WO2014174469A1 - Pharmaceutical compositions comprising a combination of sitagliptin and metformin - Google Patents
Pharmaceutical compositions comprising a combination of sitagliptin and metformin Download PDFInfo
- Publication number
- WO2014174469A1 WO2014174469A1 PCT/IB2014/060953 IB2014060953W WO2014174469A1 WO 2014174469 A1 WO2014174469 A1 WO 2014174469A1 IB 2014060953 W IB2014060953 W IB 2014060953W WO 2014174469 A1 WO2014174469 A1 WO 2014174469A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pharmaceutical composition
- sitagliptin
- metformin
- composition according
- pharmaceutically acceptable
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/155—Amidines (), e.g. guanidine (H2N—C(=NH)—NH2), isourea (N=C(OH)—NH2), isothiourea (—N=C(SH)—NH2)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4985—Pyrazines or piperazines ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2095—Tabletting processes; Dosage units made by direct compression of powders or specially processed granules, by eliminating solvents, by melt-extrusion, by injection molding, by 3D printing
Definitions
- the present invention relates to pharmaceutical compositions consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant.
- the invention farther relates to processes for the preparation of said pharmaceutical compositions,
- Sitagliptin is a dipeptidyl peptidase IV (DPP-IV) inhibitor used for the treatment of type II diabetes mellitus.
- Metformin is a widely used biguanide that is also indicated for the treatment of type II diabetes mellitus.
- a fixed-dose combination of sitagliptin and metformin is indicated to improve glycemic control in adults with type II diabetes mellitus when treatment with both sitagliptin and metformin is appropriate.
- compositions comprising sitagliptin and metformin. These compositions comprise sitagliptin and metformin in combination with lubricants including magnesium stearate, calcium stearate, stearic acid, sodium stearyl fumarate, hydrogenated castor oil, and mixtures thereof. The addition of lubricants to these compositions resulted in a slow and inconsistent dissolution profile of sitagliptin.
- This publication also discloses that the use of a surfactant, including anionic surfactants such as sodium lauryi sulfate, sodium dodscanesulfbnate, sodium oleyl sulfate, and sodium laurate mixed with stearates and iaic; cationic surfactants such as benzalkonium chlorides and aikyltrimethylammonium bromides; neutral surfactants such as glyceryl monooleate, polyoxyeihyiene sorhitan fatty acid esters, polyvinyl alcohol, and sorbitan esters; and wetting agents such as poloxarner, polyoxy ethylene castor oil derivatives, and polyoxyeihyiene stearates.
- anionic surfactants such as sodium lauryi sulfate, sodium dodscanesulfbnate, sodium oleyl sulfate, and sodium laurate mixed with stearates and iaic
- U.S. Publication No. 2012/0202820 discloses pharmaceutical compositions comprising sitagliptin and metformin in combination with a lubricant wherein the lubricant is polyethylene glycol, or mixtures of polyethylene glycol with one or more other lubricants, and comprises more than 10% by weight of the total weight of the composition.
- the present invention provides an alternate pharmaceutical composition of sitagliptin and metformin, wherein the pharmaceutical composition comprises a lubricant selected from the group consisting of glyceryl hehenate, glycery l palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof.
- a lubricant selected from the group consisting of glyceryl hehenate, glycery l palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof.
- the pharmaceutical compositions of the present invention were prepared without the use of a surfactant and were found to have a dissolution profile comparable to the marketed pharmaceutical composition of sitagliptin and metformin.
- a first aspect of the present invention provides a pharmaceutical composition consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant.
- sitagliptin includes sitagliptin and pharmaceutically acceptable salts thereof.
- the pharmaceutically acceptable salts include hydrochloride, hydrobromide, phosphate, sulphate, mesylate, besylate, tosylate, fumarate, malonate, malate, succinate, lactate, glycolate, maleate, citrate, and aspartate.
- the pharmaceutically acceptable salts of sitagliptin may be present in hydrate or anhydrate forms. The hydrate forms may be monohydrate or dihydrate forms.
- the pharmaceutically acceptable salts of sitagliptin may also be present in crystalline or amorphous forms,
- a particular example is sitagliptin phosphate, which may be present in a concentration of about 0.1% to about 30% by weight of the pharmaceutical composition.
- Sitagliptin phosphate may be used in the pharmaceutical composition in strengths of 25 mg, 50 mg, or 100 mg of sitagliptin.
- the term ''metformin includes metformin and pharmaceutically acceptable salts thereof
- the pharmaceutically acceptable salts include hydrochloride, hydrobromide, fumarate, malonate, malate, succinate, lactate, glycolate, maleate, citrate, and aspartate.
- the pharmaceutically acceptable salts of sitagliptin may be present in hydrate or anhydrate forms. They may also be present in crystalline or amorphous forms.
- a particular example is metformin hydrochloride, which may be present in a concentration of about 50% to about 90% by weight of the pharmaceutical composition. Metformin hydrochloride may be used in strengths of 500 mg, 850 mg, or 1000 mg in the
- lubricants examples include glyceryl behenate (e.g., Compritol*), glyceryl palmitostearate (e.g., Precirol ® ), stearyl alcohol, cetostearyl alcohol, or mixtures thereof.
- concentration of the lubricant may be about 0.01% to about 10% by weight of the pharmaceutical composition.
- compositions comprising sitagliptin and metformin. These compositions include tablets, capsules, pellets, pills, capiets, and granules.
- the pharmaceutical compositions may be prepared by dry granulation, wet granulation, or direct compression. Wet granulation may include rapid mixer granulation or fluid bed granulation.
- a pharmaceutical composition consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, steaiyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein more than 80% of sitagliptin is released in 15 minutes when the dissolution is performed in 900 mL of 0.025M sodium chloride solution at 75 rpm using USP type II (paddle) apparatus.
- a pharmaceutical composition consisting essentially of sitagliptin phosphate, metformin hydrochloride, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant.
- a pharmaceutical composition consisting essentially of sitagliptin phosphate, metformin hydrochloride, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the lubricant is present in an amount of about 0.01 % to about 10% by weight of the pharmaceutical composition and the pharmaceutical composition does not comprise a surfactant.
- a second aspect of the present invention provides a process for the preparation of a pharmaceutical composition consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of gly ceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant, the process comprising:
- step b) optionally, mixing the blend of step a) with one or more pharmaceutically acceptable excipients;
- step d) granulating the blend of step a) or step b) with the solution or dispersion of step c);
- step d) blending the granules of step d) with a lubricant and optionally with one or more pharmaceutically acceptable excipients;
- step f) compressing the blend of step e) into suitable sized tablets or filling the blend of step e) into suitable sized capsules.
- the granulation is carried out in a rapid mixer granulator or a fluid bed granulator.
- a third aspect of the present invention provides a process for the preparation of a pharmaceutical composition consisting essentially of sitagliptin, metformin and a lubricant selected from the group consisting of glyceryl behenate, glycery l palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant, the process comprising:
- step b) blending the granules of step b) with a lubricant and optionally one or more pharmaceutically acceptable excipients;
- step d) compressing the blend of step c) into suitable sized tablets or filling the blend of step c) into suitable sized capsules.
- the granulation is carried out in a rapid mixer granulator or a fluid bed granulator.
- suitable solvents that may be used for granulation include water, ethanol, methanol, isopropyl alcohol, dichloromethane, acetone, or mixtures thereof.
- compositions include binders, disintegrants, diluents, and antioxidants.
- the excipients may be added intragranularly and/or extragranularly in the pharmaceutical compositions.
- binders examples include cellulose or cellulose derivatives such as
- binders may be about 1 % to about 50% by weight of the pharmaceutical composition.
- disintegrants examples include sodium starch glycolate, croscarmellose, pregelatinized starch (e.g., Starch 1500*), microcrystailine cellulose, calcium carboxy methyl cellulose, low substituted hydroxy propyl cellulose, magnesium silicate, aluminum silicate, or mixtures thereof.
- concentration of disintegrants may be about 1% to about 50% by weight of the pharmaceutical composition.
- diluents examples include lactose, sucrose, maltodextrin, microcrystailine cellulose, pregelatinized starch (e.g., Starch 1500*), dextrose, mannitol, sorbitol, xyiitol, isomalt, erythritol, or mixtures thereof.
- concentration of diluents may be about 1 % to about 50% by weight of the pharmaceutical composition.
- antioxidants examples include ascorbic acid, butylated hydroxy anisole, butylated hydroxy toluene, or mixtures thereof.
- concentration of antioxidants may be about 0% to about 2% by weight of the pharmaceutical composition.
- the pharmaceutical composition may be further coated with a functional or nonfunctional coating.
- the coating composition may comprise pharmaceutically acceptable excipients such as binders, plasticizers, coloring agents, and opacifiers.
- the total weight gain after coating may be about 1% to about 10% by weight of uncoated composition.
- binders for coating include cellulose or cellulose derivatives such as methyl cellulose, hydroxy propyl methyl cellulose, hydroxy propyl cellulose, and carboxy methyl cellulose sodium; alginic acid or sodium alginate; microcrystailine cellulose; gelatin; polyvinyl pyrrolidone; copovidone; starch; pregelatinized starch; or mixtures thereof.
- plasticizers for coating include propylene glycol, triethyl citrate, tributyl citrate, dibutvl sebacate, triacetin, polyethylene glycol, dieihyl phthalate, acetylated monoglycerides, or mixtures thereof.
- opaeifiers for coating examples include titanium dioxide, talc, calcium carbonate, behenic acid, and cetyl alcohol.
- coloring agents for coating include FDA approved colorants such as iron oxide, Lake of Tartrazine, allura red, Lake of Quinoline Yellow, Lake of Erythrosine, or titanium dioxide.
- Suitable solvents for coating include ethanol, methanol, isopropyl alcohol, dichloromethane, acetone, or mixtures thereof.
- premixed film-coating may be used.
- Suitable premixes include Opadry ® coating dispersions.
- step 3 The blend of step 1 was granulated with the solution of step 2 in a rapid mixer granulator to obtain granules.
- step 3 The granules of step 3 were blended with glyceryl behenate and pregelatinized starch.
- step 4 The blend of step 4 was compressed to obtain suitable sized tablets.
- step 5 The tablets of step 5 were coated with Opadry ® coating dispersion.
- Sitagliptin phosphate monohydrate and polyvinyl pyrrolidone were dissolved in purifsed water to obtain a solution.
- step 3 The granules of step 2 were blended with glyceryl palmitostearate and
- step 3 The blend of step 3 was compressed to obtain suitable sized tablets.
- step 3 The blend of step 1 was granulated with the solution of step 2 in a rapid mixer granulator to obtain granules,
- step 3 The granules of step 3 were blended with stearyl alcohol and pregelatinized starch.
- step 4 The blend of step 4 was compressed to obtain suitable sized tablets.
- step 5 The tablets of step 5 were coated with Opadry ⁇ coating.
- Sitagliptin phosphate monohydrate and polyvinyl pyrrolidone were dissolved in purified water to obtain a solution.
- step 3 The granules of step 2 were blended with cetostearyl alcohol and pregelatinized starch.
- step 3 The blend of step 3 was compressed to obtain suitable sized tablets.
- step 4 The tablets of step 4 were coated with Opadry* J coating.
- Table 1 provides a comparison of the dissolution profile of sitagliptin in the pharmaceutical compositions of Examples 1, 2, 3, and 4 vis-a-vis Janumet ® (sitagliptin 50 mg; metformin HCl 500 mg tablet).
- the dissolution was performed in 900 mL of 0.025M sodium chloride solution at 75 rpm using the USP type II (paddle) apparatus.
- the samples were analyzed by high performance liquid chromatography (HPLC) using an Inert Sustain*" C18, 3 ⁇ , 150 mm x 4.6 mm, column and mobile phase having phosphate buffer pH 6.8 and acetonitrile in the ratio of 70:30.
- the pharmaceutical compositions (Examples 1, 2, 3, and 4) prepared without the use of a surfactant and Janumet* tablets achieved the target dissolution profile of release of more than 85% of sitagliptin within 15 minutes, indicating that Janumet* tablets and the pharmaceutical compositions (Examples 1, 2, 3, and 4) have similar dissolution profiles.
- Table 1 Percentage of sitagliptin released in the dissolution media (0.025 M sodium chloride solution).
Abstract
The present invention relates to pharmaceutical compositions consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant. The invention further relates to processes for the preparation of said pharmaceutical compositions.
Description
PHARMACEUTICAL COMPOSITIONS COMPRISING A COMBINATION OF SITAGLIPTIN AND METFORMIN
Field of the Invention
The present invention relates to pharmaceutical compositions consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant. The invention farther relates to processes for the preparation of said pharmaceutical compositions,
Background of the Invention
Sitagliptin is a dipeptidyl peptidase IV (DPP-IV) inhibitor used for the treatment of type II diabetes mellitus. Metformin is a widely used biguanide that is also indicated for the treatment of type II diabetes mellitus. A fixed-dose combination of sitagliptin and metformin is indicated to improve glycemic control in adults with type II diabetes mellitus when treatment with both sitagliptin and metformin is appropriate.
U.S. Publication No. 2009/0105265 discloses compositions comprising sitagliptin and metformin. These compositions comprise sitagliptin and metformin in combination with lubricants including magnesium stearate, calcium stearate, stearic acid, sodium stearyl fumarate, hydrogenated castor oil, and mixtures thereof. The addition of lubricants to these compositions resulted in a slow and inconsistent dissolution profile of sitagliptin. This publication also discloses that the use of a surfactant, including anionic surfactants such as sodium lauryi sulfate, sodium dodscanesulfbnate, sodium oleyl sulfate, and sodium laurate mixed with stearates and iaic; cationic surfactants such as benzalkonium chlorides and aikyltrimethylammonium bromides; neutral surfactants such as glyceryl monooleate, polyoxyeihyiene sorhitan fatty acid esters, polyvinyl alcohol, and sorbitan esters; and wetting agents such as poloxarner, polyoxy ethylene castor oil derivatives, and polyoxyeihyiene stearates.
U.S. Publication No. 2012/0202820 discloses pharmaceutical compositions comprising sitagliptin and metformin in combination with a lubricant wherein the lubricant is polyethylene glycol, or mixtures of polyethylene glycol with one or more other lubricants, and comprises more than 10% by weight of the total weight of the composition.
Summary of the Invention
The present invention provides an alternate pharmaceutical composition of sitagliptin and metformin, wherein the pharmaceutical composition comprises a lubricant selected from the group consisting of glyceryl hehenate, glycery l palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof. The pharmaceutical compositions of the present invention were prepared without the use of a surfactant and were found to have a dissolution profile comparable to the marketed pharmaceutical composition of sitagliptin and metformin.
Detailed Description of the Invention
A first aspect of the present invention provides a pharmaceutical composition consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant.
As used herein, the term "sitagliptin" includes sitagliptin and pharmaceutically acceptable salts thereof. The pharmaceutically acceptable salts include hydrochloride, hydrobromide, phosphate, sulphate, mesylate, besylate, tosylate, fumarate, malonate, malate, succinate, lactate, glycolate, maleate, citrate, and aspartate. The pharmaceutically acceptable salts of sitagliptin may be present in hydrate or anhydrate forms. The hydrate forms may be monohydrate or dihydrate forms. The pharmaceutically acceptable salts of sitagliptin may also be present in crystalline or amorphous forms, A particular example is sitagliptin phosphate, which may be present in a concentration of about 0.1% to about 30% by weight of the pharmaceutical composition. Sitagliptin phosphate may be used in the pharmaceutical composition in strengths of 25 mg, 50 mg, or 100 mg of sitagliptin.
As used herein, the term ''metformin" includes metformin and pharmaceutically acceptable salts thereof The pharmaceutically acceptable salts include hydrochloride, hydrobromide, fumarate, malonate, malate, succinate, lactate, glycolate, maleate, citrate, and aspartate. The pharmaceutically acceptable salts of sitagliptin may be present in hydrate or anhydrate forms. They may also be present in crystalline or amorphous forms. A particular example is metformin hydrochloride, which may be present in a concentration of about 50% to about 90% by weight of the pharmaceutical composition. Metformin
hydrochloride may be used in strengths of 500 mg, 850 mg, or 1000 mg in the
pharmaceutical composition.
Examples of lubricants include glyceryl behenate (e.g., Compritol*), glyceryl palmitostearate (e.g., Precirol® ), stearyl alcohol, cetostearyl alcohol, or mixtures thereof. The concentration of the lubricant may be about 0.01% to about 10% by weight of the pharmaceutical composition.
As used herein, the tenn "pharmaceutical composition" means compositions comprising sitagliptin and metformin. These compositions include tablets, capsules, pellets, pills, capiets, and granules. The pharmaceutical compositions may be prepared by dry granulation, wet granulation, or direct compression. Wet granulation may include rapid mixer granulation or fluid bed granulation.
According to one embodiment of the present invention, there is provided a pharmaceutical composition consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, steaiyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein more than 80% of sitagliptin is released in 15 minutes when the dissolution is performed in 900 mL of 0.025M sodium chloride solution at 75 rpm using USP type II (paddle) apparatus.
According to one embodiment of the present invention, there is provided a pharmaceutical composition consisting essentially of sitagliptin phosphate, metformin hydrochloride, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant.
According to another embodiment of the present invention, there is provided a pharmaceutical composition consisting essentially of sitagliptin phosphate, metformin hydrochloride, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the lubricant is present in an amount of about 0.01 % to about 10% by weight of the pharmaceutical composition and the pharmaceutical composition does not comprise a surfactant.
A second aspect of the present invention provides a process for the preparation of a pharmaceutical composition consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of gly ceryl behenate, glyceryl palmitostearate,
stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant, the process comprising:
a) blending sitagliptin and metformin;
b) optionally, mixing the blend of step a) with one or more pharmaceutically acceptable excipients;
c) dissolving or dispersing one or more pharmaceutically acceptable excipients in a suitable solvent:
d) granulating the blend of step a) or step b) with the solution or dispersion of step c);
e) blending the granules of step d) with a lubricant and optionally with one or more pharmaceutically acceptable excipients; and
f) compressing the blend of step e) into suitable sized tablets or filling the blend of step e) into suitable sized capsules.
According to one embodiment of this aspect, the granulation is carried out in a rapid mixer granulator or a fluid bed granulator.
A third aspect of the present invention provides a process for the preparation of a pharmaceutical composition consisting essentially of sitagliptin, metformin and a lubricant selected from the group consisting of glyceryl behenate, glycery l palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant, the process comprising:
a) dissolving or dispersing sitagliptin and one or more pharmaceutically
acceptable excipients in a suitable solvent;
b) granulating metformin with the solution or dispersion from step a);
c) blending the granules of step b) with a lubricant and optionally one or more pharmaceutically acceptable excipients; and
d) compressing the blend of step c) into suitable sized tablets or filling the blend of step c) into suitable sized capsules.
According to one embodiment of this aspect, the granulation is carried out in a rapid mixer granulator or a fluid bed granulator.
Examples of suitable solvents that may be used for granulation include water, ethanol, methanol, isopropyl alcohol, dichloromethane, acetone, or mixtures thereof.
Pharmaceutically acceptable excipients include binders, disintegrants, diluents, and antioxidants. The excipients may be added intragranularly and/or extragranularly in the pharmaceutical compositions.
Examples of binders include cellulose or cellulose derivatives such as
methylcellulose, hydroxy propyl methyl cellulose, hydroxy propyl cellulose, carboxy methyl cellulose sodium, polyvinyl pyrrolidone (e.g., Kollidon* 30), copovidone, sodium alginate, microcrystailine cellulose, or mixtures thereof. The concentration of binders may be about 1 % to about 50% by weight of the pharmaceutical composition.
Examples of disintegrants include sodium starch glycolate, croscarmellose, pregelatinized starch (e.g., Starch 1500*), microcrystailine cellulose, calcium carboxy methyl cellulose, low substituted hydroxy propyl cellulose, magnesium silicate, aluminum silicate, or mixtures thereof. The concentration of disintegrants may be about 1% to about 50% by weight of the pharmaceutical composition.
Examples of diluents include lactose, sucrose, maltodextrin, microcrystailine cellulose, pregelatinized starch (e.g., Starch 1500*), dextrose, mannitol, sorbitol, xyiitol, isomalt, erythritol, or mixtures thereof. The concentration of diluents may be about 1 % to about 50% by weight of the pharmaceutical composition.
Examples of antioxidants include ascorbic acid, butylated hydroxy anisole, butylated hydroxy toluene, or mixtures thereof. The concentration of antioxidants may be about 0% to about 2% by weight of the pharmaceutical composition.
The pharmaceutical composition may be further coated with a functional or nonfunctional coating. The coating composition may comprise pharmaceutically acceptable excipients such as binders, plasticizers, coloring agents, and opacifiers. The total weight gain after coating may be about 1% to about 10% by weight of uncoated composition.
Examples of binders for coating include cellulose or cellulose derivatives such as methyl cellulose, hydroxy propyl methyl cellulose, hydroxy propyl cellulose, and carboxy methyl cellulose sodium; alginic acid or sodium alginate; microcrystailine cellulose; gelatin; polyvinyl pyrrolidone; copovidone; starch; pregelatinized starch; or mixtures thereof.
Examples of plasticizers for coating include propylene glycol, triethyl citrate, tributyl citrate, dibutvl sebacate, triacetin, polyethylene glycol, dieihyl phthalate, acetylated monoglycerides, or mixtures thereof.
Examples of opaeifiers for coating include titanium dioxide, talc, calcium carbonate, behenic acid, and cetyl alcohol.
Examples of coloring agents for coating include FDA approved colorants such as iron oxide, Lake of Tartrazine, allura red, Lake of Quinoline Yellow, Lake of Erythrosine, or titanium dioxide.
Suitable solvents for coating include ethanol, methanol, isopropyl alcohol, dichloromethane, acetone, or mixtures thereof.
Alternatively, a premixed film-coating may be used. Suitable premixes include Opadry® coating dispersions.
The term "about", as used herein, refers to any value which lies within the range defined by a variation of up to ±10% of the value.
The following examples illustrate the invention but are not to be construed as limiting the scope of the invention.
EXAMPLES
Example 1 :
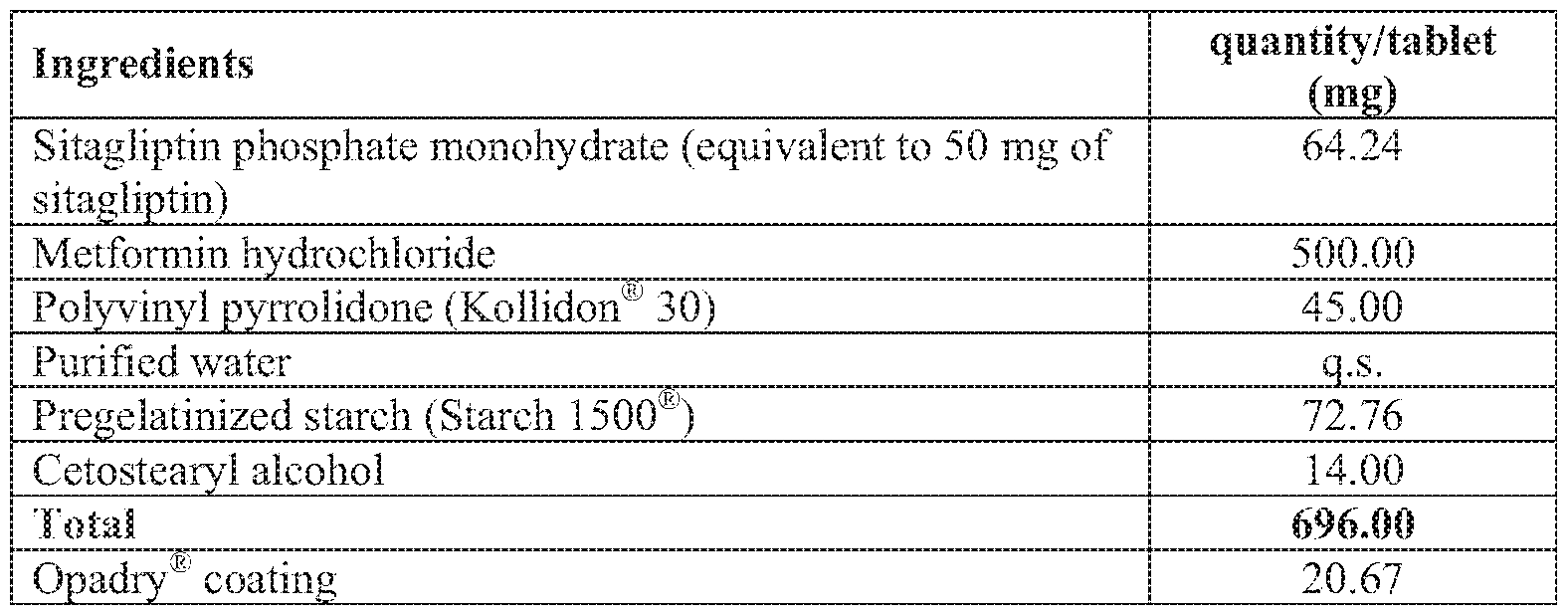
Manufacturing Process:
1. Sitagliptin phosphate monohydrate and metformin hydrochloride were blended.
2. Polyvinyl pyrrolidone was dissolved in purified water to obtain a solution.
3. The blend of step 1 was granulated with the solution of step 2 in a rapid mixer
granulator to obtain granules.
The granules of step 3 were blended with glyceryl behenate and pregelatinized starch.
The blend of step 4 was compressed to obtain suitable sized tablets.
The tablets of step 5 were coated with Opadry® coating dispersion.
Example 2:
Manufacturing Process:
1. Sitagliptin phosphate monohydrate and polyvinyl pyrrolidone were dissolved in purifsed water to obtain a solution.
2. Metformin hydrochloride was granulated with the solution of step 1 in a rapid mixer granulator.
3. The granules of step 2 were blended with glyceryl palmitostearate and
pregelatinized starch.
4. The blend of step 3 was compressed to obtain suitable sized tablets.
5. The tablets of step 4 were coated with Opadry* coating.
Example 3:
Manufacturing Process:
1. Sitagliptin phosphate monohydrate and metformin hydrochloride were blended.
2. Polyvinyl pyrrolidone was dissolved in purified water to obtain a solution.
3. The blend of step 1 was granulated with the solution of step 2 in a rapid mixer granulator to obtain granules,
4. The granules of step 3 were blended with stearyl alcohol and pregelatinized starch.
5. The blend of step 4 was compressed to obtain suitable sized tablets.
6. The tablets of step 5 were coated with Opadry^ coating.
Example 4:
1. Sitagliptin phosphate monohydrate and polyvinyl pyrrolidone were dissolved in purified water to obtain a solution.
2. Metformin hydrochloride was granulated with the solution of step 1 in a rapid mixer granulator.
3. The granules of step 2 were blended with cetostearyl alcohol and pregelatinized starch.
4. The blend of step 3 was compressed to obtain suitable sized tablets.
5. The tablets of step 4 were coated with Opadry*J coating.
As a modification to the manufacturing process steps of Examples 1 , 2, 3, and 4, the granulation step in all the examples was also carried out in a fluid bed granulator instead of a rapid mixer granulator.
Dissolution Study
Table 1 provides a comparison of the dissolution profile of sitagliptin in the pharmaceutical compositions of Examples 1, 2, 3, and 4 vis-a-vis Janumet® (sitagliptin 50 mg; metformin HCl 500 mg tablet). The dissolution was performed in 900 mL of 0.025M sodium chloride solution at 75 rpm using the USP type II (paddle) apparatus. The samples were analyzed by high performance liquid chromatography (HPLC) using an Inert Sustain*" C18, 3 μπι, 150 mm x 4.6 mm, column and mobile phase having phosphate buffer pH 6.8 and acetonitrile in the ratio of 70:30. As seen from the results, the pharmaceutical compositions (Examples 1, 2, 3, and 4) prepared without the use of a surfactant and Janumet* tablets achieved the target dissolution profile of release of more than 85% of sitagliptin within 15 minutes, indicating that Janumet* tablets and the pharmaceutical compositions (Examples 1, 2, 3, and 4) have similar dissolution profiles.
Table 1 : Percentage of sitagliptin released in the dissolution media (0.025 M sodium chloride solution).
Claims
1. A pharmaceutical composition consisting essentially of sitagliptin, metformin, and a lubricant selected from the group consisting of glyceryl behenate, glyceryl palmitostearate, stearyl alcohol, cetostearyl alcohol, or mixtures thereof, wherein the pharmaceutical composition does not comprise a surfactant.
2. The pharmaceutical composition according to claim 1, wherein the sitagliptin is sitagliptin phosphate and the metformin is metformin hydrochloride.
3. The pharmaceutical composition according to claim 1, wherein the lubricant is present in an amount of about 0.01% to about 10% by weight of the
pharmaceutical composition.
4. A process for the preparation of a pharmaceutical composition according to claim 1, wherein the process comprises the steps of:
a) blending sitagliptin and metformin;
b) optionally mixing the blend of step a) with one or more pharmaceutically acceptable excipients;
c) dissolving or dispersing one or more pharmaceutically acceptable
excipients in a suitable solvent;
d) granulating the blend of step a) or step b) with the solution or dispersion of step c);
e) blending the granules of step d) with a lubricant and optionally one or more pharmaceutically acceptable excipients; and
f) compressing the blend of step e) into suitable sized tablets or filling into suitable sized capsules.
5. A process for the preparation of a pharmaceutical composition according to claim 1, wherein the process comprises:
a) dissolving or dispersing sitagliptin and one or more pharmaceutically acceptable excipients in a suitable solvent;
b) granulating metformin with the solution or dispersion from step a);
c) blending the granules of step b) with a lubricant and optionally one or more pharmaceutically acceptable excipients; and
d) compressing the blend of step c) into suitable sized tablets or filling into suitable sized capsules.
6. The process for the preparation of a pharmaceutical composition according to claim 4 or claim 5, wherein the granulation is carried out in a rapid mixer granulator or a fluid bed granulator.
7. The pharmaceutical composition according to claim 1, wherein the pharmaceutical composition is a tablet.
8. The pharmaceutical composition according to claim 1, wherein the pharmaceutical composition is a film-coated tablet.
9. The pharmaceutical composition according to claim 1, wherein more than 80% of sitagliptin is released in 15 minutes when the dissolution is performed in 900 mL of 0.025M sodium chloride solution at 75 rpm using USP type II (paddle) apparatus.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN1226/DEL/2013 | 2013-04-25 | ||
| IN1226DE2013 | 2013-04-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014174469A1 true WO2014174469A1 (en) | 2014-10-30 |
Family
ID=50943351
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2014/060953 WO2014174469A1 (en) | 2013-04-25 | 2014-04-23 | Pharmaceutical compositions comprising a combination of sitagliptin and metformin |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2014174469A1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019203771A2 (en) | 2018-04-17 | 2019-10-24 | Sanovel Ilac Sanayi Ve Ticaret Anonim Sirketi | Solid oral pharmaceutical compositions comprising sitagliptin |
| WO2022035400A1 (en) * | 2020-08-11 | 2022-02-17 | Sanovel Ilac Sanayi Ve Ticaret Anonim Sirketi | A tablet formulation comprising sitagliptin and metformi̇n |
| WO2022074664A1 (en) * | 2020-10-05 | 2022-04-14 | V-Ensure Pharma Technologies Private Limited | An immediate release composition of sitagliptin hydrochloride |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003004009A1 (en) * | 2001-07-02 | 2003-01-16 | Geneva Pharmaceuticals, Inc. | Pharmaceutical composition |
| US20090105265A1 (en) | 2005-12-16 | 2009-04-23 | Merck & Co., Inc. | Pharmaceutical Compositions of Combinations of Dipeptidyl Peptidase-4 Inhibitors With Metformin |
| US20120202820A1 (en) | 2009-09-15 | 2012-08-09 | Ratiopharm Gmbh | Pharmaceutical composition having the active substances metformin and sitagliptin or vildagliptin |
-
2014
- 2014-04-23 WO PCT/IB2014/060953 patent/WO2014174469A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003004009A1 (en) * | 2001-07-02 | 2003-01-16 | Geneva Pharmaceuticals, Inc. | Pharmaceutical composition |
| US20090105265A1 (en) | 2005-12-16 | 2009-04-23 | Merck & Co., Inc. | Pharmaceutical Compositions of Combinations of Dipeptidyl Peptidase-4 Inhibitors With Metformin |
| US20120202820A1 (en) | 2009-09-15 | 2012-08-09 | Ratiopharm Gmbh | Pharmaceutical composition having the active substances metformin and sitagliptin or vildagliptin |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019203771A2 (en) | 2018-04-17 | 2019-10-24 | Sanovel Ilac Sanayi Ve Ticaret Anonim Sirketi | Solid oral pharmaceutical compositions comprising sitagliptin |
| WO2019203771A3 (en) * | 2018-04-17 | 2020-01-16 | Sanovel Ilac Sanayi Ve Ticaret Anonim Sirketi | Solid oral pharmaceutical compositions comprising sitagliptin |
| WO2022035400A1 (en) * | 2020-08-11 | 2022-02-17 | Sanovel Ilac Sanayi Ve Ticaret Anonim Sirketi | A tablet formulation comprising sitagliptin and metformi̇n |
| WO2022074664A1 (en) * | 2020-10-05 | 2022-04-14 | V-Ensure Pharma Technologies Private Limited | An immediate release composition of sitagliptin hydrochloride |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3089741B1 (en) | Pharmaceutical compositions comprising azd9291 | |
| JP5274261B2 (en) | Pharmaceutical composition containing low substituted hydroxypropylcellulose | |
| KR20070089207A (en) | Matrix-type controlled release preparation comprising basic substance or salt thereof, and process for production of the same | |
| MX2012012729A (en) | Immediate release formulations and dosage forms of gamma-hydroxybutyrate. | |
| WO2015097234A1 (en) | Pharmaceutical composition of dpp-iv inhibitor in combination with metformin | |
| US10207002B2 (en) | Sustained release formulation and tablets prepared therefrom | |
| PT2468361E (en) | Vildagliptin formulations | |
| CA3006192C (en) | Pharmaceutical compositions containing doravirine, tenofovir disoproxil fumarate and lamivudine | |
| WO2014068586A2 (en) | Solid oral compositions of tolvaptan | |
| KR20160000762A (en) | Composite formulation for oral administration comprising ezetimibe and rosuvastatin and a process for the preparation thereof | |
| KR20160113296A (en) | Tablet formulation for cgrp-active compounds | |
| EP2799072A1 (en) | Solid pharmaceutical composition comprising an antibiotic from the quinolone family and method for the production thereof | |
| WO2014174469A1 (en) | Pharmaceutical compositions comprising a combination of sitagliptin and metformin | |
| KR20190015329A (en) | A pharmaceutical composition of a dapagliflozin co-crystal | |
| WO2015028875A2 (en) | Unit dosage form comprising emtricitabine, tenofovir, darunavir and ritonavir and a monolithic tablet comprising darunavir and ritonavir | |
| EP3177290B1 (en) | Pharmaceutical compositions of edoxaban | |
| EP2359816B1 (en) | Aripiprazole formulations | |
| CA3135946C (en) | Enteric tablet containing dimethyl fumarate | |
| WO2011080706A1 (en) | Enhanced solubility of ziprasidone | |
| KR20110085307A (en) | Oral solid dosage form comprising poorly soluble drugs | |
| CZ2016538A3 (en) | A pharmaceutical composition comprising two different active ingredients | |
| KR20200082006A (en) | Extended release formulation containing tofacitinib or pharmaceutically acceptable salts thereof as an active ingredient and the preparation method for the same | |
| WO2022180582A1 (en) | Oral pharmaceutical composition of arsenic trioxide | |
| WO2022177983A1 (en) | Pharmaceutical compositions of cabozantinib | |
| WO2021106004A1 (en) | Pharmaceutical composition of s-adenosylmethionine |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14730583 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 32PN | Ep: public notification in the ep bulletin as address of the adressee cannot be established |
Free format text: NOTING OF LOSS OF RIGHTS PURSUANT TO RULE 112(1) EPC (EPO FORM 1205A DATED 29/04/2016) |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14730583 Country of ref document: EP Kind code of ref document: A1 |