr; 177(8):2008-2013. PMID: 7721693.
4. Arthur et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012 Oct 5;338(6103): 120-123. PMID: 22903521.
5. Aoyagi et al. Gastrointestinal urease in man. Activity of mucosal urease.
Gut. 1966 Dec;7(6):631 -635. PMID: 5957514.
6. Arai et al. Expression of the nir and nor genes for denitrification of
Pseudomonas aeruginosa requires a novel CRP/FNR-related transcriptional regulator, DNR, in addition to ANR. FEBS Lett. 1995 Aug 28;371 (1 ):73-76. PMID: 7664887.
7. Aschner et al. Manganese uptake and distribution in the central nervous system (CNS). Neurotoxicology. 1999 Apr-Jun;20(2-3): 173-180. PMID: 10385881.
8. Azorin et al. A simple animal model of hyperammonemia. Hepatology.
1989 Sep;10(3):311 -314. PMID: 2759549.
9. Bansky et al. Reversal of hepatic coma by benzodiazepene antagonists (Rol5-1788). Lancet. 1985;1 :1324-1325.
10. Basile et al. Brain concentrations of benzodiazepines are elevated in an animal model of hepatic encephalopathy. Proc Natl Acad Sci USA. 1990 Jul;87(14):5263-5267. PMID: 1973539.
11. Bearden SW, Perry RD. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol. 1999 Apr;32(2):403-414. PMID: 10231495.
12. Berk DP, Chalmers T. Deafness complicating antibiotic therapy of hepatic encephalopathy. Ann Intern Med. 1970 Sep;73(3):393-396. PMID:
5455989.
Blanc et al. Lactitol or lactulose in the treatment of chronic hepatic encephalopathy: results of a meta-analysis. Hepatology. 1992
Feb;15(2):222-228. PMID: 1531204.
Caldara et al. The arginine regulon of Escherichia coli: whole-system transcriptome analysis discovers new genes and provides an integrated view of arginine regulation. Microbiology. 2006 Nov; 152(Pt 11 ):3343-3354. PMID: 17074904.
Caldara et al. Arginine biosynthesis in Escherichia coli: experimental perturbation and mathematical modeling. J Biol Chem. 2008 Mar
7;283(10):6347-6358. PMID: 18165237.
Caldovic et al. N-acetylglutamate synthase: structure, function and defects. Mol Genet Metab. 2010;100 Suppl 1 :S13-S19. Review. PMID: 20303810. Callura et al. Tracking, tuning, and terminating microbial physiology using synthetic riboregulators. Proc Natl Acad Sci U S A. 2010 Sep
7; 107(36): 15898-15903. PMID: 20713708.
Cash et al. Current concepts in the assessment and treatment of hepatic encephalopathy. QJM. 2010 Jan;103(1 ):9-16. PMID: 19903725.
Castiglione et al. The transcription factor DNR from Pseudomonas aeruginosa specifically requires nitric oxide and haem for the activation of a target promoter in Escherichia coli. Microbiology. 2009 Sep;155(Pt 9):2838- 2844. PMID: 19477902.
Cellier et al. Resistance to intracellular infections: comparative genomic analysis of Nramp. Trends Genet. 1996 Jun;12(6):201 -204. PMID:
8928221.
Charlier et al. Arginine regulon of Escherichia coli K-12. A study of repressor-operator interactions and of in vitro binding affinities versus in vivo repression. J Mol Biol. 1992 Jul 20;226(2):367-386. PMID: 1640456.
Chiang et al. Dysregulation of C/EBPalpha by mutant Huntingtin causes the urea cycle deficiency in Huntington's disease. Hum Mol Genet. 2007 Mar 1 ;16(5):483-498. PMID: 17213233.
Collinson et al. Channel crossing: how are proteins shipped across the bacterial plasma membrane? Philos Trans R Soc Lond B Biol Sci. 2015; 370:20150025. PMID: 26370937.
Cordoba J, Minguez B. Hepatic Encephalopathy. Semin Liver Dis. 2008; 28(1 ):70-80. PMID: 18293278.
Costa et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol. 2015;13(6):343-359. PMID:
25978706.
Crabeel et al. Characterization of the Saccharomyces cerevisiae ARG7 gene encoding ornithine acetyltransferase, an enzyme also endowed with acetylglutamate synthase activity. Eur J Biochem. 1997 Dec 1 ;250(2):232- 241. PMID: 9428669.
Cuevas-Ramos et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci USA. 2010 Jun 22; 107(25): 11537-42. PMID: 20534522.
Cunin et al. Molecular basis for modulated regulation of gene expression in the arginine regulon of Escherichia coli K-12. Nucleic Acids Res. 1983 Aug 11 ;11 (15):5007-5019. PMID: 6348703.
Cunin et al. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314-52. Review. Erratum in: Microbiol Rev. 1987 Mar;51 (1 ):178. PMID: 3534538.
Danino et al. Programmable probiotics for detection of cancer in urine. Sci Transl Med. 2015 May 27;7(289):289ra84. PMID: 26019220.
Deignan et al. Contrasting features of urea cycle disorders in human patients. Mol Genet Metab. 2008 Jan;93(1 ):7-14. PMID: 17933574.
Deutscher. The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol. 2008 Apr; 11 (2):87-93. PMID: 18359269.
Diaz et al. Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology. 2013 Jun;57(6):2171 -9. PMID: 22961727.
Dinleyici et al. Saccharomyces boulardii CNCM I-745 in different clinical conditions. Expert Opin Biol Ther. 2014 Nov; 14(11 ): 1593-609. PMID: 24995675.
Doolittle. A new allele of the sparse fur gene in the mouse. J Hered. 1974 May-Jun;65(3): 194-5. PMID: 4603259.
Eckhardt et al. Isolation and characterization of mutants with a feedback resistant N-acetylglutamate synthase in Escherichia coli K 12. Mol Gen Genet. 1975 Jun 19;138(3):225-32. PMID: 1102931.
Eiglmeier et al. Molecular genetic analysis of FNR-dependent promoters. Mol Microbiol. 1989 Jul;3(7):869-78. PMID: 2677602.
Fraga et al. (2008). Real-Time PCR. Current Protocols Essential
Laboratory Techniques (10.3.1 -10.3.33). John Wiley & Sons, Inc.
Galimand et al. Positive FNR-like control of anaerobic arginine degradation and nitrate respiration in Pseudomonas aeruginosa. J Bacteriol. 1991 Mar; 173(5): 1598-606. PMID: 1900277.
Gamper et al. Anaerobic regulation of transcription initiation in the arcDABC operon of Pseudomonas aeruginosa. J Bacteriol. 1991 Aug; 173(15):4742- 50. PMID: 1906871.
Gardner et al. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339-42. PMID: 10659857.
Gorke B et al. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008 Aug;6(8):613-24. PMID: 18628769.
Haberle et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012 May 29;7:32. Review. PMID: 22642880.
Haberle J. Clinical and biochemical aspects of primary and secondary hyperammonemic disorders. Arch Biochem Biophys. 2013 Aug
15;536(2):101 -8. Review. PMID: 23628343.
Hasegawa et al. Activation of a consensus FNR-dependent promoter by DNR of Pseudomonas aeruginosa in response to nitrite. FEMS Microbiol Lett. 1998 Sep 15;166(2):213-7. PMID: 9770276.
Hodges et al. The spfash mouse: a missense mutation in the ornithine transcarbamylase gene also causes aberrant mRNA splicing. Proc Natl Acad Sci U S A. 1989 Jun;86(11 ):4142-6. PMID: 2471197.
Hoeren et al. Sequence and expression of the gene encoding the respiratory nitrous-oxide reductase from Paracoccus denitrificans. Eur J Biochem. 1993 Nov 15;218(1 ):49-57. PMID: 8243476.
Hoffmann et al. Defects in amino acid catabolism and the urea cycle. Handb Clin Neurol. 2013;113:1755-73. Review. PMID: 23622399.
Hosseini et al. Proprionate as a health-promoting microbial metabolite in the human gut. Nutr Rev. 2011 May;69(5):245-58. PMID: 21521227.
Isabella et al. Deep sequencing-based analysis of the anaerobic stimulon in Neisseria gonorrhoeae. BMC Genomics. 2011 Jan 20;12:51. PMID:
21251255.
Konieczna et al. Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci. 2012 Dec; 13(8):789-806. Review. PMID: 23305365.
Lazier et al. Hyperammonemic encephalopathy in an adenocarcinoma patient managed with carglumic acid. Curr Oncol. 2014 Oct;21 (5):e736-9. PMID: 25302046.
Leonard (2006). Disorders of the urea cycle and related enzymes. Inborn Metabolic Diseases, 4th ed (pp. 263-272). Springer Medizin Verlag
Heidelberg.
Lim et al. Nucleotide sequence of the argR gene of Escherichia coli K-12 and isolation of its product, the arginine repressor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6697-701. PMID: 3116542.
Makarova et al. Conservation of the binding site for the arginine repressor in all bacterial lineages. Genome Biol. 2001 ;2(4). PMID: 11305941.
Maas et al. Studies on the mechanism of repression of arginine
biosynthesis in Escherichia coli. Dominance of repressibility in diploids. J Mol Biol. 1964 Mar; 8: 365-70. PMID: 14168690.
Maas. The arginine repressor of Escherichia coli. Microbiol Rev. 1994 Dec;58(4):631 -40. PMID: 7854250.
Meng et al. Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH. J Bacteriol. 1992
Apr;174(8):2659-69. PMID: 1556085.
Moore et al. Regulation of FNR dimerization by subunit charge repulsion. J Biol Chem. 2006 Nov 3;281 (44):33268-75. PMID: 16959764.
Mountain et al. Cloning of a Bacillus subtilis restriction fragment
complementing auxotrophic mutants of eight Escherichia coli genes of arginine biosynthesis. Mol Gen Genet. 1984; 197(1 ):82-9. PMID: 6096675. Nagamani et al. Optimizing therapy for argininosuccinic aciduria. Mol Genet Metab. 2012 Sep; 107(1 -2): 10-4. Review. PMID: 22841516.
Nicaise et al. Control of acute, chronic, and constitutive hyperammonemia by wild-type and genetically engineered Lactobacillus plantarum in rodents. Hepatology. 2008 Oct;48(4):1184-92. PMID: 18697211.
Nicoloff et al. Two arginine repressors regulate arginine biosynthesis in Lactobacillus plantarum. J Bacteriol. 2004 Sep; 186(18):6059-69. PMID: 15342575.
Nougayrede et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006 Aug 11 ;313(5788):848-51. PMID:
16902142.
Olier et al. Genotoxicity of Escherichia coli Nissle 1917 strain cannot be dissociated from its probiotic activity. Gut Microbes. 2012 Nov- Dec;3(6):501 -9. PMID: 22895085.
Pham et al. Multiple myeloma-induced hyperammonemic encephalopathy: an entity associated with high in-patient mortality. Leuk Res. 2013
Oct;37(10): 1229-32. Review. PMID: 23932549.
Rajagopal et al. Use of inducible feedback-resistant N-acetylglutamate synthetase (argA) genes for enhanced arginine biosynthesis by genetically engineered Escherichia coli K-12 strains. Appl Environ Microbiol. 1998 May;64(5): 1805-11. PMID: 9572954.
Ray et al. The effects of mutation of the anr gene on the aerobic respiratory chain of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1997 Nov 15;156(2):227-32. PMID: 9513270.
Reister et al. Complete genome sequence of the Gram-negative probiotic Escherichia coli strain Nissle 1917. J Biotechnol. 2014 Oct 10; 187: 106-7. PMID: 25093936.
Rembacken et al. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999 Aug 21 ;354(9179):635-9. PMID: 10466665.
Remington's Pharmaceutical Sciences, 22nd ed. Mack Publishing Co.
Salmon et al. Global gene expression profiling in Escherichia coli K12. The effects of oxygen availability and FNR. J Biol Chem. 2003 Aug
8;278(32):29837-55. PMID: 12754220.
Sat et al. The Escherichia coli mazEF suicide module mediates thymineless death. J Bacteriol. 2003 Mar; 185(6): 1803-7. PMID: 12618443.
Sawers. Identification and molecular characterization of a transcriptional regulator from Pseudomonas aeruginosa PAO1 exhibiting structural and functional similarity to the FNR protein of Escherichia coli. Mol Microbiol. 1991 Jun;5(6):1469-81. PMID: 1787797.
Schneider et al. Arginine catabolism and the arginine succinyltransferase pathway in Escherichia coli. J Bacteriol. 1998 Aug; 180(16): 4278-86. PMID: 9696779.
Schultz. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008 Jul;14(7): 1012-8. Review. PMID: 18240278. Sonnenborn et al. The non-pathogenic Escherichia coli strain Nissle 1917 - features of a versatile probiotic. Microbial Ecology in Health and Disease. 2009;21 :122-58.
Suiter et al. Fitness consequences of a regulatory polymorphism in a seasonal environment. Proc Natl Acad Sci U S A. 2003 Oct
28; 100(22): 12782-6. PMID: 14555766.
Summerskill. On the origin and transfer of ammonia in the human gastrointestinal tract. Medicine (Baltimore). 1966 Nov;45(6):491 -6. PMID: 5925900.
Szwajkajzer et al. Quantitative analysis of DNA binding by the Escherichia coli arginine repressor. J Mol Biol. 2001 Oct 5;312(5):949-62. PMID:
11580241.
Tian et al. Binding of the arginine repressor of Escherichia coli K12 to its operator sites. J Mol Biol. 1992 Jul 20;226(2):387-97. PMID: 1640457.
82. Tian et al. Explanation for different types of regulation of arginine
biosynthesis in Escherichia coli B and Escherichia coli K12 caused by a difference between their arginine repressors. J Mol Biol. 1994 Jan
7;235(1 ):221 -30. PMID: 8289243.
83. Torres-Vega et al. Delivery of glutamine synthetase gene by baculovirus vectors: a proof of concept for the treatment of acute hyperammonemia. Gene Ther. 2014 Oct 23;22(1 ):58-64. PMID: 25338921.
84. Trunk et al. Anaerobic adaptation in Pseudomonas aeruginosa: definition of the Anr and Dnr regulons. Environ Microbiol. 2010 Jun; 12(6): 1719-33. PMID: 20553552.
85. Tuchman et al. Enhanced production of arginine and urea by genetically engineered Escherichia coli K-12 strains. Appl Environ Microbiol. 1997 Jan;63(1 ):33-8. PMID: 8979336.
86. Ukena et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007 Dec 12;2(12):e1308. PMID: 18074031.
87. Unden et al. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997 Jul 4;1320(3):217-34. Review. PMID: 9230919.
88. Vander Wauven et al. Pseudomonas aeruginosa mutants affected in
anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J Bacteriol. 1984 Dec; 160(3):928-34. PMID: 6438064.
89. Walker. Severe hyperammonaemia in adults not explained by liver disease.
Ann Clin Biochem. 2012 May;49(Pt 3):214-28. Review. PMID: 22349554.
90. Winteler et al. The homologous regulators ANR of Pseudomonas
aeruginosa and FNR of Escherichia coli have overlapping but distinct specificities for anaerobically inducible promoters. Microbiology. 1996 Mar; 142 ( Pt 3):685-93. PMID: 8868444.
91. Wu et al. Direct regulation of the natural competence regulator gene tfoX by cyclic AMP (cAMP) and cAMP receptor protein in Vibrios. Sci Rep. 2015 Oct 7;5:14921. PMID: 26442598.
92. Zimmermann et al. Anaerobic growth and cyanide synthesis of
Pseudomonas aeruginosa depend on anr, a regulatory gene homologous with fnr of Escherichia coli. Mol Microbiol. 1991 Jun;5(6): 1483-90. PMID: 1787798.
93. Wright O, Delmans M, Stan GB, Ellis T. GeneGuard: A modular plasmid system designed for biosafety. ACS Synth Biol. 2015 Mar 20;4(3):307-16. PMID: 24847673.
94. Liu Y, White RH, Whitman WB. Methanococci use the diaminopimelate aminotransferase (DapL) pathway for lysine biosynthesis. J Bacteriol. 2010 Jul;192(13):3304-10. PMID: 20418392.
95. Dogovski et al. (2012) Enzymology of Bacterial Lysine Biosynthesis,
Biochemistry, Prof. Deniz Ekinci (Ed.), ISBN: 978-953-51 -0076-8, InTech, Available from:
96. http://www.intechopen.com/books/biochemistry/enzymology-of-bacterial- lysine-biosynthesis.
97. Feng et al. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6061 -70. PMID: 1356964.
98. Lodeiro et al. Robustness in Escherichia coli glutamate and glutamine
synthesis studied by a kinetic model. J Biol Phys. 2008 Apr;34(1-2):91 -106. PMID: 19669495.
99. Reboul et al. Structural and dynamic requirements for optimal activity of the essential bacterial enzyme dihydrodipicolinate synthase. PLoS Comput Biol. 2012;8(6):e1002537. PMID: 22685390.
100. Saint-Girons et al. Structure and autoregulation of the metJ regulatory gene in Escherichia coli. J Biol Chem. 1984 Nov 25;259(22): 14282-5.
PMID: 6094549.
101. Shoeman et al. Regulation of methionine synthesis in Escherichia coli:
Effect of metJ gene product and S-adenosylmethionine on the expression of the metF gene. Proc Natl Acad Sci U S A. 1985 Jun;82(11 ):3601-5. PMID: 16593564.
102. van Heeswijk et al. Nitrogen assimilation in Escherichia coli: putting
molecular data into a systems perspective. Microbiol Mol Biol Rev. 2013 Dec;77(4):628-95. PMID: 24296575.
Examples
[0464] The following examples provide illustrative embodiments of the disclosure. One of ordinary skill in the art will recognize the numerous modifications and variations that may be performed without altering the spirit or scope of the disclosure. Such modifications and variations are encompassed within the scope of the disclosure. The Examples do not in any way limit the disclosure.
Arqinine Repressor Binding Sites (ARG Boxes)
Example 1. ARG box mutations
[0465] The wild-type genomic sequences comprising ArgR binding sites for each arginine biosynthesis operon in E. coli Nissle is shown in Table 3.
Modifications to those sequences are designed according to the following parameters. For each wild-type sequence, the ARG boxes are shown in italics. The ARG boxes of the arginine regulon overlap with the promoter region of each operon. The underlined sequences represent RNA polymerase binding sites and those sequences were not altered. Bases that are protected from DNA methylation during ArgR binding are highlighted, and bases that are protected from hydroxyl radical attack during ArgR binding are bolded. The
highlighted and bolded bases were the primary targets for mutations to disrupt ArgR binding.
Example 2. Lambda red recombination
[0466] Lambda red recombination is used to make chromosomal modifications, e.g., ARG box mutations. Lambda red is a procedure using recombination enzymes from a bacteriophage lambda to insert a piece of custom DNA into the chromosome of E. coli. A pKD46 plasmid is transformed into the E. coli Nissle host strain. E. coli Nissle cells are grown overnight in LB media. The overnight culture is diluted 1 :100 in 5 mL of LB media and grown until it reaches an OD6oo of 0.4-0.6. All tubes, solutions, and cuvettes are pre- chilled to 4° C. The E coli cells are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 1 mL of 4° C water. The E coli are centrifuged at 2,000 rpm for 5 min. at 4° C, the
supernatant is removed, and the cells are resuspended in 0.5 mL of 4° C water. The E coli are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 0.1 mL of 4° C water. The electroporator is set to 2.5 kV. 1 ng of pKD46 plasmid DNA is added to the E coli cells, mixed by pipetting, and pipetted into a sterile, chilled cuvette. The dry cuvette is placed into the sample chamber, and the electric pulse is applied. 1 mL of room-temperature SOC media is immediately added, and the mixture is transferred to a culture tube and incubated at 30° C for 1 hr. The cells are spread out on a selective media plate and incubated overnight at 30° C.
[0467] DNA sequences comprising the desired ARG box sequences shown in Table 3 were ordered from a gene synthesis company. For the argA operon, the mutant regulatory region comprises the following nucleic acid sequence (SEQ ID NO: 2):
gcaaaaaaacaCTTtaaaaaCTTaataatttcCTTtaatcaCTTaaagaggtgtaccgtg.
The lambda enzymes are used to insert this construct into the genome of E coli Nissle through homologous recombination. The construct is inserted into a specific site in the genome of E coli Nissle based on its DNA sequence. To insert the construct into a specific site, the homologous DNA sequence flanking the construct is identified. The homologous sequence of DNA includes
approximately 50 bases on either side of the mutated sequence. The
homologous sequences are ordered as part of the synthesized gene.
Alternatively, the homologous sequences may be added by PCR. The construct is used to replace the natural sequence upstream of argA in the E. coli Nissle genome. The construct includes an antibiotic resistance marker that may be removed by recombination. The resulting mutant argA construct comprises approximately 50 bases of homology upstream of argA, a kanamycin resistance marker that can be removed by recombination,
gcaaaaaaacaCTTtaaaaaCTTaataatttcCTTtaatcaCTTaaagaggtgtaccgtg, and approximately 50 bases of homology to argA.
[0468] In some embodiments, the ARG boxes were mutated in the argG regulatory region as described above, and a BBa_J23100 constitutive promoter was inserted into the regulatory region using lambda red recombination (SYN- UCD105). These bacteria were capable of producing arginine. In alternate embodiments, the argG regulatory region (SEQ ID NO: 16) remained ArgR- repressible (SYN-UCD104), and the bacteria were capable of producing citrulline.
Example 3. Transforming E. coli Nissle
[0469] The mutated ARG box construct is transformed into E. coli Nissle comprising pKD46. All tubes, solutions, and cuvettes are pre-chilled to 4° C. An overnight culture is diluted 1 :100 in 5 mL of LB media containing ampicillin and grown until it reaches an OD6oo of 0.1. 0.05 mL of 100X L-arabinose stock solution is added to induce pKD46 lambda red expression. The culture is grown until it reaches an OD6oo of 0.4-0.6. The E. coli cells are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are
resuspended in 1 mL of 4° C water. The E. coli are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 0.5 mL of 4° C water. The E coli are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 0.1 mL of 4° C water. The electroporator is set to 2.5 kV. 0.5 g of the mutated ARG box construct is added to the cells, mixed by pipetting, and pipetted into a sterile, chilled cuvette. The dry cuvette is placed into the sample chamber, and the
electric pulse is applied. 1 ml_ of room-temperature SOC media is immediately added, and the mixture is transferred to a culture tube and incubated at 37° C for 1 hr. The cells are spread out on an LB plate containing kanamycin and incubated overnight.
Example 4. Verifying mutants
[0470] The presence of the mutation is verified by colony PCR. Colonies are picked with a pipette tip and resuspended in 20 μΙ of cold ddH20 by pipetting up and down. 3 μΙ_ of the suspension is pipetted onto an index plate with appropriate antibiotic for use later. The index plate is grown at 37° C overnight. A PCR master mix is made using 5 μΙ_ of 10X PCR buffer, 0.6 μΙ of 10 mM dNTPs, 0.4 μΙ_ of 50 mM Mg2S04, 6.0 μΙ_ of 10X enhancer, and 3.0 μΙ_ of ddH20 (15 μΙ_ of master mix per PCR reaction). A 10 μΜ primer mix is made by mixing 2 μΙ_ of primers unique to the argA mutant construct (100 μΜ stock) into 16 μΙ_ of ddH20. For each 20 μΙ_ reaction, 15μΙ_ of the PCR master mix, 2.0 μΙ_ of the colony suspension (template), 2.0 μΙ_ of the primer mix, and 1.0 μΙ_ of Pfx Platinum DNA Pol are mixed in a PCR tube. The PCR thermocycler is programmed as follows, with steps 2-4 repeating 34 times: 1 ) 94° C at 5:00 min., 2) 94° C at 0:15 min., 3) 55° C at 0:30 min., 4) 68° C at 2:00 min., 5) 68° C at 7:00 min., and then cooled to 4° C. The PCR products are analyzed by gel electrophoresis using 10 μΙ_ of each amplicon and 2.5 μΙ_ 5X dye. The PCR product only forms if the mutation has inserted into the genome.
Example 5. Removing selection marker
[0471] The antibiotic resistance gene is removed with pCP20. Each strain with the mutated ARG boxes is grown in LB media containing antibiotics at 37° C until it reaches an OD6oo of 0.4-0.6. All tubes, solutions, and cuvettes are pre-chilled to 4° C. The cells are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 1 mL of 4° C water. The E. coli are centrifuged at 2,000 rpm for 5 min. at 4° C, the
supernatant is removed, and the cells are resuspended in 0.5 mL of 4° C water. The E. coli are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 0.1 mL of 4° C water. The
electroporator is set to 2.5 kV. 1 ng of pCP20 plasmid DNA is added to the
cells, mixed by pipetting, and pipetted into a sterile, chilled cuvette. The dry cuvette was placed into the sample chamber, and the electric pulse was applied. 1 ml_ of room-temperature SOC media is immediately added, and the mixture is transferred to a culture tube and incubated at 30° C for 1-3 hrs. The cells are spread out on an LB plate containing kanamycin and incubated overnight. Colonies that do not grow to a sufficient OD6oo overnight are further incubated for an additional 24 hrs. 200 μί of cells are spread on ampicillin plates, 200 μί of cells are spread on kanamycin plates, and both are grown at 37° C overnight. The ampicillin plate contains cells with pCP20. The kanamycin plate provides an indication of how many cells survived the electroporation. Transformants from the ampicillin plate are purified non- selectively at 43° C and allowed to grow overnight.
Example 6. Verifying transformants
[0472] The purified transformants are tested for sensitivity to ampicillin and kanamycin. A colony from the plate grown at 43° C is picked and and resuspended in 10 μί of LB media. 3 μί of the cell suspension is pipetted onto each of three plates: 1 ) an LB plate with kanamycin incubated at 37° C, which tests for the presence or absence of the kanR gene in the genome of the host strain; 2) an LB plate with ampicillin incubated at 30° C, which tests for the presence or absence of the ampR gene from the pCP20 plasmid; and 3) an LB plate without antibiotic incubated at 37° C. If no growth is observed on the kanamycin or ampicillin plates for a particular colony, then both the kanR gene and the pCP20 plasmid were lost, and the colony is saved for further analysis. The saved colonies are restreaked onto an LB plate to obtain single colonies and grown overnight at 37° C. The presence of the mutated genomic ARG box is confirmed by sequencing the argA region of the genome.
[0473] The methods for lambda red recombination, transforming E. coli Nissle, verifying the mutation, removing the selection marker, and
verifying/sequencing the transformants are repeated for each of the ARG box mutations and operons shown in Table 3. The resulting bacteria comprise mutations in each ARG box for one or more operons encoding the arginine
biosynthesis enzymes, such that ArgR binding to the ARG boxes is reduced and total ArgR binding to the regulatory region of said operons is reduced.
Example 7. Arginine feedback resistant N-acetylglutamate synthetase
(argAfbr)
[0474] In addition to the ARG box mutations described above, the E. coli Nissle bacteria further comprise an arginine feedback resistant N- acetylglutamate synthetase (argAmr, SEQ ID NO: 30) gene expressed under the control of each of the following promoters: tetracycline-inducible promoter, FNR promoter selected from SEQ ID NOs: 18-29. As discussed herein, other promoters may be used.
[0475] The argA mr gene is expressed on a high-copy plasm id, a low-copy plasmid, or a chromosome. SYN-UCD101 comprises wild-type ArgR, wild-type ArgA, tetracycline-inducible argAmr on a plasmid, and mutations in each ARG box for each arginine biosynthesis operon. The plasmid does not comprise functional ArgR binding sites, i.e., ARG boxes. SYN-UCD101 was used to generate SYN-UCD102, which comprises wild-type ArgR, wild-type ArgA, tetracycline-inducible argAfbr on a plasmid, and mutations in each ARG box for each arginine biosynthesis operon. The plasmid further comprises functional ArgR binding sites, i.e., ARG boxes. In some instances, the presence and/or build-up of functional ArgR may result in off-target binding at sites other than the ARG boxes. Introducing functional ARG boxes in this plasmid may be useful for reducing or eliminating off-target ArgR binding, i.e., by acting as an ArgR sink. SYN-UCD104 comprises wild-type ArgR, wild-type ArgA, tetracycline-inducible argAmr on a low-copy plasmid, tetracycline-inducible argG, and mutations in each ARG box for each arginine biosynthesis operon except for argG. SYN- UCD105 comprises wild-type ArgR, wild-type ArgA, tetracycline-inducible argAmr on a low-copy plasmid, constitutively expressed argG (SEQ ID NO: 17 comprising the BBa_J23100 constitutive promoter), and mutations in each ARG box for each arginine biosynthesis operon. SYN-UCD103 is a control Nissle construct.
[0476] The argAmr gene is inserted into the bacterial genome at one or more of the following insertion sites in E. coli Nissle: malE/K, araC/BAD, lacZ,
thyA, malP/T. Any suitable insertion site may be used, see, e.g., Fig. 18. The insertion site may be anywhere in the genome, e.g., in a gene required for survival and/or growth, such as thyA (to create an auxotroph); in an active area of the genome, such as near the site of genome replication; and/or in between divergent promoters in order to reduce the risk of unintended transcription, such as between AraB and AraC of the arabinose operon. At the site of insertion, DNA primers that are homologous to the site of insertion and to the argAmr construct are designed. A linear DNA fragment containing the construct with homology to the target site is generated by PCR, and lambda red recombination is performed as described above.
[0477] The resulting E. coli Nissle bacteria are genetically engineered to include nucleic acid mutations that reduce arginine-mediated repression - via ArgR binding and arginine binding to N-acetylglutamate synthetase - of one or more of the operons that encode the arginine biosynthesis enzymes, thereby enhancing arginine and/or citrulline biosynthesis.
Γ0478Ί Arginine Repressor (ArgR) sequences The wild-type argR nucleotide sequence in E. coli Nissle and the nucleotide sequence following argR deletion are shown below in Table 21 and Table 22.
Table 21. Wild-type argR nucleotide sequence
Table 22. Nucleotide sequence following argR deletion
Example 9. Deleting ArgR
[0479]A pKD46 plasmid is transformed into the E. coli Nissle host strain. E. coli Nissle cells are grown overnight in LB media. The overnight culture is diluted 1 :100 in 5 mL of LB media and grown until it reaches an OD6oo of 0.4- 0.6. All tubes, solutions, and cuvettes are pre-chilled to 4° C. The E. coli cells are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 1 mL of 4° C water. The E coli are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 0.5 mL of 4° C water. The E coli are centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 0.1 mL of 4° C water. The electroporator is set to 2.5 kV. 1 ng of pKD46 plasmid DNA is added to the E coli cells, mixed by pipetting, and pipetted into a sterile, chilled cuvette. The dry cuvette is placed into the sample chamber, and the electric pulse is applied. 1 mL of room -temperature SOC media is immediately added, and the mixture is transferred to a culture tube and incubated at 30° C for 1 hr. The cells are spread out on a selective media plate and incubated overnight at 30° C.
[0480] Approximately 50 bases of homology upstream and downstream of the ArgR gene are added by PCR to the kanamycin resistance gene in the pKD4 plasmid to generate the following KanR construct: (~50 bases upstream of ArgR) (terminator) (kanR gene flanked by FRT sites from pKD4) (DNA downstream of argR).
[0481] In some embodiments, both argR and argG genes are deleted using lambda red recombination as described above, and the bacteria are capable of producing citrulline.
Example 10. Bacterial Strains having Arginine feedback resistant N- acetylglutamate synthetase (argAfbr) and ArgR deletion
[0482] In addition to the ArgR deletion described above, the E. coli Nissle bacteria further comprise an arginine feedback resistant N-acetylglutamate synthetase (argAmr, SEQ ID NO: 30) gene expressed under the control of each of the following promoters: tetracycline-inducible promoter, FNR promoter selected from SEQ ID NOs: 18-29. As discussed herein, other promoters may be used.
[0483] The argAmr gene is expressed on a high-copy plasm id, a low-copy plasmid, or a chromosome. ArgR is deleted (AArgR) in each of SYN-UCD201 , SYN-UCD202, and SYN-UCD203. SYN-UCD201 further comprises wild-type argA, but lacks inducible argAfbr. SYN-UCD202 comprises AArgR and argA r expressed under the control of a tetracycline-inducible promoter on a high-copy plasmid. SYN-UCD203 comprises AArgR and argAmr expressed under the control of a tetracycline-inducible promoter on a low-copy plasmid. SYN- UCD204 comprises AArgR and argAmr expressed under the control of a tetracycline-inducible promoter on a low-copy plasmid. SYN-UCD205 comprises AArgR and argAmr expressed under the control of a FNR-inducible promoter (fnrS2) on a low-copy plasmid.
[0484] The argAmr gene is inserted into the bacterial genome at one or more of the following insertion sites in E. coli Nissle: malE/K, araC/BAD, lacZ, thy A, malP/T. Any suitable insertion site may be used, see, e.g., Fig. 18. The insertion site may be anywhere in the genome, e.g., in a gene required for survival and/or growth, such as thy A (to create an auxotroph); in an active area of the genome, such as near the site of genome replication; and/or in between divergent promoters in order to reduce the risk of unintended transcription, such as between AraB and AraC of the arabinose operon. At the site of insertion, DNA primers that are homologous to the site of insertion and to the argAmr construct are designed. A linear DNA fragment containing the construct with homology to the target site is generated by PCR, and lambda red recombination is performed as described above. The resulting E. coli Nissle bacteria have
deleted ArgR and inserted feedback resistant N-acetylglutamate synthetase, thereby increasing arginine or citrulline biosynthesis.
Example 11. Generation of AThyA
[0485]An auxotrophic mutation causes bacteria to die in the absence of an exogenously added nutrient essential for survival or growth because they lack the gene(s) necessary to produce that essential nutrient. In order to generate genetically engineered bacteria with an auxotrophic modification, the thyA, a gene essential for oligonucleotide synthesis was deleted. Deletion of the thyA gene in E. coli Nissle yields a strain that cannot form a colony on LB plates unless they are supplemented with thymidine.
[0486]A thyA::cam PCR fragment was amplified using 3 rounds of PCR as follows. Sequences of the primers used at a 100um concentration are found in Table 23.
Table 23. Primer Sequences
[0487] For the first PCR round, 4x50ul PCR reactions containing 1 ng pKD3 as template, 25ul 2xphusion, 0.2ul primer SR36 and SR38, and either 0, 0.2, 0.4 or 0.6ul DMSO were brought up to 50 ul volume with nuclease free water and amplified under the following cycle conditions:
[0488]step1 : 98c for 30s
[0489]step2: 98c for 10s
[0490]step3: 55c for 15s
[0491]step4: 72c for 20s
[0492] repeat step 2-4 for 30 cycles
[0493]step5: 72c for 5min
[0494] Subsequently, 5ul of each PCR reaction was run on an agarose gel to confirm PCR product of the appropriate size. The PCR product was purified from the remaining PCR reaction using a Zymoclean gel DNA recovery kit according to the manufacturer's instructions and eluted in 30ul nuclease free water.
[0495] For the second round of PCR, 1 ul purified PCR product from round 1 was used as template, in 4x50ul PCR reactions as described above except with 0.2ul of primers SR33 and SR34. Cycle conditions were the same as noted above for the first PCR reaction. The PCR product run on an agarose gel to verify amplification, purified, and eluted in 30ul as described above.
[0496] For the third round of PCR, 1 ul of purified PCR product from round 2 was used as template in 4x50ul PCR reactions as described except with primer SR43 and SR44. Cycle conditions were the same as described for rounds 1 and 2. Amplification was verified, the PCR product purified, and eluted as described above. The concentration and purity was measured using a spectrophotometer. The resulting linear DNA fragment, which contains 92 bp homologous to upstream of thyA, the chloramphenicol cassette flanked by frt sites, and 98 bp homologous to downstream of the thyA gene, was transformed into a E. coli Nissle 1917 strain containing pKD46 grown for recombineering. Following electroporation, 1 ml SOC medium containing 3mM thymidine was added, and cells were allowed to recover at 37 C for 2h with shaking. Cells were then pelleted at 10,000xg for 1 minute, the supernatant was discarded, and the cell pellet was resuspended in 10Oul LB containing 3mM thymidine and spread on LB agar plates containing 3mM thy and 20ug/ml chloramphenicol. Cells were incubated at 37 C overnight. Colonies that appeared on LB plates were restreaked. + cam 20ug/ml + or - thy 3mM. (thyA auxotrophs will only grow in media supplemented with thy 3mM).
[0497] Next, the antibiotic resistance was removed with pCP20
transformation. pCP20 has the yeast Flp recombinase gene, FLP,
chloramphenicol and ampicillin resistant genes, and temperature sensitive replication. Bacteria were grown in LB media containing the selecting antibiotic at 37°C until OD600 = 0.4 - 0.6. 1 ml_ of cells were washed as follows: cells were pelleted at 16,000xg for 1 minute. The supernatant was discarded and the pellet was resuspended in 1 ml_ ice-cold 10% glycerol. This wash step was repeated 3x times. The final pellet was resuspended in 70ul ice-cold 10% glycerol. Next, cells were electroporated with 1 ng pCP20 plasmid DNA, and 1 ml_ SOC supplemented with 3mM thymidine was immediately added to the cuvette. Cells were resuspended and transferred to a culture tube and grown at 30°C for 1 hours. Cells were then pelleted at 10,000xg for 1 minute, the supernatant was discarded, and the cell pellet was resuspended in 10Oul LB containing 3mM thymidine and spread on LB agar plates containing 3mM thy and 100ug/ml carbenicillin and grown at 30°C for 16-24 hours. Next, transformants were colony purified non-selectively (no antibiotics) at 42°C.
[0498] To test the colony-purified transformants, a colony was picked from the 42°C plate with a pipette tip and resuspended in 10 L LB. 3 L of the cell suspension was pipetted onto a set of 3 plates: Cam, (37°C; tests for the presence/absence of CamR gene in the genome of the host strain), Amp, (30°C, tests for the presence/absence of AmpR from the pCP20 plasmid) and LB only (desired cells that have lost the chloramphenicol cassette and the pCP20 plasmid), 37°C. Colonies were considered cured if there is no growth in neither the Cam or Amp plate, picked, and re-streaked on an LB plate to get single colonies, and grown overnight at 37°C.
Example 12. Quantifying ammonia
[0499] The genetically engineered bacteria described above were grown overnight in 5 mL LB. The next day, cells were pelleted and washed in M9 + glucose, pelleted, and resuspended in 3 mL M9 + glucose. Cell cultures were incubated with shaking (250 rpm) for 4 hrs and incubated aerobically or anaerobically in a Coy anaerobic chamber (supplying 90% N2, 5% CO2, 5%H2)
at 37° C. At baseline (t=0), 2 hours, and 4 hours, the OD6oo of each cell culture was measured in order to determine the relative abundance of each cell.
[0500] At t=0, 2 hrs, and 4 hrs, a 1 mL aliquot of each cell culture was analyzed on the Nova Biomedical Bioprofile Analyzer 300 in order to determine the concentration of ammonia in the media. Both SYN-UCD101 and SYN- UCD102 were capable of consuming ammonia in vitro (Fig 26). Figs. 25, 26, and 27 depict bar graphs of ammonia concentrations using SYN-UCD202, SYN-UCD204, SYN-UCD103, and blank controls.
Example 13. Quantifying arginine and citrulline
[0501] In some embodiments, the genetically engineered bacteria described above are grown overnight in LB at 37° C with shaking. The bacteria are diluted 1 : 100 in 5mL LB and grown at 37° C with shaking for 1.5 hr. The bacteria cultures are induced as follows: (1 ) bacteria comprising FNR-inducible argAmr are induced in LB at 37° C for up to 4 hrs in anaerobic conditions in a Coy anaerobic chamber (supplying 90% N2, 5% C02, 5%H2, and 20mM nitrate) at 37° C; (2) bacteria comprising tetracycline-inducible argAmr are induced with anhydrotetracycline (100 ng/mL); (3) bacteria comprising arabinose-inducible argAmr are induced with 1 % arabinose in media lacking glucose. After induction, bacterial cells are removed from the incubator and spun down at maximum speed for 5 min. The cells are resuspended in 1 mL M9 glucose, and the OD6oo is measured. Cells are diluted until the OD6oo is between 0.6-0.8. Resuspended cells in M9 glucose media are grown aerobically with shaking at 37C. 100 μί of the cell resuspension is removed and the OD6oo is measured at time = 0. A 100 μί aliquot is frozen at -20° C in a round-bottom 96-well plate for mass spectrometry analysis (LC-MS/MS). At each subsequent time point, 100 μί of the cell suspension is removed and the OD6oo is measured; a 100 μί aliquot is frozen at -20C in a round-bottom 96-well plate for mass spectrometry analysis. Samples are analyzed for arginine and/or citrulline concentrations. At each time point, normalized concentrations as determined by mass
spectrometry vs. OD6oo are used to determine the rate of arginine and/or citrulline production per cell per unit time.
[0502] In some embodiments, the genetically engineered bacteria described above are streaked from glycerol stocks for single colonies on agar. A colony is picked and grown in 3 mL LB for 4 hrs or overnight, then centrifuged for 5 min at 2,500 rcf. The cultures are washed in M9 media with 0.5% glucose. The cultures are resuspended in 3 mL of M9 media with 0.5% glucose, and the OD6oo is measured. The cultures are diluted in M9 media with 0.5% glucose, with or without ATC (100 ng/mL), with or without 20 mM glutamine, so that all of the OD6oo are between 0.4 and 0.5. A 0.5 mL aliquot of each sample is removed, centrifuged for 5 min. at 14,000 rpm, and the supernatant is removed and saved. The supernatant is frozen at -80° C, and the cell pellets are frozen at -80° C (t=0). The remaining cells are grown with shaking (250 rpm) for 4-6 hrs and incubated aerobically or anaerobically in a Coy anaerobic chamber (supplying 90% N2, 5% C02, 5%H2) at 37° C. One 0.5 mL aliquot is removed from each sample every two hours and the OD6oo is measured. The aliquots are centrifuged for 5 min at 14,000 rpm, and the supernatant is removed. The supernatant is frozen at -80° C, and the cell pellets are frozen at -80° C (t=2, 4, and 6 hrs). The samples are placed on ice, and arginine and citrulline levels are determined using mass spectrometry.
[0503] For bacterial culture supernatants, samples of 500, 100, 20, 4, and 0.8 pg/mL arginine and citrulline standards in water are prepared. In a round-bottom 96-well plate, 20 μί of sample (bacterial supernatant or standards) is added to 80 μί of water with L-Arginine-1 3C6,15N4 (Sigma) and L- Citrulline-2,3,3,4,4,5,5-d7 (CDN isotope) internal standards at a final 2 pg/mL concentration. The plate is heat-sealed with a PierceASeal foil and mixed well. In a V-bottom 96-well polypropylene plate, 5 μί of diluted samples is added to 95 μί of derivatization mix (85 μί 10 mM NaHCO3 pH 9.7 and 10 μί 10 mg/mL dansyl-chloride (diluted in acetonitrile). The plate is heat-sealed with a
ThermASeal foil and mixed well. The samples are incubated at 60°C for 45 min for derivatization and centrifuged at 4000 rpm for 5 min. In a round-bottom 96- well plate, 20 μί of the derivatized samples are added to 180 μί of water with 0.1 % formic acid. The plate is heat-sealed with a ClearASeal sheet and mixed well.
[0504]Arginine and citrulline are measured by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) using a Thermo TSQ Quantum Max triple quadrupole mass spectrometer. Table 24 below provides a summary of a LC-MS/MS method.
Table 24. a LC-MS/MS Method Summary
HPLC
Column Luna C18(2) column, 5 pm (50 x 2.1 mm)
Mobile Phase A 100% H20, 0.1 % Formic Acid)
Mobile Phase B 100% ACN, 0.1 % Formic Acid
HPLC Method Total Time (min) Flow Rate (uL/min) A% B%
0.00 400 90.0 10.0
0.50 400 90.0 10.0
2.00 400 10.0 90.0
3.25 400 10.0 90.0
3.26 400 90.0 10.0
4.30 400 90.0 10.0
Injection Volume 10μί
Tandem Mass Spectrometry
Ion Source HESI-II
Polarity Positive
SRM transitions L-Arginine: 408.1/170.1
L-Arg!nine-! 3C6,'1SN : 418.1/170.0
L-Citrulline: 409.1/170.2
L-Citrulline-2,3,3,4,4,5,5-d7: 416.1/170.1
[0505] Intracellular arginine and secreted (supernatant) arginine production in the genetically engineered bacteria in the presence or absence an ATC or anaerobic inducer is measured and compared to control bacteria of the same strain under the same conditions.
[0506] Total arginine production over 6 hrs in the genetically engineered bacteria in the genetically engineered bacteria in the presence or absence an ATC or anaerobic inducer is measured and compared to control bacteria of the same strain under the same conditions.
Example 14. Efficacy of genetically engineered bacteria in a mouse model of hyperammonemia and acute liver failure
[0507] Wild-type C57BL6/J mice are treated with thiol acetamide (TAA), which causes acute liver failure and hyperammonemia (Nicaise et al., 2008). The TAA mouse model is an industry-accepted in vivo model for HE. Mice are treated with unmodified control Nissle bacteria or Nissle bacteria engineered to produce high levels of arginine or citrulline as described above.
[0508] On day 1 , 50 mL of the bacterial cultures are grown overnight and pelleted. The pellets are resuspended in 5 mL of PBS at a final concentration of approximately 1011 CFU/mL. Blood ammonia levels in mice are measured by mandibular bleed, and ammonia levels are determined by the PocketChem Ammonia Analyzer (Arkray). Mice are gavaged with 100 μί of bacteria
(approximately 1010 CFU). Drinking water for the mice is changed to contain 0.1 mg/mL anhydrotetracycline (ATC) and 5% sucrose for palatability.
[0509] On day 2, the bacterial gavage solution is prepared as described above, and mice are gavaged with 100 μί of bacteria. The mice continue to receive drinking water containing 0.1 mg/mL ATC and 5% sucrose.
[0510] On day 3, the bacterial gavage solution is prepared as described above, and mice are gavaged with 100 μί of bacteria. The mice continue to receive drinking water containing 0.1 mg/mL ATC and 5% sucrose. Mice receive an intraperitoneal (IP) injection of 100 μί of TAA (250 mg/kg body weight in 0.5% NaCI).
[0511] On day 4, the bacterial gavage solution is prepared as described above, and mice are gavaged with 100 μί of bacteria. The mice continue to receive drinking water containing 0.1 mg/mL ATC and 5% sucrose. Mice receive another IP injection of 100 μί of TAA (250 mg/kg body weight in 0.5% NaCI). Blood ammonia levels in the mice are measured by mandibular bleed, and ammonia levels are determined by the PocketChem Ammonia Analyzer (Arkray).
[0512] On day 5, blood ammonia levels in mice are measured by mandibular bleed, and ammonia levels are determined by the PocketChem Ammonia Analyzer (Arkray). Fecal pellets are collected from mice to determine
arginine content by liquid chromatography-mass spectrometry (LC-MS).
Ammonia levels in mice treated with genetically engineered Nissle and unmodified control Nissle are compared.
Example 15. Efficacy of genetically engineered bacteria in a mouse model of hyperammonemia and UCD
[0513] Ornithine transcarbamylase is urea cycle enzyme, and mice comprising an spf-ash mutation exhibit partial ornithine transcarbamylase deficiency, which serves as a model for human UCD. Mice are treated with unmodified control Nissle bacteria or Nissle bacteria engineered to produce high levels of arginine or citrulline as described above.
[0514] 60 spf-ash mice were treated with the genetically engineered bacteria of the invention (SYN-UCD103, SYN-UCD204) or H2O control at 100ul PO QD: H2O control, normal chow (n=15); H2O control, high protein chow (n=15); SYN-UCD103, high protein chow (n=15); SYN-UCD204, high protein chow (n=15). On Day 1 , mice were weighed and sorted into groups to minimize variance in mouse weight per cage. Mice were gavaged and water with 20 mg/L ATC was added to the cages. On day 2, mice were gavaged in the morning and afternoon. On day 3, mice were gavaged in the morning and weighed, and blood was drawn 4h post-dosing to obtain baseline ammonia levels. Mice were gavaged in the afternoon and chow changed to 70% protein chow. On day 4, mice were gavaged in the morning and afternoon. On day 5, mice were gavaged in the morning and weighed, and blood was drawn 4h post- dosing to obtain ammonia levels. On days 6 and 7, mice were gavaged in the morning. On day 8, mice were gavaged in the morning and weighed, and blood was drawn 4h post-dosing to obtain ammonia levels. On day 9, mice were gavaged in the morning and afternoon. On day 10, mice were gavaged in the morning and weighed, and blood was drawn 4h post-dosing to obtain ammonia levels. On day 12, mice were gavaged in the morning and afternoon. On day 13, mice were gavaged in the morning and weighed, and blood was drawn 4h post-dosing to obtain ammonia levels. Blood ammonia levels, body weight, and survival rates are analyzed (Fig. 29).
Example 16. Efficacy of genetically engineered bacteria in a mouse model of hyperammonemia and UCD (spf-ash) maintained on a high protein diet
[0515] The hyperammonia /UCD (spf-ash) model described in Example 14 was used to assess the in vivo efficacy of genetically engineered bacteria encoding ArgAfbr driven by a fnr promoter on a low copy plasmid on ammonia levels upon administration of a high protein diet.
[0516] Two strains encoding ArgAfbr driven by a fnr promoter on a low copy plasmid, SYN-UCD206 (comprising AArgR and AThyA and argAfbr expressed under the control of a FNR-inducible promoter (fnrS2) on a low-copy plasmid) and SYN-UCD205 (comprising AArgR and argAfbr expressed under the control of a FNR-inducible promoter (fnrS2) on a low-copy plasmid) were compared to determine whether thymidine auxotrophy can influence the efficacy of ammonia removal from the blood.
[0517] Spf-ash mice were treated by oral administration with the genetically engineered bacteria (SYN-UCD205, SYN-UCD206) or H20 control. Normal or high protein chow was provided as follows: SYN-UCD205, high protein chow (n=10); SYN-UCD206, high protein chow (n=10); H20 control, normal chow (n=10); H20 control, high protein chow (n=10). For SYN-UCD205 and SYN-UCD206, a dose of 100 ul of > 1 X1010 cells/ml was administered twice a day for 12 days, with the exception of days 1 , 5, 6, and 7, where bacteria were administered once. On Day 1 , mice were weighed and
randomized. T=0 NH4 levels were determined from mandibular bleeds using the PocketChem Ammonia Analyzer (Arkray), and mice were subsequently and gavaged. On day 2, mice were gavaged in the morning and afternoon. On day 3, mice were gavaged in the morning and afternoon and the chow was changed from normal chow to 70% protein chow. On day 4, mice were gavaged in the morning and afternoon. On day 5, mice were gavaged in the morning and weighed, and blood was drawn 4h post-dosing to obtain ammonia levels. On days 8 through 12, mice were gavaged in the morning and afternoon.
[0518] As seen in Fig. 30, ammonia levels of spf-ash mice in a high protein diet were reduced 48 hours after switch to high protein chow in the SYN- UCD205 and SYN-UCD206 groups as compared to the H2O high protein diet
control group, indicating that the FNR inducible promoter can drive ArgAfbr expression, resulting in decreased ammonia levels in the blood of the mice treated with the engineered bacteria. The observed reduction in ammonia levels was similar in both SYN-UCD205 and SYN-UCD206, indicating that ThyA auxotrophy does not have a significant effect on efficacy of SYN-UCD206.
[0519] Example 17. Engineering bacterial strains using chromosomal insertions
[0520] Bacterial strains, in which ArgAfbr is integrated directly into the E. coli Nissle genome under the control of an FNR-responsive promoter at the MALEK site were constructed.
[0521] To create a vector capable of integrating the PfnrS-ArgAfbr into the chromosome at the Nissle MalE and MalK loci, Gibson assembly was used to add 1000 bp sequences of DNA homologous to the Nissle MALE/K locus to both sides of a flippase recombination target (FRT) site-flanked
chloramphenicol resistance (cmR) cassette on a knock-in knock-out (KIKO) plasmid. Gibson assembly was then used to clone the PfnrS-ArgAfbr DNA sequence between these homology arms, adjacent to the FRT-cmR-FRT site. Successful insertion of the fragment was validated by sequencing. PCR is used to amplify the entire MaIEK:: FRT-cmR-FRT:: PfnrS-ArgAfbr:: MalK region. This knock-in PCR fragment was used to transform an electrocompetent Nissle strain that contains a temperature-sensitive plasmid encoding the lambda red recombinase genes. After transformation, cells were grown for 2 hrs at 37 °C. Growth at 37 °C cures the temperature-sensitive plasmid. Transformants with successful chromosomal integration of the fragment were selected on chloramphenicol at 20 pg/mL.
[0522] In some embodiments, recombinase-based switches may be used to activate PfnrS-ArgAfbr expression. To construct a strain allowing
recombinase-based switches to regulate ArgAfbr expression, the PfnrS-driven Int5 gene and the rrnBUP-driven, recombinase site-flanked ArgAfbrare synthesized by Genewiz (Cambridge, MA). Gibson assembly is used to add 1000 bp sequences of DNA homologous to the Nissle malE and malK loci on either side of the PfnrS-lnt5, rrnBUP- ArgAfbr sequence and to clone this sequence between the homology arms. Successful insertion of the fragment
into a KIKO plasmid is validated by sequencing. PCR is used to amplify the entire PfnrS-lnt5, rrnBUP- ArgAfbr region. This knock-in PCR fragment is used to transform an electrocompetent Nissle strain expressing the lambda red recombinase genes. After transformation, cells are grown for 2 hrs at 37 °C. Transformants with successful integration of the PfnrS-ArgAfbr at the maIEK intergenic region are selected on kanamycin at 50 pg/mL. This strategy may also be used to construct a recombinase-based strain requiring T7 polymerase activity for ArgAfbr expression.
Example 18. Comparison of in vitro efficacy of chromosomal insertion and plasmid-bearing engineered bacterial strains
[0523] To compare the in vitro efficacy between engineered bacterial strains harboring a chromosomal insertion of ArgAfbr driven by an fnr inducible promoter at the maIEK locus and strains with a low copy plasmid comprising ArgAfbr driven by an fnr inducible promoter, arginine levels in the media were measured at various time points post anaerobic induction. Additionally, to assess whether auxotrophy for thymidine may have an effect on arginine production efficiency, arginine production of engineered bacterial strains with or without a ThyA deletion, comprising the fnr-ArgAfbr on a low copy plasmid or integrated on the chromosome, were compared.
[0524] Overnight cultures were diluted 1 :100 in LB and grown with shaking (250 rpm) at 37 °C. After 1.5 hrs of growth, the bacteria cultures were induced as follows: (1 ) bacteria comprising FNR-inducible argAfbr were induced in LB at 37° C for 4 hrs in anaerobic conditions in a Coy anaerobic chamber (supplying 90% N2, 5% CO2, 5%H2, and 20mM nitrate) at 37° C; (2) bacteria comprising tetracycline-inducible argAfbr were induced with anhydrotetracycline (100 ng/mL). After induction, bacteria were removed from the incubator and spun down at maximum speed for 5 min. The cells were resuspended in 1 mL M9 glucose, and the OD600 was measured. Cells were diluted until the OD600 was between 0.6-0.8. Resuspended cells in M9 glucose media were grown aerobically with shaking at 37C. 100 μί of the cell resuspension was removed and the OD600 is measured at time = 0. A 100 μί aliquot was frozen at -20° C in a round-bottom 96-well plate for mass spectrometry analysis (LC-MS/MS). At
each subsequent time point (e.g., 30, 60, and 120 min), 100 μΙ_ of the cell suspension was removed and the OD600 was measured; a 100 μί aliquot was frozen at -20C in a round-bottom 96-well plate for mass spectrometry analysis. Samples were analyzed for arginine concentrations. At each time point, normalized concentrations as determined by mass spectrometry vs. OD600 were used to determine the rate of arginine production per cell per unit time. A summary of the LC-MS/MS method is provided above.
[0525] Arginine production at 30, 60, and 120 min post induction was compared between (1 ) Syn-UCD301 (SYN825; comprising AArgR and argAfbr expressed under the control of a FNR-inducible promoter integrated into the chromosome at the maIEK locus), (2) SYN-UCD205 (comprising AArgR and argAfbr expressed under the control of a FNR-inducible promoter on a low-copy plasmid), and (3) SYN-UCD206 (comprising AArgR and AThyA and argAfbr expressed under the control of a FNR-inducible promoter on a low-copy plasmid. SYN-UCD103 was used as is a control Nissle construct and results are shown in Fig. 31A.
[0526] Fig. 31 A shows the levels of arginine production of SYN-UCD205, SYN-UCD206, and SYN-UCD301 measured at 0, 30, 60, and 120 minutes. Arginine production was comparable between all three strains, with the greatest arginine production seen with SYN-UCD301 at 120 minutes, indicating that chromosomal integration of FNR ArgA fbr results in similar levels of arginine production as seen with the low copy plasmid strains expressing the same construct, and may even slightly increase the rate of arginine production. SYN- UCD206 exhibited attenuated arginine production as compared to SYN- UCD205 and SYN-UCD-301 (lower arginine levels at 60 minutes), but reached comparable arginine production levels at 120 minutes, indicating that AThyA may have a slight attenuating effect on arginine production. No arginine production was detected for the SYN-UCD103 control.
[0527] Next, samples were prepared as described above and arginine production at 120 min post induction was compared between (1 ) SYN-UCD204 (comprising AArgR and argAfbr expressed under the control of a tetracycline- inducible promoter on a low-copy plasmid), and (2) SYN-UCD301 (comprising AArgR, CmR and argAfbr expressed under the control of a FNR-inducible
promoter integrated into the chromosome at the maIEK locus), (3) SYN- UCD302 (comprising AArgR, AThyA, CmR (chloramphenicol resistance) and argAfbr expressed under the control of a FNR-inducible promoter integrated into the chromosome at the maIEK locus), and (4) SYN-UCD303 (comprising
AArgR, AThyA, KanR (kanamycin resistance) and argAfbr expressed under the control of a FNR-inducible promoter integrated into the chromosome at the maIEK locus).
[0528] SYN-UCD106, comprising AArgR and AThyA was used as is a control Nissle construct. Results are shown in Fig. 31 B. As seen in Fig. 31 B, arginine production was elevated to between 0.7 and 0.9 umol/1X109 cells, indicating that arginine production is at similar levels in strains bearing ArgAfbr on a plasm id and strains with integrated copies of ArgAfbr.
Example 19. Efficacy of genetically engineered bacteria in a mouse model of hyperammonemia and UCD (spf-ash) maintained on a high protein diet
[0529] The hyperammonia /UCD (spf-ash) model described in Example 14 was used to assess the in vivo efficacy of genetically engineered bacteria encoding ArgAfbr driven by a fnr promoter integrated into the bacterial chromosome on ammonia levels upon administration of a high protein diet. Mice were treated with unmodified control Nissle bacteria or Nissle bacteria engineered to produce high levels of arginine or citrulline as described above.
[0530] Two strains, one with a ThyA deletion (SYN-UCD303) and one without a ThyA deletion (SYN-UCD301 ) were tested for efficacy and compared to determine whether AThyA may influence the efficacy of ammonia removal from the blood with these stains harboring chromosomal fnr-ArgAfbr.
[0531] Spf-ash mice were treated by oral administration with the genetically engineered bacteria (SYN-UCD301 , SYN-UCD303) or H20 control. Normal and high protein chow was provided as follows: SYN-UCD301 , high protein chow (n=10); SYN-UCD303, high protein chow (n=10); H20 control, normal chow (n=10); H20 control, high protein chow (n=10). For SYN- UCD301 , SYN-UCD303, and SYN-UCD106, a dose of 100 ul of > 1 X1010 cells/ml was administered twice a day for 12 days, with the exception of days 1 , 5, 6, and 7, where bacteria were administered once. Essentially the same
protocol was followed as described in Example 16, with blood being drawn on day 5 to obtain ammonia levels (Fig. 32A). On day 10, survival rates were analyzed and a time course of survival is shown in Fig. 32B.
[0532]As depicted in Fig. 32A, ammonia levels of spf-ash mice in a high protein diet were reduced in the SYN-UCD301 and SYN-UCD303 groups as compared to the H2O high protein diet control group, indicating that the FNR inducible promoter can drive ArgAfbr expression when the construct is integrated into the chromosome, resulting in decreased ammonia levels in the blood of the mice treated with the engineered bacteria. The observed reduction in ammonia levels was similar in both SYN-UCD301 and SYN-UCD303, indicating that ThyA auxotrophy does not have a significant effect on efficacy of SYN-UCD303. As seen in Fig. 32B, SYN-UCD301 and SYN-UCD303 showed prolonged survival as compared to controls. Experiments were conducted twice sequentially with similar results.
Example 20. Comparison of Efficacy at Various Doses
[0533] To determine the lowest dose which can be used, while achieving optimal arginine production in the hyperammonia /UCD (spf-ash) model described in Example 14, three doses of SYN-UCD303 were administered.
Spf-ash mice were treated by gavage with the genetically engineered bacteria (SYN-UCD303) or H2O control. For SYN-UCD303, doses of 1 X107, 1X108, 1X109, and 1 X1010 CFUswere administered in a volume of 100 ul twice a day for 12 days, with the exception of days 1 , 5, 6, and 7, where bacteria were administered once. Normal chow or high protein chow was provided as follows: SYN-UCD303 (1 X107 CFU), high protein chow (n=10); SYN-UCD303 (1 X108 CFU), high protein chow (n=10); SYN-UCD303 (1 X109 CFU), high protein chow (n=10); H2O control, normal chow (n=10); H2O control, high protein chow (n=10). Essentially the same protocol was followed as described in Example 16, with blood being drawn on day 5 to obtain ammonia levels. Blood ammonia levels were analyzed for each dose on day 5. Results are depicted in Fig. 33. Both doses of 1X108 and 1X109were sufficient to result in a significant reduction of blood ammonia levels in this model.
Example 21. Nissle residence
[0534] Unmodified E. coli Nissle and the genetically engineered bacteria of the invention may be destroyed, e.g., by defense factors in the gut or blood serum. The residence time of bacteria in vivo may be calculated. A non-limiting example using a streptomycin-resistant strain of E. coli Nissle is described below. In alternate embodiments, residence time is calculated for the genetically engineered bacteria of the invention.
[0535] C57BL/6 mice were acclimated in the animal facility for 1 week. After one week of acclimation (i.e., day 0), streptomycin-resistant Nissle (SYN- UCD103) was administered to the mice via oral gavage on days 1-3. Mice were not pre-treated with antibiotic. The amount of bacteria administered, i.e., the inoculant, is shown in Table 25. In order to determine the CFU of the inoculant, the inoculant was serially diluted, and plated onto LB plates containing streptomycin (300 pg/mL). The plates were incubated at 37°C overnight, and colonies were counted.
Table 25: CFU administered via oral gavage
[0536] On days 2-10, fecal pellets were collected from up to 6 mice (ID NOs. 1 -6; Table 14). The pellets were weighed in tubes containing PBS and homogenized. In order to determine the CFU of Nissle in the fecal pellet, the homogenized fecal pellet was serially diluted, and plated onto LB plates containing streptomycin (300 pg/mL). The plates were incubated at 37°C overnight, and colonies were counted.
[0537] Fecal pellets from day 1 were also collected and plated on LB plates containing streptomycin (300 pg/mL) to determine if there were any strains native to the mouse gastrointestinal tract that were streptomycin resistant. The time course and amount of administered Nissle still residing within the mouse gastrointestinal tract is shown in Table 26.
Fig. 34 depicts a graph of Nissle residence in vivo. Streptomycin- resistant Nissle was administered to mice via oral gavage without antibiotic pre-
treatment. Fecal pellets from six total mice were monitored post-administration to determine the amount of administered Nissle still residing within the mouse gastrointestinal tract. The bars represent the number of bacteria administered to the mice. The line represents the number of Nissle recovered from the fecal samples each day for 10 consecutive days.
Table 26. Nissle residence in vivo
Example 22. Intestinal Residence and Survival of Bacterial Strains in vivo
[0538] Localization and intestinal residence time of SYN-UCD303
(integrated fnrS inducible promoter driving ArgAfbr, kanamycin resistance, AThyA, Fig. 35C) was compared to SYN-UCD106 (AArgR, AThyA, and chloramphenicol resistance, Fig. 35B) and SYN-UCD103 (streptomycin resistant Nissle, Fig. 35A). Mice were gavaged, sacrificed at various time points, and effluents were collected from various areas of the small intestine cecum and colon.
[0539] Bacterial cultures were grown overnight and pelleted. The pellets were resuspended in PBS at a final concentration of approximately 1010
CFU/mL. Mice (C57BL6/J, 10-12 weeks old) were gavaged with 100 μΙ_ of
bacteria (approximately 109 CFU). Drinking water for the mice was changed to contain 0.1 mg/ml_ anhydrotetracycline (ATC) and 5% sucrose for palatability. At each timepoint (1 , 4, 8, 12, 24, and 30 hours post-gavage), animals (n=4) were euthanized, and intestine, cecum, and colon were removed. The small intestine was cut into three sections, and the large intestine and colon each into two sections. Each section was flushed with 0.5 ml cold PBS and collected in separate 1.5 ml tubes. The cecum was harvested, contents were squeezed out, and flushed with 0.5 ml cold PBS and collected in a 1.5 ml tube. Intestinal effluents were placed on ice for serial dilution plating.
[0540] In order to determine the CFU of bacteria in each effluent, the effluent was serially diluted, and plated onto LB plates containing kanamycin. The plates were incubated at 37°C overnight, and colonies were counted. The amount of bacteria and residence time of SYN-UCD103, SYN-UCD106, and SYN-UCD303 seen in each compartment is shown in Fig. 35. As seen in Fig. 35, all three strains behave in a similar manner. Comparing Fig. 35A with Fig. 35B, AThyA auxotrophy and AArgR does not seem to have any substantial effect on residency and transit time.
Example 23. Effect of Auxotrophy on Intestinal Residence Time
[0541]To determine if auxotrophy may have an effect on localization and time of residence, the mouse model described above is used to compare the residency of SYN-UCD303 (AThyA) with SYN-UCD304 (wild type ThyA). SYN- UCD103 (streptomycin resistant control Nissle) is administered in parallel to determine whether the Arg R deletion and argAfbr have an effect on residence.
[0542] Bacteria are prepared and mice are gavaged, euthanized and intestinal effluents collected at various time points as described in Example 22. To determine the CFU of bacteria in each effluent, the effluent is serially diluted and plated as described in Example 20. For SYN-UCD103 streptomycin containing plates and for SYN-UCD301 chloramphenicol containing plates are used.
Example 24. TAA model of Hyperammonemia
[0543] TAA treatment of mice has previously been employed in the literature to model increased blood ammonia levels associated with UCDs,
acute and chronic liver disease and HE (Wallace MC, et al., Lab Anim. 2015 Apr;49(1 Suppl):21-9. Standard operating procedures in experimental liver research: thioacetamide model in mice and rat)s. In some embodiments, a TAA- induced mouse model of hyperammonemia is employed to investigate the ability of the genetically engineered bacteria to reduce blood ammonia levels. The TAA serves as an alternative to the spf-ash model. Because spf-ash is a genetic model, the numbers of mice are limiting so developing an inducible model in wild-type mice would greatly facilitate in vivo testing of potential strains of interest.
[0544] To investigate the effects of engineered bacteria on prolonged elevations of blood ammonia, the bacteria are administered to C57BL6 mice that are also administered a dose of 300mpk thioacetamide (TAA).
[0545] C57BL6 (10 weeks old) are administered one daily dose of SYN- UCD103 or SYN-UCD303 (100 ul of > 1 X1010 cells/ml) or vehicle control.
Alternatively, mice are administered 2 daily doses of bacteria (100 ul of > 1 X1010 cells/ml) (n=5 for each treatment group), once in the AM and once in the PM. After three days of pre-dosing with the bacteria, the mice are treated intraperitoneally with thioacetamide (TAA) at 300mpk or with H20 as control. Alternatively, the mice are treated twice daily, once in the AM and once in the PM with 250mpk. The duration of the study is five days. Ammonium levels are measured and overall health survival, body weight change is monitored.
[0546] In brief, animals are acclimated for 7 days. On day 1 of the time course, animals are weighed, bled to measure baseline ammonia and collect fecal pellets (per cage), and are randomized based on initial blood ammonia levels. Animals are dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD103 or SYN-UCD303 (100ul/dose/animal). Water is changed to H2O(+)20mg/ml ATC. On day 2, animals are dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD103 or SYN-UCD303
(100ul/dose/animal). On day 3, animals are weighed and dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD103 or SYN-UCD303 (100ul/dose/animal). Additionally, animals are dosed intraperitoneally with 300mpk TAA (or saline control). Alternatively, animals are dosed with 250mpk TAA (or saline control) once in the AM and once in the PM. On day 4, animals
are weighed, bled, and blood ammonia is measured. Fecal pellets are collected per cage. Animals are dosed per oral gavage either once or twice (AM and PM) with H20, SYN-UCD103 or SYN-UCD303 (100ul/dose/animal). Animals may also be dosed with 250mpk TAA (or saline control). On day 5, animals are weighed, bled, and blood ammonia levels are measured. Fecal pellets are collected (per cage). Animals are dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD103 or SYN-UCD303 (100ul/dose/animal). Ammonium levels, bacterial load in the fecal pellets, and overall health survival, and body weight changes are monitored.
Example 25. Model of Hyperammonemia using Arginase Inhibitors
[0547] As an alternative to the genetic spf-ash model, arginase inhibitors +/- high protein chow are used as an inducible model of hyperammonemia. Arginase inhibitors fall into at least 2 classes (described in Steppan et al., Front Immunol. 2013; 4: 278. Development of Novel Arginase Inhibitors for Therapy of Endothelial Dysfunction). The first group of arginase inhibitors consisted of the boronic acid analogs of l-arginine (2)S-amino-6-hexanoic acid (ABH) and S-2- BEC both of which inhibit the catalytic activity of arginase. Another category of arginase inhibitors, that is mainly represented by N-hydroxy-l-arginine (NOHA) and N-hydroxy-nor-l-arginine (nor-NOHA), is characterized by N-hydroxy- guanidinium side chains and inhibit arginase by displacing the metal-bridging hydroxide ion of arginase with their N-hydroxy group. The model development study employs an arginase inhibitor from each group, BEC and nor-NOHA +/- high protein chow (70% protein).
[0548] To determine if engineered bacteria can change ammonium levels in the blood, inducible models using an arginase inhibitor from each group, BEC and nor-NOHA, +/- high protein chow (70% protein) (70% protein) in wild type mice are employed. C57BL6 (Female, 8weeks) are treated by oral gavage with the genetically engineered bacteria (100 ul of > 1 X1010 cells/ml) or a vehicle control and intraperitoneally either with BEC or norNOHA, and are kept either on a normal chow (n=5 per treatment group) or a high protein chow diet (n=5 per treatment group). Administration groups are as follows for SYN-UCD303 and a vehicle control-treated animals: normal chow (n=5); high protein chow
(70% protein chow; n=5); high protein chow (+)BEC (n=5); high protein chow (+)norNOHA (n=5).
[0549] On day 1 of the time course, animals are weighed, bled to measure baseline ammonia, and are randomized based on initial blood ammonia levels. Fecal pellets are collected (per cage). Animals are dosed by oral gavage with H2O, SYN-UCD303 or SYN-UCD103 (100ul/dose/animal). Water is changed to H2O(+)20mg/ml ATC. On day 2, animals are dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD303 or SYN- UCD103 (100ul/dose/animal). On day 3, animals are weighed and dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD303 or SYN- UCD103 (100ul/dose/animal). Additionally, animals are dosed intraperitoneally with BEC (25mpk) or norNOHA (100mpk) or saline control. For the high protein diet groups, the chow is changed from normal chow to 70% protein chow. On day 4, animals are weighed, bled, and blood ammonia is measured. Fecal pellets are collected per cage. Animals are dosed per oral gavage either once or twice (AM and PM) with H2O, SYN-UCD303 or SYN-UCD103
(100ul/dose/animal). Animals are dosed intraperitoneally with BEC (25mpk) or norNOHA (100mpk). On day 4, animals are dosed per oral gavage either once or twice (AM and PM) with H2O, SYN-UCD303 or SYN-UCD103
(100ul/dose/animal). Animals are weighed, bled 1 h post dose, and blood ammonia is measured. Fecal pellets are collected per cage. Animals are also dosed intraperitoneally with BEC (25mpk) or norNOHA (100mpk). On day 5, Animals are dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD303 or SYN-UCD103 (100ul/dose/animal). Animals are weighed, bled 1 h post dose, and ammonia levels are measured.
[0550] A similar study was conducted using strains SYN-UCD202, SYN- UCD204, and SYN-UCD103, and results are shown in Fig. 28.
Example 26. Effect of Proliferation Potential and Metabolic Activity on
Arginine Production
[0551] The requirement of cell division and/or active metabolism for arginine production from the genetically engineered bacteria was investigated in strain SYNUCD-303.
[0552] SYNUCD-301 were incubated with 70% isopropanol or phosphate buffered saline (PBS) as a control for 1 hour with shaking. 70% isopropanol disrupted the cellular membrane, which prevented cell division, but should allow cell metabolism. PBS incubation had no effect on the cells (not shown). After treatment, the cells were mixed at specific ratios in M9 media supplemented with 0.5% glucose and 3mM thymidine. Cells were incubated with shaking at 37 C for 2 hours. Cells can use the ammonium chloride contained in the M9 media to form arginine. Arginine concentration was measured in the media at time zero and again after the 2 hour incubation, and the amount of arginine produced per hour per billion cells was calculated. As seen in Figs. 36A and 36B., a greater ratio of isopropanol treated cells to untreated in a culture results in fewer CFUs as determined by plating, and lower levels of arginine production.
Arginine production relative to amount of bacteria present remained constant across the various cultures (Fig. 36C). These results indicate that only viable bacteria are contributing to arginine production.
Example 27. Repeat-Dose Pharmacokinetic and Pharmacodynamic Study of SYN-UCD-303 Following Daily Nasogastric Gavage Dose Administration for 28-days in Cynomolgus Monkeys (non-GLP)
[0553] To evaluate any potential toxicities arising from administration of the genetically engineered bacteria or E coli Nissle alone, the pharmacokinetics and pharmacodynamics of SYN-UCD303 and an E. coli Nissle with kanamycin resistance (SYN-UCD107) were studied following daily nasogastric gavage (NG) dose administration for 28-days to female cynomolgus monkeys.
Cynomolgus monkeys were selected because this species is closely related, both phylogenetically and physiologically, to humans and is a species
commonly used for nonclinical toxicity evaluations. The genetically engineered bacteria were administered by nasal gastric gavage, consistent with the proposed route of administration in humans. Animals overall well-being (clinical observations), weight clinical pathology (serum chemistry, hematology, and coagulation) were tracked. Fecal samples were examined for bacterial load. Plasma is analyzed for ammonia levels.
A. Materials, animals and dosing regimen:
[0554] The study was conducted in compliance with nonclinical
Laboratory Studies Good Laboratory Practice Regulations issued by the U.S. Food and Drug Administration (Title 21 of the Code of Federal Regulations, Part 58; effective June 20, 1979) and the OECD Principles on Good Laboratory Practice (C[97]186/Final; effective 1997). The animals were individually housed based on the recommendations set forth in the Guide for the Care and Use of Laboratory Animals (National Research Council 2011 ).
[0555]Animals used in the study were Female Purpose-bred, non-naive cynomolgus monkey (Macaca fascicularis) with 3 to 6 kg (at initial physical exam) 3 to 8 years (at initial physical exam) of age (SNBL USA stock, Origin: Cambodia).
[0556] For control of bias, animals were randomized to groups in a weight-stratified manner to achieve similar group body weight distributions. The number of animals selected for randomization and their weights and ages are included in Table 27.
Table 27. Animals for randomization
Animals were identified by unique tattoo numbers.
[0557] Seventeen animals were acclimated for 7 days prior to dose initiation and fifteen animals were assigned to treatment groups. Spare animals were removed from the study after Day 1.
[0558] For the duration of the study, animals were offered PMI LabDiet® Fiber-Plus® Monkey Diet 5049 biscuits twice daily. Animal were fasted for at least 2 hours prior to dose administration and fed within 1 hour post dose.
Animals also were fasted as required by specific procedures (e.g., prior to blood draws for serum chemistry, fecal collection). The diet was routinely analyzed for contaminants and found to be within manufacturer's specifications. No contaminants were expected to be present at levels that would interfere with the outcome of the study.
[0559] Fresh drinking water was provided ad libitum to all animals. The water was routinely analyzed for contaminants. No contaminants were present
at levels that would interfere with the outcome of the study. Animals were given fruits, vegetables, other dietary supplements, and cage enrichment devices throughout the course of the study.
[0560] Previously quarantined animals were acclimated to the study room for 7 days prior to initiation of dosing (day 1 ). The last dosing occurred on day 28. A stratified randomization scheme incorporating body weights was used to assign animals to study groups. Animals were assigned to groups and treated as indicated in Table 28.
Table 28. Group Assignments
Concentration of Sodium Bicarbonate: .36 M or 0.12 M (0.36 M for the control article)
[0561] SYN-UCD107 and SYN-UCD303 stocks were prepared at 1 x109 cfu/mL and 1 101 1 cfu/mL in 15% glycerol in 1X PBS with 2.2% glucose and 3 mM thymidine and were kept at 86 to -60 °C (see Table 28). PBS made in 20% glycerol with sodium bicarbonate was used as a control vehicle. Carbonate concentration was 0.36M and 0.12M for sodium bicarbonate (see Table 28). On
the day of each dosing, bacteria and vehicle control were removed from the freezer and put on ice and thawed and placed on ice until dosing.
[0562] Animals were dosed at 0, 1 *109, or 1 *1012 cfu/animal. All animals were dosed via nasal gastric gavage (NG) followed by control/vehicle flush once daily for 28-days. The concentration of bicarbonate and volume for each group is specified in Table 28. Vials were inverted at least 3 times prior to drawing the dose in the syringe. The dose site and dose time (end of flush time) was recorded. On Day 6, Animals 19, 21 , and 23 in Group 4 (SYN-UCD303, 1x109/animal) received 5 ml_ bicarbonate flush instead of 14 ml_ followed by test article dose administration.
B. Analysis
Overall Condition and Weight
[0563] Overall condition: Clinical observations were performed twice daily, beginning on Day -6 through 44. The first observation was in the AM. The second observation was no sooner than 4 hours after the AM observation.
During the dosing phase, the second observation was performed 4 hour (±10 minutes) post dose administration. Additional clinical observations were performed, as necessary.
No test article-related clinical observations were identified in this study.
[0564] Incidental and/or procedural findings, commonly noted in similarly housed animals, occurred sporadically among individuals, were noted in control animals, were also present during the acclimation phase, and did not increase in severity and incidence among dose groups. These included findings related to skin (scab/crust, wounds, abrasions, abnormal skin discoloration, bruising), hair loss, kinked tail, sunken eyes, inappetence, emesis, and urogenital discharge.
[0565] Weight: Body weights were measured on Days -6, 1 , 8, 11 , 15, 22, 29, 36, and 43 prior to the first feeding and dose administration, as applicable.
[0566] No test article-related effects on body weights were identified in this study. Decreases of 5 to 10% body weight, relative to baseline (Day 1 ), was apparent across all groups, including control, by Day 36 and determined to be study procedure-related. Compared to Day 36, an upward trend was noted in
the available body weight data on Day 43, albeit values did not completely return to baseline.
Clinical Pathology
[0567] Blood Collection: Animals were fasted overnight prior to daily dose administration and at least 4 hours prior to each series of collections that included specimens for serum chemistry and plasma bioanalysis. In these instances, associated clinical pathology evaluations were from fasted animals. Blood was collected by venipuncture from a peripheral vein of restrained, conscious animals via a single draw (if possible) and divided into appropriate tubes for analysis, as follows: Hematology: approximately 1.3 mL, K2EDTA tube; Coagulation: approximately 1.8 mL, 3.2% sodium citrate tube; Serum chemistry: approximately 1.0 mL, serum separator tube (SST); Plasma sample: approximately 1.0 mL, Lithium Heparin; The blood for clinical pathology assessment was processed to serum or plasma, or used intact, according to SNBL USA SOPs.
[0568] Whenever possible, blood was collected via a single draw and then divided appropriately. Specimen collection frequency is summarized in Table 29.
Table 29. Specimen collection frequency
Dosinq Davs 46 and Weeks 6 50
- = Not applicable
x = Number of times procedure performed within the week
[0569] Table 30 Summarizes the Clinical Pathology Assay Information.
Table 30. Clinical pathology assay information
Residual samples were discarded prior to study finalization
[0570] No test article-related effects in hematology, coagulation, and serum chemistry parameters were identified in this study.
[0571] Plasma Samples: Animals were fasted for 4 hours prior to removal of the sample. Approximately 1 mL of blood were collected from the femoral vein and transferred into 2 mL lithium heparin tubes on Days -1 and 30, and prior to dose administration on Days 2, 7, 14, and 28. After collection of the
target volume of blood in the tube, approximately 0.05 (on Days -1 and 2) or 0.1 ml_ (on Days 7, 14, 28, and 30) of mineral oil was added to cover the surface of blood in the tubes. Tubes were not inverted and were placed on wet ice.
[0572] The samples were centrifuged within 15 minutes of collection at 2 to 8 °C to obtain plasma and the plasma was maintained on dry ice prior to storage at -60 to -86°C
[0573] Analysis of specimens is conducted using a blood ammonia analyzer instrument.
[0574] Fecal Sample Collection: Two fecal samples per animal were collected at the target time points listed in Table 29. Sample collection dates and times were recorded. 50 ml_ falcon tube with approximately 5ml_ PBS were used as the container (If feces is liquid, no PBS is added). To get the fecal sample weight, pre- and post- sampling weight of container was taken. Samples were collected from the bottom of the cage from each animal. To get fresh and un-contaminated samples, remaining food was removed and the cage pan was cleaned and squeegeed to remove debris and/or water before the collection. On Days -5, 2, 4, 7, and 14, food removals and pan cleanings were performed after the first feeding and/ or dose administration. On Days 18, 20, 24, 28, 30, 35, 40, 46, and 50, the food removals and pan cleanings were performed the night before each collection. Visually fresh fecal samples were also collected in the morning before any procedures, except clinical observations, occurred.
[0575] Fecal Swab Collection: When fecal samples were not collected by the end of the scheduled day, fecal swab samples were collected with a cotton tip applicator from the rectum of animals restrained in a procedure cage.
Samples were put on wet ice immediately after the collection. Samples were stored at -20 to -15 °C until analysis. Analysis of specimens was
conducted using a PCR analytical method as described in Example 31.
[0576] Two fecal samples per animal were collected on Day -5 and after dose administration on Days 2, 4, 7, and 14, and three fecal samples per animal were collected on Days 29, 30, 35, 40, 46, and 50, and prior to dose
administration on Days 18 through 28. Fifty milliliter falcon tubes with
approximately 5 ml_ PBS were used for the collections on Days -5 through 14, and 50 mL falcon tubes with approximately 5 ml_ PBS for Tube 1 , 20 ml_ of 50%
glycerol and 10 mM thymidine for Tube 2, and 20 ml_ of 50% glycerol/PBS for Tube 3, were used for the collections on Days 18 through 50. Specimens were put on wet ice immediately after the collection and the contents of each tube collected on Days 18 through 50 were broken-up and mixed using a sterile tongue depressor.
[0577] Since no fecal samples were collected from the animals listed in Table 29 by the end of the collection day, two fecal swab samples were collected per animal. After sample collection, the cotton part of the swab was transferred to a 5 ml_ cryovial with 1 ml_ of PBS and immediately put on wet ice.
[0578] Results are shown in Fig. 37, and show that the amount of bacteria quantified from fecal samples follows a similar pattern for Kanamycin resistant control Nissle (SYN-UCD107) and SYN-UCD303. Bacteria in the fecal samples reach a level of less than 1000 bacteria/per ml of feces by day 35. Results indicate that under these dosing conditions, similar amounts of bacteria were recovered with the auxotroph SYN-UCD303 as control kanamycin resistant Nissle in the feces. Similar results have been observed in mice. In conclusion, SYN-UCD303 appeared to be present in the NHP feces at nearly the same concentration as Nissle (SYN-UCD107).
[0579] In overall conclusion, the test article, SYN-UCD303, was well tolerated by female cynomolgus monkeys after 28 days of daily NG dose administration at doses up to 1 x 1012 CFU/animal. No test article-related mortality occurred and no test article-related effects were identified upon clinical observation, body weight, and clinical pathology assessment.
Example 28. Repeat-Dose Pharmacokinetic and Pharmacodynamic Study of SYN-UCD-303 Following Daily Nasogastric Gavage Dose Administration for 28-days in Cynomolgus Monkeys (non-GLP)
[0580] Pharmacokinetics and pharmacodynamics of SYN-UCD303, SYN- UCD304, SYN-UCD305, and SYN-UCD306 and are studied following daily nasogastric gavage (NG) dose administration for 28-days to female cynomolgus monkeys essentially as described in Example 27. Cynomolgus monkeys are selected because this species is closely related, both phylogenetically and physiologically, to humans and is a species commonly used for nonclinical
toxicity evaluations. The genetically engineered bacteria are administered by nasal gastric gavage, consistent with the proposed route of administration in humans. Animals overall well-being (clinical observations), weight clinical pathology (serum chemistry, hematology, and coagulation) are tracked. Plasma is analyzed for ammonia levels, and fecal samples are examined for bacterial load.
Example 29. 4-Week Toxicity Study in Cynomolgus Monkeys with a 4-
Week Recovery (GLP)
[0581]To evaluate any potential toxicities arising from administration of the genetically engineered bacteria, the pharmacokinetics and
pharmacodynamics of SYN-UCD303 is studied following daily nasogastric gavage (NG) dose administration for 28-days to female cynomolgus monkeys under GLP conditions.
[0582] In other embodiments, the study is conducted SYN-UCD304, SYN-UCD305, and/or SYN-UCD306.
[0583] The study is conducted in compliance with nonclinical Laboratory Studies Good Laboratory Practice Regulations issued by the U.S. Food and Drug Administration (Title 21 of the Code of Federal Regulations, Part 58; effective June 20, 1979) and the OECD Principles on Good Laboratory Practice (C[97]186/Final; effective 1997). The animals are individually housed based on the recommendations set forth in the Guide for the Care and Use of Laboratory Animals (National Research Council 2011 ).
[0584]Animals are administered SYN-UCD303 or control vehicle essentially as described in the Example 27, except that all materials are manufactured under GMP standards. Dosing is tabulated in Table 31.
Additionally, animals are acclimated for 14 days and the dosing period is daily for 28 days followed by a recovery period of 28 days. Additionally, animals are euthanized at the end of the study to conduct histological analysis.
Table 31. Dosing Period and Regimen
REGULATIONS FDA GLP
aTerminal Necropsy, Day 29
bRecovery Necropsy, Day 56
[0585] Study Analysis is conducted as described in Table 32.
Hematology, Coagulation, Serum Chemistry and Plasma Samples parameters are essentially as described in Example 27, and are analyzed using the methods described in Example 27. Collection and analysis of fecal samples is essentially conducted as described in Example 27.
Table 32. Study Analysis
STOOL SAMPLE COLLECTION Once during acclimation, prior to dosing (BACTERIAL CULTURE) on Days 2, 7, and 14, Day 29, Day 33,
and Week 8
Rectal/Fecal swabs are collected via cotton tip applicator; the cotton part of the swab is transferred to a tube with an appropriate broth/media and immediately put on wet ice. Fecal samples are stored at 2 to 8 °C until time of analysis.
CYTOKINE BLOOD COLLECTIONS Once during acclimation, Days 1 , 3, 7, 14 and 28 (6 hrs post-dose), and Day 56
ARCHIVE BLOOD SAMPLE Once during acclimation, Days 1 , 3, 7, 14 COLLECTION (SAMPLE TO BE and 28 (6 hrs post-dose), and Day 56;
HELD FOR POSSIBLE ANALYSIS) Blood samples are processed to serum;
samples are stored frozen.
NECROPSY & TISSUE All animals (e.g., colon, intestine, cecum, COLLECTION liver, spleen)
ORGAN WEIGHTS All animals
TISSUE COLLECTION FOR PK/PD All animals
ASSESSMENT
HlSTOPATHOLOGY All animals
STATISTICAL ANALYSIS Comparative (Anova/Bartletts)
Example 30. 4-Week Repeat Dose Toxicity Study in Mice with a 2-Week
Recovery (GLP)
[0586] To evaluate any potential toxicities arising from administration of the genetically engineered bacteria, the pharmacokinetics and
pharmacodynamics of SYN-UCD303 is studied following daily gavage dose administration for 4 weeks followed by a two week recovery under GLP conditions.
[0587] All materials are manufactured under GLP conditions. CD-1 Mice, 6-8 weeks of age at initiation of study are acclimated for 7 days. Groups of males and female mice are studied separately. SYN-UCD303 or vehicle control are administered as described in Table 33.
[0588] In certain embodiments, the study is conducted SYN-UCD304, SYN-UCD305, and/or SYN-UCD306.
Table 33. Study Design
a Toxicity (Tox) Animals, Terminal Necropsy Day 29; b Toxicity (Tox) Animals, Recovery Necropsy Day 42
[0589] The study analysis is described in Table 34. Hematology,
Coagulation, Serum Chemistry and Plasma Samples parameters are essentially as described in Example 27, and are analyzed using the methods described in Example 27. Collection and analysis of fecal samples is essentially conducted as described in Example 27. Histology is conducted as in Example 29.
Table 34. Study Analysis
STATISTICAL ANALYSIS Comparative (Anova/Bartletts)
Example 31. Determination of Presence of Nissle in Fecal Samples of Non- human Primates
[0590]To analyze fecal samples from non-human primates (NHPs) for the presence of Nissle, the number of bacteria from NHP samples was quantified based on the quantity of DNA in the sample using qPCR. In some embodiments, this protocol is used for the analysis of fecal samples from other mammals, including, but not limited to, mice and humans.
[0591] Sample Homogenization: Fecal samples (stored at -20°C) were thawed at room temperature for 90 minutes. For solid fecal samples, the approximate solid volume was estimated, and phosphate buffered saline (PBS) was added to double the volume. For liquid fecal samples, no additional PBS was added. Samples were then vortexed for 30 seconds per tube. Fecal samples were homogenized using a disposable Pestle (Fisher 12-141 -363) in PBS in Eppendorf tubes and kept on ice for subsequent procedures.
[0592] DNA purification: The homogenized samples (250 μΙ_) were removed using sterilized filter tips (Racked Gilson Expert Sterilized Filter Tips) cut at the first gradation line and transferred to Eppendorf tubes. DNA was purified from the homogenized samples (250 μΙ) using the MoBio PowerLyzer PowerSoil DNA Isolation Kit (12855-100) following manufacturer's protocol. The amount of purified DNA recovered from each sample was quantified by measuring the OD260 of the sample on a Eppendorf BioSpectrometer Basic.
[0593] PCR reaction: Two reactions (Reaction 1 and Reaction 2) were assembled and run in triplicate. The first reaction served to quantify the amount of Nissle, and the second to quantify the total amount of bacteria present in the fecal samples. For the first qPCR reaction, purified DNA (5ng), 0.4 DL of Primer 1 (10 DM), 0.4 DL of Primer 2 (10 DM), and 10 DL of SYBR Green PCR Master Mix (Thermo Fisher Scientific: 4368577) were brought up to 20 DL with water. For the second qPCR reaction, purified DNA (5ng), 0.4 DL of Primer 3 (10 DM), 0.4 DL of Primer 4 (10 DM), and 10 DL of SYBR Green PCR Master Mix (Thermo Fisher Scientific: 4368577) were brought up to 20 DL with water.
[0594] The sequences of primers used in the reactions at a concentration of 10 DM are found in Table 35.
Table 35. Primer Sequences
[0595] For quantification of the amount of bacterial DNA amplified, a standard curve was run for each reaction. To generate the standard curve, Fragment #1 (synthesized at a set concentration) (Table 36) was diluted in 10- fold serial dilutions, resulting in the following amounts: no DNA, 1 copy of Fragment #1 , 10 copies of Fragment #1 , 100 copies of Fragment #1 , and so on, until the eighth well has 106 copies of fragment #1. Standard DNAs were then added to the qPCR reaction mix for Reaction #1.
Table 36. Standard DNA Sequence (Fragment #1)
[0596] PCR reaction conditions for standard curve, and Reactions 1 and 2 are shown in Table 37.
Table 37. PCR Reaction Conditions
[0597] As a quality control measure, the melt-curves of the test qPCR reactions were compared to the positive control for each primer set, to ensure that the melt-curve of the positive controls matched the melt-curve of the test samples. The presence and quantity of Nissle and total bacteria was
determined by analyzing CT values (Cycle threshold values) against the standard curve.
Example 32. Construction of vectors for overproducing butyrate
[0598] In addition to the ammonia conversion circuit, GABA transport circuit, GABA metabolic circuit, and/or manganese transport circuit described above, the E. coli Nissle bacteria further comprise one or more circuits for producing a gut barrier enhancer molecule.
[0599] To facilitate inducible production of butyrate in E. coli Nissle, the eight genes of the butyrate production pathway from Peptoclostridium difficile 630 (bcd2, etfB3, etfA3, thiA1 , hbd, crt2, bpt, and buk; NCBI), as well as transcriptional and translational elements, were synthesized (Gen9, Cambridge, MA) and cloned into vector pBR322. The butyrate gene cassette is placed under the control of a FNR regulatory region selected from SEQ ID NOs: 18-29 (Table 6). In certain constructs, the FNR-responsive promoter is further fused to a strong ribosome binding site sequence. For efficient translation of butyrate genes, each synthetic gene in the operon was separated by a 15 base pair ribosome binding site derived from the T7 promoter/translational start site.
[0600] In certain constructs, the butyrate gene cassette is placed under the control of an RNS-responsive regulatory region, e.g., norB, and the bacteria further comprises a gene encoding a corresponding RNS-responsive transcription factor, e.g., nsrR (see, e.g., Tables 38 and 39). In certain constructs, the butyrate gene cassette is placed under the control of an ROS- responsive regulatory region, e.g., oxyS, and the bacteria further comprises a gene encoding a corresponding ROS-responsive transcription factor, e.g., oxyR (see, e.g., Tables 14-17). In certain constructs, the butyrate gene cassette is placed under the control of a tetracycline-inducible or constitutive promoter.
Table 38. pLogic031-nsrR-norB-butyrate construct (SEQ ID NO: 79)
Description Nucleotide sequences of pLogic031-nsrR-norB-butyrate construct (SEQ ID NO: 79)
cgagtgaattaatatttgaggattgcagaatacctaaagaaaat ttacttggaaaagaaggtcaaggatttaagatagcaatgtctac tcttgatggtggtagaattggtatagctgcacaagctttaggtt tagcacaaggtgctcttgatgaaactgttaaatatgtaaaagaa agagtacaatttggtagaccattatcaaaattccaaaatacaca attccaattagctgatatggaagttaaggtacaagcggctagac accttgtatatcaagcagctataaataaagacttaggaaaacct tatggagtagaagcagcaatggcaaaattatttgcagctgaaac agctatggaagttactacaaaagctgtacaacttcatggaggat atggatacactcgtgactatccagtagaaagaatgatgagagat gctaagataactgaaatatatgaaggaactagtgaagttcaaag aatggttatttcaggaaaactattaaaatagtaagaaggagata tacatatggaggaaggatttatgaatatagtcgtttgtataaaa caagttccagatacaacagaagttaaactagatcctaatacagg tactttaattagagatggagtaccaagtataataaaccctgatg ataaagcaggtttagaagaagctataaaattaaaagaagaaatg ggtgctcatgtaactgttataacaatgggacctcctcaagcaga tatggctttaaaagaagctttagcaatgggtgcagatagaggta tattattaacagatagagcatttgcgggtgctgatacttgggca acttcatcagcattagcaggagcattaaaaaatatagattttga tattataatagctggaagacaggcgatagatggagatactgcac aagttggacctcaaatagctgaacatttaaatcttccatcaata acatatgctgaagaaataaaaactgaaggtgaatatgtattagt aaaaagacaatttgaagattgttgccatgacttaaaagttaaaa tgccatgccttataacaactcttaaagatatgaacacaccaaga tacatgaaagttggaagaatatatgatgctttcgaaaatgatgt agtagaaacatggactgtaaaagatatagaagttgacccttcta atttaggtcttaaaggttctccaactagtgtatttaaatcattt acaaaatcagttaaaccagctggtacaatatacaatgaagatgc gaaaacatcagctggaattatcatagataaattaaaagagaagt atatcatataataagaaggagatatacatatgggtaacgtttta gtagtaatagaacaaagagaaaatgtaattcaaactgtttcttt agaattactaggaaaggctacagaaatagcaaaagattatgata caaaagtttctgcattacttttaggtagtaaggtagaaggttta atagatacattagcacactatggtgcagatgaggtaatagtagt agatgatgaagctttagcagtgtatacaactgaaccatatacaa aagcagcttatgaagcaataaaagcagctgaccctatagttgta ttatttggtgcaacttcaataggtagagatttagcgcctagagt ttctgetagaatacatacaggtcttactgetgactgtacaggtc ttgcagtagctgaagatacaaaattattattaatgacaagacct gcctttggtggaaatataatggcaacaatagtttgtaaagattt cagacctcaaatgtctacagttagaccaggggttatgaagaaaa atgaacctgatgaaactaaagaagctgtaattaaccgtttcaag gtagaatttaatgatgctgataaattagttcaagttgtacaagt aataaaagaagctaaaaaacaagttaaaatagaagatgctaaga tattagtttctgctggacgtggaatgggtggaaaagaaaactta
Description Nucleotide sequences of pLogic031-nsrR-norB-butyrate construct (SEQ ID NO: 79)
gacatactttatgaattagctgaaattataggtggagaagtttc tggttctcgtgccactatagatgcaggttggttagataaagcaa gacaagttggtcaaactggtaaaactgtaagaccagacctttat atagcatgtggtatatctggagcaatacaacatatagctggtat ggaagatgctgagtttatagttgctataaataaaaatccagaag ctccaatatttaaatatgctgatgttggtatagttggagatgtt cataaagtgcttccagaacttatcagtcagttaagtgttgcaaa agaaaaaggtgaagttttagctaactaataagaaggagatatac atatgagagaagtagtaattgccagtgcagctagaacagcagta ggaagttttggaggagcatttaaatcagtttcagcggtagagtt aggggtaacagcagctaaagaagctataaaaagagctaacataa ctccagatatgatagatgaatctcttttagggggagtacttaca gcaggtcttggacaaaatatagcaagacaaatagcattaggagc aggaataccagtagaaaaaccagctatgactataaatatagttt gtggttctggattaagatctgtttcaatggcatctcaacttata gcattaggtgatgctgatataatgttagttggtggagctgaaaa catgagtatgtctccttatttagtaccaagtgcgagatatggtg caagaatgggtgatgctgcttttgttgattcaatgataaaagat ggattatcagacatatttaataactatcacatgggtattactgc tgaaaacatagcagagcaatggaatataactagagaagaacaag atgaattagctcttgcaagtcaaaataaagctgaaaaagctcaa gctgaaggaaaatttgatgaagaaatagttcctgttgttataaa aggaagaaaaggtgacactgtagtagataaagatgaatatatta agcctggcactacaatggagaaacttgctaagttaagacctgca tttaaaaaagatggaacagttactgctggtaatgcatcaggaat aaatgatggtgctgctatgttagtagtaatggctaaagaaaaag ctgaagaactaggaatagagcctcttgcaactatagtttcttat ggaacagctggtgttgaccctaaaataatgggatatggaccagt tccagcaactaaaaaagctttagaagctgctaatatgactattg aagatatagatttagttgaagctaatgaggcatttgctgcccaa tctgtagctgtaataagagacttaaatatagatatgaataaagt taatgttaatggtggagcaatagctataggacatccaataggat gctcaggagcaagaatacttactacacttttatatgaaatgaag agaagagatgctaaaactggtcttgctacactttgtataggcgg tggaatgggaactactttaatagttaagagatagtaagaaggag atatacatatgaaattagctgtaataggtagtggaactatggga agtggtattgtacaaacttttgcaagttgtggacatgatgtatg tttaaagagtagaactcaaggtgctatagataaatgtttagctt tattagataaaaatttaactaagttagttactaagggaaaaatg gatgaagctacaaaagcagaaatattaagtcatgttagttcaac tactaattatgaagatttaaaagatatggatttaataatagaag catctgtagaagacatgaatataaagaaagatgttttcaagtta ctagatgaattatgtaaagaagatactatcttggcaacaaatac ttcatcattatctataacagaaatagcttcttctactaagcgcc cagataaagttataggaatgcatttctttaatccagttcctatg atgaaattagttgaagttataagtggtcagttaacatcaaaagt
Description Nucleotide sequences of pLogic031-nsrR-norB-butyrate construct (SEQ ID NO: 79)
tacttttgatacagtatttgaattatctaagagtatcaataaag taccagtagatgtatctgaatctcctggatttgtagtaaataga atacttatacctatgataaatgaagctgttggtatatatgcaga tggtgttgcaagtaaagaagaaatagatgaagctatgaaattag gagcaaaccatccaatgggaccactagcattaggtgatttaatc ggattagatgttgttttagctataatgaacgttttatatactga atttggagatactaaatatagacctcatccacttttagctaaaa tggttagagctaatcaattaggaagaaaaactaagataggattc tatgattataataaataataagaaggagatatacatatgagtac aagtgatgttaaagtttatgagaatgtagctgttgaagtagatg gaaatatatgtacagtgaaaatgaatagacctaaagcccttaat gcaataaattcaaagactttagaagaactttatgaagtatttgt agatattaataatgatgaaactattgatgttgtaatattgacag gggaaggaaaggcatttgtagctggagcagatattgcatacatg aaagatttagatgctgtagctgctaaagattttagtatcttagg agcaaaagcttttggagaaatagaaaatagtaaaaaagtagtga tagctgctgtaaacggatttgctttaggtggaggatgtgaactt gcaatggcatgtgatataagaattgcatctgctaaagctaaatt tggtcagccagaagtaactcttggaataactccaggatatggag gaactcaaaggcttacaagattggttggaatggcaaaagcaaaa gaattaatctttacaggtcaagttataaaagctgatgaagctga aaaaatagggctagtaaatagagtcgttgagccagacattttaa tagaagaagttgagaaattagctaagataatagctaaaaatgct cagcttgcagttagatactctaaagaagcaatacaacttggtgc tcaaactgatataaatactggaatagatatagaatctaatttat ttggtctttgtttttcaactaaagaccaaaaagaaggaatgtca gctttcgttgaaaagagagaagctaactttataaaagggtaata agaaggagatatacatatgagaagttttgaagaagtaattaagt ttgcaaaagaaagaggacctaaaactatatcagtagcatgttgc caagataaagaagttttaatggcagttgaaatggctagaaaaga aaaaatagcaaatgccattttagtaggagatatagaaaagacta aagaaattgcaaaaagcatagacatggatatcgaaaattatgaa ctgatagatataaaagatttagcagaagcatctctaaaatctgt tgaattagtttcacaaggaaaagccgacatggtaatgaaaggct tagtagacacatcaataatactaaaagcagttttaaataaagaa gtaggtcttagaactggaaatgtattaagtcacgtagcagtatt tgatgtagagggatatgatagattatttttcgtaactgacgcag ctatgaacttagctcctgatacaaatactaaaaagcaaatcata gaaaatgcttgcacagtagcacattcattagatataagtgaacc aaaagttgctgcaatatgcgcaaaagaaaaagtaaatccaaaaa tgaaagatacagttgaagctaaagaactagaagaaatgtatgaa agaggagaaatcaaaggttgtatggttggtgggccttttgcaat tgataatgcagtatctttagaagcagctaaacataaaggtataa atcatcctgtagcaggacgagctgatatattattagccccagat attgaaggtggtaacatattatataaagctttggtattcttctc aaaatcaaaaaatgcaggagttatagttggggctaaagcaccaa
Description Nucleotide sequences of pLogic031-nsrR-norB-butyrate construct (SEQ ID NO: 79)
taatattaacttctagagcagacagtgaagaaactaaactaaac tcaatagctttaggtgttttaatggcagcaaaggcataataaga aggagatatacatatgagcaaaatatttaaaatcttaacaataa atcctggttcgacatcaactaaaatagctgtatttgataatgag gatttagtatttgaaaaaactttaagacattcttcagaagaaat aggaaaatatgagaaggtgtctgaccaatttgaatttcgtaaac aagtaatagaagaagctctaaaagaaggtggagtaaaaacatct gaattagatgctgtagtaggtagaggaggacttcttaaacctat aaaaggtggtacttattcagtaagtgctgctatgattgaagatt taaaagtgggagttttaggagaacacgcttcaaacctaggtgga ataatagcaaaacaaataggtgaagaagtaaatgttccttcata catagtagaccctgttgttgtagatgaattagaagatgttgcta gaatttctggtatgcctgaaataagtagagcaagtgtagtacat gctttaaatcaaaaggcaatagcaagaagatatgctagagaaat aaacaagaaatatgaagatataaatcttatagttgcacacatgg gtggaggagtttctgttggagctcataaaaatggtaaaatagta gatgttgcaaacgcattagatggagaaggacctttctctccaga aagaagtggtggactaccagtaggtgcattagtaaaaatgtgct ttagtggaaaatatactcaagatgaaattaaaaagaaaataaaa ggtaatggcggactagttgcatacttaaacactaatgatgctag agaagttgaagaaagaattgaagctggtgatgaaaaagctaaat tagtatatgaagctatggcatatcaaatctctaaagaaatagga gctagtgctgcagttcttaagggagatgtaaaagcaatattatt aactggtggaatcgcatattcaaaaatgtttacagaaatgattg cagatagagttaaatttatagcagatgtaaaagtttatccaggt gaagatgaaatgattgcattagctcaaggtggacttagagtttt aactggtgaagaagaggctcaagtttatgataactaataa
Table 39. Nucleotide sequences of pl_ogic046-nsrR-norB-butyrate construct
Description Nucleotide sequences of pLogic046-nsrR-norB-butyrate construct
(SEQ ID NO: 80)
region of norB, gagcaacatacgagccggaagcataaagtgtaaagcctggggtgccta and a atgagttgagttgaggaattataacaggaagaaatattcctcatacgc butyrogenic ttgtaattcctctatggttgttgacaattaatcatcggctcgtataat gene cassette gtataacattcatattttgtgaattttaaactctagaaataattttgt (pLogic046-nsrR- ttaactttaagaaggagatatacatatgatcgtaaaacctatggtacg caacaatatctgcctgaacgcccatcctcagggctgcaagaagggagt norB-butyrate
ggaagatcagattgaatataccaagaaacgcattaccgcagaagtcaa construct; SEQ
agctggcgcaaaagctccaaaaaacgttctggtgcttggctgctcaaa ID NO: 80).
tggttacggcctggcgagccgcattactgctgcgttcggatacggggc tgcgaccatcggcgtgtcctttgaaaaagcgggttcagaaaccaaata tggtacaccgggatggtacaataatttggcatttgatgaagcggcaaa acgcgagggtctttatagcgtgacgatcgacggcgatgcgttttcaga cgagatcaaggcccaggtaattgaggaagccaaaaaaaaaggtatcaa atttgatctgatcgtatacagcttggccagcccagtacgtactgatcc tgatacaggtatcatgcacaaaagcgttttgaaaccctttggaaaaac gttcacaggcaaaacagtagatccgtttactggcgagctgaaggaaat ctccgcggaaccagcaaatgacgaggaagcagccgccactgttaaagt tatggggggtgaagattgggaacgttggattaagcagctgtcgaagga aggcctcttagaagaaggctgtattaccttggcctatagttatattgg ccctgaagctacccaagctttgtaccgtaaaggcacaatcggcaaggc caaagaacacctggaggccacagcacaccgtctcaacaaagagaaccc gtcaatccgtgccttcgtgagcgtgaataaaggcctggtaacccgcgc aagcgccgtaatcccggtaatccctctgtatctcgccagcttgttcaa agtaatgaaagagaagggcaatcatgaaggttgtattgaacagatcac gcgtctgtacgccgagcgcctgtaccgtaaagatggtacaattccagt tgatgaggaaaatcgcattcgcattgatgattgggagttagaagaaga cgtccagaaagcggtatccgcgttgatggagaaagtcacgggtgaaaa cgcagaatctctcactgacttagcggggtaccgccatgatttcttagc tagtaacggctttgatgtagaaggtattaattatgaagcggaagttga acgcttcgaccgtatctgataagaaggagatatacatatgagagaagt agtaattgccagtgcagctagaacagcagtaggaagttttggaggagc atttaaatcagtttcagcggtagagttaggggtaacagcagctaaaga agctataaaaagagctaacataactccagatatgatagatgaatctct tttagggggagtacttacagcaggtcttggacaaaatatagcaagaca aatagcattaggagcaggaataccagtagaaaaaccagctatgactat aaatatagtttgtggttctggattaagatctgtttcaatggcatctca acttatagcattaggtgatgctgatataatgttagttggtggagctga aaacatgagtatgtctccttatttagtaccaagtgcgagatatggtgc aagaatgggtgatgctgcttttgttgattcaatgataaaagatggatt atcagacatatttaataactatcacatgggtattactgctgaaaacat agcagagcaatggaatataactagagaagaacaagatgaattagctct tgcaagtcaaaataaagctgaaaaagctcaagctgaaggaaaatttga tgaagaaatagttcctgttgttataaaaggaagaaaaggtgacactgt agtagataaagatgaatatattaagcctggcactacaatggagaaact tgctaagttaagacctgcatttaaaaaagatggaacagttactgetgg taatgcatcaggaataaatgatggtgctgctatgttagtagtaatggc
Description Nucleotide sequences of pLogic046-nsrR-norB-butyrate construct
(SEQ ID NO: 80)
taaagaaaaagctgaagaactaggaatagagcctcttgcaactatagt ttcttatggaacagctggtgttgaccctaaaataatgggatatggacc agttccagcaactaaaaaagctttagaagctgctaatatgactattga agatatagatttagttgaagctaatgaggcatttgctgcccaatctgt agctgtaataagagacttaaatatagatatgaataaagttaatgttaa tggtggagcaatagctataggacatccaataggatgctcaggagcaag aatacttactacacttttatatgaaatgaagagaagagatgctaaaac tggtcttgctacactttgtataggcggtggaatgggaactactttaat agttaagagatagtaagaaggagatatacatatgaaattagctgtaat aggtagtggaactatgggaagtggtattgtacaaacttttgcaagttg tggacatgatgtatgtttaaagagtagaactcaaggtgctatagataa atgtttagctttattagataaaaatttaactaagttagttactaaggg aaaaatggatgaagctacaaaagcagaaatattaagtcatgttagttc aactactaattatgaagatttaaaagatatggatttaataatagaagc atctgtagaagacatgaatataaagaaagatgttttcaagttactaga tgaattatgtaaagaagatactatcttggcaacaaatacttcatcatt atctataacagaaatagcttcttctactaagcgcccagataaagttat aggaatgcatttctttaatccagttcctatgatgaaattagttgaagt tataagtggtcagttaacatcaaaagttacttttgatacagtatttga attatctaagagtatcaataaagtaccagtagatgtatctgaatctcc tggatttgtagtaaatagaatacttatacctatgataaatgaagctgt tggtatatatgcagatggtgttgcaagtaaagaagaaatagatgaagc tatgaaattaggagcaaaccatccaatgggaccactagcattaggtga tttaatcggattagatgttgttttagctataatgaacgttttatatac tgaatttggagatactaaatatagacctcatccacttttagctaaaat ggttagagctaatcaattaggaagaaaaactaagataggattctatga ttataataaataataagaaggagatatacatatgagtacaagtgatgt taaagtttatgagaatgtagctgttgaagtagatggaaatatatgtac agtgaaaatgaatagacctaaagcccttaatgcaataaattcaaagac tttagaagaactttatgaagtatttgtagatattaataatgatgaaac tattgatgttgtaatattgacaggggaaggaaaggcatttgtagctgg agcagatattgcatacatgaaagatttagatgctgtagctgctaaaga ttttagtatcttaggagcaaaagcttttggagaaatagaaaatagtaa aaaagtagtgatagctgctgtaaacggatttgctttaggtggaggatg tgaacttgcaatggcatgtgatataagaattgcatctgctaaagctaa atttggtcagccagaagtaactcttggaataactccaggatatggagg aactcaaaggcttacaagattggttggaatggcaaaagcaaaagaatt aatctttacaggtcaagttataaaagctgatgaagctgaaaaaatagg gctagtaaatagagtcgttgagccagacattttaatagaagaagttga gaaattagctaagataatagctaaaaatgctcagcttgcagttagata ctctaaagaagcaatacaacttggtgctcaaactgatataaatactgg aatagatatagaatctaatttatttggtctttgtttttcaactaaaga ccaaaaagaaggaatgtcagctttcgttgaaaagagagaagctaactt tataaaagggtaataagaaggagatatacatatgagaagttttgaaga agtaattaagtttgcaaaagaaagaggacctaaaactatatcagtagc atgttgccaagataaagaagttttaatggcagttgaaatggctagaaa
Description Nucleotide sequences of pLogic046-nsrR-norB-butyrate construct
(SEQ ID NO: 80)
agaaaaaatagcaaatgccattttagtaggagatatagaaaagactaa agaaattgcaaaaagcatagacatggatatcgaaaattatgaactgat agatataaaagatttagcagaagcatctctaaaatctgttgaattagt ttcacaaggaaaagccgacatggtaatgaaaggcttagtagacacatc aataatactaaaagcagttttaaataaagaagtaggtcttagaactgg aaatgtattaagtcacgtagcagtatttgatgtagagggatatgatag attatttttcgtaactgacgcagctatgaacttagctcctgatacaaa tactaaaaagcaaatcatagaaaatgcttgcacagtagcacattcatt agatataagtgaaccaaaagttgctgcaatatgcgcaaaagaaaaagt aaatccaaaaatgaaagatacagttgaagctaaagaactagaagaaat gtatgaaagaggagaaatcaaaggttgtatggttggtgggccttttgc aattgataatgcagtatctttagaagcagctaaacataaaggtataaa tcatcctgtagcaggacgagctgatatattattagccccagatattga aggtggtaacatattatataaagctttggtattcttctcaaaatcaaa aaatgcaggagttatagttggggctaaagcaccaataatattaacttc tagagcagacagtgaagaaactaaactaaactcaatagctttaggtgt tttaatggcagcaaaggcataataagaaggagatatacatatgagcaa aatatttaaaatcttaacaataaatcctggttcgacatcaactaaaat agctgtatttgataatgaggatttagtatttgaaaaaactttaagaca ttcttcagaagaaataggaaaatatgagaaggtgtctgaccaatttga atttcgtaaacaagtaatagaagaagctctaaaagaaggtggagtaaa aacatctgaattagatgctgtagtaggtagaggaggacttcttaaacc tataaaaggtggtacttattcagtaagtgctgctatgattgaagattt aaaagtgggagttttaggagaacacgcttcaaacctaggtggaataat agcaaaacaaataggtgaagaagtaaatgttccttcatacatagtaga ccctgttgttgtagatgaattagaagatgttgctagaatttctggtat gcctgaaataagtagagcaagtgtagtacatgctttaaatcaaaaggc aatagcaagaagatatgctagagaaataaacaagaaatatgaagatat aaatcttatagttgcacacatgggtggaggagtttctgttggagctca taaaaatggtaaaatagtagatgttgcaaacgcattagatggagaagg acctttctctccagaaagaagtggtggactaccagtaggtgcattagt aaaaatgtgctttagtggaaaatatactcaagatgaaattaaaaagaa aataaaaggtaatggcggactagttgcatacttaaacactaatgatgc tagagaagttgaagaaagaattgaagctggtgatgaaaaagctaaatt agtatatgaagctatggcatatcaaatctctaaagaaataggagctag tgctgcagttcttaagggagatgtaaaagcaatattattaactggtgg aatcgcatattcaaaaatgtttacagaaatgattgcagatagagttaa atttatagcagatgtaaaagtttatccaggtgaagatgaaatgattgc attagctcaaggtggacttagagttttaactggtgaagaagaggctca agtttatgataactaataa
[0601] The gene products of the bcd2-etfA3-etfB3 genes form a complex that converts crotonyl-CoA to butyryl-CoA and may exhibit dependence on oxygen as a co-oxidant. Because the recombinant bacteria of the invention are
designed to produce butyrate in an oxygen-limited environment (e.g. the mammalian gut), that dependence on oxygen could have a negative effect of butyrate production in the gut. It has been shown that a single gene from Treponema denticola, trans-2-enoynl-CoA reductase (ter), can functionally replace this three gene complex in an oxygen-independent manner. Therefore, a second butyrate gene cassette in which the ter gene replaces the bcd2-etfA3- etfB3 genes of the first butyrate cassette is synthesized (Genewiz, Cambridge, MA). The ter gene is codon-optimized for E. coli codon usage using Integrated DNA Technologies online codon optimization tool
(https://www.idtdna.com/CodonOpt). The second butyrate gene cassette, as well as transcriptional and translational elements, is synthesized (Gen9,
Cambridge, MA) and cloned into vector pBR322. The second butyrate gene cassette is placed under control of a FNR regulatory region as described above. In certain constructs, the butyrate gene cassette is placed under the control of an RNS-responsive regulatory region, e.g., norB, and the bacteria further comprises a gene encoding a corresponding RNS-responsive transcription factor, e.g., nsrR (see, e.g., Table 38 and Table 39). In certain constructs, the butyrate gene cassette is placed under the control of an ROS-responsive regulatory region, e.g., oxyS, and the bacteria further comprises a gene encoding a corresponding ROS-responsive transcription factor, e.g., oxyR (see, e.g., Table C and Table 40).
Table 40. ROS regulated constructs, OxyR construct, Tet-regulated constructs
Description
taagatagcaatgtctactcttgatggtggtagaattggtatagctgcacaagctttag gtttagcacaaggtgctcttgatgaaactgttaaatatgtaaaagaaagagtacaattt ggtagaccattatcaaaattccaaaatacacaattccaattagctgatatggaagttaa ggtacaagcggctagacaccttgtatatcaagcagctataaataaagacttaggaaaac cttatggagtagaagcagcaatggcaaaattatttgcagctgaaacagctatggaagtt actacaaaagctgtacaacttcatggaggatatggatacactcgtgactatccagtaga aagaatgatgagagatgctaagataactgaaatatatgaaggaactagtgaagttcaaa gaatggttatttcaggaaaactattaaaatagtaagaaggagatatacatatggaggaa ggatttatgaatatagtcgtttgtataaaacaagttccagatacaacagaagttaaact agatcctaatacaggtactttaattagagatggagtaccaagtataataaaccctgatg ataaagcaggtttagaagaagctataaaattaaaagaagaaatgggtgctcatgtaact gttataacaatgggacctcctcaagcagatatggctttaaaagaagctttagcaatggg tgcagatagaggtatattattaacagatagagcatttgcgggtgctgatacttgggcaa cttcatcagcattagcaggagcattaaaaaatatagattttgatattataatagctgga agacaggcgatagatggagatactgcacaagttggacctcaaatagctgaacatttaaa tcttccatcaataacatatgctgaagaaataaaaactgaaggtgaatatgtattagtaa aaagacaatttgaagattgttgccatgacttaaaagttaaaatgccatgccttataaca actcttaaagatatgaacacaccaagatacatgaaagttggaagaatatatgatgcttt cgaaaatgatgtagtagaaacatggactgtaaaagatatagaagttgacccttctaatt taggtcttaaaggttctccaactagtgtatttaaatcatttacaaaatcagttaaacca gctggtacaatatacaatgaagatgcgaaaacatcagctggaattatcatagataaatt aaaagagaagtatatcatataataagaaggagatatacatatgggtaacgttttagtag taatagaacaaagagaaaatgtaattcaaactgtttctttagaattactaggaaaggct acagaaatagcaaaagattatgatacaaaagtttctgcattacttttaggtagtaaggt agaaggtttaatagatacattagcacactatggtgcagatgaggtaatagtagtagatg atgaagctttagcagtgtatacaactgaaccatatacaaaagcagcttatgaagcaata aaagcagctgaccctatagttgtattatttggtgcaacttcaataggtagagatttagc gcctagagtttctgctagaatacatacaggtcttactgctgactgtacaggtcttgcag tagctgaagatacaaaattattattaatgacaagacctgcctttggtggaaatataatg gcaacaatagtttgtaaagatttcagacctcaaatgtctacagttagaccaggggttat gaagaaaaatgaacctgatgaaactaaagaagctgtaattaaccgtttcaaggtagaat ttaatgatgctgataaattagttcaagttgtacaagtaataaaagaagctaaaaaacaa gttaaaatagaagatgctaagatattagtttctgctggacgtggaatgggtggaaaaga aaacttagacatactttatgaattagctgaaattataggtggagaagtttctggttctc gtgccactatagatgcaggttggttagataaagcaagacaagttggtcaaactggtaaa actgtaagaccagacctttatatagcatgtggtatatctggagcaatacaacatatagc tggtatggaagatgctgagtttatagttgctataaataaaaatccagaagctccaatat ttaaatatgctgatgttggtatagttggagatgttcataaagtgcttccagaacttatc agtcagttaagtgttgcaaaagaaaaaggtgaagttttagctaactaataagaaggaga tatacatatgagagaagtagtaattgccagtgcagctagaacagcagtaggaagttttg gaggagcatttaaatcagtttcagcggtagagttaggggtaacagcagctaaagaagct ataaaaagagctaacataactccagatatgatagatgaatctcttttagggggagtact tacagcaggtcttggacaaaatatagcaagacaaatagcattaggagcaggaataccag tagaaaaaccagctatgactataaatatagtttgtggttctggattaagatctgtttca atggcatctcaacttatagcattaggtgatgctgatataatgttagttggtggagctga aaacatgagtatgtctccttatttagtaccaagtgcgagatatggtgcaagaatgggtg atgctgcttttgttgattcaatgataaaagatggattatcagacatatttaataactat cacatgggtattactgctgaaaacatagcagagcaatggaatataactagagaagaaca agatgaattagctcttgcaagtcaaaataaagctgaaaaagctcaagctgaaggaaaat ttgatgaagaaatagttcctgttgttataaaaggaagaaaaggtgacactgtagtagat aaagatgaatatattaagcctggcactacaatggagaaacttgctaagttaagacctgc atttaaaaaagatggaacagttactgctggtaatgcatcaggaataaatgatggtgctg ctatgttagtagtaatggctaaagaaaaagctgaagaactaggaatagagcctcttgca actatagtttcttatggaacagctggtgttgaccctaaaataatgggatatggaccagt tccagcaactaaaaaagctttagaagctgctaatatgactattgaagatatagatttag ttgaagctaatgaggcatttgctgcccaatctgtagctgtaataagagacttaaatata gatatgaataaagttaatgttaatggtggagcaatagctataggacatccaataggatg
Sequence
Description
ctcaggagcaagaatacttactacacttttatatgaaatgaagagaagagatgctaaaa ctggtcttgctacactttgtataggcggtggaatgggaactactttaatagttaagaga tagtaagaaggagatatacatatgaaattagctgtaataggtagtggaactatgggaag tggtattgtacaaacttttgcaagttgtggacatgatgtatgtttaaagagtagaactc aaggtgctatagataaatgtttagctttattagataaaaatttaactaagttagttact aagggaaaaatggatgaagctacaaaagcagaaatattaagtcatgttagttcaactac taattatgaagatttaaaagatatggatttaataatagaagcatctgtagaagacatga atataaagaaagatgttttcaagttactagatgaattatgtaaagaagatactatcttg gcaacaaatacttcatcattatctataacagaaatagcttcttctactaagcgcccaga taaagttataggaatgcatttctttaatccagttcctatgatgaaattagttgaagtta taagtggtcagttaacatcaaaagttacttttgatacagtatttgaattatctaagagt atcaataaagtaccagtagatgtatctgaatctcctggatttgtagtaaatagaatact tatacctatgataaatgaagctgttggtatatatgcagatggtgttgcaagtaaagaag aaatagatgaagctatgaaattaggagcaaaccatccaatgggaccactagcattaggt gatttaatcggattagatgttgttttagctataatgaacgttttatatactgaatttgg agatactaaatatagacctcatccacttttagctaaaatggttagagctaatcaattag gaagaaaaactaagataggattctatgattataataaataataagaaggagatatacat atgagtacaagtgatgttaaagtttatgagaatgtagctgttgaagtagatggaaatat atgtacagtgaaaatgaatagacctaaagcccttaatgcaataaattcaaagactttag aagaactttatgaagtatttgtagatattaataatgatgaaactattgatgttgtaata ttgacaggggaaggaaaggcatttgtagctggagcagatattgcatacatgaaagattt agatgctgtagctgctaaagattttagtatcttaggagcaaaagcttttggagaaatag aaaatagtaaaaaagtagtgatagctgctgtaaacggatttgctttaggtggaggatgt gaacttgcaatggcatgtgatataagaattgcatctgctaaagctaaatttggtcagcc agaagtaactcttggaataactccaggatatggaggaactcaaaggcttacaagattgg ttggaatggcaaaagcaaaagaattaatctttacaggtcaagttataaaagctgatgaa gctgaaaaaatagggctagtaaatagagtcgttgagccagacattttaatagaagaagt tgagaaattagctaagataatagctaaaaatgctcagcttgcagttagatactctaaag aagcaatacaacttggtgctcaaactgatataaatactggaatagatatagaatctaat ttatttggtctttgtttttcaactaaagaccaaaaagaaggaatgtcagctttcgttga aaagagagaagctaactttataaaagggtaataagaaggagatatacatatgagaagtt ttgaagaagtaattaagtttgcaaaagaaagaggacctaaaactatatcagtagcatgt tgccaagataaagaagttttaatggcagttgaaatggctagaaaagaaaaaatagcaaa tgccattttagtaggagatatagaaaagactaaagaaattgcaaaaagcatagacatgg atatcgaaaattatgaactgatagatataaaagatttagcagaagcatctctaaaatct gttgaattagtttcacaaggaaaagccgacatggtaatgaaaggcttagtagacacatc aataatactaaaagcagttttaaataaagaagtaggtcttagaactggaaatgtattaa gtcacgtagcagtatttgatgtagagggatatgatagattatttttcgtaactgacgca gctatgaacttagctcctgatacaaatactaaaaagcaaatcatagaaaatgcttgcac agtagcacattcattagatataagtgaaccaaaagttgctgcaatatgcgcaaaagaaa aagtaaatccaaaaatgaaagatacagttgaagctaaagaactagaagaaatgtatgaa agaggagaaatcaaaggttgtatggttggtgggccttttgcaattgataatgcagtatc tttagaagcagctaaacataaaggtataaatcatcctgtagcaggacgagctgatatat tattagccccagatattgaaggtggtaacatattatataaagctttggtattcttctca aaatcaaaaaatgcaggagttatagttggggctaaagcaccaataatattaacttctag agcagacagtgaagaaactaaactaaactcaatagctttaggtgttttaatggcagcaa aggcataataagaaggagatatacatatgagcaaaatatttaaaatcttaacaataaat cctggttcgacatcaactaaaatagctgtatttgataatgaggatttagtatttgaaaa aactttaagacattcttcagaagaaataggaaaatatgagaaggtgtctgaccaatttg aatttcgtaaacaagtaatagaagaagctctaaaagaaggtggagtaaaaacatctgaa ttagatgctgtagtaggtagaggaggacttcttaaacctataaaaggtggtacttattc agtaagtgctgctatgattgaagatttaaaagtgggagttttaggagaacacgcttcaa acctaggtggaataatagcaaaacaaataggtgaagaagtaaatgttccttcatacata gtagaccctgttgttgtagatgaattagaagatgttgctagaatttctggtatgcctga aataagtagagcaagtgtagtacatgctttaaatcaaaaggcaatagcaagaagatatg ctagagaaataaacaagaaatatgaagatataaatcttatagttgcacacatgggtgga ggagtttctgttggagctcataaaaatggtaaaatagtagatgttgcaaacgcattaga
Sequence
Description
tggagaaggacctttctctccagaaagaagtggtggactaccagtaggtgcattagtaa aaatgtgctttagtggaaaatatactcaagatgaaattaaaaagaaaataaaaggtaat ggcggactagttgcatacttaaacactaatgatgctagagaagttgaagaaagaattga agctggtgatgaaaaagctaaattagtatatgaagctatggcatatcaaatctctaaag aaataggagctagtgctgcagttcttaagggagatgtaaaagcaatattattaactggt ggaatcgcatattcaaaaatgtttacagaaatgattgcagatagagttaaatttatagc agatgtaaaagtttatccaggtgaagatgaaatgattgcattagctcaaggtggactta gagttttaactggtgaagaagaggctcaagtttatgataactaataa
Nucleotide ctcgagttcattatccatcctccatcgccacgatagttcatggcgataggtagaatagc aatgaacgattatccctatcaagcattctgactgataattgctcacacgaattcattaa sequences of agaggagaaaggtaccatgatcgtaaaacctatggtacgcaacaatatctgcctgaacg pLogic046- cccatcctcagggctgcaagaagggagtggaagatcagattgaatataccaagaaacgc oxyS- attaccgcagaagtcaaagctggcgcaaaagctccaaaaaacgttctggtgcttggctg butyrate ctcaaatggttacggcctggcgagccgcattactgctgcgttcggatacggggctgcga ccatcggcgtgtcctttgaaaaagcgggttcagaaaccaaatatggtacaccgggatgg construct tacaataatttggcatttgatgaagcggcaaaacgcgagggtctttatagcgtgacgat
(SEQ ID NO: cgacggcgatgcgttttcagacgagatcaaggcccaggtaattgaggaagccaaaaaaa
82) aaggtatcaaatttgatctgatcgtatacagcttggccagcccagtacgtactgatcct gatacaggtatcatgcacaaaagcgttttgaaaccctttggaaaaacgttcacaggcaa aacagtagatccgtttactggcgagctgaaggaaatctccgcggaaccagcaaatgacg aggaagcagccgccactgttaaagttatggggggtgaagattgggaacgttggattaag cagctgtcgaaggaaggcctcttagaagaaggctgtattaccttggcctatagttatat tggccctgaagctacccaagctttgtaccgtaaaggcacaatcggcaaggccaaagaac acctggaggccacagcacaccgtctcaacaaagagaacccgtcaatccgtgccttcgtg agcgtgaataaaggcctggtaacccgcgcaagcgccgtaatcccggtaatccctctgta tctcgccagcttgttcaaagtaatgaaagagaagggcaatcatgaaggttgtattgaac agatcacgcgtctgtacgccgagcgcctgtaccgtaaagatggtacaattccagttgat gaggaaaatcgcattcgcattgatgattgggagttagaagaagacgtccagaaagcggt atccgcgttgatggagaaagtcacgggtgaaaacgcagaatctctcactgacttagcgg ggtaccgccatgatttcttagctagtaacggctttgatgtagaaggtattaattatgaa gcggaagttgaacgcttcgaccgtatctgataagaaggagatatacatatgagagaagt agtaattgccagtgcagctagaacagcagtaggaagttttggaggagcatttaaatcag tttcagcggtagagttaggggtaacagcagctaaagaagctataaaaagagctaacata actccagatatgatagatgaatctcttttagggggagtacttacagcaggtcttggaca aaatatagcaagacaaatagcattaggagcaggaataccagtagaaaaaccagctatga ctataaatatagtttgtggttctggattaagatctgtttcaatggcatctcaacttata gcattaggtgatgctgatataatgttagttggtggagctgaaaacatgagtatgtctcc ttatttagtaccaagtgcgagatatggtgcaagaatgggtgatgctgcttttgttgatt caatgataaaagatggattatcagacatatttaataactatcacatgggtattactgct gaaaacatagcagagcaatggaatataactagagaagaacaagatgaattagctcttgc aagtcaaaataaagctgaaaaagctcaagctgaaggaaaatttgatgaagaaatagttc ctgttgttataaaaggaagaaaaggtgacactgtagtagataaagatgaatatattaag cctggcactacaatggagaaacttgctaagttaagacctgcatttaaaaaagatggaac agttactgctggtaatgcatcaggaataaatgatggtgctgctatgttagtagtaatgg ctaaagaaaaagctgaagaactaggaatagagcctcttgcaactatagtttcttatgga acagctggtgttgaccctaaaataatgggatatggaccagttccagcaactaaaaaagc tttagaagctgctaatatgactattgaagatatagatttagttgaagctaatgaggcat ttgctgcccaatctgtagctgtaataagagacttaaatatagatatgaataaagttaat gttaatggtggagcaatagctataggacatccaataggatgctcaggagcaagaatact tactacacttttatatgaaatgaagagaagagatgctaaaactggtcttgctacacttt gtataggcggtggaatgggaactactttaatagttaagagatagtaagaaggagatata catatgaaattagctgtaataggtagtggaactatgggaagtggtattgtacaaacttt tgcaagttgtggacatgatgtatgtttaaagagtagaactcaaggtgctatagataaat gtttagctttattagataaaaatttaactaagttagttactaagggaaaaatggatgaa gctacaaaagcagaaatattaagtcatgttagttcaactactaattatgaagatttaaa agatatggatttaataatagaagcatctgtagaagacatgaatataaagaaagatgttt tcaagttactagatgaattatgtaaagaagatactatcttggcaacaaatacttcatca
Sequence
Description
ttatctataacagaaatagcttcttctactaagcgcccagataaagttataggaatgca tttctttaatccagttcctatgatgaaattagttgaagttataagtggtcagttaacat caaaagttacttttgatacagtatttgaattatctaagagtatcaataaagtaccagta gatgtatctgaatctcctggatttgtagtaaatagaatacttatacctatgataaatga agctgttggtatatatgcagatggtgttgcaagtaaagaagaaatagatgaagctatga aattaggagcaaaccatccaatgggaccactagcattaggtgatttaatcggattagat gttgttttagctataatgaacgttttatatactgaatttggagatactaaatatagacc tcatccacttttagctaaaatggttagagctaatcaattaggaagaaaaactaagatag gattctatgattataataaataataagaaggagatatacatatgagtacaagtgatgtt aaagtttatgagaatgtagctgttgaagtagatggaaatatatgtacagtgaaaatgaa tagacctaaagcccttaatgcaataaattcaaagactttagaagaactttatgaagtat ttgtagatattaataatgatgaaactattgatgttgtaatattgacaggggaaggaaag gcatttgtagctggagcagatattgcatacatgaaagatttagatgctgtagctgctaa agattttagtatcttaggagcaaaagcttttggagaaatagaaaatagtaaaaaagtag tgatagctgctgtaaacggatttgctttaggtggaggatgtgaacttgcaatggcatgt gatataagaattgcatctgctaaagctaaatttggtcagccagaagtaactcttggaat aactccaggatatggaggaactcaaaggcttacaagattggttggaatggcaaaagcaa aagaattaatctttacaggtcaagttataaaagctgatgaagctgaaaaaatagggcta gtaaatagagtcgttgagccagacattttaatagaagaagttgagaaattagctaagat aatagctaaaaatgctcagcttgcagttagatactctaaagaagcaatacaacttggtg ctcaaactgatataaatactggaatagatatagaatctaatttatttggtctttgtttt tcaactaaagaccaaaaagaaggaatgtcagctttcgttgaaaagagagaagctaactt tataaaagggtaataagaaggagatatacatatgagaagttttgaagaagtaattaagt ttgcaaaagaaagaggacctaaaactatatcagtagcatgttgccaagataaagaagtt ttaatggcagttgaaatggctagaaaagaaaaaatagcaaatgccattttagtaggaga tatagaaaagactaaagaaattgcaaaaagcatagacatggatatcgaaaattatgaac tgatagatataaaagatttagcagaagcatctctaaaatctgttgaattagtttcacaa ggaaaagccgacatggtaatgaaaggcttagtagacacatcaataatactaaaagcagt tttaaataaagaagtaggtcttagaactggaaatgtattaagtcacgtagcagtatttg atgtagagggatatgatagattatttttcgtaactgacgcagctatgaacttagctcct gatacaaatactaaaaagcaaatcatagaaaatgcttgcacagtagcacattcattaga tataagtgaaccaaaagttgctgcaatatgcgcaaaagaaaaagtaaatccaaaaatga aagatacagttgaagctaaagaactagaagaaatgtatgaaagaggagaaatcaaaggt tgtatggttggtgggccttttgcaattgataatgcagtatctttagaagcagctaaaca taaaggtataaatcatcctgtagcaggacgagctgatatattattagccccagatattg aaggtggtaacatattatataaagctttggtattcttctcaaaatcaaaaaatgcagga gttatagttggggctaaagcaccaataatattaacttctagagcagacagtgaagaaac taaactaaactcaatagctttaggtgttttaatggcagcaaaggcataataagaaggag atatacatatgagcaaaatatttaaaatcttaacaataaatcctggttcgacatcaact aaaatagctgtatttgataatgaggatttagtatttgaaaaaactttaagacattcttc agaagaaataggaaaatatgagaaggtgtctgaccaatttgaatttcgtaaacaagtaa tagaagaagctctaaaagaaggtggagtaaaaacatctgaattagatgctgtagtaggt agaggaggacttcttaaacctataaaaggtggtacttattcagtaagtgctgctatgat tgaagatttaaaagtgggagttttaggagaacacgcttcaaacctaggtggaataatag caaaacaaataggtgaagaagtaaatgttccttcatacatagtagaccctgttgttgta gatgaattagaagatgttgctagaatttctggtatgcctgaaataagtagagcaagtgt agtacatgctttaaatcaaaaggcaatagcaagaagatatgctagagaaataaacaaga aatatgaagatataaatcttatagttgcacacatgggtggaggagtttctgttggagct cataaaaatggtaaaatagtagatgttgcaaacgcattagatggagaaggacctttctc tccagaaagaagtggtggactaccagtaggtgcattagtaaaaatgtgctttagtggaa aatatactcaagatgaaattaaaaagaaaataaaaggtaatggcggactagttgcatac ttaaacactaatgatgctagagaagttgaagaaagaattgaagctggtgatgaaaaagc taaattagtatatgaagctatggcatatcaaatctctaaagaaataggagctagtgctg cagttcttaagggagatgtaaaagcaatattattaactggtggaatcgcatattcaaaa atgtttacagaaatgattgcagatagagttaaatttatagcagatgtaaaagtttatcc aggtgaagatgaaatgattgcattagctcaaggtggacttagagttttaactggtgaag aagaggctcaagtttatgataactaataa
Sequence
Description
Nucleotide ctcgagatgctagcaattgtgagcggataacaattgacattgtgagcggataacaagat actgagcacatcagcaggacgcactgaccttaattaaaagaattcattaaagaggagaa sequences of aggtaccatgaatattcgtgatcttgagtacctggtggcattggctgaacaccgccatt pZA22-oxyR ttcggcgtgcggcagattcctgccacgttagccagccgacgcttagcgggcaaattcgt constrcut aagctggaagatgagctgggcgtgatgttgctggagcggaccagccgtaaagtgttgtt
(SEQ ID NO: cacccaggcgggaatgctgctggtggatcaggcgcgtaccgtgctgcgtgaggtgaaag tccttaaagagatggcaagccagcagggcgagacgatgtccggaccgctgcacattggt
83) ttgattcccacagttggaccgtacctgctaccgcatattatccctatgctgcaccagac ctttccaaagctggaaatgtatctgcatgaagcacagacccaccagttactggcgcaac tggacagcggcaaactcgattgcgtgatcctcgcgctggtgaaagagagcgaagcattc attgaagtgccgttgtttgatgagccaatgttgctggctatctatgaagatcacccgtg ggcgaaccgcgaatgcgtaccgatggccgatctggcaggggaaaaactgctgatgctgg aagatggtcactgtttgcgcgatcaggcaatgggtttctgttttgaagccggggcggat gaagatacacacttccgcgcgaccagcctggaaactctgcgcaacatggtggcggcagg tagcgggatcactttactgccagcgctggctgtgccgccggagcgcaaacgcgatgggg ttgtttatctgccgtgcattaagccggaaccacgccgcactattggcctggtttatcgt cctggctcaccgctgcgcagccgctatgagcagctggcagaggccatccgcgcaagaat ggatggccatttcgataaagttttaaaacaggcggtttaaggatcccatggtacgcgtg ctagaggcatcaaataaaacgaaaggctcagtcgaaagactgggcctttcgttttatct gttgtttgtcggtgaacgctctcctgagtaggacaaatccgccgccctagacctagggg atatattccgcttcctcgctcactgactcgctacgctcggtcgttcgactgcggcgagc ggaaatggcttacgaacggggcggagatttcctggaagatgccaggaagatacttaaca gggaagtgagagggccgcggcaaagccgtttttccataggctccgcccccctgacaagc atcacgaaatctgacgctcaaatcagtggtggcgaaacccgacaggactataaagatac caggcgtttccccctggcggctccctcgtgcgctctcctgttcctgcctttcggtttac cggtgtcattccgctgttatggccgcgtttgtctcattccacgcctgacactcagttcc gggtaggcagttcgctccaagctggactgtatgcacgaaccccccgttcagtccgaccg ctgcgccttatccggtaactatcgtcttgagtccaacccggaaagacatgcaaaagcac cactggcagcagccactggtaattgatttagaggagttagtcttgaagtcatgcgccgg ttaaggctaaactgaaaggacaagttttggtgactgcgctcctccaagccagttacctc ggttcaaagagttggtagctcagagaaccttcgaaaaaccgccctgcaaggcggttttt tcgttttcagagcaagagattacgcgcagaccaaaacgatctcaagaagatcatcttat taatcagataaaatatttctagatttcagtgcaatttatctcttcaaatgtagcacctg aagtcagccccatacgatataagttgttactagtgcttggattctcaccaataaaaaac gcccggcggcaaccgagcgttctgaacaaatccagatggagttctgaggtcattactgg atctatcaacaggagtccaagcgagctctcgaaccccagagtcccgctcagaagaactc gtcaagaaggcgatagaaggcgatgcgctgcgaatcgggagcggcgataccgtaaagca cgaggaagcggtcagcccattcgccgccaagctcttcagcaatatcacgggtagccaac gctatgtcctgatagcggtccgccacacccagccggccacagtcgatgaatccagaaaa gcggccattttccaccatgatattcggcaagcaggcatcgccatgggtcacgacgagat cctcgccgtcgggcatgcgcgccttgagcctggcgaacagttcggctggcgcgagcccc tgatgctcttcgtccagatcatcctgatcgacaagaccggcttccatccgagtacgtgc tcgctcgatgcgatgtttcgcttggtggtcgaatgggcaggtagccggatcaagcgtat gcagccgccgcattgcatcagccatgatggatactttctcggcaggagcaaggtgagat gacaggagatcctgccccggcacttcgcccaatagcagccagtcccttcccgcttcagt gacaacgtcgagcacagctgcgcaaggaacgcccgtcgtggccagccacgatagccgcg ctgcctcgtcctgcagttcattcagggcaccggacaggtcggtcttgacaaaaagaacc gggcgcccctgcgctgacagccggaacacggcggcatcagagcagccgattgtctgttg tgcccagtcatagccgaatagcctctccacccaagcggccggagaacctgcgtgcaatc catcttgttcaatcatgcgaaacgatcctcatcctgtctcttgatcagatcttgatccc ctgcgccatcagatccttggcggcaagaaagccatccagtttactttgcagggcttccc aaccttaccagagggcgccccagctggcaattccgacgtctaagaaaccattattatca tgacattaacctataaaaataggcgtatcacgaggccctttcgtcttcac
Nucleotide gtaaaacgacggccagtgaattcgttaagacccactttcacatttaagttgtttttcta atccgcatatgatcaattcaaggccgaataagaaggctggctctgcaccttggtgatca sequences of aataattcgatagcttgtcgtaataatggcggcatactatcagtagtaggtgtttccct pLogic031- ttcttctttagcgacttgatgctcttgatcttccaatacgcaacctaaagtaaaatgcc
Sequence
Description
tet-butyrate ccacagcgctgagtgcatataatgcattctctagtgaaaaaccttgttggcataaaaag gctaattgattttcgagagtttcatactgtttttctgtaggccgtgtacctaaatgtac construct ttttgctccatcgcgatgacttagtaaagcacatctaaaacttttagcgttattacgta
(SEQ ID NO: aaaaatcttgccagctttccccttctaaagggcaaaagtgagtatggtgcctatctaac
84) The atctcaatggctaaggcgtcgagcaaagcccgcttattttttacatgccaatacaatgt sequence aggctgctctacacctagcttctgggcgagtttacgggttgttaaaccttcgattccga cctcattaagcagctctaatgcgctgttaatcactttacttttatctaatctagacatc encoding attaattcctaatttttgttgacactctatcattgatagagttattttaccactcccta| TetR is tcagtgatagagaaaagtgaactctagaaataattttgtttaactttaagaaggagata underlined, tacatatggatttaaattctaaaaaatatcagatgcttaaagagctatatgtaagcttc and the gctgaaaatgaagttaaacctttagcaacagaacttgatgaagaagaaagatttcctta tgaaacagtggaaaaaatggcaaaagcaggaatgatgggtataccatatccaaaagaat overlapping atggtggagaaggtggagacactgtaggatatataatggcagttgaagaattgtctaga tetR/tetA gtttgtggtactacaggagttatattatcagctcatacatctcttggctcatggcctat promoters atatcaatatggtaatgaagaacaaaaacaaaaattcttaagaccactagcaagtggag aaaaattaggagcatttggtcttactgagcctaatgctggtacagatgcgtctggccaa are boxed. caaacaactgctgttttagacggggatgaatacatacttaatggctcaaaaatatttat aacaaacgcaatagctggtgacatatatgtagtaatggcaatgactgataaatctaagg ggaacaaaggaatatcagcatttatagttgaaaaaggaactcctgggtttagctttgga gttaaagaaaagaaaatgggtataagaggttcagctacgagtgaattaatatttgagga ttgcagaatacctaaagaaaatttacttggaaaagaaggtcaaggatttaagatagcaa tgtctactcttgatggtggtagaattggtatagctgcacaagctttaggtttagcacaa ggtgctcttgatgaaactgttaaatatgtaaaagaaagagtacaatttggtagaccatt atcaaaattccaaaatacacaattccaattagctgatatggaagttaaggtacaagcgg ctagacaccttgtatatcaagcagctataaataaagacttaggaaaaccttatggagta gaagcagcaatggcaaaattatttgcagctgaaacagctatggaagttactacaaaagc tgtacaacttcatggaggatatggatacactcgtgactatccagtagaaagaatgatga gagatgctaagataactgaaatatatgaaggaactagtgaagttcaaagaatggttatt tcaggaaaactattaaaatagtaagaaggagatatacatatggaggaaggatttatgaa tatagtcgtttgtataaaacaagttccagatacaacagaagttaaactagatcctaata caggtactttaattagagatggagtaccaagtataataaaccctgatgataaagcaggt ttagaagaagctataaaattaaaagaagaaatgggtgctcatgtaactgttataacaat gggacctcctcaagcagatatggctttaaaagaagctttagcaatgggtgcagatagag gtatattattaacagatagagcatttgcgggtgctgatacttgggcaacttcatcagca ttagcaggagcattaaaaaatatagattttgatattataatagctggaagacaggcgat agatggagatactgcacaagttggacctcaaatagctgaacatttaaatcttccatcaa taacatatgctgaagaaataaaaactgaaggtgaatatgtattagtaaaaagacaattt gaagattgttgccatgacttaaaagttaaaatgccatgccttataacaactcttaaaga tatgaacacaccaagatacatgaaagttggaagaatatatgatgctttcgaaaatgatg tagtagaaacatggactgtaaaagatatagaagttgacccttctaatttaggtcttaaa ggttctccaactagtgtatttaaatcatttacaaaatcagttaaaccagctggtacaat atacaatgaagatgcgaaaacatcagctggaattatcatagataaattaaaagagaagt atatcatataataagaaggagatatacatatgggtaacgttttagtagtaatagaacaa agagaaaatgtaattcaaactgtttctttagaattactaggaaaggctacagaaatagc aaaagattatgatacaaaagtttctgcattacttttaggtagtaaggtagaaggtttaa tagatacattagcacactatggtgcagatgaggtaatagtagtagatgatgaagcttta gcagtgtatacaactgaaccatatacaaaagcagcttatgaagcaataaaagcagctga ccctatagttgtattatttggtgcaacttcaataggtagagatttagcgcctagagttt ctgctagaatacatacaggtcttactgctgactgtacaggtcttgcagtagctgaagat acaaaattattattaatgacaagacctgcctttggtggaaatataatggcaacaatagt ttgtaaagatttcagacctcaaatgtctacagttagaccaggggttatgaagaaaaatg aacctgatgaaactaaagaagctgtaattaaccgtttcaaggtagaatttaatgatgct gataaattagttcaagttgtacaagtaataaaagaagctaaaaaacaagttaaaataga agatgctaagatattagtttctgctggacgtggaatgggtggaaaagaaaacttagaca tactttatgaattagctgaaattataggtggagaagtttctggttctcgtgccactata gatgcaggttggttagataaagcaagacaagttggtcaaactggtaaaactgtaagacc agacctttatatagcatgtggtatatctggagcaatacaacatatagctggtatggaag
Sequence
Description
atgctgagtttatagttgctataaataaaaatccagaagctccaatatttaaatatgct gatgttggtatagttggagatgttcataaagtgcttccagaacttatcagtcagttaag tgttgcaaaagaaaaaggtgaagttttagctaactaataagaaggagatatacatatga gagaagtagtaattgccagtgcagctagaacagcagtaggaagttttggaggagcattt aaatcagtttcagcggtagagttaggggtaacagcagctaaagaagctataaaaagagc taacataactccagatatgatagatgaatctcttttagggggagtacttacagcaggtc ttggacaaaatatagcaagacaaatagcattaggagcaggaataccagtagaaaaacca gctatgactataaatatagtttgtggttctggattaagatctgtttcaatggcatctca acttatagcattaggtgatgctgatataatgttagttggtggagctgaaaacatgagta tgtctccttatttagtaccaagtgcgagatatggtgcaagaatgggtgatgctgctttt gttgattcaatgataaaagatggattatcagacatatttaataactatcacatgggtat tactgctgaaaacatagcagagcaatggaatataactagagaagaacaagatgaattag ctcttgcaagtcaaaataaagctgaaaaagctcaagctgaaggaaaatttgatgaagaa atagttcctgttgttataaaaggaagaaaaggtgacactgtagtagataaagatgaata tattaagcctggcactacaatggagaaacttgctaagttaagacctgcatttaaaaaag atggaacagttactgctggtaatgcatcaggaataaatgatggtgctgctatgttagta gtaatggctaaagaaaaagctgaagaactaggaatagagcctcttgcaactatagtttc ttatggaacagctggtgttgaccctaaaataatgggatatggaccagttccagcaacta aaaaagctttagaagctgctaatatgactattgaagatatagatttagttgaagctaat gaggcatttgctgcccaatctgtagctgtaataagagacttaaatatagatatgaataa agttaatgttaatggtggagcaatagctataggacatccaataggatgctcaggagcaa gaatacttactacacttttatatgaaatgaagagaagagatgctaaaactggtcttgct acactttgtataggcggtggaatgggaactactttaatagttaagagatagtaagaagg agatatacatatgaaattagctgtaataggtagtggaactatgggaagtggtattgtac aaacttttgcaagttgtggacatgatgtatgtttaaagagtagaactcaaggtgctata gataaatgtttagctttattagataaaaatttaactaagttagttactaagggaaaaat ggatgaagctacaaaagcagaaatattaagtcatgttagttcaactactaattatgaag atttaaaagatatggatttaataatagaagcatctgtagaagacatgaatataaagaaa gatgttttcaagttactagatgaattatgtaaagaagatactatcttggcaacaaatac ttcatcattatctataacagaaatagcttcttctactaagcgcccagataaagttatag gaatgcatttctttaatccagttcctatgatgaaattagttgaagttataagtggtcag ttaacatcaaaagttacttttgatacagtatttgaattatctaagagtatcaataaagt accagtagatgtatctgaatctcctggatttgtagtaaatagaatacttatacctatga taaatgaagctgttggtatatatgcagatggtgttgcaagtaaagaagaaatagatgaa gctatgaaattaggagcaaaccatccaatgggaccactagcattaggtgatttaatcgg attagatgttgttttagctataatgaacgttttatatactgaatttggagatactaaat atagacctcatccacttttagctaaaatggttagagctaatcaattaggaagaaaaact aagataggattctatgattataataaataataagaaggagatatacatatgagtacaag tgatgttaaagtttatgagaatgtagctgttgaagtagatggaaatatatgtacagtga aaatgaatagacctaaagcccttaatgcaataaattcaaagactttagaagaactttat gaagtatttgtagatattaataatgatgaaactattgatgttgtaatattgacagggga aggaaaggcatttgtagctggagcagatattgcatacatgaaagatttagatgctgtag ctgctaaagattttagtatcttaggagcaaaagcttttggagaaatagaaaatagtaaa aaagtagtgatagctgctgtaaacggatttgctttaggtggaggatgtgaacttgcaat ggcatgtgatataagaattgcatctgctaaagctaaatttggtcagccagaagtaactc ttggaataactccaggatatggaggaactcaaaggcttacaagattggttggaatggca aaagcaaaagaattaatctttacaggtcaagttataaaagctgatgaagctgaaaaaat agggctagtaaatagagtcgttgagccagacattttaatagaagaagttgagaaattag ctaagataatagctaaaaatgctcagcttgcagttagatactctaaagaagcaatacaa cttggtgctcaaactgatataaatactggaatagatatagaatctaatttatttggtct ttgtttttcaactaaagaccaaaaagaaggaatgtcagctttcgttgaaaagagagaag ctaactttataaaagggtaataagaaggagatatacatatgagaagttttgaagaagta attaagtttgcaaaagaaagaggacctaaaactatatcagtagcatgttgccaagataa agaagttttaatggcagttgaaatggctagaaaagaaaaaatagcaaatgccattttag taggagatatagaaaagactaaagaaattgcaaaaagcatagacatggatatcgaaaat tatgaactgatagatataaaagatttagcagaagcatctctaaaatctgttgaattagt ttcacaaggaaaagccgacatggtaatgaaaggcttagtagacacatcaataatactaa
Sequence
Description
aagcagttttaaataaagaagtaggtcttagaactggaaatgtattaagtcacgtagca gtatttgatgtagagggatatgatagattatttttcgtaactgacgcagctatgaactt agctcctgatacaaatactaaaaagcaaatcatagaaaatgcttgcacagtagcacatt cattagatataagtgaaccaaaagttgctgcaatatgcgcaaaagaaaaagtaaatcca aaaatgaaagatacagttgaagctaaagaactagaagaaatgtatgaaagaggagaaat caaaggttgtatggttggtgggccttttgcaattgataatgcagtatctttagaagcag ctaaacataaaggtataaatcatcctgtagcaggacgagctgatatattattagcccca gatattgaaggtggtaacatattatataaagctttggtattcttctcaaaatcaaaaaa tgcaggagttatagttggggctaaagcaccaataatattaacttctagagcagacagtg aagaaactaaactaaactcaatagctttaggtgttttaatggcagcaaaggcataataa gaaggagatatacatatgagcaaaatatttaaaatcttaacaataaatcctggttcgac atcaactaaaatagctgtatttgataatgaggatttagtatttgaaaaaactttaagac attcttcagaagaaataggaaaatatgagaaggtgtctgaccaatttgaatttcgtaaa caagtaatagaagaagctctaaaagaaggtggagtaaaaacatctgaattagatgctgt agtaggtagaggaggacttcttaaacctataaaaggtggtacttattcagtaagtgctg ctatgattgaagatttaaaagtgggagttttaggagaacacgcttcaaacctaggtgga ataatagcaaaacaaataggtgaagaagtaaatgttccttcatacatagtagaccctgt tgttgtagatgaattagaagatgttgctagaatttctggtatgcctgaaataagtagag caagtgtagtacatgctttaaatcaaaaggcaatagcaagaagatatgctagagaaata aacaagaaatatgaagatataaatcttatagttgcacacatgggtggaggagtttctgt tggagctcataaaaatggtaaaatagtagatgttgcaaacgcattagatggagaaggac ctttctctccagaaagaagtggtggactaccagtaggtgcattagtaaaaatgtgcttt agtggaaaatatactcaagatgaaattaaaaagaaaataaaaggtaatggcggactagt tgcatacttaaacactaatgatgctagagaagttgaagaaagaattgaagctggtgatg aaaaagctaaattagtatatgaagctatggcatatcaaatctctaaagaaataggagct agtgctgcagttcttaagggagatgtaaaagcaatattattaactggtggaatcgcata ttcaaaaatgtttacagaaatgattgcagatagagttaaatttatagcagatgtaaaag tttatccaggtgaagatgaaatgattgcattagctcaaggtggacttagagttttaact ggtgaagaagaggctcaagtttatgataactaataa
Nucleotide gtaaaacgacggccagtgaattcgttaagacccactttcacatttaagttgtttttcta atccgcatatgatcaattcaaggccgaataagaaggctggctctgcaccttggtgatca sequences of aataattcgatagcttgtcgtaataatggcggcatactatcagtagtaggtgtttccct pLogic046- ttcttctttagcgacttgatgctcttgatcttccaatacgcaacctaaagtaaaatgcc tet-butyrate ccacagcgctgagtgcatataatgcattctctagtgaaaaaccttgttggcataaaaag construct gctaattgattttcgagagtttcatactgtttttctgtaggccgtgtacctaaatgtac ttttgctccatcgcgatgacttagtaaagcacatctaaaacttttagcgttattacgta
(SEQ ID NO: aaaaatcttgccagctttccccttctaaagggcaaaagtgagtatggtgcctatctaac
85)The atctcaatggctaaggcgtcgagcaaagcccgcttattttttacatgccaatacaatgt sequence aggctgctctacacctagcttctgggcgagtttacgggttgttaaaccttcgattccga cctcattaagcagctctaatgcgctgttaatcactttacttttatctaatctagacatc encoding attaattcctaatttttgttgacactctatcattgatagagttattttaccactcccta
TetR is tcagtgatagagaaaagtgaactctagaaataattttgtttaactttaagaaggagata underlined, tacatatgatcgtaaaacctatggtacgcaacaatatctgcctgaacgcccatcctcag and the ggctgcaagaagggagtggaagatcagattgaatataccaagaaacgcattaccgcaga agtcaaagctggcgcaaaagctccaaaaaacgttctggtgcttggctgctcaaatggtt overlapping acggcctggcgagccgcattactgctgcgttcggatacggggctgcgaccatcggcgtg tetR/tetA tcctttgaaaaagcgggttcagaaaccaaatatggtacaccgggatggtacaataattt promoters ggcatttgatgaagcggcaaaacgcgagggtctttatagcgtgacgatcgacggcgatg cgttttcagacgagatcaaggcccaggtaattgaggaagccaaaaaaaaaggtatcaaa are boxed.
tttgatctgatcgtatacagcttggccagcccagtacgtactgatcctgatacaggtat catgcacaaaagcgttttgaaaccctttggaaaaacgttcacaggcaaaacagtagatc cgtttactggcgagctgaaggaaatctccgcggaaccagcaaatgacgaggaagcagcc gccactgttaaagttatggggggtgaagattgggaacgttggattaagcagctgtcgaa ggaaggcctcttagaagaaggctgtattaccttggcctatagttatattggccctgaag ctacccaagctttgtaccgtaaaggcacaatcggcaaggccaaagaacacctggaggcc acagcacaccgtctcaacaaagagaacccgtcaatccgtgccttcgtgagcgtgaataa
Sequence
Description
aggcctggtaacccgcgcaagcgccgtaatcccggtaatccctctgtatctcgccagct tgttcaaagtaatgaaagagaagggcaatcatgaaggttgtattgaacagatcacgcgt ctgtacgccgagcgcctgtaccgtaaagatggtacaattccagttgatgaggaaaatcg cattcgcattgatgattgggagttagaagaagacgtccagaaagcggtatccgcgttga tggagaaagtcacgggtgaaaacgcagaatctctcactgacttagcggggtaccgccat gatttcttagctagtaacggctttgatgtagaaggtattaattatgaagcggaagttga acgcttcgaccgtatctgataagaaggagatatacatatgagagaagtagtaattgcca gtgcagctagaacagcagtaggaagttttggaggagcatttaaatcagtttcagcggta gagttaggggtaacagcagctaaagaagctataaaaagagctaacataactccagatat gatagatgaatctcttttagggggagtacttacagcaggtcttggacaaaatatagcaa gacaaatagcattaggagcaggaataccagtagaaaaaccagctatgactataaatata gtttgtggttctggattaagatctgtttcaatggcatctcaacttatagcattaggtga tgctgatataatgttagttggtggagctgaaaacatgagtatgtctccttatttagtac caagtgcgagatatggtgcaagaatgggtgatgctgcttttgttgattcaatgataaaa gatggattatcagacatatttaataactatcacatgggtattactgctgaaaacatagc agagcaatggaatataactagagaagaacaagatgaattagctcttgcaagtcaaaata aagctgaaaaagctcaagctgaaggaaaatttgatgaagaaatagttcctgttgttata aaaggaagaaaaggtgacactgtagtagataaagatgaatatattaagcctggcactac aatggagaaacttgctaagttaagacctgcatttaaaaaagatggaacagttactgctg gtaatgcatcaggaataaatgatggtgctgctatgttagtagtaatggctaaagaaaaa gctgaagaactaggaatagagcctcttgcaactatagtttcttatggaacagctggtgt tgaccctaaaataatgggatatggaccagttccagcaactaaaaaagctttagaagctg ctaatatgactattgaagatatagatttagttgaagctaatgaggcatttgctgcccaa tctgtagctgtaataagagacttaaatatagatatgaataaagttaatgttaatggtgg agcaatagctataggacatccaataggatgctcaggagcaagaatacttactacacttt tatatgaaatgaagagaagagatgctaaaactggtcttgctacactttgtataggcggt ggaatgggaactactttaatagttaagagatagtaagaaggagatatacatatgaaatt agctgtaataggtagtggaactatgggaagtggtattgtacaaacttttgcaagttgtg gacatgatgtatgtttaaagagtagaactcaaggtgctatagataaatgtttagcttta ttagataaaaatttaactaagttagttactaagggaaaaatggatgaagctacaaaagc agaaatattaagtcatgttagttcaactactaattatgaagatttaaaagatatggatt taataatagaagcatctgtagaagacatgaatataaagaaagatgttttcaagttacta gatgaattatgtaaagaagatactatcttggcaacaaatacttcatcattatctataac agaaatagcttcttctactaagcgcccagataaagttataggaatgcatttctttaatc cagttcctatgatgaaattagttgaagttataagtggtcagttaacatcaaaagttact tttgatacagtatttgaattatctaagagtatcaataaagtaccagtagatgtatctga atctcctggatttgtagtaaatagaatacttatacctatgataaatgaagctgttggta tatatgcagatggtgttgcaagtaaagaagaaatagatgaagctatgaaattaggagca aaccatccaatgggaccactagcattaggtgatttaatcggattagatgttgttttagc tataatgaacgttttatatactgaatttggagatactaaatatagacctcatccacttt tagctaaaatggttagagctaatcaattaggaagaaaaactaagataggattctatgat tataataaataataagaaggagatatacatatgagtacaagtgatgttaaagtttatga gaatgtagctgttgaagtagatggaaatatatgtacagtgaaaatgaatagacctaaag cccttaatgcaataaattcaaagactttagaagaactttatgaagtatttgtagatatt aataatgatgaaactattgatgttgtaatattgacaggggaaggaaaggcatttgtagc tggagcagatattgcatacatgaaagatttagatgctgtagctgctaaagattttagta tcttaggagcaaaagcttttggagaaatagaaaatagtaaaaaagtagtgatagctgct gtaaacggatttgctttaggtggaggatgtgaacttgcaatggcatgtgatataagaat tgcatctgctaaagctaaatttggtcagccagaagtaactcttggaataactccaggat atggaggaactcaaaggcttacaagattggttggaatggcaaaagcaaaagaattaatc tttacaggtcaagttataaaagctgatgaagctgaaaaaatagggctagtaaatagagt cgttgagccagacattttaatagaagaagttgagaaattagctaagataatagctaaaa atgctcagcttgcagttagatactctaaagaagcaatacaacttggtgctcaaactgat ataaatactggaatagatatagaatctaatttatttggtctttgtttttcaactaaaga ccaaaaagaaggaatgtcagctttcgttgaaaagagagaagctaactttataaaagggt aataagaaggagatatacatatgagaagttttgaagaagtaattaagtttgcaaaagaa agaggacctaaaactatatcagtagcatgttgccaagataaagaagttttaatggcagt
Sequence
Description
tgaaatggctagaaaagaaaaaatagcaaatgccattttagtaggagatatagaaaaga ctaaagaaattgcaaaaagcatagacatggatatcgaaaattatgaactgatagatata aaagatttagcagaagcatctctaaaatctgttgaattagtttcacaaggaaaagccga catggtaatgaaaggcttagtagacacatcaataatactaaaagcagttttaaataaag aagtaggtcttagaactggaaatgtattaagtcacgtagcagtatttgatgtagaggga tatgatagattatttttcgtaactgacgcagctatgaacttagctcctgatacaaatac taaaaagcaaatcatagaaaatgcttgcacagtagcacattcattagatataagtgaac caaaagttgctgcaatatgcgcaaaagaaaaagtaaatccaaaaatgaaagatacagtt gaagctaaagaactagaagaaatgtatgaaagaggagaaatcaaaggttgtatggttgg tgggccttttgcaattgataatgcagtatctttagaagcagctaaacataaaggtataa atcatcctgtagcaggacgagctgatatattattagccccagatattgaaggtggtaac atattatataaagctttggtattcttctcaaaatcaaaaaatgcaggagttatagttgg ggctaaagcaccaataatattaacttctagagcagacagtgaagaaactaaactaaact caatagctttaggtgttttaatggcagcaaaggcataataagaaggagatatacatatg agcaaaatatttaaaatcttaacaataaatcctggttcgacatcaactaaaatagctgt atttgataatgaggatttagtatttgaaaaaactttaagacattcttcagaagaaatag gaaaatatgagaaggtgtctgaccaatttgaatttcgtaaacaagtaatagaagaagct ctaaaagaaggtggagtaaaaacatctgaattagatgctgtagtaggtagaggaggact tcttaaacctataaaaggtggtacttattcagtaagtgctgctatgattgaagatttaa aagtgggagttttaggagaacacgcttcaaacctaggtggaataatagcaaaacaaata ggtgaagaagtaaatgttccttcatacatagtagaccctgttgttgtagatgaattaga agatgttgctagaatttctggtatgcctgaaataagtagagcaagtgtagtacatgctt taaatcaaaaggcaatagcaagaagatatgctagagaaataaacaagaaatatgaagat ataaatcttatagttgcacacatgggtggaggagtttctgttggagctcataaaaatgg taaaatagtagatgttgcaaacgcattagatggagaaggacctttctctccagaaagaa gtggtggactaccagtaggtgcattagtaaaaatgtgctttagtggaaaatatactcaa gatgaaattaaaaagaaaataaaaggtaatggcggactagttgcatacttaaacactaa tgatgctagagaagttgaagaaagaattgaagctggtgatgaaaaagctaaattagtat atgaagctatggcatatcaaatctctaaagaaataggagctagtgctgcagttcttaag ggagatgtaaaagcaatattattaactggtggaatcgcatattcaaaaatgtttacaga aatgattgcagatagagttaaatttatagcagatgtaaaagtttatccaggtgaagatg aaatgattgcattagctcaaggtggacttagagttttaactggtgaagaagaggctcaa gtttatgataactaataa
[0602] In certain constructs, the butyrate gene cassette is placed under the control of a tetracycline-inducible or constitutive promoter.
In a third butyrate gene cassette, the pbt and buk genes are replaced with tesB. TesB is a thioesterase found in E. Coli that cleaves off the butyrate from butyryl- coA, thus obviating the need for pbt-buk.
[0603] In one embodiment, tesB is placed under the control of a FNR regulatory region selected from any of the sequences in Table 6. In an
alternate embodiment, tesB is placed under the control of an RNS-responsive regulatory region, e.g., norB, and the bacteria further comprises a gene
encoding a corresponding RNS-responsive transcription factor, e.g., nsrR. In yet another embodiment, tesB is placed under the control of an ROS- responsive regulatory region, e.g., oxyS, and the bacteria further comprises a
gene encoding a corresponding ROS-responsive transcription factor, e.g., oxyR. In certain constructs, the different described butyrate gene cassettes are each placed under the control of a tetracycline-inducible or constitutive promoter. For example, genetically engineered Nissle are generated
comprising a butyrate gene cassette in which the pbt and buk genes are replaced with tesB expressed under the control of a nitric oxide-responsive regulatory element. SEQ ID NO: 86 comprises a reverse complement of the nsrR repressor gene from Neisseria gonorrhoeae (underlined), intergenic region containing divergent promoters controlling nsrR and the butyrogenic gene cassette and their respective RBS (bold), and the butyrate genes (ter-thiA-hbd- crt-tesB) separated by RBS.
Table 41. SEQ ID NO: 86
SEQ ID NO: 86
ttattatcgcaccgcaatcgggattttcgattcataaagcaggtcgtaggtcggcttgt tgagcaggtcttgcagcgtgaaaccgtccagatacgtgaaaaacgacttcattgcaccg ccgagtatgcccgtcagccggcaggacggcgtaatcaggcattcgttgttcgggcccat acactcgaccagctgcatcggttcgaggtggcggacgaccgcgccgatattgatgcgtt cgggcggcgcggccagcctcagcccgccgcctttcccgcgtacgctgtgcaagaacccg cctttgaccagcgcggtaaccactttcatcaaatggcttttggaaatgccgtaggtcga ggcgatggtggcgatattgaccagcgcgtcgtcgttgacggcggtgtagatgaggacgc gcagcccgtagtcggtatgttgggtcagatacatacaacctccttagtacatgcaaaat tatttctagagcaacatacgagccggaagcataaagtgtaaagcctggggtgcctaatg agttgagttgaggaattataacaggaagaaatattcctcatacgcttgtaattcctcta tggttgttgacaattaatcatcggctcgtataatgtataacattcatattttgtgaatt ttaaactctagaaataattttgtttaactttaagaaggagatatacatatgatcgtaaa acctatggtacgcaacaatatctgcctgaacgcccatcctcagggctgcaagaagggag tggaagatcagattgaatataccaagaaacgcattaccgcagaagtcaaagctggcgca aaagctccaaaaaacgttctggtgcttggctgctcaaatggttacggcctggcgagccg cattactgetgcgttcggatacggggctgcgaccatcggcgtgtcctttgaaaaagcgg gttcagaaaccaaatatggtacaccgggatggtacaataatttggcatttgatgaagcg gcaaaacgcgagggtctttatagcgtgacgatcgacggcgatgcgttttcagacgagat caaggcccaggtaattgaggaagccaaaaaaaaaggtatcaaatttgatctgatcgtat acagcttggccagcccagtacgtactgatcctgatacaggtatcatgcacaaaagcgtt ttgaaaccctttggaaaaacgttcacaggcaaaacagtagatccgtttactggcgagct gaaggaaatctccgcggaaccagcaaatgacgaggaagcagccgccactgttaaagtta tggggggtgaagattgggaacgttggattaagcagctgtcgaaggaaggcctcttagaa gaaggctgtattaccttggcctatagttatattggccctgaagctacccaagctttgta ccgtaaaggcacaatcggcaaggccaaagaacacctggaggccacagcacaccgtctca acaaagagaacccgtcaatccgtgccttcgtgagcgtgaataaaggcctggtaacccgc gcaagcgccgtaatcccggtaatccctctgtatctcgccagcttgttcaaagtaatgaa agagaagggcaatcatgaaggttgtattgaacagatcacgcgtctgtacgccgagcgcc tgtaccgtaaagatggtacaattccagttgatgaggaaaatcgcattcgcattgatgat
tgggagttagaagaagacgtccagaaagcggtatccgcgttgatggagaaagtcacggg tgaaaacgcagaatctctcactgacttagcggggtaccgccatgatttcttagctagta acggctttgatgtagaaggtattaattatgaagcggaagttgaacgcttcgaccgtatc tgataagaaggagatatacatatgagagaagtagtaattgccagtgcagctagaacagc agtaggaagttttggaggagcatttaaatcagtttcagcggtagagttaggggtaacag cagetaaagaagctataaaaagagctaacataactccagatatgatagatgaatctctt ttagggggagtacttacagcaggtcttggacaaaatatagcaagacaaatagcattagg agcaggaataccagtagaaaaaccagctatgactataaatatagtttgtggttctggat taagatctgtttcaatggcatctcaacttatagcattaggtgatgctgatataatgtta gttggtggagctgaaaacatgagtatgtctccttatttagtaccaagtgcgagatatgg tgcaagaatgggtgatgctgcttttgttgattcaatgataaaagatggattatcagaca tatttaataactatcacatgggtattactgctgaaaacatagcagagcaatggaatata actagagaagaacaagatgaattagctcttgcaagtcaaaataaagctgaaaaagctca agctgaaggaaaatttgatgaagaaatagttcctgttgttataaaaggaagaaaaggtg acactgtagtagataaagatgaatatattaagcctggcactacaatggagaaacttgct aagttaagacctgcatttaaaaaagatggaacagttactgetggtaatgcatcaggaat aaatgatggtgctgctatgttagtagtaatggctaaagaaaaagctgaagaactaggaa tagagcctcttgcaactatagtttcttatggaacagctggtgttgaccctaaaataatg ggatatggaccagttccagcaactaaaaaagctttagaagctgctaatatgactattga agatatagatttagttgaagctaatgaggcatttgctgcccaatctgtagctgtaataa gagacttaaatatagatatgaataaagttaatgttaatggtggagcaatagctatagga catccaataggatgctcaggagcaagaatacttactacacttttatatgaaatgaagag aagagatgctaaaactggtcttgctacactttgtataggcggtggaatgggaactactt taatagttaagagatagtaagaaggagatatacatatgaaattagctgtaataggtagt ggaactatgggaagtggtattgtacaaacttttgcaagttgtggacatgatgtatgttt aaagagtagaactcaaggtgctatagataaatgtttagctttattagataaaaatttaa ctaagttagttactaagggaaaaatggatgaagctacaaaagcagaaatattaagtcat gttagttcaactactaattatgaagatttaaaagatatggatttaataatagaagcatc tgtagaagacatgaatataaagaaagatgttttcaagttactagatgaattatgtaaag aagatactatcttggcaacaaatacttcatcattatctataacagaaatagcttcttct actaagcgcccagataaagttataggaatgcatttctttaatccagttcctatgatgaa attagttgaagttataagtggtcagttaacatcaaaagttacttttgatacagtatttg aattatctaagagtatcaataaagtaccagtagatgtatctgaatctcctggatttgta gtaaatagaatacttatacctatgataaatgaagctgttggtatatatgcagatggtgt tgcaagtaaagaagaaatagatgaagctatgaaattaggagcaaaccatccaatgggac cactagcattaggtgatttaatcggattagatgttgttttagctataatgaacgtttta tatactgaatttggagatactaaatatagacctcatccacttttagctaaaatggttag agctaatcaattaggaagaaaaactaagataggattctatgattataataaataataag aaggagatatacatatgagtacaagtgatgttaaagtttatgagaatgtagctgttgaa gtagatggaaatatatgtacagtgaaaatgaatagacctaaagcccttaatgcaataaa ttcaaagactttagaagaactttatgaagtatttgtagatattaataatgatgaaacta ttgatgttgtaatattgacaggggaaggaaaggcatttgtagctggagcagatattgca tacatgaaagatttagatgctgtagctgctaaagattttagtatcttaggagcaaaagc ttttggagaaatagaaaatagtaaaaaagtagtgatagctgctgtaaacggatttgctt taggtggaggatgtgaacttgcaatggcatgtgatataagaattgcatctgctaaagct aaatttggtcagccagaagtaactcttggaataactccaggatatggaggaactcaaag gcttacaagattggttggaatggcaaaagcaaaagaattaatctttacaggtcaagtta taaaagctgatgaagctgaaaaaatagggctagtaaatagagtcgttgagccagacatt ttaatagaagaagttgagaaattagctaagataatagctaaaaatgctcagcttgcagt tagatactctaaagaagcaatacaacttggtgctcaaactgatataaatactggaatag
atatagaatctaatttatttggtctttgtttttcaactaaagaccaaaaagaaggaatg tcagctttcgttgaaaagagagaagctaactttataaaagggtaataagaaggagatat acatatgAGTCAGGCGCTAAAAAATTTACTGACATTGTTAAATCTGGAAAAAATTGAGG AAGGACTCTTTCGCGGCCAGAGTGAAGATTTAGGTTTACGCCAGGTGTTTGGCGGCCAG GTCGTGGGTCAGGCCTTGTATGCTGCAAAAGAGACCGTCCCTGAAGAGCGGCTGGTACA TTCGTTTCACAGCTACTTTCTTCGCCCTGGCGATAGTAAGAAGCCGATTATTTATGATG TCGAAACGCTGCGTGACGGTAACAGCTTCAGCGCCCGCCGGGTTGCTGCTATTCAAAAC GGCAAACCGATTTTTTATATGACTGCCTCTTTCCAGGCACCAGAAGCGGGTTTCGAACA TCAAAAAACAATGCCGTCCGCGCCAGCGCCTGATGGCCTCCCTTCGGAAACGCAAATCG CCCAATCGCTGGCGCACCTGCTGCCGCCAGTGCTGAAAGATAAATTCATCTGCGATCGT CCGCTGGAAGTCCGTCCGGTGGAGTTTCATAACCCACTGAAAGGTCACGTCGCAGAACC ACATCGTCAGGTGTGGATCCGCGCAAATGGTAGCGTGCCGGATGACCTGCGCGTTCATC AGTATCTGCTCGGTTACGCTTCTGATCTTAACTTCCTGCCGGTAGCTCTACAGCCGCAC GGCATCGGTTTTCTCGAACCGGGGATTCAGATTGCCACCATTGACCATTCCATGTGGTT CCATCGCCCGTTTAATTTGAATGAATGGCTGCTGTATAGCGTGGAGAGCACCTCGGCGT CCAGCGCACGTGGCTTTGTGCGCGGTGAGTTTTATACCCAAGACGGCGTACTGGTTGCC TCGACCGTTCAGGAAGGGGTGATGCGTAATCACAATtaa
Example 33. Construction of vectors for overproducing butyrate using a tet-inducible promoter
To facilitate inducible production of butyrate in Escherichia coli Nissle, the eight genes of the butyrate production pathway from Peptoclostridium difficile (bed, etfB, etfA, thiA, hbd, crt, bpt, and buk; NCB I), as well as
transcriptional and translational elements, were synthesized (Gen, Cambridge, MA) and cloned into vector pBR to create pLogic. As synthesized, the genes were placed under control of a tetracycline-inducible promoter, with the tet repressor (tetR) expressed constitutively, divergent from the tet-inducible synthetic butyrate operon. For efficient translation of butyrate genes, each synthetic gene in the operon was separated by a base pair ribosome binding site derived from the T promoter.
The gene products of bcd-etfA-etfB form a complex that convert crotonyl- CoA to butyryl-CoA, and may show some dependence on oxygen as a co- oxidant. Because an effective probiotic should be able to function in an oxygen- limited environment (e.g. the mammalian gut), and because it has been shown that a single gene from Treponema denticola can functionally replace this three gene complex in an oxygen-independent manner (frans-enoynl-CoA reductase; ter), we created a second plasm id capable of butyrate production in E. coli.
Inverse PCR was used to amplify the entire sequence of pLogic outside of the bcd-etfA-etfB region. The fer gene was codon optimized for E. coli codon usage using Integrated DNA technologies online codon optimization tool (https://www.idtdna.com/CodonOpt), synthesized (Genewiz, Cambridge, MA), and cloned into this inverse PCR fragment using Gibson assembly to create pLogic.
Example 34. Transforming E. coli
[0604] Each plasmid is transformed into E. coli Nissle or E. coli DH5a. All tubes, solutions, and cuvettes are pre-chilled to 4° C. An overnight culture of E. coli Nissle or E. coli DH5a is diluted 1 :100 in 5 mL of lysogeny broth (LB) and grown until it reached an OD600 of 0.4-0.6. The cell culture medium contains a selection marker, e.g., ampicillin, that is suitable for the plasmid. The E. coli cells are then centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 1 mL of 4° C water. The E. coli are again centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are resuspended in 0.5 mL of 4° C water. The E. coli are again centrifuged at 2,000 rpm for 5 min. at 4° C, the supernatant is removed, and the cells are finally resuspended in 0.1 mL of 4° C water. The electroporator is set to 2.5 kV. 0.5 g of one of the above plasmids is added to the cells, mixed by pipetting, and pipetted into a sterile, chilled cuvette. The dry cuvette is placed into the sample chamber, and the electric pulse is applied. One mL of room- temperature SOC media is immediately added, and the mixture is transferred to a culture tube and incubated at 37° C for 1 hr. The cells are spread out on an LB plate containing ampicillin and incubated overnight.
[0605] In alternate embodiments, the butyrate cassette can be inserted into the Nissle genome through homologous recombination (Genewiz,
Cambridge, MA). Organization of the constructs and nucleotide sequences are provided herein. To create a vector capable of integrating the synthesized butyrate cassette construct into the chromosome, Gibson assembly was first used to add 1000bp sequences of DNA homologous to the Nissle lacZ locus into the R6K origin plasmid pKD3. This targets DNA cloned between these homology arms to be integrated into the lacZ locus in the Nissle genome.
Gibson assembly was used to clone the fragment between these arms. PCR was used to amplify the region from this plasm id containing the entire sequence of the homology arms, as well as the butyrate cassette between them. This PCR fragment was used to transform electrocompetent Nissle-pKD46, a strain that contains a temperature-sensitive plasmid encoding the lambda red recombinase genes. After transformation, cells were grown out for 2 hours before plating on chloramphenicol at 20ug/ml_ at 37 degrees C. Growth at 37 degrees C also cures the pKD46 plasmid. Transformants containing cassette were chloramphenicol resistant and lac-minus (lac-).
Example 35. Production of butyrate in recombinant E. coli
[0606] Production of butyrate is assessed in E. coli Nissle strains containing the butyrate cassettes described above in order to determine the effect of oxygen on butyrate production. All incubations are performed at 37° C. Cultures of E. coli strains DH5a and Nissle transformed with the butyrate cassettes are grown overnight in LB and then diluted 1 :200 into 4 ml_ of M9 minimal medium containing 0.5% glucose. The cells are grown with shaking (250 rpm) for 4-6 h and incubated aerobically or anaerobically in a Coy anaerobic chamber (supplying 90% N2, 5% C02, 5%H2). One ml_ culture aliquots are prepared in 1.5 ml_ capped tubes and incubated in a stationary incubator to limit culture aeration. One tube is removed at each time point (0, 1 , 2, 4, and 20 hrs) and analyzed for butyrate concentration by LC-MS to confirm that butyrate production in these recombinant strains can be achieved in a low- oxygen environment.
Example 36. Production of butyrate in recombinant E. coli
[0607] Production of butyrate is assessed in E. coli Nissle strains containing the butyrate cassettes described above in order to determine the effect of oxygen on butyrate production. All incubations are performed at 37° C. Cultures of E. coli strains DH5a and Nissle transformed with the butyrate cassettes are grown overnight in LB and then diluted 1 :200 into 4 mL of M9 minimal medium containing 0.5% glucose. The cells are grown with shaking (250 rpm) for 4-6 h and incubated aerobically or anaerobically in a Coy anaerobic chamber (supplying 90% N2, 5% CO2, 5%H2). One mL culture
aliquots are prepared in 1.5 ml_ capped tubes and incubated in a stationary incubator to limit culture aeration. One tube is removed at each time point (0, 1 , 2, 4, and 20 hrs) and analyzed for butyrate concentration by LC-MS to confirm that butyrate production in these recombinant strains can be achieved in a low- oxygen environment.
Example 37. Production of Butyrate in Recombinant E. coli using tet- inducible promoter
[0608] Fig. 48 shows butyrate cassettes described above under the control of a tet-inducible promoter. Production of butyrate is assessed using the methods described below in Example 40. The tet-inducible cassettes tested include (1 ) tet-butyrate cassette comprising all eight genes (pLOGIC031 ); (2) tet-butyrate cassette in which the ter is substituted (pLOGIC046) and (3) tet- butyarte cassette in which tesB is substituted in place of pbt and buk genes. Fig. 51 A shows butyrate production in strains pLOGIC031 and pLOGIC046 in the presence and absence of oxygen, in which there is no significant difference in butyrate production. Enhanced butyrate production was shown in Nissle in low copy plasmid expressing pLOGIC046 which contain a deletion of the final two genes (ptb-buk) and their replacement with the endogenous E. Coli tesB gene (a thioesterase that cleaves off the butyrate portion from butyryl CoA).
[0609] Overnight cultures of cells were diluted 1 :100 in Lb and grown for 1.5 hours until early log phase was reached at which point anhydrous tet was added at a final concentration of 100ng/ml to induce plasmid expression. After 2 hours induction, cells were washed and resuspended in M9 minimal media containing 0.5% glucose at OD600=0.5. Samples were removed at indicated times and cells spun down. The supernatant was tested for butyrate production using LC-MS. Fig. 51 B shows butyrate production in strains comprising a tet- butyrate cassette having ter substitution (pLOGIC046) or the tesB substitution (ptb-buk deletion), demonstrating that the tesB substituted strain has greater butyrate production.
[0610] Fig. 52 shows the BW25113 strain of E. Coli, which is a common cloning strain and the background of the KEIO collection of E. Coli mutants. NuoB mutants having NuoB deletion were obtained. NuoB is a protein complex
involved in the oxidation of NADH during respiratory growth (form of growth requiring electron transport). Preventing the coupling of NADH oxidation to electron transport allows an increase in the amount of NADH being used to support butyrate production. Fig. 52 shows that compared with wild-type Nissle, deletion of NuoB results in grater production of butyrate.
Table 42. pl_OGIC046-tesB-butyrate pLOGIC046-tesB-butyrate : SEQ ID NO: 87
gtaaaacgacggccagtgaattcgttaagacccactttcacatttaagttgtttttcta atccgcatatgatcaattcaaggccgaataagaaggctggctctgcaccttggtgatca aataattcgatagcttgtcgtaataatggcggcatactatcagtagtaggtgtttccct ttcttctttagcgacttgatgctcttgatcttccaatacgcaacctaaagtaaaatgcc ccacagcgctgagtgcatataatgcattctctagtgaaaaaccttgttggcataaaaag gctaattgattttcgagagtttcatactgtttttctgtaggccgtgtacctaaatgtac ttttgctccatcgcgatgacttagtaaagcacatctaaaacttttagcgttattacgta aaaaatcttgccagctttccccttctaaagggcaaaagtgagtatggtgcctatctaac atctcaatggctaaggcgtcgagcaaagcccgcttattttttacatgccaatacaatgt aggctgctctacacctagcttctgggcgagtttacgggttgttaaaccttcgattccga cctcattaagcagctctaatgcgctgttaatcactttacttttatctaatctagacatc attaattcctaatttttgttgacactctatcattgatagagttattttaccactcccta tcagtgatagagaaaagtgaactctagaaataattttgtttaactttaagaaggagata tacatatgatcgtaaaacctatggtacgcaacaatatctgcctgaacgcccatcctcag ggctgcaagaagggagtggaagatcagattgaatataccaagaaacgcattaccgcaga agtcaaagctggcgcaaaagctccaaaaaacgttctggtgcttggctgctcaaatggtt acggcctggcgagccgcattactgctgcgttcggatacggggctgcgaccatcggcgtg tcctttgaaaaagcgggttcagaaaccaaatatggtacaccgggatggtacaataattt ggcatttgatgaagcggcaaaacgcgagggtctttatagcgtgacgatcgacggcgatg cgttttcagacgagatcaaggcccaggtaattgaggaagccaaaaaaaaaggtatcaaa tttgatctgatcgtatacagcttggccagcccagtacgtactgatcctgatacaggtat catgcacaaaagcgttttgaaaccctttggaaaaacgttcacaggcaaaacagtagatc cgtttactggcgagctgaaggaaatctccgcggaaccagcaaatgacgaggaagcagcc gccactgttaaagttatggggggtgaagattgggaacgttggattaagcagctgtcgaa ggaaggcctcttagaagaaggctgtattaccttggcctatagttatattggccctgaag ctacccaagctttgtaccgtaaaggcacaatcggcaaggccaaagaacacctggaggcc acagcacaccgtctcaacaaagagaacccgtcaatccgtgccttcgtgagcgtgaataa aggcctggtaacccgcgcaagcgccgtaatcccggtaatccctctgtatctcgccagct tgttcaaagtaatgaaagagaagggcaatcatgaaggttgtattgaacagatcacgcgt ctgtacgccgagcgcctgtaccgtaaagatggtacaattccagttgatgaggaaaatcg cattcgcattgatgattgggagttagaagaagacgtccagaaagcggtatccgcgttga tggagaaagtcacgggtgaaaacgcagaatctctcactgacttagcggggtaccgccat gatttcttagctagtaacggctttgatgtagaaggtattaattatgaagcggaagttga acgcttcgaccgtatctgataagaaggagatatacatatgagagaagtagtaattgcca gtgcagctagaacagcagtaggaagttttggaggagcatttaaatcagtttcagcggta gagttaggggtaacagcagctaaagaagctataaaaagagctaacataactccagatat gatagatgaatctcttttagggggagtacttacagcaggtcttggacaaaatatagcaa gacaaatagcattaggagcaggaataccagtagaaaaaccagctatgactataaatata
gtttgtggttctggattaagatctgtttcaatggcatctcaacttatagcattaggtga tgctgatataatgttagttggtggagctgaaaacatgagtatgtctccttatttagtac caagtgcgagatatggtgcaagaatgggtgatgctgcttttgttgattcaatgataaaa gatggattatcagacatatttaataactatcacatgggtattactgctgaaaacatagc agagcaatggaatataactagagaagaacaagatgaattagctcttgcaagtcaaaata aagctgaaaaagctcaagctgaaggaaaatttgatgaagaaatagttcctgttgttata aaaggaagaaaaggtgacactgtagtagataaagatgaatatattaagcctggcactac aatggagaaacttgetaagttaagacctgcatttaaaaaagatggaacagttactgetg gtaatgcatcaggaataaatgatggtgctgctatgttagtagtaatggctaaagaaaaa gctgaagaactaggaatagagcctcttgcaactatagtttcttatggaacagctggtgt tgaccctaaaataatgggatatggaccagttccagcaactaaaaaagctttagaagctg ctaatatgactattgaagatatagatttagttgaagctaatgaggcatttgctgcccaa tctgtagctgtaataagagacttaaatatagatatgaataaagttaatgttaatggtgg agcaatagctataggacatccaataggatgctcaggagcaagaatacttactacacttt tatatgaaatgaagagaagagatgctaaaactggtcttgctacactttgtataggcggt ggaatgggaactactttaatagttaagagatagtaagaaggagatatacatatgaaatt agctgtaataggtagtggaactatgggaagtggtattgtacaaacttttgcaagttgtg gacatgatgtatgtttaaagagtagaactcaaggtgctatagataaatgtttagcttta ttagataaaaatttaactaagttagttactaagggaaaaatggatgaagctacaaaagc agaaatattaagtcatgttagttcaactactaattatgaagatttaaaagatatggatt taataatagaagcatctgtagaagacatgaatataaagaaagatgttttcaagttacta gatgaattatgtaaagaagatactatcttggcaacaaatacttcatcattatctataac agaaatagcttcttctactaagcgcccagataaagttataggaatgcatttctttaatc cagttcctatgatgaaattagttgaagttataagtggtcagttaacatcaaaagttact tttgatacagtatttgaattatctaagagtatcaataaagtaccagtagatgtatctga atctcctggatttgtagtaaatagaatacttatacctatgataaatgaagctgttggta tatatgcagatggtgttgcaagtaaagaagaaatagatgaagctatgaaattaggagca aaccatccaatgggaccactagcattaggtgatttaatcggattagatgttgttttagc tataatgaacgttttatatactgaatttggagatactaaatatagacctcatccacttt tagctaaaatggttagagctaatcaattaggaagaaaaactaagataggattctatgat tataataaataataagaaggagatatacatatgagtacaagtgatgttaaagtttatga gaatgtagctgttgaagtagatggaaatatatgtacagtgaaaatgaatagacctaaag cccttaatgcaataaattcaaagactttagaagaactttatgaagtatttgtagatatt aataatgatgaaactattgatgttgtaatattgacaggggaaggaaaggcatttgtagc tggagcagatattgcatacatgaaagatttagatgctgtagctgctaaagattttagta tcttaggagcaaaagcttttggagaaatagaaaatagtaaaaaagtagtgatagctgct gtaaacggatttgctttaggtggaggatgtgaacttgcaatggcatgtgatataagaat tgcatctgctaaagctaaatttggtcagccagaagtaactcttggaataactccaggat atggaggaactcaaaggcttacaagattggttggaatggcaaaagcaaaagaattaatc tttacaggtcaagttataaaagctgatgaagctgaaaaaatagggctagtaaatagagt cgttgagccagacattttaatagaagaagttgagaaattagctaagataatagctaaaa atgctcagcttgcagttagatactctaaagaagcaatacaacttggtgctcaaactgat ataaatactggaatagatatagaatctaatttatttggtctttgtttttcaactaaaga ccaaaaagaaggaatgtcagctttcgttgaaaagagagaagctaactttataaaagggt aataagaaggagatatacatatgAGTCAGGCGCTAAAAAATTTACTGACATTGTTAAAT CTGGAAAAAATTGAGGAAGGACTCTTTCGCGGCCAGAGTGAAGATTTAGGTTTACGCCA GGTGTTTGGCGGCCAGGTCGTGGGTCAGGCCTTGTATGCTGCAAAAGAGACCGTCCCTG AAGAGCGGCTGGTACATTCGTTTCACAGCTACTTTCTTCGCCCTGGCGATAGTAAGAAG CCGATTATTTATGATGTCGAAACGCTGCGTGACGGTAACAGCTTCAGCGCCCGCCGGGT TGCTGCTATTCAAAACGGCAAACCGATTTTTTATATGACTGCCTCTTTCCAGGCACCAG
AAGCGGGTTTCGAACATCAAAAAACAATGCCGTCCGCGCCAGCGCCTGATGGCCTCCCT TCGGAAACGCAAATCGCCCAATCGCTGGCGCACCTGCTGCCGCCAGTGCTGAAAGATAA ATTCATCTGCGATCGTCCGCTGGAAGTCCGTCCGGTGGAGTTTCATAACCCACTGAAAG GTCACGTCGCAGAACCACATCGTCAGGTGTGGATCCGCGCAAATGGTAGCGTGCCGGAT GACCTGCGCGTTCATCAGTATCTGCTCGGTTACGCTTCTGATCTTAACTTCCTGCCGGT AGCTCTACAGCCGCACGGCATCGGTTTTCTCGAACCGGGGATTCAGATTGCCACCATTG ACCATTCCATGTGGTTCCATCGCCCGTTTAATTTGAATGAATGGCTGCTGTATAGCGTG GAGAGCACCTCGGCGTCCAGCGCACGTGGCTTTGTGCGCGGTGAGTTTTATACCCAAGA CGGCGTACTGGTTGCCTCGACCGTTCAGGAAGGGGTGATGCGTAATCACAATtaa
Example 38. Production of Butyrate in Recombinant E. coli
[0611] Production of butyrate is assessed in E. coli Nissle strains containing the butyrate cassettes described above in order to determine the effect of oxygen on butyrate production. All incubations are performed at 37° C. Cultures of E. coli strains DH5a and Nissle transformed with the butyrate cassettes are grown overnight in LB and then diluted 1 :200 into 4 ml_ of M9 minimal medium containing 0.5% glucose. The cells are grown with shaking (250 rpm) for 4-6 h and incubated aerobically or anaerobically in a Coy anaerobic chamber (supplying 90% N2, 5% C02, 5%H2). One ml_ culture aliquots are prepared in 1.5 ml_ capped tubes and incubated in a stationary incubator to limit culture aeration. One tube is removed at each time point (0, 1 , 2, 4, and 20 hours) and analyzed for butyrate concentration by LC-MS to confirm that butyrate production in these recombinant strains can be achieved in a low-oxygen environment.
[0612] In an alternate embodiment, overnight bacterial cultures were diluted 1 :100 into fresh LB and grown for 1.5 hrs to allow entry into early log phase. At this point, long half-life nitric oxide donor (DETA-NO;
diethylenetriamine-nitric oxide adduct) was added to cultures at a final concentration of 0.3mM to induce expression from plasmid. After 2 hours of induction, cells were spun down, supernatant was discarded, and the cells were resuspended in M9 minimal media containing 0.5% glucose. Culture
supernatant was then analyzed at indicated time points to assess levels of butyrate production. Genetically engineered Nissle comprising pLogic031-nsrR- norB-butyrate operon construct; SYN-UCD507) or (pLogic046-nsrR-norB-
butyrate operon construct; SYN-UCD508) produce significantly more butyrate as compared to wild-type Nissle.
[0613] Genetically engineered Nissle were generated comprising a butyrate gene cassette in which the pbt and buk genes are replaced with tesB (SEQ ID NO: 48) expressed under the control of a tetracycline promoter (pLOGIC046-tesB-butyrate; SEQ ID NO: 88). SEQ ID NO: 88 comprises a reverse complement of the tetR repressor (underlined), an intergenic region containing divergent promoters controlling tetR and the butyrate operon and their respective RBS (bold), and the butyrate genes (ter-thiA1-hbd-crt2-tesB) separated by RBS.
Table 43. pl_OGIC046-tesB-butyrate sequence
SEQ ID NO: 88 gtaaaacgacggccagtgaattcgttaagacccactttcacatttaagttgtttttcta atccgcatatgatcaattcaaggccgaataagaaggctggctctgcaccttggtgatca aataattcgatagcttgtcgtaataatggcggcatactatcagtagtaggtgtttccct ttcttctttagcgacttgatgctcttgatcttccaatacgcaacctaaagtaaaatgcc ccacagcgctgagtgcatataatgcattctctagtgaaaaaccttgttggcataaaaag gctaattgattttcgagagtttcatactgtttttctgtaggccgtgtacctaaatgtac ttttgctccatcgcgatgacttagtaaagcacatctaaaacttttagcgttattacgta aaaaatcttgccagctttccccttctaaagggcaaaagtgagtatggtgcctatctaac atctcaatggctaaggcgtcgagcaaagcccgcttattttttacatgccaatacaatgt aggctgctctacacctagcttctgggcgagtttacgggttgttaaaccttcgattccga cctcattaagcagctctaatgcgctgttaatcactttacttttatctaatctagacatc attaattcctaatttttgttgacactctatcattgatagagttattttaccactcccta tcagtgatagagaaaagtgaactctagaaataattttgtttaactttaagaaggagata tacatatgatcgtaaaacctatggtacgcaacaatatctgcctgaacgcccatcctcag ggctgcaagaagggagtggaagatcagattgaatataccaagaaacgcattaccgcaga agtcaaagctggcgcaaaagctccaaaaaacgttctggtgcttggctgctcaaatggtt acggcctggcgagccgcattactgctgcgttcggatacggggctgcgaccatcggcgtg tcctttgaaaaagcgggttcagaaaccaaatatggtacaccgggatggtacaataattt ggcatttgatgaagcggcaaaacgcgagggtctttatagcgtgacgatcgacggcgatg cgttttcagacgagatcaaggcccaggtaattgaggaagccaaaaaaaaaggtatcaaa tttgatctgatcgtatacagcttggccagcccagtacgtactgatcctgatacaggtat catgcacaaaagcgttttgaaaccctttggaaaaacgttcacaggcaaaacagtagatc cgtttactggcgagctgaaggaaatctccgcggaaccagcaaatgacgaggaagcagcc gccactgttaaagttatggggggtgaagattgggaacgttggattaagcagctgtcgaa ggaaggcctcttagaagaaggctgtattaccttggcctatagttatattggccctgaag ctacccaagctttgtaccgtaaaggcacaatcggcaaggccaaagaacacctggaggcc acagcacaccgtctcaacaaagagaacccgtcaatccgtgccttcgtgagcgtgaataa aggcctggtaacccgcgcaagcgccgtaatcccggtaatccctctgtatctcgccagct tgttcaaagtaatgaaagagaagggcaatcatgaaggttgtattgaacagatcacgcgt ctgtacgccgagcgcctgtaccgtaaagatggtacaattccagttgatgaggaaaatcg
cattcgcattgatgattgggagttagaagaagacgtccagaaagcggtatccgcgttga tggagaaagtcacgggtgaaaacgcagaatctctcactgacttagcggggtaccgccat gatttcttagctagtaacggctttgatgtagaaggtattaattatgaagcggaagttga acgcttcgaccgtatctgataagaaggagatatacatatgagagaagtagtaattgcca gtgcagctagaacagcagtaggaagttttggaggagcatttaaatcagtttcagcggta gagttaggggtaacagcagctaaagaagctataaaaagagctaacataactccagatat gatagatgaatctcttttagggggagtacttacagcaggtcttggacaaaatatagcaa gacaaatagcattaggagcaggaataccagtagaaaaaccagctatgactataaatata gtttgtggttctggattaagatctgtttcaatggcatctcaacttatagcattaggtga tgctgatataatgttagttggtggagctgaaaacatgagtatgtctccttatttagtac caagtgcgagatatggtgcaagaatgggtgatgctgcttttgttgattcaatgataaaa gatggattatcagacatatttaataactatcacatgggtattactgctgaaaacatagc agagcaatggaatataactagagaagaacaagatgaattagctcttgcaagtcaaaata aagctgaaaaagctcaagctgaaggaaaatttgatgaagaaatagttcctgttgttata aaaggaagaaaaggtgacactgtagtagataaagatgaatatattaagcctggcactac aatggagaaacttgetaagttaagacctgcatttaaaaaagatggaacagttactgetg gtaatgcatcaggaataaatgatggtgctgctatgttagtagtaatggctaaagaaaaa gctgaagaactaggaatagagcctcttgcaactatagtttcttatggaacagctggtgt tgaccctaaaataatgggatatggaccagttccagcaactaaaaaagctttagaagctg ctaatatgactattgaagatatagatttagttgaagctaatgaggcatttgctgcccaa tctgtagctgtaataagagacttaaatatagatatgaataaagttaatgttaatggtgg agcaatagctataggacatccaataggatgctcaggagcaagaatacttactacacttt tatatgaaatgaagagaagagatgctaaaactggtcttgctacactttgtataggcggt ggaatgggaactactttaatagttaagagatagtaagaaggagatatacatatgaaatt agctgtaataggtagtggaactatgggaagtggtattgtacaaacttttgcaagttgtg gacatgatgtatgtttaaagagtagaactcaaggtgctatagataaatgtttagcttta ttagataaaaatttaactaagttagttactaagggaaaaatggatgaagctacaaaagc agaaatattaagtcatgttagttcaactactaattatgaagatttaaaagatatggatt taataatagaagcatctgtagaagacatgaatataaagaaagatgttttcaagttacta gatgaattatgtaaagaagatactatcttggcaacaaatacttcatcattatctataac agaaatagcttcttctactaagcgcccagataaagttataggaatgcatttctttaatc cagttcctatgatgaaattagttgaagttataagtggtcagttaacatcaaaagttact tttgatacagtatttgaattatctaagagtatcaataaagtaccagtagatgtatctga atctcctggatttgtagtaaatagaatacttatacctatgataaatgaagctgttggta tatatgcagatggtgttgcaagtaaagaagaaatagatgaagctatgaaattaggagca aaccatccaatgggaccactagcattaggtgatttaatcggattagatgttgttttagc tataatgaacgttttatatactgaatttggagatactaaatatagacctcatccacttt tagctaaaatggttagagctaatcaattaggaagaaaaactaagataggattctatgat tataataaataataagaaggagatatacatatgagtacaagtgatgttaaagtttatga gaatgtagctgttgaagtagatggaaatatatgtacagtgaaaatgaatagacctaaag cccttaatgcaataaattcaaagactttagaagaactttatgaagtatttgtagatatt aataatgatgaaactattgatgttgtaatattgacaggggaaggaaaggcatttgtagc tggagcagatattgcatacatgaaagatttagatgctgtagctgctaaagattttagta tcttaggagcaaaagcttttggagaaatagaaaatagtaaaaaagtagtgatagctgct gtaaacggatttgctttaggtggaggatgtgaacttgcaatggcatgtgatataagaat tgcatctgctaaagctaaatttggtcagccagaagtaactcttggaataactccaggat atggaggaactcaaaggcttacaagattggttggaatggcaaaagcaaaagaattaatc tttacaggtcaagttataaaagctgatgaagctgaaaaaatagggctagtaaatagagt cgttgagccagacattttaatagaagaagttgagaaattagctaagataatagctaaaa atgctcagcttgcagttagatactctaaagaagcaatacaacttggtgctcaaactgat
ataaatactggaatagatatagaatctaatttatttggtctttgtttttcaactaaaga ccaaaaagaaggaatgtcagctttcgttgaaaagagagaagctaactttataaaagggt aataagaaggagatatacatatgAGTCAGGCGCTAAAAAATTTACTGACATTGTTAAAT CTGGAAAAAATTGAGGAAGGACTCTTTCGCGGCCAGAGTGAAGATTTAGGTTTACGCCA GGTGTTTGGCGGCCAGGTCGTGGGTCAGGCCTTGTATGCTGCAAAAGAGACCGTCCCTG AAGAGCGGCTGGTACATTCGTTTCACAGCTACTTTCTTCGCCCTGGCGATAGTAAGAAG CCGATTATTTATGATGTCGAAACGCTGCGTGACGGTAACAGCTTCAGCGCCCGCCGGGT TGCTGCTATTCAAAACGGCAAACCGATTTTTTATATGACTGCCTCTTTCCAGGCACCAG AAGCGGGTTTCGAACATCAAAAAACAATGCCGTCCGCGCCAGCGCCTGATGGCCTCCCT TCGGAAACGCAAATCGCCCAATCGCTGGCGCACCTGCTGCCGCCAGTGCTGAAAGATAA ATTCATCTGCGATCGTCCGCTGGAAGTCCGTCCGGTGGAGTTTCATAACCCACTGAAAG GTCACGTCGCAGAACCACATCGTCAGGTGTGGATCCGCGCAAATGGTAGCGTGCCGGAT GACCTGCGCGTTCATCAGTATCTGCTCGGTTACGCTTCTGATCTTAACTTCCTGCCGGT AGCTCTACAGCCGCACGGCATCGGTTTTCTCGAACCGGGGATTCAGATTGCCACCATTG ACCATTCCATGTGGTTCCATCGCCCGTTTAATTTGAATGAATGGCTGCTGTATAGCGTG GAGAGCACCTCGGCGTCCAGCGCACGTGGCTTTGTGCGCGGTGAGTTTTATACCCAAGA CGGCGTACTGGTTGCCTCGACCGTTCAGGAAGGGGTGATGCGTAATCACAATtaa
[0614] Overnight bacterial cultures were diluted 1 :100 into fresh LB and grown for 1.5 hrs to allow entry into early log phase. At this point, anhydrous tetracycline (ATC) was added to cultures at a final concentration of 100 ng/mL to induce expression of butyrate genes from plasmid. After 2 hours of induction, cells were spun down, supernatant was discarded, and the cells were
resuspended in M9 minimal media containing 0.5% glucose. Culture
supernatant was then analyzed at indicated time points to assess levels of butyrate production. Replacement of pbt and buk with tesB leads to greater levels of butyrate production.
[0615] Fig. 53C shows butyrate production in strains comprising an FNR- butyrate cassette syn 363 (having the ter substitution) in the presence/absence of glucose and oxygen. Fig. 53C shows that bacteria need both glucose and anaerobic conditions for butyrate production from the FNR promoter. Cells were grown aerobically or anaerobically in media containg no glucose (LB) or in media containing glucose at 0.5% (RMC). Culture samples were taken at indicaed time pints and supernatant fractions were assessed for butyrate concentration using LC-MS. These data show that SYN 363 requires glucose for butyrate production and that in the presence of glucose butyrate production can be enhanced under anaerobic conditions when under the control of the anaerobic FNR-regulated ydfZ promoter.
Example 39. In vitro Activity of Bacterial Strain Comprising Ammonia- Metabolizing and Butyrate Producing Circuits
Do determine whether ammonia uptake and conversion to arginine and production of butyrate could be accomplished in one strain, a plasmid
(Logic156) comprising a butyrate production cassette construct, was used for initial proof-of-concept experiments. The following strains were generated using the plasmid: SYN-UCD501 (comprising Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance); and SYN-UCD601 , which is SYN-UCD-305, additionally comprising Logic156 (i.e., SYN-UCD601 comprises AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance)). Arginine and butyrate production was the compared between the butyrate only producer SYN-UCD501 , the arginine only producer SYN-UCD305, and the combined butyrate/arginine producer SYN-UCD-601. Sequences for the butyrate cassette used are shown in Table 38.
[0616] Briefly, 3ml LB (containing selective antibiotics (Amp for SYN- UCD501 and SYN-UCD601 ) and 3mM thymidine for SYN-UCD-305 and SYN- UCD601 ) with bacteria from frozen glycerol stocks. Bacteria were grown overnight at 37 C with shaking. Overnight cultures were diluted 1 :100 dilution into 10ml LB (containing antibiotics and thymidine where necessary as above) in a 125ml baffled flask. Cultures were grown aerobically at 37 C with shaking for about 1.5h, and then transferred to the anaerobic chamber at 37 C for 4h. Bacteria (2X108 CFU) were added to 1 ml M9 media containing 50mM MOPS with 0.5% glucose in microcentrifuge tubes. Cells were plated to determine cell counts. The assay tubes were placed in the anaerobic chamber at 37 C. At indicated times (1 , 2, 24h), 120 ul cells were removed and pelleted at
14,000rpm for 1 m in, and 10Oul of the supernatant was transferred to a 96-well assay plate and sealed with aluminum foil, and stored at -80 C until analysis by LC-MS for arginine and butyrate concentrations (as described in Example 13 and Example 40).
[0617] Results are depicted in Fig. 53A and Fig. 53B, and show that SYN-UCD601 is able to produce similar levels of arginine as SYN-UCD305 and similar levels of butyrate as SYN-UCD501 in vitro.
Table 44. Butyrate cassette sequences
ThiA atgagagaagtagtaattgccagtgcagctagaacagcagtaggaagttttg SEQ
(polynucleotide gaggagcatttaaatcagtttcagcggtagagttaggggtaacagcagctaa ID NO: sequence) agaagctataaaaagagctaacataactccagatatgatagatgaatctctttt 92
agggggagtacttacagcaggtcttggacaaaatatagcaagacaaatagc attaggagcaggaataccagtagaaaaaccagctatgactataaatatagttt gtggttctggattaagatctgtttcaatggcatctcaacttatagcattaggtgatg ctgatataatgttagttggtggagctgaaaacatgagtatgtctccttatttagtac caagtgcgagatatggtgcaagaatgggtgatgctgcttttgttgattcaatgat aaaagatggattatcagacatatttaataactatcacatgggtattactgctgaa aacatagcagagcaatggaatataactagagaagaacaagatgaattagct cttgcaagtcaaaataaagctgaaaaagctcaagctgaaggaaaatttgatg aagaaatagttcctgttgttataaaaggaagaaaaggtgacactgtagtagat aaagatgaatatattaagcctggcactacaatggagaaacttgctaagttaag acctgcatttaaaaaagatggaacagttactgctggtaatgcatcaggaataa atgatggtgctgctatgttagtagtaatggctaaagaaaaagctgaagaacta ggaatagagcctcttgcaactatagtttcttatggaacagctggtgttgacccta
aaataatgggatatggaccagttccagcaactaaaaaagctttagaagctgct aatatgactattgaagatatagatttagttgaagctaatgaggcatttgctgccc
aatctgtagctgtaataagagacttaaatatagatatgaataaagttaatgttaat ggtggagcaatagctataggacatccaataggatgctcaggagcaagaata cttactacacttttatatgaaatgaagagaagagatgctaaaactggtcttgcta cactttgtataggcggtggaatgggaactactttaatagttaagagatag
ThiA MREVVIASAARTAVGSFGGAFKSVSAVELGVTAAKEAIK SEQ
(polypeptide RANITPDMIDESLLGGVLTAGLGQNIARQIALGAGIPVEK ID NO: sequence) PAMTINIVCGSGLRSVSMASQLIALGDADIMLVGGAENM 93
SMSPYLVPSARYGARMGDAAFVDSMIKDGLSDIFNNYH
MGITAENIAEQWNITREEQDELALASQNKAEKAQAEGK
FDEEIVPWIKGRKGDTWDKDEYIKPGTTMEKLAKLRP
AFKKDGTVTAGNASG 1 NDGAAM LVVMAKE KAE ELG IE P
LAT 1 VS YGTAG VD P Kl M G YG P VP ATKKAL E AAN MTI E D 1 D
LVEANEAFAAQSVAVIRDLNIDMNKVNVNGGAIAIGHPIG
CSGARILTTLLYEMKRRDAKTGLATLCIGGGMGTTLIVK
R
Hbd atgaaattagctgtaataggtagtggaactatgggaagtggtattgtacaaactt SEQ
(polynucleotide ttgcaagttgtggacatgatgtatgtttaaagagtagaactcaaggtgctataga ID NO: sequence) taaatgtttagctttattagataaaaatttaactaagttagttactaagggaaaaat 94
ggatgaagctacaaaagcagaaatattaagtcatgttagttcaactactaatta tgaagatttaaaagatatggatttaataatagaagcatctgtagaagacatgaa tataaagaaagatgttttcaagttactagatgaattatgtaaagaagatactatct tggcaacaaatacttcatcattatctataacagaaatagcttcttctactaagcgc ccagataaagttataggaatgcatttctttaatccagttcctatgatgaaattagtt gaagttataagtggtcagttaacatcaaaagttacttttgatacagtatttgaatta tctaagagtatcaataaagtaccagtagatgtatctgaatctcctggatttgtagt aaatagaatacttatacctatgataaatgaagctgttggtatatatgcagatggt gttgcaagtaaagaagaaatagatgaagctatgaaattaggagcaaaccat ccaatgggaccactagcattaggtgatttaatcggattagatgttgttttagctata atgaacgttttatatactgaatttggagatactaaatatagacctcatccactttta gctaaaatggttagagctaatcaattaggaagaaaaactaagataggattcta
tgattataataaataa
Hbd MKLAVIGSGTMGSGIVQTFASCGHDVCLKSRTQGAIDK SEQ
(polypeptide CLALLDKNLTKLVTKGKMDEATKAEILSHVSSTTNYEDL ID NO: sequence) KDMDLIIEASVEDMNIKKDVFKLLDELCKEDTILATNTSS 95
LSITEIASSTKRPDKVIGMHFFNPVPMMKLVEVISGQLTS
KVTFDTVFELSKSINKVPVDVSESPGFVVNRILIPMINEA
VG 1 YAD G VAS KE E 1 D E AM KLG AN H P M G P LALG D L 1 G L D V
VLAIMNVLYTEFGDTKYRPHPLLAKMVRANQLGRKTKIG
FYDYNK
Crt2 atgagtacaagtgatgttaaagtttatgagaatgtagctgttgaagtagatgga SEQ
(polynucleotide aatatatgtacagtgaaaatgaatagacctaaagcccttaatgcaataaattca ID NO: sequence) aagactttagaagaactttatgaagtatttgtagatattaataatgatgaaactatt 96
gatgttgtaatattgacaggggaaggaaaggcatttgtagctggagcagatatt gcatacatgaaagatttagatgctgtagctgctaaagattttagtatcttaggag
caaaagcttttggagaaatagaaaatagtaaaaaagtagtgatagctgctgta aacggatttgctttaggtggaggatgtgaacttgcaatggcatgtgatataaga attgcatctgctaaagctaaatttggtcagccagaagtaactcttggaataactc caggatatggaggaactcaaaggcttacaagattggttggaatggcaaaag
caaaagaattaatctttacaggtcaagttataaaagctgatgaagctgaaaaa atagggctagtaaatagagtcgttgagccagacattttaatagaagaagttga gaaattagctaagataatagctaaaaatgctcagcttgcagttagatactctaa agaagcaatacaacttggtgctcaaactgatataaatactggaatagatatag aatctaatttatttggtctttgtttttcaactaaagaccaaaaagaaggaatgtca
gctttcgttgaaaagagagaagctaactttataaaagggtaa
Crt2 MSTSDVKVYENVAVEVDGNICTVKMNRPKALNAINSKT SEQ
(polypeptide LEE LYEVFVD 1 N N D ETI DWI LTGEG KAFVAGAD IAYM KD ID NO: sequence) LDAVAAKDFSILGAKAFGEIENSKKWIAAVNGFALGGG 97
CELAMACDIRIASAKAKFGQPEVTLGITPGYGGTQRLTR
LVGMAKAKELIFTGQVIKADEAEKIGLVNRWEPDILIEEV
EKLAKIIAKNAQLAVRYSKEAIQLGAQTDINTGIDIESNLF
GLCFSTKDQKEGMSAFVEKREANFIKG
Pbt atgagaagttttgaagaagtaattaagtttgcaaaagaaagaggacctaaaa SEQ
(polynucleotide ctatatcagtagcatgttgccaagataaagaagttttaatggcagttgaaatgg ID NO: sequence) ctagaaaagaaaaaatagcaaatgccattttagtaggagatatagaaaaga 98
ctaaagaaattgcaaaaagcatagacatggatatcgaaaattatgaactgat agatataaaagatttagcagaagcatctctaaaatctgttgaattagtttcacaa ggaaaagccgacatggtaatgaaaggcttagtagacacatcaataatactaa aagcagttttaaataaagaagtaggtcttagaactggaaatgtattaagtcacg tagcagtatttgatgtagagggatatgatagattatttttcgtaactgacgcagct atgaacttagctcctgatacaaatactaaaaagcaaatcatagaaaatgcttg cacagtagcacattcattagatataagtgaaccaaaagttgctgcaatatgcg caaaagaaaaagtaaatccaaaaatgaaagatacagttgaagctaaagaa ctagaagaaatgtatgaaagaggagaaatcaaaggttgtatggttggtgggc cttttgcaattgataatgcagtatctttagaagcagctaaacataaaggtataaa tcatcctgtagcaggacgagctgatatattattagccccagatattgaaggtggt aacatattatataaagctttggtattcttctcaaaatcaaaaaatgcaggagttat agttggggctaaagcaccaataatattaacttctagagcagacagtgaagaa actaaactaaactcaatagctttaggtgttttaatggcagcaaaggcataa
Pbt (polypeptide MRSFEEVIKFAKERGPKTISVACCQDKEVLMAVEMARK SEQ
sequence) EKIANAILVGDIEKTKEIAKSIDMDIENYELIDIKDLAEASL ID NO:
KSVELVSQGKADMVMKGLVDTSIILKAVLNKEVGLRTG 99
N VLS H VAVFD VEGYD RLF FVTDAAM N LAP DTNTKKQ 11 E
N ACTVAH S L D 1 S E P KVAAI C AKE KVN P KM KDTVE AKE LE
EMYERGEIKGCMVGGPFAIDNAVSLEAAKHKGINHPVA
GRADILLAPDIEGGNILYKALVFFSKSKNAGVIVGAKAPII
LTSRADSEETKLNSIALGVLMAAKA
Buk atgagcaaaatatttaaaatcttaacaataaatcctggttcgacatcaactaaa SEQ
(polynucleotide atagctgtatttgataatgaggatttagtatttgaaaaaactttaagacattcttca ID NO: sequence) gaagaaataggaaaatatgagaaggtgtctgaccaatttgaatttcgtaaaca 100
agtaatagaagaagctctaaaagaaggtggagtaaaaacatctgaattagat gctgtagtaggtagaggaggacttcttaaacctataaaaggtggtacttattca
gtaagtgctgctatgattgaagatttaaaagtgggagttttaggagaacacgctt caaacctaggtggaataatagcaaaacaaataggtgaagaagtaaatgttc cttcatacatagtagaccctgttgttgtagatgaattagaagatgttgctagaattt ctggtatgcctgaaataagtagagcaagtgtagtacatgctttaaatcaaaag
gcaatagcaagaagatatgctagagaaataaacaagaaatatgaagatata aatcttatagttgcacacatgggtggaggagtttctgttggagctcataaaaatg gtaaaatagtagatgttgcaaacgcattagatggagaaggacctttctctccag aaagaagtggtggactaccagtaggtgcattagtaaaaatgtgctttagtgga aaatatactcaagatgaaattaaaaagaaaataaaaggtaatggcggacta gttgcatacttaaacactaatgatgctagagaagttgaagaaagaattgaagc tggtgatgaaaaagctaaattagtatatgaagctatggcatatcaaatctctaa
agaaataggagctagtgctgcagttcttaagggagatgtaaaagcaatattatt aactggtggaatcgcatattcaaaaatgtttacagaaatgattgcagatagagt taaatttatagcagatgtaaaagtttatccaggtgaagatgaaatgattgcatta gctcaaggtggacttagagttttaactggtgaagaagaggctcaagtttatgat aactaa
Buk MSKIFKILTINPGSTSTKIAVFDNEDLVFEKTLRHSSEEIG SEQ
(polypeptide KYEKVSDQFEFRKQVIEEALKEGGVKTSELDAWGRGG ID NO: sequence) LLKPIKGGTYSVSAAMIEDLKVGVLGEHASNLGGIIAKQI 101
G E E VN VP S Yl VD P WVD E L E D VAR 1 S G M P E 1 S RAS VVH A
LNQKAIARRYAREINKKYEDINLIVAHMGGGVSVGAHKN
GKIVDVANALDGEGPFSPERSGGLPVGALVKMCFSGK
YTQDEIKKKIKGNGGLVAYLNTNDAREVEERIEAGDEKA
KLVYE AM AYQ 1 S KE 1 G AS AAVLKG DVKAILLTGG 1 AYS KM
FTEMIADRVKFIADVKVYPGEDEMIALAQGGLRVLTGEE
EAQVYDN
First RBS (in TTTGTTTAACTTTAAGAAGGAGA SEQ ydfZ=RBS) ID NO:
102
Internal RBS taagaaggagatatacat SEQ between genes ID NO:
103
Butryate CATTTCCTCTCATCCCATCCGGGGTGAGAGTCTTTTC SEQ cassette under C C C C G ACTTATG G CTC ATG C ATG C ATC AAAAAAG ATG ID NO: the control of TGAGCTTGATCAAAAACAAAAAATA 1 1 1 CACTCGACA 104 the ydfZ GGAGTA 1 1 1 ATATTGCGCCCGGATCCCTCTAGAAATA
promoter ATTTTGTTTAACTTTAAGAAGGAGATATACATatgatcgt
(uppercase: aaaacctatggtacgcaacaatatctgcctgaacgcccatcctcagggctgc ydfZ promoter, aagaagggagtggaagatcagattgaatataccaagaaacgcattaccgca with RBS in gaagtcaaagctggcgcaaaagctccaaaaaacgttctggtgcttggctgctc bold; lower aaatggttacggcctggcgagccgcattactgctgcgttcggatacggggctg case: coding cgaccatcggcgtgtcctttgaaaaagcgggttcagaaaccaaatatggtac regions in the accgggatggtacaataatttggcatttgatgaagcggcaaaacgcgagggt following order: ctttatagcgtgacgatcgacggcgatgcgttttcagacgagatcaaggccca ter, thiA, hbd, ggtaattgaggaagccaaaaaaaaaggtatcaaatttgatctgatcgtataca crt2, pbt, buk, gcttggccagcccagtacgtactgatcctgatacaggtatcatgcacaaaagc separated by gttttgaaaccctttggaaaaacgttcacaggcaaaacagtagatccgtttact internal RBS ggcgagctgaaggaaatctccgcggaaccagcaaatgacgaggaagcag (uppercase and ccgccactgttaaagttatggggggtgaagattgggaacgttggattaagcag underlined) ctgtcgaaggaaggcctcttagaagaaggctgtattaccttggcctatagttata ttggccctgaagctacccaagctttgtaccgtaaaggcacaatcggcaaggc caaagaacacctggaggccacagcacaccgtctcaacaaagagaacccg tcaatccgtgccttcgtgagcgtgaataaaggcctggtaacccgcgcaagcg ccgtaatcccggtaatccctctgtatctcgccagcttgttcaaagtaatgaaaga gaagggcaatcatgaaggttgtattgaacagatcacgcgtctgtacgccgag cgcctgtaccgtaaagatggtacaattccagttgatgaggaaaatcgcattcg cattgatgattgggagttagaagaagacgtccagaaagcggtatccgcgttga tggagaaagtcacgggtgaaaacgcagaatctctcactgacttagcggggta ccgccatgatttcttagctagtaacggctttgatgtagaaggtattaattatgaag cggaagttgaacgcttcgaccgtatctgaTAAGAAGGAGATATACA
Tatgagagaagtagtaattgccagtgcagctagaacagcagtaggaagtttt ggaggagcatttaaatcagtttcagcggtagagttaggggtaacagcagcta aagaagctataaaaagagctaacataactccagatatgatagatgaatctcttt tagggggagtacttacagcaggtcttggacaaaatatagcaagacaaatagc attaggagcaggaataccagtagaaaaaccagctatgactataaatatagttt gtggttctggattaagatctgtttcaatggcatctcaacttatagcattaggtgatg ctgatataatgttagttggtggagctgaaaacatgagtatgtctccttatttagtac caagtgcgagatatggtgcaagaatgggtgatgctgcttttgttgattcaatgat aaaagatggattatcagacatatttaataactatcacatgggtattactgctgaa aacatagcagagcaatggaatataactagagaagaacaagatgaattagct cttgcaagtcaaaataaagctgaaaaagctcaagctgaaggaaaatttgatg aagaaatagttcctgttgttataaaaggaagaaaaggtgacactgtagtagat aaagatgaatatattaagcctggcactacaatggagaaacttgctaagttaag acctgcatttaaaaaagatggaacagttactgctggtaatgcatcaggaataa atgatggtgctgctatgttagtagtaatggctaaagaaaaagctgaagaacta ggaatagagcctcttgcaactatagtttcttatggaacagctggtgttgacccta aaataatgggatatggaccagttccagcaactaaaaaagctttagaagctgct aatatgactattgaagatatagatttagttgaagctaatgaggcatttgctgccc aatctgtagctgtaataagagacttaaatatagatatgaataaagttaatgttaat ggtggagcaatagctataggacatccaataggatgctcaggagcaagaata cttactacacttttatatgaaatgaagagaagagatgctaaaactggtcttgcta cactttgtataggcggtggaatgggaactactttaatagttaagagatagTAA
GAAGGAGATATACATatgaaattagctgtaataggtagtggaactat gggaagtggtattgtacaaacttttgcaagttgtggacatgatgtatgtttaaaga gtagaactcaaggtgctatagataaatgtttagctttattagataaaaatttaact aagttagttactaagggaaaaatggatgaagctacaaaagcagaaatattaa
gtcatgttagttcaactactaattatgaagatttaaaagatatggatttaataatag aagcatctgtagaagacatgaatataaagaaagatgttttcaagttactagatg aattatgtaaagaagatactatcttggcaacaaatacttcatcattatctataaca gaaatagcttcttctactaagcgcccagataaagttataggaatgcatttctttaa tccagttcctatgatgaaattagttgaagttataagtggtcagttaacatcaaaa gttacttttgatacagtatttgaattatctaagagtatcaataaagtaccagtagat gtatctgaatctcctggatttgtagtaaatagaatacttatacctatgataaatga agctgttggtatatatgcagatggtgttgcaagtaaagaagaaatagatgaag ctatgaaattaggagcaaaccatccaatgggaccactagcattaggtgattta atcggattagatgttgttttagctataatgaacgttttatatactgaatttggagata ctaaatatagacctcatccacttttagctaaaatggttagagctaatcaattagg aagaaaaactaagataggattctatgattataataaataaTAAGAAGGA
GATATACATatgagtacaagtgatgttaaagtttatgagaatgtagctgttg aagtagatggaaatatatgtacagtgaaaatgaatagacctaaagcccttaat gcaataaattcaaagactttagaagaactttatgaagtatttgtagatattaataa tgatgaaactattgatgttgtaatattgacaggggaaggaaaggcatttgtagct ggagcagatattgcatacatgaaagatttagatgctgtagctgctaaagatttta gtatcttaggagcaaaagcttttggagaaatagaaaatagtaaaaaagtagtg atagctgctgtaaacggatttgctttaggtggaggatgtgaacttgcaatggcat gtgatataagaattgcatctgctaaagctaaatttggtcagccagaagtaactct tggaataactccaggatatggaggaactcaaaggcttacaagattggttgga atggcaaaagcaaaagaattaatctttacaggtcaagttataaaagctgatga agctgaaaaaatagggctagtaaatagagtcgttgagccagacattttaatag aagaagttgagaaattagctaagataatagctaaaaatgctcagcttgcagtt agatactctaaagaagcaatacaacttggtgctcaaactgatataaatactgg aatagatatagaatctaatttatttggtctttgtttttcaactaaagaccaaaaaga aggaatgtcagctttcgttgaaaagagagaagctaactttataaaagggtaaT
AAGAAGGAGATATACATatgagaagttttgaagaagtaattaagttt gcaaaagaaagaggacctaaaactatatcagtagcatgttgccaagataaa gaagttttaatggcagttgaaatggctagaaaagaaaaaatagcaaatgcca ttttagtaggagatatagaaaagactaaagaaattgcaaaaagcatagacat ggatatcgaaaattatgaactgatagatataaaagatttagcagaagcatctct aaaatctgttgaattagtttcacaaggaaaagccgacatggtaatgaaaggct tagtagacacatcaataatactaaaagcagttttaaataaagaagtaggtctta gaactggaaatgtattaagtcacgtagcagtatttgatgtagagggatatgata gattatttttcgtaactgacgcagctatgaacttagctcctgatacaaatactaaa aagcaaatcatagaaaatgcttgcacagtagcacattcattagatataagtga accaaaagttgctgcaatatgcgcaaaagaaaaagtaaatccaaaaatgaa agatacagttgaagctaaagaactagaagaaatgtatgaaagaggagaaat caaaggttgtatggttggtgggccttttgcaattgataatgcagtatctttagaag cagctaaacataaaggtataaatcatcctgtagcaggacgagctgatatattat tagccccagatattgaaggtggtaacatattatataaagctttggtattcttctcaa aatcaaaaaatgcaggagttatagttggggctaaagcaccaataatattaact tctagagcagacagtgaagaaactaaactaaactcaatagctttaggtgtttta atggcagcaaaggcataaTAAGAAGGAGATATACATatgagcaa aatatttaaaatcttaacaataaatcctggttcgacatcaactaaaatagctgtat ttgataatgaggatttagtatttgaaaaaactttaagacattcttcagaagaaata ggaaaatatgagaaggtgtctgaccaatttgaatttcgtaaacaagtaataga agaagctctaaaagaaggtggagtaaaaacatctgaattagatgctgtagta
ggtagaggaggacttcttaaacctataaaaggtggtacttattcagtaagtgct gctatgattgaagatttaaaagtgggagttttaggagaacacgcttcaaaccta ggtggaataatagcaaaacaaataggtgaagaagtaaatgttccttcatacat agtagaccctgttgttgtagatgaattagaagatgttgctagaatttctggtatgc ctgaaataagtagagcaagtgtagtacatgctttaaatcaaaaggcaatagc aagaagatatgctagagaaataaacaagaaatatgaagatataaatcttata gttgcacacatgggtggaggagtttctgttggagctcataaaaatggtaaaata gtagatgttgcaaacgcattagatggagaaggacctttctctccagaaagaag tggtggactaccagtaggtgcattagtaaaaatgtgctttagtggaaaatatact caagatgaaattaaaaagaaaataaaaggtaatggcggactagttgcatact taaacactaatgatgctagagaagttgaagaaagaattgaagctggtgatga aaaagctaaattagtatatgaagctatggcatatcaaatctctaaagaaatag gagctagtgctgcagttcttaagggagatgtaaaagcaatattattaactggtg gaatcgcatattcaaaaatgtttacagaaatgattgcagatagagttaaatttat agcagatgtaaaagtttatccaggtgaagatgaaatgattgcattagctcaag gtggacttagagttttaactggtgaagaagaggctcaagtttatgataactaata a
[0618] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 90 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 90 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of
SEQ ID NO: 90 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 90 or a functional fragment thereof.
[0619] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 92 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 92 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of
SEQ ID NO: 92 or a functional fragment thereof, or a nucleic acid sequence
that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 92 or a functional fragment thereof.
[0620] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 94 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 94 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 94 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 94 or a functional fragment thereof.
[0621] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 96 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 96 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 96 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 96 or a functional fragment thereof.
[0622] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 98 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 98 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 98 or a functional fragment thereof, or a nucleic acid sequence
that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 98 or a functional fragment thereof.
[0623] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 100 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 100 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 100 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 100 or a functional fragment thereof.
[0624] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 104 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 104 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 104 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 104 or a functional fragment thereof.
[0625] In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 48 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 48 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 48 or a functional fragment thereof, or a nucleic acid sequence
that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 48 or a functional fragment thereof.
[0626] In alternate embodiments, pbt and buk are replaced with TesB (SEQ ID NO: 48)
In some embodiments, the butyrate cassette is driven by an inducible promoter. For example, other FNR promotors can be used in lieu of ydfZ, e.g., in SEQ ID NO: .
Non-limiting FNR promoter sequences are provided herein. In some embodiments, the genetically engineered bacteria of the invention comprise a butyrate cassette under the control of one or more of promoter sequences found in Table 6, e.g., nirB promoter, ydfZ promoter, nirB promoter fused to a strong ribosome binding site, ydfZ promoter fused to a strong ribosome binding site, fnrS, an anaerobically induced small RNA gene (fnrS promoter), nirB promoter fused to a crp binding site, and fnrS fused to a crp binding site.
In some embodiments, the butyrate cassetted is under the control of a promoter which is inducible by metabolites present in the gut. In some embodiments the butyrate cassette is induced by HE-specific molecules or metabolites indicative of liver damage, e.g., bilirubin. In some embodiments, the butyrate cassette is placed under the control of promoter, which is inducible by inflammation or an inflammatory response (e.g., RNS or ROS promoter).
[0627] In some embodiments, the genetically engineered bacteria comprise a butyrate cassette driven by a promoter induced by a molecule or metabolite, e.g., bilirubin, aspartate aminotransferase, alanine
aminotransferase, blood coagulation factors II, VII, IX, and X, alkaline phosphatase, gamma glutamyl transferase, hepatitis antigens and antibodies, alpha fetoprotein, anti-mitochondrial, smooth muscle, and anti-nuclear antibodies, iron, transferrin, ferritin, copper, ceruloplasmin, ammonia, and manganese in their blood and intestines. Promoters that respond to one of these molecules or their metabolites may be used in the genetically engineered bacteria provided herein.
In some embodiments, the butyrate cassette is inducible by arabinose and is driven by the AraBAD promoter.
Example 40. Quantification of Butyrate by LC-MS/MS
[0628] To obtain the butyrate measurements in Example 37 a LC-MS/MS protocol for butyrate quantification was used.
Sample preparation
[0629] First, fresh sodium butyrate stock solution (10mg/m), and 1000, 500, 250, 100, 20, 4 and 0.8pg/ml_ of sodium butyrate standards were prepared in water. Then, 10μΙ_ of sample (bacterial supernatants and standards) were pipetted into a V-bottom polypropylene 96-well plate, and 90μΙ_ of 67% ACN (60uL ACN+30uL water per reaction) with 4ug/ml_ of butyrate-d7 (CDN isotope) internal standard in final solution were added to each sample. The plate was heat-sealed, mixed well, and centrifuged at 4000rpm for 5 minutes. In a round- bottom 96-well polypropylene plate, 20μΙ_ of diluted samples were added to 180μΙ_ of a buffer containing 10mM MES pH4.5, 20mM EDC (N-(3- Dimethylaminopropyl)-N'-ethylcarbodiimide), and 20mM TFEA (2,2,2- trifluroethylamine). The plate was again heat-sealed and mixed well, and samples were incubated at room temperature for 1 hour.
LC-MS/MS method
[0630] Butyrate was measured by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) using a Thermo TSQ Quantum Max triple quadrupole mass spectrometer. HPLC Details are listed in Table 45 and Table 46. Tandem Mass Spectrometry details are found in Table 47.
Table 45. HPLC Details
Table 47. Tandem Mass Spectrometry Details
Example 41. Efficacy of genetically engineered bacteria producing arginine and/or butyrate in a bile duct ligation model
[0631] Ligation of the common bile duct in rodents has used as an experimental procedure in research for many years to induce liver cholestasis and fibrosis (see e.g., Tag et a., Bile Duct Ligation in Mice: Induction of Inflammatory Liver Injury and Fibrosis by Obstructive Cholestasis, Journal of Visualized Experiments, February 2015; 96; e52438, and references therein).
[0632]To determine the efficacy of a strain comprising an arginine and butyrate producing circuit in reducing symptoms of liver inflammation and fibrosis, a bile duct ligation model is used. A Nissle control (SYN-UCD107, kanamycin resistant Nissle), an arginine producing strain (SYN-UCD305), a butyrate producing strain (SYN-UCD502), and a strain producing both butyrate and arginine (SYN-UCD605) are compared in the study. ALT/AST levels, fibrosis (portal, perisinusoidal and total) and hepatic inflammation are the
primary endpoints of the study; overall animal health is the secondary endpoint of the study.
[0633] Animals (C57BL6, 8 weeks) are treated by oral gavage with either H2O control (n=12), or SYN-UCD107 (n=12; kanamycin resistant Nissle), or arginine producing SYN-UCD305 (n=12; comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and no antibiotic resistance), or butyrate producing SYN-UCD502 (n=12; comprising a PydfZ-ter butyrate cassette integrated on the chromosome) or SYN-UCD605 (n=12;
comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and PydfZ-ter butyrate cassette integrated on the chromosome, and no antibiotic resistance). Bacteria are administered at a dose of >10e10 cells/ml.
[0634] In some embodiments, SYN-UCD501 (comprising wild type ArgR, no FNR-ArgAfbr, wild type ThyA, and Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance) and SYN-UCD602 (comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance)) are used instead of SYN- UCD502 and SYN-UCD605.
[0635] On Day 0, bile duct ligation surgery is performed as described in Example 40. On day 1 , mice are weighed and randomized. Mice are gavaged with 100ul H2O, SYN-UCD107, SYN-UCD305, SYN-UCD502 or SYN-UCD605 in the AM and PM. On day two, mice are gavaged with 100ul H2O, SYN798 and SYN993-in the AM and PM. On day three, mice are gavaged with 100ul H2O, SYN-UCD107, SYN-UCD305, SYN-UCD502 or SYN-UCD605 in the AM and PM. At 4h post AM dose, blood is collected for ALT/AST analysis. On days 4-6, mice are gavaged with 100ul H2O, SYN-UCD107, SYN-UCD305, SYN-UCD502 or SYN-UCD605 in the AM and PM. On day 7, mice are gavaged with 100ul H2O, SYN-UCD107, SYN-UCD305, SYN-UCD502 or SYN-UCD605 in the AM and PM. At 4h post AM dose, blood is collected for ALT/AST analysis. On days 8-9, mice are gavaged with 100ul H2O, SYN-UCD107, SYN-UCD305, SYN- UCD502 or SYN-UCD605 in the AM and PM. On day 10, mice are gavaged with 100ul H2O, SYN-UCD107, SYN-UCD305, SYN-UCD502 or SYN-UCD605 in the AM and PM. At 4h post AM dose, blood is collected for ALT/AST analysis.
On days 11 -13, mice are gavaged with 100ul H2O, SYN-UCD107, SYN- UCD305, SYN-UCD502 or SYN-UCD605 in the AM and PM. On day 14, animals are gavaged with 100ul H2O, SYN-UCD107, SYN-UCD305, SYN- UCD502 or SYN-UCD605 in the AM. Then, 4h post dose animals are euthanized, and blood is collected by cardiac bleed for ALT/AST analysis. The liver tissue is harvested for fibrosis analysis by histological assessment.
Example 42. Bile Duct Ligation Procedure
Bile Duct Ligation
Pre-surgical Preparation:
[0636] During the complete experimentation, the animal is kept on a warming plate at a temperature of 37 °C, permanently connected to an anesthesia system, and the operational area is covered overall with fluid- impermeable, self-adhesive drapes.
[0637] The mouse is anaesthetized with inhalation of 4 vol% isoflurane in 100% oxygen at a flow rate of 4 L/min for the induction of the anesthesia. The depth of anesthetization is sufficient when the following vital criteria are reached: regular spontaneous breathing, no reflex after setting of pain stimuli between toes, and no response to pain. The abdominal fur of the mouse is shaved with an electric fur shaver and the eyes are protected from drying out by usage of eye and nose ointment. The mouse is placed on a 37 °C heated hot plate, the mouse snout is inserted in the Fluovac mask of a Fluovac anesthesia system, and the legs of the animal are fixed with stripes of silk tape. Anesthesia of the mouse is maintained by inhalation of 1.5-3 vol% isoflurane in 100% oxygen at a flow rate of 1 L/min and induct perioperative analgesia via intraperitoneal injection of buprenorphine solution (0.1 mg/kg BW dissolved in 0.9% NaCI solution).
[0638] The shaved abdominal skin is sterilized with a gauze swab that is moistened with a standard antiseptic, ready to use alcoholic solution for preoperative treatment of the skin.
Surgical Procedures:
[0639] The abdomen is opened with a midline laparotomy of a length of approximately 2 cm by cutting the cutis plus fascia at the same time with an
11.5cm surgical scissor. The connective tissue on top of the peritoneum is dissected by using the scissor as a spreader. The peritoneum is cut along the linea alba to open the peritoneal cavity. The cavity is enlarged by inserting a holding suture in the sternum, raising the filament of the suture, and fixing it on top of the Fluovac mask. The operation area is spread by inserting a Colibri retractor in the peritoneal cavity. The liver is lifted with a moisturized (0.9% NaCI solution) cotton swab so that the ventral side of it sticks to the diaphragm and the hilum is clearly visible. The bile duct is expoed by caudal movement of the gut. The bile duct is separated carefully from the flanking portal vein and hepatic artery using a micro-serrations forceps. The 5-0 suture is placed around the bile duct and secured with two surgical knots. When tying the knots the tractive force is increased continuously to ensure effective obstruction without severing the bile duct. Asecond cranial ligation is added in the same manner without dissecting the bile duct in between. The ends of the sutures are cut, the sternum lowered, and the retractor removed. The peritoneal cavity is rinsed with 0.9% NaCI solution and the abdominal organs replaced to the physiological positions. Both abdominal layers (peritoneum and cutis plus facia) are closed with separate running sutures with 6-0 Mersilk. The ends of the sutures are cut and the operation area is sterilized with a gauze swab moistened with antiseptic solution.
Postoperative Treatment and Follow-up:
[0640] The mouse is allowed to recover in a cage warmed up by an infrared lamp until the mouse is fully awake and active. Afterwards, the mouse is moved to a normal cage and provided ad libitum access to water and food. After the surgery, the animals are monitored at regular intervals and follow-up postoperative treatments are carried out with suitable analgesia (e.g.,
buprenorphine solution) following the local recommendation of the internal animal care and use committees. Animals are kept with free access to food and water ad libitum until the end of the experiment.
Example 43. TAA model of Hepatic Encephalopathy
[0641]TAA treatment of mice has previously been employed in the literature to model increased blood ammonia levels associated with UCDs,
acute and chronic liver disease and HE (Wallace MC, et al., Lab Anim. 2015 Apr;49(1 Suppl):21-9. Standard operating procedures in experimental liver research: thioacetamide model in mice and rat)s. In some embodiments, a TAA- induced mouse model of hyperammonemia is employed to investigate the duration of activity of the genetically engineered bacteria and to generate additional data to support this approach to the treatment of hepatic
encephalopathy.
[0642]To determine the efficacy of a strain comprising an arginine and butyrate producing circuit in alleviating symptoms of liver inflammation and fibrosis, Nissle control (SYN-UCD107, kanamycin resistant Nissle), an arginine producing strain (SYN-UCD305), a butyrate producing strain (SYN-UCD502), and a strain producing both butyrate and arginine (SYN-UCD605) are compared in a TAA model study. ALT/AST levels, fibrosis (portal, perisinusoidal and total) and hepatic inflammation are the primary endpoints of the study. Overall animal health is the secondary endpoint of the study.
[0643] To investigate the effects of engineered bacteria on prolonged elevations of blood ammonia, the bacteria are administered to C57BL6 mice that are also administered a dose of 300mpk thioacetamide (TAA).
[0644] C57BL6 (10 weeks old) are administered one daily dose of SYN- UCD107, or SYN-UCD305 (n=12), SYN-UCD502 (n=12), or SYN-UCD605 (n=12) (100 ul of > 1 X1010 cells/ml) or vehicle control. Alternatively, mice are administered 2 daily doses of bacteria (100 ul of > 1 X1010 cells/ml) (n=5 for each treatment group), once in the AM and once in the PM. After three days of pre-dosing with the bacteria, the mice are treated intraperitoneally with thioacetamide (TAA) at 300mpk or with H2O as control. Alternatively, the mice are treated twice daily, once in the AM and once in the PM with 250mpk. The duration of the study is five days. Ammonium levels are measured and overall health survival, body weight change is monitored.
[0645] In brief, animals are acclimated for 7 days. On day 1 of the time course, animals are weighed, bled to measure baseline ALT/AST and collect fecal pellets (per cage), and are randomized based on ALT/AST levels. Animals are dosed by oral gavage either once or twice (AM and PM) with H2O, SYN- UCD107, SYN-UCD305, SYN-UCD502, or SYN-UCD605 (100ul/dose/animal).
Water is changed to H2O(+)20mg/ml ATC. On day 2, animals are dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD107, SYN- UCD305, SYN-UCD502, or SYN-UCD605 (100ul/dose/animal). On day 3, animals are weighed and dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD107, SYN-UCD305, SYN-UCD502, or SYN-UCD605 (100ul/dose/animal). Additionally, animals are dosed intraperitoneal^ with 300mpk TAA (or saline control). Alternatively, animals are dosed with 250mpk TAA (or saline control) once in the AM and once in the PM. On day 4, animals are weighed and blood is collected for ALT/AST analysis. Fecal pellets are collected per cage. Animals are dosed per oral gavage either once or twice (AM and PM) with H2O, SYN-UCD107, SYN-UCD305, SYN-UCD502, or SYN- UCD605 (100ul/dose/animal). Animals may also be dosed with 250mpk TAA (or saline control). On day 5, animals are weighed and blood is collected for ALT/AST analysis. Fecal pellets are collected (per cage). Animals are dosed by oral gavage either once or twice (AM and PM) with H2O, SYN-UCD107, SYN- UCD305, SYN-UCD502, or SYN-UCD605 (100ul/dose/animal). ALT/AST levels, bacterial load in the fecal pellets, and overall health survival, and body weight changes are monitored. The liver tissue is harvested for fibrosis analysis by histological assessment.
Example 44. Carbontetrachloride (CCI4) Model of Hepatic Encephalopathy
[0646] CCI4 is often used to induce hepaticfibrosis and cirrhosis in animals because the underlying iochemical mechanisms and histological characteristics are similar to those observed in human liver cirrhosis (Nhung et al., Establishment of a standardized mouse model of hepatic fibrosis for biomedical research; Biomedical Research and Therapy 2014, 1 (2):43-49).
[0647] CYP2E1 is an enzyme which is expressed in perivenular hepatocytes, and which converts CCI4 into a CCI3+ radical. The accumulation of the CCI3+ radical causes centrilobular necrosis and changes the permeability of the hepatocyte plasma and mitochondrial membranes. As a result, an increase in inflammation and fibrogenesis, and extracellular matrix deposition is observed. Chronic CCI4 exposure causes the formation of nodules and fibrosis, products of the wound healing process. CCI4 treatment has been shown to
cause fibrosis after 2-4 weeks, significant bridging fibrosis after 5-7 weeks, cirrhosis after 9-11 weeks, and micronodular cirrhosis after 10-20 weeks (Nhung et al., Establishment of a standardized mouse model of hepatic fibrosis for biomedical research; Biomedical Research and Therapy 2014, 1 (2):43-49, and references therein).
[0648]To determine the efficacy of a strain comprising an arginine and butyrate producing circuit in alleviating symptoms of liver inflammation and fibrosis, a CCI4 mouse model of liver cirrhosis is used. A Nissle control (SYN- UCD107, kanamycin resistant Nissle), an arginine producing strain (SYN- UCD305), a butyrate producing strain (SYN-UCD502), and a strain producing both butyrate and arginine (SYN-UCD605) are compared in the study.
ALT/AST levels, fibrosis (portal, perisinusoidal and total) and hepatic
inflammation are the primary endpoints of the study. Overall animal health is the secondary endpoint of the study. Study duration is 8 weeks.
[0649] Animals (C57BL6, 8 weeks) are treated by oral gavage with either H2O control (n=12), or SYN-UCD107 (n=12; kanamycin resistant Nissle), or arginine producing SYN-UCD305 (n=12; comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and no antibiotic resistance), or butyrate producing SYN-UCD502 (n=12; comprising wild type ArgR, no FNR-ArgAfbr, wild type ThyA , and a PydfZ-ter butyrate cassette integrated on the chromosome) or SYN-UCD605 (n=12; comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, PydfZ-ter butyrate cassette integrated on the chromosome, and no antibiotic resistance. Bacteria are administered at a dose of >10e10 cells/ml in 100 ul.
[0650] In some embodiments, SYN-UCD501 (comprising Wild type ArgR, no FNR-ArgAfbr, wild type ThyA, and Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance) and SYN-UCD602 (comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance)) are used instead of SYN- UCD502 and SYN-UCD605.
[0651] On Day 1 , mice are weighed and randomized. Animals are gavaged with 100ul H2O, SYN-UCD107, SYN-UCD305, SYN-UCD502, or SYN- UCD605 in the AM. Additionally animals are gavaged with olive oil (sham
control) or 1 ml/kg CCL4 in olive oil (all other treatment groups). Animals are gavaged with 100ul H20, SYN-UCD107, SYN-UCD305, SYN-UCD502, or SYN- UCD605 in the PM.
[0652] Through Week 1 -8, animals are gavaged BID with 100ul H20, SYN-UCD107, SYN-UCD305, SYN-UCD502, or SYN-UCD605. Additionally, animals are gavaged 3x/week with olive oil (sham control) or 1 ml/kg CCL4 in olive oil (all other treatment groups). Animals are weighed 3x/week, and blood for ALT/AST analysis is collected 1x/week.
[0653] At the end of Week 8 animals are gavaged with 10Oul with 100ul H20, SYN-UCD107, SYN-UCD305, SYN-UCD502, or SYN-UCD605 in the AM. At 4h post dose, animals are weighed, euthanized and blood is collected by cardiac bleed for ALT/AST analysis. The liver is harvested for fibrosis analysis by histological assessment.
Example 45. Efficacy of Butyrate-Expressing Bacteria in a DSS Mouse
Model
[0654] Bacteria harboring the butyrate cassettes described above are grown overnight in LB. Bacteria are then diluted 1 :100 into LB containing a suitable selection marker, e.g., ampicillin, and grown to an optical density of 0.4- 0.5 and then pelleted by centrifugation. Bacteria are resuspended in phosphate buffered saline and 100 microliters is administered by oral gavage to mice. Damage to the gut is induced in mice by supplementing drinking water with 3% dextran sodium sulfate for 7 days prior to bacterial gavage. Mice are treated daily for 1 week and bacteria in stool samples are detected by plating stool homogenate on agar plates supplemented with a suitable selection marker, e.g., ampicillin. After 5 days of bacterial treatment, gut damage is scored in live mice using endoscopy. Endoscopic damage score is determined by assessing colon translucency, fibrin attachment, mucosal and vascular pathology, and/or stool characteristics. Mice are sacrificed and colonic tissues are isolated.
Distal colonic sections are fixed and scored for inflammation and ulceration. Colonic tissue is homogenized and measurements are made for
myeloperoxidase activity using an enzymatic assay kit and for cytokine levels (IL-1 β, TNF-α, IL-6, IFN-γ and IL-10).
Example 46. Generating a DSS-lnduced Mouse Model of HE
[0655] The genetically engineered bacteria described can be tested in the dextran sodium sulfate (DSS)-induced mouse model. The administration of DSS to animals results in chemical injury to the intestinal epithelium, allowing proinflammatory intestinal contents (e.g., luminal antigens, enteric bacteria, bacterial products) to disseminate and trigger inflammation (Low et al., 2013). To prepare mice for DSS treatment, mice are labeled using ear punch, or any other suitable labeling method. Labeling individual mice allows the investigator to track disease progression in each mouse, since mice show differential susceptibilities and responsiveness to DSS induction. Mice are then weighed, and if required, the average group weight is equilibrated to eliminate any significant weight differences between groups. Stool is also collected prior to DSS administration, as a control for subsequent assays. Exemplary assays for fecal markers of inflammation (e.g., cytokine levels or myeloperoxidase activity) are described below.
[0656] For DSS administration, a 3% solution of DSS (MP Biomedicals, Santa Ana, CA; Cat. No. 160110) in autoclaved water is prepared. Cage water bottles are then filled with 100 mL of DSS water, and control mice are given the same amount of water without DSS supplementation. This amount is generally sufficient for 5 mice for 2-3 days. Although DSS is stable at room temperature, both types of water are changed every 2 days, or when turbidity in the bottles is observed.
[0657] Acute, chronic, and resolving models of intestinal inflammation are achieved by modifying the dosage of DSS (usually 1 -5%) and the duration of DSS administration (Chassaing et al., 2014). For example, acute and resolving gut damage may be achieved after a single continuous exposure to DSS over one week or less, whereas chronic gut damage is typically induced by cyclical administration of DSS punctuated with recovery periods (e.g., four cycles of DSS treatment for 7 days, followed by 7-10 days of water).
[0658] Fig. 53D shows that butyrate produced in vivo in DSS mouse models under the control of an FNR promoter can be gut protective. LCN2 and
calprotectin are both a measure of gut barrier disruption (measure by ELISA in this assay). Fig. 53D shows that Syn 363 (ter substitution) reduces
inflammation and/or protects gut barrier as conmpared to Syn 94 (wildtype Nissle).
Example 47. Monitoring Disease Progression in Vivo
[0659] Following initial administration of DSS, stool is collected from each animal daily, by placing a single mouse in an empty cage (without bedding material) for 15-30 min. However, as DSS administration progresses and inflammation becomes more robust, the time period required for collection increases. Stool samples are collected using sterile forceps, and placed in a microfuge tube. A single pellet is used to monitor occult blood according to the following scoring system: 0, normal stool consistency with negative hemoccult; 1 , soft stools with positive hemoccult; 2, very soft stools with traces of blood; and 3, watery stools with visible rectal bleeding. This scale is used for comparative analysis of intestinal bleeding. All remaining stool is reserved for the measurement of inflammatory markers, and frozen at -20 °C.
[0660] The body weight of each animal is also measured daily. Body weights may increase slightly during the first three days following initial DSS administration, and then begin to decrease gradually upon initiation of bleeding. For mouse models of acute colitis, DSS is typically administered for 7 days. However, this length of time may be modified at the discretion of the
investigator.
Example 48. In Vivo Efficacy of Genetically Engineered Bacteria
Following DSS Induction
[0661] The genetically engineered bacteria described herein can be tested in DSS-induced animal models of HE. Bacteria are grown overnight in LB supplemented with the appropriate antibiotic. Bacteria are then diluted 1 :100 in fresh LB containing selective antibiotic, grown to an optical density of 0.4-0.5, and pelleted by centrifugation. Bacteria are then resuspended in phosphate buffered saline (PBS). Gut damage is induced in mice by
supplementing drinking water with 3% DSS for 7 days prior to bacterial gavage. On day 7 of DSS treatment, 100 μί of bacteria (or vehicle) is administered to
mice by oral gavage. Bacterial treatment is repeated once daily for 1 week, and bacteria in stool samples are detected by plating stool homogenate on selective agar plates.
[0662] After 5 days of bacterial treatment, gut damage is scored in live mice using the Coloview system (Karl Storz Veterinary Endoscopy, Goleta, CA). In mice under 1.5-2.0% isoflurane anesthesia, colons are inflated with air and approximately 3 cm of the proximal colon can be visualized (Chassaing et al., 2014). Endoscopic damage is scored by assessing colon translucency (score 0-3), fibrin attachment to the bowel wall (score 0-3), mucosal granularity (score 0-3), vascular pathology (score 0-3), stool characteristics (normal to diarrhea; score 0-3), and the presence of blood in the lumen (score 0-3), to generate a maximum score of 18. Mice are sacrificed and colonic tissues are isolated using protocols described in Examples 8 and 9. Distal colonic sections are fixed and scored for inflammation and ulceration. Remaining colonic tissue is homogenized and cytokine levels (e.g., IL-1 β, TNF-α, IL-6, IFN-γ, and IL-10), as well as myeloperoxidase activity, are measured using methods described below.
Example 49. Euthanasia Procedures for Rodent Models of HE
[0663] Four and 24 hours prior to sacrifice, 5-bromo-2'-deooxyuridine (BrdU) (Invitrogen, Waltham, MA; Cat. No. B23151 ) may be intraperitoneal^ administered to mice, as recommended by the supplier. BrdU is used to monitor intestinal epithelial cell proliferation and/or migration via
immunohistochemistry with standard anti-BrdU antibodies (Abeam, Cambridge, MA).
[0664] On the day of sacrifice, mice are deprived of food for 4 hours, and then gavaged with FITC-dextran tracer (4 kDa, 0.6 mg/g body weight). Fecal pellets are collected, and mice are euthanized 3 hours following FITC-dextran administration. Animals are then cardiac bled to collect hemolysis-free serum. Intestinal permeability correlates with fluorescence intensity of appropriately diluted serum (excitation, 488 nm; emission, 520 nm), and is measured using spectrophotometry. Serial dilutions of a known amount of FITC-dextran in mouse serum are used to prepare a standard curve.
[0665] Alternatively, intestinal inflammation is quantified according to levels of serum keratinocyte-derived chemokine (KC), lipocalin 2, calprotectin, and/or CRP-1. These proteins are reliable biomarkers of inflammatory disease activity, and are measured using DuoSet ELISA kits (R&D Systems,
Minneapolis, MN) according to manufacturer's instructions. For these assays, control serum samples are diluted 1 :2 or 1 :4 for KC, and 1 :200 for lipocalin 2. Samples from DSS-treated mice require a significantly higher dilution.
Example 42. Non-obese Diabetes (NOD) Model of Hepatic Encephalopathy
[0666] [0001 ] NOD mice can be used as an in vivo model for testing the efficacy of bacteria comprising a gut-barrier enhancing circuit, e.g., a butyrate biosynthetic cassette, as these mice exhibit a pronounced "leaky gut" phenotype, which is evidenced by a decrease in the level of tight junction proteins (e.g., occludin, zonula occludin, mucin, E-cadherin).
[0667] [0001 ] To determine the efficacy of a strain comprising an arginine and butyrate producing circuit in alleviating symptoms associated with "leaky gut", a NOD mouse model is used. NOD mice result from the autoimmune destruction of pancreatic islet β cells. A Nissle control (SYN-UCD107, kanamycin resistant Nissle), an arginine producing strain (SYN-UCD305), a butyrate producing strain (SYN-UCD502), and a strain producing both butyrate and arginine (SYN-UCD605) are compared in the study. Epithelial gut integrity (occludin and other tight junction markers) and gut inflammation (inflammatory biomarkers, e.g., IL-1A, IL-6, TNFa, IL-21 ) are the primary endpoints of the study. Overall animal health is the secondary endpoint of the study. Study duration is 8 weeks.
[0668] [0002] Animals (C57BL6, 8 weeks) are treated by oral gavage with either H2O control (n=12), or 100mM butyrate (n=12), or arginine producing SYN-UCD305 (n=12; comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and no antibiotic resistance), or butyrate producing SYN-UCD502 (n=12; comprising wild type ArgR, no FNR- ArgAfbr, wild type ThyA , and a PydfZ-ter butyrate cassette integrated on the chromosome) or SYN-UCD605 (n=12; comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, PydfZ-ter butyrate
cassette integrated on the chromosome, and no antibiotic resistance. Bacteria are administered at a dose of >10e10 cells/ml in 100 ul.
[0669] [0003] In some embodiments, SYN-UCD501 (comprising Wild type ArgR, no FNR-ArgAfbr, wild type ThyA, and Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance) and SYN-UCD602 (comprising AArgR, PfnrS- ArgAfbr integrated into the chromosome at the maIEK locus, AThyA, and Logic156 (pSC101 PydfZ-ter butyrate plasmid; amp resistance)) are used instead of SYN-UCD502 and SYN-UCD605.
[0670] [0004] On Day 1 , mice are weighed and randomized. Animals are gavaged with 100ul H2O, 100 mM butyrate, SYN-UCD305, SYN-UCD502, or SYN-UCD605 in the AM. Animals are gavaged with 100ul H2O, 100mM butyrate, SYN-UCD305, SYN-UCD502, or SYN-UCD605 in the PM. Animals are gavaged BID with 100ul H2O, 100mM butyrate, SYN-UCD305, SYN- UCD502, or SYN-UCD605. Animals are weighed daily.
[0671] [0005] On day 5, mice are fasted for 4h and then gavaged with 0.6mg/g FITC-dextran (40kD). 3h post FITC-dex administration mice are weighed, and blood is collected by cardiac bleed and colons and fecal pellets are harvested.
[0672] [0006] Haptoglobin/zonulin and Lcn2 are measured. RNA levels of TJP1 , OCLN, CLDN25, and EPCAM are measured in colon samples (increased levels of these markers indicate therapeutic effect). RNA levels of inflammatory biomarkers are measured in the blood samples (decreased levels of these biomarkers indicate therapeutic effect).
[0673]
Example 51. GABA transport and metabolic circuits
In addition to the ammonia conversion circuit described above, the E. coli Nissle bacteria further comprise one or more GABA transport and/or one or more GABA metabolic circuits. Genetically engineered strains comprising at least one GABA transport circuit comprise a gene encoding an exemplary GABA transport protein, such as GabP (SEQ ID NO: 105, Table 48; SEQ ID NO: 106, Table 49), and are constructed using methods described above. Genetically engineered strains comprising at least one GABA metabolic circuit comprise
genes encoding enzymes required for GABA catabolism, including, but not limited to GSST and SSDH, and are constructed using methods described above.
[0674] The genes encoding GabP, GSST, and SSDH are expressed under the control of a tetracycline-inducible promoter, a FNR promoter selected from SEQ ID NOs: 18-29, or a promoter induced by HE-related molecules or metabolites. The genes encoding GabP, GSST, and SSDH may be expressed under the control of the same or different promoters. As discussed herein, other promoters may be used. The genes encoding GabP, GSST, and SSDH are expressed on a high-copy plasm id, a low-copy plasm id, or a chromosome. The genes encoding GabP, GSST, and SSDH are inserted into the bacterial genome at one or more of the following insertion sites in E. coli Nissle: malE/K, araC/BAD, lacZ, thyA, malP/T. Any suitable insertion site may be used, see, e.g., Fig. 18.
Table 48 Amino acid sequence of GabP transporter (SEQ ID NO: 105)
MGQSSQPHELGGGLKSRHVTMLSIAGVIGASLFVGSSVAIAEAGPAVLLAYLFAGLLW MIMRMLAEMAVATPDTGSFSTYADKAIGRWAGYTIGWLYWWFWVLVIPLEANIAAMILH SWVPGIPIWLFSLVITLALTGSNLLSVKNYGEFEFWLALCKVIAILAFIFLGAVAISGF YPYAEVSGISRLWDSGGFMPNGFGAVLSAMLITMFSFMGAEIVTIAAAESDTPEKHIVR ATNSVIWRISIFYLCSIFVWALIPWNMPGLKAVGSYRSVLELLNIPHAKLIMDCVILL SVTSCLNSALYTASRMLYSLSRRGDAPAVMGKINRSKTPYVAVLLSTGAAFLTVWNYY APAKVFKFLIDSSGAIALLVYLVIAVSQLRMRKILRAEGSEIRLRM LYPWLTWLVIGF ITFVLWMLFRPAQQLEVISTGLLAIGIICTVPIMARWKKLVLWQKTPVHNTR
Table 49
Codon optimized polynucleotide sequence of GabP transporter (SEQ ID
NO: 106)
ATGGGACAGTCTTCACAACCACACGAACTTGGGGGTGGATTGAAATCGCGCCATGTGAC CATGTTAAGTATCGCAGGCGTGATTGGCGCCTCCTTATTTGTGGGGTCCTCCGTGGCGA TTGCAGAGGCGGGTCCGGCTGTACTTTTGGCATATCTTTTTGCGGGTTTACTGGTTGTG ATGATCATGCGCATGCTTGCCGAAATGGCTGTGGCCACGCCGGACACGGGGTCATTTTC CACTTATGCGGACAAGGCGATTGGCCGCTGGGCCGGGTACACAATCGGGTGGCTGTATT GGTGGTTCTGGGTGTTAGTTATCCCCTTGGAGGCCAACATCGCCGCAATGATTCTGCAC TCCTGGGTTCCGGGTATCCCGATCTGGCTGTTCAGCTTGGTGATCACCCTGGCACTGAC GGGCAGCAACTTATTGAGTGTGAAAAACTATGGAGAGTTTGAATTTTGGCTGGCCCTGT GTAAAGTCATTGCTATCTTGGCATTCATTTTTTTAGGAGCGGTAGCAATCAGTGGCTTC TACCCTTATGCAGAAGTTTCGGGGATTTCCCGTCTTTGGGATAGTGGCGGATTCATGCC AAACGGGTTTGGAGCTGTACTGTCAGCCATGTTGATTACCATGTTTAGCTTTATGGGTG CCGAGATCGTGACAATCGCCGCAGCCGAGAGTGATACCCCGGAAAAGCACATTGTTCGT
GCGACGAATTCGGTAATTTGGCGTATTTCGATTTTTTACTTATGCTCCATTTTCGTTGT GGTCGCCCTTATCCCCTGGAACATGCCAGGCTTAAAAGCAGTAGGCAGCTACCGCTCAG TCCTGGAATTACTGAACATTCCTCACGCGAAGTTAATTATGGATTGCGTAATCCTGTTA TCGGTAACGAGCTGCCTTAACAGTGCTCTGTACACGGCTTCACGTATGCTGTACTCTTT AAGTCGCCGTGGCGATGCACCTGCCGTTATGGGCAAGATTAACCGCAGTAAGACGCCGT ATGTAGCTGTTTTGCTGTCGACTGGAGCTGCGTTTCTTACAGTCGTAGTAAACTATTAC GCACCAGCTAAAGTTTTCAAATTCCTTATTGATTCGTCTGGGGCAATCGCACTTCTGGT GTACCTGGTCATCGCGGTGTCACAACTTCGCATGCGCAAGATCTTGCGTGCGGAGGGCA GTGAGATTCGTTTGCGTATGTGGCTGTATCCGTGGCTGACGTGGCTTGTTATTGGTTTC ATTACTTTTGTGTTGGTAGTGATGCTGTTTCGTCCAGCGCAACAGCTGGAGGTGATTTC TACCGGACTGTTGGCAATCGGCATCATCTGTACCGTCCCAATCATGGCTCGCTGGAAAA AGTTGGTCTTATGG C AGAAGAC C C C T G T AC AC AAC AC C C G T
In some embodiments, the genetically engineered bacteria comprise the nucleic acid sequence of SEQ ID NO: 106 or a functional fragment thereof. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 106 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the DNA sequence of SEQ ID NO: 106 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 106 or a functional fragment thereof.
Example 52. Manganese transport circuit
[0675] In addition to the circuits described above, the E. coli Nissle bacteria further comprise one or more manganese transport circuits.
Genetically engineered strains comprising at least one manganese transport circuit comprise a gene encoding an exemplary manganese transport protein, such as MntH (SEQ ID NO: 107, Table 50, SEQ ID NO: 108, Table 51), and are constructed using methods described above.
[0676] The gene encoding MntH is expressed under the control of a tetracycline-inducible promoter, a FNR promoter selected from SEQ ID NOs: 18-29, or a promoter induced by HE-related molecules or metabolites. As discussed herein, other promoters may be used. The gene encoding MntH is
expressed on a high-copy plasm id, a low-copy plasm id, or a chromosome. The gene encoding MntH is inserted into the bacterial genome at one or more of the following insertion sites in E. coli Nissle: malE/K, araC/BAD, lacZ, thyA, malP/T. Any suitable insertion site may be used, see, e.g., Fig. 18.
Table 50 Amino acid sequence of MntH transporter (SEQ ID NO: 107)
MTNYRVESSSGRAARKMRLALMGPAFIAAIGYIDPGNFATNIQAGASFGYQLLWVWWA N
LMAMLIQILSAKLGIATGKNLAEQIRDHYPRPVVWFYWVQAEIIAMATDLAEFIGAAIG F
KLILGVSLLQGAVLTGIATFLILMLQRRGQKPLEKVIGGLLLFVAAAYIVELIFSQPNL A
QLGKGMVIPSLPTSEAVFLAAGVLGATIMPHVIYLHSSLTQHLHGGSRQQRYSATKWDV A
IAMTIAGFVNLAMMATAAAAFHFSGHTGVADLDEAYLTLQPLLSHAAATVFGLSLVAAG L
SSTWGTLAGQVVMQGFIRFHIPLWVRRTVTMLPSFIVILMGLDPTRILVMSQVLLSFG I
ALALVPLLIFTSDSKLMGDLVNSKRVKQTGWVIWLWALNIWLLVGTALGL
Table 51 Polynucleotide sequence of MntH transporter (SEQ ID NO: 108)
ATGACCAATTATCGTGTTGAAAGTAGTAGTGGCCGCGCGGCTCGTAAAATGCGTCTGGC CTTAATGGGCCCGGCGTTTATTGCTGCGATTGGATACATTGATCCGGGCAATTTCGCTA CAAACATCCAAGCAGGTGCATCCTTCGGTTACCAGCTTCTGTGGGTAGTGGTATGGGCT AACCTGATGGCCATGCTTATTCAAATTCTTTCAGCTAAGCTTGGTATTGCCACAGGAAA GAATTTAGCCGAGCAGATTCGTGACCACTATCCCCGCCCCGTGGTCTGGTTCTATTGGG TCCAGGCAGAGATTATCGCGATGGCGACTGATTTAGCCGAATTTATTGGGGCAGCTATT GGATTTAAGCTGATCCTTGGCGTATCTCTGTTGCAAGGCGCGGTATTGACCGGAATTGC AACCTTTTTGATTCTTATGTTGCAACGTCGTGGGCAGAAGCCTCTGGAAAAAGTCATCG GCGGGTTATTGCTTTTTGTTGCCGCGGCCTACATTGTGGAACTGATCTTTTCTCAACCT AACCTGGCGCAGCTTGGTAAAGGCATGGTAATCCCGTCACTTCCTACATCTGAGGCAGT ATTCTTAGCAGCCGGCGTCTTGGGCGCAACTATCATGCCCCATGTCATCTACTTACACA GTTCTCTGACTCAGCACTTACACGGTGGGTCGCGCCAACAGCGTTACTCCGCAACAAAG TGGGACGTTGCAATTGCCATGACCATTGCCGGTTTTGTTAACCTGGCGATGATGGCCAC GGCTGCTGCCGCCTTTCATTTCAGTGGCCACACTGGTGTAGCCGATCTGGATGAGGCAT ACCTGACCTTGCAGCCTCTGTTGTCTCATGCAGCCGCCACCGTTTTTGGTTTAAGCTTA GTAGCCGCCGGCTTGAGTAGCACGGTGGTAGGCACATTGGCTGGACAGGTCGTGATGCA AGGTTTCATTCGTTTCCATATTCCGTTATGGGTACGTCGCACGGTAACGATGCTGCCGT CATTTATCGTCATCCTGATGGGATTAGACCCGACGCGCATCCTGGTAATGTCGCAAGTT TTACTGAGCTTTGGAATCGCGTTGGCCCTGGTGCCATTACTTATCTTCACTAGCGATAG TAAGTTGATGGGTGATCTTGTCAATAGCAAACGTGTGAAGCAAACAGGCTGGGTCATTG TGGTACTGGTTGTGGCCTTAAACATTTGGTTGTTAGTGGGCACGGCCCTTGGCTTG
Example 53. Circuit comprising TesB
[0677] The nucleic acid sequence of TesB is translated as follows.
Table 52 TesB sequences
[0678] In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 109 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino acid sequence of SEQ ID NO: 109 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 109 or a functional fragment thereof.
[0679] In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 91 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino
acid sequence of SEQ ID NO: 91 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 91 or a functional fragment thereof.
[0680] In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 93 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino acid sequence of SEQ ID NO: 93 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 93 or a functional fragment thereof.
[0681] In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 95 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino acid sequence of SEQ ID NO: 95 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 95 or a functional fragment thereof.
[0682] In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 97 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino acid sequence of SEQ ID NO: 97 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 97 or a functional fragment thereof.
[0683] In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 99 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino acid sequence of SEQ ID NO: 99 or a functional fragment thereof, or a nucleic
acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 99 or a functional fragment thereof.
Table 53 FNRS-fbrArgA and no antibiotic resistance
GGGGAAATGGCCTGTGTGGCAGTTCACCCGGATTAC
CGCAGTTCATCAAGGGGTGAAGTTCTGCTGGAACGC ATTGCCGCTCAGGCTAAGCAGAGCGGCTTAAGCAAA TTGTTTGTGCTGACCACGCGCAGTATTCACTGGTTC CAGGAACGTGGATTTACCCCAGTGGATATTGATTTA CTGCCCGAGAGCAAAAAGCAGTTGTACAACTACCAG CGTAAATCCAAAGTGTTGATGGCGGATTTAGGGTAA
GAAGTTCCTATACTTTCTAGAGAATAGGAACTTCGG SEQ ID AATAGGAACTTC NO: 115
KanR gene atgattgaacaagatggattgcacgcaggttctccg SEQ ID gccgcttgggtggagaggctattcggctatgactgg NO: 116 gcacaacagacaatcggctgctctgatgccgccgtg ttccggctgtcagcgcaggggcgcccggttcttttt gtcaagaccgacctgtccggtgccctgaatgaactg caggacgaggcagcgcggctatcgtggctggccacg acgggcgttccttgcgcagctgtgctcgacgttgtc actgaagcgggaagggactggctgctattgggcgaa gtgccggggcaggatctcctgtcatctcaccttgct cctgccgagaaagtatccatcatggctgatgcaatg cggcggctgcatacgcttgatccggctacctgccca ttcgaccaccaagcgaaacatcgcatcgagcgagca cgtactcggatggaagccggtcttgtcgatcaggat gatctggacgaagagcatcaggggctcgcgccagcc gaactgttcgccaggctcaaggcgcgcatgcccgac ggcgaggatctcgtcgtgacccatggcgatgcctgc ttgccgaatatcatggtggaaaatggccgcttttct ggattcatcgactgtggccggctgggtgtggcggac cgctatcaggacatagcgttggctacccgtgatatt gctgaagagcttggcggcgaa tgggctgaccgcttc ctcgtgctttacggtatcgccgctcccgattcgcag cgcatcgccttctatcgccttcttgacgagttcttc
tgagcgggactctggggttcgaaatgaccgaccaag cgacgcccaacctgccatcacgagatttcgattcca ccgccgccttctatgaaaggttgggcttcggaatcg ttttccgggacgccggctggatgatcctccagcgcg gggatctcatgctggagttcttcgcccaccccagct tcaaaagcgctct
KanR ggttgggaagccctgcaaagtaaactggatggcttt SEQ ID promoter cttgccgccaaggatctgatggcgcaggggatcaag NO: 117
atctgatcaagagacaggatgaggatcgtttcgc
CamR gene TCATCGCAGTACTGTTGTATTCATTAAGCATCTGCC SEQ ID
GACATGGAAGCCATCACAAACGGCATGATGAACCTG NO: 118 AATCGCCAGCGGCATCAGCACCTTGTCGCCTTGCGT ATAATATTTGCCCATGGTGAAAACGGGGGCGAAGAA GTTGTCCATATTGGCCACGTTTAAATCAAAACTGGT GAAACTCACCCAGGGATTGGCTGAGACGAAAAACAT ATTCTCAATAAACCCTTTAGGGAAATAGGCCAGGTT TTCACCGTAACACGCCACATCTTGCGAATATATGTG TAGAAACTGCCGGAAATCGTCGTGGTATTCACTCCA GAGCGATGAAAACGTTTCAGTTTGCTCATGGAAAAC
GGTGTAACAAGGGTGAACACTATCCCATATCACCAG
CTCACCGTCTTTCATTGCCATACGTAATTCCGGATG
AGCATTCATCAGGCGGGCAAGAATGTGAATAAAGGC
CGGATAAAACTTGTGCTTATTTTTCTTTACGGTCTT
TAAAAAGGCCGTAATATCCAGCTGAACGGTCTGGTT
ATAGGTACATTGAGCAACTGACTGAAATGCCTCAAA
ATGTTCTTTACGATGCCATTGGGATATATCAACGGT
GGTATATCCAGTGATTTTTTTCTCCAT
CamR TTTAGCTTCCTTAGCTCCTGAAAATCTCGACAACTC SEQ ID promoter AAAAAATACGCCCGGTAGTGATCTTATTTCATTATG NO: 119
GTGAAAGTTGGAACCTCTTACGTGCCGATCAACGTC TCATTTTCGCCAAAAGTTGGCCCAGGGCTTCCCGGT ATCAACAGGGACACCAGGATTTATTTATTCTGCGAA GTGATCTTCCGTCACAGGTAGGCGCGCCG
[0684] In some embodiments, the genetically engineered bacteria comprise the amino acid sequence of SEQ ID NO: 101 or a functional fragment thereof. In some embodiments, genetically engineered bacteria comprise an amino acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous to the amino acid sequence of SEQ ID NO: 101 or a functional fragment thereof, or a nucleic acid sequence that, but for the redundancy of the genetic code, encodes the same polypeptide as SEQ ID NO: 101 or a functional fragment thereof.