WO2013141239A1 - Polyamic acid and polyimide - Google Patents
Polyamic acid and polyimide Download PDFInfo
- Publication number
- WO2013141239A1 WO2013141239A1 PCT/JP2013/057819 JP2013057819W WO2013141239A1 WO 2013141239 A1 WO2013141239 A1 WO 2013141239A1 JP 2013057819 W JP2013057819 W JP 2013057819W WO 2013141239 A1 WO2013141239 A1 WO 2013141239A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- resin composition
- following formula
- polyimide
- structural unit
- Prior art date
Links
- NZOSGGNRVDMIHW-UHFFFAOYSA-N Cc1cc(S(c2cccc(C)c2)(=O)=O)ccc1 Chemical compound Cc1cc(S(c2cccc(C)c2)(=O)=O)ccc1 NZOSGGNRVDMIHW-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G73/00—Macromolecular compounds obtained by reactions forming a linkage containing nitrogen with or without oxygen or carbon in the main chain of the macromolecule, not provided for in groups C08G12/00 - C08G71/00
- C08G73/06—Polycondensates having nitrogen-containing heterocyclic rings in the main chain of the macromolecule
- C08G73/10—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
- C08G73/1046—Polyimides containing oxygen in the form of ether bonds in the main chain
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G73/00—Macromolecular compounds obtained by reactions forming a linkage containing nitrogen with or without oxygen or carbon in the main chain of the macromolecule, not provided for in groups C08G12/00 - C08G71/00
- C08G73/06—Polycondensates having nitrogen-containing heterocyclic rings in the main chain of the macromolecule
- C08G73/10—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G73/00—Macromolecular compounds obtained by reactions forming a linkage containing nitrogen with or without oxygen or carbon in the main chain of the macromolecule, not provided for in groups C08G12/00 - C08G71/00
- C08G73/06—Polycondensates having nitrogen-containing heterocyclic rings in the main chain of the macromolecule
- C08G73/10—Polyimides; Polyester-imides; Polyamide-imides; Polyamide acids or similar polyimide precursors
- C08G73/1057—Polyimides containing other atoms than carbon, hydrogen, nitrogen or oxygen in the main chain
- C08G73/1064—Polyimides containing other atoms than carbon, hydrogen, nitrogen or oxygen in the main chain containing sulfur
Definitions
- the present invention relates to a resin composition for display substrates, and in particular, a resin for display substrates capable of forming a useful polyimide film having appropriate heat resistance, transparency, linear expansion coefficient, birefringence, and appropriate flexibility. Relates to the composition.
- Polyimide resins are widely used in the field of electrical and electronic materials because of their high heat resistance, flame retardancy, and excellent electrical insulation. Specifically, it is used as a film for flexible printed wiring boards and heat-resistant adhesive tapes as a film, and as a resin varnish for semiconductor insulating films, protective films, and the like.
- display devices such as an organic EL (Electro Luminescence) display and a liquid crystal display have been required only for high definition. As such display devices are rapidly expanding their applications to information devices and the like, flexible displays using a plastic film as a substrate are attracting attention in order to satisfy new requirements such as ultra-thinness and light weight.
- an active matrix driving panel is used for a high-definition display.
- an active matrix layer including a thin film active element in addition to a matrix-like pixel electrode In order to form an active matrix layer including a thin film active element in addition to a matrix-like pixel electrode, a high temperature treatment of 200 ° C. or higher is required in the manufacturing process, and extremely accurate alignment is required. However, if the glass substrate is changed to a plastic material to make the display flexible, heat resistance and dimensional stability cannot be satisfied, and it is very difficult to directly form an active element on the glass substrate. It was.
- Patent Document 1 discloses a polyimide film for a display substrate using a polyimide obtained from an alicyclic structure-containing tetracarboxylic dianhydride and various diamines.
- Patent Document 2 discloses a polyimide film for a display substrate using a polyimide obtained from an alicyclic tetracarboxylic dianhydride having a cyclohexane skeleton and an aromatic diamine containing a sulfone group.
- the tetracarboxylic dianhydride used in Patent Document 1 also contains aromatic groups. Compared to tetracarboxylic dianhydrides that contain only alicyclic groups and do not contain any group, there is a problem that the transparency of the film obtained by intramolecular conjugation of the polyimide chain and charge transfer interaction is lowered. Moreover, since the acid dianhydride used in Patent Document 2 has a special structure called a cyclohexane skeleton having a cis structure, the versatility is poor, and the production cost of polyimide becomes high, and the resulting product becomes expensive. There is.
- the alicyclic tetracarboxylic dianhydride has a problem in that a high-molecular-weight body having sufficient film toughness may not be obtained from the viewpoint of polymerization reactivity.
- 1,2,3,4-cyclobutanetetracarboxylic dianhydride (hereinafter referred to as CBDA), which is the most popular among tetracarboxylic dianhydrides, exhibits relatively high polymerization reactivity with diamine, Due to its three-dimensional structure, imidation reaction of the polyimide precursor hardly occurs, and a higher temperature is required to complete the imidization reaction, which causes coloring of the polyimide film, and the use of CBDA as a display substrate is not advantageous. Has been pointed out (Patent Document 1 and Patent Document 2).
- the present invention has been made in view of such circumstances, and its purpose is a highly transparent film having sufficient film strength as a flexible display substrate and having heat resistance necessary for a thin film transistor (TFT) formation process.
- Another object of the present invention is to provide a high heat-resistant coating agent for display substrates capable of forming a film having low birefringence.
- the present inventor has alicyclic tetracarboxylic acid, which has been avoided in the field of display substrates due to reactivity and coloring problems as an acid dianhydride component.
- Display comprising polyamic acid or polyimide obtained by reacting dianhydride, in particular, 1,2,3,4-cyclobutanetetracarboxylic dianhydride (hereinafter also referred to as CBDA) and aromatic diamine containing a sulfone group
- dianhydride in particular, 1,2,3,4-cyclobutanetetracarboxylic dianhydride (hereinafter also referred to as CBDA) and aromatic diamine containing a sulfone group
- TFT thin film transistor
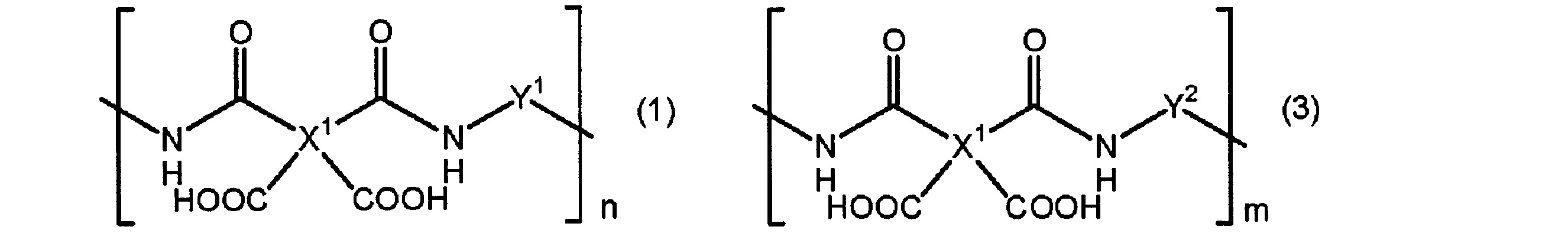
- the present invention provides a polyamic acid containing a structural unit represented by the following formula (1) and a structural unit represented by the formula (3), or a structural unit represented by the following formula (2) and It is related with the resin composition for display substrates containing the polyimide containing the structural unit represented by Formula (4).
- X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group
- Y 1 and Y 2 are different from each other and represent a divalent aromatic group or a divalent aliphatic group
- n and m represent natural numbers.
- the present invention relates to the display substrate resin composition according to the first aspect, in which X 1 represents a tetravalent group represented by the following formula (5).
- the Y 1 represents a divalent group represented by the following formula (6)
- Y 2 represents a divalent group represented by the following formula (7).
- the present invention relates to a resin composition for display substrates.
- An eighth aspect relates to a varnish, wherein the display substrate resin composition according to any one of the first to seventh aspects is dissolved in at least one solvent.
- the present invention relates to the varnish according to the eighth aspect, which has a viscosity of 0.001 to 5,000 Pa ⁇ s.
- a 10th viewpoint it is related with the cured film obtained by baking the thin film obtained using the varnish as described in an 8th viewpoint or a 9th viewpoint at the suitable temperature within the range of 100 to 450 degreeC.
- an 11th viewpoint it is related with the cured film as described in a 10th viewpoint which has a film thickness of 1.0 micrometer thru
- a 12th viewpoint it is related with the cured film as described in the 10th viewpoint or the 11th viewpoint which has self-supporting property.
- a 13th viewpoint it is related with a structure provided with at least one layer which consists of a cured film as described in any one of 10th viewpoint thru
- a 14th viewpoint it is related with the polyamic acid containing the structural unit represented by the structural unit represented by following formula (1), and Formula (3).
- X 1 represents a tetravalent group represented by the following formula (5):
- Y 1 represents a divalent group represented by the following formula (6), and Y 2 represents a divalent group represented by the following formula (7).
- the present invention relates to a polyimide containing a structural unit represented by the following formula (2) and a structural unit represented by the formula (4).
- X 1 represents a tetravalent group represented by the following formula (5):
- Y 1 represents a divalent group represented by the following formula (6), and
- Y 2 represents a divalent group represented by the following formula (7).
- n and m represent natural numbers.
- As a 16th viewpoint it is related with the composition containing the polyamic acid as described in a 14th viewpoint, and a crosslinking agent.
- the present invention relates to a varnish characterized in that the composition according to the sixteenth aspect or the seventeenth aspect is dissolved in at least one solvent.
- a 19th viewpoint it is related with the cured film obtained by baking the thin film obtained using the varnish as described in an 18th viewpoint at the appropriate temperature within the range of 100 to 450 degreeC.
- the resin composition for a display substrate of the present invention is a useful curing having required performance as a flexible display substrate, that is, sufficient heat resistance, high transparency, low birefringence, and an appropriate linear expansion coefficient and an appropriate flexibility.
- a film can be formed. Therefore, the cured film can be used for a base film for a flexible display.
- cycloaliphatic tetracarboxylic dianhydrides especially 1,2,3,4-cyclobutanetetra
- a cured film having high transparency can be obtained using carboxylic acid dianhydride), and an increase in display substrate manufacturing cost can be expected by using a special material (acid dianhydride having a special structure). it can.
- the present invention is represented by a polyamic acid containing a structural unit represented by the following formula (1) and a structural unit represented by the formula (3), or a structural unit represented by the following formula (2) and the formula (4).
- the present invention relates to a resin composition for a display substrate containing polyimide containing a structural unit.
- X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group
- Y 1 and Y 2 represent different divalent aromatic groups or divalent aliphatic groups
- n and m represent natural numbers.
- the polyamic acid having the structural unit represented by the formula (1) and the structural unit represented by the formula (3) contained in the resin composition for display substrates of the present invention is at least one acid dianhydride component. And at least two diamine components are polymerized in a solvent.
- the polyamic acid is a known method, for example, in an inert gas atmosphere such as nitrogen, the following formula (9): (Wherein X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group), and at least one acid dianhydride represented by the following formula (10): H 2 N—Y 1 —NH 2 (10) (Wherein Y 1 represents a divalent aromatic group or a divalent aliphatic group) and at least one diamine represented by the following formula (11): H 2 N—Y 2 —NH 2 (11) (Wherein Y 2 represents a divalent aromatic group or a divalent aliphatic group) and at least one diamine (wherein Y 1 and Y 2 are different divalent groups); Is dissolved in a solvent and reacted.
- formula (9) (Wherein X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group), and at least one acid dianhydride represented by the following formula (10): H 2 N
- the reaction temperature during polymerization of these acid dianhydrides and diamines is -20 to 100 ° C, preferably 20 to 60 ° C.
- the reaction time is 1 to 72 hours.
- Examples of the acid dianhydride represented by the formula (9) include pyromellitic dianhydride, 2,3,6,7-naphthalenetetracarboxylic dianhydride, 1,2,5,6. -Naphthalene tetracarboxylic dianhydride, 1,4,5,8-naphthalene tetracarboxylic dianhydride, 2,3,6,7-anthracene tetracarboxylic dianhydride, 1,2,5,6-anthracene Tetracarboxylic dianhydride, 3,3 ′, 4,4′-biphenyltetracarboxylic dianhydride, 2,2 ′, 3,3′-biphenyltetracarboxylic dianhydride, 2,3,3 ′, 4′-biphenyltetracarboxylic dianhydride, 3,3 ′, 4,4′-benzophenone tetracarboxylic dianhydride, 2,3,3 ′, 4-benzophenone
- X 1 is An acid dianhydride having a structure represented by the following formula (5), that is, 1,2,3,4-cyclobutanetetracarboxylic dianhydride (the following formula (12)) is preferable.
- Examples of the aromatic diamine represented by the formula (10) or the formula (11) include p-phenylenediamine, o-phenylenediamine, 2-methyl-1,4-phenylenediamine, 2-trifluoromethyl- 1,4-phenylenediamine, 2-methoxy-1,4-phenylenediamine, 2,5-dimethyl-1,4-phenylenediamine, 2,5-bis (trifluoromethyl) -1,4-phenylenediamine, 4 , 4'-diaminobenzanilide, 4-aminophenyl-4'-aminobenzoate, benzidine, 3,3'-dimethoxybenzidine, 3,3'-dichlorobenzidine, o-tolidine, m-tolidine, 2,2'- Bis (trifluoromethyl) benzidine, 3,3'-bis (trifluoromethyl) benzidine, octafluorobenzidine 3,3 ′, 5,5′-tetramethylbenzidine,
- p-phenylenediamine and bis [4- (3-aminophenoxy) phenyl Sulfone, bis (3-aminophenyl) sulfone and the like are preferable.
- Examples of the aliphatic diamine represented by the formula (10) or the formula (11) include 4,4′-methylenebis (cyclohexylamine), 4,4′-methylenebis (3-methylcyclohexylamine), isophoronediamine, Trans-1,4-cyclohexanediamine, cis-1,4-cyclohexanediamine, 1,4-cyclohexanebis (methylamine), 2,5-bis (aminomethyl) bicyclo [2.2.1] heptane, 2, 6-bis (aminomethyl) bicyclo [2.2.1] heptane, 3,8-bis (aminomethyl) tricyclo [5.2.1.0] decane, 1,3-diaminoadamantane, 2,2-bis (4-aminocyclohexyl) propane, 2,2-bis (4-aminocyclohexyl) hexafluoropropane, 1,3-propyl Pandiamine, 1,4-tetramethylenediamine, 1,5-pent
- aliphatic diamines from the viewpoint that the cured film obtained from the resin composition of the present invention has a sufficiently low linear expansion coefficient, it is preferable to use a diamine having a rigid and linear molecular structure, For example, trans-1,4-cyclohexanediamine is preferably used.
- one of the cured films obtained from the resin composition of the present invention has sufficiently high transparency.
- a diamine having an electron withdrawing group it is preferable to use a diamine having an electron withdrawing group.
- an electron withdrawing group is used.
- a diamine having a rigid and linear molecular structure As such a diamine, the diamine represented by the formula (10) and the formula (11) is preferably a diamine in which Y 1 and Y 2 are aromatic groups having a sulfonic acid group.
- a diamine having a divalent group represented by Y 1 represented by the following formula (6) that is, bis [4- (3-aminophenoxy) phenyl] sulfone
- a diamine having a divalent group Y 2 is expressed by the following formula (7) (i.e. bis (3-aminophenyl) sulfone) are particularly preferred.
- the solvent used in the polymerization reaction of the polyamic acid is not particularly limited.
- N, N-dimethylacetamide, N, N-diethylacetamide, N, N-dimethylformamide, N-methyl-2-pyrrolidone 3- Methoxy-N, N-dimethylpropylamide, 3-ethoxy-N, N-dimethylpropylamide, 3-propoxy-N, N-dimethylpropylamide, 3-isopropoxy-N, N-dimethylpropylamide, 3-butoxy -N, N-dimethylpropylamide, 3-sec-butoxy-N, N-dimethylpropylamide, 3-tert-butoxy-N, N-dimethylpropylamide, hexamethylphosphoramide, dimethylsulfoxide, ⁇ -butyrolactone, 1,3-dimethyl-2-imidazolidinone, 1,2-dimethyl Aprotic solvents such as xylene-bis (2-methoxyethyl)
- the ratio of the diamine component is the diamine represented by the above formula (10) (that is, the diamine having a divalent group represented by the above formula (6)) and the above formula (11).
- the molar ratio with the diamine represented is preferably 10/90 to 99/1, and obtained from the resin composition of the present invention. From the viewpoint that the cured film to be obtained has a sufficiently low linear expansion coefficient and a sufficiently high strength, 20/80 to 80/20 is more preferable.
- the weight average molecular weight of the produced polyamic acid is preferably 3,000 to 300,000 in terms of polystyrene in order to maintain the strength of the cured film obtained from the resin composition for display substrate containing polyamic acid. If the weight average molecular weight is less than 3,000, the resulting film may become brittle. On the other hand, if the weight average molecular weight exceeds 300,000, the viscosity of the polyamic acid varnish may be too high. This is because handling becomes difficult.
- n and m in the above formulas (1) and (3) are n + m, usually 6 to 180, preferably 10 to 100, more preferably 10 to 50.
- the number of repeating structural units in the polyamic acid composed of the structural unit represented by the above formula (1) and the structural unit represented by the formula (3) is represented by the formula (1). It is preferable that n and m in the above formula (3) have a relationship of n / (n + m) ⁇ 0.2 (the ratio of n is 20% or more with respect to the total number of n and m).
- the reaction solution of polyamic acid can be used as it is or diluted to be used in an imidation reaction for obtaining a polyimide described later, and this can be used as the resin composition of the present invention.
- the polyamic acid precipitated and recovered from the reaction solution can be redissolved in an appropriate solvent and used for the imidization reaction, and this can be used as the resin composition of the present invention.
- the solvent used for dilution and re-dissolution is not particularly limited as long as it can dissolve the obtained polyamic acid.
- These solvents may be used alone or in combination of two or more.
- the polyimide contained in the resin composition for display substrates of the present invention can be obtained by subjecting the polyamic acid synthesized as described above to dehydration ring closure (thermal imidization) by heating. At this time, polyamic acid can be converted to imide in a solvent and used as a solvent-soluble polyimide.
- the method of chemically ring-closing using a well-known dehydration ring-closing catalyst is also employable.
- the method by heating can be performed at an arbitrary temperature of 100 to 500 ° C., preferably 120 to 450 ° C.
- the method of chemically cyclizing can be performed, for example, in the presence of pyridine, triethylamine or the like and acetic anhydride, and the temperature at this time can be selected from -20 to 200 ° C. .
- the weight average molecular weight of the polyimide produced is preferably 3,000 to 300,000 in terms of polystyrene in order to maintain the strength of the cured film obtained from the resin composition for display substrate containing polyimide. If the weight average molecular weight is less than 3,000, the resulting film may be brittle, while if the weight average molecular weight exceeds 300,000, the viscosity of the polyimide varnish may be too high, and as a result, This is because handling becomes difficult.
- n and m in the above formulas (2) and (4) are usually n + m and are usually 6 to 180, preferably 10 to 100, more preferably 10 Thirty to fifty.
- the number of repeating structural units in the polyimide composed of the structural unit represented by the above formula (2) and the structural unit represented by the formula (4) is n in the above formula (2).
- m in the above formula (4) preferably have a relationship of n / (n + m) ⁇ 0.2 (the ratio of n is 20% or more with respect to the total number of n and m).
- the obtained polyimide can be used as a resin composition as it is or diluted with the reaction solution, or a polyimide recovered by precipitation by adding a poor solvent such as methanol or ethanol to the reaction solution is appropriately used. After re-dissolving in a solvent, it can be used as a resin composition.
- the solvent used for dilution and re-dissolution is not particularly limited as long as it can dissolve the obtained polyimide.
- N-cresol 2-pyrrolidone, N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone N-vinyl-2-pyrrolidone, N, N-dimethylacetamide, N, N-dimethylformamide, 3-methoxy-N, N-dimethylpropylamide, 3-ethoxy-N, N-dimethylpropylamide, 3-propoxy -N, N-dimethylpropylamide, 3-isopropoxy-N, N-dimethylpropylamide, 3-butoxy-N, N-dimethylpropylamide, 3-sec-butoxy-N, N-dimethylpropylamide, 3- and tert-butoxy-N, N-dimethylpropylamide, ⁇ -butyrolactone, and the like.
- solvents may be used alone or in combination of two or more.
- the ratio of the solid content in the resin composition for display substrates of the present invention is 1 to 100% by mass, or 5 to 100% by mass, or 50 to 100% by mass, or 80 to 100% by mass.
- solid content is the remaining component which removed the solvent mentioned later from all the components including the said polyamic acid of the resin composition for display substrates, and the said polyimide.
- the content of the polyamic acid or polyimide in the resin composition for display substrates of the present invention is 8 to 100% by mass, preferably 40 to 100% by mass, more preferably based on the solid content of the resin composition. Is 70 to 100% by mass.
- the resin composition for display substrates of the present invention can contain a solvent.
- a solvent examples include the solvents listed as solvents that can be used for redissolving polyamic acid and polyimide (solvents described in paragraphs [0023] and [0027]).
- cross-linking agent The resin composition for display substrates of the present invention can contain a crosslinking agent (hereinafter also referred to as a crosslinkable compound).
- the crosslinkable compound can react with an organic group contained in at least one of polyamic acid and polyimide in the step of converting the coating film (thin film) obtained using the resin composition for display substrates into a cured film. If it is a compound which has group, it will not specifically limit. Examples of such a compound include a compound containing two or more epoxy groups, a melamine derivative having a hydrogen atom of an amino group substituted by a methylol group, an alkoxymethyl group, or both, a benzoguanamine derivative, or glycoluril. Etc.
- the melamine derivative and benzoguanamine derivative may be a dimer or a trimer, or may be a mixture arbitrarily selected from a monomer, a dimer and a trimer. These melamine derivatives and benzoguanamine derivatives preferably have an average of 3 or more and less than 6 methylol groups or alkoxymethyl groups per one triazine ring. Moreover, you may use the crosslinking agent used for this invention individually or in combination of 2 or more types.
- crosslinkable compound examples include cyclohexene structures such as epolide GT-401, epolide GT-403, epolide GT-301, epolide GT-302, ceroxide 2021, and ceroxide 3000 (manufactured by Daicel Corporation).
- benzoguanamine derivative or glycoluril having a group in which the hydrogen atom of the amino group is substituted with a methylol group, an alkoxymethyl group or both, an average of 3.7 methoxymethyl groups are substituted per triazine ring.
- MX-750 MW-30 substituted with an average of 5.8 methoxymethyl groups per triazine ring (above, manufactured by Sanwa Chemical Co., Ltd.); Cymel 300, Cymel 301, Cymel 303, Cymel 350, Methoxymethylated melamines such as Cymel 370, Cymel 771, Cymel 325, Cymel 327, Cymel 703, Cymel 712, etc .; Butoxymethylated melamines such as Cymel 506 and Cymel 508; carboxyl group-containing methoxymethylated isobutoxymethylated melamines such as Cymel 1141; methoxymethylated ethoxymethylated benzoguanamines such as Cymel 1123; Methoxymethylated butoxymethylated benzoguanamine; butoxymethylated benzoguanamine such as Cymel 1128; carboxyl-containing methoxymethylated ethoxymethylated benzoguanamine such as Cymel 1125-80; butoxymethylated glycoluril such as Cymel 1170; Cy
- the content in the case of using a crosslinking agent is not particularly limited, but from the viewpoint of further improving the storage stability of the resin composition, relative to 100 parts by mass of polyamic acid or polyimide From the viewpoint that the cured film obtained from the resin composition of the present invention has a sufficiently low linear expansion coefficient, it is more preferably contained at 15 parts by mass or less. .
- a cured film comprising the resin composition for a display substrate of the present invention
- the resin composition is dissolved or dispersed in a solvent to form a varnish (film forming material), and the varnish is used as a substrate.
- a coating film (thin film) is obtained.
- the cured film is formed by baking the obtained coating film with a hotplate, oven, etc.
- the firing temperature is usually 100 to 500 ° C, preferably 120 to 450 ° C.
- Firing may be performed a plurality of times at different temperatures.
- the atmosphere is preferably nitrogen or under reduced pressure.
- the substrate include plastic (polycarbonate, polymethacrylate, polystyrene, polyester, polyolefin, epoxy, melamine, triacetyl cellulose, ABS, AS, norbornene resin, etc.), metal, wood, paper, glass, slate, and the like. Can be mentioned.
- the solvent used in the form of the varnish is not particularly limited as long as it dissolves the resin composition for display substrates.
- the solvent used in the polymerization reaction of the polyamic acid, or the re-use of polyamic acid or polyimide is used.
- the solvent etc. which were mentioned as a solvent which can be used for dissolution are mentioned. These solvents may be used alone or in combination of two or more.
- distributes a resin composition to the said solvent is arbitrary,
- the solid content is 5 to 50% by mass, preferably 10 to 40% by mass from the viewpoint of further improving the storage stability of the resin composition, and more preferably from the viewpoint of more uniformly applying the resin composition. Is 10 to 30% by mass.
- the varnish preferably has a viscosity of 0.001 to 5,000 Pa ⁇ s.
- the thickness of the cured film formed from the resin composition for display substrates or the varnish obtained from this composition is not specifically limited, For example, it is 1 to 200 micrometers, Usually 1 to 100 micrometers, Preferably it is 5 to 60 micrometers.
- Mw weight average molecular weight
- SB803HQ and SB804HQ molecular weight distribution of the polymer
- ⁇ Comparative example 2> 2.35 g (0.013 mol) of bis (3-aminophenyl) sulfone was dissolved in 14.0 g of N-methyl-2-pyrrolidone, and 2.65 g of 1,2,3,4-cyclobutanetetracarboxylic dianhydride. (0.014 mol) was added, and the mixture was reacted at 23 ° C. for 24 hours under a nitrogen atmosphere. Mw of the obtained polymer was 13,600 and molecular weight distribution was 3.1. The obtained reaction solution was used as the resin composition for display substrate of Comparative Example 2 as it was.
- the cured film produced using the display substrate resin composition of Example 1 has higher heat resistance than the cured films produced using the display substrate resin compositions of Comparative Example 1 and Comparative Example 2. Showed sex.
- the cured film obtained from the resin composition for display substrate of Comparative Example 2 can be confirmed to be partially cloudy, and the film is brittle, and the film is cracked and torn when peeled from the glass substrate. As a result, the peeled film was easily broken, and the film strength as a display substrate was insufficient.
- the cured film produced using the resin composition for display substrate of Example 1 is less susceptible to cracking and tearing when peeled from the glass substrate, and the peeled film is also difficult to break and has a sufficiently high film strength.
- a film having a uniform self-supporting property having a thickness of 30 ⁇ m could be formed.
- the cured film produced using the display substrate resin composition of Example 1 exhibited sufficiently low birefringence as a display substrate film.
- the cured film produced using the display substrate resin composition of Example 1 exhibited sufficiently high transparency as a display substrate film.
Abstract
[Problem] To provide a resin composition for a display substrate making it possible to form a useful polyimide film having high heat resistance, a suitable linear expansion coefficient, and suitable flexibility. [Solution] A resin composition for a display substrate, containing a polyamic acid comprising structural units represented by formula (1) and structural units represented by formula (3), or polyimide comprising structural units represented by formula (2) and structural units represented by formula (4). (In formulae (1) to (4), X1 represents a tetravalent aromatic group or tetravalent aliphatic group, Y1 and Y2 are different from each other and represent a divalent aromatic group or divalent aliphatic group, and n and m represent natural numbers.)
Description
本発明はディスプレイ基板用樹脂組成物に関し、詳細には適度な耐熱性、透明性、線膨張係数、複屈折、及び適度な柔軟性を有する有用なポリイミドフィルムを形成することができるディスプレイ基板用樹脂組成物に関する。
The present invention relates to a resin composition for display substrates, and in particular, a resin for display substrates capable of forming a useful polyimide film having appropriate heat resistance, transparency, linear expansion coefficient, birefringence, and appropriate flexibility. Relates to the composition.
ポリイミド樹脂は、耐熱性が高く難燃性で電気絶縁性に優れていることから、電気・電子材料分野において幅広く使用されている。具体的には、フィルムとしてフレキシブル印刷配線版や耐熱性接着テープの基材、樹脂ワニスとして半導体の絶縁皮膜、保護皮膜などに使用されている。
一方、有機EL(Electro Luminescence)ディスプレイや液晶ディスプレイなどの表示装置は、以前は高精細のみが要求されていた。こうした表示装置が情報機器などへ急速にその用途を拡大する中、例えば、超薄型・軽量化といった新たな要求を満たすために、プラスチックフィルムを基板として使用するフレキシブルディスプレイが注目されている。
従来、高精細なディスプレイには、アクティブマトリックス駆動のパネルが使用されている。マトリックス状の画素電極に加えて、薄膜アクティブ素子を含むアクティブマトリックス層を形成するには、その製造プロセスにおいて200℃以上の高温処理を必要とし、しかも、きわめて正確な位置合わせが必要である。しかし、ディスプレイのフレキシブル化のために、ガラス基板をプラスチック材料に変更すると、耐熱性、寸法安定性を満足なものとできず、その上にアクティブ素子を直に形成するのは非常に困難であった。 Polyimide resins are widely used in the field of electrical and electronic materials because of their high heat resistance, flame retardancy, and excellent electrical insulation. Specifically, it is used as a film for flexible printed wiring boards and heat-resistant adhesive tapes as a film, and as a resin varnish for semiconductor insulating films, protective films, and the like.
On the other hand, display devices such as an organic EL (Electro Luminescence) display and a liquid crystal display have been required only for high definition. As such display devices are rapidly expanding their applications to information devices and the like, flexible displays using a plastic film as a substrate are attracting attention in order to satisfy new requirements such as ultra-thinness and light weight.
Conventionally, an active matrix driving panel is used for a high-definition display. In order to form an active matrix layer including a thin film active element in addition to a matrix-like pixel electrode, a high temperature treatment of 200 ° C. or higher is required in the manufacturing process, and extremely accurate alignment is required. However, if the glass substrate is changed to a plastic material to make the display flexible, heat resistance and dimensional stability cannot be satisfied, and it is very difficult to directly form an active element on the glass substrate. It was.
一方、有機EL(Electro Luminescence)ディスプレイや液晶ディスプレイなどの表示装置は、以前は高精細のみが要求されていた。こうした表示装置が情報機器などへ急速にその用途を拡大する中、例えば、超薄型・軽量化といった新たな要求を満たすために、プラスチックフィルムを基板として使用するフレキシブルディスプレイが注目されている。
従来、高精細なディスプレイには、アクティブマトリックス駆動のパネルが使用されている。マトリックス状の画素電極に加えて、薄膜アクティブ素子を含むアクティブマトリックス層を形成するには、その製造プロセスにおいて200℃以上の高温処理を必要とし、しかも、きわめて正確な位置合わせが必要である。しかし、ディスプレイのフレキシブル化のために、ガラス基板をプラスチック材料に変更すると、耐熱性、寸法安定性を満足なものとできず、その上にアクティブ素子を直に形成するのは非常に困難であった。 Polyimide resins are widely used in the field of electrical and electronic materials because of their high heat resistance, flame retardancy, and excellent electrical insulation. Specifically, it is used as a film for flexible printed wiring boards and heat-resistant adhesive tapes as a film, and as a resin varnish for semiconductor insulating films, protective films, and the like.
On the other hand, display devices such as an organic EL (Electro Luminescence) display and a liquid crystal display have been required only for high definition. As such display devices are rapidly expanding their applications to information devices and the like, flexible displays using a plastic film as a substrate are attracting attention in order to satisfy new requirements such as ultra-thinness and light weight.
Conventionally, an active matrix driving panel is used for a high-definition display. In order to form an active matrix layer including a thin film active element in addition to a matrix-like pixel electrode, a high temperature treatment of 200 ° C. or higher is required in the manufacturing process, and extremely accurate alignment is required. However, if the glass substrate is changed to a plastic material to make the display flexible, heat resistance and dimensional stability cannot be satisfied, and it is very difficult to directly form an active element on the glass substrate. It was.
そこで、耐熱性や寸法安定性の問題を回避できるプラスチック材料として、いくつかの提案がなされている。特許文献1では脂環式構造含有テトラカルボン酸二無水物と各種ジアミンより得られるポリイミドを用いたディスプレイ基板用ポリイミドフィルムが開示されている。また特許文献2には、シクロヘキサン骨格を有する脂環式テトラカルボン酸二無水物とスルホン基を含有する芳香族ジアミンより得られるポリイミドを用いたディスプレイ基板用ポリイミドフィルムが開示されている。
Therefore, several proposals have been made as plastic materials that can avoid the problems of heat resistance and dimensional stability. Patent Document 1 discloses a polyimide film for a display substrate using a polyimide obtained from an alicyclic structure-containing tetracarboxylic dianhydride and various diamines. Patent Document 2 discloses a polyimide film for a display substrate using a polyimide obtained from an alicyclic tetracarboxylic dianhydride having a cyclohexane skeleton and an aromatic diamine containing a sulfone group.
上述したように、フレキシブルディスプレイ基板向け材料として、耐熱性の向上を図った材料の提案が為されているものの、特許文献1で使用するテトラカルボン酸二無水物は芳香族基も含むため、芳香族基を含まず脂環式基のみからなるテトラカルボン酸二無水物と比べ、ポリイミド鎖の分子内共役や電荷移動相互作用によって得られたフィルムの透明性が低くなるという問題がある。また特許文献2で使用する酸二無水物はシス構造のシクロヘキサン骨格という特殊な構造を有するために汎用性に乏しく、ポリイミドの製造コストが割高となり、ひいては得られる製品が高価になってしまうという問題がある。
また脂環式テトラカルボン酸二無水物は、そもそも重合反応性の点より十分な膜靭性を示す程の高分子量体が得られない場合があるという問題がある。その中でもテトラカルボン酸二無水物の中で最もポピュラーといえる1,2,3,4-シクロブタンテトラカルボン酸二無水物(以下CBDAと称す)はジアミンとの比較的高い重合反応性を示すものの、その立体構造から、ポリイミド前駆体のイミド化反応が起こりにくく、イミド化反応を完結するためにより高温を必要とし、これがポリイミドフィルムの着色の原因となり、ディスプレイ基板用としてCBDAの使用は有利ではない点が指摘されている(特許文献1及び特許文献2)。 As described above, although materials having improved heat resistance have been proposed as materials for flexible display substrates, the tetracarboxylic dianhydride used in Patent Document 1 also contains aromatic groups. Compared to tetracarboxylic dianhydrides that contain only alicyclic groups and do not contain any group, there is a problem that the transparency of the film obtained by intramolecular conjugation of the polyimide chain and charge transfer interaction is lowered. Moreover, since the acid dianhydride used in Patent Document 2 has a special structure called a cyclohexane skeleton having a cis structure, the versatility is poor, and the production cost of polyimide becomes high, and the resulting product becomes expensive. There is.
In addition, the alicyclic tetracarboxylic dianhydride has a problem in that a high-molecular-weight body having sufficient film toughness may not be obtained from the viewpoint of polymerization reactivity. Among them, 1,2,3,4-cyclobutanetetracarboxylic dianhydride (hereinafter referred to as CBDA), which is the most popular among tetracarboxylic dianhydrides, exhibits relatively high polymerization reactivity with diamine, Due to its three-dimensional structure, imidation reaction of the polyimide precursor hardly occurs, and a higher temperature is required to complete the imidization reaction, which causes coloring of the polyimide film, and the use of CBDA as a display substrate is not advantageous. Has been pointed out (Patent Document 1 and Patent Document 2).
また脂環式テトラカルボン酸二無水物は、そもそも重合反応性の点より十分な膜靭性を示す程の高分子量体が得られない場合があるという問題がある。その中でもテトラカルボン酸二無水物の中で最もポピュラーといえる1,2,3,4-シクロブタンテトラカルボン酸二無水物(以下CBDAと称す)はジアミンとの比較的高い重合反応性を示すものの、その立体構造から、ポリイミド前駆体のイミド化反応が起こりにくく、イミド化反応を完結するためにより高温を必要とし、これがポリイミドフィルムの着色の原因となり、ディスプレイ基板用としてCBDAの使用は有利ではない点が指摘されている(特許文献1及び特許文献2)。 As described above, although materials having improved heat resistance have been proposed as materials for flexible display substrates, the tetracarboxylic dianhydride used in Patent Document 1 also contains aromatic groups. Compared to tetracarboxylic dianhydrides that contain only alicyclic groups and do not contain any group, there is a problem that the transparency of the film obtained by intramolecular conjugation of the polyimide chain and charge transfer interaction is lowered. Moreover, since the acid dianhydride used in Patent Document 2 has a special structure called a cyclohexane skeleton having a cis structure, the versatility is poor, and the production cost of polyimide becomes high, and the resulting product becomes expensive. There is.
In addition, the alicyclic tetracarboxylic dianhydride has a problem in that a high-molecular-weight body having sufficient film toughness may not be obtained from the viewpoint of polymerization reactivity. Among them, 1,2,3,4-cyclobutanetetracarboxylic dianhydride (hereinafter referred to as CBDA), which is the most popular among tetracarboxylic dianhydrides, exhibits relatively high polymerization reactivity with diamine, Due to its three-dimensional structure, imidation reaction of the polyimide precursor hardly occurs, and a higher temperature is required to complete the imidization reaction, which causes coloring of the polyimide film, and the use of CBDA as a display substrate is not advantageous. Has been pointed out (Patent Document 1 and Patent Document 2).
本発明は、このような事情に鑑みてなされたものであり、その目的は、フレキシブルディスプレイ用基板として十分な膜強度を有し且つ薄膜トランジスタ(TFT)形成プロセスに必要な耐熱性を備えた高透明且つ低複屈折であるフィルムを形成することが可能なディスプレイ基板用高耐熱性コーティング剤を提供することである。
The present invention has been made in view of such circumstances, and its purpose is a highly transparent film having sufficient film strength as a flexible display substrate and having heat resistance necessary for a thin film transistor (TFT) formation process. Another object of the present invention is to provide a high heat-resistant coating agent for display substrates capable of forming a film having low birefringence.
本発明者は、上記目的を達成するために鋭意検討を重ねた結果、酸二無水物成分としてこれまで反応性や着色の問題によりディスプレイ基板分野ではその使用が敬遠された脂環式テトラカルボン酸二無水物、特に1,2,3,4-シクロブタンテトラカルボン酸二無水物(以下CBDAとも称す)と、スルホン基を含有する芳香族ジアミンとを反応させて得られるポリアミック酸又はポリイミドを含むディスプレイ基板用樹脂組成物から、十分な膜強度を有し且つ薄膜トランジスタ(TFT)形成プロセスに必要な耐熱性を有し、しかも驚くべきことに高い透明性を実現でき、低複屈折、適度な線膨張係数及び適度な柔軟性をも有する有用な硬化膜が得られることを見出し、本発明を完成させた。
As a result of intensive studies to achieve the above object, the present inventor has alicyclic tetracarboxylic acid, which has been avoided in the field of display substrates due to reactivity and coloring problems as an acid dianhydride component. Display comprising polyamic acid or polyimide obtained by reacting dianhydride, in particular, 1,2,3,4-cyclobutanetetracarboxylic dianhydride (hereinafter also referred to as CBDA) and aromatic diamine containing a sulfone group The resin composition for substrates has sufficient film strength, heat resistance necessary for thin film transistor (TFT) formation process, and surprisingly high transparency, low birefringence, and moderate linear expansion It was found that a useful cured film having a coefficient and moderate flexibility was obtained, and the present invention was completed.
すなわち、本発明は、第1観点として、下記式(1)で表される構造単位及び式(3)で表される構造単位を含むポリアミック酸又は下記式(2)で表される構造単位及び式(4)で表される構造単位を含むポリイミドを含有するディスプレイ基板用樹脂組成物に関する。
[式(1)乃至式(4)中、
X1は4価の芳香族基又は4価の脂肪族基を表し、
Y1及びY2は互いに異なって、2価の芳香族基又は2価の脂肪族基を表し、
n及びmは自然数を表す。]
第2観点として、前記X1が下記式(5)で表される4価の基を表す、第1観点に記載のディスプレイ基板用樹脂組成物に関する。
第3観点として、前記Y1が下記式(6)で表される2価の基を表し、Y2が下記式(7)で表される2価の基を表す、第1観点に記載のディスプレイ基板用樹脂組成物に関する。
第4観点として、前記式(1)中のnと前記式(3)中のmが、n/(n+m)≧0.2の関係式を満たす、第1観点乃至第3観点のうちいずれか1つに記載のディスプレイ基板用樹脂組成物に関する。
第5観点として、前記式(2)中のnと前記式(4)中のmが、n/(n+m)≧0.2の関係式を満たす、第1観点乃至第3観点のうちいずれか1つに記載のディスプレイ基板用樹脂組成物に関する。
第6観点として、さらに架橋剤を含む、第1観点乃至第5観点のうちいずれか1項に記載のディスプレイ基板用樹脂組成物に関する。
第7観点として、前記ポリアミック酸又はポリイミド100質量部に対して、前記架橋剤が20質量部以下で含まれる、第6観点に記載のディスプレイ基板用樹脂組成物に関する。
第8観点として、第1観点乃至第7観点のうちいずれか1つに記載のディスプレイ基板用樹脂組成物が少なくとも1種の溶剤に溶解していることを特徴とする、ワニスに関する。
第9観点として、0.001乃至5,000Pa・sの粘度を有する、第8観点に記載のワニスに関する。
第10観点として、第8観点又は第9観点に記載のワニスを用いて得られる薄膜を100℃乃至450℃の範囲内の適当な温度で焼成することにより得られる、硬化膜に関する。
第11観点として、1.0μm乃至200μmの膜厚を有する、第10観点に記載の硬化膜に関する。
第12観点として、自己支持性を有する、第10観点又は第11観点に記載の硬化膜に関する。
第13観点として、基板上に第10観点乃至第12観点のうちいずれか一項に記載の硬化膜からなる層を少なくとも一層備える、構造体に関する。
第14観点として、下記式(1)で表される構造単位及び式(3)で表される構造単位を含むポリアミック酸に関する。
[式(1)及び式(3)中、
X1は下記(5)式で表される4価の基を表し、
Y1は下記式(6)で表される2価の基を表し、Y2は下記式(7)で表される2価の基を表し
n及びmは自然数を表す。]
第15観点として、下記式(2)で表される構造単位及び式(4)で表される構造単位を含むポリイミドに関する。
[式(2)及び式(4)中、
X1は下記(5)式で表される4価の基を表し、
Y1は下記式(6)で表される2価の基を表し、Y2は下記式(7)で表される2価の基を表し
n及びmは自然数を表す。]
第16観点として、第14観点に記載のポリアミック酸と架橋剤とを含む組成物に関する。
第17観点として、第15観点に記載のポリイミドと架橋剤とを含む組成物に関する。
第18観点として、第16観点又は第17観点に記載の組成物が少なくとも1種の溶剤に溶解していることを特徴とする、ワニスに関する。
第19観点として、第18観点に記載のワニスを用いて得られる薄膜を100℃乃至450℃の範囲内の適当な温度で焼成することにより得られる、硬化膜に関する。 That is, as a first aspect, the present invention provides a polyamic acid containing a structural unit represented by the following formula (1) and a structural unit represented by the formula (3), or a structural unit represented by the following formula (2) and It is related with the resin composition for display substrates containing the polyimide containing the structural unit represented by Formula (4).
[In Formula (1) thru | or Formula (4),
X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group,
Y 1 and Y 2 are different from each other and represent a divalent aromatic group or a divalent aliphatic group,
n and m represent natural numbers. ]
As a second aspect, the present invention relates to the display substrate resin composition according to the first aspect, in which X 1 represents a tetravalent group represented by the following formula (5).
As a third aspect, the Y 1 represents a divalent group represented by the following formula (6), and Y 2 represents a divalent group represented by the following formula (7). The present invention relates to a resin composition for display substrates.
As a fourth aspect, any one of the first to third aspects in which n in the formula (1) and m in the formula (3) satisfy a relational expression of n / (n + m) ≧ 0.2. It is related with the resin composition for display substrates as described in one.
As a fifth aspect, any one of the first to third aspects in which n in the formula (2) and m in the formula (4) satisfy a relational expression of n / (n + m) ≧ 0.2. It is related with the resin composition for display substrates as described in one.
As a 6th viewpoint, it is related with the resin composition for display substrates of any one of the 1st viewpoint thru | or a 5th viewpoint further including a crosslinking agent.
As a seventh aspect, the present invention relates to the display substrate resin composition according to the sixth aspect, wherein the crosslinking agent is contained in an amount of 20 parts by mass or less with respect to 100 parts by mass of the polyamic acid or polyimide.
An eighth aspect relates to a varnish, wherein the display substrate resin composition according to any one of the first to seventh aspects is dissolved in at least one solvent.
As a ninth aspect, the present invention relates to the varnish according to the eighth aspect, which has a viscosity of 0.001 to 5,000 Pa · s.
As a 10th viewpoint, it is related with the cured film obtained by baking the thin film obtained using the varnish as described in an 8th viewpoint or a 9th viewpoint at the suitable temperature within the range of 100 to 450 degreeC.
As an 11th viewpoint, it is related with the cured film as described in a 10th viewpoint which has a film thickness of 1.0 micrometer thru | or 200 micrometers.
As a 12th viewpoint, it is related with the cured film as described in the 10th viewpoint or the 11th viewpoint which has self-supporting property.
As a 13th viewpoint, it is related with a structure provided with at least one layer which consists of a cured film as described in any one of 10th viewpoint thru | or 12th viewpoint on a board | substrate.
As a 14th viewpoint, it is related with the polyamic acid containing the structural unit represented by the structural unit represented by following formula (1), and Formula (3).
[In Formula (1) and Formula (3),
X 1 represents a tetravalent group represented by the following formula (5):
Y 1 represents a divalent group represented by the following formula (6), and Y 2 represents a divalent group represented by the following formula (7).
n and m represent natural numbers. ]
As a fifteenth aspect, the present invention relates to a polyimide containing a structural unit represented by the following formula (2) and a structural unit represented by the formula (4).
[In Formula (2) and Formula (4),
X 1 represents a tetravalent group represented by the following formula (5):
Y 1 represents a divalent group represented by the following formula (6), and Y 2 represents a divalent group represented by the following formula (7).
n and m represent natural numbers. ]
As a 16th viewpoint, it is related with the composition containing the polyamic acid as described in a 14th viewpoint, and a crosslinking agent.
As a 17th viewpoint, it is related with the composition containing the polyimide and crosslinking agent as described in a 15th viewpoint.
As an eighteenth aspect, the present invention relates to a varnish characterized in that the composition according to the sixteenth aspect or the seventeenth aspect is dissolved in at least one solvent.
As a 19th viewpoint, it is related with the cured film obtained by baking the thin film obtained using the varnish as described in an 18th viewpoint at the appropriate temperature within the range of 100 to 450 degreeC.
X1は4価の芳香族基又は4価の脂肪族基を表し、
Y1及びY2は互いに異なって、2価の芳香族基又は2価の脂肪族基を表し、
n及びmは自然数を表す。]
第2観点として、前記X1が下記式(5)で表される4価の基を表す、第1観点に記載のディスプレイ基板用樹脂組成物に関する。
第5観点として、前記式(2)中のnと前記式(4)中のmが、n/(n+m)≧0.2の関係式を満たす、第1観点乃至第3観点のうちいずれか1つに記載のディスプレイ基板用樹脂組成物に関する。
第6観点として、さらに架橋剤を含む、第1観点乃至第5観点のうちいずれか1項に記載のディスプレイ基板用樹脂組成物に関する。
第7観点として、前記ポリアミック酸又はポリイミド100質量部に対して、前記架橋剤が20質量部以下で含まれる、第6観点に記載のディスプレイ基板用樹脂組成物に関する。
第8観点として、第1観点乃至第7観点のうちいずれか1つに記載のディスプレイ基板用樹脂組成物が少なくとも1種の溶剤に溶解していることを特徴とする、ワニスに関する。
第9観点として、0.001乃至5,000Pa・sの粘度を有する、第8観点に記載のワニスに関する。
第10観点として、第8観点又は第9観点に記載のワニスを用いて得られる薄膜を100℃乃至450℃の範囲内の適当な温度で焼成することにより得られる、硬化膜に関する。
第11観点として、1.0μm乃至200μmの膜厚を有する、第10観点に記載の硬化膜に関する。
第12観点として、自己支持性を有する、第10観点又は第11観点に記載の硬化膜に関する。
第13観点として、基板上に第10観点乃至第12観点のうちいずれか一項に記載の硬化膜からなる層を少なくとも一層備える、構造体に関する。
第14観点として、下記式(1)で表される構造単位及び式(3)で表される構造単位を含むポリアミック酸に関する。
X1は下記(5)式で表される4価の基を表し、
第15観点として、下記式(2)で表される構造単位及び式(4)で表される構造単位を含むポリイミドに関する。
X1は下記(5)式で表される4価の基を表し、
第16観点として、第14観点に記載のポリアミック酸と架橋剤とを含む組成物に関する。
第17観点として、第15観点に記載のポリイミドと架橋剤とを含む組成物に関する。
第18観点として、第16観点又は第17観点に記載の組成物が少なくとも1種の溶剤に溶解していることを特徴とする、ワニスに関する。
第19観点として、第18観点に記載のワニスを用いて得られる薄膜を100℃乃至450℃の範囲内の適当な温度で焼成することにより得られる、硬化膜に関する。 That is, as a first aspect, the present invention provides a polyamic acid containing a structural unit represented by the following formula (1) and a structural unit represented by the formula (3), or a structural unit represented by the following formula (2) and It is related with the resin composition for display substrates containing the polyimide containing the structural unit represented by Formula (4).
X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group,
Y 1 and Y 2 are different from each other and represent a divalent aromatic group or a divalent aliphatic group,
n and m represent natural numbers. ]
As a second aspect, the present invention relates to the display substrate resin composition according to the first aspect, in which X 1 represents a tetravalent group represented by the following formula (5).
As a fifth aspect, any one of the first to third aspects in which n in the formula (2) and m in the formula (4) satisfy a relational expression of n / (n + m) ≧ 0.2. It is related with the resin composition for display substrates as described in one.
As a 6th viewpoint, it is related with the resin composition for display substrates of any one of the 1st viewpoint thru | or a 5th viewpoint further including a crosslinking agent.
As a seventh aspect, the present invention relates to the display substrate resin composition according to the sixth aspect, wherein the crosslinking agent is contained in an amount of 20 parts by mass or less with respect to 100 parts by mass of the polyamic acid or polyimide.
An eighth aspect relates to a varnish, wherein the display substrate resin composition according to any one of the first to seventh aspects is dissolved in at least one solvent.
As a ninth aspect, the present invention relates to the varnish according to the eighth aspect, which has a viscosity of 0.001 to 5,000 Pa · s.
As a 10th viewpoint, it is related with the cured film obtained by baking the thin film obtained using the varnish as described in an 8th viewpoint or a 9th viewpoint at the suitable temperature within the range of 100 to 450 degreeC.
As an 11th viewpoint, it is related with the cured film as described in a 10th viewpoint which has a film thickness of 1.0 micrometer thru | or 200 micrometers.
As a 12th viewpoint, it is related with the cured film as described in the 10th viewpoint or the 11th viewpoint which has self-supporting property.
As a 13th viewpoint, it is related with a structure provided with at least one layer which consists of a cured film as described in any one of 10th viewpoint thru | or 12th viewpoint on a board | substrate.
As a 14th viewpoint, it is related with the polyamic acid containing the structural unit represented by the structural unit represented by following formula (1), and Formula (3).
X 1 represents a tetravalent group represented by the following formula (5):
As a fifteenth aspect, the present invention relates to a polyimide containing a structural unit represented by the following formula (2) and a structural unit represented by the formula (4).
X 1 represents a tetravalent group represented by the following formula (5):
As a 16th viewpoint, it is related with the composition containing the polyamic acid as described in a 14th viewpoint, and a crosslinking agent.
As a 17th viewpoint, it is related with the composition containing the polyimide and crosslinking agent as described in a 15th viewpoint.
As an eighteenth aspect, the present invention relates to a varnish characterized in that the composition according to the sixteenth aspect or the seventeenth aspect is dissolved in at least one solvent.
As a 19th viewpoint, it is related with the cured film obtained by baking the thin film obtained using the varnish as described in an 18th viewpoint at the appropriate temperature within the range of 100 to 450 degreeC.
本発明のディスプレイ基板用樹脂組成物は、フレキシブルディスプレイ基板としての要求性能、すなわち、充分な耐熱性、高い透明性、低い複屈折、そして適度な線膨張係数及び適度な柔軟性を有する有用な硬化膜を形成することができる。したがって、該硬化膜は、フレキシブルディスプレイ用ベースフィルムに等に使用することができる。
特に本発明においては、膜靭性の問題や、また加熱工程を経ることによる着色のために使用が控えられてきた脂環式テトラカルボン酸二無水物(特に1,2,3,4-シクロブタンテトラカルボン酸二無水物)を用いて高い透明性を有する硬化膜を得ることができ、特殊な材料(特殊な構造を有する酸二無水物)を用いることによるディスプレイ基板製造コストの上昇の抑制が期待できる。 The resin composition for a display substrate of the present invention is a useful curing having required performance as a flexible display substrate, that is, sufficient heat resistance, high transparency, low birefringence, and an appropriate linear expansion coefficient and an appropriate flexibility. A film can be formed. Therefore, the cured film can be used for a base film for a flexible display.
In particular, in the present invention, cycloaliphatic tetracarboxylic dianhydrides (especially 1,2,3,4-cyclobutanetetra) which have been refrained from use due to the problem of film toughness and coloring due to the heating process. A cured film having high transparency can be obtained using carboxylic acid dianhydride), and an increase in display substrate manufacturing cost can be expected by using a special material (acid dianhydride having a special structure). it can.
特に本発明においては、膜靭性の問題や、また加熱工程を経ることによる着色のために使用が控えられてきた脂環式テトラカルボン酸二無水物(特に1,2,3,4-シクロブタンテトラカルボン酸二無水物)を用いて高い透明性を有する硬化膜を得ることができ、特殊な材料(特殊な構造を有する酸二無水物)を用いることによるディスプレイ基板製造コストの上昇の抑制が期待できる。 The resin composition for a display substrate of the present invention is a useful curing having required performance as a flexible display substrate, that is, sufficient heat resistance, high transparency, low birefringence, and an appropriate linear expansion coefficient and an appropriate flexibility. A film can be formed. Therefore, the cured film can be used for a base film for a flexible display.
In particular, in the present invention, cycloaliphatic tetracarboxylic dianhydrides (especially 1,2,3,4-cyclobutanetetra) which have been refrained from use due to the problem of film toughness and coloring due to the heating process. A cured film having high transparency can be obtained using carboxylic acid dianhydride), and an increase in display substrate manufacturing cost can be expected by using a special material (acid dianhydride having a special structure). it can.
[ディスプレイ基板用樹脂組成物]
本発明は、下記式(1)で表される構造単位及び式(3)で表される構造単位を含むポリアミック酸又は下記式(2)で表される構造単位及び式(4)で表される構造単位を含むポリイミドを含有するディスプレイ基板用樹脂組成物に関する。
[式(1)乃至式(4)中、
X1は4価の芳香族基又は4価の脂肪族基を表し、
Y1及びY2は互いに異なる2価の芳香族基又は2価の脂肪族基を表し、
n及びmは自然数を表す。] [Resin composition for display substrate]
The present invention is represented by a polyamic acid containing a structural unit represented by the following formula (1) and a structural unit represented by the formula (3), or a structural unit represented by the following formula (2) and the formula (4). The present invention relates to a resin composition for a display substrate containing polyimide containing a structural unit.
[In Formula (1) thru | or Formula (4),
X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group,
Y 1 and Y 2 represent different divalent aromatic groups or divalent aliphatic groups,
n and m represent natural numbers. ]
本発明は、下記式(1)で表される構造単位及び式(3)で表される構造単位を含むポリアミック酸又は下記式(2)で表される構造単位及び式(4)で表される構造単位を含むポリイミドを含有するディスプレイ基板用樹脂組成物に関する。
X1は4価の芳香族基又は4価の脂肪族基を表し、
Y1及びY2は互いに異なる2価の芳香族基又は2価の脂肪族基を表し、
n及びmは自然数を表す。] [Resin composition for display substrate]
The present invention is represented by a polyamic acid containing a structural unit represented by the following formula (1) and a structural unit represented by the formula (3), or a structural unit represented by the following formula (2) and the formula (4). The present invention relates to a resin composition for a display substrate containing polyimide containing a structural unit.
X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group,
Y 1 and Y 2 represent different divalent aromatic groups or divalent aliphatic groups,
n and m represent natural numbers. ]
<ポリアミック酸>
本発明のディスプレイ基板用樹脂組成物に含まれる前記式(1)で表される構造単位と式(3)で表される構造単位とを有するポリアミック酸は、少なくとも1種の酸二無水物成分と少なくとも2種のジアミン成分とを溶剤中で重合させることで得られる。
すなわち前記ポリアミック酸は、公知の方法、例えば、窒素などの不活性ガス雰囲気中において、下記式(9):
(式中、X1は4価の芳香族基又は4価の脂肪族基を表す。)で表される少なくとも1種の酸二無水物と、下記式(10):
H2N-Y1-NH2 (10)
(式中、Y1は2価の芳香族基又は2価の脂肪族基を表す。)で表される少なくとも1種のジアミンと、下記式(11):
H2N-Y2-NH2 (11)
(式中、Y2は2価の芳香族基又は2価の脂肪族基を表す。)で表される少なくとも1種のジアミン(但しY1とY2は異なる2価の基である)とを溶剤に溶解し、反応させることで得られる。 <Polyamic acid>
The polyamic acid having the structural unit represented by the formula (1) and the structural unit represented by the formula (3) contained in the resin composition for display substrates of the present invention is at least one acid dianhydride component. And at least two diamine components are polymerized in a solvent.
That is, the polyamic acid is a known method, for example, in an inert gas atmosphere such as nitrogen, the following formula (9):
(Wherein X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group), and at least one acid dianhydride represented by the following formula (10):
H 2 N—Y 1 —NH 2 (10)
(Wherein Y 1 represents a divalent aromatic group or a divalent aliphatic group) and at least one diamine represented by the following formula (11):
H 2 N—Y 2 —NH 2 (11)
(Wherein Y 2 represents a divalent aromatic group or a divalent aliphatic group) and at least one diamine (wherein Y 1 and Y 2 are different divalent groups); Is dissolved in a solvent and reacted.
本発明のディスプレイ基板用樹脂組成物に含まれる前記式(1)で表される構造単位と式(3)で表される構造単位とを有するポリアミック酸は、少なくとも1種の酸二無水物成分と少なくとも2種のジアミン成分とを溶剤中で重合させることで得られる。
すなわち前記ポリアミック酸は、公知の方法、例えば、窒素などの不活性ガス雰囲気中において、下記式(9):
H2N-Y1-NH2 (10)
(式中、Y1は2価の芳香族基又は2価の脂肪族基を表す。)で表される少なくとも1種のジアミンと、下記式(11):
H2N-Y2-NH2 (11)
(式中、Y2は2価の芳香族基又は2価の脂肪族基を表す。)で表される少なくとも1種のジアミン(但しY1とY2は異なる2価の基である)とを溶剤に溶解し、反応させることで得られる。 <Polyamic acid>
The polyamic acid having the structural unit represented by the formula (1) and the structural unit represented by the formula (3) contained in the resin composition for display substrates of the present invention is at least one acid dianhydride component. And at least two diamine components are polymerized in a solvent.
That is, the polyamic acid is a known method, for example, in an inert gas atmosphere such as nitrogen, the following formula (9):
H 2 N—Y 1 —NH 2 (10)
(Wherein Y 1 represents a divalent aromatic group or a divalent aliphatic group) and at least one diamine represented by the following formula (11):
H 2 N—Y 2 —NH 2 (11)
(Wherein Y 2 represents a divalent aromatic group or a divalent aliphatic group) and at least one diamine (wherein Y 1 and Y 2 are different divalent groups); Is dissolved in a solvent and reacted.
これら酸二無水物とジアミンとの重合時の反応温度は、-20乃至100℃、好ましくは20乃至60℃である。反応時間は、1乃至72時間である。
The reaction temperature during polymerization of these acid dianhydrides and diamines is -20 to 100 ° C, preferably 20 to 60 ° C. The reaction time is 1 to 72 hours.
このような式(9)で表される酸二無水物としては、例えば、ピロメリット酸二無水物、2,3,6,7-ナフタレンテトラカルボン酸二無水物、1,2,5,6-ナフタレンテトラカルボン酸二無水物、1,4,5,8-ナフタレンテトラカルボン酸二無水物、2,3,6,7-アントラセンテトラカルボン酸二無水物、1,2,5,6-アントラセンテトラカルボン酸二無水物、3,3’,4,4’-ビフェニルテトラカルボン酸二無水物、2,2’,3,3’-ビフェニルテトラカルボン酸二無水物、2,3,3’,4’-ビフェニルテトラカルボン酸二無水物、3,3’,4,4’-ベンゾフェノンテトラカルボン酸二無水物、2,3,3’,4-ベンゾフェノンテトラカルボン酸二無水物、ビス(3,4-ジカルボキシフェニル)エーテル酸二無水物、ビス(3,4-ジカルボキシフェニル)スルホン酸二無水物、ビス(3,4-ジカルボキシフェニル)メタン酸二無水物、2,2-ビス(3,4-ジカルボキシフェニル)プロパン酸二無水物、1,1,1,3,3,3-ヘキサフルオロ-2,2-ビス(3,4-ジカルボキシフェニル)プロパン酸二無水物、ビス(3,4-ジカルボキシフェニル)ジメチルシラン酸二無水物、ビス(3,4-ジカルボキシフェニル)ジフェニルシラン酸二無水物、2,3,4,5-ピリジンテトラカルボン酸二無水物、2,6-ビス(3,4-ジカルボキシフェニル)ピリジン酸二無水物等の芳香族テトラカルボン酸二無水物;1,2,3,4-シクロブタンテトラカルボン酸二無水物、1,2-ジメチル-1,2,3,4-シクロブタンテトラカルボン酸二無水物、1,2,3,4-テトラメチル-1,2,3,4-シクロブタンテトラカルボン酸二無水物、1,2,3,4-シクロペンタンテトラカルボン酸二無水物、1,2,4,5-シクロヘキサンテトラカルボン酸二無水物、3,4-ジカルボキシ-1,2,3,4-テトラヒドロ-1-ナフタレンコハク酸、5-(2,5-ジオキソテトラヒドロフリル)-3-メチル-3-シクロヘキセン-1,2-ジカルボン酸二無水物、2,3,5-トリカルボキシ-2-シクロペンタン酢酸二無水物、ビシクロ〔2.2.2〕オクト-7-エン-2,3,5,6-テトラカルボン酸二無水物、2,3,4,5-テトラヒドロフランテトラカルボン酸二無水物、3,5,6-トリカルボキシ-2-ノルボルナン酢酸二無水物等の脂環式テトラカルボン酸二無水物;及び、1,2,3,4-ブタンテトラカルボン酸二無水物等の脂肪族テトラカルボン酸の二無水物を挙げることができる。
Examples of the acid dianhydride represented by the formula (9) include pyromellitic dianhydride, 2,3,6,7-naphthalenetetracarboxylic dianhydride, 1,2,5,6. -Naphthalene tetracarboxylic dianhydride, 1,4,5,8-naphthalene tetracarboxylic dianhydride, 2,3,6,7-anthracene tetracarboxylic dianhydride, 1,2,5,6-anthracene Tetracarboxylic dianhydride, 3,3 ′, 4,4′-biphenyltetracarboxylic dianhydride, 2,2 ′, 3,3′-biphenyltetracarboxylic dianhydride, 2,3,3 ′, 4′-biphenyltetracarboxylic dianhydride, 3,3 ′, 4,4′-benzophenone tetracarboxylic dianhydride, 2,3,3 ′, 4-benzophenone tetracarboxylic dianhydride, bis (3 4-dicarboxyphenyl Ether dianhydride, bis (3,4-dicarboxyphenyl) sulfonic dianhydride, bis (3,4-dicarboxyphenyl) methanoic dianhydride, 2,2-bis (3,4-dicarboxy) Phenyl) propanoic dianhydride, 1,1,1,3,3,3-hexafluoro-2,2-bis (3,4-dicarboxyphenyl) propanoic dianhydride, bis (3,4-di Carboxyphenyl) dimethylsilane dianhydride, bis (3,4-dicarboxyphenyl) diphenylsilane dianhydride, 2,3,4,5-pyridinetetracarboxylic dianhydride, 2,6-bis (3 , 4-Dicarboxyphenyl) pyridine acid dianhydride and other aromatic tetracarboxylic dianhydrides; 1,2,3,4-cyclobutanetetracarboxylic dianhydride, 1,2-dimethyl-1,2,3 , 4-Cyclobu Tantetracarboxylic dianhydride, 1,2,3,4-tetramethyl-1,2,3,4-cyclobutanetetracarboxylic dianhydride, 1,2,3,4-cyclopentanetetracarboxylic dianhydride 1,2,4,5-cyclohexanetetracarboxylic dianhydride, 3,4-dicarboxy-1,2,3,4-tetrahydro-1-naphthalene succinic acid, 5- (2,5-dioxo Tetrahydrofuryl) -3-methyl-3-cyclohexene-1,2-dicarboxylic acid dianhydride, 2,3,5-tricarboxy-2-cyclopentaneacetic acid dianhydride, bicyclo [2.2.2] octo- 7-ene-2,3,5,6-tetracarboxylic dianhydride, 2,3,4,5-tetrahydrofurantetracarboxylic dianhydride, 3,5,6-tricarboxy-2-norbornaneacetic acid dianhydride object Alicyclic tetracarboxylic dianhydride; and it can include dianhydrides of aliphatic tetracarboxylic acids such as 1,2,3,4-butane tetracarboxylic dianhydride.
前記式(9)で表される酸二無水物においても、本発明の樹脂組成物から得られる硬化膜が十分に低い線膨張係数及び十分に高い強度を有するものとする観点から、X1が下記式(5)で表される構造である酸二無水物、すなわち、1,2,3,4-シクロブタンテトラカルボン酸二無水物(下記式(12))が好ましい。
Also in the acid dianhydride represented by the formula (9), from the viewpoint that the cured film obtained from the resin composition of the present invention has a sufficiently low linear expansion coefficient and a sufficiently high strength, X 1 is An acid dianhydride having a structure represented by the following formula (5), that is, 1,2,3,4-cyclobutanetetracarboxylic dianhydride (the following formula (12)) is preferable.
前記式(10)又は前記式(11)で表される芳香族ジアミンとしては、例えば、p-フェニレンジアミン、o-フェニレンジアミン、2-メチル-1,4-フェニレンジアミン、2-トリフルオロメチル-1,4-フェニレンジアミン、2-メトキシ-1,4-フェニレンジアミン、2,5-ジメチル-1,4-フェニレンジアミン、2,5-ビス(トリフルオロメチル)-1,4-フェニレンジアミン、4,4’-ジアミノベンズアニリド、4-アミノフェニル-4’-アミノベンゾエート、ベンジジン、3,3’-ジメトキシベンジジン、3,3’-ジクロロベンジジン、o-トリジン、m-トリジン、2,2’-ビス(トリフルオロメチル)ベンジジン、3,3’-ビス(トリフルオロメチル)ベンジジン、オクタフルオロベンジジン、3,3’,5,5’-テトラメチルベンジジン、2,2’,5,5’-テトラクロロベンジジン、ビス[4-(4-アミノフェノキシ)フェニル]スルホン、ビス[4-(3-アミノフェノキシ)フェニル]スルホン、ビス(3-アミノフェニル)スルホン、ビス(4-アミノフェニル)スルホン等が挙げられる。これらの中でも、本発明の樹脂組成物から得られる硬化膜が十分に高い透明性及び十分に高い強度を有するものとする観点から、p-フェニレンジアミン及びビス[4-(3-アミノフェノキシ)フェニル]スルホン、ビス(3-アミノフェニル)スルホン等が好ましい。
Examples of the aromatic diamine represented by the formula (10) or the formula (11) include p-phenylenediamine, o-phenylenediamine, 2-methyl-1,4-phenylenediamine, 2-trifluoromethyl- 1,4-phenylenediamine, 2-methoxy-1,4-phenylenediamine, 2,5-dimethyl-1,4-phenylenediamine, 2,5-bis (trifluoromethyl) -1,4-phenylenediamine, 4 , 4'-diaminobenzanilide, 4-aminophenyl-4'-aminobenzoate, benzidine, 3,3'-dimethoxybenzidine, 3,3'-dichlorobenzidine, o-tolidine, m-tolidine, 2,2'- Bis (trifluoromethyl) benzidine, 3,3'-bis (trifluoromethyl) benzidine, octafluorobenzidine 3,3 ′, 5,5′-tetramethylbenzidine, 2,2 ′, 5,5′-tetrachlorobenzidine, bis [4- (4-aminophenoxy) phenyl] sulfone, bis [4- (3 -Aminophenoxy) phenyl] sulfone, bis (3-aminophenyl) sulfone, bis (4-aminophenyl) sulfone, and the like. Among these, from the viewpoint that the cured film obtained from the resin composition of the present invention has sufficiently high transparency and sufficiently high strength, p-phenylenediamine and bis [4- (3-aminophenoxy) phenyl Sulfone, bis (3-aminophenyl) sulfone and the like are preferable.
前記式(10)又は前記式(11)で表わされる脂肪族ジアミンとしては、例えば、4,4’-メチレンビス(シクロヘキシルアミン)、4,4’-メチレンビス(3-メチルシクロヘキシルアミン)、イソホロンジアミン、トランス-1,4-シクロヘキサンジアミン、シス-1,4-シクロヘキサンジアミン、1,4-シクロヘキサンビス(メチルアミン)、2,5-ビス(アミノメチル)ビシクロ〔2.2.1〕ヘプタン、2,6-ビス(アミノメチル)ビシクロ〔2.2.1〕ヘプタン、3,8-ビス(アミノメチル)トリシクロ〔5.2.1.0〕デカン、1,3-ジアミノアダマンタン、2,2-ビス(4-アミノシクロヘキシル)プロパン、2,2-ビス(4-アミノシクロヘキシル)ヘキサフルオロプロパン、1,3-プロパンジアミン、1,4-テトラメチレンジアミン、1,5-ペンタメチレンジアミン、1,6-ヘキサメチレンジアミン、1,7-ヘプタメチレンジアミン、1,8-オクタメチレンジアミン、1,9-ノナメチレンジアミン等が挙げられる。前記脂肪族ジアミンの中でも、本発明の樹脂組成物から得られる硬化膜が十分に低い線膨張係数を有するものとする観点から、剛直で直線的な分子構造を有するジアミンを使用することが好ましく、例えばトランス-1,4-シクロヘキサンジアミンが好適に用いられる。
Examples of the aliphatic diamine represented by the formula (10) or the formula (11) include 4,4′-methylenebis (cyclohexylamine), 4,4′-methylenebis (3-methylcyclohexylamine), isophoronediamine, Trans-1,4-cyclohexanediamine, cis-1,4-cyclohexanediamine, 1,4-cyclohexanebis (methylamine), 2,5-bis (aminomethyl) bicyclo [2.2.1] heptane, 2, 6-bis (aminomethyl) bicyclo [2.2.1] heptane, 3,8-bis (aminomethyl) tricyclo [5.2.1.0] decane, 1,3-diaminoadamantane, 2,2-bis (4-aminocyclohexyl) propane, 2,2-bis (4-aminocyclohexyl) hexafluoropropane, 1,3-propyl Pandiamine, 1,4-tetramethylenediamine, 1,5-pentamethylenediamine, 1,6-hexamethylenediamine, 1,7-heptamethylenediamine, 1,8-octamethylenediamine, 1,9-nonamethylenediamine Etc. Among the aliphatic diamines, from the viewpoint that the cured film obtained from the resin composition of the present invention has a sufficiently low linear expansion coefficient, it is preferable to use a diamine having a rigid and linear molecular structure, For example, trans-1,4-cyclohexanediamine is preferably used.
特に前記式(10)及び前記式(11)で表わされるジアミン(これらは互いに異なるジアミンである。)において、一方は、本発明の樹脂組成物から得られる硬化膜が十分に高い透明性を有するものとする観点から、電子吸引基を有するジアミンを使用することが好ましく、また他方は、得られる硬化膜が高い透明性及び十分に低い線膨張係数を有するものとする観点から、電子吸引基を有し且つ剛直で直線的な分子構造を有するジアミンを使用することが好ましい。
このようなジアミンとして、前記式(10)及び前記式(11)で表されるジアミンは、Y1並びにY2がスルホン酸基を有する芳香族基であるジアミンが好ましい。
例えば式(10)で表されるジアミンとしては、Y1が下記式(6)で表される2価の基を有するジアミン(すなわちビス[4-(3-アミノフェノキシ)フェニル]スルホン)が特に好ましい。
また、前記式(11)で表されるジアミンとしては、Y2が下記式(7)で表される2価の基を有するジアミン(すなわちビス(3-アミノフェニル)スルホン)が特に好ましい。
In particular, in the diamines represented by the formula (10) and the formula (11) (these are diamines different from each other), one of the cured films obtained from the resin composition of the present invention has sufficiently high transparency. From the viewpoint of providing, it is preferable to use a diamine having an electron withdrawing group. On the other hand, from the viewpoint of obtaining a cured film having high transparency and a sufficiently low linear expansion coefficient, an electron withdrawing group is used. It is preferable to use a diamine having a rigid and linear molecular structure.
As such a diamine, the diamine represented by the formula (10) and the formula (11) is preferably a diamine in which Y 1 and Y 2 are aromatic groups having a sulfonic acid group.
For example, as the diamine represented by the formula (10), a diamine having a divalent group represented by Y 1 represented by the following formula (6) (that is, bis [4- (3-aminophenoxy) phenyl] sulfone) is particularly preferred. preferable.
As the diamines represented by the formula (11), a diamine having a divalent group Y 2 is expressed by the following formula (7) (i.e. bis (3-aminophenyl) sulfone) are particularly preferred.
このようなジアミンとして、前記式(10)及び前記式(11)で表されるジアミンは、Y1並びにY2がスルホン酸基を有する芳香族基であるジアミンが好ましい。
例えば式(10)で表されるジアミンとしては、Y1が下記式(6)で表される2価の基を有するジアミン(すなわちビス[4-(3-アミノフェノキシ)フェニル]スルホン)が特に好ましい。
また、前記式(11)で表されるジアミンとしては、Y2が下記式(7)で表される2価の基を有するジアミン(すなわちビス(3-アミノフェニル)スルホン)が特に好ましい。
As such a diamine, the diamine represented by the formula (10) and the formula (11) is preferably a diamine in which Y 1 and Y 2 are aromatic groups having a sulfonic acid group.
For example, as the diamine represented by the formula (10), a diamine having a divalent group represented by Y 1 represented by the following formula (6) (that is, bis [4- (3-aminophenoxy) phenyl] sulfone) is particularly preferred. preferable.
As the diamines represented by the formula (11), a diamine having a divalent group Y 2 is expressed by the following formula (7) (i.e. bis (3-aminophenyl) sulfone) are particularly preferred.
ポリアミック酸の重合反応に使用される溶剤としては特に限定されないが、例えば、N,N-ジメチルアセトアミド、N,N-ジエチルアセトアミド、N,N-ジメチルホルムアミド、N-メチル-2-ピロリドン、3-メトキシ-N,N-ジメチルプロピルアミド、3-エトキシ-N,N-ジメチルプロピルアミド、3-プロポキシ-N,N-ジメチルプロピルアミド、3-イソプロポキシ-N,N-ジメチルプロピルアミド、3-ブトキシ-N,N-ジメチルプロピルアミド、3-sec-ブトキシ-N,N-ジメチルプロピルアミド、3-tert-ブトキシ-N,N-ジメチルプロピルアミド、ヘキサメチルホスホルアミド、ジメチルスルホキシド、γ-ブチロラクトン、1,3-ジメチル-2-イミダゾリジノン、1,2-ジメトキシエタン-ビス(2-メトキシエチル)エーテル、テトラヒドロフラン、1,4-ジオキサン、ピコリン、ピリジン、アセトン、クロロホルム、トルエン、キシレン等の非プロトン性溶剤、及びフェノール、o-クレゾール、m-クレゾール、p-クレゾール、o-クロロフェノール、m-クロロフェノール、p-クロロフェノール等のプロトン性溶剤等が挙げられる。これらの溶剤は単独で又は2種類以上を組み合わせて使用してもよい。
The solvent used in the polymerization reaction of the polyamic acid is not particularly limited. For example, N, N-dimethylacetamide, N, N-diethylacetamide, N, N-dimethylformamide, N-methyl-2-pyrrolidone, 3- Methoxy-N, N-dimethylpropylamide, 3-ethoxy-N, N-dimethylpropylamide, 3-propoxy-N, N-dimethylpropylamide, 3-isopropoxy-N, N-dimethylpropylamide, 3-butoxy -N, N-dimethylpropylamide, 3-sec-butoxy-N, N-dimethylpropylamide, 3-tert-butoxy-N, N-dimethylpropylamide, hexamethylphosphoramide, dimethylsulfoxide, γ-butyrolactone, 1,3-dimethyl-2-imidazolidinone, 1,2-dimethyl Aprotic solvents such as xylene-bis (2-methoxyethyl) ether, tetrahydrofuran, 1,4-dioxane, picoline, pyridine, acetone, chloroform, toluene, xylene, and phenol, o-cresol, m-cresol, p- Examples thereof include protic solvents such as cresol, o-chlorophenol, m-chlorophenol, and p-chlorophenol. These solvents may be used alone or in combination of two or more.
上記ポリアミック酸の重合反応において、ジアミン成分の割合は、上記式(10)で表されるジアミン(すなわち上記式(6)で表される2価の基を有するジアミン)と上記式(11)で表されるジアミン(すなわち上記式(7)で表される2価の基を有するジアミン)とのモル比が、10/90乃至99/1であることが好ましく、本発明の樹脂組成物から得られる硬化膜が十分に低い線膨張係数及び十分に高い強度を有するものとする観点から、20/80乃至80/20がより好ましい。
In the polymerization reaction of the polyamic acid, the ratio of the diamine component is the diamine represented by the above formula (10) (that is, the diamine having a divalent group represented by the above formula (6)) and the above formula (11). The molar ratio with the diamine represented (that is, the diamine having a divalent group represented by the above formula (7)) is preferably 10/90 to 99/1, and obtained from the resin composition of the present invention. From the viewpoint that the cured film to be obtained has a sufficiently low linear expansion coefficient and a sufficiently high strength, 20/80 to 80/20 is more preferable.
また、上記反応において、酸二無水物成分とジアミン成分との割合は、モル比で酸二無水物成分/ジアミン成分=0.8乃至1.2であることが好ましい。通常の重縮合反応と同様に、このモル比が1に近いほど生成する重合体の重合度は大きくなる。重合度が小さすぎると、後に形成するポリイミド硬化膜の強度が不十分となり、また重合度が大きすぎるとポリイミド硬化膜形成時の作業性が悪くなる場合がある。
In the above reaction, the ratio of the acid dianhydride component to the diamine component is preferably acid dianhydride component / diamine component = 0.8 to 1.2 in terms of molar ratio. Similar to the normal polycondensation reaction, the closer the molar ratio is to 1, the higher the degree of polymerization of the polymer produced. If the degree of polymerization is too small, the strength of the polyimide cured film to be formed later will be insufficient, and if the degree of polymerization is too large, the workability at the time of forming the polyimide cured film may be deteriorated.
生成されるポリアミック酸の重量平均分子量は、ポリアミック酸を含むディスプレイ基板用樹脂組成物から得られる硬化膜の強度を維持するために、ポリスチレン換算にて3,000乃至300,000が好ましい。重量平均分子量が3,000未満では、できあがったフィルムが脆くなる可能性があり、一方、重量平均分子量が300,000を超えるとポリアミック酸のワニスの粘度が高くなり過ぎる可能性があり、その結果、取扱いが難しくなるからである。
なお、斯かる数値範囲を満たすために、上記式(1)及び式(3)中のnとmとは、n+mで通常、6乃至180であり、好ましくは10乃至100であり、より好ましくは10乃至50である。 The weight average molecular weight of the produced polyamic acid is preferably 3,000 to 300,000 in terms of polystyrene in order to maintain the strength of the cured film obtained from the resin composition for display substrate containing polyamic acid. If the weight average molecular weight is less than 3,000, the resulting film may become brittle. On the other hand, if the weight average molecular weight exceeds 300,000, the viscosity of the polyamic acid varnish may be too high. This is because handling becomes difficult.
In order to satisfy such a numerical range, n and m in the above formulas (1) and (3) are n + m, usually 6 to 180, preferably 10 to 100, more preferably 10 to 50.
なお、斯かる数値範囲を満たすために、上記式(1)及び式(3)中のnとmとは、n+mで通常、6乃至180であり、好ましくは10乃至100であり、より好ましくは10乃至50である。 The weight average molecular weight of the produced polyamic acid is preferably 3,000 to 300,000 in terms of polystyrene in order to maintain the strength of the cured film obtained from the resin composition for display substrate containing polyamic acid. If the weight average molecular weight is less than 3,000, the resulting film may become brittle. On the other hand, if the weight average molecular weight exceeds 300,000, the viscosity of the polyamic acid varnish may be too high. This is because handling becomes difficult.
In order to satisfy such a numerical range, n and m in the above formulas (1) and (3) are n + m, usually 6 to 180, preferably 10 to 100, more preferably 10 to 50.
上記のようにして、得られた上記式(1)で表される構造単位及び式(3)で表される構造単位からなるポリアミック酸における構造単位の繰り返し数は、上記式(1)中のnと上記式(3)中のmとが、n/(n+m)≧0.2(nとmの合計数に対してnの割合が20%以上)の関係であることが好ましい。
As described above, the number of repeating structural units in the polyamic acid composed of the structural unit represented by the above formula (1) and the structural unit represented by the formula (3) is represented by the formula (1). It is preferable that n and m in the above formula (3) have a relationship of n / (n + m) ≧ 0.2 (the ratio of n is 20% or more with respect to the total number of n and m).
本発明では、ポリアミック酸の反応溶液をそのまま、又は、希釈して、後述のポリイミドを得るためのイミド化反応に使用することができ、またこれを本発明の樹脂組成物とすることができる。或いは反応溶液から沈殿回収したポリアミック酸を適当な溶剤に再溶解させて、イミド化反応に使用することができ、またこれを本発明の樹脂組成物とすることができる。希釈及び再溶解に用いる溶剤は、得られたポリアミック酸を溶解させるものであれば特に限定されないが、例えば、m-クレゾール、2-ピロリドン、N-メチル-2-ピロリドン、N-エチル-2-ピロリドン、N-ビニル-2-ピロリドン、N,N-ジメチルアセトアミド、N,N-ジメチルホルムアミド、3-メトキシ-N,N-ジメチルプロピルアミド、3-エトキシ-N,N-ジメチルプロピルアミド、3-プロポキシ-N,N-ジメチルプロピルアミド、3-イソプロポキシ-N,N-ジメチルプロピルアミド、3-ブトキシ-N,N-ジメチルプロピルアミド、3-sec-ブトキシ-N,N-ジメチルプロピルアミド、3-tert-ブトキシ-N,N-ジメチルプロピルアミド、γ-ブチロラクトン等を挙げることができる。これらの溶剤は、単独で又は2種以上を組み合わせて使用してもよい。
In the present invention, the reaction solution of polyamic acid can be used as it is or diluted to be used in an imidation reaction for obtaining a polyimide described later, and this can be used as the resin composition of the present invention. Alternatively, the polyamic acid precipitated and recovered from the reaction solution can be redissolved in an appropriate solvent and used for the imidization reaction, and this can be used as the resin composition of the present invention. The solvent used for dilution and re-dissolution is not particularly limited as long as it can dissolve the obtained polyamic acid. For example, m-cresol, 2-pyrrolidone, N-methyl-2-pyrrolidone, N-ethyl-2- Pyrrolidone, N-vinyl-2-pyrrolidone, N, N-dimethylacetamide, N, N-dimethylformamide, 3-methoxy-N, N-dimethylpropylamide, 3-ethoxy-N, N-dimethylpropylamide, 3- Propoxy-N, N-dimethylpropylamide, 3-isopropoxy-N, N-dimethylpropylamide, 3-butoxy-N, N-dimethylpropylamide, 3-sec-butoxy-N, N-dimethylpropylamide, 3 -Tert-butoxy-N, N-dimethylpropylamide, γ-butyrolactone, etc. . These solvents may be used alone or in combination of two or more.
<ポリイミド>
本発明のディスプレイ基板用樹脂組成物に含まれるポリイミドは、上述のように合成したポリアミック酸を、加熱により脱水閉環(熱イミド化)して得ることができる。なお、この際、ポリアミック酸を溶剤中でイミドに転化させ、溶剤可溶性のポリイミドとして用いることも可能である。また、公知の脱水閉環触媒を使用して化学的に閉環する方法も採用することができる。加熱による方法は、100乃至500℃、好ましくは120乃至450℃の任意の温度で行うことができる。化学的に閉環する方法は、例えば、ピリジンやトリエチルアミンなどと、無水酢酸などとの存在下で行うことができ、この際の温度は、-20乃至200℃の任意の温度を選択することができる。 <Polyimide>
The polyimide contained in the resin composition for display substrates of the present invention can be obtained by subjecting the polyamic acid synthesized as described above to dehydration ring closure (thermal imidization) by heating. At this time, polyamic acid can be converted to imide in a solvent and used as a solvent-soluble polyimide. Moreover, the method of chemically ring-closing using a well-known dehydration ring-closing catalyst is also employable. The method by heating can be performed at an arbitrary temperature of 100 to 500 ° C., preferably 120 to 450 ° C. The method of chemically cyclizing can be performed, for example, in the presence of pyridine, triethylamine or the like and acetic anhydride, and the temperature at this time can be selected from -20 to 200 ° C. .
本発明のディスプレイ基板用樹脂組成物に含まれるポリイミドは、上述のように合成したポリアミック酸を、加熱により脱水閉環(熱イミド化)して得ることができる。なお、この際、ポリアミック酸を溶剤中でイミドに転化させ、溶剤可溶性のポリイミドとして用いることも可能である。また、公知の脱水閉環触媒を使用して化学的に閉環する方法も採用することができる。加熱による方法は、100乃至500℃、好ましくは120乃至450℃の任意の温度で行うことができる。化学的に閉環する方法は、例えば、ピリジンやトリエチルアミンなどと、無水酢酸などとの存在下で行うことができ、この際の温度は、-20乃至200℃の任意の温度を選択することができる。 <Polyimide>
The polyimide contained in the resin composition for display substrates of the present invention can be obtained by subjecting the polyamic acid synthesized as described above to dehydration ring closure (thermal imidization) by heating. At this time, polyamic acid can be converted to imide in a solvent and used as a solvent-soluble polyimide. Moreover, the method of chemically ring-closing using a well-known dehydration ring-closing catalyst is also employable. The method by heating can be performed at an arbitrary temperature of 100 to 500 ° C., preferably 120 to 450 ° C. The method of chemically cyclizing can be performed, for example, in the presence of pyridine, triethylamine or the like and acetic anhydride, and the temperature at this time can be selected from -20 to 200 ° C. .
生成されるポリイミドの重量平均分子量は、ポリイミドを含むディスプレイ基板用樹脂組成物から得られる硬化膜の強度を維持するために、ポリスチレン換算にて3,000乃至300,000が好ましい。重量平均分子量が3,000未満では、できあがったフィルムが脆くなる可能性があり、一方、重量平均分子量が300,000を超えるとポリイミドのワニスの粘度が高くなり過ぎる可能性があり、その結果、取扱いが難しくなるからである。
なお、斯かる数値範囲を満たすために、上記式(2)及び式(4)中のnとmとは、n+mで通常6乃至180であり、好ましくは10乃至100であり、より好ましくは10乃至50である。 The weight average molecular weight of the polyimide produced is preferably 3,000 to 300,000 in terms of polystyrene in order to maintain the strength of the cured film obtained from the resin composition for display substrate containing polyimide. If the weight average molecular weight is less than 3,000, the resulting film may be brittle, while if the weight average molecular weight exceeds 300,000, the viscosity of the polyimide varnish may be too high, and as a result, This is because handling becomes difficult.
In order to satisfy such a numerical range, n and m in the above formulas (2) and (4) are usually n + m and are usually 6 to 180, preferably 10 to 100, more preferably 10 Thirty to fifty.
なお、斯かる数値範囲を満たすために、上記式(2)及び式(4)中のnとmとは、n+mで通常6乃至180であり、好ましくは10乃至100であり、より好ましくは10乃至50である。 The weight average molecular weight of the polyimide produced is preferably 3,000 to 300,000 in terms of polystyrene in order to maintain the strength of the cured film obtained from the resin composition for display substrate containing polyimide. If the weight average molecular weight is less than 3,000, the resulting film may be brittle, while if the weight average molecular weight exceeds 300,000, the viscosity of the polyimide varnish may be too high, and as a result, This is because handling becomes difficult.
In order to satisfy such a numerical range, n and m in the above formulas (2) and (4) are usually n + m and are usually 6 to 180, preferably 10 to 100, more preferably 10 Thirty to fifty.
上記のようにして、得られた上記式(2)で表される構造単位及び式(4)で表される構造単位からなるポリイミドにおける構造単位の繰り返し数は、上記式(2)中のnと上記式(4)中のmとが、n/(n+m)≧0.2(nとmの合計数に対してnの割合が20%以上)の関係であることが好ましい。
As described above, the number of repeating structural units in the polyimide composed of the structural unit represented by the above formula (2) and the structural unit represented by the formula (4) is n in the above formula (2). And m in the above formula (4) preferably have a relationship of n / (n + m) ≧ 0.2 (the ratio of n is 20% or more with respect to the total number of n and m).
本発明では、得られたポリイミドを反応溶液ごとそのまま、又は、希釈して樹脂組成物として使用することができ、或いは反応溶液にメタノール、エタノールなどの貧溶媒を加えて沈殿回収したポリイミドを適当な溶剤に再溶解させてから、樹脂組成物として使用することができる。希釈及び再溶解に用いる溶剤は、得られたポリイミドを溶解させるものであれば特に限定されないが、例えば、m-クレゾール、2-ピロリドン、N-メチル-2-ピロリドン、N-エチル-2-ピロリドン、N-ビニル-2-ピロリドン、N,N-ジメチルアセトアミド、N,N-ジメチルホルムアミド、3-メトキシ-N,N-ジメチルプロピルアミド、3-エトキシ-N,N-ジメチルプロピルアミド、3-プロポキシ-N,N-ジメチルプロピルアミド、3-イソプロポキシ-N,N-ジメチルプロピルアミド、3-ブトキシ-N,N-ジメチルプロピルアミド、3-sec-ブトキシ-N,N-ジメチルプロピルアミド、3-tert-ブトキシ-N,N-ジメチルプロピルアミド、γ-ブチロラクトン、などが挙げられる。これらの溶剤は、単独で又は2種以上を組み合わせて使用してもよい。
In the present invention, the obtained polyimide can be used as a resin composition as it is or diluted with the reaction solution, or a polyimide recovered by precipitation by adding a poor solvent such as methanol or ethanol to the reaction solution is appropriately used. After re-dissolving in a solvent, it can be used as a resin composition. The solvent used for dilution and re-dissolution is not particularly limited as long as it can dissolve the obtained polyimide. For example, m-cresol, 2-pyrrolidone, N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone N-vinyl-2-pyrrolidone, N, N-dimethylacetamide, N, N-dimethylformamide, 3-methoxy-N, N-dimethylpropylamide, 3-ethoxy-N, N-dimethylpropylamide, 3-propoxy -N, N-dimethylpropylamide, 3-isopropoxy-N, N-dimethylpropylamide, 3-butoxy-N, N-dimethylpropylamide, 3-sec-butoxy-N, N-dimethylpropylamide, 3- and tert-butoxy-N, N-dimethylpropylamide, γ-butyrolactone, and the like. These solvents may be used alone or in combination of two or more.
<樹脂組成物>
本発明のディスプレイ基板用樹脂組成物における固形分の割合は、1乃至100質量%、又は5乃至100質量%、又は50乃至100質量%、又は80乃至100質量%である。
ここで、固形分とはディスプレイ基板用樹脂組成物の前記ポリアミック酸、前記ポリイミドを始めとする全ての成分から後述する溶剤を除去した残りの成分である。
本発明のディスプレイ基板用樹脂組成物における上記ポリアミック酸又はポリイミドの含有量は、該樹脂組成物の固形分の含有量に基づいて、8乃至100質量%、好ましくは40乃至100質量%、更に好ましくは70乃至100質量%である。 <Resin composition>
The ratio of the solid content in the resin composition for display substrates of the present invention is 1 to 100% by mass, or 5 to 100% by mass, or 50 to 100% by mass, or 80 to 100% by mass.
Here, solid content is the remaining component which removed the solvent mentioned later from all the components including the said polyamic acid of the resin composition for display substrates, and the said polyimide.
The content of the polyamic acid or polyimide in the resin composition for display substrates of the present invention is 8 to 100% by mass, preferably 40 to 100% by mass, more preferably based on the solid content of the resin composition. Is 70 to 100% by mass.
本発明のディスプレイ基板用樹脂組成物における固形分の割合は、1乃至100質量%、又は5乃至100質量%、又は50乃至100質量%、又は80乃至100質量%である。
ここで、固形分とはディスプレイ基板用樹脂組成物の前記ポリアミック酸、前記ポリイミドを始めとする全ての成分から後述する溶剤を除去した残りの成分である。
本発明のディスプレイ基板用樹脂組成物における上記ポリアミック酸又はポリイミドの含有量は、該樹脂組成物の固形分の含有量に基づいて、8乃至100質量%、好ましくは40乃至100質量%、更に好ましくは70乃至100質量%である。 <Resin composition>
The ratio of the solid content in the resin composition for display substrates of the present invention is 1 to 100% by mass, or 5 to 100% by mass, or 50 to 100% by mass, or 80 to 100% by mass.
Here, solid content is the remaining component which removed the solvent mentioned later from all the components including the said polyamic acid of the resin composition for display substrates, and the said polyimide.
The content of the polyamic acid or polyimide in the resin composition for display substrates of the present invention is 8 to 100% by mass, preferably 40 to 100% by mass, more preferably based on the solid content of the resin composition. Is 70 to 100% by mass.
<その他任意の成分:溶剤>
本発明のディスプレイ基板用樹脂組成物は、溶剤を含むことができる。そのような溶剤としては、例えば、ポリアミック酸やポリイミドの再溶解に使用可能な溶剤として挙げた溶剤(段落[0023]及び[0027]に記載された溶剤)等が挙げられる。 <Other optional components: Solvent>
The resin composition for display substrates of the present invention can contain a solvent. Examples of such a solvent include the solvents listed as solvents that can be used for redissolving polyamic acid and polyimide (solvents described in paragraphs [0023] and [0027]).
本発明のディスプレイ基板用樹脂組成物は、溶剤を含むことができる。そのような溶剤としては、例えば、ポリアミック酸やポリイミドの再溶解に使用可能な溶剤として挙げた溶剤(段落[0023]及び[0027]に記載された溶剤)等が挙げられる。 <Other optional components: Solvent>
The resin composition for display substrates of the present invention can contain a solvent. Examples of such a solvent include the solvents listed as solvents that can be used for redissolving polyamic acid and polyimide (solvents described in paragraphs [0023] and [0027]).
<その他任意の成分:架橋剤>
本発明のディスプレイ基板用樹脂組成物は、架橋剤(以下、架橋性化合物ともいう。)を含むことができる。 <Other optional components: cross-linking agent>
The resin composition for display substrates of the present invention can contain a crosslinking agent (hereinafter also referred to as a crosslinkable compound).
本発明のディスプレイ基板用樹脂組成物は、架橋剤(以下、架橋性化合物ともいう。)を含むことができる。 <Other optional components: cross-linking agent>
The resin composition for display substrates of the present invention can contain a crosslinking agent (hereinafter also referred to as a crosslinkable compound).
架橋性化合物は、そのディスプレイ基板用樹脂組成物を用いて得られる塗膜(薄膜)を、硬化膜に転換する工程で、ポリアミック酸、またはポリイミドの少なくとも一方に含有される有機基と反応し得る基を有する化合物であれば特に限定されない。そのような化合物としては、例えば、エポキシ基を2個以上含有する化合物や、アミノ基の水素原子がメチロール基、アルコキシメチル基又はその両方で置換された基を有するメラミン誘導体、ベンゾグアナミン誘導体又はグリコールウリル等が挙げられる。このメラミン誘導体及びベンゾグアナミン誘導体は、二量体又は三量体であっても良く、又、単量体、二量体及び三量体から任意に選ばれる混合物であっても良い。これらのメラミン誘導体及びベンゾグアナミン誘導体は、トリアジン環1個当たり、メチロール基又はアルコキシメチル基を平均3個以上6個未満有するものが好ましい。
また、本発明に用いられる架橋剤は、単独で又は2種以上を組み合わせて使用してもよい。 The crosslinkable compound can react with an organic group contained in at least one of polyamic acid and polyimide in the step of converting the coating film (thin film) obtained using the resin composition for display substrates into a cured film. If it is a compound which has group, it will not specifically limit. Examples of such a compound include a compound containing two or more epoxy groups, a melamine derivative having a hydrogen atom of an amino group substituted by a methylol group, an alkoxymethyl group, or both, a benzoguanamine derivative, or glycoluril. Etc. The melamine derivative and benzoguanamine derivative may be a dimer or a trimer, or may be a mixture arbitrarily selected from a monomer, a dimer and a trimer. These melamine derivatives and benzoguanamine derivatives preferably have an average of 3 or more and less than 6 methylol groups or alkoxymethyl groups per one triazine ring.
Moreover, you may use the crosslinking agent used for this invention individually or in combination of 2 or more types.
また、本発明に用いられる架橋剤は、単独で又は2種以上を組み合わせて使用してもよい。 The crosslinkable compound can react with an organic group contained in at least one of polyamic acid and polyimide in the step of converting the coating film (thin film) obtained using the resin composition for display substrates into a cured film. If it is a compound which has group, it will not specifically limit. Examples of such a compound include a compound containing two or more epoxy groups, a melamine derivative having a hydrogen atom of an amino group substituted by a methylol group, an alkoxymethyl group, or both, a benzoguanamine derivative, or glycoluril. Etc. The melamine derivative and benzoguanamine derivative may be a dimer or a trimer, or may be a mixture arbitrarily selected from a monomer, a dimer and a trimer. These melamine derivatives and benzoguanamine derivatives preferably have an average of 3 or more and less than 6 methylol groups or alkoxymethyl groups per one triazine ring.
Moreover, you may use the crosslinking agent used for this invention individually or in combination of 2 or more types.
以下に、架橋性化合物の具体例を挙げるが、これに限定されない。
エポキシ基を2個以上含有する化合物としては、エポリードGT-401、エポリードGT-403、エポリードGT-301、エポリードGT-302、セロキサイド2021、セロキサイド3000(以上、(株)ダイセル製)等のシクロヘキセン構造を有するエポキシ化合物;エピコート1001、エピコート1002、エピコート1003、エピコート1004、エピコート1007、エピコート1009、エピコート1010、エピコート828(以上、ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ)製)等のビスフェノールA型エポキシ化合物;エピコート807(ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ))製)等のビスフェノールF型エポキシ化合物;エピコート152、エピコート154(以上、ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ)製)、EPPN201、EPPN202(以上、日本化薬(株)製)等のフェノールノボラック型エポキシ化合物;ECON-102、ECON-103S、ECON-104S、ECON-1020、ECON-1025、ECON-1027(以上、日本化薬(株)製)、エピコート180S75(ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ)製)等のクレゾールノボラック型エポキシ化合物;V8000-C7(DIC(株)製)等のナフタレン型エポキシ化合物;デナコールEX-252(ナガセケムテックス(株)製)、CY175、CY177、CY179、アラルダイトCY-182、アラルダイトCY-192、アラルダイトCY-184(以上、BASF社製)、エピクロン200、エピクロン400(以上、DIC(株)製)、エピコート871、エピコート872(以上、ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ)製)、ED-5661、ED-5662(以上、セラニーズコーティング(株)製)等の脂環式エポキシ化合物;デナコールEX-611、デナコールEX-612、デナコールEX-614、デナコールEX-622、デナコールEX-411、デナコールEX-512、デナコールEX-522、デナコールEX-421、デナコールEX-313、デナコールEX-314、デナコールEX-312(以上、ナガセケムテックス(株)製)等の脂肪族ポリグリシジルエーテル化合物が挙げられる。 Although the specific example of a crosslinkable compound is given to the following, it is not limited to this.
Examples of the compound containing two or more epoxy groups include cyclohexene structures such as epolide GT-401, epolide GT-403, epolide GT-301, epolide GT-302, ceroxide 2021, and ceroxide 3000 (manufactured by Daicel Corporation). Epicoat 1001, Epicoat 1002, Epicoat 1003, Epicoat 1004, Epicoat 1007, Epicoat 1009, Epicoat 1010, Epicoat 828 (above, Japan Epoxy Resin Co., Ltd. (currently Mitsubishi Chemical Corporation, presently jER) Bisphenol A type epoxy compounds such as Epicote 807 (manufactured by Japan Epoxy Resin Co., Ltd. (currently Mitsubishi Chemical Corporation, presently jER (registered trademark) series))) Poxy compounds; Epicoat 152, Epicoat 154 (above, Japan Epoxy Resin Co., Ltd. (currently Mitsubishi Chemical Corporation, presently jER (registered trademark) series)), EPPN201, EPPN202 (above, Nippon Kayaku Co., Ltd.) Phenol novolac type epoxy compounds such as ECON-102, ECON-103S, ECON-104S, ECON-1020, ECON-1025, ECON-1027 (above, manufactured by Nippon Kayaku Co., Ltd.), Epicort 180S75 (Japan Epoxy Resin ( Cresol novolac type epoxy compounds such as (manufactured by Mitsubishi Chemical Corporation, currently jER (registered trademark) series); naphthalene type epoxy compounds such as V8000-C7 (manufactured by DIC Corporation); Denacol EX-252 ( Nagase ChemteX Corporation), CY175, C Y177, CY179, Araldite CY-182, Araldite CY-192, Araldite CY-184 (above, manufactured by BASF), Epicron 200, Epicron 400 (above, manufactured by DIC Corporation), Epicoat 871, Epicoat 872 (above, Japan) Cycloaliphatic epoxy compounds such as Epoxy Resin Co., Ltd. (currently Mitsubishi Chemical Co., Ltd., present jER (registered trademark) series), ED-5661, ED-5661 (above, Celanese Coating Co., Ltd.); Denacol EX-611, Denacol EX-612, Denacol EX-614, Denacol EX-622, Denacol EX-411, Denacol EX-512, Denacol EX-522, Denacol EX-421, Denacol EX-313, Denacol EX-314, Denacor EX-31 (Above, manufactured by Nagase ChemteX Corporation) aliphatic polyglycidyl ether compounds, and the like.
エポキシ基を2個以上含有する化合物としては、エポリードGT-401、エポリードGT-403、エポリードGT-301、エポリードGT-302、セロキサイド2021、セロキサイド3000(以上、(株)ダイセル製)等のシクロヘキセン構造を有するエポキシ化合物;エピコート1001、エピコート1002、エピコート1003、エピコート1004、エピコート1007、エピコート1009、エピコート1010、エピコート828(以上、ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ)製)等のビスフェノールA型エポキシ化合物;エピコート807(ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ))製)等のビスフェノールF型エポキシ化合物;エピコート152、エピコート154(以上、ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ)製)、EPPN201、EPPN202(以上、日本化薬(株)製)等のフェノールノボラック型エポキシ化合物;ECON-102、ECON-103S、ECON-104S、ECON-1020、ECON-1025、ECON-1027(以上、日本化薬(株)製)、エピコート180S75(ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ)製)等のクレゾールノボラック型エポキシ化合物;V8000-C7(DIC(株)製)等のナフタレン型エポキシ化合物;デナコールEX-252(ナガセケムテックス(株)製)、CY175、CY177、CY179、アラルダイトCY-182、アラルダイトCY-192、アラルダイトCY-184(以上、BASF社製)、エピクロン200、エピクロン400(以上、DIC(株)製)、エピコート871、エピコート872(以上、ジャパンエポキシレジン(株)(現 三菱化学(株)、現 jER(登録商標)シリーズ)製)、ED-5661、ED-5662(以上、セラニーズコーティング(株)製)等の脂環式エポキシ化合物;デナコールEX-611、デナコールEX-612、デナコールEX-614、デナコールEX-622、デナコールEX-411、デナコールEX-512、デナコールEX-522、デナコールEX-421、デナコールEX-313、デナコールEX-314、デナコールEX-312(以上、ナガセケムテックス(株)製)等の脂肪族ポリグリシジルエーテル化合物が挙げられる。 Although the specific example of a crosslinkable compound is given to the following, it is not limited to this.
Examples of the compound containing two or more epoxy groups include cyclohexene structures such as epolide GT-401, epolide GT-403, epolide GT-301, epolide GT-302, ceroxide 2021, and ceroxide 3000 (manufactured by Daicel Corporation). Epicoat 1001, Epicoat 1002, Epicoat 1003, Epicoat 1004, Epicoat 1007, Epicoat 1009, Epicoat 1010, Epicoat 828 (above, Japan Epoxy Resin Co., Ltd. (currently Mitsubishi Chemical Corporation, presently jER) Bisphenol A type epoxy compounds such as Epicote 807 (manufactured by Japan Epoxy Resin Co., Ltd. (currently Mitsubishi Chemical Corporation, presently jER (registered trademark) series))) Poxy compounds; Epicoat 152, Epicoat 154 (above, Japan Epoxy Resin Co., Ltd. (currently Mitsubishi Chemical Corporation, presently jER (registered trademark) series)), EPPN201, EPPN202 (above, Nippon Kayaku Co., Ltd.) Phenol novolac type epoxy compounds such as ECON-102, ECON-103S, ECON-104S, ECON-1020, ECON-1025, ECON-1027 (above, manufactured by Nippon Kayaku Co., Ltd.), Epicort 180S75 (Japan Epoxy Resin ( Cresol novolac type epoxy compounds such as (manufactured by Mitsubishi Chemical Corporation, currently jER (registered trademark) series); naphthalene type epoxy compounds such as V8000-C7 (manufactured by DIC Corporation); Denacol EX-252 ( Nagase ChemteX Corporation), CY175, C Y177, CY179, Araldite CY-182, Araldite CY-192, Araldite CY-184 (above, manufactured by BASF), Epicron 200, Epicron 400 (above, manufactured by DIC Corporation), Epicoat 871, Epicoat 872 (above, Japan) Cycloaliphatic epoxy compounds such as Epoxy Resin Co., Ltd. (currently Mitsubishi Chemical Co., Ltd., present jER (registered trademark) series), ED-5661, ED-5661 (above, Celanese Coating Co., Ltd.); Denacol EX-611, Denacol EX-612, Denacol EX-614, Denacol EX-622, Denacol EX-411, Denacol EX-512, Denacol EX-522, Denacol EX-421, Denacol EX-313, Denacol EX-314, Denacor EX-31 (Above, manufactured by Nagase ChemteX Corporation) aliphatic polyglycidyl ether compounds, and the like.
アミノ基の水素原子がメチロール基、アルコキシメチル基又はその両方で置換された基を有するメラミン誘導体、ベンゾグアナミン誘導体又はグリコールウリルとしては、トリアジン環1個当たりメトキシメチル基が平均3.7個置換されているMX-750、トリアジン環1個当たりメトキシメチル基が平均5.8個置換されているMW-30(以上、(株)三和ケミカル製);サイメル300、サイメル301、サイメル303、サイメル350、サイメル370、サイメル771、サイメル325、サイメル327、サイメル703、サイメル712等のメトキシメチル化メラミン;サイメル235、サイメル236、サイメル238、サイメル212、サイメル253、サイメル254等のメトキシメチル化ブトキシメチル化メラミン;サイメル506、サイメル508等のブトキシメチル化メラミン;サイメル1141のようなカルボキシル基含有メトキシメチル化イソブトキシメチル化メラミン;サイメル1123のようなメトキシメチル化エトキシメチル化ベンゾグアナミン;サイメル1123-10のようなメトキシメチル化ブトキシメチル化ベンゾグアナミン;サイメル1128のようなブトキシメチル化ベンゾグアナミン;サイメル1125-80のようなカルボキシル基含有メトキシメチル化エトキシメチル化ベンゾグアナミン;サイメル1170のようなブトキシメチル化グリコールウリル;サイメル1172のようなメチロール化グリコールウリル(以上、三井サイアナミッド(株)(現 サイテック インダストリーズ社)製)等が挙げられる。
As the melamine derivative, benzoguanamine derivative or glycoluril having a group in which the hydrogen atom of the amino group is substituted with a methylol group, an alkoxymethyl group or both, an average of 3.7 methoxymethyl groups are substituted per triazine ring. MX-750, MW-30 substituted with an average of 5.8 methoxymethyl groups per triazine ring (above, manufactured by Sanwa Chemical Co., Ltd.); Cymel 300, Cymel 301, Cymel 303, Cymel 350, Methoxymethylated melamines such as Cymel 370, Cymel 771, Cymel 325, Cymel 327, Cymel 703, Cymel 712, etc .; Butoxymethylated melamines such as Cymel 506 and Cymel 508; carboxyl group-containing methoxymethylated isobutoxymethylated melamines such as Cymel 1141; methoxymethylated ethoxymethylated benzoguanamines such as Cymel 1123; Methoxymethylated butoxymethylated benzoguanamine; butoxymethylated benzoguanamine such as Cymel 1128; carboxyl-containing methoxymethylated ethoxymethylated benzoguanamine such as Cymel 1125-80; butoxymethylated glycoluril such as Cymel 1170; Cymel 1172 Such as methylolated glycoluril (made by Mitsui Cyanamid Co., Ltd. (currently Cytec Industries)).
本発明のディスプレイ基板用樹脂組成物において、架橋剤を使用する場合のその含有量は特に限定されないが、樹脂組成物の保存安定性をより向上させる観点から、ポリアミック酸又はポリイミド100質量部に対して、20質量部以下で含まれることが好ましく、本発明の樹脂組成物から得られる硬化膜が十分に低い線膨張係数を有するものとする観点から、15質量部以下で含まれることがより好ましい。
In the resin composition for display substrates of the present invention, the content in the case of using a crosslinking agent is not particularly limited, but from the viewpoint of further improving the storage stability of the resin composition, relative to 100 parts by mass of polyamic acid or polyimide From the viewpoint that the cured film obtained from the resin composition of the present invention has a sufficiently low linear expansion coefficient, it is more preferably contained at 15 parts by mass or less. .
[ワニス及び硬化膜]
本発明のディスプレイ基板用樹脂組成物からなる硬化膜を形成する具体的な方法としては、まず、樹脂組成物を溶剤に溶解又は分散してワニスの形態(膜形成材料)とし、該ワニスを基板上にキャストコート法、スピンコート法、ブレードコート法、ディップコート法、ロールコート法、バーコート法、ダイコート法、インクジェット法、印刷法(凸版、凹版、平版、スクリーン印刷等)等によって塗布して塗膜(薄膜)を得る。そして得られた塗膜を、ホットプレート、オーブン等で焼成することにより硬化膜が形成される。焼成温度としては、通常100乃至500℃、好ましくは120乃至450℃である。焼成は温度を変えて複数回行なってもよい。また雰囲気は窒素または減圧下が好ましい。
また前記基板としては、例えば、プラスチック(ポリカーボネート、ポリメタクリレート、ポリスチレン、ポリエステル、ポリオレフィン、エポキシ、メラミン、トリアセチルセルロース、ABS、AS、ノルボルネン系樹脂等)、金属、木材、紙、ガラス、スレート等を挙げることができる。 [Varnish and cured film]
As a specific method for forming a cured film comprising the resin composition for a display substrate of the present invention, first, the resin composition is dissolved or dispersed in a solvent to form a varnish (film forming material), and the varnish is used as a substrate. Apply by cast coating method, spin coating method, blade coating method, dip coating method, roll coating method, bar coating method, die coating method, ink jet method, printing method (letter plate, intaglio plate, planographic plate, screen printing, etc.) A coating film (thin film) is obtained. And the cured film is formed by baking the obtained coating film with a hotplate, oven, etc. FIG. The firing temperature is usually 100 to 500 ° C, preferably 120 to 450 ° C. Firing may be performed a plurality of times at different temperatures. The atmosphere is preferably nitrogen or under reduced pressure.
Examples of the substrate include plastic (polycarbonate, polymethacrylate, polystyrene, polyester, polyolefin, epoxy, melamine, triacetyl cellulose, ABS, AS, norbornene resin, etc.), metal, wood, paper, glass, slate, and the like. Can be mentioned.
本発明のディスプレイ基板用樹脂組成物からなる硬化膜を形成する具体的な方法としては、まず、樹脂組成物を溶剤に溶解又は分散してワニスの形態(膜形成材料)とし、該ワニスを基板上にキャストコート法、スピンコート法、ブレードコート法、ディップコート法、ロールコート法、バーコート法、ダイコート法、インクジェット法、印刷法(凸版、凹版、平版、スクリーン印刷等)等によって塗布して塗膜(薄膜)を得る。そして得られた塗膜を、ホットプレート、オーブン等で焼成することにより硬化膜が形成される。焼成温度としては、通常100乃至500℃、好ましくは120乃至450℃である。焼成は温度を変えて複数回行なってもよい。また雰囲気は窒素または減圧下が好ましい。
また前記基板としては、例えば、プラスチック(ポリカーボネート、ポリメタクリレート、ポリスチレン、ポリエステル、ポリオレフィン、エポキシ、メラミン、トリアセチルセルロース、ABS、AS、ノルボルネン系樹脂等)、金属、木材、紙、ガラス、スレート等を挙げることができる。 [Varnish and cured film]
As a specific method for forming a cured film comprising the resin composition for a display substrate of the present invention, first, the resin composition is dissolved or dispersed in a solvent to form a varnish (film forming material), and the varnish is used as a substrate. Apply by cast coating method, spin coating method, blade coating method, dip coating method, roll coating method, bar coating method, die coating method, ink jet method, printing method (letter plate, intaglio plate, planographic plate, screen printing, etc.) A coating film (thin film) is obtained. And the cured film is formed by baking the obtained coating film with a hotplate, oven, etc. FIG. The firing temperature is usually 100 to 500 ° C, preferably 120 to 450 ° C. Firing may be performed a plurality of times at different temperatures. The atmosphere is preferably nitrogen or under reduced pressure.
Examples of the substrate include plastic (polycarbonate, polymethacrylate, polystyrene, polyester, polyolefin, epoxy, melamine, triacetyl cellulose, ABS, AS, norbornene resin, etc.), metal, wood, paper, glass, slate, and the like. Can be mentioned.
前記ワニスの形態において使用する溶剤としては、ディスプレイ基板用樹脂組成物を溶解させるものであれば特に限定されないが、例えば、上記ポリアミック酸の重合反応において使用される溶剤や、ポリアミック酸やポリイミドの再溶解に使用可能な溶剤として挙げた溶剤等が挙げられる。これら溶剤は単独で又は2種以上を組み合わせて使用してもよい。
また上記溶剤に樹脂組成物を溶解又は分散させる濃度は任意であるが、ディスプレイ基板用樹脂組成物と溶剤の総質量(合計質量、すなわちワニスの総質量)に対して、ディスプレイ基板用樹脂組成物の固形分の濃度は5乃至50質量%であり、樹脂組成物の保存安定性をより向上させる観点から好ましくは10乃至40質量%であり、樹脂組成物をより均一に塗布させる観点からより好ましくは10乃至30質量%である。
上記ワニスの粘度は0.001乃至5,000Pa・sであることが好ましい。
ディスプレイ基板用樹脂組成物或いは該組成物から得られるワニスから形成される硬化膜の厚さは特に限定されないが、例えば1乃至200μm、通常1乃至100μm、好ましくは5乃至60μmである。 The solvent used in the form of the varnish is not particularly limited as long as it dissolves the resin composition for display substrates. For example, the solvent used in the polymerization reaction of the polyamic acid, or the re-use of polyamic acid or polyimide is used. The solvent etc. which were mentioned as a solvent which can be used for dissolution are mentioned. These solvents may be used alone or in combination of two or more.
Moreover, the density | concentration which dissolves or disperses | distributes a resin composition to the said solvent is arbitrary, However, The resin composition for display substrates with respect to the resin composition for display substrates and the total mass (total mass, ie, total mass of varnish) The solid content is 5 to 50% by mass, preferably 10 to 40% by mass from the viewpoint of further improving the storage stability of the resin composition, and more preferably from the viewpoint of more uniformly applying the resin composition. Is 10 to 30% by mass.
The varnish preferably has a viscosity of 0.001 to 5,000 Pa · s.
Although the thickness of the cured film formed from the resin composition for display substrates or the varnish obtained from this composition is not specifically limited, For example, it is 1 to 200 micrometers, Usually 1 to 100 micrometers, Preferably it is 5 to 60 micrometers.
また上記溶剤に樹脂組成物を溶解又は分散させる濃度は任意であるが、ディスプレイ基板用樹脂組成物と溶剤の総質量(合計質量、すなわちワニスの総質量)に対して、ディスプレイ基板用樹脂組成物の固形分の濃度は5乃至50質量%であり、樹脂組成物の保存安定性をより向上させる観点から好ましくは10乃至40質量%であり、樹脂組成物をより均一に塗布させる観点からより好ましくは10乃至30質量%である。
上記ワニスの粘度は0.001乃至5,000Pa・sであることが好ましい。
ディスプレイ基板用樹脂組成物或いは該組成物から得られるワニスから形成される硬化膜の厚さは特に限定されないが、例えば1乃至200μm、通常1乃至100μm、好ましくは5乃至60μmである。 The solvent used in the form of the varnish is not particularly limited as long as it dissolves the resin composition for display substrates. For example, the solvent used in the polymerization reaction of the polyamic acid, or the re-use of polyamic acid or polyimide is used. The solvent etc. which were mentioned as a solvent which can be used for dissolution are mentioned. These solvents may be used alone or in combination of two or more.
Moreover, the density | concentration which dissolves or disperses | distributes a resin composition to the said solvent is arbitrary, However, The resin composition for display substrates with respect to the resin composition for display substrates and the total mass (total mass, ie, total mass of varnish) The solid content is 5 to 50% by mass, preferably 10 to 40% by mass from the viewpoint of further improving the storage stability of the resin composition, and more preferably from the viewpoint of more uniformly applying the resin composition. Is 10 to 30% by mass.
The varnish preferably has a viscosity of 0.001 to 5,000 Pa · s.
Although the thickness of the cured film formed from the resin composition for display substrates or the varnish obtained from this composition is not specifically limited, For example, it is 1 to 200 micrometers, Usually 1 to 100 micrometers, Preferably it is 5 to 60 micrometers.
以下、実施例を挙げて、本発明をより具体的に説明するが、本発明は下記の実施例に限定されるものではない。
Hereinafter, the present invention will be described more specifically with reference to examples. However, the present invention is not limited to the following examples.
[数平均分子量及び重量平均分子量の測定]
ポリマーの重量平均分子量(以下、Mwと略す。)と分子量分布は、東ソー(株)製GPC装置(Shodex[登録商標]カラムSB803HQ及びSB804HQ)を用い、溶出溶媒としてジメチルホルムアミドを流量0.9mL/分、カラム温度40℃の条件で測定した。なお、Mwはポリスチレン換算値とした。 [Measurement of number average molecular weight and weight average molecular weight]
The weight average molecular weight (hereinafter abbreviated as Mw) and the molecular weight distribution of the polymer were measured using a GPC apparatus (Shodex [registered trademark] columns SB803HQ and SB804HQ) manufactured by Tosoh Corporation, with dimethylformamide as the elution solvent at a flow rate of 0.9 mL / Minutes and column temperature of 40 ° C. In addition, Mw was made into the polystyrene conversion value.
ポリマーの重量平均分子量(以下、Mwと略す。)と分子量分布は、東ソー(株)製GPC装置(Shodex[登録商標]カラムSB803HQ及びSB804HQ)を用い、溶出溶媒としてジメチルホルムアミドを流量0.9mL/分、カラム温度40℃の条件で測定した。なお、Mwはポリスチレン換算値とした。 [Measurement of number average molecular weight and weight average molecular weight]
The weight average molecular weight (hereinafter abbreviated as Mw) and the molecular weight distribution of the polymer were measured using a GPC apparatus (Shodex [registered trademark] columns SB803HQ and SB804HQ) manufactured by Tosoh Corporation, with dimethylformamide as the elution solvent at a flow rate of 0.9 mL / Minutes and column temperature of 40 ° C. In addition, Mw was made into the polystyrene conversion value.
[ポリアミック酸及びディスプレイ基板用樹脂組成物(ワニス)の合成]
<実施例1>
ビス[4-(3-アミノフェノキシ)フェニル]スルホン 3.51g(0.0081モル)とビス(3-アミノフェニル)スルホン 0.504g(0.0020モル)をN-メチル-2-ピロリドン 14.0gに溶解し、1,2,3,4-シクロブタンテトラカルボン酸二無水物 1.99g(0.010モル)を添加した後、窒素雰囲気下、23℃で24時間反応させた。得られたポリマーのMwは28,700、分子量分布は2.1であった。得られた反応溶液を、そのまま、実施例1のディスプレイ基板用樹脂組成物とした。 [Synthesis of polyamic acid and resin composition for display substrate (varnish)]
<Example 1>
13. N-methyl-2-pyrrolidone containing 3.51 g (0.0081 mol) of bis [4- (3-aminophenoxy) phenyl] sulfone and 0.504 g (0.0020 mol) of bis (3-aminophenyl) sulfone After dissolving in 0 g and adding 1.99 g (0.010 mol) of 1,2,3,4-cyclobutanetetracarboxylic dianhydride, the mixture was reacted at 23 ° C. for 24 hours in a nitrogen atmosphere. The obtained polymer had Mw of 28,700 and a molecular weight distribution of 2.1. The obtained reaction solution was used as the resin composition for display substrate of Example 1 as it was.
<実施例1>
ビス[4-(3-アミノフェノキシ)フェニル]スルホン 3.51g(0.0081モル)とビス(3-アミノフェニル)スルホン 0.504g(0.0020モル)をN-メチル-2-ピロリドン 14.0gに溶解し、1,2,3,4-シクロブタンテトラカルボン酸二無水物 1.99g(0.010モル)を添加した後、窒素雰囲気下、23℃で24時間反応させた。得られたポリマーのMwは28,700、分子量分布は2.1であった。得られた反応溶液を、そのまま、実施例1のディスプレイ基板用樹脂組成物とした。 [Synthesis of polyamic acid and resin composition for display substrate (varnish)]
<Example 1>
13. N-methyl-2-pyrrolidone containing 3.51 g (0.0081 mol) of bis [4- (3-aminophenoxy) phenyl] sulfone and 0.504 g (0.0020 mol) of bis (3-aminophenyl) sulfone After dissolving in 0 g and adding 1.99 g (0.010 mol) of 1,2,3,4-cyclobutanetetracarboxylic dianhydride, the mixture was reacted at 23 ° C. for 24 hours in a nitrogen atmosphere. The obtained polymer had Mw of 28,700 and a molecular weight distribution of 2.1. The obtained reaction solution was used as the resin composition for display substrate of Example 1 as it was.
<比較例1>
ビス[4-(3-アミノフェノキシ)フェニル]スルホン 4.13g(0.0095モル)をN-メチル-2-ピロリドン 14.0gに溶解し、1,2,3,4-シクロブタンテトラカルボン酸二無水物 1.87g(0.0095モル)を添加した後、窒素雰囲気下、23℃で24時間反応させた。得られたポリマーのMwは19,800、分子量分布は2.1であった。得られた反応溶液を、そのまま、比較例1のディスプレイ基板用樹脂組成物とした。 <Comparative Example 1>
Bis [4- (3-aminophenoxy) phenyl] sulfone (4.13 g, 0.0095 mol) was dissolved in N-methyl-2-pyrrolidone (14.0 g), and 1,2,3,4-cyclobutanetetracarboxylic acid After adding 1.87 g (0.0095 mol) of anhydride, the mixture was reacted at 23 ° C. for 24 hours under a nitrogen atmosphere. Mw of the obtained polymer was 19,800 and molecular weight distribution was 2.1. The obtained reaction solution was used as the display substrate resin composition of Comparative Example 1 as it was.
ビス[4-(3-アミノフェノキシ)フェニル]スルホン 4.13g(0.0095モル)をN-メチル-2-ピロリドン 14.0gに溶解し、1,2,3,4-シクロブタンテトラカルボン酸二無水物 1.87g(0.0095モル)を添加した後、窒素雰囲気下、23℃で24時間反応させた。得られたポリマーのMwは19,800、分子量分布は2.1であった。得られた反応溶液を、そのまま、比較例1のディスプレイ基板用樹脂組成物とした。 <Comparative Example 1>
Bis [4- (3-aminophenoxy) phenyl] sulfone (4.13 g, 0.0095 mol) was dissolved in N-methyl-2-pyrrolidone (14.0 g), and 1,2,3,4-cyclobutanetetracarboxylic acid After adding 1.87 g (0.0095 mol) of anhydride, the mixture was reacted at 23 ° C. for 24 hours under a nitrogen atmosphere. Mw of the obtained polymer was 19,800 and molecular weight distribution was 2.1. The obtained reaction solution was used as the display substrate resin composition of Comparative Example 1 as it was.
<比較例2>
ビス(3-アミノフェニル)スルホン 3.35g(0.013モル)をN-メチル-2-ピロリドン 14.0gに溶解し、1,2,3,4-シクロブタンテトラカルボン酸二無水物 2.65g(0.014モル)を添加した後、窒素雰囲気下、23℃で24時間反応させた。得られたポリマーのMwは13,600、分子量分布は3.1であった。得られた反応溶液を、そのまま、比較例2のディスプレイ基板用樹脂組成物とした。 <Comparative example 2>
2.35 g (0.013 mol) of bis (3-aminophenyl) sulfone was dissolved in 14.0 g of N-methyl-2-pyrrolidone, and 2.65 g of 1,2,3,4-cyclobutanetetracarboxylic dianhydride. (0.014 mol) was added, and the mixture was reacted at 23 ° C. for 24 hours under a nitrogen atmosphere. Mw of the obtained polymer was 13,600 and molecular weight distribution was 3.1. The obtained reaction solution was used as the resin composition for display substrate of Comparative Example 2 as it was.
ビス(3-アミノフェニル)スルホン 3.35g(0.013モル)をN-メチル-2-ピロリドン 14.0gに溶解し、1,2,3,4-シクロブタンテトラカルボン酸二無水物 2.65g(0.014モル)を添加した後、窒素雰囲気下、23℃で24時間反応させた。得られたポリマーのMwは13,600、分子量分布は3.1であった。得られた反応溶液を、そのまま、比較例2のディスプレイ基板用樹脂組成物とした。 <Comparative example 2>
2.35 g (0.013 mol) of bis (3-aminophenyl) sulfone was dissolved in 14.0 g of N-methyl-2-pyrrolidone, and 2.65 g of 1,2,3,4-cyclobutanetetracarboxylic dianhydride. (0.014 mol) was added, and the mixture was reacted at 23 ° C. for 24 hours under a nitrogen atmosphere. Mw of the obtained polymer was 13,600 and molecular weight distribution was 3.1. The obtained reaction solution was used as the resin composition for display substrate of Comparative Example 2 as it was.
[耐熱性評価]
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。こうして得られた硬化膜について、TG-DTA(ブルカーエイエックスエス社製、TG/DTA2000SA)を用いて、50℃から500℃まで10℃/分にて昇温し、熱分解による5質量%の質量減少を生ずる温度を求め、耐熱性を評価した。結果を表1に示す。 [Heat resistance evaluation]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. The cured film thus obtained was heated from 50 ° C. to 500 ° C. at 10 ° C./min using TG-DTA (manufactured by Bruker AXS Co., Ltd., TG / DTA 2000SA), and 5 mass% by thermal decomposition. The temperature causing mass reduction was determined and the heat resistance was evaluated. The results are shown in Table 1.
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。こうして得られた硬化膜について、TG-DTA(ブルカーエイエックスエス社製、TG/DTA2000SA)を用いて、50℃から500℃まで10℃/分にて昇温し、熱分解による5質量%の質量減少を生ずる温度を求め、耐熱性を評価した。結果を表1に示す。 [Heat resistance evaluation]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. The cured film thus obtained was heated from 50 ° C. to 500 ° C. at 10 ° C./min using TG-DTA (manufactured by Bruker AXS Co., Ltd., TG / DTA 2000SA), and 5 mass% by thermal decomposition. The temperature causing mass reduction was determined and the heat resistance was evaluated. The results are shown in Table 1.
表1に示すように、実施例1のディスプレイ基板用樹脂組成物を用いて作製した硬化膜は、比較例1及び比較例2のディスプレイ基板用樹脂組成物を用いて作製した硬化膜より高い耐熱性を示した。
As shown in Table 1, the cured film produced using the display substrate resin composition of Example 1 has higher heat resistance than the cured films produced using the display substrate resin compositions of Comparative Example 1 and Comparative Example 2. Showed sex.
[自己支持性評価]
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。こうして得られた硬化膜について、ガラス基板から当該膜を剥離する際、及び剥離したフィルムを手で曲げたり引っ張ったりしたときのフィルムの壊れやすさ(クラック、ひび、破れなど)を目視で確認することにより、自己支持性を評価した。得られた結果を表2に示す。 [Self-supporting evaluation]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. For the cured film thus obtained, visually check the fragility (crack, crack, tear, etc.) of the film when the film is peeled from the glass substrate and when the peeled film is bent or pulled by hand. The self-supporting property was evaluated. The obtained results are shown in Table 2.
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。こうして得られた硬化膜について、ガラス基板から当該膜を剥離する際、及び剥離したフィルムを手で曲げたり引っ張ったりしたときのフィルムの壊れやすさ(クラック、ひび、破れなど)を目視で確認することにより、自己支持性を評価した。得られた結果を表2に示す。 [Self-supporting evaluation]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. For the cured film thus obtained, visually check the fragility (crack, crack, tear, etc.) of the film when the film is peeled from the glass substrate and when the peeled film is bent or pulled by hand. The self-supporting property was evaluated. The obtained results are shown in Table 2.
表2に示すように、比較例2のディスプレイ基板用樹脂組成物より得られた硬化膜は部分的に白濁が確認でき、さらにフィルムが脆く、ガラス基板から剥離する際にフィルムにクラック、破れが生じ、また剥離したフィルムも割れやすいため、ディスプレイ基板として膜強度が不十分であるとする結果となった。
一方、実施例1のディスプレイ基板用樹脂組成物を用いて作製した硬化膜は、ガラス基板から剥離する際にフィルムにクラック、破れが生じにくく、また、剥離したフィルムも割れにくく膜強度が十分高く、厚さ30μmの均一な自己支持性を有する膜を形成することができた。 As shown in Table 2, the cured film obtained from the resin composition for display substrate of Comparative Example 2 can be confirmed to be partially cloudy, and the film is brittle, and the film is cracked and torn when peeled from the glass substrate. As a result, the peeled film was easily broken, and the film strength as a display substrate was insufficient.
On the other hand, the cured film produced using the resin composition for display substrate of Example 1 is less susceptible to cracking and tearing when peeled from the glass substrate, and the peeled film is also difficult to break and has a sufficiently high film strength. A film having a uniform self-supporting property having a thickness of 30 μm could be formed.
一方、実施例1のディスプレイ基板用樹脂組成物を用いて作製した硬化膜は、ガラス基板から剥離する際にフィルムにクラック、破れが生じにくく、また、剥離したフィルムも割れにくく膜強度が十分高く、厚さ30μmの均一な自己支持性を有する膜を形成することができた。 As shown in Table 2, the cured film obtained from the resin composition for display substrate of Comparative Example 2 can be confirmed to be partially cloudy, and the film is brittle, and the film is cracked and torn when peeled from the glass substrate. As a result, the peeled film was easily broken, and the film strength as a display substrate was insufficient.
On the other hand, the cured film produced using the resin composition for display substrate of Example 1 is less susceptible to cracking and tearing when peeled from the glass substrate, and the peeled film is also difficult to break and has a sufficiently high film strength. A film having a uniform self-supporting property having a thickness of 30 μm could be formed.
[線膨張係数評価]
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。こうして得られた硬化膜について、TMA-4000SA(ブルカー・エイエックスエス社製)を用いて、50℃から300℃まで10℃/分の条件で昇温して50℃~250℃の線膨張係数を測定した。得られた結果を表3に示す。 [Evaluation of linear expansion coefficient]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. The cured film thus obtained was heated at 50 ° C. to 300 ° C. at a rate of 10 ° C./min using TMA-4000SA (manufactured by Bruker AXS Co., Ltd.), and a linear expansion coefficient of 50 ° C. to 250 ° C. Was measured. The obtained results are shown in Table 3.
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。こうして得られた硬化膜について、TMA-4000SA(ブルカー・エイエックスエス社製)を用いて、50℃から300℃まで10℃/分の条件で昇温して50℃~250℃の線膨張係数を測定した。得られた結果を表3に示す。 [Evaluation of linear expansion coefficient]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. The cured film thus obtained was heated at 50 ° C. to 300 ° C. at a rate of 10 ° C./min using TMA-4000SA (manufactured by Bruker AXS Co., Ltd.), and a linear expansion coefficient of 50 ° C. to 250 ° C. Was measured. The obtained results are shown in Table 3.
表3に示すように、実施例1のディスプレイ基板用樹脂組成物を用いて作製した硬化膜は、比較例1より得られた硬化膜と比べて線膨張係数の値が同程度乃至やや低いとする結果となり、ディスプレイ基板として適度な線膨張係数を示した。これに対し、比較例2は前述したとおり自己支持膜が得られず、線膨張係数の測定にいたらなかった。
As shown in Table 3, when the cured film produced using the display substrate resin composition of Example 1 had a value of the linear expansion coefficient comparable to or slightly lower than that of the cured film obtained from Comparative Example 1. As a result, an appropriate linear expansion coefficient as a display substrate was shown. On the other hand, as described above, the self-supporting film was not obtained in Comparative Example 2, and the linear expansion coefficient was not measured.
[複屈折評価]
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。得られた硬化膜の膜厚は何れも30μmであった。こうして得られた硬化膜の複屈折について、プリズムカプラ(METRICON社製、MODEL2010)を用いて測定した。その結果を表4に示す。 [Evaluation of birefringence]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. The film thicknesses of the obtained cured films were all 30 μm. The birefringence of the cured film thus obtained was measured using a prism coupler (manufactured by METRICON, MODEL 2010). The results are shown in Table 4.
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。得られた硬化膜の膜厚は何れも30μmであった。こうして得られた硬化膜の複屈折について、プリズムカプラ(METRICON社製、MODEL2010)を用いて測定した。その結果を表4に示す。 [Evaluation of birefringence]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. The film thicknesses of the obtained cured films were all 30 μm. The birefringence of the cured film thus obtained was measured using a prism coupler (manufactured by METRICON, MODEL 2010). The results are shown in Table 4.
表4に示すように、実施例1のディスプレイ基板用樹脂組成物を用いて作製した硬化膜は、ディスプレイ基板用フィルムとして十分に低い複屈折を示した。
As shown in Table 4, the cured film produced using the display substrate resin composition of Example 1 exhibited sufficiently low birefringence as a display substrate film.
[透明性評価]
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。得られた硬化膜の膜厚は何れも30μmであった。こうして得られた硬化膜の透明性について、全光透過率をヘーズメーター(日本電色工業社製、NDH 5000)を用いて測定した。その結果を表5に示す。 [Transparency evaluation]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. The film thicknesses of the obtained cured films were all 30 μm. With respect to the transparency of the cured film thus obtained, the total light transmittance was measured using a haze meter (NDH 5000, manufactured by Nippon Denshoku Industries Co., Ltd.). The results are shown in Table 5.
実施例1及び比較例1乃至2のディスプレイ基板用樹脂組成物をドクターブレードでガラス基板上に塗布し、120℃で10分間、続いて180℃で10分間、続いて240℃で60分間、減圧下でベークを行って硬化膜を得た。得られた硬化膜の膜厚は何れも30μmであった。こうして得られた硬化膜の透明性について、全光透過率をヘーズメーター(日本電色工業社製、NDH 5000)を用いて測定した。その結果を表5に示す。 [Transparency evaluation]
The resin compositions for display substrates of Example 1 and Comparative Examples 1 and 2 were applied onto a glass substrate with a doctor blade, and the pressure was reduced at 120 ° C. for 10 minutes, then at 180 ° C. for 10 minutes, and subsequently at 240 ° C. for 60 minutes. Baking was performed below to obtain a cured film. The film thicknesses of the obtained cured films were all 30 μm. With respect to the transparency of the cured film thus obtained, the total light transmittance was measured using a haze meter (NDH 5000, manufactured by Nippon Denshoku Industries Co., Ltd.). The results are shown in Table 5.
表5に示すように、実施例1のディスプレイ基板用樹脂組成物を用いて作製した硬化膜は、ディスプレイ基板用フィルムとして十分に高い透明性を示した。
As shown in Table 5, the cured film produced using the display substrate resin composition of Example 1 exhibited sufficiently high transparency as a display substrate film.
Claims (19)
- 下記式(1)で表される構造単位及び式(3)で表される構造単位を含むポリアミック酸又は下記式(2)で表される構造単位及び式(4)で表される構造単位を含むポリイミドを含有するディスプレイ基板用樹脂組成物。
X1は4価の芳香族基又は4価の脂肪族基を表し、
Y1及びY2は互いに異なって、2価の芳香族基又は2価の脂肪族基を表し、
n及びmは自然数を表す。] A structural unit represented by the following formula (1) and a polyamic acid containing a structural unit represented by the formula (3) or a structural unit represented by the following formula (2) and a structural unit represented by the formula (4) The resin composition for display substrates containing the containing polyimide.
X 1 represents a tetravalent aromatic group or a tetravalent aliphatic group,
Y 1 and Y 2 are different from each other and represent a divalent aromatic group or a divalent aliphatic group,
n and m represent natural numbers. ] - 前記Y1が下記式(6)で表される2価の基を表し、Y2が下記式(7)で表される2価の基を表す、請求項1に記載のディスプレイ基板用樹脂組成物。
- 前記式(1)中のnと前記式(3)中のmが、n/(n+m)≧0.2の関係式を満たす、請求項1乃至請求項3のうちいずれか1つに記載のディスプレイ基板用樹脂組成物。 4. The n according to claim 1, wherein n in the formula (1) and m in the formula (3) satisfy a relational expression of n / (n + m) ≧ 0.2. Resin composition for display substrate.
- 前記式(2)中のnと前記式(4)中のmが、n/(n+m)≧0.2の関係式を満たす、請求項1乃至請求項3のうちいずれか1つに記載のディスプレイ基板用樹脂組成物。 The n in said Formula (2) and m in said Formula (4) satisfy | fill the relational expression of n / (n + m)> = 0.2, It is any one of Claim 1 thru | or 3 characterized by the above-mentioned. Resin composition for display substrate.
- さらに架橋剤を含む、請求項1乃至請求項5のうちいずれか1項に記載のディスプレイ基板用樹脂組成物。 Furthermore, the resin composition for display substrates of any one of Claims 1 thru | or 5 containing a crosslinking agent.
- 前記ポリアミック酸又はポリイミド100質量部に対して、前記架橋剤が20質量部以下で含まれる、請求項6に記載のディスプレイ基板用樹脂組成物。 The resin composition for display substrates according to claim 6, wherein the crosslinking agent is contained in an amount of 20 parts by mass or less with respect to 100 parts by mass of the polyamic acid or polyimide.
- 請求項1乃至請求項7のうちいずれか1つに記載のディスプレイ基板用樹脂組成物が少なくとも1種の溶剤に溶解していることを特徴とする、ワニス。 A varnish, wherein the display substrate resin composition according to any one of claims 1 to 7 is dissolved in at least one solvent.
- 0.001乃至5,000Pa・sの粘度を有する、請求項8に記載のワニス。 The varnish according to claim 8, having a viscosity of 0.001 to 5,000 Pa · s.
- 請求項8又は請求項9に記載のワニスを用いて得られる薄膜を100℃乃至450℃の範囲内の適当な温度で焼成することにより得られる、硬化膜。 The cured film obtained by baking the thin film obtained using the varnish of Claim 8 or Claim 9 at the suitable temperature within the range of 100 to 450 degreeC.
- 1.0μm乃至200μmの膜厚を有する、請求項10に記載の硬化膜。 The cured film according to claim 10, having a film thickness of 1.0 μm to 200 μm.
- 自己支持性を有する、請求項10又は請求項11に記載の硬化膜。 The cured film according to claim 10 or 11, which has self-supporting property.
- 基板上に請求項10乃至請求項12のうちいずれか一項に記載の硬化膜からなる層を少なくとも一層備える、構造体。 A structure comprising at least one layer formed of the cured film according to any one of claims 10 to 12 on a substrate.
- 下記式(1)で表される構造単位及び式(3)で表される構造単位を含むポリアミック酸。
X1は下記(5)式で表される4価の基を表し、
X 1 represents a tetravalent group represented by the following formula (5):
- 下記式(2)で表される構造単位及び式(4)で表される構造単位を含むポリイミド。
X1は下記(5)式で表される4価の基を表し、
X 1 represents a tetravalent group represented by the following formula (5):
- 請求項14に記載のポリアミック酸と架橋剤とを含む組成物。 A composition comprising the polyamic acid according to claim 14 and a crosslinking agent.
- 請求項15に記載のポリイミドと架橋剤とを含む組成物。 A composition comprising the polyimide according to claim 15 and a crosslinking agent.
- 請求項16又は請求項17に記載の組成物が少なくとも1種の溶剤に溶解していることを特徴とする、ワニス。 A varnish, characterized in that the composition according to claim 16 or 17 is dissolved in at least one solvent.
- 請求項18に記載のワニスを用いて得られる薄膜を100℃乃至450℃の範囲内の適当な温度で焼成することにより得られる、硬化膜。 The cured film obtained by baking the thin film obtained using the varnish of Claim 18 at the suitable temperature within the range of 100 to 450 degreeC.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012065705A JP2015110684A (en) | 2012-03-22 | 2012-03-22 | Polyamic acid and polyimide |
| JP2012-065705 | 2012-03-22 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013141239A1 true WO2013141239A1 (en) | 2013-09-26 |
Family
ID=49222696
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/057819 WO2013141239A1 (en) | 2012-03-22 | 2013-03-19 | Polyamic acid and polyimide |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JP2015110684A (en) |
| TW (1) | TW201350522A (en) |
| WO (1) | WO2013141239A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9767853B2 (en) | 2014-07-21 | 2017-09-19 | International Business Machines Corporation | Touch screen video scrolling |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20220016917A (en) * | 2019-06-04 | 2022-02-10 | 가부시키가이샤 가네카 | Polyimide resin and manufacturing method thereof, and polyimide film and manufacturing method thereof |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62235382A (en) * | 1986-04-03 | 1987-10-15 | Hitachi Chem Co Ltd | Thermosetting adhesive film |
| JPH02219827A (en) * | 1989-02-22 | 1990-09-03 | Nissan Chem Ind Ltd | Soluble polyimide resin |
| JPH08120076A (en) * | 1994-10-25 | 1996-05-14 | Central Glass Co Ltd | Polyimide precursor composition, polyimide composition, and production thereof |
| JP2008163210A (en) * | 2006-12-28 | 2008-07-17 | New Japan Chem Co Ltd | New polyimide resin composition and its varnish |
| JP2010513591A (en) * | 2006-12-15 | 2010-04-30 | コーロン インダストリーズ,インコーポレイテッド | Polyimide resin, liquid crystal alignment film and polyimide film using the same |
| WO2011122198A1 (en) * | 2010-03-31 | 2011-10-06 | Jsr株式会社 | Polyimide precursor, resin composition containing said precursor, and method for forming a film using resin composition |
| WO2013077364A1 (en) * | 2011-11-25 | 2013-05-30 | 日産化学工業株式会社 | Resin composition for display substrates |
-
2012
- 2012-03-22 JP JP2012065705A patent/JP2015110684A/en active Pending
-
2013
- 2013-03-19 WO PCT/JP2013/057819 patent/WO2013141239A1/en active Application Filing
- 2013-03-22 TW TW102110281A patent/TW201350522A/en unknown
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62235382A (en) * | 1986-04-03 | 1987-10-15 | Hitachi Chem Co Ltd | Thermosetting adhesive film |
| JPH02219827A (en) * | 1989-02-22 | 1990-09-03 | Nissan Chem Ind Ltd | Soluble polyimide resin |
| JPH08120076A (en) * | 1994-10-25 | 1996-05-14 | Central Glass Co Ltd | Polyimide precursor composition, polyimide composition, and production thereof |
| JP2010513591A (en) * | 2006-12-15 | 2010-04-30 | コーロン インダストリーズ,インコーポレイテッド | Polyimide resin, liquid crystal alignment film and polyimide film using the same |
| JP2008163210A (en) * | 2006-12-28 | 2008-07-17 | New Japan Chem Co Ltd | New polyimide resin composition and its varnish |
| WO2011122198A1 (en) * | 2010-03-31 | 2011-10-06 | Jsr株式会社 | Polyimide precursor, resin composition containing said precursor, and method for forming a film using resin composition |
| WO2013077364A1 (en) * | 2011-11-25 | 2013-05-30 | 日産化学工業株式会社 | Resin composition for display substrates |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9767853B2 (en) | 2014-07-21 | 2017-09-19 | International Business Machines Corporation | Touch screen video scrolling |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201350522A (en) | 2013-12-16 |
| JP2015110684A (en) | 2015-06-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2019188265A1 (en) | Polyamic acid, polyamic acid solution, polyimide, polyimide film, laminate and flexible device, and method for producing polyimide film | |
| JPWO2013133168A1 (en) | Polyamic acid and polyimide | |
| JP6075564B2 (en) | Resin composition for display substrate | |
| CN108690494B (en) | Composition for forming release layer | |
| CN109642026B (en) | Polyamic acid and solution thereof, polyimide and film thereof, laminate, flexible device, and method for producing polyimide film | |
| KR102212979B1 (en) | Resin composition for display substrate, thin resin film for display substrate, and process for producing thin resin film for display substrate | |
| JP6388125B2 (en) | Method for producing resin thin film for display substrate and composition for forming resin thin film for display substrate | |
| JP2013040249A (en) | Resin composition for display substrate | |
| JP6075562B2 (en) | Resin composition for display substrate | |
| WO2014061596A1 (en) | Composition for heat-resistant substrate | |
| JP6115727B2 (en) | Resin composition for display substrate | |
| WO2013141239A1 (en) | Polyamic acid and polyimide | |
| JP2015155483A (en) | resin composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13764800 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13764800 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |