WO2015041428A1 - Organic electronic element using compound for organic electronic element and electronic device thereof - Google Patents
Organic electronic element using compound for organic electronic element and electronic device thereof Download PDFInfo
- Publication number
- WO2015041428A1 WO2015041428A1 PCT/KR2014/008399 KR2014008399W WO2015041428A1 WO 2015041428 A1 WO2015041428 A1 WO 2015041428A1 KR 2014008399 W KR2014008399 W KR 2014008399W WO 2015041428 A1 WO2015041428 A1 WO 2015041428A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- organic
- layer
- light emitting
- mmol
- Prior art date
Links
- 0 CC1(C)C(C=CC2(C3C2)N(C2=CCC(*(c4c5cccc4)C4=CC6c7ccccc7SC6C=C4)C5=C2)c2c(cccc4)c4ccc2)=C3c2c1cccc2 Chemical compound CC1(C)C(C=CC2(C3C2)N(C2=CCC(*(c4c5cccc4)C4=CC6c7ccccc7SC6C=C4)C5=C2)c2c(cccc4)c4ccc2)=C3c2c1cccc2 0.000 description 25
- MOCNGNGLTRMQQH-UHFFFAOYSA-N Brc(cc1c2c3cccc2)ccc1[n]3-c(cc1)ccc1-c1ccccc1 Chemical compound Brc(cc1c2c3cccc2)ccc1[n]3-c(cc1)ccc1-c1ccccc1 MOCNGNGLTRMQQH-UHFFFAOYSA-N 0.000 description 1
- YNZHZTUNICBTSY-UHFFFAOYSA-N CC(C)(C)[IH]c(cc1)cc2c1c1ccccc1[n]2[AlH2] Chemical compound CC(C)(C)[IH]c(cc1)cc2c1c1ccccc1[n]2[AlH2] YNZHZTUNICBTSY-UHFFFAOYSA-N 0.000 description 1
- XUPKKTPEBKMLPS-UHFFFAOYSA-N CC(C)(c1ccccc1-c1c2)c1ccc2N(C1C=C2C=CC=CC2CC1)c(cc1c2c3cccc2)ccc1[n]3-c1cc2ccccc2cc1 Chemical compound CC(C)(c1ccccc1-c1c2)c1ccc2N(C1C=C2C=CC=CC2CC1)c(cc1c2c3cccc2)ccc1[n]3-c1cc2ccccc2cc1 XUPKKTPEBKMLPS-UHFFFAOYSA-N 0.000 description 1
- NJXDAKUYTRAHCM-UHFFFAOYSA-N CC(C)(c1ccccc1-c1c2)c1ccc2Nc(cc1)ccc1-c1ccccc1 Chemical compound CC(C)(c1ccccc1-c1c2)c1ccc2Nc(cc1)ccc1-c1ccccc1 NJXDAKUYTRAHCM-UHFFFAOYSA-N 0.000 description 1
- IPWKHHSGDUIRAH-UHFFFAOYSA-N CC1(C)OB(B2OC(C)(C)C(C)(C)O2)OC1(C)C Chemical compound CC1(C)OB(B2OC(C)(C)C(C)(C)O2)OC1(C)C IPWKHHSGDUIRAH-UHFFFAOYSA-N 0.000 description 1
- YOSVAJGXTKGHLU-UHFFFAOYSA-N CC1(C)c2ccc(C(CCc(cc3)cc4c3c(C=CC(C3)N(C5C=CC=CC5)c5ccccc5)c3[s]4)c3c(-c4ccccc4)nc(-c4ccccc4)nc3)cc2-c2c1cccc2 Chemical compound CC1(C)c2ccc(C(CCc(cc3)cc4c3c(C=CC(C3)N(C5C=CC=CC5)c5ccccc5)c3[s]4)c3c(-c4ccccc4)nc(-c4ccccc4)nc3)cc2-c2c1cccc2 YOSVAJGXTKGHLU-UHFFFAOYSA-N 0.000 description 1
- XKZXLBHFFAWWBV-UHFFFAOYSA-N CC1(C)c2ccc(C(c(cc3)ccc3-c(cc3)ccc3-[n]3c4ccccc4c4ccccc34)c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)cc2-c2ccccc12 Chemical compound CC1(C)c2ccc(C(c(cc3)ccc3-c(cc3)ccc3-[n]3c4ccccc4c4ccccc34)c(cc3c4c5cccc4)ccc3[n]5-c3ccccc3)cc2-c2ccccc12 XKZXLBHFFAWWBV-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C211/00—Compounds containing amino groups bound to a carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C211/00—Compounds containing amino groups bound to a carbon skeleton
- C07C211/43—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
- C07C211/57—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings being part of condensed ring systems of the carbon skeleton
- C07C211/58—Naphthylamines; N-substituted derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C211/00—Compounds containing amino groups bound to a carbon skeleton
- C07C211/43—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
- C07C211/57—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings being part of condensed ring systems of the carbon skeleton
- C07C211/61—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton having amino groups bound to carbon atoms of six-membered aromatic rings being part of condensed ring systems of the carbon skeleton with at least one of the condensed ring systems formed by three or more rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
- C07D209/80—[b, c]- or [b, d]-condensed
- C07D209/82—Carbazoles; Hydrogenated carbazoles
- C07D209/86—Carbazoles; Hydrogenated carbazoles with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to carbon atoms of the ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
- C07D209/80—[b, c]- or [b, d]-condensed
- C07D209/82—Carbazoles; Hydrogenated carbazoles
- C07D209/88—Carbazoles; Hydrogenated carbazoles with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to carbon atoms of the ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/04—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/14—Carrier transporting layers
- H10K50/15—Hole transporting layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/80—Constructional details
- H10K50/805—Electrodes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/80—Constructional details
- H10K50/85—Arrangements for extracting light from the devices
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K59/00—Integrated devices, or assemblies of multiple devices, comprising at least one organic light-emitting element covered by group H10K50/00
- H10K59/30—Devices specially adapted for multicolour light emission
- H10K59/32—Stacked devices having two or more layers, each emitting at different wavelengths
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/621—Aromatic anhydride or imide compounds, e.g. perylene tetra-carboxylic dianhydride or perylene tetracarboxylic di-imide
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/633—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising polycyclic condensed aromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/636—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising heteroaromatic hydrocarbons as substituents on the nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/02—Ortho- or ortho- and peri-condensed systems
- C07C2603/04—Ortho- or ortho- and peri-condensed systems containing three rings
- C07C2603/06—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members
- C07C2603/10—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members containing five-membered rings
- C07C2603/12—Ortho- or ortho- and peri-condensed systems containing three rings containing at least one ring with less than six ring members containing five-membered rings only one five-membered ring
- C07C2603/18—Fluorenes; Hydrogenated fluorenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/93—Spiro compounds
- C07C2603/94—Spiro compounds containing "free" spiro atoms
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/623—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing five rings, e.g. pentacene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/626—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing more than one polycyclic condensed aromatic rings, e.g. bis-anthracene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/654—Aromatic compounds comprising a hetero atom comprising only nitrogen as heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6574—Polycyclic condensed heteroaromatic hydrocarbons comprising only oxygen in the heteroaromatic polycondensed ring system, e.g. cumarine dyes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6576—Polycyclic condensed heteroaromatic hydrocarbons comprising only sulfur in the heteroaromatic polycondensed ring system, e.g. benzothiophene
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/549—Organic PV cells
Definitions
- the present invention relates to an organic electric device using the compound for organic electric devices and an electronic device thereof.
- organic electroluminescence display (organic EL display device) using a light emitting material for the light emitting element of the display portion has been actively developed.
- the organic EL display device unlike a liquid crystal display device or the like, recombines holes and electrons injected into an anode and a cathode in a light emitting layer, thereby emitting a light emitting material containing an organic compound in the light emitting layer to realize display. Display device.
- organic electroluminescent element In an organic electroluminescent element (hereinafter referred to as an "organic EL element"), it has recently been proposed to be composed of a plurality of layers having different characteristics such as a light emitting layer and a layer for transporting carriers (holes, electrons) to the light emitting layer. .
- the hole transport layer has excellent hole transport capacity and carrier resistance.
- various hole transport materials have been proposed.
- Patent Document 1 using a carbazole derivative as a hole transport layer and an amine compound having deuterium are used as a structure in which a simple aryl group is substituted with the Patent Documents 2 and 3 amine structures used as host materials for the hole transport material and the light emitting layer.
- Patent Document 3 used as a transport material, Patent Document 4 and the like in which an amine compound having a fluorenyl group are used as a hole transport material or an injection material have been reported.
- Patent Document 0001 US Patent Application Publication No. 2007/0231503
- Patent Document 0002 International Publication No. 2012/091471
- Patent Document 0003 Korean Patent Application Publication No. 2000/0052560
- Patent Document 0004 International Publication No. 2010/110553
- An object of the present invention is to provide an organic electroluminescent device having improved device and efficiency by preventing interfacial degradation of the light emitting layer and increasing charge balance in the light emitting layer, and an organic light emitting material for realizing the same, and an electronic device using the same.
- a light emitting auxiliary layer (multiple hole transport layer) must exist between the hole transport layer and the light emitting layer. It is time to develop the auxiliary layer.
- electrons are transferred from the electron transport layer to the light emitting layer, and holes are transferred from the hole transport layer to the light emitting layer to form recombination in the light emitting layer to form excitons.
- positive polaron accumulates at the interface between the light emitting layer and the hole transport layer.
- interfacial deterioration occurs, resulting in longevity and efficiency.
- excess polaron in the light emitting layer attacks weak bonding of the light emitting material, which deforms the light emitting material, resulting in a decrease in lifespan and efficiency and a decrease in color purity. Indicates.
- the light emitting auxiliary layer should be a material having a suitable HOMO value between the light emitting layer and the hole transport layer in order to prevent the positive polaron from accumulating at the light emitting layer interface between the hole transport layer and the light emitting layer.
- it In order to increase the balance, it must be a material having hole mobility within a suitable driving voltage range (in the blue device driving voltage range of the full device).
- the present invention has been made in view of the above problems, and an object thereof is to provide an organic electroluminescent device having improved device and efficiency by preventing interfacial degradation of the light emitting layer and increasing charge balance in the light emitting layer, and an organic light emitting material for realizing the same.
- the present invention provides an organic electronic device using the compound represented by the following formula and an electronic device thereof.
- the organic material layer including at least a hole transport layer, a light emitting auxiliary layer and a light emitting layer, wherein the hole transport layer is present between the first electrode and the light emitting auxiliary layer.
- FIG. 1 is an exemplary view of an organic electroluminescent device according to the present invention.
- halo or halogen as used herein is fluorine (F), bromine (Br), chlorine (Cl) or iodine (I) unless otherwise indicated.
- alkyl or “alkyl group” has a single bond of 1 to 60 carbon atoms, unless otherwise indicated, and is a straight chain alkyl group, branched chain alkyl group, cycloalkyl (alicyclic) group, alkyl-substituted cyclo Radicals of saturated aliphatic functional groups, including alkyl groups, cycloalkyl-substituted alkyl groups.
- heteroalkyl group means that at least one of the carbon atoms constituting the alkyl group has been replaced with a heteroatom.
- alkenyl group or “alkynyl group”, unless stated otherwise, has a double or triple bond of 2 to 60 carbon atoms, and includes a straight or branched chain group, and is not limited thereto. It is not.
- cycloalkyl refers to alkyl forming a ring having 3 to 60 carbon atoms, without being limited thereto.
- alkoxyl group means an alkyl group to which an oxygen radical is attached, and unless otherwise specified, has a carbon number of 1 to 60, and is limited herein. It is not.
- alkenoxyl group means an alkenyl group to which an oxygen radical is attached, and unless otherwise stated, it is 2 to 60 It has carbon number of, It is not limited to this.
- aryloxyl group or “aryloxy group” means an aryl group to which an oxygen radical is attached, and unless otherwise specified, has a carbon number of 6 to 60, but is not limited thereto.
- aryl group and “arylene group” have a carbon number of 6 to 60 unless otherwise stated, but is not limited thereto.
- an aryl group or an arylene group means an aromatic of a single ring or multiple rings, and includes an aromatic ring formed by neighboring substituents participating in a bond or a reaction.
- the aryl group may be a phenyl group, a biphenyl group, a fluorene group, a spirofluorene group.
- aryl or "ar” means a radical substituted with an aryl group.
- an arylalkyl group is an alkyl group substituted with an aryl group
- an arylalkenyl group is an alkenyl group substituted with an aryl group
- the radical substituted with an aryl group has the carbon number described herein.
- an arylalkoxy group means an alkoxy group substituted with an aryl group
- an alkoxylcarbonyl group means a carbonyl group substituted with an alkoxyl group
- an arylcarbonylalkenyl group means an alkenyl group substituted with an arylcarbonyl group.
- the arylcarbonyl group is a carbonyl group substituted with an aryl group.
- heteroalkyl means an alkyl including one or more heteroatoms unless otherwise indicated.
- heteroaryl group or “heteroarylene group” means an aryl group or arylene group having 2 to 60 carbon atoms, each containing one or more heteroatoms, unless otherwise specified. It may include at least one of a single ring and multiple rings, and may be formed by combining adjacent functional groups.
- heterocyclic group includes one or more heteroatoms, unless otherwise indicated, and has from 2 to 60 carbon atoms, and includes at least one of single and multiple rings, heteroaliphatic rings and hetero Aromatic rings. Adjacent functional groups may be formed in combination.
- heteroatom refers to N, O, S, P or Si unless otherwise stated.
- Heterocyclic groups may also include rings comprising SO 2 in place of the carbon forming the ring.
- a “heterocyclic group” includes the following compounds.
- aliphatic as used herein means an aliphatic hydrocarbon having 1 to 60 carbon atoms
- aliphatic ring means an aliphatic hydrocarbon ring having 3 to 60 carbon atoms.
- ring refers to a fused ring consisting of an aliphatic ring having 3 to 60 carbon atoms or an aromatic ring having 6 to 60 carbon atoms or a hetero ring having 2 to 60 carbon atoms or a combination thereof. Saturated or unsaturated rings.
- heterocompounds or heteroradicals other than the aforementioned heterocompounds include, but are not limited to, one or more heteroatoms.
- carbonyl used in the present invention is represented by -COR ', wherein R' is hydrogen, an alkyl group having 1 to 20 carbon atoms, an aryl group having 6 to 30 carbon atoms, and 3 to 30 carbon atoms. Cycloalkyl group, an alkenyl group having 2 to 20 carbon atoms, an alkynyl group having 2 to 20 carbon atoms, or a combination thereof.
- ether as used herein is represented by -RO-R ', wherein R or R' are each independently of each other hydrogen, an alkyl group having 1 to 20 carbon atoms, It is an aryl group, a C3-C30 cycloalkyl group, a C2-C20 alkenyl group, a C2-C20 alkynyl group, or a combination thereof.
- substituted in the term “substituted or unsubstituted” as used in the present invention is deuterium, halogen, amino group, nitrile group, nitro group, C 1 ⁇ C 20 alkyl group, C 1 ⁇ C 20 alkoxyl group, C 1 ⁇ C 20 alkylamine group, C 1 ⁇ C 20 alkylthiophene group, C 6 ⁇ C 20 arylthiophene group, C 2 ⁇ C 20 alkenyl group, C 2 ⁇ C 20 alkynyl, C 3 ⁇ C 20 cycloalkyl group, C 6 ⁇ C 20 aryl group, of a C 6 ⁇ C 20 substituted by deuterium aryl group, a C 8 ⁇ C 20 aryl alkenyl group, a silane group, a boron Group, germanium group, and C 2 ⁇ C 20 It is meant to be substituted with one or more substituents selected from the group consist
- the substituent R 1 when a is an integer of 0, the substituent R 1 is absent, when a is an integer of 1, one substituent R 1 is bonded to any one of carbons forming the benzene ring, and a is an integer of 2 or 3 are each bonded as follows, where R 1 may be the same or different from each other, and when a is an integer from 4 to 6, it is bonded to the carbon of the benzene ring in a similar manner, while the indication of hydrogen bonded to the carbon forming the benzene ring Is omitted.
- FIG. 1 is an exemplary view of an organic electric device according to an embodiment of the present invention.
- the organic electric device 100 includes a first electrode 120, a second electrode 180, a first electrode 110, and a second electrode 180 formed on a substrate 110.
- the first electrode 120 may be an anode (anode)
- the second electrode 180 may be a cathode (cathode)
- the first electrode may be a cathode and the second electrode may be an anode.
- the organic layer may include a hole injection layer 130, a hole transport layer 140, a light emitting layer 150, an electron transport layer 160, and an electron injection layer 170 on the first electrode 120 in sequence. At this time, the remaining layers except for the light emitting layer 150 may not be formed.
- the hole blocking layer, the electron blocking layer, the light emitting auxiliary layer 151, the buffer layer 141 may be further included, and the electron transport layer 160 may serve as the hole blocking layer.
- the organic electric device according to the present invention may further include a protective layer or a light efficiency improving layer (Capping layer) formed on one surface of the at least one surface of the first electrode and the second electrode opposite to the organic material layer.
- a protective layer or a light efficiency improving layer Capping layer
- the organic electroluminescent device may be manufactured using a PVD method.
- the anode 120 is formed by depositing a metal or a conductive metal oxide or an alloy thereof on a substrate, and the hole injection layer 130, the hole transport layer 140, the light emitting layer 150, and the electron transport layer are formed thereon.
- the organic material layer including the 160 and the electron injection layer 170 it can be prepared by depositing a material that can be used as the cathode 180 thereon.
- the organic material layer is a solution or solvent process (e.g., spin coating process, nozzle printing process, inkjet printing process, slot coating process, dip coating process, roll-to-roll process, doctor blading) using various polymer materials. It can be produced in fewer layers by methods such as ding process, screen printing process, or thermal transfer method. Since the organic material layer according to the present invention may be formed in various ways, the scope of the present invention is not limited by the forming method.
- the organic electric element according to the present invention may be a top emission type, a bottom emission type or a double-sided emission type depending on the material used.
- WOLED White Organic Light Emitting Device
- Various structures for white organic light emitting devices mainly used as backlight devices have been proposed and patented. Representatively, a side-by-side method in which R (Red), G (Green) and B (Blue) light emitting parts are mutually planarized, and a stacking method in which R, G, and B light emitting layers are stacked up and down. And a color conversion material (CCM) method using photo-luminescence of an inorganic phosphor by using electroluminescence by a blue (B) organic light emitting layer and light therefrom. May also be applied to these WOLEDs.
- CCM color conversion material
- the organic electroluminescent device according to the present invention may be one of an organic electroluminescent device (OLED), an organic solar cell, an organic photoconductor (OPC), an organic transistor (organic TFT), a monochromatic or white illumination device.
- OLED organic electroluminescent device
- OPC organic photoconductor
- organic TFT organic transistor
- Another embodiment of the present invention may include a display device including the organic electric element of the present invention described above, and an electronic device including a control unit for controlling the display device.
- the electronic device may be a current or future wired or wireless communication terminal, and includes all electronic devices such as a mobile communication terminal such as a mobile phone, a PDA, an electronic dictionary, a PMP, a remote controller, a navigation device, a game machine, various TVs, and various computers.
- the organic material layer including at least a hole transport layer, a light emitting auxiliary layer, and a light emitting layer, wherein the hole transport layer is present between the first electrode and the light emitting auxiliary layer.
- Ar 0 to Ar 2 , and Ar 6 to Ar 11 are each independently i) a C 6 to C 60 aryl group; Fluorenyl group; C 2 ⁇ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; Fused ring group of an aromatic ring of C 3 ⁇ C 60 of aliphatic rings and C 6 ⁇ C 60; C 1 ⁇ C 50 Alkyl group; C 2 ⁇ C 20 Alkenyl group; Alkynyl groups of C 2 to C 20 ; C 1 -C 30 alkoxyl group; C 6 -C 30 aryloxy group; And -LN (R a ) (R b ); or ii) Ar 1 and Ar 2 may be bonded to each other to form a ring, wherein the 'ring' is an aliphatic C 3 to C 60 refers to a ring, a C 6 ⁇ C 60 aromatic, C 2 ⁇ C 60 of the heterocycle or a combination thereof is a
- Ar 4 and Ar 5 are i) independently of each other hydrogen; C 1 ⁇ C 50 Alkyl group; C 6 ⁇ C 60 Aryl group; Fluorenyl group; C 2 ⁇ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; or ii) Ar 4 and Ar 5 are bonded to each other to combine Together with fluorene it is possible to form spiro compounds.
- a, b, c, d, e, f and g are integers from 0 to 4,
- R 0 to R 6 are i) independently of each other deuterium; halogen; C 6 ⁇ C 60 Aryl group; Fluorenyl group; C 2 ⁇ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; Fused ring group of an aromatic ring of C 3 ⁇ C 60 of aliphatic rings and C 6 ⁇ C 60; C 1 ⁇ C 50 Alkyl group; C 2 ⁇ C 20 Alkenyl group; Alkynyl groups of C 2 to C 20 ; C 1 -C 30 alkoxyl group; C 6 -C 30 aryloxy group; And -LN (R a ) (R b ); or ii) when a, b, c, d, e, f and g are each an integer of 2 or more, R 0 to R 6 are each neighbor
- the substituents may be bonded to each other to form at least one ring, wherein the 'ring' is an aliphatic
- L 1 to L 6 and L are independently of each other a single bond; C 6 ⁇ C 60 arylene group; Fluorenylene groups; Fused ring group of an aromatic ring of C 3 ⁇ C 60 of aliphatic rings and C 6 ⁇ C 60; And a C 2 to C 60 heterocyclic group including at least one heteroatom of O, N, S, Si, and P; wherein L 3 to L 5 exclude a single bond.
- R a and R b are each independently a C 6 ⁇ C 60 aryl group; Fluorenyl group; Fused ring group of an aromatic ring of C 3 ⁇ C 60 of aliphatic rings and C 6 ⁇ C 60; And a C 2 -C 60 heterocyclic group including at least one heteroatom of O, N, S, Si, and P.
- the hole transport layer comprises a compound represented by the formula (1)
- the light emitting auxiliary layer is an organic electric device, characterized in that one of the compounds represented by the formula (2) to formula (4) to provide.
- the compound represented by Formula 2 of the light emitting auxiliary layer may be one of the following compounds.
- the compound represented by Formula 3 of the light emitting auxiliary layer may be one of the following compounds.
- the compound represented by Formula 4 of the light emitting auxiliary layer may be one of the following compounds.
- the compound represented by Formula 1 of the hole transport layer may be one of the following compounds.
- an organic electric device comprising a light efficiency improving layer formed on at least one side of the one side of the first electrode opposite to the organic material layer or one side of the second electrode opposite to the organic material layer
- the organic material layer is provided by any one of a spin coating process, a nozzle printing process, an inkjet printing process, a slot coating process, a dip coating process and a roll-to-roll process do.
- the organic electroluminescent device according to the present invention is at least one of an organic electroluminescent device (OLED), an organic solar cell, an organic photoconductor (OPC), an organic transistor (organic TFT), and a device for monochrome or white illumination It can be one.
- OLED organic electroluminescent device
- OPC organic photoconductor
- organic TFT organic transistor
- a device for monochrome or white illumination It can be one.
- the compound represented by Formula 1 according to the present invention may be synthesized by the reaction route of Scheme 1 below, but is not limited thereto.
- the L * is an L 5 or L 6 as defined in Ar 3 of formula (1).
- Sub 1 of Scheme 1 may be synthesized by the reaction route of Scheme 2, but is not limited thereto.
- S1 to S7 are as follows.
- Sub 2 of Scheme 1 may be synthesized by the reaction route of Scheme 12, but is not limited thereto.
- Sub 3 of Scheme 1 may be synthesized by the reaction route of Scheme 19, but is not limited thereto.
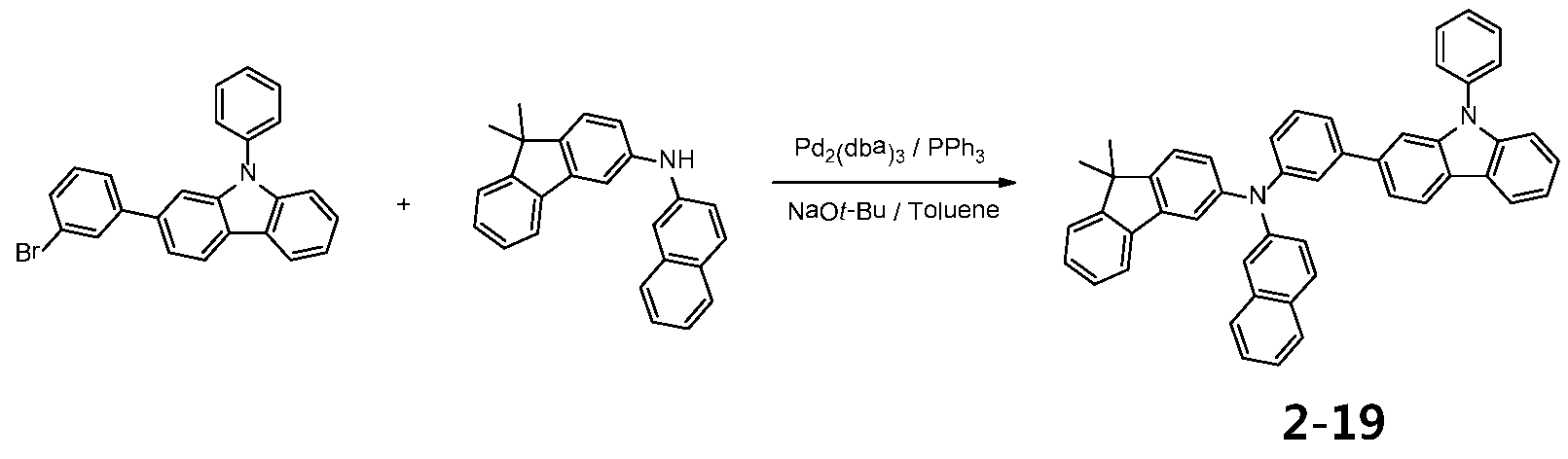
- N- (4'-bromo- [1,1'-biphenyl] -4-yl) -N-phenylnaphthalen-1-amine (10.8 g, 24 mmol) is dissolved in toluene, followed by N-phenylnaphthalen-1-amine (4.4 g, 20 mmol), Pd 2 (dba) 3 (0.05 equiv), PPh 3 (0.1 equiv) and NaO t -Bu (3 equiv) were then added and stirred under reflux at 100 ° C. for 24 hours.
- Compound represented by Formula 2 according to the present invention (Final Product) may be synthesized by the reaction route of Scheme 23, but is not limited thereto.
- Sub 4 of Scheme 23 may be synthesized by the reaction route of Scheme 24, but is not limited thereto.
- Sub 5 of Scheme 23 may be synthesized by the reaction route of Scheme 25, but is not limited thereto.
- methyl-4-chloro-2-hydroxybenzoate (100g, 537mmol) is dissolved in 1000mL of methylene chloride and triethylamine (113mL, 805mmol) is added.
- Trifluoromethanesulfonic anhydride (99 mL, 590 mmol) is added dropwise at -78 ° C and the temperature is gradually raised to room temperature. After the reaction, the mixture was extracted with water and the organic layer was removed with MgSO 4 and filtered. Remove organic solvent, dissolve with ether, separate silicagel column, remove dark color, remove solvent and vacuum dry.
- Methyl-4-chloro-2-(((trifluoromethyl) sulfonyl) oxy) benzoate (156g, 492mmol), Phenylbronicacid (60g, 492mmol), Pd (PPh 3 ) 4 (11g, 10mmol) and potassium carbonate ( 102 g, 738 mmol) and 1000 mL of DMF were added and stirred at 110 ° C. After the reaction, the mixture was extracted with ether and water, and the organic layer was removed with MgSO 4 and filtered. The organic solvent is removed, the silicagel column is separated and the solvent is removed and dried in vacuo.
- the compound represented by Chemical Formula 3 according to the present invention may be synthesized by the reaction route of Scheme 32, but is not limited thereto.
- Sub 6 of Scheme 32 may be synthesized by the reaction route of Scheme 33, but is not limited thereto.
- Sub 6-2 (16.46 g, 98.4 mmol) obtained in the above synthesis was dissolved in toluene in a round bottom flask, followed by 4-bromo-4'-iodo-1,1'-biphenyl (70.68 g, 196.9 mmol) and Pd 2 (dba) 3 (2.7 g, 3 mmol), 50% P ( t -Bu) 3 (3.8 ml, 7.9 mmol), NaO t -Bu (28.38 g, 295.3 mmol) was added and stirred at 70 ° C.
- Sub 7 of Scheme 40 may be synthesized by the reaction route of Scheme 41, but is not limited thereto.
- N 1- (naphthalen-2-yl) -N 4 , N 4 -bis (4- (naphthalen-2-yl (phenyl) amino) phenyl) -N 1- phenylbenzene-1,4-diamine (hereinafter abbreviated as "2-TNATA") was vacuum deposited to a thickness of 60 nm to form a hole injection layer, and then the compound represented by Formula 1 of the present invention on the hole injection layer Vacuum deposition to a thickness of 60nm to form a hole transport layer.
- CBP 4,4'-N, N'-dicarbazole-biphenyl
- host bis- (1-phenylisoquinolyl) iridium (III) acetylacetonate
- (piq) 2 Ir (acac)) as a dopant, and the mixture doped at 95: 5 weight was vacuum deposited to a thickness of 30 nm to form a light emitting layer.
- BAlq (1,1'-bisphenyl) -4-oleito) bis (2-methyl-8-quinolineoleito) aluminum
- BAlq 3 tris (8-quinolinol) aluminum
- LiF an alkali metal halide
- the forward bias DC voltage was applied to the organic electroluminescent devices according to Experimental Example I (Experimental Example (1) to Experimental Example 30) and Comparative Example I (Comparative Example (1) to Comparative Example (3)) prepared as described above.
- the electroluminescence (EL) characteristics were measured with a PR-650 photoresearch company, and T95 life was measured using a life-time measurement device manufactured by McScience Inc. at a luminance of 2500 cd / m 2.
- Table 10 shows device fabrication for Experimental Example I (Experimental Example (1) to Experimental Example (30)) and Comparative Example I (Comparative Example (1) to Comparative Example (3)) to which the compound according to the present invention was applied. And the evaluation result.
- a hole injection layer is formed by vacuum depositing 2-TNATA with a thickness of 60 nm on an ITO layer (anode) formed on a glass substrate, and then vacuuming the compound represented by Formula 1 of the present invention on the hole injection layer with a thickness of 60 nm. It was deposited to form a hole transport layer. Subsequently, one of the compounds represented by Formulas 2 to 4 of the present invention was vacuum-deposited to a thickness of 20 nm on the hole transport layer to form a light emitting auxiliary layer.
- LiF an alkali metal halide
- the forward bias DC voltage was applied to the organic electroluminescent devices according to Experimental Example II (Experimental Example (31) to Experimental Example (60)) and Comparative Example II (Comparative Example (4) to Comparative Example (6)) prepared as described above.
- the electroluminescence (EL) characteristics were measured with a PR-650 photoresearch company, and the T95 life was measured using a life-time measurement device manufactured by McScience Inc. at a luminance of 5000 cd / m 2.
- Table 11 shows device fabrication of Experimental Example II (Experimental Example (31) to Experimental Example (60)) and Comparative Example II (Comparative Example (4) to Comparative Example (6)) to which the compound according to the present invention was applied. And the evaluation result.
- a hole injection layer is formed by vacuum depositing 2-TNATA with a thickness of 60 nm on an ITO layer (anode) formed on a glass substrate, and then vacuuming the compound represented by Formula 1 of the present invention on the hole injection layer with a thickness of 60 nm. It was deposited to form a hole transport layer. Subsequently, one of the compounds represented by Formulas 2 to 4 of the present invention was vacuum-deposited to a thickness of 20 nm on the hole transport layer to form a light emitting auxiliary layer.
- a mixture doped with 9,10-di (naphthalen-2-yl) anthracene as a host and BD-052X (manufactured by Idemitsukosan) as a dopant at 93: 7 weight was 30 nm thick.
- Evaporation was carried out in vacuo to form a light emitting layer.
- BAlq was vacuum deposited on the emission layer to form a hole blocking layer, and Alq 3 was deposited on the hole blocking layer to 40 nm in thickness to form an electron transport layer.
- LiF an alkali metal halide
- An organic electric device was manufactured in the same manner as in Experimental Example III, except that the light emitting auxiliary layer was not formed.
- the forward bias DC voltage was applied to the organic electroluminescent devices according to Experimental Example III (Experimental Example (61) to Experimental Example 90) and Comparative Example III (Comparative Example (7) to Comparative Example (9)) prepared as described above.
- the electroluminescence (EL) characteristics were measured with a PR-650 photoresearch company, and T95 life was measured using a life measurement device manufactured by McScience Inc. at a luminance of 500 cd / m 2.
- Table 12 shows device fabrication for Experimental Example III (Experimental Example (61) to Experimental Example (90)) and Comparative Example III (Comparative Example (7) to Comparative Example (9)) to which the compound according to the present invention was applied. And the evaluation result.
- the compound of the present invention substituted with position 3 of fluorene alone is used as the light emitting auxiliary layer, it has a high T1 energy level and a deep HOMO energy level. It is believed that the light emission is performed inside the light emitting layer instead of the interface to maximize the efficiency.
- the device characteristics were described in terms of the hole transport layer and the light emitting auxiliary layer.
- the organic material layer of the organic electric element such as the light emitting layer may be used in a single or mixed with other materials. Therefore, the compounds of the present invention can be used in a single or other material mixed with other organic material layers, for example, electron injection layer, electron transport layer, hole injection layer and the light emitting layer in addition to the hole transport layer or the light emitting auxiliary layer.
Abstract
The present invention relates to an organic electronic element comprising: a first electrode; a second electrode; and an organic materials layer which is located between the first electrode and the second electrode and comprises at least a hole transport layer, a light-emitting auxiliary layer and a light-emitting layer, wherein the hole transport layer comprises a compound expressed by chemical formula (1), and the light-emitting auxiliary layer comprises at least one from among the compounds expressed by chemical formulas (2)-(4).
Description
본 발명은 유기전기소자용 화합물을 이용한 유기전기소자 및 그 전자 장치에 관한 것이다.The present invention relates to an organic electric device using the compound for organic electric devices and an electronic device thereof.
최근, 발광재료를 표시부의 발광소자에 사용한 유기일렉트로루미네센스 표시장치(Organic Electroluminescence Display: 유기 EL 표시장치)의 개발이 활발해지고 있다. 유기 EL 표시장치는 액정표시장치 등 과는 달리 양극 및 음극으로 주입된 정공 및 전자를 발광층에 있어서 재결합시킴으로써, 발광층에 있어서의 유기화합물을 포함하는 발광재료를 발광시켜 표시를 실현하는 소위 자발광형의 표시장치이다.In recent years, the development of organic electroluminescence display (organic EL display device) using a light emitting material for the light emitting element of the display portion has been actively developed. The organic EL display device, unlike a liquid crystal display device or the like, recombines holes and electrons injected into an anode and a cathode in a light emitting layer, thereby emitting a light emitting material containing an organic compound in the light emitting layer to realize display. Display device.
유기 일렉트로루미네센스소자(이하 "유기 EL 소자"라고 한다)에 있어서, 최근에는 발광층과, 상기 발광층에 캐리어(정공, 전자)를 수송하는 층 등, 특성이 다른 복수의 층으로 구성된 것이 제안되었다. In an organic electroluminescent element (hereinafter referred to as an "organic EL element"), it has recently been proposed to be composed of a plurality of layers having different characteristics such as a light emitting layer and a layer for transporting carriers (holes, electrons) to the light emitting layer. .
상기한 유기 EL 소자의 발광특성을 향상시키고 장수명화를 이루기 위해서는 정공수송층이 우수한 정공수송능력과 캐리어 내성을 가질 것이 요구된다. 이러한 관점에서 여러가지의 정공수송재료가 제안되었다. In order to improve the light emission characteristics of the organic EL device and achieve a long life, it is required that the hole transport layer has excellent hole transport capacity and carrier resistance. In this respect, various hole transport materials have been proposed.
유기 EL 소자의 각 층에 사용할 수 있는 재료로서는 방향족 아민계 화합물 등의 여러가지 화합물 등이 알려져 있다. 예를 들어, 카르바졸 유도체를 정공수송층으로 사용한 특허문헌 1과 중수소를 갖는 아민 화합물이 정공 수송재료 및 발광층의 호스트 재로로서 사용된 특허문헌 2, 3차 아민구조로 단순 아릴기가 치환된 구조로서 정공 수송재료로 사용된 특허문헌 3, 플루오레닐기를 갖는 아민 화합물이 정공 수송재료 또는 주입재료로서 사용된 특허문헌 4 등이 보고되었다. As a material which can be used for each layer of organic electroluminescent element, various compounds, such as an aromatic amine compound, etc. are known. For example, Patent Document 1 using a carbazole derivative as a hole transport layer and an amine compound having deuterium are used as a structure in which a simple aryl group is substituted with the Patent Documents 2 and 3 amine structures used as host materials for the hole transport material and the light emitting layer. Patent Document 3 used as a transport material, Patent Document 4 and the like in which an amine compound having a fluorenyl group are used as a hole transport material or an injection material have been reported.
그러나 이것들을 재료로서 사용한 유기 EL 소자는 충분한 발광수명을 갖고 있다고는 말하기 어려우며, 이에 따라 현재 더 고효율로 저전압 구동이 가능함과 동시에 발광수명이 긴 유기 EL 소자가 요구되고 있다. However, it is difficult to say that an organic EL element using these as a material has a sufficient light emission life, and therefore, an organic EL element capable of low voltage driving with high efficiency and a long light emitting lifetime is currently required.
〔선행기술문헌〕[Prior art document]
〔특허문헌〕[Patent Documents]
(특허문헌 0001) 미국특허출원공개 제2007/0231503호(Patent Document 0001) US Patent Application Publication No. 2007/0231503
(특허문헌 0002) 국제공개 제2012/091471호(Patent Document 0002) International Publication No. 2012/091471
(특허문헌 0003) 한국특허출원공개 제2000/0052560호(Patent Document 0003) Korean Patent Application Publication No. 2000/0052560
(특허문헌 0004) 국제공개 제2010/110553호(Patent Document 0004) International Publication No. 2010/110553
본 발명은 발광층 계면열화를 방지하고, 발광층 내 전하균형을 증가시킴으로써 소자 및 효율이 향상된 유기전기발광소자, 및 그것을 실현하는 유기발광재료, 이를 이용한 전자장치를 제공하는 것을 목적으로 한다. An object of the present invention is to provide an organic electroluminescent device having improved device and efficiency by preventing interfacial degradation of the light emitting layer and increasing charge balance in the light emitting layer, and an organic light emitting material for realizing the same, and an electronic device using the same.
최근 들어 유기전기발광소자에 있어서 가장 이슈화되는 것은 높은 수명의 안전성과 높은 효율이다. 이는 유기전기발광소자가 점점 대면적화 되어지면서, 가장 크게 요구되어지고 있는 부분이기도 하다. 또한 최근 유기전기발광소자에 있어서 정공수송층에서의 발광문제 및 구동전압 문제를 해결하기 위해서 정공수송층과 발광층 사이에 발광보조층(다층의 정공수송층)이 존재하여야 하며, 각각의 발광층에 따른 서로 다른 발광보조층의 개발이 필요한 시점이다.In recent years, the most issue in organic electroluminescent devices is high lifetime safety and high efficiency. This is also the most demanding part as the organic electroluminescent device becomes larger and larger. In addition, in order to solve the light emission problem and the driving voltage problem in the hole transport layer in the organic electroluminescent device, a light emitting auxiliary layer (multiple hole transport layer) must exist between the hole transport layer and the light emitting layer. It is time to develop the auxiliary layer.
일반적으로 전자수송층에서 발광층으로 전자(electron)가 전달되어지고 정공(hole)이 정공수송층에서 발광층으로 전달되어 발광층 내에서 재조합(recombination)이 이루어져 엑시톤(exciton)을 형성하게 된다. 하지만 낮은 구동전압을 만들기 위해 정공 이동도(hole mobility)가 빠른 물질을 사용할 경우 양성폴라론(Polaron)이 발광층과 정공수송층 계면에 쌓이에 되며, 이로 인해 계면열화가 발생하여, 수명 및 효율을 감소시키며, 또한 발광층 내 전하균형(Charge Balance)이 맞지 않아 발광층 내 잉여 폴라론(Polaron)이 발광물질의 약한 결합(bonding)을 공격하여 발광물질이 변형됨으로 수명 및 효율 감소, 색순도 저하 등의 현상을 나타내고 있다. In general, electrons are transferred from the electron transport layer to the light emitting layer, and holes are transferred from the hole transport layer to the light emitting layer to form recombination in the light emitting layer to form excitons. However, when a fast hole mobility material is used to make a low driving voltage, positive polaron accumulates at the interface between the light emitting layer and the hole transport layer. As a result, interfacial deterioration occurs, resulting in longevity and efficiency. In addition, due to poor balance of charge in the light emitting layer, excess polaron in the light emitting layer attacks weak bonding of the light emitting material, which deforms the light emitting material, resulting in a decrease in lifespan and efficiency and a decrease in color purity. Indicates.
따라서 발광보조층은 상기 정공수송층과 발광층 사이에 존재하여 양성 폴라론(Polaron)이 발광층 계면에 쌓이는 것을 방지하기 위해 발광층과 정공수송층 사이의 알맞은 HOMO값을 갖는 물질이어야 하며, 발광층 내 전하균형(Charge Balance)을 증가시키기 위해, 적당한 구동전압 범위 내(full device의 blue 소자 구동전압 범위 내) 정공 이동도(hole mobility)를 갖는 물질이어야 한다. Therefore, the light emitting auxiliary layer should be a material having a suitable HOMO value between the light emitting layer and the hole transport layer in order to prevent the positive polaron from accumulating at the light emitting layer interface between the hole transport layer and the light emitting layer. In order to increase the balance, it must be a material having hole mobility within a suitable driving voltage range (in the blue device driving voltage range of the full device).
하지만 이는 단순히 발광보조층 물질의 코어에 대한 구조적 특성으로 이루어질 수 없으며, 발광보조층 물질의 코어 및 sub-치환기의 특성 그리고 발광보조층과 정공수송층, 발광보조층과 발광층 간의 알맞은 조합이 이루어졌을 때 고효율 및 고수명의 소자가 구현될 수 있는 것이다.However, this cannot be achieved simply by the structural characteristics of the core of the light emitting auxiliary layer material, and when a proper combination between the light emitting auxiliary layer and the hole transport layer, the light emitting auxiliary layer and the light emitting layer is made, High efficiency and long life devices can be realized.
본 발명은 상술한 과제를 감안하여 이루어진 것으로 발광층 계면열화를 방지하고, 발광층 내 전하균형을 증가시킴으로써 소자 및 효율이 향상된 유기전기발광소자, 및 그것을 실현하는 유기발광재료를 제공하는 것을 과제로 한다.SUMMARY OF THE INVENTION The present invention has been made in view of the above problems, and an object thereof is to provide an organic electroluminescent device having improved device and efficiency by preventing interfacial degradation of the light emitting layer and increasing charge balance in the light emitting layer, and an organic light emitting material for realizing the same.
일 측면에서, 본 발명은 하기 화학식으로 표시되는 화합물을 이용한 유기전기소자 및 그 전자장치를 제공한다.In one aspect, the present invention provides an organic electronic device using the compound represented by the following formula and an electronic device thereof.
제 1전극; 제 2전극; 및 상기 제 1전극과 제 2전극 사이에 위치하며 적어도 정공수송층과 발광보조층, 발광층을 포함하는 유기물층;을 포함하는 유기전기소자에 있어서, 상기 제 1전극과 발광보조층 사이에 존재하는 정공수송층은 하기 화학식 1로 표시되는 화합물을 포함하고, 상기 정공수송층과 발광층 사이에 존재하는 발광보조층은 하기 화학식 2 내지 4로 표시되는 화합물 중 적어도 하나를 포함하는 유기전기소자를 제공한다.A first electrode; Second electrode; And an organic material layer disposed between the first electrode and the second electrode, the organic material layer including at least a hole transport layer, a light emitting auxiliary layer and a light emitting layer, wherein the hole transport layer is present between the first electrode and the light emitting auxiliary layer. Includes a compound represented by Formula 1 below, and the light emitting auxiliary layer existing between the hole transport layer and the light emitting layer provides an organic electric device including at least one of the compounds represented by Formulas 2 to 4.
<화학식 1> <화학식 2> <Formula 1> <Formula 2>
<화학식 3> <화학식 4> <Formula 3> <Formula 4>
본 발명에 따른 화합물을 이용함으로써 소자의 높은 발광효율, 낮은 구동전압, 고내열성을 달성할 수 있고, 소자의 색순도 및 수명을 크게 향상시킬 수 있다. By using the compound according to the present invention, high luminous efficiency, low driving voltage, and high heat resistance of the device can be achieved, and color purity and life of the device can be greatly improved.
도 1은 본 발명에 따른 유기전기발광소자의 예시도이다. 1 is an exemplary view of an organic electroluminescent device according to the present invention.
이하, 본 발명의 실시예를 첨부된 도면을 참조하여 상세하게 설명한다.Hereinafter, exemplary embodiments of the present invention will be described in detail with reference to the accompanying drawings.
각 도면의 구성요소들에 참조부호를 부가함에 있어서, 동일한 구성요소들에 대해서는 비록 다른 도면상에 표시되더라도 가능한 한 동일한 부호를 가지도록 하고 있음에 유의해야 한다. 또한, 본 발명을 설명함에 있어, 관련된 공지 구성 또는 기능에 대한 구체적인 설명이 본 발명의 요지를 흐릴 수 있다고 판단되는 경우에는 그 상세한 설명은 생략한다.In adding reference numerals to the components of each drawing, it should be noted that the same reference numerals are assigned to the same components as much as possible even though they are shown in different drawings. In addition, in describing the present invention, when it is determined that the detailed description of the related well-known configuration or function may obscure the gist of the present invention, the detailed description thereof will be omitted.
또한, 본 발명의 구성 요소를 설명하는 데 있어서, 제 1, 제 2, A, B, (a),(b) 등의 용어를 사용할 수 있다. 이러한 용어는 그 구성 요소를 다른 구성 요소와 구별하기 위한 것일 뿐, 그 용어에 의해 해당 구성 요소의 본질이나 차례 또는 순서 등이 한정되지 않는다. 어떤 구성 요소가 다른 구성요소에 "연결", "결합" 또는 "접속"된다고 기재된 경우, 그 구성 요소는 그 다른 구성요소에 직접적으로 연결되거나 또는 접속될 수 있지만, 각 구성 요소 사이에 또 다른 구성 요소가 "연결", "결합" 또는 "접속"될 수도 있다고 이해되어야 할 것이다.In addition, in describing the component of this invention, terms, such as 1st, 2nd, A, B, (a), (b), can be used. These terms are only for distinguishing the components from other components, and the nature, order or order of the components are not limited by the terms. If a component is described as being "connected", "coupled" or "connected" to another component, that component may be directly connected to or connected to that other component, but there may be another configuration between each component. It is to be understood that the elements may be "connected", "coupled" or "connected".
본 명세서 및 첨부된 청구의 범위에서 사용된 바와 같이, 달리 언급하지 않는 한, 하기 용어의 의미는 하기와 같다:As used in this specification and the appended claims, unless otherwise indicated, the meanings of the following terms are as follows:
본 명세서에서 사용된 용어 "할로" 또는 "할로겐"은 다른 설명이 없는 한 불소(F), 브롬(Br), 염소(Cl) 또는 요오드(I)이다.The term "halo" or "halogen" as used herein is fluorine (F), bromine (Br), chlorine (Cl) or iodine (I) unless otherwise indicated.
본 발명에 사용된 용어 "알킬" 또는 "알킬기"는 다른 설명이 없는 한 1 내지 60의 탄소수의 단일결합을 가지며, 직쇄 알킬기, 분지쇄 알킬기, 사이클로알킬(지환족)기, 알킬-치환된 사이클로알킬기, 사이클로알킬-치환된 알킬기를 비롯한 포화 지방족 작용기의 라디칼을 의미한다.As used herein, the term "alkyl" or "alkyl group" has a single bond of 1 to 60 carbon atoms, unless otherwise indicated, and is a straight chain alkyl group, branched chain alkyl group, cycloalkyl (alicyclic) group, alkyl-substituted cyclo Radicals of saturated aliphatic functional groups, including alkyl groups, cycloalkyl-substituted alkyl groups.
본 발명에 사용된 용어 "할로알킬기" 또는 "할로겐알킬기"는 다른 설명이 없는 한 할로겐으로 치환된 알킬기를 의미한다.As used herein, the term "haloalkyl group" or "halogenalkyl group" means an alkyl group substituted with halogen unless otherwise specified.
본 발명에 사용된 용어 "헤테로알킬기"는 알킬기를 구성하는 탄소 원자 중 하나 이상이 헤테로원자로 대체된 것을 의미한다.As used herein, the term "heteroalkyl group" means that at least one of the carbon atoms constituting the alkyl group has been replaced with a heteroatom.
본 발명에 사용된 용어 "알켄일기" 또는 "알킨일기"는 다른 설명이 없는 한 각각 2 내지 60의 탄소수의 이중결합 또는 삼중결합을 가지며, 직쇄형 또는 측쇄형 사슬기를 포함하며, 여기에 제한되는 것은 아니다. As used herein, the term "alkenyl group" or "alkynyl group", unless stated otherwise, has a double or triple bond of 2 to 60 carbon atoms, and includes a straight or branched chain group, and is not limited thereto. It is not.
본 발명에 사용된 용어 "시클로알킬"은 다른 설명이 없는 한 3 내지 60의 탄소수를 갖는 고리를 형성하는 알킬을 의미하며, 여기에 제한되는 것은 아니다. The term "cycloalkyl" as used herein, unless otherwise stated, refers to alkyl forming a ring having 3 to 60 carbon atoms, without being limited thereto.
본 발명에 사용된 용어 "알콕실기", "알콕시기", 또는 "알킬옥시기"는 산소 라디칼이 부착된 알킬기를 의미하며, 다른 설명이 없는 한 1 내지 60의 탄소수를 가지며, 여기에 제한되는 것은 아니다. As used herein, the term "alkoxyl group", "alkoxy group", or "alkyloxy group" means an alkyl group to which an oxygen radical is attached, and unless otherwise specified, has a carbon number of 1 to 60, and is limited herein. It is not.
본 발명에 사용된 용어 "알켄옥실기", "알켄옥시기", "알켄일옥실기", 또는 "알켄일옥시기"는 산소 라디칼이 부착된 알켄일기를 의미하며, 다른 설명이 없는 한 2 내지 60의 탄소수를 가지며, 여기에 제한되는 것은 아니다. As used herein, the term "alkenoxyl group", "alkenoxy group", "alkenyloxyl group", or "alkenyloxy group" means an alkenyl group to which an oxygen radical is attached, and unless otherwise stated, it is 2 to 60 It has carbon number of, It is not limited to this.
본 발명에 사용된 용어 "아릴옥실기" 또는 "아릴옥시기"는 산소 라디칼이 부착된 아릴기를 의미하며, 다른 설명이 없는 한 6 내지 60의 탄소수를 가지며, 여기에 제한되는 것은 아니다. As used herein, the term "aryloxyl group" or "aryloxy group" means an aryl group to which an oxygen radical is attached, and unless otherwise specified, has a carbon number of 6 to 60, but is not limited thereto.
본 발명에 사용된 용어 "아릴기" 및 "아릴렌기"는 다른 설명이 없는 한 각각 6 내지 60의 탄소수를 가지며, 이에 제한되는 것은 아니다. 본 발명에서 아릴기 또는 아릴렌기는 단일 고리 또는 다중 고리의 방향족을 의미하며, 이웃한 치환기가 결합 또는 반응에 참여하여 형성된 방향족 고리를 포함한다. 예컨대, 아릴기는 페닐기, 비페닐기, 플루오렌기, 스파이로플루오렌기일 수 있다. As used herein, the terms "aryl group" and "arylene group" have a carbon number of 6 to 60 unless otherwise stated, but is not limited thereto. In the present invention, an aryl group or an arylene group means an aromatic of a single ring or multiple rings, and includes an aromatic ring formed by neighboring substituents participating in a bond or a reaction. For example, the aryl group may be a phenyl group, a biphenyl group, a fluorene group, a spirofluorene group.
접두사 "아릴" 또는 "아르"는 아릴기로 치환된 라디칼을 의미한다. 예를 들어 아릴알킬기는 아릴기로 치환된 알킬기이며, 아릴알켄일기는 아릴기로 치환된 알켄일기이며, 아릴기로 치환된 라디칼은 본 명세서에서 설명한 탄소수를 가진다.The prefix "aryl" or "ar" means a radical substituted with an aryl group. For example, an arylalkyl group is an alkyl group substituted with an aryl group, an arylalkenyl group is an alkenyl group substituted with an aryl group, and the radical substituted with an aryl group has the carbon number described herein.
또한 접두사가 연속으로 명명되는 경우 먼저 기재된 순서대로 치환기가 나열되는 것을 의미한다. 예를 들어, 아릴알콕시기의 경우 아릴기로 치환된 알콕시기를 의미하며, 알콕실카르보닐기의 경우 알콕실기로 치환된 카르보닐기를 의미하며, 또한 아릴카르보닐알켄일기의 경우 아릴카르보닐기로 치환된 알켄일기를 의미하며 여기서 아릴카르보닐기는 아릴기로 치환된 카르보닐기이다.Also, when prefixes are named consecutively, it means that the substituents are listed in the order described first. For example, an arylalkoxy group means an alkoxy group substituted with an aryl group, an alkoxylcarbonyl group means a carbonyl group substituted with an alkoxyl group, and an arylcarbonylalkenyl group means an alkenyl group substituted with an arylcarbonyl group. Wherein the arylcarbonyl group is a carbonyl group substituted with an aryl group.
본 명세서에서 사용된 용어 "헤테로알킬"은 다른 설명이 없는 한 하나 이상의 헤테로원자를 포함하는 알킬을 의미한다. 본 발명에 사용된 용어 "헤테로아릴기" 또는 "헤테로아릴렌기"는 다른 설명이 없는 한 각각 하나 이상의 헤테로원자를 포함하는 탄소수 2 내지 60의 아릴기 또는 아릴렌기를 의미하며, 여기에 제한되는 것은 아니며, 단일 고리 및 다중 고리 중 적어도 하나를 포함하며, 이웃한 작용기기가 결합하여 형성될 수도 있다.As used herein, the term “heteroalkyl” means an alkyl including one or more heteroatoms unless otherwise indicated. As used herein, the term "heteroaryl group" or "heteroarylene group" means an aryl group or arylene group having 2 to 60 carbon atoms, each containing one or more heteroatoms, unless otherwise specified. It may include at least one of a single ring and multiple rings, and may be formed by combining adjacent functional groups.
본 발명에 사용된 용어 "헤테로고리기"는 다른 설명이 없는 한 하나 이상의 헤테로원자를 포함하고, 2 내지 60의 탄소수를 가지며, 단일 고리 및 다중 고리 중 적어도 하나를 포함하며, 헤테로지방족 고리 및 헤테로방향족 고리를 포함한다. 이웃한 작용기가 결합하여 형성될 수도 있다. As used herein, the term “heterocyclic group” includes one or more heteroatoms, unless otherwise indicated, and has from 2 to 60 carbon atoms, and includes at least one of single and multiple rings, heteroaliphatic rings and hetero Aromatic rings. Adjacent functional groups may be formed in combination.
본 명세서에서 사용된 용어 "헤테로원자"는 다른 설명이 없는 한 N, O, S, P 또는 Si를 나타낸다. The term "heteroatom" as used herein refers to N, O, S, P or Si unless otherwise stated.
또한 "헤테로고리기"는, 고리를 형성하는 탄소 대신 SO2를 포함하는 고리도 포함할 수 있다. 예컨대, "헤테로고리기"는 다음 화합물을 포함한다. "Heterocyclic groups" may also include rings comprising SO 2 in place of the carbon forming the ring. For example, a "heterocyclic group" includes the following compounds.
다른 설명이 없는 한, 본 발명에 사용된 용어 "지방족"은 탄소수 1 내지 60의 지방족 탄화수소를 의미하며, "지방족고리"는 탄소수 3 내지 60의 지방족 탄화수소 고리를 의미한다. Unless otherwise stated, the term "aliphatic" as used herein means an aliphatic hydrocarbon having 1 to 60 carbon atoms, and the "aliphatic ring" means an aliphatic hydrocarbon ring having 3 to 60 carbon atoms.
다른 설명이 없는 한, 본 발명에 사용된 용어 "고리"는 탄소수 3 내지 60의 지방족고리 또는 탄소수 6 내지 60의 방향족고리 또는 탄소수 2 내지 60의 헤테로고리 또는 이들의 조합으로 이루어진 융합 고리를 말하며, 포화 또는 불포화 고리를 포함한다.Unless otherwise stated, the term "ring" as used herein refers to a fused ring consisting of an aliphatic ring having 3 to 60 carbon atoms or an aromatic ring having 6 to 60 carbon atoms or a hetero ring having 2 to 60 carbon atoms or a combination thereof. Saturated or unsaturated rings.
전술한 헤테로화합물 이외의 그 밖의 다른 헤테로화합물 또는 헤테로라디칼은 하나 이상의 헤테로원자를 포함하며, 여기에 제한되는 것은 아니다. Other heterocompounds or heteroradicals other than the aforementioned heterocompounds include, but are not limited to, one or more heteroatoms.
다른 설명이 없는 한, 본 발명에 사용된 용어 "카르보닐"이란 -COR'로 표시되는 것이며, 여기서 R'은 수소, 탄소수 1 내지 20 의 알킬기, 탄소수 6 내지 30 의 아릴기, 탄소수 3 내지 30의 사이클로알킬기, 탄소수 2 내지 20의 알켄일기, 탄소수 2 내지 20의 알킨일기, 또는 이들의 조합인 것이다.Unless otherwise stated, the term "carbonyl" used in the present invention is represented by -COR ', wherein R' is hydrogen, an alkyl group having 1 to 20 carbon atoms, an aryl group having 6 to 30 carbon atoms, and 3 to 30 carbon atoms. Cycloalkyl group, an alkenyl group having 2 to 20 carbon atoms, an alkynyl group having 2 to 20 carbon atoms, or a combination thereof.
다른 설명이 없는 한, 본 발명에 사용된 용어 "에테르"란 -R-O-R'로 표시되는 것이며, 여기서 R 또는 R'은 각각 서로 독립적으로 수소, 탄소수 1 내지 20의 알킬기, 탄소수 6 내지 30의 아릴기, 탄소수 3 내지 30의 사이클로알킬기, 탄소수 2 내지 20의 알켄일기, 탄소수 2 내지 20의 알킨일기, 또는 이들의 조합인 것이다.Unless otherwise specified, the term "ether" as used herein is represented by -RO-R ', wherein R or R' are each independently of each other hydrogen, an alkyl group having 1 to 20 carbon atoms, It is an aryl group, a C3-C30 cycloalkyl group, a C2-C20 alkenyl group, a C2-C20 alkynyl group, or a combination thereof.
또한 명시적인 설명이 없는 한, 본 발명에서 사용된 용어 "치환 또는 비치환된"에서 "치환"은 중수소, 할로겐, 아미노기, 니트릴기, 니트로기, C1~C20의 알킬기, C1~C20의 알콕실기, C1~C20의 알킬아민기, C1~C20의 알킬티오펜기, C6~C20의 아릴티오펜기, C2~C20의 알켄일기, C2~C20의 알킨일기, C3~C20의 시클로알킬기, C6~C20의 아릴기, 중수소로 치환된 C6~C20의 아릴기, C8~C20의 아릴알켄일기, 실란기, 붕소기, 게르마늄기, 및 C2~C20의 헤테로고리기로 이루어진 군으로부터 선택되는 1개 이상의 치환기로 치환됨을 의미하며, 이들 치환기에 제한되는 것은 아니다. Also, unless stated otherwise, the term "substituted" in the term "substituted or unsubstituted" as used in the present invention is deuterium, halogen, amino group, nitrile group, nitro group, C 1 ~ C 20 alkyl group, C 1 ~ C 20 alkoxyl group, C 1 ~ C 20 alkylamine group, C 1 ~ C 20 alkylthiophene group, C 6 ~ C 20 arylthiophene group, C 2 ~ C 20 alkenyl group, C 2 ~ C 20 alkynyl, C 3 ~ C 20 cycloalkyl group, C 6 ~ C 20 aryl group, of a C 6 ~ C 20 substituted by deuterium aryl group, a C 8 ~ C 20 aryl alkenyl group, a silane group, a boron Group, germanium group, and C 2 ~ C 20 It is meant to be substituted with one or more substituents selected from the group consisting of, but not limited to these substituents.
또한 명시적인 설명이 없는 한, 본 발명에서 사용되는 화학식은 하기 화학식의 지수 정의에 의한 치환기 정의와 동일하게 적용된다.Also, unless otherwise stated, the formulas used in the present invention apply equally to the definitions of substituents based on the exponential definition of the following formulas.
여기서, a가 0의 정수인 경우 치환기 R1은 부존재하며, a가 1의 정수인 경우 하나의 치환기 R1은 벤젠 고리를 형성하는 탄소 중 어느 하나의 탄소에 결합하며, a가 2 또는 3의 정수인 경우 각각 다음과 같이 결합하며 이때 R1은 서로 동일하거나 다를 수 있으며, a가 4 내지 6의 정수인 경우 이와 유사한 방식으로 벤젠 고리의 탄소에 결합하며, 한편 벤젠 고리를 형성하는 탄소에 결합된 수소의 표시는 생략한다.Herein, when a is an integer of 0, the substituent R 1 is absent, when a is an integer of 1, one substituent R 1 is bonded to any one of carbons forming the benzene ring, and a is an integer of 2 or 3 Are each bonded as follows, where R 1 may be the same or different from each other, and when a is an integer from 4 to 6, it is bonded to the carbon of the benzene ring in a similar manner, while the indication of hydrogen bonded to the carbon forming the benzene ring Is omitted.
도 1은 본 발명에 일 실시예에 따른 유기전기소자에 대한 예시도이다.1 is an exemplary view of an organic electric device according to an embodiment of the present invention.
도 1을 참조하면, 본 발명에 따른 유기전기소자(100)는 기판(110) 상에 형성된 제 1전극(120), 제 2전극(180) 및 제 1전극(110)과 제 2전극(180) 사이에 본 발명에 따른 화합물을 포함하는 유기물층을 구비한다. 이때, 제 1전극(120)은 애노드(양극)이고, 제 2전극(180)은 캐소드(음극)일 수 있으며, 인버트형의 경우에는 제 1전극이 캐소드이고 제 2전극이 애노드일 수 있다.Referring to FIG. 1, the organic electric device 100 according to the present invention includes a first electrode 120, a second electrode 180, a first electrode 110, and a second electrode 180 formed on a substrate 110. ) Is provided with an organic material layer containing a compound according to the present invention. In this case, the first electrode 120 may be an anode (anode), the second electrode 180 may be a cathode (cathode), and in the case of an inverted type, the first electrode may be a cathode and the second electrode may be an anode.
유기물층은 제 1전극(120) 상에 순차적으로 정공주입층(130), 정공수송층(140), 발광층(150), 전자수송층(160) 및 전자주입층(170)을 포함할 수 있다. 이때, 발광층(150)을 제외한 나머지 층들이 형성되지 않을 수 있다. 정공저지층, 전자저지층, 발광보조층(151), 버퍼층(141) 등을 더 포함할 수도 있고, 전자수송층(160) 등이 정공저지층의 역할을 할 수도 있을 것이다.The organic layer may include a hole injection layer 130, a hole transport layer 140, a light emitting layer 150, an electron transport layer 160, and an electron injection layer 170 on the first electrode 120 in sequence. At this time, the remaining layers except for the light emitting layer 150 may not be formed. The hole blocking layer, the electron blocking layer, the light emitting auxiliary layer 151, the buffer layer 141 may be further included, and the electron transport layer 160 may serve as the hole blocking layer.
또한, 미도시하였지만, 본 발명에 따른 유기전기소자는 제 1전극과 제 2전극 중 적어도 일면 중 상기 유기물층과 반대되는 일면에 형성된 보호층 또는 광효율 개선층(Capping layer)을 더 포함할 수 있다. In addition, although not shown, the organic electric device according to the present invention may further include a protective layer or a light efficiency improving layer (Capping layer) formed on one surface of the at least one surface of the first electrode and the second electrode opposite to the organic material layer.
한편, 동일한 코어일지라도 어느 위치에 어느 치환기를 결합시키냐에 따라 밴드갭(band gap), 전기적 특성, 계면 특성 등이 달라질 수 있으므로, 코어의 선택 및 이에 결합된 서브(sub)-치환체의 조합도 아주 중요하며, 특히 각 유기물층 간의 에너지 준위 및 T1 값, 물질의 고유특성(이동도, 계면특성 등) 등이 최적의 조합을 이루었을 때 긴 수명과 높은 효율을 동시에 달성할 수 있다.Meanwhile, even in the same core, band gaps, electrical characteristics, and interface characteristics may vary depending on which substituents are bonded at which positions. Therefore, the selection of cores and the combination of sub-substituents bound thereto are also very significant. Importantly, long life and high efficiency can be achieved at the same time when an optimal combination of energy level and T1 value and intrinsic properties (mobility, interfacial properties, etc.) of each organic material layer is achieved.
본 발명의 일 실시예에 따른 유기전기발광소자는 PVD(physical vapor deposition) 방법을 이용하여 제조될 수 있다. 예컨대, 기판 상에 금속 또는 전도성을 가지는 금속 산화물 또는 이들의 합금을 증착시켜 양극(120)을 형성하고, 그 위에 정공주입층(130), 정공수송층(140), 발광층(150), 전자수송층(160) 및 전자주입층(170)을 포함하는 유기물층을 형성한 후, 그 위에 음극(180)으로 사용할 수 있는 물질을 증착시킴으로써 제조될 수 있다.The organic electroluminescent device according to an embodiment of the present invention may be manufactured using a PVD method. For example, the anode 120 is formed by depositing a metal or a conductive metal oxide or an alloy thereof on a substrate, and the hole injection layer 130, the hole transport layer 140, the light emitting layer 150, and the electron transport layer are formed thereon. After forming the organic material layer including the 160 and the electron injection layer 170, it can be prepared by depositing a material that can be used as the cathode 180 thereon.
또한, 유기물층은 다양한 고분자 소재를 사용하여 증착법이 아닌 용액 공정 또는 솔벤트 프로세스(solvent process), 예컨대 스핀코팅 공정, 노즐 프린팅 공정, 잉크젯 프린팅 공정, 슬롯코팅 공정, 딥코팅 공정, 롤투롤 공정, 닥터 블레이딩 공정, 스크린 프린팅 공정, 또는 열 전사법 등의 방법에 의하여 더 적은 수의 층으로 제조할 수 있다. 본 발명에 따른 유기물층은 다양한 방법으로 형성될 수 있으므로, 그 형성방법에 의해 본 발명의 권리범위가 제한되는 것은 아니다.In addition, the organic material layer is a solution or solvent process (e.g., spin coating process, nozzle printing process, inkjet printing process, slot coating process, dip coating process, roll-to-roll process, doctor blading) using various polymer materials. It can be produced in fewer layers by methods such as ding process, screen printing process, or thermal transfer method. Since the organic material layer according to the present invention may be formed in various ways, the scope of the present invention is not limited by the forming method.
본 발명에 따른 유기전기소자는 사용되는 재료에 따라 전면 발광형, 후면 발광형 또는 양면 발광형일 수 있다.The organic electric element according to the present invention may be a top emission type, a bottom emission type or a double-sided emission type depending on the material used.
WOLED(White Organic Light Emitting Device)는 고해상도 실현이 용이하고 공정성이 우수한 한편, 기존의LCD의 칼라필터 기술을 이용하여 제조될 수 있는 이점이 있다. 주로 백라이트 장치로 사용되는 백색 유기발광소자에 대한 다양한 구조들이 제안되고 특허화되고 있다. 대표적으로, R(Red),G(Green),B(Blue) 발광부들을 상호평면적으로 병렬배치(side-by-side) 방식, R,G,B 발광층이 상하로 적층되는 적층(stacking) 방식이 있고, 청색(B) 유기발광층에 의한 전계발광과 이로부터의 광을 이용하여 무기형광체의 자발광(photo-luminescence)을 이용하는 색변환물질(color conversion material, CCM) 방식 등이 있는데, 본 발명은 이러한 WOLED에도 적용될 수 있을 것이다.WOLED (White Organic Light Emitting Device) has the advantage that can be manufactured using the color filter technology of the existing LCD while being easy to realize high resolution and excellent processability. Various structures for white organic light emitting devices mainly used as backlight devices have been proposed and patented. Representatively, a side-by-side method in which R (Red), G (Green) and B (Blue) light emitting parts are mutually planarized, and a stacking method in which R, G, and B light emitting layers are stacked up and down. And a color conversion material (CCM) method using photo-luminescence of an inorganic phosphor by using electroluminescence by a blue (B) organic light emitting layer and light therefrom. May also be applied to these WOLEDs.
또한, 본 발명에 따른 유기전기소자는 유기전기발광소자(OLED), 유기태양전지, 유기감광체(OPC), 유기트랜지스터(유기 TFT), 단색 또는 백색 조명용 소자 중 하나일 수 있다.In addition, the organic electroluminescent device according to the present invention may be one of an organic electroluminescent device (OLED), an organic solar cell, an organic photoconductor (OPC), an organic transistor (organic TFT), a monochromatic or white illumination device.
본 발명의 다른 실시예는 상술한 본 발명의 유기전기소자를 포함하는 디스플레이장치와, 이 디스플레이장치를 제어하는 제어부를 포함하는 전자장치를 포함할 수 있다. 이때, 전자장치는 현재 또는 장래의 유무선 통신단말일 수 있으며, 휴대폰 등의 이동 통신 단말기, PDA, 전자사전, PMP, 리모콘, 네비게이션, 게임기, 각종 TV, 각종 컴퓨터 등 모든 전자장치를 포함한다.Another embodiment of the present invention may include a display device including the organic electric element of the present invention described above, and an electronic device including a control unit for controlling the display device. In this case, the electronic device may be a current or future wired or wireless communication terminal, and includes all electronic devices such as a mobile communication terminal such as a mobile phone, a PDA, an electronic dictionary, a PMP, a remote controller, a navigation device, a game machine, various TVs, and various computers.
이하, 본 발명의 일 측면에 따른 유기전기소자에 대하여 설명한다.Hereinafter, an organic electric device according to an aspect of the present invention will be described.
본 발명의 일 구체 예에 따르면, 제 1전극; 제 2전극; 및 상기 제 1전극과 제 2전극 사이에 위치하며 적어도 정공수송층과 발광보조층, 발광층을 포함하는 유기물층;을 포함하는 유기전자소자에 있어서, 상기 제 1전극과 발광보조층 사이에 존재하는 정공수송층은 하기 화학식 1로 표시되는 화합물을 포함하고, 상기 정공수송층과 발광층 사이에 존재하는 발광보조층은 하기 화학식 2 내지 4로 표시되는 화합물 중 적어도 하나를 포함하는 유기전기소자를 제공한다.According to one embodiment of the invention, the first electrode; Second electrode; And an organic material layer disposed between the first electrode and the second electrode, the organic material layer including at least a hole transport layer, a light emitting auxiliary layer, and a light emitting layer, wherein the hole transport layer is present between the first electrode and the light emitting auxiliary layer. Includes a compound represented by Formula 1 below, and the light emitting auxiliary layer existing between the hole transport layer and the light emitting layer provides an organic electric device including at least one of the compounds represented by Formulas 2 to 4.
<화학식 1> <Formula 1>
<화학식 2> <화학식 3> <화학식 4><Formula 2> <Formula 3> <Formula 4>
상기 화학식 1 내지 화학식 4에서,In Chemical Formulas 1 to 4,
Ar0 내지 Ar2, 및 Ar6 내지 Ar11은 i) 서로 독립적으로 C6~C60의 아릴기; 플루오렌일기; O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기; C3~C60의 지방족고리와 C6~C60의 방향족고리의 융합고리기; C1~C50의 알킬기; C2~C20의 알켄일기; C2~C20의 알킨일기; C1~C30의 알콕실기; C6~C30의 아릴옥시기; 및 -L-N(Ra)(Rb);로 이루어진 군에서 선택되거나, 또는 ii) Ar1과 Ar2는 서로 결합하여 고리를 형성할 수 있으며, 여기서 '고리'란 C3~C60의 지방족고리, C6~C60의 방향족고리, C2~C60의 헤테로고리 또는 이들의 조합인 융합 고리를 말하며, 포화 또는 불포화 고리를 포함한다.Ar 0 to Ar 2 , and Ar 6 to Ar 11 are each independently i) a C 6 to C 60 aryl group; Fluorenyl group; C 2 ~ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; Fused ring group of an aromatic ring of C 3 ~ C 60 of aliphatic rings and C 6 ~ C 60; C 1 ~ C 50 Alkyl group; C 2 ~ C 20 Alkenyl group; Alkynyl groups of C 2 to C 20 ; C 1 -C 30 alkoxyl group; C 6 -C 30 aryloxy group; And -LN (R a ) (R b ); or ii) Ar 1 and Ar 2 may be bonded to each other to form a ring, wherein the 'ring' is an aliphatic C 3 to C 60 refers to a ring, a C 6 ~ C 60 aromatic, C 2 ~ C 60 of the heterocycle or a combination thereof is a fused ring, a saturated or unsaturated ring.
Ar4 및 Ar5는 i) 서로 독립적으로 수소; C1~C50의 알킬기; C6~C60의 아릴기; 플루오렌일기; O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기;로 이루어진 군에서 선택거나, 또는 ii) Ar4와 Ar5가 서로 결합하여 이들이 결합된 플루오렌과 함께 스파이로 화합물을 형성할 수 있다.Ar 4 and Ar 5 are i) independently of each other hydrogen; C 1 ~ C 50 Alkyl group; C 6 ~ C 60 Aryl group; Fluorenyl group; C 2 ~ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; or ii) Ar 4 and Ar 5 are bonded to each other to combine Together with fluorene it is possible to form spiro compounds.
a, b, c, d, e, f 및 g는 0~4의 정수이며,a, b, c, d, e, f and g are integers from 0 to 4,
R0 내지 R6은 i) 서로 독립적으로 중수소; 할로겐; C6~C60의 아릴기; 플루오렌일기; O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기; C3~C60의 지방족고리와 C6~C60의 방향족고리의 융합고리기; C1~C50의 알킬기; C2~C20의 알켄일기; C2~C20의 알킨일기; C1~C30의 알콕실기; C6~C30의 아릴옥시기; 및 -L-N(Ra)(Rb);로 이루어진 군에서 선택되거나, 또는 ii) a, b, c, d, e, f 및 g가 각각 2 이상의 정수인 경우에 R0 내지 R6은 각각 이웃하는 치환기끼리 서로 결합하여 적어도 하나의 고리를 형성할 수 있으며, 여기서 '고리'란 C3~C60의 지방족고리, C6~C60의 방향족고리, C2~C60의 헤테로고리 또는 이들의 조합인 융합 고리를 말하며, 포화 또는 불포화 고리를 포함한다.R 0 to R 6 are i) independently of each other deuterium; halogen; C 6 ~ C 60 Aryl group; Fluorenyl group; C 2 ~ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; Fused ring group of an aromatic ring of C 3 ~ C 60 of aliphatic rings and C 6 ~ C 60; C 1 ~ C 50 Alkyl group; C 2 ~ C 20 Alkenyl group; Alkynyl groups of C 2 to C 20 ; C 1 -C 30 alkoxyl group; C 6 -C 30 aryloxy group; And -LN (R a ) (R b ); or ii) when a, b, c, d, e, f and g are each an integer of 2 or more, R 0 to R 6 are each neighbor The substituents may be bonded to each other to form at least one ring, wherein the 'ring' is an aliphatic ring of C 3 ~ C 60, an aromatic ring of C 6 ~ C 60 , a hetero ring of C 2 ~ C 60 or these It refers to a fused ring that is a combination and includes saturated or unsaturated rings.
L1 내지 L6 및 상기 L은 서로 독립적으로 단일결합; C6~C60의 아릴렌기; 플루오렌일렌기; C3~C60의 지방족고리와 C6~C60의 방향족고리의 융합고리기; 및 O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기;로 이루어진 군에서 선택되며(여기서, L3 내지 L5인 경우 단일결합은 제외함),L 1 to L 6 and L are independently of each other a single bond; C 6 ~ C 60 arylene group; Fluorenylene groups; Fused ring group of an aromatic ring of C 3 ~ C 60 of aliphatic rings and C 6 ~ C 60; And a C 2 to C 60 heterocyclic group including at least one heteroatom of O, N, S, Si, and P; wherein L 3 to L 5 exclude a single bond. ),
상기 Ra 및 Rb는 서로 독립적으로 C6~C60의 아릴기; 플루오렌일기; C3~C60의 지방족고리와 C6~C60의 방향족고리의 융합고리기; 및 O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기;로 이루어진 군에서 선택된다.R a and R b are each independently a C 6 ~ C 60 aryl group; Fluorenyl group; Fused ring group of an aromatic ring of C 3 ~ C 60 of aliphatic rings and C 6 ~ C 60; And a C 2 -C 60 heterocyclic group including at least one heteroatom of O, N, S, Si, and P.
상기 아릴기, 플루오렌일기, 헤테로고리기, 융합고리기, 알킬기, 알켄일기, 알킨일기, 알콕실기, 아릴옥시기, 아릴렌기, 플루오렌일렌기는 각각 중수소; 할로겐; 실란기; 실록산기; 붕소기; 게르마늄기; 시아노기; 니트로기; -L-N(Ra)(Rb); C1~C20의 알킬싸이오기; C1~C20의 알콕실기; C1~C20의 알킬기; C2~C20의 알켄일기; C2~C20의 알킨일기; C6~C20의 아릴기; 중수소로 치환된 C6~C20의 아릴기; 플루오렌일기; C2~C20의 헤테로고리기; C3~C20의 시클로알킬기; C7~C20의 아릴알킬기 및 C8~C20의 아릴알켄일기로 이루어진 군에서 선택된 하나 이상의 치환기로 더욱 치환될 수 있으며, 또한 이들 치환기들은 서로 결합하여 고리를 형성할 수도 있으며, 여기서 '고리'란 C3~C60의 지방족고리, C6~C60의 방향족고리, C2~C60의 헤테로고리 또는 이들의 조합인 융합 고리를 말하며, 포화 또는 불포화 고리를 포함한다.The aryl group, fluorenyl group, heterocyclic group, fused ring group, alkyl group, alkenyl group, alkynyl group, alkoxyl group, aryloxy group, arylene group, fluorenylene group, respectively; halogen; Silane group; Siloxane groups; Boron group; Germanium group; Cyano group; Nitro group; -LN (R a ) (R b ); C 1 ~ C 20 of the import alkylthio; C 1 -C 20 alkoxyl group; C 1 ~ C 20 Alkyl group; C 2 ~ C 20 Alkenyl group; Alkynyl groups of C 2 to C 20 ; C 6 -C 20 aryl group; C 6 ~ C 20 aryl group substituted with deuterium; Fluorenyl group; C 2 ~ C 20 heterocyclic group; C 3 -C 20 cycloalkyl group; It may be further substituted with one or more substituents selected from the group consisting of C 7 ~ C 20 arylalkyl group and C 8 ~ C 20 arylalkenyl group, and these substituents may combine with each other to form a ring, 'Refers to a fused ring which is an aliphatic ring of C 3 ~ C 60, an aromatic ring of C 6 ~ C 60 , a heterocyclic ring of C 2 ~ C 60 or a combination thereof, and includes a saturated or unsaturated ring.
본 발명의 또 다른 구체 예에서, 상기 정공수송층은 상기 화학식 1로 표시되는 화합물을 포함하고, 상기 발광보조층은 상기 화학식 2 내지 화학식 4로 표시되는 화합물 중 하나인 것을 특징으로 하는 유기전기소자를 제공한다.In another embodiment of the present invention, the hole transport layer comprises a compound represented by the formula (1), the light emitting auxiliary layer is an organic electric device, characterized in that one of the compounds represented by the formula (2) to formula (4) to provide.
본 발명의 또 다른 구체 예에서, 상기 발광보조층의 상기 화학식 2로 표시되는 화합물은 하기 화합물 중 하나일 수 있다.In another embodiment of the present invention, the compound represented by Formula 2 of the light emitting auxiliary layer may be one of the following compounds.
본 발명의 또 다른 구체 예에서, 상기 발광보조층의 상기 화학식 3으로 표시되는 화합물은 하기 화합물 중 하나일 수 있다.In another embodiment of the present invention, the compound represented by Formula 3 of the light emitting auxiliary layer may be one of the following compounds.
본 발명의 또 다른 구체 예에서, 상기 발광보조층의 상기 화학식 4로 표시되는 화합물은 하기 화합물 중 하나일 수 있다.In another embodiment of the present invention, the compound represented by Formula 4 of the light emitting auxiliary layer may be one of the following compounds.
본 발명의 또 다른 구체 예에서, 상기 정공수송층의 상기 화학식 1로 표시되는 화합물은 하기 화합물 중 하나일 수 있다.In another embodiment of the present invention, the compound represented by Formula 1 of the hole transport layer may be one of the following compounds.
본 발명의 또 다른 구체 예에서, 상기 제 1전극의 일측면 중 상기 유기물층과 반대되는 일측 또는 상기 제 2전극의 일측면 중 상기 유기물층과 반대되는 일측 중 적어도 하나에 형성되는 광효율 개선층을 포함하는 유기전기소자를 제공한다.In another embodiment of the present invention, comprising a light efficiency improving layer formed on at least one side of the one side of the first electrode opposite to the organic material layer or one side of the second electrode opposite to the organic material layer An organic electric device is provided.
본 발명의 또 다른 구체 예에서, 상기 유기물층은 스핀코팅 공정, 노즐 프린팅 공정, 잉크젯 프린팅 공정, 슬롯코팅 공정, 딥코팅 공정 및 롤투롤 공정 중 어느 하나에 의해 형성됨을 특징으로 하는 유기전기소자를 제공한다.In another embodiment of the present invention, the organic material layer is provided by any one of a spin coating process, a nozzle printing process, an inkjet printing process, a slot coating process, a dip coating process and a roll-to-roll process do.
본 발명의 또 다른 구체 예에서, 제 1전극; 제 2전극; 및 상기 제 1전극과 제 2전극 사이에 위치하며 적어도 정공수송층과 발광보조층, 발광층을 포함하는 유기물층;을 포함하는 유기전기소자에 있어서, 상기 제 1전극과 발광보조층 사이에 존재하는 정공수송층은 상기 화학식 1로 표시되는 화합물을 포함하고, 상기 정공수송층과 발광층 사이에 존재하는 발광보조층은 상기 화학식 2 내지 4로 표시되는 화합물 중 적어도 하나를 포함하는 유기전기소자를 포함하는 디스플레이장치; 및 상기 디스플레이장치를 구동하는 제어부;를 포함하는 전자장치를 제공한다.In another embodiment of the invention, the first electrode; Second electrode; And an organic material layer disposed between the first electrode and the second electrode, the organic material layer including at least a hole transport layer, a light emitting auxiliary layer and a light emitting layer, wherein the hole transport layer is present between the first electrode and the light emitting auxiliary layer. A display device including an organic electroluminescent device including a compound represented by Chemical Formula 1, wherein the light emitting auxiliary layer existing between the hole transport layer and the light emitting layer comprises at least one of the compounds represented by Chemical Formulas 2 to 4; And a controller for driving the display device.
본 발명의 또 다른 구체 예에서, 본 발명에 따른 유기전기소자는 유기전기발광소자(OLED), 유기태양전지, 유기감광체(OPC), 유기트랜지스터(유기 TFT), 및 단색 또는 백색 조명용 소자 중 적어도 하나일 수 있다.In another embodiment of the present invention, the organic electroluminescent device according to the present invention is at least one of an organic electroluminescent device (OLED), an organic solar cell, an organic photoconductor (OPC), an organic transistor (organic TFT), and a device for monochrome or white illumination It can be one.
이하에서, 본 발명의 유기전기소자에 포함되는 화학식 1 내지 화학식 4로 표시되는 화합물의 합성예 및 본 발명의 유기전기소자의 제조예에 관하여 실시예를 들어 구체적으로 설명하지만, 본 발명이 하기의 실시예로 한정되는 것은 아니다.Hereinafter, the synthesis examples of the compounds represented by the formulas (1) to (4) contained in the organic electroluminescent device of the present invention and the production examples of the organic electroluminescent device of the present invention will be described in detail by way of examples. It is not limited to an Example.
합성예Synthesis Example
상기 화합물의 합성은 하기와 같은 방법으로 수행하였다.Synthesis of the compound was carried out in the following manner.
Ⅰ. 화학식 1의 합성I. Synthesis of Formula 1
본 발명에 따른 화학식 1로 표시되는 화합물(Final Product)은 하기 반응식 1의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.The compound represented by Formula 1 according to the present invention may be synthesized by the reaction route of Scheme 1 below, but is not limited thereto.
<반응식 1><Scheme 1>
* 상기 L은 화학식 1의 Ar3에서 정의된 L5 또는 L6이다.The L * is an L 5 or L 6 as defined in Ar 3 of formula (1).
1. Sub 1의 합성1.Synthesis of Sub 1
상기 반응식 1의 Sub 1은 하기 반응식 2의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Sub 1 of Scheme 1 may be synthesized by the reaction route of Scheme 2, but is not limited thereto.
<반응식 2><Scheme 2>
여기서 S1 내지 S7은 다음과 같다.Here, S1 to S7 are as follows.
(1) Sub 1-1-1 합성 (L=biphenyl)(1) Sub 1-1-1 Synthesis (L = biphenyl)
<반응식 3><Scheme 3>
출발물질인 9H-carbazole (50.16g, 300mmol)에 4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3(41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 80.05g (수율: 67%)를 얻었다.Starting material 9H-carbazole (50.16g, 300mmol), 4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na 2 SO 4 (42.6g, 300mmol), K 2 CO 3 80.05 g (yield: 67%) of (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol) and nitrobenzene were obtained using the above synthesis examples.
(2) Sub 1-1-2 합성 (L=9,9-dimethyl-9H-fluorene)(2) Synthesis of Sub 1-1-2 (L = 9,9-dimethyl-9H-fluorene)
<반응식 4><Scheme 4>
출발물질인 9H-carbazole (50.16g, 300mmol)에 2-bromo-7-iodo-9,9-dimethyl-9H-fluorene (143.7g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3 (41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 88.11g (수율: 67%)를 얻었다.Starting material 9H-carbazole (50.16g, 300mmol), 2-bromo-7-iodo-9,9-dimethyl-9H-fluorene (143.7g, 360mmol), Na 2 SO 4 (42.6g, 300mmol), K 2 CO 3 (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol) and nitrobenzene were obtained using the above synthesis example to obtain 88.11 g (yield: 67%) of the product.
(3) Sub 1-1-3 합성 (L=9,9-dimethyl-9H-fluorene)(3) Synthesis of Sub 1-1-3 (L = 9,9-dimethyl-9H-fluorene)
<반응식 5>Scheme 5
출발물질인 7H-benzo[c]carbazole (65.18g, 300mmol)에 4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3 (41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 92.8g (수율: 69%)를 얻었다.Starting material 7H-benzo [c] carbazole (65.18g, 300mmol), 4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na 2 SO 4 (42.6g, 300mmol), K 2 CO 3 (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol) and nitrobenzene were obtained using the above synthesis example to give 92.8 g (yield: 69%) of the product.
(4) Sub 1-1-4 합성 (L=9,9-dimethyl-9H-fluorene)(4) Sub 1-1-4 Synthesis (L = 9,9-dimethyl-9H-fluorene)
<반응식 6><Scheme 6>
출발물질인 7H-benzo[c]carbazole (65.18g, 300mmol)에 2-bromo-7-iodo-9,9-dimethyl-9H-fluorene (143.7g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3 (41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 95.24g (수율: 65%)를 얻었다.Starting material, 7H-benzo [c] carbazole (65.18g, 300mmol), 2-bromo-7-iodo-9,9-dimethyl-9H-fluorene (143.7g, 360mmol), Na 2 SO 4 (42.6g, 300mmol) ), K 2 CO 3 (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol) and nitrobenzene were obtained using the above synthesis example to obtain 95.24 g (yield: 65%) of the product.
(5) Sub 1-1-5 합성 (L=biphenyl)(5) Sub 1-1-5 Synthesis (L = biphenyl)
<반응식 7>Scheme 7
출발물질인 11H-benzo[a]carbazole (65.18g, 300mmol)에 4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3 (41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 80.05g (수율: 62%)를 얻었다.4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na 2 SO 4 (42.6g, 300mmol), starting material 11H-benzo [a] carbazole (65.18g, 300mmol), K 2 CO 3 (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol) and nitrobenzene were obtained using the above synthesis example to obtain 80.05 g (yield: 62%) of the product.
(6) Sub 1-1-6 합성 (L=9,9-dimethyl-9H-fluorene)(6) Sub 1-1-6 Synthesis (L = 9,9-dimethyl-9H-fluorene)
<반응식 8>Scheme 8
출발물질인 5H-benzo[b]carbazole (65.18g, 300mmol)에 2-bromo-7-iodo-9,9-dimethyl-9H-fluorene (143.7g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3 (41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 93.78g (수율: 64%)를 얻었다.Starting material 5H-benzo [b] carbazole (65.18g, 300mmol), 2-bromo-7-iodo-9,9-dimethyl-9H-fluorene (143.7g, 360mmol), Na 2 SO 4 (42.6g, 300mmol) ), K 2 CO 3 (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol), and nitrobenzene were obtained using the above synthesis example to obtain 93.78 g (yield: 64%) of the product.
(7) Sub 1-1-7 합성 (L=biphenyl)(7) Sub 1-1-7 Synthesis (L = biphenyl)
<반응식 9>Scheme 9
출발물질인 9H-dibenzo[a,c]carbazole (80.2g, 300mmol)에 4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3 (41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 98.7g (수율: 66%)를 얻었다.Starting material 9H-dibenzo [a, c] carbazole (80.2g, 300mmol) to 4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na 2 SO 4 (42.6g, 300mmol) ), K 2 CO 3 (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol) and nitrobenzene were obtained using the above synthesis examples to obtain 98.7 g (yield: 66%) of the product.
(8) Sub 1-1-8 합성 (L=biphenyl)(8) Sub 1-1-8 Synthesis (L = biphenyl)
<반응식 10>Scheme 10
출발물질인 N-phenylnaphthalen-1-amine (65.8g, 300mmol)에 4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3 (41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 89.2g (수율: 66%)를 얻었다.4-bromo-4'-iodo-1,1'-biphenyl (129.2g, 360mmol), Na 2 SO 4 (42.6g, 300mmol), starting material N-phenylnaphthalen-1-amine (65.8g, 300mmol), K 2 CO 3 (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol) and nitrobenzene were obtained using the above synthesis example to give 89.2 g (yield: 66%) of the product.
(9) Sub 1-1-9 합성 (L=9,9-dimethyl-9H-fluorene)(9) Sub 1-1-9 Synthesis (L = 9,9-dimethyl-9H-fluorene)
<반응식 11>Scheme 11
출발물질인 7H-dibenzo[c,g]carbazole (80.2g, 300mmol)에 2-bromo-7-iodo-9,9-dimethyl-9H-fluorene (143.7g, 360mmol), Na2SO4 (42.6g, 300mmol), K2CO3 (41.4g, 300mmol), Cu (5.72g, 90mmol), nitrobenzene을 상기 합성예를 사용하여 생성물 98.5g (수율: 61%)를 얻었다.Starting material, 7H-dibenzo [c, g] carbazole (80.2g, 300mmol), 2-bromo-7-iodo-9,9-dimethyl-9H-fluorene (143.7g, 360mmol), Na 2 SO 4 (42.6g , 300 mmol), K 2 CO 3 (41.4 g, 300 mmol), Cu (5.72 g, 90 mmol), and nitrobenzene were obtained using the above synthesis example to obtain 98.5 g (yield: 61%) of the product.
2. Sub 2의 합성2. Synthesis of Sub 2
상기 반응식 1의 Sub 2는 하기 반응식 12의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Sub 2 of Scheme 1 may be synthesized by the reaction route of Scheme 12, but is not limited thereto.
<반응식 12>Scheme 12
(1) M2-2-1 합성 (g=0, Ar9=phenyl)(1) M2-2-1 synthesis (g = 0, Ar 9 = phenyl)
<반응식 13>Scheme 13
3-bromo-9-phenyl-9H-carbazole (45.1g, 140mmol)을 DMF 980mL에 녹인 후에, Bispinacolborate (39.1g, 154mmol), PdCl2(dppf) 촉매 (3.43g, 4.2mmol), KOAc (41.3g, 420mmol)을 순서대로 첨가한 후 24 시간 교반하여 보레이트 화합물을 합성한 후에, 얻어진 화합물을 silicagel column 및 재결정을 걸쳐서 분리한 후 보레이트 화합물을 35.2g (수율: 68 %)얻었다.After dissolving 3-bromo-9-phenyl-9H-carbazole (45.1 g, 140 mmol) in 980 mL of DMF, Bispinacolborate (39.1 g, 154 mmol), PdCl 2 (dppf) catalyst (3.43 g, 4.2 mmol), KOAc (41.3 g) , 420 mmol) were added sequentially, followed by stirring for 24 hours to synthesize the borate compound. The obtained compound was separated through a silicagel column and recrystallization to obtain 35.2 g (yield: 68%) of the borate compound.
(2) M2-2-2 합성 (g=0, Ar9=biphenyl)(2) M2-2-2 synthesis (g = 0, Ar 9 = biphenyl)
<반응식 14>Scheme 14
상기 M2-2-1과 동일한 실험방법을 통해서 40g (수율: 64%)을 얻었다.40g (yield: 64%) was obtained through the same experimental method as the M2-2-1.
(3) Sub 2-1-1 합성 [g=0, Ar9=Phenyl, L6= biphenyl (linear)](3) Sub 2-1-1 synthesis [g = 0, Ar 9 = Phenyl, L 6 = biphenyl (linear)]
<반응식 15>Scheme 15
M2-2-1 (29.5g, 80mmol)을 THF 360mL에 녹인 후에, 4-bromo-4'-iodo-1,1'-biphenyl (30.16g, 84mmol), Pd(PPh3)4 (2.8g, 2.4mmol), NaOH (9.6g, 240mmol), 물 180mL를 첨가한 후, 교반환류시킨다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 26.56g (수율: 70 %) 얻었다.After dissolving M2-2-1 (29.5 g, 80 mmol) in 360 mL of THF, 4-bromo-4'-iodo-1,1'-biphenyl (30.16 g, 84 mmol), Pd (PPh 3 ) 4 (2.8 g, 2.4 mmol), NaOH (9.6 g, 240 mmol) and 180 mL of water were added, followed by stirring under reflux. After completion of the reaction, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was purified by silicagel column and recrystallized to give 26.56g (yield: 70%) of the product.
(4) Sub 2-1-2 합성 [g=0, Ar9=Phenyl, L6= phenyl](4) Synthesis of Sub 2-1-2 [g = 0, Ar 9 = Phenyl, L 6 = phenyl]
<반응식 16>Scheme 16
M2-2-1 (29.5g, 80mmol), THF 360mL, 1-bromo-4-iodobenzene (23.8g, 84mmol), Pd(PPh3)4 (2.8g, 2.4mmol), NaOH (9.6g, 240mmol), 물 180mL를 첨가한 후, 교반환류시킨다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 22.9g (72 %) 얻었다.M2-2-1 (29.5 g, 80 mmol), THF 360 mL, 1-bromo-4-iodobenzene (23.8 g, 84 mmol), Pd (PPh 3 ) 4 (2.8 g, 2.4 mmol), NaOH (9.6 g, 240 mmol) After adding 180 mL of water, the mixture was stirred under reflux. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was purified by silicagel column and recrystallized to give 22.9g (72%).
(5) Sub 2-1-3 합성 [g=0, Ar9=Phenyl, L6= biphenyl (non-linear)](5) Synthesis of Sub 2-1-3 [g = 0, Ar 9 = Phenyl, L 6 = biphenyl (non-linear)]
<반응식 17>Scheme 17
M2-2-1 (29.5g, 80mmol)을 THF 360mL에 녹인 후에, 4'-bromo-3-iodo-1,1'-biphenyl (30.16g, 84mmol), Pd(PPh3)4 (2.8g, 2.4mmol), NaOH (9.6g, 240mmol), 물 180mL를 첨가한 후, 교반환류시킨다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 24.7g (65 %) 얻었다.M2-2-1 (29.5 g, 80 mmol) was dissolved in THF 360 mL, then 4'-bromo-3-iodo-1,1'-biphenyl (30.16 g, 84 mmol), Pd (PPh 3 ) 4 (2.8 g, 2.4 mmol), NaOH (9.6 g, 240 mmol) and 180 mL of water were added, followed by stirring under reflux. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was purified by silicagel column and recrystallized to give 24.7g (65%).
(6) Sub 2-1-4 합성 [g=0, Ar9=biphenyl, L6= biphenyl (linear)](6) Synthesis of Sub 2-1-4 [g = 0, Ar 9 = biphenyl, L 6 = biphenyl (linear)]
<반응식 18>Scheme 18
M2-2-2 (35.63g, 80mmol)를 THF 360mL에 녹인 후에, 4-bromo-4'-iodo-1,1'-biphenyl (30.16g, 84mmol), Pd(PPh3)4 (2.8g, 2.4mmol), NaOH (9.6g, 240mmol), 물 180mL를 첨가한 후, 교반환류시킨다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 29.51g (67 %) 얻었다.M2-2-2 (35.63 g, 80 mmol) was dissolved in THF 360 mL, then 4-bromo-4'-iodo-1,1'-biphenyl (30.16 g, 84 mmol), Pd (PPh 3 ) 4 (2.8 g, 2.4 mmol), NaOH (9.6 g, 240 mmol) and 180 mL of water were added, followed by stirring under reflux. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 29.51g (67%).
3. Sub 3의 합성3. Synthesis of Sub 3
상기 반응식 1의 Sub 3은 하기 반응식 19의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Sub 3 of Scheme 1 may be synthesized by the reaction route of Scheme 19, but is not limited thereto.
<반응식 19>Scheme 19
(1) Sub 3의 합성(1) Synthesis of Sub 3
[1,1'-biphenyl]-4-amine (1당량)과 4-bromo-1,1'-biphenyl (1.1당량)을 톨루엔에 넣고 Pd2(dba)3 (0.05당량), PPh3 (0.1당량), NaOt-Bu (3당량)을 각각 첨가한 뒤, 100℃에서 24시간 교반환류시킨다. ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 di([1,1'-biphenyl]-4-yl)amine을 얻었다. [1,1'-biphenyl] -4-amine (1 equiv) and 4-bromo-1,1'-biphenyl (1.1 equiv) were added to toluene and Pd 2 (dba) 3 (0.05 equiv), PPh 3 (0.1 Equivalents) and NaO t -Bu (3 equivalents), respectively, were added and then stirred at reflux for 24 hours at 100 ° C. After extracting with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was silicagel column and recrystallized to obtain di ([1,1'-biphenyl] -4-yl) amine.
한편, Sub 3의 예시는 아래와 같으나 이에 한정되는 것은 아니며, 이들의 FD/MS는 하기 [표 1]과 같다.Meanwhile, examples of Sub 3 are as follows, but are not limited thereto, and their FD / MS are shown in Table 1 below.
[표 1]TABLE 1
4. 화학식 1의 최종 생성물(Final Product) 합성4. Synthesis of Final Product of Formula 1
Sub 1 (1당량) 또는 Sub 2 (1당량)를 톨루엔에 녹인 후에, Sub 3 (1.1당량), Pd2(dba)3 (0.05당량), PPh3 (0.1당량), NaOt-Bu (3당량)을 각각 첨가한 뒤, 100℃에서 24시간 교반환류시킨다. 반응이 종료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 최종 생성물을 얻었다.Sub 1 (1 equiv) or Sub 2 (1 equiv) was dissolved in toluene, then Sub 3 (1.1 equiv), Pd 2 (dba) 3 (0.05 equiv), PPh 3 (0.1 equiv), NaO t -Bu (3 Equivalents) were added and stirred at reflux for 24 hours at 100 ° C. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain a final product.
(1) 13-17 합성(1) 13-17 synthesis
<반응식 20>Scheme 20
9-(4'-bromo-[1,1'-biphenyl]-4-yl)-9H-carbazole (9.6g, 24mmol)을 톨루엔에 녹인 후에, di([1,1'-biphenyl]-4-yl)amine (6.4g, 20mmol), Pd2(dba)3 (0.05당량), PPh3 (0.1당량), NaOt-Bu (3당량)을 각각 첨가한 뒤, 100℃에서 24시간 교반환류시킨다. 반응이 종료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 최종화합물을 12.9g (수율: 84%)을 얻었다. 9- (4'-bromo- [1,1'-biphenyl] -4-yl) -9H-carbazole (9.6 g, 24 mmol) was dissolved in toluene, followed by di ([1,1'-biphenyl] -4- yl) amine (6.4 g, 20 mmol), Pd 2 (dba) 3 (0.05 equiv), PPh 3 (0.1 equiv) and NaO t -Bu (3 equiv) were then added and stirred under reflux at 100 ° C. for 24 hours. . After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 12.9 g (yield: 84%) of the final compound.
(2) 13-32 합성(2) 13-32 synthetic
<반응식 21>Scheme 21
3-(4-bromophenyl)-9-phenyl-9H-carbazole (9.6g, 24mmol)을 톨루엔에 녹인 후에, N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-2-amine (7.2g, 20mmol), Pd2(dba)3 (0.05당량), PPh3 (0.1당량), NaOt-Bu (3당량)을 각각 첨가한 뒤, 100℃에서 24시간 교반환류시킨다. 반응이 종료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 최종화합물을 13.8g (수율: 85%)을 얻었다. 3- (4-bromophenyl) -9-phenyl-9H-carbazole (9.6 g, 24 mmol) was dissolved in toluene, followed by N-([1,1'-biphenyl] -4-yl) -9,9-dimethyl- 9H-fluoren-2-amine (7.2 g, 20 mmol), Pd 2 (dba) 3 (0.05 equiv), PPh 3 (0.1 equiv) and NaO t -Bu (3 equiv) were added respectively, followed by 24 at 100 ° C. The reaction was stirred under reflux. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 13.8 g (yield: 85%) of the final compound.
(3) 13-61 합성(3) 13-61 synthetic
<반응식 22>Scheme 22
N-(4'-bromo-[1,1'-biphenyl]-4-yl)-N-phenylnaphthalen-1-amine (10.8g, 24mmol)을 톨루엔에 녹인 후에, N-phenylnaphthalen-1-amine (4.4g, 20mmol), Pd2(dba)3 (0.05당량), PPh3 (0.1당량), NaOt-Bu (3당량)을 각각 첨가한 뒤, 100℃에서 24시간 교반환류시킨다. 반응이 종료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축 한 후 생성된 유기물을 silicagel column 및 재결정하여 최종화합물을 11.4g (수율: 81%)을 얻었다. N- (4'-bromo- [1,1'-biphenyl] -4-yl) -N-phenylnaphthalen-1-amine (10.8 g, 24 mmol) is dissolved in toluene, followed by N-phenylnaphthalen-1-amine (4.4 g, 20 mmol), Pd 2 (dba) 3 (0.05 equiv), PPh 3 (0.1 equiv) and NaO t -Bu (3 equiv) were then added and stirred under reflux at 100 ° C. for 24 hours. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallization to obtain 11.4 g (yield: 81%) of the final compound.
한편, 상기와 같은 합성예에 따라 제조된 화학식 1의 최종 생성물 중 일부분의 FD-MS 값은 하기 [표 2]와 같다.On the other hand, the FD-MS value of a part of the final product of Formula 1 prepared according to the synthesis examples as described in the following [Table 2].
[표 2]TABLE 2
Ⅱ. 화학식 2의 합성II. Synthesis of Formula 2
본 발명에 따른 화학식 2로 표시되는 화합물(Final Product)은 하기 반응식 23의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Compound represented by Formula 2 according to the present invention (Final Product) may be synthesized by the reaction route of Scheme 23, but is not limited thereto.
<반응식 23>Scheme 23
1. Sub 4의 합성1.Synthesis of Sub 4
상기 반응식 23의 Sub 4는 하기 반응식 24의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Sub 4 of Scheme 23 may be synthesized by the reaction route of Scheme 24, but is not limited thereto.
<반응식 24>Scheme 24
(1) Sub 4-2 합성(1) Sub 4-2 synthesis
3-bromo-9-phenyl-9H-carbazole (1당량)을 둥근바닥플라스크에 DMF로 녹인 후에, Bis(pinacolato)diboron (1.1당량), Pd(dppf)Cl2 (0.03당량), KOAc (3당량)를첨가하고 90℃에서 교반하였다. 반응이 완료되면 증류를 통해 DMF를 제거하고 CH2Cl2와 물로 추출하였다. 유기층을 MgSO4로 건조하고 농축한 후 생성된 화합물을silicagel column 및 재결정하여 9-phenyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole를 얻었다.3-bromo-9-phenyl-9H-carbazole (1 equiv) was dissolved in DMF in a round bottom flask, followed by Bis (pinacolato) diboron (1.1 equiv), Pd (dppf) Cl 2 (0.03 equiv), KOAc (3 equiv) ) Was added and stirred at 90 ° C. After the reaction was completed, DMF was removed by distillation and extracted with CH 2 Cl 2 and water. The organic layer was dried over MgSO 4 , concentrated, and the resulting compound was purified by silica gel column and recrystallized with 9-phenyl-3- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -9H. -carbazole was obtained.
(2) Sub 4 합성(2) Sub 4 synthesis
9-phenyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole (1당량)과 1-bromo-4-iodobenzene (1당량), Pd(PPh3)4 (0.03당량), K2CO3 (3당량)를무수 THF와 소량의 물에 녹이고 난 후, 24시간 동안 환류시켰다. 반응이 종료되면 반응물의 온도를 상온으로 식히고, CH2Cl2로 추출하고 물로 닦아주었다. 소량의 물을 무수 MgSO4로 제거하고 감압여과 후, 유기용매를 농축하여 생성된 생성물을 컬럼크로마토그래피를 이용하여 분리하여 원하는 3-(4-bromophenyl)-9-phenyl-9H-carbazole을 얻었다.9-phenyl-3- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -9H-carbazole (1 equiv) and 1-bromo-4-iodobenzene (1 equiv), Pd (PPh 3 ) 4 (0.03 equiv) and K 2 CO 3 (3 equiv) were dissolved in anhydrous THF and a small amount of water and then refluxed for 24 hours. After the reaction was completed, the temperature of the reactant was cooled to room temperature, extracted with CH 2 Cl 2 , and washed with water. After removing a small amount of water with anhydrous MgSO 4 and filtered under reduced pressure, the organic solvent was concentrated and the resulting product was separated by column chromatography to give the desired 3- (4-bromophenyl) -9-phenyl-9H-carbazole.
한편, Sub 4의 예시는 아래와 같으나 이에 한정되는 것은 아니며, 이들의 FD/MS는 하기 [표 3]과 같다.Meanwhile, examples of Sub 4 are as follows, but are not limited thereto, and their FD / MS are as shown in Table 3 below.
[표 3]TABLE 3
2. Sub 5의 합성2. Synthesis of Sub 5
상기 반응식 23의 Sub 5는 하기 반응식 25의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Sub 5 of Scheme 23 may be synthesized by the reaction route of Scheme 25, but is not limited thereto.
<반응식 25>Scheme 25
(1) Sub 5-2 합성(1) Sub 5-2 synthesis
둥근바닥플라스크에 4-chloro-2-hydroxybenzoic acid (200g, 1159mmol)과 potassium carbonate (80g, 579mmol)과 DMF 1000mL를 넣고 교반한다. 혼합물이 녹으면 상온에서 methyl iodide (172g, 1217mmol)를 적가하고 상온에서 4.5시간 후 반응을 종료한다. Ether와 물로 추출하고 유기층을 MgSO4로 물을 제거한 후 silica gel filter 한다. 여과된 유기층은 증발시킨 후 진공건조한다.In a round bottom flask, add 4-chloro-2-hydroxybenzoic acid (200g, 1159mmol), potassium carbonate (80g, 579mmol) and DMF 1000mL and stir. When the mixture is dissolved, methyl iodide (172g, 1217mmol) is added dropwise at room temperature, and the reaction is terminated after 4.5 hours at room temperature. Extract with ether and water, remove organic layer with MgSO 4 and filter with silica gel. The filtered organic layer is evaporated and dried in vacuo.
(2) Sub 5-3 합성(2) Sub 5-3 synthesis
둥근바닥플라스크에 methyl-4-chloro-2-hydroxybenzoate (100g, 537mmol)을 methylene chloride 1000mL에 녹여 Triethylamine (113mL, 805mmol)을 넣는다. -78℃에서 trifluoromethanesulfonic anhydride (99mL, 590mmol)를 적가하고 상온으로 서서히 온도를 올린다. 반응이 끝나면 물로 추출하고 유기층을 MgSO4로 물을 제거한 후 여과시킨다. 유기용매를 제거하고 ether로 녹이고 silicagel column 분리 후 어두운 색을 제거하고 용매를 제거 후 진공건조한다. In a round bottom flask, methyl-4-chloro-2-hydroxybenzoate (100g, 537mmol) is dissolved in 1000mL of methylene chloride and triethylamine (113mL, 805mmol) is added. Trifluoromethanesulfonic anhydride (99 mL, 590 mmol) is added dropwise at -78 ° C and the temperature is gradually raised to room temperature. After the reaction, the mixture was extracted with water and the organic layer was removed with MgSO 4 and filtered. Remove organic solvent, dissolve with ether, separate silicagel column, remove dark color, remove solvent and vacuum dry.
(3) Sub 5-4 합성(3) Sub 5-4 synthesis
둥근바닥플라스크에 methyl-4-chloro-2-(((trifluoromethyl)sulfonyl)oxy) benzoate (156g, 492mmol)과 Phenylbronicacid (60g, 492mmol)과 Pd(PPh3)4 (11g, 10mmol)과 potassium carbonate (102g, 738mmol), DMF 1000mL를 넣고 110℃에서 교반한다. 반응이 끝나면 ether와 물로 추출하고 유기층을 MgSO4로 물을 제거한 후 여과시킨다. 유기용매를 제거하고 silicagel column 분리 후 용매를 제거 후 진공건조한다. Methyl-4-chloro-2-(((trifluoromethyl) sulfonyl) oxy) benzoate (156g, 492mmol), Phenylbronicacid (60g, 492mmol), Pd (PPh 3 ) 4 (11g, 10mmol) and potassium carbonate ( 102 g, 738 mmol) and 1000 mL of DMF were added and stirred at 110 ° C. After the reaction, the mixture was extracted with ether and water, and the organic layer was removed with MgSO 4 and filtered. The organic solvent is removed, the silicagel column is separated and the solvent is removed and dried in vacuo.
(4) Sub 5-5 합성(4) Sub 5-5 synthesis
상기 Sub 2-4 (1당량)을 THF에 녹인 후에, 반응물의 온도를 -75℃로 낮추고methyllithium (2당량)을 넣었다. 4시간 동안 교반한 뒤 물을 넣어 희석시키고 2N HCl을 넣어준다. 반응이 완료되면 ethyl acetate와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 2-(5-bromo-[1,1'-biphenyl]-2-yl)propan-2-ol을 얻었다. After dissolving Sub 2-4 (1 equivalent) in THF, the temperature of the reaction was lowered to -75 ° C and methyllithium (2 equivalents) was added thereto. After stirring for 4 hours, dilute with water and add 2N HCl. After the reaction was completed, the mixture was extracted with ethyl acetate and water, the organic layer was dried over MgSO 4 , concentrated, and the resulting organic substance was purified by silicagel column and recrystallization with 2- (5-bromo- [1,1'-biphenyl] -2-yl) propan -2-ol was obtained.
(5) Sub 5-6 합성(5) Sub 5-6 synthesis
상기 합성에서 얻어진 2-(5-bromo-[1,1'-biphenyl]-2-yl)propan-2-ol (1당량)을 Acetic acid에 넣고, 반응물의 온도를 0℃로 낮추고 10분 동안 교반시켰다. 그 후 phosphoric acid를 추가한 후 20℃에서 1시간 교반한다. 마지막으로 Sodium hydroxide를 넣고 반응을 종료시킨다. 반응이 완료되면 ethyl acetate와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 3-bromo-9,9-dimethyl-9H-fluorene을 얻었다. 2- (5-bromo- [1,1'-biphenyl] -2-yl) propan-2-ol (1 equivalent) obtained in the above synthesis was added to Acetic acid, and the temperature of the reaction was lowered to 0 ° C. for 10 minutes. Stirred. After adding phosphoric acid, the mixture is stirred at 20 ° C for 1 hour. Finally add sodium hydroxide to terminate the reaction. After the reaction was completed, the mixture was extracted with ethyl acetate and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallization to obtain 3-bromo-9,9-dimethyl-9H-fluorene.
(6) Sub 5-7 합성(6) Sub 5-7 synthesis
3-bromo-9,9-dimethyl-9H-fluorene (1당량)을 둥근바닥플라스크에 DMF로 녹인 후에, Bis(pinacolato)diboron (1.1당량), Pd(dppf)Cl2 (0.03당량), KOAc (3당량)를 첨가하고 90℃에서 교반하였다. 반응이 완료되면 증류를 통해 DMF를 제거하고 CH2Cl2와 물로 추출하였다.유기층을 MgSO4로 건조하고 농축한 후 생성된 화합물을 silicagel column 및 재결정하여 2-(9,9-dimethyl-9H-fluoren-3-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane을 얻었다. After 3-bromo-9,9-dimethyl-9H-fluorene (1 equivalent) was dissolved in DMF in a round bottom flask, Bis (pinacolato) diboron (1.1 equiv), Pd (dppf) Cl 2 (0.03 equiv), KOAc ( 3 equivalents) was added and stirred at 90 ° C. After completion of the reaction, DMF was removed by distillation and extracted with CH 2 Cl 2 and water. The organic layer was dried over MgSO 4 , concentrated, and the resulting compound was purified by silicagel column and recrystallization, resulting in 2- (9,9-dimethyl-9H- fluoren-3-yl) -4,4,5,5-tetramethyl-1,3,2-dioxaborolane was obtained.
(7) Sub 5-8 합성(7) Sub 5-8 synthesis
2-(9,9-dimethyl-9H-fluoren-3-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1당량)과 4-bromoaniline (1당량), Pd(PPh3)4 (0.03당량), K2CO3 (3당량)를 무수 THF와 소량의 물에 녹이고 난 후, 24시간 동안 환류시켰다. 반응이 종료되면 반응물의 온도를 상온으로 식히고, CH2Cl2로 추출하고 물로 닦아주었다. 소량의 물을 무수 MgSO4로 제거하고 감압여과 후, 유기용매를 농축하여 생성된 생성물을 컬럼크로마토그래피를 이용하여 분리하여 4-(9,9-dimethyl-9H-fluoren-3-yl)aniline을 얻었다.2- (9,9-dimethyl-9H-fluoren-3-yl) -4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1 equiv), 4-bromoaniline (1 equiv), Pd ( PPh 3 ) 4 (0.03 equiv) and K 2 CO 3 (3 equiv) were dissolved in anhydrous THF and a small amount of water and then refluxed for 24 hours. After the reaction was completed, the temperature of the reactant was cooled to room temperature, extracted with CH 2 Cl 2 , and washed with water. After removing a small amount of water with anhydrous MgSO 4 and filtration under reduced pressure, the product produced by concentrating the organic solvent was separated by column chromatography to give 4- (9,9-dimethyl-9H-fluoren-3-yl) aniline. Got it.
(8) Sub 5 합성(8) Sub 5 Synthesis
상기 합성에서 얻어진 4-(9,9-dimethyl-9H-fluoren-3-yl)aniline (1당량)과bromobenzene 을 둥근바닥플라스크에 toluene으로 녹인 후에, Pd2(dba)3 (0.3당량), 50% P(t-Bu)3 (9당량), NaOt-Bu (3당량)을 첨가하고 40℃에서 교반하였다. 반응이 완료되면 CH2Cl2와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 화합물을 silicagel column 및 재결정하여 4-(9,9-dimethyl-9H-fluoren-3-yl)-N-phenylaniline를 얻었다. 4- (9,9-dimethyl-9H-fluoren-3-yl) aniline (1 equivalent) and bromobenzene obtained in the above synthesis were dissolved in toluene in a round bottom flask, followed by Pd 2 (dba) 3 (0.3 equivalent), 50 % P ( t- Bu) 3 (9 equiv), NaO t -Bu (3 equiv) were added and stirred at 40 ° C. After completion of the reaction, the mixture was extracted with CH 2 Cl 2 and water, and the organic layer was dried over MgSO 4 , concentrated, and the resulting compound was purified by silicagel column and recrystallization with 4- (9,9-dimethyl-9H-fluoren-3-yl) -N. -phenylaniline was obtained.
한편, Sub 5의 예시는 아래와 같으나 이에 한정되는 것은 아니며, 이들의 FD/MS는 하기 [표 4]와 같다.Meanwhile, examples of Sub 5 are as follows, but are not limited thereto, and their FD / MS are as shown in Table 4 below.
[표 4]TABLE 4
3. 화학식 2의 최종 생성물(Final Product) 합성3. Synthesis of Final Product of Formula 2
둥근바닥플라스크에 Sub 4 (1당량), Sub 5 (1.2당량), Pd2(dba)3 (0.05당량), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량)과 toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 최종 생성물을 얻었다.Sub 4 (1 equiv), Sub 5 (1.2 equiv), Pd 2 (dba) 3 (0.05 equiv), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv) Add toluene (10.5mL / 1mmol) and proceed with the reaction at 100 ℃. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain a final product.
(1) 1-19의 합성(1) Synthesis of 1-19
<반응식 26>Scheme 26
둥근바닥플라스크에 2-bromo-9-phenyl-9H-carbazole (7.7g, 24mmol), N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-3-amine (7.2g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 8.2g (수율: 68%) 얻었다.2-bromo-9-phenyl-9H-carbazole (7.7g, 24mmol), N-([1,1'-biphenyl] -4-yl) -9,9-dimethyl-9H-fluoren-3 in a round bottom flask -amine (7.2 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv), toluene (10.5 mL / 1 mmol) After the reaction proceeds at 100 ℃. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was purified by silicagel column and recrystallized to give 8.2g (yield: 68%) of the product.
(2) 1-37의 합성(2) synthesis of 1-37
<반응식 27>Scheme 27
둥근바닥플라스크에 3-bromo-9-(pyridin-2-yl)-9H-carbazole (7.8g, 24mmol), 9,9-dimethyl-N-phenyl-9H-fluoren-3-amine (5.7g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 7.5g (수율: 71%) 얻었다.3-bromo-9- (pyridin-2-yl) -9H-carbazole (7.8g, 24mmol), 9,9-dimethyl-N-phenyl-9H-fluoren-3-amine (5.7g, 20mmol) in round bottom flask ), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv), toluene (10.5 mL / 1 mmol), and then reacted at 100 ° C Proceed. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was purified by silicagel column and recrystallized to give 7.5g (yield: 71%) of the product.
(3) 2-19의 합성(3) Synthesis of 2-19
<반응식 28>Scheme 28
둥근바닥플라스크에 2-(3-bromophenyl)-9-phenyl-9H-carbazole (9.6g, 24mmol), 9,9-dimethyl-N-(naphthalen-2-yl)-9H-fluoren-3-amine (6.7g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 8.5g (수율: 65%) 얻었다.2- (3-bromophenyl) -9-phenyl-9H-carbazole (9.6g, 24mmol), 9,9-dimethyl-N- (naphthalen-2-yl) -9H-fluoren-3-amine ( 6.7 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv), toluene (10.5 mL / 1 mmol) The reaction proceeds at 100 ° C. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 8.5g (yield: 65%) of the product.
(4) 2-33의 합성(4) synthesis of 2-33
<반응식 29>Scheme 29
둥근바닥플라스크에 3-(4-bromophenyl)-9-phenyl-9H-carbazole (9.6g, 24mmol), N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-3-amine (7.2g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 9.8g (수율: 72%) 얻었다.3- (4-bromophenyl) -9-phenyl-9H-carbazole (9.6g, 24mmol), N-([1,1'-biphenyl] -4-yl) -9,9-dimethyl-9H in a round bottom flask -fluoren-3-amine (7.2 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv), toluene (10.5 mL / 1mmol) is added and the reaction proceeds at 100 ℃. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 9.8g (yield: 72%) of the product.
(5) 3-12의 합성(5) Synthesis of 3-12
<반응식 30>Scheme 30
둥근바닥플라스크에 3-(3'-bromo-[1,1'-biphenyl]-3-yl)-9-phenyl-9H-carbazole (11.4g, 24mmol), N-([1,1'-biphenyl]-4-yl)-9,9'-spirobi[fluoren]-3-amine (9.7g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 11.6g (수율: 66%) 얻었다.3- (3'-bromo- [1,1'-biphenyl] -3-yl) -9-phenyl-9H-carbazole (11.4 g, 24 mmol), N-([1,1'-biphenyl) in a round bottom flask ] -4-yl) -9,9'-spirobi [fluoren] -3-amine (9.7 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equivalent ), NaO t -Bu (3 equiv) and toluene (10.5mL / 1mmol) were added and the reaction was carried out at 100 ° C. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was purified by silicagel column and recrystallized to give 11.6g (yield: 66%).
(6) 4-1의 합성(6) Synthesis of 4-1
<반응식 31>Scheme 31
둥근바닥플라스크에 3-(7-bromodibenzo[b,d]thiophen-3-yl)-9-phenyl-9H-carbazole (12.1g, 24mmol), 9,9-dimethyl-N-phenyl-9H-fluoren-3-amine (5.7g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 9.1g (수율: 64%) 얻었다.3- (7-bromodibenzo [b, d] thiophen-3-yl) -9-phenyl-9H-carbazole (12.1g, 24mmol), 9,9-dimethyl-N-phenyl-9H-fluoren- in a round bottom flask 3-amine (5.7 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv), toluene (10.5 mL / 1 mmol ) And then proceed with the reaction at 100 ℃. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 9.1g (yield: 64%).
한편, 상기와 같은 합성예에 따라 제조된 본 발명의 화합물 1-1 내지 6-4의 FD-MS 값은 하기 [표 5]와 같다.On the other hand, FD-MS values of the compounds 1-1 to 6-4 of the present invention prepared according to the synthesis examples as described above are shown in [Table 5].
[표 5]TABLE 5
Ⅲ. 화학식 3의 합성III. Synthesis of Formula 3
본 발명에 따른 화학식 3으로 표시되는 화합물(Final Product)은 하기 반응식 32의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.The compound represented by Chemical Formula 3 according to the present invention may be synthesized by the reaction route of Scheme 32, but is not limited thereto.
<반응식 32> (Hal= I, Br 또는 Cl)Scheme 32 (Hal = I, Br or Cl)
1. Sub 6의 합성1.Synthesis of Sub 6
상기 반응식 32의 Sub 6은 하기 반응식 33의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Sub 6 of Scheme 32 may be synthesized by the reaction route of Scheme 33, but is not limited thereto.
<반응식 33>Scheme 33
(1) Sub 6-1 합성(1) Sub 6-1 synthesis
출발물질인 phenylboronic acid (16.52g, 135.5mmol)를 둥근바닥플라스크에 THF로 녹인 후에, 1-bromo-2-nitrobenzene (32.84g, 162.6mmol), Pd(PPh3)4 (7.83g, 6.8mmol), K2CO3 (56.18g, 406.5mmol), 물을 첨가하고 80℃에서 교반하였다. 반응이 완료되면 CH2Cl2와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 화합물을 silicagel column 및 재결정하여 생성물 24.83g (수율: 92%)를 얻었다.Starting material phenylboronic acid (16.52g, 135.5mmol) was dissolved in THF in a round bottom flask, 1-bromo-2-nitrobenzene (32.84g, 162.6mmol), Pd (PPh 3 ) 4 (7.83g, 6.8mmol) , K 2 CO 3 (56.18 g, 406.5 mmol), water was added and stirred at 80 ° C. After the reaction was completed, the mixture was extracted with CH 2 Cl 2 and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting compound was purified by silicagel column and recrystallized to give a product 24.83g (yield: 92%).
(2) Sub 6-2 합성(2) Sub 6-2 synthesis
상기 합성에서 얻어진 Sub 6-1 (24.83g, 124.6mmol)을 둥근바닥플라스크에 o-dichlorobenzene으로 녹인 후에, triphenylphosphine (81.73g, 311.6mmol)을 첨가하고 200℃에서 교반하였다. 반응이 완료되면 증류를 통해 o-dichlorobenzene을 제거하고 CH2Cl2와 물로 추출하였다. 유기층을 MgSO4로 건조하고 농축한 후 생성된 화합물을 silicagel column 및 재결정하여 생성물 16.46g (수율: 79%)를 얻었다.Sub 6-1 (24.83g, 124.6mmol) obtained in the above synthesis was dissolved in o- dichlorobenzene in a round bottom flask, triphenylphosphine (81.73g, 311.6mmol) was added and stirred at 200 ° C. After the reaction was completed, o -dichlorobenzene was removed by distillation and extracted with CH 2 Cl 2 and water. The organic layer was dried over MgSO 4 , concentrated and the resulting compound was purified by silicagel column and recrystallized to give 16.46 g (yield: 79%) of the product.
(3) Sub 6 합성(3) Sub 6 synthesis
상기 합성에서 얻어진 Sub 6-2 (16.46g, 98.4mmol)를 둥근바닥플라스크에 toluene으로 녹인 후에, 4-bromo-4'-iodo-1,1'-biphenyl (70.68g, 196.9mmol), Pd2(dba)3 (2.7g, 3mmol), 50% P(t-Bu)3 (3.8ml, 7.9mmol), NaOt-Bu (28.38g, 295.3mmol)을 첨가하고 70℃에서 교반하였다. 반응이 완료되면 CH2Cl2와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 화합물을 silicagel column 및 재결정하여 생성물 26.27g (수율: 67%)를 얻었다.Sub 6-2 (16.46 g, 98.4 mmol) obtained in the above synthesis was dissolved in toluene in a round bottom flask, followed by 4-bromo-4'-iodo-1,1'-biphenyl (70.68 g, 196.9 mmol) and Pd 2 (dba) 3 (2.7 g, 3 mmol), 50% P ( t -Bu) 3 (3.8 ml, 7.9 mmol), NaO t -Bu (28.38 g, 295.3 mmol) was added and stirred at 70 ° C. After completion of the reaction, the mixture was extracted with CH 2 Cl 2 and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting compound was purified by silicagel column and recrystallized to give 26.27 g (yield: 67%) of the product.
한편, Sub 6의 예시는 아래와 같으나, 이에 한정되는 것은 아니며, 이들의 FD-MS는 하기 [표 6]과 같다.Meanwhile, examples of Sub 6 are as follows, but are not limited thereto, and their FD-MSs are as shown in Table 6 below.
[표 6]TABLE 6
2. 화학식 3의 최종 생성물(Final Product) 합성2. Synthesis of Final Product of Formula 3
둥근바닥플라스크에 Sub 6 (1당량), Sub 5 (1.2당량), Pd2(dba)3 (0.05당량), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량)과 toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 최종 생성물을 얻었다.In a round bottom flask, Sub 6 (1 equiv), Sub 5 (1.2 equiv), Pd 2 (dba) 3 (0.05 equiv), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv) Add toluene (10.5mL / 1mmol) and proceed with the reaction at 100 ℃. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain a final product.
(1) 7-15 합성(1) 7-15 synthetic
<반응식 34>Scheme 34
9-(4'-bromo-[1,1'-biphenyl]-4-yl)-9H-carbazole (5.48g, 15.2mmol)을 둥근바닥플라스크에 toluene으로 녹인 후에, N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-3-amine (6.64g, 16.7mmol), Pd2(dba)3 (0.42g, 0.5mmol), 50% P(t-Bu)3 (0.6ml, 1.2mmol), NaOt-Bu (4.37g, 45.5mmol)을 첨가하고 100℃에서 교반하였다. 반응이 완료되면 CH2Cl2와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 화합물을 silicagel column 및 재결정하여 생성물 8.03g (수율: 78%)를 얻었다.9- (4'-bromo- [1,1'-biphenyl] -4-yl) -9H-carbazole (5.48g, 15.2mmol) was dissolved in toluene in a round bottom flask, followed by N-([1,1 ' -biphenyl] -4-yl) -9,9-dimethyl-9H-fluoren-3-amine (6.64 g, 16.7 mmol), Pd 2 (dba) 3 (0.42 g, 0.5 mmol), 50% P ( t- Bu) 3 (0.6 ml, 1.2 mmol), NaO t -Bu (4.37 g, 45.5 mmol) were added and stirred at 100 ° C. After the reaction was completed, the mixture was extracted with CH 2 Cl 2 and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting compound was purified by silicagel column and recrystallized to give the product 8.03g (yield: 78%).
(2) 7-23 합성(2) 7-23 synthetic
<반응식 35>Scheme 35
9-(4'-bromo-[1,1'-biphenyl]-4-yl)-9H-carbazole (6.19g, 18.4mmol)에 N-(9,9-dimethyl-9H-fluoren-3-yl)isoquinolin-4-amine (8.06g, 20.2mmol), Pd2(dba)3 (0.51g, 0.6mmol), 50% P(t-Bu)3 (0.7ml, 1.5mmol), NaOt-Bu (5.31g, 55.2mmol), 톨루엔을 상기 7-15 합성예를 사용하여 생성물 8.78g (수율: 73%)를 얻었다.N- (9,9-dimethyl-9H-fluoren-3-yl) to 9- (4'-bromo- [1,1'-biphenyl] -4-yl) -9H-carbazole (6.19 g, 18.4 mmol) isoquinolin-4-amine (8.06g, 20.2mmol), Pd 2 (dba) 3 (0.51g, 0.6mmol), 50% P ( t -Bu) 3 (0.7ml, 1.5mmol), NaO t -Bu (5.31 g, 55.2 mmol) and toluene were obtained using the above 7-15 synthesis example to obtain 8.78 g (yield: 73%) of product.
(3) 7-27 합성(3) 7-27 synthetic
<반응식 36>Scheme 36
9-(4'-bromo-[1,1'-biphenyl]-4-yl)-3-phenyl-9H-carbazole (4.12g, 14.4mmol)에 9,9-dimethyl-N-phenyl-9H-fluoren-3-amine (7.53g, 15.9mmol), Pd2(dba)3 (0.4g, 0.4mmol), 50% P(t-Bu)3 (0.6ml, 1.2mmol), NaOt-Bu (4.16g, 43.3mmol), 톨루엔을 상기 7-15 합성예를 사용하여 생성물 7.94g (수율: 81%)를 얻었다.9,9-dimethyl-N-phenyl-9H-fluoren to 9- (4'-bromo- [1,1'-biphenyl] -4-yl) -3-phenyl-9H-carbazole (4.12g, 14.4mmol) -3-amine (7.53g, 15.9mmol), Pd 2 (dba) 3 (0.4g, 0.4mmol), 50% P ( t -Bu) 3 (0.6ml, 1.2mmol), NaO t -Bu (4.16g , 43.3 mmol), toluene was obtained by using the above 7-15 synthesis example to obtain 7.94 g (yield: 81%) of the product.
(4) 7-36 합성(4) 7-36 synthetic
<반응식 37>Scheme 37
9-(4'-bromo-[1,1'-biphenyl]-4-yl)-3-phenyl-9H-carbazole (6.32g, 13mmol)에 N-([1,1'-biphenyl]-4-yl)-9,9-diphenyl-9H-fluoren-3-amine(6.79g, 14.3mmol), Pd2(dba)3 (0.36g, 0.4mmol), 50% P(t-Bu)3 (0.5ml, 1mmol), NaOt-Bu (3.75g, 39mmol), 톨루엔을 상기 7-15 합성예를 사용하여 생성물 8.47g (수율: 74%)를 얻었다.N-([1,1'-biphenyl] -4- to 9- (4'-bromo- [1,1'-biphenyl] -4-yl) -3-phenyl-9H-carbazole (6.32 g, 13 mmol) yl) -9,9-diphenyl-9H-fluoren-3-amine (6.79g, 14.3mmol), Pd 2 (dba) 3 (0.36g, 0.4mmol), 50% P ( t -Bu) 3 (0.5ml , 1 mmol), NaO t -Bu (3.75 g, 39 mmol) and toluene were obtained using 8.47 g (yield: 74%) of the product using the 7-15 synthesis example.
(5) 7-55 합성(5) 7-55 synthetic
<반응식 38>Scheme 38
9-(7-bromodibenzo[b,d]thiophen-3-yl)-9H-carbazole (5.26g, 14.6mmol)에 N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-3-amine (6.86g, 16mmol), Pd2(dba)3 (0.4g, 0.4mmol), 50% P(t-Bu)3 (0.6ml, 1.2mmol), NaOt-Bu (4.2g, 43.7mmol), 톨루엔을 상기 7-15 합성예를 사용하여 생성물 7.94g (수율: 77%)를 얻었다.9- (7-bromodibenzo [b, d] thiophen-3-yl) -9H-carbazole (5.26g, 14.6mmol) to N-([1,1'-biphenyl] -4-yl) -9,9- dimethyl-9H-fluoren-3-amine (6.86g, 16mmol), Pd 2 (dba) 3 (0.4g, 0.4mmol), 50% P ( t -Bu) 3 (0.6ml, 1.2mmol), NaO t- Bu (4.2 g, 43.7 mmol) and toluene were obtained using 7.94 g (yield: 77%) of the product using the 7-15 synthesis example.
(6) 7-67 합성(6) 7-67 synthetic
<반응식 39>Scheme 39
9-(4'-bromo-[1,1'-biphenyl]-4-yl)-9H-carbazole (5.93g, 13.6mmol)에 N-(4-(9,9-dimethyl-9H-fluoren-3-yl)phenyl)-[1,1'-biphenyl]-4-amine (5.94g, 14.9mmol), Pd2(dba)3 (0.37g, 0.4mmol), 50% P(t-Bu)3 (0.5ml, 1.1mmol), NaOt-Bu (3.91g, 40.7mmol), 톨루엔을 상기 7-15 합성예를 사용하여 생성물 8.08g (수율: 79%)를 얻었다.9- (4'-bromo- [1,1'-biphenyl] -4-yl) -9H-carbazole (5.93g, 13.6mmol) to N- (4- (9,9-dimethyl-9H-fluoren-3 -yl) phenyl)-[1,1'-biphenyl] -4-amine (5.94 g, 14.9 mmol), Pd 2 (dba) 3 (0.37 g, 0.4 mmol), 50% P ( t -Bu) 3 ( 0.5ml, 1.1mmol), NaO t -Bu (3.91g, 40.7mmol) and toluene were obtained using 8.7 g (yield: 79%) of the product using the 7-15 synthesis example.
한편, 상기와 같은 합성예에 따라 제조된 본 발명의 화합물 7-1 내지 7-92의 FD-MS 값은 하기 [표 7]과 같다.On the other hand, FD-MS values of the compounds 7-1 to 7-92 of the present invention prepared according to the synthesis examples as described above are shown in Table 7.
[표 7]TABLE 7
Ⅳ. 화학식 4의 합성Ⅳ. Synthesis of Formula 4
본 발명에 따른 화학식 4로 표시되는 화합물(Final Product)은 하기 반응식 40의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Compound represented by the formula (4) according to the present invention (Final Product) may be synthesized by the reaction path of the following Scheme 40, but is not limited thereto.
<반응식 40>Scheme 40
1. Sub 7의 합성1.Synthesis of Sub 7
상기 반응식 40의 Sub 7은 하기 반응식 41의 반응경로에 의해 합성될 수 있으나, 이에 한정되는 것은 아니다.Sub 7 of Scheme 40 may be synthesized by the reaction route of Scheme 41, but is not limited thereto.
<반응식 41>Scheme 41
(1) Sub 7-3 합성(1) Sub 7-3 synthesis
[1,1'-biphenyl]-4-amine (1당량)과 4-bromo-1,1'-biphenyl (1.1당량)을 톨루엔에 넣고 Pd2(dba)3 (0.05당량), PPh3 (0.1당량), NaOt-Bu (3당량)을 각각 첨가한 뒤, 100℃에서 24시간 교반환류시킨다. ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 di([1,1'-biphenyl]-4-yl)amine을 얻었다.[1,1'-biphenyl] -4-amine (1 equiv) and 4-bromo-1,1'-biphenyl (1.1 equiv) were added to toluene and Pd 2 (dba) 3 (0.05 equiv), PPh 3 (0.1 Equivalents) and NaO t -Bu (3 equivalents), respectively, were added and then stirred at reflux for 24 hours at 100 ° C. After extracting with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was silicagel column and recrystallized to obtain di ([1,1'-biphenyl] -4-yl) amine.
(2) Sub 7 합성(2) Sub 7 synthesis
di([1,1'-biphenyl]-4-yl)amine (1당량)과 1-bromo-4-iodobenzene (1.1당량)을 톨루엔에 넣고 Pd2(dba)3 (0.05당량), PPh3 (0.1당량), NaOt-Bu (3당량)을 각각 첨가한 뒤, 100℃에서 24시간 교반환류시킨다. ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 N-([1,1'-biphenyl]-4-yl)-N-(4-bromophenyl)-[1,1'-biphenyl]-4-amine을 얻었다. Di ([1,1'-biphenyl] -4-yl) amine (1 equiv) and 1-bromo-4-iodobenzene (1.1 equiv) were added to toluene and Pd 2 (dba) 3 (0.05 equiv), PPh 3 ( 0.1 equivalent), NaO t -Bu (3 equivalents) were added, and the mixture was stirred under reflux for 24 hours at 100 ° C. After extracting with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallization. 1,1'-biphenyl] -4-amine was obtained.
한편, 상기 Sub 7의 예시는 아래와 같으나, 이에 한정되는 것은 아니며, 이들의 FD-MS는 하기 [표 8]과 같다.Meanwhile, examples of Sub 7 are as follows, but are not limited thereto, and their FD-MSs are as shown in Table 8 below.
[표 8]TABLE 8
2. 화학식 4의 최종 생성물(Final Product) 합성2. Synthesis of Final Product of Formula 4
둥근바닥플라스크에 Sub 5 (1당량), Sub 7 (1.2당량), Pd2(dba)3 (0.05당량), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 최종 생성물을 얻었다.Sub 5 (1 equiv), Sub 7 (1.2 equiv), Pd 2 (dba) 3 (0.05 equiv), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv) Add toluene (10.5mL / 1mmol) and proceed with the reaction at 100 ℃. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain a final product.
(1) 8-29 합성(1) 8-29 synthetic
<반응식 42>Scheme 42
둥근바닥플라스크에 N-(4-bromophenyl)-N-phenyl-[1,1'-biphenyl]-4-amine (9.6g, 24mmol), N-(9,9-dimethyl-9H-fluoren-3-yl)pyridin-3-amine (5.7g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 7.4g (수율: 61%) 얻었다.N- (4-bromophenyl) -N-phenyl- [1,1'-biphenyl] -4-amine (9.6 g, 24 mmol) and N- (9,9-dimethyl-9H-fluoren-3- in a round bottom flask yl) pyridin-3-amine (5.7 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv), toluene ( 10.5mL / 1mmol) and then proceed with the reaction at 100 ℃. After completion of the reaction, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was purified by silicagel column and recrystallized to obtain 7.4g (yield: 61%).
(2) 9-4 합성(2) 9-4 synthesis
<반응식 43>Scheme 43
둥근바닥플라스크에 4'-bromo-N,N-diphenyl-[1,1'-biphenyl]-4-amine (9.6g, 24mmol), N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-3-amine (7.2g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 9.3g (수율: 68%) 얻었다.4'-bromo-N, N-diphenyl- [1,1'-biphenyl] -4-amine (9.6 g, 24 mmol), N-([1,1'-biphenyl] -4-yl) in a round bottom flask -9,9-dimethyl-9H-fluoren-3-amine (7.2 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv) and toluene (10.5 mL / 1 mmol) were added followed by reaction at 100 ° C. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 9.3g (yield: 68%) of the product.
(3) 10-16 합성(3) 10-16 synthetic
<반응식 44>Scheme 44
둥근바닥플라스크에 N-(4''-bromo-[1,1':4',1''-terphenyl]-4-yl)-N-phenylnaphthalen-1-amine (12.6g, 24mmol), N-([1,1'-biphenyl]-4-yl)-9,9-diphenyl-9H-fluoren-3-amine (9.7g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 13.0g (수율: 70%) 얻었다.N- (4 ''-bromo- [1,1 ': 4', 1 ''-terphenyl] -4-yl) -N-phenylnaphthalen-1-amine (12.6g, 24mmol), N- ([1,1'-biphenyl] -4-yl) -9,9-diphenyl-9H-fluoren-3-amine (9.7 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P ( After t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv) and toluene (10.5 mL / 1 mmol) were added, the reaction was performed at 100 ° C. After completion of the reaction, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic material was purified by silicagel column and recrystallized to obtain 13.0g (yield: 70%) of the product.
(4) 11-7 합성(4) 11-7 synthetic
<반응식 45>Scheme 45
둥근바닥플라스크에 7-bromo-N,N,9-triphenyl-9H-carbazol-2-amine (11.7g, 24mmol), N-(naphthalen-1-yl)-9,9'-spirobi[fluoren]-3-amine (9.2g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 10.0g (수율: 58%) 얻었다.7-bromo-N, N, 9-triphenyl-9H-carbazol-2-amine (11.7g, 24mmol), N- (naphthalen-1-yl) -9,9'-spirobi [fluoren]-in a round bottom flask 3-amine (9.2 g, 20 mmol), Pd 2 (dba) 3 (0.03-0.05 mmol), P (t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv), toluene (10.5 mL / 1 mmol ) Is added and the reaction proceeds at 100 ° C. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 10.0 g (yield: 58%) of the product.
(5) 12-4 합성(5) 12-4 synthetic
<반응식 46>Scheme 46
둥근바닥플라스크에 N-(4'-bromo-[1,1'-biphenyl]-4-yl)-N-phenylnaphthalen-1-amine (10.8g, 24mmol), N-([1,1'-biphenyl]-4-yl)-13,13-dimethyl-13H-indeno[1,2-l]phenanthren-10-amine (9.2g, 20mmol), Pd2(dba)3 (0.03~0.05mmol), P(t-Bu)3 (0.1당량), NaOt-Bu (3당량), toluene (10.5mL / 1mmol)을 넣은 후에 100℃에서 반응을 진행한다. 반응이 완료되면 ether와 물로 추출한 후 유기층을 MgSO4로 건조하고 농축한 후 생성된 유기물을 silicagel column 및 재결정하여 생성물을 10.5g (수율: 63%) 얻었다.N- (4'-bromo- [1,1'-biphenyl] -4-yl) -N-phenylnaphthalen-1-amine (10.8 g, 24 mmol) and N-([1,1'-biphenyl) in a round bottom flask ] -4-yl) -13,13-dimethyl-13H-indeno [1,2-l] phenanthren-10-amine (9.2g, 20mmol), Pd 2 (dba) 3 (0.03 ~ 0.05mmol), P ( After t-Bu) 3 (0.1 equiv), NaO t -Bu (3 equiv) and toluene (10.5 mL / 1 mmol) were added, the reaction was performed at 100 ° C. After the reaction was completed, the mixture was extracted with ether and water, the organic layer was dried over MgSO 4 and concentrated, and the resulting organic substance was purified by silicagel column and recrystallized to obtain 10.5g (yield: 63%) of the product.
한편, 상기와 같은 합성예에 따라 제조된 본 발명의 화합물 8-1 내지 12-4의 FD-MS 값은 하기 [표 9]와 같다.On the other hand, the FD-MS values of the compounds 8-1 to 12-4 of the present invention prepared according to the synthesis examples as described in the following [Table 9].
[표 9]TABLE 9
유기전기소자의 제조평가Manufacturing Evaluation of Organic Electrical Device
[실험예 1] 레드유기전기발광소자Experimental Example 1 Red Organic Light Emitting Diode
먼저, 유리 기판에 형성된 ITO층(양극) 위에 N1-(naphthalen-2-yl)-N4,N4-bis(4-(naphthalen-2-yl(phenyl)amino)phenyl)-N1-phenylbenzene-1,4-diamine (이하, "2-TNATA"로 약기함)을 60nm 두께로 진공증착하여 정공주입층을 형성한 후, 상기 정공주입층 상에 본 발명의 화학식 1로 표시되는 화합물을 60nm 두께로 진공증착하여 정공수송층을 형성하였다. 이어서, 상기 정공수송층 상에 본 발명의 화학식 2 내지 화학식 4로 표시되는 화합물 중 하나를 20nm 두께로 진공증착하여 발광보조층을 형성하였다. 이후, 상기 발광보조층 상에, 4,4'-N,N'-dicarbazole-biphenyl (이하, "CBP"로 약기함)을 호스트로, bis-(1-phenylisoquinolyl)iridium(Ⅲ) acetylacetonate (이하, "(piq)2Ir(acac)"로 약기함)를 도판트로 하여 95:5 중량으로 도핑한 혼합물을 30nm 두께로 진공증착하여 발광층을 형성하였다. 이어서, 상기 발광층 상에 (1,1'-비스페닐)-4-올레이토)비스(2-메틸-8-퀴놀린올레이토)알루미늄 (이하, "BAlq"로 약기함)을 10nm 두께로 진공증착하여 정공저지층을 형성하고, 상기 정공저지층 상에 트리스(8-퀴놀리놀)알루미늄 (이하 "Alq3"로 약기함)을 40nm 두께로 진공증착하여 전자수송층을 형성하였다. 이후, 할로젠화 알칼리 금속인 LiF를 0.2nm 두께로 증착하여 전자주입층을 형성하고, 이어서 Al을 150nm 두께로 증착하여 음극을 형성함으로써 유기전기발광소자를 제조하였다.First, on the ITO layer (anode) formed on the glass substrate, N 1- (naphthalen-2-yl) -N 4 , N 4 -bis (4- (naphthalen-2-yl (phenyl) amino) phenyl) -N 1- phenylbenzene-1,4-diamine (hereinafter abbreviated as "2-TNATA") was vacuum deposited to a thickness of 60 nm to form a hole injection layer, and then the compound represented by Formula 1 of the present invention on the hole injection layer Vacuum deposition to a thickness of 60nm to form a hole transport layer. Subsequently, one of the compounds represented by Formulas 2 to 4 of the present invention was vacuum-deposited to a thickness of 20 nm on the hole transport layer to form a light emitting auxiliary layer. Subsequently, 4,4'-N, N'-dicarbazole-biphenyl (hereinafter abbreviated as "CBP") as a host and bis- (1-phenylisoquinolyl) iridium (III) acetylacetonate (hereinafter referred to as "host") on the light emitting auxiliary layer. , abbreviated as "(piq) 2 Ir (acac)") as a dopant, and the mixture doped at 95: 5 weight was vacuum deposited to a thickness of 30 nm to form a light emitting layer. Subsequently, (1,1'-bisphenyl) -4-oleito) bis (2-methyl-8-quinolineoleito) aluminum (hereinafter abbreviated as "BAlq") was vacuum-deposited on the emission layer to a thickness of 10 nm. A hole blocking layer was formed, and tris (8-quinolinol) aluminum (hereinafter abbreviated as "Alq 3 ") was vacuum deposited to a thickness of 40 nm on the hole blocking layer to form an electron transport layer. Thereafter, LiF, an alkali metal halide, was deposited to a thickness of 0.2 nm to form an electron injection layer, and then an Al was deposited to a thickness of 150 nm to form a cathode, thereby manufacturing an organic electroluminescent device.
[비교예 Ⅰ][Comparative Example I]
발광보조층을 형성하지 않은 것을 제외하고는 상기 실험예 Ⅰ과 동일한 방법으로 유기전기소자를 제조하였다.An organic electric device was manufactured in the same manner as in Experimental Example I, except that the light emitting auxiliary layer was not formed.
이와 같이 제조된 실험예 Ⅰ(실험예(1) 내지 실험예(30)) 및 비교예 Ⅰ(비교예(1) 내지 비교예(3))에 의한 유기전기발광소자들에 순바이어스 직류전압을 가하여 포토리서치(photoresearch)사의 PR-650으로 전기발광(EL) 특성을 측정하였으며, 또한 2500cd/㎡ 기준 휘도에서 맥사이언스사에서 제조된 수명 측정 장비를 통해 T95 수명을 측정하였다.The forward bias DC voltage was applied to the organic electroluminescent devices according to Experimental Example I (Experimental Example (1) to Experimental Example 30) and Comparative Example I (Comparative Example (1) to Comparative Example (3)) prepared as described above. In addition, the electroluminescence (EL) characteristics were measured with a PR-650 photoresearch company, and T95 life was measured using a life-time measurement device manufactured by McScience Inc. at a luminance of 2500 cd / m 2.
하기 [표 10]은 본 발명에 따른 화합물을 적용한 실험예 I(실험예(1) 내지 실험예(30)) 및 비교예 Ⅰ(비교예(1) 내지 비교예(3))에 대한 소자제작 및 그 평가 결과를 나타낸다.Table 10 shows device fabrication for Experimental Example I (Experimental Example (1) to Experimental Example (30)) and Comparative Example I (Comparative Example (1) to Comparative Example (3)) to which the compound according to the present invention was applied. And the evaluation result.
[표 10]TABLE 10
[실험예 Ⅱ] 그린유기전기발광소자Experimental Example II Green Organic Light Emitting Diode
먼저, 유리 기판에 형성된 ITO층(양극) 위에 2-TNATA을 60nm 두께로 진공증착하여 정공주입층을 형성한 후, 상기 정공주입층 상에 본 발명의 화학식 1로 표시되는 화합물을 60nm 두께로 진공증착하여 정공수송층을 형성하였다. 이어서, 상기 정공수송층 상에 본 발명의 화학식 2 내지 화학식 4로 표시되는 화합물 중 하나를 20nm 두께로 진공증착하여 발광보조층을 형성하였다. 이후, 상기 발광보조층 상에, CBP를 호스트로, tris(2-phenylpyridine)-iridium (이하, "Ir(ppy)3"로 약기함)을 도판트로 하여 95:5 중량으로 도핑한 혼합물을 30nm 두께로 진공증착하여 발광층을 형성하였다. 이어서, 상기 발광층 상에 BAlq을 10nm 두께로 진공증착하여 정공저지층을 형성하고, 상기 정공저지층 상에 Alq3을 40nm 두께로 진공증착하여 전자수송층을 형성하였다. 이후, 할로젠화 알칼리 금속인 LiF를 0.2nm 두께로 증착하여 전자주입층을 형성하고, 이어서 Al을 150nm 두께로 증착하여 음극을 형성함으로써 유기전기발광소자를 제조하였다.First, a hole injection layer is formed by vacuum depositing 2-TNATA with a thickness of 60 nm on an ITO layer (anode) formed on a glass substrate, and then vacuuming the compound represented by Formula 1 of the present invention on the hole injection layer with a thickness of 60 nm. It was deposited to form a hole transport layer. Subsequently, one of the compounds represented by Formulas 2 to 4 of the present invention was vacuum-deposited to a thickness of 20 nm on the hole transport layer to form a light emitting auxiliary layer. Then, 30 nm of the mixture doped at 95: 5 weight using CBP as a host and tris (2-phenylpyridine) -iridium (hereinafter abbreviated as "Ir (ppy) 3 ") as a dopant on the light emitting auxiliary layer. Vacuum deposition was performed to a thickness to form a light emitting layer. Subsequently, BAlq was vacuum deposited on the emission layer to form a hole blocking layer, and Alq 3 was deposited on the hole blocking layer to 40 nm in thickness to form an electron transport layer. Thereafter, LiF, an alkali metal halide, was deposited to a thickness of 0.2 nm to form an electron injection layer, and then an Al was deposited to a thickness of 150 nm to form a cathode, thereby manufacturing an organic electroluminescent device.
[비교예 Ⅱ]Comparative Example II
발광보조층을 형성하지 않은 것을 제외하고는 상기 실험예 Ⅱ와 동일한 방법으로 유기전기소자를 제조하였다.An organic electric device was manufactured in the same manner as in Experimental Example II, except that the light emitting auxiliary layer was not formed.
이와 같이 제조된 실험예 Ⅱ(실험예(31) 내지 실험예(60)) 및 비교예 Ⅱ(비교예(4) 내지 비교예(6))에 의한 유기전기발광소자들에 순바이어스 직류전압을 가하여 포토리서치(photoresearch)사의 PR-650으로 전기발광(EL) 특성을 측정하였으며, 또한 5000cd/㎡ 기준 휘도에서 맥사이언스사에서 제조된 수명 측정 장비를 통해 T95 수명을 측정하였다.The forward bias DC voltage was applied to the organic electroluminescent devices according to Experimental Example II (Experimental Example (31) to Experimental Example (60)) and Comparative Example II (Comparative Example (4) to Comparative Example (6)) prepared as described above. In addition, the electroluminescence (EL) characteristics were measured with a PR-650 photoresearch company, and the T95 life was measured using a life-time measurement device manufactured by McScience Inc. at a luminance of 5000 cd / m 2.
하기 [표 11]은 본 발명에 따른 화합물을 적용한 실험예 Ⅱ(실험예(31) 내지 실험예(60)) 및 비교예 Ⅱ(비교예(4) 내지 비교예(6))에 대한 소자제작 및 그 평가 결과를 나타낸다.Table 11 shows device fabrication of Experimental Example II (Experimental Example (31) to Experimental Example (60)) and Comparative Example II (Comparative Example (4) to Comparative Example (6)) to which the compound according to the present invention was applied. And the evaluation result.
[표 11]TABLE 11
[실험예 Ⅲ] 블루유기전기발광소자Experimental Example III Blue Organic Electroluminescent Device
먼저, 유리 기판에 형성된 ITO층(양극) 위에 2-TNATA을 60nm 두께로 진공증착하여 정공주입층을 형성한 후, 상기 정공주입층 상에 본 발명의 화학식 1로 표시되는 화합물을 60nm 두께로 진공증착하여 정공수송층을 형성하였다. 이어서, 상기 정공수송층 상에 본 발명의 화학식 2 내지 화학식 4로 표시되는 화합물 중 하나를 20nm 두께로 진공증착하여 발광보조층을 형성하였다. 이후, 상기 발광보조층 상에, 9,10-di(naphthalen-2-yl)anthracene을 호스트로, BD-052X(Idemitsukosan사의 제품)를 도판트로하여 93:7 중량으로 도핑한 혼합물을 30nm 두께로 진공증착하여 발광층을 형성하였다. 이어서, 상기 발광층 상에 BAlq을 10nm 두께로 진공증착하여 정공저지층을 형성하고, 상기 정공저지층 상에 Alq3을 40nm 두께로 진공증착하여 전자수송층을 형성하였다. 이후, 할로젠화 알칼리 금속인 LiF를 0.2nm 두께로 증착하여 전자주입층을 형성하고, 이어서 Al을 150nm 두께로 증착하여 음극을 형성함으로써 유기전기발광소자를 제조하였다.First, a hole injection layer is formed by vacuum depositing 2-TNATA with a thickness of 60 nm on an ITO layer (anode) formed on a glass substrate, and then vacuuming the compound represented by Formula 1 of the present invention on the hole injection layer with a thickness of 60 nm. It was deposited to form a hole transport layer. Subsequently, one of the compounds represented by Formulas 2 to 4 of the present invention was vacuum-deposited to a thickness of 20 nm on the hole transport layer to form a light emitting auxiliary layer. Subsequently, on the light emitting auxiliary layer, a mixture doped with 9,10-di (naphthalen-2-yl) anthracene as a host and BD-052X (manufactured by Idemitsukosan) as a dopant at 93: 7 weight was 30 nm thick. Evaporation was carried out in vacuo to form a light emitting layer. Subsequently, BAlq was vacuum deposited on the emission layer to form a hole blocking layer, and Alq 3 was deposited on the hole blocking layer to 40 nm in thickness to form an electron transport layer. Thereafter, LiF, an alkali metal halide, was deposited to a thickness of 0.2 nm to form an electron injection layer, and then an Al was deposited to a thickness of 150 nm to form a cathode, thereby manufacturing an organic electroluminescent device.
[비교예 Ⅲ]Comparative Example III
발광보조층을 형성하지 않은 것을 제외하고는 상기 실험예 Ⅲ과 동일한 방법으로 유기전기소자를 제조하였다.An organic electric device was manufactured in the same manner as in Experimental Example III, except that the light emitting auxiliary layer was not formed.
이와 같이 제조된 실험예 Ⅲ(실험예(61) 내지 실험예(90)) 및 비교예 Ⅲ(비교예(7) 내지 비교예(9))에 의한 유기전기발광소자들에 순바이어스 직류전압을 가하여 포토리서치(photoresearch)사의 PR-650으로 전기발광(EL) 특성을 측정하였으며, 또한 500cd/㎡ 기준 휘도에서 맥사이언스사에서 제조된 수명 측정 장비를 통해 T95 수명을 측정하였다.The forward bias DC voltage was applied to the organic electroluminescent devices according to Experimental Example III (Experimental Example (61) to Experimental Example 90) and Comparative Example III (Comparative Example (7) to Comparative Example (9)) prepared as described above. In addition, the electroluminescence (EL) characteristics were measured with a PR-650 photoresearch company, and T95 life was measured using a life measurement device manufactured by McScience Inc. at a luminance of 500 cd / m 2.
하기 [표 12]는 본 발명에 따른 화합물을 적용한 실험예 Ⅲ(실험예(61) 내지 실험예(90)) 및 비교예 Ⅲ(비교예(7) 내지 비교예(9))에 대한 소자제작 및 그 평가 결과를 나타낸다.Table 12 shows device fabrication for Experimental Example III (Experimental Example (61) to Experimental Example (90)) and Comparative Example III (Comparative Example (7) to Comparative Example (9)) to which the compound according to the present invention was applied. And the evaluation result.
[표 12]TABLE 12
상기 [표 10] 내지 [표 12]의 결과로부터 알 수 있듯이, 화학식 1로 표시되는 화합물을 정공수송층으로 사용하고, 화학식 2 내지 화학식 4로 표시되는 화합물 중 하나를 발광보조층으로 사용한 실험예가 발광보조층을 사용하지 않은 비교예보다 유기전기발광소자의 발광효율을 현저히 개선시킴을 알 수 있다.As can be seen from the results of Tables 10 to 12, an experimental example using the compound represented by Formula 1 as the hole transport layer and using one of the compounds represented by Formulas 2 to 4 as the light emitting auxiliary layer It can be seen that the luminous efficiency of the organic electroluminescent device is remarkably improved than the comparative example without using the auxiliary layer.
이는 fluorene의 3번 위치로 치환된 본 발명의 화합물이 단독으로 발광보조층으로 사용될 경우 높은 T1 에너지 레벨과 깊은 HOMO 에너지 레벨을 갖게 되는데 이로 인해 정공과 전자가 전하균형 (charge balance)을 이루고 정공수송층 계면이 아닌 발광층 내부에서 발광이 이루어져 효율을 더 극대화시켜주기 때문인 것으로 판단된다.When the compound of the present invention substituted with position 3 of fluorene alone is used as the light emitting auxiliary layer, it has a high T1 energy level and a deep HOMO energy level. It is believed that the light emission is performed inside the light emitting layer instead of the interface to maximize the efficiency.
아울러, 전술한 소자 제작의 평가 결과에서는 정공수송층과 발광보조층 관점에서 소자 특성을 설명하였으나, 통상적으로 정공수송층이나 발광보조층으로 사용되는 재료들은 전술한 전자주입층, 전자수송층, 정공주입층 및 발광층 등 유기전기소자의 유기물층으로 단일 또는 다른 재료와 혼합으로 사용될 수 있다. 따라서 전술한 이유로 본 발명의 화합물은 정공수송층이나 발광보조층 이외에 다른 유기물층, 예를 들어 전자주입층, 전자수송층, 정공주입층 및 발광층 등에 단일 또는 다른 재료와 혼합으로 사용될 수 있다. In addition, in the evaluation results of the above-described device fabrication, the device characteristics were described in terms of the hole transport layer and the light emitting auxiliary layer. The organic material layer of the organic electric element such as the light emitting layer may be used in a single or mixed with other materials. Therefore, the compounds of the present invention can be used in a single or other material mixed with other organic material layers, for example, electron injection layer, electron transport layer, hole injection layer and the light emitting layer in addition to the hole transport layer or the light emitting auxiliary layer.
이상의 설명은 본 발명을 예시적으로 설명한 것에 불과한 것으로, 본 발명이 속하는 기술분야에서 통상의 지식을 가지는 자라면 본 발명의 본질적인 특성에서 벗어나지 않는 범위에서 다양한 변형이 가능할 것이다. 따라서, 본 명세서에 개시된 실시예들은 본 발명을 한정하기 위한 것이 아니라 설명하기 위한 것이고, 이러한 실시예에 의하여 본 발명의 사상과 범위가 한정되는 것은 아니다. 본 발명의 보호범위는 아래의 청구범위에 의하여 해석되어야 하며, 그와 동등한 범위 내에 있는 모든 기술은 본 발명의 권리범위에 포함하는 것으로 해석되어야 할 것이다.The above description is merely illustrative of the present invention, and those skilled in the art to which the present invention pertains may various modifications without departing from the essential characteristics of the present invention. Accordingly, the embodiments disclosed herein are not intended to limit the present invention but to describe the present invention, and the spirit and scope of the present invention are not limited by these embodiments. The protection scope of the present invention should be interpreted by the following claims, and all the technologies within the equivalent scope should be interpreted as being included in the scope of the present invention.
CROSS-REFERENCE TO RELATED APPLICATIONCROSS-REFERENCE TO RELATED APPLICATION
본 특허출원은 2013년 9월 17일 한국에 출원한 특허출원번호 제 10-2013-0111663 호에 대해 미국 특허법 119(a)조 (35 U.S.C § 119(a))에 따라 우선권을 주장하며, 그 모든 내용은 참고문헌으로 본 특허출원에 병합된다. 아울러, 본 특허출원은 미국 이외에 국가에 대해서도 위와 동일한 이유로 우선권을 주장하면 그 모든 내용은 참고문헌으로 본 특허출원에 병합된다.This patent application claims priority under Patent Application No. 10-2013-0111663, filed with Korea on September 17, 2013, pursuant to Article 119 (a) (35 USC § 119 (a)). All content is incorporated by reference in this patent application. In addition, if this patent application claims priority for the same reason for countries other than the United States, all its contents are incorporated into this patent application by reference.
Claims (9)
- 제 1전극; 제 2전극; 및 상기 제 1전극과 제 2전극 사이에 위치하며 적어도 정공수송층과 발광보조층, 발광층을 포함하는 유기물층;을 포함하는 유기전기소자에 있어서, A first electrode; Second electrode; And an organic material layer disposed between the first electrode and the second electrode, the organic material layer including at least a hole transport layer, a light emitting auxiliary layer, and a light emitting layer.상기 제 1전극과 발광보조층 사이에 존재하는 정공수송층은 화학식 1로 표시되는 화합물을 포함하고,The hole transport layer existing between the first electrode and the light emitting auxiliary layer includes a compound represented by Formula 1,상기 정공수송층과 발광층 사이에 존재하는 발광보조층은 하기 화학식 2 내지 4로 표시되는 화합물 중 적어도 하나를 포함하는 유기전기소자.The light emitting auxiliary layer existing between the hole transport layer and the light emitting layer comprises at least one of the compounds represented by the following formula (2) to (4).<화학식 1><Formula 1>[상기 화학식 1 내지 화학식 4에서,[In Formula 1 to Formula 4,Ar0 내지 Ar2, 및 Ar6 내지 Ar11은 i) 서로 독립적으로 C6~C60의 아릴기; 플루오렌일기; O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기; C3~C60의 지방족고리와 C6~C60의 방향족고리의 융합고리기; C1~C50의 알킬기; C2~C20의 알켄일기; C2~C20의 알킨일기; C1~C30의 알콕실기; C6~C30의 아릴옥시기; 및 -L-N(Ra)(Rb);로 이루어진 군에서 선택되거나, 또는 ii) Ar1과 Ar2는 서로 결합하여 고리를 형성하며,Ar 0 to Ar 2 , and Ar 6 to Ar 11 are each independently i) a C 6 to C 60 aryl group; Fluorenyl group; C 2 ~ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; Fused ring group of an aromatic ring of C 3 ~ C 60 of aliphatic rings and C 6 ~ C 60; C 1 ~ C 50 Alkyl group; C 2 ~ C 20 Alkenyl group; Alkynyl groups of C 2 to C 20 ; C 1 -C 30 alkoxyl group; C 6 -C 30 aryloxy group; And -LN (R a ) (R b ); or ii) Ar 1 and Ar 2 are bonded to each other to form a ring,Ar4 및 Ar5는 i) 서로 독립적으로 수소; C1~C50의 알킬기; C6~C60의 아릴기; 플루오렌일기; O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기;로 이루어진 군에서 선택거나, 또는 ii) Ar4와 Ar5가 서로 결합하여 이들이 결합된 플루오렌과 함께 스파이로 화합물을 형성하며,Ar 4 and Ar 5 are i) independently of each other hydrogen; C 1 ~ C 50 Alkyl group; C 6 ~ C 60 Aryl group; Fluorenyl group; C 2 ~ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; or ii) Ar 4 and Ar 5 are bonded to each other to combine Together with fluorene form spiro compounds,a, b, c, d, e, f 및 g는 0~4의 정수이며,a, b, c, d, e, f and g are integers from 0 to 4,R0 내지 R6은 i) 서로 독립적으로 중수소; 할로겐; C6~C60의 아릴기; 플루오렌일기; O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기; C3~C60의 지방족고리와 C6~C60의 방향족고리의 융합고리기; C1~C50의 알킬기; C2~C20의 알켄일기; C2~C20의 알킨일기; C1~C30의 알콕실기; C6~C30의 아릴옥시기; 및 -L-N(Ra)(Rb);로 이루어진 군에서 선택되거나, 또는 ii) R0 내지 R6은 이웃하는 치환기끼리 서로 결합하여 적어도 하나의 고리를 형성하며,R 0 to R 6 are i) independently of each other deuterium; halogen; C 6 ~ C 60 Aryl group; Fluorenyl group; C 2 ~ C 60 heterocyclic group containing at least one heteroatom of O, N, S, Si and P; Fused ring group of an aromatic ring of C 3 ~ C 60 of aliphatic rings and C 6 ~ C 60; C 1 ~ C 50 Alkyl group; C 2 ~ C 20 Alkenyl group; Alkynyl groups of C 2 to C 20 ; C 1 -C 30 alkoxyl group; C 6 -C 30 aryloxy group; And -LN (R a ) (R b ); or ii) R 0 to R 6 combine with each other to form at least one ring,L1 내지 L6 및 상기 L은 서로 독립적으로 단일결합; C6~C60의 아릴렌기; 플루오렌일렌기; C3~C60의 지방족고리와 C6~C60의 방향족고리의 융합고리기; 및 O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기;로 이루어진 군에서 선택되며(여기서, L3 내지 L5인 경우 단일결합은 제외함),L 1 to L 6 and L are independently of each other a single bond; C 6 ~ C 60 arylene group; Fluorenylene groups; Fused ring group of an aromatic ring of C 3 ~ C 60 of aliphatic rings and C 6 ~ C 60; And a C 2 to C 60 heterocyclic group including at least one heteroatom of O, N, S, Si, and P; wherein L 3 to L 5 exclude a single bond. ),상기 Ra 및 Rb는 서로 독립적으로 C6~C60의 아릴기; 플루오렌일기; C3~C60의 지방족고리와 C6~C60의 방향족고리의 융합고리기; 및 O, N, S, Si 및 P 중 적어도 하나의 헤테로원자를 포함하는 C2~C60의 헤테로고리기;로 이루어진 군에서 선택된다.R a and R b are each independently a C 6 ~ C 60 aryl group; Fluorenyl group; Fused ring group of an aromatic ring of C 3 ~ C 60 of aliphatic rings and C 6 ~ C 60; And a C 2 -C 60 heterocyclic group including at least one heteroatom of O, N, S, Si, and P.(상기 아릴기, 플루오렌일기, 헤테로고리기, 융합고리기, 알킬기, 알켄일기, 알킨일기, 알콕실기, 아릴옥시기, 아릴렌기, 플루오렌일렌기는 각각 중수소; 할로겐; 실란기; 실록산기; 붕소기; 게르마늄기; 시아노기; 니트로기; -L-N(Ra)(Rb); C1~C20의 알킬싸이오기; C1~C20의 알콕실기; C1~C20의 알킬기; C2~C20의 알켄일기; C2~C20의 알킨일기; C6~C20의 아릴기; 중수소로 치환된 C6~C20의 아릴기; 플루오렌일기; C2~C20의 헤테로고리기; C3~C20의 시클로알킬기; C7~C20의 아릴알킬기 및 C8~C20의 아릴알켄일기로 이루어진 군에서 선택된 하나 이상의 치환기로 더욱 치환될 수 있으며, 또한 이들 치환기들은 서로 결합하여 고리를 형성할 수도 있다)](The aryl group, fluorenyl group, heterocyclic group, fused ring group, alkyl group, alkenyl group, alkynyl group, alkoxyl group, aryloxy group, arylene group, fluorenylene group, respectively, deuterium; halogen; silane group; siloxane group; Boron group; germanium group; cyano group; nitro group; -LN (Ra) (Rb); COne~ C20Alkylthio groups; COne~ C20An alkoxyl group; COne~ C20An alkyl group; C2~ C20Alkenyl group; C2~ C20Alkynyl group of; C6~ C20of Aryl group; Deuterated C6~ C20Aryl group; Fluorenyl groups; C2~ C20Heterocyclic group of; C3~ C20Cycloalkyl group; C7~ C20Arylalkyl group and C8~ C20May be further substituted with one or more substituents selected from the group consisting of arylalkenyl groups, and these substituents may combine with each other to form a ring.]
- 제 1 항에 있어서,The method of claim 1,상기 제 1전극의 일측면 중 상기 유기물층과 반대되는 일측 또는 상기 제 2전극의 일측면 중 상기 유기물층과 반대되는 일측 중 적어도 하나에 형성되는 광효율 개선층을 포함하는 유기전기소자.And an optical efficiency improving layer formed on at least one of one side of the first electrode opposite to the organic material layer or one side of the second electrode opposite to the organic material layer.
- 제 1 항에 있어서,The method of claim 1,상기 유기물층은 스핀코팅 공정, 노즐 프린팅 공정, 잉크젯 프린팅 공정, 슬롯코팅 공정, 딥코팅 공정 및 롤투롤 공정 중 어느 하나에 의해 형성됨을 특징으로 하는 유기전기소자.The organic material layer is formed by any one of a spin coating process, a nozzle printing process, an inkjet printing process, a slot coating process, a dip coating process and a roll-to-roll process.
- 제 1 항 내지 제 7 항 중 어느 한 항의 유기전기소자를 포함하는 디스플레이장치; 및A display device comprising the organic electroluminescent element of any one of claims 1 to 7; And상기 디스플레이장치를 구동하는 제어부;를 포함하는 전자장치.And a controller for driving the display device.
- 제 8 항에 있어서,The method of claim 8,상기 유기전기소자는 유기전기발광소자, 유기태양전지, 유기감광체, 유기트랜지스터 및 단색 또는 백색 조명용 소자 중 적어도 하나인 것을 특징으로 하는 전자장치.The organic electroluminescent device is an electronic device, characterized in that at least one of an organic electroluminescent device, an organic solar cell, an organic photoconductor, an organic transistor and a device for monochrome or white illumination.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020130111663A KR102072756B1 (en) | 2013-09-17 | 2013-09-17 | Organic electronic element using a compound for organic electronic element, and an electronic device thereof |
| KR10-2013-0111663 | 2013-09-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015041428A1 true WO2015041428A1 (en) | 2015-03-26 |
Family
ID=52689049
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2014/008399 WO2015041428A1 (en) | 2013-09-17 | 2014-09-05 | Organic electronic element using compound for organic electronic element and electronic device thereof |
Country Status (2)
| Country | Link |
|---|---|
| KR (1) | KR102072756B1 (en) |
| WO (1) | WO2015041428A1 (en) |
Cited By (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016006709A1 (en) * | 2014-07-11 | 2016-01-14 | 出光興産株式会社 | Compound, material for organic electroluminescent element, organic electroluminescent element, and electronic device |
| CN105609662A (en) * | 2015-12-22 | 2016-05-25 | 成都新柯力化工科技有限公司 | Preparation method of special hole transport material for perovskite photovoltaic material |
| US20160164002A1 (en) * | 2013-07-30 | 2016-06-09 | Merck Patent Gmbh | Materials for electronic devices |
| WO2016111301A1 (en) * | 2015-01-08 | 2016-07-14 | 保土谷化学工業株式会社 | Organic electroluminescent element |
| US20170012212A1 (en) * | 2014-01-22 | 2017-01-12 | Duk San Neolux Co., Ltd. | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
| CN106565592A (en) * | 2015-12-09 | 2017-04-19 | 广东阿格蕾雅光电材料有限公司 | Organic hole transporting material |
| CN106848060A (en) * | 2016-12-26 | 2017-06-13 | 广东阿格蕾雅光电材料有限公司 | Only P-type semiconductor diode component based on asymmetric organic hole mobile material |
| CN106866499A (en) * | 2016-12-26 | 2017-06-20 | 广东阿格蕾雅光电材料有限公司 | Organic hole transport material |
| WO2017102063A1 (en) * | 2015-12-16 | 2017-06-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN107266436A (en) * | 2017-06-30 | 2017-10-20 | 长春海谱润斯科技有限公司 | A kind of organic photoelectrical material and its preparation method and application |
| CN107827873A (en) * | 2017-10-23 | 2018-03-23 | 长春海谱润斯科技有限公司 | A kind of derivative of tri-arylamine group and its organic luminescent device |
| CN107963973A (en) * | 2016-10-20 | 2018-04-27 | 东进世美肯株式会社 | Compounds and the organic luminescent device for including it |
| WO2018095392A1 (en) | 2016-11-23 | 2018-05-31 | 广州华睿光电材料有限公司 | Organic mixture, composition, and organic electronic component |
| JPWO2017122813A1 (en) * | 2016-01-14 | 2018-11-08 | 保土谷化学工業株式会社 | Organic electroluminescence device |
| CN108929234A (en) * | 2018-07-05 | 2018-12-04 | 长春海谱润斯科技有限公司 | A kind of preparation of aromatic amine derivatives and its organic electroluminescence device |
| US10170707B2 (en) | 2015-06-16 | 2019-01-01 | Idemitsu Kosan Co., Ltd. | Compound, material for organic electroluminescence element, organic electroluminescence element, and electronic device |
| JP2019506369A (en) * | 2015-12-16 | 2019-03-07 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| CN109485577A (en) * | 2017-09-13 | 2019-03-19 | 东进世美肯株式会社 | Compounds and organic luminescent device comprising it |
| JP2019510011A (en) * | 2016-03-03 | 2019-04-11 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| CN110892539A (en) * | 2017-07-27 | 2020-03-17 | Sfc株式会社 | Organic light-emitting element capable of being driven at low voltage and having high efficiency and long life |
| US11302870B2 (en) | 2016-11-02 | 2022-04-12 | Merck Patent Gmbh | Materials for electronic devices |
| US20220278285A1 (en) * | 2017-09-29 | 2022-09-01 | Duk San Neolux Co., Ltd. | Compound for organic electric element, organic electric element comprising the same, and electronic device thereof |
| US11649225B2 (en) | 2017-10-27 | 2023-05-16 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compound and organic electroluminescent device comprising the same |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10840454B2 (en) | 2013-06-14 | 2020-11-17 | Samsung Display Co., Ltd. | Organic light-emitting devices |
| US11895909B2 (en) | 2015-04-29 | 2024-02-06 | Samsung Display Co., Ltd. | Organic light-emitting device |
| EP3328825B1 (en) * | 2015-07-29 | 2023-06-28 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| KR101614738B1 (en) * | 2015-11-02 | 2016-04-22 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
| WO2017090919A1 (en) * | 2015-11-26 | 2017-06-01 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using same, and electronic device comprising same |
| KR102616579B1 (en) * | 2016-04-08 | 2023-12-22 | 삼성디스플레이 주식회사 | Organic light emitting device |
| KR102406212B1 (en) * | 2017-05-31 | 2022-06-10 | 주식회사 동진쎄미켐 | Compound for forming capping layer and organic electroluminescent divice including the same |
| WO2019083248A1 (en) * | 2017-10-27 | 2019-05-02 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compound and organic electroluminescent device comprising the same |
| CN110128330A (en) * | 2018-02-09 | 2019-08-16 | 北京鼎材科技有限公司 | Aryl amine derivatives and its application and organic electroluminescence device |
| US20230008737A1 (en) | 2020-09-30 | 2023-01-12 | Idemitsu Kosan Co.,Ltd. | Compound, material for organic electroluminescent elements, organic electroluminescent element, and electronic device |
| CN112194612A (en) * | 2020-10-15 | 2021-01-08 | 濮阳惠成电子材料股份有限公司 | Synthesis method of photoelectric material intermediate N- (7-bromo-9, 9-dimethylfluorene-2-yl) carbazole |
| KR102438159B1 (en) * | 2021-05-24 | 2022-08-30 | 덕산네오룩스 주식회사 | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030165712A1 (en) * | 2001-12-27 | 2003-09-04 | Lightronik Technology Inc. | Organic EL Device |
| KR20100054110A (en) * | 2008-11-13 | 2010-05-24 | 주식회사 엘지화학 | Organic light emitting diode having low operating voltage and method for fabricating the same |
| KR101181281B1 (en) * | 2012-03-13 | 2012-09-10 | 덕산하이메탈(주) | An organic electronic element comprising a layer for improving light efficiency, a electronic device comprising the same, and compound used for the same |
| KR20130025854A (en) * | 2011-09-02 | 2013-03-12 | 주식회사 엘엠에스 | Organic electronic device and manufacturing method of the same |
| KR20130084960A (en) * | 2012-01-18 | 2013-07-26 | 덕산하이메탈(주) | Compound for organic electronic element, organic electronic element using the same, and a electronic device thereof |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101389527B1 (en) * | 2013-09-17 | 2014-04-25 | 덕산하이메탈(주) | Organic electronic element using a compound for organic electronic element, and an electronic device thereof |
-
2013
- 2013-09-17 KR KR1020130111663A patent/KR102072756B1/en active IP Right Grant
-
2014
- 2014-09-05 WO PCT/KR2014/008399 patent/WO2015041428A1/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030165712A1 (en) * | 2001-12-27 | 2003-09-04 | Lightronik Technology Inc. | Organic EL Device |
| KR20100054110A (en) * | 2008-11-13 | 2010-05-24 | 주식회사 엘지화학 | Organic light emitting diode having low operating voltage and method for fabricating the same |
| KR20130025854A (en) * | 2011-09-02 | 2013-03-12 | 주식회사 엘엠에스 | Organic electronic device and manufacturing method of the same |
| KR20130084960A (en) * | 2012-01-18 | 2013-07-26 | 덕산하이메탈(주) | Compound for organic electronic element, organic electronic element using the same, and a electronic device thereof |
| KR101181281B1 (en) * | 2012-03-13 | 2012-09-10 | 덕산하이메탈(주) | An organic electronic element comprising a layer for improving light efficiency, a electronic device comprising the same, and compound used for the same |
Cited By (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20160164002A1 (en) * | 2013-07-30 | 2016-06-09 | Merck Patent Gmbh | Materials for electronic devices |
| US10217944B2 (en) * | 2014-01-22 | 2019-02-26 | Duk San Neolux Co., Ltd. | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
| US20170012212A1 (en) * | 2014-01-22 | 2017-01-12 | Duk San Neolux Co., Ltd. | Compound for organic electronic element, organic electronic element using the same, and an electronic device thereof |
| WO2016006709A1 (en) * | 2014-07-11 | 2016-01-14 | 出光興産株式会社 | Compound, material for organic electroluminescent element, organic electroluminescent element, and electronic device |
| WO2016006710A1 (en) * | 2014-07-11 | 2016-01-14 | 出光興産株式会社 | Compound, material for organic electroluminescent element, organic electroluminescent element, and electronic device |
| CN105658619A (en) * | 2014-07-11 | 2016-06-08 | 出光兴产株式会社 | Compound, material for organic electroluminescent element, and electronic device |
| US10516112B2 (en) | 2014-07-11 | 2019-12-24 | Idemitsu Kosan Co., Ltd. | Compound, organic electroluminescent element material, organic electroluminescent element, and electronic device |
| US10854822B2 (en) | 2014-07-11 | 2020-12-01 | Idemitsu Kosan Co., Ltd. | Compound, material for organic electroluminescent element, organic electroluminescent element, and electronic device |
| US10944057B2 (en) | 2014-07-11 | 2021-03-09 | Idemitsu Kosan Co., Ltd. | Compound, material for organic electroluminescence devices, organic electroluminescence device, and electronic equipment |
| JPWO2016111301A1 (en) * | 2015-01-08 | 2017-10-19 | 保土谷化学工業株式会社 | Organic electroluminescence device |
| WO2016111301A1 (en) * | 2015-01-08 | 2016-07-14 | 保土谷化学工業株式会社 | Organic electroluminescent element |
| US10505119B2 (en) | 2015-01-08 | 2019-12-10 | Hodogaya Chemical Co., Ltd. | Organic electroluminescence device |
| US10170707B2 (en) | 2015-06-16 | 2019-01-01 | Idemitsu Kosan Co., Ltd. | Compound, material for organic electroluminescence element, organic electroluminescence element, and electronic device |
| CN106565592A (en) * | 2015-12-09 | 2017-04-19 | 广东阿格蕾雅光电材料有限公司 | Organic hole transporting material |
| WO2017097152A1 (en) * | 2015-12-09 | 2017-06-15 | 广东阿格蕾雅光电材料有限公司 | Organic hole transport material |
| CN106565592B (en) * | 2015-12-09 | 2019-06-25 | 广东阿格蕾雅光电材料有限公司 | Organic hole transport material |
| US10374169B2 (en) | 2015-12-09 | 2019-08-06 | Guangdong Aglaia Optoelectronic Materials Co. Ltd | Organic hole transport material |
| JP2023011548A (en) * | 2015-12-16 | 2023-01-24 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| CN108368054B (en) * | 2015-12-16 | 2022-09-02 | 默克专利有限公司 | Material for organic electroluminescent device |
| TWI754625B (en) * | 2015-12-16 | 2022-02-11 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| CN108368054A (en) * | 2015-12-16 | 2018-08-03 | 默克专利有限公司 | Material for organic electroluminescence device |
| WO2017102063A1 (en) * | 2015-12-16 | 2017-06-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| JP2019506369A (en) * | 2015-12-16 | 2019-03-07 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| CN105609662A (en) * | 2015-12-22 | 2016-05-25 | 成都新柯力化工科技有限公司 | Preparation method of special hole transport material for perovskite photovoltaic material |
| TWI740881B (en) * | 2016-01-14 | 2021-10-01 | 日商保土谷化學工業股份有限公司 | Organic electroluminescent device |
| EP3404735B1 (en) * | 2016-01-14 | 2023-05-10 | Hodogaya Chemical Co., Ltd. | Organic electroluminescent device |
| JPWO2017122813A1 (en) * | 2016-01-14 | 2018-11-08 | 保土谷化学工業株式会社 | Organic electroluminescence device |
| JP7080821B2 (en) | 2016-03-03 | 2022-06-06 | メルク パテント ゲーエムベーハー | Materials for OLED devices |
| JP2019510011A (en) * | 2016-03-03 | 2019-04-11 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| US10961230B2 (en) | 2016-03-03 | 2021-03-30 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN107963973A (en) * | 2016-10-20 | 2018-04-27 | 东进世美肯株式会社 | Compounds and the organic luminescent device for including it |
| CN107963973B (en) * | 2016-10-20 | 2023-07-25 | 东进世美肯株式会社 | Novel compound and organic light emitting device comprising the same |
| US11302870B2 (en) | 2016-11-02 | 2022-04-12 | Merck Patent Gmbh | Materials for electronic devices |
| WO2018095392A1 (en) | 2016-11-23 | 2018-05-31 | 广州华睿光电材料有限公司 | Organic mixture, composition, and organic electronic component |
| CN106866499B (en) * | 2016-12-26 | 2020-01-10 | 广东阿格蕾雅光电材料有限公司 | Organic hole transport materials |
| WO2018120975A1 (en) * | 2016-12-26 | 2018-07-05 | 广东阿格蕾雅光电材料有限公司 | Organic hole transport material |
| CN106866499A (en) * | 2016-12-26 | 2017-06-20 | 广东阿格蕾雅光电材料有限公司 | Organic hole transport material |
| CN106848060A (en) * | 2016-12-26 | 2017-06-13 | 广东阿格蕾雅光电材料有限公司 | Only P-type semiconductor diode component based on asymmetric organic hole mobile material |
| CN107266436A (en) * | 2017-06-30 | 2017-10-20 | 长春海谱润斯科技有限公司 | A kind of organic photoelectrical material and its preparation method and application |
| CN110892539A (en) * | 2017-07-27 | 2020-03-17 | Sfc株式会社 | Organic light-emitting element capable of being driven at low voltage and having high efficiency and long life |
| CN110892539B (en) * | 2017-07-27 | 2023-10-31 | Sfc株式会社 | Organic light emitting element capable of low voltage driving and having high efficiency and long life characteristics |
| CN109485577A (en) * | 2017-09-13 | 2019-03-19 | 东进世美肯株式会社 | Compounds and organic luminescent device comprising it |
| CN109485577B (en) * | 2017-09-13 | 2023-12-01 | 东进世美肯株式会社 | Novel compound and organic light emitting device comprising the same |
| US20220278285A1 (en) * | 2017-09-29 | 2022-09-01 | Duk San Neolux Co., Ltd. | Compound for organic electric element, organic electric element comprising the same, and electronic device thereof |
| CN107827873B (en) * | 2017-10-23 | 2020-02-07 | 长春海谱润斯科技有限公司 | Triarylamine derivative and organic light-emitting device thereof |
| CN107827873A (en) * | 2017-10-23 | 2018-03-23 | 长春海谱润斯科技有限公司 | A kind of derivative of tri-arylamine group and its organic luminescent device |
| US11649225B2 (en) | 2017-10-27 | 2023-05-16 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compound and organic electroluminescent device comprising the same |
| CN108929234A (en) * | 2018-07-05 | 2018-12-04 | 长春海谱润斯科技有限公司 | A kind of preparation of aromatic amine derivatives and its organic electroluminescence device |
Also Published As
| Publication number | Publication date |
|---|---|
| KR102072756B1 (en) | 2020-02-03 |
| KR20150031892A (en) | 2015-03-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2015041428A1 (en) | Organic electronic element using compound for organic electronic element and electronic device thereof | |
| WO2016167491A1 (en) | Compound for organic electronic element, organic electronic element using same, and electronic apparatus | |
| WO2015182872A1 (en) | Compound for organic electronic element, and organic electronic element and electronic device using same | |
| WO2017090918A1 (en) | Compound for organic electronic element, organic electronic element using same, and electronic device comprising same | |
| WO2017095075A1 (en) | Compound for organic electric device, organic electric device using same, and electronic device | |
| WO2016003225A2 (en) | Compound for organic electronic element, organic electronic element using same, and electronic device comprising same | |
| WO2016148425A2 (en) | Compound for organic electric device, organic electric device using same, and electronic apparatus for same | |
| WO2015194791A2 (en) | Compound for organic electronic element, organic electronic element using same, and electronic device thereof | |
| WO2016122150A2 (en) | Compound for organic electronic element, organic electronic element using same, and electronic device thereof | |
| WO2015130069A1 (en) | Compound for organic electronic element, organic electronic element using same, and electronic device thereof | |
| WO2016117848A1 (en) | Compound for organic light-emitting device and organic light-emitting device comprising same | |
| WO2017082556A1 (en) | Novel organic light-emitting compound and organic light-emitting device comprising same | |
| WO2016017919A2 (en) | Condensed fluorene derivative comprising hetero ring | |
| WO2017030307A1 (en) | Compound for organic electronic element, organic electronic element using same, and electronic apparatus comprising same | |
| WO2014088284A1 (en) | Compound for organic electronic element, organic electronic element using same, and electronic device therewith | |
| WO2015178585A1 (en) | Compound for electro-organic device, electro-organic device using same, and electronic device therefor | |
| WO2015115756A1 (en) | Compound for organic electric element, organic electric element using same and electronic device thereof | |
| WO2016003202A2 (en) | Compound for organic electronic element, organic electronic element using same, and electronic device comprising same | |
| WO2016200070A2 (en) | Compound for organic electric element, organic electric element using same, and electronic device using same | |
| WO2015041416A1 (en) | Organic electrical element using compounds for organic electrical element and electronic device therefor | |
| WO2016032150A2 (en) | Compound for organic electrical element, organic electrical element using compound, and electronic device comprising same | |
| WO2013122364A2 (en) | Compound for organic electrical element, organic electrical element comprising same, and electronic device therewith | |
| WO2016129861A1 (en) | Novel compound for organic electric element, organic electric element using same, and electronic device comprising same | |
| WO2013108997A1 (en) | Compound for organic electronic device, and organic electronic device and electronic apparatus comprising same | |
| WO2015108377A1 (en) | Compound for organic electronic element, organic electronic element using same, and electronic device thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14845292 Country of ref document: EP Kind code of ref document: A1 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14845292 Country of ref document: EP Kind code of ref document: A1 |