WO2016135458A1 - Allulose syrups - Google Patents
Allulose syrups Download PDFInfo
- Publication number
- WO2016135458A1 WO2016135458A1 PCT/GB2016/050422 GB2016050422W WO2016135458A1 WO 2016135458 A1 WO2016135458 A1 WO 2016135458A1 GB 2016050422 W GB2016050422 W GB 2016050422W WO 2016135458 A1 WO2016135458 A1 WO 2016135458A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- allulose
- syrup
- allulose syrup
- weight
- dry solids
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L29/00—Foods or foodstuffs containing additives; Preparation or treatment thereof
- A23L29/30—Foods or foodstuffs containing additives; Preparation or treatment thereof containing carbohydrate syrups; containing sugars; containing sugar alcohols, e.g. xylitol; containing starch hydrolysates, e.g. dextrin
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Preparation or treatment thereof
- A23L2/52—Adding ingredients
- A23L2/60—Sweeteners
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L27/00—Spices; Flavouring agents or condiments; Artificial sweetening agents; Table salts; Dietetic salt substitutes; Preparation or treatment thereof
- A23L27/30—Artificial sweetening agents
- A23L27/33—Artificial sweetening agents containing sugars or derivatives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/125—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives containing carbohydrate syrups; containing sugars; containing sugar alcohols; containing starch hydrolysates
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/15—Vitamins
-
- C—CHEMISTRY; METALLURGY
- C13—SUGAR INDUSTRY
- C13K—SACCHARIDES OBTAINED FROM NATURAL SOURCES OR BY HYDROLYSIS OF NATURALLY OCCURRING DISACCHARIDES, OLIGOSACCHARIDES OR POLYSACCHARIDES
- C13K13/00—Sugars not otherwise provided for in this class
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2200/00—Function of food ingredients
- A23V2200/02—Antioxidant
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2200/00—Function of food ingredients
- A23V2200/20—Ingredients acting on or related to the structure
- A23V2200/212—Buffering agent
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2250/00—Food ingredients
- A23V2250/60—Sugars, e.g. mono-, di-, tri-, tetra-saccharides
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- the present invention relates to allulose syrups, use of allulose syrups in the manufacture of food or beverage products, and food and beverage products made using the allulose syrups.
- nutritive sweeteners such as sucrose (generally referred to as 'sugar' or 'table sugar'), glucose, fructose, corn syrup, high fructose corn syrup and the like.
- sucrose generally referred to as 'sugar' or 'table sugar'
- glucose fructose
- corn syrup high fructose corn syrup and the like.
- excess intake of nutritive sweeteners, such as sucrose has long been associated with an increase in diet-related health issues, such as obesity, heart disease, metabolic disorders and dental problems. This worrying trend has caused consumers to become increasingly aware of the importance of adopting a healthier lifestyle and reducing the level of nutritive sweeteners in their diet.
- Allulose also known as D- psicose. Allulose is known as a "rare sugar", since it occurs in nature in only very small amounts. It provides around 70% of the sweetness of sucrose, but only around 5% of the calories (approximately 0.2 kcal/g). It may therefore essentially be considered to be a 'zero calorie' sweetener.
- 201 1/0275138 discloses a ketose 3-epimerase derived from a microorganism of the Rhizobium genus.
- This protein shows a high specificity to D- or L- ketopentose and D- or L-ketohexose, and especially to D-fructose and D-psicose.
- This document also discloses a process for producing ketoses by using the protein.
- Korean patent no. 100832339 discloses a Sinorhizobium YB-58 strain which is capable of converting fructose into psicose (i.e. allulose), and a method of producing psicose using a fungus body of the Sinorhizobium YB-58 strain.
- Korean patent application no. 1020090098938 discloses a method of producing psicose using E. coli wherein the E. coli expresses a polynucleotide encoding a psicose 3- epimerase.
- Allulose is present in processed cane and beet molasses, steam treated coffee, wheat plant products and high fructose corn syrup.
- D-allulose is the C-3 epimer of D-fructose and the structural differences between allulose and fructose result in allulose not being metabolized by the human body to any significant extent, and thus having "zero" calories.
- allulose is thought to be a promising candidate as a replacement for nutritive sweeteners and as a sweet bulking agent, as it has essentially no calories and is reported to be sweet while maintaining similar properties to sucrose.
- allulose syrup i.e. a syrup comprising allulose and water. It has been found that allulose syrups may be susceptible to degradation over time (i.e. gradual reduction in allulose content), to color formation, to the formation of impurities (such as hydroxymethylfurfural - HMF), to crystallization, and to inadequate microbial stability.
- An object of the present invention is to provide an allulose syrup that addresses the above problems.
- the present invention provides an allulose syrup having a total dry solids content of from 50% to 80% by weight, and comprising allulose in an amount of at least 80% by weight on a dry solids basis, wherein the pH of the syrup is from 2.5 to 6.0.
- the allulose syrup has a total dry solids content of from 50% to 70% by weight, and comprises allulose in an amount of at least 80% by weight on a dry solids basis, wherein the pH of the syrup is from 2.5 to 6.0.
- the allulose syrup has a total dry solids content of from 70% to 80% by weight, and comprises allulose in an amount of at least 90% by weight on a dry solids basis, wherein the pH of the syrup is from 3.0 to 5.0.
- the total dry solids content of the allulose syrup is from 71 % to 78% by weight. In another embodiment, the total dry solids content of the allulose syrup is from 71 % to 73% by weight. In another embodiment, the total dry solids content of the allulose syrup is from 76% to 78% by weight. In another embodiment, the total dry solids content of the allulose syrup is from 50% to 71 % by weight.
- the pH of the allulose syrup is from 3.5 to 4.5. In an embodiment, the pH of the allulose syrup is from 3.8 to 4.2.
- the allulose syrup comprises allulose in an amount of at least 95% by weight on a dry solids basis. In an embodiment, the allulose syrup comprises less than 1000 ppm of HMF. In an embodiment, the allulose syrup comprises sulfur dioxide in an amount of from 0.1 to 20 ppm.
- the allulose syrup comprises sulfur dioxide in an amount of from 1 to 20 ppm.
- the allulose syrup comprises less than 10 parts per billion of isovaleraldehyde. In an embodiment, the allulose syrup comprises less than 2 parts per billion of 2- aminoacetophenone.
- the allulose syrup further comprises one or more additives.
- the one or more additives may include a stability-enhancing additive.
- the one or more additives may include an anti-oxidant.
- the one or more additives may include a buffer.
- the one or more additive may be selected from the group consisting of ascorbic acid or salts thereof; isoascorbic acid (erythorbate) or salts thereof; citric acid or salts thereof; acetic acid or salts thereof; salts of bisulfite or metabisulfite; and tocopherol acetate.
- the shelf-life of the allulose syrup as defined by maintaining an allulose content of greater than 80% by weight on a dry solids basis is at least 3, 6, 9, 12 months, or more than 12 months.
- an allulose content of greater than 80% by weight on a dry solids basis is maintained when the allulose syrup is stored for at least 3, 6, 9, 12 months, or more than 12 months.
- the shelf-life of the allulose syrup as defined by maintaining an allulose content of greater than 90% by weight on a dry solids basis is at least 3, 6, 9, 12 months, or more than 12 months. In an embodiment, the shelf-life of the allulose syrup as defined by maintaining an allulose content of greater than 95% by weight on a dry solids basis is at least 3, 6, 9, 12 months, or more than 12 months.
- the present invention provides a process for preparing an allulose syrup according to the first aspect. The process for preparing the allulose syrup includes:
- the process includes:
- the process includes:
- the dry solids content is from 70 to 78% by weight
- the allulose content of the syrup is at least 90% by weight on a dry solids basis
- the pH is controlled to between 3.5 to 4.5.
- the present invention provides the use of the allulose syrup according to the first aspect in the preparation of a food or beverage product.
- the present invention provides a food or beverage product comprising an allulose syrup according to the first aspect and at least one additional food or beverage ingredient.
- the at least one additional food or beverage ingredient includes at least one ingredient selected from the group consisting of flavorants, colorants, sweeteners other than allulose, dietary fibers, acidulants, water, and combinations thereof.
- the allulose syrup comprises 50 to 80% dry solids by weight, and greater than 80% allulose on a dry solids basis, a measured pH between 2.5 and 6.0 and a shelf life of at least 3 months.
- the allulose syrup comprises 60 to 80% dry solids by weight, and greater than 90% allulose on a dry solids basis, a measured pH between 3.0 and 5.0 and a shelf life of at least 3 months.
- the allulose syrup comprises 70 to 80% dry solids by weight, and greater than 90% allulose on a dry solids basis, a measured pH between 3.0 and 5.0 and a shelf life of at least 3 months.
- Figure 1 shows how the purity of an allulose syrup composition (initial pH 3.4) changes over time at 25 °C, 30 °C and 35 °C.
- Figure 2 shows how the color of an allulose syrup composition (initial pH 3.4) changes over time at 25 °C, 30 °C and 35 °C.
- Figure 3 shows how the amount of HMF in an allulose syrup composition (initial pH 3.4) changes over time at 25 °C, 30 °C and 35 °C.
- Figure 4 shows how the pH of an allulose syrup composition (initial pH 3.4) changes over time at 25 °C, 30 °C and 35 °C. It should be noted that the data points for storage at 25 °C are the same as for storage at 30 °C.
- Figure 5 shows how the pH of an allulose syrup composition (initial pH 4.0) changes over time at 4 °C, 25 °C, 35 °C and 50 °C.
- Figure 6 shows how the color of an allulose syrup composition (initial pH 4.0) changes over time at 4 °C, 25 °C, 35 °C and 50 °C.
- Figure 7 shows how the amount of HMF in an allulose syrup composition (initial pH 4.0) changes over time at 4 °C, 25 °C, 35 °C and 50 °C.
- Figure 8 shows how the purity of an allulose syrup composition (initial pH 4.0) changes over time at 4 °C, 25 °C and 35 °C.
- Figure 9 shows how the pH of the allulose syrup product samples of Example 2 changes over time at 40 °C.
- Figure 10 shows how the pH of the allulose syrup product samples of Example 2 changes over time at 50 °C.
- Figure 1 1 compares change in the pH of the allulose syrup product samples of Example 2 (starting pH 4.0) at 50 °C with an allulose syrup composition with an initial pH of 3.9.
- Figure 12 shows how the allulose purity of the allulose syrup product samples of Example 2 changes over time at 40 °C.
- Figure 13 shows how the allulose purity of the allulose syrup product samples of Example 2 changes over time at 50 °C.
- Figure 14 shows how the color of the allulose syrup product samples of Example 2 changes over time at 40 °C.
- Figure 15 shows how the color of the allulose syrup product samples of Example 2 changes over time at 50 °C.
- Figure 16 shows how the HMF content of the allulose syrup product samples of Example 2 changes over time at 40 °C.
- Figure 17 shows how the HMF content of the allulose syrup product samples of Example 2 changes over time at 50 °C.
- Figure 18 shows how the allulose content of the allulose syrup product samples of Example 5 changes over time at different temperature, pH and DS content.
- Figure 19 shows how the allulose content of the allulose syrup product samples of Example 5 changes over time at 25 °C.
- Figure 20 shows how the allulose content of the allulose syrup product samples of Example 5 changes over time at 35 °C.
- Figure 21 shows how the HMF content of the allulose syrup product samples of Example 5 changes over time at 25 °C.
- Figure 22 shows how the HMF content of the allulose syrup product samples of Example 5 changes over time at 35 °C.
- Figure 23 shows how the color of the allulose syrup product samples of Example 5 changes over time at 25 °C.
- Figure 24 shows how the color of the allulose syrup product samples of Example 5 changes over time at 35 °C.
- Figure 25 shows how the pH of the allulose syrup product samples of Example 6 over time is affected by additives.
- Figure 26 shows how the allulose purity of the allulose syrup product samples of Example 6 over time is affected by additives.
- Figure 27 shows how the allulose purity of the allulose syrup product samples of Example 6 over time is affected by the addition of ascorbate and isoascorbate.
- Figure 28 shows how the allulose purity of the allulose syrup product samples of Example 6 over time is affected by the addition of citrate and acetate.
- Figure 29 shows how the HMF content of the allulose syrup product samples of Example 6 over time is affected by the addition of ascorbate and isoascorbate.
- Figure 30 shows the change in allulose content at 6 months at 77% DS as modelled using DOE software according to Example 7 (each contour line represents a 2% decrease in change in allulose content from time 0).
- Figure 31 shows the change in allulose content at 6 months at 25 °C as modelled using DOE software according to Example 7.
- Figure 32 shows color change at 6 months at 77% DS as modelled using DOE software according to Example 7.
- Figure 33 shows color change at 6 months at 25 °C as modelled using DOE software according to Example 7.
- Figure 34 shows HMF formation at 6 months and 77% DS as modelled using DOE software according to Example 7.
- the present invention is based on the finding that allulose syrups with improved storage stability can be prepared by careful control of certain parameters.
- allulose refers to a monosaccharide sugar of the structure shown as a Fischer projection in below Formula I. It is also known as "D-psicose”:
- the present invention provides an allulose syrup having a total dry solids content of from 50% to 80% by weight, and comprising allulose in an amount of at least 80% by weight on a dry solids basis, wherein the pH of the syrup is from 2.5 to 6.0.
- the allulose syrup has a total dry solids content of from 70% to 80% by weight, and comprises allulose in an amount of at least 90% by weight on a dry solids basis, wherein the pH of the syrup is from 3.0 to 5.0.
- the total dry solids content of the allulose syrup is from 50% to 80% by weight.
- the total dry solids content may be 50%, 51 %, 52%, 53%, 54%, 55%, 56%, 57%, 58%, 59%, 60%, 61 %, 62%, 63%, 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71 %, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79% or 80% by weight, as well as all intermediate values.
- the total dry solids content of the allulose syrup is from 70% to 80% by weight.
- the total dry solids content may be 70%, 71 %, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79% or 80% by weight, as well as all intermediate values.
- the total dry solids content of the allulose syrup is from 71 % to 78% by weight.
- the total dry solids content of the allulose syrup is from 71 % to 73% by weight.
- the total dry solids content of the allulose syrup is from 76% to 78% by weight.
- the total dry solids content of the allulose syrup is from 50% to 70% by weight.
- compositional stability of the allulose syrup is generally highest towards the lower end of the total dry solids content range of the invention
- microbial stability is generally highest towards the higher end of the total dry solids content range of the invention. Accordingly, the selection of a suitable total dry solids content within the range of the invention can be made depending on the key attribute for the particular application.
- the pH of the allulose syrup is from 2.5 to 6.0.
- the pH of the syrup may be 2.5, 2.6, 2.7, 2.8, 2.9, 3.0, 3.1 , 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4.0, 4.1 , 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9, 5.0, 5.1 , 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9 or 6.0, as well as all intermediate values.
- the pH of the allulose syrup is from 3.0 to 5.0.
- the pH of the syrup may be 3.0, 3.1 , 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4.0, 4.1 , 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9 or 5.0 as well as all intermediate values.
- the pH of the allulose syrup is from 3.5 to 4.5.
- the pH of the syrup may be 3.5, 3.6, 3.7, 3.8, 3.9, 4.0, 4.1 , 4.2, 4.3, 4.4 or 4.5 as well as all intermediate values.
- the pH of the allulose syrup is from 3.8 to 4.2.
- the pH of the allulose syrup is about 4.0.
- the allulose syrup comprises allulose in an amount of at least 80% by weight on a dry solids basis (i.e., of the total dry solids present in the allulose syrup, at least 80% by weight is allulose).
- the allulose syrup may comprise allulose in an amount of 80%, 81 %, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91 %, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100% by weight on a dry solids basis, as well as all intermediate values.
- the allulose syrup comprises allulose in an amount of at least 90% by weight on a dry solids basis (i.e., of the total dry solids present in the allulose syrup, at least 90% by weight is allulose).
- the allulose syrup may comprise allulose in an amount of 90%, 91 %, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100% by weight on a dry solids basis, as well as all intermediate values.
- the allulose syrup comprises allulose in an amount of at least 95% by weight on a dry solids basis.
- the allulose syrup comprises less than 1000 ppm of HMF (hydroxymethylfurfural).
- the allulose syrup may comprise less than 900 ppm, less than 800 ppm, less than 700 ppm, less than 600 ppm, less than 500 ppm, less than 400 ppm, less than 300 ppm, less than 200 ppm or less than 100 ppm of HMF.
- the allulose syrup comprises more than 0.1 ppm and less than 1000 ppm of HMF (hydroxymethylfurfural), for example more than 0.1 ppm and less than 900 ppm, more than 0.1 ppm and less than 800 ppm, more than 0.1 ppm and less than 700 ppm, more than 0.1 ppm and less than 600 ppm, more than 0.1 ppm and less than 500 ppm, more than 0.1 ppm and less than 400 ppm, more than 0.1 ppm and less than 300 ppm, more than 0.1 ppm and less than 200 ppm, or more than 0.1 ppm and less than 100 ppm.
- HMF hydroxymethylfurfural
- the allulose syrup comprises sulfur dioxide in an amount of from 0.1 to 20 ppm.
- the allulose syrup comprises sulfur dioxide in an amount of from 1 to 20 ppm.
- the allulose syrup comprises less than 10 parts per billion of isovaleraldehyde. In an embodiment, the allulose syrup comprises less than 2 parts per billion of 2- aminoacetophenone.
- the allulose syrup further comprises one or more additives.
- the one or more additives may include a stability-enhancing additive.
- the one or more additives may include an anti-oxidant.
- the one or more additives may include a buffer. The incorporation of a buffer in the allulose syrup maintains the pH of the allulose within the desired range for a longer period of time, such that storage stability is further enhanced.
- the stability enhancing additives are included at around 0.01 -2.0% by weight based on the total weight of the allulose syrup.
- the stability-enhancing additive may be selected from the group consisting of ascorbic acid and salts thereof; isoascorbic acid (erythorbate) and salts thereof; citric acid and salts thereof; acetic acid and salts thereof; and salts of bisulfite and metabisulfite; and tocopherol acetate.
- suitable salts include alkali metal salts, particularly sodium and potassium salts, and especially sodium salts.
- Specific examples of stability-enhancing additives useful in the present invention include ascorbate, isoascorbate, sodium citrate, sodium acetate, tocopherol acetate and metabisulfite.
- the stability enhancing additives are included at around 0.2% by weight based on the total weight of the allulose syrup in the case of ascorbic acid or salts thereof; isoascorbic acid (erythorbate) or salts thereof; citric acid or salts thereof; acetic acid or salts thereof; and tocopherol acetate. In an embodiment, the stability enhancing additives are included at around 0.02% by weight based on the total weight of the allulose syrup in the case of salts of bisulfite or metabisulfite. The concentration of buffer included in the allulose syrup may be around 0.01-2.0% by weight based on the total weight of the allulose syrup.
- the concentration of buffer included in the allulose syrup may be around 0.2% by weight based on the total weight of the allulose syrup in the case of ascorbic acid or salts thereof; isoascorbic acid (erythorbate) or salts thereof; citric acid or salts thereof; acetic acid or salts thereof; and tocopherol acetate.
- the concentration of buffer included in the allulose syrup may be around 0.02% by weight based on the total weight of the allulose syrup in the case of salts of bisulfite or metabisulfite.
- the allulose syrup of the present invention has a shelf-life of at least 3 months.
- the allulose syrup of the present invention maintains an allulose content of at least 80% on a dry solids basis for at least 3 months, preferably at least 6 months, at least 9 months, at least 12 months or more than 12 months.
- the allulose syrup of the present invention has a shelf-life of at least 3 months.
- the allulose syrup of the present invention maintains an allulose content of at least 90% on a dry solids basis for at least 3 months, preferably at least 6 months, at least 9 months, at least 12 months or more than 12 months.
- the allulose syrup of the present invention preferably has a shelf-life of at least 6 months.
- the allulose syrup of the present invention preferably maintains an allulose content of at least 95% on a dry solids basis for at least 6 months, preferably at least 9 months, at least 12 months or more than 12 months. Allulose content is measured by standard HPLC methods such as the Sacch.03 method set forth by the corn refiners association (http://corn.org/wp-content uploads/2009/12/SACCH.03.pdf).
- the syrup has a limited amount of the following compounds: less than 1000 ppm hydroxymethylfurfural (HMF); sulphur dioxide at a concentration of less than 20 parts per million; isovaleraldehyde at a measured concentration of less than 10 parts per billion; and 2- aminoacetophenone at a concentration of less than 2 parts per billion.
- HMF hydroxymethylfurfural
- sulphur dioxide at a concentration of less than 20 parts per million
- isovaleraldehyde at a measured concentration of less than 10 parts per billion
- 2- aminoacetophenone at a concentration of less than 2 parts per billion.
- the syrup can have any of the following compounds alone or in combination thereof: a stability enhancing ingredient including one or more of: 1 ) ascorbic acid or salts thereof, 2) isoascorbic acid (erythorbate) or salts thereof, 3) citric acid or salts thereof, 4) acetic acid or salts thereof, 5) salts of bisulfite or metabisulfite, and/or 6) tocopherol acetate.
- a stability enhancing ingredient including one or more of: 1 ) ascorbic acid or salts thereof, 2) isoascorbic acid (erythorbate) or salts thereof, 3) citric acid or salts thereof, 4) acetic acid or salts thereof, 5) salts of bisulfite or metabisulfite, and/or 6) tocopherol acetate.
- the allulose syrup may have a concentration of greater than 90% (e.g. greater than 95%) with a shelf-life of at least 3, 6, 9, 12 months, or more than 12 months.
- the present invention provides a process for preparing an allulose syrup.
- the process comprises: providing an allulose syrup; adjusting the dry solids content of the allulose syrup such that it is from 50% to 80% by weight; adjusting the allulose content of the allulose syrup such that allulose is present in an amount of at least 80% by weight on a dry solids basis; and controlling the pH of the allulose syrup so that it is from 2.5 to 6.0.
- the process for preparing an allulose syrup comprises: providing an allulose syrup; adjusting the dry solids content of the allulose syrup such that it is from 60% to 80% by weight; adjusting the allulose content of the allulose syrup such that allulose is present in an amount of at least 80% by weight on a dry solids basis; and controlling the pH of the allulose syrup so that it is from 2.5 to 6.0.
- the process for preparing an allulose syrup comprises: providing an allulose syrup; adjusting the dry solids content of the allulose syrup such that it is from 70% to 80% by weight; adjusting the allulose content of the allulose syrup such that allulose is present in an amount of at least 90% by weight on a dry solids basis; and controlling the pH of the allulose syrup so that it is from 3.0 to 5.0.
- the process for preparing an allulose syrup comprises: providing an allulose syrup; adjusting the dry solids content of the allulose syrup such that it is from 70% to 78% by weight; adjusting the allulose content of the allulose syrup such that allulose is present in an amount of at least 90% by weight on a dry solids basis; and controlling the pH of the allulose syrup so that it is from 3.5 to 4.5.
- the process optionally comprises removing or avoiding the production of HMF to limit the content to less than 1000 ppm, or more preferably less than 100 ppm.
- the process optionally comprises removing or avoiding the production of isovaleraldehyde to limit the content to less than 10 parts per billion.
- the process optionally comprises removing or avoiding the production of aminoacetophenone to limit the content to less than 2 parts per billion.
- the process optionally comprises adding one or more additives to the syrup. These procedures need not be carried out in the same order recited above (for example, the pH adjustment may be performed before adjustment of the dry solids content).
- the description of the embodiments of the allulose syrup herein applies mutatis mutandis to the process for preparing an allulose syrup.
- the present invention provides the use of the allulose syrup according to the first aspect in the preparation of a food or beverage product, as well as food or beverage products made using the sweetener syrup.
- Food or beverage products which may be contemplated in the context of the present invention include baked goods; sweet bakery products (including, but not limited to, rolls, cakes, pies, pastries, and cookies); pre-made sweet bakery mixes for preparing sweet bakery products; pie fillings and other sweet fillings (including, but not limited to, fruit pie fillings and nut pie fillings such as pecan pie filling, as well as fillings for cookies, cakes, pastries, confectionary products and the like, such as fat-based cream fillings); desserts, gelatins and puddings; frozen desserts (including, but not limited to, frozen dairy desserts such as ice cream - including regular ice cream, soft serve ice cream and all other types of ice cream - and frozen non-dairy desserts such as non-dairy ice cream, sorbet and
- An allulose syrup in accordance with the present invention may be used in combination with one or more other food or beverage ingredients, including any of the food and beverage ingredients known in the art.
- additional food and beverage ingredients include, but are not limited to, flavorants, colorants, sweeteners other than allulose (including other sugars such as sucrose, fructose, allose, tagatose and other rare sugars, synthetic high intensity sweeteners such as sucralose, acesulfame K, saccharin, aspartame and the like, natural high intensity sweeteners such as Stevia and Monk Fruit Extract sweeteners and the terpene glycosides present therein, and the like), dietary fibers (including soluble dietary fibers such as soluble corn fiber and polydextrose), acidulants, water, and the like.
- Specific illustrative examples of food and beverage products which may be prepared using an allulose syrup in accordance with the invention include, but are not limited to:
- a beverage such as a carbonated or non-carbonated beverage or a juice drink comprising allulose syrup and one or more synthetic high intensity sweeteners such as sucralose;
- a beverage including a beverage concentrate, comprising an allulose syrup, a natural high intensity sweetener (such as a Stevia sweetener), and a dietary fiber (e.g., a soluble dietary fiber, such as a soluble corn fiber), and an acidulant (e.g., citric acid);
- a beverage concentrate comprising an allulose syrup, a natural high intensity sweetener (such as a Stevia sweetener), and a dietary fiber (e.g., a soluble dietary fiber, such as a soluble corn fiber), and an acidulant (e.g., citric acid);
- a yogurt such as a Greek yogurt, comprising allulose syrup (which may be free of any artificial sweeteners);
- a frozen dessert comprising allulose syrup, a dietary fiber (e.g., a soluble dietary fiber, such as a soluble corn fiber), a natural high intensity sweetener (such as a Stevia sweetener and/or a Monk Fruit Extract sweetener), and a food system stabilizer;
- a dietary fiber e.g., a soluble dietary fiber, such as a soluble corn fiber
- a natural high intensity sweetener such as a Stevia sweetener and/or a Monk Fruit Extract sweetener
- a food system stabilizer e.g., a soluble dietary fiber, such as a soluble corn fiber
- a natural high intensity sweetener such as a Stevia sweetener and/or a Monk Fruit Extract sweetener
- a cookie such as a chocolate chip cookie, comprising an allulose syrup and a corn starch;
- a confectionary such as a gummy candy, comprising an allulose syrup and a natural high intensity sweetener (e.g., a Stevia sweetener); and
- a natural high intensity sweetener e.g., a Stevia sweetener
- a flavored syrup such as a maple-flavored syrup, comprising an allulose syrup, fructose, and an acidulant (e.g., citric acid).
- an acidulant e.g., citric acid
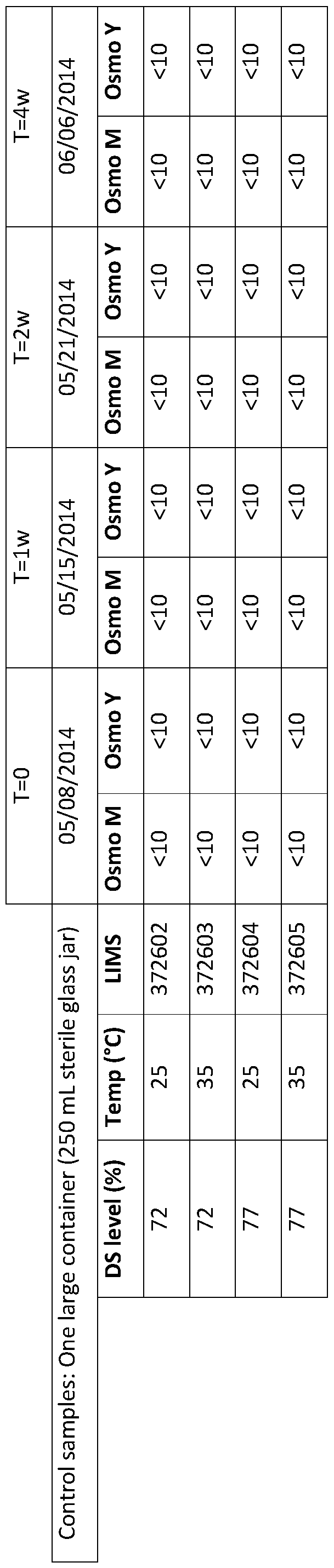
- Example 1 Each sample consisted of 3500 mL of allulose syrup in a 4 quart (4.54 liter) square plastic container. The sampling was carried out at 0 and 2 months.
- Analytical Samples were analyzed using methods known to those skilled in the art.
- the allulose composition was determined by standard HPLC methods, such as the Sacch.03 method set forth by the corn refiners association (http://corn.org/wp- content uploads/2009/12/SACCH.03.pdf).
- DS was measured by refractive index
- pH was measured at a dilution resulting in less than 40% solids
- color was analyzed by measuring the absorbance of the syrup at 450nm and subtracting the background at 600nm and dividing the result by the path length of the cuvette.
- HMF isovaleraldehyde, aminoacetophenone, were analyzed using reverse phase HPLC with UV detection.
- the HMF content increased in each sample over 2 months (Figure 3).
- the content of HMF in the sample at 35 °C increased to 180 ppm HMF after 2 months.
- the content of HMF in the 25 °C and 30 °C samples was lower.
- Samples of starting material were taken. The pH and DS were measured and recorded. One sub sample of each was taken as is, the next adjusted to pH 3.6, another to pH 4.0 and the final one to pH 4.7 using dilute HCI or sodium carbonate. One subset of starting material was diluted to 71 % DS. Another subset of the pH 4.0 batch had sodium citrate or sodium metabisulfite added. Sealed sample containers were placed into different temperature ovens at 40 °C and 50 °C. Extracts from each of the samples were removed from each oven periodically. Samples were chilled quickly in an ice bath and analyzed for carbohydrate composition, HMF, color and pH.
- Samples were analyzed to determine their DS, pH, carbohydrate composition, HMF content and color. For pH and color the samples were analyzed at a standard DS.
- the pH drift data ( Figures 9 and 10) at pH 4.0 matches the stability study of an allulose syrup product which the product pH started at 3.9 and samples were stored at 50°C (comparison in Figure 1 1). Allulose content dropped in all samples following the trend of higher temperature, lower pH and longer time resulting in faster allulose losses ( Figures 12 and 13).
- the pH 4.0 samples with additives show a similar rate of allulose loss as the pH 4.0 sample with no additive. This may be explained by the similar pH changes observed above and due to very low levels of the additives.
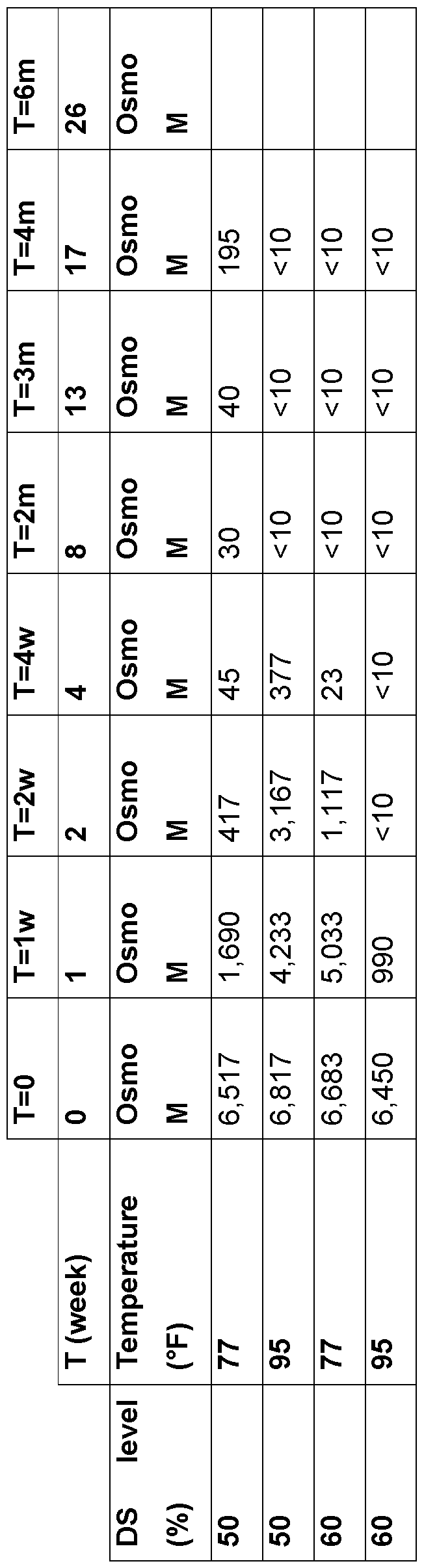
- Example 3 Crystallization stability Allulose syrups were prepared at 50, 60, 71 , 77, and 85% DS and were equilibrated at 25°C, 15°C and 4°C. These samples were seeded with ⁇ 0.1 % crystalline allulose and crystallization was monitored visually and by change in dry solids of the syrup fraction after 1 month of storage. Results:
- Microbial stability was assessed at 72% and 77% dry solids content by a challenge study with osmophilic yeasts and molds.
- Microbial stability was also assessed at 50% and 60% dry solids content by a challenge study with osmophilic yeasts and molds using the same method. At 60%DS, it took allulose syrup 2 months to completely remove viability of osmophilic yeasts and molds, and at 50% DS, viability of yeasts and molds was not removed completely even after 4 months. This suggests that 60% solids is the minimum solids concentration for allulose syrup that can reasonably be considered resistant to spoilage by microbial contamination and more ideally the concentration is 70-77% solids.
- final product stability has an optimum DS that is fairly narrow for allulose syrup.
- Lower DS reduces the rate of degradation in all parameters, however a final product DS that is below 60% DS does not maintain good microbial stability.
- Higher DS results in more rapid degradation and also crystallization. Therefore an optimal DS of 60-80% is required and more preferably a DS of 71 -78% is required for long term stability of allulose syrup and more preferably a DS of 71-73% should have the highest combined allulose content stability, microbial stability and crystalline stability. In cases where microbial stability is the key attribute necessary, 76-78% DS would have the best microbial stability.
- final product stability is optimized in a narrow range of pH, from 3.5 to 4.5 and more preferably in a pH range from 3.8 to 4.2 in order to optimize the trade-off between carbohydrate stability and color/HMF formation.
- Lower pH was shown to increase the rate of allulose content loss and HMF formation, while higher pH was shown to result in more rapid formation of color.
- Example 5 Allulose syrup stability within a narrow pH range and ambient storage temperatures This series of experiments was set up to determine allulose syrup stability within a narrow range of pH and DS at ambient temperature range of 25-35 °C.
- Additives have an effect on stability. These additives may stabilize the syrup by buffering the pH to help control at pH 4.0 and also to minimize oxidation.
- Each sample consisted of 1000 mL of syrup in a plastic container. Two gallons of this material were pH adjusted to 4.0 using 1 M sodium carbonate (NaC0 3 ), by slow and careful addition and regular pH measurement at 1 :1 dilution. This material was then split into two separate containers and one was diluted to 71 % DS (1 1.5 lbs 77% DS syrup, plus 0.97 lbs water).
- HMF HMF increased in all samples. However, one subset of additive samples displayed a substantially smaller amount of HMF increase.
- the samples with reduced HMF increase were those containing either ascorbate or isoascorbate, displaying less than half the HMF increase of the control samples (Figure 29).
- Ascorbate and isoascorbate have the ability to control HMF formation, whereas, sodium citrate and sodium acetate showed promise at controlling pH and allulose content changes. Neither MBS nor tocopherol acetate addition resulted in a significant benefit.
- Example 7 Surface response study of temperature, pH and DS:
- the acceptable stable storage conditions are further bounded in terms of solids, pH, and storage temperature when color is considered.
- colorless food ingredients are desired.
- color of the syrup was analyzed as absorbance at 450nm with background subtracted at 600nm. A change in color of more than 4 is generally considered unacceptable.
- Change in color for 77% DS allulose syrup were modeled as a response surface after 6 months storage for temperature and pH (Figure 32) we can see that both temperature and pH are critical factors. Temperature must be maintained near or below 25°C and ideally at a pH between 3.7 and 4.2.
- HMF Hydroxymethylfurfural
- Figure 34 demonstrates the modeled temperature pH response surface for change in HMF at 77%DS. HMF production is highest at low pH and high temperature and is lowest at high pH and low temperature. Less than 100ppm HMF is generally preferred for food ingredients. Thus, another pH boundary can be placed on allulose syrup: when stored at 25°C, it should be above pH 3.70.
- a syrup form that is more stable has benefits in that it can be stored for longer time periods and still be saleable, it has broader customer appeal, it can be shipped to geographic locations that require lengthy shipping and holding times. Additionally, improved product stability means that the product as used will retain a higher quality of composition and taste. This is beneficial from a calorie labelling position and final consumer product quality position.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Nutrition Science (AREA)
- Engineering & Computer Science (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Molecular Biology (AREA)
- Mycology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Organic Chemistry (AREA)
- Jellies, Jams, And Syrups (AREA)
- Non-Alcoholic Beverages (AREA)
- Seasonings (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
Abstract
Description
Claims
Priority Applications (20)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16708198.3A EP3261455B8 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| KR1020177024973A KR102283524B1 (en) | 2015-02-24 | 2016-02-19 | allulose syrup |
| CN202110500368.4A CN113317430B (en) | 2015-02-24 | 2016-02-19 | Psicose syrup |
| BR112017017941-5A BR112017017941B1 (en) | 2015-02-24 | 2016-02-19 | ALULOSE SYRUP, ITS PREPARATION PROCESS, USE AND FOOD OR BEVERAGE PRODUCT |
| EP22150616.5A EP4042873B8 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| JP2017544758A JP6982499B2 (en) | 2015-02-24 | 2016-02-19 | Allose syrup |
| KR1020217023575A KR20210096688A (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| AU2016225278A AU2016225278B2 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| CA2977617A CA2977617C (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| EP24206540.7A EP4529777A3 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| MX2017010860A MX390478B (en) | 2015-02-24 | 2016-02-19 | ALLULOSE SYRUPS. |
| IL296049A IL296049B2 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups and methods for their preparation |
| US15/552,944 US11653688B2 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| PL16708198.3T PL3261455T3 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| CN201680011915.XA CN107567283B (en) | 2015-02-24 | 2016-02-19 | Allulose syrup |
| IL254126A IL254126B2 (en) | 2015-02-24 | 2017-08-23 | Allulose syrups and methods for their preparation |
| AU2020203857A AU2020203857A1 (en) | 2015-02-24 | 2020-06-11 | Allulose syrups |
| AU2021101558A AU2021101558A4 (en) | 2015-02-24 | 2021-03-26 | Allulose syrups |
| US18/135,465 US20230248038A1 (en) | 2015-02-24 | 2023-04-17 | Allulose syrups |
| AU2023251517A AU2023251517A1 (en) | 2015-02-24 | 2023-10-20 | Allulose syrups |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562120165P | 2015-02-24 | 2015-02-24 | |
| US62/120,165 | 2015-02-24 | ||
| US201562168337P | 2015-05-29 | 2015-05-29 | |
| US62/168,337 | 2015-05-29 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/552,944 A-371-Of-International US11653688B2 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
| US18/135,465 Continuation US20230248038A1 (en) | 2015-02-24 | 2023-04-17 | Allulose syrups |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016135458A1 true WO2016135458A1 (en) | 2016-09-01 |
Family
ID=55456836
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2016/050422 Ceased WO2016135458A1 (en) | 2015-02-24 | 2016-02-19 | Allulose syrups |
Country Status (14)
| Country | Link |
|---|---|
| US (2) | US11653688B2 (en) |
| EP (3) | EP4529777A3 (en) |
| JP (3) | JP6982499B2 (en) |
| KR (2) | KR20210096688A (en) |
| CN (2) | CN113317430B (en) |
| AU (4) | AU2016225278B2 (en) |
| BR (1) | BR112017017941B1 (en) |
| CA (1) | CA2977617C (en) |
| CL (1) | CL2017002161A1 (en) |
| IL (2) | IL296049B2 (en) |
| MX (2) | MX390478B (en) |
| PL (1) | PL3261455T3 (en) |
| TW (1) | TWI699374B (en) |
| WO (1) | WO2016135458A1 (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017059352A1 (en) | 2015-10-02 | 2017-04-06 | Wm. Wrigley Jr. Company | Confections containing allulose |
| WO2018029351A1 (en) | 2016-08-12 | 2018-02-15 | Pfeifer & Langen GmbH & Co. KG | Liquid allulose composition |
| WO2018081557A2 (en) | 2016-10-28 | 2018-05-03 | Tate & Lyle Ingredients Americas Llc | Method for producing allulose crystals |
| WO2018109464A1 (en) | 2016-12-13 | 2018-06-21 | Tate & Lyle Ingredients Americas Llc | Modifying or enhancing a flavor of food and beverage products |
| FR3061414A1 (en) * | 2017-01-05 | 2018-07-06 | Roquette Freres | CRYSTALLISABLE SYRUP OF D-ALLULOSE |
| FR3061415A1 (en) * | 2017-01-05 | 2018-07-06 | Roquette Freres | NON-CRYSTALLISABLE SYRUP OF D-ALLULOSE |
| CN110049682A (en) * | 2016-12-09 | 2019-07-23 | Cj第一制糖株式会社 | Acidified milk comprising the carbohydrate containing high-content psicose |
| JP2019528775A (en) * | 2016-10-07 | 2019-10-17 | シージェイ チェイルジェダン コーポレーションCj Cheiljedang Corporation | Sweetness composition with improved taste quality containing allulose and salt and method for improving taste quality of allulose using salt |
| WO2019241583A1 (en) | 2018-06-14 | 2019-12-19 | Seattle Gummy Company | Low glycemic composition and methods of making and using thereof |
| WO2019241146A1 (en) | 2018-06-11 | 2019-12-19 | Seattle Gummy Company | Low glycemic gummy composition and methods of making and using thereof |
| US10912322B2 (en) | 2016-03-09 | 2021-02-09 | Cj Cheiljedang Corporation | Allulose-containing syrup composition and food containing same |
| EP3295808B1 (en) | 2015-05-15 | 2021-06-16 | Samyang Corporation | Saccharide mixture containing psicose with improved sweetness quality and crystallization |
| EP3261455B1 (en) | 2015-02-24 | 2022-01-26 | Tate & Lyle Ingredients Americas LLC | Allulose syrups |
| US11746392B2 (en) | 2020-11-23 | 2023-09-05 | Savanna Ingredients Gmbh | Drying of allulose crystals |
| WO2024047121A1 (en) | 2022-09-01 | 2024-03-07 | Savanna Ingredients Gmbh | Process for the preparation of a particulate allulose composition |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7031825B2 (en) | 2016-10-06 | 2022-03-08 | シージェイ チェイルジェダン コーポレーション | Tomato ketchup with improved storage stability |
| KR102004914B1 (en) | 2016-10-10 | 2019-07-30 | 씨제이제일제당 (주) | Plant-soaked solutions containing allulose and method for preparation thereof |
| US11812769B2 (en) | 2016-12-21 | 2023-11-14 | Cj Cheiljedang Corporation | Amino acid beverage containing allulose |
| WO2019004554A1 (en) * | 2017-06-30 | 2019-01-03 | 주식회사 삼양사 | Method for producing functional crystalline sweetener |
| KR102016701B1 (en) * | 2017-06-30 | 2019-09-06 | 주식회사 삼양사 | Method of preparing crystalline functional sweetener |
| EP3701808A4 (en) * | 2017-10-27 | 2021-06-02 | Samyang Corporation | ALLULOSE SYRUP AND ITS MANUFACTURING PROCESS |
| KR102215445B1 (en) * | 2017-10-31 | 2021-02-17 | 씨제이제일제당 주식회사 | Syrup comprising saccharide comprising allulose and citrus extract and method for preparation thereof |
| WO2019088632A2 (en) * | 2017-10-31 | 2019-05-09 | 씨제이제일제당 (주) | Composition, for preparing chocolate sauce, comprising allulose and use thereof |
| KR102246960B1 (en) * | 2017-11-24 | 2021-04-30 | 주식회사 삼양사 | Hot source with low calory |
| EP3753945A4 (en) * | 2018-02-12 | 2021-12-01 | Samyang Corporation | FUNCTIONAL CRYSTALLINE SWEETENER PRODUCTION PROCESS |
| MX2021008806A (en) * | 2019-01-22 | 2021-08-24 | Hershey Co | Filling composition for a confectionery product. |
| MX2022005275A (en) * | 2019-10-31 | 2022-08-17 | Samyang Corp | Improved method for manufacturing allulose. |
| KR102389709B1 (en) | 2019-11-29 | 2022-04-22 | 씨제이제일제당 주식회사 | Composition for producing allulose and method of producing allulose using thereof |
| KR102332373B1 (en) * | 2019-11-29 | 2021-11-29 | 씨제이제일제당 주식회사 | A composition comprising allulose dimer for inhibiting production of HMF |
| US20230220502A1 (en) | 2020-06-05 | 2023-07-13 | Savanna Ingredients Gmbh | Allulose syrup |
| EP4211142B1 (en) | 2020-09-07 | 2025-11-05 | Savanna Ingredients GmbH | Extrusion process for the preparation of a solid allulose composition |
| CN112285228B (en) * | 2020-10-19 | 2022-05-03 | 秦皇岛海关技术中心 | Method for identifying adulterated honey |
| KR102860681B1 (en) * | 2020-12-30 | 2025-09-16 | 주식회사 삼양사 | Allulose with improved stability |
| MX2023015530A (en) * | 2021-06-17 | 2024-03-27 | Ocean Spray Cranberries Inc | RARE SUGARS IN FOOD AND BEVERAGES. |
| CN119365086A (en) * | 2022-06-29 | 2025-01-24 | 株式会社三养社 | Allulose composition with excellent stability |
| TW202426055A (en) * | 2022-12-22 | 2024-07-01 | 美商玉米製品開發公司 | Liquid allulose syrup with improved stability |
| WO2025194080A1 (en) * | 2024-03-15 | 2025-09-18 | Santa Barbara Nutrients, Inc. | Combination of β-hydroxybutyrate and allulose for weight loss and kidney disease management |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5230742A (en) | 1987-02-02 | 1993-07-27 | A. E. Staley Manufacturing Co. | Integrated process for producing crystalline fructose and high-fructose, liquid-phase sweetener |
| US20120076908A1 (en) * | 2010-09-29 | 2012-03-29 | Matsutani Chemical Industry Co., Ltd. | Composition for improving taste of high-intensity sweetener and application thereof |
| US20140271746A1 (en) * | 2013-03-15 | 2014-09-18 | Tate & Lyle Ingredients Americas Llc | Sweetener |
| US20140271748A1 (en) * | 2013-03-15 | 2014-09-18 | Tate & Lyle Ingredients Americas Llc | Sweetener |
| US20140271747A1 (en) * | 2013-03-15 | 2014-09-18 | Tate & Lyle Ingredients Americas Llc | Sweetener |
| WO2015094342A1 (en) | 2013-12-20 | 2015-06-25 | Roquette Freres | Protein food product comprising d-allulose |
| US20160302463A1 (en) | 2013-11-22 | 2016-10-20 | Tate & Lyle Ingredients Americas Llc | Food and beverage products comprising allulose (psicose) |
| AU2016225278B2 (en) | 2015-02-24 | 2020-03-12 | Tate & Lyle Solutions Usa Llc | Allulose syrups |
Family Cites Families (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4263052A (en) | 1979-10-12 | 1981-04-21 | American Crystal Sugar Company | Production of fructose and useful by-products |
| US5039346A (en) | 1988-03-25 | 1991-08-13 | A. E. Staley Manufacturing Company | Fructose syrups and sweetened beverages |
| JP2876416B2 (en) * | 1990-01-31 | 1999-03-31 | 株式会社林原生物化学研究所 | Method for producing D-psicose |
| DE69321456T2 (en) | 1991-08-20 | 1999-06-17 | A.E. Staley Mfg. Co., Decatur, Ill. | Crystallization of fructose |
| JP4333094B2 (en) | 2002-07-16 | 2009-09-16 | 不二製油株式会社 | Concentrated crude soybean oligosaccharide liquid and process for producing the same |
| WO2005020721A1 (en) | 2003-08-25 | 2005-03-10 | Cargill, Incorporated | Beverage compositions comprising monatin and methods of making same |
| US7435564B2 (en) | 2003-09-29 | 2008-10-14 | Instituto Technologico Y De Estudios Superiores De Monterrey | Production of invert syrup from sugarcane juice using immobilized invertase |
| JP4761424B2 (en) | 2004-03-17 | 2011-08-31 | 株式会社希少糖生産技術研究所 | L-psicose crystal, method for producing the same, and sugar reagent kit |
| KR100744479B1 (en) | 2005-06-01 | 2007-08-01 | 씨제이 주식회사 | Method of producing Psychos by Psychos Epimerase |
| US20070059422A1 (en) | 2005-09-13 | 2007-03-15 | Robbins Gregory C | Disaccharide sweetener compounds, process for manufacture, and method of selecting components for dissacaride sweetener compounds based on user specific sweetener applications |
| KR101339443B1 (en) | 2005-11-15 | 2013-12-06 | 가부시기가이샤하야시바라 | Ketose 3-epimerase, process for production thereof, and use thereof |
| US8435587B2 (en) | 2005-11-23 | 2013-05-07 | The Coca-Cola Company | High-potency sweetener composition with long-chain primary aliphatic saturated alcohol and compositions sweetened therewith |
| CA2629556A1 (en) | 2005-11-23 | 2007-07-19 | The Coca Cola Company | Synthetic sweetener compositions with improved temporal profile and/or flavor profile, methods for their formulation, and uses |
| US8057840B2 (en) | 2006-01-25 | 2011-11-15 | Tate & Lyle Ingredients Americas Llc | Food products comprising a slowly digestible or digestion resistant carbohydrate composition |
| US10869494B2 (en) * | 2006-11-10 | 2020-12-22 | Matsutani Chemical Industry Co., Ltd. | Sweetener containing D-psicose and foods and drinks obtained by using the same |
| KR100832339B1 (en) | 2006-12-11 | 2008-05-26 | 솔젠트 (주) | Novel Shinorizobium spp. Converting fructose to psychose and method for producing psychose |
| JP4931741B2 (en) | 2007-09-04 | 2012-05-16 | アサマ化成株式会社 | Saccharide composition and method for producing the same |
| KR101523701B1 (en) | 2008-03-12 | 2015-06-01 | 주식회사 케이엠더블유 | Enclosure of wireless communication device |
| US9109266B2 (en) | 2009-03-30 | 2015-08-18 | Matsutani Chemical Industry Co., Ltd. | Process of producing sugar composition comprising D-psicose and D-allose via strong alkaline isomerization of D-glucose/D-fructose or alkaline pre-treatment of D-glucose/D-fructose followed by isomerization in the presence of a basic ion exchange resin |
| KR20110035805A (en) | 2009-09-30 | 2011-04-06 | 씨제이제일제당 (주) | Immobilization of Pseudomonas-Epimerase and Method for Producing Psychos using the Same |
| KR101159934B1 (en) | 2009-12-30 | 2012-06-25 | 주식회사 삼양제넥스 | Stable composition for preventing of crystalizatoin or turbid of fructooligosaccharide syrup |
| KR101189640B1 (en) * | 2010-03-26 | 2012-10-12 | 씨제이제일제당 (주) | How to Make D-Pycosy Crystals |
| JP2011200201A (en) | 2010-03-26 | 2011-10-13 | Kishoto Seisan Gijutsu Kenkyusho:Kk | Sugar paste including composite crystalline carbohydrate comprising plurality of saccharides, method for producing the same, and application of the sugar paste |
| JP2011205913A (en) | 2010-03-29 | 2011-10-20 | Kishoto Seisan Gijutsu Kenkyusho:Kk | Complex crystalline sugar of functional isomerized sugar and method for producing the same |
| CA2821116C (en) * | 2010-12-13 | 2021-01-19 | Ting Liu Carlson | Glycoside blends |
| JP5922880B2 (en) | 2011-04-28 | 2016-05-24 | 松谷化学工業株式会社 | Crystalline carbohydrate, production method and use thereof |
| WO2013039365A2 (en) | 2011-09-15 | 2013-03-21 | 씨제이제일제당(주) | Sweetener composition for alleviating diabetes, containing slowly digestible ingredient |
| JP6099870B2 (en) * | 2012-01-06 | 2017-03-22 | 松谷化学工業株式会社 | Novel sweetener containing sucrose and D-psicose |
| JP6041420B2 (en) * | 2012-02-29 | 2016-12-07 | 三井製糖株式会社 | Sugar solution and method for producing the same |
| WO2014119718A1 (en) * | 2013-01-31 | 2014-08-07 | 国立大学法人香川大学 | Processed konjak food or drink having effect of inhibiting increase in blood sugar level |
| US20140322389A1 (en) * | 2013-03-14 | 2014-10-30 | Indra Prakash | Beverages containing rare sugars |
| JP6077711B2 (en) | 2013-03-15 | 2017-02-08 | ザ プロクター アンド ギャンブル カンパニー | Personal care articles containing soluble fibers |
| JP6335883B2 (en) | 2013-04-08 | 2018-05-30 | 松谷化学工業株式会社 | Method for enhancing salty taste of food and drink, food and drink obtained by the method, and salty taste enhancer |
| CN103288887B (en) | 2013-06-04 | 2016-01-27 | 北京大学 | A kind of method being prepared ketose by aldose |
| JP6212316B2 (en) * | 2013-07-24 | 2017-10-11 | 松谷化学工業株式会社 | Method of masking off-flavor and odor of food and drink and food and drink obtained by the method |
-
2016
- 2016-02-19 KR KR1020217023575A patent/KR20210096688A/en active Pending
- 2016-02-19 AU AU2016225278A patent/AU2016225278B2/en not_active Withdrawn - After Issue
- 2016-02-19 EP EP24206540.7A patent/EP4529777A3/en active Pending
- 2016-02-19 MX MX2017010860A patent/MX390478B/en unknown
- 2016-02-19 CN CN202110500368.4A patent/CN113317430B/en active Active
- 2016-02-19 BR BR112017017941-5A patent/BR112017017941B1/en active IP Right Grant
- 2016-02-19 US US15/552,944 patent/US11653688B2/en active Active
- 2016-02-19 PL PL16708198.3T patent/PL3261455T3/en unknown
- 2016-02-19 JP JP2017544758A patent/JP6982499B2/en active Active
- 2016-02-19 EP EP16708198.3A patent/EP3261455B8/en active Active
- 2016-02-19 KR KR1020177024973A patent/KR102283524B1/en active Active
- 2016-02-19 EP EP22150616.5A patent/EP4042873B8/en active Active
- 2016-02-19 WO PCT/GB2016/050422 patent/WO2016135458A1/en not_active Ceased
- 2016-02-19 CN CN201680011915.XA patent/CN107567283B/en active Active
- 2016-02-19 CA CA2977617A patent/CA2977617C/en active Active
- 2016-02-19 IL IL296049A patent/IL296049B2/en unknown
- 2016-02-23 TW TW105105256A patent/TWI699374B/en active
-
2017
- 2017-08-23 IL IL254126A patent/IL254126B2/en unknown
- 2017-08-23 MX MX2022002697A patent/MX2022002697A/en unknown
- 2017-08-24 CL CL2017002161A patent/CL2017002161A1/en unknown
-
2020
- 2020-06-11 AU AU2020203857A patent/AU2020203857A1/en not_active Abandoned
- 2020-08-25 JP JP2020141468A patent/JP7096536B2/en active Active
-
2021
- 2021-03-26 AU AU2021101558A patent/AU2021101558A4/en not_active Expired
-
2022
- 2022-06-08 JP JP2022092690A patent/JP2022120036A/en active Pending
-
2023
- 2023-04-17 US US18/135,465 patent/US20230248038A1/en active Pending
- 2023-10-20 AU AU2023251517A patent/AU2023251517A1/en not_active Withdrawn
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5230742A (en) | 1987-02-02 | 1993-07-27 | A. E. Staley Manufacturing Co. | Integrated process for producing crystalline fructose and high-fructose, liquid-phase sweetener |
| US20120076908A1 (en) * | 2010-09-29 | 2012-03-29 | Matsutani Chemical Industry Co., Ltd. | Composition for improving taste of high-intensity sweetener and application thereof |
| US20140271746A1 (en) * | 2013-03-15 | 2014-09-18 | Tate & Lyle Ingredients Americas Llc | Sweetener |
| US20140271748A1 (en) * | 2013-03-15 | 2014-09-18 | Tate & Lyle Ingredients Americas Llc | Sweetener |
| US20140271747A1 (en) * | 2013-03-15 | 2014-09-18 | Tate & Lyle Ingredients Americas Llc | Sweetener |
| US20160302463A1 (en) | 2013-11-22 | 2016-10-20 | Tate & Lyle Ingredients Americas Llc | Food and beverage products comprising allulose (psicose) |
| WO2015094342A1 (en) | 2013-12-20 | 2015-06-25 | Roquette Freres | Protein food product comprising d-allulose |
| US20160331014A1 (en) | 2013-12-20 | 2016-11-17 | Roquette Freres | Protein food product comprising d-allulose |
| AU2016225278B2 (en) | 2015-02-24 | 2020-03-12 | Tate & Lyle Solutions Usa Llc | Allulose syrups |
Non-Patent Citations (33)
| Title |
|---|
| "Alternative sweeteners. 4th ed.", 2012, CRC PRESS, article "Specificatin and analysis", pages: 412 - 413, XP055794259 |
| AHMADREZA RAISI ET AL: "Effects of Influence Parameters on Color Formation in Glucose Syrups during Storage", AMIRKABIR / MISC, vol. 42, no. 1, 2010, pages 57 - 61, XP055794423 |
| ANONYMOUS: "GRAS Notice (GRN) No. 498", 12 June 2014 (2014-06-12), XP055449544, Retrieved from the Internet <URL:http://wayback.archive-it.org/7993/20171031042510/https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM387347.pdf> |
| BERNARD J. FREEDMAN: "Sulphur dioxide in foods and beverages: Its use as a preservative and its effect on asthma", BR J DIS CHEST., vol. 74, no. 2, April 1980 (1980-04-01), pages 128 - 134, XP023293731 |
| BERNARD J. FREEDMAN: "SULPHUR DIOXIDE IN FOODS AND BEVERAGES: ITS USE AS A PRESERVATIVE AND ITS EFFECT ON ASTHMA", BR,Y. DIS. CHEST, vol. 74, 1980, pages 128 - 134, XP023293731, DOI: 10.1016/0007-0971(80)90023-6 |
| BHAGAT SINGH ET AL: "The Role of 5-(Hydroxymethyl)-furfural in the Discoloration of Sugar Solutions", JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, vol. 70, February 1948 (1948-02-01), pages 517 - 522, XP055323191 |
| CATHLEEN M. DOBBS ET A: "Storage stability of tagatose in buffer solutions of various compositions", FOOD RESEARCH INTERNATIONAL, vol. 43, 2010, pages 382 - 386, XP026813741 |
| CATHLEEN M. DOBBS, LEONARD N. BELL: "Storage stability of tagatose in buffer solutions of various compositions", FOOD RESEARCH INTERNATIONAL, vol. 43, 2010, pages 382 - 386, XP026813741 |
| EMMA J. MCDONALD: "Stability of Dextrose Solutions of Varying pH", JOURNAL OF RESEARCH OF THE NATIONAL BUREAU AL STANDARDS, vol. 45, no. 3, September 1950 (1950-09-01), pages 200, XP055794414 |
| FDA: "Gras notification of allulose (psicose)", GRAS NOTICE (GRN) NO. 498, 2014, XP055794251, Retrieved from the Internet <URL:https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM387347.pdf> |
| HIROMICHI KATO ET AL: "Mechanisms of Browning Degradation of D-Fructose in SpecialComparison with D-Glucose-Glycine Reaction", AGR. BIOL. CHEM., vol. 33, no. 6, June 1969 (1969-06-01), pages 939 - 948, XP055793297 |
| HISAKA OSHIMA ET AL: "Factors Affecting Psicose Formation in Food Products during Cooking", FOOD SCIENCE AND TECHNOLOGY RESEARCH, vol. 20, no. 2, 2014, pages 423 - 430, XP055794358 |
| HISAKA OSHIMA, YUKA OZAKI, YUKA KITAKUBO, SHIGERU HAYAKAWA: "Decrease in the D- Psicose Content of Processed Foods Fortified with a Rare Sugar", FOOD SCIENCE AND TECHNOLOGY RESEARCH, vol. 20, no. 2, 2014, pages 415 - 421, XP055449504 |
| ISBT: "HIGH FRUCTOSE SYRUPS 42 & 55 QUALITY GUIDELINES AND ANALYTICAL PROCEDURES", ISBT - INTERNATIONAL SOCIETY OF BEVERAGE TECHNOLOGISTS, April 2013 (2013-04-01), XP055794401, Retrieved from the Internet <URL:https://www.isbt.com/> |
| J. M. DE BRUIJN ET AL: "Alkaline degradation of monosaccharides V*: Kinetics of the alkaline isomerization and degradation of monosaccharides", REEL. TRAV. CHIM. PAYS-BAS, vol. 106, no. 2, February 1987 (1987-02-01), pages 35 - 43, XP055670903 |
| JEANNE-MARIE MEMBRE ET AL: "Combined Effects of pH and Sugar on Growth Rate of Zygosaccharomyces rowcii, a Bakery Product Spoilage Yeast", APPLIED AND ENVIRONMENTAL MICROBIOLOGY, vol. 65, no. 11, November 1999 (1999-11-01), pages 4921 - 4925, XP055794418 |
| JOSEPH A. MATHEWS ET AL: "The stability of levulose in aqueos solutions of varying pH", RESEARCH PAPER RP611, PART OF BUREAU OF STANDARDS JOURNAL OF RESEARCH, vol. 11, 1 November 1933 (1933-11-01), pages 619 - 633, XP055449534 |
| JOSEPH A. MATHEWS, RICHARD F. JACKSON: "The Stability of Levulose in Aqueous Solutions of Varying pH", RESEARCH PAPER RP611, PART OF BUREAU OF STANDARDS JOURNAL OF RESEARCH, vol. 11, November 1933 (1933-11-01), pages 619 - 633, XP055449534 |
| JOSEPH B BINDER; ANTHONY V CEFALI; JACQUELINE J BLANK AND RONALD T RAINES: "Mechanistic insights on the conversion of sugars into 5-hydroxymethylfurfural", ENERGY AND ENVIRONMENTAL SCIENCE, vol. 3, 13 May 2010 (2010-05-13), pages 765 - 771, XP002728785 |
| JOSEPH B. BINDER ET AL: "Mechanistic insights on the conversion of sugars into 5-hydroxymethylfurfural", ENERGY & ENVIRONMENTAL SCIENCE, vol. 3, no. 6, June 2010 (2010-06-01), pages 765 - 771, XP002728785, DOI: 10.1039/B923961H |
| KATSUHARU YASUMATSU ET AL: "Stabilities of Enzymes in Polyhydric Alcohols", AGR. BIOL. CHEM., vol. 29, no. 7, 1954, pages 665 - 671, XP055587663, DOI: 10.1080/00021369.1965.10858443 |
| KEARSLEY M. W., DZIEDZIC S. Z: "HANDBOOK OF STARCH HYDROLYSIS PRODUCTS AND THEIR DERIVATIVES", 1995, CHAPMAN & HALL, article "5.10 Colour formation", pages: 136, 138, XP055794260 |
| KEIKO TOKUOKA ET AL: "MINIMUM WATER ACTIVITIES FOR THE GROWTH OF YEASTS ISOLATED FROM HIGH-SUGAR FOODS", J. GEN. APPL. MICROBIOL., vol. 37, 1991, pages 111 - 119, XP055794404 |
| N. J. RUSSELL ET AL: "Food Preservatives Second Edition", 2003, KLUWER ACADEMIC, article "sulfiting agent", pages: 342, XP055794370 |
| N.J. RUSSELL, G.W. GOULD: "Food Preservatives Second Edition", 2003, KLUWER ACADEMIC/ PLENUM PUBLISHERS, pages: 342, XP055449532 |
| PAMELA J. WHITE ET AL: "Corn. Chemistry and Technology. 2nd ed.", 2003, article "Table 12 and 13", pages: 660 - 661, XP055794354 |
| R. S. SHALLENBERGER & L. R. MATTICK: "Relative Stability of Glucose and Fructose at Different Acid pH", FOOD, vol. 12, 1983, pages 159 - 165, XP055449516 |
| R.S. SHALLENBERGER, L.R. MATTICK: "Relative Stability of Glucose and Fructose at Different Acid pH", FOOD CHEMISTRY, vol. 12, 1983, pages 159 - 165, XP055449516 |
| SEUNG HEE BAEK ET AL: "Maillard Browning Reaction of D-Psicose as Affected by ReactionFactors", FOOD SCI. BIOTECHNOLOGY, vol. 17, no. 6, 1 January 2008 (2008-01-01), pages 1349 - 1351, XP055449512 |
| SEUNG HEE BAEK, SO YOUNG KWON, HYEON GYU LEE, HYUNG HEE BAEK: "Maillard Browning Reaction ofd-Psicose as affected by Reaction Factors", FOOD SCIENCE AND BIOTECHNOLOGY, vol. 17, no. 6, 2008, pages 1349 - 1351, XP055449512 |
| TIKAM CHAND DAKAL ET AL: "Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii", INTERNATIONAL JOURNAL OF FOOD MICROBIOLOGY, vol. 185, 25 May 2014 (2014-05-25), pages 140 - 157, XP028877745 |
| Y. H. HUI: "Handbook of Food Products Manufacturing. Principles, Bakery, Beverages, Cereals, Cheese, Confectionary, Fats, Fruits, and Functional Foods", 2007, WILEY-INTERSCIENCE, article "Carbonated beverages", pages: 430, XP055794367 |
| Y.H. HUI: "Handbook of Food Products Manufacturing", 2007, JOHN WILEY & SONS, INC., pages: 430, XP055449522 |
Cited By (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4042873B1 (en) | 2015-02-24 | 2024-10-16 | Tate & Lyle Solutions USA LLC | Allulose syrups |
| EP3261455B1 (en) | 2015-02-24 | 2022-01-26 | Tate & Lyle Ingredients Americas LLC | Allulose syrups |
| EP4042873A1 (en) | 2015-02-24 | 2022-08-17 | Tate & Lyle Solutions USA LLC | Allulose syrups |
| EP3295808B1 (en) | 2015-05-15 | 2021-06-16 | Samyang Corporation | Saccharide mixture containing psicose with improved sweetness quality and crystallization |
| WO2017059352A1 (en) | 2015-10-02 | 2017-04-06 | Wm. Wrigley Jr. Company | Confections containing allulose |
| US10912322B2 (en) | 2016-03-09 | 2021-02-09 | Cj Cheiljedang Corporation | Allulose-containing syrup composition and food containing same |
| EP4461142A3 (en) * | 2016-08-12 | 2024-12-25 | Savanna Ingredients GmbH | Liquid allulose composition |
| WO2018029351A1 (en) | 2016-08-12 | 2018-02-15 | Pfeifer & Langen GmbH & Co. KG | Liquid allulose composition |
| JP7108601B2 (en) | 2016-08-12 | 2022-07-28 | サヴァンナ・イングレディエンツ・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング | Liquid allulose composition |
| EP3496551B1 (en) * | 2016-08-12 | 2024-10-02 | Savanna Ingredients GmbH | Liquid allulose composition |
| JP2019524139A (en) * | 2016-08-12 | 2019-09-05 | ファイファー・ウント・ランゲン・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング・ウント・コンパニー・コマンディートゲゼルシャフト | Liquid allulose composition |
| JP2019528775A (en) * | 2016-10-07 | 2019-10-17 | シージェイ チェイルジェダン コーポレーションCj Cheiljedang Corporation | Sweetness composition with improved taste quality containing allulose and salt and method for improving taste quality of allulose using salt |
| US11653684B2 (en) | 2016-10-07 | 2023-05-23 | Cj Cheiljedang Corporation | Sweetener composition with improved taste quality comprising allulose and salt and method for improving taste quality of alulose using salt |
| US12264176B2 (en) | 2016-10-28 | 2025-04-01 | Tate & Lyle Solutions Usa Llc | Method for producing allulose crystals |
| US11548907B2 (en) | 2016-10-28 | 2023-01-10 | Tate & Lyle Solutions Usa Llc | Method for producing allulose crystals |
| EP4233562A2 (en) | 2016-10-28 | 2023-08-30 | Tate & Lyle Solutions USA LLC | Method for producing allulose crystals |
| WO2018081557A2 (en) | 2016-10-28 | 2018-05-03 | Tate & Lyle Ingredients Americas Llc | Method for producing allulose crystals |
| EP3552493A4 (en) * | 2016-12-09 | 2020-06-24 | Cj Cheiljedang Corporation | FERMENTED MILK COMPRISING SACCHARIDES CONTAINING A HIGH RATE OF ALLULOSIS |
| CN110049682A (en) * | 2016-12-09 | 2019-07-23 | Cj第一制糖株式会社 | Acidified milk comprising the carbohydrate containing high-content psicose |
| WO2018109464A1 (en) | 2016-12-13 | 2018-06-21 | Tate & Lyle Ingredients Americas Llc | Modifying or enhancing a flavor of food and beverage products |
| WO2018127669A1 (en) | 2017-01-05 | 2018-07-12 | Roquette Freres | Crystallisable d-allulose syrups |
| US11439168B2 (en) | 2017-01-05 | 2022-09-13 | Roquette Freres | Non-crystallisable D-allulose syrups |
| EP3565419B1 (en) | 2017-01-05 | 2024-05-08 | Roquette Frères | Crystallisable d-allulose syrups |
| WO2018127670A1 (en) | 2017-01-05 | 2018-07-12 | Roquette Freres | Non-crystallisable d-allulose syrups |
| FR3061415A1 (en) * | 2017-01-05 | 2018-07-06 | Roquette Freres | NON-CRYSTALLISABLE SYRUP OF D-ALLULOSE |
| FR3061414A1 (en) * | 2017-01-05 | 2018-07-06 | Roquette Freres | CRYSTALLISABLE SYRUP OF D-ALLULOSE |
| US11766062B2 (en) | 2017-01-05 | 2023-09-26 | Roquette Freres | Crystallisable d-allulose syrups |
| EP4378325A3 (en) * | 2017-01-05 | 2024-08-07 | Roquette Freres | Crystallizable syrups for d-allulose |
| EP4378325A2 (en) | 2017-01-05 | 2024-06-05 | Roquette Freres | Crystallizable syrups for d-allulose |
| EP3801531A4 (en) * | 2018-06-11 | 2022-04-20 | Seattle Gummy Company | LOW GLYCEMIC GUMMY COMPOSITION AND METHODS OF MAKING AND USING THEREOF |
| WO2019241146A1 (en) | 2018-06-11 | 2019-12-19 | Seattle Gummy Company | Low glycemic gummy composition and methods of making and using thereof |
| EP3806657A4 (en) * | 2018-06-14 | 2022-03-30 | Seattle Gummy Company | LOW GLYCEMIC INDEX COMPOSITIONS AND METHODS OF MAKING AND USING THEREOF |
| WO2019241583A1 (en) | 2018-06-14 | 2019-12-19 | Seattle Gummy Company | Low glycemic composition and methods of making and using thereof |
| US11746392B2 (en) | 2020-11-23 | 2023-09-05 | Savanna Ingredients Gmbh | Drying of allulose crystals |
| US12325886B2 (en) | 2020-11-23 | 2025-06-10 | Savanna Ingredients Gmbh | Drying of allulose crystals |
| WO2024047122A1 (en) | 2022-09-01 | 2024-03-07 | Savanna Ingredients Gmbh | Process for the preparation of a particulate allulose composition |
| WO2024047121A1 (en) | 2022-09-01 | 2024-03-07 | Savanna Ingredients Gmbh | Process for the preparation of a particulate allulose composition |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2021101558A4 (en) | Allulose syrups | |
| CA2906893C (en) | Improved sweetener | |
| US20220144878A1 (en) | Liquid allulose composition | |
| AU2014229724A1 (en) | Improved sweetener | |
| GB2536304B (en) | Allulose syrups |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16708198 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 122022006954 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 2977617 Country of ref document: CA Ref document number: 2017544758 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15552944 Country of ref document: US Ref document number: MX/A/2017/010860 Country of ref document: MX Ref document number: 254126 Country of ref document: IL |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112017017941 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 20177024973 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2016225278 Country of ref document: AU Date of ref document: 20160219 Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2016708198 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 112017017941 Country of ref document: BR Kind code of ref document: A2 Effective date: 20170822 |