WO2016164487A1 - Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof - Google Patents
Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof Download PDFInfo
- Publication number
- WO2016164487A1 WO2016164487A1 PCT/US2016/026253 US2016026253W WO2016164487A1 WO 2016164487 A1 WO2016164487 A1 WO 2016164487A1 US 2016026253 W US2016026253 W US 2016026253W WO 2016164487 A1 WO2016164487 A1 WO 2016164487A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- formula
- pharmaceutically acceptable
- cycloalkyl
- extended release
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 *C(*)(C1)ON=C1c1ccc(B(O)OC2(*)*)c2c1 Chemical compound *C(*)(C1)ON=C1c1ccc(B(O)OC2(*)*)c2c1 0.000 description 4
- ULBYCANSMJONQZ-UHFFFAOYSA-N CC(C)(c1c2ccc(C(C3)=NOC3(C(F)(F)F)c(cc3Cl)cc(Cl)c3Cl)c1)OB2O Chemical compound CC(C)(c1c2ccc(C(C3)=NOC3(C(F)(F)F)c(cc3Cl)cc(Cl)c3Cl)c1)OB2O ULBYCANSMJONQZ-UHFFFAOYSA-N 0.000 description 2
- HDKWFBCPLKNOCK-UHFFFAOYSA-N Cc1c(C(NCC(NCC(F)(F)F)=O)=O)[s]c(C(C2)=NOC2(C(F)(F)F)c(cc2Cl)cc(Cl)c2Cl)c1 Chemical compound Cc1c(C(NCC(NCC(F)(F)F)=O)=O)[s]c(C(C2)=NOC2(C(F)(F)F)c(cc2Cl)cc(Cl)c2Cl)c1 HDKWFBCPLKNOCK-UHFFFAOYSA-N 0.000 description 2
- OXDDDHGGRFRLEE-UHFFFAOYSA-N O=C(CNC(c(cc1)c(cccc2)c2c1C(C1)=NOC1(C(F)(F)F)c1cc(C(F)(F)F)cc(Cl)c1)=O)NCC(F)(F)F Chemical compound O=C(CNC(c(cc1)c(cccc2)c2c1C(C1)=NOC1(C(F)(F)F)c1cc(C(F)(F)F)cc(Cl)c1)=O)NCC(F)(F)F OXDDDHGGRFRLEE-UHFFFAOYSA-N 0.000 description 1
- RCHAEZFTFQPIBZ-QHCPKHFHSA-N O=C(CNC(c1ccc(C(C2)=NO[C@]2(C(F)(F)F)c2cc(Cl)cc(Cl)c2)c2c1cccc2)=O)NCC(F)(F)F Chemical compound O=C(CNC(c1ccc(C(C2)=NO[C@]2(C(F)(F)F)c2cc(Cl)cc(Cl)c2)c2c1cccc2)=O)NCC(F)(F)F RCHAEZFTFQPIBZ-QHCPKHFHSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/42—Oxazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/42—Oxazoles
- A61K31/422—Oxazoles not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/14—Ectoparasiticides, e.g. scabicides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Definitions
- the present invention provides extended release injectable formulations comprising at least one isoxazoline active agent, a pharmaceutically acceptable polymer and a solvent; the use of these formulations against parasites (including ectoparasites (e.g., fleas or ticks) and/or endoparasites), and methods for preventing or treating parasitic infections and infestations in animals.
- ectoparasites e.g., fleas or ticks
- endoparasites e.g., endoparasites
- ectoparasites such as fleas, ticks and parasitic flies
- endoparasites such as nematodes and other worms.
- domesticated animals such as cats and dogs, are often infested with one or more of the following ectoparasites:
- - fleas e.g. Ctenocephalides spp., such as Ctenocephalides felis and the like;
- ticks e.g. Rhipicephalus spp., Ixodes spp., Dermacentor spp., Amblyomma spp., and the like
- Rhipicephalus spp. Ixodes spp.
- Dermacentor spp. Dermacentor spp.
- Amblyomma spp. and the like
- - lice e.g. Trichodectes spp., Cheyletiella spp., Linognathus spp. and the like
- Trichodectes spp. Cheyletiella spp., Linognathus spp. and the like
- Fleas are a particular problem because not only do they adversely affect the health of the animal or human, but they also cause a great deal of psychological stress. Moreover, fleas may also transmit pathogenic agents to animals and humans, such as tapeworm (Dipylidium
- ticks are also harmful to the physical and psychological health of the animal or human.
- Major diseases which may be transmitted by ticks include borreliosis (Lyme disease caused by Borrelia burgdorferi), babesiosis (or piroplasmosis caused by Babesia spp.) and rickettsioses (e.g. Rocky Mountain spotted fever).
- Ticks also release toxins which cause inflammation or paralysis in the host. Occasionally, these toxins are fatal to the host.
- farm animals are also susceptible to parasite infestations.
- cattle are affected by a large number of parasites.
- a parasite which is prevalent among cattle in some regions are ticks of the genus Rhipicephalus, especially those of the species microplus (cattle tick), decoloratus and annulatus. Ticks such as Rhipicephalus microplus (formerly Boophilus microplus) are difficult to control because they lay eggs in the pasture where farm animals graze.
- This species of ticks is considered a one-host tick and spends immature and adult stages on one animal before the female engorges and falls off the host to lay eggs in the environment. The life cycle of the tick is approximately three to four weeks.
- Rhipicephalus microplus may infest buffalo, horses, donkeys, goats, sheep, deer, pigs, and dogs.
- a heavy tick burden on animals can decrease production and damage hides as well as transmit diseases such as babesiosis (“cattle fever”) and anaplasmosis.
- helminthiasis which is caused by of parasitic worms categorized as cestodes (tapeworm), nematodes (roundworm) and trematodes (flatworm or flukes).
- cestodes tapeworm
- nematodes roundworm
- trematodes flatworm or flukes
- parasites which occur in the gastrointestinal tract of animals and humans include those from the genus Ancylostoma, Necator, Ascaris, Strongyloides, Trichinella, Capillaria, Toxocara, Toxascaris, Trichuris, Enterobius and parasites which are found in the blood or other tissues and organs such as filarial worms and the extra intestinal stages of Strongyloides, Toxocara and Trichinella.
- active agents in addition to topical and oral dosage forms, it is sometimes possible to formulate active agents as extended release injectable formulations, depending upon, for example, the physiochemical properties of the individual active agent. These properties include, for example, solubility, bioavailability, etc.
- active agents in extended release formulations comprising a pharmaceutically acceptable polymer that controls the release of the active agent in the animal's body from the formulation over an extended period of time.
- extended release formulations comprising a pharmaceutically acceptable polymer that controls the release of the active agent in the animal's body from the formulation over an extended period of time.
- the meaning of "extended release” formulations in the veterinary medicine field is the subject of the article "Terminology Challenges: Defining Modified Release Dosage Forms in Veterinary Medicine ' " by Marilyn N. Martinez, Danielle Lindquist and Sanja Modric (Journal of Pharmaceutical Sciences, vol. 99, no. 8, August 2010).
- dosage form proposed in the article“Dosage forms that are formulated in such a manner as to make the contained medicament available over an extended period of time” is consistent with the use of this term in the present application in which the formulation components induce the release characteristics of the active ingredient rather than the intrinsic properties of the active itself being responsible for the long acting nature of the formulation.
- US 6,733,767 and US 8,362,086 provide for long acting injectable formulations comprising a bioactive substance, such as, for example, an avermectin or a milbemycin and a biologically acceptable polymer.
- 5,330,768 provides for degradable polymeric matrices for the delivery of drugs by blending polymers that degrade by hydrolysis (e.g., poly(L-lactic acid), nonionic surfactants and block copolymers of polyethylene oxide and polypropylene oxide.
- polymers that degrade by hydrolysis e.g., poly(L-lactic acid), nonionic surfactants and block copolymers of polyethylene oxide and polypropylene oxide.
- Fluorinated compounds such as the some of the isoxazoline compounds provided for in the inventive formulations, often present additional challenges as compared to their non- fluorinated counterparts when formulating the compounds in extended release injectable formulations because the presence of fluorine groups make it more difficult to achieve the desired release properties of the compound from the polymeric matrices, which form the depot.
- Fluorinated organic compounds are very hydrophobic. In part, this is due to the low surface energies which make the compound less wettable. See, N. L. Jarvis and W. A. Zisman,“Surface Chemistry of Fluorochemicals”, U.S. Newcastle Research Laboratory, Washington, D.C. (1965). This property hinders the hydration of polymers such as poly(L-lactic acid), thereby delaying the degradation of the polymer and delaying the release of the fluorinated compound from the depot.
- compositions comprising isoxazoline active agents alone or in combination with other active agents described in the documents above, there is a need for veterinary compositions and methods with improved duration of efficacy, and/or bioavailability, and/or spectrum of coverage to protect animals against endoparasites and/or ectoparasites. More specifically, there is a need to develop a longer acting injectable formulation comprising an isoxazoline compound, which has good bioavailability and provides a high level of efficacy against ectoparasites (e.g., fleas and ticks) for a long duration (e.g., from three (3) up to twelve (12) months, while exhibiting reduced injection site irritation on the animal.
- ectoparasites e.g., fleas and ticks
- the present invention provides novel and inventive extended release injectable formulations for the treatment or prevention of parasite infections or infestations in an animal comprising an antiparasitic effective amount of at least one isoxazoline active agent, a pharmaceutically acceptable polymer and, optionally, a solvent.
- the extended release injectable formulations of the invention comprise an antiparasitic effective amount of at least one isoxazoline active agent, a pharmaceutically acceptable biodegradable polymer, and a solvent.
- the term“poloxamer” means a block copolymer of ethylene oxide and propylene oxide.
- poloxamers that are not co- polymerized with other pharmaceutically acceptable polymers are considered solvents or surfactants rather than a pharmaceutically acceptable polymer.
- Different grades, sources, and brands of block copolymers of ethylene oxide and propylene oxide may be used in the extended release injectable formulations of the invention.
- liquid polyethylene glycols (PEGs) that are not co-polymerized with other pharmaceutically acceptable polymers are considered to be a solvent and are not considered to be a pharmaceutically acceptable polymer
- inventive extended release injectable formulations generally show desirable bioavailability and duration of efficacy. Further, the inventive extended release formulations generally do not show undesirable irritation on the injection site of the animal.
- the compositions also provide desirable safety profiles toward the warm-blooded and avian animal recipients.
- a single administration of such formulations generally provides potent activity against one or more parasites (e.g., ectoparasites), while also tending to provide fast onset of activity, long duration
- the invention encompasses uses or veterinary uses of the isoxazoline compositions for the treatment or prevention or prophylaxis of parasitic infections and infestations of animals (either wild or domesticated), including livestock and companion animals such as cats, dogs, horses, chickens, sheep, goats, pigs, turkeys and cattle, with the aim of ridding these hosts of parasites commonly encountered by such animals.
- the invention also provides methods for the treatment or prevention of parasitic infections and infestations in animals, comprising administering an effective amount of extended release injectable formulations comprising an antiparasitic effective amount of at least one isoxazoline compound together with a pharmaceutically acceptable polymer and a solvent.
- extended release injectable formulations comprising an antiparasitic effective amount of at least one isoxazoline compound together with a pharmaceutically acceptable polymer and a solvent.
- This invention also provides for the use of an isoxazoline in the preparation of extended release injectable formulations for the treatment or prevention of an animal against parasites.
- the invention provides for extended release injectable formulations comprising antiparasitic effective amounts of at least one isoxazoline of formula (I) below, in combination and a pharmaceutically or veterinary acceptable polymer and a solvent, where variables B 1 , B 2 , B 3 , R 1 , Y and Q are defined herein and the asterisk signifies that the carbon is a quaternar carbon.
- variables A 1 , A 2 , A 3 , A 4 , A 5 , A 6 , B 1 , B 2 , B 3 , R 1 , R 2 , R 3 , R 4 , R 5 , W, n, X 1 , X 2 , X 3 , R 1 , X, Ai, A 2 , G, Y, T, R 3a and R 3b for each formula are defined herein.
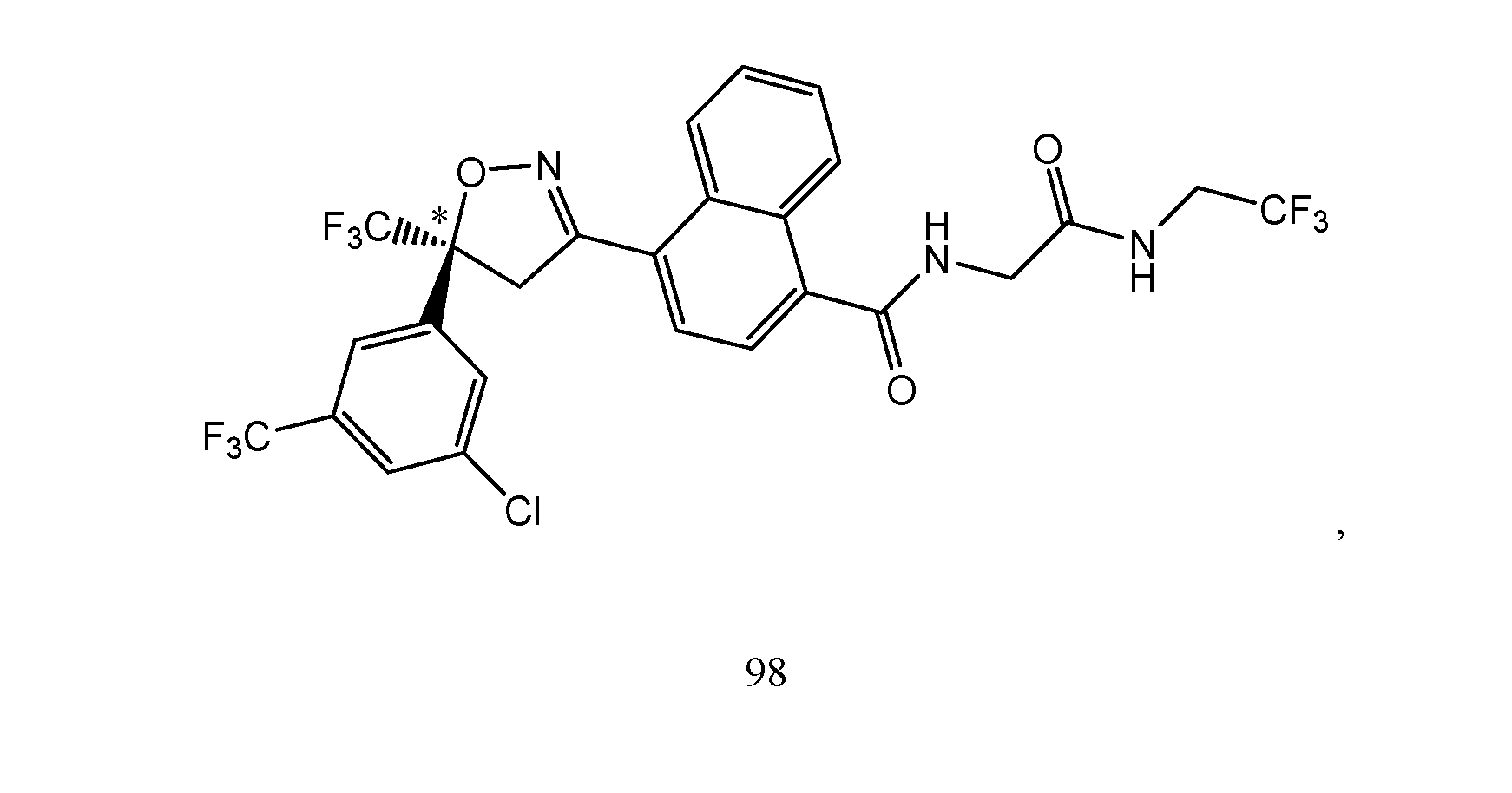
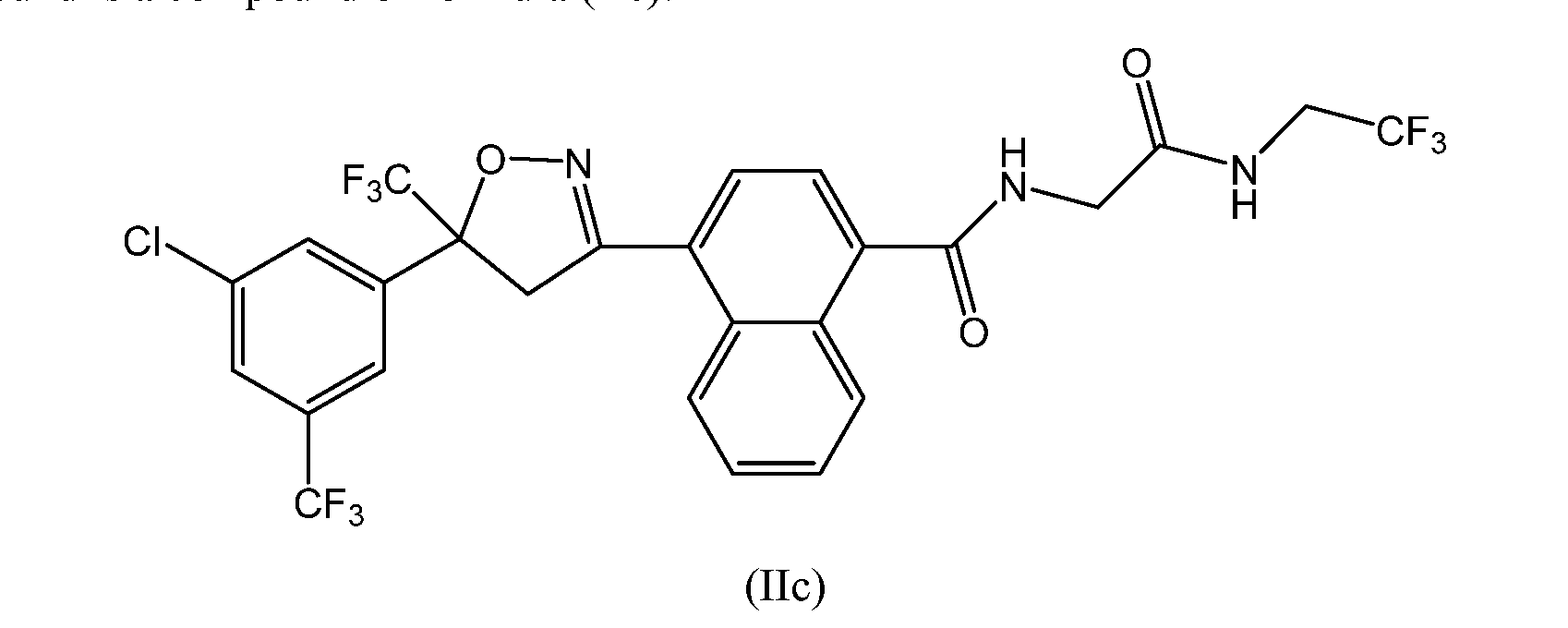
- the extended release injectable formulations and methods comprise 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]-4,5-dihydro-5-(trifluoromethyl)-3- isoxazolyl]-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-l-naphthalenecarboxamide
- the extended release injectable formulations may further comprise one or more additional active agents that are systemically active.
- Systemically-acting active agents include, but are not limited to, isoxazoline active agents of different structure, a systemically-acting neonicotinoid active agent, a systemically-acting 1-N-aiylpyrazole active agent, macrocyclic lactones such as avermectin and milbemycin compounds, a cyclic depsipeptide such as emodepside or PF1022A, or analogs thereof, benzimidazoles, imidazothiazoles, a tetrahydropyrimidine active agent, an organophosphate active agent, levamisole, a paraherquamide active agent and/or a marcfortine active agent, praziquantel, closantel, clorsulon, pyrantel, a spinosyn or spinosoid active agent, an amino acetonitrile active agent, an a
- the extended release injectable formulations comprise at least one macrocyclic lactone active agent, including, but not limited to, avermectins or milbemycins.
- the avermectin or milbemycin active agent is eprinomectin, ivermectin, abamectin, selamectin, doramectin, milbemectin, milbemycin D, milbemycin oxime, or moxidectin.
- compositions and methods comprise at least one of thiabendazole, oxibendazole, mebendazole, fenbendazole, oxfendazole, albendazole, triclabendazole, febantel, levamisole, pyrantel, morantel, praziquantel, closantel, clorsulon, an amino acetonitrile active agent, or an aryloazol-2-yl cyanoethylamino active agent.
- compositions and methods comprise at least cyclic depsipeptide active agent including, but not limited to, emodepside and PF1022A, or analogs thereof.
- the present invention provides for novel and inventive extended release injectable formulations treatment or prevention of parasite infections or infestations in an animal comprising an antiparasitic effective amount of at least one isoxazoline compound, a pharmaceutically acceptable polymer and optionally a solvent or mixture of solvents. Also provided are methods and uses for the treatment and/or prophylaxis of parasitic
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising an antiparasitic effective amount of at least one isoxazoline compound and an effective amount of at least one additional systemically-acting active agent, a pharmaceutically acceptable polymer and, optionally, a solvent or mixture of solven.
- the pharmaceutically acceptable polymer is a pharmaceutically acceptable biodegradable polymer.
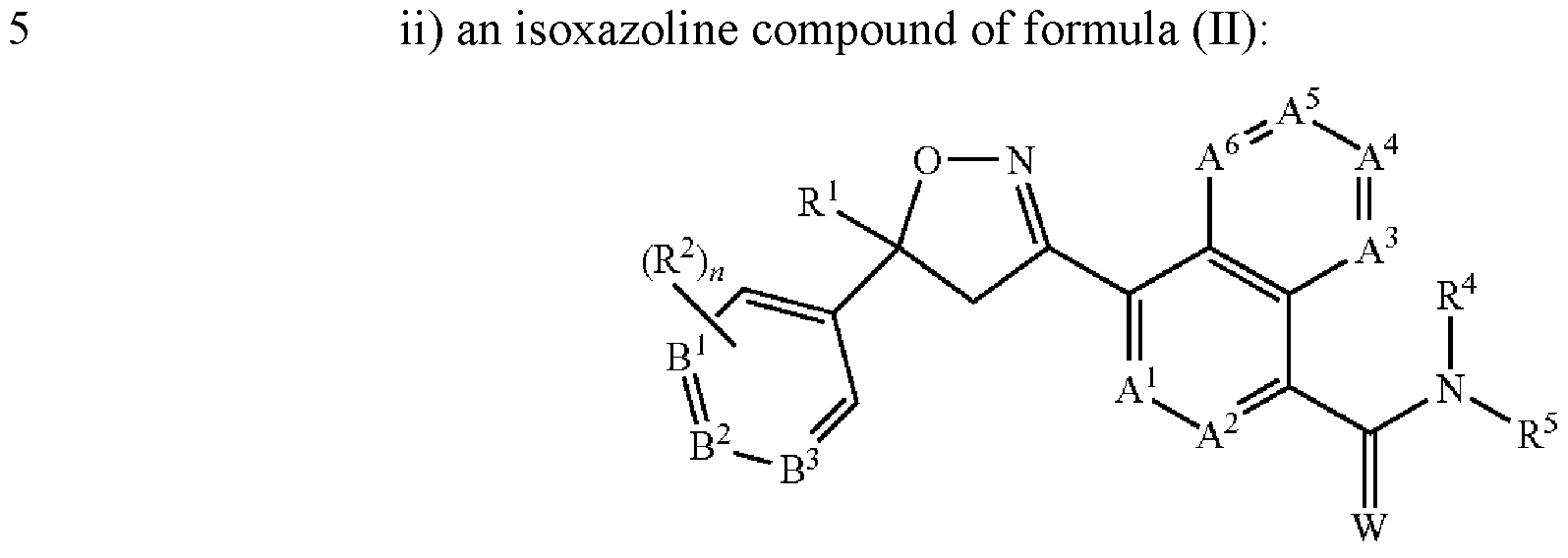
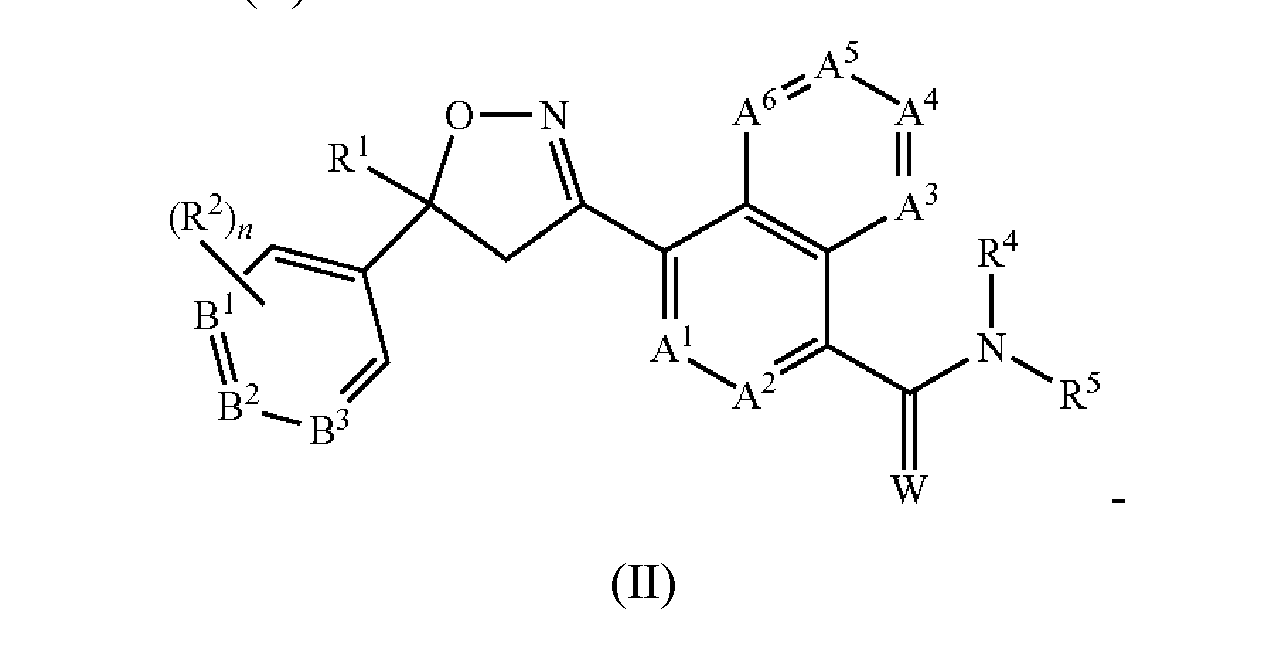
- the present invention provides for an extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising: a) an antiparasitic effective amount of at least one isoxazoline active agent, which is: i) an isoxazoline compound of formula (I):
- B 1 , B 2 and B 3 are each independently C-R or N;
- each R is independently H, halogen, cyano, -NO 2 , alkyl, haloalkyl, alkoxy, haloalkoxy, alkylthio, haloalkylthio, alkylsulfinyl, haloalkylsulfinyl, alkylsulfonyl, haloalkylsulfonyl, alkylamino, dialkylamino or alkoxycarbonyl;
- R 1 is C 1 -C 3 alkyl or C 1 -C 3 haloalkyl
- Q is X- R 2 R 3 , the group (-CH 2 -)(-CH 2 -)N-R 3 , OH, H 2 , alkoxy, haloalkoxy, alkylamino, haloalkylamino, dialkylamino, halodialkylamino, thiol, alkylthio, haloalkylthio, alkylsulfinyl, haloalkylsulfinyl, alkylsulfonyl, haloalkylsulfonyl, or an optionally substituted 5- or 6- membered carbocyclyl, heterocyclyl or heteroaryl ring;
- R 2 is H, alkyl, alkenyl, alkynyl, cycloalkyl, alkylcycloalkyl, cycloalkylalkyl, alkylcarbonyl or alkoxy carbonyl;
- R 3 is H, OR 7 , R 8 R 9 or Q 1 ; or alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, alkylcycloalkyl, cycloalkylalkyl, alkylcarbonyl, alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl or dialkylaminocarbonyl, each optionally substituted with one or more substituents independently selected from R 4 ; or
- R 2 and R 3 are taken together with the nitrogen to which they are attached to form a ring containing 2 to 6 atoms of carbon and optionally one additional atom selected from the group consisting of N, S and O, said ring optionally substituted with 1 to 4 substituents independently selected from the group consisting of alkyl, halogen,— CN,— NO 2 and alkoxy; each R 4 is independently halogen; alkyl, cycloalkyl, alkoxy, alkylthio, haloalkylthio, alkylsulfinyl, haloalkylsulfinyl, alkylsulfonyl, haloalkylsulfonyl, alkylamino, haloalkylamino, dialkylamino, dihaloalkylamino, cycloalkylamino, alkylcarbonyl, alkoxycarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl, haloal
- R 7 is H; or alkyl, alkenyl, alkynyl, cycloalkyl, alkylcycloalkyl or cycloalkylalkyl, each optionally substituted with one of more halogen;
- R 8 is H, alkyl, alkenyl, alkynyl, cycloalkyl, alkylcycloalkyl, cycloalkylalkyl, alkylcarbonyl or alkoxycarbonyl;
- R 9 is H; Q 3 ; or alkyl, alkenyl, alkynyl, cycloalkyl, alkylcycloalkyl or cycloalkylalkyl, each optionally substituted with one or more substituents independently selected from R 4 ; or
- R 8 and R 9 are taken together with the nitrogen to which they are attached to form a ring containing 2 to 6 atoms of carbon and optionally one additional atom selected from the group consisting of N, S and O, said ring optionally substituted with 1 to 4 substituents independently selected from the group consisting of alkyl, halogen,— CN,— ⁇ O 2 and alkoxy;
- Q 1 is a phenyl ring, a 5- or 6-membered heterocyclic ring, or an 8-, 9- or 10-membered fused bicyclic ring system optionally containing one to three heteroatoms selected from up to 1 O, up to 1 S and up to 3 N, each ring or ring system optionally substituted with one or more substituents independently selected from R 5 ;
- Q 2 is independently a phenyl ring or a 5- or 6-membered heterocyclic ring, each ring optionally substituted with one or more substituents independently selected from R 6 ;
- Q 3 is a phenyl ring or a 5- or 6-membered heterocyclic ring, each ring optionally substituted with one or more substituents independently selected from R 6 ;
- n 0, 1 or 2;
- Y is selected from Y-l, Y-2, Y-3, Y-4 where Z is nitrogen or CH, Y-5 or Y-6 shown below:
- the group Q is X-NR 2 R 3.
- Q is X-NR 2 R 3 wherein R 2 is H or C 1 -C 3 alkyl and R 3 is C 1 -C 3 alkyl optionally substituted by R 4 .
- Q is X-NR 2 R 3 wherein R 2 is H and R 3 is C 1 -C 3 alkyl optionally substituted by alkylthio, haloalkylthio, alkylsulfinyl, haloalkylsulfinyl, alkylsulfonyl, haloalkylsulfonyl, alkylcarbonyl, alkoxycarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl, haloalkylcarbonyl, haloalkoxycarbonyl, haloalkylaminocarbonyl or dihaloalkylaminocarbonyl.
- Q is X-NR 2 R 3 wherein R 2 is H and R 3 is C 1 -C 3 alkyl optionally substituted by alkylthio, haloalkylthio, alkylaminocarbonyl, dialkylaminocarbonyl, haloalkylaminocarbonyl or dihaloalkylaminocarbonyl.
- Q is -C(O)NHCH 2 C(O)NHCH 2 CF 3 .
- Q is -C(O)CH 2 S(O) 2 CH 3 .
- Q is -C(O)NHCH 2 CH 2 SCH 3 .
- Q is the group (- CH 2 -)(CH 2 -)N(CO)CH 2 S(O) 2 CH 3 .
- the present invention provides for an extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising: a) an antiparasitic effective amount of at least one isoxazoline active agent, which is: i) an isoxazoline compound of formula (I):
- a 1 , A 2 , A 3 , A 4 , A 5 and A 6 are independently selected from the group consisting of CR 3 and N, provided that at most 3 of A 1 , A 2 , A 3 , A 4 , A 5 and A 6 are N;
- B 1 , B 2 and B 3 are independently selected from the group consisting of CR 2 and N;
- W is O or S
- R 1 is C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7 alkylcycloalkyl or C 4 -C 7 cycloalkylalkyl, each optionally substituted with one or more substituents independently selected from R 6 ;
- each R 2 is independently H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 haloalkylsulfinyl, C 1 - C 6 alkylsulfonyl, C 1 -C 6 haloalkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 6 dialkylamino, C 2 -C 4 alkoxycarbonyl,— CN or— NO 2 ;
- each R 3 is independently H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 6 cycloalkyl, C 3 - C 6 halocycloalkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 haloalkylsulfinyl, C 1 -C 6 alkylsulfonyl, C 1 -C 6 haloalkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 6 dialkylamino,— CN or— NO 2 ;
- R 4 is H, C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7
- R 5 is H, OR 10 , R 11 R 12 or Q 1 ; or C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7 alkylcycloalkyl or C 4 -C 7 cycloalkylalkyl, each optionally substituted with one or more substituents independently selected from R 7 ; or
- R 4 and R 5 are taken together with the nitrogen to which they are attached to form a ring containing 2 to 6 atoms of carbon and optionally one additional atom selected from the group consisting of N, S and O, said ring optionally substituted with 1 to 4 substituents independently selected from the group consisting of C 1 -C 2 alkyl, halogen,— CN,— NO 2 and C 1 -C 2 alkoxy; each R 6 is independently halogen, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 alkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 alkylsulfonyl,— CN or— NO 2 ;
- each R 7 is independently halogen; C 1 -C 6 alkyl, C 3 -C 6 cycloalkyl, C 1 -C 6 alkoxy, C 1 -C 6 alkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 alkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 8 dialkylamino, C 3 -C 6 cycloalkylamino, C 2 -C 7 alkylcarbonyl, C 2 -C 7 alkoxycarbonyl, C 2 -C 7 alkylaminocarbonyl, C 3 -Cg dialkylaminocarbonyl, C 2 -C 7 haloalkylcarbonyl, C 2 -C 7 haloalkoxycarbonyl, C 2 -C 7 -C 7
- haloalkylaminocarbonyl C 3 -C 9 dihaloalkylaminocarbonyl, hydroxy,— NH 2 ,— CN or— NO 2 ; or Q 2 ;
- each R 8 is independently halogen, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 haloalkylsulfinyl, C 1 -C 6 alkylsulfonyl, C 1 -C 6 haloalkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 6 dialkylamino, C 2 -C 4 alkoxycarbonyl,— CN or— NO 2 ;
- each R 9 is independently halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 6 cycloalkyl, C 3 -C 6 halocycloalkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 haloalkylsulfinyl, C 1 -C 6 alkylsulfonyl, C 1 -C 6 haloalkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 6 dialkylamino,— CN,— NO 2 , phenyl or pyridinyl;
- R 10 is H; or C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7 alkylcycloalkyl or C 4 -C 7 cycloalkylalkyl, each optionally substituted with one of more halogen;
- R 11 is H, C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7
- alkylcycloalkyl C 4 -C 7 cycloalkylalkyl, C 2 -C 7 alkylcarbonyl or C 2 -C 7 alkoxycarbonyl;
- R 12 is H; Q 3 ; or C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7 alkylcycloalkyl or C 4 -C 7 cycloalkylalkyl, each optionally substituted with one or more substituents independently selected from R 7 ; or R 11 and R 12 are taken together with the nitrogen to which they are attached to form a ring containing 2 to 6 atoms of carbon and optionally one additional atom selected from the group consisting of N, S and O, said ring optionally substituted with 1 to 4 substituents independently selected from the group consisting of C 1 -C 2 alkyl, halogen,— CN,— NO 2 and C 1 -C 2 alkoxy;
- Q 1 is a phenyl ring, a 5- or 6-membered heterocyclic ring, or an 8-, 9- or 10-membered fused bicyclic ring system optionally containing one to three heteroatoms selected from up to 1 O, up to 1 S and up to 3 N, each ring or ring system optionally substituted with one or more substituents independently selected from R 8 ;
- each Q 2 is independently a phenyl ring or a 5- or 6-membered heterocyclic ring, each ring optionally substituted with one or more substituents independently selected from R 9 ;
- Q 3 is a phenyl ring or a 5- or 6-membered heterocyclic ring, each ring optionally substituted with one or more substituents independently selected from R 9 ;
- n 0, 1 or 2; or a pharmaceutically acceptable salt thereof; and/or
- R 1 is alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl,
- halocycloalkyl alkylcycloalkyl or cycloalkylalkyl, each which is unsubstituted or substituted with one or more of halogen, hydroxy, amino, alkyl- or di(alkyl)amino, alkyl, cycloalkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, alkoxy, haloalkoxy, alkylthio,
- X is aryl or heteroaryl, which may be unsubstituted or substituted by one or more of halogen, hydroxy, amino, alkyl- or di(alkyl)amino, alkyl, cycloalkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, alkoxy, haloalkoxy, alkylthio, haloalkylthio, R 7 S(O)-, R 7 S(O) 2 -, R 7 C(O)-, R 7 R 8 NC(O)-, R 7 OC(O)-, R 7 C(O)O-, R 7 C(O) R 8 -, -CN or -NO 2 ; oxygen; and
- a 2 is oxygen, R 2 or CR 7 R 8
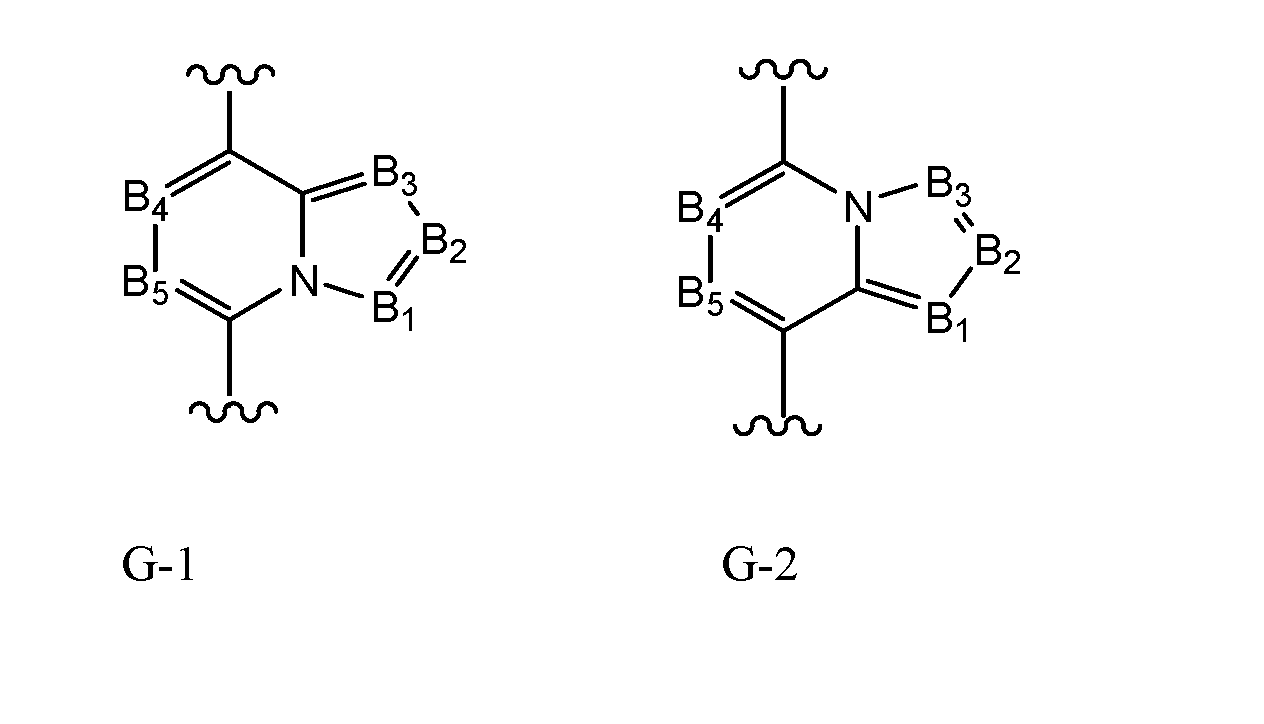
- G is G-l or G-2;
- B 1 , B 2 , B 3 , B 4 and B 5 are independently N or C-R9
- Y is hydrogen, halogen, -CN; or Y is alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, alkylcycloalkyl, cycloalkylalkyl, aryl, or heterocyclyl or heteroaryl each of which is unsubstituted or substituted with one or more of halogen, hydroxy, amino, alkyl- or di(alkyl)amino, alkyl, cycloalkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, alkoxy, haloalkoxy, alkylthio, haloalkylthio, R 7 S(O)-, R 7 S(O) 2 -, R 7 C(O)-, R 7 R 8 NC(O)-, R 7 OC(O)-,
- R 2 , R 3 are independently hydrogen, alkyl, haloalkyl, thioalkyl, alkylthioalkyl, hydroxyalkyl, alkoxyakyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, R 10 S(O)-, R 10 S(O) 2 -, R 10 C(O)-, R 10 C(S)-, R 10 R 1 1 NC(O)-, R 10 R 1 1 NC(S)- R 10 OC(O)-;
- R4, R5 and R 6 are independently hydrogen, alkyl, haloalkyl, thioalkyl, alkylthioalkyl, hydroxyalkyl, alkoxyakyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, aryl or heteroaryl;
- R 7 and R 8 are independently hydrogen, alkyl, haloalkyl, thioalkyl, alkylthioalkyl, hydroxyalkyl, alkoxyakyl, alkenyl, haloalkenyl, alkynyl or haloalkynyl;
- R9 is hydrogen, halogen, -CN, or alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, halocycloalkyl, alkylcycloalkyl or cycloalkylalkyl, each which is unsubstituted or substituted with one or more of halogen, hydroxy, amino, alkyl- or
- R 10 , R 1 1 , R 12 and R 13 are each independently hydrogen, alkyl, haloalkyl, thioalkyl, alkylthioalkyl, hydroxyalkyl, alkoxyalkyl, alkenyl, haloalkenyl, alkynyl or haloalkynyl; or R 10 together with R 1 1 form R 12
- W is O, S or R 2 ; n is 1-4; and m is 0, 1 or 2; or a pharmaceutically acceptable salt thereof; and/or iii) an isoxazoline compound of formula (IV)
- X 1 , X 2 and X 3 are independently H, halogen, C 1 -C 3 alkyl or C 1 -C 3 haloalkyl, or pharmaceutically acceptable salt thereof; and/or
- R 1 , R 2 and R 3 are independently H, CI, F or CF 3 ;
- Y is the diradical grou
- T is a C 1 -C 6 -alkyl group which is unsubstituted or substituted by halogen, cyano, nitro, amino, hydroxyl, C 1 -C 6 -alkoxy, C 1 -C 6 -haloalkoxy, C 1 -C 6 -alkylthio, C 1 -C 6 -alkylthio, carboxy, carbamoyl or C2-C 6 -alkanoyl group which may be unsubstituted or substituted in the alkyl portion by halogen or a pharmaceutical acceptable salt thereof; and/or
- R 2 is methyl, fluoromethyl, trifluoromethyl or perfluoroethyl
- R 3a and R 3b are independently selected from hydrogen, methyl, ethyl or fluoromethyl; or R 3a and R 3b together combine with the carbon to which they are attached to form a cyclopentyl ring or a cyclohexyl ring; or a pharmaceutically acceptable salt thereof;
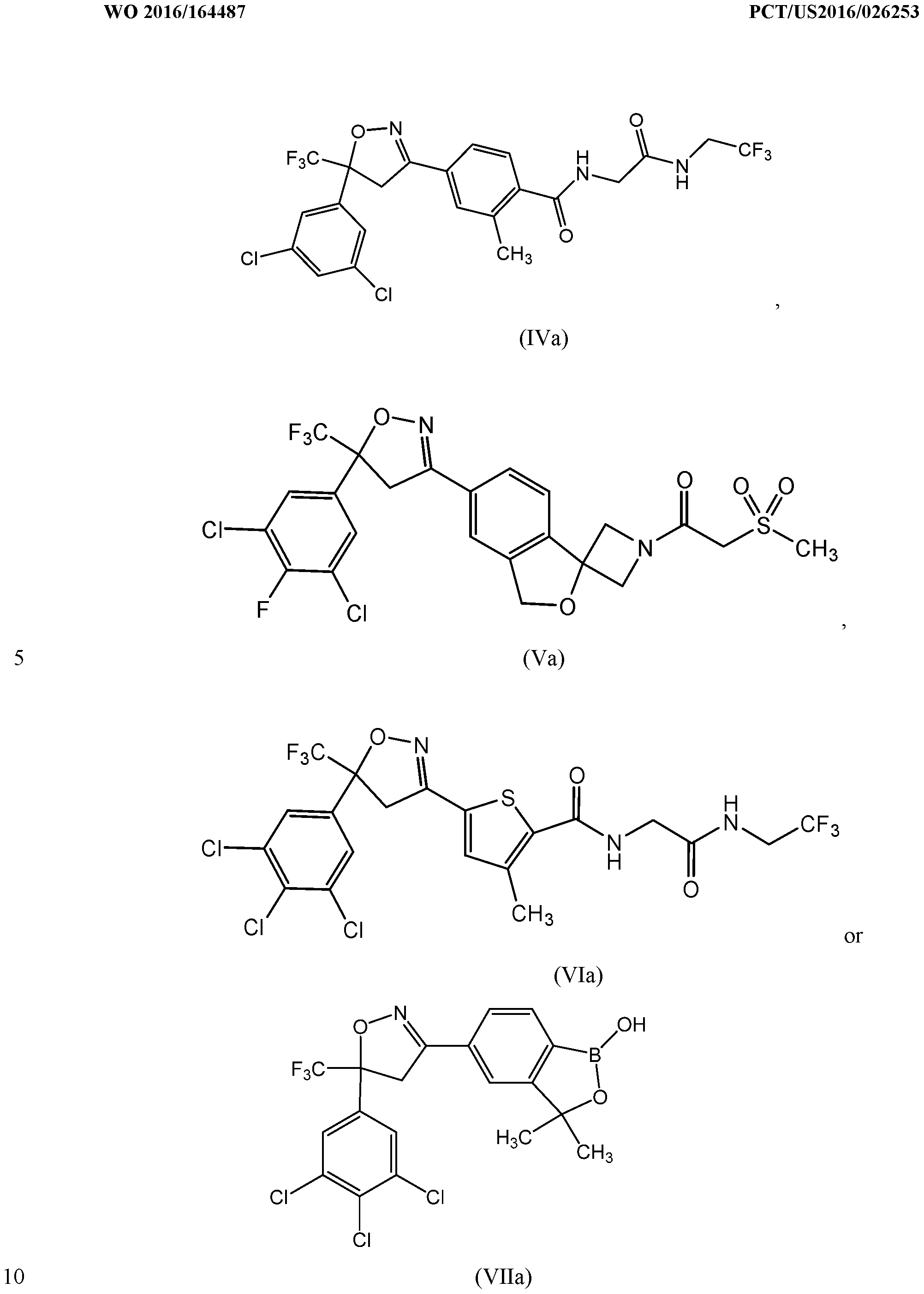
- the compound of formula (III) is disclosed in U.S. Patent No. 7,662,972 and in published U.S. Patent Application No. US 2011/0059988 Al, both incorporated herein by reference.
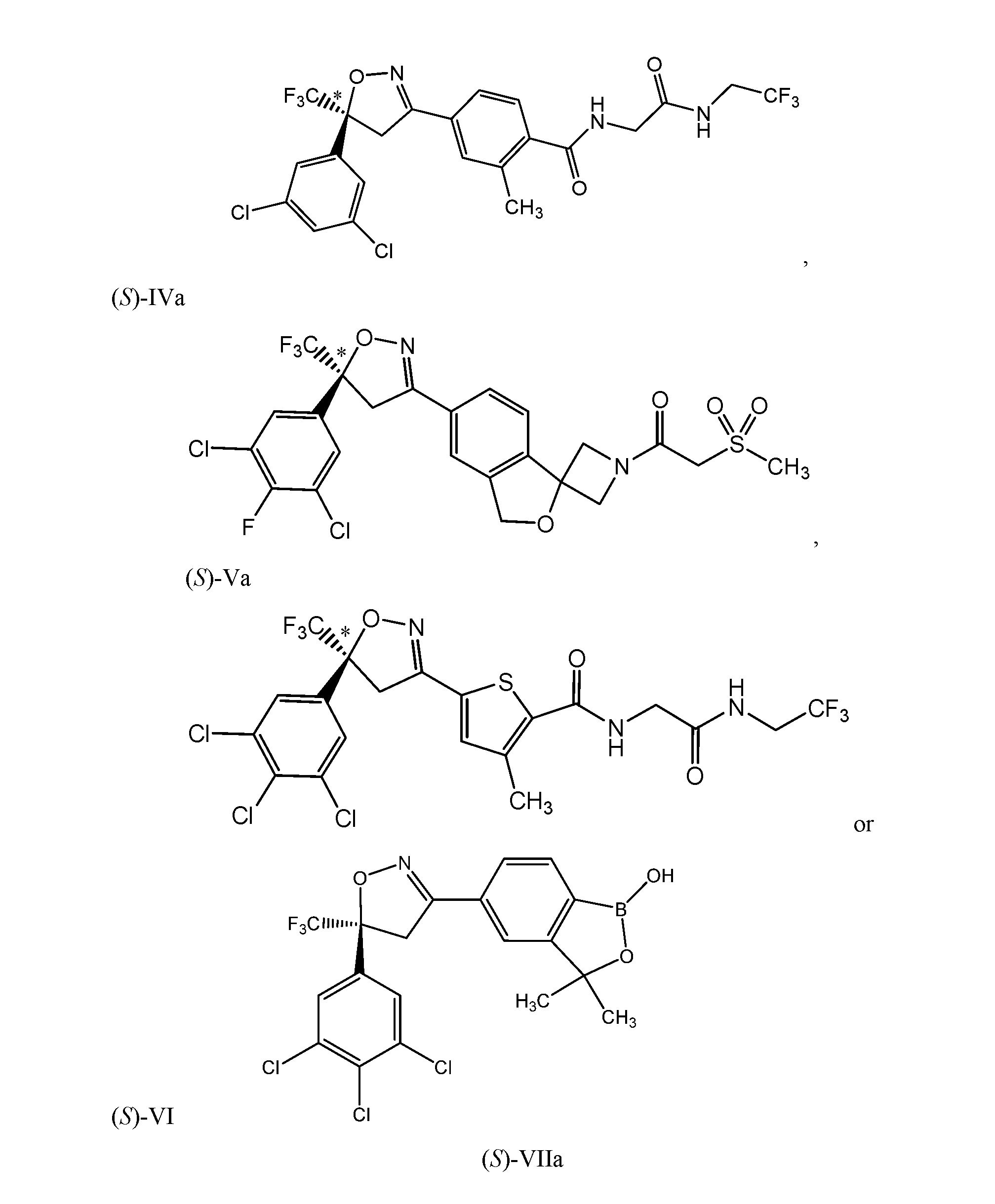
- the compound of formula (IV) is disclosed in U.S. Patent No. 8,466, 115 B2, which is incorporated herein by reference.
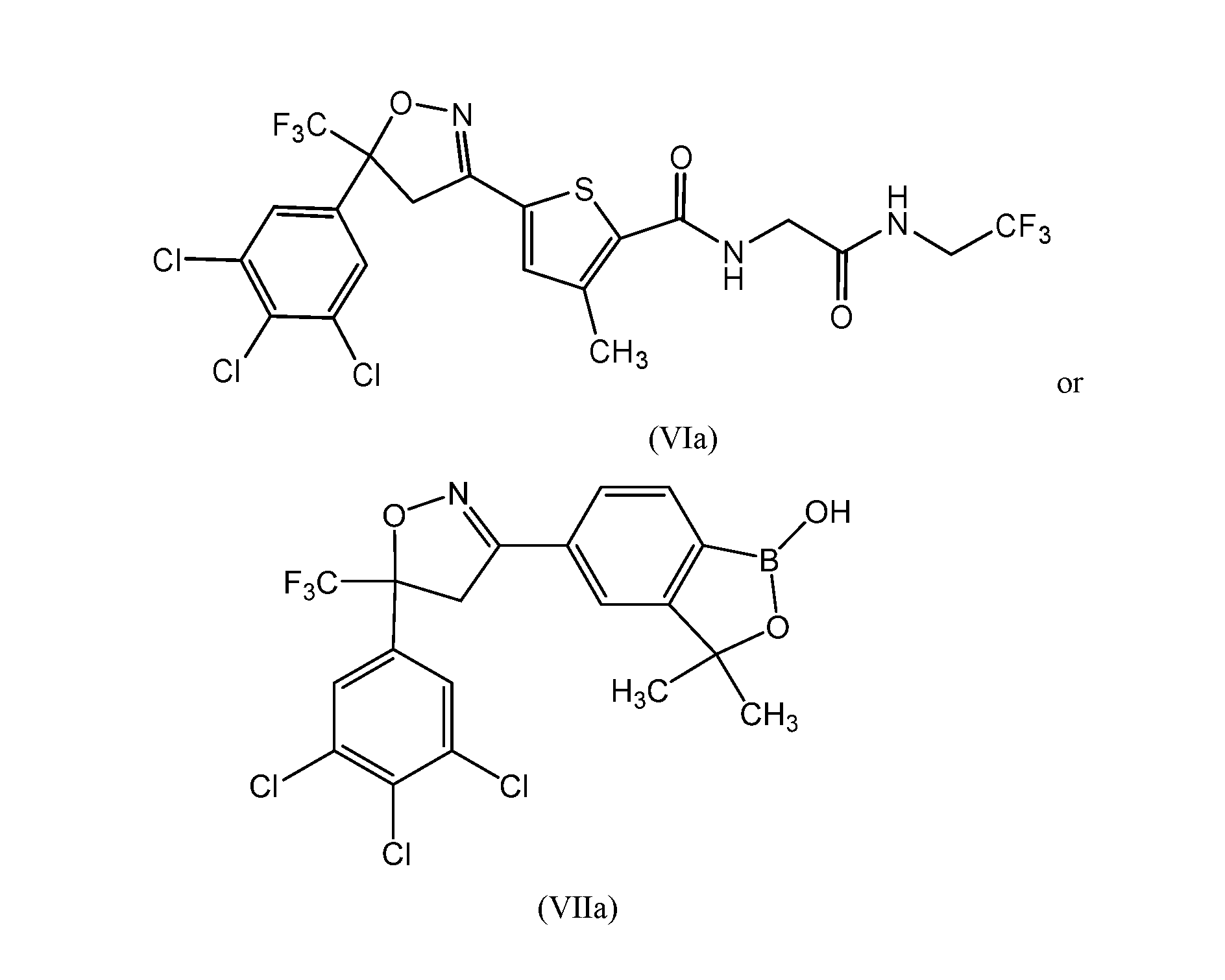
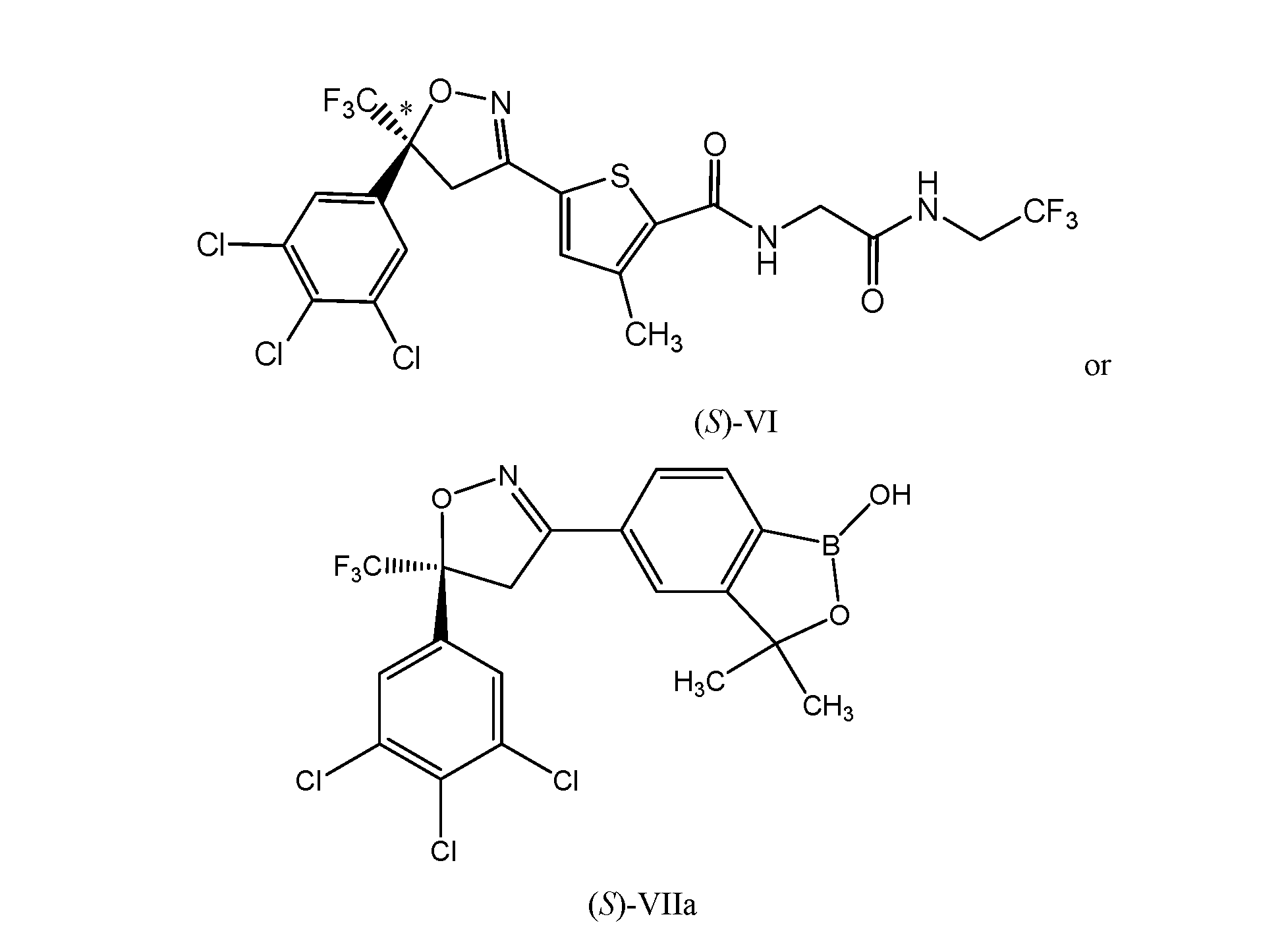
- the compound of formula (VI) is described in U.S. Patent No. 8,383,659, which is also incorporated herein by reference.
- the compound of formula (VII) is described in U.S. Patent No. 8,853,186 B2, which is incorporated herein by reference.
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- a 1 , A2, A 3 , A 4 , A 5 and A 6 are independently selected from the group consisting of CR 3 and N, provided that at most 3 of A 1 , A 2 , A 3 , A 4 , A 5 and A 6 are N;
- B 1 , B 2 and B 3 are independently selected from the group consisting of CR 2 and N;
- W is O or S
- R 1 is C 1 -C 6 alkyl, C2-C 6 alkenyl, C2-C 6 alkynyl, C 3 -C6 cycloalkyl, C 4 -C 7 alkylcycloalkyl or C 4 -C 7 cycloalkylalkyl, each optionally substituted with one or more substituents independently selected from R 6 ;
- each R 2 is independently H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 haloalkylsulfinyl, C 1 - C 6 alkylsulfonyl, C 1 -C 6 haloalkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 6 dialkylamino, C 2 -C 4 alkoxycarbonyl,— CN or— NO 2 ;
- each R 3 is independently H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C6 cycloalkyl, C 3 - C 6 halocycloalkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 haloalkylsulfinyl, C 1 -C 6 alkylsulfonyl, C 1 -C 6 haloalkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 6 dialkylamino,— CN or— NO 2 ;
- R 4 is H, C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7
- alkylcycloalkyl C 4 -C 7 cycloalkylalkyl, C 2 -C 7 alkylcarbonyl or C 2 -C 7 alkoxycarbonyl;
- R 5 is H, OR 10 , NR U R 12 or Q 1 ; or C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7 alkylcycloalkyl or C 4 -C 7 cycloalkylalkyl, each optionally substituted with one or more substituents independently selected from R 7 ; or
- R 4 and R 5 are taken together with the nitrogen to which they are attached to form a ring containing 2 to 6 atoms of carbon and optionally one additional atom selected from the group consisting of N, S and O, said ring optionally substituted with 1 to 4 substituents independently selected from the group consisting of C 1 -C 2 alkyl, halogen,— CN,— NO 2 and C 1 -C 2 alkoxy; each R 6 is independently halogen, C 1 -C 6 alkyl, C 1 -C 6 alkoxy, C 1 -C 6 alkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 alkylsulfonyl,— CN or— NO 2 ;
- each R 7 is independently halogen; C 1 -C 6 alkyl, C 3 -C 6 cycloalkyl, C 1 -C 6 alkoxy, C 1 -C 6 alkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 alkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 8 dialkylamino, C 3 -C 6 cycloalkylamino, C 2 -C 7 alkylcarbonyl, C 2 -C 7 alkoxycarbonyl, C 2 -C 7 alkylaminocarbonyl, C 3 -C 9 dialkylaminocarbonyl, C 2 -C 7 haloalkylcarbonyl, C 2 -C 7 haloalkoxycarbonyl, C 2 -C 7 haloalkylaminocarbonyl, C 3 -C 9 dihaloalkylaminocarbonyl, hydroxy,—
- each R 8 is independently halogen, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 haloalkylsulfinyl, C 1 -C 6 alkylsulfonyl, C 1 -C 6 haloalkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 6 dialkylamino, C 2 -C 4 alkoxycarbonyl,— CN or— NO 2 ;
- each R 9 is independently halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C6 cycloalkyl, C 3 -C6 halocycloalkyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkylsulfinyl, C 1 -C 6 haloalkylsulfinyl, C 1 -C 6 alkylsulfonyl, C 1 -C 6 haloalkylsulfonyl, C 1 -C 6 alkylamino, C 2 -C 6 dialkylamino,— CN,— NO 2 , phenyl or pyridinyl;
- R 10 is H; or C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7 alkylcycloalkyl or C 4 -C 7 cycloalkylalkyl, each optionally substituted with one of more halogen;R 11 is H, C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7
- alkylcycloalkyl C 4 -C 7 cycloalkylalkyl, C 2 -C 7 alkylcarbonyl or C 2 -C 7 alkoxycarbonyl;
- R 12 is H; Q 3 ; or C 1 -C 6 alkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 3 -C 6 cycloalkyl, C 4 -C 7 alkylcycloalkyl or C 4 -C 7 cycloalkylalkyl, each optionally substituted with one or more substituents independently selected from R 7 ; or
- R 11 and R 12 are taken together with the nitrogen to which they are attached to form a ring containing 2 to 6 atoms of carbon and optionally one additional atom selected from the group consisting of N, S and O, said ring optionally substituted with 1 to 4 substituents independently selected from the group consisting of C 1 -C 2 alkyl, halogen,— CN,— NO 2 and C 1 -C 2 alkoxy;
- Q 1 is a phenyl ring, a 5- or 6-membered heterocyclic ring, or an 8-, 9- or 10-membered fused bicyclic ring system optionally containing one to three heteroatoms selected from up to 1 O, up to 1 S and up to 3 N, each ring or ring system optionally substituted with one or more substituents independently selected from R 8 ;
- each Q 2 is independently a phenyl ring or a 5- or 6-membered heterocyclic ring, each ring optionally substituted with one or more substituents independently selected from R 9 ;
- Q 3 is a phenyl ring or a 5- or 6-membered heterocyclic ring, each ring optionally substituted with one or more substituents independently selected from R 9 ;
- n 0, 1 or 2
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- R 2 independently is halogen, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl
- R 4 is H or C 1 -C 6 alkyl
- R 5 is C1-C 4 alkyl optionally substituted with one or more R 7 ; and R 7 is C 2 -C 7 alkylcarbonyl, C 2 -C 7 alkoxycarbonyl, C 2 -C 7 alkylaminocarbonyl, C 3 -C 9 dialkylaminocarbonyl, C 2 -C 7 haloalkylcarbonyl, C 2 -C 7 haloalkoxycarbonyl, C 2 -C 7 haloalkylaminocarbonyl, C 3 -C 9 dihaloalkylaminocarbonyl (e.g., -CH 2 C(O) HCH 2 CF 3 ); and
- n 0, 1 or 2
- e) optionally a surfactant; and f) optionally, at least one pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for an extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising: a) an antiparasitic effective amount of an isoxazoline active agent of formul(aII b)
- X 1 , X 2 and X 3 are each independently H, halogen, C 1 -C 3 alkyl or C 1 -C 3 haloalyl or a pharmaceutically acceptable salt thereof;
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- R 1 is alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl,
- halocycloalkyl alkylcycloalkyl or cycloalkylalkyl, each which is unsubstituted or substituted with one or more of halogen, hydroxy, amino, alkyl- or di(alkyl)amino, alkyl, cycloalkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, alkoxy, haloalkoxy, alkylthio, haloalkylthio, R 7 S(O)-, R 7 S(O) 2 -, R 7 C(O)-, R 7 R 8 NC(O)-, R 7 OC(O)-, R 7 C(O)O-, R 7 C(O)NR 1 CN or -NO 2 ;
- X is aryl or heteroaryl, which may be unsubstituted or substituted by one or more of halogen, hydroxy, amino, alkyl- or di(alkyl)amino, alkyl, cycloalkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, alkoxy, haloalkoxy, alkylthio, haloalkylthio, R 7 S(O)-, R 7 S(O) 2 -, R 7 C(O)-, R 7 R 8 NC(O)-, R 7 OC(O)-, R 7 C(O)O-, R 7 C(O) R 8 -, -CN or -NO 2 ;
- a 1 is oxygen
- a 2 is oxygen, NR 2 or CR 7 R 8 ;
- G is G-l or G-2;
- B 1 , B 2 , B 3 , B 4 and B 5 are independently N or C-R9;
- Y is hydrogen, halogen, -CN; or Y is alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, alkylcycloalkyl, cycloalkylalkyl, aryl, or heterocyclyl or heteroaryl each of which is unsubstituted or substituted with one or more of halogen, hydroxy, amino, alkyl- or di(alkyl)amino, alkyl, cycloalkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, alkoxy, haloalkoxy, alkylthio, haloalkylthio, R 7 S(O)-, R 7 S(O) 2 -, R 7 C(O)-, R 7 R 8 NC(O)-, R 7 OC(O)-,
- R 2 , R-3 are independently hydrogen, alkyl, haloalkyl, thioalkyl, alkylthioalkyl, hydroxyalkyl, alkoxyalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, R 10 S(O)-, R 10 S(O) 2 -, R 10 C(O)-, R 10 C(S)-, R 10 R 1 1 NC(O)-, R 10 R 1 1 NC(S)- R 10 OC(O)-;
- R4, R5 and R 6 are independently hydrogen, alkyl, haloalkyl, thioalkyl, alkylthioalkyl, hydroxyalkyl, alkoxyalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, aryl or heteroaryl;
- R 7 and R 8 are independently hydrogen, alkyl, haloalkyl, thioalkyl, alkylthioalkyl, hydroxyalkyl, alkoxyalkyl, alkenyl, haloalkenyl, alkynyl or haloalkynyl;
- R9 is hydrogen, halogen, -CN, or alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, cycloalkyl, halocycloalkyl, alkylcycloalkyl or cycloalkylalkyl, each which is unsubstituted or substituted with one or more of halogen, hydroxy, amino, alkyl- or
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- X 1 , X 2 and X 3 are independently H, halogen, C 1 -C 3 alkyl or C 1 -C 3 haloalkyl, or a pharmaceutically acceptable salt thereof; b) at least one pharmaceutically acceptable polymer;
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- X 1 , X 2 and X 3 are independently H, halogen, C 1 -C 3 alkyl or C 1 -C 3 haloalkyl, or a pharmaceutically acceptable salt thereof;
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- T is a C 1 -C 6 -alkyl group which is unsubstituted or substituted by halogen, cyano, nitro, amino, hydroxyl, C 1 -C 6 -alkoxy, C 1 -C 6 -haloalkoxy, C 1 -C 6 -alkylthio, C 1 -C 6 -alkylthio, carboxy, carbamoyl or C 2 -C 6 -alkanoyl group which may be unsubstituted or substituted in the alkyl portion by halogen or a pharmaceutical acceptable salt thereof
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising
- Y is hydrogen, fluoro, chloro or bromo
- R 1 is phenyl substituted with 2-4 substituents selected from halogen, methyl, difluoromethyl, trifluoromethyl, methoxy, trifluoromethoxy or trifluoroethoxy;
- R 2 is methyl, fluoromethyl, trifluoromethyl or perfluoroethyl
- R 3a and R 3b are independently selected from hydrogen, methyl, ethyl or fluoromethyl; or R 3a and R 3b together combine with the carbon to which they are attached to form a cyclopentyl ring or a cyclohexyl ring; or a pharmaceutically acceptable salt thereof
- the present invention provides for extended release injectable formulations for the treatment and/or prevention (prophylaxis) of parasitic infections and infestations of animals comprising:
- e) optionally a surfactant; and f) optionally, at least one pharmaceutically acceptable additive, excipient or mixtures thereof.
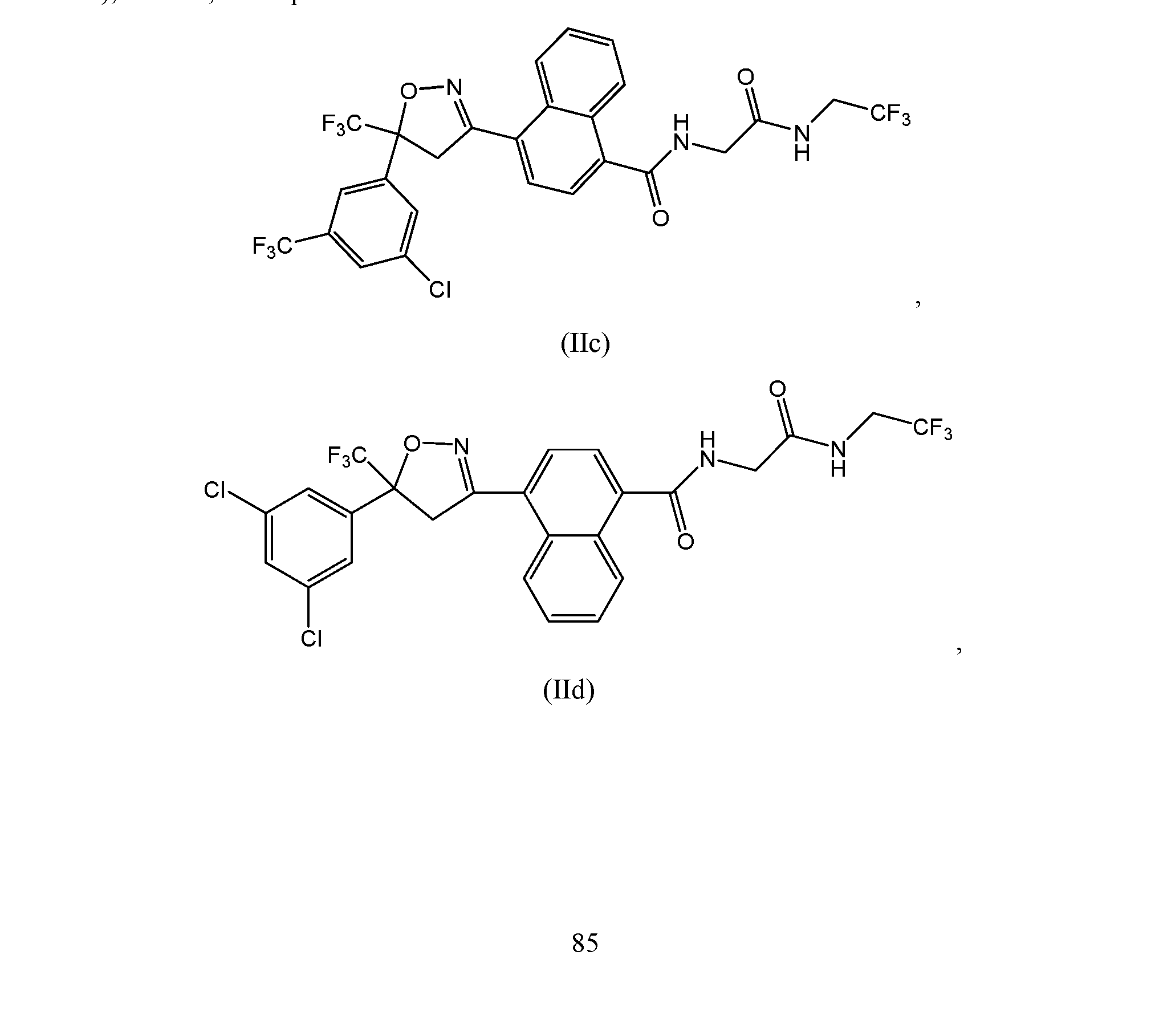
- the extended release injectable formulations of present invention comprise an antiparasitic effective amount of 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]-4,5- dihydro-5-(trifluoromethyl)-3-isoxazolyl]-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-l- naphthalanecarboxamide (Compound of formula lIc).
- the pharmaceutically acceptable polymer in the extended release injectable formulations described above may be a copolymer of polylactides and polyglycolides
- the solvent may be a single solvent, such as, for example a cyclic carbonate (e.g., ethylene carbonate or propylene carbonate) or a mixture of solvents comprising, for example, a cyclic carbonate, a glycerol ester (e.g., glycerol triacetate), and, optionally, a poloxamer (for example., P-124), which can function either as a solvent or a surfactant.
- an antioxidant is present, such as, butylated hydroxytoluene.
- the compounds of formula (I) through formula (VIla) can exist as stereoisomers since there is a chiral center in the molecule.
- the individual stereoisomers are encompassed by the structural formulas depicted herein.
- the various stereoisomers include enantiomers, diastereomers and atopisomers.
- one stereoisomer may be more active and/or may exhibit beneficial properties relative to the other enantiomer.
- the skilled person in the art knows how to separate, enrich, and/or selectively prepare a stereoisomer of the isoxazoline compounds described herein.
- the isoxazoline compounds described herein contain a chiral quaternary carbon atom in the five-membered isoxazoline ring (shown by the asterisk (*)); therefore, the compounds will contain at least two possible stereoisomers.
- the compounds of formula e) ( tIhle two possible stereoisomers resulting from the quaternary carbon are shown as formula (R)-IIc and (S) -IIc:
- the compound of formula (S)-IIc above has the (S) configuration at the chiral carbon atom and the compound of formula (R)-IIc has the (R) configuration.
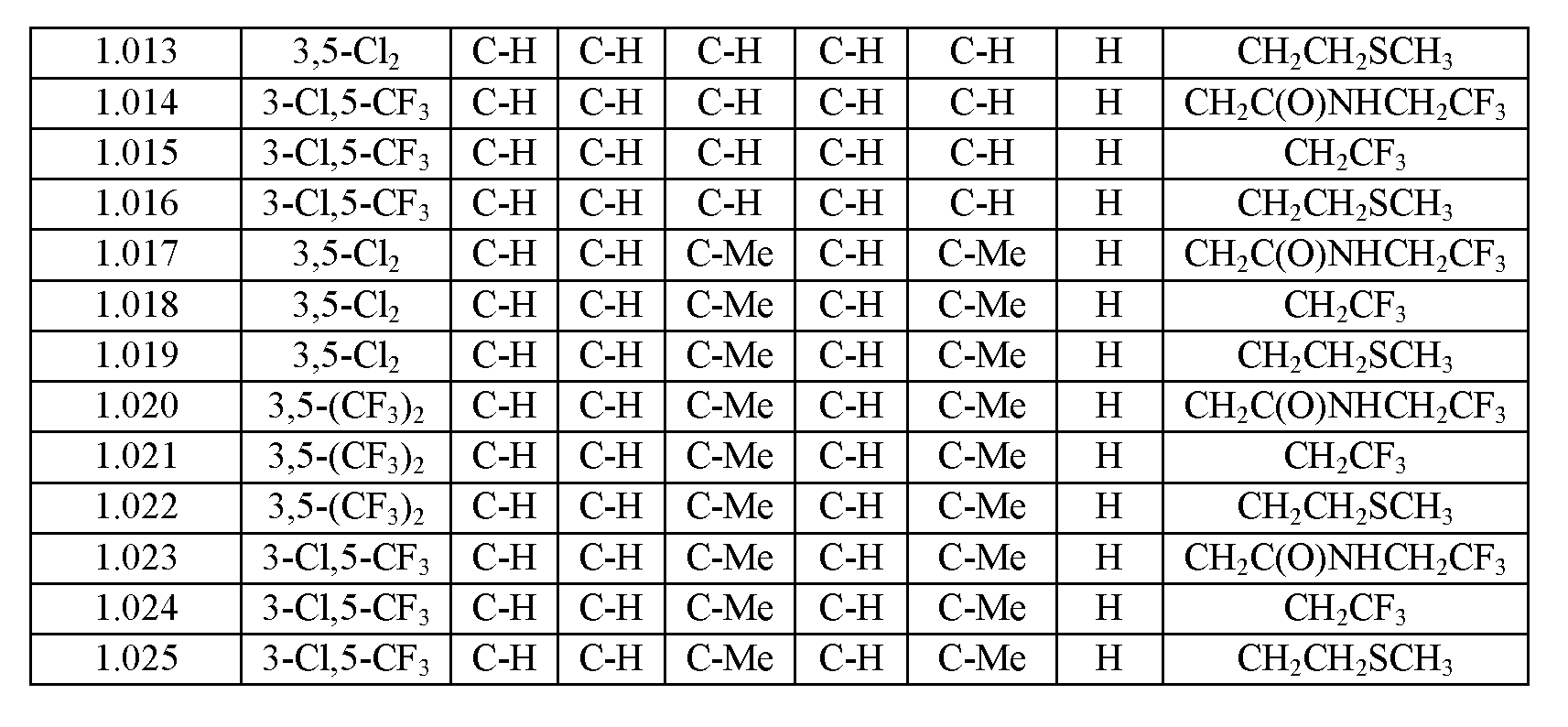
- the extended release injectable formulations of present invention comprise an antiparasitic effective amount of at least one isoxazoline of Formula (I), Formula (II), Formula (Ila), Formula (IbI), Formula (Iel), Formula (dII), Formula (Ile), Formula (Ilf), Formula (III), Formula (III- 1.1001) to Formula (III- 1.025), Formula (III- 2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla) which is enriched in one enantiomer, or a pharmaceutically acceptable salt thereof.
- the extended release injectable formulations comprise an antiparasitic effective amount of at least one isoxazoline of Formula (I), Formula (II), Formula (Ila), Formula (IIb), Formula (Ile), Formula (IdI), Formula (Iel), Formula (Ilf), Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), which is enriched an enantiomer that displays significant in vitro and in vivo activity with a favorable toxicity profile (the eutomer) whereas a compound or composition enriched in the other enantiomer displays significantly less in vitro and in vivo activity (the distomer), or a pharmaceutically acceptable salt thereof.
- the more biologically active enantiomer of the compound of Formula lie is believed to be compound of Formula ( ⁇ -IIc shown above, which has the ( ⁇ -configuration at the chiral carbon atom.
- the compounds of formulae Formula (I), Formula (II), Formula (Ila), Formula (IIb), Formula (Ile), Formula (IdI), Formula (eI)l, Formula (Ilf), Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VII) or Formula (VIla) present in the compositions of the invention are enriched in one enantiomer over the other enantiomer in a weight: weight ratio of at least 1.5 : 1.
- the compounds of Formula (I), Formula (II), Formula (Ila), Formula (IIb), Formula (IIc), Formula (lid), Formula (Ile), Formula (Ilf), Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VII) or Formula (VIla) present in the compositions of the invention are enriched in one enantiomer in a weight:weight ratio of at least 2: 1, at least 5 : 1 or at least 10: 1.
- the compounds of Formula (I), Formula (II), Formula (Ila), Formula (IIb), Formula (Ile), Formula (IdI), Formula (eI)l, Formula (Ilf), Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VII) or Formula (VIla) present in the compositions of the invention are essentially pure enantiomers.
- the invention provides extended release injectable compositions that comprise the essentially pure enantiomers of the compounds of Formula (I), Formula (II), Formula (Ila), Formula (IIb), Formula (Iel), Formula (dI)I, Formula e()Il, Formula (Ilf), Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla).
- the composition of the invention comprises a compound of formula (I), that is substantially enriched in an enantiomer.
- substantially enriched is meant wherein the weightweight ratio is at least about 1.5 : 1 or higher in favor of the desired enantiomer.
- the extended release injectable compositions of the invention comprise a compound of formula (I), that is substantially enriched in the (S)- enantiomer.
- the extended release injectable compositions of the invention comprise a compound of formula (I) that is substantially enriched in the (R)- enantiomer.
- compositions comprise a compound of formula (I), that is enriched in the (S)-enantiomer in a weightweight ratio of at least about 2: 1, (S) to (R), or greater.
- compositions of the invention comprise a compound of formula (I) that is enriched in the (S)-enantiomer in a weightweight ratio of at least about 5 : 1, (S) to (R), or greater.
- compositions of the invention comprise a compound of formula (I), that is enriched in the (S)-enantiomer in a weightweight ratio of at least about 10: 1, (S) to (R), or greater.
- the compositions of the invention comprise a compound of formula (I), that is essentially the pure (S)-enantiomer.
- compositions comprise a compound of formula (I), that is enriched in the (R)-enantiomer in a weightweight ratio is at least approximately 2: 1, (R) to (S), or greater.
- the compositions of the invention comprise a compound of formula (I), that is enriched in the (R)-enantiomer in a weight: weight ratio of at least about 5:1, (R) to (S), or greater.
- the compositions of the invention comprise a compound of formula (I), that is enriched in the (R)- enantiomer in a weight: weight ratio of at least about 10:1, (R) to (5), or greater.
- the compositions of the invention comprise a compound of formula (I) that is essentially the pure R-enantiomer.
- the composition of the invention comprises a compound of formula (II),(Il a),(IIb),(Ile), (IId),(Ile) or (Ilf) that is substantially enriched in an enantiomer.
- the extended release injectable compositions of the invention comprise a compound of formula (II), (Ila),(IIb),(IIe),(IId),(Ile) or (Ilf) that is substantially enriched in the (S)-enantiomer.
- the extended release injectable compositions of the invention comprise a compound of formula (II), (Ila),(IIb),(IIe),(IId),(Ile) or (Ilf) that is substantially enriched in the (R)-enantiomer.
- compositions comprise a compound of formula (II), (Ila), (IIb),(IIe),(IId),(Ile) or (Ilf) that is enriched in the (S)-enantiomer in a weight: weight ratio of at least about 2:1, (S) to (R), or greater.
- compositions of the invention comprise a compound of formula (II), (Ila), b()I,I e()I,l d()I,I e()Il or (Ilf) that is enriched in the (S)-enantiomer in a weight: weight ratio of at least about 5:1, (S) to (R), or greater.
- compositions of the invention comprise a compound of formula (II), (Ila),(IIb),(IIe),(IId),(Ile) or (Ilf) that is enriched in the (S)- enantiomer in a weight: weight ratio of at least about 10:1, (S) to (R), or greater.
- compositions of the invention comprise a compound of formula (II), (Ila), b),(II (lie),(IId),(Ile) or (Ilf) that is essentially the pure (S)-enantiomer.
- compositions comprise a compound of formula (II), (Ila), (IIb),(Ile), (IId),(Ile) or (Ilf) that is enriched in the (R)-enantiomer in a weigh weight ratio is at least approximately 2:1, (R) to (S), or greater.
- the compositions of the invention comprise a compound of formula (II), (Ila), b),(II (lie),(IId),(Ile) or (Ilf) that is enriched in the (R)-enantiomer in a weigh weight ratio of at least about 5:1, (R) to (S), or greater.
- compositions of the invention comprise a compound of formula (II), (Ila), (IbI), (IeI), (IId), (Ile) or (Ilf) that is enriched in the (R)-enantiomer in a weight: weight ratio of at least about 10:1, (R) to (S), or greater.
- compositions of the invention comprise a compound of formula (II), (Ila),(II b), (Ile), (IId), (Ile) or (Ilf) that is essentially the pure (R)-enantiomer.
- the composition of the invention comprises a compound of formula (lie) that is substantially enriched in an enantiomer.
- the extended release injectable compositions of the invention comprise a compound of formula e(I)I that is substantially enriched in the (S)-enantiomer.
- the extended release injectable compositions of the invention comprise a compound of formula (II e) that is substantially enriched in the (R)-enantiomer.
- this invention comprises racemic mixtures, for example, approximately equal amounts of the enantiomers of Formulae (I), Formula (II), Formula (Ila), Formula (IIb), Formula (Ile), Formula (IId), Formula (IIe), Formula (Ilf), Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VI) or Formula (VIla).
- compositions of the invention comprise compounds that have at least a 50 % enantiomeric excess.
- compositions of the invention comprise compounds that have at least a 75 % enantiomeric excess, at least a 90 % enantiomeric excess, or at least a 94 % enantiomeric excess of the more active isomer.
- the more active isomer the eutomer.
- Compounds of this invention can exist as one or more conformational isomers due to restricted rotation about the amide bond bonded to the aryl or heteroaryl ring (e.g. the amide bonded to the naphthyl group in Formula (Il e)).
- This invention comprises mixtures of conformational isomers.
- this invention includes compounds that are enriched in one conformer relative to others.
- the compositions comprise a compound of Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), that is enriched in the (S)-enantiomer in a weigh weight ratio is at least approximately 2: 1, (S) to (R), or greater.
- compositions of the invention comprise a compound of Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), that is enriched in the (S)-enantiomer in a weigh weight ratio of at least about 5 : 1, (S) to (R), or greater.
- compositions of the invention comprise a compound of Formula (III), Formula (III- 1.1001 ) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), that is enriched in the (S)- enantiomer in a weight: weight ratio of at least approximately 10: 1, (S) to (R), or greater.
- compositions of the invention comprise a compound of Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), that is essentially the pure (S)-enantiomer.
- compositions comprise a compound of Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III- 2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), that is enriched in the (R)-enantiomer in a weigh weight ratio is at least approximately 2: 1, (R) to (S), or greater.
- compositions of the invention comprise a compound of Formula (III), Formula (III- 1.1001 ) to Formula (III- 1.025), Formula (III-2.001) to Formula (III-018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), that is enriched in the (R)-enantiomer in a weight: weight ratio of at least about 5 : 1, (R) to (S), or greater.
- compositions of the invention comprise a compound of Formula (III), Formula (III-1.1001) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), that is enriched in the (R)-enantiomer in a weightweight ratio of at least approximately 10: 1, (R) to (S), or greater.
- compositions of the invention comprise a compound of Formula (III), Formula (III- 1.1001 ) to Formula (III-1.025), Formula (III-2.001) to Formula (III-2.018), Formula (IV), Formula (IVa), Formula (V), Formula (Va), Formula (VI), Formula (VIa), Formula (VII) or Formula (VIla), that is essentially the pure (R)-enantiomer.
- the extended release injectable formulations of present invention comprise an antiparasitic effective amount of at least one isoxazoline disclosed in WO 2007/079162, WO 2007/075459 and US 2009/0133319, WO 2007/070606 and US 2009/0143410, WO 2009/003075, WO 2009/002809, WO 2009/024541, WO 2005/085216 and US 2007/0066617 WO 2008/122375, WO 2014/439475 Al and WO2012 120135A1, all of which are incorporated herein by reference in their entirety.
- the extended release injectable formulations of present invention comprise an antiparasitic effective amount of at least one isoxazoline compound described in WO 2009/02451A2 and WO 201 1/075591 Al, both incorporated herein by reference in their entirety.
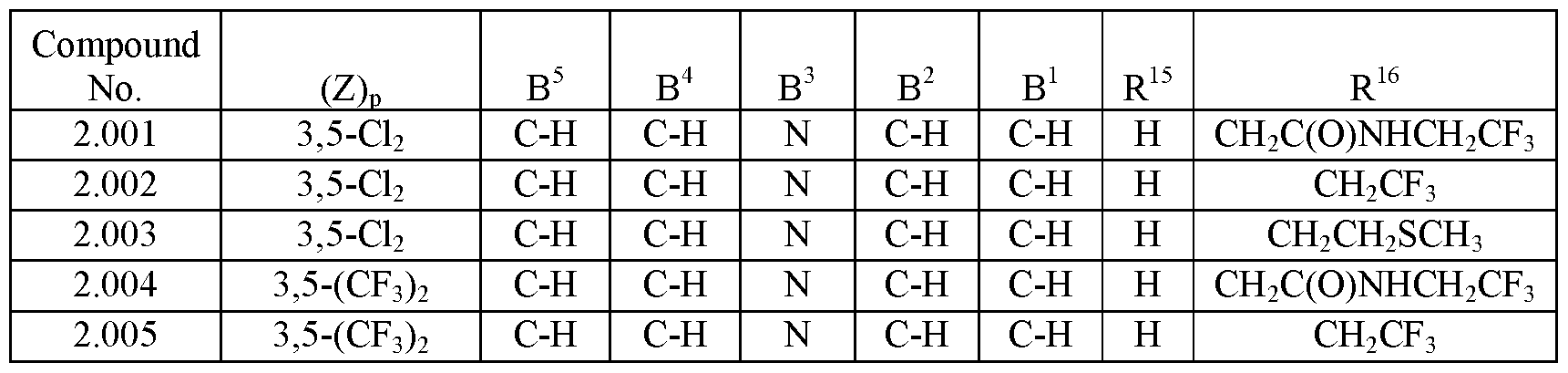
- the compositions of the invention may comprise about 5 to about 50% (w/w) of an isoxazoline active agent. In another embodiment, the compositions may comprise about 5 to about 30% (w/w) of the isoxazoline active agent. In yet other embodiments, the compositions may include about 5 to about 20% (w/w) or about 5 to about 15% (w/w) of the isoxazoline active agent. In another embodiment, the compositions of the invention may comprise about 10 to about 40% (w/w) or 10 to about 30% (w/w) of an isoxazoline active agent. In another embodiment, the compositions may comprise about 10 to about 20% of an isoxazoline active agent.
- compositions of the invention may comprise about 15% to about 40% (w/w), about 15% to about 35% (w/w) or about 15% to about 30% (w/w) of an isoxazoline compound.
- compositions of the invention will comprise about 20 to about 30% (w/w), about 20 to about 25% (w/w) or about 25 to about 30% (w/w) of the isoxazoline active agent.
- compositions of the invention comprise about 1 to about 40%
- compositions comprise about 1 to about 30% (w/w) or about 1 to about 20% (w/w) of a pharmaceutically acceptable polymer. In another embodiment, the compositions comprise about 1 to about 15% (w/w) or about 1 to about 10% (w/w) of a pharmaceutically acceptable polymer. In another embodiment, the compositions comprise about 5 to about 20% (w/w) or about 5 to about 15% (w/w) of a pharmaceutically acceptable polymer. In another embodiment, the compositions comprise about 10 to about 20% (w/w) or about 10 to about 15%> (w/w) of a pharmaceutically acceptable polymer.
- compositions comprise about 7 to about 13% (w/w) or about 8 to about 15% (w/w) of a pharmaceutically acceptable polymer.
- compositions of the invention comprise about 1 to about 7% (w/w), about 1 to about 5% (w/w) or about 3 to about 7% (w/w) of a pharmaceutically acceptable polymer.
- the compositions of the invention may comprise about 30% to about 90%) (w/w) of a solvent or mixture of solvents. In another embodiment, the compositions of the invention may comprise about 40% to about 90% (w/w) of a solvent or mixture of solvents. In yet another embodiment, the compositions comprise about 40% to about 80% (w/w), about 50% to about 80%) (w/w) or about 45% to about 80% (w/w) of a solvent or a mixture of solvents. In yet another embodiment, the compositions of the invention comprise about 60% to about 80% (w/w) or about 65% to about 80% (w/w) of a solvent or a mixture of solvents. In still another embodiment, the compositions may comprise about 65% to about 75% (w/w) or about 70% to about 80%) (w/w) of a solvent or a mixture of solvents.
- compositions of the invention may comprise about 0.01% to about 10%) (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof. In other embodiments, the compositions may comprise about 0.01% to about 5% (w/w), about 0.1% to about 10%) (w/w) or about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- compositions of the invention may comprise about 0.01% to about 5%) (w/w) of an antioxidant. In other embodiments, the compositions may comprise about 0.01%) to about 3%) (w/w) or about 0.01 to about 2% (w/w) of an antioxidant.
- the pharmaceutically acceptable polymers in the extended release injectable formulations include, but are not limited to, polylactides, polyglycolides, polycaprolactones, polyanhydrides, polyamides, polyurethanes, polyesteramides, polyorthoesters, polydioxanones, polyacetals, polyketals, polycarbonates, polyorthocarbonates, polyphosphazenes, pseudo poly(amides), polyhydroxyalcanoates, polyhydroxybutyrates, polyhydroxyvalerates, polyalkylene oxalates, polyalkylene succinates, poly(malic acid), poly(amino acids), poly(methyl vinyl ether), poly(maleic anhydride), chitin, chitosan, and copolymers, terpolymers, or combinations or mixtures therein including copolymers of polylactides, polycaprolactones, polyglycolides (e.g., poly(lactide-co-glycolide) and copolymers of poly
- the pharmaceutically acceptable polymer is a biodegradable polymer.
- the pharmaceutically acceptable biodegradable polymer can have one or more or all of the following characteristics: be bioerodible by cellular action, biodegradable by action of non-living body fluid components, soften when exposed to heat but return to the original state when cooled and are capable of substantially dissolving or dispersing in a water-miscible carrier or solvent to form a solution or dispersion.
- the polymer Upon contact with an aqueous fluid the polymer is capable of assisting in the formation of the film coated or encapsulated liquid or solid (which will contain the active agent in the present invention).
- the kinds of polymers suitable for the present composition generally include any having the foregoing characteristics.
- biodegradable polymers include, but are not limited to, polylactides, polycaprolactones, polyglycolides, polyorthoesters, polyurethanes, polyphasphazenes, pseudo poly(amides), and copolymers thereof.
- the molecular weight of a polymer is not a discreet number but can be presented in a molecular weight range.
- the average molecular weight of a polymer may be found by techniques familiar to persons of skill in the art, for example, size exclusion chromatography with molecular weight standards, or the like.
- the molecular weight range of a polymer can impact the physical characteristics of the material and the way that it interacts with the active agent. Accordingly the molecular weight range of the polymer may impact the characteristics of the extended release compositions of the invention. For example, in some embodiments depending on the active agent and solvents included, one may see an earlier release of the isoxazoline active agent when the weight average molecular weight range is from
- a combination of polymers having different average molecular weights may provide a release rate that combines the effect of the different polymers used.
- Inherent viscosity (IV) in polymer chemistry is a viscometric method for measuring molecular weight. It is defined as the ratio of the natural logarithm of the relative viscosity to the mass concentration of the polymer and is based on the flow time of a polymer solution through a narrow capillary.
- the term“low molecular weight” refers to polymer with an inherent viscosity in the range of 0.05-0.29 dL/g; the term“medium molecular weight” refers to a polymer with an inherent viscosity in the range of 0.3-0.55dL/g; and high molecular weight refers to a polymer with an inherent viscosity in the range of 0.55-1.0 dL/g.
- the pharmaceutically acceptable polymer in the extended release formulations of the invention will have an inherent viscosity of about 0.10-0.20 dL/g.
- the pharmaceutically acceptable polymer in the extended release formulations will have an inherent viscosity of about 0.35-0.50.
- the extended release injectable formulations of the invention comprise polylactides, polycaprolactones, polyglycolides and copolymers thereof.
- the compositions include a poly(lactide-co-glycolide) copolymer ("PLGA").
- PLGA copolymers may have different molecular weight ranges and may also have different weight:weight ratios of lactide to glycolide. This ratio, may affect the properties of the copolymer and the way that it interacts with the active agent.
- compositions of the invention having a higher lactide to glycolide ratio result in an increase in hydrogen bonding between the active agent and the polymer, leading to better solubility of the active agent in vivo. This effect improves the injection site reaction and allows for the extendable release injectable
- the ratio of lactide to glycolide is about 30:70 to about 99: 1. In another embodiment of the invention where the pharmaceutically acceptable polymer is PLGA, the ratio of lactide to glycolide is about 40:60 to about 80: 15. In another embodiment of the invention where the pharmaceutically acceptable polymer is PLGA, the ratio of lactide to glycolide is about 40:60 to about 60:40. In another embodiment of the invention where the pharmaceutically acceptable polymer is PLGA, the ratio of lactide to glycolide is about 70:30 to about 80:20. In another embodiment of the invention, where the pharmaceutically acceptable polymer is PLGA, the ratio of lactide to glycolide is about 50:50. In another embodiment of the invention, where the pharmaceutically acceptable polymer is PLGA, the ratio of lactide to glycolide is about 75:25.
- the amount of PLGA contained in the extended release injectable formulation of the invention is about 1% to about 30 % (w/w), In another embodiment, the compositions comprise about 1 to about 20% (w/w) of PLGA. In another embodiment, the compositions comprise about 5 to about 20%, about 8% to about 20% (w/w) or about 10 to about 20%) (w/w). In another embodiment, the compositions comprise about 5 to about 15% (w/w) of PLGA. In other embodiments, the amount of PLGA contained in the extended release injectable formulation of the invention is from about 3% to about 15% (w/w) or is from about 10% to about 15%) (w/w). In yet another embodiment, the compositions comprise about 7 to about 13% or about 8 to about 15% (w/w) of PLGA.
- the weigh weight ratio of-PLGA to the isoxazoline active agent is greater than or equal to about 1 : 1, for example, from about 1.1 : 1 to about 20: 1; e.g., about 1 : 1 to about 10: 1, about 1.1 : 1 to about 10: 1 or about 2: 1 to about 5: 1.
- the weigh weight ratio of PLGA to the isoxazoline active agent is about 1.2: 1 to about 5: 1.
- the weigh weight ratio of PLGA to the isoxazoline active agent is about 1.2: 1 to about 2: 1.
- the weigh weight ratio of PLGA to the isoxazoline active agent is about 1.2: 1 to about 1.3 : 1.
- the weigh weight ratio of PLGA to the isoxazoline active agent is about 1.5: 1 to about 1 : 1.5. In other embodiments, the ratio of the isoxazoline active agent to PLGA is from about 1.25 : 1 to about 1 : 1 :25.
- the solvents used in the extended release injectable formulations of the invention may be a single or a blend of solvents.
- Non-limiting examples of these solvents include alcohols such as ethanol, 1-propanol, isopropanol, glycol ethers (e.g., including, but limited to, diethyleneglycol monoethyl ether (DGME, Transcutol ® ), butyl diglycol, dipropylene glycol n-butyl ether, ethyleneglycol monoethyl ether, ethyleneglycol monomethyl ether, dipropylene glycol monomethyl ether, propylene glycol monomethyl ether, propylene glycol monoethyl ether, and the like), liquid polyethylene glycols (PEGs) including, but not limited to, PEG 200, PEG 300 and PEG 400; propylene glycol, glycerol, glycerol esters including glycerol triacetate (triacetin), cyclic carbonates (e
- the compositions of the invention may include one or more poloxamers as a solvent or surfactant.
- Poloxamers are a family of synthetic block copolymers of ethylene oxide and propylene oxide. Poloxamers may be liquid, a milky white paste or a powder and are represented by the followin structure: where a is an integer between 2 and 130 and b is an integer between 15 and 67 (see, US 3,740,421). Poloxamer are available from commercial sources such as BASF and Croda.
- the amount of poloxamer, when present is from about 0.5% to about 20 (w/w).
- the compositions may have, when present, about 1% to about 20% (w/w), about 1% to about 10% (w/w) or from about 1 to about 5% (w/w).
- the amount of poloxamer, when present is from about 1 % to about 3 % (w/w).
- compositions of the invention comprise a solvent or mixture of solvents that is miscible with water.
- Solvents that are miscible with water are well known and include certain alcohols, liquid polyethylene glycols (PEGs), certain poloxamers, glycols and glycol ethers and polar aprotic solvents.
- Alcohols that are miscible with water include, but are not limited to ethanol, isopropanol, «-propanol, Solketal (isopropylidene glycerol) or glycerol formal.
- Polar aprotic solvents include, but are not limited to, amides such as dimethylacetamide, dimethylformamide, 2-pyrrolidone, N-alkylpyrrolidones such as N-methylpyrrolidone and N- octylpyrrolidone, dimethylisosorbide, dimethylsulfoxide, cyclic carbonates including propylene carbonate and ethylene carbonate, and certain ketones such as acetone and the like.
- Glycol ethers include, but are not limited to, diethyleneglycol monoethyl ether (DGME, Transcutol®), butyl diglycol, dipropylene glycol n-butyl ether, ethyleneglycol monoethyl ether, ethyleneglycol monomethyl ether, dipropylene glycol monomethyl ether, propylene glycol monomethyl ether, propylene glycol monoethyl ether, and the like.
- DGME diethyleneglycol monoethyl ether
- Transcutol® diethyleneglycol monoethyl ether
- the extended release formulations of the invention comprise a polar protic solvent including, but not limited to, an alcohol such as ethanol, isopropanol or a glycol or glycol ether.
- the extended release injectable formulations of the invention comprise a polar aprotic solvent such as N-methylpyrrolidone, dimethyl isosorbide, dimethylacetamide or propylene carbonate.
- a polar aprotic solvent such as N-methylpyrrolidone, dimethyl isosorbide, dimethylacetamide or propylene carbonate.
- the compositions of the invention include non-water miscible solvents (e.g. not completely miscible with water, although they may have some solubility in water).
- these solvents include 1-butanol, 2-butanol, 1-pentanol, 3-pentanol, benzyl alcohol, methylethylketone (MEK), triacetin, lipids, triglycerides including medium chain triglycerides such C 8 -C 10 triglycerides such as capric/caprilic triglycerides, propylene glycol derivatives (e.g.
- propylene glycol monolaurate caprylocaproyl polyoxyl-8 glycerides (Labrasol) (non-ionic water dispersible surfactant, isopropyl myristate, oils such as castor oil, soybean oil or other vegetable oils or derivatives thereof such as epoxidized or hydrogenated vegetable oils such as epoxidized soybean oil or hydrogenated castor oil, or a mixture of at least two of these solvents.
- Labrasol non-ionic water dispersible surfactant, isopropyl myristate, oils such as castor oil, soybean oil or other vegetable oils or derivatives thereof such as epoxidized or hydrogenated vegetable oils such as epoxidized soybean oil or hydrogenated castor oil, or a mixture of at least two of these solvents.
- the composition of the invention may include neutral oils as a solvent.
- Neutral oils are triglycerides of fractionated plant fatty acids with chain lengths of C 8 to C 10 .
- Two commercially available products are known as MIGLYOL® 810 and MIGLYOL®812.
- the neutral oil is a triglyceride of fractionated plant fatty acids with chain lengths of C 8 and C 10 combined with linoleic acid (about 4-5 %).
- a commercially available product is known as MIGLYOL® 818.
- the neutral oil is a glycerin ester of fractionated plant fatty acids with chain lengths of C 8 and C 10 combined with succinic acid.
- a commercially available product is known as MIGLYOL® 829.
- the neutral oil is a propylene glycol fatty acid ester.
- the neutral oil may be a propylene glycol diester of saturated plant fatty acids with chain lengths of C 8 and C 10 .
- a commercially available product is known as MIGLYOL® 840 (propylene glycol dicaprylate/dicaprate).
- the solvent may be a mixture of two or more neutral oils.
- blends of solvents may be used as the solvent of the extended release injectable formulations.
- the compositions of the invention may contain a blend of a water-miscible solvent with a solvent that is not water miscible.
- the solvent may be a mixture of a cyclic carbonate such as propylene carbonate with triacetin.
- a water-miscible solvent and a non-water miscible solvent are possible.
- the water-miscible solvent in the solvent blend may be a water-miscible alcohol such as ethanol or isopropanol, glycerol formal or Solketal, an amide such as 2-pyrrolidone, N-methylpyrrolidone, dimethylisosorbide or dimethylacetamide, a glycol such as propylene glycol, glycerol or a glycol ether.
- a water-miscible alcohol such as ethanol or isopropanol, glycerol formal or Solketal
- an amide such as 2-pyrrolidone, N-methylpyrrolidone, dimethylisosorbide or dimethylacetamide
- a glycol such as propylene glycol, glycerol or a glycol ether.
- the non-water miscible solvent in the solvent blend may be triacetin, benzyl alcohol, a triglyceride including C 8 -C 10 triglycerides such as capric/caprilic triglycerides, propylene glycol derivatives (e.g. propylene glycol monolaurate), caprylocaproyl polyoxyl-8 glycerides (Labrasol); a propylene glycol fatty acid diester, and the like.
- a triglyceride including C 8 -C 10 triglycerides such as capric/caprilic triglycerides
- propylene glycol derivatives e.g. propylene glycol monolaurate
- caprylocaproyl polyoxyl-8 glycerides Labrasol
- a propylene glycol fatty acid diester and the like.
- the solvent may be a blend of a water-miscible solvent and a non- water miscible solvent in a weight: weight ratio of between about 10 to 1 to about 1 to 10, water- miscible solvent to non-water miscible solvent.
- the weigh weight ratio of the water-miscible solvent to non-water miscible solvent may be from about 5 to 1 to about 1 to 1.
- the weigh weight ratio of the water-miscible solvent to non-water miscible solvent may be from about 3 to 1 to about 1 to 1.
- the weigh weight ratio of the water-miscible solvent to non-water miscible solvent may be from about 3 to 1 to about 2 to 1 or about 2 to 1 to about 1 to 1.
- the solvent may be a blend of a water-miscible solvent and a non-water miscible solvent in a weigh weight ratio of about 1 to 2 or about 1 to 3, water- miscible solvent to non-water miscible solvent.
- the weigh weight ratio of the water-miscible solvent to non-water miscible solvent may be from about 1 to about 5, 1 to about 7.
- the solvent may be a blend of cyclic carbonate (e.g., propylene carbonate) and glycerol ester (e.g., triacetin) in a weigh weight ratio of between about 10 to 1 to about 1 to 1, cyclic carbonate (e.g., propylene carbonate) to glycerol ester (e.g., triacetin).
- the solvent may be a blend of cyclic carbonate (e.g., propylene carbonate) and glycerol ester (e.g., triacetin) in a weigh weight ratio of between about 5 to 1 to about 1 to 1, cyclic carbonate (e.g.
- the solvent may be a blend of cyclic carbonate (e.g., propylene carbonate) and glycerol ester (e.g., triacetin) in a weigh weight ratio of between about 3 to 1 to about 1 to 1, cyclic carbonate (e.g. propylene carbonate) to glycerol ester (e.g., triacetin).
- the solvent may be a blend of cyclic carbonate (e.g., propylene carbonate) and glycerol ester (e.g., triacetin) in a weightweight ratio of between about 2 to 1 to about 1 to 1 or about 3 : 1 to about 2: 1, cyclic carbonate (e.g. propylene carbonate) to glycerol ester (e.g., triacetin).
- the range for the weightweight ratio of cyclic carbonate (e.g. propylene carbonate) to glycerol ester (e.g., triacetin) is 1.5: 1 to about 15: 1 or from about 2: 1 to about 6: 1.
- Surfactants may be present in the inventive formulations at concentrations of about 0.1 % to about 10% (w/w), about 1 % to about 10% (w/w) or about 5% to about 10% (w/w). More typically, surfactants may be present at concentrations of about 0.1 % to about 5% (w/w) or about 1 to about 5% (w/w).
- surfactants examples include, but are not limited to, glyceryl monooleate, polyoxyethylene sorbitan fatty acid esters, sorbitan esters30 including sorbitan monooleate (Span ® 20), polyvinyl alcohol, polysorbates including polysorbate 20 and polysorbate 80, d- ⁇ -tocopheryl polyethylene glycol 1000 succinate (TPGS), sodium lauryl sulfate, co-polymers of ethylene oxide and propylene oxide (e.g.
- poloxamers such as LUTROL ® F87 and the like
- polyethylene glycol castor oil derivatives including polyoxyl 35 castor oil (Cremophor ® EL), polyoxyl 40 hydrogenated castor oil (Cremophor RH 40), polyoxyl 60 hydrogenated castor oil (Cremophor RH60); propylene glycol monolaurate (LAUROGLYCOL ® ); glyceride esters including glycerol caprylate/caprate (CAPMUL ® MCM), polyglycolized glycerides (GELUCIRE ® ), PEG 300 caprylic/capric glycerides (Softigen ® 767), PEG 400 caprylic/capric glycerides (Labrasol ® ), PEG 300 oleic glycerides (Labrafil® M-1944CS), PEG 300 linoleic glycerides (Labrafil ® M-2125CS); polyethylene glycol stearates and polyethylene
- Polyethylene glycol stearates are mixtures of mono- and distearate esters of mixed polyoxyethylene polymers.
- Polyethylene glycol hydroxystearate is a mixture of mono- and diesters of hydroxystearic acid with polyethylene glycols.
- One polyethylene glycol hydroxystearate that may be used in the compositions is polyethylene glycol 12-hydroxy stearate.
- the inventive formulations may include the surfactant polyethylene glycol 15 12-hydroxy stearate (Kolliphor ® HS 15 from BASF), a mixture of mono- and diesters of 12-hydroxy stearic acid with 15 moles of ethylene oxide. Again, these compounds, as well as their amounts are well known in the art.
- the inventive formulations may include polyoxyl 35 castor oil (Kolliphor ® EL) as a surfactant.
- the inventive formulations may include polyoxyl 40 hydrogenated castor oil (Kolliphor ® RH 40) or polyoxyl 60 hydrogenated castor oil as surfactants.
- the formulations of the invention may also include a combination of surfactants.
- the inventive formulations may contain other inert ingredients such as antioxidants, preservatives, or pH stabilizers. These compounds are well known in the formulation art.
- Antioxidants such as vitamin E, alpha tocopherol, ascorbic acid, ascorbyl palmitate, citric acid, fumaric acid, malic acid, sodium ascorbate, sodium metabi sulfate, sodium metabisulfite, n- propyl gallate, BHA (butylated hydroxy anisole), BHT (butylated hydroxy toluene), BHA and citric acid, monothioglycerol, tert-butyl hydroquinone (TBHQ), and the like, may be added to the present formulation.
- the antioxidants are generally added to the formulation in amounts of from about 0.01 to about 5.0%, based upon total weight of the formulation, with about 0.05 to about 2.0% being especially preferred.
- the formulation preferably contains about 0.05 to about 1.0% (w/w) of an antioxidant.
- Preservatives such as the parabens (methylparaben, ethylparaben, butylparaben and/or propylparaben), are suitably used in the formulation in amounts ranging from about 0.01 to about 2.0%, with about 0.05 to about 1.0% being especially preferred.
- preservatives include benzalkonium chloride, benzethonium chloride, benzoic acid, benzyl alcohol, bronopol, cetrimide, chlorhexidine, chlorobutanol, chlorocresol, cresol, imidurea, phenol, phenoxyethanol, phenylethyl alcohol, phenylmercuric acetate, phenylmercuric borate, phenylmercuric nitrate, potassium sorbate, sodium benzoate, sodium propionate, sorbic acid, thimerosal, and the like. Preferred ranges for these compounds include from about 0.01 to about 5%.
- Buffering systems include, for example, systems selected from the group consisting of acetic acid/acetate, malic acid/malate, citric acid/citrate, tartaric acid/tartrate, lactic acid/lactate, phosphoric acid/phosphate, glycine/glycimate, tris, glutamic acid/glutamates and sodium carbonate.
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- d) optionally, about 0.01% to about 2.0% (w/w) of an antioxidant; e) optionally, about 0.1 % to about 10% (w/w) of a surfactant; and
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla);
- compositions comprise an isoxazoline active agent which is enriched in the more active enantiomer (the eutomer)
- present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- compositions comprise an isoxazoline active agent which is enriched in the more active enantiomer (the eutomer)
- present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- compositions comprise an isoxazoline active agent which is enriched in the more active enantiomer (the eutomer)
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- compositions comprise an isoxazoline active agent which is enriched in the more active enantiomer (the eutomer)
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- compositions comprise an isoxazoline active agent which is enriched in the more active enantiomer (the eutomer)
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- compositions comprise an isoxazoline active agent which is enriched in the more active enantiomer (the eutomer)
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- compositions comprise an isoxazoline active agent which is enriched in the more active enantiomer (the eutomer)
- the present invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- compositions comprise an isoxazoline active agent enriched in the more active enantiomer (the eutomer)
- the invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- compositions comprise an isoxazoline active agent enriched in the more active enantiomer (the eutomer)
- the invention provides for extended release injectable formulations for the treatment and/or prevention of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla) enriched in the (S)-enantiomer;
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), such as, a compound of the formula: (VIla) or a pharmaceutically acceptable salt thereof,
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula: or a pharmaceutically acceptable salt thereof,
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- the water miscible solvent is selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol including ethanol, isopropanol, glycerol formal and Solketal; and an amide and the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, a triglyceride and a propylene glycol ester ;
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- the water miscible solvent is selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol including ethanol, isopropanol, glycerol formal and Solketal; and an amide and the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, a triglyceride and a propylene glycol ester ;
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- Vila), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VIla), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VI), such as, a compound of the formula:
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VI), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VI), such as, a compound of the formula:
- d) optionally, about 0.01% to about 2.0% (w/w) of an antioxidant; e) optionally, about 0.1 % to about 10% (w/w) of a surfactant; and
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VI), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VI), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VI), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides for extended release injectable formulations for the treatment and/or prophylaxis of parasitic infections and infestations of animals comprising:
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of Formulae I- VI), such as, a compound of the formula:
- f) optionally, about 0.01% to about 5.0% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- Another embodiment of the present invention is an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting essentially of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- a pharmaceutically acceptable polymer b) a pharmaceutically acceptable polymer; c) at least one solvent or a mixture of solvents;
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting essentially of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- the water miscible solvent is selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide
- the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting essentially of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting essentially of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- the water miscible solvent is selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide
- the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting essentially of: a) an antiparasitic effective amount of at least one isoxazoline active agent, such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- a water miscible solvent selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide and the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting essentially of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- a water miscible solvent selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide and the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- e) optionally, aboutO. l % to about 10% (w/w) of a surfactant; and f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof
- Another embodiment of the present invention is an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- the water miscible solvent is selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide
- the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- d) optionally, about 0.01% to about 2% (w/w) of an antioxidant; e) optionally, about 0.1 % to about 10% (w/w) 0.1 % to about 10% (w/w) of a surfactant; and
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- the water miscible solvent is selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide
- the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- a PLGA polymer b) about 5% to about 20% (w/w) of a PLGA polymer; c) about 40% to about 90% (w/w) of a mixture of a water miscible solvent and a water immiscible solvent, wherein the water miscible solvent is selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide and the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting of:
- an antiparasitic effective amount of at least one isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- a water miscible solvent selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide and the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the present invention provides an extended release injectable formulation for the treatment and/or prevention of parasitic infections and infestations of animals consisting of: a) an antiparasitic effective amount of at least one isoxazoline active agent, such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- an isoxazoline active agent such as, for example, any of the isoxazoline compounds provided for in the embodiments above (e.g., a compound of any of Formulae I- VIla), and optionally at least one additional active agent as identified in this application;
- a water miscible solvent selected from the group consisting of a cyclic carbonate, dimethylisosorbide, dimethylsulfoxide, 2-pyrrolidone, N-methylpyrrolidone, N-octylpyrrolidone, a liquid polyethylene glycol, a poloxamer, an alcohol, Solketal and an amide and the water immiscible solvent is selected from the group consisting of benzyl alcohol, a glycerol ester, glycerol formal, a triglyceride, a propylene glycol ester and glycerol formal;
- f) optionally, about 0.1% to about 5% (w/w) of a pharmaceutically acceptable additive, excipient or mixtures thereof.
- the pharmaceutically acceptable polymer in the extended release injectable formulations described above may be a copolymer of a polylactides and polyglycolides and the solvent may be a single solvent, such as, for example a cyclic carbonate (e.g., ethylene carbonate or propylene carbonate) or a mixture of solvents comprising, for example, a cyclic carbonate, a glycerol ester (e.g., glycerol triacetate), and, optionally, a poloxamer (for example, P-124), which can function either as a solvent or a surfactant.
- the extended release injectable formulations, described above may further include an antioxidant, such as, butylated hydroxytoluene (BHT).
- BHT butylated hydroxytoluene
- any of the extended release injectable formulations provided for above wherein: the ratio of PLGA to the isoxazoline active agent to the copolymer of polylactides and polyglycolides is about 1.5: 1 to about 1 : 1.5 (weightweight); the weight average molecular weight of the copolymer of polylactides and polyglycolides is about 5 kDa to about 20 kDa; and the concentration of the copolymer of polylactides and polyglycolides is about 8 % (w/w) to about 20% (w/w) (e.g., 12.5% (w/w) or 13% (w/w)).
- any of the extended release injectable formulations provided for above may further comprise 0.5% (w/w) to about 20% (w/w) of poloxamer (e.g., about 1% (w/w) to about
- the copolymer of polylactides and polyglycolides may have a lactide to glycolide ratio of about 75:25 (weight:weight).
- the extended release formulations of the invention are prepared by adding to the solvent or solvent mixture, any non-polymer excipients (e.g. if present antioxidants, surfactants, etc.), followed by addition of the active ingredient(s) with mixing.
- any non-polymer excipients e.g. if present antioxidants, surfactants, etc.
- the pharmaceutically acceptable polymer(s) are added with mixing until completely dissolved.
- the composotions may be prepared by other appropriate processes known in the art as long as the resulting formulation is a homogeneous liquid formulation suitable for use.
- extended release or“extended release formulation” or“extended release composition” as used herein means a dosage form that is formulated in such a manner to make the active agent(s) contained therein to be available over an extended period of time due to the interaction of the formulation components in combination with the natural pharmacokinetic or pharmacodynamic characteristics of the active agent(s).
- This definition is consistent with the use of the term known and accepted in the veterinary field as described in the article“Terminology Challenges: Defining Modified Release Dosage Forms in Veterinary Medicine” by Marilyn N. Martinez, Danielle Lindquist and Sanja Modric (Journal of Pharmaceutical Sciences, vol.99, no. 8, August 2010).
- the extended release formulations according to the present invention would be understood to provide an efficacy of at least 90% against fleas and/or ticks for at least 3 months as described herein.
- animal is used herein to include all mammals, birds and fish and also include all vertebrate animals.
- Animals include, but are not limited to, cats, dogs, cattle, chickens, cows, deer, goats, horses, llamas, pigs, sheep and yaks. It also includes an individual animal in all stages of development, including embryonic and fetal stages. In some embodiments, the animal will be a non-human animal.
- essentially pure is used herein to indicate that a compound or an enantiomer is at least about 90% (w/w) pure, at least about 95% (w/w), or at least about 98% (w/w) pure, or higher.
- alkyl refers to saturated straight, branched, cyclic, primary, secondary or tertiary hydrocarbons, including those having 1 to 20 atoms.
- alkyl groups will include C 1 -C 12 , C 1 -C 10 , C 1 -C 8 , C 1 -C 6 or C 1 -C 4 alkyl groups.
- C 1 -C 10 alkyl examples include, but are not limited to, methyl, ethyl, propyl, 1-methylethyl, butyl, 1-methylpropyl, 2- methylpropyl, 1, 1-dimethylethyl, pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl, 2,2- dimethylpropyl, 1-ethylpropyl, hexyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3- dimethylbutyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl, 1-ethylbutyl, 2- ethylbutyl, 1, 1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1
- Cyclic alkyl groups or "cycloalkyl", which are encompassed by alkyl include those with 3 to 10 carbon atoms having single or multiple condensed rings.
- cycloalkyl groups include C 4 -C 7 or C 3 -C 4 cyclic alkyl groups.
- Non-limiting examples of cycloalkyl groups include adamantyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl and the like.
- alkyl groups described herein can be unsubstituted or substituted with one or more moieties selected from the group consisting of alkyl, halo, haloalkyl, hydroxyl, carboxyl, acyl, acyloxy, amino, alkyl- or dialkylamino, amido, arylamino, alkoxy, aryloxy, nitro, cyano, azido, thiol, imino, sulfonic acid, sulfate, sulfonyl, sulfanyl, sulfinyl, sulfamoyl, ester, phosphonyl, phosphinyl, phosphoryl, phosphine, thioester, thioether, acid halide, anhydride, oxime, hydrazine, carbamate, phosphoric acid, phosphate, phosphonate, or any other viable functional group that does not inhibit the biological activity of the compounds of the invention,
- alkyl such as “alkylcycloalkyl,” “cycloalkylalkyl,” “alkylamino,” or “dialkylamino” will be understood to comprise an alkyl group as defined above linked to the other functional group, where the group is linked to the compound through the last group listed, as understood by those of skill in the art.
- alkenyl refers to both straight and branched carbon chains which have at least one carbon-carbon double bond.
- alkenyl groups may include C 2 -C 20 alkenyl groups.
- alkenyl includes C 2 -C 12 , C 2 -C 10 , C 2 -C 8 , C 2 -C 6 or C 2 -C 4 alkenyl groups.
- the number of double bonds is 1-3, in another embodiment of alkenyl, the number of double bonds is one or two. Other ranges of carbon- carbon double bonds and carbon numbers are also contemplated depending on the location of the alkenyl moiety on the molecule.
- C 2 -C 10 -alkenyl groups may include more than one double bond in the chain. Examples include, but are not limited to, ethenyl, 1-propenyl, 2-propenyl, 1- methyl-ethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1 -methyl- 1-propenyl, 2-m ethyl- 1-propenyl, 1- methyl-2-propenyl, 2-methyl-2-propenyl; 1-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 1- m ethyl- 1-butenyl, 2-methyl- 1-butenyl, 3 -methyl- 1-butenyl, l-methyl-2-butenyl, 2-methyl-2- butenyl, 3-methyl-2-butenyl, l-methyl-3-butenyl, 2-methyl-3-butenyl, 3-methyl-3-butenyl, 1,1- dimethyl-2-propenyl,
- alkynyl refers to both straight and branched carbon chains which have at least one carbon-carbon triple bond.
- the number of triple bonds is 1-3; in another embodiment of alkynyl, the number of triple bonds is one or two.
- alkynyl groups include from C 2 -C 20 alkynyl groups.
- alkynyl groups may include C 2 -C 12 , C 2 -C 10 , C 2 -C8, C 2 -C6 or C 2 -C 4 alkynyl groups.
- Other ranges of carbon-carbon triple bonds and carbon numbers are also contemplated depending on the location of the alkenyl moiety on the molecule.
- C 2 -C 10 -alkynyl refers to a straight-chain or branched unsaturated hydrocarbon group having 2 to 10 carbon atoms and containing at least one triple bond, such as ethynyl, prop-l-yn-l-yl, prop-2-yn-l-yl, n-but-l-yn- 1-yl, n-but-l-yn-3-yl, n-but-l-yn-4-yl, n-but-2-yn-l-yl, n-pent-l-yn-l-yl, n-pent-l-yn-3-yl, n- pent-l-yn-4-yl, n-pent-l-yn-5-yl, n-pent-2-yn-l-yl, n-pent-2-yn-4-yl, n-pent-2-yn-5-yl, 3- methyl
- haloalkyl refers to an alkyl group, as defined herein, which is substituted by one or more halogen atoms.
- C 1 -C 4 -haloalkyl includes, but is not limited to, chloromethyl, bromomethyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl, trifluoromethyl, chlorofluoromethyl, di chlorofluoromethyl, chlorodifluoromethyl, 1-chloroethyl, 1-bromoethyl, 1-fluoroethyl, 2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-2- fluoroethyl, 2-chloro-2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl, pentafluoroethyl and the like
- haloalkenyl refers to an alkenyl group, as defined herein, which is substituted by one or more halogen atoms.
- haloalkynyl refers to an alkynyl group, as defined herein, which is substituted by one or more halogen atoms.
- Alkoxy refers to alkyl-O-, wherein alkyl is as defined above.
- alkenyloxy refers to the groups alkenyl- 0-, alkynyl-O-, haloalkyl-O-, haloalkenyl-O-, haloalkynyl-O-, cycloalkyl-O-, cycloalkenyl-O-, halocycloalkyl-O-, and halocycloalkenyl-O-, respectively, wherein alkenyl, alkynyl, haloalkyl, haloalkenyl, haloalkynyl, cycloalkyl, cycloalkenyl, halocycloalkyl, and halocycloalkenyl-O-, respectively, wherein alkenyl, alkynyl, haloalkyl, haloalkenyl, haloalkynyl, cycloalkyl, cycloalkenyl, halocycloalkyl, and halocycloal
- C 1 -C 6 -alkoxy examples include, but are not limited to, methoxy, ethoxy, C 2 H 5 -CH 2 O-, (CH 3 ) 2 CHO-, n-butoxy, C 2 H 5 -CH(CH 3 )O-, (CH 3 ) 2 CH-CH 2 0- ; (CH 3 ) 3 CO-, n- pentoxy, 1-methylbutoxy, 2-methylbutoxy, 3-methylbutoxy, 1, 1-dimethylpropoxy,
- alkylthio refers to alkyl-S-, wherein alkyl is as defined above.
- haloalkylthio refers to haloalkyl-S- and cycloalkyl-S- where haloalkyl and cycloalkyl are as defined above.
- alkyl sulfinyl refers to alkyl-S(O)-, wherein alkyl is as defined above.
- haloalkylsulfinyl refers to haloalkyl-S(O)- where haloalkyl is as defined above.
- alkyl sulfonyl refers to alkyl-S(O) 2 -, wherein alkyl is as defined above.
- haloalkylsulfonyl refers to haloalkyl-S(O) 2 - where haloalkyl is as defined above.
- alkylamino and dialkylamino refer to alkyl- H- and (alkyl) 2 N- where alkyl is as defined above.
- haloalkylamino refers to haloalkyl- H- where haloalkyl is as defined above.
- alkylcarbonyl alkoxycarbonyl
- alkylaminocarbonyl alkylaminocarbonyl
- dialkylaminocarbonyl refer to alkyl-C(O)-, alkoxy-C(O)-, alkylamino-C(O)- and dialkylamino-C(O)- where alkyl, alkoxy, alkylamino and dialkylamino are as defined above.
- haloalkylcarbonyl refers to the groups haloalkyl-C(O)-, haloalkoxy-C(O)-, haloalkylamino-C(O)- and dihaloalkylamino-C(O)- where haloalkyl, haloalkoxy, haloalkylamino and dihaloalkylamino are as defined above.
- Aryl refers to a monovalent aromatic carbocyclic group of from 6 to 14 carbon atoms having a single ring or multiple condensed rings.
- aryl groups include C 6 - C 10 aryl groups.
- Aryl groups include, but are not limited to, phenyl, biphenyl, naphthyl, tetrahydronaphthyl, phenylcyclopropyl and indanyl.
- Aryl groups may be unsubstituted or substituted by one or more moieties selected from halogen, cyano, nitro, hydroxy, mercapto, amino, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, haloalkyl, haloalkenyl, haloalkynyl, halocycloalkyl, halocycloalkenyl, alkoxy, alkenyloxy, alkynyloxy, haloalkoxy, haloalkenyloxy, haloalkynyloxy, cycloalkoxy, cycloalkenyloxy, halocycloalkoxy, halocycloalkenyloxy, alkylthio, haloalkylthio, cycloalkylthio, halocycloalkylthio, alkyl sulfinyl, alkenylsulfinyl, al
- aralkyl or "arylalkyl” refers to an aryl group that is bonded to the parent compound through a diradical alkylene bridge, (-CH 2 -) n , where n is 1-12 and where "aryl” is as defined above.
- Heteroaryl refers to a monovalent aromatic group of from 1 to 15 carbon atoms, preferably from 1 to 10 carbon atoms, having one or more oxygen, nitrogen, and sulfur heteroatoms within the ring, preferably 1 to 4 heteroatoms, or 1 to 3 heteroatoms.
- the nitrogen and sulfur heteroatoms may optionally be oxidized.
- Such heteroaryl groups can have a single ring (e.g., pyridyl or furyl) or multiple condensed rings provided that the point of attachment is through a heteroaryl ring atom.
- heteroaryls include pyridyl, piridazinyl, pyrimidinyl, pyrazinyl, triazinyl, pyrrolyl, indolyl, quinolinyl, isoquinolinyl, quinazolinyl, quinoxalinnyl, furanyl, thienyl, furyl, pyrrolyl, imidazolyl, oxazolyl, isoxazolyl, isothiazolyl, pyrazolyl benzofuranyl, and benzothienyl.
- Heteroaryl rings may be unsubstituted or substituted by one or more moieties as described for aryl above.
- the term "heteroarylene" (where the heteroaryl group is a bridging group) should be construed accordingly.
- Heterocyclyl refers to fully saturated or unsaturated, cyclic groups, for example, 3 to 7 membered monocyclic or 4 to 7 membered monocyclic; 7 to 11 membered bicyclic, or 10 to 15 membered tricyclic ring systems, which have one or more oxygen, sulfur or nitrogen heteroatoms in ring, preferably 1 to 4 or 1 to 3 heteroatoms.
- the nitrogen and sulfur heteroatoms may optionally be oxidized and the nitrogen heteroatoms may optionally be quaternized.
- the heterocyclic group may be attached at any heteroatom or carbon atom of the ring or ring system and may be unsubstituted or substituted by one or more moieties as described for aryl groups above.
- Exemplary monocyclic heterocyclic groups include, but are not limited to, pyrrolidinyl, pyrrolyl, pyrazolyl, oxetanyl, pyrazolinyl, imidazolyl, imidazolinyl, imidazolidinyl, oxazolyl, oxazolidinyl, isoxazolinyl, isoxazolyl, thiazolyl, thiadiazolyl, thiazolidinyl, isothiazolyl, isothiazolidinyl, furyl, tetrahydrofuryl, thienyl, oxadiazolyl, piperidinyl, piperazinyl, 2- oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolodinyl, 2-oxoazepinyl, azepinyl, 4-piperidonyl, pyridinyl, pyrazinyl, pyrimi
- bicyclic heterocyclic groups include, but are not limited to, indolyl, benzothiazolyl, benzoxazolyl, benzodioxolyl, benzothienyl, quinuclidinyl, quinolinyl, tetra- hydroisoquinolinyl, isoquinolinyl, benzimidazolyl, benzopyranyl, indolizinyl, benzofuryl, chromonyl, coumarinyl, benzopyranyl, cinnolinyl, quinoxalinyl, indazolyl, pyrrolopyridyl, furopyridinyl (such as furo[2,3-c]pyridinyl, furo[3,2-b]pyridinyl]or furo[2,3-b]pyridinyl), dihydroisoindolyl, dihydroquinazolinyl (such as 3,4-dihydro-4-oxo-qui
- Exemplary tricyclic heterocyclic groups include carbazolyl, benzidolyl, phenanthrolinyl, acridinyl, phenanthridinyl, xanthenyl, and the like.
- Halogen means the atoms fluorine, chlorine, bromine and iodine.
- halo refers to all degrees of substitutions from a single substitution to a perhalo substitution (e.g. as illustrated with methyl as chloromethyl (-CH 2 Cl), dichloromethyl (-CHCl 2 ), trichloromethyl (-CCl 3 )).
- the weigh weight ratio is at least approximately 1.05 or higher in favor of one enantiomer over the other.
- the weigh weight ratio is at least approximately 1.05 or higher in favor of the enantiomer that displays significant in vitro and in vivo activity (the eutomer).
- compositions of the invention may exist and be isolated as optically active and racemic forms.
- Compounds having one or more chiral centers, including at a sulfur atom may be present as single enantiomers or diastereomers or as mixtures of enantiomers and/or diastereomers.
- sulfoxide compounds may be optically active and may exist as single enantiomers or racemic mixtures.
- compounds within the compositions of the invention may include one or more chiral centers, which results in a theoretical number of optically active isomers.
- compositions within the compositions of the invention may comprise up to 2 n optical isomers.
- the present invention encompasses compositions comprising the specific enantiomers or diastereomers of each compound as well as mixtures of different enantiomers and/or diastereomers of the compounds of the invention that possess the useful properties described herein.
- the invention encompasses compositions comprising one or more conformational isomers (e.g. rotamers) as well as mixtures of conformational isomers.
- Conformational isomers of the isoxazoline compounds may be produced by a restriction of rotation about the amide bond bonded to the aryl or heteroaryl ring (e.g.
- optically active forms can be prepared by, for example, resolution of the racemic forms by selective crystallization techniques, by synthesis from optically active precursors, by chiral synthesis, by chromatographic separation using a chiral stationary phase or by enzymatic resolution.
- compositions of the invention may exist as hydrates or solvates, in which a certain stoichiometric amount of water or a solvent is associated with the molecule in the crystalline form.
- the compositions of the invention may include hydrates and solvates of the active agents.
- the compositions of the invention may include up to 15% (w/w), up to 20% (w/w), or up to 30% (w/w) of a particular solid form.
- acid or base salts contemplated within the scope of the invention.
- the term "acid salt” contemplates salts of the compounds with all pharmaceutically acceptable inorganic or organic acids.
- Inorganic acids include mineral acids such as hydrohalic acids such as hydrobromic acid and hydrochloric acid, sulfuric acid, phosphoric acids and nitric acid.
- Organic acids include all pharmaceutically acceptable aliphatic, alicyclic and aromatic carboxylic acids, dicarboxylic acids, tricarboxylic acids and fatty acids.
- the acids are straight chain or branched, saturated or unsaturated C 1 -C 2 o aliphatic carboxylic acids, which are optionally substituted by halogen or by hydroxyl groups, or C 6 -C 12 aromatic carboxylic acids.
- acids are carbonic acid, formic acid, acetic acid, propionic acid, isopropionic acid, valeric acid, a-hydroxy acids such as glycolic acid and lactic acid, chloroacetic acid, benzoic acid, methane sulfonic acid, and salicylic acid.
- dicarboxylic acids include oxalic acid, malic acid, succinic acid, tartaric acid, fumaric acid, and maleic acid.
- a tricarboxylic acid is citric acid.
- Fatty acids include all pharmaceutically acceptable saturated or unsaturated aliphatic or aromatic carboxylic acids having 4 to 24 carbon atoms. Examples include butyric acid, isobutyric acid, sec-butyric acid, lauric acid, palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, and phenylsteric acid.
- Other acids include gluconic acid, glycoheptonic acid and lactobionic acid.
- base salt contemplates salts of the compounds with all pharmaceutically acceptable inorganic or organic bases, including hydroxides, carbonates or bicarbonates of alkali metal or alkaline earth metals. Salts formed with such bases include, for example, the alkali metal and alkaline earth metal salts, including, but not limited to, as the lithium, sodium, potassium, magnesium or calcium salts. Salts formed with organic bases include the common hydrocarbon and heterocyclic amine salts, which include, for example, ammonium salts ( H4 + ), alkyl- and dialkylammonium salts, and salts of cyclic amines such as the morpholine and piperidine salts.
- the extended release injectable formulations of present invention comprise an effective amount of at least one isoxazoline or a pharmaceutically acceptable salt thereof in combination at least one other active agent.
- the extended release injectable compositions comprise an effective amount of at least one isoxazoline compound of formula (I) to (VIla), or a pharmaceutically acceptable salt thereof, in combination with at least one other active agent that is systemically-active.
- Additional veterinary/pharmaceutical active ingredients may be used with the compositions of the invention.
- the additional active agents may include, but are not limited to, acaricides, anthelmintics, anti-parasitics and insecticides.
- Anti-parasitic agents can include both ectoparasiticidal and/or endoparasiticidal agents.
- Veterinary pharmaceutical agents that may be included in the compositions of the invention are well-known in the art (see e.g. Plumb ' Veterinary Drug Handbook, 5 th Edition, ed. Donald C. Plumb, Blackwell Publishing, (2005) or The Merck Veterinary Manual, 9 th Edition, (January 2005)) and include but are not limited to acarbose, acepromazine maleate, acetaminophen, acetazolamide, acetazolamide sodium, acetic acid, acetohydroxamic acid, acetylcysteine, acitretin, acyclovir, albendazole, albuterol sulfate, alfentanil, allopurinol, alprazolam, altrenogest, amantadine, amikacin sulfate, aminocaproic acid, aminopentamide hydrogen sulfate, aminophylline/theophylline, amiodarone, amitriptyline
- arylpyrazole compounds such as phenylpyrazoles, known in the art may be combined with the isoxazoline compounds in the extended release injectable compositions of the invention.
- arylpyrazole compounds include but are not limited to fipronil, pyriprole, ethiprole and those described in U.S. Patent Nos.6,001,384; 6,010,710; 6,083,519; 6,096,329; 6,174,540; 6,685,954 and 6,998,131 (all of which are incorporated herein by reference, each assigned to Merial, Ltd., Duluth, GA).
- one or more macrocyclic lactones or lactams which act as an acaricide, anthelmintic agent and/or insecticide, can be added to the compositions of the invention.
- the macrocyclic lactones include, but are not limited to, avermectins such as abamectin, dimadectin, doramectin, emamectin, eprinomectin, ivermectin, latidectin, lepimectin, selamectin and ML-1,694,554, and milbemycins such as milbemectin, milbemycin D, milbemycin oxime, moxidectin and nemadectin. Also included are the 5-oxo and 5-oxime derivatives of said avermectins and milbemycins.
- the macrocyclic lactone compounds are known in the art and can easily be obtained commercially or through synthesis techniques known in the art. Reference is made to the widely available technical and commercial literature.
- avermectins ivermectin and abamectin
- doramectin “Veterinary Parasitology”, vol. 49, No.
- milbemycins reference may be made, inter alia, to Davies H.G. et al., 1986, “Avermectins and Milbemycins”, Nat. Prod. Rep., 3, 87-121, Mrozik H. et al., 1983, Synthesis of Milbemycins from Avermectins, Tetrahedron Lett., 24, 5333-5336, U.S. Patent No.4,134,973 and EP 0677054.
- Macrocyclic lactones are either natural products or are semi-synthetic derivatives thereof.
- the structure of the avermectins and milbemycins are closely related, e.g., by sharing a complex 16-membered macrocyclic lactone ring.
- the natural product avermectins are disclosed in U.S. Patent No. 4,310,519 and the 22,23-dihydro avermectin compounds are disclosed in U.S. Patent No.4,199,569. Mention is also made of U.S. Patent Nos. 4,468,390, 5,824,653, EP 0 007 812 A1, U.K. Patent Specification 1 390 336, EP 0 002 916, and New Zealand Patent No. 237 086,
- the invention provides the extended release formulations of the present invention comprising 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]-4,5-dihydro-5- (trifluoromethyl)-3-isoxazolyl]-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-l- naphthalanecarboxamide (Compound of formula lie) in combination with a macrocyclic lactone active agent.
- the invention provides the extended release formulations of the present invention comprising 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]-4,5-dihydro-5- (trifluoromethyl)-3-isoxazolyl]-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-l- naphthalanecarboxamide (Compound of formula lie) in combination with ivermectin, eprinomectin, selamectin, milbemycin oxime or moxidectin.
- the invention provides the extended release formulations of the present invention comprising 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]-4,5-dihydro-5- (trifluoromethyl)-3-isoxazolyl]-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-l- naphthalanecarboxamide enriched in the (S)-enantiomer or as the substantially pure (S)- enantiomer (Compound of formula (S)-IIc) in combination with a macrocyclic lactone active agent.
- the invention provides the extended release formulations of the present invention comprising 4-[5-[3-chloro-5-(trifluoromethyl)phenyl]-4,5-dihydro-5- (trifluoromethyl)-3-isoxazolyl]-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-l- naphthalanecarboxamide enriched in the (S)-enantiomer or as the substantially pure (S)- enantiomer (Compound of formula (S) -IIc) in combination with ivermectin, eprinomectin, selamectin, milbemycin oxime or moxidectin.
- the invention comprises an extended release injectable formulation comprising an isoxazoline compound in combination with systemically- acting compounds from a class of acaricides or insecticides known as insect growth regulators (IGRs).
- IGRs insect growth regulators
- Compounds belonging to this group are well known to the practitioner and represent a wide range of different chemical classes. These compounds all act by interfering with the development or growth of the insect pests.
- Insect growth regulators are described, for example, in U.S. Patent Nos. 3,748,356, 3,818,047, 4,225,598, 4,798,837, 4,751,225, EP 0 179 022 or U.K. 2 140 010 as well as U.S. Patent Nos. 6,096,329 and 6,685,954 (all incorporated herein by reference).
- the IGR is a compound that mimics juvenile hormone.
- juvenile hormone mimics include azadirachtin, diofenolan, fenoxycarb, hydroprene, kinoprene, methoprene, pyriproxyfen, tetrahydroazadirachtin and 4-chloro-2(2-chloro-2-methyl-propyl)-5- (6-iodo-3-pyridylmethoxy)pyridazine-3(2H)-one.
- the extended release injectable formulations of present invention comprise an effective amount of at least one isoxazoline of Formula (I) to (VI), or a pharmaceutically acceptable salt thereof, in combination with methoprene or pyriproxyfen.
- the IGR compound is a chitin synthesis inhibitor.
- Chitin synthesis inhibitors include chlorofluazuron, cyromazine, diflubenzuron, fluazuron, flucycloxuron, flufenoxuron, hexaflumoron, lufenuron, tebufenozide, teflubenzuron, triflumoron, novaluron, l-(2,6-difluorobenzoyl)-3-(2-fluoro-4-(trifluoromethyl)phenylurea, l-(2,6-difluoro- benzoyl)-3-(2-fluoro-4-(l,l,2,2-tetrafluoroethoxy)-phenylurea and l-(2,6-difluorobenzoyl)-3-(2- fluoro-4-trifluoromethyl)phenylurea.
- adulticide insecticides and acaricides can also be added to the extended release formulations of the present invention.
- these include pyrethrins (which include cinerin I, cinerin II, jasmolin I, jasmolin II, pyrethrin I, pyrethrin II and mixtures thereof) and pyrethroids, and carbamates including, but are not limited to, benomyl, carbanolate, carbaryl, carbofuran, meththiocarb, metolcarb, promacyl, propoxur, aldicarb, butocarboxim, oxamyl, thiocarboxime and thiofanox.
- the compositions can include permethrin in combination with an isoxazoline active agent.
- the extended release injectable formulations of the present invention may include one or more antinematodal agents including, but not limited to, active
- benzimidazoles including, but not limited to, thiabendazole, cambendazole, parbendazole, oxibendazole, mebendazole, flubendazole, fenbendazole, oxfendazole, albendazole, cyclobendazole, febantel, thiophanate and its o,o- dimethyl analogue may be included in the compositions.
- the extended release injectable formulations of the present invention may include an imidazothiazole compounds including, but not limited to, tetramisole, levamisole and butamisole.
- the extended release formulations of the present invention may include tetrahydropyrimidine active agents including, but not limited to, pyrantel, oxantel, and morantel.
- Suitable organophosphate active agents include, but are not limited to, coumaphos, trichlorfon, haloxon, naftalofos and dichlorvos, heptenophos, mevinphos, monocrotophos, TEPP, and tetrachlorvinphos.
- the extended release injectable formulations of the present invention may include the antinematodal compounds phenothiazine and piperazine as the neutral compound or in various salt forms, diethylcarbamazine, phenols such as disophenol, arsenicals such as arsenamide, ethanolamines such as bephenium, thenium closylate, and methyridine; cyanine dyes including pyrvinium chloride, pyrvinium pamoate and dithiazanine iodide; isothiocyanates including bitoscanate, suramin sodium, phthalofyne, and various natural products including, but not limited to, hygromycin B, ⁇ -santonin and kainic acid.
- the antinematodal compounds phenothiazine and piperazine as the neutral compound or in various salt forms, diethylcarbamazine, phenols such as disophenol, arsenicals such as arsenamide, ethanolamines such as
- the extended release injectable formulations of the present invention of the invention may include antitrematodal agents.
- Suitable antitrematodal agents include, but are not limited to, the miracils such as miracil D and mirasan; praziquantel, epsiprantel, clonazepam and its 3-methyl derivative, oltipraz, lucanthone, hycanthone, oxamniquine, amoscanate, niridazole, nitroxynil, various bisphenol compounds known in the art including hexachlorophene, bithionol, bithionol sulfoxide and menichlopholan; various salicylanilide compounds including tribromsalan, oxyclozanide, clioxanide, rafoxanide, brotianide, bromoxanide and closantel; triclabendazole, diamfenetide, clorsulon, he
- Anticestodal compounds may also be advantageously used in the extended release formulations of the present invention of the invention including, but not limited to, praziquantel,
- the extended release injectable formulations of the present invention may include other active agents that are effective against arthropod parasites.
- Suitable active agents include, but are not limited to, bromocyclen, chlordane, DDT, endosulfan, lindane, methoxychlor, toxaphene, bromophos, bromophos-ethyl, carbophenothion, chlorfenvinphos, chlorpyrifos, crotoxyphos, cythioate, diazinon, dichlorenthion, diemthoate, dioxathion, ethion, famphur, fenitrothion, fenthion, fospirate, iodofenphos, malathion, naled, phosalone, phosmet, phoxim, propetamphos, ronnel, stirofos, allethrin, cyhalothrin, cypermethrin
- An antiparasitic agent that can be combined with an isoxazoline compounds in the extended release formulations of the present invention can be a biologically active peptide or protein including, but not limited to, depsipeptides, which act at the neuromuscular junction by stimulating presynaptic receptors belonging to the secretin receptor family resulting in the paralysis and death of parasites.
- the depsipeptide is emodepside (see Willson et al., Parasitology, Jan. 2003, 126(Pt l):79-86).
- the depsipeptide is PF1022A or a derivative thereof.
- the extended release injectable formulations of the present invention may comprise an active agent from the neonicotinoid class of pesticides.
- the neonicotinoids bind and inhibit insect specific nicotinic acetylcholine receptors.
- the neonicotinoid insecticidal agent that can be combined with an isoxazoline compound to form an extended release injectable formulation of the invention is imidacloprid.
- Imidacloprid is a well-known neonicotinoid active agent and is the key active ingredient in the topical parasiticide products Advantage ® , Advantage ® II, K9 Advantix ® , and K9 Advantix ® II sold by Bayer Animal Health and the oral soft-chewable formulation AdvantusTM from Piedmont
- the extended release injectable formulations of the present invention may comprise nitenpyram, another active agent of the neonicotinoid class of pesticides.
- Nitenpyram has the following chemical structure and is the active ingredient in the oral product CAPSTARTM Tablets sold by Novartis Animal Health.
- Nitenpyram is active against adult fleas when given daily as an oral tablet. Nitenpyram works by interfering with normal nerve transmission and leads to the death of the insect. Nitenpyram has a very fast onset of action against fleas. For example, CAPSTARTM Tablets begin to act against fleas in as early as 30 minutes after administration and is indicated for use as often as once a day. However, nitenpyram is only known to be effective when administered orally as a systemic parasiticide, as with CAPSTARTM Tablets.
- an insecticidal agent that can be combined with the extended release formulations of the present invention is a semicarbazone, such as metaflumizone.
- the extended release injectable formulations of the present invention may advantageously include a combination of isoxazoline compounds known in the art. These active agents are described in WO 2007/079162, WO 2007/075459 and US 2009/0133319, WO 2007/070606 and US 2009/0143410, WO 2009/003075, WO 2009/002809, WO 2009/024541, WO 2005/085216 and US 2007/0066617 and WO 2008/122375, all of which are incorporated herein by reference in their entirety.
- nodulisporic acid and its derivatives may be added to the extended release formulations of the present invention.
- These compounds are used to treat or prevent infections in humans and animals and are described, for example, in U.S. Patent No. 5,399,582, 5,962,499, 6,221,894 and 6,399,786, all of which are hereby incorporated by reference in their entirety.
- the formulations may include one or more of the known nodulisporic acid derivatives in the art, including all stereoisomers, such as those described in the patents cited above.
- anthelmintic compounds of the amino acetonitrile class (AAD) of compounds such as monepantel (ZOLVIX), and the like may be added to the. the extended release formulations of the present invention
- AAD amino acetonitrile class
- ZOLVIX monepantel
- compositions of the invention may also include aryloazol-2-yl cyanoethylamino compounds such as those described in US Patent No. 8,088,801 to Soll et al., which is incorporated herein in its entirety, and thioamide derivatives of these compounds, as described in U.S. Patent No.7,964,621, which is incorporated herein by reference.
- the extended release injectable formulations of the present invention may also be combined with paraherquamide compounds and derivatives of these compounds, including derquantel (see Ostlind et al., Research in Veterinary Science, 1990, 48, 260-61; and Ostlind et al., Medical and Veterinary Entomology, 1997, 11, 407-408).
- the paraherquamide family of compounds is a known class of compounds that include a spirodioxepino indole core with activity against certain parasites (see Tet. Lett.1981, 22, 135; J. Antibiotics 1990, 43, 1380, and J. Antibiotics 1991, 44, 492).
- marcfortines A-C structurally related marcfortine family of compounds, such as marcfortines A-C, are also known and may be combined with the formulations of the invention (see J. Chem. Soc.– Chem. Comm. 1980, 601 and Tet. Lett.1981, 22, 1977). Further references to the paraherquamide derivatives can be found, for example, in WO 91/09961, WO 92/22555, WO 97/03988, WO 01/076370, WO 09/004432, U.S. Patent 5,703,078 and U.S. Patent 5,750,695, all of which are hereby incorporated by reference in their entirety.
- the compositions may include a spinosyn active agent produced by the soil actinomycete Saccharopolyspora spinosa (see, for example Salgado V.L. and Sparks T.C.,“The Spinosyns: Chemistry, Biochemistry, Mode of Action, and Resistance,” in Comprehensive Molecular Insect Science, vol. 6, pp. 137-173, 2005) or a semi- synthetic spinosoid active agent.
- a spinosyn active agent produced by the soil actinomycete Saccharopolyspora spinosa (see, for example Salgado V.L. and Sparks T.C.,“The Spinosyns: Chemistry, Biochemistry, Mode of Action, and Resistance,” in Comprehensive Molecular Insect Science, vol. 6, pp. 137-173, 2005) or a semi- synthetic spinosoid active agent.
- the spinosyns are typically referred to as factors or components A, B, C, D, E, F, G, H, J, K, L, M, N, 0, P, Q, R, S, T, U, V, W, or Y, and any of these components, or a combination thereof, may be used in the compositions of the invention.
- the spinosyn compound may be a 5,6,5-tricylic ring system, fused to a 12-membered macro cyclic
- spinosyn compounds including 21-butenyl spinosyn produced by Saccharopolyspora pagona, which may be used in the compositions of the invention, may be produced via fermentation by conventional techniques known in the art.
- Other spinosyn compounds that may be used in the compositions of the invention are disclosed in U.S. Patent Nos. 5,496,931; 5,670,364; 5,591,606; 5,571,901; 5,202,242; 5,767,253; 5,840,861; 5,670,486; 5,631, 155 and 6,001,981, all incorporated by reference herein in their entirety.
- the spinosyn compounds may include, but are not limited to, spinosyn A, spinosyn D, spinosad, spinetoram, or combinations thereof.
- Spinosad is a combination of spinosyn A and spinosyn D
- spinetoram is a combination of 3'-ethoxy- 5,6-dihydro spinosyn J and 3'-ethoxy spinosyn L.
- the additional active agent is included in the extended release formulations of the present invention in an amount of between about 0.1 ⁇ g and about 1000 mg. More typically, the additional active agent may be included in an amount of about 10 ⁇ g to about 500 mg, about 1 mg to about 300 mg, about 10 mg to about 200 mg or about 10 mg to about 100 mg.
- the additional active agent may be included in the composition to deliver a dose of about 5 ⁇ g/kg to about 50 mg/kg per weight of the animal. In other embodiments, the additional active agent may be present in an amount sufficient to deliver a dose of about 0.01 mg/kg to about 30 mg/kg, about 0.1 mg/kg to about 20 mg/kg, or about 0.1 mg/kg to about 10 mg/kg of weight of animal. In other embodiments, the additional active agent may be present in a dose of about 5 ⁇ g/kg to about 200 ⁇ g/kg or about 0.1 mg/kg to about 1 mg/kg of weight of animal. In still another embodiment of the invention, the additional active agent is included in a dose between about 0.5 mg/kg to about 50 mg/kg.
- the extended release injectable formulations of the present invention which include at least an isoxazoline active agent, a pharmaceutically acceptable polymer and a solvent, have been surprisingly discovered to be stable and effective against a broad spectrum of ectoparasites, and possibly also endoparasites if another active is included, for an extended period of time; e.g., a period from three (3) up to twelve (12) months or longer, while exhibiting favorable properties with respect to the site of injection.
- Dosage forms may contain from about 0.5 mg to about 5 g of a combination of active agents. More typically, the amount of active agent(s) in the compositions of the invention will be from about 1 mg to about 3 g. In another embodiment, the amount of active agent(s) in the
- compositions will be from about 20 mg to about 3 g. In another embodiment, the amount of active agent(s) present in the compositions will be from about 20 mg to about 2 g, about 20 mg to about 1.5 g or about 20 mg to about 1 g. In other embodiments, the amount of active agent(s) in the compositions will be from about 20 mg to about 500 mg, about 30 mg to about 200 mg or about 50 mg to about 200 mg. In still another embodiment, the amount of active agent(s) present in the compositions will be from about 50 mg to about 2 g, about 50 mg to about 1 g or about 50 mg to about 500 mg. In yet another embodiment of the invention, the about of active agent(s) present will be from about 100 mg to about 2 g, about 100 mg to about 1 g or about 100 mg to about 500 mg.
- compositions of the invention are made by mixing the appropriate amount of the active agents, pharmaceutically acceptable polymer, a solvent and, optionally, an antioxidant, pharmaceutically acceptable additive and/or excipient to form a formulation of the invention.
- the formulations of the present invention can be obtained by following the method of making these forms described above by the description of making these forms found in general formulation text known to those in the art, e.g. Remington– The Science and Practice of Pharmacy (21 st Edition) (2005), Goodman & Gilman’s The Pharmacological Basis of Therapeutics (11 th Edition) (2005) and Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (8 th Edition), edited by Allen et al., Lippincott Williams & Wilkins, (2005). Methods of Treatment
- a method for preventing or treating a parasite infestation/infection in an animal comprising administering to the animal an extended release injectable formulation comprising an effective amount of at least one isoxazoline compound, a pharmaceutically acceptable polymer and a solvent.
- the formulations of the invention have long-lasting efficacy against ectoparasites (e.g. fleas and ticks) and in
- compositions include an additional active agent they may also be active against endoparasites that harm animals.
- methods for the treatment or prevention of a parasitic infestation or infection in a domestic animal comprise administering an extended release injectable formulation comprising an effective amount of at least one isoxazoline active agent to the animal.
- Ectoparasites against which the methods and compositions of the invention are effective include, but are not limited to, fleas, ticks, mites, mosquitoes, flies and lice.
- the inventive formulations include one or more additional active agents that are active against internal parasites the compositions and methods of the invention may also be effective against endoparasites including, but not limited to, cestodes, nematodes, hookworms and roundworms of the digestive tract of animals and humans.
- the ectoparasite is one or more insect or arachnid including those of the genera Ctenocephalides, Rhipicephalus, Dermacentor, Ixodes, Amblyomma, Haemaphysalis, Hyalomma, Sarcoptes, Psoroptes, Otodectes, Chorioptes, Hypoderma, Damalinia, Linognathus, Haematopinus, Solenoptes, Trichodectes, and Felicola.
- the ectoparasite is from the genera Ctenocephalides, Rhipicephalus, Dermacentor and/or Ixodes .
- the ectoparasites treated include but are not limited to fleas, ticks, mites, mosquitoes, flies, lice, blowfly and combinations thereof. Specific examples include, but are not limited to, cat and dog fleas (Ctenocephalides sp. such as Ctenocephalides felis, Ctenocephalides canis, and the like), ticks (Rhipicephalus sp., Ixodes sp., Dermacentor sp., Amblyomma sp.
- ectoparasites include but are not limited to the tick genus
- Rhipicephalus especially those of the species microplus (cattle tick), decoloratus and annulatus; myiasis such as Dermatobia hominis (known as Berne in Brazil) and Cochliomyia hominivorax (greenbottle); sheep myiasis such as Lucilia sericata, Lucilia cuprina (known as blowfly strike in Australia, New Zealand and South Africa). Flies proper, namely those whose adult constitutes
- the parasite such as Haematobia irritans (horn fly) and Stomoxys calcitrans (stable fly); lice such as Linognathus vituli, etc.; and mites such as Sarcoptes scabiei and Psoroptes ovis.
- Haematobia irritans horn fly
- Stomoxys calcitrans stable fly
- lice such as Linognathus vituli, etc.
- mites such as Sarcoptes scabiei and Psoroptes ovis.
- the composition can also be used to treat against endoparasites such as those helminths selected from the group consisting of Anaplocephala, Ancylostoma, Necator, Ascaris, Capillaria, Cooperia, Dipylidium, Dirofilaria, Echinococcus, Enterobius, Fasciola, Haemonchus, Oesophagostomum, Ostertagia, Toxocara, Strongyloides, Toxascaris, Trichinella, Trichuris, Angiostrongylus and Trichostrongylus, among others.
- endoparasites such as those helminths selected from the group consisting of Anaplocephala, Ancylostoma, Necator, Ascaris, Capillaria, Cooperia, Dipylidium, Dirofilaria, Echinococcus, Enterobius, Fasciola, Haemonchus, Oesophagostomum, Ostertagia, Toxocara, Strongyloid
- the invention provides methods for the treatment and prevention of parasitic infections and infestations of animals (either wild or domesticated), including livestock and companion animals such as cats, dogs, horses, birds including chickens, sheep, goats, pigs, deer, turkeys and cattle, with the aim of ridding these hosts of parasites commonly encountered by such animals.
- livestock and companion animals such as cats, dogs, horses, birds including chickens, sheep, goats, pigs, deer, turkeys and cattle, with the aim of ridding these hosts of parasites commonly encountered by such animals.
- the invention provides methods and compositions for the treatment or prevention of parasitic infections and infestations in companion animals including, but not limited to, cats and dogs.
- the methods and compositions are particularly effective for preventing or treating parasitic infestations of cats and dogs with fleas and ticks.
- the methods and compositions of the invention are used for the treatment or prevention of parasitic infections and infestations in cattle or sheep.
- livestock animals such as cattle or sheep
- the methods and compositions are particularly effective against Rhipicephalus (formerly Boophilus) microplus, Haematobia irritans (horn fly), Stomoxys calcitrans (stable fly), and sheep myiasis such as Lucilia sericata, Lucilia cuprina (known as blowfly strike in Australia, New Zealand and South Africa).
- treating or “treat” or “treatment” are intended to mean the administration of an extended release formulation of the present invention to an animal that has a parasitic infestation for the eradication of the parasite or the reduction of the number of the parasites infesting the animal undergoing treatment. It is noted that the compositions of the invention may be used to prevent such a parasitic infestation.
- the parasitic infection or infestation has occurred in order to keep said infection or infestation from occurring.
- the formulations of the invention are administered in parasiticidally effective amounts which are which are suitable to control the parasite in question to the desired extent, as described below.
- the compounds and compositions of the invention can be applied against a single pest or combinations thereof.
- antiparasitic effective amount is intended a sufficient amount of a composition of the invention to eradicate or reduce the number of parasites infesting the animal.
- an effective amount of the active agent achieves at least 70% efficacy (% reduction vs. control) against the target parasite.
- an effective amount of the active agent achieves at least 80%, or at least 90% efficacy against the target pests.
- an effective amount of the active agent will achieve at least 95%, at least 98% or 100% efficacy against the target parasites.
- a dose of from about 0.001 to about 100 mg per kg of body weight given as a single dose or in divided doses for a period of from 1 to 5 days will be satisfactory but, of course, there can be instances where higher or lower dosage ranges are indicated, and such are within the scope of this invention. It is well within the routine skill of the practitioner to determine a particular dosing regimen for a specific host and parasite.
- the dose of the isoxazoline active agent administered from the extended release injectable formulations of the invention is between about 0.1 to about 50 mg per kg of body weight. More typically the dose of the isoxazoline active agent administered is about 0.5 to about 40 mg/kg or about 0.5 to about 30 mg/kg body weight. In another embodiment, the dose of the isoxazoline active agent administered is about 10 to about 40 mg/kg, about 15 to about 35 mg/kg or about 20 to about 30 mg/kg of body weight. In another embodiment, the dose of the isoxazoline active agent will be about 20 to about 25 mg/kg of body weight.
- the dose administered may be lower depending on the animal and the isoxazoline administered.
- the composition comprises the more active enantiomer of the isoxazoline compounds a lower dose may be administered.
- the dose is from about 0.1 to about 30 mg/kg of body weight. In another embodiment, the dose may be from about 0.1 to about 20 mg/kg or about 0.1 to about 10 mg/kg
- the dose may be from about 1 to about 20 mg/kg of body weight or about 1 to about 10 mg/kg. In yet another embodiment, the dose may be from about 5 to about 20 mg/kg or about 10 to about 20 mg/kg of body weight. In another embodiment, the dose may be from about 10 to about 30 mg/kg of body weight.
- doses of the isoxazoline active agent administered may be about 0.1 to about 40 mg/kg of body weight. More typically the doses administered will be about 1 to about 30 mg/kg, about 1 to about 20 mg/kg or about 1 to about 10 mg/kg of bodyweight. In yet another embodiment, the dose may be from about 10 to about 25 mg/kg, about 15 to about 30 mg/kg of body weight or about 20 to about 30 mg/kg of body weight.
- the extended release formulations of the present invention comprising an isoxazoline compound has an efficacy against fleas and/or ticks of at least about 90.0% or higher for about 3 months, or longer.
- the extended release formulations of the present invention provide an efficacy against fleas and/or ticks of at least 95.0% or higher for about, 3 months or longer.
- the extended release formulations of the invention provide an efficacy against fleas and/or ticks of at least 90% or higher for about 6 months or longer.
- the extended release formulations of the invention provide an efficacy against fleas and/or ticks of at least 95% or higher for about 6 months or longer.
- the extended release formulations of the invention provide an efficacy against fleas and/or ticks of at least 90% or higher for about 9 months or longer. In yet another embodiment, the extended release formulations of the invention provide an efficacy against fleas and/or ticks of at least 90% or higher for about 12 months or longer. In another embodiment, the extended release formulations of the present invention provide an efficacy against fleas and/or ticks in cats and dogs of at least about 90% for two months, or longer. In another embodiment, the extended release formulations of the present invention efficacy against fleas and/or ticks in cats and dogs of about 95% for about 3 months, or longer. In still another embodiment, the compositions provide an efficacy of about 95% for about 5 months or longer. In another aspect of the invention, a kit for the treatment or prevention of a parasitic infestation in an animal is provided, which comprises an extended release formulation of the
- the following extended release injectable formulations were prepared by mixing the following ingredients. Unless indicated otherwise, the concentrations of each component is percent (%) weight per weight (w/w) and MW refers to weight average molecular weight.
- MMW Medium molecular weight
- LMW Low molecular weight
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Dermatology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
Abstract
Description
Claims
Priority Applications (15)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2016246598A AU2016246598B2 (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
| EP16720949.3A EP3280413A1 (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
| CN201680031640.6A CN108055825B (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising isoxazoline active agents, methods and uses thereof |
| SG11201708068PA SG11201708068PA (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
| NZ736374A NZ736374B2 (en) | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof | |
| MX2017012848A MX2017012848A (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof. |
| CA2981797A CA2981797C (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
| JP2017553068A JP6824900B2 (en) | 2015-04-08 | 2016-04-06 | Extended-release injectable formulations containing isooxazoline active agents, methods and their use |
| EA201792235A EA036272B1 (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
| KR1020177032205A KR102593069B1 (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
| BR112017021073A BR112017021073A2 (en) | 2015-04-08 | 2016-04-06 | prolonged release injectable compositions comprising an isoxazoline active agent, its methods and uses |
| IL254813A IL254813B (en) | 2015-04-08 | 2017-10-01 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
| CONC2017/0010065A CO2017010065A2 (en) | 2015-04-08 | 2017-10-04 | Injectable extended release formulations comprising an active isoxazoline agent |
| AU2020210266A AU2020210266B2 (en) | 2015-04-08 | 2020-07-30 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
| AU2022202035A AU2022202035A1 (en) | 2015-04-08 | 2022-03-24 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562144871P | 2015-04-08 | 2015-04-08 | |
| US62/144,871 | 2015-04-08 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016164487A1 true WO2016164487A1 (en) | 2016-10-13 |
Family
ID=57072717
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2016/026253 Ceased WO2016164487A1 (en) | 2015-04-08 | 2016-04-06 | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof |
Country Status (17)
| Country | Link |
|---|---|
| US (2) | US20170020848A1 (en) |
| EP (1) | EP3280413A1 (en) |
| JP (2) | JP6824900B2 (en) |
| KR (1) | KR102593069B1 (en) |
| CN (1) | CN108055825B (en) |
| AR (1) | AR104212A1 (en) |
| AU (3) | AU2016246598B2 (en) |
| BR (1) | BR112017021073A2 (en) |
| CA (1) | CA2981797C (en) |
| CL (1) | CL2017002536A1 (en) |
| CO (1) | CO2017010065A2 (en) |
| EA (2) | EA202090762A1 (en) |
| IL (1) | IL254813B (en) |
| MX (2) | MX2017012848A (en) |
| SG (2) | SG10201912724SA (en) |
| UY (1) | UY36610A (en) |
| WO (1) | WO2016164487A1 (en) |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017147352A1 (en) * | 2016-02-24 | 2017-08-31 | Merial, Inc. | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| WO2018039508A1 (en) | 2016-08-25 | 2018-03-01 | Merial, Inc. | Method for reducing unwanted effects in parasiticidal treatments |
| WO2019091936A1 (en) | 2017-11-07 | 2019-05-16 | Intervet International B.V. | Injectable isoxazoline pharmaceutical compositions and their use against parasite infestation |
| CN111032634A (en) * | 2017-04-05 | 2020-04-17 | 勃林格殷格翰动物保健美国公司 | The crystal form of (S)-Aflana |
| WO2020225143A1 (en) | 2019-05-03 | 2020-11-12 | Intervet International B.V. | Injectable pharmaceutical compositions and uses thereof |
| WO2021233967A1 (en) | 2020-05-20 | 2021-11-25 | Intervet International B.V. | Injectable pharmaceutical compositions and uses thereof |
| US11648238B2 (en) * | 2017-12-12 | 2023-05-16 | Intervet Inc. | Implantable isoxazoline pharmaceutical compositions and uses thereof |
| RU2799591C2 (en) * | 2017-11-07 | 2023-07-07 | Интервет Интернэшнл Б.В. | Injectable isoxazoline pharmaceutical compositions and their use |
| US11858904B2 (en) | 2017-11-07 | 2024-01-02 | Intervet Inc. | Process for preparing large size isoxazoline particles |
| US11878968B2 (en) | 2021-07-09 | 2024-01-23 | Plexium, Inc. | Aryl compounds and pharmaceutical compositions that modulate IKZF2 |
| US12304903B2 (en) | 2020-07-24 | 2025-05-20 | Elanco Us Inc. | Process for making an isoxazoline compound and intermediate thereof |
| US12497370B2 (en) | 2019-12-18 | 2025-12-16 | Elanco Tiergesundheit Ag | Isoxazoline derivatives |
| WO2025257633A1 (en) | 2024-06-12 | 2025-12-18 | Boehringer Ingelheim Vetmedica Gmbh | Long-acting castor oil-containing injectable formulations and methods of use thereof |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PL3609470T3 (en) | 2017-04-13 | 2024-04-15 | Ceva Santé Animale | Implant for treating parasites infestations |
| TWI812673B (en) * | 2018-02-12 | 2023-08-21 | 美商富曼西公司 | Naphthalene isoxazoline compounds for controlling invertebrate pests |
| AR116524A1 (en) * | 2018-10-04 | 2021-05-19 | Elanco Tiergesundheit Ag | HELMINTOS TREATMENT POTENTIAL |
| AR118215A1 (en) * | 2019-03-01 | 2021-09-22 | Boehringer Ingelheim Animal Health Usa Inc | INJECTABLE CLORSULON COMPOSITIONS, ITS METHODS AND USES |
| CN116897044A (en) * | 2020-12-21 | 2023-10-17 | 勃林格殷格翰动物保健有限公司 | Parasiticide rings containing isoxazoline compounds |
| CN118477040B (en) * | 2024-04-11 | 2025-02-25 | 河北威远药业有限公司 | A kind of preparation method of flurellana solution |
Citations (57)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3740421A (en) | 1966-09-19 | 1973-06-19 | Basf Wyandotte Corp | Polyoxyethylene-polyoxypropylene aqueous gels |
| GB1390336A (en) | 1972-06-08 | 1975-04-09 | Sankyo Co | Antibiotic b-41 |
| US3950360A (en) | 1972-06-08 | 1976-04-13 | Sankyo Company Limited | Antibiotic substances |
| US4134973A (en) | 1977-04-11 | 1979-01-16 | Merck & Co., Inc. | Carbohydrate derivatives of milbemycin and processes therefor |
| EP0002916A2 (en) | 1977-12-19 | 1979-07-11 | Merck & Co. Inc. | A milbemycin compound or a mixture thereof for use in the treatment of helminthic infections and anthelmintic compositions containing such compounds |
| EP0007812A1 (en) | 1978-08-02 | 1980-02-06 | Merck & Co. Inc. | Sugar derivatives of C-076 compounds, their preparation and their use as antiparasitic agents |
| US4199569A (en) | 1977-10-03 | 1980-04-22 | Merck & Co., Inc. | Selective hydrogenation products of C-076 compounds and derivatives thereof |
| US4310519A (en) | 1976-04-19 | 1982-01-12 | Merck & Co., Inc. | Novel substances and process for their production |
| US4427663A (en) | 1982-03-16 | 1984-01-24 | Merck & Co., Inc. | 4"-Keto-and 4"-amino-4"-deoxy avermectin compounds and substituted amino derivatives thereof |
| US4468390A (en) | 1981-02-23 | 1984-08-28 | Sankyo Company, Limited | Anthelmintic composition and the use thereof |
| US4742060A (en) | 1985-02-04 | 1988-05-03 | Nihon Tokushu Noyaku Seizo K. K. | Heterocyclic compounds |
| US4855317A (en) | 1987-03-06 | 1989-08-08 | Ciba-Geigy Corporation | Insecticides and parasiticides |
| US4859657A (en) | 1986-07-02 | 1989-08-22 | Ciba-Geigy Corporation | Pesticides |
| US4871719A (en) | 1987-03-24 | 1989-10-03 | Ciba-Geigy Corporation | Composition for controlling parasites in productive livestock |
| US4874749A (en) | 1987-07-31 | 1989-10-17 | Merck & Co., Inc. | 4"-Deoxy-4-N-methylamino avermectin Bla/Blb |
| US4963582A (en) | 1986-03-25 | 1990-10-16 | Sankyo Company Limited | Macrolide compounds, their preparation and their use |
| US4978677A (en) | 1986-03-06 | 1990-12-18 | Ciba-Geigy Corporation | C(29)-carbonyloxymilbemycin derivatives for controlling parasitic pets of animals and plants |
| US5055596A (en) | 1987-11-03 | 1991-10-08 | Beecham Group P.L.C. | Derivatives of avermectin and milbemycin |
| US5077308A (en) | 1989-02-16 | 1991-12-31 | Merck & Co., Inc. | Avermectin ketal derivatives useful as antiparasitic |
| NZ237086A (en) | 1991-02-12 | 1993-07-27 | Ancare Distributors | Veterinary anthelminitic drench compositions containing praziquantel and at least one other anthelmintic compound; method of treatment |
| US5330768A (en) | 1991-07-05 | 1994-07-19 | Massachusetts Institute Of Technology | Controlled drug delivery using polymer/pluronic blends |
| EP0667054A1 (en) | 1992-10-30 | 1995-08-16 | Electric Power Research Institute, Inc | Harmonic controller for an active power line conditioner |
| EP0677054A1 (en) | 1993-01-18 | 1995-10-18 | Pfizer Inc. | New antiparasitic agents related to the milbemycins and avermectins |
| US5824653A (en) | 1994-11-28 | 1998-10-20 | Virbac S.A. | Anthelmintic compositions for equidae |
| EP0892060A2 (en) | 1997-07-18 | 1999-01-20 | Smithkline Beecham Corporation | The Chlamydia trachomatis Glutamyl-tRNAGln amidotransferase subunit ratA |
| US20020064547A1 (en) * | 1998-03-19 | 2002-05-30 | Rey T. Chern | Liquid polymeric compositions for controlled release of bioactive substances |
| WO2007070606A2 (en) | 2005-12-14 | 2007-06-21 | E. I. Du Pont De Nemours And Company | Isoxazolines for controlling invertebrate pests |
| WO2007075459A2 (en) | 2005-12-16 | 2007-07-05 | E. I. Du Pont De Nemours And Company | 5-aryl isoxazolines for controlling invertebrate pests |
| WO2007079162A1 (en) | 2005-12-30 | 2007-07-12 | E. I. Du Pont De Nemours And Company | Isoxazolines for controlling invertebrate pests |
| WO2007123855A2 (en) | 2006-04-20 | 2007-11-01 | E. I. Du Pont De Nemours And Company | Pyrazolines for controlling invertebrate pests |
| WO2008154528A2 (en) | 2007-06-13 | 2008-12-18 | E. I. Du Pont De Nemours And Company | Isoxazoline insecticides |
| WO2009002809A2 (en) | 2007-06-26 | 2008-12-31 | E. I. Du Pont De Nemours And Company | Naphthalene isoxazoline invertebrate pest control agents |
| WO2010003923A1 (en) | 2008-07-09 | 2010-01-14 | Basf Se | Pestcidal active mixtures comprising isoxazoline compounds i |
| US7662972B2 (en) | 2004-03-05 | 2010-02-16 | Nissan Chemical Industries, Ltd. | Isoxazoline-substituted benzamide compound and pesticide |
| US20100137372A1 (en) | 2007-03-29 | 2010-06-03 | Hideki Ihara | Isoxazoline compounds and their use in pest control |
| US20100179194A1 (en) | 2007-04-10 | 2010-07-15 | Bayer Corpscience Ag | Insecticidal aryl isoxazoline derivatives |
| US20100254960A1 (en) | 2007-10-03 | 2010-10-07 | E. I. Dupont Nemours And Company | Naphthalene isoxazoline compounds for control of invertebrate pests |
| US20110059988A1 (en) | 2007-08-17 | 2011-03-10 | Intervet International B.V. | Isoxazoline compositions and their use as antiparasitics |
| US20110086886A2 (en) | 2006-08-15 | 2011-04-14 | Bayer Cropscience Ag | Insecticidal isoxazolines |
| US7951828B1 (en) | 2005-09-02 | 2011-05-31 | Nissan Chemical Industries, Ltd. | Isoxazoline-substituted benzamide compound and pesticide |
| WO2011075591A1 (en) * | 2009-12-17 | 2011-06-23 | Merial Limited | Anti parasitic dihydroazole compounds and compositions comprising same |
| US20110245274A1 (en) * | 2008-12-18 | 2011-10-06 | Novartis Ag | Isoxazolines derivatives and their use as pesticide |
| WO2011149749A1 (en) | 2010-05-27 | 2011-12-01 | E.I. Du Pont De Nemours And Company | Crystalline form of 4- [5 - [3 -chloro-5 - (trifluoromethyl) phenyl] -4, 5 - dihydro - 5 - (trifluoromethyl) -3 - isoxazolyl] -n- [2-0x0-2- [ ( 2, 2, 2 - trifluoroethyl) amino] ethyl] -1- naphthalenecarboxamide |
| WO2012089623A1 (en) | 2010-12-27 | 2012-07-05 | Intervet International B.V. | Topical localized isoxazoline formulation comprising glycofurol |
| WO2012120399A1 (en) | 2011-03-10 | 2012-09-13 | Pfizer Inc. | Spirocyclic isoxazoline derivatives as antiparasitic agents |
| WO2012120135A1 (en) | 2011-03-10 | 2012-09-13 | Novartis Ag | Isoxazole derivatives |
| US8362086B2 (en) | 2005-08-19 | 2013-01-29 | Merial Limited | Long acting injectable formulations |
| WO2013039948A1 (en) | 2011-09-12 | 2013-03-21 | Merial Limited | Parasiticidal compositions comprising an isoxazoline active agent, methods and uses thereof |
| US20130095126A1 (en) * | 2010-06-18 | 2013-04-18 | Jean-Luc Perret | Use of substituted heterocyclic compounds to control sea lice on fish |
| US20130131016A1 (en) * | 2011-11-21 | 2013-05-23 | Anacor Pharmaceuticals, Inc. | Boron-containing small molecules |
| US20130143956A1 (en) * | 2011-12-02 | 2013-06-06 | Merial Limited | Long-acting injectable moxidectin formulations and novel moxidectin crystal forms |
| WO2013119442A1 (en) | 2012-02-06 | 2013-08-15 | Merial Limited | Parasiticidal oral veterinary compositions comprising systemically-acting active agents, methods and uses thereof |
| US20130324538A1 (en) * | 2011-02-10 | 2013-12-05 | Noëlle Gauvry | Isoxazoline derivatives for controlling invertebrate pests |
| WO2014039475A1 (en) | 2012-09-07 | 2014-03-13 | Zoetis Llc | Spirocyclic isoxazoline parasiticidal combinations |
| WO2014039484A1 (en) | 2012-09-07 | 2014-03-13 | Zoetis Llc | Spirocyclic isoxazolines as antiparasitic agents |
| WO2014189837A1 (en) | 2013-05-20 | 2014-11-27 | Zoetis Llc | Long-acting spiro-isoxazoline antiparasitic compositions |
| WO2015048371A1 (en) * | 2013-09-30 | 2015-04-02 | Zoetis Llc | Long-acting spiro-isoxazoline formulations |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AR088669A1 (en) * | 2011-11-21 | 2014-06-25 | Lilly Co Eli | DERIVATIVES OF DIHYDRODIBENZO [C] [1,2] OXABOROL AND DIHYDROISOXAZOL USEFUL FOR THE CONTROL OF ECTOPARASITES |
-
2016
- 2016-04-06 SG SG10201912724SA patent/SG10201912724SA/en unknown
- 2016-04-06 MX MX2017012848A patent/MX2017012848A/en unknown
- 2016-04-06 US US15/092,491 patent/US20170020848A1/en not_active Abandoned
- 2016-04-06 WO PCT/US2016/026253 patent/WO2016164487A1/en not_active Ceased
- 2016-04-06 CN CN201680031640.6A patent/CN108055825B/en active Active
- 2016-04-06 AU AU2016246598A patent/AU2016246598B2/en active Active
- 2016-04-06 EA EA202090762A patent/EA202090762A1/en unknown
- 2016-04-06 SG SG11201708068PA patent/SG11201708068PA/en unknown
- 2016-04-06 EA EA201792235A patent/EA036272B1/en not_active IP Right Cessation
- 2016-04-06 CA CA2981797A patent/CA2981797C/en active Active
- 2016-04-06 JP JP2017553068A patent/JP6824900B2/en active Active
- 2016-04-06 KR KR1020177032205A patent/KR102593069B1/en active Active
- 2016-04-06 BR BR112017021073A patent/BR112017021073A2/en not_active Application Discontinuation
- 2016-04-06 EP EP16720949.3A patent/EP3280413A1/en active Pending
- 2016-04-08 AR ARP160100953A patent/AR104212A1/en active IP Right Grant
- 2016-04-08 UY UY0001036610A patent/UY36610A/en active IP Right Grant
-
2017
- 2017-10-01 IL IL254813A patent/IL254813B/en active IP Right Grant
- 2017-10-04 CO CONC2017/0010065A patent/CO2017010065A2/en unknown
- 2017-10-05 MX MX2022010831A patent/MX2022010831A/en unknown
- 2017-10-06 CL CL2017002536A patent/CL2017002536A1/en unknown
-
2020
- 2020-07-30 AU AU2020210266A patent/AU2020210266B2/en active Active
- 2020-11-20 JP JP2020193357A patent/JP2021050206A/en active Pending
-
2021
- 2021-05-24 US US17/327,929 patent/US20210299104A1/en active Pending
-
2022
- 2022-03-24 AU AU2022202035A patent/AU2022202035A1/en not_active Abandoned
Patent Citations (65)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3740421A (en) | 1966-09-19 | 1973-06-19 | Basf Wyandotte Corp | Polyoxyethylene-polyoxypropylene aqueous gels |
| GB1390336A (en) | 1972-06-08 | 1975-04-09 | Sankyo Co | Antibiotic b-41 |
| US3950360A (en) | 1972-06-08 | 1976-04-13 | Sankyo Company Limited | Antibiotic substances |
| US4310519A (en) | 1976-04-19 | 1982-01-12 | Merck & Co., Inc. | Novel substances and process for their production |
| US4134973A (en) | 1977-04-11 | 1979-01-16 | Merck & Co., Inc. | Carbohydrate derivatives of milbemycin and processes therefor |
| US4199569A (en) | 1977-10-03 | 1980-04-22 | Merck & Co., Inc. | Selective hydrogenation products of C-076 compounds and derivatives thereof |
| EP0002916A2 (en) | 1977-12-19 | 1979-07-11 | Merck & Co. Inc. | A milbemycin compound or a mixture thereof for use in the treatment of helminthic infections and anthelmintic compositions containing such compounds |
| EP0007812A1 (en) | 1978-08-02 | 1980-02-06 | Merck & Co. Inc. | Sugar derivatives of C-076 compounds, their preparation and their use as antiparasitic agents |
| US4468390A (en) | 1981-02-23 | 1984-08-28 | Sankyo Company, Limited | Anthelmintic composition and the use thereof |
| US4427663A (en) | 1982-03-16 | 1984-01-24 | Merck & Co., Inc. | 4"-Keto-and 4"-amino-4"-deoxy avermectin compounds and substituted amino derivatives thereof |
| US4742060A (en) | 1985-02-04 | 1988-05-03 | Nihon Tokushu Noyaku Seizo K. K. | Heterocyclic compounds |
| US4978677A (en) | 1986-03-06 | 1990-12-18 | Ciba-Geigy Corporation | C(29)-carbonyloxymilbemycin derivatives for controlling parasitic pets of animals and plants |
| US4963582A (en) | 1986-03-25 | 1990-10-16 | Sankyo Company Limited | Macrolide compounds, their preparation and their use |
| US4859657A (en) | 1986-07-02 | 1989-08-22 | Ciba-Geigy Corporation | Pesticides |
| US4920148A (en) | 1987-03-06 | 1990-04-24 | Ciba-Geigy Corporation | Insecticides and parasiticides |
| US4855317A (en) | 1987-03-06 | 1989-08-08 | Ciba-Geigy Corporation | Insecticides and parasiticides |
| US4871719A (en) | 1987-03-24 | 1989-10-03 | Ciba-Geigy Corporation | Composition for controlling parasites in productive livestock |
| US4973711A (en) | 1987-03-24 | 1990-11-27 | Ciba-Geigy Corporation | Composition for controlling parasites in productive livestock |
| US4874749A (en) | 1987-07-31 | 1989-10-17 | Merck & Co., Inc. | 4"-Deoxy-4-N-methylamino avermectin Bla/Blb |
| US5055596A (en) | 1987-11-03 | 1991-10-08 | Beecham Group P.L.C. | Derivatives of avermectin and milbemycin |
| US5077308A (en) | 1989-02-16 | 1991-12-31 | Merck & Co., Inc. | Avermectin ketal derivatives useful as antiparasitic |
| NZ237086A (en) | 1991-02-12 | 1993-07-27 | Ancare Distributors | Veterinary anthelminitic drench compositions containing praziquantel and at least one other anthelmintic compound; method of treatment |
| US5330768A (en) | 1991-07-05 | 1994-07-19 | Massachusetts Institute Of Technology | Controlled drug delivery using polymer/pluronic blends |
| EP0667054A1 (en) | 1992-10-30 | 1995-08-16 | Electric Power Research Institute, Inc | Harmonic controller for an active power line conditioner |
| EP0677054A1 (en) | 1993-01-18 | 1995-10-18 | Pfizer Inc. | New antiparasitic agents related to the milbemycins and avermectins |
| US5824653A (en) | 1994-11-28 | 1998-10-20 | Virbac S.A. | Anthelmintic compositions for equidae |
| EP0892060A2 (en) | 1997-07-18 | 1999-01-20 | Smithkline Beecham Corporation | The Chlamydia trachomatis Glutamyl-tRNAGln amidotransferase subunit ratA |
| US20020064547A1 (en) * | 1998-03-19 | 2002-05-30 | Rey T. Chern | Liquid polymeric compositions for controlled release of bioactive substances |
| US6733767B2 (en) | 1998-03-19 | 2004-05-11 | Merck & Co., Inc. | Liquid polymeric compositions for controlled release of bioactive substances |
| US7662972B2 (en) | 2004-03-05 | 2010-02-16 | Nissan Chemical Industries, Ltd. | Isoxazoline-substituted benzamide compound and pesticide |
| US8362086B2 (en) | 2005-08-19 | 2013-01-29 | Merial Limited | Long acting injectable formulations |
| US7951828B1 (en) | 2005-09-02 | 2011-05-31 | Nissan Chemical Industries, Ltd. | Isoxazoline-substituted benzamide compound and pesticide |
| WO2007070606A2 (en) | 2005-12-14 | 2007-06-21 | E. I. Du Pont De Nemours And Company | Isoxazolines for controlling invertebrate pests |
| WO2007075459A2 (en) | 2005-12-16 | 2007-07-05 | E. I. Du Pont De Nemours And Company | 5-aryl isoxazolines for controlling invertebrate pests |
| WO2007079162A1 (en) | 2005-12-30 | 2007-07-12 | E. I. Du Pont De Nemours And Company | Isoxazolines for controlling invertebrate pests |
| WO2007123855A2 (en) | 2006-04-20 | 2007-11-01 | E. I. Du Pont De Nemours And Company | Pyrazolines for controlling invertebrate pests |
| US20110086886A2 (en) | 2006-08-15 | 2011-04-14 | Bayer Cropscience Ag | Insecticidal isoxazolines |
| US20100137372A1 (en) | 2007-03-29 | 2010-06-03 | Hideki Ihara | Isoxazoline compounds and their use in pest control |
| US20100179194A1 (en) | 2007-04-10 | 2010-07-15 | Bayer Corpscience Ag | Insecticidal aryl isoxazoline derivatives |
| US20100179195A1 (en) | 2007-06-13 | 2010-07-15 | E.I. Dupont De Nemours And Company | Isoxazoline insecticides |
| WO2008154528A2 (en) | 2007-06-13 | 2008-12-18 | E. I. Du Pont De Nemours And Company | Isoxazoline insecticides |
| WO2009002809A2 (en) | 2007-06-26 | 2008-12-31 | E. I. Du Pont De Nemours And Company | Naphthalene isoxazoline invertebrate pest control agents |
| US20100254959A1 (en) * | 2007-06-26 | 2010-10-07 | E. I. Du Pont De Nemours And Company | Naphthalene isoxazoline invertebrate pest control agents |
| US20110059988A1 (en) | 2007-08-17 | 2011-03-10 | Intervet International B.V. | Isoxazoline compositions and their use as antiparasitics |
| US20100254960A1 (en) | 2007-10-03 | 2010-10-07 | E. I. Dupont Nemours And Company | Naphthalene isoxazoline compounds for control of invertebrate pests |
| WO2010003923A1 (en) | 2008-07-09 | 2010-01-14 | Basf Se | Pestcidal active mixtures comprising isoxazoline compounds i |
| US20110245274A1 (en) * | 2008-12-18 | 2011-10-06 | Novartis Ag | Isoxazolines derivatives and their use as pesticide |
| WO2011075591A1 (en) * | 2009-12-17 | 2011-06-23 | Merial Limited | Anti parasitic dihydroazole compounds and compositions comprising same |
| WO2011149749A1 (en) | 2010-05-27 | 2011-12-01 | E.I. Du Pont De Nemours And Company | Crystalline form of 4- [5 - [3 -chloro-5 - (trifluoromethyl) phenyl] -4, 5 - dihydro - 5 - (trifluoromethyl) -3 - isoxazolyl] -n- [2-0x0-2- [ ( 2, 2, 2 - trifluoroethyl) amino] ethyl] -1- naphthalenecarboxamide |
| US20130137735A1 (en) * | 2010-05-27 | 2013-05-30 | E.I. Du Pont De Nemours And Company | Crystalline form of 4- [5 - [3 -chloro-5 - (trifluoromethyl) phenyl] -4, 5 - dihydro - 5 - (trifluoromethyl) -3 - isoxazolyl] -n- [2-0x0-2- [ ( 2, 2, 2 - trifluoroethyl) amino] ethyl] -1- naph-thalenecarboxamide |
| US20130095126A1 (en) * | 2010-06-18 | 2013-04-18 | Jean-Luc Perret | Use of substituted heterocyclic compounds to control sea lice on fish |
| WO2012089623A1 (en) | 2010-12-27 | 2012-07-05 | Intervet International B.V. | Topical localized isoxazoline formulation comprising glycofurol |
| US20130324538A1 (en) * | 2011-02-10 | 2013-12-05 | Noëlle Gauvry | Isoxazoline derivatives for controlling invertebrate pests |
| WO2012120135A1 (en) | 2011-03-10 | 2012-09-13 | Novartis Ag | Isoxazole derivatives |
| US8466115B2 (en) | 2011-03-10 | 2013-06-18 | Zoetis Llc | Spirocyclic isoxazoline derivatives as antiparasitic agents |
| WO2012120399A1 (en) | 2011-03-10 | 2012-09-13 | Pfizer Inc. | Spirocyclic isoxazoline derivatives as antiparasitic agents |
| US20130345221A1 (en) * | 2011-03-10 | 2013-12-26 | Novartis Ag | Isoxazole derivatives |
| WO2013039948A1 (en) | 2011-09-12 | 2013-03-21 | Merial Limited | Parasiticidal compositions comprising an isoxazoline active agent, methods and uses thereof |
| US20130131016A1 (en) * | 2011-11-21 | 2013-05-23 | Anacor Pharmaceuticals, Inc. | Boron-containing small molecules |
| US20130143956A1 (en) * | 2011-12-02 | 2013-06-06 | Merial Limited | Long-acting injectable moxidectin formulations and novel moxidectin crystal forms |
| WO2013119442A1 (en) | 2012-02-06 | 2013-08-15 | Merial Limited | Parasiticidal oral veterinary compositions comprising systemically-acting active agents, methods and uses thereof |
| WO2014039475A1 (en) | 2012-09-07 | 2014-03-13 | Zoetis Llc | Spirocyclic isoxazoline parasiticidal combinations |
| WO2014039484A1 (en) | 2012-09-07 | 2014-03-13 | Zoetis Llc | Spirocyclic isoxazolines as antiparasitic agents |
| WO2014189837A1 (en) | 2013-05-20 | 2014-11-27 | Zoetis Llc | Long-acting spiro-isoxazoline antiparasitic compositions |
| WO2015048371A1 (en) * | 2013-09-30 | 2015-04-02 | Zoetis Llc | Long-acting spiro-isoxazoline formulations |
Non-Patent Citations (32)
| Title |
|---|
| "Ansel's Pharmaceutical Dosage Forms and Drug Delivery Systems", 2005, LIPPINCOTT WILLIAMS & WILKINS |
| "International Nonproprietary Names for Pharmaceutical Substances (INN", WHO DRUG INFORMATION, vol. 17, no. 4, 2003, pages 263 - 286 |
| "The Merck Index", 1996, MERCK & CO., INC |
| "The Merck Veterinary Manual", January 2005 |
| ALBERS-SCHONBERG ET AL.: "Avermectins Structure Determination", J. AM. CHEM. SOC., vol. 103, 1981, pages 4216 - 4221 |
| DAVIES H.G. ET AL.: "Avermectins and Milbemycins", NAT. PROD. REP., vol. 3, 1986, pages 87 - 121 |
| GOODMAN; GILMAN'S: "The Pharmacological Basis of Therapeutics", 2005 |
| GREENE ET AL.: "Protective Groups in Organic Synthesis", 1999, JOHN WILEY AND SONS |
| HATEFI A ET AL: "Biodegradable injectable in situ forming drug delivery systems", JOURNAL OF CONTROLLED RELEASE, ELSEVIER, AMSTERDAM, NL, vol. 80, no. 1-3, 23 April 2002 (2002-04-23), pages 9 - 28, XP004348621, ISSN: 0168-3659, DOI: 10.1016/S0168-3659(02)00008-1 * |
| HIRENKUMAR K. MAKADIA ET AL: "Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier", POLYMERS, vol. 3, no. 4, 26 August 2011 (2011-08-26), pages 1377 - 1397, XP055282506, DOI: 10.3390/polym3031377 * |
| J. ANTIBIOTICS, vol. 43, 1990, pages 1380 |
| J. ANTIBIOTICS, vol. 44, 1991, pages 492 |
| J. CHEM. SOC. - CHEM. COMM., 1980, pages 601 |
| KAMINSKY ET AL., NATURE, vol. 452, 13 March 2008 (2008-03-13), pages 176 - 181 |
| LETENDRE ET AL., VETERINARY PARASITOLOGY, vol. 201, 2014, pages 190 - 197 |
| M.H. FISCHER; H. MROZIK; WILLIAM C. CAMPBELL: "Ivermectin and Abamectin", 1989, SPRINGER VERLAG |
| MARILYN N. MARTINEZ; DANIELLE LINDQUIST; SANJA MODRIC: "Terminology Challenges: Defining Modified Release Dosage Forms in Veterinary Medicine", JOURNAL OF PHARMACEUTICAL SCIENCES, vol. 99, no. 8, August 2010 (2010-08-01) |
| MATSCHKE C ET AL: "Sustained-release injectables formed in situ and their potential use for veterinary products", JOURNAL OF CONTROLLED RELEASE, ELSEVIER, AMSTERDAM, NL, vol. 85, no. 1-3, 13 December 2002 (2002-12-13), pages 1 - 15, XP004397760, ISSN: 0168-3659, DOI: 10.1016/S0168-3659(02)00266-3 * |
| MROZIK H. ET AL.: "Synthesis of Milbemycins from Avermectins", TETRAHEDRON LETT., vol. 24, 1983, pages 5333 - 5336 |
| N. L. JARVIS; W. A. ZISMAN: "Surface Chemistry of Fluorochemicals", 1965, U.S. NAVAL RESEARCH LABORATORY |
| OSTLIND ET AL., MEDICAL AND VETERINARY ENTOMOLOGY, vol. 11, 1997, pages 407 - 408 |
| OSTLIND ET AL., RESEARCH IN VETERINARY SCIENCE, vol. 48, 1990, pages 260 - 261 |
| PLUMB: "Veterinary Drug Handbook", 2005, BLACKWELL PUBLISHING |
| REMINGTON: "The Science and Practice of Pharmacy", 2005 |
| SAGER ET AL., VETERINARY PARASITOLOGY, vol. 159, 2009, pages 49 - 54 |
| SALGADO V.L.; SPARKS T.C.: "The Spinosyns: Chemistry, Biochemistry, Mode of Action, and Resistance", COMPREHENSIVE MOLECULAR INSECT SCIENCE, vol. 6, 2005, pages 137 - 173 |
| SHI Y ET AL: "Recent advances in intravenous delivery of poorly water-soluble compounds", EXPERT OPINION ON DRUG DELIVERY, INFORMA HEALTHCARE, GB, vol. 6, no. 12, 1 January 2009 (2009-01-01), pages 1261 - 1282, XP009159343, ISSN: 1742-5247 * |
| SUSANNE KILP ET AL: "Pharmacokinetics of fluralaner in dogs following a single oral or intravenous administration", PARASITES & VECTORS, BIOMED CENTRAL LTD, LONDON UK, vol. 7, no. 1, 7 March 2014 (2014-03-07), pages 85, XP021179157, ISSN: 1756-3305, DOI: 10.1186/1756-3305-7-85 * |
| TET. LETT., vol. 22, 1981, pages 135 |
| TET. LETT., vol. 22, 1981, pages 1977 |
| VETERINARY PARASITOLOGY, vol. 49, no. 1, July 1993 (1993-07-01), pages 5 - 15 |
| WILLSON ET AL., PARASITOLOGY, vol. 126, January 2003 (2003-01-01), pages 79 - 86 |
Cited By (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EA036716B1 (en) * | 2016-02-24 | 2020-12-11 | БЁРИНГЕР ИНГЕЛЬХАЙМ ЭНИМАЛ ХЕЛТ ЮЭсЭй ИНК. | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| EP3763211A1 (en) * | 2016-02-24 | 2021-01-13 | Boehringer Ingelheim Animal Health USA Inc. | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| US11497732B2 (en) | 2016-02-24 | 2022-11-15 | Boehringer Ingelheim Animal Health USA Inc. | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| AU2017223828B2 (en) * | 2016-02-24 | 2019-09-26 | Boehringer Ingelheim Vetmedica Gmbh | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| AU2019236686B2 (en) * | 2016-02-24 | 2021-01-28 | Boehringer Ingelheim Vetmedica Gmbh | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| WO2017147352A1 (en) * | 2016-02-24 | 2017-08-31 | Merial, Inc. | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| US12083100B1 (en) | 2016-02-24 | 2024-09-10 | Boehringer Ingelheim Animal Health USA Inc. | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| AU2021202118B2 (en) * | 2016-02-24 | 2022-03-17 | Boehringer Ingelheim Vetmedica Gmbh | Antiparasitic isoxazoline compounds, long-acting injectable formulations comprising them, methods and uses thereof |
| WO2018039508A1 (en) | 2016-08-25 | 2018-03-01 | Merial, Inc. | Method for reducing unwanted effects in parasiticidal treatments |
| CN111032634A (en) * | 2017-04-05 | 2020-04-17 | 勃林格殷格翰动物保健美国公司 | The crystal form of (S)-Aflana |
| EP4316522A2 (en) | 2017-11-07 | 2024-02-07 | Intervet International B.V. | Injectable isoxazoline pharmaceutical compositions and their use against parasite infestation |
| WO2019091936A1 (en) | 2017-11-07 | 2019-05-16 | Intervet International B.V. | Injectable isoxazoline pharmaceutical compositions and their use against parasite infestation |
| RU2799591C2 (en) * | 2017-11-07 | 2023-07-07 | Интервет Интернэшнл Б.В. | Injectable isoxazoline pharmaceutical compositions and their use |
| US11858904B2 (en) | 2017-11-07 | 2024-01-02 | Intervet Inc. | Process for preparing large size isoxazoline particles |
| US11648238B2 (en) * | 2017-12-12 | 2023-05-16 | Intervet Inc. | Implantable isoxazoline pharmaceutical compositions and uses thereof |
| WO2020225143A1 (en) | 2019-05-03 | 2020-11-12 | Intervet International B.V. | Injectable pharmaceutical compositions and uses thereof |
| RU2829447C2 (en) * | 2019-05-03 | 2024-10-30 | Интервет Интернэшнл Б.В. | Pharmaceutical compositions for injections and use thereof |
| US12497370B2 (en) | 2019-12-18 | 2025-12-16 | Elanco Tiergesundheit Ag | Isoxazoline derivatives |
| WO2021233967A1 (en) | 2020-05-20 | 2021-11-25 | Intervet International B.V. | Injectable pharmaceutical compositions and uses thereof |
| US12304903B2 (en) | 2020-07-24 | 2025-05-20 | Elanco Us Inc. | Process for making an isoxazoline compound and intermediate thereof |
| US11878968B2 (en) | 2021-07-09 | 2024-01-23 | Plexium, Inc. | Aryl compounds and pharmaceutical compositions that modulate IKZF2 |
| WO2025257633A1 (en) | 2024-06-12 | 2025-12-18 | Boehringer Ingelheim Vetmedica Gmbh | Long-acting castor oil-containing injectable formulations and methods of use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| MX2022010831A (en) | 2022-09-29 |
| UY36610A (en) | 2016-10-31 |
| AU2022202035A1 (en) | 2022-04-14 |
| EP3280413A1 (en) | 2018-02-14 |
| SG10201912724SA (en) | 2020-02-27 |
| AU2020210266B2 (en) | 2022-03-10 |
| AU2020210266A1 (en) | 2020-08-20 |
| KR102593069B1 (en) | 2023-10-23 |
| JP2018510897A (en) | 2018-04-19 |
| EA036272B1 (en) | 2020-10-21 |
| NZ736374A (en) | 2024-02-23 |
| JP6824900B2 (en) | 2021-02-03 |
| IL254813B (en) | 2020-03-31 |
| MX2017012848A (en) | 2018-01-15 |
| CN108055825B (en) | 2021-03-30 |
| EA201792235A1 (en) | 2018-01-31 |
| BR112017021073A2 (en) | 2018-07-03 |
| CA2981797A1 (en) | 2016-10-13 |
| EA202090762A1 (en) | 2020-09-08 |
| AU2016246598A1 (en) | 2017-11-02 |
| KR20170134700A (en) | 2017-12-06 |
| AU2016246598B2 (en) | 2020-07-23 |
| US20170020848A1 (en) | 2017-01-26 |
| US20210299104A1 (en) | 2021-09-30 |
| IL254813A0 (en) | 2017-12-31 |
| AR104212A1 (en) | 2017-07-05 |
| SG11201708068PA (en) | 2017-10-30 |
| CN108055825A (en) | 2018-05-18 |
| CL2017002536A1 (en) | 2018-04-20 |
| JP2021050206A (en) | 2021-04-01 |
| CA2981797C (en) | 2023-11-21 |
| CO2017010065A2 (en) | 2018-01-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2020210266B2 (en) | Extended release injectable formulations comprising an isoxazoline active agent, methods and uses thereof | |
| US11484528B2 (en) | Long-acting injectable formulations comprising an isoxazoline active agent, methods and uses thereof | |
| EP3419424B1 (en) | Long-acting injectable formulations comprising antiparasitic isoxazoline compounds and use thereof | |
| AU2016202105B2 (en) | Parasiticidal compositions comprising an isoxazoline active agent, methods and uses thereof | |
| WO2018039508A1 (en) | Method for reducing unwanted effects in parasiticidal treatments | |
| WO2025257633A1 (en) | Long-acting castor oil-containing injectable formulations and methods of use thereof | |
| HK1257959B (en) | Long-acting injectable formulations comprising antiparasitic isoxazoline compounds and use thereof | |
| EA040232B1 (en) | USE OF INJECTABLE DELAYED RELEASE PREPARATIONS CONTAINING ISOXAZOLINE ACTIVE SUBSTANCE | |
| BR112017018442B1 (en) | LONG-ACTING INJECTABLE COMPOSITIONS COMPRISING AN ISOXAZOLINE ACTIVE AGENT, METHODS AND USES THEREOF |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16720949 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11201708068P Country of ref document: SG |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 254813 Country of ref document: IL |
|
| REEP | Request for entry into the european phase |
Ref document number: 2016720949 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2981797 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: NC2017/0010065 Country of ref document: CO |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2017/012848 Country of ref document: MX |
|
| ENP | Entry into the national phase |
Ref document number: 2017553068 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112017021073 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 2016246598 Country of ref document: AU Date of ref document: 20160406 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 20177032205 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 201792235 Country of ref document: EA |
|
| ENP | Entry into the national phase |
Ref document number: 112017021073 Country of ref document: BR Kind code of ref document: A2 Effective date: 20170929 |