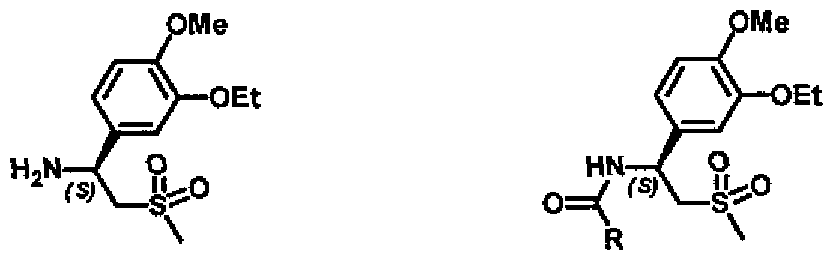WO2016192694A1 - A process for preparing the key intermediate of apremilast, using enzymatic resolution of the racemic amines - Google Patents
A process for preparing the key intermediate of apremilast, using enzymatic resolution of the racemic amines Download PDFInfo
- Publication number
- WO2016192694A1 WO2016192694A1 PCT/CZ2016/000061 CZ2016000061W WO2016192694A1 WO 2016192694 A1 WO2016192694 A1 WO 2016192694A1 CZ 2016000061 W CZ2016000061 W CZ 2016000061W WO 2016192694 A1 WO2016192694 A1 WO 2016192694A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- amine
- ethyl
- ethoxy
- methoxyphenyl
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P41/00—Processes using enzymes or microorganisms to separate optical isomers from a racemic mixture
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C317/00—Sulfones; Sulfoxides
- C07C317/26—Sulfones; Sulfoxides having sulfone or sulfoxide groups and nitrogen atoms, not being part of nitro or nitroso groups, bound to the same carbon skeleton
- C07C317/28—Sulfones; Sulfoxides having sulfone or sulfoxide groups and nitrogen atoms, not being part of nitro or nitroso groups, bound to the same carbon skeleton with sulfone or sulfoxide groups bound to acyclic carbon atoms of the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P13/00—Preparation of nitrogen-containing organic compounds
- C12P13/02—Amides, e.g. chloramphenicol or polyamides; Imides or polyimides; Urethanes, i.e. compounds comprising N-C=O structural element or polyurethanes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P41/00—Processes using enzymes or microorganisms to separate optical isomers from a racemic mixture
- C12P41/006—Processes using enzymes or microorganisms to separate optical isomers from a racemic mixture by reactions involving C-N bonds, e.g. nitriles, amides, hydantoins, carbamates, lactames, transamination reactions, or keto group formation from racemic mixtures
- C12P41/007—Processes using enzymes or microorganisms to separate optical isomers from a racemic mixture by reactions involving C-N bonds, e.g. nitriles, amides, hydantoins, carbamates, lactames, transamination reactions, or keto group formation from racemic mixtures by reactions involving acyl derivatives of racemic amines
Definitions
- the invention relates to a preparation method of (iS)-l-(3-ethoxy-4-methoxyphenyl)- 2-(methylsulfonyl)-ethyl-amine (S)-l, or its N-acyl derivatives of general formula (S)-2:
- the amine (S)-l is the key intermediate in the synthesis of (5)- ⁇ 2-[l-(3-ethoxy-4- methoxyphenyl)-2-methylsulfonylemyl]-4-acetylaminoisoindolin-l,3-dione 3, known as Apremilast.
- the amine (S)-l, or its corresponding salts are subjected to condensation with 3-acetamidophthalic anhydride 4, providing the desired product 3 (Scheme 7).
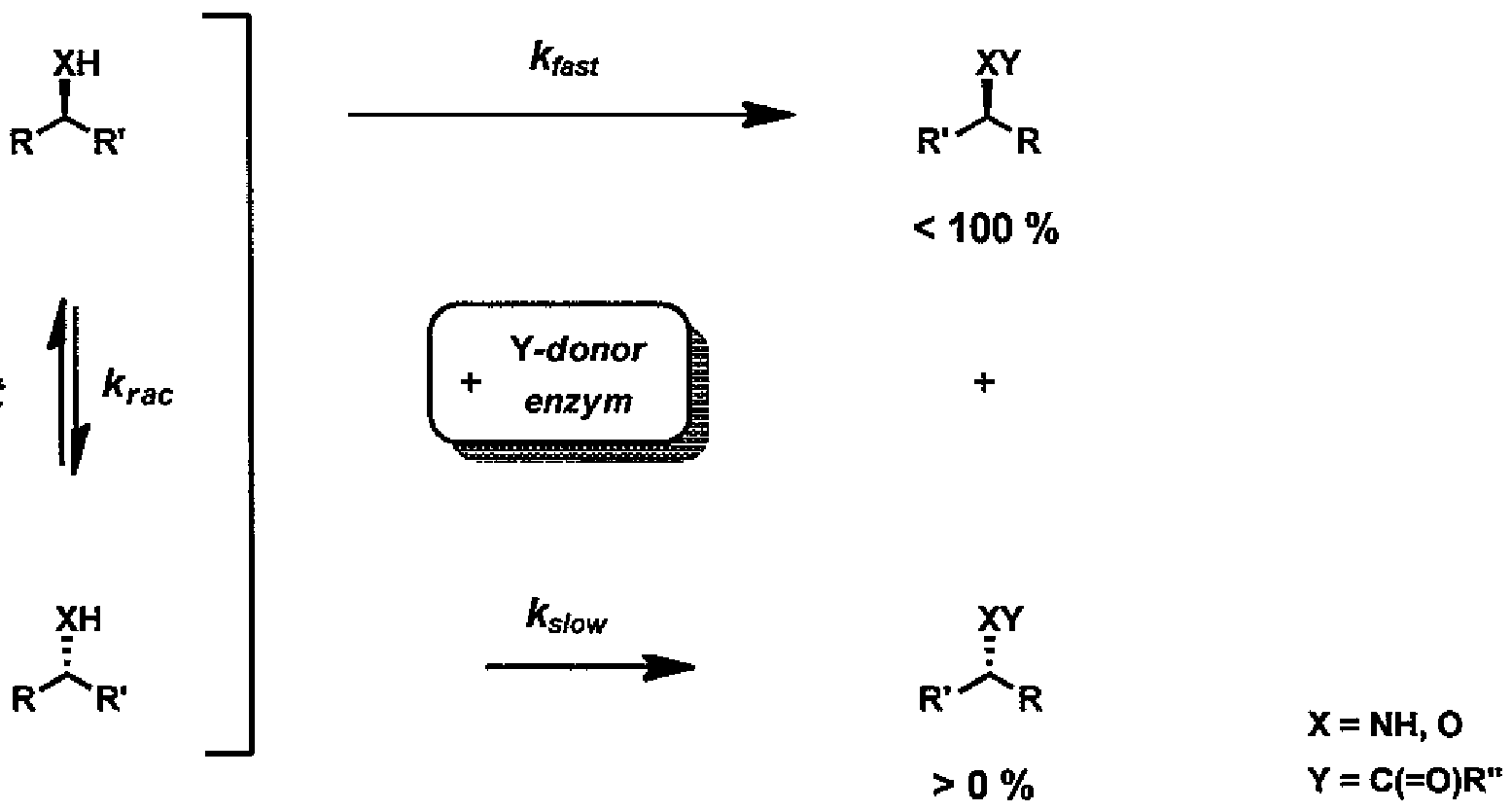
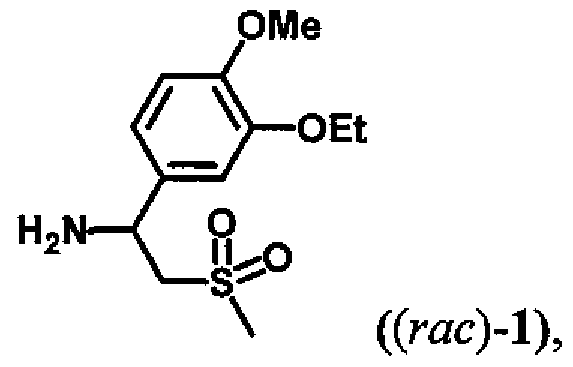
- Racemic 1 -(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl-amine (rac)-l actually consists of an equimolar mixture (1:1) of two opposite enantiomers (S)-l and (R)-l. Reactions catalyzed by enzymes are generally characterized by high stereoselectivity with respect to the substrates. Therefore, in the presence of a suitable enzyme and the acyl donor 5 derivatization of just one of the two enantiomers preferentially occurs.
- the product of this reaction is either a mixture of the desired chiral amine (S)-l and N-acyl derivative (R)-2 (method /, Scheme 1), or a mixture of the amine (R)-l and N-acyl derivative of the desired amine (S)-2 (method ii, Scheme 1).
- the present invention represents a very efficient, economically advantageous and in addition environment-friendly preparation method of the key intermediate of the synthesis of Apremilast 3, i.e. (5)-l-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl- amine
- Apremilast 3 ⁇ Scheme 1 is an orally available inhibitor of phosphodiesterase 4 (PDE4), which inhibits spontaneous production of the tumor necrosis factor a (TNF- ⁇ ), thus exhibiting an anti-inflammatory activity. It can be used for the treatment of psoriatic arthritis and it is also being tested for the treatment of other inflammatory diseases.
- PDE4 phosphodiesterase 4
- Apremilast 3 was first described as a racemic mixture of active pharmaceutical ingredients (WO 2000/25777 Al; EP 1126839 B). A few years later, in an application (WO 2003/080049) a particular enantiomer, (5)-isomer, commonly only referred to as Apremilast 3 was described, which is the carrier of the biological activity as such. Thus, it is the chiral amine (S)-l that is the key intermediate for the synthesis of Apremilast 3.
- Dynamic Kinetic Resolution (DKR, Figure 2) belongs to well-established and frequently used methods not only in the laboratory, but also in the industrial scale (P. Hoyos, V. Pace, A. R. Alcantara: Adv. Synth. Catal. 2012, 354, 2585-2611; A. Schmid, J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, B. Witholt: Nature 2001, 409, 258-268; A. Kamal, M. A. Azhar, T. Krishnaji, M. S. Malik, S. Azeeza: Coord. Chem. Rev. 2008, 252, 569-592; O. Kirk, and M. W.
- Enzymes that are generally usable for enzymatic resolution or for DKR belong to the wider group of so-called hydrolases (international identification EC 3), comprising e.g. lipases, esterases, peptidases etc.
- hydrolases international identification EC 3
- enzymes immobilized on solid carriers can be advantageously used as they make it possible to carry out the above mentioned reactions also in organic solvents without the presence of water or other additives.
- One of the most commonly used immobilized enzymes is Novozym 435®, which is lipase B of the Candida antarctica (CAL-B) yeast, usually bound to polymers of the acrylate type.
- Suitable N-acyl donors 5 are most frequently esters of carboxylic acids 5a, less frequently free carboxylic acids 5b and their corresponding anhydrides 5c, or more rarely also derivatives of carbonic acid 5d ( Figure 3) (C. E. Hoben, L. Kanupp, J.-E. Backvall: Tetrahedron Lett. 2008, 49, 977-979).
- racemization catalysts especially those of ruthenium, iridium, palladium, rhodium or vanadium ( Figure 4), or even pure transition metals, possibly adsorbed on suitable carriers (e.g. Pd/C, Pd/CaC0 3 , Pd/BaS0 4 , Rh/C, Rh/Al 2 0 3 etc.).
- suitable carriers e.g. Pd/C, Pd/CaC0 3 , Pd/BaS0 4 , Rh/C, Rh/Al 2 0 3 etc.
- Rz alkyls and aryls (also substituted in any manner)
- R 3 Me, Et, t-Bu, PhCH z , allyl, and the like
- Ar Ph " shv0 8 10 7b
- Ar 4-MeOC 6 H 4
- Ar Ph, PhCH 2 , 4-CF 3 C 6 H 4 , 4-F-C 6 H 4
- the invention provides a process for preparing ( i S)-l-(3-ethoxy-4-methox phenyl)-2- (methylsulfonyl)-ethyl-amine (S)-l, or its N-acyl derivatives of the general formula (S)-2, generally using enzymatic resolution of the corresponding racemic amine (rac)-l (Scheme 3)
- Racemic 1 -(3-ethoxy-4-memoxyphenyl)-2-(methylsulfonyl)-ethyl-amine (rac)-l consists of the equimolar mixture (1 :1) of two opposite enantiomers and In the presence of a suitable acyl donor 5 and a suitable enzyme from the group of so-called hydrolases (international identification EC 3), comprising e.g. Upases, esterases, peptidases etc., derivatization of one of both the enantiomers preferentially occurs.
- the product of such a reaction is either a mixture of the desired chiral amine and N-acyl derivative (R)- 2 (method i, Scheme 3), or a mixture of the amine (R)-l and N-acyl derivative of the desired amine (5)-2 (method ⁇ , Scheme 3).
- Suitable acyl-donors 5 ( Figure 3 and Figure 5) usable for enzymatic resolution of the racemic amine ⁇ rac)- ⁇ are generally the following compounds: a) esters of carboxylic acids 5a, b) carboxylic acids 5b, c) anhydrides of carboxylic acids 5c or d) esters of carbonic acid (referred to as carbonates) 5 d.
- R ⁇ R 2 is R 3 independently stand for H (except 5c and 5d), a Ci-Ci alkyl, aryl or heteroaryl with one or more heteroatoms, wherein all these groups may be further substituted by any functional groups.
- any functional group refers to: (a) halogens, (b) hydroxy, alkoxy or aryloxy groups, (c) amino and nitro groups, (d) CHO and acyl groups (i.e. ketones), (e) derivatives of carboxylic acids.
- the R 2 group may also stand for any alkenyl group (i.e. so-called enol esters).
- the esters 5a the enol esters can also be preferentially used, i.e. compounds wherein R 2 is a vinyl or isopropenyl.
- Suitable carbonates 5d are compounds wherein R 3 independently stands for a Ci-Cig alkyl, aryl or heteroaryl with one or more heteroatoms, wherein all these groups may be further substituted by any functional groups in the sense of the above mentioned definition.
- R 3 is a -Ce alkyl, phenyl, benzyl or allyl.
- Enzymes usable for the above mentioned enzymatic resolution ⁇ Scheme 3) can be any enzymes belonging to the wider group of so-called hydrolases (international identification EC 3), comprising e.g. lipases, esterases, peptidases etc.
- hydrolases international identification EC 3
- enzymes immobilized on solid carriers can be advantageously used as they also make it possible to carry out the above mentioned reactions in organic solvents without the presence of water or other additives.
- the following enzymes can especially be advantageously used: Novozym 435® (E.C. 3.1.1.3; lipase B from Candida antarctica CAL-B yeast, bound to a polymer of the acrylate type), Subtilisin (E.C.
- Enzymatic resolution also offers another prospective advantage, which is the above described enzymatic dynamic kinetic resolution ( Figure 2).
- Figure 2 In the presence of suitable racemization catalysts, continuous mutual conversion of the enantiomers ( ⁇ S -1 and (R)-l can be achieved with the use of this method, which makes it possible to theoretically achieve up to 100% yield of the desired isomer, or its derivative while without the presence of racemization catalysts the maximum yield can only be 50%. In terms of industrial production and its economy this fact also represents a substantial advantage.
- the invention provides a process for preparing (S)-l-(3-ethoxy-4-methoxyphenyl)-2- (methylsulfonyl)-ethyl-amine (S)-l, or its iV-acyl derivatives of the general formula (S)-2, generally using enzymatic resolution of the corresponding racemic amine (mc)-l.
- the invention provides a process for preparing (5)-l-(3-ethoxy-4-methoxyphenyl)-2- (methylsulfonyl)-ethyI-amine (S)-l, or its N-acyl derivatives of general formula (S)-2, generally using the enzymatic dynamic kinetic resolution ( Figure 2, Scheme 5), i.e.
- Still another aspect of the invention provides conversion of the derivatives of general formula (S)-2 to the chiral amine (S)-l and its use for the preparation of apremilast 3 (Scheme 1).
- Racemic 1 -(3-emoxy-4-methoxyphenyl)-2-(memylsulfonyl)-emyl-amine (rac)-l consists of the equimolar mixture (1:1) of two opposite enantiomers (S -l and (R)-l.
- R, CH 3 ;
- Suitable acyl-donors 5 ⁇ Figure 6) usable for enzymatic resolution of the racemic amine (rac)- 1 are generally the following compounds: a) esters of carboxylic acids 5a, b) carboxylic acids 5b, c) anhydrides of carboxylic acids 5c or d) esters of carbonic acid (referred to as carbonates) 5d.
- R ,R , R independently stand for H (except 5c and 5d), a Q-C ⁇ alkyl, aryl or heteroaryl with one or more heteroatoms wherein all these groups may be further substituted by any functional groups.
- any functional group refers to: (a) halogens, (b) hydroxy, alkoxy or aryloxy groups, (c) amino and nitro groups, (d) CHO and acyl groups (i.e. ketones), (e) derivatives of carboxylic acids.
- the R 2 group may also stand for any alkenyl group (i.e. enol esters).
- esters of the general formula 5a in the particular case esters Saa - Saf, or carbonates of the general formula 5d, in particular the compounds 5da - Sdc, can be preferably used ⁇ Figure 6).
- All the above mentioned acyl donors 5a and 5b are liquid substances, commonly commercially available and, in addition, inexpensive, so they can be advantageously used not only as acyl donors, but also as reaction media (solvents), which are, moreoverm environment-friendly.
- the enzymes usable for the above mentioned enzymatic resolution ⁇ Scheme 3) can be any enzymes belonging to the wider group of hydrolases (international identification EC 3), comprising e.g. lipases, esterases, peptidases etc.
- hydrolases international identification EC 3
- enzymes immobilized on solid carriers can be advantageously used as they also make it possible to carry out the above mentioned reactions in organic solvents without the presence of water or other additives.
- the following enzymes can especially be advantageously used: Novozym 435® (E.C. 3.1.1.3; lipase B from Candida antarctica CAL-B yeast, bound to a polymer of the acrylate type), Subtilisin (E.C.
- the esters of carboxylic acids 5a preferably especially the esters of methoxyacetic acid 5aa and 5ab
- Immobilized lipases are highly resistant to the environment of common solvents as well as to elevated temperatures.
- the above mentioned reactions ⁇ Scheme 6) can be conducted either without the presence of any solvents (the acyl donors 5 serve as a solvent at the same time), or in a wide range of commonly available solvents.
- Applicable solvents comprise aliphatic or aromatic hydrocarbons and their halo derivatives, ethers, alcohols, derivatives of carboxylic acids (e.g. amides and nitriles), or sulfur derivatives, such as sulfones and sulfoxides.
- especially ethers can be used as f-butyl methyl ether (MTBE), cyclopentyl methyl ether (CPME), or cyclic 2-memyltetxahydrofuran ( eTHF).
- DMC dimethyl carbonate
- the reaction itself can be carried out in a wide temperature range.
- the temperature of the reaction mixture mainly influences the reaction rate: with an increased temperature the reaction gets accelerated, i.e. the reaction time gets shorter. It is suitable to carry out the above mentioned reactions at elevated temperatures (depending on the use of a possible solvent and its physical characteristics), preferably in the temperature range of 50 to 125°C.
- the so-called “Shvo catalysts” 7a and 7b can mainly be used in combination with the acyl donors 5aa, 5ab and 5da and with immobilized lipase Novozym 435® as the enzyme (Scheme 7).
- the respective derivatives (S)-2 of the desired amine (S)-l can be obtained in chemical yields of up to 90% (i.e. 40% of the undesired enantiomer (R)-l transformed) while the chiral purity achieves up to 97%.
- either strong acids, or strong bases can be used equally successfully for the conversion to the free amine (S)-l ⁇ Scheme 8).
- Suitable acids are any strong mineral acids, preferably HC1, HBr or H 2 S0 4 .
- the strong bases for example, hydroxides of alkali metals or alkaline earth metals can be used, preferably LiOH, NaOH, KOH and Ba(OH) 2 . Further, also some carbonates, preferably Na 2 C0 3 and K 2 C0 3 .
- the reactions can be conducted in a wide temperature range, preferably at the temperatures from 50 to 150°C, depending on the used agent and solvent.
- solvents for both the approaches mainly protic polar solvents can be used, including water, or their mixtures with aprotic polar solvents, preferably, e.g., with ethers such as dimethoxyethane, tetrahydrofuran and dioxane.
- the chiral purity (general stereometric purity) of all the said compounds was determined by means of high-performance liquid chromatography (HPLC equipped with a UV/VIS detector). The analyses were conducted in a Chiracel® OZ-3 column (150x4.6 mm; 3 ⁇ stationary phase) using a hexane/ethanol 75:25 mixture as the mobile phase (temperature 35 °C; flow rate 1.2 ml/min; detection at 280 nm).
- Novozym 435 Lipase B from Candida antarctica yeast (E.C. 3.1.1.3; CAL-B) immobilized on methacrylate polymer (>5000 U/g).
Abstract
The invention relates to a preparation method of (S)-l-(3-ethoxy-4-methoxyphenyl)- 2-(methylsulfonyl)-ethyl-amine (S)-l, or its N-acyl derivatives of general formula (S)-2. The amine (S)-l is the key intermediate in the synthesis of (S)-{2-[l-(3-ethoxy-4- methoxyphenyi)-2-methyisulfonylethyl]-4-acetylaminoisoindolin-l ?3-dione, known as Apremilast. In this synthesis, the amine (S)-l, or its respective salts, are subjected to condensation with 3-acetamidophthalic anhydride 4, providing the desired product 3 (Scheme 1).
Description
A process for preparing the key intermediate of apremilast, using enzymatic resolution of the racemic amines
Technical Field
The invention relates to a preparation method of (iS)-l-(3-ethoxy-4-methoxyphenyl)- 2-(methylsulfonyl)-ethyl-amine (S)-l, or its N-acyl derivatives of general formula (S)-2:
(S>-2
Figure 1
The amine (S)-l is the key intermediate in the synthesis of (5)-{2-[l-(3-ethoxy-4- methoxyphenyl)-2-methylsulfonylemyl]-4-acetylaminoisoindolin-l,3-dione 3, known as Apremilast. In this synthesis, the amine (S)-l, or its corresponding salts, are subjected to condensation with 3-acetamidophthalic anhydride 4, providing the desired product 3 (Scheme 7).
Racemic 1 -(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl-amine (rac)-l actually consists of an equimolar mixture (1:1) of two opposite enantiomers (S)-l and (R)-l. Reactions catalyzed by enzymes are generally characterized by high stereoselectivity with respect to the substrates. Therefore, in the presence of a suitable enzyme and the acyl donor 5 derivatization of just one of the two enantiomers preferentially occurs. The product of this reaction is either a mixture of the desired chiral amine (S)-l and N-acyl derivative (R)-2 (method /, Scheme 1), or a mixture of the amine (R)-l and N-acyl derivative of the desired amine (S)-2 (method ii, Scheme 1).
Scheme 1 In the first case (method i), the optically pure amine (S)-l can, after separation from the respective derivative (R)-2, be directly used for the synthesis of Apremilast 3. In the second case (method ii), after enzymatic resolution and separation of the products, the desired amine (5)-l is chemically released from the corresponding derivative (S)-2.
The said enzymatic resolution only makes it possible to achieve max. 50% yield because the contents of each of the two opposite enantiomers S)-l and (R)-l in the racemic amine (rac)-\ are just 50%. However, in the presence of a suitable racemization catalyst continuous mutual conversion of the (S) and (R) isomers occurs, which makes it possible to theoretically achieve up to 100% yield of the desired isomer, or its derivative. This method is generally known as the enzymatic dynamic kinetic resolution (hereinafter only: DKR; Figure 2).
Thus, the present invention represents a very efficient, economically advantageous and in addition environment-friendly preparation method of the key intermediate of the synthesis of Apremilast 3, i.e. (5)-l-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl- amine
catalyst
Figure 2 Background Art
Apremilast 3 {Scheme 1) is an orally available inhibitor of phosphodiesterase 4 (PDE4), which inhibits spontaneous production of the tumor necrosis factor a (TNF-α), thus exhibiting an anti-inflammatory activity. It can be used for the treatment of psoriatic arthritis and it is also being tested for the treatment of other inflammatory diseases.
Apremilast 3 was first described as a racemic mixture of active pharmaceutical ingredients (WO 2000/25777 Al; EP 1126839 B). A few years later, in an application (WO 2003/080049) a particular enantiomer, (5)-isomer, commonly only referred to as Apremilast 3 was described, which is the carrier of the biological activity as such. Thus, it is the chiral amine (S)-l that is the key intermediate for the synthesis of Apremilast 3. An application (WO 2003/080049) describes the procedure of chiral resolution of the racemic amine (rac)-l with the use of N-acetyl-L-leucine and subsequent use of the corresponding salt 6 for the synthesis of Apremilast 3 (Scheme 2).
Using this procedure, the desired enantiomer (S)-l was isolated in the yield of 44%, or 89% (based on the theoretical content of 50% of formula (S)-l in the racemic amine (rac)-X). Also, later applications (US 2008/0234359 Al; EP 2431371 Al) make use of the use N- acetyl-L-leucine, or derivatives of chiral amino acids in general, for chiral resolution of the racemic amine (rac)-l and subsequent use of the corresponding salt 6 for synthesis of Apremilast 3 (Scheme 2).
Besides the above mentioned ones, a number of asymmetrical syntheses of the desired enantiomer of formula (5)-l have been described that use chiral catalysts of transition metals, e.g. rhodium (US2013/217919 Al; US2014/81032 Al). However, these procedures are not suitable for industrial production of (5)-l-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)- ethylamine (S)-l, or Apremilast 3, in economic terms.
At present, Dynamic Kinetic Resolution (DKR, Figure 2) belongs to well-established and frequently used methods not only in the laboratory, but also in the industrial scale (P. Hoyos, V. Pace, A. R. Alcantara: Adv. Synth. Catal. 2012, 354, 2585-2611; A. Schmid, J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, B. Witholt: Nature 2001, 409, 258-268; A. Kamal, M. A. Azhar, T. Krishnaji, M. S. Malik, S. Azeeza: Coord. Chem. Rev. 2008, 252, 569-592; O. Kirk, and M. W. Christensen: Org. Process Res. Dev. 2002, 6, 446-451). In the industrial scale, this method is also used for a number of amines of the benzyl type, i.e. for compounds that are structurally close to the racemic amine (rac)-l.
Enzymes that are generally usable for enzymatic resolution or for DKR belong to the wider group of so-called hydrolases (international identification EC 3), comprising e.g. lipases, esterases, peptidases etc. Besides free enzymes or cell cultures, enzymes immobilized on solid carriers can be advantageously used as they make it possible to carry
out the above mentioned reactions also in organic solvents without the presence of water or other additives. One of the most commonly used immobilized enzymes is Novozym 435®, which is lipase B of the Candida antarctica (CAL-B) yeast, usually bound to polymers of the acrylate type.
Suitable N-acyl donors 5 (Scheme 1; Y-donor in Figure 2) are most frequently esters of carboxylic acids 5a, less frequently free carboxylic acids 5b and their corresponding anhydrides 5c, or more rarely also derivatives of carbonic acid 5d (Figure 3) (C. E. Hoben, L. Kanupp, J.-E. Backvall: Tetrahedron Lett. 2008, 49, 977-979).
In the case of DKR, complexes of some transition metals are most frequently used as the racemization catalysts, especially those of ruthenium, iridium, palladium, rhodium or vanadium (Figure 4), or even pure transition metals, possibly adsorbed on suitable carriers (e.g. Pd/C, Pd/CaC03, Pd/BaS04, Rh/C, Rh/Al203 etc.).
i = Me, Et, CF3, MeOCH2, and the like
Rz = alkyls and aryls (also substituted in any manner)
R3 = Me, Et, t-Bu, PhCHz, allyl, and the like
Figure 3
7a Ar = Ph "shv0 8 10 7b Ar = 4-MeOC6H4 Ar = Ph, PhCH2, 4-CF3C6H4, 4-F-C6H4
. -iOSi tis
Ph3SiCT V OSiPI¾
11 X = CI, I 12
Figure 4
Disclosure of Invention
The invention provides a process for preparing (iS)-l-(3-ethoxy-4-methox phenyl)-2- (methylsulfonyl)-ethyl-amine (S)-l, or its N-acyl derivatives of the general formula (S)-2, generally using enzymatic resolution of the corresponding racemic amine (rac)-l (Scheme 3)
Scheme 3
Racemic 1 -(3-ethoxy-4-memoxyphenyl)-2-(methylsulfonyl)-ethyl-amine (rac)-l consists of the equimolar mixture (1 :1) of two opposite enantiomers and In the presence of a suitable acyl donor 5 and a suitable enzyme from the group of so-called hydrolases (international identification EC 3), comprising e.g. Upases, esterases, peptidases etc., derivatization of one of both the enantiomers preferentially occurs. Then, the product of such a reaction is either a mixture of the desired chiral amine and N-acyl derivative (R)- 2 (method i, Scheme 3), or a mixture of the amine (R)-l and N-acyl derivative of the desired amine (5)-2 (method κ, Scheme 3).
Suitable acyl-donors 5 (Figure 3 and Figure 5) usable for enzymatic resolution of the racemic amine {rac)-\ are generally the following compounds: a) esters of carboxylic acids 5a, b) carboxylic acids 5b, c) anhydrides of carboxylic acids 5c or d) esters of carbonic acid (referred to as carbonates) 5 d. In the case of all these compounds, R^R2 is R3 independently stand for H (except 5c and 5d), a Ci-Ci alkyl, aryl or heteroaryl with one or more heteroatoms, wherein all these groups may be further substituted by any functional groups. For the purposes of this invention, the term "any functional group" refers to: (a) halogens, (b) hydroxy, alkoxy or aryloxy groups, (c) amino and nitro groups, (d) CHO and acyl groups (i.e. ketones), (e) derivatives of carboxylic acids. In the case of the esters 5a, the R2 group may also stand for any alkenyl group (i.e. so-called enol esters).
Figure 5
The derivatives 5a-5c can advantageously be used, wherein R stands for the 0¾.ηΧη group (n = 1 to 3), wherein X is any Ci-Cis alkyl, any halogen (F, CI, Br and I), or alkoxy group OR4, wherein R4 stands for any Ci-Qs alkyl or C6-C10 aryl. Concerning the esters 5a, the enol esters can also be preferentially used, i.e. compounds wherein R2 is a vinyl or isopropenyl. Suitable carbonates 5d are compounds wherein R3 independently stands for a Ci-Cig alkyl, aryl or heteroaryl with one or more heteroatoms, wherein all these groups may be further substituted by any functional groups in the sense of the above mentioned
definition. Preferably, especially the carbonates 5d, wherein R3 is a -Ce alkyl, phenyl, benzyl or allyl.
Enzymes usable for the above mentioned enzymatic resolution {Scheme 3) can be any enzymes belonging to the wider group of so-called hydrolases (international identification EC 3), comprising e.g. lipases, esterases, peptidases etc. Besides free enzymes or cell cultures, enzymes immobilized on solid carriers can be advantageously used as they also make it possible to carry out the above mentioned reactions in organic solvents without the presence of water or other additives. Out of immobilized enzymes, the following enzymes can especially be advantageously used: Novozym 435® (E.C. 3.1.1.3; lipase B from Candida antarctica CAL-B yeast, bound to a polymer of the acrylate type), Subtilisin (E.C. 3.4.21.62; protease from Bacillus species, covalently bound on an amino-acrylate polymer), or Penicilin-G amidase (E.C. 3.5.1.11; amidase from Escherichia coli, immobilized on an epoxy-acrylate polymer).
It has been unexpectedly found out that especially the carbonates 5d (R3 = C1-C3) provide, in the presence of the immobilized lipase Novozym 435®, the desired enantiomer of the amine in the form of the respective carbamates (S)-2a (Scheme 4), in high chemical yields and in addition with high chiral purity. A clear advantage of this transformation is the possibility to use the respective carbonates 5d not only as the acyl donors, but also directly as the reaction media. In addition, the carbonates 5d are commonly commercially available, cheap and entirely environment-friendly compounds. In terms of industrial production, its economy and environmental aspects, these are all significant innovative elements.
(rac)-1 5d (S)-2a Scheme 4
Enzymatic resolution also offers another prospective advantage, which is the above described enzymatic dynamic kinetic resolution (Figure 2). In the presence of suitable racemization catalysts, continuous mutual conversion of the enantiomers (<S -1 and (R)-l can be achieved with the use of this method, which makes it possible to theoretically achieve up
to 100% yield of the desired isomer, or its derivative while without the presence of racemization catalysts the maximum yield can only be 50%. In terms of industrial production and its economy this fact also represents a substantial advantage.
It has been surprisingly found out that under the conditions of enzymatic resolution of the racemic amine (rac)-X, in the presence of suitable racemization catalysts, mutual conversion of the two enantiomers (S)-l and (R)-l (represented in the racemic amine in the molar ratio of 1:1) can be efficiently achieved, i.e. the chemical yield of the desired enantiomer (5)-l in the form of the respective derivatives (5)-2 can exceed 50% (Scheme 5). Especially complexes of some transition metals, preferably complexes of ruthenium - referred to as the Shvo catalysts 7a and 7b (Figure 4) have proved to be suitable racemization catalysts for this transformation (Figure 4).
Scheme 5
Detailed description of the invention
The invention provides a process for preparing (S)-l-(3-ethoxy-4-methoxyphenyl)-2- (methylsulfonyl)-ethyl-amine (S)-l, or its iV-acyl derivatives of the general formula (S)-2, generally using enzymatic resolution of the corresponding racemic amine (mc)-l. In another aspect, the invention provides a process for preparing (5)-l-(3-ethoxy-4-methoxyphenyl)-2- (methylsulfonyl)-ethyI-amine (S)-l, or its N-acyl derivatives of general formula (S)-2, generally using the enzymatic dynamic kinetic resolution (Figure 2, Scheme 5), i.e. enzymatic resolution in the presence of any racemization catalysts. Still another aspect of the invention provides conversion of the derivatives of general formula (S)-2 to the chiral amine (S)-l and its use for the preparation of apremilast 3 (Scheme 1).
Racemic 1 -(3-emoxy-4-methoxyphenyl)-2-(memylsulfonyl)-emyl-amine (rac)-l consists of the equimolar mixture (1:1) of two opposite enantiomers (S -l and (R)-l. In the presence of a suitable acyl-donor 5 and a suitable enzyme from the group of hydrolases (international identification EC 3), derivatization of preferentially only one of the two enantiomers occurs. Such reaction produces either a mixture of the desired chiral amine (5)-l and the N-acyl derivative (R -2 (method i, Scheme 3), or a mixture of the amine (R)-l and N- acyl derivative of the desired amine (S)-2 (method ii, Scheme 3).
Saa 1 = MeOCH2; R2 = Me 5da R3 = Me
Sab R, = MeOCH2; R2 = /-Pr 5db j = Et
5ac Rf = CH3; R2 = Et 5dc R3 = CH2Ph
5ad R, = CH3; R2 = /-Pr
5ae R, = CH3; R2 = CH=CH2
5ae R, = CH3; R2 = C(CH3)=CH2
Saf R1 = CF3; R2 = Et
Figure 6
Suitable acyl-donors 5 {Figure 6) usable for enzymatic resolution of the racemic amine (rac)- 1 are generally the following compounds: a) esters of carboxylic acids 5a, b) carboxylic acids 5b, c) anhydrides of carboxylic acids 5c or d) esters of carbonic acid (referred to as carbonates) 5d. In the case of all these compounds, R ,R , R independently stand for H (except 5c and 5d), a Q-C^ alkyl, aryl or heteroaryl with one or more heteroatoms wherein all these groups may be further substituted by any functional groups. For the purposes of this invention, the term "any functional group " refers to: (a) halogens, (b) hydroxy, alkoxy or aryloxy groups, (c) amino and nitro groups, (d) CHO and acyl groups (i.e. ketones), (e) derivatives of carboxylic acids. In the case of the esters 5a, the R2 group may also stand for any alkenyl group (i.e. enol esters).
Either esters of the general formula 5a, in the particular case esters Saa - Saf, or carbonates of the general formula 5d, in particular the compounds 5da - Sdc, can be preferably used {Figure 6). All the above mentioned acyl donors 5a and 5b are liquid substances, commonly commercially available and, in addition, inexpensive, so they can be
advantageously used not only as acyl donors, but also as reaction media (solvents), which are, moreoverm environment-friendly.
The enzymes usable for the above mentioned enzymatic resolution {Scheme 3) can be any enzymes belonging to the wider group of hydrolases (international identification EC 3), comprising e.g. lipases, esterases, peptidases etc. Besides free enzymes or cell cultures, enzymes immobilized on solid carriers can be advantageously used as they also make it possible to carry out the above mentioned reactions in organic solvents without the presence of water or other additives. Out of immobilized enzymes, the following enzymes can especially be advantageously used: Novozym 435® (E.C. 3.1.1.3; lipase B from Candida antarctica CAL-B yeast, bound to a polymer of the acrylate type), Subtilisin (E.C. 3.4.21.62; protease from Bacillus species, covalently bound on an amino-acrylate polymer), or Penicilin-G amidase (E.C. 3.5.1.11; amidase from Escherichia coli, immobilized on an epoxy-acrylate polymer).
It has been surprisingly found out that especially the carbonates 5d (R3 = Cx-C^), preferably the dimethyl carbonate 5da, and also the esters of carboxylic acids 5a, preferably especially the esters of methoxyacetic acid 5aa and 5ab, provide, in the presence of immobilized lipases, preferably the immobilized lipase Novozym 435®, the desired enantiomer (S)-l in the form of the respective derivatives (S)-2aa, or (5)-2ba {Scheme 6) in high chemical yields and in addition with high chemical purity.
Scheme 6
Immobilized lipases are highly resistant to the environment of common solvents as well as to elevated temperatures. Thus, the above mentioned reactions {Scheme 6) can be conducted either without the presence of any solvents (the acyl donors 5 serve as a solvent at
the same time), or in a wide range of commonly available solvents. Applicable solvents comprise aliphatic or aromatic hydrocarbons and their halo derivatives, ethers, alcohols, derivatives of carboxylic acids (e.g. amides and nitriles), or sulfur derivatives, such as sulfones and sulfoxides. Advantageously, especially ethers can be used as f-butyl methyl ether (MTBE), cyclopentyl methyl ether (CPME), or cyclic 2-memyltetxahydrofuran ( eTHF).
However, in economical and environmental terms it is even more advantageous to carry out the above mentioned enzymatic resolution (Scheme 6) without the use of solvents. Especially, dimethyl carbonate (DMC) can be preferably used, which serves, in the particular case, not only as an acyl donor 5, but also as a solvent. DMC is well-known and frequently recommended as a cheap and at the same time environment-friendly solvent.
The reaction itself can be carried out in a wide temperature range. The temperature of the reaction mixture mainly influences the reaction rate: with an increased temperature the reaction gets accelerated, i.e. the reaction time gets shorter. It is suitable to carry out the above mentioned reactions at elevated temperatures (depending on the use of a possible solvent and its physical characteristics), preferably in the temperature range of 50 to 125°C.
It has been surprisingly found out that under the conditions of enzymatic resolution of the racemic amine (rac)~l, in the presence of suitable racemization catalysts, mutual conversion of the two enantiomers (S)-l and (R)-l (represented in the racemic amine in the molar ratio of 1 :1) can be efficiently achieved, i.e. the chemical yield of the desired enantiomer (S)-l in the form of the respective derivatives (S)-2 can exceed 50% (Scheme 5). A number of complexes of transition metals as well as pure transition metals, both free, or adsorbed on various carriers, have turned out to be suitable racemization catalysts for this transformation (Figure 4).
Advantageously, the so-called "Shvo catalysts" 7a and 7b can mainly be used in combination with the acyl donors 5aa, 5ab and 5da and with immobilized lipase Novozym 435® as the enzyme (Scheme 7). Under these conditions, the respective derivatives (S)-2 of the desired amine (S)-l can be obtained in chemical yields of up to 90% (i.e. 40% of the undesired enantiomer (R)-l transformed) while the chiral purity achieves up to 97%.
Scheme 7
To be able to use the protected derivatives (S)-2 for the preparation of apremilast 3 {Scheme 1), it is first necessary to convert them, in a suitable way, to the desired free amine (S)-l (method ii, Scheme 1), The RCO acyl groups belong to groups that are commonly used to protect amines. Thanks to this, a number of methods and agents have been described, enabling carrying out such transformations even under very moderate conditions.
In the particular case of the derivatives (5)-2aa and (S)-2ba, either strong acids, or strong bases can be used equally successfully for the conversion to the free amine (S)-l {Scheme 8). Suitable acids are any strong mineral acids, preferably HC1, HBr or H2S04. As the strong bases, for example, hydroxides of alkali metals or alkaline earth metals can be used, preferably LiOH, NaOH, KOH and Ba(OH)2. Further, also some carbonates, preferably Na2C03 and K2C03.
(S)-2aa (S)-2ba (SH
Scheme 8
In both the above mentioned cases (use of acids or bases), the reactions can be conducted in a wide temperature range, preferably at the temperatures from 50 to 150°C,
depending on the used agent and solvent. As the solvents, for both the approaches mainly protic polar solvents can be used, including water, or their mixtures with aprotic polar solvents, preferably, e.g., with ethers such as dimethoxyethane, tetrahydrofuran and dioxane. Suitable protic solvents are: a) water; b) alcohols of the general formula R OH, wherein R4 stands for any Ci-Cg alkyl (branched as well as unbranched); c) diols of the general formula HO-R5-OH, wherein Rs stands for (CHR6)n, wherein n = 2-4 and R6 independently stands for H, a CrC3 alkyl or CH2OH; d) diols of the general formula HO-R5-OH, wherein R5 stands for [(C]¾)n Z(CH2)n]m, wherein independently: n = 1-4, m = 1-4 and Z stands for O, S or NR7, wherein R7 is H or any Ci-C8 alkyl, possibly substituted by another hydroxy group; e) liquid carboxylic acids of the general formula R8COOH, wherein R8 stands for H or any C\- Cg alkyl. Out of the above mentioned protic solvents, especially water, methanol, ethanol, propanols, butanols, ethylene glycol, propylene glycols, diethanolamine and triethanolamine can be advantageously used.
In the case of the carbamate (S)-2aa, other specific agents can also be used for its transformation to the free amine (S)-l, such as trialkyl halo silanes of the general formula (R9)3SiX, wherein R9 independently represents a Ci-C6 alkyl and X may be Cl, Br or I. Especially, trimethyl silyl iodide MesSil (TMSI) can be preferably used; the reaction itself can be conducted in various polar solvents, preferably in dichloromethane or in acetonitrile.
The invention is clarified in a more detailed way using the examples below. These examples, which illustrate the improvement of the procedure in accordance with the invention, only have an illustrative character and do not restrict the scope of the invention in any respect.
Experimental part
General:
The chemical purity of all the said compounds was determined by means of high- performance liquid chromatography (HPLC equipped with a UV/VIS detector). The analyses were conducted in an XSelect® HSS C18 SB column (100x4.6 mm; 2.5 μηι stationary phase) using an acetonitrile/10 mM phosphate buffer mixture (pH = 2.5) as the mobile phase (temperature 45 °C; flow 0.8 ml/min; detection at 230 run).
The chiral purity (general stereometric purity) of all the said compounds was determined by means of high-performance liquid chromatography (HPLC equipped with a
UV/VIS detector). The analyses were conducted in a Chiracel® OZ-3 column (150x4.6 mm; 3 μιη stationary phase) using a hexane/ethanol 75:25 mixture as the mobile phase (temperature 35 °C; flow rate 1.2 ml/min; detection at 280 nm).
The term "laboratory temperature" refers, for the purposes of the text below and above, to the temperature range from 22 to 26°C. Unless indicated otherwise, the term "equivalent" (or abbreviated "equiv.") always means "molar ratio" in the text and tables below. The indication ee means "enantiomeric excess" (in percent) of a pure isomer (R or S) in its mixture with the racemate (RS) mixture, and its calculation is based on the equation: ee = (\R - S\)/(R + S)- 100 = \%R -%S\ [%].
Abbreviations and names of the chemical compounds:
(rac)-l 1 -(3 -Emoxy-4-memoxyphenyl)-2-(memylsulfonyl)-ethyl-amine
(iS)-l (5)- 1 -(3-Emoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl-amine
(if )-l (R)- 1 -(3 -Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl-amine
2aa Methyl ( 1 -(3 -ethoxy-4-methoxyphenyl)-2-(methyl sulfonyl)ethyl)carbamate
(5)-2aa Methyl (S)-(l -(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)- carbamate
(S)-2ba (5)-N-(l-(3-emoxy-4-memoxyphenyl)-2-(memylsulfonyl)-ethyl)-2- methoxyacetamide
Novozym 435 Lipase B from Candida antarctica yeast (E.C. 3.1.1.3; CAL-B) immobilized on methacrylate polymer (>5000 U/g).
TMSI Trimethyl silyl iodide
DMC Dimethyl carbonate
MeTHF 2-Methyltetrahydrofuran
MTBE /-Butyl methyl ether
CPME Cyclopentyl methyl ether
Examples
Example 1
Methyl (5 -( 1 -(3 -emoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)carbamate (5)-2aa
0.13 g (0.48 mmol) of the racemic amine {rac)-\ is suspended in a mixture of CPME (0.4 ml) and dimethyl carbonate (0.1 ml). Novozym 435 (60 mg) is added to the white
suspension and the mixture is maintained at a temperature of 85-90°C, being moderately stirred. After 43 hours, the reaction mixture contains 44% of the starting amine 1 and 53% of the carbamate 2aa. The reaction mixture is filtered in a hot state and the filtrate is concentrated at a reduced pressure. The concentrated product is stirred up in 2M aqueous HC1 and extracted with 2 x 3 ml of dichloromethane. The combined organic extracts are concentrated. The process provides 75 mg of the carbamate 2aa (47%) containing 92% of (S)-2aa.
Example 2
Methyl (S)-(l-(3-emoxy-4-memoxyphenyl)-2-(methylsulfonyl)ethyl)carbamate (S)-2aa
0.13 g (0.48 mmol) of the racemic amine (rac)~l is suspended in a mixture of CPME (0.4 ml) and dimethyl carbonate (0.1 ml). Novoz m 435 (60 mg) and 5% Pd on CaC03 (16 mg) are added to the white suspension. The mixture is maintained at 80°C, being moderately stirred. After 24 hours, the reaction mixture contains 45% of the starting amine 1 and 53% of the carbamate 2aa. The reaction mixture is filtered in a hot state and the filtrate is concentrated at a reduced pressure. The concentrated product is stirred up in 2M aqueous HC1 and extracted with 2 x 3 ml of dichloromethane. The combined organic extracts are concentrated. The process provides 75 mg of the carbamate 2aa (47%) containing 82% of (5)-2aa.
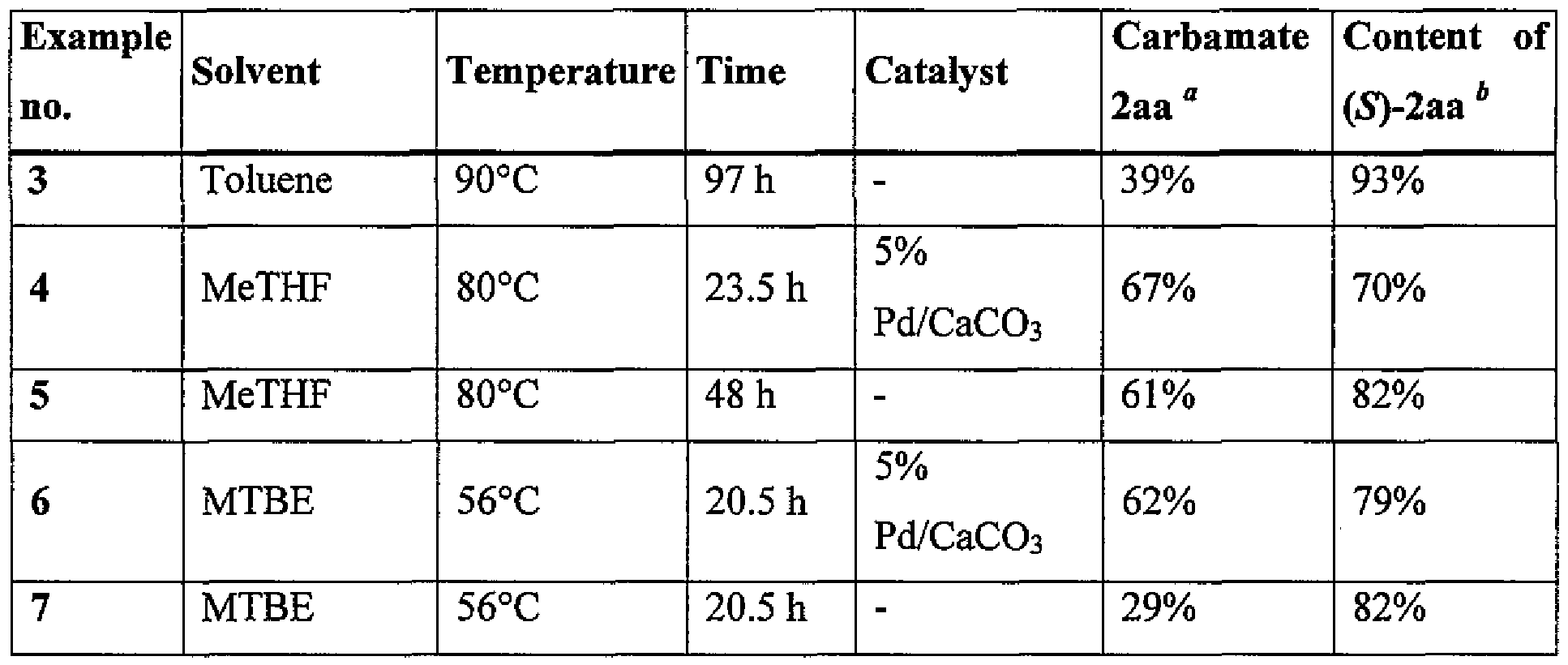
Examples 3-7
Methyl (5)-(l-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)carbamate (S)-2aa The procedure described in Example 1 was precisely repeated in the subsequent experiments. All, and the only, changes (solvent type, temperature, reaction time, or addition of a catalyst) are specified in Table 1.
Table 1. a Content of the carbamate 2aa in the reaction mixture (according to HPLC);
* Content of (S)-2aa in the isolated carbamate 2aa (according to HPLC).
Example 8
Methyl (5)-( 1 -(3-emoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)carbamate (5)-2aa
0.13 g (0.48 mmol) of the racemic amine (rac)-\ is suspended in dimethyl carbonate (0.5 ml). Novozym 435 (60 mg) is added to the white suspension. The mixture is maintained at 70°C, being moderately stirred. After 40 hours, the reaction mixture contains 25% of the carbamate 2aa. The reaction mixture is filtered in a hot state and the filtrate is concentrated at a reduced pressure. The concentrated product is stirred up in 2M aqueous HC1 and extracted with 2 x 3 ml of dichloromethane. The combined organic extracts are concentrated. The process provides 35 mg of the carbamate 2aa (22%) containing 92% of (.S)-2aa.
Example 9
Methyl (<S)-(1 -(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)carbamate (S)-2aa
0.13 g (0.48 mmol) of the racemic amine (rac)-l is suspended in dimethyl carbonate (0.5 ml). Novozym 435 (60 mg) is added to the white suspension. The mixture is maintained at 90°C, being moderately stirred. After 18 hours, the reaction mixture contains 44% of the carbamate 2aa. The reaction mixture is filtered in a hot state and the filtrate is concentrated at a reduced pressure. The concentrated product is stirred up in methanol (5 ml). The white
crystalline product is aspirated and dried. The process provides 44 mg of the carbamate 2aa (28%) containing 92% of (S)-2aa.
Example 10
Methyl (5)-(l-(3-emoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)carbaniate (S)-2aa
0.13 g (0.48 mmol) of the racemic amine (rac)-l is suspended in dimethyl carbonate (0.5 ml). Novozym 435 (100 mg) is added to the white suspension. The mixture is maintained at 90°C, being moderately stirred. After 5 hours, the reaction mixture contains 46% of the carbamate 2aa. The reaction mixture is filtered in a hot state and the filtrate is concentrated at a reduced pressure. The concentrated product is stirred up in methanol (5 ml). The white crystalline product is aspirated and dried. The process provides 40 mg of the carbamate 2aa (25%) containing 97% of (S)-2aa. Example 11
Methyl (5 -(l-(3-emoxy-4-memoxyphenyl)-2-(methylsulfonyl)ethyl)carbarnate (S)-2aa
0.13 g (0.48 mmol) of the racemic amine {rac)-\ is suspended in dimethyl carbonate (0.5 ml). Novozym 435 (100 mg) and 52 mg (0,048 mmol) of the racemization catalyst 7a are added to the white suspension (Figure 4). The mixture is maintained at 90°C, being moderately stirred. After 5 hours, the reaction mixture contains 90% of the carbamate 2aa. The reaction mixture is filtered in a hot state and the filtrate is concentrated at a reduced pressure. The concentrated product is stirred up in methanol. The white crystalline product is aspirated and dried. The process provides 108 mg of the carbamate 2aa (68%) containing 97% of(S)-2aa.
Example 12
(S)-N-( 1 -(3 -ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl)-2-methoxyacetamide (S)- 2ba
50 mg of Novozym 435, 20 ml of CPME; 0.87 ml (8.757 mmol) of methyl methoxy acetate 5aa are added to 0.342 g (1.251 mmol) of the racemic amine (rac)-l and the reaction mixture is stirred at 105 °C for 37 h. The resulting mixture containing 48% of the respective amide 2ba is filtered, concentrated at a reduced pressure on an evaporator and the product is
isolated with the use of column chromatography on silica gel (an ethyl acetate/hexane mixture as the mobile phase). The process provides 199 mg (46%) of the crystalline compound (S)-2ba with the chiral purity of ee 97% and chemical purity of 99.3% (HPLC). Example 13
{S)-N-( 1 -(3 -emoxy-4-memoxyphenyl)-2-(memylsulfonyl)-ethyl)-2-methoxyacetamide (5)- 2ba
50 mg of Novoz m 435, 20 ml of CPME and 0.121 ml (8.757 mmol) of isopropyl methoxy acetate Sab are added to 0.342 g (1.251 mmol) of the racemic amine {rac)-\ and the reaction mixture is stirred at 105°C for 37 h. The resulting mixture containing 47% of the respective amide 2ba is filtered, concentrated at a reduced pressure on an evaporator and the product is isolated with the use of column chromatography on silica gel (an ethyl acetate/hexane mixture as the mobile phase). The process provides 194 mg (45%) of the crystalline compound (S)-2ba with the chiral purity of ee 95% and chemical purity of 99.2% (HPLC).
Example 14
(S)-N-(l-(3-emoxy-4-memoxyphenyl)-2-(^ (S)- 2ba
50 mg of Novozym 435, 136 mg (0.1251 mmol) of the racemization catalyst 7a {Figure 4), 20 ml of CPME and 0.87 ml (8.757 mmol) of methyl methoxy acetate 5aa are added to 0.342 g (1.251 mmol) of the racemic amine (rac)-l. The reaction mixture is stirred at 105°C for 37 h. The resulting mixture is filtered, concentrated at a reduced pressure on an evaporator and the product is isolated with the use of column chromatography on silica gel (an ethyl acetate/hexane mixture as the mobile phase). The process provides 346 mg (80%) of the crystalline compound (5)-2ba with the chiral purity of ee 91% and chemical purity of 99.4% (HPLC).
Example 15
(S)-N-( 1 -(3 -ethoxy-4-methoxyphenyl)-2-(memylsulfonyl)-emyl)-2-methoxyacetamide (5)- 2ba 50 mg of Novoz m 435, 136 mg (0.1251 mmol) of the racemization catalyst 7b
(Figure 4), 20 ml of CPME and 0.87 ml (8,757 mmol) of methyl methoxy acetate 5aa are added to 0.342 g (1.251 mmol) of the racemic amine (mc)-l. The reaction mixture is stirred at 105°C for 37 h. The resulting mixture is filtered, concentrated at a reduced pressure on an evaporator and the product is isolated with the use of column chromatography on silica gel (an ethyl acetate/hexane mixture as the mobile phase). The process provides 389 mg (90%) of the crystalline compound (S)-2ba with the chiral purity of ee 97% and chemical purity of 99.4% (HPLC).
Example 16
(S)- 1 -(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl-amine (S)-l
0.5 g of triethanolamine and 0.5 ml of a 50% (by weight) aqueous solution of NaOH are added to 345 mg (1 mmol) of the amide (5)-2ba from Example 15. The mixture is gradually heated up in a flask to 120°C and at this temperature the heating and stirring continues for another 6 hours. After cooling to the laboratory temperature the reaction mixture is diluted with water (5 ml) and extracted with toluene (3 5 ml). The combined toluene phases are washed with water (2 5 ml) and salt brine (1 5 ml). After evaporation at a reduced pressure on an evaporator the amount of 243 mg (89%) of the crystalline amine (S)-l is obtained with the chiral purity of ee 97% and the chemical purity of 99.7% (HPLC).
Example 17
(5)-l -(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl-amine
2 ml of ethylene glycol and 0.5 ml of a 50% (by weight) aqueous solution of KOH are added to 331 mg (1 mmol) of the carbamate (5)-2aa obtained according to the process of Example 11. The mixture is heated in a flask at 100°C for 12 hours. After cooling to the laboratory temperature the reaction mixture is diluted with water (10 ml) and extracted with MTBE (3 x 5 ml). The combined ether phases are washed with water (2 x 5 ml) and salt brine (1 5 ml). After evaporation at a reduced pressure on an evaporator the amount of 249 mg
(91%) of the crystalline amine (S)-l is obtained with the chiral purity of ee 92% and the chemical purity of 99.5% (HPLC).
Example 18
(5 -l-(3-Emoxy-4-methoxyphenyl)-2-(memylsulfonyl)-emyl-amine (S)-l
215 μΐ TMSI (1.5 mmol) are added to a solution of 331 mg (1 mmol) of the carbamate (S)-2aa obtained with the use of the process of Example 11 in 10 ml of dry DCM and the mixture is heated up to boil for 12 hours. The reaction mixture is then concentrated at a reduced pressure on an evaporator, diluted with water (10 ml) and after neutralization with a 1M aqueous solution of NaOH it is extracted with MTBE (3 x 5 ml). The combined ether phases are washed with water (2 5 ml) and salt brine (1 x 5 ml). After evaporation at a reduced pressure on an evaporator the amount of 211 mg (77%) of the crystalline amine (S)-l is obtained with the chiral purity of ee 94% and the chemical purity of 99.7% (HPLC).
Example 19
(jS)-l-(3-Emoxy-4-memoxyphenyl)-2-(methylsulfonyl)-ethyl-amine (S)-l
2 ml of a commercial solution of HBr in acetic acid (33% solution - by weight) are added to 331 mg (1 mmol) of the carbamate (S)-2aa obtained with the use of the process of Example 11 and the mixture is stirred at 25 °C for 24 hours. The reaction mixture is concentrated at a reduced pressure on an evaporator, diluted with water (10 ml) and after neutralization with a 1M aqueous solution of NaOH it is extracted with MTBE (3 x 5 ml). The combined ether phases are washed with water (2 x 5 ml) and salt brine (1 5 ml). After evaporation at a reduced pressure on an evaporator the amount of 257 mg (94%) of the crystalline amine (S)-l is obtained with the chiral purity of ee 94% and the chemical purity of 99.2% (HPLC).
Example 20
(S)- { 2- [ 1 -(3 -ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl] -4-acetylaminoisoindoline- 1,3- dione (3) 137 g (0.5 mmol; 97% ee) of (S)-l-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)- ethylamine (S)-l (see Example 16), 108 mg (0.525 mmol) of 3-acetamidophthalic anhydride 4 and 5 ml of glacial acetic acid were charged into a 25ml flask. The mixture was refluxed overnight and then cooled down to the laboratory temperature. Then, the mixture was concentrated at a reduced pressure and the residue was dissolved in 25 ml of ethyl acetate. The obtained solution was washed with water (2 x 5 ml), saturated aqueous solution of NaHCC«3 (2 x 5 ml), salt brine (2 x ml) and dried with sodium sulfate. The solvent was evaporated at a reduced pressure and the rest was crystallized from a mixture of ethanol / acetone 2:1 (by volume). The separated crystals were isolated by filtration on frit, washed with ethanol and dried at a reduced pressure (0.5 kPa) at 60°C. The process provided 194 mg otApremilast 1 (yield 84%, 97% ee, HPLC 99.8%)
Example 21
(S)- { 2- [ 1 -(3 -ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl] -4-acetylaminoisoindoline- 1,3- dione (3)
1.21 g (4.43 mmol; 97% ee) of (S)-l-(3-ethoxy-4-methoxyphenyl)-2- (methylsulfonyl)-ethylamine (S)-l (obtained using the process of Example 16), 954 mg (4.65 mmol) of 3-acetamidophthalic anhydride 4 and 18 ml of glacial acetic acid were charged into a 50ml flask. The mixture was refluxed for 3 h and cooled down to 20°C. After that, 35 ml of water was gradually added, under continuous stirring the mixture was inoculated with crystals of Apremilast 3 (10 mg) and stirred at 20°C for another 15 hours. The separated crystals were aspirated, washed with a mixture of acetic acid - water (volume ratio 2:5) and dried at a reduced pressure. The amount of 1.875 g of yellowish crystals of the product 3 was obtained (yield 92%, 98% ee, HPLC 99.1%).
Claims
1. A process for preparing apremilast, (S)~{2-[l-(3-ethoxy-4-methoxyphenyl)-2- memylsmfonylethyl]-4-acetylaniinoisoindoline-l,3-dione of formula 3
wherein R is H, a Ci-Cig alkyl, C6-Q4 aryl or Ci-Cg heteroaryl with one or more heteroatoms, or a C1-C18 alkoxy group, wherein all said groups can be further substituted by any functional groups; the donor is OH, any Ct-Cig alkoxy, C1-C9 aryloxy, CrQs alkylthio group or a Cj- Ci8 acyloxy group;
in the presence of an enzyme from the group of hydrolases, preferably in the presence of a racemization catalyst,
or (ii) iV-acylated amine of formula (S)-2,
((^-2) which is converted, by treatment with a base or an acid in the presence of a solvent, to the chiral amine (S)-l, wherein the amine (S)-l obtained in step (i) or (ii) is further subjected to a reaction with 3-acetamidophthalic anhydride of formula 4,
2. The process according to claim 1, characterized in that the N-acyl donor is selected from esters of carboxylic acids of formula 5a, carboxylic acids of formula 5b, anhydrides of carboxylic acids of formula 5c or derivatives of carbonic acid of formula 5d, preferably in the form of esters of carboxylic acids of formula 5a and derivatives of carbonic acid of formula 5d, wherein l, R2, R3 are independently H, except formulae 5c and 5d, a Q-Cis alkyl, aryl or heteroaryl with one or more heteroatoms, wherein all these groups may also by further substituted by any functional groups.
3. The process according to claims 1 and 2, characterized in that the N-acyl donor is an ester of carboxylic acids of formula 5a and is selected from methyl methoxyacetate, isopropyl methoxyacetate, ethyl acetate, isopropyl acetate, vinyl acetate, isopropenyl acetate or ethyl trifiuoroacetate, preferably methyl methoxyacetate.
4. The process according to claims 1 and 2, characterized in that the N-acyl donor is a derivative of carbonic acid of formula 5d, selected from dimethyl carbonate, diethyl carbonate or dibenzyl carbonate, preferably dimethyl carbonate.
5. The process according to claims 1-4, characterized in that the enzyme from the group of hydrolases is selected from lipases, esterases or peptidases, which are free or immobilized on solid carriers, the enzyme preferably being Novozym 435, i.e. lipase B from Candida Antarctica yeast, preferably bound to polymers of the acrylate type, Subtilisin or Penicilin-G amidase.
6. The process according to claims 1-5, characterized in that the reaction of the racemic amine of formula (rac)-l with the N-acyl donor of formula 5 is carried out in the presence of a racemization catalyst, which is a transition metal, selected from Ru, Ir, Pd, Pt or Rh, which is free or adsorbed on a carrier, selected from active carbon, aluminium oxide and calcium carbonate, or said catalyst is complexes of transition metals selected from Ru, Ir, Pd, Rh or V, preferably complexes of transition metals, especially the Shvo catalysts.
7. The process for preparing apremilast according to any one of the preceding claims, characterized in that it comprises a reaction of the racemic amine of formula (rac)-i with dimethyl carbonate under treatment with Novozym 435, preferably in the presence of a racemization catalyst, producing methyl-(S)-(l-(3-ethoxy-4-methox phenyl)-2- (methylsulfonyl)ethyl)carbamate of formula (S)-2aa.
8. (iS -(l-(3-emoxy-4-memoxyphenyl)-2-(memylsulfonyl)emyl)carbam (S)-2aa.
9. The process for preparing apremilast according to any one of the preceding claims, characterized in that it comprises a reaction of the racemic amine of formula (rac)-l with methyl methoxyacetate or isopropyl methoxyacetate under treatment with Novozym 435, preferably in the presence of a racemization catalyst, producing (5 -iV-(l-(3-ethoxy-4- methoxyphenyl)-2-(methylsulfonyl)ethyl)-2-methoxyacetamide of formula (S)-2ba.
10. (S)-N-( 1 -(3 -emoxy-4-methoxyphenyl)-2-(memylsulfonyl)-e1ihyl)-2-methoxyacetamide (S)-2ba.
11. Use of (5)-(l-(3-ethoxy-4-methoxyphenyi)-2-(methylsulfonyl)ethyl)carbamate of formula (S)-2aa or ^5)-N-(l-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)-ethyl)-2- methoxyacetamide of formula (5)-2ba for the preparation of apremilast.
12. The process for preparing apremilast according to claims 1-7 and 9, characterized in that the N-acyled amine of formula (5)-2 is converted, in the presence of a solvent, to the free amine (5)-l by treatment with an acid selected from hydrochloric, hydrobromic, and sulfuric acid, or a base selected from LiOH, KOH, NaOH, Ba(OH)2, Na2C03, and K2C03.
13. The process according to claim 12, characterized in that the N-acyled amine of formula (S)-2 is selected from methyl-(5)-(l-(3-ethoxy-4-methoxyphenyl)-2- (methylsulfonyl)ethyl)carbamate or (S)-N-( 1 -(3-ethoxy-4-methoxyphenyl)-2- (methylsulfonyl)-ethyl)-2-methoxyacetamide.
14. The process for preparing apremilast according to claims 1 to 7, 9 and 12, characterized in that the solvent is selected from protic polar solvents such as water, CI -8 alcohols, diols, C1-C8 carboxylic acids or their mixtures with aprotic polar solvents selected from ethers such as dimethoxyethane, THF and dioxane, a preferred solvent being water, methanol, ethanol, ethylene glycol, propylene glycol, diethanolamine, dimethoxyethane, THF, dioxane or their mixtures.
15. The process according to any one of claims 1 to 7, 9 and 12 to 14, characterized in that the reaction of the amine of formula (S)-l with 3-acetamidophthalic anhydride of formula 4 is conducted in the presence of water, an organic solvent, or their mixtures, said organic solvent being preferably selected from the group comprising C2-C5 carboxylic acids, especially acetic acid, nitriles of C2-C5 carboxylic acids, especially acetonitrile, and polar aprotic solvents such as dimethyl foraiamide, dimethyl acetamide, dimethyl sulfoxide, and further hydrocarbons, preferably e.g. toluene, xylenes, α,α,α-trifluorotoluene, chlorobenzene and n-butyl chloride.
16. The process for preparing apremilast according to claim 1, characterized in that it comprises a reaction of the racemic amine (rac)-l with dimethyl carbonate, under treatment with Novozym 435, producing methyl (S)-(l-(3-ethoxy-4-methoxyphenyl)-2- (methylsulfonyl)ethyl)carbamate of formula (iS)-2aa, preferably in the presence of a racemization catalyst, , the resulting carbamate being converted, with the use of hydrobromic acid in the presence of acetic acid, to the chiral amine of formula (S -l, which provides, through the subsequent reaction with 3-acetamidophthalic anhydride of formula 4 in glacial acetic acid, the desired (S)-{2-[l-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4- acetylaminoisoindolin-l,3-dione of formula 3.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CZPV2015-383 | 2015-06-05 | ||
| CZ2015-383A CZ2015383A3 (en) | 2015-06-05 | 2015-06-05 | Process for preparing apremilast key intermediate employing enzymatic cleavage of racemic amines |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016192694A1 true WO2016192694A1 (en) | 2016-12-08 |
Family
ID=56463986
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CZ2016/000061 WO2016192694A1 (en) | 2015-06-05 | 2016-06-02 | A process for preparing the key intermediate of apremilast, using enzymatic resolution of the racemic amines |
Country Status (2)
| Country | Link |
|---|---|
| CZ (1) | CZ2015383A3 (en) |
| WO (1) | WO2016192694A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017033116A1 (en) | 2015-08-26 | 2017-03-02 | Glenmark Pharmaceuticals Limited | Process for preparation of apremilast |
| IT201600083132A1 (en) * | 2016-08-05 | 2018-02-05 | Dipharma Francis Srl | PROCEDURE FOR THE PREPARATION OF AN INHIBITOR OF THE PHOSPHODIESTERASE 4 |
| IT201700020784A1 (en) * | 2017-02-23 | 2018-08-23 | Dipharma Francis Srl | PROCEDURE FOR THE PREPARATION OF A SELECTIVE INHIBITOR OF THE PHOSPHODIESTERASE 4 |
| US20220348542A1 (en) * | 2021-04-26 | 2022-11-03 | Amgen Inc. | Process for synthesizing apremilast |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5981267A (en) * | 1996-01-24 | 1999-11-09 | The Scripps Research Institute | Enantioselection of amines using homocarbonates with hydrolase |
| WO2000025777A1 (en) | 1998-10-30 | 2000-05-11 | Celgene Corporation | SUBSTITUTED PHENETHYLSULFONES AND METHOD OF REDUCING TNFαLEVELS |
| WO2003080049A1 (en) | 2002-03-20 | 2003-10-02 | Celgene Corporation | (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof |
| EP1870475A1 (en) * | 2006-06-21 | 2007-12-26 | Evonik Degussa GmbH | Process for the preparation of optically active amines and amides employing a hydrolase and sulfonyl acetic acid ester as the acyl donor |
| US20080234359A1 (en) | 2002-03-20 | 2008-09-25 | Muller George W | Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof |
| EP2431371A1 (en) | 2009-05-14 | 2012-03-21 | Tianjin Hemay Bio-Tech Co., Ltd. | Thiophene derivatives |
| US20130217919A1 (en) | 2012-02-21 | 2013-08-22 | Celgene Corporation | Asymmetric synthetic processes for the preparation of aminosulfone compounds |
| US20140081032A1 (en) | 2012-09-14 | 2014-03-20 | Celgene Corporation | Processes for the preparation of isoindole compounds and isotopologues thereof |
-
2015
- 2015-06-05 CZ CZ2015-383A patent/CZ2015383A3/en unknown
-
2016
- 2016-06-02 WO PCT/CZ2016/000061 patent/WO2016192694A1/en active Application Filing
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5981267A (en) * | 1996-01-24 | 1999-11-09 | The Scripps Research Institute | Enantioselection of amines using homocarbonates with hydrolase |
| WO2000025777A1 (en) | 1998-10-30 | 2000-05-11 | Celgene Corporation | SUBSTITUTED PHENETHYLSULFONES AND METHOD OF REDUCING TNFαLEVELS |
| EP1126839B1 (en) | 1998-10-30 | 2007-01-03 | Celgene Corporation | Substituted phenethylsulfones and method of reducing tnf-alpha and pde-iv levels |
| WO2003080049A1 (en) | 2002-03-20 | 2003-10-02 | Celgene Corporation | (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione: methods of using and compositions thereof |
| US20080234359A1 (en) | 2002-03-20 | 2008-09-25 | Muller George W | Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof |
| EP1870475A1 (en) * | 2006-06-21 | 2007-12-26 | Evonik Degussa GmbH | Process for the preparation of optically active amines and amides employing a hydrolase and sulfonyl acetic acid ester as the acyl donor |
| EP2431371A1 (en) | 2009-05-14 | 2012-03-21 | Tianjin Hemay Bio-Tech Co., Ltd. | Thiophene derivatives |
| US20130217919A1 (en) | 2012-02-21 | 2013-08-22 | Celgene Corporation | Asymmetric synthetic processes for the preparation of aminosulfone compounds |
| US20140081032A1 (en) | 2012-09-14 | 2014-03-20 | Celgene Corporation | Processes for the preparation of isoindole compounds and isotopologues thereof |
Non-Patent Citations (6)
| Title |
|---|
| A. KAMAL; M. A. AZHAR; T. KRISHNAJI; M. S. MALIK; S. AZEEZA, COORD. CHEM. REV., vol. 252, 2008, pages 569 - 592 |
| A. SCHMID; J. S. DORDICK; B. HAUER; A. KIENER; M. WUBBOLTS; B. WITHOLT, NATURE, vol. 409, 2001, pages 258 - 268 |
| C. E. HOBEN, L.; KANUPP, J.-E. BACKVALL, TETRAHEDRON LETT., vol. 49, 2008, pages 977 - 979 |
| HOBEN ET AL: "Practical chemoenzymatic dynamic kinetic resolution of primary amines via transfer of a readily removable benzyloxycarbonyl group", TETRAHEDRON LETTERS, PERGAMON, GB, vol. 49, no. 6, 8 December 2007 (2007-12-08), pages 977 - 979, XP022417897, ISSN: 0040-4039, DOI: 10.1016/J.TETLET.2007.12.017 * |
| O. KIRK; M. W. CHRISTENSEN, ORG. PROCESS RES. DEV., vol. 6, 2002, pages 446 - 451 |
| P. HOYOS; V. PACE; A. R. ALCANTARA, ADV. SYNTH. CATAL., vol. 354, 2012, pages 2585 - 2611 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017033116A1 (en) | 2015-08-26 | 2017-03-02 | Glenmark Pharmaceuticals Limited | Process for preparation of apremilast |
| EP3341359A4 (en) * | 2015-08-26 | 2019-04-24 | Glenmark Pharmaceuticals Limited | Process for preparation of apremilast |
| IT201600083132A1 (en) * | 2016-08-05 | 2018-02-05 | Dipharma Francis Srl | PROCEDURE FOR THE PREPARATION OF AN INHIBITOR OF THE PHOSPHODIESTERASE 4 |
| IT201700020784A1 (en) * | 2017-02-23 | 2018-08-23 | Dipharma Francis Srl | PROCEDURE FOR THE PREPARATION OF A SELECTIVE INHIBITOR OF THE PHOSPHODIESTERASE 4 |
| US20220348542A1 (en) * | 2021-04-26 | 2022-11-03 | Amgen Inc. | Process for synthesizing apremilast |
| WO2022232037A1 (en) * | 2021-04-26 | 2022-11-03 | Amgen Inc. | Process for synthesizing apremilast |
Also Published As
| Publication number | Publication date |
|---|---|
| CZ2015383A3 (en) | 2016-12-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Mitsukura et al. | Asymmetric synthesis of chiral cyclic amine from cyclic imine by bacterial whole-cell catalyst of enantioselective imine reductase | |
| Koszelewski et al. | Asymmetric synthesis of optically pure pharmacologically relevant amines employing ω‐transaminases | |
| WO2016192694A1 (en) | A process for preparing the key intermediate of apremilast, using enzymatic resolution of the racemic amines | |
| JP5096435B2 (en) | Process for producing optically active α-methylcysteine derivative | |
| CN109836362A (en) | A kind of method preparing chiral (2S, 3R)-D-4-methylsulfonylphserine serine ethyl ester | |
| TWI628158B (en) | New process and intermediates | |
| JPS61239899A (en) | Biotechnological production of optically active alpha-arylalkanoic acid | |
| EP1992609A1 (en) | A process for the preparation of a (S)(+)-3-(aminomethyl)-5-methylhexanoic acid | |
| KR20080036060A (en) | The use of enzymatic resolution for the preparation of intermediates of pregabalin | |
| WO2004022766A1 (en) | PROCESS FOR PRODUCING L-α-METHYLCYSTEINE DERIVATIVE | |
| WO2006015885A1 (en) | Amino acid dehydrogenase-derived biocatalysts | |
| WO2005073388A1 (en) | Processes for producing optically active 1-substituted 2-methylpyrrolidine and intermediate therefor | |
| CN103045667A (en) | Preparation method of S-(+)-2-aminobutanamide hydrochloride | |
| AU2001230192B2 (en) | Method for the enzymatic resolution of the racemates of aminomethyl-aryl-cyclohexanol derivatives | |
| JPWO2004108944A1 (en) | Method for producing optically active chromancarboxylic acid ester | |
| EP1724253A2 (en) | Production method of optically active diphenylalanine compounds | |
| Hacking et al. | Lipase catalysed acylation of hydroxylamine and hydrazine derivatives | |
| US8546114B2 (en) | Processes for the preparation of optically active cyclopentenones and cyclopentenones prepared therefrom | |
| JP5010266B2 (en) | Process for producing optically active biphenylalanine compound or salt thereof and ester thereof | |
| CN1121383C (en) | Process for recycle of waste product of diltiazem synthesis | |
| EP2218788B1 (en) | Process for the preparation of optically active cyclopentenones | |
| US20050014818A1 (en) | Process for producing optically active chroman derivative and intermediate | |
| KR20070076549A (en) | Processes for the preparations of optically active cyclopentenones and cyclopentenones prepared therefrom | |
| US8828691B2 (en) | Process for resolving cyclopropyl diesters | |
| Gupta et al. | An expedient chemo-enzymatic method for the synthesis of optically active masked 1, 2-amino alcohols |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16740956 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16740956 Country of ref document: EP Kind code of ref document: A1 |