WO2016055602A1 - Production of melonal or 2,6-dimethylhept-5-enal via baeyer-villiger oxidation - Google Patents
Production of melonal or 2,6-dimethylhept-5-enal via baeyer-villiger oxidation Download PDFInfo
- Publication number
- WO2016055602A1 WO2016055602A1 PCT/EP2015/073341 EP2015073341W WO2016055602A1 WO 2016055602 A1 WO2016055602 A1 WO 2016055602A1 EP 2015073341 W EP2015073341 W EP 2015073341W WO 2016055602 A1 WO2016055602 A1 WO 2016055602A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- water
- dienal
- dimethylocta
- formate
- equivalents
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C45/00—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds
- C07C45/51—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by pyrolysis, rearrangement or decomposition
- C07C45/54—Preparation of compounds having >C = O groups bound only to carbon or hydrogen atoms; Preparation of chelates of such compounds by pyrolysis, rearrangement or decomposition of compounds containing doubly bound oxygen atoms, e.g. esters
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C67/00—Preparation of carboxylic acid esters
- C07C67/39—Preparation of carboxylic acid esters by oxidation of groups which are precursors for the acid moiety of the ester
Definitions
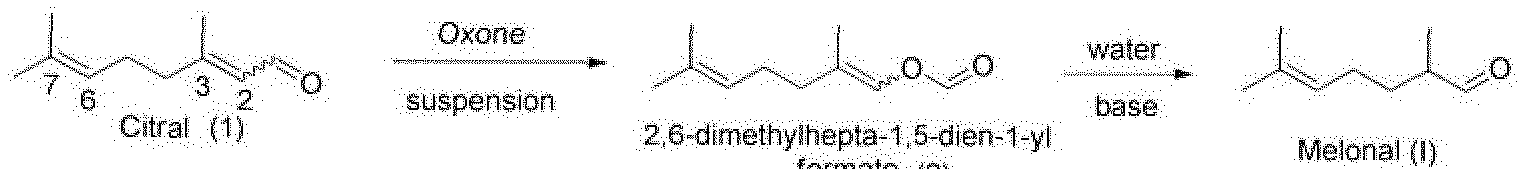
- the present invention is concerned with a novel process for the production of 2,6- dimethylhept-5-enal, also known as Melonal, which is an important, aldehydic, melon- like odorant for the fragrance industry.
- the present invention relates to a process for the synthesis Melonal by Bayer-Villiger oxidation of (E/Z)-3,7-dimethylocta- 2,6-dienal, also known as Citral, in the presence of potassium peroxymonosulfate, via the intermediate 2,6-dimethylhepta-1 ,5-dien-1 -yl formate.
- heterogeneous catalysts need to be prepared in a separate process. Accordingly, there remains a need for a simple and cheap process for the production of Melonal in an olfactory pure form.
- Melonal can be prepared starting from citral by Bayer- 5 Villiger oxidation with potassium peroxymonosulfate (KHS0 5 ) via the intermediate 2,6- dimethylhepta-1 ,5-dien-1-yl formate, resulting in high yields and high purity of the reaction product.
- KHS0 5 potassium peroxymonosulfate
- a process comprising the oxidation of 3,7- 1 dimethylocta-2,6-dienal (1 ) in the presence of potassium peroxymonosulfate suspended in a solvent selected from amides, such as ⁇ , ⁇ -dimethyl formamide (D F),N,N- dimethyl acetamide (DMAC), N-methylpyrrolidinone (NMP), ketones with about 0.5 to 5 equivalents of water (e.g. 1.5 equivalents of water), such as acetone with 0.5-5.0 equivalent of water (e.g. 1.5 equivalents of water), esters with about 0.5 to 5
- amides such as ⁇ , ⁇ -dimethyl formamide (D F),N,N- dimethyl acetamide (DMAC), N-methylpyrrolidinone (NMP), ketones with about 0.5 to 5 equivalents of water (e.g. 1.5 equivalents of water), such as acetone with 0.5-5.0 equivalent of water (e.g. 1.5 equivalents of water), esters with about 0.5 to 5
- KHS0 5 potassium peroxymonosulfate
- the triple salt with 0 the formula 2KHS0 5 ⁇ KHS0 4 ⁇ K 2 S0 4 (MW 615), which is, e.g., commercially available under the trade name Oxone ® ).
- Oxone ® a source of potassium peroxymonosulfate
- the potassium peroxymonsulfate is either added at once in form of a suspension or part of the potassium peroxymonsulfate may be successively added to the reaction.
- the amount of potassium peroxymonosulfate is at least 0.6 5 equivalents of Oxone ® suspended in an amide solvent, such as such as N,N-dimethyl formamide (DMF),N,N-dimethyl acetamide (DMAC), N-methylpyrrolidinone (NMP).
- an amide solvent such as such as N,N-dimethyl formamide (DMF),N,N-dimethyl acetamide (DMAC), N-methylpyrrolidinone (NMP).
- the reactions may be carried out at different temperatures, preferably from about 0°C to about 60°C, e.g. at room temperature or at about WC to about 40°C.
- Oxone ® 33,9 g, 55.0 mmol
- ethyl acetate 120 ml
- water 1.80 g, 100 mmol
- 3,7 ⁇ Dimethylocta-2,6-dienal (15.2 g, 100 mmol) was added dropwise in 5 min. The addition was not exothermal.
- the slurry reaction mixture was vigorously stirred at room temperature for 20 hours. The reaction was monitored by GC (results are given, in the Table 1 below).
- Example 5 1 Oxone ® in acetonitrile
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Disclosed is a process for the production of 2,6-dimethylhept-5-enal by Baeyer-Villiger oxidation of 3,7-dimethylocta-2,6-dienal in the presence of potassium peroxymonosulfate suspended in a solvent selected followed by hydrolysis.
Description
PRODUCTION OF MELONAL OR 2,6-DIMETHYLHEPT-5-ENAL VIA
BAEYER-VILLIGER OXIDATION
The present invention is concerned with a novel process for the production of 2,6- dimethylhept-5-enal, also known as Melonal, which is an important, aldehydic, melon- like odorant for the fragrance industry. In particular, the present invention relates to a process for the synthesis Melonal by Bayer-Villiger oxidation of (E/Z)-3,7-dimethylocta- 2,6-dienal, also known as Citral, in the presence of potassium peroxymonosulfate, via the intermediate 2,6-dimethylhepta-1 ,5-dien-1 -yl formate. Up to now 2,6-dimethyl-5-heptenal is industrially produced by Darzens reaction from 6- methyl-hept-5-en-2-one. This reaction requires alkylation with chloro acetic esters to giyctdesters' followed by ftydrolysis rtcf decarboxylation leading to the aldehyde: This approach is not atom economic as an alcohol (ROH), a chloride salt, as well as carbon dioxide is eliminated from the reactants.
Some years ago Corma at al (J. Catal. 2005, 234. 96) reported a heterogeneous Baeyer-Villiger reaction of citral (1 ) applying zeolites and other mosoprous materials followed by hydrolysis of the intermediate formate ester. Unfortunately not only 2,6- di methyl hepta-1 ,5-dien-1-yl formate (2), but also olfactory disturbing side products are generated, such as 6-methylhept-5-en-2-one, citral-6,7-epoxide, citral-2,3-epoxide and various cyclization products of citral, as shown Scheme 1.
Scheme 1 :
Furthermore, such heterogeneous catalysts need to be prepared in a separate process.
Accordingly, there remains a need for a simple and cheap process for the production of Melonal in an olfactory pure form.
Surprisingly, inventors found that Melonal can be prepared starting from citral by Bayer- 5 Villiger oxidation with potassium peroxymonosulfate (KHS05) via the intermediate 2,6- dimethylhepta-1 ,5-dien-1-yl formate, resulting in high yields and high purity of the reaction product.
It was found that only if a suspension of - potassium peroxymonosulfate in amides, i o ketones with water, nitrites with water, or esters with water is used, the reaction resulted in 2,6-dimethylhepta-1 ,5-dien-1-yi formate in high yields and high purity of the reaction product.
Thus there is provided in a first aspect a process comprising the oxidation of 3,7- 1 dimethylocta-2,6-dienal (1 ) in the presence of potassium peroxymonosulfate suspended in a solvent selected from amides, such as Ν,Ν-dimethyl formamide (D F),N,N- dimethyl acetamide (DMAC), N-methylpyrrolidinone (NMP), ketones with about 0.5 to 5 equivalents of water (e.g. 1.5 equivalents of water), such as acetone with 0.5-5.0 equivalent of water (e.g. 1.5 equivalents of water), esters with about 0.5 to 5
0 equivalents of water (e.g. 1.5 equivalents of water), such as ethyl acetate with 0.5-5.0 equivalent of water (e.g.1 5 equivalents of water), and nitriles with about 0.5 to 5 equivalents of water (e.g.1 .5 equivalents of water), such as acetonitrile with 0.5-5.0 equivalent of water (e.g. 1 .5 equivalents of water), resulting in 2,6-dimethylhepta-1 ,5- dien-1 -yl formate (2). In a subsequent step the formate (2) is hydrolyzed to melonal (I). 5
ormate (2)
As a source of potassium peroxymonosulfate (KHS05) one may use the triple salt with 0 the formula 2KHS05 · KHS04 · K2S04 (MW 615), which is, e.g., commercially available under the trade name Oxone®). Preferably about 1 - 3 equivalents of KHS05 (or 0 5 - 1 .5 equivalents of Oxone®) based on the substrate (1 ) are used. The potassium
peroxymonsulfate is either added at once in form of a suspension or part of the potassium peroxymonsulfate may be successively added to the reaction.
In one embodiment the amount of potassium peroxymonosulfate is at least 0.6 5 equivalents of Oxone® suspended in an amide solvent, such as such as N,N-dimethyl formamide (DMF),N,N-dimethyl acetamide (DMAC), N-methylpyrrolidinone (NMP).
As is well established, by "equivalent(s)" or "eq." is meant mol equivalents based on the substrate (3,7-dimethylocta-2,6-dienal (1)).
10
The reactions may be carried out at different temperatures, preferably from about 0°C to about 60°C, e.g. at room temperature or at about WC to about 40°C.
1 5 The invention is now further described with reference to the following non-limiting examples. These examples are for the purpose of illustration only and it is understood that variations and modifications can be made by one skilled in the art.
All products described in the examples were obtained starting from (E/Z)-3,7- 20 dimethylocta-2,6-dienal (1). The first reaction product of the Baeyer-Villiger reaction, 2,6-dimethylhepta-1 ,5-dien-1-yl formate (2), is a mixture of E/Z isomers.
The reported NMR spectra were measured in CDCI3 at 300 MHz if not otherwise stated; chemical shifts (δ) are reported in ppm downfield from TMS; coupling constants J in Hz. 25 The GC/MS analyses were run using a HB-5 column, if not stated otherwise. All purified products were purified by distillation in vacuo and isolated as colorless oils.The purity was confirmed by GC/MS.
-^o Example 1.1 : Oxone^ in DMF
To the suspension of Oxone® (24 3 g, 39 5 mmol, 0.60 eq.) in DMF {70.0 ml) with a water bath at 23°C was added 3.7-dimethylocta-2,6-dienal (10.0 g, 65.7 mmol) in 10 minutes and the mixture was stirred for 90 minutes (conversion of 3,7-dimethylocta- 2,6-dienal: 93%), during which time the inside temperature reached 32°C and then
35 dropped to room temperature.
Another 4.0 g Oxone® (6.5 mmol, 0.10 eq.) was added and the mixture was stirred at 25°C for 1 hour (3,7-dimethylocta-2,6-dienal was converted completely.).
The mixture was filtered through a small pad of silica gel. Water (200 g) was added to the filtrate. The mixture was extracted with isohexane (3*150 mL). The combined organic phase was dried over MgSO„ and concentrated. The residue (9.5 g) was distilled by Kugelrohr to furnish 2,6-dimethylhepta-1 ,5-dien-1-yl formate (7.7 g, 42,7 mmol, 65% yield) (Mixtures of E/2 isomers in a ratio of 72:28) as a colorless liquid .
1H N R (CDCI3): δ = 1.61 (S, 3 H, CH3), 1.69 (s, 3 H, CH3), 1.71 , 1.66 (s, 3 H, CH3), 1.97-2.22 (m, 4 H, ~CH2-CH2-). 5.03-5.14 (m, 1 H, C-CH=C), 6.94, 6.98 (s, 1 H, C=C CH-O). (E)-isomer (major): 13C NMR (CDCI3): 6 = 13.7 (q), 17.7 (q), 25.6 (q), 26.1 (t), 34.0 (t), 123'.4 {a)V'T24.5' '(s), T28 J '(ø*), 132':2 '{s), T58', T (d). '"©-isomer (major): MS:' m/z (%) = 44 (49), 55 (1 1), 69 (100), 81 (22), 107 (8), 122 (32) [M+ - CHO], Example 1.2:
The procedure as described in Example 1.1) was repeated with varying amounts of Oxone®. The results are given in Table 2 below.
Example 2.1 : Oxone® in DMAC
To a solution of 3,7-dimethylocta-2,6-dienal (6.0 g, 39.4 mmol, 1.0 eq. ) in DMAC (50 ml) was added Oxone® (19.4 g, 31.5 mmol, 0.80 eq.) in one portion. The suspension was stirred at room temperature for 90 minutes. GC analysis indicated 3,7- dimethylocta-2,6-dienal was converted completely. The mixture was filtered and the solid was washed with 100 mL petroleum ether. The filtrate was washed with water (150 mL). The aqueous phase was extracted by petroleum ether (2*150 mL). The combined organic phase was dried over MgS04 and concentrated, and the residue (4.2 g) was distilled by Kugelrohr distillation to furnish 2,6-dimethylhepta-1 ,5-dien-1-yl formate (3.6 g, 21.4 mmol, 54% yield) as a colorless liquid .
Example 2 2: Oxone® in NMP
To the suspension of Oxone® (28.3 g, 46.0 mmol, 0.70 eq.) in NMP (70 ml) with a water bath at 23°C was added 3,7-dimethylocta-2,6-dienal (10 g, 65.7 mmol, 1.0 eq.) in 5 minutes and the mixture was stirred for 180 minutes (conversion of 3,7-dimethylocta-
2,6-dienal: 85%), Another 4.0 g Oxone® (6.5 mmol, 0.10 eq.) was added and the mixture was stirred at 2SX for 1 hour (conversion of 3,7-dimethylocta-2,6-dienal: 95%). The mixture was filtered through a small pad of silica gel. Water (2.00 g) was added to the filtrate. The mixture was extracted with isohexane (3* 50 mL). The combined organic phase was dried over gS0 and concentrated. The residue (9.5 g) was distilled by Kugelrohr to furnish 2,6-dimethylhepta-1 ,5-dien-1-yl formate (6,1 g, 33,8 mmol, 51% yield} as a colorless liquid.
Example 3.1 : Oxone® in acetone
In a 250 mL three-necked round-bottomeed flask was added Oxone® (33.9 g, 55.0 mrrrot) n acetone (80· ml) to give a white suspension.' With vigorously stirring, "water (1.80 g, 100 mmol) was added to the suspension. 3.7-Dimethylocta-2,6-dienal (15.2 g, 100 mmol) was added dropwise in 5 minutes. The addition was not exothermal. The slurry reaction mixture was vigorously stirred at room temperature for 4 hours. The reaction was monitored by GC. After one hour the conversion of 3,7-dimethylocta-2,6- dienal was 49% (GC selectivity = (desired product) / (desired product + all other byproducts) = 88%). After 4 hours, GC conversion was 81.8% (GC selectivity = (desired product) / (desired product + all other by-products) = 82.5%). The conversion stopped and the reaction mixture was filtered and the white solid was washed with 3*10 mL acetone. The combined filtrates were tested by peroxide content test paper. The peroxide content was 10 ppm. Sodium bisulfite (5.2 g, 5.0 mmol) and water (10 mL) were added to the acetone solution. The mixture was stirred at room temperature until peroxide test indicated negative result (about 30 minutes). gS0 (5.0 g) was added and the suspension was filtered. The filtrate was concentrated to give 20.0 g yellow cloudy liquid. The liquid was dissolved in MTBE (100 mL), washed once with brine (50 mL) and dried by MgSO„. Concentration gave 17.5 g light yellow liquid. Kugelrohr distillation gave a colorless liquid (10.6 g) (boiling point: 75-85 degree/0.25 mbar). GC purity of the 2.6-dimethylhepta-1 ,5-dien-1-yl formate is 90% and with 1.5% of melonal and with some others are unreacted citral. Yield 57%.
Example 3.2 - 3.3;
The procedure as described in Example 3.1) was repeated with varying amounts of water. The results are given in Table 2 below.
ExgfflpJeA 1 Oxone® in .ethyl, acetate
In a 250 mL three-necked round-bottomeed flask was added Oxone® (33,9 g, 55.0 mmol) in ethyl acetate (120 ml) to give a white suspension. With vigorously stirring, water (1.80 g, 100 mmol) was added to the suspension. 3,7~Dimethylocta-2,6-dienal (15.2 g, 100 mmol) was added dropwise in 5 min. The addition was not exothermal. The slurry reaction mixture was vigorously stirred at room temperature for 20 hours. The reaction was monitored by GC (results are given, in the Table 1 below). After 20 hours additional 9.0 g, 4.0 g and 4.0 g of Oxone® were successively added in portions to the reaction mixture within 4 hours. The reaction mixture was filtered and the filtrate was washed once with sodium bisulfite ( 0.4 g, 10.0 mmol) in water (80 mL). After the washing, the peroxide test paper indicated negative result. The organic phase was further washed- Once -witft-brine (SO ml), -dried by Mg-SQ-4-(5-.©-g-)-a-nd concentration- to remove the solvent resulting in a light yellow liquid (18,0 g) of the organic phase.
Kugelrohr distillation gave a colorless liquid (12.6 g). Boiling point: 75-85 degree/0,25 mbar. GC purity of the 2,&-dimethylhepta-1 ,5-dien-1-yl formate product is 95% and with some of melonal and unreacted citral. Yield 71 %.
Table 1 :
Example 4.2:
The procedure as described in Example 4.1) was repeated in the absence of water. The results are given in Table 2 below.
Example 5:1 Oxone® in acetonitrile
In a 250 mL three-necked round-bottomeed flask was added Oxone® (33.9 g, 55.0 mmol) in acetonitrile (80 ml) to give a white suspension. With vigorously stirring, water (1.80 g, 100 mmo!) was added to the suspension, 3,7-Dimethylocta-2,6-dienal (15.2 g, 100 mmol) was added dropwise in 5 minutes. The slurry reaction mixture was vigorously stirred at room temperature for 4 hours. The reaction was monitored by GC. After one hour the conversion of 3,7-dimethylocta-2,6-dienal was 82%. The reaction mixture was filtered and the white solid was washed with 3*10 mL acetonitrile. The filtrate was concentrated to give 18.0 g yellow cloudy liquid. The liquid was dissolved in MTBE (100 mL), washed once with brine (50 mL) and dried by MgS04. Concentration gave 17.5 g light yellow liquid. Kugelrohr distillation gave 2,6-dimethylhepta-1 ,5-dier 1- yfT©rmate'*'as*a*c©teftess liquid (7.6 ~g)* (boiling' point: 75-85 *degreei¾ 25*mter). GG* purity of the 2,6-dimethylhepta-1 ,5-dien-1-yl formate is 80% and with some others are unreacted citral. Yield 36%.
Example 5.2:
The procedure as described in Example 5.1) was repeated in the absence of water. The results are given in Table 2 below.
Example 6 (comparison): Oxone in methanol
Following the general procedure as described in Example 1 , To the suspension of Oxone® (24.3 g, 39.5 mmol, 0.60 eq.) in methanol (70 ml) was added 3,7-dimethylocta- 2,6-dienal (10 g, 65.7 mmol, 1.0 eq.) in 10 minutes and the mixture was stirred at 23°C for 120 minutes (conversion of 3,7-dimethylocta-2,6-dienal was 78%). The reaction was a very comple mixture and the GC selectivity (GC selectivity = (desired product) / (desired product + all other by-products) of the 2,6-dimethylhepta-1 ,5-dien-1-yl formate was lower than 5%.
Table 2: Summary of the results {Example 1 - 8)
Water Oxone® Time Conversion Yield entry solvent
_Je¾J 1) _ (hours) (%)
1 DMF 0.55 1.0 79 n. d.
2 DMF - 0.60 1.0 93 n. d.
3 DMF - 0.70 2.5 100 65
4 DMAC _ 0.80 1.5 100 54
5 N P - 0.80 4.0 95 51
8 Acetone - 0.55 1.0 5.0 n. d.
7 Acetone 1.0 0 55 1.0 49 n. d.
Acetone †:0~ 0.55 4.0 82 57
9 Acetone 10 0.55 1.0 90 <10
10 Ethyl acetate 0.55 2.0 0 n. d.
11 Ethyl acetate 1.0 0.55 1.0 10.0 n. d
12 Ethyl acetate 1.0 0.55 20 75 62
13 Ethyl acetate 1.0 0.80 30 92 71
14 CH3CN - 0 55 1.0 3.7 <1
15 CH3CN 1.0 0.55 1.0 82 36
16 Methanol 0.60 2.0 78 <5
1) Mol equivalents based on 3,7-dimethylocta-2,6-dienal (1 )
2) n. d. = not determined
As can be seen from the results above, the addition of water is important for the process in solvents such as acetone, ethyl acetate and acetonitrile. But when water content is too larger (greater than 10 equivalent), the process gave poor selectivity of the desired 2,6-dimethylhepta-1 ,5-dien-1-yl formate product.
Example 7: Conversion, of 2, 6-dimethylhepta-1.5-dien-1-yl formate to melonal The 2,6-dimethylhepta-1,5-dien-1-yl formate obtained according to one of the processes as described above was hydrolysized using NaOH solution resulting in melonal (99% purity).
Claims
1. A process for the production of 2,6-dimethylhept-5-enai by Baeyer-Villiger oxidation of 3,7-dimethylocta-2,6-dienal in the presence of potassium peroxymonosulfate suspended in a solvent selected from amides, ketones with 0.5 - 5.0 equivalents of water, esters with 0.5 - 5,0 equivalents of water and nitriles with 0.5 - 5,0 equivalents of water, resulting in 2,6-dimethylhepta-1 ,5-dien-1-yl formate, followed by hydrolysis.
2. A process according to claim 1 wherein the amides are selected from N,N-di methyl formamide (DMF),N,N-dimethyl acetamide (D AC), N-methylpyrrolidinone (NMP).
3. A process according to claim 1 wherein the ketone is acetone.
4. A process according to claim 1 wherein the ester is ethyl acetate.
5. A process according to claim 1 wherein the nitrile is acetonitrile.
6. A process according to claim 1 wherein the resulting 2,6-dimethylhepta-1 ,5-dien-1-yl formate is hydrolysed in the presence of NaOH.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/CN2014/088266 WO2016054797A1 (en) | 2014-10-10 | 2014-10-10 | Process |
| CNPCT/CN2014/088266 | 2014-10-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016055602A1 true WO2016055602A1 (en) | 2016-04-14 |
Family
ID=54288798
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2014/088266 WO2016054797A1 (en) | 2014-10-10 | 2014-10-10 | Process |
| PCT/EP2015/073341 WO2016055602A1 (en) | 2014-10-10 | 2015-10-09 | Production of melonal or 2,6-dimethylhept-5-enal via baeyer-villiger oxidation |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2014/088266 WO2016054797A1 (en) | 2014-10-10 | 2014-10-10 | Process |
Country Status (1)
| Country | Link |
|---|---|
| WO (2) | WO2016054797A1 (en) |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003060852A2 (en) * | 2002-01-15 | 2003-07-24 | Michigan State University | Catalytic osmium-assisted oxidative cleavage of olefins |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8987497B2 (en) * | 2012-11-05 | 2015-03-24 | Basf Se | Process for the oxidation of organic carbonyl compounds |
-
2014
- 2014-10-10 WO PCT/CN2014/088266 patent/WO2016054797A1/en active Application Filing
-
2015
- 2015-10-09 WO PCT/EP2015/073341 patent/WO2016055602A1/en active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003060852A2 (en) * | 2002-01-15 | 2003-07-24 | Michigan State University | Catalytic osmium-assisted oxidative cleavage of olefins |
Non-Patent Citations (3)
| Title |
|---|
| B.R. TRAVIS ET AL.: "Facile oxidation of aldehydes to acids and estes with Oxone", ORGANIC LETTERS, vol. 5, no. 7, 3 August 2003 (2003-08-03), pages 1031 - 1034, XP002752189 * |
| CORMA A ET AL: "A new, alternative, halogen-free synthesis for the fragrance compound Melonal using zeolites and mesoporous materials as oxidation catalysts", JOURNAL OF CATALYSIS, ACADEMIC PRESS, DULUTH, MN, US, vol. 234, no. 1, 15 August 2005 (2005-08-15), pages 96 - 100, XP004997345, ISSN: 0021-9517, DOI: 10.1016/J.JCAT.2005.06.006 * |
| CORMA, J. CATAL., vol. 234, 2005, pages 96 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2016054797A1 (en) | 2016-04-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Kimel et al. | The rearrangement of allyl-type esters of β-keto acids | |
| Becker et al. | Synthesis of Ninhydrin1, 2 | |
| JPS6113449B2 (en) | ||
| CN107108445B (en) | Intermediate compounds for the production of perfuming ingredients | |
| US5489694A (en) | Preparation of R/S-γ-lipoic acid or R/S-α-lipoic acid | |
| CA2505094A1 (en) | Continuous process for producing pseudoionones and ionones | |
| WO2016055602A1 (en) | Production of melonal or 2,6-dimethylhept-5-enal via baeyer-villiger oxidation | |
| JP5442001B2 (en) | Method for producing high purity terminal olefin compound | |
| US6984742B2 (en) | Method for preparing polyprenyl compounds | |
| JP2019112380A (en) | Method for producing 3,7-dimethyl-7-octenol and method for producing 3,7-dimethyl-7-octenyl carboxylate compound | |
| JP4687464B2 (en) | Process for producing tetrahydropyran-4-one and pyran-4-one | |
| JPWO2006075596A1 (en) | Method for producing 2-allylcarboxylic acid compound | |
| WO2015110505A1 (en) | Process for the production of 2,6-dimethylhept-5-enal by baeyer-villiger oxidation | |
| JP4520203B2 (en) | Method for producing polyprenyl compound | |
| JP7279702B2 (en) | Method for producing aromatic hydroxy compound | |
| KR101613065B1 (en) | Synthetic method of dihydroquinoline derivatives | |
| JP2682687B2 (en) | New thiophene compounds and their manufacture | |
| EP3470388A1 (en) | Synthesis of aliphatic alcohols as aroma chemicals | |
| CN109195942B (en) | Method for preparing polyssantalol compounds | |
| EP0568621A1 (en) | Process for the production of cyanoacetic acid | |
| JPH1059892A (en) | Production of alpha,beta-unsaturated aldehyde | |
| US3093692A (en) | Preparation of cyclohexene fluoride-1 | |
| JP4545478B2 (en) | Piran production method | |
| JP2843430B2 (en) | Cyclic sesquiterpene and method for producing the same | |
| SU602490A1 (en) | Method of preparing 4-tretbutylpyrocatechol |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15777942 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15777942 Country of ref document: EP Kind code of ref document: A1 |